Abstract
Adaptation of bacterial cells to diverse habitats relies on the ability of RNA polymerase to respond to various regulatory signals. Some of these signals are conserved throughout evolution, whereas others are species specific. In this study we present a comprehensive comparative analysis of RNA polymerases from two distantly related bacterial species, Escherichia coli and Bacillus subtilis, using a panel of in vitro transcription assays. We found substantial species-specific differences in the ability of these enzymes to escape from the promoter and to recognize certain types of elongation signals. Both enzymes responded similarly to other pause and termination signals and to the general E. coli elongation factors NusA and GreA. We also demonstrate that, although promoter recognition depends largely on the ς subunit, promoter discrimination exhibited in species-specific fashion by both RNA polymerases resides in the core enzyme. We hypothesize that differences in signal recognition are due to the changes in contacts made between the β and β′ subunits and the downstream DNA duplex.
Live bacteria are found in a variety of habitats, ranging from underwater volcano craters to arctic seas to the interior of plant and animal cells. The ability of bacterial species to thrive in an extraordinary wide range of conditions depends on diverse molecular mechanisms that adjust gene expression patterns according to nutrient flux and fluctuations of macroscopic parameters such as temperature, pressure, and pH.
DNA-dependent RNA polymerase (RNAP) is the main target for the regulation of gene expression and is composed of several subunits in all cellular organisms. Four subunits of eubacterial RNAP (α2ββ′) comprise the core enzyme that is capable of basic polymerization activity in vitro, but requires the specificity factor (ς) to initiate transcription from a promoter (44, 65). Orthologs of bacterial core subunits have been identified in Archaea and Eucarya (35), suggesting conservation of the basic architecture and function of RNAPs in all forms of life.
A substantial amount of mechanistic and structural information regarding transcription in bacteria has been accumulated, but most of this information is derived from only a few model organisms. The disparity between the bacterial diversity and the scope of detailed transcriptional analysis is quite staggering. Out of the estimated 106 to 109 free-living and parasitic bacterial species (13), studies of only three RNAPs, from Escherichia coli, Bacillus subtilis, and Thermus aquaticus, together account for most of the information accumulated in the field to date. Moreover, none of these three data sets is complete. The high-resolution structural information is available only for the T. aquaticus RNAP, which has not been studied mechanistically. In contrast, the E. coli enzyme has served as the model for the majority of the biochemical and biophysical studies in transcription but has proven refractory to structural analysis except for the fragments of its smallest, α and ς, subunits (17, 29, 42, 71). The study of B. subtilis RNAP, which has provided the paradigm for the interplay of the ς and anti-ς factors in eubacterial genome regulation, has yet to yield structural or mechanistic information comparable to that accumulated about T. aquaticus and E. coli enzymes, respectively.
The scarcity and incongruity of information regarding eubacterial RNAPs notwithstanding, it is often assumed that these enzymes share substantial similarity if not essential identity of their structural and mechanistic characteristics (70). In fact, the overall shape of core RNAP is conserved between distantly related bacteria: the structure of T. aquaticus enzyme at 3.3 Å and the structure of E. coli RNAP at 12 Å are essentially superimposable (70). In addition, the amino acid residues implicated in catalysis and contacts to the nucleic acids by the E. coli RNAP are appropriately positioned within the T. aquaticus structure. The common features of the transcription mechanism are thought to be encoded within the evolutionary conserved regions (70), whereas the variable protein segments located on the surface of the enzyme likely mediate interactions with regulatory proteins (48, 60).
However, the regulatory inputs that affect RNAP behavior are not limited to ancillary proteins (extrinsic signals) but also include various RNA and DNA sequences (template-specified intrinsic signals) (44), which make contacts to the highly conserved regions of the core enzyme (30, 70). Although at least some of these intrinsic signals appear to be recognized in vitro by RNAPs from phage to humans (47), regulatory signals in vivo are likely to exhibit certain species-specific differences. For example, the U-rich sequences that play an essential role in transcription termination in E. coli can be replaced without the loss of function in GC-rich Streptomyces sp. (28). This suggests that functional diversity can exist even within the conserved regions of RNAP, and thus enzymes from evolutionary distant species could respond differently to regulatory nucleic acid signals. To address this question, we compared properties of highly purified enzymes from E. coli and B. subtilis using a panel of in vitro transcription assays. We report that these enzymes exhibit an amalgam of common and idiosyncratic characteristics. Both RNAPs responded to the E. coli elongation factors NusA and GreA and recognized a subset of pause and termination signals similarly. In contrast, promoter-utilization patterns and recognition of the hairpin-dependent pause sites, as well as some arrest and termination signals, were significantly different. Our findings suggest that bacterial RNAPs, despite functional and sequence similarities, could exhibit dramatic differences at the level of catalysis and signal recognition and urge prudence in generalizing conclusions obtained with any single enzyme.
MATERIALS AND METHODS
Sources of proteins and reagents.
Oligonucleotides were obtained from Operon Technologies (Alameda, Calif.), nucleoside triphosphates (NTPs) were from Pharmacia (Piscataway, N.J.), [α-32P]CTP and [α-32P]UTP were from NEN (Boston, Mass.), and other chemicals were from Sigma (St. Louis, Mo.). E. coli core RNAP (2), NusA (58), ς70 (19), GreA (16), and B. subtilis core RNAP (1) were purified as described elsewhere.
DNA templates.
Plasmid pIA226 was constructed by cloning a PCR-generated cassette encoding a unique BbsI site between the BsaBI and XbaI sites of pDW3 (16) to generate pCM101, followed by cloning of the his pause signal and the surrounding sequences from pCL102 (2) between BbsI and SphI sites of pCM101. Plasmid pIA253 was constructed by cloning of the synthetic oligonucleotide cassette (top strand, TCCTCGGCTTTTTTTTTCGCG; bottom strand, GATCCGCGAAAAAAAAGCCG) between the BbsI and BamHI sites of pCM101. Sequences of the transcribed regions in the vicinity of the studied regulatory signals are indicated in each figure; the complete sequences will be made available upon request. Linear templates for in vitro transcription were generated by PCR amplification.
Halted complex formation.
Elongation complexes were formed with a 40 nM concentration of linear DNA template and 50 nM RNAP holoenzyme in 20 to 100 μl of transcription buffer (20 mM Tris-HCl, 20 mM NaCl, 10 mM MgCl2, 14 mM 2-mercaptoethanol, 0.1 mM EDTA; pH 7.9). On the T7A1 promoter templates, elongation complexes can be halted at positions indicated in figure legends when transcription is initiated in the absence of UTP, with ApU at 150 μM, ATP and GTP at 2.5 μM, and CTP at 1 μM, with 32P derived from [α-32P]CTP (3,000 Ci/mmol) (34). On the λ PR promoter templates (pIA253 and pIA226) elongation complexes can be halted at position A26 when transcription is initiated in the absence of CTP, with ApU at 150 μM, ATP and GTP at 2.5 μM, and UTP at 1 μM, with 32P derived from [α-32P]UTP (3,000 Ci/mmol). Halted complexes were formed for 15 min at 37°C and stored on ice prior to use as described previously (34).
Abortive initiation assays.
Reactions were assembled on ice in 50 μl of transcription buffer with ApU at 150 μM, ATP and CTP at 20 μM, and 10 μCi of [α-32P]CTP (3,000 Ci/mmol). Linear pRL550 (16) DNA template was at 40 nM, and RNAP holoenzyme containing ς70 was at 50 nM. The E. coli GreA protein was added to 800 nM where indicated. Transcription was initiated by shifting samples to 37°C. Samples (5 μl) were removed at the times indicated above each lane and after a final 5-min incubation with 500 μM concentrations of each NTP (chase) and quenched by the addition of an equal volume of STOP buffer (10 M urea, 20 mM EDTA, 45 mM Tris-borate; pH 8.3).
Single-round pause assays.
