1. Introduction
Chronic pain conditions occur in 30% of individuals and cost the U.S. $635 billion dollars per year in health care costs and lost wages [30]. Exercise, as a non-pharmacological therapeutic tool, is prescribed as a first-line treatment option for many chronic pain conditions including fibromyalgia, low back pain, migraine, and peripheral neuropathies, with strong evidence supporting exercise effectiveness in reducing pain in both clinical and animal research [3;9;10;17;18;24;42;54;67;68]. In the rehabilitation setting, both aerobic and resistance training exercise programs are recommended for chronic low back pain, osteoarthritis, and fibromyalgia [10;15–18;65]. Similarly, animal studies demonstrate exercise-induced pain relief (analgesia) following aerobic exercise programs such as running or swimming for models of neuropathic, inflammatory, and musculoskeletal pain [11–14;47;55]. Aerobic exercise is shown to produce analgesic effects through supraspinal, spinal, neuroimmune, and peripheral changes within the body [52;54]. While the mechanism of aerobic-based exercise-induced analgesia is widely studied, little research has focused on animal models of resistance training for pain relief. Prior studies into the analgesic effects of resistance training were performed in pain-free animals and thus translatability is limited for the pain population. Further these studies used electric shock to encourage participation in the exercise task which can produce stress [21]. Stress can alter nociception and result in either hyperalgesia or analgesia depending on the condition [19;20], thus confounding interpretation of results. Thus, a resistance exercise model that relies on intrinsic motivation rather than electrical shocks is needed. Understanding mechanisms of resistance training exercise could lead to more effective prescription and dosing for chronic pain populations and potentially to the discovery of new pharmacological targets for pain reduction.
Resistance training acutely increases testosterone contributing to its anabolic effects to increase muscle growth and strength in both males and females [34;76]. More recently, several studies show that testosterone is protective in animal models of pain including inflammatory and stress-induced pain [26;36]. Similarly, our laboratory demonstrated that testosterone protects against development of widespread hyperalgesia in both male and female mice [51]. In female mice, administration of testosterone protected animals from development of widespread muscle hyperalgesia, while orchiectomy of male mice results in development of widespread muscle hyperalgesia [31;51]. Further, androgen receptors transcriptionally increase expression of mu-opioid and cannabinoid type-1 receptors in trigeminal ganglia, and blockade of androgen receptors prevents the analgesia produced by DAMGO, a synthetic opioid peptide, in male mice using a model of inflammatory pain [49;50]. Lastly, in women with fibromyalgia (n=12), an uncontrolled trial of a transdermal testosterone gel, improved muscle pain, stiffness and fatigue [77]. In hypogonadal or androgen deficient men, testosterone treatment reduced experimentally induced and self-reported pain [4;38]. This evidence suggests testosterone has protective effects against chronic pain in both male and females led to the hypothesis that in an animal model of resistance training, analgesia would be produced through continual activation of androgen receptors throughout the exercise program.
2. Materials and Methods:
2.1. Mice
A total of 266 C57BL/6J mice (134 male, 132 female) (20–30 g) (8 weeks of age) (Jackson Laboratories, Bar Harbor, ME) were used in the following studies. Unless noted, all mice were housed 4 to a cage on a 12-hour light-dark cycle with access to food and water ad libitum. All experiments conducted were approved by the University of Iowa Animal Care and Use Committee and were carried out in accordance with National Institute of Health guidelines. All animals were randomly allocated to groups through use of a random number generator in blocks of 4 stratified by sex. Males and female mice were evenly distributed, and multiple replicates were done for each experiment. In experiments where animals were housed 4 per cage, resistance trained and sedentary mice were housed in the same cage.
2.2. Resistance Training Protocol and Strength Testing
Animals spontaneously climbed a custom built 1-meter ladder at an 80° incline with 1-centimeter square wire mesh overlaying the ladder. Small weights (5–40 grams (g)) (Lakeshore, Carson, CA, USA) in plastic bags were attached to the animal’s tail via a foam lined human nose clip (7.5g) (Harvard Apparatus, Holliston, MA, USA). Mice were acclimated twice per day over a two-day span prior to training. Twenty-four hours after acclimation, mice were assessed for strength. Animals performed a 1 repetition maximum (1RM) to determine the highest amount of weight each animal could successfully carry up the ladder one time. During 1RM testing, each animal’s first attempt started with a weight equal to 100% of their bodyweight. After each successful climb, 3 g was added until the mouse failed the test. Failure was defined as turning around to climb down ladder, falling off ladder, or the elapsing of 60 seconds without climbing up the ladder. Two minutes of rest were given between 1RM attempts. Following a 30-minute break after 1 RM testing, mice were tested for grip strength. Mice were placed on a grip force device (Chatillon, Largo, FL, USA) and pulled by their tail to measure front paw and back paw grip strength. Front paw and back paw grip strength were calculated by averaging three trials.
After acclimation and strength assessment, animals were placed into one of three groups: either resistance training with weight, resistance training without weight or a sedentary group according to the randomization schedule. In the resistance training with and without weight groups, resistance training was completed three days a week (Monday, Wednesday, Friday) for eight weeks. During training sessions, animals climbed the ladder six times with one-minute rest between each climb. In animals who trained with weight, each animal was prescribed individualized training loads based on pre-training 1 RM assessments. During week one, animals carried a load 70% of their initial 1RM. This load was increased by 5% of their initial 1 RM each week in order to maintain progressive overload (i.e. wk 2: 75%, wk 3: 80%…wk 8: 105%) throughout the 8 weeks [1;46]. As a control, a group of mice performed the resistance training program without weight attached to their tails by climbing the ladder six times with one-minute rest between each climb. In order to maintain similar conditions between exercise and controls groups, sedentary mice were brought to the exercise room and left in their cages during the training sessions. On the last day of the training session in week eight, all mice underwent post-training 1RM and grip strength testing. In total, 127 animals completed 8 weeks of resistance training. Three of the 127 mice did not successfully complete the 8 weeks of resistance training due to refusal to climb and were removed from subsequent analysis.
2.3. Muscle Cross Sectional Area
To determine if strength training resulted in muscle hypertrophy, we analyzed cross sectional area of muscle fibers from the gastrocnemius and biceps brachii muscles. Animals were euthanized with sodium pentobarbital (100 mg/kg, i.p.) and the left bicep brachii and left gastrocnemius muscle were harvested. Muscles were fixed in 10% neutral buffered formalin for 72 hours and then embedded in paraffin. Muscles were cut in cross section at 4μm and stained with hematoxylin and eosin (H&E). For muscle cross sectional area analysis, eight tissue sections were analyzed per animal. The entire muscle section was imaged and tiled to provide one image per section. Within each section, 300 muscle fibers were systematically selected for analysis. Muscle cross sectional area was manually determined through utilization of the freehand measure tool on ImageJ software (NIH, Bethesda, MD, USA); the individual performing this was blinded to treatment group.
2.4. Nuclear Magnetic Resonance
To determine if resistance training altered body composition, we utilized nuclear magnetic resonance to determine body fat percentage and lean body mass. To determine body compositions, animals were briefly placed into a polycarbonate restraint tube and placed in a Bruker minispec L50 nuclear magnetic resonance device (Bruker, Billerica, MA, USA).
2.5. Activity-Induced Muscle Pain Model
Activity-induced muscle pain was produced through a combination of intramuscular (i.m.) acidic saline injections coupled with muscle fatigue. On day one, mice were anesthetized with 2–4% isoflurane and given a 20 μl i.m. injection of pH 5.0±0.05 saline into the left gastrocnemius muscle. On day five, fatigue was induced in the same muscle using electrically simulated muscle contractions as previously described [31]. Briefly, mice were deeply anesthetized with 2–4% isoflurane. Needle electrodes were inserted into the left gastrocnemius muscle in a parallel orientation with the muscle fibers. Pre-fatigue maximum force production was quantified using three, 100Hz stimulus trains at 7V. To induce gastrocnemius fatigue, submaximal contractions were delivered with a duty cycle of 47% (3.75 s on, 4.25 sec off) using trains of stimulations at 40 Hz and an amplitude of 7V for six minutes. Post-fatigue maximum force production was measured following the six minutes of fatiguing contractions via three additional maximum force contractions (i.e., 100 Hz stimulation trains). The animal’s left hindpaw was positioned on a 3-D printed force plate to measure the isometric gastrocnemius contraction forces throughout the fatiguing protocol. Data was collected and analyzed using Labview software (National Instruments, Austin, TX, USA). Fatigue was operationally defined as the decline in maximum force production from baseline following the six minutes of fatiguing stimulation. This protocol results in approximately 60% reduction in muscle force and a significant decrease in muscle pH [31]. Immediately following the muscle fatigue protocol animals were given their second 20 μl i.m. injection of pH 5.0±0.5 saline into the left gastrocnemius muscle. This paradigm results in a sex-dependent pain phenotype with males developing unilateral muscle hyperalgesia and females developing bilateral muscle hyperalgesia [31].
2.6. Behavioral Assessment
We assessed muscle withdrawal thresholds (MWT) by applying force sensitive tweezers to the gastrocnemius muscle as previously described [69]. All mice were acclimated in four, five-minute sessions over a two-day period by placing them in a gardener’s glove with their hindlimbs placed in extension with light manual squeezing of the gastrocnemius muscle. On testing days, the gastrocnemius muscle was squeezed with force sensitive tweezers until the animal withdrew its hindlimb. We tested each animal bilaterally and the average of three trials was used to determine MWT for each limb. To prevent behavioral sensitization to testing, 5 minutes was allowed to elapse between each MWT assessment. A decrease from baseline in MWT is interpreted as muscle hyperalgesia. All testers were blinded to treatment group during behavioral assessments. One experimenter performed the resistance training while a second experimenter performed MWT testing.
