Abstract
The dinB gene of Escherichia coli is known to be involved in the untargeted mutagenesis of λ phage. Recently, we have demonstrated that this damage-inducible and SOS-controlled gene encodes a novel DNA polymerase, DNA Pol IV, which is able to dramatically increase the untargeted mutagenesis of F′ plasmid. At the amino acid level, DNA Pol IV shares sequence homologies with E. coli UmuC (DNA Pol V), Rev1p, and Rad30p (DNA polymerase η) of Saccharomyces cerevisiae and human Rad30A (XPV) proteins, all of which are involved in translesion DNA synthesis. To better characterize the Pol IV-dependent untargeted mutagenesis, i.e., the DNA Pol IV mutator activity, we analyzed the genetic requirements of this activity and determined the forward mutation spectrum generated by this protein within the cII gene of λ phage. The results indicated that the DNA Pol IV mutator activity is independent of polA, polB, recA, umuDC, uvrA, and mutS functions. The analysis of more than 300 independent mutations obtained in the wild-type or mutS background revealed that the mutator activity clearly promotes single-nucleotide substitutions as well as one-base deletions in the ratio of about 1:2. The base changes were strikingly biased for substitutions toward G:C base pairs, and about 70% of them occurred in 5′-GX-3′ sequences, where X represents the base (T, A, or C) that is mutated to G. These results are discussed with respect to the recently described biochemical characteristics of DNA Pol IV.
Stability of genetic information is a key element in the maintenance of proper cell biology and the perpetuation of species. On the other hand, evolution obviously proceeds with the help of mutations. It is thus of great interest to understand the mechanisms underlying DNA replication fidelity and its modulation. In both eukaryotic and prokaryotic organisms, DNA replication is a highly accurate process, allowing only one error among 109 to 1010 incorporation events (8). However, this low error frequency of DNA replication may be enhanced either upon modification of the substrate molecule by endogenous or exogenous DNA-damaging agents, i.e., targeted mutagenesis, or through modulation of the replication fidelity in the absence of any DNA damage, i.e., untargeted mutagenesis.
In the bacterium Escherichia coli, the induction of the so-called SOS system allows the organism to cope with adverse conditions in various ways. One of the consequences of its activation is an increase in both targeted and untargeted mutagenesis (for a review, see reference 12). SOS-dependent targeted mutagenesis in E. coli relies on an increase in the frequency of DNA synthesis past DNA lesions, i.e., translesion synthesis. Although their precise mechanisms are not yet established, two distinct translesion synthesis pathways have been described to date. One relies on the activity, together with the replicative polymerase III, of the UmuDC and RecA proteins (12), whereas the other occurs independently of these accessory proteins but requires another, yet unidentified, SOS function termed Npf (17, 34, 43).
It has also been demonstrated that activation of the SOS response results in the increase of untargeted mutagenesis through the accumulation of mutations during replication of DNA that has not been exposed to any exogenous DNA-damaging agent (61). Here again, two distinct pathways can be distinguished on the basis of their specific genetic requirements. One is the so-called SOS mutator activity observed on chromosomal or episomal DNA in constitutively SOS-activated cells. This pathway requires functional recA and umuDC genes (3, 60). Strong evidence supports the notion that this mutator activity results from a transient decrease in the replication fidelity of damage-free DNA (10). The other pathway was first observed when mutations in undamaged λ bacteriophages grown in UV-preirradiated E. coli cells were measured (4, 15, 32, 62). This mutagenesis, which is called λ untargeted mutagenesis (λ UTM), has been shown to occur independently of the umuDC function, instead requiring functional uvrABC, polA, and dinB genes (2, 7, 33, 62). As for the SOS mutator activity, some evidence supports the notion that λ UTM results from a transient decrease in the replication fidelity of damage-free DNA (32).
More recently, Kim et al. (24) have shown that the expression of DinB from a low-copy-number plasmid (pYG782) is sufficient to dramatically increase the untargeted mutagenesis on F′ plasmids in E. coli. This effect, subsequently termed the DinB mutator activity, has been shown to rely on the recently discovered DNA polymerase activity of DinB (DNA Pol IV [57]). Pol IV mediates template-directed DNA replication and lacks a 3′-to-5′ exonuclease (proofreading) activity, and its replication mode is strictly distributive. In addition, it is prone to elongate bulged (misaligned) primer/template structures in vitro. Interestingly, at the amino acid level, DNA Pol IV shares homologies with UmuC of E. coli (also known as DNA Pol V [56]), Rev1p of Saccharomyces cerevisiae (30, 44), and DNA polymerase η of S. cerevisiae (Rad30p) and humans (also known as hRad30A or XP-V [18–20, 35–37, 51]), all of which are endowed with a DNA polymerase or a nucleotidyltransferase activity and involved in translesion DNA synthesis (11). It appears that human cells possess at least four DinB-related proteins, i.e., DNA polymerase η (hRad30A or XP-V), hRad30B, hRev1, and hDinB (14, 18, 21, 31, 36, 38, 45a).
To better characterize the DinB mutator activity in vivo, we wished to analyze the genetic requirements and mutational specificity of the DNA Pol IV mutator activity. Concerning genetic requirements, we show here that the Pol IV mutator activity acts independently of the umuDC, recA, polA, polB, and uvrA functions. Moreover, Pol IV-induced errors are correctable by the mismatch repair machinery, suggesting that they most probably represent true replication errors arising upon replication of damage-free DNA. The mutational specificity of the Pol IV mutator activity was determined by analyzing 323 cII mutants recovered from either wild-type or mismatch repair-deficient E. coli strains transformed with a low-copy-number plasmid expressing dinB (pYG782) or the control vector (pWKS30). As previously observed in another system (24), the expression of dinB greatly enhances −1 frameshift mutagenesis in this forward mutation assay. However, it also strongly promotes single nucleotide substitutions with an obvious specificity for substitutions toward G:C base pairs. Altogether, these results are discussed in terms of possible mechanisms by which DNA Pol IV mediates untargeted mutagenesis.
MATERIALS AND METHODS
Media, bacterial strains, and plasmids.
L broth (1% Bacto Tryptone, 0.5% yeast extract, 1% NaCl [pH 7.4]) was used throughout this study. L agar contained 1.5% agar in L broth. Top agar consisted of L broth plus 0.6% agar. If necessary, ampicillin (50 μg ml−1), tetracycline (15 μg ml−1), kanamycin (20 μg ml−1), chloramphenicol (20 μg ml−1), or rifampin (100 μg ml−1) was added. All bacterial strains and plasmids used are listed in Table 1 (see the indicated references for detailed genotypes). P1vir was used for general transduction (41). Plasmid pWKS30, a pSC101 derivative containing the multiple cloning site of pBluescript II SK (59), was used for the construction of pYG782, the Pol IV-expressing vector used throughout this study. In this construction, the dinB gene is transcribed from the vector Plac promoter (24). pMQ339 is a pACYC184 derivative containing the mutL gene (64).
TABLE 1.
Bacterial strains and plasmids used
| Strain or plasmid | Relevant genotype | Source, construction, or reference |
|---|---|---|
| Strainsa | ||
| AB1157 | Laboratory stock | |
| YG2243 | Δ(recA-srlR)306::Tn10 | P1(GW6752) (45) × AB1157 |
| YG2244 | ΔumuDC595::cat | P1(RW120) (63) × AB1157 |
| YG2246 | uvrA277::Tn10 | P1(CGSC no. 6661b) × AB1157 |
| YG2237 | mutS::Tn10 | P1(BMH 71-18 mutSc) × AB1157 |
| YG2239 | mutL::Tn10 | P1(GW3733) (46) × AB1157 |
| YG2238 | polA | P1(HRS7052d) × AB1157 |
| YG2241 | ΔpolB::kan | P1(HRS6700d) × AB1157 |
| G1217 | 16 | |
| G1225 | hflA::Tn5 hflB29::Tn10 | 16 |
| Plasmids | ||
| pWKS30 | 24 | |
| pYG782 | dinB | 24 |
| pMQ339 | mutL | 64 |
Constructed by transduction with P1 phage grown in the strain indicated in parentheses and by selecting for the appropriate antibiotic resistance marker.
