Abstract
Resistance training is a promising strategy to promote healthy cognitive aging; however, the brain mechanisms by which resistance training benefits cognition have yet to be determined. Here, we examined the effects of a 12-week resistance training program on resting brain activity and cerebrovascular function in 20 healthy older adults (14 females, mean age 69.1 years). In this single group clinical trial, multimodal 3 T magnetic resonance imaging was performed at 3 time points: baseline (preceding a 12-week control period), pre-intervention, and post-intervention. Along with significant improvements in fluid cognition (d = 1.27), 4 significant voxelwise clusters were identified for decreases in resting brain activity after the intervention (Cerebellum, Right Middle Temporal Gyrus, Left Inferior Parietal Lobule, and Right Inferior Parietal Lobule), but none were identified for changes in resting cerebral blood flow. Using a separate region of interest approach, we provide estimates for improved cerebral blood flow, compared with declines over the initial control period, in regions associated with cognitive impairment, such as hippocampal blood flow (d = 0.40), and posterior cingulate blood flow (d = 0.61). Finally, resistance training had a small countermeasure effect on the age-related progression of white matter lesion volume (rank-biserial = −0.22), a biomarker of cerebrovascular disease. These proof-of-concept data support larger trials to determine whether resistance training can attenuate or even reverse salient neurodegenerative processes.
Keywords: fALFF, fMRI, cerebral blood flow, vascular compliance, arterial spin labeling, white matter hyperintensities
Introduction
Although the beneficial effects of physical activity on cognition and dementia risk are well-accepted, 1 the conclusions are primarily based on studies of aerobic exercise and the evidence for other forms of exercise like resistance training is scarce. Resistance training (RT) interventions have been shown to improve fluid cognition in both healthy older adults and patients with mild cognitive impairment (MCI).2,3 These improvements hold promise in the multifaceted effort to delay or even prevent cognitive decline and dementia. However, a clear understanding of the neurophysiological processes that underlie these benefits has not been established. While most of the magnetic resonance imaging (MRI) literature has focused on aerobic exercise interventions, 4 RT likely has distinct brain mechanisms of benefit that have yet to be fully determined. 5
Functional brain changes may underpin the unique cognitive benefits of RT. 6 While some RT studies have characterized functional plasticity during executive function and associative memory tasks,7,8 the effects on resting brain activity are unclear. Suo et al 9 found decreased resting-state functional connectivity within the default mode network; however, there were no correlations with cognitive outcomes. Because functional connectivity calculations do not directly provide information on the amplitude of brain activity, an alternative approach is to use the fractional amplitude of low-frequency fluctuations (fALFF) which measures the relative contribution of low frequency fluctuations within a specific frequency band to the whole detectable frequency range. 10 Rather than estimating the connectivity (or temporal synchrony) between different brain regions, this technique quantifies the differences in resting-state activity across the whole brain, providing a measure of regional neuronal activity amplitude. 11 Since these techniques are based on blood-oxygenation-level-dependent (BOLD) functional MRI (fMRI), they are physiologically driven by the cerebrovascular (CV) system.
CV function is a major component of brain health, responsible for securing adequate blood supply to the brain in resting and active conditions. Mounting evidence suggests that CV dysfunction may precede, and even cause, neuronal dysfunction and cognitive impairment.12,13 For example, reductions in global cerebral blood flow (CBF) are associated with accelerated cognitive decline and increased risk of dementia. 14 While aerobic training studies suggest that improved resting CBF is a significant mechanism of cognitive enhancement, 15 this has not been tested after RT. For example, regional CBF is increased after only 12 weeks of aerobic training in healthy older adults, and these changes are associated with improvements in memory.16,17 RT is known to improve systemic vascular function and cardiovascular health,18,19 so improvements in CV function are plausible. Furthermore, changes in CBF patterns during resistance exercise, although complex, do seem to present an adaptive stimulus. 20 Intervention studies are thus warranted. Specifically, investigations are needed to test and extend previous cross-sectional evidence linking RT with greater global CBF in older women. 21
Global and regional CBF can be assessed by arterial spin labeling (ASL), a dynamic noninvasive MRI technique that uses arterial blood water as an endogenous tracer for quantitative measurements. 22 New ASL methodologies have been developed for various different aspects of CV function. 23 For example, intracranial vascular compliance (VC) is an essential buffering mechanism for continuous blood flow to the brain, whereas the opposite, for example, increased pulsatility and resistance, is associated with decreased cognitive function and believed to cause long-term brain damage. 24 By synchronizing dynamic ASL with the cardiac cycle, VC can be calculated as the ratio of the change in cerebral blood volume (CBV) between systole and diastole to the change in arterial blood pressure (BP) between systole and diastole. 25 Using these ASL techniques allows novel investigations of the effects of RT on dynamic CV function.
Another important marker of CV dysfunction, specifically of small-vessel disease, is white matter lesion (WML) volume. WMLs are structural markers of ischemic and inflammatory brain tissue that are visualized as hyperintense signals on T2-weighted FLAIR (Fluid-attenuated inversion recovery) MRI 26 and strongly implicated in the pathogenesis of vascular cognitive impairment and dementia. 27 Furthermore, the presence of WMLs interacts with resting CBF, such that hypoperfusion-related rate of cognitive decline and risk of dementia increase with greater severity of WML volume. 14 A similar interaction effect may occur in reverse with exercise. RT has demonstrated countermeasure effects against the normal progression of WMLs in both healthy older adults and patients with MCI,9,28 accompanied by improved executive functions.
The purpose of this investigation was to estimate the effects of 12-week of high-intensity RT on resting brain activity and CV function (measured via multimodal 3T MRI) and to explore potential associations between brain changes and cognitive improvements. These proof-of-concept findings are presented in a practical manner to support the design of larger randomized controlled trials. We hypothesized that 12-week of periodized RT performed 3 days per week can improve resting brain activity, CBF, VC, and WML volume in healthy older adults 60 to 80 years of age.
Methods
Study design
Using a single arm pre-post design, the effects of a 12-week periodized RT intervention on fluid cognition and CV function were compared to that of an initial 12-week control period. Participants served as their own controls and completed all assessments at 3 time points: baseline, pre-intervention, and post-intervention. The full protocol for this mechanistic proof-of-concept clinical trial has been described in detail previously. 2 This simple yet rigorous and well-designed study was approved by the USC Health Sciences Review Board (#HS-17-00770) and registered with ClinicalTrials.gov (ID: NCT03982550). All procedures were conducted at the University of Southern California (USC, Los Angeles, CA) Health Sciences Campus. Participants were asked to not change their eating or exercise habits outside of the study and were encouraged to continue their normal activities.
Written informed consent was obtained from all potential participants assessed for eligibility. Primary considerations for inclusion/exclusion criteria were to ensure that participants were healthy, interested, and available to participate, without any contraindications to RT or MRI. Sample size was determined via an a priori power analysis using the effect size (ES) (d = 0.71) of RT on global fluid cognition, 3 alpha of 0.05, power of 0.80, within-subjects pre-post design, and anticipated attrition rate of 10%. Twenty healthy older adults (14 Female, mean age 69.1 ± 5.8 years, age range 60.1-79.7 years, mean height 166.7 ± 8.9 cm, mean weight 72.3 ± 10.1 kg, baseline mini mental state examination score 28.0 ± 1.6) were enrolled in the study and all completed the required number of training sessions (>90%) and assessments at baseline, pre-intervention, and post-intervention. Recruitment took place between July 2018 and May 2019, and the last participant finished post-intervention testing in December 2019. There were no participant drop-outs or severe adverse events. The COVID-19 pandemic prevented 1-year follow-up testing.
