Abstract
Background
Disease resistance phenotypes are associated with immune regulatory functions and immune tolerance and have implications for both the livestock industry and human health. Microbiota plays an essential role in regulating immunity and autoimmunity in the host organism, but the influence of host-microbiota interactions on disease resistance phenotypes remains unclear. Here, multiomics analysis was performed to identify potential regulatory mechanisms of disease resistance at both the microbiome and host levels in two pig breeds.
Results
Acute colitis models were established in Min pigs and Yorkshire pigs, and control and diseased individuals were compared. Compared with Yorkshire pigs under the same nutritional and management conditions, Min pigs exhibited strong disease resistance, as indicated by a low disease activity index (DAI) and a low histological activity index (HAI). Microbiota sequencing analysis showed that potentially harmful microbes Desulfovibrio, Bacteroides and Streptococcus were enriched in diseased individuals of the two breeds. Notably, potentially beneficial microbes, such as Lactobacillus, Clostridia and Eubacterium, and several genera belonging to Ruminococcaceae and Christensenellaceae were enriched in diseased Min pigs and were found to be positively associated with the microbial metabolites related to intestinal barrier function. Specifically, the concentrations of indole derivatives and short-chain fatty acids were increased in diseased Min pigs, suggesting beneficial action in protecting intestinal barrier. In addition, lower concentrations of bile acid metabolites and short-chain fatty acids were observed in diseased Yorkshire pigs, which were associated with increased potentially harmful microbes, such as Bilophila and Alistipes. Concerning enrichment of the immune response, the increase in CD4+ T cells in the lamina propria improved supervision of the host immunity response in diseased Min pigs, contributing to the maintenance of Th2-type immune superiority and immune tolerance patterns and control of excessive inflammation with the help of potentially beneficial microbes. In diseased Yorkshire pigs, more terms belonging to biological processes of immunity were enriched, including Toll-like receptors signalling, NF-κB signalling and Th1 and Th17-type immune responses, along with the increases of potentially harmful microbes and damaged intestinal barrier.
Conclusions
Cumulatively, the results for the two pig breeds highlight that host-microbiota crosstalk promotes a disease resistance phenotype in three ways: by maintaining partial PRR nonactivation, maintaining Th2-type immune superiority and immunological tolerance patterns and recovering gut barrier function to protect against colonic diseases.
Video abstract.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40168-022-01303-1.
Keywords: Disease resistance, Microbiota, Metabolome, Immunity response, Intestinal barrier function
Background
The gut harbours a diverse community of microbiota that exhibit large between-individual variations [1], which next-generation sequencing has linked to host phenotypes [2]. Millions of years of coevolution between host and microbiota have led to a mutualistic relationship in which microbiota likely mediate host physiological suitability [3]. Specifically, host physiology can be altered by microbiota-induced cell signalling and proliferation, leading to changes in the mucosa, barrier function and immune response and thereby affecting phenotype [4–6]. Given the broad range of effects of microbiota on host physiology and the induction and function of the immune system, it is unsurprising that the combination of host and microbiota largely drives the development of disease resistance phenotypes. The immune response to disease is an essential indicator of the host’s disease resistance phenotype, which differs depending on microbiota composition. Host-microbiota interactions have been implicated in various gut-related diseases, including diarrhoea, enterotoxaemia and inflammatory bowel disease (IBD) [7–10]. These diseases are characterised by microbial dysbiosis and perturbations of host homeostasis, but the roles of changes in the microbiota in different disease resistance phenotypes have not been entirely determined. Moreover, whether and to what extent the gut microbiota drives diseases or is altered in response to disease remains unclear. Intervention studies targeting different disease resistance phenotypes are required to address this gap. Given the importance and complexity of microbiota across different pig breeds, there is increasing interest in identifying the composition of the microbiota and their functional contribution to achieve a better understanding of the mechanism of disease resistance phenotype formation.
The Yorkshire pig is a common commercial pig breed worldwide and is found in most countries with pig breeding industries. Pedigreed pigs of this breed are adaptable to various environments and display a certain resistance to diseases [11], and the difference in the disease resistance phenotype compared with native breeds may not be small [12]. Min pigs are a native Chinese breed with stronger disease resistance than Yorkshire pigs. Previous studies have demonstrated that compared with other breeds, Min pigs reach the peak of diarrhoea later in the weaning period and have a lower diarrhoeal burden [13]. The more excellent disease resistance of Min pigs compared with other breeds, such as Yorkshire pigs, might offer a route to characterise efficient microbiomes. Recently, we found that Min pig faecal microbiota transfer could modulate the intestinal microbiota and increase immunoglobulins and antimicrobial peptides in Yorkshire piglets [14], suggesting that the microbiota of Min pigs can enhanced the health of Yorkshire pigs by affecting host physiology. This may also partially explain why the disease resistance of Min pigs is stronger than that of Yorkshire pigs. While we have identified an association of the microbiota with diseases, how certain core bacteria in Min pigs contribute to shaping the disease resistance phenotype compared with Yorkshire pigs under the same nutrition and management remains unclear. We hypothesised that Yorkshire pigs might lack important host-microbiota interactions, resulting in weak host adaptability under pressure from diseases and inflammatory immune stimuli. DSS-induced colitis is a well-established model of acute colitis with ulceration resembling ulcerative colitis in humans [15, 16]. Ulcerative colitis, a type of IBD, can be viewed as an autoimmune disease strongly influenced by disruptions in host-microbiota homoeostasis [17]. Therefore, in this study, we employed the DSS-induced colitis model to identify certain commensal bacteria and their metabolites in Min pigs and Yorkshire pigs that may affect host physiological suitability and subsequently affect IBD susceptibility. The current study provides fundamental information about the potential links between gut microbiota and host phenotypes and may contribute to screening for core intestinal microbial candidates associated with host health.
Methods
Animals and experimental design
All animal procedures were performed in compliance with the approval of the Institutional Animal Care and Use Committee of Northeast Agricultural University (certification number NEAU-[2011]-9). Sixteen Min pigs (50 days old) and sixteen Yorkshire pigs (50 days old) in the same physiological state were selected from a commercial farm and individually housed in stainless steel metabolism crates with automatic troughs and drinking water nipples for free access to water. Animals received the same diet. After 10 days of acclimation, the pigs of each breed were randomised into 2 treatments administered via gavage for 5 days: CON (control, sterile saline) and DSS (4% DSS in 100 mL of water). The first dose of DSS water had a volume of 200 mL and was preceded by a 12-h fasting period. The dose of DSS was chosen by referring to Bassaganya et al. [18]. All pigs were slaughtered at 66 days of age. Samples were collected from each pig immediately after slaughter. Colon tissue was rinsed and stored immediately at −80 °C until further analysis. Colon contents were collected and stored at −80 °C until microbiota and metabolite analyses.
Phenotypic clinical evaluation
Disease Activity Index (DAI)
Following DSS treatment, the disease activity index (DAI) was monitored on a daily basis. The DAI score was calculated as (body weight loss score + stool consistency score + haematochezia score)/3. The body weight loss score ranged from 0 to 3: 0 = stable; 1 = mild loss (lost > 1% to < 5%); 2 = moderate loss (lost > 5% to < 10%); and 3 = severe loss (lost > 10%). The stool consistency score ranged from 0 to 3: 0 = normal, 1 = soft, 2 = fluid and 3 = completely liquid. The haematochezia score was determined by the colloidal gold method.
H&E staining
The severity of colonic lesions was scored macroscopically and histologically. Colon samples were fixed in 4% paraformaldehyde, embedded in paraffin and sectioned and stained with haematoxylin and eosin (H&E) for histological examination to determine the severity of inflammation and the extent of mucosal and crypt damage. Images were captured at 100× magnification using a US Moticam 3000 photomicrography imaging system.
Organ index
The spleen and colon were collected and evaluated by the organ index formula: organ index (%) = (organ weight/body weight) × 100%.
Sequencing of microbiota from colonic digesta samples and data analysis
DNA extraction
According to the manufacturer’s instructions, total genomic DNA was extracted from colonic digesta using the E.Z.N.A. ®Stool DNA Kit (D4015, Omega, Inc., USA).
PCR amplification and microbiota sequencing
The V3-V4 region of the bacterial 16S rDNA gene was amplified using the primers 341F (5′-CCTACGGGNGGCWGCAG-3′) and 805R (5′-GACTACHVGGGTATCTAATCC-3′) combined with barcode sequences. Then, the amplicon pools were prepared for sequencing, and the size and quantity of the amplicon library were assessed on an Agilent 2100 Bioanalyzer (Agilent, USA) and with the Library Quantification Kit for Illumina (Kapa Biosciences, Woburn, MA, USA), respectively. The libraries were sequenced on the NovaSeq PE250 platform.
Bioinformatics analysis
The sequences obtained by sequencing were subjected to quality filtering and modification. Alpha diversity and beta diversity were calculated by random normalisation to the same sequences. Then, according to the SILVA (release 132) classifier, feature abundance was normalised using the relative abundance of each sample. LEfSe was performed to assess differences in abundance. Phenotypes of bacterial communities were predicted using BugBase. The microbiome functions were determined by the KEGG profiles by combining the resulting metagenomic data with biodiversity data.
Colonic metabolite measurements and bioinformatics analysis
The collected samples were thawed on ice, and metabolites were extracted with 50% methanol buffer. Then, all samples were analysed by LC-MS according to the instructions for the system, and a TripleTOF 5600 Plus high-resolution tandem mass spectrometer (SCIEX, UK) was used to detect metabolites eluted from the column. The online KEGG database was used to annotate the metabolites by matching the exact molecular mass data (m/z) of samples with those from a database. We used an in-house fragment spectrum library of metabolites to validate the metabolite identification. Student t-tests were conducted to detect differences in metabolite concentrations between the two phenotypes. The P-value was adjusted for multiple tests using an FDR (false discovery rate). Supervised PLS-DA was conducted to discriminate the different variables between groups. The VIP value was calculated. A VIP cut-off value of 1.0 was used to select important features. For SCFA analysis, phosphoric acid (0.5% v/v) solution was added to the colonic digesta samples, then vortexed for 10 min, ultrasonicated for 5 min and centrifuged at 12,000 r/min and 4 °C for 10 min. Then, the supernatant was collected, and MTBE (containing internal standard) solution was added. After centrifugation at 12,000 r/min for 10 min at 4 °C, the supernatant was aspirated into a sampling bottle for GC–MS/MS analysis.
RNA-seq analysis from colon samples and data analysis
RNA isolation and purification
Total RNA was isolated and purified using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s procedure. The amount and purity of each RNA sample were quantified using a NanoDrop ND-1000 (NanoDrop, Wilmington, DE, USA).
cDNA library construction and bioinformatics analysis
The cDNA library was constructed, and we performed 150 bp paired-end sequencing on an Illumina NovaSeq 6000 (LC Bio, Hangzhou, China). Gene expression levels were estimated by calculating the value of fragments per kilobase of exon per million reads mapped (FPKM). We selected genes with a fold change > 2 and P-value < 0.05. Then, we analysed the enrichment of differentially expressed genes using GO enrichment analysis and the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis.
Characterisation and quantification of genes and proteins related to the immune response and intestinal barrier
Immunohistochemistry
Colon tissue pieces embedded in wax were sliced and dried. The sections were dewaxed in xylene and rehydrated in a gradient of alcohol to distilled water. Then, the sections were placed in antigen retrieval solution and heated for 10 min under low heat. After cooling, the samples were washed with 2% PBST and incubated with a blocking solution for 30 min. Next, the sections were incubated with primary antibody overnight, followed by incubation with secondary antibody overnight. After washing, the sections were incubated with ABC reagents for 30 min. The DAB chromogenic reagent was dripped onto the slice until the colour deepened to the correct hue. The sections were counterstained with haematoxylin, dehydrated and sealed. Finally, the sections were observed using a microscope, and images were acquired at 200× magnification. IHC quantification was performed using a parameter called the mean optical density detected using Image-Pro Plus (version 6.0, Rockville, MD, USA). This parameter was obtained by dividing the cumulative optical density value of each point on the picture by the area of the target distribution.