Obtained from the E. coli Genetic Stock Center, Yale University.
Purchased from TaKaRa Biomedicals (Japan).
Kindly provided by Hiroshi Iwasaki (Osaka University, Japan). The polA mutation of strain HRS7052 was derived from 5′ Exo on F′ plasmid described by Joyce and Grindley (22). The strain should have 5′-3′ exonuclease activity of Pol I but lack 3′-5′ exonuclease and polymerase activities.
Mutation assays.
To measure the frequency of appearance of rifampin-resistant (Rifr) colonies, at least two fresh transformants were independently resuspended into 3 ml of liquid L broth medium and grown overnight at 37°C with shaking. Aliquots of appropriate dilutions of these saturated cultures were then plated on agar L broth plates containing ampicillin to determine the total cell count and on plates containing rifampin (100 μg ml−1) to determine the frequency of Rifr mutant colonies. λ cII mutation frequencies were determined as follow. Overnight cultures of AB1157 derivative cells harboring the empty vector pWKS30 or the Pol IV expression vector pYG782 were concentrated by a factor of 2 in 10 mM MgSO4. These host strains were then infected with 106 PFU of untreated λ phage (λ cIts857). After 15 min of incubation at room temperature, 2 ml of L broth was added to these mixtures, and the cells were grown at 37°C with agitation until lysis occurred. After clearing the lysate by the addition of a few drops of chloroform and additional incubation at 37°C for 15 min, aliquots were centrifuged (7 min, 12,000 rpm), and 500 μl of the supernatant was saved in a fresh Eppendorf tube containing 25 μl of chloroform. Ten-microliter aliquots of the appropriate dilutions of these primary lysates were used to infect G1217 (the hfl+ nonselecting strain) and G1225 (the hfl mutant selecting strain) in order to determine the titer of the lysate and the λ cII mutation frequency, respectively, as described by Jakubczak et al. (16). Plaques were counted after 36 to 48 h of incubation at 25°C. G1217 and G1225 strains were purchased from Epicentre Technologies as part of the MutaPlax cIISelect kit.
Mutational spectrum determination.
λ cII mutants were selected as described above, using G1225 (hfl) and independent primary lysates resulting from infection of either AB1157 or YG2237, harboring either pWKS30 or pYG782. One plaque per independent lysate was then toothpicked and resuspended into 20 μl of 1× Pfu PCR buffer and boiled for 10 min in the PCR apparatus. The PCR mixture was then completed, and 25 cycles (15 s at 94°C, 25 s at 53°C, and 40 s at 72°C) were performed. The final composition of the PCR mixture was 1× Pfu buffer with MgSO4, 15 pmol of each primer, 200 μM each deoxynucleoside triphosphate (TaKaRa, Shiga, Japan), and 1.25 U of cloned Pfu polymerase (Stratagene, La Jolla, Calif.). The PCR products were directly sequenced using a SequiTherm Long-Read cycle sequencing kit (Epicentre Technologies) and an ALF Red automatic sequencer (Pharmacia). The primer sequences used were the same as described by Jakubczak et al. (16) and were purchased from Pharmacia with a nonlabeled or Cy5-end-labeled oligonucleotides for sequencing purposes.
RESULTS
Experimental system.
Originally, the λ UTM phenomenon was described and analyzed through the measurement of λ clear-plaque mutation frequency of undamaged bacteriophage particles grown in UV-preirradiated E. coli cells (4). Recently, Kim et al. (24) have shown that UV preirradiation of the recipient cells is not needed if DNA Pol IV is expressed from the low-copy-number plasmid pYG782. In the present study, we investigated the genetic requirements and mutational specificity of the Pol IV mutator activity by the use of two forward mutational assays, i.e., the λ cII gene inactivation assay (cII assay [16]) and the rifampin resistance assay. The latter employs the rpoB gene encoding the β subunit of RNA polymerase in the chromosome of E. coli as a reporter gene for mutation and scores exclusively base substitution mutations (52). All mutagenesis experiments were carried out with cells harboring plasmid pYG782 expressing dinB or its corresponding empty vector pWKS30 as a control. No DNA-damaging treatment was applied to either the bacteria or the λ particles.
Genetic requirements of the Pol IV mutator activity.
In the wild-type strain, the introduction of plasmid pYG782 carrying the dinB gene induces a dramatic increase in both cII and Rifr mutation frequencies (24- and 87-fold, respectively [Table 2]). Although some variations in the amplitude of this increase are observed, we show here that this mutator activity is independent of all gene functions tested (Table 2). The results showing the independence of the Pol IV mutator activity upon the recA, umuDC, and uvrA genes is consistent with the results obtained previously but using a target gene located on an F′ plasmid (24). Results in Table 2 also demonstrate the independence of this activity on polA and polB gene functions.
TABLE 2.
Genetic requirements of the DNA Pol IV mutator activitya
| Strain (relevant genotype) |
cII mutation frequency (10−5)
|
Ratioa | Rif. mutation frequency (10−8)
|
Ratio | ||
|---|---|---|---|---|---|---|
| pWKS30 | pYG782 | pWKS30 | pYG782 | |||
| AB1157 (wild type) | 5 | 121 | 24 | 5 | 436 | 87 |
| YG2238 (polA) | 3 | 77 | 26 | 4 | 249 | 62 |
| YG2241 (ΔpolB) | 4 | 133 | 33 | 3 | 446 | 149 |
| YG2223 (ΔrecA) | 6 | 532 | 89 | 2 | 217 | 108 |
| YG2228 (ΔumuDC) | 4 | 73 | 18 | 2 | 313 | 156 |
| YG2226 (uvrA) | 4 | 46 | 11 | 5 | 175 | 35 |
| YG2237 (mutS) | 14 | 250 | 18 | 362 | 1,714 | 5 |
Mutation frequencies were measured in the indicated genetic backgrounds as described in Materials and Methods. Each value represents the average of at least two independent experiments.
Ratio of mutation frequency measured in strain harboring plasmid pYG782 to that in the isogenic strain transformed with control plasmid pWKS30.
It is known that λ DNA is poorly subjected to mismatch repair (mediated by the MutHLS proteins), most probably due to the undermethylation of its DNA which results from its rapid lytic life cycle (3, 4, 50). This is exemplified here by the low, i.e., threefold (14/5), increase in spontaneous cII mutation frequency observed in a mismatch repair-deficient strain (Table 2, compare wild-type and mutS strains in pWKS30 column), whereas in the same conditions, the Rifr mutation frequency increased more than 70-fold. The introduction of plasmid pYG782 in the mutS strain leads to an additional 18-fold increase in cII mutation frequency, clearly ruling out the possibility that the Pol IV mutator activity observed here proceeds through direct inactivation of the mismatch repair pathway. Intriguingly, the Pol IV mutator effect, expressed as the ratio of mutation frequencies indicated in Table 2, is more pronounced in the Rifr mutation assay than in the cII assay, except in the case of the mismatch repair-deficient background (35- to 156-fold increase in the Rifr assay versus 11- to 89-fold increase in the cII assay). It is thus possible that the apparent greater mutator effect on the rpoB gene is partly due to the suppression of mismatch repair functions. This suppression might result from sequestration of the mismatch repair proteins bound to mismatches, including one-base frameshift intermediates, generated by DNA Pol IV all over the genome. In fact, the Pol IV mutator effect on the rpoB gene was increased only fivefold (1,714/362) in the mutS background. This is probably because the rifampin mutation assay scores only base changes, and the most frequently observed mutations associated with Pol IV expression are frameshifts (reference 24 and this study).