All 12-week control periods took place before the RT intervention to ensure that results were not confounded by detraining effects or long-term cognitive benefits of RT. 29 The periodized and progressive RT program (Table 1) emphasized development of total-body strength and all sessions (3 days per week for 12 weeks) were supervised by an exercise specialist. The 1-hour training sessions alternated between lower body (leg press, leg extension, leg curl) focus and upper body (chest press, lat pulldown, seated row, and seated shoulder press) focus. Training loads were individually progressed in a safe and effective manner using autoregulatory progression. 30 While the sets and repetitions were fixed for each week, supervised autoregulation allowed participants to progress loads at their own pace based on maximum performance on the last set of each exercise. If participants missed training sessions during the 12-week intervention, they were allowed to complete up to 6 sessions during a 2-week buffer period. All post-intervention study procedures were conducted at least 48 hours after the last training session and within 2 weeks.
Table 1.
Linear periodization model used to maximize strength gains.
| 12-Week resistance training intervention | ||||||
|---|---|---|---|---|---|---|
| Weeks | 1-2 | 3-4 | 5-6 | 7-8 | 9-10 | 11-12 |
| Sets | 3 | 3 | 4 | 3 | 4 | 3 |
| Repetitions | 10 | 8 | 6 | 6 | 4 | 4 |
Cognitive testing
All cognitive assessments were administered by the same trained investigator (TRM) using the NIH Toolbox® Cognition Battery (NIHTB-CB, Version 1.21) application. The NIHTB-CB provides a standard set of comprehensive assessment tools that have been normed and validated in participants ages 3 to 85, and ensures that assessment methods and results can be used for comparisons across existing and future studies. 31 Three test versions were used to reduce practice effects and were administered in random order for each participant. The NIHTB-CB tests 5 fluid cognition subdomains (inhibitory control and attention, episodic memory, working memory, executive function, and processing speed) to yield a fluid cognition composite score. 32 We analyzed uncorrected scores, standardized to the population with a normative mean of 100 and a standard deviation of 15.
Data acquisition
MRI data were acquired on a Siemens 3T Magnetom Prisma (Erlangen, Germany) using a 20-channel head coil. Padding was placed around the head to minimize motion during scans. Electrocardiogram (ECG) leads were placed on the participants’ chest prior to scans. Before and after the VC scans, brachial blood pressure was recorded using an MR compatible cuff sphygmomanometer. Participants were asked to stay as still as possible without falling asleep.
A sagittal scout image was used as a localizer, and brain structure was assessed using a T1-weighted accelerated sagittal 3D MPRAGE (Magnetization-Prepared Rapid Acquisition with Gradient Echo) scan (TR/TE: 2300/2.95 ms; TI: 900 ms; Flip Angle: 9°; slice thickness 1.2 mm without gap; field of view read: 270 mm; resolution 256). In addition, T-2 weighted sagittal 3D FLAIR (Fluid Attenuated Inversion Recovery; TR/TE: 5000/388 ms; TI: 1800 ms) was acquired with an interleaved series and anterior to posterior phase encoding. Each slab had 176 slices (1.0 mm thickness) and a field of view read of 256 mm.
An ECG-triggered time resolved phase contrast MRI was performed to measure the blood flow velocity in the internal carotid arteries. A single axial slice of 5.0 mm thickness at the level of C1/C2 was imaged with the following parameters: TR/TE = 49.25/4.86 ms, FOV read = 200 mm, flip angle = 15°, matrix = 192 × 192, Velocity encoding = 100 cm/s, 23 phases with an interval of 50 ms, scan time of 2 minutes. The time delays at peak systole and early diastole were identified in each individual subject (on average 150 ms and 400 ms following the trigger, respectively). These time delays were used as the ECG trigger to synchronize 2 separate multi-phase balanced steady-state free precession (bSSFP) ASL scans (pulsed spin labeling) with the peak systolic and early diastolic phases. 25 The flow-sensitive alternating inversion recovery (FAIR) scheme was implemented for spin labeling, which was immediately followed by a cine bSSFP readout train with the following parameters: TR/TE = 73.2/1.83 ms, FOV read = 220 mm, flip angle = 40°, matrix = 96 × 96, slice thickness = 8.0 mm, centric ordering k-space acquisition with 20 lines per segment, and 29 phases from 150 to 2250 ms with an interval of 75 ms. The interval between inversion pulses was approximately 3 seconds covering 3 to 4 heartbeats. An oblique axial slice of 5-mm thickness (Parallel to AC–PC) at the level of internal capsule was imaged. In each cine bSSFP ASL scan, 8 pairs of label/control acquisitions were collected which took approximately 3 minutes. The 2 bSSFP ASL scans at systole and diastole took approximately 6 minutes.
Following the 2 ECG-triggered dynamic bSSFP ASL scans, a 5-minute resting-state perfusion MRI scan was performed using a multi-delay pseudo-continuous ASL (pCASL) sequence with background suppressed 3D gradient and spin echo readout. 33 A M0 scan was acquired at the beginning of the pCASL sequence. Fifty-four 2.5 mm transverse slices were acquired to cover the whole brain with the following imaging parameters: TR/TE = 4100 ms/36.76 ms, FOV read = 240 mm, flip angle = 120°, matrix = 64 × 64. The tagging plane was positioned 90 mm inferior to the center of the imaging slab with a labeling duration of 1500 ms and 5 post-labeling delays totaling 1800 ms. Thirty pairs of label and control acquisitions were performed.
Last, a resting-state fMRI scan was performed using T2* echo-planar imaging with an interleaved BOLD sequence (TR/TE = 800/37 ms, flip angle = 52°, slice thickness = 2.0 mm, 72 slices, 5.75 minutes). The field of view read was 208 mm with each voxel size 2.0 × 2.0 × 2.0 mm. Participants were asked to keep their eyes open for the duration of the fMRI scan. All ASL and fMRI data from one participant were removed because of excess noise, likely due to head movement during the MRI.
Voxelwise data processing
Resting-state functional MRI scans were preprocessed using fMRIPrep 1.5.8, 34 which is based on Nipype 1.4.1. 35 In short, images were motion and slice timing corrected. Signal noise was removed using FSL-Fix, where noise components were identified based on a “HCP-2000” training set available with FSL-fix package (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FIX). fALFF was determined using AFNI’s RSFC package within a frequency range 0.01 to 0.027 Hz, known as the slow-5 band. Frequency results were smoothed with a 6 mm full width at half maximum gaussian kernel. Significant changes in fALFF amplitude were evaluated in a voxelwise repeated measures analysis in SPM12. Baseline, pre-intervention, and post-intervention scans were included in the longitudinal analysis and modeled as categorical variables of interest. A separate intercept was also fit for each participant to account for repeated measures. Each statistic map was first thresholded using a cluster defining threshold of P = .005, and the surviving clusters were then cluster corrected using a familywise error of P = .05 with a cluster extent threshold of 348 voxels.
