Abstract
The most efficient and safe source of medications is the natural and traditional medications which are produced from plants and herbs. In this study, Sisymbrium officinale (S. officinale) was tested to explore its total phenolic and flavonoids contents. Antioxidant, antimicrobial, and anticancer activities were assessed as well. S. officinale was bought from a local Palestinian market, air-dried, and extracted with 99% ethanol with the aid of ultrasonication. The extract was tested on three types of bacteria using well diffusion method. The anti-microbial testing included three different types of bacteria, two gram-positive bacteria, Streptococcus and Staphylococcus and E. coli as a gram-negative bacterium. Antioxidant activity of the plant extract was conducted using DPPH method, while total phenolic and flavonoids contents were performed using a well-known assay chemical method. Anticancer activity of the extract was conducted against two cancer cell lines (breast (MCF7) and colon (HCT116) cancer cell lines). Results showed that the extract is rich polyphenolic and flavonoids and has strong antioxidant activity reflected by inhibition of free radicals (DPPH) (193.7 ± 3.4). The plant extract showed also strong antimicrobial activity against both E. coli and Streptococcus bacteria with of inhibition of 10 and 14 mm respectively. The extract of this plant also showed anticancer activity (about 6%) against MCF7 (breast cancer cell line).
Keywords: S. officinale, Plant extract, Antioxidants, Antimicrobial, Anticancer, Activity
S. officinale; Plant extract; Antioxidants; Antimicrobial; Anticancer; Activity.
1. Introduction
Traditional medicine is known as the knowledge, skills, and practices based on previously done theories and experiments on maintaining health and finding treatments for physical and mental illness through the use of herbs [1]. The Medicinal plant is the use of plants in herbalism and for medicinal purposes. Plants have been a source for medical use for so many years. Herbal medicine varies from using traditional to popular medicines that were extracted from herbs and plants. However, herbal medicine is evaluated according to its safety, efficiency, and efficacy. Therefore, every extracted herb must be investigated and studied professionally before putting it to public use.
Chemical drugs have so many toxic side effects, the side effects of the chemotherapeutic drugs in the long-term cause serious health complications, also many types of bacteria start developing resistance against synthetic antibiotics drugs, new treatments should be developed and investigated instead, the most efficient and safe source of medications is the natural and traditional medications which are produced from plants and herbs. A medicinal plant is any plant that contains one or more substances in its organs that might be used for therapeutic purposes, or investigated in the synthesis of useful drugs [2]. Plant extracts can be an alternative to manufactured drugs and act as an antimicrobial agent such as; Artemisia annua which has antimalarial activity [3]. Medicinal plants can play vital roles in the prevention of diseases. Several studies were done on different plants and the results were promising. Sisymbrium officinale (commonly known as hedge mustard) is a medical and herbal plant that was used since the Greeks' age in medicine (Figure 1). It can also be used in cuisines such as salads and mustard sauces. However, S. officinale was not only used for culinary preparations. It was mainly used as an herb in traditional medicine in southwest Asia, Arabia, northwestern India, and northeast Africa. It helped in treating respiratory tract diseases, such as pharyngitis, laryngitis, cough, aphonia, common cold, sore throat, and other asthma [4].
Figure 1.
Sisymbrium officinale, Hedge Mustard leaves (fresh) and dried plant.
S. officinale is an annual or biennial herbaceous plant from the family Brassica Mediterranean area [5]. It commonly grows on disturbed sites such as roadsides, wastelands, disturbed habitats, and field margins [6]. The wild plants were always important in the traditional medicine history in the Mediterranean region. Some researchers tested the anti-inflammatory antioxidant and antimicrobial activities of S. officinale [7]. However, despite its traditional use, this plant was not investigated deeply.
S. officinale has a single stem erect branched at right angles, and opaque green or purplish [4]. This stem showed antimicrobial, muscle relaxant, antimutagenic, and antioxidant activities in some researches [8]. S. officinale starts to emerge from late autumn until early spring [9]. It has been used for many centuries but only has been studied and explored scientifically in the last decades. It possesses interesting therapeutic properties, especially for throat diseases and that is why it is commonly called “the herb of singers''.