Nature of the mutations induced by DNA Pol IV.
Different lines of evidence have suggested that the λ UTM phenomenon results from replication of nondamaged DNA (4). More recently, participation of DinB (Pol IV) in UV mutagenesis has also been investigated (24), and the results were negative. Here, using the Rifr assay, we addressed the nature of the mutations promoted by Pol IV by analyzing the interactions between the mismatch repair pathway and Pol IV mutator activity. As depicted in Table 3, expression of the MutL protein through the introduction of plasmid pMQ339 in the cell efficiently minimized the Pol IV mutator effect in both wild-type and mutL backgrounds. On the other hand, providing additional MutL protein to a mutS strain did not affect Pol IV mutator activity, indicating that the pMQ339 effect observed in the wild-type and mutL strains indeed results from the enhancement of the mismatch repair capacities of the cell but not from some side effect of the MutL expression from pMQ339. In addition, the Pol IV mutator activity was not enhanced in a strain defective in the general nucleotide excision repair pathway mediated by the UvrABC proteins (Table 2, uvrA strain). Taken together, these findings suggest that a vast majority of the mutations caused by Pol IV are not due to the processing of cryptic DNA lesions but instead represent an amplification of true DNA replication errors.
TABLE 3.
Interactions between mismatch repair functions and DNA Pol IV mutator activity
| Strain (relevant genotype)a | pMQ339b | Rifr/108 cells (SD)c |
|---|---|---|
| AB1157 | − | 622 (53) |
| AB1157 | + | 39 (7) |
| YG2239 (mutL) | + | 40 (10) |
| YG2237 (mutS) | + | 1,692 (218) |
All harbor plasmid pYG782.
Bears the sequence coding for the MutL protein (64).
Mutation frequencies were measured as described in Materials and Methods. Data are averages of two independent experiments.
Mutational specificity of the DNA Pol IV mutator activity.
Using the positive selection system described by Jakubczak et al. (16) adapted to a bacterial study, we determined the sequence of a total of 323 independent mutants within the 294-bp-long cII gene of λ phage. Briefly, E. coli host strains transformed with plasmid pYG782 or the corresponding empty vector are infected with intact λ phage particles and grown until complete lysis has occurred (primary lysates). The resulting lysates are then used to infect the E. coli indicator strain that carries the hflA and hflB mutations. These infection mixtures are plated onto LB-agar plates and incubated at 25°C for 36 to 48 h. Under such conditions, only cII mutant phages are able to enter the lytic cycle and consequently form plaques on the hfl lawn. To ensure independence of such mutants, a single plaque per primary lysate is then used to amplify the entire cII gene by PCR. These PCR products are then directly sequenced. To our knowledge, this is the first extensive mutational spectrum study of untargeted mutagenesis using the cII gene as a target.
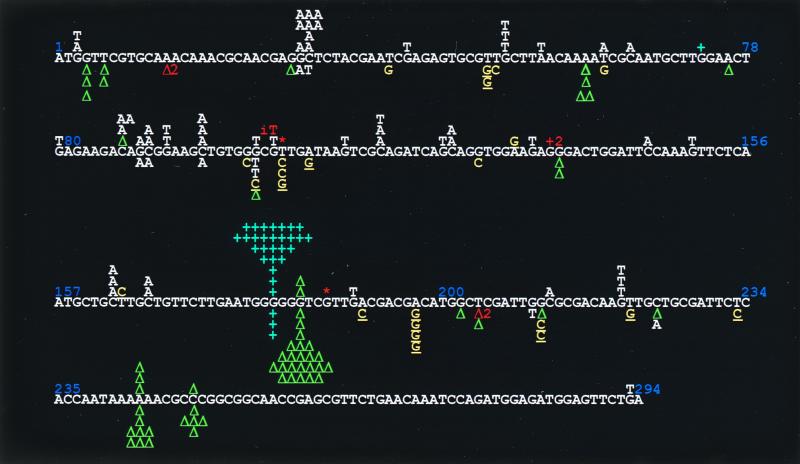
We first analyzed the spectrum of 93 spontaneous mutations isolated from the wild-type strain AB1157 transformed with the empty vector plasmid pWKS30 (Fig. 1). The spectrum is composed of 58% base substitutions and 40% frameshifts mutations. One deletion of 84 bp was also observed between short direct repeats. Among base substitutions, transversions largely dominate, and almost all (93%) are G:C to T:A mutations. These mutations may eventually result from the replication of some endogenously generated DNA lesion such as 8-oxo-2′-deoxyguanosine, an abundant oxidative lesion known to efficiently induce such mutations (5, 58). Frameshift mutations are also frequently observed in runs (two or more identical base pairs), with 32 out of 34 frameshifts (94%) occurring in two distinct runs of six identical bp, 84% (27/32) of these being +1 G:C observed within the six-G:C run at positions 179 to 184.
FIG. 1.
cII mutational spectra determined in E. coli AB1157 (wild-type) strain. Mutants recovered in AB1157 transformed with the pWKS30 or pYG782 vector are indicated above or below, respectively, the cII coding sequence. Base substitutions to G:C and A:T are represented in yellow and white, respectively. The G:C-directed substitutions in the sequence of 5′-GX-3′, where X represents the base that is changed to G, is underlined. Blue +, +1 frameshift mutation; green triangle, −1 frameshift mutation. Other mutations are colored in orange: −2 deletion at positions 16 to 18; T insertion (iT) between positions 101 and 102; 84-bp deletion between the GTT direct repeat marked by *; +2 addition at positions 134 and 135 and -CT or -TC deletion at positions 203 to 205. Position 200 is T of a TGG sequence.
Eighty-nine mutations have been determined in the same strain but harboring plasmid pYG782 (Fig. 1). The spectrum is now composed of 34% base substitutions and 66% frameshifts mutations. The 24-fold increase in the cII mutation frequency resulting from Pol IV expression is accompanied with a drastic modification in the quality and distribution of the mutations. Here, transitions and transversions are almost equally represented (13 and 20%, respectively, of total mutations), and G:C to T:A transversions no longer dominate (Table 4). Rather, substitutions with G:C represent 70% of all base substitutions. More strikingly, frameshift mutations are now vastly dominated by 1-bp deletion events that account for 58% of the total mutations and for 95% of the frameshift mutations observed in the runs. This feature is especially well exemplified by the six-G:C hot spot at positions 179 to 184. These clear modifications in the mutation spectrum undoubtedly reveal specificities of the Pol IV mutator effect.
TABLE 4.
