Abstract
Demyelination results from the pathological loss of myelin and is a hallmark of many neurodegenerative diseases. Despite the prevalence of demyelinating diseases, there are no disease modifying therapies that prevent the loss of myelin or promote remyelination. This review aims to summarize studies in the field that highlight the importance of nuclear hormone receptors in the promotion and maintenance of myelination and the relevance of nuclear hormone receptors as potential therapeutic targets for demyelinating diseases. These nuclear hormone receptors include the estrogen receptor, progesterone receptor, androgen receptor, vitamin D receptor, thyroid hormone receptor, peroxisome proliferator-activated receptor, liver X receptor, and retinoid X receptor. Pre-clinical studies in well-established animal models of demyelination have shown a prominent role of these nuclear hormone receptors in myelination through their promotion of oligodendrocyte maturation and development. The activation of the nuclear hormone receptors by their ligands also promotes the synthesis of myelin proteins and lipids in mouse models of demyelination. There are limited clinical studies that focus on how the activation of these nuclear hormone receptors could alleviate demyelination in patients with diseases such as Multiple sclerosis (MS). However, the completed clinical trials have reported improved clinical outcome in MS patients treated with the ligands of some of these nuclear hormone receptors. Together, the positive results from both clinical and pre-clinical studies point to nuclear hormone receptors as promising therapeutic targets to counter demyelination.
Keywords: myelin, demyelination, hormone receptors, nuclear receptors, steroid hormones
Introduction
Nuclear hormone receptors are uniquely activated by hormones and hormone-like ligands to carry out various functions in nucleus such as transcriptional regulation [1, 2]. These receptors include the estrogen receptor, progesterone receptor, androgen receptor, vitamin D receptor, thyroid hormone receptor, peroxisome proliferator-activated receptor, liver X receptor, and retinoid X receptor, which share similar structures and have functions in metabolism, reproduction, and development [2]. Recent studies have also implicated these receptors in demyelinating diseases. In this review, we will highlight the current findings on the roles of nuclear hormone receptors in lipid metabolism, myelin maintenance, and oligodendrocyte-specific functions as well as their clinical relevance to demyelinating diseases.
Estrogen Receptor
The estrogen receptor (ER) is a member of the nuclear receptor superfamily that is activated by estrogens, including estrone, estradiol, and estriol [3]. The ER was discovered in 1958 and was the first receptor known to be modulated by hormones [4, 5]. The ER subtypes, ERα and ERβ, are encoded by several genes, including ESR1 and ESR2 [3]. The ESR1 gene, located on chromosome 6, encodes three isoforms of ERα, while the ESR2 gene, located on chromosome 14, encodes five isoforms of ERβ [6]. ERs have the capacity to alter signaling pathways in transcriptional regulation–dependent and –independent manners [7]. The transcriptional activity of ERs is most commonly activated by their binding to estrogen. The most common estrogen that binds to ERs and activates their transcriptional activity is 17β-estradiol. Once such estrogens bind to ERs, the activated ER complex translocate to the nucleus, where it binds to estrogen response elements [3, 7]. On the other hand, the main mediator of ER non-transcriptional activity was found to be G protein-coupled estrogen receptor (GPER1), a membrane estrogen receptor [3, 7–9]. Occasionally, non-transcriptional activity can also be mediated by the nuclear ERs [3, 7]. This non-transcriptional activity of ERs often results in cAMP regulation and protein-kinase activation of signaling cascades that can indirectly change the expression of estrogen target genes [3, 7]. Both types of ER signaling pathways modulate biological processes that include the development of breast tissue and sexual organs, reproduction, bone density, cholesterol mobilization, control of inflammation, and brain function [3, 7].
In the brain, ERs play a critical role by regulating cognition, body temperature, and sexual behavior through neuronal modulation[10]. ER expression in the brain is well documented in neurons, astrocytes, oligodendrocytes,[11–13]. [14, 15]. More specifically, ERβ promotes neurogenesis and modulates neuroendocrine regulation of stress response, inflammation, anxiety, and depression behaviors[16, 17]. The ventral spinal cord is characterized by the absence of neuronal cell bodies, having only fibers and glial cells, opening questions about [18]. Supporting this notion is the role of ERs in oligodendrocyte differentiation, myelination, and remyelination. The estradiol-ER axis was found to activate the pAkt/mTOR pathway in oligodendrocytes, a pathway known[19]. These findings are supported by studies in which the ERβ agonist chloroindazole increased the proliferation of oligodendrocyte precursor cells (OPCs) and the remyelination of axons of the neocortex and spinal cord in experimental autoimmune encephalomyelitis (EAE),[20, 21]. Chloroindazole treatment also improved clinical disease and motor performance in EAE and cuprizone (CPZ)-induced demyelinating mouse models while upregulating cholesterol-synthesis pathways in oligodendrocytes[20]. In addition, the estradiol-ER axis promotes myelination in peripheral fibers and remyelination by enhancing the immunomodulatory properties and pleiotropic efficacy of adipose-derived mesenchymal stem cells[22]. In contrast to the positive roles of estrogen-ERs in remyelination and myelin maintenance, Theoharides et al. reported that 17β-estradiol negatively impacted myelin by inducing mast cell secretion[23]. After mast cells were exposed to estradiol, myelin basic protein (MBP) displayed structural irregular similar those present in initial demyelination in[23]. This phenotype was more pronounced in Lewis rats, which are susceptible to the development of EAE, [23]. In general, there is strong evidence supporting the roles of ERs in remyelination and neuroprotection, making this receptor a potential targetable route for demyelinating diseases.
Estrogen receptor involvement in MS has been explored at the clinical level through treatments using multiple estrogens to target ERβ. A clinical trial in 2002 administered estriol, a naturally occurring estrogen during pregnancy, to MS patients [24]. The estriol doses significantly ameliorated the clinical symptoms and reduced relapse in patients with primary progressive MS [24–26]. A phase 2 randomized clinical trial of women with relapsing-remitting MS reported a reduction in MS relapse over a 2-year period of estriol treatment [26]. The study also reported an acceptable risk-benefit ratio of estriol administration in many healthy women [26]. This result highlights the safety profile of estriol compared to some of the current anti-inflammatory disease-modifying therapies commercially available for MS [26]. In conclusion, estrogen replacement therapy is a potential treatment for demyelinating and neurodegenerative diseases.
While ER agonist administration has been established to alleviate demyelinating deficits, their use for patient treatment, unfortunately, comes with challenges. Side effects of ER agonist treatment include the feminization of male patients and potential carcinogenicity due to increased activity of the ERα receptor [27, 28]. However, compared with previous ER ligands, chloroindazole permits a lower incidence of these side effects [20, 29–31].
Overall, the ER and its respective ligands have important roles in maintaining bodily processes, including the development of the reproductive system [3, 7]. Studies over the years have also indicated the ER to have regulatory roles in the brain, specifically in oligodendrocyte differentiation and myelin maintenance, making it an attractive target for treating demyelinating diseases [25, 32–36]. Estrogen treatment in demyelinating mouse models and clinical trials of MS patients have demonstrated ameliorative effects. Although treatment does produce adverse effects, the investigations previously mentioned consolidate the notion of ERs as a potential effective treatment for MS.
Progesterone Receptor
The progesterone receptor (PR) is another nuclear steroid hormone receptor expressed primarily in female reproductive tissues and the central nervous system (CNS). PRs have two isoforms, PR-A and PR-B, located on chromosome 11q22 [37]. In response to progesterone binding, PRs regulate multiple gene networks involved in the development of female sexual phenotypes [38–40]. The primary ligand for PR, progesterone, is a lipophilic molecule that can cross the blood-brain barrier or be directly synthesized in the brain, where it can be further metabolized into active neurosteroids [41–43]. Neural progesterone is known to be synthesized in glia, oligodendrocytes, astrocytes, and neurons [44–50].
The progesterone-PR axis regulates the proliferation of neural progenitor cells [51–53], sexual differentiation, and CNS tumor development [54–61]. PR activation also has neuroprotective and anti-inflammatory roles in the CNS [62–64]. In addition, the progesterone-PR axis enhances developmental myelination through the regulation of OPC proliferation and maturation, and through increased MBP expression in the cerebellum [62–65]. Furthermore, PRs act in a cell-autonomous manner in oligodendrocytes to promote remyelination. This phenomenon was demonstrated in several studies where activation of PRs improved remyelination in multiple demyelinating models by increasing myelin protein upregulation, oligodendrocyte differentiation, and proliferation [66–69]. PRs also promote remyelination by increasing astrocyte proliferation and inducing the polarization of microglia from a pro-inflammatory to anti-inflammatory state [68]. Altogether, the progesterone-PR axis plays a critical role in myelination by promoting both de novo myelination and remyelination through its regulation of various brain cell types. However, more research is needed to further elucidate the PR mechanism of action in myelination.
Independent from the progesterone-PR axis, progesterone itself has been observed to have a role in the remyelination process. Progesterone restores mRNA levels of neurosteroidogenic proteins and enzymes in the EAE mouse model which may reinforce the effects of exogenous progesterone [70]. In addition, progesterone derivatives, such as allopregnanolone, seem to be a promising form of treatment for demyelination. Noorbakhsh et al. reported the protective effect of allopregnanolone treatment in an EAE model via attenuating neuroinflammation, decreasing axonal injury, and restoring myelin basic protein expression [71].
Although progesterone displays promising pre-clinical results for treating demyelination, there is only one clinical trial in which the hormone is investigated as a treatment for MS [72, 73]. In this clinical trial, MS female patients were administered progestin, a synthetic progesterone analog, in combination with estriol at pregnancy levels after the post-partum period [73]. In the study, the patients who were given the combinatory treatment had less cortical gray matter atrophy than the patients in the placebo group. However, two main limitations of this clinical trial were that the autonomous function of progesterone could not be addressed and only a small number of patients participated, leaving the results inconclusive. Therefore, there is a need to further understand the impact of this hormone receptor at a clinical level. Overall, PR is an attractive target to further explore as a potential treatment for MS.
Androgen Receptor
The androgen receptor (AR) is also part of the nuclear hormone receptor superfamily. The AR has two isoforms, ARα and ARβ, and one splice variant, AR-V7, encoded by a single-copy gene located on chromosome Xq11.2-q12 [74, 75]. Androgens, including testosterone, are the activating ligands for AR [75]. Once activated, the AR heterodimerizes and translocates to the nucleus, where it acts as a transcription factor that regulates essential genes involved in the development and maintenance of male sexual phenotypes and the development of the nervous system [76–78]. When inactive, ARs are sequestered in the cytoplasm by multiple chaperones [79]. Furthermore, ARs are expressed in brain cell types including neurons, astrocytes, oligodendrocytes, and microglia, where they exert protective functions [80–82]. In a recent study of the testosterone-AR impact on remyelination, after demyelination of the CNS it was observed that the male gonad, testosterone, and ARs promoted astrocyte recruitment and myelin regeneration by oligodendrocytes [83]. Furthermore, testosterone treatment ameliorated demyelination in multiple demyelinating mouse models through the generation of OPCs and mature oligodendrocytes to increase the formation of new myelin in the brain [83, 84].
To date, only one clinical trial has been completed to assess the effect of testosterone treatment on MS patients [85, 86]. The clinical trial results showed that deficiency in testosterone serum levels is present in one-third of male MS patients and correlates with increased MS disability. Testosterone treatment of male MS patients significantly slowed gray matter atrophy during 1 year of treatment compared to pretreatment [87]. Currently, a one-armed clinical trial using testosterone treatment for MS is ongoing and is expected to be completed by August 2022 [88]. That trial aims to determine the effects of testosterone treatment on cognitive function, quality of life, and neuronal damage in male MS patients with low testosterone [88].
While both clinical and pre-clinical studies support the use of androgens to treat demyelinating diseases, androgen therapy has faced multiple obstacles. Androgen therapy has undesired side effects that include cardiovascular risk in some patients and masculinization in female patients [89, 90]. The limited understanding of the biological mechanism of this therapy has also caused a debate on whether it is a safe treatment route for MS. Moreover, there are sparse androgen clinical trials owing to limited funding and low enrollment in initial clinical trials, which has caused the withdrawal of three clinical trials and the termination of one.
