Abstract
Background
Current cholesterol guidelines have recommended very low low‐density lipoprotein cholesterol (LDL‐C) treatment targets for people at high risk of cardiovascular disease (CVD). However, recent observational studies indicated that very low LDL‐C levels may be associated with increased mortality and other adverse outcomes. The association between LDL‐C levels and long‐term risk of overall and cardiovascular mortality among the U.S. general population remains to be determined.
Methods and Results
This prospective cohort study included a nationally representative sample of 14 035 adults aged 18 years or older, who participated in the National Health and Nutrition Examination Survey III 1988–1994. LDL‐C levels were divided into 6 categories: <70, 70–99.9, 100–129.9, 130–159.9, 160–189.9 and ≥190 mg/dL. Deaths and underlying causes of deaths were ascertained by linkage to death records through December 31, 2015. Weighted Cox proportional hazards regression models were used to estimate the hazard ratios (HR) of mortality outcomes and its 95% CIs. During 304 025 person‐years of follow up (median follow‐up 23.2 years), 4458 deaths occurred including 1243 deaths from CVD. At baseline, mean age was 41.5 years and 51.9% were women. Very low and very high levels of LDL‐C were associated with increased mortality. After adjustment for age, sex, race and ethnicity, education, socioeconomic status, lifestyle factors, C‐reactive protein, body mass index, and other cardiovascular risk factors, individuals with LDL‐C<70 mg/dL, compared to those with LDL‐C 100–129.9 mg/dL, had HRs of 1.45 (95% CI, 1.10–1.93) for all‐cause mortality, 1.60 (95% CI, 1.01–2.54) for CVD mortality, and 4.04 (95% CI, 1.83–8.89) for stroke‐specific mortality, but no increased risk of coronary heart disease mortality. Compared with those with LDL‐C 100–129.9 mg/dL, individuals with LDL‐C≥190 mg/dL had HRs of 1.49 (95% CI, 1.09–2.02) for CVD mortality, and 1.63 (95% CI, 1.12–2.39) for coronary heart disease mortality, but no increased risk of stroke mortality.
Conclusions
Both very low and very high LDL‐C levels were associated with increased risks of CVD mortality. Very low LDL‐C levels was also associated with the high risks of all‐cause and stroke mortality. Further investigation is needed to elucidate the optimal range of LDL‐C levels for CVD health in the general population.
Keywords: all‐cause mortality, cardiovascular disease, cohort study, general population, low low‐density lipoprotein cholesterol
Subject Categories: Lipids and Cholesterol
Nonstandard Abbreviations and Acronyms
- ASCVD
atherosclerotic cardiovascular disease
- DBP
diastolic blood pressure
- HDL‐C
high density lipoprotein cholesterol
- ICH
intracerebral hemorrhage
- IPR
income‐to‐poverty ratios
- IS
ischemic stroke
- LDL‐C
low‐density lipoprotein cholesterol
- NCHS
National Center for Health Statistics
- NDI
National Death Index
- NHANES
National Health and Nutrition Examination Survey
- SBP
systolic blood pressure
- TC
total cholesterol
Clinical Perspective.
What Is New?
In a nationally representative cohort with a median follow‐up of 23.2 years, we examined the associations between low‐density lipoprotein cjolesterol (LDL‐C) levels and long‐term risk of overall and cardiovascular death among U.S. adults.
Very low LDL‐C levels <70 mg/dL was associated with increased risks of all‐cause, cardiovascular disease and stroke mortality. Moreover, very high LDL‐C levels ≥190 mg/dL were also associated with increased cardiovascular disease and coronary heart disease mortality.
What Are the Clinical Implications?
These findings indicated the adverse outcomes of both very high and very low LDL‐C levels, providing a new light of lipid control in clinic and lifestyle.
Further investigation is needed to elucidate the optimal range of LDL‐C levels for cardiovascular disease health in the general population.
Cardiovascular disease (CVD) remains the leading cause of death worldwide. Elevated low‐density lipoprotein cholesterol (LDL‐C) levels are clearly associated with increased risks of CVD death and incident CVD events in multiple cohort studies. 1 , 2 , 3 Among U.S. adults, LDL‐C showed a slowly decreasing trend due to healthy lifestyles and extensive use of lipid‐lowering therapy. 4 , 5 LDL‐C plays a causal role in atherosclerotic CVD (ASCVD), with a slightly more than 20% risk reduction in major vascular events per 1.0 mmol/L decrease of LDL‐C in trials of statins and other LDL‐C lowering drugs. 6 , 7 , 8 FOURIER (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Evelvated Risk) trial with PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibiting monoclonal antibodies added to background statin therapy had mean on‐treatment LDL‐C levels as low as 30 mg/dL. 9 In these short‐term trials (median duration 2–2.6 years), no untoward adverse effects were reported.
However, recent observational studies have indicated the possible harms of very low LDL‐C. 10 , 11 , 12 A cohort study, including 347 941 subjects with the mean follow up of 5.64 years, found that the LDL‐C less than 70 mg/dL were associated higher risk of all‐cause mortality and CVD mortality when compared with LDL‐C 100–129 mg/dL group. 13 Two prospective studies showed the strong association between low LDL‐C levels and risks of intracerebral hemorrhage (ICH) over 9 to 19 years. 11 , 12 Evidence from clinical trials has demonstrated that benefits of LDL‐C lowering therapy on all‐cause and CVD mortality are observed in individuals with baseline LDL‐C≥100 mg/dL, but not in individuals with LDL‐C<100 mg/dL over treatment periods of up to 7 years. 14 However, the relationship between very low levels of circulating LDL‐C and the risk of long‐term mortality outcomes have not been well elucidated. This information should be worth considering in determination of the optimal target when lowering LDL‐C level.
This study evaluated the association between the LDL‐C levels and risks of all‐cause and CVD mortality in a nationally representative cohort with up to 27 years of follow up in general population.
Methods
Data Availability
The data from National Health and Nutrition Examination Survey (NHANES) are publicly available and can be accessed directly at https://wwwn.cdc.gov/Nchs/Nhanes.
Study Population
NHANES was conducted by the National Center for Health Statistics (NCHS) at the Centers for Disease Control and Prevention, NHANES III was conducted in 1988 to 1994, in which participants were selected by a multistage stratified probability cluster sampling and represented the total civilian. Details of NHANES III procedures, interviewing, questionnaires and data collection, quality control techniques, survey design, nonresponse, and sample weighting have been described extensively. 15 NHANES has been approved by the NCHS Ethics Review Board. Written informed consent was obtained from all participants.
As shown in Figure, in this study we included participants at baseline according to the following criteria 1 : aged 18 years and older 2 ; exactly using Friedewald equation to calculate LDL‐C levels, requesting information on high density lipoprotein cholesterol (HDL‐C) and total cholesterol (TC), and triglycerides <400 mg/dL 3 ; without CVD or cancer diseases at baseline. Besides, we also excluded participants who died within 12 months from the baseline. Finally, 14 035 subjects were included as the analytical sample.
Figure 1. Flow chart of participants in this study.
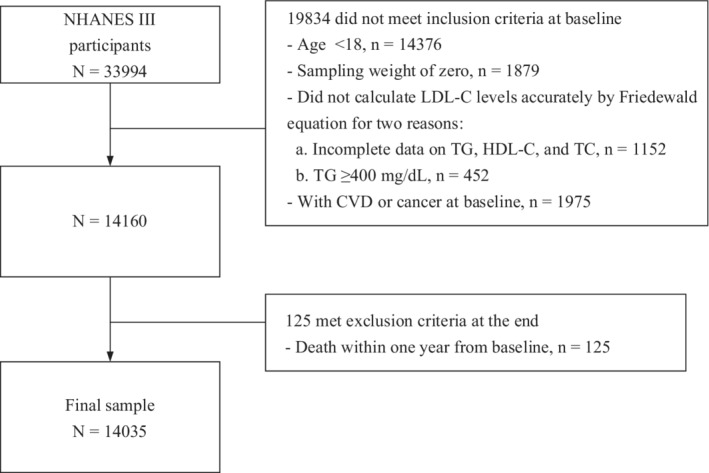
HDL‐C indicates high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; NHANES III, National Health and Nutrition Examination Survey III; TC, total cholesterol; and TG, triglyceride.