Specificity of DNA Pol IV mutator activity
| Change | Mutation frequency (10−5)a
|
|||||
|---|---|---|---|---|---|---|
| AB1157/pWKS30 | AB1157/pYG782 | Ratiob | YG2237/pWKS30 | YG2237/pYG782 | Ratiob | |
| Base substitutions | ||||||
| G:C to T:A | 2.6 (47) | 8.2 (6) | 3.1 | 1.6 (8) | 3.7 (1) | 2.3 |
| G:C to A:T | 0.2 (4) | 4.1 (3) | 20 | 2.4 (12) | 7.3 (2) | 3 |
| A:T to T:A | 0.1 (2) | <12c | ||||
| A:T to G:C | 0.1 (2) | 12.3 (9) | 123 | 1.6 (8) | 44 (12) | 27.5 |
| A:T to C:G | 9.5 (7) | >170c | 29.4 (8) | >147c | ||
| G:C to C:G | 6.85 (5) | >121c | 3.7 (1) | >18c | ||
| Frameshifts | ||||||
| +1 in runs | 1.6 (28) | 4.1 (3) | 2.6 | 6.9 (35) | 22 (6) | 3.2 |
| −1 in runs | 0.3 (6) | 71 (52) | 237 | 1.8 (9) | 136 (37) | 85 |
| −1 in nonruns | 0.06 (1) | 2.7 (2) | 45 | 0.2 (1) | 3.7 (1) | 18.5 |
| Othersd | 0.1 (2) | 2.7 (2) | 27 | (0) | (0) | |
| Total | 5 (92) | 121 (89) | 14 (73) | 250 (68) | ||
Calculated using the corresponding cII mutation frequency presented in Table 1 and the relative frequency of each specific mutation recovered in the related spectrum. AB1157, wild-type strain; YG2237, mutS strain; pWKS30, empty vector; pYG782, dinB-carrying vector. Numbers of observed events are given in parentheses.
Ratio of mutation frequency in the strain harboring pYG782 to that in the strain harboring pWKS30.
Default value calculated on the basis of one observed event.
Insertion, 2-bp deletion, or addition.
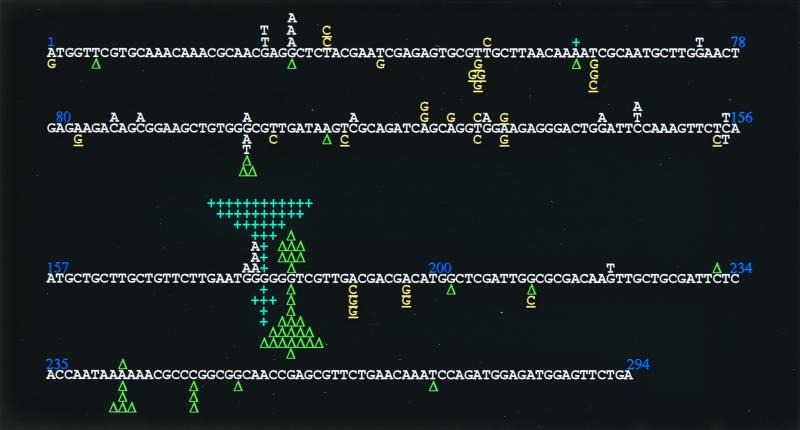
Although mismatch repair has only a small effect on the cII mutation frequencies induced by Pol IV (Table 2), we carried out the same analysis in a mismatch repair-deficient background (Fig. 2). Since the Pol IV-induced mutations most probably result from true replication errors (see above), analyzing the mutational spectrum in a mismatch repair-deficient background allows one to directly assess the specificity of these induced mutational events. In the mutS background (YG2237 strain), 39% of the mutations harboring the vector plasmid pWKS30 were base substitutions and 61% were frameshifts in runs. Among base substitutions, transitions are now dominant (71%), as expected from the specificity of the mismatch repair pathway (for a review see reference 12). Frameshift mutations were almost exclusively observed at the six-G:C hot spot, at which site 80% of them were a single-base-pair addition event. When plasmid pYG782 is introduced in strain YG2237, base substitutions are almost equally distributed between transitions and transversions (20 and 15%, respectively), and −1 deletion events now dominate the frameshift part of the spectrum (86%) as well as the whole spectrum (56%).
FIG. 2.
cII mutational spectra determined in E. coli YG2237 (mutS). Mutants recovered in YG2237 strain transformed with pWKS30 or pYG782 are indicated above or below, respectively, the cII coding sequence. Base substitutions to G:C and A:T represented in yellow and white, respectively. The G:C-directed substitutions in the sequence 5′-GX-3′, where X represents the base that is changed to G, is underlined. Blue +, +1 frameshift mutation; green triangle, −1 frameshift mutation. Position 200 is T of a TGG sequence.
To further analyze these two sets of data, we calculated the mutation frequencies of each type of mutation in the different genetic backgrounds. The mutation frequencies presented in Table 4 were obtained by multiplying the relative frequency of each specific event in one spectrum by the corresponding cII mutation frequency determined previously (Table 2). Finally, the Pol IV-induced enhancement factor for each mutation was calculated by dividing the value obtained in the strain harboring plasmid pYG782 by that obtained in the strain harboring the control vector (Table 4). It appears that although none of the mutation frequencies seems to be decreased by the presence of plasmid pYG782, Pol IV strongly enhances specific types of mutations. Among base substitutions, A:T to G:C transitions, A:T to C:G transversions, and G:C to C:G transversions were preferentially promoted in both backgrounds (Table 4). Although only one G:C-to-C:G event was recovered in the mutS background, five of them were observed in the wild-type background plasmid transformed with pYG782, and none were recovered from strains harboring the control plasmid. It thus clearly appears that Pol IV expression specifically enhances the A:T-to-G:C, A:T-to-C:G, and C:G-to-G:C changes. In other words, Pol IV promotes base substitutions toward G:C base pairs. Since both A:T and G:C are affected, this preference does not seem to rely on the nature of the original template base, as would be expected for a specific DNA lesion-induced mechanism. Rather, this bias may reflect a mechanistical specificity of the Pol IV-mediated base substitution mutagenesis (see Discussion).
Concerning the frameshift mutagenesis, it is extremely clear that Pol IV exclusively promotes 1-bp deletion events (Table 4). This confirms previous findings that either in the λ UTM assay (62) or in the lacZ reversion assay of F′ plasmid (24), 1-bp deletions in runs of six or more identical base pairs are the predominant dinB-dependent mutations recovered. However, the present study reveals that Pol IV enhances frameshifts not only in long runs such as the six-G:C sequence (280- and 42-fold increases in wild-type and mutS backgrounds, respectively) but also in shorter runs such as three G:C runs (195- and >110-fold increases in wild-type and mutS backgrounds, respectively), two G:C runs (>136- and >73-fold increases), two A:T runs (>68- and >36-fold increases), and nonrun sequences (45- and 18.5-fold increases).
Sequence specificity of Pol IV-induced mutations.
In an attempt to gain further insight into the mechanism by which Pol IV promotes mutagenesis, we looked for an eventual sequence context specificity. As described above, the patterns of mutation induction by Pol IV were very similar in both wild-type and mismatch repair-deficient strains. For the purpose of this analysis, we combined the mutational events observed in both strains. When base substitutions are considered, it appears that 70% (14/20) of the Pol IV-mediated A:T-to-G:C transitions occurred within 5′-GA-3′ sequences where A is mutated to G (Fig. 1 and 2). If one looks for the same type of mutations in strains harboring the control plasmid, it appears that only 20% (2/10) occurred in such a sequence context. In the case of A:T-to-C:G transversions, 69% (11/16) occurred in 5′-GT-3′ sequences where T is mutated to G. Finally, 67% (4/6) of G:C-to-C:G transversions occurred in 5′-GC-3′ sequences where C is mutated to G. In summary, 69% (29/42) of the whole G:C-directed substitutions enhanced by Pol IV occurred within 5′-GX-3′ sequences, where X represents A, T, or C that is mutated to G.
