Abstract
Background
Older adults experience considerable muscle and bone loss that are closely interconnected. The efficacy of progressive resistance training programs to concurrently reverse/slow the age-related decline in muscle strength and bone mineral density (BMD) in older adults remains unclear.
Objectives
We aimed to quantify concomitant changes in lower-body muscle strength and BMD in older adults following a progressive resistance training program and to determine how these changes are influenced by mode (resistance only vs. combined resistance and weight-bearing exercises), frequency, volume, load, and program length.
Methods
MEDLINE/PubMed and Embase databases were searched for articles published in English before 1 June, 2021. Randomized controlled trials reporting changes in leg press or knee extension one repetition maximum and femur/hip or lumbar spine BMD following progressive resistance training in men and/or women ≥ 65 years of age were included. A random-effects meta-analysis and meta-regression determined the effects of resistance training and the individual training characteristics on the percent change (∆%) in muscle strength (standardized mean difference) and BMD (mean difference). The quality of the evidence was assessed using the Cochrane risk-of-bias tool (version 2.0) and Grading of Recommendation, Assessment, Development, and Evaluation (GRADE) criteria.
Results
Seven hundred and eighty studies were identified and 14 were included. Progressive resistance training increased muscle strength (∆ standardized mean difference = 1.1%; 95% confidence interval 0.73, 1.47; p ≤ 0.001) and femur/hip BMD (∆ mean difference = 2.77%; 95% confidence interval 0.44, 5.10; p = 0.02), but not BMD of the lumbar spine (∆ mean difference = 1.60%; 95% confidence interval − 1.44, 4.63; p = 0.30). The certainty for improvement was greater for muscle strength compared with BMD, evidenced by less heterogeneity (I2 = 78.1% vs 98.6%) and a higher overall quality of evidence. No training characteristic significantly affected both outcomes (p > 0.05), although concomitant increases in strength and BMD were favored by higher training frequencies, increases in strength were favored by resistance only and higher volumes, and increases in BMD were favored by combined resistance plus weight-bearing exercises, lower volumes, and higher loads.
Conclusions
Progressive resistance training programs concomitantly increase lower-limb muscle strength and femur/hip bone mineral density in older adults, with greater certainty for strength improvement. Thus, to maximize the efficacy of progressive resistance training programs to concurrently prevent muscle and bone loss in older adults, it is recommended to incorporate training characteristics more likely to improve BMD.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40279-022-01675-2.
Key Points
| Progressive resistance training programs concomitantly increase muscle strength and bone mineral density in older adults and, therefore, may be used to prevent muscle and bone loss in old age |
| Most evidence demonstrated an increase in muscle strength irrespective of differences within common training characteristics whereas bone mineral density improvement was more uncertain |
| To maximize dual improvements in muscle and bone strength with progressive resistance training programs for older adults, it may be beneficial to complete three sessions per week, incorporate weight-bearing/impact loading exercises (e.g., jumping, stepping), perform one or two sets per exercise, and adopt a load corresponding to 75–80% 1 repetition maximum |
Introduction
Life expectancy almost doubled in the last 100 years owing to advances in technology and medical treatments [1], with the global number of people aged over 65 years projected to rise from 703 million in 2019 to 1.5 billion by the year 2050 [2]. Unfortunately, aging is associated with the development of many chronic diseases, including sarcopenia (the loss of muscle mass, strength, and function) and osteoporosis (severe bone loss), which respectively costs the USA ~ $40 billion [3] and ~ $17 billion [4] annually in healthcare. Between the ages of 65 and 80 years, the annual percentage loss in muscle strength is ~ 1–4% for both sexes [5, 6], whereas the decline in bone mineral density (BMD) is accelerated in women (~ 1–3% vs ~ 0.25–1.5% for men) [5]. The reduction in muscle strength and BMD with age decreases the capacity to perform activities of daily living and increases the susceptibility to falls and fractures [7, 8]. The factors associated with age-related osteoporosis and sarcopenia are multi-faceted [9, 10] and range from lifestyle (e.g., inactivity, nutritional intake) [11, 12], psychosocial (e.g., self-efficacy, resiliency) [13], and biological factors (e.g., genetic, inflammatory, hormonal) [14–17].
Skeletal muscle and bone are closely interconnected via mechanical and endocrine functions, which are highly sensitive to physical activity [18, 19]. During physical activity, external (gravitational and inertial) and internal (skeletal muscle contraction) mechanical loads stimulate dose-dependent changes in bone formation [20–22], and skeletal muscle releases various growth factors and myokines known to influence muscle protein synthesis and bone turnover rate (e.g., insulin-like growth factor-1, interleukin-6) [23]. As such, long-term physical exercise training is a cost-effective and non-pharmacological approach to limit the health and economic burden of sarcopenia and osteoporosis in older adults.
Previous meta-analyses have reported beneficial effects of progressive resistance training for increasing muscle strength [24–27] and BMD [28–31] in older adults, with complementary benefits such as increased muscle mass [24, 27], improved functional capacity [32, 33], and a reduced fall and fracture risk [34]. However, previous meta-analyses have only focused on muscle strength or BMD independently, with only a recent systematic review reporting a potential benefit of progressive resistance training for improving muscle strength and BMD in older adults with low muscle and bone mass [35]. As such, it remains unclear if progressive resistance training may be used to concomitantly reverse/slow the age-related decline in muscle strength and BMD in older adults. Moreover, it remains unknown how dual changes in muscle strength and BMD may be influenced by training characteristics such as mode (resistance-only training using weighted machines/pulleys and/or free weights vs. combined resistance training and weight-bearing/impact-loading exercises such as jumping, agility and/or balance), frequency (sessions per week), volume (sets and repetitions), load (% one repetition maximum [1RM]), and program length (total weeks of training). This information may elucidate optimal progressive resistance training guidelines for the concurrent treatment of sarcopenia and osteoporosis in older adults, which is of significant clinical value.
The purpose of this systematic review and meta-analysis is to examine randomized controlled trials that investigated the effects of progressive resistance training programs on concomitant changes in lower-body muscle strength and BMD in older adults over the age of 65 years. Furthermore, a sub-group meta-regression aimed to determine how dual changes in muscle strength and BMD are affected by training mode, frequency, volume, load, and program length, so that exercise prescription guidelines for dual benefits could be provided.
Methods
Protocol and Registration
The protocol of this systematic review and meta-analysis was registered in the Prospero database (https://www.crd.york.ac.uk/prospero/, registration number: 220210) and prepared in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [36].
Eligibility Criteria
Eligibility criteria were based on the PICO approach: (i) male and female participants with a mean age ≥ 65 years; (ii) randomized controlled trials examining the effect of progressive resistance training only or resistance plus weight-bearing/impact-loading training of ≥ 4 weeks duration against a non-training prescribed control group; (iii) changes in BMD for hip, lumbar spine and/or femur (no limitations on assessment method); and (iv) changes in muscle strength of the lower limbs assessed via leg press/knee extension 1RM or isometric/isokinetic knee extension strength. Studies were excluded if participants had cancer, were rehabilitating from acute orthopedic surgery (within ≤ 6 months), were administered hormone replacement therapy as part of the study intervention, were actively losing weight during the study period, or were judged as having a high risk of bias. Only peer-reviewed journal articles published in English and matching the eligibility criteria were considered for analysis.
Information Sources and Search Strategy
A literature search in electronic databases PubMed/MEDLINE via EBSCOhost and Embase via Ovid retrieved articles published in English before 1 June, 2021. The reference lists of all included studies were also screened for eligibility. A combination of MeSH/Emtree and free-text terms were included in our Boolean search syntax: (geriatrics OR aged OR older adults OR elderly) AND (resistance training OR resistance exercise OR strength training) AND (muscle strength OR sarcopenia OR muscle mass OR muscle power) AND (bone mineral density OR osteoporosis OR bone strength OR osteopenia).
Study Selection
All records retrieved from the literature search were compiled into an Endnote library and imported into COVIDENCE software for screening (https://www.covidence.org/). Titles and abstracts of potential articles for inclusion were screened against the eligibility criteria by two independent reviewers (SO and CG or MW). When title and abstract screening implied inclusion, the full-text article was then screened by two independent reviewers (SO and CG or MW). If it was unclear whether an article met the inclusion criteria during the full-text screening process, study authors were contacted for clarification via e-mail. Any disagreements on inclusion were resolved when consensus was reached through discussions with a third reviewer (IL).
Data Collection
The following information was manually extracted from each individual study included in the analysis and entered into a Microsoft Excel spreadsheet by the first author (SO): (i) full article reference; (ii) participant characteristics including sex, age (years), body mass (kg), height (cm), and body mass index [BMI] (kg/m2); (iii) general training description including exercises performed (upper and/or lower body), equipment used (resistance machines, free weights, weighted vests, resistance bands), whether training sessions were supervised and training attendance; (iv) training specifics including mode (resistance only or resistance plus weight-bearing/impact-loading), frequency (# per week), volume (sets and repetitions per exercise), load (% 1RM), and program length (weeks), and; (v) pre-exercise and post-exercise intervention mean ± standard deviation (SD) measures for the primary outcomes including muscle strength (leg press 1RM, knee extension 1RM, or maximal isometric/isokinetic knee extension force) and BMD (femoral neck, total hip, thigh, inter-trochanteric region, trochanter, Ward’s triangle, or lumbar spine); and (vi) statistical significance for changes in secondary outcomes including body composition, functional performance, falls, and self-efficacy.
A second reviewer (CG) validated the extracted data in-person with the first author (SO) by cross-referencing the spreadsheet against printed hard-copy versions of the included studies. If a study reported multiple outcome measures for muscle strength, leg press 1RM was chosen as the preferred outcome for analysis because of its superior representation of overall lower-limb muscle strength (n = 5); if leg press 1RM was not reported, then maximal isometric/isokinetic knee extension force was used (n = 7). If a study reported BMD for multiple sites on the femur, the femoral neck was chosen as the preferred outcome for analysis as it was the most reported across articles (n = 8); if femoral neck BMD was not reported, total hip (n = 2) or proximal one-third thigh (n = 1) BMD was used. Where standard errors (SEs) were reported, the SD was calculated using the equation . The mean changes in muscle strength and BMD were calculated by subtracting the post-intervention mean score from the pre-intervention mean score, whereas the SD of the change was calculated using a correlation coefficient (Corr = 0.52 for muscle strength [37] and 0.97 for BMD [38]) and the equation:
Data were pooled together [39] if there was more than one training intervention group [40–42], if data on male and female individuals were reported separately [43], if non-exercise groups were supplemented with placebo or vitamin D/calcium [44, 45], and if data were reported for the left and right leg separately [46].
Risk-of-Bias and Quality Assessment
Two assessors (SO and MW) independently assessed the risk of bias for each outcome measure using the Cochrane risk-of-bias tool (version 2.0) [47]. Where any differences between assessors were observed, discussions between the authors were conducted to arrive at agreement. In addition to a risk of bias, inconsistency, indirectness, imprecision, and publication bias were assessed using the Grading of Recommendation, Assessment, Development, and Evaluation (GRADE) approach [48] to evaluate the overall quality of the evidence.
Statistical Analysis
Statistical analyses were performed using Stata software (version 16.1; Stata Corporation, College Station, TX, USA) and RStudio (version 1.2.5042, 2020; RStudio, Inc., Boston, MA, USA). Effect size was expressed as Hedges’ g standardized mean difference (SMD) between intervention and control for muscle strength, and as the mean difference (MD) between intervention and control for BMD. A random-effects multi-variate meta-analysis using restricted maximum likelihood was performed on percent change (∆%) in muscle strength and BMD. Because of unknown within-study correlations, Riley’s model was used to estimate an overall correlation between concomitant changes in the outcomes [49]. A univariate meta-analysis was also performed separately for each primary outcome. A random-effects meta-regression was performed using restricted maximum likelihood estimation to determine how muscle strength and BMD were affected by resistance training characteristics (mode, frequency, volume, load, and program length) and population characteristics (when differences were identified between studies). Heterogeneity (quantified as I2 measure) larger than 60% was considered substantial [48], and p < 0.05 was regarded as statistically significant. A small study effect was evaluated using funnel plots and Egger’s test. When a small study effect was observed, a sensitivity analysis trim-and-fill method was performed.
