Key Points
Question
Is aspirin monotherapy noninferior to enoxaparin in preventing symptomatic venous thromboembolism (VTE) within 90 days following primary total hip or knee arthroplasty performed for osteoarthritis?
Findings
In this cluster-randomized, crossover trial that included 9711 patients, treatment with aspirin vs enoxaparin resulted in symptomatic VTE (including below-knee VTE) in 3.45% vs 1.82% of patients, respectively. The difference failed to meet the noninferiority margin of 1% and was statistically significant for superiority of enoxaparin.
Meaning
In patients undergoing hip or knee arthroplasty for osteoarthritis, aspirin compared with enoxaparin resulted in a significantly higher rate of symptomatic VTE, including below-knee VTE.
Abstract
Importance
There remains a lack of randomized trials investigating aspirin monotherapy for symptomatic venous thromboembolism (VTE) prophylaxis following total hip arthroplasty (THA) or total knee arthroplasty (TKA).
Objective
To determine whether aspirin was noninferior to enoxaparin in preventing symptomatic VTE after THA or TKA.
Design, Setting, and Participants
Cluster-randomized, crossover, registry-nested trial across 31 hospitals in Australia. Clusters were hospitals performing greater than 250 THA or TKA procedures annually. Patients (aged ≥18 years) undergoing hip or knee arthroplasty procedures were enrolled at each hospital. Patients receiving preoperative anticoagulation or who had a medical contraindication to either study drug were excluded. A total of 9711 eligible patients were enrolled (5675 in the aspirin group and 4036 in the enoxaparin group) between April 20, 2019, and December 18, 2020. Final follow-up occurred on August 14, 2021.
Interventions
Hospitals were randomized to administer aspirin (100 mg/d) or enoxaparin (40 mg/d) for 35 days after THA and for 14 days after TKA. Crossover occurred after the patient enrollment target had been met for the first group. All 31 hospitals were initially randomized and 16 crossed over prior to trial cessation.
Main Outcomes and Measures
The primary outcome was symptomatic VTE within 90 days, including pulmonary embolism and deep venous thrombosis (DVT) (above or below the knee). The noninferiority margin was 1%. Six secondary outcomes are reported, including death and major bleeding within 90 days. Analyses were performed by randomization group.
Results
Enrollment was stopped after an interim analysis determined the stopping rule was met, with 9711 patients (median age, 68 years; 56.8% female) of the prespecified 15 562 enrolled (62%). Of these, 9203 (95%) completed the trial. Within 90 days of surgery, symptomatic VTE occurred in 256 patients, including pulmonary embolism (79 cases), above-knee DVT (18 cases), and below-knee DVT (174 cases). The symptomatic VTE rate in the aspirin group was 3.45% and in the enoxaparin group was 1.82% (estimated difference, 1.97%; 95% CI, 0.54%-3.41%). This failed to meet the criterion for noninferiority for aspirin and was significantly superior for enoxaparin (P = .007). Of 6 secondary outcomes, none were significantly better in the enoxaparin group compared with the aspirin group.
Conclusions and Relevance
Among patients undergoing hip or knee arthroplasty for osteoarthritis, aspirin compared with enoxaparin resulted in a significantly higher rate of symptomatic VTE within 90 days, defined as below- or above-knee DVT or pulmonary embolism. These findings may be informed by a cost-effectiveness analysis.
Trial Registration
ANZCTR Identifier: ACTRN12618001879257
This randomized clinical trial assesses the effect of postsurgery aspirin compared with enoxaparin on incidence of symptomatic venous thromboembolism within 90 days after surgery among patients undergoing hip or knee arthroplasty.
Introduction
Approximately 1.5 million hip and knee arthroplasty procedures are performed in the United States each year.1,2 Symptomatic venous thromboembolism (VTE) occurs in approximately 2% of patients following these procedures, even with strategies to prevent VTE.3,4 Due to their low cost, perceived safety, ease of administration, and evidence from observational studies, the use of aspirin-based therapies for thromboprophylaxis has increased between 2010 and 2021.5,6
However, limited evidence exists regarding the safety and efficacy of aspirin when used as a sole prophylactic agent.7 The few randomized trials have lacked sufficient statistical power or have used an alternative form of prophylaxis for the immediate postoperative period prior to aspirin prescription,4,8 which is not consistent with how aspirin is typically used to prevent VTE after hip or knee arthroplasty in many institutions. The CRISTAL randomized trial was a registry-nested study that used a cluster-randomized, crossover, noninferiority design to assess the effects of aspirin compared with low-molecular-weight heparin to prevent symptomatic VTE in patients undergoing hip or knee arthroplasty. This randomized trial aimed to determine if aspirin monotherapy was noninferior to low-molecular-weight heparin (enoxaparin) in preventing symptomatic VTE within 90 days for patients undergoing primary total hip or total knee arthroplasty for osteoarthritis. A noninferiority design was chosen, as aspirin would represent a less expensive, easier to administer alternative if shown to be noninferior to enoxaparin.
Methods
Trial Design
This cluster-randomized, crossover, noninferiority trial was performed across 31 hospitals in Australia. It was nested within the Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR). The study protocol and statistical analysis plan are available in Supplement 1 and Supplement 2, respectively.9,10 A cluster-randomized design was selected as the most efficient, pragmatic method of enrolling patients. The trial was approved by the Sydney Local Health District human research ethics committee (lead) and by all relevant ethics committees prior to commencement.
