Abstract
Many consumers are turning to kratom ( Mitragyna speciosa ) to self-manage pain and opioid addiction. In the United States, an array of capsules, powders, and loose-leaf kratom products are readily available. Additionally, several online sites supply live kratom plants. A prerequisite to establishing quality control and quality assurance standards for the kratom industry, or understanding how alkaloid levels effect clinical outcomes, is the identification and quantitation of major and minor alkaloid constituents within available products and preparations. To this end, an ultra-high performance liquid chromatography-high resolution mass spectrometry method was developed for the analysis of 8 indole alkaloids (7-hydroxymitragynine, ajmalicine, paynantheine, mitragynine, speciogynine, isopaynantheine, speciociliatine, and mitraciliatine) and 6 oxindole alkaloids (isomitraphylline, isospeciofoleine, speciofoline, corynoxine A, corynoxeine, and rhynchophylline) in US-grown kratom plants and commercial products. These commercial products shared a qualitatively similar alkaloid profile, with 12 – 13 detected alkaloids and high levels of the indole alkaloid mitragynine (13.9 ± 1.1 – 270 ± 24 mg/g). The levels of the other major alkaloids (paynantheine, speciociliatine, speciogynine, mitraciliatine, and isopaynantheine) and the minor alkaloids varied in concentration from product to product. The alkaloid profile of US-grown M. speciosa “Rifat” showed high levels of the indole alkaloid speciogynine (7.94 ± 0.83 – 11.55 ± 0.18 mg/g) and quantifiable levels of isomitraphylline (0.943 ± 0.033 – 1.47 ± 0.18 mg/g). Notably, the alkaloid profile of a US-grown M. speciosa seedling was comparable to the commercial products with a high level of mitragynine (15.01 ± 0.20 mg/g). This work suggests that there are several M. speciosa chemotypes.
Key words: kratom, Mitragyna speciosa, Rubiaceae, indole alkaloid, oxindole alkaloid, chemotype, UPLC-HRMS
Abbreviations
- BEH
ethylene bridged hybrid
- BLQ
below the lower limit of quantification
- CH 3 CN
acetonitrile
- CHCl 3
chloroform
- CYP
cytochrome P450
- ECD
electronic circular dichroism spectroscopy
- HESI
heated electrospray ionization
- IT
injection time
- LLOD
lower limit of detection
- LLOQ
lower limit of quantitation
- PDA
photodiode array detector
- PVDF
polyvinylidene fluoride
- RE
relative error
- RSD
relative standard deviation
- SE
standard error
- SERS
surface-enhanced Raman spectroscopy
- SFC
supercritical fluid chromatography
- ULOQ
upper limit of quantitation
Introduction
Chronic pain affects an estimated 50 million adults in the United States (US) and is a prominent reason for seeking medical care 1 . The prevalence of pain and the highly addictive nature of opiates, the major therapeutics for chronic pain, have, in part, led to the current opioid crisis surging through the US 2 . An estimated 2 million Americans suffer from substance use disorders related to prescription opioids, and more than 130 die each day from opioid overdose 3 . In light of these concerns, an increasing number of US consumers are turning to plant-based medicines (i.e., botanical dietary supplements) as an alternative way to treat chronic pain 4 . Among the most popular of these is Mitragyna speciosa (Korth.) Havil. (Rubiaceae), commonly referred to as kratom 5 , the use of which has risen dramatically in the US over the last decade 5 , 6 .
Kratom is an evergreen tree native to Southeast Asia 7 , 8 , where its medicinal use was interwoven into Thai and Malaysian cultures. Kratom exhibits a complex pharmacology illustrated by its traditional use both to thwart pain 7 , 9 and ameliorate opioid addiction 7 . The earliest literature reports of kratom refer to its seemingly contradictory use by Malay and Thai people as a sedative opium substitute 10 , an aid to opium-use cessation, and as a stimulant to combat fatigue and increase productivity 11 . These divergent applications could be explained by the mixture of structurally diverse alkaloids present in kratom leaf material, which exhibit differential binding affinities to neurochemical receptors and elicit concentration dependent effects 7 , 12 , 13 . While a body of literature largely attributes the effects of kratom to the major indole alkaloid constituent, mitragynine ( 12 ) ( Fig. 1 ), and the minor alkaloid, 7-hydroxymitragynine ( 2 ), the plant also produces at least fifty-eight other alkaloids with diverse structures 14 .
Fig. 1.
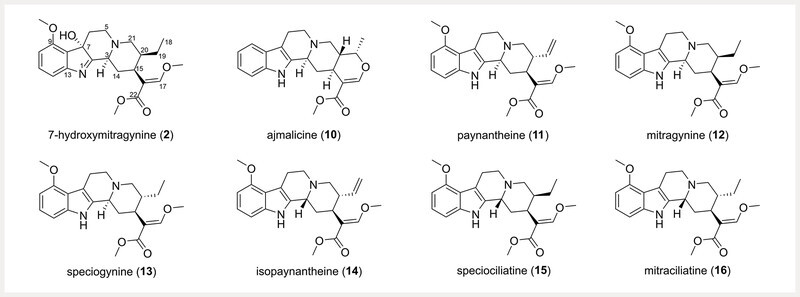
Structures of the indole alkaloids 2, 10 , and 11 – 16 . The numerical order of the compounds corresponds to their elution order via ultra-high performance liquid chromatography in Fig. 3 .
Recent literature is beginning to evaluate the pharmacological importance of the other major and minor alkaloids present in M. speciosa 15 , 16 , 17 , 18 . Investigation of the opioid and adrenergic binding affinities of five kratom alkaloids, including 2, 12 , speciociliatine ( 15 ), corynantheidine, and 9-hydroxycorynantheidine, revealed that 15 exhibited stronger binding affinity to κ - and μ -opioid receptors than 12 19 ; interestingly, 12 and 15 only differ by their configuration at position C-3 14 . In the same study, the indole alkaloids corynantheidine and 9-hydroxycorynantheidine showed measurable, albeit weaker, affinity to μ -opioid receptors than 12 . Mitraciliatine ( 16 ), a diastereomer of 12 with opposite configurations at the C-3 and C-20 positions, shows μ -opioid receptor partial agonism and κ -opioid receptor full agonism at both mouse and human receptors 18 . Our recent analysis of over 50 commercial kratom products identified two different chemotypes with either a high or low abundance of the oxindole alkaloid speciofoline ( 6 ) ( Fig. 2 ) 20 . While 6 does not exhibit measurable binding affinity at the μ -, δ -, or κ -opioid receptors, it does inhibit important cytochrome P450 enzymes involved in drug metabolism (i.e., CYP2C9, CYP2D6, CYP3A) 20 . Speciociliatine ( 15 ), a diastereomer of 12 also present in commercial kratom products, was shown recently in a preclinical pharmacokinetic study to exhibit a higher systemic exposure and lower clearance compared to 12 and corynantheidine 21 .
Fig. 2.
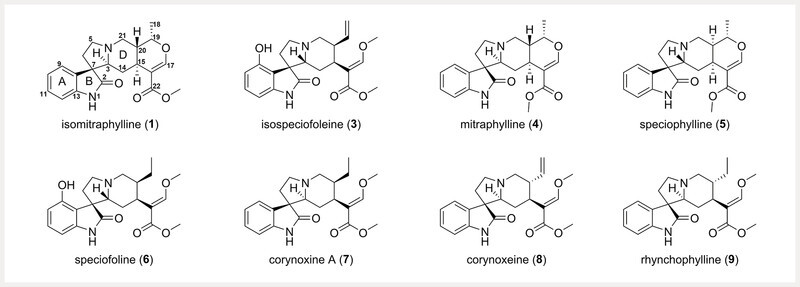
Structures of the oxindole alkaloids 1 and 3 – 9 . The numerical order of the compounds corresponds to their elution order via ultra-high performance liquid chromatography in Fig. 3 .
The reports of variable biological effects of kratom alkaloids substantiate the need for further preclinical and clinical studies to determine the pharmacological and pharmacokinetic properties of consumer-utilized kratom products and the lesser studied kratom alkaloids contained therein. A prerequisite to such studies is the thorough identification and quantitation of the major and minor alkaloid constituents within commercially available products, traditional preparations, and those utilized for preclinical or clinical investigations. Analytical and bioanalytical methods over the last century have focused on the identification and quantification of 12 and 2 from different matrices (e.g., plant material, commercial products, blood, and urine) 22 , 23 with varying chromatographic [e.g., HPLC, UHPLC, GC, and supercritical fluid chromatography (SFC)] and detection techniques [e.g., UV/DAD, MS, ELISA, and surface-enhanced Raman spectroscopy (SERS)] (see Table 1S , Supporting Information, for full comparison of methods and pertinent references). Recent efforts have pushed beyond the examination of 12 into the detection and quantification of several alkaloids present in M. speciosa and commercial kratom products. Kikura-Hanajiri et al. 24 applied LC-ESI-MS to simultaneously quantify five kratom alkaloids [ 2 , paynantheine ( 11 ), 12 , speciogynine ( 13 ), and 15 ]. Wang et al. 25 compared three different chromatographic techniques (HPLC-DAD/MS, GC-MS, and SFC-DAD) to analyze eight kratom alkaloids [ 2 , corynoxine A ( 7 ), 11, 12, 13 , isopaynantheine ( 14 ), 15 , and corynoxine B]. Several methods arising from the Avery and McCurdy groups utilize a triple quadrupole mass spectrometer for UHPLC-MS/MS of kratom leaf extracts and commercial products. Initially, their method quantified simultaneously ten kratom alkaloids [ 2 , mitraphylline ( 4 ), 7, 11, 12, 13, 15 , corynoxine B, corynantheidine, and isocorynantheidine] in leaf extracts and commercial products 26 . Later, Jeng-Yeou Chear et al. 17 quantified Malaysian M. speciosa samples using the method reported by Sharma et al. 26 , including an additional four alkaloids [isospeciofoline, mitragynine oxindole B, speciociliatine- N (4)-oxide, and ajmalicine ( 10 )] in the standard mixture. Recently, Kamble et al. used an optimized method with a shorter runtime to quantify 11 kratom alkaloids ( 2, 4, 7, 10, 11, 12, 13, 15 , corynoxine B, corynantheidine, and isospeciofoline) in rat plasma 16 .
