Abstract
Age-related declines in cognitive control, an ability critical in most daily tasks, threaten individual independence. We previously showed in both older and younger adults that transcranial alternating current stimulation (tACS) can improve cognitive control, with effects observed across neural regions distant from the stimulated site and frequencies outside the stimulated range. Here, we assess network-level changes in neural activity that extend beyond the stimulated site and evaluate anatomical pathways that subserve these effects. We investigated the potential to rescue cognitive control in aging using prefrontal (F3-F4) theta (6 Hz) or control (1 Hz) tACS while older adults engaged in a cognitive control video game intervention on three consecutive days. Functional connectivity was assessed with EEG by measuring daily changes in frontal-posterior phase-locking values (PLV) from the tACS-free baseline. Structural connectivity was measured using MRI diffusion tractography data collected at baseline. Theta tACS improved multitasking performance, and individual gains reflected a dissociation in daily PLV changes, where theta tACS strengthened PLV and control tACS reduced PLV. Strengthened alpha-beta PLV in the theta tACS group correlated positively with inferior longitudinal fasciculus and corpus callosum body integrity, and further explained multitasking gains. These results demonstrate that theta tACS can improve cognitive control in aging by strengthening functional connectivity, particularly in higher frequency bands. However, the extent of functional connectivity gains is limited by the integrity of structural white matter tracts. Given that advanced age is associated with decreased white matter integrity, results suggest that the deployment of tACS as a therapeutic is best prior to advanced age.
Keywords: Aging, Cognitive control, Diffusion tractography, EEG, Functional connectivity, tACS
1. Introduction
Cognitive control refers to high-level executive functions and complex goal-directed processes (Mackie et al., 2013; Badre and Nee, 2018; Braver, 2012). It is essential to most daily activities and this vital process declines throughout the lifespan, eventually leading to loss of independence. In aging, the inability to ignore distractors, particularly while multitasking, increasingly adds to declining cognitive control (Gazzaley et al., 2008; McNab et al., 2015). Seniors who suffer from cognitive decline due to mild cognitive impairment and Alzheimer’s disease account for one of the largest financial burdens on society, in addition to billions in hours of assistance provided by families (Hurd et al., 2013). Indeed, successfully targeting cognitive decline in aging could lead to a larger economic impact than disease eradication (Scott et al., 2021). Therefore, the need to restore lost cognitive control abilities and maintain independence in the rapidly aging population is critical to public health.
Here, we investigate how augmenting cognitive training with noninvasive neurostimulation affects brain connectivity underlying cognitive control in aging. Synchronous neural activities are a core mechanism of connectivity in long-range brain networks, with the phase of cortical oscillations reflecting the timing of neuronal activity (Fries, 2005; Fries, 2015; Sauseng and Klimesch, 2008). Vital functions (e.g., vision, attention) are made possible through phase synchrony between distant, but functionally related neurons (Sauseng and Klimesch, 2008; Rizzolatti et al., 2018), with distinct sensory and cognitive functions operating in distinct frequency ranges (VanRullen, 2016). During cognitive control, frontal midline theta (3–7 Hz) oscillations, posited to be generated by the anterior cingulate (Cavanagh and Frank, 2014) in synchrony with the hippocampus (Buzsáki, 2002), enable communication across brain regions (Benchenane et al., 2011). Likewise, age-related cognitive declines in cognitive control are linked to declines in frontal midline theta oscillations (Cavanagh and Frank, 2014). This important link highlights frontal midline theta oscillations as a target to combat age-related cognitive decline. Elucidating the mechanisms by which such intervention improves cognition in aging holds the potential to increase quality of life through remediation of lost cognitive abilities and prophylaxis of decline in vulnerable aging populations.
Age-related cognitive decline is associated with decreased cortical volume (Jernigan et al., 2001), particularly in prefrontal cortex (PFC) (Raz et al., 2010), a critical region supporting cognitive control. Additionally, deterioration of white matter integrity is common in older adults (Salat et al., 2005; Pagani et al., 2008; Bennett et al., 2012), especially in longer tracts (Yang et al., 2016), thereby reducing the quality of anatomical connections throughout the brain. Given these anatomical changes, it is not surprising that functional connectivity also changes across the lifespan (Davis et al., 2008), as both local and inter-regional dynamics are disrupted in aging (Courtney and Hinault, 2021). Because structural and functional connectivity are vital to maintaining cognition (Hausman et al., 2021; Chan et al., 2021; Changeux et al., 2021), interventions seeking to improve cognition in aging should identify and target connectivity mechanisms (Grover et al., 2021). The implications for identifying such mechanisms are immense, as abnormal patterns of connectivity are also a fundamental feature of many clinical disorders (Bullmore and Sporns, 2009).
One approach to synchronize brain rhythms is through noninvasive transcranial alternating current stimulation (tACS) (Grover et al., 2021). TACS entrains neural oscillations at specific frequencies (Herrmann et al., 2013) and is safe, tolerable, and cost effective (Chaieb et al., 2014). When tACS successfully synchronizes brain rhythms, functional connectivity is increased (K.T. Jones et al., 2020; K.T. Jones et al., 2020; Kim et al., 2021; Jones et al., 2017; Reinhart and Nguyen, 2019), and results show promise for enhancing (Alekseichuk et al., 2016) and rescuing (Reinhart and Nguyen, 2019) working memory across the lifespan. In younger adults, PFC theta tACS also shows promise for improving sustained attention, related to strengthened alpha (8–12 Hz) connectivity in the dorsal attention network (Rostami et al., 2021). Improved inhibitory control has also been observed following PFC theta tACS, however, only when the theta frequency matches an individual’s peak frequency (Klírová et al., 2021), similar to results from working memory studies (Reinhart and Nguyen, 2019). The recent successes of tACS, when optimized to affect brain networks, set the groundwork for developing therapeutics for individuals with clinical diagnoses that implicate cognitive control.
We previously demonstrated the utility of a cognitive control video game training paradigm in healthy older adults, in which improved multitasking performance was linked to increased theta activity in medial PFC (i.e., frontal midline theta) and synchrony between medial PFC and posterior regions (Anguera et al., 2013). Given the observed medial PFC theta effects and importance of frontal midline theta in cognitive control (Cavanagh and Frank, 2014), we next investigated whether a single session of sham-controlled theta tACS to medial PFC would enhance cognitive control in young adults (Hsu et al., 2019; Hsu et al., 2017). Participants who received theta tACS (6 Hz), compared to control tACS (sham), showed enhanced performance, and increased broadband EEG power – particularly in posterior regions within the beta (12–30 Hz) band. In a follow-up study, we applied theta tACS to medial PFC during cognitive control training in older adults (Zanto et al., 2021). Participants who received theta tACS (6 Hz) showed greater behavioral gains than those who received control tACS (1 Hz) and, similar to young adults, exhibited increased posterior beta band activity. Yet, group differences were not as robust as in young adults. Using individualized models of the tACS-induced electrical field, we found that age-related cortical atrophy attenuated the amount of stimulation that reached the cortex, explaining some of the older adults’ variability in performance outcomes.
Our previous report assessing effects of theta tACS in aging focused on pre-registered hypotheses relating tACS’ effects on performance to regional neuroanatomy and neurophysiology (Zanto et al., 2021). Here, we recognize the importance of network-level analysis considering known variability in brain connectivity among older adults (Davis et al., 2008; Courtney and Hinault, 2021; Chan et al., 2021; Hinault et al., 2021). Therefore, we report new analyses to characterize how structural and functional connectivity contribute to tACS’ effects during cognitive control training in aging, and the frequency specificity of effects. We narrowed the scope of our analyses to investigate the behavioral and inter-regional neural changes observed during training, and between active theta and control tACS groups receiving the same length of training and stimulation. Based on our previous results demonstrating broadband changes in posterior spectral activity following frontal theta tACS (Hsu et al., 2019; Hsu et al., 2017), we tested the hypothesis that individual cognitive control gains following frontal theta tACS would be associated with increased frontal-posterior broadband functional connectivity, which in turn would be associated with the integrity of long-range white matter tracts. Supporting this hypothesis are recent reports linking preserved white matter in older adults with preserved cognitive control mediated by long-range functional connectivity in the alpha and gamma bands (Hinault et al., 2021; Hinault et al., 2020). Our findings: 1) provide mechanistic insight into how frontal tACS affects long-range neural synchrony, 2) facilitate applied research seeking to rescue cognitive control in older adults at risk of further decline (Grover et al., 2021), and 3) inform models of electrophysiological brain networks in aging (Sadaghiani et al., 2022).
