Abstract
All tRNAs have numerous modifications, lack of which often results in growth defects in the budding yeast Saccharomyces cerevisiae and neurological or other disorders in humans. In S. cerevisiae, lack of tRNA body modifications can lead to impaired tRNA stability and decay of a subset of the hypomodified tRNAs. Mutants lacking 7-methylguanosine at G46 (m7G46), N2,N2-dimethylguanosine (m2,2G26), or 4-acetylcytidine (ac4C12), in combination with other body modification mutants, target certain mature hypomodified tRNAs to the rapid tRNA decay (RTD) pathway, catalyzed by 5’-3’ exonucleases Xrn1 and Rat1, and regulated by Met22. The RTD pathway is conserved in the phylogenetically distant fission yeast Schizosaccharomyces pombe for mutants lacking m7G46. In contrast, S. cerevisiae trm6/gcd10 mutants with reduced 1-methyladenosine (m1A58) specifically target pre-tRNAiMet(CAU) to the nuclear surveillance pathway for 3’-5’ exonucleolytic decay by the TRAMP complex and nuclear exosome. We show here that the RTD pathway has an unexpected major role in the biology of m1A58 and tRNAiMet(CAU) in both S. pombe and S. cerevisiae. We find that S. pombe trm6Δ mutants lacking m1A58 are temperature sensitive due to decay of tRNAiMet(CAU) by the RTD pathway. Thus, trm6Δ mutants had reduced levels of tRNAiMet(CAU) and not of eight other tested tRNAs, overexpression of tRNAiMet(CAU) restored growth, and spontaneous suppressors that restored tRNAiMet(CAU) levels had mutations in dhp1/RAT1 or tol1/MET22. In addition, deletion of cid14/TRF4 in the nuclear surveillance pathway did not restore growth. Furthermore, re-examination of S. cerevisiae trm6 mutants revealed a major role of the RTD pathway in maintaining tRNAiMet(CAU) levels, in addition to the known role of the nuclear surveillance pathway. These findings provide evidence for the importance of m1A58 in the biology of tRNAiMet(CAU) throughout eukaryotes, and fuel speculation that the RTD pathway has a major role in quality control of body modification mutants throughout fungi and other eukaryotes.
Author summary
tRNA modifications are highly conserved, and their lack frequently results in growth defects in the yeast Saccharomyces cerevisiae and neurological disorders in humans. In S. cerevisiae lack of 1-methyladenosine at N58 (m1A58) in the tRNA body is lethal due to 3’-5’ decay of pre-tRNAiMet by the nuclear surveillance pathway. By contrast, lack of any of three other body modifications causes growth defects due to 5’-3’ decay of specific hypomodified tRNAs by the rapid tRNA decay (RTD) pathway. Despite their importance, little is known about either tRNAiMet quality control or tRNA decay pathways in eukaryotes other than S. cerevisiae.
Here we show an unexpected role of the RTD pathway in quality control of tRNAiMet lacking m1A58 in the phylogenetically distant yeast species Schizosaccharomyces pombe and S. cerevisiae. We find that S. pombe trm6Δ mutants, lacking m1A58, are temperature sensitive due to decay of tRNAiMet(CAU) primarily by the RTD pathway. Furthermore, re-investigation of S. cerevisiae trm6 mutants revealed a significant role of the RTD pathway, in addition to the nuclear surveillance pathway, in decay of tRNAiMet(CAU). Our results suggest that throughout eukaryotes the RTD pathway has a major role in decay of hypomodified tRNAs and that m1A58 is crucial to tRNAiMet(CAU) biology.
Introduction
tRNAs are central to the process of translation, a role that is enabled by their extensive and highly conserved post-transcriptional modifications [1–3]. Lack of any of a number of modifications causes growth defects in the budding yeast Saccharomyces cerevisiae [3], as well as a number of neurological or mitochondrial disorders in humans [4, 5]. Lack of modifications in and around the anticodon loop (ACL) frequently reduces the efficiency and/or fidelity of mRNA decoding [6–8], disrupts reading-frame maintenance [9, 10], or decreases charging efficiency and/or fidelity [11]. Lack of modifications in the main tRNA body (outside the ACL) often results in altered folding [12] or reduced tRNA stability, leading to degradation of a subset of the hypomodified tRNAs by one of two characterized decay pathways [13–15].
The rapid tRNA decay (RTD) pathway degrades a subset of the tRNA species lacking any of several body modifications. Degradation of tRNAs by the RTD pathway is catalyzed by the 5’-3’ exonucleases Rat1 and Xrn1, and inhibited by a met22Δ mutation [16] due to accumulation of the Met22 substrate adenosine 3’,5’ bisphosphate (pAp) [17, 18]. In S. cerevisiae, lack of m7G46, m2,2G26, or ac4C12 is known to trigger RTD, and is associated with temperature sensitivity, particularly in combination with lack of other tRNA body modifications. Thus, an S. cerevisiae trm8Δ trm4Δ mutant (lacking m7G46 and m5C), is temperature sensitive due to decay of mature tRNAVal(AAC) by the RTD pathway [14, 16]. Similarly, an S. cerevisiae tan1Δ trm44Δ mutant (lacking ac4C12 and Um44) and a trm1Δ trm4Δ mutant (lacking m2,2G26 and m5C) are each temperature sensitive due to decay of tRNASer(CGA) and tRNASer(UGA) [16, 19, 20]. Moreover, each of the corresponding trm8Δ, tan1Δ, and trm1Δ single mutants has an RTD signature, as their temperature sensitivity is suppressed by a met22Δ mutation, and is associated with decay of one or more hypomodified tRNA substrates [20].
In addition, recent results show that the RTD pathway also acts on a subset of tRNA species lacking m7G46 in the phylogenetically distant fission yeast Schizosaccharomyces pombe. Thus, the temperature sensitivity of S. pombe trm8Δ mutants is due to decay of tRNATyr(GUA) and to some extent tRNAPro(AGG), and both the decay and the temperature sensitivity are suppressed by mutations in the RAT1 ortholog dhp1 [15].
The other major decay pathway that targets tRNAs lacking a body modification in S. cerevisiae is the nuclear surveillance pathway, which degrades the precursor of initiator tRNA (pre-tRNAiMet(CAU)) lacking m1A58 [13, 21]. Degradation by this pathway is catalyzed by Trf4 of the TRAMP complex, which oligoadenylates the 3’ end of pre-tRNAiMet(CAU), followed by its 3’-5’ exonucleolytic degradation by Rrp6 and Rrp44 of the nuclear exosome [13, 22–25]. The m1A58 modification is found on numerous tRNA species in S. cerevisiae, but tRNAiMet(CAU) is uniquely different from other tRNAs due in part to its non-canonical nucleotides at A20, A54, and A60 and an unusual substructure involving these residues and m1A58 [26], presumably accounting for its unique sensitivity to decay in strains lacking m1A58 [13]. In addition, the nuclear surveillance pathway is also known to target about 50% of all tRNA transcripts, possibly due to stochastic errors during transcription, mis-folding, or natural competition between decay and processing [27].
Understanding the quality control of tRNAiMet(CAU) is crucial because of its central role in translation initiation. In this role, tRNAiMet(CAU) is a component of the eukaryotic ternary complex that binds the 40S ribosome subunit to form the 43S pre-initiation complex, which in turn binds capped mRNAs and scans their sequence for an appropriate AUG start codon [28]. Moreover, tRNAiMet(CAU) is only involved in translation initiation and has its own dedicated set of factors for its delivery to the 40S subunit [29], whereas all other tRNAs participate only in elongation, and their delivery only involves the elongation factor eEF-1A [30], which does not participate in translation initiation. In addition, tRNAiMet(CAU) levels are important in regulating the general amino acid control pathway (integrated stress response in humans) by regulation of translation of the transcription factor Gcn4 (human ATF4), which re-programs gene expression in the cell [31, 32]. Furthermore, in human breast epithelial cells, overexpression of tRNAiMet(CAU) causes increased cell proliferation and metabolic activity [33], and in mouse, elevated expression of tRNAiMet(CAU) stimulates cell migration and drives melanoma invasion [34].
Despite the importance of tRNAiMet(CAU) levels in S. cerevisiae and humans, it is not clear how tRNAiMet(CAU) levels are regulated in eukaryotes. Although there is compelling evidence in S. cerevisiae that the nuclear surveillance pathway targets pre-tRNAiMet(CAU) for decay in trm6/gcd10 or trm61/gcd14 mutants with reduced m1A58, available information in other eukaryotes suggests the possibility of alternative pathways. HeLa cells that are heat shocked at 43°C undergo decay of tRNAiMet(CAU) by Xrn1 and Rat1 over several hours, but it is not clear why decay occurred, as there was no obvious change in the modifications or physical stability of the remaining tRNAiMet(CAU) [35]. Moreover, tRNAiMet(CAU) levels in human cells were upregulated by knockdown of ALKBH1, which has an m1A-demethylase activity, and glucose starvation led to increased ALKBH1, linked to reduced tRNAiMet(CAU) and reduced translation [36]. Although the mechanisms by which tRNAiMet(CAU) levels are regulated are not known, it is clear that there is a link between m1A58 modification status and tRNAiMet(CAU) levels in eukaryotes. Thus, depletion of TRM6 or TRM61 in human cells results in reduced levels of tRNAiMet(CAU) and slow growth, which is partially rescued by overexpression of tRNAiMet(CAU) [37]. Similarly, Arabidopsis thaliana trm61 mutants have reduced tRNAiMet(CAU) levels, which is associated with shortened siliques [38].
To address the evolutionary role and mechanisms by which m1A58 influences tRNAiMet(CAU) levels and function, we have compared m1A58 biology in the fission yeast S. pombe with that in S. cerevisiae, which diverged ~600 million years ago (Mya) [39]. We show here that the RTD pathway has an unexpected major role in the biology of m1A58 and tRNAiMet(CAU) in both S. pombe and S. cerevisiae. We find that S. pombe trm6Δ mutants lack m1A58 and are temperature sensitive due to the decay of tRNAiMet(CAU) by the RTD pathway, as spontaneous suppressors that restored tRNAiMet(CAU) levels had mutations in the RAT1 ortholog dhp1 or the MET22 ortholog tol1, whereas mutation of the TRF4 ortholog cid14 did not suppress the growth defect. Moreover, we found a major role of the RTD pathway in S. cerevisiae TRM6 biology, as mutation of the RTD pathway components MET22, RAT1, or XRN1 each suppressed the temperature sensitivity of S. cerevisiae trm6-504 mutants and restored tRNAiMet(CAU) levels. Furthermore, we found that the lethality of S. cerevisiae trm6Δ mutants was suppressed by inhibition of both the RTD pathway and the nuclear surveillance pathway, but not by inhibition of either pathway alone.
Thus, our results show the importance of tRNAiMet(CAU) as a target for m1A58 modification by Trm6:Trm61 across evolutionarily distant fungal species. Our results also uncover an unexpected conserved evolutionary role of the RTD pathway in tRNAiMet(CAU) quality control in trm6 mutants of both fungal species, as previously found for all three other body modification mutants studied in S. cerevisiae [16, 20], and the only other body modification mutant studied in S. pombe [15]. These findings fuel speculation that the RTD pathway has a major role in quality control of other body modification mutants in these organisms, as well as in metazoans.
Results
S. pombe trm6Δ and trm61Δ mutants are temperature sensitive, and lack m1A58 in their tRNA
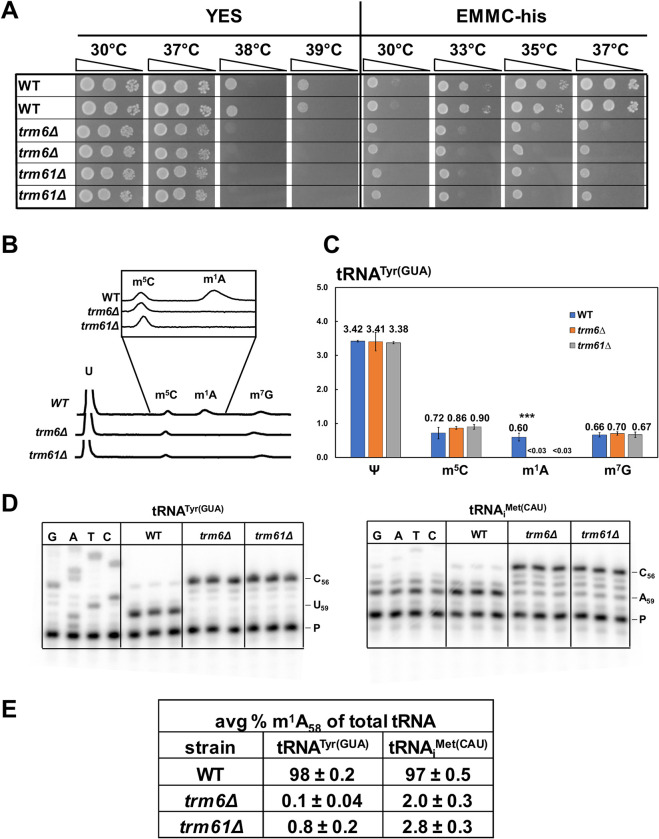
To begin analysis of the biology of S. pombe Trm6 and Trm61, we investigated the growth phenotype of trm6Δ and trm61Δ mutants which, unlike the corresponding S. cerevisiae mutants, are reported to be viable [40]. To guard against background mutations that might have accumulated in the deletion collection, we first re-made the trm6Δ and trm61Δ mutants in a wild type (WT) strain, using appropriate kanamycin resistance cassettes, and then compared the growth of two independent trm6Δ and trm61Δ transformants relative to the WT parent. Each tested S. pombe trm6Δ and trm61Δ mutant grew nearly as well as WT at lower temperatures, but was temperature sensitive on rich (YES) media at 38°C, and on minimal complete media lacking histidine (EMMC-His) at 33°C (Fig 1A). As expected if these trm6Δ and trm61Δ phenotypes were due to the corresponding deletions, the growth defects were fully complemented after introduction of an S. pombe [leu2+] plasmid expressing Ptrm6 trm6+ or Ptrm61 trm61+ respectively (S1A and S1B Fig).
