Abstract
Obesity hypoventilation syndrome (OHS) is defined as daytime hypercapnia in obese individuals in the absence of other underlying causes. In the United States, OHS is present in 10%–20% of obese patients with obstructive sleep apnea and is linked to hypoventilation during sleep. OHS leads to high cardiorespiratory morbidity and mortality, and there is no effective pharmacotherapy. The depressed hypercapnic ventilatory response plays a key role in OHS. The pathogenesis of OHS has been linked to resistance to an adipocyte-produced hormone, leptin, a major regulator of metabolism and control of breathing. Mechanisms by which leptin modulates the control of breathing are potential targets for novel therapeutic strategies in OHS. Recent advances shed light on the molecular pathways related to the central chemoreceptor function in health and disease. Leptin signaling in the nucleus of the solitary tract, retrotrapezoid nucleus, hypoglossal nucleus, and dorsomedial hypothalamus, and anatomical projections from these nuclei to the respiratory control centers, may contribute to OHS. In this review, we describe current views on leptin-mediated mechanisms that regulate breathing and CO2 homeostasis with a focus on potential therapeutics for the treatment of OHS.
Keywords: sleep, ventilation, chemoreceptor, mechanisms
Statement of Significance.
Obesity hypoventilation syndrome (OHS) leads to high cardiorespiratory morbidity and mortality. There is no pharmacotherapy for OHS. Leptin resistance is implicated in the pathogenesis of OHS. Leptin stimulates control of breathing and relieves OHS in obese rodents. In this review, we discuss the respiratory neurobiology of leptin and the relevance of leptin signaling in specific brain areas to the pathogenesis of OHS.
Introduction
Obesity causes significant respiratory morbidity, including sleep-disordered breathing (SDB) [1]. Obstructive sleep apnea (OSA) is the most common type of SDB [2–6]. OSA is defined as an intermittent upper airway collapse caused by impaired upper airway anatomy and reduced upper airway dilator muscle tone during sleep [7, 8]. OSA is manifested by recurrent inspiratory flow limitation, obstructive apneas, and hypopneas that cause intermittent hypoxemia and hypercapnia, multiple arousals, and sleep fragmentation. The prevalence of OSA varies based on the threshold of the apnea-hypopnea index, defined as a number of apneas and hypopneas per hour of sleep. Using an apnea-hypopnea index ≥ 5 events/hour, approximately 54 million Americans (33.2% of the adult US population) have OSA [9]. Moderate-severe OSA, defined as an apnea-hypopnea index ≥ 15 events/hour, is present in 23.7 million Americans (14.5% of the adult US population). The global burden of OSA has been estimated between 425 million to 936 million people [9]. Obesity is by far the most common risk factor for OSA. The prevalence of OSA in obese individuals exceeds 50% [2, 4, 5, 10, 11].
Another obesity-induced type of SDB is obesity hypoventilation syndrome (OHS). OHS is defined as daytime hypercapnia (arterial carbon dioxide partial pressure, PaCO2 ≥ 45 mmHg at sea level) in obese patients (body mass index ≥ 30 kg/m2) in the absence of an alternative explanation for hypoventilation [12]. OHS is present in 10%–20% of obese OSA patients [13]. Although the prevalence of OHS in the community is unknown, it can be estimated. According to the Centers for Disease Control and Prevention, 8% of the US adult population has severe obesity (body mass index ≥ 40 kg/m2) [14]. According to the most conservative estimates, 50% of adults with severe obesity have OSA and approximately 10% of the patients with severe obesity and OSA have OHS, the prevalence of OHS in the general adult population would be approximately 0.4% (one out of 260). Approximately 70% of OHS patients have severe OSA [15], defined by an apnea-hypopnea index ≥ 30 events/hour. Continuous positive airway pressure can be effective in reversing hypercapnia in patients with OHS and concomitant severe OSA [16]. Noninvasive ventilation, typically delivered as bilevel positive airway pressure or volume-targeted pressure support, is the treatment of choice for patients with OHS with mild OSA or no OSA. Noninvasive ventilation is also widely used in OHS patients recovering from acute hypercapnic respiratory failure, and in those with residual hypercapnia despite adequate continuous positive airway pressure treatment [17]. Although patients with OHS have better adherence to positive airway pressure therapy (continuous positive airway pressure or noninvasive ventilation) compared to eucapnic OSA, adherence in many patients remains suboptimal leading to persistent hypercapnia [18]. Untreated or suboptimally treated OHS leads to high morbidity and mortality [19] with an all-cause mortality of 24% after 18 months of follow-up [15], 18% at 1 year, and 31.3% at 3 years [20, 21]. While some of these patients were treated with positive airway pressure therapy after hospital discharge, such high mortality rates should be an impetus to explore alternative or complementary effective therapies. A thorough understanding of the mechanisms of disease will be critical in accelerating the discovery of effective and safe pharmacotherapeutic approaches to OHS and OSA.
Leptin and Leptin Resistance
Several mechanisms have been implicated in the pathogenesis of OHS including respiratory muscle weakness, small lung volumes, and disbalance between CO2 production and elimination [19]. Nevertheless, impaired control of breathing plays a key role. Research in respiratory neurobiology of leptin and leptin resistance has been facilitated by the development of animal models of OHS [22–25]. Effects of leptin on the control of breathing were discovered in the 1990s using animal models of leptin-deficient and leptin-resistant obesity [22, 26]. Clinical data suggest that OSA may increase leptin levels and aggravate leptin resistance [27].
Resistance to respiratory effects of leptin, an adipocyte-produced hormone regulating metabolism and control of breathing, is implicated in the OSA and OHS [19, 28, 29]. Major development occurred in respiratory neurobiology over the last two decades [30], but the novel fundamental findings have not been translated into clinical advances. In this review, we attempt to connect physiology literature on respiratory effects of leptin and state-of-the-art respiratory neurobiology.
Leptin is a 16-kDa protein encoded by the ob gene, which was discovered by Dr. Jeffrey Friedman’s laboratory in 1994 [31]. Leptin is predominantly produced by adipocytes and plays a role as a pleiotropic hormone suppressing appetite, increasing metabolic rate [32–34], stimulating control of breathing [22, 26, 35], and improving upper airway patency during sleep [24]. Leptin-deficient ob/ob mice are severely obese, hyperphagic, hypometabolic, and their obesity is treatable by leptin. Ob/ob mice hypoventilate during sleep and wakefulness, have a higher PaCO2 and lower hypercapnic ventilatory sensitivity, recurrent hypopneas during REM sleep treatable by leptin [23, 26, 36]. Leptin deficiency in humans also leads to severe obesity treatable by leptin, but it is exceedingly rare [37].
There are six isoforms of leptin receptors, but all central metabolic and respiratory effects of leptin in the brain occur via its action on the long isoforms Ob-Rb or LEPRb [22, 35, 38–41]. After binding to this receptor, leptin activates receptor-associated Janus kinase 2 and phosphorylates signal transducer and activator of transcription 3 (STAT3). pSTAT3 dimerizes and translocates to the nucleus where it activates proopiomelanocortin gene transcription [28, 42]. Leptin alters neuronal excitability in several cell types due to a myriad of cellular mechanisms. Leptin hyperpolarizes neuropeptide Y-expressing neurons in the hypothalamic arcuate nucleus via activation of ATP-sensitive potassium channels [43]. Leptin depolarizes hippocampal neurons by activating transient receptor potential-canonical channels [44]. Despite the growing evidence of the role of leptin in the control of breathing, the cellular mechanisms are not fully understood.