2. Materials and methods
2.1. Participants
40 older adults (26 females/14 males; M ± SD age, 6 Hz: 66.45 ± 4.99 years; 1 Hz: 67.85 ± 5.41 years) completed a week-long cognitive control training paradigm. All participants were between 60 and 78 years old, had no history of neurological or psychiatric disease (e.g., seizures), no history of brain tumors, were not taking medications that modulate brain excitability (e.g., neuroleptic, anti-depressant, stimulant, hypnotic), had no amblyopia, strabismus, or color blindness, and did not have a pacemaker. Prior to enrollment, all participants completed an in-person neuropsychological screener that included: California Verbal Learning Test-II, animal fluency, digit symbol, Patient Health Questionnaire, Delis-Kaplan Executive Functioning System Trails, Number and Number-Letter, Stroop, Measurement of Everyday Cognition, Ishihara Color Deficiency test, physical assessments (chair sitting and standing speed), hearing, and visual acuity. All participants scored within 2 SD of standardized scores on each of the 12 of neuropsychological and physical function assessments. Montreal Cognitive Assessment (MoCA (Nasreddine et al., 2005)) scores ranged from 23 to 30 and were not significantly different between tACS groups (M ± SD, 6 Hz: 27.05 ± 2.50; 1 Hz: 26.05 ± 2.67; p = 0.25; Supplementary Figure 1). All participants signed informed consent documents approved by the University of California, San Francisco Institutional Review Board. Participants received $20 per hour for participation and $50 bonus for completing the study.
2.2. NeuroRacer paradigm
The NeuroRacer cognitive control video game was developed using the OpenGL Utility Toolkit (GLUT; http://www.opengl.org/resources/libraries/glut/) to serve as a multitasking challenge that assesses visual discrimination (sign matching) while simultaneously performing visuomotor tracking (driving a car; see (Anguera et al., 2013) for details). The visuomotor tracking driving task required participants to control a constantly moving car in the center of the road within yellow and red boundaries at a fixed speed as the road turned horizontally and moved up and down hills. The visual discrimination sign matching task required participants to press a button with their right thumb on the same controller (Logitech controller, USA). Participants were instructed to respond only to signs with green circle targets and ignore all other distractor non-targets (i.e., signs with blue and red objects, pentagons and squares, and circles that were not green). The signs were presented above the car every 2–3 s, with the window to respond and be counted as correct determined during the thresholding session on the first day.
2.3. Experimental procedure
Participants completed a week-long training paradigm (Fig. 1; see (Zanto et al., 2021) for additional details). The first day (Monday) began with structural MRI (see MRI methods Section 2.6). Participants then completed the adaptive NeuroRacer thresholding procedure, where they were assessed on their skill level independently for the visuomotor and visual discrimination tasks that would be performed at the same time during NeuroRacer training. The difficulty levels were thresholded to maintain ~75–80% accuracy on each of the tasks and corresponded to a window to respond between 250 and 1000 ms (M ± SD, 6 Hz: 431 ± 47 ms; 1 Hz: 545 ± 41 ms). Finally, participants completed two runs each of the visuomotor and visual discrimination tasks in isolation at their respective difficulty levels to assess reaction time (RT) measures prior to training and neurostimulation. The second, third, and fourth days (Tuesday-Thursday) began with setting up the dual EEG/tACS device paired with all subsequent tasks. Each day, participants completed 16 runs of NeuroRacer, with a 30-minute break between runs 8 and 9. The one-day follow-up (Friday) session began with setup of the EEG and included 8 runs of NeuroRacer without tACS.
Fig. 1.
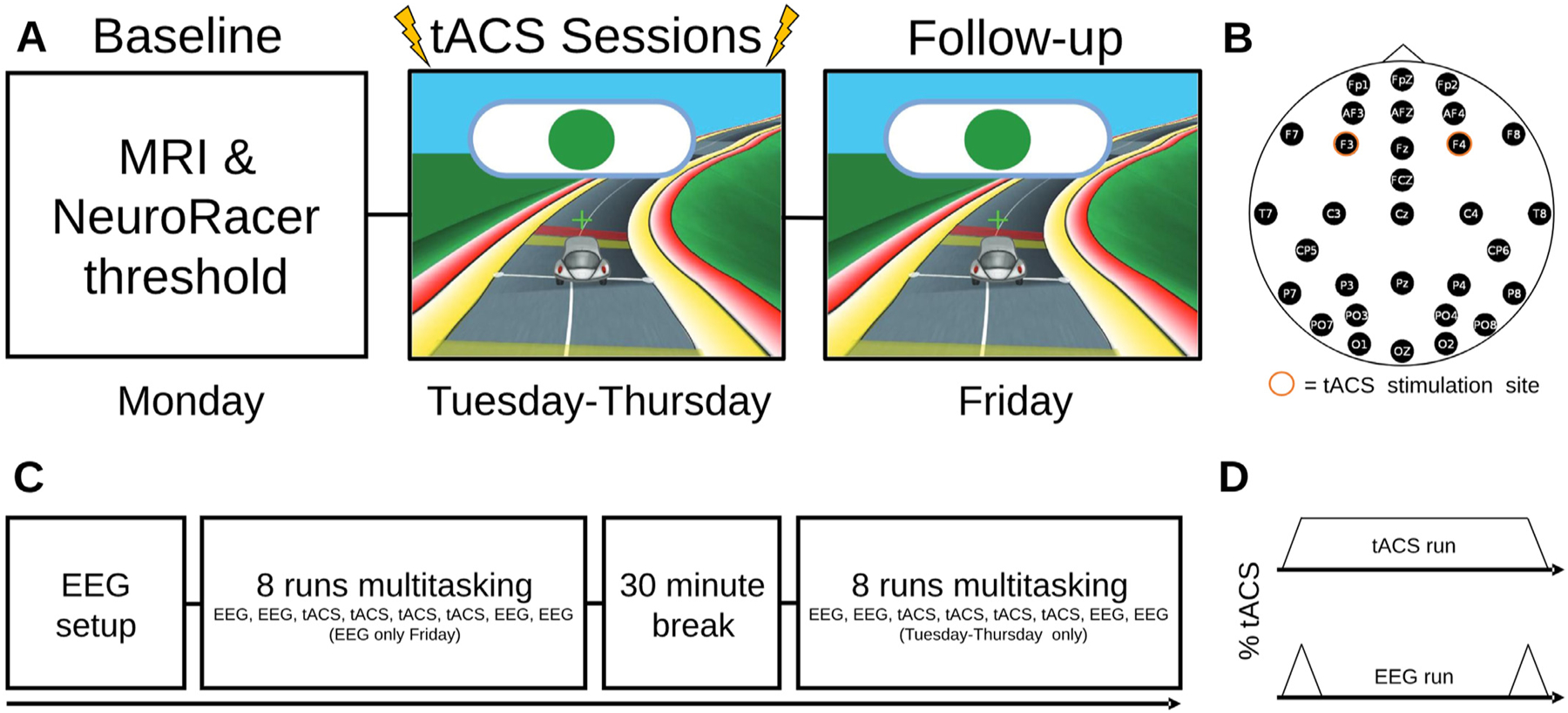
Experimental paradigm. A) Order of tasks from Monday to Friday. The Monday session contained baseline NeuroRacer threshold and MRI measures with no tACS. The following three days contained eight runs of EEG and tACS NeuroRacer multitasking assessments. Friday contained eight runs of NeuroRacer with EEG and no tACS. B) EEG electrode locations marked in black with the tACS stimulation locations highlighted in orange (F3-F4). During non-tACS runs, the stimulating electrodes recorded EEG activity. C) Timeline of tasks within each training session. The Friday session contained only eight runs of NeuroRacer. D) The timing of stimulation during NeuroRacer runs with tACS (top) and with EEG recorded (bottom).
2.4. Neurostimulation protocol
Participants were randomly assigned to one of two tACS conditions (6 Hz or 1 Hz) so that each group had 20 participants. TACS was delivered through a Starstim device (Neuroelectrics, Spain) with 3.14 cm2 electrodes placed above bilateral PFC (F3, F4; 10–20 system) at 2 mA peak-to-peak with current ramping up and down over 10 s at the beginning and end of stimulation. For stimulation runs, tACS was delivered while participants engaged in NeuroRacer training. On the days where tACS was applied (Tuesday-Thursday; tACS 1–3), both groups received tACS during runs 3–6 and 11–14 each day. For runs without tACS (runs 1–2, 7–10, 15–16), participants experienced 10 s of ramping up and then immediately back down, at which time the EEG was recorded to be free of tACS artifacts. After each of the 16 runs, participants filled out a survey rating potential side effects on a scale from 0 to 10: headache, neck pain, scalp pain, tingling, itching, burning sensation, alertness, sleepiness, trouble concentrating, acute mood change, and phosphenes (see Supplementary Table 1 in (Zanto et al., 2021)).
2.5. EEG acquisition and preprocessing
Electrophysiological data were collected with a gel electrode Starstim 32-channel system (Neuroelectrics, Spain) with a sampling rate of 500 Hz. During set up, channels were visually inspected and adjusted as needed before beginning data collection. Channels were monitored during NeuroRacer and adjusted prior to each run if they were distorted for any reason. One electrode was used as an electrooculogram (EOG) channel and the remaining 31 channels were distributed across the scalp (P8, T8, CP6, F8, AF4, C4, P4, AFz, Fp2, Fp1, Fpz, Fz, F4, Cz, PO8, PO3, O1, Oz, O2, PO4, Pz, PO7, FCz, P3, C3, F3, AF3, F7, CP5, T7, P7; 10–20 system; Fig. 1B). The stimulating electrodes (F3, F4) acted as EEG during the NeuroRacer runs where EEG was recorded without tACS.