All enrolled patients provided written consent for provision and use of their data, but the ethics committee did not require specific written informed consent for use of either study drug because both drugs were commonly used for prophylaxis throughout Australia4 prior to trial commencement (Supplement 1, pp 20-27).
Trial Participants
The clusters were defined as the participating hospitals, which were eligible if they had performed more than 250 hip or knee arthroplasty procedures in the year prior to recruitment and agreed to follow the trial protocol. All adult patients (aged ≥18 years) at participating hospitals undergoing hip or knee arthroplasty procedures were eligible for inclusion. Patients ineligible to receive either study drug were those who were receiving preoperative anticoagulants (specifically, a direct oral anticoagulant, warfarin, or dual antiplatelet therapy) or those with a medical contraindication (allergy or bleeding disorder precluding anticoagulation). Preoperative single antiplatelet therapy for preexisting medical conditions was permitted in both groups and continued preoperatively and postoperatively per local hospital practice and beyond the 14- or 35-day prophylaxis period as indicated by a patient’s condition. If patients were randomized to the aspirin VTE prophylaxis group and were already taking aspirin, they did not receive a double dose of aspirin; they received aspirin according to the study dosage. If patients were randomized to enoxaparin, they received their usual aspirin dose along with enoxaparin.
The protocol was applied to all patients undergoing any hip or knee arthroplasty procedure. However, as prespecified in the protocol and statistical analysis plan, this study was restricted to patients undergoing primary total hip or total knee arthroplasty for osteoarthritis who were eligible to receive the study drug. These represent the majority of hip or knee arthroplasty procedures, and differences in patient comorbidities associated with other diagnoses (eg, fracture, tumor) could affect the risk of postoperative VTE.11
Randomization and Blinding
Each hospital was allocated to consecutive periods of a standard protocol of enoxaparin or aspirin, with the treatment order being randomized. Hospitals were randomized in permuted blocks of 4 by statisticians from the South Australian Health and Medical Research Institute (SAHMRI), independent of study investigators. The randomization sequence was generated using an online application and provided to an unblinded data manager from SAHMRI. Each hospital was randomly allocated to a treatment sequence by SAHMRI staff the week prior to enrolling patients. Hospitals were advised to cross over once the sample size for the first treatment group was met, which was monitored by registry staff.
Participating hospitals and patients were not masked to treatment allocation. Study investigators and the data and safety monitoring board (DSMB) were masked to treatment assignment during the trial and all analyses. A final manuscript with masked treatment assignment (labeled A and B) was completed and agreed to by all investigators prior to unmasking (eAppendix 5 in Supplement 3).
Interventions and Assessments
Patients in the aspirin group received aspirin, 100 mg/d, orally for 35 days after hip arthroplasty and for 14 days after knee arthroplasty, beginning within 24 hours postoperatively. Patients in the enoxaparin group received enoxaparin, 40 mg/d, subcutaneously for the same time periods, with the dose reduced to 20 mg for patients weighing less than 50 kg and for patients with an estimated glomerular filtration rate less than 30 mL/min/1.73 m2. All patients received intraoperative and postoperative intermittent pneumatic compression calf devices until mobile, received compression stockings, and were offered mobilization on day 0 or day 1 postoperatively.
Data collected by the registry at the time of surgery included age, sex, body mass index (calculated as weight in kilograms divided by height in meters squared), American Society of Anesthesiologists classification,12 type of joint replacement, indication for surgery, and implant type. Additional data were collected from patients using online data entry through the registry’s clinical trials platform and were entered by patients. Patients received a web link via email or text message to complete online data collection at 90 days postoperatively (Supplement 1, p 46). Patients who did not respond were contacted at 95, 100, and 105 days. Data collection forms were reviewed by registry staff and all patients who responded “yes” to having had a VTE, a secondary operation within 90 days or 6 months, a major bleeding event within 90 days, or a joint-related readmission within 90 days had this outcome verified through written documentation from treating physicians. These results were verified by the trial outcome verification committee. For the primary outcome, the date and type of VTE (below- or above-knee deep venous thrombosis [DVT] or pulmonary embolism) and side of DVT (left or right) were recorded. Mortality data were collected through linkage between the registry and the National Death Index.
Patients at each hospital were audited to assess inpatient drug adherence (correct drug and dose prescribed, administered once daily, given within 24 hours of surgery, and a discharge prescription supplied). The aim was to audit 20 patients from each treatment group in each hospital. A further 178 patients selected randomly were audited after discharge at the end of the drug treatment period to assess postdischarge adherence (eAppendix 6 and eAppendix 7 in Supplement 3).
Outcome Measures
The primary outcome was symptomatic VTE within 90 days of surgery. Screening tests for VTE were not performed in asymptomatic patients. Secondary outcomes were joint-related readmission, joint-related reoperation, major bleeding events (those resulting in readmission, reoperation, or death) and mortality within 90 days, joint-related reoperation within 6 months of surgery, and adherence rates as assessed by the audits. Adherence was defined as completion of all drug doses. Prespecified secondary outcomes not reported herein were changes in the Oxford Hip Score, the Oxford Knee Score, the EuroQol Visual Analog Scale score, and the EuroQol 5 Dimensions score from baseline to 6 months postoperatively.13,14
Sample Size Calculation
The sample size used an event rate of 2% and a noninferiority margin of 1% (based on the current available literature),4,8 a statistical power of 90%, and a 1-sided significance level of .025. For a cluster-randomized crossover trial with an intracluster correlation of 0.01, an interperiod correlation of 0.008, and 31 clusters, this yielded 11 160 patients (180 patients per group for each cluster).15,16 Due to uncertainty in the accuracy of the above parameters, the aim was to enroll 251 eligible patients per group from each cluster, yielding a sample size of 15 562. This allowed for a 27% loss to follow-up.
