Abstract
Introduction
Multisystem inflammatory syndrome (MIS-C) is a rare paediatric hyper-inflammatory disorder that occurs following SARS-CoV-2 infection. Acute kidney injury (AKI) occurs in approximately one-quarter to one-third of the patients with MIS-C and is associated with poor prognosis in critically ill children. This systematic review is aimed to evaluate the incidence of AKI, mortality, and the need for kidney replacement therapy (KRT) in patients with MIS-C.
Methods
We searched databases from Medline, EMBASE, Cochrane Register, and Google Scholar from December 2019 to December 2021 with our search strategy. Studies meeting the following criteria were included in this systematic review: (1) articles on AKI in MIS-C; (2) studies providing AKI in MIS-C and COVID-19 infection separately; (3) studies reporting outcomes such as mortality, KRT, serum creatinine; length of hospital/ICU stay.
Quality assessment
The quality of the included studies was independently assessed by using the National Heart Lung and Blood Institute (NHLBI) quality assessment tool for cohort studies and case series.
Statistical analysis
Outcomes and their 95% confidence intervals (CI) were reported if a meta-analysis of these outcomes was conducted. Heterogeneity was reported using I2 statistics, and heterogeneity ≥ 50% was considered high. We used Baujat’s plot for the contribution of each study toward overall heterogeneity. In sensitivity analysis, the summary estimates were assessed by repeating meta-analysis after omitting one study at a time. Forest plots were used for reporting outcomes in each study and with their 95% CI. All statistical tests were performed using R software version 4.0.3.
Results
A total of 24 studies were included in this systematic review and of these, 11 were included in the meta-analysis. The pooled proportion of patients with MIS-C developing AKI was 20% (95% CI: 14–28%, I2 = 80%). Pooled proportion of death in children with MIS-C was 4% (95% CI: 1–14%; I2 = 93%). The odds of death in patients with AKI were 4.68 times higher than in patients without AKI (95% CI: 1.06–20.7%; I2 = 17%). The overall pooled proportion of MIS-C-induced AKI patients requiring KRT was 15% (95% CI: 4–42%; I2 = 91%).
Conclusion
Approximately one-fifth of children with MIS-C develop AKI which is associated with higher odds of death.
PROSPERO registration: CRD42022306170
Graphical abstract
A higher resolution version of the Graphical abstract is available as Supplementary information
Supplementary Information
The online version contains supplementary material available at 10.1007/s00467-022-05701-3.
Keywords: Multisystem inflammatory syndrome in children (MIS-C), Acute kidney injury (AKI), Meta-analysis
Introduction
Severe acute respiratory syndrome coronavirus (SARS-CoV-2) infection is typically characterized by lower respiratory tract involvement that leads to acute respiratory distress syndrome in patients with moderate to severe COVID-19 disease. Multisystem inflammatory syndrome (MIS-C) is a rare paediatric hyper-inflammatory disorder following SARS-CoV-2 infection that manifests as fever, gastrointestinal symptoms, cardiac dysfunction, and shock [1, 2]. Incidence of MIS-C is nearly 3.16 cases per 10,000 SARS-CoV-2-infected persons [3]. Different scientific societies, like the Centers for Disease Control (CDC), World Health Organization (WHO), and Royal College of Paediatric and Child Health (RCPCH), have proposed diagnostic criteria [4–6]. MIS-C is thought to result from infection-related autoimmunity [7]. Reports from different parts of the world have shown an overlapping clinical picture of MIS-C with incomplete Kawasaki disease or toxic shock syndrome (TSS) [8] and multisystem involvement, including acute kidney injury (AKI) [9].
AKI occurs in approximately 25 to 33% of the patients with MIS-C [10, 11] and is associated with poor prognosis in critically ill children [10]. The mechanism of AKI in SARS-CoV-2 patients is multifactorial, including dehydration, poor cardiac output, cytokine storm, the direct cytopathic effect of the virus on renal tubular cells, and the use of nephrotoxic drugs. However, the mechanism implicated in AKI development in MIS-C patients is chiefly due to renal hypoperfusion [11, 12].
There is a lack of data on AKI incidence, its effect on mortality, and the requirement of kidney replacement therapy (KRT) in children with MIS-C [10, 11, 13]. This systematic review is aimed to evaluate the incidence of AKI, mortality, and the need for KRT in patients with MIS-C.
Methods
Data sources
We searched databases from Medline, EMBASE, Cochrane Register, and Google Scholar from December 2019 to December 2021. The following search strategy was used for the extraction of data ‘((((((acute kidney injury) OR (acute renal failure)) OR (renal failure)) AND (multisystem inflammatory syndrome)) OR (MIS-C)) OR (pediatric multisystem inflammatory syndrome)) OR (PIMS covid)’.
Inclusion criterion
Studies meeting the following criteria were included in this systematic review: (1) articles on AKI in MIS-C; (2) studies providing AKI in MIS-C and COVID-19 infection separately; (3) studies reporting outcomes such as mortality, KRT, serum creatinine; length of hospital/ICU stay. Studies were excluded if they were case reports, case series with the inclusion of < 10 patients, review articles, letters, or commentaries.
Data extraction
Two authors (AT and RKP) independently reviewed the literature, title, abstract, and full-text article. Any disagreement during the data extraction was resolved by discussing with the third author (GCB). Data related to AKI incidence, overall mortality, the need for KRT, and length of hospital stay were calculated.
Quality assessment
The quality of the included studies was independently assessed by two authors (RKP, AK) using the National Heart Lung and Blood Institute (NHLBI) quality assessment tool for cohort studies and case series. Any disagreement was resolved by consulting with a third author (GCB).
Statistical analysis
The systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA). Outcomes and their 95% confidence intervals (CI) were reported if a meta-analysis of these outcomes was conducted. Heterogeneity was reported using I2 statistics, and heterogeneity ≥ 50% was considered high. In case of high heterogeneity, the random-effects model was used. We used Baujat’s plot for the contribution of each study toward overall heterogeneity [14]. In sensitivity analysis, the summary estimates were assessed by repeating meta-analysis after omitting one study at a time. The influence of an individual study on overall results was identified by using various statistical tests, including studentized residuals, difference in fits (DFFITS), Cooks distance, covariance ratio, tau square, and the contribution of each study in the Q, H2 test statistics value and the weights assigned to these studies [15]. Forest plots were used for reporting outcomes in each study with their 95% CI. All statistical tests were performed using R software version 4.0.3 and Revan 5.4.