Fig 1. S. pombe trm6Δ mutants and trm61Δ mutants are temperature sensitive and lack m1A58.
(A) S. pombe trm6Δ and trm61Δ mutants are temperature sensitive on YES and EMMC-his media. S. pombe trm6Δ mutants, trm61Δ mutants, and WT cells were grown overnight in YES media at 30°C, diluted to OD600 ~0.5, serially diluted 10-fold in YES media, and then 2 μL were spotted onto plates containing YES or EMMC-his media and incubated at the indicated temperatures for 3 days. (B) tRNATyr(GUA) from S. pombe trm6Δ and trm61Δ mutants has no detectable m1A, as measured by HPLC separation of nucleosides. S. pombe trm6Δ mutants, trm61Δ mutants, and WT cells were grown in biological triplicate in YES media at 30°C and tRNATyr(GUA) was purified, and digested to nucleosides, and then nucleosides were separated by HPLC as described in Materials and Methods. (C) Quantification of levels of modified nucleosides of purified tRNATyr(GUA) in S. pombe trm6Δ, trm61Δ, and WT strains. The chart shows average moles/mol of nucleosides with associated standard deviations; WT, blue; trm6Δ, orange; trm61Δ, gray. (D) tRNATyr(GUA) and tRNAiMet(CAU) from S. pombe trm6Δ and trm61Δ mutants have little or no detectable m1A58. Bulk RNA from the growth for Fig 1B was analyzed by poison primer extension assay, as described in Materials and Methods, with primer OMT 775 (complementary to tRNAiMet(CAU) nt 76–61) and primer OMT 477 (complementary to tRNATyr(GUA) 76–61) in the presence of ddGTP. The poison primer extension produces a stop at C56 for both tRNAiMet(CAU) and tRNATyr(GUA), and the presence of m1A58 results in a stop at N59. A sequencing ladder is shown at the left. (E) Quantification of poison primer extension of tRNATyr(GUA) and tRNAiMet(CAU). For each primer extension, the signals at N59 and C56 were first corrected by subtraction of the signals at A58 and N57 respectively.
Because the Trm6:Trm61 complex is essential in S. cerevisiae for m1A58 modification of substrate tRNAs [21, 41], we examined S. pombe trm6Δ and trm61Δ mutants for m1A58, to guard against the possibility that the mutants were alive because there is another protein that can catalyze some m1A58 modification. Examination by HPLC of the nucleoside composition of purified tRNATyr(GUA) from trm6Δ and trm61Δ mutants revealed that m1A levels were less than 0.03 moles/mole, compared to 0.60 moles/mole in WT cells, whereas levels of Ψ, m5C, and m7G were very similar in the tRNATyr(GUA) from both mutant and WT cells (Fig 1B and 1C). Poison primer extension of tRNATyr(GUA) from WT bulk RNA showed a complete block at U59 (98%) due to the presence of m1A58, which was virtually undetectable in trm6Δ and trm61Δ mutants (0.1% for trm6Δ and 0.8% in trm61Δ) (Fig 1D and 1E). Similarly, poison primer extension showed that tRNAiMet(CAU) was nearly completely modified with m1A58 in WT cells (97%), but not visibly modified in trm6Δ and trm61Δ mutants (although quantification with the high background gave 2.0% for trm6Δ and 2.8% in trm61Δ). Furthermore, analysis of bulk tRNA modifications revealed that m1A modification was less than 0.03% of the levels of cytidine in the trm6Δ mutant, compared to 2.5% for WT, whereas levels of Ψ were similar in both strains (16.8% vs 17.9%), as were levels of m5C, m2G, m7G, and inosine (I) (S2 Fig). These results show that S. pombe trm6+ and trm61+ are required for all detectable m1A58 modification of cytoplasmic tRNAs.
S. pombe trm6Δ mutants are temperature sensitive due to reduced levels of tRNAiMet(CAU)
Since the temperature sensitive growth defect of S. cerevisiae trm6-504 mutants is caused by decreased levels of tRNAiMet(CAU) [21], we analyzed levels of tRNAiMet(CAU) and other tRNAs in S. pombe trm6Δ mutants at low and high temperatures to determine if this property was conserved. We sampled cells grown in rich media in triplicate at 30°C and at 3-hour intervals after shift to 38.5°C, and analyzed tRNA levels by northern blot hybridization. We quantified tRNA levels by normalizing relative to tRNAGly(GCC) at the corresponding temperature and time point, and then relative to the normalized amount in WT cells at 30°C. Note that, as shown previously [15], levels of the usual standards 5S and 5.8S rRNA are significantly affected by temperature changes in S. pombe, and tRNAGly(GCC) levels were not affected in WT cells. Note also that in this and all other temperature shift experiments described in this report, cells were shown quantitatively to survive the temperature shift, based on spot tests of the cells on plates.
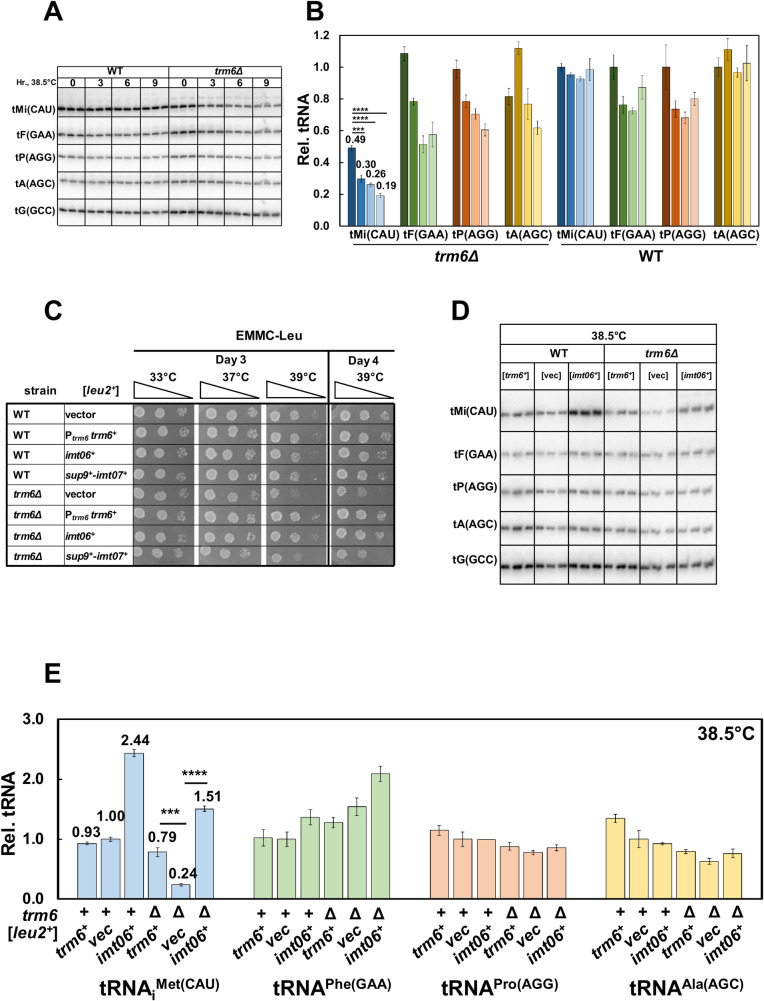
The northern analysis revealed that tRNAiMet(CAU) levels were substantially reduced in the S. pombe trm6Δ mutants, both at 30°C and 38.5°C. At 30°C, tRNAiMet(CAU) levels were 49% of those in WT cells, whereas each of the other eight tRNAs had levels between 82% and 121% of those in WT cells (Figs 2A, 2B and S3). At 38.5°C, tRNAiMet(CAU) levels decreased further in the trm6Δ mutants, to 30% of WT levels after 3 hours, whereas levels of each of the other 8 tRNAs ranged from 78% to 114% of WT levels. Furthermore, although tRNA levels generally decreased after longer times at 38.5°C, tRNAiMet(CAU) levels decreased the most, to 19% of those in WT after 9 hours, compared to 53% to 90% for the other eight tRNAs. These results indicate that S. pombe trm6Δ mutants are associated with reduced levels of tRNAiMet(CAU) at 30°C, and with further reduced tRNAiMet(CAU) levels at 38.5°C.
Fig 2. S. pombe trm6Δ temperature sensitivity is associated with reduced tRNAiMet(CAU) levels.
(A) Northern analysis of tRNAs in S. pombe trm6Δ and WT cells before and after shift from 30°C to 38.5°C. Strains were grown in YES media at 30°C, shifted to 38.5°C for 9 hours as described in Materials and Methods, and RNA was isolated at the indicated times, and analyzed by northern blotting, with probes as indicated. tMi(CAU), tRNAiMet(CAU); tF(GAA), tRNAPhe(GAA); tP(AGG), tRNAPro (AGG); tA(AGC), tRNAAla(AGC); tG(GCC), tRNAGly(GCC). (B) Quantification of tRNA levels in S. pombe trm6Δ and WT cells at 30°C and 38.5°C. The bar chart depicts relative levels of tRNA species at each temperature, relative to their levels in the WT strain at 30°C (each itself first normalized to levels of the control tG(GCC)). For each tRNA, the dark shade indicates 30°C, and progressively lighter shades indicate time points (3, 6, 9 hours) Standard deviations for each tRNA measurement are indicated. The statistical significance of tRNA levels was evaluated using a two-tailed Student’s t-test assuming equal variance. ns, not significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p<0.0001. tMi(CAU), blue; tF(GAA), green; tP(AGG), orange; tA(AGC), yellow. (C) Overproduction of tRNAiMet(CAU) suppresses the ts growth defects of S. pombe trm6Δ mutants. Strains with plasmids as indicated were grown overnight in EMMC-Leu media at 30°C and analyzed for growth as in Fig 1A on indicated plates and temperatures. (D) Overproduction of tRNAiMet(CAU) restores tRNAiMet(CAU) levels in S. pombe trm6Δ mutants. Strains containing plasmids as indicated were grown in EMMC-Leu at 30°C and shifted to 38.5°C for 8 hours as described in Materials and Methods, and RNA from cells grown at 38.5°C was isolated and analyzed by northern blotting as in Fig 2A. (E) Quantification of tRNA levels in S. pombe trm6Δ mutants and WT strains overproducing tRNAiMet(CAU). tRNA levels were quantified as in Fig 2B.
To test if the temperature sensitivity of S. pombe trm6Δ mutants is caused by decreased levels of tRNAiMet(CAU), we examined suppression of the trm6Δ growth defect upon tRNAiMet(CAU) overexpression. In S. pombe, only one of the four genes encoding tRNAiMet(CAU) is expressed in the usual manner, as a stand-alone tRNA gene. By contrast, the other three tRNAiMet(CAU) genes are expressed in tandem with a tRNASer species as a tRNASer-tRNAiMet dimeric transcript, which is then processed into single tRNAs [42], similar to the tRNAArg-tRNAAsp tandem genes in S. cerevisiae [43, 44]. To test for suppression of the trm6Δ growth defect, we overexpressed either the stand-alone SPBTRNAMET.06 (imt06+) gene, or the tandem tRNASer-tRNAiMet gene pair with sup9+ and SPCTRNAMET.07 (imt07+), each on a [leu2+] plasmid. We found that the temperature sensitive growth defect of S. pombe trm6Δ mutants on EMMC-leu media was completely suppressed by expression of the stand-alone imt06+ gene, growing identically to that of an S. pombe trm6Δ [Ptrm6 trm6+] strain at high temperature (Fig 2C), whereas expression of imt07+ from the tRNASer-tRNAiMet tandem gene only modestly suppressed the trm6Δ temperature sensitivity. Consistent with this result, northern analysis showed that levels of tRNAiMet(CAU) are much higher in strains overexpressing imt06+ than in strains overexpressing imt07+ (S4 Fig). Similarly, the temperature sensitivity of the S. pombe trm61Δ strain was completely suppressed by expression of the stand-alone imt06+ gene (S5 Fig). As expected, northern analysis showed that trm6Δ strains expressing [imt06+ leu2+] had substantially more tRNAiMet(CAU) than the trm6Δ [leu2+] vector control strains at 38.5°C (6.3 fold; relative levels of 1.51 vs 0.24) while the levels of other tested tRNAs remained unchanged (Fig 2D and 2E). These results suggest strongly that the temperature sensitivity of S. pombe trm6Δ mutants is caused by the loss of tRNAiMet(CAU) and that this tRNA is the major physiologically important tRNA substrate of Trm6:Trm61 methyltransferase.