Results
Study Selection
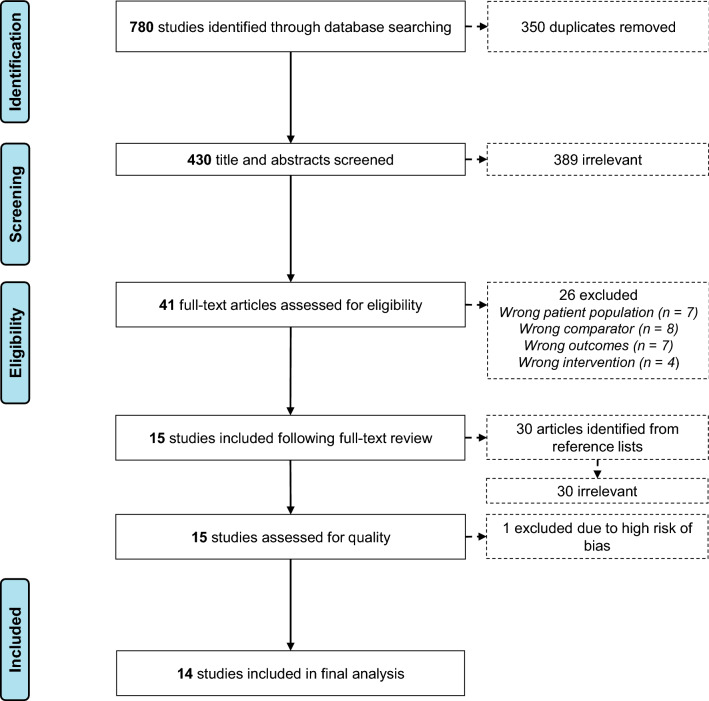
A flow diagram of the study selection process is presented in Fig. 1. Overall, 780 studies were identified in the initial database search. Following removal of duplicates (n = 350), 430 titles and abstracts were screened against the inclusion criteria, and 389 studies were irrelevant. A full-text review of the remaining 41 studies excluded a further 26 studies because of a wrong patient population (n = 7), a wrong comparator (n = 8), wrong outcomes (n = 7), or a wrong intervention (n = 4). Fifteen studies were included following a full-text review. Screening of reference lists identified 30 potential articles; however, none of these met the inclusion criteria. Of the 15 included studies, one study was excluded because of a high risk of bias (Electronic Supplementary Material [ESM]). A total of 14 studies were included in the final meta-analysis.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of the study selection process
Several studies appeared to meet the inclusion criteria but were excluded. This included two non-progressive resistance training studies [50, 51], two studies that prescribed low-intensity supervised exercise programs to the control group (e.g., stretching, walking) [52, 53], three studies that prescribed different doses of whey protein to the control and intervention groups [54–56], and one study that reported three repetition maximum strength outcomes [57].
Study Characteristics
The training and participant characteristics of included studies are detailed in Table 1. A total of 1130 participants across the fourteen studies were included. Of the twelve studies that reported sex [40, 41, 43–46, 58–63], 944/1022 (92%) were female. The mean age across studies was 70 ± 6.1 years (range 65–77 years). Most studies described participants as being apparently healthy, not engaged in regular physical activity, and having no/limited previous resistance training experience, although one study classified participants as being mild to moderately frail [63] and another study only included participants who had experienced a fall in the previous twelve months [45]. Of the nine studies reporting BMI, according to World Health Organization classification ranges [64], three included participants with normal BMI [40, 41, 65], five included participants who were overweight [44, 46, 60–62], and one included participants who were obese [63]. Five studies supplemented participants with varying doses of calcium (range 500–1500 mg per day) and vitamin D (range 200–1000 IU per day) [40, 44, 45, 59, 63]; in this instance, control and exercise groups were supplemented equally [46, 60–62].
Table 1.
Individual studies examining the combined effect of resistance training on lower-body muscle strength and bone mineral density in older adults
| References | Sex (M/F) | Age (years) | Mass (kg) | Sup; Att | Mode | Length (weeks) | Freq. (week−1) | Load (% 1RM) | Sets (#) | Reps (#) | Strength outcome | BMD outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pruitt et al. [40] |
C: 0/11 G1: 0/7 G2: 0/8 |
C: 69.6 ± 4.2 G1: 67.6 ± 1.4 G2: 67.0 ± 0.5 |
C: 63.8 ± 9.1 G1: 61.5 ± 4.6 G2: 64.5 ± 9.2 |
Y; 65% | RES | 52 | 3 |
G1: 40% G2: 80% |
G1: 3 G2: 2 |
G1: 14 G2: 7 |
G1: ↑ LP, KE G2: ↑ LP, KE |
G1: ↔ FN, LS, Hip, WT G2: ↔ FN, LS, Hip, WT |
| Taaffe et al. [41] |
C: 0/7 G1: 0/7 G2: 0/7 |
C: 69.6 ± 3.4 G1: 67.6 ± 1.3 G2: 67.0 ± 0.5 |
C: 63.8 ± 7.1 G1: 61.5 ± 4.5 G2: 63.4 ± 9.3 |
Y; 79% | RES | 52 | 3 |
G1: 40% G2: 80% |
G1: 3 G2: 2 |
G1: 14 G2: 7 |
G1: ↑ LP, KE G2: ↑ LP, KE |
G1: ↔ TH G2: ↑ TH |
| McCartney et al. [43] |
C: 21/35 G1: 29/28 |
Ca: 68.2 ± 5.3 G1a: 68.1 ± 4.5 |
Ca: 70.4 ± 13.2 G1a: 72.2 ± 11.1 |
Y; 85% | RES | 84 | 2 | 80% | 3 | 12 | ↑ LP | ↓ LSb |
| Taaffe et al. [65] |
C: 12 G1: 11 G2: 12 G3: 11 (sex not reported) |
C: 68.9 ± 3.6 G1: 68.5 ± 3.6 G2: 69.4 ± 3.0 G3: 71.0 ± 4.1 |
C: 80.4 ± 10.3 G1: 70.2 ± 14.4 G2: 70.3 ± 8.9 G3: 72.4 ± 13.0 |
Y; 98% | RES | 24 |
G1: 1 G2: 2 G3: 3 |
80% | 3 | 8 |
G1: ↑ LP, KE G2: ↑ LP, KE G3: ↑ LP, KE |
G1: ↔ LS, Hip G2: ↔ LS, Hip G3: ↔ LS, Hip |
| Rhodes et al. [58] |
C: 0/22 G1: 0/22 |
C: 68.2 ± 3.5 G1: 68.8 ± 3.2 |
C: 61.7 ± 12.9 G1: 68.4 ± 12.0 |
MN; 85% | RES | 52 | 3 | 75% | 3 | 8 | ↑ LP, KE | ↔ FN, LS, T, WT |
| Vincent and Braith [42] |
C: 16 G1: 24 G2: 22 (sex not reported) |
C: 71.0 ± 5 G1: 67.6 ± 6 G2: 66.6 ± 7 |
C: 71.0 ± 14 G1: 74.4 ± 16 G2: 74.8 ± 15 |
Y; ≥ 85% | RES | 26 | 3 |
G1: 50% G2: 80% |
1 |
G1: 13 G2: 8 |
G1: ↔ LP; ↑ KE G2: ↑ LP, KE |
G1: ↔ FN, LS, WT G2: ↑ FN; ↔ LS, WT |
| Jessup et al. [59] |
C: 0/9 G1: 0/9 |
C: 69.4 ± 4.2 G1: 69.1 ± 2.8 |
C: 84.2 ± 17.7 G1: 78.0 ± 9.2 |
Y; N/A | RES + WB | 32 | 3 | 75% | 1 | 8—10 | ↑ LP, KEc | ↑ FN; ↔ LS |
| Bunout et al. [44] |
C: 5/43 G1: 4/44 |
C: 77.0 ± 4.5 G1: 77.0 ± 4.1 |
C: 65.0 ± 11.2 G1: 66.3 ± 10.7 |
Y; 53% | RES + WB | 39 | 2 | Elastic bands | 3 | 10 | ↑ KE | ↔ FN, LS |
| Karinkanta et al. [60] |
C: 0/37 G1: 0/37 G2: 0/36 |
C: 72.0 ± 2.1 G1: 72.7 ± 2.5 G2: 72.9 ± 2.2 |
C: 74.3 ± 10.8 G1: 74.3 ± 11.0 G2: 69.4 ± 10.6 |
G1: Y; 74% G2: Y; 67% |
G1: RES G2: RES + WB |
52 | 3 | 75 – 80% | 3 | 8—10 |
G1: ↑ KE G2: ↑ KE |
G1: ↔ FN G2: ↔ FN |
| Bocalini et al. [61] |
C: 0/12 G1: 0/13 |
C: 64 ± 8 G1: 66 ± 9 |
C: 69.1 ± 2.2 G1: 67.9 ± 1.3 |
Y; N/A | RES | 24 | 3 | 60 – 70% | 3 | 10—12 | ↑ KE | ↑ FN, LS |
| Marques et al. [62] |
