Abstract
Dietary fibers contribute to structure and storage reserves of plant foods and fundamentally impact human health, partly by involving the intestinal microbiota, notably in the colon. Considerable attention has been given to unraveling the interaction between fiber type and gut microbiota utilization, focusing mainly on single, purified fibers. Studying these fibers in isolation might give us insights into specific fiber effects, but neglects how dietary fibers are consumed daily and impact our digestive tract: as intrinsic structures that include the cell matrix and content of plant tissues. Like our ancestors we consume fibers that are entangled in a complex network of plants cell walls that further encapsulate and shield intra-cellular fibers, such as fructans and other components from immediate breakdown. Hence, the physiological behavior and consequent microbial breakdown of these intrinsic fibers differs from that of single, purified fibers, potentially entailing unexplored health effects. In this mini-review we explain the difference between intrinsic and isolated fibers and discuss their differential impact on digestion. Subsequently, we elaborate on how food processing influences intrinsic fiber structure and summarize available human intervention studies that used intrinsic fibers to assess gut microbiota modulation and related health outcomes. Finally, we explore current research gaps and consequences of the intrinsic plant tissue structure for future research. We postulate that instead of further processing our already (extensively) processed foods to create new products, we should minimize this processing and exploit the intrinsic health benefits that are associated with the original cell matrix of plant tissues.
Keywords: intact plant cells, plant cell wall, minimal processing, colonic microbiota, gut health, short-chain fatty acids (SCFA)
Introduction
Human health is substantially influenced by the food we eat. One food component that especially gained attention during recent years is the indigestible backbone of our plant foods: dietary fibers that cannot be directly utilized by our body. Dietary fibers appear to be an all-round talent reducing all-cause-mortality and protecting against different types of cancer, type 2 diabetes and cardiovascular diseases (1). The hypothesis that fibers have a crucial place in maintaining human health is not new. Already in the 1960’s, Denis Burkitt developed the dietary fiber hypothesis placing dietary fibers at the origin of numerous diseases occurring in high-income countries with a Western lifestyle (2, 3). At that time, many beneficial effects of fibers were linked to gut microbiota-independent effects. These were based on physicochemical properties of fibers, like retaining water, which increases stool bulk and speeds up transit time, or bile-acid binding, which reduces cholesterol levels (2). Gut microbiota-dependent health benefits of fibers where hypothesized, but could not be determined as the present culture-independent high-throughput tools and mechanistic insights were lacking (2, 4, 5). This has changed during the last 25 years (3). As dietary fibers are not broken down by human endogenous enzymes these are passed down to the lower gut where they are utilized by the gut microbiota, mainly consisting of bacteria residing in the colon (6). The gut bacteria thereby produce various metabolites, most importantly short-chain fatty acids (SCFA) that mediate some of the microbiota-dependent effects of fibers (7–9). These effects can range from locally interacting with epithelial and immune cells affecting barrier function and gut homeostasis (8–10) to peripherally impacting organs either by receptor-binding or by exerting direct effects (7, 11). As a consequence, modulating gut microbiota activity and composition by fiber intake holds potential new therapeutic avenues. In this context, distinct fiber types have been studied to elucidate their specific microbiota-dependent and -independent effects on human health (12). This has led to a continuous effort to further narrow down the underlying fiber-microbiota interactions in vitro and to understand the specific molecular make-up of fibers as for instance leading to the immunomodulatory potential of different fiber chain lengths and structural features (13, 14). These efforts, however, follow a reductionist approach that considers fibers as loose entities (15–17). This single-fiber-approach is in line with Western food processing and food design practices but neglects the original form in which fibers are present and consumed during our evolution: as part of whole foods and often only minimally processed.
Here we elaborate on the need to change our understanding of dietary fibers to unlock their full potential to modulate gut microbiota in relation to human health. We explain how dietary fibers exist in nature and discuss how this holistic view differs from the current approaches. Finally, we explore the existing intervention studies of intrinsic fibers in relation to human health and discuss potential consequences for future research avenues.
Current view of dietary fibers: single, isolated components and their impact
Dietary fiber is an umbrella term for a group of polymers that are structurally and chemically very different. Numerous sugar molecules, such as glucose, xylose, mannose, galactose, arabinose and rhamnose are linked together by various glycosidic bonds following a specific or random pattern creating linear or branched structures that can be further decorated with phenolic acids or linked to other compounds (18, 19). For instance, inulin and starch consist of linear repetitions of a single molecule (fructose and glucose monomers, respectively). In contrast, rhamnogalacturonan – a type of pectin – is a highly branched molecule with various side-chains of different monomers (20). What makes them dietary fibers is the common trait of their bonds not being broken by human endogenous enzymes.
The enormous variation in fiber structures brings along physicochemical properties that are often classified into “soluble versus insoluble”. This classification, despite being widely used, is only based on fiber content analysis of foods rather than functional behavior of the fibers in the human gut (21–25). Functional fiber properties are therefore better described in terms like bulking, viscosity and fermentability (23). Fermentation of the different fibers by the gut microbiota requires microbes to have at their disposal a set of enzymes that can break down the specific chemical linkages and types of molecules (6, 26). An often-observed feature of microbial communities is functional redundancy, meaning that taxonomically different members of the gut microbiota can perform similar functions, e.g. break down the same type of fiber (6, 27, 28). Microbes within the gut microbiota community can assist each other via cross-feeding, with primary degraders cleaving polymers into smaller compounds that can be further broken down by others and finally be converted into SCFA (6, 28–30). Since the vast majority of the gut microbiota consists of mainly anaerobic bacteria, these are the main contributors of the fiber degradation, occasionally supported by the action of methanogens that convert generated hydrogen into methane (29, 31). While some of these bacteria are adapted to metabolize a wide range of polymers, others appear to be more specialized (6, 28). Moreover, specific bacteria have been coined to be so-called “keystone” species as they are unique players in the metabolic networks for the breakdown of compounds or the production of specific metabolites (6).
Multiple intervention studies with isolated, single fibers have been reviewed elsewhere (32–38). The emerging picture is that i) a range of different fibers are able to stimulate a more diverse range of gut bacteria (39), and ii) chemically and structurally complex fibers can be used to specifically target bacteria relevant for human health (40–42). Using this approach, efforts have been made to define differences in the fine structure of fibers and relate these to the specific gut microbiota response and health outcomes (12, 39, 43). For instance, in a recent human trial wheat bread was enriched with a variety of fibers like wheat dextrin, micronized wheat bran, oat flakes and bran, inulin, locust bean gum and pectin, which to some degree decreased cholesterol, insulin and HOMA-IR levels and these changes were linked to an increase in the gut microbiota able to break glycosidic bonds (44). Similarly, another recent human trial in overweight individuals linked the gut microbiota’s ability to ferment arabinoxylan but not crystalline cellulose to an observed increase in satiety (45). Moreover, in vitro assessment of different fast-fermentable fiber types indicated a delayed fermentation rate for some fibers when presented in a mix instead of alone (46). A delayed fermentation rate is hypothesized but not yet shown to be a desired feature for the therapeutic application of fibers in the treatment of irritable bowel syndrome (23). All these studies have as a common principle that they rely on single fibers or fiber extracts that have been isolated from the plant tissue they originate from. Understanding the isolated effects of these single fiber types has its place in determining trophic chain interactions between microbes (29) and to unravel underlying mechanisms for specific fiber applications in e.g. the medical field (47). However, looking at fibers as isolated components overlooks one aspect of dietary fibers: how we eat them.
Intrinsic fibers: complexly intertwined three-dimensional structures
When we discuss dietary fibers, we mainly think of them as single, loose compounds. However, this contrasts with their “natural form” - the form already recognized by Burkitt to entail the crucial health benefits of fibers. The bulk of fibers we eat are not single, isolated fibers, but part of plant foods like vegetables, fruits, seeds, nuts, legumes and grains (22, 48–51). We consume the tissues of these plants, which are made up from a matrix of plant cells (52). The backbone of this matrix are dietary fibers that are complexly intertwined into a three-dimensional network creating plant cell walls (52). These plant cells can further encapsulate other fibers that are stored in the vacuoles, which are either fructans or starch serving the plant as reserve for growth (53, 54). During recent years, awareness has risen that the existence of this three-dimensional plant cell matrix has important consequences for digestibility and health while very likely exerting different effects than the single, isolated fibers. For this purpose, a distinction was made between isolated fibers and fibers in their natural, three-dimensional form. The latter fibers were termed “intrinsic fibers” (22) referring to these as being an intrinsic part of the plant cell wall.
Make-up of intrinsic fibers
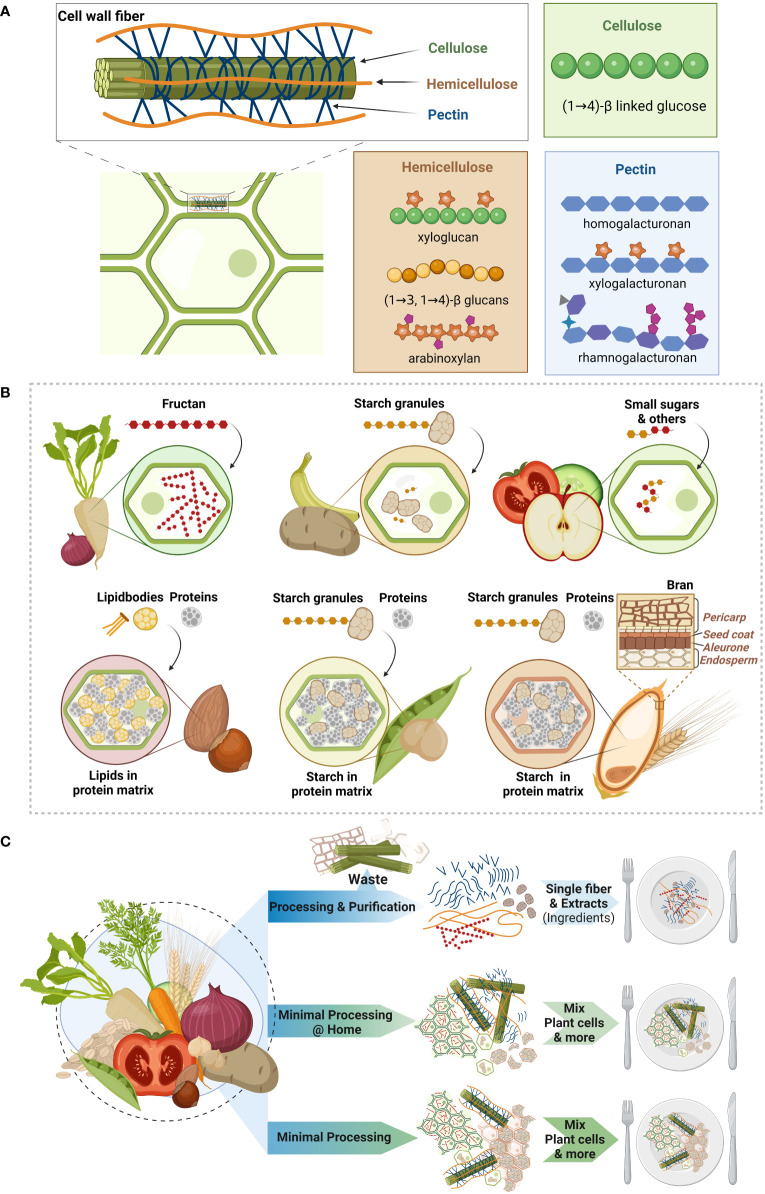
The make-up of plant cells follows a common principle, which consists of three main building blocks: cellulose, hemicellulose and pectin ( Figure 1A ) (52, 55). Cellulose is considered as basic scaffold of the cell wall, being a linear polymer of β(1→4) linked glucose units that align into rigid microfibrils. These microfibrils are reinforced by hemicellulose ( Figure 1A ), which is a group of structurally very diverse polymers with either glucose-xylose (xyloglucans), a glucose (mixed-linkage glucans) or xylose (xylan) or mannan backbones (24, 56). To these backbones other sugar molecules and also phenolic acids can be linked, such as the hemicellulose type arabinoxylan (56, 57). Finally, pectin serves like a filler giving the cellulose-hemicellulose scaffold stability, controlling permeability and together creating a matrix that can retain water ( Figure 1A ) (55, 58). Similarly to hemicellulose, also pectin is a group of structurally diverse polymers, which have a linear galacturonic acid backbone (homogalacturonans) or a galacturonic acid and rhamnose backbone (rhamnogalacturonans) that can be further decorated with side-chains forming complexly branched polymers ( Figure 1A ) (20, 24, 59). It is essential to understand that these different polymers are physically entangled and partly also chemically bound to each other, despite the fact that their precise organization is still not fully understood (55). These complex structures form together three-dimensional plant cells that are glued together by pectin, which fills the intercellular space between connecting plant cells (55). Besides the fiber polymers, plant cell walls also contain small amounts of proteins (arabinogalactan proteins) and minerals (52, 55). Finally, certain specialized cell-types are reinforced with lignin, which is a dietary fiber consisting not of sugars but of different types of phenolic phytochemicals that are complexly linked (52, 55). While all plants share these common aspects of the plant cell wall, their make-up differs depending on the type of plant (e.g. monocots versus dicots; see below), its maturation or ripeness and especially food processing (18, 52, 58). Even within plants, differences in cell types exist between the tissue we consume (such as leaf, root, fruit, stem, seed) and within these tissues (52, 56).
