Significance
Protein expression in nonnative cell types is critical for successful gene therapy. Here, we explore the expression of α1-antrypsin (AAT), a gene therapy target, in a variety of cell types and assess the ability of chemical methods to boost its expression. While AAT was efficiently secreted by hepatocytes, its native host, it was poorly expressed in myoblasts and myotubes, although its secretion could be augmented by treatment with the proteostasis regulator suberoylanilide hydroxamic acid, a histone deacetylase inhibitor. The use of proteostasis regulators thus provides a potential strategy to optimize protein expression for a variety of therapeutics.
Keywords: endoplasmic reticulum, proteostasis regulator, serpin, protein quality control, proteostasis
Abstract
Heterologous expression of proteins is used widely for the biosynthesis of biologics, many of which are secreted from cells. In addition, gene therapy and messenger RNA (mRNA) vaccines frequently direct the expression of secretory proteins to nonnative host cells. Consequently, it is crucial to understand the maturation and trafficking of proteins in a range of host cells including muscle cells, a popular therapeutic target due to the ease of accessibility by intramuscular injection. Here, we analyzed the production efficiency for α1-antitrypsin (AAT) in Chinese hamster ovary cells, commonly used for biotherapeutic production, and myoblasts (embryonic progenitor cells of muscle cells) and compared it to the production in the major natural cells, liver hepatocytes. AAT is a target protein for gene therapy to address pathologies associated with insufficiencies in native AAT activity or production. AAT secretion and maturation were most efficient in hepatocytes. Myoblasts were the poorest of the cell types tested; however, secretion of active AAT was significantly augmented in myoblasts by treatment with the proteostasis regulator suberoylanilide hydroxamic acid, a histone deacetylase inhibitor. These findings were extended and validated in myotubes (mature muscle cells) where AAT was transduced using an adeno-associated viral capsid transduction method used in gene therapy clinical trials. Overall, our study sheds light on a possible mechanism to enhance the efficacy of gene therapy approaches for AAT and, moreover, may have implications for the production of proteins from mRNA vaccines, which rely on the expression of viral glycoproteins in nonnative host cells upon intramuscular injection.
Secretory pathway clients include both membrane and soluble proteins with final destinations within the secretory/endocytic pathway, the plasma membrane, or the extracellular environment. These proteins comprise approximately one-third of the human proteome. They are first targeted to the endoplasmic reticulum (ER) where they fold, assemble, and receive modifications prior to further trafficking in the secretory pathway to their final destinations (1). The diversity and load of secretory pathway cargo (i.e., the secretory pathway proteome) are species and cell type dependent. The ER comprises a significant fraction of the cellular volume for pancreatic acinar and plasma cells, both of which support the production and secretion of large amounts of proteins each day (2). With the explosive growth in the use of biologics as therapeutics, as well as advances in gene therapy and messenger RNA (mRNA) vaccines, all approaches where proteins are commonly not expressed in their natural biosynthetic host cells, it is important to understand the efficiency of heterologous expression of proteins in various cell types and how this can be optimized to produce active protein.
Proteases are key players in physiological processes ranging from tissue remodeling in whole organisms to regulation of the cell cycle. Because poorly controlled protein degradation can damage tissues and cells, this process must be under tight temporal and spatial regulation. Protease inhibitors, particularly the inhibitory serpins, are key to this regulation, and inadequate levels of these protease inhibitors can lead to disease. The most abundant protease inhibitor in human plasma is the serpin α1-antitrypsin (AAT), which mainly regulates the serine proteases human neutrophil elastase (HNE), proteinase 3, and cathepsin G, which are released by leukocytes (3). High AAT plasma levels (at least 11 μM [4, 5]) help ensure that these proteases are active at sites of inflammation and infection and that active forms of the proteases do not freely circulate. Mutations in AAT can lead to low AAT plasma levels and excessive cleavage of lung elastin, primarily by action of HNE, resulting in debilitating lung disease (6, 7). AAT augmentation therapy provides some redress to such AAT deficiencies; however, this cumbersome and expensive treatment slows but does not halt lung disease progression (8, 9).
In addition to lowering circulating AAT levels, some mutations, such as the relatively common Z (Glu342Lys) mutation (AAT-Z), lead to AAT misfolding and accumulation in the ER (7, 10, 11). ER-retained AAT-Z can be cleared by proteasomal and lysosomal degradation (12–14). However, clearance is not always efficient, and accumulation of AAT polymers can result in cell death. Because hepatocytes are the major source of circulating AAT, AAT-Z and other mutations can lead to liver damage and disease (15). AAT augmentation therapy does not offset liver damage, and liver transplantation is currently the only available treatment for severe liver disease caused by AAT misfolding (7, 15). Thus, better treatments for the AAT deficiencies are clearly needed.
Treatments under development include gene therapy approaches to express wild-type AAT (AAT-M) in patients with AAT deficiencies and small molecule pharmacological chaperones or chemical chaperone therapies aimed at rescuing the folding of AAT mutants (16–19). Genetic approaches include silencing to lower the production of pathogenic AAT mutants with a goal of reducing liver damage, gene therapy to increase circulating levels of AAT-M to reduce lung damage, or combinations of the two approaches in order to treat both pathologies. Some current gene therapy approaches target hepatocytes (18, 20–22), the major AAT producer, and others are directed toward cells not specialized for secretion such as skeletal muscle, which provide ease in accessibility (21–24). Several groups have used recombinant adeno-associated virus (rAAV) as delivery vehicles for protein expression. The first rAAV gene therapy fully approved by the European Medicines Agency was alipogene tiparvovec, a rAAV1 vector injected into skeletal muscle for ectopic expression of lipoprotein lipase (25). It is worth noting that rAAV vectors have been associated with liver toxicity and that the hepatotoxicity of rAAV-AAT vectors was increased in nonhuman primates with preexisting liver disease, a situation that could be encountered in AAT-deficient patients (26–28). These approaches often use strong, nonnative promoters to maximize AAT-M production, and successful therapy depends on the proper folding and maturation of AAT-M when overexpressed in a variety of cell types. In addition, while some gene therapies may be directly injected into a tissue such as the skeletal muscle–targeted AAT gene therapy (29), approaches that are delivered systemically may affect a variety of cell types. Similarly, chemical chaperones would be administered systemically, preferably in pill form, and while hepatocytes may be the major target of such therapeutics, many cell types other than hepatocytes secrete AAT, and these small molecules are likely to also affect these cells (30–37).
Secretory proteins such as AAT fold and mature in the ER where they interact with the protein quality-control machinery. This quality-control machinery is abundant in secretory cell types such as hepatocytes that make and secrete large quantities of proteins and reduced in cell types not specialized for secretion such as skeletal muscle myofibrils (38). These differences are likely to impact the efficiency of AAT maturation and may modulate the effects of gene therapies and chemical chaperones currently under development. To investigate how cell type and associated abundance and composition of the protein quality-control network impact AAT folding and misfolding, we investigated the maturation, secretion, and activity of AAT expressed in a hepatocyte-derived human hepatocarcinoma cell line (Huh-7); Chinese hamster ovary (CHO) cells that are commonly used for biotherapeutic production of secreted proteins; and to mimic skeletal muscle-based gene therapy, mouse myoblasts and myotubes using the murine C2C12 cell line (39). In agreement with the muscle-based human gene therapy trials where patients homozygous for the Z mutation showed increased but subtherapeutic levels of circulating wild type AAT-M, secretion of AAT from C2C12 cells was low. We therefore also investigated the ability of various small-molecule protein homeostasis (proteostasis) modulators to effectively boost AAT secretion in C2C12 cells. Altogether, these results demonstrate that myoblasts and myotubes, although not optimal hosts for AAT production, can be enhanced using a possible combinatorial therapy including the proteostasis regulator SAHA.
Results
Efficient Secretion of Endogenous AAT from Huh-7 Cells.
