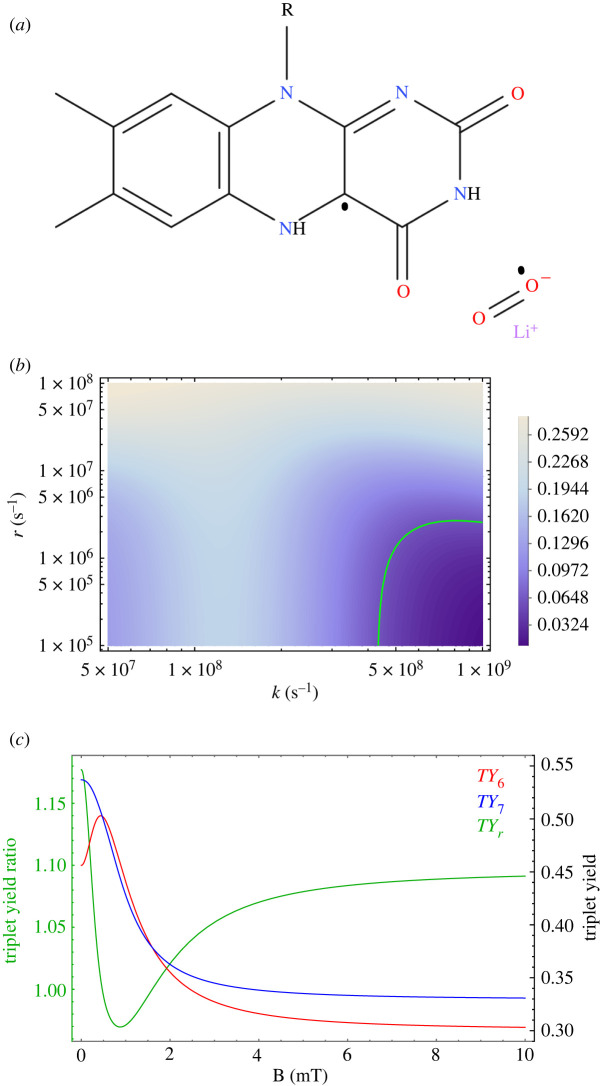
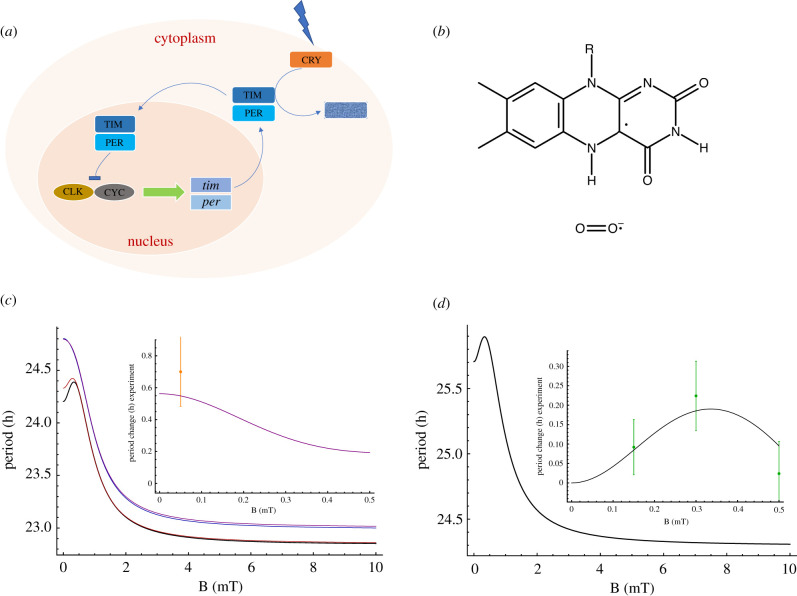
Abstract
Hundreds of studies have found that weak magnetic fields can significantly influence various biological systems. However, the underlying mechanisms behind these phenomena remain elusive. Remarkably, the magnetic energies implicated in these effects are much smaller than thermal energies. Here, we review these observations, and we suggest an explanation based on the radical pair mechanism, which involves the quantum dynamics of the electron and nuclear spins of transient radical molecules. While the radical pair mechanism has been studied in detail in the context of avian magnetoreception, the studies reviewed here show that magnetosensitivity is widespread throughout biology. We review magnetic field effects on various physiological functions, discussing static, hypomagnetic and oscillating magnetic fields, as well as isotope effects. We then review the radical pair mechanism as a potential unifying model for the described magnetic field effects, and we discuss plausible candidate molecules for the radical pairs. We review recent studies proposing that the radical pair mechanism provides explanations for isotope effects in xenon anaesthesia and lithium treatment of hyperactivity, magnetic field effects on the circadian clock, and hypomagnetic field effects on neurogenesis and microtubule assembly. We conclude by discussing future lines of investigation in this exciting new area of quantum biology.
Keywords: magnetic field effects in biology, isotope effects in biology, reactive oxygen species, radical pair mechanism, quantum biology, spin chemistry
1. Introduction
Sensitivity to weak magnetic fields is abundant throughout biology, as discussed in numerous review articles [1–24]. Effects of either static or oscillating weak magnetic fields have been reported on the circadian clock, electron transfer in cryptochrome, stem cells, calcium concentration, the brain’s functions such as action potentials, reactive oxygen species (ROS), development, neuronal activities, DNA, memory, anxiety, analgaesia, genetics and many other functions (see §2). Despite the wealth of observations, thus far, there is no clear explanation for the mechanism behind these phenomena. This is mainly due to the fact that the corresponding energies for such effects are far smaller than thermal energies.
However, there is a promising quantum physics (or spin chemistry) concept that can account for the effects of such weak fields, namely the radical pair mechanism [25,26]. This mechanism, which is an example of the emerging field of quantum biology [27–31], has been studied in significant detail in the comparatively narrow context of bird magnetoreception [32–39], where it is accepted as one of the leading potential explanations for how birds sense magnetic fields, and in particular the Earth’s magnetic field, for the purpose of navigation. It is known that birds and amphibians, and in all likelihood other vertebrates, have not one but two magnetoreception mechanisms, a magnetite-based detector that provides the high sensitivity necessary for sensing weak spatial gradients in the magnetic field [40,41] and a light-dependent magnetic compass that underlies a magnetic map sense [42]. The latter is thought to be based on the radical pair mechanism [43,44].
The radical pair mechanism involves magnetically sensitive intermediate molecules, so-called radical pairs [25,43,45–49]. The key ingredient is the spin correlation between two unpaired electrons, one on the donor molecule and the other on the acceptor molecule. Depending on the initial spin configuration of the donor and acceptor molecules, this initial spin correlation of the radical pair will be either a singlet (S) or a triplet (T) state, which are, respectively, spin-0 and spin-1 states (see §3.1 for further discussion). Due to the spin interactions with its environment (in particular with external magnetic fields and with nearby nuclear spins), the state of the radical pair will oscillate between S and T states [26,50]. Each spin state, S and T, can lead to different reaction products, providing an example of spin chemistry [51,52]. The energies induced by the above-mentioned magnetic fields are hundreds of thousands of times smaller than thermal energies, kBT (kB is Boltzmann constant and T is temperature), which are associated with motions, rotation and vibrations in biological environments. In thermal equilibrium, the energies required to alter the rate or yield of a chemical transformation should be at least comparable to kBT. Due to this, the radical pair mechanism was originally ignored in the context of physiology. However, the situation differs in systems far from thermal equilibrium, which is the case for radical pairs [43]. Sensitivity to weak magnetic fields is one of the key properties of radical pair reactions. Nowadays, many research laboratories study the role of radical pairs in (bio)chemical reactions [26,52–56].
Recent studies have proposed roles for radical pairs beyond avian magnetoreception, in particular in xenon-induced anaesthesia [57], lithium effects on mania [58], magnetic field and lithium effects on the circadian clock [59], and hypomagnetic field effects on microtubule reorganization [60] and neurogenesis [61] (where hypomagnetic fields are fields much weaker than that of the Earth). Here, we suggest that the radical pair mechanism is in fact quite common in biology, and that it may provide an explanation for many of the weak magnetic field effects on physiological functions that have been observed.
This paper, which is part review and part perspective article, is organized as follows. Section 2 briefly surveys studies reporting effects of low-intensity magnetic fields on biological systems, including effects of static (§2.1), hypomagnetic (§2.2) and oscillating (§2.3) magnetic fields. We further survey studies on isotope effects in biology from a spin perspective. In §3, we discuss how the radical pair mechanism can account for static, hypomagnetic and oscillating magnetic field effects. Section 3.4 reviews possible candidate molecules for radical pair formation in biological systems. In §4, we review the above-mentioned recent studies on the possible biological roles of radical pairs beyond avian magnetoreception. Section 5 discusses important directions for further investigation.
2. Magnetosensitivity in biology
There is a considerable amount of research investigating magnetic field effects on biological functions [22,62–70]. In the following, we review the effects of low-intensity magnetic fields on biology. We organize this section based on the type of magnetic fields, namely static magnetic fields, hypomagnetic fields and oscillating magnetic fields. Isotope effects in biology, which can be related to nuclear magnetic moments, are also discussed at the end of this section.
2.1. Static magnetic field
2.1.1. Cryptochrome
In the context of avian magnetoreception in animals, the canonical proteins are cryptochromes [43,48]. Maeda et al. demonstrated that photo-induced flavin–tryptophan radical pairs in cryptochrome are magnetically sensitive [71]. Moreover, Ahmad et al. observed that hypocotyl growth inhibition in higher plants are sensitive to the magnetic field, where such responses are linked to cryptochrome-dependent signalling pathways [72]. Sheppard et al. reported that magnetic fields of a few millitesla could influence photo-induced electron transfer reactions in Drosophila cryptochrome [73]. Further, Marley et al. showed that a static magnetic field of 100 mT substantially affected seizure response in Drosophila larvae in a cryptochrome-dependent manner [74]. In addition, using a transgenic approach, Foley et al. showed that human cryptochrome-2 has the molecular capability to function as a light-sensitive magnetosensor [75]. Applying a 0.5 mT magnetic field, Ahmad and co-workers reported that cryptochrome responses were enhanced by the magnetic field, including dark-state processes following the cryptochrome photoreduction step [76,77]. Further, there have been extensive studies on the radical pair mechanism for cryptochrome(s) [43,47]. Table 1 summarizes static magnetic field effects on various biological functions.
Table 1.
Static magnetic field effects on different biological functions.
| system | magnetic field | references |
|---|---|---|
| cryptochrome | ||
| cryptochrome responses enhanced | 0.5 mT | Pooam et al. [76] |
| cryptochrome responses enhanced | 0.5 mT | Hammad et al. [77] |
| seizure response in Drosophila (cryptochrome-dependent) | further, 100 mT | Marley et al. [74] |
| photo-induced electron transfer reactions in Drosophila cryptochrome | a few mT | Sheppard et al. [73] |
| body size increase and in Drosophila melanogaster | 0.4–0.7 mT | Giorgi et al. [78] |
| decrease in wing size in Drosophila melanogaster | 35 mT | Stamenkovi-Radak et al. [79] |
| circadian clock | ||
| circadian clock in Drosophila melanogaster | <0.5 mT | Yoshii et al. [80] |
| stem cell | ||
| stem cell-mediated growth | <1 mT | Huizen et al. [81] |
| proliferation/migration/differentiation in human dental pulp stem cells | 1/2/4 mT | Zheng et al. [82] |
| bone stem cells in vitro | 0.5–30 mT | Abdolmaleki et al. [83–85] |
| calcium | ||
| Ca2+ influx | 0.6 mT | Fanelli et al. [86] |
| myosin phosphorylation in a cell-free preparation (Ca2+-dependent) | 0.2 mT | Markov & Pilla [87] |
| Ca2+ concentration/morphology in cell lines | 6 mT | Tenuzzo et al. [88] |
| Ca2+ concentration in in vitro aged human lymphocytes | 6 mT | Tenuzzo et al. [89] |
| cell shape, cell surface, sugar residues, cytoskeleton and apoptosis | 6 mT | Chionna et al. [90] |
| neurons and brain | ||
| blocked sensory neuron action potentials in the somata of adult mouse | 10 mT | McLean et al. [91] |
| symptomatic diabetic neuropathy | 50 mT | Weintraub et al. [92] |
| ROS | ||
| increased intercellular ROS in human neuroblastoma cells | 2.2 mT | Calabro et al. [93] |
| increased intercellular ROS in human neuroblastoma cells | 31.7–232 mT | Vergallo et al. [94] |
| increased H2O2 level in embryoid bodies | 1–10 mT | Bekhite et al. [95] |
| ROS increase in mouse cardiac progenitor cells | 0.2–5 mT | Bekhite et al. [96] |
| elevated H2O2 in diploid embryonic lung fibroblast cell | 230–250 mT | Sullivan et al. [97] |
| increase of H2O2 in the human fibrosarcoma cancer cell | 45−60 μT | Martino& Castello [98] |
| increased H2O2 production of human peripheral blood neutrophils | 60 mT | Poniedzialek et al. [99] |
| ROS levels in cancer cells | 10 mT | Verdon [100] |
| type 2 diabetes via regulating cellular ROS | 3 mT | Carter et al. [101,102] |
| ROS changes in stem cell-mediated growth | <1 mT | Huizen et al. [81] |
| mitochondrial electron transport chain activity | 0–1.93 mT | Sheu et al. [103] |
| others | ||
| flavin adenine dinucleotide photochemistry | <20 mT | Antill et al. [104] |
| enzymatic ATP production | 80 mT | Buchachenko et al. [105] |
| chlorophyll fluorescence/nutrient content of Hordeum vulgare L. | 20/42/125/250 mT | Ercan et al. [106] |
| antioxidant defense system of plant cells | 10/30 mT | Sahebjamei et al. [107] |
| enhance the killing effect of adriamycin on K562 cells. | 8.8 mT | Hao et al. [108] |
| regeneration and plant growth of shoot tips | 2.9–4.6 mT | Atak et al. [109] |
| accelerated loss of integrity of plasma membrane during apoptosis | 6 mT | Teodori et al. [110] |
| macrophagic differentiation in human pro-monocytic U937 cells | 6 mT | Pagliara et al. [111] |
| cell proliferation and cell death balance | 0.5 mT | Buemi et al. [112] |
| growth and sporulation of phytopathogenic microscopic fungi | 1 mT | Nagy et al. [113] |
2.1.2. Genetics
It is known that exposure to magnetic fields has genetic consequences [114]. Giorgi et al. showed that chronic exposure to magnetic fields (0.4–0.7 mT) increased the body size and induced lethal mutations in populations of Drosophila melanogaster [78]. Furthermore, a magnetic field of 35 mT decreased the wing size in Drosophila melanogaster [79] (table 1).
2.1.3. Circadian clock
It has been shown that magnetic fields can modulate the circadian clock [115–117]. Yoshii et al. [80] showed that the effects of static magnetic fields affected the circadian clock of Drosophila and reported that exposure to these fields slowed down the clock rhythms in the presence of blue light, with a maximal change at 300 μT, and reduced effects at both lower and slightly higher field strengths. We discuss this observation further from the perspective of the radical pair mechanism in §4.3 (table 1).
2.1.4. Stem cells
Static magnetic fields have been commonly used in medicine as a tool to increase wound healing, bone regeneration and as a component of magnetic resonance techniques. However, recent data have shed light on deeper mechanisms of static magnetic field action on physiological properties of different cell populations, including stem cells. It is known that static magnetic fields can increase wound healing and bone regeneration [8]. Huizen et al. reported that weak magnetic fields (less than 1 mT) alter stem cell-mediated growth, where changes in ROS were implicated [81]. The authors suggested that the radical pair mechanism may be the potential explanation for their observations. Zheng et al. showed that a static magnetic field of 1, 2 or 4 mT regulated proliferation, migration, and differentiation of human dental pulp stem cells [82]. It is also known that applied static magnetic fields (0.5–30 mT) affect stem cells in vitro [83–85] (table 1).
2.1.5. Calcium
Fanelli et al. reported that magnetic fields allow the indefinite survival and replication of the cells hit by apoptogenic agents. The anti-apoptosis effect was found to be mediated by the ability of the fields to increase Ca2+ influx from the extracellular medium. In that experiment, the geomagnetic field was not shielded. They found 0.6 mT to be the minimal intensity required to detect an anti-apoptotic effect [86]. Moreover, it has been shown that weak static magnetic fields can influence myosin phosphorylation in a cell-free preparation in a Ca2+-dependent manner [87]. Tenuzzo and colleagues observed that exposure to a 6 mT static magnetic field influenced Ca2+ concentration and bcl-2, bax, p53 and hsp70 expression in freshly isolated and in vitro aged human lymphocytes [89]. Further, Chionna et al. showed that exposure to a static magnetic field of 6 mT of Hep G2 cells resulted in time-dependent modifications in cell shape, cell surface, sugar residues, cytoskeleton and apoptosis [90]. They reported that after 24 h exposure, the cells had a less flat shape due to partial detachment from the culture dishes. They further observed that microfilaments and microtubules were modified in a time-dependent manner. They also suggested that the induced apoptosis was likely due to the increment of Ca2+ during exposure. In another study, Tenuzzo and co-workers showed that cell viability, proliferation, intracellular Ca2+ concentration and morphology in several primary cultures and cell lines can be influenced by a 6 mT magnetic field [88] (table 1).
2.1.6. Neurons and brain
Exposure to static magnetic fields can have impacts on various brain functions. McLean et al. reported that a static magnetic field in the 10 mT range blocked sensory neuron action potentials in the somata of adult mouse dorsal root ganglion neurons in monolayer dissociated cell culture [91]. It has also been shown that exposure to a transcranial static magnetic field over the supplementary motor area can modulate resting-state activity and motor behaviour associated with modulation of both local and distant functionally connected cortical circuits [118]. Static magnetic field exposure can also affect the production of melatonin [119–122], the pineal gland [123,124], and cause functional alterations in immature cultured rat hippocampal neurons [125]. Further, Dileone et al. observed that an applied transcranial static magnetic field can induce dopamine-dependent changes of cortical excitability in patients with Parkinson’s disease [126]. In addition, neuron firing frequency can also be affected by static magnetic field intensity [127,128]. There exist a considerable number of studies indicating the effects of applied magnetic field on pain sensitivity (nociception) and pain inhibition (analgesia) [129]. Additionally, it has been known that a static magnetic field (50 mT) can influence symptomatic diabetic neuropathy [92] (table 1).
2.1.7. Reactive oxygen species
ROS are the collection of derivatives of molecular oxygen that occur in biology, which can be categorized into two types, free radicals and non-radical species. The non-radical species are hydrogen peroxide (H2O2), organic hydroperoxides (ROOH), singlet molecular oxygen (1O2), electronically excited carbonyl, ozone (O3), hypochlorous acid (HOCl, and hypobromous acid HOBr). Free radical species are superoxide anion radical (O•−2), hydroxyl radical (•OH), peroxyl radical (ROO•) and alkoxyl radical (RO•) [130]. Any imbalance of ROS can lead to adverse effects. H2O2 and O•−2 are the main redox signalling agents. It is now well known that ROS are essential for physiology as functional signalling entities. H2O2 plays a crucial role in redox regulation of biological functions, where its intracellular concentration is under tight control. The cellular concentration of H2O2 is about 10−8 M, which is almost a thousand times more than that of O•−2. Transmembrane NADPH oxidases (NOXs) [131,132] and the mitochondrial electron transport chain (ETC) [133,134] are the major sources of O·−2 and H2O2.
In a considerable number of studies, magnetic field effects in biology are accompanied with oxidative stress [15,135,136], which is an imbalance between oxidants and antioxidants in favour of the oxidants, leading to a disruption of redox signalling and control and/or molecular damage. [137–139]. Studies found that exposure to static magnetic fields of 2.2 mT [93] and 31.7–232 mT [94] increased the intercellular ROS in human neuroblastoma cells. Furthermore, De Nicola et al. observed that the intracellular ROS level in human monocyte tumour cells was raised when exposed to a static magnetic field [140]. Further, Bekhite et al. reported that static magnetic field exposure (1–10 mT) increased the H2O2 level in embryoid bodies [95]. Later, the same group found an induced increase of ROS in cardiac progenitor cells derived from mouse cells by a 0.2–5 mT static magnetic field, where ROS was suggested to be generated by NADPH oxidase [96]. Sullivan et al. reported that 230–250 mT of a magnetic field elevated H2O2 in diploid embryonic lung fibroblast cell [97]. Upon exposure to 45–60 μT, Martino and Castello observed an increase of H2O2 in the human fibrosarcoma cancer cell, which can be suppressed by reducing the geomagnetic field’s strength [98]. Further studies show that exposure to a 60 mT magnetic field increased H2O2 production of human peripheral blood neutrophils [99]. It has also been reported that the effects of an applied magnetic field of 10 mT on DOXO-induced toxicity and proliferation rate of cancer cells are correlated to ROS levels [100]. Furthermore, Carter et al. observed that a 3 mT static magnetic field can influence type 2 diabetes via regulating cellular ROS [101,102]. Pooam et al. showed that applying a low intensity static magnetic field modulated ROS generation in HEK293 cells. The authors suggested that the radical pair mechanism may explain that observation [141]. In a recent work, Sheu and co-workers reported that static low intensity magnetic fields can regulate mitochondrial ETC activity and thus enhance mitochondrial respiration [103]. They observed that exposure to magnetic fields of 0–1.93 mT of mitochondria isolated from adult rat hearts produced a bell-shape increase in the respiratory control ratio with a maximum at 0.50 mT and a return to baseline at 1.50 mT. It was further observed that the magnetic field affected only the activity of the complexes 2, 3 and 5 but not 1 of the mitochondrial ETC and several enzymes of the tricarboxylic acid cycle. The authors suggested that the low intensity magnetic field effects on the mitochondrial respiratory activity may be explained by the radical pair mechanism. Huizen and co-workers showed that weak magnetic fields (less than 1 mT) changed stem cell-mediated growth, where changes in ROS were implicated [81].
2.1.8. Others
Ikeya et al. reported that exposure to magnetic fields influenced autofluorescence in cells involving flavins [142]. Studies also showed that static magnetic fields can affect the photoactivation reaction of E. coli DNA photolyase [143]. Moreover, Giachello et al. observed that applying static magnetic fields on blue light activated cryptochromes in Drosophila neurons resulted in an elevation of action potential firing [144]. Further, it is also known that the chemiluminescence intensity in Madin–Darby canine kidney cells is magnetic field dependent [145], where ROS are implicated. In solutions, flavin adenine dinucleotide is the key cofactor of cryptochrome. Antill and co-workers showed that flavin adenine dinucleotide photochemistry in solution is magnetic field sensitive (less than 20 mT) even at physiological pH and higher [104].
Buchachenko et al. reported that applying 80 mT static magnetic field affected enzymatic ATP production [105]. Recently, Ercan et al. showed that exposure to magnetic fields (20, 42, 125 and 250 mT) can affect the magnetic properties, germination, chlorophyll fluorescence and nutrient content of barley (Hordeum vulgare L.) [106]. Further, it is observed that exposure to magnetic fields (10 and 30 mT) can deteriorate the antioxidant defence system of plant cells [107]. Hao et al. reported that exposure to an 8.8 mT static magnetic field can enhance the killing effect of adriamycin on K562 cells [108]. It is also observed that exposure to magnetic fields (2.9–4.6 mT) of soya bean tissue culture enhances the regeneration and plant growth of shoot tips [109]. Teodori et al. showed that exposure of HL-60 cells to a 6 mT static magnetic field accelerated loss of integrity of plasma membrane during apoptosis [110]. It has been shown that exposure of human pro-monocytic U937 cells to a static magnetic field (6 mT) decreased the degree of macrophagic differentiation [111]. Buemi et al. report that exposure to a 0.5 mT magnetic field of renal cell cultures and cortical astrocyte cultures from rats influenced cell proliferation and cell death balance [112]. They concluded that such magnetic field effects were cell type-dependent. It has been shown that exposure to magnetic fields (1 mT) significantly affected growth and sporulation of phytopathogenic microscopic fungi [113].
Surma et al. found that the application of a weak static magnetic field with intensities only a few times that of the geomagnetic field can accelerate the development of skeletal muscle cells, resulting in the formation of multinuclear hypertrophied myotubes [146]. They further reported that these effects were accompanied by a 1.5- to 3.5-fold rise in the concentration of intracellular [Ca2+]i.
2.2. Hypomagnetic field
Earth’s geomagnetic field, ranging from approximately 24 to 66 μT depending on latitude [147], can have critical roles in numerous biological processes. Shielding the geomagnetic field, called hypomagnetic field, is known to cause biological effects [19,21,23,148–152].
It has also been suggested that the apparent cycle of mass extinction on Earth [153] may be related to the geomagnetic field fluctuation [154]. Decades ago, the first studies on the effects of hypomagnetic field on humans were conducted, motivated by the concerns around the health of astronauts in outer space [155–158]. These studies concluded that exposure to hypomagnetic fields had adverse effects on human health. Besides hypomagnetic field effects on animal and human cells and tissues, deprivation in geomagnetic field can influence the development of plants as well [151,152]. The geomagnetic field seems to play essential roles in living organisms, and diminishing or removing it could result in adverse consequences.
It was shown that exposure to hypomagnetic fields decreased the size and number of Staphylococcus aureus [159]. Exposure to hypomagnetic fields can also influence early developmental processes of newts (Cynops pyrrhogaster) [160], early embryogenesis [161,162], development of Xenopus [163], cryptochrome-related hypocotyl growth and flowering of Arabidopsis [164,165], development and reproduction of brown planthopper [166], mortality [167] and anhydrobiotic abilities [168] in tardigrades.
It was observed that the circadian clock in fiddler crabs and other organisms [169], including human [170] and birds [171] can be influenced by exposure to hypomagnetic fields.
Zhang et al. showed that long-term exposure to hypomagnetic fields adversely influenced adult hippocampal neurogenesis in mice [172]. They further observed that these effects were accompanied by reductions in ROS levels. Moreover, Wang et al. observed that exposure to hypomagnetic fields (10–100 nT) caused disorders in tubulin self-assembly [173]. They show that the absorbance for monitoring tubulin self-assembly was altered by exposure to hypomagnetic fields. We discuss both these observations from the perspective of the radical pair mechanism in the following (see §§4.4 and 4.5). Furthermore, Baek et al. reported that exposure to hypomagnetic fields influenced DNA methylation in vitro in mouse embryonic stem cell (ESC) culture [174]. Upon exposure to a hypomagnetic field ESC morphology remained undifferentiated while under exposure to the geomagnetic field, ESCs exhibited differentiation. Moreover, Ikenaga and co-workers reported that genetic mutation in Drosophila during space flight [175]. Further, Martino and co-workers reported that reducing the geomagnetic field to 6–13 μT resulted in significantly altered cell cycle rates for multiple cancer-derived cell lines [176]. Belyavskaya observed that hypomagnetic conditions included reduction of the meristem, disruption of protein synthesis and accumulation of lipids, reduction in organelle growth, the amount of phytoferritin in plastids and crista in mitochondria [177]. Further, the effects of zero magnetic field on human VH-10 fibroblasts and lymphocytes were observed by Belyaev et al. [178]. They concluded that exposure to hypomagnetic fields caused hypercondensation and decondensation of chromatin. Studies conducted by NASA revealed that exposure to hypomagnetic fields decreased enzyme activity in cells obtained from mice [179].
Yan et al. show that reducing the magnetic field to less than 0.5 μT significantly lengthened larval and pupal development durations, increased male longevity, and reduced pupal weight, female reproduction, and the relative expression level of the vitellogenin gene in Mythimna separata [180]. In addition, they observed that exposure to the hypomagnetic field had adverse effects on the mating ratio of M. separata adults. They further reported that moths in the hypomagnetic conditions had less flight activity late in the night compared to the control group. They suggest that the latter may be related to the circadian rhythm of M. separata.
Sarimov et al. reported that hypomagnetic conditions influence human cognitive processes [181]. They concluded that exposure to hypomagnetic fields resulted in an increased number of errors and extension of the time required to complete the tasks compared to normal conditions.
Wang and co-workers showed that exposure to hypomagnetic fields induced cell proliferation of SH-SY5Y cells in a glucose-dependent manner [182]. They suggested that lactate dehydrogenase was a direct response to cell proliferation under hypomagnetic conditions. The authors further proposed that the up-regulation of anaerobic glycolysis and repression of oxidative stress shifted cellular metabolism more towards the Warburg effect commonly observed in cancer metabolism. Table 2 summarizes hypomagnetic field effects observed on various physiological functions.
Table 2.
Hypomagnetic field effects on different biological functions.
| system | references |
|---|---|
| development | |
| decrease in size and number of Staphylococcus aureus | Rosenbach [159] |
| changes of tinctorial, morphological, cultural and biochemical properties in bacteria | Eerkin et al. [183] |
| newt (Cynops pyrrhogaster)—early developmental processes | Asashima et al. [160] |
| inhibition of early embryogenesis | Osipenko [161,162] |
| Xenopus embryos—development | Mo et al. [163] |
| Arabidopsis—cryptochrome-related hypocotyl growth and flowering | Xu et al. [164,165] |
| brown planthopper—development and reproduction | Wan et al. [166] |
| increased mortality in tardigrades | Erdmann et al. [167] |
| inhibition of anhydrobiotic abilities in tardigrades | Erdmann et al. [168] |
| developmental and behavioural effects in moths | Yan et al. [180] |
| cell proliferation in SH-SY5Y cells, ROS implicated | Wang et al. [182] |
| circadian system | |
| fiddler crabs and other organisms—circadian clock | Brown [169] |
| human—circadian rhythms | Waver et al. [170] |
| bird—circadian clock | Bliss & Heppner [171] |
| mice—circadian rhythm/increases algesia | Mo et al. [184] |
| neurons and brain | |
| inhibition of stress-induced analgesia in male mice | Seppia et al. [185] |
| hamster—GABA in cerebellum and basilar nucleus | Junfeng et al. [186] |
| mice—amnesia | Choleris et al. [187] |
| chick—long-term memory | Wang et al. [188] |
| impairment in learning abilities and memory of adult male mice | Wang et al. [189] |
| Drosophila—amnesia | Zhang et al. [190] |
| mice—analgesia | Prato et al. [191] |
| golden hamster—noradrenergic activities in the brainstem | Zhang et al. [192] |
| human cognitive processes | Sarimov et al. [181] |
| purified tubulin from calf brain—assembly | Wang et al. [173] |
| chickens needed additional noradrenaline for memory consolidation | Xiao et al. [193] |
| human—cognitive processes | Binhi & Sarimov [194] |
| human neuroblastoma cell—proliferation | Mo et al. [195] |
| human neuroblastoma cells—actin assembly and inhibits cell motility | Mo et al. [196] |
| human neuroblastoma cell—H2O2 production | Zhang et al. [197] |
| anxiety in adult male mice | Ding et al. [198] |
| mouse—proliferation of mouse neural progenitor and stem cells | Fu et al. [199] |
| DNA | |
| genetic mutations in Drosophila during space flight | Ikenaga et al. [175] |
| mouse ESCs culture—DNA methylation | Baek et al. [174] |
| human bronchial epithelial cells—DNA repair process | Xue et al. [200] |
| others | |
| decreased enzyme activity in cells obtained from mice | Conley [179] |
| Ca2+ balance in meristem cell of pea roots | Belyavskaya [177] |
| ability to change colour in Xenopus laevis | Leucht [201] |
| chromatin hypercondensation/decondensation in human fibroblasts/lymphocytes | Belyaev et al. [178] |
| increased protoplasts fusion | Nedukha et al. [202] |
| decreasing certain elements in rats’ hair | Tombarkiewicz [203] |
| cancer-derived cell lines—cell cycle rates | Martino et al. [176] |
| human fibrosarcoma cancer cells—H2O2 production | Martino et al. [204] |
| mouse primary skeletal muscle cell—ROS levels | Fu et al. [205] |
| invertebrates and fish—calcium-dependent proteases | Kantserova et al. [206] |
2.3. Oscillating magnetic field
2.3.1. Low-frequency
The effects of oscillating magnetic fields on biological functions are abundant [207–215], and are often correlated with modulation of ROS levels [216–218]. In this section, we review several studies on extremely low-frequency (less than 3 kHz) magnetic fields on various biological functions.
Sherrard and co-workers showed that exposure of the cerebellum to low-intensity repetitive transcranial magnetic stimulation (LI-rTMS) (10 mT) modulated behaviour and Purkinje cell morphology [219,220]. Recently, the same group reported that LI-rTMS (2 mT) induced axon growth and synapse formation providing olivocerebellar reinnervation in the cerebellum [221]. The authors concluded that cryptochrome was required for the magnetosensitivity of the neurons, which was consistent with ROS production by activated cryptochrome [222]. In a recent study, the team showed that LI-rTMS (10 mT and 10 Hz) evoked neuronal firing during the stimulation period and induced durable attenuation of synaptic activity and spontaneous firing in cortical neurons of rats in vivo [223].
Contalbrigo et al. showed that magnetic fields (less than 1 mT, 50 Hz) influenced some haematochemical parameters of circadian rhythms in Sprague–Dawley rats [224]. Further, Fedele et al. reported that a 300 μT magnetic field (3–50 Hz) induced changes in two locomotor phenotypes, circadian period and activity levels via modulating cryptochrome in Drosophila [225]. Moreover, it has been shown that exposure to a magnetic field of an 0.1 mT and 50 Hz alters clock gene expressions [226].
Manikonda et al. applied magnetic fields (50 and 100 μT, 50 Hz) to the cerebellum, hippocampus and cortex of rat brains. They observed that H2O2 increased in the descending order of cerebellum, hippocampus and cortex. In that work, 100 μT induced more oxidative stress compared to 50 μT [227]. Furthermore, Özgün et al. reported that exposure to a magnetic field (1 mT, 50 Hz) in vitro induced human neuronal differentiation through N-methyl-d-aspartate (NMDA) receptor activation [228]. They observed that the magnetic field enhanced intracellular Ca2+ levels. The authors concluded that NMDA receptors (NMDARs) are essential for magnetosensitivity in such phenomena. It is also known that a combination of static (27–37 μT) and time varying (13/114 μT, 7/72 Hz) magnetic fields directly interact with the Ca2+ channel protein in the cell membrane [229]. It has also been reported that exposure to greater than 5 mT (50 Hz) magnetic fields may promote X-ray-induced mutations in hamster ovary K1 cells [230]. Koyama et al. showed that exposure to a magnetic field of 5 mT (60 Hz) promoted damage induced by H2O2, resulting in an increase in the number of mutations in plasmids in E. coli [231]. Studies of extremely low-frequency magnetic field effects (less than 1000 Hz) on various biological functions are shown in tables 3 and 4.
Table 3.
