Abstract
The merger of photoredox and transition-metal catalysis has evolved as a robust platform in organic synthesis over the past decade. The stereoselective 1,4-functionalization of 1,3-enynes, a prevalent synthon in synthetic chemistry, could afford valuable chiral allene derivatives. However, tremendous efforts have been focused on the ionic reaction pathway. The radical-involved asymmetric 1,4-functionalization of 1,3-enynes remains a prominent challenge. Herein, we describe the asymmetric three-component 1,4-dialkylation of 1,3-enynes via dual photoredox and chromium catalysis to provide chiral allenols. This method features readily available starting materials, broad substrate scope, good functional group compatibility, high regioselectivity, and simultaneous control of axial and central chiralities. Mechanistic studies suggest that this reaction proceeds through a radical-involved redox-neutral pathway.
Subject terms: Synthetic chemistry methodology, Asymmetric catalysis, Photocatalysis
In contrast to the better-studied ionic pathway, radical-involved asymmetric 1,4-functionalization of 1,3-enynes remains a prominent challenge. Here, the authors describe the asymmetric three-component 1,4-dialkylation of 1,3-enynes via dual photoredox and chromium catalysis to provide chiral allenols.
Introduction
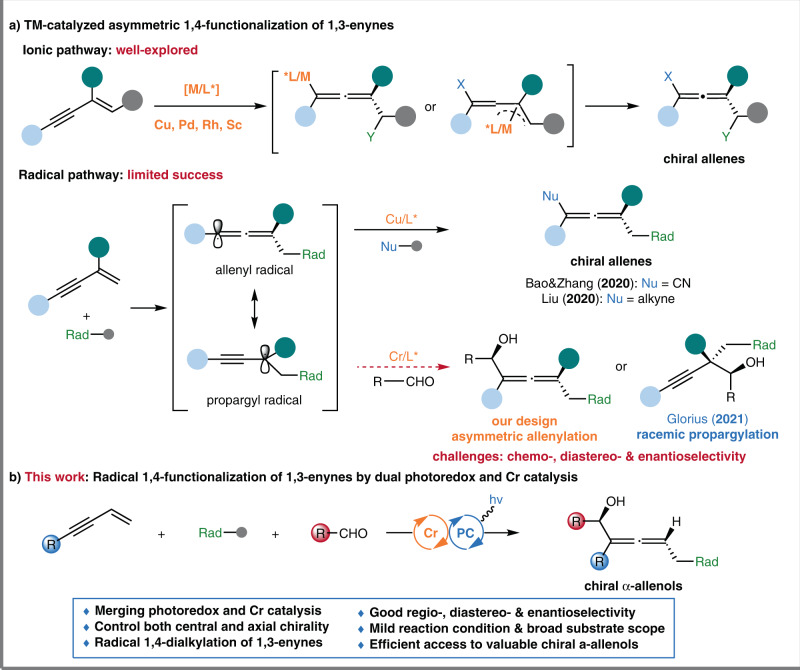
1,3-Enynes serve as a class of fundamental building blocks with diverse reactivity patterns, including 1,2-, 3,4-, and 1,4-functionalization1–4. Particularly, the asymmetric 1,4-functionalization of 1,3-enynes provides quick access to chiral allenes, which not only widely occur in natural products and pharmaceuticals5,6 but also represent one of the most versatile building blocks for the synthesis of complex molecules7,8. Various transition-metal complexes (TM = Pd, Cu, Rh, Sc, etc.) have proved to be able to achieve the asymmetric 1,4-functionalization of 1,3-enynes, involving hydrosilylation9,10, hydroborylation11, hydroamination12, hydrocarbonization13–15, dicarbonization16, etc.17–19. These transformations generally proceeded via an ionic pathway with allenyl or homoallenyl metal intermediates and mainly formed only one axial chirality (Fig. 1a)3. On the other hand, the radical 1,4-functionalization of 1,3-enynes via allenyl or propargylic radicals has attracted much attention recently20–29, but only limited success has been achieved in their asymmetric versions. In 2020, the Bao and Zhang groups30, and Liu group31 independently reported the elegant Cu-catalyzed enantioselective synthesis of chiral allenes via the radical 1,4-dicarbonization of 1,3-enynes. Compared to the ionic pathway, these radical reactions could proceed under mild conditions and afford densely functionalized complexes via a multicomponent manner, which expanded the chemical space for the functionalization of 1,3-enynes. Thus, further exploration of new reaction patterns involving radicals could facilitate efficient access to valuable chiral allenes.
Fig. 1. Catalytic asymmetric 1,4-functionalization of 1,3-enynes.
a Transition-metal catalyzed asymmetric 1,4-functionalization of 1,3-enynes. b This work: radical 1,4-functionalization of 1,3-enynes by dual photoredox and chromium catalysis. Rad alkyl radical precursors.
The Nozaki–Hiyama–Kishi reaction32 is one of the most reliable C–C bond construction approaches with various applications in synthesis chemistry33–36. However, conventional NHK reactions are generally limited to reductive processes, and stoichiometric amounts of metal reductants and strong Lewis acids (e.g., chlorosilanes and Schwartz’s reagent) must be employed to turn over the chromium catalytic cycle33. Recent breakthroughs in dual photoredox and chromium catalysis37–42 have enabled redox-neutral NHK reactions43–46. However, the photocatalytic transformations are limited to asymmetric allylations, reported by the Glorius group47, Kanai group48,49. To the best of our knowledge, the asymmetric radical 1,4-functionalization of 1,3-enynes via merging photoredox and Cr catalysis remains underdeveloped.
As our ongoing efforts in Cr-catalyzed radical-involved reactions50, we anticipate that the propargyl radical, which is in equilibrium with the allenyl radical, could be captured by a chiral chromium complex, and subsequent nucleophilic addition to the aldehyde affords the enantioenriched products (Fig. 1a, bottom). To achieve this goal, several challenges have to be addressed: (1) the regioselectivity control of 1,4-functionalization versus 1,2-functionalization; (2) the proper choice of radical precursors and photocatalysts to maintain the catalytic cycle; (3) the inhibition of quickly occurring side reactions from reactive radical intermediates or organochromium complexes.