Humans are always exposed to many radiations and causing oxidative damage to DNA, protein, and lipids and thus causing chromosomal mutations. For example oxidative stress activities may lead to other diseases such as cancer, diabetes, and even neurological and cardiovascular diseases. S. officinale was used in many experiments that proved its antioxidant activity. S. officinale can block biochemical reactions which potentially leads to genetic material damage. These antioxidative activities can support the consumption of the plant by people who work in highly polluted environments or even smokers [10].
The increased use of antimicrobial agents in clinical practices leads to bacterial resistance against antibiotic agents, where this mechanism is developed by bacteria to promote such resistance to survive [11]. However, a high MIC above the susceptibility threshold to an antibiotic will be reported as resistance. Bacteria also may develop resistance through gaining the resistance genes from other bacteria or in developing a mutation that results in the reduction of antibiotic component [9].
Cancer is widely cured by targeted therapy such as radiation and chemotherapy. However, the side effects of chemotherapy in the long-term cause serious concerns [12]. It may affect the patient by causing nausea and vomiting through the shot-run, and ulceration, anorexia, malabsorption, anemia, and sepsis in the long run [13]. On the other hand, the overuse of antibiotics has been a serious issue the past decade causing microbial, and even cancerous diseases to be resistant to drugs [14]. Therefore, innovative and alternative therapeutic strategies must be found through investigating the use of herbs to reduce the use of chemotherapy and antibiotics.
Despite the significance of Sisymbrium officinale being a natural herbal plant and its use to cure many respiratory tract diseases, its antioxidant and biological activities are not thoroughly investigated. The objectives of this work are therefore to investigate antioxidant, antimicrobial and anticancer activities of S. officinale as well as its total phenolic and flavonoids contents.
2. Materials and methods
2.1. Plant material
The plant S. officinale was bought from the local market in Jerusalem Palestine in January 2021. The plant was air-dried (Figure 1) in a shade room for 16 days and crushed to obtain a fine powder. The dried plant material was stored in a plastic bag in the refrigerator, until extraction.
2.2. Extraction
About 3 g of dried S. officinale leaves were soaked in 50 ml of ethanol (99%) and placed in the ultrasonicater for 90 min. The mixture was then filtered by passing through Whatman filter paper. After that, the ethanol solvent was evaporated using rotary evaporator at reduced pressure. The obtained viscous crude extract was collected and transferred into small bottles and stored in the fridge to be used for analysis. For analysis, the extract was dissolved in a minimum amount of ethanol to get a concentration of 1.0 mg per ml.
2.3. Determination of total phenolic content
A volume of 1.8 ml of Folin-Ciocalteu (5ml of Folin-Ciocalteu reagent were diluted with 50 ml distilled water) was added to 50μl of sample extract. Then 1.2 ml of sodium carbonate (7.5%) was added after 5 min. The mixture was left for 120 min and absorbance was measured at 760 nm. Calibration curve using gallic acid was used for total phenolic content determination, and the results of total phenolic content in the extracts were expressed as mg gallic acid per gram of crude extract.
2.4. Determination of total flavonoid content
To 100 μl of crude extract, 4 ml of distilled water, 0.3 ml of 10% AlCl3, and 0.3 ml of 5%NaNO2 were added. After 6 min, 2 ml of 1 N NaOH, and 2.5 ml of distilled water were added to the mixture. The absorbance was then measured at 510 nm. Calibration curve using Catechin was used for total flavonoids content determination, and the results of total flavonoids content in the extracts were expressed as mg catechin per gram of crude extract.
2.5. Antioxidant activity (free radical scavenging activity using DPPH)
The stable 1,1-diphenyl-2-picryl hydrazyl radical (DPPH) assay is focused on the calculation of antioxidants' scavenging capacity against the stable radical DPPH. 4 ml aliquot of 0.0634 mM of DPPH solution in methanol was mixed with aliquots of extract (50 μL) at several concentrations (0.1–2 mg/ml), then incubated at 25 °C for 30 min in the dark area. A positive standard using vitamin C was prepared by the same procedure. The absorbance of the different samples and standards was measured at 515 nm. The scavenging ability of DPPH radicals was assessed according to scavenging activity (%) = Abs (Control) – Abs (Sample)/Abs (Control) × 100, where Abs control is the absorbance of the control (it is containing all reagents except the plant extract), and Abs (sample) is the absorbance of the tested plant extract. The extract concentration that gives 50% inhibition (IC50) is calculated based on the plot of inhibition (%) against extract concentration and compared with the IC50 of vitamin C value as a positive control.