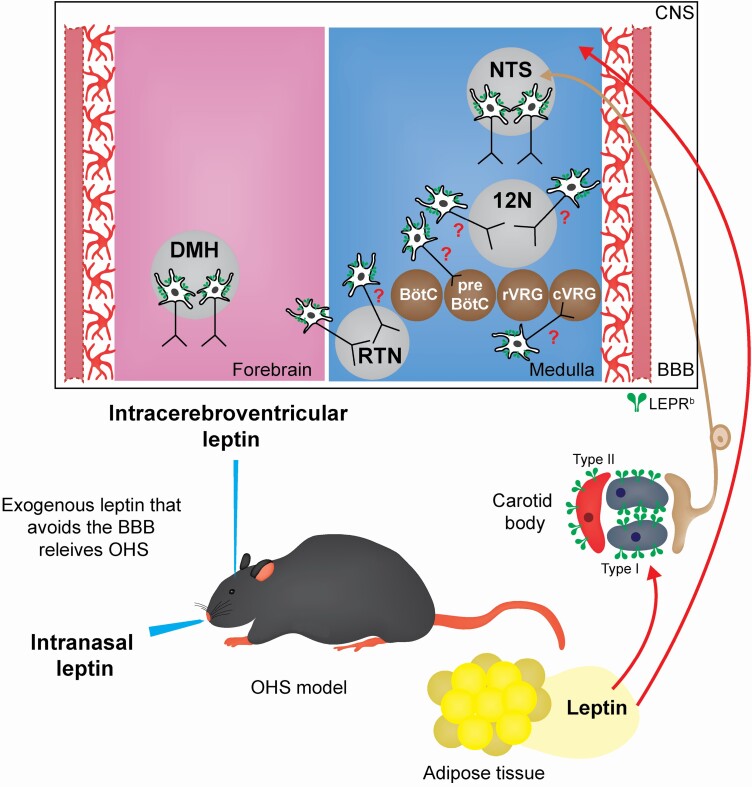
In the most common form of human obesity caused by positive energy balance, leptin levels are increased in proportion to the adipose mass [45, 46]. Obese people and mice with diet-induced obesity remain hyperphagic, despite high leptin levels, and are resistant to the beneficial respiratory and metabolic effects of leptin. A previous study suggests that leptin may play a role in linking ventilation to metabolism [47]. Obese patients and rodents develop SDB and are resistant to the respiratory effects of the hormone. The resistance to leptin in obesity may contribute to a variety of respiratory diseases, including OHS [19]. Resistance to central effects of leptin is attributed to limited permeability of the blood-brain barrier to leptin and impaired LEPRb signaling [46–51]. LEPRb dysfunction is mediated by several mechanisms including upregulation of the suppressor of cytokine signaling 3 and protein tyrosine phosphatase 1B [52–54]. Our study in diet-induced obese mice suggests that resistance to central respiratory effects of leptin on hypercapnic sensitivity occurs at the blood-brain barrier level [25]. Leptin-deficient ob/ob and leptin-resistant mice with diet-induced obesity have increased upper airway collapsibility, inspiratory airflow limitation, and OSA as well as OHS [23, 24]. Systemic (subcutaneous or intraperitoneal) leptin abolished OSA and OHS in ob/ob mice [23], but had no effect in diet-induced obese mice [55]. In contrast, intranasal leptin, which delivers leptin to the brain [56], circumvented the blood-brain barrier and abolished OSA and OHS in obese mice [55]. Therefore, LEPRb in the brain is an important therapeutic target in OSA and OHS, but the localization of leptin-sensitive respiratory neurons remains uncertain (Figure 1).
Figure 1.
Schematic representation of leptin-mediated neural targets in obesity hypoventilation syndrome (OHS). The experimental models of OHS allowed the study of the effects of leptin on the control of breathing acting in leptin receptor (LEPRb) positive cells. Brain blood barrier (BBB). Central nervous system (CNS). Nucleus tractus solitarii (NTS). Retrotrapezoid nucleus (RTN). Hypoglossal nucleus (12 N). Dorsomedial hypothalamus (DMH). pre-Bötzinger complex (pre-BötC). Bötzinger complex (BötC). Caudal ventral respiratory group (cVRG). Rostral ventral respiratory group (vVRG).
Leptin in Experimental Models of Obesity Hypoventilation Syndrome
Studies in animal models of obesity showed that perturbation of leptin pathways compromises the control of breathing. Leptin-deficient obesity is very uncommon in humans, but studies in the only rodent model of leptin-deficient obesity, ob/ob mice, provided significant insight in the physiology of OHS and SDB in general [26, 36]. Ob/ob mice have a recessive mutation in the ob gene, which prevents leptin biosynthesis [31]. Ob/ob mice weigh on average 58.2 ± 3.6g at 16 weeks of age. Exogenous leptin administration reversed the effects of the ob gene mutation resulting in decreased food intake, increased energy expenditure, and weight loss [57–59]. Ob/ob mice hypoventilate during sleep [26, 36] and leptin infusion increased minute ventilation in wakefulness, NREM, and specifically in REM sleep, which was independent of the food intake, body weight, and CO2 production [26]. Intracerebroventricular administration of leptin markedly improved baseline ventilation and hypercapnic ventilatory responses in ob/ob mice and this effect was attributed to leptin deficiency rather than obesity [60]. Microinjection of leptin into the ventrolateral medulla of ob/ob mice increased minute ventilation, tidal volume, and respiratory response to hypercapnia [22]. Subcutaneous administration of leptin in ob/ob mice increased inspiratory airflow and minute ventilation in flow-limited and nonflow limited breaths in REM as well as NREM sleep as a result of increased tidal volume [23]. Intracerebroventricular administration of leptin to the lateral versus fourth ventricle of ob/ob mice showed that both routes of leptin administration increased minute ventilation during nonflow-limited breathing during sleep, while inspiratory flow limitation and obstructive hypopneas were attenuated by leptin administration to the lateral but not to the fourth cerebral ventricle. Given that the cerebrospinal fluid flow is rostrocaudal, these findings indicate that leptin relieves upper airway obstruction in sleep apnea by activating the forebrain and leptin upregulates ventilatory control through sites of action located in the medulla [24].
In contrast to leptin deficiency, leptin-resistant obesity is very common. There are multiple rodent models of leptin-resistant obesity. LEPRb-deficient, db/db mice have spontaneous point mutations in the gene encoding the leptin receptor leading to leptin resistance [61, 62]. Db/db mice weigh on average 54.3 ± 3.8g at 16 weeks of age. In db/db mice, exogenous leptin administration caused no significant changes in food intake and body weight [63]. Db/db obese mice hypoventilate with decreased minute ventilation during sleep and elevated PaCO2 while awake [35].
Agouti yellow (Ay) mice model moderate obesity. Ay mice have a dominant mutation in the Agouti locus [64]. The agouti gene is also known to be involved in the inhibition of the melanocortin-4-receptor (MC4R) which is involved in the downstream pathway of leptin that leads to decreased hunger, diminished fat storage in adipocytes, and increased energy expenditure [65]. Ay mice weigh on average 39 ± 2g at 16 weeks of age. Exogenous leptin administration showed little to no changes in food intake and body weight [66, 67]. Baseline ventilation of Ay mice was significantly lower compared to control mice across all sleep/wake stages. The hypoxic ventilatory response was not affected in Ay mice, while hypercapnic sensitivity was depressed during NREM sleep, but not during wakefulness or REM sleep [68].
New Zealand Obese mice model polygenic-spontaneous obesity [69]. New Zealand Obese mice were generated by inbreeding from a mixed population with selection for obesity. New Zealand Obese mice weigh on average 67 ± 0.4 g at 16 weeks of age [70]. New Zealand Obese mice are resistant to the metabolic effects of leptin and they are hyperphagic, obese, and have decreased energy expenditure when administered leptin [33, 71, 72]. New Zealand Obese mice are predisposed to SDB due to altered upper airway anatomy marked by an increased size of the tongue, lateral pharyngeal walls, soft palate, and parapharyngeal fat pads, which leads to a reduction in the upper airway size and flow limitation [73–75]. Systemic leptin receptor blockade in New Zealand Obese mice did not affect minute ventilation during NREM and REM sleep and hypoxic ventilatory response [76].