Extremely low-frequency (less than 3 kHz) magnetic field effects on memory, stress, pain, dopamine, serotonin, melatonine, genetics and calcium flux.
| system | magnetic field and frequency | references |
|---|---|---|
| memory | ||
| rat—acquisition and maintenance of memory | 2 mT, 50 Hz | Liu et al. [232] |
| rat—memory and corticosterone level | 0.2 mT, 50 Hz | Mostafa et al. [233] |
| spatial recognition memory in mice | 0.6/0.9/1.1/2 mT, 25/50 Hz | Fu et al. [234] |
| spatial memory disorder/hippocampal damage in Alzheimer’s disease rat model | 400 μT, 50 Hz | Liu et al. [235] |
| recognition memory task/hippocampal spine density in mice | 1 mT, 50 Hz | Zhao et al. [236] |
| human hippocampal slices—semantic memory | 1 μT, 5 min on/5 min off | Richards et al. [237] |
| stress | ||
| behaviour/anxiety in rats | 520 μT, 50 Hz | Balassa et al. [238] |
| benzodiazepine system in hyperalgesia in rats | 0.5/1/2 mT, 60 Hz | Jeong et al. [239] |
| anxiogenic effect in adult rats | 2 mT, 50 Hz | Liu et al. [240] |
| anxiety level and spatial memory of adult rats | 2 mT, 50 Hz | He et al. [241] |
| stress-related behaviour of rats | 10 mT, 50 Hz | Korpinar et al. [242] |
| depression and corticosterone secretion in mice | 1.5/3 mT, 60 Hz | Kitaoka et al. [243] |
| anxiety, memory and electrophysiological properties of male rats | 4 mT, <60 Hz | Rostami et al. [244] |
| induction of anxiety via NMDA activation in mice | 1 mT, 50 Hz | Salunke et al. [245] |
| pain | ||
| mice—pain thresholds | 2 mT, 60 Hz | Jeong et al. [246] |
| snail—analgesia | 141−414 μT, 30 & 60 Hz | Prato et al. [247] |
| human—analgesia/EEG | 200 μT, <500 Hz | Cook et al. [248] |
| attenuate chronic neuropathic pain in rats | 1 mT, 1/10/20/40 Hz | Mert et al. [249] |
| mice—inhibition of morphine-induced analgesia | 0.15-9 mT, 0.5 Hz | Kavaliers & Osscnkopp [250] |
| dopamine/serotonin/melatonin | ||
| rat frontal cortex—dopamine and serotonin level | 1.8–3.8 mT, 10 Hz | Siero et al. [251] |
| rat brain—serotonin and dopamine receptors activity | 0.5 mT, 50 Hz | Janac et al. [252] |
| rat—central dopamine receptor | 1.8–3.8 mT, 10 Hz | Siero et al. [253] |
| rat—plasma and pineal melatonin levels | 1/5/50/250 μT, 50 Hz | Kato et al. [254] |
| human—melatonin concentration | 2.9 mT, 40 Hz | Karasek et al. [255] |
| genetic | ||
| rat brain cells—increases DNA strand breaks | 0.5 mT, 60 Hz | Lai & Singh [256,257] |
| human HL-60 cells-steady—state levels of some mRNAs | 8 μT, 60 Hz | Karabakhtsian et al. [258] |
| hamster ovary K1cells—promotion in X-ray-induced mutations | >5 mT, 50 Hz | Miyakoshi et al. [230] |
| HL-60 cells—CREB DNA binding activation | 0.1 mT, 50 Hz | Zhou et al. [259] |
| plasmids in E. coli—increase in the number of mutations | 5 mT, 60 Hz | Komaya et al. [231] |
| genetic analysis of circadian responses in Drosophila | 300 μT, 3–50 Hz | Fedele et al. [225] |
| epigenetic modulation of adult hippocampal neurogenesis in mice | 1 mT, 50 Hz | Leone et al. [260] |
| circadian gene expression in human fibroblast cell | 0.1 mT, 50 Hz | Manzella et al. [226] |
| epigenetic modulation in human neuroblastoma cells | 1 mT, 50 Hz | Consales et al. [261] |
| calcium | ||
| lymphocyte—calcium signal transduction | 42.1 μT, 16 Hz | Yost & Liburdy [262] |
| T cell—intracellular calcium oscillations | 0.1 mT, 50 Hz | Lindströum et al. [263] |
| rat pituitary cells—Ca2+ influx | 50 μT, 50 Hz | Barbier et al. [264] |
| Ca2+ channel protein in the cell membrane | 13/114 μT, 7/72 Hz | Baurus Koch et al. [229] |
| human skin fibroblast populations—intracellular calcium oscillations | 8 mT, 20 Hz | Löschinger et al. [265] |
| osteoblasts cells—intracellular calcium levels | 0.8 mT, 50 Hz | Zhang et al. [266] |
| C2C12 muscle cells—calcium handling and increasing H2O2 | 1 mT, 50 Hz | Morabito et al. [267] |
| rat ventricle cells—intracellular Ca2+ | 0.2 mT, 50 Hz | Sert et al. [268] |
| mesenchymal stem cells—Ca2+ intake | 1 mT, 50 Hz | Özgün & Garipcan [269] |
| brain tissue—radiation-induced efflux of Ca2+ ions | μT, 15/45 Hz | Blackman et al. [270] |
| rat hippocampus—Ca2+ signalling and NMDA receptor functions | 50/100 μT, <300 Hz | Manikonda et al. [271] |
| entorhinal cortex neurons—calcium dynamics | 1/3 mT, 50 Hz | Luo et al. [272] |
Table 4.
Extremely low-frequency (less than 3 kHz) magnetic field effects on reactive oxygen species (ROS) levels.
| system | magnetic field | references |
|---|---|---|
| ROS | ||
| ageing via ROS involvement in brain of mongolian gerbils | 0.1/0.25/0.5 mT, 50 Hz | Selakovi et al. [273] |
| hippocampus mitochondria via increasing H2O2 in mice | 8 mT, 50 Hz | Duan et al. [274] |
| neural differentiation/H2O2 elevation in mesenchymal stem cells | 1 mT, 50 Hz | Park et al. [275] |
| H2O2 production in neuroblastoma cell | 2 ± 0.2 mT, 75 ± 2 Hz | Osera et al. [276] |
| pro-Parkinson’s disease toxin MPP+/H2O2 increase in SH-SY5Y cells | 1 mT, 50 Hz | Benassi et al. [277] |
| rat peritoneal neutrophils-oxidative burst | 0.1 mT, 60 Hz | Roy et al. [278] |
| cortical synaptosomes of Wistar rats-oxidative stress | 0.7 mT, 60 Hz | Túnez et al. [279] |
| pro-oxidant effects of H2O2 in human neuroblastoma cells | 2 mT, 75 Hz | Falone et al. [280] |
| reducing hypoxia/inflammation damage ROS-mediated in neuron-like and microglial cells | 1.5 ± 0.2 mT, 75 Hz | Vincenzi et al. [281] |
| mouse brain-antioxidant defense system | 1.2 mT, 60 Hz | Lee et al. [282] |
| rat-cortical neurons-redox and trophic response/reducing ROS | 1 mT, 50 Hz | DiLoreto et al. [283] |
| human monocytes-cell activating capacity/ROS modulation | 1 mT, 50 Hz | Lupke et al. [284] |
| HL-60 leukaemia cells-proliferation/DNA damage implicating ROS | 1 mT, 50 Hz | Wolf et al. [285] |
| human monocytes-alteration of 986 genes/modulating ROS | 1 mT, 50 Hz | Lupke et al. [286] |
| prostate cancer cells-apoptosis through ROS | 0.2 mT, 60 Hz | Koh et al. [287] |
| K562 cells-O·−2 formation and HSP70 induction | 0.025–0.1 mT, 50 Hz | Mannerling et al. [288] |
| K562 Cells-differentiation via increasing O·−2 production | 5 mT, 50 Hz | AySe et al. [289] |
| K562 leukaemia cell-number of apoptotic cells via increasing O·−2 production | 1 mT, 50 Hz | Garip & Akan [290] |
| PC12 cells-H2O2 increase | 1 mT, 50 Hz | Morabito et al. [291] |
| carcinoma cells-cisplatin via increasing H2O2 | 1 mT, 50 Hz | Bułdak et al. [292] |
| human carcinoma cells-morphology and biochemistry implicating ROS | 0.1 mT, 100&217 Hz | Sadeghipour et al. [293] |
| rats- DNA strand breaks in brain cells by modulating ROS | 0.1–0.5 mT, 60 Hz | Lai & Singh [294] |
| cardiomyocytes-injury treatment implicating ROS | 4.5 mT, 15 Hz | Ma et al. [295] |
| genomic instability/oxidative processes in human neuroblastoma cells | 100 μT, 50 Hz | Luukkonen et al. [296] |
| expression of NOS and O·−2 in human SH-SY5Y cells | 1 mT, 50 Hz | Reale et al. [297] |
| ROS-related autophagy in mouse embryonic fibroblasts | 2 mT, 50 Hz | Chen et al. [298] |
| healing via reducing ROS production in artificial skin wounds | <40 μT, 100 Hz | Ferroni et al. [299] |
| apoptosis via oxidative stress in human osteosarcoma cells | 1 mT, 50 Hz | Yang et al. [300] |
| increase O·−2 in erythro-leukemic cells | 1 mT, 50 Hz | Patruno et al. [301] |
| Genomic instability/H2O2 increase in SH-SY5Y cells | 100 μT, 50 Hz | Kesari et al. [302] |
| NOX-produced ROS in hAECs | 0.4 mT, 50 Hz | Feng et al. [303] |
| mitochondrial permeability via increasing H2O2 in human aortic endothelial cells | 0.4 mT, 50 Hz | Feng et al. [304] |
| apoptotic via mitochondrial O·−2 release in human aortic endothelial cells | 0.4 mT, 50 Hz | Feng et al. [305] |
| antioxidant activity implicating H2O2 in human keratinocyte cells | 25 − 200 μT, 1–50 Hz | Calcabrini et al. [306] |
| antioxidative defense mechanisms via ROS in human osteoblasts | 2 − 282 μT, 16 Hz, | Ehnert et al. [307] |
| astrocytic differentiation implicating ROS in human bone stem cells | 1 mT, 50 Hz | Jeong et al. [308] |
| reduce mitochondrial O·−2 production in human neuroblastoma cells | 100 μT, 50 Hz | Höytö et al. [309] |
| ROS production in human cryptochrome | 1.8 mT, <100 Hz | Sherrard et al. [222] |
| proliferation by decreasing intracellular ROS levels in human cells | 10 mT, 60 Hz | Song et al. [310] |
| cytotoxic effect in by raising intracellular ROS in human GBM cells | 1–58 mT, 350 Hz | Helekar et al. [311] |
2.3.2. Medium/high-frequency
In this section, we review several studies on medium/high-frequency (greater than 3 kHz) magnetic field effects on various physiological functions (table 5). Usselman et al. reported that oscillating magnetic fields at Zeeman resonance (1.4 MHz and 50 μT) influenced relative yields of cellular O·−2 and H2O2 products in human umbilical vein endothelial cells [340]. Considering a radical pair in [FADH...O·−2] form, the authors suggested that coherent electron spin dynamics may explain their observation. Moreover, Friedman et al. observed that a 875 MHz magnetic field increased ROS production, which was mediated by membrane-associated NOX in HeLa cells and rats [341]. Castello and colleagues showed that exposure of fibrosarcoma HT1080 cells to weak radio frequency (5/10 MHz) combined with a 45 μT static magnetic field modulated the number of cells and significantly increased H2O2 production [342]. Martino and Castello showed that exposure of cultured yeast and isolated mitochondria to magnetic fields (150 μT; 45 μT and a parallel 10 MHz RF; 45 μT and a perpendicular 10 MHz RF) modulated the production of extracellular, intracellular, and mitochondrial O·−2 and H2O2 [343]. They concluded that complex I of the ETC is involved in H2O2 production. Table 6 summarizes a few medium/high-frequency magnetic field effects observed in various experiments.
Table 5.
Extremely low-frequency (less than 3 kHz) magnetic field effects on different biological functions.
| system | magnetic field | references |
|---|---|---|
| others | ||
| neuroendocrine cell—proliferation and death | <1 mT, 50 Hz | Grassi et al. [312] |
| cortices of mice—neuronal differentiation of neural stem/progenitor cells | 1 mT, 50 Hz | Piacentini et al. [313] |
| hippocampal slices—excitability in hippocampal neurons | 15 mT, 0.16 Hz | Ahmed & Wieraszko [314] |
| human—EEG alpha activity | 200 μT, 300 Hz | Cook et al. [315,316] |
| rat—neuroprotective effects | 0.1/0.3/0.5 mT, 15 Hz | Yang et al. [317] |
| rat—neuroprotective effects on Huntington’s disease | 0.7 mT, 60 Hz | Tasset et al. [318] |
| synaptic efficacy in rat brain slices | 0.5/3 mT, 50 Hz | Balassa et al. [319] |
| global cerebral ischaemia/pituitary ACTH and TSH cells in gerbils | 0.5 mT, 50 Hz | Balind et al. [320] |
| neurotrophic factor expression in rat dorsal root ganglion neurons | 1 mT, 50 Hz | Li et al. [321] |
| visual cortical circuit topography and BDNF in mice | ∼10 mT, <10 Hz | Makowiecki et al. [322] |
| hippocampal long-term potentiation in rat | 100 μT, 50 Hz | Komaki et al. [323] |
| neuronal GABAA current in rat cerebellar granule neurons | 1 mT, 50 Hz | Yang et al. [324] |
| central nervous regeneration in planarian Girardia sinensis | 200 mT, 60 Hz | Chen et al. [325] |
| neuronal differentiation and neurite outgrowth in embryonic neural stem cells | 1 mT, 50 Hz | Ma et al. [326] |
| synaptic transmission and plasticity in mammalian central nervous synapse | 1 mT, 50 Hz | Sun et al. [327] |
| human—pineal gland function | <μT, 60 Hz | Wilson et al. [328] |
| rat—electrically kindled seizures | 0.1 mT, 60 Hz | Ossenkopp & Cain [329] |
| rat—central cholinergic systems | 1 mT, 60 Hz | Lai et al. [330] |
| deer mice—spatial learning | 0.1 mT, 60 Hz | Kavaliers et al. [331] |
| T-cell receptor—signalling pathway | 0.15 mT, 50 Hz | Lindström et al. [332] |
| enhances locomotor activity via activation of dopamine D1-like receptors in mice | 0.3/2.4 mT, 60 Hz | Shin et al. [333] |
| rat pituitary ACTH cells | 0.5 mT, 50 Hz | Balind et al. [334] |
| actin cytoskeleton reorganization in human amniotic cells | 0.4 mT, 50 Hz | Wu et al. [335] |
| reduces hypoxia and inflammation in damage microglial cells | 1.5 mT, 50 Hz | Vincenzi et al. [281] |
| pluripotency and neuronal differentiation in mesenchymal stem cells | 20 mT, 50 Hz | Haghighat et al. [336] |
| proliferation and differentiation in osteoblast cells | 5 mT, 15 Hz | Tong et al. [337] |
| reduced hyper-inflammation triggered by COVID-19 in human | 10 mT, 300 Hz | Pooam et al. [338] |
| proliferation and regeneration in planarian Schmidtea mediterranea | 74 μT, 30 Hz | Ermakov et al. [339] |
Table 6.
Medium/High-frequency (greater than 3 kHz) magnetic field effects on biological functions.
| system | magnetic field and frequency | references |
|---|---|---|
| ROS production and DNA damage in human SH-SY5Y neuroblastoma cells | 872 MHz | Luukkonen et al. [344] |
| ROS level in human ejaculated semen | 870 MHz | Agarwal et al. [345] |
| ROS production and DNA damage in human spermatozoa | 1.8 GHz | Iuliis et al. [346] |
| ROS levels and DNA fragmentation in astrocytes | 900 MHz | Campisi et al. [347] |
| ROS formation and apoptosis in human peripheral blood mononuclear cell | 900 MHz | Lu et al. [348] |
| ROS elevation in Drosophila | 1.88–1.90 GHz | Manta et al. [349] |
| ROS modulation in rat pulmonary arterial smooth muscle cells | 7 MHz | Usselman et al. [350] |
| bioluminescence and oxidative response in HEK cells | 940 MHz | Sefidbakht et al. [351] |
| electrical network activity in brain tissue | <150 MHz | Gramowski-Voß et al. [352] |
| ROS production in human umbilical vein endothelial cells | 50 μT, 1.4 MHz | Usselman et al. [340] |
| insect circadian clock | 420 μT, RF | Bartos et al. [353] |
| tinnitus, migraine and non-specific in human | 100 KHz to 300 GHz | Röösli et al. [354] |
| magnetic compass orientation in night-migratory songbird | 75–85 MHz | Leberecht et al. [355] |
2.4. Isotope effects
Atomic nuclei contain protons and neutrons. The number of protons determines the element (e.g. carbon, oxygen etc.), and the number of neutrons determines the isotope of the desired element. Some isotopes are stable, i.e. they preserve the number of protons and neutrons during chemical reactions. It has been shown that using different isotopes of the element in certain chemical reactions results in different outcomes. Such observations have been seen in many chemical reactions [356–363] including biological processes [45,364–368]. Inheriting quantum properties, not only do different isotopes of an element have different masses, but they can also have different spins. For that reason, isotope effects in (bio)chemical reactions can be regarded from two distinct points of view: mass-dependency and spin-dependency. Thiemens et al. observed mass-independent isotope effects as a deviation of isotopic distribution in reaction products [369–373]. Furthermore, in 1976 Buchachenko and colleagues by applying magnetic fields detected the first mass-independent isotope effect, which chemically discriminated isotopes by their nuclear spins and nuclear magnetic moments [374]. Since then, the term ‘magnetic isotope effect’ was dubbed for such phenomena as they are controlled by electron-nuclear hyperfine coupling in the paramagnetic species. Moreover, isotope effects have been observed for a great variety of chemical and biochemical reactions involving oxygen, silicon, sulfur, germanium, tin, mercury, magnesium, calcium, zinc and uranium [65,367,368,375–381]. In this review, we focus on isotope effects from a spin perspective, see table 7.
Table 7.
Spin-dependent isotope effects on different biological functions.
| system | isotope | spin, I | references |
|---|---|---|---|
| parenting/offspring development in rat | 6Li, 7Li | 1, 3/2 | Sechzer et al. [382] |
| hyperactivity in rat | 6Li, 7Li | 1, 3/2 | Ettenberg et al. [383] |
| anaesthetic potency in mice | 129Xe, 131Xe, 132Xe, 134Xe | 1/2, 3/2, 0, 0 | Li et al. [384] |
| ATP production in purified pig skeletal muscle PGK | 24Mg, 25Mg, 26Mg | 0, 5/2, 0 | Buchachenko et al. [385] |
| DNA synthesis in HL-60 human myeloid leukaemia cells | 64Zn, 67Zn | 0, 5/2 | Buchachenko et al. [386] |
| DNA synthesis in HL-60 human myeloid leukaemia cells | 24Mg, 25Mg, 26Mg | 0, 5/2, 0 | Buchachenko et al. [387] |
| DNA synthesis in HL-60 human myeloid leukaemia cells | 40Ca, 43Ca | 0, 7/2 | Bukhvostov et al. [388] |
In 1986 Sechzer and co-workers reported that lithium administration results in different parenting behaviours and potentially delayed offspring development in rats [382]. Their findings were not quantitative; however, it was observed that different lithium isotopes exhibited different impacts. Moreover, in 2020, Ettenberg et al. [383] conducted an experiment demonstrating an isotope effect of lithium on rat hyperactivity. Lithium has two stable isotopes, 6Li and 7Li, possessing different nuclear spin angular momentum, I6 = 1 and I7 = 3/2, respectively. In that work, the mania phase was induced by sub-anaesthetic doses of ketamine. The authors reported that produced a longer suppression of hyperactivity in an animal model of mania compared to 7Li. We further discuss this phenomenon from the point of view of the radical pair mechanism in §4.2.
Li and co-workers reported that xenon (Xe)-induced anaesthesia in mice is isotope-dependent. They used four different Xe isotopes, 129Xe, 131Xe, 132Xe and 134Xe with nuclear spins of 1/2, 3/2, 0 and 0, respectively [384]. The results fell into two groups, isotopes with spin and isotopes without spin, such that isotopes of xenon with non-zero nuclear spin had lower anaesthetic potency than isotopes with no nuclear spin. The results of this work are discussed from the perspective of the radical pair mechanism in §4.1.
Buchachenko et al. observed that magnesium-25 (25Mg) controlled phosphoglycerate kinase (PGK) [385]. 25Mg has a nuclear spin of 5/2, while 24Mg is spin-less. The authors reported that ATP production was more than twofold in the presence of 25Mg compared to 24Mg. They suggested that the nuclear spin of Mg was the key factor for such an observation. In another study, the same group reported that 25Mg reduced enzymatic activity in DNA synthesis compared to 24Mg. They concluded that DNA synthesis is magnetic field-dependent [387,389]. In the same system, they further observed that if Mg2+ ion is replaced by stable isotopes of calcium ion, 40Ca2+ and 43Ca2+ (with nuclear spins of 0, 7/2, respectively), the enzyme catalytic reactions will be isotope-dependent, such that 43Ca2+promoted enzyme hyper-suppression leading to a residual synthesis of shorted DNA fragments compared to 40Ca2+ [388]. They repeated the same experiment but this time instead of Mg2+ ion stable isotopes of zinc, 64Zn2+ and 67Zn2+ (with nuclear spins of 0, 5/2, respectively) were used. The authors reported that 67Zn2+ suppressed DNA synthesis a few times more than 64Zn2+ [386].
3. The radical pair mechanism
3.1. Spin and radical pairs
Spin is an inherently quantum property that emerges from Dirac’s relativistic quantum mechanics [390,391], and is described by two numbers, S and ms, respectively, the spin quantum number and the spin projection quantum number. Electrons, protons and neutrons have spins of S = 1/2. Having an angular momentum characteristic, spin can be coupled not only with external magnetic fields but also with other spin in its vicinity. For instance, coupling of two electrons spins, SA and SB, results in a total spin of ST, which has a quantum number of either S = 1 or S = 0. The latter case is called a singlet state, with ms = 0, and the former is called a triplet state, with ms = 0, ±1 [392].
| 3.1 |
| 3.2 |
| 3.3 |
| 3.4 |
where ⊗ is the tensor product.
Radicals are molecules with an odd number of electrons in the outer shell [393,394]. A pair of radicals can be formed by breaking a chemical bond or electron transfer between two molecules. It is important to note that in reactions of organic molecules, spin is usually a conserved quantity, which is essential for magnetic field effect in biochemical reactions. For example, a radical pair can be created if a bond between a pair of molecules [A · · · D] breaks or an electron is transferred from D to A, [A−. · · · D.+] (D and A denote donor and acceptor molecules). A radical pair may be in a superposition of singlet and triplet states, depending on the parent molecule’s spin configuration. Assuming that the initial state of the electron pairs before separation was a singlet (triplet), the recombination of unpaired electrons can only happen if they stayed in a singlet (triplet) [395].
If the radical pairs are formed in singlet (triplet) states, the initial spin density matrix reads as follows:
| 3.5 |
| 3.6 |
| 3.7 |
| 3.8 |
| 3.9 |
where and are the singlet and triplet projection operators, respectively, M is the nuclear spin multiplicity, Ii is the spin angular momentum of ith nucleus and is the identity matrix. S is entangled. The T projector is not entangled, even though |T0〉 is an entangled state.
3.2. Interactions
3.2.1. Zeeman interaction
The interaction between the unpaired electron spins on each radical and the external magnetic field is essential for generating MFEs. This interaction is called the Zeeman effect [396]. The nuclear spins of radical molecules also experience applied magnetic fields; however, as nuclear magnetogyric ratios are much smaller than that of the electrons, these interactions are negligible. The Zeeman interaction is defined in the following form:
| 3.10 |
where μB, , g-tensor and B are the Bohr magneton, the spin operators of electron, the interaction coupling and applied magnetic field, respectively. Here, we focus on magnetic field interactions with relatively low field strengths. In such cases, it is possible to assume that the g-tensor equals to ge of free electron, and hence,
| 3.11 |
where γe and h are the electron magnetogyric ratio and the Planck constant, respectively.
3.2.2. Hyperfine interaction
Similar to electron–electron spin coupling, electron spins can couple to the nuclear spins, called hyperfine interactions [397]. This interaction consists of two contributions, isotropic and anisotropic interactions. The former is also called Fermi contact term, which results from the magnetic interaction of the electron and nuclear spins when the electron is within the nucleus. The overall hyperfine interaction can be defined as follows:
| 3.12 |
where ai and are the hyperfine coupling tensor and nuclear spin of ith nucleus. The anisotropic components of the hyperfine interactions are only relevant when the radicals are immobilized and aligned [25]. Neglecting the anisotropic component of the hyperfine interaction, the hyperfine Hamiltonian has the following form:
| 3.13 |
where ai is the isotropic hyperfine coupling constant and can be calculated as
| 3.14 |
μ0 is the vacuum permeability, γn is the nuclear magnetogyric ratio and is the electron probability density at the nucleus [398].
3.2.3. Exchange interaction
The electrons on radicals are identical in quantum calculations. This indistinguishability of electrons on radical pairs can be introduced via the exchange interaction [399]. It is generally assumed to weaken exponentially with increasing radical pair separation. The exchange interaction can prevent singlet–triplet interconversion, as discussed later. However, recent studies show that this term is negligible [400] in the magnetic field effects on pigeon cryptochrome [401].
3.2.4. Dipolar interaction
As spins are magnetic moments, the radical pairs also influence each other by a dipolar interaction [402]. This interaction can suppress singlet–triplet interconversion in the radical pair dynamics. However, studies on avian magnetoreception suggest that under certain conditions exchange and dipolar interactions can be neglected [43,403–406].
3.2.5. Other contributions
It is thought that after a first re-encounter, radicals either react or diffuse apart forever [407]. In the context of birds’ magnetoreception, for this contribution, an exponential model is used [43,408].
High electron density on an atom of a radical can lead to have a higher anisotropic g-value compared to the case with lower electron density, called the spin-orbit effect, which results in the non-radiative transition between two electronic states with different spin multiplicity (e.g. singlet and triplet)—intersystem crossing, which can play important roles in chemical reactions [409–412].
3.3. Spin dynamics of radical pairs
The sensitivity of certain reactions to weak magnetic fields relies on the oscillations between singlet and triplet states of radical pairs, also known as ‘quantum beats’ [26]. If the radicals are separated enough spatially, having the same energies, singlet and triplet will undergo a coherent interconversion process, quantum beating. The interconversion is tuned by the magnetic fields experienced by the electrons, including Zeeman and hyperfine interactions. At low magnetic fields, the main drive for S–T interconversion is due to the hyperfine interactions. Obeying selection rules, the singlet and triplet yields will follow different chemical pathways, which depend on the timing of the coherent spin dynamics [413]. These quantum beats have just recently been observed directly [414].
The fractional singlet yield resulting from the radical pair mechanism throughout the reaction can be normally defined by using the Liouville–von Neumann equation [50]
| 3.15 |
where and are the spin density and Hamiltonian operators, respectively. [ · , · ] denotes the commutator.
For instance, the probability of finding the radical pairs in singlet states at some later time is determined by Hamiltonian using equation (3.15)
| 3.16 |
where Tr is trace.
The probability depends on other contributions, including kinetic reactions, spin relaxation, vibration and rotation of radical pairs, which can be introduced to equation (3.15).
3.3.1. Static magnetic field
Static magnetic field effects have been extensively studied in the context of birds’ magnetosensitivity [46,48]. However, the applications of these models can be extended to other magnetic field effects reviewed in §2.1. Assuming that the spin of the radical pairs start off from a singlet state, equation (3.16) can be rewritten as
| 3.17 |
where |m〉 and |n〉 are eigenstates of with corresponding eigenenergies of ωm and ωn, respectively.
Spin relaxation can be introduced phenomenologically [408,415] such that
| 3.18 |
where r denotes the spin relaxation rate. Following the work of Timmel et al. [50], the chemical fate of the radical pair can be modelled separating spin-selective reactions of the singlet and triplet pairs, as shown in figure 1. For simplicity, it is assumed that k = kS = kT, where kS and kT are the singlet and triplet reaction rates, respectively. The final singlet yield, , for periods much greater than the radical pair lifetime reads as follows:
| 3.19 |
where the fractional triplet yield can be calculated as .
Figure 1.
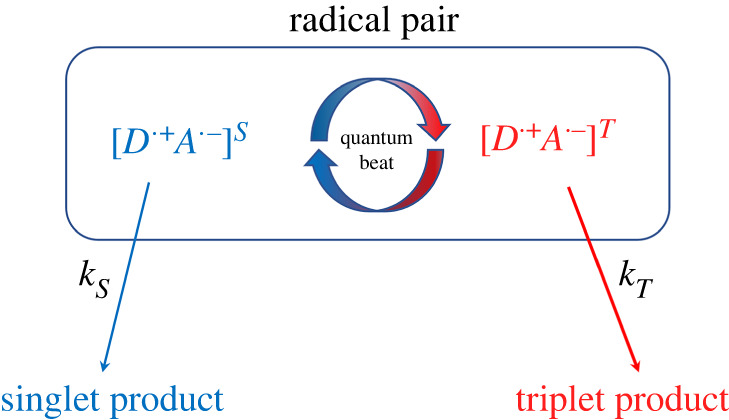
A simple schematic presentation of donor (D)–acceptor (A) radical pair reaction undergoing intersystem crossing between singlet (S) and triplet (T) states. Each state takes different chemical pathways via distinct reaction rates to produces S and T products with kS and kT, respectively, for S and T states.
In §4, we briefly review recent studies that suggest the radical pair mechanism may explain xenon-induced anaesthesia, lithium effects on hyperactivity, magnetic field and lithium effects on circadian clock, and hypomagnetic field effects on neurogenesis and microtubule reorganization. In these studies, for simplicity, only Zeeman and isotropic hyperfine interactions are considered. For a pair of radicals, the Hamiltonian reads
| 3.20 |
where and are the spin operators of radical electrons on A·− and D.+, respectively, and are the nuclear spin operators on the acceptor and donor radical molecule, aA and aB are the isotropic hyperfine coupling constants, NA and ND are the number of nuclei coupled to electron A and D, respectively, and ω is the Larmor precession frequency of the electrons due to the Zeeman effect.
3.3.2. Hypomagnetic field
Although hypomagnetic fields belong to the static magnetic field category, the effects due to extremely low magnetic field are often particularly significant compared to other magnetic field effects.
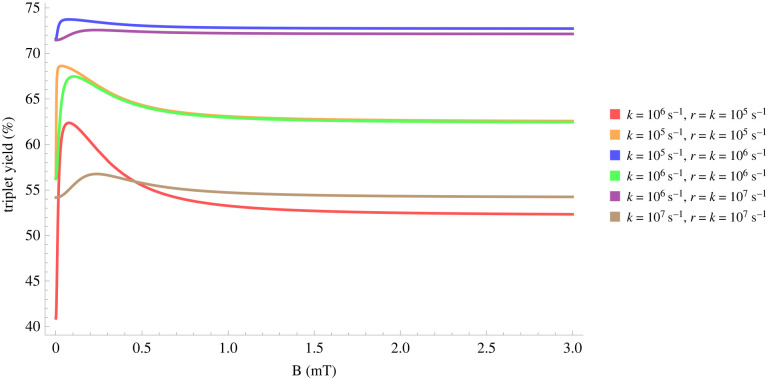
Using equation (3.19), it can be shown that for different relaxation and reactions rates, the hypomagnetic field effects are significant, as shown in figure 2.
Figure 2.
Triplet yield vs applied magnetic field for different reaction and spin relaxation rates for a simple model of a radical pair. In this model, one of the radicals is coupled to a nucleus with a hyperfine coupling constant of 1 mT. For different values of the rates, one can see a pronounced dip near zero field, together with a maximum close to the value of the geomagnetic field (around 0.05 mT)
3.3.3. Extremely low-frequency magnetic field
Given the short lifetime of radical pairs compared to the low frequency of the applied magnetic field, in general, the extremely low-frequency magnetic field can be treated as static during the lifetime of a radical pair [408,416]. Depending on the phase of oscillation, α ∈ (0, π), each radical pair therefore experiences a different, effectively static, magnetic field whose field strength is B. Assuming that B0 and B1(t) are parallel, the net effect of the oscillating field is an average over α, such that
| 3.21 |
and
| 3.22 |
where B0 and B1 indicate the static magnetic field and the amplitude of the oscillating magnetic field, respectively. Such theoretical model can be applied to the magnetic field effects reviewed in §2.3.1.
3.3.4. Medium/high-frequency magnetic field
For the cases of medium/high-frequency magnetic fields, a general approach is to integrate equation (3.15), using, for example, a fourth-order Runge–Kutta scheme. It is shown that high-frequency magnetic effects can be accounted for by the radical pair mechanism [417–419]. For instance, if the magnetic field has the following form:
| 3.23 |
the corresponding Hamiltonian can be transformed into a rotating reference frame where it becomes a time-independent Hamiltonian [420]. To do so, one could use a unitary transformation matrix
| 3.24 |
such that
| 3.25 |
Where H′ is the time-independent Hamiltonian and is the time derivative of T(t). After some algebra, one can obtain
| 3.26 |
3.4. Candidate radical pairs
It is now well known that in biology electron-transfer reactions can take place at reasonable rates even when the reactants are separated far beyond ‘collisional’ distances [421,422]. A radical pair can be formed by breaking a chemical bond or electron transfer between two molecules. Electron transfer between proteins is facilitated by the formation of a complex of the reacting proteins, which may be accompanied by conformational changes in the proteins. For that, the reactants must reach each other to build up the coupling of their electronic orbitals. The most used approach to rationalize and predict the rate of electron transfer processes is Marcus electron transfer theory [423]. Determining realistic radical pair candidates for the magnetosensitivity of physiological function, however, is still an interesting challenge. Here, we briefly review a few plausible radical pairs that maybe be relevant for the magnetosensitivity in biology.
3.4.1. Cryptochrome-based radical pairs
In the context of songbird avian magnetoreception, the cryptochrome proteins are the canonical magnetosensitive agent [48,424,425]. Cryptochromes are classified as flavoproteins. They play an important role in the circadian clock, where the circadian function can be either light-dependent or -independent. Kutta et al. showed that Type II animal cryptochromes lack the structural features to securely bind the photoactive flavin cofactor [426]. The circadian clock regulates photoreceptor sensitivity in the compound eye of insects and retinas of vertebrates, potentially including the sensitivity of specialized photo-magnetoreceptors. In flies, photo-magnetoreceptors are likely to be an unusual class of photoreceptors, i.e. retinula R7y cells [427]. It is thought that, in cryptochromes and photolyases, photoreduction of FAD is through three consecutive electron transfers along a conserved triad of tryptophan (Trp) residues to give FAD·− and TrpH.+ approximately 2 nm distant from each other [428–431]. In cryptochrome-4a, sequentially four radical pair states are formed by the progressive transfer of an electron along a chain of four tryptophan residues to the photo-excited flavin. In a recent study, Hore and co-workers suggest that, based on spin dynamics, while the third radical pair is mainly responsible for magnetic sensing, the fourth could enhance initiation of magnetic signalling particularly if the terminal tryptophan radical can be reduced by a nearby tyrosine (Tyr) [432]. They concluded that this arrangement may play an essential role in sensing and signalling functions of the protein. It is also suggested that Tyr can be the donor instead of the fourth Trp [429]. It is also found based on spin dynamics analysis that a radical pair in the form of [FAD·− and Tyr.] can provide sensitivity to the direction of the magnetic field [433].
Alternative radical pairs to [FAD·− · · · TrpH.+] have been suggested. In 2009, Ritz and Schulten showed that exposure to low-intensity oscillating magnetic fields disoriented European robins [434]. Interestingly the frequency of the applied magnetic field in that experiment was equal to the Larmor frequency (approx. 1.4 MHz) of a free electron spin in the geomagnetic field. Magnetic fields with the same amplitude but different frequencies had much less impact on the birds’ magnetic compass. Theoretical analysis suggests that such phenomenon may be explained if one of the radicals were free from internal magnetic interactions [435–438], which implies that such an observation is not compatible with the radical pair model based on [FAD·− · · · TrpH.+]. Various authors have suggested that the superoxide radical is the most plausible radical under such circumstances [434,435,439–443]; this is also consistent with animal magnetoreception in the dark [444–446], as it was suggested that during the backreaction, a radical pair is formed between flavin and an O2 and that the radical pair reaction responds significantly to reorientation in the geomagnetic field [438,439,447–449]. Such a radical pair could be generated without further absorption of light in the form of . However, deciding the more realistic radical pair between and [FAD·− · · · TrpH.+] to explain avian magnetoreception is still a matter of active debate [446,450–452]. The radical pair involving superoxide demands more reliable evidence.
3.4.2. Beyond cryptochrome-based radical pairs
Flavin-dependent enzymes are ubiquitous in biology. The isoalloxazine ring of the flavin cofactor (figure 3) can undergo thermally driven redox chemistry. The different redox states of flavin play essential roles in various electron transfer processes and consequently are crucial for a variety of important biological functions, including energy production, oxidation, DNA repair, RNA methylation, apoptosis, protein folding, cytoskeleton dynamics, detoxification, neural development, biosynthesis, the circadian clock, photosynthesis, light emission and biodegradation [422,454–465]. Different forms of transient radical pair intermediates can be created during reactions catalysed by flavin-dependent enzymes, including [466–468].
Figure 3.
