Abstract
Obesity is a complex metabolic condition considered a worldwide public health crisis, and a deeper mechanistic understanding of obesity-associated diseases is urgently needed. Obesity comorbidities include many associated cancers and are estimated to account for 20% of female cancer deaths in the USA. Breast cancer, in particular, is associated with obesity and is the focus of this review. The exact causal links between obesity and breast cancer remain unclear. Still, interactions have emerged between body mass index, tumor molecular subtype, genetic background, and environmental factors that strongly suggest obesity influences the risk and progression of certain breast cancers. Supportive preclinical research uses various diet-induced obesity models to demonstrate that weight loss, via dietary interventions or changes in energy expenditure, reduces the onset or progression of breast cancers. Ongoing and future studies are now aimed at elucidating the underpinning mechanisms behind weight-loss-driven observations to improve therapy and outcomes in patients with breast cancer and reduce risk. This review aims to summarize the rapidly emerging literature on obesity and weight loss strategies with a focused discussion of bariatric surgery in both clinical and preclinical studies detailing the complex interactions between metabolism, immune response, and immunotherapy in the setting of obesity and breast cancer.
Keywords: Immunotherapy, PD-L1, Obesity, High fat diet, Adiposity, Caloric restriction
Introduction
Obesity is one of the most prevalent diseases in westernized societies, identified as a public health crisis both in the USA by the Surgeon General and worldwide by the World Health Organization [1, 2]. Based on body mass index (BMI; kg/m2), two-thirds of adults are considered obese (BMI > 30) or overweight (BMI 25–30), with one in three Americans identified as experiencing moderate (class 1: BMI 30 to 35 and class 2: BMI 35 to 40) to severe (class 3: BMI > 40) obesity [3, 4]. Surveys show increasing incidence, especially in women, and disproportionately high incidence in minorities [5–9]. Since a landmark publication by Calle et al. in 2003, obesity has increasingly been associated with specific types of cancer [10–12]. Currently, thirteen cancers are associated with obesity in terms of increased risk as defined by the American Institute for Cancer Research (AICR) [13], including postmenopausal breast cancer, colorectal cancer, endometrial/uterine cancer, esophageal adenocarcinoma, gall bladder cancer, gastric cancer, hepatocellular cancer, meningioma, multiple myeloma, ovarian cancer, pancreatic cancer, renal cancer, and thyroid cancer [11, 14]. Obesity is a significant, modifiable risk factor in nearly 20% of total US female cancer deaths and 30% of postmenopausal breast cancer cases [12, 15, 16]. Of particular interest for this review is obesity-linked breast cancer. With breast cancer being the most common malignancy and the second leading cause of cancer death in females [12, 17–26], understanding the links between obesity, adipose tissue, inflammation, immune response, and potential therapeutic interventions is critical to improving patient outcomes. This review will focus on the role of obesity in impacting breast cancer risk, prognoses, and survival, emphasizing the impacts of weight loss by bariatric surgery.
Obesity and breast cancer
Obesity and breast cancer remain a complex conundrum that truly highlights the importance of including clinically relevant data in analyses such as tumor subtypes and menopausal status, ensuring adequate representation of diverse populations, predominantly minority and younger patients, and measuring several metrics to quantify obesity in complementary ways. For example, it is well established that obesity is a metabolically dysregulated state that increases the risk for postmenopausal breast cancer, primarily represented by the more prevalent luminal A subtype (ER-positive and/or PR-positive, HER2-negative [27]). In contrast, obesity in premenopausal women has been reported to be protective or have a null effect on breast cancer risk, typically when breast cancer is considered without accounting for subtypes [28–37]. However, many early studies were limited by lack of minority inclusion, few younger participants, a single measure of obesity, and estrogen receptor (ER) positivity determination only from immunohistochemistry (IHC) data. When studies include mainly white, older women who are more likely to present with luminal A and ER-positive subtypes, these studies may not be powered to detect the impacts of obesity on other breast cancer subtypes that strike younger or minority women. The decreased cost of sequencing has greatly increased the ability to quantify molecular subtypes.
Likewise, while BMI is convenient for extensive studies and retrospective analyses drawing from patient medical records with relatively little measurement error, BMI is fraught with limitations. Most notably, BMI lacks the ability to account for muscle mass compared to adiposity and fails to capture the compartmentalization of visceral versus central adiposity [38]. Other measures of obesity, including waist-to-hip ratio (WHR) and waist circumference, as well as more invasive measures such as DEXA scan or MRI, are complementary or improved metric options that are increasingly utilized in many studies. In addition, evaluation of obesity at cancer diagnosis may not be indicative of lifetime obesity exposure, hence some studies record obesity at a younger age along with current obesity status. Each strategy has pros and cons, influenced mainly by measurement error or cost. Likewise, the extent (classes 1–3) and duration of obesity are critical to consider. In sum, while BMI is convenient for population-based studies, including additional obesity measures adds to the ability to examine many important associations.
Once investigative teams began to oversample or enroll a disproportionately high percentage of minorities and younger women [27], include multiple breast cancer subtypes by additional IHC markers [39] or transcriptomic analysis, record pre- or postmenopausal status at the time of diagnosis, and use multiple measures of obesity, our understanding of obesity’s impact on risk became even more complex. In studies such as the Carolina Breast Cancer Study (CBCS) or consortia such as AMBER (African American Breast Cancer Epidemiology and Risk, which includes CBCS), obesity in premenopausal women was reported to be a risk factor for breast cancer, especially for triple-negative breast cancer (TNBC) [34, 40–43]. In contrast, several studies have not supported these findings, showing null results or moderately reduced risk ratios for obesity risk in premenopausal women. There is also evidence that overweight BMI reduces all-cause mortality of patients, promoting longer lifespans than normal-weight counterparts and reducing comorbidities [44], although controversy exists. Notably, a direct comparison of BMI to WHR suggests that WHR may better predict risk in African American women that is not detected by BMI alone, masking risk in many previous studies that only report BMI [40]. These findings exemplify the complexity of tumor subtypes, pre-or post-menopausal cancer, consideration of race and ethnicity, age when obese, duration of obesity, type of adiposity (visceral versus central), and use of BMI, WHR, or other quantification, which are all critical to consider in designing and evaluating studies of cancer risk. Furthermore, there is evidence that critically ill patients with obesity experience lower mortality rates [45]. This phenomenon is called the “obesity paradox.”
While the impact of obesity on cancer risk is complex, it is abundantly clear that obesity negatively impacts cancer outcomes and survival. In breast cancer patients, women with increased BMI have a higher risk of invasion [46, 47], distant metastases [48–50], tumor recurrence [51, 52], impaired delivery of systemic therapies [22, 53], and mortality [12, 54–60]. Thus, obesity represents a modifiable risk factor and is a target for cancer prevention measures and to improve cancer outcomes [61–63]. The exact molecular mechanisms linking obesity to breast cancer initiation (i.e., risk) or progression (i.e., outcomes) remain poorly characterized and are of great interest to the research community. As other recent reviews have examined the broad relationships between obesity, cancer, and the immune system [11, 64–66], this review will focus on the importance of modifying obesity-mediated breast cancer through various methods, focusing on weight loss induced by bariatric surgery. The field has much work ahead to integrate the many complex avenues of crosstalk in obesity and cancer. Still, there is great promise in identifying causal and targetable interactions in the obese tumor microenvironment.
Weight loss and breast cancer
Benefits of weight loss
Obesity is one of the few modifiable breast cancer risk factors [67], and weight loss has been proven to lower the risk of many cancers [68]. The risk reduction is most pronounced with intentional weight loss of ≥ 5% basal body weight among postmenopausal women [69, 70]. To date, most studies examining breast cancer risk are conducted with breast cancer survivors to measure recurrence rates as opposed to naïve patients being diagnosed with breast cancer for the first time. Weight loss improves overall prognosis [71] and reduces the risk of recurrence when combined with increased exercise [72–74], although not every study supports these conclusions [75]. Indeed, among breast cancer survivors, reduction in cancer recurrence resulting from weight loss varies by molecular subtype, though further analysis is needed to describe the differences [76]. Reduced risk of recurrence may be linked to weight loss-associated effects on circulating sex hormone levels [77]. In murine models, weight loss by caloric restriction has proven remarkably successful in reducing breast cancer progression, with intermittent caloric restriction proving more effective for lowering tumor incidence and size than chronic caloric restriction [78, 79]. Likewise, time-restricted feeding or time-restricted eating, also known as intermittent fasting, is successful for weight loss, tumor initiation inhibition, and tumor size reduction in both humans and mouse models [80]. Our group and others commonly use the relatively simple switch in dietary exposure from a high fat to a low fat diet to induce rapid and sustained weight loss [81, 82]. However, a diet switch approach includes both a change in dietary fat exposure and weight loss, which are difficult to disentangle when investigating mechanisms. Overall, findings support that weight loss by various weight loss methods such as dietary changes, caloric restriction, and increased exercise is beneficial in reducing both risk and recurrence, with the impact of bariatric surgery discussed in detail below.
Lifestyle-induced weight loss and breast cancer
Lifestyle-induced weight loss relies on dietary changes, increased exercise, and similar adaptations to daily routines focused on lowering body adiposity and increasing physical activity. The Look AHEAD studies, for example, found that significant weight loss could be achieved and maintained over eight years. However, these results were most feasible when patients received a personalized lifestyle intervention plan and attended frequent check-ins with healthy lifestyle professionals [83]. However, a majority of studies suggest poor adherence to weight loss programs, with significant numbers of patients regaining lost weight or gaining weight over their starting point [84]. As introduced above, many studies on lifestyle-induced weight loss have been performed on breast cancer survivors because these cohorts typically show excellent study retention rates and adherence to lifestyle intervention plans [85]. Indeed, at-home intervention studies and supervised exercise programs have shown healthy changes in dietary behaviors and improved quality of life, along with weight loss and increased physical activity in long-term survivors [86, 87]. With regard to exercise alone, risk in murine models was first examined with supportive evidence showing that exercise reduces breast cancer incidence [88–90]. Indeed, voluntary exercise reduces tumor incidence and growth in mouse models as well as pre-operative breast cancer patients [91, 92].
Like obesity, exercise and dietary changes induce varied and complex benefits that can be linked to reduced cancer risk or progression from immunity to growth factors to adipokines to metabolism. For example, exercise upregulates anti-tumor immunity and downregulates immunosuppressive cells [92]. Others have reported that exercise intervention alone leads to reduced circulating levels of insulin, IGF-1, and leptin, improved natural killer (NK) cell cytotoxicity, or elevated adiponectin in patients [87, 93]. In patients, a healthy diet correlates to a reduction in circulatory estradiol and other sex hormones that promote oncogenesis [94], while fasting was associated with a decreased hemoglobin A1c and reduced risk of breast cancer recurrence [95].
In sum, published work supports the idea that healthy exercise habits and dietary choices are vital to reducing breast cancer risk or recurrence. This research area is expanding, with multiple randomized controlled clinical trials underway [74]. One such study, a large-scale, randomized phase III trial known as the Breast Cancer Weight Loss (BWEL, NCT02750826) trial, will examine the effects of weight loss due to lifestyle changes on disease-free survival among breast cancer patients [96]. Similar lifestyle changes have been shown to improve outcomes for other diseases associated with obesity as a modifiable risk factor, such as type 2 diabetes mellitus and associated cardiovascular disease [97]. Further study must be completed to draw any conclusions about reducing risk or improving survival with specific successful weight loss and lifestyle intervention approaches that are sustainable.
Surgically induced weight loss and benefits
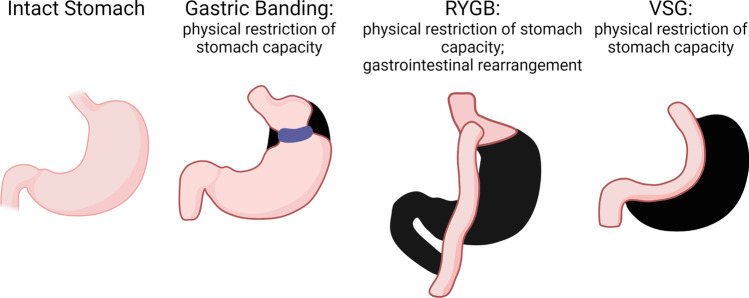
The most effective method for long-term, sustained weight loss in adolescents and adults is bariatric surgery when combined with healthy lifestyle changes [98–102]. Surgical methods for weight loss were first introduced in the 1950s [103] yet did not gain wide prevalence in the USA or worldwide until the 1990s, when obesity rates and associated health conditions had risen to high enough levels to be considered an epidemic by the CDC and the WHO [1, 2, 104]. Historically, bariatric surgeries fall into two categories: restrictive or malabsorptive. Standard procedures performed today include adjustable gastric banding (AGB), vertical sleeve gastrectomy (VSG), and Roux-en-Y gastric bypass (RYGB) [105] (Fig. 1). AGB and VSG are restrictive gastric surgeries that primarily restrict or limit food intake. The resectional nature of VSG is also characterized by increased gastric emptying and hormone changes, leading to sustained, long-term weight loss. The RYGB, in contrast, is both a restriction of stomach size, leaving only a small pouch, as well as a dramatic rearrangement of the gut with impacts on gut peptides and gastric emptying. Gastric banding was widely utilized but has fallen out of favor in the past five years due to adverse side effects, such as failure to sustain long-term weight loss, band slippage, and perforation [106, 107]. The VSG, performed by removing the fundus and greater curvature of the stomach laparoscopically [108], is a technically more straightforward operation than the RYGB. In the USA, the VSG increased in prevalence from 11% to 70% of all bariatric surgeries performed from 2006 to 2015, while RYGB decreased proportionally [109]. While several studies show no significant difference in weight loss achieved and sustained by RYGB and VSG patients [110–112], others have shown that RYGB is more effective at supporting long-term weight loss than VSG [113]. Additionally, several studies have shown RYGB superior to VSG in comorbidity resolution, especially regarding diabetes [114]. However, the decreased level of invasiveness involved in VSG and its lower operative complexity have made it more appealing as an initial approach for many surgeons and patients.
Fig. 1.
Common bariatric surgery approaches. Compared with the normal intact stomach, laparoscopic adjustable gastric banding (AGB) maintains the gastric cavity but restricts the fundus and cardia just below the esophagus to slow the rate of feeding and induce more rapid satiety. AGB was widely utilized but has fallen out of favor in the past 5 years due to adverse side effects, such as failure to sustain long-term weight loss, band slippage, and perforation. Roux-en-Y gastric bypass (RYGB) is both a restriction of stomach size leaving only a small pouch, as well as a dramatic rearrangement of the gut with impacts on gut peptides and gastric emptying. RYGB involves division of the upper gastric cavity from the lower, where the remaining duodenal pancreatic and hepatic secretions are diverted free of digesta and are anastomosed at a distal region of jejunum. Vertical sleeve gastrectomy (VSG) involves removal of the fundus and greater curvature portion of the stomach, leaving a sleeve that connects the esophagus to the duodenum. VSG is characterized by increased gastric emptying and hormone changes
Benefits of bariatric surgery include weight loss as well as many molecular changes that improve health. For instance, RYGB has five principle results, known as the BRAVE effects: bile flow alteration, reduction of gastric size, anatomical gut rearrangement with the altered flow of nutrients, vagal manipulation, and enteric gut hormone modulation [115]. Many of these effects are shared by the VSG procedure, except for anatomical gut rearrangement and possibly vagal manipulation. Both of these procedures are associated with an overall lack of hunger and improved gastric emptying [116]. These principles, primarily modulation of the gut microbiome, hormone signaling, and activity of various metabolic pathways, may be the true causes of both weight loss and many of the beneficial side effects of bariatric surgery, such as reducing comorbidities [116, 117]. Interestingly, in some patients, some benefits are rapidly induced after surgery, indicating they are not necessarily a result of the weight loss per se [118]. Additional mechanisms mediating the benefits of bariatric surgery include changes in endocrine signaling, microbiome, bile acid metabolism, and impacts on neuronal pathways [119–121].