2.7. Blood Measurements
2.7.1. Lactate Assessment
To determine if the strength training was anaerobic, we measured blood lactate levels using a hand-held lactate device (Arkray, Minneapolis, MN, USA). Animals were placed in a custom-built flat bottom rodent restrainer with their tails exposed. The tail vein was punctured with a 23-gauge needle to draw blood. The tail was then massaged until a large drop of blood formed. The blood was absorbed by the lactate strip into the device which renders an instant reading of blood lactate levels.
2.7.2. Testosterone Assessment
To determine if the strength training acutely increased testosterone, we measured blood levels of total testosterone after a single strength training session. Animals were anesthetized with pentobarbital (50 mg/ml) and blood was collected via a cardiac blood draw. Blood was placed in 3.0 mL serum blood collection tubes (BD vacutainer, Franklin Lakes, NJ) and allowed to clot for 30 minutes. The tubes were then centrifuged at 1500 RPM for 10 minutes at room temperature in order to separate serum. Serum samples were tested for total testosterone concentration levels in duplicate with a rat/mouse enzyme linked immunosorbent assay (ELISA) testosterone kit (MyBioSource, Inc. San Diego, CA).
2.8. Blockade of Androgen Receptors
2.8.1. Flutamide Injections
To determine if strength training produced analgesic effects through activation of androgen receptors, a single injection of flutamide was delivered systemically (i.p.) 24 hours after induction of the activity-induced pain model in resistance-trained animals. On the day of testing, flutamide was dissolved in ethanol/saline (2:1) and then diluted to desired concentration. Animals received either i.p. injection of flutamide (1, 3, or 10mg/kg) or vehicle and MWT testing was reassessed 30 and 120 minutes after injection. Flutamide solutions were prepared fresh daily to ensure drug concentrations were at desired levels. These doses were chosen based on prior literature showing effects at these doses [50]. The behavioral testing experimenter was blinded to drug dose or vehicle.
2.8.2. Flutamide Pellets
To test if repeated strength training activated androgen receptors to produce analgesia, we implanted slow release flutamide pellets to block activity during the training sessions. Slow release flutamide pellets (200 mg flutamide/pellet, 60-day release; Innovative Research of America, Sarasota, FL, USA) or control pellets were implanted subcutaneously. This dose was chosen based on prior literature that demonstrated flutamide’s ability to block exercise-induced peripheral nerve regeneration [73]. Animals were deeply anesthetized with 2–4% isoflurane. A small incision was made at the nape of the neck and the pellet was implanted via this incision. The incision was stitched closed with a synthetic non-absorbable monofilament silk suture. Following surgeries animals were moved to single cage housing for the reminder of the experiment in order to protect the surgical site. The experimenter was blinded to drug or vehicle.
2.9. Experimental Design and Statistical Analysis
All data is presented as mean±SEM. Statistical analyses were performed using SPSS Version 25.0 (IBM SPSS, Inc. Chicago, IL). For each experiment preliminary data was used to calculate appropriate samples sizes with power set at 0.80 and significance set at 0.05. To characterize the sex-dependent pain phenotype, we analyzed baseline and 24-hour MWT data from the animals across all experiments who did not perform the resistance training prior to induction of the activity-induced pain model (n=28 male, n=28 female); this included animals from the sedentary group (Experiment 2), and the post-treatment resistance trained and sedentary groups (Experiment 3). We used a mixed model repeated measure ANOVA with 2 dependent factors (time and side) and 1 independent factor (sex). For post hoc testing as appropriate, we performed a paired samples t-test between baseline and 24-hour MWT values. We also analyzed the change score between baseline and 24h MWT using a mixed model repeated measure ANOVA for side and sex. Tukey post hoc test was performed to determine difference in changes scores between side and sex. To determine if the resistance training program altered baseline MWT values, we analyzed pre and post 8 weeks of training MWT date from naïve animals across all experiments (n=28 male, n=28 female); this included animals from the exercised groups in Experiments 1 and 4. We utilized a mixed model repeated measures ANOVA for time, side, and sex. Statistical analysis for each experiment is presented below.
2.9.1. Experiment 1: Validation of weighted ladder climbing as a resistance training model
This experiment tested if a weighted ladder climbing exercise program was reflective of resistance training-based exercise through assessment of strength, muscle hypertrophy, and acute changes in blood lactate. For strength assessments, we tested if 8 weeks of the resistance training program increased strength and produced muscle hypertrophy. Mice were grouped into resistance training (n=8 male, n=8 female) or sedentary groups (n=8 male, n=8 female). One repetition max, grip strength, and body weights were assessed at baseline and again following the 8-week training period. For 1RM, grip strength, and body weight data, change scores were determined for each animal by subtracting post training session values from baseline values and an independent samples t-test was used to determine differences between resistance trained and sedentary groups. In a small cohort of resistance trained (n=2 male, n=2 female) and sedentary (n=2 male, n=2 female) animals we analyzed total body fat percentage and lean mass after 8 weeks of training. Differences in 8-week body fat percentage and total lean body mass were analyzed with a t-test.
An additional group of mice was used to test for muscle hypertrophy. Mice were randomly divided into the resistance training program group (n=4 male, n=4 female) or sedentary group (n=4 male, n=4 female). Following 8 weeks of training, biceps brachii and gastrocnemius muscles were harvested following the last training session for muscle cross section area analysis. During harvesting, two bicep brachii muscles were damaged and removed from analysis. For cross sectional area analysis, an independent samples t-test was performed to determine differences between trained and sedentary groups.
We also tested if a single bout of the resistance training program acutely increased lactate levels in blood to determine if animals were undergoing intensive exercise. Lactate was analyzed immediately after the first training session (n=7 male, n=6 female) or from those that were sedentary (n=4 male, n=6 female). For blood lactate analysis an independent samples t-test was performed to determine differences between trained and sedentary groups.
2.9.2. Experiment 2: Ability of resistance training to protect against chronic muscle pain
This experiment tested if 8 weeks of resistance training could protect against development of chronic muscle pain in both male and female mice. Animals were randomly grouped into resistance training (n=8 male, n=8 female) or sedentary (n=8 male, n=8 female). One repetition max, grip strength, and MWT were assessed at baseline and again following the 8-week training period. Following post-training assessments, the activity-induced muscle pain model was induced and MWT were assessed at 24hr, 48hr, 72hr, 1wk, 2wk, 3wk, and 4wk after induction of the model. For MWT data, a repeated measures ANOVA with baseline as a covariate was performed to determine differences between resistance trained and sedentary groups. The activity-induced pain model produces a robust sex dependent pain phenotype in which males develop unilateral pain and females develop bilateral pain [31;51]. Thus, we analyzed MWT values from each sex independently using a repeated measures ANOVA. To determine if there was a correlation between amount of weight lifted and protection against muscle pain, we correlated volume of weight lifted with behavior by determining Pearson correlation coefficients. Volume for each animal was calculated by summing the product of resistance training weight, sets, and reps for each week. Pearson correlation coefficients were determined between total volume and 24hr MWT values and between total volume and the change score of baseline and 24hr MWT values. Analysis was done on both the ipsilateral and contralateral limb and was performed for each sex independently.
2.9.3. Experiment 3: The ability of the resistance training program to reverse chronic muscle pain
This experiment tested if the resistance training program could reverse chronic muscle pain after it has been induced. Animals were randomly divided into resistance training (n=10 male, n=10 female) or sedentary (n=10 male, n=10 female) groups. Baseline 1RM and MWT were assessed and then the activity-induced muscle pain model was applied. MWT was assessed 24 hours after induction of the activity-induced muscle pain model to confirm hyperalgesia was present. Animals then initiated the resistance training program and MWT were reassessed weekly for 4 weeks. On days where MWT testing and resistance training both occur, MWT were assessed prior to resistance training. The MWT time course data was analyzed with a repeated measures ANOVA with the 24-hour timepoint used as a covariate. To determine if there was an association between amount of weight lifted and alleviation of muscle pain, Pearson correlation coefficients were calculated between total volume and 4wk MWT values and between total volume and the change score of 24hr and 4wk MWT values. Analysis was done on both the ipsilateral and contralateral limb and was performed for males only since females did not see alleviation of muscle pain.
2.9.4. Experiment 4: The role of testosterone in analgesia produced by resistance training
2.9.4.1. Effect of resistance training on testosterone concentrations
This experiment tested if the resistance training program acutely elevates blood levels of testosterone. Animals were randomly assigned to either the resistance training program group or sedentary group. We performed a time course analysis by harvesting blood at 5 (n=10 male, n=10 female), 15 (n=6 male, n=6 female), and 30 minutes (n=6 male, n=6 female) following the first training session and from a sedentary group (n=10 male, n=10 female). To control for variability in hormone levels, all blood draws were taken at similar times in the morning and all animals were the same age. For blood testosterone assessment, a one-way ANOVA with post hoc comparison to the sedentary group was performed to determine differences across the time course. Since male and female mice have different basal levels of testosterone [51], we analyzed testosterone concentrations from each sex independently
2.9.4.2. Effect of androgen receptor antagonist flutamide following induction of activity-induced pain model
This experiment tested if continual activation of the androgen receptor is required for the maintenance of resistance training induced analgesia. All animals were assessed for baseline 1RM and MWT and underwent the standard 8-week resistance training program followed by induction of the activity-induced pain model. Resistance training induced protection against muscle pain was assessed via MWT 24hr after induction of pain model. Animals were then randomly divided into 4 groups which received one of the following i.p. injections: flutamide 1mg/kg (n=4 male, n=4 female), 3mg/kg (n=4 male, n=4 female), or 10mg/kg (n=6 male, n=6 female); or vehicle (n=6 male, n=6 female). Following drug administration, MWTs were reassessed at 30 and 120 minutes following the injection. In order to determine differences between drug treatment groups, the MWT thresholds were analyzed via a one-way ANOVA with a Tukey post hoc test at the 30-minute time point. We only analyzed the 30-minute timepoint because no group differences were observed at the 120-minute timepoint.