Perfusion images were generated from 3D pCASL data by pairwise subtraction between the control and label pairs, after head motion correction. Quantitative CBF maps were calculated based on a standard one compartment model, 36 assuming a labeling efficiency of 0.77 by taking into account the loss of efficiency by 2 background suppression pulses, 33 and blood T1 of 1650 ms at 3 T. 37 The structural 3D MPRAGE scan was segmented into white matter and gray matter masks (including cortical and subcortical segmentations) using SPM8 (https://www.fil.ion.ucl.ac.uk/spm/). Mean CBF was extracted from these masks, thresholded for probabilities above 80% for each subject. 38 Using the same 3 time point model described above for fALFF, we evaluated changes in CBF across the whole brain. No cluster corrected significant regions were identified.
Region of interest data processing
Regions of interest (ROIs) were defined to further examine effect sizes and correlations. The significant whole-brain derived voxelwise clusters identified for the changes in fALFF were used as ROIs. Additional ROIs were pre-defined based on salient biomarkers of neurodegenerative diseases 39 and previous evidence of RT-induced brain morphological changes.9,29,40,41 Specific ROIs for CBF analyses included the hippocampus, anterior cingulate, posterior cingulate, putamen, insula, frontal lobe, occipital lobe, parietal lobe, temporal lobe, and caudate nucleus. These were extracted from the quantitative CBF maps described above.
Our VC measures provided additional global ROIs for CV function. Arterial CBV maps were generated from dynamic bSSFP ASL sequences after image outlier rejection. 42 Both within-scan and inter-scan outlier rejections were performed instead of motion correction to preserve the original signal intensity. Perfusion-weighted image series were first generated by pair-wise subtraction of control and label acquisitions at each inversion time (TI), and the time course of perfusion-weighted signals of the entire slice were extracted at each TI for each measurement. The mean perfusion-weighted signal and the standard deviation were calculated at each TI across the measurements. If the time course of the perfusion weighted signal of all the time points (TIs) in one measurement was beyond one standard deviation from the mean, the ASL data at all time points of that measurement were excluded. The calculation of CBV involves the ratio of the sum concentrations of labeled spins between the artery and each pixel, as a function of T1 relaxation time of arterial blood. 42 Two regions of interest were defined: large arteries with CBV > 5% total slice volume and small arteries/arterioles with CBV of 1.5% to 5% of the total slice volume. VC was then calculated as the difference between relative CBV (% of total volume) at systole and relative CBV at diastole divided by the difference between systolic and diastolic blood pressure. 25
WMLs were extracted from FLAIR images using the lesion prediction algorithm 43 as implemented in the Lesion Segmentation Toolbox (V3.0.0, https://www.applied-statistics.de/lst.html) for SPM12. This algorithm consists of a binary classifier in the form of a logistic regression model trained on data from patients with multiple sclerosis. A lesion belief map is used as covariates for the model, as well as spatial covariates that account for voxel specific changes in lesion probability. Each participant’s corresponding MPRAGE image was added as a reference for coregistration before lesion segmentation. Parameters of the model provide an estimate for the lesion probability of each voxel in the image. The main output of this segmentation process was total WML volume across the whole brain.
Statistical analyses for ROIs
To confirm the reliability of our measures, the control period—differences between baseline and pre-intervention—was evaluated using 2-way mixed effects intraclass correlation coefficients (ICCs) with absolute agreement. Estimates less than 0.50, between 0.50 and 0.75, between 0.75 and 0.90, and greater than 0.90 were classified as poor, moderate, good, and excellent test-retest reliability, respectively. 44
Effect sizes were calculated for all outcomes to determine the magnitude of differences between control (baseline to pre-intervention) and intervention (pre-intervention to post-intervention) periods. For normally distributed data, an adapted Cohen’s d effect size was calculated—mean changes from pre- to post-intervention were subtracted by the mean changes from baseline to pre-intervention, then divided by the average standard deviation of those changes.45,46 Effect sizes of 0.2, 0.5, 0.8, and 1.2 were classified as small, medium, large, and very large, respectively. 47
Relationships between changes in outcome variables were analyzed to explore agreement and potential mechanisms of benefit. Only raw changes from pre- to post-intervention were used so that effects were not washed out by percent changes. These raw changes often have high kurtosis when a small sample size (N = 20) is used. Therefore, Spearman rank correlation coefficients were chosen to reduce effect variability. 48 Spearman’s rho values of 0.1, 0.3, and greater than 0.5 were classified as small, medium, and large associations, 45 respectively.
Results
Cognition
Cognitive outcomes, including each individual measure, have been described in detail previously. 2 Briefly, fluid cognition composite scores had good reliability (ICC = 0.77) from baseline to pre-intervention, with very minimal practice effects (+0.5 ± 5.7 Standard Units), and significantly increased from pre- to post-intervention (+7.7 ± 5.5 Standard Units, paired t-test P < .01, d = 1.27). Note that, as expected, there were no changes in crystallized cognition composite scores. 2 Only fluid cognition composite scores were used for correlations with the following brain MRI measures.
fALFF
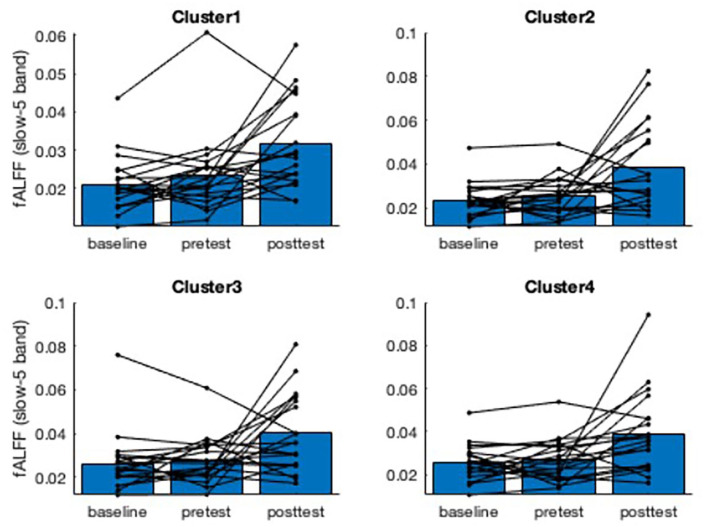
The whole brain analysis yielded 4 distinct clusters where fALFF increased following the RT intervention [t-statistic = 2.7-5.0]. The clusters are shown in Figure 1 along with spaghetti plots for each individual participant’s fALFF values. Group level statistics are presented in Table 2. The fALFF values had moderate reliability from baseline to pre-intervention (ICC range: 0.58 to 0.75). Pre- to post-intervention changes ranged from +33% (Cluster 1) to +50% (Cluster 2), which were all considered medium effect sizes compared to the control period. These increases in fALFF slow-5 band are interpreted as an overall decrease in resting brain activity.
Figure 1.
Spaghetti plots (N = 19) of fALFF values within 4 significant voxelwise clusters, shown in axial brain slices. Cluster 1 is in the Cerebellum, Cluster 2 is in the Right Middle Temporal Gyrus, Cluster 3 is in the Left Inferior Parietal Lobule, and Cluster 4 is in the Right Inferior Parietal Lobule. The group mean fALFF values are shown in blue for each time point. The results support the voxelwise analysis indicating an increase in fALFF after the RT intervention, but not during the initial control period. Increases in fALFF suggest reduced resting brain activity in that region of interest.
Table 2.