Results
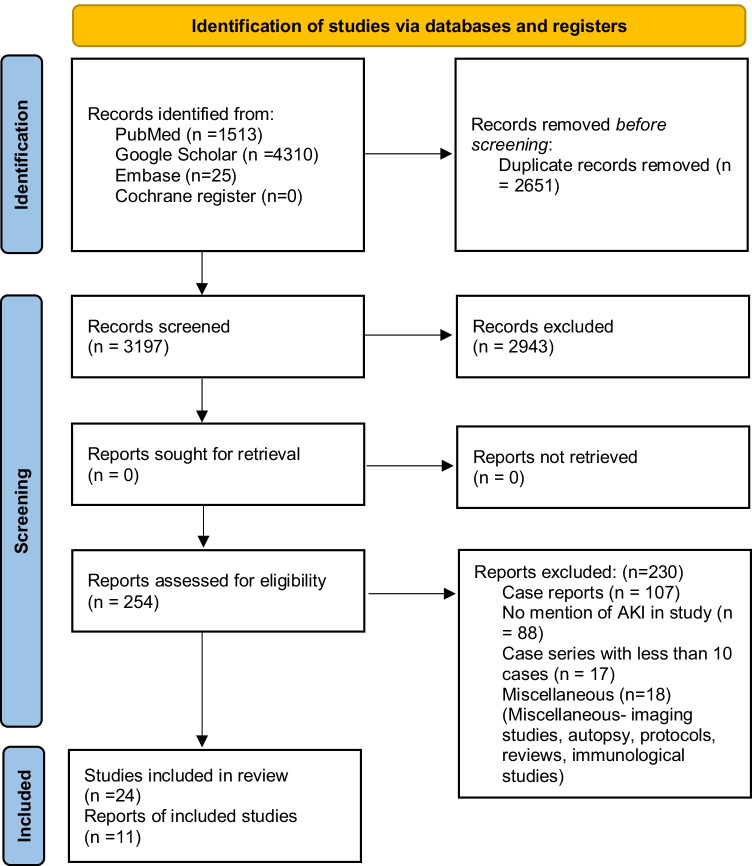
The search strategy yielded 5848 records. After removing duplicates, 3197 records were screened. After reading the title and abstract, 2943 were excluded. After going through the full text of 254 studies, 230 records were excluded due to the following reasons: case reports (n = 107), studies did not mention AKI (n = 88), case series with less than 10 cases (n = 17), and miscellaneous (n = 18). Finally, 24 studies were included in the systematic review — of these, 11 were included in the meta-analysis (Fig. 1). Multiple single-centre and multicentric studies published during the time period were considered for this meta-analysis, and there was a considerable overlap of data. To avoid duplication of data, we only included the nationwide multicentric studies which had collected data from single-centre studies, after confirming with the respective authors.
Fig. 1.
PRISMA flow diagram describing the inclusion of studies
A total of 24 studies with 6186 children were included in the systematic review, and of these, 11 studies with 4947 children were included in the meta-analysis. Fourteen of these studies were multicentric [10, 11, 16–27], and 10 were single-centre studies [12, 28–36]. Twelve studies were conducted in the USA [10, 12, 16, 21, 23, 24, 27–29, 31, 32, 36], 2 in the UK [11, 19], 2 in Spain [18, 20], and 1 each in Columbia, Brazil, and Pakistan, respectively [17, 22, 25]. Three studies were conducted in India [30, 33, 35], and 2 were conducted in Turkey [26, 34]. Fourteen studies were retrospective [10, 16, 21, 22, 24, 26–29, 31, 32, 34–36], 7 were prospective [11, 12, 17–20, 33], while 3 were prospective as well as retrospective [23, 25, 30]. Study characteristics are described in Table 1. A total of 11 studies were included in meta-analysis. The detailed qualitative analysis of the studies is provided in Supplementary Table 1 and Supplementary Table 2.
Table 1.
Characteristics of included studies
| S. no. | Study title | Study author | Country | Multicentric/single centre | Prospective/retrospective | Median age/sample size/invasive mechanical ventilation (IMV)/shock | IVIG/steroids/antiplatelet/anticoagulants | AKI/KRT/mortality | Study quality (NHLBI tool) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | COVID-19-associated multisystem inflammatory syndrome in children — United States, March–July 2020 | Godfred-Cato S et al. [16] | USA | Multicentric | Retrospective |
Median age: 8 years Sample size: 570 IMV: 69 Shock: 202 |
IVIG: 424 Steroids: 331 Antiplatelet drugs: 309 Anticoagulants: 233 |
AKI: 105 KRT: 2 Mortality: 10 |
Fair |
| 2 | Distinct clinical and immunological features of SARS-CoV-2-induced multisystem inflammatory syndrome in children | Lee PY et al. [28] | USA | Single centre | Retrospective |
Median age: 9 years Sample size: 28 IMV: 0 Shock: 15 |
IVIG: 20 Steroids: 17 Antiplatelet drugs: 19 Anticoagulants: 18 |
AKI: 6 KRT: not mentioned Mortality: 0 |
Fair |
| 3 | Severe manifestations of SARS-CoV-2 in children and adolescents: from COVID-19 pneumonia to multisystem inflammatory syndrome: a multicentre study in pediatric intensive care units in Spain | García-Salido A et al. [18] | Spain | Multicentric | Prospective |
Median age: 9.4 years Sample size: 74 IMV: 6 Shock: 38 |
IVIG: 23 Steroids: 36 Antiplatelet drugs: not mentioned Anticoagulants: not mentioned |
AKI: 9 KRT: 0 Mortality: 0 |
Good |
| 4 | Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study | Davies P et al. [19] | UK | Multicentric | Prospective |
Median age: 11 years Sample size: 78 IMV: 36 Shock: 68 |
IVIG: 59 Steroids: 57 Antiplatelet drugs: 45 Anticoagulants: 45 |
No mention of AKI, but 1 patient received KRT Mortality: 2 |
Low |
| 5 | Acute kidney injury in pediatric inflammatory multisystem syndrome temporally associated with severe acute respiratory syndrome Coronavirus-2 pandemic: experience from PICUs across United Kingdom | Deep A et al. [11] | UK | Multicentric | Prospective |
Median age: 11 years Sample size: 116 IMV: 41 Shock: 71 |
Not mentioned |
AKI: 48 KRT: 3 Mortality: 2 |
Good |
| 6 | Imaging findings in multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease (COVID-19) | Blumfield E et al. [29] | USA | Single centre | Retrospective |
Mean age: 9.2 years Sample size: 16 IMV: 1 Shock: 10 |
IVIG: 5 Steroids: 10 Antiplatelet drugs: not mentioned Anticoagulants: not mentioned |
AKI: 5 KRT: not mentioned Mortality: 0 |