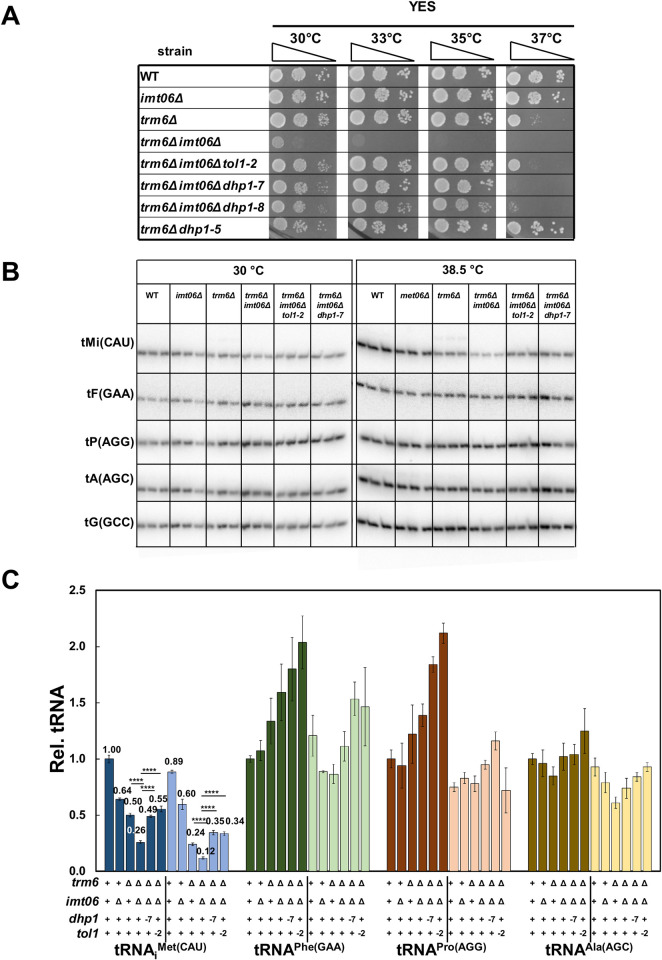
Mutations in the Rapid tRNA decay pathway restore growth and tRNAiMet(CAU) levels in S. pombe trm6Δ mutants
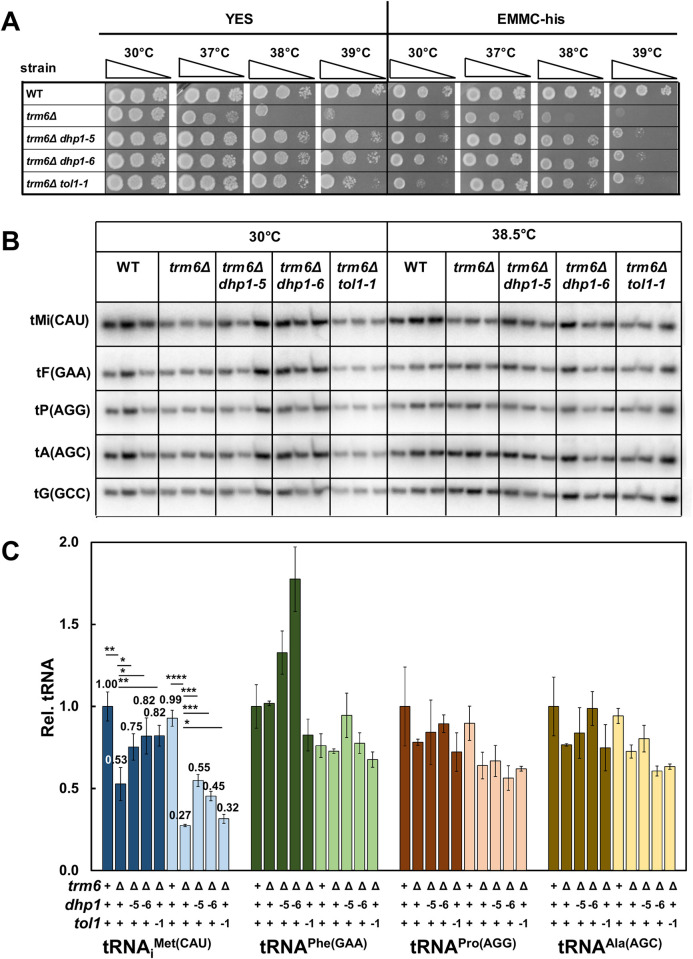
To identify potential mechanisms that contribute to the loss of tRNAiMet(CAU) in S. pombe trm6Δ mutants, we isolated and analyzed spontaneous suppressors of the temperature sensitivity. Among 25 temperature resistant suppressors from 14 cultures, we found three with increased levels of tRNAiMet(CAU) but not of a control tRNA, and whole genome sequencing revealed that two of these had mutations in dhp1+ (dhp1-5 and dhp1-6) and one had a mutation in tol1+ (tol1-1). dhp1+ is the ortholog of S. cerevisiae RAT1, which encodes one of the two 5’-3’ exonucleases involved in RTD, and tol1+ is the ortholog of S. cerevisiae MET22, deletion of which inhibits RTD by inhibiting 5’-3’ exonucleases [16–18].
Growth analysis on plates showed that the trm6Δ dhp1-5 and trm6Δ dhp1-6 mutants were nearly as healthy at high temperatures as the WT strain on both YES and EMMC-his media, whereas the trm6Δ tol1-1 mutant was slightly less healthy at higher temperatures (Fig 3A). Northern analysis of tRNA from strains grown at 30°C and after temperature shift to 38.5°C showed that the dhp1 and tol1 suppressors substantially restored tRNAiMet(CAU) levels at both high and low temperatures, without affecting any of a number of other tRNAs (Fig 3B and 3C). At 38.5°C, tRNAiMet(CAU) levels increased from 27% in trm6Δ strains (relative to WT at 30°C) to 55%, 45%, and 32% in the trm6Δ dhp1-5, trm6Δ dhp1-6, and trm6Δ tol1-1 mutants respectively, with no significant change in the levels of tRNAPhe(GAA), tRNAPro(AGG), and tRNAAla(AGC) (Fig 3B and 3C). This increase in tRNAiMet(CAU) levels at 38.5°C accounts for the temperature resistance of the strains, and reflects the weaker suppression in the trm6Δ tol1-1 strain. At 30°C, tRNAiMet(CAU) levels also increased, from 53% of WT in the trm6Δ strains to 75%, 82%, and 82% in the trm6Δ dhp1-5, trm6Δ dhp1-6, and trm6Δ tol1-1 mutants, again with little change in the levels of other tRNAs. Thus, it appears that the observed decay is occurring at both temperatures.
Fig 3. Spontaneous suppressors of S. pombe trm6Δ mutants with mutations in dhp1 and tol1 restore growth and increase tRNAiMet(CAU) levels of S. pombe trm6Δ mutants.
(A) Spontaneous suppressors of S. pombe trm6Δ mutants with mutations in dhp1 and tol1 restore growth at high temperatures. Strains as indicated were grown overnight in YES media at 30°C and analyzed for growth as in Fig 1A on indicated plates and temperatures. (B) Spontaneous suppressors of S. pombe trm6Δ mutants with mutations in dhp1 and tol1 restore tRNAiMet(CAU) levels after growth at 38.5°C. Strains were grown in YES media at 30°C and shifted to 38.5°C for 8 hours as described in Materials and Methods, and RNA was isolated and analyzed by northern blotting as in Fig 2A. (C) Quantification of tRNA levels of S. pombe trm6Δ dhp1 and tol1 mutants. tRNA levels were quantified as in Fig 2B, with dark and light shades as indicated for 30°C and 38.5°C.
Two lines of evidence suggest that the dhp1 mutations were responsible for the suppression in the trm6Δ dhp1 mutants. First, the isolation of two different dhp1 missense mutations in genetically independent suppressors argues strongly that the relevant suppressing mutation is that in dhp1. Whole genome sequencing typically results in only a few mutations, and finding two independent suppressors with different dhp1 mutations would be highly unlikely to occur by chance. Furthermore, like S. cerevisiae RAT1, dhp1+ is an essential gene in S. pombe, and thus there are limited mutations that would reduce function without killing the cell. Indeed, alignments show that neither of the dhp1 mutations is completely conserved, although each is likely to be important; the S737P (dhp1-5) mutation is predicted to disrupt the central portion of an α-helix, and the Y669C (dhp1-6) mutation is within a highly conserved block of amino acids (S6A and S6B Fig). Second, complementation experiments showed that introduction of an additional chromosomal copy of dhp1+ at ura4+ restored the temperature sensitivity of the trm6Δ dhp1-5 mutant, to a level similar to that of a trm6Δ control strain. The increased gene dosage of dhp1+ had no effect on growth of the WT strain, and a barely detectable inhibitory effect on growth of the trm6Δ strain (S6C Fig), which we attribute to the 2-fold overproduction of Dhp1 and the presumed sensitivity of the trm6Δ strain to any further reduction in tRNAiMet(CAU) levels. We therefore conclude that the isolated dhp1 mutations were responsible for the suppression of the temperature sensitive growth defect of S. pombe trm6Δ mutants.
Similarly, we infer that the tol1-1 mutation is responsible for suppression because expression of Ptol1 tol1+ on a [leu2+] plasmid restored temperature sensitivity to the trm6Δ tol1-1 strain, with no effect on growth of the trm6Δ or the WT strain (S7 Fig). The tol1-A151D (tol1-1) point mutation is located in a highly conserved region of the essential tol1+ gene [45], presumably resulting in a partial loss of function variant (S8A and S8B Fig).
The discovery of dhp1 and tol1 mutations as suppressors of the S. pombe trm6Δ temperature sensitivity demonstrates the involvement of the RTD pathway in decay of tRNAiMet(CAU) lacking m1A58 in S. pombe. However, this result was unexpected, because it is well established in S. cerevisiae that trm6 mutants trigger decay of pre-tRNAiMet(CAU) by the nuclear surveillance pathway in vivo [13, 22, 24, 46] and in vitro [47].
The exacerbated growth defect of an S. pombe trm6Δ imt06Δ mutant is due to further reduction in tRNAiMet(CAU) levels, and suppressors of the growth defect are in the RTD pathway
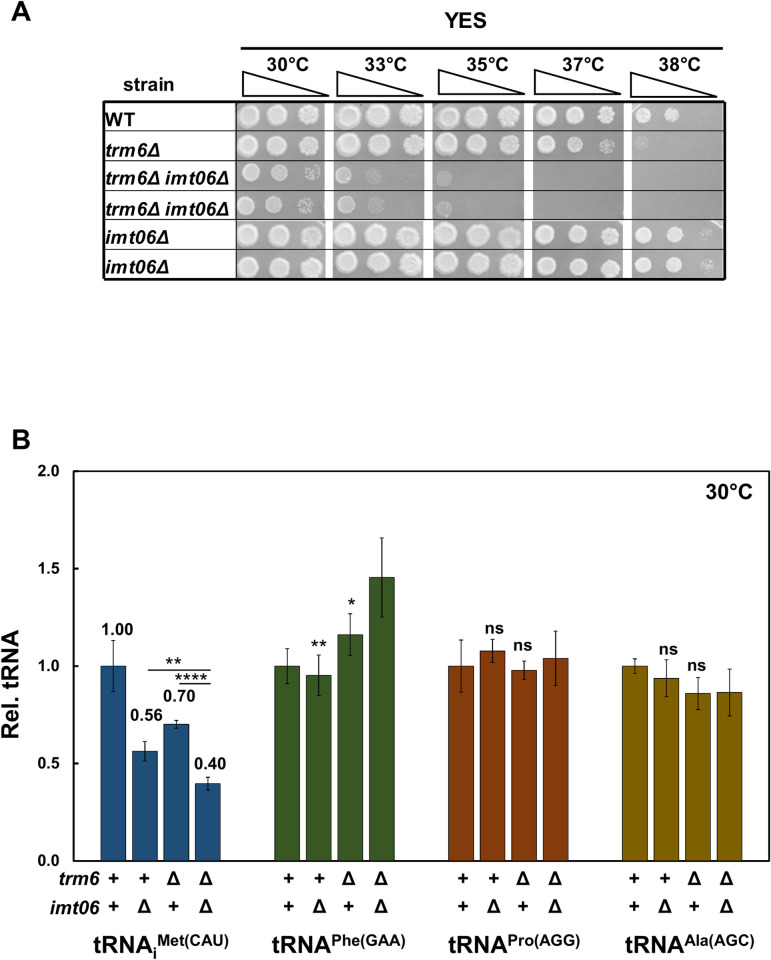
To obtain a more robust set of suppressors of the trm6Δ temperature sensitivity, we further reduced tRNAiMet(CAU) levels in the trm6Δ mutants by introduction of an imt06Δ mutation, decreasing the number of tRNAiMet(CAU) genes from four to three. As anticipated, the resulting trm6Δ imt06Δ strain grew very poorly at 30°C, and was temperature sensitive at higher temperatures, not growing at all at 37°C, whereas the imt06Δ mutant had no growth defect at any tested temperature (Figs 4A and S9A). Moreover, the trm6Δ imt06Δ growth defect was strictly due to the loss of tRNAiMet(CAU) because the trm6Δ imt06Δ strains expressing both an integrated copy of imt06+ and a [leu2+ imt06+] plasmid grew as well as WT on YES media at all temperatures up to 39°C (S9B Fig). As anticipated, tRNAiMet(CAU) levels in the trm6Δ imt06Δ strain at 30°C were substantially reduced from those in the trm6Δ mutants (40% vs 70% of WT), consistent with the 44% reduction in tRNAiMet(CAU) levels in the imt06Δ strains (Figs 4B and S9C). As also expected, the levels of other tested tRNAs in these strains were virtually unaffected. These results show a prominent synthetic growth defect in the S. pombe trm6Δ imt06Δ strain, due only to reduced levels of tRNAiMet(CAU).