2.6. HPLC analysis
The HPLC analysis of the extracts was conducted on the ODS column of Waters (XBridge, 4.6 ID x 150 mm, 5 μm). The mobile phase is a mixture of water (0.5 percent) acetic acid (solvent A) and acetonitrile (solvent B) (80:20, v/v). 100% (solvent A) decreased to 70% (solvent A) in 40 min, then to 40% (solvent A) in 20 min and eventually to 10% (solvent A) in 2 min and would remain there for 6 min and then return to initial conditions in 2 min. The HPLC system shall be balanced with the initial acidic mobile water phase (solvent A) for 7 min before the next sample is injected. With a 0.45 μm PTFE filter, all samples were filtered. The range of PDA wavelengths is 210–500 nm. Flow rate is 1 ml/min. The volume of injection is 20 μL and the temperature of the column is 25 °C.
2.7. Antimicrobial activity using well diffusion method
The well diffusion method was used to test the antimicrobial activity of the S. officinale extracts against three different types of bacteria, two gram-positive bacterium (Streptococcus, and Staphylococcus aureus) and 1-g negative bacteria (E. coli). Müller-Hinton agar (Oxoid, UK) was prepared and poured into different sterilized petri dishes, the bacterial dilutions were prepared using the 0.5 McFarland turbidity standard and streaked separately on different agar plates. DMSO was used as negative control against the bacterial cell lines. Wells were made in the agar plates using aseptic techniques in order to add the S. officinale plant extract. The petri dishes were then incubated at 37 °C for 24 h, before measuring the zone of inhibition.
2.8. Anti-cancer activity
Anti-cancer activity was performed using two types of cancer cell lines, the breast (MCF7) and colon (HCT116) cancer cell lines. The cancer cells were cultured in RPMI media and incubated for 24 h before the treatment with the S. officinale plant extract. 100μl of the extracts were diluted with DMSO and then added separately at four different concentrations (50 μg/ml, 100 μg/ml, 200 μg/ml, and 400 μg/ml), in addition to a negative control of DMSO. After the treatment, the cells were incubated for 24, and 48 h before assessing the effect of the different concentrations of the plant extract on the different cancer cell lines.
2.9. Statistical analysis
All data analysis were performed using the Microsoft excel 2013. Results were reported as mean of three samples ±standard deviation (SD).
3. Results and discussion
3.1. Total phenolic contents (TPC)
Total phenolic content of the S. officinale extract was determined using Folin method using calibration curve of different concentrations of gallic acid and the results are expressed as mg gallic acid per gram of crude extract (Table 1). TPC of S. officinale plant extracts when extracted with ethanol was found to be 101.3 ± 2.4 mg/g. These results show that the plant ethanolic extract is rich in polyphenolic compounds.
Table 1.
Total phenolic and flavonoids contents, and antioxidant activity of ethanolic extracts of S. officinale.
| Concentration of extract | Total phenolic content (mg gallic acid/g) | Total flavonoids content (mg ruting/g) | Antioxidant activity (μmol Vitamin C∖g) |
|---|---|---|---|
| 0.2 mg/ml | 101.3 ± 2.4 | 29.3 ± 1.2 | 193.7 ± 3.4 |
3.2. Total flavonoid content (TFC)
Total flavonoids in the extracts were determined using aluminum chloride method using calibration curve of different concentrations of catechin and the results are expressed as mg catechin per gram of crude extract. TFC for the plant material, when extracted with ethanol extract, was found to be 29.3 ± 1.2 mg catechin/g, which shows that the plant is rich in flavonoids (Table 1).