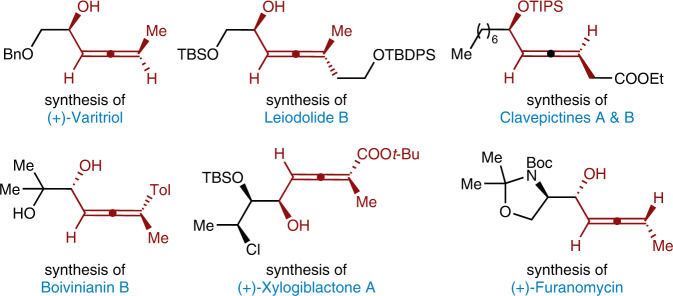
Herein, we describe the three-component asymmetric radical 1,4-functionalization of 1,3-enynes by merging photoredox and chromium catalysis (Fig. 1b). This reaction proceeds efficiently in a redox-neutral manner without an external reductant. And two C–C bonds are simultaneously constructed to provide chiral α-allenols with both a stereogenic center and a stereogenic axis, which serve as essential building blocks in total synthesis (Fig. 2) 51. Furthermore, the application of versatile and readily accessible materials, including 1,3-enyne, aldehyde, radical precursors, endow the reaction with significant advantages in practical utility.
Fig. 2. The importance of chiral allenols.
Bn benzyl, TBS tert-butyldimethylsilyl, Me methyl, TIPS triisopropylsilyl, Et ethyl, Tol p-methylphenyl, t-Bu tert-butyl, Boc tert-butoxylcarbonyl.
Results
Reaction optimization
With the idea in mind, we initially explored the three-component reaction of benzaldehyde, 1,3-enyne 1, and DHP ester 2 (Table 1). After detailed investigations of a series of reaction parameters, we determined that the merger of a chiral chromium/cyano-bisoxazoline ((S,R)-L1) and a photocatalyst 4-CzIPN could achieve the chemoselective allenylation reaction in good yield and high diastereoselectivity and enantioselectivity under visible-light irradiation (entry 1). Control experiments establish that CrCl2, 4-CzIPN, and light are critical for this allenylation reaction under these conditions (entries 2–4). A slight decrease in enantioselectivity was detected when using a similar anionic ligand L2 (entry 5). Other chiral nitrogen-containing ligands are not effective for this reaction under similar conditions (entries 6–10). In the case of L6, the homopropargylic alcohol was isolated in 1:3 ratio vs the allenol (entry 9). The reaction also performed well in DME, CH3CN, or EtOAc, furnishing the desired chiral allenol only with a slight decrease in yield and dr (entries 11–13). The photocatalyst [Ir(dF(CF3)ppy)2(dtbpy)]PF6 also led to the allenol but with a slight erosion in d.r. and ee (entry 14). Decreasing the catalyst loading to 5 mol% CrCl2 and 6 mol% (S,R)-L1 led to a drop in yield (entry 15). When increasing the concentration from 0.05 M to 0.1 M, the d.r. decreased from 20:1 to 12:1 (entry 16). And the yield or dr of the allenylation product was only modestly diminished, if 1.2 equivalent of 1,3-enyne 1 and DHP ester 2 are used (entries 17&18). However, adding 1.0 equivalent water to the reaction mixture inhibits the formation of α-allenol 3 (entry 19). The addition of 1.0 mL air to the reaction vessel has a deleterious effect (entry 20). These results indicated that the reaction was sensitive to moisture and air, probably due to the involvement of unstable alkyl chromium complexes.
Table 1.
Effect of reaction parameters
| Entry | Variation from “standard conditions” | Yielda [%] | drb | eec [%] |
|---|---|---|---|---|
| 1 | None | >95 | 20:1 | 94 |
| 2 | Without CrCl2 | <2 | – | – |
| 3 | Without 4-CzIPN | <2 | – | – |
| 4 | Without blue LED | <2 | – | – |
| 5 | L2, instead of (S,R)-L1 | >95 | 20:1 | 92 |
| 6 | L3, instead of (S,R)-L1 | 84 | 3.1:1 | 47 |
| 7 | L4, instead of (S,R)-L1 | 68 | 1.6:1 | 19 |
| 8 | L5, instead of (S,R)-L1 | 52 | 5.5:1 | 34 |
| 9d | L6, instead of (S,R)-L1 | 71 | 1.8:1 | 46 |
| 10 | L7, instead of (S,R)-L1 | 79 | 2.8:1 | 93 |
| 11 | DME, instead of THF | 72 | 10:1 | 94 |
| 12 | MeCN, instead of THF | 85 | 20:1 | 95 |
| 13 | EtOAc, instead of THF | >95 | 20:1 | 94 |
| 14 | [Ir(dF(CF3)ppy)2(dtbpy)]PF6 instead of 4-CzIPN | >95 | 11:1 | 88 |
| 15 | 5 mol% CrCl2, 6 mol% (S,R)-L1 | 74 | 20:1 | 94 |
| 16 | 0.1 M, instead of 0.05 M, in THF | >95 | 12:1 | 93 |
| 17 | 1.2, instead of 1.5, equiv 1 and 2 | 80 | 20:1 | 94 |
| 18 | 1.2, instead of 1.5, equiv 1 | 90 | 18:1 | 93 |
| 19 | 1.0 equiv H2O was added | <2 | – | – |
| 20 | 1 mL air (added via syringe) | 55 | 14:1 | 87 |
aYields were determined via 1H NMR analysis with 1,3,5-trimethoxybenzene as the internal standard.
bDrs were determined via 1H NMR analysis of the crude product.
cEe was determined via HPLC analysis.
dHomopropargylic alcohol by-product was observed in 1:3 ratio vs the allenol 3.