Low |
| 7 | Evidence of thrombotic microangiopathy in children with SARS-CoV-2 across the spectrum of clinical presentations | Diorio C et al. [12] | USA | Single centre | Prospective |
Median age: 9 years Sample size: 55 IMV: 4 Shock: Not mentioned (ionotropic support in 20 cases) |
Not mentioned |
AKI: 5 KRT: 0 Mortality: 0 |
Fair |
| 8 | Epidemiological and clinical profile of pediatric inflammatory multisystem syndrome —temporally associated with SARS-CoV-2 (PIMS-TS) in Indian children | Dhanalakshmi K et al. [39] | India | Single centre | Prospective and retrospective |
Median age: 6 years Sample size: 19 IMV: 0 Shock: 10 |
IVIG: 15 Steroids: 11 Antiplatelet drugs: 16 Anticoagulants: not mentioned |
AKI: 3 KRT: not mentioned Mortality: 0 |
Low |
| 9 | Multi-inflammatory syndrome in children related to severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) in Spain | Moraleda C et al. [20] | Spain | Multicentric | Prospective |
Median age: 7.6 years Sample size: 31 IMV: 6 Shock: 15 |
IVIG: 20 Steroids: 21 Antiplatelet drugs: not mentioned Anticoagulants: not mentioned |
AKI: 4 KRT: not mentioned Mortality: 1 |
Good |
| 10 | Multisystem inflammatory syndrome in children associated with novel coronavirus SARS-CoV-2: presentations to a pediatric emergency department in Michigan | Sethuraman U et al. [31] | USA | Single centre | Retrospective |
Median age: 6 years Sample size: 34 IMV: 8 Shock: 13 |
IVIG: 34 Steroids: 0 Antiplatelet drugs: 34 Anticoagulants: 0 |
AKI: 10 KRT: not mentioned Mortality: 0 |
Fair |
| 11 | Acute hepatitis is a prominent presentation of the multisystem inflammatory syndrome in children: a single-center report | Cantor A et al. [32] | USA | Single centre | Retrospective |
Median age: not provided Sample size: 44 IMV: 1 Shock: 22 |
Not mentioned |
AKI: 7 KRT: 1 Mortality: 0 |
Fair |
| 12 | Multisystem inflammatory syndrome in children: clinical features and management-intensive care experience from a pediatric public hospital in Western India | Shobhavat L et al. [33] | India | Single centre | Prospective |
Median age: 7 years Sample size: 21 IMV: 7 Shock: 20 |
IVIG: 11 Steroids: 18 Antiplatelet drugs: not mentioned Anticoagulants: 21 |
AKI: 8 KRT: not mentioned Mortality: 3 |
Low |
| 13 | Acute kidney injury in pediatric patients hospitalized with acute COVID-19 and multisystem inflammatory syndrome in children associated with COVID-19 | Basalely A et al. [10] | USA | Multicentric | Retrospective |
Median age: 7.5 years Sample size: 152 IMV: 1 Shock: not mentioned (25 patients received ionotropic/vasopressor support) |
IVIG: 40 Steroids: 26 Antiplatelet drugs: not mentioned Anticoagulants: not mentioned |
AKI: 10 KRT: 0 Mortality: 0 |
Fair |
| 14 | Clinical features and outcome of MIS-C patients: an experience from Central Anatolia | Alkan G et al. [34] | Turkey | Single centre | Retrospective |
Median age: 94.5 months Sample size: 36 IMV: not mentioned Shock: 11 |
IVIG: 36 Steroids: 36 Antiplatelet drugs: 36 Anticoagulants: 36 |
AKI: 5 KRT: not mentioned Mortality: 0 |
Fair |
| 15 | Unusual clinical manifestations and outcome of multisystem inflammatory syndrome in children (MIS-C) in a tertiary care hospital of North India | Gupta Dch S et al. [35] | India | Single centre | Retrospective |
Median age: not provided Sample size: 41 IMV: 13 Shock: 13 |
IVIG: 0 Steroids: 16 Antiplatelet drugs: not mentioned Anticoagulants: not mentioned |
AKI: 6 KRT: not mentioned Mortality: 13 |
Low |
| 16 | Multisystem inflammatory syndrome in children (MIS-C) during SARS-CoV-2 pandemic in Brazil: a multicenter, prospective cohort study | Lima-Setta F et al. [17] | Brazil | Multicentric | Prospective |
Median age: 6.2 years Sample size: 56 IMV: 6 Shock: 33 |
IVIG: 50 Steroids: 31 Antiplatelet drugs: 25 Anticoagulants: 29 |
AKI: 5 KRT: not mentioned Mortality: 1 |
Fair |
| 17 | Characteristics, cardiac involvement, and outcomes of multisystem inflammatory syndrome of childhood associated with severe acute respiratory syndrome coronavirus 2 Infection | Capone CA et al. [21] | USA | Multicentric | Retrospective |
Median age: 8.6 years Sample size: 33 IMV: 6 Shock: 25 |
IVIG: 33 Steroids: 23 Antiplatelet drugs: 29 Anticoagulants: 14 |
AKI: 23 KRT: not mentioned Mortality: 0 |
Fair |
| 18 | Multisystem inflammatory syndrome in U.S. children and adolescents | Feldstein LR et al. [23] | USA | Multicentric | Prospective and retrospective |