Fig 4. Deletion of one of the four S. pombe genes encoding tRNAiMet(CAU) in an S. pombe trm6Δ mutant exacerbates its growth defect and further reduces tRNAiMet(CAU) levels.
(A) Deletion of the imt06+ gene encoding tRNAiMet(CAU) in an S. pombe trm6Δ mutant severely exacerbates its growth. Strains were grown overnight in YES media at 30°C and analyzed for growth as in Fig 1A on indicated plates and temperatures for 4 days. (B) Quantification of tRNAiMet(CAU) levels in S. pombe trm6Δ imt06Δ mutants at 30°C. tRNA levels were quantified as in Fig 2B.
Analysis of spontaneous suppressors of the severe growth defect of trm6Δ imt06Δ mutants revealed additional mutations in the RTD pathway. Of fifteen suppressors isolated from six independent cultures of trm6Δ imt06Δ strains after plating on YES media at 35°C, eleven had increased tRNAiMet(CAU) levels at both 30°C and 38.5°C, relative to a control tRNA, and whole genome sequencing of nine of these eleven suppressors revealed eight with dhp1 mutations (six alleles; dhp1-7 to dhp1-12), and one with a tol1 mutation (tol1-2). All six new dhp1 mutations and the tol1-2 mutation were in conserved regions of the respective proteins (S10 Fig). Growth comparisons showed that the dhp1-7, dhp1-8, and tol1-2 mutations each efficiently rescued the trm6Δ imt06Δ growth defect at 35°C, with the trm6Δ imt06Δ tol1-2 strain growing almost as well as the original trm6Δ strain at 37°C (Fig 5A). Moreover, the growth phenotype of the trm6Δ imt06Δ tol1-2 mutant was fully complemented upon introduction of a [leu2+ Ptol1 tol1+] plasmid (S11 Fig). Consistent with the growth suppression, tRNAiMet(CAU) levels were increased from 26% of WT in the trm6Δ imt06Δ mutant at 30°C to 49% and 55% in the corresponding dhp1-7 and the tol1-2 suppressors, and from 12% at 38.5°C to 35% and 34% in the suppressors, whereas control tRNA levels were largely unchanged at each temperature (Fig 5B and 5C).
Fig 5. Spontaneous suppressors of S. pombe trm6Δ imt06Δ mutants with mutations in dhp1 and tol1 restore growth and increase tRNAiMet(CAU) levels.
(A) Spontaneous suppressors of S. pombe trm6Δ imt06Δ mutants with mutations in dhp1 and tol1 suppress the growth defect. Strains were grown overnight in YES media at 30°C and analyzed for growth as in Fig 1A on indicated plates and temperatures. (B) Spontaneous suppressors of S. pombe trm6Δ imt06Δ mutants with mutations in dhp1 and tol1 restore tRNAiMet(CAU) levels at low and high temperatures. Strains were grown in YES media at 30°C and shifted to 38.5°C for 6 hours as described in Materials and Methods, and RNA was isolated and analyzed by northern blotting as in Fig 2A. (C) Quantification of tRNAiMet(CAU) levels in S. pombe trm6Δ imt06Δ dhp1-7 and trm6Δ imt06Δ tol1-2 mutants. tRNA levels were quantified as in Fig 2B.
These findings underscore the crucial role of Dhp1 and Tol1, and thus of the RTD pathway, in quality control of tRNAiMet(CAU) in S. pombe trm6Δ mutants. Indeed, the isolation of eight genetically distinct dhp1/rat1 alleles (two in trm6Δ mutants and six in trm6Δ imt06Δ mutants) and two distinct tol1/met22 alleles (one each in the trm6Δ mutant and the trm6Δ imt06Δ mutant) argues that the genetic landscape of trm6Δ suppressors has been nearly saturated, particularly considering that both dhp1 and tol1 are essential in S. pombe.
Further examination suggests the lack of participation of the Trf4 ortholog Cid14 of the nuclear surveillance pathway [48–50] in quality control of tRNAiMet(CAU) in S. pombe trm6Δ mutants. A cid14Δ mutation was introduced into WT and trm6Δ strains and independent isolates were confirmed by PCR (Materials and Methods), after which the resulting strains were shown to be sensitive to 5-fluorouracil (5-FU) (S12A and S12B Fig), as previously reported [48, 51]. We observed little, if any, suppression of the trm6Δ growth defect in the trm6Δ cid14Δ strains (S13A Fig), and only very minor restoration of tRNAiMet(CAU) levels at high temperature, relative to levels in trm6Δ mutants (21% vs 18%, compared to 39% in the trm6Δ dhp1-5 strain) (S13B and S13C Fig). Thus, we infer that tRNAiMet(CAU) is degraded in S. pombe trm6Δ and trm6Δ imt06Δ mutants primarily by the RTD pathway, and not appreciably by the TRAMP complex of the nuclear surveillance pathway.
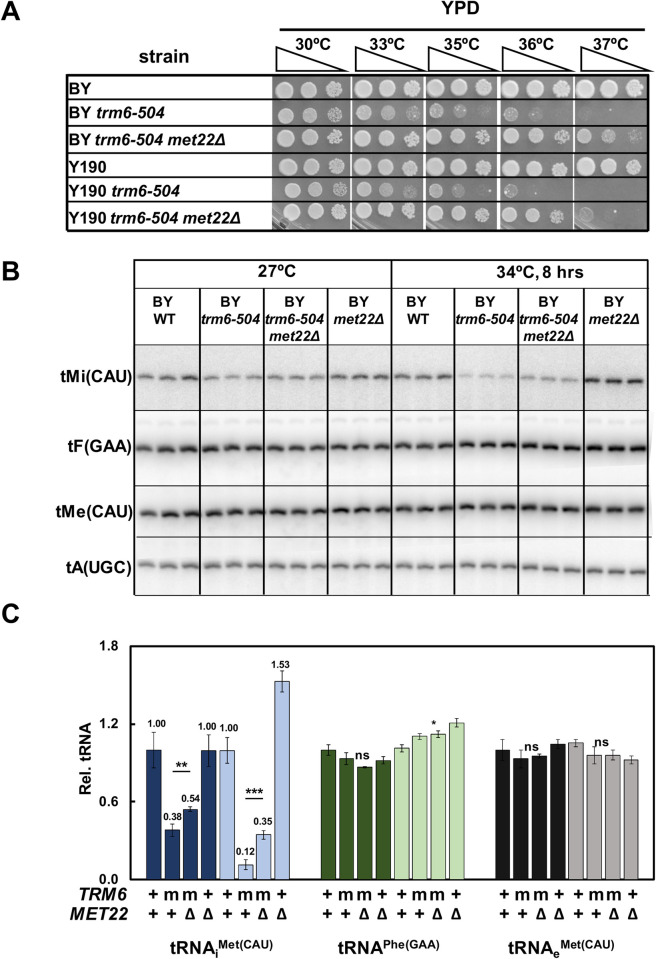
A met22Δ mutation substantially suppresses the S. cerevisiae trm6-504 temperature sensitivity and partially restores tRNAiMet(CAU) levels at low and high temperatures
Because of our discovery of the predominant role of the RTD pathway in tRNAiMet(CAU) quality control in S. pombe trm6Δ mutants, we examined the participation of the RTD pathway in tRNAiMet(CAU) quality control in the well-studied S. cerevisiae trm6-504ts mutant, which had previously been shown to trigger tRNAiMet(CAU) decay by the nuclear surveillance pathway [13, 22, 23]. We found that deletion of MET22 substantially suppressed the temperature sensitive growth defect of an S. cerevisiae trm6-504ts mutant, both in its original background (Y190) and in the BY4741 (BY) background, with obvious suppression at 36°C in both backgrounds and at 37°C in the BY background (Fig 6A). Consistent with the growth suppression, tRNAiMet(CAU) levels were increased from 12% of WT in the BY trm6-504 strain to 35% in the met22Δ derivative at 34°C, and from 38% to 54% at 27°C, with little effect on other tested tRNAs (Fig 6B and 6C). Similar restoration of tRNAiMet(CAU) levels was observed in the met22Δ derivative of the original Y190 trm6-504 strain, with no effect on other tested tRNAs (S14 Fig). These results show that MET22 regulates tRNAiMet(CAU) levels in trm6-504 strains regardless of their genetic background and suggest the involvement of the RTD pathway in tRNAiMet(CAU) quality control in S. cerevisiae trm6-504 mutants.
Fig 6. The temperature sensitivity and reduced tRNAiMet(CAU) levels in S. cerevisiae trm6-504 mutants are suppressed by a met22Δ mutation.
(A) A met22Δ mutation substantially suppresses the S. cerevisiae trm6-504 temperature sensitivity. Strains were grown overnight in YPD media at 30°C and analyzed for growth on indicated plates and temperatures. BY; standard BY4741 WT strain background; Y190, background of original trm6-504 mutant (B) A met22Δ mutation substantially restores tRNAiMet(CAU) levels in S. cerevisiae trm6-504 mutants. Strains were grown in YPD media at 27°C and shifted to 34°C for 8 hours as described in Materials and Methods, and RNA was isolated and analyzed by northern blotting. (C) Quantification of northern analysis of tRNAiMet(CAU) levels in S. cerevisiae BY trm6-504 mutants. tRNA levels were quantified as in Fig 2B. m, trm6-504 mutant.
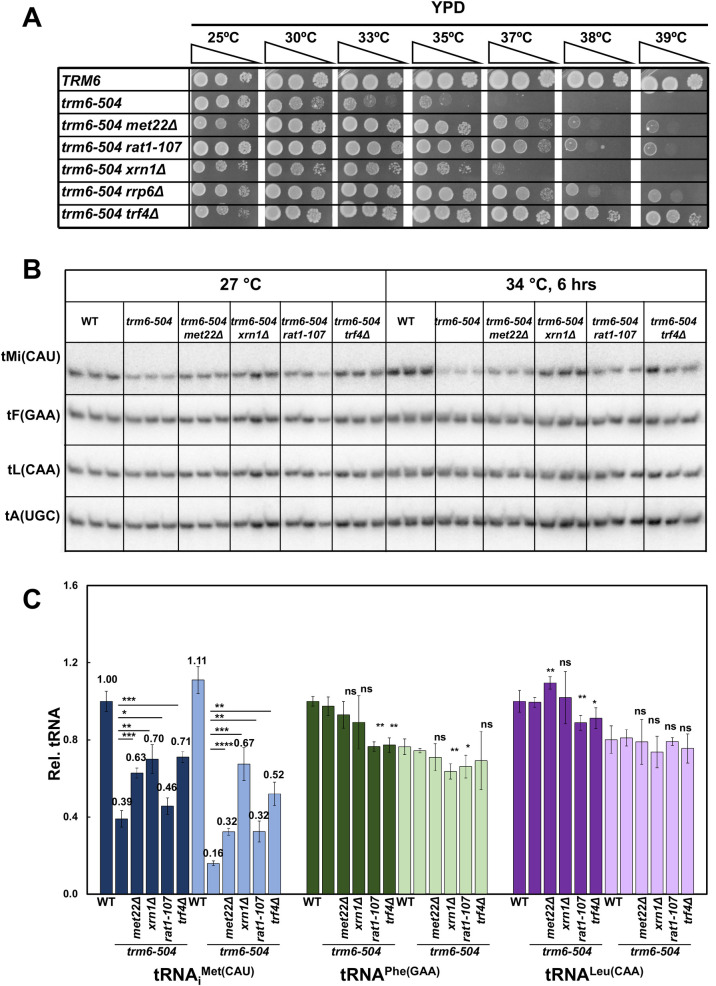
Each of the RTD pathway exonucleases has a significant role in tRNAiMet(CAU) quality control in S. cerevisiae BY trm6-504 mutants
Although the restoration of growth and tRNAiMet(CAU) levels in a BY trm6-504 met22Δ mutant suggested the involvement of the RTD pathway, we sought to provide additional evidence by directly testing the roles of the RTD pathway exonucleases Rat1 and Xrn1 in tRNAiMet(CAU) quality control in the trm6-504 mutant. As Rat1 is essential [52], we tested the role of Rat1 using the rat1-107 mutation, which we had previously isolated as a suppressor of RTD in trm8Δ trm4Δ mutants [16].