Substrate scope
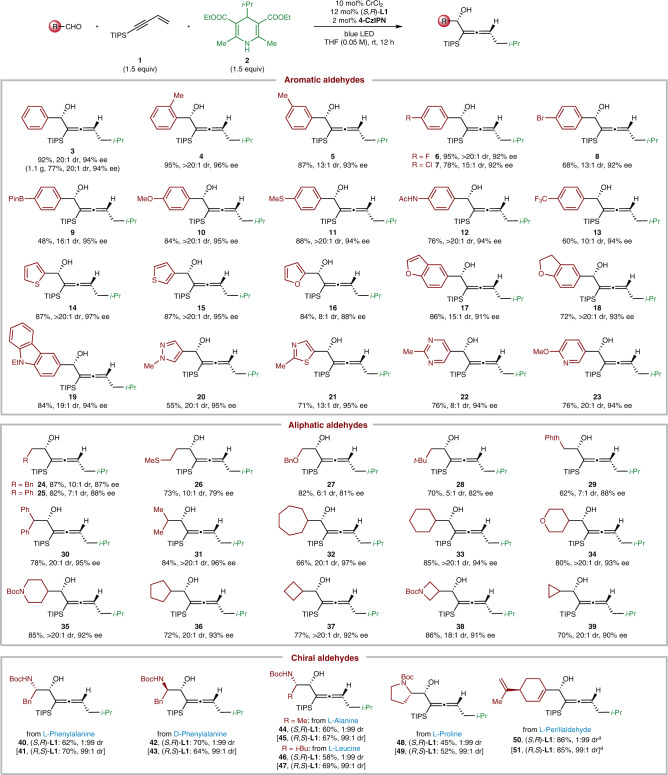
We next explored the aldehyde scope (Fig. 3). Gratifyingly, a broad array of aromatic and aliphatic aldehydes serve as effective reaction partners, affording the chiral α-allenols in high yields, good diastereoselectivities, and enantioselectivities (Fig. 3, 3–51). On a gram scale (1.10 g product), the 1,4-functionalization of 1,3-enyne to product 3 proceeded in 77% yield and 94% ee. A variety of functional groups are compatible with this method, including an aryl halide (e.g., fluoride, chloride, bromide), boronate, methoxy, thioether, amide, carboxylate ester, CF3, furan, thiophene, benzofuran and N-alkylated carbazole (Fig. 3, 6–19). To our delight, heteroaromatic aldehydes, with N, O, or S in the aromatic ring, could react well with the 1,3-enyne and DHP ester under the optimal condition, affording the enantioenriched products efficiently (Fig. 3, 14–23). Notably, N-heteroaromatic rings are widespread in pharmaceuticals and natural products52. However, the reactivity of N-heteroaromatic aldehydes is rarely demonstrated in previous NHK reactions. As disclosed in recent studies, they generally led to poor yields, including our stereoconvergent allenylation reaction (20–23)50,53–55. Aliphatic aldehydes, substituted with diverse primary or secondary alkyl chains, participated efficiently in this 1,4-functionalization of 1,3-enynes (24–39). However, moderate diastereoselectivities (5:1 dr to 10:1 dr) were generally observed in the cases of primary aliphatic aldehydes (24–29), probably resulting from the reduced steric hindrance in comparison with secondary alkyl aldehydes (30–39).
Fig. 3. The scope of aldehydes in the 1,4-functionalization of 1,3-enynes.
a2.0 equiv of DHP ester and 2.0 equiv of 1,3-enyne were used. i-Pr isopropyl, Bpin boronic acid pinacol ester, Ac acetyl.
Naturally occurring α-amino acids are readily available and act as prevalent feedstocks in asymmetric synthesis56. We were delighted to find that the chiral α-amino aldehydes, derived from natural amino acids, served as effective substrates under the standard condition for synthesizing chiral amino alcohols with continuous two stereogenic centers and one chiral axis (Fig. 3, 40–49). As indicated by the single-crystal structure for products 12 and 42 (see Supplementary Information), the chiral chromium catalyst, rather than existing stereocentres on the chiral aldehydes, predominantly determines the stereochemistry of the allenylation products 40–49. It is noteworthy that chiral amino alcohols are prevalent synthons in pharmaceuticals and asymmetric catalysis57. Finally, the reactivity of α,β-unsaturated aldehydes was tested, and the desired chiral α-allenols were obtained in high yields and diastereoselectivities after increasing the equivalents of 1,3-enyne 1 and DHP ester 2 (Fig. 3, 50 and 51).
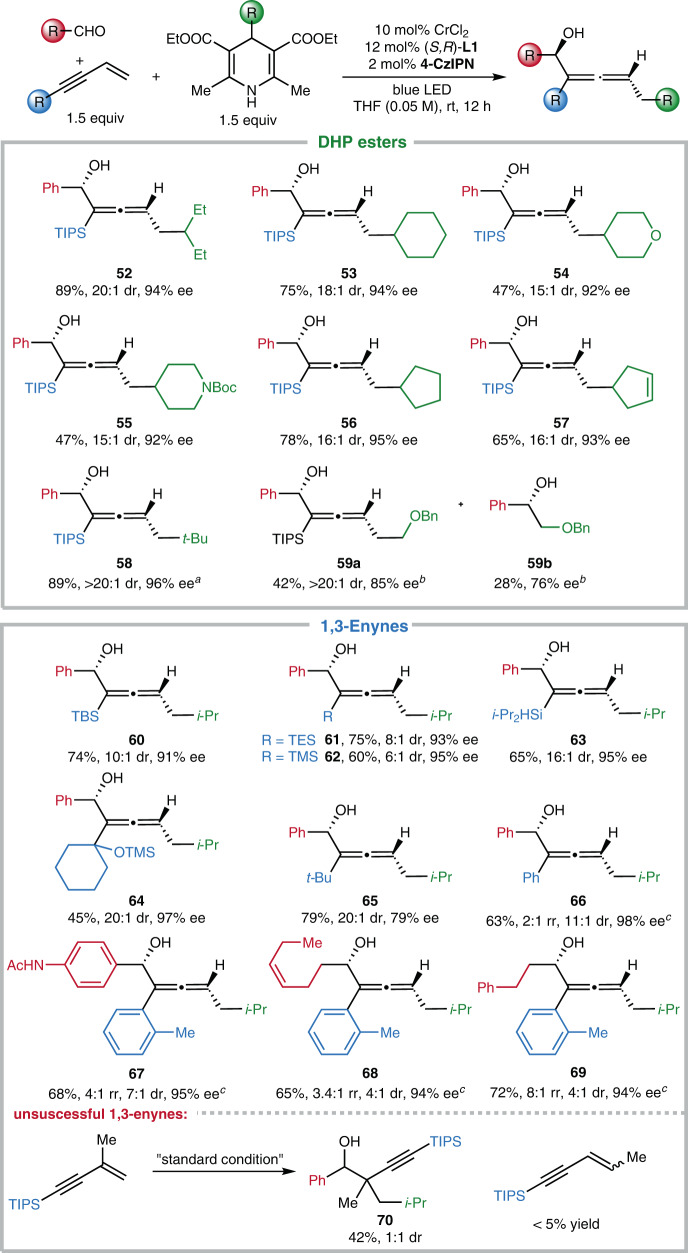
With respect to the DHP esters and 1,3-enynes, the scope of this method is also fairly broad (Fig. 4, 52–69). For example, moderate to good yields and high diastereo- and enantioselectivities are achieved for the alkyl radical precursors with various alkyl substituents, such as cyclohexyl, oxacyclohexyl, azacyclohexyl, cyclopentyl, cyclopentenyl, and tert-butyl (52–58). However, using DHP ester with a primary alkyl substituent furnished the desired allenol 59a in moderate yield (42%, >20:1 d.r., 85% ee), accompanied by 28% direct alkylation product 59b in 76% ee. These results indicate that the single electron reduction of the primary alkyl radical by CrII/L could compete with its addition to 1,3-enynes. 1,3-Enynes, bearing different acetylenic substituents varying from silyl, alkyl to aryl groups, all reacted smoothly with aryl or alkyl aldehydes and DHP ester 1 to furnish the chiral products efficiently (60–69). We found that the use of TMS and TES substituted enynes slightly decreased diastereoselectivity (61, 62), probably due to the variation of steric hindrance. And 1,3-enynes with an aryl group led to the allenols in high enantioselectivity, albeit with moderate regio- and diastereoselectivity (66–69). However, the current optimal condition does apply to 1,3-enynes bearing substituents on the C=C bond (Fig. 4, bottom). The use of triisopropyl(3-methylbut-3-en-1-yn-1-yl)silane gave the propargylation product 70 predominantly with poor diastereoselectivity.