Considering the −1 deletion events in short runs, 86% (12/14) of those observed in three C:G runs occurred within 5′-GCCC-3′ sequences, and 70% (7/10) of deletions in two C:G runs occurred within 5′-GCC-3′ sequences. Finally, 80% (4/5) of the frameshift mutations detected in two A:T runs occurred in 5′-GTT-3′ sequences. The three one-base deletion events in nonrun sequences observed in strains harboring plasmid pYG782 also occurred in 5′-GX-3′ sequences where X represents the deleted base. Altogether, these findings suggest a bias for mutations occurring in sequences with a guanine base at the 5′ position of the mutated bases. As discussed below, this may shed some light on the possible mechanisms by which DNA Pol IV promotes mutagenesis.
DISCUSSION
This study was conducted to further characterize the previously described DinB mutator activity which relies on the DNA polymerase activity of this protein (i.e., DNA Pol IV [57]). We show here that Pol IV does not require the functions provided by the umuDC, recA, polA, polB, or uvrA gene to promote untargeted mutagenesis (Table 2). The independence of the umuDC and recA functions is consistent with the known genetic requirements for the λ UTM (2, 32, 62) and the DinB-mediated untargeted mutagenesis observed in the lacZ gene on F′ plasmid (24). The independence of this mutagenic pathway of umuDC and recA distinguishes it genetically from the classical umuDC-dependent SOS mutator activity (3). On the other hand, Maenhaut-Michel and Caillet-Fauquet have shown that λ UTM is not observable in uvr strains or cells that are deficient in DNA polymerase I activity (33). In addition, UV irradiation of the host cells is required for λ UTM even if the SOS system is derepressed (3). In this study, however, the results clearly indicate that functional uvrA and polA genes are not necessary for the DNA Pol IV (DinB)-mediated untargeted mutagenesis (Table 2). Thus, we suggest that polymerase I activity and uvr functions play indirect rather than direct mechanistic roles in the λ UTM pathway. For example, the induction of dinB might be inefficient in the polA or uvr background, and some UV-inducible but not SOS-regulated gene, such as groEL (25), may be involved in the stabilization of either DNA Pol IV itself or a not fully characterized molecular partner of the polymerase (55).
The Pol IV mutator activity is observable on either λ phage or chromosomal DNA (the present work) and also on F′ episomal DNA (24). It thus appears that the Pol IV mutator activity is general and does not depend on the nature of the target DNA. We used the positive selection system provided by the inactivation of the λ cII gene functions to determine the specificity of the Pol IV mutator effect in a forward mutational assay (16). This selection system is very simple and appears to be quite effective since we noticed that of all the DNA of the selected plaques carried a mutation in the target gene. The ease of recovery of the target and of its sequencing also render this mutational system quite attractive. All classes of point mutations have been detected, and the recovered mutations are well distributed along the entire sequence, with perhaps the exception of the 70-nucleotide-long C-terminal part, where few base changes have been observed. Among a total of 137 base changes scored in this study, 70 represent different base substitutions, distributed over 61 different sites (in 42 out of the 98 codons). In addition, the six-G:C sequence at positions 179 to 184 clearly represent a mutational hot spot which may be useful for frameshift mutagenesis studies.
We demonstrate here that the Pol IV-induced mutations are susceptible to mismatch repair as are the mutations induced during λ UTM (4), suggesting that Pol IV most probably acts at the replication fork (at least on hemimethylated DNA) and on undamaged DNA to promote true replication errors. As mentioned earlier, DNA Pol IV is a strictly distributive DNA polymerase (57). This feature implicates Pol IV in short DNA synthesis rather than in replication of the whole chromosome of E. coli or the whole λ phage DNA. It is suggested that DNA Pol III holoenzyme is synthesized poorly from terminal mispairs during chromosome replication (10, 42, 48, 49). In general, purine:purine mispairs are worse substrates than purine:pyrimidine or pyrimidine:pyrimidine mispairs for extension (23, 40). Hence, we speculate that DNA Pol IV has access to the replication fork where DNA Pol III holoenzyme stalls and dissociates after it has created poorly extendable terminal mismatches. Once DNA Pol IV has access to the primer/template DNA, it carries out short DNA synthesis. This synthesis may be highly mutagenic because Pol IV lacks proofreading activity and because of its purely distributive mode of replication, as discussed below. Following dissociation of Pol IV, reassociation of the replicative Pol III holoenzyme could take place downstream from the original dissociation site and resume processive synthesis. This model could be summarized as a DNA polymerase switch (6).
The characteristics of the Pol IV-induced mutational spectrum described in this study offer clues to the mechanisms by which DNA Pol IV mediates untargeted mutagenesis (Fig. 1 and 2; Table 4). For the production of frameshifts during DNA replication, two general models have been proposed: (i) the misincorporation plus realignment model (1, 29) and (ii) the Streisinger slippage model (53, 54). The former can occur favorably if the misincorporated base is complementary to the next, i.e., 5′, template base; the misalignment allowing further synthesis to proceed from a correctly paired 3′ terminus. This type of mechanism is generally associated with frameshifts occurring at nonreiterated sequences in specific sequence contexts and with poorly processive DNA polymerases (1, 27). In contrast, frameshifts thought to be generated through the Streisinger slippage model are associated with runs of identical bases, and their probability of occurrence increases with the length of the run (28, 54). Slippage errors in runs are generated during processive DNA replication (13).
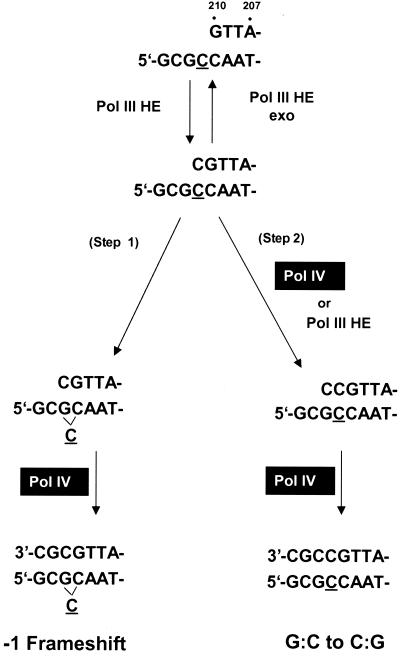
In this study, −1 frameshift mutations are the predominant mutations observed in the cII gene when DNA Pol IV is expressed (Table 4; Fig. 1 and 2). Among them, the six-G:C sequence at positions 179 to 184 is the most sensitive site for Pol IV mutator activity. Such hot spots in runs are generally considered an indication for the Streisinger slippage model. Thus, it seems that the frameshifts produced within the six-G:C hot spot result from direct slippage errors by DNA Pol IV. The slippage errors may be enhanced when Pol IV interacts with β subunit of Pol III holoenzyme (55). Besides run sequences, frameshift mutagenesis was efficiently promoted by Pol IV in short runs or even in nonrun sequences. In addition, the mutations in short runs or nonrun sequences occur predominantly in a specific sequence context, i.e., sequences harboring a 5′ G next to the mutated base. Thus, it seems possible that some of Pol IV-mediated frameshift mutagenesis occur by the misincorporation-realignment mechanism. Unlike processive DNA synthesis, only a single nucleotide is incorporated during each encounter of DNA Pol IV with the template/primer DNA (57). This feature may increase the realignment probability of the DNA strands, thereby promoting the formation of a 1-bp looped-out structure (Fig. 3, step 1). Our recent in vitro studies have highlighted the propensity of DNA Pol IV to extend, within a three-G:C context, a preexisting terminal mismatch through the formation of a 1-bp loop in the template strand (57).
FIG. 3.