Figure 1.
Intrinsic make-up of dietary fibers in plant foods (A) Dietary fibers are the backbone of plant foods that we consume, like grains, legumes, fruits, vegetables, nuts and seeds. The intrinsic make-up of plant tissue is created by the plant cell wall fibers cellulose, hemicellulose and pectin. Cellulose (green) forms fibrils, which are stabilized by hemicellulose (orange) and further strengthened by pectin (blue) forming a complexly intertwined three-dimensional structure. While cellulose is a structurally simple molecule made of glucose molecules that are β(1→4) linked, hemicellulose and pectin are two groups of structurally very diverse cell wall fibers. An abundant hemicellulose in dicot plants (like legumes) is xyloglucan, while monocot plants (grains) have higher amounts of mixed linkage β-glucans (e.g. oats) and arabinoxylans (e.g. wheat). Pectin can either consist of linear chains of monomers (homogalacturonan), that can be esterified and further decorated with other sugars (xylogalacturonan) or of several molecules with side-chains made of other compounds (rhamnogalacturonans). (B) The complex cell wall fiber structure encapsulates various other nutrients in the plant vacuole like storage carbohydrates, which can be either fructan – a dietary fiber with a fructose backbone - or starch. Starch is stored in starch granules, which are found in vegetables and fruits like potatoes and (unripe) bananas. Other fruits and vegetables’ plant cells contain vacuoles mainly filled with water and other nutrients (e.g. small sugars). Nuts contain lipid bodies that are embedded within the vacuole in a protein matrix, while in legumes starch granules are embedded in the protein matrix. Finally, in contrast to the beforementioned dicot plants, grains, which are monocots, have different plant cell walls but also have starch granules embedded in a protein matrix. This starch-protein matrix is surrounded by the so-called aleurone layer, the seed coat and the pericarp that form together the so called bran. (C) Dietary fibers can be processed in various ways that either disintegrate or maintain the three-dimensional plant tissue matrix. Common approaches in the industry are to extract and purify dietary fibers into single fibers creating (new) ingredients that are used to create new food products. Cooking and other domestic processing types maintain the tissue matrix to different extents but can generally provide intact plant cell wall structures. Finally, we propose that similar to domestic processing, plant foods should be minimally processed such that the plant cell wall structure and overall plant tissue matrix are maintained. The latter opens avenues for new, convenient and minimally processed fiber products that exploit the health benefits of the intrinsic plant structure, while aligning with the need for more sustainably produced, plant-based food solutions. Created with BioRender.com.
As hemicellulose and pectin are groups of very heterogenous polymers, the specific types of these fibers that are present in the plant cell walls distinguish different plant types (52, 58). Vegetables, fruits, nuts, seeds and legumes belong to the dicots, that have so-called type I cell walls, while grains are monocots having type II cell walls. Type I cell walls of dicots contain xyloglucan as the most abundant hemicellulose and the hemi-/cellulose network is further stabilized by pectin, which has been reported to make up a third of the cell wall weight (18, 52). In contrast type II cell walls of monocots, have no or very low amounts of pectin and the abundant hemicelluloses are β-glucan and arabinoxylan (52, 58). Rice is one of the grains that contains some pectin, while oats and barley are particularly rich in β-glucan, and wheat, maize and rye in arabinoxylan (57). Another distinguishing feature of grains is the coating that covers the grain kernel. This outer coating consists of several layers known as the aleurone layer, the seed coat and the pericarp, which together form what we know as “bran” (see Figure 1B ) (60). Bran is therefore not a homogenous compound but contains different cell types that have a different ratio of hemicellulose types than the starchy endosperm (57).
The three-dimensional capsules that are created by the cell wall fibers contain the plant cell with its vacuole in which other nutrients like lipids, proteins and carbohydrates as well as phytochemicals are stored ( Figure 1B ) (53). Depending on the plant type, the encapsulated contents can profoundly differ. For instance, plants store reserve carbohydrates in their vacuoles. These can either be fructans (54), or starch, which is stored in the form of starch granules ( Figure 1B ). While fructans classify as dietary fibers as their glycosidic bonds cannot be broken down by human endogenous enzymes, most types of starch can be digested with some resistant to digestion due to their physical state (resistant starch). Many vegetables and fruits have plant cells whose vacuoles are filled with mainly water, which maintains the internal pressure (turgor) and keeps an open cell structure that provides the food product its characteristic crispiness/crunchiness ( Figure 1B ). The vacuoles also contain other small molecules, such as sugars and phytochemicals (53). In legumes and nuts the vacuoles are filled with a protein matrix, embedding starch granules and lipid bodies, respectively ( Figure 1B ). Finally, the starch granules in grains are also embedded in a protein matrix within the cell walls ( Figure 1B ).
The three-dimensional make-up of plant cells, the physical and chemical entanglement and the encapsulation of other fibers and nutrients have important consequences for the gut microbes’ ability to access the individual fibers. Food processing can substantially influence and destroy these intrinsic structures and is used to extract, isolate and purify single fibers. Moreover, in contrast to these isolated fibers, during upper gut digestion the cell wall fibers do not simply dissolve and become freely available as isolated components but remain to a considerable extent within their intertwined matrix. Hence, the make-up of the plant food cell matrix, food processing and digestion influence how intrinsic dietary fibers are altered in the gut and fermented by the gut microbiota.
Food processing impacts the intrinsic fiber make-up
Whether a fiber can be classified as intrinsic fiber and how further digestive processes influence its digestive fate depends largely on its processing. As intrinsic fibers are an intrinsic part of the plant cell wall whole, raw foods, like unprocessed apples, bananas, cucumbers, tomatoes etc. contain by definition intrinsic fibers. However, any form of processing - industrial as well as domestic - can alter this three-dimensional structure (19, 24, 52, 58, 61, 62) and whether a fiber is still an intrinsic fiber needs careful checking of the applied methodology.
In general, food processing has as aim to preserve foods, increase digestibility or extract compounds that can be used to create new foodstuffs. To do so, a plethora of techniques can be applied which include mechanical (physical), chemical, thermal and enzymatic processing as well as high pressure, ultrasound and microwave technologies (61, 63). While industrial processing often aims at extracting single fibers for specific food applications, domestic processing is usually gentle and does not fully disintegrate the intrinsic fiber structures ( Figure 1C ). The effects of different processing techniques on the physicochemical properties (64) of fibers as well as cell wall integrity and overall cell structure has been reviewed by others (19, 24, 52, 58). Hence, here we give only a small overview of these techniques to provide a basic understanding of their consequences for intrinsic fibers.
Possibly the most gentle food processing techniques in terms of safeguarding the overall intrinsic tissue structure are drying, freezing and freeze-drying, which aim to conserve food products. While drying leads to shrinkage of the cell tissue, freezing and freeze-drying maintain the open cell structure, but the respective plastic changes of drying and water crystals formed during freezing can induce cracks in the cell wall (65). However, the extent of the caused damage depends on the applied conditions. Consequently, despite cell wall cracks the overall tissue is largely maintained reducing the possible release of intracellular components. Similarly, hydrothermal processing can maintain the overall tissue structure, while inducing more profound damage to cell walls mainly by affecting pectin (24). Boiling or steaming can result in swelling of cell walls and dissolution of pectin (24, 52). When pectin leaks out of the intercellular space it weakens the overall cohesion between cells. This can then lead to separation of plant cells, but also to cell wall rupture. Moreover, leaking of pectin increases the space between the fibers within cell walls, which is called cell wall porosity (24, 52, 58). Dry thermal treatments like baking, roasting or popping are more violent processing types, fundamentally damaging the cell wall, but can also maintain overall tissue structure (52).
Mechanical (physical) destruction, like milling/grinding, generally break cell walls releasing the encapsulated contents (52, 58, 66). The finer the particle size, the less likely it is that whole plant cells are still present and the more likely that parts of the three-dimensional organization of cell walls have undergone physical destruction. Fine milling of the starchy endosperm of grains for instance leads to complete disruption of plant cells, breaking the protein-starch matrix and releasing the enclosed starch granules ( Figure 1B ) (19). Similarly, the complex structure of bran can be substantially destructed with fine milling (49). In this context we want to highlight that despite the suggestive name, whole grain products do not necessarily provide intrinsic fibers (35, 67). The reason is that for the production of whole grain products, first the components bran, germ and the starchy endosperm are separated and each of these components is milled (25, 67). After processing, these fractions are reconstituted together, which allows the product to be defined as whole grain (67). Hence, when we read about whole grain products, we refer to the processed and reconstituted fractions of the whole grain instead of the intact grains (67–69). The intact cells in grains have, however, specific effects on starch accessibility and the gelatinization during heating and thereby impact whether starch escapes upper gut digestion becoming available to the colonic microbiota (52, 70).