Fig. 4. The scope of DHP esters and 1,3-enynes in the 1,4-functionalization of 1,3-enynes.
a4-(tert-butyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarbonitrile was used as radical precursor. b3.0 equiv of corresponding 1,3-enyne were used. cThe yield is for allenol product, and the minor regioisomer refers to the propargylation product from the 1,2-functionalization. TMS trimethylsilyl, TES triethylsilyl.
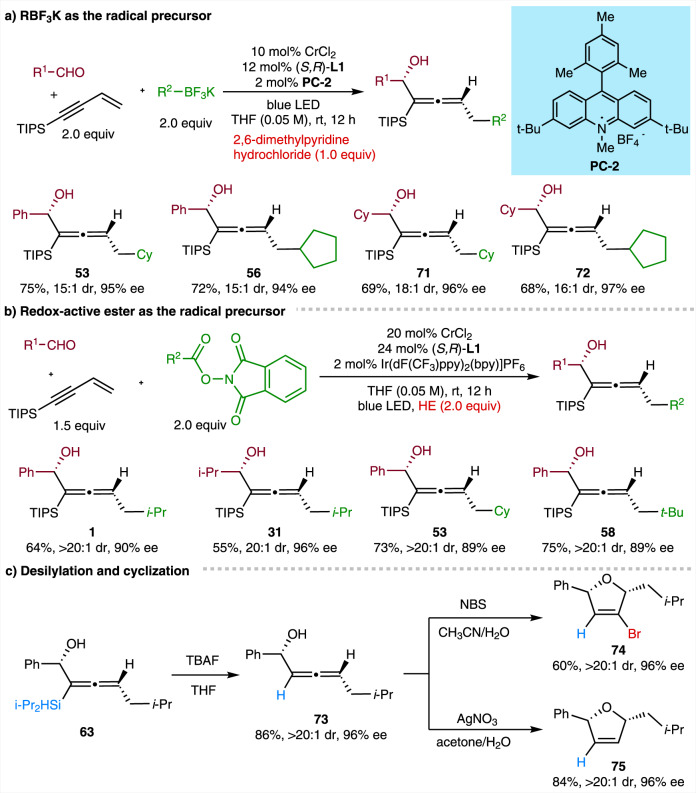
Organotrifluoroborates, featuring tetracoordinate boron with strong boron-fluoride bonds, are generally stable toward numerous regents that are often problematic for other trivalent organoborons, and thus have been widely used in Suzuki-Miyaura couplings58. Moreover, organotrifluoroborates also prove to be suitable radical precursors for C–C bond construction via photoredox catalysis59,60. In this context, we applied them as radical precursors to our newly developed method. After further evaluation of different reaction parameters, we determined an optimal condition with the acridine tetrafluoroborate (PC-2) as the photocatalyst and 2,6-dimethylpyridine hydrochloride as the dissociation reagent. Thus, the representative secondary organotrifluoroborates engaged well in the 1,4-functionalization of enynes with aryl and aliphatic aldehydes to efficiently afford the desired coupling products (Fig. 5a, 53, 56, 71, and 72). N-(Acyloxy)phthalimides (NHPI esters) are widely available from carboxylic acids, and have proved to be priviliged alkyl radical precursors in decarboxylitive cross-couplings61,62. Gratifyingly, NHPI esters also work well under a slightly modified condition with Hantzsch ester as the reductant, furnishing the desired allenols in moderate to good yield and high stereoselectivity (Fig. 5b, 1, 31, 53, and 58).
Fig. 5. Exploration of other radical precursors and representative synthetic applications.
a RBF3K as the radical precursor. b Redox-active ester as the radical precusor. c Desilylation and cyclization. NBS N-bromosuccinimide.
Synthetic application
Product transformations were performed to demonstrate the synthetic utility of our newly developed method (Fig. 5c). Chiral α-allenols serve as suitable building blocks in the synthesis of enantioenriched dihydrofurans63. The desilylation reaction of 63 proceeded smoothly, affording the chiral α-allenol 73 without losing diastereomeric or enantiomeric excess. The stereoselective electrophilic cyclization of 73 furnished 2,5-dihydrofurans 74 and 75 with good efficiency in axial-to-central chirality transfer (Fig. 5c).