Exposure Measurement
Blood collection and blood lipid profile analyses including total cholesterol, triglycerides, high‐density lipoprotein cholesterol were described previously. 16 LDL‐C was calculated by the Friedewald equation [LDL‐C=TC − HDL‐C − (TG/5)], 17 except the individuals with triglycerides ≥400 mg/dL because of the inexact calculation. In the present study, fasting blood samples (i.e., a more than 9‐hour fast) were collected from 6212 subjects and non‐fasting blood samples were collected from 7823 subjects. Previous studies show that whether subjects are fasting or not, Friedewald equation has an excellent accuracy in LDL‐C calculation. 18 , 19 , 20 Therefore, in the main analysis, we used LDL‐C levels regardless of fasting status. LDL‐C levels were categorized according to Adult Treatment Panel III guidelines as follow: <70, 70–99.9, 100–129.9, 130–159.9, 160–189.9, and ≥190 mg/dL. 21
Outcome Ascertainment
We used the NHANES III Public‐use Linked Mortality File through 31 December 2015, which was linked by the NCHS to the National Death Index (NDI) with a probabilistic matching algorithm to determine the mortality status. 22 Previous studies have proved that the cause‐specific mortality in the NDI have the accurate results in death of classification and relatively small possibility of misclassification. 23 , 24 Data about underlying cause of death were used for case definition according to the International Classification of Diseases, Ninth Revision (ICD‐9) through 1998, and the remainder for case definition according to the Tenth Revision (ICD‐10). In order to adjust for changes between the 2 coding systems, final cause of deaths occurring prior to 1999 were recoded into comparable ICD‐10‐based underlying cause of death groups. 25 The all‐cause and CVD mortality were the primary outcomes in this study. We defined deaths from cardiovascular diseases as death from either coronary heart diseases or stroke. The NCHS classified mortality from coronary heart diseases (codes I00‐I09, I11, I13, and I20‐I51), stroke (i.e., cerebrovascular disease) (codes I60‐I69), and cancer (i.e., malignant neoplasms) (codes C00‐C97) according to ICD‐10 (Table S1). People who survived were administratively censored on 31 December, 2015. Follow‐up time for each person was calculated as the difference between the NHANES III examination date and the last known date alive or censored from the NHANES III mortality file.
Covariate Assessment
Information on age, sex, race and ethnicity, education level, marital status, family income, smoking status, alcoholic intake, and physical activity was collected using standardized questionnaires during interviews. Race and ethnicity was classified as non‐Hispanic White, non‐Hispanic Black, Mexican American, or other. Education level were categorized as less than high school, high school, and college or above. Marital status was categorized as married (married and living as married), widowed, divorced, and single (never married and separated). The family income‐to‐poverty ratios (IPR) was computed as a ratio of family income to poverty threshold values. The family income was set as the midpoint of the observed family income category in the Family Questionnaire in NHANES III. 26 The poverty threshold values are produced annually by the Census Bureau. 27 A higher IPR represents a higher family income status. The IPR were categorized as ≤1.30, 1.31–3.50, and >3.50 according to previous report. 28 Participants were categorized as non‐smoker, past smokers, and current smokers based on their responses to questions about smoking at least 100 cigarettes during their lifetime and whether they were currently smoking. The alcohol intake of participants was estimated based on 24‐hour dietary recall. 29 For physical activity, the inactive group was defined as those with no reported leisure time physical activity, active group was defined as those who had recommended levels of physical activity 30 (i.e., self‐reported leisure time moderate activity (metabolic equivalents [METs] ranging from 3 to 6) of 5 or more times per week or leisure time vigorous activity (METs >6) 3 or more times per week), and insufficiently active group was defined as those who were not inactive and did not meet the criteria for recommended levels of physical activity. CRP (C‐reactive protein) is a risk factor for CVD. CRP levels were categorized as <3.0 and ≥3.0 mg/L according to previous studies. 31 , 32 Comorbidities and treatment at baseline also need to be included (obesity, hypertension, diabetes, respiratory diseases, liver diseases, cholesterol‐lowering drugs). Measurements of height and weight were performed following a standardized protocol, and body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m2, <25, 25–29.9, ≥30). Hypertension was defined as any participant who had systolic blood pressure (SBP) level ≥130 mm Hg and diastolic blood pressure (DBP) level ≥80 mm Hg, or were taking antihypertensive medication according to the 2017 ACC/AHA Hypertension Guideline. 33 Diabetes was defined as any participant who had diagnosed diabetes or were taking insulin or were taking diabetes pills, or had a hemoglobin A1c level ≥6.5% or a fasting plasma glucose level ≥126 mg/dL. 34 Respiratory diseases were defined as any participant who had a diagnosed history of asthma, chronic bronchitis, or emphysema. Liver diseases were defined as participants with hepatic steatosis. Chronic kidney disease was defined as any participant who had estimated glomerular filtration rate (eGFR) <60 mL/min per 1.73 m2. 35 Cholesterol‐lowering drug was defined as participants who took prescribed medicine at baseline.
Statistical Analysis
All statistical analyses accounted for the complex, multistage, stratified, and cluster‐sampling design (including oversampling of certain subpopulations) of NHANES by using sample weights, strata, and primary sampling units embedded in the NHANES data. We used SDPSTRA6 to reflect stratum, SDPPSU6 to reflect primary sampling units, and the WTPFHX6 to reflect sample final weight for all analyses. Means and proportions of baseline characteristics were compared by using logistic regression for categorical variables and linear regression for continuous variables. The associations between LDL‐C levels and all‐cause and cardiovascular mortality were investigated using Cox proportional hazards regression models. The final models included the social‐demographic characteristics (age, sex, race and ethnicity, education, marital status, family income level), lifestyle factors (smoking status, alcohol intake, physical activity), inflammatory state (CRP), history of diseases (obesity, hypertension, diabetes, respiratory diseases, liver diseases, chronic kidney disease), and lipids‐lowering drugs use. The missing values of categorical variables (education, marital status, family income level, inflammatory state, history of diseases, and lipids‐lowering drugs use) were imputed by extra category with dummy coding. The alcohol intake as continuous variable was multiply imputed based on 5 replications using the Markov‐chain Monte Carlo method. We used the levels of 100–129 mg/dL as the referent group, because this group presented lowest risk of CVD mortality than other groups. Model assumptions were evaluated by testing the proportional hazards assumption for all analyses. The models including covariates were as followed: age, sex and race and ethnicity (Model 1); Model 1 plus education level, marital status, family income level, smoking status, alcohol intake, physical activity (Model 2); model 2 plus CRP level (Model 3); model 3 plus BMI, hypertension, diabetes, respiratory diseases, liver disease, chronic kidney disease, cholesterol‐lowering drugs (Model 4). Several analyses were performed to verify the robustness of results. First, in order to avoid bias of imputation for missing data on covariates, we performed the complete‐case analysis (n=9962). Second, given the confounding from cholesterol‐lowering drugs, we used the population who have no cholesterol‐lowering drugs use to assess the associations of LDL‐C with mortality outcomes (n=13 740). Third, we also explored the associations between LDL‐C levels and mortality outcomes by excluding the participants who have comorbidities (obesity, hypertension, diabetes, respiratory diseases, liver diseases, chronic kidney disease). All statistical analyses were conducted using survey modules of SAS software version 9.4 (SAS Institute, Cary, NC). Two‐sided P‐values <0.05 were considered statistically significant.
Results
The baseline characteristics were presented in Tables 1 and 2. In the 14 035 participants aged 18 years and older, the mean age was 41.5 years (SE, 0.39 years) and 51.9% were women at baseline. Participants within the lowest LDL‐C category (<70 mg/dL; 5.6%) were more likely to be young (mean age 33.0 years), have lower levels of education and income, and to be unmarried, physically active, moderate or heavy drinkers, current or nonsmokers, less likely to be non‐Hispanic White or past smokers, have hypertension, and take cholesterol‐lowering drugs than those with cholesterol levels of 100–129.9 mg/dL (referent). Those in the highest LDL‐C category (≥190 mg/dL; 5.6%) were older (mean age 52.4 years), and more likely to be non‐Hispanic White, have lower levels of education and income, to be married, past smokers, physically inactive, have hypertension, and take cholesterol‐lowering drugs than those with cholesterol levels of 100–129.9 mg/dL. Trends were seen for increasing rates of obesity, hypertension, cholesterol‐lowering drugs, higher triglycerides and HDL‐C, and lower alcohol intake were observed for increasing LDL‐C categories.
