Abstract
The metabolically active retina obtains essential lipids by endogenous biosynthesis and from the systemic circulation. Clinical studies provide limited and sometimes conflicting evidence as to the relationships between circulating lipid levels and the development and progression of diabetic retinopathy in people with diabetes. Cardiovascular-system-focused clinical trials that also evaluated some retinal outcomes demonstrate the potential protective power of lipid-lowering therapies in diabetic retinopathy and some trials with ocular primary endpoints are in progress. Although triacylglycerol-lowering therapies with fibrates afforded some protection against diabetic retinopathy, the effect was independent of changes in traditional blood lipid classes. While systemic LDL-cholesterol lowering with statins did not afford protection against diabetic retinopathy in most clinical trials, and none of the trials focused on retinopathy as the main outcome, data from very large database studies suggest the possible effectiveness of statins. Potential challenges in these studies are discussed, including lipid-independent effects of fibrates and statins, modified lipoproteins and retinal-specific effects of lipid-lowering drugs. Dysregulation of retinal-specific cholesterol metabolism leading to retinal cholesterol accumulation and potential formation of cholesterol crystals are also addressed.
Keywords: Cholesterol, Cholesterol crystals, Diabetic retinopathy, Dyslipidaemia, Fibrate, Hyperreflective crystalline deposits, Lipids, Review, Statin
Introduction
Hyperglycaemia is a major risk factor for diabetic retinopathy and improving blood glucose control substantially protects against diabetic retinopathy onset and progression in people with type 1 or type 2 diabetes [1, 2]. Furthermore, metabolic memory exists for blood glucose levels and diabetic retinopathy [3–5]. While not as strong or consistent as the links between glucose and diabetic retinopathy, and between lipids and CVD, evidence from clinical studies suggests associations between traditional lipid profiles and diabetic retinopathy [6]. There is also evidence of metabolic memory of some vascular beds for lipids and lipid-lowering drugs [7], likely modulated by epigenetics [8].
In this review we discuss associations between traditional blood lipid levels and diabetic retinopathy, challenges in this research area, and the effects of lipid-lowering drugs on diabetic retinopathy, including a meta-analysis of relevant trials. We then discuss the potential retinal-specific mechanisms of lipid-lowering and of immune cells in diabetic retinopathy, and the role of the bone marrow in vascular repair, before providing a summary and suggesting future directions.
Associations between circulating lipid levels and diabetic retinopathy
Since recognition of the association between circulating lipid levels and retinal hard exudates in the 1950s [9, 10], cross-sectional and longitudinal studies have evaluated associations between traditional lipid classes, total cholesterol, triacylglycerols (TGs), HDL-cholesterol and (usually calculated) LDL-cholesterol, and the novel prothrombotic proatherogenic lipoprotein(a). Some illustrative studies are summarised in Table 1 [11–28]. Associations are not always consistent, possibly relating to differences in study size, diabetes type and duration, definitions of diabetic retinopathy, lipid assays and other factors (discussed next).
Table 1.
Clinical studies demonstrating the diversity of associations between circulating traditional lipid profiles and diabetic retinopathy
| Study | Diabetes type | Type of DR | n | Blood lipids evaluated | Results |
|---|---|---|---|---|---|
| Cross-sectional studies | |||||
| Brown et al [12] | T1D+T2D | Exudative DR | 31 | TC, TG | Increased serum TG in participants with vs without DR |
| Sacks et al [23] | T1D+T2D | DR (PDR, moderate or severe DMO) or ETDRS scale ≥20 | 2535 | TC, TG, LDL-C, HDL-C | Higher TG and lower HDL with vs without DR, but not once adjusted for hypertension and HbA1c |
| Raman et al [22] (SN-DREAMS) | T2D | CSMO, non-CSMO | 1414 | TC, TG, HDL-C, LDL-C | High serum LDL-C, non-HDL-C and HDL-C ratio related to non-CSMO High serum TC related to CSMO |
| Benarous et al [11] | T1D+T2D | NPDR (mild, moderate, severe), PDR, DMO (mild, moderate, CSMO) | 500 | TC, TG, LDL-C, HDL-C, non-HDL-C | Serum lipids independently associated with CSMO only No associations with DR, mild or moderate DMO, or macular thickness |
| Wong et al [26] (MESA) | T1D+T2D | DR, DMO, CSMO STDR |
778 | TC, TG, LDL-C, HDL-C | No associations with any DR, DMO or CSMO |
| Cetin et al [13] | Not specified | NPDR, PDR, DME | 199 | TC, HDL-C, LDL-C, VLDL-C, TG | Serum lipid levels not associated with severity of DR or DMO |
| Tan et al [25] (SEED study) | T2D | DR, severe non-PDR, STDR, DMO, CSMO, | 2877 | TC, LDL-C | Higher TC and LDL-C associated with lower risk of any type of DR |
| Guerci et al [16] | T1D | DR, NPDR, PDR | 341 | Lp(a) | Higher Lp(a) levels were associated with more severe DR, and Lp(a) >300 mg/l (30 mg/dl) was associated with higher PDR |
| Longitudinal studies | |||||
| Dodson and Gibson [15] (7 years) | T2D Hypertension | DR with exudative maculopathy | 52 | TC, HDL-C, HDL2, LDL-C, VLDL-C, TG | Higher HDL2 subfraction with exudative maculopathy |
| Klein etal [18] (WESDR; 30 years) | T1D | PDR, DMO | 903 | TC, HDL-C | Serum lipids not associated with incidence of PDR or macular oedema, nor was statin use |
| Chew et al [14] (ETDRS; 5 years) | T2D+T1D | Hard exudates | 2709 | TC, TG, HDL-C, LDL-C, VLDL-C | High TC, TG and LDL-C associated with higher risk of hard exudates |
| Miljanovic et al [20] (DCCT; 6.5 years) | T1D | CSMO, hard exudate, DR progression, PDR | 1441 | TC, LDL-C, HDL-C, TG, TC / HDL | Higher serum lipids associated with higher risk of CSMO and retinal hard exudates No lipids associated with DR progression or development of PDR after adjustment for HbA1c |
| Klein et al [17] (WESDR substudy; 5 years) | T1D | DR severity, PDR, hard exudate incidence and progression, DMO | 251 | TC/HDL | Univariate analyses: TC/HDL-C was associated with all incident retinal lesions Multivariate analyses: no association was significant |
| Morton et al [21] (ADVANCE trial 5 years) | T2D | New or worsening DR | 11,400 | Baseline HDL-C | HDL-C levels not related to DR |
| Lloyd et al [19] (EDC; 2 years) | T1D | DR (Airlie House Classification) Progression, PDR | 657 | TC, TG, LDL-C, HDL-C | TG and LDL-C predictive DR progression and of development of PDR |
| Singh et al [24] (DiaGene; 6.97 years) | T2D | NPDR, PDR | 1886 | Plasma Lp(a) levels Two SNPs modulating Lp(a) levels |
No association between Lp(a) levels or SNPs with incident or prevalent DR |
| Meta-analyses | |||||
| Yau et al [27] (META-EYE) | T1D+T2D | DR, PDR, STDR, DMO, CSMO | 22,896 | TC | Higher TC associated with higher prevalence of DMO |
| Zhou et al [28] | T1D+T2D | DR | 4366 | TC, TG, LDL-C, HDL-C | TC, TG,HDL-C: no difference betweenDR vs no DR LDL-C: higher in DR vs no DR |
CSMO, clinically significant macular oedema; DR, diabetic retinopathy; EDC (Pittsburgh) Epidemiology of Diabetes Complications; HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol; Lp(a), lipoprotein(a); MESA, Multi-ethnic Study of Atherosclerosis; META-EYE, Meta-Analysis for Eye Disease; NPDR, non-proliferative diabetic retinopathy; SEED, Singapore Epidemiology of Eye Diseases; SN-DREAMS, Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetic Study; T1D, type 1 diabetes; T2D, type 2 diabetes; TC, total cholesterol; WESDR, Wisconsin Epidemiologic Study of Diabetic Retinopathy
Challenges in evaluating the roles of lipids and lipid-lowering drugs in retinopathy
Many factors make it difficult to discern the relative importance of lipids and lipid-lowering drugs in diabetic retinopathy [6]. Likely confounding factors include interactions between lipids, blood glucose levels and other retinopathy risk factors such as obesity, renal dysfunction and smoking. Other challenges are the long duration over which retinopathy develops and the potential for different lipid effects on the different stages (e.g. initiation or progression) of diabetic retinopathy. Differences between studies in the type of diabetes, diabetes duration, retinopathy status at initial visit and duration of follow-up and in the definitions of diabetic retinopathy and methods used to assess it may also contribute. Furthermore, most clinical studies use relatively simple measures of circulating lipoproteins, such as total, LDL-cholesterol and HDL-cholesterol and TG levels for what are complex quantitative and qualitative changes in lipoproteins. Additionally, circulating lipid levels do not best reflect extravasated and modified lipids, such as in the retina, nor intra-retinal lipid metabolism, which may be more pathogenic than unmodified lipoproteins in plasma. Often only a single measure of blood lipid levels is evaluated in relationship to diabetic retinopathy status (or only a few measures) and there may be little consideration of confounding effects such as blood glucose levels, metabolic memory for glucose, lipids and some drugs [7, 8], unmeasured genetics, epigenetic effects and other yet to be determined factors.
Of further importance to understanding the potential role of lipid-lowering drugs in ameliorating diabetic retinopathy is the direct and indirect effects of these drugs. Their pleiotropic effects are common to most lipid-lowering drug classes, in spite of different mechanisms of action and predominant lipid targets. A novel approach to help elucidate the role of lipids in diabetic retinopathy are Mendelian randomisation studies, in which the impact of genes involved in high or low lipid levels are related to the condition of interest [29], and a recent study is described herein.
Effects of lipid-lowering drugs
Lipid-lowering drugs are broadly classified by their predominant lipid target. Unlike CVD, there is a relative paucity of evidence and sometimes inconsistent evidence regarding the effect of lipid-lowering drugs on diabetic retinopathy. Basic science studies can be very helpful in preclinical drug testing and in elucidating mechanisms [30–39] but results do not always translate to the human setting. We now summarise key lipid-lowering drug trials that have evaluated diabetic retinopathy endpoints and highlight evidence gaps.
TG-lowering and LDL-lowering drugs
As yet there are no large placebo-controlled diabetic retinopathy primary endpoint trial results related to the (TG-lowering) fibrates, fish oils or eicosapentaenoic acid (EPA). Apart from some early small intervention studies (1–50 participants) with 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) [40–43], there are no large, randomised controlled trials for (LDL-lowering) drugs with a diabetic retinopathy primary endpoint. The LDL-lowering drug classes of interest are as follows: statins; ezetimibe; resins; proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors; and bempedoic acid (the first in the LDL-lowering drug class of ATP citrate lysase inhibitors, which acts upstream of HMG-CoA reductase) [44].
Fibrates
In an early (1968) trial, a 3 year study of clofibrate in 23 individuals with exudative diabetic retinopathy and 25 control individuals, clofibrate significantly reduced exudate severity but did not improve other retinal vascular lesions or visual acuity; there was no association between exudate severity or improvement and serum lipid levels [45]. Subsequently, there have been much larger RCTs of fenofibrate, though retinopathy was not the main focus of these trials. The peroxisome proliferator-activated receptor alpha (PPARα)-agonist fenofibrate has positive diabetic retinopathy-related findings from two major placebo-controlled RCTs in type 2 diabetes, the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) trial [46] and the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Lipid Eye Trial [47].
The FIELD trial was a cardiovascular trial wherein microvascular complications were not the primary endpoint, thus not all participants had retinal imaging, ocular coherence tomography (OCT) was not used, and the then-standard therapy for sight-threatening diabetic retinopathy (STDR) was retinal laser photocoagulation. In the FIELD trial (n = 9795), comicronised fenofibrate 200 mg once daily for a median of 5 years significantly reduced STDR incidence requiring retinal laser therapy by 31%, with all STDR events being verified by adjudicating ophthalmologists. Similar benefits were seen for proliferative diabetic retinopathy (PDR) and diabetic macular oedema (DMO). In the FIELD ophthalmology substudy (n = 1012), which included serial retinal images, the composite exploratory endpoint (two-step progression of Early Treatment Diabetic Retinopathy Study (ETDRS) severity scale grade, DMO or laser photocoagulation) was significantly reduced by 34% by fenofibrate allocation, with results driven by the pre-existence of diabetic retinopathy [46].
In the ACCORD Eye Lipid trial (n = 1593), simvastatin therapy was combined with fenofibrate 160 mg daily or placebo for a mean of 4 years. Simvastatin with fenofibrate group had significantly reduced diabetic retinopathy progression (by ≥3 ETDRS severity scale steps based on grading of seven-field stereoscopic fundus photo of all participants or need for laser or vitrectomy therapy) by 40% compared with simvastatin with placebo group [47]. Participants were also examined by an ophthalmologist or optometrist at baseline and at 4 years.