We found that mutation of each of the RTD exonucleases efficiently suppressed both the temperature sensitivity and the reduced tRNAiMet(CAU) levels of the BY trm6-504 mutant. Whereas the trm6-504 mutant was impaired for growth at 33°C and above, the trm6-504 rat1-107 strain had healthy growth at 37°C and visible growth at 39°C, which was similar to that of the trm6-504 met22Δ strain, and the trm6-504 xrn1Δ strain grew up to 37°C (Fig 7A), despite the known growth defect of xrn1Δ mutants [16]. This growth suppression by mutation of each RTD component was nearly as efficient as that due to mutation of the nuclear surveillance components RRP6 or TRF4 (Fig 7A) [13]. Moreover, consistent with the suppression results, the temperature dependent decay of tRNAiMet(CAU) in trm6-504 mutants was efficiently suppressed by mutation of RTD components. Thus, after 6-hour temperature shift to 34°C, relative tRNAiMet(CAU) levels in trm6-504 mutants were restored from 16% to 32%, 67%, and 32% by met22Δ, xrn1Δ, and rat1-107 mutations respectively, comparable to the 52% observed in a trm6-504 trf4Δ mutant (Fig 7B and 7C). Significant suppression of tRNAiMet(CAU) levels was also found at 27°C. A parsimonious interpretation of these results is that all components of the RTD pathway are involved in tRNAiMet(CAU) quality control in trm6-504 mutants, and that the nuclear surveillance pathway and RTD pathway each contribute substantially to this tRNAiMet(CAU) quality control.
Fig 7. The RTD and nuclear surveillance pathways are each involved in tRNAiMet(CAU) quality control in S. cerevisiae trm6-504 mutants.
(A) The S. cerevisiae trm6-504 mutant growth defect is substantially suppressed by mutations in individual components of the RTD and nuclear surveillance pathways. Strains were grown overnight in YPD media at 30°C and analyzed for growth on indicated plates and temperatures. (B) tRNAiMet(CAU) levels are substantially restored in S. cerevisiae trm6-504 strains with mutations in individual components of the RTD and nuclear surveillance pathways. Strains were grown in YPD at 27°C and shifted to 34°C for 6 hours as described in Materials and Methods, and RNA was isolated and analyzed by northern blotting. (C) Quantification of tRNAiMet(CAU) levels in S. cerevisiae trm6-504 strains with mutations in the RTD and nuclear surveillance pathways. Dark and light colors indicate growth at 27°C and 34°C.
tRNAiMet(CAU) in S. cerevisiae trm6-504 mutants and suppressors is fully modified to m1A58 at both low and high temperatures
As trm6-504 mutants are known to have reduced, but not absent, m1A modification levels [13], we wanted to determine if m1A levels were altered in tRNAiMet(CAU) as a result of the temperature shift in trm6-504 mutants. By using poison primer extension to measure m1A58 modification, we found that A58 of tRNAiMet(CAU) was nearly fully modified at both 27°C and 34°C in both trm6-504 mutants (96% and 94%) and WT strains (98% and 97%) (S15 Fig), although tRNAiMet(CAU) levels are reduced in trm6-504 mutants. By contrast, A58 of tRNAPhe(GAA) was substantially hypomodified at low temperature in trm6-504 mutants compared to WT strains (25% vs 83%), and also at high temperature (27% vs 68%) (S15 Fig). As tRNAiMet(CAU) is 96% modified at low temperature and present at 39% of WT levels, whereas tRNAPhe(GAA) is 25% modified and present at 97% of WT levels, these findings suggest that tRNAiMet(CAU) is the preferred substrate of Trm6:Trm61. In addition, comparison of the tRNAPhe(GAA) modification levels in trm6-504 mutants at low and high temperature suggests that there is little or no temperature-dependent reduction in the Trm6:Trm61 methyltransferase activity.
To further assess the connection between m1A58 modification and tRNA decay, we measured m1A levels in tRNAiMet(CAU) in trm6-504 strains with mutations in the nuclear surveillance or the RTD pathway. We found that tRNAiMet(CAU) was still nearly fully modified to m1A58 at 34°C in trm6-504 mutants with suppressing mutations in any of the components of the RTD pathway (met22Δ, rat1-107 or xrn1Δ) or the nuclear surveillance pathway (trf4Δ or rrp6Δ), with modification levels ranging from 92.6% to 95.2%, compared to 97.6% in WT cells (S16A and S16C Fig). As anticipated, A58 modification of tRNAPhe(GAA) was similarly reduced in trm6-504 mutants and in derivatives with suppressing mutations in the RTD or nuclear surveillance pathway, compared to WT cells (S16B and S16C Fig). Thus, although tRNAiMet(CAU) decay at 34°C is inhibited in trm6-504 strains with mutations in the nuclear surveillance or the RTD pathway, the nearly complete modification of the remaining, undegraded tRNAiMet(CAU) in all of these trm6-504 derivative strains argues for competition between the Trm6:Trm61 enzyme and the decay pathways.
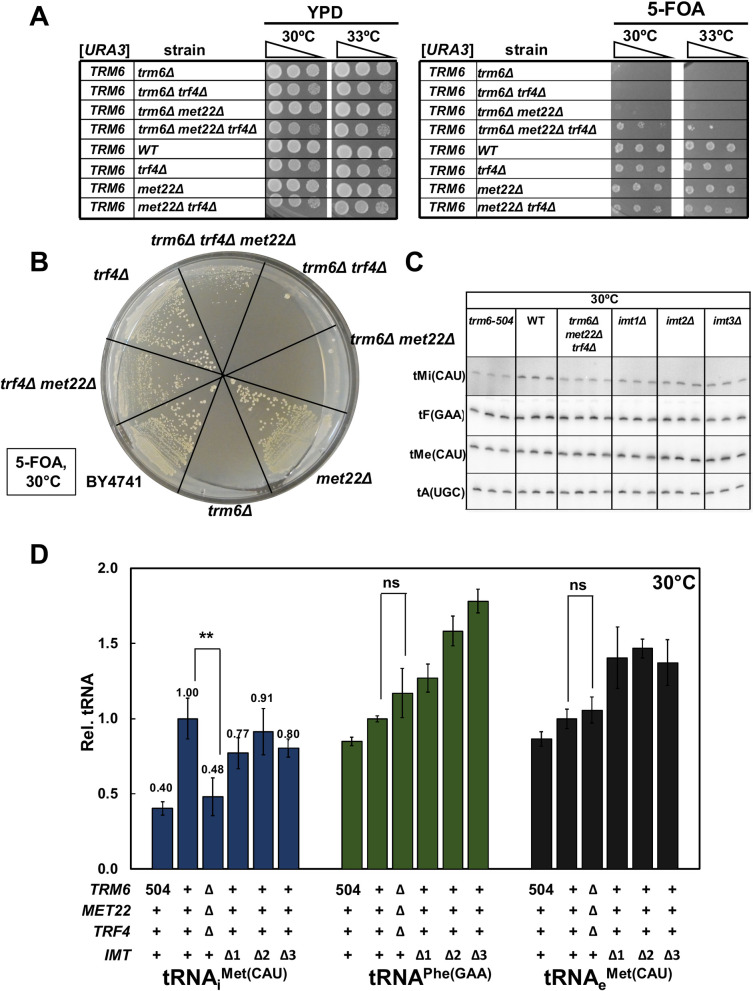
The lethality of S. cerevisiae trm6Δ mutants is suppressed by mutation of both the RTD and the nuclear surveillance pathways, but not either one alone
To separate the effects of the decay pathways from the presumed competition with Trm6:Trm61, we determined if the lethality of S. cerevisiae trm6Δ mutants could be rescued by inhibition of either or both of the RTD and nuclear surveillance pathways. As previously shown, the S. cerevisiae trm6Δ lethality is due to lack of tRNAiMet(CAU) [21], as a trm6Δ [TRM6 URA3] [2μ IMT1 LEU2] strain was healthy when the URA3 plasmid was selected against on media containing 5-FOA, but the corresponding strain with a [2μ LEU2] vector died (S17 Fig). We found that deletion of both MET22 and TRF4 suppressed the lethality of the S. cerevisiae trm6Δ mutant on 5-FOA-containing media at 30°C and 33°C, but neither single deletion could rescue the lethality of the trm6Δ mutant alone (Fig 8A and 8B). Thus, we conclude that both the RTD pathway and the nuclear surveillance pathway significantly contribute to tRNAiMet(CAU) quality control in mutants lacking m1A58 modification. As tRNAiMet(CAU) levels in trm6Δ met22Δ trf4Δ strains were only 48% of WT levels, comparable to the tRNAiMet(CAU) levels in trm6-504 mutants and somewhat less than tRNAiMet(CAU) levels in mutants lacking one of the four IMT genes (Fig 8C and 8D), we infer that tRNAiMet(CAU) lacking m1A58 modification is still being degraded in this strain.
Fig 8. The lethality of an S. cerevisiae trm6Δ mutant is suppressed by deletion of both MET22 and TRF4, but not by either deletion alone, and results in modest tRNAiMet(CAU) levels.
(A) Growth test on plates of an S. cerevisiae trm6Δ [URA3 TRM6] strain with a met22Δ and/or trf4Δ mutation. Strains were grown overnight in YPD media at 30°C, diluted to OD600~3, serially diluted 10-fold in water, and 2 μL were spotted onto YPD or 5-FOA plates as indicated, and grown for 4 days. (B) Pie streak growth test of an S. cerevisiae trm6Δ [URA3 TRM6] strain with a met22Δ and/or trf4Δ mutation. Strains were grown overnight in YPD media at 30°C, and then 2 μl of each of the cultures was streaked on 5-FOA plates for single colonies, and then incubated at 30°C for 7 days. (C) Northern analysis of tRNAiMet(CAU) in S. cerevisiae trm6Δ met22Δ trf4Δ mutants. Strains were grown in YPD at 30°C for 6 hours as described in Materials and Methods, and RNA was isolated and analyzed by northern blotting. (D) Quantification of tRNAiMet(CAU) levels in S. cerevisiae trm6Δ met22Δ trf4Δ mutants. tRNA levels were quantified as in Fig 2B.
Discussion
We have provided strong evidence that the rapid tRNA decay pathway has a major role in decay of tRNAiMet(CAU) lacking m1A58 in both S. pombe and S. cerevisiae. In S. pombe, dhp1 and tol1 mutations in the RTD pathway suppress the temperature sensitivity and decay of tRNAiMet(CAU) in trm6Δ and trm6Δ imt06Δ mutants. In S. cerevisiae, met22, rat1, and xrn1 mutations in the RTD pathway suppress the temperature sensitivity and tRNAiMet(CAU) decay in trm6-504 mutants, and a met22Δ mutation is required, together with a trf4Δ mutation in the nuclear surveillance pathway, to restore viability of S. cerevisiae trm6Δ mutants.
The finding that reduced tRNAiMet(CAU) levels are the cause of the defect in both S. pombe and S. cerevisiae trm6 mutants lacking m1A58 is consistent with the unique structural properties of eukaryotic tRNAiMet(CAU) in the D-loop and the T-loop. In this region, eukaryotic initiator tRNA differs from that of canonical elongator tRNAs in the absence of N17, the replacement of canonical residues with A20, A54, and A60, and a unique substructure involving these residues and m1A58, as well as G57 and A59 [26, 53]. As Trm6:Trm61 are conserved in eukaryotes [38, 54], we infer that tRNAiMet(CAU) levels will be similarly subject to decay in other eukaryotes lacking m1A58.
These findings establish that the RTD pathway acts on all body modification mutants that have been shown to result in decay of tRNAs in fungi, including S. cerevisiae mutants lacking m7G46, ac4C12, or m2,2G26, particularly in combination with other body modification mutants [16, 20], S. pombe mutants lacking m7G46 [15], and now mutants lacking m1A58 in both organisms. This set of results suggests that the RTD pathway will mediate decay of other body modification mutants in S. pombe and S. cerevisiae. Furthermore, as S. cerevisiae and S. pombe diverged about 600 Mya [39], these findings suggest that body modification mutants in other eukaryotes will also undergo decay by the RTD pathway. In support of this suggestion, we note that Rat1/Dhp1, Met22/Tol1, and Xrn1 of the RTD pathway are all conserved in eukaryotes [55, 56], and that WT HeLa cells (without a modification defect) that are incubated at 43°C undergo decay of tRNAiMet(CAU) by Rat1 and Xrn1 [35]. As a subset of hypomodified tRNAs are known to be reduced in mammalian cells lacking m7G46 [57, 58] or m5C [59], it is likely that tRNA decay is occurring in these cells, and based on our results, we speculate that this decay is due to the RTD pathway.
It was surprising to find that the decay of tRNAiMet(CAU) in S. pombe trm6Δ mutants was not due to the nuclear surveillance pathway, because of its well established role in decay of tRNAiMet(CAU) in S. cerevisiae trm6-504 mutants [13, 22, 23]. We argued above that in S. pombe, tRNAiMet(CAU) lacking m1A58 is primarily degraded by the RTD pathway, because we had essentially saturated the genetic landscape of suppressors of S. pombe trm6Δ or trm6Δ imt06Δ strains with mutations in dhp1 or tol1 of the RTD pathway, and because a cid14Δ mutation in the nuclear surveillance pathway did not restore growth or tRNAiMet(CAU) levels to an S. pombe trm6Δ mutant. It is known that the other components of the nuclear surveillance pathway are present and functional in S. pombe [48, 49, 60, 61]. It is possible that the lack of participation of the nuclear surveillance pathway in decay of tRNAiMet(CAU) in S. pombe trm6Δ mutants is due in some way to the structure of three of the four tRNAiMet(CAU) genes, each of which is present as a dimeric tRNA gene, and expressed as a tandem tRNASer- tRNAiMet transcript that is then processed into individual tRNAs [42]. Alternatively, it is possible that the lack of participation of the nuclear surveillance pathway in S. pombe trm6Δ mutants is due to some, as yet unappreciated, difference in the structure or folding between S. pombe and S. cerevisiae tRNAiMet(CAU), or to differences in the activity of the nuclear surveillance pathway.