To conclude, recent publications have demonstrated the critical role of the AR during remyelination in both female and male subjects at the pre-clinical level and have suggested that the testosterone-AR axis modulates various stages of myelin maintenance and homeostasis. However, targeting the testosterone-AR axis as a potential treatment for demyelinating diseases faces multiple limitations. At a pre-clinical level, there is still a need to elucidate the mechanism of how androgens impact demyelination and promote remyelination. A cell-specific mouse model is absent, limiting understanding of the AR’s cell-autonomous role in demyelinating mouse models [80–82]. In addition to the pre-clinical limitations, at a clinical level, androgen therapy lacks complete clinical trials and lacks conclusive results of its efficacy for MS patients. Therefore, there is a clear need for more research on the potential of using AR as a therapeutic target for demyelinating diseases.
Vitamin D Receptor
The vitamin D receptor (VDR) is a chromatin-associated nuclear receptor that interacts with the active form of vitamin D and has a crucial role in actions of vitamin D [91]. Vitamin D is transformed into its active form, 1,25(OH)2 D3, in a sequential process by the enzymes vitamin D3-25-OHase and 25-OHD3-1α-OHase [92]. The gene that encodes the VDR is located on chromosome 12 [91]. Once activated by its ligand, the VDR heterodimerizes with the retinoid X receptor (RXR) to regulate the transcription of numerous genes that contain the vitamin D response element [93]. The target genes of the VDR are involved in cellular processes such as calcium homeostasis, cell proliferation, differentiation, and immune responses [94–96].
VDR is highly expressed in various organs such as the gut organs and kidney, but it is also expressed in the brain [97, 98]. Specifically, VDR expression is found in both neurons and glial cells, where it plays an essential role in oligodendrocyte differentiation and myelination [98, 99]. To support its function in myelination, Sakai et al. reported that the loss of the VDR in mice leads to reduced myelination in the peripheral nervous system (PNS) [100]. Additionally, vitamin D administration to Schwann cells, the myelinating cells of the PNS, was found to increase MBP expression [100]. Similar trends were found in the CNS, where activation of the VDR resulted in increased neural stem cell proliferation and oligodendrocyte differentiation [101]. On the other hand, the inhibition of VDR signaling leads to decreased MBP expression in oligodendrocytes, impaired OPC differentiation, and reduced myelination and remyelination [102–104]. Taken together, the current findings in the field support the active role of VDR in both PNS and CNS myelination.
In addition to promoting myelination by increasing oligodendrocyte differentiation, the VDR is also involved in remyelination. In rodent models of demyelination, VDR activation increased myelin proteins, reduced demyelination, and promoted axon growth and neural stem cell differentiation and migration to promote remyelination [105–107]. However, the VDR also acts independently of oligodendrocytes to promote remyelination by reducing microglia activation and macrophage infiltration [107, 108]. Overall, the VDR performs non-cell-autonomous functions to enhance remyelination.
In support of its important functions in myelination and remyelination, the VDR has been reported to be dysregulated in neurological diseases. For example, a deficiency in vitamin D is linked to both demyelinating diseases and cognitive decline [109, 110]. The VDR’s involvement in these diseases is also evidenced by its enriched binding in the promoter regions of genes involved in autoimmunity and MS [111]. Additionally, specific polymorphisms in the VDR gene and other vitamin D metabolism genes have been associated with increased MS risk [109, 110, 112]. Recently, epidemiological studies have demonstrated that vitamin D deficiency increased the risk for developing MS [113, 114]. In contrast, high levels of vitamin D were associated with lower risk of developing motor disability over the course of 10 years [113–117]. Vitamin D also plays a key role in cognitive function, as lower serum vitamin D levels were associated with poorer cognitive performance and increased risk for cognitive impairment [118–121]. Together, these findings highlight the active role the VDR pathway plays in the development of cognitive dysfunction and demyelinating diseases.
Given the myriad studies that have demonstrated the protective effects of vitamin D against MS, many researchers have encouraged its use to treat MS [122]. However, using vitamin D to target VDR has several challenges [96]. First, a high dose of vitamin D can lead to hypercalcemia [123]. Second, the VDR is known to have ligand-independent and non-genomic functions and carries out unclear epigenetic modification and regulation of other DNA-bound transcription factors [124–127]. Therefore, a thorough understanding of the function of both the VDR, and vitamin D will allow more precise development of therapeutics to treat demyelinating diseases.
Thyroid Hormone Receptors
Thyroid hormones (THs) are produced by the thyroid gland and are important regulatory hormones that have critical roles in physiology and development [128]. Thyroid hormone receptors (TRs) are ligand-activated transcription factors belonging to the superfamily of nuclear hormone receptors. The major THs 3,5,3´-triiodo-L-thyronine (T3) and L-thyroxine (T4) can bind to TRs and activate transcription. TRs can bind to thyroid hormone response elements as monomers, homodimers, or heterodimers with RXRs, the latter being the most common form of this binding [129].
TRs are encoded by two genes: TRα by THRA and TRβ by THRB [130]. Each gene alternatively splices itself and generates several isoforms: TRα−1, TRα−2, and TRα−3 from the TRα gene and TRβ−1, TRβ−2, and TRβ−3 from the TRβ gene [131]. These isoforms differ in binding affinity, tissue expression levels, and temporal expression throughout and after development. TRα−1, a spliced isoform of TRα, comprises about 80% of all TR expression in the adult vertebrate brain and is actively involved in both oligodendrocyte differentiation and myelination. Its loss was observed to delay oligodendrocyte differentiation in the optic nerve in rats [132, 133]. On the other hand, although developing OPCs in rodents do not express TRβ, enhancing its expression can result in accelerated differentiation [132, 134]. Interestingly, as OPCs continue to differentiate towards myelinating oligodendrocytes, TRα expression decreases and TRβ expression is relied on to support permanent oligodendrocyte maturation [135, 136]. Overall, TRs have established involvement in oligodendrocyte differentiation and maturation; however, THs may have a more important role in the context of oligodendrocyte maintenance.
Loss of THs has been shown to have more detrimental consequences in neuronal development and myelination compared to the TRs themselves. For example, loss of the TH T3 induces a hypothyroid condition affecting neuronal differentiation and myelination [137–140]. Cytoplasmic MBP mRNA concentration was described to be modulated by T3 and T4 during early myelination [141]. In addition, low to absent THs resulted in a reduction and delayed aggregation of myelin-associated glycoprotein mRNA expression in the cerebral cortex [142, 143]. Several studies have also demonstrated that TH administration aids in restoring MBP expression and remyelinating oligodendrocytes in EAE and CPZ-treated demyelinating models [144, 145].
In a study of the role of TH in demyelinating diseases such as MS, THs were supplemented during the remission period of a CPZ mouse model, which improved remyelination and led to recovery of rodent body weight and physical behaviors [146]. Treatment with THs after CPZ-induced impairment resulted in enhanced differentiation and proliferation of OPCs in the mouse corpus callosum. In most MS patients, remyelination during the remission period is insufficient, allowing more lesions to accrue and potentiating relapse, progressing the severity of the disease. These findings detail that TH treatment during remission is a significant therapeutic strategy for alleviating MS.
While it has been established that TH administration can improve and enhance OPC differentiation and remyelination in demyelinating models, TH treatment does come with some challenges. The condition of excess circulating THs, otherwise known as hyperthyroidism, can result in osteoporosis, changes in heart and skeletal muscle, increased heart rate and body temperature, and unwanted changes in mood and mental health [147, 148]. Achieving the benefits of TH treatment without the off-target effects when using endogenous TH is a challenge; however, new therapeutic strategies such as a hydrogel-based delivery system for local delivery or the use of thyromimetics, which are selective synthetic TH analogs, in injured demyelinated sites have demonstrated reduced adverse effects and enhanced remyelination [149–151].
Overall, THs and TRs play an important role in oligodendrocyte and myelin maintenance. As TH administration for demyelinating diseases comes with its limitations, more research is needed to safely target the hormone without adverse effects.
Peroxisome Proliferator-Activated Receptors
Peroxisome proliferator-activated receptors (PPARs) are activated by a diverse group of substances called the peroxisome proliferators [152]. Peroxisome proliferators were first discovered to cause extensive proliferation of peroxisomes in rodent hepatocytes [152]. Natural PPAR ligands include fatty acids and 15-deoxy-delta 12,14-prostaglandin J2 (15d-PGJ2) [153–155]. Once activated, PPAR dimerizes with the RXR to regulate the expression of genes that contain the PPAR response elements [156, 157]. PPARs can also regulate transcription in a DNA binding–independent manner by interacting with signal transducers and transcriptional activators [158].
There are three different isoforms of PPARs—PPARα, PPARβ/δ, and PPARγ—which all play major functions in lipid metabolism [152]. The three isoforms are functionally similar but expressed by distinct genes on different chromosomes [159–163]; specifically, PPARα, PPARβ/δ, and PPARγ are encoded on chromosomes 22, 6, and 3, respectively. In addition, the PPAR isoforms have differential relative expression throughout the human body and are involved in various aspects of lipid metabolism, such as fatty acid oxidation and desaturation and lipid transport and storage [152, 160, 164–171].
While all PPARs are co-expressed, PPARβ/δ has the highest expression in the brain [165]. Specifically, PPARβ/δ has high expression in oligodendrocytes, where it regulates myelination. The role of PPARβ/δ in myelination was demonstrated by several studies where the loss of PPARβ/δ led to reduced myelination of the corpus callosum [165, 166, 172]. In addition, PPARβ/δ activation by agonists induced the expression of oligodendrocyte differentiation markers, promoted oligodendrocyte survival, and increased oligodendrocyte elaboration [173–176]. The role of PPARβ/δ in oligodendrocyte differentiation was further supported by PPARβ/δ’s regulation of myelin-specific genes and its temporal expression during oligodendrocyte maturation [172, 177]. Additionally, PPARβ/δ promotes myelination through lipid metabolism. Specifically, Zhou et al. and others have shown that the inhibition or reduced activation of PPARβ/δ resulted in decreased expression of myelin lipid enzymes such as lignocerolyl-CoA synthase and stearoyl-CoA desaturases [171, 178]. Together, these findings demonstrate that PPARβ/δ positively regulates oligodendrocyte differentiation and lipid metabolism to promote myelination.
Notably, PPARα has low expression in oligodendrocytes. Therefore, the PPARα isoform has been the least implicated in oligodendrocyte differentiation and myelination [165, 176]. Conversely, PPARγ was found to promote oligodendrocyte differentiation through the regulation of oxidative stress, myelin composition, and even mitochondrial function [179–186]. Given the co-expression of PPARs, there is crosstalk between these nuclear receptors in the form of a positive feedback loop, with PPARβ/δ being the master regulator [187]. Therefore, PPARs might work synergistically to promote oligodendrocyte differentiation and myelination.
PPARs also have protective actions in demyelinating and neurological diseases. Specifically, mouse models of demyelination have shown positive effects of enhancement of PPAR activity on disease development and progression. In the EAE demyelination model, studies found reduced recruitment of PPARβ/δ to the PLP promoter region, while the activation of PPARβ/δ restored expression of PLP and MOG in the EAE mouse spinal cord, resulting in reduced demyelination [176, 188, 189]. PPARβ/δ also controls CNS inflammation, as its loss led to increased T helper cell expansion and cytokine production as well as more severe demyelination in the EAE model [190]. In addition, PPARγ loss is known to exacerbate clinical symptoms in the EAE model, while PPARγ agonists reduce the incidence and severity of demyelination through anti-inflammatory mechanisms [191–194]. Given the appreciable expression of PPARy in immune cells, similar protective anti-inflammatory effects are seen in other neurological assaults, such as traumatic brain injury and stroke[178, 186, 195, 196]. Together, these studies support the importance of PPARs in preventing demyelination through both anti-inflammatory pathways and promotion of the synthesis of essential myelin proteins.
