Abstract
Transcription initiation by the ς54-RNA polymerase holoenzyme requires an enhancer-binding protein that is thought to contact ς54 to activate transcription. To identify potential enhancer-binding protein contact sites in ς54, we compared the abilities of wild-type and truncated forms of Salmonella enterica serovar Typhimurium ς54 to interact with the enhancer-binding protein DctD in a chemical cross-linking assay. Removal of two regions in the amino-terminal portion of ς54, residues 57 to 105 and residues 144 to 179, prevented cross-linking, but removal of either region alone did not. In addition, deletion of 56 amino-terminal residues of ς54 (region I) reduced the affinity of the protein for a fork junction DNA probe.
Transcription initiation by ς54-RNA polymerase holoenzyme (ς54-holoenzyme) requires an enhancer-binding protein (19, 20, 22). ς54-Holoenzyme binds to promoters with consensus sequences in the −12 and −24 regions to form a closed complex. Enhancer-binding proteins generally bind to sites upstream of the promoter and contact ς54-holoenzyme through DNA looping (21, 23). Productive intermediates between enhancer-binding proteins and ς54-holoenzyme lead to isomerization of the closed complex to an open complex that can initiate transcription. Enhancer-binding proteins must hydrolyze ATP or GTP to catalyze open-complex formation (20, 28).
Interactions between ς54-holoenzyme and enhancer-binding proteins are transient, and little is known about the nature of these interactions. The C4-dicarboxylic acid transport protein D (DctD) is an enhancer-binding protein from Sinorhizobium meliloti that can be cross-linked to ς54 and the β subunit of RNA polymerase (16). Some mutant forms of DctD that fail to activate transcription also fail to cross-link to these subunits (27), suggesting that DctD contacts these subunits of ς54-holoenzyme to catalyze open-complex formation. To identify the region of ς54 that interacts with DctD, we generated a series of truncated ς54 proteins and assessed their abilities to cross-link with DctD.
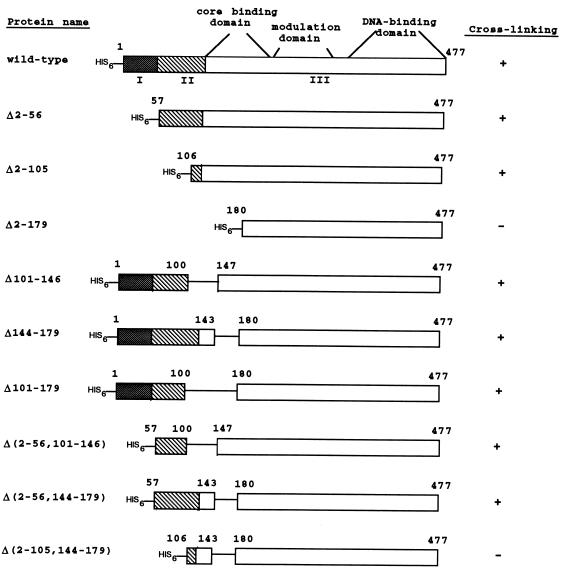
Deletion of 179 amino acid residues from the amino terminus of ς54 disrupts cross-linking to DctD.
The cross-linking reagent succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate (sulfo-SMCC) can cross-link Sinorhizobium meliloti DctD and Salmonella enterica serovar Typhimurium ς54 (16). Sulfo-SMCC is a heterobifunctional cross-linking reagent that has a maleimide group and an N-hydroxysuccinimide ester group, which react preferentially with sulfhydryl groups and primary amines, respectively, and are linked by a relatively long and flexible spacer arm. Cys-307 of S. enterica serovar Typhimurium ς54 is critical for cross-linking to DctD (16), presumably because it is surface exposed and reacts readily with the maleimide group of sulfo-SMCC. For the cross-linking experiments in this study, we used DctD(Δ1–142), which is a truncated, constitutively active form of DctD (17). As in previous studies, these cross-linking assays were done with purified ς54 proteins and DctD(Δ1–142) in the absence of core RNA polymerase and DNA (16, 27).
