Abstract
Toxoplasma gondii is an obligate intracellular parasite and the causative agent of Toxoplasmosis. A key to understanding and treating the disease lies with determining how the parasite can survive and replicate within cells of its host. Proteins released from specialized secretory vesicles, named the dense granules (DGs), have diverse functions that are critical for adapting the intracellular environment, and are thus key to survival and pathogenicity. In this review, we describe the current understanding and outstanding questions regarding dense granule biogenesis, trafficking, and regulation of secretion. In addition, we provide an overview of dense granule protein (“GRA”) function upon secretion, with a focus on proteins that have recently been identified.
Keywords: Toxoplasma gondii, dense granule, host-pathogen interactions, vesicle secretion
THE Apicomplexan phylum consists of a diverse collection of obligate intracellular parasites of significant medical and veterinary importance. Apicomplexa include Plasmodium spp., the causative agent of malaria that is responsible for approximately half a million deaths per year (WHO Malaria report 2020), Cryptosporidium which can cause life-threatening diarrheal disease (Khalil et al. 2018), and Toxoplasma gondii, the focus of this review and the causative agent of toxoplasmosis (Weiss and Dubey 2009). Parasites in this phylum have diverse and complex lifecycles but the intracellular portion of their lifecycle usually involves replication within a specialized vacuole called the parasitophorous vacuole (PV) (Aly et al. 2009; Blader et al. 2015; Francia and Striepen 2014).
In T. gondii, three types of secretory vesicles play vital roles in parasite invasion into the host cell, replication within the PV, and parasite egress. Micronemes are small, elongated vesicles located predominately at the parasite’s apical end. They are secreted in a calcium dependent manner and are vital for motility of extracellular parasites, host cell invasion and egress (Bisio and Soldati-Favre 2019; Lourido et al. 2012; Lourido et al. 2010; Paredes-Santos et al. 2012). Rhoptries are club-shaped organelles containing proteins necessary for invasion and modification of host cells, including a number of important virulence factors (Bradley and Sibley 2007). Secretion of rhoptry proteins is tightly controlled and only triggered after strong attachment to the host cell (Alexander et al. 2006; Sparvoli and Lebrun 2021). Dense granules (DG) were first described in Toxoplasma in 1968 (Sheffield and Melton 1968). These 200nm membrane enclosed vesicles have an electron dense crystalline protein core and are vital for intracellular survival.
During invasion, the PV is formed from host plasma membrane (PM). Host proteins are removed from the newly formed PV membrane (PVM) which presents a physical boundary between the parasite and host cell cytosol (Mordue et al. 1999; Schwab et al. 1994). Proteins released from the dense granules immediately after invasion are vital for modifying the PV and intracellular environment of the host. Understanding the function of dense granule proteins (collectively termed “GRA” proteins) and the mechanisms by which dense granules are synthesized, transported, and triggered for secretion is fundamental for understanding infection.
Dense Granule biogenesis, transport, and secretion
GRA proteins are synthesized in the ER and proteolytically processed in the Golgi
The majority of GRA proteins, like the proteins destined for the micronemes and rhoptries, contain an N-terminal ER-targeting signal peptide, and thus enter the secretory pathway via synthesis and translocation at the rough endoplasmic reticulum (Table S1). As in other eukaryotes, the signal peptide is thought to be removed co-translationally by a signal peptidase, however the function of a putative signal peptidase in T. gondii (TgME49_280740) has not been verified experimentally.
Many dense granule proteins contain a single transmembrane (TM) domain (Table S1). In other eukaryotes, proteins containing both a signal peptide and a TM domain are inserted into the ER membrane during synthesis and subsequently trafficked to either the plasma membrane or the membrane of another compartment within the endomembrane system. Expression of the TM-domain containing protein GRA5 in mammalian cells resulted in the protein trafficking to the plasma membrane (Gendrin et al. 2008). In parasites, GRA5 and other TM domain-containing GRA proteins are translocated across the ER membrane into the lumen and trafficked through the endomembrane system, which involves export from the ER, trafficking through the Golgi to dense granules, and finally secreted from the parasite (Fig. 1) (Braun et al. 2008; Gendrin et al. 2008; Lecordier et al. 1999; Mercier et al. 2005). Thus, there must be parasite specific mechanisms to facilitate GRA protein trafficking to the ER lumen and ensure GRA proteins are not co-translationally inserted into the ER membrane. GRA proteins form higher ordered aggregates that may mask the TM domains preventing premature membrane insertion (Braun et al. 2008) and a recently identified chaperone (GRA45) also appears to play a role.
Figure 1.
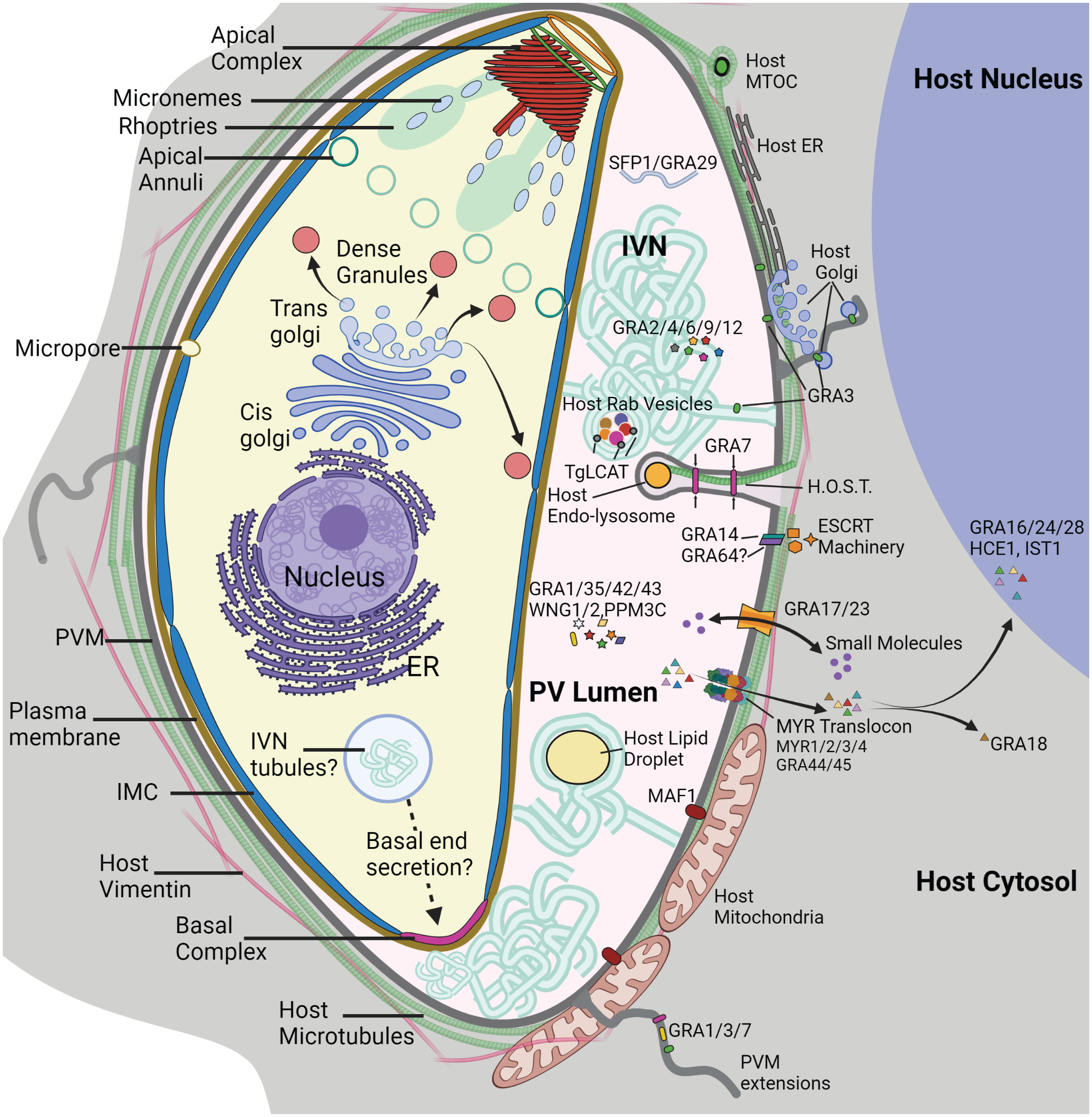
Illustration of parasite (tachyzoite stage) and parasitophorous vacuole morphology
GRA45 has structural similarity to the alpha crystalline domain (ACD) small heat shock proteins and may aid the trafficking of other GRA proteins through the secretory pathway (Wang et al. 2020). When key residues in the ACD domain of GRA45 were mutated, trafficking of GRA7 through T. gondii’s endomembrane system was retarded and aberrant GRA protein aggregates were formed. In addition, in GRA45 knockout parasites, a number of GRA proteins that normally associate with PVM were retained in the PV lumen which would indicate misfolding or aberrant protein-protein interactions and perturbed membrane insertion (Wang et al. 2020). Thus, it is likely a combination of aggregation and chaperone binding that masks the TM domains and prevents their insertion into membranes within the secretory pathway (Braun et al. 2008; Gendrin et al. 2008; Wang et al. 2020).
Once synthesis and translocation into the ER lumen is complete, proteins are trafficked between organelles in the endomembrane pathway via transport vesicles. Conserved, membrane-associated protein complexes drive vesicle budding from the donor compartment, vesicle transport and then tethering, docking and membrane fusion with the target membrane. SNARE complexes control vesicle docking and membrane fusion at the target membrane, and are composed of four coiled-coil domain containing proteins: Three Q SNAREs on the target membrane, designated Qa, Qb and Qc according to their position in the complex and one vesicle associated R-SNARE (Fasshauer et al. 1998; Sauvola and Littleton 2021). In T. gondii, a SNARE complex at the cis-Golgi composed of syntaxin 5 (TgStx5: Qa), TgGS27 (Qb), TgBet1 (Qc), and TgSec22b (R) control ER to Golgi anterograde trafficking (Cao et al. 2021) (Fig. 2). TgGS27 knockdown parasites exhibited impaired formation of the micronemes and rhoptries, and although the trafficking of proteins to the dense granules was not addressed directly in this study, it is probable that DG trafficking would also be impacted by the loss of this SNARE complex (Cao et al. 2021).
