Abstract
Tribolium castaneum is one of the major pests of stored grains which causes extensive damages. To control this insect pest many synthetic chemical pesticides are used. However, continuous usage of synthetic fumigants causes pest resurgence, toxic residues, genetic resistance in pests, environmental contamination and health hazards etc., To avert these problems, essential oils are used as bio-fumigants to control the stored pests. They could act as best alternatives to synthetic fumigant in closed environment. Hence, the present study aimed to evaluate the pesticidal activity of Callistemon citrinus oil against Tribolium castaneum. GC-MS analysis of C. citrinus essential oil (EO) showed 10 compounds; among them, the major constituent was eucalyptol (1, 8-cineole) at 40.44%. The lethal concentration (LC50) values were 37.05 μL/L (adults) and 144.31 μL/L (larvae) at 24 and 48 hrs respectively. Exposure to C. citrinus EO significantly reduced the beetle fecundity, ovicidal activity, egg hatchability, larvae survival and emergence of adult. The effect of EO on enzymatic activity of T. castaneum adults was examined using Acetylcholinesterase, α-Carboxylesterase, β-Carboxylesterase, Glutathione-S-Transferase, Acid and Alkaline phosphatase assays. The results indicated that the activity of detoxification enzymes drastically varied when compared with control. This EO had toxicant effects on all stages of the life of T. castaneum.
Introduction
Insect infestation on stored grains, pulses, and their processed products is a major problem that results in significant economic losses and reduces the quality as well as the quantity of stored products. Stored grains can be infested by several insect pests that cause severe damage. Storage pests alone damaged 14–17 million tonnes of food grains and nearly 15 insect species were listed as major stored grain pests in India [1]. Among them, Tribolium castaneum Herbst 1797 (Coleoptera: Tenebrionidae) was listed in the major pest category of stored grains, it is predominantly found in tropical countries. Both larvae and adults of T. castaneum feed on grains, seeds and flour.
Fumigation is an effective method of pest management in stored grains. This method is used to control all stages of insects in stored grains and is cost effective, rapidly kills insects and leaves no residue [2]. The commonly used synthetic fumigants in stored grains are ethylene dichloride, carbon bisulphide, methyl bromide and phosphine etc. However, Methyl bromides has ozone depleting properties [3–5]; insect resistance to phosphine has been documented [6, 7]. Application of fumigants leads to increasing pest resurgence, deleterious effects on beneficial organisms, as well as raising the levels of toxicity [8]. To address these problems, biodegradable plant products have been evaluated for insecticidal properties.
Interestingly, plant leaves and oils have been used as a stored grain protectants. More recently, essential oils (EOs) derived from plants have been receiving more attention. Several studies have shown that plant essential oils are having great potential to control the pests of stored grains [9–12]. EOs have been extracted majorly from the families Myrtaceae, Lauraceae, Umbelliferae, Lamiaceae, Asteraceae and conifers [13, 14].
EO can exhibit toxic repellent, pupicidal, ovicidal, and oviposition deterrent activities against insect pests of stored grains [15–20]. Callistemon citrinus (Curtis) belongs to the family Myrtaceae, commonly known as bottle brush. The C. citrinus essential oil is highly aromatic in nature. Literature survey showed that the essential oil of C. citrinus possesses insecticidal and repellent activity against Callosobruchus maculatus, adulticidal activity against Rhyzopertha dominica and also the plant was used as antimicrobial, deodorants, perfumes and antifungal agent [21–24]. T. castaneum (Red flour beetle) is the major pest of stored products like dry fruit, broken cereals, pulses and flour. Both adult and larvae cause severe damages to the stored products which could reduce the seed viability, loss of nutrition, contamination of grains with toxic microbes and pathogens. Hence the present investigation was aimed to evaluate the toxic effect of C. citrinus EO against T. castaneum.
Materials and methods
Culture of insect
T. castaneum, was maintained in Insectarium, Department of Zoology, University of Madras The culture was maintained in wheat flour in a plastic container (35 x 20 cm) at room temperature 28±2˚ C and R.H. of 65–70%. For experiments, the flour was sieved by siever gently; adult and larvae were separated by fine brush without damaging.
Chemicals
α and β naphthol, α and β naphthyl acetate, 1-chloro-2,4-dinitrobenzene (CDNB), Glutathione Reduced (GSH), 5,5-Dithiobis(2-Nitro Benzoic Acid) extrapure (DTNB), Acetylthiocholine iodide, p-nitrophenyl phosphate, p-Nitrophenol were used for the present study and were of analytical grade. They were purchased from Sigma-Aldrich, Himedia and Sisco Research Laboratories Pvt. Ltd. (India).
Essential oil extraction
C. citrinus (Bottle brush) leaves were collected from the campus of University of Madras (13.01140° N, 80.24006° E). The plant was identified by Muniappan Ayyanar, plant taxonomist, A.V.V.M. Sri Pushpam College (Autonomous), Poondi, Thanjavur (Tamilnadu, India). The oil was extracted from the freshly-collected leaves by hydro-distillation using Clevenger Apparatus with 500 g of leaves and 5000 ml of distilled water.
GC-MS analysis
Gas chromatography–mass spectrometry (GC-MS) -2010 Plus, GCMS-TQ8040 was used to identify the composition of oil. The parameter used were: Column temperature (60°C) with 1 min holding time, injection (240°C) with 10 minutes holding time, injection mode Split, [GCMS-TQ8040] Ion Source Temp: 230.00°C; Interface Temp.: 280.00°C; Solvent Cut Time: 2.50 min; Detector Gain Mode: Relative to the Tuning Result Detector Gain: 0.94 kV +0.20 kV;[MS Table], Start Time: 2.50min;End Time: 29.00min; Acq. Mode: Q3 Scan; Event Time: 0.300sec; Scan Speed: 1666 Start m/z: 50.00; End m/z: 500.00; sample preparation for injection: 10ppm in ethyl acetate; sample injected volume: 2μL; compound identification method: GCMS Tripple quadrapole (Acq. Mode: Q3 Scan).
Fumigation toxicity
Fumigation toxicity of C. citrinus EO was tested in the laboratory by filter paper method at 28 ± 2°C and 60–70% RH [25]. Different ranges of concentrations such as 40, 80, 120, 160, 200, 240, 280, and 320 μl/L were evaluated for fumigation toxicity on adults and larvae, respectively. 2–3 days old adults (10/replicate) and 3rd instar larvae (10/replicate) were released in a glass bottle (7 X 3.5 cm) along with 2 g of wheat flour as feed. The EO was poured on Whatman No. 1 filter paper (2 cm dia) and it was adhered inside the screw cap of 100 mL glass container and then closed. Separate control without EO was maintained. Five replications were made for all treatments and controls. Mortality of adults and larvae was recorded after 3, 6, 9, 12, 24, 36, and 48 hrs of the commencements of treatment. Dead insects were counted; if there was no antennal or leg movement, it was considered dead.
Repellency test
The larval repellency of EO was measured using the diet impregnation method as described by Stefanazzi et al. [26]. Five grams of feed medium were mixed with different concentrations such as of 5, 10, 15, and 20 μL of EO individually in each Petri plate (10 cm X 1.5 cm). Twenty-five 3rd instar larvae were introduced in each treatment and replicated five times. After 2, 4, 6, 12 and 24 hrs of treatment the number of larvae present in the treated and control diets were counted.
The adult repellency of C. citrinus was evaluated with the help of glass Y tube olfactometer (Stem and both arms measured about 21 cm and 2.5 cm diameter). Two grams of feed medium were mixed with different concentrations such as 5, 10, 15, and 20 μL of EO individually in vial (25 mL) and attached into one arm of the olfactometer. Medium without EO was used as control and was attached into the other arm of Y-tube. Then fifty freshly-emerged adults were released into the stem of the olfactometer. The number of beetles found in each arm was recorded after 24 hrs [27]. The percentage of repellent activity was calculated using the formula of Karemu et al. [28].
Fecundity and knock-down effect of EO on adult
Ten 4 to 6 days old unsexed adults were released into glass vial (100 mL) with 2 g of wheat flour. Filter paper (Whatman No. 1) discs measuring about 2 cm dia, were impregnated with different concentrations (5, 10, 20 and 30 μL/L) of C. citrinus EO. After 24 hrs, knocked down adults were counted and the live adults were transferred to new Petri plate with feed. The Petri plate was carefully examined and the number of eggs laid in control and treatments for a period of 2 days was recorded by using compound microscope. Five replications were used for each treatment and the control group.