Reduction in all-cause mortality after bariatric surgery ranges widely by study from 30 to 60% [122]. Patients eligible for bariatric surgery are characterized as those with BMI > 40 kg/m2 or 35–39.9 kg/m2 in combination with one or more obesity-associated comorbidities [3, 123]. Bariatric surgery results in sustained weight loss and decreased risk for multiple obesity-associated conditions [114, 124–128]. In the long term, bariatric surgery is also more cost-effective when compared to the financial burden of obesity-associated comorbidities [129], especially for women [130]. Despite these benefits, in 2018, bariatric surgery procedures were undertaken by less than 1% of the eligible population [3, 123]. There are several potential explanations for the low percentage of patients willing to undergo bariatric surgery. Bariatric surgery is an intensive procedure with long-term follow-up and lifestyle changes required. Patients must be healthy enough to experience such a surgery. Public distrust in the safety of such operations persists in the general population, partly because this surgery is intended for communities that are already more vulnerable to adverse complications of surgical procedures [131]. Other significant barriers include a lack of knowledge of the low risk yet high benefit of surgery by both patients and potential referring practitioners, a lack of insurance coverage, especially in public health plans, and the stigmatization of obesity, including the public perception of obesity as a lifestyle choice and not a disease. Currently, mortality rates are very low for all types of bariatric surgery, decreasing tenfold since the 1990s, and fall within accepted operative mortality rates at 0.3% for experienced hospitals [118, 123, 132]. Indeed, compared to the early 1990s, when open surgeries were the primary approach, laparoscopic surgery approaches now dominate bariatric surgery, which both improves outcomes and reduces recovery time [133]. Interestingly, benefits after bariatric surgery appear to be skewed towards women in the current research, as the cancer rates among men remained essentially unchanged [134]. The sex disparity in cancer risk could have biological underpinnings, but it should be noted that more women have undergone bariatric surgery and were available for analysis. Indeed, from 2002 to 2011, 80.7% of bariatric surgery patients in the USA were women [135]. Further study is necessary to determine the impact of bariatric surgery on male cancer risk and outcomes. Future studies are imminent, with the percentage of male patients receiving bariatric surgery increasing [135].
In sum, while bariatric surgeries are greatly increasing in prevalence, research is lagging to fully estimate the benefits of these approaches. As of 2022, 58.8% of studies had a follow-up time of just 1–2 years, which is a limitation to understanding the full impacts of these surgeries [136]. Thus, longer-term studies are needed to examine how and to what extent the underlying mechanisms of the benefits of bariatric surgery persist over many years.
Bariatric surgery and breast cancer
Recent bariatric surgery studies have sparked great interest in the cancer field. Several retrospective studies reported reduced risk for some types of cancer as long-term benefits of bariatric surgery [134, 137, 138]. The decrease in all-cause cancer mortality varies up to 60% [122]. The SPLENDID study (Surgical Procedures and Long-term Effectiveness in Neoplastic Disease Incidence and Death) had a median follow-up of 6.1 years and examines a composite obesity-associated cancer score of 13 cancer types as the primary endpoint. SPLENDID results showed that bariatric surgery was associated with a 32% reduction in obesity-associated cancer and a 48% overall cancer-related mortality [139].
Regarding breast cancer specifically, bariatric surgery reduced breast cancer risk among postmenopausal women compared to non-surgical controls, with the most significant impact on risk reduction in ER-negative tumors, with a 64% decrease in risk [120, 134, 140]. Moderate declines in ER-positive [141] and HER2-positive breast cancer rates were reported [142]. An additional benefit to bariatric surgery is that subsequent cancers appear less aggressive or detected earlier. When breast cancer is diagnosed in patients after bariatric surgery, it is often diagnosed early as stage I with decreased prevalence of stage III or IV breast cancer [143]. Furthermore, bariatric surgery following remission from breast cancer resulted in sustained weight loss comparable to that of patients without a history of cancer. This may lengthen the disease-free survival time for breast cancer survivors [144]. In sum, bariatric surgery is promising for maintaining long-term benefits associated with weight loss with increasing evidence of reduced risk in some cancers, especially obesity-associated cancers. However, the benefits of bariatric surgery may not be so clear-cut. A potential complicating factor to the conclusion that bariatric surgery is protective against all obesity-associated cancers is evidence of an increased risk of colorectal cancer (CRC) after bariatric surgery [145]. Other studies have shown no increased risk of CRC associated with bariatric surgery [146]. This apparent discrepancy in risk of CRC likely reflects how the pathogenesis of cancer is heterogeneous and can be affected by different insults such as IBD, inherited germline mutations, and other factors. Further investigation is required to fully understand the benefits and risks of this life-changing surgery, especially concerning cancer risk of primary tumor development compared to recurrence. Table 1 highlights current clinical trials active or completed during this review.
Table 1.
Clinical trials from clinicaltrials.org. The nine cancer-associated bariatric surgery human clinical trials are summarized by title, status, and conditions examined
| NCT number | Title | Status | Conditions |
|---|---|---|---|
| NCT03946423 | BAriaTric Surgery After Breast Cancer Treatment (BATS) | Not yet recruiting | Early-stage breast cancer|obesity |
| NCT04008563 | B-FiERCE—Bariatric Surgery for Fertility-Sparing Treatment of Atypical Hyperplasia and Grade 1 Cancer of the Endometrium | Not yet recruiting | Endometrial cancer|atypical hyperplasia|bariatric surgery candidate |
| NCT04170335 | Effects of Bariatric Surgery on Breast Density Improvement and Impact on Breast Cancer Risk in Severe Obese Patients | Recruiting | Breast cancer|morbid obesity|bariatric surgery candidate |
| NCT04839614 | Concurrent Laparoscopic Hysterectomy and Weight Loss Surgery in Obese Patients with Endometrial Carcinoma or Endometrial Intraepithelial Neoplasia | Recruiting | Endometrial carcinoma|obesity|EIN|endometrial intraepithelial neoplasia|endometrial cancer stage I |
| NCT04284943 | STARDOM- Surgical TreAtment for Obesity-Related Disease and Onco-Metabolic Surgery | Recruiting | Gastric cancer|diabetes mellitus, type 2 |
| NCT01047735 | TRIABETES- The TRIABETES—ARMMS-T2D Study: A Randomized Trial to Compare Surgical and Medical Treatments for Type 2 Diabetes | Active, not recruiting | Type 2 diabetes mellitus|obesity |
| NCT01922778 | Screening for Endometrial Abnormalities in Overweight and Obese Women | Completed | Endometrial cancer |
| NCT02681120 | Pilot Study of the Effect of Weight Loss on Breast Tissue and Blood Biomarkers in Women at Increased Risk for Breast Cancer | Completed | Breast neoplasms|obesity |
| NCT04345328 | IMPORTUNE- Impact of Bariatric Surgery on the Gut Environment | Completed | Bariatric surgery candidate |
Modeling obesity in various mouse strains
Human cohorts offer insight into the effects of bariatric surgery and weight loss on cancer and other conditions. Still, current studies have been primarily retrospective, and this type of analysis is limited in the questions that can be answered. To be able to examine the mechanisms responsible for the benefits of weight loss, in vivo models are beneficial. Since the mid-1950s, researchers have been developing rodent models to mimic diet-induced obesity (DIO). Naturally occurring mutations in rodents and the development of transgenic mice complement DIO models, but single gene mutant mice are often not the most accurate representation of DIO observed in humans. Therefore, DIO and obesity-related conditions have been induced in murine models for decades by feeding the animals high fat diets; the effects are well-characterized [147, 148]. However, variability in diet, timing, and mouse genetics across studies often leads to inconsistent DIO findings [149].
To best conduct diet studies in pre-clinical models, it is vital to use defined, controlled diets that are matched on important dietary factors such as protein and micronutrient content. A major concern from a nutritional standpoint is that many researchers use chow to keep mice lean as the standard diet control as compared to DIO mice fed a defined high fat diet. This is adequate and understandably cheaper to maintain lean mice, but this approach is not ideal as a proper control. Micronutrients, fiber, and dietary components like phytoestrogens vary from lot to lot in chow, creating extreme variability in studies and introducing mediators that impact cancer outcomes [149]. Murine diets from commercial sources range from 45 to 60% kcal derived from fat, with 10% fat content typically used as the matched control for the high fat diets [149]. The main component in current commercial high fat diets is a high percentage of lard, or pig fat. However, lard is now consumed by Americans at much lower levels than were common decades ago [150, 151]. Still, lard-based diets remain the staple of pre-clinical DIO studies. Some dietary approaches modulate the fatty acid content [152]. Additional methods attempt to mimic the typical American or “Western Diet” more closely with a mixed diet of human foods, called the Cafeteria Diet [153–155], which varies widely from lab to lab. Others add cholesterol to a high fat diet to mimic Western Diet. Therefore, it is essential to note dietary interventions in manuscripts. Fortunately, investigators and journals pay increasing attention to diet details, exposures, and timing of diet intervention, which, taken together, will add to the clarity of our findings.
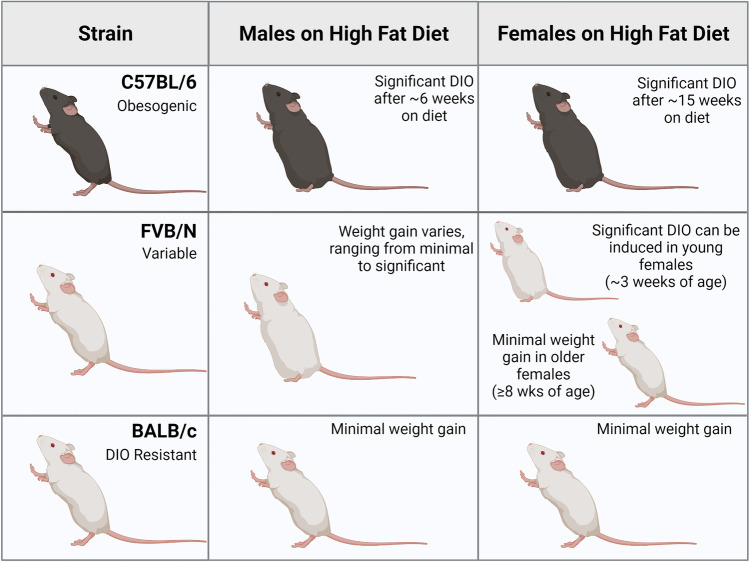
A critical factor in DIO studies is that mouse strains exhibit different extents of weight gain when exposed to high fat diets, just like individuals respond to various diets differently (Fig. 2). C57BL/6 mice are the classic strain used for many DIO studies because both sexes are readily obesogenic. C57BL/6 mice are highly susceptible to weight gain and tend to overeat on high fat diets [156]. Males tend to gain weight faster than females, with the male mice showing significant differences in weight gain between high and low fat diets after 6 weeks of diet, while females require 15 weeks to demonstrate such differences [157]. Notably, there can be variability in the C57BL/6 strain with some mice non-responsive to DIO weight gain. C57BL/6 variability in DIO is often attributed to differences in energy expenditure, ketogenic pathway regulation, or microbiomes [158]. Likewise, care should be taken to consider vendor and colony maintenance for consistency within studies. Mice from Jackson Labs, Inc. (C57BL/6 J) differ genetically from the NIH subline of C57BL/6 N (also sold at Jackson Labs), for example, with J or N in the strain name. The same is true for mice purchased from Taconic Inc. vs. Charles River Labs, Inc vs. Jackson, etc. [159], and the microbiome from these vendors also varies at baseline [160, 161]. Furthermore, mouse microbiomes may change for several reasons post-shipment, including transport from one building to another. These considerations are crucial for microbiome-dependent studies, as reviewed in Hugenholtz and de Vos [162]. Taken together, C57BL/6 mice are highly reliable obesogenic models but careful attention to study design should be implemented before and during DIO studies.
Fig. 2.
Diet-induced obesity varies by strain and sex. The susceptibility of murine models to diet-induced obesity (DIO) varies based on sex and background strain. C57BL/6 animals are highly obesogenic, where both sexes become obese, but males are more responsive to DIO compared to females with a more rapid and greater extent of adiposity observed. FVB/N animals exhibit highly variable weight gain based on the sex, age, and study in response to DIO. BALB/c animals are DIO-resistant regardless of sex or age
FVB/N mice are a strain commonly used for cancer studies but rarely used in DIO studies. In contrast to the C57BL/6 strain, the FVB/N strain shows significantly greater variability in responsiveness to DIO [150]. For example, substantial weight gain in FVB/N males has been published [163, 164]. In contrast, Boudina et al. showed resistance to high fat DIO and weight gain in FVB/N males [165]. Likewise, Devlin et al. reported females with no high fat diet-induced weight gain or increases in adiposity in FVB/N mice carried out to 20 weeks by body mass or body fat quantification [156]. In contrast, Zhu et al. published that a high fat diet following dimethylbenzanthracene (DMBA) exposure resulted in weight gain in FVB/N female mice carried out to 37 weeks of age [166]. Our group has extensive experience with a genetic model of cancer in the FVB/N strain. We reported moderate weight gain and increases in adiposity, with obesity-like detection of crown-like structures in adipose, and elevated obesity-associated endocrine factors, including leptin, using a transgenic breast cancer model in the FVB/N strain (C3(1)-T antigen, or C3(1)-Tag) [81, 82, 167–169]. We reported that obesity-like changes were reduced in C3(1)-Tag FVB/N mice by weight loss once obese mice were switched from a high fat diet to a low fat diet [81, 169]. Age and extent of exposure are critical to consider as well in DIO studies. In one study, young female mice introduced to a high fat diet at around 3 weeks of age gained significant amounts of weight, similar to that of age-matched C57BL/6 mice, when compared to low fat diet controls; however, when a high fat diet was introduced at 8 weeks of age, the response to diet was much less dramatic [170]. Mild weight gain was detected in older FVB/N female mice exposed to a high fat diet [171], but the difference in weight gain between low and high fat diet-fed mice was inconsequential compared to that observed in C57BL/6 female mice at the same age. Thus, FVB/N mice remain relatively controversial, with results across the spectrum of DIO. Depending on the study, FVB/N are generally perceived to be either resistant to DIO, moderately susceptible to becoming overweight but perhaps not frank obesity, or an adequate model for DIO. In sum, the model must be tested in each lab’s specific animal facility with diet details and timing of dietary exposure adequately controlled for adequate determination of DIO in FVB/N mice.
Interestingly, a third strain commonly used in cancer studies is BALB/c. It is well-established that BALB/c mice display minimal to no weight gain on a high fat diet compared to C57BL/6 and FVB/N strains. Indeed, the strain is often termed “obesity-resistant” [172]. In male BALB/c mice, high fat diet induced minimal weight gain but to a far lesser degree than that measured in C57BL/6 mice [173]. However, increases in colon cancer progression and metastases were shown in BALB/c males consuming a high fat diet, suggesting impacts of diet alone in the absence of DIO [174]. High fat diet induced changes in metabolite profiles despite the lack of significant weight gain [175]. In female BALB/c mice, a high fat diet increased tumor progression and metastases following injection with 4T1 breast cancer cells [176, 177], while others have shown that HFD (with no weight gain) did not increase 4T1 progression [178]. Taken together, high fat diet in the DIO-resistant BALB/c needs further study. Like other strains, timing of diet intervention is critical. The introduction of a high fat diet during pubertal mammary gland development, which is 3 to 6 weeks of age, led to weight gain and changes to the mammary duct development in C57BL/6 but not BALB/C female mice of the same age [179]. However, a high fat diet during this pubertal period in BALB/c females reduced carcinogen-induced tumor latency. High fat diet elevated inflammatory factors, supporting the importance of diet exposure in the absence of obesity and understanding critical windows of exposure susceptibility [180]. In sum, the BALB/c model is excellent for studying diet-induced cancer risk or progression but fails to induce frank DIO. Thus, if the intention is to research obesity per se, the BALB/c strain is a poor model, especially when investigating weight loss impacts after dietary interventions or bariatric surgery.