Figure 2.
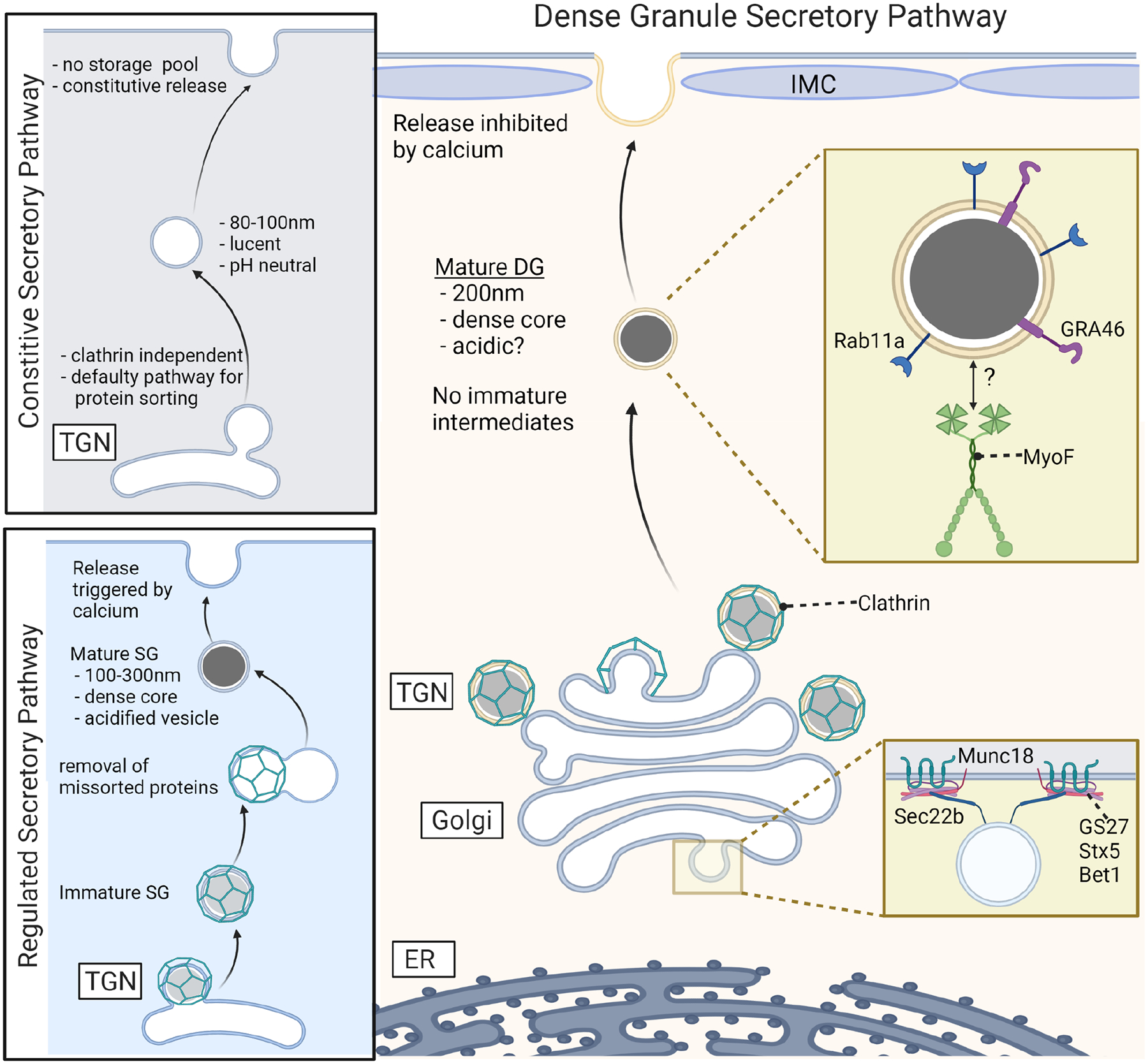
Model of dense granule biogenesis and trafficking compared to constitutive and regulated secretory pathways in higher eukaryotes.
SNARE complex assembly and function is regulated by the Sec1/Munc18 family of proteins (Wang et al. 2017). Depletion of the Golgi localized Sec1/Munc18 family protein, SLY1, impairs the trafficking of proteins destined for all three secretory vesicles, most likely by regulating ER to Golgi trafficking. Although a direct interaction between SLY1 and the TgGS27 SNARE complex has not been demonstrated (Bisio et al. 2020; Cao et al. 2021; Demircioglu et al. 2014; Ossig et al. 1991) (Fig. 2).
DG formation at the TGN
Upon arrival in the Golgi, GRA proteins containing a TEXEL motif (Toxoplasma export motif) with the amino acids RRLxxx are further proteolytically processed by the aspartyl protease, ASP5 (Table S1) (Coffey et al. 2015; Coffey et al. 2016; Coffey et al. 2018; Curt-Varesano et al. 2016; Hammoudi et al. 2015). In ASP5 knockout parasites, a number GRA proteins, the functions of which will be discussed in depth below, were mislocalized upon release from the parasite. Specifically, GRA16 failed to be exported from the PV (Coffey et al. 2015; Curt-Varesano et al. 2016) and GRA44, MYR1 and MAF1 failed to localize to the PVM (Coffey et al. 2015; Coffey et al. 2018) indicating that loss of ASP5 severely impacts the parasite’s ability to modulate the intracellular environment of the host cell and control host signaling pathways, resulting in a decreased parasite fitness (Coffey et al. 2015; Hammoudi et al. 2015). Surprisingly, mutation of TEXEL motifs in MYR1 and GRA44 did not affect protein insertion into the PV or protein function (Blakely et al. 2020; Cygan et al. 2021). One interpretation of this conflicting data is that proteolysis is critical for the function of some but not all GRA’s cleaved by ASP5. For example, if the function of a molecular chaperone such as GRA45 is perturbed by loss of ASP5, then this could lead to the secretion of mis-folded or aggregated GRA proteins whose activity is GRA45 dependent. In further support of this idea, translocation of GRA24 across the PV membrane was dependent on ASP5 even though it does not contain a TEXEL motif and is not cleaved by the protease (Coffey et al. 2015; Curt-Varesano et al. 2016; Hammoudi et al. 2015).
Mature dense granules bud directly from the trans-Golgi network (TGN) and are morphologically similar to dense core vesicles in neuroendocrine cells that are part of the regulated secretory pathway in mammalian cells. Despite these similarities, dense granule formation and trafficking has aspects of both the constitutive and regulated secretory pathways (CSP and RSP, respectively) (Fig. 2) (reviewed by Gondré-Lewis et al. 2012). Briefly, vesicles in the CSP in mammalian cells are formed at the medial and trans-Golgi in a clathrin independent manner, are electron lucent and ~100nm in size (Orci et al. 1987; Walworth and Novick 1987). There is no specific sorting signal, and these vesicles serve as the “default” pathway for secretion. Constitutive secretory vesicles (CSVs) have no storage pool and are continually secreted; thus, the rate of secretion is dependent on the rate of synthesis. It is estimated that the total time from formation to secretion is approximately 30 minutes (Gondré-Lewis et al. 2012). In contrast, vesicles in the RSP in mammalian cells form initially as immature vesicles that bud from the TGN in a clathrin dependent manner. Removal of mistargeted proteins, and vesicle maturation involving acidification of the vesicle and crystallization of protein contents, occurs before secretion. Pools of mature secretory vesicles are retained in the cell cortex until a secretagogue (usually increased intracellular calcium) triggers vesicle fusion with the plasma membrane (Gondré-Lewis et al. 2012; Park and Loh 2008; Verhage and Sørensen 2008; Wang and Hsu 2006).
Similar to RSP’s, DG formation at the TGN is clathrin-dependent (Pieperhoff et al. 2013). T. gondii has a single clathrin heavy chain (CHC) that is localized predominately at the TGN. Expression of a dominant negative CHC fragment leads to accumulation of a DG marker in the ER and Golgi, as well as disrupting trafficking to the rhoptries and micronemes indicating that CHC is required for vesicle formation at the TGN. However, deletion of the clathrin adaptor AP1 that links the vesicle membrane to the clathrin cage, resulted in mis-sorting of microneme and rhoptry proteins but was not required for formation of the dense granules (Ngô et al. 2003; Venugopal et al. 2017). Thus, the adaptors targeting CHC to dense granules budding from the TGN have not yet been identified. The direct budding of DGs from the TGN is distinct from the more complex trafficking pathway of proteins destined for the micronemes and rhoptries, which traffic through one or more post-Golgi compartments before delivery to mature rhoptry and microneme compartments (reviewed by Venugopal and Marion 2018).