In both the FIELD and the ACCORD Eye Lipid trials, results were consistent regarding fenofibrate showing similar magnitude of benefit in diabetic retinopathy which was independent of both baseline lipids and changes in traditional lipid classes and without benefit to visual acuity [46, 47].
Cultured retinal cell and diabetic animal models support potentially protective mechanisms of fibrates being due to a combination of anti-inflammatory effects, suppression of vascular endothelial growth factor (VEGF) via modulation of the PPARα–Wnt–β-catenin signalling pathway, outer blood–retina barrier (BRB) breakdown, neuroprotection and anti-apoptotic effects on retinal microvascular cells [30, 31, 33, 35–39, 48]. Fenofibrate has been shown to reverse the adverse effects of hyperglycaemia-induced metabolic memory in endothelial cells [7, 8] through a sirtuin (silent mating type information regulation 2 homolog) 1 (SIRT1)-dependent mechanism [49]. Benefits of the intraocular administration of fibrate have been demonstrated [31, 50] but as yet there are no human studies using this route. Ocular delivery may increase retinal drug levels, reduce toxic systemic drug levels and side effects and enable treatment in those for whom the systemic drug is contraindicated (e.g. individuals with end stage renal disease). Based on non-primary endpoint retinal outcome data, fenofibrate is approved in 19 (mainly low and low-middle income) countries in which intraocular anti-VEGF agents are less-widely available for use. However, it has not been approved by major regulators (European Medicines Agency [EMA], Food and Drug Administration [FDA]) for type 2 diabetes with diabetic retinopathy.
As yet, there are no completed fibrate trials specifically addressing diabetic retinopathy in type 1 or type 2 diabetes, although several trials with primary endpoints related to diabetic retinopathy are in progress: the Fenofibrate and Microvascular End-points (FAME-1) Eye trial in adults with type 1 diabetes and existent diabetic retinopathy (ETDRS severity scale 35–53) (results anticipated in 3 years) [51]; the Lowering Events in Non-Proliferative Retinopathy in Scotland (LENS) trial in people with type1 and type 2 diabetes [52, 53]; and the Fenofibrate for Prevention of Diabetic Retinopathy Worsening trial in the USA [54]. As well as fenofibrate studies, retinopathy-related data related to other more recently developed fibrates, such as fenofibric acid and pemafibrate, are merited.
Omega-3 fatty acids
Omega-3 fatty acids, found in fish, nuts, fish oils or purified EPA supplements, also lower TGs. There are very limited data relating the effects of fish oils or EPA supplements to diabetic retinopathy. Most studies have focused on CVD, as did the Prevención con Dieta Mediterránea (PREDIMED) study testing Mediterranean diets supplemented with extra virgin olive oil or nuts vs a control diet [55]. In this type 2 diabetes trial (n = 3482), 75% of the participants met the dietary long-chain ω-3 polyunsaturated fatty acid (LCω3PUFA) recommendation (≥500 mg/day) and over 6 years of follow-up there were 69 new-onset STDR cases, with (on adjusted analyses) a 48% reduced risk of incident STDR in those meeting vs. not meeting the dietary target. However, these data are observational and robust clinical trials using omega-3 fatty acid and EPA supplements with detailed primary diabetic retinopathy endpoints are merited.
Statins
HMG-CoA reductase inhibitors (statins) have major primary and secondary cardioprotective effects in diabetes [56] but a meta-analysis of 13 trials (n = 91,140; mean follow-up of 4 years) revealed that statins can significantly increase the risk of new-onset type 2 diabetes (OR 1.09; 95% CI 1.02, 1.17). People with statin-induced new-onset diabetes should be screened for diabetic retinopathy, and the overall cardiovascular and death risk/benefit ratio for most people offered statin therapy remains favourable [57].
Early relatively small statin studies (1–50 participants with diabetes) suggested statin benefit for retinopathy, including protection against late-stage diabetic retinopathy complications and visual acuity loss [23, 41, 58]. In early statin CVD trials, such as the primary CVD prevention Collaborative Atorvastatin Diabetes Study (CARDS) trial in type 2 diabetes [59], no diabetic retinopathy benefit was evident [59]. These relatively small trials of short duration designed for cardiovascular primary endpoints are not conclusive for a condition such as diabetic retinopathy that develops very slowly. Large observational studies, usually with more potent statins, do support some statin benefit for diabetic retinopathy but no consistent improvement in visual acuity [60–65]. Indeed, several studies support different aspects of statin benefit in the development or progression of diabetic retinopathy: the Longitudinal Health Insurance Database in Taiwan adults with type 2 diabetes (n = 37,894) [64]; a Taiwanese population-based cohort (n = 219,359) with type 2 diabetes and dyslipidaemia [65]; a large USA health insurance claims database study of adults with type 2 diabetes (n = 269,782) [61]; and a Japanese prospective clinical practice study of 40- to 75-year-old individuals with type 1 and type 2 diabetes (n = 363 and n = 5489, respectively) [63]. However, not all observational studies evaluating statins and risk of diabetic retinopathy result in positive findings [60].
Potential mechanisms by which statins may reduce retinopathy risk, other than their lipid benefits, include anti-inflammatory, antioxidant, anti-platelet, anti-clotting, vasodilatory, immunomodulatory, cell-signalling, epigenetic and stem-cell effects [66]. Other lipid-lowering drugs, such as the fibrates, have similar pleiotropic effects [37].
Meta-analysis of statin and/or fibrate trials for diabetic retinopathy endpoints
A recent systematic review and meta-analysis evaluated statin and/or fibrate RCTs for prevention and progression of diabetic retinopathy using Cochrane guidelines, the PRISMA Statement and GRADE approach regarding evidence certainty [67]. Included were four fibrate trials, three statin trials and one fibrate plus statin trial, with eight for diabetic retinopathy therapy and four for diabetic retinopathy prevention. Without increased adverse events in 1309 participants, fibrates were associated with a 45% risk reduction of DMO incidence. There were no other positive outcomes. Trial quality was not ranked highly in this meta-analysis [68], nor were 13 trials in a separate similar meta-analysis [67].
Potential retinal-specific mechanisms of lipid lowering in diabetic retinopathy
As described above, lipid-modifying therapies (in particular fenofibrate) may have some protective effects against diabetic retinopathy but existent trials are limited by retinopathy not being the main endpoint. Moreover, the connection between the benefits of lipid-lowering drugs in the retina and the effect of these drugs on traditional blood lipid classes is not established, suggesting that non-traditional lipoprotein-related measures, such as lipoprotein subclasses, modified lipoproteins and/or retinal-specific effects could be at play.
Cholesterol input into retina
The retina is a highly specialised organ in which lipid levels are tightly regulated independently of their systemic levels. Under normal conditions, retinal cholesterol levels are controlled by a balance of input and output mechanisms [69]. Cholesterol input in the retina includes local biosynthesis [69–75] and uptake from the circulation (Fig. 1). Local cholesterol biosynthesis is the primary source of cholesterol in the retina. Most retinal cells express the enzymes involved in cholesterol biosynthesis, with the highest levels found in Muller cells and photoreceptor inner segments (Fig. 1). Unique to the retina are the BRBs separating the neuroretina from the systemic circulation, requiring specialised cholesterol transport mechanisms [70, 71]. The inner BRB is comprised of retinal endothelial cells (RECs) connected by tight junctions and is virtually impermeable to cholesterol under normal conditions (Fig. 1). Retinal pigment epithelium (RPE) cells, also connected by tight junctions, make up the outer BRB (Fig. 1). LDL receptors (LDLRs) and scavenger receptors class B (SR-B) type 1 and type 3 (CD36) [70, 72, 76–81] expressed on the basal membrane of the RPE provide the mechanism for controlled cholesterol-rich lipoprotein particle uptake from the circulation [74, 82] through the outer BRB (Fig. 1). Once in the RPE, cholesterol is transported through the apical membrane by ATP-binding cassette subfamily A member 1 (ABCA1) and ATP-binding cassette subfamily G member 1 (ABCG1) transporters and delivered by lipoproteins to the neural retina [75, 76, 83–86].
Fig. 1.
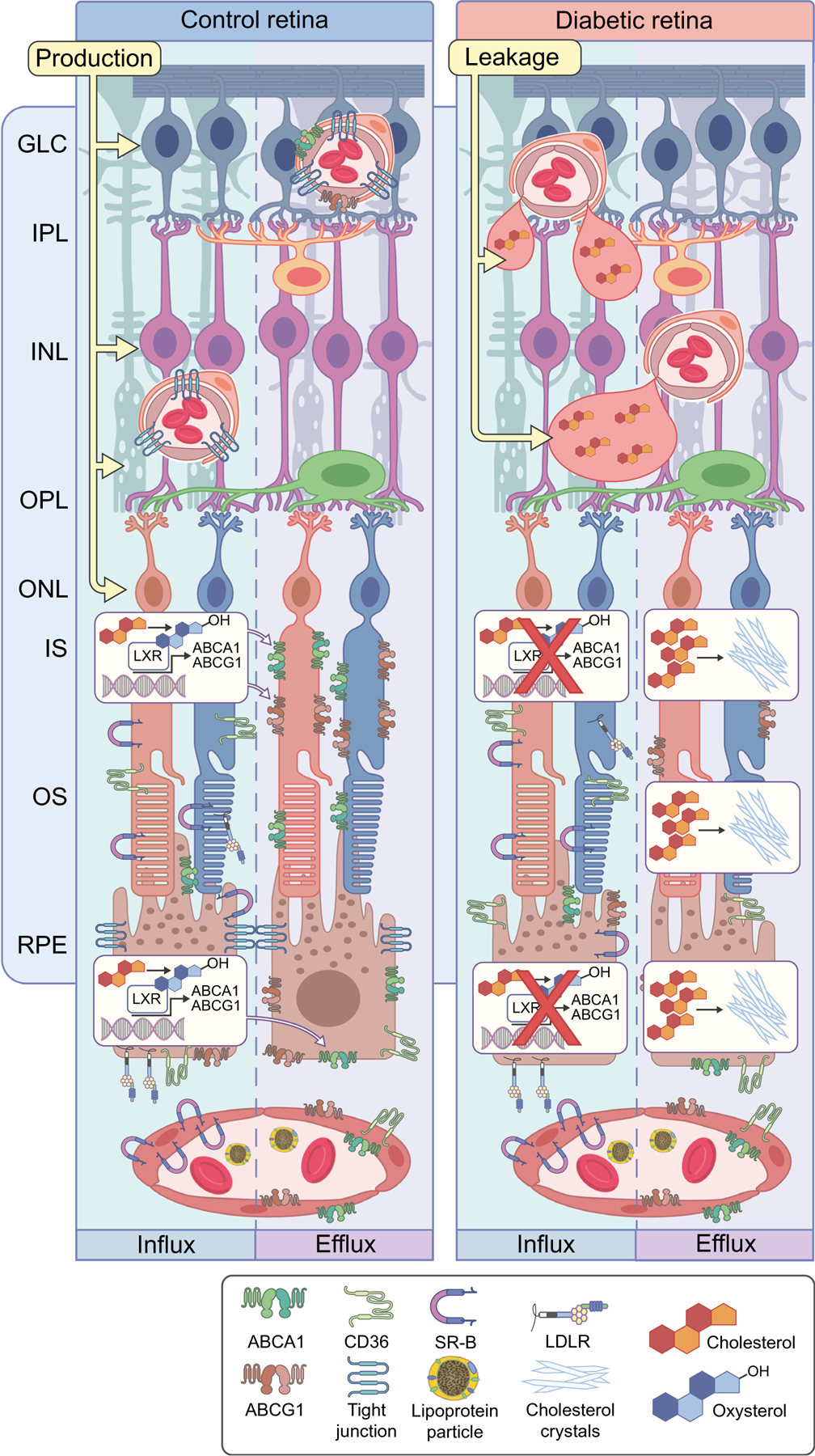
Dysregulation of cholesterol metabolism leading to cholesterol accumulation and formation of hyperreflective crystalline deposits (CCs) in diabetic retina. Cholesterol plays an important role in normal retinal function, and the balance of retinal cholesterol production, uptake by RPE cells and elimination is tightly controlled. At the outer BRB, cholesterol uptake from circulation by RPE cells through CD36, SR-B and LDLR is normally balanced by oxidation to oxysterols, LXR activation and production of ABCA1 and ABCG1, which control cholesterol efflux through RPE cells. Reduced production of oxysterols, decreased LXR expression and activity and thus decreased expression of ABC cassette cholesterol efflux transporters, collectively lead to cholesterol accumulation in the neuroretina. At high concentrations, cholesterol has the propensity to crystalise, forming proinflammatory and pro-atherogenic CCs. The endothelial cells of the inner BRB are not permeable to cholesterol uptake; however, endothelial cells express ABC cassette cholesterol efflux transporters. Inner BRB breakdown in diabetes leads to leaky blood vessels. Coupled with decreased expression of ABC transporters, this can lead to accumulation of cholesterol in the inner retina. GLC, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; IS, photoreceptor inner segment; ONL, outer nuclear layer; OPL, outer plexiform layer; OS, photoreceptor outer segment. This figure is available as part of a downloadable slideset
Cholesterol output from retina
In a healthy retina, cholesterol input is balanced by cholesterol removal. Cholesterol efflux is aided by the ABCA1 and ABCG1 transporters on the RPE cells and endothelial cells, which deliver cholesterol to lipoprotein particles in the choroidal or retinal circulation through a process called reverse cholesterol transport [75, 76, 83–86]. In addition to reverse cholesterol transport, cholesterol is removed from the retina through metabolism to more soluble oxysterols by cytochrome P450s, 27A1 and 46A1 [75, 76, 83–87]. Oxysterols are rapidly diffused into the systemic circulation and are delivered to the liver for conversion to bile acids. Oxysterols are the activating ligands for liver X receptor (LXR).