Median age: 8.3 years Sample size: 186 IMV: 37 Shock: not mentioned (90 patients received vasopressor support) |
IVIG: 144 Steroids: 91 Antiplatelet drugs: not mentioned Anticoagulants: 87 |
AKI: 10 KRT: not mentioned Mortality: 4 |
Low |
| 19 | Kawasaki disease-like features in 10 pediatric COVID-19 cases: a retrospective study | Falah NU et al. [22] | Pakistan | Multicentric | Retrospective |
Mean age: 6 years Sample size: 10 IMV: 0 Shock: 6 |
IVIG: 9 Steroids: 3 Antiplatelet drugs: 7 Anticoagulants: not mentioned |
AKI: 1 KRT: not mentioned Mortality: 0 |
Fair |
| 20 | Multisystem inflammatory syndrome in children in New York State | Dufort EM et al. [24] | USA | Multicentric | Prospective |
Median age: not provided Sample size: 99 IMV: 10 Shock: 10 |
IVIG: 69 Steroids: 63 Antiplatelet drugs: not mentioned Anticoagulants: not mentioned |
AKI: 10 KRT: not mentioned Mortality: 2 |
Low |
| 21 | Acute kidney injury in COVID-19-associated multisystem inflammatory syndrome in children (MIS-C) | Marissa Lipton et al. [36] | USA | Single centre | Retrospective |
Median age: 7 years Sample size: 57 IMV: 1 Shock: not mentioned (18 patients received vasopressors) |
IVIG: 21 Steroids: 26 Antiplatelet drugs: not mentioned Anticoagulants: not mentioned |
AKI: 26 KRT: 1 Mortality: 0 |
Good |
| 22 | Mortality and clinical characteristics of multisystem inflammatory syndrome in children (MIS-C) associated with covid-19 in critically ill patients: an observational multicentre study (MISCO study) | Lorena Acevedo et al. [25] | Colombia | Multicentric | Prospective and retrospective |
Median age: 7 years Sample size: 78 IMV: 27 Shock: 68 |
IVIG: 71 Steroids: 55 Antiplatelet drugs: 34 Anticoagulants: 34 |
AKI: 23 KRT: 9 Mortality: 7 |
Fair |
| 23 | Clinical features and outcomes of 76 patients with COVID-19-related multi-system inflammatory syndrome in children | Fatih Haslak et al. [26] | Turkey | Multicentric | Retrospective |
Mean age: 8.17 years Sample size: 76 IMV: 3 Shock: not mentioned (18 patients received vasopressors) |
IVIG: 27 Steroids: 26 Antiplatelet drugs: not mentioned Anticoagulants: 23 |
AKI: 8 KRT: 4 Mortality: 1 |
Good |
| 24 | Multisystem inflammatory syndrome in children—United States, February 2020–July 2021 | Miller AD et al. [27] | USA | Multicentric | Retrospective |
Median age: 9 years Sample size: 4470 IMV: 419 Shock: 2018 |
IVIG: 3772 Steroids: 3428 Antiplatelet drugs: not mentioned Anticoagulants: not mentioned |
AKI: 849 KRT: 42 Mortality: 37 |
Fair |
Abbreviations: AKI acute kidney injury, KRT kidney replacement therapy, IVIG intravenous immunoglobulin
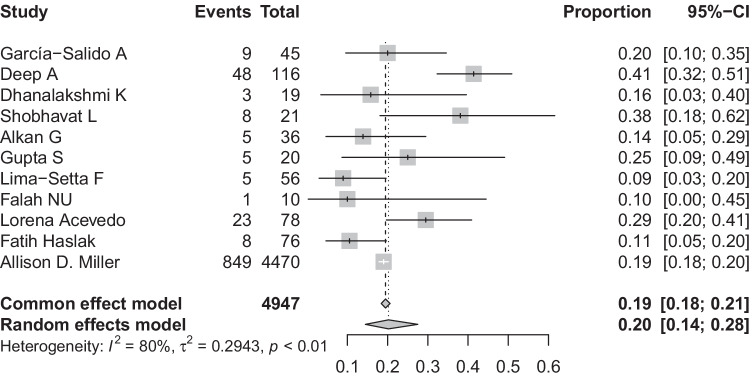
The overall proportion of MIS-C children developing AKI
All studies reported the proportion of the children developing AKI. The pooled proportion of patients with MIS-C developing AKI was 20% (95% CI: 14–28%, I2 = 80%) (Fig. 2) (11 studies, 4947 patients). As there was unexplained heterogeneity, we used the Baujat plot and identified 2 studies (Deep A and Miller AD) [11, 27] as outliers (Supplementary Fig. 1). However, no study was found to significantly influence the heterogeneity (Supplementary Fig. 2) on performing influential analysis. Visual inspection of the funnel plot for publication bias was symmetrical, and Egger’s test was non-significant (p = 0.62) (Supplementary Fig. 3). A subgroup analysis was done for incidence of AKI based on sample size, geography, definition of AKI used (KDIGO versus others), and multicentric versus single-centre studies. Incidence of AKI in MIS-C patients in studies with large sample size [19% (95% CI: 18–20%2)] was almost similar to incidence of AKI in studies with small sample size [21% (95% CI: 14–30%; I2 = 76%)] (Supplementary Fig. 4). Comparing incidence of AKI in MIS-C patients based on geography, we found that non-Asian studies had higher incidence of AKI [23% (95% CI: 14–35%; I2 = 90%)] as compared to Asian studies [18% (95% CI: 11–28%; I2 = 48%)] (Supplementary Fig. 5). The incidence of AKI was also higher in studies which used the KDIGO definition of AKI [24% (95% CI: 14–37%; I2 = 93%)] as compared to studies which used some other definition of AKI [18% (95% CI: 12–27%; I2 = 39%)] (Supplementary Fig. 6). There was a slightly higher proportion of children developing AKI in single-centre studies [23% (95% CI: 13–36%; I2 = 38%)] as compared to multicentric studies [20% (95% CI: 13–29%; I2 = 87%)] (Supplementary Fig. 7).
Fig. 2.