Hepatocytes are the major physiological producer of circulating AAT. Thus, the secretion efficiency of AAT from Huh-7 cells provides a baseline by which to judge the efficiency of AAT secretion by other cell types. The amino acid sequence of AAT wild type (referred to as AAT-M) varies among humans with at least four major variants termed M1V, M1A, M2, and M3. For AAT-M1, the V (valine) or A (alanine) refers to the polymorphism at position 213 (40). M2 (R101H and E376D) and M3 (E376D) denote additional double and single--site mutations, respectively (41–43). The endogenous AAT sequence in Huh-7 cells was determined by extracting the total RNA, generating complementary DNA (cDNA), and sequencing with primers designed for AAT (SI Appendix, Table S1). The AAT amino acid sequence in Huh-7 cells was determined to be M1V, the most common human AAT sequence (44). The M1V sequence was therefore used for all subsequent experiments and designated here as AAT-M.
To determine the secretion efficiency of endogenous AAT-M in Huh-7 cells, a pulse-chase metabolic labeling experiment was performed. Huh-7 cells were pulsed with [35S]-Met/Cys for 30 min and chased for the indicated times with cold amino acids. Cell lysates, media, and detergent (Triton X-100) insoluble cell fractions were then collected and analyzed for AAT content by immunoprecipitation (IP) with anti-AAT antibodies, sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and autoradiography (Fig. 1 A and B).
Fig. 1.
Huh-7 cells efficiently secrete active AAT M1V. (A) Huh-7 cells were pulsed with media containing [35S]-Met/Cys for 30 min and chased with nonradioactive media for the indicated times. Cell lysates (lanes 1 to 8), media (lanes 9 to 16), and detergent insoluble (lanes 17 to 24) fractions were collected. AAT was isolated by IP with anti-AAT antibodies. Samples were resolved by SDS-PAGE and visualized by autoradiography. No significant amounts of AAT were observed in the detergent-insoluble fraction (lanes 17 to 24); therefore, it was not plotted. (B) Band intensities on the SDS-PAGE gels were quantified and plotted over time. The points are the means of three independent biological replicates, and the error bars are the SDs. Secreted fraction of AAT was calculated as media [AAT/total AAT (cell lysate + media + detergent insoluble)] × 100. (C) Cell lysates (lanes 1 to 3) and medium (lanes 4 to 6) immunoprecipitated AAT were treated with glycosidases EndoH (lanes 2 and 5) and PNGaseF (lanes 3 and 6). AAT with complex (AATComp) or high mannose (AATHM) carbohydrates are designated along with deglycosylated AAT (AATDG). (D) The activity of AAT in the media was determined by treatment with HNE and quantified by dividing the complex amount (HNE-AAT, lane 2) by total AAT (lane 2 reactive AAT plus unreactive AAT) and multiplying by 100. Inhibition of elastase by AAT results in the formation of the higher-molecular-weight AAT-elastase covalent conjugate. The points are the means of three independent biological replicates, and the error bars are the SDs. Molecular weight markers (kDa) are shown on the right of all gels.
Endogenous AAT-M secretion in Huh-7 cells was rapid and efficient. The half-time of secretion was 25 min. The maximum efficiency of secretion approached 92%, plateauing after 90 min of chase. There was essentially no AAT found in the detergent-insoluble fraction over the 4-h time course, supporting the high level of efficient secretion (Fig. 1A, lanes 17 to 24). In the cell lysate, AAT-M appeared as a discrete band of ∼50 kDa corresponding to the high-mannose ER glycoform (AATHM) that was sensitive to both endoglycosidase H (EndoH) and PNGaseF digestion (Fig. 1A, lane 1; and Fig. 1C, lanes 1 to 3), consistent with AAT containing glycans with ER glycoforms (45). AAT in the media migrated as a slower and broader band due to the addition of complex sugars in the Golgi as verified by its resistance to EndoH (AATComp; Fig. 1A, lanes 10 to 16; and Fig. 1C, lanes 4 and 5).
Since inactive inhibitory serpins may be secreted from cells (46, 47), a gel-based assay was used to determine the activity of AAT secreted into the media. Attack at the solvent-accessible reactive center loop (RCL) of a serpin by a target protease leads to the formation of an inactive higher-molecular-weight covalent conjugate between the serpin and the inhibited protease. A serpin without inhibitory activity will instead act as a protease substrate and yields cleaved products observed as lower molecular weight bands on a gel (39, 48). The efficient formation of the covalent conjugate between AAT and HNE was therefore used to assess the activity of the secreted AAT and demonstrated that 82% of AAT secreted into the media from Huh-7 cells was active (Fig. 1D). Thus, Huh-7 cells efficiently and rapidly secrete endogenously expressed active AAT into the media.
Secretion of Exogenously Expressed AAT Is Cell Type Dependent.
The exogenous expression of AAT in a variety of cell types was followed to compare their ability to serve as suitable hosts for the efficient production of active AAT with the efficiency of the natural host cells Huh-7. To this end, the production and secretion of myc-tagged AAT-M overexpressed in Huh-7 cells, CHO, and C2C12 cells were monitored (Fig. 2 A–F). The pulse-chase experiments were performed similarly to those described in Fig. 1 for endogenous AAT except cells were transfected with AAT-M cDNA for 20 h prior to performing the 30-min pulse and tagged AAT was isolated using anti-myc antibodies.
Fig. 2.
Secretion of overexpressed AAT-M in CHO, Huh-7, and C2C12 myoblast. AAT-M was exogenously expressed in Huh-7 (A and B), CHO (C and D), and C2C12 myoblast (E and F) cells for 20 h; pulsed with media containing [35S]-Met/Cys for 30 min; chased with nonradioactive media for the indicated times; and processed and quantified as described in Fig. 1. (G) Medium samples were collected after 20 h of transfection, and AAT was isolated by IP and treated with HNE before resolving by SDS-PAGE and immunoblotting to visualize the active HNE-AAT complex. (H) The points are the means of three independent biological replicates, and the error bars are the SDs.
Similar to the result with endogenously expressed AAT-M, Huh-7 cells supported the efficient trafficking of exogenous and overexpressed AAT-M, reaching a maximum of 88% secretion after 2 h of chase (Fig. 2 A and B). Although no AAT was found to accumulate in the detergent-insoluble fraction, the half-time of exogenous AAT-M secretion was modestly increased from 25 min for the endogenous protein to 39 min. Huh-7, therefore, served as a suitable source for the efficient production and secretion of overexpressed AAT.
CHO cells have been widely used as a host for the biosynthesis of biologics that traverse the secretory pathway (49, 50). This includes the production of biologics used in enzyme replacement therapy for a variety of lysosomal storage and blood clotting diseases (51–53). The timing for heterologously expressed AAT-M secretion in CHO cells was similar to that in Huh-7 cells (Fig. 2 C and D, 38-min half-time); however, the yield decreased to 75% with 5% of the protein accumulating in the detergent-insoluble fraction. This secretion level was further reduced when AAT-M was overexpressed in C2C12 myoblast cells where only 55% was secreted, and the detergent-insoluble fraction increased further to 13% (Fig. 2 E and F). When the activity of AAT-M expressed in the three different cell types was compared by monitoring the formation of the elastase-AAT inhibitory complex (Fig. 2 G and H), the activity levels were indistinguishable with levels ranging from 85 to 95%. In summary, the natural host hepatocytes supported the most efficient production of active AAT-M, followed by CHO cells (SI Appendix, Table S2). The mouse myoblast cell line C2C12 was the poorest host with only roughly half of the AAT-M being secreted even after 3 h of chase. The quality-control process appears to be monitoring AAT-M efficiently in all cell types, as regardless of the cell type, AAT-M that traffics to the media is highly active.
The Trafficking of the Common Disease Mutant AAT-Z (E342K) Is Cell Type Dependent.
The abundance and type of quality-control machinery can be cell type dependent. AAT-Z is prone to form homopolymers, and although cellularly retained AAT-Z can be degraded by the proteasome or lysosome, nonnative polymers may still accumulate in the ER and can result in hepatocyte cell death (7, 10–14). We therefore characterized the ability of the various cell types to properly sort polymergenic AAT-Z by following its trafficking in Huh-7, CHO, and C2C12 cells.