Growth inhibition effects
Fifty unsexed adult beetles (4–6 days old) were released in a Petri plate containing 5 g of wheat flour. After 48 hrs, the adult beetles were removed and number of eggs laid in each Petri plate was counted. Subsequently, the filter paper treated with different concentrations (5, 10, 20, 30 μL) of EO was placed on the lid of the Petri plate. Filter paper disc devoid of any volatiles was used as control. The experiments were replicated five times for both treatments and control. The eggs hatched in each Petri plate was recorded daily; the hatched larvae were maintained continuously on wheat flour. The larval survivability and per cent adult emergence (F1) were recorded.
Sample preparation for biochemical studies
Adult insects (2–3 days old) were treated with sub-lethal concentrations (5, 10 and 20 μL/L) of EO as mentioned in fumigation toxicity. The live insects were used in the biochemical analysis which consisted of three replicates. Treated adults (10 individuals for each concentration) were transferred separately and homogenized with 500 μL of ice-cold phosphate buffer (20 mM, pH 7.0) using a Teflon, hand homogenizer to estimate the total protein, esterase, phosphatase, and Glutathione-S-Transferase activities. The homogenates were centrifuged at 15,000 rpm at 4°C for 20 min. and the clear supernatants were stored at -20°C until used. The supernatants were used for both qualitative and quantitative analyses of biochemical studies. The concentration of total protein content was estimated by the method of Bradford [29]. Three replicates were maintained for all the enzymes studies.
Acetylcholinesterase assays
Acetylcholinesterase activity of homogenate was spectrophotometrically measured by the method of Ellman [30] and Ikezawa and Taguchi [31] using the substrate of acetylthiocholine iodide. Each aliquot of whole body homogenate of adult (100 μL) was mixed with 800 μL of sodium phosphate buffer (pH 7.5), 50 μL of 10 mM DTNB reagent and 50 μL of 12.5 mM acetylthiocholine iodide. After the incubation period for 5 min at room temperature, the optical density of the sample was read at 400 nm using spectrophotometer (Lark LI-UV-2500) against suitable reagent blank.
Carboxylesterase assays
The activity of α and β carboxylesterase activity of the whole body homogenate of adult was detected by the method of Asperen [32] and Koodalingam et al. [33] with slight modification. The 100 μL of homogenate was incubated with 900 μL of sodium phosphate buffer (20 mM pH 7.0) containing 250 μM of α- or β- naphthyl acetate for 30 min at room temperature. After incubation, 400 μL of 0.3% fast blue B in 3.3% SDS were added to stop the enzymatic reaction and incubated further for 15 min at room temperature. The optical density of the sample was read at 588 nm in spectrophotometer against the respective blank reagent.
Acid and alkaline phosphatases assay
Acid and alkaline phosphatase levels of whole body homogenate of adult were measured by the method of Asakura [34] with suitable modification. The acid phosphatase activity was measured by adding of 50 μL of homogenate with 450 μL of 50 mM Sodium acetate buffer (pH 4.0) and 500 μL of 15 mM p-nitrophenyl phosphate. It was incubated, in water bath at 37°C for 15 min.
The alkaline phosphatase level in the homogenate was measured by adding 50 μL of homogenate with 450 μl of 50 mM Tris-HCl buffer (pH 8.0) and 500 μl of 15 mM p-nitrophenyl phosphate and incubated in water bath for 15 min at 37°C. Both the acid and alkaline phosphatase reactions were stopped by adding of 100 μL of 0.5 N NaOH solution and centrifuged (Refrigerated Centrifuge—SIGMA 1-15K, Germany) at 3000 rpm for 5 min and optical density of the sample was measured at 440 nm against suitable blank.
Glutathione-S-Transferase activity
Glutathione-S-Transferase level of whole body homogenate of adult was measured by the method of Brogden and Barber [35]. 50 μL of homogenate was placed in microtiter plate. Then 200 μL of GSH/CDNB working solution was added. The plate was incubated for 10 min at room temperature and optical density of the sample was measured at 340 nm against suitable blank.
Qualitative analysis of biochemical constituents
The profiles of protein, alpha and beta carboxylesterase of whole body homogenate of adult were analysed in non-denaturing condition by discontinuous polyacrylamide gel electrophoresis (PAGE). It was performed by using 4% stacking gel (pH 6.8) and 8% separating gel (pH 8.8) in Tris-glycine buffer (pH 8.3). Homogenate sample (50 μL) of treated and control adult was electrophoresed at 4 mA per sample and at 10°C. After electrophoresis, the gel was stained with suitable staining to detect the biochemical constituents. For protein profile, the gel was stained with Coomassie brilliant blue R-250 for the detection of bands.
Detection of α- and β- carboxylesterase activity
According to Kirkeby and Moe [36] and Argentine and James [37], the protein bands were detected from electrophoresed gel with alpha and beta carboxylesterase activity. For alpha/beta carboxylesterase, the gel was first incubated with phosphate buffer (20 mM, pH 7.0) for 15 min at room temperature. After decanting the buffer, the gel was re-incubated with freshly prepared α /β -naphthyl acetate + fast blue B solution for 30 min at room temperature. Then the gel was washed with double distilled water to remove excess stains from the gel and stored at 7% acetic acid.
Detection of acid or alkaline phosphatase activity
The gel was first incubated for 15 min at room temperature with 50 mM acetate buffer (pH 4.6) and 50 mM Tris-HCL buffer (pH 8.0) for acid and alkaline phosphatase activity respectively. After decanting the buffer, the gel was re-incubated with freshly prepared 50 mM acetate buffer (pH 4.6; contains α-naphthyl phosphate + fast blue B solution) and 50 mM Tris-HCL buffer (pH 8.0; contains α-naphthyl phosphate + fast blue B solution) for acid and alkaline phosphatase activity respectively for 2 hrs at 37°C on water bath [38]. Then the gels were washed with double distilled water to remove excess stains and stored at 7% acetic acid.
Statistical analysis
Statistical analyses were done using the original data. The probit analysis was done for fumigation toxicity. The original data was subjected to homogeneity test (Levene Statistic) and normality test (Shapiro–Wilk test); based on the results, parametric test (Tukeys test), and non-parametric test (Kruskal Wallis test) were used. Significant difference between the treatment groups was calculated by ANOVA. Parametric test (Tukey’s test) was used for number of eggs laid, ovicidal, egg hatchability and larval survival, Non-Parametric test (Kruskal wallis test) was used for fumigation toxicity, larvicidal activity, knockdown and adult emergence (F1 generation) and for repellency Chi-Square test was used by SPSS-20.
Results
Oil yield
Initially the oil was whitish in colour but later it turned into pale yellow. The yield of EO was 0.65% v/w.
Chemical composition of EO
Chemical composition of C. citrinus EO was analysed by GC-MS and it identified 10 different compounds in varying quantities (ranging between 0.59 to 40.44%). The total composition of the compounds were 100%. Among the 10 compounds, eucalyptol represented the major constituent (40.44%), followed by linalool (27.35%), and α- Pinene (17.36%) (Table 1).
Table 1. Chemical Constituents and composition (%) of essential oil from Callistemon citrinus.
| Peak No. | Chemical Constituents | Retention Time (min) | Area% |
|---|---|---|---|
| 1 | α.-Pinene | 5.690 | 17.46 |
| 2 | Camphene | 5.955 | 0.59 |
| 3 | β.-Pinene | 6.412 | 5.83 |
| 4 | β.-Myrcene | 6.627 | 3.22 |
| 5 | β.-Pinene | 6.852 | 0.93 |
| 6 | Eucalyptol | 7.315 | 40.44 |
| 7 | gamma.-Terpinene | 7.738 | 2.32 |
| 8 | .alpha.-Methyl-.alpha.-[4-methyl-3-pentenyl]oxiranemethanol | 7.936 | 0.92 |
| 9 | 2-Carene | 8.165 | 0.96 |
| 10 | Linalool | 8.419 | 27.34 |
Fumigation toxicity study
At 160 μL/L EO showed 100% adult beetle mortality at 9 hrs of treatment. At the lowest concentration (40 μL/L), EO exhibited 50% mortality after 24 hrs of exposure, and there was a gradual increase in insect mortality while increasing concentration of EO (Table 2). Maximum larvicidal activity of 95.78% was observed at 320 μL/L concentration at 48 hrs after exposure period (Table 3). The lethal concentration (LC50) of EO against T. castaneum adults was 37.05 μL/L at 24 h of exposure and against larvae it was 144.31 μL/L at 48 h of exposure (Table 4). The fumigation toxicity of C. citrinus EO was concentration and time dependent against T. castaneum larvae, however, the larvae, appeared to be more tolerant to EO than adults.