Pooled incidence of AKI in patients with MIS-C
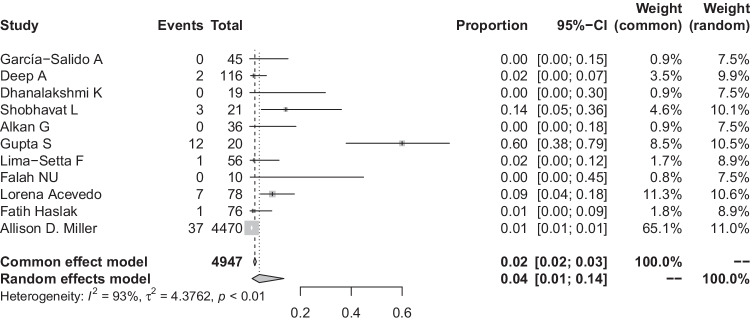
Mortality
All studies reported death in children with MIS-C. Pooled proportion of death in children with MIS-C was 4% (95% CI: 1–14%; I2 = 93%) (Fig. 3) (11 studies, 4947 patients). Due to unexplained heterogeneity in results, the Baujat plot was used, which identified studies (Miller AD, Gupta S) [27, 35] as outliers (Supplementary Fig. 8). However, on performing influential analysis, we could not find any study significantly contributing towards heterogeneity (Supplementary Fig. 9). Visual inspection of the funnel plot revealed asymmetry (Supplementary Fig. 10), but Egger’s test for publication bias was not significant (p = 0.27). Subgroup analysis of mortality was done based on sample size (large versus small), geography (Asian versus non-Asian studies), definition of AKI used (KDIGO versus others), and multicentric versus single-centre studies. The subgroup of smaller studies was compared with a single large study by Miller et al. and showed higher mortality in studies with smaller sample size [5% (95% CI: 2–15%; I2 = 83%)] than the study with larger sample size [1% (95% CI: 1–1%2)], and this result was significant (Supplementary Fig. 11). Mortality was also higher in Asian studies [7% (95% CI: 1–32%; I2 = 84%)] as compared to non-Asian studies [2% (95%CI: 1–7%; I2 = 88%)] (Supplementary Fig. 12). Mortality was less in studies which used KDIGO definition of AKI [2% (95% CI: 0–9%; I2 = 91%)] as compared to studies which used other definitions of AKI [6% (95% CI: 1–26%; I2 = 83%)] (Supplementary Fig. 13). The subgroup analysis based on multicentric versus single-centre study showed higher pooled mortality in multi-centre studies [2% (95% CI: 1–6%; I2 = 83%)] as compared to single-centre studies [12% (95% CI: 2–52 %; I2 = 85%)] (Supplementary Fig. 14).
Fig. 3 .
Overall mortality in patients with MIS-C
Risk of death in MIS-C patients with and without AKI
Four studies (Deep A, Acevedo L, Haslak F, Shobhavat L) [11, 25, 26, 33] provided data on AKI in deceased patients. The odds of death in patients with AKI were 4.68 times higher than in patients without AKI (95% CI: 1.06–20.7; I2 = 17%) (Fig. 4).
Fig. 4.
Comparison of death in MIS-C patients with and without AKI
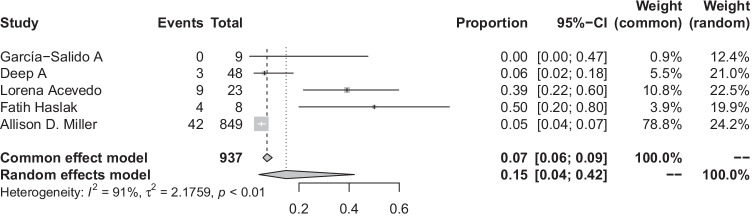
Requirement of kidney replacement therapy
Five studies reported this outcome (Deep A, Garcia-Salido A, Acevedo L, Haslak F, Miller AD) [11, 18, 25–27]. The overall pooled proportion of MIS-C-induced AKI patients requiring KRT was 15% (95% CI: 4–42%; I2 = 91%) (Fig. 5) (5 studies, 4785 patients). We used Baujat’s plot and identified a study (Miller AD) [27] as influencing the heterogeneity. However, influential analysis did not reveal significant heterogeneity (Supplementary Figs. 15 and 16).
Fig. 5.
Pooled incidence of the need for kidney replacement therapy in patients with MIS-C
Discussion
Our analysis found that one-fifth of the children with MIS-C develop AKI and have higher odds of death as compared to children with MIS-C without AKI. The incidence of AKI in pediatric patients is variable, ranging from 5 to 37% in ICU with variable requirement of KRT ranging from 20 to 23% [37, 38]. Thus, we see that patients with MIS-C have a similar risk of AKI when compared to pediatric patients in ICU due to other causes and their need for KRT may be slightly less (15%), as evident by our results. Our analysis of subgroups provided further insight into the incidence of AKI. We found that studies which used KDIGO criteria for diagnosis of AKI had higher incidence of AKI when compared to studies which used other criteria for AKI, which is in agreement with the previous literature.
Over the last decade, there has been a substantial change in the definition and staging of AKI. In the present review, out of 24 studies included for qualitative synthesis, only 21 used the term AKI. However, no definition was provided by 11 of these studies (Godfred-Cato S, Lima Setta F, Falah NU, Haslak F, Miller AD, Lee PY, Blumfield E, Sethuraman U, Cantor A, Shobhavat L, Dhanlakshmi K) [16, 17, 22, 26–29, 31–33, 39], 5 studies (Basalely A, Diorio C, Acevedo L, Lipton M, Capone CA) [10, 12, 25, 36, 40] used the KDIGO definition for AKI, while 4 studies used other definitions. Deep et al. used age-specific upper limit of reference values according to guidelines from the British Association of Pediatric Nephrology for AKI due to lack of previously known baseline creatinine values in their patients [11]. Garcia-Salido et al. defined AKI as serum creatinine values two times the upper normal reference for age and sex [18]. One study each used the terms ‘renal failure’, ‘prerenal insufficiency’, and ‘renal impairment’ (Moraleda C, Alkan G, Gupta S) [20, 34, 35].
The study by Miller et al. was the largest, including patients from all over the USA, and the study period encompassed all three waves of COVID-19. This study had 4470 cases of MIS-C, with 849 of them developing AKI, but only 42 of them required KRT. The study also provided data about AKI in each of the three waves, with 106 patients (of 649) developing AKI in the first wave, 166 patients (of 769) developing AKI in the second wave, and 577 patients (of 3052) developing AKI in the third wave. KRT was required in 5, 4, and 33 patients in each wave, respectively [27].
Godfred-cato et al. used latent class analysis to divide patients into three classes. Class 1 patients had the highest number of organ systems involved, while class 2 patients had predominantly respiratory involvement, and class 3 patients had manifestations most similar to Kawasaki disease. This report used the term AKI, but no definition was provided. Most patients with AKI (77 of 105) were in class 1, few were in class 2 (28 of 105), while no patient in class 3 developed AKI [16].