C: 0/24 G1: 0/23 |
C: 67.9 ± 5.9 G1: 67.3 ± 5.2 |
not reported | Y; 78% | RES | 32 | 3 | 75 – 80% | 2 | 6—8 | ↑ KE | ↑ T, Hip; ↔ FN, IT |
| Marques et al. [46] |
C: 0/30 G1: 0/30 |
C: 68.2 ± 5.7 G1: 70.1 ± 5.4 |
not reported | Y; 72% | RES + WB | 32 | 2 | Elastic bands | 1—3 | 8—15 | ↔ KE | ↑ FN |
| Villareal et al. [63] |
C: 9/18 G1: 10/16 |
C: 69 ± 4 G1: 70 ± 4 |
C: 101 ± 16.3 G1: 99.2 ± 17.4 |
Y; 88% | RES + WB | 52 | 3 | 65 – 80% | 1—2 | 8—12 | ↑ LP, KE | ↑ Hip; ↔ LS |
| Uusi-Rasi et al. [45] |
C: 0/204 G1: 0/205 |
C: 74.0 ± 3.0 G1: 74.5 ± 2.9 |
C: 72.5 ± 12.7 G1: 72.0 ± 10.6 |
Y; 73% | RES + WB | 52 | 2 | 60 – 75% | 2 | 8—12 | ↑ KE | ↔ FN, LS |
| Vincent and Braith [42] |
C: 16 G1: 24 G2: 22 (sex not reported) |
C: 71.0 ± 5 G1: 67.6 ± 6 G2: 66.6 ± 7 |
C: 71.0 ± 14 G1: 74.4 ± 16 G2: 74.8 ± 15 |
Y; ≥ 85% | RES | 26 | 3 |
G1: 50% G2: 80% |
1 |
G1: 13 G2: 8 |
G1: ↔ LP; ↑ KE G2: ↑ LP, KE |
G1: ↔ FN, LS, WT G2: ↑ FN; ↔ LS, WT |
| Jessup et al. [59] |
C: 0/9 G1: 0/9 |
C: 69.4 ± 4.2 G1: 69.1 ± 2.8 |
C: 84.2 ± 17.7 G1: 78.0 ± 9.2 |
Y; N/A | RES + WB | 32 | 3 | 75% | 1 | 8—10 | ↑ LP, KEc | ↑ FN; ↔ LS |
| Bunout et al. [44] |
C: 5/43 G1: 4/44 |
C: 77.0 ± 4.5 G1: 77.0 ± 4.1 |
C: 65.0 ± 11.2 G1: 66.3 ± 10.7 |
Y; 53% | RES + WB | 39 | 2 | Elastic bands | 3 | 10 | ↑ KE | ↔ FN, LS |
| Karinkanta et al. [60] |
C: 0/37 G1: 0/37 G2: 0/36 |
C: 72.0 ± 2.1 G1: 72.7 ± 2.5 G2: 72.9 ± 2.2 |
C: 74.3 ± 10.8 G1: 74.3 ± 11.0 G2: 69.4 ± 10.6 |
G1: Y; 74% G2: Y; 67% |
G1: RES G2: RES + WB |
52 | 3 | 75 – 80% | 3 | 8—10 |
G1: ↑ KE G2: ↑ KE |
G1: ↔ FN G2: ↔ FN |
| Bocalini et al. [61] |
C: 0/12 G1: 0/13 |
C: 64 ± 8 G1: 66 ± 9 |
C: 69.1 ± 2.2 G1: 67.9 ± 1.3 |
Y; N/A | RES | 24 | 3 | 60 – 70% | 3 | 10—12 | ↑ KE | ↑ FN, LS |
| Marques et al. [62] |
C: 0/24 G1: 0/23 |
C: 67.9 ± 5.9 G1: 67.3 ± 5.2 |
not reported | Y; 78% | RES | 32 | 3 | 75 – 80% | 2 | 6—8 | ↑ KE | ↑ T, Hip; ↔ FN, IT |
| Marques et al. [46] |
C: 0/30 G1: 0/30 |
C: 68.2 ± 5.7 G1: 70.1 ± 5.4 |
not reported | Y; 72% | RES + WB | 32 | 2 | Elastic bands | 1—3 | 8—15 | ↔ KE | ↑ FN |
| Villareal et al. [63] |
C: 9/18 G1: 10/16 |
C: 69 ± 4 G1: 70 ± 4 |
C: 101 ± 16.3 G1: 99.2 ± 17.4 |
Y; 88% | RES + WB | 52 | 3 | 65 – 80% | 1—2 | 8—12 | ↑ LP, KE | ↑ Hip; ↔ LS |
| Uusi-Rasi et al. [45] |
C: 0/204 G1: 0/205 |
C: 74.0 ± 3.0 G1: 74.5 ± 2.9 |
C: 72.5 ± 12.7 G1: 72.0 ± 10.6 |
Y; 73% | RES + WB | 52 | 2 | 60 – 75% | 2 | 8—12 | ↑ KE | ↔ FN, LS |
Sup supervised, Att attendance rate, Freq. frequency, C control group, G1 intervention group 1, G2 intervention group 2, G3 intervention group 3, RES resistance training only, RES + WB resistance plus weight-bearing/impact-loading, ↑ statistical improvement post-training compared to control group (p ≤ 0.05), ↓ statistical decrement post-training compared to control group (p ≤ 0.05), ↔ no statistical difference post-training compared to the control group (p > 0.05), LP leg press, KE knee extension, FN femoral neck, LS lumbar spine, WT Ward's triangle, TH proximal 1/3rd thigh, T trochanter, IT inter-trochanteric region, Y yes, MN mostly no—participants supervised during initial three months of twelve-month program
aEstimated from McCartney et al. [53]
bBMD assessed via dual photon absorptiometry rather than dual energy x-ray
cSum of multiple exercises including LP and KE
Training programs were supervised by members of the research team or physical therapists in thirteen studies [40–46, 59–63, 65], whereas one study supervised participants during the initial three months of a twelve-month program [58]. Mean training attendance was 77% (range 53–98%). Eight studies [40–43, 58, 61, 62, 65] utilized exclusively resistance training-only exercises using weighted/pulley machines and/or free weights (i.e., push, pull), five studies [44–46, 59, 63] utilized combined resistance training plus weight-bearing/impact-loading exercises such as jumping, agility (e.g., change of direction, sideways movements), balance (e.g., heel-to-toe), or aerobic (e.g., step-ups, squats, stair climbing), and one study [60] compared resistance training-only and combined resistance plus weight-bearing/impact-loading programs. Mean program length was 43 ± 17 weeks (range 24–84 weeks). Training frequency was three sessions per week for nine studies [40–42, 58–63], two sessions per week for four studies [43–46], and one study examined the effect of one vs. two vs. three sessions per week [65]. In one study [45], training frequency was reduced from twice per week to once per week in year two of a two-year program, and hence data after only the first year were examined. Resistance training intensity was a load between 60 and 80% of 1RM for nine studies [43, 45, 58–63, 65], and three studies examined the effect of 40% 1RM [40, 41] or 50% 1RM [42] vs. 80% 1RM. Two studies used elastic resistance bands and, therefore, load could not be quantified [44, 46]. The number of completed sets per exercise was three for six studies [43, 44, 58, 60, 61, 65], two for two studies [45, 62], one for two studies [42, 59] and ranged between one and three sets for four studies [40, 41, 46, 63]. Repetitions ranged from six to fifteen per exercise for seven studies [45, 46, 59–63] whereas others completed twelve [43], ten [44], or eight repetitions [58, 65]; two studies performed seven or fourteen repetitions depending on the load [40, 41].
