Abstract
Protein post-translational modifications (PTMs) profoundly influence protein functions and play crucial roles in essentially all cell biological processes. The diverse realm of PTMs and their crosstalk is linked to many critical signaling events involved in neoplastic transformation, carcinogenesis and metastasis. The pathological roles of various PTMs are implicated in all aspects of cancer hallmark functions, cancer metabolism and regulation of tumor microenvironment. Study of PTMs has become an important area in cancer research to understand cancer biology and discover novel biomarkers and therapeutic targets. With a limited scope, this review attempts to discuss some PTMs of high frequency with recognized importance in cancer biology, including phosphorylation, acetylation, glycosylation, palmitoylation and ubiquitination, as well as their implications in clinical applications. These protein modifications are among the most abundant PTMs and profoundly implicated in carcinogenesis.
Keywords: post-translational modifications, cancer, tumorigenesis, pathological role, signaling pathways, proteomics
1. Introduction
Protein post-translational modifications (PTMs) are chemical changes on amino acid side chains or protein terminuses. An enormous number of proteoforms can be generated by more than 200 known PTMs (Deribe et al., 2010; Duan and Walther, 2015; Olsen and Mann, 2013), profoundly influencing the complexity of proteomes and vast biological functions. A proteome-wide PTM study on the Swiss-Prot protein database suggested that the top five most prevalent PTMs observed experimentally were phosphorylation, acetylation, glycosylation, amidation and hydroxylation, whereas the top five putative PTMs were glycosylation, phosphorylation, acetylation, methylation and palmitoylation (Khoury et al., 2011). PTMs constitute a central mechanism that is beyond the genetic code to regulate cellular functional machinery in response to developmental or/and environmental stimuli. The majority of these modifications are catalyzed by various enzymes through highly regulated complex pathways, while some of them are driven by non-enzymatic chemical reactions, reflecting the convoluted influences of both genomic, transcriptomic and environmental factors on PTM status (Harmel and Fiedler, 2018; Trougakos et al., 2013; Walsh et al., 2005).
It is well known that PTMs are profoundly implicated in tumorigenesis and immune modulation, and have been emerging as important targets for early detection and therepeutic treatment of cancer (Benton et al., 2017; Chandler et al., 2019; Chang et al., 2016; Chang and Ding, 2018; De et al., 2014; Gong et al., 2016; Hsu et al., 2015; Jeusset and McManus, 2019; Ko and Dixon, 2018; Lan and Wang, 2019; Leeming et al., 2011; Mereiter et al., 2019; Munkley and Elliott, 2016; Naro and Sette, 2013; Popovic et al., 2014; Resh, 2017; Singh et al., 2017; Watanabe and Osada, 2012; Wu et al., 2019). Figure 1 examplifies various types of PTMs, their influences on protein functions and implications in tumorigenesis. Enzymatic PTMs are pathway driven, and occur at specific motifs in protein sequence, involving various modifications, such as chemical groups (e.g. phosphorylation, acetylation, etc), glycans (glycosylation), lipids (lipidation) or polypeptides (ubiquitination, SUMOylation). While non-enzymatic PTMs (e.g. glycation, oxidation, etc) are conventionally considered as a proteome-wide phenomena, mounting evidences have suggested that their occurrences can also be selective, and influenced by many factors, such as protein structure, amino acid neighboring preference and other local conditions (Harmel and Fiedler, 2018; Trougakos et al., 2013). These modifications can have significant impacts on proteins in many aspects, including activity, stability, functions, structure, localization, trafficking, signaling transduction, and their interactions with partner biomolecules (Chen et al., 2020; Krueger and Srivastava, 2006; Lothrop et al., 2013; Olsen and Mann, 2013; von Stechow and Olsen, 2017). The pathological implications of PTMs are involved in all cancer hallmarks, including sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, activating invasion and metastasis (Hanahan and Weinberg, 2000, 2011; Sanchez-Vega et al., 2018) (Fouad and Aanei, 2017; Pan et al., 2020; Senga and Grose, 2021; Wu et al., 2019). PTMs also play essential roles in regulating tumor microenvironment, such as remodeling of extracellular matrix (ECM) for cancer invasion and chronic inflammation (Chandler et al., 2019; Chang and Ding, 2018; Leeming et al., 2011). Targeting PTMs of critical proteins or pathways represents an emerging strategy to improve early detection and therapeutic treatment of cancer.
Fig. 1. Protein PTMs, biological functions and implication in tumorigenesis.
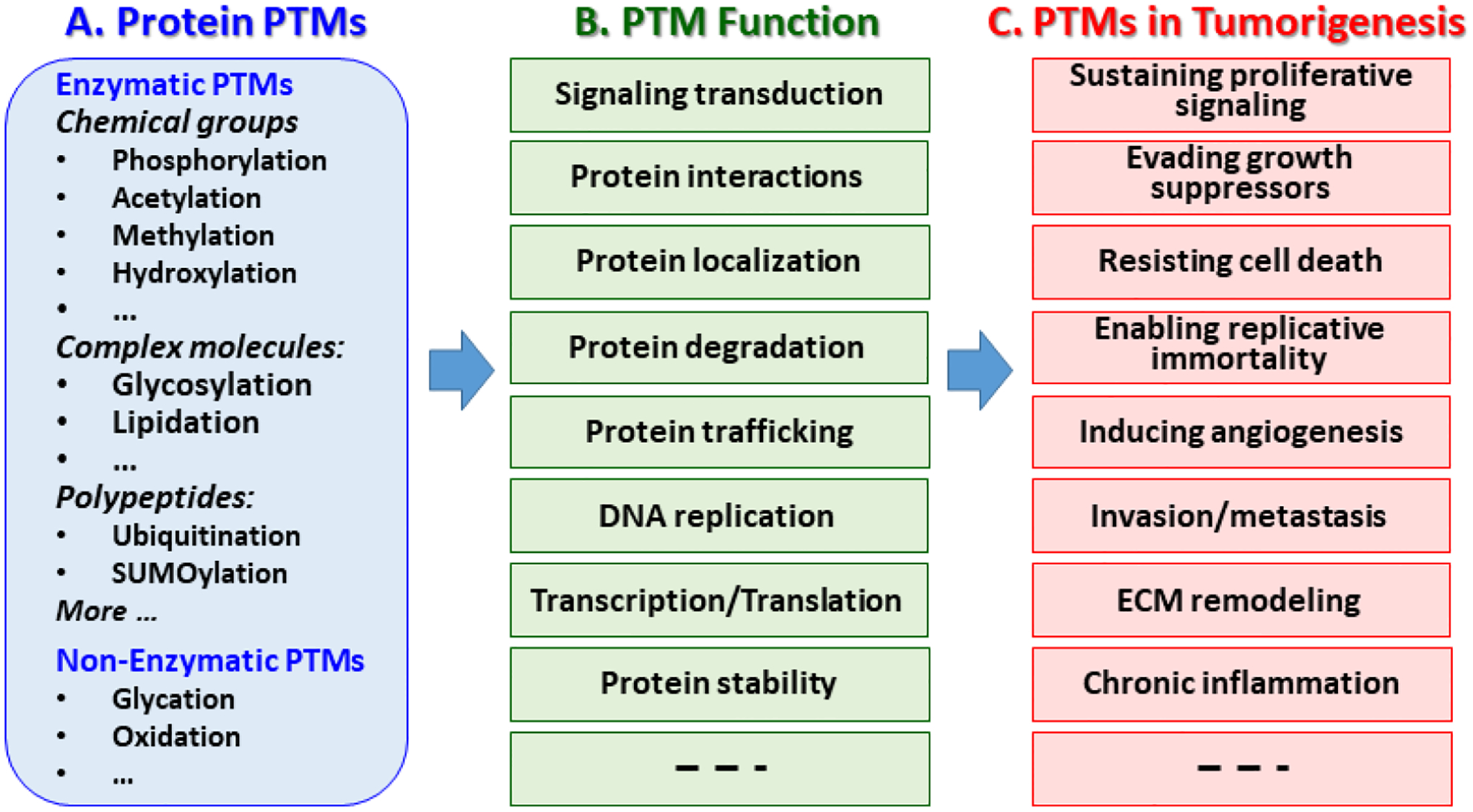
A) Well-known examples of PTMs, B) Influences of PTMs on protein biological functions, C) Pathogenesis implications of PTMs in cancer.
Enormous amount of information has been presented in the literature in studying PTMs relevant to cancer (Brooks and Gu, 2003; Chen et al., 2020; Krueger and Srivastava, 2006; Leeming et al., 2011; Meng et al., 2021; Wang et al., 2020; Wu et al., 2019). With the limited scope and space of this review, we attempt to discuss herein some PTMs of high frequency with recognized importance in cancer biology, including phosphorylation, acetylation, glycosylation, palmitoylation, and ubiquitination, as well as their implications in clinical applications. These protein modifications are among the most abundant PTMs and profoundly implicated in carcinogenesis.
2. Phosphorylation
Phosphorylation is a prevalent and reversible modification on amino acid residues of serine (Ser), threonine (Thr) and tyrosine (Tyr), which are catalyzed by kinases. Many kinases have been identified to be the oncogenic drivers, and their constitutive activities can result in aberrant phosphorylation of target substrates that activate or deactivate signaling pathways implicated in various types of cancer (Brooksbank, 2001; Buday and Vas, 2020; Choudhary and Mann, 2010; Ruprecht and Lemeer, 2014; Ubersax and Ferrell, 2007; Xu et al., 2019). Here we will discuss several driver phosphorylation abnormalities commonly involved in the cancer hallmarks (Hanahan and Weinberg, 2000, 2011).
2.1. RTK/RAS pathway.
The mitogen-activated protein kinase (MAPK) cascades are the major signaling pathways that regulate cellular growth and survival. The main canonical pathway, RTK/RAS/RAF/MEK/ERK pathway, transmits signals through sequential phosphorylation/activation of downstream protein kinases that eventually leads to transcription of genes that encode proteins involved in the regulation of essential cellular functions, including cell proliferation, differentiation and stress responses (Cseh et al., 2014; Santarpia et al., 2012). This pathway is highly regulated by regulator proteins and negative feedback mechanisms. Dysregulation of this pathway could induce a wide array of diseases including cancer. Oncogenic mutations in various components of the RTK/RAS/RAF/MEK/ERK pathway have been found in various cancers, with mutations of RAS and RAF being the most common in all cancer types.
Under normal cellular homeostasis, the Ras protein is cycling through GDP state (inactivation) and GTP state (activation) depending on the external stimuli or its interacting proteins. Oncogenic RAS mutations enable mutant Ras protein to be constitutively activated and continuously turned “on” leading to hyperphosphorylation and hyperactivation of the RAS/RAF/MEK/ERK pathway that contributes to the pathogenesis of several cancer types. Since the discovery of RAS as transforming oncogenes 40 years ago, there has been continuously ongoing interest in Ras research. An interesting finding from recent studies revealed a novel pathological role of argonaute 2 (AGO2), a Ras interacting protein, in promoting tumor progression in multiple mouse models of KRAS-driven pancreatic cancer and non–small cell lung cancer (Shankar et al., 2020; Tien et al., 2021). KRAS-AGO2 interaction is critical for PDAC progression, whereas disruption of this interaction by phosphorylation of AGO2 prevents progression to invasive tumor (Shankar et al., 2020; Tien et al., 2021).
Similarly, oncogenic mutation of RAF (mostly BRAF V600E mutation) induces constitutional activation of RAF kinase without stimulation, thereby leading to hyperactivation of the downstream signaling cascade. Alterations in this pathway are widespread across all tumor types. In an analysis of over 9000 TCGA tumors, the RAS/RAF/MEK/ERK pathway was the most frequently genetically altered pathway among the oncogenic signaling pathways, affecting about 46% of all the tumor samples analyzed (Sanchez-Vega et al., 2018).
Upstream of Ras are membrane receptors that initiate the signal transduction upon activation by extracellular stimuli. As the major membrane receptors of the MAPK signaling, tyrosine kinases (RTKs) undergo autophosphorylation after engagement of growth factor ligands and subsequently activate downstream signaling proteins to propagate signaling pathways. Mutations leading to constitutive activation of RTKs in the absence of upstream stimuli may thus induce oncogenesis. For example, epidermal growth factor receptor (EGFR) gene mutations predominantly occur in its tyrosine kinase domain, leading to the mutant EGFR protein staying at constitutive phosphorylated stage, thereby activating its downstream signaling and conferring oncogenic properties. In a recent large scale proteogenomic analysis of lung cancer tissues, several ALK driver chromosomal rearrangements and missense mutations of STK11 were identified (Gillette et al., 2020). ALK is a RTK that activate multiple downstream signaling cascades, including RAS/MAPK and PI3K, to promote cell proliferation and survival. Oncogenic fusion protein EML4-ALK resulting from chromosomal rearrangements are constitutively activated and can directly phosphorylate its downstream proteins without ligand binding to induce oncogenesis. STK11 is an intracellular kinase that functions as a tumor suppressor by phosphorylating and activating AMP-activated protein kinase (AMPK) to maintain cell polarity and inhibit cell proliferation and energy metabolism. Missense mutations in STK11 inactivate the kinase activity of STK11 and abolish its tumor suppressor activity, thereby contributing to tumorigenesis(Li et al., 2015).