In summary, mechanical processing, either domestic or industrial, has major detrimental effects on the intrinsic fiber structure and for instance, dried, pulverized fibers likely do not behave like dried fibers that have undergone minor mechanical destruction. Nevertheless, in these powdered preparations, cell-wall parts might still be present, but their particle size might be very small, increasing the accessibility for gut bacteria. Finally, extrusion, a processing technique where food is subjected to high pressure, high shear and high temperature, is one of the most violent processing techniques (62) as it can completely degrade even the rigid cellulose fibrils (71).
In order to produce single, isolated fibers further extensive extraction techniques are needed that separate the specific fibers from the three-dimensional plant cell matrix (61, 63). Some of these fibers are water-extractable like certain arabinoxylans, which are hence termed “soluble”, while others need the application of enzymatic, gravitational and/or chemical treatments in order to be extracted (57, 63). During these extensive extraction processes, a variety of waste streams are created that might still contain valuable residual fibers and intracellular components. However, the integrity of these structures depends on the harshness of the applied processing. Therefore, fiber products that are based on waste streams might or might not contribute intrinsic fibers. While in some cases the waste streams can be valorized (72, 73), in many cases the production of fibers have a considerable carbon foot print and do not contribute to a circular economy (61, 74).
It is crucial to realize that when we investigate the isolated behavior of single dietary fibers, we in fact study fibers that have undergone extensive processing in order to be released, separated and purified from plant cells (61). It is highly doubtable, that the effects and behaviors of these isolated fibers sufficiently reflect that of intrinsic fibers (52). To illustrate this, we will below describe the impact of intrinsic fibers on digestibility in vitro followed by insights from human interventions.
Implications of intrinsic fibers on digestion
It is clear that food processing substantially impacts how fibers behave in the gut during digestion (24). While the behavior of isolated, single fibers has extensively been studied, the digestive fate of plant tissues is far less understood. The limited research that assessed plant tissue and single plant cells has focused more on the upper gut mainly relying on in vitro models and animal in vivo models. There are some studies that assessed human ileostomy effluents (with carrots) (75) or ileal samples (with white beans) (76), but very few studies followed the digestion of intrinsic fibers until the colon (77). Here, we will only highlight the main consequences of upper gut digestion for intrinsic fiber structures and refer the reader to the comprehensive reviews by others (19, 24, 52, 58, 78).
Our general understanding of digestion is the breakdown of food matrices into their building blocks and subsequent absorption. However, when consuming plant food tissues with an intact cell matrix, the constituents will not simply dissolve as they are intertwined within the cell wall and in this form not readily water-soluble under physiological conditions (24, 25). Similarly, encapsulated compounds cannot dissolve directly into the gut environment and will be shielded from immediate digestion, delaying their breakdown. In general, the orogastric processes lead to size reduction of plant tissue fractions, and dissolution of certain water-soluble pectins (52). During chewing, plant tissues are degraded into smaller sizes, which depending on the physical state of the plant type (hard, soft), ripeness and preparation methods (like cooking), happens by cell rupture (hard foods) or separation along the cell walls (soft foods) (24, 52, 58). Hence, a mix of differently sized particles containing intact and broken cells and their contents arrives in the stomach. There, further particle breakdown can occur by the mechanical gastric forces. It is generally believed that particles smaller than a few millimeters pass unchanged into the small intestine and, hence, intact plant cells can arrive into the small intestine. Larger structures are retained for further size reduction (called gastric sieving) (58). However, whether this also applies to soft plant tissue is not clear. Pectin can leak from the intercellular spaces (24, 52, 58) and thereby reduce overall cohesion within plant particles. Whether also water-extractable hemicelluloses, like arabinoxylan, can leak out is not known but rather unlikely (24, 57). That means that plant particles with intact cells arrive in the colon. Indeed this has been confirmed from analysis of ileostomy effluents and ileal samples (75, 76). The mixture of broken and intact cells is available to the gastric and small intestinal digestive enzymes to be degraded further, but released fructans and certain resistant starches will resist digestion. Due to pectin leakage cell wall porosity might increase. However, based on available literature, the diffusion of enzymes into intact cells and the diffusion of nutrients out of the cell is believed to be limited by the cell wall pore size (52, 58). Interestingly, the presence of plant cell wall material has been shown to reduce enzymatic breakdown of starch due to adsorption of α-amylase to the plant cell material (19, 52, 58).
In cases of mild food processing, the fractions of intact plant tissue that we swallow likely arrive in the colon in a rather intact state, mainly affected by chewing and dissolution of pectin ( Figure 1C ). Hence, the bacteria in the colon are confronted with a mix of plant tissue particles, intact plant cells and broken cell wall material and its contents. Dissolved fibers, like released fructans or starch (and possibly leaked pectin) can be directly used by the gut bacteria. However, since bacteria are too large to diffuse into intact plant cells, these must spatially interact with the plant tissue, to access intertwined polymers such as pectins and hemicelluloses. All these digestive aspects make it obvious that the breakdown and consequent physiological behavior of intrinsic fibers cannot or can only partly be explained by that of their isolated single counterparts (49, 52).
Gut microbial intrinsic fiber breakdown
The number of studies that have investigated the microbial breakdown of intrinsic fibers in the colon is impressively low especially regarding human in vivo studies. From in vitro studies we have a fair understanding of which type of bacteria and bacterial enzymes are generally involved in metabolizing specific isolated fiber types (38, 79). The different enzymes employed by gut bacteria can be classified according to carbohydrate-active enzymes classes (CAZymes; see www.cazy.org (80)), and bacteria differ in the number and type of enzymes they have, as thoroughly reviewed elsewhere (26). The most abundant microbial enzyme class are glycoside hydrolases, cleaving neighboring sugar and non-sugar moieties. Other less abundant enzymes are polysaccharide lyases specialized in the cleavage of uronic acid moieties as present in pectins, and carbohydrate esterases cleaving off esterified groups as present in pectins and hemicelluloses. These enzymes may be assisted by other so-called carbohydrate binding modules or auxiliary activities (80). The bacterial enzymes involved in storage carbohydrate breakdown are relatively well established, like the GH13 subclass for starch and the GH32 subclass for fructan utilization (26), and the discovery of enzyme systems to disintegrate the cell wall fibers pectin (81) and hemicellulose is ongoing (82). However, our knowledge on how gut bacteria interact with the most robust compound of the plant cell wall, the cellulose microfibrils, is still very limited (6, 29). While amorphous cellulose is believed to be partly utilized by employing a complex enzyme system, crystalline cellulose has been reported to be not fermentable (6, 29). Bacteria were found to adhere to cell walls on almond particles (77) and coarse wheat bran (83) recovered from human feces, which indicates that bacteria possibly use the crystalline cellulose backbone for particle adherence (29, 84). A small set of studies tried to identify the bacterial communities colonizing these plant tissue fractions (84–87). Bacteria recovered from plant particles in human feces differed in the detected phyla compared to the liquid fraction (84, 85). Moreover, the type of recovered particles (bran versus resistant starch, differently processed brans) impacted which bacteria adhered to them (86, 87). Based on these observations the breakdown of plant cell walls is believed to be initiated by primary degraders able to interact with the insoluble plant cell material. Subsequently, material is released from cell wall compounds which other bacteria use to cross-feed (6, 29, 84). Some studies have addressed these plant cell-bacteria biofilms and the action of bacterial enzymatic systems that would be needed to degrade cell walls (29, 88, 89). However, how exactly the spatial interaction of these cooperations would look like and how plant cells are exactly opened-up by gut bacteria is unclear. Future research distinguishing between particulate and liquid fecal phases and using imaging techniques combined with identifying bacterial communities will offer exciting insights into these aspects.
It is generally believed that fermentable fibers are readily metabolized in the proximal colon. However, if readily fermentable fibers are not in isolation but either part of plant cell walls or encapsulated by them, the fermentation rate is likely to be slowed down. This has indeed been supported by the results of in vitro experiments. For instance, in vitro assessment of plant cell-encapsulated cereal starches revealed that microbial enzymatic activity was first directed towards cell wall fiber degradation (pectin, xylan and cellulose) followed later by the slow degradation of starch and involving different microbial communities (90). Accessible intracellular material from broken cells also impacts the fermentation kinetics of plant cell walls, as demonstrated in vitro with differently processed wheat bran. A reduced SCFA production was found in wheat bran from which remaining starch fractions had been enzymatically removed (87). In the same experiment, the effect of fine milling was demonstrated with micronized (very finely milled) bran leading to higher initial SCFA production in vitro compared to unmodified bran. This observation had been made previously and attributed to a higher bacteria-to-surface ratio with smaller particle sizes (91). However, at the end of the fermentations, SCFA levels were similar (87, 91, 92), but the ratios between SCFA differed, with larger bran particles producing more butyrate (91, 92). This is particularly interesting since more extensive processing of bran, such as extrusion, is considered desirable as it makes the constituents more accessible for microbial fermentation by degrading the three-dimensional bran structure. However, this may reduce the potentially health-promoting butyrate production (57, 93, 94). In summary, the intrinsic structural features of the plant cells likely slow down fiber fermentation, inducing a lag phase (58), but do not necessarily reduce the absolute amount of SCFA produced. Consequently, there is a gradual release of SFCA, which means that SCFA production is not restricted to the proximal colon but spread throughout the whole colon, including its distal parts benefitting local, mucosal health. This likely translates into beneficial systemic, peripheral effects as distal SCFA infusion in vivo has shown to induce more pronounced effects on biomarkers than proximal (95). Also delayed fermentation has been proposed as a desired feature of fibers in the treatment of irritable bowel syndrome but lacks presently experimental verification (23). In summary, the delayed fermentation of intrinsic fibers presents a highly relevant feature that isolated, single fibers do not have.
Insights from existing human studies
Whether the observed features of intrinsic fibers in vitro also occur in vivo and how these effects are reflected in health outcomes is not yet clear. To advance our insight, we summarized human intervention studies that investigated the effect of intrinsic fibers on gut microbiota composition and activity and/or related metabolic and bowel function outcomes ( Table 1 ; extended version in Supplementary Table S1 ). We included randomized-controlled trials (no acute testing, patient-control or single-arm designs) published during the last 20 years. These trials either assessed diets based on whole foods or used one specific food (including vegetables, fruits and nuts) either in its fresh, cooked or dried form. If whole foods were further processed during the study to be incorporated into meals we did include those as well, but excluded pulverized versions of food products as the mechanical processing extensively destroys the plant matrix. Similarly, we excluded fruit and vegetable pastes, juices or extracts as these underwent extended processing. For each study we have presented the processing type based on the reported information as to understand the impact on the intrinsic fiber structure. We did not include studies using waste-stream products as the level of information on the applied processing is generally insufficient to decide whether intrinsic structures are maintained or not. Based on provided information on fold-changes in relative abundance of gut microbial taxa we estimated the modulatory potential to be either small (<1.5-fold), moderate (1.5 - 2.5-fold) or large (>2.5-fold), which is summarized in an extended version of Table 1 ( Supplementary Table S1 ).
Table 1.
