Abstract
Insulin resistance (IR) is a chronic pathological condition that is related to reduce the rates of glucose uptake, especially in the liver, muscle, and adipose tissue as target tissues. Metabolic syndrome and type 2 diabetes mellitus can occur following progression of the disease. The majority of prior research has applied that some cations such as magnesium (Mg2+) have important physiological role in insulin metabolism. Mg2+ is the fourth most abundant mineral in the human body that gets involved as a cofactor of various enzymes in several metabolic events, such as carbohydrate oxidation, and it has a fundamental role in glucose transporting mechanism of the cell membrane. This cation has numerous duties in the human body such as regulation of insulin secretion in pancreatic beta-cells and phosphorylation of the insulin receptors in target cells and also gets involved in other downstream signal kinases as intracellular cation. On this basis, intracellular Mg2+ balancing is vital for adequate carbohydrate metabolism. This paper summarizes the present knowledge about the therapeutic effects of Mg2+ in reducing IR in liver, muscle, and pancreases with different mechanisms. For this, the search was performed in Google Scholar, PubMed, Scopus, and Web of Science by insulin resistance, skeletal muscle, liver, pancreases, magnesium, Mg2+, and inflammation keywords.
Keywords: Diabetes, glucose, insulin resistance, magnesium
Introduction
Type 2 diabetes (T2D) is very common in human societies and the most important feature of this disease is insulin resistance (IR). The complications of this disease can severely affect the quality of human life. On the other hand, long-term use of insulin leads to increased IR, so today there is a need for alternative drugs or supplements that, in addition to lowering blood sugar, maintain the integrity of cell pathways and also maintain the survival of pancreatic beta cells. In this regard, in the present study, we decided to investigate the effects of magnesium (Mg2+) as a complementary drug to reduce IR on important issues involved in glucose metabolism.
Insulin Resistance
Insulin resistance is a chronic pathological condition that is related to reduce the rates of glucose uptake, especially in the liver, muscle, and adipose tissue as target tissues, and it occurs when insulin receptors (INRs) lose their sensitivity to insulin.[1] Insulin controls energy homeostasis and glucose metabolism by stimulating glucose uptake from skeletal muscle and, to a lesser degree, liver and adipose tissue. It was reported in the literature that a family of glucose transport proteins, which are expressed in specific tissues and known as glucose transporter (GLUTs), contributes to the glucose uptake process. In adipose tissue and skeletal muscle, GLUT4 is the main isoform of GLUTs that participate in insulin-stimulated glucose uptake based on translocation of GLUT4 from an intracellular pool to the plasma membrane.[2,3] Hence, IR and type 2 diabetes in rodents and humans are associated with defects at the level of GLUT4 content in skeletal muscles and adipose tissue[2,3] that leads to enhancing the concentration of insulin in the circulatory system as a compensatory mechanism.[4,5] Following these conditions, IR occurs with downregulating INR and desensitizing postreceptor pathways.[6] IR is widely recognized as an important risk factor for cardiovascular disease, metabolic syndrome (MetS), obesity, cancer, and T2D.[5,7]
Diabetes Mellitus
DM is a common metabolic disorder that can change people’s life due to high morbidity and mortality. This disease is associated with pancreatic dysfunction in insulin secretion or low insulin-directed fostering of glucose by target cells which cause hyperglycemia in the blood. DM is divided into two general types: insulin-dependent diabetes mellitus (IDDM) or type 1 and non-IDDM or type 2. IDDM is known as an autoimmune disorder because T-lymphocytes reactivity against pancreatic β-cells induced hypoinsulinemia and thus hyperglycemia.[8,9] T2D as a chronic disease is characterized by hyperglycemia due to impaired insulin secretion, insulin function, or both.[10,11]
Chronic hyperglycemia is the main risk factor for heart disease, stroke, kidney disease, blindness, and amputation.[10,12] Unfortunately, the World Health Organization reported that the prevalence of diabetes is rapidly increasing all around the world and there has been an epidemic increase in mortality from T2D.[10] It is expected that the number of people suffering from diabetes will reach 25%–28% by 2050. To overcome this situation, some approaches have been made to find some medications that may increase insulin sensitivity and improve T2D complications. Quite recently, considerable attention has been paid to magnesium as a potential option for balancing glucose uptake.[13]
Mg2+
Mg2+ is the fourth most important element in the human body and the second most abundant intracellular cation,[1] with 99% distribution in the intracellular compartment and only 1% distribution in the extracellular fluid.[14] The normal serum of Mg2+ concentration is reported to be in the range of 0.76–1.15 mmol/L.[1] Mg2+ involves in more than 300 enzymatic reactions and numerous physiological processes by acting as a cofactor for many enzymes such as energy metabolism, glucose transport across cell membrane, hepatic gluconeogenesis, pancreatic functions, insulin secretion, and action in pancreatic cells and target tissues through interaction with receptors of this hormone.[5,15,16,17] On this basis, intracellular Mg2+ balancing is vital for adequate carbohydrate metabolism.[10,18] Studies have indicated that daily Mg2+ supplements may improve glycemic response among T2D patients[10] and also prevent MetS.[19] With this aim in mind, in this paper, we reviewed Mg2+ performance in improving IR.
The Role of Mg2+ in Improving Insulin Resistance and Blood Glucose
There have been numerous studies that investigate the benefits of Mg2+ supplementation in IR and diabetic subjects. Nevertheless, contradictory opinions have been presented in the literature throughout the years. For instance, a number of studies have found that Mg2+ supplementation has beneficial effects on metabolic control in individuals with T2D,[3,20,21] while other observations provided insufficient data about the effects of Mg2+ supplementation on T2D.[10,22] Guerrero-Romero has stated that the intake of supplements containing 50 mL MgCl2 for 16 weeks remarkably improved homeostatic model assessment for IR (HOMA-IR), fasting blood sugar (FBS), and hemoglobin A1c (HbA1c) in individuals with T2D.[23] More recent evidence highlights that higher levels of Mg2+ serum are associated with a greater degree of sensitivity to insulin,[1,24,25] and this justifies the improvement of the glycemic control indicators after Mg2+ supplementation. However, this improvement could be interpreted by different mechanisms.[10] More recent evidence reveals that increasing Mg2+ intake is related to not only lowering fasting glucose (FG) and insulin[10] but also decreasing the risk of T2D.[21,26] Considering the positive effects of Mg2+ on mechanisms involved in IR, the prescription of a healthy Mg2+-rich diet can be effective for individuals with MetS and T2D. In addition, a meta-analysis was conducted on T2D individuals highlighting that Mg2+ supplementation improves glycemic control in T2D patients.[27] Different doses of Mg2+ have been suggested by researchers in diabetic patients. ELDerawi et al. have indicated that daily administration of 250 mg of Mg2+ in T2D patients group improved HbA1c, insulin levels (ILs), C-peptide, and HOMA-IR and subsequently reduced IR and also improved the glycemic control indicators after 3 months of intervention.[10] As well, a meta-analysis that was carried out in 2006 to evaluate the effect of oral Mg2+ supplementation on glycemic control revealed that a median Mg2+ dose of 360 mg/day was associated with significantly lower FG in treatment groups, suggesting improved glucose control.[27] A recent small, randomized, placebo-controlled trial in obese, nondiabetic, IR individuals demonstrated that 365 mg/day of Mg2+ for 6 months significantly lowered FG, fasting insulin (FI), and IR and improved insulin sensitivity.[28,29] Solati et al. have demonstrated that oral Mg2+ supplementation, during 4 months or more, reduced the FG concentrations as compared with people who received Mg2+ supplementation for less than 4 months. They also stated that Mg2+ supplementation is related to significant decrease in low-density lipoprotein (LDL) and cholesterol,[30] as mentioned by other studies.[31] In explaining this issue, it should be stated that Mg2+ increases the activity of lipoprotein lipase enzyme as described by Rayssiguier and Gueux. In other words, increased secretion of catecholamines due to Mg2+ deficiency in the body is associated with increases in lipolysis.[32] With increasing lipolysis and subsequent enhancement in plasma free fatty acids, a series of reactions lead to an increase in very LDL and the synthesis and secretion of triglycerides (TGs) and also enhance plasma TG concentration.[30,32] Table 1 lists a number of studies that have examined the role of Mg2+ in improving IR.
