Abstract
European wildcats (Felis silvestris silvestris) have not been investigated in large numbers for blood-associated pathogens in Germany, because wildcats, being a protected species, may not be hunted, and the collection of samples is therefore difficult. Thus, spleen tissue and whole blood from 96 wildcats from Germany found as roadkill or dead from other causes in the years 1998–2020 were examined for the prevalence of blood associated pathogens using molecular genetic tools. PCR was used to screen for haemotrophic Mycoplasma spp., Hepatozoon spp., Cytauxzoon spp., Bartonella spp., Filarioidea, Anaplasmataceae, and Rickettsiales, and positive samples were subsequently sequenced. Phylogenetic analyses were performed for Mycoplasma spp. and Hepatozoon spp. by calculating phylogenetic trees and DNA haplotype networks. The following pathogens were found: Candidatus Mycoplasma haematominutum (7/96), Mycoplasma ovis (1/96), Hepatozoon silvestris (34/96), Hepatozoon felis (6/96), Cytauxzoon europaeus (45/96), and Bartonella spp. (3/96). This study elucidates the prevalence of blood-associated pathogens in wildcats from Germany.
Keywords: Vector-borne disease, Mycoplasma, Hepatozoon, Cytauxzoon, Bartonella
Graphical abstract
Highlights
-
•
European wildcats from Germany carry different blood-associated pathogens.
-
•
Pathogens can also affect domestic cats.
-
•
Transmision by vectors or other transmisison routes are possible.
1. Introduction
Arthropod vectors are responsible for the transmission of several blood-associated parasites and bacteria, which can cause disease in wild and domestic animals, as well as in humans. Their relevance is increasing due to climate change driven migration into regions that are more temperate. Other factors promoting the introduction and spread of vector-borne pathogens (VBP) include, for instance, globalization, habitat change, loss of biodiversity, and pollution (Harrus and Baneth, 2005; Aguirre, 2009). Different wildlife species have different effects on the density of vectors (Takumi et al., 2019), and the role of wildlife in the transmission of VBP to humans and pets is not yet fully elucidated (Mackenstedt et al., 2015). Wild carnivores are important for the maintenance of the sylvatic cycle, therefore understanding the epidemiology of their VBP is crucial (Battisti et al., 2020). Wild canids and felids can spread disease-causing pathogens to their domestic counterparts and vice versa. The close relationship of pet dogs and cats to humans increases the risk of the emergence of zoonotic disease (Otranto et al., 2015).
The European wildcat (Felis silvestris silvestris) is closely related to the domestic cat (Felis silvestris catus) and inhabits pristine forests in different parts of Europe, with one main population group in Germany (Mattucci et al., 2016). Since the 17th their population has been significantly reduced as a result of human activities century, and the species is now listed as endangered (Heddergott et al., 2018; Meinig et al., 2020). However, species protection and habitat restoration has enabled the wildcat to thrive again, and there is now an estimated population size of 5000–10000 individuals (Balzer et al., 2018; European Topic Centre on Biological Diversity, 2019). Nonetheless, anthropogenic habitat fragmentation and possible genetic introgression from domestic cats still threaten their genetic integrity, and conservation measures are still essential (Hertwig et al., 2009; Eckert et al., 2010; Witzenberger and Hochkirch, 2014; Mattucci et al., 2016). Understanding the prevalence of potential pathogens in wildcat populations can be important in this context (Poirson and Dutilleul, 2014).
Only few studies on blood-associated parasites and bacteria in these animals are available due to their secretive lifestyle and, compared to the ubiquitous red fox (Vulpes vulpes), small population size, making it difficult to obtain samples from these animals (Hodžić et al., 2018a). Apicomplexan parasites such as Hepatozoon spp., Cytauxzoon spp., and Babesia spp. are frequently found in wildcats (Zaeemi et al., 2015; Gallusová et al., 2016; Veronesi et al., 2016; Hodžić et al., 2018a; Hornok et al., 2022). Hepatozoon spp. use a wide range of vertebrates as intermediate hosts and hematophagous invertebrates as definitive hosts and vectors. The transmission to their vertebrate host is mainly through ingestion of the vector but can also happen through predation via tissue cysts or transplacental transmission (Nordgren and Craig, 1984; Johnson et al., 2009; Baneth et al., 2013). Among the Hepatozoidae, Hepatozoon felis is the predominant species found in cats and wildcats, but H. silvestris and have also been described (Giannelli et al., 2017a; Hodžić et al., 2017). The vectors of feline hepatozoonosis are unknown (Hodžić et al., 2017). Among the family Theileriidae, Cytauxzoon felis, C. manul, and three newly described species, namely C. europaeus, C. otrantorum, and C. banethi, have been reported from felids. The tick species Amblyomma americanum and Dermacentor variabilis were identified as vectors for C. felis in America (Blouin et al., 1984; Reichard et al., 2010). The vector is not known for European Cytauxzoon spp., but Ixodes ricinus has been suggested due to its high abundance (Panait et al., 2021b). Bacterial pathogens transmitted by vectors to domestic cats include Bartonella spp., Anaplasma spp., Rickettsia spp., and Mycoplasma spp. (Lappin, 2018). Of these, Mycoplasma spp. has also been reported in wildcats (Willi et al., 2007; Hodžić et al., 2018a). Candidatus Mycoplasma haemominutum was first described by Foley and Pedersen (2001); it affects domestic cats as well as wild felids (Foley and Pedersen, 2001; Willi et al., 2007; Cerreta et al., 2022). Oren (2017) suggested correcting the name to Candidatus Mycoplasma haematominutum for the sake of linguistic accuracy.
Reports of Filarioidea in wildcats are only sporadic and the role of wildcats in the maintenance of the sylvatic cycle of Filarioidea, such as Dirofilaria immitis, is considered to be low (Penezić et al., 2014; Ionică et al., 2017).
In the present study, we screened blood and spleen tissue of wildcats from Germany for blood-associated pathogens using molecular genetic methods, to estimate the possibility of pathogen transmission between wildcats and domestic cats by vectors or other transmission routes.
2. Materials and methods
2.1. Sample collection
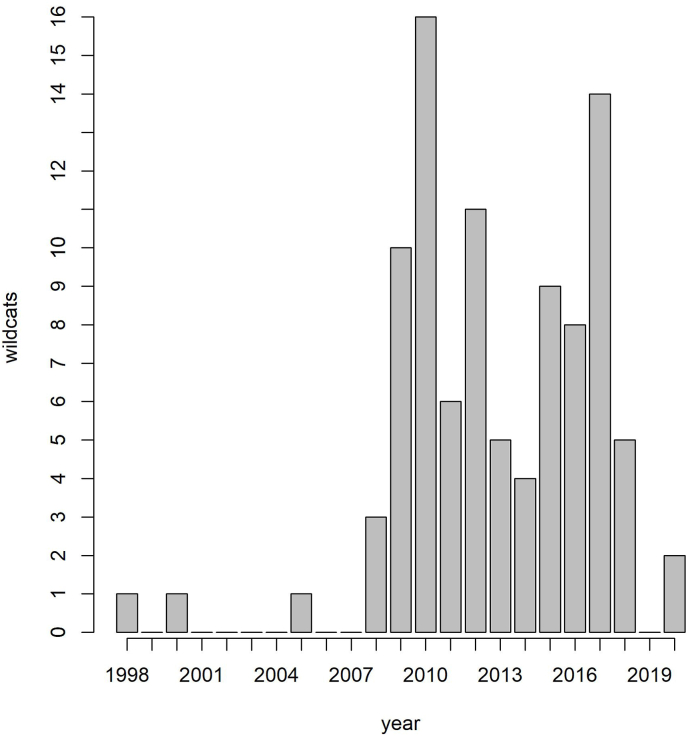
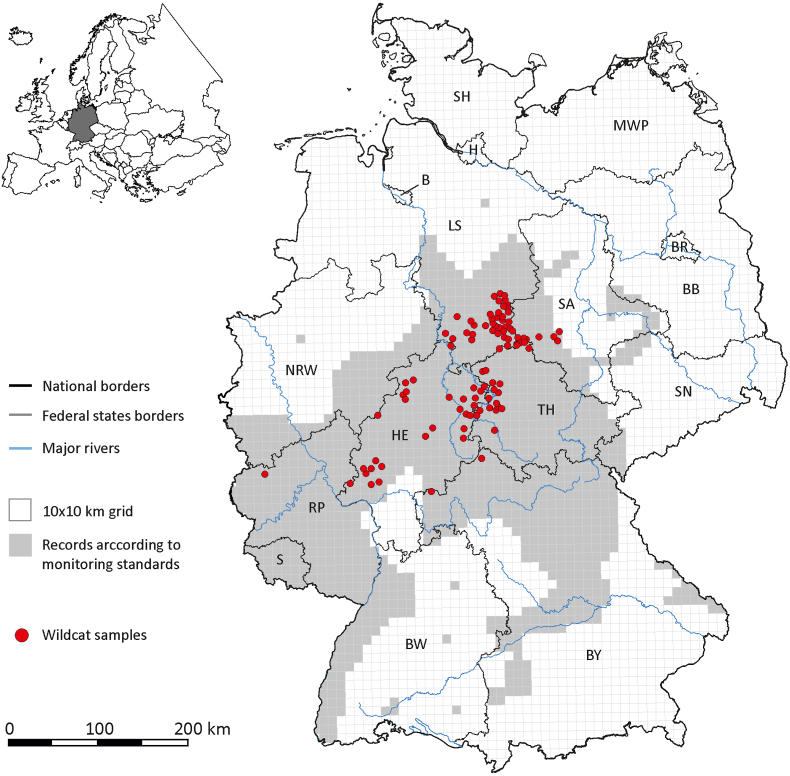
Between 1998 and 2020, 96 wildcats found as roadkill or dead from other causes were collected in Germany (Fig. 1) and stored at −20 °C until necropsy. The individuals originated from six federal states: Bavaria (n = 2), Hesse (n = 30), Lower Saxony (n = 37), Rhineland-Palatinate (n = 1), Saxony-Anhalt (n = 4), and Thuringia (n = 22) (Fig. 2). During dissection, 1–5 ml of blood were collected from 55 individuals and spleen samples from 41 individuals. The samples were stored at −20 °C until final processing at the University of Veterinary Medicine, Vienna. The cats were classified as wildcats according to the intestinal index (Braunschweig, 1963) and cranial index (Schauenberg, 1969). Individuals, where classification was not clear or not possible due to severe destruction, were genetically tested (Steyer et al., 2016). The age determination of the wildcats was based on the growth lines in the enamel of a mandibular canine (Ansorge, 1995; Heddergott et al., 2016). According to Piechocki and Stiefel (1988), the cats were assigned to two age classes: juvenile/subadult (≤24 months; none or one growth line) and adult (≥25 months; two or more growth lines).