2.9.4.3. Effect of androgen receptor antagonist, flutamide, applied throughout resistance training program
This experiment tested if antagonism of androgen receptors during resistance training via slow release flutamide pellets could block the analgesic effects of the resistance training program. Animals were randomly divided into receiving either flutamide (200mg) (n=10 male, n=10 female) or control pellets (n=10 male, n=10 female). Baseline 1RM and MWT were assessed followed by subcutaneous pellet implantation. Animals were given 4 days of recovery between surgery and initiation of the resistance training program. The experiment was designed so that the 60-day pellets would expire on the last day of the training session. Following the 8-week training program, 1RM and MWTs were reassessed, followed by induction of the activity-induced muscle pain model. Muscle withdrawal thresholds were assessed 24 hours after the induction of the pain model. Differences between groups at the 24-hour time point were analyzed with a univariate analysis with baseline MWT used as a covariate.
3. Results
3.1. Activity-induced muscle pain phenotype
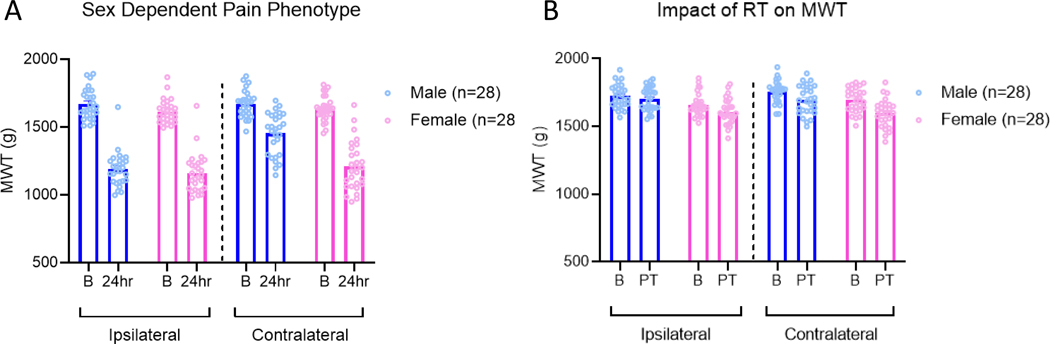
After induction of the activity-induced pain model, there were significant differences in the MWTs ipsilaterally and contralaterally that differed by sex (time x side x sex interaction: F1,54=43.8, p<0.01)(Fig. 1A). Post-hoc analysis showed a significant decrease in MWT values at the 24-hour time point in each sex on both the ipsilateral and contralateral side (p<0.01). Baseline and 24-hour change score values revealed a significant difference between ipsilateral and contralateral values (F1,54=68.74, p<0.01) that also differed by sex (F1,54=7.10, p=0.01). In females the contralateral MWT decreased by 425.64±31.53g which was significantly greater than the decrease observed in male mice (216.65±21.17g)(F3,111= 19.42, p<0.01; post-hoc Tukey’s p<0.01). There were no differences between the male ipsilateral, female ipsilateral, and female contralateral MWT change scores (p=0.48–0.91). This data demonstrates that the activity-induced muscle pain model produces equal decreases in MWT values on the ipsilateral side between males and females, however there is greater decrease in MWT in the contralateral side in females when compared with males.
Figure 1.
Representation of the sex dependent pain phenotype produced by the activity-induced pain model. On the ipsilateral side, both male and female mice show an equivalent reduction in muscle withdrawal threshold (MWT) 24 hours after induction of the pain model. On the contralateral side, both sexes show a reduction in MWT values, however this reduction is greater in females (A). Representation of the impact of 8 weeks of resistance training on baseline MWT values. 8 weeks of resistance training produced no biologically significant impact on MWT values in male or female mice. B=baseline, PT=Post Training; Data are mean±SEM.
3.2. Effect of resistance training on muscle pain thresholds
To determine if the resistance training program changed baseline pain behaviors, we measured MWT before and after 8 weeks of resistance training. There was a significant effect for time (F1,54=21.79, p<0.01) but not for side (F1,54=2.29, p=0.14) or sex (time X sex: F1,54=1.80, p=0.19; side X sex: F1,54=0.06, p=0.81). On the ipsilateral side, the average MWT value between pre and post resistance training decreased by 37.12±12.12g while the contralateral side decreased by 80.05±17.11g (Figure 1B). Despite the statistically significant main effect for time, the changes in MWTs over the 8-week training program may not be biologically significant.
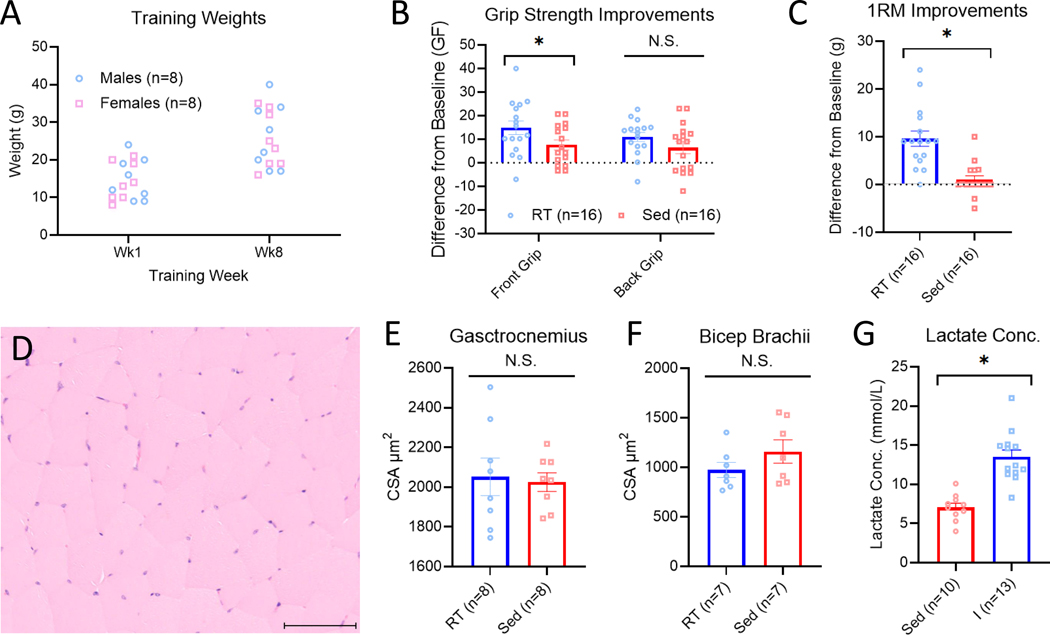
3.3. Experiment 1 – Resistance training increases strength and lactate but not muscle hypertrophy
To validate the resistance training model, we examined grip strength, 1RM measures, cross sectional area of muscle, body weight and acute changes in blood lactate. The variability of weight lifted for each animal and how they increased over time during the resistance training program is displayed in Figure 2A. Strength measures, cross sectional area and lactate measures showed no differences between sex and thus the data are presented combining male and female mice. For forepaw grip strength, resistance trained mice had a significantly greater increase in strength (14.89±2.87g) compared with sedentary animals (8.43±2.10g)(t30=2.05, p=0.05)(Fig. 2B). Resistance trained mice also had a significantly greater increase in 1RM (9.61±1.59g) when compared with sedentary animals (1.02±0.84g)(t30=4.75, p<0.01)(Fig 2C). However, there was no difference between resistance trained (11.00±1.91g) and sedentary animals (6.47±2.60g) hindpaw grip strength (t30=1.39 p=0.172)(Fig. 2B). There were no differences in changes in body weights over the 8 week training program between resistance trained (4.13±0.67g) and sedentary animals (4.71±0.54g)(t30=0.66, p=0.51). Lastly, in a small subset of resistance trained (n=2 male, n=2 female) and sedentary (n=2 male, n=2 female) animals we analyzed total body fat percentage and lean mass after 8 weeks of training. There were no significant difference in body fat percentage between resistance trained (6.31±1.81%) and sedentary animals (8.43±1.72%) (t6=1.70, p=0.14) and no difference in total lean mass between resistance trained (18.05±2.14g) and sedentary animals (18.35±1.93g)(t6=0.10, p=0.92).
Figure 2.
Validation of weighted ladder climbing as a resistance training-based exercise program. Weights lifted during week 1 and week 8 of the resistance training program (A). Animals who underwent eight weeks of resistance training (RT) had significantly greater improvements in front paw grip strength but not back paw grip strength when compared with sedentary (Sed) animals (B). Animals who underwent eight weeks of RT also had a significantly greater increase in 1 repetition maximum (1RM) when compared with sedentary animals. (C) Representative depiction of H&E-stained muscle cross sections used for cross sectional area analysis. Line denotes 100μm (D). Animals who underwent eight weeks of RT had no significant difference in gastrocnemius (E) and bicep brachii (F) muscle cross sectional area when compared with sedentary animals. There was a significant elevation of lactate immediately following a single exercise bout when compared with sedentary animals (G). I=Immediately; *p<0.05 compared with sedentary group. Data are mean±SEM.
To determine if resistance training resulted in muscle hypertrophy, we collected gastrocnemius and bicep brachii muscles after 8 weeks of training and determined muscle cross sectional area between trained and sedentary animals (Fig. 2D). For the gastrocnemius muscle there was no significant difference in cross sectional area between resistance trained (2052.36±94.68μm2) and sedentary animals (2025.26±47.20μm2)(t14=0.25, p=0.80)(Fig. 2E). Similarly, for the biceps brachii muscle there was no significant difference in cross sectional area between resistance trained (974.50±76.98 μm2) and sedentary animals (1159.81±118.40μm2) (t12=1.31, p=0.21)(Fig. 2F).