Group means for fALFF values at baseline, pre-intervention, and post-intervention (N = 19).
| Measure | ICC^ (3,1) | Baseline | Pre-Intervention | Post-Intervention | ES^^ (d) |
|---|---|---|---|---|---|
| fALFF Cluster 1 | 0.70 | 0.021 ± 0.007 | 0.024 ± 0.009 | 0.032 ± 0.011 | 0.60 |
| fALFF Cluster 2 | 0.58 | 0.023 ± 0.007 | 0.026 ± 0.008 | 0.039 ± 0.019 | 0.74 |
| fALFF Cluster 3 | 0.75 | 0.026 ± 0.013 | 0.027 ± 0.010 | 0.040 ± 0.017 | 0.76 |
| fALFF Cluster 4 | 0.60 | 0.025 ± 0.008 | 0.027 ± 0.009 | 0.039 ± 0.018 | 0.79 |
No changes were expected over the 12-week control period. Thus, test-retest reliability was calculated with baseline and pre-intervention values. The effects of the 12-week periodized RT intervention are evident via changes from pre- to post-intervention and calculated effect sizes. Mean ± SD.
Two-way mixed intraclass correlation coefficient (ICC) with absolute agreement calculated using baseline and pre-intervention data.
Adapted Cohen’s d effect size calculated by subtracting the mean changes from pre- to post-intervention by the mean changes from baseline to pre-intervention and dividing by the average standard deviation of those changes.
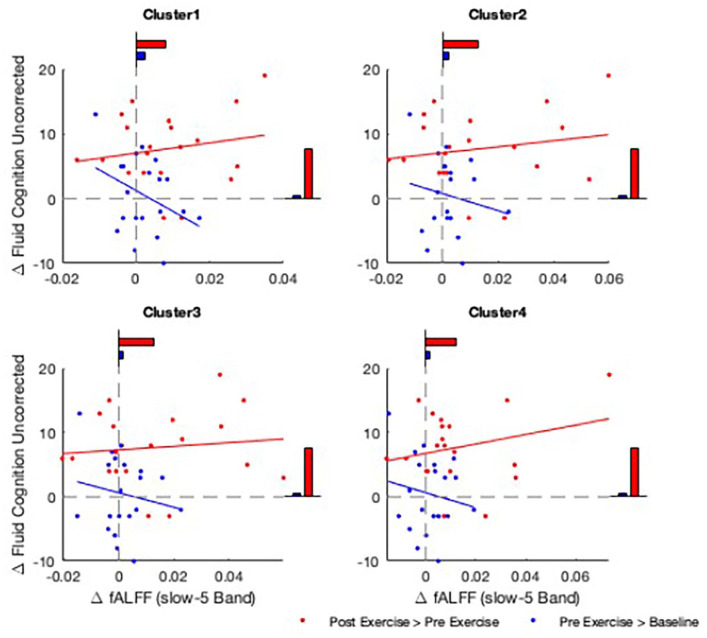
Also, within these 4 clusters, there appeared to be a relationship between changes in fALFF and changes in fluid cognition. Figure 2 plots these relationships, comparing the control and intervention periods. Note the distinct regression slopes between the control and intervention periods. Spearman’s rho for the pre- to post-intervention changes ranged from +0.18 to +0.26. This suggests that reduced resting brain activity was positively correlated with improved fluid cognition.
Figure 2.
Relationship between changes in fluid cognition and changes in resting brain activity measures in fALFF slow-5 band. Clusters with significantly higher fALFF at rest after intervention are shown (Clusters 1-4), with the change in fALFF (x-axis) and change in fluid cognition (y-axis) shown for each participant. Change during the control period (blue) and intervention period (red) are shown. Average change in fluid cognition and fALFF are shown as bar plots, respectively.
Cerebral blood flow
When CBF values were evaluated via whole brain analysis, no cluster corrected significant regions were identified. To explore agreement with fALFF results, we assessed CBF in the 4 significant clusters identified in the fALFF voxelwise analysis. However, there were no significant pre- to post-intervention changes in CBF within these 4 clusters.
CBF values in pre-defined ROIs are presented in Table 3. Reliability over the control period was moderate for global CBF measures and most regional CBF measures (hippocampus, anterior cingulate, posterior cingulate, putamen, amygdala, insula, occipital lobe, and temporal lobe), though a few regions (caudate nucleus, frontal lobe, and parietal lobe) had poor reliability. The changes in global CBF were mixed. Whole brain CBF slightly increased from pre- to post-intervention (+1.9 ± 17.9%), with a small increase in gray matter CBF (+3.4 ± 19.8%) but not white matter CBF (−0.2 ± 16.7%) after the intervention. Because effect sizes were calculated comparing the intervention period to the control period, and CBF slightly decreased from baseline to pre-intervention (eg, whole brain: −1.4 ± 14.0%), the estimates generated show even more pronounced positive effects (Table 3). Small to medium positive effect sizes were observed in the hippocampus, anterior cingulate, posterior cingulate, putamen, insula, occipital lobe, and temporal lobe, primarily due to an attenuation or reversal of declines over the control period. For example, hippocampal CBF increased an average + 1.9 ± 22.9% from pre- to post-intervention, but that contrasts the average 5.8 ± 14.1% decline from baseline to pre-intervention. Despite the variability of these changes the effect size is promising (adapted d = 0.40). Other regions had similar variability in changes from pre- to post-intervention; for example, anterior cingulate CBF (+3.3 ± 17.6%), posterior cingulate CBF (+5.6 ± 15.1%), and putamen CBF (+6.6 ± 20.7%). The remaining regions examined (the frontal lobe, caudate nucleus, and parietal lobe) had negligible effect sizes due to similar changes over the control and intervention periods. Frontal lobe CBF and parietal lobe CBF continued slight increases over control to intervention periods, and caudate nucleus CBF continued slight declines over control to intervention periods.
Table 3.
Group means for cerebral blood flow measures at baseline, pre-intervention, and post-intervention (N = 19).
| Measure | Baseline | Pre-intervention | Post-intervention | ICC^ (3,1) | Effect size^^ (d) |
|---|---|---|---|---|---|
| Global cerebral blood flow | |||||
| Whole Brain CBF | 37.3 ± 6.6 | 36.6 ± 7.4 | 36.7 ± 7.6 | 0.69 | 0.12 |
| Gray Matter CBF | 40.1 ± 7.6 | 38.8 ± 8.5 | 39.5 ± 8.7 | 0.61 | 0.22 |
| White Matter CBF | 34.1 ± 5.7 | 34.1 ± 6.3 | 33.8 ± 6.3 | 0.73 | −0.04 |
| Regional cerebral blood flow | |||||
| Hippocampal CBF | 37.4 ± 7.7 | 35.1 ± 8.0 | 35.3 ± 9.9 | 0.75 | 0.40 |
| Anterior Cingulate CBF | 44.1 ± 8.3 | 41.9 ± 9.1 | 42.7 ± 9.3 | 0.61 | 0.38 |
| Posterior Cingulate CBF | 43.8 ± 9.9 | 40.7 ± 9.6 | 42.2 ± 8.3 | 0.57 | 0.61 |
| Putamen CBF | 41.6 ± 7.2 | 39.2 ± 9.8 | 41.3 ± 11.1 | 0.58 | 0.56 |
| Caudate Nucleus CBF | 35.7 ± 7.9 | 34.7 ± 8.6 | 34.1 ± 8.5 | 0.35 | 0.03 |
| Insula CBF | 45.8 ± 7.2 | 42.3 ± 8.8 | 43.6 ± 10.3 | 0.51 | 0.69 |
| Frontal Lobe CBF | 37.2 ± 6.7 | 37.6 ± 7.3 | 38.3 ± 7.9 | 0.36 | 0.03 |
| Occipital Lobe CBF | 39.1 ± 11.1 | 36.4 ± 13.9 | 38.9 ± 13.2 | 0.54 | 0.45 |
| Parietal Lobe CBF | 35.9 ± 9.3 | 36.8 ± 10.6 | 38.2 ± 10.6 | 0.30 | 0.05 |
| Temporal Lobe CBF | 40.5 ± 7.4 | 37.7 ± 9.6 | 39.1 ± 9.6 | 0.51 | 0.50 |
Abbreviation: CBF, cerebral blood flow, (ml/100 g/min).