Diet-induced obese mice are C57BL/6J mice fed with high-fat diet to induce obesity. These mice weigh on average 43 ± 0.3 g at 16 weeks of age. Diet-induced obese mice are leptin resistant due to the poor permeability of the blood-brain barrier for leptin [49, 77–79]. These mice have inspiratory flow limitation and hypoventilate during sleep, which leads to high PaCO2 in wakefulness [25]. Diet-induced obese mice do not respond to intraperitoneal leptin due to poor permeability of the blood-brain barrier, while intranasal leptin increases ventilation in NREM and REM sleep [55].
Zucker rats are the most widely used rat model of genetic obesity. Zucker rats have a missense mutation in the leptin receptor leading to leptin sensitivity [80]. Zucker rats weigh on average 500 g at 16 weeks of age [81]. Intracerebroventricular injection of leptin in Zucker rats did not reduce food intake and body weight [82, 83]. These animals have blunted hypercapnic ventilatory response [84]. The data on the hypoxic ventilatory response are contradictory with one group of investigators reporting no effect [84], whereas others report that hypoxic ventilatory response was reduced and this reduction was abolished by carotid body denervation [85]. Diet-induced obese Sprague-Dawley and Wistar rats have also been used as a model of leptin resistance. When leptin was administered through intracerebroventricular injection, obese Sprague-Dawley rats decreased food intake [86].
Leptin plays a key role in the pathogenesis of OSA through central regulation of upper airway patency [87]. Animal models of leptin deficiency and leptin resistance have defects in upper airway structural and neuromuscular control leading to increased pharyngeal collapsibility and flow-limited breathing [23, 24]. The occurrence of upper airway obstruction in animal models of aberrant leptin signaling and the stimulating effects of exogenous leptin on the upper airway suggests that the impaired leptin axis plays a role in the pathogenesis of OSA [24]. However, the involvement of leptin in OHS has not received the same attention in the literature.
Multiple mechanisms contribute to the development of OHS, including aberrant pulmonary mechanics, upper airway closure during sleep, and leptin resistance [88–90]. During hypercapnic ventilatory response test, patients with OHS have blunted ventilatory responses compared to obese patients without OHS [91], which indicates that they have an attenuation in the central respiratory drive responsiveness, particularly during sleep. Clinically, OHS patients suffer from poor quality of life, have higher healthcare expenses, and are prone to develop pulmonary hypertension and early mortality [19]. Experimental models shed light on mechanisms that cause hypoventilation during sleep, higher awake CO2, increased upper airway collapsibility, inspiratory airflow limitation, and a decrease in CO2 central chemosensitivity [25]. Novel plethysmographic recording methods allow monitoring high-fidelity airflow with the addition of continuous pulse oximetry and respiratory effort signals measured continuously during sleep in mice [74]. These recording methods demonstrated recurrent hypopneas with oxyhemoglobin desaturations in leptin-deficient ob/ob mice and diet-induced obese mice during sleep, which indicated that the mouse models human OSA in addition to OHS [24, 25].
Leptin, Neural Control of Breathing and Central Chemoreceptors in OHS
Breathing is generated by neurons located in the ventral medulla. This respiratory network is represented in Figure 1. The respiratory network includes a ventral respiratory group (VRG) composed of four subdivisions: Bötzinger complex (BötC, expiratory neurons); pre-Bötzinger complex (pre-BötC, inspiratory neurons); the rostral portion of the ventral respiratory group (rVRG, bulbospinal inspiratory neurons), and caudal portion of the ventral respiratory group (cVRG, bulbospinal expiratory neurons). Respiratory neurons located in the brainstem are synaptically connected to other regions of the brain integrating in the network that coordinates the contraction and relaxation of thoracic and abdominal muscles and generates the eupneic respiratory pattern [92, 93]. PaCO2 and pH, in turn, are precisely regulated in a narrow range even in the presence of environmental challenges [94]. PaCO2 is determined by the ratio of CO2 production and CO2 elimination by the lungs. Central respiratory chemoreceptors are specific neuronal and glial cells that detect small changes of pH/PaCO2 [95] and are involved in the regulation of breathing. The hypercapnia in OHS is entirely due to alveolar hypoventilation [19]. The lack of compensatory hyperventilation to higher PaCO2 suggests that OHS may lead to impaired hypercapnic sensitivity. Next, we will discuss putative sites at which leptin may act to regulate breathing and CO2 homeostasis (Fig. 1).
Leptin, the nucleus of the solitary tract and OHS
Neurons with heterogeneous properties and functions are found in the nucleus tractus solitarii (NTS). NTS is located in the dorsolateral medulla, extending from the level of the caudal portion of the facial nucleus to the caudal portion of the pyramidal decussation [96]. NTS is a crucial region of the brainstem that processes afferent information from peripheral chemoreceptors in the carotid bodies and is also involved in the modulation of breathing [97]. LEPRbs have been detected in the NTS neurons [98, 99].
A single administration of leptin in the ventrolateral NTS increased the activity of the inspiratory muscles [40] as well as hypercapnic ventilatory responses in anesthetized rats [41]. A recent study took advantage of the state-of-the-art optogenetic approach to activate specifically LEPRb positive NTS neurons in anesthetized and mechanically ventilated mice [47]. This study documented that the stimulation of LEPRb positive NTS neurons transiently increased phrenic nerve burst amplitude and induced a transient depression in phrenic nerve burst frequency [47]. The mechanisms by which leptin acts in the NTS were thought to be via activation of the sodium leak channel depolarizing a subset of glutamatergic (VGluT2) LEPRb positive NTS neurons expressing the neuropeptide galanin. This study has also demonstrated that VGluT2-expressing LEPRb positive NTS neurons project to the brainstem inspiratory premotor region (rVRG) and dorsomedial hypothalamus (DMH), which are putative regions involved in the control of breathing and metabolism [47]. Using anesthetized and freely moving mice, Yu et al [100]. have shown that chemogenetic (designer receptor exclusively activated by designer drugs, DREADD) and optogenetic stimulation of LEPRb positive NTS neurons also activate breathing. The authors suggested that leptin augments breathing via the NTS–lateral parabrachial nuclei–pre-Bötzinger complex circuit. The above studies in anesthetized and awake animals showed that leptin acts in the NTS to stimulate breathing, but they did not examine the duration of the leptin’s effect and relevance of these findings for SDB.
Given this evidence, we hypothesized that leptin acts on LEPRb positive neurons in the NTS to increase ventilation and maintain upper airway patency during sleep. We expressed DREADD selectively in LEPRb positive NTS neurons and performed sleep recordings in diet-induced obese mice, which demonstrate SDB remarkably similar to human OHS and OSA [25, 55]. Activation of LEPRb positive NTS neurons throughout sleep failed to stimulate control of breathing and upper airway function and did not relieve SDB [101] (Table 1). Thus, it appears that although leptin signaling in the NTS stimulates breathing, this effect appears to be transient and may not be relevant for long-term therapeutic action of this hormone in SDB.
Table 1.