As part of a project to study the potential for interactions between herbal medicines and drugs 27 , our team recently generated a suite of kratom alkaloid reference standards 14 . The purpose was to incorporate these standards into a validated analytical method to quantify the indole and oxindole alkaloid constituents of commercially available kratom material. Compared to the published analytical methods for kratom alkaloid quantitation, this method was designed to leverage the superior resolving power of a hybrid quadruple-orbitrap mass spectrometer and a suite of both oxindole and indole alkaloid standards to generate a more comprehensive method for quantifying the major and minor alkaloids in kratom. Toward this goal, we developed a method that identifies and quantifies 14 kratom alkaloids, including all four mitragynine diastereomers ( 12, 13, 15, 16 ) ( Fig. 1 ), and 5 oxindole alkaloids ( 1, 3, 6, 8 , and 9 ) that have not previously been quantified in kratom ( Fig. 2 ). The method was then employed to describe the indole and oxindole alkaloid variability between commercial kratom products and living kratom plants, ultimately, to identify if US-grown kratom plants could exhibit a similar alkaloid profile to commercial kratom material purportedly originating from Southeast Asia.
Results and Discussion
Our initial aim was to develop a method capable of quantifying both indole and oxindole alkaloids simultaneously, including several compounds that are known to M. speciosa but never included in prior quantification studies. Ultimately, a UPLC-HRMS method was developed for the quantitative analysis of 14 kratom alkaloids, including 8 indole alkaloids (i.e., 2, 10, 11, 12, 13, 14, 15 , and 16 ) and 6 oxindole alkaloids [i.e., isomitraphylline ( 1 ), isospeciofoleine ( 3 ), 6, 7 , corynoxeine ( 8 ), and rhynchophylline ( 9 )], in complex extracts.
The methodology benefits from the comprehensive nature of examining a suite of both indole and oxindole alkaloids, including all four mitragynine diastereomers ( 12, 13, 15, 16 ), and 5 oxindole alkaloids ( 1, 3, 6, 8 , and 9 ) which have not previously been quantified in kratom materials.
Method development began with the analysis of kratom alkaloids in both positive and negative electron ionization modes and UPLC separation using various chromatographic conditions. Positive mode ionization was suited for this analysis, but the chromatography proved to be a unique challenge. Kratom extracts were initially screened using a BEH C18 column and a binary mobile phase consisting of 0.1% formic acid in H 2 O and CH 3 CN ( Fig. 1S , Supporting Information); however, the resolution between peaks was inadequate. Optimal chromatographic resolution was achieved by evaluating several chromatographic conditions, including alternative mobile phases [e.g., aqueous phase (A): water with added formic acid or ammonium formate; organic phase (B): MeOH or CH 3 CN with added formic acid or ammonium formate] and alternative stationary phases (e.g., C18, amide, biphenyl, and pentafluorophenyl). The combination that showed the best chromatographic separation was the Kinetex F5 column (Phenomenex) with a binary mobile phase consisting of 0.1% formic acid in H 2 O and CH 3 CN at a flow rate of 0.6 mL/min. The alkaloids eluted sequentially over the course of 20.0 minutes with three primary groupings, specifically: compounds 1, 2, 3, 4, and 5 (6.11, 6.94, 7.00, 7.12, and 7.26 min, respectively) in the first group; compounds 6, 7, 8, 9 , and 10 (8.94, 9.12, 9.25, 9.95, and 10.23 min, respectively) in the second group; followed by the internal standard (12.41 min) and compounds 11, 12, 13, 14, 15 , and 16 (12.39, 12.44, 12.75, 12.85, 13.01, 13.15 min, respectively) forming the last group ( Figs. 3 and 4 ).
Fig. 3.
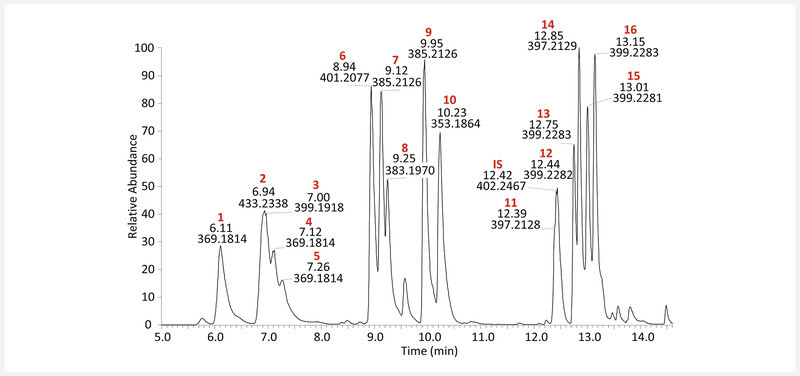
Base peak MS chromatogram of an equimolar mixture of kratom alkaloids at 313 ng/mL. Structures corresponding to each peak ( Figs. 1 and 2 ) were assigned by matching retention time, fragmentation pattern, and accurate mass with authentic standards. Compounds are numbered in order of elution.
Fig. 4.
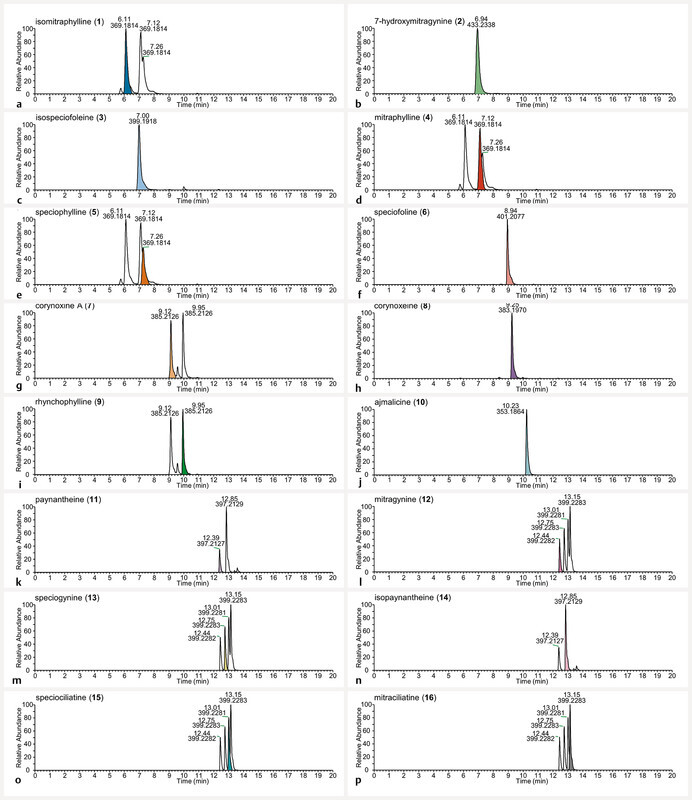
Extracted ion chromatograms of the protonated molecular ions m/z 369.1809 (panels a, d , and e ), 433.2333 (panel b ), 399.1915 (panel c ), 401.2071 (panel f ), 385.2122 (panels g and i ), 383.1965 (panel h ), 353.1860 (panel j ), 397.2122 (panels k and n ), and 399.2278 (panels l, m, o , and p ) at 313 ng/mL, the central concentration of the analyzed calibration solutions. The diastereomers in the standard mixture share the same m/z value, thus several panels ( a, d, e, g, i, k – p ) have more than one peak present. The colors distinguish the alkaloid ( 1 – 16 ) being referenced by the panel name (e.g., isomitraphylline ( 1 ) is denoted by the color blue in panel a ).
The analytical method was then validated for linearity, precision, accuracy, repeatability, and sensitivity. A nine- (alkaloids 2, 3, 7, 9, 11, 13, 14, 15 and 16 ) or ten-point (alkaloids 1, 6, 8, 10 and 12 ) calibration curve was plotted using a weighted (1/x 2 ) least squares regression model. The curves were linear across a concentration range of 9.77 – 2500 ng/mL and 9.77 – 5000 ng/mL, respectively, with a coefficient of determination greater than 0.992 ( Table 1 ). The repeatability (intraday) and intermediate precision (interday) of the method was confirmed by evaluating the precision (RSD) at either nine or ten different concentrations for each alkaloid ( Table 2S , Supporting Information). Intraday and interday accuracy was evaluated by calculating the relative error (RE), which is the percent difference between the measured concentration and the nominal concentration of each standard. Both interday and intraday RE and RSD were all below the acceptable cut-off value of 20% ( Table 2S , Supporting Information) 28 . To evaluate potential matrix effects, an internal standard of heavy labeled 12 (mitragynine- d 3 ) was added to each of the samples at a final concentration of 125 µg/mL. The recovery of mitragynine- d 3 was determined using the standard curve of 12 ( Table 3S , Supporting Information). Recoveries ranged from 80 – 96%, suggesting minimal matrix interference. Analysis of the standard mixture (9.77 ng/mL) at the start of each run verified a resolution greater than 2 for all isomeric compounds included in the quantitation, except isomers 15 and 16 with an adequate resolution of greater than 1. While compounds 4 and 5 were omitted from quantitative validation due to poor chromatographic resolution and indistinguishable fragmentation spectra, this is the first method wherein both 4 and 5 are included and are identifiable. Our method surpasses previous methods by differentiating the isomers 1, 4 , and 5 , and quantifying isomer 1 .