We initially generated three amino-terminally truncated ς54 proteins that had deletions of residues 2 to 56 (Δ2–56), 2 to 105 (Δ2–105), and 2 to 179 (Δ2–179) (Fig. 1). All deletions were generated by using PCR to introduce a unique NdeI site at the desired position of rpoN, the gene encoding ς54. The NdeI site was used to clone the truncated rpoN alleles into the expression vector pCyt3 (provided by Elliot Altman, University of Georgia), which placed the rpoN alleles under the control of the Escherichia coli lac promoter/operator. The rpoN alleles were also cloned into the expression vector pET-28a(+) (Novagen), which resulted in attachment of a hexahistidine tag at the amino terminus of each truncated ς54 protein.
FIG. 1.
Deletion mutants of S. enterica serovar Typhimurium ς54. The three functional regions of ς54 are indicated as I (stippled boxes), II (hatched boxes), and III (open boxes). The core-binding, modulation, and DNA-binding domains within region III are noted. Residues corresponding to the amino-terminal or internal deletions are also indicated. The ability of each protein to cross-link to DctD(Δ1–142) is indicated as a positive (+) or negative (−) result.
Hexahistidine-tagged ς54 proteins were overexpressed in E. coli BL21 (DE3) [F− ompT (lon) hsdSB gal λDE3::lacI lacUV5-gene 1 (T7 polymerase)] by growing the cells in Luria-Bertani broth supplemented with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). Cells were harvested, resuspended in 50 mM Tris-acetate (pH 8.2)–200 mM KCl–1 mM EDTA–1 mM dithiothreitol, and lysed in a French pressure cell at 12,000 lb/in2. Like the full-length hexahistidine-tagged ς54 protein (15), the truncated hexahistidine-tagged ς54 proteins were in the insoluble fraction following centrifugation of the cell lysates. The insoluble fractions containing the hexahistidine-tagged ς54 proteins were washed with a solution containing 1 M NaCl and 1% Triton X-100, after which the hexahistidine-tagged proteins were solubilized in 50 mM Tris-HCl (pH 8.0)–50 mM NaCl–0.1 mM EDTA–1 mM dithiothreitol–5% glycerol–1% sarcosyl as described previously (6). The hexahistidine-tagged ς54 proteins were then purified by affinity chromatography with nickel-nitrilotriacetic acid resin as described previously (15). Preparations of the hexahistidine-tagged ς54 proteins were >90% homogeneous as judged from Coomassie blue-stained sodium dodecyl sulfate-polyacrylamide gels containing samples of the preparations (data not shown).
The Δ2–56 mutant lacked region I of ς54. Region I, which consists of approximately amino acid residues 1 to 56, has important roles in transcriptional activation (4, 7, 13, 14, 26, 30). A ς54 protein lacking region I still binds core RNA polymerase and directs polymerase to promoter DNA, but the resulting holoenzyme cannot respond to enhancer-binding protein to form an open complex that is capable of transcription initiation (4, 26). Region I-deleted holoenzyme, however, can initiate transcription in the absence of an enhancer-binding protein under solution conditions that permit transient DNA melting or from a premelted heteroduplex template (4, 26). Region I appears to prevent polymerase from undergoing isomerization to form an open complex in the absence of an enhancer-binding protein (4). Region I has a weak core-binding activity, suggesting that it exerts influence on core by direct protein-protein interactions (9).
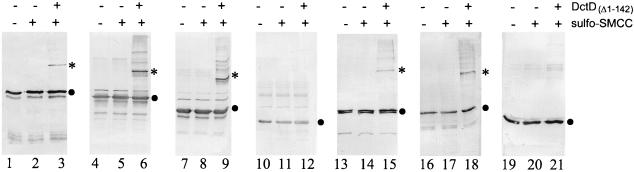
The Δ2–56 mutant cross-linked to DctD(Δ1–142) (Fig. 2, lane 6), indicating that region I is not required for contact with this enhancer-binding protein. These data suggest that despite its requirement for responsiveness to enhancer-binding protein, region I does not interact directly with that protein. Consistent with this suggestion, Buck and colleagues showed that a polypeptide containing region I inhibited transcriptional activation by ς54-holoenzyme in a manner that was noncompetitive with respect to the enhancer-binding protein (10).