To determine if lactate levels were significantly elevated following a single resistance training bout, we measured lactate immediately after a training session and compared to lactate levels from sedentary animals. Lactate levels were significantly higher following the exercise program (12.96±0.63 mmol/L) when compared with sedentary controls (7.06±0.53 mmol/L)(p<0.01)(Fig. 2G). Taken together this data demonstrates that the resistance training program results in significant increases in strength and blood lactate levels without muscle hypertrophy.
3.4. Experiment 2 – Resistance training protects against onset of widespread muscle pain
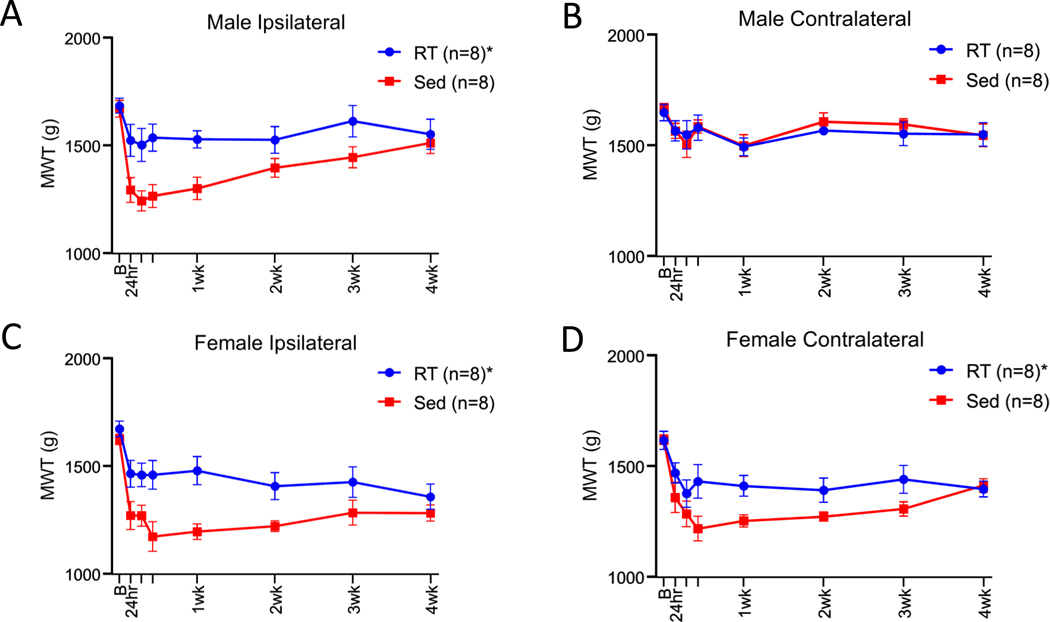
To determine if resistance training could protect against development of activity-induced muscle pain, we compared MWT before and after induction of the activity-induced muscle pain model between resistance trained and sedentary mice. As stated above, sedentary male mice showed a unilateral decrease in MWT while sedentary female mice showed a bilateral decrease in MWT 24 hours after induction of the activity induced pain model [31](Fig. 1). In males, the expected decrease in MWT ipsilaterally did not occur in the resistance trained animals and the MWT was significantly higher than sedentary mice (F1,13 =7.31, p=0.018). As expected, the contralateral MWT in males remained at baseline after induction of the pain model, and thus there were no differences between sedentary and exercised animals (F1,13 =0.07, p=0.79)(Fig. 3A+B). In females, the expected decrease in MWT did not occur ipsilaterally or contralaterally in resistance trained mice. In females, the MWT time course was significantly higher in the resistance trained group when compared to the sedentary group ipsilaterally (F1,13 =7.55, p=0.017) and contralaterally (F1,13 =5.55, p=0.035)(Fig. 3C+D). To determine if there was an association between amount of weight lifted during resistance training and the degree of protection against muscle hyperalgesia, we correlated volume of resistance training throughout 8 weeks with MWT. There was no significant correlation between volume lifted and ipsilateral 24hr MWT (r=−0.22 male; r=0.06 female), ipsilateral 24hr MWT change from baseline (r=−0.14 male; r=−0.33 female), contralateral 24hr MWT (r=−0.27 male; r=−0.05 female) and contralateral 24hr MWT change from baseline (r=−0.35 male; r=−0.40 female)(p=0.32–0.91).
Figure 3.
Line graphs show group differences in muscle withdrawal threshold (MWT). Male mice who underwent eight weeks of resistance training (RT) had significantly higher MWT when compared with sedentary (Sed) animals on the ipsilateral (A) but not on the contralateral (B) side. Female mice who underwent eight weeks of RT had significantly higher MWT when compared with sedentary animals on the ipsilateral (C) and contralateral (D) side. *p<0.05 compared with sedentary group; B=baseline; Data are mean±SEM.
In a subset of animals who participated in the resistance training program we quantified gastrocnemius muscle fatigue produced by the activity-induced pain model to determine if a lack of muscle fatigue was preventing the decrease in MWT. Gastrocnemius muscle fatigue was determined by the percent decline of electrically stimulated isometric contractions before and after 6 minutes of fatiguing gastrocnemius muscle contractions. There was no significant difference in gastrocnemius muscle fatigue between animals who performed the resistance training program (66.13±2.46 %decline) when compared with sedentary animals (69.25±1.82 %decline)(t14=1.01, p=0.32)(Supplemental Fig. 1). This data taken together suggests performing 8 weeks of the resistance training program protects against development of muscle hyperalgesia which is not caused by a lack of muscle fatigue during induction of the activity-induced pain model.
To determine if weight was required for the protective effects of the resistance training program, a separate group of animals climbed the ladder without weight attached to their tail for 8 weeks. Performing 8 weeks of the resistance training program without weight prior to induction of muscle pain failed to protect against the onset of hyperalgesia when compared with sedentary controls. In males, there was no significant difference in MWT values on the ipsilateral (F1,9 =5.0, p=0.052) and contralateral limb (F1,9 =0.82, p=0.28)(Supplemental Fig. 2A+B). Similarly, in females, there was no significant difference in MWT values on both the ipsilateral (F1,9 =1.36, p=0.27) and contralateral limb (F1,9 =1.96, p=0.19)(Supplemental Fig. 2C+D). This data suggests that climbing the resistance training ladder with weight is required for protection against activity-induced muscle hyperalgesia.
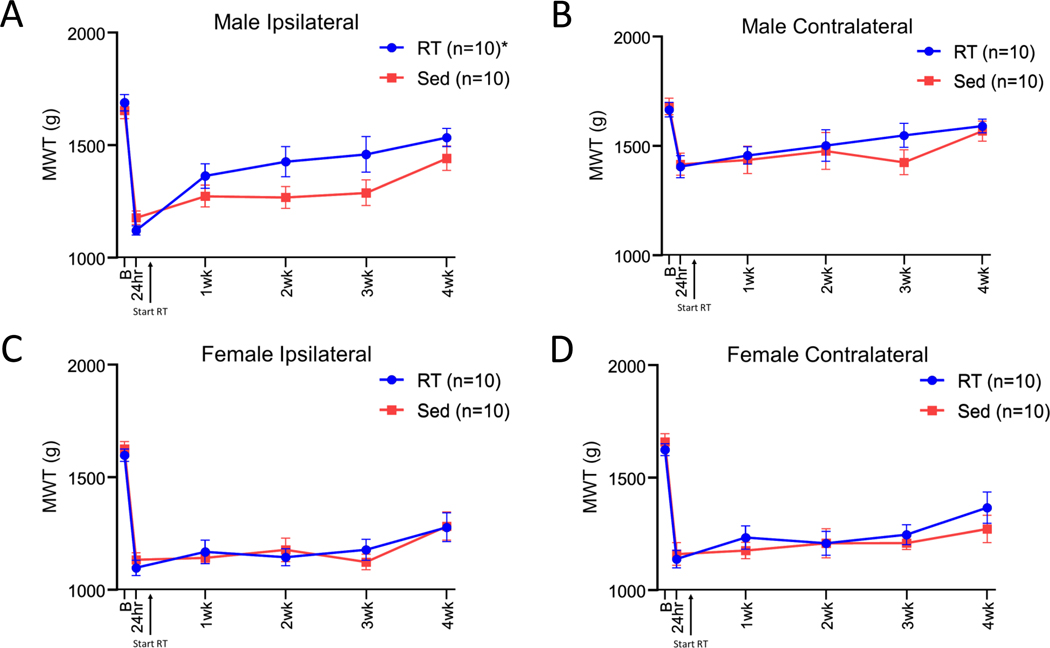
3.5. Experiment 3 – Resistance training alleviates activity-induced muscle pain in male but not female mice
To determine if the resistance training program could reverse the decrease in MWT produced by the activity-induced pain model, we initiated the resistance training program after induction of the model. In males, resistance training significantly increased the MWT when compared to sedentary animals ipsilaterally (F1,17 =12.46, p<0.01) but not contralaterally (F1,17 =1.50, p=0.23) (Fig. 4A+B). However, in females there was not a significant difference in MWT values between resistance trained and sedentary animals on the ipsilateral (F1,17 =1.07, p=0.315) or contralateral limb (F1,17 =2.16, p=0.16)(Fig. 4C+D). In males, there was not a significant correlation between total volume of weight lifted and ipsilateral 4wk MWT (r=−0.30), ipsilateral 4wk MWT change score from 24hr (r=−0.31), contralateral 4wk MWT (r=−0.12) and contralateral 4wk MWT change score from 24hr (r=−0.09) (p=0.39–0.81). This data demonstrates that the resistance training program alleviated activity-induced muscle hyperalgesia in male but not female mice.
Figure 4.