No changes were expected over the 12-week control period. Thus, test-retest reliability was calculated with baseline and pre-intervention values. The effects of the 12-week periodized RT intervention are evident via changes from pre- to post-intervention and calculated effect sizes. Mean ± SD.
Two-way mixed intraclass correlation coefficient (ICC) with absolute agreement calculated using baseline and pre-intervention data.
Adapted Cohen’s d effect size calculated by subtracting the mean changes from pre- to post-intervention by the mean changes from baseline to pre-intervention and dividing by the average standard deviation of those changes.
Unexpectedly, pre- to post-intervention changes in CBF measures had negative (or zero) correlations with changes in fluid cognition. For example, spearman’s rho for CBF in the whole brain, gray matter, hippocampus, anterior cingulate, posterior cingulate, and putamen were −0.27, −0.17, −0.26, −0.06, −0.04, and −0.17, respectively. This suggests that participants with the greatest increases in CBF tended to have the smallest increases in fluid cognition.
Vascular compliance
Intracranial VC data from 3 participants were removed because 2 had offset imaging planes and one had excess noise, likely due to head movement during the MRI (N = 17). Data from the remaining 17 participants were not normally distributed (Shapiro-Wilk P < .05). In addition, caution should be taken with interpretations because of the very poor measurement reliability from baseline to pre-intervention (large artery VC ICC = 0.46, small artery VC ICC = 0.10). Large and small artery VC did not change from pre- to post-intervention (median = −1.6 %, interquartile range [IQR] = 175.6%; and median = −3.0 %,IQR = 166.7%, respectively). Matched-pairs rank-biserial effect sizes were negligible (−0.8 and 0.08, respectively) because similar decreases were observed from baseline to pre-intervention.
White matter lesion volume
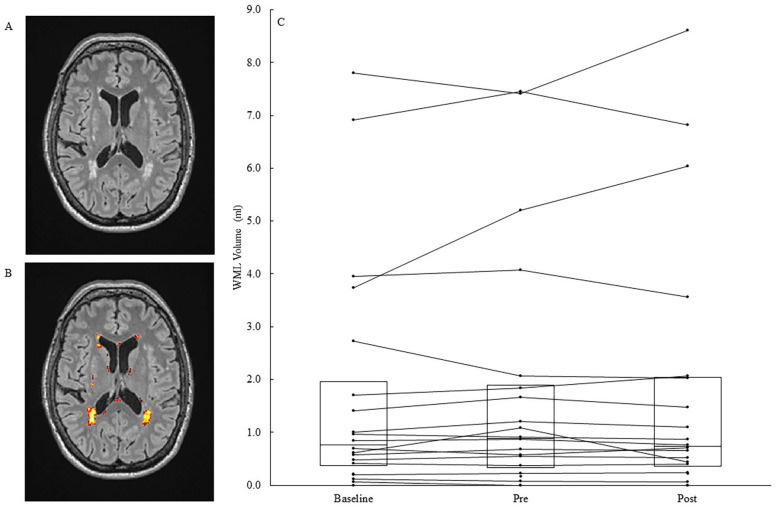
Whole brain WML volume (N = 20) had excellent reliability from baseline to pre-intervention (ICC = 0.98), however the data were not normally distributed (Shapiro-Wilk P < .05). Median WML volume slightly decreased from pre- to post-intervention (median = −3.7 %,IQR = 22.4%). The promising effect size (rank-biserial = −0.22) indicated a reversal of the WML volume progression from baseline to pre-intervention (median = +5.3%,IQR = 26.3%). An example of WML volume segmentation and individual participant data are shown in Figure 3. Unexpectedly, pre- to post- intervention changes in WML volume had a small positive correlation with changes in fluid cognition (spearman’s rho = .44).
Figure 3.
(A) Transverse slice of a co-registered T2-weighted FLAIR (Fluid-attenuated inversion recovery) image. (B) White matter lesions (WML) in the same slice are segmented via the lesion prediction algorithm and shown in yellow, orange, and red. (C) Whole brain WML volume in 20 participants at baseline, pre-intervention, and post-intervention. The boxes show Q1, median, and Q3, respectively, and the marked lines show individual participant data.
Discussion
In this mechanistic proof-of-concept clinical trial, we report exploratory findings of RT’s effects on the brain, assessed via multimodal 3T MRI. Along with significant improvements in fluid cognition, we provide the first estimates of changes in resting-state brain activity and CV function. Specifically, we found medium decreases in resting fALLF, small to medium improvements in global and regional resting CBF, and small countermeasure effects on whole brain WML volume progression. These benefits were notably observed in putative markers of dementia despite normal age-related declines over the initial control period. Thus, RT may attenuate or even reverse salient neurodegenerative processes.
We found novel increases in fALFF slow-5 band within 4 distinct clusters after the intervention, which suggests that RT decreases regional resting brain activity. 10 In clinical settings, this fALFF measure has shown to be sensitive to cognitive impairment. For example, patients with amnestic mild cognitive impairment had widespread decreases in fALFF slow-5 band in several brain regions,49,50 including parietal regions consistent with 2 of our clusters. In addition, gradual decreases in fALFF have been found in several regions along the Alzheimer’s disease continuum when comparing normal aging, subjective cognitive decline, mild cognitive impairment, and Alzheimer’s disease dementia. 51 This includes bilateral cerebellum regions consistent with one of our clusters. It is important to note that the previous studies also identified regions where increased fALFF was associated with cognitive impairment; however, these were mainly in limbic and midbrain regions where no significant clusters were found in the current study. Finally, the clinical decreases in fALFF were associated with decreased fluid cognitive functions. 51 We observed the opposite after the RT intervention—increases in fALFF showed small positive correlations with improvements in fluid cognition, the clinical target for the intervention. Although the relationship between spontaneous functional activity and cognitive impairment remains unclear, our findings suggest that improved fALFF may partially mediate RT-induced cognitive enhancement. Future studies are warranted to test this hypothesis.
A positive effect of RT on CBF would be a clinically significant benefit. This measure is strongly supported in the literature, as declines increase the risk of Alzheimer’s disease and vascular dementia.14,52 We hypothesized that RT could improve resting CBF, as outlined previously. 20 Small positive effects were observed in global CBF, a good marker for aging and neurodegenerative diseases, 52 but regional quantifications are likely more sensitive for understanding the effects on cognition. Resting CBF in regions like the hippocampus, precuneus, superior temporal lobe, and orbitofrontal regions have shown associations with aging and cognition. 23 Accordingly, regional CBF increases after aerobic training were found primarily in the hippocampus and anterior cingulate, associated with improvements in memory16,17 and executive function. 53 Similarly, we observed positive CBF effects after RT in regions involved in neurodegenerative processes. 54 These included the posterior cingulate, putamen, insula, temporal lobe, hippocampal, anterior cingulate, and occipital lobe (Table 3). The positive effect sizes typically reflected a small increase in regional CBF from pre- to post-intervention that contrasted a moderate decrease over the initial control period. We interpret this as an attenuation/reversal effect of RT against normal age-related declines. These data provide the first indication for potential dynamic CV plasticity after RT.