In summary, PPARs play a pivotal role in the regulation of myelination through the promotion of oligodendrocyte differentiation, synthesis of myelin lipids and proteins, and alleviation of inflammation. Despite these compelling findings, the treatment of neurological diseases through the activation of PPARs with agonists has been difficult. The commercially available PPAR agonists have poor solubility across the blood-brain barrier and are actively effluxed from the brain [197]. There is also a dearth of clinical trials that focus on the direct activation of PPARs to reduce demyelinating symptoms. These challenges highlight the need for more exploration of the development and clinical applications of PPAR-targeted therapies.
Liver X Receptor
Liver X receptors (LXRs) are transcription factors belonging to the nuclear receptor superfamily. Natural oxysterols, which are oxidized forms of cholesterol, were identified as the physiological ligands for LXRs [198–200]. The LXR subfamily consists of two isoforms, LXRα (NR1H3) and LXRβ (NR1H2). LXR retains a classic ligand binding structure conserved within the nuclear receptor family and forms an obligate heterodimer with RXRs, which binds to target DNA binding domains that contain LXR-responsive elements [201–203]. The LXR/RXR complex is also known as the “permissive” heterodimer since it can be activated by ligands of either receptor [204].
LXRα is expressed in tissues that are heavily involved in lipid metabolism, which include liver, intestine, adipose tissue, kidney, and adrenal glands, as well as in macrophages, whereas LXRβ is expressed ubiquitously, but predominantly in the liver and in the brain [199, 205]. Moreover, LXRs and their target genes are essential for lipid and cholesterol homeostasis and transport [206–210]. Cholesterol is vital for myelin composition and necessary for the structure and functionality of both PNS and CNS [199].
In studies of myelination and remyelination in the cerebellum, LXRs and their respective ligands exhibit transcriptional control of myelin genes in oligodendrocytes [211]. For example, double knockout of LXRα and LXRβ demonstrated thin myelin sheaths and decreased myelin gene expression, which contributed to deficits in motor coordination and spatial learning. Further, activation of LXRs in lysolecithin-induced demyelination promoted remyelination and maturation of oligodendroglia cells in vitro. This study proved that LXRs can also be activators of remyelination. The role of LXRs in myelin regulation was also investigated across various ages and different regions of the CNS, including the corpus callosum, cerebellum, optic nerve, and the spinal cord [212]. Activation of LXRs in adult mice resulted in significantly increased myelin gene expression in the cerebellum only, whereas double knockout of LXRs resulted in reduced myelin gene expression across all regions besides the corpus callosum. Dysfunctional LXRs in younger mice showed reduced mRNA expression of myelin genes in the optic nerve and cerebellum, yet it was, interestingly, increased in the spinal cord. These results further detail how each isoform is differentially implicated in myelin expression across ages and CNS regions. Owing to its activity in myelination, the LXR pathway displays a potential role in demyelinating diseases.
In a progressive EAE mouse model, RNA sequencing of spinal cord identified a significant downregulation of the LXR/RXR pathway and related genes compared with levels in healthy, normal mice [213]. Furthermore, Mailleux et al. investigated whether LXRs are activated in MS lesions. It was discovered that after myelin phagocytosis, macrophages had increased LXR ligand expression [214]. Local microglia and invading macrophages demonstrated an increased level of LXRα and the LXR target genes apolipoprotein E (APOE) and ABCA1 in active lesions. The formation of oxysterol 27OHC predominantly occurs 5 days after myelin uptake and processing. The consistent presence and formation of oxysterols is argued to be the reason why LXR activation is seen in myelin phagocytosis in active MS lesions.
A 5-year follow-up study investigated whether expression levels in apolipoproteins and oxysterols over time relate to progression of MS in patients [215]. Compared to healthy individuals, MS patients’ apolipoprotein expression was positively associated with oxysterol levels. Moreover, certain changes in apolipoproteins and oxysterols correlated with MS progression. Relapsing-remitting MS patients that had increases in the oxysterol 24S-hydroxycholesterol and the apolipoprotein ApoB and reduction in 7-ketocholesterol unfortunately progressed to secondary progressive MS. This study further establishes evidence that LXRs and their respective targets play a role in demyelinating diseases.
These discoveries reveal an interesting dynamic between the interaction of LXRs and their ligands in demyelinating diseases, from stimulating remyelination to possibly regulating MS pathogenesis, yet LXR’s role in myelination remains to be fully understood.
Retinoid X Receptor
Retinoid X Receptors (RXRs) are ligand-inducible transcription factors from the superfamily of nuclear receptors. The first natural ligand candidates for RXR were 9-cis-retinoic acid and polyunsaturated fatty acids, such as oleic acid and arachidonic acid [216]. RXRs regularly associate with other transcription factors from the nuclear receptor superfamily, including the PPARs, VDR, LXR, and TR. While heterodimerization partnering is necessary for high-affinity binding to their target genes, RXRs can act as a homodimer to activate transcription with similar reporter genes [216–218].
RXRs are encoded by three different genes: RXRα by RXRA (NR2B1), RXRβ by RXRB (NR2B2), and RXRγ by RXRG (NR2B3). Expression of RXRs in tissues vary between these isoforms. RXRα is expressed in the kidney, liver, intestine, and epidermis, RXRβ expression is ubiquitous, and RXRɣ is expressed in brain and muscle [219]. RXR has a wide variety of functions in the nervous system, including as a morphogen for neural tube development and a differentiation factor for CNS neurons [220–222]. RXR and its isoforms were even found to be transcriptionally upregulated in Schwann cells in the PNS through both axonal contact and treatment with retinoic acid [222].
Besides its various roles in neural development in the CNS and PNS, RXRs have been associated with demyelinating diseases through the anti-inflammatory response from immune cells, and RXR agonist treatment alleviates demyelinating models by immune modulation; however, the exact role of RXRs in myelination in those diseases is not well known [223–225]. In studying RXR function, Huang et al. provided new insights on their pivotal role in myelination. RXRγ expression was observed to be upregulated in rat cerebellum 2 to 3 weeks after focal demyelination. RXRγ knockdown by RNA interference or treatment with specific antagonists in mice impaired OPC differentiation. Interestingly, levels of RXRγ transcripts were increased in remyelinating lesions compared to demyelinating lesions. Moreover, administering the RXR ligand 9-cis-retinoic acid improved CNS remyelination in demyelinated cerebellar samples [103, 226]. Another study depicted similar results with the RXR agonist bexarotene, which promoted myelination and enhanced the expression of oligodendrocyte developmental markers in the hippocampus and cortex of older mice [227]. While these investigations have demonstrated RXR activity in myelination through agonists, further studies would be needed to elucidate RXRs’ exact function.
RXR agonist treatment has resulted in some challenges due to differences in responses and adverse effects like hypothyroidism and increased blood triglycerides [228, 229]. As an example, Kruczek et al. found that activation of RXRs by various agonists in the CPZ-induced demyelination model did not affect the severity of demyelination and did not protect oligodendrocytes from CPZ damage [224]. However, consistent with its immune modulation interaction, 9-cis-retinoic acid reduced microglia activity in demyelination, while all-trans retinoic acid enhanced the microglia activity. Novel treatment strategies have been used to stimulate and promote remyelination for MS. Recently, electrotherapy acupuncture, also known as electroacupuncture, in a rat model of spinal cord demyelination elicited a significant increase in endogenous OPCs and promoted oligodendrocytes that expressed RXRγ [230]. Moreover, the use of RXR agonists in conjunction with electroacupuncture treatment led to enhanced OPC differentiation.
Although RXRs play a role in demyelinating diseases immunologically, additional investigations would need to be conducted to elucidate the extent of RXR function in demyelinating diseases in relation to myelination.
Sex Differences in Nuclear Hormone Receptors
Nuclear hormone receptors are regulated by steroid hormones, and diseases that implicate these receptors are also known to have gender disparities; for example, women are more susceptible to developing MS and Alzheimer’s disease compared to men [87, 231–234].
The gender differences observed between the demyelinating CNS pathologies support pre-clinical and clinical investigation of the role of the sex hormones and their corresponding receptors in demyelination. The ER was an early treatment candidate for MS, as the estrogen-ER axis is upregulated during the last trimester of pregnancy, a period in which women suffering demyelinating diseases display clinical improvement, including an 80% reduction in relapse rate, followed by a high rate of relapse once pregnancy is over [235, 236]. In addition, estrogen-ER is known to upregulate immunomodulatory factors with known neuroprotective effects such as cortisol, progesterone, vitamin D, early pregnancy factor, and α-fetoprotein [25].
Also widely studied is PR, known to regulate critical molecular and cellular processes of CNS development, during which the receptor is differentially expressed between sexes [237]. PR is involved in other neuroprotective-related actions in the brain and spinal cord injury, inflammation, and demyelination [48, 50, 237, 238]. Along with PR’s role in brain development, many neurodegenerative disease patients display alteration in levels of progesterone and its metabolites. When progesterone and PR levels are altered, patients and study subjects under neurodegenerative conditions have worse outcomes than their healthy counterparts [234].
The androgen-AR axis is known to regulate the sexual dimorphism in CNS white matter, as the density of oligodendrocytes and myelin sheath thickness are greater in males than their female counterparts [72, 84]. This hormonal axis is also involved in sex disparity expression in different demyelinating diseases. A key role of the brain AR in the sexual phenotype of myelin was demonstrated by its conditional deletion, upon which testosterone levels were significantly lower in women with MS than in controls [81]. In the CPZ model, castrated male and female mice exhibited more severe demyelination, while female mice pretreated with the AR ligand dihydrotestosterone showed a less severe demyelinating phenotype [84].
VDR distribution is known to overlap with sex hormone receptors such as estradiol, progesterone, and ARs [239]. Several studies have shown coregulation of VDR and ER activities: vitamin D has a stronger transcriptional impact in females, and both vitamin D and estradiol work synergistically to enhance estradiol synthesis and VDR expression [240–245]. The VDR also regulates the synthesis of testosterone, as its increased stability resulted in a positive correlation between testosterone and serum vitamin D levels [246]. PPARs also have gender-specific functions, as reduced corpus callosum myelination and changes in brain phospholipid content due to loss of the PPARβ isoform were more predominant in female mice [166, 247].
Thyroid-related medical problems are more common in females than in males. Age-related thyroid dysfunction is also more common in women compared to men [248]. In an animal study, male and female mice showed differential behaviors under the conditions of hyperthyroidism and hypothyroidism [249]. Under hyperthyroidism, females showed increased locomotor activity, whereas males showed a decline in motor coordination when compared to their respective controls. Interestingly, under hypothyroidism, male mice had a higher water intake and higher levels of serum cholesterol compared to female mice. Levels of T3 and T4 in the brain have also been found to be sex specific. According to a study comparing male and female finches in early development, female fledglings had increased T4 compared to males and had different expression peak periods [250].
Sex differences in LXR expression were observed at a gestational level. Gestational diabetes can change the development of offspring; thus, Kruse et al. evaluated whether LXR expression in the hypothalamus and hippocampus differed between offspring from diabetic and non-diabetic mothers during the offspring’s early and late development [251]. LXRβ demonstrated an increase in expression in the hypothalamus in adult male offspring of diabetic mothers. On the other hand, there were no long-term changes observed in LXR expression in female offspring of diabetic mothers compared to those of non-diabetic mothers. Additionally, no alterations were observed in the hippocampus between the non-diabetic and diabetic groups across sexes. It is speculated that energy and glucose homeostasis would be affected by the increase of LXRβ expression found in male diabetic offspring.
Finally, differences in RXR expression across sexes have been uncovered in hepatic lipid processing in the liver and in neutrophil signaling after myocardial infarction [252, 253]. However, sex differences in RXR expression in the PNS and CNS are not known. More investigations would be needed to understand whether there are RXR disparities between the sexes in the nervous system.