Table 1.
Baseline Demographic and Lifestyle Characteristics of the Study Population According to the LDL‐C Level
| Characteristics | LDL‐C levels | P value | |||||
|---|---|---|---|---|---|---|---|
| <70 mg/dL | 70–99.9 mg/dL | 100–129.9 mg/dL | 130–159.9 mg/dL | 160–189.9 mg/dL | ≥190 mg/dL | ||
| No. of participants | 790 | 3071 | 4481 | 3326 | 1585 | 782 | … |
| Age, y | 33.00 (0.61) | 35.24 (0.43) | 40.16 (0.50) | 45.00 (0.54) | 49.25 (0.69) | 52.42 (0.85) | < 0.001 |
| Sex, % | 0.002 | ||||||
| Male | 46.47 (2.75) | 45.67 (1.16) | 45.80 (1.28) | 51.69 (1.25) | 52.23 (1.70) | 48.11 (2.63) | |
| Female | 53.53 (2.75) | 54.33 (1.16) | 54.20 (1.28) | 48.31 (1.25) | 47.77 (1.70) | 51.89 (2.63) | |
| Race and ethnicity, % | 0.002 | ||||||
| Non‐Hispanic White | 72.07 (2.46) | 71.84 (2.03) | 74.52 (1.68) | 77.63 (1.28) | 78.49 (2.14) | 76.00 (2.38) | |
| Non‐Hispanic Black | 14.69 (1.31) | 12.42 (0.95) | 11.21 (0.74) | 9.95 (0.73) | 9.63 (0.93) | 12.11 (1.32) | |
| Mexican‐American | 6.55 (0.86) | 6.38 (0.73) | 6.04 (0.54) | 4.88 (0.38) | 3.72 (0.40) | 3.94 (0.71) | |
| Other | 6.69 (1.88) | 9.36 (1.49) | 8.23 (1.17) | 7.54 (0.86) | 8.16 (1.82) | 7.95 (1.66) | |
| Education, % | 0.003 | ||||||
| Less than high school | 3.50 (0.85) | 4.26 (0.48) | 4.46 (0.46) | 4.92 (0.47) | 4.79 (0.72) | 7.74 (1.22) | |
| High school | 55.74 (2.09) | 50.89 (2.14) | 52.06 (1.39) | 53.51 (1.50) | 58.86 (1.70) | 55.15 (3.13) | |
| College or above | 40.44 (2.24) | 44.37 (2.24) | 43.08 (1.45) | 41.13 (1.61) | 36.05 (1.82) | 36.82 (3.18) | |
| Marital status, % | <0.001 | ||||||
| Married | 48.34 (3.57) | 54.49 (1.15) | 64.10 (1.10) | 69.83 (1.10) | 70.96 (1.54) | 68.60 (2.75) | |
| Widowed | 1.82 (0.45) | 3.57 (0.45) | 4.57 (0.33) | 6.66 (0.52) | 7.91 (0.84) | 12.43 (1.84) | |
| Divorced | 9.70 (1.74) | 8.15 (1.01) | 7.12 (0.57) | 8.36 (0.70) | 8.32 (1.10) | 7.78 (1.61) | |
| Single | 40.14 (3.38) | 33.67 (1.41) | 24.15 (0.93) | 15.07 (0.91) | 12.64 (1.25) | 10.95 (1.76) | |
| Ratio of family income to poverty, % | < 0.001 | ||||||
| ≤1.30 | 23.95 (2.79) | 19.53 (1.35) | 17.34 (0.96) | 13.6 (1.01) | 16.75 (1.68) | 16.73 (1.85) | |
| 1.31–3.50 | 41.87 (2.82) | 42.13 (1.71) | 42.26 (1.42) | 43.56 (1.85) | 40.92 (2.15) | 46.01 (2.46) | |
| >3.50 | 28.96 (3.25) | 32.08 (1.74) | 34.33 (1.65) | 36.52 (2.18) | 33.75 (1.87) | 30.63 (2.12) | |
| Missing | 5.23 (0.97) | 6.27 (0.77) | 6.07 (0.49) | 6.32 (0.75) | 8.58 (1.13) | 6.62 (1.08) | |
| Smoking status, % | <0.001 | ||||||
| Non‐smoker | 51.96 (2.70) | 50.47 (1.46) | 48.57 (1.10) | 45.40 (1.52) | 44.53 (2.33) | 43.52 (2.13) | |
| Past smoker | 15.45 (1.90) | 17.63 (0.99) | 22.70 (0.96) | 27.39 (1.19) | 27.07 (1.74) | 28.33 (1.77) | |
| Current smoker | 32.59 (2.04) | 31.89 (1.50) | 28.73 (1.20) | 27.20 (1.50) | 28.39 (1.97) | 28.15 (2.52) | |
| Alcohol intake, g/day | 15.77 (1.93) | 14.99 (1.32) | 12.16 (0.88) | 9.93 (1.04) | 8.33 (1.03) | 6.83 (1.09) | 0.007 |
| Physical activity*, % | 0.001 | ||||||
| Inactive | 15.44 (2.29) | 12.69 (1.22) | 12.59 (0.85) | 13.26 (0.85) | 18.92 (1.76) | 14.28 (1.69) | |
| Insufficient | 41.09 (3.07) | 44.23 (1.43) | 45.26 (1.30) | 47.78 (1.36) | 44.80 (1.71) | 45.47 (2.53) | |
| Recommended level | 43.47 (2.66) | 43.08 (1.63) | 42.15 (1.52) | 38.96 (1.55) | 36.28 (2.08) | 40.25 (2.49) | |
| Lipids | |||||||
| Triglyceride, mg/dL | 137.89 (1.66) | 162.08 (0.58) | 190.16 (0.50) | 220.04 (0.46) | 249.79 (0.67) | 292.33 (1.58) | <0.001 |
| LDL‐C, mg/dL | 57.97 (0.69) | 87.17 (0.22) | 114.89 (0.19) | 143.69 (0.25) | 172.15 (0.32) | 212.07 (0.98) | <0.001 |
| HDL‐C, mg/dL | 57.15 (0.98) | 54.10 (0.52) | 51.52 (0.44) | 48.53 (0.36) | 48.24 (0.48) | 47.90 (1.35) | <0.001 |
| Triglyceride, mg/dL | 113.82 (5.88) | 104.04 (2.20) | 118.76 (2.13) | 139.10 (1.97) | 147.01 (2.16) | 161.83 (4.22) | <0.001 |
Values are means (SE) for continuous variables or percentages (SE) for categorical variables and are weighted except No. of participants. Numbers may not add to 100% due to missing data. HDL‐C indicates high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; and TC, total cholesterol.
Insufficient activity was defined where the sum of (weekly frequency of moderate activity/5) + (weekly frequency of vigorous activity/3) is <1.
Table 2.