Table 1.
Studies that have examined the role of magnesium in improving insulin resistance
| Protective effects of Mg2+ | ||||
|---|---|---|---|---|
|
| ||||
| Source | Doses | Time of administration | Effect | References |
| MgSO4 | 1 or 2 mg/kg diet | 4 weeks | Mg2+ supplementation improved glucose tolerance, lowered BG levels, lipid perturbations and HOMA-IR index. Mg2+, on the other hand, was able to increase insulin sensitivity and INR and GLUT4 | [2] |
| MgSO4 | 10 g/l drinking water | 12 weeks | MgSO4 lowered BG, glucose tolerance and HbA1c relative to the diabetic group. Also Mg2+ increased GIR in diabetics | [3] |
| Elemental high-potency, highly absorbable magnesium (oxide, gluconate, lactate) | 250 mg/d tablet | 3 months | Administration of Mg2+ indicated a significant improvement in HbA1C, insulin levels, C-peptide, HOMA-IR and HOMA-β% | [10] |
| MgCl | 300 mg/day (50 gr of MgCl2 by 1000 ml of solution) | 12 weeks | Mg2+-supplemented subjects significantly increased their serum Mg2+ levels and reduced HOMA-IR index | [23] |
| Mg2+ supplementation | 360 mg/day | 4–16 weeks | Oral Mg2+ supplementation was effective in reducing plasma fasting glucose levels and raising HDL cholesterol in patients with T2D diabetes | [27] |
| Mg–aspartate–hydrochloride | 365 mg/day | 6 months | Mg2+ supplementation resulted in a significant improvement of fasting plasma glucose and some insulin sensitivity indices. Mg2+ prevent IR and subsequently T2D diabetes | [28] |
| MgSO4 | 300 mg/d | 3 months | Administration of Mg2+ significantly improved fasting BG, 2 h postprandial glucose, lipid profile, and hepatic enzymes | [30] |
| Magnesium oxide | 250 mg/day | 6 weeks | Mg2+ significantly improved glycemic control and lipid profiles | [31] |
| MgSO4 | 10 g/l of | 16 weeks | Administration of MgSO4 improved IPGTT, lowered BG levels, and decreased FoxO1 and PEPCK genes and proteins expression in muscle and liver | [57] |
IR: Insulin resistance, HOMA-IR: Homeostatic model assessment for IR, BG: Blood glucose, INR: Insulin receptor, HbA1c: Hemoglobin A1c, GLUT: Glucose transporter, T2D: Type 2 diabetes mellitus, HDL: High-density lipoprotein, Mg2+: Magnesium, MgSO4: Magnesium sulfate IPGTT: Intraperitoneal glucose tolerance test: for measures the clearance of an intraperitoneally injected glucose load from the body. It is used to detect disturbances in glucose metabolism that can be linked to condition such as diabetes or metabolic syndrome., GIR: Glucose infusion rate: shows the rate at which glucose enters the cell and is inversely related to IR
Taken together, these findings suggest that Mg2+ supplementation in the diet of people with IR can help enhance insulin sensitivity. In the following of this study, we reviewed the effectiveness of Mg2+ in reducing IR and mechanisms involved in this process.
Search Strategy
To identify relevant studies, the search was performed in Google Scholar, PubMed, Scopus, and Web of Science. The search was carried out from 1983 until 2021. The keywords used in the search were insulin resistance, skeletal muscle, liver, pancreases, magnesium, Mg2+, and inflammation without language or date restrictions. The title and abstract of all the articles were studied and those describing mechanisms of IR and therapeutic effects of Mg2+ in reducing IR were finally selected.
The Role of Mg2+ on Insulin Secretion from Pancreatic Beta-Cells
Some studies found evidence about the significant effect of Mg2+ on insulin secretion and BG control in diabetic and nondiabetic patients,[1,6] whereas other studies have found no significant association between normal intracellular Mg2+ concentrations and insulin secretion.[33,34,35,36] Therefore, based on some studies, Mg2+ has a fundamental role in the function of many enzymes in these metabolic pathways.[37,38]
As an initial step in insulin secretion, glucose uptake into the pancreatic β-cell via GLUT2. In cellular metabolism, adenosine triphosphate (ATP) is generated through glycolytic pathway from glucose.[39] In the path of ATP synthesis in beta-cells, glucose converts to glucose-6-phosphate (G6P) by glucokinase (GK). The rate of GK activity depends on magnesium ATP (MgATP).
On the other hand, with the binding of ATP to the Kir6.2 subunits, the KATP channels are closed and opening of these channels depends on the binding of MgATP to the sulfonylurea receptor subunit (SUR1). After closing KATP channels, pancreatic beta-cell membranes depolarize. These events stimulate electrical activity that opens the voltage-gated calcium (Ca2+) (L-type) channels to stimulate insulin secretion.[1,11] The role of Mg2+ in the regulation of various stages of insulin secretion is discussed below.
Glucokinase
In the path of ATP synthesis in beta-cells, glucose converts to G6P by GK. The rate of GK activity depends on MgATP.[11,40]
Glycolysis
After converting glucose to G6P via glycolysis and the Krebs cycle, the level of ATP significantly increases. In this metabolic process, MgATP acts as a cofactor for numerous enzymes.[11,25] A number of studies have found that Mg2+ acts as an essential cofactor in all ATP-transfer reactions. In addition, this cation is known as rate-limiting enzyme of glycolysis.[41,42]
KATP Channel
The first investigations into KATP channels in 1984 found that these channels are the important regulators of the membrane potential in pancreatic beta-cells.[43] The pancreatic KATP channel is composed of four Kir6.2 subunits and four SUR1 subunits. The activity of these channels is controlled by the intracellular ATP-to-adenosine diphosphate (ADP) ratio. In the presence of Mg2+, binding of MgATP and MgADP to the SUR1 subunits promotes channel opening. High glucose levels stimulate glycolysis that shifts the balance toward ATP, which simultaneously reduces the level of MgADP and consequently closed KATP channels that increased insulin secretion.[11,25,44] The role of Mg2+ on insulin secretion can be justified by its Ca2+ antagonism, and this role may be dependent on the cytosolic Ca2+/Mg2+ ratio and not exclusively on Mg2+ concentration.[25,45,46]
L-Type Ca2+ Channel
Following the increasing ATP levels and closure of KATP channels, the pancreatic beta-cell membrane becomes depolarized. This causes activating Ca2+ influx via the voltage-dependent L-type Ca2+ channel.[11] It has now been suggested that hypomagnesemia decreases the expression of L-type Ca2+ channels that reduces insulin secretion indirectly.[47] Another plausible role of Mg2+ is controlling L-type Ca2+ channel opening via blocking Ca2+ uptake into adipocytes.[5]
Insulin Vesicle Release
Insulin-containing vesicles exocytose out of the cell by increasing Ca2+ levels and binding this cation to these vesicles.[25] Mg2+ blocks L-type Ca2+ channels, thus regulating insulin secretion.[11] Atwater et al. have mentioned that Ca2+/Mg2+ ratio is the most important factor in induced insulin secretion in perfused rat pancreas and mouse islets.