Human intrinsic fiber studies.
| Intrinsic fiber | Processing | Study design | Gut microbiota composition | Microbiota activity | Metabolic markers | Bowel function | Reference |
|---|---|---|---|---|---|---|---|
| Whole diets | |||||||
| Mediterranean diet | Whole foods; incorporated into meals @Home | RCT, 1 year, parallel (dietary advice & provided foods) | Yes ‡ | – | Yes | – | (96, 97) |
| Nordic diet | Whole foods; incorporated into meals @Home | RCT, 18 or 24 weeks, parallel (dietary advice & provided foods) | Not assessed | – | Yes | – | (98, 99) |
| Macrobiotic diet | Whole foods; incorporated into meals by cooks | RCT, 3 weeks, parallel (controlled diet) | Yes ‡ | – | Yes | – | (100) |
| Bran | |||||||
| Wheat bran (20 g/day) | Coarse vs fine | RCT, 4 weeks, parallel | – | – | – | Yes | (101) |
| Wheat bran (12-22 g/day) | Raw vs cooked | RCT, 2 weeks, cross-over | – | – | – | Yes (raw) | (102) |
| Wheat bran (20 g/day) | Reduced in size | RCT, 4 weeks, parallel (normal/obese) | No change | No change | Yes (obese) | No change | (103) |
| Grains | |||||||
| Barley (75 g/day) | Whole kernels, boiled, in bread (no milling) | RCT, 4 weeks, cross-over | – | – | Yes | – | (104) |
| Barley vs brown rice vs mix of both (60 g/day) | Whole kernels, cooked | RCT, 28 days, cross-over | Yes, moderate Δfold | – | Yes (mix) | – | (105) |
| Coix (160 g/day) | Whole kernels, cooked | RCT, 1 week, parallel | Yes, small Δfold | – | Yes | – | (106) |
| Nuts | |||||||
| Walnut (42 g/day) | Whole | RCT, 3 weeks, cross-over | Yes, moderate changes | – | Yes | – | (107) |
| Almond (57 g/day) | Whole, roasted | RCT, 6 weeks, parallel | Yes, small & large Δfold | – | - | – | (108) |
| Almond (42 g/day) | Whole raw, whole roasted, chopped roasted, almond butter | RCT, 3 weeks, cross-over | Yes, moderate to large Δfold (most chopped) | – | - | – | (109) |
| Almond or pistachio (43 or 85 g/day) | Whole | RCT, 2.5 weeks, cross-over | Yes ‡, stronger pistachio effect | – | – | – | (110) |
| Legumes & Seeds | |||||||
| Chickpea or raffinose (200g vs 5 g/day) | Canned; incorporated into soups & desserts | RCT, 3 weeks, cross-over | Yes ‡ | No change | – | – | (111) |
| Linseed, sunflower & sesame seed, wheat grain, haricot & kidney bean, chickpea | Whole vs ground; incorporated into meals (no milling) | RCT, 1 week, cross-over, (controlled diet) | – | Yes | – | Yes | (112) |
| Vegetables | |||||||
| Broccoli, cauliflower +/- green & red cabbage(~ 800 g/day) | Raw and incorporated in soup or microwaved | RCT, 2 weeks, cross-over (controlled diet) | Yes ‡ | – | – | – | (113) |
| Broccoli and cauliflower (168 +/- 300 soup g/day) | Frozen & steamed or incorporated into soup | RCT, 2 weeks, cross-over | Yes ‡ | – | – | – | (114) |
| Chicory root (30 g/day) | Dried, cut into cubes (3 g) | RCT, 3 weeks, parallel | Yes, large Δfold | Yes | Yes | Yes | (115) |
| Fruits | |||||||
| Avocado (1 piece/day) | Wholefood | RCT, 12-weeks, parallel (hypocaloric diet) | Yes, moderate to large Δfold | – | Yes | – | (116) |
| Avocado (140-175 g/day) | Whole food, part of meal | RCT, 12 weeks, parallel (partly controlled diet) | Yes, small Δfold | Yes | Yes | – | (117) |
| Mango (300 g/day) | Whole food | RCT, 4 weeks, parallel | – | Yes | Yes | Yes | (118) |
| Kiwi (2 pieces/day) | Whole food | RCT, 3 days, cross-over | – | – | – | Yes | (119) |
| Date (~50 g/day) | Dried | RCT, 3 weeks, cross-over | No change | Yes | – | Yes | (120) |
| Prune (80 or 120 g/day) | Dried | RCT, 4 weeks, parallel | Yes, small Δfold | No change | – | Yes | (121) |
| Raisin (120 g/day) | Dried | RCT, 3 weeks, cross-over | – | Yes | – | Yes | (122) |
RCT, randomized-controlled trials; Δfold, fold changes in relative abundances; ‡ no information on fold changes provided. We selected randomized-controlled trails that have either used diets or single whole foods to assess their modulatory potential on gut microbiota and/or related health effects. These trials were published during the past 20 years, except for two of the bran studies. Even though bran does not necessarily classify as intrinsic fibers (depending on its processing), we did include some old and recent bran studies due to the long and ongoing interest in this type of plant food. Details of the trials are provided in the Supplemental Table S1 .
Whole food interventions are particularly relevant in the context of intrinsic fibers as subjects do not consume one but a variety of differently processed intrinsic fibers. Most of these studies ( Table 1 , Diets) have in common that they emphasize whole grains, fruits and vegetables often combined with legumes and nuts (96), specific types of grains and berries (98) as well as dairy and animal products, while others can be more restricted (macrobiotic diet) (100). These diets have been linked to numerous beneficial health outcomes, like improvements in markers of cognitive function (97), inflammation (97), lipid (98–100) and glucose metabolism (98, 100), and in subjects adhering to these diets these changes were associated with increased levels of gut microbial taxa involved in fiber breakdown and short-chain fatty acid production (e.g. Faecalibacterium spp.; Supplementary Table S1 ).
Although strictly speaking wheat bran is not necessarily an intrinsic fiber as its intactness largely depends on the degree of processing, we also included old and recent wheat bran studies ( Table 1 , Bran). Bran has been investigated in the 1970’s and 1980’s for its modulatory effect on lipid metabolism and bowel function and current approaches potentially provide insights into underlying gut microbiota modulation. Especially the early studies assessed processing effects, like different milling degrees and concluded that only coarse bran reduced transit time and intraluminal pressure ( Supplementary Table S1 ) (101). Also hydrothermal processing (cooking) impaired effectiveness of wheat bran and only raw but not cooked bran influenced bowel function ( Supplementary Table S1 ) (102). Surprisingly, with the available high-throughput technologies only very limited effects of bran on gut microbiota composition and activity were observed in a recent study with bran of different particle sizes ( Supplementary Table S1 ) (103). However, also in bread-based interventions and acute settings few effects are found (123, 124), likely linked to the applied processing (fine milling, and enzymatic treatment). Moreover, bran and enclosed starch might have a synergistic function in the whole kernel, as in isolation they have been found to distinctly and differently impact gut microbiota (125, 126). For instance, intake of resistant starch increased the relative levels of Ruminococcaeae and decreased members of the Lachnospiraceae family, while wheat bran induced the opposite effect (125, 126). In contrast, two whole-kernel barely interventions with or without brown rice (104, 105) and a coix seed intervention (106) did modulate gut microbiota response by increasing health-associated fiber-degraders such as Bifidobacterium, Roseburia or Faecalibacterium spp. and further impacted metabolic outcomes, like decreasing inflammatory markers ( Table 1 , Grains; Supplementary Table S1 ). This indicates that the whole kernel of grains likely acts differently than the separated, milled and reconstituted kernel fractions in whole-grain products. Moreover, this confirms the notion described above that whole grain products do not necessarily provide intrinsic fibers (35, 67).
Nuts are another increasingly studied source of intrinsic fibers as their plant cells are filled with lipid bodies ( Figure 1B ). Information on lipid-degrading gut bacteria is limited and, generally, the amount of fat reaching the colon is believed to be low as most fats we consume are of animal origin or extracted from plants (oil) (127). Lipid bodies in nuts, however, resist upper gut digestion (77, 128, 129). Consequently, studying the impact of nuts on the gut microbiota is of interest and a considerable number of studies assessed the modulatory potential of especially walnuts and almonds in various processed forms, as has been reviewed comprehensively (130). Indeed, nuts generally appear to exert moderate effects on various taxa, yet these effects are not consistent within and between nut types ( Supplementary Table S1 ). One study assessed the effect of different almonds preparations and as expected their most processed form, which was almond butter, had only a small to moderate modulatory effect on the affected taxa when consumed ( Supplementary Table S1 ) (109). In contrast, chopped and roasted almonds exerted the largest modulatory effect on the affected taxa (109). It is tempting to hypothesize that increased particles size improves but roasting impairs the efficiency of the intrinsic matrix disintegration in the upper gut (128, 129), which in turn impacts gut microbial breakdown. Unfortunately, these nut studies did generally not assess other parameters like fecal SCFA or bowel function ( Table 1 , Nuts).
To the best of our knowledge only one human study tried to assess the difference between isolated and intrinsic fiber structure in adult volunteers (111). In this study canned chickpeas were compared to raffinose ( Table 1 , Legumes & Seeds). While the intactness of the plant cell structure was likely already impaired in the canned chickpeas, we hypothesize that this was even further reduced by mechanical processing, as both fiber types were incorporated into soups and desserts. Both fibers slightly affected the gut microbiota composition compared to control, with the suggestion that chickpea induced a decrease of ammonium-producing strains (111). Another study assessed a variety of whole seeds, legumes and wheat grains mixed together and compared them against their ground form incorporated into meals (112). Both types of meals improved bowel function but only the meals with unground seeds, legumes and grains increased fecal butyrate as well as total SCFA ( Supplementary Table S1 ) (112).
Vegetables have been rarely investigated in their intrinsic form and fruits are mainly assessed dried, but not fresh ( Table 1 , Vegetables). This might be due to the fact that more attention has been given to their phytochemicals, which require cell breakage to be released from the vacuole. Two studies assessing cooked cruciferous vegetables prepared in various dishes observed modulation of the gut microbiota composition, but did not indicate how affected taxa related to fecal SCFA levels and other outcomes ( Supplementary Table S1 ) (113, 114). Recently, we reported on the intake of dried chicory root cubes (approximately 3 mm rib), as a palatable preparation of this root vegetable high in inulin-type fructans (115). The product had major effects on gut microbiota composition and activity, inducing a butyrogenic trophic chain including Bifidobacterium and Anaerostipes spp. and improved bowel function ( Supplementary Table S1 ). Of note, fecal SCFA levels were highly increased to an extent never observed with isolated inulin. While the variation of blood glucose levels was reduced by the dried chicory root, fasting plasma markers were only slightly impacted, which we found to relate to baseline gut microbiota composition (115). Interestingly, a single-arm trial, using meals based on inulin-rich vegetables (e.g. onion, Jerusalem artichoke, leeks), did not observe changes in fecal SCFA levels despite a major increase in Bifidobacterium spp. (131). This could relate to the low dosage (~15 g/day fresh vegetable) and damage to cell walls by cooking, releasing encapsulated inulin already in the proximal colon.