Conclusion
Overall, nuclear hormone receptors have a prominent role in myelination in addition to a variety of functions in development, metabolism, and reproduction. As most of these receptors are activated by hormones, there is a gender disparity in their functions. Interestingly, myelination itself is impacted by gender, which confers sex-based susceptibility in demyelinating disorders. In myelination, nuclear hormone receptors function by activating pathways that promote oligodendrocyte differentiation and increase the expression of both myelin lipids and myelin proteins (Figure 1). They also promote myelination through cell non-autonomous pathways by enhancing anti-inflammatory signals, which attenuates the immune response. Although we discussed the nuclear hormone receptors individually, they demonstrate functional convergence within myelin regulation via dimer pairing and pathways. While most of the receptors can form homodimers, some of them preferentially form heterodimers with RXR to regulate similar genes and pathways in a context dependent manner. One such pathway that the receptor function converges on is the regulation of lipid metabolism. Specifically, receptors ERβ, PR, AR, VDR, LXRs, and PPARs were found to regulate the transcription of proteins involved in myelin lipid synthesis and lipid transport. The convergence of these receptors in multiple pathways highlights some redundancy in their regulation of myelination. Given their proven role in myelination from pre-clinical and clinical studies, targeting nuclear hormone receptors is a promising therapeutic strategy to counter demyelinating diseases.
Figure 1.
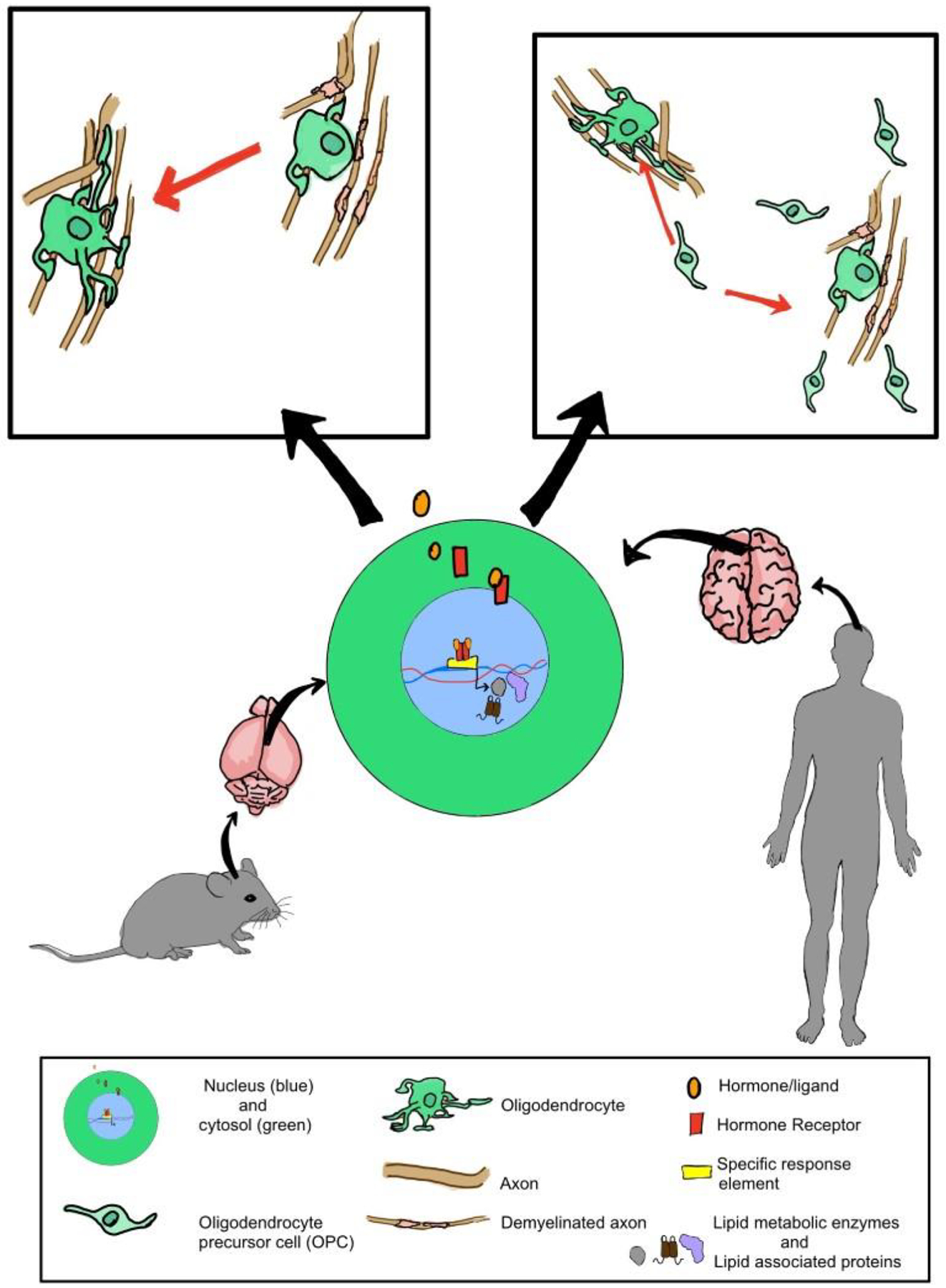
Overview of the role of nuclear hormone receptors in regulating myelination. The activation of nuclear hormone receptors by their respective ligands results in the transcription of genes involved in lipid metabolism, proliferation and differentiation of oligodendrocyte precursor cells and maintenance of myelination.
Acknowledgments
We thank Scientific Publications, Research Medical Library at MD Anderson for editorial assistance. JH is supported by The University of Texas Rising STARs Award, the Sidney Kimmel Scholar Award, the Sontag Foundation Distinguished Scientist Award, and the Sabin Foundation. RZV is supported by the NIH Diversity Supplement. TM is supported by the Dean’s Excellence Scholarship and the Roberta M and Jean M Worsham Endowed Fellowship. BP is supported by the TL1 Fellowship.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Mangelsdorf DJ, et al. , The nuclear receptor superfamily: the second decade. Cell, 1995. 83(6): p. 835–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sever R and Glass CK, Signaling by nuclear receptors. Cold Spring Harb Perspect Biol, 2013. 5(3): p. a016709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuentes N and Silveyra P, Estrogen receptor signaling mechanisms. Adv Protein Chem Struct Biol, 2019. 116: p. 135–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jensen EV, et al. , Estrogen-binding substances of target tissues. Science, 1967. 158(3800): p. 529–30. [DOI] [PubMed] [Google Scholar]
- 5.Jensen EV, et al. , A two-step mechanism for the interaction of estradiol with rat uterus. Proc Natl Acad Sci U S A, 1968. 59(2): p. 632–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagel A, et al. , Clinical and Biological Significance of ESR1 Gene Alteration and Estrogen Receptors Isoforms Expression in Breast Cancer Patients. Int J Mol Sci, 2019. 20(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yaşar P, et al. , Molecular mechanism of estrogen-estrogen receptor signaling. Reprod Med Biol, 2017. 16(1): p. 4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacenik D, et al. , G protein-coupled estrogen receptor mediates anti-inflammatory action in Crohn’s disease. Scientific Reports, 2019. 9(1): p. 6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu S, et al. , G Protein-Coupled Estrogen Receptor: A Potential Therapeutic Target in Cancer. Frontiers in Endocrinology, 2019. 10(725). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Z, et al. , The Effects of Estrogens on Neural Circuits That Control Temperature. Endocrinology, 2021. 162(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keller H, et al. , Fatty acids and retinoids control lipid metabolism through activation of peroxisome proliferator-activated receptor-retinoid X receptor heterodimers. Proc Natl Acad Sci U S A, 1993. 90(6): p. 2160–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Platania P, et al. , Differential expression of estrogen receptors alpha and beta in the spinal cord during postnatal development: localization in glial cells. Neuroendocrinology, 2003. 77(5): p. 334–340. [DOI] [PubMed] [Google Scholar]
- 13.Baker AE, Brautigam VM, and Watters JJ, Estrogen modulates microglial inflammatory mediator production via interactions with estrogen receptor β. Endocrinology, 2004. 145(11): p. 5021–5032. [DOI] [PubMed] [Google Scholar]
- 14.Mhyre A and Dorsa D, Estrogen activates rapid signaling in the brain: role of estrogen receptor α and estrogen receptor β in neurons and glia. Neuroscience, 2006. 138(3): p. 851–858. [DOI] [PubMed] [Google Scholar]
- 15.Ábrahám IM, et al. , Estrogen receptor β mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. Journal of neuroscience, 2003. 23(13): p. 5771–5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang JM, Liu L, and Brinton RD, Estradiol-17β-induced human neural progenitor cell proliferation is mediated by an estrogen receptor β-phosphorylated extracellularly regulated kinase pathway. Endocrinology, 2008. 149(1): p. 208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oyola MG, et al. , Anxiolytic effects and neuroanatomical targets of estrogen receptor-β (ERβ) activation by a selective ERβ agonist in female mice. Endocrinology, 2012. 153(2): p. 837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warfvinge K, et al. , Estrogen receptors α, β and GPER in the CNS and trigeminal system-molecular and functional aspects. The Journal of Headache and Pain, 2020. 21(1): p. 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar S, et al. , Estrogen receptor β ligand therapy activates PI3K/Akt/mTOR signaling in oligodendrocytes and promotes remyelination in a mouse model of multiple sclerosis. Neurobiol Dis, 2013. 56: p. 131–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore SM, et al. , Multiple functional therapeutic effects of the estrogen receptor β agonist indazole-Cl in a mouse model of multiple sclerosis. Proceedings of the National Academy of Sciences, 2014. 111(50): p. 18061–18066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karim K, Giribabu N, and Salleh N, Marantodes pumilum Var Alata (Kacip Fatimah) ameliorates derangement in RANK/RANKL/OPG pathway and reduces inflammation and oxidative stress in the bone of estrogen-deficient female rats with type-2 diabetes. Phytomedicine, 2021. 91: p. 153677. [DOI] [PubMed] [Google Scholar]
- 22.Gu Y, et al. , 17β-estradiol enhances schwann cell differentiation via the ERβ-ERK1/2 signaling pathway and promotes remyelination in injured sciatic nerves. Frontiers in pharmacology, 2018. 9: p. 1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Theoharides T, et al. , Synergistic action of estradiol and myelin basic protein on mast cell secretion and brain myelin changes resembling early stages of demyelination. Neuroscience, 1993. 57(3): p. 861–871. [DOI] [PubMed] [Google Scholar]
- 24.Sicotte NL, et al. , Treatment of multiple sclerosis with the pregnancy hormone estriol. Ann Neurol, 2002. 52(4): p. 421–8. [DOI] [PubMed] [Google Scholar]
- 25.Gold SM and Voskuhl RR, Estrogen treatment in multiple sclerosis. J Neurol Sci, 2009. 286(1–2): p. 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Voskuhl RR, et al. , Estriol combined with glatiramer acetate for women with relapsing-remitting multiple sclerosis: a randomised, placebo-controlled, phase 2 trial. Lancet Neurol, 2016. 15(1): p. 35–46. [DOI] [PubMed] [Google Scholar]
- 27.Tavani A and La Vecchia C, The adverse effects of hormone replacement therapy. Drugs Aging, 1999. 14(5): p. 347–57. [DOI] [PubMed] [Google Scholar]
- 28.Stanford JL and Thomas DB, Exogenous progestins and breast cancer. Epidemiologic reviews, 1993. 15(1): p. 98–107. [DOI] [PubMed] [Google Scholar]
- 29.Crawford D, et al. , Functional recovery of callosal axons following demyelination: a critical window. Neuroscience, 2009. 164(4): p. 1407–1421. [DOI] [PubMed] [Google Scholar]
- 30.Khalaj AJ, et al. , Nudging oligodendrocyte intrinsic signaling to remyelinate and repair: Estrogen receptor ligand effects. The Journal of steroid biochemistry and molecular biology, 2016. 160: p. 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karim H, et al. , Analogues of ERβ ligand chloroindazole exert immunomodulatory and remyelinating effects in a mouse model of multiple sclerosis. Scientific reports, 2019. 9(1): p. 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Acs P, et al. , 17beta-estradiol and progesterone prevent cuprizone provoked demyelination of corpus callosum in male mice. Glia, 2009. 57(8): p. 807–14. [DOI] [PubMed] [Google Scholar]
- 33.Cheong RY, et al. , Effects of Neuron-Specific Estrogen Receptor (ER) α and ERβ Deletion on the Acute Estrogen Negative Feedback Mechanism in Adult Female Mice. Endocrinology, 2014. 155(4): p. 1418–1427. [DOI] [PubMed] [Google Scholar]
- 34.Han S, et al. , Estrogen receptor variant ER-alpha36 is involved in estrogen neuroprotection against oxidative toxicity. Neuroscience, 2015. 310: p. 224–41. [DOI] [PubMed] [Google Scholar]
- 35.Yilmaz C, et al. , Neurosteroids as regulators of neuroinflammation. Front Neuroendocrinol, 2019. 55: p. 100788. [DOI] [PubMed] [Google Scholar]
- 36.Spence RD and Voskuhl RR, Neuroprotective effects of estrogens and androgens in CNS inflammation and neurodegeneration. Front Neuroendocrinol, 2012. 33(1): p. 105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horwitz KB and Sartorius CA, 90 YEARS OF PROGESTERONE: Progesterone and progesterone receptors in breast cancer: past, present, future. J Mol Endocrinol, 2020. 65(1): p. T49–t63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conneely OM, et al. , Reproductive functions of progesterone receptors. Recent Prog Horm Res, 2002. 57: p. 339–55. [DOI] [PubMed] [Google Scholar]
- 39.Conneely OM, Mulac-Jericevic B, and Lydon JP, Progesterone-dependent regulation of female reproductive activity by two distinct progesterone receptor isoforms. Steroids, 2003. 68(10–13): p. 771–8. [DOI] [PubMed] [Google Scholar]
- 40.Lydon JP, et al. , Reproductive phenotpes of the progesterone receptor null mutant mouse. J Steroid Biochem Mol Biol, 1996. 56(1–6 Spec No): p. 67–77. [DOI] [PubMed] [Google Scholar]
- 41.Baulieu EE, et al. , Progesterone as a neurosteroid: actions within the nervous system. Cell Mol Neurobiol, 1996. 16(2): p. 143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schumacher M, et al. , Local synthesis and dual actions of progesterone in the nervous system: neuroprotection and myelination. Growth hormone & IGF research, 2004. 14: p. 18–33. [DOI] [PubMed] [Google Scholar]