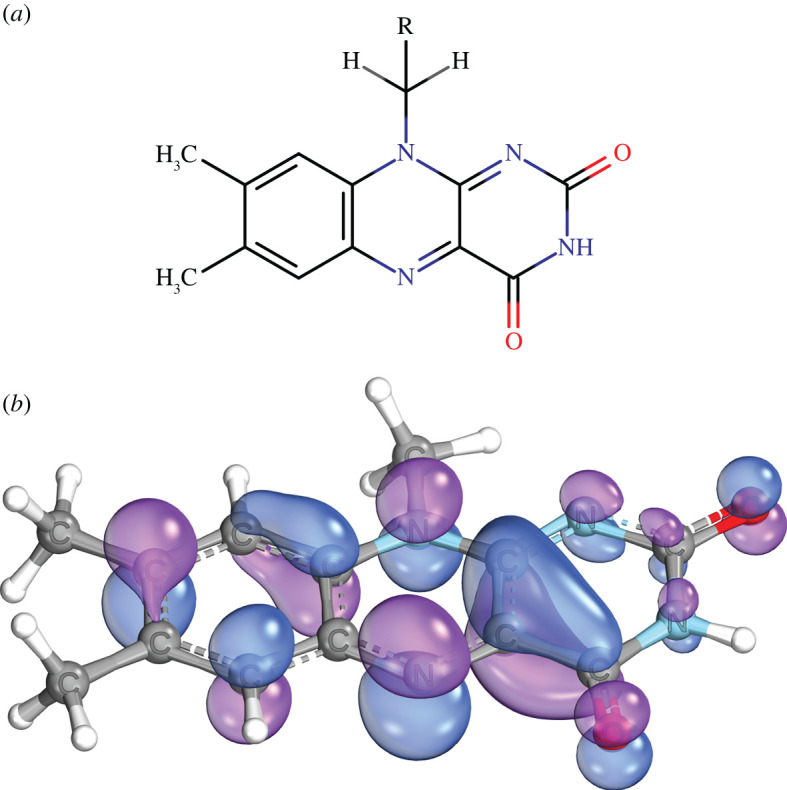
Molecular structure and orbitals of the flavin radical. (a) Structure of flavin adenine dinucleotide (FAD). R denotes the adenosine diphosphate group and the rest of the ribityl chain. (b) Representations of the molecular orbitals that contain the unpaired electron in a flavin anion radical. Blue and purple indicate parts of the wave function with opposite signs. ORCA package used to calculate the HOMO using PBE0/def2-TZVP [453]. Image rendered using IboView [v20211019-RevA].
Although cryptochrome is the main protein for avian magnetoreception, there exist many observational challenges for the canonical cryptochrome-centric radical pair mechanism. In a recent work, Bradlaugh and co-workers observed that the FAD binding domain and the Trp chain in cryptochrome are not required for magnetic field responses at the single neuron and organismal level in Drosophila. They further reported that an increase in FAD intracellular concentration enhanced neuronal sensitivity to blue light in the presence of a magnetic field. The authors concluded that the magnetosensitivity in cells may be well explained based on non-cryptochrome-dependent radical pair models [117]. However, the question whether fruit flies use a magnetic compass demands more experimental evidence.
It is known that near the tetrodotoxin binding site in Na+ channels there are tryptophan residues. Similarly, in the pore-forming region of voltage-sensitive Na+ channels, Tyr and tryptophan residues are located. It is suggested that gating these channel proteins may depend on the electron transfer between these residues, and hence formation of radicals [469]. This form of electron transfer is also proposed to play a key role in DNA photolyase [470].
Many physiological and pathological processes involve protein oxidation [471], icluding important residues such as Trp, Tyr, histidine (His) and proline (Pro). It is known that a radical pair in the form of can be created [472]. The superoxide radical may also be formed in a spin correlated manner with other partners, including tetrahydrobiopterin [473–475]. In addition, it was shown that an electron transfer process can occur between Trp and superoxide [476,477]. However, as discussed above, the radical pairs involving superoxide is a matter of debate. It was also suggested that in PGK phosphorylation a radical pair [RO. · · · Mg(H2O)n.+] complex can be formed [385].
4. Studies of the potential role of radical pairs in the brain
In this section, we briefly review recent studies that suggest that the radical pair mechanism may explain isotope effects in xenon-induced anaesthesia, and lithium effects on hyperactivity, magnetic field and lithium effects on the circadian clock, and hypomagnetic field effects on neurogenesis and microtubule reorganization.
4.1. Xenon anaesthesia
Xenon is a well-known general anaesthetic used for several species, including Drosophila, mice and humans [478]. Despite its simple structure (a single atom), the exact underlying mechanism by which it exerts its anaesthetic effects remains unclear. Turin et al. showed that when xenon acts anaesthetically on Drosophila, specific electron spin resonance (ESR) signals can be observed [479]. The same authors proposed that the anaesthetic action of xenon may involve some form of electron transfer. Moreover, Li et al. showed experimentally that isotopes of xenon with non-zero nuclear spin had reduced anaesthetic potency in mice compared with isotopes with no nuclear spin [384]. These findings are consistent with the hypothesis of radical pair creation in xenon-induced anaesthesia.
Franks and co-workers identified the NMDA subtype of glutamate receptor [480] as a target for xenon anaesthesia [478,481]. They further showed that xenon exerted its effects by inhibiting NMDARs by competing with the co-agonist glycine at the glycine-binding site on the GluN1 subunit [482]. Subsequently, the same group identified that xenon interacts with a small number of amino acids at the predicted binding site of the NMDAR [483]. Using grand canonical Monte Carlo method, they showed that xenon at the binding site can interact with tryptophan and phenylalanine, as shown in figure 4a. However, due to redox inactivity, it is highly unlikely that phenylalanine can be involved in the electron transfer process [484,485]. Meanwhile, tryptophan is redox active and hence can feasibly be involved in electron transfer and hence the formation of radical pairs, as seen in the context of cryptochrome magnetoreception [43]. In addition, it is known that tryptophan residues of the NMDAR play key roles in channel gating [486,487]. Moreover, exposure to low-intensity magnetic fields activates the NMDAR [228,245,271].
Figure 4.
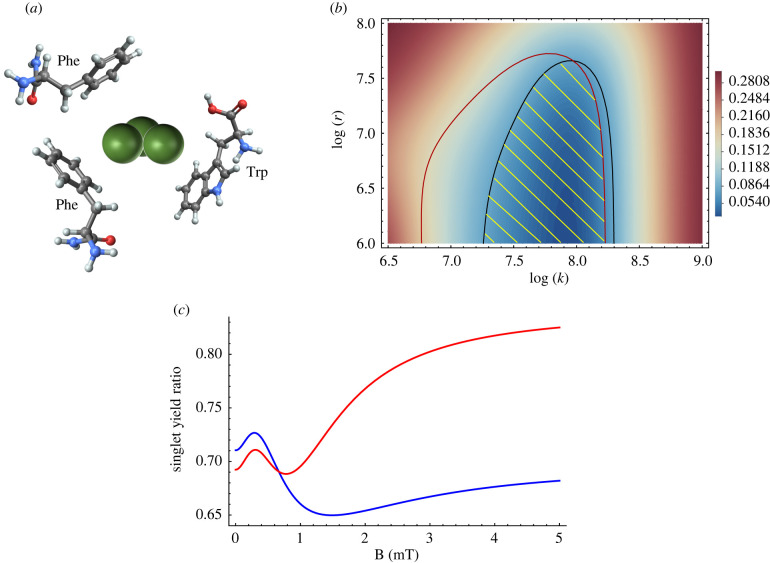
Radical pair explanation for isotope effects in xenon-induced anaesthesia. (a) Schematic presentation of the interaction of xenon (green spheres) with aromatic rings of tryptophan (Trp) and phenylalanine (Phe) at the glycine-binding site of the NMDAR [483]. (b) The dependence of the agreement between relative anaesthetic potency and singlet yield ratio on the relationship between relaxation rate, r, and reaction rate, k. The radical pair model can explain the experimentally derived relative anaesthetic potency of xenon, shaded in yellow. (c) Predicted dependence of the anaesthetic potency as given by the singlet yield ratio, based on the radical pair model of 129Xe/130Xe (blue) and 131Xe/130Xe (red) on an external magnetic field [57].
It is also known that ROS are implicated in the activation of the NMDARs [482,488–492]. Moreover, Turin and Skoulakis [493] reported that oxygen gas was necessary for observing spin changes during xenon-induced anaesthesia in Drosophila. Motivated by these observations, the authors [57] suggested that the electron transfer related to xenon’s anaesthetic action that is evidenced by Turin et al. [479] plays a role in the recombination dynamics of a naturally occurring radical pair (see §3.4 for further discussion). Using equations (3.19) and (3.20), they showed that for isotopes of xenon with a non-zero nuclear spin, this nuclear spin couples with (at least one of) the electron spins of such a radical pair, affecting the reaction yields of the radical pair and hence xenon’s anaesthetic action. The radical pair was assumed to start off from a singlet state. Such a mechanism is consistent with the experimental results of Li et al. [384] that xenon isotopes with non-zero nuclear spin have reduced anaesthetic potency compared to isotopes with zero nuclear spin, as shown in figure 4b. The authors also provide an experimental test for the validity of their model (figure 4c). It predicts that under a static magnetic field the anaesthetic potency of xenon may be significantly different than that observed by Li et al. [384], as shown in figure 4c.
4.2. Lithium effects on hyperactivity
Lithium (Li) is the most well-known treatment for bipolar illness [494–499]. Despite its frequent clinical use, the mechanism by which Li exerts its effects remains elusive [500]. Ettenberg and co-workers [383] showed that Li effects on the manic phase in rats are isotope-dependent. They used sub-anaesthetic doses of ketamine to induce hyperactivity which was then treated with lithium. They observed that 6Li produced a longer suppression of mania compared to 7Li. Further, there is a considerable amount of evidence that oxidative stress [130] is implicated in both bipolar disorder [501–509] and its Li treatment [510–513].
Bipolar disorder is also correlated with irregularities in circadian rhythms [514–517]. In addition, it is well known that Li influences the circadian rhythms that are disrupted in patients with bipolar disorders [518–531]. Further, Osland et al. reported that Li significantly enhanced the expression of Per2 and Cry1, while Per3, Cry2, Bmal1, E4BP4 and Rev-Erb-α expression was decreased [532]. However, the exact mechanisms and pathways behind this therapy are incompletely known. It has been shown that Li can exert it effects via a direct action on the suprachiasmatic nucleus (SCN), a circadian pacemaker in the brain [533–536]. Cryptochromes are key proteins for the circadian clock [537] and SCN’s intercellular networks development, which subserves coherent rhythm expression [538]. Furthermore, it is also shown that cryptochrome is associated with bipolar disorder disease [539–542]. In the context of animal magnetoreception, cryptochromes are the canonical magnetic sensing proteins (See §3.4) [43], with flavin radicals playing a key role. Moreover, it has been shown that circadian rhythms are susceptible to magnetic fields at low intensities [115–117,169–171,224,226,353], where cryptochromes [80,225] are implicated. It has also been observed that cryptochromes play key roles in alteration of ROS levels through exposure to magnetic fields [76,141,222,543]. Based on these facts, a new study suggests [59] that Li’s nuclear spin influences the recombination dynamics of S–T interconversion in the naturally occurring radical pairs (figure 5a). These pairs are initially in singlet states, and due to the different nuclear spins, each isotope of Li alters these dynamics differently. Using equations (3.19) and (3.20), the authors showed that a radical pair model could provide results consistent with the experimental finding of Ettenberg and colleagues [383], as shown in figure 5b. In that work, it was assumed that the fractional triplet yield of the radical pairs is correlated with lithium potency. They further predict a magnetic field dependence of the effectiveness of lithium, which provides one potential experimental test of their hypothesis, as shown in figure 5c.
Figure 5.
Radical pair explanation for isotope effects in lithium treatment for hyperactivity. (a) Flavinsemiquinone (FADH.) and lithium superoxide radical pair . (b) The dependence of the agreement between the total travelled distance ratio, TDr, and the triplet yield ratio, TYr of 7Li over 6Li on the radical pair reaction rate, k, and the radical pair spin-coherence relaxation rate, r. The green line indicates the ranges smaller than the experimental uncertainty. (c) The dependence of the triplet yield (red, 6Li; blue, 7Li) and triplet yield ratio 7Li/6Li (green) on an external magnetic field, calculated based on the radical pair model [58].
Furthermore, the authors suggested that the proposed mechanism for Li effects is also plausible via different pathways. Li may exert its effect via competing with magnesium in inhibiting glycogen synthase kinase-3 (GSK-3) [544–546], which is regulated by phosphorylation of inhibitory serine residues [547–549]. GSK-3 phosphorylates the clock components including PER2, CRY1, CLOCK, BMAL1 and REV-ERBα [550–557]. In such cases, the radical pairs could be formed in a [RO. · · · Li(H2O)n.] complex (see §3.4), where RO. is the protein oxy-anion, similar to [65,385,558,559].
4.3. Magnetic field and lithium effects on the circadian clock
The circadian clock is essential for the regulation of a variety of physiological and behavioural processes in nearly all organisms, including Neurospora [560], Arabidopsis [561], Drosophila [562], mouse [563] and humans [564–566]. It is known that the disruption of the circadian clock can be detrimental for many physiological functions, including depression [567,568], metabolic and cardiovascular diseases [569], and cancer [570,571]. It is also known that the circadian clock controls physiological processes such as brain metabolism, ROS homeostasis, hormone secretion, autophagy and stem cell proliferation, which are correlated with ageing, memory formation, and neurodegenerative and sleep disorders [572–576]. In Drosophila, the circadian clock regulates the timing of eclosion, courtship, rest, activity and feeding; it also influences daytime colour [577] and temperature preference [578]. Regardless of the differences in the molecular components of the circadian clocks, their organization, features, and the molecular mechanism that give rise to rhythmicity are very alike across organisms [579].
Environmental zeitgebers such as light, food and temperature can influence the circadian clock’s rhythmicity [580]. The circadian clock is also susceptible to magnetic field exposures [23,74,171,224–226,353,581,582] (see also §2.1.3). Yoshii et al. reported the effects of static magnetic fields with different intensities, [0, 150, 300, 500] μT, on the period changes of Drosophila’s circadian clock under blue light illumination [583]. They showed that the period was altered significantly depending on the strength of the magnetic field, with a maximum change at 300 μT. In that work, the geomagnetic field was shielded, and arrhythmic flies were excluded from the analysis. As discussed in §4.2, the disruption of the circadian clock is associated with bipolar disorders, for which Li is the first-line treatment. Li’s effects on bipolar disorder are isotope-dependent. Dokucu et al. [584] reported that Li lengthened the period of Drosophila’s circadian clock. However, the exact mechanism behind these phenomena is still mostly unknown. Further, ROS homeostasis is correlated to the circadian rhythms [585–591].
A recent study suggests that a radical pair model based on (figure 6b), similar to §4.2, may explain the magnetic field and lithium effects on Drosophila’s circadian clock [59]. Following the work of Tyson et al. [592], the authors used a simple mathematical model for Drosophila’s circadian clock, as shown in figure 6a (for more detailed models see [593]). Similar to the work of Player et al. [594], they introduced the effects of applied magnetic fields and hyperfine interactions on the circadian clock process by modifying the corresponding rate representing the role of cryptochrome’s light activation and, hence, proteolysis of protein. Based on these models and using equations (3.19) and (3.20), they reproduced the experimental findings of the magnetic field [583] and lithium effects [584] on Drosophila’s circadian clock, as shown in figure 6c,d. The proposed model in that work predicts that lithium influences the clock in an isotope-dependent manner and magnetic fields and hyperfine interactions modulate oxidative stress in the circadian clock.
Figure 6.
Radical pair explanation for magnetic field and lithium effects on the circadian clock. (a) A simple model of the circadian clock feedback loop in Drosophila. CLOCK (CLK) and CYCLE (CYC) proteins promote the tim and per genes. PER and TIM proteins first accumulate in the cytoplasm and then enter into the nucleus to block their gene transcription. Upon light absorption CRY binds to TIM and this results in the degradation of TIM [59]. (b) Flavinsemiquinone (FADH.) and superoxide radical pair . The dependence of the period of Drosophila’s circadian clock calculated by the radical pair model on the static magnetic field strength B with (c) and without (d) lithium effects. Higher magnetic field intensities shorten the period of the circadian clock. (c) The effects of Li [purple], 6Li [red], 7Li [blue] and zero Li [black]. The inset indicates the comparison between the effects of Li on the period of the clock calculated by the radical pair model [purple line] and the experimental findings [orange dots with error bars] of [584]. (d) The comparison between the dependence of the period on the applied magnetic field calculated by the radical pair model [black line in the inset of plot (d)] and the experimental findings [green dots with error-bars] of [583]. The results from the radical pair model fit the experimental data within the experimental uncertainty.
4.4. Hypomagnetic field effects on microtubule reorganization
Single-cell organisms perform cognitive activities predominantly by cytoskeletal microtubules and are inhibited by anaesthetic gases even in the absence of synapses or networks [595]. Linganna and colleagues showed that modulation of microtubule stability is a mechanism of action for these anaesthetics [596]. Bernard reported that anaesthetics act directly on cytoplasm, depending on cytoskeletal proteins’ dynamics comprising actin filaments and microtubules [597]. Further, Eckenhoff and co-workers found that anaesthetics bind to actin and tubulin [598,599]. In another study, they show that microtubules play key roles in the action of anaesthetics on protein reaction networks involved in neuronal growth, proliferation, division and communication [600]. Despite the low binding affinity of anaesthetics to tubulin compared to membrane protein, the abundance of tubulin is much more than membrane protein sites. It thus seems plausible that our conscious state of mind is intertwined with microtubules and their dynamics.
In recent decades, it has been proposed that quantum physics may explain the mystery of consciousness. In particular, the holistic character of quantum entanglement might shed more light on the binding problem [601]. Penrose & Hameroff proposed that quantum computations in microtubules may be the basis for consciousness [602–604]. It was suggested that electron resonance transfer among tryptophan residues in tubulin in a quantum electronic process could play a role in consciousness [605]. Computational models show that anaesthetic molecules might bind in the same regions and hence result in loss of consciousness [606]. In a recent work, Zhang and co-workers observed a connection between electronic states and vibrational states in tubulin and microtubules [607]. However, quantum electronic coherence beyond ultrafast timescales has been recently challenged experimentally [30]. In contrast, the coherence of quantum spins can be preserved for much longer timescales [608]. Similarly, Fisher has proposed that phosphorus nuclear spins could be entangled in networks of Posner molecules which could form the basis of a quantum mechanism for neural processing in the brain [609]; however, this sort of spin-based model also demands more supporting evidence [610].
A considerable amount of evidence indicates that magnetic fields can influence microtubules [88,611–617]. Wang and colleagues showed that shielding the geomagnetic field caused tubulin assembly disorder [173]. All these observations point to the magnetosensitivity of microtubules for wide ranges of magnetic field strengths. Further, studies suggest that oxidative stress plays important roles in regulating actin and microtubule dynamics [618]. Microtubules contain tryptophan, Tyr and phenylalanine residues which are susceptible to oxidation. Further, it is also known that the stability of polymerized microtubules is susceptible to changes in zinc ion concentration in neurons [619].
Magnetosensitivty of chemical reactions often involve radical molecules [46]. (See also §3.1.) Using equations (3.19) and (3.20) and a simple kinetic model [619] for dynamics of microtubules, a recent study [60] suggests that a radical pair model in the form of , similar to [57] (see §4.1), may explain the hypomagnetic field effects on microtubule reorganization reported in [173]. They further predict that the effect of zinc on the microtubule density exhibits isotopic dependence, as shown in figure 7.
Figure 7.
Radical pair explanation for hypomagnetic field effects on microtubule organization. (a) Schematic presentation of tryptophan ring and superoxide radicals. (b) The dependence of microtubule density on the applied static magnetic field according to a radical pair model based on complex. The hypomagnetic field causes a strong decrease in microtubule density. The maximum microtubule density occurs around the geomagnetic field. (c) The radical pair model prediction of the microtubule density ratio in the geomagnetic field compared to hypomagnetic field. (d) The predicted dependence of microtubule density on administration of Zn (with zero nuclear spin) [red] and 67Zn (with nuclear spin of ) [blue] as a function of applied magnetic field based on the RP complex of [60].
4.5. Hypomagnetic field effects on neurogenesis
In a recent work, Zhang and co-workers showed that shielding the geomagnetic field for a long period (several weeks) decreased neurogenesis in the hippocampal region in mice [172]. They observed that the neurogenesis impairment was through decreasing adult neuronal stem cell proliferation, altering cell lineages in critical development stages of neurogenesis, impeding dendritic development of newborn neurons in the adult hippocampus, and resulting in impaired cognition. Using transcriptome analysis and endogenous ROS in situ labelling via hydroethidine, they reported that the hypomagnetic fields reduced levels of ROS [130]. The authors further revealed that such a reduction in reactive oxygen species can be compensated by pharmacological inhibition of ROS removal via diethyldithiocarbamate, which rescued defective adult hippocampal neurogenesis in hypomagnetic field-exposed mice.
Moreover, it is known that the cellular production of ROS is susceptible to magnetic field exposure [136,227,620–637]. ROS play vital roles in biology. The mitochondrial ETC and an enzyme family termed NADPH oxidase are two main cellular sources of ROS [130]. The latter is a flavin-containing enzyme. NADPH oxidase enzymes transport electrons from NADPH, through flavin adenine dinucleotide, across the plasma membrane to O2 to produce O−2 [638].
Based on these findings, a recent study [61] suggests that a radical pair model may explain the modulation of ROS production and the attenuation of adult hippocampal neurogenesis in a hypomagnetic field, observed by Zhang and colleagues [172]. The authors proposed that the reduction of the geomagnetic field influences the spin dynamics of the naturally occurring radical pairs in the form of , similar to other studies [58,59,446] (see also §§4.2 and 4.3). They further predict the effects of applied magnetic fields and oxygen isotopic substitution on hippocampal neurogenesis (figure 8)
Figure 8.
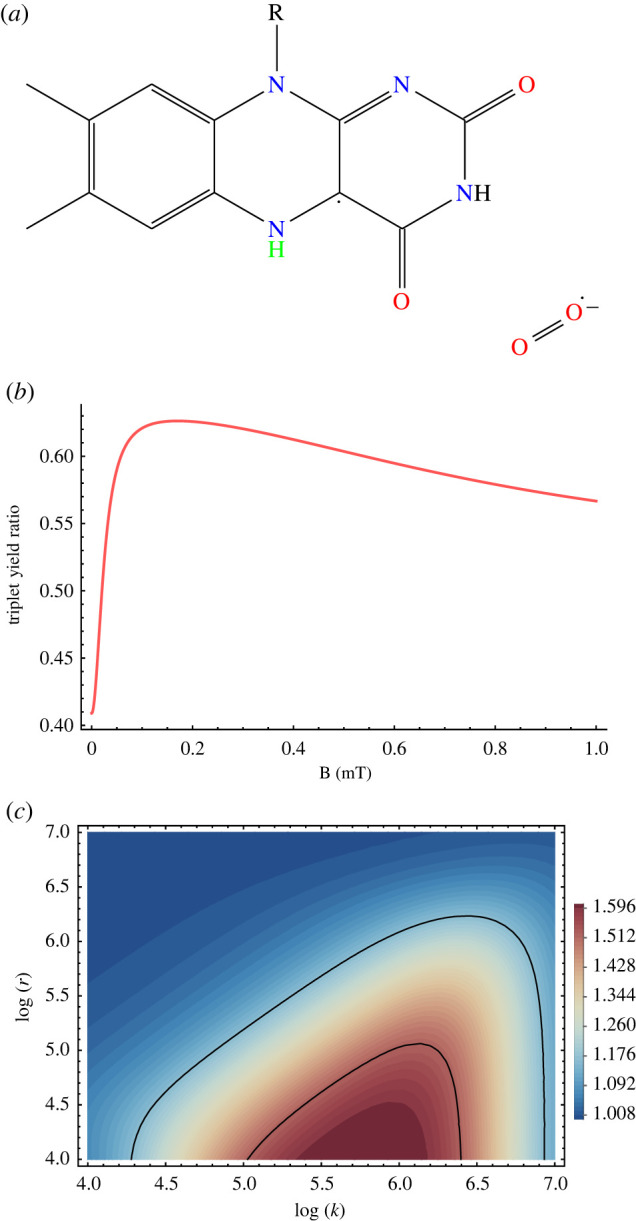
Radical pair explanation for hypomagnetic field effects on hippocampal neurogenesis. (a) radical pair. (b) The dependence of the triplet yield of the radical pair model for singlet-born radical pair on external magnetic field [61]. (c) Triplet yield ratio (geomagnetic field to hypomagnetic field) for singlet-born radical pair in the plan of reaction rate (k) and relaxation rate (r). The region between the solid black lines is in agreement with the experimental range for the ratio of the numbers of BrdU+ cells after eight weeks, observed in [172].
5. Conclusion and outlook
The effects of weak magnetic fields in biology are abundant, including in plants, fungi, animals and humans. The corresponding energies for such effects are far below thermal energies. So far, there is no explanation for such phenomena. However, quantum biology provides a promising explanation for these effects, namely the radical pair mechanism. Here, we have reviewed numerous studies on the biological effects of weak magnetic fields (static and oscillating), as well as related isotope effects. We then reviewed the radical pair mechanism and proposed that it can provide a unified model for weak magnetic field and isotope effects on biology. We discussed candidate radical pairs that may be formed in biological environments. We reviewed recent studies that propose that the radical pair mechanism may explain xenon-induced anaesthesia, lithium effects on mania, magnetic field and lithium effects on the circadian clock, and hypomagnetic field effects on neurogenesis and microtubule reorganization. These recent studies provide avenues for testing the proposed models. For instance, it is proposed that, in xenon anaesthesia, applying magnetic fields over 1mT will increase the anaesthetic potency difference between 129Xe and 131Xe [57]. Similarly, it is predicted that for mania treatment by 6Li and 7Li [58] exposure to hypomagnetic and magnetic fields greater than 3 mT will magnify the difference in the potency of these two isotopes. Moreover, it is predicted that exposure of the circadian clock to magnetic fields >mT will shorten the period of the clock [59]. Another study suggests that exposure to magnetic fields greater than the geomagnetic field will reduce microtubule assembly [60]. Further, it is also predicted that hippocampal neurogenesis [61], the circadian clock [59] and microtubule reorganization [60] will be isotope-dependent using different isotopes of oxygen, lithium and zinc, respectively.
It should be noted that the radical pair models used in the studies that we reviewed in §4 are simplified, partly because the exact radical pair molecules involved in these systems are still unknown [117]. This is the case even in the context of avian magnetoreception, where the proposed radical pairs include flavin–tryptophan, flavin–tyrosin and flavin–superoxide among others [43,433]. More realistic models of the radical pairs may provide further insight into the underlying mechanism behind these phenomena. This might involve including multiple nuclei, dipolar, and exchange interactions in the models. It should also be pointed out that including these interactions can reduce the predicted effect size [61,440]. However, this may be balanced by potential amplification effects in the biological systems [59,594].
It has been pointed out that due to fast molecular rotation, free superoxide has a short spin relaxation lifetime on the order of 1 ns, which means a high spin relaxation rate r [440,446], which is consistent with the scarcity of observations of superoxide radicals by ESR spectroscopy. The required relaxation rates in the discussed projects in §4 are significantly lower than this expected value. However, it has also been argued that the spin relaxation of free superoxide can be reduced if the molecular symmetry is lowered and the angular momentum is quenched by the biological environment [440,446]. Such conditions might occur if the superoxide molecule is tightly bound [446]. It has also been suggested that the involvement of scavenger species around superoxide can reduce its spin relaxation rate [404–406]. These suggested mechanisms are more complex than the simple radical pair mechanism discussed in this review.
Going beyond these already published proposals, it would be of interest to investigate the roles of radical pairs to help explain magnetic field effects on a large variety of physiological functions, including NMDAR activation [228,245], DNA/RNA methylation [174], dopamine dynamics [251,252], flavin autofluorescence [142], epigenetics [260,261] and many others. As discussed earlier in this review, for each of these systems, there are naturally occurring radical pairs that can conceivably act as magnetosensitive agents. However, in all of the mentioned systems, it remains a major open challenge to definitively identify the magnetic sensitive radical pairs as well as the relevant chemical reactions and corresponding kinetic rates. This challenge will require multi-disciplinary collaborations including biologists, chemists and quantum physicists.
It should be noted that reproducibility of weak magnetic field effects in biology has been a challenge [3,31,408,639–641]. There are several studies reporting failed attempts at independent replications of magnetic field effects in biological systems [5,642–646]. However, this problem is not confined to this particular area of the life sciences. For example, a recent analysis of high-impact cancer studies concluded that only five out of 53 papers could be fully reproduced [647]. A lot of these issues are likely due to the complexity of biological systems [648]. Despite these challenges, it seems unlikely that all of the hundreds of magnetic field effects on biological systems that have been reported are erroneous. One of our main goals in writing the present review was to make the scientific community aware of how many of such studies there are, and how far they go beyond the specific and much more well-known context of avian magnetoreception.
Low level (graeter than 10 nT) radio frequencies from ambient anthropogenic sources present in and around laboratory settings have been observed to influence magnetic compass responses in animals as different a song-birds, murine rodents and amphipods [451,649,650]. Further, it is shown that changes in radio frequencies exposure, not just the presence or absence of an RF field, can alter responses to the static field [650,651]. This may also contribute to the reproducibility issues of magnetosensitivity in biology.
A considerable amount of evidence indicates that shielding the geomagnetic field has direct biological consequences, which in some cases could be detrimental. This could also be pertinent for the quest of life on other planets without a magnetic field, including Mars [652,653]. In a similar vein, nowadays almost all species are exposed to magnetic fields at different intensities and frequencies originated by manufactured devices [70,354,654–658]. The effects of magnetic fields on physiological functions are inevitable and could be detrimental. Thus this review and perspective is pertinent to the debate on the putative adverse health effects of environmental magnetic fields. Understanding the underlying mechanism should help to clarify many of these issues.
It would be of interest to further investigate the role of cryptochrome proteins in magnetic sensitivity in biology. However, it is equally important to search for candidate molecules other than cryptochromes that could be involved in magnetosensitivity involving a radical pair mechanism.
It is also of interest to explore other potential mechanisms for magnetosensitivity beyond the radical pair mechanism, such as magnetites. The high sensitivity necessary to detect spatial variation in the inclination (approx. 0.01° km−1) or intensity (3–5 nT km−1) may be relevant to the effects that are discussed in this review [41]. It is well established that migratory birds and sea turtles use a magnetic map for navigation. However, a recent study suggests that a short-range, high-resolution map may be used by vertebrates that move only a few kilometres (newts, deer mice) [659]; this may help explain claims over the years that temporal fluctuations in the magnetic field could provide a zeitgeber for the entrainment of circadian rhythms. The link between high sensitivity responses to the magnetic field and circadian rhythmicity might be relevant to some of the ‘non-specific’ effects discussed in this review. Another interesting avenue for magnetosensitivity is the involvement of scavenger species in the radical pair mechanism, which leads to radical triads [404–406,660].
From a quantum perspective, it would also be of interest to explore the relevance of quantum entanglement [661] in the radical pair models for various magnetic field effects on biological functions [662–664]. This could be particularly interesting in the context of neuroscience, where it has been suggested that the brain might use quantum effects such as entanglement for information processing purposes [605,609,665].
Studying magnetic field and isotope effects in biology is a rich and important interdisciplinary field. The potential essential involvement of quantum effects related to the radical pair mechanism provides an exciting new avenue for further investigation, with the promise of revealing a common underlying mechanism for many of these effects.
Acknowledgements
The authors would like to thank Rishabh, D. Salahub, J. Phillips, W. Nicola, T. Craddock, A. Jones, M. Ahmad, D. Wallace, A. Lewis, W. Beane, R. Sherrard, M. Lohof, J. Mariani and D. Oblak for their input.
Contributor Information
Hadi Zadeh-Haghighi, Email: hadi.zadehhaghighi@ucalgary.ca.
Christoph Simon, Email: csimo@ucalgary.ca.
Data accessibility
This article has no additional data.
Authors' contributions
H.Z.-H.: conceptualization, formal analysis, investigation, methodology, software, visualization, writing—original draft; C.S.: conceptualization, funding acquisition, resources, supervision, writing—review and editing. All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interest.
Funding
This work was supported by the Natural Sciences and Engineering Research Council of Canada.