FIG. 2.
DctD(Δ1–142) cross-linking assays. Cross-linking reactions were carried out and visualized by immunoblotting with antiserum directed against S. enterica serovar Typhimurium ς54 as described previously (16). The black dots to the right of the gels indicate the positions of ς54 proteins, and the asterisks indicate the positions of major cross-linked products. In addition to cross-reacting with antiserum directed against ς54, these cross-linked products also cross-reacted with antiserum directed against DctD (reference 16 and data not shown). The presence (+) or absence (−) of sulfo-SMCC and DctD(Δ1–142) is indicated above each lane. The hexahistidine-tagged ς54 proteins used were the wild type (lanes 1 to 3), Δ2–56 (lanes 4 to 6), Δ2–105 (lanes 7 to 9), Δ2–179 (lanes 10 to 12), Δ101–179 (lanes 13 to 15), Δ(2–56, 144–179) (lanes 16 to 18), and Δ(2–105, 144–179) (lanes 19 to 21).
The Δ2–105 mutant lacked region I and most of region II. Region II of S. enterica serovar Typhimurium ς54 spans residues 50 to 120 and is very acidic. Deletions within region II appear to reduce the rate of open-complex formation (29). Like the region I-deleted holoenzyme, holoenzyme containing ς54 deleted for regions I and II binds promoter DNA and initiates transcription from a premelted heteroduplex template in the absence of enhancer-binding protein (2). The Δ2–105 mutant cross-linked to DctD(Δ1–142) (Fig. 2, lane 9), indicating that regions I and II are dispensible for contact with DctD(Δ1–142).
The Δ2–179 mutant lacked regions I and II and part of region III. Region III is responsible for core binding and DNA binding and also enhancer responsiveness (5, 8, 11, 24, 25, 30). Core binding by ς54 appears to involve more than one region of the protein. The minimal core-binding domain, which spans residues 120 to 215, has the highest affinity for core RNA polymerase and likely directs formation of the holoenzyme (9). The DNA-binding determinants are located between residues 329 and 477, while a modulation domain that stimulates the DNA-binding activity of the DNA-binding domain lies within residues 180 to 306 (3, 6, 18, 24).
The Δ2–179 mutant failed to cross-link to DctD(Δ1–142) (Fig. 2, lane 12), suggesting that a sequence between residues 106 and 179 is required for interactions with DctD(Δ1–142). To define this sequence more precisely, we generated internal deletions within ς54. We initially deleted residues 101 to 146 and residues 144 to 179 by using unique restriction sites within rpoN. Both of these mutant proteins, Δ101–146 and Δ144–179, cross-linked to DctD(Δ1–142) (data not shown). When we deleted residues 101 to 179, the resulting protein could also be cross-linked to DctD(Δ1–142) (Fig. 2, lane 15).
Regions II and III appear to be involved in interactions with DctD(Δ1–142).
Because Δ101–179 cross-linked to DctD(Δ1–142) but Δ2–179 did not, we reasoned that a region between residues 2 and 100 compensated for the loss of residues 101 to 179. To test this hypothesis, we generated amino-terminal deletions in the mutant proteins Δ101–146 and Δ144–179. The double-deletion mutants Δ(2–56, 101–146) (data not shown) and Δ(2–56, 144–179) (Fig. 2, lane 18) cross-linked to DctD(Δ1–142). However, when residues 2 to 105 were deleted in addition to residues 144 to 179, the resulting protein, Δ(2–105, 144–179), failed to cross-link to DctD(Δ1–142) (Fig. 2, lane 21). These data suggest that a sequence at around residues 57 to 105 and a second sequence at around residues 144 to 179 are involved in interactions with DctD(Δ1–142), but only one of these sequences is needed for cross-linking.
In vivo DNA-binding activities of ς54 deletion mutants.
All of the mutant proteins that we generated contained intact DNA-binding and modulation domains. We wanted to verify that the two deletion mutants that failed to cross-link to DctD(Δ1–142) retained their DNA-binding activities, as this would imply that the DNA-binding and modulation domains of these mutant proteins were folded correctly. The modulation domain contains Cys-307, which is critical for cross-linking of ς54 to DctD(Δ1–142) by sulfo-SMCC (16).