Three studies met the eligibility criteria but did not report both outcome measures in sufficient detail, and information could not be retrieved via e-mail correspondence with study authors [43, 44, 59]. As such, these three studies were omitted from the meta-analysis.
Risk-of-Bias and Quality Assessment
A detailed risk-of-bias analysis is provided in the ESM. Thirteen studies had an overall low risk of bias, one study had some concerns [42], and one study had a high risk of bias [66] and was excluded from the quantitative analysis. The overall quality of the evidence was high for muscle strength, moderate for femur/hip BMD, and very low for lumbar spine BMD (Table 2).
Table 2.
GRADE analysis of the overall quality of the evidence
| Certainty assessment | № of patients | Main Effects | Overall Certainty | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| № of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Progressive resistance training | Non-exercise control | Absolute (95% CI) |
|
| Muscle Strength (follow-up: range 24 weeks to 52 weeks; assessed with: leg press or knee extension 1RMAX) | ||||||||||
| 11 | Randomised trials | Not serious | Not seriousa | Not serious | Not serious | Undetected | 498 | 406 |
Mean 1.1% SMD higher (0.73 higher to 1.47 higher) |
⨁⨁⨁⨁ High |
| Femur/hip bone mineral density (follow-up: range 24 weeks to 52 weeks; assessed with: DXA) | ||||||||||
| 11 | Randomised trials | Not serious | Seriousb | Not serious | Not serious | Undetected | 501 | 402 |
Mean 2.77% g/cm3 higher (0.44 higher to 5.1 higher) |
⨁⨁⨁◯ Moderate |
| Lumbar spine bone mineral density (follow-up: range 24 weeks to 52 weeks; assessed with: DXA) | ||||||||||
| 10 | Randomised trials | Not serious | Very seriousb,c | Not serious | Serious d | Undetected | 447 | 390 |
Mean 1.6% g/cm3 higher (1.44 lower to 4.63 higher) |
⨁◯◯◯ Very low |
DXA dual X-ray absorptiometry, SMD standardized mean difference
aAlthough substantial overall heterogeneity reported, this was downgraded to moderate when considering training mode
bConsiderable heterogeneity that could not be explained by individual training characteristics
cLarge differences in beneficial effects
dThe lower limit of the 95% confidence interval contradicts the benefit of the intervention
Concomitant Changes in Muscle Strength and BMD Following Resistance Training
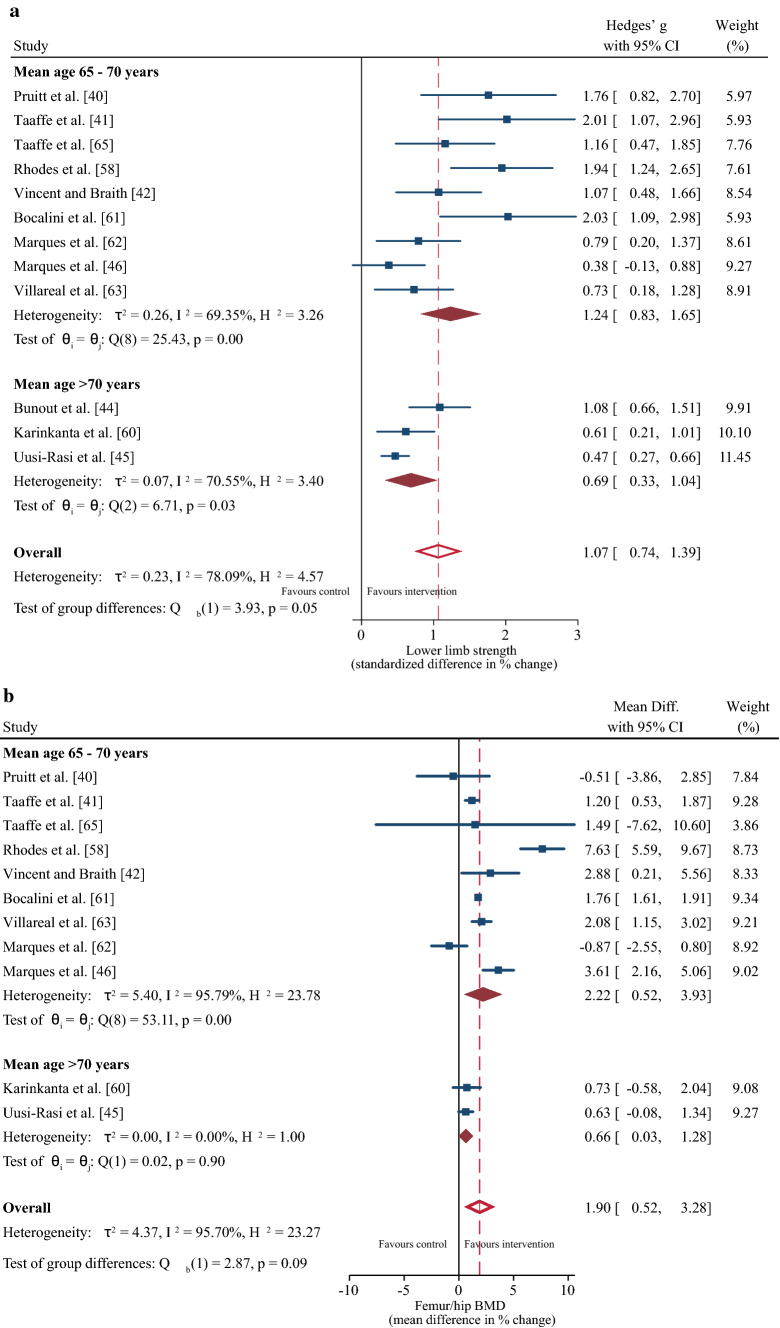
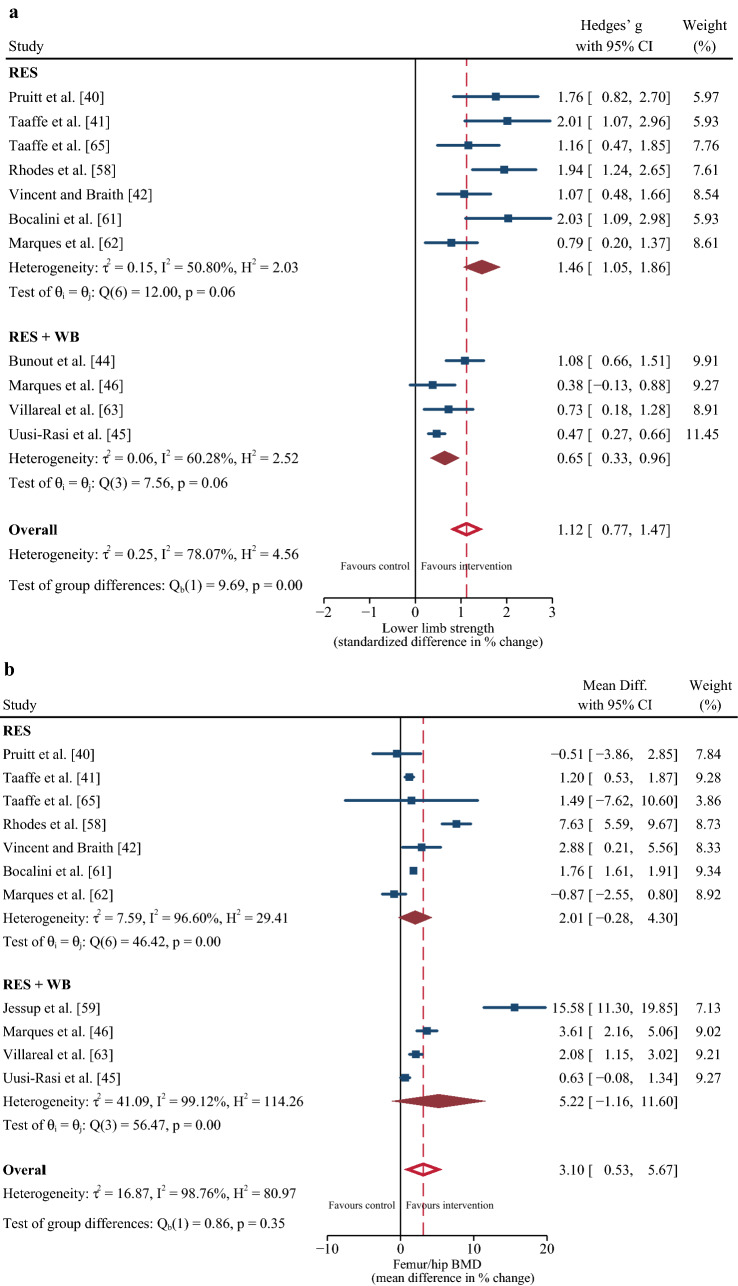
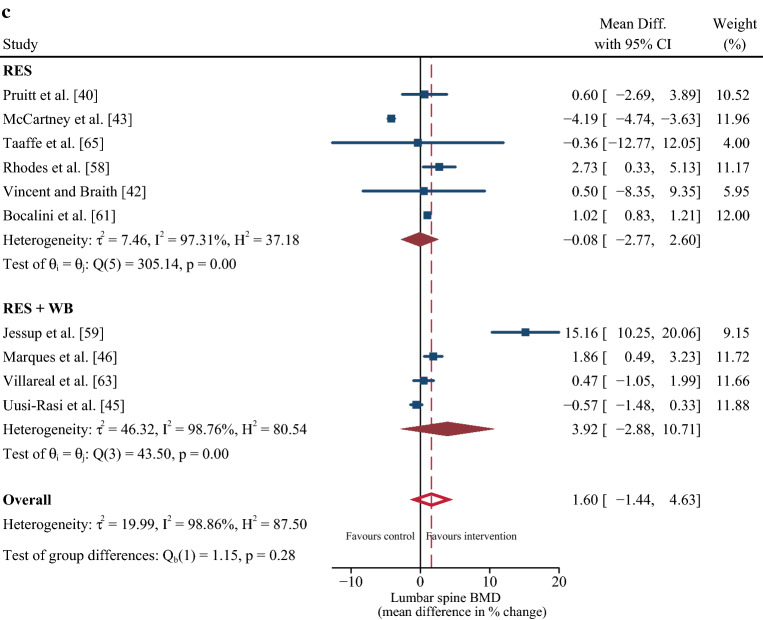
Eleven studies [40–42, 45, 46, 58, 60–63, 65] were included in the multi-variate meta-analysis of combined changes in muscle strength (control n = 406; intervention n = 498) and femur/hip BMD (control n = 402; intervention n = 501). Progressive resistance training programs concomitantly increased muscle strength (∆ SMD = 1.1%; 95% confidence interval [CI] 0.73, 1.47; p ≤ 0.001) and femur/hip BMD (∆ MD = 2.77%; 95% CI 0.44, 5.10; p = 0.02) with a Riley’s correlation of r = 0.28 (Fig. 2). When muscle strength was reported as changes in leg press 1RM [41, 42, 58, 63, 65], the pooled MD was 25.06% (95% CI 16.87, 33.25; p ≤ 0.001). The likelihood for positive change in muscle strength was more certain than femur/hip BMD, evidenced by lower heterogeneity (I2 = 78.1% vs 98.6%), a higher lower limit of the prediction interval (> ∆ 0% vs ~ ∆ − 5%) (Fig. 2]), and higher overall quality of the evidence (high vs moderate) (Table 2). From ten studies [40, 42, 43, 45, 46, 58, 59, 61, 63, 65] that included lumbar spine BMD (control n = 390; intervention n = 447), no change in this outcome was detected following the resistance training intervention (∆ MD = 1.60%; 95% CI − 1.44, 4.63; p = 0.30).
Fig. 2.
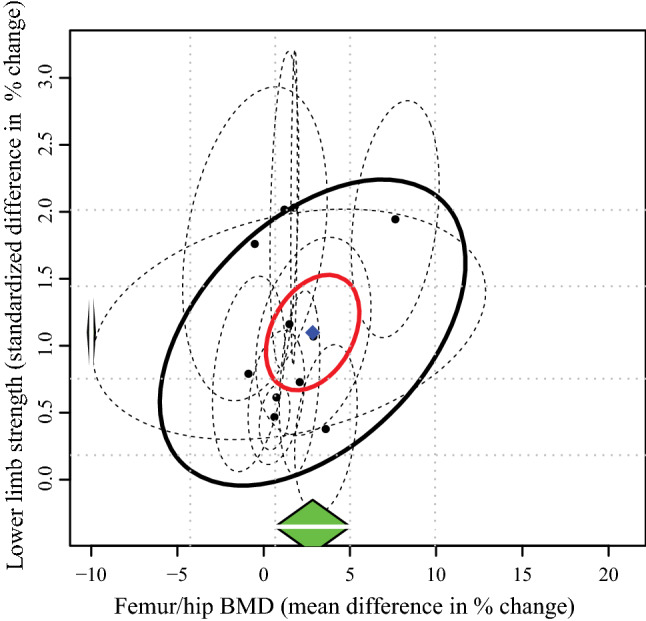
Correlation between changes in lower-limb muscle strength and femur/bone mineral density (BMD) for each individual study (black dots) and their 95% confidence intervals (dashed ellipses). The green diamonds show the estimated pooled change for each outcome separately, while the blue diamond shows the overall combined effect of the two outcomes. The red ellipse represents the 95% confidence interval of the combined effect, whereas the black ellipse represents the prediction interval for future studies
Effect of Participant Characteristics on Concomitant Changes in Muscle Strength and BMD Following Resistance Training
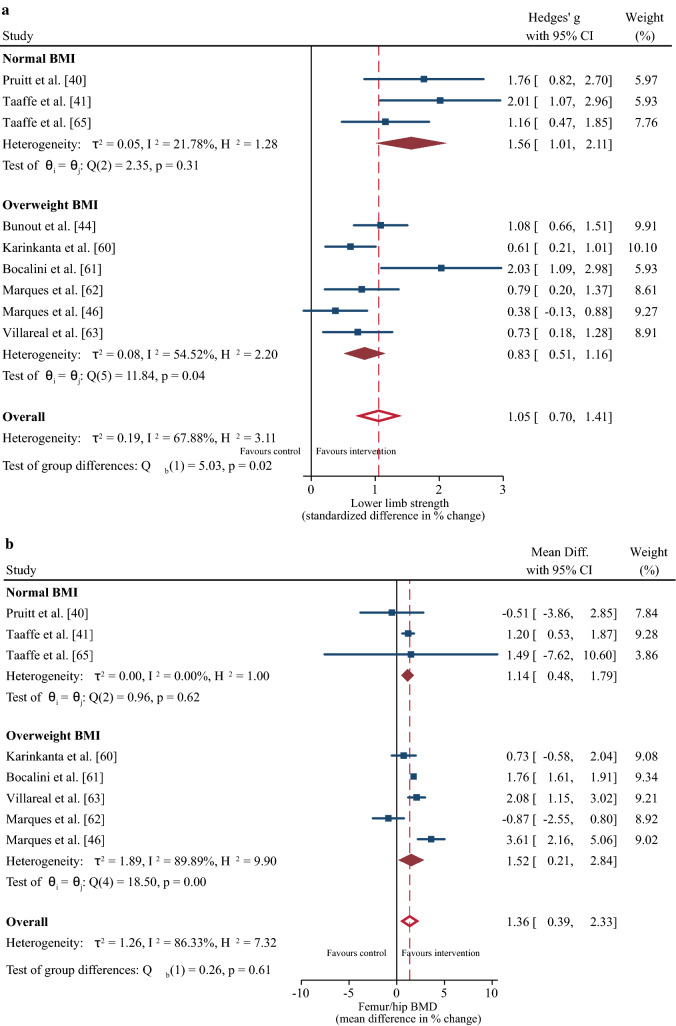
From the differences in participant characteristics identified in Sect. 3.2, a sub-group meta-regression determined the effects of age (mean age 65–70 years vs. > 70 years) and BMI (normal BMI vs. overweight BMI) on strength and BMD outcomes. Age had no significant effect on the positive change in strength or femur/hip BMD following the resistance training intervention (both p ≥ 0.05), although the magnitude of the increase tended to be greater for the 65- to 70-year-old group (Fig. 3a, b). Participants with a normal BMI demonstrated greater improvements in muscle strength (∆ SMD = 1.05%; 95% CI 0.7, 1.41; p = 0.02) but no difference in BMD compared to the overweight group (Fig. 4a, b, c).
Fig. 3.
Sub-group meta-regression for the effect of age on changes in muscle strength (a) and femur/hip bone mineral density (b). Lumbar spine bone mineral density was omitted because there was only one study in the > 70-year-old age group [45]. CI confidence interval, Diff difference
Fig. 4.