References
- 1.Ketchen E, Porter W, Bolton N. 1978. The biological effects of magnetic fields on man. Am. Ind. Hyg. Assoc. J. 39, 1-11. ( 10.1080/0002889778507706) [DOI] [PubMed] [Google Scholar]
- 2.Barnes FS, Greenebaum B (eds). 2018. Handbook of biological effects of electromagnetic fields. Boca Raton, FL: CRC Press. [Google Scholar]
- 3.Jones AR. 2016. Magnetic field effects in proteins. Mol. Phys. 114, 1691-1702. ( 10.1080/00268976.2016.1149631) [DOI] [Google Scholar]
- 4.Dini L, Abbro L. 2005. Bioeffects of moderate-intensity static magnetic fields on cell cultures. Micron 36, 195-217. ( 10.1016/j.micron.2004.12.009) [DOI] [PubMed] [Google Scholar]
- 5.Albuquerque WWC, Costa RMPB, de Salazar e Fernandes T, Porto ALF. 2016. Evidences of the static magnetic field influence on cellular systems. Prog. Biophys. Mol. Biol. 121, 16-28. ( 10.1016/j.pbiomolbio.2016.03.003) [DOI] [PubMed] [Google Scholar]
- 6.Fan Y, Ji X, Zhang L, Zhang X. 2021. The analgesic effects of static magnetic fields. Bioelectromagnetics 42, 115-127. ( 10.1002/bem.22323) [DOI] [PubMed] [Google Scholar]
- 7.Shupak NM, Prato FS, Thomas AW. 2003. Therapeutic uses of pulsed magnetic-field exposure: a review. URSI Radio Sci. Bull. 2003, 9-32. [Google Scholar]
- 8.Marycz K, Kornicka K, Röcken M. 2018. Static magnetic field (SMF) as a regulator of stem cell fate—new perspectives in regenerative medicine arising from an underestimated tool. Stem Cell Rev. Rep. 14, 785-792. ( 10.1007/s12015-018-9847-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gartzke J, Lange K. 2002. Cellular target of weak magnetic fields: ionic conduction along actin filaments of microvilli. Am. J. Physiol.-Cell Physiol. 283, C1333-C1346. ( 10.1152/ajpcell.00167.2002) [DOI] [PubMed] [Google Scholar]
- 10.McKay JC, Prato FS, Thomas AW. 2007. A literature review: the effects of magnetic field exposure on blood flow and blood vessels in the microvasculature. Bioelectromagnetics 28, 81-98. ( 10.1002/bem.20284) [DOI] [PubMed] [Google Scholar]
- 11.Markov MS. 2007. Magnetic field therapy: a review. Electromagn. Biol. Med. 26, 1-23. ( 10.1080/15368370600925342) [DOI] [PubMed] [Google Scholar]
- 12.Davanipour Z, Sobel E. 2009. Long-term exposure to magnetic fields and the risks of Alzheimer’s disease and breast cancer: further biological research. Pathophysiology 16, 149-156. ( 10.1016/j.pathophys.2009.01.005) [DOI] [PubMed] [Google Scholar]
- 13.Radhakrishnan R. 2019. Magnetic field regulates plant functions, growth and enhances tolerance against environmental stresses. Physiol. Mol. Biol. Plants 25, 1107-1119. ( 10.1007/s12298-019-00699-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saunders R. 2005. Static magnetic fields: animal studies. Prog. Biophys. Mol. Biol. 87, 225-239. ( 10.1016/j.pbiomolbio.2004.09.001) [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Zhang X. 2017. Magnetic fields and reactive oxygen species. Int. J. Mol. Sci. 18, 2175. ( 10.3390/ijms18102175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vergallo C, Dini L. 2018. Comparative analysis of biological effects induced on different cell types by magnetic fields with magnetic flux densities in the range of 1–60 mT and frequencies up to 50 Hz. Sustainability 10, 2776. ( 10.3390/su10082776) [DOI] [Google Scholar]
- 17.Nyakane NE, Markus ED, Sedibe MM. 2019. The effects of magnetic fields on plants growth: a comprehensive review. ETP Int. J. Food Eng. 5, 79-87. ( 10.18178/ijfe.5.1.79-87) [DOI] [Google Scholar]
- 18.Sarraf M, Kataria S, Taimourya H, Santos LO, Menegatti RD, Jain M, Ihtisham M, Liu S. 2020. Magnetic field (MF) applications in plants: an overview. Plants 9, 1139. ( 10.3390/plants9091139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maffei ME. 2014. Magnetic field effects on plant growth, development, and evolution. Front. Plant Sci. 5, 445. ( 10.3389/fpls.2014.00445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villa M, Mustarelli P, Caprotti M. 1991. Minireview biological effects of magnetic fields. Life Sci. 49, 85-92. ( 10.1016/0024-3205(91)90021-3) [DOI] [PubMed] [Google Scholar]
- 21.Binhi VN, Prato FS. 2017. Biological effects of the hypomagnetic field: an analytical review of experiments and theories. PLoS ONE 12, e0179340. ( 10.1371/journal.pone.0179340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Yarema K, Xu A. 2017. Biological effects of static magnetic fields. Singapore: Springer. [Google Scholar]
- 23.Xue X, Ali YF, Luo W, Liu C, Zhou G, Liu NA. 2021. Biological effects of space hypomagnetic environment on circadian rhythm. Front. Physiol. 12, 643943. ( 10.3389/fphys.2021.643943) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mo W, Liu Y, Bartlett PF, He R. 2014. Transcriptome profile of human neuroblastoma cells in the hypomagnetic field. Sci. China Life Sci. 57, 448-461. ( 10.1007/s11427-014-4644-z) [DOI] [PubMed] [Google Scholar]
- 25.Schulten K, Swenberg CE, Weller A. 1978. A biomagnetic sensory mechanism based on magnetic field modulated coherent electron spin motion. Zeitschrift für Physikalische Chemie 111, 1-5. ( 10.1524/zpch.1978.111.1.001) [DOI] [Google Scholar]
- 26.Steiner UE, Ulrich T. 1989. Magnetic field effects in chemical kinetics and related phenomena. Chem. Rev. 89, 51-147. ( 10.1021/cr00091a003) [DOI] [Google Scholar]
- 27.Mohseni M, Omar Y, Engel GS, Plenio MB. (eds) 2009. Quantum effects in biology. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 28.Lambert N, Chen YN, Cheng YC, Li CM, Chen GY, Nori F. 2012. Quantum biology. Nat. Phys. 9, 10-18. ( 10.1038/nphys2474) [DOI] [Google Scholar]
- 29.Ball P. 2011. Physics of life: the dawn of quantum biology. Nature 474, 272-274. ( 10.1038/474272a) [DOI] [PubMed] [Google Scholar]
- 30.Cao J, et al. 2020. Quantum biology revisited. Sci. Adv. 6, eaaz4888. ( 10.1126/sciadv.aaz4888) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim Y, et al. 2021. Quantum biology: an update and perspective. Q. Rep. 3, 80-126. ( 10.3390/quantum3010006) [DOI] [Google Scholar]
- 32.Wiltschko R, Wiltschko W. 1995. Magnetic orientation in animals. Berlin, Germany: Springer. [Google Scholar]
- 33.Wiltschko W, Wiltschko R. 1972. Magnetic compass of European robins. Science 176, 62-64. ( 10.1126/science.176.4030.62) [DOI] [PubMed] [Google Scholar]
- 34.Cochran WW, Mouritsen H, Wikelski M. 2004. Migrating songbirds recalibrate their magnetic compass daily from twilight cues. Science 304, 405-408. ( 10.1126/science.1095844) [DOI] [PubMed] [Google Scholar]
- 35.Zapka M, et al. 2009. Visual but not trigeminal mediation of magnetic compass information in a migratory bird. Nature 461, 1274-1277. ( 10.1038/nature08528) [DOI] [PubMed] [Google Scholar]
- 36.Wiltschko W. 2010. Über den einfluß statischer magnetfelder auf die zugorientierung der rotkehlchen (Erithacus rubecula). Zeitschrift für Tierpsychologie 25, 537-558. ( 10.1111/j.1439-0310.1968.tb00028.x) [DOI] [PubMed] [Google Scholar]
- 37.Mouritsen H. 2018. Long-distance navigation and magnetoreception in migratory animals. Nature 558, 50-59. ( 10.1038/s41586-018-0176-1) [DOI] [PubMed] [Google Scholar]
- 38.Wiltschko R, Nießner C, Wiltschko W. 2021. The magnetic compass of birds: the role of cryptochrome. Front. Physiol. 12, 667000. ( 10.3389/fphys.2021.667000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mouritsen H. 2022. Magnetoreception in birds and its use for long-distance migration. In Sturkie’s Avian Physiology, pp. 233–256. London, UK: Elsevier. ( 10.1016/b978-0-12-819770-7.00040-2) [DOI]
- 40.Kirschvink J. 2001. Magnetite-based magnetoreception. Curr. Opin Neurobiol. 11, 462-467. ( 10.1016/s0959-4388(00)00235-x) [DOI] [PubMed] [Google Scholar]
- 41.Freake MJ, Muheim R, Phillips JB. 2006. Magnetic maps in animals: a theory comes of age? Q Rev. Biol. 81, 327-347. ( 10.1086/511528) [DOI] [PubMed] [Google Scholar]
- 42.Nimpf S, Keays DA. 2022. Myths in magnetosensation. iScience 25, 104454. ( 10.1016/j.isci.2022.104454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hore PJ, Mouritsen H. 2016. The radical-pair mechanism of magnetoreception. Annu. Rev. Biophys. 45, 299-344. ( 10.1146/annurev-biophys-032116-094545) [DOI] [PubMed] [Google Scholar]
- 44.Wiltschko R, Wiltschko W. 2019. Magnetoreception in birds. J. R. Soc. Interface 16, 20190295. ( 10.1098/rsif.2019.0295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grissom CB. 1995. Magnetic field effects in biology: a survey of possible mechanisms with emphasis on radical-pair recombination. Chem. Rev. 95, 3-24. ( 10.1021/cr00033a001) [DOI] [Google Scholar]
- 46.Rodgers CT, Hore PJ. 2009. Chemical magnetoreception in birds: the radical pair mechanism. Proc. Natl Acad. Sci. USA 106, 353-360. ( 10.1073/pnas.0711968106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hiscock HG, Worster S, Kattnig DR, Steers C, Jin Y, Manolopoulos DE, Mouritsen H, Hore PJ. 2016. The quantum needle of the avian magnetic compass. Proc. Natl Acad. Sci. USA 113, 4634-4639. ( 10.1073/pnas.1600341113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu J, et al. 2021. Magnetic sensitivity of cryptochrome 4 from a migratory songbird. Nature 594, 535-540. ( 10.1038/s41586-021-03618-9) [DOI] [PubMed] [Google Scholar]
- 49.Wong SY, Frederiksen A, Hanic M, Schuhmann F, Grüning G, Hore PJ, Solov’yov IA. 2021. Navigation of migratory songbirds: a quantum magnetic compass sensor. Neuroforum 27, 141-150. ( 10.1515/nf-2021-0005) [DOI] [Google Scholar]
- 50.Timmel C, Till U, Brocklehurst B, Mclauchlan K, Hore P. 1998. Effects of weak magnetic fields on free radical recombination reactions. Mol. Phys. 95, 71-89. ( 10.1080/00268979809483134) [DOI] [PubMed] [Google Scholar]
- 51.Schulten K, Staerk H, Weller A, Werner H-J, Nickel B. 1976. Magnetic field dependence of the geminate recombination of radical ion pairs in polar solvents. Zeitschrift für Physikalische Chemie 101, 371-390. ( 10.1524/zpch.1976.101.1-6.371) [DOI] [Google Scholar]
- 52.Hore PJ, Ivanov KL, Wasielewski MR. 2020. Spin chemistry. J. Chem. Phys. 152, 120401. ( 10.1063/5.0006547) [DOI] [PubMed] [Google Scholar]
- 53.Woodward JR. 2002. Radical pairs in solution. Prog. React. Kinetics Mech. 27, 165-207. ( 10.3184/007967402103165388) [DOI] [Google Scholar]
- 54.Rodgers CT. 2009. Magnetic field effects in chemical systems. Pure Appl. Chem. 81, 19-43. ( 10.1351/pac-con-08-10-18) [DOI] [Google Scholar]
- 55.Zhang Y, Liang C, Wu J, Liu H, Zhang B, Jiang Z, Li S, Xu P. 2020. Recent advances in magnetic field-enhanced electrocatalysis. ACS Appl. Energy Mater. 3, 10 303-10 316. ( 10.1021/acsaem.0c02104) [DOI] [Google Scholar]
- 56.Beretta G, Mastorgio AF, Pedrali L, Saponaro S, Sezenna E. 2019. The effects of electric, magnetic and electromagnetic fields on microorganisms in the perspective of bioremediation. Rev. Environ. Sci. Bio/Technol. 18, 29-75. ( 10.1007/s11157-018-09491-9) [DOI] [Google Scholar]
- 57.Smith J, Zadeh-Haghighi H, Salahub D, Simon C. 2021. Radical pairs may play a role in xenon-induced general anesthesia. Sci. Rep. 11, 1-13. ( 10.1038/s41598-021-85673-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zadeh-Haghighi H, Simon C. 2021. Entangled radicals may explain lithium effects on hyperactivity. Sci. Rep. 11, 1-10. ( 10.1038/s41598-021-91388-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zadeh-Haghighi H, Simon C. 2022. Radical pairs can explain magnetic field and lithium effects on the circadian clock. Sci. Rep. 12, 269. ( 10.1038/s41598-021-04334-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zadeh-Haghighi H, Simon C. 2022. Radical pairs may play a role in microtubule reorganization. Sci. Rep. 12, 1-11. ( 10.1038/s41598-022-10068-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rishabh R, Zadeh-Haghighi H, Salahub D, Simon C. 2022. Radical pairs may explain reactive oxygen species-mediated effects of hypomagnetic field on neurogenesis. PLoS Comput. Biol. 18, e1010198. ( 10.1371/journal.pcbi.1010198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Repacholi MH, Greenebaum B. 1999. Interaction of static and extremely low frequency electric and magnetic fields with living systems: health effects and research needs. Bioelectromagnetics 20, 133-160. () [DOI] [PubMed] [Google Scholar]
- 63.Galland P, Pazur A. 2005. Magnetoreception in plants. J. Plant Res. 118, 371-389. ( 10.1007/s10265-005-0246-y) [DOI] [PubMed] [Google Scholar]
- 64.Pazur A, Schimek C, Galland P. 2007. Magnetoreception in microorganisms and fungi. Open Life Sci. 2, 597-659. ( 10.2478/s11535-007-0032-z) [DOI] [Google Scholar]
- 65.Buchachenko AL. 2014. Magnetic field-dependent molecular and chemical processes in biochemistry, genetics and medicine. Russ. Chem. Rev. 83, 1-12. ( 10.1070/rc2014v083n01abeh004335) [DOI] [Google Scholar]
- 66.Lai H. 2019. Exposure to static and extremely-low frequency electromagnetic fields and cellular free radicals. Electromagn. Biol. Med. 38, 231-248. ( 10.1080/15368378.2019.1656645) [DOI] [PubMed] [Google Scholar]
- 67.Guerra MF, Lacoste MG, Anzulovich AC, Makinistian L. 2019. Magnetic fields, cancer and circadian rhythms: hypotheses on the relevance of intermittence and cycling. Proc. R. Soc. B 286, 20192337. ( 10.1098/rspb.2019.2337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Binhi VN, Rubin AB. 2022. Theoretical concepts in magnetobiology after 40 years of research. Cells 11, 274. ( 10.3390/cells11020274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bertea CM, Narayana R, Agliassa C, Rodgers CT, Maffei ME. 2015. Geomagnetic field (Gmf) and plant evolution: investigating the effects of gmf reversal on Arabidopsis thaliana development and gene expression. J. Vis. Exp. 105, e53286. ( 10.3791/53286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maffei ME. 2022. Magnetic fields and cancer: epidemiology, cellular biology, and theranostics. Int. J. Mol. Sci. 23, 1339. ( 10.3390/ijms23031339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maeda K, et al. 2012. Magnetically sensitive light-induced reactions in cryptochrome are consistent with its proposed role as a magnetoreceptor. Proc. Natl Acad. Sci. USA 109, 4774-4779. ( 10.1073/pnas.1118959109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ahmad M, Galland P, Ritz T, Wiltschko R, Wiltschko W. 2006. Magnetic intensity affects cryptochrome-dependent responses in Arabidopsis thaliana. Planta 225, 615-624. ( 10.1007/s00425-006-0383-0) [DOI] [PubMed] [Google Scholar]
- 73.Sheppard DMW, et al. 2017. Millitesla magnetic field effects on the photocycle of an animal cryptochrome. Sci. Rep. 7, 1-17. ( 10.1038/srep42228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marley R, Giachello CNG, Scrutton NS, Baines RA, Jones AR. 2014. Cryptochrome-dependent magnetic field effect on seizure response in Drosophila larvae. Sci. Rep. 4, 1-14. ( 10.1038/srep05799) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Foley LE, Gegear RJ, Reppert SM. 2011. Human cryptochrome exhibits light-dependent magnetosensitivity. Nat. Commun. 2, 1-3. ( 10.1038/ncomms1364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pooam M, Arthaut LD, Burdick D, Link J, Martino CF, Ahmad M. 2018. Magnetic sensitivity mediated by the arabidopsis blue-light receptor cryptochrome occurs during flavin reoxidation in the dark. Planta 249, 319-332. ( 10.1007/s00425-018-3002-y) [DOI] [PubMed] [Google Scholar]
- 77.Hammad M, Albaqami M, Pooam M, Kernevez E, Witczak J, Ritz T, Martino C, Ahmad M. 2020. Cryptochrome mediated magnetic sensitivity in arabidopsis occurs independently of light-induced electron transfer to the flavin. Photochem. Photobiol. Sci. 19, 341-352. ( 10.1039/c9pp00469f) [DOI] [PubMed] [Google Scholar]
- 78.Giorgi G, Guerra D, Pezzoli C, Cavicchi S, Bersani F. 1992. Genetic effects of static magnetic fields. body size increase and lethal mutations induced in populations of Drosophila melanogaster after chronic exposure. Genet. Sel. Evol. 24, 393. ( 10.1186/1297-9686-24-5-393) [DOI] [Google Scholar]
- 79.Stamenković-Radak M, Kitanović I, Prolić Z, Tomišić I, Stojković B, Andjelković M. 2001. Effect of a permanent magnetic field on wing size parameters in Drosophila melanogaster. Bioelectromagnetics 22, 365-369. ( 10.1002/bem.63) [DOI] [PubMed] [Google Scholar]
- 80.Yoshii T, Ahmad M, Helfrich-Förster C. 2009. Cryptochrome mediates light-dependent magnetosensitivity of Drosophila’s circadian clock. PLoS Biol. 7, e1000086. ( 10.1371/journal.pbio.1000086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huizen AVV, et al. 2019. Weak magnetic fields alter stem cell-mediated growth. Sci. Adv. 5, eaau7201. ( 10.1126/sciadv.aau7201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zheng L, Zhang L, Chen L, Jiang J, Zhou X, Wang M, Fan Y. 2018. Static magnetic field regulates proliferation, migration, differentiation and YAP/TAZ activation of human dental pulp stem cells. J. Tissue Eng. Regen. Med. 12, 2029-2040. ( 10.1002/term.2737) [DOI] [PubMed] [Google Scholar]
- 83.Tavasoli Z, Abdolmaleki P, Mowla SJ, Ghanati F, Sarvestani AS. 2009. Investigation of the effects of static magnetic field on apoptosis in bone marrow stem cells of rat. Environmentalist 29, 220-224. ( 10.1007/s10669-008-9210-4) [DOI] [Google Scholar]
- 84.Jouni FJ, Abdolmaleki P, Movahedin M. 2013. Investigation on the effect of static magnetic field up to 15 mT on the viability and proliferation rate of rat bone marrow stem cells. In Vitro Cell. Dev. Biol. Anim. 49, 212-219. ( 10.1007/s11626-013-9580-x) [DOI] [PubMed] [Google Scholar]
- 85.Jouni FJ, Abdolmaleki P, Behmanesh M, Movahedin M. 2014. An in vitro study of the impact of 4mT static magnetic field to modify the differentiation rate of rat bone marrow stem cells into primordial germ cells. Differentiation 87, 230-237. ( 10.1016/j.diff.2014.06.001) [DOI] [PubMed] [Google Scholar]
- 86.Fanelli C, Coppola S, Barone R, Colussi C, Gualandi G, Volpe P, Ghibelli L. 1999. Magnetic fields increase cell survival by inhibiting apoptosis via modulation of Ca2+ influx. FASEB J. 13, 95-102. ( 10.1096/fasebj.13.1.95) [DOI] [PubMed] [Google Scholar]
- 87.Markov M, Pilla A. 1997. Weak static magnetic field modulation of myosin phosphorylation in a cell-free preparation: calcium dependence. Bioelectrochem. Bioenerg. 43, 233-238. ( 10.1016/s0302-4598(96)02226-x) [DOI] [Google Scholar]
- 88.Tenuzzo B, Chionna A, Panzarini E, Lanubile R, Tarantino P, Jeso BD, Dwikat M, Dini L. 2006. Biological effects of 6 mT static magnetic fields: a comparative study in different cell types. Bioelectromagnetics 27, 560-577. ( 10.1002/bem.20252) [DOI] [PubMed] [Google Scholar]
- 89.Tenuzzo B, Vergallo C, Dini L. 2009. Effect of 6 mT static magnetic field on the bcl-2, bax, p53 and hsp70 expression in freshly isolated and in vitro aged human lymphocytes. Tissue Cell 41, 169-179. ( 10.1016/j.tice.2008.09.004) [DOI] [PubMed] [Google Scholar]
- 90.Chionna A, Tenuzzo B, Panzarini E, Dwikat MB, Abbro L, Dini L. 2005. Time dependent modifications of hep G2 cells during exposure to static magnetic fields. Bioelectromagnetics 26, 275-286. ( 10.1002/bem.20081) [DOI] [PubMed] [Google Scholar]
- 91.McLean MJ, Holcomb RR, Wamil AW, Pickett JD, Cavopol AV. 1995. Blockade of sensory neuron action potentials by a static magnetic field in the 10 mT range. Bioelectromagnetics 16, 20-32. ( 10.1002/bem.2250160108) [DOI] [PubMed] [Google Scholar]
- 92.Weintraub MI, et al. 2003. Static magnetic field therapy for symptomatic diabetic neuropathy: a randomized, double-blind, placebo-controlled trial. Arch. Phys. Med. Rehabil. 84, 736-746. ( 10.1016/s0003-9993(03)00106-0) [DOI] [PubMed] [Google Scholar]
- 93.Calabrò E, Condello S, Curro M, Ferlazzo N, Caccamo D, Magazu S, Ientile R. 2013. Effects of low intensity static magnetic field on FTIR spectra and ros production in SH-SY5Y neuronal-like cells. Bioelectromagnetics 34, 618-629. ( 10.1002/bem.21815) [DOI] [PubMed] [Google Scholar]
- 94.Vergallo C, Ahmadi M, Mobasheri H, Dini L. 2014. Impact of inhomogeneous static magnetic field (31.7–232.0 mT) exposure on human neuroblastoma SH-SY5Y cells during cisplatin administration. PLoS ONE 9, e113530. ( 10.1371/journal.pone.0113530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bekhite MM, Finkensieper A, Abou–Zaid FA, El-Shourbagy IK, Omar KM, Figulla HR, Sauer H, Wartenberg M. 2010. Static electromagnetic fields induce vasculogenesis and chondro-osteogenesis of mouse embryonic stem cells by reactive oxygen species-mediated up-regulation of vascular endothelial growth factor. Stem Cells Dev. 19, 731-743. ( 10.1089/scd.2008.0266) [DOI] [PubMed] [Google Scholar]
- 96.Bekhite MM, Figulla H-R, Sauer H, Wartenberg M. 2013. Static magnetic fields increase cardiomyocyte differentiation of Flk − 1+ cells derived from mouse embryonic stem cells via Ca2+ influx and ROS production. Int. J. Cardiol. 167, 798-808. ( 10.1016/j.ijcard.2012.02.020) [DOI] [PubMed] [Google Scholar]
- 97.Sullivan K, Balin AK, Allen RG. 2010. Effects of static magnetic fields on the growth of various types of human cells. Bioelectromagnetics 32, 140-147. ( 10.1002/bem.20624) [DOI] [PubMed] [Google Scholar]
- 98.Martino CF, Castello PR. 2011. Modulation of hydrogen peroxide production in cellular systems by low level magnetic fields. PLoS ONE 6, e22753. ( 10.1371/journal.pone.0022753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Poniedziałek B, Rzymski P, Karczewski J, Jaroszyk F, Wiktorowicz K. 2013. Reactive oxygen species (ROS) production in human peripheral blood neutrophils exposed in vitro to static magnetic field. Electromagn. Biol. Med. 32, 560-568. ( 10.3109/15368378.2013.773910) [DOI] [PubMed] [Google Scholar]
- 100.Verdom BH, Abdolmaleki P, Behmanesh M. 2018. The static magnetic field remotely boosts the efficiency of doxorubicin through modulating ROS behaviors. Sci. Rep. 8, 1-12. ( 10.1038/s41598-018-19247-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Carter CS, et al. 2020. Exposure to static magnetic and electric fields treats type 2 diabetes. Cell Metab. 32, 561-574.e7. ( 10.1016/j.cmet.2020.09.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yu B, Liu J, Cheng J, Zhang L, Song C, Tian X, Fan Y, Lv Y, Zhang X. 2021. A static magnetic field improves iron metabolism and prevents high-fat-diet/streptozocin-induced diabetes. The Innovation 2, 100077. ( 10.1016/j.xinn.2021.100077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sheu S-S, Beutner G, Yuh H-J, Goldenberg I, Moss A. 2022. Low intensity magnetic fields stimulate the electron transport chain in heart mitochondria. Biophys. J. 121, 508a. ( 10.1016/j.bpj.2021.11.224) [DOI] [Google Scholar]
- 104.Antill LM, Woodward JR. 2018. Flavin adenine dinucleotide photochemistry is magnetic field sensitive at physiological pH. J. Phys. Chem. Lett. 9, 2691-2696. ( 10.1021/acs.jpclett.8b01088) [DOI] [PubMed] [Google Scholar]
- 105.Buchachenko AL, Kuznetsov DA. 2008. Magnetic field affects enzymatic ATP synthesis. J. Am. Chem. Soc. 130, 12868-12869. ( 10.1021/ja804819k) [DOI] [PubMed] [Google Scholar]
- 106.Ercan I, Tombuloglu H, Alqahtani N, Alotaibi B, Bamhrez M, Alshumrani R, Ozcelik S, Kayed TS. 2022. Magnetic field effects on the magnetic properties, germination, chlorophyll fluorescence, and nutrient content of barley (Hordeum vulgare L.). Plant Physiol. Biochem. 170, 36-48. ( 10.1016/j.plaphy.2021.11.033) [DOI] [PubMed] [Google Scholar]
- 107.Sahebjamei H, Abdolmaleki P, Ghanati F. 2006. Effects of magnetic field on the antioxidant enzyme activities of suspension-cultured tobacco cells. Bioelectromagnetics 28, 42-47. ( 10.1002/bem.20262) [DOI] [PubMed] [Google Scholar]
- 108.Hao Q, Wenfang C, Xia A, Qiang W, Ying L, Kun Z, Runguang S. 2010. Effects of a moderate-intensity static magnetic field and adriamycin on K562 cells. Bioelectromagnetics 32, 191-199. ( 10.1002/bem.20625) [DOI] [PubMed] [Google Scholar]
- 109.Atak Ç, Çelik O, Olgun A, Alikamanoğlu S, Rzakoulieva A. 2007. Effect of magnetic field on peroxidase activities of soybean tissue culture. Biotechnol. Biotechnol. Equip. 21, 166-171. ( 10.1080/13102818.2007.10817438) [DOI] [Google Scholar]
- 110.Teodori L, Grabarek J, Smolewski P, Ghibelli L, Bergamaschi A, De Nicola M, Darzynkiewicz Z. 2002. Exposure of cells to static magnetic field accelerates loss of integrity of plasma membrane during apoptosis. Cytometry 49, 113-118. ( 10.1002/cyto.10160) [DOI] [PubMed] [Google Scholar]
- 111.Pagliara P, Lanubile R, Dwikat M, Abbro L, Dini L. 2009. Differentiation of monocytic U937 cells under static magnetic field exposure. Eur. J. Histochem. 49, 75. ( 10.4081/930) [DOI] [PubMed] [Google Scholar]
- 112.Buemi M, et al. 2001. Cell proliferation/cell death balance in renal cell cultures after exposure to a static magnetic field. Nephron 87, 269-273. ( 10.1159/000045925) [DOI] [PubMed] [Google Scholar]
- 113.Nagy P, Fischl G. 2004. Effect of static magnetic field on growth and sporulation of some plant pathogenic fungi. Bioelectromagnetics 25, 316-318. ( 10.1002/bem.20015) [DOI] [PubMed] [Google Scholar]
- 114.McCann J, Dietrich F, Rafferty C. 1998. The genotoxic potential of electric and magnetic fields: an update. Mutat. Res./Rev. Mutat. Res. 411, 45-86. ( 10.1016/s1383-5742(98)00006-4) [DOI] [PubMed] [Google Scholar]
- 115.Close JP. 2014. The compass within the clock—part 1: the hypothesis of magnetic fields as secondary zeitgebers to the circadian system-logical and scientific objections. Hypothesis 12, e1. ( 10.5779/hypothesis.v12i1.359) [DOI] [Google Scholar]
- 116.Close JP. 2014. The compass within the clock—part 2: does cryptochrome radical-pair based signalling contribute to the temperature-robustness of circadian systems? Hypothesis 12, e3. ( 10.5779/hypothesis.v12i1.360) [DOI] [Google Scholar]
- 117.Bradlaugh AA, Fedele G, Munro AL, Hansen CN, Kyriacou CP, Jones AR, Rosato E, Baines RA. 2021. Essential elements of radical pair magnetosensitivity in Drosophila. 2021.10.29.466426. ( 10.1101/2021.10.29.466426). [DOI]
- 118.Pineda-Pardo JA, Obeso I, Guida P, Dileone M, Strange BA, Obeso JA, Oliviero A, Foffani G. 2019. Static magnetic field stimulation of the supplementary motor area modulates resting-state activity and motor behavior. Commun. Biol. 2, 1-13. ( 10.1038/s42003-019-0643-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Welker HA, Semm P, Willig RP, Commentz JC, Wiltschko W, Vollrath L. 1983. Effects of an artificial magnetic field on serotonin N-acetyltransferase activity and melatonin content of the rat pineal gland. Exp. Brain Res. 50–50, 426-432. ( 10.1007/bf00239209) [DOI] [PubMed] [Google Scholar]
- 120.Reiter RJ, Richardson BA. 1992. Magnetic field effects on pineal indoleamine metabolism and possible biological consequences. FASEB J. 6, 2283-2287. ( 10.1096/fasebj.6.6.1544540) [DOI] [PubMed] [Google Scholar]
- 121.Reiter RJ. 1993. Static and extremely low frequency electromagnetic field exposure: reported effects on the circadian production of melatonin. J. Cell. Biochem. 51, 394-403. ( 10.1002/jcb.2400510403) [DOI] [PubMed] [Google Scholar]
- 122.Reiter RJ. 1995. Reported biological consequences related to the suppression of melatonin by electric and magnetic field exposure. Integr. Physiol. Behav. Sci. 30, 314-330. ( 10.1007/bf02691604) [DOI] [PubMed] [Google Scholar]
- 123.Semm P, Schneider T, Vollrath L. 1980. Effects of an Earth-strength magnetic field on electrical activity of pineal cells. Nature 288, 607-608. ( 10.1038/288607a0) [DOI] [PubMed] [Google Scholar]
- 124.Lerchl A, Nonaka KO, Reiter RJ. 1991. Pineal gland ‘magnetosensitivity’ to static magnetic fields is a consequence of induced electric currents (eddy currents). J. Pineal Res. 10, 109-116. ( 10.1111/j.1600-079x.1991.tb00826.x) [DOI] [PubMed] [Google Scholar]
- 125.Hirai T, Yoneda Y. 2003. Functional alterations in immature cultured rat hippocampal neurons after sustained exposure to static magnetic fields. J. Neurosci. Res. 75, 230-240. ( 10.1002/jnr.10819) [DOI] [PubMed] [Google Scholar]
- 126.Dileone M, et al. 2017. Dopamine-dependent changes of cortical excitability induced by transcranial static magnetic field stimulation in Parkinson’s disease. Sci. Rep. 7, 1-7. ( 10.1038/s41598-017-04254-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Azanza M. 1995. Neuron firing frequency dependence on the static magnetic field intensity. J. Magn. Magn. Mater. 140–144, 1464-1465. ( 10.1016/0304-8853(94)00904-x) [DOI] [Google Scholar]
- 128.Spasić S, Nikolić L, Mutavdžić D, Šaponjić J. 2011. Independent complexity patterns in single neuron activity induced by static magnetic field. Comput. Methods Programs Biomed. 104, 212-218. ( 10.1016/j.cmpb.2011.07.006) [DOI] [PubMed] [Google Scholar]
- 129.Del Seppia C, Ghione S, Luschi P, Ossenkopp KP, Choleris E, Kavaliers M. 2007. Pain perception and electromagnetic fields. Neurosci. Biobehav. Rev. 31, 619-642. ( 10.1016/j.neubiorev.2007.01.003) [DOI] [PubMed] [Google Scholar]
- 130.Sies H, Jones DP. 2020. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 21, 363-383. ( 10.1038/s41580-020-0230-3) [DOI] [PubMed] [Google Scholar]
- 131.Bedard K, Krause K-H. 2007. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 87, 245-313. ( 10.1152/physrev.00044.2005) [DOI] [PubMed] [Google Scholar]
- 132.Moghadam ZM, Henneke P, Kolter J. 2021. From flies to men: ROS and the NADPH oxidase in phagocytes. Front. Cell Dev. Biol. 9, 628991. ( 10.3389/fcell.2021.628991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Murphy MP. 2008. How mitochondria produce reactive oxygen species. Biochem. J. 417, 1-13. ( 10.1042/bj20081386) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hernansanz-Agustın P, Enrıquez JA. 2021. Generation of reactive oxygen species by mitochondria. Antioxidants 10, 415. ( 10.3390/antiox10030415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Okano H. 2008. Effects of static magnetic fields in biology: role of free radicals. Front Biosci. 13, 6106-6125. ( 10.2741/3141) [DOI] [PubMed] [Google Scholar]
- 136.Ghodbane S, Lahbib A, Sakly M, Abdelmelek H. 2013. Bioeffects of static magnetic fields: oxidative stress, genotoxic effects, and cancer studies. BioMed Res. Int. 2013, 1-12. ( 10.1155/2013/602987) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sies H, Berndt C, Jones DP. 2017. Oxidative stress. Annu. Rev. Biochem. 86, 715-748. ( 10.1146/annurev-biochem-061516-045037) [DOI] [PubMed] [Google Scholar]
- 138.Sies H. 2017. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol. 11, 613-619. ( 10.1016/j.redox.2016.12.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sies H. 2020. Oxidative stress: concept and some practical aspects. Antioxidants 9, 852. ( 10.3390/antiox9090852) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.De Nicola M, Cordisco S, Cerella C, Albertini MC, D’AlesSio M, Accorsi A, Bergamaschi A, Magrini A, Ghibelli L. 2006. Magnetic fields protect from apoptosis via redox alteration. Ann. N Y Acad. Sci. 1090, 59-68. ( 10.1196/annals.1378.006) [DOI] [PubMed] [Google Scholar]