Table 2. Fumigation toxicity (%) of essential oil from C. citrinus against T. castaneum-adult.
| Concentrations (μL/L) | After Exposure (h) | ||||
|---|---|---|---|---|---|
| 3 | 6 | 9 | 12 | 24 | |
| 40 | 0.0±0.0a | 12.0±2.00a | 30.0±3.16a | 36.0±2.45a | 50.0±1.75a |
| 80 | 0.0±0.0a | 16.0±2.45a | 58.0±2.00ab | 68.0±3.74ab | 81.3±1.93ab |
| 120 | 0.0±0.0a | 30.0±3.16ab | 72.0±3.74ab | 82.0±2.00ab | 91.6±2.12ab |
| 160 | 8.36±3.74b | 48.0±3.74b | 100.0±0.00b | 100.0±0.00b | 100.0±0.00b |
| 200 | 22.0 ±2.00b | 64.0±2.45b | 100.0±0.00b | 100.0±0.00b | 100.0±0.00b |
| ANOVA | **p≤0.04 from 40, 80 and 120 μL/L | **p≤0.05 from 40 & 80 μL/L * p≤0.05 from 40 μL/L | **p≤0.01 from 40 μL/L | **p≤0.01 from 40 μL/L | **p≤0.01 from 40 μL/L |
Mean of five replication ± SE; within the column same alpha bête do not significant by Kruskal-Wallis Test
Table 3. Larvicidal activity (%) of essential oil from C. citrinus against T. Castaneum.
| Concen- trations (μL/L) | After Exposure (hrs) | ||||||
|---|---|---|---|---|---|---|---|
| 3 | 6 | 9 | 12 | 24 | 36 | 48 | |
| 40 | 0.0±0.0 | 0.0±0.0a | 2.0±2.00a | 4.0±2.45a | 6.0±2.45a | 10.2±3.16a | 16.9±2.38a |
| 80 | 0.0±0.0 | 0.0±0.0a | 4.0±2.45a | 14.0±2.45a | 18.2±3.62a | 22.9±1.84ab | 31.6±3.99ab |
| 120 | 0.0±0.0 | 0.0±0.0a | 6.0±2.45 abc | 18.0±3.74bc | 22.4±1.93bc | 26.9±3.69b | 42.2±3.76bc |
| 160 | 0.0±0.0 | 6.0±2.45ab | 10.0±3.16abcd | 22.0±2.00bc | 26.2±3.77bc | 31.1±2.87bc | 50.9±3.07c |
| 200 | 0.0±0.0 | 8.0±2.00ab | 14.0±2.45bcd | 26.0±2.45bcd | 30.4±2.82bce | 35.1±3.47bcd | 55.3±3.85c |
| 240 | 0.0±0.0 | 12.0±2.00bc | 16.0±2.45cde | 30.0±3.16cde | 34.7±2.26cd | 41.8±1.09cd | 70.4±3.39d |
| 280 | 0.0±0.0 | 14.0±2.45bc | 20.0±3.16de | 36.0±2.45de | 40.7±2.66de | 47.8±3.34d | 80.9±2.06d |
| 320 | 0.0±0.0 | 18.0±3.74c | 26.0±2.45e | 42.0±2.00e | 49.1±2.51e | 64.7±2.00e | 95.8±2.59e |
| Anova | *Df = 39, | Df = 39, F-11.55, p≤0.00 | Df = 39, F-10.23, p≤0.00, | Df = 39, F-21.39, p≤0.00 | Df = 39, F-22.75, p≤0.00 | Df = 39, F-34.24, p≤0.00 | Df = 39, F-65.64, p≤0.00 |
*No homogeneity; Mean of five replication ± SE; within the column same alpha bête do not significant by Tukey Test Test (p ≤ 0.05).
Table 4. Lethal concentration of essential oil from C. citrinus against T. castaneum adult and larvae.
| Stage of the insect | LC50 | 95%Feducial level | LC90 (μL/L) | 95%Feducial level | Chi-square | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | ||||
| (μL/L)24 hrs after treatment | |||||||
| Adult | 37.05 | 14.09 | 51.02 | 102.82 | 89.70 | 123.41 | 3.74 |
| 48 hrs after treatment | |||||||
| Larva | 144.31 | 123.32 | 162.85 | 321.62 | 289.44 | 369.08 | 11.01 |
Repellency test
The maximum repellency (93.3%) was observed at 20 μL concentration after 24 hrs observation. Lowest concentration showed 30% repellent activity against T. castaneum larvae at 24 hrs (Table 3). The highest concentration exhibited time related repellent activity by Chi-Square test (χ2–72, df-36, p≤0.00). Other concentrations did not show significant activity.
A 100% adult repellent activity was observed at 20 μL concentration after 24 hrs through Y-arm olfactometer. The lower concentration of EO exhibited more than 31.1% repellent activity (Table 5). The results indicated that EO exhibited good repellent potential against both larvae and adults. There was no significant effect found between the treatments by Chi-Square test (χ2–36, df-27, p≤0.12).
Table 5. Repellent activity (%) of essential oil from C. citrinus against T. Castaneum.
| Concentration (μl/L) | After Exposure (hrs) | Chi-Square test | ||||||
|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 12 | 24 | ||||
| Larvae | χ2 | df | p value | |||||
| 5 | 24.3±2.9 | 24.3±2.9 | 25.3±3.6 | 26.3±3.9 | 30.5±3.1 | 23.29 | 28 | 0.72 |
| 10 | 40.9±2.1 | 41.4±1.7 | 41.4±1.7 | 42.4±1.6 | 45.6±3.0 | 15.67 | 16 | 0.48 |
| 15 | 61.4±1.3 | 61.8±1.1 | 63.2±2.0 | 63.2±2.0 | 64.5±1.9 | 18.46 | 20 | 0.56 |
| 20 | 70.2±1.3 | 74.4±1.6 | 81.7±1.7 | 82.1±1.5 | 93.3±3.1 | 72.00 | 36 | 0.00 |
| Adult-repellent activity (%) | Concentration (μl/L) | |||||||
| 5 | 10 | 15 | 20 | - | χ2 | df | p value | |
| 31.1±1.95 | 64.8±1.6 | 89.1±1.4 | 100±0.0 | - | 36.00 | 27 | 0.12 | |
Mean of five replication ± SE
Fecundity and knock-down activity
The control beetle group laid 5.8 eggs per individual on an average. In EO treated group, concentrations of 20 and 30 μL/L showed 2.6 and 1.4 eggs. In terms of per cent reduction, 20 and 30 μL/L concentrations showed 55.17 and 75.86% reduction in fecundity, respectively. C. citrinus EO significantly reduced oviposition activity at 20 and 30 μL/L (Table 6) Knockdown effect was increased according to increasing concentration. Maximum knockdown activity of 35.5% was observed at 30 μL/L which was statistically significant from the control (Kruskal-Wallis).
Table 6. Oviposition deterrent (number of eggs/insect/day) activity of essential oil from C. citrinus on T. Castaneum.
| Concentrations (μL/L) | #Number of Eggs laid | Knockdown (%) |
|---|---|---|
| Control | 5.8±0.37d | 0.0±0.0 |
| 5 | 4.6±0.51d | 0.0±0.0 |
| 10 | 3.8±0.20c | 5.5±0.49 |
| 20 | 2.6±0.24b | 24.0±0.61 |
| 30 | 1.4±0.24a | 35.5±0.93* |
| Anova | Df-24, F-26.14, p≤0.00 | Kruskal-Wallis Test ** p≤0.00 (control & 5 μL/L) (5 μL/L) |
Mean of five replication ± SE (n = 50); within the column same alpha bête do not significant by Kruskal-Wallis Test (p ≤ 0.05) (Knock down); Tukeys test (#)
Growth effects
Ovicidal activity and egg hatchability
The ovicidal effect of C. citrinus EO was studied against T. castaneum at four different concentrations. Maximum ovicidal activity of 91.49% was observed at 30 μL/L concentration of EO. All the concentrations showed statistically (Df-24, F-16924.3, p≤0.00) different activity from the control (Table 7).