Table 1 Parameters of calibration curves for each alkaloid.
| Analyte | Slope (± SE a ) × 10 3 | Intercept (± SE) × 10 5 | r 2 | LLOD b (ng/mL) | Linear Range of Quantitation (ng/mL) |
|---|---|---|---|---|---|
| a Standard error; b Lower limit of detection | |||||
| isomitraphylline ( 1 ) | 729.9 (9.0) | − 11.2 (2.4) | 0.996 | 1.1 | 9.77 – 5000 |
| 7-hydroxymitragynine ( 2 ) | 647 (11) | − 9.9 (2.8) | 0.993 | 0.67 | 9.77 – 2500 |
| isospeciofoleine ( 3 ) | 307.1 (5.0) | − 4.9 (1.3) | 0.993 | 0.79 | 9.77 – 2500 |
| speciofoline ( 6 ) | 1274 (15) | − 24.5 (4.1) | 0.996 | 0.66 | 9.77 – 5000 |
| corynoxine A ( 7 ) | 1261 (16) | − 26.9 (4.1) | 0.996 | 0.69 | 9.77 – 2500 |
| corynoxeine ( 8 ) | 817 (11) | − 20.7 (2.8) | 0.995 | 0.77 | 9.77 – 5000 |
| rhynchophylline ( 9 ) | 1915 (27) | − 33.6 (6.8) | 0.995 | 1.0 | 9.77 – 2500 |
| ajmalicine ( 10 ) | 1024 (17) | − 12.0 (4.6) | 0.992 | 1.0 | 9.77 – 5000 |
| paynantheine ( 11 ) | 658.0 (7.3) | − 2.4 (1.8) | 0.997 | 1.5 | 9.77 – 2500 |
| mitragynine ( 12 ) | 917 (11) | − 1.3 (2.8) | 0.996 | 1.4 | 9.77 – 5000 |
| speciogynine ( 13 ) | 1009 (16) | − 23.1 (4.1) | 0.994 | 1.3 | 9.77 – 2500 |
| isopaynantheine ( 14 ) | 668 (12) | − 5.7 (2.9) | 0.993 | 0.94 | 9.77 – 2500 |
| speciociliatine ( 15 ) | 1110 (16) | − 21.1 (4.1) | 0.995 | 0.59 | 9.77 – 2500 |
| mitraciliatine ( 16 ) | 1514 (23) | − 28.2 (5.8) | 0.994 | 0.70 | 9.77 – 2500 |
The landscape of commercially available kratom products in the US has grown significantly in the past decade. Currently, a vast array of powders, capsules, extracts, and loose-leaf kratom products are readily available to consumers via online sites and local retailers. The utility of the validated method was demonstrated by choosing a small sampling of products of different formulations and quantifying the indole and oxindole alkaloids contained therein ( Table 2 ). While powders representative of several different chemotypes (e.g., high/low speciofoline) were included in the group, this sampling does not epitomize the vast landscape of kratom materials and yet unknown chemotypes. Alkaloid content was measured by preparing methanolic extracts of two powdered plant products (K51 and K52), a loose-leaf product (K49), a liquid product (K76), and an encapsulated powder (K77) ( Tables 2 and 3 ). Alkaloid quantities revealed that 12 was the major alkaloidal constituent (0.53 ± 0.12 – 270 ± 24 mg/g of powdered material) ( Table 2 and Fig. 2S , Supporting Information), which is consistent with the literature 17 , 24 , 26 , 29 . The order of the next most abundant alkaloids varied among the samples with the general trend being 11, 15, 13, 16, 14, 7 , and 2 with 5.79 ± 0.91 – 70.4 ± 5.2, 3.68 ± 0.32 – 41.7 ± 3.2, 3.18 ± 0.13 – 33.4 ± 2.7, 0.647 ± 0.035 – 4.75 ± 0.47, 0.512 ± 0.010 – 3.80 ± 0.26, 0.2322 ± 0.0044 – 11.40 ± 0.84, and 0.1240 ± 0.0014 – 1.10 ± 0.17 mg/g of dry material, respectively. Comparable to our previous analysis, K52 had a higher concentration of 6 (2.51 ± 0.19 mg/g of material) than the other two leaf products (K49 and K51), where the concentration of 6 was below the lower limit of quantitation (K49) and 0.1222 ± 0.0020 mg/g of material (K51), respectively 14 , 20 .
Table 2 Alkaloid content in commercial products and dried leaves (24 h maceration in MeOH), mg of compound/g of powder a (± SD b ).
| Sample | isomitraphylline (1) | 7-hydroxymitragynine (2) | isospeciofoleine (3) | speciofoline (6) | corynoxine A (7) | corynoxeine (8) | rhynchophylline (9) | ajmalicine (10) | paynantheine (11) | mitragynine (12) | speciogynine (13) | isopaynantheine (14) | speciociliatine (15) | mitraciliatine (16) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples K49, K51, K52, and K77 are commercial kratom products that were purchased for this study. Sample K55 and K59 are cultivated kratom plants. Quantities were calculated by preparing methanolic extracts (24 h maceration) in triplicate and analyzing by LC-MS. a Quantity is denoted as mg of compound per g of dried kratom leaf or product material (± SD) and reflects the average of triplicate extractions; b Standard deviation of triplicate extractions, each analyzed separately. Italicized = BLQ, below the linear range and above the calculated lower limit of quantitation (LLOQ). – = below calculated LLOQ; No value = not detected or below lower limit of detection (LLOD) | ||||||||||||||
| K49 | 0.1590 (0.0077) | – | – | – | – | – | 5.79 (0.91) | 26.5 (4.0) | 3.42 (0.52) | 0.85 (0.13) | 9.4 (1.7) | 1.174 (0.079) | ||
| K51 | 0.1240 (0.0014) | – | 0.1222 (0.0020) | 0.2322 (0.0044) | – | – | – | 5.86 (0.26) | 19.48 (0.81) | 3.18 (0.13) | 0.512 (0.010) | 5.12 (0.26) | 0.647 (0.035) | |
| K52 | 0.1383 (0.0053) | 0.382 (0.034) | 2.51 (0.19) | 1.73 (0.13) | 0.290 (0.017) | – | – | 9.94 (0.88) | 13.9 (1.1) | 3.84 (0.32) | 1.20 (0.11) | 3.68 (0.32) | 1.08 (0.12) | |
| K55 | 1.47 (0.18) | – | 0.451 (0.077) | – | 0.239 (0.034) | 0.192 (0.015) | 1.46 (0.19) | 0.53 (0.12) | 7.94 (0.83) | – | 0.185 (0.029) | 0.872 (0.073) | ||
| K59 | 0.943 (0.033) | – | 0.205 (0.045) | – | – | 0.648 (0.053) | 2.418 (0.020) | 2.076 (0.068) | 11.55 (0.18) | 0.269 (0.018) | 0.862 (0.027) | 1.93 (0.13) | ||
| K77 | 1.10 (0.17) | 1.675 (0.051) | 5.90 (0.48) | 11.40 (0.84) | 1.217 (0.043) | – | – | 70.4 (5.2) | 270 (24) | 33.4 (2.7) | 3.80 (0.26) | 41.7 (3.2) | 4.75 (0.47) | |
Table 3 Alkaloid content in commercial products (24 h maceration in MeOH or H 2 O), a liquid product, and an alkaloidal partition, mg of compound/g of dried extract a (± SD b ).
| Sample | isomitraphylline (1) | 7-hydroxymitragynine (2) | isospeciofoleine (3) | speciofoline (6) | corynoxine A (7) | corynoxeine (8) | rhynchophylline (9) | ajmalicine (10) | paynantheine (11) | mitragynine (12) | speciogynine (13) | isopaynantheine (14) | speciociliatine (15) | mitraciliatine (16) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples K49, K51, K52, K76, and K77 are commercial kratom products that were purchased for this study. K51-2 is the alkaloidal partition from a CHCl 3 –MeOH extract of K51. K51-3 is a hot water (tea) extract of K51. Quantities were calculated by preparing extracts in triplicate and analyzing by LC-MS. a Quantity is denoted as mg of compound per g of dried extract (± SD) and reflects the average of triplicate extractions; b Standard deviation of triplicate extractions, each analyzed separately; Italicized = BLQ, below the linear range and above the calculated lower limit of quantitation (LLOQ); – = below calculated LLOQ. No value = not detected or below lower limit of detection (LLOD) | ||||||||||||||
| K49 | 0.449 (0.025) | – | – | – | – | – | 16.3 (2.7) | 75 (12) | 9.7 (1.6) | 2.41 (0.39) | 26.4 (5.0) | 3.31 (0.25) | ||
| K51 | 0.4585 (0.0069) | – | 0.4516 (0.0030) | 0.859 (0.014) | – | – | – | 21.65 (0.80) | 72.0 (2.7) | 11.76 (0.41) | 1.893 (0.052) | 18.93 (0.76) | 2.39 (0.11) | |
| K51-2 | 10.3 (1.6) | – | 3.05 (0.18) | 8.81 (0.62) | 2.686 (0.015) | – | – | 102 (12) | 387 (48) | 57.7 (7.1) | 10.9 (1.3) | 106 (12) | 13.1 (1.7) | |
| K51-3 | 0.0939 (0.0028) | 0.0528 (0.0021) | 0.1114 (0.0076) | 0.275 (0.036) | 0.0949 (0.0051) | – | – | 4.3 (0.39) | 15.3 (1.4) | 2.18 (0.16) | 0.301 (0.032) | 3.20 (0.34) | 0.377 (0.049) | |
| K52 | 0.420 (0.014) | 1.160 (0.097) | 7.62 (0.53) | 5.27 (0.37) | 0.881 (0.047) | – | – | 30.2 (2.5) | 42.2 (3.2) | 11.68 (0.92) | 3.65 (0.30) | 11.17 (0.91) | 3.28 (0.33) | |
| K76 | – | 0.694 (0.013) | 1.776 (0.041) | 2.711 (0.016) | 0.6096 (0.0054) | – | – | 14.43 (0.11) | 54.89 (0.37) | 7.08 (0.13) | 1.013 (0.034) | 8.87 (0.17) | 1.232 (0.022) | |
| K77 | 2.88 (0.38) | 4.40 (0.22) | 15.5 (1.5) | 30.0 (2.7) | 3.20 (0.17) | – | – | 185 (17) | 709 (76) | 87.8 (8.5) | 10.00 (0.86) | 110 (10) | 12.45 (0.98) | |
The encapsulated powder (K77) had the highest concentration of alkaloids among the commercial products ( Table 2 and 3 ; Fig. 2S and 3S , Supporting Information). The ten-fold higher concentration of alkaloids in K77 suggests that the capsules were prepared from the alkaloidal fraction of kratom. Indeed, the alkaloid concentrations in K77 were comparable to those measured in an alkaloidal fraction prepared from product K51 (K51-2). This observation was supported by the prominent yellow color of the K77 powder, which closely resembled the color of the K51 alkaloidal fraction (K51-2). The alkaloid quantities of the liquid product (K76) were comparable to the methanolic extracts of K49, K51, and K52, suggesting the liquid product was an alcoholic extract of kratom material ( Table 3 and Fig. 3S , Supporting Information).