Model for Pol IV-induced mutagenesis leading to a 1-bp deletion and single-nucleotide substitution. The primer strand of a replication intermediate is extended by DNA Pol III holoenzyme (Pol III HE). The model assumes that Pol III HE dissociates from DNA when it creates a 3′-terminal C:C mismatch. If the matched primer terminus having an extrahelical C is efficiently extended by Pol IV, a 1-bp deletion will be generated (step 1). DNA Pol IV may also catalyze the direct extension from the mismatched primer terminus (step 2), thereby generating a G:C-to-C:G transversion mutation. The DNA sequences are those at positions 208 to 215 of the cII gene of λ phage, where the two types of mutations are observed. The underlined base is position 211 of the cII gene of λ phage shown in Fig. 1 and 2.
Base substitutions account for about one-third of the total mutations induced by Pol IV. Intriguingly, upon expression of DNA Pol IV in the wild-type strain, base changes toward G:C (i.e., A:T to G:C, T:A to G:C, and C:G to G:C) are enhanced 121- to 170-fold (18- to 147-fold in the mutS strain [Table 4]) and largely outnumber other base substitutions. Assuming the aforementioned DNA polymerase switch model, this bias may be explained by a strong difficulty for Pol III holoenzyme to elongate particular terminal mismatches. This difficulty will result in an increased probability for Pol III to dissociate, thus giving a chance to the proofreading deficient Pol IV to elongate the mismatch (Fig. 3, step 2). We noticed that about 70% of these base substitutions occurred within a 5′-GX-3′ sequence context where X represents the mutated base. The 5′-proximal neighbor effect in the template strand is described for base replacements by other DNA polymerases (9, 26, 28, 29). Thus, the sequence context may suggest a particular ease for DNA Pol IV to extend a mismatched primer terminus by the incorporation of a C residue. Interestingly, the mutations observed at positions 210 and 211 of the cII gene are a 1-bp deletion, i.e., 5′-GC-3′ to 5′-G-3′, and a base change, i.e., 5′-GC-3′ to 5′-GG-3′, in both wild-type and mutS backgrounds. Competition may occur between the pathway leading to a 1-bp deletion (Fig. 3, step 1) and that leading to a base substitution (Fig. 3, step 2) at the same site. Nevertheless, 5′-GX-3′ sequence (the lower sequence in Fig. 3) might act as a primer strand, and the opposite strand might be the template. In that case, an A insertion opposite G at position 210 would allow ready slippage on T209, causing a −1 frameshift, and G misinsertion opposite G211 might yield a G:C-to-C:G transversion. It is reported that 5′ base in the primer strand (G212 in this case) affects the misinsertion rate (at position 211) through stacking interactions (39, 47). Thus, more focused mutational and biochemical studies are needed to elucidate the role of this sequence context.
In a previous paper, Kim et al. reported that base changes as well as frameshifts are induced by DinB (DNA Pol IV) expression in the lacZ gene on F′ plasmid (24). However, the extent of induction of these base changes observed in the F′ system is much lower than the extent of induction of base changes in the cII gene in the present study, although the amplitudes of frameshift induction in the run of six G:C are comparable between the lacZ and cII assays. Notably, the G:C-directed base substitutions are poorly detected in the lacZ reversion assay: the frequency of A:T to G:C, T:A to G:C, and C:G to G:C changes are enhanced 4, 17, and 4 times, respectively, by Pol IV expression. Since the lacZ assay is a reversion assay, only a specific type of mutation in a specific sequence context can revert the phenotype from LacZ− to LacZ+. E. coli CC101 detects T:A-to-G:C base changes in the sequence 5′-AATTAG-3′ where the underlined T is the target base. Similarly, strains CC103 and CC106 detect C:G to G:C and A:T to G:C within the sequences 5′-AATCAG-3′ and 5′-AATAAG-3′, respectively. None of these sequences include 5′-GX-3′, which is the sequence favored for base substitutions generated by DNA Pol IV. Thus, the G:C-directed base changes could be induced in the lacZ gene by Pol IV but are poorly detectable in the lacZ reversion assay because of the sequence context. In this respect, a forward mutation assay such as that using the cII gene in this study reflects more genuinely the mutation spectra generated by DNA Pol IV than the reversion assay.
This study represents an in vivo analysis of the mutational specificity of DNA Pol IV (DinB), which belongs to the ubiquitous family of the very recently discovered novel DNA polymerases involved in mutagenesis (reviewed in reference 11). The data obtained should help to direct future studies aimed at better characterizing the biochemical specificities of this novel DNA polymerase and the molecular basis of mutagenesis.
ACKNOWLEDGMENTS
We thank Genevieve Maenhaut-Michel for providing the λ cIts857 phage stock, Hiroshi Iwasaki for the gift of the HRS strains, and M. G. Marinus for plasmid pMQ339. We are grateful to Su-Ryang Kim for construction of the mutS and mutL strains.
This work was supported by grant-in-aids for Scientific Research on Priority Areas (08280104) from the Ministry of Education, Science, Sports and Culture of Japan and from the Human Frontier Science Program (RG0351/1998-M). J.W. had a postdoctoral position supported by a fellowship from the Science and Technology Agency (Japan).
REFERENCES
- 1.Bebenek K, Kunkel T A. Frameshift errors initiated by nucleotide misincorporation. Proc Natl Acad Sci USA. 1990;87:4946–4950. doi: 10.1073/pnas.87.13.4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brotcorne-Lannoye A, Maenhaut-Michel G. Role of RecA protein in untargeted UV mutagenesis of bacteriophage lambda: evidence for the requirement for the dinB gene. Proc Natl Acad Sci USA. 1986;83:3904–3908. doi: 10.1073/pnas.83.11.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caillet-Fauquet P, Maenhaut-Michel G. Nature of the SOS mutator activity: genetic characterization of untargeted mutagenesis in Escherichia coli. Mol Gen Genet. 1988;213:491–498. doi: 10.1007/BF00339621. [DOI] [PubMed] [Google Scholar]
- 4.Caillet F P, Maenhaut M G, Radman M. SOS mutator effect in E. coli mutants deficient in mismatch correction. EMBO J. 1984;3:707–712. doi: 10.1002/j.1460-2075.1984.tb01873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng K C, Cahill D S, Kasai H, Nishimura S, Loeb L A. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G→T and A→C substitutions. J Biol Chem. 1992;267:166–172. [PubMed] [Google Scholar]
- 6.Cordonnier A M, Fuchs R P P. Replication of damaged DNA: molecular defect in xeroderma pigmentosum variant cells. Mutat Res. 1999;435:111–119. doi: 10.1016/s0921-8777(99)00047-6. [DOI] [PubMed] [Google Scholar]