2.2. Wnt-β-catenin pathway.
Wnt signaling pathway, a key cascade regulating cell stemness and cell-fate specification, is tightly activated and deactivated by phosphorylation and other PTMs. In the resting cell, the cytosolic β-catenin is deactivated through phosphorylation by a multi-protein destructing complex, and further degraded by ubiquitination. When Wnt ligands bind to the surface receptor Frizzled (FZD) and co- receptor low-density lipoprotein receptor-related protein (LRP), this trimeric complex then triggers phosphorylation of LRP, which further phosphorylates and recruits the components of the β-catenin destructing complex, leading to dissociation of the destruction complex. As a result, the phosphorylation of β-catenin is blocked, and β-catenin is then stabilized and accumulated, which then enters the nucleus where it promotes the transcription of Wnt target genes (Bugter et al., 2021). In this signaling cascade, phosphorylation plays the primary force in both activating LRP at Wnt-on state, and inactivating β-catenin at Wnt-off state. Wnt pathway is subject to numerous negative feedback regulations to maintain balanced signaling. Mutations causing sustained Wnt–β-catenin activation occur in most major cancer types, being most prominent in colorectal cancer, where over 90% colorectal cancers display mutations in this pathway (Sanchez-Vega et al., 2018).
2.3. Cell cycle pathway.
Cell cycle progression is regulated through a series of cell cycle regulators, whose expression levels are regulated at the post-translational level, mainly through Cyclin-dependent kinases (CDKs) mediated phosphorylation or ubiquitination (Liu et al., 2021). When there is a mitogenic signal, cell cycle progression is initiated through phosphorylation of Rb proteins by CDKs, which then release transcriptional factor E2F to promote transcription of downstream targets, leading to transition from G1 to S phase. When there are DNA double-strand breaks, protein kinase ATM (ataxia telangiectasia mutated) is recruited to phosphorylate several cell cycle regulators which then activate the DNA damage checkpoint, leading to cell cycle arrest, DNA repair or apoptosis. Dysregulation in cell cycle pathway, especially in the cell cycle checkpoints, is one of the major mechanisms for cancer cells to sustain uncontrolled proliferative signaling. Up to 45% of the over 9000 TCGA tumors had alterations in cell cycle pathway, only next to the RAS pathway which had the highest alteration frequency (Sanchez-Vega et al., 2018).
2.4. Other pathways relevant to hallmarks of cancer.
Phosphorylation is a very common modification of proteins that can rapidly switch proteins between activated and inactivated states. In the study analyzing the oncogenic signaling pathway of over 9000 tumor samples (Sanchez-Vega et al., 2018), the top ten most frequently altered pathways include RTK/RAS, cell cycle, PI3K, p53, Notch, Wnt, Myc, Hippo, TGFβ, and Nrf2 pathways. In addition to Ras, Wnt, and cell cycle pathways, almost all other frequently altered oncogenic signaling pathways found in cancers also involve changes in phosphorylation of key proteins, affecting their stability and activity (Sanchez-Vega et al., 2018).
2.5. Targeting phosphorylation as a cancer therapeutic strategy.
For nearly four decades, Ras was thought to be an “undruggable” cancer target. Although there are some recent breakthrough targeting a specific mutation (KRASG12C), no clinically effective therapies directly targeting Ras for RAS-mutant cancers have been developed yet (Stalnecker and Der, 2020). It is now clear that Ras proteins are also regulated by a vast array PTMs. Phosphorylation at several residues of Ras proteins have recently been shown to regulate Ras activities (Campbell and Philips, 2021). Currently, several small molecule inhibitors targeting various Ras phosphorylation sites are under preclinical studies or clinical trials (Campbell and Philips, 2021) (Table 1). Due to the critical role of RTKs in cancer development, substantial interests have been on targeting oncogenic mutations of RTKs for cancer therapeutics. Today, there are many FDA approved targeted therapies on RTKs (e.g. EGFR, HER2/HER3, ALK), using either RTK inhibitors or monoclonal antibody to combat the phosphorylation and constitutive activation of RTKs (Table 1). Inhibitors targeting other members of the MAPK cascade are also in various development stages, including preclinical and clinical studies, while some are already FDA approved (Table 1) (Yip and Papa, 2021).
Table 1.
Selected drugs or compounds trageting PTMs.
| Gene/protein Target | Targeted Modification | Drugs | Cancer | Phase |
|---|---|---|---|---|
| 20S proteasome | Ubiquitination | Bortezomib, Carfilzomib, | Myeloma, Lymphoma, Lung, Pancreas | FDA approved |
| ALK | Phosphorylation | Alectinib, Brigatinib, Ceritinib, Crizotinib, Lorlatinib | Lung | FDA approved |
| BRAF | Phosphorylation | Dabrafenib, Encorafenib, Vemurafenib | Melanoma, Lung, Colon, Rectum | FDA approved |
| CDK4/6 | Phosphorylation | Abemaciclib, Ribociclib, and Palbociclib | Breast | FDA approved |
| CRBN | Ubiquitination | Thalidomide, Lenalidomide, Pomalidomide | Myeloma | FDA approved |
| EGFR | Phosphorylation | Afatinib, Almonertinib, Dacomitinib, Erlotinib, Gefitinib, Osimertinib, Amivantamab, Mobocertinib, Cetuximab, Panitumumab | Lung, Head and neck, Colon, Rectum | FDA approved |
| HDAC | Acetylation | Vorinostat, Belinostat, Panobinostat, and Chidamide, Romidepsin | Lymphoma, Myeloma | FDA approved |
| HER2/HER3 | Phosphorylation | Lapatinib, Neratinib, Tucatinib, Trastuzumab and Pertuzumab | Lung, Breast | FDA approved |
| Mdm2 | Ubiquitination | PRIMA | Liver, Pancreas | FDA approved |
| MEK1/2 | Phosphorylation | Binimetinib, Trametinib, and Cobimetinib | Melanoma, Lung | FDA approved |
| PD-L1 | Glycosylation | gPD-L1 | Breast | Preclinical |
| PI3K | Phosphorylation | Copanlisib | Lymphomas | FDA approved |
| PI3K | Phosphorylation | Copanlisib | Breast | Phase II |
| Ras/SHP2 | Phosphorylation | TNO155, JAB-3068, RMC-4630 | Solid tumors | Phase 1, 2a |
| Wnt/LRP6 | Phosphorylation | Salinomycin, Rottlerin, and Monensin | Preclinical | |
| Wnt/SRSF | Phosphorylation | SM08502 | Solid tumors | Phase 1 |
| Wnt/β-catenin | Ubiquitination | MSAB | Colon | Preclinical |
For cell cycle and DNA damage repair pathways, inhibitors targeting CDK4/6 activation have made significant progresses in breast cancer treatment, with three FDA-approved CDK4/6 inhibitors in clinics (Table 1) (Yip and Papa, 2021). Due to the critical role of WNT–β-catenin signaling in cancer development, especially in colorectal cancer, substantial efforts have been directed in developing therapeutics targeting this pathway. Numerous Wnt pathway antagonists, monoclonal antibodies and small molecule inhibitors have been developed, although none of these drugs has been clinically approved yet (Table 1) (Jung and Park, 2020). On-target toxicity is a common challenge in blockage of Wnt pathway that needs to be addressed in the development of Wnt targeting therapy.
The success of recent development in targeted therapies has revolutionized the treatment of cancer patients and indicated a new era of precision medicine (Du and Lovly, 2018). Despite these exciting advancements, inherent limitations on drug toxicity and drug resistance still cause treatment failure. Novel approaches have been shown to effectively overcome acquired resistance, including the development of next-generation drugs and combinational use of therapy (Du and Lovly, 2018).
3. Acetylation
Lysine acetylation is a ubiquitous PTM that extensively presents across the entire proteome, contributing to the regulation of a wide range of cellular functions for maintaining cellular homeostasis. More than 35,000 acetylation sites have been reported to date (Choudhary et al., 2009; Gil et al., 2017; Kim et al., 2006; Lundby et al., 2012; Zhao et al., 2010), providing an enormous functional diversity that may be implicated in the tumorigenesis or the manifestations of malignancies (Ali et al., 2018; Hu et al., 2022; Liu et al., 2017; Shvedunova and Akhtar, 2022; Wang and Zhao, 2022). We attempted to exemplify the complex biology of acetylation in cancer by overviewing its implications in histone regulation, metabolic pathways, oncoproteins and tumor suppressors.
As one of the major epigenetics systems, histone proteins can have various PTMs, including acetylation, methylation, ubiquitination, phosphorylation, and SUMOylation, all of which can affect the chromatin conformation and subsequent gene expression. Among these PTMs, alterations in histone acetylation play important roles in cancer development and progress (Fullgrabe et al., 2011; Harachi et al., 2021). Histone acetylation may occur on all core histone proteins. The well-studied histone H4 acetylation on lysine 16 (H4K16ac) is usually localized at the promoters and enhancers of genes that its acetylation state can activate gene transcription. Removal of the H4K16ac mark could decouple the binding of transcriptional factors at the promoters and subsequently inactivate gene transcription. Furthermore, loss of H4K16ac might also affect chromatin conformation and contribute to genome instability. Studies have evidenced that reduction in H4K16ac is associated with various cancer types and chemoresistance of cancer cells, and correlated with tumor progression (Elsheikh et al., 2009; Hajji et al., 2010). The global reduction of H4K16ac, together with loss of trimethylation of histone H4 at lysine 20 (H4K20me3) and DNA hypomethylation at repetitive DNA sequences are often considered as a common hallmark of malignant transformation (Fraga et al., 2005). In breast cancer, low or absent H4K16ac might be an early sign of cancer development, while moderate to low levels of H3K9ac, H3K18ac and H4K12ac were associated with poorer prognostic subtypes (Elsheikh et al., 2009). Downregulations of H3K9ac, H3K18ac and H4K16ac were strongly correlated with the risk of prostate cancer recurrence (Seligson et al., 2005). In the case of non-small cell lung carcinoma (NSCLC), upregulation of H4K5ac and H4K8ac and downregulation of H4K12ac, H4K16ac and H2AK5ac were correlated with the progression of NSCLC (Barlesi et al., 2007; Van Den Broeck et al., 2008), while reduction in H3K9ac was associated with better survival (Barlesi et al., 2007).
Since histone acetylation directly impacts gene transcription and is involved in the process of tumorigenesis, small molecules targeting histone acetylation are being developed as potential anti-cancer therapeutic drugs. Currently, there are five FDA approved histone deacetylase (HDAC) inhibitors for cancer treatment, with many more inhibitors targeting histone acetylation are still under clinical trials (Table 1) (Wu et al., 2020).
Lysine acetylation of non-histone substrates, including many oncoproteins, tumor suppressors and important enzymes involved in cancer metabolism, is significantly implicated in tumorigenesis (Harachi et al., 2021; Kaypee et al., 2016). The regulatory effects of acetylation on these proteins are multifaceted. For example, by competing lysine residues with ubiquitination, which mediates proteins for proteasomal degradation, acetylation can influence protein stability of p53, SMAD7 and c-MYC (Gronroos et al., 2002; Ito et al., 2002; Patel et al., 2004). Acetylation can also influence protein-protein interactions (Cohen et al., 2004) and subcellular localization of proteins (di Bari et al., 2006). Mutations of KRAS are common for the development of many cancers. A study showed that acetylation of KRAS at K104 attenuated its transforming activity and was a negative regulatory modification (Yang et al., 2012). For tumor suppressor p53, acetylation at K305, K370, K372, K373, K381, K382 and K320 increases its DNA-binding ability and activates its transcription target genes (Glozak and Seto, 2007).
Acetylation also influences the function of many key proteins in the regulation of tumor metabolism. Up to 20% of mitochondrial proteins have acetylation that may affect numerous metabolic pathways, such as the TCA cycle, urea cycle, and oxidative phosphorylation (Harachi et al., 2021). Alterations in the mitochondrial acetylation could contribute to the aerobic glycolysis and other metabolic reprogramming phenomenon observed in cancer. For example, acetylation of glyceraldehyde 3-phosphate dehydrogenase (GAPDH), an essential enzyme in glycolysis, at K254 was found to accelerate tumor growth (Li et al., 2014). The reduction in acetylation of lactate dehydrogenase (ALDH-A) at K5 during the initiation of pancreatic cancer was found to promote tumor metabolism and cancer progression (Zhao et al., 2013).
4. Glycosylation
Most secretory and membrane-bound proteins produced by mammalian cells contain covalently linked glycans with diverse structures (Rudd and Dwek, 1997). Glycosylation plays a pivotal role in many biological processes, such as protein folding, cell adhesion and trafficking, cell signaling, pathogen recognition and immune response (Haltiwanger and Lowe, 2004; Helenius and Aebi, 2001; Kudelka et al., 2020; Laubli and Borsig, 2019; Marth and Grewal, 2008; Ohtsubo and Marth, 2006; Peixoto et al., 2019; Rudd et al., 2001; Woods et al., 1994). The most common forms of glycosylation are N-linked and O-linked glycosylation (Bertozzi and Kiessling, 2001; Khoury et al., 2011; Varki et al., 2015). While O-linked glycans are linked to the hydroxyl group on serine (Ser) or threonine (Thr) residues, N-linked glycans are attached to the amide group of asparagine residues in a consensus Asn-X-Ser/Thr sequence (X can be any amino acid except proline) (Bause, 1983; Bertozzi and Kiessling, 2001; Khoury et al., 2011; Varki et al., 2015). Glycan synthesis is not template bound but involves the concerted action of glycosidases, glycosyltransferases, and glycan-modifying enzymes. Environmental factors, immune pressure and altered metabolic mechanisms can lead to genetic and epigenetic modification of these enzymes and result in altered glycan biosynthesis and protein glycosylation that drive several key biological processes in cancer (Kannagi et al., 2010; Pinho and Reis, 2015). The known aberrant glycosylation implicated in cancer may include changes in global sialylation and fucosylation, N- and O-linked glycan branching, O-glycan truncation (Arnold et al., 2008; Christiansen et al., 2014; Hakomori, 2002), as well as abnormal glycosylation occupancy (Pan et al., 2014).