Product K51 is being used in a Phase 1 clinical trial to evaluate the pharmacokinetics of kratom alkaloids (Clinical Trials.gov, NCT04392011, 2020). As part of the trial, participants consume a slurry of tea composed of 2 g of kratom dry leaf powder in 240 mL of hot water. This method of use mimics the typical consumption of kratom tea by US consumers. Considering this, a hot water extract of K51 was prepared (K51-3) for quantifying the alkaloidal constituents. Using this validated method, 13 kratom alkaloids were detected in the hot water infusion. The compounds 12, 11, 15, 13, 16, 14, 7, 6, 8, 2 , and 3 were quantified at 15.3 ± 1.4, 4.3 ± 0.39, 3.20 ± 0.34, 2.18 ± 0.16, 0.377 ± 0.049, 0.301 ± 0.032, 0.275 ± 0.036, 0.1114 ± 0.00076, 0.0949 ± 0.0051, 0.0939 ± 0.0028, and 0.0528 ± 0.0021 mg per g of dry extract, respectively. Compounds 9 and 10 were detected in the tea but were found below the LLOQ. One caveat to comparing the alkaloid levels from this specific water extraction to the tea preparation consumed in the referenced clinical trial is that the clinical trial participants consume both the liquid tea and the residual dregs (powder). The alkaloid levels reported here refer specifically to the water extractable amounts and may, therefore, be lower than the quantities ingested in the clinical study.
Quantitative analysis of leaves from a US kratom grower (K59) and a young kratom plant obtained from the same supplier and grown in Greensboro, NC (K55) yielded alkaloid profiles vastly different from the commercial products ( Table 2 and Fig. 2S , Supporting Information). Compound 13 was the most abundant alkaloid in both samples (K55 7.94 and K59 11.55 mg/g powdered leaf material), with 12 content greater in K59 (2.076 ± 0.068 mg/g powdered leaf material) than K55 (0.53 mg/g powdered leaf material). Notably, both samples contained the oxindole alkaloid 1 , which was not detected in the commercial products.
The low levels of 12 observed in K55 and K59 were similar to previous analyses of US-grown M. speciosa . Specifically, a young (< 5 years old) M. speciosa plant grown in the gardens of the University of Mississippi yielded the oxindole alkaloid 4 as the major constituent 30 , Florida grown M. speciosa cuttings yielded low levels of 12 with 4 and 13 as the major constituents 31 , and a young M. speciosa plant grown in New York had the least amount of 12 per g of dried plant material compared to commercial kratom products 32 . The identity of the young kratom plants were not specified in the referenced articles, but it can be speculated that they are clones arising from either the “Rifat”, “Bumblebee”, or “Malay” plants available from several online suppliers of live kratom plants. Lesiak and Musah 2016 reported using direct analysis in real time-high resolution mass spectrometry (DART-HRMS) to analyze M. speciosa leaf samples 33 . The analysis compared a young “Rifat” plant to commercial kratom leaf and powder, wherein the relative intensity of the protonated mitragynine ion ([M + H] + = 399.2284) was 100% in the commercial samples, and 64.1% in the live “Rifat” sample. The protonated mitraphylline ion ([M + H] + = 369.1814) was the most abundant ion in the live “Rifat” sample (100% relative intensity) but was undetected in the commercial samples. The DART-HRMS method does not distinguish between diastereomers, thus the m/z of 369.1814 may represent any one of the stereoisomers of mitraphylline (i.e., speciophylline or isomitraphylline).
Previous studies hypothesized that compared to commercially obtained kratom samples, US-grown M. speciosa were a chemical variant (chemotype) 17 , 32 , a genetic variant (genotype) 31 , or that a key environmental factor responsible for biosynthesis of 12 was not present in their study 31 . Assuming that previous work done on US-grown M. speciosa was conducted using rooted cuttings of a “Rifat” plant, we tested the hypothesis that the ratio of 12 to 13 in M. speciosa “Rifat” is due to underlying chemotype differences and not due to growing conditions. To test this hypothesis, we quantified the indole and oxindole alkaloid levels of a rooted cutting of M. speciosa “Rifat” (K64) and a M. speciosa seedling (purportedly from Mempawah, West Kalimantan, Indonesia) (K68) that were cultivated under the same growing conditions in Greensboro, North Carolina, USA ( Table 4 , Fig. 5 , and Fig. 4S , Supporting Information). For this analysis we performed a rapid methanolic extraction utilizing 30 min of ultrasound sonication. Other literature has established sonication as a rapid and useful method to extract alkaloids from M. speciosa 24 , 34 . This method allows a higher throughput of plant samples, which will prove useful in future analyses of many kratom plants and products. The 12 / 13 ratio for K64 was 1 : 8 (0.962 ± 0.010 : 7.81 ± 0.33 mg/g powdered leaf material), while that of K68 was 3 : 1 (15.01 ± 0.20 : 4.52 ± 0.23 mg/g powdered leaf material). The 12 / 13 ratio of K64 resembled that of K59 (1 : 5, 2.782 ± 0.041 : 12.69 ± 0.42 mg/g powdered leaf material), which was obtained from a different supplier of M. speciosa “Rifat” leaf material. Conversely, the 12 / 13 ratio of K68 was similar to the commercial products K51 and K52 (6 : 1, 16.60 ± 0.89 : 2.95 ± 0.17 mg/g powdered material and 4 : 1, 16.84 ± 0.14 : 4.560 ± 0.010 mg/g powdered material, respectively) ( Table 4 ). This is the first literature example of a US-grown M. speciosa plant exhibiting a high-mitragynine alkaloid profile equivalent to Southeast Asian kratom products. Moreover, these results substantiate the claim that the M. speciosa “Rifat” plants used in this study (and likely previous studies in the literature) are a different chemotype (i.e., chemical phenotype) than M. speciosa plants growing in Southeast Asia.
Table 4 Alkaloid content in commercial products and dried leaves (30 min sonication in MeOH), mg of compound/g of powder a (± SD b ).
| Sample | isomitraphylline (1) | 7-hydroxymitragynine (2) | isospeciofoleine (3) | speciofoline (6) | corynoxine A (7) | corynoxeine (8) | rhynchophylline (9) | ajmalicine (10) | paynantheine (11) | mitragynine (12) | speciogynine (13) | isopaynantheine (14) | speciociliatine (15) | mitraciliatine (16) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples K51 and K52 are commercial kratom products that were purchased for this study. Sample K55, K59, K64, and K68 are cultivated kratom plants. Quantities were calculated by preparing methanolic extracts (30 min sonication) in triplicate and analyzing by LC-MS. a Quantity is denoted as mg of compound per g of dried kratom leaf or product material (± SD) and reflects the average of triplicate extractions; b Standard deviation of triplicate extractions, each analyzed separately; Italicized = BLQ, below the linear range and above the calculated lower limit of quantitation (LLOQ); – = below calculated LLOQ. No value = not detected or below lower limit of detection (LLOD) | ||||||||||||||
| K51 | – | – | 0.1241 (0.0029) | 0.1900 (0.0055) | 0.1212 (0.0014) | – | – | 4.97 (0.25) | 16.56 (0.89) | 2.86 (0.18) | 0.387 (0.019) | 4.32 (0.24) | 0.611 (0.060) | |
| K52 | 0.01288 (0.00034) | 0.2271 (0.0050) | 3.091 (0.043) | 1.680 (0.042) | 0.2500 (0.0029) | 0.1561 (0.0026) | – | 11.00 (0.23) | 16.84 (0.14) | 4.560 (0.010) | 1.5074 (0.0050) | 6.13 (0.23) | 1.639 (0.057) | |
| K59 | 1.230 (0.054) | 0.01348 (0.00030) | – | 0.113 (0.012) | – | 0.0480 (0.0053) | 0.883 (0.024) | 3.034 (0.043) | 2.782 (0.041) | 12.69 (0.42) | 0.251 (0.011) | 1.536 (0.078) | 2.522 (0.078) | |
| K64 | 0.853 (0.032) | – | – | 0.1556 (0.0054) | – | 0.0525 (0.0022) | 0.0665 (0.0015) | 1.535 (0.049) | 0.962 (0.010) | 7.81 (0.33) | 0.1052 (0.0079) | 0.2181 (0.0069) | 1.1042 (0.0081) | |
| K68 | 0.476 (0.022) | 0.01450 (0.00062) | 0.01985 (0.00019) | 0.1904 (0.0079) | 0.4240 (0.0037) | 0.1778 (0.0051) | 0.0262 (0.0020) | 3.53 (0.10) | 15.01 (0.20) | 4.52 (0.23) | 0.0411 (0.0017) | 0.4539 (0.0047) | 0.400 (0.026) | |
Fig. 5.