The results emphasize that stimulation of insulin secretion depends on decreasing in physiological Mg2+ concentrations, but insulin secretion was inhibited only by decreased Ca2+ levels. In this regard, as long as the Ca2+/Mg2+ ratio remains constant, the amount of insulin secretion does not change. Decreasing the level of Mg2+ without changing the level of Ca2+ changes this ratio, which can affect insulin secretion.[48]
Effects of Mg2+ on Insulin Signaling Kinases Activity
A growing body of literature has been introduced Ras–MAPK pathway as a regulator for gene expression and insulin-associated mitogenic effects. Some metabolic processes such as glucose uptake mobilization, lipogenesis, and also glycogen and protein synthesis are stimulated by PI3K/Akt kinase pathway. With the binding of insulin to the INR, plasma membrane subunits of the INR (insulin receptor substrate [IRS] and Shc proteins) undergo phosphorylation that changes receptor conformation.[1] Shc phosphorylation activates the Ras–MAPK pathway. IRS phosphorylation, on the other hand, triggers a cascade of events through the phosphoinositide 3-kinases-protein kinases (PI3K–Akt) pathway which eventually leads to the production of a second messenger phosphatidylinositol-3,4,5-triphosphate (PIP3). PIP3 induces phosphorylation of 3-phosphoinositide-dependent protein kinase-1 that activates downstream Akt. Akt has an essential role in the regulation of glucose transport, lipid synthesis, gluconeogenesis, and glycogen synthesis.[1,49]
In this regard, MgATP acts as a phosphorylation factor for INR tyrosine kinase (IRTK) and other signal kinases that initiate a cascade of phosphorylation events. On the other hand, Mg2+ has a central role in the regulation of glucose control and metabolic effects of insulin via interacting with TK of INR and other enzymes. Evidence highlights that Mg2+ level is the important factor in inducing downstream signal kinases after binding the insulin to the INR.[1] Moreover, Mg2+ by binding to the regulatory site of the IRTK induces regulatory influence. The affinity of this site to MgATP is dependent on free Mg2+ concentration.[1,6] A growing body of studies reveals that a decrease in Mg2+ concentration is associated with an increase in IR.[50,51] In IR cell models, there is an impaired response of the TK to insulin stimulation.[50,51,52] In addition, Mg2+ acts as a limiting factor in carbohydrate metabolism. Because Mg2+ is necessary for phosphorylation activities of some enzymes such as glutathione peroxidase, superoxide dismutase, and catalase.[50,52] It is by now generally accepted that Mg2+ has a central role for autophosphorylation of the b-subunits of the INR. In this regard, two Mg2+ ions bind to the TK domain that enhancing the affinity of TK for ATP molecules.[11,53] Siddiqui et al. demonstrated that enhancing intracellular Mg2+ concentration improves some biological processes such as TK activity, glucose transport, and consequently insulin secretion.[54]
Effects of Mg2+ on Glucose Metabolism in the Muscle
Skeletal muscle uptake approximately 80% of dietary glucose via GLUT4.[11,55] Many literatures highlights that Mg2+ could stimulate GLUT4 gene expression.[2,56] For example, a recent study in diabetic rats has indicated that oral Mg2+ supplementation enhanced muscle GLUT4 gene expression that decreases serum glucose levels to the normal range.[57] A group of researchers from Korea has been proved that diabetic mice fed with Mg2+-rich seawater have shown enhanced GLUT1 and GLUT4 expression in the skeletal muscle.[58] Insulin and also insulin-like growth factor 1 induced Akt cascade through PI3K by phosphorylation of IRS1.[59,60] The Akt protein kinase is consists of three isoforms that have a major contributor to tumor initiation and also tumor metastasis. These isoforms have been found in rodent muscle while insulin is a ligand for Akt1 and to a lesser extent Akt2.[59,61] Previous research showed that Mg2+ administration increases Akt2 transcription ~2.6 fold higher in the diabetic animal case group, compared with the diabetic control group via enhancing in IRS1 gene expression.[59] Some scientists demonstrate that Mg2+ administration in diabetic animal models for 16 weeks enhances Akt2 transcription.[59] Following research in this area, researchers stated that Mg2+ supplementation increases INR and GLUT4 level, whereas before Mg2+ therapy, the levels of these proteins were low in uncontrolled diabetic conditions.[2] One investigation not only confirmed other studies that demonstrated that Mg2+ induces glucose metabolism in muscle cells by transporting GLUT4-containing vesicles to the plasma membranes.[2] This result was also confirmed by experiments performed on rats by Morakinyo et al.[2] A study in 2019 showed that daily administration of 1000 mg/kg MgSO4 was associated with an increase in GLUT4 gene expression in all the diabetic groups.[8] Solaimani et al. provided further evidence that magnesium sulfate (MgSO4) supplementation increase GLUT4 mRNA expression about 23%.[56]
Various approaches have been suggested that nuclear receptors called peroxisome proliferator-activated receptors (PPARs) play a controlling role in glucose metabolism in diabetics.[62,63] PPAR has three isoforms including PPAR-α, PPAR-β/δ, and PPAR-γ[63,64] that expressed in adipose, muscle, and liver tissues.[63] Each isoform plays different roles in vivo.[63,65] For instance, PPAR-γ is mainly synthesized in white adipose tissue and muscle[63,66] and has essential roles in glycemic control via decreasing plasma ILs and increasing insulin sensitivity.[63,67] In addition, its ligand increases the expression of GLUT4 mRNA and fatty acid storage in fat tissues. Scientists believe that PPAR-γ agonist receptor therapy improves muscle glucose homeostasis by inducing insulin cell signaling. It was reported in the literature that MgSO4 supplementation for 16 weeks in diabetic rats enhances PPAR-γ transcription and expression.[63] These studies provide support for the central role of the Akt2 gene in glucose homeostasis after Mg2+ therapy. This is because the intracellular level of Mg2+ concentration is inversely related to BG levels. In summary, Mg2+ mediates effective metabolic control by autophosphorylation and stimulation of insulin.