3.3. Antioxidant activity
An antioxidant is a term that is used for a chemical that prevents oxygen consumption. However, antioxidants act as a radical scavenger, electron donor, hydrogen donor, peroxide decomposer, enzyme inhibitor, synergist, and metal chelating agent [15]. Free radicals and oxidants play a dual role since they can be harmful and helpful to the body. They could be produced either in situ from normal cell metabolism or ex-situ from pollution, smoking, radiation, and medications. When an overload of free radicals cannot be destroyed it would be oxidative stress. Oxidative stress may cause many illnesses such as autoimmune disorders, cancer, aging, cardiovascular and neurodegenerative diseases. However, the body counteracts oxidative stress through several mechanisms which are either naturally produced in situ or ex situ gained from food or supplements [15].
Antioxidant Activity accounts for the availability of antioxidants like phenolic compounds and flavonoids as efficient oxygen radical scavengers. Phenolic antioxidant activity is primarily due to their redox properties which make them serve as reduction agents, donors of hydrogen and singlet oxygen quenchers. Results showed that the plant extract has strong antioxidant activity reflected by DPPH test (193.7 ± 3.4 μmol Vitamin C/g) which also implies that this extract is rich with antioxidants reflected by inhibition of the free radical DPPH. Results were expressed as μmol vitamin C per gram extract. Different concentrations of Vitamin C were prepared for the calibration curve.
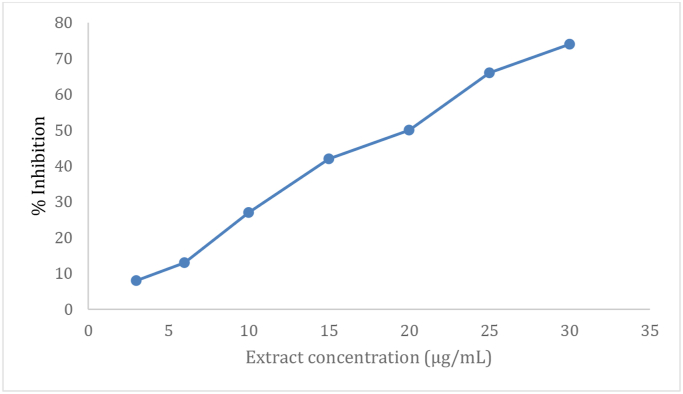
The % inhibition of DPPH was also calculated. Results showed that the DPPH radical scavenging activity increased in a concentration-dependent manner at 3–30 μg/mL with 8–74% inhibition of DPPH (Figure 2). 20 μg/ml S. officinale extract was associated with 50% inhibition of the DPPH radical, whereas the ascorbic acid (as a reference) was associated with a 50% inhibition of the DPPH radical, at 6.2 μg/ml concentration.
Figure 2.
Inhibition (%) of DPPH against extract concentration of S. officinale.
3.4. HPLC-PDA profiles of the extracts
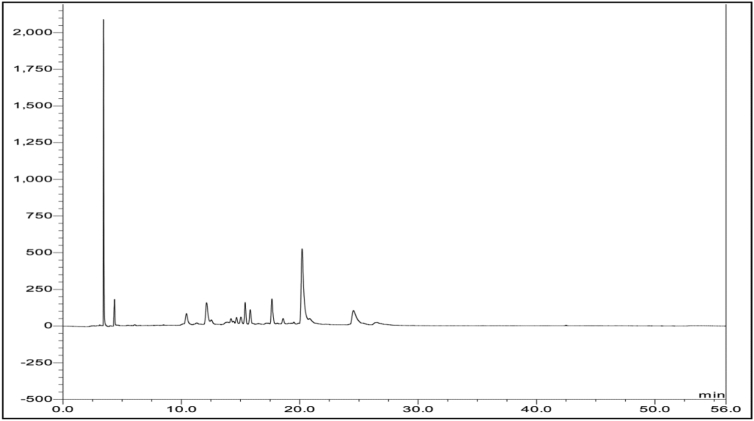
Figure 3 shows the chromatogram of the crude extract of S. officinale extract at 340 nm. This wavelength was selected because the major peaks showed maximum absorption at this wavelength. The eluted compounds were detected in the range of 3-5- and 10-25-minutes indicating compounds with different polarities.