Targets of respiratory effects of leptin in animal models of OHS
| Region | References | Animal model of OHS | Methods | Evidences |
|---|---|---|---|---|
| Fourth ventricle | Bassi et al., 2012 [60] | Leptin-deficient (ob/ob) mice | Mice received leptin into the fourth ventricle for four consecutive days. Baseline ventilation and ventilatory responses to CO2 were measured using plethysmography. | Central leptin increased ventilation and ventilatory response to hypercapnia Subcutaneous leptin administration did not change ventilation and ventilatory response to hypercapnia. lean pair-weighted ob/ob mice have impaired ventilatory response. |
| Ventrolateral medulla | Bassi et al., 2014 [22] | ob/ob mice | Mice received microinjections of leptin for 3 days in the Ventrolateral medulla. Ventilatory responses to CO2 were measured using plethysmography. | Increased minute ventilation, tidal volume, and ventilatory response to hypercapnia |
| Fourth ventricle and lateral ventricles | Yao et al., 2016 [24] | ob/ob mice | ICV administration of leptin followed by polysomnographic recording | Leptin administration in the fourth ventricle and lateral ventricles increased minute ventilation during nonflow-limited breathing in sleep. Inspiratory flow limitation were relieved by leptin administration to the lateral but not to the fourth cerebral ventricle |
| Carotid bodies | Ribeiro et al., 2018 [102] | Diet-induced obese Wistar rats | Intravenous leptin administration on ventilatory parameters in vivo. Leptin aplication on carotid sinus nerve activity ex vivo. | Leptin increases minute ventilation at the baseline and during hypoxia in control rats. In high-fat model, the effect of leptin in ventilation is blunted. High-fat rats presented an increased frequency of carotid cinus nerve at tha baseline, which is not affected by leptin. |
| Carotid bodies | Yuan et al., 2018 [85] |
Obese Zucker rats | Ventilation was assessed in conscious obese Zucker rats or lean littermates that received an injection of leptin at the baseline and during hypoxia in control animais and rats with carotid body denervation. The expression of pSTAT3, as well as K + channel TASK-1 was evaluated in the carotid bodies. |
Leptin signaling increases hypoxic ventilatory responses.Leptin administration is associated with changes in expression of pSTAT3 and TASK channels in the carotid bodies. |
| (?) | Berger et al., 2019 [55] | Diet-induced obese mice | A single dose of leptin or vehicle were administered intranasally or intraperitoneally, followed by sleep studies | Intranasal, but not intraperitoneal, leptin decreased the number of oxygen desaturation events in REM sleep, and increased ventilation in non-REM and REM sleep. |
| Carotid bodies | Caballero‐Eraso et al., 2019 [103] | C57BL/6J mice and LEPRb‐deficient db/db mice | Subcutaneous leptin infusion followed by baseline minute ventilation and the hypoxic ventilatory response (HVR) to 10% O2 measurementens in C57BL/6J mice before and after carotid bodies denervation. Expression of LEPRb in the carotid bodies of db/db mice followed by recording of breathing during sleep and wakefulness and on HVR | Leptin acts on LEPRb in the carotid bodies to stimulate breathing and HVR. |
| DMH | Pho et al., 2021 [35] | db/db mice | Mice were infected with Ad-LepRb or control Ad-mCherry virus into the DMH. Ventilation was measured during sleep as well as CO2 production after intracerebroventricular (ICV) of leptin or vehicle | After leptin receptor expression in DMH of db/db mice, ICV leptin increased inspiratory flow, tidal volume, and minute ventilation during NREM sleep |
| NTS | Amorim et al., 2021 [101] | LEPRb-Cre diet-induced obese mice | Designer receptors exclusively activated by designer drugs were selectively expressed in the LEPRb positive neurons of the NTS The effect of DREADD ligand, J60, on tongue muscle activity and breathing during sleep was measured. | Activation of LEPRb positive NTS neurons did not stimulate breathing or upper airway muscles during NREM and REM sleep |
Leptin, the retrotrapezoid nucleus (RTN) and OHS
The RTN is very sensitive to hypercapnia and is considered the major site through which ventilation is regulated by CO2 and pH. RTN neurons are glutamatergic, can be identifiable by the presence of Neuromedin B mRNA [95] and their intrinsic properties are consistent with those expected for specialized central respiratory chemoreceptors. Selective stimulation of RTN neurons increased breathing without affecting arousals [104], a critical feature in the management of OSA and OHS. Previous studies showed that administration of leptin for three consecutive days into the rostral ventrolateral medulla, where the RTN is located, increased baseline ventilation and the ventilatory response to CO2 in ob/ob mice [22] (Table 1). Wei et al., examined if impaired leptin signaling in the RTN leads to obesity-related hypoventilation [105]. The authors concluded that obesity-related impairment of HCVR is closely associated with the disordered cellular leptin signaling in the RTN, even though breathing during sleep was not measured. In contrast, acute exposure to leptin did not change the activity of RTN chemosensitive neurons or astrocytes in brainstem slices from rats [28]. LEPRbs were not detected in the ventral medulla [99], where RTN neurons are located, hence it is likely that leptin does not stimulate RTN neurons directly. Rather these central respiratory neurons may receive inputs from LEPRbs neurons elsewhere. Taken together, current evidence indicates that monosynaptic or polysynaptic projections from LEPRb positive neurons elsewhere in the brain to the RTN may be necessary for stimulation of breathing during sleep and wakefulness. These RTN-projecting LEPRb neurons have to be identified in the future for leptin-targeted approach to OHS therapy.
Leptin, the dorsomedial hypothalamus (DMH) and OHS
Initial studies in our laboratory suggested that leptin may regulate control of breathing and upper airway function during sleep by acting in the DMH [24]. In a follow-up study we demonstrated that LEPRb expression in the DMH of db/db mice followed by intracerebroventricular leptin administration increased inspiratory flow, tidal volume, and minute ventilation during NREM sleep [35] (Table 1). DMH neurons are key regulators of metabolism. Notably, these neurons project to hypoglossal motoneurons [24] and may also be involved in maintaining upper airway patency. Altogether, our findings are consistent with the hypothesis that leptin stimulates control of breathing and CO2 sensitivity, and improves upper airway patency during sleep by acting on LEPRb positive DMH neurons and their projections to respiratory neurons in the brainstem. Whether or not stimulation of DMH neurons in obese animals and, eventually in humans, may lead to relieving OHS, is an important area for future investigation.
One potential pathway by which leptin may regulate breathing and metabolism in the DMH is via the melanocortin-4-receptor (MC4R) [106]. MC4R is a G protein-coupled receptor that binds α-melanocyte-stimulating hormone and is downstream of the leptin pathway [107]. The majority of MC4Rs are localized in the paraventricular nuclei and the DMH [108]. Mutations in the MC4R gene are the most frequent cause of monogenic obesity [109]. We found that LEPRb positive DMH neurons express MC4R [35]. Thus, the MC4R is a highly relevant therapeutic target given that MC4R deficiency predisposes to SDB [110]; MC4R agonists have been effective in the treatment of obesity not limited to MC4R deficiency [111, 112], and the MC3/4 competitive antagonist SHU9119 [113] blocked effects of leptin on hypercapnic chemoreflex [114]. Altogether, these findings are consistent with the hypothesis the LEPRb positive DMH neurons that express MC4R are a possible therapeutic target stimulating breathing in OHS.
Leptin, the hypoglossal nucleus and OSA
Leptin replacement significantly increases ventilation during flow-limited breathing in ob/ob mice, indicating that leptin treatment improves upper airway patency with a potential to treat OSA [23]. Genioglossus muscle is the principal upper airway dilator, which plays a major role in the maintenance of upper airway patency during sleep. This muscle is innervated by the hypoglossal nerve originating from the hypoglossal nucleus in the medulla. Hypoglossal motoneurons are a major drug target in OSA [115]. We have performed histological and in vitro electrophysiological studies of hypoglossal motoneurons in Leprb-Cre mice. The application of the μ-opioid receptor agonist DAMGO significantly reduced excitatory postsynaptic currents (EPSC) frequency in hypoglossal motoneurons, but the focal application of leptin was restored EPSC frequency in these neurons [116]. Confocal microscopy showed that hypoglossal motoneurons did not express LEPRb, but were synaptically connected to LEPRb positive neurons [55]. These findings suggest that leptin acts on hypoglossal motoneurons presynaptically. Taken together with our in vivo sleep recording after intranasal leptin administration in mice, our findings imply that the LEPRb positive neurons projecting to the hypoglossal nucleus may promote upper airway patency and serve as a drug target in OSA. Precise localization and molecular characteristics of this population of LEPRb positive neurons in the brainstem remain unknown and is a participant of ongoing investigation in our laboratory.