Deep et al. found that severe AKI in MIS-C was associated with nephrotoxic drugs, high BMI, and high ferritin values. However, on multivariate analysis, only association with hyperferritinemia was significant [11]. Basalely et al. also described the use of nephrotoxic drugs as a potential exacerbator of AKI in MIS-C [10]. The authors also found an association between a greater need for vasoactive medication and a longer duration of ICU stay among MIS-C patients who had AKI compared to those without AKI [10]. Similar findings were reported by Deep et al., who suggested that the duration of ICU stay and mechanical ventilation were longer in MIS-C patients who had severe AKI [11].
A peek into the mechanism of renal injury was provided by Diorio et al., who suggested a combination of viral infection of the cell along with complement activation and vascular injury as the cause. Elevations in sC5b9 were independent of other MIS-C markers and associated with renal injury [12].
Overall mortality in MIS-C patients in the present study was 4%. On subgroup analysis, we found that single-centre studies had slightly higher mortality rates than multicentric studies. Similarly, studies which used KDIGO definition had lower mortality when compared to studies that used other definitions of AKI, and Asian studies had higher mortality than non-Asian studies. However, none of these differences were significant. But we found significantly low mortality in studies with larger sample size when compared with studies of smaller sample size. Mortality in PICU varies from 2 to 30% across countries based on availability of resources and common diseases leading to ICU admissions [41–44]. The present analysis also showed that mortality among MIS-C patients increases by 4.68 times if the patients develop AKI. Thus, mortality among MIS-C patients with AKI is comparable to mortality among PICU patients having AKI due to other causes. Previous studies in the pediatric age group have reported high mortality ranging from 11 to 36% in children with AKI [35, 37, 38, 45].
The strengths of this systematic review are as follows: (1) this is the first systematic review along with a meta-analysis describing incidence, mortality, and need for KRT in patients with MIS-C; and (2) the use of rigorous statistical methods to explore the heterogeneity among included studies.
A possible limitation can be considered because of different definitions of AKI being used in different studies. Also, not all studies provided data about the cause of death, and as such, mortality specific to AKI in MIS-C could vary if these data are included. Another limitation that should be considered is the heterogenous ways the different retrospective studies were performed. Leak of prospective data is another limitation of this systematic review.
Conclusion
Approximately 20% of children with MIS-C develop AKI which is associated with higher odds of death.
Supplementary Information
(PPTX 42 kb)
Baujat plot for unexplained heterogeneity in pooled incidence of AKI (PDF 5 kb)
Influential analysis for unexplained heterogeneity in pooled incidence of AKI (PDF 11 kb)
Funnel plot for overall AKI. (PDF 11 kb)
Incidence of AKI in studies with small sample size versus studies with large sample size. (PDF 6 kb)
Incidence of AKI in Asian studies versus non-Asian studies. (PDF 6 kb)
Incidence of AKI in studies using KDIGO definition of AKI versus studies using other definitions of AKI. (PDF 6 kb)
Incidence of AKI in multicentric and single centre studies. (PDF 7 kb)
Baujat plot for heterogeneity in overall mortality. (PDF 5 kb)
Influential analysis for heterogeneity in overall mortality. (PDF 11 kb)
Funnel plot for overall mortality. (PDF 10 kb)
Comparison of incidence of overall mortality in studies with small sample size versus studies with large sample size. (PDF 6 kb)
Comparison of incidence of overall mortality in Asian versus non-Asian studies. (PDF 6 kb)
Comparison of incidence of overall mortality in studies using KDIGO definition of AKI versus studies using other definitions of AKI. (PDF 6 kb)
Comparison of incidence of overall mortality in multicentric and single centre studies. (PDF 6 kb)
Baujat plot for heterogeneity in need for kidney replacement therapy. (PDF 4 kb)
Influential analysis for heterogeneity in need for kidney replacement therapy. (PDF 8 kb)
(DOCX 31 kb)
(DOCX 20.8 kb)
(DOCX 25.1 kb)
Acknowledgements
The authors would like to thank Mr Amit Gupta, senior librarian, AIIMS Bhopal, for helping in search strategy and providing full text of articles.
Author contribution
GCB and RP conceptualized the review; AT, AK, MA, and RP extracted the data from the studies and wrote the initial draft of the manuscript; GCB performed statistical analysis; GCB and SM revised the manuscript and contrived the final draft. All authors read and approved the final version of the manuscript.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rowley AH. Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat Rev Immunol. 2020;20:453–454. doi: 10.1038/s41577-020-0367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kabeerdoss J, Pilania RK, Karkhele R, Kumar TS, Danda D, Singh S. Severe COVID-19, multisystem inflammatory syndrome in children, and Kawasaki disease: immunological mechanisms, clinical manifestations and management. Rheumatol Int. 2021;41:19–32. doi: 10.1007/s00296-020-04749-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Payne AB, Gilani Z, Godfred-Cato S, Belay ED, et al. Incidence of multisystem inflammatory syndrome in children among US persons infected with SARS-CoV-2. JAMA Netw Open. 2021;4:e2116420. doi: 10.1001/jamanetworkopen.2021.16420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(2021) HAN Archive - 00432 | Health Alert Network (HAN). https://emergency.cdc.gov/han/2020/han00432.asp. Accessed 14 Nov 2021
- 5.Multisystem inflammatory syndrome in children and adolescents with COVID-19. https://www.who.int/publications-detail-redirect/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19. Accessed 14 Nov 2021
- 6.(2020) Rapid risk assessment: Paediatric inflammatory multisystem syndrome and SARS -CoV-2 infection in children. In: European Centre for Disease Prevention and Control. https://www.ecdc.europa.eu/en/publications-data/paediatric-inflammatory-multisystem-syndrome-and-sars-cov-2-rapid-risk-assessment. Accessed 14 Nov 2021