Our finding that dhp1 and tol1 mutations significantly restore tRNAiMet(CAU) levels at 30°C and 38.5°C in S. pombe trm6Δ and trm6Δ imt06Δ mutants underscores that the RTD pathway is active at all temperatures in S. pombe, as we also found in S. cerevisiae trm6-504 mutants, and previously found in S. cerevisiae mutants lacking m7G46 and m5C [16] and in fully modified variants of tRNATyr [62]. The relatively healthy growth of S. pombe trm6Δ mutants and the poor growth of trm6Δ imt06Δ mutants at 30°C, with tRNAiMet(CAU) levels at ~50% and 26% of WT respectively, is consistent with prior results that S. cerevisiae strains are healthy with three of four tRNAiMet(CAU) genes, generally grow slowly with two of four genes, and can survive with as little as 40% of WT fully modified tRNAiMet(CAU) [63, 64].
Our results provide evidence that the nuclear surveillance and the RTD pathways in S. cerevisiae are in competition with the Trm6:Trm61 m1A methyltransferase, as tRNAiMet(CAU) was fully modified in S. cerevisiae trm6-504 mutants at both 27°C and 34°C, and inhibition of either decay pathway resulted in more tRNAiMet(CAU) that was fully modified. Moreover, as Trm6:Trm61 is a nuclear enzyme [21], and Xrn1 is cytoplasmic [65], the increased levels of fully modified tRNAiMet(CAU) in trm6-504 xrn1Δ mutants argues that unmodified tRNAiMet(CAU) is not immediately degraded, but rather that tRNAiMet(CAU) goes to the cytoplasm without m1A and returns to the nucleus for another chance at m1A modification by Trm6:Trm61. This second chance for m1A modification is analogous to the second chance pathway suggested earlier for tRNAs lacking m2,2G26 [66].
Our finding that tRNAPhe(GAA) in S. cerevisiae trm6-504 mutants had very similar m1A58 modification at both 27°C and 34°C suggests that Trm6:Trm61 is not temperature sensitive in this strain. If so, the reduced tRNAiMet(CAU) levels at high temperature in trm6-504 mutants would imply that tRNAiMet(CAU) lacking m1A58 is itself temperature sensitive, perhaps becoming partially unfolded at high temperature. One could envision a model in which tRNAiMet(CAU) lacking m1A58 is functioning in the cell cytoplasm (consistent with the viability of S. pombe trm6Δ mutants and of S. cerevisiae trm6Δ trf4Δ met22Δ mutants), but is in equilibrium with a state in which the tertiary structure is partially or completely unfolded due to lack of the modification [21, 26]. As tertiary structure unfolding precedes unfolding of the individual helices of tRNA [67], the disrupted tertiary structure due to lack of m1A58 could lead to unfolding of the acceptor stem and increased availability of the 5’ end to the RTD pathway. This model is very similar to that we proposed previously to explain the increased Xrn1 susceptibility of S. cerevisiae tRNASer(CGA) lacking ac4C12 and Um44 and for tRNAVal(AAC) lacking m7G46 and m5C49 [68], and could be tested in subsequent experiments.
Materials and methods
Yeast strains
All S. pombe and S. cerevisiae strains with integrated markers that are described in this work were made in biological triplicate. S. pombe strains are shown in S1 Table. S. pombe trm6Δ::KanMX strains were constructed in the S. pombe WT strain derived from SP286 (ade6-M210/ade6-M216, leu1-32/leu1-32, ura4-D18/ura4-D18 h+/h+) by PCR amplification of the trm6Δ::kanMX cassette from the S. pombe trm6Δ::kanMX strain of the genomic knockout collection [40], followed by linear transformation using lithium acetate [69], and PCR screening of transformants for the presence of the deletion. Other S. pombe deletion strains were made similarly, but the DNA containing the drug marker (KanMX or HygR) was obtained by Gibson assembly of ~ 500 nt 5’ of the target site, the drug marker, and ~ 500 nt 3’ of the target site [70]. The dhp1+ and imt06+ genes were integrated at the chromosomal ura4-D18 locus using a single ura4+integrating vector containing the corresponding gene under its promoter [71]. S. cerevisiae deletion strains are shown in S2 Table, and were constructed by linear transformation with PCR amplified DNA from the appropriate knockout strain, followed by PCR amplification to confirm the knockout.
The S. cerevisiae trm6-504 mutant strain was obtained in two ways. We obtained the original trm6-504 (Y200) and its WT parent (Y190) from Dr. James Anderson. The BY trm6-504 strain was reconstructed essentially as previously described [72], with three DNA components constructed in a plasmid vector: first, nt 893–1434 of the TRM6 coding sequence (containing the C1292G mutation of the trm6-504 mutant) and 204 nt of the 3’ UTR; second, K. lactis URA5; third, nt 1384–1434 of the TRM6 coding region. The DNA construct was removed from the vector, transformed into S. cerevisiae WT cells by linear transformation, confirmed by PCR, and then strains were plated onto media containing 5-FOA to select for Ura- cells obtained by homologous recombination, which were sequence verified.
Plasmids
Plasmids used in this study are listed in S3 Table. The S. pombe plasmid expressing S. pombe Ptrm6 trm6+, S. pombe Ptrm61 trm61+, and Ptol1+ tol1+ contained ~ 1000 bp and 500 bp of flanking 5’ and 3’ DNA and were cloned into a pREP3X-derived plasmid, removing the Pnmt1 promoter, as described [15]. Plasmids expressing S. pombe tRNA genes contained ~ 300 bp of flanking 5’ and 3’ DNA. The S. pombe ura4+ single integrating vectors [71] expressing imt06+ or Pdhp1+ dhp1+ were constructed similarly.
Yeast media and growth conditions
S. pombe strains were grown in rich (YES) media or Edinburgh minimal complete (EMMC) media, or corresponding dropout media, as described [15]. For temperature shift experiments, cells were grown in YES or EMMC-leu media at 30°C to OD600 ~ 0.5, and then diluted to OD600 0.1 in pre-warmed media at the desired temperature, and grown to OD600 ~ 0.5, and then aliquots were chilled, harvested at 4°C, washed with ice cold water, frozen on dry ice, and stored at -80°C. S. cerevisiae strains were grown in rich (YPD) media or minimal complete (SDC) media as described [15], and temperature shift experiments were performed as described for S. pombe. All experiments with measurements were performed in biological triplicate.
Spontaneous suppressor isolation
Spontaneous suppressors of S. pombe trm6Δ and trm6Δ imt06Δ mutants were isolated by growing cultures of individual colonies in YES media at 30°C, followed by plating 107 cells on YES and EMMC plates at 39°C (for trm6Δ mutants) and on YES plates at 35°C (for trm6Δ imt06Δ mutants).
Bulk RNA preparation and northern blot analysis
For northern analysis, biological triplicates were grown in parallel, aliquots of 3–5 OD were harvested, and then bulk RNA was prepared with glass beads and phenol as described [73], resolved on a 10% polyacrylamide (19:1), 7M urea, 1X TBE gel, transferred to Amersham Hybond-N+ membrane (Cytiva, Marlborough, MA cat# RPN303B), and hybridized with 5’ 32P-labeled DNA probes (S4 Table) as described [14], followed by exposure and imaging on an Amersham Typhoon phosphorimager (Cytiva, Marlborough, MA), and quantification using Image Quant v5.2
Isolation and purification of bulk tRNA
S. pombe WT and trm6Δ mutant strains were grown to ~ 0.5 OD in YES media at 30°C, and then bulk low molecular weight RNA was extracted from ~ 300 OD of pellets by using hot phenol [74], and resolved on an 8% polyacrylamide (19:1) 7M urea, 1X TBE gel to purify bulk tRNA by elution of tRNA from the excised gel slice.
Isolation and purification of tRNATyr(GUA)
tRNATyr(GUA) was purified from S. pombe WT strains, and trm6Δ and trm61Δ mutant strains using 1 mg of bulk RNA (prepared using hot phenol), and the 5’-biotinylated oligonucleotide (TDZ 365; tY(GUA) 76–64; 5’ TGGTCTCCTGAGCCAGAATCGAACTA 3’), as described [74].
HPLC analysis of nucleosides
Purified tRNATyr(GUA) (~ 1.25 μg) was digested to nucleosides by treatment with P1 nuclease, followed by phosphatase, as described [74], and nucleosides were analyzed by HPLC (Waters, Millford, MA) at pH 7.0 as described [75]. To quantify nucleosides in bulk tRNA, relative amounts of modified nucleosides were compared to cytidine.
Poison primer extension assays
Oligomers used for primer extension are shown in S5 Table. Primers were 5’-end labeled essentially as described [74], with excess label removed using a MicroSpin G-25 chromatography column (Cytiva, Marlborough, MA cat#27532501), and poison primer extension was done as described [76], in 10 μL reactions containing 2 U AMV reverse transcriptase (Promega, Madison, WI cat# M5101), 1X AMV RT buffer, 1 mM ddNTP, and 1 mM of the other three dNTPs. Following extension for 1 h at 50°C, aliquots were resolved on a 15% polyacrylamide gel (29:1) containing 7 M urea in 1× TBE, and the gel was dried on a Model 583 Biorad gel dryer, exposed and analyzed on an Amersham Typhoon phosphorimager, and quantified using Image Quant v5.2.
Whole genome sequencing
Whole genome sequencing was performed by the University of Rochester Genomics Center at 25–50 fold coverage of the genome, and reads were compared to the corresponding parent strain, and to the reference genome.
Supporting information
(A) The temperature sensitivity of an S. pombe trm6Δ mutant is complemented by [Ptrm6 trm6+ leu2+] on EMMC-leu media. WT and trm6Δ cells expressing Ptrm6 trm6+ were grown overnight in EMMC-leu media 30°C and analyzed for growth at the indicated temperatures. (B) The temperature sensitivity of S. pombe trm61Δ is complemented by [Ptrm61 trm61+ leu2+] on EMMC-leu media. WT and trm61Δ cells expressing Ptrm61 trm61+ were grown overnight in EMMC-leu media 30°C and analyzed for growth.
(PDF)
(A,B) Bulk tRNA from S. pombe trm6Δ mutants have no detectable m1A. S. pombe trm6Δ mutants and WT cells were grown in biological triplicate in YES media at 30°C and bulk tRNA was purified, digested to nucleosides, and analyzed for modifications by HPLC as described in Materials and Methods. (A) A trace of the A258 nm of eluted nucleosides of bulk tRNA. (B) Quantification of levels of modified nucleosides of purified tRNATyr(GUA). The bar chart depicts the average moles/mol of nucleosides (expressed as a percentage of the moles of cytidine), with associated standard deviation; WT, gray; S. pombe trm6Δ, red. The data is also tabulated in the table below (C).
(PDF)
(A) Northern blot. Full analysis is shown of tRNAs analyzed in the northern blot shown in Fig 2A. (B,C) Quantification of tRNA levels. The bar chart depicts relative levels of tRNA species at each temperature, relative to their levels in WT at 30°C.
(PDF)
(A) Northern blot. Strains were grown and analyzed as in Fig 2D. (B) Quantification of tRNA levels. tRNA levels were quantified as in Fig 2B.
(PDF)
Strains with plasmids as indicated were grown overnight in EMMC-Leu media at 30°C and analyzed for growth as in Fig 1A on indicated plates and temperatures.
(PDF)
(A) Alignment of regions around the dhp1-5 (S737P) and dhp1-6 (Y669C) mutations. S. pombe Dhp1 was aligned with putative Rat1/Dhp1 orthologs from 12 evolutionarily distinct eukaryotes, using Multalin [77]; http://multalin.toulouse.inra.fr/multalin/). red, more than 80% conservation; blue 40% - 80% conservation. (B) Location of dhp1-5 (S737P) and dhp1-6 (Y669C) mutations mapped onto the S. pombe structure [78]. magenta, residues in the catalytic center; blue, residues interacting with Rai1. (C) Expression of Pdhp1 dhp1+ integrated in the chromosome restores temperature sensitive growth of the S. pombe trm6Δ dhp1-5 mutant. WT, trm6Δ, and trm6Δ dhp1-5 cells expressing a chromosomally integrated copy of Pdhp1 dhp1+ in the ura4+ locus or the control vector integrant, were grown overnight in YES media at 30°C, and analyzed for growth.
(PDF)
trm6Δ tol1-1 mutants, trm6Δ mutants and WT strains were transformed with either [Ptol1 tol1+ leu2+] or empty vector, grown in EMMC-leu, and spotted.
(PDF)
(A) Alignment of the regions around the tol1-1 (A151D) mutation. S. pombe Tol1 was aligned with putative Tol1 orthologs from 12 evolutionarily distinct eukaryotes, as in S5 Fig. red, more than 80% conservation; blue 40% - 80% conservation. (B) Location of tol1-1 (A151D) mapped onto the structure of the S. cerevisiae ortholog Met22 [79]. orange, active site residues.
(PDF)
(A) Deletion of the imt06 gene encoding tRNAiMet(CAU) in an S. pombe trm6Δ mutant severely exacerbates its growth. Strains from the growth test in Fig 4A are shown after 2 days of growth (B) Complementation of trm6Δ imt06Δ growth defect with an integrated imt06 and a [leu2+ imt06+] plasmid. trm6Δ imt06Δ and WT cells expressing tRNAiMet(CAU) from a chromosomally integrated copy of imt06+ and from a [leu2+ imt06+] plasmid, and controls were grown overnight in EMMC or EMMC-leu media at 30°C, and analyzed for growth. (C) Levels of tRNAiMet(CAU) are significantly reduced in S. pombe trm6Δ imt06Δ mutants at 30°C. The Northern blot from Fig 4B is shown.