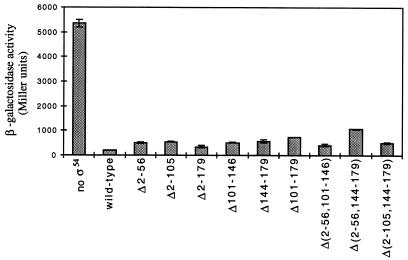
We examined the abilities of the deletion mutants to repress transcription from a phage P22 ant′-′lacZ reporter gene in which the ς54-dependent Sinorhizobium meliloti nifH promoter overlapped the ant promoter (1). When ς54 was overexpressed from a plasmid in an S. enterica serovar Typhimurium strain carrying a chromosomal copy of the ant′-′lacZ fusion, expression from this reporter gene was reduced ∼25-fold (Fig. 3). All of the deletion mutants that we generated repressed transcription from the ant′-′lacZ reporter gene when overexpressed in the same strain, indicating that they retained their DNA-binding activities. The degree to which these mutant forms of ς54 repressed transcription was not as great as that of wild-type ς54, as the mutant proteins reduced expression from the ant′-′lacZ reporter gene by 5- to 15-fold (Fig. 3). Several of the mutant ς54 proteins were expressed at lower levels than wild-type ς54, however, and so these repression assays are not completely indicative of the abilities of these mutant ς54 proteins to repress transcription from the ant′-′lacZ reporter gene (data not shown). Some of the deletions extended into the core-binding domain, and these mutant proteins may have repressed transcription by binding directly to the nifH promoter rather than by directing polymerase to this promoter. Although the mutant ς54 proteins retained their DNA-binding activities, none of them complemented the rpoN mutation, as indicated by their failure to confer glutamine prototrophy.
FIG. 3.
Repression of the ant′-′lacZ reporter gene by the ς54 deletion mutants. S. enterica serovar Typhimurium strain TRH107 (15) was transformed with derivatives of pCyt3 bearing the various rpoN alleles. Following the overexpression of the ς54 proteins in this strain by the addition of 0.1 mM IPTG, β-galactosidase activities were determined as described previously (15). Values shown are averages of data from three assays, and for each sample 1 standard deviation is indicated by an error bar.
In vitro DNA- and core-binding activities of ς54 deletion mutants.
We examined the abilities of the deletion proteins to bind to promoter DNA in vitro by using a 21-bp double-stranded probe that spanned the −29 to −9 region of the Sinorhizobium meliloti nifH promoter and a fork junction probe that spanned the same region but had a 5′ overhang on the template strand corresponding to residues −11 to −9. Guo and Gralla (12) showed that E. coli ς54 formed a heparin-resistant complex with this fork junction DNA probe.
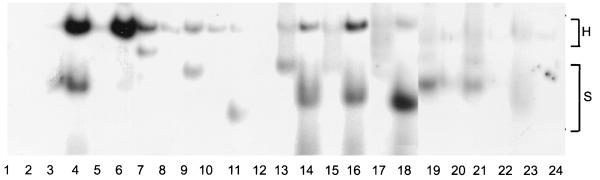
Holoenzyme formed with wild-type ς54 or full-length hexahistidine-tagged ς54 had a much higher affinity for the fork junction probe than for the double-stranded probe (Fig. 4, lanes 3 to 6). In contrast, mutant ς54 proteins with amino-terminal deletions had higher affinities for the double-stranded probe, both as free ς54 and when associated with RNA polymerase (Fig. 4, lanes 7 to 12 and 19 to 24). The three original amino-terminal deletion mutants, Δ2–56, Δ2–105, and Δ2–179, appeared to bind the double-stranded probe better than wild-type ς54 did, both as free ς54 and the holoenzyme forms. These data indicate that the ς54 mutant proteins retained their DNA-binding activities, consistent with the repression observed at the ant′-′lacZ reporter gene. These data also demonstrate that region I is required for efficient binding to the fork junction probe but not the double-stranded probe. This is consistent with our previous observation that certain amino acid substitutions within region I reduce the affinity of ς54 for the fork junction probe (15). Casaz and Buck (7) reported that deletion of region I of ς54 affects the conformation of the carboxy-terminal DNA-binding domain, which may account for the reduced affinities of the region I deletion mutants for the fork junction probe.