Leptin and Peripheral Chemoreception in OHS
Carotid bodies are the main peripheral chemoreceptors located in the bifurcation of the carotid arteries. Carotid bodies are comprised of the oxygen-sensing glomus cells or type I cells and glia-like type II cells [117]. The peripheral chemoreceptors are cells highly specialized in sensing not only O2 but also glucose, hormones, osmolarity, proinflammatory cytokines, and leptin. The chemoreceptor mechanism of carotid bodies in response to hypoxia is related to the closure of K + channels of glomus cells and increased intracellular Ca + 2. The first synaptic contact of carotid bodies afferents in the brain is located in the NTS, which in turn leads to a stimulation of breathing. LEPRb receptors are abundantly expressed in the carotid bodies type I and type II cells [103]. Our publication has shown that leptin signals via LEPRb in carotid body type I cells increases carotid sinus nerve activity, augments the hypoxic ventilatory response, and increases blood pressure. The effects of leptin on minute ventilation and HVR were abolished when the carotid bodies were denervated [103]. Expression of LEPRb in the carotid bodies of db/db mice stimulated minute ventilation at baseline and hypoxic conditions while awake and augmented ventilation during REM and NREM sleep [103]. We have also shown that cardiovascular effects of leptin are mediated via carotid bodies LEPRb and downstream transient receptor potential melastatin 7 (TRPM7) channels [118]. However, the role of leptin signaling in the carotid body in OHS in diet-induced obesity remains unclear [85, 102].
Is Leptin the Only Respiratory Hormone?
Leptin stimulates breathing and is a promising therapeutic target for OSA and OHS. However, other hormones are implicated in the control of breathing and upper airway function. Orexin, an excitatory neuropeptide released from neurons in the hypothalamus, functions in a wide array of biological regulations of sleep, energy balance, and breathing [119, 120]. Previous studies documented that orexin plays a critical role in CO2 sensitivity [121]. Upper airway obstruction increased hypothalamic orexin levels in rats suggesting that this peptide is critical for respiratory homeostasis [122]. In addition, intranasal oxytocin, a peptide implicated in the control of autonomic function, decreased the duration of obstructive events and oxygen desaturations in patients with OSA [123]. Upper airway obstruction decreases growth hormone, which may stunt growth in children and adolescents [124]. Ghrelin, a hormone secreted by the stomach, induces hunger and plays a role in meal initiation. In obese humans, ghrelin levels are increased [125]. Interestingly, nonobese patients with OSA appear to have higher levels of ghrelin than obese OSA patients, but continuous positive airway pressure treatment does not seem to affect ghrelin levels in both populations [126]. Ghrelin administration increased the acute hypoxic ventilatory response in rats exposed to chronic hypoxia [127]. Thyrotropin-releasing hormone and its analogs increase genioglossus electromyography activity, which may improve upper airway patency during sleep [128]. Taken together, current literature suggests that hormones other than leptin have a therapeutic potential for OHS and OSA.
Clinical Implications and Perspectives
Animal data strongly suggest that leptin and the LEPRb signaling pathway in respsry gasrydfdsfsdfdfdsfsrsdkljjskdbvksjcbvjfbvhkfbvkjfvbljfsvbljfbvkjfvjfviratory neurons is a promising target for future drug development in OHS. Recombinant human leptin (Metreleptin) in subcutaneous injections has been approved by US Food and Drug Administration to treat metabolic complications of leptin deficiency in patients with congenital or acquired generalized lipodystrophy [129]. Intranasal formulations of leptin should be tested in proof-of-concepts clinical trials. Leptin receptor agonists freely penetrating the blood-brain barrier have been developed and shown to be effective in rodent diet-induced obesity. These agonists may have a therapeutic potential in OHS [130, 131]. Additional preclinical studies are also needed (1) to characterize leptin signaling in respiratory neurons in more detail; (2) to characterize the long-term efficacy and safety of LEPRb modulations in preclinical models.
Contributor Information
Mateus R Amorim, Department of Medicine, Division of Pulmonary and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
O Aung, Department of Medicine, Division of Pulmonary and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Babak Mokhlesi, Department of Internal Medicine, Division of Pulmonary, Critical Care, and Sleep Medicine, Rush University Medical Center, Chicago, IL, USA.
Vsevolod Y Polotsky, Department of Medicine, Division of Pulmonary and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Funding
This research was funded by grants from the National Institutes of Health (R01 HL128970, R01 HL133100, R01 HL13892, R61 HL156240—VYP); American Heart Association funded Postdoctoral Fellowship Award (#915167—MRA).
Disclosure Statement
None declared.
References
- 1. Leinum CJ, et al. . Sleep disordered breathing and obesity: pathophysiology, complications and treatment. Nutr Clin Pract. 2009;24(6):675–687. doi: 10.1177/0884533609351532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Young T, et al. . The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704 [DOI] [PubMed] [Google Scholar]
- 3. Vgontzas AN, et al. . Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia [see comments]. J Clin Endocrinol Metab. 2000;85(0021-972X):1151–1158. [DOI] [PubMed] [Google Scholar]
- 4. Tufik S, et al. . Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010;11(5):441–446. doi: 10.1016/j.sleep.2009.10.005 [DOI] [PubMed] [Google Scholar]
- 5. Peppard PE, et al. . Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heinzer R, et al. . Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. doi: 10.1016/S2213-2600(15)00043-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gastaut H, et al. . Polygraphic study of diurnal and nocturnal (hypnic and respiratory) episodal manifestations of Pickwick syndrome. Rev Neurol (Paris). 1965;112(6):568–579. [PubMed] [Google Scholar]
- 8. Mesarwi OA, et al. . Metabolic dysfunction in obstructive sleep apnea: a critical examination of underlying mechanisms. Sleep Biol Rhythms. 2015;13(1):2–17. doi: 10.1111/sbr.12078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benjafield AV, et al. . Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687–698. doi: 10.1016/S2213-2600(19)30198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Punjabi NM, et al. . Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med. 2002;165(5):677–682. doi: 10.1164/ajrccm.165.5.2104087 [DOI] [PubMed] [Google Scholar]
- 11. Young T, et al. . Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. [DOI] [PubMed] [Google Scholar]
- 12. Mokhlesi B, et al. . Assessment and management of patients with obesity hypoventilation syndrome. Proc Am Thorac Soc. 2008;5(2):218–225. doi: 10.1513/pats.200708-122MG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Balachandran JS, et al. . Obesity hypoventilation syndrome epidemiology and diagnosis. Sleep Med Clin. 2014;9(3):341–347. doi: 10.1016/j.jsmc.2014.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hales CM, et al. . Differences in obesity prevalence by demographic characteristics and urbanization level among adults in the United States, 2013-2016. JAMA. 2018;319(23):2419–2429. doi: 10.1001/jama.2018.7270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Masa JF, et al. . Obesity hypoventilation syndrome. Eur Respir Rev. 2019;28(151):180097. doi: 10.1183/16000617.0097-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Masa JF, et al. . Long-term clinical effectiveness of continuous positive airway pressure therapy versus non-invasive ventilation therapy in patients with obesity hypoventilation syndrome: a multicentre, open-label, randomised controlled trial. Lancet. 2019;393(10182):1721–1732. doi: 10.1016/S0140-6736(18)32978-7 [DOI] [PubMed] [Google Scholar]