Dysregulation of retinal cholesterol in diabetes
LXR downregulation
In diabetes, LXR is downregulated leading to dysregulation of retinal cholesterol metabolism and increased proinflammatory signalling. Moreover, there is reduced oxysterol production due to a decrease in cytochrome P450 27A1 and cytochrome P450 46A1 in the diabetic retina, further reducing LXR activity. Diabetes-induced disruption of the LXR signalling and decrease of oxysterol production, result in diminished cholesterol removal and increased cholesterol accumulation in the retina [88–90] (Fig. 1). In addition to cholesterol dyshomeostasis in the diabetic retina, the breakdown of the outer and inner BRBs (Fig. 1) in diabetes further promotes cholesterol accumulation in the retina. Activation of LXR in animal models of diabetes restores cholesterol transport, leads to normalisation of cholesterol homeostasis and repression of inflammatory genes, such as those coding for inducible nitric oxide synthase (iNOS), IL-1β, intercellular adhesion molecule-1 (ICAM-1), and C-C motif chemokine ligand 2 (CCL2) in the retina [89], and prevents the formation of diabetes-induced acellular capillaries.
Cholesterol crystal formation
Disruption in cholesterol homeostasis and metabolism leads to a high cholesterol concentration in the diabetic retina, comparable with the levels that have been previously shown to lead to spontaneous formation of cholesterol crystals (CCs) in atherosclerotic plaques [91–93]. Accumulation of cholesterol in the diabetic retina thus creates a favourable environment for the formation of CCs [94–101] (Fig. 1). Indeed, the crystalline structures consistent with CCs (hyperreflective crystalline deposits) were recently identified by spectral-domain OCT in the retina and choroid of individuals with diabetes [102], age-related macular degeneration [103–105] and Coats disease [106] and are associated with diabetic retinopathy progression. The presence of hyperreflective deposits has been proposed as a novel prognostic biomarker in diabetic retinopathy [102, 106–111]. Although cholesterol is likely to be the main component of crystalline structures in hyperreflective deposits, a positive identification of these deposits cannot be made based on OCT alone. Moreover, CCs are often overlooked in traditional scanning electron microscopy and immunohistochemistry as the ethanol used as a dehydrating agent in standard tissue preparations can dissolve the CCs, masking their presence and their potential involvement in pathogenic mechanisms.
Since CCs are very stable physiologically and are not easily amenable to dissolving in vivo, they become a source of chronic inflammation. CCs are recognised by the innate immune system as foreign bodies because of their shape, firmness and inability to be dissolved [97, 112–123]. CCs can activate all three complement pathways (classical [121], lectin [124] and alternative [115, 123]) in the extracellular space and can activate intracellular complement system [125–128]. Emerging experimental and clinical evidence supports the link between the complement system and diabetic vascular complications, including diabetic retinopathy and atherosclerosis [129–154].
Membrane attack complex is the final step in the activation of complement. Increased membrane attack complex deposition is found in eyes with diabetic retinopathy but not in those without [148] and is believed to be the result of reduced levels of complement regulatory proteins and sustained activation of the alternative complement pathway [147]. Circulating extracellular vesicles, such as exosomes, activate the complement system via the classical pathway and contribute to microvascular damage in diabetic retinopathy [155]. Moreover, genetic association studies have linked complement genes to diabetic retinopathy, as exemplified by the finding that intronic SNP rs2269067 in the C5 gene is a risk factor for PDR in the Chinese Han population [139]. CCs were shown to activate the complement factor 5a (C5a) and C5a receptor (C5aR) pathways leading to the upregulation of complement receptor 3 (CR3) [121]. CR3 (CD11b/CD18), a β2 integrin, is a highly versatile pattern-recognition receptor that activates leucocytes, mediates phagocytosis and promotes leucocyte transmigration [156]. CR3 and C5aR link CC to leucocyte activation [157]. CC-induced cytokine/chemokine production occurs through interaction of C5a with C5aR [157].
Importantly, CCs also induce inflammation via the NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome, which mediates the formation of activated IL-1β [97, 158], a key inflammatory mediator in diabetic retinopathy. HDL, the only known element in the body that can dissolve CCs [96] is often reduced and/or dysfunctional in diabetes and has poor accessibility to deep tissue sites such as the retina. Thus, diabetic individuals are particularly at risk for developing CCs.
Potential immune cell-specific mechanisms of lipid lowering in diabetic retinopathy
In addition to retinal-specific effects described above, lipid-lowering drugs can affect the retina through specific effects on immune cells. Lipid dysregulation can contribute to low-grade chronic inflammation [159, 160] and VEGF receptor 2 (VEGFR2) activation, resulting in increased retinal endothelial permeability and cell injury [161].
Support for the modulation of immune cell behaviour in the prevention of atheromatous disease exists; however, for diabetic retinopathy evidence is lacking. Statins have a major primary and secondary cardioprotective effect in diabetes [56], yet diabetic individuals exhibit impaired plaque regression [162], possibly reflecting differences in statin effects on CCs by diabetes status. Clinical studies provide support for the inflammatory hypothesis of atherosclerosis [163], including the recent Canakinumab Anti-Inflammatory Thrombosis Outcome Study (CANTOS) showing that controlling vascular inflammation independent of lipid lowering could lower the rates of recurrent cardiovascular events [164]. However, none of these trials had diabetic retinopathy progression as an endpoint and thus assessment of the impact of controlling inflammation on diabetic retinopathy is lacking.
Hypercholesterolaemia increases the levels of circulating CCs [99, 165, 166]. As described above, CCs can activate the innate immune system [167] and can trigger the NLRP3 inflammasome [168]. CCs, when interacting with innate immune receptors, can enhance tissue damage and initiate inflammatory responses though secretion of IL-1 and IL-18 [169–171]. Hypercholesterolaemia is associated with increased proliferation and mobilisation of haematopoietic stem cells (HSCs) [172] and can directly regulate the function of HSCs through epigenetic reprogramming. This can occur through the upregulation or downregulation of different epigenetic enzymes (such as histone demethylases and methylases) or the upregulation of the granulocyte-macrophage colony stimulating factor (GM-CSF) receptor. GM-CSF receptor upregulation results in the differentiation of HSCs towards the monocyte lineage [173, 174] (Fig. 2). Inflammasome activation promotes neutrophil recruitment and neutrophil extracellular trap formation (NETosis) (Fig. 2) in atherosclerotic plaques, leading to the development of advanced atherosclerotic lesions [175]. Neutrophil expansion, activation and mobilisation from the bone marrow is the result of hypercholesterolaemia [176, 177]. Yet only in preclinical studies has the impact of changes in the bone marrow been examined regarding diabetic retinopathy [178–180].
Fig. 2.
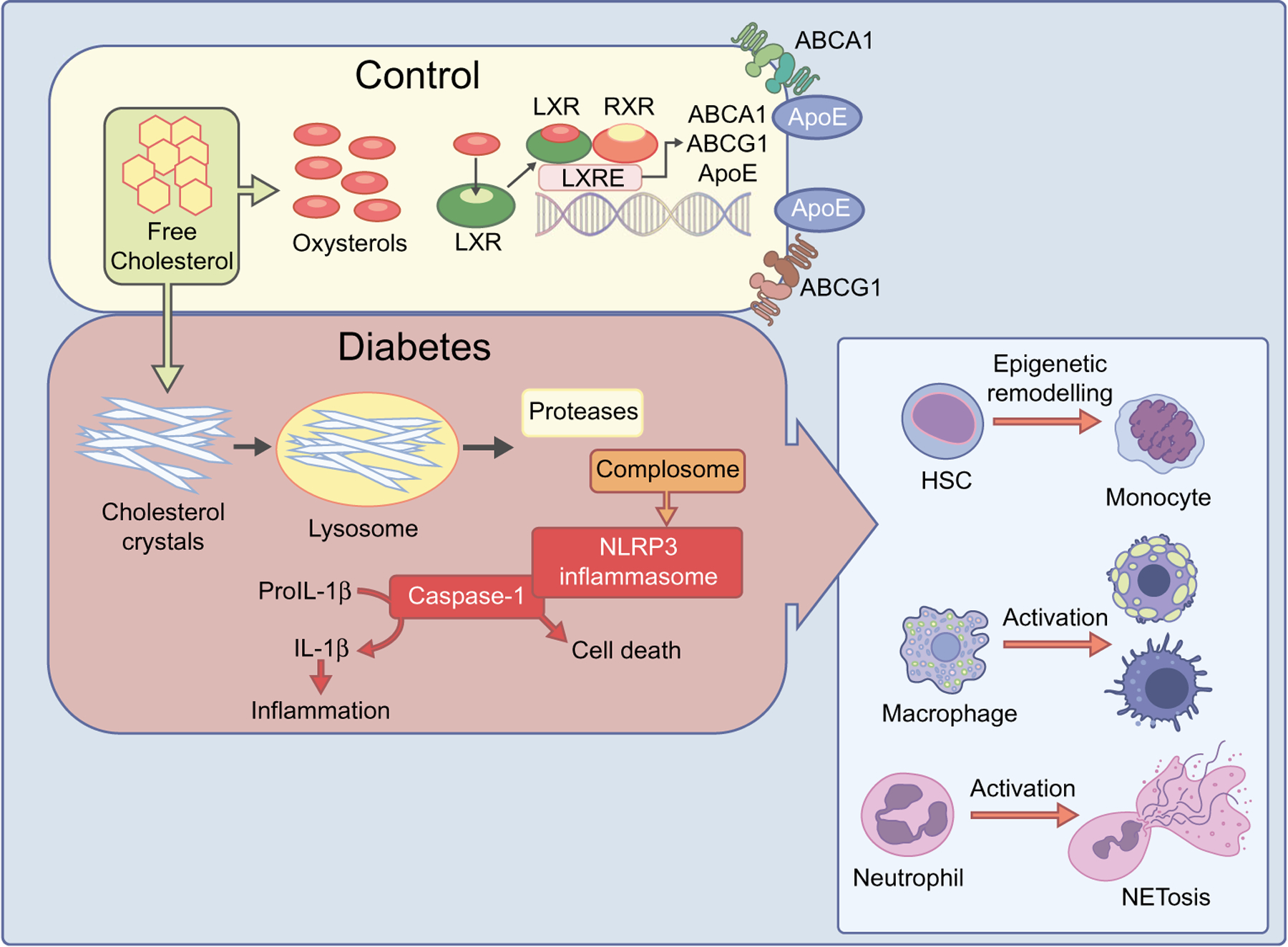
Dysregulation of cholesterol metabolism in diabetes and immune system. In a healthy retina (control), oxidation of cholesterol to oxysterols, LXR activation and production of ABCA1 and ABCG1 maintains cholesterol homeostasis. However, in diabetes, the disruption of these processes can lead to hypercholesterolaemia and CC formation, which can activate the innate immune system and trigger the NLRP3 inflammasome. CCs, when interacting with innate immune receptors, can enhance tissue damage and initiate inflammatory responses though secretion of IL-1β and inflammation. This can lead to HSC epigenetic reprogramming resulting in the differentiation of HSC towards the monocyte lineage, neutrophil activation and NETosis, and macrophage activation and foam cell formation. ApoE, apolipoprotein E. This figure is available as part of a downloadable slideset
LXR is a critical modulator of cholesterol metabolism (Figs 2, 3). Its activation is a key cellular pathway regulating intracellular cholesterol and plays an important role in inflammation and disease pathogenesis [181]. LXR is a major regulator of ABCA1 and ABG1 transporter expression (Figs 2, 3). In addition to its role in cholesterol removal, LXR has pronounced anti-inflammatory properties through the inhibition of NF-κB signalling (Fig. 3). Since diabetes is associated with chronic systemic inflammation, these clinical findings support the notion that activating Toll-like receptors (TLRs) can worsen cholesterol homeostasis. Thus, bacterial and viral particles that activate TLR3/4 profoundly inhibit both the expression of LXR target genes and the ability of macrophages to efflux excess cholesterol [182]. The activation of TLRs 3/4 suppresses the activity of LXR on corresponding genes in macrophages, thus reducing cholesterol efflux and contributing to hypercholesterolaemia-related inflammation [182]. The effect of TLRs occurs through the viral response transcription factor interferon regulatory factor 3 (IRF3). Expression and activation of IRF3 in macrophages is necessary and sufficient to block the transcriptional activity of LXR on the ABCA1 promoter and to inhibit cholesterol efflux from macrophages [182]. These findings provide an unexpected mechanism whereby microbial infection may contribute to cardiometabolic disease, such as type 2 diabetes, by interference with LXR-dependent cholesterol metabolism.