FIG. 4.
Gel mobility shift assays with ς54 deletion mutants. The double-stranded probe was used in the odd-numbered lanes, and the fork junction probe was used in the even-numbered lanes. All reaction mixtures contained 5 nM DNA and 300 nM E. coli core RNA polymerase, and ς54 proteins were present at 600 nM in lanes 3 to 18 and at 1 μM in lanes 19 to 24. Reactions were carried out as described previously (15), and products were visualized by exposing the 5% native polyacrylamide gels to X-ray film for 24 h. Lanes 1 and 2 contained core RNA polymerase only. The ς54 proteins used were as follows: wild-type ς54 (lanes 3 and 4), hexahistidine-tagged wild-type ς54 (lanes 5 and 6), Δ2–56 (lanes 7 and 8), Δ2–105 (lanes 9 and 10), Δ2–179 (lanes 11 and 12), Δ101–146 (lanes 13 and 14), Δ144–179 (lanes 15 and 16), Δ101–179 (lanes 17 and 18), Δ(2–56, 101–146) (lanes 19 and 20), Δ(2–56, 144–179) (lanes 21 and 22), and Δ(2–105, 144–179) (lanes 23 and 24). Wild-type ς54-holoenzyme shifted >50% of the fork junction probe. Free probe is not shown. H, the shifted species with holoenzyme bound to the DNA probe; S, the shifted species with ς54 bound to the DNA probe.
The mutant ς54 proteins retarded the mobility of the DNA probes to different degrees, which may reflect altered mobilities of the various mutant proteins in the native gel. Complexes of the mutant forms of ς54-holoenzyme and the DNA probes, however, had the same mobility. For each mutant protein, we confirmed that the faster-migrating species was due to free ς54 by omitting core from the gel mobility shift assay (data not shown). Interestingly, the three mutant proteins with internal deletions, Δ101–146, Δ144–179, and Δ101–179, retarded the mobility of the double-stranded probe to a greater extent than they retarded the fork junction probe. A possible explanation for this observation is that these mutant forms of ς54 bend the double-stranded probe more than they bend the fork junction probe.
The gel mobility shift assays also allowed us to assess the abilities of the mutant ς54 proteins to bind to core RNA polymerase. In the gel shift assays with the deletion mutants Δ2–56, Δ2–105, and Δ2–179, the amount of double-stranded probe shifted by holoenzyme decreased with increases in the extent of the deletion. It was surprising that Δ2–179 yielded a holoenzyme-shifted species at all given that the deletion extended into the minimal core-binding domain. Core protects sequences within the DNA-binding and modulation domains from hydroxyl radical cleavage (7), suggesting possible interactions of core with these regions of ς54. It is possible that the double-stranded DNA probe stabilized interactions between core and these regions in the Δ2–179 mutant protein. The three mutant proteins with internal deletions, Δ101–146, Δ144–179, and Δ101–179, also retained some core-binding activity, although their affinities for core appeared to be greatly reduced compared to that of wild-type ς54.
The three double-deletion mutants bound poorly to both the fork junction probe and core. All of these mutant proteins shifted the double-stranded probe as free ς54, although the shifted species with Δ(2–105, 144–173) resulted in a fairly diffuse band. It is unclear why the double-deletion mutants appeared to have lower affinities for core than did the Δ2–179 mutant. It is possible that the sequences that remained at the amino termini of these deletion mutants interfered with core binding.
Taken together, the in vivo and in vitro DNA-binding assays indicate that the ς54 deletion mutants retained their DNA-binding activities and, in some cases, their core-binding activities. We infer from these results that the DNA-binding and modulation domains of the ς54 deletion mutants were folded correctly. Therefore, it seems likely that the failure of Δ2–179 and Δ(2–105, 144–179) to cross-link with DctD(Δ1–142) was due to the removal of protein sequences important for specific interactions with this enhancer-binding protein.
Acknowledgments
We thank Ellen Neidle for comments on the manuscript.