Preprocessing was conducted using custom MATLAB (MathWorks, Natick, MA) scripts with the FieldTrip toolbox (Oostenveld et al., 2011). Raw EEG data were first band-passed through a 0.5–50 Hz two-pass Butterworth infinite impulse response filter. NeuroRacer data were then segmented into 2-s epochs around sign onset and demeaned, detrended, and re-referenced to the average EEG signal (Hsu et al., 2019; Hsu et al., 2017). Channels with excessive noise (2 SD above the mean of all channels; average removed: 4.71, SD: 2.59, IQR: 4) and trials with excessive noise (±500 μV) were removed prior to independent components analysis (ft_componentanalysis.m). Next, we removed components contaminated by eye blinks and muscle movements (average removed: 2.27, SD: 1.49, IQR: 2; ft_rejectcomponent.m). Trials were then marked for rejection if residual noise was detected ±75 μV (average trials removed per run: 27.05, SD: 22.01, IQR: 29.19). All trials and channels marked for rejection were reviewed by the authors to ensure that thresholds were appropriate. Following trial rejection, missing channels were interpolated with the average of neighboring channels (ft_channelrepair.m).
We opted not to perform a scalp surface Laplacian filter for consistency with the prior pre-registered report of these data (Zanto et al., 2021), and because the Laplacian can reduce long-distance coherence measures (Srinivasan et al., 2007; Nunez et al., 1994). As described below (Sections 2.7.2 and 2.7.4), analyses were performed on changes in the EEG from baseline. This is a common approach in EEG studies of change within individuals, and one we previously employed to analyze stimulation effects regardless of EEG headcap density or preprocessing routine (K.T. Jones et al., 2020; Jones et al., 2017; Johnson et al., 2022). Additional results are based on correlation between baseline EEG and behavior. No claims are made based on the anatomical source of the EEG signal.
2.6. MRI data collection
All data were collected by a Siemens 3T MAGNETOM Trio MRI. Prior to all baseline measurements and tACS (Monday), high-resolution T1 (1.4 × 1.4 × 1.4 mm voxel size, FOV = 270 ×270 mm, TR = 2300 ms, TE = 2.75 ms, FA = 9°) and T2 (1.4 × 1.4 × 1.4 mm voxel size, FOV = 270 ×270 mm, TR = 4000 ms, TE = 207 ms, FA = 160°) scans were acquired. Next, diffusion-weighted imaging (DWI) data were acquired as 10 initial non-diffusion weighted images (b = 0 s/mm2) followed by 96 diffusion-weighted images (b = 2500s/mm2, TR = 2420, TE = 72.2, FA = 85°).
2.7. Analyses
2.7.1. Behavior
To assess NeuroRacer performance gains throughout training and at the follow-up, we calculated RT for all target trials. Of note, our previous report of these data focused on the pre-registered measure of d-prime (d’) as an index of discrimination ability, which were only counted within an individually pre-determined (thresholded) RT window, and all responses outside of this window were marked incorrect. Here, we focused on target detection RT to fully capture the behavioral improvements throughout the study without any binary correct or incorrect limitation. Because all participants received verum stimulation during the first two NeuroRacer runs on the initial training day (Tuesday), we averaged the mean RT scores on these runs to serve as a baseline for training changes. Performance on all NeuroRacer runs where tACS was not applied were averaged together to obtain a measure of performance for each tACS day (Tuesday-Thursday: tACS 1–3) and the one-day follow-up (Friday). This approach ensured that tACS artifacts did not contaminate the EEG signal and enabled comparable assessments of change in behavioral and EEG data.
2.7.2. EEG
To assess the effects of tACS and cognitive control training on functional connectivity, phase locking values (PLV) (Lachaux et al., 1999) were computed for the sessions without tACS (runs 1–2, 7–10, 15–16). PLV represents the phase covariance between two signals that occur in the same time window and only accounts for phase, which makes PLV independent of differences in amplitude. PLV was selected for consistency with prior research on aging from other research groups (Hinault et al., 2021; Hinault et al., 2020), and with our prior neurostimulation and cognitive training research (K.T. Jones et al., 2020; Jones et al., 2017; Anguera et al., 2013; Johnson et al., 2022). Spectral decomposition was performed per channel using a multitapering approach (Mitra and Pesaran, 1999) as implemented in FieldTrip (Oostenveld et al., 2011). The 2-s trial data segments were zero-padded to 10 s to minimize filtering-induced edge artifacts (Zanto et al., 2021), and the multitaper time-frequency spectrum was calculated by sliding a 500-ms window in 10-ms increments at each frequency (3–50 Hz, 1/4 Hz fractional band-width, rounded up). The time window for PLV analyses was limited to 300–500 ms following sign onset, as in previous analyses of NeuroRacer (Anguera et al., 2013; Hsu et al., 2019; Hsu et al., 2017). PLV was computed between all pairs of frontal (AFz, AF3, AF4, Fz, F3, F4) and posterior (Pz, P3, P4, P7, P8, PO3, PO4, PO7, PO8, Oz, O1, O2) channels and then averaged across channels, resulting in one PLV value per time and frequency. Of note, PLV was analyzed only between frontal and posterior regions because our hypotheses are related to long-distance coherence and local measures of PLV are prone to volume conduction effects. For analysis, PLV was then averaged across the 200-ms multitasking epoch in canonical frequency bands: theta (3–7 Hz), alpha (8–12 Hz), beta (13–29 Hz), and gamma (30–50 Hz). Critically, we report PLV change scores (by subtracting the multitasking baseline NeuroRacer runs) and statistics to assess tACS’ effects and control for confounds of volume conduction, which does not vary between days.
2.7.3. Diffusion-weighted imaging
DWI data were preprocessed using the Functional MRI of the Brain software library (FSL; Analysis Group, FMRIB, Oxford, United Kingdom (Smith et al., 2004)). The Brain Extraction Tool was used to remove non-cortical tissue. Eddy Correct was applied to correct for head movement and eddy current distortions. Next, DTIfit was applied to calculate fractional anisotropy (FA) maps per individual. The FA maps were input in to Tract-based Spatial Statistics (TBSS) to create a mean FA skeleton (Smith et al., 2006) by aligning to a common space (FM-RIB58_FA; FA > 0.2 threshold). White matter masks were created for tracts of interest with Johns Hopkins University’s white matter atlas in FSL (Mori et al., 2008). We analyzed eight candidate tracts, four association tracts (within-hemisphere, anterior-posterior: superior longitudinal fasciculus (SLF), inferior longitudinal fasciculus (ILF), fronto-occipital fasciculus (FOF), cingulum (CG)) and four commissural tracts (between-hemisphere, left-right: anterior commissure (AC), corpus callosum body (CCB), forceps major (FMa), forceps minor (FMi)). Association tracts were selected given our previous observations that frontal theta tACS affects posterior spectral activity during NeuroRacer (Hsu et al., 2019; Hsu et al., 2017; Zanto et al., 2021) and commissural tracts were selected because tACS was applied bilaterally.
2.7.4. Statistical analyses
Unless stated otherwise, all analyses were performed with JASP statistical software (Love et al., 2019). Analyses focused on RT cost, which is calculated as the difference between multitasking RT and baseline single task RT (pre-tACS). RT cost is a sensitive measure of decrements in RT when attention is divided between two tasks and reducing RT cost represents improved multitasking performance. By focusing on RT cost, this approach also controls for differences between individual pre-tACS thresholds.
To investigate the relationship between age and white matter tracts across all participants, we conducted stepwise linear regressions with age as the dependent variable and FA in the four association (SLF, ILF, FOF, CG) or four commissural (AC, CCB, FMa, FMi) tracts as predictors, respectively. In addition, we conducted exploratory analyses of FA between groups using randomise (Winkler et al., 2014), the FSL non-parametric permutation inference tool to measure any potential group differences in baseline FA. Multiple comparisons were corrected using threshold-free cluster enhancement of p < 0.05. To investigate baseline relationships between PLV and multitasking RT across all participants, we conducted stepwise linear regressions with raw baseline multitasking RT as the dependent variable and baseline PLV in the four frequency bands (theta, alpha, beta, and gamma) as predictors.
Group differences in RT cost were determined by a 5 session (baseline, tACS 1, tACS 2, tACS 3, follow-up) × 2 group (6 Hz, 1 Hz) mixed repeated-measures ANOVA (rm-ANOVA) and post-hoc independent samples t-tests contingent on a significant interaction between session and group. These analyses identified the session of maximal group differences (i.e., theta tACS effects) and established that session as the focus of all subsequent analyses. Group-level changes in PLV (calculated as change from the multitasking baseline NeuroRacer runs) were determined by a 4 frequency (theta, alpha, beta, gamma) × 2 group (6 Hz, 1 Hz) mixed rm-ANOVA and post-hoc independent samples t-tests contingent on a significant interaction between frequency and group. In the case of a frequency main effect, post-hoc testing contrasted each pair of frequency bands.