Last, examining patient-derived cell lines or xenografts (PDXs) in the obese setting introduces another layer of complexity. Immunocompromised murine models are necessary to avoid rejection of human cell lines or patient-derived samples. However, there are immunocompromised mouse strains, such as severe combined immunodeficiency (SCID) mice [181], that may be susceptible to DIO, including obesogenic (C57BL/6 J or C3H/HeJ), or DIO resistant (BALB/cBy), or NOD/ShiLtSz background, which is variable in response to HFD depending on background [182, 183]. NSG mice are from Jackson Labs on the background NOD/ShiLtJ and are commonly used in PDX studies, but their response to HFD appears variable. These strains are just some of the available options for PDX studies. The genetic background of the strain used must be noted and DIO responsiveness carefully considered based on the research question.
Overall, numerous studies use the three primary mouse strains discussed here, C57BL/6, FVB/N, and BALB/c, as models of DIO and diet-induced weight gain or resistance. There are multiple preclinical models of DIO to examine cancer risk and progression using carcinogen initiation, transgenic oncogene expression, syngeneic cell implantation, and more, which will be highly informative to the field. Primary study endpoints include obesity per se, specific dietary impacts, and the extent of weight gain or adiposity impacting cancer outcomes. The intention of the study design should inform model selection.
Modeling bariatric surgery in preclinical setting
DIO and dietary interventions to induce weight loss, such as changing diet, timed feeding, fasting, and caloric restriction, are increasingly common in cancer outcomes studies. However, examining bariatric surgery after DIO is a little studied area that holds great interest based on the epidemiological findings about bariatric surgery and cancer risk discussed above. One major limiting factor is the skill set necessary to perform these complex, expensive, and time-consuming surgeries. Despite the technical challenges of mouse survival surgery, multiple groups have employed surgical models throughout the last decade to examine a wide range of obesity-associated conditions ranging from diabetes to cardiovascular disease and even gut-brain crosstalk. This field is growing immensely with over 170 publications on PubMed in the last year alone using rodent models of bariatric surgery. A selected subset of manuscripts published since 2010 are summarized in Table 2.
Table 2.
Rodent models of bariatric surgery highlighted in this review. The results of 20 preclinical studies from 2010 to 2021 are summarized based on the rodent model, sex included, type of bariatric intervention, primary outcomes, and study results [184–187, 189–194, 196–199, 202, 203, 205, 206, 312, 313]
| Study | Model | Sex | Surgical techniques | Primary outcomes | Study results |
|---|---|---|---|---|---|
| Stepfather et al. 2010 | Long-Evans rats | Male, female | VSG | Weight loss | Weight loss is not sustained over time |
| Chambers et al. 2011 | Long-Evans rats | Male | RGB, VSG | Weight loss and glucose regulation | Both methods had similar weight loss and improvement in glucose regulation |
| Yin et al. 2011 | C57BL/6, FVB/N mice | Not specified | Gastric banding, VSG, RYGB, modified RYGB, biliopancreatic diversion | Comparison of weight loss induced by multiple bariatric surgery procedures | RYGB and biliopancreatic diversion are the most effective but also have the most side effects |
| Asian et al. 2012 | Long-Evans rats | Female | RYGB | Effects of estradiol on weight loss and efficacy of satiation signaling | Estradiol increases weight loss and satiation signaling |
| Yin et al. 2012 | C57BL/6 mice | Not specified | Duodenal-jejunal bypass, gastric banding, VSG, modified RYGB, biliopancreatic diversion | Comparison of weight loss induced by multiple bariatric surgery procedures | Duodenal-jejunal bypass is more effective than VSG, but more side effects |
| Brinckerhoff et al. 2013 | Sprague Dawley rats | Female | VSG | Weight loss, adiponectin, and leptin levels | VSG results in weight loss and improvement of adiponectin and leptin levels |
| Grayson et al. 2013 | Long-Evans rats | Female | VSG | Effects on the female reproductive cycle and their offspring | VSG improved the reproductive process, but offspring on HFD were predisposed to glucose intolerance and obesity |
| Liou et al. 2013 | C57BL/6 J mice | Male | RYGB | Adiposity and gut microbiome composition | Shifts in microbiome composition contribute to decreased adiposity |
| Saedi et al. 2013 | Long-Evans and Goto-Kakizaki rats | Male | RYGB | Weight loss, glucose metabolism | RYGB in rodents improves glucose metabolism as seen in diabetic humans |
| Ryan et al. 2014 | C57BL/6 J FXR KO and W.T. mice | Male | VSG | Effects of FXR signaling on feeding, glucose tolerance, and gut microbiome composition | Bile acid signaling plays a critical role in modulating the results of VSG surgery |
| Hao et al. 2016 | C57BL/6 J mice | Male | RYGB | Body weight, composition, food intake, and energy expenditure | Weight loss is achieved through increased energy expenditure, not decreased food intake |
| Frikke-Schmidt et al. 2017 | C57BL/6 J mice | Male | VSG | Adipocyte tissue leukocyte profile | VSG causes changes in adipose immune populations, regardless of weight loss |
| Grayson et al. 2017 | Long-Evans rats | Male, female | VSG | Sex differences in fat loss, hepatic gene expression, and cholesterol metabolism | Males see a reduction in hepatic lipids while females see an increase in the hepatic inflammatory pathway; VLDL was decreased in females alone |
| Hao et al. 2017 | C57BL/6 J mice | Male | RYGB, VSG | RYGB vs. VSG in weight loss, food intake, energy expenditure, and glycemic control | RYGB is more effective in inducing weight loss and improving glycemic control than VSG |
| Spann et al. 2018 | Long-Evans rats | Female | VSG | Effects of VSG on DIO dams | VSG reduces T cell populations, increases IL-1β, and increases placental permeability contributing to fetal demise |
| Spann et al. 2019 | Long-Evans rats | Female | VSG | Effects on the immune system of offspring | The immune system is compromised early in life but rebounds after weaning |
| Ye et al. 2020 | C57BL/6 J mice | Male | RGB, VSG | Changes in energy balance and browning of visceral fat | RYGB specifically increases resting metabolic rate and fat browning compared to VSG |
| Chaudhari et al. 2021 A | C57BL/6 J mice | Male | VSG | Changes in bile acid levels, glucose metabolism | VSG causes an increase of a TGR5 agonist, indicating the role of bile acid signaling |
| Chaudhari et al. 2021 B | C57BL/6 J mice | Male | VSG | Changes in the gut microbiome, bile acid levels, changes in bile acid signaling | VSG induces microbiome shifts that increase bile acid signaling, and this can be replicated in germ-free animals following FMT from VSG animals |
| Stevenson et al. 2021 | C57BL/6 mice | Male | RYGB, VSG | Post-op effects of HFD on adipose tissue composition, weight alterations, and metabolic dysregulation | RYGB causes lasting improvement in these outcomes, while VSG is comparable to sham control |
Both rat and mouse models have been used to examine the effects of surgical weight loss following DIO. In 2010, Stefater et al. described an early method for reviewing the weight loss induced by bariatric surgery in both male and female rats. They showed that in the long term, weight rebounded due to changes in eating habits resulting in a lack of changes in overall calorie intake [184]. The same group then compared the efficacy of RYGB against VSG in Long-Evans rats; males were fed a 41% high fat, butter oil-based diet and subjected to RYGB, VSG, or accompanying sham procedures to show that both RYGB and VSG resulted in similar patterns of weight loss and glucose regulation [185]. Later work by Saeidi et al. demonstrated that the improvements to glucose metabolism observed in humans with diabetes are recapitulated in rats following RYGB [186].
In 2011, Yin et al. developed five types of bariatric surgery in C57BL/6 and FVB/N mice to treat DIO resulting from a 60% high fat diet [187]. This study was likely conducted in males, though not specified. The surgical models included gastric banding, sleeve gastrectomy, RYGB, a modified RYGB better adapted to mouse survival, and biliopancreatic diversion [187]. Biliopancreatic diversion combines sleeve gastrectomy with an intestinal bypass in a highly invasive method previously reserved for individuals with BMI > 50 kg/m2 [188]. Recent innovations to decrease complexity make this procedure more attractive to patients and surgeons. Although biliopancreatic diversion and RYGB resulted in the most pronounced weight loss, a higher risk of side effects, such as anemia, was observed in the mice undergoing these procedures, which mirrors the results in patients. Further development in 2012 led to the implementation of the duodenal-jejunal bypass procedure in mice, which involves the removal of the duodenum from the gastrointestinal tract performed on some patients [189]. Like RYGB and biliopancreatic diversion, the duodenal-jejunal bypass procedure also showed worsened side effects for the mice than the sleeve gastrectomy. Despite the concerns for anemia, multiple studies have successfully used the RYGB surgical approach in preclinical models to examine its effects on estradiol metabolism, body composition, and the nervous system [190–192]. More recent studies have used C57BL/6 mice to compare the efficacy of RYGB versus VSG as it relates to sustained weight loss and glycemic control [193–195], with RYGB producing better results. However, the VSG was effective with significant weight loss with minimal side effects, and it is often used today in preclinical studies. Interestingly, VSG can also alter the immune systems of male C57BL/6 mice in a weight-independent manner [196], and many of the surgery results are influenced by bile acid signaling [197–200].
A significant factor to consider in interpreting studies, especially about cancers primarily or solely impacting women, such as breast, ovarian, or endometrial cancer, is that most published DIO bariatric surgery studies are completed in male rodents. There are several pros to using male rodents. First, male rodents gain much more weight on a high fat diet and gain that weight faster than females, as noted above. Second, large commercial labs like Jackson Labs, Inc. sell obese males on a high fat diet which makes completing DIO studies much more rapid and feasible. Unfortunately, Jackson Labs does not provide the same DIO service for female mice. This means that each study on female mice must be completed in house over many months to induce DIO. Third, since most publications use male mice, it is possible to compare ongoing studies to previously published work. A significant con when studying diseases specific to females is that females do not gain as much weight as males. To induce sufficient DIO, researchers must maintain mice on a high fat diet for extended periods, often 4–5 months. Some researchers remove the ovary to cause weight gain, but ovariectomy is used to generate a postmenopausal state, which may not be ideal for what the study design intends. Likewise, since Jackson Labs does not stock DIO females, responding to reviewers or implementing new therapies in DIO mice takes a long time. These long-term studies to induce DIO in female mice are costly. Furthermore, female DIO studies typically need larger sample sizes (N) to have sufficient statistical power due to lower weight gain compared to low fat diet control. Last, there are fewer established experimental paradigms in the literature to aid in study design or compare ongoing studies to existing literature. Yet including female DIO studies is imperative for reasons detailed below, where we focus on further studies regarding the VSG.
As of 2010, women were more likely to undergo bariatric surgery than men, as discussed above [201]. However, a model to study bariatric surgery on female rodents with DIO was yet to be published at that time. In 2013, Brinckerhoff et al. published that female Sprague Dawley rats fed a 60% high fat diet that underwent VSG causing weight loss, reducing leptin levels, and improving adiponectin concentrations compared to sham surgery controls [202]. Grayson et al. demonstrated that VSG improves many of the comorbidities of metabolic syndrome in female Long-Evans rats [203], just as had been previously reported in males. However, this group later showed that females and males respond differently to the surgery, with females showing changes in the regulation of lipid metabolism genes that are not observed in male rats [204]. Spann et al. also performed VSG on Long-Evans female rats fed a 40% high fat diet to examine the effects of bariatric surgery on the immune systems of mother and offspring [205, 206]. Mothers with VSG-induced reversal of DIO had lower levels of circulating T cells and increased placental permeability, ultimately leading to a high percentage of fetal demise [205]. Surviving offspring of female dams with previous VSG experienced early decreases in immune competency before rebounding later in life [206]. VSG on rats is easier simply due to the large size of the animal, but bariatric surgery on female mice has also been conducted. We have reported in C57BL/6 J female mice, that DIO induced significant weight gain, increased adiposity, and leptin compared to lean low fat fed controls, which was lost in DIO mice after VSG [207]. In our study, we aimed to mimic epidemiologic findings of reduced breast cancer risk after bariatric surgery in patients discussed above. We reported that DIO induced breast cancer progression using a syngeneic transplant model. Importantly, after weight loss by VSG, tumor progression was reduced. We showed that responsiveness to immune checkpoint blockade (ICB) immunotherapy was increased after VSG, but not in DIO mice, suggesting unique changes after bariatric surgery that primed for elevated efficacy of therapy [207]. In sum, despite limitations of extended time and costs using female mice to reach the extremes of obesity on a high fat diet compared to male mice, the use of female mice in DIO studies undergoing bariatric surgery is increasing. However, studies involving female models of bariatric surgery are still in the minority, comprising only 19% of the papers published on PubMed at the time of this review. With increasing interest from the National Cancer Institute (NCI) in benefits of bariatric surgery on cancer risk and outcomes, it is likely that increasing bariatric surgery studies on both male and female pre-clinical models are imminent.
Mechanisms of improved metabolic outcomes associated with surgical weight loss
Bariatric surgery and metabolic signaling
Obesity is a mediator of dysfunction in normal adipose tissue, mammary gland fat pads, or the breast [9, 208–212]. Of this dysfunction, there are three common mechanisms related to obesity-associated cancer risk: impaired insulin and metabolic signaling, altered sex hormone metabolism, and dysregulated inflammatory conditions [213]. Bariatric surgery and other weight loss methods may impact each of these mechanisms, though the mechanistic details are under active investigation [214]. It is currently unclear whether certain benefits of bariatric surgery are associated with the surgery itself or the resulting weight loss.
Increased insulin sensitivity was noted as a beneficial outcome of bariatric surgery as early in the development and characterization process as 1949 [215]. Further investigation has shown that changes to insulin sensitivity are merely a single component in the more notable changes to glucose metabolism correlated with bariatric surgery that is highly beneficial to reversing some of the more commonly obesity-associated comorbidities: type 2 diabetes mellitus, hyperlipidemia, and hypertension [118]. As intestinal hormones are stimulated following bariatric surgery to enhance insulin secretion, metabolic regulation is shifted to improve glucose metabolism [215]. Furthermore, increased insulin sensitivity is linked to shifts in immune phenotype, promoting anti-inflammatory aspects of immunity such as Th2 differentiation and systemic decrease in reactive oxygen species (ROS) [216]. These shifts in ROS production are significant in terms of cancer risk and promotion; ROS is a known inducer of DNA damage and genetic instability (discussed below). Likewise, insulin resistance has been connected to the development and prognosis of breast cancer [217–219]. Therefore, surgery-associated reductions in glucose and insulin, improvements in insulin sensitivity, and impacts on various pathways, including immune and oxidative stress, are mediators of the metabolic benefit associated with bariatric surgery. Recent reviews have reported on further potential mechanisms such as incretin hormone responses, bile acid and bile acid receptor signaling, and microbiota changes [119, 220, 221], not discussed in detail herein.
Leptin is an adipokine released by adipocytes involved in a range of cellular functions, from proliferation and angiogenesis to differentiation and inflammation [219, 222]. Increased serum levels have been associated with breast cancer occurrence and tumor aggression across all subtypes [222, 223], primarily in obese and/or postmenopausal women [224]. Leptin promotes tumor initiation, development, proliferation, and metastasis through various signaling mechanisms [225, 226]. Leptin also induces ROS production by activating NADPH oxidases and influences the production of pro-inflammatory cytokines [227]. Therefore, decreased leptin is an essential mediator of beneficial outcomes associated with bariatric surgery and reduced adiposity [184, 202].