- 43.Jacobsen BM and Horwitz KB, Progesterone receptors, their isoforms and progesterone regulated transcription. Mol Cell Endocrinol, 2012. 357(1–2): p. 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jung-Testas I, et al. , Estrogen-inducible progesterone receptor in primary cultures of rat glial cells. Experimental cell research, 1991. 193(1): p. 12–19. [DOI] [PubMed] [Google Scholar]
- 45.Labombarda F, et al. , Immunocytochemical evidence for a progesterone receptor in neurons and glial cells of the rat spinal cord. Neuroscience letters, 2000. 288(1): p. 29–32. [DOI] [PubMed] [Google Scholar]
- 46.Marin-Husstege M, et al. , Oligodendrocyte progenitor proliferation and maturation is differentially regulated by male and female sex steroid hormones. Developmental neuroscience, 2004. 26(2–4): p. 245–254. [DOI] [PubMed] [Google Scholar]
- 47.Pivot XB, et al. , Activity of ixabepilone in oestrogen receptor-negative and oestrogen receptor-progesterone receptor-human epidermal growth factor receptor 2-negative metastatic breast cancer. European Journal of Cancer, 2009. 45(17): p. 2940–2946. [DOI] [PubMed] [Google Scholar]
- 48.Lacroix-Fralish ML, et al. , Differential regulation of neuregulin 1 expression by progesterone in astrocytes and neurons. Neuron glia biology, 2006. 2(4): p. 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chesik D and De Keyser J, Progesterone and dexamethasone differentially regulate the IGF-system in glial cells. Neuroscience letters, 2010. 468(3): p. 178–182. [DOI] [PubMed] [Google Scholar]
- 50.Sakamoto H, Ukena K, and Tsutsui K, Effects of progesterone synthesized de novo in the developing Purkinje cell on its dendritic growth and synaptogenesis. Journal of Neuroscience, 2001. 21(16): p. 6221–6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barha CK, et al. , Progesterone treatment normalizes the levels of cell proliferation and cell death in the dentate gyrus of the hippocampus after traumatic brain injury. Experimental neurology, 2011. 231(1): p. 72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bali N, et al. , Differential responses of progesterone receptor membrane component-1 (Pgrmc1) and the classical progesterone receptor (Pgr) to 17β-estradiol and progesterone in hippocampal subregions that support synaptic remodeling and neurogenesis. Endocrinology, 2012. 153(2): p. 759–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bali N, Morgan TE, and Finch CE, Pgrmc1: new roles in the microglial mediation of progesterone-antagonism of estradiol-dependent neurite sprouting and in microglial activation. Frontiers in neuroscience, 2013. 7: p. 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Camacho-Arroyo I, et al. , Sex hormones and proteins involved in brain plasticity. Vitamins and Hormones, 2020. 114: p. 145–165. [DOI] [PubMed] [Google Scholar]
- 55.Weisz J and Ward IL, Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology, 1980. 106(1): p. 306–316. [DOI] [PubMed] [Google Scholar]
- 56.Petz LN, et al. , Differential regulation of the human progesterone receptor gene through an estrogen response element half site and Sp1 sites. The Journal of steroid biochemistry and molecular biology, 2004. 88(2): p. 113–122. [DOI] [PubMed] [Google Scholar]
- 57.Hosie AM, et al. , Endogenous neurosteroids regulate GABA A receptors through two discrete transmembrane sites. Nature, 2006. 444(7118): p. 486–489. [DOI] [PubMed] [Google Scholar]
- 58.Verger A, Perdomo J, and Crossley M, Modification with SUMO: a role in transcriptional regulation. EMBO reports, 2003. 4(2): p. 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yawno T, et al. , Inhibition of neurosteroid synthesis increases asphyxia-induced brain injury in the late gestation fetal sheep. Neuroscience, 2007. 146(4): p. 1726–1733. [DOI] [PubMed] [Google Scholar]
- 60.Li H-D, et al. , A translocator protein 18 kDa agonist protects against cerebral ischemia/reperfusion injury. Journal of neuroinflammation, 2017. 14(1): p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piña-Medina AG, et al. , Progesterone promotes cell migration, invasion and cofilin activation in human astrocytoma cells. Steroids, 2016. 105: p. 19–25. [DOI] [PubMed] [Google Scholar]
- 62.Trabert B, et al. , Is estrogen plus progestin menopausal hormone therapy safe with respect to endometrial cancer risk? International Journal of cancer, 2013. 132(2): p. 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Nicola AF, et al. , Neurosteroidogenesis and progesterone anti-inflammatory/neuroprotective effects. Journal of neuroendocrinology, 2018. 30(2): p. e12502. [DOI] [PubMed] [Google Scholar]
- 64.González-Orozco JC, Moral-Morales AD, and Camacho-Arroyo I, Progesterone through progesterone receptor b isoform promotes rodent embryonic oligodendrogenesis. Cells, 2020. 9(4): p. 960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chan JR, Phillips LJ 2nd, and Glaser M, Glucocorticoids and progestins signal the initiation and enhance the rate of myelin formation. Proc Natl Acad Sci U S A, 1998. 95(18): p. 10459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schumacher M, et al. , Progesterone synthesis in the nervous system: implications for myelination and myelin repair. Front Neurosci, 2012. 6: p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.El-Etr M, et al. , Progesterone and nestorone promote myelin regeneration in chronic demyelinating lesions of corpus callosum and cerebral cortex. Glia, 2015. 63(1): p. 104–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aryanpour R, et al. , Progesterone therapy induces an M1 to M2 switch in microglia phenotype and suppresses NLRP3 inflammasome in a cuprizone-induced demyelination mouse model. International immunopharmacology, 2017. 51: p. 131–139. [DOI] [PubMed] [Google Scholar]
- 69.Labombarda F, et al. , Effects of progesterone on oligodendrocyte progenitors, oligodendrocyte transcription factors, and myelin proteins following spinal cord injury. Glia, 2009. 57(8): p. 884–897. [DOI] [PubMed] [Google Scholar]
- 70.Garay L, et al. , Progesterone treatment modulates mRNA OF neurosteroidogenic enzymes in a murine model of multiple sclerosis. J Steroid Biochem Mol Biol, 2017. 165(Pt B): p. 421–429. [DOI] [PubMed] [Google Scholar]
- 71.Noorbakhsh F, et al. , Impaired neurosteroid synthesis in multiple sclerosis. Brain, 2011. 134(Pt 9): p. 2703–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ghoumari AM, et al. , Roles of Progesterone, Testosterone and Their Nuclear Receptors in Central Nervous System Myelination and Remyelination. Int J Mol Sci, 2020. 21(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vukusic S, et al. , The Prevention of Post-Partum Relapses with Progestin and Estradiol in Multiple Sclerosis (POPART’MUS) trial: rationale, objectives and state of advancement. J Neurol Sci, 2009. 286(1–2): p. 114–8. [DOI] [PubMed] [Google Scholar]
- 74.Brown CJ, et al. , Androgen receptor locus on the human X chromosome: regional localization to Xq11–12 and description of a DNA polymorphism. Am J Hum Genet, 1989. 44(2): p. 264–9. [PMC free article] [PubMed] [Google Scholar]
- 75.Gao W, Bohl CE, and Dalton JT, Chemistry and structural biology of androgen receptor. Chem Rev, 2005. 105(9): p. 3352–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sato T, et al. , Brain masculinization requires androgen receptor function. Proceedings of the National Academy of Sciences, 2004. 101(6): p. 1673–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perrin JS, et al. , Growth of white matter in the adolescent brain: role of testosterone and androgen receptor. Journal of Neuroscience, 2008. 28(38): p. 9519–9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sarkey S, et al. , Classical androgen receptors in non-classical sites in the brain. Hormones and behavior, 2008. 53(5): p. 753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Heemers HV and Tindall DJ, Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocrine reviews, 2007. 28(7): p. 778–808. [DOI] [PubMed] [Google Scholar]
- 80.Liva SM and Voskuhl RR, Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. The Journal of Immunology, 2001. 167(4): p. 2060–2067. [DOI] [PubMed] [Google Scholar]
- 81.Fargo KN, et al. , Neuroprotective actions of androgens on motoneurons. Frontiers in neuroendocrinology, 2009. 30(2): p. 130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raskin K, et al. , Conditional inactivation of androgen receptor gene in the nervous system: effects on male behavioral and neuroendocrine responses. Journal of Neuroscience, 2009. 29(14): p. 4461–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bielecki B, et al. , Unexpected central role of the androgen receptor in the spontaneous regeneration of myelin. Proceedings of the National Academy of Sciences, 2016. 113(51): p. 14829–14834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hussain R, et al. , The neural androgen receptor: a therapeutic target for myelin repair in chronic demyelination. Brain, 2013. 136(1): p. 132–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Metzger-Peter K, et al. , The TOTEM RRMS (Testosterone treatment on neuroprotection and Myelin repair in relapsing remitting multiple sclerosis) trial: Study protocol for a randomized, double-blind, placebo-controlled trial. Trials, 2020. 21(1): p. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sicotte NL, et al. , Testosterone treatment in multiple sclerosis: a pilot study. Archives of neurology, 2007. 64(5): p. 683–688. [DOI] [PubMed] [Google Scholar]
- 87.Kurth F, et al. , Neuroprotective effects of testosterone treatment in men with multiple sclerosis. Neuroimage Clin, 2014. 4: p. 454–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bove R, et al. , Low testosterone is associated with disability in men with multiple sclerosis. Mult Scler, 2014. 20(12): p. 1584–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grech A, Breck J, and Heidelbaugh J, Adverse effects of testosterone replacement therapy: an update on the evidence and controversy. Ther Adv Drug Saf, 2014. 5(5): p. 190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Osterberg EC, Bernie AM, and Ramasamy R, Risks of testosterone replacement therapy in men. Indian J Urol, 2014. 30(1): p. 2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brumbaugh PF and Haussler MR, 1 Alpha,25-dihydroxycholecalciferol receptors in intestine. I. Association of 1 alpha,25-dihydroxycholecalciferol with intestinal mucosa chromatin. J Biol Chem, 1974. 249(4): p. 1251–7. [PubMed] [Google Scholar]
- 92.Garcion E, et al. , New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab, 2002. 13(3): p. 100–5. [DOI] [PubMed] [Google Scholar]
- 93.Barsony J and Prufer K, Vitamin D receptor and retinoid X receptor interactions in motion. Vitam Horm, 2002. 65: p. 345–76. [DOI] [PubMed] [Google Scholar]
- 94.Meyer MB, et al. , The human transient receptor potential vanilloid type 6 distal promoter contains multiple vitamin D receptor binding sites that mediate activation by 1,25-dihydroxyvitamin D3 in intestinal cells. Mol Endocrinol, 2006. 20(6): p. 1447–61. [DOI] [PubMed] [Google Scholar]