Mechanistic observations
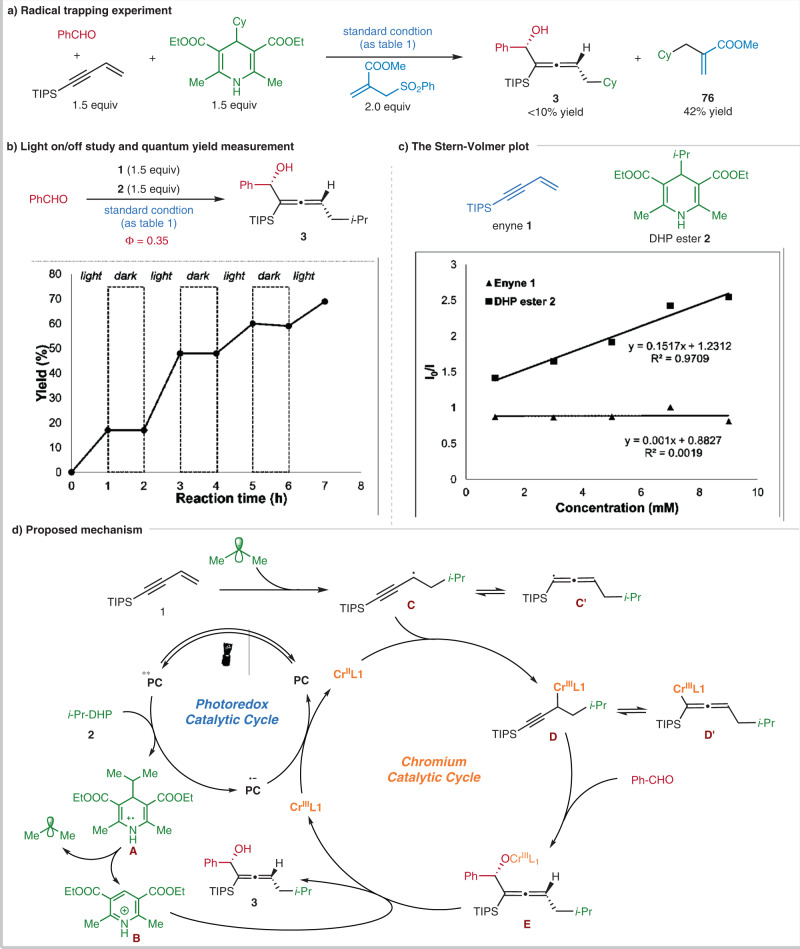
A series of conventional experiments were conducted to provide insights into the reaction mechanism (Fig. 6a–c). The addition of 2 equiv of an allyl sulfone under the standard condition led to an adduct 76 in 42% yield, with a trace amount of desired product 3, which suggested that the reaction might involve the formation of cyclohexyl radical from the DHP ester (Fig. 6a). According to a reported method64, the quantum yield of this model reaction was determined to be 0.35. Moreover, the direct correlation between photolysis and product formation is demonstrated by an interval light-dark reaction (Fig. 6b). These results indicate that the radical 1,4-functionalization process undergoes a photoredox, instead of a radical-chain, pathway. As shown in Fig. 6c, the Stern–Volmer luminescence quenching studies proved that the DHP ester, rather than the 1,3-enyne, quenches the excited-state photocatalyst 4-CzIPN, suggesting a reductive quenching pathway.
Fig. 6. Mechanistic investigations.
a Radical trapping experiment. b Light on/off and quantum yield mearsurement. c The Stern–Volmer plot. d Proposed mechanism for the 1,4-functionalizaton of 1,3-enynes.
According to our observations and previous reports44,46,47, a putative mechanism is proposed in Fig. 6d with the model reaction as an example. The excited-state photocatalyst PC* 4-CzIPN* (E1/2(*PC/PC˙ˉ) = 1.35 V vs. SCE in MeCN)65 is reductively quenched by the DHP ester 2 (E1/2 = 1.10 V vs. SCE in MeCN)66, generating the reduced photocatalyst PC˙ˉ and the radical cation A. The rapid fragmentation of intermediate A affords the isopropyl radical and the pyridinium B. The isopropyl radical could either reversibly add to the low valent CrII/L1 to generate an off-cycle alkyl CrIII/L1 complex67, or add to the terminus of 1,3-enyne 1 to forge the propargyl radical C, which is in equilibrium with the allenyl radical C’. The radical capture by CrII/L1 leads to two equilibrated species, the propargyl chromium D and allenyl chromium D’. Subsequent nucleophilic attack to benzaldehyde is proposed via a six-member cyclic manner68, affording intermediate E. We believe that the isomerization between intermediates D and D’ is faster than the subsequent nucleophilic addition to aldehydes. So the regioselectivity might be determined in the nucleophilic carbonyl addition step via a possible Zimmerman-Traxler transition state. As observed in the scope study, the steric hindrance of the acetylenic substituents of 1,3-enynes is critical for the high regioselectivity, which favors the allenylation product formation from the propargyl Cr D, instead of the allenyl D’. The dissociation of the O–Cr bond in E by the pyridinium B, provides chiral allenol 3. Finally, the CrIII/L1 is reduced to CrII/L1 (E1/2 = –0.65 V vs. SCE in H2O, E1/2 = –0.51 V vs. SCE in DMF)47 by the reduced photocatalyst PC˙ˉ (E1/2 (PC/PC˙ˉ) = –1.21 V vs. SCE in MeCN) 65, which closes the catalytic cycle.
In conclusion, we described a three-component asymmetric radical 1,4-functionalization of 1,3-enynes via dual photoredox and chromium catalysis. The key to success is using DHP esters under photoredox conditions, thus obviating stoichiometric amounts of metal reductants and dissociation reagents in conventional catalytic NHK reactions. The present method exhibits broad substrate scope with good functional group compatibility, providing efficient access to valuable chiral α-allenols from the readily available starting material. Given the importance of allenols and the growing interest in metallaphotoredox catalysis37, we anticipate that our protocol will find broad utility in organic synthesis and facilitate the current endeavors to develop dual catalytic systems.
Methods
General procedure for radical 1,4-functionalization of 1,3-enynes with aldehydes and DHP esters
In a nitrogen-filled glovebox, an oven-dried 20 mL vial with a magnetic stir bar, were charged with the CrCl2 (5.0 mg, 0.04 mmol, 10 mol%) and (S,R)-L1 (23.2 mg, 0.048 mmol, 12 mol%). Then 8.0 mL THF was added via syringe. The vial was closed with a PTFE septum cap and then stirred at room temperature for 2 hours. Next, to the prepared catalyst solution were added the 1,3-enynes (0.6 mmol, 1.5 equiv), the aldehydes (0.4 mmol, 1.0 equiv), the DHP esters (0.6 mmol, 1.5 equiv), and photocatalyst 4-CzIPN (6.4 mg, 0.008 mmol, 2 mol%) sequentially. Then the vial was closed with a PTFE septum cap and taken out of the glovebox. The reaction was irradiated with two 20 W 160-440 nm LED for 12 h (tube 5 cm away from lights, fans for cooling, 30–35 °C). After that, the reaction mixture was concentrated and run through a short silica gel pad with hexanes/EtOAc (3:1) as the eluent. Then the solvent was removed under the reduced pressure. The diastereoselectivity was determined via 1H NMR analysis of the crude reaction mixture. The residue was purified by flash chromatography to provide the desired product, and the ee was determined via HPLC/SFC analysis.
Supplementary information
Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (22171231, Z.W.), the China Postdoctoral Science Foundation (2021M692879, F.-H.Z.), and Zhejiang Leading Innovative and Entrepreneur Team Introduction Program (2020R01004). We thank Instrumentation and Service Center for Molecular Science and Physical Sciences at Westlake University for the assistance work in measurement/data interpretation. We thank Dr. Xiaohuo Shi, Dr. Yinjuan Chen, Dr. Zhong Chen, and Dr. Danyu Gu from Instrumentation and Service Center for Molecular Sciences at Westlake University for assistance in mechanistic study.