- 7.Fabi M, Filice E, Biagi C, Andreozzi L, Palleri D, Mattesini BE, Rizzello A, Gabrielli L, Ghizzi C, Di Luca D, Caramelli F, De Fanti A, Lanari M. Multisystem inflammatory syndrome following SARS-CoV-2 infection in children: one year after the onset of the pandemic in a high-incidence area. Viruses. 2021;13:2022. doi: 10.3390/v13102022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang L, Tang K, Levin M, Irfan O, Morris SK, Wilson K, Klein JD, Bhutta ZA. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis. 2020;20:e276–e288. doi: 10.1016/S1473-3099(20)30651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irfan O, Muttalib F, Tang K, Jiang L, Lassi ZS, Bhutta Z. Clinical characteristics, treatment and outcomes of paediatric COVID-19: a systematic review and meta-analysis. Arch Dis Child. 2021;106:440–448. doi: 10.1136/archdischild-2020-321385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basalely A, Gurusinghe S, Schneider J, Shah SS, Siegel LB, Pollack G, Singer P, Castellanos-Reyes LJ, Fishbane S, Jhaveri KD, Mitchell E, Merchant K, Capone C, Gefen AM, Steinberg J, Sethna CB. Acute kidney injury in pediatric patients hospitalized with acute COVID-19 and multisystem inflammatory syndrome in children associated with COVID-19. Kidney Int. 2021;100:138–145. doi: 10.1016/j.kint.2021.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deep A, Upadhyay G, du Pré P, Lillie J, et al. Acute kidney injury in pediatric inflammatory multisystem syndrome temporally associated with severe acute respiratory syndrome coronavirus-2 pandemic: experience from PICUs across United Kingdom. Crit Care Med. 2020;48:1809–1818. doi: 10.1097/CCM.0000000000004662. [DOI] [PubMed] [Google Scholar]
- 12.Diorio C, McNerney KO, Lambert M, Paessler M, et al. Evidence of thrombotic microangiopathy in children with SARS-CoV-2 across the spectrum of clinical presentations. Blood Adv. 2020;4:6051–6063. doi: 10.1182/bloodadvances.2020003471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pilania RK, Dokania S, Kumar A, Ahmad R, Malik S, Bhatt GC. Acute renal failure requiring renal replacement therapy: unusual presentation of multisystem inflammatory syndrome in children. J Paediatr Child Health. 2021;57:1724–1725. doi: 10.1111/jpc.15715. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viechtbauer W, Cheung MW-L. Outlier and influence diagnostics for meta-analysis. Res Synth Methods. 2010;1:112–125. doi: 10.1002/jrsm.11. [DOI] [PubMed] [Google Scholar]
- 16.Godfred-Cato S, Bryant B, Leung J, Oster ME et al (2020) COVID-19-associated multisystem inflammatory syndrome in children — United States, March–July 2020. MMWR Morb Mortal Wkly Rep 69:1074–1080 [DOI] [PMC free article] [PubMed]
- 17.Lima-Setta F, de Magalhães-Barbosa MC, Rodrigues-Santos G, Figueiredo EADN, et al. Multisystem inflammatory syndrome in children (MIS-C) during SARS-CoV-2 pandemic in Brazil: a multicenter, prospective cohort study. J Pediatr (Rio J) 2021;97:354–361. doi: 10.1016/j.jped.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.García-Salido A, de Carlos Vicente JC, Belda Hofheinz S, Balcells Ramírez J, et al. Severe manifestations of SARS-CoV-2 in children and adolescents: from COVID-19 pneumonia to multisystem inflammatory syndrome: a multicentre study in pediatric intensive care units in Spain. Crit Care. 2020;24:666. doi: 10.1186/s13054-020-03332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies P, Evans C, Kanthimathinathan HK, Lillie J, et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc Health. 2020;4:669–677. doi: 10.1016/S2352-4642(20)30215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moraleda C, Serna-Pascual M, Soriano-Arandes A, Simó S, et al. Multi-inflammatory syndrome in children related to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Spain. Clin Infect Dis. 2021;72:e397–e401. doi: 10.1093/cid/ciaa1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Capone CA, Subramony A, Sweberg T, Schneider J, et al. Characteristics, cardiac involvement, and outcomes of multisystem inflammatory syndrome of childhood associated with severe acute respiratory syndrome coronavirus 2 infection. J Pediatr. 2020;224:141–145. doi: 10.1016/j.jpeds.2020.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falah NU, Hashmi S, Ahmed Z, Jaan A, Akhtar A, Khalid F, Farooque U, Shera MT, Ali S, Javed A. Kawasaki disease-like features in 10 pediatric COVID-19 cases: a retrospective study. Cureus. 2020;12:e11035. doi: 10.7759/cureus.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feldstein LR, Rose EB, Horwitz SM, Collins JP, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acevedo L, Piñeres-Olave BE, Niño-Serna LF, Vega LM, et al. Mortality and clinical characteristics of multisystem inflammatory syndrome in children (MIS-C) associated with covid-19 in critically ill patients: an observational multicenter study (MISCO study) BMC Pediatr. 2021;21:516. doi: 10.1186/s12887-021-02974-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haslak F, Barut K, Durak C, Aliyeva A, et al. Clinical features and outcomes of 76 patients with COVID-19-related multi-system inflammatory syndrome in children. Clin Rheumatol. 2021;40:4167–4178. doi: 10.1007/s10067-021-05780-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller AD, Zambrano LD, Yousaf AR, Abrams JY, Meng L, Wu MJ, Melgar M, Oster ME, Godfred Cato SE, Belay ED, Campbell AP; MIS-C Surveillance Authorship Group (2021) Multisystem inflammatory syndrome in children—United States, February 2020-July 2021. Clin Infect Dis. 10.1093/cid/ciab1007 [DOI] [PubMed]
- 28.Lee PY, Day-Lewis M, Henderson LA, Friedman KG, et al. Distinct clinical and immunological features of SARS-CoV-2-induced multisystem inflammatory syndrome in children. J Clin Invest. 2020;130:5942–5950. doi: 10.1172/JCI141113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blumfield E, Levin TL, Kurian J, Lee EY, Liszewski MC. Imaging findings in multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease (COVID-19) AJR Am J Roentgenol. 2021;216:507–517. doi: 10.2214/AJR.20.24032. [DOI] [PubMed] [Google Scholar]