Halted complexes were formed as described above in 50 μl of transcription buffer. Transcription was restarted by the addition of nucleotides (at the concentrations indicated in figure legends) and heparin to 100 μg/ml. When present, elongation factors NusA and GreA were added simultaneously with the nucleotides to final concentrations of 50 and 800 nM, respectively. Samples were removed at the times shown in the figures and after a final 5-min incubation with a 250 μM concentration of each NTP (Chase) and then quenched as described above. Pause half-life (the time during which half of the complexes reenter the elongation pathway) and pause efficiency (fraction of transcribing RNAP molecules that pause) were determined by nonlinear regression analysis as described previously (34).
Termination assays.
Templates encoding different Rho-independent terminators were described previously (16, 53); the sequences of the terminator regions with dyad symmetry elements (underlined) and 8 nucleotides (nt) of the following “U-tracks” (lowercase) were as follows: T7Te, GGCUCACCUUCGGGUGGGCCuuucugcg, plasmid pAR1707 (53); T3Te, GGCUCACCUUCACGGGUGGGCCuuucuucg, plasmid pCPG T3Te (53); rrnB T1, GGCACAGUCGAAAGACUGGGCCuuucguuu, plasmid pCPG rrnB T1 (53); λ tR2, GGCCUGCUGGUAAUCGCAGGCCuuuuuauu, plasmid pCPG λ tR2 (53); P14, GCCUCCGGUCGGAGGCuuuugacu, plasmid pCPG P14 (53); BS7, CAGCCGUUGCCAGAAAGAGGCACGGCUGuuuuuauu, plasmid pCPG BS7 (53); tonB, GCCUCCGACCGGAGGCuuuugacu, plasmid pCPG tonB (53); and his T, GCCCCCGGAAGAUGCAUCUUCCGGGGGCuuuuuuuu, plasmid pGF104 (16). Halted [32P]CTP-labeled A20 elongation complexes were prepared as described above in 20 μl of transcription buffer with a 40 nM concentration of linear DNA templates and a 50 nM concentration of RNAP holoenzymes containing ς70. Elongation was restarted by the addition of NTPs to 400 μM each, KCl to 100 mM, and heparin to 25 μg/ml. Reactions were incubated at 37°C for 15 min and stopped by the addition of an equal volume of the STOP buffer. RNA products were analyzed on 5% denaturing gels, and the termination efficiencies were determined as described previously (53).
Sample analysis.
Samples were heated for 2 min at 90°C and separated by electrophoresis in denaturing acrylamide (19:1) gels (7 M urea, 0.5× Tris-borate-EDTA) of various concentrations (5 to 20%). RNA products were visualized and quantified using a Molecular Dynamics (Piscataway, N.J.) PhosphorImaging System, ImageQuant Software, and Microsoft Excel (34).
RESULTS
Core-specific differences in utilization of λ PR and T7A1 phage promoters.
The formation of open complexes at most E. coli promoters is essentially irreversible (25). In contrast, at the majority of promoters, B. subtilis enzyme forms unusually unstable open complexes that are in equilibrium with closed complexes and, in turn, with free RNAP (12, 57, 69). Consequently, B. subtilis core RNAP complexed with its major ς factor (ςA) utilizes E. coli promoters poorly (26, 46), in spite of the essential identity of the −35 and −10 consensus promoter elements recognized by ςA and its E. coli homolog, ς70 (23, 24). This suggests that the differences between the two enzymes are unlikely to be solely encoded within the ς factors. In fact, in the E. coli promoter, utilization can be affected by mutations not only in ς but also in β and β′ subunits (5, 50, 59, 73). In addition, B. subtilis core RNAP frequently copurifies with a dispensable δ subunit, which affects in vitro transcription properties of the enzyme dramatically, apparently by binding to RNAP and reducing stability of the RNAP-DNA complexes (39). However, the B. subtilis core preparation we used in this study did not contain δ factor (1), and therefore the differences between the two enzymes could not be due to the presence of δ.
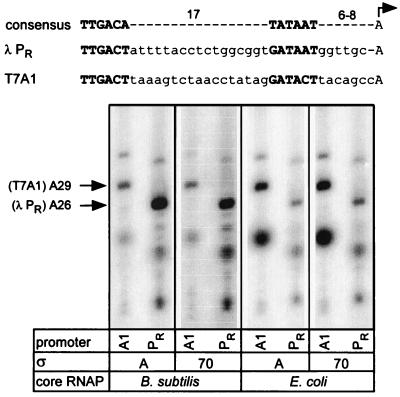
We compared the ability of both RNAPs to form ternary elongation complexes (TECs) on templates carrying two strong bacteriophage promoters, T7A1 and λ PR (Fig. 1). We found that B. subtilis ςA holoenzyme (EBS · ςA) readily utilized λ PR promoter but was defective in the formation of halted complexes at T7A1 promoter. This pattern of promoter utilization is in sharp contrast to the E. coli ς70 holoenzyme (EEC · ς70), which preferred T7A1 promoter to λ PR. In order to determine whether the promoter discrimination activity resided in the polymerase core or ς components, we prepared holoenzymes containing heterologous ς factors (EBS · ς70 and EEC · ςA). This reciprocal substitution of the ς factors did not alter the pattern of promoter preference for each of the two cores used, leading to the conclusion that differential utilization of T7A1 and λ PR promoters appears to be determined principally by the core RNAP (Fig. 1).
FIG. 1.
Promoter selectivity of the E. coli and B. subtilis RNA polymerases. (Top) The consensus E. coli promoter is aligned with λ PR and T7A1 promoters with the −35 and −10 hexamers shown in boldface, and the transcription start site is indicated with a bent arrow. (Bottom) Elongation complexes were formed for 15 min at 37°C on the linear DNA templates encoding either λ PR (PR, pIA226) or T7A1 (A1, pCL102) promoter with holoenzymes composed of the core and ς components indicated below each lane in the presence of limited subset of NTP substrates (see Materials and Methods). The positions of halted RNA transcripts originating from each promoter are indicated on the left. Bands below the A26 and A29 RNAs correspond to abortive products.
Studies by Henkin and Sonenshein (26), who isolated mutations that increased transcription from the E. coli lacUV5 promoter by B. subtilis RNAP, also suggest the relative importance of the Bacillus core interaction with the promoter DNA. In addition to mutations that improved the −35 and the extended −10 motifs and thus increased transcription by both E. coli and B. subtilis enzymes, several mutants were isolated that did not affect promoter activity in E. coli significantly but increased promoter activity up to eightfold in Bacillus. These substitutions were located around the start site (at positions −5 to +4), the region that apparently interacts with the core RNAP (20, 30, 59).
Having established that the origin of the major ς subunit has little impact on the species-specific properties of the holoenzyme, we used only ς70 in all following experiments.
Bacillus RNAP is less prone to abortive transcription.
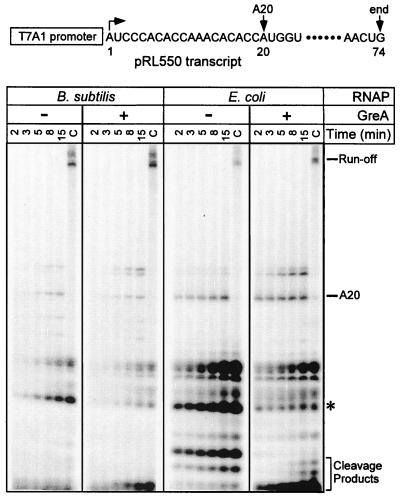
Formation of stable promoter complexes frequently results in increased production of abortive products (8, 15), probably because RNAP cannot release its contacts with the promoter DNA and clear the promoter. Conversely, if Bacillus RNAP forms less-stable open complexes, it would be expected to have fewer problems during initiation at promoters limited for escape. To test this hypothesis, we utilized a plasmid encoding the T7A1 promoter followed by an unfavorable initial transcribed sequence that inhibits promoter escape by E. coli RNAP in vitro (pRL550) (16). In the presence of initiating ApU dinucleotide and two NTP substrates (A and C), EEC · ς70 produced a large amount of abortive transcripts (Fig. 2) and a small amount of the A20 halted complex (longer products result from transcript slippage at this promoter [16]). Upon addition of all four NTPs (C lanes), the 74-nt runoff transcript is synthesized. The addition of a 20-fold molar excess of the cleavage factor GreA (to 800 nM) alleviated the escape defect moderately. In contrast, EBS · ς70 produced fewer abortive transcripts, both in the absence and in the presence of GreA. With both enzymes, GreA preferentially targeted the same abortive product, which is indicated by an asterisk in Fig. 2.