Fig. 3.
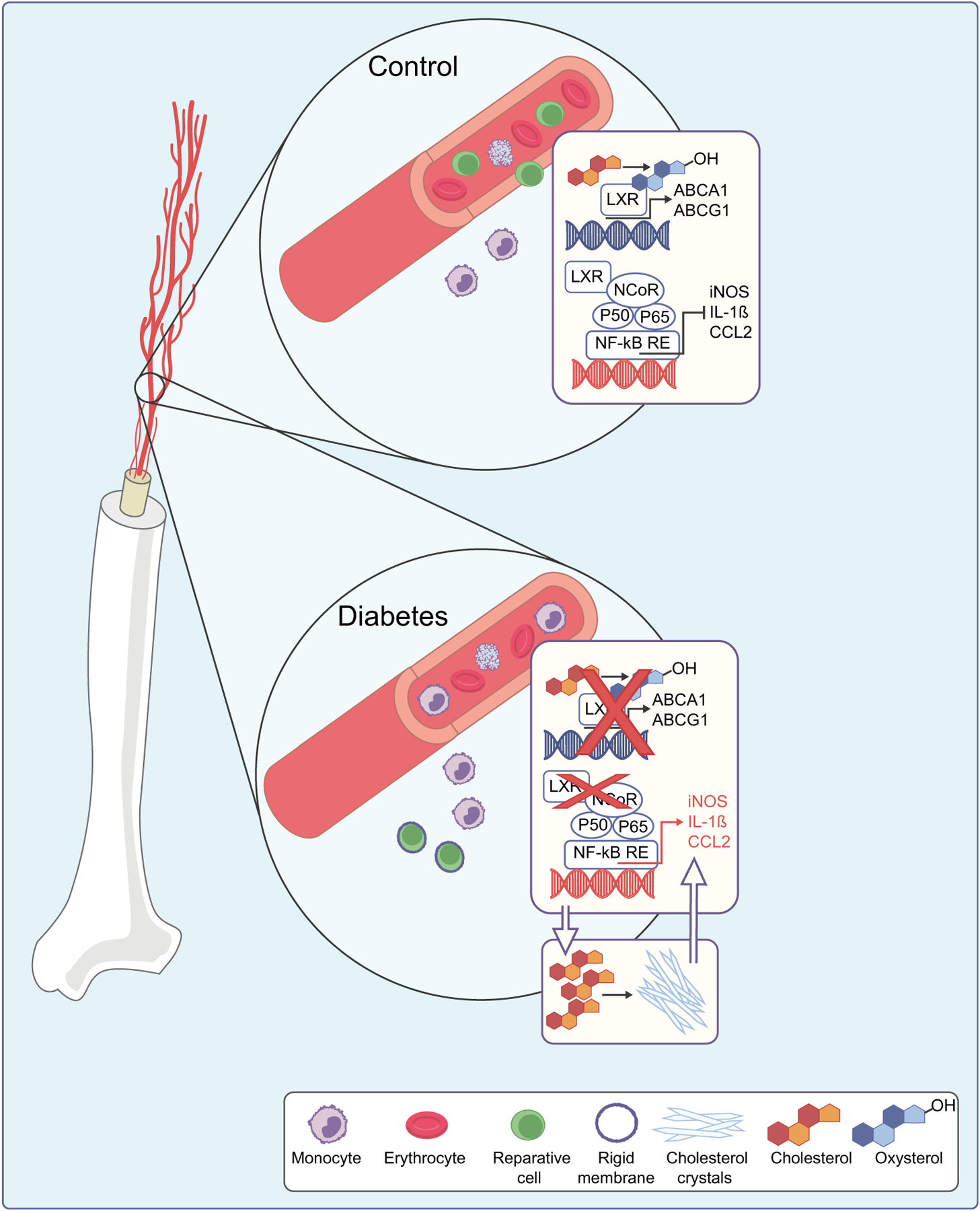
Dysregulation of cholesterol metabolism in diabetes in bone marrow-derived cells. In control cells, LXR activation leads to increased expression of cholesterol efflux transporters ABCA1 and ABCG1, and inhibition of expression of NF-κB-controlled inflammatory factors. Inhibition of LXR in diabetes leads to cholesterol accumulation, CC formation and increased inflammation. Increased membrane rigidity of reparative cells due to cholesterol accumulation further exacerbates diabetes-induced damage. NCoR, nuclear receptor corepressor; RE, response element. This figure is available as part of a downloadable slideset
Macrophage LXR signalling pathway is an important determinant of vascular damage including in the retina [89, 90, 180, 183] as LXR interferes with the action of NF-κβ on the promoters of inflammatory genes. LXR thus serves to integrate lipid metabolic and innate immune functions in macrophages (Figs 2, 3).
The diabetic bone marrow and vascular reparative dysfunction
Low-level chronic inflammation as seen in type 2 diabetes can alter the homeostatic balance of most tissues, including the bone marrow microenvironment [184]. Myeloidosis, defined as an increase in the proportion of myeloid-derived leucocytes, is a common feature in diabetic bone marrow and promotes the systemic inflammatory phenotype [185]. Bone marrow supernatant fractions from diabetic mice displayed significant increases in the levels of secreted proinflammatory proteins, including TNF-α, IL-3 and CCL-2, promoting differentiation towards myeloid cells. Bone marrow is also the source of vascular reparative cells, which are called myeloid angiogenic cells. In disease states such as diabetes and CVD myeloid angiogenic cells are reduced in number and function, thus leading to compromised endothelial recovery [186–188].
The molecular defect in these cells results in their compromised function [180, 189–194]. Migration of myeloid angiogenic cells is impacted by cell rigidity, which is markedly increased in the cells of diabetic individuals (Fig. 3). In diabetic dyslipidaemia, cholesterol accumulates in the plasma membrane, decreasing fluidity and thereby suppressing the ability of cells to transduce ligand-activated signalling pathways. N,N-dimethyl-3β-hydroxy-cholenamide (DMHCA), a selective LXR agonist, specifically activates the cholesterol efflux arm of the LXR pathway with minimal stimulation of TG synthesis by the liver. DMHCA rejuvenates membrane fluidity in myeloid angiogenic cells and reduces the proinflammatory microenvironment of the diabetic bone marrow. Using single-cell RNA sequencing on HSCs from untreated and DMHCA-treated diabetic mice, it was found that DMHCA increased the expression of several LXR target genes, confirming that DMHCA directly modulates these primitive cells. An increase in immediate early gene expression in HSCs was also observed, as the DMHCA-enhanced formation of membrane microdomains amplifies the transduction of intercellular signalling. Together, these findings suggest that DMHCA treatment normalises the bone marrow microenvironment. Thus, through modulation of LXR and other key signalling pathways, cholesterol homeostasis plays a critical role in immune function and in vascular repair. Loss of the function of bone marrow-derived myeloid angiogenic cells can lead to inadequate repair of injured retinal capillaries, leading to vasodegeneration and development of diabetic retinopathy [195–199]. One area of ongoing research involves pharmacological strategies that normalise cholesterol synthesis and membrane fluidity of myeloid angiogenic cells to improve diabetic retinopathy outcomes.
Summary and future directions
Lipids are implicated in the pathogenesis of diabetic vascular complications, including retinopathy; lipid-lowering drugs may be protective, though large robust clinical trials and observational studies with detailed eye-related primary endpoints and lipid and lipoprotein-related measures are still merited. Studies with more recently available lipid-modulating drugs and of ocular drug delivery are also of interest. Usually, two or more trials with positive results will be required to change clinical practice and gain regulatory approval and, importantly, such treatments must then be translated into clinical practice. Recognition of effects specific to bone marrow, immune cells and the retina could help design more specific treatments. Further understanding of the accumulation of CCs and hyperreflective crystalline deposits generation in the diabetic retina as well as circulating cells could result in a novel prognostic biomarker and a novel therapeutic target in diabetic retinopathy.
Supplementary Material
Authors’ relationships and activities
AJJ is an investigator on the FIELD trial (post completion of its intervention stage), FAME-1 Eye and DCCT/EDIC trials and collaborates with Prof Emily Chew of the NEI on an unrelated study. The FAME-1 Eye trial, of which she is Co-Study Chairperson has been funded by the NHMRC (Australia), JDRF Australia and Abbott Europe for the investigator-initiated FAME-1 Eye trial. FIELD related biomarkers studies are funded by NHMRC Australia. Unrelated to this work AJJ is an Honorary Board Member of the International Diabetes Federation Western Pacific Region Executive Council, of the NGO Insulin For Life and an Honorary Member of the Diabetes Australia Research Advisory Council. Unrelated to this work she is an Advisory Board member for Abbott Diabetes, Medtronic, Sanofi-Aventis and Amgen. Unrelated to this work she has received peer-reviewed funding from the NHRMC, Medical Research Future Fund, JDRF International, JDRF Australia, Diabetes Australia Research Programme and investigator-initiated funding from Medtronic and Sanofi-Aventis. JVB is an Associate Editor of Diabetologia and consultant of Ceramedix Inc.
Abbreviations
- ABCA1
ATP-binding cassette subfamily A member 1
- ABCG1
ATP-binding cassette subfamily G member 1
- ACCORD
Action to Control Cardiovascular Risk in Diabetes
- BRB
Blood–retina barrier
- CC
Cholesterol crystal
- C5a
Complement factor 5a
- C5aR
Complement factor 5a receptor
- CR3
Complement receptor 3
- DMHCA
N,N-dimethyl-3β-hydroxy-cholenamide
- DMO
Diabetic macular oedema
- EPA
Eicosapentaenoic acid
- ETDRS
Early Treatment Diabetic Retinopathy Study
- FIELD
Fenofibrate Intervention and Event Lowering in Diabetes
- GM-CSF
Granulocyte-macrophage colony stimulating factor
- HMG-CoA
3-hydroxy-3-methylglutaryl coenzyme A
- HSC
Haematopoietic stem cell
- IRF3
Interferon regulatory factor 3
- LDLR
LDL receptor
- LENS
Lowering Events in Non-Proliferative Retinopathy in Scotland
- LXR
Liver X receptor
- NETosis
Neutrophil extracellular trap formation
- NLRP3
NOD-like receptor family pyrin domain containing 3
- OCT
Ocular coherence tomography
- PDR
Proliferative diabetic retinopathy
- PPARα
Peroxisome proliferator-activated receptor alpha
- REC
Retinal endothelial cell
- RPE
Retinal pigment endothelium
- SR-B
Scavenger receptor class b
- STDR
Sight-threatening diabetic retinopathy
- TLR
Toll-like receptors
- TG
Triacylglycerol
- VEGF
Vascular endothelial growth factor
Footnotes
Supplementary Information The online version contains a slideset of the figures for download, which is available to authorised users, available at https://doi.org/10.1007/s00125-022-05655-z.