- 17. Mokhlesi B, et al. . Evaluation and management of obesity hypoventilation syndrome. An official american thoracic society clinical practice guideline. Am J Respir Crit Care Med. 2019;200(3):e6–e24. doi: 10.1164/rccm.201905-1071ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wearn J, et al. . Adherence to positive airway pressure therapy in obesity hypoventilation syndrome. Sleep Med Clin. 2021;16(1):43–59. doi: 10.1016/j.jsmc.2020.10.009 [DOI] [PubMed] [Google Scholar]
- 19. Mokhlesi B. Obesity hypoventilation syndrome: a state-of-the-art review. Respir Care. 2010;55(10):1347–1362 [PubMed] [Google Scholar]
- 20. Carrillo A, et al. . Noninvasive ventilation in acute hypercapnic respiratory failure caused by obesity hypoventilation syndrome and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(12):1279–1285. doi: 10.1164/rccm.201206-1101OC [DOI] [PubMed] [Google Scholar]
- 21. Marik PE, et al. . The clinical characteristics and hospital and post-hospital survival of patients with the obesity hypoventilation syndrome: analysis of a large cohort. Obes Sci Pract. 2016;2(1):40–47. doi: 10.1002/osp4.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bassi M, et al. . Leptin into the ventrolateral medulla facilitates chemorespiratory response in leptin-deficient (ob/ob) mice. Acta Physiol (Oxf). 2014;211(1):240–248. doi: 10.1111/apha.12257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pho H, et al. . The effect of leptin replacement on sleep-disordered breathing in the leptin-deficient ob/ob mouse. J Appl Physiol (1985). 2016;120(1):78–86. doi: 10.1152/japplphysiol.00494.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yao Q, et al. . Localizing effects of leptin on upper airway and respiratory control during sleep. Sleep. 2016;39(5):1097–1106. doi: 10.5665/sleep.5762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fleury Curado T, et al. . Sleep-disordered breathing in C57BL/6J mice with diet-induced obesity. Sleep. 2018;41(8). doi: 10.1093/sleep/zsy089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O’donnell CP, et al. . Leptin prevents respiratory depression in obesity. Am J Respir Crit Care Med. 1999;159(5 Pt 1):1477–1484. doi: 10.1164/ajrccm.159.5.9809025 [DOI] [PubMed] [Google Scholar]
- 27. Imayama I, et al. . Role of leptin in obstructive sleep apnea. Ann Am Thorac Soc. 2017;14(11):1607–1621. doi: 10.1513/AnnalsATS.201702-181FR [DOI] [PubMed] [Google Scholar]
- 28. Bassi M, et al. . Facilitation of breathing by leptin effects in the central nervous system. J Physiol. 2016;594(6):1617–1625. doi: 10.1113/JP270308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Phipps PR, et al. . Association of serum leptin with hypoventilation in human obesity. Thorax. 2002;57(1):75–76. doi: 10.1136/thorax.57.1.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Del Negro CA, et al. . Breathing matters. Nat Rev Neurosci. 2018;19(6):351–367. doi: 10.1038/s41583-018-0003-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang Y, et al. . Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0 [DOI] [PubMed] [Google Scholar]
- 32. Friedman JM. Leptin at 14 y of age: an ongoing story. Am J Clin Nutr. 2009;89(3):973S–979S. doi: 10.3945/ajcn.2008.26788B [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Halaas JL, et al. . Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269(5223):543–546. [DOI] [PubMed] [Google Scholar]
- 34. Spiegelman BM, et al. . Obesity and the regulation of energy balance. Cell. 2001;104(4):531–543. [DOI] [PubMed] [Google Scholar]
- 35. Pho H, et al. . Leptin receptor expression in the dorsomedial hypothalamus stimulates breathing during NREM sleep in db/db mice. Sleep. 2021. 44(6). doi: 10.1093/sleep/zsab046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tankersley C, et al. . Modified control of breathing in genetically obese (ob/ob) mice. J Appl Physiol (1985). 1996;81(2):716–723. doi: 10.1152/jappl.1996.81.2.716 [DOI] [PubMed] [Google Scholar]
- 37. Farooqi IS, et al. . Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341(12):879–884. doi: 10.1056/NEJM199909163411204 [DOI] [PubMed] [Google Scholar]
- 38. Friedman JM. Leptin, leptin receptors, and the control of body weight. Nutr Rev. 1998;56(2 Pt 2):s38–s46. [DOI] [PubMed] [Google Scholar]
- 39. Adya R, et al. . Differential effects of leptin and adiponectin in endothelial angiogenesis. Finucane FM, ed. J Diabetes Res. 2015;2015:648239. doi: 10.1155/2015/648239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Inyushkin AN, et al. . Respiratory responses to microinjections of leptin into the solitary tract nucleus. Neurosci Behav Physiol. 2009;39(3):231–240. doi: 10.1007/s11055-009-9124-8 [DOI] [PubMed] [Google Scholar]
- 41. Inyushkina EM, et al. . Mechanisms of the respiratory activity of leptin at the level of the solitary tract nucleus. Neurosci Behav Physiol. 2010;40(7):707–713. doi: 10.1007/s11055-010-9316-2 [DOI] [PubMed] [Google Scholar]
- 42. Farooqi IS, et al. . 20 years of leptin: human disorders of leptin action. J Endocrinol. 2014;223(1):T63–T70. doi: 10.1530/JOE-14-0480 [DOI] [PubMed] [Google Scholar]
- 43. van den Top M, et al. . Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci. 2004;7(5):493–494. doi: 10.1038/nn1226 [DOI] [PubMed] [Google Scholar]
- 44. Dhar M, et al. . Leptin-induced spine formation requires TrpC channels and the CaM Kinase cascade in the hippocampus. J Neurosci. 2014;34(30):10022–10033. doi: 10.1523/JNEUROSCI.2868-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maffei M, et al. . Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1(11):1155–1161. doi: 10.1038/nm1195-1155 [DOI] [PubMed] [Google Scholar]
- 46. Considine RV, et al. . Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334(5):292–295. doi: 10.1056/NEJM199602013340503 [DOI] [PubMed] [Google Scholar]
- 47. Do J, et al. . A leptin-mediated neural mechanism linking breathing to metabolism. Cell Rep. 2020;33(6):108358. doi: 10.1016/j.celrep.2020.108358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schwartz MW, et al. . Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med. 1996;2(5):589–593. doi: 10.1038/nm0596-589 [DOI] [PubMed] [Google Scholar]
- 49. Banks WA, et al. . Impaired transport of leptin across the blood-brain barrier in obesity. Peptides. 1999;20(11):1341–1345. doi: 10.1016/s0196-9781(99)00139-4 [DOI] [PubMed] [Google Scholar]
- 50. Wauman J, et al. . Leptin receptor signaling: pathways to leptin resistance. Front Biosci (Landmark Ed). 2011;16:2771–2793. [DOI] [PubMed] [Google Scholar]
- 51. Pan H, et al. . Advances in understanding the interrelations between leptin resistance and obesity. Physiol Behav. 2014;130:157–169. doi: 10.1016/j.physbeh.2014.04.003 [DOI] [PubMed] [Google Scholar]
- 52. Zhou Y, et al. . Leptin signaling and leptin resistance. Front Med. 2013;7(2):207–222. doi: 10.1007/s11684-013-0263-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kaszubska W, et al. . Protein tyrosine phosphatase 1B negatively regulates leptin signaling in a hypothalamic cell line. Mol Cell Endocrinol. 2002;195(1-2):109–118. doi: 10.1016/s0303-7207(02)00178-8 [DOI] [PubMed] [Google Scholar]
- 54. Bjorbak C, et al. . SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J Biol Chem. 2000;275(51):40649–40657. doi: 10.1074/jbc.M007577200 [DOI] [PubMed] [Google Scholar]
- 55. Berger S, et al. . Intranasal leptin relieves sleep-disordered breathing in mice with diet-induced obesity. Am J Respir Crit Care Med. 2019;199(6):773–783. doi: 10.1164/rccm.201805-0879OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Freire C, et al. . Intranasal leptin improves survival after opioid overdose in a mouse model. J Transl Med. 2021;19(1):134. doi: 10.1186/s12967-021-02803-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pelleymounter MA, et al. . Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269(5223):540–543. doi: 10.1126/science.7624776 [DOI] [PubMed] [Google Scholar]