The modifications of carbohydrates may be cell-, protein- and glycosylation site-specific, and involve incomplete synthesis or neo-synthesis of glycans (Hakomori, 2002; Hakomori and Kannagi, 1983). Increased sialylation, such as sialyl Lewis a (SLea) and SLex), have been associated with many cancers (Kannagi et al., 2008). While SLea is the antigen of CA19–9 clinical assay for pancreatic and other GI-tract cancers for therapeutic monitoring (Indellicato et al., 2020; Luo et al., 2021), the expression levels of SLex, which is a ligand for selectins (Rosen and Bertozzi, 1994), have been correlated with poor cancer survival (Amado et al., 1998; Baldus et al., 1998). Core fucosylation is the addition of α1,6-fucose to the innermost GlcNAc residue of N-glycans. Increase of core fucosylation through the action of Fuc-TVIII have been associated with multiple cancers, including breast, lung and liver cancer (Hutchinson et al., 1991; Liu et al., 2011; Potapenko et al., 2010). Core fucosylated α-fetoprotein (AFP-L3) is a clinical biomarker for the early detection of hepatocellular carcinoma (HCC) (Aoyagi et al., 1985). The increased expression of complex β1,6-branched N-linked glycan are frequently associated with malignant transformation (Dennis et al., 1987). The abnormal expression of shortened or truncated O-linked glycan, including disaccharide Thomsen–Friedenreich antigen (T antigen) and the monosaccharide GalNAc (Tn), as well as their sialylated forms (ST and STn, respectively), have been associated with many cancers (Pinho et al., 2007; Radhakrishnan et al., 2014).
In addition to their direct implication in malignancy, glycans also profoundly influence protein functionality (Helenius and Aebi, 2001), altering many biological processes in cancer, such as inflammation, immune surveillance, cell adhesion, extracellular modification, inter- and intracellular signaling, and cellular metabolism (Pinho and Reis, 2015). Proteomic study and network analysis have found a group of cancer associated glycoproteins, including MUC5AC, CEACAM5, IGFBP3 and LGALS3BP, substantially hyper-glycosylated in pancreatic ductal adenocarcinoma tissue, and their increased activity of N-glycosylation was implicated in TGF-β, TNF and NF-kappa-B pathways (Pan et al., 2014). Mucins are a heavily glycosylated protein family that is well known to be associated with epithelial cancers, such as colorectal and pancreatic cancers. The glycan component can make up more than 50% of the molecular weight of a mucin glycoprotein, modulating their functionality in tumorigenesis as well as cancer cell interaction with the tumor microenvironment (Andrianifahanana et al., 2006; Chugh et al., 2015; Kudelka et al., 2015; Nagata et al., 2007; Pan et al., 2016). E-cadherin is a tumor suppressor protein and a biomarker for the epithelial mesenchymal transition (EMT). The addition of GnT-V-mediated β1,6GlcNAc-branched N-glycans to E-cadherin compromises cell-cell adhesion, promoting cancer invasiveness and metastases (Guo et al., 2003; Pinho et al., 2013). In cancer metabolism and signaling, increased O-GlcNAcylation crosstalk with phosphorylation can stabilize c-MYC, a cancer suppressor protein, contributing to oncogenesis (Itkonen et al., 2013).
The majority of the cell-surface receptors are transmembrane glycoproteins, such as epithelial growth factor receptor (EGFR), IGF receptor (IGFR), fibroblast growth factor receptor (FGFR), TGF β receptor (TGFβR) and integrins. Increased complex N-glycan number and branching affects the stability and retention of receptors at cell surface for proper functioning by modulating the interaction of branched N-glycans with galectin-3 and galectin-1, thus altering the signaling (Lau et al., 2007). In addition, aberrant glycosylation on cancer cell surface can modulate their interactions with various lectins, which mediate immune and inflammatory responses, and help tumor cells escape immune surveillance (Liu and Rabinovich, 2005).
Immunosuppressive ligand, programmed death-ligand 1 (PD-L1), is frequently overexpressed and heavily glycosylated in cancer tissues. Studies found that the glycosylation in PD-L1 was required for proper interaction with receptor programmed cell death protein-1 (PD-1) in triple-negative breast cancer (Hsu et al., 2018; Li et al., 2018). Antibody specifically targeting the glycosylated PD-L1 was able to block PD-L1/PD-1 interaction and promote PD-L1 internalization and degradation, providing a potential approach targeting protein glycosylation to enhance immune checkpoint therapy (Li et al., 2018).
Given the pivotal role of protein glycosylation in many biological processes and the tendency of glycoprotein entering circulation, malignancy associated glycoproteins represent promising biomarkers for diagnostics and prognostics. In fact, many currently FDA-approved cancer biomarkers are glycoproteins or glycosylation assays, including: a-fetoprotein (AFP) in liver cancer; prostate-specific antigen (PSA) in prostate cancer; cancer antigen 125 (CA125) and human epididymis protein 4 (HE4) in ovarian cancer; carcinogenic embryonic antigen (CEA) in colorectal cancer, CA 19–9 in pancreatic cancer, HER2/NEU and CA15–3 and CA27–29 in breast cancer, and thyroglobulin (Tg) in thyroid cancer (Kirwan et al., 2015).
5. Palmitoylation
Protein lipidation is the covalent addition of various lipids to the residues of proteins, including cysteine, serine, lysine, or histidine (Nadolski and Linder, 2007). Protein lipidation increases the hydrophobicity of target proteins and affects their conformation and stability, binding affinity to membranes, subcellular localizations, and association with other biomolecules. Therefore, lipidation can regulate protein function, and is mechanistically linked to lipid metabolism and cellular energy homeostasis. Among the diverse types of lipidation, palmitoylation is the most common and best studied lipid modification (Linder and Deschenes, 2007). Protein palmitoylation has been associated with various cancer, and aberrantly palmitoylated proteins may indicate their functional changes in cancer (Chen et al., 2018; Chen et al., 2020; Yang et al., 2010). In addition, fatty acid synthase, the enzyme system which catalyzes palmitate synthesis, is frequently upregulated in cancers and has been identified as a potential therapeutic target (Fhu and Ali, 2020; Menendez and Lupu, 2007).
Palmitoylation as well as depalmitoylation is implicated in cancer metabolism and the physiological state of mitochondria. Palmitoylation of CD36 increases fatty acid uptake and oxidation, facilitating liver cancer development (Zhao et al., 2018). Palmitoylation of estrogen receptor α and GLUT4 increases glucose uptake in estrogen receptor-positive breast cancer cells and CHO-IR cells, respectively (Garrido et al., 2013; Ren et al., 2015). Palmitoylation of TMX1 and CKAP4 increases mitochondrial respiration (Harada et al., 2020; Raturi et al., 2016). Reversely, depalmitoylation of KRAS4A (Amendola et al., 2019) and PRDX5 (Cao et al., 2019) increases glycolytic flux and mitochondrial redox buffering capacity, respectively. These studies demonstrated that palmitoylation, as well as other lipidations, play a crucial role in impacting cancer metabolism. In cancer development, protein lipidation is a potent regulator of apoptotic calcium signaling (Chen and Boehning, 2017). Studies have also suggested that lipidation of Wnt proteins, which regulates cell proliferation and differentiation, are implicated in intestinal carcinogenesis and cancer (Kaemmerer and Gassler, 2016). Some nonspecific inhibitors of protein palmitoylation have been developed for research. However, at present, no therapeutic drugs have been developed to target the palmitoylation status of specific target proteins, such as Ras proteins (Cox et al., 2015).
6. Ubiquitination and SUMOylation
Ubiquitination is the addition of the evolutionally conserved ubiquitin protein to target proteins (Hershko and Ciechanover, 1998). The multistep process is mediated by ubiquitin activating enzymes (E1), ubiquitin conjugating enzymes (E2) and ubiquitin ligases (E3). In addition to its important role in proteasome degradation, the physiological functions of ubiquitination also involve non-proteolytic signaling, such as regulation of inflammatory pathway, autophagy, DNA repair, T cell receptor signaling and enzymatic activity (Bhattacharjee and Nandi, 2017; Kattah et al., 2017; Martin-Vicente et al., 2017). In cancer, ubiquitination plays a pivotal role in tumorigenic pathways by regulating the activity or degradation of tumor promoting or suppressor proteins. CDKs are the primary drivers of cell cycle transition and its activities are regulated through ubiquitination of the key regulators, including cyclins, CDK inhibitors (CKIs), other kinases and phosphatases (Nakayama and Nakayama, 2006). Deregulation of ubiquitination-proteasome system (UPS), such as ubiquitination status of cyclins, might result in uncontrolled proliferation, migration and genomic instability in cancer (Dong et al., 2018; Liu et al., 2019; Shan et al., 2009). The dynamic and reversible ubiquitination of tumor suppressor p53 is a central mechanism that controls p53 regulation. In cancer cells, p53 undergoes ubiquitination by interacting with the RING finger E3 ubiquitin protein ligase MDM2, and is subsequently subject to degradation by UPS (Kussie et al., 1996). In a cancer-associated inflammatory response, ubiquitination is utilized to regulate various components of the TNF-induced signaling complexes by ubiquitin conjugation and deubiquitination. Ubiquitination of RIPK1, a key regulator of TNF-mediated apoptosis, necroptosis and inflammatory pathways, on lysine 377 in human was shown to be critical for NF-kB activation (Li et al., 2006). In TGF-β signaling cascade, higher protein ubiquitination and accelerated degradation of downstream SMAD4 can lead to inhibition of transcriptional response of TGF-β (Wan et al., 2005).
Similar to ubiquitination, SUMOylation is the covalent attachment of small ubiquitin-like modifier (SUMO) to the lysine residues in target proteins (Seeler and Dejean, 2017). Known SUMO isoforms include SUMO1, SUMO2/3 and SUMO4. In cancer, many oncoproteins and tumor suppressors are functionally regulated via SUMOylation. For example, BRCA1 is a tumor suppress gene associated with a high risk of breast and ovarian cancer, and its activity is regulated by the SUMO pathway. BRCA1 is SUMOylated in response to genotoxic stress, and co-localizes at sites of DNA damage with SUMO1, SUMO2/3 and the SUMO-conjugating enzyme Ubc9 (Morris et al., 2009). SUMO pathway plays an important role in many aspects of carcinogenesis, including DNA damage response, cancer cell proliferation, invasion, metastasis and apoptosis.
Targeting the dysregulated UPS is an active area of therapeutic development against cancer. Many inhibitors have been developed targeting different components of the UPS, including the proteasome, E3, E1, E2, and DUBs (Deng et al., 2020). Currently, there are five FDA approved ubiquitination targeting drugs and many more are under preclinical studies and clinical trials for cancer treatment (Table 1) (Deng et al., 2020).
7. PTM crosstalk
PTM crosstalk or PTM code is the complex and dynamic interplay between multiple PTMs to influence the actions of each other (Huang et al., 2019; Lothrop et al., 2013; Minguez et al., 2015; Minguez et al., 2012; Wu et al., 2019). PTM crosstalk can occur in the fashion of either intraprotein or interprotein, between the same or different types of modifications. Regardless of the actions, PTM crosstalk can orchestrate sophisticated interactions of PTMs to influence the functions, signaling pathways and regulation of protein networks in tunorigenesis. For instance, Ras/MAPK, TGF-β/Smad, PD-L1/PD-1 and TP53 pathways are among the most important regulatory signaling pathways involved in various cancers and metastasis. In the RAS/MAPK pathway, KRAS and other signal mediators are subject to multiple PTMs, including phosphorylation, ubiquitination, farnesylation, proteolysis, methylation and palmitoylation (Ahearn et al., 2011; Laude and Prior, 2008). These PTMs regulate the trafficking and localization of KRAS into the membrane, and influence KRAS activity and signaling. Dysregulation of the TGF-β signaling pathway is commonly implicated at cancer initiation and progression. Many signaling mediators in the TGF-β pathway are subject to extensive PTMs (Xu et al., 2016), including phosphorylation and ubiquitination, which are critical for the initiation and regulation of the signal transduction into the nucleus. As discussed in above, the key T-cell checkpoint ligand, PD-L1, which shows increased positivity in multiple cancers (Teng et al., 2015; van der Woude et al., 2017), is subject to extensive regulation by PTMs, including phosphorylation,N-linked glycosylation, acetylation and ubiquitination (Hsu et al., 2018; Li et al., 2016). Aberrant alterations of PTMs directly affect PD-L1 protein stabilization, subcellular localization and PD-L1-mediated immune resistance (Hsu et al., 2018). The activation/inactivation of tumor suppressor p53 functions, which regulates cellular responses to various stress signals, is modulated by a wide spectrum of PTMs, including phosphorylation, ubiquitination, acetylation, methylation, SUMOylation and neddylation (Bode and Dong, 2004; Dai and Gu, 2010). These are a few examples among the numerous PTM crosstalks implicated in cancer. As an emerging field in PTM studies, PTM crosstalk in many diseases, including cancer, remains largely unexplored.