Like vesicles in the CSP, numerous lines of evidence indicate that dense granules appear to be the default pathway for secretory protein trafficking. Removal of the GPI anchor signal sequence from the surface protein SAG1 results in mis-targeting to the dense granules (Heaslip et al. 2016; Striepen et al. 1998). While expression of E. coli alkaline phosphatase and β-lactamase with an N-terminal signal peptide results in trafficking to the dense granules and secretion into the PV lumen (Karsten et al. 1998). Similarly, when the first 68 amino acids of the microneme protein MIC3 were tagged with GFP, MIC3 was directed to the dense granules (Striepen et al. 2001). Thus, unlike rhoptry proteins which must contain a dileucine (LL) motif or a tyrosine-based sorting signal (Yxxɸ motif), there appears to be no requisite sequence for trafficking to the dense granules (Hoppe et al. 2000; Ngô et al. 2003)
Proteins in the RSP are sorted at the TGN by aggregation that is induced by low pH, calcium, or zinc as demonstrated by electron microscopy and pulse chase experiments. After sorting, the vesicles are acidified further by proton pumps on the vesicle membrane (Chanat and Huttner 1991; Germanos et al. 2021; Gondré-Lewis et al. 2012). These protein aggregates are responsible for the electron dense appearance of granules in electron microscopy images, for which this class of vesicles is named (i.e., dense granules or large dense core vesicles (DG or LDCV)). GRA proteins do form electron dense aggregates in T. gondii (Labruyere et al. 1999; Wang et al. 2020) but it is not understood when along the secretory pathway this occurs, or what environmental conditions within the granule lumen facilitate aggregate formation. The pH of the dense granule lumen has not been investigated thoroughly. DAMP (3-(2,4-dinitroanilino)-3’amino-N-methyldipropylamine), which accumulates in acidic cellular compartments, only labeled the rhoptries, suggesting that the dense granules were not acidic (Shaw et al. 1998), however, this question warrants further investigation. Outstanding questions include: What is the pH of the DG lumen and what is the concentration of calcium and zinc? If the DG are acidic, what proton pumps are responsible for acidification? Does acidification or calcium and zinc ions play a role in protein aggregation?
Mechanisms of dense granule transport
In many cell types, secretory vesicles are trafficked from the Golgi to the plasma membrane in an active, motor-driven manner. In mammalian cells, kinesin motion on microtubule tracks controls anterograde vesicle motion (Hirokawa et al. 2009), while in budding and fission yeast, vesicle transport is driven by myosin V movement on actin tracks (Hammer and Sellers 2011). Live cell imaging of GFP labeled dense granules demonstrated that DG movements are highly dynamic and DGs exhibit long directed, motor driven movements predominately along the parasite periphery. Depolymerization of F-actin with cytochalasin D or conditional knockdown of an unconventional myosin motor, MyoF, significantly reduced directed vesicle movement. Depolymerization of parasite microtubules had no effect on directed movement indicating dense granule motion is an acto-myosin dependent process (Heaslip et al. 2016). Interestingly, dense granule secretion is not affected by the loss of MyoF, suggesting that this protein is exclusively involved in vesicle transport and is not required for the secretion event itself. One surprising finding that emerged from the analysis of dense granule movements was that granules do not appear to be tethered at or near plasma membrane release sites, on the contrary vesicle movement along the periphery appeared almost random with movements being bidirectionally towards both the apical and basal ends of the parasite. Frequent direction changes were observed (Heaslip et al. 2016), suggesting that the purpose of vesicle movement was to increase the chances of a granule encountering a granule release site.
In order to control the motion of membrane bound cargo, transport motors typically bind to adaptor proteins on the surface of a vesicle/organelle with their C-terminal cargo binding domains. Diffraction limited images of fluorescently labeled MyoF and dense granules indicate that MyoF is not enriched on the granule surface (Heaslip et al. 2016). Moreover, no MyoF tail binding proteins have been identified to date, and so it is not clear if MyoF mediates dense granule transport through a direct interaction with the granule surface. Elucidating MyoFs mechanism of action is complicated by fact that MyoF is also required for apicoplast (a non-photosynthetic plasmid organelle) inheritance, transport of Rab6 vesicles, movement of ER tubules and positioning of the post-Golgi compartments (Carmeille et al. 2021). Future studies focused on identifying MyoF interacting proteins are crucial for a complete understanding of the mechanisms of dense granule movement.
Dense Granule protein secretion
There are many outstanding questions in regards to the mechanism, regulation and location of GRA protein secretion.
Is GRA protein secretion regulated or constitutive?
As discussed in detail above, DG biogenesis has components of both the CSP and RSP, and whether DG secretion is regulated or constitutive has been the subject of debate in the literature (Chaturvedi et al. 1999; Coppens et al. 1999). Numerous lines of evidence support the idea that DG secretion is regulated. First, secretion from DGs was not affected by treatment with brefeldin A, which inhibits trafficking between the ER and Golgi and results in decreased secretion from CSVs in mammalian cells (Coppens et al. 1999). DG release was originally thought to be independent of calcium. However, it has recently been shown that the dense granule secretion is negatively regulated by calcium (Katris et al. 2019). Secretion is also triggered by incubation in bovine serum albumin (BSA) or fetal calf serum (FCS) so it is possible there is a yet unidentified, secretagogue which induces secretion from DGs (Coppens et al. 1999).
GRA proteins accumulate in the PV within minutes of invasion and it has been proposed that this rapid accumulation represents an upregulation or burst of secretion immediately after invasion (Carruthers and Sibley 1997; Dubremetz et al. 1993; Sibley et al. 1995). However, GRAs are also secreted from extracellular parasites, and a direct comparison of the DG secretion rates from extracellular, newly invaded, and replicating parasites has not yet been achieved. Changes in the rate of secretion in different cellular environments would provide evidence for regulated secretion.
How do DG traverse the IMC to reach the plasma membrane?
Despite extensive imaging of intracellular T. gondii by electron microscopy, there are only a few images which appear to depict dense granule secretion events, probably due to the quick and transient nature of this cellular process (Dubremetz et al. 1993; Paredes-Santos et al. 2013). These images show dense granule secretion occurring via direct fusion with the parasite plasma membrane through gaps in the inner membrane complex (IMC). Intriguingly, where the “gaps” in the IMC are located is not understood. Large gaps in the IMC at the apical and basal ends are plugged by the apical and basal complexes respectively (Dos Santos Pacheco et al. 2020; Morano and Dvorin 2021) (Fig. 1), and are not thought to be involved in DG secretion. In addition, the two other characterized gaps in the IMC are the apical annuli and the micropore. The parasite contains a single micropore, an invagination in the PM thought to be the site of endocytosis rather than secretion (Nichols et al. 1994). Each parasite also contains 5–6 apical annuli, ring shaped structures with a diameter between 200–400nm and located ~1.5μm from the apical complex on the lateral sides of the parasite (Engelberg et al. 2020; Hu et al. 2006) (Fig. 1). The imaging that exists indicate that dense granule secretion may occur from the apical lateral sides of the parasite at a location consistent with positioning of the apical annuli (Dubremetz et al. 1993; Paredes-Santos et al. 2013; Sibley et al. 1995). However, there has been no direct evidence that secretion of the dense granules occurs at this position.
Few proteins have been identified that are essential for dense granule secretion. The small GTPase Rab11a is found on a subset of DGs and is required for both dense granule transport and secretion (Venugopal et al. 2020), although the mechanism by which Rab11a controls secretion has not been elucidated. Only one other protein is known to localize in the DG membrane, GRA46, and the function of this protein has not been defined (Coffey et al. 2018). Thus, additional studies are needed to generate a catalog of DG membrane-associated proteins whose functions in transport and secretion could then be investigated.
Live cell imaging is one possible methodology that could be utilized to address many of the outstanding questions in regard to dense granule secretion. Individual DG secretion events at the parasite PM have never been visualized. One major limitation to imaging dense granule secretion events is the accumulation of secreted fluorophore in the PV upon secretion making imaging of any subsequent secretion events challenging. Overcoming this technical limitation will enable the identification of the DG secretion site, quantification of GRA secretion rates, and characterization of proteins important for this cellular process.
Functions of Dense Granule (GRA) Proteins
GRA proteins play pivotal roles in mediating host-parasite interactions. Upon secretion, GRA proteins will either remain in the PV lumen, interact with the intravacuolar network (IVN, described below), be inserted into the PVM, or secreted out of the PVM into the host cell. The PV is only permeable to molecules less than ~1kDa (Schwab et al. 1994), therefore the ionic composition and pH of the cytosol and PV space are likely equivalent but the parasite is not in direct contact with the vast majority of cytosolic proteins or organelles. Thus, the PV is the interface for many host-parasite interactions. Over 60 GRA proteins have been identified to date, first through the generation of antibodies against the parasite lysates or secreted antigens (Carey et al. 2000; Cesbron-delauw et al. 1989; Leriche and Dubremetz 1991), and more recently from BioID proteomics and CRISPR-Cas9 functional characterization studies (Table S1) (Bai et al. 2018; Cygan et al. 2021; Nadipuram et al. 2016). Only a fraction of these proteins have a well characterized role in parasite infection. In this section we will focus on discussing new insights into GRA protein function and describe recently identified GRA proteins. A comprehensive description of all GRA proteins is not possible due to space constrains but we refer the reader to a number of other excellent reviews (Clough and Frickel 2017; Panas and Boothroyd 2021;Saeij and Frickel 2017;Mercier and Cesbron-Delauw 2015) .
Nutrient acquisition
Morphology and Biogenesis of the IVN:
The PVM is a physical barrier between the host cell and parasite that must be overcome for the parasite to scavenge nutrients and lipids from the host. GRA proteins induce a number of dramatic alterations to the PVM that are vital for nutrient acquisition. The intravacuolar network (IVN) is an extensive membranous tubular network that at times is continuous with the PVM (Coppens et al. 2000;Sibley et al. 1995) (Fig. 1). Newly invaded parasites contain an ~400nm intracellular compartment filled with membranous tubules that could be a source of lipids for initial IVN formation (Romano et al. 2017). This compartment was only recently seen in electron microscope images and no biomarkers for this compartment have been identified leaving many outstanding questions regarding the biogenesis, regulation, and mechanisms of IVN tubule secretion. The IVN forms within minutes of invasion with tubular membranes emanating from the parasites’ basal end (Sibley et al. 1995). Shortly thereafter, dense granule proteins including GRA2, GRA4, GRA6, GRA9, and GRA12 associate with the IVN (Cesbron-Delauw et al. 2008; Mercier et al. 2002; Rommereim et al. 2016; Travier et al. 2008). Deletion of IVN associated GRA4, GRA9 and GRA12 did not lead to any apparent alterations in the IVN structure, while GRA2 and GRA6 knockout parasites exhibit defects in IVN morphology (Cesbron-Delauw et al. 2008; Mercier et al. 2002; Travier et al. 2008).The IVN expands as parasites replicate, and while the parasite may contribute some lipids for initial formation of the IVN, the host is the major source of lipids for IVN expansion (Caffaro and Boothroyd 2011). PtdSer decarboxylase (TgPSD1) is a protein that is secreted by dense granules and localizes in the PV (Gupta et al. 2012). TgPSD1 decarboxylates phosphatidylserine (PtdSer) to create phosphatidylethanolamine (PtdEtn), an important building block for membrane biogenesis. While it has not been directly observed to aid in expansion of the parasite plasma membrane or the PV membrane, it does have the capacity for carboxylase activity on membranes (Gupta et al. 2012).