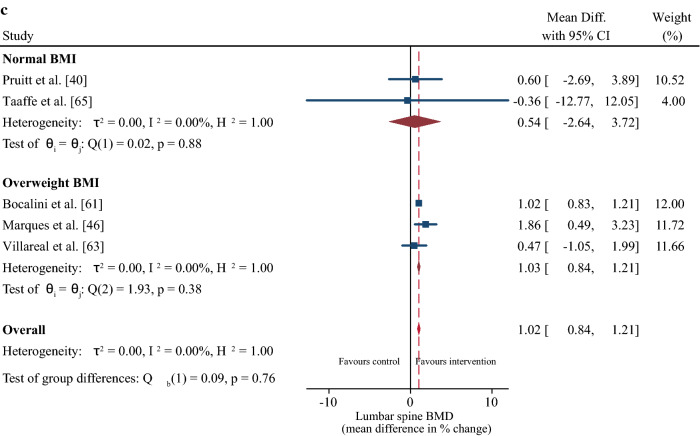
Sub-group meta-regression for the effect of body mass index (BMI) on changes in muscle strength (a), femur/hip bone mineral density (b), and lumbar spine bone mineral density (c). Body mass index was classified according to World Health Organization classification ranges [64]. CI confidence interval, Diff difference
Effect of Resistance Training Characteristics on Concomitant Changes in Muscle Strength and BMD
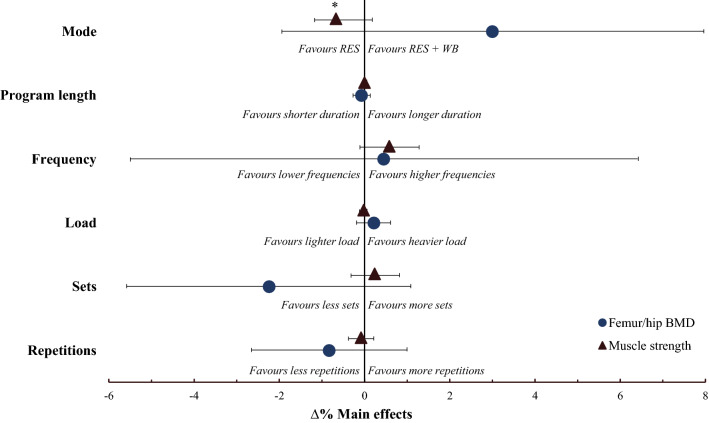
None of the individual training characteristics showed a significant combined effect on both muscle strength and BMD, presumably because of significant heterogeneity and noticeably large 95% CIs for BMD (Fig. 5 and Table 3). However, similar positive main effects on muscle strength and femur/hip BMD were observed with higher training frequencies, whereas differences in the magnitude and direction of the main effect for muscle strength and femur/hip BMD were observed for mode, volume (sets and repetitions), and load. For example, strength improvements were significantly better following resistance training only (Fig. 6a) and enhanced with a higher number of sets, whereas improvements in femur/hip BMD were enhanced following resistance plus weight-bearing/impact-loading training, lower volumes, and higher loads. Program length had a minimal effect on both outcomes.
Fig. 5.
Effect of the different training characteristics on the combined changes in muscle strength (∆% standardized mean difference) and bone mineral density [BMD] (∆% mean difference) with 95% confidence intervals when entered into the multi-variate model one at a time. *Significant main effect (p < 0.05). RES resistance training only, RES + WB combined resistance training plus weight-bearing/impact-loading exercises. The confidence interval for program length and load effect on muscle strength is hidden behind the main effect symbol
Table 3.
Output statistics from the univariate sub-group meta-regression for the effect of the different training characteristics on changes in muscle strength, femur/hip BMD, and lumbar spine BMD
| Outcome | Training characteristic | Studies (#) | Coefficient (95% CI) | I2 (%) | R2 (%) | Z score | p value |
|---|---|---|---|---|---|---|---|
| Muscle strength | Mode (RES vs RES + WB) | 12 | − 0.786 (− 1.236, − 0.335) | 50.6 | 70.1 | − 3.42 | 0.001 |
| Training frequency (3 vs 2) | 12 | 0.513 (− 0.119, 1.145) | 72.9 | 13.5 | 1.59 | 0.111 | |
| Duration | 12 | − 0.0004 (− 0.03, 0.03) | 79.1 | 0 | − 0.03 | 0.978 | |
| Load | 10 | − 0.043 (− 0.097, 0.012) | 76.3 | 12.4 | − 1.54 | 0.124 | |
| Volume | |||||||
| Sets | 12 | 0.347 (− 0.132, 0.827) | 76.0 | 7.4 | 1.42 | 0.156 | |
| Reps | 12 | − 0.043 (− 0.315, 0.229) | 80.0 | 0 | − 0.31 | 0.757 | |
| Femur/hip BMD | Mode (RES vs RES + WB) | 12 | 2.052 (− 2.846, 6.951) | 98.2 | 0 | 0.82 | 0.411 |
| Training frequency (3 vs 2) | 12 | 1.158 (− 4.776, 7.093) | 98.5 | 0 | 0.38 | 0.702 | |
| Duration | 12 | − 0.059 (− 0.264, 0.146) | 97.6 | 0 | − 0.57 | 0.570 | |
| Load | 11 | 0.171 (− 0.230, 0.572) | 98.8 | 0 | 0.83 | 0.404 | |
| Volume | |||||||
| Sets | 12 | − 2.065 (− 5.404, 1.273) | 97.8 | 0 | − 1.21 | 0.225 | |
| Reps | 12 | − 0.793 (− 2.637, 1.051) | 98 | 0 | − 0.84 | 0.399 | |
| Lumbar spine BMD | Mode (RES vs RES + WB) | 10 | 3.578 (− 2.564, 9.721) | 98.7 | 0 | 1.14 | 0.254 |
| Training frequency (3 vs 2) | 12 | 4.149 (− 1.755, 10.054) | 97.2 | 10.6 | 1.38 | 0.168 | |
| Duration | 10 | − 0.127 (− 0.272, 0.018) | 96.3 | 27.0 | − 1.72 | 0.086 | |
| Load | 9 | 0.025 (− 0.564, 0.615) | 97.9 | 0 | 0.08 | 0.934 | |
| Volume | |||||||
| Sets | 10 | − 3.44 (− 7.104, 0.222) | 98.2 | 25.6 | − 1.84 | 0.066 | |
| Reps | 10 | − 0.759 (− 3.269, 1.751) | 97.9 | 0 | − 0.59 | 0.553 |
Significant values are in bold. RES resistance training only, RES + WB resistance plus weight-bearing/impact-loading exercises
Fig. 6.
Sub-group meta-regression for the effect of exercise mode on changes in muscle strength (a), femur/hip bone mineral density [BMD] (b), and lumbar spine BMD (c) following training interventions. CI confidence interval, Diff difference, RES resistance training only, RES + WB combined resistance training plus weight-bearing/impact-loading exercises
Secondary Outcomes
Changes in secondary outcomes following the exercise intervention are detailed in the ESM. Briefly, the following changes were reported for the intervention group compared with the control group: (i) lean body mass increased for 3/5 studies [62, 63, 65], (ii) muscle hypertrophy increased for 2/2 studies [41, 43], (iii) functional performance increased for 10/11 studies [43–46, 59–63, 65], (iv) number of injurious falls decreased for 1/1 study [45], and (v) self-efficacy increased for 2/3 studies [60, 63].
Small Study Effect
A small study effect was observed for the strength outcome (Egger’s test p < 0.001) [ESM]. The trim-and-fill method imputed three additional studies, and pooled Hedges’ g was slightly smaller than initial results (∆ SMD = 0.84%; 95% CI 0.45, 1.23 vs ∆ SMD = 1.07; 95% CI 0.74, 1.39) [ESM]. While there was some asymmetry in funnel plots for both femur/hip and lumbar spine BMD outcomes, Egger’s test was not significant (ESM).
Discussion
We investigated the effect of progressive resistance training programs on concomitant changes in muscle strength and BMD in older adults and report that: (i) progressive resistance training concomitantly increased muscle strength and femur/hip BMD, but not lumbar spine BMD, (ii) larger heterogeneity and uncertainty for positive adaptation was reported for femur/hip BMD over muscle strength, (iii) the strongest determinant for concomitant increases in muscle strength and femur/hip BMD was a higher training frequency, and (iv) opposite main effects on muscle strength and femur/hip BMD were observed for resistance training mode, load, and volume.
Progressive Resistance Training and Concomitant Changes in Muscle Strength and BMD
The loss of muscle and bone is an inevitable part of the aging process. As such, effective interventions that can mitigate both muscle and bone loss have an important clinical relevance. We report that progressive resistance training improved muscle strength in 13/14 studies (∆ SMD = 1.12% or MD 25.06% when measured via leg press 1RM) [40–45, 58–63, 65], while femur/hip BMD was improved in 6/12 studies (∆ MD = 2.77%) [42, 46, 59, 61–63]. The magnitude of the increase is clinically relevant considering the positive association between muscle strength and functional capacity [67] and the inverse relationship between BMD and fracture risk [68] in older adults, and was greater than reported by a previous meta-analysis evaluating the effects of other non-pharmacological interventions on muscle and bone strength including whole-body vibration [69], Tai Chi [50], and aerobic training [31]. The reduced likelihood for significant increases in BMD compared to muscle strength is perhaps due to the slower physiological response of bone to mechanical loading [70] and/or that bone requires more time and more novel loading as well as higher dynamic strain rates to maximize positive adaptation [20–22]. However, despite the reduced likelihood for significant increases in BMD of the femur/hip, some suggest that maintenance in BMD could be clinically relevant [71]. Indeed, some studies that failed to identify changes in BMD following resistance training reported significant improvements in mobility (e.g., timed up and go, chair stand, figure of 8 running) [44, 60, 65], enhanced endurance [43], and a reduced risk of injurious falls [45] (ESM).
Nine out of ten studies showed that the exercise training protocols did not improve BMD of the lumbar spine [40, 42–45, 58, 59, 63, 65]. In fact, one study reported a significant decrease in BMD at this site compared with a control group; although, the authors could not physiologically explain this response [43]. The lack of change in lumbar spine BMD was somewhat surprising considering that five of the studies that reported no change had included specific back strengthening exercises (e.g., lumbar extension, seated row, latissimus pull-down) [40, 42, 45, 59, 65]. However, these exercises were completed in a seated or prone position, which would considerably offset external load and strain placed through the lumbar spine, which is essential for triggering an osteogenic response. As such, it is possible that more extensive compound exercises performed in the standing position and that promote gravitational loading through the lumbar spine may be necessary for improving BMD at this site [72].
The magnitude of the increase in muscle strength and BMD following resistance training was not significantly affected by age (65–70 years vs. > 70 years), although the 65- to 70-year-old group tended to exhibit a greater improvement compared with the > 70-year-old group. The somewhat reduced capacity for the resistance training intervention to stimulate muscle and bone strength adaptation in the > 70-year-old group may stem from the accelerated decline in neuromuscular structure and quality with advancing age [73], which would subsequently reduce internal bone stress required to stimulate bone formation [20–22]. However, it is important to acknowledge that progressive resistance training remains an effective strategy for increasing muscle strength [24] and bone formation [74] into very old age (> 75 years).
Participants in the normal BMI range exhibited greater improvements in muscle strength compared with their overweight counterparts, whereas the change in BMD was not different between the groups. This result supports the blunting effect of excess adipose tissue on strength adaptations to resistance training [75], which evolve from impairments in muscle protein metabolism and reduced muscle quality [76, 77]. Thus, we support the recommendation of combining progressive resistance training with a weight management program (including ~ 1 g of high-quality protein per kilogram of body weight per day) and moderate-to-high-intensity aerobic weight-bearing exercises (e.g., walking, stair climbing) to maximize concomitant improvements in muscle strength and BMD in overweight individuals [63].
Effect of Individual Training Characteristics on Concomitant Changes in Strength and BMD
The sub-group meta-regression did not detect significant effects of the individual training characteristics on concomitant changes in muscle strength and femur/hip BMD, likely because of considerable heterogeneity in the outcomes. As such, it is difficult to make clear recommendations in terms of the effect of training mode, frequency, volume, load, and program length on concomitant changes in muscle strength and BMD. However, as muscle strength increased irrespective of differences within the common training characteristics, whereas positive adaptation for femur/hip BMD was more heterogeneous and uncertain, we recommend that programs adopt characteristics more likely to improve femur/hip BMD. Despite a lack of statistical significance and wide CIs for the effects of the individual training characteristics on femur/hip BMD, the direction of the main effects may indicate that higher training frequencies enhance both outcomes, whereas mode, volume, and load may differentially affect strength and BMD.
Resistance training frequencies of three times per week seemed to enhance concomitant improvements in muscle strength and femur/hip BMD compared with two times per week. Previous reviews and original studies have reported that higher training frequencies increase muscle cross-sectional area [78] and strength [79–81], although these effects are minimized when equated for weekly training volume [79, 80]. In terms of the bone response, higher training frequencies seem to facilitate BMD improvements following completion of weight-bearing/impact-loading programs [82, 83], but have less effect following resistance training programs [65, 84], likely contributing to the wide CIs reported for the frequency effect on femur/hip BMD. As such, frequency effects on muscle may depend on volume, whereas frequency effects on BMD may be more dependent on mode.