Quantitative RT-PCR analysis of gene expression
Briefly, total RNA was isolated using TRIzol reagent (Takara Biomedical Technology Co., Ltd., Beijing, China) and then reverse transcribed into cDNA using the PrimeScript™ RT reagent Kit and gDNA Eraser Kit (Takara Biomedical Technology, Beijing, China) following the manufacturer’s protocol. Next, mRNA levels were detected by standard real-time polymerase chain reaction (RT-PCR). RT-PCR was performed using a 7500 real-time PCR system (Applied Biosystems) with the SYBR Green mix kit (Takara Biomedical Technology, Beijing, China) to measure mRNA expression, with β-actin as a reference gene. Relative quantification of gene amplification by RT-PCR was performed using cycle threshold (CT) values. The relative mRNA expression of the selected genes was normalised to the control gene β-actin and determined using the 2−ΔΔCt method [19]. All primer sequences are listed in Supplementary Table S1.
Enzyme-linked immunosorbent assay
The cytokines in colon homogenate and TJ proteins in mucosal tissue were analysed using an enzyme-linked immunosorbent assay (ELISA) kit (Hnybio, Shanghai, China) according to the manufacturer’s instructions.
Statistical analysis
Phenotypic clinical evaluation, colonic SCFA concentration and immune cell and key immune factor content data were analysed using SPSS statistical software with the t-test. The results are presented as the mean and standard error of the mean (SEM). Statistical significance was based on a P-value < 0.05 (*P < 0.05, **P < 0.01 and ***P < 0.001). Microbial families and genera were compared using linear discriminant analysis effect size (LEfSe), and significant differences were denoted by an LDA score > 2 and P-value < 0.05. For metabolome data, partial least squares discriminant analysis (PLS-DA) and t-tests were performed between control and diseased individuals of the two breeds, with P < 0.05 and VIP > 1 indicating significantly different metabolites.
Results
Pig breeds exhibit varying degrees of phenotypic characteristics of disease resistance
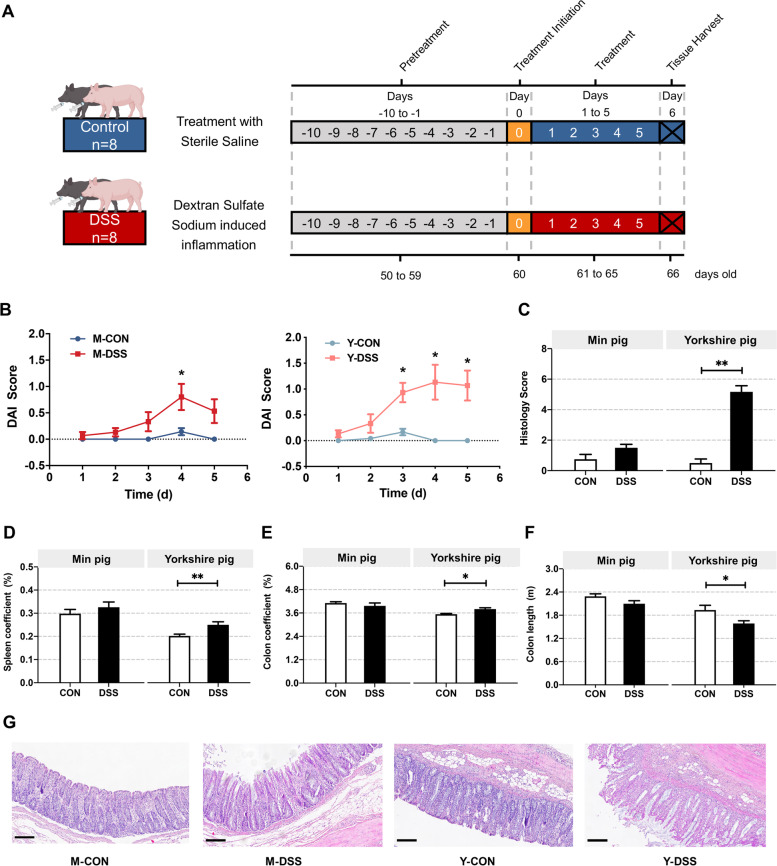
The unambiguously different disease resistance phenotypes of Min and Yorkshire pigs may be related to gut microbiota composition and host gut biological function. For this reason, it is imperative to first determine if there are distinct phenotypic differences between Min pigs and Yorkshire pigs. Therefore, we compared control Min pigs with acute colitis models of Min pigs (M-CON vs. M-DSS) and control Yorkshire pigs with acute colitis models of Yorkshire pigs (Y-CON vs. Y-DSS) (Fig. 1a). To enable a more intuitive comparison of two pig breeds, the disease activity index (DAI) and histological activity index (HAI) evaluation were used to compare disease activity between control individuals and DSS-induced colitis models. The DAI and HAI were increased in M-DSS and Y-DSS pigs compared with M-CON and Y-CON pigs, confirming the successful establishment of experimental colitis. Although impairments were observed in both M-DSS and Y-DSS pigs, Y-DSS pigs exhibited earlier and more severe organismal damage, as indicated by high body weight loss, stool consistency and haematochezia scores and extensive mucosal disease and crypt loss (P < 0.01) (Fig. 1b, c and g). These observations suggested differences in disease resistance between the two pig breeds. An important element of immunophenotype is the proportion of the colon and spleen, an immune organ containing abundant lymphocytes, in overall body weight. Significant spleen and colon enlargement were observed only in Y-DSS pigs, in addition to significantly shorter colon length (P < 0.05), indicating severe inflammation (Fig. 1d–f).
Fig. 1.
Response of Min pigs and Yorkshire pigs to DSS-induced colitis. a Min pigs and Yorkshire pigs were administered CON (control, sterile saline) or DSS (4% DSS in 100 mL of water) via gavage for 5 days. The first dose had a volume of 200 mL. b Plot of the disease activity index (DAI) values of Min pigs and Yorkshire pigs against the number of days (n = 8 samples/group). DAI values were calculated as (weight loss rate score + stool consistency score + bloody stool score)/3. c Histological activity index (HAI) values of Min pigs and Yorkshire pigs after DSS treatment. Comparison of d spleen coefficient, e colon coefficient and f colon length in Min pigs and Yorkshire pigs after DSS treatment (n = 8 samples/group). g Distal colon inflammation and histopathology score of Min pigs and Yorkshire pigs after DSS treatment (n = 5 samples/group). Scale bar, 200 μm. Data are presented as the mean ± SEM and were analysed by the t-test; significance is reported as *P < 0.05, **P < 0.01
Colonic microbiota and metabolites undergo dramatic remodelling between control and diseased individuals in both two pig breeds
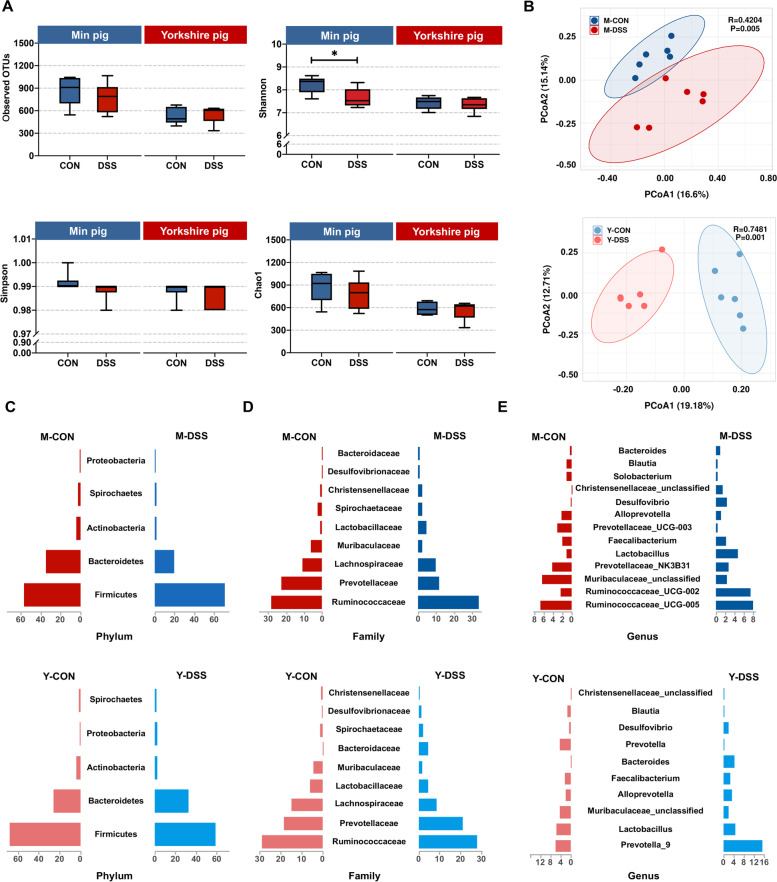
Intrigued by the results of the phenotypic characterisation, we next sought to determine the bacterial composition of the microbiota in control and diseased individuals of the two pig breeds to elucidate any compositional differences. We calculated α-diversity using the richness and Shannon indices as a measure of species diversity across the samples. α-diversity did not differ between Y-CON and Y-DSS pigs but was slightly lower (although still high) in M-DSS pigs than in M-CON pigs (Fig. 2a). Compared with control pigs, pigs with DSS-induced colitis showed significant differences in β-diversity, a measure of compositional similarity, as measured by unweighted UniFrac distances. It is known that pigs with DSS-induced colitis could alter the gut microbiota, and our findings further supported significant changes in bacterial composition in M-DSS and Y-DSS pigs (Fig. 2b). To further understand the microbial dissimilarities between control and diseased individuals of the two breeds, we evaluated the taxonomic composition of the colonic microbiota. The clusters of samples that significantly separated the microbiota according to breed and treatment are shown in Fig. 2c, d and e and Supplementary Fig. 1. M-CON pigs had a diverse community of different bacterial phyla in the colon, whereas M-DSS pigs displayed an increase in Firmicutes and major reductions in Bacteroidetes and Spirochaetes (Fig. 2c). A detailed investigation of the families belonging to Firmicutes revealed increases in Ruminococcaceae, Christensenellaceae and Lactobacillaceae in the colons of M-DSS pigs. Among families belonging to Bacteroidetes, Prevotellaceae and Muribaculaceae decreased, while Bacteroidaceae increased. Different results were obtained for Yorkshire pigs (Fig. 2d). At the phylum level, Y-DSS pigs displayed a reduction in Firmicutes and increases in Bacteroidetes, Proteobacteria and Spirochaetes compared with Y-CON pigs (Fig. 2c). The decreased abundance of Firmicutes included a wide range of families, including Lachnospiraceae, Christensenellaceae and Lactobacillaceae, and the increased abundance of Bacteroidetes was represented by increases in the families Prevotellaceae and Bacteroidaceae (Fig. 2d).
Fig. 2.