Fig. 1.
Distribution of wildcat samples in total number of wildcats (y-axis) collected per year (x-axis).
Fig. 2.
Geographic origin of the 96 European wildcats (Felis silvestris) from Germany included in this study. The gray area represents the geographic distribution of wildcats in Germany according to the National FFH Report 2019, plotted on the 10 × 10 km reference grid ETRS89-LAEA5210 EEA according to a compilation of the German Federal Agency for Nature Conservation (BfN) and monitoring data of the federal states (Bundesamt für Naturschutz, 2020). Abbreviations: Brandenburg (BB), Bremen (B), Berlin (BR), Baden-Württemberg (BW), Bavaria (BY), Hamburg (H), Hesse (HE), Mecklenburg-West Pomerania (MWP), Lower Saxony (LS), North Rhine-Westphalia (NRW), Rhineland-Palatinate (RP), Schleswig-Holstein (SH), Saarland (S), Saxony (SN), Saxony-Anhalt (SA) and Thuringia (TH).
2.2. DNA extraction, PCR amplification, and sequencing
DNA was isolated from spleen samples and whole blood using the QIAGEN DNeasy Blood and Tissue kit (QIAGEN, Hilden, Germany). Samples were incubated at 56 °C overnight and processed according to the manufacturer's protocol. Samples were screened for the presence of various blood-associated pathogens using specific broad-range PCR assays (Table 1) targeting the following fragments: Mycoplasma spp. Within the 16 S rRNA gene, and if positive, a larger fragment of the 16 S rRNA gene; Piroplasmida and other Apicomplexa within the 18 S rRNA gene, and if positive, for Hepatozoon spp. or Cytauxzoon spp. a larger fragment of the 18 S rRNA gene; and for Cytauxzoon spp. additionally the cytochrome b gene (CytB); Bartonella spp. Within the 16 S–23 S rRNA gene, and if positive, the citrate synthase gene (gltA); Rickettsia spp. the 23 S–5S rRNA gene; Anaplasmataceae within the 16 S rRNA gene; and Filarioidea targeting a fragment of the mitochondrial cytochrome c oxidase subunit I gene (COI). Positive and negative controls were used to validate results. PCR products were analyzed by electrophoresis in 2% agarose gels stained with Midori Green Advance DNA stain (Nippon Genetics Europe, Germany). Positive samples were sent to a commercial company (LGC Genomics GmbH, Germany) for sequencing using amplification primers.
Table 1.
Oligonucleotide sequences of primers used in the present study.
| Target organism (genetic marker) | Primer sequences (5′→3′) | Product size | Reference | |
|---|---|---|---|---|
| Mycoplasma spp. (16 S rRNA) | HBT-F: ATA CGG CCC ATA TTC CTA CG | 600 bp | Criado-Fornelio et al. (2003) | |
| HBT-R: TGC TCC ACC ACT TGT TCA | ||||
| Mycoplasma spp. (16 S rRNA) | UNI_16 S_mycF: GGC CCA TAT TCC TAC GGG AAG CAG CAG T | 1000 bp | Volokhov et al. (2011) | |
| UNI_16 S_mycR: TAG TTT GAC GGG CGG TGT ACA AGA CCT G | ||||
| Hepatozoon spp. (18 S rRNA) | H14Hepa18SFw: GAA ATA ACA ATA CAA GGC AGT TAA AAT GCT | 620 bp | Hodžić et al. (2015) | |
| H14Hepa18SRv: GTG CTG AAG GAG TCG TTT ATA AAG A | ||||
| Piroplasmida (18 S rRNA) | BTH-1F: CCT GAG AAA CGG CTA CCA CAT CT | 700 bp | Zintl et al. (2011) | |
| BTH-1R: TTG CGA CCA TAC TCC CCC CA | ||||
| GF2: GTC TTG TAA TTG GAA TGA TGG | 561 bp | |||
| GR2: CCA AAG ACT TTG ATT TCT CTC | ||||
| Cytauxzoon spp. (18 S rRNA) | 7549 F: GTC AGG ATC CTG GGT TGA TCC TGC CAG | 1726 bp | Millán et al. (2007) | |
| 7548 R: GAC TGA ATT CGA CTT CTC CTT CCT TTA AG | ||||
| Cyt-SSU-F2: CAT GGA TAA CCG TGC TAA TTG | 1335 bp | Panait et al. (2021b) | ||
| Cyt-SSU-R4: AGG ATG AAC TCG ATG AAT GCA | ||||
| Cytauxzoon spp. (CytB) | Cytaux_cytb_F1: CTT AAC CCA ACT CAC GTA CC | 1434 bp | Schreeg et al. (2013) | |
| Cytaux_cytb_R3: GGT TAA TCT TTC CTA TTC CTT ACG | ||||
| Cytaux_cytb_Finn: ACC TAC TAA ACC TTA TTC AAG CRT T | 1333 bp | Panait et al. (2021b) | ||
| Cytaux_cytb_Rinn: AGA CTC TTA GAT GYA AAC TTC CC | ||||
| Bartonella spp. (16 S–23 S rRNA) | bartgd_for: GAT GAT GAT CCC AAG CCT TC | 179 bp | Jensen et al. (2000) | |
| B1623_rev: AAC CAA CTG AGC TAC AAG CC | ||||
| Bartonella spp. (gltA) | BhCS.781p: GGG GAC CAG CTC ATG GTG G | 379 bp | Norman et al. (1995) | |
| BhCS.1137n: AT GCA AAA AGA ACA GTA AAC A | ||||
| Rickettsia spp. (23 S–5S rRNA) | ITS-F: GAT AGG TCG GGT GTG GAA G | 350-550 bp | Vitorino et al. (2003) | |
| ITS-R: TCG GGA TGG GAT CGT GTG | ||||
| Anaplasmataceae (16 S rRNA) | EHR16SD_for: GGT ACC YAC AGA AGA AGT CC | 345 bp | Parola et al. (2000) | |
| EHR16SR_rev: TAG CAC TCA TCG TTT ACA GC | ||||
| Filarioidea (COI) | H14FilaCOIFw: GCC TAT TTT GAT TGG TGG TTT TGG | 724 bp | Hodžić et al. (2015) | |
| H14FilaCOIRv: AGC AAT AAT CAT AGT AGC AGC ACT AA | ||||
Note: Supplementary data associated with this article.
2.3. Phylogenetic analysis
The 18 S rRNA sequences of C. europaeus and the 16 S–23 S rRNA sequences of Bartonella spp. Were analyzed using the BLAST function on NCBI GenBank. For C. europaeus the CytB sequences were compared to those of Cytauxzoon spp. published by Panait et al. (2021b) to determine the species. For phylogenetic analysis, nucleotide sequences available on the NCBI GenBank database were searched by using the BLAST function, using one of the sequences obtained for each organism. The organism group was specified as Mycoplasma (taxid:2093) for the Mycoplasma spp. sequences and Adeleorina (taxid:75,740) for the Hepatozoon spp. sequences, with the number of maximum target sequences set to 5000. The sequences were aligned and sorted using the default option (FFT–NS–2) in MAFFT v.7.311 (Katoh and Standley, 2013) and sequences not covering the fragment of the sequences obtained in this study were excluded. All sequences featuring obvious sequencing errors and ambiguity characters were removed from the alignment and were excluded from the analysis. The chosen sequences included selected Mycoplasma spp. (based on their similarity in the alignment) and Hepatozoon spp. as well as other members of the suborder Adeleorina. Sequences used for analysis were uploaded to GenBank (GenBank accession numbers: ON202709-ON202711, ON180678-ON180682, OL415842-OL415874, ON380442-ON380486, ON855993-ON856037, and OL697395-OL697397).