Of all the individual training characteristics, training mode had the largest effect on strength and bone adaptations. Traditional resistance training programs were significantly better for improving muscle strength, whereas adding a weight-bearing impact-loading component appeared to be better for improving femur/hip BMD. High-volume resistance training has been advocated as the most important factor for facilitating improvements in muscle strength and size [85]. In contrast, resistance plus weight-bearing/impact-loading protocols have been suggested to be superior for BMD improvements [86]. Altogether, the evidence suggests that to maximize combined gains in muscle strength and BMD, it seems necessary to maintain resistance training volume while incorporating weight-bearing impact-loading exercises into the program. However, adding weight-bearing exercises to a resistance training session would (in most cases) reduce training volume and limit strength adaptations [25, 87]. As such, one potential approach would be to perform resistance and weight-bearing/impact-loading activities on alternate days to mitigate a reduction in within-session resistance training volume. Indeed, 4/6 resistance plus weight-bearing/impact-loading studies included in this review combined these activities into a single session [46, 59, 61, 63], which likely decreased total resistance training volume and exacerbated differences in the strength response between the training modes. Conversely, Karinkanta et al. [60] reported no statistical difference in strength and BMD between resistance-only and resistance plus weight-bearing/impact-loading training when modes were performed on alternating days [60].
Increasing the number of completed sets seemed to improve muscle strength, whereas fewer sets may be better for improving femur/hip BMD. The expression of signaling pathways known to promote myofibrillar protein synthesis (e.g., insulin-like growth factor 1, Akt/mTOR) is highly sensitive to changes in resistance training volume [88], which likely explains increased strength with an increased number of completed sets. Moreover, it is possible that higher training volumes stimulate positive neuromuscular adaptations such as motor unit remodeling and a type IIa fiber type shift [89]. However, the mechanosensitivity of bone declines soon after a stimulus is initiated, meaning that if the load is adequate, increasing volume provides no additional osteogenic benefit [90, 91]. In support of this, Taaffe et al. [65] reported no additional benefit to BMD when resistance training at 80%1RM was completed once, twice, or three times per week. Moreover, Cunha et al. [87] reported that three sets of resistance training increased muscle strength compared with one set, but had no additional benefit to BMD in osteosarcopenic women [87]. Despite this, of the eight studies included in this review that evaluated the effect of one or two sets per exercise on strength and BMD outcomes [40–42, 45, 46, 59, 62, 63], all (but one that used elastic bands [46]) reported increased muscle strength and six reported increased femur/hip BMD [41, 42, 46, 59, 62, 63]. This is in contrast to the eight studies that evaluated the effect of three sets per exercise [40, 41, 43, 44, 58, 60, 61, 65], which all reported increased muscle strength but only one reported an increase in femur/hip BMD [61]. Indeed, meta-regression showed that training volume had the second largest main effect on femur/hip BMD behind training mode and, therefore, is an important variable to consider when targeting bone formation with progressive resistance training. Although a physiological explanation for the observed favorable effect of lower training volumes on femur/hip BMD is unclear, a higher volume of resistance training may induce fatigue and require a reduction in the external load, subsequently reducing bone strain and the osteogenic response [18, 92]. Unfortunately, none of the included studies specified if the load remained constant within a session or if repetitions were completed to failure, which could provide insight into participant fatigue development during training sessions.
The external load (%1RM) had minimal effect on muscle strength, whereas external loads of 75–80% 1RM seemed better for improving the BMD response. Previous meta-analyses have advocated higher external loads for facilitating strength adaptations in older men and women [25]. Reasons for discrepancy may evolve from a higher percentage of female individuals in our study (92% vs ~ 50/50 split). Higher intensity loads tend to favor older male individuals compared with female individuals [27], potentially because women display a failure to downregulate myostatin after resistance loading compared with age-matched men [93], and older men exhibit greater anti-inflammatory benefits in response to external loads compared with women [94]. In terms of BMD, heightened mechanical loads stimulate bone modeling and remodeling to increase bone mass and bone stiffness [92, 95], and the higher loads advocated here are in agreeance with current recommendations for optimizing BMD in older adults [96]. However, a recent meta-analysis by Souza et al. [97] reported similar effects of high (≥ 70% 1RM) and low (< 70% 1RM) load resistance training on BMD in male and female adults aged ≥ 45 years. Taken together, it is possible that the effects of the external load on BMD may be influenced by hormonal changes with age (i.e., middle-aged vs old age) and/or if resistance training is performed in conjunction with weight-bearing exercises.
Limitations
Limitations of the evidence include a lack of specific details pertaining to the rate of mechanical loading/movement velocity [98], time under tension [25], contraction type [99], and whether repetitions were completed to failure [100], some of which may explain some of the reported heterogeneity and lack of significant effects for the individual training characteristics. Moreover, seven studies reported range values for load, sets, and/or repetitions [45, 46, 59–63] and two studies utilized elastic bands during resistance training [44, 46], likely increasing inter-individual variability in the strength and BMD responses. Last, the underrepresentation of older male participants (8% of the total sample) makes it difficult to determine whether similar concomitant changes in muscle strength and BMD following progressive resistance training exist between the sexes. A sub-group meta-regression to determine sex differences was not conducted as sufficient data were not available for male and female individuals separately.
The PRISMA guidelines [36] were adhered to in preparation of this review, although some limitations of the processes should be acknowledged. For example, the omission of gray literature, conference abstracts, and peer-reviewed articles not published in English poses some risk of publication bias. Moreover, if a single study included multiple intervention groups with different training characteristics (e.g., control vs. varying loads or frequencies) [40–42, 65], it was necessary to pool these data into a single intervention group, potentially influencing sub-group meta-regression. However, the results of the sub-group analysis were in line with the overall conclusions from each of these studies.
Future Directions
Future research needs to carefully consider and report specific details pertaining to exercise training principles beyond mode, frequency, volume, load, and program length, so that the actual effects of these variables on concomitant changes in muscle strength and BMD can be clearly defined. Although maximal strength is a primary indicator of skeletal muscle health and function in older adults, the ability to produce high forces at fast contraction velocities (i.e., power) may be a better predictor of function [101] and fatigue [102]. Moreover, although BMD may explain 60–70% of total bone strength [103], bone architecture [104] and matrix components [105] are also crucial for bone strength and may better predict fracture risk [106]. Future research may consider combining techniques such as force–velocity profiling and quantitative computed tomography [107] or magnetic resonance imaging [108] to concurrently assess changes in muscle strength, contraction velocity, and power production as well as trabecular architecture and matrix components such as mineral, collagen, water, and non-collagenous proteins. An increase in the representation of male participants in future research is also warranted.
Conclusions
Progressive resistance training programs concomitantly increase lower-limb muscle strength and femur/hip BMD in older adults. However, whereas improvements in muscle strength occur regardless of manipulation to well-known training characteristics, positive adaptations in femur/hip BMD are less certain. As such, to promote concomitant increases in muscle strength and BMD, we recommend adopting training characteristics more likely to facilitate improvements in BMD, which may include resistance training with a weight-bearing/impact-loading component, training frequency three times weekly, training volume of one or two sets per exercise, and an external load of 75–80% 1RM.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Declarations
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. No sources of funding were used to assist in the preparation of this article.
Conflict of interest
Steven O'Bryan, Catherine Giuliano, Mary Woessner, Sara Vogrin, Cassandra Smith, Gustavo Duque and Itamar Levinger declare that they have no conflicts of interest relevant to the content of this review.
Author contributions
GD, SO, CG, MW and IL conceived the idea for the review. SO, CG and MW conducted study selection. SO and MW conducted quality assessment. SO and CG conducted data extraction. SV performed statistical analysis. SO drafted the initial manuscript. All authors contributed to data interpretation and reviewing the manuscript. All authors read and approved the final manuscript.
Availability of data and material
Datasets for this review can be made available from the corresponding author on reasonable request.
References
- 1.Woessner MN, Tacey A, Levinger-Limor A, et al. The evolution of technology and physical inactivity: the good, the bad, and the way forward. Front Public Health. 2021;9:655491. doi: 10.3389/fpubh.2021.655491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United Nations, Department of Economic and Social Affairs, Population Division. World population ageing 2019. 2020.