- 95.Nurminen V, Seuter S, and Carlberg C, Primary Vitamin D Target Genes of Human Monocytes. Front Physiol, 2019. 10: p. 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pike JW, Genome-wide principles of gene regulation by the vitamin D receptor and its activating ligand. Mol Cell Endocrinol, 2011. 347(1–2): p. 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Taymans SE, et al. , The human vitamin D receptor gene (VDR) is localized to region 12cen-q12 by fluorescent in situ hybridization and radiation hybrid mapping: genetic and physical VDR map. J Bone Miner Res, 1999. 14(7): p. 1163–6. [DOI] [PubMed] [Google Scholar]
- 98.Eyles DW, et al. , Intracellular distribution of the vitamin D receptor in the brain: comparison with classic target tissues and redistribution with development. Neuroscience, 2014. 268: p. 1–9. [DOI] [PubMed] [Google Scholar]
- 99.Eyles DW, et al. , Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat, 2005. 29(1): p. 21–30. [DOI] [PubMed] [Google Scholar]
- 100.Sakai S, et al. , Vitamin D receptor signaling enhances locomotive ability in mice. J Bone Miner Res, 2015. 30(1): p. 128–36. [DOI] [PubMed] [Google Scholar]
- 101.Shirazi HA, et al. , 1,25-Dihydroxyvitamin D3 suppressed experimental autoimmune encephalomyelitis through both immunomodulation and oligodendrocyte maturation. Exp Mol Pathol, 2017. 102(3): p. 515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Baas D, et al. , Persistence of oligodendrocyte precursor cells and altered myelination in optic nerve associated to retina degeneration in mice devoid of all thyroid hormone receptors. Proc Natl Acad Sci U S A, 2002. 99(5): p. 2907–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang JK, et al. , Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat Neurosci, 2011. 14(1): p. 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.de la Fuente AG, et al. , Vitamin D receptor-retinoid X receptor heterodimer signaling regulates oligodendrocyte progenitor cell differentiation. J Cell Biol, 2015. 211(5): p. 975–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gomez-Pinedo U, et al. , Vitamin D increases remyelination by promoting oligodendrocyte lineage differentiation. Brain Behav, 2020. 10(1): p. e01498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Goudarzvand M, et al. , Vitamins E and D3 attenuate demyelination and potentiate remyelination processes of hippocampal formation of rats following local injection of ethidium bromide. Cell Mol Neurobiol, 2010. 30(2): p. 289–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wergeland S, et al. , Dietary vitamin D3 supplements reduce demyelination in the cuprizone model. PLoS One, 2011. 6(10): p. e26262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Matías-Guíu J, et al. , Vitamin D and remyelination in multiple sclerosis. Neurología (English Edition), 2018. 33(3): p. 177–186. [DOI] [PubMed] [Google Scholar]
- 109.Lu M, Taylor BV, and Korner H, Genomic Effects of the Vitamin D Receptor: Potentially the Link between Vitamin D, Immune Cells, and Multiple Sclerosis. Front Immunol, 2018. 9: p. 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Scazzone C, et al. , Vitamin D and Genetic Susceptibility to Multiple Sclerosis. Biochem Genet, 2021. 59(1): p. 1–30. [DOI] [PubMed] [Google Scholar]
- 111.Ramagopalan SV, et al. , A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome Res, 2010. 20(10): p. 1352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen XL, et al. , Vitamin D receptor gene polymorphisms and the risk of multiple sclerosis: An updated meta-analysis. Microb Pathog, 2017. 110: p. 594–602. [DOI] [PubMed] [Google Scholar]
- 113.Nielsen NM, et al. , Neonatal vitamin D status and risk of multiple sclerosis: A population-based case-control study. Neurology, 2017. 88(1): p. 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wesnes K, et al. , Low vitamin D, but not tobacco use or high BMI, is associated with long-term disability progression in multiple sclerosis. Mult Scler Relat Disord, 2021. 50: p. 102801. [DOI] [PubMed] [Google Scholar]
- 115.Correale J, Ysrraelit MC, and Gaitán MI, Vitamin D-mediated immune regulation in multiple sclerosis. J Neurol Sci, 2011. 311(1–2): p. 23–31. [DOI] [PubMed] [Google Scholar]
- 116.Goldberg P, Fleming MC, and Picard EH, Multiple sclerosis: decreased relapse rate through dietary supplementation with calcium, magnesium and vitamin D. Med Hypotheses, 1986. 21(2): p. 193–200. [DOI] [PubMed] [Google Scholar]
- 117.Simpson S Jr., et al. , Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann Neurol, 2010. 68(2): p. 193–203. [DOI] [PubMed] [Google Scholar]
- 118.Darwish H, et al. , Serum 25-hydroxyvitamin D predicts cognitive performance in adults. Neuropsychiatr Dis Treat, 2015. 11: p. 2217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Llewellyn DJ, Langa KM, and Lang IA, Serum 25-hydroxyvitamin D concentration and cognitive impairment. J Geriatr Psychiatry Neurol, 2009. 22(3): p. 188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Llewellyn DJ, et al. , Vitamin D and cognitive impairment in the elderly U.S. population. J Gerontol A Biol Sci Med Sci, 2011. 66(1): p. 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Darwish H, et al. , Effect of Vitamin D Replacement on Cognition in Multiple Sclerosis Patients. Sci Rep, 2017. 7: p. 45926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lincoln MR, Schneider R, and Oh J, Vitamin D as disease-modifying therapy for multiple sclerosis? Expert Rev Clin Immunol, 2021. 17(7): p. 691–693. [DOI] [PubMed] [Google Scholar]
- 123.Atkinson SA and Fleet JC, Canadian recommendations for vitamin D intake for persons affected by multiple sclerosis. J Steroid Biochem Mol Biol, 2020. 199: p. 105606. [DOI] [PubMed] [Google Scholar]
- 124.Huet T, et al. , A vitamin D receptor selectively activated by gemini analogs reveals ligand dependent and independent effects. Cell Rep, 2015. 10(4): p. 516–26. [DOI] [PubMed] [Google Scholar]
- 125.Dimitrov V and White JH, Species-specific regulation of innate immunity by vitamin D signaling. J Steroid Biochem Mol Biol, 2016. 164: p. 246–253. [DOI] [PubMed] [Google Scholar]
- 126.Hii CS and Ferrante A, The Non-Genomic Actions of Vitamin D. Nutrients, 2016. 8(3): p. 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee SM and Pike JW, The vitamin D receptor functions as a transcription regulator in the absence of 1,25-dihydroxyvitamin D(3). J Steroid Biochem Mol Biol, 2016. 164: p. 265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kester MH, et al. , Iodothyronine levels in the human developing brain: major regulatory roles of iodothyronine deiodinases in different areas. J Clin Endocrinol Metab, 2004. 89(7): p. 3117–28. [DOI] [PubMed] [Google Scholar]
- 129.Lazar MA, Berrodin TJ, and Harding HP, Differential DNA binding by monomeric, homodimeric, and potentially heteromeric forms of the thyroid hormone receptor. Mol Cell Biol, 1991. 11(10): p. 5005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brent GA, The molecular basis of thyroid hormone action. N Engl J Med, 1994. 331(13): p. 847–53. [DOI] [PubMed] [Google Scholar]
- 131.Zhang J and Lazar MA, The mechanism of action of thyroid hormones. Annu Rev Physiol, 2000. 62: p. 439–66. [DOI] [PubMed] [Google Scholar]
- 132.Billon N, et al. , Role of thyroid hormone receptors in timing oligodendrocyte differentiation. Dev Biol, 2001. 235(1): p. 110–20. [DOI] [PubMed] [Google Scholar]
- 133.Billon N, et al. , Normal timing of oligodendrocyte development depends on thyroid hormone receptor alpha 1 (TRalpha1). Embo j, 2002. 21(23): p. 6452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Baas D, et al. , Expression of alpha and beta thyroid receptors during oligodendrocyte differentiation. Neuroreport, 1994. 5(14): p. 1805–8. [DOI] [PubMed] [Google Scholar]
- 135.Sarliève LL, Rodríguez-Peña A, and Langley K, Expression of thyroid hormone receptor isoforms in the oligodendrocyte lineage. Neurochem Res, 2004. 29(5): p. 903–22. [DOI] [PubMed] [Google Scholar]
- 136.Calza L, Fernandez M, and Giardino L, Cellular approaches to central nervous system remyelination stimulation: thyroid hormone to promote myelin repair via endogenous stem and precursor cells. J Mol Endocrinol, 2010. 44(1): p. 13–23. [DOI] [PubMed] [Google Scholar]
- 137.Muñoz A, et al. , Thyroid hormone receptor/c-erbA: control of commitment and differentiation in the neuronal/chromaffin progenitor line PC12. J Cell Biol, 1993. 121(2): p. 423–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bernal J, Thyroid hormone receptors in brain development and function. Nat Clin Pract Endocrinol Metab, 2007. 3(3): p. 249–59. [DOI] [PubMed] [Google Scholar]
- 139.Namba N, et al. , Clinical phenotype and endocrinological investigations in a patient with a mutation in the MCT8 thyroid hormone transporter. Eur J Pediatr, 2008. 167(7): p. 785–91. [DOI] [PubMed] [Google Scholar]
- 140.Horn S and Heuer H, Thyroid hormone action during brain development: more questions than answers. Mol Cell Endocrinol, 2010. 315(1–2): p. 19–26. [DOI] [PubMed] [Google Scholar]
- 141.Shanker G, Campagnoni AT, and Pieringer RA, Investigations on myelinogenesis in vitro: developmental expression of myelin basic protein mRNA and its regulation by thyroid hormone in primary cerebral cell cultures from embryonic mice. J Neurosci Res, 1987. 17(3): p. 220–4. [DOI] [PubMed] [Google Scholar]
- 142.Rodriguez-Peña A, et al. , Neonatal hypothyroidism affects the timely expression of myelin-associated glycoprotein in the rat brain. J Clin Invest, 1993. 91(3): p. 812–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ibarrola N and Rodríguez-Peña A, Hypothyroidism coordinately and transiently affects myelin protein gene expression in most rat brain regions during postnatal development. Brain Res, 1997. 752(1–2): p. 285–93. [DOI] [PubMed] [Google Scholar]
- 144.Fernandez M, et al. , Thyroid hormone administration enhances remyelination in chronic demyelinating inflammatory disease. Proc Natl Acad Sci U S A, 2004. 101(46): p. 16363–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Franco PG, et al. , Thyroid hormones promote differentiation of oligodendrocyte progenitor cells and improve remyelination after cuprizone-induced demyelination. Exp Neurol, 2008. 212(2): p. 458–67. [DOI] [PubMed] [Google Scholar]
- 146.Zhang M, et al. , Thyroid hormone alleviates demyelination induced by cuprizone through its role in remyelination during the remission period. Exp Biol Med (Maywood), 2015. 240(9): p. 1183–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Scanlan TS, Thyroid hormone analogues: useful biological probes and potential therapeutic agents. Ann Endocrinol (Paris), 2008. 69(2): p. 157–9. [DOI] [PubMed] [Google Scholar]
- 148.Li L, et al. , Abnormal brain functional connectivity leads to impaired mood and cognition in hyperthyroidism: a resting-state functional MRI study. Oncotarget, 2017. 8(4): p. 6283–6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Scanlan TS, Sobetirome: a case history of bench-to-clinic drug discovery and development. Heart Fail Rev, 2010. 15(2): p. 177–82. [DOI] [PubMed] [Google Scholar]