To investigate the contributions of individual white matter microstructure to changes in PLV, we conducted a stepwise linear regression with change in each PLV frequency band separately as the dependent variable and FA in the four association and four commissural tracts as predictors, respectively. These analyses were conducted per tACS group to identify the tracts contributing to increased PLV in the theta tACS group (Hsu et al., 2017). Next, to investigate the combined contribution of functional and structural connectivity measures to behavioral gains in the theta tACS group, we conducted two multiple regressions with RT cost change as the dependent variable, and both PLV changes in the four PLV frequencies and the single associative or commissural tract that was identified from the proceeding linear regression as predictors. Finally, to investigate the contribution of PLV changes to behavioral gains regardless of tACS treatment, we conducted a stepwise linear regression across all participants with RT cost change as the dependent variable and changes in PLV in the four frequency bands as predictors.
Stepwise regression was used to extract the strongest predictor of the dependent variable, always selected from four predictors (FA in association or commissural tracts, PLV by frequency band), if one existed. This principled exploration enabled significance testing while minimizing multiple comparisons. For each stepwise regression, we investigated the impact of the predictor on the dependent variable using models generated in steps by removing the predictor with the lowest correlation with the dependent variable and p > 0.1. This procedure continues at each step by further removing predictors and only stops when there are no more predictors left in the model that satisfy the elimination criterion of p > 0.1. We report the outcome of models where predictors were significant at p < 0.05 and provide statistics for the final model. For each stepwise and multiple regression, we report the statistic (t), significance (p), unstandardized regression coefficient (b), 95% confidence interval (CI), variance explained by the predictor(s) in the final model (R2), and the standardized residual (Std. Residual) to assess the normal distribution of the data with the cutoff of ±3. Bootstrapping determined unstandardized regression coefficients (b) and 95% CI (1000 iterations), as implemented in JASP.
3. Results
3.1. Baseline measures
We first investigated the relationship between age and white matter integrity. Stepwise linear regression with the four association tracts (SLF, ILF, FOF, CG) as predictors revealed a significant negative association between age and FA in the ILF (t = −2.53, p = 0.016, b = −403.15, 95% CI = [−712.31, −47.67], R2 = 0.16, Std. Residual = [−1.93, 1.52]). Stepwise linear regression with the four commissural tracts (AC, CCB, FMa, FMi) as predictors also revealed significant negative associations between age and FA in the AC (t = −2.56, p = 0.015, b = −255.58, 95% CI = [−412.30, −50.31]) and FMa (t = −2.16, p = 0.038, b = −340.91, 95% CI = [−595.02, −16.40], R2 = 0.31, Std. Residual = [−2.05, 1.67]). These results are consistent with literature on age-related declines in white matter microstructure (Salat et al., 2005; Pagani et al., 2008; Bennett et al., 2012; Yang et al., 2016). For completeness, all age-FA correlations are provided in Supplementary Table 1. Importantly, the output of randomize demonstrated that there were no FA differences between tACS groups at the threshold of p = 0.05.
Next, we assessed whether baseline multitasking performance (raw RT, prior to multitasking training) was associated with baseline PLV. Stepwise regression with multitasking RT as the dependent variable and PLV in the four frequency bands (theta, alpha, beta, gamma) as predictors revealed a significant negative association between baseline RT and theta PLV (t = −2.19, p = 0.035, b = −0.27, 95% CI = [−0.52, −0.07], R2 = 0.12, Std. Residual = [−1.75, 2.37]; Fig. 2A), supporting our previous findings (Anguera et al., 2013) and the choice of theta as an effective frequency to apply to medial PFC (Hsu et al., 2019; Hsu et al., 2017; Zanto et al., 2021). There was no significant stepwise correlation with RT cost, meaning that theta PLV predicts multitasking RT, but not the extent that RT is specifically affected by performing two tasks simultaneously.
Fig. 2.
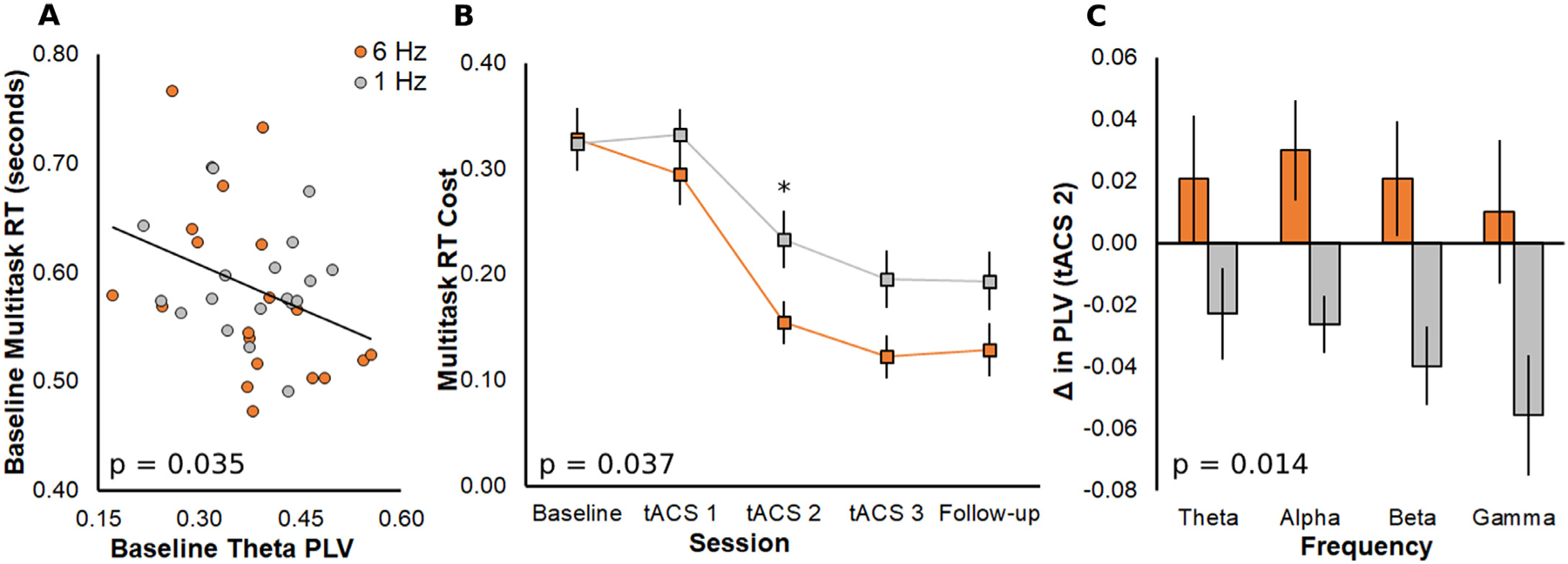
A) Data included in stepwise regression model across all participants on the dependent variable of baseline multitasking RT with the significant predictor of baseline theta PLV. P value generated from significant stepwise model. The black trendline represents both groups and participants are color-coded for the tACS treatment that they will later receive (orange, 6 Hz; gray, 1 Hz). B) Cost of multitasking compared to single task on RT at each session. The group that received 6 Hz tACS had faster and overall greater gains than the group that received 1 Hz tACS. P value generated from time × group interaction. Follow-up analyses revealed a significant group difference in RT cost change on tACS 2 (denoted by small asterisk). C) Group change in each PLV frequency band on tACS 2. Theta tACS increased PLV and control tACS decreased PLV. P value generated from main effect of tACS group.
3.2. Theta tACS improves multitasking
To determine the session of maximal multitasking gains after theta compared to control tACS, we conducted a mixed rm-ANOVA with daily RT cost as the dependent variable, the within-subjects factor of session (baseline, tACS 1, tACS 2, tACS 3, follow-up), and the between-subjects factor of tACS group (6 Hz, 1 Hz). The rm-ANOVA demonstrated a main effect of session (F4, 152 = 60.26, p < 0.001, ƞp2 = 0.61), such that performance improved following multitasking training. Importantly, a significant session × group interaction (F4, 152 = 2.62, p = 0.037, ƞp2 = 0.06) confirmed that 6-Hz tACS led to greater gains than 1-Hz tACS (Fig. 2B; Supplementary Table 2). The between-subjects main effect of group was not significant (p = 0.10). We conducted independent-samples t-tests to determine daily group differences and observed maximal group differences on tACS 2 (Wednesday; t38 = 2.19, p = 0.035, Cohen’s d = 0.69). The tACS 3 effect was marginally significant (t38 = 1.98, p = 0.055, Cohen’s d = 0.63). RT cost did not differ between groups at baseline (p = 0.91), or significantly at tACS 1 (p = 0.12) or the follow-up session (p = 0.12). For this reason, we used data from tACS 2 as the exemplar session to identify the connectivity measures underlying group differences in behavioral gains.
3.3. Theta tACS increases functional connectivity
To determine whether theta tACS increased functional connectivity in the EEG, we conducted a mixed rm-ANOVA with the within-subjects factor of PLV frequency (change from the multitasking baseline NeuroRacer runs) and the between subjects-factor of tACS group, using tACS 2 as the exemplar session. The rm-ANOVA revealed significant main effects of frequency (F3, 108 = 3.05, p = 0.032, ƞp2 = 0.08) and group (F1, 36 = 6.71, p = 0.014, ƞp2 = 0.16), with no frequency × group interaction (p = 0.691). Alpha PLV increased more than gamma PLV (t = 2.83, p = 0.034, Cohen’s d = 0.46; other p ≥ 0.114). As shown in Fig. 2C, theta tACS increased PLV and control tACS decreased PLV. The same mixed rm-ANOVA with the within-subjects factor of baseline PLV confirmed that there was no significant group difference prior to the tACS intervention (F1, 36 = 1.15, p = 0.290; ƞp2 = 0.03).