Figure 3.
HPLC-PDA chromatogram of crude extract of S. officinale at 340 nm.
3.5. Antimicrobial activity
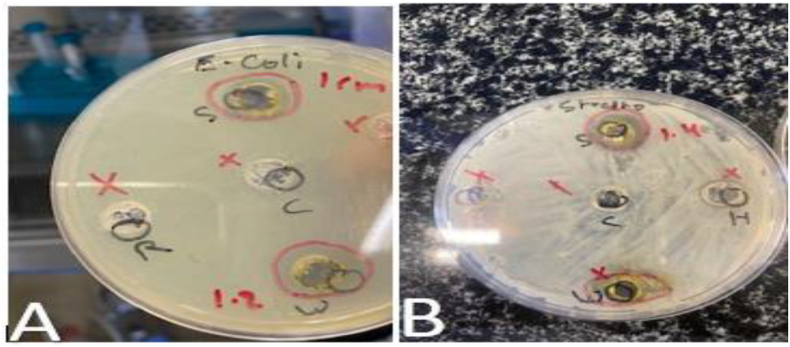
In order to test the antimicrobial activity of S. officinale extract, a well diffusion method was used. Results showed strong antibacterial activity of S. officinale extracts against E. coli and Streptococcus with 10 mm, and 14 mm zone of inhibition, respectively (Figure 4). On the other hand, no activity was observed for S. officinale plant extract against Staphylococcus bacteria.
Figure 4.
Antimicrobial activity of S. officinale (A): the plate of E. coli bacteria (S) shows (10 mm) zone of inhibition. (B): the plate of Streptococcus bacteria (S) shows (14 mm) zones of inhibition.
3.6. Anticancer activity
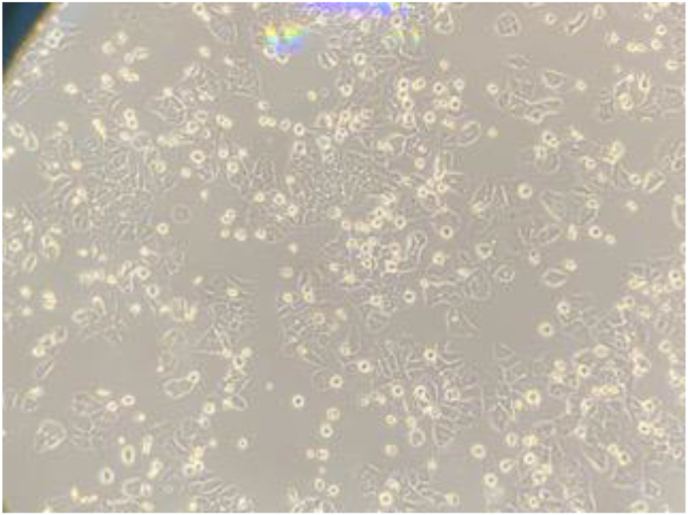
Anticancer activity was noticed at 100μL of the plant extract on the MCF7 (breast cancer cells) with 6% cancer cell death (Figure 5). However, no results were shown on the 50μL, 200 μL, or 400 μL concentrations. No anticancer results were shown on the HCT116 (colon cancer cells) at all concentrations studied of the plant extract. Negative control using DMSO was used for the two cancer cell lines investigated in this study, where results show no anticancer activity against these two cancer cell lines.
Figure 5.
Anticancer activity of S. officinale on MCF7 (breast cancer cells) showing 6% of breast cancer cell death.
4. Discussion
The tests of total phenolic content, flavonoids, and antioxidant activity of ethanolic extracts of dried S. officinale prove that there are medicinally active constituents in the studied ethanolic extract of S. officinale. It has been observed that the plant has a good antioxidant activity, and the extract is rich in flavonoids and phenolic compounds. Additionally, the results of the HPLC were shown in the chromatogram of the S. officinale plant extracts at 340 nm, which revealed mixed polarities of compounds in the plant's extract.