FIG. 2.
Abortive initiation assays. The pRL550 template shown on the top encodes the T7A1 promoter followed by a mutant initial transcribed sequence that has been shown to impede promoter escape by the E. coli RNAP in vitro (16). The transcription start site is indicated by a bent arrow; the positions of halted A20 RNA and the template end are also shown. Multiple-round abortive initiation assays were carried out with either EEC · ς70 or EBS · ς70, in the absence or presence of the E. coli GreA protein. Aliquots were withdrawn at the times indicated above each lane. After the last aliquot was withdrawn, the sample was incubated with NTPs (at 500 μM each) for 5 min at 37°C to generate the chase sample (the last lane in each panel [i.e., lanes C]). Positions of different RNA species are indicated on the right. The major GreA-sensitive RNA product is shown with a star.
B. subtilis fails to pause at the hairpin-dependent sites.
Progression of RNAP along the template is not monotonous and is interrupted by pause, arrest, and termination signals (65). Pausing by the E. coli RNAP is mediated by two distinct classes of signals. The class I pause signals are distinguished by the presence of a stable RNA hairpin, which appears to delay escape from the pause via direct interaction with RNAP (67). In contrast, class II pause signals do not encode RNA secondary structures; at these sites reverse translocation (backtracking) of RNAP along the template slows nucleotide addition by removing the 3′ end from the active site (3). In E. coli, transcriptional pausing is thought to play an important regulatory role during the expression of genes regulated by attenuation (33). Interestingly, in B. subtilis the corresponding operons are also regulated by attenuation, but the attenuation mechanisms do not rely on transcriptional pausing (see Discussion). We therefore wanted to find out if Bacillus RNAP would recognize heterologous pause sites from E. coli.
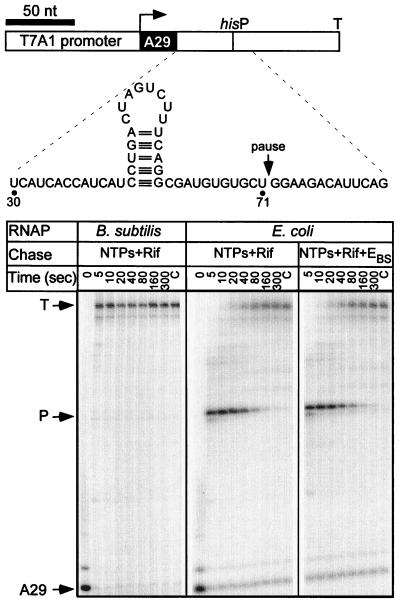
The best-characterized class I pause is the his pause site, a multicomponent signal that depends in large part on the formation of a nascent RNA hairpin 11 nt upstream from the pause site (Fig. 3). In addition to the hairpin, three components control recognition of the his pause: the ∼14-bp downstream DNA, the 11-nt 3′-proximal RNA segment between the hairpin and the transcript 3′ end, and the bases in the active site (9). We used EBS · ς70 and EEC · ς70 holoenzymes to transcribe the template that encodes the his pause signal (Fig. 3). The E. coli RNAP recognized the his site with 80% efficiency and a half-life of 50 s (see Materials and Methods for definitions). In contrast, B. subtilis enzyme failed to pause at the his and the related trp sites (Fig. 3 and data not shown).
FIG. 3.
Recognition of the his pause site. (Top) Linear pCL102 DNA template is drawn to scale with the positions of T7A1 promoter, transcription start and his pause sites, and the his terminator from the attenuator region of the E. coli his biosynthetic operon (33) indicated. The structure of the RNA transcript in the vicinity of the pause site is depicted in the inset. (Bottom) Halted A29 TECs were formed with either EEC · ς70 or EBS · ς70 RNAP and challenged with NTPs (20 μM GTP and 150 μM ATP, CTP, and UTP) and rifampin (Rif) at 50 μg/ml. In the rightmost panel, EBS · ς70 was added to the reaction (to 50 nM) simultaneously with NTPs and rifampin. Aliquots were withdrawn at the times indicated above each lane, followed by the high NTP chase (C lanes as described for Fig. 2. The positions of the halted (A29), paused (P), and terminated (T) transcripts are indicated with arrows.
The inability of the Bacillus RNAP to pause at the his site could be explained in different ways. First, the Bacillus enzyme preparation may contain an unidentified factor (in substoichiometric amounts and hence not detected by protein gel analysis) that possesses antipausing activity. To test this possibility, halted E. coli TECs were incubated with NTPs and rifampin (to prevent transcription reinitiation) in the presence of B. subtilis RNAP. We found that pausing at the his pause site was not suppressed (Fig. 3), indicating that an antipausing factor is either absent in B. subtilis RNAP or is unable to affect the E. coli enzyme activity.
Second, the failure to recognize the his pause site could be due to the lower Km for the NTP addition; the generally faster transcription rate by Bacillus enzyme (data not shown) is consistent with this explanation. However, the lower Km for the incoming GTP cannot fully explain the lack of pausing at the his pause site since B. subtilis enzyme recognized the P RNA pause site (which also occurs between U and G residues, see below), although with a lower half-life and efficiency compared to E. coli RNAP.
Finally, Bacillus RNAP may be unable to recognize other components of the his pause signal: the RNA hairpin, the 3′-proximal RNA, or the downstream DNA. Among these three components, the RNA hairpin is thought to play the critical role in slowing elongation at the pause site: at least in E. coli, the pause half-life can be reduced by 20-fold when the hairpin formation is prevented (2). The his pause hairpin has been shown to contact the β subunit of E. coli RNAP between residues 904 and 950 (67); this interaction apparently stabilizes the paused TEC and delays RNAP escape from the pause site. Since the corresponding region of the B. subtilis β subunit differs from the E. coli β at several positions, the inability of B. subtilis RNAP to pause at the class I sites could be due (at least in part) to its inability to interact with RNA hairpins. To test this possibility, we replaced amino acid residues 849 through 935 of the E. coli β with the homologous fragment of the B. subtilis β. This hybrid enzyme recognized the his pause site in vitro similarly to the wild-type E. coli RNAP (data not shown). Therefore, the differences in contacts to other components of the his pause signal are likely responsible for the inability of Bacillus RNAP to pause at this site.
Bacillus RNAP pauses at hairpin-independent pause sites.
Unlike class I pause signals that depend on a combination of correctly positioned components, class II pause signals could arise fortuitously in response to sequences that induce backtracking by RNAP. However, certain class II pause signals could play important regulatory roles. Interestingly, phage, bacterial, and mammalian RNAPs all recognize one of the class II sites as an elongation block in vitro (47), suggesting that diverse enzymes respond similarly to variations in the thermodynamic stability of the RNA-DNA hybrid. Thus, it seemed likely that the B. subtilis RNAP would recognize the class II sites that induce pausing by its E. coli counterpart.