There are some studies that investigated various effects of fresh fruits like avocado, mango and kiwi and dried fruits like prunes, raisin and dates ( Table 1 , Fruits). Similarly to nuts, avocados are plant foods rich in fat, but despite their high fat content have been found to decrease circulating triglyceride levels and concomitantly increase fecal fat and bile acid output (116, 117). While fiber can exert these positive effects on lipid metabolism by microbiota-independent effects and the small intestinal microbiota is known to impact bile acid metabolism (8, 132), the role of the colonic microbiota is not yet understood. As avocado intake was reported to increase levels of bacteria normally related to a diet high in fat from animal foods (e.g. Bilophila (133)) and associated with negative health outcomes, future studies will shed light onto the microbiota-mediated health benefits of these intrinsic fibers ( Supplementary Table S1 ) (116, 117). Many other fruit studies have focused on the application of intrinsic fibers to stimulate bowel function, and for a comprehensive overview hereof we refer the reader to others (134). Unfortunately, the majority of these fruit studies did not address the gut microbiota and its products, such as butyrate and other SCFA (119, 135, 136). One recent study compared mango to an equal fiber dose of psyllium ( Supplementary Table S1 ) (118). Psyllium likely relieves constipation by microbiota-independent effects as only a minimal impact on gut microbiota composition and SCFA production has been reported (137). In contrast, the mango fruit improved bowel function and also increased fecal SCFA and decreased IL-6 levels ( Supplementary Table S1 ) (118). Also intake of dried raisins improved bowel function and increased fecal SCFA ( Supplementary Table S1 ) (122). These results suggests that (dried) fruits that are metabolizable by the gut microbiota in the colon have microbiota-dependent health impacts related to their intrinsic fibers.
Overall, human intervention studies assessing intrinsic fibers confirm that these fibers impact the gut microbiota in various ways despite the possible physical barrier and complexity of the plant cell matrix. The majority of the reported effects on gut microbiota composition and activity is small to moderate, with exception of the dried chicory root particles that had major effects ( Table 1 , Vegetables). Moreover, few studies assessed changes in gut microbiota composition and activity together with metabolic markers and bowel function, which makes the translation of the observed effects challenging. In future it is important to investigate the effect of processing including particle size of intrinsic fibers evaluated against isolated, single fibers.
Conclusions and considerations for future research
Modulating the gut microbiota using dietary fibers is an exciting field likely resulting in new therapeutic avenues to maintain and improve human health. Research on fiber-microbiota interactions has followed for years a reductionist approach based on the concept that isolated fibers are needed to understand how dietary fibers impact human health. However, during digestion dietary fibers do not simply dissolve from the plant tissues making them available to the gut microbiota as single components. Instead, plant tissue fractions are maintained and their complexly intertwined cell walls and encapsulated fructans and starch polymers arrive in the colon. These intrinsic fibers likely slow down colonic bacterial fermentation as the gut bacteria cannot spatially access all cell wall fibers and encapsulated contents. Thereby fiber-derived SCFA production is likely spread throughout the entire length of the colon, notably the distal colon with described health benefits (95, 138). However, how these processes evolve is barely understood. Research assessing intact plant tissue fractions and cells has focused mainly on the upper gut. Few studies have assessed the further breakdown of intrinsic fibers in the colon and are mainly based on in vitro or animal in vivo data. Hence, there is a clear lack of human in vivo data on the utilization of intrinsic fibers by the gut microbiota. Future research should focus on understanding (i) how intrinsic fiber structures differ from isolated single fibers in their fermentation kinetics, (ii) how the gut microbiota spatially colonizes intrinsic fiber particles and cooperates with other bacteria in the liquid and mucosal environment, and (iii) how intrinsic fibers from different plant sources and their processing affects microbial breakdown and related human health outcomes. Finally, with the shift from animal-based to more sustainable, minimally-processed plant-based diets we should put considerable effort in the understanding how plant cell-encapsulated proteins and fats affect the gut microbiota in the distal colon in contrast to animal-derived equivalents.
Food processing can fundamentally affect the intrinsic fiber structure ( Figure 1C ). Elucidating how different domestic preparation techniques (e.g. raw versus cooked in water versus steamed vegetables) affect health status by modulating the gut microbiota is an exciting field that has rarely been explored (75, 139). In this context it is also important to be reminded that food processing per se is not health-detrimental. Certain foods are barely digestible without any processing and for specific populations, e.g. those suffering from malnutrition or diseases, food processing is crucial. However, in the Western population that consumes an abundance of highly (over)processed foods, the increased digestibility has resulted in negative health outcomes related to obesity and welfare diseases (140). Unsurprisingly, focusing on assessing the isolated effects of fibers and relying on them to create new food designs stimulates food processing and the production of waste streams but not necessarily promotes the development of healthy foods. As fibers in their unextracted, minimally processed form of the intrinsic plant cell matrix provide health benefits by naturally encapsulating ingredients and slowing down dietary fiber fermentation, we need to rethink the way we use dietary fibers in healthy food design. Hence, we postulate that future food designs should rather reduce the extent of food processing and move towards exploiting the intrinsic plant cell matrix, which we find in any plant food ( Figures 1A–C ). By doing so, we might not only reduce the level of food processing, but also reduce waste and create new healthy products that are in line with more sustainable and plant-based oriented diets.
Author contributions
M-LP and WMdV conceptualization; M-LP literature search, writing initial draft; WMdV supervision, critical revision. All authors contributed to the article and approved the submitted version
Funding
Part of this work was supported by the Innovation Program Microbiology of Wageningen University (IPM-4 2022, Small Science Seeds).
Acknowledgments
We thank Frederik S. Kaper from WholeFiber BV for his technological insights on fiber structures and processing effects. We are also grateful to Prof. dr. Edith Feskens and Prof. dr. Hauke Smidt for the stimulating discussions.
Conflict of interest
WV provided scientific advice to WholeFiber BV.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.954845/full#supplementary-material
Abbreviations
SCFA, short-chain fatty acids.
References
- 1. Reynolds A, Mann J, Cummings J, Winter N, Mete E, Te Morenga L. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet (2019) 393:434–45. doi: 10.1016/S0140-6736(18)31809-9 [DOI] [PubMed] [Google Scholar]
- 2. Cummings J, Engineer A. Denis Burkitt and the origins of the dietary fibre hypothesis. Nutr Res Rev (2017) 31:1–15. doi: 10.1017/S0954422417000117 [DOI] [PubMed] [Google Scholar]
- 3. O’Keefe SJ. The association between dietary fibre deficiency and high-income lifestyle-associated diseases: Burkitt’s hypothesis revisited. Lancet Gastroenterol Hepatol (2019) 4:984–96. doi: 10.1016/S2468-1253(19)30257-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zoetendal EG, Rajilić-Stojanović M, De Vos WM. High-throughput diversity and functionality analysis of the gastrointestinal tract microbiota. Gut (2008) 57:1605–15. doi: 10.1136/GUT.2007.133603 [DOI] [PubMed] [Google Scholar]
- 5. De Vos WM, Tilg H, Van Hul M, Cani PD. Recent advances in basic science gut microbiome and health: mechanistic insights. Gut (2022) 71:1020–32. doi: 10.1136/gutjnl-2021-326789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes (2012) 3:289–306. doi: 10.4161/gmic.19897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Canfora EE, Jocken JWE, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol (2015) 11:577–91. doi: 10.1038/nrendo.2015.128 [DOI] [PubMed] [Google Scholar]
- 8. Gasaly N, de Vos P, Hermoso MA. Impact of bacterial metabolites on gut barrier function and host immunity: a focus on bacterial metabolism and its relevance for intestinal inflammation. Front Immunol (2021) 12:658354. doi: 10.3389/fimmu.2021.658354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Venegas DP, de la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, et al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol (2019) 10:2019.00277. doi: 10.3389/fimmu.2019.00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li M, van Esch BCAM, Wagenaar GTM, Garssen J, Folkerts G, Henricks PAJ. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur J Pharmacol (2018) 831:52–9. doi: 10.1016/J.EJPHAR.2018.05.003 [DOI] [PubMed] [Google Scholar]
- 11. Blaak EE, Canfora EE, Theis S, Frost G, Groen AK, Mithieux G, et al. Short chain fatty acids in human gut and metabolic health. Benef Microbes (2020) 11:411–55. doi: 10.3920/BM2020.0057 [DOI] [PubMed] [Google Scholar]
- 12. Deehan EC, Yang C, Perez-Muñoz ME, Nguyen NK, Cheng CC, Triador L, et al. Precision microbiome modulation with discrete dietary fiber structures directs short-chain fatty acid production. Cell Host Microbe (2020) 27:389–404.e6. doi: 10.1016/J.CHOM.2020.01.006 [DOI] [PubMed] [Google Scholar]
- 13. Fransen F, Sahasrabudhe NM, Elderman M, Bosveld M, El Aidy S, Hugenholtz F, et al. β2→1-fructans modulate the immune system in vivo in a microbiota-dependent and -independent fashion. Front Immunol (2017) 8:154. doi: 10.3389/fimmu.2017.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beukema M, Faas MM, de Vos P. The effects of different dietary fiber pectin structures on the gastrointestinal immune barrier: impact via gut microbiota and direct effects on immune cells. Exp Mol Med 2020 529 (2020) 52:1364–76. doi: 10.1038/s12276-020-0449-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eastwood MA, Kay RM. An hypothesis for the action of dietary fiber along the gastrointestinal tract. Am J Clin Nutr (1979) 32:364–7. doi: 10.1093/AJCN/32.2.364 [DOI] [PubMed] [Google Scholar]