Table 7. Bioefficacy of essential oil from C. citrinus against different life stages of T. Castaneum.
| Concentrations—(μL/L) | Ovicidal activity (%) | Egg hatchability (%) | Larval survival (%) | *Adult emergence of F1 generation (%) |
|---|---|---|---|---|
| Control | 10.85±0.25a | 89.15±0.25e | 86.96±1.23e | 80.58±1.15 |
| 5 | 43.45±0.23b | 56.55±0.24d | 70.67±0.57d | 53.45±0.27 |
| 10 | 56.70±0.36c | 43.29±0.36c | 60.61±1.41c | 36.14±0.90 |
| 20 | 78.69±0.15d | 21.31±0.15b | 51.04±1.22b | 21.82±0.67 |
| 30 | 91.49±0.13e | 8.51±0.13a | 42.54±1.32a | 5.15±2.10**# |
| ANOVA | Df-24, F-16924.3, p≤0.00 | Df-24, F-16924.3, p≤0.00 | Df-24, F-210.12, p≤0.00 | Kruskal-Wallis Test ** p≤0.00 (control) #p≤0.013 (5 μL/L) |
Mean of five replication ± SE; within the column same alpha bête do not significantly by Tukeys test (P ≤ 0.05)
The 5 μL/L concentration exhibited egg hatchability of 56.55% while the control exhibited 89.15% egg hatchability. The minimum egg hatchability of 8.51% was recorded at 30 μL/L concentration (Table 7). The treatment showed significant difference from the control by Tukeys test (p≤0.00).
Larval survival and adult emergence
Larval survival and adult emergence (F1 generation) were 86.96% and 80.58%, respectively in the control. In contrast, at 30 μL/L concentration EO exhibited larval survival of 42.54%, and it was notably lower than the control. Significant reduction of larval survival was recorded in all the treatments when compared to the control. The 30 μL/L treatment allowed 5.15% of adults to emerge when compared to the control. A significant reduction in adult emergence (F1) 5.15% was observed at 30 μL/L (Table 7). The 30 μL/L concentration was statistically significant from other treatments and the control.
Quantitative analysis of biochemical constituents
Based on the obtained results, sub-lethal concentrations (5, 10 and 20 μL/L) were used to study the impact of C. citrinus EO on various biochemical constituents in adult T. castaneum beetles. Results indicated that the biochemical constituents measured in T. castaneum adult beetles significantly varied after exposure to sub-lethal concentrations of EO for 24 hrs. The total protein content of T. castaneum adult was highly and significantly reduced 6.19 mg/mL, 5.72 mg/mL, 5.32 mg/mL in adults exposed to different concentrations of 5, 10 and 20 μL/L of EO, respectively relative to the control value of 7.43 mg/mL. The higher concentration was statistically different from other treatments and control. Acetylcholinesterase activity dramatically increased in the 10 and 20 μL/L, treatments when compared to the control; while, at 20 μL/L concentration treatment, there was reduced Acetylcholinesterase activity. The highest concentration statistically and significantly influenced enzyme activity when compared to the control (Kruskal-Wallis Test p ≤ 0.028) (Table 8).
Table 8. Biochemical effect of essential oil of C. citrinus on T. castaneum.
| BIOCHEMICAL STUDIES | Concentrations–(μL/L) | ANOVA | |||
|---|---|---|---|---|---|
| Control | 5 | 10 | 20 | ||
| Total protein (μg protein/μl of homogenate | 7.43±0.07 | 6.19±0.03 | 5.72±0.15 | 5.32±0.21* | Kruskal-Wallis Test p≤0.022 |
| Acetylcholinesterase (μM of AcT Hydrolysed/min/mg of protein) | 3.30±0.04 | 3.68±0.05 | 3.74±0.04 | 2.93±0.06* | Kruskal-Wallis Test (p ≤ 0.039) |
| α-Carboxylesterase (μM of α-Naphthol released/min/mg of protein) | 67.06±1.83 | 85.46±1.13 | 99.38±2.25 | 110.59±2.69* | Kruskal-Wallis Test (p ≤ 0.013) |
| β-carboxylesterase (μM of β-Naphthol released/min/mg of protein) | 0.056±0.0002 | 0.137±0.0009 | 0.137±0.0007 | 0.133±0.0015 | Kruskal-Wallis Test (p ≤ 0.055) |
| Acid-phosphatase (mM p-nitrophenol released/min/mg of protein) | 0.86±0.02 | 0.84±0.04 | 0.73±0.10 | 0.60±0.11 | Tukeys Test (p ≤ 0.137) |
| Alkaline-phosphatase (mM p-nitrophenol released/min/mg of protein) | 0.42±0.02 | 0.30±0.03 | 0.71±0.06 | 0.14±0.04* | Kruskal-Wallis Test (p ≤ 0.013) |
| Glutathione-S-Transferase (μM of GS-CDNB conjugated/min/mg of protein) | 0.219±0.002 | 0.365±0.003 | 0.333±0.001 | 0.376±0.009 | Kruskal-Wallis Test (p ≤ 0.027) |
* Significant from control
The level of α-Carboxylesterase activity was significantly increased at three of the selected sub-lethal concentrations of EO, compared to the control, but statistically significant activity was found at 20 μL/L. β-carboxylesterase activity level was also drastically elevated in the 5, 10 and 20 μL/L treatments compared to the control; however, no significant difference was observed between the different sub-lethal treatments (Table 8).
Exposure of T. castaneum adults to C. citrinus EO resulted in decreased level of acid phosphatase activity at selected concentrations when compared to the control group. Alkaline phosphatase activity was significantly reduced in 5 μL/L treatment and drastically elevated in the 10 μL/L treatment. Significantly lower activity was recorded in the 20 μL/L treatment when compared to the control. Glutathione-S-Transferase levels gradually increased in the 5, 10 and 20 μL/L treatments, relative to the control (Table 8).