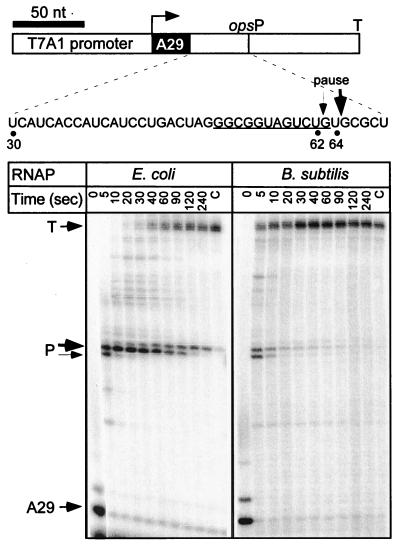
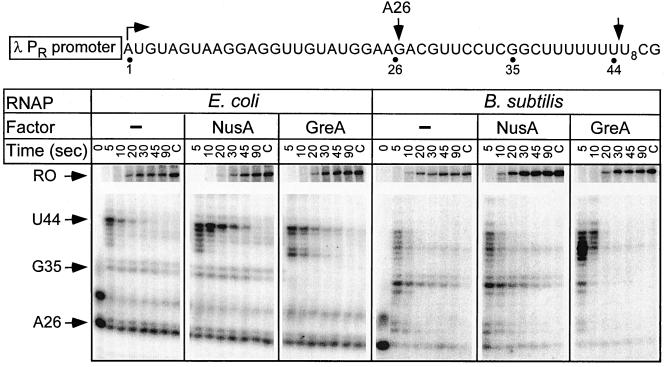
Two such sites have been characterized: the ops signal (3) and the U-track (21, 43). E. coli RNAP paused at the pheP ops signal after the addition of U62 and U64 residues (Fig. 4). Bacillus RNAP also recognized the ops pause site, but with a lower half-life and efficiency. In E. coli, the ops pause sites are thought to recruit elongation factor RfaH to the ternary complex; however, these sites are unlikely to have a similar regulatory function in B. subtilis since RfaH is absent in this species (27). In addition, Bacillus RNAP was less prone to arrest at A29 (Fig. 3 and 4) and A26 positions (Fig. 5), as shown by the comparison of the amounts of nonchaseable RNA species (lanes C in Fig. 3 to 5). In contrast, the U-tracks that specify the pause after termination hairpins appear to play essential role in both bacterial species: the E. coli (21, 43) and B. subtilis (6) enzymes have been shown to pause at the U-tracks following the terminator hairpins in vitro. We cloned the U8-track (without the preceding hairpin) under control of λ PR promoter (Fig. 5) and tested its recognition by E. coli and B. subtilis enzymes in the presence of the E. coli elongation factors NusA and GreA. Both RNAPs paused at multiple positions within the U-track (from U2 through U7; the pause positions were mapped by “walking” RNAP through this region; data not shown); however, the pausing window appeared to be shifted upstream with the Bacillus enzyme. The strongest pausing by E. coli RNAP occurred between the residues U7 and U8 and was enhanced in the presence of NusA (Fig. 5). In contrast, GreA preferentially enhanced pausing after U2. Bacillus RNAP paused weakly and evenly in the absence of elongation factors; GreA significantly enhanced pausing after U4, whereas NusA had little if any effect (Fig. 5). Interestingly, RNA release at the terminator in the attenuator region of the B. subtilis pyr operon also occurs after the U4 position following the hairpin (40), compared to U7 at many E. coli terminators (11). The slight differences in response of both RNAPs to NusA and GreA could be due to the fact that these proteins are only moderately conserved between the two species, with 37 and 40% identities, respectively. In addition, B. subtilis NusA protein is missing a large C-terminal region compared to its E. coli ortholog; however, this part of the protein appears to be largely dispensable in vivo (64).
FIG. 4.
Recognition of the ops pause site. (Top) Linear pIA251 DNA template is drawn to scale; the positions of T7A1 promoter, transcription start and ops pause sites, and the his terminator are indicated. The RNA sequence in the vicinity of the pause sites (major at U64, minor at U62) is also shown. (Bottom) Transcription complexes halted at position A29 were incubated with NTPs (20 μM GTP and 150 μM ATP, CTP, and UTP) in the presence of heparin (at 100 μg/ml). Aliquots were withdrawn at times indicated above each lane, followed by the high NTP chase (lanes C) as described in Fig. 2. The positions of the halted (A29), paused (P), and terminated (T) transcripts are indicated.
FIG. 5.
Pausing at the U-track signal. The pIA253 template shown on the top encodes the λ PR promoter followed by a 26-nt C-less cassette and a stretch of eight consecutive U residues (positions 38 to 45). The positions of the transcription start site, the halted A26 RNA, and the major E. coli pause site are indicated with arrows. Transcription complexes halted at position A26 were challenged with NTPs (20 μM UTP and 150 μM ATP, CTP, and GTP) and heparin (at 100 μg/ml); the E. coli NusA or GreA proteins were added where indicated. Aliquots were withdrawn at the times indicated above each lane, followed by the high NTP chase (lanes C) in the last lane of each panel. The positions of the A26, G35, and U44 RNA transcripts are indicated with arrows. The portion of the gel between the U-track and the terminator (T) has been deleted to conserve space.
Both enzymes recognize the pause site in B. subtilis P RNA transcript.
We recently reported that the rate and the pathway of folding of the B. subtilis P RNA ribozyme is drastically affected by pausing during in vitro transcription by E. coli RNAP (52). Addition of the E. coli NusA protein accelerated folding more than 10-fold. We mapped a major pause site to position U55 of the wild-type P RNA (I.A. and R.L., unpublished observations) and demonstrated that pausing at this site is significantly enhanced by NusA (52). Substitutions that eliminated pausing affected the folding pathway, suggesting an important regulatory role for this signal. However, the biological relevance of this observation relies on the assumption that B. subtilis RNAP displays congruent elongation behavior. We therefore set out to determine if B. subtilis enzyme recognizes this pause site in vitro.
The P RNA pause site is structurally similar to the his pause site: it is preceded by an RNA hairpin at the distance of 12 nt (Fig. 6), which is 1 to 2 nt longer than typical for the hairpin-dependent pause sites in E. coli (9). However, we recently found that pausing at this site by E. coli RNAP is hairpin independent, and the role of the hairpin appears to be limited to the recruitment of NusA (I.A. and R.L., unpublished observations). Both E. coli and B. subtilis RNAPs paused at U72 (Fig. 6). Addition of the E. coli NusA protein increased the pause half-life and efficiency; however, this effect was much more pronounced with the E. coli enzyme, suggesting that interactions of NusA with B. subtilis RNAP may be inadequate. Although these observations support our previous conclusion about the importance of pausing in determining the RNA folding pathway (52), the in vivo role of the P RNA pause signal remains to be established.
FIG. 6.
Recognition of the pause site in the B. subtilis P RNA transcript. (Top) Linear pIA199 DNA template is drawn to scale with the positions of T7A1 promoter, transcription start and pause sites, and position of the template end indicated; position 17 corresponds to the +1 of P RNA. Structure of the RNA transcript in the vicinity of the pause site (at U73) is also shown. (Bottom) Pause assays were performed with either EEC · ς70 or EBS · ς70. Transcription complexes halted at position G16 were challenged with NTPs (20 μM GTP and 150 μM ATP, CTP, and UTP) and heparin in the absence or presence of the E. coli NusA protein (at 50 nM). Aliquots were withdrawn at the times indicated above each lane; the chase samples (lanes C) were generated as described in Fig. 5. The positions of the pause (P) and runoff (end) transcripts are indicated with arrows.
B. subtilis and E. coli enzymes differ in recognition of termination signals.
Similar to the his pause site, bacterial intrinsic terminators are multicomponent signals. The RNA hairpin is variable in the length of the stem and the size of the loop and is followed by a stretch of three or more U residues; mutations in both affect termination in vivo and in vitro (55). Efficiency of terminators can also be affected by changes in the DNA region immediately following the termination position (62), the identity of the promoter-proximal sequences (18, 63), and the region immediately upstream of the terminator hairpin (54).