Statistical Analyses
All analyses were performed using SAS version 9.4 (SAS Institute Inc) and R version 4.1.0 (R Foundation for Statistical Computing) . Patients were analyzed according to their randomization group and no as-treated analyses were performed, as there were no verified data available (outside of the audits) to determine treatment adherence. Multiple imputation for missing data used the R package “jomo” to account for clustering through multilevel joint modeling.17 Inverse probability weighting for missing data was used as a sensitivity analysis for the primary outcome.18
The analysis for the primary outcome tested the between-group difference of patients developing a symptomatic VTE within 90 days for noninferiority at a margin of 1%. Between-group differences were estimated for the primary and secondary outcomes using cluster summary methods.19,20 The unit of analysis was considered to be the hospital, with outcomes reported at the individual level. The same statistical methods were used for the primary and secondary outcomes, for the subgroup and sensitivity analyses. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary outcomes should be interpreted as exploratory.
For the primary outcome, 95% confidence intervals were examined to determine noninferiority and if superiority could be concluded. Confidence intervals were examined for superiority for secondary outcomes and subgroup analyses.
Subgroup analyses for the primary outcome were performed according to whether a patient underwent total hip or total knee arthroplasty, underwent a bilateral or unilateral procedure, and had a history of VTE or not and whether there was preoperative use of a single antiplatelet agent or not. Sensitivity analyses were performed to determine the effect of (1) site surgical volume; (2) site enrollment rates; and (3) sites that required multiple audits on the primary outcome. The median value was used to dichotomize groups. A post hoc analysis investigating the time to VTE occurrence for each group was performed using the Kaplan-Meier method, including the log-rank P value and a number-at-risk table.
Interim Analysis
The trial was monitored by a trial management committee (eAppendix 1 in Supplement 3). No interim analysis was planned. However, due to concerns raised from 1 ethics committee regarding a serious adverse event, an independent DSMB was assembled and an interim analysis was performed. The DSMB applied the Haybittle-Peto stopping rule of a 2-sided significance level of .001 to detect a between-group superiority difference for the primary outcome from the initial trial protocol (Supplement 1), modified to account for incomplete cluster crossover.21,22 This stopping rule was chosen because it does not require adjustment of the significance threshold for subsequent analyses. After the first interim analysis (September 2020), the DSMB recommended trial continuation and a second analysis 3 months later. After the second interim analysis (December 2020), the DSMB recommended stopping patient enrollment, as the stopping rule had been met (eAppendixes 2-4 in Supplement 3). The statistical analysis plan reflected these changes and was published in July 2021, prior to the final analyses, which were carried out in September 2021.
Results
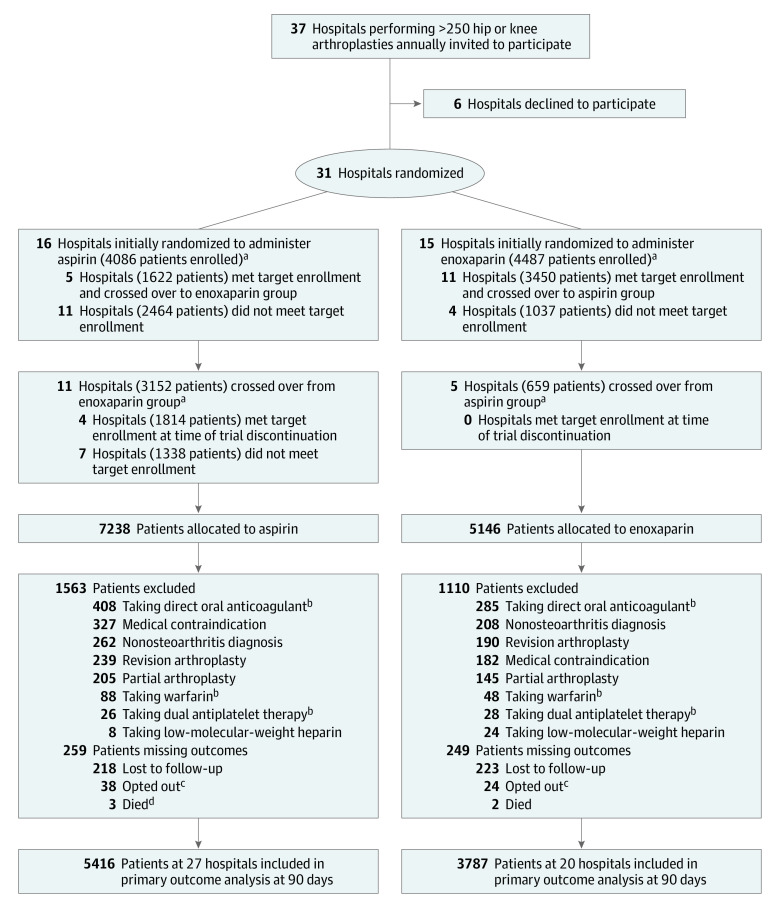
Thirty-one hospitals were randomized between April 15, 2019, and August 12, 2019 (Figure 1). There were 21 public and 10 private hospitals included, and the median number of hip and knee arthroplasty procedures at these hospitals performed in the year prior to study commencement was 580 (eTable 1 in Supplement 3). All 31 hospitals were initially randomized, 16 to administer aspirin and 15 to administer enoxaparin. Between April 15, 2019, and December 18, 2020, 16 of 31 hospitals completed enrollment for their initially assigned therapy and crossed over prior to trial cessation (11 crossed over to aspirin and 5 to enoxaparin), and the remaining 15 hospitals did not cross over.