To provide an overview of the diversity of haplotypes, Maximum Likelihood (ML) and Bayesian Inference (BI) trees were calculated for each organism based on alignments, including 158 sequences (975 nucleotide positions) for Mycoplasma spp. and 537 sequences (585 nucleotide positions) for Hepatozoon spp. Alignment gaps were removed using TrimAl v.1.3 (http://phylemon2.bioinfo.cipf.es/; Sánchez et al., 2011) and sequences were collapsed to haplotypes using DAMBE v.7.0.5.1 (Xia and Xie, 2001), leaving 84 haplotypes (969 nucleotide positions) for Mycoplasma spp. and 183 haplotypes (539 nucleotide positions) for Hepatozoon spp. As outgroup for Mycoplasma spp. one sequence of Mycoplasma pneumonia (GenBank accession number: NR041751) and for Hepatozoon spp. two sequences of Adelina bambarooniae (GenBank accession numbers: AF494058, AF494059) were used. ML bootstrap consensus trees (1000 replicates) were calculated using the W-IQ-TREE web server (http://iqtree.cibiv.univie.ac.at/; Trifinopoulos et al., 2016) applying the models TIM3+F + I + G4 for Mycoplasma spp. and K81u (K3P)+F + I + G4 for Hepatozoon spp., which were suggested as best fit for the data set in the model test according to the Bayesian inference criterion (BIC). The BI trees were calculated using MrBayes v.3.2.7 (Ronquist et al., 2012), applying the next complex model GTR + G + I, because the same models were not available in this program. The analysis was run for 106 generations (Number of chains: 4), sampling every thousandth tree. The first 25% of trees were discarded as burn-in and a 50% majority-rule consensus tree was calculated based on the remaining 7500 trees.
Median-joining haplotype networks were calculated with Network 10.2.0.0 (Fluxus Technology Ltd., Suffolk, UK), applying the default settings. If only one haplotype was present, pie charts were created in Excel (2016) (Microsoft Corporation, Redmond, USA). Networks and pie charts were graphically prepared and provided with information on the countries and hosts in Network Publisher v.2.1.2.3 (Fluxus Technology Ltd., Suffolk, UK) and finalized with CorelDRAW 2021 (Corel, Ottawa, Canada). Calculation of p-distances was performed with MEGA version 11 (Tamura et al., 2021).
2.4. Statistical analysis
Binary logistic regression was conducted to test the association between detection of pathogens (summarized per genus) and tissue investigated, age and sex of the animals (each fitted as fixed categorical effects with two levels), and over time. Effects were considered statistically significant if P < 0.05. No multiple testing was necessary. Statistical analysis was performed using R version 4.2.0 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
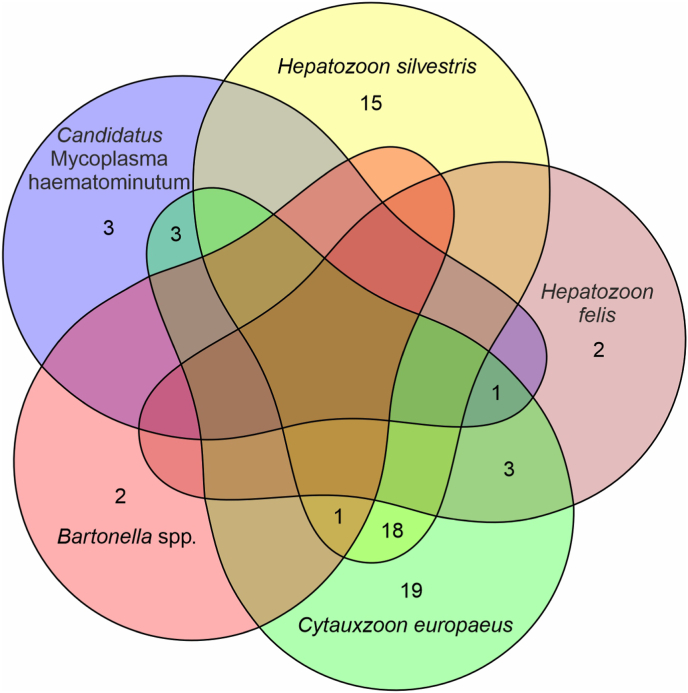
All individuals included in this study were European wildcats (Felis silvestris). The data set evaluated in this study consisted of 61 males and 35 females (comprising 25 juveniles/subadults, 70 adults, and one of unknown age). Pathogens detected were Candidatus Mycoplasma haematominutum (n = 7; 7.29%), Mycoplasma ovis (n = 1; 1.04%), Hepatozoon silvestris (n = 34; 35.42%), H. felis (n = 6; 6.25%), Cytauxzoon europaeus (n = 45, 46.88%) and Bartonella spp. (n = 3; 3.13%). All PCRs for Filarioidea, Anaplasmataceae, and Rickettsiales were negative (Fig. 3). In total, pathogens were found in 67/96 (69.79%) wildcats. One pathogen only was documented in 40/96 (41.97%) cats, two different pathogens were found in 25/96 (26.04%), and three different pathogens were detected in 2/96 (2.08%) animals (Fig. 4). Logistic regression did not detect any association between pathogen occurrence and tissue investigated, and age and sex of the animals. There was a statistically significant increase in detecting C. europaeus (P = 0.038, McFadden R2 = 0.05) over time, but no other association over time was detected.
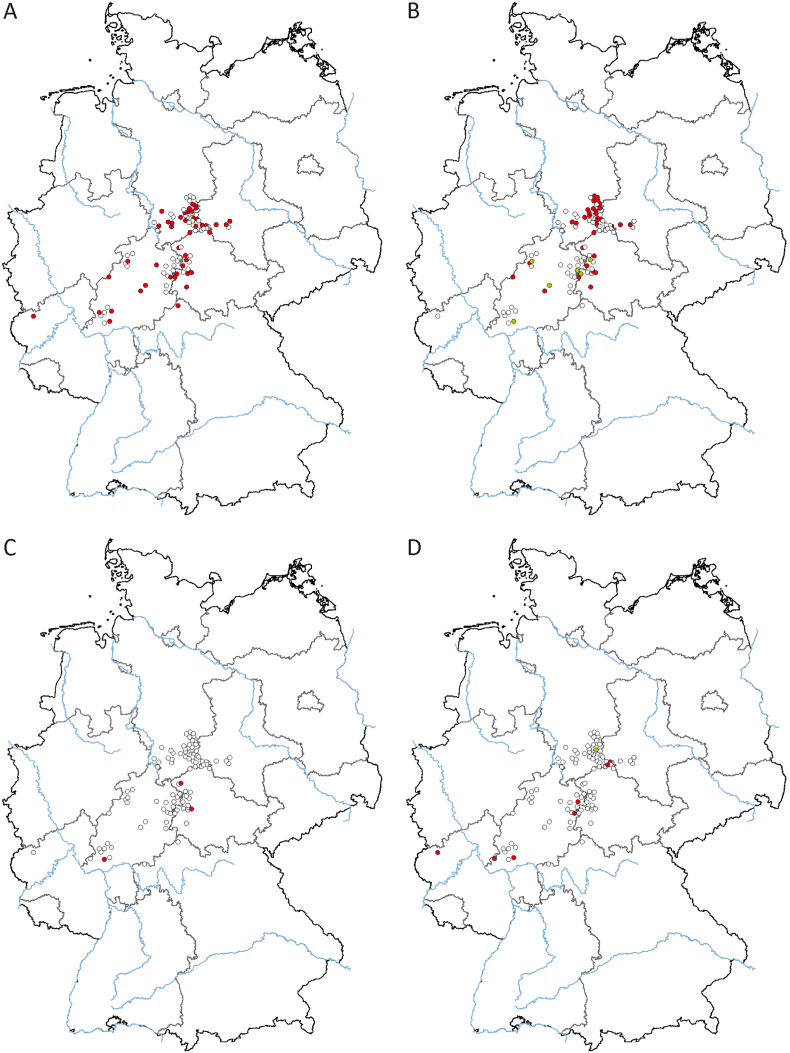
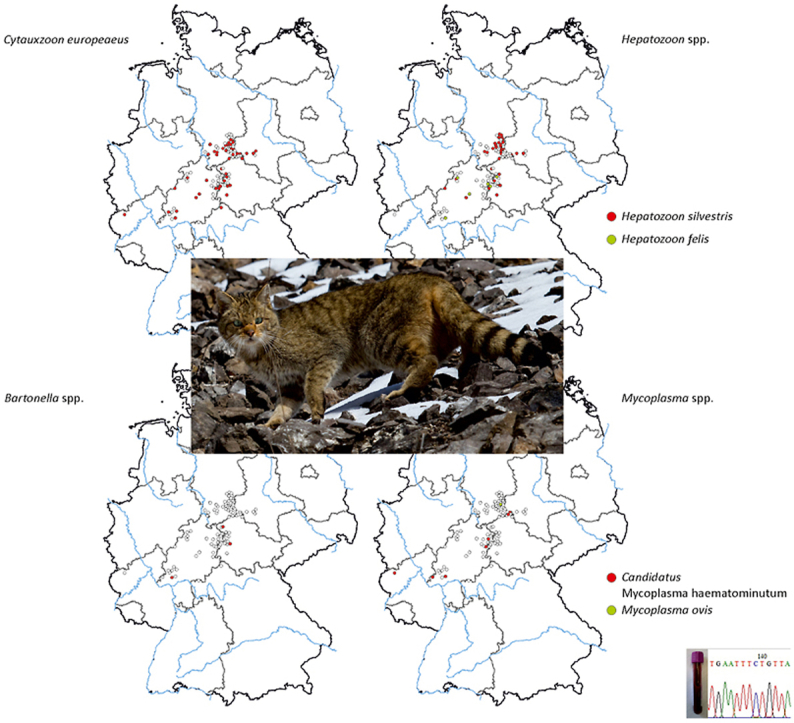
Fig. 3.
Geographical distribution of uninfected (white dots) and infected European wildcats (Felis silvestris) from Germany according to detected pathogens. A: red dots represent detection of Cytauxzoon europaeus; B: red dots represent detection of Hepatozoon silvestris, green dots represent detection of Hepatozoon felis; C: red dots represent detection of Bartonella spp.; D: red dots represent detection of Candidatus Mycoplasma haematominutum; green dots represent detection of Mycoplasma ovis; blue lines represent major rivers; and black lines represent borders of federal states. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 4.