Distribution of Cardiovascular Risk Factors of the Study Population According to the LDL‐C Level
| Risk factors | LDL‐C levels | P value | |||||
|---|---|---|---|---|---|---|---|
| <70 mg/dL | 70–99.9 mg/dL | 100–129.9 mg/dL | 130–159.9 mg/dL | 160–189.9 mg/dL | ≥190 mg/dL | ||
| CRP, % | <0.001 | ||||||
| 0.00–2.99 mg/L | 78.85 (2.52) | 79.63 (1.31) | 73.81 (1.41) | 69.88 (1.62) | 68.04 (2.28) | 67.38 (3.05) | |
| ≥3.00 mg/L | 19.75 (2.51) | 19.37 (1.31) | 25.36 (1.47) | 29.09 (1.72) | 31.28 (2.26) | 31.50 (3.08) | |
| BMI categories, % | <0.001 | ||||||
| <25.0 | 62.57 (3.01) | 64.30 (1.37) | 50.13 (1.35) | 35.92 (1.60) | 28.86 (1.92) | 33.17 (2.60) | |
| 25.0–29.9 | 26.16 (2.86) | 22.60 (1.33) | 28.33 (1.19) | 37.55 (1.26) | 46.08 (1.98) | 39.78 (1.95) | |
| ≥30.0 | 11.25 (1.52) | 13.05 (0.93) | 21.49 (1.35) | 26.42 (1.26) | 24.91 (1.44) | 27.00 (2.70) | |
| Hypertension, % | 20.34 (2.51) | 23.34 (1.15) | 32.85 (1.30) | 46.40 (1.36) | 54.48 (2.14) | 53.81 (3.17) | <0.001 |
| SBP, mm Hg | 115.21 (0.68) | 115.36 (0.38) | 119.09 (0.57) | 123.41 (0.41) | 126.44 (0.65) | 129.70 (0.92) | <0.001 |
| DBP, mm Hg | 69.27 (0.58) | 71.02 (0.30) | 73.12 (0.28) | 75.49 (0.28) | 76.74 (0.35) | 77.14 (0.56) | <0.001 |
| Pulse pressure, mm Hg | 45.95 (0.68) | 44.33 (0.43) | 45.96 (0.48) | 47.92 (0.37) | 49.70 (0.55) | 52.55 (0.78) | 0.003 |
| Diabetes, % | 7.12 (1.99) | 4.43 (0.54) | 6.66 (0.47) | 8.24 (0.69) | 8.15 (0.75) | 8.39 (1.53) | 0.001 |
| Fasting glucose, mg/dL | 97.59 (3.78) | 93.07 (0.66) | 95.88 (0.61) | 98.87 (0.64) | 98.92 (0.72) | 101.65 (1.38) | 0.575 |
| Respiratory diseases, % | 0.887 | ||||||
| Yes | 13.72 (2.41) | 11.00 (0.81) | 11.83 (0.70) | 12.09 (0.92) | 11.85 (1.33) | 11.54 (2.13) | |
| No | 86.28 (2.41) | 89.00 (0.81) | 88.17 (0.70) | 87.91 (0.92) | 88.15 (1.33) | 88.46 (2.13) | |
| Liver diseases, % | 0.013 | ||||||
| Yes | 35.49 (4.57) | 27.73 (1.54) | 30.76 (1.67) | 34.80 (1.80) | 35.80 (2.40) | 33.50 (4.00) | |
| No | 64.51 (4.57) | 72.27 (1.54) | 69.24 (1.67) | 65.20 (1.80) | 64.20 (2.40) | 66.50 (4.00) | |
| Chronic kidney disease, % | 5.95 (0.99) | 7.95 (0.72) | 10.52 (0.74) | 16.19 (0.99) | 22.33 (1.43) | 27.12 (2.37) | <0.001 |
| eGFR, mL/min per 1.73 m2 | 85.73 (1.08) | 82.74 (0.58) | 79.14 (0.54) | 75.58 (0.54) | 72 (0.64) | 69.9 (0.91) | <0.001 |
| Cholesterol‐lowering drugs, % | <0.001 | ||||||
| Yes | 0.17 (0.14) | 0.53 (0.22) | 1.37 (0.30) | 3.08 (0.42) | 4.80 (0.63) | 4.05 (0.93) | |
| No | 99.83 (0.14) | 99.47 (0.22) | 98.63 (0.30) | 96.92 (0.42) | 95.20 (0.63) | 95.95 (0.93) | |
Values are means (SE) for continuous variables or percentages (SE) for categorical variables and are weighted except No. of participants. Numbers may not add to 100% due to missing data. BMI indicates body mass index; CRP, C‐reactive protein; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; LDL‐C, low‐density lipoprotein cholesterol; and SBP, systolic blood pressure.
During the median follow‐up duration of 23.2 years, 4458 participants died, including 1243 deaths from cardiovascular diseases, 948 deaths from coronary heart diseases (CHD), and 295 deaths from stroke. As shown in Table 3, in fully adjusted models, when compared to participants with LDL‐C of 100–129 mg/dL, those with LDL‐C<70 mg/dL had a higher risk of all‐cause mortality (HR, 1.45 [95%CI, 1.10–1.93]). For CVD mortality, those with LDL‐C<70 mg/dL (HR, 1.60 [95%CI, 1.01–2.54]) and LDL‐C ≥190 mg/dL (HR, 1.49 [95%CI, 1.09–2.02]) were associated with increased risks, these with LDL‐C levels of 70–99.9, 130–159.9, and 160–189.9 mg/dL have risks of 1.28 (95%CI, 0.86–1.90), 1.19 (95%CI, 0.87–1.62), and 1.30 (95%CI, 0.98–1.72). The results also showed that LDL‐C level was not associated with risk of cancer mortality. Compared with LDL‐C level of 100–129.9 mg/dL, these in LDL‐C<70 mg/dL was associated with higher risk of non‐CVD and non‐cancer mortality (HR, 1.63 [95%CI, 1.08–2.46]).
Table 3.
Associations of LDL‐C Levels With All‐Cause, CVD, Cancer, and Non‐Cardiovascular and Non‐Cancer Mortality
| LDL‐C levels | ||||||
|---|---|---|---|---|---|---|
| <70 mg/dL | 70–99.9 mg/dL | 100–129.9 mg/dL | 130–159.9 mg/dL | 160–189.9 mg/dL | ≥190 mg/dL | |
| All‐cause mortality | ||||||
| Deaths/person‐years | 191/17217 | 692/68895 | 1238/98192 | 1236/71276 | 681/33030 | 620/15415 |
| Model 1 | 1.72 (1.27–2.32)* | 1.05 (0.88–1.25) | 1 (ref) | 0.92 (0.83–1.02) | 0.88 (0.76–1.02) | 1.07 (0.89–1.27) |
| Model 2 | 1.52 (1.14–2.02)* | 1.02 (0.86–1.21) | 1 (ref) | 0.93 (0.84–1.02) | 0.86 (0.75–0.99)* | 1.05 (0.87–1.27) |
| Model 3 | 1.52 (1.14–2.03)* | 1.02 (0.86–1.22) | 1 (ref) | 0.92 (0.83–1.01) | 0.86 (0.75–0.99)* | 1.06 (0.88–1.29) |
| Model 4 | 1.45 (1.10–1.93)* | 1.03 (0.86–1.22) | 1 (ref) | 0.92 (0.83–1.02) | 0.88 (0.76–1.01) | 1.08 (0.88–1.32) |
| CVD mortality | ||||||
| Deaths/person‐years | 41/17217 | 166/68895 | 317/98192 | 350/71276 | 236/33030 | 133/15415 |
| Model 1 | 1.88 (1.19–2.98)* | 1.26 (0.84–1.87) | 1 (ref) | 1.16 (0.84–1.58) | 1.28 (0.97–1.70) | 1.44 (1.06–1.98)* |
| Model 2 | 1.66 (1.05–2.62)* | 1.24 (0.84–1.83) | 1 (ref) | 1.17 (0.86–1.59) | 1.27 (0.97–1.67) | 1.43 (1.06–1.93)* |
| Model 3 | 1.65 (1.04–2.62)* | 1.24 (0.83–1.83) | 1 (ref) | 1.17 (0.86–1.58) | 1.27 (0.96–1.67) | 1.45 (1.08–1.95)* |
| Model 4 | 1.60 (1.01–2.54)* | 1.28 (0.86–1.90) | 1 (ref) | 1.19 (0.87–1.62) | 1.30 (0.98–1.72) | 1.49 (1.09–2.02)* |
| Cancer mortality | ||||||
| Deaths/person‐years | 42/17217 | 152/68895 | 267/98192 | 276/71276 | 131/33030 | 82/15415 |
| Model 1 | 1.11 (0.63–1.96) | 0.77 (0.54–1.10) | 1 (ref) | 0.89 (0.63–1.26) | 0.74 (0.48–1.15) | 0.91 (0.61–1.35) |
| Model 2 | 1.01 (0.58–1.76) | 0.76 (0.53–1.09) | 1 (ref) | 0.89 (0.65–1.22) | 0.71 (0.46–1.08) | 0.89 (0.59–1.34) |
| Model 3 | 1.02 (0.59–1.77) | 0.76 (0.53–1.09) | 1 (ref) | 0.89 (0.65–1.22) | 0.70 (0.46–1.07) | 0.90 (0.59–1.36) |
| Model 4 | 1.02 (0.58–1.79) | 0.76 (0.52–1.09) | 1 (ref) | 0.88 (0.64–1.20) | 0.71 (0.46–1.08) | 0.90 (0.59–1.36) |
| Non‐CVD and non‐cancer mortality | ||||||
| Deaths/person‐years | 108/17217 | 374/68895 | 654/98192 | 610/71276 | 314/33030 | 201/15415 |
| Model 1 | 1.98 (1.30–3.00)* | 1.11 (0.90–1.36) | 1 (ref) | 0.84 (0.71–1.00)* | 0.79 (0.65–0.95)* | 0.99 (0.80–1.23) |
| Model 2 | 1.74 (1.16–2.61)* | 1.08 (0.89–1.31) | 1 (ref) | 0.85 (0.73–0.99)* | 0.77 (0.65–0.93)* | 0.97 (0.78–1.23) |
| Model 3 | 1.73 (1.15–2.60)* | 1.08 (0.89–1.31) | 1 (ref) | 0.84 (0.72–0.98)* | 0.77 (0.65–0.92)* | 0.99 (0.79–1.24) |
| Model 4 | 1.63 (1.08–2.46)* | 1.08 (0.89–1.32) | 1 (ref) | 0.85 (0.72–0.99)* | 0.80 (0.67–0.95)* | 1.01 (0.81–1.27) |
Values are n or hazard ratio (95% CI) and are weighted except No. of deaths/person years. CVD indicates cardiovascular disease; and LDL‐C, low‐density lipoprotein cholesterol.