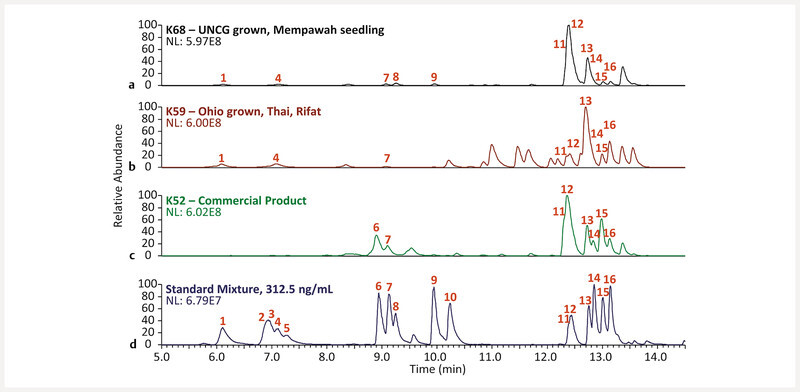
Base peak MS chromatograms of, from top to bottom, (panel a) methanolic extracts of a UNCG grown kratom seedling (K68), (panel b) an Ohio grown Thai-Rifat cutting, (panel c) a commercial kratom powder (K52), and (panel d) an equimolar mixture of kratom alkaloids at 313 ng/mL. Structures corresponding to each peak ( Figs. 1 and 2 ) were assigned by matching retention time, fragmentation pattern, and accurate mass with authentic standards. Compounds are numbered in order of elution. NL is the normalization level, i.e., the base peak intensity.
The scientific evidence supporting the existence of M. speciosa chemotypes is growing. In addition to literature reports of alkaloid levels varying considerably between geographical localities, such as Thailand 13 , 35 , Malaysia 13 , 17 , 35 , Indonesia 35 , and the Philippines 36 , a recent report has shown significant alkaloid variation from M. speciosa within the same Malaysian plantation 17 . Further research is needed to reveal the breadth of M. speciosa chemical diversity in wild and/or cultivated plants and the prevalence of different chemotypes in kratom products circulating in the worldwide market. Toward that goal, this quantitative method provides an effective tool, and may be used for future efforts in quality control, quality assurance, and standardization of kratom materials.
This method would also prove useful to the chemical analysis of kratom products prior to pharmacological and clinical studies. The literature on the pharmacology of kratom continues to expand with several studies evaluating the “major” constituents of kratom such as 12, 11, 13 and 15 12 , 19 , studies focused on the metabolic products of kratom alkaloids such as 2 and mitragynine pseudoindoxyl 37 , 38 , and a few recent studies that have characterized the pharmacological contributions of low abundance alkaloids such as 4 , isorhynchophylline, 6, 7 , corynoxine B, 14, 16 , and corynantheidine 17 , 18 , 19 , 20 , 39 .
Pharmacological analyses were not incorporated into this study; however, it is worth noting that the powder and loose-leaf products K49, K51, and K52 are composed of alkaloid quantities slightly higher (due to alcoholic extraction), but comparable to traditional tea preparations (i.e., 37.8 mg of 12 per g of extract) 15 , 40 . Thus, tea-based preparations of these products would likely mimic the pharmacological activity of teas consumed in Southeast Asia. Alternatively, the alkaloid quantities of the commercial capsules far exceed those used traditionally. Thus, these products may pose a heightened health risk as compared to kratom tea, particularly given that adverse interactions can occur when kratom alkaloids are co-consumed with other pharmaceutical and illicit substances 41 .
The commercial products evaluated herein, K51 and K52, have comparable levels of 12 ; however, K52 has a greater concentration of the other alkaloids (e.g., 6, 7 , and 11 ). The oxindole alkaloid 7 is reported to have high binding affinity to μ -opioid receptors and exhibit antinociceptive activity equal to morphine 17 , 18 . Alkaloids 6 and 11 have both been shown to moderately inhibit CYP2D6, while 6 also has inhibitory activity against CYP3A and CYP2C9, which may affect the pharmacokinetics and pharmacodynamics of the other alkaloids 20 , 42 . Pharmacokinetic differences between 12 administered alone (as an HCl salt), as an organic extract, as a lyophilized kratom tea, or as a commercial liquid sample have been reported in the literature 16 .
Chemical analysis of the living kratom samples K55, K59, K64, and K68 shows that there are, at least, two different chemotypes in kratom plants being cultivated within the US. The high- 12 producing variety (K68) exhibits a chemical profile resembling the commercial products obtained from Southeast Asia, thus the biological activity may also be similar. The high- 13 producing variety (K55, K59, and K4), with low levels of 12 , could be predicted to exhibit weaker analgesic effects, based on the lower binding affinity of 13 compared to 12 12 , 39 . However, a recent analysis by Buckhalter et al. demonstrated that “Rifat” kratom (purchased from the same supplier as K55 and K59) does exhibit antidepressant-like and analgesic effects 43 . This activity may be from the two major constituents, 13 and 3-isoajmalicine, or may be attributed to metabolism products, as has been shown with 7-hydroxyspeciogynine 44 and 9- O -desmethylspeciogynine (i.e., gambirine) 45 . Further experiments would be needed to verify any predictions of biological activity based on differences in alkaloid content.
Collectively, the results presented herein build on previous literature to show that kratom is a botanical supplement composed of a variable mixture of alkaloids. The precise composition and quantitative amounts of each alkaloid may determine the cumulative biological effects of the whole. Therefore, comparative pharmacological evaluation of disparate mixtures of kratom alkaloids in both in vitro and in vivo models is imperative to fully elucidate the pharmacological potential of products containing M. speciosa . Quantitative analysis using the method described herein could serve as a useful aspect of such biological studies.
Materials and Methods
Materials and chemicals
Six of the indole alkaloids ( 11, 12, 13, 14, 15 , and 16 ) and four of the oxindole alkaloids ( 3, 6, 7 and 8 ) were isolated from commercial kratom powders and characterized in detail using proton ( 1 H) and carbon ( 13 C) nuclear magnetic resonance spectroscopy (NMR), high-resolution electrospray ionization mass spectrometry (HRESIMS), and electronic circular dichroism spectroscopy (ECD) data, as described previously 14 , and were all of a high purity (≥ 98%) as determined by ultra-high-performance liquid chromatography-ultraviolet spectroscopy (UHPLC-UV) analysis. Corynoxine B was not included as a reference standard due to instability, as noted previously 14 .
The indole alkaloids [ 2 (> 98%, Clearsynth) and 10 (≥ 98%, Adipogen)] and the oxindole alkaloids [ 1 (97.5%, BOC Sciences), speciophylline ( 5 ) (90.3%, Chromadex), 4 (91.6%, Chromadex), and 9 (> 98%, Carbosynth)] were all of a high purity. The purity and identity of these standards were verified by 1 H NMR, 13 C NMR, HRESIMS, and ECD. Data obtained with these methods were consistent with literature values ( Table 4S , Supporting Information).
Mitragynine- d 3 (purity ≥ 98%; internal standard) was purchased from Sigma-Aldrich. Optima LC-MS grade acetonitrile (CH 3 CN), formic acid, methanol (MeOH), and water (H 2 O) were purchased from Fisher Scientific. Chemical grade chloroform (CHCl 3 ) was purchased from Fisher Scientific.
Constituent levels were quantified in MeOH extracts obtained from five commercial kratom products, which were termed “Green Maeng Da”, “Yellow Indonesian”, “White Jongkong”, Kratom Extract, and “ Mitragyna speciosa Botanical Extract” by the suppliers, and were internally coded as K49, K51, K52, K76, and K77, respectively ( Table 5S , Supporting Information). Product K49 was a dried and cut leaf material, K51 and K52 were powdered leaf material, K76 was a liquid extract, and K77 was a capsule containing powdered kratom extract. Three powdered, dry leaf samples (coded as K55, K59, and K68) from live M. speciosa plants were also included in the analysis. Powdered, dry leaf material (coded as K55) was obtained from a young (ca. 9-month-old), cultivated M. speciosa plant (“Rifat”), as previously described 20 . Fresh leaves obtained from a 7-year-old cultivated M. speciosa plant (“Rifat”), generously donated by the same supplier as the K55 plant, were lyophilized, powdered (high-speed grinder, Newtry, Amazon), and sifted (400 µm) to yield sample K59. A 2-month-old rooted cutting (coded as K64) from a 4-year-old M. speciosa tree (“Rifat”-Thai) and an approximately 8-month-old M. speciosa seedling (purportedly from Mempawah, West Kalimantan, Indonesia) (coded as K68) were purchased from a US-based online vendor (Texas Coast Botanicals LLC). Upon receipt, the plants were re-potted in a 3-gal container using Happy Frog potting soil (FoxFarm Soil & Fertilizer Co.) and exposed to 24 h full spectrum LED light (GHodec, Amazon) with relative humidity maintained at > 90% for 4 weeks. The plants were then transferred to a grow tent (VIVOSUN) and grown with a 24 h photoperiod of indirect light from a full spectrum LED light source (BP-1000, Bloom Plus, Amazon). The temperature and humidity were maintained above 15 °C and 60%, respectively. At 6 months, 3 leaves were harvested, dried at 25 °C, powdered using a high-speed grinder (Newtry, Amazon) and sifted (400 µm). Samples K49, K51, K52, and K55 were previously identified as M. speciosa based on DNA barcoding and maximum likelihood phylogenetic analysis 14 , 20 . A BLAST search in the NCBI GenBank database using the Internal Transcribed Spacer (ITS) region from K64 and K68 showed these samples had ≥ 99% sequence similarity with M. speciosa ( Fig. 5S and 6S , Supporting Information). Phylogenetic analysis using the ITS region also placed K64 and K68 in a strongly supported clade (≥ 78% PhyML bootstrap support) with M. speciosa sequences ( Fig. 7S , Supporting Information). All living specimens grown at UNCG were also identified morphologically by authors M. K. or P. K. M.