Interestingly, there were no associations between changes in fALFF and changes in CBF despite the indirect physiological relationship between these measures. The fALFF approach reflects the regional intensity of spontaneous fluctuations in BOLD signal, 10 and BOLD contrast depends not only on blood oxygenation but also on CBF and volume. However, this is a complex response controlled by several parameters, 55 which may explain the lack of association. In addition, fALFF is calculated as a relative measure of activity across the whole brain, whereas CBF is an absolute measure of blood flow in units of ml/100 g/min. Finally, both measures, particularly CBF, can be confounded by physiological noise. 56 While these measures have been used together as potential markers for cognitive impairment, 57 the mechanistic relationship between fALFF and CBF or oxygen metabolism still needs further evaluation in future studies.
It is important to note that the changes in resting CBF all had negative (or zero) correlations with changes in fluid cognition. Although this was unexpected, it may suggest that resting CBF benefits do not directly contribute to cognitive improvements. Instead, RT-induced cognitive enhancement may be mediated by other factors, such as the motor learning or skill acquisition aspects of RT. 58 Since CV plasticity contributes to aerobic training induced cognitive enhancement,16,17 this may be a distinct mechanism between exercise modalities. Regardless of function, improvements in resting CBF as a result of RT would likely promote overall brain health during late aging.
Beneficial changes were also observed in WML volume, a marker of small vessel disease with high prevalences in older adults and strong implications in the pathogenesis of vascular cognitive impairment and dementia. 59 While WML volume is expected to steadily increase with normal aging, we only observed a median increase after the 12-week control period and not after the 12-week intervention, which showed a median decrease. These promising results support previous findings and suggest that RT has a countermeasure effect on WML volume progression.9,28 However, the mechanisms of this protective effect and relevance for cognition are currently unclear. 27 The underlying physiology of the imaging marker would suggest a reversal of interstitial fluid accumulations that are represented by hyperintensities, thus halting long-term demyelination and axonal damage. 60
An unexpected association was found between WML volume and changes in fluid cognition. The positive correlation indicates that greater improvements in fluid cognition occurred with smaller benefits to WML volume. Again, this may suggest that CV adaptations occur independently from cognitive enhancement after RT intervention. However, WML volume is commonly associated with impaired mobility and falls, 61 both known to be impacted by RT, and a previous RT study reported associations between attenuated WML volume progression and maintained gait speed. 28 Therefore, benefits to WML volume may still contribute to other widespread benefits of RT and to overall brain health during late aging.
Less convincing are the changes in VC, the results of which were mixed and unreliable. First, most VC measures suggested that VC was unchanged after the intervention. Since VC is necessary to buffer pulsatile pressures for continuous blood flow to the brain, 62 a decrease would mean that RT leads to arterial stiffening, thus causing increased pulsatility and resistance. 24 However, we find that unlikely given the positive changes in CBF and WML volume. Could this be an adaptation to or a continuation of the high pulsatile pressures that occur during RT? 63 We also find this unlikely because a minimum of 48 hours buffer separated the last training session and the first testing session. The high pulsatility during exercise might even be beneficial, driving neurotrophic factors into the brain for neuroplasticity. 64 Why then did we find no changes in VC? Perhaps RT was unable to attenuate the normal decreases in VC with aging. 65 Alternatively, our VC measures may not be reliable enough for repeated testing since some IQRs were large and reliability was poor. This could be due to MRI flow acquisition parameters, the assumptions in defining arteries by CBV (such as the state of smooth muscles in smaller arteries), or the use of brachial blood pressure to estimate pressure in cerebral arteries. 25 To our knowledge, this is the first intervention study to use this VC technique as a repeated measure. Since heart rate can affect CBF pulsatility, 66 participants’ nervousness in the MRI may have also confounded the results, but there were no changes in resting heart rate during the MRI.
Limitations
One limitation of this study was the use of a single group. Because this was a proof-of-concept study for potential mechanisms, the design was determined based on feasibility and study funding. Having a single group allowed us to address practicality while maintaining a thorough design. A randomized crossover was opposed so that results were not confounded by detraining effects or long-term cognitive benefits of RT. 29 A separate active control group is needed in future studies to accurately estimate the effects of training and establish causal relationships. In addition, larger sample sizes and longer training durations would increase the likelihood of observing positive effects versus controls, 6 if they exist.
Another limitation was the high variability of our data. This is normal for functional and dynamic scans because they are more affected by movement, natural physiological variability, and potential modifiers such as nervousness 67 than structural scans such as T-2 weighted FLAIR used for WML volume (which had excellent reliability over the control period). Most of the dynamic ASL measures had only moderate reliability over the control period, so their results should be interpreted with some caution. Changing the sizes of ROIs may decrease the variability, but we chose to focus on standard segmentations for this pilot study. Caution is especially warranted for VC data, which had poor reliability. The bSSFP ASL scans were only performed using single 2D slices. To improve the accuracy of measurements, multiple repetitions needed to be acquired, which add up to a relatively long scan time. This raises the importance of motion control with image outlier rejection, especially since older adults tend to move more during the scan. Three dimensional acquisitions are being developed for future studies to improve imaging efficiency and motion correction in volumetric measurements.
A final limitation was the ROI-based analyses. Since our small sample size may have caused us to miss significant clusters throughout the brain, our approach provided a more focused examination of RT’s effects in the context of aging. In addition, we chose standard segmentation approaches to help generalize findings. For example, 0.6 and 0.8 thresholds from the tissue probability maps were used for gray matter and white matter segmentation, according to a previous study, 38 which could induce some partial volume effects when calculating mean CBF, especially in gray matter. However, using higher thresholds or partial volume corrections likely would not affect the overall results. Future studies will need to characterize the multidimensional nature of changes.39,68 Furthermore, because our findings are exploratory, they do not conclusively prove that RT causes structural or functional plasticity or that these changes are drivers of cognitive enhancement. Instead, the findings from this clinical trial provide important insight into the efficacy of RT on improving fluid cognition and a greater understanding of the underlying mechanisms. Larger studies are needed to confirm these findings and re-test associations with fluid cognition. 69 A major benefit of conducting these small proof-of-concept clinical trials is generating these preliminary signals of efficacy that can redirect investigators toward the most important research questions and the most promising study designs.
Conclusion
RT may provide benefits for healthy brain aging. Larger randomized controlled trials are needed to confirm the novel supportive evidence for positive RT-induced brain plasticity through improvements in resting brain activity and CV function. These were observed in established measures, with the clinical relevancy of regional improvements (ie, the hippocampus and posterior cingulate) supported by previous evidence. Since the effect sizes seemed to reflect an attenuation or reversal of normal age-related declines over the initial control period, future studies should similarly examine training-induced changes in the context of aging. From a practitioner’s standpoint, developing strategies to improve fluid cognition and overall brain health may preserve independent functioning and quality of life in older adults. In addition, establishing the efficacy of different types of exercise interventions will expand the exercise prescription options for older adults.
Acknowledgments
We thank our participants and student volunteers in the Clinical Exercise Research Center for their hard work and dedication throughout the study.