Figure 1. Participant Flow in a Trial of Anticoagulation After Hip or Knee Arthroplasty.
aA total of 1333 patients declined to have their data collected after enrollment; therefore, group allocation was not recorded. These patients were not included in any analysis and are not included in the tallies represented here.
bPatients taking dabigatran, rivaroxaban, apixaban, edoxaban, betrixaban, warfarin, or dual antiplatelet therapy preoperatively were excluded from the primary analysis.
cPatients who opted out chose to withdraw after initially consenting and prior to 90-day follow-up.
dOne additional patient who died recorded a venous thromboembolic event prior to death and therefore was not considered to be missing an outcome.
A total of 13 717 patients were enrolled. Of these, 9711 patients underwent total hip or knee arthroplasty for osteoarthritis and were eligible to receive the study drug. In the aspirin group (n = 5675), the median age was 67 years, 56.5% were female, the median body mass index was 30.5, and 276 (5.2%) had a history of VTE (Table 1). In the enoxaparin group (n = 4036), the median age was 68 years, 57.1% were female, the median body mass index was 30.6, and 240 (6.3%) had a history of VTE. There were 9203 patients (94.7%) included in the final analysis, 5416 in the aspirin group (from 27 hospitals) and 3787 in the enoxaparin group (from 20 hospitals).
Table 1. Baseline Patient Characteristics.
| Characteristics | Aspirin (n = 5675) | Enoxaparin (n = 4036) |
|---|---|---|
| Age, median (IQR), y | 67.0 (61.0-74.0) | 68.0 (61.0-74.0) |
| Body mass index, median (IQR)a | 30.5 (26.9-35.1) | 30.6 (26.9-34.9) |
| Sex, No. (%) | ||
| Female | 3208 (56.5) | 2303 (57.1) |
| Male | 2467 (43.5) | 1733 (42.9) |
| American Society of Anesthesiologists classification, No. (%)b | ||
| I | 315 (5.6) | 201 (5.0) |
| II | 3219 (56.9) | 2221 (55.1) |
| III | 2074 (36.7) | 1582 (39.2) |
| IV | 47 (0.8) | 29 (0.7) |
| Previous deep venous thrombosis or pulmonary embolism, No. (%) | 276 (5.2) | 240 (6.3) |
| Preoperative antiplatelet therapy, No. (%) | ||
| Aspirin | 817 (15.4) | 584 (15.2) |
| Other single antiplatelet agent | 67 (1.3) | 50 (1.3) |
| Other agent (unspecified) | 197 (3.7) | 133 (3.5) |
| Joint replacement, No. (%) | ||
| Unilateral total knee arthroplasty | 2973 (52.4) | 2113 (52.4) |
| Unilateral total hip arthroplasty | 2066 (36.4) | 1494 (37.0) |
| Bilateral total knee arthroplasty | 547 (9.6) | 385 (9.5) |
| Bilateral total hip arthroplasty | 89 (1.6) | 44 (1.1) |
| Prosthesis, No. (%) | ||
| Cemented | 2781 (49.0) | 2067 (51.2) |
| Hybrid | 1454 (25.6) | 1088 (27.0) |
| Uncemented | 1440 (25.4) | 881 (21.8) |
Calculated as weight in kilograms divided by height in meters squared.
As determined by anesthesiologist on the day of surgery. This classification assesses and communicates a patient’s preanesthesia medical comorbidities and ranges from I to VI, with I being healthy and VI being brain death.
The median patient enrollment rate for all hospitals was 66%. Baseline characteristics by treatment group were similar for enrolled and nonenrolled patients (eTable 2 in Supplement 3).
Primary Outcome
Imputation for missing data was performed, but there was no difference between the imputed and complete case analyses, so complete case analyses are reported (eTables 4-6 in Supplement 3). Symptomatic VTE within 90 days occurred in 187 of 5416 patients (3.45%) in the aspirin group and in 69 of 3787 patients (1.82%) in the enoxaparin group (estimated difference, 1.97%; 95% CI, 0.54%-3.41%) (Table 2). This failed to meet the criterion for noninferiority for aspirin and was significantly superior for enoxaparin (P = .007) (eFigure in Supplement 3).