Co-infection scheme of detected pathogens, excluding M. ovis. Numbers represent counts of European wildcats (Felis silvestris) with respective pathogen(s) detected.
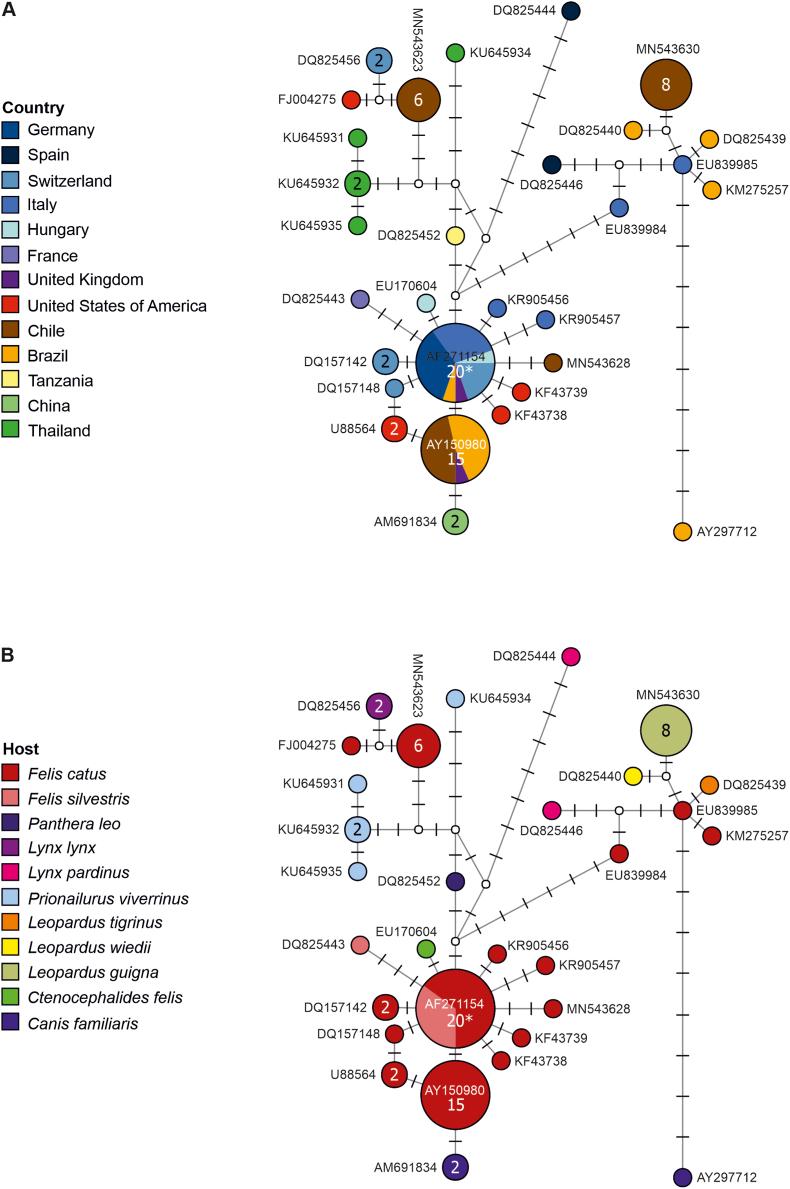
The genetic analysis of Candidatus Mycoplasma haematominutum revealed one haplotype identical to a haplotype found in domestic cats in Switzerland, Italy, Hungary, the United Kingdom, and Brazil (Fig. 5). This haplotype was placed within the clade (BI posterior probability (BI pp): 1; ML bootstrap value (ML bs): 100) of other Candidatus Mycoplasma haematominutum sequences in the consensus tree (Suppl. 1). The sequence of M. ovis obtained in this study showed 100% identity to a M. ovis sequence found in a goat (Capra hircus) from China (GenBank accession number: KU983745).
Fig. 5.
Median Joining haplotype network of the 16 S rRNA sequences (983 nucleotide positions) of Candidatus Mycoplasma haematominutum showing the geographical distribution (A) and the reported hosts (B). Circles represent haplotypes; numbers within the circles represent the number of individuals, if no number is shown, then only one individual is represented; labels next to circles specify representative GenBank accession numbers of the haplotypes, white circles represent intermediate nodes; bars on branches interconnecting haplotypes represent the number of substitutions; and asterisks mark haplotypes containing the individuals obtained in the present study.
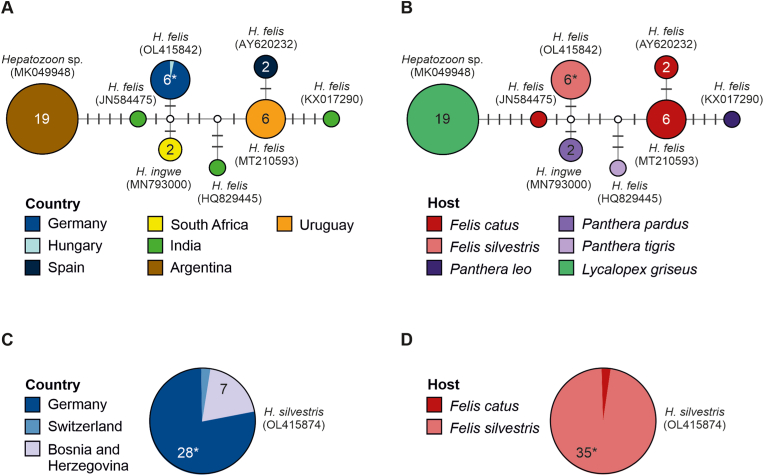
The sequences of H. silvestris were 100% identical to the haplotype detected in wildcats in Bosnia and Herzegovina and one domestic cat in Switzerland. The sequences of H. felis were 100% identical to the haplotype found in a wildcat from Hungary (Fig. 6). In the consensus tree, H. felis and H. silvestris were placed in a clade (BI pp: 0.65; ML bs: 75) together with Hepatozoon spp. mainly found in Canidae, Suidae, and Mustelidae (Suppl. 2). Within that clade, the two species were not closely related, and most sequences of H. felis were placed in a separate clade in both the BI tree (BI pp: 0.65) and the consensus tree (BI pp: 0.93; ML bs: 99), except for two sequences that fell outside this clade. Two H. felis sequences were placed in another distinct clade (BI pp: 0.92; ML bs: 99) together with H. luiperdjie.
Fig. 6.
Median Joining haplotype network of the 18 S rRNA sequences (561 nucleotide positions) of Hepatozoon felis (A, B) and pie chart of the 18 S rRNA gene (572 nucleotide positions) of Hepatozoon silvestris (C, D) showing the geographical distribution (A, C) and the reported hosts (B, D). Circles represent haplotypes; numbers within the circles represent the number of individuals, if no number is shown, then only one individual is represented; labels next to circles specify organism name and representative GenBank accession numbers of the haplotypes, white circles represent intermediate nodes; bars on branches interconnecting haplotypes represent the number of substitutions; and asterisks mark haplotypes containing the individuals obtained in the present study.
The 18S rRNA sequences of C. europaeus showed high similarity to other European Cytauxzoon spp. (99.77–100% identity to C. europaeus with the GenBank accession number MT904044). The alignment of CytB sequences with C. europaeus (GenBank accession number: MT916191), C. otrantorum (GenBank accession number: MT916204), C. banethi (GenBank accession number: MT916193), and C. felis (GenBank accession number: MT916203) showed the highest similarity to C. europaeus with a p-distance < 0.011.
The three Bartonella spp. sequences obtained in this study were distinct from each other and showed 100% identity to Bartonella sp. found in a yellow-necked mouse (Apodemus flavicollis) from Slovakia (GenBank accession number: KX267683), 100% identity to Bartonella sp. found in a common mole (Microtus arvalis) from Poland (GenBank accession number: GU338968) and 100% identity to Bartonella sp. found in a bank vole (Clethrionomys glareolus) from Slovakia (GenBank accession number: KX267679), respectively.
4. Discussion
The large sample size of the present study allowed us to obtain an overview of blood-borne pathogens harboured by wildcats in Germany. The pathogens detected and their prevalence are comparable to those obtained by studies on wildcats from other parts of Europe using molecular genetic tools (Willi et al., 2007, 2022; Gallusová et al., 2016; Veronesi et al., 2016; Hodžić et al., 2018a; Panait et al., 2021b).
Our results of Hepatozoon spp. are comparable with the results in wildcats from Bosnia and Herzegovina, where H. felis (3/9), H. silvestris (2/9), and unidentified Hepatozoon species (2/9) were detected (Hodžić et al., 2018a). In the same study, Cytauxzoon sp. (10/18) was found, which is consistent with results in wildcats from Romania (9/12) and with our study (Gallusová et al., 2016; Hodžić et al., 2018a). Cytauxzoon sp. Was also shown to be present in wildcats in Italy (4/21), albeit with a lower prevalence (Veronesi et al., 2016). Panait et al. (2021b) analyzed European Cytauxzoon spp. in more detail and described three new species. They found C. europaeus in wildcats from Germany (30/46), Romania (9/31), the Czech Republic (5/11), and Luxembourg (9/13). This is also comparable with the findings of C. europaeus in wildcats from France (10/34) and with our results (Willi et al., 2022).