8. Mass spectrometry-based PTM analysis
Mass spectrometry (MS)-based proteomics is currently the best available tool to meet the challenges for proteome-wide PTM analysis, offering unprecedented resolution and throughput for precise site mapping and structure identification, qualitatively or quantitatively (Olsen and Mann, 2013). The compositional and structural modifications in various amino acid residues due to PTMs can be dramatically different and may require different strategies for analysis. Furthermore, the enormous complexity and vast dynamic range of protein abundances in a proteome pose additional challenges in PTM analysis, as many peptides with PTMs are low abundance and subject to low sensitivity for MS detection. Using phosphorylation, glycosylation, ubiquitination, acetylation and palmitoylation as examples, Figure 2 illustrates the generic workflow for a PTM characterization. The concerted approaches typically comprise of several integrated modules, including sample preparation, PTM enrichment, chemical/biological cleavage or derivatization, separation strategy and mass spectrometric acquisition, followed by corresponding bioinformatics analysis.
Fig. 2.
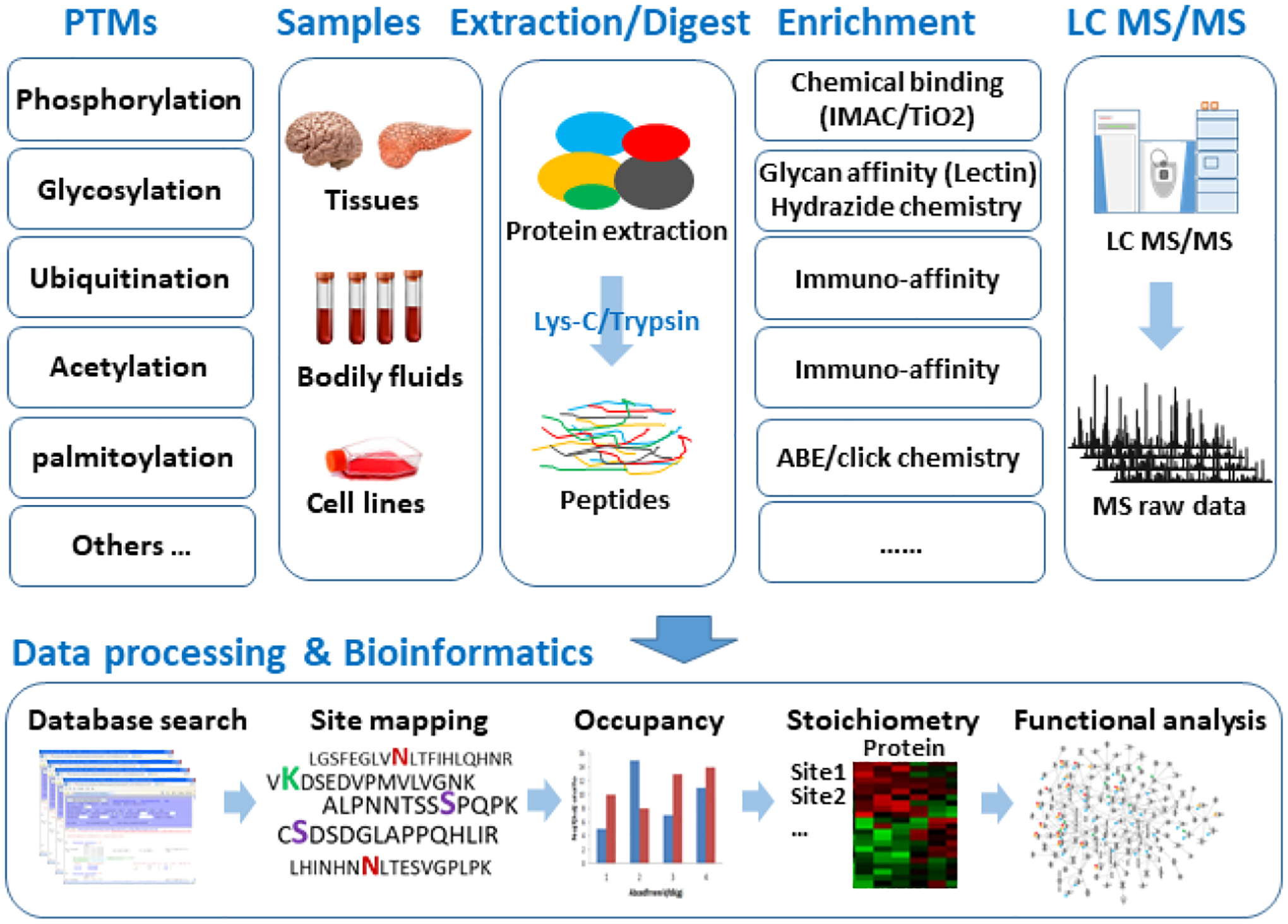
A generic workflow of integrated PTM analysis, using glycosylation, phosphorylation, ubiquitination, acetylation and palmitoylation as examples.
For many PTMs with small universal chemical groups, such as phosphorylation, and acetylation, tens of thousands of sites can now be precisely located and confidently identified in the sequence of the protein in complex biological samples (Gil et al., 2017; Jensen, 2004; Ke et al., 2016; Low et al., 2020; Pagel et al., 2015; Paulo and Schweppe, 2021; Polat and Ozlu, 2014; Riley and Coon, 2016; Roux and Thibault, 2013; Simithy et al., 2018; Trost et al., 2010; Zhao and Jensen, 2009). Nonetheless, a comprehensive, quantitative analysis of these PTMs has never been trivial, and may involve multiple steps of optimizations in purification, enrichment and quantification prior to MS analysis. For instance, phosphoproteomics may be enhanced by strategies for phosphoprotein or phosphopeptide enrichment, such as IMAC, TiO2 or HILIC, as well as MS technique of soft fragmentation, such as electron-transfer dissociation (ETD) (Junger and Aebersold, 2014; Riley et al., 2017).
For PTMs with complex and heterogeneous structures, such as glycosylation, ubiquitination, SUMOylation, lipidation, various strategies have been developed to overcome the analytical hurdles. Below we will briefly discuss several chemical or biological methods that are often utilized to proteolyze or derivatize these PTMs to generate specific mass tags that can be recognized by MS analysis.
A glycoproteomic analysis is complicated not only by the variety of carbohydrates, but also by the complex glycosidic linkage of the glycan to the protein. Various methods have been developed for enrichment of glycoproteins or glycopeptides to enhance a glycoproteomic analysis, such as lectin affinity, hydrazide chemistry, hydrophilic interaction and size-exclusion (Alagesan et al., 2020; Narimatsu et al., 2018; Pan et al., 2011; Peng et al., 2021; Riley et al., 2021). Conventional technologies can separately profile the deglycosylated glycoproteins using a proteomic approach (Liu et al., 2013; Pan et al., 2011; Sjostrom et al., 2015; Wollscheid et al., 2009; Zhang et al., 2003; Zhang et al., 2007), or identify the cleaved glycan structures using glycomics (Morelle and Michalski, 2005; Orlando, 2010; Shriver et al., 2004; Vercoutter-Edouart et al., 2008; Wuhrer, 2013; Wuhrer et al., 2009). However, a technical hurdle still exists in integrating the information together to define the glycoforms with site-specific glycan structures. With the recent advances in MS and bioinformatics, hybrid fragmentation technologies, such as stepped higher energy collisional dissociation (HCD) or electron-transfer/higher energy collision dissociation (EThcD), have been increasingly applied for intact glycopeptide analysis to obtain in situ information on site-specific glycoforms (Bollineni et al., 2018; Brunner et al., 2015; Gaunitz et al., 2017; Hoffmann et al., 2016; Riley and Coon, 2018; Thaysen-Andersen et al., 2016; Ye et al., 2019; Zhu et al., 2020).
The analysis of ubiquitination is enhanced by targeting the diglycyllysine Lys-ε-Gly-Gly (K-ε-GG) remnant produced by tryptic digestion of proteins, which cleaves ubiquitinated lysine side-chains (Kang and Yi, 2011; Udeshi et al., 2013). The K-ε-GG remnants in ubiquitinated lysine residues not only serve as a mass tag for precise mapping of ubiquitination site, but also can be used for enrichment of ubiquitinated peptides using antibodies that recognize K-ε-GG. In SUMOylation analysis, tryptic digestion of small ubiquitin-like polypeptides produce large branched peptides instead of K-ε-GG. These large remnants of SUMO conjugates drastically complicate the fragmentation pattern of the SUMOylated peptides, hampering the identification of SUMO acceptor sites in target proteins (Filosa et al., 2013; Hendriks et al., 2014; Lamoliatte et al., 2014; Tammsalu et al., 2015). A recent effort has demonstrated a method for proteome-wide detection of endogenous SUMOylation using α-lytic protease, which digests SUMO conjugates and generates SUMO-remnant K-ε-GG for MS analysis (Lumpkin et al., 2017).
Methods have also been developed to enrich and characterize protein palmitoylation, including hydroxylamine-mediated acyl-biotin exchange (ABE) and click chemistry based approaches (Collins et al., 2017; Drisdel and Green, 2004; Forrester et al., 2011; Hang et al., 2007; Martin and Cravatt, 2009; Yang et al., 2010). Using combinations of these approaches enabled MS identification of many palmitoylated proteins in various biological samples (Collins et al., 2017; Rodenburg et al., 2017; Thinon et al., 2018; Wang and Schey, 2018; Won and Martin, 2018).
Proteome-wide analysis of protein PTMs has become a unique domain in MS-based proteomics. Quantitative assessment of PTM status, and precise identification of PTM sites and detailed structures, especially those with complex and heterogeneous structures, still remain challenging and require integrated approaches that involve multiple technical aspects. In the human proteome, while a complete inventory of modification sites has not been established for any PTM, methods and strategies have been developed for many PTMs, and hundreds and thousands of PTM sites have been identified. The analysis of PTMs has emerged as a vibrant field in cancer research.
Conclusion Remarks
The implications of various PTMs in tumorigenesis, metastasis, and anti-cancer strategies have been increasingly recognized. Altered status of many PTMs are associated with cancer hallmarks and manifestations. This is an important emerging area in cancer research, which could lead to novel mechanisms and therapeutic strategies to facilitate early detection and treatment of cancer. Mounting efforts have been driven towards investigating dynamic occupancy or stoichiometry of site-specific PTMs and their interplays to establish PTM landscapes implicated in cancer. Given that PTMs afford another layer of regulation for the core proteins, exploiting PTMs for development of diagnostic markers and therapeutic targets is intriguing and has shown to be successful. It is expected that the advances in mass spectrometry and bioinformatics, as well as perspectives from systems biology, will facilitate PTM studies in cancer biology and clinical applications.
Funding:
This work was supported in part by federal fund from the National Institutes of Health (NIH) under grant R01CA211892 and R01CA180949, the Cancer Prevention & Research Institute of Texas (CPRIT) (RP210111), and Rochelle and Max Levit endowment fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest.