Another obesity-associated factor impacted by bariatric surgery is estrogen signaling. Increased levels of white adipose tissue (WAT) are accompanied by increased aromatase activity [219]. Enhanced aromatase activity results in the rise of circulating sex hormones such as estradiol and other estrogens. As mentioned previously, weight loss by lifestyle intervention has been shown to reduce these circulating levels [77, 94], as does bariatric surgery-induced weight loss [228]. Lowered estrogen exposure reduces the risk of developing ER-positive breast cancer [225]. However, a concern after bariatric surgery is decreased bone density, which could also be attributed to reduced estrogen levels [229, 230]. Therefore, bariatric surgery-induced weight loss and reductions in adiposity would be beneficial in reducing overall estrogen concentrations, but this reduction comes with potential side effects that must be monitored. Interestingly, as noted above, the most significant impact on risk reduction after bariatric surgery was in ER-negative patients where estrogen signaling is not occurring in tumors due to low or absent ER expression. These complex interactions must be further studied to understand the benefits of bariatric surgery.
Bariatric surgery and the immune system
Excess amounts of WAT have a remarkable impact on the immune system systemically and in tissues such as adipose depots [231–234]. Obesity impacts various immune cells, altering the proliferation of resident cells or recruiting them to adipose depots such as the epithelium surrounding the mammary gland or the mammary fat pad, resulting in an imbalance of pro-inflammatory and regulatory or anti-inflammatory mechanisms that can impair protective immunity to increase cancer risk and progression [231, 232, 235, 236]. As the extent of WAT increases and adipocytes expand with triglyceride storage, oxygen requirements exceed availability, leading adipocytes to become hypertrophic and undergo apoptosis [237]. These elevated levels of adipocyte expansion and cell death trigger innate immune responses and stimulate a pro-inflammatory immune state [238]. In the past two decades, the increase in our understanding of myeloid lineages, tissue-specific cell phenotypes, and innate and adaptive immune cell changes between preclinical models and humans has been immense [232]. The current understanding is that low-level, chronic (“smoldering”) inflammation resulting from obesity can cause oxidative stress, lipid peroxidation, and DNA damage as well as poor DNA repair, which results in genetic instability [67, 239, 240]; together, these factors can predispose cells to cancer initiation. Genetic instability (i.e., DNA integrity and stability) is necessary for tumorigenesis [208].
However, a sometimes-complicated notion is that obesity also leads to compensatory immunosuppressive mechanisms to avoid a constant, full-blown pro-inflammatory response, similar to controlling any immune reaction, to maintain homeostasis. These mechanisms include reduced absolute numbers of activated CD8 + T cells, T cell suppression by PD-1 [241, 242], T cell exhaustion [243], and dysfunctional NK cells [244]. Thus, obesity induces a complex and dynamic state of pro-inflammatory immune cell content and function with an influx of monocyte-derived “M1”-like macrophages, combined with reductions in immunomodulatory/regulatory or dysfunctional immune cells such as regulatory T cells (Tregs), “M2”-like macrophages, or NK Cells, together with an induction of checkpoint ligands (discussed below) [245–248], and elevated immunosuppressive cells such as immature monocytes or myeloid-derived suppressive cells (MDSCs) [249–253]. Peripheral Treg levels are reduced in correlation with increasing adiposity in humans [254, 255]. Fat resident Tregs, however, are a specific subtype of Treg cells that are abundant in adipose tissue [256], are increased in obesity [254], and likely perpetuate the obese phenotype. Paradigm shifting work described the importance of myeloid cells in adipose tissue which established adipose tissue and not merely a storage depot but an active immune depot. Macrophage infiltration to WAT was reported in back-to-back seminal publications in 2003 by Xu and Weisberg [257, 258]. Over the past two decades, a large body of work has shown that macrophages in obese adipose display a mixed phenotype that is dependent upon the extent and duration of obesity, specific adipose depot, site of origin of myeloid precursor, and severity of insulin resistance [259, 260]. Together, obesity’s chronic inflammation may increase the risk of immune-associated conditions such as cancer while suppressing levels of immunity that may protect against these conditions, or failed protective immunity.
Since obesity induces a pro-oncogenic state, it follows that weight loss may improve or reverse effects. Weight loss, specifically bariatric surgery, has been shown to reverse many of the impacts of obesity on the immune system, particularly low-grade inflammation and oxidative stress in both mouse models [261] and human studies [262–264]. Data on weight loss-induced changes to immunity after bariatric surgery is emerging. There is evidence that leukocyte infiltration of adipose tissue remains elevated above baseline for as long as 12 months following bariatric surgery in patients [265]. However, others have reported that surgically induced weight loss was shown to reverse this enrichment 3 months after gastric bypass in a human cohort [266]. Further, bariatric surgery decreased monocyte content and shifted circulatory T lymphocytes from Th2 to more pro-inflammatory Th1 [262, 267, 268]. Bariatric surgery has been indicated to restore pre-obesity ratios of M1/M2-like macrophages [269]. This polarization shift may be related to weight loss-induced decreases in the expression of molecules involved in macrophage chemotaxis and relief of the tissue hypoxia commonly found in obese patients [270]. Multiple studies have also described tissue-specific shifts in neutrophil populations, with increased neutrophils found in splenic and adipose tissue after bariatric surgery [265, 271–273]. Considerable work has been done to examine the effects of obesity, weight loss, and bariatric surgery on the B cell compartment to restore obesity-associated dysfunction [272–277]. With regard to cancer risk, studies must understand how bariatric surgery will impact specific immune subtypes, which depots (circulating, WAT, breast/mammary gland, or other tissues) will be affected, and how long these changes will persist after surgery. The advances in flow cytometry, reduced RNA sequencing costs, and increasing single-cell sequencing with advanced bioinformatics will transform our understanding of changes to immune cells with weight loss by bariatric surgery to help inform our understanding of cancer risk and impacts on cancer progression.
Another factor that impacts the immune system is the gut or extra-intestinal microbiome [119]. Bariatric surgery has been shown to increase the gut microbial richness and diversity, which is lost in obesity, though not increased to the levels observed in lean patients [278]. In this aspect, not all bariatric surgeries are created equal, with RYGB exhibiting more substantial alterations than gastric banding procedures [279]. Taking probiotics may help enhance the effects of bariatric surgery on microbiome richness. However, probiotics have only been examined in a few studies showing conflicting results between RYGB, where an improvement of microbiome diversity was reported following probiotic treatment, and VSG, which saw no such effects [280]. The increase in diversity may result from reduced comorbidities allowing for discontinuance of some medications [278] or the dietary and lifestyle changes encouraged in post-surgery patients [280, 281]. One study in a rat model observed that the changes in the microbiota occurred independently of weight loss following RYGB, indicating that other mechanisms beyond obesity and weight loss may be at work [282]. The impact of the gut and extra-intestinal microbes on cancer is a rapidly emerging field, mainly because gut microbes associate with and impact the response to immune checkpoint inhibitors [283–289]. Microbes and their microbially derived peptides and metabolites affect the enteric and systemic immune system, including the tumor microenvironment; the role of bariatric surgery, adaptive and innate immunity, and changes in the microbiome are areas of active study.
Overall, bariatric surgery may decrease the expression of pro-inflammatory cytokines and shift the phenotype of adaptive and innate immune cells towards the anti-inflammatory Th2 cells or M2-like phenotypes. This shift from a pro-inflammatory state to a regulatory state benefits tissue homeostasis. It reduces levels of cytokines and growth factors, improving comorbidities associated with obesity, such as insulin resistance. However, this loss of pro-inflammatory cells and increased regulatory cells is an apparent recipe for failed protective immune surveillance and increased cancer risk or progression through weak anti-tumor immunity. Yet, in long-term retrospective studies detailed above, epidemiologic evidence suggests the opposite: bariatric surgery reduces subsequent cancer risk. This implies that multiple factors associated with weight loss impact cancer risk. It is currently unclear as to whether the reversal of immune-mediated changes is entirely due to weight loss or instead due to the impacts of bariatric surgery, which are currently under active study. The investigation of alterations to metabolic pathways (i.e., reduced insulin, leptin, and estrogen) and varied immune cells, phenotypes, microbiome, and changes with time after bariatric surgery with weight loss are critical to examine.
Bariatric surgery and immunotherapy
Obesity serves as a unique challenge for immunotherapy. Current strategies in immunotherapy target immunosuppressive proteins such as PD-1, PD-L1, and CTLA-4, among others. Immune checkpoint proteins are immune-inhibitory and depend on the crosstalk between the cancer cells, T cells, MDSCs, tumor-associated macrophages (TAMs), NK cells, and more [246, 290–294]. For the past decade, immune checkpoint blockade (ICB) has been a revolutionary intervention in some cancers such as melanoma, with just moderate effects in others, which is often due to the “cold” or immune excluded nature of some tumor microenvironments. Indeed, PD-1 and PD-L1 expression is correlated with worse outcomes in breast cancer patients [290, 295–302]. However, greater expression of these immune checkpoint proteins is also associated with improved responsiveness to immunotherapy. For example, combination therapy of nab-paclitaxel with ICB atezolizumab (anti-PD-L1 treatment) was efficacious for a minority of TNBC patients exhibiting high PD-L1 expression [303].
A limiting factor with ICB therapy is toxicity, including immune-related adverse events (irAEs) [66, 304]. Activation of the immune system by ICB in the obese state, which is already associated with inflammation, must be administered carefully to avoid exacerbating inflammatory mediators to inducing therapy-limiting complications [305]. In one study, a model of obese mice reported evidence of lethal cytokine storms in response to immunotherapy treatment. However, this study was not a cancer immunotherapy study [306]. Indeed, published studies in murine models have not demonstrated elevated lethality in obese mice [207, 307].
In fact, in obese patients, obesity has proven favorable in ICB treatment, as reviewed previously [65, 308]. PD-1 and PD-L1 expression or ligand-positive immune cells are increased by obesity, which likely allows for better efficacy of anti-PD-L1 treatments [245–247]. Obesity improves responsiveness to immunotherapy in melanoma and other cancers, but has not been reported for breast cancer [309–311]. One potential underlying mechanism is elevated inflammatory cytokines in obesity that stabilize PD-L1 or PD-1 [207, 248]. Thus, inflammation associated with the obese tumor microenvironment may synergistically increase PD-L1 or PD-1. We have begun to investigate the impact of VSG on breast cancer and immunotherapy. VSG itself induced PD-L1 which allowed for highly effective anti-PD-L1 immunotherapy in pre-clinical studies [207]. How obesity and weight loss, specifically by bariatric surgery, impacts the tumor microenvironment and systemic anti-tumor immunity, especially immune checkpoint ligands and immune cells, and response to ICB is under active investigation.
Conclusions and future directions
With this review, we have highlighted the complexity of obesity’s impacts on risk and prognoses in epidemiological studies, preclinical models, and clinical trials. Obesity is associated with chronic low-level inflammation and hypoxia that can lead to genomic instability, elevated production of growth factors and adipokines such as estrogen and leptin, altered microbiota, and pro-inflammatory signaling combined with elevated immunosuppression. In general, obesity elevates cancer risk, which is highly consistent in postmenopausal breast cancer studies. Impacts on premenopausal breast cancer risk in different studies are varied from elevated risk of obesity to null to protective, likely depending on breast cancer subtype or methods to measure obesity. As specific subtypes and a more detailed evaluation of obesity metrics with the inclusion of menopausal status, race, and ethnicity are undertaken, we believe that the picture will become more evident. It is certain that obesity negatively impacts patient outcomes, recurrence, and survival in most settings. Weight loss before cancer onset or after treatment is beneficial. These benefits of weight loss are summarized in Fig. 3. While lifestyle interventions can reduce obesity and overweight, they often do not reach the critical levels necessary to induce positive changes and are difficult to maintain. Recent evidence suggests a long-term benefit of bariatric surgery that extends to greatly reduced cancer risks. Preclinical models, while sometimes variable, are beginning to disentangle the potential underlying mechanisms associated with dietary or bariatric surgery-associated weight loss. The impact of therapy, such as immunotherapy, on obesity-driven cancers and outcomes after lifestyle-associated weight loss or bariatric surgery is only beginning to be examined in patient populations and using preclinical models by our group and others. A deeper understanding of the immune cells, tissue signaling cascades, and cellular mechanisms underpinning these risks will lead to the identification of new targeted interventions aimed at improving outcomes. Future studies will ideally provide insight into biomarkers of obesity, immunosurveillance, and protective immunity for those at greater risk of cancer or point towards novel directions to improve therapeutic approaches in cancer patients.
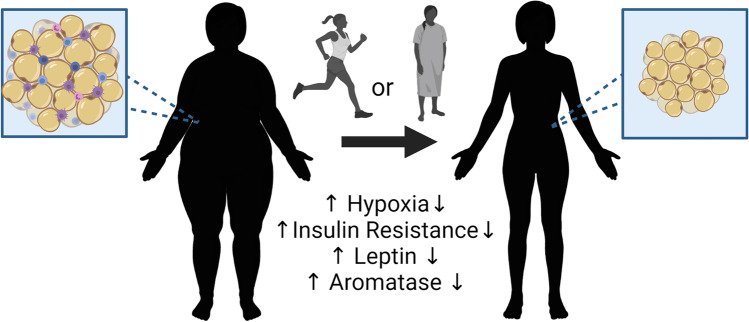
Fig. 3.
Benefits of weight loss by lifestyle or surgical intervention. The benefits associate with weight loss through lifestyle modifications or bariatric surgery include alterations in the mammary adipose with decreased hypoxia, insulin resistance, leptin release, and aromatase expression. These changes are associated with alterations in resident and non-resident immune cell populations that may influence the risk of onset and progression in the setting of breast cancer
Acknowledgements
We would like to acknowledge Boston W. Simmons for his help in editing this manuscript.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Organization, W.H. Controlling the global obesity epidemic. [Webpage] [cited 2022 01/28/2022]; Available from: https://www.who.int/activities/controlling-the-global-obesity-epidemic.
- 2.Services, U.S.D.o.H.a.H., The Surgeon General’s call to action to prevent and decrease overweight and obesity. 2001, Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General. [PubMed]
- 3.Altieri MS, et al. Examining the rates of obesity and bariatric surgery in the United States. Obesity Surgery. 2021;31(11):4754–4760. doi: 10.1007/s11695-021-05628-y. [DOI] [PubMed] [Google Scholar]
- 4.Prevention, C.f.D.C.a. Defining adult overweight & obesity. Overweight & obesity 2022 June 3, 2022; Available from: https://www.cdc.gov/obesity/basics/adult-defining.html.