Huh-7 cells secreted 40% of AAT-Z (Fig. 3 A and B), although it was largely inactive (Fig. 3G). The Triton-insoluble fraction persisted at ∼7% throughout the chase. The half-time of secretion was delayed by over an hour compared to AAT-M.
Fig. 3.
Trafficking of overexpressed AAT-Z in CHO, Huh-7, and C2C12 cells. AAT-Z was exogenously expressed in Huh-7 (A and B), CHO (C and D), and C2C12 myoblasts (E and F) for 20 h; pulsed with media containing [35S]-Met/Cys for 30 min; chased with nonradioactive media for the indicated times; and processed and quantified as described in Fig. 1. (G) Medium samples were collected after 20 h of transfection, and AAT-Z was isolated by IP and treated with HNE before resolving by SDS-PAGE and immunoblotting to visualize the active AAT-elastase complex.
CHO and C2C12 myoblasts cells displayed retarded and decreased levels of secretion (Fig. 3 C–F; 20 and 18%, respectively). To a large extent, this diminished level of secretion was caused by dramatic increases in the detergent-insoluble fractions to 40 to 45% of the total. In addition, a decrease in total AAT-Z (lysate+media+insoluble) was observed with time in CHO and C2C12 myoblasts but not with Huh-7 cells, indicative of AAT-Z degradation (SI Appendix, Fig. 1). Only slight activity was observed for Huh-7-overexpressed AAT-Z in the media, whereas no activity was detected for the protein secreted from either CHO or C2C12 cells (Fig. 3G). Altogether, while Huh-7 served as a better host for the secretion of AAT-Z to the media and minimization of detergent-insoluble aggregates, these cells were still unable to produce significant active protein with the mutant variant. However, because AAT-Z is less stable than AAT-M, processing steps required for the assay may have played a role in reducing the observed activity.
Proteostasis Regulator SAHA Increases AAT-M Secretion Efficiency in Muscle Cells.
A clinical trial of injection-based muscle-targeted gene therapy has shown sustained AAT-M secretion, but AAT-M plasma levels are still subtherapeutic (23). These results correlate with our finding that C2C12 myoblasts are suboptimal hosts for AAT secretion (Fig. 2 E and F), and we therefore explored whether secretion of properly folded and active AAT-M could be enhanced by known proteostasis regulators (Fig. 4). To this end, we analyzed the effects of a panel of proteostasis regulators on AAT-M secretion and its activity upon arriving in the media.
Fig. 4.
Treatment with SAHA increases the maturation efficiency of active AAT-M in C2C12 myoblasts. (A) C2C12 myoblasts were transfected with an AAT-M plasmid and grown for 24 h. The medium was replaced and the cells were grown for an additional 24 h in the presence of DMSO alone or one of nine small molecules dissolved in DMSO as indicated. Small molecule compounds include verapamil, dantrolene, and diltiazem, which increase the Ca2+ concentration in the ER (56); Tg, which nonspecifically induces UPR by decreasing the Ca2+ concentration in the ER (57); compounds 5 (comp5) and 147 (comp147), which up-regulate the ATF6 arm of the UPR (60); SAHA, an HDAC inhibitor (17); salubrinal, an inhibitor of eIF2α phosphorylation (61); and Bix, a small molecule that is reported to induce BiP expression (63, 64). Immunoblots of AAT-M secreted into the media following treatment in the absence (−) or presence (+) of the AAT target protease HNE are shown. (B) Amounts of AAT-M that accumulated in the media were quantified and compared to DMSO control levels that were set at 100%. The mean secretion efficiency (bars) relative to DMSO alone from four biological replicates with the SD given by the error bars. (C) HNE-AAT complex formation was quantified as described in Fig. 1 from at least four biological replicates with the SD given by the error bars. (D) C2C12 cell lysates were immunoblotted for HDAC7, SEL1L, BiP, and GAPDH (loading control) to determine their cellular expression levels after treatment with the various proteostasis regulators for 24 h. (E) Levels of HDAC7, SEL1L, and BiP were quantified from four biological replicates, indicated by color, with error bars representing the SDs.
Small molecules that increase the calcium concentration in the ER and thus affect quality-control components, such as the calcium-dependent lectin chaperones calnexin and calreticulin and the ER-resident Hsp70 chaperone BiP, also activate the ER stress response (54–56). These stress activators that increase calcium levels include verapamil, dantrolene, and diltiazem. In contrast, thapsigargin (Tg) nonspecifically induces the unfolded protein response by decreasing the ER Ca2+ concentration (57). However, modulation of ER calcium levels failed to have any impact on the secretion of AAT-M in C2C12 myoblasts (Fig. 4 A and B).
Up-regulation of the ATF6 arm of the unfolded protein response has been reported to increase the degradation of AAT-Z and thus preserve cell health (58, 59). However, treating C2C12 muscle myoblasts with two small molecules, namely, Comp5 and Comp147, that up-regulate ATF6 (60) did not have a significant effect on AAT-M secretion or activity (Fig. 4 A and B). In addition, salubrinal, an inhibitor of eIF2α phosphatase and another stress response activator in eukaryotic cells (61), did not influence AAT-M secretion.
BiP, the ER Hsp70 family member, is a central player in ER homeostasis and regulation of the ER stress response (62). Bix (BiP protein inducer X, 1-(3,4-dihydroxy-phenyl)-2-thiocyanate-ethanone) is a small molecule that has been reported to specifically induce BiP expression without influencing the expression of other ER chaperones (63, 64). The treatment of C2C12 cells with Bix was found to modestly increase the secretion of AAT-M (Fig. 4 A and B) despite the fact that a significant increase in BiP expression was not observed.
Histone deacetylases (HDACs) support the posttranslational deacetylation of histones, as well as other luminal and cytoplasmic proteins (65–72). The small molecule HDAC inhibitor suberoylanilide hydroxamic acid (SAHA; the Food and Drug Administration–approved drug veronistat [Zolinza]) has been shown to increase the cellular secretion of active AAT-Z from a lung cell line and the secretion of both AAT-Z and -M from a colon carcinoma cell line, thus reducing accumulation in the ER, suggesting that its effects may be independent of cell line (17, 73). Treatment of C2C12 cells with SAHA for 20 h significantly augmented the level of AAT-M secreted into the media (by 75%; Fig. 4A, lane 15 compared to lane 1, and Fig. 4B). AAT-M localized to the media appeared to be properly interrogated by the cellular quality-control process as the activity was independent of treatments, ranging from 52 to 83% active (Fig. 4C).
The correction ability of SAHA for both AAT-Z and CFTR-ΔF508 appears to be mediated mainly through the silencing of HDAC7 (17, 74, 75). As expected, SAHA diminished the level of HDAC7 expression by 84% (Fig. 4 D and E). The action of SAHA may be more complex as several of the other small molecules tested also significantly reduced HDAC7 levels but did not enhance AAT secretion. Furthermore, SAHA did not influence the expression of two unfolded protein response (UPR)-induced factors, namely, SEL1L and BiP. Altogether, the deacetylase inhibitor SAHA diminished HDAC7 levels significantly and enhanced the secretion of AAT in myoblasts.