Shifts in the colonic microbiota composition of Min pigs and Yorkshire pigs after DSS treatment. a Comparison of the observed OTUs and Shannon, Simpson and Chao1 indices of the gut microbiota between control and diseased Min pigs and Yorkshire pigs (n = 6 samples/group). b Principal coordinate analysis (PCoA) plot of the microbial compositional profiles between control and diseased Min pigs and Yorkshire pigs (n = 6 samples/group). Relative abundance of the colonic microbiota at the c phylum, d family and e genus levels in Min pigs and Yorkshire pigs after DSS treatment (n = 6 samples/group)
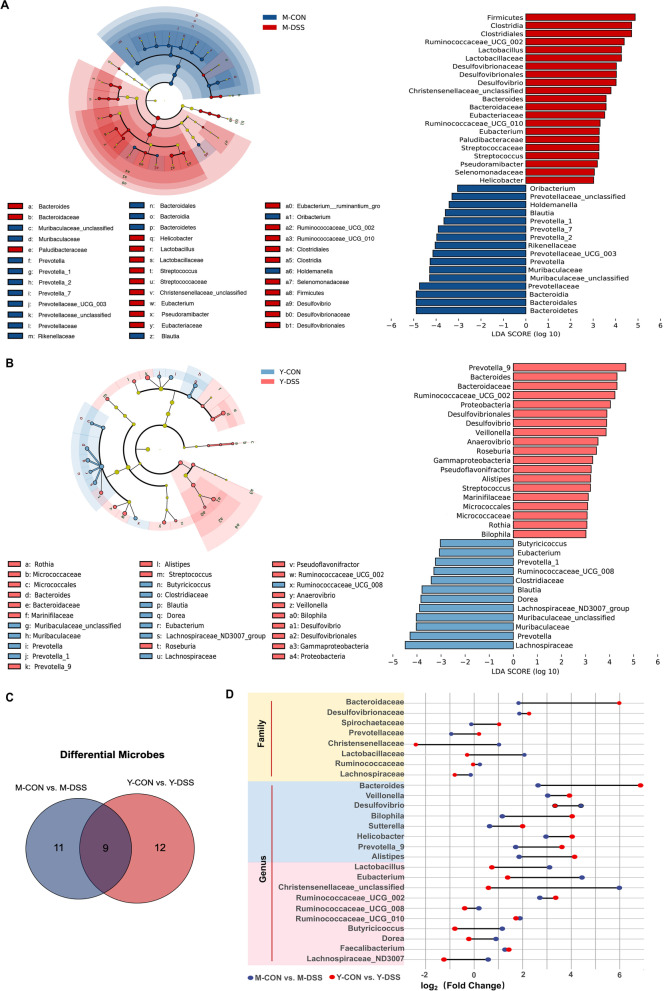
To identify specific bacterial genera that were characteristic of the two breeds, linear discriminant analysis effect size (LEFSe) was used to evaluate further the differences in bacterial composition between animals (Fig. 3a and b). In our study, differential abundance tests identified similarities in the dysbiosis patterns of the Min pigs and Yorkshire pigs. Our data indicated that Bacteroides, Desulfovibrio and Streptococcus, which were associated with intestinal inflammation [20, 21], were enriched in diseased individuals of two breeds, while Muribaculaceae and Blautia decreased in diseased individuals of two breeds (Fig. 3c and d). Another critical characteristic of dysbiosis in Yorkshire pigs was that diseased individuals demonstrated enrichment of Gammaproteobacteria, Bilophila, Veillonella and Prevotellaceae genera and depletion of Eubacterium, Dorea, Butyricicoccus and Lachnospiraceae. Of interest, many potentially beneficial microbes, such as Ruminococcaceae_UCG-02, Ruminococcaceae_UCG-10, Lactobacillus, Clostridia, Eubacterium and unclassified Christensenellaceae, were increased in M-DSS pigs (Fig. 3d) but not Y-DSS pigs.
Fig. 3.
Differences in microbial abundance between control and diseased Min pigs and Yorkshire pigs. Cladogram. LDA distribution. Linear discriminate analysis effect size (LEfSe) was used to analyse the differences in microbial abundance in a Min pigs and b Yorkshire pigs after DSS treatment (n = 6 samples/group). c Venn diagram for differential microbes in the comparisons M-CON vs. M-DSS and Y-CON vs. Y-DSS (n = 6). d Fold changes of differential microbes in the comparisons M-CON vs. M-DSS and Y-CON vs. Y-DSS (n = 6)
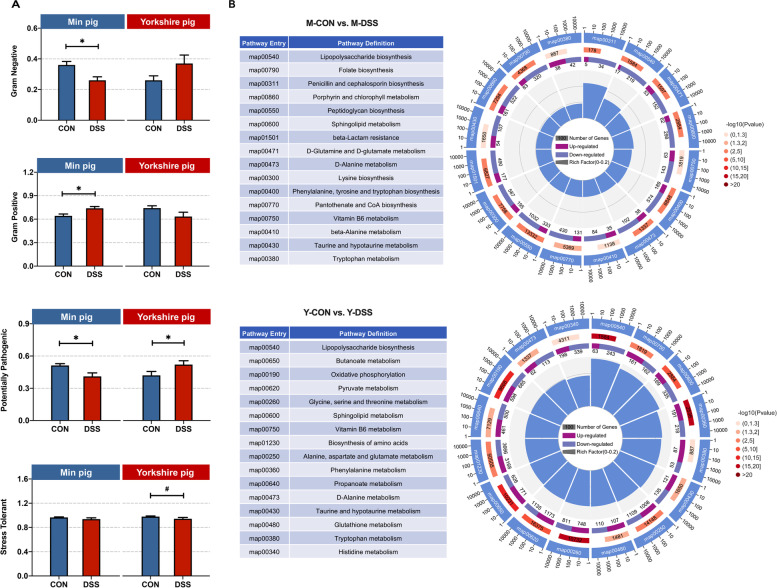
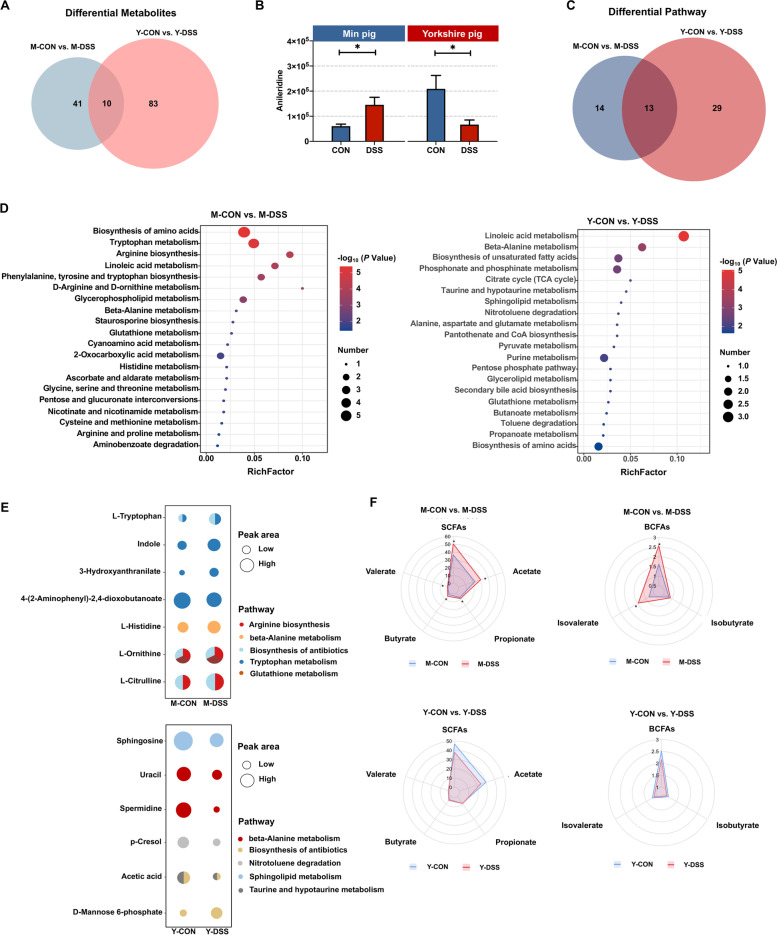
The data from the two breeds indicated that commensal bacteria of Min pigs might play important roles in the resistance to acute colitis. The phenotype prediction results based on the bacterial communities closely matched our experimental findings (Fig. 4a). Gram-negative bacteria were the most common co-pathogens [22]. In M-DSS pigs, the drastic decrease in the abundance of gram-negative bacteria was accompanied by an increase in gram-positive bacteria (P < 0.05), suggesting that potential pathogenicity decreased. In addition, the prediction results also revealed that potential pathogenicity decreased in M-DSS pigs but increased in Y-DSS pigs. The functions of the microbiome were determined by evaluating the Kyoto Encyclopedia of Genes and Genomes (KEGG) profiles (Fig. 4b). In the KEGG profiles, several third-level categories were considered microbial metabolic pathways, with “lipopolysaccharide biosynthesis”, “sphingolipid metabolism”, “beta-alanine metabolism”, “taurine and hypotaurine metabolism” and “tryptophan metabolism” being the most abundant in the two breeds. Some pathways, such as “butanoate metabolism”, were enriched in Yorkshire pigs.
Fig. 4.
Differential functions of the microbiome between control and diseased Min pigs and Yorkshire pigs. a Bacterial community phenotypes of control and diseased Min pigs and Yorkshire pigs were predicted using BugBase (n = 6 samples/group). b Comparison of microbial KEGG modules between M-CON and M-DSS pigs and Y-CON and Y-DSS pigs (n = 6)
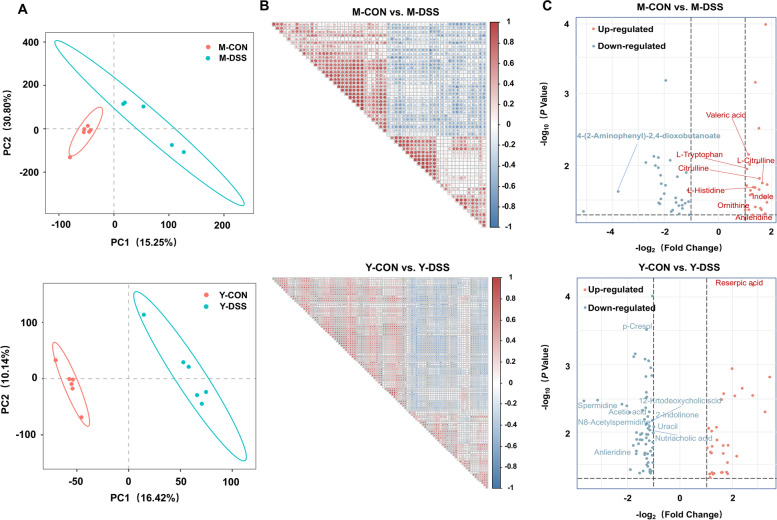
To gain further insight into the metabolic changes in the two breeds after DSS treatment, untargeted metabolomics analysis of colon content was performed. A total of 1578 compounds were identified in the colon metabolome. Precise results were obtained with partial least squares discriminant analysis (PLS-DA), which indicated a significant change in colonic metabolites after DSS treatment (Fig. 5a). The metabolites in each comparison were correlated with each other (Fig. 5b). Univariate analysis of fold change, t-test and variable importance in projection (VIP) filtering of the expressed metabolites indicated that 51 metabolites were significantly different in Min pigs, including 26 that were significantly higher and 25 that were relatively low in M-DSS pigs (Fig. 5c and Supplementary Fig. 2). A total of 93 metabolites were significantly different in Y-DSS pigs compared with Y-CON pigs, including 31 upregulated and 62 downregulated metabolites (Fig. 5c).
Fig. 5.