Effects of Mg2+ on Glucose Metabolism in the Liver
Scientists believe that the main reason for the rapid rise in BG is the high production of glucose by the liver.[57,68] According to research findings, Mg2+ is an essential factor in the function of liver enzymes. For instance, it has a fundamental function in gluconeogenesis and glycogenesis pathways in the liver. In this regard, seawater Mg2+ administration in diabetic mice model decreases phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) mRNA expression.[25] Findings from one study demonstrated that gluconeogenic genes such as PEPCK are inhibited by Mg2+57] and hepatic glucose production is diminished by Mg2+ supplementation to maintain BG levels near normal.[8,25] In the same way, Mg2+ supplement therapy has positive effects on the ISI–HOMA and fasting plasma glucose, as an indicator of hepatic IR and hepatic glucose production, respectively.[28,69,70] Mg2+ acts as a significant cofactor for catalytic activity of phosphofructokinase-1 (PFK-1). This enzyme is a key enzyme and rate-limiting step that catalyzes glycolysis.[8,11] In general, it can be stated that Mg2+ has cofactor activity for a large number of enzymes that involve in the glycolytic pathway as kinases or phosphorylase. By enhancing the level of intracellular Mg2+, these enzymes keep their activated form that this situation reduces the risk of hyperglycemia by increasing the ATP production through converting glucose to pyruvate and eventually ATP via citric acid pathway. Results from one study support the idea that the activated form of protein kinase (AMPK) system acts as a key factor in regulating energy balance in the cell and consequently the whole body. AMPK inhibits gluconeogenic gene expression and hepatic glucose production and thus maintains BG concentrations at a normal level. Mg2+ can activate AMPK in a Ca2+-dependent manner that activates PFK-1 in the glycolytic pathway.[8,71] In addition, this cation can decrease the effect of glucagon in the liver.[13,25] Glucagon upregulates hepatic glucose production, glycogenolysis, gluconeogenesis, fatty acid oxidation, and lipolysis.[25] In diabetic animals model, Mg2+ supplementation downregulates the glucagon receptor that leads to improve insulin sensitivity.[71]
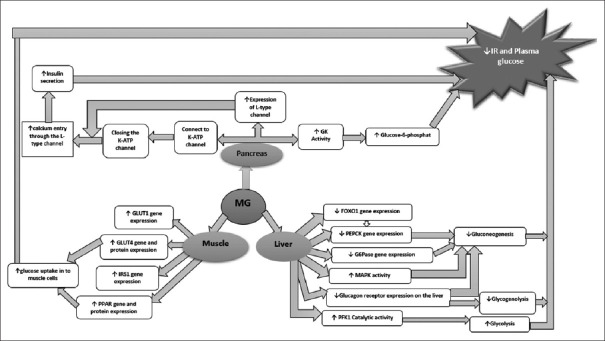
Forkhead box protein O1 (FoxO1) is a transcription factor that increases hepatic glucose production by regulating target gene expression.[25,72] This factor is controlled by Akt-mediated phosphorylation at three highly conserved Akt phosphorylation sites (Thr-24, Ser-253, and Ser-316) on its genome.[25,73] Phosphorylation of this factor causes it to leave the nucleus and thereby inactivating transcription of gluconeogenic enzymes. This situation increases the chance of glucose uptake by cells and ultimately balances glucose levels. On the other hand, FoxO1 binds to insulin-response elements in the promoters of PEPCK and G6Pc genes and increases the expression of these genes.[73,74] In this regard, overexpression of FoxO1 induces gluconeogenesis, hyperglycemia, and IR. More recent evidence reveals that Mg2+ supplementation in diabetic animal models decreases the expression of hepatic FoxO1 and consequently suppresses gluconeogenic genes, such as PEPCK.[25,57] Figure 1 summarizes the role of Mg2+ in reducing IR in the pancreas, muscle, and liver.
Figure 1.
Role of magnesium in reducing insulin resistance in the pancreas, muscle, and liver. GLUT1: Glucose transporter 1, GLUT4: Glucose transporter 4, IRS1: Insulin receptor substrate 1, Mg: Magnesium, PPAR: Peroxisome proliferator-activated receptors, FOXO1: Forkhead box protein O1, FEPCK: Phosphoenolpyruvate carboxykinase, G6Pase: Glucose 6-phosphatase, MAPK: Mitogen-activated protein kinase, PFK-1: Phosphofructokinase-1, GK: Glucokinase
Effects of Mg2+ on Low-Grade Systemic Inflammation
Inflammation is a key factor in the development of IR.[1,75] Interleukin-1 (IL-1) is an inflammatory cytokine that secretes by multiple tissues, particularly adipose tissue. This factor reduces the expression of IRS-1 and GLUT4 that eventually causes IR.[1,76] Production of IL-1 regulated by diet-induced metabolic stress. IL-1 is involved in the production of another inflammatory factor called IL-6 that in turn causes IR by inhibiting the PI3K pathway.[75,77]
A major factor involved in the production and secretion of some inflammatory mediators such as IL-1, IL-6, IL-8, IL-18, and cycloxygenase-2 is a mediator called tumor necrosis factor-alpha (TNFα). TNFα induces gene expression of the mentioned factors. Some recent studies have suggested positive associations between the serum level of TNFα and pathophysiology of IR. The authors of these studies have believed that TNFα is one of the most important factors in the development of IR.[77] Inhibition of TNFα stimulates some pathways such as nuclear factor kappa B (NF-κB) and c-Jun NH2-terminal kinases (JNK).[1,26,77] On the other hand, some studies provide several reasons for the role of TNFα in the development of diabetes and its complications.[63]
It has been suggested that hyperglycemia induces NFKB gene expression as a proinflammatory agent which leads to IR.[63]
It was reported in one article that drinking Mg2+-enriched water could not inhibit the expression of the NFKB gene in animal diabetic models, but Mg2+ administration for 16 weeks could enhance GLUT4 expression and consequently decrease BG level via decreasing NFKB protein.[63] Activated NFKB and JNK induce ser307 phosphorylation in IRS-1 that leads to blocking Tyr phosphorylation of IRS-1.[77] In addition, TNFα could decrease Akt activity and GLUT4 gene expression simultaneously which consider as important pro-inflammatory factors.[78] The majority of prior suggested that Mg2+ therapy reduces production of some pro-inflammatory factors such as interleukins (IL1, IL-6), TNF, vascular cell adhesion molecule-1, and plasminogen activator inhibitor-1. Furthermore, this cation can enhance production and activity of the antioxidant enzymes such as glutathione peroxidase, superoxide dismutase, catalase, and also cellular and tissue levels of some antioxidants such as glutathione, Vitamin C, Vitamin E, and selenium.[1,25,79] According to the above and based on the results of studies, Mg2+ decreases the development of chronic low-grade inflammation by blocking the production of inflammatory mediators.[79]
Effects of Mg2+ on Oxidative Stress
Persistent and prolonged chronic hyperglycemia promotes progressive accumulation of nonenzymatic glycation of proteins and oxygen-free radical production[80,81,82,83,84] that leads to an increase in oxidative stress (OS) and also can produce permanent chemical alterations in proteins.[81] It has been proven that overproduction of reactive oxygen species (ROS) and inadequate antioxidant protection create metabolic and cellular imbalance that plays an important role in the development of DM.[85] Mg2+ therapy can decrease oxidative damage by reducing ROS production.[86] This element can prevent the OH formation from hydrogen peroxide via counteracting the redox-active transition metals.[87] In diabetic individuals, the concentrations of free radicals are higher than normal.[88,89] Damage caused by ROS excessive production for repair causes inflammation. This involves the activation of the NF-κB pathway.[90] Mg2+ therapy blocks the production of pro-inflammatory cytokines by inhibiting NF-κB production.[91] Furthermore, Mg2+ plays a critical role as an anti-inflammatory agent and acts as a Ca2+ antagonist.[46] In the light of previous studies on the relationship between Mg2+ and mitochondrial function, it has been proven that Mg2+ improves mitochondrial function through increasing ATP production, decreasing the mitochondrial production of ROS and intracellular Ca2+ overload, and repolarizing the mitochondrial membranes, as well as by reducing OS.[25]
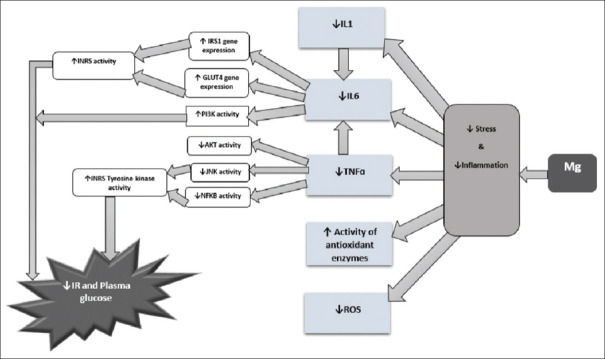
Figure 2 shows the role of Mg2+ in reducing inflammation and OS, which leads to a decrease in IR.