- 58. Muzzin P, et al. . Woo SLC. Correction of obesity and diabetes in genetically obese mice by leptin gene therapy. Proc Natl Acad Sci USA. 1996;93(25):14804–14808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Harris RB, et al. . A leptin dose-response study in obese (ob/ob) and lean (+/?) mice. Endocrinology. 1998;139(1):8–19. doi: 10.1210/endo.139.1.5675 [DOI] [PubMed] [Google Scholar]
- 60. Bassi M, et al. . Central leptin replacement enhances chemorespiratory responses in leptin-deficient mice independent of changes in body weight. Pflugers Arch. 2012;464(2):145–153. doi: 10.1007/s00424-012-1111-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chen H, et al. . Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84(3):491–495. doi: 10.1016/s0092-8674(00)81294-5 [DOI] [PubMed] [Google Scholar]
- 62. Lee GH, et al. . Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379(6566):632–635. doi: 10.1038/379632a0 [DOI] [PubMed] [Google Scholar]
- 63. Campfield LA, et al. . Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269(5223):546–549. doi: 10.1126/science.7624778 [DOI] [PubMed] [Google Scholar]
- 64. Yen TT, et al. . Obesity, diabetes, and neoplasia in yellow A(vy)/- mice: ectopic expression of the agouti gene. FASEB J. 1994;8(8):479–488. doi: 10.1096/fasebj.8.8.8181666 [DOI] [PubMed] [Google Scholar]
- 65. Seeley RJ, et al. . Melanocortin receptors in leptin effects. Nature. 1997;390(6658):349. doi: 10.1038/37016 [DOI] [PubMed] [Google Scholar]
- 66. Rahmouni K, et al. . Selective resistance to central neural administration of leptin in agouti obese mice. Hypertension. 2002;39(2 Pt 2):486–490. doi: 10.1161/hy0202.102836 [DOI] [PubMed] [Google Scholar]
- 67. Ebihara K, et al. . Involvement of agouti-related protein, an endogenous antagonist of hypothalamic melanocortin receptor, in leptin action. Diabetes. 1999;48(10):2028–2033. doi: 10.2337/diabetes.48.10.2028 [DOI] [PubMed] [Google Scholar]
- 68. Polotsky VY, et al. . Impact of interrupted leptin pathways on ventilatory control. J Appl Physiol (1985). 2004;96(3):991–998. doi: 10.1152/japplphysiol.00926.2003 [DOI] [PubMed] [Google Scholar]
- 69. Bielschowsky M, et al. . Origin of inbred NZ mouse strains. Cancer Res. 1970;30(3):834–836. [PubMed] [Google Scholar]
- 70. John C, et al. . Sex differences in cardiac mitochondria in the New Zealand obese mouse. Front Endocrinol (Lausanne). 2018;9:732. doi: 10.3389/fendo.2018.00732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Igel M, et al. . Hyperleptinemia, leptin resistance, and polymorphic leptin receptor in the New Zealand obese mouse. Endocrinology. 1997;138(10):4234–4239. doi: 10.1210/endo.138.10.5428 [DOI] [PubMed] [Google Scholar]
- 72. Wator L, et al. . Impaired leptin activity in New Zealand Obese mice: model of angiogenesis. Genes Nutr. 2008;3(3-4):177–180. doi: 10.1007/s12263-008-0103-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Brennick MJ, et al. . Altered upper airway and soft tissue structures in the New Zealand Obese mouse. Am J Respir Crit Care Med. 2009;179(2):158–169. doi: 10.1164/rccm.200809-1435OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hernandez AB, et al. . Novel whole body plethysmography system for the continuous characterization of sleep and breathing in a mouse. J Appl Physiol (1985). 2012;112(4):671–680. doi: 10.1152/japplphysiol.00818.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Baum DM, et al. . New Zealand obese mice as a translational model of obesity-related obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2018;198(10):1336–1339. doi: 10.1164/rccm.201801-0162LE [DOI] [PubMed] [Google Scholar]
- 76. Kim LJ, et al. . Leptin receptor blockade attenuates hypertension, but does not affect ventilatory response to hypoxia in a model of polygenic obesity. Front Physiol. 2021;12:688375. doi: 10.3389/fphys.2021.688375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Van Heek M, et al. . Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest. 1997;99(3):385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Golden PL, et al. . Human blood-brain barrier leptin receptor. Binding and endocytosis in isolated human brain microvessels. J Clin Invest. 1997;99(1):14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Souza-Almeida G, et al. . Peripheral leptin signaling persists in innate immune cells during diet-induced obesity. J Leukoc Biol. 2021;109(6):1131–1138. doi: 10.1002/JLB.3AB0820-092RR [DOI] [PubMed] [Google Scholar]
- 80. Phillips MS, et al. . Leptin receptor missense mutation in the fatty Zucker rat. Nat Genet. 1996;13(1):18–19. doi: 10.1038/ng0596-18 [DOI] [PubMed] [Google Scholar]
- 81. Vasselli JR. Patterns of hyperphagia in the Zucker obese rat: a role for fat cell size and number? Brain Res Bull. 1985;14(6):633–641. doi: 10.1016/0361-9230(85)90113-3 [DOI] [PubMed] [Google Scholar]
- 82. Seeley RJ, et al. . Intraventricular leptin reduces food intake and body weight of lean rats but not obese Zucker rats. Horm Metab Res. 1996;28(12):664–668. doi: 10.1055/s-2007-979874 [DOI] [PubMed] [Google Scholar]
- 83. Wang T, et al. . Responses of lean and obese Zucker rats to centrally administered leptin. Physiol Behav. 1998;65(2):333–341. doi: 10.1016/s0031-9384(98)00173-5 [DOI] [PubMed] [Google Scholar]
- 84. Farkas GA, et al. . Pulmonary ventilation and mechanics in morbidly obese Zucker rats. Am J Respir Crit Care Med. 1994;150(2):356–362. doi: 10.1164/ajrccm.150.2.8049815 [DOI] [PubMed] [Google Scholar]
- 85. Yuan F, et al. . Leptin signaling in the carotid body regulates a hypoxic ventilatory response through altering TASK channel expression. Front Physiol. 2018;9:249. doi: 10.3389/fphys.2018.00249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Fam BC, et al. . Modulation of central leptin sensitivity and energy balance in a rat model of diet-induced obesity. Diabetes Obes Metab. 2007;9(6):840–852. doi: 10.1111/j.1463-1326.2006.00653.x [DOI] [PubMed] [Google Scholar]
- 87. Berger S, et al. . Leptin and leptin resistance in the pathogenesis of obstructive sleep apnea: a possible link to oxidative stress and cardiovascular complications. Oxid Med Cell Longevity. 2018;2018:1–8. doi: 10.1155/2018/5137947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Piper AJ, et al. . Obesity hypoventilation syndrome: mechanisms and management. Am J Respir Crit Care Med. 2011;183(3):292–298. doi: 10.1164/rccm.201008-1280CI [DOI] [PubMed] [Google Scholar]
- 89. Powers MA. The obesity hypoventilation syndrome. Respir Care. 2008;53(12):1723–1730. [PubMed] [Google Scholar]
- 90. Olson AL, et al. . The obesity hypoventilation syndrome. Am J Med. 2005;118(9):948–956. doi: 10.1016/j.amjmed.2005.03.042 [DOI] [PubMed] [Google Scholar]
- 91. Sampson MG, et al. . Neuromechanical properties in obese patients during carbon dioxide rebreathing. Am J Med. 1983;75(1):81–90. doi: 10.1016/0002-9343(83)91171-3 [DOI] [PubMed] [Google Scholar]
- 92. Gauda EB, et al. . Leptin: master regulator of biological functions that affects breathing. Compr Physiol. 2020;10(3):1047–1083. doi: 10.1002/cphy.c190031 [DOI] [PubMed] [Google Scholar]
- 93. Smith JC, et al. . Paton JFR. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol. 2007;98(6):3370–3387. doi: 10.1152/jn.00985.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Dempsey JA, et al. . Exercise and chemoreception. Am Rev Respir Dis. 1984;129(2 Pt 2):S31–S34. doi: 10.1164/arrd.1984.129.2P2.S31 [DOI] [PubMed] [Google Scholar]
- 95. Guyenet PG, et al. . The retrotrapezoid nucleus: central chemoreceptor and regulator of breathing automaticity. Trends Neurosci. 2019;42(11):807–824. doi: 10.1016/j.tins.2019.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994;74(2):323–364. doi: 10.1152/physrev.1994.74.2.323 [DOI] [PubMed] [Google Scholar]