(PDF)
The alignment of S. pombe Dhp1 was done as in S5A Fig. (B) Location of dhp1 suppressor mutations mapped onto the S. pombe structure [78]. magenta, residues in the catalytic center; blue, residues interacting with Rai1. (C) Alignment of the regions around the tol1-2 (A297D) mutation. The alignment of S. pombe Tol1 was done as in S7 Fig. (D) Location of tol1-2 (A297D) mapped onto the structure of the S. cerevisiae ortholog Met22 [79]. orange, active site residues.
(PDF)
WT, trm6Δ imt06Δ, and trm6Δ imt06Δ tol1-2 cells expressing Ptol1 tol1+ or a vector [80] were grown overnight in EMMC-Leu media at 30°C, and analyzed for growth
(PDF)
(A) Analysis of growth of cid14Δ strains on YES media with or without 5-FU. Strains were grown overnight in YES media at 30°C and analyzed for growth as in Fig 1A on indicated plates and temperatures. (B) Complementation of the 5-FU sensitivity of cid14Δ strains. Strains were grown overnight in EMMC-Leu media at 30°C and analyzed for growth on indicated plates and temperatures.
(PDF)
(A) A cid14Δ mutation does not suppress the growth defect of S. pombe trm6Δ mutants. Strains were grown overnight in YES media at 30°C and analyzed for growth as in Fig 1A on indicated plates and temperatures. (B,C) A cid14Δ mutation has only a minimal effect on tRNAiMet(CAU) levels in S. pombe trm6Δ mutants.
(PDF)
Strains were grown in YPD at 27°C and shifted to 33°C for 6 hours as described in Materials and Methods, and RNA was isolated and analyzed by northern blotting. (A) Northern Blot. (B) Quantification of northern. B; standard BY4741 WT strain background; Y, Y190 background of original trm6-504 mutant; m, trm6-504 mutant.
(PDF)
(A) Primer extension analysis of m1A58 modification in tRNAiMet(CAU) and tRNAPhe(GAA). Bulk RNA from S. cerevisiae trm6-504 mutants and WT cells grown for Fig 7B was analyzed by poison primer extension assay, as described in Materials and Methods, with the P1 primer (complementary to tRNAiMet(CAU) nt 76–61) and P2 primer (complementary to tRNAPhe(GAA) 76–60) in the presence of ddCTP, producing a stop at G57 for both tRNAiMet(CAU) and tRNAPhe(GAA), and a stop at N59 for m1A58. (B) Quantification of the poison primer extension. Values were calculated by first subtracting background levels, as in Fig 1E.
(PDF)
(A) Primer extension analysis of m1A58 modification in tRNAiMet(CAU). Bulk RNA from the growth done for Fig 7B was analyzed by poison primer extension assay with the P1 primer in the presence of ddATP, producing a stop at U55 or at A59 for m1A58. (B) Primer extension analysis of m1A58 modification in tRNAPhe(GAA). Bulk RNA from the growth done for Fig 7B was analyzed by poison primer extension assay with the P2 primer in the presence of ddCTP, producing a stop at G57 and for m1A58 at U59. (C) Quantification of the data from (A) and (B).
(PDF)
S. cerevisiae WT, trm6-504, and trm6Δ strains containing [2μ PGALTRM6 URA3] plasmid [81] and [2μ IMT1 LEU2] plasmids or empty vector, as indicated, were grown overnight in SD-leu media at 30°C and analyzed by spotting on SD-Leu media containing 5-FOA. Then cells from the 5-FOA plates were streaked for colonies, inoculated into SD-Leu media and grown overnight, and re-spotted on SD-Leu media.
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We thank Jeffrey Pleiss for S. pombe strains and James Anderson for the S. cerevisiae trm6-504 and background strain. We also thank Elizabeth Grayhack, Thareendra de Zoysa, Alayna Hauke, Erin Marcus, and other members of the Phizicky and Grayhack labs for valuable discussions and comments during the course of this work, and Elizabeth Grayhack for critical reading of the manuscript.
Data Availability
All relevant data are within the manuscript and its supporting information files.
Funding Statement
This research was supported by Grant GM052347, awarded to EMP from the National Institute of General Medical Sciences of the National Institutes of Health (https://www.nigms.nih.gov/). MT was partially supported by NIH Training Grant T32 GM068411 in Cellular, Biochemical and Molecular Sciences. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Phizicky EM, Hopper AK. tRNA biology charges to the front. Genes Dev. 2010;24(17):1832–60. doi: 10.1101/gad.1956510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boccaletto P, Machnicka MA, Purta E, Piatkowski P, Baginski B, Wirecki TK, et al. MODOMICS: a database of RNA modification pathways. 2017 update. Nucleic Acids Res. 2018;46(D1):D303–D7. doi: 10.1093/nar/gkx1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hopper AK. Transfer RNA post-transcriptional processing, turnover, and subcellular dynamics in the yeast Saccharomyces cerevisiae. Genetics. 2013;194(1):43–67. doi: 10.1534/genetics.112.147470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramos J, Fu D. The emerging impact of tRNA modifications in the brain and nervous system. Biochim. Biophys. Acta Gene Regul. Mech. 2019;1862(3):412–28. doi: 10.1016/j.bbagrm.2018.11.007 [DOI] [PubMed] [Google Scholar]
- 5.Suzuki T. The expanding world of tRNA modifications and their disease relevance. Nat Rev Mol. Cell. Biol. 2021;22(6):375–92. doi: 10.1038/s41580-021-00342-0 [DOI] [PubMed] [Google Scholar]
- 6.Muramatsu T, Nishikawa K, Nemoto F, Kuchino Y, Nishimura S, Miyazawa T, et al. Codon and amino-acid specificities of a transfer RNA are both converted by a single post-transcriptional modification. Nature. 1988;336(6195):179–81. doi: 10.1038/336179a0 [DOI] [PubMed] [Google Scholar]
- 7.Johansson MJ, Esberg A, Huang B, Bjork GR, Bystrom AS. Eukaryotic wobble uridine modifications promote a functionally redundant decoding system. Mol. Cell. Biol. 2008;28(10):3301–12. doi: 10.1128/MCB.01542-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nedialkova DD, Leidel SA. Optimization of Codon Translation Rates via tRNA Modifications Maintains Proteome Integrity. Cell. 2015;161(7):1606–18. doi: 10.1016/j.cell.2015.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urbonavicius J, Qian O, Durand JMB, Hagervall TG, Bjork GR. Improvement of reading frame maintenance is a common function for several tRNA modifications. EMBO J. 2001;20(17):4863–73. doi: 10.1093/emboj/20.17.4863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Yacoubi B, Hatin I, Deutsch C, Kahveci T, Rousset JP, Iwata-Reuyl D, et al. A role for the universal Kae1/Qri7/YgjD (COG0533) family in tRNA modification. EMBO J. 2011;30(5):882–93. doi: 10.1038/emboj.2010.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Putz J, Florentz C, Benseler F, Giege R. A single methyl group prevents the mischarging of a tRNA. Nat. Struct. Biol. 1994;1(9):580–2. doi: 10.1038/nsb0994-580 [DOI] [PubMed] [Google Scholar]
- 12.Helm M, Giege R, Florentz C. A Watson-Crick base-pair-disrupting methyl group (m1A9) is sufficient for cloverleaf folding of human mitochondrial tRNALys. Biochemistry. 1999;38(40):13338–46. doi: 10.1021/bi991061g [DOI] [PubMed] [Google Scholar]
- 13.Kadaba S, Krueger A, Trice T, Krecic AM, Hinnebusch AG, Anderson J. Nuclear surveillance and degradation of hypomodified initiator tRNAMet in S. cerevisiae. Genes Dev. 2004;18(11):1227–40. doi: 10.1101/gad.1183804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexandrov A, Chernyakov I, Gu W, Hiley SL, Hughes TR, Grayhack EJ, et al. Rapid tRNA decay can result from lack of nonessential modifications. Mol. Cell. 2006;21(1):87–96. doi: 10.1016/j.molcel.2005.10.036 [DOI] [PubMed] [Google Scholar]
- 15.De Zoysa T, Phizicky EM. Hypomodified tRNA in evolutionarily distant yeasts can trigger rapid tRNA decay to activate the general amino acid control response, but with different consequences. PLoS Genet. 2020;16(8):e1008893. doi: 10.1371/journal.pgen.1008893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chernyakov I, Whipple JM, Kotelawala L, Grayhack EJ, Phizicky EM. Degradation of several hypomodified mature tRNA species in Saccharomyces cerevisiae is mediated by Met22 and the 5’-3’ exonucleases Rat1 and Xrn1. Genes Dev. 2008;22(10):1369–80. doi: 10.1101/gad.1654308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dichtl B, Stevens A, Tollervey D. Lithium toxicity in yeast is due to the inhibition of RNA processing enzymes. EMBO J. 1997;16(23):7184–95. doi: 10.1093/emboj/16.23.7184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yun JS, Yoon JH, Choi YJ, Son YJ, Kim S, Tong L, et al. Molecular mechanism for the inhibition of DXO by adenosine 3’,5’-bisphosphate. Biochem. Biophys. Res. Commun. 2018;504(1):89–95. doi: 10.1016/j.bbrc.2018.08.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotelawala L, Grayhack EJ, Phizicky EM. Identification of yeast tRNA Um(44) 2’-O-methyltransferase (Trm44) and demonstration of a Trm44 role in sustaining levels of specific tRNA(Ser) species. RNA. 2008;14(1):158–69. doi: 10.1261/rna.811008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dewe JM, Whipple JM, Chernyakov I, Jaramillo LN, Phizicky EM. The yeast rapid tRNA decay pathway competes with elongation factor 1A for substrate tRNAs and acts on tRNAs lacking one or more of several modifications. RNA. 2012;18(10):1886–96. doi: 10.1261/rna.033654.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson J, Phan L, Cuesta R, Carlson BA, Pak M, Asano K, et al. The essential Gcd10p-Gcd14p nuclear complex is required for 1-methyladenosine modification and maturation of initiator methionyl-tRNA. Genes Dev. 1998;12(23):3650–62. doi: 10.1101/gad.12.23.3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kadaba S, Wang X, Anderson JT. Nuclear RNA surveillance in Saccharomyces cerevisiae: Trf4p-dependent polyadenylation of nascent hypomethylated tRNA and an aberrant form of 5S rRNA. RNA. 2006;12(3):508–21. doi: 10.1261/rna.2305406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Jia H, Jankowsky E, Anderson JT. Degradation of hypomodified tRNA(iMet) in vivo involves RNA-dependent ATPase activity of the DExH helicase Mtr4p. RNA. 2008;14(1):107–16. doi: 10.1261/rna.808608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaCava J, Houseley J, Saveanu C, Petfalski E, Thompson E, Jacquier A, et al. RNA degradation by the exosome is promoted by a nuclear polyadenylation complex. Cell. 2005;121(5):713–24. doi: 10.1016/j.cell.2005.04.029 [DOI] [PubMed] [Google Scholar]
- 25.Vanacova S, Wolf J, Martin G, Blank D, Dettwiler S, Friedlein A, et al. A new yeast poly(A) polymerase complex involved in RNA quality control. PLoS Biol. 2005;3(6):e189. doi: 10.1371/journal.pbio.0030189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basavappa R, Sigler PB. The 3 A crystal structure of yeast initiator tRNA: functional implications in initiator/elongator discrimination. EMBO J. 1991;10(10):3105–11. doi: 10.1002/j.1460-2075.1991.tb07864.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gudipati RK, Xu Z, Lebreton A, Seraphin B, Steinmetz LM, Jacquier A, et al. Extensive degradation of RNA precursors by the exosome in wild-type cells. Mol. Cell. 2012;48(3):409–21. doi: 10.1016/j.molcel.2012.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136(4):731–45. doi: 10.1016/j.cell.2009.01.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aitken CE, Lorsch JR. A mechanistic overview of translation initiation in eukaryotes. Nat. Struct. Mol. Biol. 2012;19(6):568–76. doi: 10.1038/nsmb.2303 [DOI] [PubMed] [Google Scholar]
- 30.Sasikumar AN, Perez WB, Kinzy TG. The many roles of the eukaryotic elongation factor 1 complex. Wiley Interdiscip Rev RNA. 2012;3(4):543–55. doi: 10.1002/wrna.1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hinnebusch AG. Translational regulation of GCN4 and the general amino acid control of yeast. Annu. Rev. Microbiol. 2005;59:407–50. doi: 10.1146/annurev.micro.59.031805.133833 [DOI] [PubMed] [Google Scholar]