3.4. Individual changes in functional connectivity are related to brain structure
We next investigated relationships between structural and functional connectivity by assessing the contribution of baseline white matter FA to changes in PLV, using tACS 2 as the exemplar session. Specifically, we sought to identify the white matter tracts supporting increased PLV in the theta tACS group (see Fig. 2C). For this reason, we conducted separate stepwise linear regressions on the 6 Hz and 1 Hz tACS groups (Hsu et al., 2017). In the 6 Hz tACS group, stepwise regression with changes in theta PLV as the dependent variable and the four association tracts as predictors revealed a significant positive association with FA in the ILF (t = 2.81, p = 0.012, b = 10.06, 95% CI = [5.12, 13.31], R2 = 0.33, Std. Residual = [−1.94, 1.47]). Significant positive associations with FA in the ILF were also observed with the dependent variable of changes in alpha PLV (t = 3.13, p = 0.007, b = 8.81, 95% CI = [2.76, 14.12], R2 = 0.38, Std. Residual = [−1.66, 1.39]), beta PLV (t = 3.98, p = 0.001, b = 10.88, 95% CI = [4.99, 16.49], R2 = 0.50, Std. Residual = [−2.33, 1.78]), and gamma PLV (t = 2.69, p = 0.016, b = 11.03, 95% CI = [2.08, 20.66], R2 = 0.31, Std. Residual = [−2.78, 1.66]). Repeating this approach with the four commissural tracts as predictors revealed significant positive associations with FA in the CCB and the dependent variable of changes in theta PLV (t = 3.41, p = 0.004, b = 8.79, 95% CI = [4.38, 16.70], R2 = 0.42, Std. Residual = [−1.68, 1.44]), alpha PLV (t = 3.16, p = 0.006, b = 7.21, 95% CI = [1.29, 11.80], R2 = 0.38, Std. Residual = [−1.87, 1.32]), and beta PLV (t = 3.34, p = 0.004, b = 7.83, 95% CI = [2.29, 12.81], R2 = 0.41, Std. Residual = [−2.30, 1.80]). As shown in Fig. 3, participants with the largest increase in PLV (post-tACS) had the highest FA values at baseline (pre-tACS). In contrast, the same analyses for the 1 Hz group returned no significant associations. For completeness, all PLV-FA correlations are provided in Supplementary Table 1.
Fig. 3.
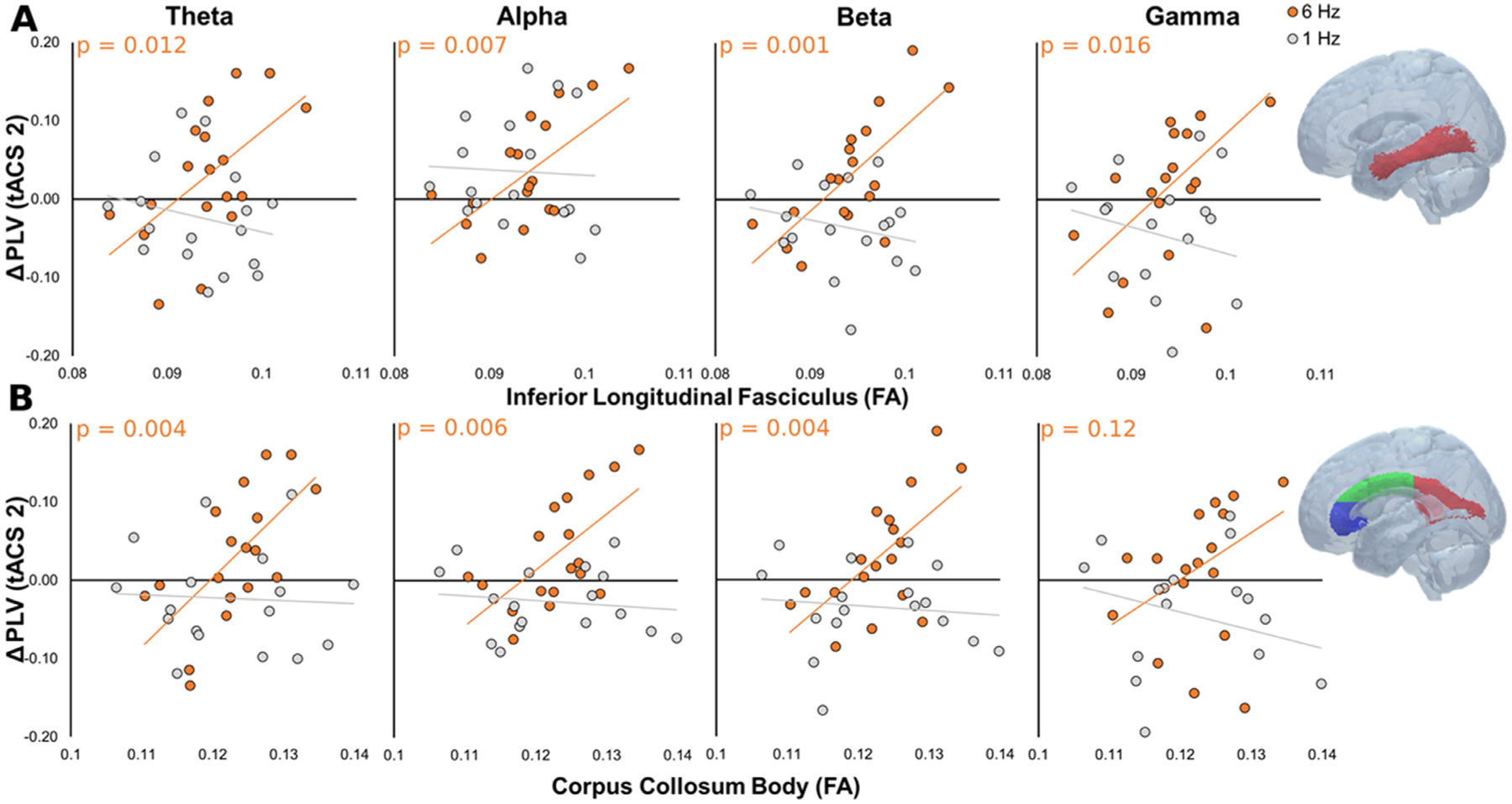
Changes in theta, alpha, beta, and gamma PLV as a function of baseline ILF (A) and CCB (B) integrity. P values represent the significance of the predictor of ILF or CCB FA on PLV change in the final model for the 6 Hz group. Insets indicate the tract masks in MNI space, adapted from (Ivanova et al., 2016). Note that the CCB is shown in green.
To ensure that baseline FA and not baseline PLV predicted tACS-induced PLV increases, we performed confirmatory analyses of covariance (ANCOVAs), one per PLV frequency with ILF or CCB FA as a covariate. First, we replaced each PLV change score dependent variable with tACS 2 PLV and entered baseline PLV as a covariate. Second, we entered group as the between-subjects factor. There was a significant interaction of group and FA in each ANCOVA on the ILF tract (theta F1, 30 = 14.27, p < 0.001, ƞp2 = 0.32, alpha F1, 30 = 12.07, p < 0.001, ƞp2 = 0.29, and beta F1, 30 = 12.62, p = 0.001, ƞp2 = 0.30, gamma F1, 30 = 5.88, p = 0.022, ƞp2 = 0.16) and on the CCB tract (theta F1, 30 = 16.65, p < 0.001, ƞp2 = 0.36, alpha F1, 30 = 11.53, p = 0.002, ƞp2 = 0.28, and beta F1, 30 = 9.82, p = 0.004, ƞp2 = 0.25, gamma F1, 30 = 4.51, p = 0.042, ƞp2 = 0.13).
3.5. Individual changes in functional connectivity related to brain structure predict multitasking gains
Post hoc multiple regression analysis examined whether multitasking gains in the 6 Hz group were dually predicted by changes in PLV and individual brain structure. Multiple regression was conducted with RT cost change as the dependent variable and PLV changes in the four frequency bands, ILF or CCB FA, and PLV-FA interactions as predictors. To assess the dual contribution of brain structure (white matter FA) and function (PLV), we focused on only the interactions of PLV changes and FA (Johnson et al., 2022). The ILF model revealed that individuals with greater ILF FA at baseline showed greater multitasking gains and strengthening of PLV in the alpha band (t = 2.56, p = 0.034, b = 798.68, 95% CI = [−2442.16, 11,759.65], R2 = 0.74, Std. Residual = [−1.39, 1.91]). The interaction with strengthening of PLV in the beta band was marginally significant (t = −2.29, p = 0.051, b = −706.54, 95% CI = [−110,028.94, 1482.53]). The CCB model revealed that individuals with greater CCB FA at baseline showed greater multitasking gains and strengthening of PLV in the alpha (t = 3.42, p = 0.009, b = 734.09, 95% CI = [−807.95, 17,265.38], R2 = 0.78, Std. Residual = [−1.49, 1.69]) and beta bands (t = −2.67, p = 0.028, b = −584.31, 95% CI = [−7923.12, 2441.01]). No other interactions reached significance in either model (p > 0.13). Together, changes in alpha and beta PLV, and FA in the ILF and CCB account for 65.1% of the variability in multitasking gains on tACS 2.