Table 2. Primary and Secondary Outcomes for Patients Undergoing Primary Total Hip or Knee Arthroplasty for a Diagnosis of Osteoarthritis Who Were Eligible to Receive the Study Drug.
| Outcomes | No./total (%) | Estimated treatment difference, % (95% CI)a | P valueb | |
|---|---|---|---|---|
| Aspirin (n = 5416) | Enoxaparin (n = 3787) | |||
| Primary outcome | ||||
| Any symptomatic venous thromboembolism within 90 d | 187/5416 (3.5) | 69/3787 (1.8) | 1.97 (0.54 to 3.41)c | .007 |
| Components of primary outcomed | ||||
| Pulmonary embolism within 90 d | 58/5416 (1.1) | 21/3787 (0.6) | 0.44 (−0.19 to 1.08) | .17 |
| Any deep venous thrombosis within 90 d | 140/5416 (2.6) | 50/3787 (1.3) | 1.61 (0.54 to 2.68) | .003 |
| Both pulmonary embolism and deep venous thrombosis within 90 d | 11/5416 (0.2) | 2/3787 (0.1) | 0.10 (−0.10 to −0.30) | .32 |
| Above-knee deep venous thrombosis within 90 de | 12/5415 (0.2) | 6/3787 (0.2) | 0.06 (−0.11 to 0.23) | .49 |
| Below-knee deep venous thrombosis within 90 d | 129/5415 (2.4) | 45/3787 (1.2) | 1.49 (0.48 to 2.50) | .004 |
| Secondary outcomes | ||||
| Death within 90 df | 4/5675 (0.1) | 2/4036 (0.1) | 0.05 (−0.05 to 0.15) | .36 |
| Major bleeding within 90 d | 17/5401 (0.3) | 15/3779 (0.4) | −0.05 (−0.35 to 0.25) | .75 |
| Readmission within 90 d | 130/5403 (2.4) | 85/3782 (2.3) | 0.6 (−0.19 to 1.39) | .13 |
| Reoperation within 90 d | 116/5412 (2.1) | 73/3787 (1.9) | 0.67 (−0.12 to 1.46) | .10 |
| Reoperation within 6 mo | 175/5086 (3.4) | 120/3535 (3.4) | 0.16 (−0.82 to 1.14) | .75 |
| Drug adherence | 521/614 (85) | 491/569 (86) | −0.99 (−0.91 to 1.08) | .85 |
Risk differences were calculated based on unadjusted cluster summaries.
All P values are for superiority.
The upper limit of the 95% CI (3.41%) was above the noninferiority margin of 1%; therefore, noninferiority of aspirin was not achieved.
Below-knee deep venous thrombosis was any thrombus distal to the popliteal vein. Above-knee deep venous thrombosis was any thrombus proximal to the popliteal vein. Pulmonary embolism was any thrombus in the pulmonary arterial tree.
One patient in the aspirin group with a verified deep venous thrombosis did not have a location (above or below the knee) specified.
Mortality data for those lost to follow-up or who opted out were determined through the National Death Index.
Secondary Outcomes
Death within 90 days occurred in 4 of 5675 patients (0.07%) in the aspirin group and 2 of 4036 patients (0.05%) in the enoxaparin group (estimated difference, 0.05%; 95% CI, −0.05% to 0.15%) (Table 2). Major bleeding events occurred in 17 of 5401 patients (0.31%) in the aspirin group and in 15 of 3779 patients (0.40%) in the enoxaparin group (estimated difference, −0.05%; 95% CI, −0.25% to 0.04%). Joint-related reoperation within 90 days occurred in 116 of 5412 patients (2.1%) in the aspirin group and in 73 of 3787 patients (1.9%) in the enoxaparin group, and within 6 months, in 175 of 5086 patients (3.4%) in the aspirin group and in 120 of 3535 patients (3.4%) in the enoxaparin group. Joint-related readmission within 90 days occurred in 130 of 5403 patients (2.4%) in the aspirin group and in 85 of 3782 patients (2.3%) in the enoxaparin group.
There were 1005 patients audited for inpatient drug adherence (543 in the aspirin group and 462 in the enoxaparin group) and 178 patients audited for postdischarge drug adherence (71 in the aspirin group and 107 in the enoxaparin group). Overall adherence rates were 85% (521 of 614 patients) for the aspirin group and 86% (491 of 569 patients) for the enoxaparin group. The mean numbers of postdischarge drug doses missed were 2.5 and 3.4 in the aspirin and enoxaparin groups, respectively.
Subgroup and Sensitivity Analyses
Subgroup analyses for the primary outcome (Figure 2) demonstrated a lower symptomatic VTE rate for the enoxaparin group compared with the aspirin group in patients undergoing total hip arthroplasty (estimated difference, 1.9%; 95% CI, 0.0%-3.9%), undergoing total knee arthroplasty (estimated difference, 2.1%; 95% CI, 0.6%-3.5%), with a history of VTE (estimated difference, 6.0%; 95% CI, 0.4%-11.5%), and without a history of VTE (estimated difference, 1.7%; 95% CI, 0.4%-3.0%).
Figure 2. Subgroup Analyses of the Primary Outcome of Symptomatic Venous Thromboembolism (Deep Venous Thrombosis or Pulmonary Embolism) Within 90 Days.