The antioxidant properties of the plant S. officinale extracts might be connected to the chemical composition which includes bioactive phytochemicals, high levels of phenolic acids, and flavonoids. S. officinale could be considered as a good source of antioxidants that will help in the prevention of many diseases that are caused by oxidative stress e.g. diabetes mellitus, carcinomas, neurodegenerative disorders and cardiovascular diseases.
Recently, many studies were done about phenolic and flavonoid-rich natural diets with antioxidants activities. Natural phenolic and flavonoid compounds hold an aromatic ring bearing with at least one hydroxyl group for this reason it is considered as a plant secondary metabolite [16]. The phenolic compounds are considered a good electron donor because their hydroxyl group can contribute to antioxidant actions directly. According to previous investigations phenolic compounds prevent oxidative disease burden, exhibit free radical inhibition, and peroxide decomposition [16]. Recent studies have shown that the consumption of leafy plant vegetables that contain phenolic and flavonoid compounds with potent antioxidant activity is associated with lowering the incidence of cancer, cardiovascular diseases, diabetes, and neurodegenerative diseases [17].
Antimicrobial activity has shown strong activity against E. coli, and Streptococcus bacteria, but no activity against Staphylococcus bacteria. This result shows the selectivity of this extract against 1-g positive bacteria (Streptococcus bacteria) and gram-negative bacteria (E. coli). Previous studies showed antimicrobial activity for both gram-positive and gram-negative bacteria and especially for E. coli bacteria [18]. The well diffusion method was done in this experiment using different concentrations of the S. officinale extract. More investigations should be done for using the S. officinale plant as an antimicrobial agent. S. officinale plant extracts have been also used for microbial infections and as a source of antioxidants. Since pathogenic microorganisms have been building resistance to many antibiotics and antimicrobial medications, new drug discovery is needed. Ethyl acetate extracts of S. officinale had also a good antimicrobial activity [18, 19].
The anticancer activity was shown for the breast cancer cells on a concentration of 100μL of the plant extraction. Previous studies revealed the anticancer activity of the S. officinale plant extract [20]. The S. officinale plant extract is promising for the anticancer activities since it showed a 6% of cell death when it was incubated for 24 hrs, after the addition of 100μL of the plant extract.
5. Conclusion
Traditional medicine is widely used to cure diverse diseases, and now researchers are exploiting it in new research and medical drug synthesis. S. officinale was used in traditional medicine for wounds and some diseases for a long time. However, serious issues have been evolving lately such as bacteria gaining resistance against antibiotics. Therefore, scientists were encouraged to look for new medicine. Our research about the S. officinale plant through our experiments and data analysis suggests that the leaf part of the plant can be used as an antioxidant, anti-bacterial, and anticancer agent. The completion of this study provided further information about the antioxidant, antimicrobial, and anticancer activity of S. officinale in particular. We recommend further investigations on the leaves of S. officinale which has shown a potential anticancer activity by causing apoptosis to stop cell-proliferation. Also, antioxidant activity was observed highly in this research. The S. officinale has shown also an antimicrobial activity against the E. coli and the Streptococcus bacteria.
Declarations
Author contribution statement
Mahmoud Khalid; Mousa Amayreh; Saadi Sanduka: Performed the experiments; Wrote the paper.
Zaidoun Salah: Analyzed and interpreted the data; Wrote the paper.
Fuad Al-Rimawi; Abdulkareem A. Alanezi: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ghassab M. Al-Mazaideh; Fadel Wedian: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Mohammed Helmy Faris Shalayel; Fawaz Alasmari: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Researchers supporting project number (RSP2022R235), King Saud University, Riyadh, Saudi Arabia.
Data availability statement
The authors are unable or have chosen not to specify which data has been used.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Authors wish to acknowledge King Saud University, Al-Quds University, University of Hafr Al Batin and Yarmouk University for providing facilities and encouragement.