- 150.Tancevski I, Rudling M, and Eller P, Thyromimetics: a journey from bench to bed-side. Pharmacol Ther, 2011. 131(1): p. 33–9. [DOI] [PubMed] [Google Scholar]
- 151.Shultz RB, et al. , Local delivery of thyroid hormone enhances oligodendrogenesis and myelination after spinal cord injury. J Neural Eng, 2017. 14(3): p. 036014. [DOI] [PubMed] [Google Scholar]
- 152.Wahli W, Braissant O, and Desvergne B, Peroxisome proliferator activated receptors: transcriptional regulators of adipogenesis, lipid metabolism and more. Chem Biol, 1995. 2(5): p. 261–6. [DOI] [PubMed] [Google Scholar]
- 153.Liu Y, et al. , The Role of PPAR-δ in Metabolism, Inflammation, and Cancer: Many Characters of a Critical Transcription Factor. International journal of molecular sciences, 2018. 19(11): p. 3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Reddy AT, et al. , Identification and Molecular Characterization of Peroxisome Proliferator-Activated Receptor δ as a Novel Target for Covalent Modification by 15-Deoxy-Δ(12,14)-prostaglandin J(2). ACS Chem Biol, 2018. 13(12): p. 3269–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schoonjans K, Staels B, and Auwerx J, Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. Journal of Lipid Research, 1996. 37(5): p. 907–925. [PubMed] [Google Scholar]
- 156.Berger J and Moller DE, The mechanisms of action of PPARs. Annu Rev Med, 2002. 53: p. 409–35. [DOI] [PubMed] [Google Scholar]
- 157.Jpenberg A, et al. , Polarity and specific sequence requirements of peroxisome proliferator-activated receptor (PPAR)/retinoid X receptor heterodimer binding to DNA. A functional analysis of the malic enzyme gene PPAR response element. J Biol Chem, 1997. 272(32): p. 20108–17. [DOI] [PubMed] [Google Scholar]
- 158.Barbier O, et al. , Pleiotropic actions of peroxisome proliferator-activated receptors in lipid metabolism and atherosclerosis. Arterioscler Thromb Vasc Biol, 2002. 22(5): p. 717–26. [DOI] [PubMed] [Google Scholar]
- 159.Auboeuf D, et al. , Tissue distribution and quantification of the expression of mRNAs of peroxisome proliferator-activated receptors and liver X receptor-alpha in humans: no alteration in adipose tissue of obese and NIDDM patients. Diabetes, 1997. 46(8): p. 1319–27. [DOI] [PubMed] [Google Scholar]
- 160.Fajas L, et al. , The organization, promoter analysis, and expression of the human PPARgamma gene. J Biol Chem, 1997. 272(30): p. 18779–89. [DOI] [PubMed] [Google Scholar]
- 161.Sher T, et al. , cDNA cloning, chromosomal mapping, and functional characterization of the human peroxisome proliferator activated receptor. Biochemistry, 1993. 32(21): p. 5598–604. [DOI] [PubMed] [Google Scholar]
- 162.Skogsberg J, et al. , Characterization of the human peroxisome proliferator activated receptor delta gene and its expression. Int J Mol Med, 2000. 6(1): p. 73–81. [DOI] [PubMed] [Google Scholar]
- 163.Moreno M, et al. , PPARs: Nuclear Receptors Controlled by, and Controlling, Nutrient Handling through Nuclear and Cytosolic Signaling. PPAR Res, 2010. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Braissant O, et al. , Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology, 1996. 137(1): p. 354–66. [DOI] [PubMed] [Google Scholar]
- 165.Cullingford TE, et al. , Distribution of mRNAs encoding the peroxisome proliferator-activated receptor alpha, beta, and gamma and the retinoid X receptor alpha, beta, and gamma in rat central nervous system. J Neurochem, 1998. 70(4): p. 1366–75. [DOI] [PubMed] [Google Scholar]
- 166.Peters JM, et al. , Growth, adipose, brain, and skin alterations resulting from targeted disruption of the mouse peroxisome proliferator-activated receptor beta(delta). Mol Cell Biol, 2000. 20(14): p. 5119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fajas L, Debril MB, and Auwerx J, Peroxisome proliferator-activated receptor-gamma: from adipogenesis to carcinogenesis. J Mol Endocrinol, 2001. 27(1): p. 1–9. [DOI] [PubMed] [Google Scholar]
- 168.Gupta RA, et al. , Target genes of peroxisome proliferator-activated receptor gamma in colorectal cancer cells. J Biol Chem, 2001. 276(32): p. 29681–7. [DOI] [PubMed] [Google Scholar]
- 169.Mandard S, Müller M, and Kersten S, Peroxisome proliferator-activated receptor alpha target genes. Cell Mol Life Sci, 2004. 61(4): p. 393–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ahmadian M, et al. , PPARγ signaling and metabolism: the good, the bad and the future. Nat Med, 2013. 19(5): p. 557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhou X, et al. , Mature myelin maintenance requires Qki to coactivate PPARβ-RXRα-mediated lipid metabolism. J Clin Invest, 2020. 130(5): p. 2220–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Granneman J, Skoff R, and Yang X, Member of the peroxisome proliferator-activated receptor family of transcription factors is differentially expressed by oligodendrocytes. J Neurosci Res, 1998. 51(5): p. 563–73. [DOI] [PubMed] [Google Scholar]
- 173.Saluja I, Granneman JG, and Skoff RP, PPAR delta agonists stimulate oligodendrocyte differentiation in tissue culture. Glia, 2001. 33(3): p. 191–204. [PubMed] [Google Scholar]
- 174.Vittoria Simonini M, et al. , Regulation of oligodendrocyte progenitor cell maturation by PPARdelta: effects on bone morphogenetic proteins. ASN Neuro, 2010. 2(1): p. e00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sakuma S, et al. , Synthesis of a novel human PPARdelta selective agonist and its stimulatory effect on oligodendrocyte differentiation. Bioorg Med Chem Lett, 2011. 21(1): p. 240–4. [DOI] [PubMed] [Google Scholar]
- 176.Jana M, et al. , Gemfibrozil, a lipid-lowering drug, increases myelin genes in human oligodendrocytes via peroxisome proliferator-activated receptor-beta. J Biol Chem, 2012. 287(41): p. 34134–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Magge SS and Guardiola-Diaz H, Characterization of the mouse peroxisome proliferator-activated receptor delta gene. Biochem Biophys Res Commun, 2002. 290(1): p. 230–5. [DOI] [PubMed] [Google Scholar]
- 178.Cimini A, et al. , TNFalpha downregulates PPARdelta expression in oligodendrocyte progenitor cells: implications for demyelinating diseases. Glia, 2003. 41(1): p. 3–14. [DOI] [PubMed] [Google Scholar]
- 179.Roth AD, et al. , PPAR gamma activators induce growth arrest and process extension in B12 oligodendrocyte-like cells and terminal differentiation of cultured oligodendrocytes. J Neurosci Res, 2003. 72(4): p. 425–35. [DOI] [PubMed] [Google Scholar]
- 180.Paintlia AS, et al. , IL-4-induced peroxisome proliferator-activated receptor gamma activation inhibits NF-kappaB trans activation in central nervous system (CNS) glial cells and protects oligodendrocyte progenitors under neuroinflammatory disease conditions: implication for CNS-demyelinating diseases. J Immunol, 2006. 176(7): p. 4385–98. [DOI] [PubMed] [Google Scholar]
- 181.Sim FJ, et al. , Statin treatment of adult human glial progenitors induces PPAR gamma-mediated oligodendrocytic differentiation. Glia, 2008. 56(9): p. 954–62. [DOI] [PubMed] [Google Scholar]
- 182.Bernardo A, et al. , Peroxisome proliferator-activated receptor-gamma agonists promote differentiation and antioxidant defenses of oligodendrocyte progenitor cells. J Neuropathol Exp Neurol, 2009. 68(7): p. 797–808. [DOI] [PubMed] [Google Scholar]
- 183.Paintlia AS, et al. , Activation of PPAR-gamma and PTEN cascade participates in lovastatin-mediated accelerated differentiation of oligodendrocyte progenitor cells. Glia, 2010. 58(14): p. 1669–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Minghetti L, et al. , Nonenzymatic oxygenated metabolites of alpha-linolenic acid B1- and L1-phytoprostanes protect immature neurons from oxidant injury and promote differentiation of oligodendrocyte progenitors through PPAR-gamma activation. Free Radic Biol Med, 2014. 73: p. 41–50. [DOI] [PubMed] [Google Scholar]
- 185.De Nuccio C, et al. , Peroxisome proliferator activated receptor-gamma agonists protect oligodendrocyte progenitors against tumor necrosis factor-alpha-induced damage: Effects on mitochondrial functions and differentiation. Exp Neurol, 2015. 271: p. 506–14. [DOI] [PubMed] [Google Scholar]
- 186.Han L, et al. , Rosiglitazone Promotes White Matter Integrity and Long-Term Functional Recovery After Focal Cerebral Ischemia. Stroke, 2015. 46(9): p. 2628–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Leisewitz AV, et al. , A PPARs cross-talk concertedly commits C6 glioma cells to oligodendrocytes and induces enzymes involved in myelin synthesis. J Cell Physiol, 2008. 217(2): p. 367–76. [DOI] [PubMed] [Google Scholar]
- 188.Polak PE, et al. , Protective effects of a peroxisome proliferator-activated receptor-beta/delta agonist in experimental autoimmune encephalomyelitis. J Neuroimmunol, 2005. 168(1–2): p. 65–75. [DOI] [PubMed] [Google Scholar]
- 189.Kanakasabai S, et al. , Peroxisome proliferator-activated receptor delta agonists inhibit T helper type 1 (Th1) and Th17 responses in experimental allergic encephalomyelitis. Immunology, 2010. 130(4): p. 572–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Dunn SE, et al. , Peroxisome proliferator-activated receptor delta limits the expansion of pathogenic Th cells during central nervous system autoimmunity. J Exp Med, 2010. 207(8): p. 1599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Feinstein DL, et al. , Peroxisome proliferator-activated receptor-gamma agonists prevent experimental autoimmune encephalomyelitis. Ann Neurol, 2002. 51(6): p. 694–702. [DOI] [PubMed] [Google Scholar]
- 192.Natarajan C and Bright JJ, Peroxisome proliferator-activated receptor-gamma agonists inhibit experimental allergic encephalomyelitis by blocking IL-12 production, IL-12 signaling and Th1 differentiation. Genes Immun, 2002. 3(2): p. 59–70. [DOI] [PubMed] [Google Scholar]
- 193.Natarajan C, et al. , Peroxisome proliferator-activated receptor-gamma-deficient heterozygous mice develop an exacerbated neural antigen-induced Th1 response and experimental allergic encephalomyelitis. J Immunol, 2003. 171(11): p. 5743–50. [DOI] [PubMed] [Google Scholar]
- 194.Raikwar HP, et al. , PPARgamma antagonists reverse the inhibition of neural antigen-specific Th1 response and experimental allergic encephalomyelitis by Ciglitazone and 15-deoxy-Delta12,14-prostaglandin J2. J Neuroimmunol, 2006. 178(1–2): p. 76–86. [DOI] [PubMed] [Google Scholar]
- 195.He J, et al. , Bexarotene promotes microglia/macrophages - Specific brain - Derived Neurotrophic factor expression and axon sprouting after traumatic brain injury. Exp Neurol, 2020. 334: p. 113462. [DOI] [PubMed] [Google Scholar]
- 196.Pontis S, et al. , N-Acylethanolamine Acid Amidase contributes to disease progression in a mouse model of multiple sclerosis. Pharmacol Res, 2020. 160: p. 105064. [DOI] [PubMed] [Google Scholar]