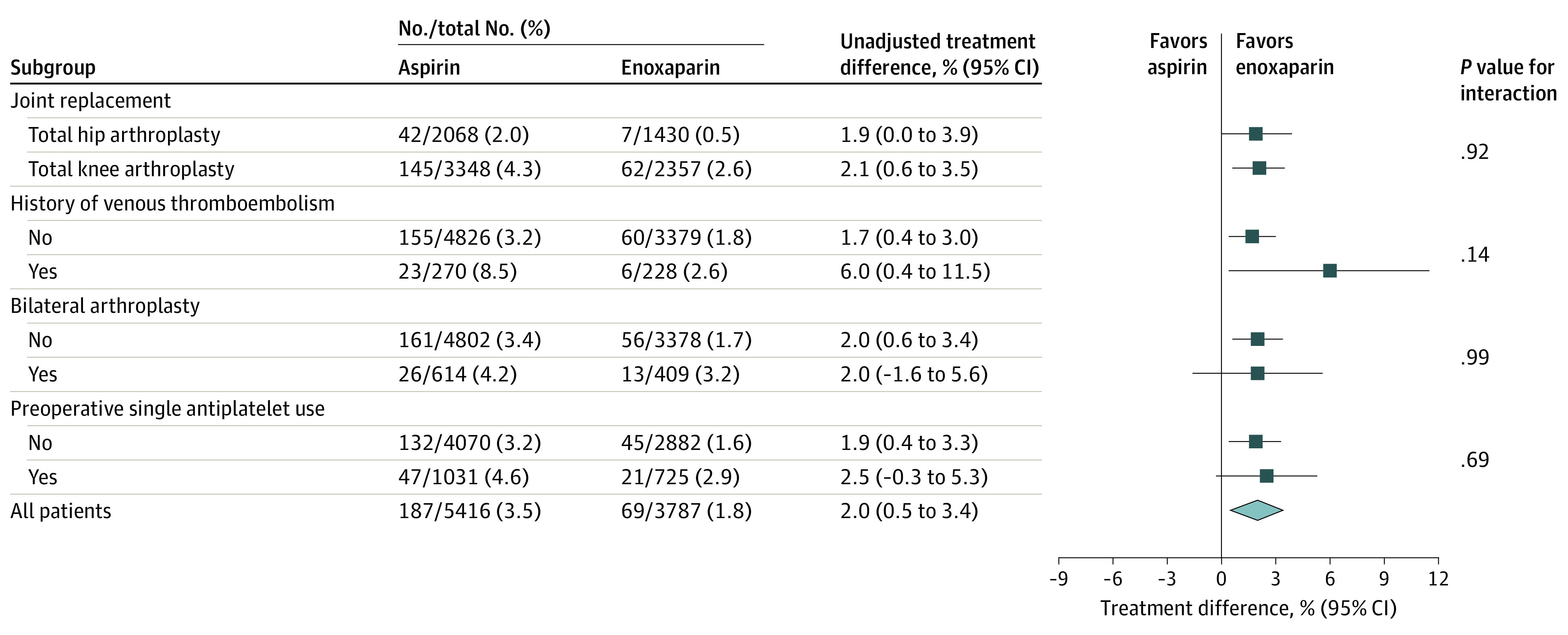
Sensitivity analyses for the primary outcome (eTable 3 in Supplement 3) demonstrated lower symptomatic VTE rates in the enoxaparin group compared with the aspirin group, despite between-cluster differences in surgical volume, enrollment rate, and number of adherence audits. These differences were significant for higher-volume sites (1.61%; 95% CI, 0.23%-2.99%), sites with higher enrollment rates (2.39%; 95% CI, 0.54%-4.23%), and sites that did not require multiple adherence audits (2.11%; 95% CI, 0.07%-4.15%).
Post Hoc Outcomes
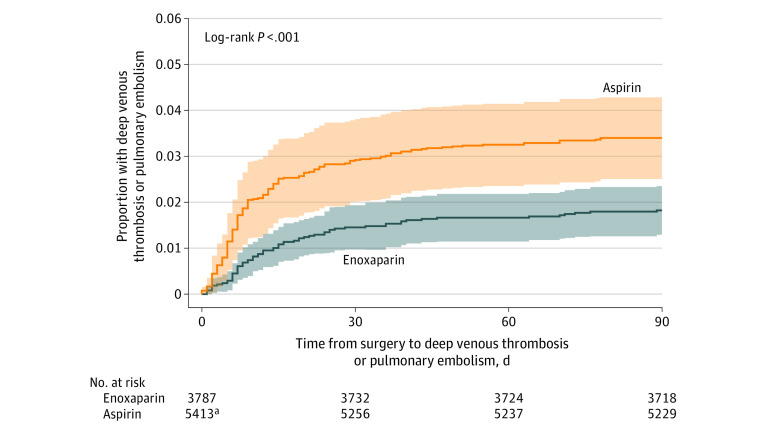
The median time to diagnosis of symptomatic VTE was 7.5 days (IQR, 5-19 days) in the aspirin group and 12 days (IQR, 7-25 days) in the enoxaparin group (Figure 3).
Figure 3. Kaplan-Meier Analysis of Time to Venous Thromboembolism (Deep Venous Thrombosis or Pulmonary Embolism) Occurrence by Randomized Group.
Shaded areas indicate 95% confidence intervals. Median time to venous thromboembolism in the aspirin group was 7.5 days (IQR, 5-9 days); median time to venous thromboembolism in the enoxaparin group was 12 days (IQR, 7-25 days).
aThree patients in the aspirin group who experienced a venous thromboembolism did not have a date of event recorded and are excluded from this analysis.
Discussion
In this cluster-randomized crossover trial, enoxaparin was more effective than aspirin for preventing symptomatic VTE within 90 days following primary total hip and knee arthroplasty for osteoarthritis. Although the study was designed as a noninferiority trial, the results demonstrated statistical superiority of enoxaparin compared with aspirin.