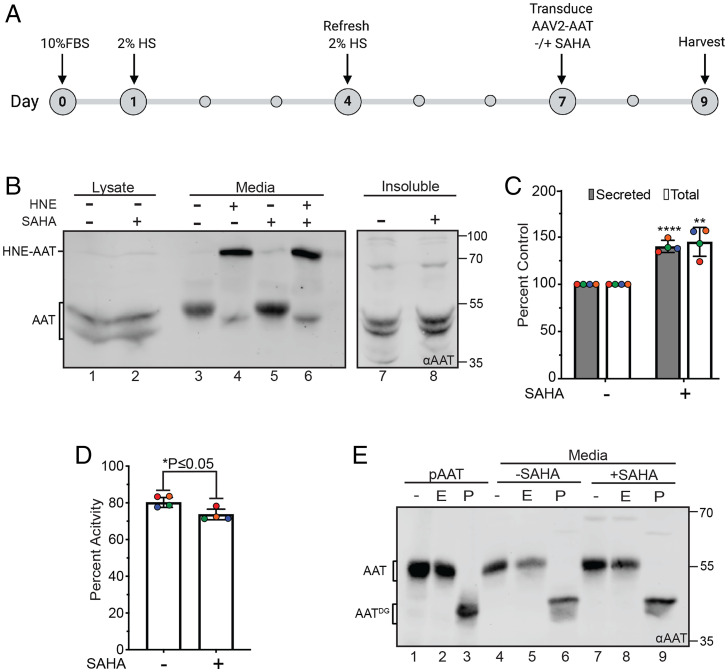
Myoblasts differentiate into mature myotubes (76), and gene therapy administration often targets myotubes. Therefore, we wanted to determine if SAHA could also augment the secretion of AAT-M in myotubes. Myotubes are refractory to transfection with plasmids. However, AAV capsids have been used effectively in clinical trials as a vehicle to deliver AAT-M cDNA intramuscularly for AAT-M expression (77). Therefore, AAV capsids encapsulating AAT-M cDNA were employed to express AAT-M in myotubes in the absence and presence of SAHA. After 5 d of treatment with horse serum, myoblasts differentiate into myotubes (76) (Fig. 5A). At this time, the medium was exchanged as the horse serum contains an unknown factor that inactivated AAT-M. After transduction for 2 h, the myotubes were treated with fresh media in the absence or presence of SAHA for 2 d. The medium was collected after 2 d and probed for AAT-M secretion and activity.
Fig. 5.
AAV transduction of myotubes supports efficient secretion of active AAT that is increased by SAHA treatment. (A) Timeline of myotube differentiation and transduction of AAT. Cells were plated and differentiated in DMEM containing 2% horse serum (HS) for 7 d. On day 7, cells were transduced with AAV2 containing the AAT plasmid, treated with or without SAHA for 2 d, and harvested on day 9. (B) Immunoblot of AAT expression and activity produced by differentiated myotubes ± SAHA. AAT from the lysate (lanes 1 and 2), medium fractions (lanes 3 and 5), and detergent insoluble pellet (lanes 7 and 8) were probed using an αAAT antibody for cells treated with or without SAHA. A fraction of the media from each sample was tested for activity in its ability to form a complex when incubated with HNE (lanes 4 and 6). (C) Quantification of AAT levels for secreted and total protein from B. Increase in the secreted protein was determined by dividing the amount of AAT in lane 5 by lane 3. The fold increase in total protein was determined by adding AAT in all fractions for each condition (lanes 1, 3, and 7 for − SAHA and lanes 2, 5, and 8 for + SAHA). The total amount for + SAHA was divided by the amount calculated for − SAHA. (D) Percent activity of AAT produced by myotubes ± SAHA from B. Activity of AAT was measured in lane 4 (− SAHA) and lane 6 (+ SAHA). The amount of the complex (HNE-AAT) was divided by the total amount of protein in each lane (HNE-AAT + noncomplexed AAT) and multiplied by 100 to obtain a percentage. Error bars represent the SD of four biological replicates (indicated by color). **** represents P ≤ 0.0001, ** represents P ≤ 0.01, and * represents P ≤ 0.05 for both C and D. (E) Commercial purified AAT (pAAT) (lanes 1 to 3), AAT from the media of untreated cells (lanes 4 to 6), and + SAHA (lanes 7 to 9) were immunoblotted and probed using an αAAT to determine glycan sensitivity. All treatments were compared to undigested protein (−; lanes 1, 4, and 7). Samples were treated with EndoH (E; lanes 2, 5, and 8), a glycosidase unable to digest complex/hybrid glycans, and a lack of digestion suggests protein trafficking to the Golgi. All three samples were also treated with PNGaseF (P; lanes 3, 6, and 9), an enzyme that completely digests glycans regardless of type.
C2C12 myotubes were efficiently transduced by the AAV2 capsids containing AAT cDNA (Fig. 5B). SAHA treatment increased the secretion of AAT-M by 40% (Fig. 5 B and C). The secreted AAT-M found in media was EndoH resistant (Fig. 5E), arguing that it efficiently traversed the Golgi, and the percentage of active AAT-M in the media was similar regardless of the presence of SAHA (Fig. 5D). The slight decrease in AAT-M activity upon SAHA treatment may be due to the modest increase in insoluble AAT-M observed (Fig. 5B, lanes 7 and 8). Taking into consideration both the increased level of AAT in the media and the percentage activity of the secreted protein, overall SAHA treatment boosted AAT-M activity in the media by 30%. Therefore, SAHA was effective at increasing both the level of secreted AAT-M and the protease inhibitor activity in the media.
Discussion
The ability of different cell types to express active protein via their secretory pathways is highly variable. The expression of proteins in heterologous sources is a common mechanism for gene therapy, production of biologics, and most recently the effectiveness of mRNA vaccines. While our study focuses on the expression of the gene therapy target AAT in different cell types and its optimization with proteostasis regulators, the lessons learned here have a general impact on the strategies and development of a variety of therapeutic avenues for a wide range of diseases. We determined that the secretion efficiency of the serine protease inhibitor AAT varied widely in different cell lines. Hepatocytes (Huh-7), the natural biosynthetic host for the majority of circulating AAT, were found to be the superior cell type for expressing properly folded and active AAT. In terms of production levels of active AAT, CHO cells, which are a work horse cell line for the commercial protein production of therapeutic glycoproteins or glycobiologics, were the next best. Strikingly, muscle myoblasts and myotubes, which are the targets of many gene therapy protocols, were poor hosts for AAT production, and even for AAT-M, 13% of the protein accumulated in the detergent-insoluble fraction in myoblasts, an indication of unsuccessful folding. However, the level of secretion of AAT in myoblasts and myotubes could be significantly boosted by treatment with the proteostasis regulator SAHA. Intramuscular injections for gene therapy trials involve intramuscular injections and the transduction of nearby myotubes (77). Here, we used an AAV capsid system similar to that used in clinical trials to efficiently transduce the myotubes.
AAT is primarily synthesized in the liver under normal physiological conditions. After trafficking through the secretory pathway of hepatocytes, it is secreted into the serum, where it then circulates throughout the body helping to regulate tissue proteolysis and the innate immune system by inhibition of some microbial Ser proteases. AAT deficiency alters the protease:anti-protease balance leading to dysregulated proteolysis, particularly in the lungs (7), and low AAT levels along with extracellular AAT polymers may have additional proinflammatory effects (78, 79). Thus, for AAT deficiency disease, low AAT levels in serum can result in emphysema due to inflammation and degradation of interstitial elastin within the lungs. In addition, the build-up of misfolded protein in the liver can lead to cirrhosis.
The effectiveness of gene therapy is limited by the production ability of the transduced cells and the duration of expression. Recombinant AAV capsids have been used as delivery vehicles for protein expression upon intramuscular injections. Murine studies have shown that skeletal myofibers can be successfully transduced by an AAV to express active human erythropoietin and therapeutic levels of leptin (80, 81). Song et al. (77) found that mouse muscle transduced with AAT using an AAV showed sustained secretion of AAT over 15 wk with serum levels reaching over 800 mg/mL. Human trials involving multiple numerous intramuscular injections using AAV-based platforms for the expression of wild-type AAT in patients with AAT deficiency due to the AAT-Z mutation showed wild-type AAT production (29, 82), which even 5 y after injection was sustained at 2.5 to 3% of the therapeutic serum level (23). Methods to augment glycoprotein expression in muscle cells could make gene therapy involving intramuscular injections more effective.
There is a possibility that enhancements to the protein secretion and maturation machinery in cells could overcome the cell type limitations in the expression of active protein. For example, CHO cells are the most frequently used mammalian cell system for the manufacturing of recombinant protein therapeutics. These cells have been extensively bioengineered to optimize protein production by overexpressing or knocking out factors that involve posttranslational modifications, such as glycosylation, or factors involved in metabolism or protein maturation including molecular chaperones (49, 83). BiP or PDI overexpression in yeast Saccharomyces cerevisiae supported a twofold to eightfold increase in secretion for single chain antibody fragments (84). Alternatively, overexpression of chaperones such as BiP can reduce secretion in mammalian cells by favoring binding and ER retention (85). The balance of ER factors is critical for efficient protein production by the secretory pathway and is maintained by UPR.