Model 1: adjusted for age, sex, and race and ethnicity.
Model 2: model 1+ education level, marital status, family income level, smoking status, alcohol intake, and physical activity.
Model 3: model 2+ C‐reactive protein level.
Model 4: model 3+ BMI, hypertension, diabetes, respiratory diseases, liver diseases, chronic kidney disease and cholesterol‐lowering drugs.
P < 0.05.
As shown in Table 4, we further evaluated the associations of LDL‐C levels with CHD‐specific and stroke‐specific mortality separately. Compared with LDL‐C 100–129.9 mg/dL, those in LDL‐C<70, 70–99.9, 130–159.9, 160–189.9, and LDL‐C≥190 mg/dL have multivariable adjusted HRs of 1.08 (95%CI, 0.58–2.02), 1.32 (95%CI, 0.86–2.00), 1.22 (95%CI, 0.87–1.72), 1.32 (95%CI, 0.95–1.82), and 1.63 (95%CI, 1.12–2.39) for CHD mortality, respectively. Using LDL‐C 100–129.9 mg/dL as a reference, the LDL‐C<70 mg/dL was significantly associated with stroke mortality with HR of 4.04 (95%CI, 1.83–8.89), those in LDL‐C 70–99.9, 130–159.9, 160–189.9, and LDL‐C≥190 mg/dL have the adjusted HRs of 1.16 (95%CI, 0.54–2.49), 1.07 (95%CI, 0.63–1.82), 1.22 (95%CI, 0.76–1.94), and 1.01 (95%CI, 0.54–1.89) for stroke mortality, respectively.
Table 4.
Associations of LDL‐C Levels With Coronary Heart Disease and Stroke Mortality
| LDL‐C levels | ||||||
|---|---|---|---|---|---|---|
| <70 mg/dL | 70–99.9 mg/dL | 100–129.9 mg/dL | 130–159.9 mg/dL | 160–189.9 mg/dL | ≥190 mg/dL | |
| CHD mortality | ||||||
| Deaths/person‐years | 26/17217 | 129/68895 | 241/98192 | 282/71276 | 166/33030 | 104/15415 |
| Model 1 | 1.33 (0.70–2.51) | 1.30 (0.85–1.98) | 1 (ref) | 1.18 (0.85–1.65) | 1.29 (0.94–1.76) | 1.59 (1.08–2.33)* |
| Model 2 | 1.14 (0.62–2.10) | 1.27 (0.85–1.92) | 1 (ref) | 1.20 (0.86–1.66) | 1.28 (0.94–1.75) | 1.57 (1.08–2.28)* |
| Model 3 | 1.13 (0.61–2.09) | 1.27 (0.84–1.92) | 1 (ref) | 1.19 (0.86–1.66) | 1.28 (0.93–1.75) | 1.58 (1.09–2.30)* |
| Model 4 | 1.08 (0.58–2.02) | 1.32 (0.86–2.00) | 1 (ref) | 1.22 (0.87–1.72) | 1.32 (0.95–1.82) | 1.63 (1.12–2.39)* |
| Stroke mortality | ||||||
| Deaths/person‐years | 15/17217 | 37/68895 | 76/98192 | 68/71276 | 70/33030 | 29/15415 |
| Model 1 | 3.94 (1.64–9.51)* | 1.12 (0.50–2.51) | 1 (ref) | 1.06 (0.62–1.81) | 1.25 (0.78–2.02) | 0.98 (0.53–1.84) |
| Model 2 | 3.75 (1.61–8.70)* | 1.10 (0.51–2.40) | 1 (ref) | 1.08 (0.64–1.80) | 1.22 (0.76–1.97) | 0.98 (0.53–1.81) |
| Model 3 | 3.78 (1.64–8.72)* | 1.10 (0.50–2.40) | 1 (ref) | 1.07 (0.64–1.78) | 1.21 (0.75–1.97) | 1.00 (0.55–1.82) |
| Model 4 | 4.04 (1.83–8.89)* | 1.16 (0.54–2.49) | 1 (ref) | 1.07 (0.63–1.82) | 1.22 (0.76–1.94) | 1.01 (0.54–1.89) |
Values are n or hazard ratio (95% CI) and are weighted except No. of deaths/person years. CVD indicates cardiovascular disease; and LDL‐C, low‐density lipoprotein cholesterol.
Model 1: adjusted for age, sex, and race and ethnicity.
Model 2: model 1+ education level, marital status, family income level, smoking status, alcohol intake, and physical activity.
Model 3: model 2+ C‐reactive protein level.
Model 4: model 3+ BMI, hypertension, diabetes, respiratory diseases, liver diseases, chronic kidney disease and cholesterol‐lowering drugs.
P < 0.05.
Sensitivity analyses with complete‐case analysis were performed, the results were similar to previous findings (Table S2). In addition, we also assess the associations of LDL with mortality by excluding participants taking prescribed medicine to lower cholesterol (Table S3) and with comorbidities, including hypertension, diabetes, respiratory diseases, liver diseases, and chronic kidney disease (data not shown) and yielded similar results for mortalities outcomes.
Discussion
In a nationally representative cohort with a median follow‐up of 23.2 years, we found very low LDL‐C levels <70 mg/dL was associated with increased risks of all‐cause, CVD and stroke mortality. Moreover, very high LDL‐C levels ≥190 mg/dL were also associated with increased CVD and CHD mortality.
Current cholesterol guidelines (2019 ESC/EAS and 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline) recommend reducing LDL‐C levels for the management of ASCVD. 36 , 37 Our findings that very high LDL‐C levels were significantly associated with high risk of CVD and CHD mortality are consistent with previous studies. 1 , 2 A cohort study of 36 375 participants with low CVD risk reported that using LDL‐C<100 mg/dL as a reference, LDL‐C>160 mg/dL was associated with increased risks of CVD and CHD mortality. 1 Another large prospective study including 6 cohorts showed the strong association between high level of young adult LDL‐C and CHD risks in later life. 2 For the lipids‐lowering trials, Cholesterol Treatment Trialists' Collaboration meta‐analyses evaluated the prevention effect of statins on CVD events in older people, 6 people at low risk of vascular disease, 38 and both men and women, 39 which supported the net benefits of statins use and reduction in LDL‐C level. Another large‐scale clinical trial also showed that ezetimibe could further reduce the LDL‐C level and improve the cardiovascular outcomes when combined with the statin therapy. 40 Moreover, a meta‐analysis of LDL‐C lowering drugs demonstrated participants with baseline LDL‐C>160 mg/dL had the most reduction in CVD and all‐cause mortality. 14 In line with these findings, the present study also underscores the adverse outcome from exposure to very high LDL‐C levels.