Rickettsiales such as Anaplasma phagocytophilum are widespread tick-borne pathogens in many mammals in Europe, and have also been reported in domestic cats from Germany. However, we did not detect these pathogens in our study (Stuen, 2007; Morgenthal et al., 2012; Bergmann et al., 2015; Bergmann and Hartmann, 2017; Schäfer et al., 2022). Likewise, García-Pérez et al. (2016) did not detect Anaplasmataceae in wildcats from Spain (0/8), although the sample size may have been too small for detection. This is not the case with the sample size in our study. Considering that serological detection revealed a higher prevalence than molecular detection in studies conducted in domestic cats, our results may suggest few active infections with this pathogen, rather than an absence of infection in wildcats (Morgenthal et al., 2012; Schäfer et al., 2022).
Candidatus Mycoplasma haematominutum was detected in our study as well as in wildcats from France (6/13), where Candidatus Mycoplasma turicensis (11/31) was also found (Willi et al., 2007). In the study by Hodžić et al. (2018a) mentioned above Mycoplasma spp., which were genetically distinct from Candidatus Mycoplasma haematominutum, were found in wildcats from Bosnia and Herzegovina (4/18). Surprisingly, we detected M. ovis in a wildcat in our study. Since M. haemofelis was used as a positive control, contamination of the sample is unlikely. This pathogen is usually found in sheep and other small ruminants, but it was also recently reported in horses from Iran (Kalantari et al., 2020). M. ovis was likely only transiently present in the blood of the one wildcat from our study, as there are no other reports of M. ovis in carnivores to the authors’ knowledge.
Bartonella spp. sequences obtained in our study were distinct from each other but were all 100% identical to sequences found in rodents. It is also possible that these pathogens were temporarily present in the blood, or that transmission to wildcats occurred through predation. This hypothesis, however, would need further investigation. Generally, fleas are known to play an important role in the spread of Bartonella spp. and are also discussed as vectors for haemotrophic Mycoplasma spp. (Millán et al., 2021). In fact, Candidatus Mycoplasma haematominutum was detected in cat fleas (Ctenocephalides felis) in Hungary (Hornok et al., 2010). Findings of Millán et al. (2021) support this theory, as co-infection of Bartonella spp. and haemotrophic Mycoplasma spp. often occur, although ingestion of the cat flea does not seem to play a role in transmission (Woods et al., 2006). This co-infection was not detected in our study, possibly due to the low prevalence of Bartonella spp. In the same review, Millán et al. (2021) state that detection of haemotrophic Mycoplasma spp. is also dependent on the tissue investigated, being more prevalent in blood compared to spleen tissue, probably because the pathogen is eliminated more quickly from the spleen compared to the blood. Likewise, in our study, Mycoplasma spp. Was detected more often in the blood compared to spleen tissue, although this difference was not statistically significant. Modelling of transmission pathways for Candidatus Mycoplasma haematominutum suggests a concurrent role of vectors as well as direct transmission (Kellner et al., 2018).
Similarly, there are known routes of transmission for Piroplasmida, for example by hard ticks, but they are not fully elucidated for all species (Giannelli et al., 2017b; Thomas et al., 2018). Based on the overlapping distribution of the pathogens detected in our study, Ixodes ricinus, Dermacentor reticulatus, Ixodes hexagonus, and Ixodes inopinatus could act as possible vectors (Rubel et al., 2021), but other routes of transmission are also suggested to play a role, such as direct transmission through bites or diaplacentar transmission (Hornok et al., 2013; Hodžić et al., 2018a, 2018b). The high number co-infections with C. europaus and H. silvestris might indicate a common transmission route for these pathogens, but since these pathogens were the most prevalent, a high rate of co-infection is expected.
Although climate change is considered to promote the distribution of vectors (Harrus and Baneth, 2005; Aguirre, 2009; Cunze et al., 2022), for most pathogens we found no evidence of a trend over time for possible or definite vector transmission. However, it could be that a trend in this and other transmission routes, was not yet detectable, but will become apparent as climate change progresses. In that case, this study will provide essential baseline data. Nevertheless, an increase over time was observed in C. europaeus, although unknown confounding factors may not have been accounted for in the model, as indicated by the low McFadden R2. Although Willi et al. (2022) demonstrated that C. europaeus could be detected in wildcat samples between 1995 and 1996 and for this reason do not consider this pathogen as emerging, an increase in prevalence in wildcats over time may have led to spill over into domestic cats and therefore explain the recent more frequent detection in domestic cats Legroux et al. (2017); Panait et al. (2021b); Willi et al. (2022).
Interestingly, H. silvestris was more widespread in central Germany than H. felis, which was detected more in the western part of Germany. This distribution might reflect the separation of the German wildcat population into a western and central population (Mattucci et al., 2016).
The phylogenetic analysis of Mycoplasma spp. and Hepatozoon spp. sequences focused on generating a network to illustrate the distribution of haplotypes according to hosts and countries with closely related sequences. For this reason, M. haemofelis and Candidatus Mycoplasma turicense were not included in our phylogenetic tree, due to their dissimilarity, although these pathogens have been found in wild felids and their phylogenetic relation was described by Willi et al. (2007). For Hepatozoon spp., the BI tree only supported the clade of H. felis with a posterior probability of 0.65, and the clade was not supported in the ML tree. However due to the high similarity of the sequences this clade was chosen for the network analysis.
The H. felis haplotype found in the present study and in a wildcat from Hungary was not closely related to the only other haplotype found in Europe in domestic cats from Spain (Criado-Fornelio et al., 2006; Hornok et al., 2022). Furthermore, Hornok et al. (2022) described two distinct genotypes of H. felis in wildcats from Hungary. Comparison with the consensus tree calculated in the present study shows that these genotypes refer to the clade containing the H. felis sequences obtained here, and to the clade containing sequences of H. luiperdjie described by van As et al. (2020) in a leopard (Panthera pardus). These findings support the hypothesis that H. felis is not a phylogenetically well-defined species, but rather a species complex that requires further investigation (Hodžić et al., 2017; van As et al., 2020; Hornok et al., 2022).
Apart from one report in wildcats from France (Willi et al., 2007), which had a different haplotype, this is the first report of this Candidatus Mycoplasma haematominutum lineage in wildcats. All sequences from the present study belong to the same haplotype, which is also the major haplotype reported, but has only been detected in domestic cats until now (Tasker et al., 2001; Willi et al., 2006; Hornok et al., 2008; Aquino et al., 2014). The haplotype of H. silvestris reported in the present study is identical to the only haplotype reported so far in wildcats from Bosnia and Herzegovina, and also in a domestic cat from Switzerland (Hodžić et al., 2017; Kegler et al., 2018). This indicates that wildcats and domestic cats do share blood-associated pathogens.
The clinical impact of the pathogens detected in wildcats is unknown. Although there are case reports in domestic cats with severe disease (Hornok et al., 2008; Legroux et al., 2017; Kegler et al., 2018; Basso et al., 2019), the high prevalence of Candidatus Mycoplasma haematominutum, Hepatozoon spp., and C. europaeus more likely indicate asymptomatic infection in wildcats. This theory is supported by other studies reporting high prevalence without clinical disease (Willi et al., 2006; Grillini et al., 2021).
Hodžić et al. (2018a), as well as a study performed in Romania (Panait et al., 2021a), described the presence of Babesia spp. in wildcats, yet we failed to detect this parasite in the present study. In our study, Piroplasmida and other Apicomplexa were detected by a nested PCR, and detection of Hepatozoon spp. or Cytauxzoon spp. might have interfered with the detection of Babesia spp. This interpretation is contradicted by the fact that in the study by Hodžić et al. (2018a) the same method was used, and Babesia sp. Was detected in a sample that was also positive for Cytauxzoon sp. Another explanation might be that Babesia spp. associated with felids are not yet widespread in Europe (Penzhorn and Oosthuizen, 2020). Similarly, Filarioidea, such as Dirofilaria spp. Were not detected in our study, which is most likely due to the fact that though Dirofilaria spp. has been described in mosquitoes and vertebrate hosts in Germany, the prevalence is not considered to be high (Fuehrer et al., 2021). Romania is a highly endemic country for Dirofilaria spp., and D. immitis has been detected in a wildcat there (Ionică et al., 2017).
In conclusion, this study provides information on the prevalence of blood-associated pathogens in wildcats from Germany. Considering that the wildcat is an endangered species, the data can be of importance for wildlife conservation. The results are also valuable for veterinarians, as free-ranging domestic cats roam in the same area as wildcats and are therefore at risk of infection. However, additional studies are needed to elucidate the route of transmission and the clinical impact of these pathogens.
Data availability statement
The data presented in this study are contained within the article and supplementary material (Supplementary file).
Funding
KH was supported by the project Nr. CZ.02.1.01/0.0/0.0/16_019/0000787 ‘Fighting Infectious Diseases' provided by the Ministry of Education, Youth and Sports of the Czech Republic. All other researchers received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
None.
Acknowledgments
The authors thank Marlies Dolezal for her valuable advice on the statistical analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2022.08.012.
Contributor Information
Maria Sophia Unterköfler, Email: Maria.Unterkoefler@vetmeduni.ac.at.
Josef Harl, Email: Josef.Harl@vetmeduni.ac.at.
Bita Shahi Barogh, Email: Bita.ShahiBarogh@vetmeduni.ac.at.
Joachim Spergser, Email: Joachim.Spergser@vetmeduni.ac.at.
Kristýna Hrazdilová, Email: Kristyna@Hrazdilova.cz.
Franz Müller, Email: franzgersfeld@web.de.
Diana Jeschke, Email: Diana.Jeschke@senckenberg.de.
Ole Anders, Email: Ole.Anders@npharz.de.
Peter Steinbach, Email: PSteinbach@web.de.
Hermann Ansorge, Email: Hermann.Ansorge@senckenberg.de.