Colon metabolome changes. a Partial least squares discriminant analysis (PLS-DA) of metabolite composition in Min pigs and Yorkshire pigs after DSS treatment (n = 6 samples/group). b Correlations of the metabolites between control and diseased Min pigs and Yorkshire pigs (n = 6 samples/group). c Changes in colonic metabolites in Min pigs and Yorkshire pigs after DSS treatment (n = 6 samples/group)
Interestingly, the Venn diagram revealed 10 differential metabolites in common between the two comparisons (M-CON vs. M-DSS, Y-CON vs. Y-DSS) (Fig. 6a). Nine of the common differential metabolites showed the same trends between control and diseased individuals of the two breeds; the remaining metabolite, anileridine, showed opposing directions. Anileridine was significantly higher in M-DSS pigs but significantly lower in Y-DSS pigs than in the corresponding controls (Fig. 6b). To determine the functions of the altered metabolites, we performed KEGG enrichment analysis (Fig. 6c and d), and the results were in basic agreement with the microbiome functions. The common pathways enriched in comparisons of M-CON vs. M-DSS and Y-CON vs. Y-DSS included linoleic acid metabolism, biosynthesis of amino acids, glutathione metabolism and beta-alanine metabolism. Notably, the significantly altered metabolites related to beta-alanine metabolism exhibited opposing trends of change between the two breeds. These metabolites increased in M-DSS pigs, indicating activation of this pathway but decreased in Y-DSS pigs. The specific pathways enriched in M-DSS pigs compared with M-CON pigs were arginine biosynthesis, tryptophan metabolism and biosynthesis of antibiotics. Specifically, we found that some intermediate metabolites involved in the biosynthesis of antibiotics, such as ornithine, L-citrulline and L-tryptophan, were significantly increased in M-DSS pigs. All significantly altered metabolites involved in tryptophan metabolism were upregulated in M-DSS pigs (Fig. 6e), suggesting that the metabolome of Min pigs may undergo a superior adaptive adjustment by increasing the accessibility of tryptophan to the host. The specific pathways enriched in Y-DSS pigs compared with Y-CON pigs were sphingolipid metabolism, nitrotoluene degradation and taurine and hypotaurine metabolism. The major intermediate metabolites involved in these pathways, such as spermidine, sphingosine and acetic acid, were downregulated in Y-DSS pigs compared with Y-CON pigs (Fig. 6e). In addition to the relative concentrations of colonic small molecules that were identified by metabolomics, the absolute concentrations of the total short-chain fatty acids (SCFAs), acetate, propionate, butyrate, valerate, isobutyrate and isovalerate were quantified (Fig. 6f). Acetate, propionate, butyrate, valerate and isovalerate were significantly higher in M-DSS pigs than in M-CON pigs (P < 0.05) but lower in Y-DSS pigs than in Y-CON pigs, potentially indicating a relatively lower self-adjustment capacity of Yorkshire pigs during acute colitis.
Fig. 6.
Comparison of colonic metabolites in control and diseased Min pigs and Yorkshire pigs. a Venn diagram for differential metabolites in the comparisons M-CON vs. M-DSS and Y-CON vs. Y-DSS. b Common differential metabolites between control and diseased Min pigs and Yorkshire pigs. c Venn diagram for differential pathways of the comparisons M-CON vs. M-DSS and Y-CON vs. Y-DSS. d Pathway enrichment analysis was performed using the significantly different metabolites between control and diseased Min pigs and Yorkshire pigs (n = 6 samples/group). e The major pathways of significantly different metabolites in Min pigs and Yorkshire pigs after DSS treatment. f Concentrations of short-chain fatty acids (SCFAs) in Min pigs and Yorkshire pigs after DSS treatment (n = 8 samples/group)
Spearman’s correlation coefficient (R > 0.50, P < 0.05) assessed the correlation between the colonic microbiota and metabolites. In Min pigs (Supplementary Fig. 3a), positive correlations were observed mainly between potentially beneficial microbes Lactobacillus, Eubacterium, Blautia, Ruminococcaceae_UCG_002 and Christensenellaceae and acetate, L-tryptophan, butyrate, benzoic acid and anileridine (0.58 < R < 0.93, P < 0.05). These microbiota and metabolites are involved in the biosynthesis of amino acids, biosynthesis of antibiotics, linoleic acid metabolism, glycerophospholipid metabolism and tryptophan metabolism. The Spearman’s rank correlation network showed that potentially beneficial microbes might assist in metabolite recovery and support normal gut function (Supplementary Fig. 3c). In Yorkshire pigs (Supplementary Fig. 3b), negative correlations mainly existed between potentially harmful microbes Veillonella, Alistipes, Bacteroides, Bilophila and Desulfovibrio and fatty acyls, phenols, imidazopyridine, isoflavonoids, sterol lipids, carboxylic acids and derivatives (0.58 < R < 0.96, P < 0.05), which are involved in the biosynthesis of antibiotics, taurine and hypotaurine metabolism, butanoate metabolism, linoleic acid metabolism, secondary bile acid biosynthesis and beta-alanine metabolism. The correlation network was built from the Spearman’s nonparametric rank correlation coefficient between microbiota and the above metabotypes (Supplementary Fig. 3d). Potentially harmful microbes were negatively correlated with most of the above metabotypes, including metabolites such as N8-acetylspermidine, spermidine, 12-ketodeoxycholic acid, nutriacholic acid, reserpic acid, kanzonol and valerate. These data suggested that the changes in microbes in Yorkshire pigs were associated with metabolic dyshomeostasis and interfered with normal intestinal function.
Gut barrier function responds differently to DSS treatment between Min and Yorkshire pigs
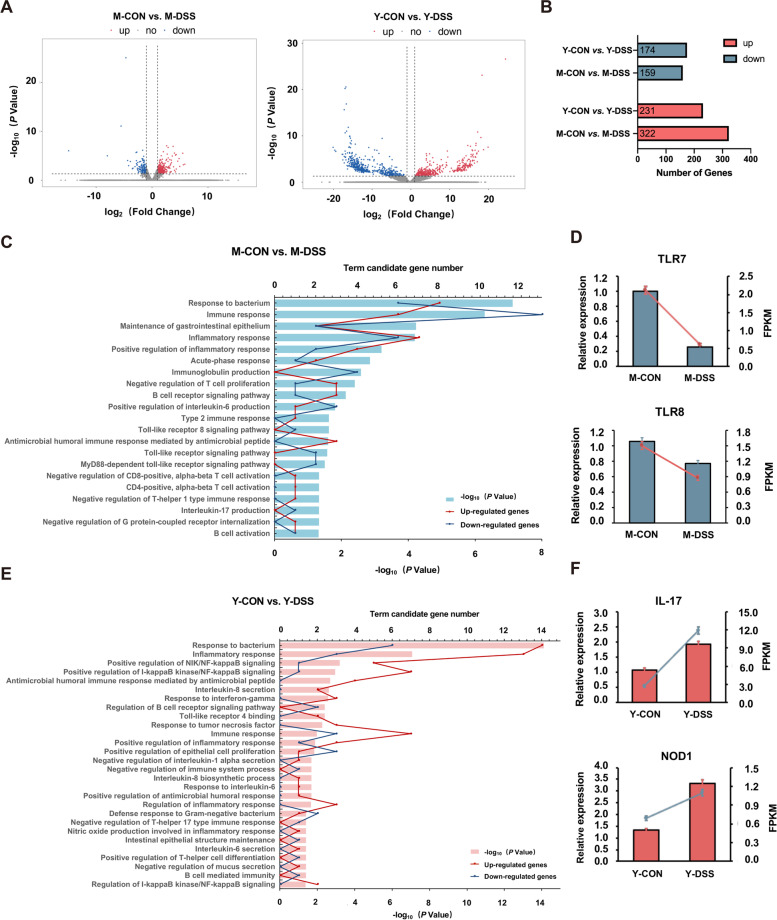
Gut barrier function comprises three major parts: a mechanical barrier, an ecological barrier and an immunological barrier [23]. Disorganisation of the ecological barrier (microbial dysbiosis) often leads to dysfunction of the mechanical barrier and immunological barrier. To explore the underlying mechanisms of core bacterial protection of the host colon, the colon gene expression profiles of the two breeds were quantified by RNA-seq analysis. Figure 7a shows the volcano plots generated to visualise the distribution of expressed genes between control and diseased Min pigs and Yorkshire pigs. In total, 322 genes were upregulated and 159 genes were downregulated in M-DSS pigs compared with M-CON pigs, and 231 genes were upregulated and 174 genes were downregulated in Y-DSS pigs compared with Y-CON pigs (Fig. 7b). To gain insight into the biological processes of the immunological barrier and mechanical barrier that were differentially regulated between control and diseased Min and Yorkshire pigs, gene ontology (GO) enrichment analysis was performed (Fig. 7c–f). Compared with M-CON pigs, several terms involved in immunity, such as response to bacterium, immune response, inflammatory response, acute-phase response, type-2 immune response, CD4-positive, alpha-beta T-cell activation, negative regulation of T-helper 1 type immune response, negative regulation of CD8-positive, alpha-beta T-cell activation, antimicrobial humoral immune response mediated by antimicrobial peptide, toll-like receptor signalling pathway and MyD88-dependent toll-like receptor signalling pathway, were enriched in M-DSS pigs. The expression trends of the TLR7 and TLR8 genes identified by RNA-seq analysis were validated by qRT-PCR, which was downregulated in M-DSS pigs (P < 0.05) (Fig. 7d). In Y-DSS pigs, GO analysis showed that the following terms belonging to biological processes of immunity and the intestinal structure were enriched compared with Y-CON pigs: response to bacterium, inflammatory response, positive antimicrobial humoral immune response mediated by antimicrobial peptide, Toll-like receptor 4 binding and immune response. A few pathways were enriched in Y-DSS pigs but not M-DSS pigs, such as regulation of NIK/NF-κB signalling and positive regulation of I-κB kinase/NF-κB signalling. The genes in these pathways exhibited strong upregulation in Y-DSS pigs (P < 0.05), which indicated that inflammation was exacerbated in Yorkshire pigs. Some pathways involved in the immune response, including interleukin-8 secretion, interleukin-6 secretion, response to tumour necrosis factor, response to interferon-gamma and T-helper 17 type immune response, were also activated in Yorkshire pigs. These results indicated that the Th17-type immune response, an important driver of autoimmune disease with inflammatory properties, was dominant in Yorkshire pigs [24]. Matching the altered genes to the KEGG database indicated that the IL-17 signalling pathway was enriched in Yorkshire pigs (Supplementary Fig. 4).
Fig. 7.