Professional secretory cells such as pancreatic acinar cells can secrete huge amounts of proteins daily because of their expansive ER (86). B cell development involves the remodeling of the ER and expansion of the ER machinery to increase the biosynthetic capacity for increased antibody production for a plasma cell (2). This ER expansion observed for the transition of B cells to plasma cells involves the activation of UPR pathways. Therefore, we tested whether pharmacological activation of UPR in myoblasts could increase the expression of biologically active AAT. ER stress activators that increase (verapamil, dantrolene, and diltiazem) or decrease (Tg) Ca2+ concentration in the ER did not influence AAT-M secretion in C2C12 myoblasts (Fig. 4). This lack of Ca2+ concentration dependence is consistent with results from AAT expression in CHO-K1 cells where expression of AAT mutants did not significantly perturb Ca2+ signaling (87). Muscle cells also contain calcium-rich sarcoplasmic reticulum (SR), which would also be disrupted by calcium modulation. Up-regulation of the ATF6 arm of the UPR pathway has been shown to increase the degradation of AAT-Z in hepatoma cells (Hepa 1-6 cells) (58, 59). However, treatment of C2C12 myoblasts with the ATF6 activators Comp5 and Comp147 did not impact AAT-M secretion. While Bix appeared to slightly increase AAT-M secretion, the only proteostasis regulator that was found to significantly impact AAT-M secretion was SAHA.
SAHA treatment nearly doubled the level of AAT-M secreted by C2C12 myoblasts (Fig. 4 A and B). As an HDAC inhibitor, SAHA treatment diminished the level of HDAC7 in C2C12 cells (Fig. 4 D and E). Similar fractions of active AAT were observed in the media regardless of the cell types in which AAT was produced. Since myotubes could not be transfected, AAV capsids were used to efficiently transduce mature myotubes with AAT. This also simulated the expression method used in clinical trials (23, 29, 77). SAHA boosted AAT secretion from myotubes by 40% and overall activity in the media (considering both amount and percent active AAT) by 30%, demonstrating that SAHA treatment is an effective strategy to increase the production of active protease inhibitor in muscle cells.
Previous work from the Balch laboratory has shown that SAHA treatment pharmacologically and genetically increases the secretion of AAT-Z in HCT116 cells (17, 73). SAHA was proposed to act by targeting an early folding intermediate of AAT-Z and altering its binding to the carbohydrate-dependent ER calnexin, allowing for its secretion. Calnexin has been shown to be involved in the ER retention of misfolded AAT-Z and its eventual sorting for ER lysosomal-associated degradation (12–14). Future studies are needed to understand if the effect of SAHA on AAT is mediated through calnexin possibly by the inhibition of an ER deacetylase or some other mechanism (17).
We found that hepatocytes were better equipped to deal with polymerization-prone AAT-Z than other cell types likely because hepatocytes are the natural host for AAT. Secretion of AAT-Z in Huh-7 cells reached 40% after a 3 h of chase, while the amount of the detergent insoluble fraction that accumulate was minimal (Fig. 3). In contrast, secretion levels of AAT-Z in CHO and C2C12 myoblasts only reached ∼20%, as the detergent insoluble fraction increased to over 40%. Therefore, hepatocytes optimally responded to AAT-Z expression, diminishing the level of detergent insoluble protein, while C2C12 myoblasts remained the weakest with large insoluble fractions and only 18% of AAT-Z secreted into the media.
Muscle cells possess an SR that is responsible for the regulation of calcium and myofibril contractions (55, 88–90). The SR is believed to be a subcompartment of the ER and the endomembrane system. However, the protein biosynthetic role of the SR is poorly defined. Whether proteins can be directly translocated into the SR upon translation by ribosomes or must first be translocated into the ER before diffusing to the continuous SR lumen and membrane is not fully understood. The architecture of the ER and SR are even more obscured when considering protein expression in multinucleated myotubes, as nuclear membranes are contiguous with the ER membranes. More work is needed to decipher the roles of the SR in the production of secreted proteins like AAT.
Ongoing studies in our group are focused on the analysis of the critical ER factors involved in AAT maturation and quality control and how the activities of these factors may be modulated to augment secretion for gene therapy or relax retention in the case of the overzealous retention of potentially active mutant variants. Previous work has pinpointed calnexin as a key player in AAT maturation and quality control (12–14, 74). More direct ways of facilitating the actions of these factors are being explored. In addition, with recent advances in mRNA technologies using delivery mechanisms such as lipid nanoparticles for intramuscular injections, especially in the case of vaccines (91, 92), our work may provide strategies to control the protein production capacity of secretory pathway cargo in muscle cells.
MATERIALS AND METHODS
Molecular Biology.
Sequencing endogenous Huh-7 AAT.
The sequence of endogenous AAT encoded by the SerpinA1 gene in Huh-7 cells was determined by performing a total RNA extraction (Qiagen RNeasy kit) and then synthesizing cDNA using the AMV First Strand cDNA Synthesis Kit (New England BioLabs) and an oligo d(T) anchored primer, d(T)23VN, that anneals at the start of polyA mRNA tails. The cDNA was then used as a template in a PCR using the SerpinA1 forward and reverse primers (SI Appendix, Table S1) to amplify the SERPINA1 gene including the 5′ untranslated region. The resulting PCR product was sequenced by Genewiz using the SERPINA1 forward primer and endoaat forward and reverse primers (SI Appendix, Table S3). Endogenous AAT has the M1V (Arg101, Val213, Glu376) sequence.
Sequences of the SerpinA1 gene in the pcDNA vector.
The M1V sequence was used in the pcDNA plasmid (Thermofisher V79020) for expression of wild-type AAT with a C-terminal myc tag in order to differentiate endogenous AAT from transfected AAT. The Z, Glu342Lys, mutation was introduced into the M1V background in the pcDNA vector by site-directed mutagenesis using E342K forward and E342K reverse primers (SI Appendix, Table S3) and the C-terminal myc tag was replaced with a C-terminal 3XFLAG tag using pCDNA-Z-Flag-his-1,2,3,4,5 primers (SI Appendix, Table S3). Full AAT DNA and protein sequences are provided in SI Appendix, Table S4. C-terminal tags have been commonly employed with AAT. C-terminal myc and FLAG-tagged AAT have been previously characterized (93, 94).
Cell Biology.
Cell culture, transfections, differentiation, and transduction.
Huh-7 cells were acquired from Japanese Collection of Research Bioresources cell bank (lot 7222016) and maintained in Dulbecco’s modified Eagle medium (DMEM) containing 1g/L D-glucose, 10% New Zealand fetal bovine serum (FBS) (Gibco, A3160901), and 1% penicillin-streptomycin (Pen/Strep) (Gibco, 15140-122). CHO-K1 cells were acquired from ATCC (lot 62960170) and maintained in MEM Alpha(1×)+GlutaMAX (Gibco, 32561-037), 10% FBS (Gibco, 16000-044), and 1% Pen/Strep. C2C12 myoblasts were acquired from Dr. Lawrence Schwartz (University of Massachusetts, Amherst) and maintained in DMEM containing 10% FBS. All cell lines were confirmed to be mycoplasma free by testing for mycoplasma contamination using a universal mycoplasma detection kit (ATCC, catalog number 30-1012K).
Near-confluent cells on 3.5-mm plates were transfected with AAT cDNA. One microgram of plasmid and 60 μL Opti-MEM with 2.5 μg poly(ethylenimine) for CHO cells and 2 μL Lipofectamine 2000 (ThermoFisher, 11668027) for C2C12 myoblasts and 2 μL GeneXPlus (ATCC, ACS-4004) for Huh-7 cells were used. Opti-MEM was utilized to grow cells following transfection.