3.6. Individual changes in functional connectivity track multitasking gains regardless of tACS treatment
Finally, we sought to determine whether increased PLV was associated with improvements in multitasking across all participants regardless of tACS frequency. Post hoc stepwise regression with multitasking RT as the dependent variable and PLV in the four frequency bands as predictors revealed a significant negative association between changes in RT cost and changes in alpha PLV across all participants (t = −2.48, p = 0.018, b = −0.76, 95% CI = [−1.35, −0.24], R2 = 0.15, Std. Residual = [−2.97, 1.57]; Fig. 4). This result demonstrates that strengthening alpha PLV underpins multitasking gains in aging and suggests a functional mechanism of behavioral performance improvement that is not specific to tACS.
Fig. 4.
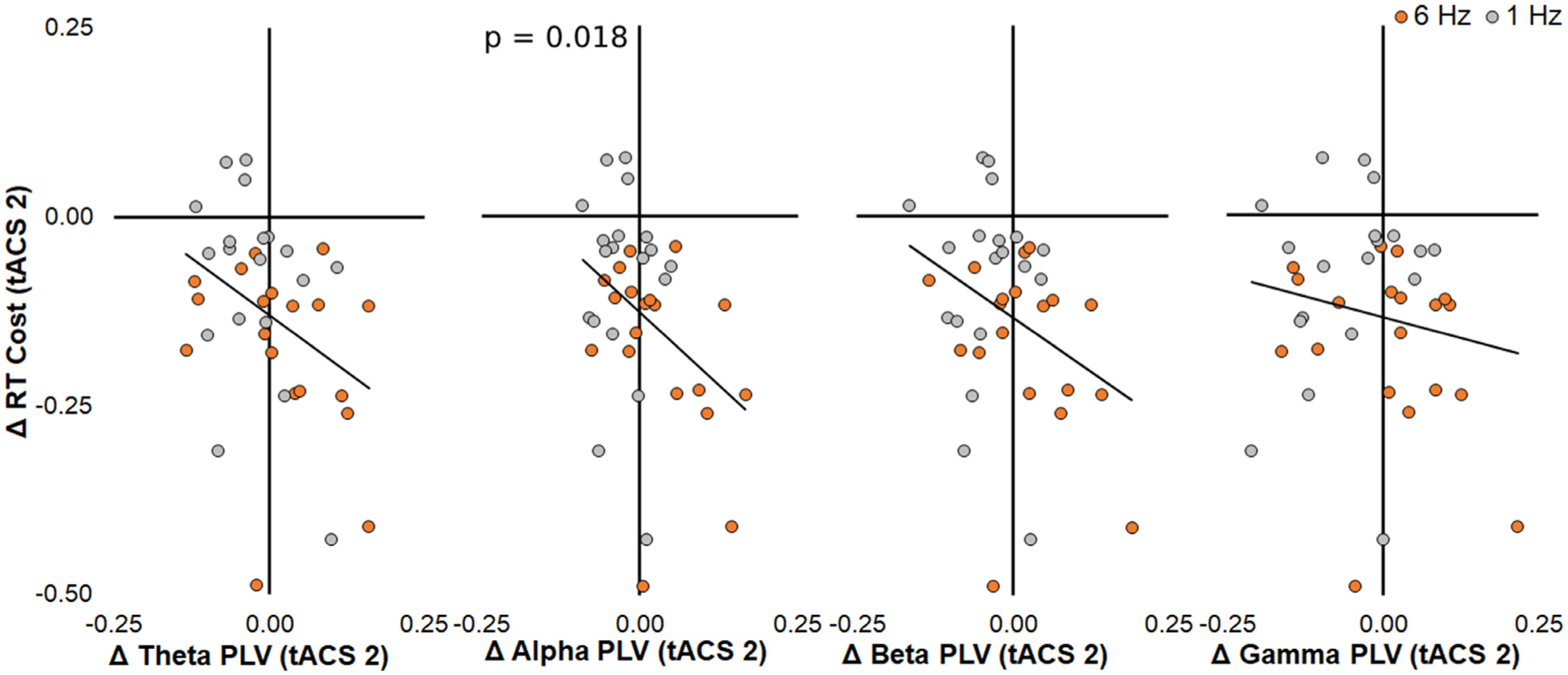
Data included in stepwise regression model across all participants on the dependent variable of RT cost change and PLV change in each frequency predictors. Only alpha PLV was a significant predictor of change in RT cost. The black trendline represents both groups and participants are color-coded for visualization purposes only of the tACS treatment that they received (orange, 6 Hz; gray, 1 Hz). P value listed only for the significant predictor.
4. Discussion
We present neural measures that emphasizes the role of multi-modal brain connectivity in cognitive control (multitasking) gains in older adults following tACS. First, we confirmed that advanced age in this sample was associated with decreased white matter integrity. Prior to our tACS intervention, we also observed that theta PLV predicted multitasking RT at baseline, supporting our previous findings (Anguera et al., 2013) and the choice of theta as an effective frequency to apply to medial PFC (Hsu et al., 2019; Hsu et al., 2017; Zanto et al., 2021). Next, tACS applied at theta (6 Hz) led to greater improvements in multitasking ability compared to control (1 Hz) tACS. Theta tACS increased frontal-posterior functional connectivity, as measured by changes in PLV from baseline, whereas control tACS decreased PLV. Individual increases in broadband functional connectivity following theta tACS correlated positively with white matter integrity, particularly within the ILF (theta-gamma bands; occipital-temporal association tract) and CCB (theta-beta bands, major between-hemisphere commissural tract). Importantly, individual multitasking gains following theta tACS were dually predicted by changes in alpha and beta PLV and baseline FA in the ILF and CCB. Together, these functional and structural factors help explain how theta tACS rescues multitasking in aging. Therefore, these results demonstrate that tACS-related improvements in multitasking are associated with increased frontal-posterior functional connectivity, and increased connectivity was greater in those with greater white matter integrity at baseline.
This finding extends recent reports associating individual differences in cognitive control in older adults with white matter integrity, mediated by functional connectivity (specifically PLV) in the alpha and gamma bands (Hinault et al., 2021; Hinault et al., 2020). We propose that theta tACS may improve cognitive control in vulnerable aging populations by strengthening functional connectivity, particularly in the alpha-beta range, and the extent of gains is limited by individual structural white matter integrity. Given that advanced age was associated with decreased white matter integrity across all participants, we speculate that the deployment of tACS as a therapeutic may be best utilized as a preventative measure before age-related structural deterioration. However, we note that the reported interactions of white matter integrity and PLV on behavior should be treated as preliminary given the sample size and that further research is needed to replicate these results. The sample size of 20 participants within the theta tACS group limited analysis of full factorial effects examining age, white matter integrity, and PLV on behavior.
Cognitive training and neurostimulation interventions are plagued by subtle and unreliable outcomes (Horvath et al., 2015; Zinke et al., 2014; Simons et al., 2016; Jacobson et al., 2012; Medina and Cason, 2017; Richmond et al., 2011). Our previous results inform some of this variability by accounting for individual differences in regional neuroanatomy and neurophysiology (Zanto et al., 2021). However, recent advances in understanding the nuanced mechanisms of cognition, such as functional connectivity (Ju and Bassett, 2020; Buzsáki and Draguhn, 2004), and how structural and functional connectivity are impacted in age and disease (Bennett et al., 2012; Bullmore and Sporns, 2009; Hinault et al., 2021; Hinault et al., 2020; Persson et al., 2006; Madden et al., 2007), illuminate the importance of maintaining long-range brain networks for prophylaxis of cognitive decline. Here, we demonstrate that both long-range functional connectivity and white matter integrity influence the extent of neurostimulation-linked cognitive control training gains in aging. These outcomes, taken together with other recent reports of success in targeting connectivity-based mechanisms of cognition (Grover et al., 2021; K.T. Jones et al., 2020; Jones et al., 2017; Reinhart and Nguyen, 2019; Alekseichuk et al., 2016), are shown to be reliable.
Our initial report from these data demonstrated that regional neuroanatomy and neurophysiology both predict the extent to which tACS can rescue cognitive control (Zanto et al., 2021), which is relevant to this study by highlighting the importance of preserved brain structure for tACS efficacy in aging. Here, the observed structure-function relationship in brain connectivity extends theories of neurocognitive aging based on associations between preserved structural MRI metrics and cognitive capabilities. Specifically, many older adults maintain performance on cognitive tasks even as they reach advanced age, with posterior-anterior functional shifts observed as a compensatory mechanism (Davis et al., 2008; Park and Reuter-Lorenz, 2009), and alterations in alpha power and connectivity linked to age-related cognitive decline (Lejko et al., 2020; Ishii et al., 2017). Our findings extend this literature by demonstrating a link between older adults’ brain health, as indexed by brain connectivity (Chan et al., 2021), and tACS efficacy at improving cognitive performance (cf. transcranial direct current stimulation efficacy in (Johnson et al., 2022)). In multitasking, individual behavioral gains were maximal in older adults with greater ILF and CBB integrity at baseline and greater increases in alpha-beta functional connectivity during training.