- 16. Hoffmann I. Transcending reductionism in nutrition research. Am J Clin Nutr (2003) 78:514S–6S. doi: 10.1093/AJCN/78.3.514S [DOI] [PubMed] [Google Scholar]
- 17. Mozaffarian D, Rosenberg I, Uauy R. History of modern nutrition science–implications for current research, dietary guidelines, and food policy. Br Med J (2018) 361:k2392. doi: 10.1136/bmj.k2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Louis P, Solvang M, Duncan SH, Walker AW, Mukhopadhya I. The impact of nutrition on the gut microbiome dietary fibre complexity and its influence on functional groups of the human gut microbiota. Proc Nutr Soc (2021) 80:386–97. doi: 10.1017/S0029665121003694 [DOI] [Google Scholar]
- 19. Li C, Hu Y, Zhang B. Plant cellular architecture and chemical composition as important regulator of starch functionality in whole foods. Food Hydrocoll (2021) 117:106744. doi: 10.1016/J.FOODHYD.2021.106744 [DOI] [Google Scholar]
- 20. Zdunek A, Pieczywek PM, Cybulska J. The primary, secondary, and structures of higher levels of pectin polysaccharides. Compr Rev Food Sci Food Saf (2021) 20:1101–17. doi: 10.1111/1541-4337.12689 [DOI] [PubMed] [Google Scholar]
- 21. McRorie JW, McKeown NM. Understanding the physics of functional fibers in the gastrointestinal tract: an evidence-based approach to resolving enduring misconceptions about insoluble and soluble fiber. J Acad Nutr Diet (2017) 117:251–64. doi: 10.1016/j.jand.2016.09.021 [DOI] [PubMed] [Google Scholar]
- 22. Augustin LSA, Aas A-M, Astrup A, Atkinson FS, Baer-Sinnott S, Barclay AW, et al. Dietary fibre consensus from the international carbohydrate quality consortium (ICQC). Nutrients (2020) 12:2553. doi: 10.3390/nu12092553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. So D, Gibson PR, Muir JG, Yao CK. Dietary fibres and IBS: translating functional characteristics to clinical value in the era of personalised medicine. Gut (2021) 70:2383–94. doi: 10.1136/gutjnl-2021-324891 [DOI] [PubMed] [Google Scholar]
- 24. Capuano E. The behavior of dietary fiber in the gastrointestinal tract determines its physiological effect. Crit Rev Food Sci Nutr (2017) 57:3543–64. doi: 10.1080/10408398.2016.1180501 [DOI] [PubMed] [Google Scholar]
- 25. Williams BA, Mikkelsen D, Flanagan BM, Gidley MJ. “Dietary fibre”: moving beyond the “soluble/insoluble” classification for monogastric nutrition, with an emphasis on humans and pigs. J Anim Sci Biotechnol (2019) 10:45. doi: 10.1186/s40104-019-0350-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. El Kaoutari A, Armougom F, Gordon JI, Raoult D, Henrissat B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat Rev Microbiol (2013) 11:497–504. doi: 10.1038/nrmicro3050 [DOI] [PubMed] [Google Scholar]
- 27. Louca S, Polz MF, Mazel F, Albright MBN, Huber JA, O’Connor MI, et al. Function and functional redundancy in microbial systems. Nat Ecol Evol 2018 26 (2018) 2:936–43. doi: 10.1038/s41559-018-0519-1 [DOI] [PubMed] [Google Scholar]
- 28. Reichardt N, Vollmer M, Holtrop G, Farquharson FM, Wefers D, Bunzel M, et al. Specific substrate-driven changes in human faecal microbiota composition contrast with functional redundancy in short-chain fatty acid production. ISME J (2017) 12:610–22. doi: 10.1038/ismej.2017.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Flint HJ, Bayer EA, Rincon MT, Lamed R, White BA. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol (2008) 6:121–31. doi: 10.1038/nrmicro1817 [DOI] [PubMed] [Google Scholar]
- 30. Baxter NT, Schmidt AW, Venkataraman A, Kim KS, Waldron C, Schmidt TM. Dynamics of human gut microbiota and short-chain fatty acids in response to dietary interventions with three fermentable fibers. mBio (2019) 10:e02566-18. doi: 10.1128/mBio.02566-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rajilić-Stojanović M, de Vos WM. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol Rev (2014) 38:996–1047. doi: 10.1111/1574-6976.12075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Le Bastard Q, Chapelet G, Javaudin F, Lepelletier D, Batard E, Montassier E. The effects of inulin on gut microbial composition: a systematic review of evidence from human studies. Eur J Clin Microbiol Infect Dis (2019) 39:403–13. doi: 10.1007/s10096-019-03721-w [DOI] [PubMed] [Google Scholar]
- 33. Armet AM, Deehan EC, Thöne JV, Hewko SJ, Walter J. The effect of isolated and synthetic dietary fibers on markers of metabolic diseases in human intervention studies: a systematic review. Adv Nutr (2020) 11:420–38. doi: 10.1093/advances/nmz074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes (2017) 8:172–84. doi: 10.1080/19490976.2017.1290756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jefferson A, Adolphus K. The effects of intact cereal grain fibers, including wheat bran on the gut microbiota composition of healthy adults: A systematic review. Front Nutr (2019) 6:33. doi: 10.3389/fnut.2019.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Flint HJ, Scott KP, Louis P, Duncan SH. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol 2012 910 (2012) 9:577–89. doi: 10.1038/nrgastro.2012.156 [DOI] [PubMed] [Google Scholar]
- 37. So D, Whelan K, Rossi M, Morrison M, Holtmann G, Kelly JT, et al. Dietary fiber intervention on gut microbiota composition in healthy adults: A systematic review and meta-analysis. Am J Clin Nutr (2018) 107:965–83. doi: 10.1093/ajcn/nqy041 [DOI] [PubMed] [Google Scholar]
- 38. Rastall RA, Diez-Municio M, Forssten SD, Hamaker B, Meynier A, Moreno FJ, et al. Structure and function of non-digestible carbohydrates in the gut microbiome. Benef Microbes (2022) 13:95–168. doi: 10.3920/BM2021.0090 [DOI] [PubMed] [Google Scholar]
- 39. Chung SF, Walker AW, Vermeiren J, Sheridan PO, Bosscher D, Garcia-Campayo V, et al. Impact of carbohydrate substrate complexity on the diversity of the human colonic microbiota. FEMS Microbiol Ecol (2019) 95:201. doi: 10.1093/femsec/fiy201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cantu-Jungles TM, Hamaker BR. New view on dietary fiber selection for predictable shifts in gut microbiota. MBio (2020) 11:e02179–19. doi: 10.1128/mBio.02179-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chung WSF, Walker AW, Louis P, Parkhill J, Vermeiren J, Bosscher D, et al. Modulation of the human gut microbiota by dietary fibres occurs at the species level. BMC Biol (2016) 14:3. doi: 10.1186/s12915-015-0224-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hamaker BR, Tuncil YE. A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J Mol Biol (2014) 426:3838–50. doi: 10.1016/J.JMB.2014.07.028 [DOI] [PubMed] [Google Scholar]
- 43. Cantu-Jungles TM, Bulut N, Chambry E, Ruthes A, Iacomini M, Keshavarzian A, et al. Dietary fiber hierarchical specificity: The missing link for predictable and strong shifts in gut bacterial communities. MBio (2021) 12:e0102821. doi: 10.1128/MBIO.01028-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ranaivo H, Thirion F, Béra-Maillet C, Guilly S, Simon C, Sothier M, et al. Increasing the diversity of dietary fibers in a daily-consumed bread modifies gut microbiota and metabolic profile in subjects at cardiometabolic risk. Gut Microbes (2022) 14(1):e2044722. doi: 10.1080/19490976.2022.2044722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Deehan EC, Zhang Z, Riva A, Armet AM, Perez-Muñoz ME, Nguyen NK, et al. Elucidating the role of the gut microbiota in the physiological effects of dietary fiber. Microbiome 2022 101 (2022) 10:1–22. doi: 10.1186/S40168-022-01248-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tuncil YE, Nakatsu CH, Kazem AE, Arioglu-Tuncil S, Reuhs B, Martens EC, et al. Delayed utilization of some fast-fermenting soluble dietary fibers by human gut microbiota when presented in a mixture. J Funct Foods (2017) 32:347–57. doi: 10.1016/j.jff.2017.03.001 [DOI] [Google Scholar]
- 47. Sorbara MT, Pamer EG. Microbiome-based therapeutics. Nat Rev Microbiol 2022 206 (2022) 20:365–80. doi: 10.1038/s41579-021-00667-9 [DOI] [PubMed] [Google Scholar]
- 48. Hansen NW, Sams A. The microbiotic highway to health - new perspective on food structure, gut microbiota, and host inflammation. Nutrients (2018) 10:1590. doi: 10.3390/nu10111590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Qin W, Sun L, Miao M, Zhang G. Plant-sourced intrinsic dietary fiber: Physical structure and health function. Trends Food Sci Technol (2021) 118:341–55. doi: 10.1016/j.tifs.2021.09.022 [DOI] [Google Scholar]
- 50. Grundy MML, Edwards CH, Mackie AR, Gidley MJ, Butterworth PJ, Ellis PR. Re-evaluation of the mechanisms of dietary fibre and implications for macronutrient bioaccessibility, digestion and postprandial metabolism. Br J Nutr (2016) 116:816–33. doi: 10.1017/S0007114516002610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. An Y, Lu W, Li W, Pan L, Lu M, Cesarino I, et al. Dietary fiber in plant cell walls-the healthy carbohydrates. Food Qual Saf (2022) 6:1–17. doi: 10.1093/fqsafe/fyab037 [DOI] [Google Scholar]
- 52. Holland C, Ryden P, Edwards CH, Grundy MML. Plant cell walls: Impact on nutrient bioaccessibility and digestibility. Foods (2020) 9:16. doi: 10.3390/FOODS9020201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Eisenach C, Francisco R, Martinoia E. Plant vacuoles. CURBIO (2015) 25:R136–7. doi: 10.1016/j.cub.2014.11.056 [DOI] [PubMed] [Google Scholar]
- 54. Yoshida M. Fructan structure and metabolism in overwintering plants. Plants (2021) 10(5):1–11. doi: 10.3390/PLANTS10050933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Albersheim P, Darvill A, Roberts K, Sederoff R, Staehelin A. Plant cell Walls : From chemistry to biology. New York: Garland Science; (2010). doi: 10.1201/9780203833476 [DOI] [Google Scholar]
- 56. Scheller HV, Ulvskov P. Hemicelluloses. Annu Rev Plant Biol (2010) 61:263–89. doi: 10.1146/ANNUREV-ARPLANT-042809-112315 [DOI] [PubMed] [Google Scholar]
- 57. Collins HM, Burton RA, Topping DL, Liao M-L, Bacic A, Fincher GB. Variability in fine structures of noncellulosic cell wall polysaccharides from cereal grains: Potential importance in human health and nutrition. Cereal Chem (2010) 87:272–82. doi: 10.1094/CCHEM-87-4-0272 [DOI] [Google Scholar]
- 58. Xiong W, Devkota L, Zhang B, Muir J, Dhital S. Intact cells: “Nutritional capsules” in plant foods. Compr Rev Food Sci Food Saf (2022) 21:1198–217. doi: 10.1111/1541-4337.12904 [DOI] [PubMed] [Google Scholar]
- 59. Mohnen D, Keegstra K, Pauly M. Pectin structure and biosynthesis. Curr Opin Plant Biol (2008) 11:266–77. doi: 10.1016/j.pbi.2008.03.006 [DOI] [PubMed] [Google Scholar]
- 60. Zitterman A. DIETARY FIBER | bran. In: Caballero B, editor. Encyclopedia of food sciences and nutrition. San Diego, CA, London: Academic Press; (2003). p. 1844–50. doi: 10.1016/B0-12-227055-X/00346-1 [DOI] [Google Scholar]