Lipid acquisition:
T. gondii scavenges a wide variety of lipids from the host cell. Sphingolipids are acquired by engulfing a variety of host-Golgi derived Rab-associated vesicles (Romano et al. 2013; Romano et al. 2017). Intact vesicles are concentrated within the IVN and vesicle uptake is reduced in GRA2 and GRA6 double knockout parasites. Until recently, it was unclear how vesicles traversed the PVM. A recent study using both light and electron microscopy revealed double membraned vesicular structures in the PV lumen, indicating that the host derived vesicles become surrounded with PVM before internal budding into the PVM lumen (Romano et al. 2017). The association with yet another IVN associated dense granule protein, TgLCAT (a lecithin-cholesterol acyltransferase), with internalized vesicles suggests this protein plays a role in destabilization of the PV derived membrane surrounding the Rab vesicles before uptake into the parasite (Pszenny et al. 2016; Romano et al. 2017).
T. gondii is an auxotroph for cholesterol (Blader and Koshy 2014; Coppens et al. 2000). This vital lipid is acquired from host endosomal and lysosomal vesicles using PVM tubules distinct from the IVN (Fig. 1) (Coppens et al. 2000; Coppens et al. 2006). These tubule invaginations, termed “Host Organelle-Sequestering Tubulo-structures” (HOST) are ~100nm in diameter. The lumen is continuous with the host cytosol and contains host microtubules that may act as a track for vesicle transport to the HOST tip. The HOSTs contain transverse striations in TEM images indicating proteinaceous collars with potential roles in tubule formation, stability, or constriction (Coppens et al. 2006). Lysosomal vesicles are also found in the PV, surrounded by PV-derived membrane, so the proposed mechanism of uptake is similar to what is described above whereby vesicles undergo internal budding into the PV lumen (Coppens et al. 2006).
It has been proposed that GRA7 is a component of the proteinaceous collars associated with the HOSTs. Recombinant GRA7 can tubulate liposomes in vitro and GRA7 knockouts exhibit decreased growth rates in nutrient depleted media. However, it was not directly determined if loss of GRA7 affected HOST formation (Coppens et al. 2006). GRA7 along with GRA3 and GRA14 are found in filamentous extensions (termed PVM projections, PVMPs) which extend into the host cytosol (Dubremetz et al. 1993; Dunn et al. 2020). PVMPs may also play a role in vesicle or organelle association with the PVM although their function is poorly defined.
Another major source of lipids for the parasite comes from host neutral lipid droplets (LD) (Hu et al. 2017; Nolan et al. 2017). Infected cells have more lipid droplets compared to uninfected cells (Hu et al. 2017; Nolan et al. 2017). This increase in lipid droplet production is dependent on an unidentified parasite factor secreted into the host (Hu et al. 2017). Host LDs are found both surrounding the PV and within the PV lumen, and neutral lipids from the host are incorporated into organellar membranes of the parasite. As was the case with other vesicle types described above loss of GRA2, GRA6 and LCAT resulted in decreased LD uptake (Nolan et al. 2017).
Acquisition of soluble proteins from the host cytosol:
In 2014 Dou and colleagues (Dou et al. 2014) demonstrated that parasites can acquire soluble proteins from the host, as evidenced by the uptake in fluorescent proteins from the host cytosol into the parasite. Reductions in host protein uptake were seen in GRA2 and GRA6 knockout parasites, again implicating the IVN in this process (Dou et al. 2014; Rommereim et al. 2016). Expression of a dominant negative variant of VASP4A, a component of the host ESCRT (Endosomal Sorting Complex Required for Transport) decreased protein uptake to the parasite. GRA14 and GRA64, two single TM domain containing dense granule proteins, bind ESCRT components, and while loss of GRA14 resulted in reduced host protein uptake, loss of GRA64 had no effect (Mayoral., unpubl. data BioRxiv: 2021.11.02.467042; Rivera-Cuevas et al. 2021). The connection, if any, between ESCRT mediated uptake of host material and other uptake mechanisms at the PVM/IVN, should be explored to understand this process in more depth..
Diffusion between PV lumen and cytosol:
The PV is only permeable to molecules less than ~1kDa (Schwab et al. 1994). Two proteins, GRA17 and GRA23, form pores within the PVM which allow for the diffusion of small molecules between the host cytosol and PV lumen (Schwab et al. 1994; Gold et al. 2015). Loss of GRA17 led to swelling of PV, possibly due to osmolarity differences between the PV lumen and cytosol. In addition, GRA17 knockout parasites exhibited growth defects that could be attributed either to nutrient deprivation or inability of the parasite to remove toxic byproducts from the PV (Gold et al. 2015).
Host organelles and cytoskeleton associate with the PV
Host Mitochondria association (HMA):
Host mitochondria association (HMA) has been extensively studied (Blank et al. 2021; Crawford et al. 2006; Jones et al. 1972; Li et al. 2022; Pernas et al. 2014) Mitochondria that associate with the PV have an approximately 3-fold increase in surface area compared to cytosolic mitochondria from infected cells due to enhanced mitochondrial fusion mediated by the outer mitochondrial membrane (OMM) proteins mitofusin 1 (Mfn1) and mitofusin 2 (Mfn2) (Pernas et al. 2014; Pernas et al. 2018). Mitochondria play a role in host defenses against infection by increasing the uptake of fatty acids (FA) and thereby reducing the pool of FA available to the parasite (Pernas et al. 2018), a process that is also dependent on Mfn1 and Mfn2 (Li et al. 2022; Pernas et al. 2018).
Not all strains of T. gondii display HMA; type I and III strains exhibit HMA while type II stains do not (Pernas et al. 2014). By analyzing the differential gene expression between the three strains, Pernas and colleagues identified the dense granule protein Mitochondria Association Factor 1 (MAF1) as necessary and sufficient for HMA (Blank et al. 2021; Pernas et al. 2014). MAF1 contains a transmembrane domain and is inserted into the PVM. The C-terminus exposed to the host cytosol binds (either directly or indirectly) the OMM protein TOM70 (Blank et al. 2021; Li et al. 2022). HMA induces shedding of the OMM and decreases expression of Mfn1 and Mfn2 thereby counteracting the host defenses designed to limit parasite uptake of FA (Blank et al. 2021; Li et al. 2022). In addition, infection with HMA+ strains have altered cytokine production compared to HMA- strains. Surprisingly, expression of MAF1 in the MAF1-negative type II strain did not alter parasite growth in culture or parasite burden in mice (Pernas et al. 2014).
Host Golgi and ER association with the PV:
In addition to mitochondria, host ER and fragmented Golgi stacks associate with PV periphery (Coppens et al. 2000; Romano et al. 2013; Sinai et al. 1997). GRA3 associates with Golgi purified from CHO cells, and also binds phosphatidylinositol lipids via its C-terminus (Deffieu et al. 2019). GRA3 knockouts display reduced entry of host Golgi vesicles into the PV supporting a potential role in lipid scavenging from the host (Deffieu et al. 2019), whereas recruitment of Golgi stacks to the PV was not perturbed. GRA3 knockout parasites exhibited no growth defects in cell culture but exhibited attenuated virulence in vivo (Craver and Knoll 2007).
Host cytoskeleton recruitment:
The PV is frequently located adjacent to the host nucleus and centrosome and becomes encased in host microtubules and vimentin intermediate filaments (Halonen and Weidner 1994; Walker et al. 2008). Vimentin may play a beneficial role in retaining the PV in a host perinuclear region (Halonen and Weidner 1994), and by extension in closer proximity to host perinuclear organelles (ER, MTOC, Golgi). Bundling of host microtubules around the PV may provide a highway for organelle trafficking to the PV by microtubule associated motor protein. The mechanism by which microtubules and vimentin associate with the PV is not known. However, given the role of GRA proteins in organelle recruitment, it is possible that as yet unidentified GRA proteins also mediate the recruitment of cytoskeletal elements to the PV. Further work will be required to determine the mechanism and function of the PV-host cytoskeletal association.
Mechanism of GRA protein export from the PV
A number of dense granule proteins are exported out of the PV into the host cell cytosol and nucleus, including GRA16 (Bougdour et al. 2013), GRA24 (Braun et al. 2013), IST1 (Gay et al. 2016), GRA18 (He et al. 2018), and TEEGR/HCE1 (Braun et al. 2019; Panas et al. 2019). As described in more detail below, these proteins play a variety of roles in regulating host cell gene expression and immune response pathways. MYR1, MYR2 and MYR3 (short for c-Myc regulation), were identified in a mutagenesis screen designed to identify the effector responsible for inducing upregulation of host c-myc (Franco et al. 2016; Marino et al. 2018). As it turned out, these proteins were not the direct effectors of c-myc induction but components of the translocon that controls export of the c-myc effector and other GRA proteins to the host cytosol. All three proteins contain TM domains and are localized to the PVM. MYR1 is cleaved by the protease ASP5 (described in more detail above in the section entitled “DG formation at the TGN) before secretion and both fragments interact directly with MYR3 (Marino et al. 2018). Four additional components of the translocon, GRA44, GRA45, PPM3C, ROP17 and MYR4, were subsequently identified by immunoprecipitation (Blakely et al. 2020; Cygan et al. 2020; Mayoral et al. 2020; Panas et al. 2019; Wang et al. 2020). Knockout of all MYR components resulted in decreased parasite replication and blocked effector export into the host cytosol. GRA45, the putative chaperone describe above, is needed for membrane association of MYR components (Wang et al. 2020). The other components, GRA44, PPM3C, and a rhoptry protein ROP17 could play a role in regulating translocation or effector activity through phosphorylation. ROP17, a serine/threonine protein kinase residing on the host cytosol side of the PVM, is speculated to phosphorylate one or multiple components of the translocon as ROP17 kinase activity is needed for its role in translocation (Panas et al. 2019). GRA44 contains a putative phosphatase domain that interacts directly with GRA45 (Coffey et al. 2018), however the targets of GRA44 phosphatase activity have not been identified (Blakely et al. 2020). PPM3C is also a phosphatase and in its absence, PPM3C target proteins GRA16 and GRA28 exhibited changes in phosphorylation, and were not exported into the host (Mayoral et al. 2020). While phosphorylation of MYR1 was also altered in the PPM3C knockout, a global defect in effector export was not observed as GRA24 and IST1 export was unaffected. PPM3C knockout parasites exhibited growth defects in vitro and reduced virulence in mice. It has yet to be determined which components of the MYR translocon are structural and which are regulatory. Cryo-EM studies, like those performed with the Plasmodium translocon (see below), would be extremely informative.