Footnotes
Author Contributions: Concept/Design: TRM, LY, DD, JP, JJK, CL, ETS. Data acquisition: TRM, LY, DD, JP, ETS. Data processing/analysis: TRM, AH, LY, MLR, DD, JP, JJK, CL. Interpretation of results: TRM, AH, LY, DD, JP, JJK, MLR, CL, ETS. Writing: TRM, AH.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Southern California Clinical and Translational Science Institute (SC-CTSI, https://sc-ctsi.org/, grant number UL1TR001855 to ETS and TRM).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Significance Statement: Resistance training appears to decrease resting regional brain activity and may also improve markers of cerebrovascular function.
Understanding these neurophysiological processes that underlie exercise-induced cognitive enhancement will help us develop therapies for the healthy cognitive aging.
ORCID iDs: Timothy R Macaulay  https://orcid.org/0000-0003-4961-2035
https://orcid.org/0000-0003-4961-2035
Amy Hegarty  https://orcid.org/0000-0002-1491-6760
https://orcid.org/0000-0002-1491-6760
References
- 1. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Macaulay TR, Pa J, Kutch JJ, et al. 12 weeks of strength training improves fluid cognition in older adults: a nonrandomized pilot trial. PLoS One. 2021;16:e0255018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Landrigan J-F, Bell T, Crowe M, Clay OJ, Mirman D. Lifting cognition: a meta-analysis of effects of resistance exercise on cognition. Psychol Res. 2020;84:1167-1183. [DOI] [PubMed] [Google Scholar]
- 4. Haeger A, Costa AS, Schulz JB, Reetz K. Cerebral changes improved by physical activity during cognitive decline: a systematic review on MRI studies. NeuroImage Clin. 2019;23:101933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barha CK, Galea LA, Nagamatsu LS, Erickson KI, Liu-Ambrose T. Personalising exercise recommendations for brain health: considerations and future directions. Br J Sports Med. 2017;51:636-639. [DOI] [PubMed] [Google Scholar]
- 6. Herold F, Törpel A, Schega L, Müller NG. Functional and/or structural brain changes in response to resistance exercises and resistance training lead to cognitive improvements - a systematic review. Eur Rev Aging Phys Activity. 2019;16:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu-Ambrose T, Nagamatsu LS, Voss MW, Khan KM, Handy TC. Resistance training and functional plasticity of the aging brain: a 12-month randomized controlled trial. Neurobiol Aging. 2012;33:1690-1698. [DOI] [PubMed] [Google Scholar]
- 8. Nagamatsu LS, Handy TC, Hsu CL, Voss M, Liu-Ambrose T. Resistance training promotes cognitive and functional brain plasticity in seniors with probable mild cognitive impairment. Arch Intern Med. 2012;172:666-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suo C, Singh MF, Gates N, et al. Therapeutically relevant structural and functional mechanisms triggered by physical and cognitive exercise. Mol Psychiatry. 2016;21:1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zou Q-H, Zhu C-Z, Yang Y, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods. 2008;172:137-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yu R, Chien YL, Wang HL, et al. Frequency-specific alternations in the amplitude of low-frequency fluctuations in schizophrenia. Hum Brain Mapp. 2014;35:627-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Corriveau RA, Bosetti F, Emr M, et al. The science of vascular contributions to cognitive impairment and dementia (VCID): a framework for advancing research priorities in the cerebrovascular biology of cognitive decline. Cell Mol Neurobiol. 2016;36:281-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672-2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wolters FJ, Zonneveld HI, Hofman A, et al. Cerebral perfusion and the risk of dementia: a population-based study. Circulation. 2017;136:719-728. [DOI] [PubMed] [Google Scholar]
- 15. Pereira AC, Huddleston DE, Brickman AM, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci. 2007;104:5638-5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maass A, Düzel S, Goerke M, et al. Vascular hippocampal plasticity after aerobic exercise in older adults. Mol Psychiatry. 2015;20:585-593. [DOI] [PubMed] [Google Scholar]
- 17. Chapman SB, Aslan S, Spence JS, et al. Shorter term aerobic exercise improves brain, cognition, and cardiovascular fitness in aging. Front Aging Neurosci. 2013;5:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Williams MA, Haskell WL, Ades PA, et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, physical activity, and metabolism. Circulation. 2007;116:572-584. [DOI] [PubMed] [Google Scholar]
- 19. Ashor AW, Lara J, Siervo M, et al. Exercise modalities and endothelial function: a systematic review and dose-response meta-analysis of randomized controlled trials. Sports Med. 2015;45:279-296. [DOI] [PubMed] [Google Scholar]
- 20. Macaulay TR, Fisher BE, Schroeder ET. Potential indirect mechanisms of cognitive enhancement after long-term resistance training in older adults. Phys Ther. 2020;100:907-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu X, Jerskey BA, Cote DM, et al. Cerebrovascular perfusion among older adults is moderated by strength training and gender. Neurosci Lett. 2014;560:26-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Detre JA, Wang J, Wang Z, Rao H. Arterial spin-labeled perfusion MRI in basic and clinical neuroscience. Curr Opin Neurol. 2009;22:348-355. [DOI] [PubMed] [Google Scholar]
- 23. Zhang N, Gordon ML, Goldberg TE. Cerebral blood flow measured by arterial spin labeling MRI at resting state in normal aging and Alzheimer’s disease. Neurosci Biobehav Rev. 2017;72:168-175. [DOI] [PubMed] [Google Scholar]
- 24. Beishon L, Haunton VJ, Panerai RB, Robinson TG. Cerebral hemodynamics in mild cognitive impairment: a systematic review. J Alzheimers Dis. 2017;59:369-385. [DOI] [PubMed] [Google Scholar]
- 25. Yan L, Liu CY, Smith RX, et al. Assessing intracranial vascular compliance using dynamic arterial spin labeling. Neuroimage. 2016;124:433-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dichgans M, Leys D. Vascular cognitive impairment. Circ Res. 2017;120:573-591. [DOI] [PubMed] [Google Scholar]
- 27. Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol. 2015;11:157-165. [DOI] [PubMed] [Google Scholar]
- 28. Bolandzadeh N, Tam R, Handy TC, et al. Resistance training and white matter lesion progression in older women: Exploratory analysis of a 12-Month randomized controlled trial.. J Am Geriatr Soc. 2015;63:2052-2060. [DOI] [PubMed] [Google Scholar]
- 29. Best JR, Chiu BK, Liang Hsu C, Nagamatsu LS, Liu-Ambrose T. Long-term effects of resistance exercise training on cognition and brain volume in older women: results from a randomized controlled trial. J Int Neuropsychol Soc. 2015;21:745-756. [DOI] [PubMed] [Google Scholar]
- 30. Mann JB, Thyfault JP, Ivey PA, Sayers SP. The effect of autoregulatory progressive resistance exercise vs. Linear periodization on strength improvement in college athletes. J Strength Cond Res. 2010;24:1718-1723. [DOI] [PubMed] [Google Scholar]