Preparation of calibration standards
A 1 mg/mL primary stock solution was prepared for each of the kratom alkaloids [ 1, 3, 4, 5, 6, 7, 8 , rhynchopylline ( 9 ), 10, 11, 12, 13, 14, 15 , and 16 ] by dissolving an accurately weighed quantity of each standard in an appropriate volume of MeOH. Compound 2 was purchased and used as a 100 µg/mL solution in MeOH. A combined stock of the 16 kratom alkaloids was prepared with the concentration of each constituent at 20 µg/mL in MeOH. The combined stock was further diluted with MeOH and H 2 O to form a combined stock of 10 µg/mL of each alkaloid in MeOH–H 2 O (90 : 10, v/v). A mitragynine- d 3 internal standard was prepared at 0.25 µg/mL in MeOH–H 2 O (90 : 10, v/v). A working stock was prepared by diluting the 10 µg/mL combined stock two-fold with the internal standard. This combined working stock (5000 ng/mL) was then serially diluted two-fold with a 1 : 1 (v/v) mixture of the internal standard and the dilution solution (MeOH–H 2 O, 90 : 10, v/v) to produce calibration curve solutions containing 2500, 1250, 625, 313, 156, 78.1, 39.1, 19.5, and 9.77 ng/mL of each analyte and 125 ng/mL of mitragynine- d 3 .
UPLC-HRMS quantitative analysis
Chromatographic analyses of the kratom alkaloids were conducted utilizing a Waters Acquity Ultra-Performance Liquid Chromatography (UPLC) system (Waters) coupled to a Q Exactive Plus Hybrid Quadrupole-Orbitrap mass spectrometer (Thermo Fisher Scientific). This system was operated using Thermo Scientific Xcalibur software version 3.0 (Thermo Fisher Scientific). UPLC system consisted of the following modules: a sample manager, photodiode array detector (PDA), column manager, and binary solvent manager. Chromatographic separation was achieved using a Kinetex F5 column (Phenomenex, 100 mm × 2.1 mm, 1.7 µm) at a column temperature of 35 °C, and a binary mobile phase consisting of 0.1% formic acid (A) and 0.1% formic acid in CH 3 CN (B). Samples were eluted from the column at a flow rate of 0.6 mL/min using the following gradient: 95% A and 5% B were held isocratically for 1.0 min, followed by a linear decrease in solvent A from 95% to 88% over 2.0 min, a slight decrease to 87% in 4.0 min, a decrease to 75% in 4.0 min, a linear decrease to 55% in 4.0 min, and a sharp decrease to 0% in 1.0 min. The gradient was held at 0% A and 100% B for 0.9 min followed by a sharp increase to the 95% A and 5% B starting conditions over 0.1 min. The column was re-equilibrated at the starting conditions for 3.0 min. Total analysis time per sample was 20.0 minutes ( Fig. 3 ). All samples and standards were analyzed in triplicate using 2 µL injections via a 10 µL sample loop. The strong needle wash was comprised of isopropanol; the weak needle wash consisted of H 2 O–CH 3 CN (90 : 10, v/v). Isopropanol was injected between each sample to minimize carryover.
The Q Exactive Plus was equipped with a heated electrospray ionization (HESI) source operated in positive ionization mode using the following parameters: spray voltage of 3.5 kV, heater temperature of 450 °C, capillary temperature of 275 °C, S-Lens RF level of 50, sheath gas, auxiliary gas, and spare gas of 55, 15, and 3 (arbitrary units), respectively. Nitrogen was used as the source gas and as the collision gas.
Detection of the alkaloids was achieved using one full scan event followed by up to five data-dependent scans, based on the top five most abundant ions found on the inclusion list ( Table 6S , Supporting Information). The full scan event included a mass range from m/z 250 to 1200 with a resolving power of 35 000, an AGC target of 1.0 × 10 6 , and a maximum injection time (IT) of 50 milliseconds. The data dependent acquisition occurred with a resolving power of 17 500, an AGC target of 1E5, a maximum IT of 50 milliseconds, an isolation window of 1.8 Da, a collision energy of 43, and an intensity threshold of 1.6 × 10 5 .
The spectrometer was calibrated weekly using Thermo Scientific Pierce LTQ Velos ESI Positive Ion Calibration Solution (Thermo Fisher Scientific).
Data acquisition and quantitative analysis of the alkaloids were accomplished using Thermo Scientific Xcalibur software version 3.0 (Thermo Fisher Scientific). All calibration curves were generated in Xcalibur Quan Browser. The measured uncertainty for the quantified alkaloids was reported with two significant figures, and the mean was rounded to the same decimal as the uncertainty 46 . Retention times, accurate mass, and MS-MS fragmentation spectra were compared with those of the known standards to confirm identities of the alkaloids in the M. speciosa samples.
Method validation
The analytical method to quantify indole and oxindole alkaloids was validated using guidance from the Association of Official Analytical Collaboration (AOAC) International guidelines for single-laboratory validation of chemical methods for dietary supplements and botanicals for linearity, precision, accuracy, repeatability, and sensitivity 47 , 48 .
Extracted-ion chromatograms were plotted for each of the alkaloids using the calculated m/z with a mass tolerance of 5.0 ppm. Peak integration was performed using peak picking algorithms built into the Thermo Scientific Xcalibur software. Settings used for peak integration are included in Table 7S (Supporting Information). The peak area was then plotted against the standard concentration and a weighted (1/x 2 ) least-squares regression was performed to determine the linear portion of the calibration curve.
Precision and accuracy were determined by calculating the percent RSD and percent RE, respectively, for replicate injections 49 , 50 . RSD is defined as the percent of the standard deviation divided by the mean of sample replicates, where the standard deviation is the square root of the sum of squared residuals divided by the degrees of freedom 47 . In this work, RE is defined as the percent difference between the average measured concentration of three replicate injections of each standard concentration, and the nominal concentration of that standard.
Repeatability was evaluated based on the RSD and RE for triplicate analysis in a single day (i.e., triplicate injections of the standard solution at set concentrations), while intermediate precision was determined based on the interday RSD and RE of standard solutions at set concentrations (freshly prepared each day of analysis). Thus, intraday RSD and RE (repeatability) was determined using triplicate injections of the same solution, while interday RSD and RE (intermediate precision) used triplicate injections from each of the replicate solutions prepared and analyzed on the three separate days (total of 9 injections).
System suitability was verified at the start and end of each analysis by analyzing a reverse-phase HPLC Gradient System Diagnostics Mix (Sigma-Aldrich). System suitability was also addressed by analyzing the lowest concentration of the calibration curve solution of alkaloids (9.77 ng/mL) at the start of each analysis. The AOAC half-height equation was used to determine the resolution of isomers in the system suitability mixture 48 . Additionally, mitragynine- d 3 was included as an internal control in all standard concentrations and samples to monitor consistency in retention times, linearity of instrument response, and to evaluate potential matrix effects.
The lower limit of detection (LLOD) was defined as the estimated lowest concentration that would give a measurable response 51 . This value was calculated according to Equation 1, where x̄ is the average measured response and s is the standard deviation of the measured response of three experimental replicates of the lowest measured concentration, 9.77 ng/mL. The lower limit of quantitation (LLOQ) and upper limit of quantitation (ULOQ) were defined as the lowest and highest concentration where the given analyte could be measured with an intraday accuracy between 80 – 120%. The linear dynamic range for each alkaloid was defined as being in the range between (and including) the LLOQ and the ULOQ. The linearity of all standard curves was verified by each having an R 2 > 0. 990. An extrapolated lower limit of quantitation was calculated as three times the LLOD. Analytes beneath the linear range and above the extrapolated lower limit of quantitation were quantified and denoted as below the lower limit of quantification (BLQ).
LLOD = 3.3 × (((lowest measured concentration)/ x̄ ) × s )
Preparation and analysis of commercial kratom products and M. speciosa leaf samples
Triplicate extractions of the cut leaf and powdered kratom materials K49, K51, K52, K55, and K77 were conducted by adding 50 mg of kratom material and 5 mL of MeOH to a 20 mL scintillation vial, consistent with previously published methods 24 , 27 . The mixtures were shaken 24 h at 20 °C and 150 rpm, decanted, and dried under a stream of nitrogen. Triplicate extractions of the powdered kratom products and M. speciosa leaf samples K51, K52, K59, K64, and K68 were conducted with a similar solvent to powder ratio (1 : 10), subjected to 30 minutes of sonication (FS110 Ultrasonic Cleaner, Fisher Scientific), decanted, and dried under a stream of nitrogen. An aqueous preparation of K51 (coded K51-3) was included in our quantitative analysis by adding 5 mL of 90 °C pure H 2 O (NANOpure, Barnstead) to 50 mg powdered material in a 20 mL scintillation vial, performed in triplicate. The mixture was shaken 24 h at 20 °C and 150 rpm, decanted, and dried under a stream of nitrogen. The liquid kratom product K76 was filtered using 0.22 µm PDVF syringe filters (Fisherbrand, Fisher Scientific) and dried under a stream of nitrogen. In addition to the methanolic and aqueous preparations of K51, an alkaloidal fraction was prepared using the method described by Flores-Bocanegra et al. 14 . Briefly, 10 g of powdered material was macerated with 20 mL of 10% aqueous potassium hydroxide and 180 mL of CHCl 3 and MeOH (1 : 1, v/v) for 24 h at room temperature. The mixture was filtered, and the solvent was evaporated under reduced pressure. The majority of the dried extract was reconstituted in a 400 mL mixture of 1 M HCl and hexanes (1 : 1, v/v), filtered through a cotton plug into a separatory funnel, and partitioned. The aqueous layer was separated and basified (pH 9) with dropwise addition of concentrated NH 4 OH, and the alkaloids were extracted with CHCl 3 (200 mL). The organic phase was washed with neutral H 2 O and dried under reduced pressure and a stream of nitrogen to yield the alkaloidal fraction (35.6 mg) (K51-2).
The dried methanolic extracts of the commercial products and M. speciosa leaf samples, K51 tea, and K51 alkaloidal fraction were reconstituted to 2 mg/mL in an appropriate volume of Optima LC-MS grade MeOH (Fisher Scientific), sonicated, and centrifuged. Samples K76, K77, and K51 alkaloidal fraction were further diluted to 20 µg/mL prior to sample preparation, due to their high concentration of alkaloids. All samples (50 µL) were diluted with 450 µL of Optima LC-MS grade MeOH and 500 µL of the internal standard solution and analyzed with freshly prepared calibration standards (i.e., the primary stock solutions used to make the calibration standards were prepared from dry material within 24 h of the analysis) using the validated UPLC-HRMS method.