This work was supported by award MCB-963054 to T.R.H. from the National Science Foundation.
REFERENCES
- 1.Ashraf S I, Kelly M T, Wang Y-K, Hoover T R. Genetic analysis of the Rhizobium meliloti nifH promoter, using the P22 challenge phage system. J Bacteriol. 1997;179:2356–2362. doi: 10.1128/jb.179.7.2356-2362.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cannon W, Chaney M, Buck M. Characterisation of holoenzyme lacking ςN regions I and II. Nucleic Acids Res. 1999;27:2478–2486. doi: 10.1093/nar/27.12.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cannon W, Claverie-Martin F, Austin S, Buck M. Identification of a DNA-contacting surface in the transcription factor sigma-54. Mol Microbiol. 1994;11:227–236. doi: 10.1111/j.1365-2958.1994.tb00303.x. [DOI] [PubMed] [Google Scholar]
- 4.Cannon W, Gallegos M-T, Casaz P, Buck M. Amino-terminal sequences of ςN (ς54) inhibit RNA polymerase isomerization. Genes Dev. 1999;13:357–370. doi: 10.1101/gad.13.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cannon W, Missailidis S, Smith C, Cottier A, Austin S, Moore M, Buck M. Core RNA polymerase and promoter DNA interactions of purified domains of ςN: bipartite functions. J Mol Biol. 1995;248:781–803. doi: 10.1006/jmbi.1995.0260. [DOI] [PubMed] [Google Scholar]
- 6.Cannon W V, Chaney M K, Wang X-Y, Buck M. Two domains within ςN (ς54) cooperate for DNA binding. Proc Natl Acad Sci USA. 1997;94:5006–5011. doi: 10.1073/pnas.94.10.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casaz P, Buck M. Region I modifies DNA-binding domain conformation of sigma 54 within the holoenzyme. J Mol Biol. 1999;285:507–514. doi: 10.1006/jmbi.1998.2328. [DOI] [PubMed] [Google Scholar]
- 8.Chaney M, Buck M. The sigma 54 DNA-binding domain includes a determinant of enhancer responsiveness. Mol Microbiol. 1999;33:1200–1209. doi: 10.1046/j.1365-2958.1999.01566.x. [DOI] [PubMed] [Google Scholar]
- 9.Gallegos M-T, Buck M. Sequences in ςN determining holoenzyme formation and properties. J Mol Biol. 1999;288:539–553. doi: 10.1006/jmbi.1999.2704. [DOI] [PubMed] [Google Scholar]
- 10.Gallegos M-T, Cannon W, Buck M. Functions of the ς54 region I in trans and implications for transcription activation. J Biol Chem. 1999;274:25285–25290. doi: 10.1074/jbc.274.36.25285. [DOI] [PubMed] [Google Scholar]
- 11.Guo Y, Gralla J D. DNA-binding determinants of sigma 54 as deduced from libraries of mutations. J Bacteriol. 1997;179:1239–1245. doi: 10.1128/jb.179.4.1239-1245.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo Y, Gralla J D. Promoter opening via a DNA fork junction binding activity. Proc Natl Acad Sci USA. 1998;95:11655–11660. doi: 10.1073/pnas.95.20.11655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsieh M, Gralla J D. Analysis of the N-terminal leucine heptad and hexad repeats of sigma 54. J Mol Biol. 1994;239:15–24. doi: 10.1006/jmbi.1994.1347. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh M, Tintut Y, Gralla J D. Functional roles for the glutamines within the glutamine-rich region of the transcription factor ς54. J Biol Chem. 1994;269:373–378. [PubMed] [Google Scholar]
- 15.Kelly M T, Hoover T R. Mutant forms of Salmonella typhimurium ς54 defective in initiation but not promoter binding activity. J Bacteriol. 1999;181:3351–3357. doi: 10.1128/jb.181.11.3351-3357.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J H, Hoover T R. Protein crosslinking studies suggest that Rhizobium meliloti C4-dicarboxylic acid transport protein D, a ς54-dependent transcriptional activator, interacts with ς54 and the β subunit of RNA polymerase. Proc Natl Acad Sci USA. 1995;92:9702–9706. doi: 10.1073/pnas.92.21.9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J H, Scholl D, Nixon B T, Hoover T R. Constitutive ATP hydrolysis and transcriptional activation by a stable, truncated form of Rhizobium meliloti DCTD, a ς54-dependent transcriptional activator. J Biol Chem. 1994;269:20401–20409. [PubMed] [Google Scholar]