Figure 2.
Role of magnesium in reducing inflammation and oxidative stress. INR: Insulin receptor, NFKB: Nuclear factor-kappa B, JNK: c-Jun N-terminal kinases, TNFα: Tumor necrosis factor-alpha, IL1: Interleukin-1, IL6: Interleukin-6, PI3K: Phosphoinositide 3-kinases, GLUT4: Glucose transporter 4, AKT (PKB): Protein kinase B, ROS: Reactive oxygen species, Mg: Magnesium
Effects of Mg2+ on Key Mg2+-Dependent Enzymes of Carbohydrate and Energy Metabolism
Mg2+ acts as a rate-accelerating factor in numerous metabolic pathways because Mg2+ or MgATP has a crucial role as a cofactor for many enzymes such as gluconeogenesis enzymes in the liver PEPCK, fructose-1,6-bisphosphatase, pyruvate carboxylase (PC), and G6Pase.[1,92] For example, in the path of ATP synthesis in beta-cells, glucose converts to G6P by GK and the rate of GK activity depends on MgATP concentration.[93]
On the other hand, glycogen synthase kinase 3 (GSK3) is a potential regulator for glycogen synthase (GS) activity. Insulin signaling activates PI3K and Akt that lead to enhancing the inhibitory serine phosphorylation of GSK3.[94] Mg2+ ions bind to GSK3 that neutralizing the negative charge on the aspartic acid side chains.[95] In addition, Mg2+ has an important regulatory role in numerous metabolic activities such as glycolysis and the Krebs cycle.[96,97] It regulates glycolytic enzymes, such as hexokinase, PFK, phosphoglycerate kinase, and pyruvate kinase.[96,98]
Mg2+ is involved in increasing the activity of three important mitochondrial dehydrogenases: isocitrate dehydrogenase and 2-oxoglutarate dehydrogenase complex that stimulated directly by Mg2+ isocitrate complex and free Mg2+, respectively, and pyruvate dehydrogenase complex that is stimulated indirectly by the effect of Mg2+ on pyruvate dehydrogenase phosphatase. Studies show that Mg2+ is a rate limiting factor for oxidative phosphorylation when 2-oxoglutarate is the oxidizable substrate.
Conclusions
Based on the research reviewed in this article, it can be concluded that normal Mg2+ serum level is essential for optimal functioning of many enzymes in insulin secretion and also glucose and energy metabolism. Mg2+ is associated with improvement in beta-cell function, decreasing IR, accelerating glucose tolerance, and ultimately, clinical improvement of T2D. Oral Mg2+ supplementation and appropriate dietary patterns improve insulin sensitivity and metabolic control in individuals with T2D, suggesting that Mg2+ is an important factor in the etiology and management of this widespread socially significant disease. In addition, it is worth highlighting that Mg2+ acts as the insulin sensitizer by regulating TK activity of the receptor of this hormone and autophosphorylation of this receptor b-subunit, with ensuing phosphorylation of its substrates mediators, and favors the manifestation of IR. Mg2+ improves glucose consumption and glucose tolerance, at least in part, via stimulation of GLUT4 gene expression and translocation and also suppression of the gluconeogenesis pathway and glucagon receptor gene expression by targeting the liver and muscle. According to the results of our study and the previous ones, we can conclude that not only Mg2+ supplementation can be helpful in diabetes control, but also the effective dosage and duration of supplementation, and the patients who need the supplementation should be considered. Therefore, Mg2+ is also recommended as an inexpensive, easy-to-use, natural adjuvant therapy for patients with T2D.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to express their gratitude to Isfahan University of Medical Sciences, Isfahan, Iran.
References
- 1.Kostov K. Effects of magnesium deficiency on mechanisms of insulin resistance in type 2 diabetes: Focusing on the processes of insulin secretion and signaling. Int J Mol Sci. 2019;20:1351. doi: 10.3390/ijms20061351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morakinyo AO, Samuel TA, Adekunbi DA. Magnesium upregulates insulin receptor and glucose transporter-4 in streptozotocin-nicotinamide-induced type-2 diabetic rats. Endocr Regul. 2018;52:6–16. doi: 10.2478/enr-2018-0002. [DOI] [PubMed] [Google Scholar]
- 3.Rezazadeh H, Sharifi MR, Sharifi M, Soltani N. Magnesium sulfate improves insulin resistance in high fat diet induced diabetic parents and their offspring. Eur J Pharmacol. 2021;909:174418. doi: 10.1016/j.ejphar.2021.174418. [DOI] [PubMed] [Google Scholar]
- 4.Chutia H, Lynrah KG. Association of serum magnesium deficiency with insulin resistance in type 2 diabetes mellitus. J Lab Physicians. 2015;7:75–8. doi: 10.4103/0974-2727.163131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morais JB, Severo JS, de Alencar GR, de Oliveira AR, Cruz KJ, Marreiro DD, et al. Effect of magnesium supplementation on insulin resistance in humans: A systematic review. Nutrition. 2017;38:54–60. doi: 10.1016/j.nut.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Günther T. The biochemical function of Mg2+ in insulin secretion, insulin signal transduction and insulin resistance. Magnes Res. 2010;23:5–18. doi: 10.1684/mrh.2009.0195. [DOI] [PubMed] [Google Scholar]
- 7.Samuel VT, Shulman GI. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J Clin Invest. 2016;126:12–22. doi: 10.1172/JCI77812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fapohunda O, Balogun O. Oral magnesium supplementation modulates hepatic and intestinal expression of some carbohydrate metabolizing genes in type 2 diabetic rats. Int J Mol Biol Open Access. 2019;4:189–94. [Google Scholar]
- 9.Tan SY, Mei Wong JL, Sim YJ, Wong SS, Mohamed Elhassan SA, Tan SH, et al. Type 1 and 2 diabetes mellitus: A review on current treatment approach and gene therapy as potential intervention. Diabetes Metab Syndr. 2019;13:364–72. doi: 10.1016/j.dsx.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 10.ELDerawi WA, Naser IA, Taleb MH, Abutair AS. The effects of oral magnesium supplementation on glycemic response among type 2 diabetes patients. Nutrients. 2018;11:E44. doi: 10.3390/nu11010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gommers LM, Hoenderop JG, Bindels RJ, de Baaij JH. Hypomagnesemia in type 2 diabetes: A vicious circle? Diabetes. 2016;65:3–13. doi: 10.2337/db15-1028. [DOI] [PubMed] [Google Scholar]
- 12.Giri B, Dey S, Das T, Sarkar M, Banerjee J, Dash SK. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. Biomed Pharmacother. 2018;107:306–28. doi: 10.1016/j.biopha.2018.07.157. [DOI] [PubMed] [Google Scholar]
- 13.Sohrabipour S, Sharifi MR, Sharifi M, Talebi A, Soltani N. Effect of magnesium sulfate administration to improve insulin resistance in type 2 diabetes animal model: Using the hyperinsulinemic-euglycemic clamp technique. Fundam Clin Pharmacol. 2018;32:603–16. doi: 10.1111/fcp.12387. [DOI] [PubMed] [Google Scholar]