Qualitative analysis of biochemical constituents
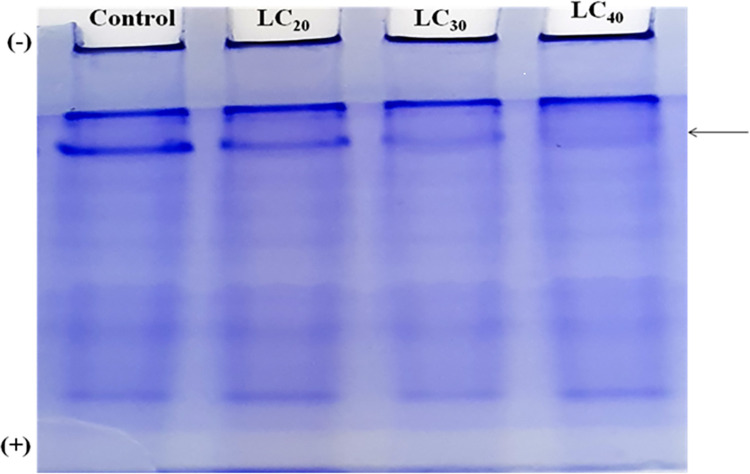
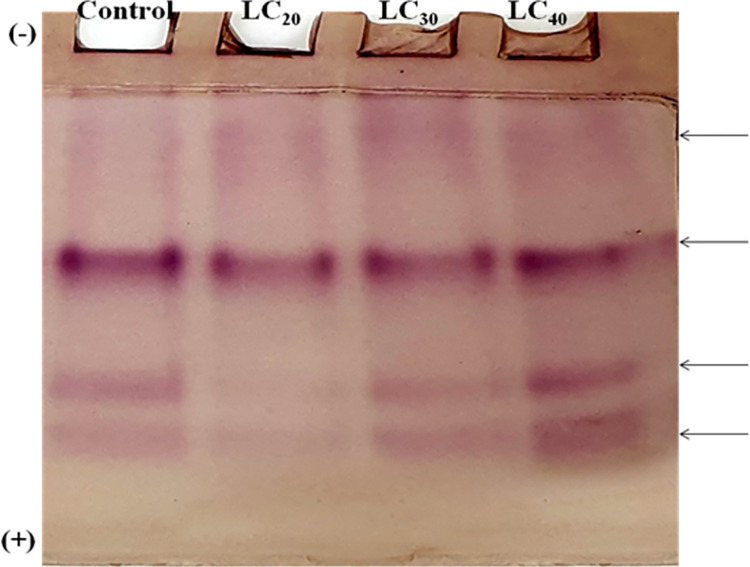
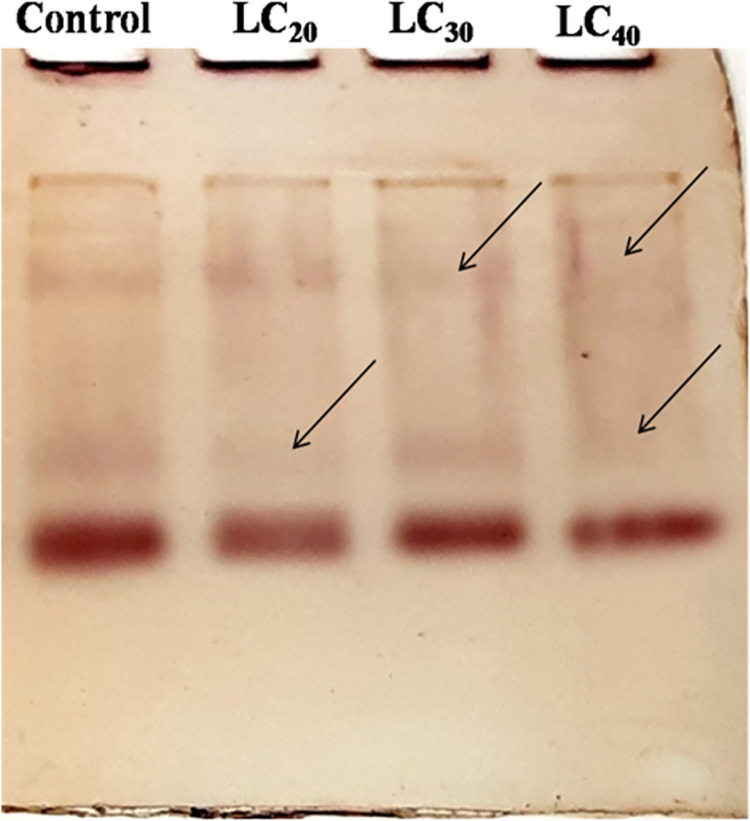
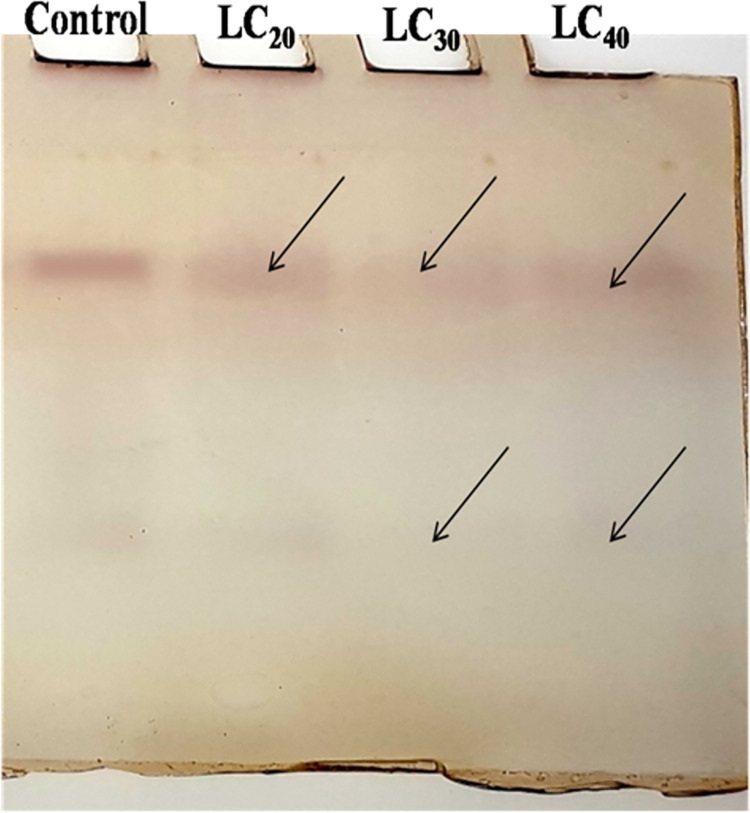
A qualitative analysis of total proteins was done using the native PAGE method. Protein extracted from T. castaneum adults treated with sub-lethal concentrations of C. citrinus EO showed a reduction in the number protein bands, compared to the control (Fig 1). The intensity of the esterase band of β-Carboxylesterase isoenzyme was modulated by the concentration of EO. The intensity of the band was lowered in the treatment but increased gradually as the concentration of EO increased. The two lower isoenzyme bands decreased in their intensity, relative to the control, at the lower concentrations of EO and gradually increased when beetles were exposed to higher concentration of EO (Fig 2). Electrophoretic analysis of acid and alkaline phosphatase enzyme activity in adult beetles was slightly affected by exposure to the range of sub-lethal concentrations of EO used in the experiment (Figs 3 and 4).
Fig 1. Qualitative analysis of total protein in native PAGE, of T. castaneum adult after treatment with essential oil of C. Citrinus.
Fig 2. Qualitative analysis of isoenzyme of β-Carboxylesterases of T. castaneum adult after treatment with essential oil of C. Citrinus.
Fig 3. Qualitative analysis of acid phosphatises of T. castaneum adult after treatment with essential oil of C. Citrons.
Fig 4. Qualitative analysis of alkaline phosphatases T. castaneum adult after treatment with essential oil of C. Citrinus.
Discussion
Botanical pesticides are generally biodegradable, and have the potential to eradicate pests without causing harm to the environment and non-target organisms. Essential oils are volatile in nature; they are easy to use and kill the pests of stored grains. Hence, in the present study, EO extracted from C. citrinus leaves was tested for its potential against the larvae and adult beetles of T. castaneum.
GC-MS analysis of C. citrinus revealed ten different compounds, in which eucalyptol (40.44%) was the major constituent, followed by linalool, and α- Pinene. However, the same plant from Ethiopia contained 76.9% of eucalyptol and from Western Himalayas it contained only 9.8% eucalyptol [39, 40]. These data clearly indicate that the level and type of constituents in EO extracted from this plant varies depending on where the plant was collected, and most likely the climate and ecology of that region playing a role. These results are in accordance with Misharina [41], Souza and Vendramim [42] and Isikber et al. [43] who indicated that the variations in extracted compounds, could be associated with geographical location, collection time, amount of sunlight, length of storage, temperature, and extraction methods.
Fumigation is one of the most effective, practically feasible, and rapid methods that can be used to protect feedstuffs, stored food grains and other agricultural products from pest infestation [44, 45]. In the present study, fumigation activity of EO extracted from leaves of C. citrinus showed 100% mortality in adult beetles of T. castaneum at 9 hrs after treatment at a concentration of 160 μL/L. Similar results were obtained from Coriandrum sativum seed oil, where mortality in C. maculatus and T. confusum increased with increasing concentrations of EO from 43 to 357 μL/L air [46]. The EO used in our study exhibited different levels of toxicity to larvae vs. adult beetles. In general, fumigation toxicity was lower against larvae than adult beetles. Earlier, Huang et al. [47] and Isikber [43] have reported similar results.
In the present study, a significant reduction in oviposition was recorded when adult beetles were exposed to different concentrations of EO. Similarly, Moura et al. [20] also reported that EO derived from Vanillosn opsis arborea reduced the level of oviposition when compared to the control. A previous report indicated decreased oviposition (28%) at 5.2 mg/cm2 concentration when the adult beetles were exposed to EO derived from leaves of C. loga. The reduction in oviposition was probably due to physically weakened insects as well as lesser surviving insects [48].