- 18.Merrick M, Chambers S. The helix-turn-helix motif of ς54 is involved in recognition of the −13 promoter region. J Bacteriol. 1992;174:7221–7226. doi: 10.1128/jb.174.22.7221-7226.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merrick M J. In a class of its own—the RNA polymerase sigma factor ς54 (ςN) Mol Microbiol. 1993;10:903–909. doi: 10.1111/j.1365-2958.1993.tb00961.x. [DOI] [PubMed] [Google Scholar]
- 20.Popham D, Szeto D, Keener J, Kustu S. Function of a bacterial activator protein that binds to transcriptional enhancers. Science. 1989;243:629–635. doi: 10.1126/science.2563595. [DOI] [PubMed] [Google Scholar]
- 21.Rippe K, Guthold M, von Hippel P H, Bustamante C. Transcriptional activation via DNA-looping: visualization of intermediates in the activation pathway of E. coli RNA polymerase ς54 holoenzyme by scanning force microscopy. J Mol Biol. 1997;270:125–138. doi: 10.1006/jmbi.1997.1079. [DOI] [PubMed] [Google Scholar]
- 22.Sasse-Dwight S, Gralla J D. Probing the Escherichia coli glnALG upstream activation mechanism in vivo. Proc Natl Acad Sci USA. 1988;85:8934–8938. doi: 10.1073/pnas.85.23.8934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su W, Porter S, Kustu S, Echols H. DNA-looping and enhancer activity: association between DNA-bound NTRC activator and RNA polymerase at the bacterial glnA promoter. Proc Natl Acad Sci USA. 1990;87:5504–5508. doi: 10.1073/pnas.87.14.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor M, Butler R, Chambers S, Casimiro M, Badii F, Merrick M. The RpoN-box motif of the RNA polymerase sigma factor ςN plays a role in promoter recognition. Mol Microbiol. 1996;22:1045–1054. doi: 10.1046/j.1365-2958.1996.01547.x. [DOI] [PubMed] [Google Scholar]
- 25.Tintut Y, Gralla J D. PCR mutagenesis identifies a polymerase-binding sequence of sigma 54 that includes a sigma 70 homology region. J Bacteriol. 1995;177:5818–5825. doi: 10.1128/jb.177.20.5818-5825.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J T, Syed A, Gralla J D. Multiple pathways to bypass the enhancer requirement of sigma 54 RNA polymerase: roles for DNA and protein determinants. Proc Natl Acad Sci USA. 1997;94:9538–9543. doi: 10.1073/pnas.94.18.9538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y-K, Lee J H, Brewer J M, Hoover T R. A conserved region in the ς54-dependent activator DctD is involved in both binding to RNA polymerase and coupling ATP hydrolysis to activation. Mol Microbiol. 1997;26:373–386. doi: 10.1046/j.1365-2958.1997.5851955.x. [DOI] [PubMed] [Google Scholar]
- 28.Weiss D S, Batut J, Klose K E, Keener J, Kustu S. The phosphorylated form of the enhancer-binding protein NTRC has an ATPase activity that is essential for activation of transcription. Cell. 1991;67:155–167. doi: 10.1016/0092-8674(91)90579-n. [DOI] [PubMed] [Google Scholar]
- 29.Wong C, Gralla J D. A role for the acidic trimer repeat region of transcription factor ς54 in setting the rate and temperature dependence of promoter melting in vivo. J Biol Chem. 1992;267:24762–24768. [PubMed] [Google Scholar]
- 30.Wong C, Tintut Y, Gralla J D. The domain structure of sigma 54 as determined by analysis of a set of deletion mutants. J Mol Biol. 1994;236:81–90. doi: 10.1006/jmbi.1994.1120. [DOI] [PubMed] [Google Scholar]