Contributorsʼ Statement
Conception and design of the studies: P. K. Manwill, H. A. Raja, D. A. Todd, N. B. Cech, N. H. Oberlies, L. Flores-Bocanegra, M. Khin. Data collection: P. K. Manwill, M. Khin, L. Flores-Bocanegra, H. A. Raja, D. A. Todd. Analysis and data interpretations: P. K. Manwill, H. A. Raja, L. Flores-Bocanegra, D. A. Todd, N. B. Cech, M. Khin. Provision of key study materials: L. Flores-Bocanegra. Manuscript drafting: P. K. Manwill, H. A. Raja, D. A. Todd, N. B. Cech, N. H. Oberlies, M. Khin. Critical revisions of manuscript: P. K. Manwill, H. A. Raja, D. A. Todd, N. B. Cech, N. H. Oberlies.
Acknowledgements
This project was supported by the National Institutes of Health/National Center for Complementary and Integrative Health via the Center of Excellence for Natural Product Drug Interaction Research (NaPDI Center, U54 AT008909), including an Administrative Supplement for Validation Studies of Analytical Methods for Dietary Supplements and Natural Products provided by the Office of Dietary Supplements. Mass spectrometry data were collected in the Triad Mass Spectrometry Facility at The University of North Carolina at Greensboro.
Conflict of Interest The authors declare that they have no conflict of interest.
Dedicated to Professor Dr. A. Douglas Kinghorn on the occasion of his 75th birthday.
Supporting Information
The following are available in the Supporting Information: List of publications covering the analytical analysis of kratom plants and products ( Table 1S ), LC-MS chromatograms comparing a generic screening method using a BEH C18 column with the developed method using a Kinetex F5 column ( Fig. 1S ), precision and accuracy of the method for kratom alkaloid quantification ( Table 2S ), concentration and percent recovery of internal standard ( Table 3S ), base peak MS chromatograms representing kratom materials quantified in Tables 2 , 3 and 4 ( Fig. 2S, 3S , and 4S , respectively), literature references for purchased indole and oxindole alkaloid standards ( Table 4S ), the commercial kratom products and M. speciosa specimens analyzed in this study ( Table 5S ), graphical overview of BLAST results in NCBI GenBank database using the Internal Transcribed Spacer (ITS) region for K64 ( Fig. 5S ) and K68 ( Fig. 6S ), phylogram showing that K64 and K68 group with other M. speciosa ( Fig. 7S ), the precursor ion inclusion list used for MS 2 analysis ( Table 6S ), and the constrain peak width settings used for peak integration ( Table 7S ).
References
- 1.Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, Kerns R, Von Korff M, Porter L, Helmick C. Prevalence of chronic pain and high-impact chronic pain among adults – United States, 2016. Morb Mortal Wkly Rep. 2018;67:1001–1006. doi: 10.15585/mmwr.mm6736a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dasgupta N, Beletsky L, Ciccarone D. Opioid crisis: No easy fix to its social and economic determinants. Am J Public Health. 2018;108:182–186. doi: 10.2105/AJPH.2017.304187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson N, Kariisa M, Seth P, Smith H, Davis N L. Drug and opioid-involved overdose deaths – United States, 2017–2018. Morb Mortal Wkly Rep. 2020;69:290–297. doi: 10.15585/mmwr.mm6911a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward J, Rosenbaum C, Hernon C, McCurdy C R, Boyer E W. Herbal medicines for the management of opioid addiction. CNS Drugs. 2011;25:999–1007. doi: 10.2165/11596830-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Boyer E W, Babu K M, Macalino G E, Compton W. Self-treatment of opioid withdrawal with a dietary supplement, kratom. Am J Addict. 2007;16:352–356. doi: 10.1080/10550490701525368. [DOI] [PubMed] [Google Scholar]
- 6.Coe M A, Pillitteri J L, Sembower M A, Gerlach K K, Henningfield J E. Kratom as a substitute for opioids: Results from an online survey. Drug Alcohol Depend. 2019;202:24–32. doi: 10.1016/j.drugalcdep.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Hassan Z, Muzaimi M, Navaratnam V, Yusoff N HM, Suhaimi F W, Vadivelu R, Vicknasingam B K, Amato D, von Hörsten S, Ismail N IW, Jayabalan N, Hazim A I, Mansor S M, Müller C P. From kratom to mitragynine and its derivatives: Physiological and behavioural effects related to use, abuse, and addiction. Neurosci Biobehav Rev. 2013;37:138–151. doi: 10.1016/j.neubiorev.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Brown P N, Lund J A, Murch S J. A botanical, phytochemical and ethnomedicinal review of the genus Mitragyna Korth.: Implications for products sold as kratom . J Ethnopharmacol. 2017;202:302–325. doi: 10.1016/j.jep.2017.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Saingam D, Assanangkornchai S, Geater A F, Balthip Q. Pattern and consequences of krathom ( Mitragyna speciosa Korth.) use among male villagers in southern Thailand: A qualitative study . Int J Drug Policy. 2013;24:351–358. doi: 10.1016/j.drugpo.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Wray L. “Biak”: An opium substitute. J Fed Malay States Mus. 1907;2:53–56. [Google Scholar]
- 11.Cinosi E, Martinotti G, Simonato P, Singh D, Demetrovics Z, Roman-Urrestarazu A, Bersani F S, Vicknasingam B, Piazzon G, Li J H, Yu W J, Kapitány-Fövény M, Farkas J, Di Giannantonio M, Corazza O. Following “the roots” of kratom ( Mitragyna speciosa ): The evolution of an enhancer from a traditional use to increase work and productivity in southeast asia to a recreational psychoactive drug in western countries . BioMed Res Int. 2015;968786:1–11. doi: 10.1155/2015/968786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kruegel A C, Gassaway M M, Kapoor A, Váradi A, Majumdar S, Filizola M, Javitch J A, Sames D. Synthetic and receptor signaling explorations of the Mitragyna alkaloids: Mitragynine as an atypical molecular framework for opioid receptor modulators . J Am Chem Soc. 2016;138:6754–6764. doi: 10.1021/jacs.6b00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takayama H. Chemistry and pharmacology of analgesic indole alkaloids from the rubiaceous plant, Mitragyna speciosa. Chem Pharm Bull. 2004;52:916–928. doi: 10.1248/cpb.52.916. [DOI] [PubMed] [Google Scholar]
- 14.Flores-Bocanegra L, Raja H A, Graf T N, Augustinovic M, Wallace E D, Hematian S, Kellogg J J, Todd D A, Cech N B, Oberlies N H. The chemistry of kratom [ Mitragyna speciosa ]: Updated characterization data and methods to elucidate indole and oxindole alkaloids . J Nat Prod. 2020;83:2165–2177. doi: 10.1021/acs.jnatprod.0c00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson L L, Harris H M, Eans S O, Brice-Tutt A C, Cirino T J, Stacy H M, Simons C A, León F, Sharma A, Boyer E W, Avery B A, McLaughlin J P, McCurdy C R. Lyophilized kratom tea as a therapeutic option for opioid dependence. Drug Alcohol Depend. 2020;216:108310. doi: 10.1016/j.drugalcdep.2020.108310. [DOI] [PubMed] [Google Scholar]
- 16.Kamble S H, Berthold E C, King T I, Raju Kanumuri S R, Popa R, Herting J R, Leon F, Sharma A, McMahon L R, Avery B A, McCurdy C R. Pharmacokinetics of eleven kratom alkaloids following an oral dose of either traditional or commercial kratom products in rats. J Nat Prod. 2021;84:1104–1112. doi: 10.1021/acs.jnatprod.0c01163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chear N J, Leon F, Sharma A, Kanumuri S RR, Zwolinski G, Abboud K A, Singh D, Restrepo L F, Patel A, Hiranita T, Ramanathan S, Hampson A J, McMahon L R, McCurdy C R. Exploring the chemistry of alkaloids from Malaysian Mitragyna speciosa (kratom) and the role of oxindoles on human opioid receptors . J Nat Prod. 2021;84:1034–1043. doi: 10.1021/acs.jnatprod.0c01055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chakraborty S, Uprety R, Daibani A E, Rouzic V L, Hunkele A, Appourchaux K, Eans S O, Nuthikattu N, Jilakara R, Thammavong L, Pasternak G W, Pan Y X, McLaughlin J P, Che T, Majumdar S. Kratom alkaloids as probes for opioid receptor function: Pharmacological characterization of minor indole and oxindole alkaloids from kratom. ACS Chem Neurosci. 2021;12:2661–2678. doi: 10.1021/acschemneuro.1c00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Obeng S, Kamble S H, Reeves M E, Restrepo L F, Patel A, Behnke M, Chear N JY, Ramanathan S, Sharma A, León F, Hiranita T, Avery B A, McMahon L R, McCurdy C R. Investigation of the adrenergic and opioid binding affinities, metabolic stability, plasma protein binding properties, and functional effects of selected indole-based kratom alkaloids. J Med Chem. 2019;63:433–439. doi: 10.1021/acs.jmedchem.9b01465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Todd D A, Kellogg J J, Wallace E D, Khin M, Flores-Bocanegra L, Tanna R S, McIntosh S, Raja H A, Graf T N, Hemby S E, Paine M F, Oberlies N H, Cech N B. Chemical composition and biological effects of kratom ( Mitragyna speciosa ): In vitro studies with implications for efficacy and drug interactions . Sci Rep. 2020;10:19158. doi: 10.1038/s41598-020-76119-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berthold E C, Kamble S H, Raju K S, King T I, Popa R, Sharma A, León F, Avery B A, McMahon L R, McCurdy C R. Preclinical pharmacokinetic study of speciociliatine, a kratom alkaloid, in rats using an UPLC-MS/MS method. J Pharm Biomed Anal. 2021;194:113778. doi: 10.1016/j.jpba.2020.113778. [DOI] [PubMed] [Google Scholar]