- 61. Tejada-Ortigoza V, Garcia-Amezquita LE, Serna-Saldívar SO, Welti-Chanes J. Advances in the functional characterization and extraction processes of dietary fiber. Food Eng Rev 2015 83 (2015) San Diego, Calif.;London: 8:251–71. doi: 10.1007/S12393-015-9134-Y [DOI] [Google Scholar]
- 62. Hu X, Zhang G, Hamaker BR, Miao M. The contribution of intact structure and food processing to functionality of plant cell wall-derived dietary fiber. Food Hydrocoll (2022) 127:107511. doi: 10.1016/J.FOODHYD.2022.107511 [DOI] [Google Scholar]
- 63. Maphosa Y, Jideani VA. Dietary fiber extraction for human nutrition-a review dietary fiber extraction for human nutrition-a review. Food Rev Int (2015) 32:98–115. doi: 10.1080/87559129.2015.1057840 [DOI] [Google Scholar]
- 64. Guillon F, Champ M. Structural and physical properties of dietary fibres, and consequences of processing on human physiology. Food Res Int (2000) 33:233–45. doi: 10.1016/S0963-9969(00)00038-7 [DOI] [Google Scholar]
- 65. Lewicki PP, Pawlak G. Effect of drying on microstructure of plant tissue. Dry Technol (2003) 21:657–83. doi: 10.1081/DRT-120019057 [DOI] [Google Scholar]
- 66. Zhang N, Ju Z, Zuo T. Time for food: The impact of diet on gut microbiota and human health. Nutrition (2018) 51–52:80–5. doi: 10.1016/j.nut.2017.12.005 [DOI] [PubMed] [Google Scholar]
- 67. Van Der Kamp JW. Whole grain definition: New perspectives for inclusion of grains and processing but not for analysis. Cer Foods Wld Plexus (2012) 15–16 10.1094/CPLEX-2013-1001-08B [DOI] [Google Scholar]
- 68. Bach Knudsen KE. Microbial degradation of whole-grain complex carbohydrates and impact on short-chain fatty acids and health. Adv Nutr (2015) 6:206–13. doi: 10.3945/AN.114.007450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Williams BA, Grant LJ, Gidley MJ, Mikkelsen D. Gut fermentation of dietary fibres: Physico-chemistry of plant cell walls and implications for health. Int J Mol Sci (2017) 18:2203. doi: 10.3390/IJMS18102203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Do DT, Singh J, Oey I, Singh H. Modulating effect of cotyledon cell microstructure on in vitro digestion of starch in legumes. Food Hydrocoll (2019) 96:112–22. doi: 10.1016/j.foodhyd.2019.04.063 [DOI] [Google Scholar]
- 71. Dang TT, Vasanthan T. Modification of rice bran dietary fiber concentrates using enzyme and extrusion cooking. Food Hydrocoll (2019) 89:773–82. doi: 10.1016/J.FOODHYD.2018.11.024 [DOI] [Google Scholar]
- 72. Sabater C, Calvete-Torre I, Villamiel M, Moreno FJ, Margolles A, Ruiz L. Vegetable waste and by-products to feed a healthy gut microbiota: Current evidence, machine learning and computational tools to design novel microbiome-targeted foods. Trends Food Sci Technol (2021) 118:399–417. doi: 10.1016/J.TIFS.2021.10.002 [DOI] [Google Scholar]
- 73. Campos DA, Gómez-García R, Vilas-Boas AA, Madureira AR, Pintado MM, Cardoso SM, et al. Management of fruit industrial by-products - a case study on circular economy approach. Molecules (2020) 25:320. doi: 10.3390/molecules25020320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhu Z, He J, Liu G, Barba FJ, Koubaa M, Ding L, et al. Recent insights for the green recovery of inulin from plant food materials using non-conventional extraction technologies: A review. Innov Food Sci Emerg Technol (2016) 33:1–9. doi: 10.1016/j.ifset.2015.12.023 [DOI] [Google Scholar]
- 75. Tydeman EA, Parker ML, Wickham MSJ, Rich GT, Faulks RM, Gidley MJ, et al. Effect of carrot (Daucus carota) microstructure on carotene bioaccessibilty in the upper gastrointestinal tract. 1. In vitro simulations of carrot digestion. J Agric Food Chem (2010) 58:9847–54. doi: 10.1021/jf101034a [DOI] [PubMed] [Google Scholar]
- 76. Noah L, Guillon F, Bouchet B, Buléon A, Molis C, Gratas M, et al. Digestion of carbohydrate from white beans (Phaseolus vulgaris l.) in healthy humans. J Nutr (1998) 128:977–85. doi: 10.1093/JN/128.6.977 [DOI] [PubMed] [Google Scholar]
- 77. Ellis PR, Kendall CWC, Ren Y, Parker C, Pacy JF, Waldron KW, et al. Role of cell walls in the bioaccessibility of lipids in almond seeds. Am J Clin Nutr (2004) 80:604–13. doi: 10.1093/AJCN/80.3.604 [DOI] [PubMed] [Google Scholar]
- 78. Do DT, Singh J, Oey I, Singh H. Biomimetic plant foods: Structural design and functionality. Trends Food Sci Technol (2018) 82:46–59. doi: 10.1016/j.tifs.2018.09.010 [DOI] [Google Scholar]
- 79. Flint HJ, Duncan SH, Scott KP, Louis P. Interactions and competition within the microbial community of the human colon: Links between diet and health: Minireview. Environ Microbiol (2007) 9:1101–11. doi: 10.1111/j.1462-2920.2007.01281.x [DOI] [PubMed] [Google Scholar]
- 80. Drula E, Garron ML, Dogan S, Lombard V, Henrissat B, Terrapon N. The carbohydrate-active enzyme database: functions and literature. Nucleic Acids Res (2022) 50:D571–7. doi: 10.1093/NAR/GKAB1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Despres J, Forano E, Lepercq P, Comtet-Marre S, Jubelin G, Yeoman CJ, et al. Unraveling the pectinolytic function of bacteroides xylanisolvens using a RNA-seq approach and mutagenesis. BMC Genomics (2016) 17:147. doi: 10.1186/s12864-016-2472-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhang M, Chekan JR, Dodd D, Hong PY, Radlinsk L, Revindran V, et al. Xylan utilization in human gut commensal bacteria is orchestrated by unique modular organization of polysaccharide-degrading enzymes. Proc Natl Acad Sci U.S.A. (2014) 111:E3708–17. doi: 10.1073/pnas.1406156111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Schel JHN, Stasse-Wolthuis M, Katan MB, Willemse MTM. Structural changes of wheat bran after human digestion. Meded Landbouwhogesch Wageningen (1980) 80:9. [Google Scholar]
- 84. De Paepe K, Kerckhof FM, Verspreet J, Courtin CM, Van de Wiele T. Inter-individual differences determine the outcome of wheat bran colonization by the human gut microbiome. Environ Microbiol (2017) 19:3251–67. doi: 10.1111/1462-2920.13819 [DOI] [PubMed] [Google Scholar]
- 85. Walker AW, Duncan SH, Harmsen HJM, Holtrop G, Welling GW, Flint HJ. The species composition of the human intestinal microbiota differs between particle-associated and liquid phase communities. Environ Microbiol (2008) 10:3275–83. doi: 10.1111/J.1462-2920.2008.01717.X [DOI] [PubMed] [Google Scholar]
- 86. Leitch ECMW, Walker AW, Duncan SH, Holtrop G, Flint HJ. Selective colonization of insoluble substrates by human faecal bacteria. Environ Microbiol (2007) 9:667–79. doi: 10.1111/j.1462-2920.2006.01186.x [DOI] [PubMed] [Google Scholar]
- 87. De Paepe K, Verspreet J, Rezaei MN, Martinez SH, Meysman F, Van De Walle D, et al. Modification of wheat bran particle size and tissue composition affects colonisation and metabolism by human faecal microbiota. Food Funct (2019) 10:379–96. doi: 10.1039/C8FO01272E [DOI] [PubMed] [Google Scholar]
- 88. Ze X, Ben David Y, Laverde-Gomez JA, Dassa B, Sheridan PO, Duncan SH, et al. Unique organization of extracellular amylases into amylosomes in the resistant starch-utilizing human colonic firmicutes bacterium ruminococcus bromii. MBio (2015) 6:e01058–15. doi: 10.1128/mBio.01058-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ben David Y, Dassa B, Borovok I, Lamed R, Koropatkin NM, Martens EC, et al. Ruminococcal cellulosome systems from rumen to human. Environ Microbiol (2015) 17:3407–26. doi: 10.1111/1462-2920.12868 [DOI] [PubMed] [Google Scholar]
- 90. Warren FJ, Fukuma NM, Mikkelsen D, Flanagan BM, Williams BA, Lisle AT, et al. Food starch structure impacts gut microbiome composition. mSphere (2018) 3:e00086–18. doi: 10.1128/mSphere.00086-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Stewart ML, Slavin JL. Particle size and fraction of wheat bran influence short-chain fatty acid production in vitro . Br J Nutr (2009) 102:1404–7. doi: 10.1017/S0007114509990663 [DOI] [PubMed] [Google Scholar]
- 92. Tuncil YE, Thakkar RD, Marcia ADR, Hamaker BR, Lindemann SR. Divergent short-chain fatty acid production and succession of colonic microbiota arise in fermentation of variously-sized wheat bran fractions. Sci Rep (2018) 8:16655. doi: 10.1038/S41598-018-34912-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Demuth T, Betschart J, Nyström L. Structural modifications to water-soluble wheat bran arabinoxylan through milling and extrusion. Carbohydr Polym (2020) 240:116328. doi: 10.1016/J.CARBPOL.2020.116328 [DOI] [PubMed] [Google Scholar]
- 94. Roye C, Henrion M, Chanvrier H, de Roeck K, de Bondt Y, Liberloo I, et al. Extrusion-cooking modifies physicochemical and nutrition-related properties of wheat bran. Foods (2020) 9:738. doi: 10.3390/FOODS9060738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. van der Beek CM, Canfora EE, Lenaerts K, Troost FJ, Damink S, Holst JJ, et al. Distal, not proximal, colonic acetate infusions promote fat oxidation and improve metabolic markers in overweight/obese men. Clin Sci (Lond) (2016) 130:2073–82. doi: 10.1042/cs20160263 [DOI] [PubMed] [Google Scholar]
- 96. Berendsen A, Santoro A, Pini E, Cevenini E, Ostan R, Pietruszka B, et al. A parallel randomized trial on the effect of a healthful diet on inflammageing and its consequences in European elderly people: Design of the NU-AGE dietary intervention study. Mech Ageing Dev (2013) 134:523–30. doi: 10.1016/J.MAD.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 97. Ghosh TS, Rampelli S, Jeffery IB, Santoro A, Neto M, Capri M, et al. Mediterranean Diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut (2020) 69:1218–28. doi: 10.1136/GUTJNL-2019-319654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Uusitupa M, Hermansen K, Savolainen MJ, Schwab U, Kolehmainen M, Brader L, et al. Effects of an isocaloric healthy Nordic diet on insulin sensitivity, lipid profile and inflammation markers in metabolic syndrome – a randomized study (SYSDIET). J Intern Med (2013) 274:52–66. doi: 10.1111/JOIM.12044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gürdeniz G, Uusitupa M, Hermansen K, Savolainen MJ, Schwab U, Kolehmainen M, et al. Analysis of the SYSDIET healthy Nordic diet randomized trial based on metabolic profiling reveal beneficial effects on glucose metabolism and blood lipids. Clin Nutr (2022) 41:441–51. doi: 10.1016/j.clnu.2021.12.031 [DOI] [PubMed] [Google Scholar]
- 100. Candela M, Biagi E, Soverini M, Consolandi C, Quercia S, Severgnini M, et al. Modulation of gut microbiota dysbioses in type 2 diabetic patients by macrobiotic ma-pi 2 diet. Br J Nutr (2016) 116:80–93. doi: 10.1017/S0007114516001045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kirwan WO, Smith AN, Mcconnell AA, Mitchell WD, Eastwood MA. Action of different bran preparations on colonic function. Br Med J (1972) 4:187–9. doi: 10.1136/bmj.4.5938.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wyman JB, Heaton KW, Manning AP, Wicks ACB. The effect on intestinal transit and the feces of raw and cooked bran in different doses. Am J Clin Nutr (1976) 29:1474–9. doi: 10.1093/AJCN/29.12.1474 [DOI] [PubMed] [Google Scholar]