RNA-seq analysis of the colon of Min pigs and Yorkshire pigs. a Volcano plot of differentially expressed genes between control and diseased Min pigs and Yorkshire pigs. b Numbers of upregulated and downregulated differentially expressed genes in Min pigs and Yorkshire pigs after DSS treatment. GO functional enrichment analysis of differentially expressed genes and qRT–PCR validation of the biological process of immunity and intestinal structure in c–d Min pigs and e–f Yorkshire pigs after DSS treatment
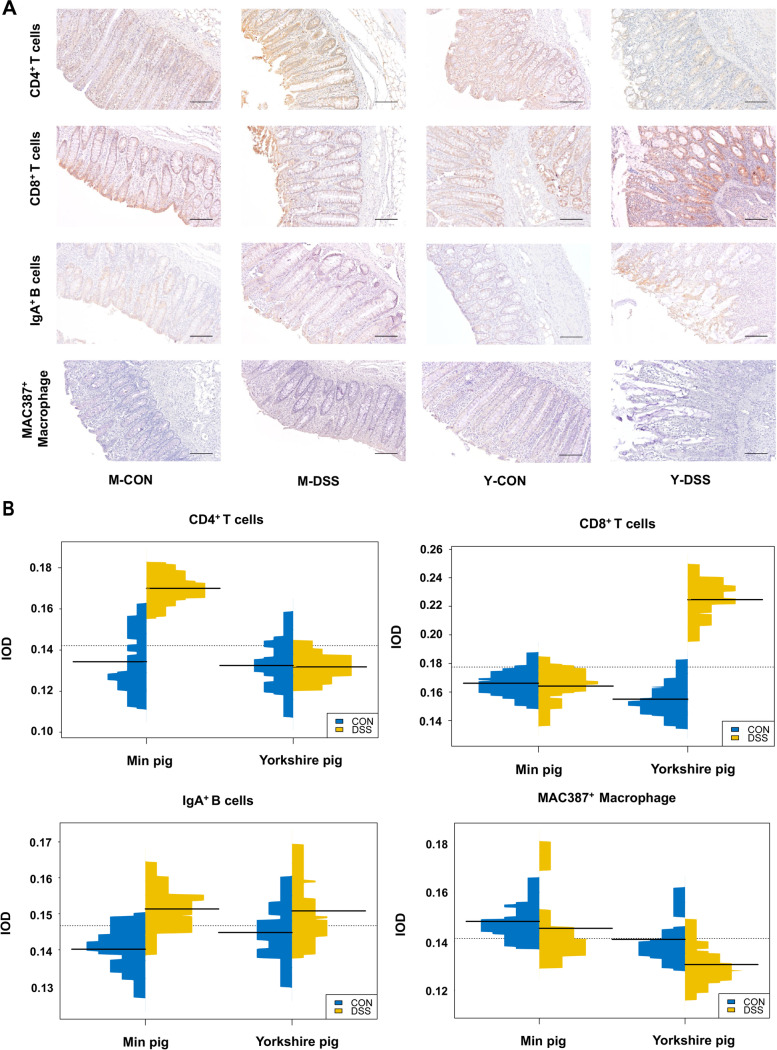
Since several terms involved in the mechanical barrier and immunological barrier were enriched in RNA-seq analysis, immune-related biological processes and pathways were analysed in the colon samples. We validated the activation of these biological processes and identified their consequences. For the immunological barrier, the most striking difference in immune cells was that CD4+ T cells were significantly higher in M-DSS pigs than in M-CON pigs (P < 0.05). Conversely, CD8+ T cells were significantly higher in Y-DSS pigs than in Y-CON pigs (P < 0.05) (Fig. 8a and b).
Fig. 8.
Changes in immune cells in the pig colon after DSS treatment. a Immunohistochemical staining (IHC) shows the numbers and distribution of CD4+ T cells, CD8+ T cells, IgA+ B cells and MAC387+ macrophages in control and diseased Min pigs and Yorkshire pigs (n = 4 samples/group). b Qualitative comparisons of the numbers of immune cells. The results are expressed as integrated optical density (IOD)
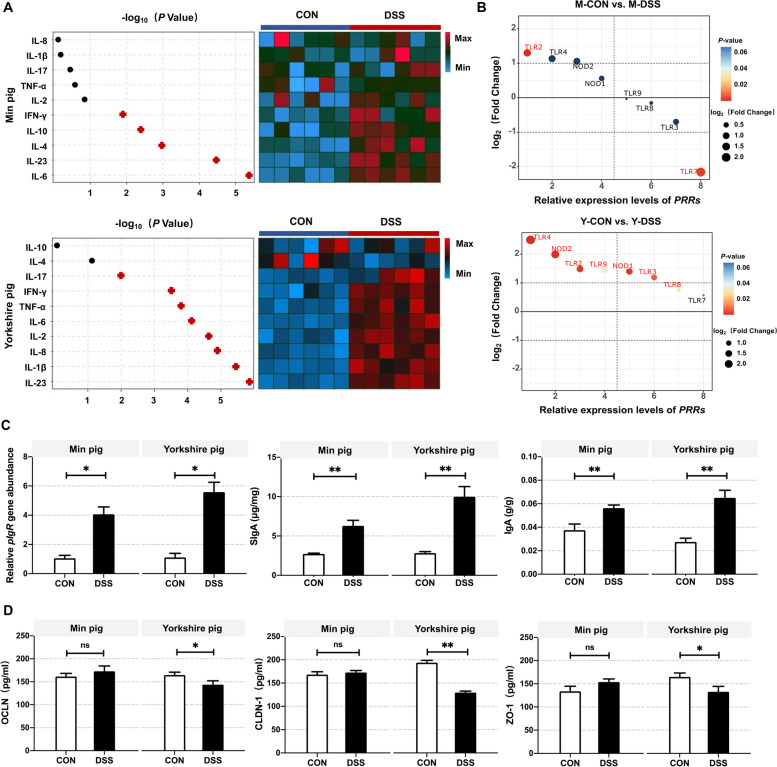
Among inflammatory cytokines, IL-4, IL-6, IL-10, IL-23 and IFN-γ levels were significantly increased in M-DSS pigs (P < 0.05). IL-4, IL-6 and IL-10 are the primary regulators of type-2 immune response [25, 26]. IL-1β, IL-2, IL-8, IL-17, IL-23, IFN-γ and TNF-α levels were increased in Y-DSS pigs compared with Y-CON pigs (P < 0.01) (Fig. 9a). Among PRR genes, TLR2 was significantly downregulated in M-DSS pigs (P < 0.05), while TLR7 was downregulated (P < 0.05). TLR2, TLR3, TLR4, TLR8, NOD1 and NOD2 were significantly upregulated in Y-DSS pigs (P < 0.05) (Fig. 9b). Both immunoglobulin A (IgA) and secreted IgA (SIgA) were increased in the colons of M-DSS and Y-DSS pigs (P < 0.05). Although polymeric immunoglobulin receptor (pIgR) levels were increased in M-DSS and Y-DSS pigs compared with the corresponding controls (P < 0.05), the increase was larger in Y-DSS pigs (Fig. 9c). With respect to the mechanical barrier, unlike Min pigs, tight junction (TJ) proteins such as occludin (OCLN), claudin (CLDN-1) and zonula occludens (ZO-1) were downregulated in Y-DSS pigs (P < 0.05) (Fig. 9d). Taken together, these results strongly suggest that Yorkshire pigs are more susceptible to inflammatory stimuli, leading to sustained inflammation.
Fig. 9.
Analysis of the response of the gut barrier to inflammation based on transcriptome data. a Heat map showing the changes in inflammatory cytokines associated with different types of immunity in colon homogenate isolated from Min pigs and Yorkshire pigs after DSS treatment (n = 6 samples/group). b Relative expression levels of pattern recognition receptors (PRRs) in Min pigs and Yorkshire pigs after DSS treatment as detected by qRT-PCR (n = 6 samples/group). c Confirmation of autoantibody secretion in Min pigs and Yorkshire pigs after DSS treatment. d To evaluate the mechanical barrier, tight junction (TJ) proteins in Min pigs and Yorkshire pigs treated with DSS were quantified by ELISA
Discussion
Within the gut, the colon is home to the densest and most metabolically active community (comprising more than 1013 individual microbial cells) [27]. Although colitogenic pathobionts promote IBD development, commensal bacteria are also crucial for reducing IBD susceptibility [7]. The protective effect of commensal bacteria is evident from studies using germ-free mice, which are more susceptible to DSS-induced colitis than conventionally housed mice [28, 29]. The microbiota may have a major role in gut health, including the maturation of host immune responses, protection against enteric pathogen proliferation and response to or modification of specific drugs [6]. In the present study, diseased Min pigs exhibited mild organismal damage, as indicated by lower values of the body weight loss, stool consistency and haematochezia scores and limited mucosal disease and crypt loss, bolstering interest in whether changes in microbiota were associated with disease resistance. Important host-microbiota interactions in Min pigs are likely crucial for host physiology and the disease phenotype. We integrated microbiota sequencing, metabolomics and transcriptomics to investigate the host-microbiota crosstalk mechanisms that contribute to disease resistance.
In this study, all results for the acute colitis models were analysed separately for Min pigs and Yorkshire pigs. Progress to date indicates that IBD is a polymicrobial disease in which a combination of various gut microbial factors, abnormal immune responses and a weakened intestinal mucosal barrier leads to aberrant host-microbial interactions [30]. Intriguingly, the disease responses of the two breeds were not identical, although both exhibited inflammation. Consistent with many previous assessments of changes in microbiota in IBD [20, 31, 32], Y-DSS pigs exhibited a reduction in Firmicutes and increases in Bacteroidetes, Proteobacteria and Spirochaetes with Y-CON pigs. Surprisingly, however, similar changes were not observed in Min pigs. In contrast to Yorkshire pigs, M-DSS pigs displayed an increase in Firmicutes and major reductions in Bacteroidetes and Spirochaetes. At the genus level, more specific shifts were clearly observed. The higher incidence of populations of members of Bacteroidetes and Proteobacteria in the colonic mucosa of IBD patients has been reported [20, 21]. Our data demonstrated that Bacteroides and Desulfovibrio, as members of Bacteroidetes and Proteobacteria, respectively, were enriched in diseased individuals of the two breeds. Still, the extent and severity of these changes were less pronounced in Min pigs than in Yorkshire pigs. Increases in these bacteria are a frequent finding in IBD-associated dysbiosis, suggesting that these bacteria might function as potentially harmful microbes and play roles in the pathogenesis of the disease. A possible mechanism by which members of Bacteroidetes and Prevotella influence the severity of colitis is by removing sulphate from mucin with sulfatases, resulting in mucin degradation [33–35]. In addition, Desulfovibrio, as a sulphate-reducing bacteria (SRB), increases the concentration of hydrogen sulphide, eroding the intestinal epithelium [36]. Therefore, dysbiosis of intestinal microbiota might contribute to inflammation by an impairment of intestinal barrier. In our study, downregulated OCLN, CLDN-1 and ZO-1 levels were observed in Y-DSS pigs, which were consistent with our inference of intestinal barrier alteration with the role of microbes. Thus, it seems plausible to infer that more aggravative compromised intestinal barrier and more aggressive translocation of bacteria in Yorkshire pigs might influence each other, together exacerbating inflammation.
Additional key characteristics of dysbiosis in Y-DSS pigs were enrichment of Gammaproteobacteria, Bilophila, Veillonella, Sutterella, Alistipes and Prevotella and depletion of Blautia, Eubacterium, Dorea, Butyricicoccus and Lachnospiraceae. According to the Spearman’s rank correlation network, we found that the increases in Bilophila, Alistipes and Bacteroidetes in the colons of Yorkshire pigs were inversely correlated with the changes in acetate, deoxycholic acid and nutriacholic acid. The higher abundances of bile-tolerant microbes (Bilophila, Alistipes and Bacteroidetes) indicated that these bacteria might be the main contributors to the disruption of bile acid metabolism [37–39]. As supportive evidence, taurine and hypotaurine metabolism, involved in bile acid metabolism, were enriched both in KEGG profiles of microbiota and metabolite in Yorkshire pigs. Furthermore, members of Blautia, Eubacterium, Dorea, Butyricicoccus and Lachnospiraceae are among the main producers of SCFAs [40, 41]. SCFAs, particularly butyrate, have beneficial effects on the regulation of intestinal immune function and inhibition of intestinal inflammation [42, 43]. Depletion of these bacteria in Yorkshire pigs might be an influential factor to reduce SCFA concentrations and enlarge intestinal inflammation; these findings were reinforced by the results for inflammatory cytokine levels and mucosal barrier function. Remarkably, many potentially beneficial microbes, such as Lactobacillus, Ruminococcaceae genera, Eubacterium and Christensenellaceae genera belonging to Firmicutes, were increased in M-DSS pigs. The increased abundances of several Ruminococcaceae genera and Eubacterium could increase SCFA concentrations, thus protecting intestinal barrier function and exerting anti-inflammatory properties in the host [44]. Moreover, since Christensenellaceae is considered a potential probiotic that is negatively correlated with inflammation and metabolic-related diseases [45], the higher abundance of Christensenellaceae in M-DSS pigs suggests that defence against disease may be triggered in Min pigs. The more interesting finding, however, was the role of Lactobacillus in Min pigs. Lactobacillus expresses tryptophanase and is the most important commensal that metabolises tryptophan [46]. Lactobacillus protects the integrity of the intestinal epithelial barrier damaged by pathogenic bacteria and reduces the number of potentially pathogenic bacteria [47, 48]. Consistent with other studies, restoration of mechanical function and enrichment of tryptophan metabolites were observed in Min pigs. The data herein of microbiota and microbial pathways suggested that Min pigs can adjust the commensal bacteria to achieve disease remission.