In Plasmodium falciparum the machinery for GRA protein export into the PV has been extensively investigated and there are a number of significant differences between Toxoplasma and Plasmodium translocons that are worth highlighting. The majority of Plasmodium proteins transported past the PVM have both a signal sequence and a N-terminal Plasmodium export element (PEXEL) found about 15–30 residues downstream of the signal sequence (PEXEL: RxLxE/Q/D or relaxed PEXEL: RxLxEE) (Boddey et al. 2016; Boddey and Cowman 2013; Boddey et al. 2010; Russo et al. 2010; Sleebs et al. 2014). Only a very small number of exported proteins in P. falciparum parasites do not have a PEXEL motif. Most of these PEXEL-negative export proteins (PNEPs) also do not contain an ER-signal sequence but it has been shown that the first 20 amino acids at the N-terminus of PNEPs and one or more transmembrane domains are enough to promote export (Heiber et al. 2013; Boddey and Cowman 2013; Hiller et al. 2004). The aspartyl protease Plasmepsin V (PM5), which is a homolog of TgASP5 and localized in the ER, cleaves the PEXEL motif after the leucine residue, leaving the protein with an acetylated xE/Q/D N-terminal motif (Boddey and Cowman 2013; Boddey et al. 2010; Russo et al. 2010; Sleebs et al. 2014). PNEPs or proteins with newly exposed N-terminal PEXEL motif are targeted to Plasmodium translocon of exported proteins (PTEX) embedded in the PVM (Koning-Ward et al. 2009; Marti and Spielmann 2013). PTEX is composed of the 3 core proteins EXP2, PTEX150, HSP101, as well as 2 other proteins PTEX88 and TRX2 (Elsworth et al. 2014). EXP2 is a heptameric complex in which each monomer contains a single transmembrane domain (Ho et al. 2018). EXP2 and PTEX150 form an interdigitating protein conducting channel through which effector proteins are translocated through the PVM into the host (Ho et al. 2018). HSP101 is an AAA ATPase that binds and provides the energy needed for protein translocation and plays a role in unfolding proteins prior to translocation (Bullen et al. 2012; Chisholm et al. 2018; Elsworth et al. 2014; Ho et al. 2018; Sanders et al. 2019). Refolding of effector proteins happens in the host, although the mechanisms behind this process are not fully understood. Host proteins and exported parasite chaperones, such as HSP40 and HSP70, are thought to play a role (Hakamada et al. 2020; Külzer et al. 2012). GRA17 and GRA23 are related to Plasmodium EXP2. However, as described above these proteins do not play a role in protein translocation in Toxoplasma, but rather form a pore in the PVM to allow diffusion of small molecules (Gold et al. 2015). The function of two additional PTEX components, PTEX88 and TRX2, are less well defined. In P. berghei, PTEX88 and TRX2 are nonessential while in P. falciparum TRX2 knockouts exhibited a reduction in protein export (Chisholm et al. 2018; Elsworth et al. 2014; Matthews et al. 2013; Matz et al. 2013).
Phosphorylation of GRA Proteins in the PV
Proteomic analysis has demonstrated that a large number of GRA proteins are phosphorylated upon secretion, many of which are differentially phosphorylated in tachyzoites and bradyzoites (Treeck et al. 2011; Young et al. 2020). The effect of phosphorylation in regulating GRA function has only begun to be appreciated. A number of kinases and phosphatases are secreted from the dense granules, including GRA44 and PPM3C described above, and the newly characterized WNG1 and WNG2 kinases (Beraki et al. 2019). Originally thought to be rhoptry kinases targeted to the host cytosol (Peixoto et al. 2010), ROP34 and ROP35 have been reclassified as it has recently been demonstrated that they are secreted from the dense granules and localize to the PV lumen (Beraki et al. 2019; Coffey et al. 2018). Phosphoproteomic analysis identified a number of GRA proteins that exhibited differential phosphorylation patterns in WNG1 knockout parasites (previously ROP35) including GRA6. In the absence of WNG1, GRA6 was not inserted into the IVN and remained in the PV lumen which lead to defects in IVN development. WNG2 (previously ROP34) contributes to parasite virulence in vivo, however the targets of WNG2 have not been identified (Coffey et al. 2018).
Strand forming proteins within the PV have recently been described that are separate from the IVN. However the role it plays in the parasite life cycle is unclear (Young et al. 2020). These strands form through interactions between the C-termini of two dense granule proteins, strand-forming protein (SFP1), and GRA29 (Young et al. 2020). Phosphorylation negatively regulates the formation of the SFP1-GRA29 strands (Young et al. 2020). The enzymes responsible for this phosphorylation have not been identified.
Regulation of host cell cycle
T. gondii infection results in significant transcriptional changes in infected cells and dense granule proteins play pivotal roles in mediating this response.
Cell cycle control:
A number of GRA proteins have been characterized that alter the signaling pathways controlling the host cell cycle. T. gondii infection leads to host cell cycle arrest in G2 and inhibiting the G1/S transition led to decreased parasite proliferation (Brunet et al. 2008). The combined action of two dense granule effectors (HCE1/TEERG and GRA16) controls G2 arrest. Identified through bioinformatic approaches, GRA16, localizes to the host nucleus after translocation from the PV where it interacts with host proteins PP2A and HAUSP (Bougdour et al. 2013). HAUSP (herpesvirus-associated ubiquitin-specific protease) binds to the transcription factor and tumor suppressor p53 (Li et al. 2002). In healthy cells, p53 levels are low due to continuous degradation by the proteasome. Upon infection or DNA damage HAUSP deubiquitinates p53 leading to p53 stabilization, nuclear accumulation and transcription of p53 responsive genes. HAUSP-GRA16 interactions leads to p53 stabilization which leads to decreased levels of Cyclin B, a factor which controls the G2/M transition of the cell cycle. (Bougdour et al. 2013). TEEGR/HCE1 is also trafficked to the nucleus, where it binds to the E2F-DP1 transcription factor (Braun et al. 2019; Panas et al. 2019) leading to increased cyclin E expression, which drives infected cells through the G1/S cell cycle transition. It is not understood why arresting cells in G2 confers a proliferation advantage to parasites and warrants further investigation.
PP2A, the second host protein that binds to GRA16, is a component of the holoenzyme that controls phosphorylation and stability of c-myc, a vital transcription factor that controls the expression of a wide variety of genes involved in cell cycle regulation, and apoptosis (Bougdour et al. 2013; Panas and Boothroyd 2020). T. gondii infection upregulates c-myc expression (Franco et al. 2014) but significantly reduced c-myc expression was observed in GRA16 knockout parasites. In addition, expression of TgGRA16 in Neospora caninum conferred this parasite with the ability to upregulate c-myc (Panas and Boothroyd 2020).
Regulation of immune response pathways
Parasite proteins that alter the host immune response can be broadly categorized into two groups: Those found at the PVM that limit PV destruction by both host Immunity-Related GTPases (IRGs) and Guanylate Binding Proteins (GBPs) (Guevara, et al. 2021); and those that are secreted into the host nucleus and cytosol to influence immune signaling and transcriptional pathways (Hakimi et al. 2017).
Modulation of IRG and GBP:
The mediators of host IRG and GBP inactivation are predominately secreted from the rhoptries (Saeij et al. 2006; Taylor et al. 2006). The active kinase ROP18 is secreted into the host cell during invasion where it complexes with inactive pseudokinases ROP5 (Behnke et al. 2011; Reese et al. 2011) and ROP8/2 on the cytosolic side of the PV to phosphorylate IRGs and perturb their recruitment to PV. The demonstration that GRA7 binds to this rhoptry complex was one of the first examples of an interaction between rhoptry and dense granule proteins (Alaganan et al. 2014; Dunn et al. 2020). These proteins have distinctive and complementary roles in host immune evasion (Alaganan et al. 2014).
Manipulation of immune signaling pathways:
Dense granule proteins manipulate key immune-related signaling pathways including the NF-kB, IFN-γ, and p38 MAPK. GRAs have opposing activity on the pro-inflammatory NF-kB pathway. In type II strains, GRA15 induces nuclear translocation of NF-kB resulting in upregulation of pro-inflammatory cytokines including IL-12 and IL-1β (Rosowski et al. 2011). A second dense granule protein, the previously mentioned TEEGR/HCE1, which binds E2F-DP1, represses the expression of a subset of NF-kB effectors (Braun et al. 2019; Panas et al. 2019). These opposing effects can be explained by the parasites need to limit cytokine activation and prevent tissue damage, after all the parasite transmission on the host depends on host survival and parasite encystation in tissues of the host.
Independent of the NF-kB signaling, parasites also control IL-12 production by manipulating the MAPK pathway (Braun et al. 2013). GRA24 localizes to the host nucleus upon secretion from the PV where it binds directly with the MAP kinase p38a where it facilitates p38 autophosphorylation in the absence of the typical kinase cascade that controls MAPK activation (Cuadrado and Nebreda 2010). This results in increased IL-12 production.