- 141.Pooam M, Jourdan N, Esawi ME, Sherrard RM, Ahmad M. 2020. HEK293 cell response to static magnetic fields via the radical pair mechanism may explain therapeutic effects of pulsed electromagnetic fields. PLoS ONE 15, e0243038. ( 10.1371/journal.pone.0243038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ikeya N, Woodward JR. 2021. Cellular autofluorescence is magnetic field sensitive. Proc. Natl Acad. Sci. USA 118, e2018043118. ( 10.1073/pnas.2018043118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Henbest KB, Maeda K, Hore PJ, Joshi M, Bacher A, Bittl R, Weber S, Timmel CR, Schleicher E. 2008. Magnetic-field effect on the photoactivation reaction of Escherichia coli DNA photolyase. Proc. Natl Acad. Sci. USA 105, 14 395-14 399. ( 10.1073/pnas.0803620105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Giachello CN, Scrutton NS, Jones AR, Baines RA. 2016. Magnetic fields modulate blue-light-dependent regulation of neuronal firing by cryptochrome. J. Neurosci. 36, 10 742-10 749. ( 10.1523/jneurosci.2140-16.2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cheun BS, Yi SH, Baik KY, Lim JK, Yoo JS, Shin HW, Soh KS. 2007. Biophoton emission of mdck cell with hydrogen peroxide and 60 Hz AC magnetic field. J. Environ. Biol. 28, 735-740. [PubMed] [Google Scholar]
- 146.Surma SV, Belostotskaya GB, Shchegolev BF, Stefanov VE. 2014. Effect of weak static magnetic fields on the development of cultured skeletal muscle cells. Bioelectromagnetics 35, 537-546. ( 10.1002/bem.21876) [DOI] [PubMed] [Google Scholar]
- 147.Alken P, et al. 2021. International geomagnetic reference field: the thirteenth generation. Earth, Planets Space 73, 49. ( 10.1186/s40623-020-01288-x) [DOI] [Google Scholar]
- 148.Belyavskaya N. 2004. Biological effects due to weak magnetic field on plants. Adv. Space Res. 34, 1566-1574. ( 10.1016/j.asr.2004.01.021) [DOI] [PubMed] [Google Scholar]
- 149.Zhang B, Tian L. 2020. Reactive oxygen species: potential regulatory molecules in response to hypomagnetic field exposure. Bioelectromagnetics 41, 573-580. ( 10.1002/bem.22299) [DOI] [PubMed] [Google Scholar]
- 150.Zhang Z, Xue Y, Yang J, Shang P, Yuan X. 2021. Biological effects of hypomagnetic field: ground-based data for space exploration. Bioelectromagnetics 42, 516-531. ( 10.1002/bem.22360) [DOI] [PubMed] [Google Scholar]
- 151.da Silva JAT, Dobránszki J. 2015. Magnetic fields: how is plant growth and development impacted? Protoplasma 253, 231-248. ( 10.1007/s00709-015-0820-7) [DOI] [PubMed] [Google Scholar]
- 152.Tsetlin VV, Moisa SS, Levinskikh MA, Nefedova EL. 2016. Effect of very small doses of ionizing radiation and hypomagnetic field change physiological characteristics of higher plant seeds. Aerosp. Environ. Med. 50, 51-58. ( 10.21687/0233-528x-2016-50-6-51-58) [DOI] [PubMed] [Google Scholar]
- 153.Raup DM, Sepkoski JJ. 1984. Periodicity of extinctions in the geologic past. Proc. Natl Acad. Sci. USA 81, 801-805. ( 10.1073/pnas.81.3.801) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lipowski A, Lipowska D. 2006. Long-term evolution of an ecosystem with spontaneous periodicity of mass extinctions. Theory Biosci. 125, 67-77. ( 10.1016/j.thbio.2006.01.001) [DOI] [PubMed] [Google Scholar]
- 155.Becker RO. 1963. Relationship of geomagnetic environment to human biology. N. Y. State J. Med. 63, 2215-2219. [PubMed] [Google Scholar]
- 156.Beischer DE. 1971. The null magnetic field as reference for the study of geomagnetic directional effects in animals and man. Ann. N Y Acad. Sci. 188, 324-330. ( 10.1111/j.1749-6632.1971.tb13107.x) [DOI] [PubMed] [Google Scholar]
- 157.Beischer DE, Miller EF -II, Knepton JC. 1967. Exposure of man to low intensity magnetic fields in a coil system, vol. 1018. Pensacola, FL Naval Aerospace Medical Institute. Naval Aviation Medical Center. [Google Scholar]
- 158.Dubrov AP. 1978. The geomagnetic field and life. New York, NY: Springer US. [Google Scholar]
- 159.Rosenbach AJF. 1884. Mikro-organismen bei den Wund-infections-krankheiten des Menschen. Wiesbaden, Germany: JF Bergmann. [Google Scholar]
- 160.Asashima M, Shimada K, Pfeiffer CJ. 1991. Magnetic shielding induces early developmental abnormalities in the newt, Cynops pyrrhogaster. Bioelectromagnetics 12, 215-224. ( 10.1002/bem.2250120403) [DOI] [PubMed] [Google Scholar]
- 161.Osipenko MA, Mezhevikina LM, Krasts IV, Iashin VA, Novikov VV, Fesenko EE. 2008. Influence of ‘zero’ magnetic field on the growth of embryonic cells and primary embryos of mouse in vitro. Biofizika 53, 705-712. [PubMed] [Google Scholar]
- 162.Osipenko MA, Mezhevikina LM, Krasts IV, Yashin VA, Novikov VV, Fesenko EE. 2008. Deterioration of murine embryonic fibroblasts and early embryos upon magnetic field deprivation. Biophysics 53, 317-321. ( 10.1134/s0006350908040167) [DOI] [PubMed] [Google Scholar]
- 163.Mo W, Liu Y, Cooper HM. 2011. Altered development of Xenopus embryos in a hypogeomagnetic field. Bioelectromagnetics 33, 238-246. ( 10.1002/bem.20699) [DOI] [PubMed] [Google Scholar]
- 164.Xu C, Yin X, Lv Y, Wu C, Zhang Y, Song T. 2012. A near-null magnetic field affects cryptochrome-related hypocotyl growth and flowering in Arabidopsis. Adv. Space Res. 49, 834-840. ( 10.1016/j.asr.2011.12.004) [DOI] [Google Scholar]
- 165.Xu C, Wei S, Lu Y, Zhang Y, Chen C, Song T. 2013. Removal of the local geomagnetic field affects reproductive growth in Arabidopsis. Bioelectromagnetics 34, 437-442. ( 10.1002/bem.21788) [DOI] [PubMed] [Google Scholar]
- 166.Wan GJ, Jiang SL, Zhao ZC, Xu JJ, Tao XR, Sword GA, Gao YB, Pan WD, Chen FJ. 2014. Bio-effects of near-zero magnetic fields on the growth, development and reproduction of small brown planthopper, Laodelphax striatellus and brown planthopper, Nilaparvata lugens. J. Insect. Physiol. 68, 7-15. ( 10.1016/j.jinsphys.2014.06.016) [DOI] [PubMed] [Google Scholar]
- 167.Erdmann W, Idzikowski B, Kowalski W, Kosicki JZ, Kaczmarek Ł. 2021. Tolerance of two anhydrobiotic tardigrades Echiniscus testudo and Milnesium inceptum to hypomagnetic conditions. PeerJ 9, e10630. ( 10.7717/peerj.10630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Erdmann W, Idzikowski B, Kowalski W, Szymanski B, Kosicki JZ, Kaczmarek L. 2017. Can the tardigrade Hypsibius dujardini survive in the absence of the geomagnetic field? PLoS ONE 12, e0183380. ( 10.1371/journal.pone.0183380.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Brown FA. 1960. Response to pervasive geophysical factors and the biological clock problem. Cold Spring Harb. Symp. Quant. Biol. 25, 57-71. ( 10.1101/sqb.1960.025.01.007) [DOI] [Google Scholar]
- 170.Wever R. 1970. The effects of electric fields on circadian rhythmicity in men. Life Sci. Space Res. 8, 177-187. [PubMed] [Google Scholar]
- 171.Bliss VL, Heppner FH. 1976. Circadian activity rhythm influenced by near zero magnetic field. Nature 261, 411-412. ( 10.1038/261411a0) [DOI] [PubMed] [Google Scholar]
- 172.Zhang B, Wang L, Zhan A, Wang M, Tian L, Guo W, Pan Y. 2021. Long-term exposure to a hypomagnetic field attenuates adult hippocampal neurogenesis and cognition. Nat. Commun. 12, 1-17. ( 10.1038/s41467-021-21468-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang DL, Wang XS, Xiao R, Liu Y, He RQ. 2008. Tubulin assembly is disordered in a hypogeomagnetic field. Biochem. Biophys. Res. Commun. 376, 363-368. ( 10.1016/j.bbrc.2008.08.156) [DOI] [PubMed] [Google Scholar]
- 174.Baek S, Choi H, Park H, Cho B, Kim S, Kim J. 2019. Effects of a hypomagnetic field on DNA methylation during the differentiation of embryonic stem cells. Sci. Rep. 9, 1-10. ( 10.1038/s41598-018-37372-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ikenaga M, Yoshikawa I, Kojo M, Ayaki T, Ryo H, Ishizaki K, Kato T, Yamamoto H, Hara R. 1997. Mutations induced in Drosophila during space flight. Biol. Sci. Space 11, 346-350. ( 10.2187/bss.11.346) [DOI] [PubMed] [Google Scholar]
- 176.Martino CF, Portelli L, McCabe K, Hernandez M, Barnes F. 2010. Reduction of the earth’s magnetic field inhibits growth rates of model cancer cell lines. Bioelectromagnetics 31, 649-655. ( 10.1002/bem.20606) [DOI] [PubMed] [Google Scholar]
- 177.Belyavskaya N. 2001. Ultrastructure and calcium balance in meristem cells of pea roots exposed to extremely low magnetic fields. Adv. Space Res. 28, 645-650. ( 10.1016/s0273-1177(01)00373-8) [DOI] [PubMed] [Google Scholar]
- 178.Belyaev IY, Alipov YD, Harms-Ringdahl M. 1997. Effects of zero magnetic field on the conformation of chromatin in human cells. Biochim. Biophys. Acta 1336, 465-473. ( 10.1016/s0304-4165(97)00059-7) [DOI] [PubMed] [Google Scholar]
- 179.Conley CC. 1970. A review of the biological effects of very low magnetic fields. Washington, DC: National Aeronautics and Space Administration. [Google Scholar]
- 180.Yan MM, Zhang L, Cheng YX, Sappington TW, Pan WD, Jiang XF. 2021. Effect of a near-zero magnetic field on development and flight of oriental armyworm (Mythimna separata). J. Integr. Agric. 20, 1336-1345. ( 10.1016/s2095-3119(20)63287-7) [DOI] [Google Scholar]
- 181.Sarimov RM, Binhi VN, Milyaev VA. 2008. The influence of geomagnetic field compensation on human cognitive processes. Biophysics 53, 433-441. ( 10.1134/s0006350908050205) [DOI] [PubMed] [Google Scholar]
- 182.Wang GM, Fu JP, Mo WC, Zhang HT, Liu Y, He RQ. 2022. Shielded geomagnetic field accelerates glucose consumption in human neuroblastoma cells by promoting anaerobic glycolysis. Biochem. Biophys. Res. Commun. 601, 101-108. ( 10.1016/j.bbrc.2022.01.114) [DOI] [PubMed] [Google Scholar]
- 183.Verkin B, Bondarenko S, Sheremet V, Tsutsaeva A, Safonova T. 1976. The effects of weak magnetic fields on bacteria. Mikrobiologiia 45, 1067-1070. [PubMed] [Google Scholar]
- 184.Mo WC, Fu JP, Ding HM, Liu Y, Hua Q, He RQ. 2015. Hypomagnetic field alters circadian rhythm and increases algesia in adult male mice. Prog. Biochem. Biophys. 42, 639-646. [Google Scholar]
- 185.Del Seppia C, Luschi P, Ghione S, Crosio E, Choleris E, Papi F. 2000. Exposure to a hypogeomagnetic field or to oscillating magnetic fields similarly reduce stress-induced analgesia in C57 male mice. Life Sci. 66, 1299-1306. ( 10.1016/s0024-3205(00)00437-9) [DOI] [PubMed] [Google Scholar]
- 186.Junfeng L, Qijiu W, Qian W, Jinchang J, Haiqiang J, Yunfang L. 2001. Effect of magnetic free field space (MFFS) on gaba, glycine and taurine of cortex, cerebellum and basilar nucleus in hamster. Sheng wu hua xue yu Sheng wu wu li jin Zhan 28, 358-361. [Google Scholar]
- 187.Choleris E, Del Seppia C, Thomas AW, Luschi P, Ghione S, Moran GR, Prato FS. 2002. Shielding, but not zeroing of the ambient magnetic field reduces stress-induced analgesia in mice. Proc. R. Soc. Lond. B 269, 193-201. ( 10.1098/rspb.2001.1866) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang X, Xu M, Li B, Li D, Jiang J. 2003. Long-term memory was impaired in one-trial passive avoidance task of day-old chicks hatching from hypomagnetic field space. Chin. Sci. Bull. 48, 2454-2457. ( 10.1360/03wc0231) [DOI] [Google Scholar]
- 189.Wang X, Xu M, Li B, Li D, Jiang J. 2003. The taste of one-day-old chicks incubated in hypomagnetic field avoids long-term memory impairment. Sci. Bull. 48, 2042-2045. [Google Scholar]
- 190.Zhang B, Lu H, Xi W, Zhou X, Xu S, Zhang K, Jiang J, Li Y, Guo A. 2004. Exposure to hypomagnetic field space for multiple generations causes amnesia in Drosophila melanogaster. Neurosci. Lett. 371, 190-195. ( 10.1016/j.neulet.2004.08.072) [DOI] [PubMed] [Google Scholar]
- 191.Prato FS, Robertson JA, Desjardins D, Hensel J, Thomas AW. 2005. Daily repeated magnetic field shielding induces analgesia in CD-1 mice. Bioelectromagnetics 26, 109-117. ( 10.1002/bem.20056) [DOI] [PubMed] [Google Scholar]
- 192.Zhang X, Li J-F, Wu Q-J, Li B, Jiang J-C. 2007. Effects of hypomagnetic field on noradrenergic activities in the brainstem of golden hamster. Bioelectromagnetics 28, 155-158. ( 10.1002/bem.20290) [DOI] [PubMed] [Google Scholar]
- 193.Xiao Y, Wang Q, Xu M-L, Jiang J-C, Li B. 2009. Chicks incubated in hypomagnetic field need more exogenous noradrenaline for memory consolidation. Adv. Space Res. 44, 226-232. ( 10.1016/j.asr.2009.04.013) [DOI] [Google Scholar]
- 194.Binhi VN, Sarimov RM. 2009. Zero magnetic field effect observed in human cognitive processes. Electromagn. Biol. Med. 28, 310-315. ( 10.3109/15368370903167246) [DOI] [PubMed] [Google Scholar]
- 195.chuan Mo W, Liu Y, Bartlett PF. 2013. Magnetic shielding accelerates the proliferation of human neuroblastoma cell by promoting G1-phase progression. PLoS ONE 8, e54775. ( 10.1371/journal.pone.0054775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mo WC, Zhang ZJ, Wang DL, Liu Y, Bartlett PF, He RQ. 2016. Shielding of the geomagnetic field alters actin assembly and inhibits cell motility in human neuroblastoma cells. Sci. Rep. 6, 1-17. ( 10.1038/srep22624) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhang HT, Zhang ZJ, Mo WC, Hu PD, Ding HM, Liu Y, Hua Q, He RQ. 2017. Shielding of the geomagnetic field reduces hydrogen peroxide production in human neuroblastoma cell and inhibits the activity of CuZn superoxide dismutase. Protein Cell 8, 527-537. ( 10.1007/s13238-017-0403-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ding H, et al. 2018. Hypomagnetic fields cause anxiety in adult male mice. Bioelectromagnetics 40, 27-32. ( 10.1002/bem.22155) [DOI] [PubMed] [Google Scholar]
- 199.Fu J-P, Mo W-C, Liu Y, Bartlett PF, He R-Q. 2016. Elimination of the geomagnetic field stimulates the proliferation of mouse neural progenitor and stem cells. Protein Cell 7, 624-637. ( 10.1007/s13238-016-0300-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Xue X, Ali YF, Liu C, Hong Z, Luo W, Nie J, Li B, Jiao Y, Liu NA. 2020. Geomagnetic shielding enhances radiation resistance by promoting DNA repair process in human bronchial epithelial cells. Int. J. Mol. Sci. 21, 9304. ( 10.3390/ijms21239304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Leucht T. 1987. Effects of weak magnetic fields on background adaptation in xenopus laevis. Naturwissenschaften 74, 192-194. ( 10.1007/bf00372927) [DOI] [PubMed] [Google Scholar]
- 202.Nedukha O, Kordyum E, Bogatina N, Sobol M, Vorobyeva T, Ovcharenko Y. 2007. The influence of combined magnetic field on the fusion of plant protoplasts. J. Gravit. Physiol. 14, P117-P118. [PubMed] [Google Scholar]
- 203.Tombarkiewicz B. 2008. Effect of long-term geomagnetic field deprivation on the concentration of some elements in the hair of laboratory rats. Environ. Toxicol. Pharmacol. 26, 75-79. ( 10.1016/j.etap.2008.02.003) [DOI] [PubMed] [Google Scholar]
- 204.Martino CF, Castello P. 2012. Modulation of H2O2 production in vitro by low level magnetic fields. FASEB J. 26, e22753. ( 10.1096/fasebj.26.1_supplement.783.3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Fu J-P, Mo W-C, Liu Y, He R-Q. 2016. Decline of cell viability and mitochondrial activity in mouse skeletal muscle cell in a hypomagnetic field. Bioelectromagnetics 37, 212-222. ( 10.1002/bem.21968) [DOI] [PubMed] [Google Scholar]
- 206.Kantserova NP, Krylov VV, Lysenko LA, Ushakova NV, Nemova NN. 2017. Effects of hypomagnetic conditions and reversed geomagnetic field on calcium-dependent proteases of invertebrates and fish. Izvestiya, Atmos. Oceanic Phys. 53, 719-723. ( 10.1134/s0001433817070040) [DOI] [Google Scholar]
- 207.Lai H. 2021. Genetic effects of non-ionizing electromagnetic fields. Electromagn. Biol. Med. 40, 264-273. ( 10.1080/15368378.2021.1881866) [DOI] [PubMed] [Google Scholar]
- 208.Klimek A, Rogalska J. 2021. Extremely low-frequency magnetic field as a stress factor—really detrimental?—Insight into literature from the last decade. Brain Sci. 11, 174. ( 10.3390/brainsci11020174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Karimi A, Moghaddam FG, Valipour M. 2020. Insights in the biology of extremely low-frequency magnetic fields exposure on human health. Mol. Biol. Rep. 47, 5621-5633. ( 10.1007/s11033-020-05563-8) [DOI] [PubMed] [Google Scholar]
- 210.Pall ML. 2013. Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J. Cell. Mol. Med. 17, 958-965. ( 10.1111/jcmm.12088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Riancho J, Sanchez de la Torre JR, Paz-Fajardo L, Limia C, Santurtun A, Cifra M, Kourtidis K, Fdez-Arroyabe P. 2020. The role of magnetic fields in neurodegenerative diseases. Int. J. Biometeorol. 65, 107-117. ( 10.1007/s00484-020-01896-y) [DOI] [PubMed] [Google Scholar]
- 212.Chervyakov AV, Chernyavsky AY, Sinitsyn DO, Piradov MA. 2015. Possible mechanisms underlying the therapeutic effects of transcranial magnetic stimulation. Front. Hum. Neurosci. 9, 303. ( 10.3389/fnhum.2015.00303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Walleczek J. 1992. Electromagnetic field effects on cells of the immune system: the role of calcium signaling 1. FASEB J. 6, 3177-3185. ( 10.1096/fasebj.6.13.1397839) [DOI] [PubMed] [Google Scholar]
- 214.Funk RHW, Fähnle M. 2021. A short review on the influence of magnetic fields on neurological diseases. Front. Biosci.-Scholar 13, 181. ( 10.52586/s561) [DOI] [PubMed] [Google Scholar]
- 215.Moretti J, Rodger J. 2022. A little goes a long way: neurobiological effects of low intensity rTMS and implications for mechanisms of rTMS. Curr. Res. Neurobiol. 3, 100033. ( 10.1016/j.crneur.2022.100033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Schuermann D, Mevissen M. 2021. Manmade electromagnetic fields and oxidative stress-biological effects and consequences for health. Int. J. Mol. Sci. 22, 3772. ( 10.3390/ijms22073772) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nazıroğlu M, Tokat S, Demirci S. 2012. Role of melatonin on electromagnetic radiation-induced oxidative stress and Ca2+ signaling molecular pathways in breast cancer. J. Recept. Signal Transduction 32, 290-297. ( 10.3109/10799893.2012.737002) [DOI] [PubMed] [Google Scholar]
- 218.Simko M. 2007. Cell type specific redox status is responsible for diverse electromagnetic field effects. Curr. Med. Chem. 14, 1141-1152. ( 10.2174/092986707780362835) [DOI] [PubMed] [Google Scholar]
- 219.Morellini N, Grehl S, Tang A, Rodger J, Mariani J, Lohof AM, Sherrard RM. 2014. What does low-intensity rTMS do to the cerebellum? Cerebellum 14, 23-26. ( 10.1007/s12311-014-0617-9) [DOI] [PubMed] [Google Scholar]
- 220.Dufor T, et al. 2019. Neural circuit repair by low-intensity magnetic stimulation requires cellular magnetoreceptors and specific stimulation patterns. Sci. Adv. 5, eaav9847. ( 10.1126/sciadv.aav9847) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lohof AM, Dufor T, Sherrard RM. 2022. Neural circuit repair by low-intensity rTMS. Cerebellum, 1-5. ( 10.1007/s12311-021-01354-4) [DOI] [PubMed] [Google Scholar]
- 222.Sherrard RM, et al. 2018. Low-intensity electromagnetic fields induce human cryptochrome to modulate intracellular reactive oxygen species. PLoS Biol. 16, e2006229. ( 10.1371/journal.pbio.2006229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Boyer M, Baudin P, Stengel C, Valero-Cabre A, Lohof AM, Charpier S, Sherrard RM, Mahon S. 2022. In vivo low-intensity magnetic pulses durably alter neocortical neuron excitability and spontaneous activity. bioRxiv. [DOI] [PubMed]
- 224.Contalbrigo L, Stelletta C, Falcioni L, Casella S, Piccione G, Soffritti M, Morgante M. 2009. Effects of different electromagnetic fields on circadian rhythms of some haematochemical parameters in rats. Biomed. Environ. Sci. 22, 348-353. ( 10.1016/s0895-3988(09)60067-2) [DOI] [PubMed] [Google Scholar]
- 225.Fedele G, et al. 2014. Genetic analysis of circadian responses to low frequency electromagnetic fields in Drosophila melanogaster. PLoS Genet. 10, e1004804. ( 10.1371/journal.pgen.1004804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Manzella N, et al. 2015. Circadian gene expression and extremely low-frequency magnetic fields: an in vitro study. Bioelectromagnetics 36, 294-301. ( 10.1002/bem.21915) [DOI] [PubMed] [Google Scholar]
- 227.Manikonda PK, Rajendra P, Devendranath D, Gunasekaran B, Channakeshava AS, Sashidhar RB, Subramanyam C. 2014. Extremely low frequency magnetic fields induce oxidative stress in rat brain. Gen. Physiol. Biophys. 33, 81-90. ( 10.4149/gpb_2013059) [DOI] [PubMed] [Google Scholar]
- 228.Özgün A, Marote A, Behie LA, Salgado A, Garipcan B. 2019. Extremely low frequency magnetic field induces human neuronal differentiation through NMDA receptor activation. J. Neural Transm. 126, 1281-1290. ( 10.1007/s00702-019-02045-5) [DOI] [PubMed] [Google Scholar]
- 229.Koch CB, Sommarin M, Persson B, Salford L, Eberhardt J. 2003. Interaction between weak low frequency magnetic fields and cell membranes. Bioelectromagnetics 24, 395-402. ( 10.1002/bem.10136) [DOI] [PubMed] [Google Scholar]
- 230.Miyakoshi J, Koji Y, Wakasa T, Takebe H. 1999. Long-term exposure to a magnetic field (5 mT at 60 Hz) increases X-ray-induced mutations. J. Radiat. Res. 40, 13-21. ( 10.1269/jrr.40.13) [DOI] [PubMed] [Google Scholar]
- 231.Koyama S, Nakahara T, Hirose H, Ding GR, Takashima Y, Isozumi Y, Miyakoshi J. 2004. ELF electromagnetic fields increase hydrogen peroxide (H2O2)-induced mutations in pTN89 plasmids. Mut. Res./Genet. Toxicol. Environ. Mutagenesis 560, 27-32. ( 10.1016/j.mrgentox.2004.01.012) [DOI] [PubMed] [Google Scholar]
- 232.Liu T, Wang S, He L, Ye K. 2008. Chronic exposure to low-intensity magnetic field improves acquisition and maintenance of memory. NeuroReport 19, 549-552. ( 10.1097/wnr.0b013e3282f8b1a0) [DOI] [PubMed] [Google Scholar]
- 233.Mostafa RM, Mostafa YM, Ennaceur A. 2002. Effects of exposure to extremely low-frequency magnetic field of 2 g intensity on memory and corticosterone level in rats. Physiol. Behav. 76, 589-595. ( 10.1016/s0031-9384(02)00730-8) [DOI] [PubMed] [Google Scholar]
- 234.Fu Y, Wang C, Wang J, Lei Y, Ma Y. 2008. Long-term exposure to extremely low-frequency magnetic fields impairs spatial recognition memory in mice. Clin. Exp. Pharmacol. Physiol. 35, 797-800. ( 10.1111/j.1440-1681.2008.04922.x) [DOI] [PubMed] [Google Scholar]
- 235.Liu X, et al. 2015. Improvement of spatial memory disorder and hippocampal damage by exposure to electromagnetic fields in an Alzheimer’s disease rat model. PLoS ONE 10, e0126963. ( 10.1371/journal.pone.0126963) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zhao QR, Lu JM, Yao JJ, Zhang ZY, Ling C, Mei YA. 2015. Neuritin reverses deficits in murine novel object associative recognition memory caused by exposure to extremely low-frequency (50 Hz) electromagnetic fields. Sci. Rep. 5, 1-13. ( 10.1038/srep11768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Richards PM, Persinger MA, Koren SA. 1996. Modification of semantic memory in normal subjects by application across the temporal lobes of a weak (1 microt) magnetic field structure that promotes long-term potentiation in hippocampal slices. Electro- Magnetobiol. 15, 141-148. ( 10.3109/15368379609009830) [DOI] [Google Scholar]
- 238.Balassa T, Szemerszky R, Bárdos G. 2009. Effect of short-term 50 Hz electromagnetic field exposure on the behavior of rats. Acta Physiol. Hung. 96, 437-448. ( 10.1556/aphysiol.96.2009.4.4) [DOI] [PubMed] [Google Scholar]
- 239.Jeong JH, Choi KB, Moon NJ, Park ES, Sohn UD. 2005. Benzodiazepine system is involved in hyperalgesia in rats induced by the exposure to extremely low frequency magnetic fields. Arch. Pharm. Res. 28, 238-242. ( 10.1007/bf02977722) [DOI] [PubMed] [Google Scholar]
- 240.Liu T, Wang S, He L, Ye K. 2008. Anxiogenic effect of chronic exposure to extremely low frequency magnetic field in adult rats. Neurosci. Lett. 434, 12-17. ( 10.1016/j.neulet.2008.01.019) [DOI] [PubMed] [Google Scholar]
- 241.He LH, Shi HM, Liu TT, Xu Y-C, Ye KP, Wang S. 2011. Effects of extremely low frequency magnetic field on anxiety level and spatial memory of adult rats. Chin. Med. J. 124, 3362-3366. [PubMed] [Google Scholar]
- 242.Korpinar MA, Kalkan MT, Tuncel H. 2012. The 50 Hz (10 mT) sinusoidal magnetic field: effects on stress-related behavior of rats. Bratislava Med. J. 113, 521-524. ( 10.4149/bll_2012_117) [DOI] [PubMed] [Google Scholar]
- 243.Kitaoka K, Kitamura M, Aoi S, Shimizu N, Yoshizaki K. 2012. Chronic exposure to an extremely low-frequency magnetic field induces depression-like behavior and corticosterone secretion without enhancement of the hypothalamic–pituitary–adrenal axis in mice. Bioelectromagnetics 34, 43-51. ( 10.1002/bem.21743) [DOI] [PubMed] [Google Scholar]
- 244.Rostami A, Shahani M, Zarrindast MR, Semnanian S, Roudsari MR, Tavirani MR, Hasanzadeh H. 2016. Effects of 3 Hz and 60 Hz extremely low frequency electromagnetic fields on anxiety-like behaviors, memory retention of passive avoidance and electrophysiological properties of male rats. J. Lasers Med. Sci. 7, 120-125. ( 10.15171/jlms.2016.20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Salunke BP, Umathe SN, Chavan JG. 2013. Involvement of NMDA receptor in low-frequency magnetic field-induced anxiety in mice. Electromagn. Biol. Med. 33, 312-326. ( 10.3109/15368378.2013.839453) [DOI] [PubMed] [Google Scholar]
- 246.Jeong JH, Choi KB, Yi BC, Chun CH, Sung KY, Sung JY, Gimm YM, Huh IH, Sohn UD. 2000. Effects of extremely low frequency magnetic fields on pain thresholds in mice: roles of melatonin and opioids. J. Auton. Pharmacol. 20, 259-264. ( 10.1046/j.1365-2680.2000.00189.x) [DOI] [PubMed] [Google Scholar]
- 247.Prato FS, Kavaliers M, Thomas A. 2000. Extremely low frequency magnetic fields can either increase or decrease analgaesia in the land snail depending on field and light conditions. Bioelectromagnetics 21, 287-301. () [DOI] [PubMed] [Google Scholar]
- 248.Cook CM, Thomas AW, Prato FS. 2004. Resting EEG is affected by exposure to a pulsed ELF magnetic field. Bioelectromagnetics 25, 196-203. ( 10.1002/bem.10188) [DOI] [PubMed] [Google Scholar]
- 249.Mert T, Kurt AH, Altun I, Celik A, Baran F, Gunay I. 2017. Pulsed magnetic field enhances therapeutic efficiency of mesenchymal stem cells in chronic neuropathic pain model. Bioelectromagnetics 38, 255-264. ( 10.1002/bem.22038) [DOI] [PubMed] [Google Scholar]
- 250.Kavaliers M, Ossenkopp K-P. 1987. Calcium channel involvement in magnetic field inhibition of morphine-induced analgesia. Naunyn-Schmiedebergs Arch. Pharmacol. 336, 308-315. ( 10.1007/bf00172683) [DOI] [PubMed] [Google Scholar]
- 251.Siero A, et al. 2004. Alternating extremely low frequency magnetic field increases turnover of dopamine and serotonin in rat frontal cortex. Bioelectromagnetics 25, 426-430. ( 10.1002/bem.20011) [DOI] [PubMed] [Google Scholar]
- 252.Janać B, Tovilović G, Tomić M, Prolić Z, Radenović L. 2009. Effect of continuous exposure to alternating magnetic field (50 Hz, 0.5 mT) on serotonin and dopamine receptors activity in rat brain. Gen. Physiol. Biophys. 28, 41-46. [PubMed] [Google Scholar]
- 253.Sieron A, Brus R, Szkilnik R, Plech A, Kubanski N, Cieslar G. 2001. Influence of alternating low frequency magnetic fields on reactivity of central dopamine receptors in neonatal 6-hydroxydopamine treated rats. Bioelectromagnetics 22, 479-486. ( 10.1002/bem.76) [DOI] [PubMed] [Google Scholar]
- 254.Kato M, Honma K-I, Shigemitsu T, Shiga Y. 1993. Effects of exposure to a circularly polarized 50-Hz magnetic field on plasma and pineal melatonin levels in rats. Bioelectromagnetics 14, 97-106. ( 10.1002/bem.2250140203) [DOI] [PubMed] [Google Scholar]
- 255.Karasek M, Woldanska-Okonska M, Czernicki J, Zylinska K, Swietoslawski J. 1998. Chronic exposure to 2.9 mT, 40 Hz magnetic field reduces melatonin concentrations in humans. J. Pineal Res. 25, 240-244. ( 10.1111/j.1600-079x.1998.tb00393.x) [DOI] [PubMed] [Google Scholar]
- 256.Lai H, Singh NP. 1997. Acute exposure to a 60 Hz magnetic field increases DNA strand breaks in rat brain cells. Bioelectromagnetics 18, 156-165. () [DOI] [PubMed] [Google Scholar]
- 257.Lai H, Singh NP. 1997. Melatonin and N-tert-butyl-α-phenylnitrone block 60-Hz magnetic field-induced DNA single and double strand breaks in rat brain cells. J. Pineal Res. 22, 152-162. ( 10.1111/j.1600-079x.1997.tb00317.x) [DOI] [PubMed] [Google Scholar]
- 258.Karabakhtsian R, Broude N, Shalts N, Kochlatyia S, Goodman R, Henderson AS. 1994. Calcium is necessary in the cell response to EM fields. FEBS Lett. 349, 1-6. ( 10.1016/0014-5793(94)00618-0) [DOI] [PubMed] [Google Scholar]
- 259.Zhou J, Yao G, Zhang J, Chang Z. 2002. CREB DNA binding activation by a 50-Hz magnetic field in HL60 cells is dependent on extra- and intracellular Ca2+ but not PKA, PKC, ERK, or p38 MAPK. Biochem. Biophys. Res. Commun. 296, 1013-1018. ( 10.1016/s0006-291x(02)02022-3) [DOI] [PubMed] [Google Scholar]
- 260.Leone L, Fusco S, Mastrodonato A, Piacentini R, Barbati SA, Zaffina S, Pani G, Podda MV, Grassi C. 2014. Epigenetic modulation of adult hippocampal neurogenesis by extremely low-frequency electromagnetic fields. Mol. Neurobiol. 49, 1472-1486. ( 10.1007/s12035-014-8650-8) [DOI] [PubMed] [Google Scholar]
- 261.Consales C, et al. 2017. Fifty-hertz magnetic field affects the epigenetic modulation of the miR-34b/c in neuronal cells. Mol. Neurobiol. 55, 5698-5714. ( 10.1007/s12035-017-0791-0) [DOI] [PubMed] [Google Scholar]
- 262.Yost M, Liburdy R. 1992. Time-varying and static magnetic fields act in combination to alter calcium signal transduction in the lymphocyte. FEBS Lett. 296, 117-122. ( 10.1016/0014-5793(92)80361-j) [DOI] [PubMed] [Google Scholar]
- 263.Lindströum E, Lindströum P, Berglund A, Mild KH, Lundgren E. 1993. Intracellular calcium oscillations induced in a t-cell line by a weak 50 Hz magnetic field. J. Cell. Physiol. 156, 395-398. ( 10.1002/jcp.1041560223) [DOI] [PubMed] [Google Scholar]
- 264.Barbier E, Veyret B, Dufy B. 1996. Stimulation of Ca22+ influx in rat pituitary cells under exposure to a 50 Hz magnetic field. Bioelectromagnetics 17, 303-311. () [DOI] [PubMed] [Google Scholar]
- 265.Löschinger M, Thumm S, Hämmerle H, Rodemann HP. 1999. Induction of intracellular calcium oscillations in human skin fibroblast populations by sinusoidal extremely low-frequency magnetic fields (20 Hz, 8 mT) is dependent on the differentiation state of the single cell. Radiat. Res. 151, 195. ( 10.2307/3579770) [DOI] [PubMed] [Google Scholar]
- 266.Zhang X, Liu X, Pan L, Lee I. 2010. Magnetic fields at extremely low-frequency (50Hz, 0.8mT) can induce the uptake of intracellular calcium levels in osteoblasts. Biochem. Biophys. Res. Commun. 396, 662-666. ( 10.1016/j.bbrc.2010.04.154) [DOI] [PubMed] [Google Scholar]
- 267.Morabito C, Guarnieri S, Fanò G, Mariggiò MA. 2010. Effects of acute and chronic low frequency electromagnetic field exposure on PC12 cells during neuronal differentiation. Cell. Physiol. Biochem. 26, 947-958. ( 10.1159/000324003) [DOI] [PubMed] [Google Scholar]
- 268.Sert C, Söker S, Deniz M, Nergiz Y. 2011. Intracellular Ca2+ levels in rat ventricle cells exposed to extremely low frequency magnetic field. Electromagn. Biol. Med. 30, 14-20. ( 10.3109/15368378.2011.566773) [DOI] [PubMed] [Google Scholar]
- 269.Özgün A, Garipcan B. 2021. Magnetic field-induced Ca2+ intake by mesenchymal stem cells is mediated by intracellular Zn2+ and accompanied by a Zn2+ influx. Biochim. Biophys. Acta Mol. Cell Res. 1868, 119062. ( 10.1016/j.bbamcr.2021.119062) [DOI] [PubMed] [Google Scholar]
- 270.Blackman CF, Benane SG, Rabinowitz JR, House DE, Joines WT. 1985. A role for the magnetic field in the radiation-induced efflux of calcium ions from brain tissue in vitro. Bioelectromagnetics 6, 327-337. ( 10.1002/bem.2250060402) [DOI] [PubMed] [Google Scholar]
- 271.Manikonda PK, Rajendra P, Devendranath D, Gunasekaran B, Aradhya RSS, Sashidhar RB, Subramanyam C. 2007. Influence of extremely low frequency magnetic fields on Ca2+ signaling and NMDA receptor functions in rat hippocampus. Neurosci. Lett. 413, 145-149. ( 10.1016/j.neulet.2006.11.048) [DOI] [PubMed] [Google Scholar]
- 272.Luo FL, Yang N, He C, Li HL, Li C, Chen F, Xiong JX, Hu ZA, Zhang J. 2014. Exposure to extremely low frequency electromagnetic fields alters the calcium dynamics of cultured entorhinal cortex neurons. Environ. Res. 135, 236-246. ( 10.1016/j.envres.2014.09.023) [DOI] [PubMed] [Google Scholar]