Author contributions
F.-H.Z. and X.G. contributed equally to this work. F.-H.Z. and Z.W. conceived this work; F.-H.Z., X.G., and X.Z. designed and conducted all the experiments; F.-H.Z. and Z.W. wrote the manuscript.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The data relating to the materials and methods, experimental procedures, HPLC/SFC spectra, mechanism research, and NMR spectra are available in the Supplementary Information. The crystallographic data for compounds 12 and 42 are available free of charge from the CCDC under reference numbers 2130059 and 2130062. All other data are available from the authors upon request.
Competing interests
The authors declare no competing interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Feng-Hua Zhang, Xiaochong Guo.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-32614-4.
References
- 1.Aubert C, Buisine O, Malacria M. The behavior of 1,n-enynes in the presence of transition metals. Chem. Rev. 2002;102:813–834. doi: 10.1021/cr980054f. [DOI] [PubMed] [Google Scholar]
- 2.Holmes M, Schwartz LA, Krische MJ. Intermolecular metal-catalyzed reductive coupling of dienes, allenes, and enynes with carbonyl compounds and imines. Chem. Rev. 2018;118:6026–6052. doi: 10.1021/acs.chemrev.8b00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu L, Greßies S, Chen P, Liu G. Recent advances and perspectives in transition metal‐catalyzed 1,4‐functionalizations of unactivated 1,3‐enynes for the synthesis of allenes. Chin. J. Chem. 2019;38:91–100. doi: 10.1002/cjoc.201900277. [DOI] [Google Scholar]
- 4.Dherbassy Q, et al. Copper-catalyzed functionalization of enynes. Chem. Sci. 2020;11:11380–11393. doi: 10.1039/D0SC04012F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffmann-Röder A, Krause N. Synthesis and properties of allenic natural products and pharmaceuticals. Angew. Chem. Int. Ed. 2004;43:1196–1216. doi: 10.1002/anie.200300628. [DOI] [PubMed] [Google Scholar]
- 6.Yu S, Ma S. Allenes in catalytic asymmetric synthesis and natural product syntheses. Angew. Chem. Int. Ed. 2012;51:3074–3112. doi: 10.1002/anie.201101460. [DOI] [PubMed] [Google Scholar]
- 7.Ma S. Some typical advances in the synthetic applications of allenes. Chem. Rev. 2005;105:2829–2872. doi: 10.1021/cr020024j. [DOI] [PubMed] [Google Scholar]
- 8.Campolo D, Gastaldi S, Roussel C, Bertrand MP, Nechab M. Axial-to-central chirality transfer in cyclization processes. Chem. Soc. Rev. 2013;42:8434–8466. doi: 10.1039/c3cs60182j. [DOI] [PubMed] [Google Scholar]
- 9.Han JW, Tokunaga N, Hayashi T. Palladium-catalyzed asymmetric hydrosilylation of 4-substituted 1-buten-3-ynes. catalytic asymmetric synthesis of axially chiral allenylsilanes. J. Am. Chem. Soc. 2001;123:12915–12916. doi: 10.1021/ja017138h. [DOI] [PubMed] [Google Scholar]
- 10.Wang M, et al. Synthesis of highly substituted racemic and enantioenriched allenylsilanes via copper-catalyzed hydrosilylation of (Z)-2-alken-4-ynoates with silylboronate. J. Am. Chem. Soc. 2015;137:14830–14833. doi: 10.1021/jacs.5b08279. [DOI] [PubMed] [Google Scholar]
- 11.Huang Y, Del Pozo J, Torker S, Hoveyda AH. Enantioselective synthesis of trisubstituted allenyl-B(pin) compounds by phosphine-Cu-catalyzed 1,3-enyne hydroboration. insights regarding stereochemical integrity of Cu-allenyl intermediates. J. Am. Chem. Soc. 2018;140:2643–2655. doi: 10.1021/jacs.7b13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adamson NJ, Jeddi H, Malcolmson SJ. Preparation of chiral allenes through Pd-catalyzed intermolecular hydroamination of conjugated enynes: enantioselective synthesis enabled by catalyst design. J. Am. Chem. Soc. 2019;141:8574–8583. doi: 10.1021/jacs.9b02637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao Q, et al. Efficient synthesis of chiral trisubstituted 1,2-allenyl ketones by catalytic asymmetric conjugate addition of malonic esters to enynes. Angew. Chem. Int. Ed. 2016;55:1859–1863. doi: 10.1002/anie.201509455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang SQ, Wang YF, Zhao WC, Lin GQ, He ZT. Stereodivergent synthesis of tertiary fluoride-tethered allenes via copper and palladium dual catalysis. J. Am. Chem. Soc. 2021;143:7285–7291. doi: 10.1021/jacs.1c03157. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura T, Makino H, Nagaosa M, Hayashi T. Rhodium-catalyzed enantioselective 1,6-addition of arylboronic acids to enynamides: asymmetric synthesis of axially chiral allenylsilanes. J. Am. Chem. Soc. 2010;132:12865–12867. doi: 10.1021/ja1066509. [DOI] [PubMed] [Google Scholar]
- 16.Law C, Kativhu E, Wang J, Morken JP. Diastereo- and enantioselective 1,4-difunctionalization of borylenynes by catalytic conjunctive cross-coupling. Angew. Chem. Int. Ed. 2020;59:10311–10315. doi: 10.1002/anie.202001580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bayeh-Romero L, Buchwald SL. Copper hydride catalyzed enantioselective synthesis of axially chiral 1,3-disubstituted allenes. J. Am. Chem. Soc. 2019;141:13788–13794. doi: 10.1021/jacs.9b07582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao Y, et al. Enantioselective synthesis of multisubstituted allenes by cooperative Cu/Pd-catalyzed 1,4-arylboration of 1,3-enynes. Angew. Chem. Int. Ed. 2020;59:1176–1180. doi: 10.1002/anie.201912703. [DOI] [PubMed] [Google Scholar]
- 19.Qian H, Yu X, Zhang J, Sun J. Organocatalytic enantioselective synthesis of 2,3-allenoates by intermolecular addition of nitroalkanes to activated enynes. J. Am. Chem. Soc. 2013;135:18020–18023. doi: 10.1021/ja409080v. [DOI] [PubMed] [Google Scholar]