Line graphs show group differences for muscle withdrawal thresholds (MWT). Males who initiated resistance training (RT) after induction of the pain model had significantly elevated MWT on the ipsilateral (A) but not contralateral side (B) when compared with sedentary (Sed) animals. Females who initiated the RT program after induction of the pain model saw no difference in MWT values when compared with sedentary animals on both the ipsilateral (C) and contralateral (D) side. *p<0.05 compared with sedentary group,; B=baseline; Data are mean±SEM.
3.6. Experiment 4 – The role of testosterone and androgen receptor signaling in resistance training induced protection against muscle hyperalgesia
3.6.1. Resistance training increases testosterone acutely in male but not female mice
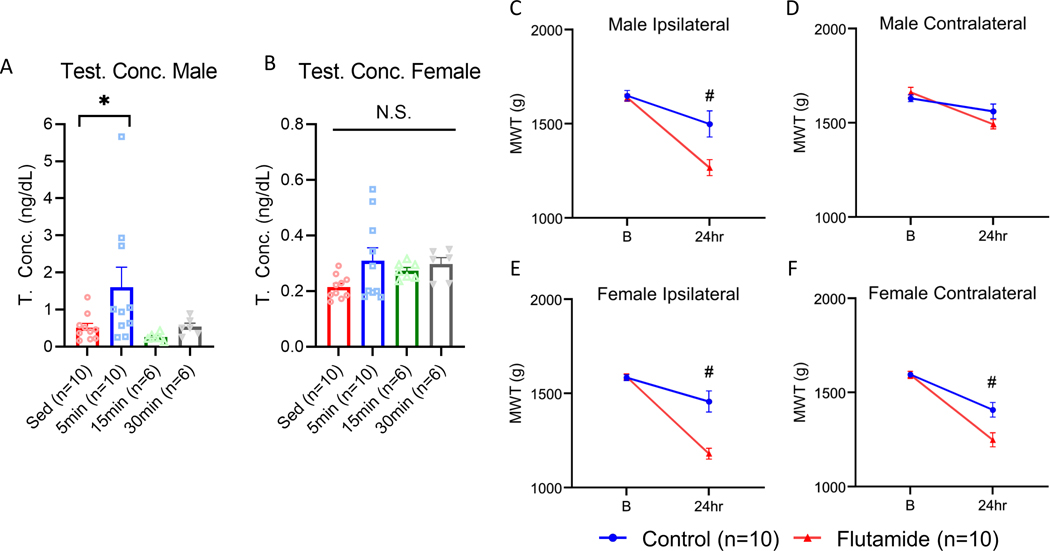
To determine if a single resistance training session increased testosterone, we analyzed testosterone concentrations from serum at 5, 15, and 30 minutes after a resistance training session and from sedentary controls. In males there was a significant group difference for testosterone concentrations (F3,28 =3.17, p=0.03) with post hoc analysis demonstrating a significant difference between sedentary (0.51±0.11ng/dL) and 5 minutes post training (1.60±0.53ng/dL)(p=0.05) but not between any other time points (p=0.20–0.41)(Fig. 5A). In females there was no significant group difference for testosterone concentrations (F3,28 =2.13, p=0.11)(Fig. 5B).
Figure 5.
Role of testosterone and androgen receptor signaling in resistance training-based protection against muscle hyperalgesia. Males saw a significant elevation in testosterone concentration 5 minutes after the resistance training bout when compared with sedentary (Sed) animals (A). Females saw no significant changes in testosterone concentration after a resistance training bout (B). Line graphs show group differences for MWT. All animals performed eight weeks of resistance training with slow release flutamide (200 mg/pellet, 60-day release) or control pellets implanted subcutaneously. In males with flutamide pellets, MWT values were significantly lower 24 hours after induction of the model on the ipsilateral (C) but not the contralateral (D) side. In females with flutamide pellets, MWT values were significantly lower 24 hours after induction of the model on the ipsilateral (E) and contralateral (F) side. *p<0.05 compared with Sed, #p<0.05 when compared with Flutamide; B=baseline; Data are mean±SEM.
3.6.2. Flutamide administration after induction of activity-induced muscle pain has no effect on resistance training induced protection against muscle hyperalgesia
To determine if activation of androgen receptors is required for the maintenance of resistance training analgesic effects on hyperalgesia, we applied the androgen receptor antagonist flutamide to trained animals after induction of muscle pain. A single injection of flutamide (1, 3, 10mg/kg) had no effect on the analgesia produced by resistance training in male (ipsilateral F3,19 =0.269, p=0.84; contralateral F3,19 =1.00, p=0.42) or female mice (ipsilateral F3,19 =1.13, p=0.36; contralateral limb F3,19 =0.16, p=0.91)(Supplemental Fig. 3 A–D). This data illustrates that continual activation of androgen receptors is not required for the maintenance of resistance training induced protection against muscle pain.
3.6.3. Flutamide administration during resistance training blocked the exercise-induced muscle hyperalgesia protection
To determine if activation of androgen receptors during resistance training is required for prevention of the activity-induced muscle hyperalgesia, we blocked androgen receptors during the resistance training program using flutamide pellets. In males, the administration flutamide throughout the training interval resulted in significantly lower MWTs on the ipsilateral side (1266.0±42.41g) compared to controls (1498.33±69.47g) (F1,19=7.84, p=0.01) (Fig. 5C). There was no difference observed on the contralateral side in males animals who received flutamide (1560.53±38.78) vs control pellets (1491.76±24.33)(F1,19=2.07, p=0.16)(Fig. 5D). In females, on the ipsilateral side, the MWT was significantly lower in animals who received flutamide (1179.23±28.88g) compared with animals who received control pellets (1456.63±56.23g)(F1,19=18.32, p<0.01)(Fig. 5E). Similarly, on the contralateral side in females, the MWT was significantly lower in animals who received flutamide (1248.30±37.24g) compared with animals who received control pellets (1407.23±38.77g)(F1,19=8.30, p=0.01)(Fig. 5F). This data suggest that activation of androgen receptors is required for the protection against activity-induced muscle pain that is produced by the resistance training program.
4. Discussion
4.1. Overview
The current study is the first to show an animal model of resistance training protects against development of activity-induced muscle hyperalgesia, in male and female mice, and produces analgesia through activation of androgen receptors. However, there was a clear sex difference in reversal of existing muscle hyperalgesia - resistance training was only effective in male mice. The current study is consistent with two prior studies showing a single bout resistance training produces analgesia in uninjured male mice [27;29]. Thus, this new animal model of resistance training can be used to probe the underlying mechanisms of a clinically used form of exercise.
Of note, prior studies examining analgesic effects of exercise in animal models of pain primarily evaluated aerobic exercise tasks. Interestingly, voluntary running wheel showed a delayed development of hyperalgesia in our animal model of chronic muscle pain (pH 4 injections). It is unclear if voluntary running wheel completed prevented hyperalgesia in the activity-induced pain model as observed for resistance training. A large body of literature now shows multiple underlying mechanisms for aerobic exercise-induced analgesia including alterations in the peripheral and central nervous system and the immune system (add other references)[52]. Whether these same mechanisms, or novel mechanisms, are found for resistance training remains to be determined.
4.2. Validation of weighted ladder climbing as resistance training-based exercise
The current study showed acute elevation of blood lactate following a single exercise bout and increases in strength (grip force and 1RM) after 8 weeks of training. Elevated lactate suggests anaerobic metabolism commonly seen following intense exercise, including resistance training [72]. The increases in strength are consistent with prior studies using weighted ladder climbing which show increases in strength [39;59]. However, there was no muscle hypertrophy of the gastrocnemius or biceps brachii muscles after resistance training, as might be expected with strengthening exercise. Prior work using weighted ladder climbing demonstrated mixed results for muscle hypertrophy [35;39;40;59], measured by muscle weight. Resistance training increased weight of the flexor hallucis longus [35], quadriceps [59], and soleus [40] but not the gastrocnemius [39;59]. The lack of hypertrophy could be related to dosing. Those that report increases used maximal training loads each day [35] or trained animals for 12–18 weeks [40;59]. In contrast, we utilized a progressive increase in training loads starting at 70% of 1RM on week 1 and we trained animals for 8 weeks. Of note, despite a lack of muscle hypertrophy, there are increases in muscle strength and protection against muscle hyperalgesia. This suggests adaptations with resistance training are sufficient to prevent development of hyperalgesia, and the underlying mechanisms are not dependent on trophic muscle changes. Further, since strength increases were only demonstrated in the upper extremity it suggests analgesic effects were systemic since hyperalgesia was prevented in the lower extremity.
4.3. Androgen receptors mediate the protective effects of resistance training on muscle hyperalgesia
A notable finding in the current study is resistance-training dependence on androgen receptor activation (i.e., testosterone) to protect against development of activity-induced hyperalgesia. Further, this protection is not reversed with blockade of androgen receptors after training has occurred. These data suggest that androgen receptors activation initiates long-term effects that maintain analgesia or prevent events that initiate development of muscle hyperalgesia. Similarly, prior studies by us and others, show testosterone can protect against development of hyperalgesia in both male and female mice [51]. For example, in the same activity-induced pain model, testosterone pellets in female mice prevent development of contralateral hyperalgesia, while blockade of androgen receptors (with flutamide) in male mice results in contralateral hyperalgesia [51;64]. These data suggest activation of androgen receptors is protective in both male and female mice, similar to that observed with resistance training in the current study.
The current study administered flutamide systemically; thus, the site of androgen receptors activation to produce the analgesic effect is unknown. Activation of androgen receptors mediates several biological pathways which could produce antinociception. In trigeminal ganglia, activation of androgen receptors in males transcriptionally increases expression of mu-opioid and cannabinoid type-1 receptors which have antinociceptive effects [2;49;50;63;78]. Activation of androgen receptors, in males, modulates peripheral immune system function by decreasing pro-inflammatory cytokines [8], increasing anti-inflammatory cytokines [61], and shifting resident macrophages to an anti-inflammatory M2 phenotype [5;6]. It is unclear if the same mechanisms are activated by testosterone in males and females. Further, many central nervous system sites that modulate nociception contain androgen receptors in both sexes [57], and can dynamically influence expression of neurotransmitters and their receptors by directly modulating gene expression or through membrane bound receptors [25]. In humans, lower blood testosterone levels in females are correlated with lower activation of brain regions responsible for descending pain inhibition [74]. Prior studies show that mu-opioid, cannabinoid type-1, and immune mechanisms can underlie the analgesic effects of aerobic exercise [14;28;53]; it is unclear if these same pathways are involved in resistance-training induced analgesia. As with aerobic exercise, the antinociceptive effects of resistance training are likely multifactorial.