- 3.Goates S, Du K, Arensberg M, et al. Economic impact of hospitalizations in US adults with sarcopenia. J Frailty Aging. 2019;8(2):93–99. doi: 10.14283/jfa.2019.10. [DOI] [PubMed] [Google Scholar]
- 4.Tsai AJ. Disparities in osteoporosis by race/ethnicity, education, work status, immigrant status, and economic status in the United States. Eur J Intern Med. 2019;64:85–89. doi: 10.1016/j.ejim.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Kim KM, Lim S, Oh TJ, et al. Longitudinal changes in muscle mass and strength, and bone mass in older adults: gender-specific associations between muscle and bone losses. J Gerontol. 2017;73(8):1062–1069. doi: 10.1093/gerona/glx188. [DOI] [PubMed] [Google Scholar]
- 6.Frontera WR, Hughes VA, Fielding RA, et al. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol. 2000;88(4):1321–1326. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- 7.Fischer S, Kapinos KA, Mulcahy A, et al. Estimating the long-term functional burden of osteoporosis-related fractures. Osteoporos Int. 2017;28(10):2843–2851. doi: 10.1007/s00198-017-4110-4. [DOI] [PubMed] [Google Scholar]
- 8.Rudäng R, Zoulakis M, Sundh D, et al. Bone material strength is associated with areal BMD but not with prevalent fractures in older women. Osteoporos Int. 2016;27(4):1585–1592. doi: 10.1007/s00198-015-3419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tieland M, Trouwborst I, Clark BC. Skeletal muscle performance and ageing. J Cachexia Sarcopenia Muscle. 2018;9(1):3–19. doi: 10.1002/jcsm.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Almeida M, Manolagas S, et al. Chapter 12: aging and bone. In: Bilezikian JP, et al., editors. Principles of bone biology. 4. Cambridge: Academic Press; 2020. pp. 275–292. [Google Scholar]
- 11.Fiatarone MA, O'Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330(25):1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 12.Santos L, Elliott-Sale KJ, Sale C. Exercise and bone health across the lifespan. Biogerontology. 2017;18(6):931–946. doi: 10.1007/s10522-017-9732-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitten LJ. Psychological frailty in the aging patient. Nestle Nutr Inst Workshop Ser. 2015;83:45–53. doi: 10.1159/000382060. [DOI] [PubMed] [Google Scholar]
- 14.Garatachea N, Lucía A. Genes and the ageing muscle: a review on genetic association studies. Age. 2013;35(1):207–233. doi: 10.1007/s11357-011-9327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Degens H. The role of systemic inflammation in age-related muscle weakness and wasting. Scand J Med Sci Sports. 2010;20(1):28–38. doi: 10.1111/j.1600-0838.2009.01018.x. [DOI] [PubMed] [Google Scholar]
- 16.Visser M, Deeg DJ, Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88(12):5766–5772. doi: 10.1210/jc.2003-030604. [DOI] [PubMed] [Google Scholar]
- 17.Corrado A, Cici D, Rotondo C, et al. Molecular basis of bone aging. Int J Mol Sci. 2020;21(10):3679. doi: 10.3390/ijms21103679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tagliaferri C, Wittrant Y, Davicco M-J, et al. Muscle and bone, two interconnected tissues. Ageing Res Rev. 2015;21:55–70. doi: 10.1016/j.arr.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Gomarasca M, Banfi G, Lombardi G. Myokines: the endocrine coupling of skeletal muscle and bone. Adv Clin Chem. 2020;94:155–218. doi: 10.1016/bs.acc.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 20.O'Connor J, Lanyon L, MacFie H. The influence of strain rate on adaptive bone remodelling. J Biomech. 1982;15(10):767–781. doi: 10.1016/0021-9290(82)90092-6. [DOI] [PubMed] [Google Scholar]
- 21.Rubin CT, Lanyon LE. Regulation of bone formation by applied dynamic loads. J Bone Joint Surg Am. 1984;66(3):397–402. doi: 10.2106/00004623-198466030-00012. [DOI] [PubMed] [Google Scholar]
- 22.Lanyon L. Using functional loading to influence bone mass and architecture: objectives, mechanisms, and relationship with estrogen of the mechanically adaptive process in bone. Bone. 1996;18(1):S37–43. doi: 10.1016/8756-3282(95)00378-9. [DOI] [PubMed] [Google Scholar]
- 23.Lombardi G, Sanchis-Gomar F, Perego S, et al. Implications of exercise-induced adipo-myokines in bone metabolism. Endocrine. 2016;54(2):284–305. doi: 10.1007/s12020-015-0834-0. [DOI] [PubMed] [Google Scholar]
- 24.Grgic J, Garofolini A, Orazem J, et al. Effects of resistance training on muscle size and strength in very elderly adults: a systematic review and meta-analysis of randomized controlled trials. Sports Med. 2020;50(11):1983–1999. doi: 10.1007/s40279-020-01331-7. [DOI] [PubMed] [Google Scholar]
- 25.Borde R, Hortobágyi T, Granacher U. Dose-response relationships of resistance training in healthy old adults: a systematic review and meta-analysis. Sports Med. 2015;45(12):1693–1720. doi: 10.1007/s40279-015-0385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guizelini PC, de Aguiar RA, Denadai BS, et al. Effect of resistance training on muscle strength and rate of force development in healthy older adults: a systematic review and meta-analysis. Exp Gerontol. 2018;102:51–58. doi: 10.1016/j.exger.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 27.Jones MD, Wewege MA, Hackett DA, et al. Sex differences in adaptations in muscle strength and size following resistance training in older adults: a systematic review and meta-analysis. Sports Med. 2021;51(3):503–517. doi: 10.1007/s40279-020-01388-4. [DOI] [PubMed] [Google Scholar]
- 28.Wolff I, Van Croonenborg J, Kemper H, et al. The effect of exercise training programs on bone mass: a meta-analysis of published controlled trials in pre-and postmenopausal women. Osteoporos Int. 1999;9(1):1–12. doi: 10.1007/s001980050109. [DOI] [PubMed] [Google Scholar]
- 29.Kemmler W, Shojaa M, Kohl M, et al. Effects of different types of exercise on bone mineral density in postmenopausal women: a systematic review and meta-analysis. Calcif Tissue Int. 2020;107(5):409–439. doi: 10.1007/s00223-020-00744-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marques EA, Mota J, Carvalho J. Exercise effects on bone mineral density in older adults: a meta-analysis of randomized controlled trials. Age. 2012;34(6):1493–1515. doi: 10.1007/s11357-011-9311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahimi GRM, Smart NA, Liang MT, et al. The impact of different modes of exercise training on bone mineral density in older postmenopausal women: a systematic review and meta-analysis research. Calcif Tissue Int. 2020;106(6):577–590. doi: 10.1007/s00223-020-00671-w. [DOI] [PubMed] [Google Scholar]
- 32.Steib S, Schoene D, Pfeifer K. Dose-response relationship of resistance training in older adults: a meta-analysis. Med Sci Sports Exerc. 2010;42(5):902–914. doi: 10.1249/MSS.0b013e3181c34465. [DOI] [PubMed] [Google Scholar]
- 33.Varahra A, Rodrigues IB, MacDermid JC, et al. Exercise to improve functional outcomes in persons with osteoporosis: a systematic review and meta-analysis. Osteoporos Int. 2018;29(2):265–286. doi: 10.1007/s00198-017-4339-y. [DOI] [PubMed] [Google Scholar]
- 34.García-Hermoso A, Ramirez-Vélez R, de Sáez AML, et al. Safety and effectiveness of long-term exercise interventions in older adults: a systematic review and meta-analysis of randomized controlled trials. Sports Med. 2020;50(6):1095–1106. doi: 10.1007/s40279-020-01259-y. [DOI] [PubMed] [Google Scholar]
- 35.Atlihan R, Kirk B, Duque G. Non-pharmacological interventions in osteosarcopenia: a systematic review. J Nutr Health Aging. 2021;25(1):25–32. doi: 10.1007/s12603-020-1537-7. [DOI] [PubMed] [Google Scholar]
- 36.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teixeira LE, Silva KN, Imoto AM, et al. Progressive load training for the quadriceps muscle associated with proprioception exercises for the prevention of falls in postmenopausal women with osteoporosis: a randomized controlled trial. Osteoporos Int. 2010;21(4):589–596. doi: 10.1007/s00198-009-1002-2. [DOI] [PubMed] [Google Scholar]
- 38.Gualano B, Macedo AR, Alves CR, et al. Creatine supplementation and resistance training in vulnerable older women: a randomized double-blind placebo-controlled clinical trial. Exp Gerontol. 2014;53:7–15. doi: 10.1016/j.exger.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Higgins JP, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. New York: John Wiley & Sons; 2019. [Google Scholar]
- 40.Pruitt LA, Taaffe DR, Marcus R. Effects of a one-year high-intensity versus low-intensity resistance training program on bone mineral density in older women. J Bone Miner Res. 1995;10(11):1788–1795. doi: 10.1002/jbmr.5650101123. [DOI] [PubMed] [Google Scholar]
- 41.Taaffe DR, Pruitt L, Pyka G, et al. Comparative effects of high- and low-intensity resistance training on thigh muscle strength, fiber area, and tissue composition in elderly women. C Physiol. 1996;16(4):381–392. doi: 10.1111/j.1475-097X.1996.tb00727.x. [DOI] [PubMed] [Google Scholar]
- 42.Vincent KR, Braith RW. Resistance exercise and bone turnover in elderly men and women. Med Sci Sports Exerc. 2002;34(1):17–23. doi: 10.1097/00005768-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 43.McCartney N, Hicks AL, Martin J, et al. A longitudinal trial of weight training in the elderly: continued improvements in year 2. J Gerontol A Biol Sci Med Sci. 1996;51(6):B425–B433. doi: 10.1093/gerona/51A.6.B425. [DOI] [PubMed] [Google Scholar]
- 44.Bunout D, Barrera G, Leiva L, et al. Effects of vitamin D supplementation and exercise training on physical performance in Chilean vitamin D deficient elderly subjects. Exp Gerontol. 2006;41(8):746–752. doi: 10.1016/j.exger.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Uusi-Rasi K, Patil R, Karinkanta S, et al. Exercise and vitamin D in fall prevention among older women: a randomized clinical trial. JAMA Intern Med. 2015;175(5):703–711. doi: 10.1001/jamainternmed.2015.0225. [DOI] [PubMed] [Google Scholar]
- 46.Marques EA, Mota J, Machado L, et al. Multicomponent training program with weight-bearing exercises elicits favorable bone density, muscle strength, and balance adaptations in older women. Calcif Tissue Int. 2011;88(2):117–129. doi: 10.1007/s00223-010-9437-1. [DOI] [PubMed] [Google Scholar]
- 47.Sterne JA, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 48.Schünemann H, Brożek J, Guyatt G, et al. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. The GRADE Working Group. Updated October 2013.
- 49.Riley RD, Thompson JR, Abrams KR. An alternative model for bivariate random-effects meta-analysis when the within-study correlations are unknown. Biostat. 2008;9(1):172–186. doi: 10.1093/biostatistics/kxm023. [DOI] [PubMed] [Google Scholar]
- 50.Woo J, Hong A, Lau E, et al. A randomised controlled trial of Tai Chi and resistance exercise on bone health, muscle strength and balance in community-living elderly people. Age Ageing. 2007;36(3):262–268. doi: 10.1093/ageing/afm005. [DOI] [PubMed] [Google Scholar]
- 51.Englund U, Littbrand H, Sondell A, et al. A 1-year combined weight-bearing training program is beneficial for bone mineral density and neuromuscular function in older women. Osteoporos Int. 2005;16(9):1117–1123. doi: 10.1007/s00198-004-1821-0. [DOI] [PubMed] [Google Scholar]
- 52.do Nascimento MA, Gerage AM, Januario RS, et al. Resistance training with dietary intake maintenance increases strength without altering body composition in older women. J Sport Med Phys Fit. 2016;13:1–20. doi: 10.23736/S0022-4707.16.06730-X. [DOI] [PubMed] [Google Scholar]
- 53.McCartney N, Hicks AL, Martin J, et al. Long-term resistance training in the elderly: effects on dynamic strength, exercise capacity, muscle, and bone. J Gerontol A Biol Sci Med Sci. 1995;50(2):B97–104. doi: 10.1093/gerona/50A.2.B97. [DOI] [PubMed] [Google Scholar]
- 54.Kemmler W, Kohl M, Fröhlich M, et al. Effects of high-intensity resistance training on fitness and fatness in older men with osteosarcopenia. Front Physiol. 2020;11:1014. doi: 10.3389/fphys.2020.01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kemmler W, Kohl M, Jakob F, et al. Effects of high intensity dynamic resistance exercise and whey protein supplements on osteosarcopenia in older men with low bone and muscle mass: final results of the randomized controlled FrOST Study. Nutrients. 2020;12(8):2341. doi: 10.3390/nu12082341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kemmler W, Kohl M, Fröhlich M, et al. Effects of high-intensity resistance training on osteopenia and sarcopenia parameters in older men with osteosarcopenia: one-year results of the randomized controlled Franconian Osteopenia and Sarcopenia Trial (FrOST) J Bone Miner Res. 2020;35(9):1634–1644. doi: 10.1002/jbmr.4027. [DOI] [PubMed] [Google Scholar]