- 14.Fang X, Liang C, Li M, Montgomery S, Fall K, Aaseth J, et al. Dose-response relationship between dietary magnesium intake and cardiovascular mortality: A systematic review and dose-based meta-regression analysis of prospective studies. J Trace Elem Med Biol. 2016;38:64–73. doi: 10.1016/j.jtemb.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Thomas AE, Inagadapa PJ, Jeyapal S, Merugu NM, Kalashikam RR, Manchala R. Maternal magnesium restriction elevates glucocorticoid stress and inflammation in the placenta and fetus of WNIN rat dams. Biol Trace Elem Res. 2018;181:281–7. doi: 10.1007/s12011-017-1058-3. [DOI] [PubMed] [Google Scholar]
- 16.Schutten JC, Joosten MM, de Borst MH, Bakker SJ. Magnesium and blood pressure: A physiology-based approach. Adv Chronic Kidney Dis. 2018;25:244–50. doi: 10.1053/j.ackd.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Alghobashy AA, Alkholy UM, Talat MA, Abdalmonem N, Zaki A, Ahmed IA, et al. Trace elements and oxidative stress in children with type 1 diabetes mellitus. Diabetes Metab Syndr Obes. 2018;11:85–92. doi: 10.2147/DMSO.S157348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mooren FC. Magnesium and disturbances in carbohydrate metabolism. Diabetes Obes Metab. 2015;17:813–23. doi: 10.1111/dom.12492. [DOI] [PubMed] [Google Scholar]
- 19.Uwitonze AM, Razzaque MS. Role of magnesium in vitamin D activation and function. J Am Osteopath Assoc. 2018;118:181–9. doi: 10.7556/jaoa.2018.037. [DOI] [PubMed] [Google Scholar]
- 20.Spiga R, Mannino GC, Mancuso E, Averta C, Paone C, Rubino M, et al. Are circulating Mg2+ levels associated with glucose tolerance profiles and incident type 2 diabetes? Nutrients. 2019;11:2460. doi: 10.3390/nu11102460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piuri G, Zocchi M, Della Porta M, Ficara V, Manoni M, Zuccotti GV, et al. Magnesium in obesity, metabolic syndrome, and type 2 diabetes. Nutrients. 2021;13:320. doi: 10.3390/nu13020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gommers LM, Hill TG, Ashcroft FM, de Baaij JH. Low extracellular magnesium does not impair glucose-stimulated insulin secretion. PLoS One. 2019;14:e0217925. doi: 10.1371/journal.pone.0217925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guerrero-Romero F, Tamez-Perez HE, González-González G, Salinas-Martínez AM, Montes-Villarreal J, Treviño-Ortiz JH, et al. Oral magnesium supplementation improves insulin sensitivity in non-diabetic subjects with insulin resistance. A double-blind placebo-controlled randomized trial. Diabetes Metab. 2004;30:253–8. doi: 10.1016/s1262-3636(07)70116-7. [DOI] [PubMed] [Google Scholar]
- 24.Dominguez LJ, Barbagallo M, Sowers JR, Resnick LM. Magnesium responsiveness to insulin and insulin-like growth factor I in erythrocytes from normotensive and hypertensive subjects. J Clin Endocrinol Metab. 1998;83:4402–7. doi: 10.1210/jcem.83.12.5327. [DOI] [PubMed] [Google Scholar]
- 25.Feng J, Wang H, Jing Z, Wang Y, Cheng Y, Wang W, et al. Role of magnesium in type 2 diabetes mellitus. Biol Trace Elem Res. 2020;196:74–85. doi: 10.1007/s12011-019-01922-0. [DOI] [PubMed] [Google Scholar]
- 26.Dou M, Ma Y, Ma AG, Han L, Song MM, Wang YG, et al. Combined chromium and magnesium decreases insulin resistance more effectively than either alone. Asia Pac J Clin Nutr. 2016;25:747–53. doi: 10.6133/apjcn.092015.48. [DOI] [PubMed] [Google Scholar]
- 27.Song Y, He K, Levitan EB, Manson JE, Liu S. Effects of oral magnesium supplementation on glycaemic control in Type 2 diabetes: A meta-analysis of randomized double-blind controlled trials. Diabet Med. 2006;23:1050–6. doi: 10.1111/j.1464-5491.2006.01852.x. [DOI] [PubMed] [Google Scholar]
- 28.Mooren FC, Krüger K, Völker K, Golf SW, Wadepuhl M, Kraus A. Oral magnesium supplementation reduces insulin resistance in non-diabetic subjects – A double-blind, placebo-controlled, randomized trial. Diabetes Obes Metab. 2011;13:281–4. doi: 10.1111/j.1463-1326.2010.01332.x. [DOI] [PubMed] [Google Scholar]
- 29.Hruby A, Meigs JB, O’Donnell CJ, Jacques PF, McKeown NM. Higher magnesium intake reduces risk of impaired glucose and insulin metabolism and progression from prediabetes to diabetes in middle-aged Americans. Diabetes Care. 2014;37:419–27. doi: 10.2337/dc13-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solati M, Ouspid E, Hosseini S, Soltani N, Keshavarz M, Dehghani M. Oral magnesium supplementation in type II diabetic patients. Med J Islam Repub Iran. 2014;28:67. [PMC free article] [PubMed] [Google Scholar]
- 31.Maktabi M, Jamilian M, Amirani E, Chamani M, Asemi Z. The effects of magnesium and vitamin E co-supplementation on parameters of glucose homeostasis and lipid profiles in patients with gestational diabetes. Lipids Health Dis. 2018;17:163. doi: 10.1186/s12944-018-0814-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rayssiguier Y, Gueux E. Magnesium and lipids in cardiovascular disease. J Am Coll Nutr. 1986;5:507–19. doi: 10.1080/07315724.1986.10720153. [DOI] [PubMed] [Google Scholar]
- 33.Navarrete-Cortes A, Ble-Castillo JL, Guerrero-Romero F, Cordova-Uscanga R, Juárez-Rojop IE, Aguilar-Mariscal H, et al. No effect of magnesium supplementation on metabolic control and insulin sensitivity in type 2 diabetic patients with normomagnesemia. Magnes Res. 2014;27:48–56. doi: 10.1684/mrh.2014.0361. [DOI] [PubMed] [Google Scholar]
- 34.de Valk HW, Verkaaik R, van Rijn HJ, Geerdink RA, Struyvenberg A. Oral magnesium supplementation in insulin-requiring Type 2 diabetic patients. Diabet Med. 1998;15:503–7. doi: 10.1002/(SICI)1096-9136(199806)15:6<503::AID-DIA596>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 35.Gullestad L, Jacobsen T, Dolva LO. Effect of magnesium treatment on glycemic control and metabolic parameters in NIDDM patients. Diabetes Care. 1994;17:460–1. doi: 10.2337/diacare.17.5.460. [DOI] [PubMed] [Google Scholar]
- 36.Johansen K, Dagogo-Jack S. Non-insulin-dependent diabetes mellitus. In: Diabetes Guide. Berlin Heidelberg, Springer: 1992. pp. 79–83. [Google Scholar]
- 37.Shigematsu M, Tomonaga S, Shimokawa F, Murakami M, Imamura T, Matsui T, et al. Regulatory responses of hepatocytes, macrophages and vascular endothelial cells to magnesium deficiency. J Nutr Biochem. 2018;56:35–47. doi: 10.1016/j.jnutbio.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 38.Hardy S, Kostantin E, Wang SJ, Hristova T, Galicia-Vázquez G, Baranov PV, et al. Magnesium-sensitive upstream ORF controls PRL phosphatase expression to mediate energy metabolism. Proc Natl Acad Sci U S A. 2019;116:2925–34. doi: 10.1073/pnas.1815361116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Z, Wang J, Sun W, Archibong E, Kahkoska AR, Zhang X, et al. Synthetic beta cells for fusion-mediated dynamic insulin secretion. Nat Chem Biol. 2018;14:86–93. doi: 10.1038/nchembio.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dhanawat A, Mohanty L, Khatua P, Panda SS, Maheshwari G. Correlation of fasting serum magnesium with glycaemic and nephropathy status in type 2 diabetes mellitus. J Clin Diagn Res. 2020;14:21–25. [Google Scholar]