Egg hatchability was drastically reduced because the vapour of EO diffused through the permeable membranes of insect eggs into the chorion and vitelline membrane [49, 50]. The diffusion of EO vapours into the eggs results in a disruption in normal physiological and biochemical processes [51]. The ovicidal activity observed in the present study confirmed the above statements, as the tested EO resulted in 91.49% ovicidal activity in T. castaneum. The reduction of the F1 generation could be due to the toxicity of EO to all life stages of the insect, from eggs to adults, via., both fumigant and possibly by stomach action [52]. In the present study, a drastic reduction (94.85%) was observed in adult emergence when the eggs were exposed to EO.
Proteins are the most abundant organic compounds in the insect body as they provide structure, energy and catalyse chemical reactions in the form of enzymes [53, 54]. Decreasing protein content commonly occurs when the insects are treated with lethal compounds [55]. Insects degrade the protein content into amino acid and release energy to compensate the lowering energy level during stress condition [56]. Reductions in protein levels were observed in the present study and has been previously reported that protein content was reduced due to the toxicity of plant products [27, 57–59].
In the present study, AChE activity was inhibited at the higher concentrations of C. citrinus oil. Saponins were able to inhibit AChE and the inhibition increased with increasing concentration [60]. Inhibition of AChE results in the accumulation of acetylcholine at cholinergic synapses and causes hyper excitation of cholinergic pathways [61].
Carboxylesterase activity can be altered by plant secondary metabolites. Phenolic glycoside significantly increased the level of Carboxylesterase in Lymantria dispar [62]. A higher level of CarE activity was recorded in Sitobion avenae fed on diet with high indole alkaloid content [63]. In the present study, both α- CarE and β- CarE levels increased in adult beetles exposed to C. citrinus EO.
Hydrolytic cleavage of phosphoric acid esters is catalysed by Phosphatase enzymes that are classified into "acid" or "alkaline" phosphatases based on their pH [64]. Acid phosphatases are a lysosomal marker enzyme whose active site is in the gut of insects [65–68]. Alkaline phosphatases are a brush border membrane marker [69]. Exposure to plant compounds reduced the acid and alkaline phosphatises content in Cnaphalocrocis medinalis larvae [70, 71]. Similarly, C. citrinus EO also reduced both acid and alkaline phosphatase activity in a concentration dependent manner.
Glutathione S-transferases are the enzymes that catalyse the detoxification of insecticides typically after the phase-I metabolic process [72]. In the present study, elevated GST levels were observed in adult beetles exposed to the higher concentrations of C. citrinus EO. Shojaei et al. [73] reported that adults of T. castaneum exposed to Artemisia dracunculus EO enhanced the level of Glutathione-S-Transferase in a concentration dependent manner.
The present study clearly indicated that the EO possessed wide range of biological activities which included fumigant, repellent, oviposition and growth inhibitory activities, and acted up on all insect development stages. Hence, this EO should be prepared as a formulation and further regulatory studies (ecotoxicological effects, genetic toxicological effects and acute toxicity studies along with stability studies) should be conducted for safety assessment. Then it could be considered for used in stored product pest management.
Supporting information
(PPTX)
(PPTX)
(PDF)
Acknowledgments
The authors also deeply thanks to Rev. Fr. Dr. S. Ignacimuthu, Director, Xavier Research Foundation, St Xavier’s College, Palayamkottai, Tamil Nadu for valuable comments to improve early version of manuscript
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The present study was funded by the DST (DST-PURSE Phase II Programme), India, New Delhi sanctioned to Dr. M. Jayakumar Assistant Professor University of Madras. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ramaraju K. Post-harvest loss in food grains at 6%: ICAR. The economic times Business News; 2013. [Google Scholar]
- 2.Graver JEVS. Guide to Fumigation under Gas-Proof Sheets. Food and Agricultural Organization of the United Nations, Rome: 2004. publication code CoP006.pp 1–176. [Google Scholar]
- 3.WMO Scientific assessment of ozone depletion: World Meteological Organization Report. 25. World Meteological Organization, UN, Geneva, 1991.
- 4.Anonymous, Regulatory action under the clean air act on methyl bromide. United States Environmental Protection Agency, Office of Air Radiation Stratospheric Protection Division, Washington, DC, 1993. [Google Scholar]
- 5.Ogendo JO, Kostyukovsky M, Ravid U, Matasyoh JC, Deng AL, et al. Bioactivity of OcimumGratissimum L. oil and two of its constituents against five insect pests attacking stored food products. J Stored Prod Res. 2008; 44(4): 328. [Google Scholar]
- 6.Bell CH, Wilson SM. Phosphine tolerance and resistance in Trogoderma granarium Everts (Coleoptera: Dermestidae). J Stored Prod Res. 1995; 31(3): 199–205. 10.1016/0022-474X(95)00012-V. [DOI] [Google Scholar]
- 7.Alzahrani SM, Ebert PR. Stress pre-conditioning with temperature, UV and gamma radiation induces tolerance against phosphine toxicity. PLoS ONE. 2018; 13(4): e0195349. doi: 10.1371/journal.pone.0195349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garry V, Griffith J, Danzl T, Nelson R, Whoston E. Human genotoxicity: pesticide applicators and phosphine. Sci. 1989; 246: 251–255. doi: 10.1126/science.2799386 [DOI] [PubMed] [Google Scholar]
- 9.Jayakumar M, Seenivasan SP, Rehman F, Ignacimuthu S. Fumigant effect of some essential oils against pulse beetle, Callosobruchus maculatus (Fab.) (Coleoptera: Bruchidae). African Entomol. 2017; 25(1);193–199. [Google Scholar]
- 10.Janaki S, Zandi-Sohani N, Ramezani L, Szumny A. Chemical composition and insecticidal efficacy of Cyperus rotundus essential oil against three stored product pests. Inter Biodeter Biodegradation. 2018; 13: 93–98. 10.1016/j.ibiod.2018.06.008. [DOI] [Google Scholar]
- 11.Rajkumar V, Gunasekaran C, Christy IK, Dharmaraj J, Chinnaraj P. Toxicity, antifeedant and biochemical efficacy of Mentha piperita L. essential oil and their major constituents against stored grain pest. Pesticide Biochem Physiol. 2019; 156; 138–144. 10.1016/j.pestbp.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, Pang X, Guo S, Zhang W. Geng Z. Chemical Composition and Bioactivities of Alpinia Katsumadai Hayata seed essential oil against three stored product insects. J Essential Oil Bearing Plants. 2019; 22(2): 504–515. 10.1080/0972060X.2019.1611482. [DOI] [Google Scholar]
- 13.Regnault-Roger C. The potential of botanical essential oils for insect pest control. Integrated Pest Management Reviews 1997; 2(1): 25–34. 10.1023/A:101847222. [DOI] [Google Scholar]
- 14.Regnault-Roger C, Vincent C, Arnason JT. Essential oils in insect control: low-risk products in a high-stakes world. Ann Rev Entomol. 2012; 57: 405–424. 10.1146/annurev-ento-120710-100554. [DOI] [PubMed] [Google Scholar]
- 15.Koutsaviti A, Antonopoulou V, Vlassi A, Antonatos S, Michaelakis A. Chemical composition and fumigant activity of essential oils from six plant families against Sitophilus oryzae (Col: Curculionidae). J Pest Sci. 2018; 91(2): 873–886. 10.1007/s10340-017-0934-0. [DOI] [Google Scholar]