The gut microbiota can produce diverse metabolites via anaerobic fermentation of exogenous undigested dietary components that reach the colon as well as endogenous compounds that are generated by microbes and the host. Microbial metabolites can access and interact with host cells to affect immune and inflammatory responses. Strong associations between colonic microbiota and metabolites were found by pairing metabolomics with microbiota sequencing analysis, showing changed levels of indole derivatives, bile acid, SCFAs and other metabolites. Previous studies have implicated that specific classes of metabolites, notably bile acids, SCFAs and tryptophan metabolites, were linked to the pathogenesis of IBD [37, 49]. We observed reduced SCFA concentrations and inhibition of taurine and hypotaurine metabolism in Yorkshire pigs. Min pigs exhibited a starkly different trend than Yorkshire pigs. In Min pigs, acetate, propionate, butyrate, valerate and isovalerate levels were enhanced, which were associated with the alteration of indigenous microbiota, and thus attenuated mucosal inflammation. In addition, tryptophan metabolism was promoted in Min pigs, which correspond to the increased abundances of Lactobacillus and other potentially beneficial microbes, indicating the gut microbiota can reduce tryptophan excretion and liberated more tryptophan to the host. Gut commensal bacteria can utilise tryptophan to ameliorate DSS-induced colitis by promoting colonic goblet cell differentiation and inducing mucin gene expression [37, 46]. These changes might explain the higher disease resistance of Min pigs compared with Yorkshire pigs. Additionally, the data on colonic metabolites that correlated negatively with disease activity showed that anileridine (spasmolytic action in the intestine [50]) and spermidine (protective role in colitis and colon carcinogenesis [51]) were decreased in Y-DSS pigs, suggesting an out-of-control state. Anileridine content was increased in M-DSS pigs, further supporting the ability of Min pigs to resist acute colitis. Overall, the results of the present study indicated that the beneficial gut microbes in Min pigs heal the colon and recover metabolites, given the good correlation between the gut microbiota and metabolites.
Microbial imbalance can directly contribute to gut barrier dysfunction and an immature immune system [52]. Gut barrier dysfunction is also strongly correlated with inflammation [53]. To further study the potential links of the gut microbiota with the host immune response and intestinal barrier function in the two pig breeds, RNA-seq analysis of the colons of Min pigs and Yorkshire pigs was performed. GO analyses showed enrichment of pathways involved in immune function, including the immune response, inflammatory response and antimicrobial humoral immune response mediated by antimicrobial peptides, in both Min and Yorkshire pigs, suggesting a response to exogenous stimuli. Although the responses were similar, the colonic inflammatory response was more active in Y-DSS pigs than in M-DSS pigs. Specifically, several PRRs signalling and downstream inflammation-related pathways, such as Toll-like receptor 4 binding, NOD-like receptor signalling, NIK/NF-κB, I-κB kinase/NF-κB signalling and proinflammatory cytokines secretion pathways, were enriched in Yorkshire pigs but not Min pigs. In agreement with the GO enrichment results, the increase of some NLR and TLR genes and their downstream proinflammatory factors were observed in Y-DSS pigs but not M-DSS pigs, which were confirmed by Elisa analysis and real-time PCR. It has been reported that PRRs, such as TLR4, NOD1 and NOD2, initiate inflammatory responses by sensing microbe-associated molecular patterns (MAMP) [54, 55]. PRRs serve the function of distinguishing potentially beneficial microbes and potentially harmful microbes, with different consequences for the host (inflammation versus immune homeostasis) [56]. Moreover, recent data suggested that the context in which the host receptors (PRRs) receive MAMP stimulation determines the quality of the inflammatory and immune response [57, 58]. DSS notoriously disrupts intestinal barrier integrity, allowing commensal microbiota to intestinal epithelium [59, 60]. In Y-DSS pigs, with the increase of potentially harmful microbes, such as Spirochaetes [61], microbial ligand signals were received in the presence of intestinal barrier damage and thus might activate PRRs signalling and downstream inflammation-related pathways. In M-DSS pigs, many potentially beneficial symbiotic microbes increased, such as Lactobacillus, suggesting that some MAMPs promoted beneficial outcomes. Under such circumstances, PRRs including TLR2, TLR3, TLR4, TLR8, NOD1 and NOD2 exhibited nonactivation compared with M-CON pigs, eliciting tolerant responses. Of course, some PRRs, such as TLR2, were activated via sensing MAMPs. Generally, TLR2 mediates the immune response to gram-positive cell wall peptidoglycan but does not affect the expression of NF-κB and MyD88 [62], shaping beneficial host immune responses. Therefore, compared to Yorkshire pigs, Min pigs might have evolved a more established strategy to modulate the structure of the colonic microbiota and manipulate PRRs pathways, resulting in less inflammation.
The gut microbiota contributes to the development of normal immunity but, when dysregulated, can promote autoimmunity and affect the immunological barrier through effects on lymphocytes [63]. GO analyses showed that the Th17-type immune response was activated, and that a plethora of proinflammatory factors was secreted in Y-DSS pigs. In addition, Th1 and cytotoxic CD8+ T-cell (Tc) cytokines (IFN-γ and TNF-α) and Th17 cytokines (IL-17) were increased, suggesting that cell-mediated immunity was promoted in Y-DSS pigs via Th1, Th17 and Tc immune responses. Regarding a possible correlation with microbiota, Prevotellaceae genera were increased in Y-DSS pigs, which could stimulate epithelial cell production of IL-6, IL-8 and chemokine ligand 20 (CCL20) and promote the mucosal Th17 immune response and neutrophil recruitment [64]. Stimulation of most TLRs leads to Th1 rather than Th2 differentiation [65]. The levels of some NLR and TLR genes increased in Y-DSS pigs, which were consistent with the activation of Th1 and Th17-type immune responses. Furthermore, CD8+ T-cell levels were markedly increased in the lamina propria of Yorkshire pigs. Although CD8+ T cells build a protective barrier against attack by surrounding host cells, their proliferation indicates that numerous pathogens are attacking. Accumulating evidence suggests that colitis is characterised by an expansion of colonic CD8+ T cells [66–68]. Distinct from the increase in CD8+ T cells in Y-DSS pigs, CD8+ T cells remained at steady-state levels in M-DSS pigs, but an increase in CD4+ T cells was observed. CD4+ T cells reflect immune status; higher levels indicate better immune performance. CD4+ T cells can be categorised into Th1 and Th2 cells, which drive different immune responses [69]. GO enrichment analyses of genes under immunity terms revealed CD4 T-cell activation, and acute-phase response was upregulated in Min pigs, indicating acceleration of the end of the inflammatory phase of type-1 immune response and initiation of the Th2-type immune-mediated repair phase. Characterisation and quantification of CD4+ T cells and Th2 cytokines may also be robust evidence for supporting our interpretation of the initiation of Th2-type immune-mediated repair phase. To date, investigations found Clostridia clusters IV and XVIII [70, 71] promote CD4+ regulatory T-cell (Treg) differentiation and Lactobacillus plantarum D-alanylated teichoic acid [72] signals through TLR2 to modulate Treg cells, which are negative regulators of inflammation. Moreover, Lactobacillus reuteri, together with tryptophan, can reprogram intraepithelial CD4+ T cells into tolerant immunoregulatory T cells [73]. The relative abundances of these genera (Clostridia, Lactobacillus) were increased in M-DSS pigs. Additionally, tryptophan metabolism and TLR2 activation were promoted in M-DSS pigs. These data provided a possible explanation that resident bacteria in Min pigs modulated the immune response to remain tolerant and control excessive inflammatory reactions.
IgA is a major class of immunoglobulins that is produced in mucosal tissues, including in the intestine. In the intestinal lumen, IgA is produced as polymeric IgA at high concentrations [7]. Polymeric IgA is transported via pIgR, which is expressed on intestinal epithelial cells and is released into the intestinal lumen as SIgA [74]. Although the levels of pIgR, SIgA and IgA increased in both M-DSS and Y-DSS pigs, the change was more significant in Y-DSS pigs, suggesting a more robust adaptive immune response in Yorkshire pigs compared with Min pigs. Recent studies have shown a substantial correlation between gut mechanical barrier dysfunction and IBD [75]. The intestinal mechanical barrier is also strongly correlated with the microbiota and its metabolites [76]. On the one hand, numerous bacterial products regulate gut barrier function by activating the TLR and NLR pathways [77]. On the other hand, microbial metabolites (such as SCFAs, secondary bile acids and indole derivatives) produced in the gut directly influence gut barrier integrity by interacting with immune cells [78]. In this study, common functional terms related to intestinal structure and trefoil factors in the maintenance of gastrointestinal epithelium were upregulated in M-DSS pigs, indicating repair of intestinal mucosal damage in Min pigs. In addition, the analyses of colonic microbiota and metabolites and TJ proteins in the mechanical barrier provided a further explanation of the intestinal mechanical barrier dysfunction in Yorkshire pigs and intestinal mechanical barrier restoration in Min pigs. In Y-DSS pigs, depletion of members of Blautia, Eubacterium, Dorea, Butyricicoccus and Lachnospiraceae could be associated with the reduction in SCFA concentrations, resulting in obstruction of the epithelial barrier. Decreases in secondary bile acids as a factor may further accentuated gut barrier dysfunction. In M-DSS pigs, enrichment of Lactobacillus and promotion of tryptophan metabolism might increase indole derivative levels, which could enhance intestinal barrier function. At the same time, the increases of SCFA production might result from the increase in several potentially beneficial microbes, maintaining colonic OCLN and CLDN levels. In addition, TLR2 activation was increased in M-DSS pigs. TLR2 enhances the integrity of the intestinal epithelial barrier through protein kinase C (PKC) [79]. These data suggested that adjustment of microbial community structures and subsequent metabolite recovery in Min pigs may protect the intestinal barrier in this IBD model.
Conclusions
Here, an effect of breeds characterised by different disease resistance on host-microbiota interaction was discovered using microbiota sequencing, metabolomics, transcriptomics and other analyses (Fig. 10). Our study suggested that partial PRR nonactivation was maintained in M-DSS pigs with the increases of potentially beneficial microbes and stabilisation of intestinal barrier. Our data also suggested that the increase in CD4+ T cells in M-DSS pigs but not in Y-DSS pigs might be relevant to the increases in Lactobacillus and tryptophan metabolism, thus retaining Th2-type immune superiority and an immune tolerance pattern. Furthermore, association analyses revealed a positive association between Lactobacillus, Christensenellaceae, Eubacterium and SCFA concentrations, suggesting beneficial action in protecting intestinal barrier in Min pigs. Growing evidence suggests host-microbiota interaction has been linked to IBD disease progression; thus, the results of this study may contribute to screening for core intestinal microbial candidates associated with host health and support a shift in thinking about anti-inflammatory strategies from improving immunity to immunosuppression.