- 7.Devoret R, Coquerelle T. Multiplication du phage chez des bacteries depourvues d'activite enzymatique de reparation des lesions produites par l'ultraviolet. Bull Soc Chim Biol (Paris) 1965;47:1726–1728. [PubMed] [Google Scholar]
- 8.Drake J W, Charlesworth B, Charlesworth D, Crow J F. Rates of spontaneous mutation. Genetics. 1998;148:1667–1686. doi: 10.1093/genetics/148.4.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Efrati E, Tocco G, Eritja R, Wilson S H, Goodman M F. Abasic translesion synthesis by DNA polymerase beta violates the “A-rule.” Novel types of nucleotide incorporation by human DNA polymerase beta at an abasic lesion in different sequence contexts. J Biol Chem. 1997;272:2559–2569. doi: 10.1074/jbc.272.4.2559. [DOI] [PubMed] [Google Scholar]
- 10.Fijalkowska I J, Dunn R L, Schaaper R M. Genetic requirements and mutational specificity of the Escherichia coli SOS mutator activity. J Bacteriol. 1997;179:7435–7445. doi: 10.1128/jb.179.23.7435-7445.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedberg E C, Gerlach V L. Novel DNA polymerases offer clues to the molecular basis of mutagenesis. Cell. 1999;98:413–416. doi: 10.1016/s0092-8674(00)81970-4. [DOI] [PubMed] [Google Scholar]
- 12.Friedberg E C, Walker G C, Siede W. DNA repair and mutagenesis. Washington, D.C.: ASM Press; 1995. [Google Scholar]
- 13.Fujii S, Akiyama M, Aoki K, Sugaya Y, Higuchi K, Hiraoka M, Miki Y, Saitoh N, Yoshiyama K, Ihara K, Seki M, Ohtsubo E, Maki H. DNA replication errors produced by the replicative apparatus of Escherichia coli. J Mol Biol. 1999;289:835–850. doi: 10.1006/jmbi.1999.2802. [DOI] [PubMed] [Google Scholar]
- 14.Gerlach V L, Aravind L, Gotway G, Schultz R A, Koonin E V, Friedberg E C. Human and mouse homologs of Escherichia coli DinB (DNA polymerase IV), members of the UmuC/DinB superfamily. Proc Natl Acad Sci USA. 1999;96:11922–11927. doi: 10.1073/pnas.96.21.11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ichikawa-Ryo H, Kondo S. Indirect mutagenesis in phage lambda by ultraviolet preirradiation of host bacteria. J Mol Biol. 1975;97:77–92. doi: 10.1016/s0022-2836(75)80023-4. [DOI] [PubMed] [Google Scholar]
- 16.Jakubczak J L, Merlino G, French J E, Muller W J, Paul B, Adhya S, Garges S. Analysis of genetic instability during mammary tumor progression using a novel selection-based assay for in vivo mutations in a bacteriophage lambda transgene target. Proc Natl Acad Sci USA. 1996;93:9073–9078. doi: 10.1073/pnas.93.17.9073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janel-Bintz R, Maenhaut-Michel G, Fuchs R P P. MucAB but not UmuDC proteins enhance −2 frameshift mutagenesis induced by N-2-acetylaminofluorene at alternating GC sequences. Mol Gen Genet. 1994;245:279–285. doi: 10.1007/BF00290107. [DOI] [PubMed] [Google Scholar]
- 18.Johnson R E, Kondratick C M, Prakash S, Prakash L. hRAD30 mutations in the variant form of xeroderma pigmentosum. Science. 1999;285:263–265. doi: 10.1126/science.285.5425.263. [DOI] [PubMed] [Google Scholar]
- 19.Johnson R E, Prakash S, Prakash L. Efficient bypass of a thymine-thymine dimer by yeast DNA polymerase, Pol η. Science. 1999;283:1001–1004. doi: 10.1126/science.283.5404.1001. [DOI] [PubMed] [Google Scholar]
- 20.Johnson R E, Prakash S, Prakash L. Requirement of DNA polymerase activity of yeast Rad30 protein for its biological function. J Biol Chem. 1999;274:15975–15977. doi: 10.1074/jbc.274.23.15975. [DOI] [PubMed] [Google Scholar]
- 21.Johnson R E, Prakash S, Prakash L. The human DINB1 gene encodes the DNA polymerase θ. Proc Natl Acad Sci USA. 2000;97:3838–3843. doi: 10.1073/pnas.97.8.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joyce C M, Grindley N D. Method for determining whether a gene of Escherichia coli is essential: application to the polA gene. J Bacteriol. 1984;158:636–643. doi: 10.1128/jb.158.2.636-643.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joyce C M, Sun X C, Grindley N D. Reactions at the polymerase active site that contribute to the fidelity of Escherichia coli DNA polymerase I (Klenow fragment) J Biol Chem. 1992;267:24485–24500. [PubMed] [Google Scholar]
- 24.Kim S R, Maenhaut-Michel G, Yamada M, Yamamoto Y, Matsui K, Sofuni T, Nohmi T, Ohmori H. Multiple pathways for SOS-induced mutagenesis in Escherichia coli: an overexpression of dinB/dinP results in strongly enhancing mutagenesis in the absence of any exogenous treatment to damage DNA. Proc Natl Acad Sci USA. 1997;94:13792–13797. doi: 10.1073/pnas.94.25.13792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krueger J H, Walker G C. groEL and dnaK genes of Escherichia coli are induced by UV irradiation and nalidixic acid in an htpR+-dependent fashion. Proc Natl Acad Sci USA. 1984;81:1499–1503. doi: 10.1073/pnas.81.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunkel T A. The mutational specificity of DNA polymerase-beta during in vitro DNA synthesis. Production of frameshift, base substitution, and deletion mutations. J Biol Chem. 1985;260:5787–5796. [PubMed] [Google Scholar]
- 27.Kunkel T A. The mutational specificity of DNA polymerases-alpha and -gamma during in vitro DNA synthesis. J Biol Chem. 1985;260:12866–12874. [PubMed] [Google Scholar]
- 28.Kunkel T A. Misalignment-mediated DNA synthesis errors. Biochemistry. 1990;29:8003–8011. doi: 10.1021/bi00487a001. [DOI] [PubMed] [Google Scholar]
- 29.Kunkel T A, Soni A. Mutagenesis by transient misalignment. J Biol Chem. 1988;263:14784–14789. [PubMed] [Google Scholar]
- 30.Larimer F W, Perry J R, Hardigree A A. The REV1 gene of Saccharomyces cerevisiae: isolation, sequence, and functional analysis. J Bacteriol. 1989;171:230–237. doi: 10.1128/jb.171.1.230-237.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin W, Xin H, Zhang Y, Wu X, Yuan F, Wang Z. The human REV1 gene codes for a DNA template-dependent dCMP transferase. Nucleic Acids Res. 1999;27:4468–4475. doi: 10.1093/nar/27.22.4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maenhaut-Michel G, Caillet-Fauquet P. Effect of umuC mutations on targeted and untargeted ultraviolet mutagenesis in bacteriophage lambda. J Mol Biol. 1984;177:181–187. doi: 10.1016/0022-2836(84)90064-0. [DOI] [PubMed] [Google Scholar]
- 33.Maenhaut-Michel G, Caillet-Fauquet P. Genetic control of the UV-induced SOS mutator effect in single- and double-stranded DNA phages. Mutat Res. 1990;230:241–254. doi: 10.1016/0027-5107(90)90062-9. [DOI] [PubMed] [Google Scholar]
- 34.Maenhaut-Michel G, Janel B R, Fuchs R P P. A umuDC-independent SOS pathway for frameshift mutagenesis. Mol Gen Genet. 1992;235:373–380. doi: 10.1007/BF00279383. [DOI] [PubMed] [Google Scholar]
- 35.Masutani C, Araki M, Yamada A, Kusumoto R, Nogimori T, Maekawa T, Iwai S, Hanaoka F. Xeroderma pigmentosum variant (XP-V) correcting protein from HeLa cells has a thymine dimer bypass DNA polymerase activity. EMBO J. 1999;18:3491–3501. doi: 10.1093/emboj/18.12.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masutani C, Kusumoto R, Yamada A, Dohmae N, Yokoi M, Yuasa M, Araki M, Iwai S, Takio K, Hanaoka F. The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase η. Nature. 1999;399:700–704. doi: 10.1038/21447. [DOI] [PubMed] [Google Scholar]