While reducing LDL‐C has become a consensus for CVD prevention and management, increasing evidence from recent studies suggested potential risk of very low LDL‐C levels. Consistent with our findings in the US general population, a prospective study including 2 cohorts consisting of non‐statin users from South Korea found that LDL‐C<70 mg/dL were associated with higher risks of mortality, with a 95% increased risk of all‐cause mortality and a 102% increased risk of CVD mortality in one cohort, and a 81% increased risk of all‐cause mortality in another one, as compared with LDL‐C 100–129 mg/dL. 13 A cohort study among participants with higher risk of coronary events showed that in participants with hs‐CRP ≥2 mg/dL, LDL‐C<70 mg/dL is associated with increased all‐cause mortality (HR, 1.37 [95%CI, 1.07–1.74]) when compared with LDL ≥70 mg/dL. 10 A systemic review including 28 cohorts of older people showed the inverse association between lowest LDL‐C tertile or quartile and high all‐cause mortality in 16 cohort studies representing 92% of the number of participants. 41 However, the scope of lowest LDL‐C tertile or quartile cannot be identified in this study.
For specific type of CVD, a meta‐analysis reported the low level of LDL‐C was associated with higher risk of ICH stroke. 42 Recently, a cohort study found that women with LDL‐C<70 mg/dL, as compared with those with LDL‐C between 100 and 129.9 mg/dL, had 2.17 times the risk of ICH. 11 A cohort study of Chinese population also showed that participants LDL‐C<70 mg/dL was associated with increased risk of ICH when compared with those with LDL‐C ranged 70–100 mg/dL. 12 In accordance with these evidences, our findings indicated increased stroke death risk in very low LDL‐C level group, the high stroke incidence may strengthen the association between them. However, recent results from 6 Chinese cohort showed no association of LDL‐C with incident hemorrhagic stroke. 43 Furthermore, low levels of LDL‐C are also observed in individuals with PCSK9 variants (Y142X, C679X, and R46L). 44 , 45 Previous studies also demonstrated the reduction in LDL‐C levels due to PCSK9 variants is safe and associated with decreased risks of heart disease. 44 , 46 More studies on different races and population deserve attention in the future.
Importantly, the stroke types, ICH and ischemic stroke (IS), seem to have different associations with LDL‐C levels. A large nested case–control study with Mendelian randomization analyses showed that low LDL‐C levels were associated with a higher risk of ICH and a lower risk of IS. 47 A large clinical trial using atorvastatin in patients with recent stroke or transient ischemic attack showed that 80 mg of atorvastatin per day decreased LDL‐C from 132 to 73 mg/dL, reduced overall incidence of cardiovascular events, but increased incidence of ICH. 48 Meta‐analysis on clinical trials of statin and non‐statin LDL‐C lowering therapies showed the positive effect of lowering‐lipid therapies on decreasing CHD and IS, even though there is always a doubt on adverse events of ICH, these data indicated the cardiovascular events would be further reduced when LDL‐C levels are controlled to be very low. 49 , 50 However, the primary outcome of these clinical trials based on incidence of cardiovascular events rather than CVD or all‐cause mortality. Another meta‐analysis of clinical trials found that among patients with baseline LDL‐C<100 mg/dL, LDL‐C lowering therapies was not associated with all‐cause mortality (RR, 1.00 [95%CI, 0.95–1.06]) and CVD mortality (RR, 0.99 [95%CI, 0.92–1.06]). 14 Similar to that in all‐cause death and CVD death, results from present study did not support the net benefits of very low LDL‐C levels for reducing overall stroke and CVD. There might be some reasons why our results differed from these studies which supported the net effect of low LDL‐C levels on CVD risks. First, ICH is a serious disease and have a higher fatality rate than IS, 51 death as primary outcome may strengthen the relationship between low level of LDL‐C and overall stroke. Second, most of clinical trials have a short follow‐up period no longer than 5 years, it is limited for observing the occurrence of ICH even though with very low LDL‐C levels by lipid‐lowering therapies. Third, lowering‐lipid therapies apply to the groups who are more likely to have ASCVD, as compared with general population, it can play a more positive role in delaying the progression of the ASCVD.
In addition, non‐HDL‐C level, accounting for the small dense LDL phenotype and triglyceride remnants, was increasingly mentioned in guidelines 37 , 52 for the management of blood lipids. The previous large‐scale population‐based study found the positive associations between the non‐HDL‐C level and all‐cause and CVD mortality. 3 A previous cohort study based on 30 554 Japanese individuals also showed the inverse association between the non‐HDL‐C level and intracerebral hemorrhage (ICH), 53 and positive association between the non‐HDL‐C level and CHD events. 53 These epidemiological evidences highlighted the risks of high non‐HDL‐C level for CHD and ischemic stroke, 53 , 54 Given study revealing the risks of low non‐HDL‐C level for ICH events and stroke mortality is sparce, appropriate level of non‐HDL‐C for population needed to be fully clarified in future studies.
Taken together, the evidence from numerous cohorts and clinical trials suggested that both very low and very high LDL‐C levels are linked to an increased risk of CVD events, it is important to maintain a moderate level of LDL‐C by healthy lifestyle and lipid‐lowering medications. The cholesterol guidelines recommend different cholesterol managements based on risks of ASCVD events. 52 Although CHD and IS were included in ASCVD events, ICH was not, 55 persisting in very low level of LDL‐C for reducing risk of ASCVD, which may increase the risk of ICH. Given the steadily descending trend of LDL‐C levels and increasing use of lipid‐lowering medications in U.S. adults, 4 , 5 the potential harmful effect of very low LDL‐C levels deserves attention and should be considered in the development of risk assessment tool and future clinical guidelines on blood cholesterol goal.
Furthermore, a clinical trial among older adults without baseline CVD demonstrated that statin treatment did not improve the adverse outcomes (eg, overall deaths, CVD deaths, CHD deaths and stroke deaths) for primary cardiovascular prevention. 56 Guidelines also showed lower strength recommendations in older adults for statin uses than in younger adults. 37 , 52 A meta‐analysis on benefits of statin in primary prevention, including 24 674 elderly subjects, demonstrated statin use did not significantly reduce the risk of all‐cause and CVD mortality. 57 Another meta‐analysis of clinical trials showed that for people without vascular disease, net benefits of statins on cardiovascular events were found in adults younger than 70 years of age, not in adults older than 70 years. 6 Notably, advanced age was the risk factor of the adverse outcomes of statin use, including diabetes 58 and muscle symptoms. 59 These studies highlighted that the role of age and statins use on adverse outcomes needed to be further investigated in future studies.
The main strengthens of this study is based on nationally representative sample, suggesting that present findings could be better extrapolated to the general population. Moreover, with more than 20 years of observation, this study allowed determining and quantifying the long‐term risk associated with LDL‐C levels. Additionally, a variety of confounding factors provided from NAHNES III data were controlled for the more accurate estimates. The prevalence of lipid‐lowering therapy was 3.4% in NHANES III, with little impact on LDL‐C level among US. adults, 4 hence the associations between LDL‐C and outcomes were less confounded by lipid‐lowering therapies.
This study has several limitations. First, given the observational nature of this study, we could not establish causality between LDL‐C levels and the risk of mortality. Second, the LDL‐C levels were only available at baseline. The single measurement of LDL‐C indeed not reflected the average levels in the long follow‐up. Third, the timing of baseline samples in this study was before the widespread uptake of the lipids lowering drugs, such as statins use. Temporal increasing trends in statin utilization may attenuate the increased risks of CVD and CHD death in those with the highest LDL‐C levels at baseline, thus present study may underestimate the risk of high LDL‐C levels for CVD and CHD mortality. 4 Fourth, NHANES data could not provide the number of deaths from stroke subtypes (ICH and IS), which may limit the power to identify the association between the LDL‐C level and mortality from subtypes of stroke. Fifth, although we have adjusted for many potential confounders in this study, we are unable to completely rule out residual confounding from unmeasured factors and a small amount of missing data.