- 197.Chamberlain S, et al. , An Exploratory Phase IIa Study of the PPAR delta/gamma Agonist T3D-959 Assessing Metabolic and Cognitive Function in Subjects with Mild to Moderate Alzheimer’s Disease. J Alzheimers Dis, 2020. 73(3): p. 1085–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Janowski BA, et al. , Structural requirements of ligands for the oxysterol liver X receptors LXRalpha and LXRbeta. Proc Natl Acad Sci U S A, 1999. 96(1): p. 266–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Courtney R and Landreth GE, LXR Regulation of Brain Cholesterol: From Development to Disease. Trends Endocrinol Metab, 2016. 27(6): p. 404–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Baranowski M, Biological role of liver X receptors. J Physiol Pharmacol, 2008. 59 Suppl 7: p. 31–55. [PubMed] [Google Scholar]
- 201.Willy PJ, et al. , LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev, 1995. 9(9): p. 1033–45. [DOI] [PubMed] [Google Scholar]
- 202.Svensson S, et al. , Crystal structure of the heterodimeric complex of LXRalpha and RXRbeta ligand-binding domains in a fully agonistic conformation. Embo j, 2003. 22(18): p. 4625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Varga G and Su C, Classification and predictive modeling of liver X receptor response elements. BioDrugs, 2007. 21(2): p. 117–24. [DOI] [PubMed] [Google Scholar]
- 204.Mouzat K, et al. , Regulation of Brain Cholesterol: What Role Do Liver X Receptors Play in Neurodegenerative Diseases? Int J Mol Sci, 2019. 20(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wang L, et al. , Liver X receptors in the central nervous system: from lipid homeostasis to neuronal degeneration. Proc Natl Acad Sci U S A, 2002. 99(21): p. 13878–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Edwards PA, Kennedy MA, and Mak PA, LXRs; oxysterol-activated nuclear receptors that regulate genes controlling lipid homeostasis. Vascul Pharmacol, 2002. 38(4): p. 249–56. [DOI] [PubMed] [Google Scholar]
- 207.Ulven SM, et al. , LXR is crucial in lipid metabolism. Prostaglandins Leukot Essent Fatty Acids, 2005. 73(1): p. 59–63. [DOI] [PubMed] [Google Scholar]
- 208.Beaven SW and Tontonoz P, Nuclear receptors in lipid metabolism: targeting the heart of dyslipidemia. Annu Rev Med, 2006. 57: p. 313–29. [DOI] [PubMed] [Google Scholar]
- 209.Cermenati G, et al. , Liver X receptors, nervous system, and lipid metabolism. J Endocrinol Invest, 2013. 36(6): p. 435–43. [DOI] [PubMed] [Google Scholar]
- 210.Wang B and Tontonoz P, Liver X receptors in lipid signalling and membrane homeostasis. Nat Rev Endocrinol, 2018. 14(8): p. 452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Meffre D, et al. , Liver X receptors alpha and beta promote myelination and remyelination in the cerebellum. Proc Natl Acad Sci U S A, 2015. 112(24): p. 7587–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Shackleford GG, et al. , Liver X Receptors differentially modulate central myelin gene mRNA levels in a region-, age- and isoform-specific manner. J Steroid Biochem Mol Biol, 2017. 169: p. 61–68. [DOI] [PubMed] [Google Scholar]
- 213.Sevastou I, et al. , Characterisation of Transcriptional Changes in the Spinal Cord of the Progressive Experimental Autoimmune Encephalomyelitis Biozzi ABH Mouse Model by RNA Sequencing. PLoS One, 2016. 11(6): p. e0157754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Mailleux J, et al. , Active liver X receptor signaling in phagocytes in multiple sclerosis lesions. Mult Scler, 2018. 24(3): p. 279–289. [DOI] [PubMed] [Google Scholar]
- 215.Fellows Maxwell K, et al. , Oxysterols and apolipoproteins in multiple sclerosis: a 5 year follow-up study. J Lipid Res, 2019. 60(7): p. 1190–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Dawson MI and Xia Z, The retinoid X receptors and their ligands. Biochim Biophys Acta, 2012. 1821(1): p. 21–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Vivat-Hannah V, et al. , Separation of retinoid X receptor homo- and heterodimerization functions. Mol Cell Biol, 2003. 23(21): p. 7678–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Szanto A, et al. , Retinoid X receptors: X-ploring their (patho)physiological functions. Cell Death Differ, 2004. 11 Suppl 2: p. S126–43. [DOI] [PubMed] [Google Scholar]
- 219.Nohara A, Kobayashi J, and Mabuchi H, Retinoid X receptor heterodimer variants and cardiovascular risk factors. J Atheroscler Thromb, 2009. 16(4): p. 303–18. [DOI] [PubMed] [Google Scholar]
- 220.Chiang MY, et al. , An essential role for retinoid receptors RARbeta and RXRgamma in long-term potentiation and depression. Neuron, 1998. 21(6): p. 1353–61. [DOI] [PubMed] [Google Scholar]
- 221.van Neerven S, Kampmann E, and Mey J, RAR/RXR and PPAR/RXR signaling in neurological and psychiatric diseases. Prog Neurobiol, 2008. 85(4): p. 433–51. [DOI] [PubMed] [Google Scholar]
- 222.Latasa MJ, et al. , Retinoic acid regulates myelin formation in the peripheral nervous system. Glia, 2010. 58(12): p. 1451–64. [DOI] [PubMed] [Google Scholar]
- 223.Royal W 3rd, Gartner S, and Gajewski CD, Retinol measurements and retinoid receptor gene expression in patients with multiple sclerosis. Mult Scler, 2002. 8(6): p. 452–8. [DOI] [PubMed] [Google Scholar]
- 224.Davina Kruczek TC, Cordian Beyer, Markus Kipp, Jörg Mey, Activation of Nuclear Receptors RAR, RXR, and LXR Does Not Reduce Cuprizone-Induced Demyelination in Mice. Nuclear Receptor Research, 2015. 2: p. 9. [Google Scholar]
- 225.Chandraratna RA, Noelle RJ, and Nowak EC, Treatment with retinoid X receptor agonist IRX4204 ameliorates experimental autoimmune encephalomyelitis. Am J Transl Res, 2016. 8(2): p. 1016–26. [PMC free article] [PubMed] [Google Scholar]
- 226.Huang JK, et al. , Retinoid X receptors as a potential avenue for regenerative medicine in multiple sclerosis. Expert Rev Neurother, 2011. 11(4): p. 467–8. [DOI] [PubMed] [Google Scholar]
- 227.Santos-Gil DF, Arboleda G, and Sandoval-Hernandez AG, Retinoid X receptor activation promotes re-myelination in a very old triple transgenic mouse model of Alzheimer’s disease. Neurosci Lett, 2021. 750: p. 135764. [DOI] [PubMed] [Google Scholar]
- 228.Merk D, Chances and challenges of retinoid X receptor gamma targeting for regenerative multiple sclerosis treatment. Future Med Chem, 2015. 7(18): p. 2411–3. [DOI] [PubMed] [Google Scholar]
- 229.Cunniffe N and Coles A, Promoting remyelination in multiple sclerosis. J Neurol, 2021. 268(1): p. 30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Yang XH, et al. , Effects of electroacupuncture and the retinoid X receptor (RXR) signalling pathway on oligodendrocyte differentiation in the demyelinated spinal cord of rats. Acupunct Med, 2017. 35(2): p. 122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Grytten N, et al. , A 50-year follow-up of the incidence of multiple sclerosis in Hordaland County, Norway. Neurology, 2006. 66(2): p. 182–6. [DOI] [PubMed] [Google Scholar]
- 232.Alonso A and Hernán MA, Temporal trends in the incidence of multiple sclerosis: a systematic review. Neurology, 2008. 71(2): p. 129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Gold SM and Voskuhl RR, Estrogen and testosterone therapies in multiple sclerosis. Prog Brain Res, 2009. 175: p. 239–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ysrraelit MC and Correale J, Impact of sex hormones on immune function and multiple sclerosis development. Immunology, 2019. 156(1): p. 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Vukusic S and Confavreux C, [Multiple sclerosis and pregnancy]. Rev Neurol (Paris), 2006. 162(3): p. 299–309. [DOI] [PubMed] [Google Scholar]
- 236.Vukusic S and Confavreux C, Pregnancy and multiple sclerosis: the children of PRIMS. Clin Neurol Neurosurg, 2006. 108(3): p. 266–70. [DOI] [PubMed] [Google Scholar]
- 237.González-Orozco JC and Camacho-Arroyo I, Progesterone Actions During Central Nervous System Development. Front Neurosci, 2019. 13: p. 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.De Nicola AF, et al. , Progesterone neuroprotection in traumatic CNS injury and motoneuron degeneration. Frontiers in neuroendocrinology, 2009. 30(2): p. 173–187. [DOI] [PubMed] [Google Scholar]
- 239.Prüfer K, et al. , Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal cord. J Chem Neuroanat, 1999. 16(2): p. 135–45. [DOI] [PubMed] [Google Scholar]
- 240.Speeckaert M, et al. , Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clin Chim Acta, 2006. 372(1–2): p. 33–42. [DOI] [PubMed] [Google Scholar]
- 241.Subramanian S, et al. , Contribution of GPR30 for 1,25 dihydroxyvitamin D(3) protection in EAE. Metab Brain Dis, 2012. 27(1): p. 29–35. [DOI] [PubMed] [Google Scholar]
- 242.Spanier JA, et al. , Vitamin D and estrogen synergy in Vdr-expressing CD4(+) T cells is essential to induce Helios(+)FoxP3(+) T cells and prevent autoimmune demyelinating disease. J Neuroimmunol, 2015. 286: p. 48–58. [DOI] [PubMed] [Google Scholar]
- 243.Pasing Y, et al. , Changes in the human transcriptome upon vitamin D supplementation. J Steroid Biochem Mol Biol, 2017. 173: p. 93–99. [DOI] [PubMed] [Google Scholar]
- 244.Liu H, et al. , Defining vitamin D receptor expression in the brain using a novel VDR(Cre) mouse. J Comp Neurol, 2021. 529(9): p. 2362–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Spach KM and Hayes CE, Vitamin D3 confers protection from autoimmune encephalomyelitis only in female mice. J Immunol, 2005. 175(6): p. 4119–26. [DOI] [PubMed] [Google Scholar]
- 246.Laczmanski L, et al. , Association between vitamin D concentration and levels of sex hormones in an elderly Polish population with different genotypes of VDR polymorphisms (rs10735810, rs1544410, rs7975232, rs731236). Gene, 2015. 559(1): p. 73–6. [DOI] [PubMed] [Google Scholar]
- 247.Rosenberger TA, Hovda JT, and Peters JM, Targeted disruption of peroxisomal proliferator-activated receptor beta (delta) results in distinct gender differences in mouse brain phospholipid and esterified FA levels. Lipids, 2002. 37(5): p. 495–500. [DOI] [PubMed] [Google Scholar]
- 248.Baksi S and Pradhan A, Thyroid hormone: sex-dependent role in nervous system regulation and disease. Biology of Sex Differences, 2021. 12(1): p. 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Rakov H, et al. , Sex-specific phenotypes of hyperthyroidism and hypothyroidism in mice. Biology of sex differences, 2016. 7(1): p. 36–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Yamaguchi S, et al. , Sex Differences in Brain Thyroid Hormone Levels during Early Post-Hatching Development in Zebra Finch (Taeniopygia guttata). PloS one, 2017. 12(1): p. e0169643–e0169643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kruse MS, et al. , Sex differences in LXR expression in normal offspring and in rats born to diabetic dams. J Endocrinol, 2014. 222(1): p. 53–60. [DOI] [PubMed] [Google Scholar]
- 252.Kosters A, et al. , Sexually dimorphic genome-wide binding of retinoid X receptor alpha (RXRα) determines male-female differences in the expression of hepatic lipid processing genes in mice. PloS one, 2013. 8(8): p. e71538–e71538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.DeLeon-Pennell KY, et al. , LXR/RXR signaling and neutrophil phenotype following myocardial infarction classify sex differences in remodeling. Basic Res Cardiol, 2018. 113(5): p. 40. [DOI] [PMC free article] [PubMed] [Google Scholar]