References
- 1.The Diabetes Control Complications Trial Research Group, Nathan DM, Genuth S et al. (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329 (14):977–986. 10.1056/NEJM199309303291401 [DOI] [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study Group (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK prospective diabetes study (UKPDS) group. Lancet 352(9131):837–853. 10.1016/S0140-6736(98)07019-6 [DOI] [PubMed] [Google Scholar]
- 3.Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group, Lachin JM, White NH et al. (2015) Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow-up in the DCCT/EDIC. Diabetes 64(2):631–642. 10.2337/db14-0930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA (2008) 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 359(15):1577–1589. 10.1056/NEJMoa0806470 [DOI] [PubMed] [Google Scholar]
- 5.White NH, Sun W, Cleary PA et al. (2008) Prolonged effect of intensive therapy on the risk of retinopathy complications in patients with type 1 diabetes mellitus: 10 years after the diabetes control and complications trial. Arch Ophthalmol 126(12):1707–1715. 10.1001/archopht.126.12.1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jenkins AJ, Joglekar MV, Hardikar AA, Keech AC, O’Neal DN, Januszewski AS (2015) Biomarkers in diabetic retinopathy. Rev Diabet Stud 12(1–2):159–195. 10.1900/RDS.2015.12.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jermendy G (2012) Vascular memory: can we broaden the concept of the metabolic memory? Cardiovasc Diabetol 11:44. 10.1186/1475-2840-11-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy MA, Zhang E, Natarajan R (2015) Epigenetic mechanisms in diabetic complications and metabolic memory. Diabetologia 58(3):443–455. 10.1007/s00125-014-3462-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keiding NR, Mann GV, Root HF, Lawry EY, Marble A (1952) Serum lipoproteins and cholesterol levels in normal subjects and in young patients with diabetes in relation to vascular complications. Diabetes 1(6):434–440. 10.2337/diab.1.6.434 [DOI] [PubMed] [Google Scholar]
- 10.Kissebah AH, Kohner EM, Lewis B, Siddiq YK, Lowy C, Fraser TR (1975) Plasma-lipids and glucose/insulin relationship in non-insulin-requiring diabetics with and without retinopathy. Lancet 1(7916):1104–1108. 10.1016/s0140-6736(75)92497-6 [DOI] [PubMed] [Google Scholar]
- 11.Benarous R, Sasongko MB, Qureshi S et al. (2011) Differential association of serum lipids with diabetic retinopathy and diabetic macular edema. Invest Ophthalmol Vis Sci 52(10):7464–7469. 10.1167/iovs.11-7598 [DOI] [PubMed] [Google Scholar]
- 12.Brown GC, Ridley M, Haas D, Lucier AC, Sarin LK (1984) Lipemic diabetic retinopathy. Ophthalmology 91(12):1490–1495. 10.1016/s0161-6420(84)34098-2 [DOI] [PubMed] [Google Scholar]
- 13.Cetin EN, Bulgu Y, Ozdemir S et al. (2013) Association of serum lipid levels with diabetic retinopathy. Int J Ophthalmol 6(3):346–349. 10.3980/j.issn.2222-3959.2013.03.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chew EY, Klein ML, Ferris FL 3rd et al. (1996) Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy. Early treatment diabetic retinopathy study (ETDRS) report 22. Arch Ophthalmol 114(9):1079–1084. 10.1001/archopht.1996.01100140281004 [DOI] [PubMed] [Google Scholar]
- 15.Dodson PM, Gibson JM (1991) Long-term follow-up of and underlying medical conditions in patients with diabetic exudative maculopathy. Eye (Lond) 5(Pt 6):699–703. 10.1038/eye.1991.128 [DOI] [PubMed] [Google Scholar]
- 16.Guerci B, Meyer L, Sommer S et al. (1999) Severity of diabetic retinopathy is linked to lipoprotein (a) in type 1 diabetic patients. Diabetes Metab 25(5):412–418 [PubMed] [Google Scholar]
- 17.Klein BE, Klein R, Moss SE (1999) Is serum cholesterol associated with progression of diabetic retinopathy or macular edema in persons with younger-onset diabetes of long duration? Am J Ophthalmol 128(5):652–654. 10.1016/s0002-9394(99)00222-6 [DOI] [PubMed] [Google Scholar]
- 18.Klein BE, Myers CE, Howard KP, Klein R (2015) Serum lipids and proliferative diabetic retinopathy and macular edema in persons with long-term type 1 diabetes mellitus: the Wisconsin epidemiologic study of diabetic retinopathy. JAMA Ophthalmol 133(5):503–510. 10.1001/jamaophthalmol.2014.5108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lloyd CE, Klein R, Maser RE, Kuller LH, Becker DJ, Orchard TJ (1995) The progression of retinopathy over 2 years: the Pittsburgh epidemiology of diabetes complications (EDC) study. J Diabetes Complicat 9(3):140–148. 10.1016/1056-8727(94)00039-q [DOI] [PubMed] [Google Scholar]
- 20.Miljanovic B, Glynn RJ, Nathan DM, Manson JE, Schaumberg DA (2004) A prospective study of serum lipids and risk of diabetic macular edema in type 1 diabetes. Diabetes 53(11):2883–2892. 10.2337/diabetes.53.11.2883 [DOI] [PubMed] [Google Scholar]
- 21.Morton J, Zoungas S, Li Q et al. (2012) Low HDL cholesterol and the risk of diabetic nephropathy and retinopathy: results of the ADVANCE study. Diabetes Care 35(11):2201–2206. 10.2337/dc12-0306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raman R, Rani PK, Kulothungan V, Rachepalle SR, Kumaramanickavel G, Sharma T (2010) Influence of serum lipids on clinically significant versus nonclinically significant macular edema: SN-DREAMS report number 13. Ophthalmology 117(4): 766–772. 10.1016/j.ophtha.2009.09.005 [DOI] [PubMed] [Google Scholar]
- 23.Sacks FM, Hermans MP, Fioretto P et al. (2014) Association between plasma triglycerides and high-density lipoprotein cholesterol and microvascular kidney disease and retinopathy in type 2 diabetes mellitus: a global case-control study in 13 countries. Circulation 129(9):999–1008. 10.1161/CIRCULATIONAHA.113.002529 [DOI] [PubMed] [Google Scholar]
- 24.Singh SS, Rashid M, Lieverse AG et al. (2020) Lipoprotein(a) plasma levels are not associated with incident microvascular complications in type 2 diabetes mellitus. Diabetologia 63(6): 1248–1257. 10.1007/s00125-020-05120-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan GS, Gan A, Sabanayagam C et al. (2018) Ethnic differences in the prevalence and risk factors of diabetic retinopathy: the Singapore epidemiology of eye diseases study. Ophthalmology 125(4):529–536. 10.1016/j.ophtha.2017.10.026 [DOI] [PubMed] [Google Scholar]
- 26.Wong TY, Klein R, Islam FM et al. (2006) Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol 141(3):446–455. 10.1016/j.ajo.2005.08.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yau JW, Rogers SL, Kawasaki R et al. (2012) Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35(3):556–564. 10.2337/dc11-1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Y, Wang C, Shi K, Yin X (2018) Relationship between dyslipidemia and diabetic retinopathy: a systematic review and meta-analysis. Medicine (Baltimore) 97(36):e12283. 10.1097/MD.0000000000012283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haase CL, Tybjaerg-Hansen A, Nordestgaard BG, Frikke-Schmidt R (2015) HDL cholesterol and risk of type 2 diabetes: a Mendelian randomization study. Diabetes 64(9):3328–3333. 10.2337/db14-1603 [DOI] [PubMed] [Google Scholar]
- 30.Al-Shabrawey M, Bartoli M, El-Remessy AB et al. (2008) Role of NADPH oxidase and Stat3 in statin-mediated protection against diabetic retinopathy. Invest Ophthalmol Vis Sci 49(7):3231–3238. 10.1167/iovs.08-1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Q, Qiu F, Zhou K et al. (2017) Pathogenic role of microRNA-21 in diabetic retinopathy through downregulation of PPARalpha. Diabetes 66(6):1671–1682. 10.2337/db16-1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, Hu Y, Lin M et al. (2013) Therapeutic effects of PPARalpha agonists on diabetic retinopathy in type 1 diabetes models. Diabetes 62(1):261–272. 10.2337/db11-0413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deng G, Moran EP, Cheng R et al. (2017) Therapeutic effects of a novel agonist of peroxisome proliferator-activated receptor alpha for the treatment of diabetic retinopathy. Invest Ophthalmol Vis Sci 58(12):5030–5042. 10.1167/iovs.16-21402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding L, Cheng R, Hu Y et al. (2014) Peroxisome proliferator-activated receptor alpha protects capillary pericytes in the retina. Am J Pathol 184(10):2709–2720. 10.1016/j.ajpath.2014.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Q, Zhang F, Zhang X et al. (2018) Fenofibrate ameliorates diabetic retinopathy by modulating Nrf2 signaling and NLRP3 inflammasome activation. Mol Cell Biochem 445(1–2):105–115. 10.1007/s11010-017-3256-x [DOI] [PubMed] [Google Scholar]
- 36.Liu Q, Zhang X, Cheng R, Ma JX, Yi J, Li J (2019) Salutary effect of fenofibrate on type 1 diabetic retinopathy via inhibiting oxidative stress-mediated Wnt/beta-catenin pathway activation. Cell Tissue Res 376(2):165–177. 10.1007/s00441-018-2974-z [DOI] [PubMed] [Google Scholar]
- 37.Noonan JE, Jenkins AJ, Ma JX, Keech AC, Wang JJ, Lamoureux EL (2013) An update on the molecular actions of fenofibrate and its clinical effects on diabetic retinopathy and other microvascular end points in patients with diabetes. Diabetes 62(12):3968–3975. 10.2337/db13-0800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pearsall EA, Cheng R, Matsuzaki S et al. (2019) Neuroprotective effects of PPARalpha in retinopathy of type 1 diabetes. PLoS One 14(2):e0208399. 10.1371/journal.pone.0208399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng Z, Chen H, Wang H et al. (2010) Improvement of retinal vascular injury in diabetic rats by statins is associated with the inhibition of mitochondrial reactive oxygen species pathway mediated by peroxisome proliferator-activated receptor gamma coactivator 1alpha. Diabetes 59(9):2315–2325. 10.2337/db10-0638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banerjee S, Denniston AK, Gibson JM, Dodson PM (2004) Does cardiovascular therapy affect the onset and recurrence of preretinal and vitreous haemorrhage in diabetic eye disease? Eye (Lond) 18(8):821–825. 10.1038/sj.eye.6701338 [DOI] [PubMed] [Google Scholar]
- 41.Gordon B, Chang S, Kavanagh M et al. (1991) The effects of lipid lowering on diabetic retinopathy. Am J Ophthalmol 112(4):385–391. 10.1016/s0002-9394(14)76244-0 [DOI] [PubMed] [Google Scholar]
- 42.Gupta A, Gupta V, Thapar S, Bhansali A (2004) Lipid-lowering drug atorvastatin as an adjunct in the management of diabetic macular edema. Am J Ophthalmol 137(4):675–682. 10.1016/j.ajo.2003.11.017 [DOI] [PubMed] [Google Scholar]
- 43.Sen K, Misra A, Kumar A, Pandey RM (2002) Simvastatin retards progression of retinopathy in diabetic patients with hypercholesterolemia. Diabetes Res Clin Pract 56(1):1–11. 10.1016/s0168-8227(01)00341-2 [DOI] [PubMed] [Google Scholar]
- 44.Burke AC, Telford DE, Huff MW (2019) Bempedoic acid: effects on lipoprotein metabolism and atherosclerosis. Curr Opin Lipidol 30(1):1–9. 10.1097/MOL.0000000000000565 [DOI] [PubMed] [Google Scholar]
- 45.Duncan LJ, Cullen JF, Ireland JT, Nolan J, Clarke BF, Oliver MF (1968) A three-year trial of atromid therapy in exudative diabetic retinopathy. Diabetes 17(7):458–467. 10.2337/diab.17.7.458 [DOI] [PubMed] [Google Scholar]
- 46.Keech AC, Mitchell P, Summanen PA et al. (2007) Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet 370(9600): 1687–1697. 10.1016/S0140-6736(07)61607-9 [DOI] [PubMed] [Google Scholar]
- 47.Accord Study Group, Accord Eye Study Group, Chew EY et al. (2010) Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med 363(3):233–244. 10.1056/NEJMoa1001288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fu D, Yu JY, Connell AR, Hookham MB, McLeese RH, Lyons TJ (2020) Effects of modified low-density lipoproteins and Fenofibrate on an outer blood-retina barrier model: implications for diabetic retinopathy. J Ocul Pharmacol Ther 36(10):754–764. 10.1089/jop.2020.0068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao S, Li J, Wang N et al. (2015) Feno fi brate suppresses cellular metabolic memory of high glucose in diabetic retinopathy via a sirtuin 1-dependent signalling pathway. Mol Med Rep 12(4): 6112–6118. 10.3892/mmr.2015.4164 [DOI] [PubMed] [Google Scholar]