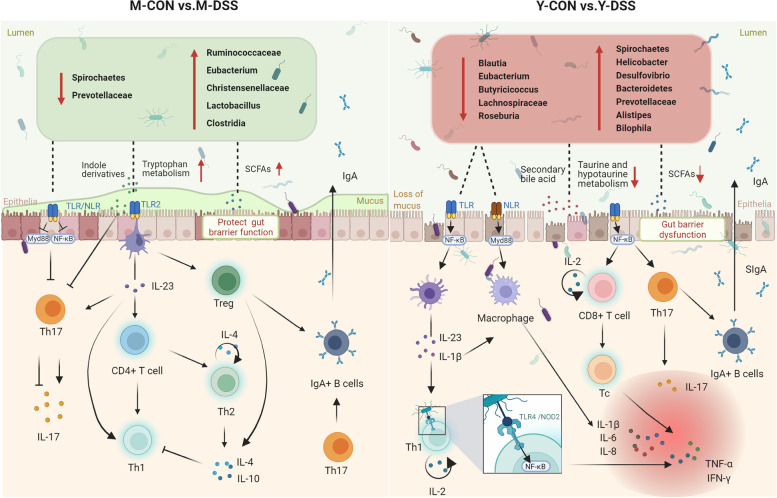
Fig. 10.
Host-microbiota interaction-mediated resistance to inflammatory bowel disease in Min pigs and Yorkshire pigs. The comparison of the responses of the two breeds to disease and inflammatory immune stimuli showed that colon microbial taxonomies, functions and metabolites and their interactions with host physiological suitability were associated with resistance. Many potentially beneficial microbes, such as Lactobacillus, Clostridia, Eubacterium and several Ruminococcaceae and Christensenellaceae genera, were increased in diseased Min pigs, which were positively correlated with the improvement of microbial metabolites, including indole derivatives and short-chain fatty acids, and thus may recover the intestinal barrier. In addition, core bacteria altered in abundance, and partial PRR nonactivation was maintained, thereby effectively inhibiting the inflammatory response. The increase in CD4+ T cells in the lamina propria improved the supervision of the host immunity response, contributing to maintenance of Th2-type immune superiority and immune tolerance patterns and controlling excessive inflammatory reactions. In Yorkshire pigs, concentrations of bile acid metabolites and short-chain fatty acids were lower in diseased individuals, associated with the increases in potentially harmful microbes, such as Bilophila, Alistipes and Bacteroidetes, and the depletion of potentially beneficial microbes, such as Blautia, Eubacterium, Dorea, Butyricicoccus, Lachnospiraceae and Roseburia. The increases in potentially harmful microbes and damaged intestinal barrier in Yorkshire pigs might be a factor in activating PRRs, thereby enriching Toll-like receptor 4 binding, NF-κB signalling and T-helper 1- and 17-type immune responses
Supplementary Information
Additional file 1: Figure S1. Sankey diagrams of the phylum, family, and genus affiliations of the microbiota in Min pigs and Yorkshire pigs.
Additional file 2: Figure S2. Metabolite changes in pig colonic digesta in M-CON vs. M-DSS pigs and Y-CON vs. Y-DSS pigs.
Additional file 3: Figure S3. Spearman correlation analysis between gut microbiota and metabolites. The heatmap and correlation network plots show the Spearman correlation coefficients of the comparison groups a,c M-CON vs. M-DSS and b,d Y-CON vs. Y-DSS.
Additional file 4: Figure S4. Additional data for RNA-seq analysis. KEGG enrichment analysis of colon genes in M-CON vs. M-DSS and Y-CON vs. Y-DSS.
Additional file 5: Table S1. qRT–PCR primer sequences.
Acknowledgements
We acknowledge the members of the Institute of Animal Nutrition of Northeast Agricultural University (Harbin, China) for their assistance with sampling and data analysis.
Authors’ contributions
BS, QM and XZ conceived and designed the research. XZ, XF and ZG performed the research. XZ, LJ and XW analysed the data. XZ wrote the manuscript, and all other authors revised the manuscript. The authors read and approved the final manuscript.
Funding
This work was supported by the Major Program of Heilongjiang Province of China (2021ZX12B08-02).
Availability of data and materials
The datasets supporting the conclusions of this article were deposited in the NCBI Sequence Read Archive database under the accession numbers PRJNA797567 (RNA-seq raw data) and PRJNA799232 (microbiota raw sequencing data). All other data are contained within the main manuscript and supplemental files.
Declarations
Ethics approval and consent to participate
All animal experimental procedures used in this experiment were approved by the Institutional Animal Care and Use Committee of Northeast Agricultural University (certification number NEAU-[2011]-9).
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Baoming Shi, Email: shibaoming1974@163.com.
Qingwei Meng, Email: qingweimeng1990@163.com.
References
- 1.Zhernakova A, Kurilshikov A, Bonder MJ, Tigchelaar EF, Schirmer M, Vatanen T, Mujagic Z, Vila AV, Falony G, Vieira-Silva S, et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 2016;352:565–569. doi: 10.1126/science.aad3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen LM, Collij V, Jaeger M, van den Munckhof ICL, Vila AV, Kurilshikov A, Gacesa R, Sinha T, Oosting M, Joosten LAB, et al. Gut microbial co-abundance networks show specificity in inflammatory bowel disease and obesity. Nat Commun. 2020;11:1–12. doi: 10.1038/s41467-019-13993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen L, Wang D, Garmaeva S, Kurilshikov A, Vich Vila A, Gacesa R, Sinha T, Lifelines Cohort S, Segal E, Weersma RK, et al. The long-term genetic stability and individual specificity of the human gut microbiome. Cell. 2021;184:2302–2315. doi: 10.1016/j.cell.2021.03.024. [DOI] [PubMed] [Google Scholar]
- 4.Ijssennagger N, Belzer C, Hooiveld GJ, Dekker J, van Mil SW, Muller M, Kleerebezem M, van der Meer R. Gut microbiota facilitates dietary heme-induced epithelial hyperproliferation by opening the mucus barrier in colon. Proc Natl Acad Sci U S A. 2015;112:10038–10043. doi: 10.1073/pnas.1507645112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schirmer M, Garner A, Vlamakis H, Xavier RJ. Microbial genes and pathways in inflammatory bowel disease. Nat Rev Microbiol. 2019;17:497–511. doi: 10.1038/s41579-019-0213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamada N, Seo SU, Chen GY, Nunez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 8.Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330:1768–1773. doi: 10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim CH. Immune regulation by microbiome metabolites. Immunology. 2018;154:220–229. doi: 10.1111/imm.12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fairbrother JM, Nadeau E, Gyles CL. Escherichia coli in postweaning diarrhea in pigs: an update on bacterial types, pathogenesis, and prevention strategies. Anim Health Res Rev. 2005;6:17–39. doi: 10.1079/AHR2005105. [DOI] [PubMed] [Google Scholar]
- 11.Getmantseva L, Bakoev S, Bakoev N, Karpushkina T, Kostyunina O. Mitochondrial DNA diversity in large white pigs in Russia. Animals. 2020;10:1365. doi: 10.3390/ani10081365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeong HS, Kim DW, Chun SY, Sung S, Kim HJ, Cho S, Kim H, Oh SJ. Native pig and chicken breed database: NPCDB. Asian-Australas J Anim Sci. 2014;27:1394–1398. doi: 10.5713/ajas.2014.14059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma QQ, Jiao WJ, Wang ZY, Wu CX, Shan AS, Wang YB, Cai JM. Tissue specificity and species superiority of cathelicidin gene expression in Chinese indigenous Min pigs. Livest Sci. 2014;161:36–40. doi: 10.1016/j.livsci.2014.01.004. [DOI] [Google Scholar]
- 14.Teng T, Gao F, He W, Fu HY, Guo J, Bai GD, Shi BM. An early fecal microbiota transfer improves the intestinal conditions on microflora and immunoglobulin and antimicrobial peptides in piglets. J Agric Food Chem. 2020;68:4830–4843. doi: 10.1021/acs.jafc.0c00545. [DOI] [PubMed] [Google Scholar]
- 15.Ghia JE, Li N, Wang H, Collins M, Deng Y, El-Sharkawy RT, Cote F, Mallet J, Khan WI. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology. 2009;137:1649–1660. doi: 10.1053/j.gastro.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 16.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-H. [DOI] [PubMed] [Google Scholar]
- 17.Pu ZC, Han CZ, Zhang WW, Xu MD, Wu ZJ, Liu YH, Wu M, Sun H, Xie HT. Systematic understanding of the mechanism and effects of arctigenin attenuates inflammation in dextran sulfate sodium-induced acute colitis through suppression of NLRP3 inflammasome by SIRT1. Am J Transl Res. 2019;11:3992–4009. [PMC free article] [PubMed] [Google Scholar]
- 18.Bassaganya-Riera J, Hontecillas R. CLA and n-3 PUFA differentially modulate clinical activity and colonic PPAR-responsive gene expression in a pig model of experimental IBD. Clin Nutr. 2006;25:454–465. doi: 10.1016/j.clnu.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Lucke K, Miehlke S, Jacobs E, Schuppler M. Prevalence of Bacteroides and Prevotella spp. in ulcerative colitis. J Med Microbiol. 2006;55:617–624. doi: 10.1099/jmm.0.46198-0. [DOI] [PubMed] [Google Scholar]
- 21.Ni J, Shen TCD, Chen EZ, Bittinger K, Bailey A, Roggiani M, Sirota-Madi A, Friedman ES, Lin LCA, Nissim I, et al. A role for bacterial urease in gut dysbiosis and Crohn’s disease. Sci Transl Med. 2017;9:eaah6888. doi: 10.1126/scitranslmed.aah6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imai Y, Meyer KJ, Iinishi A, Favre-Godal Q, Green R, Manuse S, Caboni M, Mori M, Niles S, Ghiglieri M, et al. A new antibiotic selectively kills Gram-negative pathogens. Nature. 2019;576:459–464. doi: 10.1038/s41586-019-1791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinross JM, Darzi AW, Nicholson JK. Gut microbiome-host interactions in health and disease. Genome Med. 2011;3:1–12. doi: 10.1186/gm228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Shea JJ, Jones RG. Autoimmunity Rubbing salt in the wound. Nature. 2013;496:437–439. doi: 10.1038/nature11959. [DOI] [PubMed] [Google Scholar]
- 25.Plugis NM, Weng N, Zhao Q, Palanski BA, Maecker HT, Habtezion A, Khosla C. Interleukin 4 is inactivated via selective disulfide-bond reduction by extracellular thioredoxin. Proc Natl Acad Sci U S A. 2018;115:8781–8786. doi: 10.1073/pnas.1805288115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang HE, Reinhardt RL, Bando JK, Sullivan BM, Ho IC, Locksley RM. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat Immunol. 2011;13:58–66. doi: 10.1038/ni.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164:337–340. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 28.Saha S, Jing XF, Park SY, Wang SY, Li XN, Gupta D, Dziarski R. Peptidoglycan recognition proteins protect mice from experimental colitis by promoting normal gut flora and preventing induction of interferon-gamma. Cell Host Microbe. 2010;8:147–162. doi: 10.1016/j.chom.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 31.Lloyd-Price J, Arze C, Ananthakrishnan AN, Schirmer M, Avila-Pacheco J, Poon TW, Andrews E, Ajami NJ, Bonham KS, Brislawn CJ, et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569:655–662. doi: 10.1038/s41586-019-1237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai HH, Dwarakanath AD, Hart CA, Milton JD, Rhodes JM. Increased faecal mucin sulphatase activity in ulcerative colitis: a potential target for treatment. Gut. 1995;36:570–576. doi: 10.1136/gut.36.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright DP, Rosendale DI, Robertson AM. Prevotella enzymes involved in mucin oligosaccharide degradation and evidence for a small operon of genes expressed during growth on mucin. FEMS Microbiol Lett. 2000;190:73–79. doi: 10.1111/j.1574-6968.2000.tb09265.x. [DOI] [PubMed] [Google Scholar]