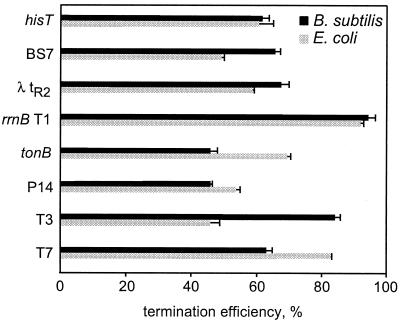
We tested in vitro efficiencies of a collection of terminators (16, 53) ranging from strong (hisT, rrnB T1, and T7) to relatively weak (T3, P14, BS7, tonB, and λ tR2) by E. coli and B. subtilis RNAPs under identical conditions (Fig. 7). We found that B. subtilis enzyme was as proficient as its E. coli counterpart (<10% difference) in terminating transcription at the rrnB T1, P14, and his terminators and actually terminated transcription better at the BS7, λ tR2, and T3 sites. In contrast, it displayed reduced termination efficiency at T7 and tonB terminators (Fig. 7). Terminators annotated in the B. subtilis genome (31) frequently possess perfect U-stretches, and a relatively low efficiency of the Bacillus phage SPO1 TL2 terminator has been suggested to result from substitutions in this region (6). Thus, reduced recognition of the T7 and tonB terminators by the Bacillus RNAP could be attributed to the fact that both signals have non-U residues following the hairpin (UUUCUGCG for T7 and UUUGACU for tonB). In E. coli, deletions in the U-track dramatically reduce termination efficiency at some sites (41, 53); the T7 terminator is, however, one of the strongest intrinsic terminators in vivo and in vitro. On the other hand, the 3′-proximal region of the T3 terminator (UUUCUUCG) differs from the T7 sequence in just one position, but T3 is a mediocre release site in vitro (53, 62). In turn, the P14 and tonB terminators share identical sequences between the hairpin and the release point. Altogether, it seems unlikely that sequences of the 3′-proximal regions could solely account for the differences in termination observed between the two enzymes.
FIG. 7.
Recognition of Rho-independent terminators. The efficiency of termination at several previously characterized terminators (see Materials and Methods for sequences) by B. subtilis (black bars) and E. coli (gray bars) enzymes is depicted graphically. Each value represents an average of four or five independent measurements; the error bars correspond to one standard deviation.
DISCUSSION
In this study we compare in vitro transcription by RNAPs from two bacterial species: gram-negative E. coli and gram-positive B. subtilis, reconstituted from highly purified components with their cognate or heterologous major ς subunits. We report that these enzymes profoundly differ in their recognition of several regulatory sequences, including promoters, hairpin-dependent pauses, certain intrinsic terminators, and arrest signals, and yet respond similarly to other elongation signals. The differences between the E. coli and B. subtilis enzymes are determined by the core component of the enzyme in vitro, but the exact structural determinants responsible for the altered signal recognition are difficult to pinpoint due to a substantial divergence of these two proteins. Compared to E. coli, B. subtilis enzyme contains multiple amino acid changes, including those in regions implicated in pausing, termination, and open complex stability (32, 68, 73), and is missing three so-called dispensable regions (two in β and one in β′); overall, the two RNAPs are only about 50% identical. Differences in the recognition of nucleic acid signals by B. subtilis and E. coli enzymes could be mediated by the same part of RNAP. Alternatively, each class of signals (initiation, elongation, and termination) could be specifically affected by a separate region of RNAP. We favor the former proposition and argue that the observed differences in signal processing by the E. coli and B. subtilis RNAPs could result from changes in the part of RNAP that makes contacts with the DNA in front of the active site.
Altered downstream DNA contacts could explain many differences in signal recognition.
Interactions between E. coli RNAP and the downstream duplex DNA affect the recognition of various regulatory signals, such as promoters (26, 37), pause sites (36), and intrinsic terminators (62). For several reasons, we think that these interactions could account for many differences between the B. subtilis and E. coli enzymes during transcription. First, altered contacts to the downstream DNA duplex could explain why, unlike E. coli RNAP, Bacillus enzyme is unable to protect the downstream DNA from the DNase I digestion (49, 69) and forms unstable open complexes at many promoters (12, 57, 69). Substitutions that confer open complex instability in the E. coli enzyme (5, 59, 73) are located not only in the downstream clamp, which interacts with the duplex DNA directly (30), but also in the rifampin-binding pocket that interacts with the RNA-DNA hybrid (30). These regions are proposed to be linked allosterically, so that the altered contacts in one region could lead to changes in the other (45).
Second, strong interactions between RNAP and the template DNA could inhibit escape from some promoters. These interactions could be mediated by the upstream promoter regions in contact with the α and ς subunits (15) or by the DNA downstream from the active site of RNAP (37) that interacts with β and β′ (30). In this study we demonstrate that, in contrast to E. coli RNAP, Bacillus RNAP is able to clear a promoter that is limited for escape due to an unfavorable transcribed region that traps E. coli enzyme (Fig. 2). In E. coli, altered interactions between RNAP and this promoter region could lead (either directly or indirectly) to a tight binding to the downstream DNA, whereas in B. subtilis the lack of contacts between the β and β′ subunits and the downstream DNA could facilitate escape from the promoter. However, the B. subtilis and E. coli RNAPs are both hindered at a promoter that strongly interacts with the α and ς subunits (I. Artsimovitch, T. Gaal, R. L. Gourse, and R. Landick, unpublished observations; see also reference 7), suggesting that α- and ς-mediated promoter contacts are similar between the two enzymes.
Third, B. subtilis and E. coli RNAPs responded differently to pause and termination signals whose recognition is sensitive to changes in the downstream DNA region (36, 62). Most strikingly, Bacillus enzyme did not pause at the E. coli class I pause sites (Fig. 3). In addition, B. subtilis RNAP terminated transcription at the T3 terminator more efficiently than the E. coli enzyme (Fig. 7). The low efficiency of RNA release at the T3 terminator in vitro by E. coli RNAP has been attributed to DNA sequences in the region between 3 and 7 nt downstream from the release site (62); mutations in this region convert the strong T7 terminator into a weak T3-like signal. Altered downstream contacts could explain the differential utilization of the T3 terminator, as well as the failure of Bacillus RNAP to pause at the class I pause sites, whose recognition is also affected by the downstream DNA sequences (36). A favorable downstream DNA sequence could enhance pausing and thus suppress such a defect by inducing backtracking of RNAP in the vicinity of a pause-termination site. The idea that similar interactions could control both promoter utilization and elongation is supported by observations that mutations that reduce the open complex stability (5) also reduce pausing at the his pause site (I. Artsimovitch, M. Bartlett, R. L. Gourse, and R. Landick, unpublished observations).
The differences in recognition of these nucleic acid signals could be due to substitutions within the conserved regions or to the deletions and/or insertions of large variable regions. An attractive hypothesis is that the downstream contacts could be altered, either directly or indirectly, by the dispensable regions of the E. coli β and β′ subunits that are adjacent to parts of RNAP forming the downstream DNA clamp (30) but are absent in Bacillus. A large deletion in the E. coli β subunit that includes dispensable region I has been reported to recapitulate some of the B. subtilis RNAP properties (59). In addition, we found that deletion of the β′ dispensable region (positions 943 to 1130 in the E. coli enzyme) also leads to a “Bacillus-like” behavior in vitro by reducing significantly both open complex stability and the his pause half-life (manuscript in preparation).
Class I transcriptional pausing may be nonessential in Bacillus.
Pausing is thought to play an important regulatory role during attenuation in amino acid and nucleotide biosynthetic operons in E. coli (33). The hairpin-dependent pause sites in the leader regions of the his and trp operons delay RNAP until the arrival of the ribosome, which in turn controls the expression of downstream genes by affecting the competition between terminator and antiterminator (33). Although an attenuation mechanism is implicated in regulation of the Bacillus trp operon, there is no evidence for the role of transcriptional pausing in this process (4). Instead, a trans-acting protein called TRAP in the presence of tryptophan binds to the nascent RNA and either destabilizes the antiterminator hairpin or blocks the ribosome-binding site (4, 61). Another attenuation mechanism, which in E. coli utilizes pausing at tandem U residues to regulate production of pyrimidine nucleotides (33), in Bacillus also relies on the trans-acting protein, PyrR (40). Interestingly, the requirement for two general elongation factors that affect pausing and termination, NusA and NusG, in Bacillus is also quite distinct from that in E. coli. In E. coli, NusG is essential, whereas NusA could be deleted if Rho function is inhibited (72). In B. subtilis, where Rho-dependent termination appears to be not very widespread, NusG is readily dispensable, whereas NusA is essential even when Rho-dependent termination is blocked (27).