- 5.Flegal KM, et al. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dietze EC, et al. Triple-negative breast cancer in African-American women: Disparities versus biology. Nature Reviews Cancer. 2015;15(4):248–254. doi: 10.1038/nrc3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plank-Bazinet JL, et al. A report of the Women’s Health Congress Workshop on the health of women of color: A critical intersection at the corner of sex/gender and race/ethnicity. Journal of Women's Health (Larchmt) 2016;25(1):4–10. doi: 10.1089/jwh.2015.5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staiano AE, et al. Uncovering physiological mechanisms for health disparities in type 2 diabetes. Ethnicity and Disease. 2015;25(1):31–37. [PMC free article] [PubMed] [Google Scholar]
- 9.Sundaram S, Johnson AR, Makowski L. Obesity, metabolism and the microenvironment: Links to cancer. Journal of Carcinogenesis. 2013;12:19. doi: 10.4103/1477-3163.119606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutaria S, et al. Is obesity associated with depression in children? Systematic review and meta-analysis. Archives of Disease in Childhood. 2019;104(1):64–74. doi: 10.1136/archdischild-2017-314608. [DOI] [PubMed] [Google Scholar]
- 11.Perry RJ, Shulman GI. Mechanistic links between obesity, insulin, and cancer. Trends Cancer. 2020;6(2):75–78. doi: 10.1016/j.trecan.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calle EE, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. New England Journal of Medicine. 2003;348(17):1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 13.Lauby-Secretan B, et al. Body fatness and cancer—Viewpoint of the IARC working group. New England Journal of Medicine. 2016;375(8):794–798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clinton SK, Giovannucci EL, Hursting SD. The World Cancer Research Fund/American Institute for Cancer Research third expert report on diet, nutrition, physical activity, and cancer: Impact and future directions. Journal of Nutrition. 2020;150(4):663–671. doi: 10.1093/jn/nxz268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reeves GK, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: Cohort study. BMJ. 2007;335(7630):1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Picon-Ruiz M, et al. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA: A Cancer Journal for Clinicians. 2017;67(5):378–397. doi: 10.3322/caac.21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Association, A.M. AMA adopts new policies on second day of voting at annual meeting. 2013; Available from: http://www.ama-assn.org/ama/pub/news/news/2013/2013-06-18-new-ama-policies-annual-meeting.page.
- 18.Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362(9382):419–427. doi: 10.1016/S0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 19.Bliss, J.M. & Gray, R. (2003) Breast cancer and hormone-replacement therapy: The Million Women Study.Lancet 362(9392):1328–9; author reply 1330–1. [DOI] [PubMed]
- 20.DeSantis C, et al. Breast cancer statistics, 2011. CA: A Cancer Journal for Clinicians. 2011;61(6):409–418. doi: 10.3322/caac.20134. [DOI] [PubMed] [Google Scholar]
- 21.Jemal A, et al. Global cancer statistics. CA: A Cancer Journal for Clinicians. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 22.Ligibel, J.A., et al. (2014). American Society of Clinical Oncology position statement on obesity and cancer.Journal of Clinical Oncology. [DOI] [PMC free article] [PubMed]
- 23.Marsden J, A'Hern R. The Million Women Study and breast cancer. British Menopause Society Journal. 2003;9(3):95–97. doi: 10.1177/136218070300900302. [DOI] [PubMed] [Google Scholar]
- 24.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: A Cancer Journal for Clinicians. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 25.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: A Cancer Journal for Clinicians. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 26.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: A Cancer Journal for Clinicians. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 27.Carey LA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 28.Keum, N., et al. (2015). Adult weight gain and adiposity-related cancers: A dose-response meta-analysis of prospective observational studies. Journal of the National Cancer Institute 107(2). [DOI] [PubMed]
- 29.Lega, I.C. & L.L. Lipscombe, (2020). Review: Diabetes, obesity, and cancer-pathophysiology and clinical implications.Endocrine Reviews 41(1) [DOI] [PubMed]
- 30.Xia X, et al. Body mass index and risk of breast cancer: A nonlinear dose-response meta-analysis of prospective studies. Science and Reports. 2014;4:7480. doi: 10.1038/srep07480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Premenopausal Breast Cancer Collaborative Group et al. Association of body mass index and age with subsequent breast cancer risk in premenopausal women. JAMA Oncology. 2018;4(11):e181771. doi: 10.1001/jamaoncol.2018.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.His M, et al. Anthropometry, body shape in early-life and risk of premenopausal breast cancer among Latin American women: Results from the PRECAMA study. Science and Reports. 2020;10(1):2294. doi: 10.1038/s41598-020-59056-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tran TXM, et al. Association of the interaction between mammographic breast density, body mass index, and menopausal status with breast cancer risk among Korean women. JAMA Network Open. 2021;4(12):e2139161. doi: 10.1001/jamanetworkopen.2021.39161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schairer C, et al. Risk factors for inflammatory breast cancer and other invasive breast cancers. Journal of the National Cancer Institute. 2013;105(18):1373–1384. doi: 10.1093/jnci/djt206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodwin PJ, et al. Insulin- and obesity-related variables in early-stage breast cancer: Correlations and time course of prognostic associations. Journal of Clinical Oncology. 2012;30(2):164–171. doi: 10.1200/JCO.2011.36.2723. [DOI] [PubMed] [Google Scholar]
- 36.Jiralerspong S, Goodwin PJ. Obesity and breast cancer prognosis: Evidence, challenges, and opportunities. Journal of Clinical Oncology. 2016;34(35):4203–4216. doi: 10.1200/JCO.2016.68.4480. [DOI] [PubMed] [Google Scholar]
- 37.Lohmann AE, et al. Association of obesity with breast cancer outcome in relation to cancer subtypes: A meta-analysis. Journal of the National Cancer Institute. 2021;113(11):1465–1475. doi: 10.1093/jnci/djab023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pischon T, et al. Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC) Journal of the National Cancer Institute. 2006;98(13):920–931. doi: 10.1093/jnci/djj246. [DOI] [PubMed] [Google Scholar]
- 39.Benefield HC, et al. Epidemiology of basal-like and luminal breast cancers among black women in the AMBER Consortium. Cancer Epidemiology, Biomarkers and Prevention. 2021;30(1):71–79. doi: 10.1158/1055-9965.EPI-20-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bandera EV, et al. Obesity, body fat distribution, and risk of breast cancer subtypes in African American women participating in the AMBER Consortium. Breast Cancer Research and Treatment. 2015;150(3):655–666. doi: 10.1007/s10549-015-3353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kerlikowske, K., et al. (2017). Risk factors that increase risk of estrogen receptor-positive and -negative breast cancer.Journal of the National Cancer Institute 109(5). [DOI] [PMC free article] [PubMed]
- 42.McCarthy AM, et al. Relationship of established risk factors with breast cancer subtypes. Cancer Medicine. 2021;10(18):6456–6467. doi: 10.1002/cam4.4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: New mechanistic insights from epidemiology. Nature Reviews Cancer. 2015;15(8):484–498. doi: 10.1038/nrc3967. [DOI] [PubMed] [Google Scholar]
- 44.Pes, G.M., et al., (2019). Overweight: A protective factor against comorbidity in the elderly.International Journal of Environmental Research and Public Health 16(19). [DOI] [PMC free article] [PubMed]
- 45.Falls C, Melander S. Obesity in the critical care setting. Nursing Clinics of North America. 2021;56(4):573–581. doi: 10.1016/j.cnur.2021.08.002. [DOI] [PubMed] [Google Scholar]
- 46.Gillespie EF, et al. Obesity and angiolymphatic invasion in primary breast cancer. Annals of Surgical Oncology. 2010;17(3):752–759. doi: 10.1245/s10434-009-0797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neuhouser ML, et al. Overweight, obesity, and postmenopausal invasive breast cancer risk: A secondary analysis of the Women’s Health Initiative randomized clinical trials. JAMA Oncology. 2015;1(5):611–621. doi: 10.1001/jamaoncol.2015.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ewertz M, et al. Effect of obesity on prognosis after early-stage breast cancer. Journal of Clinical Oncology. 2011;29(1):25–31. doi: 10.1200/JCO.2010.29.7614. [DOI] [PubMed] [Google Scholar]
- 49.Osman MA, Hennessy BT. Obesity correlation with metastases development and response to first-line metastatic chemotherapy in breast cancer. Clin Med Insights Oncol. 2015;9:105–112. doi: 10.4137/CMO.S32812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mazzarella L, et al. Obesity increases the incidence of distant metastases in oestrogen receptor-negative human epidermal growth factor receptor 2-positive breast cancer patients. European Journal of Cancer. 2013;49(17):3588–3597. doi: 10.1016/j.ejca.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 51.Sestak I, et al. Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: An exploratory analysis from the ATAC trial. Journal of Clinical Oncology. 2010;28(21):3411–3415. doi: 10.1200/JCO.2009.27.2021. [DOI] [PubMed] [Google Scholar]
- 52.Biglia N, et al. Body mass index (BMI) and breast cancer: Impact on tumor histopathologic features, cancer subtypes and recurrence rate in pre and postmenopausal women. Gynecological Endocrinology. 2013;29(3):263–267. doi: 10.3109/09513590.2012.736559. [DOI] [PubMed] [Google Scholar]
- 53.Anders CK, et al. The evolution of triple-negative breast cancer: From biology to novel therapeutics. American Society of Clinical Oncology Educational Book. 2016;35:34–42. doi: 10.1200/EDBK_159135. [DOI] [PubMed] [Google Scholar]
- 54.Dawood S, et al. Prognostic value of body mass index in locally advanced breast cancer. Clinical Cancer Research. 2008;14(6):1718–1725. doi: 10.1158/1078-0432.CCR-07-1479. [DOI] [PubMed] [Google Scholar]
- 55.Azrad M, Demark-Wahnefried W. The association between adiposity and breast cancer recurrence and survival: A review of the recent literature. Current Nutrition Reports. 2014;3(1):9–15. doi: 10.1007/s13668-013-0068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chan DS, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Annals of Oncology. 2014;25(10):1901–1914. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Widschwendter P, et al. The influence of obesity on survival in early, high-risk breast cancer: Results from the randomized SUCCESS A trial. Breast Cancer Research. 2015;17:129. doi: 10.1186/s13058-015-0639-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.von Drygalski A, et al. Obesity is an independent predictor of poor survival in metastatic breast cancer: Retrospective analysis of a patient cohort whose treatment included high-dose chemotherapy and autologous stem cell support. Int J Breast Cancer. 2011;2011:523276. doi: 10.4061/2011/523276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loi S, et al. Obesity and outcomes in premenopausal and postmenopausal breast cancer. Cancer Epidemiology, Biomarkers and Prevention. 2005;14(7):1686–1691. doi: 10.1158/1055-9965.EPI-05-0042. [DOI] [PubMed] [Google Scholar]
- 60.Abrahamson PE, et al. General and abdominal obesity and survival among young women with breast cancer. Cancer Epidemiology, Biomarkers and Prevention. 2006;15(10):1871–1877. doi: 10.1158/1055-9965.EPI-06-0356. [DOI] [PubMed] [Google Scholar]
- 61.Friedenreich CM, Ryder-Burbidge C, McNeil J. Physical activity, obesity and sedentary behavior in cancer etiology: Epidemiologic evidence and biologic mechanisms. Molecular Oncology. 2021;15(3):790–800. doi: 10.1002/1878-0261.12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lammert J, Grill S, Kiechle M. Modifiable lifestyle factors: Opportunities for (hereditary) breast cancer prevention - A narrative review. Breast Care (Basel) 2018;13(2):109–114. doi: 10.1159/000488995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goding Sauer A, et al. Current prevalence of major cancer risk factors and screening test use in the United States: Disparities by education and race/ethnicity. Cancer Epidemiology, Biomarkers and Prevention. 2019;28(4):629–642. doi: 10.1158/1055-9965.EPI-18-1169. [DOI] [PubMed] [Google Scholar]
- 64.Kulkarni A, Bowers LW. The role of immune dysfunction in obesity-associated cancer risk, progression, and metastasis. Cellular and Molecular Life Sciences. 2021;78(7):3423–3442. doi: 10.1007/s00018-020-03752-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Woodall, M.J., et al. (2020). The effects of obesity on anti-cancer immunity and cancer immunotherapy. Cancers (Basel) 12(5). [DOI] [PMC free article] [PubMed]
- 66.Naik A, Monjazeb AM, Decock J. The obesity paradox in cancer, tumor immunology, and immunotherapy: Potential therapeutic implications in triple negative breast cancer. Frontiers in Immunology. 2019;10:1940. doi: 10.3389/fimmu.2019.01940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bhardwaj P, Brown KA. Obese adipose tissue as a driver of breast cancer growth and development: Update and emerging evidence. Frontiers in Oncology. 2021;11:638918. doi: 10.3389/fonc.2021.638918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Avgerinos KI, et al. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism. 2019;92:121–135. doi: 10.1016/j.metabol.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 69.Birks S, et al. A systematic review of the impact of weight loss on cancer incidence and mortality. Obesity Reviews. 2012;13(10):868–891. doi: 10.1111/j.1467-789X.2012.01010.x. [DOI] [PubMed] [Google Scholar]
- 70.Chlebowski RT, et al. Weight loss and breast cancer incidence in postmenopausal women. Cancer. 2019;125(2):205–212. doi: 10.1002/cncr.31687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seiler A, et al. Obesity, dietary factors, nutrition, and breast cancer risk. Current Breast Cancer Reports. 2018;10(1):14–27. doi: 10.1007/s12609-018-0264-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ligibel JA, Goodwin PJ. NEW and RENEW: Building the case for weight loss in breast cancer. Journal of Clinical Oncology. 2012;30(19):2294–2296. doi: 10.1200/JCO.2012.42.5496. [DOI] [PubMed] [Google Scholar]
- 73.Pierce JP. Diet and breast cancer prognosis: Making sense of the Women’s Healthy Eating and Living and Women’s Intervention Nutrition Study trials. Current Opinion in Obstetrics and Gynecology. 2009;21(1):86–91. doi: 10.1097/GCO.0b013e32831da7f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chlebowski RT, Reeves MM. Weight loss randomized intervention trials in female cancer survivors. Journal of Clinical Oncology. 2016;34(35):4238–4248. doi: 10.1200/JCO.2016.69.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pierce JP, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: The Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA. 2007;298(3):289–298. doi: 10.1001/jama.298.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chlebowski RT, et al. Dietary fat reduction and breast cancer outcome: Interim efficacy results from the Women’s Intervention Nutrition Study. Journal of the National Cancer Institute. 2006;98(24):1767–1776. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 77.Patterson RE, et al. The effects of metformin and weight loss on biomarkers associated with breast cancer outcomes. Journal of the National Cancer Institute. 2018;110(11):1239–1247. doi: 10.1093/jnci/djy040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lv M, et al. Roles of caloric restriction, ketogenic diet and intermittent fasting during initiation, progression and metastasis of cancer in animal models: A systematic review and meta-analysis. PLoS One. 2014;9(12):e115147. doi: 10.1371/journal.pone.0115147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hursting SD, et al. Calorie restriction, aging, and cancer prevention: Mechanisms of action and applicability to humans. Annual Review of Medicine. 2003;54:131–152. doi: 10.1146/annurev.med.54.101601.152156. [DOI] [PubMed] [Google Scholar]