- 32.Dever TE, Yang W, Astrom S, Bystrom AS, Hinnebusch AG. Modulation of tRNA(iMet), eIF-2, and eIF-2B expression shows that GCN4 translation is inversely coupled to the level of eIF-2.GTP.Met-tRNA(iMet) ternary complexes. Mol. Cell.Biol. 1995;15(11):6351–63. doi: 10.1128/MCB.15.11.6351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pavon-Eternod M, Gomes S, Rosner MR, Pan T. Overexpression of initiator methionine tRNA leads to global reprogramming of tRNA expression and increased proliferation in human epithelial cells. RNA. 2013;19(4):461–6. doi: 10.1261/rna.037507.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Birch J, Clarke CJ, Campbell AD, Campbell K, Mitchell L, Liko D, et al. The initiator methionine tRNA drives cell migration and invasion leading to increased metastatic potential in melanoma. Biol Open. 2016;5(10):1371–9. doi: 10.1242/bio.019075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watanabe K, Miyagawa R, Tomikawa C, Mizuno R, Takahashi A, Hori H, et al. Degradation of initiator tRNAMet by Xrn1/2 via its accumulation in the nucleus of heat-treated HeLa cells. Nucleic Acids Res. 2013;41(8):4671–85. doi: 10.1093/nar/gkt153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu F, Clark W, Luo G, Wang X, Fu Y, Wei J, et al. ALKBH1-Mediated tRNA Demethylation Regulates Translation. Cell. 2016;167(3):816–28 e16. doi: 10.1016/j.cell.2016.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Macari F, El-Houfi Y, Boldina G, Xu H, Khoury-Hanna S, Ollier J, et al. TRM6/61 connects PKCalpha with translational control through tRNAi(Met) stabilization: impact on tumorigenesis. Oncogene. 2016;35(14):1785–96. doi: 10.1038/onc.2015.244 [DOI] [PubMed] [Google Scholar]
- 38.Tang J, Jia P, Xin P, Chu J, Shi DQ, Yang WC. The Arabidopsis TRM61/TRM6 complex is a bona fide tRNA N1-methyladenosine methyltransferase. J. Exp. Bot. 2020;71(10):3024–36. doi: 10.1093/jxb/eraa100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parfrey LW, Lahr DJ, Knoll AH, Katz LA. Estimating the timing of early eukaryotic diversification with multigene molecular clocks. Proc Natl Acad Sci U S A. 2011;108(33):13624–9. doi: 10.1073/pnas.1110633108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim DU, Hayles J, Kim D, Wood V, Park HO, Won M, et al. Analysis of a genome-wide set of gene deletions in the fission yeast Schizosaccharomyces pombe. Nat. Biotechnol. 2010;28(6):617–23. doi: 10.1038/nbt.1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson J, Phan L, Hinnebusch AG. The Gcd10p/Gcd14p complex is the essential two-subunit tRNA(1- methyladenosine) methyltransferase of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A. 2000;97(10):5173–8. doi: 10.1073/pnas.090102597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mao J, Schmidt O, Soll D. Dimeric transfer RNA precursors in S. pombe. Cell. 1980;21(2):509–16. doi: 10.1016/0092-8674(80)90488-2 [DOI] [PubMed] [Google Scholar]
- 43.Seufert W, Jentsch S. Nucleotide sequence of two tRNA(Arg)-tRNA(Asp) tandem genes linked to duplicated UBC genes in Saccharomyces cerevisiae. Nucleic Acids Res. 1990;18(6):1638. doi: 10.1093/nar/18.6.1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt O, Mao J, Ogden R, Beckmann J, Sakano H, Abelson J, et al. Dimeric tRNA precursors in yeast. Nature. 1980;287(5784):750–2. doi: 10.1038/287750a0 [DOI] [PubMed] [Google Scholar]
- 45.Miyamoto R, Sugiura R, Kamitani S, Yada T, Lu Y, Sio SO, et al. Tol1, a fission yeast phosphomonoesterase, is an in vivo target of lithium, and its deletion leads to sulfite auxotrophy. J Bacteriol. 2000;182(13):3619–25. doi: 10.1128/JB.182.13.3619-3625.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Houseley J, Tollervey D. The nuclear RNA surveillance machinery: The link between ncRNAs and genome structure in budding yeast? Biochim. Biophys. Acta. 2008;1779(4):239–46. doi: 10.1016/j.bbagrm.2007.12.008 [DOI] [PubMed] [Google Scholar]
- 47.Schneider C, Anderson JT, Tollervey D. The exosome subunit Rrp44 plays a direct role in RNA substrate recognition. Mol. Cell. 2007;27(2):324–31. doi: 10.1016/j.molcel.2007.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Win TZ, Draper S, Read RL, Pearce J, Norbury CJ, Wang SW. Requirement of fission yeast Cid14 in polyadenylation of rRNAs. Mol. Cell. Biol. 2006;26(5):1710–21. doi: 10.1128/MCB.26.5.1710-1721.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buhler M, Moazed D. Transcription and RNAi in heterochromatic gene silencing. Nat. Struct. Mol. Biol. 2007;14(11):1041–8. doi: 10.1038/nsmb1315 [DOI] [PubMed] [Google Scholar]
- 50.Keller C, Woolcock K, Hess D, Buhler M. Proteomic and functional analysis of the noncanonical poly(A) polymerase Cid14. RNA. 2010;16(6):1124–9. doi: 10.1261/rna.2053710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu L, Yao F, Ma Y, Liu Q, Chen S, Hayafuji T, et al. Genetic evidence for involvement of membrane trafficking in the action of 5-fluorouracil. Fungal Genet Biol. 2016;93:17–24. doi: 10.1016/j.fgb.2016.05.007 [DOI] [PubMed] [Google Scholar]
- 52.Amberg DC, Goldstein AL, Cole CN. Isolation and characterization of RAT1: an essential gene of Saccharomyces cerevisiae required for the efficient nucleocytoplasmic trafficking of mRNA. Genes Dev. 1992;6(7):1173–89. doi: 10.1101/gad.6.7.1173 [DOI] [PubMed] [Google Scholar]
- 53.Kolitz SE, Lorsch JR. Eukaryotic initiator tRNA: finely tuned and ready for action. FEBS Lett. 2010;584(2):396–404. doi: 10.1016/j.febslet.2009.11.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ozanick SG, Bujnicki JM, Sem DS, Anderson JT. Conserved amino acids in each subunit of the heteroligomeric tRNA m1A58 Mtase from Saccharomyces cerevisiae contribute to tRNA binding. Nucleic Acids Res. 2007;35(20):6808–19. doi: 10.1093/nar/gkm574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagarajan VK, Jones CI, Newbury SF, Green PJ. XRN 5’—>3’ exoribonucleases: structure, mechanisms and functions. Biochim. Biophys. Acta. 2013;1829(6–7):590–603. doi: 10.1016/j.bbagrm.2013.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hudson BH, York JD. Roles for nucleotide phosphatases in sulfate assimilation and skeletal disease. Adv. Biol. Regul. 2012;52(1):229–38. doi: 10.1016/j.advenzreg.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin S, Liu Q, Lelyveld VS, Choe J, Szostak JW, Gregory RI. Mettl1/Wdr4-Mediated m(7)G tRNA Methylome Is Required for Normal mRNA Translation and Embryonic Stem Cell Self-Renewal and Differentiation. Mol. Cell. 2018;71(2):244–55 e5. doi: 10.1016/j.molcel.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Orellana EA, Liu Q, Yankova E, Pirouz M, De Braekeleer E, Zhang W, et al. METTL1-mediated m(7)G modification of Arg-TCT tRNA drives oncogenic transformation. Mol Cell. 2021;81(16):3323–38 e14. doi: 10.1016/j.molcel.2021.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tuorto F, Liebers R, Musch T, Schaefer M, Hofmann S, Kellner S, et al. RNA cytosine methylation by Dnmt2 and NSun2 promotes tRNA stability and protein synthesis. Nat Struct Mol Biol. 2012;19(9):900–5. doi: 10.1038/nsmb.2357 [DOI] [PubMed] [Google Scholar]
- 60.Huang Y, Bayfield MA, Intine RV, Maraia RJ. Separate RNA-binding surfaces on the multifunctional La protein mediate distinguishable activities in tRNA maturation. Nat Struct Mol Biol. 2006;13(7):611–8. doi: 10.1038/nsmb1110 [DOI] [PubMed] [Google Scholar]
- 61.Weick EM, Puno MR, Januszyk K, Zinder JC, DiMattia MA, Lima CD. Helicase-Dependent RNA Decay Illuminated by a Cryo-EM Structure of a Human Nuclear RNA Exosome-MTR4 Complex. Cell. 2018;173(7):1663–77 e21. doi: 10.1016/j.cell.2018.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guy MP, Young DL, Payea MJ, Zhang X, Kon Y, Dean KM, et al. Identification of the determinants of tRNA function and susceptibility to rapid tRNA decay by high-throughput in vivo analysis. Genes Dev. 2014;28(15):1721–32. doi: 10.1101/gad.245936.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bystrom AS, Fink GR. A functional analysis of the repeated methionine initiator tRNA genes (IMT) in yeast. Mol. Genet. Genet. 1989;216(2–3):276–86. doi: 10.1007/BF00334366 [DOI] [PubMed] [Google Scholar]
- 64.von Pawel-Rammingen U, Astrom S, Bystrom AS. Mutational analysis of conserved positions potentially important for initiator tRNA function in Saccharomyces cerevisiae. Mol. Cell. Biol. 1992;12(4):1432–42. doi: 10.1128/mcb.12.4.1432-1442.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Johnson AW. Rat1p and Xrn1p are functionally interchangeable exoribonucleases that are restricted to and required in the nucleus and cytoplasm, respectively. Mol. Cell. Biol. 1997;17(10):6122–30. doi: 10.1128/MCB.17.10.6122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kramer EB, Hopper AK. Retrograde transfer RNA nuclear import provides a new level of tRNA quality control in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A. 2013;110(52):21042–7. doi: 10.1073/pnas.1316579110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shelton VM, Sosnick TR, Pan T. Altering the intermediate in the equilibrium folding of unmodified yeast tRNAPhe with monovalent and divalent cations. Biochemistry. 2001;40(12):3629–38. doi: 10.1021/bi002646+ [DOI] [PubMed] [Google Scholar]
- 68.Whipple JM, Lane EA, Chernyakov I, D’Silva S, Phizicky EM. The yeast rapid tRNA decay pathway primarily monitors the structural integrity of the acceptor and T-stems of mature tRNA. Genes Dev. 2011;25(11):1173–84. doi: 10.1101/gad.2050711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bahler J, Wu JQ, Longtine MS, Shah NG, McKenzie A 3rd, Steever AB, et al. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 1998;14(10):943–51. doi: [DOI] [PubMed] [Google Scholar]
- 70.Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA 3rd, Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods. 2009;6(5):343–5. doi: 10.1038/nmeth.1318 [DOI] [PubMed] [Google Scholar]
- 71.Vjestica A, Marek M, Nkosi PJ, Merlini L, Liu G, Berard M, et al. A toolbox of stable integration vectors in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 2020;133(1). doi: 10.1242/jcs.240754 [DOI] [PubMed] [Google Scholar]
- 72.Houston L, Platten EM, Connelly SM, Wang J, Grayhack EJ. Frameshifting at collided ribosomes is modulated by elongation factor eEF3 and by integrated stress response regulators Gcn1 and Gcn20. RNA. 2022;28(3):320–39. doi: 10.1261/rna.078964.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Elder RT, Loh EY, Davis RW. RNA from the yeast transposable element Ty1 has both ends in the direct repeats, a structure similar to retrovirus RNA. Proc. Natl. Acad. Sci. U. S. A. 1983;80(9):2432–6. doi: 10.1073/pnas.80.9.2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jackman JE, Montange RK, Malik HS, Phizicky EM. Identification of the yeast gene encoding the tRNA m1G methyltransferase responsible for modification at position 9. RNA. 2003;9(5):574–85. doi: 10.1261/rna.5070303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guy MP, Podyma BM, Preston MA, Shaheen HH, Krivos KL, Limbach PA, et al. Yeast Trm7 interacts with distinct proteins for critical modifications of the tRNAPhe anticodon loop. RNA. 2012;18(10):1921–33. doi: 10.1261/rna.035287.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Payea MJ, Hauke AC, De Zoysa T, Phizicky EM. Mutations in the anticodon stem of tRNA cause accumulation and Met22-dependent decay of pre-tRNA in yeast. RNA. 2020;26(1):29–43. doi: 10.1261/rna.073155.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Corpet F. Multiple sequence alignment with hierarchical clustering. Nucl. Acids Res. 1988;16:10881–90. doi: 10.1093/nar/16.22.10881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xiang S, Cooper-Morgan A, Jiao X, Kiledjian M, Manley JL, Tong L. Structure and function of the 5’—>3’ exoribonuclease Rat1 and its activating partner Rai1. Nature. 2009;458(7239):784–8. doi: 10.1038/nature07731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Albert A, Yenush L, Gil-Mascarell MR, Rodriguez PL, Patel S, Martinez-Ripoll M, et al. X-ray structure of yeast Hal2p, a major target of lithium and sodium toxicity, and identification of framework interactions determining cation sensitivity. J. Mol. Biol. 2000;295(4):927–38. doi: 10.1006/jmbi.1999.3408 [DOI] [PubMed] [Google Scholar]
- 80.Forsburg SL. Comparison of Schizosaccharomyces pombe expression systems. Nucleic Acids Res. 1993;21(12):2955–6. doi: 10.1093/nar/21.12.2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gelperin DM, White MA, Wilkinson ML, Kon Y, Kung LA, Wise KJ, et al. Biochemical and genetic analysis of the yeast proteome with a movable ORF collection. Genes Dev. 2005;19(23):2816–26. doi: 10.1101/gad.1362105 [DOI] [PMC free article] [PubMed] [Google Scholar]