Hans-Peter Fuehrer, Email: Hans-Peter.Fuehrer@vetmeduni.ac.at.
Mike Heddergott, Email: Mike-Heddergott@web.de.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Aguirre A.A. Wild canids as sentinels of ecological health: a conservation medicine perspective. Parasites Vectors. 2009;2(Suppl. 1):S7. doi: 10.1186/1756-3305-2-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge H. In: Methoden Feldökol. Säugetierforsch. Wiss. Beitr. Stubbe M., Stubbe A., Heidecke D., editors. Univ. Halle; Halle: 1995. Notizen zur Altersbestimmung nach Wachstumslinien an Säugetierschädeln; pp. 95–102. [Google Scholar]
- Aquino L.C., Hicks C.A.E., Scalon M.C., Da Lima M.G.M., Lemos M.d.S., Paludo G.R., Helps C.R., Tasker S. Prevalence and phylogenetic analysis of haemoplasmas from cats infected with multiple species. J. Microbiol. Methods. 2014;107:189–196. doi: 10.1016/j.mimet.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzer S., Mölich T., Streif S., Tiesmeyer A., Thein J., Nowak C. Status der Wildkatze in deutschland. Nat. Landsch. 2018;93:146–152. [Google Scholar]
- Baneth G., Sheiner A., Eyal O., Hahn S., Beaufils J.-P., Anug Y., Talmi-Frank D. Redescription of Hepatozoon felis (Apicomplexa: Hepatozoidae) based on phylogenetic analysis, tissue and blood form morphology, and possible transplacental transmission. Parasites Vectors. 2013;6:102. doi: 10.1186/1756-3305-6-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso W., Görner D., Globokar M., Keidel A., Pantchev N. First autochthonous case of clinical Hepatozoon felis infection in a domestic cat in Central Europe. Parasitol. Int. 2019;72 doi: 10.1016/j.parint.2019.101945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battisti E., Zanet S., Khalili S., Trisciuoglio A., Hertel B., Ferroglio E. Molecular survey on vector-borne pathogens in alpine wild carnivorans. Front. Vet. Sci. 2020;7:1. doi: 10.3389/fvets.2020.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann M., Englert T., Stuetzer B., Hawley J.R., Lappin M.R., Hartmann K. Prevalence of selected rickettsial infections in cats in Southern Germany. Comp. Immunol. Microbiol. Infect. Dis. 2015;42:33–36. doi: 10.1016/j.cimid.2015.08.003. [DOI] [PubMed] [Google Scholar]
- Bergmann M., Hartmann K. Vector-borne diseases in cats in Germany. Tierarztl. Prax. Ausg. K. Kleintiere Heimtiere. 2017;45:329–335. doi: 10.15654/TPK-160874. [DOI] [PubMed] [Google Scholar]
- Blouin E.F., Kocan A.A., Glenn B.L., Kocan K.M., Hair J.A. Transmission of Cytauxzoon felis kier, 1979 from bobcats, Felis rufus (schreber), to domestic cats by Dermacentor variabilis (say) J. Wildl. Dis. 1984;20:241–242. doi: 10.7589/0090-3558-20.3.241. [DOI] [PubMed] [Google Scholar]
- Braunschweig A. von. Untersuchungen an Wildkatzen und diesen ähnlichen Hauskatzen. Z. Jagdwiss. 1963;9:109–112. [Google Scholar]
- Bundesamt für Naturschutz Nationaler FFH Bericht. 2020. https://www.bfn.de/themen/natura-2000/berichte-monitoring/nationaler-ffh-bericht.html
- Cerreta A.J., Yang T.S., Ramsay E.C., Birkenheuer A.J., Rahoi D., Qurollo B., Wilson J., Cushing A.C. Detection of vector-borne infections in lions and tigers at two zoos in Tennessee and Oklahoma, USA. J. Zoo Wildl. Med. 2022;53:50–59. doi: 10.1638/2020-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado-Fornelio A., Martinez-Marcos A., Buling-Saraña A., Barba-Carretero J.C. Presence of Mycoplasma haemofelis, Mycoplasma haemominutum and piroplasmids in cats from southern Europe: a molecular study. Vet. Microbiol. 2003;93:307–317. doi: 10.1016/s0378-1135(03)00044-0. [DOI] [PubMed] [Google Scholar]
- Criado-Fornelio A., Ruas J.L., Casado N., Farias N.A.R., Soares M.P., Müller G., Brumt J.G.W., Berne M.E.A., Buling-Saraña A., Barba-Carretero J.C. New molecular data on mammalian Hepatozoon species (Apicomplexa: Adeleorina) from Brazil and Spain. J. Parasitol. 2006;92:93–99. doi: 10.1645/GE-464R.1. [DOI] [PubMed] [Google Scholar]
- Cunze S., Glock G., Kochmann J., Klimpel S. Ticks on the move-climate change-induced range shifts of three tick species in Europe: current and future habitat suitability for Ixodes ricinus in comparison with Dermacentor reticulatus and Dermacentor marginatus. Parasitol. Res. 2022;121:2241–2252. doi: 10.1007/s00436-022-07556-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert I., Suchentrunk F., Markov G., Hartl G.B. Genetic diversity and integrity of German wildcat (Felis silvestris) populations as revealed by microsatellites, allozymes, and mitochondrial DNA sequences. Mamm. Biol. 2010;75:160–174. [Google Scholar]
- European Topic Centre on Biological Diversity Article 17 web tool on biogeographical assessments of conservation status of species and habitats under Article 17 of the Habitats Directive. Europäische Umweltagentur. 2019. https://nature-art17.eionet.europa.eu/article17/
- Foley J.E., Pedersen N.C. ‘Candidatus Mycoplasma haemominutum’, a low-virulence epierythrocytic parasite of cats. Int. J. Syst. Evol. Microbiol. 2001;51:815–817. doi: 10.1099/00207713-51-3-815. [DOI] [PubMed] [Google Scholar]
- Fuehrer H.-P., Morelli S., Unterköfler M.S., Bajer A., Bakran-Lebl K., Dwużnik-Szarek D., Farkas R., Grandi G., Heddergott M., Jokelainen P., Knific T., Leschnik M., Miterpáková M., Modrý D., Petersen H.H., Skírnisson K., Vergles Rataj A., Schnyder M., Strube C. Dirofilaria spp. and Angiostrongylus vasorum: current risk of spreading in central and northern Europe. Pathogens. 2021;10:1268. doi: 10.3390/pathogens10101268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallusová M., Jirsová D., Mihalca A.D., Gherman C.M., D'Amico G., Qablan M.A., Modrý D. Cytauxzoon infections in wild felids from carpathian-danubian-pontic space: further evidence for a different Cytauxzoon species in European felids. J. Parasitol. 2016;102:377–380. doi: 10.1645/15-881. [DOI] [PubMed] [Google Scholar]
- García-Pérez A.L., Oporto B., Espí A., del Cerro A., Barral M., Povedano I., Barandika J.F., Hurtado A. Anaplasmataceae in wild ungulates and carnivores in northern Spain. Ticks Tick Borne Dis. 2016;7:264–269. doi: 10.1016/j.ttbdis.2015.10.019. [DOI] [PubMed] [Google Scholar]
- Giannelli A., Latrofa M.S., Nachum-Biala Y., Hodžić A., Greco G., Attanasi A., Annoscia G., Otranto D., Baneth G. Three different Hepatozoon species in domestic cats from southern Italy. Ticks Tick Borne Dis. 2017;8:721–724. doi: 10.1016/j.ttbdis.2017.05.005. [DOI] [PubMed] [Google Scholar]
- Giannelli A., Lia R.P., Annoscia G., Buonavoglia C., Lorusso E., Dantas-Torres F., Baneth G., Otranto D. Rhipicephalus turanicus, a new vector of Hepatozoon canis. Parasitology. 2017;144:730–737. doi: 10.1017/S003118201600250X. [DOI] [PubMed] [Google Scholar]
- Grillini M., Simonato G., Tessarin C., Dotto G., Traversa D., Cassini R., Marchiori E., Di Frangipane Regalbono A. vol. 10. 2021. Cytauxzoon sp. and Hepatozoon spp; p. 1214. (Domestic Cats: A Preliminary Study in North-Eastern Italy. Pathogens). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrus S., Baneth G. Drivers for the emergence and re-emergence of vector-borne protozoal and bacterial diseases. Int. J. Parasitol. 2005;35:1309–1318. doi: 10.1016/j.ijpara.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Heddergott M., Pohl D., Steinbach P., Salazar L.C., Müller F., Frantz A.C. Determinants and effects of sinus worm Skrjabingylus nasicola (Nematoda: metastrongyloidae) infestation in invasive American mink Neovison vison in Germany. Parasitol. Res. 2016;115:3449–3457. doi: 10.1007/s00436-016-5107-1. [DOI] [PubMed] [Google Scholar]
- Heddergott M., Steeb S., Osten-Sacken N., Steinbach P., Schneider S., Pir J.P., Müller F., Pigneur L.-M., Frantz A.C. Serological survey of feline viral pathogens in free-living European wildcats (Felis s. silvestris) from Luxembourg. Arch. Virol. 2018;163:3131–3134. doi: 10.1007/s00705-018-3972-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertwig S.T., Schweizer M., Stepanow S., Jungnickel A., Böhle U.-R., Fischer M.S. Regionally high rates of hybridization and introgression in German wildcat populations (Felis silvestris , Carnivora, Felidae) J. Zool. Syst. Evol. Res. 2009;47:283–297. [Google Scholar]
- Hodžić A., Alić A., Duscher G.G. High diversity of blood-associated parasites and bacteria in European wild cats in Bosnia and Herzegovina: a molecular study. Ticks Tick Borne Dis. 2018;9:589–593. doi: 10.1016/j.ttbdis.2018.01.017. [DOI] [PubMed] [Google Scholar]