For myotube differentiation a week prior to transduction, 2.5E5 C2C12 myoblasts were plated onto a 3-cm plate with DMEM (Sigma, D5796) completed with 10% FBS (Gibco 16000-044). Plates were placed in a 37 °C and 5% CO2 incubator overnight. On day 1, the medium was aspirated and replaced with 1.5 mL of DMEM containing 2% horse serum (Gibco, 26050-070) to begin differentiation into myotubes. Plates were then incubated at 37 °C and 5% CO2 for 3 d. On day 4, the medium was aspirated and replaced with 1.5 mL of DMEM and 2% horse serum. The plates were then incubated for an additional 3 d at 37 °C and 5% CO2 to complete the differentiation.
On day 7, the cells were ready for transduction. AAV capsids containing the AAT plasmid was prepared as previously reported (95, 96). A representative plate was used to determine the density of cells needed for multiplicity of infection (MOI) calculations. Prior to transduction, the medium was removed and replaced with 1.5 mL of serum-free DMEM. At this point, AAV2 capsids containing the AAT plasmid wre added to each plate at a MOI of 2.5E5 viral particles per cell. The plates were then returned to a 37 °C and 5% CO2 incubator for 2 h. The medium was again removed and replaced with 1.5 mL of Opti-MEM (Gibco, 31985-070) for the protein expression. Following this media exchange, 1.5 μL of 5 mM SAHA (Sigma, SML0061) was added to the treatment plates before incubating them at 37 °C and 5% CO2 for an additional 2 d. After this incubation, the media and cells were collected to measure AAT expression, glycoforms, and activity.
AAT IP and immunoblots.
Endogenous AAT was immunoprecipitated from Huh-7 cell lysate, Triton X-100 insoluble cellular fractions using polyclonal rabbit anti-human AAT antibody (Agilent Dako, catalog number A 0012, lot 20028622) (97). Transfected AAT was immunoprecipitated using an anti-myc mouse monoclonal antibody for M1V (Cell Signaling, 9B11, lot24) or an anti-FLAG monoclonal antibody (Sigma, F1804, lot SLBQ6349V). Additional details can be found in SI Appendix.
AAT pulse-chase, activity, and endoglycosidase assays.
Full details for the pulse-chase activity and endoglycosidase assay methods can be found in SI Appendix.
Proteostasis regulators.
C2C12 myoblasts were treated with a series of proteostasis regulators for 24 h including verapamil (3 µM; Cayman, 14288), dantrolene (25 µM; Sigma-Aldrich, D9175), and diltiazem (10 µM, TOCRIS,0685) that increase the Ca2+ concentration in the ER (56), while Tg (3 µM; ThermoFisher, T7458) decreases it. Regulators that activate the ATF6 arm of the UPR pathway include compound 5 (comp5) (10 µM; Life Chemicals, HS:3822000000) and compound 147 (comp147) (10 µM; Chembridge, 6538059) (60). SAHA (5 µM; Sigma-Aldrich, SML0061), an HDAC inhibitor (17); Bix (10 µM; SML1073 from Sigma-Aldrich), a small molecule that is reported to specifically induce BiP expression (63, 64); and salubrinal (20 µM; Sigma-Aldrich, SML0951), an inhibitor of eIF2α phosphorylation thereby maintaining activation of the PERK UPR pathway (61), were also employed.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Alpha-1 Foundation (to D.N.H., A.G., and L.M.G.). This work was also supported by awards from the NIH (GM086874 to D.N.H., GM118161 to L.M.G., and HL158506-01 to T.R.F.) and a Chemistry-Biology Interface program training grant (T32GM008515 to K.P.G.). We would also like to acknowledge the University of Massachusetts Chan School of Medicine Vector Core Facility and Qiushi Tang for the synthesis and shipment of the AAV2-AAT capsids. This research was performed while A.G. was employed at the University of Massachusetts Amherst. The opinions expressed in this article are the author's own and do not reflect the view of the NIH, the Department of Health and Human Services, or the United States government.
Footnotes
Reviewers: L.H., St. Jude Children's Research Hospital; J.K., Scripps; and J.B., University of Pittsburgh.
Competing interest statement: The authors declare a competing interest. T.R.F. serves on the scientific advisory board of Ferring Ventures, S.A., and was a scientific founder of Applied Genetic Technologies Corporation. The other authors declare no competing or financial interests.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2206103119/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Adams B. M., Oster M. E., Hebert D. N., Protein quality control in the endoplasmic reticulum. Protein J. 38, 317–329 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Anken E., Bakunts A., Hu C. A., Janssens S., Sitia R., Molecular evaluation of endoplasmic reticulum homeostasis meets humoral immunity. Trends Cell Biol. 31, 529–541 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gettins P. G. W., Serpin structure, mechanism, and function. Chem. Rev. 102, 4751–4804 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Campos M. A., et al. , The biological effects of double-dose alpha-1 antitrypsin augmentation therapy. A pilot clinical trial. Am. J. Respir. Crit. Care Med. 200, 318–326 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gadek J. E., Klein H. G., Holland P. V., Crystal R. G., Replacement therapy of alpha 1-antitrypsin deficiency. Reversal of protease-antiprotease imbalance within the alveolar structures of PiZ subjects. J. Clin. Invest. 68, 1158–1165 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eriksson S., Studies in alpha 1-antitrypsin deficiency. Acta Med. Scand. Suppl. 432, 1–85 (1965). [PubMed] [Google Scholar]
- 7.Greene C. M., et al. , α1-antitrypsin deficiency. Nat. Rev. Dis. Primers 2, 16051 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Chapman K. R., et al. ; RAPID Trial Study Group, Intravenous augmentation treatment and lung density in severe α1 antitrypsin deficiency (RAPID): A randomised, double-blind, placebo-controlled trial. Lancet 386, 360–368 (2015). [DOI] [PubMed] [Google Scholar]
- 9.McElvaney N. G., et al. ; RAPID Extension Trial Group, Long-term efficacy and safety of α1 proteinase inhibitor treatment for emphysema caused by severe α1 antitrypsin deficiency: An open-label extension trial (RAPID-OLE). Lancet Respir. Med. 5, 51–60 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Bazzan E., et al. , α1-antitrypsin polymerizes in alveolar macrophages of smokers with and without α1-antitrypsin deficiency. Chest 154, 607–616 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Lomas D. A., Evans D. L., Finch J. T., Carrell R. W., The mechanism of Z alpha 1-antitrypsin accumulation in the liver. Nature 357, 605–607 (1992). [DOI] [PubMed] [Google Scholar]
- 12.Fregno I., et al. , ER-to-lysosome-associated degradation of proteasome-resistant ATZ polymers occurs via receptor-mediated vesicular transport. EMBO J. 37, e99259 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fregno I., Fasana E., Soldà T., Galli C., Molinari M., N-glycan processing selects ERAD-resistant misfolded proteins for ER-to-lysosome-associated degradation. EMBO J. 40, e107240 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qu D., Teckman J. H., Omura S., Perlmutter D. H., Degradation of a mutant secretory protein, alpha1-antitrypsin Z, in the endoplasmic reticulum requires proteasome activity. J. Biol. Chem. 271, 22791–22795 (1996). [DOI] [PubMed] [Google Scholar]