We also demonstrated that males, but not females, show an acute increase in serum testosterone concentrations immediately after a single exercise bout, consistent with clinical findings in males [44;45;56;71]. However, in females the results are equivocal with some observing increases in testosterone [23;33;62;75] and others no change [43;56;58] in response to resistance training. In males, Leydig cells in the testes are responsible for the acute increase in testosterone following resistance training [48]. However, in females, testosterone is produced from the ovaries, adrenal cortex, and through the conversion of testosterone precursors in peripheral tissues [76]. Thus, the lack of ability to show increases in testosterone in females could be related to the lack of specialized cells dedicated to testosterone production, or local release at key sites in the peripheral or central nervous system [76]. Despite the lack of acute increase in testosterone in females, we demonstrated that blockade of the androgen receptors through flutamide prevented the resistance training induced protection against muscle pain.
4.4. Sex differences in resistance training analgesia after development of muscle hyperalgesia
While we show that 8 weeks of prior resistance training can protect against pain in both sexes, while resistance training reduced hyperalgesia when initiated after induction of the model only in males. Few studies have examined sex differences in the effectiveness of exercise programs for preventing and alleviating pain. Of these, there are conflicting results. Voluntary wheel running prevents onset of hyperalgesia in both male and female mice prior to induction of acidic saline or activity-induced muscle pain models [14;53;55;66]. However, voluntary wheel running alleviated mechanical hyperalgesia only in females in an experimental autoimmune encephalomyelitis [60]. Future studies should examine underlying mechanisms for exercise-induced analgesia in both sexes for similarities and differences.
Given sex differences in pain phenotype in the activity-induced pain model, it is possible that different underlying mechanisms in males and females account for the sensitivity to resistance training. For example, we showed females, but not males, have increases in serotonin transporters (SERT) in the rostral ventromedial medulla (RVM) after induction of the activity-induced pain model [51]. The RVM is a main location for facilitation and inhibition of nociception and increases in SERT in females demonstrate alterations in central nervous system pain pathways. The presence of centrally mediated pain could make resistance training less effective. Clinically, nociplastic symptoms are related to poorer treatment outcomes in response to both conservative and surgical interventions [7;22;32;37;41;70]. Interestingly, aerobic exercise training uses serotonin to produce analgesia and reduces SERT expression in the RVM [11;14;55]. It is unclear if resistance training has the same effects on altered central processing of nociception. It is likely that different mechanisms underly the analgesia produced by different forms of exercise, i.e. aerobic vs. strengthening exercise.
4.5. Clinical Relevance
We are the first to demonstrate the ability of an animal model of resistance training to prevent or alleviate muscle pain. This information provides a scientific basis for the use of strength training as a therapeutic tool clinically for individuals with chronic musculoskeletal pain. Since both aerobic and resistance training-based exercise have been shown to produce analgesia [9;10;18;52], clinicians may want to consider patient preferences when prescribing exercise modes. Next, the lower extremity was protected against development of muscle pain despite strength increases only evident in the upper extremity suggesting resistance training produced systemic alterations that led to the protection against muscle pain. Thus, resistance training exercise prescriptions do not need to be targeted at the painful muscle groups for therapeutic benefits. Lastly, we saw the resistance training program was more effective at preventing than alleviating muscle pain. This suggests that exercise should be continued in the absence of symptoms to prevent against future development of musculoskeletal pain.
5. Summary
In summary, the current study demonstrated that an animal model of resistance training can be used for the study of exercise-induced analgesia. We showed that 8 weeks of resistance training protects against the development of activity-induced muscle pain and is mediated through activation of androgen receptors. However, resistance training was only able to alleviate muscle in pain in male mice.
Supplementary Material
Supplemental Figure 1 Group differences for muscle fatigue produced by electrical stimulation. Muscle fatigue was measured by percent decline of electrically stimulated maximum force contractions before and after six minutes of fatiguing contractions. Animals who participated in eight weeks of the resistance training (RT) saw similar levels of muscle fatigue when compared with sedentary (Sed) animals. P>0.05, t-test; Data are mean±SEM.
Supplemental Figure 2 Line graphs show group differences in muscle withdrawal threshold (MWT). Male mice who underwent eight weeks of resistance training without weight (RT w/o Weight) had no significant difference in MWT values when compared with sedentary (Sed) animals on the ipsilateral (A) and contralateral (B) side. Female mice who underwent eight weeks of RT without weight had no significant difference in MWT values when compared with sedentary animals on the ipsilateral (C) and contralateral (D) side. Repeated measures ANOVA using time points 24 hours through 4 weeks with baseline as a covariate; B=baseline; Data are mean±SEM.
Supplemental Figure 3 Effect of androgen receptor antagonist flutamide on resistance training induced protection against muscle hyperalgesia. Line graphs show group differences for muscle withdrawal threshold (MWT) after acute administration of flutamide. Flutamide (1, 3, 10 mg/kg, or vehicle, i.p.) was administered 24-hour after induction of the activity-induced pain model (PUT an arrow on the graph after the 24h time period or a bar underneath the 30min and 120min with words drug or vehicle under it). MWT were reassessed 30 and 120 minutes after flutamide injection. No significant differences in MWT data were observed on either the ipsilateral or contralateral side in male and female mice after flutamide when compared to vehicle. (A-D).
Acknowledgements
Supported by the National Institutes of Health [AR073187, GM067795] and by a Promotion of Doctoral Studies 1 Scholarship from the Foundation for Physical Therapy Research.
Footnotes
Conflict of interest statement
The authors have no conflicts of interest to declare.
References:
- [1].American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc 2009;41(3):687–708. [DOI] [PubMed] [Google Scholar]
- [2].Bai X, Zhang X, Li Y, Lu L, Li B, He X. Sex Differences in Peripheral Mu-Opioid Receptor Mediated Analgesia in Rat Orofacial Persistent Pain Model. PLoS One 2015;10(3):e0122924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Barber M, Pace A. Exercise and Migraine Prevention: a Review of the Literature. Curr Pain Headache Rep 2020;24(8):39. [DOI] [PubMed] [Google Scholar]
- [4].Basaria S, Travison TG, Alford D, Knapp PE, Teeter K, Cahalan C, Eder R, Lakshman K, Bachman E, Mensing G, Martel MO, Le D, Stroh H, Bhasin S, Wasan AD, Edwards RR. Effects of testosterone replacement in men with opioid-induced androgen deficiency: a randomized controlled trial. Pain 2015;156(2):280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Becerra-Diaz M, Song M, Heller N. Androgen and Androgen Receptors as Regulators of Monocyte and Macrophage Biology in the Healthy and Diseased Lung. Front Immunol 2020;11:1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Becerra-Díaz M, Strickland AB, Keselman A, Heller NM. Androgen and Androgen Receptor as Enhancers of M2 Macrophage Polarization in Allergic Lung Inflammation. J Immunol 2018;201(10):2923–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bennett EE, Walsh KM, Thompson NR, Krishnaney AA. Central Sensitization Inventory as a Predictor of Worse Quality of Life Measures and Increased Length of Stay Following Spinal Fusion. World Neurosurg 2017;104:594–600. [DOI] [PubMed] [Google Scholar]
- [8].Bianchi VE. The Anti-Inflammatory Effects of Testosterone. J Endocr Soc 2019;3(1):91–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bidonde J, Busch AJ, Bath B, Milosavljevic S. Exercise for adults with fibromyalgia: an umbrella systematic review with synthesis of best evidence. Curr Rheumatol Rev 2014;10(1):45–79. [DOI] [PubMed] [Google Scholar]
- [10].Bidonde J, Busch AJ, Schachter CL, Overend TJ, Kim SY, Goes SM, Boden C, Foulds HJ. Aerobic exercise training for adults with fibromyalgia. Cochrane Database Syst Rev 2017;6:Cd012700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bobinski F, Ferreira TAA, Cordova MM, Dombrowski PA, da Cunha C, Santo C, Poli A, Pires RGW, Martins-Silva C, Sluka KA, Santos ARS. Role of brainstem serotonin in analgesia produced by low-intensity exercise on neuropathic pain after sciatic nerve injury in mice. Pain 2015;156(12):2595–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bobinski F, Martins DF, Bratti T, Mazzardo-Martins L, Winkelmann-Duarte EC, Guglielmo LG, Santos AR. Neuroprotective and neuroregenerative effects of low-intensity aerobic exercise on sciatic nerve crush injury in mice. Neuroscience 2011;194:337–348. [DOI] [PubMed] [Google Scholar]