- 50.Qiu F, Meng T, Chen Q et al. (2019) Fenofibrate-loaded biodegradable nanoparticles for the treatment of experimental diabetic retinopathy and Neovascular age-related macular degeneration. Mol Pharm 16(5):1958–1970. 10.1021/acs.molpharmaceut.8b01319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.University of Sydney. The Fenofibrate And Microvascular Events in Type 1 Diabetes Eye. (FAME 1 EYE). Identifier: NCT01320345. Available from https://clinicaltrials.gov/ct2/show/NCT01320345. Accessed June 26 2021
- 52.University of Oxford. LENS trial. Identifier: NCT03439345. Available from https://clinicaltrials.gov/ct2/show/NCT03439345. Accessed June 26 2021
- 53.University of Oxford, University of Glasgow. The LENS trial. Available from https://www.ctsu.ox.ac.uk/lens. Accessed June 26 2021
- 54.Jaeb Center for Health Research. Fenofibrate for Prevention of Diabetic Retinopathy Worsening. Identifier: NCT04661358. Available from https://clinicaltrials.gov/ct2/show/NCT04661358. Accessed June 26 2021
- 55.Sala-Vila A, Diaz-Lopez A, Valls-Pedret C et al. (2016) Dietary marine omega-3 fatty acids and incident sight-threatening retinopathy in middle-aged and older individuals with type 2 diabetes: prospective investigation from the PREDIMED trial. JAMA Ophthalmol 134(10):1142–1149. 10.1001/jamaophthalmol.2016.2906 [DOI] [PubMed] [Google Scholar]
- 56.Cholesterol Treatment Trialists’ (CTT) Collaborators, Kearney PM, Blackwell L et al. (2008) Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 371(9607):117–125. 10.1016/S0140-6736(08)60104-X [DOI] [PubMed] [Google Scholar]
- 57.Sattar N, Preiss D, Murray HM et al. (2010) Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 375(9716):735–742. 10.1016/S0140-6736(09)61965-6 [DOI] [PubMed] [Google Scholar]
- 58.The Diabetes Atorvastatin Lipid Intervention Study Group (2001) The effect of aggressive versus standard lipid lowering by atorvastatin on diabetic dyslipidemia: the DALI study: a double-blind, randomized, placebo-controlled trial in patients with type 2 diabetes and diabetic dyslipidemia. Diabetes Care 24(8):1335–1341. 10.2337/diacare.24.8.1335 [DOI] [PubMed] [Google Scholar]
- 59.Colhoun HM, Betteridge DJ, Durrington PN et al. (2004) Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the collaborative atorvastatin diabetes study (CARDS): multicentre randomised placebo-controlled trial. Lancet 364(9435):685–696. 10.1016/S0140-6736(04)16895-5 [DOI] [PubMed] [Google Scholar]
- 60.Zhang J, McGwin G Jr (2007) Association of statin use with the risk of developing diabetic retinopathy. Arch Ophthalmol 125(8): 1096–1099. 10.1001/archopht.125.8.1096 [DOI] [PubMed] [Google Scholar]
- 61.Vail D, Callaway NF, Ludwig CA, Saroj N, Moshfeghi DM (2019) Lipid-lowering medications are associated with lower risk of retinopathy and ophthalmic interventions among United States patients with diabetes. Am J Ophthalmol 207:378–384. 10.1016/j.ajo.2019.05.029 [DOI] [PubMed] [Google Scholar]
- 62.Tuuminen R, Loukovaara S (2016) Statin medication in patients with epiretinal membrane is associated with low intravitreal EPO, TGF-beta-1, and VEGF levels. Clin Ophthalmol 10:921–928. 10.2147/OPTH.S105686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kawasaki R, Kitano S, Sato Y et al. (2019) Factors associated with non-proliferative diabetic retinopathy in patients with type 1 and type 2 diabetes: the Japan diabetes complication and its prevention prospective study (JDCP study 4). Diabetol Int 10(1):3–11. 10.1007/s13340-018-0357-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang EY, Chen TH, Garg SJ et al. (2019) Association of Statin Therapy with Prevention of vision-threatening diabetic retinopathy. JAMA Ophthalmol 137(4):363–371. 10.1001/jamaophthalmol.2018.6399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jeng CJ, Hsieh YT, Yang CM, Yang CH, Lin CL, Wang IJ (2018) Diabetic retinopathy in patients with dyslipidemia: development and progression. Ophthalmol Retina 2(1):38–45. 10.1016/j.oret.2017.05.010 [DOI] [PubMed] [Google Scholar]
- 66.Danesh FR, Kanwar YS (2004) Modulatory effects of HMG-CoA reductase inhibitors in diabetic microangiopathy. FASEB J 18(7): 805–815. 10.1096/fj.03-0839rev [DOI] [PubMed] [Google Scholar]
- 67.Mozetic V, Pacheco RL, Latorraca COC, Riera R (2019) Statins and/or fibrates for diabetic retinopathy: a systematic review and meta-analysis. Diabetol Metab Syndr 11:92. 10.1186/s13098-019-0488-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mozetic V, Leonel L, Leite Pacheco R et al. (2019) Reporting quality and adherence of randomized controlled trials about statins and/or fibrates for diabetic retinopathy to the CONSORT checklist. Trials 20(1):729. 10.1186/s13063-019-3868-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fliesler SJ, Bretillon L (2010) The ins and outs of cholesterol in the vertebrate retina. J Lipid Res 51(12):3399–3413. 10.1194/jlr.R010538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fliesler SJ, Florman R, Rapp LM, Pittler SJ, Keller RK (1993) In vivo biosynthesis of cholesterol in the rat retina. FEBS Lett 335(2):234–238. 10.1016/0014-5793(93)80736-e [DOI] [PubMed] [Google Scholar]
- 71.Fliesler SJ, Keller RK (1995) Metabolism of [3H]farnesol to cholesterol and cholesterogenic intermediates in the living rat eye. Biochem Biophys Res Commun 210(3):695–702. 10.1006/bbrc.1995.1715 [DOI] [PubMed] [Google Scholar]
- 72.Fliesler SJ, Florman R, Keller RK (1995) Isoprenoid lipid metabolism in the retina: dynamics of squalene and cholesterol incorporation and turnover in frog rod outer segment membranes. Exp Eye Res 60(1):57–69. 10.1016/s0014-4835(05)80084-3 [DOI] [PubMed] [Google Scholar]
- 73.Fliesler SJ, Keller RK (1997) Isoprenoid metabolism in the vertebrate retina. Int J Biochem Cell Biol 29(6):877–894. 10.1016/s1357-2725(97)00018-6 [DOI] [PubMed] [Google Scholar]
- 74.Lin JB, Mast N, Bederman IR et al. (2016) Cholesterol in mouse retina originates primarily from in situ de novo biosynthesis. J Lipid Res 57(2):258–264. 10.1194/jlr.M064469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zheng W, Reem RE, Omarova S et al. (2012) Spatial distribution of the pathways of cholesterol homeostasis in human retina. PLoS One 7(5):e37926. 10.1371/journal.pone.0037926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pikuleva IA, Curcio CA (2014) Cholesterol in the retina: the best is yet to come. Prog Retin Eye Res 41:64–89. 10.1016/j.preteyeres.2014.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Elner VM (2002) Retinal pigment epithelial acid lipase activity and lipoprotein receptors: effects of dietary omega-3 fatty acids. Trans Am Ophthalmol Soc 100:301–338 [PMC free article] [PubMed] [Google Scholar]
- 78.Tserentsoodol N, Sztein J, Campos M et al. (2006) Uptake of cholesterol by the retina occurs primarily via a low density lipoprotein receptor-mediated process. Mol Vis 12:1306–1318 [PubMed] [Google Scholar]
- 79.Duncan KG, Hosseini K, Bailey KR et al. (2009) Expression of reverse cholesterol transport proteins ATP-binding cassette A1 (ABCA1) and scavenger receptor BI (SR-BI) in the retina and retinal pigment epithelium. Br J Ophthalmol 93(8):1116–1120. 10.1136/bjo.2008.144006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rudolf M, Winkler B, Aherrahou Z, Doehring LC, Kaczmarek P, Schmidt-Erfurth U (2005) Increased expression of vascular endothelial growth factor associated with accumulation of lipids in Bruch’s membrane of LDL receptor knockout mice. Br J Ophthalmol 89(12):1627–1630. 10.1136/bjo.2005.071183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Houssier M, Raoul W, Lavalette S et al. (2008) CD36 deficiency leads to choroidal involution via COX2 down-regulation in rodents. PLoS Med 5(2):e39. 10.1371/journal.pmed.0050039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Anderson KW, Mast N, Hudgens JW, Lin JB, Turko IV, Pikuleva IA (2016) Mapping of the allosteric site in cholesterol hydroxylase CYP46A1 for Efavirenz, a drug that stimulates enzyme activity. J Biol Chem 291(22):11876–11886. 10.1074/jbc.M116.723577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mast N, Reem R, Bederman I et al. (2011) Cholestenoic acid is an important elimination product of cholesterol in the retina: comparison of retinal cholesterol metabolism with that in the brain. Invest Ophthalmol Vis Sci 52(1):594–603. 10.1167/iovs.10-6021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pikuleva IA (2008) Cholesterol-metabolizing cytochromes P450: implications for cholesterol lowering. Expert Opin Drug Metab Toxicol 4(11):1403–1414. 10.1517/17425255.4.11.1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zheng W, Mast N, Saadane A, Pikuleva IA (2015) Pathways of cholesterol homeostasis in mouse retina responsive to dietary and pharmacologic treatments. J Lipid Res 56(1):81–97. 10.1194/jlr.M053439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Omarova S, Charvet CD, Reem RE et al. (2012) Abnormal vascularization in mouse retina with dysregulated retinal cholesterol homeostasis. J Clin Invest 122(8):3012–3023. 10.1172/JCI63816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Meaney S, Bodin K, Diczfalusy U, Bjorkhem I (2002) On the rate of translocation in vitro and kinetics in vivo of the major oxysterols in human circulation: critical importance of the position of the oxygen function. J Lipid Res 43(12):2130–2135. 10.1194/jlr.m200293-jlr200 [DOI] [PubMed] [Google Scholar]
- 88.Hammer SS, Busik JV (2017) The role of dyslipidemia in diabetic retinopathy. Vis Res 139:228–236. 10.1016/j.visres.2017.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hammer SS, Beli E, Kady N et al. (2017) The mechanism of diabetic retinopathy pathogenesis unifying key lipid regulators, Sirtuin 1 and liver X receptor. EBioMedicine 22:181–190. 10.1016/j.ebiom.2017.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saadane A, Mast N, Trichonas G et al. (2019) Retinal vascular abnormalities and microglia activation in mice with deficiency in cytochrome P450 46A1-mediated cholesterol removal. Am J Pathol 189(2):405–425. 10.1016/j.ajpath.2018.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leung H, Wang JJ, Rochtchina E, Wong TY, Klein R, Mitchell P (2005) Dyslipidaemia and microvascular disease in the retina. Eye (Lond) 19(8):861–868. 10.1038/sj.eye.6701668 [DOI] [PubMed] [Google Scholar]
- 92.Lyons TJ, Jenkins AJ, Zheng D et al. (2006) Nuclear magnetic resonance-determined lipoprotein subclass profile in the DCCT/EDIC cohort: associations with carotid intima-media thickness. Diabet Med 23(9):955–966. 10.1111/j.1464-5491.2006.01905.x [DOI] [PubMed] [Google Scholar]
- 93.Tikhonenko M, Lydic TA, Opreanu M et al. (2013) N-3 polyunsaturated fatty acids prevent diabetic retinopathy by inhibition of retinal vascular damage and enhanced endothelial progenitor cell reparative function. PLoS One 8(1):e55177. 10.1371/journal.pone.0055177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Abela GS (2010) Cholesterol crystals piercing the arterial plaque and intima trigger local and systemic inflammation. J Clin Lipidol 4(3):156–164. 10.1016/j.jacl.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 95.Abela GS, Kalavakunta JK, Janoudi A et al. (2017) Frequency of cholesterol crystals in culprit coronary artery aspirate during acute myocardial infarction and their relation to inflammation and myocardial injury. Am J Cardiol 120(10):1699–1707. 10.1016/j.amjcard.2017.07.075 [DOI] [PubMed] [Google Scholar]
- 96.Adams CW, Abdulla YH (1978) The action of human high density lipoprotein on cholesterol crystals. Part 1. Light-microscopic observations. Atherosclerosis 31(4):465–471. 10.1016/0021-9150(78)90142-9 [DOI] [PubMed] [Google Scholar]
- 97.Bakke SS, Aune MH, Niyonzima N et al. (2017) Cyclodextrin reduces cholesterol crystal-induced inflammation by modulating complement activation. J Immunol 199(8):2910–2920. 10.4049/jimmunol.1700302 [DOI] [PubMed] [Google Scholar]