Reference List
- Ahearn IM, Haigis K, Bar-Sagi D, Philips MR, 2011. Regulating the regulator: posttranslational modification of RAS. Nat. Rev. Mol. Cell Biol 13 (1), 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagesan K, Hoffmann M, Rapp E, Kolarich D, 2020. Glycoproteomics Technologies in Glycobiotechnology. Adv Biochem Eng Biotechnol. [DOI] [PubMed] [Google Scholar]
- Ali I, Conrad RJ, Verdin E, Ott M, 2018. Lysine Acetylation Goes Global: From Epigenetics to Metabolism and Therapeutics. Chem Rev 118 (3), 1216–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amado M, Carneiro F, Seixas M, Clausen H, Sobrinho-Simoes M, 1998. Dimeric sialyl-Le(x) expression in gastric carcinoma correlates with venous invasion and poor outcome. Gastroenterology 114 (3), 462–470. [DOI] [PubMed] [Google Scholar]
- Amendola CR, Mahaffey JP, Parker SJ, Ahearn IM, Chen WC, Zhou M, Court H, Shi J, Mendoza SL, Morten MJ, Rothenberg E, Gottlieb E, Wadghiri YZ, Possemato R, Hubbard SR, Balmain A, Kimmelman AC, Philips MR, 2019. KRAS4A directly regulates hexokinase 1. Nature 576 (7787), 482–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrianifahanana M, Moniaux N, Batra SK, 2006. Regulation of mucin expression: mechanistic aspects and implications for cancer and inflammatory diseases. Biochim Biophys Acta 1765 (2), 189–222. [DOI] [PubMed] [Google Scholar]
- Aoyagi Y, Isemura M, Suzuki Y, Sekine C, Soga K, Ozaki T, Ichida F, 1985. Fucosylated alpha-fetoprotein as marker of early hepatocellular carcinoma. Lancet 2 (8468), 1353–1354. [DOI] [PubMed] [Google Scholar]
- Arnold JN, Saldova R, Hamid UM, Rudd PM, 2008. Evaluation of the serum N-linked glycome for the diagnosis of cancer and chronic inflammation. Proteomics 8 (16), 3284–3293. [DOI] [PubMed] [Google Scholar]
- Baldus SE, Zirbes TK, Monig SP, Engel S, Monaca E, Rafiqpoor K, Hanisch FG, Hanski C, Thiele J, Pichlmaier H, Dienes HP, 1998. Histopathological subtypes and prognosis of gastric cancer are correlated with the expression of mucin-associated sialylated antigens: Sialosyl-Lewis(a), Sialosyl-Lewis(x) and sialosyl-Tn. Tumour Biol 19 (6), 445–453. [DOI] [PubMed] [Google Scholar]
- Barlesi F, Giaccone G, Gallegos-Ruiz MI, Loundou A, Span SW, Lefesvre P, Kruyt FA, Rodriguez JA, 2007. Global histone modifications predict prognosis of resected non small-cell lung cancer. J Clin Oncol 25 (28), 4358–4364. [DOI] [PubMed] [Google Scholar]
- Bause E, 1983. Structural requirements of N-glycosylation of proteins. Studies with proline peptides as conformational probes. Biochem. J 209 (2), 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton CB, Fiskus W, Bhalla KN, 2017. Targeting Histone Acetylation: Readers and Writers in Leukemia and Cancer. Cancer J 23 (5), 286–291. [DOI] [PubMed] [Google Scholar]
- Bertozzi CR, Kiessling LL, 2001. Chemical glycobiology. Science 291 (5512), 2357–2364. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee S, Nandi S, 2017. DNA damage response and cancer therapeutics through the lens of the Fanconi Anemia DNA repair pathway. Cell Commun Signal 15 (1), 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode AM, Dong Z, 2004. Post-translational modification of p53 in tumorigenesis. Nat. Rev. Cancer 4 (10), 793–805. [DOI] [PubMed] [Google Scholar]
- Bollineni RC, Koehler CJ, Gislefoss RE, Anonsen JH, Thiede B, 2018. Large-scale intact glycopeptide identification by Mascot database search. Sci Rep 8 (1), 2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks CL, Gu W, 2003. Ubiquitination, phosphorylation and acetylation: the molecular basis for p53 regulation. Curr Opin Cell Biol 15 (2), 164–171. [DOI] [PubMed] [Google Scholar]
- Brooksbank C, 2001. Phosphorylation. The key to staying faithful. Nat Rev Mol Cell Biol 2 (3), 167. [DOI] [PubMed] [Google Scholar]
- Brunner AM, Lossl P, Liu F, Huguet R, Mullen C, Yamashita M, Zabrouskov V, Makarov A, Altelaar AF, Heck AJ, 2015. Benchmarking multiple fragmentation methods on an orbitrap fusion for top-down phospho-proteoform characterization. Anal Chem 87 (8), 4152–4158. [DOI] [PubMed] [Google Scholar]
- Buday L, Vas V, 2020. Novel regulation of Ras proteins by direct tyrosine phosphorylation and dephosphorylation. Cancer Metastasis Rev 39 (4), 1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugter JM, Fenderico N, Maurice MM, 2021. Mutations and mechanisms of WNT pathway tumour suppressors in cancer. Nat Rev Cancer 21 (1), 5–21. [DOI] [PubMed] [Google Scholar]
- Campbell SL, Philips MR, 2021. Post-translational modification of RAS proteins. Curr Opin Struct Biol 71, 180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Qiu T, Kathayat RS, Azizi SA, Thorne AK, Ahn D, Fukata Y, Fukata M, Rice PA, Dickinson BC, 2019. ABHD10 is an S-depalmitoylase affecting redox homeostasis through peroxiredoxin-5. Nat Chem Biol 15 (12), 1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler KB, Costello CE, Rahimi N, 2019. Glycosylation in the Tumor Microenvironment: Implications for Tumor Angiogenesis and Metastasis. Cells 8 (6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PC, Campbell M, Robertson ES, 2016. Human Oncogenic Herpesvirus and Posttranslational Modifications - Phosphorylation and SUMOylation. Front Microbiol 7, 962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SC, Ding JL, 2018. Ubiquitination and SUMOylation in the chronic inflammatory tumor microenvironment. Biochim. Biophys. Acta Rev. Cancer 1870 (2), 165–175. [DOI] [PubMed] [Google Scholar]
- Chen B, Sun Y, Niu J, Jarugumilli GK, Wu X, 2018. Protein Lipidation in Cell Signaling and Diseases: Function, Regulation, and Therapeutic Opportunities. Cell Chem Biol 25 (7), 817–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Boehning D, 2017. Protein Lipidation As a Regulator of Apoptotic Calcium Release: Relevance to Cancer. Front Oncol 7, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Liu S, Tao Y, 2020. Regulating tumor suppressor genes: post-translational modifications. Signal Transduct Target Ther 5 (1), 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M, 2009. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325 (5942), 834–840. [DOI] [PubMed] [Google Scholar]
- Choudhary C, Mann M, 2010. Decoding signalling networks by mass spectrometry-based proteomics. Nat Rev Mol Cell Biol 11 (6), 427–439. [DOI] [PubMed] [Google Scholar]
- Christiansen MN, Chik J, Lee L, Anugraham M, Abrahams JL, Packer NH, 2014. Cell surface protein glycosylation in cancer. Proteomics 14 (4–5), 525–546. [DOI] [PubMed] [Google Scholar]
- Chugh S, Gnanapragassam VS, Jain M, Rachagani S, Ponnusamy MP, Batra SK, 2015. Pathobiological implications of mucin glycans in cancer: Sweet poison and novel targets. Biochim Biophys Acta 1856 (2), 211–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HY, Lavu S, Bitterman KJ, Hekking B, Imahiyerobo TA, Miller C, Frye R, Ploegh H, Kessler BM, Sinclair DA, 2004. Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. Mol Cell 13 (5), 627–638. [DOI] [PubMed] [Google Scholar]
- Collins MO, Woodley KT, Choudhary JS, 2017. Global, site-specific analysis of neuronal protein S-acylation. Sci Rep 7 (1), 4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox AD, Der CJ, Philips MR, 2015. Targeting RAS Membrane Association: Back to the Future for Anti-RAS Drug Discovery? Clin Cancer Res 21 (8), 1819–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cseh B, Doma E, Baccarini M, 2014. “RAF” neighborhood: protein-protein interaction in the Raf/Mek/Erk pathway. FEBS Lett 588 (15), 2398–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Gu W, 2010. p53 post-translational modification: deregulated in tumorigenesis. Trends Mol. Med 16 (11), 528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De SF, Sandri S, Ferrarini G, Pagliarello I, Sartoris S, Ugel S, Marigo I, Molon B, Bronte V, 2014. The emerging immunological role of post-translational modifications by reactive nitrogen species in cancer microenvironment. Front Immunol 5, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Meng T, Chen L, Wei W, Wang P, 2020. The role of ubiquitination in tumorigenesis and targeted drug discovery. Signal Transduct Target Ther 5 (1), 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis JW, Laferte S, Waghorne C, Breitman ML, Kerbel RS, 1987. Beta 1–6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science 236 (4801), 582–585. [DOI] [PubMed] [Google Scholar]
- Deribe YL, Pawson T, Dikic I, 2010. Post-translational modifications in signal integration. Nat Struct Mol Biol 17 (6), 666–672. [DOI] [PubMed] [Google Scholar]
- di Bari MG, Ciuffini L, Mingardi M, Testi R, Soddu S, Barila D, 2006. c-Abl acetylation by histone acetyltransferases regulates its nuclear-cytoplasmic localization. EMBO Rep 7 (7), 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L, Yu L, Bai C, Liu L, Long H, Shi L, Lin Z, 2018. USP27-mediated Cyclin E stabilization drives cell cycle progression and hepatocellular tumorigenesis. Oncogene 37 (20), 2702–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drisdel RC, Green WN, 2004. Labeling and quantifying sites of protein palmitoylation. Biotechniques 36 (2), 276–285. [DOI] [PubMed] [Google Scholar]
- Du Z, Lovly CM, 2018. Mechanisms of receptor tyrosine kinase activation in cancer. Mol Cancer 17 (1), 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan G, Walther D, 2015. The roles of post-translational modifications in the context of protein interaction networks. PLoS Comput Biol 11 (2), e1004049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsheikh SE, Green AR, Rakha EA, Powe DG, Ahmed RA, Collins HM, Soria D, Garibaldi JM, Paish CE, Ammar AA, Grainge MJ, Ball GR, Abdelghany MK, Martinez-Pomares L, Heery DM, Ellis IO, 2009. Global histone modifications in breast cancer correlate with tumor phenotypes, prognostic factors, and patient outcome. Cancer Res 69 (9), 3802–3809. [DOI] [PubMed] [Google Scholar]
- Fhu CW, Ali A, 2020. Fatty Acid Synthase: An Emerging Target in Cancer. Molecules 25 (17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa G, Barabino SM, Bachi A, 2013. Proteomics strategies to identify SUMO targets and acceptor sites: a survey of RNA-binding proteins SUMOylation. Neuromolecular Med 15 (4), 661–676. [DOI] [PubMed] [Google Scholar]
- Forrester MT, Hess DT, Thompson JW, Hultman R, Moseley MA, Stamler JS, Casey PJ, 2011. Site-specific analysis of protein S-acylation by resin-assisted capture. J Lipid Res 52 (2), 393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad YA, Aanei C, 2017. Revisiting the hallmarks of cancer. Am J Cancer Res 7 (5), 1016–1036. [PMC free article] [PubMed] [Google Scholar]
- Fraga MF, Ballestar E, Villar-Garea A, Boix-Chornet M, Espada J, Schotta G, Bonaldi T, Haydon C, Ropero S, Petrie K, Iyer NG, Perez-Rosado A, Calvo E, Lopez JA, Cano A, Calasanz MJ, Colomer D, Piris MA, Ahn N, Imhof A, Caldas C, Jenuwein T, Esteller M, 2005. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat Genet 37 (4), 391–400. [DOI] [PubMed] [Google Scholar]
- Fullgrabe J, Kavanagh E, Joseph B, 2011. Histone onco-modifications. Oncogene 30 (31), 3391–3403. [DOI] [PubMed] [Google Scholar]
- Garrido P, Moran J, Alonso A, Gonzalez S, Gonzalez C, 2013. 17beta-estradiol activates glucose uptake via GLUT4 translocation and PI3K/Akt signaling pathway in MCF-7 cells. Endocrinology 154 (6), 1979–1989. [DOI] [PubMed] [Google Scholar]
- Gaunitz S, Nagy G, Pohl NL, Novotny MV, 2017. Recent Advances in the Analysis of Complex Glycoproteins. Anal Chem 89 (1), 389–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil J, Ramirez-Torres A, Encarnacion-Guevara S, 2017. Lysine acetylation and cancer: A proteomics perspective. J Proteomics 150, 297–309. [DOI] [PubMed] [Google Scholar]
- Gillette MA, Satpathy S, Cao S, Dhanasekaran SM, Vasaikar SV, Krug K, Petralia F, Li Y, Liang WW, Reva B, Krek A, Ji J, Song X, Liu W, Hong R, Yao L, Blumenberg L, Savage SR, Wendl MC, Wen B, Li K, Tang LC, MacMullan MA, Avanessian SC, Kane MH, Newton CJ, Cornwell M, Kothadia RB, Ma W, Yoo S, Mannan R, Vats P, Kumar-Sinha C, Kawaler EA, Omelchenko T, Colaprico A, Geffen Y, Maruvka YE, da Veiga Leprevost F, Wiznerowicz M, Gumus ZH, Veluswamy RR, Hostetter G, Heiman DI, Wyczalkowski MA, Hiltke T, Mesri M, Kinsinger CR, Boja ES, Omenn GS, Chinnaiyan AM, Rodriguez H, Li QK, Jewell SD, Thiagarajan M, Getz G, Zhang B, Fenyo D, Ruggles KV, Cieslik MP, Robles AI, Clauser KR, Govindan R, Wang P, Nesvizhskii AI, Ding L, Mani DR, Carr SA, Clinical Proteomic Tumor Analysis, C., 2020. Proteogenomic Characterization Reveals Therapeutic Vulnerabilities in Lung Adenocarcinoma. Cell 182 (1), 200–225 e235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glozak MA, Seto E, 2007. Histone deacetylases and cancer. Oncogene 26 (37), 5420–5432. [DOI] [PubMed] [Google Scholar]