- 57.Gianoudis J, Bailey CS, Ebeling PR, et al. Effects of a targeted multimodal exercise program incorporating high-speed power training on falls and fracture risk factors in older adults: a community-based randomized controlled trial. J Bone Miner Res. 2014;29(1):182–191. doi: 10.1002/jbmr.2014. [DOI] [PubMed] [Google Scholar]
- 58.Rhodes EC, Martin AD, Taunton JE, et al. Effects of one year of resistance training on the relation between muscular strength and bone density in elderly women. Br J Sports Med. 2000;34(1):18–22. doi: 10.1136/bjsm.34.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jessup JV, Horne C, Vishen R, et al. Effects of exercise on bone density, balance, and self-efficacy in older women. Biol Res Nurs. 2003;4(3):171–180. doi: 10.1177/1099800402239628. [DOI] [PubMed] [Google Scholar]
- 60.Karinkanta S, Heinonen A, Sievänen H, et al. A multi-component exercise regimen to prevent functional decline and bone fragility in home-dwelling elderly women: randomized, controlled trial. Osteoporos Int. 2007;18(4):453–462. doi: 10.1007/s00198-006-0256-1. [DOI] [PubMed] [Google Scholar]
- 61.Bocalini DS, Serra AJ, Dos Santos L. Moderate resistive training maintains bone mineral density and improves functional fitness in postmenopausal women. J Aging Res. 2010;2010:760818. doi: 10.4061/2010/760818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marques EA, Wanderley F, Machado L, et al. Effects of resistance and aerobic exercise on physical function, bone mineral density, OPG and RANKL in older women. Exp Gerontol. 2011;46(7):524–532. doi: 10.1016/j.exger.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 63.Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364(13):1218–1229. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Diet, nutrition and the prevention of chronic diseases. Report of a WHO Study Group. WHO Technical Report Series, No. 797. 2003. Geneva: World Health Organisation; 1990. [PubMed]
- 65.Taaffe DR, Duret C, Wheeler S, et al. Once-weekly resistance exercise improves muscle strength and neuromuscular performance in older adults. J Am Geriatr Soc. 1999;47(10):1208–1214. doi: 10.1111/j.1532-5415.1999.tb05201.x. [DOI] [PubMed] [Google Scholar]
- 66.Bocalini DS, Serra J, dos Santos L, et al. Strength training preserves the bone mineral density of postmenopausal women without hormone replacement therapy. J Aging Health. 2009;21(3):519–527. doi: 10.1177/0898264309332839. [DOI] [PubMed] [Google Scholar]
- 67.Dulac MC, Carvalho LP, Aubertin-Leheudre M. Functional capacity depends on lower limb muscle strength rather than on abdominal obesity in active postmenopausal women. Menopause. 2018;25(2):176–181. doi: 10.1097/GME.0000000000000970. [DOI] [PubMed] [Google Scholar]
- 68.Faulkner KG. Bone matters: are density increases necessary to reduce fracture risk? J Bone Miner Res. 2000;15(2):183–187. doi: 10.1359/jbmr.2000.15.2.183. [DOI] [PubMed] [Google Scholar]
- 69.Lau RW, Liao L-R, Yu F, et al. The effects of whole body vibration therapy on bone mineral density and leg muscle strength in older adults: a systematic review and meta-analysis. Clin Rehabil. 2011;25(11):975–988. doi: 10.1177/0269215511405078. [DOI] [PubMed] [Google Scholar]
- 70.Zehnacker CH, Bemis-Dougherty A. Effect of weighted exercises on bone mineral density in post menopausal women a systematic review. J Geriatr Phys Ther. 2007;30(2):79–88. doi: 10.1519/00139143-200708000-00007. [DOI] [PubMed] [Google Scholar]
- 71.Clarke BL, Khosla S. Physiology of bone loss. Radiol Clin North Am. 2010;48(3):483–495. doi: 10.1016/j.rcl.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Judex S, Carlson KJ. Is bone’s response to mechanical signals dominated by gravitational loading. Med Sci Sports Exerc. 2009;41(11):2037–2043. doi: 10.1249/MSS.0b013e3181a8c6e5. [DOI] [PubMed] [Google Scholar]
- 73.Power GA, Dalton BH, Rice CL. Human neuromuscular structure and function in old age: a brief review. J Sport Health Sci. 2013;2(4):215–226. doi: 10.1016/j.jshs.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu-Ambrose TY, Khan KM, Eng JJ, et al. Both resistance and agility training increase cortical bone density in 75-to 85-year-old women with low bone mass: a 6-month randomized controlled trial. J Clin Densitom. 2004;7(4):390–398. doi: 10.1385/JCD:7:4:390. [DOI] [PubMed] [Google Scholar]
- 75.de Oliveira SA, Dutra MT, de Moraes WMAM, et al. Resistance training-induced gains in muscle strength, body composition, and functional capacity are attenuated in elderly women with sarcopenic obesity. Clin Interv Aging. 2018;13:411–417. doi: 10.2147/CIA.S156174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guillet C, Delcourt I, Rance M, et al. Changes in basal and insulin and amino acid response of whole body and skeletal muscle proteins in obese men. J Clin Endocrinol Metab. 2009;94(8):3044–3050. doi: 10.1210/jc.2008-2216. [DOI] [PubMed] [Google Scholar]
- 77.Hamrick MW, McGee-Lawrence ME, Frechette DM. Fatty infiltration of skeletal muscle: mechanisms and comparisons with bone marrow adiposity. Front Endocrinol. 2016;7:69. doi: 10.3389/fendo.2016.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wernbom M, Augustsson J, Thomeé R. The influence of frequency, intensity, volume and mode of strength training on whole muscle cross-sectional area in humans. Sports Med. 2007;37(3):225–264. doi: 10.2165/00007256-200737030-00004. [DOI] [PubMed] [Google Scholar]
- 79.Ralston GW, Kilgore L, Wyatt FB, et al. Weekly training frequency effects on strength gain: a meta-analysis. Sports Med. 2018;4(1):1–24. doi: 10.1186/s40798-018-0149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grgic J, Schoenfeld BJ, Davies TB, et al. Effect of resistance training frequency on gains in muscular strength: a systematic review and meta-analysis. Sports Med. 2018;48(5):1207–1220. doi: 10.1007/s40279-018-0872-x. [DOI] [PubMed] [Google Scholar]
- 81.Richardson DL, Duncan MJ, Jimenez A, et al. Effects of movement velocity and training frequency of resistance exercise on functional performance in older adults: a randomised controlled trial. Eur J Sport Sci. 2019;19(2):234–246. doi: 10.1080/17461391.2018.1497709. [DOI] [PubMed] [Google Scholar]
- 82.Kemmler W, von Stengel S. Exercise frequency, health risk factors, and diseases of the elderly. Arch Phys Med Rehabil. 2013;94(11):2046–2053. doi: 10.1016/j.apmr.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 83.Bailey CA, Brooke-Wavell K. Optimum frequency of exercise for bone health: randomised controlled trial of a high-impact unilateral intervention. Bone. 2010;46(4):1043–1049. doi: 10.1016/j.bone.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 84.Ashe MC, Gorman E, Khan KM, et al. Does frequency of resistance training affect tibial cortical bone density in older women? A randomized controlled trial. Osteoporos Int. 2013;24(2):623–632. doi: 10.1007/s00198-012-2000-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Figueiredo VC, de Salles BF, Trajano GS. Volume for muscle hypertrophy and health outcomes: the most effective variable in resistance training. Sports Med. 2018;48(3):499–505. doi: 10.1007/s40279-017-0793-0. [DOI] [PubMed] [Google Scholar]
- 86.Zhao R, Zhao M, Xu Z. The effects of differing resistance training modes on the preservation of bone mineral density in postmenopausal women: a meta-analysis. Osteoporos Int. 2015;26(5):1605–1618. doi: 10.1007/s00198-015-3034-0. [DOI] [PubMed] [Google Scholar]
- 87.Cunha PM, Ribeiro AS, Tomeleri CM, et al. The effects of resistance training volume on osteosarcopenic obesity in older women. J Sport Sci. 2018;36(14):1564–1571. doi: 10.1080/02640414.2017.1403413. [DOI] [PubMed] [Google Scholar]
- 88.Burd NA, West DW, Staples AW, et al. Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men. PLoS ONE. 2010;5(8):e12033. doi: 10.1371/journal.pone.0012033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lavin KM, Roberts BM, Fry CS, et al. The importance of resistance exercise training to combat neuromuscular aging. Physiology (Bethseda) 2019;34(2):112–122. doi: 10.1152/physiol.00044.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Robling AG, Burr DB, Turner CH. Recovery periods restore mechanosensitivity to dynamically loaded bone. J Exp Biol. 2001;204(19):3389–3399. doi: 10.1242/jeb.204.19.3389. [DOI] [PubMed] [Google Scholar]
- 91.Cullen DM, Smith RT. Akhter MP Bone-loading response varies with strain magnitude and cycle number. J Appl Physiol. 2001;91(5):1971–1976. doi: 10.1152/jappl.2001.91.5.1971. [DOI] [PubMed] [Google Scholar]
- 92.Frost HM. Bone's mechanostat: a 2003 update. Anat Rec. 2003;275(2):1081–1101. doi: 10.1002/ar.a.10119. [DOI] [PubMed] [Google Scholar]
- 93.Kim J-S, Cross JM, Bamman MM. Impact of resistance loading on myostatin expression and cell cycle regulation in young and older men and women. Am J Physiol Endocrinol Metab. 2005;288(6):E1110–E1119. doi: 10.1152/ajpendo.00464.2004. [DOI] [PubMed] [Google Scholar]
- 94.Forti LN, Van Roie E, Njemini R, et al. Load-specific inflammation mediating effects of resistance training in older persons. J Am Med Dir Assoc. 2016;17(6):547–552. doi: 10.1016/j.jamda.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 95.Hughes JM, Castellani CM, Popp KL, et al. The central role of osteocytes in the four adaptive pathways of bone's mechanostat. Exerc Sport Sci Rev. 2020;48(3):140–148. doi: 10.1249/JES.0000000000000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Daly RM, Dalla Via J, Duckham RL, et al. Exercise for the prevention of osteoporosis in postmenopausal women: an evidence-based guide to the optimal prescription. Braz J Phys Ther. 2019;23(2):170–180. doi: 10.1016/j.bjpt.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Souza D, Barbalho M, Ramirez-Campillo R, et al. High and low-load resistance training produce similar effects on bone mineral density of middle-aged and older people: a systematic review with meta-analysis of randomized clinical trials. Exp Gerontol. 2020;138:110973. doi: 10.1016/j.exger.2020.110973. [DOI] [PubMed] [Google Scholar]
- 98.von Stengel S, Kemmler W, Kalender WA, et al. Differential effects of strength versus power training on bone mineral density in postmenopausal women: a 2-year longitudinal study. Br J Sports Med. 2007;41(10):649–655. doi: 10.1136/bjsm.2006.033480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Clark LA, Russ DW, Tavoian D, et al. Heterogeneity of the strength response to progressive resistance exercise training in older adults: contributions of muscle contractility. Exp Gerontol. 2021;152:111437. doi: 10.1016/j.exger.2021.111437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Davies T, Orr R, Halaki M, et al. Effect of training leading to repetition failure on muscular strength: a systematic review and meta-analysis. Sports Med. 2016;46(4):487–502. doi: 10.1007/s40279-015-0451-3. [DOI] [PubMed] [Google Scholar]
- 101.McKinnon NB, Connelly DM, Rice CL, et al. Neuromuscular contributions to the age-related reduction in muscle power: mechanisms and potential role of high velocity power training. Ageing Res Rev. 2017;35:147–154. doi: 10.1016/j.arr.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 102.Christie A, Snook EM, Kent-Braun JA. Systematic review and meta-analysis of skeletal muscle fatigue in old age. Med Sci Sports Exerc. 2011;43(4):568. doi: 10.1249/MSS.0b013e3181f9b1c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ammann P, Rizzoli R. Bone strength and its determinants. Osteoporos Int. 2003;14(3):13–18. doi: 10.1007/s00198-002-1345-4. [DOI] [PubMed] [Google Scholar]
- 104.Koehne T, Vettorazzi E, Küsters N, et al. Trends in trabecular architecture and bone mineral density distribution in 152 individuals aged 30–90 years. Bone. 2014;66:31–38. doi: 10.1016/j.bone.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 105.Burr DB. Changes in bone matrix properties with aging. Bone. 2019;120:85–93. doi: 10.1016/j.bone.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 106.Mikolajewicz N, Bishop N, Burghardt AJ, et al. HR-pQCT measures of bone microarchitecture predict fracture: systematic review and meta-analysis. J Bone Miner Res. 2020;35(3):446–459. doi: 10.1002/jbmr.3901. [DOI] [PubMed] [Google Scholar]
- 107.Geusens P, Chapurlat R, Schett G, et al. High-resolution in vivo imaging of bone and joints: a window to microarchitecture. Nat Rev Rheumatol. 2014;10(5):304–313. doi: 10.1038/nrrheum.2014.23. [DOI] [PubMed] [Google Scholar]
- 108.Soldati E, Rossi F, Vicente J, et al. Survey of MRI usefulness for the clinical assessment of bone microstructure. Int J Mol Med Sci. 2021;22(5):2509. doi: 10.3390/ijms22052509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.