Evolutionary basis of differential signal recognition.
The differences between the E. coli and B. subtilis RNAPs likely result from combination of random genetic drift, adaptation to different habitats, and specialization to transcribing a particular genome. We cannot estimate the contribution of drift to the divergence between the two enzymes, but we can contemplate the roles of environment and genome architecture in shaping of these molecular machines during evolution. Many free-living prokaryotes live at the extremes of pressure, temperature, salinity, and pH (66) and could demonstrate a number of molecular adaptations to a particular range of conditions. For instance, a strong amino acid substitution bias has been reported between related mesophilic and thermophilic microorganisms (22). However, similar adaptations could not explain the differences between RNAPs from B. subtilis and E. coli, since their habitats tracts fall within the same temperature and pressure range.
Adaptation of RNAPs could also arise in response to particular characteristics of their DNA templates. For example, the molecular “adaptations” toward transcribing short, precisely terminated and long, stochastically terminated messages by RNAP III and RNAP II, respectively, has been proposed to explain the structural differences between their paralogous subunits Rpc11 and Rpb9 (10). We asked whether the properties of the E. coli and B. subtilis genomes could provide an explanation for the differences between the two enzymes. One of the most comprehensive surveys carried out to date indeed argues that the B. subtilis and E. coli genomes are substantially different from each other—in fact, the most polar out of eight genomes analyzed (56)—and could contribute to divergent molecular evolution of their cognate RNAPs.
Although at present we cannot suggest a detailed mechanism linking genomic differences with a particular elongation behavior of the E. coli and B. subtilis RNAPs, a possible explanation could lie in a differential requirement for the resolution of collisions with the replication machinery. In B. subtilis, 75% of genes are transcribed in the direction of the leading strand, whereas the E. coli genes are distributed essentially equally between leading and lagging strands. The head-on collisions are thought to be more disadvantageous to an organism than codirectional encounters, and heavily transcribed genes are oriented in the direction of the leading strand of the DNA replication fork (reference 38 and references therein). It is plausible that interactions between the E. coli RNAP and the downstream DNA allow the enzyme to switch from its original template to a newly synthesized DNA strand during head-on collisions (38), whereas Bacillus RNAP could be more prone to delays in resolution of these collisions. Studies of head-on collisions in vitro have demonstrated that the replication fork can bypass stalled E. coli TEC, whereas B. subtilis TEC presents an effective barrier to its progression (14, 38).
Prospects.
In this work we argue that transcriptional properties of the E. coli and B. subtilis RNAPs are not identical, and we propose that the differences in signal recognition between the two enzymes could be mediated by interactions with the downstream DNA. The availability of the high resolution structure for T. aquaticus RNAP (70) allows modeling of interactions between protein and nucleic acid components of the transcriptional machinery, even when the data are derived from other source species. Some of these interactions seem to be well conserved among bacteria since a good agreement has been reported between structural data obtained for T. aquaticus RNAP and biochemical data collected for its E. coli counterpart (51, 70). However, our findings indicate that other important interactions may differ substantially if relatively distant species were analyzed. Studies of RNAPs from other bacteria would allow for better appreciation of evolutionary plasticity of transcriptional machinery and shed light on its underlying mechanisms.
ACKNOWLEDGMENTS
We thank Rick Gourse and John Helmann for critical reading of the manuscript and helpful suggestions for its improvement. We also acknowledge a generous gift of purified B. subtilis ςA from Bradley Pietz.
This work was supported by grants from U.S. Department of Agriculture (to R.L.) and National Institutes of Health (to R.L. and R.R.B.).
REFERENCES
- 1.Anthony L, Artsimovitch I, Svetlov V, Landick R, Burgess R R. Rapid purification of the His(6)-tagged Bacillus subtilis core RNA polymerase. Protein Expr Purif. 2000;19:350–354. doi: 10.1006/prep.2000.1272. [DOI] [PubMed] [Google Scholar]
- 2.Artsimovitch I, Landick R. Interaction of a nascent RNA structure with RNA polymerase is required for hairpin-dependent transcriptional pausing but not for transcript release. Genes Dev. 1998;12:3110–3122. doi: 10.1101/gad.12.19.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Artsimovitch I, Landick R. Pausing by bacterial RNA polymerase is mediated by mechanistically distinct classes of signals. Proc Natl Acad Sci USA. 2000;97:7090–7095. doi: 10.1073/pnas.97.13.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babitzke P. Regulation of tryptophan biosynthesis: Trp-ing the TRAP or how Bacillus subtilis reinvented the wheel. Mol Microbiol. 1997;26:1–9. doi: 10.1046/j.1365-2958.1997.5541915.x. [DOI] [PubMed] [Google Scholar]
- 5.Bartlett M S, Gaal T, Ross W, Gourse R L. RNA polymerase mutants that destabilize RNA polymerase-promoter complexes alter NTP-sensing by rrn P1 promoters. J Mol Biol. 1998;279:331–345. doi: 10.1006/jmbi.1998.1779. [DOI] [PubMed] [Google Scholar]
- 6.Brennan S M, Geiduschek E P. Regions specifying transcriptional termination and pausing in the bacteriophage SP01 terminal repeat. Nucleic Acids Res. 1983;11:4157–4175. doi: 10.1093/nar/11.12.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camacho A, Salas M. Effect of mutations in the “extended −10” motif of three Bacillus subtilis sigmaA-RNA polymerase-dependent promoters. J Mol Biol. 1999;286:683–693. doi: 10.1006/jmbi.1998.2526. [DOI] [PubMed] [Google Scholar]
- 8.Carpousis A J, Gralla J D. Cycling of ribonucleic acid polymerase to produce oligonucleotides during initiation in vitro at the lac UV5 promoter. Biochemistry. 1980;19:3245–3253. doi: 10.1021/bi00555a023. [DOI] [PubMed] [Google Scholar]
- 9.Chan C, Wang D, Landick R. Multiple interactions stabilize a single paused transcription intermediate in which hairpin to 3′ end spacing distinguishes pause and termination pathways. J Mol Biol. 1997;268:54–68. doi: 10.1006/jmbi.1997.0935. [DOI] [PubMed] [Google Scholar]
- 10.Chedin S, Riva M, Schultz P, Sentenac A, Carles C. The RNA cleavage activity of RNA polymerase III is mediated by an essential TFIIS-like subunit and is important for transcription termination. Genes Dev. 1998;12:3857–3871. doi: 10.1101/gad.12.24.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.d'Aubenton Carafa Y, Brody E, Thermes C. Prediction of Rho-independent Escherichia coli transcription terminators. A statistical analysis of their RNA stem-loop structures. J Mol Biol. 1990;216:835–858. doi: 10.1016/s0022-2836(99)80005-9. [DOI] [PubMed] [Google Scholar]
- 12.Dobinson K F, Spiegelman G B. Effect of the delta subunit of Bacillus subtilis RNA polymerase on initiation of RNA synthesis at two bacteriophage phi 29 promoters. Biochemistry. 1987;26:8206–8213. doi: 10.1021/bi00399a028. [DOI] [PubMed] [Google Scholar]