- Gong F, Chiu LY, Miller KM, 2016. Acetylation Reader Proteins: Linking Acetylation Signaling to Genome Maintenance and Cancer. PLoS. Genet 12 (9), e1006272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronroos E, Hellman U, Heldin CH, Ericsson J, 2002. Control of Smad7 stability by competition between acetylation and ubiquitination. Mol Cell 10 (3), 483–493. [DOI] [PubMed] [Google Scholar]
- Guo HB, Lee I, Kamar M, Pierce M, 2003. N-acetylglucosaminyltransferase V expression levels regulate cadherin-associated homotypic cell-cell adhesion and intracellular signaling pathways. J Biol Chem 278 (52), 52412–52424. [DOI] [PubMed] [Google Scholar]
- Hajji N, Wallenborg K, Vlachos P, Fullgrabe J, Hermanson O, Joseph B, 2010. Opposing effects of hMOF and SIRT1 on H4K16 acetylation and the sensitivity to the topoisomerase II inhibitor etoposide. Oncogene 29 (15), 2192–2204. [DOI] [PubMed] [Google Scholar]
- Hakomori S, 2002. Glycosylation defining cancer malignancy: new wine in an old bottle. Proc. Natl. Acad. Sci. U. S. A 99 (16), 10231–10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakomori S, Kannagi R, 1983. Glycosphingolipids as tumor-associated and differentiation markers. J Natl Cancer Inst 71 (2), 231–251. [PubMed] [Google Scholar]
- Haltiwanger RS, Lowe JB, 2004. Role of glycosylation in development. Annu. Rev. Biochem 73, 491–537. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA, 2000. The hallmarks of cancer. Cell 100 (1), 57–70. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA, 2011. Hallmarks of cancer: the next generation. Cell 144 (5), 646–674. [DOI] [PubMed] [Google Scholar]
- Hang HC, Geutjes EJ, Grotenbreg G, Pollington AM, Bijlmakers MJ, Ploegh HL, 2007. Chemical probes for the rapid detection of Fatty-acylated proteins in Mammalian cells. J Am Chem Soc 129 (10), 2744–2745. [DOI] [PubMed] [Google Scholar]
- Harachi M, Masui K, Cavenee WK, Mischel PS, Shibata N, 2021. Protein Acetylation at the Interface of Genetics, Epigenetics and Environment in Cancer. Metabolites 11 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T, Sada R, Osugi Y, Matsumoto S, Matsuda T, Hayashi-Nishino M, Nagai T, Harada A, Kikuchi A, 2020. Palmitoylated CKAP4 regulates mitochondrial functions through an interaction with VDAC2 at ER-mitochondria contact sites. J Cell Sci 133 (21). [DOI] [PubMed] [Google Scholar]
- Harmel R, Fiedler D, 2018. Features and regulation of non-enzymatic post-translational modifications. Nat Chem Biol 14 (3), 244–252. [DOI] [PubMed] [Google Scholar]
- Helenius A, Aebi M, 2001. Intracellular functions of N-linked glycans. Science 291 (5512), 2364–2369. [DOI] [PubMed] [Google Scholar]
- Hendriks IA, D’Souza RC, Yang B, Verlaan-de Vries M, Mann M, Vertegaal AC, 2014. Uncovering global SUMOylation signaling networks in a site-specific manner. Nat Struct Mol Biol 21 (10), 927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A, 1998. The ubiquitin system. Annu Rev Biochem 67, 425–479. [DOI] [PubMed] [Google Scholar]
- Hoffmann M, Marx K, Reichl U, Wuhrer M, Rapp E, 2016. Site-specific O-Glycosylation Analysis of Human Blood Plasma Proteins. Mol Cell Proteomics 15 (2), 624–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu JM, Li CW, Lai YJ, Hung MC, 2018. Posttranslational Modifications of PD-L1 and Their Applications in Cancer Therapy. Cancer Res 78 (22), 6349–6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YC, Hsieh YH, Liao CC, Chong LW, Lee CY, Yu YL, Chou RH, 2015. Targeting post-translational modifications of histones for cancer therapy. Cell Mol. Biol. (Noisy. -le-grand) 61 (6), 69–84. [PubMed] [Google Scholar]
- Hu M, He F, Thompson EW, Ostrikov KK, Dai X, 2022. Lysine Acetylation, Cancer Hallmarks and Emerging Onco-Therapeutic Opportunities. Cancers (Basel) 14 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang KY, Lee TY, Kao HJ, Ma CT, Lee CC, Lin TH, Chang WC, Huang HD, 2019. dbPTM in 2019: exploring disease association and cross-talk of post-translational modifications. Nucleic Acids Res 47 (D1), D298–D308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson WL, Du MQ, Johnson PJ, Williams R, 1991. Fucosyltransferases: differential plasma and tissue alterations in hepatocellular carcinoma and cirrhosis. Hepatology 13 (4), 683–688. [PubMed] [Google Scholar]
- Indellicato R, Zulueta A, Caretti A, Trinchera M, 2020. Complementary Use of Carbohydrate Antigens Lewis a, Lewis b, and Sialyl-Lewis a (CA19.9 Epitope) in Gastrointestinal Cancers: Biological Rationale Towards A Personalized Clinical Application. Cancers (Basel) 12 (6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itkonen HM, Minner S, Guldvik IJ, Sandmann MJ, Tsourlakis MC, Berge V, Svindland A, Schlomm T, Mills IG, 2013. O-GlcNAc transferase integrates metabolic pathways to regulate the stability of c-MYC in human prostate cancer cells. Cancer Res 73 (16), 5277–5287. [DOI] [PubMed] [Google Scholar]
- Ito A, Kawaguchi Y, Lai CH, Kovacs JJ, Higashimoto Y, Appella E, Yao TP, 2002. MDM2-HDAC1-mediated deacetylation of p53 is required for its degradation. EMBO J 21 (22), 6236–6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen ON, 2004. Modification-specific proteomics: characterization of post-translational modifications by mass spectrometry. Curr Opin Chem Biol 8 (1), 33–41. [DOI] [PubMed] [Google Scholar]
- Jeusset LM, McManus KJ, 2019. Developing Targeted Therapies That Exploit Aberrant Histone Ubiquitination in Cancer. Cells 8 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YS, Park JI, 2020. Wnt signaling in cancer: therapeutic targeting of Wnt signaling beyond beta-catenin and the destruction complex. Exp Mol Med 52 (2), 183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junger MA, Aebersold R, 2014. Mass spectrometry-driven phosphoproteomics: patterning the systems biology mosaic. Wiley Interdiscip Rev Dev Biol 3 (1), 83–112. [DOI] [PubMed] [Google Scholar]
- Kaemmerer E, Gassler N, 2016. Wnt Lipidation and Modifiers in Intestinal Carcinogenesis and Cancer. Cancers (Basel) 8 (7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C, Yi GS, 2011. Identification of ubiquitin/ubiquitin-like protein modification from tandem mass spectra with various PTMs. BMC Bioinformatics 12 Suppl 14, S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannagi R, Sakuma K, Miyazaki K, Lim KT, Yusa A, Yin J, Izawa M, 2010. Altered expression of glycan genes in cancers induced by epigenetic silencing and tumor hypoxia: clues in the ongoing search for new tumor markers. Cancer Sci 101 (3), 586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannagi R, Yin J, Miyazaki K, Izawa M, 2008. Current relevance of incomplete synthesis and neo-synthesis for cancer-associated alteration of carbohydrate determinants--Hakomori’s concepts revisited. Biochim Biophys Acta 1780 (3), 525–531. [DOI] [PubMed] [Google Scholar]
- Kattah MG, Malynn BA, Ma A, 2017. Ubiquitin-Modifying Enzymes and Regulation of the Inflammasome. J Mol Biol 429 (22), 3471–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaypee S, Sudarshan D, Shanmugam MK, Mukherjee D, Sethi G, Kundu TK, 2016. Aberrant lysine acetylation in tumorigenesis: Implications in the development of therapeutics. Pharmacol Ther 162, 98–119. [DOI] [PubMed] [Google Scholar]
- Ke M, Shen H, Wang L, Luo S, Lin L, Yang J, Tian R, 2016. Identification, Quantification, and Site Localization of Protein Posttranslational Modifications via Mass Spectrometry-Based Proteomics. Adv Exp Med Biol 919, 345–382. [DOI] [PubMed] [Google Scholar]
- Khoury GA, Baliban RC, Floudas CA, 2011. Proteome-wide post-translational modification statistics: frequency analysis and curation of the swiss-prot database. Sci. Rep 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, Cheng T, Kho Y, Xiao H, Xiao L, Grishin NV, White M, Yang XJ, Zhao Y, 2006. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell 23 (4), 607–618. [DOI] [PubMed] [Google Scholar]
- Kirwan A, Utratna M, O’Dwyer ME, Joshi L, Kilcoyne M, 2015. Glycosylation-Based Serum Biomarkers for Cancer Diagnostics and Prognostics. Biomed Res Int 2015, 490531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko PJ, Dixon SJ, 2018. Protein palmitoylation and cancer. EMBO Rep 19 (10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger KE, Srivastava S, 2006. Posttranslational protein modifications: current implications for cancer detection, prevention, and therapeutics. Mol Cell Proteomics 5 (10), 1799–1810. [DOI] [PubMed] [Google Scholar]
- Kudelka MR, Ju T, Heimburg-Molinaro J, Cummings RD, 2015. Simple sugars to complex disease--mucin-type O-glycans in cancer. Adv Cancer Res 126, 53–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudelka MR, Stowell SR, Cummings RD, Neish AS, 2020. Intestinal epithelial glycosylation in homeostasis and gut microbiota interactions in IBD. Nat Rev Gastroenterol Hepatol 17 (10), 597–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kussie PH, Gorina S, Marechal V, Elenbaas B, Moreau J, Levine AJ, Pavletich NP, 1996. Structure of the MDM2 oncoprotein bound to the p53 tumor suppressor transactivation domain. Science 274 (5289), 948–953. [DOI] [PubMed] [Google Scholar]
- Lamoliatte F, Caron D, Durette C, Mahrouche L, Maroui MA, Caron-Lizotte O, Bonneil E, Chelbi-Alix MK, Thibault P, 2014. Large-scale analysis of lysine SUMOylation by SUMO remnant immunoaffinity profiling. Nat Commun 5, 5409. [DOI] [PubMed] [Google Scholar]
- Lan R, Wang Q, 2019. Deciphering structure, function and mechanism of lysine acetyltransferase HBO1 in protein acetylation, transcription regulation, DNA replication and its oncogenic properties in cancer. Cell Mol. Life Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau KS, Partridge EA, Grigorian A, Silvescu CI, Reinhold VN, Demetriou M, Dennis JW, 2007. Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell 129 (1), 123–134. [DOI] [PubMed] [Google Scholar]
- Laubli H, Borsig L, 2019. Altered Cell Adhesion and Glycosylation Promote Cancer Immune Suppression and Metastasis. Front Immunol 10, 2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laude AJ, Prior IA, 2008. Palmitoylation and localisation of RAS isoforms are modulated by the hypervariable linker domain. J. Cell Sci 121 (Pt 4), 421–427. [DOI] [PubMed] [Google Scholar]
- Leeming DJ, Bay-Jensen AC, Vassiliadis E, Larsen MR, Henriksen K, Karsdal MA, 2011. Post-translational modifications of the extracellular matrix are key events in cancer progression: opportunities for biochemical marker development. Biomarkers 16 (3), 193–205. [DOI] [PubMed] [Google Scholar]
- Li CW, Lim SO, Chung EM, Kim YS, Park AH, Yao J, Cha JH, Xia W, Chan LC, Kim T, Chang SS, Lee HH, Chou CK, Liu YL, Yeh HC, Perillo EP, Dunn AK, Kuo CW, Khoo KH, Hsu JL, Wu Y, Hsu JM, Yamaguchi H, Huang TH, Sahin AA, Hortobagyi GN, Yoo SS, Hung MC, 2018. Eradication of Triple-Negative Breast Cancer Cells by Targeting Glycosylated PD-L1. Cancer Cell 33 (2), 187–201 e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CW, Lim SO, Xia W, Lee HH, Chan LC, Kuo CW, Khoo KH, Chang SS, Cha JH, Kim T, Hsu JL, Wu Y, Hsu JM, Yamaguchi H, Ding Q, Wang Y, Yao J, Lee CC, Wu HJ, Sahin AA, Allison JP, Yu D, Hortobagyi GN, Hung MC, 2016. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat. Commun 7, 12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Kobayashi M, Blonska M, You Y, Lin X, 2006. Ubiquitination of RIP is required for tumor necrosis factor alpha-induced NF-kappaB activation. J Biol Chem 281 (19), 13636–13643. [DOI] [PubMed] [Google Scholar]
- Li N, Huang D, Lu N, Luo L, 2015. Role of the LKB1/AMPK pathway in tumor invasion and metastasis of cancer cells (Review). Oncol Rep 34 (6), 2821–2826. [DOI] [PubMed] [Google Scholar]