References
- 1.Benzie I.F.F., Wachtel-Galor S. Herbal Medicine: Biomolecular and Clinical Aspects. CRC Press/Taylor & Francis Copyright © 2011 by Taylor and Francis Group, LLC.; Boca Raton (FL: 2011. Herbal medicine: biomolecular and clinical aspects. [PubMed] [Google Scholar]
- 2.Sofowora A., Ogunbodede E., Onayade A. The role and place of medicinal plants in the strategies for disease prevention. Afr. J. Tradit., Complementary Altern. Med. : AJTCAM. 2013;10(5):210–229. doi: 10.4314/ajtcam.v10i5.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houghton P.J. The role of plants in traditional medicine and current therapy. J. Altern. Compl. Med. 1995;1(2):131–143. doi: 10.1089/acm.1995.1.131. [DOI] [PubMed] [Google Scholar]
- 4.Rahman M., Khatun A. Brassicaceae mustards: traditional and agronomic uses in Australia and New Zealand. Molecules. 2018;23(1):E231. doi: 10.3390/molecules23010231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Sotto A., et al. Pharmacological and phytochemical study on a Sisymbrium officinale Scop. extract. J. Ethnopharmacol. 2010;127(3):731–736. doi: 10.1016/j.jep.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura E. Architecture and growth of an annual plant Chenopodium album in different light climates. Ecol. Res. 2009;25:383–393. [Google Scholar]
- 7.Amodeo V., et al. Chenopodium album L. And Sisymbrium officinale (L.) Scop.: phytochemical content and in vitro antioxidant and anti-inflammatory potential. Plants. 2019;8(11):505. doi: 10.3390/plants8110505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zucca P., Bellot S., Rescigno A. The modern use of an ancient plant: exploring the antioxidant and nutraceutical potential of the Maltese mushroom (Cynomorium coccineum L.) Antioxidants. 2019;8(8):289. doi: 10.3390/antiox8080289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zorzan M., et al. Sisymbrium officinale, the plant of singers: a review of its properties and uses. Planta Med. 2020;86(5):307–311. doi: 10.1055/a-1088-9928. [DOI] [PubMed] [Google Scholar]
- 10.Ben Attia I., et al. Chemical composition and antioxidant potential differences between Cynomorium coccineum L. Growing in Italy and in Tunisia: effect of environmental stress. Diversity. 2018;10(3):53. [Google Scholar]
- 11.Ventola C.L. The antibiotic resistance crisis: part 1: causes and threats. P T. 2015;40(4):277–283. [PMC free article] [PubMed] [Google Scholar]
- 12.Nurgali K., Jagoe R.T., Abalo R. Editorial: adverse effects of cancer chemotherapy: anything new to improve tolerance and reduce Sequelae? Front. Pharmacol. 2018;9:245. doi: 10.3389/fphar.2018.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cinausero M., et al. New frontiers in the pathobiology and treatment of cancer regimen-related mucosal injury. Front. Pharmacol. 2017;8:354. doi: 10.3389/fphar.2017.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shallcross L.J., Davies D.S.C. Antibiotic overuse: a key driver of antimicrobial resistance. Br. J. Gen. Pract.: J. Roy. Coll. Gen. Pract. 2014;64(629):604–605. doi: 10.3399/bjgp14X682561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pham-Huy L.A., He H., Pham-Huy C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. : I.J.B.S. 2008;4(2):89–96. [PMC free article] [PubMed] [Google Scholar]
- 16.Choudhary R.K., Swarnkar P.L. Antioxidant activity of phenolic and flavonoid compounds in some medicinal plants of India. Nat. Prod. Res. 2011;25(11):1101–1109. doi: 10.1080/14786419.2010.498372. [DOI] [PubMed] [Google Scholar]
- 17.Aryal S., et al. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants. 2019;8(4):96. doi: 10.3390/plants8040096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egharevba G.O., et al. Antidiabetic, antioxidant and antimicrobial activities of extracts of Tephrosia bracteolata leaves. Heliyon. 2019;5(8) doi: 10.1016/j.heliyon.2019.e02275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandrich L., Caputo E. Brassicaceae-derived anticancer agents: towards a green approach to beat cancer. Nutrients. 2020;12(3):868. doi: 10.3390/nu12030868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamza A.A., Hassanin S.O., Hamza S., et al. Polyphenolic-enriched olive leaf extract attenuated doxorubicin-induced cardiotoxicity in rats via suppression of oxidative stress and inflammation. JoBAZ. 2021;82:54. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors are unable or have chosen not to specify which data has been used.