- 13.Dykhuizen D E. Santa Rosalia revisited: why are there so many species of bacteria? Antonie Leeuwenhoek. 1998;73:25–33. doi: 10.1023/a:1000665216662. [DOI] [PubMed] [Google Scholar]
- 14.Elias-Arnanz M, Salas M. Resolution of head-on collisions between the transcription machinery and bacteriophage phi29 DNA polymerase is dependent on RNA polymerase translocation. EMBO J. 1999;18:5675–5682. doi: 10.1093/emboj/18.20.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellinger T, Behnke D, Bujard H, Gralla J D. Stalling of Escherichia coli RNA polymerase in the +6 to +12 region in vivo is associated with tight binding to consensus promoter elements. J Mol Biol. 1994;239:455–465. doi: 10.1006/jmbi.1994.1388. [DOI] [PubMed] [Google Scholar]
- 16.Feng G, Lee D N, Wang D, Chan C L, Landick R. GreA-induced transcript cleavage in transcription complexes containing Escherichia coli RNA polymerase is controlled by multiple factors, including nascent transcript location and structure. J Biol Chem. 1994;269:22282–22294. [PubMed] [Google Scholar]
- 17.Gaal T, Ross W, Blatter E E, Tang H, Jia X, Krishnan V V, Assa-Munt N, Ebright R H, Gourse R L. DNA-binding determinants of the alpha subunit of RNA polymerase: novel DNA-binding domain architecture. Genes Dev. 1996;10:16–26. doi: 10.1101/gad.10.1.16. [DOI] [PubMed] [Google Scholar]
- 18.Goliger J A, Yang X J, Guo H C, Roberts J W. Early transcribed sequences affect termination efficiency of Escherichia coli RNA polymerase. J Mol Biol. 1989;205:331–341. doi: 10.1016/0022-2836(89)90344-6. [DOI] [PubMed] [Google Scholar]
- 19.Gribskov M, Burgess R R. Overexpression and purification of the sigma subunit of Escherichia coli RNA polymerase. Gene. 1983;26:109–118. doi: 10.1016/0378-1119(83)90180-4. [DOI] [PubMed] [Google Scholar]
- 20.Guo Y, Gralla J D. Promoter opening via a DNA fork junction binding activity. Proc Natl Acad Sci USA. 1998;95:11655–11660. doi: 10.1073/pnas.95.20.11655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gusarov I, Nudler E. The mechanism of intrinsic transcription termination. Mol Cell. 1999;3:495–504. doi: 10.1016/s1097-2765(00)80477-3. [DOI] [PubMed] [Google Scholar]
- 22.Haney P, Badger J, Buldak G, Reich C, Woese C, Olsen G. Thermal adaptation analyzed by comparison of protein sequences from mesophilic and extremely thermophilic Methanococcus species. Proc Natl Acad Sci USA. 1999;96:3578–3583. doi: 10.1073/pnas.96.7.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawley D K, McClure W R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983;11:2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helmann J D. Compilation and analysis of Bacillus subtilis sigma A-dependent promoter sequences: evidence for extended contact between RNA polymerase and upstream promoter DNA. Nucleic Acids Res. 1995;23:2351–2360. doi: 10.1093/nar/23.13.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helmann J D, deHaseth P L. Protein-nucleic acid interactions during open complex formation investigated by systematic alteration of the protein and DNA binding partners. Biochemistry. 1999;38:5959–5967. doi: 10.1021/bi990206g. [DOI] [PubMed] [Google Scholar]
- 26.Henkin T M, Sonenshein A L. Mutations of the Escherichia coli lacUV5 promoter resulting in increased expression in Bacillus subtilis. Mol Gen Genet. 1987;209:467–474. doi: 10.1007/BF00331151. [DOI] [PubMed] [Google Scholar]
- 27.Ingham C, Dennis J, Furneaux P. Autogenous regulation of transcription termination factor Rho and the requirement for Nus factors in Bacillus subtilis. Mol Microbiol. 1999;31:651–663. doi: 10.1046/j.1365-2958.1999.01205.x. [DOI] [PubMed] [Google Scholar]
- 28.Ingham C J, Hunter I S, Smith M C. Rho-independent terminators without 3′ poly-U tails from the early region of actinophage oC31. Nucleic Acids Res. 1995;23:370–376. doi: 10.1093/nar/23.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeon Y H, Negishi T, Shirakawa M, Yamazaki T, Fujita N, Ishihama A, Kyogoku Y. Solution structure of the activator contact domain of the RNA polymerase alpha subunit. Science. 1995;270:1495–1497. doi: 10.1126/science.270.5241.1495. [DOI] [PubMed] [Google Scholar]
- 30.Korzheva N, Mustaev A, Kozlov M, Malhotra M, Nikiforov V, Goldfarb A, Darst S. A structural model of transcription elongation. Science. 2000;289:619–625. doi: 10.1126/science.289.5479.619. [DOI] [PubMed] [Google Scholar]
- 31.Kunst F, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 32.Landick R, Stewart J, Lee D. Amino acid changes in conserved regions of the β-subunit of Escherichia coli RNA polymerase alter transcription pausing and termination. Genes Dev. 1990;4:1623–1636. doi: 10.1101/gad.4.9.1623. [DOI] [PubMed] [Google Scholar]
- 33.Landick R, Turnbough C, Jr, Yanofsky C. Transcription attenuation. In: Neidhardt F, et al., editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: ASM Press; 1996. pp. 1263–1286. [Google Scholar]
- 34.Landick R, Wang D, Chan C. Quantitative analysis of transcriptional pausing by RNA polymerase: the his leader pause site as a paradigm. Methods Enzymol. 1996;274:334–352. doi: 10.1016/s0076-6879(96)74029-6. [DOI] [PubMed] [Google Scholar]
- 35.Langer D, Hain J, Thuriaux P, Zillig W. Transcription in archaea: similarity to that in eucarya. Proc Natl Acad Sci USA. 1995;92:5768–5772. doi: 10.1073/pnas.92.13.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee D N, Phung L, Stewart J, Landick R. Transcription pausing by Escherichia coli RNA polymerase is modulated by downstream DNA sequences. J Biol Chem. 1990;265:15145–15153. [PubMed] [Google Scholar]
- 37.Levin J R, Blake J J, Ganunis R A, Tullius T D. The roles of specific template nucleosides in the formation of stable transcription complexes by Escherichia coli RNA polymerase. J Biol Chem. 2000;275:6885–6893. doi: 10.1074/jbc.275.10.6885. [DOI] [PubMed] [Google Scholar]
- 38.Liu B, Alberts B M. Head-on collision between a DNA replication apparatus and RNA polymerase transcription complex. Science. 1995;267:1131–1137. doi: 10.1126/science.7855590. [DOI] [PubMed] [Google Scholar]
- 39.Lopez de Saro F Y N, Helmann J. Expression, abundance, and RNA polymerase binding properties of the delta factor of Bacillus subtilis. J Biol Chem. 1999;274:15953–15958. doi: 10.1074/jbc.274.22.15953. [DOI] [PubMed] [Google Scholar]
- 40.Lu Y, Turner R J, Switzer R L. Function of RNA secondary structures in transcriptional attenuation of the Bacillus subtilis pyr operon. Proc Natl Acad Sci USA. 1996;93:14462–14467. doi: 10.1073/pnas.93.25.14462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lynn S P, Kasper L M, Gardner J F. Contributions of RNA secondary structure and length of the thymidine tract to transcription termination at the thr operon attenuator. J Biol Chem. 1988;263:472–479. [PubMed] [Google Scholar]
- 42.Malhotra A, Severinova E, Darst S A. Crystal structure of a sigma 70 subunit fragment from E. coli RNA polymerase. Cell. 1996;87:127–136. doi: 10.1016/s0092-8674(00)81329-x. [DOI] [PubMed] [Google Scholar]
- 43.McDowell J C, Roberts J W, Jin D J, Gross C. Determination of intrinsic transcription termination efficiency by RNA polymerase elongation rate. Science. 1994;266:822–825. doi: 10.1126/science.7526463. [DOI] [PubMed] [Google Scholar]