Conclusion
Among U.S. adults, both very low and very high LDL‐C levels were associated with increased risks of CVD mortality. Very low LDL‐C levels was also associated with the increased risks of all‐cause and stroke mortality. These findings indicated the adverse outcomes of both very high and very low LDL‐C levels, providing a new light of lipid control in clinic and lifestyle.
Sources of Funding
None.
Disclosures
None.
Supporting information
Tables S1–S3
Acknowledgments
The authors thank the participants and staff of the National Health and Nutrition Examination Survey III 1988‐1994 for their valuable contributions.
This paper was sent to Kwok Leung Ong, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplementary Material for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.023690
For Sources of Funding and Disclosures, see page 10.
Contributor Information
Shuang Rong, Email: rongshuangwust@yeah.net.
Wei Bao, Email: drwbao@hotmail.com.
References
- 1. Abdullah SM, Defina LF, Leonard D, Barlow CE, Radford NB, Willis BL, Rohatgi A, McGuire DK, de Lemos JA, Grundy SM, et al. Long‐term association of low‐density lipoprotein cholesterol with cardiovascular mortality in individuals at low 10‐year risk of atherosclerotic cardiovascular disease. Circulation. 2018;138:2315–2325. doi: 10.1161/CIRCULATIONAHA.118.034273 [DOI] [PubMed] [Google Scholar]
- 2. Zhang Y, Vittinghoff E, Pletcher MJ, Allen NB, Zeki Al Hazzouri A, Yaffe K, Balte PP, Alonso A, Newman AB, Ives DG, et al. Associations of blood pressure and cholesterol levels during young adulthood with later cardiovascular events. J Am Coll Cardiol. 2019;74:330–341. doi: 10.1016/j.jacc.2019.03.529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brunner FJ, Waldeyer C, Ojeda F, Salomaa V, Kee F, Sans S, Thorand B, Giampaoli S, Brambilla P, Tunstall‐Pedoe H, et al. Application of non‐HDL cholesterol for population‐based cardiovascular risk stratification: results from the multinational cardiovascular risk consortium. Lancet. 2019;394:2173–2183. doi: 10.1016/S0140-6736(19)32519-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carroll MD, Kit BK, Lacher DA, Shero ST, Mussolino ME. Trends in lipids and lipoproteins in US adults, 1988‐2010. JAMA. 2012;308:1545–1554. doi: 10.1001/jama.2012.13260 [DOI] [PubMed] [Google Scholar]
- 5. Rosinger A, Carroll MD, Lacher D, Ogden C. Trends in total cholesterol, triglycerides, and low‐density lipoprotein in US adults, 1999‐2014. JAMA Cardiol. 2017;2:339–341. doi: 10.1001/jamacardio.2016.4396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cholesterol Treatment Trialists' Collaboration . Efficacy and safety of statin therapy in older people: a meta‐analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393:407–415. doi: 10.1016/S0140-6736(18)31942-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, Braunwald E, Sabatine MS. Association between lowering LDL‐C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta‐analysis. JAMA. 2016;316:1289–1297. doi: 10.1001/jama.2016.13985 [DOI] [PubMed] [Google Scholar]
- 8. Gencer B, Marston NA, Im K, Cannon CP, Sever P, Keech A, Braunwald E, Giugliano RP, Sabatine MS. Efficacy and safety of lowering LDL cholesterol in older patients: a systematic review and meta‐analysis of randomised controlled trials. Lancet. 2020;396:1637–1643. doi: 10.1016/S0140-6736(20)32332-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Giugliano RP, Pedersen TR, Park JG, De Ferrari GM, Gaciong ZA, Ceska R, Toth K, Gouni‐Berthold I, Lopez‐Miranda J, Schiele F, et al. Clinical efficacy and safety of achieving very low LDL‐cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial. Lancet. 2017;390:1962–1971. doi: 10.1016/S0140-6736(17)32290-0 [DOI] [PubMed] [Google Scholar]
- 10. Penson PE, Long DL, Howard G, Toth PP, Muntner P, Howard VJ, Safford MM, Jones SR, Martin SS, Mazidi M, et al. Associations between very low concentrations of low density lipoprotein cholesterol, high sensitivity C‐reactive protein, and health outcomes in the reasons for geographical and racial differences in stroke (REGARDS) study. Eur Heart J. 2018;39:3641–3653. doi: 10.1093/eurheartj/ehy533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rist PM, Buring JE, Ridker PM, Kase CS, Kurth T, Rexrode KM. Lipid levels and the risk of hemorrhagic stroke among women. Neurology. 2019;92:e2286–e2294. doi: 10.1212/WNL.0000000000007454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ma C, Gurol ME, Huang Z, Lichtenstein AH, Wang X, Wang Y, Neumann S, Wu S, Gao X. Low‐density lipoprotein cholesterol and risk of intracerebral hemorrhage: a prospective study. Neurology. 2019;93:e445–e457. doi: 10.1212/WNL.0000000000007853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sung KC, Huh JH, Ryu S, Lee JY, Scorletti E, Byrne CD, Kim JY, Hyun DS, Ko SB. Low levels of low‐density lipoprotein cholesterol and mortality outcomes in non‐statin users. J Clin Med. 2019;8. doi: 10.3390/jcm8101571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Navarese EP, Robinson JG, Kowalewski M, Kolodziejczak M, Andreotti F, Bliden K, Tantry U, Kubica J, Raggi P, Gurbel PA. Association between baseline LDL‐C level and Total and cardiovascular mortality after LDL‐C lowering: a systematic review and meta‐analysis. JAMA. 2018;319:1566–1579. doi: 10.1001/jama.2018.2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. Series 1: programs and collection procedures. Vital Health Stat 1. 1994;32:1–407. [PubMed] [Google Scholar]
- 16. Myers GL, Cooper GR, Winn CL, Smith SJ. The centers for disease control‐National Heart, lung and blood institute lipid standardization program. An approach to accurate and precise lipid measurements. Clin Lab Med. 1989;9:105–135. doi: 10.1016/S0272-2712(18)30645-0 [DOI] [PubMed] [Google Scholar]
- 17. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. doi: 10.1093/clinchem/18.6.499 [DOI] [PubMed] [Google Scholar]
- 18. Schectman G, Patsches M, Sasse EA. Variability in cholesterol measurements: comparison of calculated and direct LDL cholesterol determinations. Clin Chem. 1996;42:732–737. doi: 10.1093/clinchem/42.5.732 [DOI] [PubMed] [Google Scholar]
- 19. Nauck M, Warnick GR, Rifai N. Methods for measurement of LDL‐cholesterol: a critical assessment of direct measurement by homogeneous assays versus calculation. Clin Chem. 2002;48:236–254. doi: 10.1093/clinchem/48.2.236 [DOI] [PubMed] [Google Scholar]
- 20. Mora S, Rifai N, Buring JE, Ridker PM. Comparison of LDL cholesterol concentrations by Friedewald calculation and direct measurement in relation to cardiovascular events in 27,331 women. Clin Chem. 2009;55:888–894. doi: 10.1373/clinchem.2008.117929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC Jr, Stone NJ, Coordinating Committee of the National Cholesterol Education Program . Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. J Am Coll Cardiol. 2004;44:720–732. doi: 10.1016/j.jacc.2004.07.001 [DOI] [PubMed] [Google Scholar]
- 22. National Center for Health Statistics . The Linkage of National Center for Health Statistics Survey Data to the National Death Index — 2019 Linked Mortality File (LMF): Linkage Methodology and Analytic Considerations. Hyattsville, MD: National Center for Health Statistics Division of Analysis and Epidemiology; 2022. [Google Scholar]
- 23. Skopp NA, Smolenski DJ, Schwesinger DA, Johnson CJ, Metzger‐Abamukong MJ, Reger MA. Evaluation of a methodology to validate National Death Index retrieval results among a cohort of U.S. service members. Ann Epidemiol. 2017;27:397–400. doi: 10.1016/j.annepidem.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 24. Lash TL, Silliman RA. A comparison of the National Death Index and Social Security Administration databases to ascertain vital status. Epidemiology. 2001;12:259–261. doi: 10.1097/00001648-200103000-00021 [DOI] [PubMed] [Google Scholar]
- 25. National Center for Health Statistics . Office of analysis and epidemiology, public‐use linked mortality file, 2015.