- 80.Das M, et al. Time-restricted feeding normalizes hyperinsulinemia to inhibit breast cancer in obese postmenopausal mouse models. Nature Communications. 2021;12(1):565. doi: 10.1038/s41467-020-20743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qin Y, et al. Weight loss reduces basal-like breast cancer through kinome reprogramming. Cancer Cell International. 2016;16:26. doi: 10.1186/s12935-016-0300-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sundaram S, et al. Obesity-mediated regulation of HGF/c-Met is associated with reduced basal-like breast cancer latency in parous mice. PLoS One. 2014;9(10):e111394. doi: 10.1371/journal.pone.0111394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Look ARG. Eight-year weight losses with an intensive lifestyle intervention: The look AHEAD study. Obesity (Silver Spring) 2014;22(1):5–13. doi: 10.1002/oby.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lemstra M, et al. Weight loss intervention adherence and factors promoting adherence: A meta-analysis. Patient Preference and Adherence. 2016;10:1547–1559. doi: 10.2147/PPA.S103649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Demark-Wahnefried W, et al. Reach out to enhance wellness home-based diet-exercise intervention promotes reproducible and sustainable long-term improvements in health behaviors, body weight, and physical functioning in older, overweight/obese cancer survivors. Journal of Clinical Oncology. 2012;30(19):2354–2361. doi: 10.1200/JCO.2011.40.0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morey MC, et al. Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: A randomized controlled trial. JAMA. 2009;301(18):1883–1891. doi: 10.1001/jama.2009.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dieli-Conwright CM, et al. Effects of aerobic and resistance exercise on metabolic syndrome, sarcopenic obesity, and circulating biomarkers in overweight or obese survivors of breast cancer: A randomized controlled trial. Journal of Clinical Oncology. 2018;36(9):875–883. doi: 10.1200/JCO.2017.75.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goh J, et al. Exercise training in transgenic mice is associated with attenuation of early breast cancer growth in a dose-dependent manner. PLoS One. 2013;8(11):e80123. doi: 10.1371/journal.pone.0080123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hojman P, et al. Exercise-induced muscle-derived cytokines inhibit mammary cancer cell growth. American Journal of Physiology Endocrinology and metabolism. 2011;301(3):E504–E510. doi: 10.1152/ajpendo.00520.2010. [DOI] [PubMed] [Google Scholar]
- 90.Murphy EA, et al. Benefits of exercise training on breast cancer progression and inflammation in C3(1)SV40Tag mice. Cytokine. 2011;55(2):274–279. doi: 10.1016/j.cyto.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pedersen L, et al. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metabolism. 2016;23(3):554–562. doi: 10.1016/j.cmet.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 92.Ligibel JA, et al. Impact of a pre-operative exercise intervention on breast cancer proliferation and gene expression: Results from the Pre-Operative Health and Body (PreHAB) Study. Clinical Cancer Research. 2019;25(17):5398–5406. doi: 10.1158/1078-0432.CCR-18-3143. [DOI] [PubMed] [Google Scholar]
- 93.Ballard-Barbash R, et al. Physical activity, biomarkers, and disease outcomes in cancer survivors: A systematic review. Journal of the National Cancer Institute. 2012;104(11):815–840. doi: 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Campbell KL, et al. Reduced-calorie dietary weight loss, exercise, and sex hormones in postmenopausal women: Randomized controlled trial. Journal of Clinical Oncology. 2012;30(19):2314–2326. doi: 10.1200/JCO.2011.37.9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Marinac CR, et al. Prolonged nightly fasting and breast cancer prognosis. JAMA Oncology. 2016;2(8):1049–1055. doi: 10.1001/jamaoncol.2016.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ligibel JA, et al. Randomized phase III trial evaluating the role of weight loss in adjuvant treatment of overweight and obese women with early breast cancer (Alliance A011401): Study design. NPJ Breast Cancer. 2017;3:37. doi: 10.1038/s41523-017-0040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alkhatib, A., et al. (2017). Functional foods and lifestyle approaches for diabetes prevention and management. Nutrients 9(12). [DOI] [PMC free article] [PubMed]
- 98.Mitchell JE, et al. Postoperative behavioral variables and weight change 3 years after bariatric surgery. JAMA Surgery. 2016;151(8):752–757. doi: 10.1001/jamasurg.2016.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sarwer DB, et al. Preoperative eating behavior, postoperative dietary adherence, and weight loss after gastric bypass surgery. Surgery for Obesity and Related Diseases. 2008;4(5):640–646. doi: 10.1016/j.soard.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ryder JR, et al. Factors associated with long-term weight-loss maintenance following bariatric surgery in adolescents with severe obesity. International Journal of Obesity (Lond) 2018;42(1):102–107. doi: 10.1038/ijo.2017.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Malhotra S, et al. Bariatric surgery in the treatment of adolescent obesity: Current perspectives in the United States. Expert Review of Endocrinology and Metabolism. 2021;16(3):123–134. doi: 10.1080/17446651.2021.1914585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Soriano-Maldonado A, et al. Physical exercise following bariatric surgery in women with morbid obesity: Study protocol clinical trial (SPIRIT compliant) Medicine (Baltimore) 2020;99(12):e19427. doi: 10.1097/MD.0000000000019427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Baker, M.T., (2011). The history and evolution of bariatric surgical procedures.Surgical Clinics of North America 91(6):1181–201, viii. [DOI] [PubMed]
- 104.Pories, W.J., et al. (1995). Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Annals of Surgery 222(3):339–50; discussion 350–2. [DOI] [PMC free article] [PubMed]
- 105.Arterburn DE, et al. Benefits and risks of bariatric surgery in adults: A review. JAMA. 2020;324(9):879–887. doi: 10.1001/jama.2020.12567. [DOI] [PubMed] [Google Scholar]
- 106.Buchwald H. The evolution of metabolic/bariatric surgery. Obesity Surgery. 2014;24(8):1126–1135. doi: 10.1007/s11695-014-1354-3. [DOI] [PubMed] [Google Scholar]
- 107.Campos GM, et al. Changes in utilization of bariatric surgery in the United States from 1993 to 2016. Annals of Surgery. 2020;271(2):201–209. doi: 10.1097/SLA.0000000000003554. [DOI] [PubMed] [Google Scholar]
- 108.Melissas J, et al. Sleeve gastrectomy-a “food limiting” operation. Obesity Surgery. 2008;18(10):1251–1256. doi: 10.1007/s11695-008-9634-4. [DOI] [PubMed] [Google Scholar]
- 109.Alalwan AA, et al. US national trends in bariatric surgery: A decade of study. Surgery. 2021;170(1):13–17. doi: 10.1016/j.surg.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 110.Gronroos S, et al. Effect of laparoscopic sleeve gastrectomy vs Roux-en-Y gastric bypass on weight loss and quality of life at 7 years in patients with morbid obesity: The SLEEVEPASS randomized clinical trial. JAMA Surgery. 2021;156(2):137–146. doi: 10.1001/jamasurg.2020.5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Salminen P, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: The SLEEVEPASS randomized clinical trial. JAMA. 2018;319(3):241–254. doi: 10.1001/jama.2017.20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Peterli R, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux-en-Y gastric bypass on weight loss in patients with morbid obesity: The SM-BOSS randomized clinical trial. JAMA. 2018;319(3):255–265. doi: 10.1001/jama.2017.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schauer PR, et al. Bariatric surgery versus intensive medical therapy for diabetes - 5-Year outcomes. New England Journal of Medicine. 2017;376(7):641–651. doi: 10.1056/NEJMoa1600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Buchwald H, Buchwald JN, McGlennon TW. Systematic review and meta-analysis of medium-term outcomes after banded Roux-en-Y gastric bypass. Obesity Surgery. 2014;24(9):1536–1551. doi: 10.1007/s11695-014-1311-1. [DOI] [PubMed] [Google Scholar]
- 115.Ashrafian H, et al. Diabetes resolution and hyperinsulinaemia after metabolic Roux-en-Y gastric bypass. Obesity Reviews. 2011;12(5):e257–e272. doi: 10.1111/j.1467-789X.2010.00802.x. [DOI] [PubMed] [Google Scholar]
- 116.Evers SS, Sandoval DA, Seeley RJ. The physiology and molecular underpinnings of the effects of bariatric surgery on obesity and diabetes. Annual Review of Physiology. 2017;79:313–334. doi: 10.1146/annurev-physiol-022516-034423. [DOI] [PubMed] [Google Scholar]
- 117.Varela-Rodriguez, B.M., et al. (2020). Beneficial effects of bariatric surgery-induced by weight loss on the proteome of abdominal subcutaneous adipose tissue.Journal of Clinical Medicine 9(1). [DOI] [PMC free article] [PubMed]
- 118.Buchwald H, et al. Bariatric surgery: A systematic review and meta-analysis. JAMA. 2004;292(14):1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 119.Sipe LM, et al. Microbiome, bile acids, and obesity: How microbially modified metabolites shape anti-tumor immunity. Immunological Reviews. 2020;295(1):220–239. doi: 10.1111/imr.12856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ashrafian H, et al. Metabolic surgery and cancer: Protective effects of bariatric procedures. Cancer. 2011;117(9):1788–1799. doi: 10.1002/cncr.25738. [DOI] [PubMed] [Google Scholar]
- 121.Gimeno RE, Briere DA, Seeley RJ. Leveraging the gut to treat metabolic disease. Cell Metabolism. 2020;31(4):679–698. doi: 10.1016/j.cmet.2020.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Quail, D. F., & Dannenberg, A. J. (2018). The obese adipose tissue microenvironment in cancer development and progression.Nature Reviews Endocrinology. [DOI] [PMC free article] [PubMed]
- 123.Liu N, Funk LM. Bariatric surgery trends in the U.S.: 1% is the loneliest number. Annals of Surgery. 2020;271(2):210–211. doi: 10.1097/SLA.0000000000003714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bjornstad P, et al. Five-year kidney outcomes of bariatric surgery differ in severely obese adolescents and adults with and without type 2 diabetes. Kidney International. 2020;97(5):995–1005. doi: 10.1016/j.kint.2020.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Derderian SC, et al. Influence of weight loss on obesity-associated complications after metabolic and bariatric surgery in adolescents. Obesity (Silver Spring) 2020;28(12):2397–2404. doi: 10.1002/oby.23038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mollan SP, et al. Effectiveness of bariatric surgery vs community weight management intervention for the treatment of idiopathic intracranial hypertension: A randomized clinical trial. JAMA Neurology. 2021;78(6):678–686. doi: 10.1001/jamaneurol.2021.0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Purnell JQ, et al. Diabetes remission status during seven-year follow-up of the longitudinal assessment of bariatric surgery study. Journal of Clinical Endocrinology and Metabolism. 2021;106(3):774–788. doi: 10.1210/clinem/dgaa849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sjostrom L, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. New England Journal of Medicine. 2007;357(8):741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 129.Picot, J., et al. (2009). The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: A systematic review and economic evaluation. Health Technology Assessment 13(41):1–190, 215–357, iii-iv. [DOI] [PubMed]
- 130.Ward ZJ, et al. Association of body mass index with health care expenditures in the United States by age and sex. PLoS One. 2021;16(3):e0247307. doi: 10.1371/journal.pone.0247307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sikorski C, et al. Attitudes towards bariatric surgery in the general public. Obesity Surgery. 2013;23(3):338–345. doi: 10.1007/s11695-012-0767-0. [DOI] [PubMed] [Google Scholar]
- 132.Nguyen, N.T., et al. (2004). The relationship between hospital volume and outcome in bariatric surgery at academic medical centers. Annals of Surgery 240(4):586–93; discussion 593–4. [DOI] [PMC free article] [PubMed]
- 133.Varela JE, Nguyen NT. Laparoscopic sleeve gastrectomy leads the U.S. utilization of bariatric surgery at academic medical centers. Surgery for Obesity and Related Diseases. 2015;11(5):987–90. doi: 10.1016/j.soard.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 134.Schauer DP, et al. Bariatric surgery and the risk of cancer in a large multisite cohort. Annals of Surgery. 2019;269(1):95–101. doi: 10.1097/SLA.0000000000002525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Young MT, Phelan MJ, Nguyen NT. A decade analysis of trends and outcomes of male vs female patients who underwent bariatric surgery. Journal of the American College of Surgeons. 2016;222(3):226–231. doi: 10.1016/j.jamcollsurg.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 136.Rives-Lange, C., et al. (2022). Seventy years of bariatric surgery: A systematic mapping review of randomized controlled trials. Obesity Reviews e13420 [DOI] [PubMed]
- 137.Schauer DP, et al. Association between weight loss and the risk of cancer after bariatric surgery. Obesity (Silver Spring) 2017;25(Suppl 2):S52–S57. doi: 10.1002/oby.22002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Aminian, A.W., R.; Al-Kurd, A.; Tu, C.; Milinovich, A.; Kroh, M.; Rosenthal, R. J.; Brethauer, S. A.; Schauer, P. R.; Kattan, M. W.; Brown, J. C.; Berger, N. A.; Abraham, J.; Nissen, S. E. (2022). Association of bariatric surgery with cancer risk and mortality in adults with obesity. JAMA, [DOI] [PMC free article] [PubMed]
- 139.Aminian, A., et al. (2022). Association of bariatric surgery with cancer risk and mortality in adults with obesity. JAMA, [DOI] [PMC free article] [PubMed]
- 140.Wiggins T, Antonowicz SS, Markar SR. Cancer risk following bariatric surgery-systematic review and meta-analysis of national population-based cohort studies. Obesity Surgery. 2019;29(3):1031–1039. doi: 10.1007/s11695-018-3501-8. [DOI] [PubMed] [Google Scholar]
- 141.Feigelson HS, et al. Bariatric surgery is associated with reduced risk of breast cancer in both premenopausal and postmenopausal women. Annals of Surgery. 2020;272(6):1053–1059. doi: 10.1097/SLA.0000000000003331. [DOI] [PubMed] [Google Scholar]
- 142.Heshmati K, et al. Association of bariatric surgery status with reduced HER2+ breast cancers: A retrospective cohort study. Obesity Surgery. 2019;29(4):1092–1098. doi: 10.1007/s11695-018-03701-7. [DOI] [PubMed] [Google Scholar]
- 143.Lovrics O, et al. The effect of bariatric surgery on breast cancer incidence and characteristics: A meta-analysis and systematic review. American Journal of Surgery. 2021;222(4):715–722. doi: 10.1016/j.amjsurg.2021.03.016. [DOI] [PubMed] [Google Scholar]
- 144.Zhang S, et al. The impact of bariatric surgery on breast cancer recurrence: Case series and review of literature. Obesity Surgery. 2020;30(2):780–785. doi: 10.1007/s11695-019-04099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bruno DS, Berger NA. Impact of bariatric surgery on cancer risk reduction. Ann Transl Med. 2020;8(Suppl 1):S13. doi: 10.21037/atm.2019.09.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bailly L, et al. Colorectal cancer risk following bariatric surgery in a nationwide study of French individuals with obesity. JAMA Surgery. 2020;155(5):395–402. doi: 10.1001/jamasurg.2020.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Surwit RS, et al. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988;37(9):1163–1167. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- 148.Winzell MS, Ahren B. The high-fat diet-fed mouse: A model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53(Suppl 3):S215–S219. doi: 10.2337/diabetes.53.suppl_3.S215. [DOI] [PubMed] [Google Scholar]
- 149.Engber D. What models eat. Nature Medicine. 2018;24(6):692–695. doi: 10.1038/s41591-018-0055-1. [DOI] [PubMed] [Google Scholar]
- 150.Lai M, Chandrasekera PC, Barnard ND. You are what you eat, or are you? The challenges of translating high-fat-fed rodents to human obesity and diabetes. Nutrition and Diabetes. 2014;4:e135. doi: 10.1038/nutd.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Blasbalg TL, et al. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. American Journal of Clinical Nutrition. 2011;93(5):950–962. doi: 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ford NA, et al. Omega-3-acid ethyl esters block the protumorigenic effects of obesity in mouse models of postmenopausal basal-like and claudin-low breast cancer. Cancer Prevention Research (Philadelphia, Pa.) 2015;8(9):796–806. doi: 10.1158/1940-6207.CAPR-15-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Johnson AR, et al. Cafeteria diet-induced obesity causes oxidative damage in white adipose. Biochemical and Biophysical Research Communications. 2016;473(2):545–550. doi: 10.1016/j.bbrc.2016.03.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sampey BP, et al. Metabolomic profiling reveals mitochondrial-derived lipid biomarkers that drive obesity-associated inflammation. PLoS One. 2012;7(6):e38812. doi: 10.1371/journal.pone.0038812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sampey BP, et al. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: Comparison to high-fat diet. Obesity (Silver Spring) 2011;19(6):1109–1117. doi: 10.1038/oby.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Devlin MJ, et al. Differential effects of high fat diet and diet-induced obesity on skeletal acquisition in female C57BL/6J vs FVB/NJ Mice. Bone Rep. 2018;8:204–214. doi: 10.1016/j.bonr.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yang Y, et al. Variations in body weight, food intake and body composition after long-term high-fat diet feeding in C57BL/6J mice. Obesity (Silver Spring) 2014;22(10):2147–2155. doi: 10.1002/oby.20811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Boulange CL, et al. Early metabolic adaptation in C57BL/6 mice resistant to high fat diet induced weight gain involves an activation of mitochondrial oxidative pathways. Journal of Proteome Research. 2013;12(4):1956–1968. doi: 10.1021/pr400051s. [DOI] [PubMed] [Google Scholar]