- [13].Bobinski F, Teixeira JM, Sluka KA, Santos ARS. Interleukin-4 mediates the analgesia produced by low-intensity exercise in mice with neuropathic pain. Pain 2018;159(3):437–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Brito RG, Rasmussen LA, Sluka KA. Regular physical activity prevents development of chronic muscle pain through modulation of supraspinal opioid and serotonergic mechanisms. Pain Rep 2017;2(5):e618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Brosseau L, Taki J, Desjardins B, Thevenot O, Fransen M, Wells GA, Mizusaki Imoto A, Toupin-April K, Westby M, Álvarez Gallardo IC, Gifford W, Laferrière L, Rahman P, Loew L, De Angelis G, Cavallo S, Shallwani SM, Aburub A, Bennell KL, Van der Esch M, Simic M, McConnell S, Harmer A, Kenny GP, Paterson G, Regnaux J-P, Lefevre-Colau M-M, McLean L. The Ottawa panel clinical practice guidelines for the management of knee osteoarthritis. Part three: aerobic exercise programs. Clinical rehabilitation 2017;31(5):612–624. [DOI] [PubMed] [Google Scholar]
- [16].Brosseau L, Taki J, Desjardins B, Thevenot O, Fransen M, Wells GA, Mizusaki Imoto A, Toupin-April K, Westby M, Álvarez Gallardo IC, Gifford W, Laferrière L, Rahman P, Loew L, De Angelis G, Cavallo S, Shallwani SM, Aburub A, Bennell KL, Van der Esch M, Simic M, McConnell S, Harmer A, Kenny GP, Paterson G, Regnaux J-P, Lefevre-Colau M-M, McLean L. The Ottawa panel clinical practice guidelines for the management of knee osteoarthritis. Part two: strengthening exercise programs. Clinical rehabilitation 2017;31(5):596–611. [DOI] [PubMed] [Google Scholar]
- [17].Busch AJ, Webber SC, Brachaniec M, Bidonde J, Bello-Haas VD, Danyliw AD, Overend TJ, Richards RS, Sawant A, Schachter CL. Exercise therapy for fibromyalgia. Curr Pain Headache Rep 2011;15(5):358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Busch AJ, Webber SC, Richards RS, Bidonde J, Schachter CL, Schafer LA, Danyliw A, Sawant A, Dal Bello-Haas V, Rader T, Overend TJ. Resistance exercise training for fibromyalgia. Cochrane Database Syst Rev 2013(12):Cd010884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Butler RK, Finn DP. Stress-induced analgesia. Prog Neurobiol 2009;88(3):184–202. [DOI] [PubMed] [Google Scholar]
- [20].Carruba MO, Nisoli E, Garosi V, Sacerdote P, Panerai AE, Da Prada M. Catecholamine and serotonin depletion from rat spinal cord: effects on morphine and footshock induced analgesia. Pharmacological research 1992;25(2):187–194. [DOI] [PubMed] [Google Scholar]
- [21].Contarteze RV, Manchado Fde B, Gobatto CA, De Mello MA. Stress biomarkers in rats submitted to swimming and treadmill running exercises. Comp Biochem Physiol A Mol Integr Physiol 2008;151(3):415–422. [DOI] [PubMed] [Google Scholar]
- [22].Coombes BK, Bisset L, Vicenzino B. Cold hyperalgesia associated with poorer prognosis in lateral epicondylalgia: a 1-year prognostic study of physical and psychological factors. Clin J Pain 2015;31(1):30–35. [DOI] [PubMed] [Google Scholar]
- [23].Copeland JL, Consitt LA, Tremblay MS. Hormonal responses to endurance and resistance exercise in females aged 19–69 years. J Gerontol A Biol Sci Med Sci 2002;57(4):B158–165. [DOI] [PubMed] [Google Scholar]
- [24].Courneya KS, McKenzie DC, Mackey JR, Gelmon K, Friedenreich CM, Yasui Y, Reid RD, Vallerand JR, Adams SC, Proulx C, Dolan LB, Wooding E, Segal RJ. Subgroup effects in a randomised trial of different types and doses of exercise during breast cancer chemotherapy. Br J Cancer 2014;111(9):1718–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Davey RA, Grossmann M. Androgen Receptor Structure, Function and Biology: From Bench to Bedside. Clin Biochem Rev 2016;37(1):3–15. [PMC free article] [PubMed] [Google Scholar]
- [26].Fanton LE, Macedo CG, Torres-Chávez KE, Fischer L, Tambeli CH. Activational action of testosterone on androgen receptors protects males preventing temporomandibular joint pain. Pharmacology Biochemistry and Behavior 2017;152:30–35. [DOI] [PubMed] [Google Scholar]
- [27].Galdino G, Romero T, Silva JF, Aguiar D, Paula AM, Cruz J, Parrella C, Piscitelli F, Duarte I, Di Marzo V, Perez A. Acute resistance exercise induces antinociception by activation of the endocannabinoid system in rats. Anesth Analg 2014;119(3):702–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Galdino G, Romero TR, Silva JF, Aguiar DC, de Paula AM, Cruz JS, Parrella C, Piscitelli F, Duarte ID, Di Marzo V, Perez AC. The endocannabinoid system mediates aerobic exercise-induced antinociception in rats. Neuropharmacology 2014;77:313–324. [DOI] [PubMed] [Google Scholar]
- [29].Galdino GS, Duarte ID, Perez AC. Participation of endogenous opioids in the antinociception induced by resistance exercise in rats. Braz J Med Biol Res 2010;43(9):906–909. [DOI] [PubMed] [Google Scholar]
- [30].Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain 2012;13(8):715–724. [DOI] [PubMed] [Google Scholar]
- [31].Gregory NS, Gibson-Corley K, Frey-Law L, Sluka KA. Fatigue-enhanced hyperalgesia in response to muscle insult: induction and development occur in a sex-dependent manner. Pain 2013;154(12):2668–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gwilym SE, Oag HC, Tracey I, Carr AJ. Evidence that central sensitisation is present in patients with shoulder impingement syndrome and influences the outcome after surgery. J Bone Joint Surg Br 2011;93(4):498–502. [DOI] [PubMed] [Google Scholar]
- [33].Häkkinen K, Pakarinen A, Kraemer WJ, Newton RU, Alen M. Basal concentrations and acute responses of serum hormones and strength development during heavy resistance training in middle-aged and elderly men and women. J Gerontol A Biol Sci Med Sci 2000;55(2):B95–105. [DOI] [PubMed] [Google Scholar]
- [34].Hayes LD, Grace FM, Baker JS, Sculthorpe N. Exercise-induced responses in salivary testosterone, cortisol, and their ratios in men: a meta-analysis. Sports Med 2015;45(5):713–726. [DOI] [PubMed] [Google Scholar]
- [35].Hornberger TA, Jr., Farrar RP. Physiological hypertrophy of the FHL muscle following 8 weeks of progressive resistance exercise in the rat. Can J Appl Physiol 2004;29(1):16–31. [DOI] [PubMed] [Google Scholar]
- [36].Ji Y, Hu B, Li J, Traub RJ. Opposing Roles of Estradiol and Testosterone on Stress-Induced Visceral Hypersensitivity in Rats. J Pain 2018;19(7):764–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jull G, Sterling M, Kenardy J, Beller E. Does the presence of sensory hypersensitivity influence outcomes of physical rehabilitation for chronic whiplash?--A preliminary RCT. Pain 2007;129(1–2):28–34. [DOI] [PubMed] [Google Scholar]
- [38].Kato Y, Shigehara K, Kawaguchi S, Izumi K, Kadono Y, Mizokami A. Efficacy of testosterone replacement therapy on pain in hypogonadal men with chronic pain syndrome: A subanalysis of a prospective randomised controlled study in Japan (EARTH study). Andrologia 2020;52(9):e13768. [DOI] [PubMed] [Google Scholar]
- [39].Kim HJ, So B, Choi M, Kang D, Song W. Resistance exercise training increases the expression of irisin concomitant with improvement of muscle function in aging mice and humans. Exp Gerontol 2015;70:11–17. [DOI] [PubMed] [Google Scholar]
- [40].Kim JS, Yoon DH, Kim HJ, Choi MJ, Song W. Resistance exercise reduced the expression of fibroblast growth factor-2 in skeletal muscle of aged mice. Integr Med Res 2016;5(3):230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kim SH, Yoon KB, Yoon DM, Yoo JH, Ahn KR. Influence of Centrally Mediated Symptoms on Postoperative Pain in Osteoarthritis Patients Undergoing Total Knee Arthroplasty: A Prospective Observational Evaluation. Pain Pract 2015;15(6):E46–53. [DOI] [PubMed] [Google Scholar]
- [42].Kleckner IR, Kamen C, Gewandter JS, Mohile NA, Heckler CE, Culakova E, Fung C, Janelsins MC, Asare M, Lin PJ, Reddy PS, Giguere J, Berenberg J, Kesler SR, Mustian KM. Effects of exercise during chemotherapy on chemotherapy-induced peripheral neuropathy: a multicenter, randomized controlled trial. Support Care Cancer 2018;26(4):1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kraemer WJ, Fleck SJ, Dziados JE, Harman EA, Marchitelli LJ, Gordon SE, Mello R, Frykman PN, Koziris LP, Triplett NT. Changes in hormonal concentrations after different heavy-resistance exercise protocols in women. J Appl Physiol (1985) 1993;75(2):594–604. [DOI] [PubMed] [Google Scholar]
- [44].Kraemer WJ, Häkkinen K, Newton RU, McCormick M, Nindl BC, Volek JS, Gotshalk LA, Fleck SJ, Campbell WW, Gordon SE, Farrell PA, Evans WJ. Acute hormonal responses to heavy resistance exercise in younger and older men. Eur J Appl Physiol Occup Physiol 1998;77(3):206–211. [DOI] [PubMed] [Google Scholar]
- [45].Kraemer WJ, Marchitelli L, Gordon SE, Harman E, Dziados JE, Mello R, Frykman P, McCurry D, Fleck SJ. Hormonal and growth factor responses to heavy resistance exercise protocols. J Appl Physiol (1985) 1990;69(4):1442–1450. [DOI] [PubMed] [Google Scholar]
- [46].Kraemer WJ, Ratamess NA. Fundamentals of resistance training: progression and exercise prescription. Med Sci Sports Exerc 2004;36(4):674–688. [DOI] [PubMed] [Google Scholar]
- [47].Kuphal KE, Fibuch EE, Taylor BK. Extended swimming exercise reduces inflammatory and peripheral neuropathic pain in rodents. J Pain 2007;8(12):989–997. [DOI] [PubMed] [Google Scholar]