- 16.Upadhyay N, Dwivedy AK, Kumar M, Prakash B. Dubey NK. Essential oils as eco-friendly alternatives to synthetic pesticides for the control of Tribolium castaneum (Herbst)(Coleoptera: Tenebrionidae). J Essential Oil Bearing Plants. 2018; 21(2): 282–297 10.1080/0972060X.2018.1459875. [DOI] [Google Scholar]
- 17.Wagan TA, Cai W, Hua H. Repellency, toxicity, and anti-oviposition of essential oil of Gardenia jasminoides and its four major chemical components against whiteflies and mites. Sci Reports. 2018; 8(1): 1–12. 10.1038/s41598-018-27366-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alcala-Orozco M, Caballero-Gallardo K, Stashenko EE, Olivero-Verbel J. Repellent and fumigant actions of the essential oils from Elettaria cardamomum (L.) Maton, Salvia officinalis (L.) Linnaeus, and Lippia origanoides (V.) Kunth against Tribolium castaneum and Ulomoides dermestoides. J Essential Oil Bearing Plants. 2019; 22(1): 18–30. 10.1080/0972060X.2019.1585966. [DOI] [Google Scholar]
- 19.Joshi R, Tiwari SN. Fumigant toxicity and repellent activity of some essential oils against stored grain pest Rhyzopertha dominica (Fabricius). J Pharmacognosy Phytochem. 2019; 8(4): 59–62. [Google Scholar]
- 20.da Silva Moura E, Faroni LR, Zanuncio JC, Heleno FF, Prates LH. Insecticidal activity of Vanillosmopsis arborea essential oil and of its major constituent α-bisabolol against Callosobruchus maculatus (Coleoptera: Chrysomelidae). Scientific reports. 2019; 9(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zandi-Sohani N, Hojjati M, Carbonell-Barrachina ÁA. Insecticidal and repellent activities of the essential oil of Callistemon citrinus (Myrtaceae) against Callosobruchus maculatus (F.)(Coleoptera: Bruchidae). Neotropical entomology. 2013; 42(1): 89–94. doi: 10.1007/s13744-012-0087-z [DOI] [PubMed] [Google Scholar]
- 22.Ramachandran M, Krishnaveni T, Jayakumar M. Fumigation toxicity and evaluation of detoxifying enzymes of Rhyzopertha dominica (coleoptera: bostrichidae) exposed to essential oil of Callistemon citrinus (curtis) skeels. Pestology. 2020; XLIV (1): 38–41. [Google Scholar]
- 23.Shaha A, Salunkhe V. Development and validation of a high performance thin layer chromatographic method for determination of 1,8-cineole in Callistemon citrinus. Pharmacognosy Research. 2014; 6(2):143. doi: 10.4103/0974-8490.129034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Netala S, Penmetsa R, Nakka S, Polisetty BL. Pharmacognistic study of Callistemon citrinus L. bark. International Journal of Pharmacy and Pharmaceutical Sciences. 2015; 7:427–430. [Google Scholar]
- 25.Negahban M, Moharramipour S. Fumigant toxicity of Eucalyptus intertexta, Eucalyptus sargentii and Eucalyptus camaldulensis against stored product beetles. J Appl Entomol. 2007; 131(4): 256–261. 10.1111/j.1439-0418.2007.01152.x [DOI] [Google Scholar]
- 26.Stefanazzi N, Stadler T, Ferrero A. Composition and toxic, repellent and feeding deterrent activity of essential oils against the stored‐grain pests Tribolium castaneum (Coleoptera: Tenebrionidae) and Sitophilus oryzae (Coleoptera: Curculionidae). Pest management science. 2011; 67(6): 639–46. doi: 10.1002/ps.2102 [DOI] [PubMed] [Google Scholar]
- 27.Moses JP, Nattudurai G, Baskar K, Arokiyaraj S, Jayakumar M. Efficacy of essential oil from Clausena anisata and its impact on biochemical changes of Sitophilus oryzae. Environ Sci Pollut Res. 2020; 27: 23215–23221 doi: 10.1007/s11356-020-08928-5 [DOI] [PubMed] [Google Scholar]
- 28.Karemu CK, Ndung’u MW, Githua M. Repellent effects of essential oils from selected eucalyptus species and their major constituents against Sitophilus zeamais (Coleoptera: Curculionidae). Int J Trop Insect Sci. 2013; 33: 188–194. 10.1017/S1742758413000179. [DOI] [Google Scholar]
- 29.Bradford MM. A Rapid and Sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analy Biochem. 1976; 72: 248–254 doi: 10.1006/abio.1976.9999 [DOI] [PubMed] [Google Scholar]
- 30.Ellman GL, Courtney KD, Andres V. Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961; 7: 88–95. doi: 10.1016/0006-2952(61)90145-9 [DOI] [PubMed] [Google Scholar]
- 31.Ikezawa H, Taguchi R. Phosphatidylinositol-specific phospholipase C from Bacillus cereus and Bacillus thuringiensis. Methods Enzymol. 1981; 71: 731–741. 10.1016/0076-6879(81)71086-3. [DOI] [Google Scholar]
- 32.Asperen KV. A study of house fly esterase by means of a sensitive colorimetric method. J Insect Physiol. 1962; 8: 401–416. 10.1016/0022-1910(62)90074-4. [DOI] [Google Scholar]
- 33.Koodalingam A, Deepalakshmi R, Ammu M, Rajalakshmi A. Effects of NeemAzal on marker enzymes and hemocyte phagocytic activity of larvae and pupae of the vector mosquito Aedes aegypti. J Asia-Pacific Entomol. 2014; 17: 175–181. 10.1016/j.aspen.2013.12.007. [DOI] [Google Scholar]
- 34.Asakura K. Phosphatase activity in the larva of the euryhaline mosquito, Aedes togoi Theobold, with special reference to sea water adaptation. J Exp Mar Biol Ecol. 1978; 31: 325–337, 10.1016/0022-0981(78)90067-9. [DOI] [Google Scholar]
- 35.Brogden WG, Barber AM. Microplate assay of glutathione-S-transferase activity for resistance detection in single mosquito triturates. Comp Biochem Physiol. 1990; 96: 339–342. doi: 10.1016/0305-0491(90)90385-7 [DOI] [PubMed] [Google Scholar]
- 36.Kirkeby S, Moe D. Hydrolyses of α-naphthyl acetate, β-naphthyl acetate, and acetyl-DL-phenylalanine β-naphthyl ester. Acta Histochem. 1983; 72: 225–231. doi: 10.1016/s0065-1281(83)80058-0 [DOI] [PubMed] [Google Scholar]
- 37.Argentine JA, James AA. Characterization of a salivary gland-specific esterase in the vector mosquito, Aedes aegypti. Insect Biochem Mol Biol. 1995; 25: 621–630. doi: 10.1016/0965-1748(94)00103-o [DOI] [PubMed] [Google Scholar]
- 38.Houk EJ, Hardy JL. Alkaline phosphatases of the mosquito, Culex tarsalis coquillett. Comp Biochem Physiol. 1984; 78(2): 303–310. doi: 10.1016/0305-0491(84)90034-8 [DOI] [PubMed] [Google Scholar]
- 39.Aweke N, Yeshanew S. Chemical composition and antibacterial activity of essential oil of Callistemon citrinus from Ethiopia. European Journal of Medicinal Plants. 2016; 17(1): 1–7. 10.9734/EJMP/2016/27987. [DOI] [Google Scholar]
- 40.Kumar D, Sukapaka M, Babu GDK, Padwad Y. Chemical Composition and In vitro cytotoxicity of essential oils from leaves and flowers of Callistemon citrinus from Western Himalayas. PloS One 2015; 10(8): p.e0133823. doi: 10.1371/journal.pone.0133823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Misharina TA. Influence of the Duration and Conditions of Storage on the Composition of the Essential Oil from Coriander from Coriander Seeds. Appl Biochem Microbiol. 2001; 37: 622–628. 10.1023/A:1012315403828. [DOI] [Google Scholar]
- 42.Souza APD. Vendramim JD. Insecticidal activity of aqueous extracts of Meliaceae plants on the silverleaf whitefly, Bemisia tabaci (Genn.) Biotype B (Hemiptera: Aleyrodidae). Neotrop Entomol. 2001; 30(1); 133–137. 10.1590/S1519-566X2001000100019. [DOI] [Google Scholar]
- 43.Isikber AA, Alma MH, Kanat M, Karci A. Fumigant toxicity of essential oils from Laurus nobilis and Rosmarinus officinalis against all life stages of Tribolium confusum. Phytoparasitica. 2006; 34(2): 167–177. 10.1007/BF02981317. [DOI] [Google Scholar]
- 44.Shaaya E, Kostjukovski M, Eilberg J, Sukprakarn C. Pliant oils as fumigants and contact insecticides for the control of stored-product insects. J Stored Prod Res. 1997; 33: 7–15. doi: 10.1016/S0022-474X(96)00032-X [DOI] [Google Scholar]
- 45.Emekci M. Section: fumigation, modified atmospheres and hermetic storage. In 10th Int Work Conf Stored Prod Prot Lisbon, Portugal, 2010.