How this structure-function relationship relates to tACS efficacy reveals a mechanism of cognitive control that may be targeted to combat the deleterious effects of age-related cognitive decline. Given that age-related deteriorations of white matter integrity (Pagani et al., 2008; Yang et al., 2016) and functional connectivity (Davis et al., 2008; Chan et al., 2021) contribute to cognitive decline (Hinault et al., 2021), any effort that maintains or rescues brain connectivity should combat cognitive decline. Although this study was limited by the spatial resolution of scalp EEG, necessitating use of a composite functional connectivity measure that is not linked to specific frontal and posterior brain regions, two white matter tracts consistently emerged as predictors of individual gains in PLV, the ILF and CCB. Given the visual detection requirements of NeuroRacer, it is logical that we observed effects in the ILF, the occipital-temporal association tract which supports object processing along the ventral visual steam (Herbet et al., 2018). Likewise, because we applied bilateral PFC stimulation, it is logical that we also observed effects in the CCB, the major between-hemisphere commissural tract. Finally, we note that although white matter integrity decreased with advancing age in several tracts in our sample, including ILF, the relationship was not significant in the CCB. This suggests that tACS-based interventions in advanced age may be efficacious when stimulating across both hemispheres, capitalizing on the resilience of the CCB to age-related degradation (Voineskos et al., 2012) and its role in facilitating tACS-related changes in functional connectivity. Additional research will need to directly test this hypothesis.
It is worth noting that theta tACS increased PLV in not only the theta band, but also alpha through gamma bands (Veniero et al., 2015). Indeed, in our previous research employing theta tACS and NeuroRacer, we consistently observed that frontal theta tACS led to broadband increases in spectral activity with maximal effects in posterior beta power (Hsu et al., 2019; Hsu et al., 2017; Zanto et al., 2021). The present results extend findings from posterior activity to frontal-posterior functional connectivity, illuminate the alpha and beta bands, and link such changes to multitasking gains. Particularly relevant to the present results, previous research associates sustained attention gains after PFC theta tACS to increased theta power and alpha PLV in the dorsal attention network (Rostami et al., 2021). Increased frontal-posterior connectivity may also reflect changes in the default mode network (Alves et al., 2019), as previous research associates working memory gains after PFC theta tACS to increased connectivity across default mode regions (Abellaneda-Pérez et al., 2020). Collectively, our results are consistent with research demonstrating that theta tACS can entrain brain networks according to the demands posed by the task, linking theta tACS during cognitive control to neural regions distant from the stimulated site and frequencies outside the stimulated range. Yet, it is clear that additional research is needed to understand the specific role of the different frequencies of functional connectivity associated with cognitive control, and why manipulation of frontal theta activity resulted in network-level changes in different frequency bands.
Last, we note that if our sample had included a larger, more heterogeneous population, such as those with mild cognitive impairment, it is possible that baseline neuroanatomical factors may have predicted both disease severity and responsiveness to neurostimulation intervention. Indeed, there are many neurological conditions that lead to cognitive control deficits, such as traumatic brain injury, stroke, and age-related mild cognitive impairment, and that could therefore benefit from interventions which strengthen brain networks. In addition, aging research often differentiates between younger- and oldest-old adults (Merenstein and Bennett, 2022). We enrolled younger-old adults (60–78 years) and not the oldest-old cohorts (80+ years). Had we included a more comprehensive sample of ages and cognitive abilities, we speculate that structure-function relationships would be of even greater importance in predicting intervention efficacy. Our results indicate that interventions seeking to improve cognitive control should target functional connectivity and account for individual brain structure. Future research should examine how older adults with greater cortical atrophy or weaker white matter microstructure could still benefit from targeted interventions, such as those of much longer duration (Mackey et al., 2012) or which tailor the dose and frequency of stimulation (Reinhart and Nguyen, 2019; Zanto et al., 2021; Indahlastari et al., 2021), with the goal of rescuing cognition in those who need it most.
In sum, the current study presents a nuanced mechanism by which cognitive training and neurostimulation interact with individual brain structure to affect functional connectivity, leading to cognitive control gains in aging. Given the growing number of older adults at-risk of cognitive decline, identifying alternatives or complements to costly pharmacological interventions would return a significant financial and time-resource benefit to society (Scott et al., 2021). Once protocols are successfully optimized to yield reliable outcomes, the potential for neurostimulation interventions to scale to the public is high.
Supplementary Material
Acknowledgements
This work was supported by the National Science Foundation grant #1829473 and National Institute on Aging grants R21AG062395 and R03AG065966. ELJ is supported by the National Institute of Neurological Disorders and Stroke grant R00NS115918. We would like to thank Andy Baird, David Ziegler, Elizabeth Pierce, Hobbes Jones, and Keith Johnson for their important contributions to this work.
Footnotes
Competing Interests
AG is a scientific advisor for Neuroelectrics, which makes the neurostimulation device employed in the current study. As such, AG was not involved in data collection or analysis. TZ is a scientific advisor for HUMM, which makes a neurostimulation device not used in the current study.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi: 10.1016/j.neuroimage.2022.119547.
References
- Abellaneda-Pérez K, et al. , 2020. Differential tDCS and tACS effects on working memory-related neural activity and resting-state connectivity. Front. Neurosci doi: 10.3389/fnins.2019.01440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alekseichuk I, Turi Z, Amador de Lara G, Antal A, Paulus W, 2016. Spatial working memory in humans depends on theta and high gamma synchronization in the prefrontal cortex. Curr. Biol 26, 1513–1521. [DOI] [PubMed] [Google Scholar]
- Alves PN, et al. , 2019. An improved neuroanatomical model of the default-mode network reconciles previous neuroimaging and neuropathological findings. Commun. Biol 2, 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anguera JA, et al. , 2013. Video game training enhances cognitive control in older adults. Nature 501, 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Nee DE, 2018. Frontal Cortex and the Hierarchical Control of Behavior. Trends Cogn. Sci 22, 170–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchenane K, Tiesinga PH, Battaglia FP, 2011. Oscillations in the prefrontal cortex: a gateway to memory and attention. Curr. Opin. Neurobiol 21, 475–485. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Motes MA, Rao NK, Rypma B, 2012. White matter tract integrity predicts visual search performance in young and older adults. Neurobiol. Aging 33, 433 e21–433.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, 2012. The variable nature of cognitive control: a dual mechanisms framework. Trends Cogn. Sci 16, 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Sporns O, 2009. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci 10, 186–198. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Draguhn A, 2004. Neuronal oscillations in cortical networks. Science (80-.) 304 1926 LP–1929. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, 2002. Theta Oscillations in the Hippocampus. Neuron 33, 325–340. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, Frank MJ, 2014. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. (Regul. Ed.) 18, 414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaieb L, et al. , 2014. Safety of 5kHz tACS. Brain Stimul 7, 92–96. [DOI] [PubMed] [Google Scholar]
- Chan MY, et al. , 2021. Long-term prognosis and educational determinants of brain network decline in older adult individuals. Nat. Aging 1, 1053–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J-P, Goulas A, Hilgetag CC, 2021. A connectomic hypothesis for the hominization of the brain. Cereb. Cortex 31, 2425–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney SM, Hinault T, 2021. When the time is right: temporal dynamics of brain activity in healthy aging and dementia. Prog. Neurobiol 203, 102076. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R, 2008. Que PASA? The posterior-anterior shift in aging. Cereb. Cortex 18, 1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P, 2005. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn. Sci 9, 474–480. [DOI] [PubMed] [Google Scholar]
- Fries P, 2015. Rhythms for Cognition: communication through Coherence. Neuron 88, 220–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, et al. , 2008. Age-related top-down suppression deficit in the early stages of cortical visual memory processing. Proc. Natl. Acad. Sci. U. S. A doi: 10.1073/pnas.0806074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover S, Nguyen JA, Reinhart RMG, 2021. Synchronizing brain rhythms to improve cognition. Annu. Rev. Med 72, 29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausman HK, et al. , 2021. Cingulo-opercular and frontoparietal control network connectivity and executive functioning in older adults. GeroScience doi: 10.1007/s11357-021-00503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbet G, Zemmoura I, Duffau H, 2018. Functional anatomy of the inferior longitudinal fasciculus: from historical reports to current hypotheses. Front. Neuroanat 12, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann CS, Rach S, Neuling T, Strüber D, 2013. Transcranial alternating current stimulation: a review of the underlying mechanisms and modulation of cognitive processes. Front. Hum. Neurosci 7, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinault T, Kraut M, Bakker A, Dagher A, Courtney SM, 2020. Disrupted neural synchrony mediates the relationship between white matter integrity and cognitive performance in older adults. Cereb. Cortex 30, 5570–5582. [DOI] [PubMed] [Google Scholar]