- 98.Baumer Y, McCurdy S, Weatherby TM et al. (2017) Hyperlipidemia-induced cholesterol crystal production by endothelial cells promotes atherogenesis. Nat Commun 8(1):1129. 10.1038/s41467-017-01186-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grebe A, Latz E (2013) Cholesterol crystals and inflammation. Curr Rheumatol Rep 15(3):313. 10.1007/s11926-012-0313-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thacker SG, Zarzour A, Chen Y et al. (2016) High-density lipoprotein reduces inflammation from cholesterol crystals by inhibiting inflammasome activation. Immunology 149(3):306–319. 10.1111/imm.12638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vedre A, Pathak DR, Crimp M, Lum C, Koochesfahani M, Abela GS (2009) Physical factors that trigger cholesterol crystallization leading to plaque rupture. Atherosclerosis 203(1):89–96. 10.1016/j.atherosclerosis.2008.06.027 [DOI] [PubMed] [Google Scholar]
- 102.Yiu G, Welch RJ, Wang Y, Wang Z, Wang PW, Haskova Z (2020) Spectral-domain OCT predictors of visual outcomes after Ranibizumab treatment for macular edema resulting from retinal vein occlusion. Ophthalmol Retina 4(1):67–76. 10.1016/j.oret.2019.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li M, Dolz-Marco R, Messinger JD et al. (2019) Clinicopathologic correlation of aneurysmal type 1 neovascularization in age-related macular degeneration. Ophthalmol Retina 3(2):99–111. 10.1016/j.oret.2018.08.008 [DOI] [PubMed] [Google Scholar]
- 104.Li M, Dolz-Marco R, Huisingh C et al. (2019) Clinicopathologic correlation of geographic atrophy secondary to age-related macular degeneration. Retina 39(4):802–816. 10.1097/IAE.0000000000002461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pang CE, Messinger JD, Zanzottera EC, Freund KB, Curcio CA (2015) The onion sign in Neovascular age-related macular degeneration represents cholesterol crystals. Ophthalmology 122(11): 2316–2326. 10.1016/j.ophtha.2015.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ong SS, Cummings TJ, Freedman SF (2018) Cholesterol crystals in the anterior segment in Coats’ disease. Ophthalmol Retina 2(8): 791. 10.1016/j.oret.2018.04.015 [DOI] [PubMed] [Google Scholar]
- 107.Niu S, Yu C, Chen Q et al. (2017) Multimodality analysis of hyperreflective foci and hard exudates in patients with diabetic retinopathy. Sci Rep 7(1):1568. 10.1038/s41598-017-01733-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mimouni M, Segev O, Dori D, Geffen N, Flores V, Segal O (2017) Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with macular edema secondary to vein occlusion. Am J Ophthalmol 182:160–167. 10.1016/j.ajo.2017.08.005 [DOI] [PubMed] [Google Scholar]
- 109.Lloyd CW, Liu M, Kitchens J, Wang PW, Haskova Z (2019) Baseline characteristics associated with early visual acuity gains after ranibizumab treatment for retinal vein occlusion. BMC Ophthalmol 19(1):11. 10.1186/s12886-018-1012-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tang F, Qin X, Lu J, Song P, Li M, Ma X (2020) Optical coherence tomography predictors of short-term visual acuity in eyes with macular edema secondary to retinal vein occlusion treated with intravitreal Conbercept. Retina 40(4):773–785. 10.1097/IAE.0000000000002444 [DOI] [PubMed] [Google Scholar]
- 111.Waldstein SM, Wright J, Warburton J, Margaron P, Simader C, Schmidt-Erfurth U (2016) Predictive value of retinal morphology for visual acuity outcomes of different Ranibizumab treatment regimens for Neovascular AMD. Ophthalmology 123(1):60–69. 10.1016/j.ophtha.2015.09.013 [DOI] [PubMed] [Google Scholar]
- 112.Arbore G, Kemper C (2016) A novel “complement-metabolism-inflammasome axis” as a key regulator of immune cell effector function. Eur J Immunol 46(7):1563–1573. 10.1002/eji.201546131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Freeley S, Kemper C, Le Friec G (2016) The “ins and outs” of complement-driven immune responses. Immunol Rev 274(1):16–32. 10.1111/imr.12472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hammerschmidt DE, Greenberg CS, Yamada O, Craddock PR, Jacob HS (1981) Cholesterol and atheroma lipids activate complement and stimulate granulocytes. A possible mechanism for amplification of ischemic injury in atherosclerotic states. J Lab Clin Med 98(1):68–77 [PubMed] [Google Scholar]
- 115.Hasselbacher P, Hahn JL (1980) Activation of the alternative pathway of complement by microcrystalline cholesterol. Atherosclerosis 37(2):239–245. 10.1016/0021-9150(80)90009-X [DOI] [PubMed] [Google Scholar]
- 116.Niyonzima N, Halvorsen B, Sporsheim B et al. (2017) Complement activation by cholesterol crystals triggers a subsequent cytokine response. Mol Immunol 84:43–50. 10.1016/j.molimm.2016.09.019 [DOI] [PubMed] [Google Scholar]
- 117.Nymo S, Niyonzima N, Espevik T, Mollnes TE (2014) Cholesterol crystal-induced endothelial cell activation is complement-dependent and mediated by TNF. Immunobiology 219(10):786–792. 10.1016/j.imbio.2014.06.006 [DOI] [PubMed] [Google Scholar]
- 118.Pilely K, Bakke SS, Palarasah Y et al. (2019) Alpha-cyclodextrin inhibits cholesterol crystal-induced complement-mediated inflammation: a potential new compound for treatment of atherosclerosis. Atherosclerosis 283:35–42. 10.1016/j.atherosclerosis.2019.01.034 [DOI] [PubMed] [Google Scholar]
- 119.Pilely K, Fumagalli S, Rosbjerg A et al. (2017) C-reactive protein binds to cholesterol crystals and co-localizes with the terminal complement complex in human atherosclerotic plaques. Front Immunol 8:1040. 10.3389/fimmu.2017.01040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pilely K, Rosbjerg A, Genster N et al. (2016) Cholesterol crystals activate the lectin complement pathway via Ficolin-2 and mannose-binding lectin: implications for the progression of atherosclerosis. J Immunol 196(12):5064–5074. 10.4049/jimmunol.1502595 [DOI] [PubMed] [Google Scholar]
- 121.Samstad EO, Niyonzima N, Nymo S et al. (2014) Cholesterol crystals induce complement-dependent inflammasome activation and cytokine release. J Immunol 192(6):2837–2845. 10.4049/jimmunol.1302484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Seifert PS, Kazatchkine MD (1987) Generation of complement anaphylatoxins and C5b-9 by crystalline cholesterol oxidation derivatives depends on hydroxyl group number and position. Mol Immunol 24(12):1303–1308. 10.1016/0161-5890(87)90125-8 [DOI] [PubMed] [Google Scholar]
- 123.Vogt W, von Zabern I, Damerau B, Hesse D, Luhmann B, Nolte R (1985) Mechanisms of complement activation by crystalline cholesterol. Mol Immunol 22(2):101–106. 10.1016/S0161-5890(85)80003-1 [DOI] [PubMed] [Google Scholar]
- 124.Kiyotake R, Oh-Hora M, Ishikawa E, Miyamoto T, Ishibashi T, Yamasaki S (2015) Human Mincle binds to cholesterol crystals and triggers innate immune responses. J Biol Chem 290(42): 25322–25332. 10.1074/jbc.M115.645234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Arbore G, Kemper C, Kolev M (2017) Intracellular complement - the complosome - in immune cell regulation. Mol Immunol 89:2–9. 10.1016/j.molimm.2017.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liszewski MK, Kolev M, Le Friec G et al. (2013) Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity 39(6):1143–1157. 10.1016/j.immuni.2013.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Reichhardt MP, Meri S (2018) Intracellular complement activation-An alarm raising mechanism? Semin Immunol 38: 54–62. 10.1016/j.smim.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 128.Tam JC, Bidgood SR, McEwan WA, James LC (2014) Intracellular sensing of complement C3 activates cell autonomous immunity. Science 345(6201):1256070. 10.1126/science.1256070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Clark SJ, Bishop PN (2018) The eye as a complement dysregulation hotspot. Semin Immunopathol 40(1):65–74. 10.1007/s00281-017-0649-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liao WL, Lin JM, Liu SP et al. (2018) Loss of response gene to complement 32 (RGC-32) in diabetic mouse retina is involved in retinopathy development. Int J Mol Sci 19(11):3629. 10.3390/ijms19113629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chrzanowska M, Modrzejewska A, Modrzejewska M (2018) New insight into the role of the complement in the most common types of retinopathy-current literature review. Int J Ophthalmol 11(11): 1856–1864. 10.18240/ijo.2018.11.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen M, Luo C, Zhao J, Devarajan G, Xu H (2019) Immune regulation in the aging retina. Prog Retin Eye Res 69:159–172. 10.1016/j.preteyeres.2018.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Manoharan N, Patnaik JL, Olson JL (2019) Increased complement levels in human vitreous aspirates of proliferative diabetic retinopathy and retinal detachment eyes. Retina 39(11):2212–2218. 10.1097/IAE.0000000000002288 [DOI] [PubMed] [Google Scholar]
- 134.Schori C, Trachsel C, Grossmann J, Zygoula I, Barthelmes D, Grimm C (2018) The proteomic landscape in the vitreous of patients with age-related and diabetic retinal disease. Invest Ophthalmol Vis Sci 59(4):AMD31–AMD40. 10.1167/iovs.18-24122 [DOI] [PubMed] [Google Scholar]
- 135.Hokazono K, Belizario FS, Portugal V, Messias-Reason I, Nisihara R (2018) Mannose binding lectin and Pentraxin 3 in patients with diabetic retinopathy. Arch Med Res 49(2):123–129. 10.1016/j.arcmed.2018.06.003 [DOI] [PubMed] [Google Scholar]
- 136.Rasmussen KL, Nordestgaard BG, Nielsen SF (2018) Complement C3 and risk of diabetic microvascular disease: a cohort study of 95202 individuals from the general population. Clin Chem 64(7):1113–1124. 10.1373/clinchem.2018.287581 [DOI] [PubMed] [Google Scholar]
- 137.Balaiya S, Zhou Z, Chalam KV (2017) Characterization of vitreous and aqueous proteome in humans with proliferative diabetic retinopathy and its clinical correlation. Proteomics Insights 8: 1178641816686078. 10.1177/1178641816686078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yang MM, Wang J, Ren H et al. (2016) Genetic investigation of complement pathway genes in type 2 diabetic retinopathy: An inflammatory perspective. Mediat Inflamm 2016:1313027. 10.1155/2016/1313027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xu D, Yi H, Yu S, Li X, Qiao Y, Deng W (2016) Association of complement C5 gene polymorphisms with proliferative diabetic retinopathy of type 2 diabetes in a Chinese Han population. PLoS One 11(3):e0149704. 10.1371/journal.pone.0149704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xu H, Chen M (2016) Targeting the complement system for the management of retinal inflammatory and degenerative diseases. Eur J Pharmacol 787:94–104. 10.1016/j.ejphar.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang J, Yang MM, Li YB, Liu GD, Teng Y, Liu XM (2013) Association of CFH and CFB gene polymorphisms with retinopathy in type 2 diabetic patients. Mediat Inflamm 2013:748435. 10.1155/2013/748435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fujita T, Hemmi S, Kajiwara M et al. (2013) Complement-mediated chronic inflammation is associated with diabetic microvascular complication. Diabetes Metab Res Rev 29(3):220–226. 10.1002/dmrr.2380 [DOI] [PubMed] [Google Scholar]
- 143.Yanai R, Thanos A, Connor KM (2012) Complement involvement in neovascular ocular diseases. Adv Exp Med Biol 946: 161–183. 10.1007/978-1-4614-0106-3_10 [DOI] [PubMed] [Google Scholar]
- 144.Flyvbjerg A (2010) Diabetic angiopathy, the complement system and the tumor necrosis factor superfamily. Nat Rev Endocrinol 6(2):94–101. 10.1038/nrendo.2009.266 [DOI] [PubMed] [Google Scholar]
- 145.Jha P, Bora PS, Bora NS (2007) The role of complement system in ocular diseases including uveitis and macular degeneration. Mol Immunol 44(16):3901–3908. 10.1016/j.molimm.2007.06.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ostergaard J, Hansen TK, Thiel S, Flyvbjerg A (2005) Complement activation and diabetic vascular complications. Clin Chim Acta 361(1–2):10–19. 10.1016/j.cccn.2005.04.028 [DOI] [PubMed] [Google Scholar]
- 147.Zhang J, Gerhardinger C, Lorenzi M (2002) Early complement activation and decreased levels of glycosylphosphatidylinositol-anchored complement inhibitors in human and experimental diabetic retinopathy. Diabetes 51(12):3499–3504. 10.2337/diabetes.51.12.3499 [DOI] [PubMed] [Google Scholar]
- 148.Gerl VB, Bohl J, Pitz S, Stoffelns B, Pfeiffer N, Bhakdi S (2002) Extensive deposits of complement C3d and C5b-9 in the choriocapillaris of eyes of patients with diabetic retinopathy. Invest Ophthalmol Vis Sci 43(4):1104–1108 [PubMed] [Google Scholar]