- 46.Khani A, Rahdari T. Chemical composition and insecticidal activity of essential oil from Coriandrum sativum seeds against Tribolium confusum and Callosobruchus maculatus. ISRN Pharmaceutics 2012. doi: 10.5402/2012/263517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang Y, Chen SX, Ho SH. Bioactivities of methyl allyl disulfide and diallyl trisulfide from essential oil of garlic to two species of stored-product pests, Sitophilus zeamais (Coleoptera: Curculionidae) and Tribolium castaneum (Coleoptera: Tenebrionidae). J Econ Entomol. 2000; 93: 537–543. doi: 10.1603/0022-0493-93.2.537 [DOI] [PubMed] [Google Scholar]
- 48.Tripathi AK, Prajapati V, Verma N, Bahl JR, Bansal RP, et al. Bioactivities of the leaf essential oil of Curcuma longa (var. Ch-66) on three species of stored-product beetles (Coleoptera). J Econ Entomol. 2002; 95: 183–189. doi: 10.1603/0022-0493-95.1.183 [DOI] [PubMed] [Google Scholar]
- 49.Ho SH, Koh L, Ma Y, Huang Y, Sim KY. The oil of garlic, Allium sativum L. (Amaryllidaceae), as a potential grain protectant against Tribolium castaneum (Herbst) and Sitophilus zeamais Motsch. Postharvest Biol Technol. 1996; 9: 41–48. 10.1016/0925-5214(96)00018-X. [DOI] [Google Scholar]
- 50.Mondal M, Khalequzzaman M. Ovicidal activity of essential oils against red flour beetle, Tribolium castaneum (Coleoptera: Tenebrionidae). J Bio-Sci. 2009; 17: 57–62. 10.3329/jbs.v17i0.7102. [DOI] [Google Scholar]
- 51.Gurusubramanian G, Krishna SS. The effects of exposing eggs of four cotton insect pests to volatiles of Allium sativum (Liliaceae). Bull Entomol Res. 1996; 86(1): 29–31. 10.1017/S0007485300052160. [DOI] [Google Scholar]
- 52.Huang Y, Tan JMWL, Kini RM, Ho SH. Toxic and antifeedant action of nutmeg oil against Tribolium castaneum (Herbst) and Sitophilus zeamais Motsch. J Stored Prod Res. 1997; 33: 289–298. 10.1016/S0022-474X(97)00009-X. [DOI] [Google Scholar]
- 53.Ross DC, Brown TM. Inhibition of larval growth in Spodoptera frugiperda by sublethal dietary concentrations of insecticides. J Agric Food Chem. 1982; 30(1): 193–196. 10.1021/jf00109a045. [DOI] [Google Scholar]
- 54.Chapman RF. The insects: structure and function. 4 edition, Cambridge university press. United States of America, New York, 1998. [Google Scholar]
- 55.Nathan SS, Choi MY, Seo HY, Paik CH, Kalivani K. et al. Effect of azadirachtin on acetylcholinesterase (AChE) activity and histology of the brown planthopper Nilaparvata lugens (Stål). Ecotoxicol Environ Saf. 2008; 70: 244–250. doi: 10.1016/j.ecoenv.2007.07.005 [DOI] [PubMed] [Google Scholar]
- 56.Nath BS, Suresh A, Varma BM, Kumar RPS. Changes in protein metabolism in hemolymph and fat body of the silkworm, Bombyx mori (Lepidoptera: Bombycidae) in response to organophosphorus insecticides toxicity. Ecotox Environ Safety. 1997; 36; 169–173 doi: 10.1006/eesa.1996.1504 [DOI] [PubMed] [Google Scholar]
- 57.Baskar K, Muthu C, Ignacimuthu S. Effect of pectolinaringenin, a flavonoid from Clerodendrum phlomidis L. on total protein, glutathione S- transferase and esterase activities of Earias vittella Fab. and Helicoverpa armigera Hub. Phytoparasitica. 2014; 42: 323–331. [Google Scholar]
- 58.Baskar K, Ignacimuthu S, Jayakumar M. Toxic Effects of Couroupita guianensis against Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae). Neotrop Entomol. 2015; 44; 84–91. doi: 10.1007/s13744-014-0260-7 [DOI] [PubMed] [Google Scholar]
- 59.Baskar K, Ananthi J, Ignacimuthu S. Toxic effect of Solanum xanthocarpum Sch & Wendle on Helicoverpa armigera (Hub.) and Culex quinquefasciatus (Say.). Environ Sci Pollu Res. 2018; 25; 2774–2782. [DOI] [PubMed] [Google Scholar]
- 60.Sami AJ, Bilal S, Khalid M, Nazir MT, Shakoori ARA. Comparative Study of Inhibitory Properties of Saponins (derived from Azadirachta indica) for Acetylcholinesterase of Tribolium castaneum and Apis mellifera. Pakistan J Zool. 2018; 50(2): 725–733. doi: 10.17582/journal.pjz/2018.50.2.725.733 [DOI] [Google Scholar]
- 61.Boyer S, Zhang H, Lempérière G. A review of control methods and resistance mechanisms in stored-product insects. Bull Entomol Res. 2012; 102(2): 213–229. doi: 10.1017/S0007485311000654 [DOI] [PubMed] [Google Scholar]
- 62.Lindroth RL. Adaptations of quaking aspen for defense against damage by herbivores and related environmental agents. USDA Forest Service Proceedings 2001, RMRS-P-18, 273–284. [Google Scholar]
- 63.Cai QN, Zhang QW, Cheo M. Contribution of indole alkaloids to Sitobion avenae (F.) resistance in wheat. J Appl Entomol. 2004; 128: 517–521. 10.1111/j.1439-0418.2004.00770.x [DOI] [PubMed] [Google Scholar]
- 64.Bergmeyer HU. Methods of enzymatic analysis. Methods of enzymatic analysis. 1963. [Google Scholar]
- 65.Sridhara S, Bhat JV. Alkaline and acid phosphatases of the silkworm, Bombyx mori L. J Insect Physiol. 1963; 9: 693–701. 10.1016/0022-1910(63)90012-X. [DOI] [PubMed] [Google Scholar]
- 66.Srivastava JV, Saxena SC. On the alkaline and acid phosphatase in the alimentary tract of Periplaneta americana L. J Appl Entmol Zool. 1963; 2: 85–92. doi: 10.1303/aez.2.85 [DOI] [Google Scholar]
- 67.Csikos G, Sass M. Changes of acid phosphatase content and activity in the fat body and the hemolymph of the flesh fly Neobellieria (Sarcophaga) bullata during metamorphosis. Arch Insect Biochem Physiol. 1997; 34: 369–390. . [DOI] [Google Scholar]
- 68.Ferreira C, Terra WR. Intracellular distribution of hydrolases in midgut caeca cells from an insect with emphasis on plasma membrane-bound enzymes. Comp Biochem Physiol. 1980; 66B: 467–473. 10.1016/0305-0491(80)90235-7. [DOI] [Google Scholar]
- 69.Wolfersberger MG. Enzymology of plasma membranes of insect intestinal cells. Am Zool. 1994; 24: 187–197 10.1093/icb/24.1.187. [DOI] [Google Scholar]
- 70.Nathan SS, Choi MY, Paik CH, Seo HY. Food consumption, utilization and detoxification enzyme activity of the rice leaf folder larvae after treatment with Dysoxylum triterpenes. Pestic Biochem Physiol. 2007; 88: 260–267. 10.1016/j.pestbp.2006.12.004. [DOI] [Google Scholar]
- 71.Nathan SS. Effects of Melia azedarach on nutritional physiology and enzyme activities of the rice leaf folder Cnaphalocrocis medinalis (Guenée) (Lepidoptera: Pyralidae). Pestic Biochem Physiol. 2006; 84: 98–108. 10.1016/j.pestbp.2005.05.006. [DOI] [Google Scholar]
- 72.Kranthi KR. Insecticide Resistance -Monitoring, Mechanisms and Management Manual’. Published by CICR, Nagpur, India: and ICAC, Washington, 2005. [Google Scholar]
- 73.Shojaei A, Talebi K, Sharifian I, Ahsaei SM. Evaluation of detoxifying enzymes of Tribolium castaneum and Tribolium confusum (Col.: Tenebrionidae) exposed to essential oil of Artemisia dracunculus L. Biharean Biologist 2017; 11(1): 5–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PPTX)
(PPTX)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.