IFN-γ is another central player in immune signaling. Pathways induced by IFN-γ culminate in nuclear translocation of the transcription factor STAT1 and upregulation of STAT1 responsive genes. Upon T. gondii infection STAT1 dependent transcription is inhibited by the dense granule effector IST1 (Inhibitor of STAT1 Transcriptional activity). In the absence of IST1, T. gondii survival in activated macrophages is reduced (Olias et al. 2016). Export of IST1 from bradyzoites protects infected cells from IFN- γ mediated cell death (Seizova et al. 2022).
Antigen presentation:
Antigen presentation is a vital immune-related process that leads to the destruction of infected cells. Infected cells present protein fragments on their surface via MHC-1 pathway for recognition by CD8+ T cells. PV localized dense granule and rhoptry proteins have the ability to suppress antigen recognition in host CD8+ T cells, while also being processed for presentation (Blanchard et al. 2008; Feliu et al. 2013; Frickel and Hunter 2021; Gregg et al. 2011; Grover et al. 2014; Rommereim et al. 2019). In the absence of ROP18, ROP5 and GRA7, the PV is targeted for host cell destruction by host IRG’s leading to increased antigen presentation (Rommereim et al. 2019). PV associated proteins are also processed for presentation from intact PV’s although it is not understood how proteins are released into the cytosol for degradation and entry into the MHC-1 pathway. However, GRA2, GRA3 and GRA12 play a role in mitigating presentation, as antigen presentation is increased in knockout parasites, although the mechanisms underpinning this phenomenon are not understood (Rommereim et al. 2019; Lopez et al. 2015). In GRA2 knockout parasites, IVN formation is disrupted leading to increased GRA6 association to the PVM and increased GRA6 presentation via the MHC-1 pathway. Thus, the IVN may limit antigen presentation by modulating MHC-1 processing of PVM bound proteins(Lopez et al. 2015).
Bradyzoite cyst formation and maintenance
GRA protein function has been predominately investigated in the fast-replicating tachyzoite form of the parasite, which is responsible for the acute infection and vital for parasite dissemination into peripheral organs. In the presence of a functioning immune system tachyzoites differentiate into the slow-growing, cyst-forming bradyzoites which can have lifelong persistence within skeletal, heart, and brain tissue (Di-Cristina et al. 2008; Watts et al. 2015). GRA proteins play vital roles in cyst formation and maintenance. During differentiation, a cyst wall forms on the luminal side of the PVM. The cyst wall consists of a dense outer layer, and looser filamentous layer underneath. The cyst lumen (also referred to as the cyst matrix) contains filamentous strands, vesicles and tubule structures termed the intracyst network (ICN) that is equivalent to the tachyzoite IVN (Lemgruber et al. 2011; Tu et al. 2020).
During the tachyzoite-to-bradyzoite transition, many IVN associated GRA proteins including GRA1, GRA2, GRA4, GRA6, GRA9, and GRA12 relocalize from the IVN to the cyst wall early in differentiation (Guevara et al. 2019; Tu et al. 2020). GRA2 expression is necessary for GRA4 and GRA6 localization to the cyst periphery (Guevara et al. 2019) and deletions of all but GRA1 resulted in slower cyst maturation (Guevara et al. 2019; Guevara et al. 2021). The localization of SFP1 and GRA29 also exhibit distinct localizations in tachyzoites compared to bradyzoites. In bradyzoites, these proteins do not form strands and are dispersed throughout the PV (Young et al. 2020). MAG1, first characterized over 25 years ago, is present in the PV lumen of tachyzoites. Upon differentiation, MAG1 expression is upregulated and localizes to the cyst wall and matrix, where it is thought to have a structural role (Parmley et al. 1994; Tomita et al. 2021).
Recent proteomic, transcriptomic and interactome approaches have identified a plethora of bradyzoite specific GRA proteins (Buchholz et al. 2011; Nadipuram et al. 2020; Tu et al. 2020), including MCP3, CST2, CST3, CST4, CST6 (Tu et al. 2019), GRA55–59 (Nadipuram et al. 2020), and CST7–9 (Tu et al. 2020). MCP3 knockouts appear to form smaller cysts with no obvious fitness loss (Tu et al. 2020), CST2 knockouts have attenuated virulence and do not establish a measurable cyst burden in mice (Tu et al. 2019), and GRA55 knockouts have a lower cyst burden in mice (Nadipuram et al. 2020). Bradyzoite pseudokinase-1 (BPK1) associates with the cyst wall and interacts with other cyst components MAG1, MCP4, GRA8 and GRA9. BPK1 knockout parasites formed smaller cysts that were more susceptible to pepsin digestion, leading to reduced efficiency of oral infection (Buchholz et al. 2013).
While knockouts of some of the cyst wall components have a demonstrated role in cyst formation or maintenance, the exact roles these proteins play, be they regulatory or structural, is not understood. In addition, many GRA proteins exhibit differential phosphorylation in the bradyzoite stage vs. tachyzoite stages (Guevara et al. 2019; Young et al. 2020). Whether phosphorylation regulates GRA function or influences the redistribution of GRA proteins from the IVN to the cyst, and within the cyst wall during cyst maturation requires further investigation.
The export of effector proteins from bradyzoite cysts into the host cell is not well understood. In parasites induced to differentiate in cell culture, MYR1 was present on the bradyzoite membrane at both early (1 day) and late (7 days) stages of differentiation, allowing for the export of effectors from cysts. GRA16, GRA24, GRA28, and IST1 accumulated in the host early after induction but only IST1 was present in the host at later time points (Mayoral et al. 2020). However, it’s not clear if this change in effector accumulation was due to changes in the MYR complex activity or due to changes in effector expression (Mayoral et al. 2020). The export of IST1 throughout early and late stages of bradyzoite infection has recently been confirmed to be MYR-dependent, supporting a role of the MYR complex in specific effector export during the bradyzoite stage (Seizova et al. 2022). Contrasting this, evidence of MAG1 secretion in both bradyzoites and tachyzoites in a MYR independent manner has been observed. The mechanism behind this MYR independent secretion warrants further investigation (Tomita et al. 2021). In addition, GRA17 is found in the cyst membrane and postulated to provide a similar role in diffusion of solutes from the host as described in tachyzoites (Gold et al. 2015; Paredes-Santos et al. 2019).
While GRA proteins are secreted from bradyzoites, it is unclear if there are differences in DG biogenesis, granule transport dynamics, and regulation of secretion between tachyzoites and bradyzoites.
Parasite Egress/Calcium homeostasis
GRA1 was the first dense granule protein identified. It’s found in the PV lumen upon release and contains a predicted EF-hand calcium binding domain (Cesbron-delauw et al. 1989). It’s predicted to be essential for parasite growth in culture, however its function and putative role in calcium homeostasis have not been elucidated (Sidik et al. 2016).
Parasite egress from the PV is triggered by increased intracellular calcium and secretion of proteins from the micronemes. However, four GRA proteins have been implicated in egress (Bisio et al. 2019; LaFavers et al. 2017; Okada et al. 2013; Schultz and Carruthers 2018). GRA41 is associated with the IVN and GRA41 knockout parasites show defects in IVN formation and exhibit a dysregulation in take up of extracellular calcium (LaFavers et al. 2017). However, GRA41 is not thought to bind calcium directly so the dysregulation may be an indirect consequence of loss of GRA41. GRA22 knockout parasites also exhibit defects in the timing of egress (Okada et al. 2013). Interestingly, GRA22 also does not contain putative calcium binding domains, so the mechanism by which this protein controls egress is not understood. TgLCAT knockout parasites do not display proper egress phenotypes, with its catalytic activity being required (Schultz and Carruthers 2018). Lastly, Diacylglycerol Kinase 2 (DGK2) is secreted into the PV and produces phosphatidic acid, which acts as a signaling molecule for egress (Bisio et al. 2019).
Concluding Remarks:
Dense granules proteins are vital for regulating the intracellular environment of host cells. Less than a decade ago, only 25 GRA proteins had been identified (Mercier and Cesbron-Delauw 2015). Thanks to recently developed proteomic and transcriptomic approaches and CRISPR-Cas9 technology, that has made tagging and deletion of genes more efficient, the identification and characterization of GRA proteins has accelerated (Bai et al. 2018; Mayoral et al. 2021; Nadipuram et al. 2020; Naor et al. 2018; Shen et al. 2014; Sidik et al. 2014; Sidik et al. 2016). However, the function and mechanism of action of many GRA proteins is poorly understood, particularly in the bradyzoite stage of the parasite lifecycle, and there are undoubtedly more GRA proteins yet to be discovered.
A more complete understanding of dense granule biology will require a range of experimental approaches: additional proteomic analysis for further identification of GRA lumen and membrane associated proteins, live cell imaging to visualize DG secretion to directly measure rates of dense granule secretion from both tachyzoites and bradyzoites, biochemical and biophysical approaches to characterize GRA protein properties and protein-protein interactions, and in vivo studies to access the roles of GRA proteins in parasite dissemination in the host and cyst formation. Collectively, this will lead to a more complete understanding of T. gondii pathogenicity.
Supplementary Material
SUPPORTING INFORMATION
Table S1. List of known GRA proteins. * indicates proteins shown to be cleaved by ASP5. Accession numbers and CRISPR scores were obtained from ToxoDB (www.ToxoDB.org) (Amos et al. 2022).