- 31. Weintraub S, Dikmen SS, Heaton RK, et al. The cognition battery of the NIH toolbox for assessment of neurological and behavioral function: Validation in an adult sample. J Int Neuropsychol Soc. 2014;20:567-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heaton RK, Akshoomoff N, Tulsky D, et al. Reliability and validity of composite scores from the NIH Toolbox Cognition Battery in adults. J Int Neuropsychol Soc. 2014;20:588-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kilroy E, Apostolova L, Liu C, Yan L, Ringman J, Wang DJ. Reliability of two-dimensional and three-dimensional pseudo-continuous arterial spin labeling perfusion MRI in elderly populations: comparison with 15O-water positron emission tomography. J Magn Reson Imaging. 2014;39:931-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Esteban O, Markiewicz CJ, Blair RW, et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods. 2019;16:111-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gorgolewski K, Burns CD, Madison C, et al. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front Neuroinformatics. 2011;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang J, Zhang Y, Wolf RL, Roc AC, Alsop DC, Detre JA. Amplitude-modulated continuous arterial spin-labeling 3.0-T perfusion MR imaging with a single coil: feasibility study. Radiology. 2005;235:218-228. [DOI] [PubMed] [Google Scholar]
- 37. Lu H, Clingman C, Golay X, van Zijl PCM. Determining the longitudinal relaxation time (T1) of blood at 3.0 Tesla. Magn Reson Med. 2004;52:679-682. [DOI] [PubMed] [Google Scholar]
- 38. Jann K, Shao X, Ma SJ, et al. Evaluation of cerebral blood flow measured by 3D PCASL as biomarker of vascular cognitive impairment and dementia (VCID) in a cohort of elderly latinx subjects at risk of small vessel disease. Front Neurosci. 2021;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Frisoni GB, Fox NC, Jack CR, Jr, Scheltens P, Thompson PM. The clinical use of structural MRI in Alzheimer disease. Nat Rev Neurol. 2010;6:67-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu-Ambrose T, Nagamatsu LS, Graf P, Beattie BL, Ashe MC, Handy TC. Resistance training and executive functions: a 12-month randomized controlled trial. Arch Intern Med. 2010;170:170-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Palmer HS, Håberg AK, Fimland MS, et al. Structural brain changes after 4 wk of unilateral strength training of the lower limb. J Appl Physiol. 2013;115:167-175. [DOI] [PubMed] [Google Scholar]
- 42. Yan L, Li C, Kilroy E, Wehrli FW, Wang DJJ. Quantification of arterial cerebral blood volume using multiphase-balanced SSFP-based ASL. Magn Reson Med. 2012;68:130-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schmidt P. Bayesian Inference for Structured Additive Regression Models for Large-Scale Problems With Applications to Medical Imaging. LMU; 2017. [Google Scholar]
- 44. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cohen J. Statistical power analysis. Curr Dir Psychol Sci. 1992;1:98-101. [Google Scholar]
- 46. Morris SB. Estimating effect sizes from pretest-posttest-control group designs. Organ Res Methods. 2008;11:364-386. [Google Scholar]
- 47. Sawilowsky SS. New effect size rules of thumb. J Mod Appl Stat Methods. 2009;8:597-599. [Google Scholar]
- 48. de Winter JC, Gosling SD, Potter J. Comparing the Pearson and Spearman correlation coefficients across distributions and sample sizes: A tutorial using simulations and empirical data. Psychol Methods. 2016;21:273-290. [DOI] [PubMed] [Google Scholar]
- 49. Han Y, Wang J, Zhao Z, et al. Frequency-dependent changes in the amplitude of low-frequency fluctuations in amnestic mild cognitive impairment: a resting-state fMRI study. Neuroimage. 2011;55:287-295. [DOI] [PubMed] [Google Scholar]
- 50. Han Y, Lui S, Kuang W, Lang Q, Zou L, Jia J. Anatomical and functional deficits in patients with amnestic mild cognitive impairment. PLoS One. 2012;7:e28664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang L, Yan Y, Wang Y, et al. Gradual disturbances of the amplitude of low-frequency fluctuations (ALFF) and fractional ALFF in Alzheimer spectrum. Front Neurosci. 2018;12:975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hays CC, Zlatar ZZ, Wierenga CE. The utility of cerebral blood flow as a biomarker of preclinical Alzheimer’s disease. Cell Mol Neurobiol. 2016;36:167-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kleinloog JPD, Mensink RP, Ivanov D, Adam JJ, Uludağ K, Joris PJ. Aerobic exercise training improves cerebral blood flow and executive function: A randomized, controlled cross-over trial in sedentary older men. Front Aging Neurosci. 2019;11:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lehmann M, Crutch SJ, Ridgway GR, et al. Cortical thickness and voxel-based morphometry in posterior cortical atrophy and typical Alzheimer’s disease. Neurobiol Aging. 2011;32:1466-1476. [DOI] [PubMed] [Google Scholar]
- 55. Logothetis NK, Pfeuffer J. On the nature of the BOLD fMRI contrast mechanism. Magn Reson Imaging. 2004;22:1517-1531. [DOI] [PubMed] [Google Scholar]
- 56. Holiga Sambataro F, Luzy C, et al. Test-retest reliability of task-based and resting-state blood oxygen level dependence and cerebral blood flow measures. PLoS One. 2018;13:e0206583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li P, Mu J, Ma X, et al. Neurovascular coupling dysfunction in end-stage renal disease patients related to cognitive impairment. J Cereb Blood Flow Metab. 2021;41:2593-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tomporowski PD, Pesce C. Exercise, sports, and performance arts benefit cognition via a common process. Psychol Bull. 2019;145:929-951. [DOI] [PubMed] [Google Scholar]
- 59. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689-701. [DOI] [PubMed] [Google Scholar]
- 60. Wardlaw JM, Valdés Hernández MC, Muñoz-Maniega S. What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J Am Heart Assoc. 2015;4:001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zheng JJ, Delbaere K, Close JC, Sachdev PS, Lord SR. Impact of white matter lesions on physical functioning and fall risk in older people: a systematic review. Stroke. 2011;42:2086-2090. [DOI] [PubMed] [Google Scholar]
- 62. Avolio A, Kim MO, Adji A, et al. Cerebral haemodynamics: effects of systemic arterial pulsatile function and hypertension. Curr Hypertens Rep. 2018;20:20. [DOI] [PubMed] [Google Scholar]
- 63. Edwards MR, Martin DH, Hughson RL. Cerebral hemodynamics and resistance exercise. Med Sci Sports Exerc. 2002;34:1207-1211. [DOI] [PubMed] [Google Scholar]
- 64. El-Sayes J, Harasym D, Turco CV, Locke MB, Nelson AJ. Exercise-induced neuroplasticity: a mechanistic model and prospects for promoting plasticity. Neuroscientist. 2019;25:65-85. [DOI] [PubMed] [Google Scholar]
- 65. Zarrinkoob L, Ambarki K, Wåhlin A, et al. Aging alters the dampening of pulsatile blood flow in cerebral arteries. J Cereb Blood Flow Metab. 2016;36:1519-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. de Riva N, Budohoski KP, Smielewski P, et al. Transcranial Doppler pulsatility index: what it is and what it isn’t. Neurocrit Care. 2012;17:58-66. [DOI] [PubMed] [Google Scholar]
- 67. Clement P, Mutsaerts H-J, Václavů L, et al. Variability of physiological brain perfusion in healthy subjects – a systematic review of modifiers. Considerations for multi-center ASL studies. J Cereb Blood Flow Metab. 2018;38:1418-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bozzali M, Serra L, Cercignani M. Quantitative MRI to understand Alzheimer’s disease pathophysiology. Curr Opin Neurol. 2016;29:437-444. [DOI] [PubMed] [Google Scholar]
- 69. Liu-Ambrose T, Dao E, Crockett RA, et al. Reshaping the path of vascular cognitive impairment with resistance training: a study protocol for a randomized controlled trial. Trials. 2021;22:217. [DOI] [PMC free article] [PubMed] [Google Scholar]