- [48].Kvorning T, Andersen M, Brixen K, Schjerling P, Suetta C, Madsen K. Suppression of testosterone does not blunt mRNA expression of myoD, myogenin, IGF, myostatin or androgen receptor post strength training in humans. J Physiol 2007;578(Pt 2):579–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lee KS, Asgar J, Zhang Y, Chung MK, Ro JY. The role of androgen receptor in transcriptional modulation of cannabinoid receptor type 1 gene in rat trigeminal ganglia. Neuroscience 2013;254:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lee KS, Zhang Y, Asgar J, Auh QS, Chung MK, Ro JY. Androgen receptor transcriptionally regulates mu-opioid receptor expression in rat trigeminal ganglia. Neuroscience 2016;331:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lesnak JB, Inoue S, Lima L, Rasmussen L, Sluka KA. Testosterone protects against the development of widespread muscle pain in mice. Pain 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lesnak JB, Sluka KA. Mechanism of exercise-induced analgesia: what we can learn from physically active animals. PAIN Reports 2020;5(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Leung A, Gregory NS, Allen LA, Sluka KA. Regular physical activity prevents chronic pain by altering resident muscle macrophage phenotype and increasing interleukin-10 in mice. Pain 2016;157(1):70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lima LV, Abner TSS, Sluka KA. Does exercise increase or decrease pain? Central mechanisms underlying these two phenomena. J Physiol 2017;595(13):4141–4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lima LV, DeSantana JM, Rasmussen LA, Sluka KA. Short-duration physical activity prevents the development of activity-induced hyperalgesia through opioid and serotoninergic mechanisms. Pain 2017;158(9):1697–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Linnamo V, Pakarinen A, Komi PV, Kraemer WJ, Häkkinen K. Acute hormonal responses to submaximal and maximal heavy resistance and explosive exercises in men and women. J Strength Cond Res 2005;19(3):566–571. [DOI] [PubMed] [Google Scholar]
- [57].Loyd DR, Murphy AZ. Androgen and estrogen (alpha) receptor localization on periaqueductal gray neurons projecting to the rostral ventromedial medulla in the male and female rat. J Chem Neuroanat 2008;36(3–4):216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Marx JO, Ratamess NA, Nindl BC, Gotshalk LA, Volek JS, Dohi K, Bush JA, Gómez AL, Mazzetti SA, Fleck SJ, Häkkinen K, Newton RU, Kraemer WJ. Low-volume circuit versus high-volume periodized resistance training in women. Med Sci Sports Exerc 2001;33(4):635–643. [DOI] [PubMed] [Google Scholar]
- [59].Matheny RW, Merritt E, Zannikos SV, Farrar RP, Adamo ML. Serum IGF-I-deficiency does not prevent compensatory skeletal muscle hypertrophy in resistance exercise. Exp Biol Med (Maywood) 2009;234(2):164–170. [DOI] [PubMed] [Google Scholar]
- [60].Mifflin KA, Yousuf MS, Thorburn KC, Huang J, Perez-Munoz ME, Tenorio G, Walter J, Ballanyi K, Drohomyrecky PC, Dunn SE, Kerr BJ. Voluntary wheel running reveals sex-specific nociceptive factors in murine experimental autoimmune encephalomyelitis. Pain 2019;160(4):870–881. [DOI] [PubMed] [Google Scholar]
- [61].Mohamad NV, Wong SK, Wan Hasan WN, Jolly JJ, Nur-Farhana MF, Ima-Nirwana S, Chin KY. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male 2019;22(2):129–140. [DOI] [PubMed] [Google Scholar]
- [62].Nindl BC, Kraemer WJ, Gotshalk LA, Marx JO, Volek JS, Bush FA, Häkkinen K, Newton RU, Fleck SJ. Testosterone responses after resistance exercise in women: influence of regional fat distribution. Int J Sport Nutr Exerc Metab 2001;11(4):451–465. [DOI] [PubMed] [Google Scholar]
- [63].Niu KY, Zhang Y, Ro JY. Effects of gonadal hormones on the peripheral cannabinoid receptor 1 (CB1R) system under a myositis condition in rats. Pain 2012;153(11):2283–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Paige C, Barba-Escobedo PA, Mecklenburg J, Patil M, Goffin V, Grattan DR, Dussor G, Akopian AN, Price TJ. Neuroendocrine Mechanisms Governing Sex Differences in Hyperalgesic Priming Involve Prolactin Receptor Sensory Neuron Signaling. J Neurosci 2020;40(37):7080–7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Qaseem A, Wilt TJ, McLean RM, Forciea MA, Clinical Guidelines Committee of the American College of P. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Annals of internal medicine 2017;166(7):514–530. [DOI] [PubMed] [Google Scholar]
- [66].Sabharwal R, Rasmussen L, Sluka KA, Chapleau MW. Exercise prevents development of autonomic dysregulation and hyperalgesia in a mouse model of chronic muscle pain. Pain 2016;157(2):387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Searle A, Spink M, Ho A, Chuter V. Exercise interventions for the treatment of chronic low back pain: a systematic review and meta-analysis of randomised controlled trials. Clin Rehabil 2015;29(12):1155–1167. [DOI] [PubMed] [Google Scholar]
- [68].Sluka KA, Frey-Law L, Hoeger Bement M. Exercise-induced pain and analgesia? Underlying mechanisms and clinical translation. Pain 2018;159 Suppl 1:S91–s97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Sluka KA, Rasmussen LA. Fatiguing exercise enhances hyperalgesia to muscle inflammation. Pain 2010;148(2):188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Smart KM, Blake C, Staines A, Doody C. Self-reported pain severity, quality of life, disability, anxiety and depression in patients classified with ‘nociceptive’, ‘peripheral neuropathic’ and ‘central sensitisation’ pain. The discriminant validity of mechanisms-based classifications of low back (±leg) pain. Man Ther 2012;17(2):119–125. [DOI] [PubMed] [Google Scholar]
- [71].Smilios I, Pilianidis T, Karamouzis M, Tokmakidis SP. Hormonal responses after various resistance exercise protocols. Med Sci Sports Exerc 2003;35(4):644–654. [DOI] [PubMed] [Google Scholar]
- [72].Spurway NC. Aerobic exercise, anaerobic exercise and the lactate threshold. Br Med Bull 1992;48(3):569–591. [DOI] [PubMed] [Google Scholar]
- [73].Thompson NJ, Sengelaub DR, English AW. Enhancement of peripheral nerve regeneration due to treadmill training and electrical stimulation is dependent on androgen receptor signaling. Developmental Neurobiology 2014;74(5):531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Vincent K, Warnaby C, Stagg CJ, Moore J, Kennedy S, Tracey I. Brain imaging reveals that engagement of descending inhibitory pain pathways in healthy women in a low endogenous estradiol state varies with testosterone. Pain 2013;154(4):515–524. [DOI] [PubMed] [Google Scholar]
- [75].Vingren JL, Kraemer WJ, Hatfield DL, Volek JS, Ratamess NA, Anderson JM, Hakkinen K, Ahtiainen J, Fragala MS, Thomas GA, Ho JY, Maresh CM. Effect of resistance exercise on muscle steroid receptor protein content in strength-trained men and women. Steroids 2009;74(13–14):1033–1039. [DOI] [PubMed] [Google Scholar]
- [76].Vingren JL, Kraemer WJ, Ratamess NA, Anderson JM, Volek JS, Maresh CM. Testosterone physiology in resistance exercise and training: the up-stream regulatory elements. Sports medicine (Auckland, NZ) 2010;40(12):1037–1053. [DOI] [PubMed] [Google Scholar]
- [77].White HD, Brown LA, Gyurik RJ, Manganiello PD, Robinson TD, Hallock LS, Lewis LD, Yeo KT. Treatment of pain in fibromyalgia patients with testosterone gel: Pharmacokinetics and clinical response. Int Immunopharmacol 2015;27(2):249–256. [DOI] [PubMed] [Google Scholar]
- [78].Zhang X, Zhang Y, Asgar J, Niu KY, Lee J, Lee KS, Schneider M, Ro JY. Sex differences in mu-opioid receptor expression in trigeminal ganglia under a myositis condition in rats. Eur J Pain 2014;18(2):151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 Group differences for muscle fatigue produced by electrical stimulation. Muscle fatigue was measured by percent decline of electrically stimulated maximum force contractions before and after six minutes of fatiguing contractions. Animals who participated in eight weeks of the resistance training (RT) saw similar levels of muscle fatigue when compared with sedentary (Sed) animals. P>0.05, t-test; Data are mean±SEM.
Supplemental Figure 2 Line graphs show group differences in muscle withdrawal threshold (MWT). Male mice who underwent eight weeks of resistance training without weight (RT w/o Weight) had no significant difference in MWT values when compared with sedentary (Sed) animals on the ipsilateral (A) and contralateral (B) side. Female mice who underwent eight weeks of RT without weight had no significant difference in MWT values when compared with sedentary animals on the ipsilateral (C) and contralateral (D) side. Repeated measures ANOVA using time points 24 hours through 4 weeks with baseline as a covariate; B=baseline; Data are mean±SEM.
Supplemental Figure 3 Effect of androgen receptor antagonist flutamide on resistance training induced protection against muscle hyperalgesia. Line graphs show group differences for muscle withdrawal threshold (MWT) after acute administration of flutamide. Flutamide (1, 3, 10 mg/kg, or vehicle, i.p.) was administered 24-hour after induction of the activity-induced pain model (PUT an arrow on the graph after the 24h time period or a bar underneath the 30min and 120min with words drug or vehicle under it). MWT were reassessed 30 and 120 minutes after flutamide injection. No significant differences in MWT data were observed on either the ipsilateral or contralateral side in male and female mice after flutamide when compared to vehicle. (A-D).