- 26. NHANES III household adult data file documention.
- 27. Prior HHS Poverty Guidelines and Federal Register References.
- 28. Ogden CL, Carroll MD, Fakhouri TH, Hales CM, Fryar CD, Li X, Freedman DS. Prevalence of obesity among youths by household income and education level of head of household ‐ United States 2011‐2014. MMWR Morb Mortal Wkly Rep. 2018;67:186–189. doi: 10.15585/mmwr.mm6706a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. National Center for Health Statistics . Examination File, The Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. 1996. [Google Scholar]
- 30. Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, Buchner D, Ettinger W, Heath GW, King AC, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–407. doi: 10.1001/jama.1995.03520290054029 [DOI] [PubMed] [Google Scholar]
- 31. Wulaningsih W, Holmberg L, Ng T, Rohrmann S, Van Hemelrijck M. Serum leptin, C‐reactive protein, and cancer mortality in the NHANES III. Cancer Med. 2016;5:120–128. doi: 10.1002/cam4.570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C‐reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131 [DOI] [PubMed] [Google Scholar]
- 33. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and Management of High Blood Pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2018;138:e426–e483. [DOI] [PubMed] [Google Scholar]
- 34. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988‐2012. JAMA. 2015;314:1021–1029. doi: 10.1001/jama.2015.10029 [DOI] [PubMed] [Google Scholar]
- 35. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wilson PWF, Polonsky TS, Miedema MD, Khera A, Kosinski AS, Kuvin JT. Systematic review for the 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the Management of Blood Cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;139:e1144–e1161. doi: 10.1161/CIR.0000000000000626 [DOI] [PubMed] [Google Scholar]
- 37. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 38. Cholesterol Treatment Trialists C, Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, Voysey M, Gray A, Collins R, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta‐analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. doi: 10.1016/S0140-6736(12)60367-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cholesterol Treatment Trialists C, Fulcher J, O'Connell R, Voysey M, Emberson J, Blackwell L, Mihaylova B, Simes J, Collins R, Kirby A, et al. Efficacy and safety of LDL‐lowering therapy among men and women: meta‐analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385:1397–1405. doi: 10.1016/S0140-6736(14)61368-4 [DOI] [PubMed] [Google Scholar]
- 40. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, et al. Ezetimibe added to statin therapy after acute coronary syndromes. New Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489 [DOI] [PubMed] [Google Scholar]
- 41. Ang A, Pullar JM, Currie MJ, Vissers MCM. Vitamin C and immune cell function in inflammation and cancer. Biochem Soc Trans. 2018;46:1147–1159. doi: 10.1042/BST20180169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang X, Dong Y, Qi X, Huang C, Hou L. Cholesterol levels and risk of hemorrhagic stroke: a systematic review and meta‐analysis. Stroke. 2013;44:1833–1839. doi: 10.1161/STROKEAHA.113.001326 [DOI] [PubMed] [Google Scholar]
- 43. Gu X, Li Y, Chen S, Yang X, Liu F, Li Y, Li J, Cao J, Liu X, Chen J, Shen C, Yu L, Huang J, Lam TH, Fang X, He Y, Zhang X, Lu X, Wu S, Gu D Association of Lipids with Ischemic and Hemorrhagic Stroke: a prospective cohort study among 267 500 Chinese. Stroke 2019:STROKEAHA119026402, 50, 3376, 3384, DOI: 10.1161/STROKEAHA.119.026402. [DOI] [PubMed] [Google Scholar]
- 44. Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. New Engl J Med. 2006;354:1264–1272. doi: 10.1056/NEJMoa054013 [DOI] [PubMed] [Google Scholar]
- 45. Tsai CW, North KE, Tin A, Haack K, Franceschini N, Saroja Voruganti V, Laston S, Zhang Y, Best LG, MacCluer JW, et al. Both rare and common variants in PCSK9 influence plasma low‐density lipoprotein cholesterol level in American Indians. J Clin Endocrinol Metab. 2015;100:E345–E349. doi: 10.1210/jc.2014-3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Benn M, Nordestgaard BG, Grande P, Schnohr P, Tybjaerg‐Hansen A. PCSK9 R46L, low‐density lipoprotein cholesterol levels, and risk of ischemic heart disease: 3 independent studies and meta‐analyses. J Am Coll Cardiol. 2010;55:2833–2842. doi: 10.1016/j.jacc.2010.02.044 [DOI] [PubMed] [Google Scholar]
- 47. Sun L, Clarke R, Bennett D, Guo Y, Walters RG, Hill M, Parish S, Millwood IY, Bian Z, Chen Y, et al. Causal associations of blood lipids with risk of ischemic stroke and intracerebral hemorrhage in Chinese adults. Nat Med. 2019;25:569–574. doi: 10.1038/s41591-019-0366-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Amarenco P, Bogousslavsky J, Callahan A III, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, et al. High‐dose atorvastatin after stroke or transient ischemic attack. New Engl J Med. 2006;355:549–559. doi: 10.1056/NEJMoa061894 [DOI] [PubMed] [Google Scholar]
- 49. Sabatine MS, Wiviott SD, Im K, Murphy SA, Giugliano RP. Efficacy and safety of further lowering of low‐density lipoprotein Cholesterol in patients starting with very low levels: a meta‐analysis. JAMA Cardiol. 2018;3:823–828. doi: 10.1001/jamacardio.2018.2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cholesterol Treatment Trialists C, Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Collaborators GBDLRoS , Feigin VL, Nguyen G, Cercy K, Johnson CO, Alam T, Parmar PG, Abajobir AA, Abate KH, Abd‐Allah F, et al. Global, regional, and country‐specific lifetime risks of stroke, 1990 and 2016. New Engl J Med. 2018;379:2429–2437. doi: 10.1056/NEJMoa1804492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella‐Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the Management of Blood Cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:e285–e350. doi: 10.1016/j.jacc.2018.11.003 [DOI] [PubMed] [Google Scholar]
- 53. Saito I, Yamagishi K, Kokubo Y, Yatsuya H, Iso H, Sawada N, Inoue M, Tsugane S. Non‐high‐density lipoprotein cholesterol and risk of stroke subtypes and coronary heart disease: the Japan public health center‐based prospective (JPHC) study. J Atheroscler Thromb. 2020;27:363–374. doi: 10.5551/jat.50385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wu J, Chen S, Liu L, Gao X, Zhou Y, Wang C, Zhang Q, Wang A, Hussain M, Sun B, et al. Non‐high‐density lipoprotein cholesterol vs low‐density lipoprotein cholesterol as a risk factor for ischemic stroke: a result from the Kailuan study. Neurol Res. 2013;35:505–511. doi: 10.1179/1743132813Y.0000000206 [DOI] [PubMed] [Google Scholar]
- 55. Goff DC Jr, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 56. Han BH, Sutin D, Williamson JD, Davis BR, Piller LB, Pervin H, Pressel SL, Blaum CS, Group ACR . Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults: the ALLHAT‐LLT randomized clinical trial. JAMA Intern Med. 2017;177:955–965. doi: 10.1001/jamainternmed.2017.1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Savarese G, Gotto AM Jr, Paolillo S, D'Amore C, Losco T, Musella F, Scala O, Marciano C, Ruggiero D, Marsico F, et al. Benefits of statins in elderly subjects without established cardiovascular disease: a meta‐analysis. J Am Coll Cardiol. 2013;62:2090–2099. doi: 10.1016/j.jacc.2013.07.069 [DOI] [PubMed] [Google Scholar]
- 58. Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW, et al. Statins and risk of incident diabetes: a collaborative meta‐analysis of randomised statin trials. Lancet. 2010;375:735–742. doi: 10.1016/S0140-6736(09)61965-6 [DOI] [PubMed] [Google Scholar]
- 59. Thompson PD, Panza G, Zaleski A, Taylor B. Statin‐associated side effects. J Am Coll Cardiol. 2016;67:2395–2410. doi: 10.1016/j.jacc.2016.02.071 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Data Availability Statement
The data from National Health and Nutrition Examination Survey (NHANES) are publicly available and can be accessed directly at https://wwwn.cdc.gov/Nchs/Nhanes.