- 44.Mooney R, Artsimovitch I, Landick R. Information processing by RNA polymerase: recognition of regulatory signals during RNA chain elongation. J Bacteriol. 1998;180:3265–3275. doi: 10.1128/jb.180.13.3265-3275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mooney R A, Landick R. RNA polymerase unveiled. Cell. 1999;98:687–690. doi: 10.1016/s0092-8674(00)81483-x. [DOI] [PubMed] [Google Scholar]
- 46.Moran C P J, Lang N, LeGrice S F, Lee G, Stephens M, Sonenshein A L, Pero J, Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186:339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- 47.Mote J J, Reines D. Recognition of a human arrest site is conserved between RNA polymerase II and prokaryotic RNA polymerases. J Biol Chem. 1998;273:16843–16852. doi: 10.1074/jbc.273.27.16843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nechaev S, Severinov K. Inhibition of Escherichia coli RNA polymerase by bacteriophage T7 gene 2 protein. J Mol Biol. 1999;289:815–826. doi: 10.1006/jmbi.1999.2782. [DOI] [PubMed] [Google Scholar]
- 49.Nechaev, S., M. Tchlenov, and K. Severinov. Dissection of two hallmarks of the open promoter complex by mutation in an RNA polymerase core subunit. J. Biol. Chem., in press. [DOI] [PubMed]
- 50.Nomura T, Ishihama A, Kajitani M, Takahashi T, Nakada N, Yoshinaga K. Promoter selectivity of Escherichia coli RNA polymerase. II. Altered promoter selection by mutant holoenzymes. Mol Gen Genet. 1984;193:8–16. doi: 10.1007/BF00327407. [DOI] [PubMed] [Google Scholar]
- 51.Opalka N, Mooney R A, Richter C, Severinov K, Landick R, Darst S A. Direct localization of a beta-subunit domain on the three-dimensional structure of Escherichia coli RNA polymerase. Proc Natl Acad Sci USA. 2000;97:617–622. doi: 10.1073/pnas.97.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan T, Artsimovitch I, Fang X, Landick R, Sosnick T. Folding of a large ribozyme during transcription and the effect of the elongation factor NusA. Proc Natl Acad Sci USA. 1999;96:9545–9550. doi: 10.1073/pnas.96.17.9545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reynolds R, Bermúdez-Cruz R M, Chamberlin M J. Parameters affecting transcription termination by Escherichia coli RNA polymerase. Analysis of 13 rho-independent terminators. J Mol Biol. 1992;224:31–51. doi: 10.1016/0022-2836(92)90574-4. [DOI] [PubMed] [Google Scholar]
- 54.Reynolds R, Chamberlin M J. Parameters affecting transcription termination by Escherichia coli RNA polymerase. II. Construction and analysis of hybrid terminators. J Mol Biol. 1992;224:53–63. doi: 10.1016/0022-2836(92)90575-5. [DOI] [PubMed] [Google Scholar]
- 55.Richardson J, Greenblatt J. Control of RNA chain elongation and termination. In: Neidhardt F, et al., editors. Escherichia coli and Salmonella: cellular and molecular biology. Vol. 1. Washington, D.C.: ASM Press; 1996. pp. 822–848. [Google Scholar]
- 56.Rocha E P, Danchin A, Viari A. Analysis of long repeats in bacterial genomes reveals alternative evolutionary mechanisms in Bacillus subtilis and other competent prokaryotes. Mol Biol Evol. 1999;16:1219–1230. doi: 10.1093/oxfordjournals.molbev.a026212. [DOI] [PubMed] [Google Scholar]
- 57.Rojo F, Nuez B, Mencia M, Salas M. The main early and late promoters of Bacillus subtilis phage phi 29 form unstable open complexes with sigma A-RNA polymerase that are stabilized by DNA supercoiling. Nucleic Acids Res. 1993;21:935–940. doi: 10.1093/nar/21.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmidt M, Chamberlin M. Amplification and isolation of Escherichia coli nusA protein and studies of its effect on in vitro RNA chain elongation. Biochemistry. 1984;23:197–203. doi: 10.1021/bi00297a004. [DOI] [PubMed] [Google Scholar]
- 59.Severinov K, Darst S A. A mutant RNA polymerase that forms unusual open promoter complexes. Proc Natl Acad Sci USA. 1997;94:13481–13486. doi: 10.1073/pnas.94.25.13481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Severinov K, Kashlev M, Severinova E, Bass I, McWilliams K, Kutter E, Nikiforov V, Snyder L, Goldfarb A. A non-essential domain of Escherichia coli RNA polymerase required for the action of the termination factor Alc. J Biol Chem. 1994;269:14254–14259. [PubMed] [Google Scholar]
- 61.Sudershana S, Du H, Mahalanabis M, Babitzke P. A 5′ RNA stem-loop participates in the transcription attenuation mechanism that controls expression of the Bacillus subtilis trpEDCFBA operon. J Bacteriol. 1999;181:5742–5749. doi: 10.1128/jb.181.18.5742-5749.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Telesnitsky A, Chamberlin M. Terminator-distal sequences determine the in vitro efficiency of the early terminators of bacteriophages T3 and T7. Biochemistry. 1989;28:5210–5218. doi: 10.1021/bi00438a044. [DOI] [PubMed] [Google Scholar]
- 63.Telesnitsky A, Chamberlin M J. Sequences linked to prokaryotic promoters can affect the efficiency of downstream termination sites. J Mol Biol. 1989;205:315–330. doi: 10.1016/0022-2836(89)90343-4. [DOI] [PubMed] [Google Scholar]
- 64.Tsugawa A, Saito M, Court D L, Nakamura Y. nusA amber mutation that causes temperature-sensitive growth of Escherichia coli. J Bacteriol. 1988;170:908–915. doi: 10.1128/jb.170.2.908-915.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Uptain S, Kane C, Chamberlin M. Basic mechanisms of transcript elongation and its regulation. Annu Rev Biochem. 1997;66:117–172. doi: 10.1146/annurev.biochem.66.1.117. [DOI] [PubMed] [Google Scholar]
- 66.Walsh M, Seckbach J. The versatility of microorganisms. In: Seckbach J, editor. Enigmatic microorganisms and life in extreme environments. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1999. pp. 155–162. [Google Scholar]
- 67.Wang D, Landick R. Preferential interaction of the his pause RNA hairpin with RNA polymerase β subunit residues 904–950 correlates with strong transcriptional pausing. Proc Natl Acad Sci USA. 1997;94:8433–8438. doi: 10.1073/pnas.94.16.8433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weilbaecher R, Hebron C, Feng G, Landick R. Termination-altering amino acid substitutions in the β′ subunit of Escherichia coli RNA polymerase identify regions involved in RNA chain elongation. Genes Dev. 1994;8:2913–2917. doi: 10.1101/gad.8.23.2913. [DOI] [PubMed] [Google Scholar]
- 69.Whipple F W, Sonenshein A L. Mechanism of initiation of transcription by Bacillus subtilis RNA polymerase at several promoters. J Mol Biol. 1992;223:399–414. doi: 10.1016/0022-2836(92)90660-c. [DOI] [PubMed] [Google Scholar]
- 70.Zhang G, Campbell E A, Minakhin L, Richter C, Severinov K, Darst S A. Crystal structure of Thermus aquaticus core RNA polymerase at 3.3 Å resolution. Cell. 1999;98:811–824. doi: 10.1016/s0092-8674(00)81515-9. [DOI] [PubMed] [Google Scholar]
- 71.Zhang G, Darst S A. Structure of the Escherichia coli RNA polymerase alpha subunit amino-terminal domain. Science. 1998;281:262–266. doi: 10.1126/science.281.5374.262. [DOI] [PubMed] [Google Scholar]
- 72.Zheng C, Friedman D. Reduced Rho-dependent transcription termination permits NusA-independent growth of Escherichia coli. Proc Natl Acad Sci USA. 1994;91:7543–7547. doi: 10.1073/pnas.91.16.7543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou Y N, Jin D J. The rpoB mutants destabilizing initiation complexes at stringently controlled promoters behave like “stringent” RNA polymerases in Escherichia coli. Proc Natl Acad Sci USA. 1998;95:2908–2913. doi: 10.1073/pnas.95.6.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]