- 273.Selaković V, Balind SR, Radenović L, Prolić Z, Janać B. 2013. Age-dependent effects of ELF-MF on oxidative stress in the brain of Mongolian gerbils. Cell Biochem. Biophys. 66, 513-521. ( 10.1007/s12013-012-9498-z) [DOI] [PubMed] [Google Scholar]
- 274.Duan Y, Wang Z, Zhang H, He Y, Lu R, Zhang R, Sun G, Sun X. 2013. The preventive effect of lotus seedpod procyanidins on cognitive impairment and oxidative damage induced by extremely low frequency electromagnetic field exposure. Food Funct. 4, 1252. ( 10.1039/c3fo60116a) [DOI] [PubMed] [Google Scholar]
- 275.Park JE, Seo YK, Yoon HH, Kim CW, Park JK, Jeon S. 2013. Electromagnetic fields induce neural differentiation of human bone marrow derived mesenchymal stem cells via ROS mediated EGFR activation. Neurochem. Int. 62, 418-424. ( 10.1016/j.neuint.2013.02.002) [DOI] [PubMed] [Google Scholar]
- 276.Osera C, Amadio M, Falone S, Fassina L, Magenes G, Amicarelli F, Ricevuti G, Govoni S, Pascale A. 2015. Pre-exposure of neuroblastoma cell line to pulsed electromagnetic field prevents H2O2-induced ROS production by increasing MnSOD activity. Bioelectromagnetics 36, 219-232. ( 10.1002/bem.21900) [DOI] [PubMed] [Google Scholar]
- 277.Benassi B, Filomeni G, Montagna C, Merla C, Lopresto V, Pinto R, Marino C, Consales C. 2015. Extremely low frequency magnetic field (ELF-MF) exposure sensitizes SH-SY5y cells to the pro-Parkinson’s disease toxin MPP+. Mol. Neurobiol. 53, 4247-4260. ( 10.1007/s12035-015-9354-4) [DOI] [PubMed] [Google Scholar]
- 278.Roy S, Noda Y, Eckert V, Traber MG, Mori A, Liburdy R, Packer L. 1995. The phorbol 12-myristate 13- acetate (PMA)-induced oxidative burst in rat peritoneal neutrophils is increased by a 0.1 mT (60 Hz) magnetic field. FEBS Lett. 376, 164-166. ( 10.1016/0014-5793(95)01266-X) [DOI] [PubMed] [Google Scholar]
- 279.Túnez I, Montilla P, Medina FJ, Drucker-Colın R. 2006. Effect of transcranial magnetic stimulation on oxidative stress induced by 3-nitropropionic acid in cortical synaptosomes. Neurosci. Res. 56, 91-95. ( 10.1016/j.neures.2006.05.012) [DOI] [PubMed] [Google Scholar]
- 280.Falone S, Marchesi N, Osera C, Fassina L, Comincini S, Amadio M, Pascale A. 2016. Pulsed electromagnetic field (PEMF) prevents pro-oxidant effects of H2O2 in SK-n-BE(2) human neuroblastoma cells. Int. J. Radiat. Biol. 92, 281-286. ( 10.3109/09553002.2016.1150619) [DOI] [PubMed] [Google Scholar]
- 281.Vincenzi F, Ravani A, Pasquini S, Merighi S, Gessi S, Setti S, Cadossi R, Borea PA, Varani K. 2016. Pulsed electromagnetic field exposure reduces hypoxia and inflammation damage in neuron-like and microglial cells. J. Cell. Physiol. 232, 1200-1208. ( 10.1002/jcp.25606) [DOI] [PubMed] [Google Scholar]
- 282.Lee B-C, et al. 2004. Effects of extremely low frequency magnetic field on the antioxidant defense system in mouse brain: a chemiluminescence study. J. Photochem. Photobiol., B 73, 43-48. ( 10.1016/j.jphotobiol.2003.10.003) [DOI] [PubMed] [Google Scholar]
- 283.Di Loreto S, Falone S, Caracciolo V, Sebastiani P, D’Alessandro A, Mirabilio A, Zimmitti V, Amicarelli F. 2009. Fifty hertz extremely low-frequency magnetic field exposure elicits redox and trophic response in rat-cortical neurons. J. Cell. Physiol. 219, 334-343. ( 10.1002/jcp.21674) [DOI] [PubMed] [Google Scholar]
- 284.Lupke M, Rollwitz J, Simkó M. 2004. Cell activating capacity of 50 Hz magnetic fields to release reactive oxygen intermediates in human umbilical cord blood-derived monocytes and in Mono Mac 6 cells. Free Radic. Res. 38, 985-993. ( 10.1080/10715760400000968) [DOI] [PubMed] [Google Scholar]
- 285.Wolf FI, Torsello A, Tedesco B, Fasanella S, Boninsegna A, D’Ascenzo M, Grassi C, Azzena GB, Cittadini A. 2005. 50-Hz extremely low frequency electromagnetic fields enhance cell proliferation and DNA damage: possible involvement of a redox mechanism. Biochim. Biophys. Acta Mol. Cell Res. 1743, 120-129. ( 10.1016/j.bbamcr.2004.09.005) [DOI] [PubMed] [Google Scholar]
- 286.Lupke M, Frahm J, Lantow M, Maercker C, Remondini D, Bersani F, Simkó M. 2006. Gene expression analysis of ELF-MF exposed human monocytes indicating the involvement of the alternative activation pathway. Biochim. Biophys. Acta Mol. Cell Res. 1763, 402-412. ( 10.1016/j.bbamcr.2006.03.003) [DOI] [PubMed] [Google Scholar]
- 287.Koh EK, Ryu BK, Jeong DY, Bang IS, Nam MH, Chae KS. 2008. A 60-Hz sinusoidal magnetic field induces apoptosis of prostate cancer cells through reactive oxygen species. Int. J. Radiat. Biol. 84, 945-955. ( 10.1080/09553000802460206) [DOI] [PubMed] [Google Scholar]
- 288.Mannerling A-C, Simkó M, Mild KH, Mattsson M-O. 2010. Effects of 50-Hz magnetic field exposure on superoxide radical anion formation and HSP70 induction in human k562 cells. Radiat. Environ. Biophys. 49, 731-741. ( 10.1007/s00411-010-0306-0) [DOI] [PubMed] [Google Scholar]
- 289.AyŞe I-G, Zafer A, Şule O, IŞil I-T, Kalkan T. 2010. Differentiation of K562 cells under ELF-EMF applied at different time courses. Electromagn. Biol. Med. 29, 122-130. ( 10.3109/15368378.2010.502451) [DOI] [PubMed] [Google Scholar]
- 290.Garip A, Akan Z. 2010. Effect of ELF-EMF on number of apoptotic cells; correlation with reactive oxygen species and HSP. Acta Biol. Hung. 61, 158-167. ( 10.1556/ABiol.61.2010.2.4) [DOI] [PubMed] [Google Scholar]
- 291.Morabito C, Rovetta F, Bizzarri M, Mazzoleni G, Fanò G, Mariggiò MA. 2010. Modulation of redox status and calcium handling by extremely low frequency electromagnetic fields in C2C12 muscle cells: a real-time, single-cell approach. Free Radical Biol. Med. 48, 579-589. ( 10.1016/j.freeradbiomed.2009.12.005) [DOI] [PubMed] [Google Scholar]
- 292.Bułdak RJ, et al. 2012. Short-term exposure to 50 Hz ELF-EMF alters the cisplatin-induced oxidative response in AT478 murine squamous cell carcinoma cells. Bioelectromagnetics 33, 641-651. ( 10.1002/bem.21732) [DOI] [PubMed] [Google Scholar]
- 293.Sadeghipour R, Ahmadian S, Bolouri B, Pazhang Y, Shafiezadeh M. 2012. Effects of extremely low-frequency pulsed electromagnetic fields on morphological and biochemical properties of human breast carcinoma cells (T47D). Electromagn. Biol. Med. 31, 425-435. ( 10.3109/15368378.2012.683844) [DOI] [PubMed] [Google Scholar]
- 294.Lai H, Singh NP. 2004. Magnetic-field-induced DNA strand breaks in brain cells of the rat. Environ. Health Perspect. 112, 687-694. ( 10.1289/ehp.6355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Ma S, Zhang Z, Yi F, Wang Y, Zhang X, Li X, Yuan Y, Cao F. 2013. Protective effects of low-frequency magnetic fields on cardiomyocytes from ischemia reperfusion Injury via ROS and NO/ONOO-. Oxid. Med. Cell. Longev. 2013, 1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Luukkonen J, Liimatainen A, Juutilainen J, Naarala J. 2014. Induction of genomic instability, oxidative processes, and mitochondrial activity by 50 Hz magnetic fields in human SH-SY5Y neuroblastoma cells. Mutat. Res./Fundam. Mol. Mechan. Mutagen. 760, 33-41. ( 10.1016/j.mrfmmm.2013.12.002) [DOI] [PubMed] [Google Scholar]
- 297.Reale M, Kamal MA, Patruno A, Costantini E, D’Angelo C, Pesce M, Greig NH. 2014. Neuronal cellular responses to extremely low frequency electromagnetic field exposure: implications regarding oxidative stress and neurodegeneration. PLoS ONE 9, e104973. ( 10.1371/journal.pone.0104973) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Chen Y, Hong L, Zeng Y, Shen Y, Zeng Q. 2014. Power frequency magnetic fields induced reactive oxygen species-related autophagy in mouse embryonic fibroblasts. Int. J. Biochem. Cell Biol. 57, 108-114. ( 10.1016/j.biocel.2014.10.013) [DOI] [PubMed] [Google Scholar]
- 299.Ferroni L, Bellin G, Emer V, Rizzuto R, Isola M, Gardin C, Zavan B. 2015. Treatment by therapeutic magnetic resonance (TMRTM) increases fibroblastic activity and keratinocyte differentiation in an in vitro model of 3D artificial skin. J. Tissue Eng. Regen. Med. 11, 1332-1342. ( 10.1002/term.2031) [DOI] [PubMed] [Google Scholar]
- 300.Yang M-l, Ye Z-m. 2015. Extremely low frequency electromagnetic field induces apoptosis of osteosarcoma cells via oxidative stress. J. Zhejiang University (Medical Science) 44, 323-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Patruno A, Tabrez S, Pesce M, Shakil S, Kamal MA, Reale M. 2015. Effects of extremely low frequency electromagnetic field (ELF-EMF) on catalase, cytochrome P450 and nitric oxide synthase in erythro-leukemic cells. Life Sci. 121, 117-123. ( 10.1016/j.lfs.2014.12.003) [DOI] [PubMed] [Google Scholar]
- 302.Kesari KK, Luukkonen J, Juutilainen J, Naarala J. 2015. Genomic instability induced by 50 Hz magnetic fields is a dynamically evolving process not blocked by antioxidant treatment. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 794, 46-51. ( 10.1016/j.mrgentox.2015.10.004) [DOI] [PubMed] [Google Scholar]
- 303.Feng B, Dai A, Chen L, Qiu L, Fu Y, Sun W. 2016. NADPH oxidase-produced superoxide mediated a 50-Hz magnetic field-induced epidermal growth factor receptor clustering. Int. J. Radiat. Biol. 92, 596-602. ( 10.1080/09553002.2016.1206227) [DOI] [PubMed] [Google Scholar]
- 304.Feng B, Qiu L, Ye C, Chen L, Fu Y, Sun W. 2016. Exposure to a 50-Hz magnetic field induced mitochondrial permeability transition through the ROS/GSK-3β signaling pathway. Int. J. Radiat. Biol. 92, 148-155. ( 10.3109/09553002.2016.1135261) [DOI] [PubMed] [Google Scholar]
- 305.Feng B, Ye C, Qiu L, Chen L, Fu Y, Sun W. 2016. Mitochondrial ROS release and subsequent akt activation potentially mediated the anti-apoptotic effect of a 50-Hz magnetic field on FL cells. Cell. Physiol. Biochem. 38, 2489-2499. ( 10.1159/000445599) [DOI] [PubMed] [Google Scholar]
- 306.Calcabrini C, et al. 2016. Effect of extremely low-frequency electromagnetic fields on antioxidant activity in the human keratinocyte cell line NCTC 2544. Biotechnol. Appl. Biochem. 64, 415-422. ( 10.1002/bab.1495) [DOI] [PubMed] [Google Scholar]
- 307.Ehnert S, et al. 2017. Extremely low frequency pulsed electromagnetic fields cause antioxidative defense mechanisms in human osteoblasts via induction of - and H2O2. Sci. Rep. 7, 1-11. ( 10.1038/s41598-017-14983-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Jeong W-Y, Kim J-B, Kim H-J, Kim C-W. 2017. Extremely low-frequency electromagnetic field promotes astrocytic differentiation of human bone marrow mesenchymal stem cells by modulating SIRT1 expression. Biosci. Biotechnol. Biochem. 81, 1356-1362. ( 10.1080/09168451.2017.1308243) [DOI] [PubMed] [Google Scholar]
- 309.Höytö A, Herrala M, Luukkonen J, Juutilainen J, Naarala J. 2017. Cellular detection of 50 Hz magnetic fields and weak blue light: effects on superoxide levels and genotoxicity. Int. J. Radiat. Biol. 93, 646-652. ( 10.1080/09553002.2017.1294275) [DOI] [PubMed] [Google Scholar]
- 310.Song K, Im SH, Yoon YJ, Kim HM, Lee HJ, Park GS. 2018. A 60 Hz uniform electromagnetic field promotes human cell proliferation by decreasing intracellular reactive oxygen species levels. PLoS ONE 13, e0199753. ( 10.1371/journal.pone.0199753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Helekar SA, Hambarde S, Ijare OB, Pichumani K, Baskin DS, Sharpe MA. 2021. Selective induction of rapid cytotoxic effect in glioblastoma cells by oscillating magnetic fields. J. Cancer Res. Clin. Oncol. 147, 3577-3589. ( 10.1007/s00432-021-03787-0) [DOI] [PubMed] [Google Scholar]
- 312.Grassi C, D’Ascenzo M, Torsello A, Martinotti G, Wolf F, Cittadini A, Azzena GB. 2004. Effects of 50 Hz electromagnetic fields on voltage-gated Ca2+ channels and their role in modulation of neuroendocrine cell proliferation and death. Cell Calcium 35, 307-315. ( 10.1016/j.ceca.2003.09.001) [DOI] [PubMed] [Google Scholar]
- 313.Piacentini R, Ripoli C, Mezzogori D, Azzena GB, Grassi C. 2008. Extremely low-frequency electromagnetic fields promote in vitro neurogenesis via upregulation of cav1-channel activity. J. Cell. Physiol. 215, 129-139. ( 10.1002/jcp.21293) [DOI] [PubMed] [Google Scholar]
- 314.Ahmed Z, Wieraszko A. 2008. The mechanism of magnetic field-induced increase of excitability in hippocampal neurons. Brain Res. 1221, 30-40. ( 10.1016/j.brainres.2008.05.007) [DOI] [PubMed] [Google Scholar]
- 315.Cook C, Saucier D, Thomas A, Prato F. 2006. Exposure to ELF magnetic and ELF-modulated radiofrequency fields: the time course of physiological and cognitive effects observed in recent studies (2001–2005). Bioelectromagnetics 27, 613-627. ( 10.1002/bem.20247) [DOI] [PubMed] [Google Scholar]
- 316.Cook C, Saucier D, Thomas A, Prato F. 2009. Changes in human EEG alpha activity following exposure to two different pulsed magnetic field sequences. Bioelectromagnetics 30, 9-20. ( 10.1002/bem.20434) [DOI] [PubMed] [Google Scholar]
- 317.Yang Y, Li L, Wang YG, Fei Z, Zhong J, Wei LZ, Long QF, Liu WP. 2012. Acute neuroprotective effects of extremely low-frequency electromagnetic fields after traumatic brain injury in rats. Neurosci. Lett. 516, 15-20. ( 10.1016/j.neulet.2012.03.022) [DOI] [PubMed] [Google Scholar]
- 318.Tasset I, et al. 2012. Neuroprotective effects of extremely low-frequency electromagnetic fields on a Huntington’s disease rat model: effects on neurotrophic factors and neuronal density. Neuroscience 209, 54-63. ( 10.1016/j.neuroscience.2012.02.034) [DOI] [PubMed] [Google Scholar]
- 319.Balassa T, Varró P, Elek S, Drozdovszky O, Szemerszky R, Világi I, Bárdos G. 2013. Changes in synaptic efficacy in rat brain slices following extremely low-frequency magnetic field exposure at embryonic and early postnatal age. Int. J. Dev. Neurosci. 31, 724-730. ( 10.1016/j.ijdevneu.2013.08.004) [DOI] [PubMed] [Google Scholar]
- 320.Rauš Balind S, Manojlovic-Stojanoski M, Šošic-Jurjevic B, Selakovic V, Miloševic V, Petkovic B. 2019. An extremely low frequency magnetic field and global cerebral ischemia affect pituitary ACTH and TSH cells in gerbils. Bioelectromagnetics 41, 91-103. ( 10.1002/bem.22237) [DOI] [PubMed] [Google Scholar]
- 321.Li Y, Yan X, Liu J, Li L, Hu X, Sun H, Tian J. 2014. Pulsed electromagnetic field enhances brain-derived neurotrophic factor expression through L-type voltage-gated calcium channel- and Erk-dependent signaling pathways in neonatal rat dorsal root ganglion neurons. Neurochem. Int. 75, 96-104. ( 10.1016/j.neuint.2014.06.004) [DOI] [PubMed] [Google Scholar]
- 322.Makowiecki K, Harvey AR, Sherrard RM, Rodger J. 2014. Low-intensity repetitive transcranial magnetic stimulation improves abnormal visual cortical circuit topography and upregulates BDNF in mice. J. Neurosci. 34, 10 780-10 792. ( 10.1523/JNEUROSCI.0723-14.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Komaki A, Khalili A, Salehi I, Shahidi S, Sarihi A. 2014. Effects of exposure to an extremely low frequency electromagnetic field on hippocampal long-term potentiation in rat. Brain Res. 1564, 1-8. ( 10.1016/j.brainres.2014.03.041) [DOI] [PubMed] [Google Scholar]
- 324.Yang G, Ren Z, Mei Y-A. 2015. Exposure to 50 Hz magnetic field modulates GABA a currents in cerebellar granule neurons through an EP receptor-mediated PKC pathway. J. Cell. Mol. Med. 19, 2413-2422. ( 10.1111/jcmm.12626) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Chen Q, et al. 2016. Early exposure of rotating magnetic fields promotes central nervous regeneration in planarian Girardia sinensis. Bioelectromagnetics 37, 244-255. ( 10.1002/bem.21971) [DOI] [PubMed] [Google Scholar]
- 326.Ma Q, et al. 2016. Extremely low-frequency electromagnetic fields promote in vitro neuronal differentiation and neurite outgrowth of embryonic neural stem cells via up-regulating TRPC1. PLoS ONE 11, e0150923. ( 10.1371/journal.pone.0150923) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.cheng Sun Z, et al. 2016. Extremely low frequency electromagnetic fields facilitate vesicle endocytosis by increasing presynaptic calcium channel expression at a central synapse. Sci. Rep. 6, 1-11. ( 10.1038/s41598-016-0001-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Wilson BW, et al. 1990. Evidence for an effect of ELF electromagnetic fields on human pineal gland function. J. Pineal Res. 9, 259-269. ( 10.1111/j.1600-079X.1990.tb00901.x) [DOI] [PubMed] [Google Scholar]
- 329.Ossenkopp K-P, Cain DP. 1988. Inhibitory effects of acute exposure to low-intensity 60-Hz magnetic fields on electrically kindled seizures in rats. Brain Res. 442, 255-260. ( 10.1016/0006-8993(88)91510-7) [DOI] [PubMed] [Google Scholar]
- 330.Lai H, Carino MA, Horita A, Guy AW. 1993. Effects of a 60 Hz magnetic field on central cholinergic systems of the rat. Bioelectromagnetics 14, 5-15. ( 10.1002/bem.2250140104) [DOI] [PubMed] [Google Scholar]
- 331.Kavaliers M, Ossenkopp KP, Prato FS, Innes DGL, Galea LAM, Kinsella DM, Perrot-Sinal TS. 1996. Spatial learning in deer mice: sex differences and the effects of endogenous opioids and 60 Hz magnetic fields. J. Comp. Physiol. A 179, 715-724. ( 10.1007/BF00216135) [DOI] [PubMed] [Google Scholar]
- 332.Lindström E, Mild KH, Lundgren E. 1998. Analysis of the T cell activation signaling pathway during ELF magnetic field exposure, p56lck and [Ca2+]i-measurements. Bioelectrochem. Bioenerg. 46, 129-137. ( 10.1016/S0302-4598(98)00063-4) [DOI] [Google Scholar]
- 333.Shin EJ, Jeong JH, Kim HJ, Jang CG, Yamada K, Nabeshima T, Kim HC. 2007. Exposure to extremely low frequency magnetic fields enhances locomotor activity via activation of dopamine D1-like receptors in mice. J. Pharmacol. Sci. 105, 367-371. ( 10.1254/jphs.SC0070348) [DOI] [PubMed] [Google Scholar]
- 334.Rauš Balind S, Manojlovic-Stojanoski M, Miloševic V, Todorovic D, Nikolic L, Petkovic B. 2014. Short- and long-term exposure to alternating magnetic field (50 Hz, 0.5 mT) affects rat pituitary ACTH cells: stereological study. Environ. Toxicol. 31, 461-468. ( 10.1002/tox.22059) [DOI] [PubMed] [Google Scholar]
- 335.Wu X, et al. 2014. Weak power frequency magnetic field acting similarly to EGF stimulation, induces acute activations of the EGFR sensitive actin cytoskeleton motility in human amniotic cells. PLoS ONE 9, e87626. ( 10.1371/journal.pone.0087626) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Haghighat N, Abdolmaleki P, Parnian J, Behmanesh M. 2017. The expression of pluripotency and neuronal differentiation markers under the influence of electromagnetic field and nitric oxide. Mol. Cell. Neurosci. 85, 19-28. ( 10.1016/j.mcn.2017.08.005) [DOI] [PubMed] [Google Scholar]
- 337.Tong J, Sun L, Zhu B, Fan Y, Ma X, Yu L, Zhang J. 2017. Pulsed electromagnetic fields promote the proliferation and differentiation of osteoblasts by reinforcing intracellular calcium transients. Bioelectromagnetics 38, 541-549. ( 10.1002/bem.22076) [DOI] [PubMed] [Google Scholar]
- 338.Pooam M, Aguida B, Drahy S, Jourdan N, Ahmad M. 2021. Therapeutic application of light and electromagnetic fields to reduce hyper-inflammation triggered by COVID-19. Commun. Integr. Biol. 14, 66-77. ( 10.1080/19420889.2021.1911413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Ermakov A, Afanasyeva V, Ermakova O, Blagodatski A, Popov A. 2022. Effect of weak alternating magnetic fields on planarian regeneration. Biochem. Biophys. Res. Commun. 592, 7-12. ( 10.1016/j.bbrc.2021.12.096) [DOI] [PubMed] [Google Scholar]
- 340.Usselman RJ, Chavarriaga C, Castello PR, Procopio M, Ritz T, Dratz EA, Singel DJ, Martino CF. 2016. The quantum biology of reactive oxygen species partitioning impacts cellular bioenergetics. Sci. Rep. 6, 1-6. ( 10.1038/srep38543) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Friedman J, Kraus S, Hauptman Y, Schiff Y, Seger R. 2007. Mechanism of short-term ERK activation by electromagnetic fields at mobile phone frequencies. Biochem. J. 405, 559-568. ( 10.1042/BJ20061653) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Castello PR, Hill I, Sivo F, Portelli L, Barnes F, Usselman R, Martino CF. 2014. Inhibition of cellular proliferation and enhancement of hydrogen peroxide production in fibrosarcoma cell line by weak radio frequency magnetic fields. Bioelectromagnetics 35, 598-602. ( 10.1002/bem.21858) [DOI] [PubMed] [Google Scholar]
- 343.Martino CF, Castello P. 2013. Modulation of cellular and mitochondrial reactive oxygen species production by external magnetic fields.The FASEB Journal 27, ( 10.1096/fasebj.27.1_supplement.578.1) [DOI] [Google Scholar]
- 344.Luukkonen J, Hakulinen P, Mäki-Paakkanen J, Juutilainen J, Naarala J. 2009. Enhancement of chemically induced reactive oxygen species production and DNA damage in human SH-SY5Y neuroblastoma cells by 872MHz radiofrequency radiation. Mutat. Res./Fundam. Mol. Mechan. Mutagen. 662, 54-58. ( 10.1016/j.mrfmmm.2008.12.005) [DOI] [PubMed] [Google Scholar]
- 345.Agarwal A, Desai NR, Makker K, Varghese A, Mouradi R, Sabanegh E, Sharma R. 2009. Effects of radiofrequency electromagnetic waves (RF-EMW) from cellular phones on human ejaculated semen: an in vitro pilot study. Fertil. Steril. 92, 1318-1325. ( 10.1016/j.fertnstert.2008.08.022) [DOI] [PubMed] [Google Scholar]
- 346.Iuliis GND, Newey RJ, King BV, Aitken RJ. 2009. Mobile phone radiation induces reactive oxygen species production and DNA damage in human spermatozoa in vitro. PLoS ONE 4, e6446. ( 10.1371/journal.pone.0006446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Campisi A, Gulino M, Acquaviva R, Bellia P, Raciti G, Grasso R, Musumeci F, Vanella A, Triglia A. 2010. Reactive oxygen species levels and DNA fragmentation on astrocytes in primary culture after acute exposure to low intensity microwave electromagnetic field. Neurosci. Lett. 473, 52-55. ( 10.1016/j.neulet.2010.02.018) [DOI] [PubMed] [Google Scholar]
- 348.Lu Y-S, Huang B-T, Huang Y-X. 2012. Reactive oxygen species formation and apoptosis in human peripheral blood mononuclear cell induced by 900 MHz mobile phone radiation. Oxid. Med. Cell. Longev. 2012, 1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Manta AK, Stravopodis DJ, Papassideri IS, Margaritis LH. 2013. Reactive oxygen species elevation and recovery in Drosophila bodies and ovaries following short-term and long-term exposure to DECT base EMF. Electromagn. Biol. Med. 33, 118-131. ( 10.3109/15368378.2013.791991) [DOI] [PubMed] [Google Scholar]
- 350.Usselman RJ, Hill I, Singel DJ, Martino CF. 2014. Spin biochemistry modulates reactive oxygen species (ROS) production by radio frequency magnetic fields. PLoS ONE 9, e93065. ( 10.1371/journal.pone.0093065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Sefidbakht Y, et al. 2014. Effects of 940 MHz EMF on bioluminescence and oxidative response of stable luciferase producing HEK cells. Photochem. Photobiol. Sci. 13, 1082-1092. ( 10.1039/C3PP50451D) [DOI] [PubMed] [Google Scholar]
- 352.Gramowski-Voss A, Schwertle HJ, Pielka AM, Schultz L, Steder A, Juegelt K, Axmann J, Pries W. 2015. Enhancement of cortical network activity in vitro and promotion of GABAergic neurogenesis by stimulation with an electromagnetic field with a 150 MHz carrier wave pulsed with an alternating 10 and 16 Hz modulation. Front. Neurol. 6, 158. ( 10.3389/fneur.2015.00158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Bartos P, Netusil R, Slaby P, Dolezel D, Ritz T, Vacha M. 2019. Weak radiofrequency fields affect the insect circadian clock. J. R. Soc. Interface 16, 20190285. ( 10.1098/rsif.2019.0285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Röösli M, et al. 2021. The effects of radiofrequency electromagnetic fields exposure on tinnitus, migraine and non-specific symptoms in the general and working population: a protocol for a systematic review on human observational studies. Environ. Int. 157, 106852. ( 10.1016/j.envint.2021.106852) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Leberecht B, et al. 2022. Broadband 75–85 MHz radiofrequency fields disrupt magnetic compass orientation in night-migratory songbirds consistent with a flavin-based radical pair magnetoreceptor. J. Comp. Physiol. A 208, 97-106. ( 10.1007/s00359-021-01537-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Bigeleisen J. 1965. Chemistry of isotopes. Science 147, 463-471. ( 10.1126/science.147.3657.463) [DOI] [PubMed] [Google Scholar]
- 357.Zel’dovich YB, Buchachenko AL, Frankevich EL. 1988. Magnetic-spin effects in chemistry and molecular physics. Soviet Phys. Uspekhi 31, 385-408. ( 10.1070/PU1988v031n05ABEH003544) [DOI] [Google Scholar]
- 358.Wolfsberg M, Hook WA, Paneth P, Rebelo LPN. 2009. Isotope effects. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 359.Faure G. 1977. Principles of isotope geology. New York, NY: John Wiley and Sons, Inc. [Google Scholar]
- 360.Hoefs J, Hoefs J. 2009. Stable isotope geochemistry, vol. 285. New York, NY: Springer. [Google Scholar]
- 361.Fry B. 2006. Stable isotope ecology, vol. 521. New York, NY: Springer. [Google Scholar]
- 362.Van Hook WA. 2011. Isotope effects in chemistry. Nukleonika 56, 217-240. [Google Scholar]
- 363.Buchachenko AL. 2001. Magnetic isotope effect:nuclear spin control of chemical reactions. J. Phys. Chem. A 105, 9995-10011. ( 10.1021/jp011261d) [DOI] [Google Scholar]
- 364.Cook PF. 1991. Enzyme mechanism from isotope effects. Boca Raton, FL: CRC Press. [Google Scholar]
- 365.Kohen A, Limbach H-H. 2005. Isotope effects in chemistry and biology. Boca Raton, FL: CRC Press. [Google Scholar]
- 366.Buchachenko A. 2009. Magnetic isotope effect in chemistry and biochemistry. New York, NY: Nova Science Publishers. [Google Scholar]
- 367.Buchachenko AL, Kuznetsov DA, Breslavskaya NN. 2012. Chemistry of enzymatic ATP synthesis: an insight through the isotope window. Chem. Rev. 112, 2042-2058. ( 10.1021/cr200142a) [DOI] [PubMed] [Google Scholar]
- 368.Koltover VK. 2021. Nuclear spin catalysis in biochemical physics. Russ. Chem. Bull. 70, 1633-1639. ( 10.1007/s11172-021-3264-6) [DOI] [Google Scholar]
- 369.Thiemens MH, Heidenreich JE. 1983. The mass-independent fractionation of oxygen: a novel isotope effect and its possible cosmochemical implications. Science 219, 1073-1075. ( 10.1126/science.219.4588.1073) [DOI] [PubMed] [Google Scholar]
- 370.Thiemens MH. 1999. Mass-independent isotope effects in planetary atmospheres and the early solar system. Science 283, 341-345. ( 10.1126/science.283.5400.341) [DOI] [PubMed] [Google Scholar]
- 371.Thiemens MH, Savarino J, Farquhar J, Bao H. 2001. Mass-independent isotopic compositions in terrestrial and extraterrestrial solids and their applications. Acc. Chem. Res. 34, 645-652. ( 10.1021/ar960224f) [DOI] [PubMed] [Google Scholar]
- 372.Thiemens MH. 2006. History and applications of mass-independent isotope effects. Annu. Rev. Earth and Planet. Sci. 34, 217-262. ( 10.1146/annurev.earth.34.031405.125026) [DOI] [Google Scholar]
- 373.Thiemens MH, Chakraborty S, Dominguez G. 2012. The physical chemistry of mass-independent isotope effects and their observation in nature. Annu. Rev. Phys. Chem. 63, 155-177. ( 10.1146/annurev-physchem-032511-143657) [DOI] [PubMed] [Google Scholar]
- 374.Buchachenko A, Galimov E, Ershov V, Nikiforov G, Pershin A. 1976. Isotopic enrichment induced by magnetic interactions in chemical reactions. Doklady Akademii Nauk Sssr 228, 379-381. [Google Scholar]
- 375.Buchachenko AL. 2013. Mass-independent isotope effects. J. Phys. Chem. B 117, 2231-2238. ( 10.1021/jp308727w) [DOI] [PubMed] [Google Scholar]
- 376.Buchachenko AL. 2014. Magnetic control of enzymatic phosphorylation. J. Phys. Chem. Biophys. 2, 000. ( 10.4172/2161-0398.1000142) [DOI] [Google Scholar]
- 377.Bukhvostov A, Napolov J, Buchachenko A, Kuznetsov D. 2014. A new platform for anti-cancer experimental pharmacology: the DNA repair enzyme affected. Brit. J. Pharmacol. Toxicol. 5, 35-41. ( 10.19026/bjpt.5.5414) [DOI] [Google Scholar]
- 378.Buchachenko A, Bukhvostov A, Ermakov K, Kuznetsov D. 2019. Nuclear spin selectivity in enzymatic catalysis: a caution for applied biophysics. Arch. Biochem. Biophys. 667, 30-35. ( 10.1016/j.abb.2019.04.005) [DOI] [PubMed] [Google Scholar]
- 379.Arkhangelskaya EY, Vorobyeva NY, Leonov SV, Osipov AN, Buchachenko AL. 2020. Magnetic isotope effect on the repair of radiation-induced DNA damage. Russ. J. Phys. Chem. B 14, 314-317. ( 10.1134/s1990793120020177) [DOI] [Google Scholar]
- 380.Buchachenko AL, Bukhvostov AA, Ermakov KV, Kuznetsov DA. 2020. A specific role of magnetic isotopes in biological and ecological systems. physics and biophysics beyond. Prog. Biophys. Mol. Biol. 155, 1-19. ( 10.1016/j.pbiomolbio.2020.02.007) [DOI] [PubMed] [Google Scholar]
- 381.Letuta UG. 2021. Magnesium magnetic isotope effects in microbiology. Arch. Microbiol. 203, 1853-1861. ( 10.1007/s00203-021-02219-4) [DOI] [PubMed] [Google Scholar]
- 382.Sechzer JA, Lieberman KW, Alexander GJ, Weidman D, Stokes PE. 1986. Aberrant parenting and delayed offspring development in rats exposed to lithium. Biol. Psychiatry 21, 1258-1266. ( 10.1016/0006-3223(86)90308-2) [DOI] [PubMed] [Google Scholar]
- 383.Ettenberg A, Ayala K, Krug JT, Collins L, Mayes MS, Fisher MP. 2020. Differential effects of lithium isotopes in a ketamine-induced hyperactivity model of mania. Pharmacol. Biochem. Behav. 190, 172875. ( 10.1016/j.pbb.2020.172875) [DOI] [PubMed] [Google Scholar]
- 384.Li N, et al. 2018. Nuclear spin attenuates the anesthetic potency of xenon isotopes in mice. Anesthesiology 129, 271-277. ( 10.1097/aln.0000000000002226) [DOI] [PubMed] [Google Scholar]
- 385.Buchachenko AL, Kouznetsov DA, Orlova MA, Markarian AA. 2005. Magnetic isotope effect of magnesium in phosphoglycerate kinase phosphorylation. Proc. Natl Acad. Sci. USA 102, 10 793-10 796. ( 10.1073/pnas.0504876102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Buchachenko AL, Chekhonin VP, Orlov AP, Kuznetsov DA. 2010. Zinc-related magnetic isotope effect in the enzymatic ATP synthesis: a medicinal potential of the nuclear spin selectivity phenomena. Int. J. Med. Mol. Adv. Sci 6, 34-37. ( 10.3923/ijmmas.2010.34.37) [DOI] [Google Scholar]
- 387.Buchachenko AL, Orlov AP, Kuznetsov DA, Breslavskaya NN. 2013. Magnetic control of the DNA synthesis. Chem. Phys. Lett. 586, 138-142. ( 10.1016/j.cplett.2013.07.056) [DOI] [Google Scholar]
- 388.Bukhvostov AA, Shatalov OA, Buchachenko AL, Kuznetsov DA. 2013. 43Ca2+–paramagnetic impact on DNA polymerase beta function as it relates to a molecular pharmacology of leukemias. Der Pharmacia Lett. 5, 18-26. [Google Scholar]
- 389.Buchachenko AL, Orlov AP, Kuznetsov DA, Breslavskaya NN. 2013. Magnetic isotope and magnetic field effects on the DNA synthesis. Nucleic Acids Res. 41, 8300-8307. ( 10.1093/nar/gkt537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Dirac PAM. 1928. The quantum theory of the electron. Proc. R. Soc. Lond. A 117, 610-624. ( 10.1098/rspa.1928.0023) [DOI] [Google Scholar]
- 391.Ohanian HC. 1986. What is spin? Am. J. Phys. 54, 500-505. ( 10.1119/1.14580) [DOI] [Google Scholar]
- 392.Sakurai JJ, Commins ED. 1995. Modern quantum mechanics, revised edition. Am. J. Phys. 63, 93-95. ( 10.1119/1.17781) [DOI] [Google Scholar]
- 393.Salikhov KM, Molin YN, Sagdeev R, Buchachenko A. 1984. Spin polarization and magnetic effects in radical reactions. New York, NY: Elsevier. [Google Scholar]
- 394.Gerson F, Huber W. 2003. Electron spin resonance spectroscopy of organic radicals. New York, NY: Wiley. [Google Scholar]
- 395.Hayashi H. 2004. Introduction to dynamic spin chemistry. Singapore: World Scientific. [Google Scholar]
- 396.Wertz J. 2012. Electron spin resonance: elementary theory and practical applications. Berlin, Germany: Springer Science & Business Media. [Google Scholar]
- 397.Atkins PW, Friedman RS. 2011. Molecular quantum mechanics. Oxford, UK: Oxford University Press. [Google Scholar]