- 20.Wang F, et al. Divergent synthesis of CF3-substituted allenyl nitriles by ligand-controlled radical 1,2- and 1,4-addition to 1,3-enynes. Angew. Chem. Int. Ed. 2018;57:7140–7145. doi: 10.1002/anie.201803668. [DOI] [PubMed] [Google Scholar]
- 21.Zhang K-F, et al. Nickel-catalyzed carbofluoroalkylation of 1,3-enynes to access structurally diverse fluoroalkylated allenes. Angew. Chem. Int. Ed. 2019;58:5069–5074. doi: 10.1002/anie.201813184. [DOI] [PubMed] [Google Scholar]
- 22.Zhu X, et al. Copper-catalyzed radical 1,4-difunctionalization of 1,3-enynes with alkyl diacyl peroxides and N-fluorobenzenesulfonimide. J. Am. Chem. Soc. 2019;141:548–559. doi: 10.1021/jacs.8b11499. [DOI] [PubMed] [Google Scholar]
- 23.Song Y, Fu C, Ma S. Copper-catalyzed syntheses of multiple functionalizatized allenes via three-component reaction of enynes. ACS Catal. 2021;11:10007–10013. doi: 10.1021/acscatal.1c02140. [DOI] [Google Scholar]
- 24.Chen Y, Wang J, Lu Y. Decarboxylative 1,4-carbocyanation of 1,3-enynes to access tetra-substituted allenes via copper/photoredox dual catalysis. Chem. Sci. 2021;12:11316–11321. doi: 10.1039/D1SC02896K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, Ma R, Sun J, Zheng G, Zhang Q. NHC and visible light-mediated photoredox co-catalyzed 1,4-sulfonylacylation of 1,3-enynes for tetrasubstituted allenyl ketones. Chem. Sci. 2022;13:3169–3175. doi: 10.1039/D1SC06100C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L, et al. 1,4-Alkylcarbonylation of 1,3-enynes to access tetra-substituted allenyl ketones via an NHC-catalyzed radical relay. ACS Catal. 2021;11:13363–13373. doi: 10.1021/acscatal.1c03861. [DOI] [Google Scholar]
- 27.Chen Y, Zhu K, Huang Q, Lu Y. Regiodivergent sulfonylarylation of 1,3-enynes via nickel/photoredox dual catalysis. Chem. Sci. 2021;12:13564–13571. doi: 10.1039/D1SC04320J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y-Q, et al. Radical acylalkylation of 1,3-enynes to access allenic ketones via N-heterocyclic carbene organocatalysis. J. Org. Chem. 2022;87:5229–5241. doi: 10.1021/acs.joc.2c00037. [DOI] [PubMed] [Google Scholar]
- 29.Li C, et al. Selective 1,4-arylsulfonation of 1,3-enynes via photoredox/nickel dual catalysis. Org. Chem. Front. 2022;9:788–794. doi: 10.1039/D1QO01653A. [DOI] [Google Scholar]
- 30.Zeng Y, et al. Copper-catalyzed enantioselective radical 1,4-difunctionalization of 1,3-enynes. J. Am. Chem. Soc. 2020;142:18014–18021. doi: 10.1021/jacs.0c06177. [DOI] [PubMed] [Google Scholar]
- 31.Dong XY, et al. Copper-catalyzed asymmetric coupling of allenyl radicals with terminal alkynes to access tetrasubstituted allenes. Angew. Chem. Int. Ed. 2021;60:2160–2164. doi: 10.1002/anie.202013022. [DOI] [PubMed] [Google Scholar]
- 32.Okude Y, Hirano S, Hiyama T, Nozaki H. Grignard-type carbonyl addition of allyl halides by means of chromous salt. A chemospecific synthesis of homoallyl alcohols. J. Am. Chem. Soc. 1977;99:3179–3181. doi: 10.1021/ja00451a061. [DOI] [Google Scholar]
- 33.Fürstner A. Carbon–carbon bond formations involving organochromium(III) reagents. Chem. Rev. 1999;99:991–1046. doi: 10.1021/cr9703360. [DOI] [PubMed] [Google Scholar]
- 34.Hargaden GC, Guiry PJ. The development of the asymmetric Nozaki–Hiyama–Kishi reaction. Adv. Synth. Catal. 2007;349:2407–2424. doi: 10.1002/adsc.200700324. [DOI] [Google Scholar]
- 35.Zhang G, Tian Q. Recent advances in the asymmetric Nozaki–Hiyama–Kishi reaction. Synthesis. 2016;48:4038–4049. doi: 10.1055/s-0036-1589457. [DOI] [Google Scholar]
- 36.Gil A, Albericio F, Alvarez M. Role of the Nozaki-Hiyama-Takai-Kishi reaction in the synthesis of natural products. Chem. Rev. 2017;117:8420–8446. doi: 10.1021/acs.chemrev.7b00144. [DOI] [PubMed] [Google Scholar]
- 37.Chan AY, et al. Metallaphotoredox: the merger of photoredox and transition metal catalysis. Chem. Rev. 2022;122:1485–1542. doi: 10.1021/acs.chemrev.1c00383. [DOI] [PubMed] [Google Scholar]
- 38.Lu F-D, et al. Recent advances in transition-metal-catalysed asymmetric coupling reactions with light intervention. Chem. Soc. Rev. 2021;50:12808–12827. doi: 10.1039/D1CS00210D. [DOI] [PubMed] [Google Scholar]
- 39.Zhao K, Knowles RR. Contra-thermodynamic positional isomerization of olefins. J. Am. Chem. Soc. 2022;144:137–144. doi: 10.1021/jacs.1c11681. [DOI] [PubMed] [Google Scholar]
- 40.Liu J, et al. Photoredox-enabled chromium-catalyzed alkene diacylations. ACS Catal. 2022;12:1879–1885. doi: 10.1021/acscatal.1c05672. [DOI] [Google Scholar]
- 41.Huang H-M, Bellotti P, Glorius F. Merging carbonyl addition with photocatalysis. Acc. Chem. Res. 2022;55:1135–1147. doi: 10.1021/acs.accounts.1c00799. [DOI] [PubMed] [Google Scholar]
- 42.Zhang H-H, Chen H, Zhu C, Yu S. A review of enantioselective dual transition metal/photoredox catalysis. Sci. China Chem. 2020;63:637–647. doi: 10.1007/s11426-019-9701-5. [DOI] [Google Scholar]
- 43.Kanai M, Mitsunuma H, Katayama Y. Recent progress in chromium-mediated carbonyl addition reactions. Synthesis. 2021;54:1684–1694. [Google Scholar]
- 44.Schwarz JL, Schäfers F, Tlahuext-Aca A, Lückemeier L, Glorius F. Diastereoselective allylation of aldehydes by dual photoredox and chromium catalysis. J. Am. Chem. Soc. 2018;140:12705–12709. doi: 10.1021/jacs.8b08052. [DOI] [PubMed] [Google Scholar]
- 45.Schwarz JL, Kleinmans R, Paulisch TO, Glorius F. 1,2-Amino alcohols via Cr/photoredox dual-catalyzed addition of α-amino carbanion equivalents to carbonyls. J. Am. Chem. Soc. 2020;142:2168–2174. doi: 10.1021/jacs.9b12053. [DOI] [PubMed] [Google Scholar]