- 159.Mekada K, Yoshiki A. Substrains matter in phenotyping of C57BL/6 mice. Experimental Animals. 2021;70(2):145–160. doi: 10.1538/expanim.20-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ivanov II, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mims TS, et al. The gut mycobiome of healthy mice is shaped by the environment and correlates with metabolic outcomes in response to diet. Communications Biology. 2021;4(1):281. doi: 10.1038/s42003-021-01820-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hugenholtz F, de Vos WM. Mouse models for human intestinal microbiota research: A critical evaluation. Cellular and Molecular Life Sciences. 2018;75(1):149–160. doi: 10.1007/s00018-017-2693-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Metlakunta AS, Sahu M, Sahu A. Hypothalamic phosphatidylinositol 3-kinase pathway of leptin signaling is impaired during the development of diet-induced obesity in FVB/N mice. Endocrinology. 2008;149(3):1121–1128. doi: 10.1210/en.2007-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nascimento-Sales, M., et al. (2017). Is the FVB/N mouse strain truly resistant to diet-induced obesity? Physiological Reports 5(9). [DOI] [PMC free article] [PubMed]
- 165.Boudina S, et al. Early mitochondrial adaptations in skeletal muscle to diet-induced obesity are strain dependent and determine oxidative stress and energy expenditure but not insulin sensitivity. Endocrinology. 2012;153(6):2677–2688. doi: 10.1210/en.2011-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhu Y, et al. Pubertally initiated high-fat diet promotes mammary tumorigenesis in obesity-prone FVB mice similarly to obesity-resistant BALB/c mice. Translational Oncology. 2017;10(6):928–935. doi: 10.1016/j.tranon.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cozzo AJ, et al. cMET inhibitor crizotinib impairs angiogenesis and reduces tumor burden in the C3(1)-Tag model of basal-like breast cancer. Springerplus. 2016;5:348. doi: 10.1186/s40064-016-1920-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sundaram S, et al. Role of HGF in obesity-associated tumorigenesis: C3(1)-TAg mice as a model for human basal-like breast cancer. Breast Cancer Research and Treatment. 2013;142(3):489–503. doi: 10.1007/s10549-013-2741-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sundaram S, et al. Weight loss reversed obesity-induced HGF/c-Met pathway and basal-like breast cancer progression. Frontiers in Oncology. 2014;4:175. doi: 10.3389/fonc.2014.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chamberlin T, D'Amato JV, Arendt LM. Obesity reversibly depletes the basal cell population and enhances mammary epithelial cell estrogen receptor alpha expression and progenitor activity. Breast Cancer Research. 2017;19(1):128. doi: 10.1186/s13058-017-0921-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hillers-Ziemer, L.E., et al. (2020). Obesity promotes cooperation of cancer stem-like cells and macrophages to enhance mammary tumor angiogenesis. Cancers (Basel) 12(2). [DOI] [PMC free article] [PubMed]
- 172.Leibowitz SF, et al. Phenotypic profile of SWR/J and A/J mice compared to control strains: Possible mechanisms underlying resistance to obesity on a high-fat diet. Brain Research. 2005;1047(2):137–147. doi: 10.1016/j.brainres.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 173.Buckley TN, et al. High-fat feeding disrupts daily eating behavior rhythms in obesity-prone but not in obesity-resistant male inbred mouse strains. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2021;320(5):R619–R629. doi: 10.1152/ajpregu.00150.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Park H, et al. A high-fat diet increases angiogenesis, solid tumor growth, and lung metastasis of CT26 colon cancer cells in obesity-resistant BALB/c mice. Molecular Carcinogenesis. 2012;51(11):869–880. doi: 10.1002/mc.20856. [DOI] [PubMed] [Google Scholar]
- 175.Kim HY, et al. Lysophospholipid profile in serum and liver by high-fat diet and tumor induction in obesity-resistant BALB/c mice. Nutrition. 2014;30(11–12):1433–1441. doi: 10.1016/j.nut.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 176.Kim EJ, et al. Biological features of core networks that result from a high-fat diet in hepatic and pulmonary tissues in mammary tumour-bearing, obesity-resistant mice. British Journal of Nutrition. 2013;110(2):241–255. doi: 10.1017/S0007114512004965. [DOI] [PubMed] [Google Scholar]
- 177.Kim M, et al. Benzyl isothiocyanate suppresses high-fat diet-stimulated mammary tumor progression via the alteration of tumor microenvironments in obesity-resistant BALB/c mice. Molecular Carcinogenesis. 2015;54(1):72–82. doi: 10.1002/mc.22159. [DOI] [PubMed] [Google Scholar]
- 178.Gourgue F, et al. Obesity and triple-negative-breast-cancer: Is apelin a new key target? Journal of Cellular and Molecular Medicine. 2020;24(17):10233–10244. doi: 10.1111/jcmm.15639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Olson LK, et al. Pubertal exposure to high fat diet causes mouse strain-dependent alterations in mammary gland development and estrogen responsiveness. International Journal of Obesity . 2010;34(9):1415–1426. doi: 10.1038/ijo.2010.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhao Y, et al. Pubertal high fat diet: Effects on mammary cancer development. Breast Cancer Research. 2013;15(5):R100. doi: 10.1186/bcr3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lo JC, et al. Obesity does not promote tumorigenesis of localized patient-derived prostate cancer xenografts. Oncotarget. 2016;7(30):47650–47662. doi: 10.18632/oncotarget.10258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Friedline RH, et al. Genetic ablation of lymphocytes and cytokine signaling in nonobese diabetic mice prevents diet-induced obesity and insulin resistance. The FASEB Journal. 2016;30(3):1328–1338. doi: 10.1096/fj.15-280610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Xue Y, et al. Maternal obesity induces gut inflammation and impairs gut epithelial barrier function in nonobese diabetic mice. Journal of Nutritional Biochemistry. 2014;25(7):758–764. doi: 10.1016/j.jnutbio.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Stefater MA, et al. Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology. 2010;138(7):2426–36, 2436 e1-3. doi: 10.1053/j.gastro.2010.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chambers AP, et al. Similar effects of roux-en-Y gastric bypass and vertical sleeve gastrectomy on glucose regulation in rats. Physiology and Behavior. 2011;105(1):120–123. doi: 10.1016/j.physbeh.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Saeidi N, et al. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science. 2013;341(6144):406–410. doi: 10.1126/science.1235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yin DP, et al. Assessment of different bariatric surgeries in the treatment of obesity and insulin resistance in mice. Annals of Surgery. 2011;254(1):73–82. doi: 10.1097/SLA.0b013e3182197035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Feng JJ, Gagner M. Laparoscopic biliopancreatic diversion with duodenal switch. Semin Laparosc Surg. 2002;9(2):125–129. doi: 10.1053/slas.2002.126332. [DOI] [PubMed] [Google Scholar]
- 189.Yin, D.P., et al. (2012). Mouse models of bariatric surgery. Current Protocols in Mouse Biology 2012. [DOI] [PMC free article] [PubMed]
- 190.Asarian L, et al. Estradiol increases body weight loss and gut-peptide satiation after Roux-en-Y gastric bypass in ovariectomized rats. Gastroenterology. 2012;143(2):325–7 e2. doi: 10.1053/j.gastro.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 191.Hao Z, et al. Body composition, food intake, and energy expenditure in a murine model of Roux-en-Y gastric bypass surgery. Obesity Surgery. 2016;26(9):2173–2182. doi: 10.1007/s11695-016-2062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ye Y, et al. Endocannabinoid receptor-1 and sympathetic nervous system mediate the beneficial metabolic effects of gastric bypass. Cell Reports. 2020;33(4):108270. doi: 10.1016/j.celrep.2020.108270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hao Z, et al. RYGB produces more sustained body weight loss and improvement of glycemic control compared with VSG in the diet-induced obese mouse model. Obesity Surgery. 2017;27(9):2424–2433. doi: 10.1007/s11695-017-2660-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Stevenson M, et al. RYGB is more effective than VSG at protecting mice from prolonged high-fat diet exposure: An occasion to roll up our sleeves? Obesity Surgery. 2021;31(7):3227–3241. doi: 10.1007/s11695-021-05389-8. [DOI] [PubMed] [Google Scholar]
- 195.Stevenson M, et al. Surgical mouse models of vertical sleeve gastrectomy and Roux-en Y gastric bypass: A review. Obesity Surgery. 2019;29(12):4084–4094. doi: 10.1007/s11695-019-04205-8. [DOI] [PubMed] [Google Scholar]
- 196.Frikke-Schmidt H, et al. Weight loss independent changes in adipose tissue macrophage and T cell populations after sleeve gastrectomy in mice. Molecular Metabolism. 2017;6(4):317–326. doi: 10.1016/j.molmet.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chaudhari SN, et al. A microbial metabolite remodels the gut-liver axis following bariatric surgery. Cell Host and Microbe. 2021;29(3):408–424 e7. doi: 10.1016/j.chom.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Chaudhari SN, et al. Bariatric surgery reveals a gut-restricted TGR5 agonist with anti-diabetic effects. Nature Chemical Biology. 2021;17(1):20–29. doi: 10.1038/s41589-020-0604-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ryan KK, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509(7499):183–188. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ridlon JM. Bariatric surgery stirs symbionts to counteract diabesity by CA(7)Sting a liver-generated bile acid into the mix. Cell Host and Microbe. 2021;29(3):320–322. doi: 10.1016/j.chom.2021.02.011. [DOI] [PubMed] [Google Scholar]
- 201.Martin M, et al. Socioeconomic disparities in eligibility and access to bariatric surgery: A national population-based analysis. Surgery for Obesity and Related Diseases. 2010;6(1):8–15. doi: 10.1016/j.soard.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 202.Brinckerhoff TZ, et al. Metabolic effects of sleeve gastrectomy in female rat model of diet-induced obesity. Surgery for Obesity and Related Diseases. 2013;9(1):108–112. doi: 10.1016/j.soard.2011.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Grayson BE, et al. Improved rodent maternal metabolism but reduced intrauterine growth after vertical sleeve gastrectomy. Science Translational Medicine. 2013;5(199):199ra112. doi: 10.1126/scitranslmed.3006505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Grayson BE, et al. Bariatric surgery emphasizes biological sex differences in rodent hepatic lipid handling. Biology of Sex Differences. 2017;8:4. doi: 10.1186/s13293-017-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Spann RA, et al. Rodent vertical sleeve gastrectomy alters maternal immune health and fetoplacental development. Clinical Science (London, England) 2018;132(2):295–312. doi: 10.1042/CS20171416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Spann RA, et al. Altered immune system in offspring of rat maternal vertical sleeve gastrectomy. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2019;317(6):R852–R863. doi: 10.1152/ajpregu.00230.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sipe LM, et al. Response to immune checkpoint blockade improved in pre-clinical model of breast cancer after bariatric surgery. eLife. 2022;11:e79143. doi: 10.7554/eLife.79143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hanahan D, Robert A. Weinberg, Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 209.Bissell MJ, Hines WC. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nature Medicine. 2011;17(3):320–329. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Correia AL, Bissell MJ. The tumor microenvironment is a dominant force in multidrug resistance. Drug Resist Updat. 2012;15(1–2):39–49. doi: 10.1016/j.drup.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gatenby RAG, Brown RJ, Joel S. Of cancer and cave fish. Nature Publishing Group. 2011;11(4):237–238. doi: 10.1038/nrc3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Johnson AR, Milner JJ, Makowski L. The inflammation highway: Metabolism accelerates inflammatory traffic in obesity. Immunologic Reviews. 2012;249(1):218–238. doi: 10.1111/j.1600-065X.2012.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zheng J, et al. Obesity-associated digestive cancers: A review of mechanisms and interventions. Tumour Biology. 2017;39(3):1010428317695020. doi: 10.1177/1010428317695020. [DOI] [PubMed] [Google Scholar]
- 214.Courcoulas, A.P., (2022). Bariatric surgery and cancer risk. JAMA. [DOI] [PubMed]
- 215.Douros JD, Tong J, D'Alessio DA. The effects of bariatric surgery on islet function, insulin secretion, and glucose control. Endocrine Reviews. 2019;40(5):1394–1423. doi: 10.1210/er.2018-00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Villarreal-Calderon JR, et al. Interplay between the adaptive immune system and insulin resistance in weight loss induced by bariatric surgery. Oxidative Medicine and Cellular Longevity. 2019;2019:3940739. doi: 10.1155/2019/3940739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Srinivasan M, et al. A systematic review: Does insulin resistance affect the risk and survival outcome of breast cancer in women? Cureus. 2022;14(1):e21712. doi: 10.7759/cureus.21712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Pan K, et al. Insulin resistance and breast cancer incidence and mortality in postmenopausal women in the Women’s Health Initiative. Cancer. 2020;126(16):3638–3647. doi: 10.1002/cncr.33002. [DOI] [PubMed] [Google Scholar]
- 219.Sanchez-Jimenez F, et al. Obesity and breast cancer: Role of leptin. Frontiers in Oncology. 2019;9:596. doi: 10.3389/fonc.2019.00596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Koliaki C, et al. The role of bariatric surgery to treat diabetes: Current challenges and perspectives. BMC Endocrine Disorders. 2017;17(1):50. doi: 10.1186/s12902-017-0202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sinclair P, Brennan DJ, le Roux CW. Gut adaptation after metabolic surgery and its influences on the brain, liver and cancer. Nature Reviews Gastroenterology and Hepatology. 2018;15(10):606–624. doi: 10.1038/s41575-018-0057-y. [DOI] [PubMed] [Google Scholar]
- 222.Crean-Tate KK, Reizes O. Leptin regulation of cancer stem cells in breast and gynecologic cancer. Endocrinology. 2018;159(8):3069–3080. doi: 10.1210/en.2018-00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Iyengar NM, et al. Systemic correlates of white adipose tissue inflammation in early-stage breast cancer. Clinical Cancer Research. 2016;22(9):2283–2289. doi: 10.1158/1078-0432.CCR-15-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zhao C, et al. Cancer-associated adipocytes: Emerging supporters in breast cancer. Journal of Experimental and Clinical Cancer Research. 2020;39(1):156. doi: 10.1186/s13046-020-01666-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Atoum MF, Alzoughool F, Al-Hourani H. Linkage between obesity leptin and breast cancer. Breast Cancer (Auckl) 2020;14:1178223419898458. doi: 10.1177/1178223419898458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Maroni, P. (2020). Leptin, adiponectin, and Sam68 in bone metastasis from breast cancer. International Journal of Molecular Sciences 21(3). [DOI] [PMC free article] [PubMed]
- 227.Di Domenico M, et al. The role of oxidative stress and hormones in controlling obesity. Front Endocrinol (Lausanne) 2019;10:540. doi: 10.3389/fendo.2019.00540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hage MP, El-Hajj Fuleihan G. Bone and mineral metabolism in patients undergoing Roux-en-Y gastric bypass. Osteoporosis International. 2014;25(2):423–39. doi: 10.1007/s00198-013-2480-9. [DOI] [PubMed] [Google Scholar]
- 229.Luhrs AR, et al. Determining changes in bone metabolism after bariatric surgery in postmenopausal women. Surgical Endoscopy. 2020;34(4):1754–1760. doi: 10.1007/s00464-019-06922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Rinonapoli, G., et al. (2021). Obesity and bone: A complex relationship. International Journal of Molecular Sciences 22(24). [DOI] [PMC free article] [PubMed]