- 22.Lo Faro A F, Di Trana A, La Maida N, Tagliabracci A, Giorgetti R, Busardò F P. Biomedical analysis of new psychoactive substances (NPS) of natural origin. J Pharm Biomed Anal. 2020;179:112945. doi: 10.1016/j.jpba.2019.112945. [DOI] [PubMed] [Google Scholar]
- 23.Kerrigan S, Basiliere S. Kratom: A systematic review of toxicological issues. WIREs Forensic Sci. 2022;4:e1420. [Google Scholar]
- 24.Kikura-Hanajiri R, Kawamura M, Maruyama T, Kitajima M, Takayama H, Goda Y. Simultaneous analysis of mitragynine, 7-hydroxymitragynine, and other alkaloids in the psychotropic plant “kratom” ( Mitragyna speciosa ) by LC-ESI-MS . Forensic Toxicol. 2009;27:67–74. [Google Scholar]
- 25.Wang M, Carrell E J, Ali Z, Avula B, Avonto C, Parcher J F, Khan I A. Comparison of three chromatographic techniques for the detection of mitragynine and other indole and oxindole alkaloids in Mitragyna speciosa (kratom) plants . J Sep Sci. 2014;37:1411–1418. doi: 10.1002/jssc.201301389. [DOI] [PubMed] [Google Scholar]
- 26.Sharma A, Kamble S H, León F, Chear N JY, King T I, Berthold E C, Ramanathan S, McCurdy C R, Avery B A. Simultaneous quantification of ten key Kratom alkaloids in Mitragyna speciosa leaf extracts and commercial products by ultra‐performance liquid chromatography−tandem mass spectrometry . Drug Test Anal. 2019;11:1162–1171. doi: 10.1002/dta.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kellogg J J, Paine M F, McCune J S, Oberlies N H, Cech N B. Selection and characterization of botanical natural products for research studies: a NaPDI center recommended approach. Nat Prod Rep. 2019;36:1196–1221. doi: 10.1039/c8np00065d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Veterinary Medicine (CVM) Bioanalytical method validation: Guidance for industry. Rockville, MD: Food and Drug Administration; 2018: 1–41Accessed March 22, 2022 at:https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf
- 29.Mudge E M, Brown P N. Determination of mitragynine in Mitragyna speciosa raw materials and finished products by liquid chromatography with UV detection: Single-laboratory validation . J AOAC Int. 2017;100:18–24. doi: 10.5740/jaoacint.16-0220. [DOI] [PubMed] [Google Scholar]
- 30.León F, Habib E, Adkins J E, Furr E B, McCurdy C R, Cutler S J. Phytochemical characterization of the leaves of Mitragyna speciosa grown in USA . Nat Prod Commun. 2009;4:907–910. [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang M, Sharma A, León F, Avery B, Kjelgren R, McCurdy C R, Pearson B J. Effects of nutrient fertility on growth and alkaloidal content in Mitragyna speciosa (kratom) . Front Plant Sci. 2020;11:597696. doi: 10.3389/fpls.2020.597696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fowble K L, Musah R A. A validated method for the quantification of mitragynine in sixteen commercially available kratom ( Mitragyna speciosa ) products . Forensic Sci Int. 2019;299:195–202. doi: 10.1016/j.forsciint.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 33.Lesiak A D, Musah R A. Rapid high-throughput species identification of botanical material using direct analysis in real time high resolution mass spectrometry. J Visualized Exp. 2016;116:e54197. doi: 10.3791/54197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orio L, Alexandru L, Cravotto G, Mantegna S, Barge A. UAE, MAE, SFE-CO2 and classical methods for the extraction of Mitragyna speciosa leaves . Ultrason Sonochem. 2012;19:591–595. doi: 10.1016/j.ultsonch.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Boffa L, Ghè C, Barge A, Muccioli G, Cravotto G. Alkaloid profiles and activity in different Mitragyna speciosa strains . Nat Prod Commun. 2018;13:1111–1116. [Google Scholar]
- 36.Uy S P, jr., Seville S S, Jaranilla J LM, Desamito Y KS, Barbacena R P, Narceda R JA. Determination of psychoactive mitragynine drug in suspected kratom species collected from various geographical areas in the Philippines: A pilot study on existing local plant-based new psychoactive substance (NPS) Arab J Forensic Sci. 2019;1:1358–1366. [Google Scholar]
- 37.Kamble S H, León F, King T I, Berthold E C, Lopera-Londoño C, Siva Rama Raju K, Hampson A J, Sharma A, Avery B A, McMahon L R, McCurdy C R. Metabolism of a kratom alkaloid metabolite in human plasma increases its opioid potency and efficacy. ACS Pharmacology & Translational Science. 2020;3:1063–1068. doi: 10.1021/acsptsci.0c00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chakraborty S, Uprety R, Slocum S T, Irie T, Le Rouzic V, Li X, Wilson L L, Scouller B, Alder A F, Kruegel A C, Ansonoff M, Varadi A, Eans S O, Hunkele A, Allaoa A, Kalra S, Xu J, Pan Y X, Pintar J, Kivell B M, Pasternak G W, Cameron M D, McLaughlin J P, Sames D, Majumdar S. Oxidative metabolism as a modulator of kratomʼs biological actions. J Med Chem. 2021;64:16553–16572. doi: 10.1021/acs.jmedchem.1c01111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ellis C R, Racz R, Kruhlak N L, Kim M T, Zakharov A V, Southall N, Hawkins E G, Burkhart K, Strauss D G, Stavitskaya L. Evaluating kratom alkaloids using PHASE. PLoS One. 2020;15:e0229646. doi: 10.1371/journal.pone.0229646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh D, Yeou Chear N J, Narayanan S, Leon F, Sharma A, McCurdy C R, Avery B A, Balasingam V. Patterns and reasons for kratom ( Mitragyna speciosa ) use among current and former opioid poly-drug users . J Ethnopharmacol. 2020;249:e112462. doi: 10.1016/j.jep.2019.112462. [DOI] [PubMed] [Google Scholar]
- 41.Babin J K. http:// http://
- 42.Kamble S H, Sharma A, King T I, Berthold E C, León F, Meyer P KL, Kanumuri S RR, McMahon L R, McCurdy C R, Avery B A. Exploration of cytochrome P450 inhibition mediated drug-drug interaction potential of kratom alkaloids. Toxicol Lett. 2020;319:148–154. doi: 10.1016/j.toxlet.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buckhalter S, Soubeyrand E, Ferrone S AE, Rasmussen D J, Manduca J D, Al-Abdul-Wahid M S, Frie J A, Khokhar J Y, Akhtar T A, Perreault M L. The antidepressant-like and analgesic effects of kratom alkaloids are accompanied by changes in low frequency oscillations but not Δ FosB accumulation . Front Pharmacol. 2021;12:696461. doi: 10.3389/fphar.2021.696461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gutridge A M, Chakraborty S, Varga B R, Rhoda E S, French A R, Blaine A T, Royer Q H, Cui H, Yuan J, Cassell R J, Szabó M, Majumdar S, van Rijn R M. Evaluation of kratom opioid derivatives as potential treatment option for alcohol use disorder. Front Pharmacol. 2021;12:764885. doi: 10.3389/fphar.2021.764885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.León F, Obeng S, Mottinelli M, Chen Y, King T I, Berthold E C, Kamble S H, Restrepo L F, Patel A, Gamez-Jimenez L R, Lopera-Londoño C, Hiranita T, Sharma A, Hampson A J, Canal C E, McMahon L R, McCurdy C R. Activity of Mitragyna speciosa (“kratom”) alkaloids at serotonin receptors . J Med Chem. 2021;64:13510–13523. doi: 10.1021/acs.jmedchem.1c00726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harris D C. New York, NY: W. H. Freeman; 2015. Quantitative Chemical Analysis. 9th ed. [Google Scholar]
- 47.AOAC . Rockville, MD, USA: AOAC International; 2016. Appendix F: Guidelines for Standard Method Performance Requirements; pp. 1–18. [Google Scholar]
- 48.AOAC . Rockville, MD, USA: AOAC International; 2019. Appendix K: Guidelines for Dietary Supplements and Botanicals; pp. 1–32. [Google Scholar]
- 49.VanderMolen K M, Cech N B, Paine M F, Oberlies N H. Rapid quantitation of furanocoumarins and flavonoids in grapefruit juice using ultra-performance liquid chromatography. Phytochem Anal. 2013;24:654–660. doi: 10.1002/pca.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Graf T N, Cech N B, Polyak S J, Oberlies N H. A validated UHPLC-tandem mass spectrometry method for quantitative analysis of flavonolignans in milk thistle ( Silybum marianum ) extracts . J Pharm Biomed Anal. 2016;126:26–33. doi: 10.1016/j.jpba.2016.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wells G, Prest H, Russ C W., IVSignal, Noise, and Detection Limits in Mass SpectrometryAccessed January 7, 2022 at:https://www.agilent.com/cs/library/technicaloverviews/public/5990-7651EN.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following are available in the Supporting Information: List of publications covering the analytical analysis of kratom plants and products ( Table 1S ), LC-MS chromatograms comparing a generic screening method using a BEH C18 column with the developed method using a Kinetex F5 column ( Fig. 1S ), precision and accuracy of the method for kratom alkaloid quantification ( Table 2S ), concentration and percent recovery of internal standard ( Table 3S ), base peak MS chromatograms representing kratom materials quantified in Tables 2 , 3 and 4 ( Fig. 2S, 3S , and 4S , respectively), literature references for purchased indole and oxindole alkaloid standards ( Table 4S ), the commercial kratom products and M. speciosa specimens analyzed in this study ( Table 5S ), graphical overview of BLAST results in NCBI GenBank database using the Internal Transcribed Spacer (ITS) region for K64 ( Fig. 5S ) and K68 ( Fig. 6S ), phylogram showing that K64 and K68 group with other M. speciosa ( Fig. 7S ), the precursor ion inclusion list used for MS 2 analysis ( Table 6S ), and the constrain peak width settings used for peak integration ( Table 7S ).