In contrast to results reported herein, a recent randomized clinical trial of 3424 participants undergoing total hip or knee arthroplasty reported that aspirin was noninferior to rivaroxaban for preventing VTE after surgery. However, both groups received rivaroxaban for 5 days prior to randomization to either aspirin or continued rivaroxaban.4
Guidelines from a recent international consensus meeting on VTE prophylaxis provided a “strong” recommendation for use of aspirin as prophylaxis; however, this was based on a network meta-analysis that primarily included observational and retrospective studies and did not distinguish between symptomatic and asymptomatic VTE.23 The rate of aspirin use for VTE prophylaxis following primary total hip or knee arthroplasty has increased in the United States from 2012 to 2021.5
In interpreting the study findings, it is important to note that most of the difference in rates of VTE events between the enoxaparin and aspirin groups was due to differences in rates of below-knee DVT. Below-knee DVT represents a less clinically important form of VTE compared with above-knee DVT or pulmonary embolism, and the clinical importance of these findings remains uncertain.
Limitations
This study has several limitations. First, the loss to follow-up was 5.2%, and outcomes may have been missed. Second, a low patient enrollment rate was noted for some participating hospitals, raising the possibility that some hospitals enrolled patients in a selective manner, for example based on a patient’s risk of VTE. However, the sensitivity analyses demonstrated consistent results despite variations in enrollment rates, and the baseline characteristics of enrolled and nonenrolled patients by treatment allocation were similar. Third, hospitals were not blinded to treatment allocation, which may have prompted an increased rate of diagnostic testing after crossover to aspirin. However, rates of diagnostic testing were not recorded. Fourth, despite a higher rate of pulmonary embolism in the aspirin group, the study may not have had adequate statistical power to detect a significant between-group difference for this outcome. Fifth, the early cessation of the trial led to less precise results and confidence intervals, and the results of other outcomes may have differed if the trial had not been stopped early. Sixth, data on race and ethnicity were not collected. Seventh, the trial was limited to patients who were undergoing knee or hip arthroplasty for osteoarthritis. Although most patients undergoing total hip or knee arthroplasty have osteoarthritis,11 the results may not be generalizable to people undergoing knee or hip arthroplasty for other reasons. Eighth, among patients already taking aspirin at the start of the trial, those randomized to aspirin did not take additional aspirin, while those randomized to enoxaparin continued aspirin in addition to enoxaparin. This characteristic of the trial may have influenced the finding of superiority for enoxaparin. However, only approximately 15% of participants were taking aspirin at the start of the trial. Ninth, while the findings of superiority for enoxaparin met statistical significance, a cost-effectiveness analysis is needed to better understand the clinical relevance of the trial results.
Conclusions
Among patients undergoing hip or knee arthroplasty for osteoarthritis, aspirin compared with enoxaparin resulted in a significantly higher rate of symptomatic VTE within 90 days, defined as below- or above-knee DVT or pulmonary embolism. These findings may be informed by a cost-effectiveness analysis.
Trial Protocol
Statistical Analysis Plan
eAppendix 1. Collaborators and Participating Institutions
eAppendix 2. Letter Recommending Early Trial Cessation
eAppendix 3. Results of Second Interim Analysis
eAppendix 4. Second Interim Analysis – Update on Patients With a Reported History of VTE
eAppendix 5. Minutes From Meeting to Unblind Investigators
eAppendix 6. Audit Process for Inpatient Adherence
eAppendix 7. Audit Process for Post-discharge Adherence
eAppendix 8. Audit Process for “Absence” of VTE Audit
eFigure. Non-inferiority Diagram for Primary Outcome
eTable 1. Baseline Features of Participating Clusters
eTable 2. Comparison of Enrolled and Non-enrolled Patients
eTable 3. Sensitivity Analyses
eTable 4. Comparison of Complete Case and Imputed Analyses for Primary Outcome
eTable 5. Comparison of Complete Case and Imputed Analyses for Secondary Outcomes
eTable 6. Comparison of Complete Case and Imputed Analyses for Subgroup Analyses
Data Sharing Statement
References
- 1.Sloan M, Premkumar A, Sheth NP. Projected volume of primary total joint arthroplasty in the US, 2014 to 2030. J Bone Joint Surg Am. 2018;100(17):1455-1460. doi: 10.2106/JBJS.17.01617 [DOI] [PubMed] [Google Scholar]
- 2.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi: 10.2106/00004623-200704000-00012 [DOI] [PubMed] [Google Scholar]
- 3.An VV, Phan K, Levy YD, Bruce WJ. Aspirin as thromboprophylaxis in hip and knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty. 2016;31(11):2608-2616. doi: 10.1016/j.arth.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 4.Anderson DR, Dunbar M, Murnaghan J, et al. Aspirin or rivaroxaban for VTE prophylaxis after hip or knee arthroplasty. N Engl J Med. 2018;378(8):699-707. doi: 10.1056/NEJMoa1712746 [DOI] [PubMed] [Google Scholar]