- 35.Luis AS, Jin CS, Pereira GV, Glowacki RWP, Gugel SR, Singh S, Byrne DP, Pudlo NA, London JA, Basle A, et al. A single sulfatase is required to access colonic mucin by a gut bacterium. Nature. 2021;598:332–337. doi: 10.1038/s41586-021-03967-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kushkevych I, Dordevic D, Vitezova M. Possible synergy effect of hydrogen sulfide and acetate produced by sulfate-reducing bacteria on inflammatory bowel disease development. J Adv Res. 2021;27:71–78. doi: 10.1016/j.jare.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lavelle A, Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17:223–237. doi: 10.1038/s41575-019-0258-z. [DOI] [PubMed] [Google Scholar]
- 38.Devkota S, Wang YW, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in IL10-/- mice. Nature. 2012;487:104–110. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mukherjee A, Lordan C, Ross RP, Cotter PD. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes. 2020;12:1802866. doi: 10.1080/19490976.2020.1802866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi H, Chang Y, Yuan G, Xiong W, Tang QJ. Dietary fucoidan of Acaudina molpadioides alters gut microbiota and mitigates intestinal mucosal injury induced by cyclophosphamide. Food Funct. 2017;8:3383–3393. doi: 10.1039/C7FO00932A. [DOI] [PubMed] [Google Scholar]
- 42.Sun M, Wu W, Liu Z, Cong Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J Gastroenterol. 2017;52:1–8. doi: 10.1007/s00535-016-1242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–119. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- 44.Xue CY, Lv H, Li Y, Dong N, Wang YH, Zhou JL, Shi BM, Shan AS. Oleanolic acid reshapes the gut microbiota and alters immune-related gene expression of intestinal epithelial cells. J Sci Food Agric. 2022;102:764–773. doi: 10.1002/jsfa.11410. [DOI] [PubMed] [Google Scholar]
- 45.Waters JL, Ley RE. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol. 2019;17:1–11. doi: 10.1186/s12915-019-0699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCarville JL, Chen GY, Cuevas VD, Troha K, Ayres JS. Microbiota metabolites in health and disease. Annu Rev Immunol. 2020;38:147–170. doi: 10.1146/annurev-immunol-071219-125715. [DOI] [PubMed] [Google Scholar]
- 47.Ma DW, Chen YD, Chen TT. Vaginal microbiota transplantation for the treatment of bacterial vaginosis: a conceptual analysis. FEMS Microbiol Lett. 2019;366:fnz025. doi: 10.1093/femsle/fnz025. [DOI] [PubMed] [Google Scholar]
- 48.Yu QH, Yuan LX, Deng J, Yang Q. Lactobacillus protects the integrity of intestinal epithelial barrier damaged by pathogenic bacteria. Front Cell Infect Mi. 2015;5:26. doi: 10.3389/fcimb.2015.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schirmer M, Garner A, Vlamakis H, Xavier RJ. Microbial genes and pathways in inflammatory bowel disease. Nat Rev Microbiol. 2019;17:497–511. doi: 10.1038/s41579-019-0213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tuominen M. Comparison of the effect of anileridine and pethidine on the intracholedochal pressure during constant fluid perfusion. Acta Anaesthesiol Scand. 1976;20:177–182. doi: 10.1111/j.1399-6576.1976.tb05025.x. [DOI] [PubMed] [Google Scholar]
- 51.Gobert AP, Latour YL, Asim M, Barry DP, Allaman MM, Finley JL, Smith TM, McNamara KM, Singh K, Sierra JC, et al. Protective role of spermidine in colitis and colon carcinogenesis. Gastroenterology. 2022;162:813–827. doi: 10.1053/j.gastro.2021.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gonzalez-Gonzalez M, Diaz-Zepeda C, Eyzaguirre-Velasquez J, Gonzalez-Arancibia C, Bravo JA, Julio-Pieper M. Investigating gut permeability in animal models of disease. Front Physiol. 2019;9:1962. doi: 10.3389/fphys.2018.01962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martini E, Krug SM, Siegmund B, Neurath MF, Becker C. Mend your fences The epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell Mol Gastroenter. 2017;4:33–46. doi: 10.1016/j.jcmgh.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang K, Lin WJ, Zhang JT, Zhao YW, Wang XQ, Zhao M. Effect of Toll-like receptor 4 on depressive-like behaviors induced by chronic social defeat stress. Brain Behav. 2020;10:e01525. doi: 10.1002/brb3.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fritz JH, Le Bourhis L, Sellge G, Magalhaes JG, Fsihi H, Kufer TA, Collins C, Viala J, Ferrero RL, Girardin SE, Philpott DJ. Nod1-mediated innate immune recognition of peptidoglycan contributes to the onset of adaptive immunity. Immunity. 2007;26:445–459. doi: 10.1016/j.immuni.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 56.Chu H, Mazmanian SK. Innate immune recognition of the microbiota promotes host-microbial symbiosis. Nat Immunol. 2013;14:668–675. doi: 10.1038/ni.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kubinak JL, Round JL. Toll-like receptors promote mutually beneficial commensal-host interactions. PLoS Pathog. 2012;8:e1002785. doi: 10.1371/journal.ppat.1002785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014;104:15–25. doi: 10.1002/0471142735.im1525s104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Borton MA, Sabag-Daigle A, Wu J, Solden LM, O'Banion BS, Daly RA, Wolfe RA, Gonzalez JF, Wysocki VH, Ahmer BMM, Wrighton KC. Chemical and pathogen-induced inflammation disrupt the murine intestinal microbiome. Microbiome. 2017;5:1–15. doi: 10.1186/s40168-017-0264-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dennis VA, Dixit S, O'Brien SM, Alvarez X, Pahar B, Philipp MT. Live Borrelia burgdorferi spirochetes elicit inflammatory mediators from human monocytes via the toll-like receptor signaling pathway. Infect Immun. 2009;77:1238–1245. doi: 10.1128/IAI.01078-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yeh ED. Characterisation of breast lesions with proton MR spectroscopy. AJR Am J Roentgenol. 2003;181:1273–1274. doi: 10.2214/ajr.181.5.1811273. [DOI] [PubMed] [Google Scholar]
- 63.Sprouse ML, Bates NA, Felix KM, Wu HJ. Impact of gut microbiota on gut-distal autoimmunity: a focus on T cells. Immunology. 2019;156:305–318. doi: 10.1111/imm.13037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C, Rostron T, Cerundolo V, Pamer EG, Abramson SB, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013;2:e01202. doi: 10.7554/eLife.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 66.Punit S, Dube PE, Liu CY, Girish N, Washington MK, Polk DB. Tumor necrosis factor receptor 2 restricts the pathogenicity of CD8+ T cells in mice with colitis. Gastroenterology. 2015;149:993–1005. doi: 10.1053/j.gastro.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schreiber S, MacDermott RP, Raedler A, Pinnau R, Bertovich MJ, Nash GS. Increased activation of isolated intestinal lamina propria mononuclear cells in inflammatory bowel disease. Gastroenterology. 1991;101:1020–1030. doi: 10.1016/0016-5085(91)90729-5. [DOI] [PubMed] [Google Scholar]
- 68.Jamwal DR, Marati RV, Harrison CA, Midura-Kiela MT, Figliuolo Paz VR, Besselsen DG, Ghishan FK, Kiela PR. Total CD3 T cells are necessary and sufficient to induce colitis in immunodeficient mice with dendritic cell-specific deletion of TGFbR2: a novel ibd model to study CD4 and CD8 T-cell interaction. Inflamm Bowel Dis. 2020;26:229–241. doi: 10.1093/ibd/izz191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roybal KT, Williams JZ, Morsut L, Rupp LJ, Kolinko I, Choe JH, Walker WJ, McNally KA, Lim WA. Engineering T cells with customized therapeutic response programs using synthetic notch receptors. Cell. 2016;167:419–432. doi: 10.1016/j.cell.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 72.Smelt MJ, de Haan BJ, Bron PA, van Swam I, Meijerink M, Wells JM, Kleerebezem M, Faas MM, de Vos P. The impact of Lactobacillus plantarum WCFS1 teichoic acid D-alanylation on the generation of effector and regulatory T-cells in healthy mice. PLoS One. 2013;8:e63099. doi: 10.1371/journal.pone.0063099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cervantes-Barragan L, Chai JN, Tianero MD, Di Luccia B, Ahern PP, Merriman J, Cortez VS, Caparon MG, Donia MS, Gilfillan S, et al. Lactobacillus reuteri induces gut intraepithelial CD4+ CD8αα+ T cells. Science. 2017;357:806–810. doi: 10.1126/science.aah5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Strugnell RA, Wijburg OLC. The role of secretory antibodies in infection immunity. Nat Rev Microbiol. 2010;8:656–667. doi: 10.1038/nrmicro2384. [DOI] [PubMed] [Google Scholar]
- 75.Gopalakrishnan V, Helmink BA, Spencer CN, Reuben A, Wargo JA. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell. 2018;33:570–580. doi: 10.1016/j.ccell.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ghosh S, Whitley CS, Haribabu B, Jala VR. Regulation of intestinal barrier function by microbial metabolites. Cell Mol Gastroenter. 2021;11:1463–1482. doi: 10.1016/j.jcmgh.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ong HS, Yim HCH. Microbial factors in inflammatory diseases and cancers. Adv Exp Med Biol. 2017;1024:153–174. doi: 10.1007/978-981-10-5987-2_7. [DOI] [PubMed] [Google Scholar]
- 78.Prochazkova P, Roubalova R, Dvorak J, Tlaskalova-Hogenova H, Cermakova M, Tomasova P, Sediva B, Kuzma M, Bulant J, Bilej M, et al. Microbiota, microbial metabolites, and barrier function in a patient with anorexia nervosa after fecal microbiota transplantation. Microorganisms. 2019;7:338. doi: 10.3390/microorganisms7090338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kurashima Y, Kiyono H. Mucosal ecological network of epithelium and immune cells for gut homeostasis and tissue healing. Annu Rev Immunol. 2017;35:119–147. doi: 10.1146/annurev-immunol-051116-052424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Sankey diagrams of the phylum, family, and genus affiliations of the microbiota in Min pigs and Yorkshire pigs.
Additional file 2: Figure S2. Metabolite changes in pig colonic digesta in M-CON vs. M-DSS pigs and Y-CON vs. Y-DSS pigs.
Additional file 3: Figure S3. Spearman correlation analysis between gut microbiota and metabolites. The heatmap and correlation network plots show the Spearman correlation coefficients of the comparison groups a,c M-CON vs. M-DSS and b,d Y-CON vs. Y-DSS.
Additional file 4: Figure S4. Additional data for RNA-seq analysis. KEGG enrichment analysis of colon genes in M-CON vs. M-DSS and Y-CON vs. Y-DSS.
Additional file 5: Table S1. qRT–PCR primer sequences.
Data Availability Statement
The datasets supporting the conclusions of this article were deposited in the NCBI Sequence Read Archive database under the accession numbers PRJNA797567 (RNA-seq raw data) and PRJNA799232 (microbiota raw sequencing data). All other data are contained within the main manuscript and supplemental files.