- 41.Radin JN, Kelliher JL, Solórzano PK, Grim KP, Ramezanifard R, Slauch JM, et al. Metal-independent variants of phosphoglycerate mutase promote resistance to nutritional immunity and retention of glycolysis during infection. PLoS Pathog. 2019;15:e1007971. doi: 10.1371/journal.ppat.1007971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dibaba DT, Xun P, Fly AD, Yokota K, He K. Dietary magnesium intake and risk of metabolic syndrome: A meta-analysis. Diabet Med. 2014;31:1301–9. doi: 10.1111/dme.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ashcroft FM, Harrison DE, Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature. 1984;312:446–8. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- 44.Cunha AR, Umbelino B, Correia ML, Neves MF. Magnesium and vascular changes in hypertension. Int J Hypertens 2012. 2012:754250. doi: 10.1155/2012/754250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giménez-Mascarell P, Schirrmacher CE, Martínez-Cruz LA, Müller D. Novel aspects of renal magnesium homeostasis. Front Pediatr. 2018;6:77. doi: 10.3389/fped.2018.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: Implications for health and disease. Physiol Rev. 2015;95:1–46. doi: 10.1152/physrev.00012.2014. [DOI] [PubMed] [Google Scholar]
- 47.Shimaoka T, Wang Y, Morishima M, Miyamoto S, Ono K. Hypomagnesemic down-regulation of L-type Ca (2+) channel in cardiomyocyte as an arrhythmogenic substrate in rats. Pathophysiology. 2015;22:87–93. doi: 10.1016/j.pathophys.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Atwater I, Frankel BJ, Rojas E, Grodsky GM. Beta cell membrane potential and insulin release; role of calcium and calcium: magnesium ratio. Q J Exp Physiol. 1983;68:233–45. doi: 10.1113/expphysiol.1983.sp002715. [DOI] [PubMed] [Google Scholar]
- 49.Wang CC, Chen HJ, Chan DC, Chiu CY, Liu SH, Lan KC. Low-dose acrolein, an endogenous and exogenous toxic molecule, inhibits glucose transport via an inhibition of Akt-regulated GLUT4 signaling in skeletal muscle cells. Int J Mol Sci. 2021;22:7228. doi: 10.3390/ijms22137228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kostov K, Halacheva L. Role of magnesium deficiency in promoting atherosclerosis, endothelial dysfunction, and arterial stiffening as risk factors for hypertension. Int J Mol Sci. 2018;19:1724. doi: 10.3390/ijms19061724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cutler DA, Pride SM, Cheung AP. Low intakes of dietary fiber and magnesium are associated with insulin resistance and hyperandrogenism in polycystic ovary syndrome: A cohort study. Food Sci Nutr. 2019;7:1426–37. doi: 10.1002/fsn3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Al Alawi AM, Majoni SW, Falhammar H. Magnesium and human health: Perspectives and research directions. Int J Endocrinol 2018. 2018:9041694. doi: 10.1155/2018/9041694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vicario PP, Bennun A. Separate effects of Mg2+, MgATP, and ATP4- on the kinetic mechanism for insulin receptor tyrosine kinase. Arch Biochem Biophys. 1990;278:99–105. doi: 10.1016/0003-9861(90)90236-r. [DOI] [PubMed] [Google Scholar]
- 54.Siddiqui MU, Ali I, Zakariya M, Asghar SP, Ahmed MR, Ibrahim GH. Frequency of hypomagnesemia in patients with uncontrolled type ii diabetes mellitus. Pak Armed Forces Med J. 2016;66:845–50. [Google Scholar]
- 55.Mi J, He W, Lv J, Zhuang K, Huang H, Quan S. Effect of berberine on the HPA-axis pathway and skeletal muscle GLUT4 in type 2 diabetes mellitus rats. Diabetes Metab Syndr Obes. 2019;12:1717–25. doi: 10.2147/DMSO.S211188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Solaimani H, Soltani N, MaleKzadeh K, Sohrabipour S, Zhang N, Nasri S, et al. Modulation of GLUT4 expression by oral administration of Mg (2+) to control sugar levels in STZ-induced diabetic rats. Can J Physiol Pharmacol. 2014;92:438–44. doi: 10.1139/cjpp-2013-0403. [DOI] [PubMed] [Google Scholar]
- 57.Barooti A, Kamran M, Kharazmi F, Eftakhar E, Malekzadeh K, Talebi A, et al. Effect of oral magnesium sulfate administration on blood glucose hemostasis via inhibition of gluconeogenesis and FOXO1 gene expression in liver and muscle in diabetic rats. Biomed Pharmacother. 2019;109:1819–25. doi: 10.1016/j.biopha.2018.10.164. [DOI] [PubMed] [Google Scholar]
- 58.Ha BG, Park JE, Cho HJ, Shon YH. Stimulatory effects of balanced deep sea water on mitochondrial biogenesis and function. PLoS One. 2015;10:e0129972. doi: 10.1371/journal.pone.0129972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kamran M, Kharazmi F, Malekzadeh K, Talebi A, Khosravi F, Soltani N. Effect of long-term administration of oral magnesium sulfate and insulin to reduce streptozotocin-induced hyperglycemia in rats: The role of Akt2 and IRS1 gene expressions. Biol Trace Elem Res. 2019;190:396–404. doi: 10.1007/s12011-018-1555-z. [DOI] [PubMed] [Google Scholar]
- 60.Lan S, Albinsson S. Regulation of IRS-1, insulin signaling and glucose uptake by miR-143/145 in vascular smooth muscle cells. Biochem Biophys Res Commun. 2020;529:119–25. doi: 10.1016/j.bbrc.2020.05.148. [DOI] [PubMed] [Google Scholar]
- 61.Jaiswal N, Gavin MG, Quinn WJ, 3rd, Luongo TS, Gelfer RG, Baur JA, et al. The role of skeletal muscle Akt in the regulation of muscle mass and glucose homeostasis. Mol Metab. 2019;28:1–13. doi: 10.1016/j.molmet.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405:421–4. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- 63.Khosravi F, Kharazmi F, Kamran M, Malekzadeh K, Talebi A, Soltani N. The role of PPAR-γ and NFKB genes expression in muscle to improve hyperglycemia in STZ-induced diabetic rat following magnesium sulfate administration. Int J Physiol Pathophysiol Pharmacol. 2018;10:124–31. [PMC free article] [PubMed] [Google Scholar]
- 64.Habor A. Peroxisome proliferator activated receptors. Farmacia. 2010;58:13. [Google Scholar]