Acknowledgments
This work was funded by the National institute of general medical sciences, 1R35GM138316-01 awarded to ATH
Literature Cited
- Alaganan Aditi, Fentress Sarah J., Tang Keliang, Wang Qiuling, and Sibley L. David. 2014. “Toxoplasma GRA7 Effector Increases Turnover of Immunity-Related GTPases and Contributes to Acute Virulence in the Mouse.” Proceedings of the National Academy of Sciences of the United States of America 111 (3): 1126–31. 10.1073/pnas.1313501111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander David L., Arastu-Kapur Shirin, Dubremetz Jean-Francois, and Boothroyd John C.. 2006. “Plasmodium falciparum AMA1 Binds a Rhoptry Neck Protein Homologous to TgRON4, a Component of the Moving Junction in Toxoplasma gondii.” Eukaryotic Cell 5 (7): 1169–73. 10.1128/EC.00040-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly Ahmed S. I., Vaughan Ashley M., and Kappe Stefan H. I.. 2009. “Malaria Parasite Development in the Mosquito and Infection of the Mammalian Host.” Annual Review of Microbiology 63: 195–221. 10.1146/annurev.micro.091208.073403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos Beatrice, Aurrecoechea Cristina, Barba Matthieu, Barreto Ana, Basenko Evelina Y, Bażant Wojciech, Belnap Robert, Blevins Ann S, Böhme Ulrike, Brestelli John, Brunk Brian P, Caddick Mark, Callan Danielle, Campbell Lahcen, Christensen Mikkel B, Christophides George K, Crouch Kathryn, Davis Kristina, DeBarry Jeremy, Doherty Ryan, Duan Yikun, Dunn Michael, Falke Dave, Fisher Steve, Flicek Paul, Fox Brett, Gajria Bindu, Giraldo-Calderón Gloria I, Harb Omar S, Harper Elizabeth, Hertz-Fowler Christiane, Hickman Mark J, Howington Connor, Hu Sufen, Humphrey Jay, Iodice John, Jones Andrew, Judkins John, Kelly Sarah A, Kissinger Jessica C, Kwon Dae Kun, Lamoureux Kristopher, Lawson Daniel, Li Wei, Lies Kallie, Lodha Disha, Long Jamie, MacCallum Robert M, Maslen Gareth, McDowell Mary Ann, Nabrzyski Jaroslaw, Roos David S, Rund Samuel S C, Schulman Stephanie Wever, Shanmugasundram Achchuthan, Sitnik Vasily, Spruill Drew, Starns David, Stoeckert Christian J, Shah Tomko Sheena, Wang Haiming, Warrenfeltz Susanne, Wieck Robert, Wilkinson Paul A, Xu Lin, and Zheng Jie. 2022. “VEuPathDB: The Eukaryotic Pathogen, Vector and Host Bioinformatics Resource Center.” Nucleic Acids Research 50 (D1): D898–911. 10.1093/nar/gkab929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai Takashi, Miura Satoshi, Sibley L. David, Okabayashi Hironori, and Takeuchi Tsutomu. 1995. “Biochemical and Molecular Characterization of Nucleoside Triphosphate Hydrolase Isozymes from the Parasitic Protozoan Toxoplasma gondii.” Journal of Biological Chemistry 270 (19): 11391–97. 10.1074/jbc.270.19.11391. [DOI] [PubMed] [Google Scholar]
- Bai Meng Jie, Wang Jin Lei, Elsheikha Hany M., Liang Qin Li, Chen Kai, Nie Lan Bi, and Zhu Xing Quan. 2018. “Functional Characterization of Dense Granule Proteins in Toxoplasma gondii RH Strain Using CRISPR-Cas9 System.” Frontiers in Cellular and Infection Microbiology 8 (AUG): 1–9. 10.3389/fcimb.2018.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke MS, Khan A, Wootton JC, Dubey JP, Tang K, and Sibley LD. 2011. “Virulence Differences in Toxoplasma Mediated by Amplification of a Family of Polymorphic Pseudokinases.” Proceedings of the National Academy of Sciences 108 (23): 9631–36. 10.1073/pnas.1015338108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beraki Tsebaot, Hu Xiaoyu, Broncel Malgorzata, Young Joanna C., O’Shaughnessy William J., Borek Dominika, Treeck Moritz, and Reese Michael L.. 2019. “Divergent Kinase Regulates Membrane Ultrastructure of the Toxoplasma Parasitophorous Vacuole.” Proceedings of the National Academy of Sciences 116 (13): 6361–70. 10.1073/pnas.1816161116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisio Hugo, Chaabene Rouaa Ben, Sabitzki Ricarda, Maco Bohumil, Marq Jean Baptiste, Gilberger Tim-Wolf, Spielmann Tobias, and Soldati-Favre Dominique. 2020. “The ZIP Code of Vesicle Trafficking in Apicomplexa: SEC1/Munc18 and SNARE Proteins.” MBio 11 (5). 10.1128/mBio.02092-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisio Hugo, Lunghi Matteo, Brochet Mathieu, and Soldati-Favre Dominique. 2019. “Phosphatidic Acid Governs Natural Egress in Toxoplasma gondii via a Guanylate Cyclase Receptor Platform.” Nature Microbiology 4 (3): 420–28. 10.1038/s41564-018-0339-8. [DOI] [PubMed] [Google Scholar]
- Bisio Hugo, and Soldati-Favre Dominique. 2019. “Signaling Cascades Governing Entry into and Exit from Host Cells by Toxoplasma gondii.” Annual Review of Microbiology 73: 579–99. 10.1146/annurev-micro-020518-120235. [DOI] [PubMed] [Google Scholar]
- Blader Ira J., Coleman Bradley I., Chen Chun-Ti Ti, and Gubbels Marc-Jan Jan. 2015. “Lytic Cycle of Toxoplasma gondii: 15 Years Later.” Annual Review of Microbiology 69 (1): 463–85. 10.1146/annurev-micro-091014-104100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blader Ira J., and Koshy Anita A.. 2014. “Toxoplasma gondii Development of Its Replicative Niche: In Its Host Cell and Beyond.” Eukaryotic Cell 13 (8): 965–76. 10.1128/EC.00081-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely William J., Holmes Michael J., and Arrizabalaga Gustavo. 2020. “The Secreted Acid Phosphatase Domain-Containing GRA44 from Toxoplasma gondii Is Required for c-Myc Induction in Infected Cells.” Edited by Mitchell Aaron P.. MSphere 5 (1): 1–18. 10.1128/mSphere.00877-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard Nicolas, Gonzalez Federico, Schaeffer Marie, Joncker Nathalie T, Cheng Tiffany, Shastri Anjali J, Robey Ellen A, and Shastri Nilabh. 2008. “Immunodominant, Protective Response to the Parasite Toxoplasma gondii Requires Antigen Processing in the Endoplasmic Reticulum.” Nature Immunology 9 (8): 937–44. 10.1038/ni.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank Matthew L., Xia Jing, Morcos Mary M., Sun Mai, Cantrell Pamela S., Liu Yang, Zeng Xuemei, Powell Cameron J., Yates Nathan, Boulanger Martin J., and Boyle Jon P.. 2021. “Toxoplasma gondii Association with Host Mitochondria Requires Key Mitochondrial Protein Import Machinery.” Proceedings of the National Academy of Sciences 118 (12): e2013336118. 10.1073/pnas.2013336118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddey Justin A., and Cowman Alan F.. 2013. “Plasmodium Nesting: Remaking the Erythrocyte from the Inside Out.” Annual Review of Microbiology 67 (1): 243–69. 10.1146/annurev-micro-092412-155730. [DOI] [PubMed] [Google Scholar]
- Boddey Justin A., Hodder Anthony N., Günther Svenja, Gilson Paul R., Patsiouras Heather, Kapp Eugene A., Andrew Pearce J, de Koning-Ward Tania F., Simpson Richard J., Crabb Brendan S., and Cowman Alan F.. 2010. “An Aspartyl Protease Directs Malaria Effector Proteins to the Host Cell.” Nature 463 (7281): 627–31. 10.1038/nature08728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddey Justin A., O’Neill Matthew T., Lopaticki Sash, Carvalho Teresa G., Hodder Anthony N., Nebl Thomas, Wawra Stephan, van West Pieter, Ebrahimzadeh Zeinab, Richard Dave, Flemming Sven, Spielmann Tobias, Przyborski Jude, Babon Jeff J., and Cowman Alan F.. 2016. “Export of Malaria Proteins Requires Co-Translational Processing of the PEXEL Motif Independent of Phosphatidylinositol-3-Phosphate Binding.” Nature Communications 7 (1): 10470. 10.1038/ncomms10470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougdour Alexandre, Durandau Eric, Brenier-Pinchart Marie Pierre, Ortet Philippe, Barakat Mohamed, Kieffer Sylvie, Curt-Varesano Aurélie, Curt-Bertini Rose Laurence, Bastien Olivier, Coute Yohann, Pelloux Hervé, and Hakimi Mohamed Ali. 2013. “Host Cell Subversion by Toxoplasma GRA16, an Exported Dense Granule Protein That Targets the Host Cell Nucleus and Alters Gene Expression.” Cell Host and Microbe 13 (4): 489–500. 10.1016/j.chom.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Bradley Peter J., and Sibley L. David. 2007. “Rhoptries: An Arsenal of Secreted Virulence Factors.” Current Opinion in Microbiology 10 (6): 582–87. 10.1016/j.mib.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun Laurence, Brenier-Pinchart Marie-Pierre, Hammoudi Pierre-Mehdi, Cannella Dominique, Kieffer-Jaquinod Sylvie, Vollaire Julien, Josserand Véronique, Touquet Bastien, Couté Yohann, Tardieux Isabelle, Bougdour Alexandre, and Hakimi Mohamed-Ali. 2019. “The Toxoplasma Effector TEEGR Promotes Parasite Persistence by Modulating NF-KB Signalling via EZH2.” Nature Microbiology 4 (7): 1208–20. 10.1038/s41564-019-0431-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun Laurence, Brenier-Pinchart Marie Pierre, Yogavel Manickam, Curt-Varesano Aurélie, Curt-Bertini Rose Laurence, Hussain Tahir, Kieffer-Jaquinod Sylvie, Coute Yohann, Pelloux Hervé, Tardieux Isabelle, Sharma Amit, Belrhali Hassan, Bougdour Alexandre, and Hakimi Mohamed Ali. 2013. “A Toxoplasma Dense Granule Protein, GRA24, Modulates the Early Immune Response to Infection by Promoting a Direct and Sustained Host P38 MAPK Activation.” Journal of Experimental Medicine 210 (10): 2071–86. 10.1084/jem.20130103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun Laurence, Travier Laetitia, Kieffer Sylvie, Musset Karine, Garin Jérôme, Mercier Corinne, and Cesbron-Delauw Marie-France. 2008. “Purification of Toxoplasma Dense Granule Proteins Reveals That They Are in Complexes throughout the Secretory Pathway.” Molecular and Biochemical Parasitology 157 (1): 13–21. 10.1016/j.molbiopara.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Brunet Julie, Pfaff Alexander W, Abidi Ahmed, Unoki Motoko, Nakamura Yusuke, Guinard Marie, Klein Jean-Paul, Candolfi Ermanno, and Mousli Marc. 2008. “Toxoplasma gondii Exploits UHRF1 and Induces Host Cell Cycle Arrest at G2 to Enable Its Proliferation.” Cellular Microbiology 10 (4): 908–20. 10.1111/j.1462-5822.2007.01093.x. [DOI] [PubMed] [Google Scholar]