- 30.Dhanalakshmi K, Venkataraman A, Balasubramanian S, Madhusudan M, Amperayani S, Putilibai S, Sadasivam K, Ramachandran B, Ramanan AV. Epidemiological and clinical profile of pediatric inflammatory multisystem syndrome — temporally associated with SARS-CoV-2 (PIMS-TS) in Indian Children. Indian Pediatr. 2020;57:1010–1014. doi: 10.1007/s13312-020-2025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sethuraman U, Kannikeswaran N, Ang J, Singer A, Miller J, Haddad R, Stankovic C. Multisystem inflammatory syndrome in children associated with novel coronavirus SARS-CoV-2: presentations to a pediatric emergency department in Michigan. Am J Emerg Med. 2021;39:164–167. doi: 10.1016/j.ajem.2020.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cantor A, Miller J, Zachariah P, DaSilva B, Margolis K, Martinez M. Acute hepatitis is a prominent presentation of the multisystem inflammatory syndrome in children: a single-center report. Hepatology. 2020;72:1522–1527. doi: 10.1002/hep.31526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shobhavat L, Solomon R, Rao S, Bhagat I, Prabhu S, Prabhu S, Chandrakar M, Bodhanwala M. Multisystem inflammatory syndrome in children: clinical features and management-intensive care experience from a pediatric public hospital in Western India. Indian J Crit Care Med. 2020;24:1089–1094. doi: 10.5005/jp-journals-10071-23658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alkan G, Sert A, Oz SKT, Emiroglu M, Yılmaz R. Clinical features and outcome of MIS-C patients: an experience from Central Anatolia. Clin Rheumatol. 2021;40:4179–4189. doi: 10.1007/s10067-021-05754-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta S, Chopra N, Singh A, Gera R, Chellani H, Pandey R, Arora BS. Unusual clinical manifestations and outcome of multisystem inflammatory syndrome in children (MIS-C) in a tertiary care hospital of North India. J Trop Pediatr. 2021;67:fmaa127. doi: 10.1093/tropej/fmaa127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipton M, Mahajan R, Kavanagh C, Shen C, Batal I, Dogra S, Jain NG, Lin F, Uy NS. AKI in COVID-19–associated multisystem inflammatory syndrome in children (MIS-C) Kidney360. 2021;2:611–618. doi: 10.34067/KID.0005372020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bouchard J, Acharya A, Cerda J, Maccariello ER, Madarasu RC, Tolwani AJ, Liang X, Fu P, Liu ZH, Mehta RL. A prospective international multicenter study of AKI in the intensive care unit. Clin J Am Soc Nephrol. 2015;10:1324–1331. doi: 10.2215/CJN.04360514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Restrepo JM, Mondragon MV, Forero-Delgadillo JM, Lasso RE, Zemanate E, Bravo Y, Castillo GE, Tetay S, Cabal N, Calvache JA. Acute renal failure in children. Multicenter prospective cohort study in medium-complexity intensive care units from the Colombian southeast. PLoS One. 2020;15:e0235976. doi: 10.1371/journal.pone.0235976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dhanalakshmi K, Venkataraman A, Balasubramanian S, Madhusudan M, Amperayani S, Putilibai S, Sadasivam K, Ramachandran B, Ramanan AV. Epidemiological and clinical profile of pediatric inflammatory multisystem syndrome — temporally associated with SARS-CoV-2 (PIMS-TS) in Indian Children. Indian Pediatr. 2020;57:1010–1014. doi: 10.1007/s13312-020-2025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Capone CA, Subramony A, Sweberg T, Schneider J, Shah S, Rubin L, Schleien C, Northwell Health COVID-19 Research Consortium. Epstein S, Johnson JC, Kessel A, Misra N, Mitchell E, Palumbo N, Rajan S, Rocker J, Williamson K, Davidson KW. Characteristics, cardiac involvement, and outcomes of multisystem inflammatory syndrome of childhood associated with severe acute respiratory syndrome coronavirus 2 Infection. J Pediatr. 2020;224:141–145. doi: 10.1016/j.jpeds.2020.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teshager NW, Amare AT, Tamirat KS. Incidence and predictors of mortality among children admitted to the pediatric intensive care unit at the University of Gondar comprehensive specialised hospital, northwest Ethiopia: a prospective observational cohort study. BMJ Open. 2020;10:e036746. doi: 10.1136/bmjopen-2019-036746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Punchak M, Hall K, Seni A, Buck WC, DeUgarte DA, Hartford E, Kelly RB, Muando VI. Epidemiology of disease and mortality from a PICU in Mozambique. Pediatr Crit Care Med. 2018;19:e603–e610. doi: 10.1097/PCC.0000000000001705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burns JP, Sellers DE, Meyer EC, Lewis-Newby M, Truog RD. Epidemiology of death in the pediatric intensive care unit at five U.S. teaching hospitals. Crit Care Med. 2014;42:2101–2108. doi: 10.1097/CCM.0000000000000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siddiqui N-R, Ashraf Z, Jurair H, Haque A. Mortality patterns among critically ill children in a pediatric intensive care unit of a developing country. Indian J Crit Care Med. 2015;19:147–150. doi: 10.4103/0972-5229.152756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Safder OY, Alhasan KA, Shalaby MA, Khathlan N, Al Rezgan SA, Albanna AS, Kari JA. Short-term outcome associated with disease severity and electrolyte abnormalities among critically ill children with acute kidney injury. BMC Nephrol. 2019;20:89. doi: 10.1186/s12882-019-1278-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PPTX 42 kb)
Baujat plot for unexplained heterogeneity in pooled incidence of AKI (PDF 5 kb)
Influential analysis for unexplained heterogeneity in pooled incidence of AKI (PDF 11 kb)
Funnel plot for overall AKI. (PDF 11 kb)
Incidence of AKI in studies with small sample size versus studies with large sample size. (PDF 6 kb)
Incidence of AKI in Asian studies versus non-Asian studies. (PDF 6 kb)
Incidence of AKI in studies using KDIGO definition of AKI versus studies using other definitions of AKI. (PDF 6 kb)
Incidence of AKI in multicentric and single centre studies. (PDF 7 kb)
Baujat plot for heterogeneity in overall mortality. (PDF 5 kb)
Influential analysis for heterogeneity in overall mortality. (PDF 11 kb)
Funnel plot for overall mortality. (PDF 10 kb)
Comparison of incidence of overall mortality in studies with small sample size versus studies with large sample size. (PDF 6 kb)
Comparison of incidence of overall mortality in Asian versus non-Asian studies. (PDF 6 kb)
Comparison of incidence of overall mortality in studies using KDIGO definition of AKI versus studies using other definitions of AKI. (PDF 6 kb)
Comparison of incidence of overall mortality in multicentric and single centre studies. (PDF 6 kb)
Baujat plot for heterogeneity in need for kidney replacement therapy. (PDF 4 kb)
Influential analysis for heterogeneity in need for kidney replacement therapy. (PDF 8 kb)
(DOCX 31 kb)
(DOCX 20.8 kb)
(DOCX 25.1 kb)