- 65.Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454:470–7. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- 66.Quinn CE, Hamilton PK, Lockhart CJ, McVeigh GE. Thiazolidinediones: Effects on insulin resistance and the cardiovascular system. Br J Pharmacol. 2008;153:636–45. doi: 10.1038/sj.bjp.0707452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pujimulyani D, Yulianto WA, Setyowati A, Arumwardana S, Sari Widya Kusuma H, Adhani Sholihah I, et al. Hypoglycemic activity of Curcuma mangga Val. extract via modulation of GLUT4 and PPAR-γ mRNA expression in 3T3-L1 adipocytes. J Exp Pharmacol. 2020;12:363–9. doi: 10.2147/JEP.S267912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Armoni M, Harel C, Karnieli E. Transcriptional regulation of the GLUT4 gene: From PPAR-gamma and FOXO1 to FFA and inflammation. Trends Endocrinol Metab. 2007;18:100–7. doi: 10.1016/j.tem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 69.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–70. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 70.Jeong JW, Lee B, Kim DH, Jeong HO, Moon KM, Kim MJ, et al. Mechanism of action of magnesium lithospermate B against aging and obesity-induced ER stress, insulin resistance, and inflammsome formation in the liver. Molecules. 2018;23:2098. doi: 10.3390/molecules23092098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen MJ, Yan X, Chen YQ, Zhao C. Phytochemicals for non-insulin diabetes mellitus: A minireview on plant-derived compounds hypoglycemic activity. J Food Nutr Sci. 2017;5:23–7. [Google Scholar]
- 72.Maiese K. FoxO transcription factors and regenerative pathways in diabetes mellitus. Curr Neurovasc Res. 2015;12:404–13. doi: 10.2174/1567202612666150807112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sato T, Watanabe Y, Nishimura Y, Inoue M, Morita A, Miura S. Acute fructose intake suppresses fasting-induced hepatic gluconeogenesis through the AKT-FoxO1 pathway. Biochem Biophys Rep. 2019;18:100638. doi: 10.1016/j.bbrep.2019.100638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gross DN, Wan M, Birnbaum MJ. The role of FOXO in the regulation of metabolism. Curr Diab Rep. 2009;9:208–14. doi: 10.1007/s11892-009-0034-5. [DOI] [PubMed] [Google Scholar]
- 75.Chen L, Chen R, Wang H, Liang F. Mechanisms linking inflammation to insulin resistance. Int J Endocrinol 2015. 2015:508409. doi: 10.1155/2015/508409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ghanbari M, Momen Maragheh S, Aghazadeh A, Mehrjuyan SR, Hussen BM, Abdoli Shadbad M, et al. Interleukin-1 in obesity-related low-grade inflammation: From molecular mechanisms to therapeutic strategies. Int Immunopharmacol. 2021;96:107765. doi: 10.1016/j.intimp.2021.107765. [DOI] [PubMed] [Google Scholar]
- 77.Rehman K, Akash MS. Mechanisms of inflammatory responses and development of insulin resistance: How are they interlinked? J Biomed Sci. 2016;23:87. doi: 10.1186/s12929-016-0303-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khodabandehloo H, Gorgani-Firuzjaee S, Panahi G, Meshkani R. Molecular and cellular mechanisms linking inflammation to insulin resistance and β-cell dysfunction. Transl Res. 2016;167:228–56. doi: 10.1016/j.trsl.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 79.Liu M, Dudley SC., Jr Magnesium, oxidative stress, inflammation, and cardiovascular disease. Antioxidants (Basel) 2020;9:907. doi: 10.3390/antiox9100907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ribeiro MC, Avila DS, Barbosa NB, Meinerz DF, Waczuk EP, Hassan W, et al. Hydrochlorothiazide and high-fat diets reduce plasma magnesium levels and increase hepatic oxidative stress in rats. Magnes Res. 2013;26:32–40. doi: 10.1684/mrh.2013.0334. [DOI] [PubMed] [Google Scholar]
- 81.Maiese K, Morhan SD, Chong ZZ. Oxidative stress biology and cell injury during type 1 and type 2 diabetes mellitus. Curr Neurovasc Res. 2007;4:63–71. doi: 10.2174/156720207779940653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Beverly JK, Budoff MJ. Atherosclerosis: Pathophysiology of insulin resistance, hyperglycemia, hyperlipidemia, and inflammation. J Diabetes. 2020;12:102–4. doi: 10.1111/1753-0407.12970. [DOI] [PubMed] [Google Scholar]
- 83.Khot V, Upadhye S, Kothali B, Apte A, Kulkarni A, Patil A, et al. Free radicals, oxidative stress and diseases an overview. Am. J. PharmTech Res. 2018;8:59–67. [Google Scholar]
- 84.Olawale F, Aninye II, Ajaja UI, Nwozo SO. Long-term hyperglycemia impairs hormonal balance and induces oxidative damage in ovaries of streptozotocin-induced diabetic wistar rat. Niger J Physiol Sci. 2020;35:46–51. [PubMed] [Google Scholar]
- 85.Volpe CM, Villar-Delfino PH, Dos Anjos PM, Nogueira-Machado JA. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018;9:119. doi: 10.1038/s41419-017-0135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morais JB, Severo JS, Santos LR, de Sousa Melo SR, de Oliveira Santos R, de Oliveira AR, et al. Role of magnesium in oxidative stress in individuals with obesity. Biol Trace Elem Res. 2017;176:20–6. doi: 10.1007/s12011-016-0793-1. [DOI] [PubMed] [Google Scholar]
- 87.Jamilian M, Mirhosseini N, Eslahi M, Bahmani F, Shokrpour M, Chamani M, et al. The effects of magnesium-zinc-calcium-vitamin D co-supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. BMC Pregnancy Childbirth. 2019;19:107. doi: 10.1186/s12884-019-2258-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sajjan NB, Choudhari AS, Desai GM, Dharapur M, Wali VV. Evaluation of association of serum magnesium with dyslipidaemia in diabetic nephropathy – A case control study. Natl J Med Res. 2014;4:318–21. [Google Scholar]
- 89.Pavithra D, Praveen D, Chowdary PR, Aanandhi MV. A review on role of Vitamin E supplementation in type 2 diabetes mellitus. Drug Invent Today. 2018;10:236–40. [Google Scholar]
- 90.Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond) 2013;124:139–52. doi: 10.1042/CS20120198. [DOI] [PubMed] [Google Scholar]
- 91.Lin CY, Tsai PS, Hung YC, Huang CJ. L-type calcium channels are involved in mediating the anti-inflammatory effects of magnesium sulphate. Br J Anaesth. 2010;104:44–51. doi: 10.1093/bja/aep336. [DOI] [PubMed] [Google Scholar]
- 92.Voma C, Romani A. Role of Magnesium in the Regulation of Hepatic Glucose Homeostasis. London, UK: InTech; 2014. pp. 95–111. [Google Scholar]
- 93.Nakamura A, Omori K, Terauchi Y. Glucokinase activation or inactivation: Which will lead to the treatment of type 2 diabetes? Diabetes Obes Metab. 2021;23:2199–206. doi: 10.1111/dom.14459. [DOI] [PubMed] [Google Scholar]
- 94.Beurel E, Grieco SF, Jope RS. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol Ther. 2015;148:114–31. doi: 10.1016/j.pharmthera.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Medina M, Wandosell F. Deconstructing GSK-3: The fine regulation of its activity. Int J Alzheimers Dis. 2011;2011:479249. doi: 10.4061/2011/479249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Garfinkel L, Garfinkel D. Magnesium regulation of the glycolytic pathway and the enzymes involved. Magnesium. 1985;4:60–72. [PubMed] [Google Scholar]
- 97.Debnath B, Das T, Dhore R, Chakrabarti A, Kumar P, Acharjee S. Role of some critical minerals in nutrient metabolism academia. edu. 2018;4:17–19. [Google Scholar]
- 98.Pilchova I, Klacanova K, Tatarkova Z, Kaplan P, Racay P. The involvement of Mg2+ in regulation of cellular and mitochondrial functions. Oxid Med Cell Longev. 2017;2017:6797460. doi: 10.1155/2017/6797460. [DOI] [PMC free article] [PubMed] [Google Scholar]