- Li T, Liu M, Feng X, Wang Z, Das I, Xu Y, Zhou X, Sun Y, Guan KL, Xiong Y, Lei QY, 2014. Glyceraldehyde-3-phosphate dehydrogenase is activated by lysine 254 acetylation in response to glucose signal. J Biol Chem 289 (6), 3775–3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder ME, Deschenes RJ, 2007. Palmitoylation: policing protein stability and traffic. Nat Rev Mol Cell Biol 8 (1), 74–84. [DOI] [PubMed] [Google Scholar]
- Liu B, Liu Y, Wang Y, Xie C, Gan M, Han T, Cao J, Wang J, 2019. CyclinB1 deubiquitination by USP14 regulates cell cycle progression in breast cancer. Pathol Res Pract 215 (10), 152592. [DOI] [PubMed] [Google Scholar]
- Liu FT, Rabinovich GA, 2005. Galectins as modulators of tumour progression. Nat Rev Cancer 5 (1), 29–41. [DOI] [PubMed] [Google Scholar]
- Liu J, Peng Y, Wei W, 2021. Cell cycle on the crossroad of tumorigenesis and cancer therapy. Trends Cell Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Li S, Wu N, Cho KS, 2017. Acetylation and deacetylation in cancer stem-like cells. Oncotarget 8 (51), 89315–89325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Huttenhain R, Surinova S, Gillet LC, Mouritsen J, Brunner R, Navarro P, Aebersold R, 2013. Quantitative measurements of N-linked glycoproteins in human plasma by SWATH-MS. Proteomics 13 (8), 1247–1256. [DOI] [PubMed] [Google Scholar]
- Liu YC, Yen HY, Chen CY, Chen CH, Cheng PF, Juan YH, Chen CH, Khoo KH, Yu CJ, Yang PC, Hsu TL, Wong CH, 2011. Sialylation and fucosylation of epidermal growth factor receptor suppress its dimerization and activation in lung cancer cells. Proc Natl Acad Sci U S A 108 (28), 11332–11337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lothrop AP, Torres MP, Fuchs SM, 2013. Deciphering post-translational modification codes. FEBS Lett 587 (8), 1247–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low TY, Mohtar MA, Lee PY, Omar N, Zhou H, Ye M, 2020. Widening the Bottleneck of Phosphoproteomics: Evolving Strategies for Phosphopeptide Enrichment. Mass Spectrom Rev. [DOI] [PubMed] [Google Scholar]
- Lumpkin RJ, Gu H, Zhu Y, Leonard M, Ahmad AS, Clauser KR, Meyer JG, Bennett EJ, Komives EA, 2017. Site-specific identification and quantitation of endogenous SUMO modifications under native conditions. Nat. Commun 8 (1), 1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundby A, Lage K, Weinert BT, Bekker-Jensen DB, Secher A, Skovgaard T, Kelstrup CD, Dmytriyev A, Choudhary C, Lundby C, Olsen JV, 2012. Proteomic analysis of lysine acetylation sites in rat tissues reveals organ specificity and subcellular patterns. Cell Rep 2 (2), 419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Jin K, Deng S, Cheng H, Fan Z, Gong Y, Qian Y, Huang Q, Ni Q, Liu C, Yu X, 2021. Roles of CA19–9 in pancreatic cancer: Biomarker, predictor and promoter. Biochim Biophys Acta Rev Cancer 1875 (2), 188409. [DOI] [PubMed] [Google Scholar]
- Marth JD, Grewal PK, 2008. Mammalian glycosylation in immunity. Nat Rev Immunol 8 (11), 874–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Vicente M, Medrano LM, Resino S, Garcia-Sastre A, Martinez I, 2017. TRIM25 in the Regulation of the Antiviral Innate Immunity. Front Immunol 8, 1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BR, Cravatt BF, 2009. Large-scale profiling of protein palmitoylation in mammalian cells. Nat Methods 6 (2), 135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez JA, Lupu R, 2007. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 7 (10), 763–777. [DOI] [PubMed] [Google Scholar]
- Meng F, Liang Z, Zhao K, Luo C, 2021. Drug design targeting active posttranslational modification protein isoforms. Med Res Rev 41 (3), 1701–1750. [DOI] [PubMed] [Google Scholar]
- Mereiter S, Balmana M, Campos D, Gomes J, Reis CA, 2019. Glycosylation in the Era of Cancer-Targeted Therapy: Where Are We Heading? Cancer Cell 36 (1), 6–16. [DOI] [PubMed] [Google Scholar]
- Minguez P, Letunic I, Parca L, Garcia-Alonso L, Dopazo J, Huerta-Cepas J, Bork P, 2015. PTMcode v2: a resource for functional associations of post-translational modifications within and between proteins. Nucleic Acids Res 43 (Database issue), D494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguez P, Parca L, Diella F, Mende DR, Kumar R, Helmer-Citterich M, Gavin AC, van Noort V, Bork P, 2012. Deciphering a global network of functionally associated posttranslational modifications. Mol Syst Biol 8, 599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelle W, Michalski JC, 2005. Glycomics and mass spectrometry. Curr. Pharm. Des 11 (20), 2615–2645. [DOI] [PubMed] [Google Scholar]
- Morris JR, Boutell C, Keppler M, Densham R, Weekes D, Alamshah A, Butler L, Galanty Y, Pangon L, Kiuchi T, Ng T, Solomon E, 2009. The SUMO modification pathway is involved in the BRCA1 response to genotoxic stress. Nature 462 (7275), 886–890. [DOI] [PubMed] [Google Scholar]
- Munkley J, Elliott DJ, 2016. Hallmarks of glycosylation in cancer. Oncotarget 7 (23), 35478–35489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadolski MJ, Linder ME, 2007. Protein lipidation. FEBS J 274 (20), 5202–5210. [DOI] [PubMed] [Google Scholar]
- Nagata K, Horinouchi M, Saitou M, Higashi M, Nomoto M, Goto M, Yonezawa S, 2007. Mucin expression profile in pancreatic cancer and the precursor lesions. J Hepatobiliary. Pancreat. Surg 14 (3), 243–254. [DOI] [PubMed] [Google Scholar]
- Nakayama KI, Nakayama K, 2006. Ubiquitin ligases: cell-cycle control and cancer. Nat Rev Cancer 6 (5), 369–381. [DOI] [PubMed] [Google Scholar]
- Narimatsu H, Kaji H, Vakhrushev SY, Clausen H, Zhang H, Noro E, Togayachi A, Nagai-Okatani C, Kuno A, Zou X, Cheng L, Tao SC, Sun Y, 2018. Current Technologies for Complex Glycoproteomics and Their Applications to Biology/Disease-Driven Glycoproteomics. J Proteome Res 17 (12), 4097–4112. [DOI] [PubMed] [Google Scholar]
- Naro C, Sette C, 2013. Phosphorylation-mediated regulation of alternative splicing in cancer. Int. J. Cell Biol 2013, 151839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo K, Marth JD, 2006. Glycosylation in cellular mechanisms of health and disease. Cell 126 (5), 855–867. [DOI] [PubMed] [Google Scholar]
- Olsen JV, Mann M, 2013. Status of large-scale analysis of post-translational modifications by mass spectrometry. Mol. Cell Proteomics 12 (12), 3444–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando R, 2010. Quantitative glycomics. Methods Mol. Biol 600, 31–49. [DOI] [PubMed] [Google Scholar]
- Pagel O, Loroch S, Sickmann A, Zahedi RP, 2015. Current strategies and findings in clinically relevant post-translational modification-specific proteomics. Expert Rev Proteomics 12 (3), 235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan S, Brentnall TA, Chen R, 2016. Glycoproteins and glycoproteomics in pancreatic cancer. World J Gastroenterol 22 (42), 9288–9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan S, Brentnall TA, Chen R, 2020. Proteome alterations in pancreatic ductal adenocarcinoma. Cancer Lett 469, 429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan S, Chen R, Aebersold R, Brentnall TA, 2011. Mass spectrometry based glycoproteomics--from a proteomics perspective. Mol. Cell Proteomics 10 (1), R110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan S, Chen R, Tamura Y, Crispin DA, Lai LA, May DH, McIntosh MW, Goodlett DR, Brentnall TA, 2014. Quantitative glycoproteomics analysis reveals changes in N-glycosylation level associated with pancreatic ductal adenocarcinoma. J Proteome Res 13 (3), 1293–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JH, Du Y, Ard PG, Phillips C, Carella B, Chen CJ, Rakowski C, Chatterjee C, Lieberman PM, Lane WS, Blobel GA, McMahon SB, 2004. The c-MYC oncoprotein is a substrate of the acetyltransferases hGCN5/PCAF and TIP60. Mol Cell Biol 24 (24), 10826–10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulo JA, Schweppe DK, 2021. Advances in quantitative high-throughput phosphoproteomics with sample multiplexing. Proteomics 21 (9), e2000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto A, Relvas-Santos M, Azevedo R, Santos LL, Ferreira JA, 2019. Protein Glycosylation and Tumor Microenvironment Alterations Driving Cancer Hallmarks. Front Oncol 9, 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W, Gutierrez Reyes CD, Gautam S, Yu A, Cho BG, Goli M, Donohoo K, Mondello S, Kobeissy F, Mechref Y, 2021. MS-based glycomics and glycoproteomics methods enabling isomeric characterization. Mass Spectrom Rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho S, Marcos NT, Ferreira B, Carvalho AS, Oliveira MJ, Santos-Silva F, Harduin-Lepers A, Reis CA, 2007. Biological significance of cancer-associated sialyl-Tn antigen: modulation of malignant phenotype in gastric carcinoma cells. Cancer Lett 249 (2), 157–170. [DOI] [PubMed] [Google Scholar]
- Pinho SS, Figueiredo J, Cabral J, Carvalho S, Dourado J, Magalhaes A, Gartner F, Mendonfa AM, Isaji T, Gu J, Carneiro F, Seruca R, Taniguchi N, Reis CA, 2013. E-cadherin and adherens-junctions stability in gastric carcinoma: functional implications of glycosyltransferases involving N-glycan branching biosynthesis, N-acetylglucosaminyltransferases III and V. Biochim Biophys Acta 1830 (3), 2690–2700. [DOI] [PubMed] [Google Scholar]
- Pinho SS, Reis CA, 2015. Glycosylation in cancer: mechanisms and clinical implications. Nat. Rev. Cancer 15 (9), 540–555. [DOI] [PubMed] [Google Scholar]
- Polat AN, Ozlu N, 2014. Towards single-cell LC-MS phosphoproteomics. Analyst 139 (19), 4733–4749. [DOI] [PubMed] [Google Scholar]
- Popovic D, Vucic D, Dikic I, 2014. Ubiquitination in disease pathogenesis and treatment. Nat. Med 20 (11), 1242–1253. [DOI] [PubMed] [Google Scholar]
- Potapenko IO, Haakensen VD, Luders T, Helland A, Bukholm I, Sorlie T, Kristensen VN, Lingjaerde OC, Borresen-Dale AL, 2010. Glycan gene expression signatures in normal and malignant breast tissue; possible role in diagnosis and progression. Mol Oncol 4 (2), 98–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan P, Dabelsteen S, Madsen FB, Francavilla C, Kopp KL, Steentoft C, Vakhrushev SY, Olsen JV, Hansen L, Bennett EP, Woetmann A, Yin G, Chen L, Song H, Bak M, Hlady RA, Peters SL, Opavsky R, Thode C, Qvortrup K, Schjoldager KT, Clausen H, Hollingsworth MA, Wandall HH, 2014. Immature truncated O-glycophenotype of cancer directly induces oncogenic features. Proc Natl Acad Sci U S A 111 (39), E4066–4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raturi A, Gutierrez T, Ortiz-Sandoval C, Ruangkittisakul A, Herrera-Cruz MS, Rockley JP, Gesson K, Ourdev D, Lou PH, Lucchinetti E, Tahbaz N, Zaugg M, Baksh S, Ballanyi K, Simmen T, 2016. TMX1 determines cancer cell metabolism as a thiol-based modulator of ER-mitochondria Ca2+ flux. J Cell Biol 214 (4), 433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren W, Sun Y, Du K, 2015. Glut4 palmitoylation at Cys223 plays a critical role in Glut4 membrane trafficking. Biochem Biophys Res Commun 460 (3), 709–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh MD, 2017. Palmitoylation of proteins in cancer. Biochem. Soc. Trans 45 (2), 409–416. [DOI] [PubMed] [Google Scholar]
- Riley NM, Bertozzi CR, Pitteri SJ, 2021. A Pragmatic Guide to Enrichment Strategies for Mass Spectrometry-Based Glycoproteomics. Mol Cell Proteomics 20, 100029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley NM, Coon JJ, 2016. Phosphoproteomics in the Age of Rapid and Deep Proteome Profiling. Anal Chem 88 (1), 74–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley NM, Coon JJ, 2018. The Role of Electron Transfer Dissociation in Modern Proteomics. Anal Chem 90 (1), 40–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley NM, Hebert AS, Durnberger G, Stanek F, Mechtler K, Westphall MS, Coon JJ, 2017. Phosphoproteomics with Activated Ion Electron Transfer Dissociation. Anal Chem 89 (12), 6367–6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenburg RNP, Snijder J, van de Waterbeemd M, Schouten A, Granneman J, Heck AJR, Gros P, 2017. Stochastic palmitoylation of accessible cysteines in membrane proteins revealed by native mass spectrometry. Nat Commun 8 (1), 1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen SD, Bertozzi CR, 1994. The selectins and their ligands. Curr Opin Cell Biol 6 (5), 663–673. [DOI] [PubMed] [Google Scholar]
- Roux PP, Thibault P, 2013. The coming of age of phosphoproteomics--from large data sets to inference of protein functions. Mol Cell Proteomics 12 (12), 3453–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd PM, Dwek RA, 1997. Glycosylation: heterogeneity and the 3D structure of proteins. Crit Rev. Biochem. Mol. Biol 32 (1), 1–100. [DOI] [PubMed] [Google Scholar]