- 149.Lhotta K, Auinger M, Kronenberg F, Irsigler K, Konig P (1996) Polymorphism of complement C4 and susceptibility to IDDM and microvascular complications. Diabetes Care 19(1):53–55. 10.2337/diacare.19.1.53 [DOI] [PubMed] [Google Scholar]
- 150.Weller M, Clausen R, Bresgen M, Heimann K, Wiedemann P (1990) Immunoglobulin G, complement factor C3 and lymphocytes in proliferative intraocular disorders. Int Ophthalmol 14(4): 277–283. 10.1007/BF00159864 [DOI] [PubMed] [Google Scholar]
- 151.Costa B, Belmonte MA, Callizo J, Pastor RM, Huguet R, Richart C (1988) Serum C4 concentration, diabetes mellitus and diabetic microangiopathy. Med Clin (Barc) 91(19):728–731 [PubMed] [Google Scholar]
- 152.Arora S, Gauri LA, Saxena HC (1988) A study of plasma C4 concentration and its possible association with microvascular complications in type I (insulin dependent) and type II (non-insulin dependent) diabetes. J Assoc Physicians India 36(7):425–427 [PubMed] [Google Scholar]
- 153.McCann VJ, McCluskey J, Kay PH, Zilko PJ, Christiansen FT, Dawkins RL (1983) HLA and complement genetic markers in diabetic retinopathy. Diabetologia 24(3):221. 10.1007/BF00250171 [DOI] [PubMed] [Google Scholar]
- 154.Powell ED, Field RA (1966) Studies on salicylates and complement in diabetes. Diabetes 15(10):730–733. 10.2337/diab.15.10.730 [DOI] [PubMed] [Google Scholar]
- 155.Huang C, Fisher KP, Hammer SS, Navitskaya S, Blanchard GJ, Busik JV (2018) Plasma exosomes contribute to microvascular damage in diabetic retinopathy by activating the classical complement pathway. Diabetes 67(8):1639–1649. 10.2337/db17-1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ehlers MR (2000) CR3: a general purpose adhesion-recognition receptor essential for innate immunity. Microbes Infect 2(3):289–294. 10.1016/S1286-4579(00)00299-9 [DOI] [PubMed] [Google Scholar]
- 157.An G, Ren G, An F, Zhang C (2014) Role of C5a-C5aR axis in the development of atherosclerosis. Sci China Life Sci 57(8):790–794. 10.1007/s11427-014-4711-5 [DOI] [PubMed] [Google Scholar]
- 158.Rajamaki K, Lappalainen J, Oorni K et al. (2010) Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One 5(7):e11765. 10.1371/journal.pone.0011765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Asare-Bediako B, Noothi SK, Li Calzi S et al. (2020) Characterizing the retinal phenotype in the high-fat diet and Western diet mouse models of prediabetes. Cells 9(2):464. 10.3390/cells9020464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Eid S, Sas KM, Abcouwer SF et al. (2019) New insights into the mechanisms of diabetic complications: role of lipids and lipid metabolism. Diabetologia 62(9):1539–1549. 10.1007/s00125-019-4959-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cao X, Guo Y, Wang Y et al. (2020) Effects of high-fat diet and Apoe deficiency on retinal structure and function in mice. Sci Rep 10(1):18601. 10.1038/s41598-020-75576-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hiro T, Kimura T, Morimoto T et al. (2010) Diabetes mellitus is a major negative determinant of coronary plaque regression during statin therapy in patients with acute coronary syndrome–serial intravascular ultrasound observations from the Japan assessment of Pitavastatin and atorvastatin in acute coronary syndrome trial (the JAPAN-ACS trial). Circ J 74(6):1165–1174. 10.1253/circj.cj-09-0766 [DOI] [PubMed] [Google Scholar]
- 163.Geovanini GR, Libby P (2018) Atherosclerosis and inflammation: overview and updates. Clin Sci (Lond) 132(12):1243–1252. 10.1042/CS20180306 [DOI] [PubMed] [Google Scholar]
- 164.Ridker PM, Everett BM, Thuren T et al. (2017) Antiinflammatory therapy with Canakinumab for atherosclerotic disease. N Engl J Med 377(12):1119–1131. 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 165.Abela GS, Aziz K (2005) Cholesterol crystals cause mechanical damage to biological membranes: a proposed mechanism of plaque rupture and erosion leading to arterial thrombosis. Clin Cardiol 28(9):413–420. 10.1002/clc.4960280906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Duewell P, Kono H, Rayner KJ et al. (2010) NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464(7293):1357–1361. 10.1038/nature08938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Taborda NA, Blanquiceth Y, Urcuqui-Inchima S, Latz E, Hernandez JC (2019) High-density lipoproteins decrease Proinflammatory activity and modulate the innate immune response. J Interf Cytokine Res 39(12):760–770. 10.1089/jir.2019.0029 [DOI] [PubMed] [Google Scholar]
- 168.Menini S, Iacobini C, Vitale M, Pugliese G (2020) The Inflammasome in chronic complications of diabetes and related metabolic disorders. Cells 9(8):1812. 10.3390/cells9081812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nowarski R, Gagliani N, Huber S, Flavell RA (2013) Innate immune cells in inflammation and cancer. Cancer Immunol Res 1(2):77–84. 10.1158/2326-6066.CIR-13-0081 [DOI] [PubMed] [Google Scholar]
- 170.Henao-Mejia J, Elinav E, Thaiss CA, Flavell RA (2014) Inflammasomes and metabolic disease. Annu Rev Physiol 76: 57–78. 10.1146/annurev-physiol-021113-170324 [DOI] [PubMed] [Google Scholar]
- 171.Jin C, Flavell RA (2013) Innate sensors of pathogen and stress: linking inflammation to obesity. J Allergy Clin Immunol 132(2): 287–294. 10.1016/j.jaci.2013.06.022 [DOI] [PubMed] [Google Scholar]
- 172.Soehnlein O, Swirski FK (2013) Hypercholesterolemia links hematopoiesis with atherosclerosis. Trends Endocrinol Metab 24(3):129–136. 10.1016/j.tem.2012.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tall AR, Yvan-Charvet L (2015) Cholesterol, inflammation and innate immunity. Nat Rev Immunol 15(2):104–116. 10.1038/nri3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Drechsler M, Megens RT, van Zandvoort M, Weber C, Soehnlein O (2010) Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation 122(18):1837–1845. 10.1161/CIRCULATIONAHA.110.961714 [DOI] [PubMed] [Google Scholar]
- 175.Westerterp M, Fotakis P, Ouimet M et al. (2018) Cholesterol efflux pathways suppress Inflammasome activation, NETosis, and Atherogenesis. Circulation 138(9):898–912. 10.1161/CIRCULATIONAHA.117.032636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Farah R, Shurtz-Swirski R, Dorlechter F (2010) Primed polymorphonuclear leukocytes constitute a possible link between inflammation and oxidative stress in hyperlipidemic patients: effect of statins. Minerva Cardioangiol 58(2):175–181 [PubMed] [Google Scholar]
- 177.Rothe G, Gabriel H, Kovacs E et al. (1996) Peripheral blood mono-nuclear phagocyte subpopulations as cellular markers in hypercholesterolemia. Arterioscler Thromb Vasc Biol 16(12):1437–1447. 10.1161/01.atv.16.12.1437 [DOI] [PubMed] [Google Scholar]
- 178.Duan Y, Beli E, Li Calzi S et al. (2018) Loss of angiotensin-converting enzyme 2 exacerbates diabetic retinopathy by promoting bone marrow dysfunction. Stem Cells 36(9):1430–1440. 10.1002/stem.2848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Duan Y, Prasad R, Feng D et al. (2019) Bone marrow-derived cells restore functional integrity of the gut epithelial and vascular barriers in a model of diabetes and ACE2 deficiency. Circ Res 125(11):969–988. 10.1161/CIRCRESAHA.119.315743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Vieira CP, Fortmann SD, Hossain M et al. (2020) Selective LXR agonist DMHCA corrects retinal and bone marrow dysfunction in type 2 diabetes. JCI Insight 5(13):137230. 10.1172/jci.insight.137230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Glaria E, Letelier NA, Valledor AF (2020) Integrating the roles of liver X receptors in inflammation and infection: mechanisms and outcomes. Curr Opin Pharmacol 53:55–65. 10.1016/j.coph.2020.05.001 [DOI] [PubMed] [Google Scholar]
- 182.Castrillo A, Joseph SB, Vaidya SA et al. (2003) Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol Cell 12(4):805–816. 10.1016/s1097-2765(03)00384-8 [DOI] [PubMed] [Google Scholar]
- 183.Hazra S, Rasheed A, Bhatwadekar A et al. (2012) Liver X receptor modulates diabetic retinopathy outcome in a mouse model of streptozotocin-induced diabetes. Diabetes 61(12):3270–3279. 10.2337/db11-1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ferraro F, Lymperi S, Mendez-Ferrer S et al. (2011) Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Sci Transl Med 3(104):104ra101. 10.1126/scitranslmed.3002191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hazra S, Jarajapu YP, Stepps V et al. (2013) Long-term type 1 diabetes influences haematopoietic stem cells by reducing vascular repair potential and increasing inflammatory monocyte generation in a murine model. Diabetologia 56(3):644–653. 10.1007/s00125-012-2781-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chambers SEJ, O’Neill CL, Guduric-Fuchs J et al. (2018) The Vasoreparative function of myeloid Angiogenic cells is impaired in diabetes through the induction of IL1beta. Stem Cells 36(6): 834–843. 10.1002/stem.2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Medina RJ, Barber CL, Sabatier F et al. (2017) Endothelial progenitors: a consensus statement on nomenclature. Stem Cells Transl Med 6(5):1316–1320. 10.1002/sctm.16-0360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Medina RJ, O’Neill CL, O’Doherty TM et al. (2011) Myeloid angiogenic cells act as alternative M2 macrophages and modulate angiogenesis through interleukin-8. Mol Med 17(9–10):1045–1055. 10.2119/molmed.2011.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bhatwadekar AD, Duan Y, Korah M et al. (2017) Hematopoietic stem/progenitor involvement in retinal microvascular repair during diabetes: implications for bone marrow rejuvenation. Vis Res 139:211–220. 10.1016/j.visres.2017.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chakravarthy H, Navitskaya S, O’Reilly S et al. (2016) Role of acid sphingomyelinase in shifting the balance between Proinflammatory and reparative bone marrow cells in diabetic retinopathy. Stem Cells 34(4):972–983. 10.1002/stem.2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chakravarthy H, Beli E, Navitskaya S et al. (2016) Imbalances in mobilization and activation of pro-inflammatory and vascular reparative bone marrow-derived cells in diabetic retinopathy. PLoS One 11(1):e0146829. 10.1371/journal.pone.0146829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bhatwadekar AD, Yan Y, Stepps V et al. (2015) miR-92a corrects CD34+ cell dysfunction in diabetes by modulating Core circadian genes involved in progenitor differentiation. Diabetes 64(12): 4226–4237. 10.2337/db15-0521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cogle CR, Wise E, Meacham AM et al. (2014) Detailed analysis of bone marrow from patients with ischemic heart disease and left ventricular dysfunction: BM CD34, CD11b, and clonogenic capacity as biomarkers for clinical outcomes. Circ Res 115(10): 867–874. 10.1161/CIRCRESAHA.115.304353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Freeman MS, Thomas JR, Zipper JA (1989) Clavicular division technique. A new approach for lengthening the pectoralis flap. Arch Otolaryngol Head Neck Surg 115(2):224–227. 10.1001/archotol.1989.01860260098022 [DOI] [PubMed] [Google Scholar]
- 195.Guthrie SM, Curtis LM, Mames RN, Simon GG, Grant MB, Scott EW (2005) The nitric oxide pathway modulates hemangioblast activity of adult hematopoietic stem cells. Blood 105(5):1916–1922. 10.1182/blood-2004-09-3415 [DOI] [PubMed] [Google Scholar]
- 196.Grant MB, May WS, Caballero S et al. (2002) Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med 8(6):607–612. 10.1038/nm0602-607 [DOI] [PubMed] [Google Scholar]
- 197.Caballero S, Sengupta N, Afzal A et al. (2007) Ischemic vascular damage can be repaired by healthy, but not diabetic, endothelial progenitor cells. Diabetes 56(4):960–967. 10.2337/db06-1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Guerin E, Sheridan C, Assheton D et al. (2008) SDF1-alpha is associated with VEGFR-2 in human choroidal neovascularisation. Microvasc Res 75(3):302–307. 10.1016/j.mvr.2007.12.001 [DOI] [PubMed] [Google Scholar]
- 199.Otani A, Kinder K, Ewalt K, Otero FJ, Schimmel P, Friedlander M (2002) Bone marrow-derived stem cells target retinal astrocytes and can promote or inhibit retinal angiogenesis. Nat Med 8(9): 1004–1010. 10.1038/nm744 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