- 15.Patel D., Teckman J. H., Alpha-1-antitrypsin deficiency liver disease. Clin. Liver Dis. 22, 643–655 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Bouchecareilh M., Conkright J. J., Balch W. E., Proteostasis strategies for restoring alpha1-antitrypsin deficiency. Proc. Am. Thorac. Soc. 7, 415–422 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouchecareilh M., Hutt D. M., Szajner P., Flotte T. R., Balch W. E., Histone deacetylase inhibitor (HDACi) suberoylanilide hydroxamic acid (SAHA)-mediated correction of α1-antitrypsin deficiency. J. Biol. Chem. 287, 38265–38278 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Remih K., Amzou S., Strnad P., Alpha1-antitrypsin deficiency: New therapies on the horizon. Curr. Opin. Pharmacol. 59, 149–156 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Liddle J., et al. , The development of highly potent and selective small molecule correctors of Z α1-antitrypsin misfolding. Bioorg. Med. Chem. Lett. 41, 127973 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Borel F., et al. , Survival advantage of both human hepatocyte xenografts and genome-edited hepatocytes for treatment of α-1 antitrypsin deficiency. Mol. Ther. 25, 2477–2489 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiuchiolo M. J., Crystal R. G., Gene therapy for alpha-1 antitrypsin deficiency lung disease. Ann. Am. Thorac. Soc. 13 (suppl. 4), S352–S369 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C., Xiao P., Gray S. J., Weinberg M. S., Samulski R. J., Combination therapy utilizing shRNA knockdown and an optimized resistant transgene for rescue of diseases caused by misfolded proteins. Proc. Natl. Acad. Sci. U.S.A. 108, 14258–14263 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mueller C., et al. , 5 Year expression and neutrophil defect repair after gene therapy in alpha-1 antitrypsin deficiency. Mol. Ther. 25, 1387–1394 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loring H. S., Flotte T. R., Current status of gene therapy for α-1 antitrypsin deficiency. Expert Opin. Biol. Ther. 15, 329–336 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Gaudet D., et al. , Long-term retrospective analysis of gene therapy with alipogene tiparvovec and its effect on lipoprotein lipase deficiency-induced pancreatitis. Hum. Gene Ther. 27, 916–925 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Flotte T. R., et al. , Apparently nonspecific enzyme elevations after portal vein delivery of recombinant adeno-associated virus serotype 2 vector in hepatitis C virus-infected chimpanzees. Hum. Gene Ther. 19, 681–689 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nathwani A. C., et al. , Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 365, 2357–2365 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manno C. S., et al. , Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat. Med. 12, 342–347 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Flotte T. R., et al. , Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing α1-antitrypsin: Interim results. Hum. Gene Ther. 22, 1239–1247 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cichy J., Potempa J., Travis J., Biosynthesis of alpha1-proteinase inhibitor by human lung-derived epithelial cells. J. Biol. Chem. 272, 8250–8255 (1997). [DOI] [PubMed] [Google Scholar]
- 31.du Bois R. M., et al. , Human neutrophils express the alpha 1-antitrypsin gene and produce alpha 1-antitrypsin. Blood 77, 2724–2730 (1991). [PubMed] [Google Scholar]
- 32.Geboes K., et al. , Morphological identification of alpha-I-antitrypsin in the human small intestine. Histopathology 6, 55–60 (1982). [DOI] [PubMed] [Google Scholar]
- 33.Hafeez W., Ciliberto G., Perlmutter D. H., Constitutive and modulated expression of the human alpha 1 antitrypsin gene. Different transcriptional initiation sites used in three different cell types. J. Clin. Invest. 89, 1214–1222 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mornex J. F., et al. , Expression of the alpha-1-antitrypsin gene in mononuclear phagocytes of normal and alpha-1-antitrypsin-deficient individuals. J. Clin. Invest. 77, 1952–1961 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perlmutter D. H., et al. , The alpha 1-antitrypsin gene is expressed in a human intestinal epithelial cell line. J. Biol. Chem. 264, 9485–9490 (1989). [PubMed] [Google Scholar]
- 36.Van’t Wout E. F. A., et al. , Function of monocytes and monocyte-derived macrophages in α1-antitrypsin deficiency. Eur. Respir. J. 45, 365–376 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Venembre P., et al. , Secretion of alpha 1-antitrypsin by alveolar epithelial cells. FEBS Lett. 346, 171–174 (1994). [DOI] [PubMed] [Google Scholar]
- 38.Uhlen M., et al. , Towards a knowledge-based human protein atlas. Nat. Biotechnol. 28, 1248–1250 (2010). [DOI] [PubMed] [Google Scholar]
- 39.Loebermann H., Tokuoka R., Deisenhofer J., Huber R., Human alpha 1-proteinase inhibitor. Crystal structure analysis of two crystal modifications, molecular model and preliminary analysis of the implications for function. J. Mol. Biol. 177, 531–557 (1984). [PubMed] [Google Scholar]
- 40.Nukiwa T., et al. , Characterization of the M1(Ala213) type of alpha 1-antitrypsin, a newly recognized, common “normal” alpha 1-antitrypsin haplotype. Biochemistry 26, 5259–5267 (1987). [DOI] [PubMed] [Google Scholar]
- 41.Curiel D., Laubach V., Vogelmeier C., Wurts L., Crystal R. G., Characterization of the sequence of the normal alpha-1-antitrypsin M3 allele and function of the M3 protein. Am. J. Respir. Cell Mol. Biol. 1, 471–477 (1989). [DOI] [PubMed] [Google Scholar]
- 42.Graham A., et al. , Characterisation of the alpha-1-antitrypsin M3 gene, a normal variant. Hum. Genet. 85, 381–382 (1990). [DOI] [PubMed] [Google Scholar]
- 43.Nukiwa T., Brantly M. L., Ogushi F., Fells G. A., Crystal R. G., Characterization of the gene and protein of the common alpha 1-antitrypsin normal M2 allele. Am. J. Hum. Genet. 43, 322–330 (1988). [PMC free article] [PubMed] [Google Scholar]
- 44.UniProt Consortium, UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 49 (D1), D480–D489 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Halaban R., et al. , Endoplasmic reticulum retention is a common defect associated with tyrosinase-negative albinism. Proc. Natl. Acad. Sci. U.S.A. 97, 5889–5894 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fra A., et al. , Polymers of Z α1-antitrypsin are secreted in cell models of disease. Eur. Respir. J. 47, 1005–1009 (2016). [DOI] [PubMed] [Google Scholar]
- 47.Adams B. M., Ke H., Gierasch L. M., Gershenson A., Hebert D. N., Proper secretion of the serpin antithrombin relies strictly on thiol-dependent quality control. J. Biol. Chem. 294, 18992–19011 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Löbermann H., Lottspeich F., Bode W., Huber R., Interaction of human alpha 1-proteinase inhibitor with chymotrypsinogen A and crystallization of a proteolytically modified alpha 1-proteinase inhibitor. Hoppe Seylers Z. Physiol. Chem. 363, 1377–1388 (1982). [DOI] [PubMed] [Google Scholar]
- 49.Tripathi N. K., Shrivastava A., Recent developments in bioprocessing of recombinant proteins: Expression hosts and process development. Front. Bioeng. Biotechnol. 7, 420 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walsh G., Biopharmaceutical benchmarks 2018. Nat. Biotechnol. 36, 1136–1145 (2018). [DOI] [PubMed] [Google Scholar]
- 51.Parenti G., Andria G., Ballabio A., Lysosomal storage diseases: From pathophysiology to therapy. Annu. Rev. Med. 66, 471–486 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Parenti G., Medina D. L., Ballabio A., The rapidly evolving view of lysosomal storage diseases. EMBO Mol. Med. 13, e12836 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pittman D. D., Tomkinson K. N., Kaufman R. J., Post-translational requirements for functional factor V and factor VIII secretion in mammalian cells. J. Biol. Chem. 269, 17329–17337 (1994). [PubMed] [Google Scholar]
- 54.Lamb H. K., et al. , The affinity of a major Ca2+ binding site on GRP78 is differentially enhanced by ADP and ATP. J. Biol. Chem. 281, 8796–8805 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Michalak M., Robert Parker J. M., Opas M., Ca2+ signaling and calcium binding chaperones of the endoplasmic reticulum. Cell Calcium 32, 269–278 (2002). [DOI] [PubMed] [Google Scholar]