- Hinault T, et al. , 2021. Age-related differences in network structure and dynamic synchrony of cognitive control. Neuroimage 236, 118070. [DOI] [PubMed] [Google Scholar]
- Horvath JC, Forte JD, Carter O, 2015. Evidence that transcranial direct current stimulation (tDCS) generates little-to-no reliable neurophysiologic effect beyond MEP amplitude modulation in healthy human subjects: a systematic review. Neuropsychologia 66, 213–236. [DOI] [PubMed] [Google Scholar]
- Hsu W, Zanto T, van Schouwenburg M, Gazzaley A, 2017. Enhancement of multitasking performance and neural oscillations by transcranial alternating current stimulation. PLoS ONE 12, e0178579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu WY, Zanto TP, Gazzaley A, 2019. Parametric effects of transcranial alternating current stimulation on multitasking performance. Brain Stimul 12, 73–83. [DOI] [PubMed] [Google Scholar]
- Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM, 2013. Monetary costs of dementia in the United States. N. Engl. J. Med 368, 1326–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indahlastari A, et al. , 2021. Individualized tDCS modeling predicts functional connectivity changes within the working memory network in older adults. Brain Stimul. 14, 1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii R, et al. , 2017. Healthy and pathological brain aging: from the perspective of oscillations, functional connectivity, and signal complexity. Neuropsychobiology 75, 151–161. [DOI] [PubMed] [Google Scholar]
- Ivanova MV, et al. , 2016. Diffusion-tensor imaging of major white matter tracts and their role in language processing in aphasia. Cortex 85, 165–181. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Koslowsky M, Lavidor M, 2012. TDCS polarity effects in motor and cognitive domains: a meta-analytical review. Exp. Brain Res doi: 10.1007/s00221-011-2891-9. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, et al. , 2001. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol. Aging 22, 581–594. [DOI] [PubMed] [Google Scholar]
- Johnson EL, Arciniega H, Jones KT, Kilgore-Gomez A, Berryhill ME, 2022. Individual predictors and electrophysiological signatures of working memory enhancement in aging. Neuroimage 250, 118939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KT, Peterson DJ, Blacker KJ, Berryhill ME, 2017. Frontoparietal neurostimulation modulates working memory training benefits and oscillatory synchronization. Brain Res doi: 10.1016/j.brainres.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KT, Johnson EL, Tauxe ZS, Rojas DC, 2020a. Modulation of auditory gamma-band responses using transcranial electrical stimulation. J. Neurophysiol 123, 2504–2514. [DOI] [PubMed] [Google Scholar]
- Jones KT, Johnson EL, Berryhill ME, 2020b. Frontoparietal theta-gamma interactions track working memory enhancement with training and tDCS. Neuroimage doi: 10.1016/j.neuroimage.2020.116615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju H, Bassett DS, 2020. Dynamic representations in networked neural systems. Nat. Neurosci 23, 908–917. [DOI] [PubMed] [Google Scholar]
- Kim K, Sherwood MS, McIntire LK, McKinley RA, Ranganath C, 2021. Transcranial direct current stimulation modulates connectivity of left dorsolateral prefrontal cortex with distributed cortical networks. J. Cogn. Neurosci 33, 1381–1395. [DOI] [PubMed] [Google Scholar]
- Klírová M, et al. , 2021. Modulating inhibitory control processes using individualized high definition theta transcranial alternating current stimulation (HD θ-tACS) of the anterior cingulate and medial prefrontal cortex. Front. Syst. Neurosc.i 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaux JP, Rodriguez E, Martinerie J, Varela FJ, 1999. Measuring phase synchrony in brain signals. Hum. Brain Mapp 8, 194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejko N, Larabi DI, Herrmann CS, Aleman A, Ćurčić-Blake B, 2020. Alpha power and functional connectivity in cognitive decline: a systematic review and meta-analysis. J. Alzheimers. Dis 78, 1047–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love J, et al. , 2019. JASP: graphical statistical software for common statistical designs. J. Stat. Softw 88, 1–17. [Google Scholar]
- Mackey A, Whitaker K, Bunge S, 2012. Experience-dependent plasticity in white matter microstructure: reasoning training alters structural connectivity. Front. Neuroanat 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie MA, Van Dam NT, Fan J, 2013. Cognitive control and attentional functions. Brain Cogn. doi: 10.1016/j.bandc.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, et al. , 2007. Adult age differences in the functional neuroanatomy of visual attention: a combined fMRI and DTI study. Neurobiol. Aging 28, 459–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab F, et al. , 2015. Age-related changes in working memory and the ability to ignore distraction. Proc. Natl. Acad. Sci. U. S. A doi: 10.1073/pnas.1504162112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina J, Cason S, 2017. No evidential value in samples of transcranial direct current stimulation (tDCS) studies of cognition and working memory in healthy populations. Cortex doi: 10.1016/j.cortex.2017.06.021. [DOI] [PubMed] [Google Scholar]
- Merenstein JL, Bennett IJ, 2022. Bridging patterns of neurocognitive aging across the older adult lifespan. Neurosci. Biobehav. Rev 135, 104594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra PP, Pesaran B, 1999. Analysis of dynamic brain imaging data. Biophys. J doi: 10.1016/S0006-3495(99)77236-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, et al. , 2008. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine ZS, et al. , 2005. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Nunez PL, et al. , 1994. A theoretical and experimental study of high resolution EEG based on surface Laplacians and cortical imaging. Electroencephalogr. Clin. Neurophysiol 90, 40–57. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen J.M.FieldTrip, 2011. Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani E, Agosta F, Rocca MA, Caputo D, Filippi M, 2008. Voxel-based analysis derived from fractional anisotropy images of white matter volume changes with aging. Neuroimage 41, 657–667. [DOI] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P, 2009. The adaptive brain: aging and neurocognitive scaffolding. Annu. Rev. Psychol 60, 173–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, et al. , 2006. Structure–function correlates of cognitive decline in aging. Cereb. Cortex 16, 907–915. [DOI] [PubMed] [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U, 2010. Trajectories of brain aging in middle-aged and older adults: regional and individual differences. Neuroimage 51, 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart RMG, Nguyen JA, 2019. Working memory revived in older adults by synchronizing rhythmic brain circuits. Nat. Neurosci 22, 820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond LL, Morrison AB, Chein JM, Olson IR, 2011. Working memory training and transfer in older adults. Psychol. Aging doi: 10.1037/a0023631. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fabbri-Destro M, Caruana F, Avanzini P, 2018. System neuroscience: past, present, and future. CNS Neurosci. Ther 24, 685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami R, et al. , 2021. 6Hz transcranial alternating current stimulation of mPFC improves sustained attention and modulates alpha phase synchronization and power in dorsal attention network. Cogn. Neurosci 12, 1–13. [DOI] [PubMed] [Google Scholar]
- Sadaghiani S, Brookes MJ, Baillet S, 2022. Connectomics of human electrophysiology. Neuroimage 247, 118788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, et al. , 2005. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol. Aging 26, 1215–1227. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, 2008. What does phase information of oscillatory brain activity tell us about cognitive processes? Neurosci. Biobehav. Rev 32, 1001–1013. [DOI] [PubMed] [Google Scholar]
- Scott AJ, Ellison M, Sinclair DA, 2021. The economic value of targeting aging. Nat. Aging 1, 616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons DJ, et al. , 2016. Do ‘Brain-Training’ Programs Work? Psychol. Sci. Public Interes 17, 103–186. [DOI] [PubMed] [Google Scholar]
- Smith SM, et al. , 2004. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23, S208–S219. [DOI] [PubMed] [Google Scholar]
- Smith SM, et al. , 2006. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Winter WR, Ding J, Nunez PLEEG, coherence, M.E.G., 2007. Measures of functional connectivity at distinct spatial scales of neocortical dynamics. J. Neurosci. Methods 166, 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRullen R, 2016. Perceptual Cycles. Trends Cogn. Sci 20, 723–735. [DOI] [PubMed] [Google Scholar]
- Veniero D, Vossen A, Gross J, Thut G, 2015. Lasting EEG/MEG aftereffects of rhythmic transcranial brain stimulation: level of control over oscillatory network activity. Front. Cell. Neurosci 9, 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineskos AN, et al. , 2012. Age-related decline in white matter tract integrity and cognitive performance: a DTI tractography and structural equation modeling study. Neurobiol. Aging 33, 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE, 2014. Permutation inference for the general linear model. Neuroimage doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang AC, Tsai S-J, Liu M-E, Huang C-C, Lin C-P, 2016. The association of aging with white matter integrity and functional connectivity Hubs. Front Aging Neurosci 8, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, et al. , 2021. Individual differences in neuroanatomy and neurophysiology predict effects of transcranial alternating current stimulation. Brain Stimul. Basic, Transl. Clin. Res. Neuromodulat 14, 1317–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinke K, et al. , 2014. Working memory training and transfer in older adults: effects of age, baseline performance, and training gains. Dev. Psychol 50, 304–315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