- 103. Deroover L, Vázquez-Castellanos JF, Vandermeulen G, Luypaerts A, Raes J, Courtin CM, et al. Wheat bran with reduced particle size increases serum SCFAs in obese subjects without improving health parameters compared with a maltodextrin placebo. Am J Clin Nutr (2021) 114:1328–41. doi: 10.1093/ajcn/nqab196 [DOI] [PubMed] [Google Scholar]
- 104. Nilsson A, Johansson-Boll E, Sandberg J, Björck I. Gut microbiota mediated benefits of barley kernel products on metabolism, gut hormones, and inflammatory markers as affected by co-ingestion of commercially available probiotics: a randomized controlled study in healthy subjects. Clin Nutr ESPEN (2016) 15:49–56. doi: 10.1016/J.CLNESP.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 105. Martínez I, Lattimer JM, Hubach KL, Case JA, Yang J, Weber CG, et al. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J (2013) 7:269–80. doi: 10.1038/ismej.2012.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Jinnouchi M, Miyahara T, Suzuki Y. Coix seed consumption affects the gut microbiota and the peripheral lymphocyte subset profiles of healthy male adults. Nutrients (2021) 13:4079. doi: 10.3390/NU13114079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Holscher HD, Guetterman HM, Swanson KS, An R, Matthan NR, Lichtenstein AH, et al. Walnut consumption alters the gastrointestinal microbiota, microbially derived secondary bile acids, and health markers in healthy adults: a randomized controlled trial. J Nutr (2018) 148:861–7. doi: 10.1093/JN/NXY004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Dhillon J, Li Z, Ortiz RM. Almond snacking for 8 wk increases alpha-diversity of the gastrointestinal microbiome and decreases bacteroides fragilis abundance compared with an isocaloric snack in college freshmen. Curr Dev Nutr (2019) 3:nzz079. doi: 10.1093/CDN/NZZ079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Holscher HD, Taylor AM, Swanson KS, Novotny JA, Baer DJ. Almond consumption and processing affects the composition of the gastrointestinal microbiota of healthy adult men and women: A randomized controlled trial. Nutr (2018) 10:126. doi: 10.3390/NU10020126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ukhanova M, Wang X, Baer DJ, Novotny JA, Fredborg M, Mai V. Effects of almond and pistachio consumption on gut microbiota composition in a randomised cross-over human feeding study. Br J Nutr (2014) 111:2146–52. doi: 10.1017/S0007114514000385 [DOI] [PubMed] [Google Scholar]
- 111. Fernando WMU, Hill JE, Zello GA, Tyler RT, Dahl WJ, Van Kessel AG. Diets supplemented with chickpea or its main oligosaccharide component raffinose modify faecal microbial composition in healthy adults. Benef Microbes (2010) 1:197–207. doi: 10.3920/BM2009.0027 [DOI] [PubMed] [Google Scholar]
- 112. Hovey AL, Jones GP, Devereux HM, Walker KZ. Whole cereal and legume seeds increase faecal short chain fatty acids compared to ground seeds. Asia Pacific J Clin Nutr (2003) 12:477–82. [PubMed] [Google Scholar]
- 113. Li F, Hullar MAJ, Schwarz Y, Lampe JW. Human gut bacterial communities are altered by addition of cruciferous vegetables to a controlled fruit- and vegetable-free diet. J Nutr (2009) 139:1685. doi: 10.3945/JN.109.108191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kellingray L, Tapp HS, Saha S, Doleman JF, Narbad A, Mithen RF. Consumption of a diet rich in brassica vegetables is associated with a reduced abundance of sulphate-reducing bacteria: A randomised crossover study. Mol Nutr Food Res (2017) 61:1600992. doi: 10.1002/MNFR.201600992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Puhlmann M-L, Jokela R, Van Dongen KCW, Bui TPN, Van Hangelbroek RWJ, Smidt H, et al. Dried chicory root improves bowel function, benefits intestinal microbial trophic chains and increases faecal and circulating short chain fatty acids in subjects at risk for type 2 diabetes. Gut Microbiome (2022) 3:e4. doi: 10.1017/GMB.2022.4 [DOI] [Google Scholar]
- 116. Henning SM, Yang J, Woo SL, Lee RP, Huang J, Rasmusen A, et al. Hass avocado inclusion in a weight-loss diet supported weight loss and altered gut microbiota: a 12-week randomized, parallel-controlled trial. Curr Dev Nutr (2019) 3:1–9. doi: 10.1093/CDN/NZZ068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Thompson SV, Bailey MA, Taylor AM, Kaczmarek JL, Mysonhimer AR, Edwards CG, et al. Avocado consumption alters gastrointestinal bacteria abundance and microbial metabolite concentrations among adults with overweight or obesity: a randomized controlled trial. J Nutr (2021) 151:753–62. doi: 10.1093/JN/NXAA219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Venancio VP, Kim H, Sirven MA, Tekwe CD, Honvoh G, Talcott ST, et al. Polyphenol-rich mango (Mangifera indica l.) ameliorate functional constipation symptoms in humans beyond equivalent amount of fiber. Mol Nutr Food Res (2018) 62:e1701034. doi: 10.1002/MNFR.201701034 [DOI] [PubMed] [Google Scholar]
- 119. Wilkinson-Smith V, Dellschaft N, Ansell J, Hoad C, Marciani L, Gowland P, et al. Mechanisms underlying effects of kiwifruit on intestinal function shown by MRI in healthy volunteers summary background: Chronic constipation affects approximately 17% of the population. Aliment Pharmacol Ther (2019) 49:759–768. doi: 10.1111/apt.15127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Eid N, Osmanova H, Natchez C, Walton G, Costabile A, Gibson G, et al. Impact of palm date consumption on microbiota growth and large intestinal health: a randomised, controlled, cross-over, human intervention study. Br J ofNutrition (2015) 114:1226–36. doi: 10.1017/S0007114515002780 [DOI] [PubMed] [Google Scholar]
- 121. Lever E, Scott SM, Louis P, Emery PW, Whelan K. The effect of prunes on stool output, gut transit time and gastrointestinal microbiota: A randomised controlled trial. Clin Nutr (2019) 38:165–73. doi: 10.1016/J.CLNU.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 122. Spiller GA, Story JA, Furumoto EJ, Chezem JC, Spiller M. Effect of tartaric acid and dietary fibre from sun-dried raisins on colonic function and on bile acid and volatile fatty acid excretion in healthy adults. Br J Nutr (2003) 90:803–7. doi: 10.1079/BJN2003966 [DOI] [PubMed] [Google Scholar]
- 123. Boets E, Gomand SV, Deroover L, Preston T, Vermeulen K, De Preter V, et al. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: a stable isotope study. J Physiol (2017) 595:541–55. doi: 10.1113/jp272613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Lappi J, Salojärvi J, Kolehmainen M, Mykkänen H, Poutanen K, de Vos WM, et al. Intake of whole-grain and fiber-rich rye bread versus refined wheat bread does not differentiate intestinal microbiota composition in Finnish adults with metabolic syndrome. J Nutr (2013) 143:648–55. doi: 10.3945/jn.112.172668 [DOI] [PubMed] [Google Scholar]
- 125. Salonen A, Lahti L, Salojärvi J, Holtrop G, Korpela K, Duncan SH, et al. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J (2014) 8:2218–30. doi: 10.1038/ismej.2014.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, Ze X, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J (2010) 5:220–30. doi: 10.1038/ismej.2010.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Oliphant K, Allen-Vercoe E. Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome (2019) 7:91. doi: 10.1186/s40168-019-0704-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Grundy MML, Wilde PJ, Butterworth PJ, Gray R, Ellis PR. Impact of cell wall encapsulation of almonds on in vitro duodenal lipolysis. Food Chem (2015) 185:405. doi: 10.1016/J.FOODCHEM.2015.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Grundy MML, Carrière F, Mackie AR, Gray DA, Butterworth PJ, Ellis PR. The role of plant cell wall encapsulation and porosity in regulating lipolysis during the digestion of almond seeds. Food Funct (2016) 7:69–78. doi: 10.1039/C5FO00758E [DOI] [PubMed] [Google Scholar]
- 130. Fitzgerald E, Lambert K, Stanford J, Neale EP. The effect of nut consumption (tree nuts and peanuts) on the gut microbiota of humans: a systematic review. Br J Nutr (2021) 125:508–20. doi: 10.1017/S0007114520002925 [DOI] [PubMed] [Google Scholar]
- 131. Hiel S, Bindels LB, Pachikian BD, Kalala G, Broers V, Zamariola G, et al. Effects of a diet based on inulin-rich vegetables on gut health and nutritional behavior in healthy humans. Am J Clin Nutr (2019) 109:1683–95. doi: 10.1093/ajcn/nqz001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Staley C, Weingarden AR, Khoruts A, Sadowsky MJ. Interaction of gut microbiota with bile acid metabolism and its influence on disease states. Appl Microbiol Biotechnol (2017) 101:47. doi: 10.1007/S00253-016-8006-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature (2014) 505:559–63. doi: 10.1038/nature12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Katsirma Z, Dimidi E, Rodriguez-Mateos A, Whelan K. Fruits and their impact on the gut microbiota, gut motility and constipation. Food Funct (2021) 12:8850–66. doi: 10.1039/D1FO01125A [DOI] [PubMed] [Google Scholar]
- 135. Chang C-C, Lin Y-T, Bs Y-TL, Liu Y-S, Liu J-F. Kiwifruit improves bowel function in patients with irritable bowel syndrome with constipation. Asia Pac J Clin Nutr (2010) 19:451–7. [PubMed] [Google Scholar]
- 136. Chan AOO, Leung G, Tong T, Wong NYH. Increasing dietary fiber intake in terms of kiwifruit improves constipation in Chinese patients. World J Gastroenterol (2007) 13:4771–5. doi: 10.3748/wjg.v13.i35.4771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Jalanka J, Major G, Murray K, Singh G, Nowak A, Kurtz C, et al. The effect of psyllium husk on intestinal microbiota in constipated patients and healthy controls. Int J Mol Sci (2019) 20:433. doi: 10.3390/ijms20020433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Neis EPJG, van Eijk HMH, Lenaerts K, Olde Damink SWM, Blaak EE, Dejong CHC, et al. Distal versus proximal intestinal short-chain fatty acid release in man. Gut (2019) 68:764–5. doi: 10.1136/gutjnl-2018-316161 [DOI] [PubMed] [Google Scholar]
- 139. Peírez-Burillo S, Pastoriza S, Jimeínez-Hernaíndez N, D’Auria G, Francino MP, Rufiaín-Henares JA. Effect of food thermal processing on the composition of the gut microbiota. J Agric Food Chem (2018) 66:11500–9. doi: 10.1021/acs.jafc.8b04077 [DOI] [PubMed] [Google Scholar]
- 140. Monteiro CA. Nutrition and health. the issue is not food, nor nutrients, so much as processing. Public Health Nutr (2009) 12:729–31. doi: 10.1017/S1368980009005291 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.