- Hodžić A., Alić A., Fuehrer H.-P., Harl J., Wille-Piazzai W., Duscher G.G. A molecular survey of vector-borne pathogens in red foxes (Vulpes vulpes) from Bosnia and Herzegovina. Parasites Vectors. 2015;8:88. doi: 10.1186/s13071-015-0692-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodžić A., Alić A., Prašović S., Otranto D., Baneth G., Duscher G.G. Hepatozoon silvestris sp. nov.: morphological and molecular characterization of a new species of Hepatozoon (Adeleorina: Hepatozoidae) from the European wild cat (Felis silvestris silvestris) Parasitology. 2017;144:650–661. doi: 10.1017/S0031182016002316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodžić A., Mrowietz N., Cézanne R., Bruckschwaiger P., Punz S., Habler V.E., Tomsik V., Lazar J., Duscher G.G., Glawischnig W., Fuehrer H.-P. Occurrence and diversity of arthropod-transmitted pathogens in red foxes (Vulpes vulpes) in western Austria, and possible vertical (transplacental) transmission of Hepatozoon canis. Parasitology. 2018;145:335–344. doi: 10.1017/S0031182017001536. [DOI] [PubMed] [Google Scholar]
- Hornok S., Boldogh S.A., Takács N., Kontschán J., Szekeres S., Sós E., Sándor A.D., Wang Y., Tuska-Szalay B. Molecular epidemiological study on ticks and tick-borne protozoan parasites (Apicomplexa: Cytauxzoon and Hepatozoon spp.) from wild cats (Felis silvestris), Mustelidae and red squirrels (Sciurus vulgaris) in central Europe, Hungary. Parasites Vectors. 2022;15:174. doi: 10.1186/s13071-022-05271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornok S., Meli M.L., Gönczi E., Ignits E., Willi B., Lutz H., Hofmann-Lehmann R. First molecular identification of ‘Candidatus mycoplasma haemominutum’ from a cat with fatal haemolytic anaemia in Hungary. Acta Vet. Hung. 2008;56:441–450. doi: 10.1556/AVet.56.2008.4.2. [DOI] [PubMed] [Google Scholar]
- Hornok S., Meli M.L., Perreten A., Farkas R., Willi B., Beugnet F., Lutz H., Hofmann-Lehmann R. Molecular investigation of hard ticks (Acari: ixodidae) and fleas (Siphonaptera: pulicidae) as potential vectors of rickettsial and mycoplasmal agents. Vet. Microbiol. 2010;140:98–104. doi: 10.1016/j.vetmic.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Hornok S., Tánczos B., Fernández de Mera I.G., La Fuente J. de, Hofmann-Lehmann R., Farkas R. High prevalence of Hepatozoon-infection among shepherd dogs in a region considered to be free of Rhipicephalus sanguineus. Vet. Parasitol. 2013;196:189–193. doi: 10.1016/j.vetpar.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Ionică A.M., Matei I.A., D'Amico G., Ababii J., Daskalaki A.A., Sándor A.D., Enache D.V., Gherman C.M., Mihalca A.D. Filarioid infections in wild carnivores: a multispecies survey in Romania. Parasites Vectors. 2017;10:332. doi: 10.1186/s13071-017-2269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen W.A., Fall M.Z., Rooney J., Kordick D.L., Breitschwerdt E.B. Rapid identification and differentiation of Bartonella species using a single-step PCR assay. J. Clin. Microbiol. 2000;38:1717–1722. doi: 10.1128/jcm.38.5.1717-1722.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E.M., Panciera R.J., Allen K.E., Sheets M.E., Beal J.D., Ewing S.A., Little S.E. Alternate pathway of infection with Hepatozoon americanum and the epidemiologic importance of predation. J. Vet. Intern. Med. 2009;23:1315–1318. doi: 10.1111/j.1939-1676.2009.0375.x. [DOI] [PubMed] [Google Scholar]
- Kalantari M., Sharifiyazdi H., Ghane M., Nazifi S. The occurrence of hemotropic Mycoplasma ovis-like species in horses. Prev. Vet. Med. 2020;175 doi: 10.1016/j.prevetmed.2019.104877. [DOI] [PubMed] [Google Scholar]
- Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegler K., Nufer U., Alic A., Posthaus H., Olias P., Basso W. Fatal infection with emerging apicomplexan parasite Hepatozoon silvestris in a domestic cat. Parasites Vectors. 2018;11:428. doi: 10.1186/s13071-018-2992-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner A., Carver S., Scorza V., McKee C.D., Lappin M., Crooks K.R., VandeWoude S., Antolin M.F. Transmission pathways and spillover of an erythrocytic bacterial pathogen from domestic cats to wild felids. Ecol. Evol. 2018;8:9779–9792. doi: 10.1002/ece3.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappin M.R. Update on flea and tick associated diseases of cats. Vet. Parasitol. 2018;254:26–29. doi: 10.1016/j.vetpar.2018.02.022. [DOI] [PubMed] [Google Scholar]
- Legroux J.-P., Halos L., René-Martellet M., Servonnet M., Pingret J.-L., Bourdoiseau G., Baneth G., Chabanne L. First clinical case report of Cytauxzoon sp. infection in a domestic cat in France. BMC Vet. Res. 2017;13:81. doi: 10.1186/s12917-017-1009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenstedt U., Jenkins D., Romig T. The role of wildlife in the transmission of parasitic zoonoses in peri-urban and urban areas. Int. J. Parasitol. Parasites Wildl. 2015;4:71–79. doi: 10.1016/j.ijppaw.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattucci F., Oliveira R., Lyons L.A., Alves P.C., Randi E. European wildcat populations are subdivided into five main biogeographic groups: consequences of Pleistocene climate changes or recent anthropogenic fragmentation? Ecol. Evol. 2016;6:3–22. doi: 10.1002/ece3.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinig H., Boy P., Dähne M., Hutterer R., Lang J. Rote Liste und Gesamtartenliste der Säugetiere (Mammalia) Deutschlands. Naturschutz und Biologische Vielfalt. 2020;170:73. [Google Scholar]
- Millán J., Di Cataldo S., Volokhov D.V., Becker D.J. Worldwide occurrence of haemoplasmas in wildlife: insights into the patterns of infection, transmission, pathology and zoonotic potential. Transbound. Emerg. Dis. 2021;68:3236–3256. doi: 10.1111/tbed.13932. [DOI] [PubMed] [Google Scholar]
- Millán J., Naranjo V., Rodríguez A., La Lastra J.M.P. de, Mangold A.J., La Fuente J. de. Prevalence of infection and 18S rRNA gene sequences of Cytauxzoon species in Iberian lynx (Lynx pardinus) in Spain. Parasitology. 2007;134:995–1001. doi: 10.1017/S003118200700248X. [DOI] [PubMed] [Google Scholar]
- Morgenthal D., Hamel D., Arndt G., Silaghi C., Pfister K., Kempf V.A.J., Kohn B. Prevalence of haemotropic mycoplasma spp., Bartonella spp. and Anaplasma phagocytophilum in cats in berlin/brandenburg (northeast Germany) Berl. Münchener Tierärztliche Wochenschr. 2012;125:418–427. [PubMed] [Google Scholar]
- Nordgren R.M., Craig T.M. Experimental transmission of the Texas strain of Hepatozoon canis. Vet. Parasitol. 1984;16:207–214. doi: 10.1016/0304-4017(84)90038-4. [DOI] [PubMed] [Google Scholar]
- Norman A.F., Regnery R., Jameson P., Greene C., Krause D.C. Differentiation of Bartonella-like isolates at the species level by PCR-restriction fragment length polymorphism in the citrate synthase gene. J. Clin. Microbiol. 1995;33:1797–1803. doi: 10.1128/jcm.33.7.1797-1803.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren A. A plea for linguistic accuracy - also for Candidatus taxa. Int. J. Syst. Evol. Microbiol. 2017;67:1085–1094. doi: 10.1099/ijsem.0.001715. [DOI] [PubMed] [Google Scholar]
- Otranto D., Cantacessi C., Pfeffer M., Dantas-Torres F., Brianti E., Deplazes P., Genchi C., Guberti V., Capelli G. The role of wild canids and felids in spreading parasites to dogs and cats in Europe. Part I: Protozoa and tick-borne agents. Vet. Parasitol. 2015;213:12–23. doi: 10.1016/j.vetpar.2015.04.022. [DOI] [PubMed] [Google Scholar]
- Panait L.C., Hrazdilová K., Ionică A.M., Deak G., Chişamera G.B., Adam C., Gherman C.M., Mihalca A.D. Babesia pisicii n. sp. and Babesia canis infect European wild cats, Felis silvestris, in Romania. Microorganisms. 2021;9:1474. doi: 10.3390/microorganisms9071474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panait L.C., Mihalca A.D., Modrý D., Juránková J., Ionică A.M., Deak G., Gherman C.M., Heddergott M., Hodžić A., Veronesi F., Reichard M., Zieman E.A., Nielsen C.K., Jiménez-Ruiz F.A., Hrazdilová K. Three new species of Cytauxzoon in European wild felids. Vet. Parasitol. 2021;290 doi: 10.1016/j.vetpar.2021.109344. [DOI] [PubMed] [Google Scholar]
- Parola P., Roux V., Camicas J.L., Baradji I., Brouqui P., Raoult D. Detection of ehrlichiae in African ticks by polymerase chain reaction. Trans. R. Soc. Trop. Med. Hyg. 2000;94:707–708. doi: 10.1016/s0035-9203(00)90243-8. [DOI] [PubMed] [Google Scholar]