- 97. Zoccal DB, et al. . Colombari DSA, Colombari E. The nucleus of the solitary tract and the coordination of respiratory and sympathetic activities. Front Physiol. 2014;5:1–12. doi: 10.3389/fphys.2014.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mercer JG, et al. . Localization of leptin receptor (Ob-R) messenger ribonucleic acid in the rodent hindbrain. Endocrinology. 1998;139(1):29–34. doi: 10.1210/endo.139.1.5685 [DOI] [PubMed] [Google Scholar]
- 99. Scott MM, et al. . Leptin targets in the mouse brain. J Comp Neurol. 2009;514(5):518–532. doi: 10.1002/cne.22025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Yu H, et al. . A neural circuit mechanism controlling breathing by leptin in the nucleus tractus solitarii. Neurosci Bull. 2022;38(2):149–165. doi: 10.1007/s12264-021-00742-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Amorim MR, et al. . The effect of DREADD activation of leptin receptor positive neurons in the nucleus of the solitary tract on sleep disordered breathing. Int J Mol Sci . 2021;22(13):6742. doi: 10.3390/ijms22136742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ribeiro MJ, et al. . High fat diet blunts the effects of leptin on ventilation and on carotid body activity. J Physiol. 2018;596(15):3187–3199. doi: 10.1113/JP275362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Caballero-Eraso C, et al. . Leptin acts in the carotid bodies to increase minute ventilation during wakefulness and sleep and augment the hypoxic ventilatory response. J Physiol. 2019;597(1):151–172. doi: 10.1113/JP276900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Burke PGR, et al. . Selective optogenetic stimulation of the retrotrapezoid nucleus in sleeping rats activates breathing without changing blood pressure or causing arousal or sighs. J Appl Physiol (1985). 2015;118(12):1491–1501. doi: 10.1152/japplphysiol.00164.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wei Z, et al. . Disordered Leptin signaling in the retrotrapezoid nucleus is associated with the impaired hypercapnic ventilatory response in obesity. Life Sci. 2020;257:117994. doi: 10.1016/j.lfs.2020.117994 [DOI] [PubMed] [Google Scholar]
- 106. Farooqi IS, et al. . Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348(12):1085–1095. doi: 10.1056/NEJMoa022050 [DOI] [PubMed] [Google Scholar]
- 107. Tao YX. Constitutive activity in melanocortin-4 receptor: biased signaling of inverse agonists. Adv Pharmacol. 2014;70:135–154. doi: 10.1016/B978-0-12-417197-8.00005-5 [DOI] [PubMed] [Google Scholar]
- 108. Kim MS, et al. . Hypothalamic localization of the feeding effect of agouti-related peptide and alpha-melanocyte-stimulating hormone. Diabetes. 2000;49(2):177–182. doi: 10.2337/diabetes.49.2.177 [DOI] [PubMed] [Google Scholar]
- 109. Wangensteen T, et al. . Mutations in the melanocortin 4 receptor (MC4R) gene in obese patients in Norway. Exp Clin Endocrinol Diabetes. 2009;117(6):266–273. doi: 10.1055/s-0028-1102942 [DOI] [PubMed] [Google Scholar]
- 110. Pillai S, et al. . Severe obstructive sleep apnea in a child with melanocortin-4 receptor deficiency. J Clin Sleep Med. 2014;10(1):99–101. doi: 10.5664/jcsm.3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Collet TH, et al. . Evaluation of a melanocortin-4 receptor (MC4R) agonist (Setmelanotide) in MC4R deficiency. Mol Metab. 2017;6(10):1321–1329. doi: 10.1016/j.molmet.2017.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kühnen P, et al. . Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. N Engl J Med. 2016;375(3):240–246. doi: 10.1056/NEJMoa1512693 [DOI] [PubMed] [Google Scholar]
- 113. Doering SR, et al. . Melanocortin antagonist tetrapeptides with minimal agonist activity at the mouse melanocortin-3 receptor. ACS Med Chem Lett. 2015;6(2):123–127. doi: 10.1021/ml500340z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Bassi M, et al. . Activation of the brain melanocortin system is required for leptin-induced modulation of chemorespiratory function. Acta Physiol (Oxf). 2015;213(4):893–901. doi: 10.1111/apha.12394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Fleury Curado T, et al. . Designer receptors exclusively activated by designer drugs approach to treatment of sleep-disordered breathing. Am J Respir Crit Care Med. 2021;203(1):102–110. doi: 10.1164/rccm.202002-0321OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Freire C, et al. . Intranasal Leptin Prevents Opioid-induced Sleep-disordered Breathing in Obese Mice. Am J Respir Cell Mol Biol. 2020;63(4):502–509. doi: 10.1165/rcmb.2020-0117OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kumar P, et al. . Peripheral chemoreceptors: function and plasticity of the carotid body. Compr Physiol. 2012;2(1):141–219. doi: 10.1002/cphy.c100069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Shin MK, et al. . Leptin induces hypertension acting on transient receptor potential melastatin 7 channel in the carotid body. Circ Res. 2019;125(11):989–1002. doi: 10.1161/CIRCRESAHA.119.315338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wang W, et al. . Orexin: a potential role in the process of obstructive sleep apnea. Peptides. 2013;42:48–54. doi: 10.1016/j.peptides.2013.01.001 [DOI] [PubMed] [Google Scholar]
- 120. Nattie E, et al. . Respiration and autonomic regulation and orexin. Prog Brain Res. 2012;198:25–46. doi: 10.1016/B978-0-444-59489-1.00004-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Nakamura A, et al. . Vigilance state-dependent attenuation of hypercapnic chemoreflex and exaggerated sleep apnea in orexin knockout mice. J Appl Physiol (1985). 2007;102(1):241–248. doi: 10.1152/japplphysiol.00679.2006 [DOI] [PubMed] [Google Scholar]
- 122. Tarasiuk A, et al. . Role of orexin in respiratory and sleep homeostasis during upper airway obstruction in rats. Sleep. 2014;37(5):987–998. doi: 10.5665/sleep.3676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Jain V, et al. . Intranasal oxytocin increases respiratory rate and reduces obstructive event duration and oxygen desaturation in obstructive sleep apnea patients: a randomized double blinded placebo controlled study. Sleep Med. 2020;74:242–247. doi: 10.1016/j.sleep.2020.05.034 [DOI] [PubMed] [Google Scholar]
- 124. Tarasiuk A, et al. . Role of growth hormone-releasing hormone in sleep and growth impairments induced by upper airway obstruction in rats. Eur Respir J. 2011;38(4):870–877. doi: 10.1183/09031936.00197610 [DOI] [PubMed] [Google Scholar]
- 125. Klok MD, et al. . The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev. 2007;8(1):21–34. doi: 10.1111/j.1467-789X.2006.00270.x [DOI] [PubMed] [Google Scholar]
- 126. Sánchez-de-la-Torre M, et al. . The influence of obesity and obstructive sleep apnea on metabolic hormones. Sleep Breath. 2012;16(3):649–656. doi: 10.1007/s11325-011-0552-7 [DOI] [PubMed] [Google Scholar]
- 127. Schwenke DO, et al. . Exogenous ghrelin accentuates the acute hypoxic ventilatory response after two weeks of chronic hypoxia in conscious rats. Acta Physiol (Oxf). 2010;200(3):279–287. doi: 10.1111/j.1748-1716.2010.02142.x [DOI] [PubMed] [Google Scholar]
- 128. Liu WY, et al. . Differential activating effects of thyrotropin-releasing hormone and its analog taltirelin on motor output to the tongue musculature in vivo. Sleep. 2020;43(9). doi: 10.1093/sleep/zsaa053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Mosbah H, et al. . Therapeutic indications and metabolic effects of metreleptin in patients with lipodystrophy syndromes: real-life experience from a national reference network. Diabetes Obes Metab. 2022;24(8):1565–1577. doi: 10.1111/dom.14726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Knappe D, et al. . Drug development-targeted screening of leptin agonist glycopeptides. Int J Pept Res Ther. 2008;14(3):247–254. doi: 10.1007/s10989-008-9139-y [DOI] [Google Scholar]
- 131. Kovalszky I, et al. . Leptin-based glycopeptide induces weight loss and simultaneously restores fertility in animal models. Diabetes Obes Metab. 2010;12(5):393–402. doi: 10.1111/j.1463-1326.2009.01170.x [DOI] [PubMed] [Google Scholar]