- 5.Abdel MP, Meneghini RM, Berry DJ. Current practice trends in primary hip and knee arthroplasties among members of the American Association of Hip and Knee Surgeons: an update during the COVID-19 pandemic. J Arthroplasty. 2021;36(7S):S40-S44. doi: 10.1016/j.arth.2021.01.080 [DOI] [PubMed] [Google Scholar]
- 6.Mirkazemi C, Bereznicki LR, Peterson GM. Comparing Australian orthopaedic surgeons’ reported use of thromboprophylaxis following arthroplasty in 2012 and 2017. BMC Musculoskelet Disord. 2019;20(1):57. doi: 10.1186/s12891-019-2409-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farey JE, An VVG, Sidhu V, Karunaratne S, Harris IA. Aspirin versus enoxaparin for the initial prevention of venous thromboembolism following elective arthroplasty of the hip or knee: a systematic review and meta-analysis. Orthop Traumatol Surg Res. 2021;107(1):102606. doi: 10.1016/j.otsr.2020.04.002 [DOI] [PubMed] [Google Scholar]
- 8.Anderson DR, Dunbar MJ, Kahn SR. Aspirin versus low-molecular-weight heparin after total hip arthroplasty. Ann Intern Med. 2013;159(7):502-503. doi: 10.7326/0003-4819-159-7-201310010-00018 [DOI] [PubMed] [Google Scholar]
- 9.Sidhu VS, Graves SE, Buchbinder R, et al. CRISTAL: protocol for a cluster randomised, crossover, non-inferiority trial of aspirin compared to low molecular weight heparin for venous thromboembolism prophylaxis in hip or knee arthroplasty, a registry nested study. BMJ Open. 2019;9(11):e031657. doi: 10.1136/bmjopen-2019-031657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sidhu VS, Kelly TL, Pratt N, et al. CRISTAL (a cluster-randomised, crossover, non-inferiority trial of aspirin compared to low molecular weight heparin for venous thromboembolism prophylaxis in hip or knee arthroplasty, a registry nested study): statistical analysis plan. Trials. 2021;22(1):564. doi: 10.1186/s13063-021-05486-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Australian Orthopaedic Association National Joint Replacement Registry . Annual Report 2021: Hip, Knee & Shoulder Arthroplasty Annual Report. Accessed October 10, 2021. https://aoanjrr.sahmri.com/annual-reports-2021
- 12.American Society of Anesthesiologists . ASA Physical Status Classification System. Accessed October 10, 2021. https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system
- 13.Murray DW, Fitzpatrick R, Rogers K, et al. The use of the Oxford Hip and Knee Scores. J Bone Joint Surg Br. 2007;89(8):1010-1014. doi: 10.1302/0301-620X.89B8.19424 [DOI] [PubMed] [Google Scholar]
- 14.EuroQol Group . EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199-208. doi: 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 15.Kelly T-L, Pratt N. A note on sample size calculations for cluster randomised crossover trials with a fixed number of clusters. Stat Med. 2019;38(18):3342-3345. doi: 10.1002/sim.8191 [DOI] [PubMed] [Google Scholar]
- 16.Giraudeau B, Ravaud P, Donner A. Sample size calculation for cluster randomized cross-over trials. Stat Med. 2008;27(27):5578-5585. doi: 10.1002/sim.3383 [DOI] [PubMed] [Google Scholar]
- 17.Carpenter J, Kenward M. Multiple Imputation and its Application. John Wiley & Sons; 2013. doi: 10.1002/9781119942283 [DOI] [Google Scholar]
- 18.Hughes RA, Heron J, Sterne JAC, Tilling K. Accounting for missing data in statistical analyses: multiple imputation is not always the answer. Int J Epidemiol. 2019;48(4):1294-1304. doi: 10.1093/ije/dyz032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forbes AB, Akram M, Pilcher D, Cooper J, Bellomo R. Cluster randomised crossover trials with binary data and unbalanced cluster sizes: application to studies of near-universal interventions in intensive care. Clin Trials. 2015;12(1):34-44. doi: 10.1177/1740774514559610 [DOI] [PubMed] [Google Scholar]
- 20.Turner RM, White IR, Croudace T; PIP Study Group . Analysis of cluster randomized cross-over trial data: a comparison of methods. Stat Med. 2007;26(2):274-289. doi: 10.1002/sim.2537 [DOI] [PubMed] [Google Scholar]
- 21.Haybittle JL. Repeated assessment of results in clinical trials of cancer treatment. Br J Radiol. 1971;44(526):793-797. doi: 10.1259/0007-1285-44-526-793 [DOI] [PubMed] [Google Scholar]
- 22.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient, I: introduction and design. Br J Cancer. 1976;34(6):585-612. doi: 10.1038/bjc.1976.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The ICM-VTE Hip & Knee Delegates . Recommendations from the ICM-VTE: hip & knee. J Bone Joint Surg Am. 2022;104(suppl 1):180-231. doi: 10.2106/JBJS.21.01529 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eAppendix 1. Collaborators and Participating Institutions
eAppendix 2. Letter Recommending Early Trial Cessation
eAppendix 3. Results of Second Interim Analysis
eAppendix 4. Second Interim Analysis – Update on Patients With a Reported History of VTE
eAppendix 5. Minutes From Meeting to Unblind Investigators
eAppendix 6. Audit Process for Inpatient Adherence
eAppendix 7. Audit Process for Post-discharge Adherence
eAppendix 8. Audit Process for “Absence” of VTE Audit
eFigure. Non-inferiority Diagram for Primary Outcome
eTable 1. Baseline Features of Participating Clusters
eTable 2. Comparison of Enrolled and Non-enrolled Patients
eTable 3. Sensitivity Analyses
eTable 4. Comparison of Complete Case and Imputed Analyses for Primary Outcome
eTable 5. Comparison of Complete Case and Imputed Analyses for Secondary Outcomes
eTable 6. Comparison of Complete Case and Imputed Analyses for Subgroup Analyses
Data Sharing Statement