- Penezić A., Selaković S., Pavlović I., Ćirović D. First findings and prevalence of adult heartworms (Dirofilaria immitis) in wild carnivores from Serbia. Parasitol. Res. 2014;113:3281–3285. doi: 10.1007/s00436-014-3991-9. [DOI] [PubMed] [Google Scholar]
- Penzhorn B.L., Oosthuizen M.C. Babesia species of domestic cats: molecular characterization has opened pandora's box. Front. Vet. Sci. 2020;7:134. doi: 10.3389/fvets.2020.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechocki R., Stiefel A. Über die Altersstruktur der Verluste der Wildkatze (Felis s. silvestris Schreber 1777) Hercynia NF. 1988:235–258. [Google Scholar]
- Poirson C., Dutilleul S. Coordination Mammalogique du Nord de la France, pour le Conseil Régional Nord-Pas de Calais; 2014. Plan régional de restauration du chat forestier (Felis silvestris) et de la martre des pins (Martes martes) en Nord—Pas de Calais. [Google Scholar]
- Reichard M.V., Edwards A.C., Meinkoth J.H., Snider T.A., Meinkoth K.R., Heinz R.E., Little S.E. Confirmation of Amblyomma americanum (Acari: ixodidae) as a vector for Cytauxzoon felis (piroplasmorida: Theileriidae) to domestic cats. J. Med. Entomol. 2010;47:890–896. doi: 10.1603/me10013. [DOI] [PubMed] [Google Scholar]
- Ronquist F., Teslenko M., van der Mark P., Ayres D.L., Darling A., Höhna S., Larget B., Liu L., Suchard M.A., Huelsenbeck J.P. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubel F., Brugger K., Chitimia-Dobler L., Dautel H., Meyer-Kayser E., Kahl O. Atlas of ticks (Acari: argasidae, ixodidae) in Germany. Exp. Appl. Acarol. 2021;84:183–214. doi: 10.1007/s10493-021-00619-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez R., Serra F., Tárraga J., Medina I., Carbonell J., Pulido L., María A. de, Capella-Gutíerrez S., Huerta-Cepas J., Gabaldón T., Dopazo J., Dopazo H. Phylemon 2.0: a suite of web-tools for molecular evolution, phylogenetics, phylogenomics and hypotheses testing. Nucleic Acids Res. 2011;39:W470–W474. doi: 10.1093/nar/gkr408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer I., Kohn B., Müller E. Anaplasma phagocytophilum in domestic cats from Germany, Austria and Switzerland and clinical/laboratory findings in 18 PCR-positive cats (2008-2020) J. Feline Med. Surg. 2022;24:290–297. doi: 10.1177/1098612X211017459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauenberg P. L’identification du chat forestier d'Europe Felis s. silvestris Schreber, 1777, par une méthode ostéométrique. Revue suisse de Zool. 1969;76:441–453. [PubMed] [Google Scholar]
- Schreeg M.E., Marr H.S., Tarigo J., Cohn L.A., Levy M.G., Birkenheuer A.J. Pharmacogenomics of Cytauxzoon felis cytochrome b: implications for atovaquone and azithromycin therapy in domestic cats with cytauxzoonosis. J. Clin. Microbiol. 2013;51:3066–3069. doi: 10.1128/JCM.01407-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyer K., Kraus R.H.S., Mölich T., Anders O., Cocchiararo B., Frosch C., Geib A., Götz M., Herrmann M., Hupe K., Kohnen A., Krüger M., Müller F., Pir J.B., Reiners T.E., Roch S., Schade U., Schiefenhövel P., Siemund M., Simon O., Steeb S., Streif S., Streit B., Thein J., Tiesmeyer A., Trinzen M., Vogel B., Nowak C. Large-scale genetic census of an elusive carnivore, the European wildcat (Felis s. silvestris) Conserv. Genet. 2016;17:1183–1199. [Google Scholar]
- Stuen S. Anaplasma phagocytophilum - the most widespread tick-borne infection in animals in Europe. Vet. Res. Commun. 2007;31(Suppl. 1):79–84. doi: 10.1007/s11259-007-0071-y. [DOI] [PubMed] [Google Scholar]
- Takumi K., Sprong H., Hofmeester T.R. Impact of vertebrate communities on Ixodes ricinus-borne disease risk in forest areas. Parasites Vectors. 2019;12:434. doi: 10.1186/s13071-019-3700-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Kumar S. MEGA11: molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021;38:3022–3027. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasker S., Helps C.R., Belford C.J., Birtles R.J., Day M.J., Sparkes A.H., Gruffydd-Jones T.J., Harbour D.A. 16S rDNA comparison demonstrates near identity between an United Kingdom Haemobartonella felis strain and the American California strain. Vet. Microbiol. 2001;81:73–78. doi: 10.1016/s0378-1135(01)00331-5. [DOI] [PubMed] [Google Scholar]
- Thomas J.E., Ohmes C.M., Payton M.E., Hostetler J.A., Reichard M.V. Minimum transmission time of Cytauxzoon felis by Amblyomma americanum to domestic cats in relation to duration of infestation, and investigation of ingestion of infected ticks as a potential route of transmission. J. Feline Med. Surg. 2018;20:67–72. doi: 10.1177/1098612X17691172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifinopoulos J., Nguyen L.-T., Haeseler A. von, Minh B.Q. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016;44:W232–W235. doi: 10.1093/nar/gkw256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van As M., Netherlands E.C., Smit N.J. Molecular characterisation and morphological description of two new species of Hepatozoon Miller, 1908 (Apicomplexa: Adeleorina: Hepatozoidae) infecting leukocytes of African leopards Panthera pardus pardus (L.) Parasites Vectors. 2020;13:222. doi: 10.1186/s13071-020-3933-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronesi F., Ravagnan S., Cerquetella M., Carli E., Olivieri E., Santoro A., Pesaro S., Berardi S., Rossi G., Ragni B., Beraldo P., Capelli G. First detection of Cytauxzoon spp. infection in European wildcats (Felis silvestris silvestris) of Italy. Ticks Tick Borne Dis. 2016;7:853–858. doi: 10.1016/j.ttbdis.2016.04.003. [DOI] [PubMed] [Google Scholar]
- Vitorino L., Zé-Zé L., Sousa A., Bacellar F., Tenreiro R. rRNA intergenic spacer regions for phylogenetic analysis of Rickettsia species. Ann. N. Y. Acad. Sci. 2003;990:726–733. doi: 10.1111/j.1749-6632.2003.tb07451.x. [DOI] [PubMed] [Google Scholar]
- Volokhov D.V., Norris T., Rios C., Davidson M.K., Messick J.B., Gulland F.M., Chizhikov V.E. Novel hemotrophic mycoplasma identified in naturally infected California sea lions (Zalophus californianus) Vet. Microbiol. 2011;149:262–268. doi: 10.1016/j.vetmic.2010.10.026. [DOI] [PubMed] [Google Scholar]
- Willi B., Boretti F.S., Baumgartner C., Tasker S., Wenger B., Cattori V., Meli M.L., Reusch C.E., Lutz H., Hofmann-Lehmann R. Prevalence, risk factor analysis, and follow-up of infections caused by three feline hemoplasma species in cats in Switzerland. J. Clin. Microbiol. 2006;44:961–969. doi: 10.1128/JCM.44.3.961-969.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willi B., Filoni C., Catão-Dias J.L., Cattori V., Meli M.L., Vargas A., Martínez F., Roelke M.E., Ryser-Degiorgis M.-P., Leutenegger C.M., Lutz H., Hofmann-Lehmann R. Worldwide occurrence of feline hemoplasma infections in wild felid species. J. Clin. Microbiol. 2007;45:1159–1166. doi: 10.1128/JCM.02005-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willi B., Meli M.L., Cafarelli C., Gilli U.O., Kipar A., Hubbuch A., Riond B., Howard J., Schaarschmidt D., Regli W., Hofmann-Lehmann R. Cytauxzoon europaeus infections in domestic cats in Switzerland and in European wildcats in France: a tale that started more than two decades ago. Parasites Vectors. 2022;15:19. doi: 10.1186/s13071-021-05111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzenberger K.A., Hochkirch A. The genetic integrity of the ex situ population of the European wildcat (Felis silvestris silvestris) is seriously threatened by introgression from domestic cats (Felis silvestris catus) PLoS One. 2014;9 doi: 10.1371/journal.pone.0106083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods J.E., Wisnewski N., Lappin M.R. Attempted transmission of Candidatus Mycoplasma haemominutum and Mycoplasma haemofelis by feeding cats infected Ctenocephalides felis. Am. J. Vet. Res. 2006;67:494–497. doi: 10.2460/ajvr.67.3.494. [DOI] [PubMed] [Google Scholar]
- Xia X., Xie Z. DAMBE: software package for data analysis in molecular biology and evolution. J. Hered. 2001;92:371–373. doi: 10.1093/jhered/92.4.371. [DOI] [PubMed] [Google Scholar]
- Zaeemi M., Razmi G.R., Khoshnegah J. The first detection of Cytauxzoon felis in a wild cat (Felis silvestris) in Iran. Comp. Clin. Pathol. 2015;24:181–184. [Google Scholar]
- Zintl A., Finnerty E.J., Murphy T.M., Waal T. de, Gray J.S. Babesias of red deer (Cervus elaphus) in Ireland. Vet. Res. 2011;42:7. doi: 10.1186/1297-9716-42-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are contained within the article and supplementary material (Supplementary file).