- 398.Improta R, Barone V. 2004. Interplay of electronic, environmental, and vibrational effects in determining the hyperfine coupling constants of organic free radicals. Chem. Rev. 104, 1231-1254. ( 10.1021/cr960085f) [DOI] [PubMed] [Google Scholar]
- 399.Illas F, Moreira IPR, de Graaf C, Barone V. 2000. Magnetic coupling in biradicals, binuclear complexes and wide-gap insulators: a survey of ab initio wave function and density functional theory approaches. Theor. Chem. Accounts: Theory, Comput., Model. (Theoretica Chimica Acta) 104, 265-272. ( 10.1007/s002140000133) [DOI] [Google Scholar]
- 400.Nohr D, Paulus B, Rodriguez R, Okafuji A, Bittl R, Schleicher E, Weber S. 2017. Determination of radical–radical distances in light-active proteins and their implication for biological magnetoreception. Angew. Chem. Int. Ed. 56, 8550-8554. ( 10.1002/anie.201700389) [DOI] [PubMed] [Google Scholar]
- 401.Hochstoeger T, et al. 2020. The biophysical, molecular, and anatomical landscape of pigeon CRY4: a candidate light-based quantal magnetosensor. Sci. Adv. 6, eabb9110. ( 10.1126/sciadv.abb9110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Ernst RR, Bodenhausen G, Wokaun A. 1987. Principles of nuclear magnetic resonance in one and two dimensions, vol. 14. Oxford, UK: Clarendon Press. [Google Scholar]
- 403.Efimova O, Hore P. 2008. Role of exchange and dipolar interactions in the radical pair model of the avian magnetic compass. Biophys. J. 94, 1565-1574. ( 10.1529/biophysj.107.119362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Babcock NS, Kattnig DR. 2021. Radical scavenging could answer the challenge posed by electron–electron dipolar interactions in the cryptochrome compass model. JACS Au 1, 2033-2046. ( 10.1021/jacsau.1c00332) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Kattnig DR, Hore PJ. 2017. The sensitivity of a radical pair compass magnetoreceptor can be significantly amplified by radical scavengers. Sci. Rep. 7, 1-12. ( 10.1038/s41598-017-09914-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Kattnig DR. 2017. Radical-pair-based magnetoreception amplified by radical scavenging: resilience to spin relaxation. J. Phys. Chem. B 121, 10215-10227. ( 10.1021/acs.jpcb.7b07672) [DOI] [PubMed] [Google Scholar]
- 407.Brocklehurst B, McLauchlan KA. 1996. Free radical mechanism for the effects of environmental electromagnetic fields on biological systems. Int. J. Radiat. Biol. 69, 3-24. ( 10.1080/095530096146147) [DOI] [PubMed] [Google Scholar]
- 408.Hore P. 2019. Upper bound on the biological effects of 50/60 Hz magnetic fields mediated by radical pairs. eLife 8, e44179. ( 10.7554/elife.44179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Hameka H. 1967. Part 1 spin-orbit coupling and intersystem crossing. In The triplet state (eds AB Zahlan, GM Androes, CA Hutchison, HF Hameka, GW Robinson, FW Heineken, JH van der Waals), pp. 1–62. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 410.Khudyakov IV, Serebrennikov YA, Turro NJ. 1993. Spin-orbit coupling in free-radical reactions: on the way to heavy elements. Chem. Rev. 93, 537-570. ( 10.1021/cr00017a023) [DOI] [Google Scholar]
- 411.Li H, Kamasah A, Matsika S, Suits AG. 2018. Intersystem crossing in the exit channel. Nat. Chem. 11, 123-128. ( 10.1038/s41557-018-0186-5) [DOI] [PubMed] [Google Scholar]
- 412.Marian CM. 2021. Understanding and controlling intersystem crossing in molecules. Annu. Rev. Phys. Chem. 72, 617-640. ( 10.1146/annurev-physchem-061020-053433) [DOI] [PubMed] [Google Scholar]
- 413.Hore PJ. 2021. Radical quantum oscillations. Science 374, 1447-1448. ( 10.1126/science.abm9261) [DOI] [PubMed] [Google Scholar]
- 414.Mims D, Herpich J, Lukzen NN, Steiner UE, Lambert C. 2021. Readout of spin quantum beats in a charge-separated radical pair by pump-push spectroscopy. Science 374, 1470-1474. ( 10.1126/science.abl4254) [DOI] [PubMed] [Google Scholar]
- 415.Bagryansky VA, Borovkov VI, Molin YN. 2007. Quantum beats in radical pairs. Russ. Chem. Rev. 76, 493-506. ( 10.1070/rc2007v076n06abeh003715) [DOI] [Google Scholar]
- 416.Scaiano JC, Mohtat N, Cozens FL, McLean J, Thansandote A. 1994. Application of the radical pair mechanism to free radicals in organized systems: can the effects of 60 Hz be predicted from studies under static fields? Bioelectromagnetics 15, 549-554. ( 10.1002/bem.2250150608) [DOI] [PubMed] [Google Scholar]
- 417.Canfield J, Belford R, Debrunner P, Schulten K. 1995. A perturbation treatment of oscillating magnetic fields in the radical pair mechanism using the liouville equation. Chem. Phys. 195, 59-69. ( 10.1016/0301-0104(95)00049-t) [DOI] [Google Scholar]
- 418.Timmel C, Hore P. 1996. Oscillating magnetic field effects on the yields of radical pair reactions. Chem. Phys. Lett. 257, 401-408. ( 10.1016/0009-2614(96)00466-6) [DOI] [Google Scholar]
- 419.Hiscock HG, Kattnig DR, Manolopoulos DE, Hore PJ. 2016. Floquet theory of radical pairs in radiofrequency magnetic fields. J. Chem. Phys. 145, 124117. ( 10.1063/1.4963793) [DOI] [PubMed] [Google Scholar]
- 420.Canfield J, Belford R, Debrunner P, Schulten K. 1994. A perturbation theory treatment of oscillating magnetic fields in the radical pair mechanism. Chem. Phys. 182, 1-18. ( 10.1016/0301-0104(93)e0442-x) [DOI] [Google Scholar]
- 421.McLendon G, Hake R. 1992. Interprotein electron transfer. Chem. Rev. 92, 481-490. ( 10.1021/cr00011a007) [DOI] [Google Scholar]
- 422.Moser CC, Page CC, Farid R, Dutton PL. 1995. Biological electron transfer. J. Bioenerg. Biomembr. 27, 263-274. ( 10.1007/bf02110096) [DOI] [PubMed] [Google Scholar]
- 423.Marcus R, Sutin N. 1985. Electron transfers in chemistry and biology. Biochim. Biophys. Acta Rev. Bioenerg. 811, 265-322. ( 10.1016/0304-4173(85)90014-x) [DOI] [Google Scholar]
- 424.Dodson CA, Hore P, Wallace MI. 2013. A radical sense of direction: signalling and mechanism in cryptochrome magnetoreception. Trends Biochem. Sci. 38, 435-446. ( 10.1016/j.tibs.2013.07.002) [DOI] [PubMed] [Google Scholar]
- 425.Mouritsen H, Hore P. 2012. The magnetic retina: light-dependent and trigeminal magnetoreception in migratory birds. Curr. Opin Neurobiol. 22, 343-352. ( 10.1016/j.conb.2012.01.005) [DOI] [PubMed] [Google Scholar]
- 426.Kutta RJ, Archipowa N, Johannissen LO, Jones AR, Scrutton NS. 2017. Vertebrate cryptochromes are vestigial flavoproteins. Sci. Rep. 7, 1-11. ( 10.1038/srep44906) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Phillips JB, Jorge PE, Muheim R. 2010. Light-dependent magnetic compass orientation in amphibians and insects: candidate receptors and candidate molecular mechanisms. J. R. Soc. Interface 7, S241-S256. ( 10.1098/rsif.2009.0459.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Cailliez F, Müller P, Firmino T, Pernot P. 2016. Energetics of photoinduced charge migration within the tryptophan tetrad of an animal (6–4) photolyase. J. Am. Chem. Soc. 138, 1904-1915. ( 10.1021/jacs.5b10938) [DOI] [PubMed] [Google Scholar]
- 429.Giovani B, Byrdin M, Ahmad M, Brettel K. 2003. Light-induced electron transfer in a cryptochrome blue-light photoreceptor. Nat. Struct. Mol. Biol. 10, 489-490. ( 10.1038/nsb933) [DOI] [PubMed] [Google Scholar]
- 430.Müller P, Yamamoto J, Martin R, Iwai S, Brettel K. 2015. Discovery and functional analysis of a 4th electron-transferring tryptophan conserved exclusively in animal cryptochromes and (6-4) photolyases. Chem. Commun. 51, 15 502-15 505. ( 10.1039/c5cc06276d) [DOI] [PubMed] [Google Scholar]
- 431.Zeugner A, Byrdin M, Bouly JP, Bakrim N, Giovani B, Brettel K, Ahmad M. 2005. Light-induced electron transfer in Arabidopsis cryptochrome-1 correlates with in vivo function. J. Biol. Chem. 280, 19437-19440. ( 10.1074/jbc.c500077200) [DOI] [PubMed] [Google Scholar]
- 432.Wong SY, Wei Y, Mouritsen H, Solov’yov IA, Hore PJ. 2021. Cryptochrome magnetoreception: four tryptophans could be better than three. J. R. Soc. Interface 18, 20210601. ( 10.1098/rsif.2021.0601) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Hong G, Pachter R, Essen L-O, Ritz T. 2020. Electron transfer and spin dynamics of the radical-pair in the cryptochrome from Chlamydomonas reinhardtii by computational analysis. J. Chem. Phys. 152, 065101. ( 10.1063/1.5133019) [DOI] [PubMed] [Google Scholar]
- 434.Ritz T, Adem S, Schulten K. 2000. A model for photoreceptor-based magnetoreception in birds. Biophys. J. 78, 707-718. ( 10.1016/s0006-3495(00)76629-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Müller P, Ahmad M. 2011. Light-activated cryptochrome reacts with molecular oxygen to form a flavin–superoxide radical pair consistent with magnetoreception. J. Biol. Chem. 286, 21033-21040. ( 10.1074/jbc.m111.228940) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Nießner C, Denzau S, Peichl L, Wiltschko W, Wiltschko R. 2014. Magnetoreception in birds: I. Immunohistochemical studies concerning the cryptochrome cycle. J. Exp. Biol. 217, 4221-4224. ( 10.1242/jeb.110965) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Nießner C, Denzau S, Stapput K, Ahmad M, Peichl L, Wiltschko W, Wiltschko R. 2013. Magnetoreception: activated cryptochrome 1a concurs with magnetic orientation in birds. J. R. Soc. Interface 10, 20130638. ( 10.1098/rsif.2013.0638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Ritz T, Wiltschko R, Hore PJ, Rodgers CT, Stapput K, Thalau P, Timmel CR, Wiltschko W. 2009. Magnetic compass of birds is based on a molecule with optimal directional sensitivity. Biophys. J. 96, 3451-3457. ( 10.1016/j.bpj.2008.11.072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Maeda K, Henbest KB, Cintolesi F, Kuprov I, Rodgers CT, Liddell PA, Gust D, Timmel CR, Hore PJ. 2008. Chemical compass model of avian magnetoreception. Nature 453, 387-390. ( 10.1038/nature06834) [DOI] [PubMed] [Google Scholar]
- 440.Hogben HJ, Efimova O, Wagner-Rundell N, Timmel CR, Hore P. 2009. Possible involvement of superoxide and dioxygen with cryptochrome in avian magnetoreception: origin of Zeeman resonances observed by in vivo EPR spectroscopy. Chem. Phys. Lett. 480, 118-122. ( 10.1016/j.cplett.2009.08.051) [DOI] [Google Scholar]
- 441.Solov’yov IA, Schulten K. 2009. Magnetoreception through cryptochrome may involve superoxide. Biophys. J. 96, 4804-4813. ( 10.1016/j.bpj.2009.03.048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Lee AA, Lau JC, Hogben HJ, Biskup T, Kattnig DR, Hore PJ. 2014. Alternative radical pairs for cryptochrome-based magnetoreception. J. R. Soc. Interface 11, 20131063. ( 10.1098/rsif.2013.1063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.van Wilderen LJ, Silkstone G, Mason M, van Thor JJ, Wilson MT. 2015. Kinetic studies on the oxidation of semiquinone and hydroquinone forms of arabidopsis cryptochrome by molecular oxygen. FEBS Open Bio. 5, 885-892. ( 10.1016/j.fob.2015.10.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Netušil R, Tomanová K, Chodáková L, Chvalová D, Doležel D, Ritz T, Vácha M. 2021. Cryptochrome-dependent magnetoreception in a heteropteran insect continues even after 24 h in darkness. J. Exp. Biol. 224, jeb243000. ( 10.1242/jeb.243000) [DOI] [PubMed] [Google Scholar]
- 445.Hiscock HG, Hiscock TW, Kattnig DR, Scrivener T, Lewis AM, Manolopoulos DE, Hore PJ. 2019. Navigating at night: fundamental limits on the sensitivity of radical pair magnetoreception under dim light. Q Rev. Biophys. 52, e9. ( 10.1017/s0033583519000076) [DOI] [PubMed] [Google Scholar]
- 446.Player TC, Hore PJ. 2019. Viability of superoxide-containing radical pairs as magnetoreceptors. J. Chem. Phys. 151, 225101. ( 10.1063/1.5129608) [DOI] [PubMed] [Google Scholar]
- 447.Solovyov IA, Chandler DE, Schulten K. 2008. Exploring the possibilities for radical pair effects in cryptochrome. Plant Signal. Behav. 3, 676-677. ( 10.4161/psb.3.9.5809) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Bouly J-P, et al. 2007. Cryptochrome blue light photoreceptors are activated through interconversion of flavin redox states. J. Biol. Chem. 282, 9383-9391. ( 10.1074/jbc.m609842200) [DOI] [PubMed] [Google Scholar]
- 449.Prabhakar R, Siegbahn PEM, Minaev BF, Ågren H. 2002. Activation of triplet dioxygen by glucose oxidase:spin-orbit coupling in the superoxide ion. J. Phys. Chem. B 106, 3742-3750. ( 10.1021/jp014013q) [DOI] [Google Scholar]
- 450.Schwarze S, Schneider NL, Reichl T, Dreyer D, Lefeldt N, Engels S, Baker N, Hore PJ, Mouritsen H. 2016. Weak broadband electromagnetic fields are more disruptive to magnetic compass orientation in a night-migratory songbird (Erithacus rubecula) than strong narrow-band fields. Front. Behav. Neurosci. 10, 55. ( 10.3389/fnbeh.2016.00055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Engels S, et al. 2014. Anthropogenic electromagnetic noise disrupts magnetic compass orientation in a migratory bird. Nature 509, 353-356. ( 10.1038/nature13290) [DOI] [PubMed] [Google Scholar]
- 452.Hiscock HG, Mouritsen H, Manolopoulos DE, Hore P. 2017. Disruption of magnetic compass orientation in migratory birds by radiofrequency electromagnetic fields. Biophys. J. 113, 1475-1484. ( 10.1016/j.bpj.2017.07.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Neese F. 2011. The ORCA program system. WIREs Comput. Mol. Sci. 2, 73-78. ( 10.1002/wcms.81) [DOI] [Google Scholar]
- 454.Massey V. 2000. The chemical and biological versatility of riboflavin. Biochem. Soc. Trans. 28, 283-296. ( 10.1042/bst0280283) [DOI] [PubMed] [Google Scholar]
- 455.Joosten V, van Berkel WJ. 2007. Flavoenzymes. Curr. Opin Chem. Biol. 11, 195-202. ( 10.1016/j.cbpa.2007.01.010) [DOI] [PubMed] [Google Scholar]
- 456.Romero E, Castellanos JRG, Gadda G, Fraaije MW, Mattevi A. 2018. Same substrate, many reactions: oxygen activation in flavoenzymes. Chem. Rev. 118, 1742-1769. ( 10.1021/acs.chemrev.7b00650) [DOI] [PubMed] [Google Scholar]
- 457.Walsh CT, Wencewicz TA. 2013. Flavoenzymes: versatile catalysts in biosynthetic pathways. Nat. Prod. Rep. 30, 175-200. ( 10.1039/c2np20069d) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Fraaije MW, Mattevi A. 2000. Flavoenzymes: diverse catalysts with recurrent features. Trends Biochem. Sci. 25, 126-132. ( 10.1016/s0968-0004(99)01533-9) [DOI] [PubMed] [Google Scholar]
- 459.Vanoni M, Vitali T, Zucchini D. 2013. MICAL, the flavoenzyme participating in cytoskeleton dynamics. Int. J. Mol. Sci. 14, 6920-6959. ( 10.3390/ijms14046920) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Vitali T, Maffioli E, Tedeschi G, Vanoni MA. 2016. Properties and catalytic activities of MICAL1, the flavoenzyme involved in cytoskeleton dynamics, and modulation by its CH, LIM and C-terminal domains. Arch. Biochem. Biophys. 593, 24-37. ( 10.1016/j.abb.2016.01.016) [DOI] [PubMed] [Google Scholar]
- 461.Hamdane D, Grosjean H, Fontecave M. 2016. Flavin-dependent methylation of RNAs: complex chemistry for a simple modification. J. Mol. Biol. 428, 4867-4881. ( 10.1016/j.jmb.2016.10.031) [DOI] [PubMed] [Google Scholar]
- 462.Udhayabanu T, Manole A, Rajeshwari M, Varalakshmi P, Houlden H, Ashokkumar B. 2017. Riboflavin responsive mitochondrial dysfunction in neurodegenerative diseases. J. Clin. Med. 6, 52. ( 10.3390/jcm6050052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Zwang TJ, Tse ECM, Zhong D, Barton JK. 2018. A compass at weak magnetic fields using thymine dimer repair. ACS Central Sci. 4, 405-412. ( 10.1021/acscentsci.8b00008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Husen P, Nielsen C, Martino CF, Solov’yov IA. 2019. Molecular oxygen binding in the mitochondrial electron transfer flavoprotein. J. Chem. Inf. Model. 59, 4868-4879. ( 10.1021/acs.jcim.9b00702) [DOI] [PubMed] [Google Scholar]
- 465.Lukacs A, Tonge PJ, Meech SR. 2022. Photophysics of the blue light using flavin domain. Acc. Chem. Res. 55, 402-414. ( 10.1021/acs.accounts.1c00659) [DOI] [PubMed] [Google Scholar]
- 466.Gebicki J, Marcinek A, Zielonka J. 2004. Transient species in the stepwise interconversion of NADH and NAD+. Acc. Chem. Res. 37, 379-386. ( 10.1021/ar030171j) [DOI] [PubMed] [Google Scholar]
- 467.Fukuzumi S, Kotani H, Lee Y-M, Nam W. 2008. Sequential electron-transfer and proton-transfer pathways in hydride-transfer reactions from dihydronicotinamide adenine dinucleotide analogues to non-heme oxoiron(IV) complexes and p-chloranil. detection of radical cations of NADH analogues in acid-promoted hydride-transfer reactions. J. Am. Chem. Soc. 130, 15 134-15 142. ( 10.1021/ja804969k) [DOI] [PubMed] [Google Scholar]
- 468.Yuasa J, Yamada S, Fukuzumi S. 2008. Detection of a radical cation of an NADH analogue in two-electron reduction of a protonated p-quinone derivative by an NADH analogue. Angew. Chem. Int. Ed. 47, 1068-1071. ( 10.1002/anie.200704136) [DOI] [PubMed] [Google Scholar]
- 469.Lee C-Y. 1992. A possible biological role of the electron transfer between tyrosine and tryptophan. FEBS Lett. 299, 119-123. ( 10.1016/0014-5793(92)80228-9) [DOI] [PubMed] [Google Scholar]
- 470.Aubert C, Mathis P, Eker APM, Brettel K. 1999. Intraprotein electron transfer between tyrosine and tryptophan in DNA photolyase from Anacystis nidulans. Proc. Natl Acad. Sci. USA 96, 5423-5427. ( 10.1073/pnas.96.10.5423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Stadtman ER, Levine RL. 2006. Protein oxidation. Ann. NY Acad. Sci. 899, 191-208. ( 10.1111/j.1749-6632.2000.tb06187.x) [DOI] [PubMed] [Google Scholar]
- 472.Houée-Lévin C, Bobrowski K, Horakova L, Karademir B, Schöneich C, Davies MJ, Spickett CM. 2015. Exploring oxidative modifications of tyrosine: an update on mechanisms of formation, advances in analysis and biological consequences. Free Radic. Res. 49, 347-373. ( 10.3109/10715762.2015.1007968) [DOI] [PubMed] [Google Scholar]
- 473.Eberlein G, Bruice TC, Lazarus RA, Henrie R, Benkovic SJ. 1984. The interconversion of the 5, 6, 7, 8-tetrahydro-, 6, 7, 8-dihydro-, and radical forms of 6, 6, 7, 7-tetramethyldihydropterin. a model for the biopterin center of aromatic amino acid mixed function oxidases. J. Am. Chem. Soc. 106, 7916-7924. ( 10.1021/ja00337a047) [DOI] [Google Scholar]
- 474.Adams JD, Klaidman LK, Ribeiro P. 1997. Tyrosine hydroxylase: mechanisms of oxygen radical formation. Redox Rep. 3, 273-279. ( 10.1080/13510002.1997.11747123) [DOI] [PubMed] [Google Scholar]
- 475.Roberts KM, Fitzpatrick PF. 2013. Mechanisms of tryptophan and tyrosine hydroxylase. IUBMB Life 65, 350-357. ( 10.1002/iub.1144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.McCormick JP, Thomason T. 1978. Near-ultraviolet photooxidation of tryptophan. proof of formation of superoxide ion. J. Am. Chem. Soc. 100, 312-313. ( 10.1021/ja00469a068) [DOI] [Google Scholar]
- 477.Saito I, Matsuura T, Inoue K. 1981. Formation of superoxide ion from singlet oxygen. Use of a water-soluble singlet oxygen source. J. Am. Chem. Soc. 103, 188-190. ( 10.1021/ja00391a035) [DOI] [Google Scholar]
- 478.Franks NP, Dickinson R, de Sousa SLM, Hall AC, Lieb WR. 1998. How does xenon produce anaesthesia? Nature 396, 324-324. ( 10.1038/24525) [DOI] [PubMed] [Google Scholar]
- 479.Turin L, Skoulakis EMC, Horsfield AP. 2014. Electron spin changes during general anesthesia in Drosophila. Proc. Natl Acad. Sci. USA 111, E3524-E3533. ( 10.1073/pnas.1404387111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Traynelis SF, et al. 2010. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 62, 405-496. ( 10.1124/pr.109.002451) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.de Sousa SLM, Dickinson R, Lieb WR, Franks NP. 2000. Contrasting synaptic actions of the inhalational general anesthetics isoflurane and xenon. Anesthesiology 92, 1055-1066. ( 10.1097/00000542-200004000-00024) [DOI] [PubMed] [Google Scholar]
- 482.Dickinson R, Peterson BK, Banks P, Simillis C, Martin JCS, Valenzuela CA, Maze M, Franks NP. 2007. Competitive inhibition at the glycine site of the N-methyl-d-aspartate receptor by the anesthetics xenon and isoflurane. Anesthesiology 107, 756-767. ( 10.1097/01.anes.0000287061.77674.71) [DOI] [PubMed] [Google Scholar]
- 483.Armstrong SP, et al. 2012. Identification of two mutations (F758W and F758Y) in the N-methyl-d-aspartate receptor glycine-binding site that selectively prevent competitive inhibition by xenon without affecting glycine binding. Anesthesiology 117, 38-47. ( 10.1097/aln.0b013e31825ada2e) [DOI] [PubMed] [Google Scholar]
- 484.Byrdin M, Villette S, Eker APM, Brettel K. 2007. Observation of an intermediate tryptophanyl radical in W306F mutant DNA photolyase from Escherichia coli supports electron hopping along the triple tryptophan chain. Biochemistry 46, 10072-10077. ( 10.1021/bi700891f) [DOI] [PubMed] [Google Scholar]
- 485.Li YF, Heelis PF, Sancar A. 1991. Active site of DNA photolyase: tryptophan-306 is the intrinsic hydrogen atom donor essential for flavin radical photoreduction and DNA repair in vitro. Biochemistry 30, 6322-6329. ( 10.1021/bi00239a034) [DOI] [PubMed] [Google Scholar]
- 486.Williams K, Pahk AJ, Kashiwagi K, Masuko T, Nguyen ND, Igarashi K. 1998. The selectivity filter of the N-methyl-d-aspartate receptor: a tryptophan residue controls block and permeation of Mg2+. Mol. Pharmacol. 53, 933-941. [PubMed] [Google Scholar]
- 487.Buck D, Howitt S, Clements J. 2000. NMDA channel gating is influenced by a tryptophan residue in the M2 domain but calcium permeation is not altered. Biophys. J. 79, 2454-2462. ( 10.1016/s0006-3495(00)76488-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Furukawa H. 2003. Mechanisms of activation, inhibition and specificity: crystal structures of the NMDA receptor NR1 ligand-binding core. EMBO J. 22, 2873-2885. ( 10.1093/emboj/cdg303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Aizenman E, Hartnett KA, Reynoldst IJ. 1990. Oxygen free radicals regulate NMDA receptor function via a redox modulatory site. Neuron 5, 841-846. ( 10.1016/0896-6273(90)90343-e) [DOI] [PubMed] [Google Scholar]
- 490.Girouard H, Wang G, Gallo EF, Anrather J, Zhou P, Pickel VM, Iadecola C. 2009. NMDA receptor activation increases free radical production through nitric oxide and NOX2. J. Neurosci. 29, 2545-2552. ( 10.1523/jneurosci.0133-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Betzen C, White R, Zehendner CM, Pietrowski E, Bender B, Luhmann HJ, Kuhlmann CR. 2009. Oxidative stress upregulates the NMDA receptor on cerebrovascular endothelium. Free Radical Biol. Med. 47, 1212-1220. ( 10.1016/j.freeradbiomed.2009.07.034) [DOI] [PubMed] [Google Scholar]
- 492.Dukoff DJ, Hogg DW, Hawrsyh PJ, Buck LT. 2014. Scavenging ROS dramatically increases NMDA receptor whole cell currents in painted turtle cortical neurons. J. Exp. Biol. 217, 3346-3355. ( 10.1242/jeb.105825) [DOI] [PubMed] [Google Scholar]
- 493.Turin L, Skoulakis EM. 2018. Electron spin resonance (EPR) in Drosophila and general anesthesia. Methods Enzymol. 603, 115–128. ( 10.1016/bs.mie.2018.01.020) [DOI]
- 494.Berridge MJ, Downes C, Hanley MR. 1989. Neural and developmental actions of lithium: a unifying hypothesis. Cell 59, 411-419. ( 10.1016/0092-8674(89)90026-3) [DOI] [PubMed] [Google Scholar]
- 495.Klein PS, Melton DA. 1996. A molecular mechanism for the effect of lithium on development. Proc. Natl Acad. Sci. USA 93, 8455-8459. ( 10.1073/pnas.93.16.8455) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Geddes JR, Miklowitz DJ. 2013. Treatment of bipolar disorder. Lancet 381, 1672-1682. ( 10.1016/s0140-6736(13)60857-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Grande I, Berk M, Birmaher B, Vieta E. 2016. Bipolar disorder. Lancet 387, 1561-1572. ( 10.1016/s0140-6736(15)00241-x) [DOI] [PubMed] [Google Scholar]
- 498.Vieta E, et al. 2018. Bipolar disorders. Nat. Rev. Dis. Primers 4, 1-16. ( 10.1038/nrdp.2018.8) [DOI] [PubMed] [Google Scholar]
- 499.Burdick KE, et al. 2020. The association between lithium use and neurocognitive performance in patients with bipolar disorder. Neuropsychopharmacology 45, 1743-1749. ( 10.1038/s41386-020-0683-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Jope RS. 1999. Anti-bipolar therapy: mechanism of action of lithium. Mol. Psychiatry 4, 117-128. ( 10.1038/sj.mp.4000494) [DOI] [PubMed] [Google Scholar]
- 501.Berk M, et al. 2011. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci. Biobehav. Rev. 35, 804-817. ( 10.1016/j.neubiorev.2010.10.001) [DOI] [PubMed] [Google Scholar]
- 502.Andreazza AC, Kauer-Sant’Anna M, Frey BN, Bond DJ, Kapczinski F, Young LT, Yatham LN. 2008. Oxidative stress markers in bipolar disorder: a meta-analysis. J. Affect. Disord. 111, 135-144. ( 10.1016/j.jad.2008.04.013) [DOI] [PubMed] [Google Scholar]
- 503.Yumru M, Savas HA, Kalenderoglu A, Bulut M, Celik H, Erel O. 2009. Oxidative imbalance in bipolar disorder subtypes: a comparative study. Progr. Neuro-Psychopharmacol. Biol. Psychiat. 33, 1070-1074. ( 10.1016/j.pnpbp.2009.06.005) [DOI] [PubMed] [Google Scholar]
- 504.Steckert AV, Valvassori SS, Moretti M, Dal-Pizzol F, Quevedo J. 2010. Role of oxidative stress in the pathophysiology of bipolar disorder. Neurochem. Res. 35, 1295-1301. ( 10.1007/s11064-010-0195-2) [DOI] [PubMed] [Google Scholar]
- 505.Wang J-F, Shao L, Sun X, Young LT. 2009. Increased oxidative stress in the anterior cingulate cortex of subjects with bipolar disorder and schizophrenia. Bipolar Disord. 11, 523-529. ( 10.1111/j.1399-5618.2009.00717.x) [DOI] [PubMed] [Google Scholar]
- 506.Salim S. 2014. Oxidative stress and psychological disorders. Curr. Neuropharmacol. 12, 140-147. ( 10.2174/1570159x11666131120230309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Ng F, Berk M, Dean O, Bush AI. 2008. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int. J. Neuropsychopharmacol. 11, 851-876. ( 10.1017/s1461145707008401) [DOI] [PubMed] [Google Scholar]
- 508.Lee SY, Lee SJ, Han C, Patkar AA, Masand PS, Pae CU. 2013. Oxidative/nitrosative stress and antidepressants: targets for novel antidepressants. Progr. Neuro-Psychopharmacol. Biol. Psychiat. 46, 224-235. ( 10.1016/j.pnpbp.2012.09.008) [DOI] [PubMed] [Google Scholar]
- 509.Brown NC, Andreazza AC, Young LT. 2014. An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res. 218, 61-68. ( 10.1016/j.psychres.2014.04.005) [DOI] [PubMed] [Google Scholar]
- 510.Machado-Vieira R. 2011. Effects of lithium on oxidative stress parameters in healthy subjects. Mol. Med. Rep. 5, 680-682. ( 10.3892/mmr.2011.732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Machado-Vieira R, et al. 2007. Oxidative stress parameters in unmedicated and treated bipolar subjects during initial manic episode: a possible role for lithium antioxidant effects. Neurosci. Lett. 421, 33-36. ( 10.1016/j.neulet.2007.05.016) [DOI] [PubMed] [Google Scholar]
- 512.Frey BN, et al. 2006. Effects of lithium and valproate on amphetamine-induced oxidative stress generation in an animal model of mania. J. Psychiatry Neurosci. 31, 326-332. [PMC free article] [PubMed] [Google Scholar]
- 513.de Sousa RT, Zarate CA Jr, Zanetti MV, Costa AC, Talib LL, Gattaz WF, Machado-Vieira R. 2014. Oxidative stress in early stage bipolar disorder and the association with response to lithium. J. Psychiatr. Res. 50, 36-41. ( 10.1016/j.jpsychires.2013.11.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Takahashi JS, Hong H-K, Ko CH, McDearmon EL. 2008. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat. Rev. Genet. 9, 764-775. ( 10.1038/nrg2430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Lee H-J. 2019. Circadian misalignment and bipolar disorder. Chronobiol. Med. 1, 132-136. ( 10.33069/cim.2019.0027) [DOI] [Google Scholar]
- 516.Porcu A, Gonzalez R, McCarthy MJ. 2019. Pharmacological manipulation of the circadian clock: a possible approach to the management of bipolar disorder. CNS Drugs 33, 981-999. ( 10.1007/s40263-019-00673-9) [DOI] [PubMed] [Google Scholar]
- 517.Fang L, Yu Q, Yin F, Yu J, Zhang Y, Zhang Y, Zhu D, Qin X. 2021. Combined cortisol and melatonin measurements with detailed parameter analysis can assess the circadian rhythms in bipolar disorder patients. Brain Behav. 11, e02186. ( 10.1002/brb3.2186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Engelmann W. 1973. A slowing down of circadian rhythms by lithium ions. Zeitschrift für Naturforschung C 28, 733-736. ( 10.1515/znc-1973-11-1214) [DOI] [PubMed] [Google Scholar]
- 519.Delius K, Günderoth-Palmowski M, Krause I, Engelmann W. 1984. Effects of lithium salts on the behaviour and the orcadian system of Mesocricetus auratus W. J. Interdiscip. Cycle Res. 15, 289-299. ( 10.1080/09291018409359861) [DOI] [Google Scholar]
- 520.Possidente B, Exner RH. 1986. Gene-dependent effect of lithium on circadian rhythms in mice (Mus musculus). Chronobiol. Int. 3, 17-21. ( 10.3109/07420528609083155) [DOI] [PubMed] [Google Scholar]
- 521.Klemfuss H. 1992. Rhythms and the pharmacology of lithium. Pharmacol. Ther. 56, 53-78. ( 10.1016/0163-7258(92)90037-z) [DOI] [PubMed] [Google Scholar]
- 522.Moreira J, Geoffroy PA. 2016. Lithium and bipolar disorder: impacts from molecular to behavioural circadian rhythms. Chronobiol. Int. 33, 351-373. ( 10.3109/07420528.2016.1151026) [DOI] [PubMed] [Google Scholar]
- 523.Geoffroy PA, et al. 2017. Lithium response in bipolar disorders and core clock genes expression. World J. Biol. Psychiatry 19, 619-632. ( 10.1080/15622975.2017.1282174) [DOI] [PubMed] [Google Scholar]
- 524.McCarthy MJ, et al. 2018. Chronotype and cellular circadian rhythms predict the clinical response to lithium maintenance treatment in patients with bipolar disorder. Neuropsychopharmacology 44, 620-628. ( 10.1038/s41386-018-0273-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Wei H, Landgraf D, Wang G, McCarthy MJ. 2018. Inositol polyphosphates contribute to cellular circadian rhythms: implications for understanding lithium’s molecular mechanism. Cell. Signal. 44, 82-91. ( 10.1016/j.cellsig.2018.01.001) [DOI] [PubMed] [Google Scholar]
- 526.Papiol S, Heilbronner U, Hou L, McCarthy M, Nievergelt C, Byrne E, McMahon F, Schulze T. 2019. Comprehensive evaluation of enrichment for circadian clock gene sets in psychiatric traits: specific enrichment in clinical response to lithium. Eur. Neuropsychopharmacol. 29, S932. ( 10.1016/j.euroneuro.2017.08.270) [DOI] [Google Scholar]
- 527.Sawai Y, Okamoto T, Muranaka Y, Nakamura R, Matsumura R, Node K, Akashi M. 2019. In vivo evaluation of the effect of lithium on peripheral circadian clocks by real-time monitoring of clock gene expression in near-freely moving mice. Sci. Rep. 9, 1-12. ( 10.1038/s41598-019-47053-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Andrabi M, et al. 2019. Lithium acts to modulate abnormalities at behavioral, cellular, and molecular levels in sleep deprivation-induced mania-like behavior. Bipolar Disord. 22, 266-280. ( 10.1111/bdi.12838) [DOI] [PubMed] [Google Scholar]
- 529.Sanghani HR, et al. 2020. Patient fibroblast circadian rhythms predict lithium sensitivity in bipolar disorder. Mol. Psychiatry 26, 5252-5265. ( 10.1038/s41380-020-0769-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Xu N, Shinohara K, Saunders KEA, Geddes JR, Cipriani A. 2021. Effect of lithium on circadian rhythm in bipolar disorder: a systematic review and meta-analysis. Bipolar Disord. 23, 445-453. ( 10.1111/bdi.13070) [DOI] [PubMed] [Google Scholar]
- 531.Federoff M, et al. 2021. Correction of depression-associated circadian rhythm abnormalities is associated with lithium response in bipolar disorder. Bipolar Disord. 0, 0-0. ( 10.1111/bdi.13162) [DOI] [PubMed] [Google Scholar]
- 532.Osland TM, Fernø J, Håvik B, Heuch I, Ruoff P, Lærum OD, Steen VM. 2010. Lithium differentially affects clock gene expression in serum-shocked NIH-3t3 cells. J. Psychopharmacol. 25, 924-933. ( 10.1177/0269881110379508) [DOI] [PubMed] [Google Scholar]