- 231.Lengyel E, et al. Cancer as a matter of fat: The crosstalk between adipose tissue and tumors. Trends Cancer. 2018;4(5):374–384. doi: 10.1016/j.trecan.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Cozzo AJ, Fuller AM, Makowski L. Contribution of adipose tissue to development of cancer. Comprehensive Physiology. 2017;8(1):237–282. doi: 10.1002/cphy.c170008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Rathmell JC. Obesity, immunity, and cancer. New England Journal of Medicine. 2021;384(12):1160–1162. doi: 10.1056/NEJMcibr2035081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Saha, S.K., et al. (2017). Correlation between oxidative stress, nutrition, and cancer initiation. International Journal of Molecular Sciences 18(7). [DOI] [PMC free article] [PubMed]
- 235.Franceschi C, et al. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nature Reviews Endocrinology. 2018;14(10):576–590. doi: 10.1038/s41574-018-0059-4. [DOI] [PubMed] [Google Scholar]
- 236.Caslin HL, et al. Adipose tissue macrophages: Unique polarization and bioenergetics in obesity. Immunological Reviews. 2020;295(1):101–113. doi: 10.1111/imr.12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Quail DF, Dannenberg AJ. The obese adipose tissue microenvironment in cancer development and progression. Nature Reviews. Endocrinology. 2019;15(3):139–154. doi: 10.1038/s41574-018-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Holm, J. B., Rosendahl, A. H., Borgquist, S. (2021). Local biomarkers involved in the interplay between obesity and breast cancer. Cancers (Basel) 13(24). [DOI] [PMC free article] [PubMed]
- 239.Kompella P, Vasquez KM. Obesity and cancer: A mechanistic overview of metabolic changes in obesity that impact genetic instability. Molecular Carcinogenesis. 2019;58(9):1531–1550. doi: 10.1002/mc.23048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wlodarczyk, M., Nowicka, G., (2019). Obesity, DNA damage, and development of obesity-related diseases. International Journal of Molecular Sciences 20(5). [DOI] [PMC free article] [PubMed]
- 241.Ringel AE, et al. Obesity shapes metabolism in the tumor microenvironment to suppress anti-tumor immunity. Cell. 2020;183(7):1848–1866 e26. doi: 10.1016/j.cell.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Le CT, et al. PD-1 Blockade reverses obesity-mediated T cell priming impairment. Frontiers in Immunology. 2020;11:590568. doi: 10.3389/fimmu.2020.590568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Neumann, S., et al. (2021). Obesity has a systemic effect on immune cells in naive and cancer-bearing mice. International Journal of Molecular Sciences 22(16). [DOI] [PMC free article] [PubMed]
- 244.Weihe P, et al. Childhood obesity and cancer risk in adulthood. Current Obesity Reports. 2020;9(3):204–212. doi: 10.1007/s13679-020-00387-w. [DOI] [PubMed] [Google Scholar]
- 245.Yang S, et al. PD-1, PD-L1 and PD-L2 expression in mouse prostate cancer. American Journal of Clinical and Experimental Urology. 2016;4(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- 246.Del Corno, M., et al. (2016). Visceral fat adipocytes from obese and colorectal cancer subjects exhibit distinct secretory and omega6 polyunsaturated fatty acid profiles and deliver immunosuppressive signals to innate immunity cells. Oncotarget, [DOI] [PMC free article] [PubMed]
- 247.Shirakawa K, et al. Abstract 11105: Obesity accelerates T cell senescence in visceral adipose tissue. Circulation. 2015;132(Suppl 3):A11105–A11105. [Google Scholar]
- 248.Lim SO, et al. Deubiquitination and stabilization of PD-L1 by CSN5. Cancer Cell. 2016;30(6):925–939. doi: 10.1016/j.ccell.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Sanchez-Pino MD, et al. Obesity-associated myeloid immunosuppressive cells, key players in cancer risk and response to immunotherapy. Obesity (Silver Spring) 2021;29(6):944–953. doi: 10.1002/oby.23108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Fernandez-Ruiz JC, et al. Myeloid-derived suppressor cells show different frequencies in diabetics and subjects with arterial hypertension. Journal of Diabetes Research. 2019;2019:1568457. doi: 10.1155/2019/1568457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Friedrich K, et al. Perturbation of the monocyte compartment in human obesity. Frontiers in Immunology. 2019;10:1874. doi: 10.3389/fimmu.2019.01874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Xia S, et al. Gr-1+ CD11b+ myeloid-derived suppressor cells suppress inflammation and promote insulin sensitivity in obesity. Journal of Biological Chemistry. 2011;286(26):23591–23599. doi: 10.1074/jbc.M111.237123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Okwan-Duodu D, et al. Obesity-driven inflammation and cancer risk: Role of myeloid derived suppressor cells and alternately activated macrophages. American Journal of Cancer Research. 2013;3(1):21–33. [PMC free article] [PubMed] [Google Scholar]
- 254.Donninelli G, et al. Distinct blood and visceral adipose tissue regulatory T cell and innate lymphocyte profiles characterize obesity and colorectal cancer. Frontiers in Immunology. 2017;8:643. doi: 10.3389/fimmu.2017.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Agabiti-Rosei C, et al. Decreased circulating T regulatory lymphocytes in obese patients undergoing bariatric surgery. PLoS One. 2018;13(5):e0197178. doi: 10.1371/journal.pone.0197178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Chen X, Wu Y, Wang L. Fat-resident Tregs: An emerging guard protecting from obesity-associated metabolic disorders. Obesity Reviews. 2013;14(7):568–578. doi: 10.1111/obr.12033. [DOI] [PubMed] [Google Scholar]
- 257.Weisberg SP, et al. Obesity is associated with macrophage accumulation in adipose tissue. The Journal of Clinical Investigation. 2003;112(12):1796–1808. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Xu H, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. The Journal of Clinical Investigation. 2003;112(12):1821–1830. doi: 10.1172/JCI200319451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Sun S, et al. Mechanisms of inflammatory responses in obese adipose tissue. Annual Review of Nutrition. 2012;32:261–286. doi: 10.1146/annurev-nutr-071811-150623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Diedrich J, Gusky HC, Podgorski I. Adipose tissue dysfunction and its effects on tumor metabolism. Hormone Molecular Biology and Clinical Investigation. 2015;21(1):17–41. doi: 10.1515/hmbci-2014-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Ahn CH, et al. Vertical sleeve gastrectomy induces distinctive transcriptomic responses in liver, fat and muscle. Science and Reports. 2021;11(1):2310. doi: 10.1038/s41598-021-81866-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Viardot A, Lord RV, Samaras K. The effects of weight loss and gastric banding on the innate and adaptive immune system in type 2 diabetes and prediabetes. Journal of Clinical Endocrinology and Metabolism. 2010;95(6):2845–2850. doi: 10.1210/jc.2009-2371. [DOI] [PubMed] [Google Scholar]
- 263.Rao SR. Inflammatory markers and bariatric surgery: A meta-analysis. Inflammation Research. 2012;61(8):789–807. doi: 10.1007/s00011-012-0473-3. [DOI] [PubMed] [Google Scholar]
- 264.Poitou C, et al. Bariatric surgery induces disruption in inflammatory signaling pathways mediated by immune cells in adipose tissue: A RNA-Seq study. PLoS One. 2015;10(5):e0125718. doi: 10.1371/journal.pone.0125718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Hagman DK, et al. The short-term and long-term effects of bariatric/metabolic surgery on subcutaneous adipose tissue inflammation in humans. Metabolism. 2017;70:12–22. doi: 10.1016/j.metabol.2017.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Cancello R, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54(8):2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 267.Aguilar EG, Murphy WJ. Obesity induced T cell dysfunction and implications for cancer immunotherapy. Current Opinion in Immunology. 2018;51:181–186. doi: 10.1016/j.coi.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Ignacio RM, Kim CS, Kim SK. Immunological profiling of obesity. Journal of Lifestyle Medicine. 2014;4(1):1–7. doi: 10.15280/jlm.2014.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Hess DA, et al. Vascular risk reduction in obesity through reduced granulocyte burden and improved angiogenic monocyte content following bariatric surgery. Cell Reports Medicine. 2020;1(2):100018. doi: 10.1016/j.xcrm.2020.100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Kabon B, et al. Obesity decreases perioperative tissue oxygenation. Anesthesiology. 2004;100(2):274–280. doi: 10.1097/00000542-200402000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Kratz M, et al. Improvements in glycemic control after gastric bypass occur despite persistent adipose tissue inflammation. Obesity (Silver Spring) 2016;24(7):1438–1445. doi: 10.1002/oby.21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Himel AR, et al. Splenectomy fails to attenuate immuno-hematologic changes after rodent vertical sleeve gastrectomy. Experimental Biology and Medicine (Maywood, N.J.) 2019;244(13):1125–1135. doi: 10.1177/1535370219857991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Harris DA, et al. Weight and organ specific immune cell profiling of sleeve gastrectomy in mice. Metabolism. 2021;118:154729. doi: 10.1016/j.metabol.2021.154729. [DOI] [PubMed] [Google Scholar]
- 274.Zhang C, et al. Improvements in humoral immune function and glucolipid metabolism after laparoscopic sleeve gastrectomy in patients with obesity. Surgery for Obesity and Related Diseases. 2019;15(9):1455–1463. doi: 10.1016/j.soard.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 275.DeFuria J, et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proceedings of the National Academy of Sciences U S A. 2013;110(13):5133–5138. doi: 10.1073/pnas.1215840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Winer DA, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nature Medicine. 2011;17(5):610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Winer DA, et al. The intestinal immune system in obesity and insulin resistance. Cell Metabolism. 2016;23(3):413–426. doi: 10.1016/j.cmet.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 278.Debedat J, Clement K, Aron-Wisnewsky J. Gut microbiota dysbiosis in human obesity: Impact of bariatric surgery. Current Obesity Reports. 2019;8(3):229–242. doi: 10.1007/s13679-019-00351-3. [DOI] [PubMed] [Google Scholar]
- 279.Ilhan ZE, et al. Distinctive microbiomes and metabolites linked with weight loss after gastric bypass, but not gastric banding. ISME Journal. 2017;11(9):2047–2058. doi: 10.1038/ismej.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Ciobarca, D., et al. (2020). Bariatric surgery in obesity: Effects on gut microbiota and micronutrient status. Nutrients 12(1). [DOI] [PMC free article] [PubMed]
- 281.Santos JM, et al. Pre and post-operative alterations of the gastrointestinal microbiome following bariatric surgery. Cureus. 2021;13(2):e13057. doi: 10.7759/cureus.13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Haange SB, et al. Gastric bypass surgery in a rat model alters the community structure and functional composition of the intestinal microbiota independently of weight loss. Microbiome. 2020;8(1):13. doi: 10.1186/s40168-020-0788-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Langan EA, et al. Does the gastrointestinal microbiome contribute to the ‘obesity paradox’ in melanoma survival? British Journal of Dermatology. 2018;179(1):225–226. doi: 10.1111/bjd.16681. [DOI] [PubMed] [Google Scholar]
- 284.Gopalakrishnan V, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Frankel AE, et al. Metagenomic shotgun sequencing and unbiased metabolomic profiling identify specific human gut microbiota and metabolites associated with immune checkpoint therapy efficacy in melanoma patients. Neoplasia. 2017;19(10):848–855. doi: 10.1016/j.neo.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Vetizou M, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Matson V, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359(6371):104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Routy B, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 289.Iida N, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342(6161):967–970. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Dawood S, Rugo HS. Targeting the host immune system: PD-1 and PD-L1 antibodies and breast cancer. Current Opinion in Supportive and Palliative Care. 2016;10(4):336–342. doi: 10.1097/SPC.0000000000000243. [DOI] [PubMed] [Google Scholar]
- 291.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: Past, present, and future. The Journal of Clinical Investigation. 2015;125(9):3384–3391. doi: 10.1172/JCI80011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Josefsson A, et al. Imaging, biodistribution, and dosimetry of radionuclide-labeled PD-L1 antibody in an immunocompetent mouse model of breast cancer. Cancer Research. 2016;76(2):472–479. doi: 10.1158/0008-5472.CAN-15-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Beatson R, et al. The mucin MUC1 modulates the tumor immunological microenvironment through engagement of the lectin Siglec-9. Nature Immunology. 2016;17(11):1273–1281. doi: 10.1038/ni.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Wu B, et al. Adipose PD-L1 modulates PD-1/PD-L1 checkpoint blockade immunotherapy efficacy in breast cancer. Oncoimmunology. 2018;7(11):e1500107. doi: 10.1080/2162402X.2018.1500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Mittendorf EA, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunology Research. 2014;2(4):361–370. doi: 10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Muenst S, et al. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Research and Treatment. 2013;139(3):667–676. doi: 10.1007/s10549-013-2581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Ali HR, et al. PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes. Annals of Oncology. 2015;26(7):1488–1493. doi: 10.1093/annonc/mdv192. [DOI] [PubMed] [Google Scholar]
- 298.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Guo L, et al. PD-L1 expression and CD274 gene alteration in triple-negative breast cancer: Implication for prognostic biomarker. Springerplus. 2016;5(1):805. doi: 10.1186/s40064-016-2513-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Li Z, et al. PD-L1 expression is associated with tumor FOXP3(+) regulatory T-cell infiltration of breast cancer and poor prognosis of patient. Journal of Cancer. 2016;7(7):784–793. doi: 10.7150/jca.14549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Muenst S, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Research and Treatment. 2014;146(1):15–24. doi: 10.1007/s10549-014-2988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Nanda R, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: Phase Ib KEYNOTE-012 Study. Journal of Clinical Oncology. 2016;34(21):2460–2467. doi: 10.1200/JCO.2015.64.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Schmid P, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. New England Journal of Medicine. 2018;379(22):2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 304.Larkin J, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. New England Journal of Medicine. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Zhao C, et al. Current landscape: The mechanism and therapeutic impact of obesity for breast cancer. Frontiers in Oncology. 2021;11:704893. doi: 10.3389/fonc.2021.704893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Mirsoian A, et al. Adiposity induces lethal cytokine storm after systemic administration of stimulatory immunotherapy regimens in aged mice. Journal of Experimental Medicine. 2014;211(12):2373–2383. doi: 10.1084/jem.20140116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Pingili AK, et al. Immune checkpoint blockade reprograms systemic immune landscape and tumor microenvironment in obesity-associated breast cancer. Cell Reports. 2021;35(12):109285. doi: 10.1016/j.celrep.2021.109285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Canter RJ, et al. Obesity as an immune-modifying factor in cancer immunotherapy. Journal of Leukocyte Biology. 2018;104(3):487–497. doi: 10.1002/JLB.5RI1017-401RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Richtig G, et al. Body mass index may predict the response to ipilimumab in metastatic melanoma: An observational multi-centre study. PLoS One. 2018;13(10):e0204729. doi: 10.1371/journal.pone.0204729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.McQuade JL, et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: A retrospective, multicohort analysis. The lancet Oncology. 2018;19(3):310–322. doi: 10.1016/S1470-2045(18)30078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Cortellini A, et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: When overweight becomes favorable. Journal for Immunotherapy of Cancer. 2019;7(1):57. doi: 10.1186/s40425-019-0527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Greenlee H, et al. Association between body mass index and cancer survival in a pooled analysis of 22 clinical trials. Cancer Epidemiology, Biomarkers and Prevention. 2017;26(1):21–29. doi: 10.1158/1055-9965.EPI-15-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Liou AP, et al. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Science Translational Medicine. 2013;5(178):178ra41. doi: 10.1126/scitranslmed.3005687. [DOI] [PMC free article] [PubMed] [Google Scholar]