- Rudd PM, Elliott T, Cresswell P, Wilson IA, Dwek RA, 2001. Glycosylation and the immune system. Science 291 (5512), 2370–2376. [DOI] [PubMed] [Google Scholar]
- Ruprecht B, Lemeer S, 2014. Proteomic analysis of phosphorylation in cancer. Expert Rev Proteomics 11 (3), 259–267. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vega F, Mina M, Armenia J, Chatila WK, Luna A, La KC, Dimitriadoy S, Liu DL, Kantheti HS, Saghafinia S, Chakravarty D, Daian F, Gao Q, Bailey MH, Liang WW, Foltz SM, Shmulevich I, Ding L, Heins Z, Ochoa A, Gross B, Gao J, Zhang H, Kundra R, Kandoth C, Bahceci I, Dervishi L, Dogrusoz U, Zhou W, Shen H, Laird PW, Way GP, Greene CS, Liang H, Xiao Y, Wang C, Iavarone A, Berger AH, Bivona TG, Lazar AJ, Hammer GD, Giordano T, Kwong LN, McArthur G, Huang C, Tward AD, Frederick MJ, McCormick F, Meyerson M, Cancer Genome Atlas Research, N., Van Allen EM, Cherniack AD, Ciriello G, Sander C, Schultz N, 2018. Oncogenic Signaling Pathways in The Cancer Genome Atlas. Cell 173 (2), 321–337 e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarpia L, Lippman SM, El-Naggar AK, 2012. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin Ther Targets 16 (1), 103–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeler JS, Dejean A, 2017. SUMO and the robustness of cancer. Nat Rev Cancer 17 (3), 184–197. [DOI] [PubMed] [Google Scholar]
- Seligson DB, Horvath S, Shi T, Yu H, Tze S, Grunstein M, Kurdistani SK, 2005. Global histone modification patterns predict risk of prostate cancer recurrence. Nature 435 (7046), 1262–1266. [DOI] [PubMed] [Google Scholar]
- Senga SS, Grose RP, 2021. Hallmarks of cancer-the new testament. Open Biol 11 (1), 200358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan J, Zhao W, Gu W, 2009. Suppression of cancer cell growth by promoting cyclin D1 degradation. Mol Cell 36 (3), 469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar S, Tien JC, Siebenaler RF, Chugh S, Dommeti VL, Zelenka-Wang S, Wang XM, Apel IJ, Waninger J, Eyunni S, Xu A, Mody M, Goodrum A, Zhang Y, Tesmer JJ, Mannan R, Cao X, Vats P, Pitchiaya S, Ellison SJ, Shi J, Kumar-Sinha C, Crawford HC, Chinnaiyan AM, 2020. An essential role for Argonaute 2 in EGFR-KRAS signaling in pancreatic cancer development. Nat Commun 11 (1), 2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriver Z, Raguram S, Sasisekharan R, 2004. Glycomics: a pathway to a class of new and improved therapeutics. Nat. Rev. Drug Discov 3 (10), 863–873. [DOI] [PubMed] [Google Scholar]
- Shvedunova M, Akhtar A, 2022. Modulation of cellular processes by histone and non-histone protein acetylation. Nat Rev Mol Cell Biol. [DOI] [PubMed] [Google Scholar]
- Simithy J, Sidoli S, Garcia BA, 2018. Integrating Proteomics and Targeted Metabolomics to Understand Global Changes in Histone Modifications. Proteomics 18 (18), e1700309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Ram M, Kumar R, Prasad R, Roy BK, Singh KK, 2017. Phosphorylation: Implications in Cancer. Protein J 36 (1), 1–6. [DOI] [PubMed] [Google Scholar]
- Sjostrom M, Ossola R, Breslin T, Rinner O, Malmstrom L, Schmidt A, Aebersold R, Malmstrom J, Nimeus E, 2015. A Combined Shotgun and Targeted Mass Spectrometry Strategy for Breast Cancer Biomarker Discovery. J Proteome Res 14 (7), 2807–2818. [DOI] [PubMed] [Google Scholar]
- Stalnecker CA, Der CJ, 2020. RAS, wanted dead or alive: Advances in targeting RAS mutant cancers. Sci Signal 13 (624). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammsalu T, Matic I, Jaffray EG, Ibrahim AF, Tatham MH, Hay RT, 2015. Proteome-wide identification of SUMO modification sites by mass spectrometry. Nat. Protoc 10 (9), 1374–1388. [DOI] [PubMed] [Google Scholar]
- Teng MW, Ngiow SF, Ribas A, Smyth MJ, 2015. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res 75 (11), 2139–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaysen-Andersen M, Packer NH, Schulz BL, 2016. Maturing Glycoproteomics Technologies Provide Unique Structural Insights into the N-glycoproteome and Its Regulation in Health and Disease. Mol Cell Proteomics 15 (6), 1773–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thinon E, Fernandez JP, Molina H, Hang HC, 2018. Selective Enrichment and Direct Analysis of Protein S-Palmitoylation Sites. J Proteome Res 17 (5), 1907–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien JC, Chugh S, Goodrum AE, Cheng Y, Mannan R, Zhang Y, Wang L, Dommeti VL, Wang X, Xu A, Hon J, Kenum C, Su F, Wang R, Cao X, Shankar S, Chinnaiyan AM, 2021. AGO2 promotes tumor progression in KRAS-driven mouse models of non-small cell lung cancer. Proc Natl Acad Sci U S A 118 (20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost M, Bridon G, Desjardins M, Thibault P, 2010. Subcellular phosphoproteomics. Mass Spectrom Rev 29 (6), 962–990. [DOI] [PubMed] [Google Scholar]
- Trougakos IP, Sesti F, Tsakiri E, Gorgoulis VG, 2013. Non-enzymatic post-translational protein modifications and proteostasis network deregulation in carcinogenesis. J Proteomics 92, 274–298. [DOI] [PubMed] [Google Scholar]
- Ubersax JA, Ferrell JE Jr., 2007. Mechanisms of specificity in protein phosphorylation. Nat Rev Mol Cell Biol 8 (7), 530–541. [DOI] [PubMed] [Google Scholar]
- Udeshi ND, Mertins P, Svinkina T, Carr SA, 2013. Large-scale identification of ubiquitination sites by mass spectrometry. Nat Protoc 8 (10), 1950–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Broeck A, Brambilla E, Moro-Sibilot D, Lantuejoul S, Brambilla C, Eymin B, Gazzeri S, 2008. Loss of histone H4K20 trimethylation occurs in preneoplasia and influences prognosis of non-small cell lung cancer. Clin Cancer Res 14 (22), 7237–7245. [DOI] [PubMed] [Google Scholar]
- van der Woude LL, Gorris MAJ, Halilovic A, Figdor CG, de Vries IJM, 2017. Migrating into the Tumor: a Roadmap for T Cells. Trends Cancer 3 (11), 797–808. [DOI] [PubMed] [Google Scholar]
- Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, Seeberger PH, 2015. Essentials of Glycobiology Cold Spring Harbor (NY). [PubMed]
- Vercoutter-Edouart AS, Slomianny MC, Dekeyzer-Beseme O, Haeuw JF, Michalski JC, 2008. Glycoproteomics and glycomics investigation of membrane N-glycosylproteins from human colon carcinoma cells. Proteomics 8 (16), 3236–3256. [DOI] [PubMed] [Google Scholar]
- von Stechow L, Olsen JV, 2017. Proteomics insights into DNA damage response and translating this knowledge to clinical strategies. Proteomics 17 (3–4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh CT, Garneau-Tsodikova S, Gatto GJ Jr., 2005. Protein posttranslational modifications: the chemistry of proteome diversifications. Angew Chem Int Ed Engl 44 (45), 7342–7372. [DOI] [PubMed] [Google Scholar]
- Wan M, Huang J, Jhala NC, Tytler EM, Yang L, Vickers SM, Tang Y, Lu C, Wang N, Cao X, 2005. SCF(beta-TrCP1) controls Smad4 protein stability in pancreatic cancer cells. Am. J. Pathol 166 (5), 1379–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WH, Yuan T, Qian MJ, Yan FJ, Yang L, He QJ, Yang B, Lu JJ, Zhu H, 2020. Post-translational modification of KRAS: potential targets for cancer therapy. Acta Pharmacol Sin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhao J, 2022. Targeted Cancer Therapy Based on Acetylation and Deacetylation of Key Proteins Involved in Double-Strand Break Repair. Cancer Manag Res 14, 259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Schey KL, 2018. Proteomic Analysis of S-Palmitoylated Proteins in Ocular Lens Reveals Palmitoylation of AQP5 and MP20. Invest Ophthalmol Vis Sci 59 (13), 5648–5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Osada H, 2012. Phosphorylation-dependent protein-protein interaction modules as potential molecular targets for cancer therapy. Curr. Drug Targets 13 (13), 1654–1658. [DOI] [PubMed] [Google Scholar]
- Wollscheid B, Bausch-Fluck D, Henderson C, O’Brien R, Bibel M, Schiess R, Aebersold R, Watts JD, 2009. Mass-spectrometric identification and relative quantification of N-linked cell surface glycoproteins. Nat. Biotechnol 27 (4), 378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won SJ, Martin BR, 2018. Temporal Profiling Establishes a Dynamic S-Palmitoylation Cycle. ACS Chem Biol 13 (6), 1560–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RJ, Edge CJ, Dwek RA, 1994. Protein surface oligosaccharides and protein function. Nat. Struct. Biol 1 (8), 499–501. [DOI] [PubMed] [Google Scholar]
- Wu D, Qiu Y, Jiao Y, Qiu Z, Liu D, 2020. Small Molecules Targeting HATs, HDACs, and BRDs in Cancer Therapy. Front Oncol 10, 560487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Huang R, Yuan L, 2019. Crosstalk of intracellular post-translational modifications in cancer. Arch. Biochem. Biophys 676, 108138. [DOI] [PubMed] [Google Scholar]
- Wuhrer M, 2013. Glycomics using mass spectrometry. Glycoconj. J 30 (1), 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuhrer M, de Boer AR, Deelder AM, 2009. Structural glycomics using hydrophilic interaction chromatography (HILIC) with mass spectrometry. Mass Spectrom. Rev 28 (2), 192–206. [DOI] [PubMed] [Google Scholar]
- Xu P, Lin X, Feng XH, 2016. Posttranslational Regulation of Smads. Cold Spring Harb. Perspect. Biol 8 (12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Won JY, Kim CH, Kim DE, Yim H, 2019. Roles of the Phosphorylation of Transcriptional Factors in Epithelial-Mesenchymal Transition. J Oncol 2019, 5810465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang MH, Nickerson S, Kim ET, Liot C, Laurent G, Spang R, Philips MR, Shan Y, Shaw DE, Bar-Sagi D, Haigis MC, Haigis KM, 2012. Regulation of RAS oncogenicity by acetylation. Proc Natl Acad Sci U S A 109 (27), 10843–10848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Di Vizio D, Kirchner M, Steen H, Freeman MR, 2010. Proteome scale characterization of human S-acylated proteins in lipid raft-enriched and non-raft membranes. Mol Cell Proteomics 9 (1), 54–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Mao Y, Clausen H, Vakhrushev SY, 2019. Glyco-DIA: a method for quantitative O-glycoproteomics with in silico-boosted glycopeptide libraries. Nat Methods 16 (9), 902–910. [DOI] [PubMed] [Google Scholar]
- Yip HYK, Papa A, 2021. Signaling Pathways in Cancer: Therapeutic Targets, Combinatorial Treatments, and New Developments. Cells 10 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Li XJ, Martin DB, Aebersold R, 2003. Identification and quantification of N-linked glycoproteins using hydrazide chemistry, stable isotope labeling and mass spectrometry. Nat. Biotechnol 21 (6), 660–666. [DOI] [PubMed] [Google Scholar]
- Zhang H, Liu AY, Loriaux P, Wollscheid B, Zhou Y, Watts JD, Aebersold R, 2007. Mass spectrometric detection of tissue proteins in plasma. Mol. Cell Proteomics 6 (1), 64–71. [DOI] [PubMed] [Google Scholar]
- Zhao D, Zou SW, Liu Y, Zhou X, Mo Y, Wang P, Xu YH, Dong B, Xiong Y, Lei QY, Guan KL, 2013. Lysine-5 acetylation negatively regulates lactate dehydrogenase A and is decreased in pancreatic cancer. Cancer Cell 23 (4), 464–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Zhang C, Luo X, Wang P, Zhou W, Zhong S, Xie Y, Jiang Y, Yang P, Tang R, Pan Q, Hall AR, Luong TV, Fan J, Varghese Z, Moorhead JF, Pinzani M, Chen Y, Ruan XZ, 2018. CD36 palmitoylation disrupts free fatty acid metabolism and promotes tissue inflammation in non-alcoholic steatohepatitis. J Hepatol 69 (3), 705–717. [DOI] [PubMed] [Google Scholar]
- Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, Yao J, Zhou L, Zeng Y, Li H, Li Y, Shi J, An W, Hancock SM, He F, Qin L, Chin J, Yang P, Chen X, Lei Q, Xiong Y, Guan KL, 2010. Regulation of cellular metabolism by protein lysine acetylation. Science 327 (5968), 1000–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Jensen ON, 2009. Modification-specific proteomics: strategies for characterization of post-translational modifications using enrichment techniques. Proteomics 9 (20), 4632–4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Qiu C, Gryniewicz-Ruzicka CM, Keire DA, Ye H, 2020. Multiplexed Comparative Analysis of Intact Glycopeptides Using Electron-Transfer Dissociation and Synchronous Precursor Selection Based Triple-Stage Mass Spectrometry. Anal Chem 92 (11), 7547–7555. [DOI] [PubMed] [Google Scholar]