- 56.Ong D. S. T., Mu T.-W., Palmer A. E., Kelly J. W., Endoplasmic reticulum Ca2+ increases enhance mutant glucocerebrosidase proteostasis. Nat. Chem. Biol. 6, 424–432 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P., Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc. Natl. Acad. Sci. U.S.A. 87, 2466–2470 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Plate L., Wiseman R. L., Regulating secretory proteostasis through the unfolded protein response: From function to therapy. Trends Cell Biol. 27, 722–737 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith S. E., et al. , Activating transcription factor 6 limits intracellular accumulation of mutant α(1)-antitrypsin Z and mitochondrial damage in hepatoma cells. J. Biol. Chem. 286, 41563–41577 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Plate L., et al. , Small molecule proteostasis regulators that reprogram the ER to reduce extracellular protein aggregation. eLife 5, e15550 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boyce M., et al. , A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science 307, 935–939 (2005). [DOI] [PubMed] [Google Scholar]
- 62.Pobre K. F. R., Poet G. J., Hendershot L. M., The endoplasmic reticulum (ER) chaperone BiP is a master regulator of ER functions: Getting by with a little help from ERdj friends. J. Biol. Chem. 294, 2098–2108 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fu Y.-L., et al. , Remodeling the endoplasmic reticulum proteostasis network restores proteostasis of pathogenic GABAA receptors. PLoS One 13, e0207948 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kudo T., et al. , A molecular chaperone inducer protects neurons from ER stress. Cell Death Differ. 15, 364–375 (2008). [DOI] [PubMed] [Google Scholar]
- 65.Behnke J., Mann M. J., Scruggs F.-L., Feige M. J., Hendershot L. M., Members of the Hsp70 family recognize distinct types of sequences to execute ER quality control. Mol. Cell 63, 739–752 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cook C., Petrucelli L., Tau triage decisions mediated by the chaperone network. J. Alzheimers Dis. 33 (suppl. 1), S145–S151 (2013). [DOI] [PubMed] [Google Scholar]
- 67.Hutt D. M., Balch W. E., Expanding proteostasis by membrane trafficking networks. Cold Spring Harb. Perspect. Biol. 5, a013383 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu P., et al. , Posttranslational modification and beyond: Interplay between histone deacetylase 6 and heat-shock protein 90. Mol. Med. 27, 110 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mayer M. P., Le Breton L., Hsp90: Breaking the symmetry. Mol. Cell 58, 8–20 (2015). [DOI] [PubMed] [Google Scholar]
- 70.Mollapour M., Neckers L., Post-translational modifications of Hsp90 and their contributions to chaperone regulation. Biochim. Biophys. Acta 1823, 648–655 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Murphy P. J. M., Morishima Y., Kovacs J. J., Yao T.-P., Pratt W. B., Regulation of the dynamics of hsp90 action on the glucocorticoid receptor by acetylation/deacetylation of the chaperone. J. Biol. Chem. 280, 33792–33799 (2005). [DOI] [PubMed] [Google Scholar]
- 72.Pulya S., et al. , HDAC6 as privileged target in drug discovery: A perspective. Pharmacol. Res. 163, 105274 (2021). [DOI] [PubMed] [Google Scholar]
- 73.Wang C., Bouchecareilh M., Balch W. E., Measuring the effect of histone deacetylase inhibitors (HDACi) on the secretion and activity of alpha-1 antitrypsin. Methods Mol. Biol. 1639, 185–193 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bouchecareilh M., Balch W. E., Proteostasis, an emerging therapeutic paradigm for managing inflammatory airway stress disease. Curr. Mol. Med. 12, 815–826 (2012). [DOI] [PubMed] [Google Scholar]
- 75.Hutt D. M., et al. , Reduced histone deacetylase 7 activity restores function to misfolded CFTR in cystic fibrosis. Nat. Chem. Biol. 6, 25–33 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yu C.-Y., et al. , A muscle-targeting peptide displayed on AAV2 improves muscle tropism on systemic delivery. Gene Ther. 16, 953–962 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Song S., et al. , Sustained secretion of human alpha-1-antitrypsin from murine muscle transduced with adeno-associated virus vectors. Proc. Natl. Acad. Sci. U.S.A. 95, 14384–14388 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Belchamber K. B. R., Walker E. M., Stockley R. A., Sapey E., Monocytes and macrophages in alpha-1 antitrypsin deficiency. Int. J. Chron. Obstruct. Pulmon. Dis. 15, 3183–3192 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McCarthy C., Reeves E. P., McElvaney N. G., The role of neutrophils in alpha-1 antitrypsin deficiency. Ann. Am. Thorac. Soc. 13 (suppl. 4), S297–S304 (2016). [DOI] [PubMed] [Google Scholar]
- 80.Kessler P. D., et al. , Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc. Natl. Acad. Sci. U.S.A. 93, 14082–14087 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Murphy J. E., et al. , Long-term correction of obesity and diabetes in genetically obese mice by a single intramuscular injection of recombinant adeno-associated virus encoding mouse leptin. Proc. Natl. Acad. Sci. U.S.A. 94, 13921–13926 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brantly M. L., et al. , Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc. Natl. Acad. Sci. U.S.A. 106, 16363–16368 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fischer S., Handrick R., Otte K., The art of CHO cell engineering: A comprehensive retrospect and future perspectives. Biotechnol. Adv. 33, 1878–1896 (2015). [DOI] [PubMed] [Google Scholar]
- 84.Shusta E. V., Raines R. T., Plückthun A., Wittrup K. D., Increasing the secretory capacity of Saccharomyces cerevisiae for production of single-chain antibody fragments. Nat. Biotechnol. 16, 773–777 (1998). [DOI] [PubMed] [Google Scholar]
- 85.Dorner A. J., Kaufman R. J., The levels of endoplasmic reticulum proteins and ATP affect folding and secretion of selective proteins. Biologicals 22, 103–112 (1994). [DOI] [PubMed] [Google Scholar]
- 86.Matlin K. S., Caplan M. J., The secretory pathway at 50: A golden anniversary for some momentous grains of silver. Mol. Biol. Cell 28, 229–232 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Malintan N. T., Buckingham S. D., Lomas D. A., Sattelle D. B., Calcium signalling in mammalian cell lines expressing wild type and mutant human α1-antitrypsin. Sci. Rep. 9, 17293 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Glembotski C. C., Roles for the sarco-/endoplasmic reticulum in cardiac myocyte contraction, protein synthesis, and protein quality control. Physiology (Bethesda) 27, 343–350 (2012). [DOI] [PubMed] [Google Scholar]
- 89.Rossi D., Barone V., Giacomello E., Cusimano V., Sorrentino V., The sarcoplasmic reticulum: An organized patchwork of specialized domains. Traffic 9, 1044–1049 (2008). [DOI] [PubMed] [Google Scholar]
- 90.Sitia R., Meldolesi J., Endoplasmic reticulum: A dynamic patchwork of specialized subregions. Mol. Biol. Cell 3, 1067–1072 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hassett K. J., et al. , Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol. Ther. Nucleic Acids 15, 1–11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Heinz F. X., Stiasny K., Distinguishing features of current COVID-19 vaccines: Knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines 6, 104 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Laffranchi M., Berardelli R., Ronzoni R., Lomas D. A., Fra A., Heteropolymerization of α-1-antitrypsin mutants in cell models mimicking heterozygosity. Hum. Mol. Genet. 27, 1785–1793 (2018). [DOI] [PubMed] [Google Scholar]
- 94.Zhang Y., et al. , LMAN1-MCFD2 complex is a cargo receptor for the ER-Golgi transport of α1-antitrypsin. Biochem. J. 479, 839–855 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Su Q., Sena-Esteves M., Gao G., Production of recombinant adeno-associated viruses (rAAVs) by transient transfection. Cold Spring Harb. Protoc. 2020, 095596 (2020). [DOI] [PubMed] [Google Scholar]
- 96.Su Q., Sena-Esteves M., Gao G., Purification of recombinant adeno-associated viruses (rAAVs) by cesium chloride gradient sedimentation. Cold Spring Harb Protoc 2020, 095604 (2020). [DOI] [PubMed] [Google Scholar]
- 97.Kalsheker N. A., Warner J. T., Watkins G. L., Phenotyping alpha-1-antitrypsin (alpha 1-AT) variants by isoelectric focusing in agarose and immunoblotting. Clin. Chim. Acta 148, 157–160 (1985). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.