- 37.McDonald J P, Levine A S, Woodgate R. The Saccharomyces cerevisiae RAD30 gene, a homologue of Escherichia coli dinB and umuC, is DNA damage inducible and functions in a novel error-free postreplication repair mechanism. Genetics. 1997;147:1557–1568. doi: 10.1093/genetics/147.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDonald J P, Rapic-Otrin V, Epstein J A, Broughton B C, Wang X, Lehmann A R, Wolgemuth D J, Woodgate R. Novel human and mouse homologs of Saccharomyces cerevisiae DNA polymerase eta. Genomics. 1999;60:20–30. doi: 10.1006/geno.1999.5906. [DOI] [PubMed] [Google Scholar]
- 39.Mendelman L V, Boosalis M S, Petruska J, Goodman M F. Nearest neighbor influences on DNA polymerase insertion fidelity. J Biol Chem. 1989;264:14415–14423. [PubMed] [Google Scholar]
- 40.Mendelman L V, Petruska J, Goodman M F. Base mispair extension kinetics. Comparison of DNA polymerase alpha and reverse transcriptase. J Biol Chem. 1990;265:2338–2346. [PubMed] [Google Scholar]
- 41.Miller J H. A short course in bacterial genetics. A laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 42.Mo J Y, Schaaper R M. Fidelity and error specificity of the α catalytic subunit of Escherichia coli DNA polymerase III. J Biol Chem. 1996;271:18947–18953. doi: 10.1074/jbc.271.31.18947. [DOI] [PubMed] [Google Scholar]
- 43.Napolitano R L, Lambert I B, Fuchs R P P. SOS factors involved in translesion synthesis. Proc Natl Acad Sci USA. 1997;94:5733–5738. doi: 10.1073/pnas.94.11.5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nelson J R, Lawrence C W, Hinkle D C. Deoxycytidyl transferase activity of yeast REV1 protein. Nature. 1996;382:729–731. doi: 10.1038/382729a0. [DOI] [PubMed] [Google Scholar]
- 45.Nohmi T, Battista J R, Dodson L A, Walker G C. RecA-mediated cleavage activates UmuD for mutagenesis: mechanistic relationship between transcriptional derepression and posttranslational activation. Proc Natl Acad Sci USA. 1988;85:1816–1820. doi: 10.1073/pnas.85.6.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45a.Ogi T, Kato T, Jr, Kato T, Ohmori H. Mutation enhancement by DINB1, a mammalian homologue of the Escherichia coli mutagenesis protein DinB. Genes Cells. 1999;4:607–618. doi: 10.1046/j.1365-2443.1999.00289.x. [DOI] [PubMed] [Google Scholar]
- 46.Pang P P, Lundberg A S, Walker G C. Identification and characterization of the mutL and mutS gene products of Salmonella typhimurium LT2. J Bacteriol. 1985;163:1007–1015. doi: 10.1128/jb.163.3.1007-1015.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petruska J, Goodman M F. Influence of neighboring bases on DNA polymerase insertion and proofreading fidelity. J Biol Chem. 1985;260:7533–7539. [PubMed] [Google Scholar]
- 48.Pham P T, Olson M W, McHenry C S, Schaaper R M. The base substitution and frameshift fidelity of Escherichia coli DNA polymerase III holoenzyme in vitro. J Biol Chem. 1998;273:23575–23584. doi: 10.1074/jbc.273.36.23575. [DOI] [PubMed] [Google Scholar]
- 49.Pham P T, Olson M W, McHenry C S, Schaaper R M. Mismatch extension by Escherichia coli DNA polymerase III holoenzyme. J Biol Chem. 1999;274:3705–3710. doi: 10.1074/jbc.274.6.3705. [DOI] [PubMed] [Google Scholar]
- 50.Pukkila P J, Peterson J, Herman G, Modrich P, Meselson M. Effects of high levels of DNA adenine methylation on methyl-directed mismatch repair in Escherichia coli. Genetics. 1983;104:571–582. doi: 10.1093/genetics/104.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roush A A, Suarez M, Friedberg E C, Radman M, Siede W. Deletion of the Saccharomyces cerevisiae gene RAD30 encoding an Escherichia coli DinB homolog confers UV radiation sensitivity and altered mutability. Mol Gen Genet. 1998;257:686–692. doi: 10.1007/s004380050698. [DOI] [PubMed] [Google Scholar]
- 52.Severinov K, Soushko M, Goldfarb A, Nikiforov V. RifR mutations in the beginning of the Escherichia coli rpoB gene. Mol Gen Genet. 1994;244:120–126. doi: 10.1007/BF00283512. [DOI] [PubMed] [Google Scholar]
- 53.Streisinger G, Okada Y, Emrich J, Newton J, Tsugita A, Terzaghi E, Inouye M. Frameshift mutations and the genetic code. Cold Spring Harbor Symp Quant Biol. 1966;31:77–84. doi: 10.1101/sqb.1966.031.01.014. [DOI] [PubMed] [Google Scholar]
- 54.Streisinger G, Owen J. Mechanisms of spontaneous and induced frameshift mutation in bacteriophage T4. Genetics. 1985;109:633–659. doi: 10.1093/genetics/109.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang M, Pham P, Shen X, Taylor J S, O'Donnell M, Woodgate R, Goodman M. Roles of E. coli DNA polymerases IV and V in lesion-targeted and untargeted SOS mutagenesis. Nature. 2000;404:1014–1018. doi: 10.1038/35010020. [DOI] [PubMed] [Google Scholar]
- 56.Tang M J, Shen X, Frank E G, O'Donnell M, Woodgate R, Goodman M F. UmuD′2C is an error-prone DNA polymerase, Escherichia coli pol V. Proc Natl Acad Sci USA. 1999;96:8919–8924. doi: 10.1073/pnas.96.16.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wagner J, Gruz P, Kim S R, Yamada M, Matsui K, Fuchs R P, Nohmi T. The dinB gene encodes a novel E. coli DNA polymerase, DNA pol IV, involved in mutagenesis. Mol Cell. 1999;4:281–286. doi: 10.1016/s1097-2765(00)80376-7. [DOI] [PubMed] [Google Scholar]
- 58.Wagner J, Kamiya H, Fuchs R P P. Leading versus lagging strand mutagenesis induced by 7,8-dihydro-8-oxo-2′-deoxyguanosine in Escherichia coli. J Mol Biol. 1997;265:302–309. doi: 10.1006/jmbi.1996.0740. [DOI] [PubMed] [Google Scholar]
- 59.Wang R F, Kushner S R. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene. 1991;100:195–199. [PubMed] [Google Scholar]
- 60.Witkin E M, Kogoma T. Involvement of the activated form of RecA protein in SOS mutagenesis and stable DNA replication in Escherichia coli. Proc Natl Acad Sci USA. 1984;81:7539–7543. doi: 10.1073/pnas.81.23.7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Witkin E M, Wermundsen I E. Targeted and untargeted mutagenesis by various inducers of SOS functions in Escherichia coli. Cold Spring Harbor Symp Quant Biol. 1979;43:881–886. doi: 10.1101/sqb.1979.043.01.095. [DOI] [PubMed] [Google Scholar]
- 62.Wood R D, Hutchinson F. Non-targeted mutagenesis of unirradiated lambda phage in Escherichia coli host cells irradiated with ultraviolet light. J Mol Biol. 1984;173:293–305. doi: 10.1016/0022-2836(84)90122-0. [DOI] [PubMed] [Google Scholar]
- 63.Woodgate R. Construction of a umuDC operon substitution mutation in Escherichia coli. Mutat Res Lett. 1992;281:221–225. doi: 10.1016/0165-7992(92)90012-7. [DOI] [PubMed] [Google Scholar]
- 64.Wu T H, Marinus M G. Dominant negative mutator mutations in the mutS gene of Escherichia coli. J Bacteriol. 1994;176:5393–5400. doi: 10.1128/jb.176.17.5393-5400.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]