- 46.Huang HM, Bellotti P, Daniliuc CG, Glorius F. Radical carbonyl propargylation by dual catalysis. Angew. Chem. Int. Ed. 2021;60:2464–2471. doi: 10.1002/anie.202011996. [DOI] [PubMed] [Google Scholar]
- 47.Schwarz JL, Huang H-M, Paulisch TO, Glorius F. Dialkylation of 1,3-dienes by dual photoredox and chromium catalysis. ACS Catal. 2020;10:1621–1627. doi: 10.1021/acscatal.9b04222. [DOI] [Google Scholar]
- 48.Mitsunuma H, Tanabe S, Fuse H, Ohkubo K, Kanai M. Catalytic asymmetric allylation of aldehydes with alkenes through allylic C(sp3)–H functionalization mediated by organophotoredox and chiral chromium hybrid catalysis. Chem. Sci. 2019;10:3459–3465. doi: 10.1039/C8SC05677C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanabe S, Mitsunuma H, Kanai M. Catalytic allylation of aldehydes using unactivated alkenes. J. Am. Chem. Soc. 2020;142:12374–12381. doi: 10.1021/jacs.0c04735. [DOI] [PubMed] [Google Scholar]
- 50.Zhang F-H, Guo X, Zeng X, Wang Z. Catalytic enantioconvergent allenylation of aldehydes with propargyl halides. Angew. Chem. Int. Ed. 2022;61:e202117114. doi: 10.1002/anie.202117114. [DOI] [PubMed] [Google Scholar]
- 51.Alonso JM, Almendros P. Deciphering the chameleonic chemistry of allenols: breaking the taboo of a onetime esoteric functionality. Chem. Rev. 2021;121:4193–4252. doi: 10.1021/acs.chemrev.0c00986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meanwell, N. A. in Advances inHeterocyclic Chemistry Vol. 123 (eds Scriven, E. F. V. & Ramsden, C. A.) 245–361 (Academic Press, 2017).
- 53.Ni S, et al. A radical approach to anionic chemistry: synthesis of ketones, alcohols, and amines. J. Am. Chem. Soc. 2019;141:6726–6739. doi: 10.1021/jacs.9b02238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao Y, et al. Electrochemical Nozaki–Hiyama–Kishi coupling: scope, applications, and mechanism. J. Am. Chem. Soc. 2021;143:9478–9488. doi: 10.1021/jacs.1c03007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matos JLM, Vasquez-Cespedes S, Gu J, Oguma T, Shenvi RA. Branch-selective addition of unactivated olefins into imines and aldehydes. J. Am. Chem. Soc. 2018;140:16976–16981. doi: 10.1021/jacs.8b11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hughes, A. B. Amino Acids, Peptides and Proteins in Organic Chemistry, Analysis and Function of Amino Acids and Peptides (John Wiley & Sons, 2013).
- 57.Ager DJ, Prakash I, Schaad DR. 1,2-Amino alcohols and their heterocyclic derivatives as chiral auxiliaries in asymmetric synthesis. Chem. Rev. 1996;96:835–876. doi: 10.1021/cr9500038. [DOI] [PubMed] [Google Scholar]
- 58.Molander GA, Ellis N. Organotrifluoroborates: protected boronic acids that expand the versatility of the suzuki coupling reaction. Acc. Chem. Res. 2007;40:275–286. doi: 10.1021/ar050199q. [DOI] [PubMed] [Google Scholar]
- 59.Tellis JC, Primer DN, Molander GA. Single-electron transmetalation in organoboron cross-coupling by photoredox/nickel dual catalysis. Science. 2014;345:433–436. doi: 10.1126/science.1253647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yasu Y, Koike T, Akita M. Visible light-induced selective generation of radicals from organoborates by photoredox catalysis. Adv. Synth. Catal. 2012;354:3414–3420. doi: 10.1002/adsc.201200588. [DOI] [Google Scholar]
- 61.Qin T, et al. A general alkyl-alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents. Science. 2016;352:801–805. doi: 10.1126/science.aaf6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parida SK, et al. Single electron transfer-induced redox processes involving N-(Acyloxy)phthalimides. ACS Catal. 2021;11:1640–1683. doi: 10.1021/acscatal.0c04756. [DOI] [Google Scholar]
- 63.Ma S. Transition metal-catalyzed/mediated reaction of allenes with a nucleophilic functionality connected to the α-carbon atom. Acc. Chem. Res. 2003;36:701–712. doi: 10.1021/ar020133k. [DOI] [PubMed] [Google Scholar]
- 64.Cismesia MA, Yoon TP. Characterizing chain processes in visible light photoredox catalysis. Chem. Sci. 2015;6:5426–5434. doi: 10.1039/C5SC02185E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luo J, Zhang J. Donor–acceptor fluorophores for visible-light-promoted organic synthesis: photoredox/Ni dual catalytic C(sp3)–C(sp2) cross-coupling. ACS Catal. 2016;6:873–877. doi: 10.1021/acscatal.5b02204. [DOI] [Google Scholar]
- 66.Cheng J-P, et al. Heterolytic and homolytic N−H bond dissociation energies of 4-substituted hantzsch 2,6-dimethyl-1,4-dihydropyridines and the effect of one-electron transfer on the N−H bond activation. J. Org. Chem. 2000;65:3853–3857. doi: 10.1021/jo991145v. [DOI] [PubMed] [Google Scholar]
- 67.MacLeod KC, Conway JL, Patrick BO, Smith KM. Exploring chromium(III)-alkyl bond homolysis with CpCr[(ArNCMe)2CH](R) complexes. J. Am. Chem. Soc. 2010;132:17325–17334. doi: 10.1021/ja1083392. [DOI] [PubMed] [Google Scholar]
- 68.Inoue M, Nakada M. Studies into asymmetric catalysis of the Nozaki-Hiyama allenylation. Angew. Chem. Int. Ed. 2005;45:252–255. doi: 10.1002/anie.200502871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data relating to the materials and methods, experimental procedures, HPLC/SFC spectra, mechanism research, and NMR spectra are available in the Supplementary Information. The crystallographic data for compounds 12 and 42 are available free of charge from the CCDC under reference numbers 2130059 and 2130062. All other data are available from the authors upon request.