- 533.LeSauter J, Silver R. 1993. Lithium lengthens the period of circadian rhythms in lesioned hamsters bearing SCN grafts. Biol. Psychiatry 34, 75-83. ( 10.1016/0006-3223(93)90259-g) [DOI] [PubMed] [Google Scholar]
- 534.Abe M, Herzog ED, Block GD. 2000. Lithium lengthens the circadian period of individual suprachiasmatic nucleus neurons. NeuroReport 11, 3261-3264. ( 10.1097/00001756-200009280-00042) [DOI] [PubMed] [Google Scholar]
- 535.Yoshikawa T, Honma S. 2016. Lithium lengthens circadian period of cultured brain slices in area specific manner. Behav. Brain Res. 314, 30-37. ( 10.1016/j.bbr.2016.07.045) [DOI] [PubMed] [Google Scholar]
- 536.Vadnie CA, McClung CA. 2017. Circadian rhythm disturbances in mood disorders: insights into the role of the suprachiasmatic nucleus. Neural Plast. 2017, 1-28. ( 10.1155/2017/1504507) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.van der Horst GTJ, et al. 1999. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 398, 627-630. ( 10.1038/19323) [DOI] [PubMed] [Google Scholar]
- 538.Welsh DK, Takahashi JS, Kay SA. 2010. Suprachiasmatic nucleus: cell autonomy and network properties. Annu. Rev. Physiol. 72, 551-577. ( 10.1146/annurev-physiol-021909-135919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Lavebratt C, et al. 2010. CRY2 is associated with depression. PLoS ONE 5, e9407. ( 10.1371/journal.pone.0009407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Schnell A, Sandrelli F, Ranc V, Ripperger JA, Brai E, Alberi L, Rainer G, Albrecht U. 2015. Mice lacking circadian clock components display different mood-related behaviors and do not respond uniformly to chronic lithium treatment. Chronobiol. Int. 32, 1075-1089. ( 10.3109/07420528.2015.1062024) [DOI] [PubMed] [Google Scholar]
- 541.Hühne A, Volkmann P, Stephan M, Rossner M, Landgraf D. 2020. An in-depth neurobehavioral characterization shows anxiety-like traits, impaired habituation behavior, and restlessness in male Cryptochrome-deficient mice. Genes, Brain Behav. 19, e12661. ( 10.1111/gbb.12661) [DOI] [PubMed] [Google Scholar]
- 542.Sokolowska E, et al. 2020. The circadian gene Cryptochrome 2 influences stress-induced brain activity and depressive-like behavior in mice. Genes, Brain Behav. 20, e12708. ( 10.1111/gbb.12708) [DOI] [PubMed] [Google Scholar]
- 543.Sherrard RM, et al. 2018. Low-intensity electromagnetic fields induce human cryptochrome to modulate intracellular reactive oxygen species. PLoS Biol. 16, e2006229. ( 10.1371/journal.pbio.2006229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Stambolic V, Ruel L, Woodgett JR. 1996. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr. Biol. 6, 1664-1669. ( 10.1016/s0960-9822(02)70790-2) [DOI] [PubMed] [Google Scholar]
- 545.Ryves W, Harwood AJ. 2001. Lithium inhibits glycogen synthase kinase-3 by competition for magnesium. Biochem. Biophys. Res. Commun. 280, 720-725. ( 10.1006/bbrc.2000.4169) [DOI] [PubMed] [Google Scholar]
- 546.Iwahana E, Akiyama M, Miyakawa K, Uchida A, Kasahara J, Fukunaga K, Hamada T, Shibata S. 2004. Effect of lithium on the circadian rhythms of locomotor activity and glycogen synthase kinase-3 protein expression in the mouse suprachiasmatic nuclei. Eur. J. Neurosci. 19, 2281-2287. ( 10.1111/j.0953-816x.2004.03322.x) [DOI] [PubMed] [Google Scholar]
- 547.Fang X, Yu SX, Lu Y, Bast RC Jr, Woodgett JR, Mills GB. 2000. Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc. Natl Acad. Sci. USA 97, 11 960-11 965. ( 10.1073/pnas.220413597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Jope RS, Johnson GV. 2004. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem. Sci. 29, 95-102. ( 10.1016/j.tibs.2003.12.004) [DOI] [PubMed] [Google Scholar]
- 549.Beurel E, Grieco SF, Jope RS. 2015. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol. Ther. 148, 114-131. ( 10.1016/j.pharmthera.2014.11.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Yin L, Wang J, Klein PS, Lazar MA. 2006. Nuclear receptor rev-erbα is a critical lithium-sensitive component of the circadian clock. Science 311, 1002-1005. ( 10.1126/science.1121613) [DOI] [PubMed] [Google Scholar]
- 551.Iitaka C, Miyazaki K, Akaike T, Ishida N. 2005. A role for glycogen synthase kinase-3β in the mammalian circadian clock. J. Biol. Chem. 280, 29 397-29 402. ( 10.1074/jbc.m503526200) [DOI] [PubMed] [Google Scholar]
- 552.Harada Y, Sakai M, Kurabayashi N, Hirota T, Fukada Y. 2005. Ser-557-phosphorylated mCRY2 is degraded upon synergistic phosphorylation by glycogen synthase kinase-3β. J. Biol. Chem. 280, 31 714-31 721. ( 10.1074/jbc.m506225200) [DOI] [PubMed] [Google Scholar]
- 553.Yin L, Joshi S, Wu N, Tong X, Lazar MA. 2010. E3 ligases Arf-bp1 and Pam mediate lithium-stimulated degradation of the circadian heme receptor Rev-erbα. Proc. Natl Acad. Sci. USA 107, 11 614-11 619. ( 10.1073/pnas.1000438107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Sahar S, Zocchi L, Kinoshita C, Borrelli E, Sassone-Corsi P. 2010. Regulation of BMAL1 protein stability and circadian function by GSK3β-mediated phosphorylation. PLoS ONE 5, e8561. ( 10.1371/journal.pone.0008561) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Spengler ML, Kuropatwinski KK, Schumer M, Antoch M. 2009. A serine cluster mediates BMAL1-dependent CLOCK phosphorylation and degradation. Cell Cycle 8, 4138-4146. ( 10.4161/cc.8.24.10273) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Besing RC, Rogers CO, Paul JR, Hablitz LM, Johnson RL, McMahon LL, Gamble KL. 2017. GSK3 activity regulates rhythms in hippocampal clock gene expression and synaptic plasticity. Hippocampus 27, 890-898. ( 10.1002/hipo.22739) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Breit A, Miek L, Schredelseker J, Geibel M, Merrow M, Gudermann T. 2018. Insulin-like growth factor-1 acts as a zeitgeber on hypothalamic circadian clock gene expression via glycogen synthase kinase-3β signaling. J. Biol. Chem. 293, 17 278-17 290. ( 10.1074/jbc.ra118.004429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Buchachenko A, Shchegoleva L, Breslavskaya N. 2009. Paramagnetic complexes of magnesium as mediators in enzymatic ATP synthesis: DFT calculations of magnetic parameters. Chem. Phys. Lett. 483, 77-80. ( 10.1016/j.cplett.2009.10.044) [DOI] [Google Scholar]
- 559.Buchachenko AL, Kuznetsov DA, Breslavskaya NN. 2010. Ion-radical mechanism of enzymatic ATP synthesis: DFT calculations and experimental control. J. Phys. Chem. B 114, 2287-2292. ( 10.1021/jp909992z) [DOI] [PubMed] [Google Scholar]
- 560.Loros JJ, Denome SA, Dunlap JC. 1989. Molecular cloning of genes under control of the circadian clock in neurospora. Science 243, 385-388. ( 10.1126/science.2563175) [DOI] [PubMed] [Google Scholar]
- 561.Harmer SL, Hogenesch JB, Straume M, Chang HS, Han B, Zhu T, Wang X, Kreps JA, Kay SA. 2000. Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science 290, 2110-2113. ( 10.1126/science.290.5499.2110) [DOI] [PubMed] [Google Scholar]
- 562.Beaver LM, Gvakharia BO, Vollintine TS, Hege DM, Stanewsky R, Giebultowicz JM. 2002. Loss of circadian clock function decreases reproductive fitness in males of Drosophila melanogaster. Proc. Natl Acad. Sci. USA 99, 2134-2139. ( 10.1073/pnas.032426699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Peek CB, et al. 2013. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science 342, 1243417. ( 10.1126/science.1243417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Ashbrook LH, Krystal AD, Fu Y-H, Ptáček LJ. 2019. Genetics of the human circadian clock and sleep homeostat. Neuropsychopharmacology 45, 45-54. ( 10.1038/s41386-019-0476-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Roenneberg T, Merrow M. 2016. The circadian clock and human health. Curr. Biol. 26, R432-R443. ( 10.1016/j.cub.2016.04.011) [DOI] [PubMed] [Google Scholar]
- 566.Takahashi JS. 2016. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 18, 164-179. ( 10.1038/nrg.2016.150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567.Roybal K, et al. 2007. Mania-like behavior induced by disruption of CLOCK. Proc. Natl Acad. Sci. USA 104, 6406-6411. ( 10.1073/pnas.0609625104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Taillard J, Sagaspe P, Philip P, Bioulac S. 2021. Sleep timing, chronotype and social jetlag: impact on cognitive abilities and psychiatric disorders. Biochem. Pharmacol. 0, 114438. ( 10.1016/j.bcp.2021.114438) [DOI] [PubMed] [Google Scholar]
- 569.Crnko S, Pré BCD, Sluijter JPG, Laake LWV. 2019. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat. Rev. Cardiol. 16, 437-447. ( 10.1038/s41569-019-0167-4) [DOI] [PubMed] [Google Scholar]
- 570.Battaglin F, et al. 2021. Clocking cancer: the circadian clock as a target in cancer therapy. Oncogene 40, 3187-3200. ( 10.1038/s41388-021-01778-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Sancar A, Gelder RNV. 2021. Clocks, cancer, and chronochemotherapy. Science 371, eabb0738. ( 10.1126/science.abb0738) [DOI] [PubMed] [Google Scholar]
- 572.Kondratova AA, Kondratov RV. 2012. The circadian clock and pathology of the ageing brain. Nat. Rev. Neurosci. 13, 325-335. ( 10.1038/nrn3208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Kondratova A, Antoch MP, Kondratov RV. 2010. Circadian clock proteins control adaptation to novel environment and memory formation. Aging 2, 285-297. ( 10.18632/aging.100142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Kyriacou CP, Hastings MH. 2010. Circadian clocks: genes, sleep, and cognition. Trends Cogn. Sci. 14, 259-267. ( 10.1016/j.tics.2010.03.007) [DOI] [PubMed] [Google Scholar]
- 575.Liang C, et al. 2020. Stabilization of heterochromatin by CLOCK promotes stem cell rejuvenation and cartilage regeneration. Cell Res. 31, 187-205. ( 10.1038/s41422-020-0385-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Maiese K. 2021. Cognitive impairment and dementia: gaining insight through circadian clock gene pathways. Biomolecules 11, 1002. ( 10.3390/biom11071002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Lazopulo S, Lazopulo A, Baker JD, Syed S. 2019. Daytime colour preference in Drosophila depends on the circadian clock and TRP channels. Nature 574, 108-111. ( 10.1038/s41586-019-1571-y) [DOI] [PubMed] [Google Scholar]
- 578.Allada R, Chung BY. 2010. Circadian organization of behavior and physiology in Drosophila. Annu. Rev. Physiol. 72, 605-624. ( 10.1146/annurev-physiol-021909-135815) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Tataroglu O, Emery P. 2014. Studying circadian rhythms in Drosophila melanogaster. Methods 68, 140-150. ( 10.1016/j.ymeth.2014.01.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Patke A, Young MW, Axelrod S. 2019. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 21, 67-84. ( 10.1038/s41580-019-0179-2) [DOI] [PubMed] [Google Scholar]
- 581.Lewczuk B, Redlarski G, Zak A, Ziólkowska N, Przybylska-Gornowicz B, Krawczuk M. 2014. Influence of electric, magnetic, and electromagnetic fields on the circadian system: current stage of knowledge. BioMed Res. Int. 2014, 1-13. ( 10.1155/2014/169459) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Vanderstraeten J, Burda H, Verschaeve L, Brouwer CD. 2015. Could magnetic fields affect the circadian clock function of cryptochromes? Testing the basic premise of the cryptochrome hypothesis (ELF magnetic fields). Health Phys. 109, 84-89. ( 10.1097/hp.0000000000000292) [DOI] [PubMed] [Google Scholar]
- 583.Yoshii T, Ahmad M, Helfrich-Förster C. 2009. Cryptochrome mediates light-dependent magnetosensitivity of Drosophila’s circadian clock. PLoS Biol. 7, e1000086. ( 10.1371/journal.pbio.1000086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Dokucu ME, Yu L, Taghert PH. 2005. Lithium- and valproate-induced alterations in circadian locomotor behavior in Drosophila. Neuropsychopharmacology 30, 2216-2224. ( 10.1038/sj.npp.1300764) [DOI] [PubMed] [Google Scholar]
- 585.Lai AG, Doherty CJ, Mueller-Roeber B, Kay SA, Schippers JH, Dijkwel PP. 2012. CIRCADIAN CLOCK-ASSOCIATED 1 regulates ROS homeostasis and oxidative stress responses. Proc. Natl Acad. Sci. USA 109, 17129-17134. ( 10.1073/pnas.1209148109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Gyöngyösi N, Káldi K. 2014. Interconnections of reactive oxygen species homeostasis and circadian rhythm in Neurospora crassa. Antioxid. Redox Signal. 20, 3007-3023. ( 10.1089/ars.2013.5558) [DOI] [PubMed] [Google Scholar]
- 587.Jiménez A, Sevilla F, Martı MC. 2021. Reactive oxygen species homeostasis and circadian rhythms in plants. J. Exp. Bot. 72, 5825-5840. ( 10.1093/jxb/erab318) [DOI] [PubMed] [Google Scholar]
- 588.Ndiaye MA, Nihal M, Wood GS, Ahmad N. 2014. Skin, reactive oxygen species, and circadian clocks. Antioxid. Redox Signal. 20, 2982-2996. ( 10.1089/ars.2013.5645) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Manella G, Asher G. 2016. The circadian nature of mitochondrial biology. Front. Endocrinol. 7, 162. ( 10.3389/fendo.2016.00162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590.de Goede P, Wefers J, Brombacher EC, Schrauwen P, Kalsbeek A. 2018. Circadian rhythms in mitochondrial respiration. J. Mol. Endocrinol. 60, R115-R130. ( 10.1530/jme-17-0196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Mezhnina V, Ebeigbe OP, Poe A, Kondratov RV. 2022. Circadian control of mitochondria in ROS homeostasis. Antioxid. Redox Signal. 0, 0-0. ( 10.1089/ars.2021.0274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 592.Tyson JJ, Hong CI, Thron CD, Novak B. 1999. A simple model of circadian rhythms based on dimerization and proteolysis of PER and TIM. Biophys. J. 77, 2411-2417. ( 10.1016/s0006-3495(99)77078-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Leloup J-C, Gonze D, Goldbeter A. 1999. Limit cycle models for circadian rhythms based on transcriptional regulation in Drosophila and Neurospora. J. Biol. Rhythms 14, 433-448. ( 10.1177/074873099129000948) [DOI] [PubMed] [Google Scholar]
- 594.Player TC, Baxter EDA, Allatt S, Hore PJ. 2021. Amplification of weak magnetic field effects on oscillating reactions. Sci. Rep. 11, 1-9. ( 10.1038/s41598-021-88871-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Craddock TJA, Hameroff SR, Ayoub AT, Klobukowski M, Tuszynski JA. 2015. Anesthetics act in quantum channels in brain microtubules to prevent consciousness. Curr. Top. Med. Chem. 15, 523-533. ( 10.2174/1568026615666150225104543) [DOI] [PubMed] [Google Scholar]
- 596.Linganna RE, Levy WJ, Dmochowski IJ, Eckenhoff RG, Speck RM. 2015. Taxane modulation of anesthetic sensitivity in surgery for nonmetastatic breast cancer. J. Clin. Anesth. 27, 481-485. ( 10.1016/j.jclinane.2015.05.001) [DOI] [PubMed] [Google Scholar]
- 597.Perouansky M. 2012. The quest for a unified model of anesthetic action. Anesthesiology 117, 465-474. ( 10.1097/aln.0b013e318264492e) [DOI] [PubMed] [Google Scholar]
- 598.Xi J, Liu R, Asbury GR, Eckenhoff MF, Eckenhoff RG. 2004. Inhalational anesthetic-binding proteins in rat neuronal membranes. J. Biol. Chem. 279, 19628-19633. ( 10.1074/jbc.m313864200) [DOI] [PubMed] [Google Scholar]
- 599.Pan JZ, Xi J, Tobias JW, Eckenhoff MF, Eckenhoff RG. 2006. Halothane binding proteome in human brain cortex. J. Proteome Res. 6, 582-592. ( 10.1021/pr060311u) [DOI] [PubMed] [Google Scholar]
- 600.Pan JZ, Xi J, Eckenhoff MF, Eckenhoff RG. 2008. Inhaled anesthetics elicit region-specific changes in protein expression in mammalian brain. PROTEOMICS 8, 2983-2992. ( 10.1002/pmic.200800057) [DOI] [PubMed] [Google Scholar]
- 601.Simon C. 2019. Can quantum physics help solve the hard problem of consciousness? J. Conscious. Stud. 26, 204-218. [Google Scholar]
- 602.Hameroff SR, Craddock TJA, Tuszynski JA. 2014. Quantum effects in the understanding of consciousness. J. Integr. Neurosci. 13, 229-252. ( 10.1142/s0219635214400093) [DOI] [PubMed] [Google Scholar]
- 603.Stuart H. 1998. Quantum computation in brain microtubules? The Penrose–Hameroff ‘orch OR‘ model of consciousness. Phil. Trans. R. Soc. Lond. Ser. A 356, 1869-1896. ( 10.1098/rsta.1998.0254) [DOI] [Google Scholar]
- 604.Hagan S, Hameroff SR, Tuszyński JA. 2002. Quantum computation in brain microtubules: decoherence and biological feasibility. Phys. Rev. E 65, 061901. ( 10.1103/physreve.65.061901) [DOI] [PubMed] [Google Scholar]
- 605.Hameroff S, Nip A, Porter M, Tuszynski J. 2002. Conduction pathways in microtubules, biological quantum computation, and consciousness. Biosystems 64, 149-168. ( 10.1016/s0303-2647(01)00183-6) [DOI] [PubMed] [Google Scholar]
- 606.Craddock TJ, Freedman H, Barakat KH, Damaraju S, Hameroff S, Tuszynski JA. 2012. Computational predictions of volatile anesthetic interactions with the microtubule cytoskeleton: implications for side effects of general anesthesia. PLoS ONE 7, e37251. ( 10.1371/journal.pone.0037251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Zhang W, Craddock TJ, Li Y, Swartzlander M, Alfano RR, Shi L. 2022. Fano resonance line shapes in the raman spectra of tubulin and microtubules reveal quantum effects. Biophys. Rep. 2, 100043. ( 10.1016/j.bpr.2021.100043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Hu H, Wu M. 2004. Spin-mediated consciousness theory: possible roles of neural membrane nuclear spin ensembles and paramagnetic oxygen. Med. Hypotheses 63, 633-646. ( 10.1016/j.mehy.2004.04.002) [DOI] [PubMed] [Google Scholar]
- 609.Fisher MP. 2015. Quantum cognition: the possibility of processing with nuclear spins in the brain. Ann. Phys. 362, 593-602. ( 10.1016/j.aop.2015.08.020) [DOI] [Google Scholar]
- 610.Chen R, Li N, Qian H, Zhao R-H, Zhang S-H. 2020. Experimental evidence refuting the assumption of phosphorus-31 nuclear-spin entanglement-mediated consciousness. J. Integr. Neurosci. 19, 595-600. ( 10.31083/j.jin.2020.04.250) [DOI] [PubMed] [Google Scholar]
- 611.Vassilev PM, Dronzine RT, Vassileva MP, Georgiev GA. 1982. Parallel arrays of microtubles formed in electric and magnetic fields. Biosci. Rep. 2, 1025-1029. ( 10.1007/bf01122171) [DOI] [PubMed] [Google Scholar]
- 612.Glade N, Tabony J. 2005. Brief exposure to high magnetic fields determines microtubule self-organisation by reaction–diffusion processes. Biophys. Chem. 115, 29-35. ( 10.1016/j.bpc.2004.12.048) [DOI] [PubMed] [Google Scholar]
- 613.Bras W, Diakun GP, Diaz JF, Maret G, Kramer H, Bordas J, Medrano FJ. 1998. The susceptibility of pure tubulin to high magnetic fields: a magnetic birefringence and X-ray fiber diffraction study. Biophys. J. 74, 1509-1521. ( 10.1016/s0006-3495(98)77863-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Zhang L, et al. 2017. 27 T ultra-high static magnetic field changes orientation and morphology of mitotic spindles in human cells. eLife 6, e22911. ( 10.7554/elife.22911) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Qian A-R, et al. 2009. Large gradient high magnetic field affects the association of MACF1 with actin and microtubule cytoskeleton. Bioelectromagnetics 30, 545-555. ( 10.1002/bem.20511) [DOI] [PubMed] [Google Scholar]
- 616.Luo Y, Ji X, Liu J, Li Z, Wang W, Chen W, Wang J, Liu Q, Zhang X. 2016. Moderate intensity static magnetic fields affect mitotic spindles and increase the antitumor efficacy of 5-FU and taxol. Bioelectrochemistry 109, 31-40. ( 10.1016/j.bioelechem.2016.01.001) [DOI] [PubMed] [Google Scholar]
- 617.Wu X, Du J, Song W, Cao M, Chen S, Xia R. 2018. Weak power frequency magnetic fields induce microtubule cytoskeleton reorganization depending on the epidermal growth factor receptor and the calcium related signaling. PLoS ONE 13, e0205569. ( 10.1371/journal.pone.0205569) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Wilson C, González-Billault C. 2015. Regulation of cytoskeletal dynamics by redox signaling and oxidative stress: implications for neuronal development and trafficking. Front. Cell. Neurosci. 9, 381. ( 10.3389/fncel.2015.00381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Craddock TJ, Tuszynski JA, Chopra D, Casey N, Goldstein LE, Hameroff SR, Tanzi RE. 2012. The zinc dyshomeostasis hypothesis of Alzheimer’s disease. PLoS ONE 7, e33552. ( 10.1371/journal.pone.0033552) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620.Emre M, Cetiner S, Zencir S, Unlukurt I, Kahraman I, Topcu Z. 2010. Oxidative stress and apoptosis in relation to exposure to magnetic field. Cell Biochem. Biophys. 59, 71-77. ( 10.1007/s12013-010-9113-0) [DOI] [PubMed] [Google Scholar]
- 621.Goraca A, Ciejka E, Piechota A. 2010. Effects of extremely low frequency magnetic field on the parameters of oxidative stress in heart. J. Physiol. Pharmacol. 61, 333. [PubMed] [Google Scholar]
- 622.Amara S, Abdelmelek H, Garrel C, Guiraud P, Douki T, Ravanat JL, Favier A, Sakly M, Rhouma KB. 2007. Zinc supplementation ameliorates static magnetic field-induced oxidative stress in rat tissues. Environ. Toxicol. Pharmacol. 23, 193-197. ( 10.1016/j.etap.2006.09.001) [DOI] [PubMed] [Google Scholar]
- 623.Amara S, Abdelmelek H, Garrel C, Guiraud P, Douki T, Ravanat JL, Favier A, Sakly M, Rhouma KB. 2006. Influence of static magnetic field on cadmium toxicity: study of oxidative stress and DNA damage in rat tissues. J. Trace Elem. Med. Biol. 20, 263-269. ( 10.1016/j.jtemb.2006.07.002) [DOI] [PubMed] [Google Scholar]
- 624.Hajnorouzi A, Vaezzadeh M, Ghanati F. 2011. Growth promotion and a decrease of oxidative stress in maize seedlings by a combination of geomagnetic and weak electromagnetic fields. J. Plant Physiol. 168, 1123-1128. ( 10.1016/j.jplph.2010.12.003) [DOI] [PubMed] [Google Scholar]
- 625.Kthiri A, Hidouri S, Wiem T, Jeridi R, Sheehan D, Landouls A. 2019. Biochemical and biomolecular effects induced by a static magnetic field in Saccharomyces cerevisiae: evidence for oxidative stress. PLoS ONE 14, e0209843. ( 10.1371/journal.pone.0209843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Kamalipooya S, Abdolmaleki P, Salemi Z, Javani Jouni F, Zafari J, Soleimani H. 2017. Simultaneous application of cisplatin and static magnetic field enhances oxidative stress in HeLa cell line. In Vitro Cell. Dev. Biol. - Anim. 53, 783-790. ( 10.1007/s11626-017-0148-z) [DOI] [PubMed] [Google Scholar]
- 627.Politański P, et al. 2010. Static magnetic field affects oxidative stress in mouse cochlea. Int. J. Occup. Med. Environ. Health 23, 377. ( 10.2478/v10001-010-0041-4) [DOI] [PubMed] [Google Scholar]
- 628.Ghodbane S, Lahbib A, Ammari M, Mohsen Sakly HA. 2015. Does static magnetic field-exposure induced oxidative stress and apoptosis in rat kidney and muscle? Effect of vitamin E and selenium supplementations. Gen. Physiol. Biophys. 34, 23-32. ( 10.4149/gpb_2014027) [DOI] [PubMed] [Google Scholar]
- 629.Ahn H, Shin K, Lee H. 2020. Effects of pulsed magnetic field on the hemolysis of erythrocytes exposed to oxidative stress. Adv. Exp. Med. Biol. 1232, 263-269. ( 10.1007/978-3-030-34461-0_33) [DOI] [PubMed] [Google Scholar]
- 630.Akdag MZ, Dasdag S, Ulukaya E, Uzunlar AK, Kurt MA, Taskin A. 2010. Effects of extremely low-frequency magnetic field on caspase activities and oxidative stress values in rat brain. Biol. Trace Elem. Res. 138, 238-249. ( 10.1007/s12011-010-8615-3) [DOI] [PubMed] [Google Scholar]
- 631.Amara S, Douki T, Garrel C, Favier A, Ben Rhouma K, Sakly M, Abdelmelek H. 2010. Effects of static magnetic field and cadmium on oxidative stress and DNA damage in rat cortex brain and hippocampus. Toxicol. Ind. Health 27, 99-106. ( 10.1177/0748233710381887) [DOI] [PubMed] [Google Scholar]
- 632.Cui Y, Ge Z, Rizak JD, Zhai C, Zhou Z, Gong S, Che Y. 2012. Deficits in water maze performance and oxidative stress in the hippocampus and striatum induced by extremely low frequency magnetic field exposure. PLoS ONE 7, e32196. ( 10.1371/journal.pone.0032196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Chu LY, et al. 2012. Extremely low frequency magnetic field induces oxidative stress in mouse cerebellum. Gen. Physiol. Biophys. 30, 415-421. ( 10.4149/gpb_2011_04_415) [DOI] [PubMed] [Google Scholar]
- 634.Zielinski J, Ducray AD, Moeller AM, Murbach M, Kuster N, Mevissen M. 2020. Effects of pulse-modulated radiofrequency magnetic field (RF-EMF) exposure on apoptosis, autophagy, oxidative stress and electron chain transport function in human neuroblastoma and murine microglial cells. Toxicol. In Vitro 68, 104963. ( 10.1016/j.tiv.2020.104963) [DOI] [PubMed] [Google Scholar]
- 635.Mert T, Sahin E, Yaman S, Sahin M. 2020. Pulsed magnetic field treatment ameliorates the progression of peripheral neuropathy by modulating the neuronal oxidative stress, apoptosis and angiogenesis in a rat model of experimental diabetes. Arch. Physiol. Biochem. 0, 1-8. ( 10.1080/13813455.2020.1788098) [DOI] [PubMed] [Google Scholar]
- 636.Ciejka E, Kleniewska P, Skibska B, Goraca A. 2011. Effects of extremely low frequency magnetic field on oxidative balance in brain of rats. J. Physiol. Pharmacol. 62, 657. [PubMed] [Google Scholar]
- 637.Coballase-Urrutia E, Navarro L, Ortiz JL, Verdugo-Díaz L, Gallardo JM, Hernández ME, Estrada-Rojo F. 2018. Static magnetic fields modulate the response of different oxidative stress markers in a restraint stress model animal. BioMed Res. Int. 2018, 1-9. ( 10.1155/2018/3960408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Terzi A, Suter DM. 2020. The role of NADPH oxidases in neuronal development. Free Radical Biol. Med. 154, 33-47. ( 10.1016/j.freeradbiomed.2020.04.027) [DOI] [PubMed] [Google Scholar]
- 639.Hore PJ. 2012. Are biochemical reactions affected by weak magnetic fields? Proc. Natl Acad. Sci. USA 109, 1357-1358. ( 10.1073/pnas.1120531109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Crumpton MJ. 2005. The bernal lecture 2004 are low-frequency electromagnetic fields a health hazard? Phil. Trans. R. Soc. B 360, 1223-1230. ( 10.1098/rstb.2005.1663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Lacy-hulbert A, Metcalfe JC, Hesketh R. 1998. Biological responses to electromagnetic fields. FASEB J. 12, 395-420. ( 10.1096/fasebj.12.6.395) [DOI] [PubMed] [Google Scholar]
- 642.Jones AR, Scrutton NS, Woodward JR. 2006. Magnetic field effects and radical pair mechanisms in enzymes:a reappraisal of the horseradish peroxidase system. J. Am. Chem. Soc. 128, 8408-8409. ( 10.1021/ja060463q) [DOI] [PubMed] [Google Scholar]
- 643.Jones AR, Hay S, Woodward JR, Scrutton NS. 2007. Magnetic field effect studies indicate reduced geminate recombination of the radical pair in substrate-bound adenosylcobalamin-dependent ethanolamine ammonia lyase. J. Am. Chem. Soc. 129, 15 718-15 727. ( 10.1021/ja077124x) [DOI] [PubMed] [Google Scholar]
- 644.Harris SR, Henbest KB, Maeda K, Pannell JR, Timmel CR, Hore PJ, Okamoto H. 2009. Effect of magnetic fields on cryptochrome-dependent responses in Arabidopsis thaliana. J. R. Soc. Interface 6, 1193-1205. ( 10.1098/rsif.2008.0519) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645.Crotty D, Silkstone G, Poddar S, Ranson R, Prina-Mello A, Wilson MT, Coey JMD. 2011. Reexamination of magnetic isotope and field effects on adenosine triphosphate production by creatine kinase. Proc. Natl Acad. Sci. USA 109, 1437-1442. ( 10.1073/pnas.1117840108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Mattsson M-O, Simkó M. 2012. Is there a relation between extremely low frequency magnetic field exposure, inflammation and neurodegenerative diseases? A review of in vivo and in vitro experimental evidence. Toxicology 301, 1-12. ( 10.1016/j.tox.2012.06.011) [DOI] [PubMed] [Google Scholar]
- 647.Kaiser J. 2021. More than half of high-impact cancer lab studies could not be replicated in controversial analysis. Science 374, 000. ( 10.1126/science.acx9770) [DOI] [Google Scholar]
- 648.Buchachenko A. 2015. Why magnetic and electromagnetic effects in biology are irreproducible and contradictory? Bioelectromagnetics 37, 1-13. ( 10.1002/bem.21947) [DOI] [PubMed] [Google Scholar]
- 649.Tomanova K, Vacha M. 2016. The magnetic orientation of the antarctic amphipod Gondogeneia antarctica is cancelled by very weak radiofrequency fields. J. Exp. Biol. 11, 1717-1724. ( 10.1242/jeb.132878) [DOI] [PubMed] [Google Scholar]
- 650.Phillips J, et al. 2022. Why is it so difficult to study magnetic compass orientation in murine rodents? J. Comp. Physiol. A 208, 197-212. ( 10.1007/s00359-021-01532-z) [DOI] [PubMed] [Google Scholar]
- 651.Landler L, Painter MS, Youmans PW, Hopkins WA, Phillips JB. 2015. Spontaneous magnetic alignment by yearling snapping turtles: rapid association of radio frequency dependent pattern of magnetic input with novel surroundings. PLoS ONE 10, e0124728. ( 10.1371/journal.pone.0124728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652.McKay DS, et al. 1996. Search for past life on Mars: possible relic biogenic activity in martian meteorite ALH84001. Science 273, 924-930. ( 10.1126/science.273.5277.924) [DOI] [PubMed] [Google Scholar]
- 653.Hyodo R, Usui T. 2021. Searching for life on Mars and its moons. Science 373, 742-742. ( 10.1126/science.abj1512) [DOI] [PubMed] [Google Scholar]
- 654.Khan MW, Juutilainen J, Naarala J, Roivainen P. 2021. Residential extremely low frequency magnetic fields and skin cancer. Occup. Environ. Med. 79, 49-54. ( 10.1136/oemed-2021-107776) [DOI] [PubMed] [Google Scholar]
- 655.Burch JB, Reif JS, Yost MG, Keefe TJ, Pitrat CA. 1999. Reduced excretion of a melatonin metabolite in workers exposed to 60 Hz magnetic fields. Am. J. Epidemiol. 150, 27-36. ( 10.1093/oxfordjournals.aje.a009914) [DOI] [PubMed] [Google Scholar]
- 656.Zastko L, Makinistian L, Tvarožná A, Ferreyra FL, Belyaev I. 2021. Mapping of static magnetic fields near the surface of mobile phones. Sci. Rep. 11, 1-10. ( 10.1038/s41598-021-98083-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657.Adair RK. 2000. Static and low-frequency magnetic field effects: health risks and therapies. Rep. Prog. Phys. 63, 415. ( 10.1088/0034-4885/63/3/204) [DOI] [Google Scholar]
- 658.Touitou Y, Selmaoui B. 2012. The effects of extremely low-frequency magnetic fields on melatonin and cortisol, two marker rhythms of the circadian system. Dialogues Clin. Neurosci. 14, 381-399. ( 10.31887/dcns.2012.14.4/ytouitou) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 659.Diego-Rasilla FJ, Phillips JB. 2021. Evidence for the use of a high-resolution magnetic map by a short-distance migrant, the alpine newt (Ichthyosaura alpestris). J. Exp. Biol. 224, jeb238345. ( 10.1242/jeb.238345) [DOI] [PubMed] [Google Scholar]
- 660.Ramsay J, Kattnig DR. 2022. Radical triads, not pairs, may explain effects of hypomagnetic fields on neurogenesis. arXiv. ( 10.48550/ARXIV.2206.08192) [DOI]
- 661.Wootters WK. 1998. Entanglement of formation of an arbitrary state of two qubits. Phys. Rev. Lett. 80, 2245-2248. ( 10.1103/physrevlett.80.2245) [DOI] [Google Scholar]
- 662.Gauger EM, Rieper E, Morton JJL, Benjamin SC, Vedral V. 2011. Sustained quantum coherence and entanglement in the avian compass. Phys. Rev. Lett. 106, 040503. ( 10.1103/physrevlett.106.040503) [DOI] [PubMed] [Google Scholar]
- 663.Pauls JA, Zhang Y, Berman GP, Kais S. 2013. Quantum coherence and entanglement in the avian compass. Phys. Rev. E 87, 062704. ( 10.1103/physreve.87.062704) [DOI] [PubMed] [Google Scholar]
- 664.Zhang Y, Berman GP, Kais S. 2014. Sensitivity and entanglement in the avian chemical compass. Phys. Rev. E 90, 042707. ( 10.1103/physreve.90.042707) [DOI] [PubMed] [Google Scholar]
- 665.Kumar S, Boone K, Tuszyński J, Barclay P, Simon C. 2016. Possible existence of optical communication channels in the brain. Sci. Rep. 6, 1-3. ( 10.1038/srep36508) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.