- Buchholz Kerry R., Bowyer Paul W., and Boothroyd John C.. 2013. “Bradyzoite Pseudokinase 1 Is Crucial for Efficient Oral Infectivity of the Toxoplasma gondii Tissue Cyst.” Eukaryotic Cell 12 (3): 399–410. 10.1128/EC.00343-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz Kerry R., Fritz Heather M., Chen Xiucui, Durbin-Johnson Blythe, Rocke David M., Ferguson David J., Conrad Patricia A., and Boothroyd John C.. 2011. “Identification of Tissue Cyst Wall Components by Transcriptome Analysis of in Vivo and in Vitro Toxoplasma gondii Bradyzoites.” Eukaryotic Cell 10 (12): 1637–47. 10.1128/EC.05182-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen Hayley E., Charnaud Sarah C., Kalanon Ming, Riglar David T., Dekiwadia Chaitali, Kangwanrangsan Niwat, Torii Motomi, Tsuboi Takafumi, Baum Jacob, Ralph Stuart A., Cowman Alan F., de Koning-Ward Tania F., Crabb Brendan S., and Gilson Paul R.. 2012. “Biosynthesis, Localization, and Macromolecular Arrangement of the Plasmodium falciparum Translocon of Exported Proteins (PTEX).” Journal of Biological Chemistry 287 (11): 7871–84. 10.1074/jbc.M111.328591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffaro Carolina E., and Boothroyd John C.. 2011. “Evidence for Host Cells as the Major Contributor of Lipids in the Intravacuolar Network of Toxoplasma-Infected Cells.” Eukaryotic Cell 10 (8): 1095–99. 10.1128/EC.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Shinuo, Yang Juan, Fu Jiawen, Chen Heming, and Jia Honglin. 2021. “The Dissection of SNAREs Reveals Key Factors for Vesicular Trafficking to the Endosome-like Compartment and Apicoplast via the Secretory System in Toxoplasma gondii.” MBio 12 (4): e0138021. 10.1128/mBio.01380-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey Kimberly L., Donahue Carolyn G., and Ward Gary E.. 2000. “Identification and Molecular Characterization of GRA8, a Novel, Proline-Rich, Dense Granule Protein of Toxoplasma gondii.” Molecular and Biochemical Parasitology 105 (1): 25–37. 10.1016/S0166-6851(99)00160-7. [DOI] [PubMed] [Google Scholar]
- Carmeille Romain, Schiano Lomoriello Porfirio, Devarakonda Parvathi M., Kellermeier Jacob A., and Heaslip Aoife T.. 2021. “Actin and an Unconventional Myosin Motor, TgMyoF, Control the Organization and Dynamics of the Endomembrane Network in Toxoplasma gondii.” Edited by Gubbels Marc-Jan. PLOS Pathogens 17 (2): e1008787. 10.1371/journal.ppat.1008787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers VB, and Sibley LD. 1997. “Sequential Protein Secretion from Three Distinct Organelles of Toxoplasma gondii Accompanies Invasion of Human Fibroblasts.” European Journal of Cell Biology 73 (2): 114–23. http://www.ncbi.nlm.nih.gov/pubmed/9208224. [PubMed] [Google Scholar]
- Cesbron-Delauw MF, Guy B, Torpier G, Pierce RJ, Lenzen G, Cesbron JY, Charif H, Lepage P, Darcy F, Lecocq JP, and Capron A. 1989. “Molecular Characterization of a 23-Kilodalton Major Antigen Secreted by Toxoplasma gondii.” Proceedings of the National Academy of Sciences of the United States of America 86 (19): 7537–41. 10.1073/pnas.86.19.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesbron-Delauw Marie-France, Gendrin Claire, Travier Laetitia, Ruffiot Pauline, and Mercier Corinne. 2008. “Apicomplexa in Mammalian Cells: Trafficking to the Parasitophorous Vacuole.” Traffic 9 (5): 657–64. 10.1111/j.1600-0854.2008.00728.x. [DOI] [PubMed] [Google Scholar]
- Chanat Eric, and Huttner Wieland B.. 1991. “Milieu-Induced, Selective Aggregation of Regulated Secretory Proteins in the Trans-Golgi Network.” Journal of Cell Biology 115 (6): 1505–19. 10.1083/jcb.115.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi S, Qi H, Coleman D, Rodriguez A, Hanson PI, Striepen B, Roos DS, and Joiner KA. 1999. “Constitutive Calcium-Independent Release of Toxoplasma gondii Dense Granules Occurs through the NSF/SNAP/SNARE/Rab Machinery.” The Journal of Biological Chemistry 274 (4): 2424–31. 10.1074/jbc.274.4.2424. [DOI] [PubMed] [Google Scholar]
- Chen Jia, Li Zhong-Yuan, Zhou Dong-Hui, Liu Guo-Hua, and Zhu Xing-Quan. 2012. “Genetic Diversity among Toxoplasma gondii Strains from Different Hosts and Geographical Regions Revealed by Sequence Analysis of GRA5 Gene.” Parasites & Vectors 5 (1): 279. 10.1186/1756-3305-5-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm Scott A., Kalanon Ming, Nebl Thomas, Sanders Paul R., Matthews Kathryn M., Dickerman Benjamin K., Gilson Paul R., and Koning-Ward Tania F.. 2018. “The Malaria PTEX Component PTEX88 Interacts Most Closely with HSP101 at the Host–Parasite Interface.” The FEBS Journal 285 (11): 2037–55. 10.1111/febs.14463. [DOI] [PubMed] [Google Scholar]
- Clough Barbara, and Frickel Eva-Maria. 2017. “The Toxoplasma Parasitophorous Vacuole: An Evolving Host–Parasite Frontier.” Trends in Parasitology 33 (6): 473–88. 10.1016/j.pt.2017.02.007. [DOI] [PubMed] [Google Scholar]
- Coffey Michael J., Dagley Laura F., Seizova Simona, Kapp Eugene A., Infusini Giuseppe, Roos David S., Boddey Justin A., Webb Andrew I., and Tonkin Christopher J.. 2018. “Aspartyl Protease 5 Matures Dense Granule Proteins That Reside at the Host-Parasite Interface in Toxoplasma gondii.” Edited by David Sibley L. MBio 9 (5): 1–21. 10.1128/mbio.01796-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey Michael J., Jennison Charlie, Tonkin Christopher J., and Boddey Justin A.. 2016. “Role of the ER and Golgi in Protein Export by Apicomplexa.” Current Opinion in Cell Biology 41 (August): 18–24. 10.1016/j.ceb.2016.03.007. [DOI] [PubMed] [Google Scholar]
- Coffey Michael J., Sleebs Brad E., Uboldi Alessandro D., Garnham Alexandra, Franco Magdalena, Marino Nicole D, Panas Michael W, Ferguson David JP., Enciso Marta, O’Neill Matthew T., Lopaticki Sash, Stewart Rebecca J., Dewson Grant, Smyth Gordon K., Smith Brian J., Masters Seth L., Boothroyd John C., Boddey Justin A., and Tonkin Christopher J.. 2015. “An Aspartyl Protease Defines a Novel Pathway for Export of Toxoplasma Proteins into the Host Cell.” ELife 4 (November). 10.7554/eLife.10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppens Isabelle, Andries Marie, Liu Jinli L., and Cesbron-Delauw Marie France. 1999. “Intracellular Trafficking of Dense Granule Proteins in Toxoplasma gondii and Experimental Evidences for a Regulated Exocytosis.” European Journal of Cell Biology 78 (7): 463–72. 10.1016/S0171-9335(99)80073-9. [DOI] [PubMed] [Google Scholar]
- Coppens Isabelle, Dunn Joe Dan, Romano Julia D., Pypaert Marc, Zhang Hui, Boothroyd John C., and Joiner Keith A.. 2006. “Toxoplasma gondii Sequesters Lysosomes from Mammalian Hosts in the Vacuolar Space.” Cell 125 (2): 261–74. 10.1016/j.cell.2006.01.056. [DOI] [PubMed] [Google Scholar]
- Coppens Isabelle, Sinai Anthony P., and Joiner Keith A.. 2000. “Toxoplasma gondii Exploits Host Low-Density Lipoprotein Receptor-Mediated Endocytosis for Cholesterol Acquisition.” Journal of Cell Biology 149 (1): 167–80. 10.1083/jcb.149.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craver Mary Patricia J., and Knoll Laura J.. 2007. “Increased Efficiency of Homologous Recombination in Toxoplasma gondii Dense Granule Protein 3 Demonstrates That GRA3 Is Not Necessary in Cell Culture but Does Contribute to Virulence.” Molecular and Biochemical Parasitology 153 (2): 149–57. 10.1016/j.molbiopara.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Crawford Michael J., Thomsen-Zieger Nadine, Ray Manisha, Schachtner Joachim, Roos David S., and Seeber Frank. 2006. “Toxoplasma gondii Scavenges Host-Derived Lipoic Acid despite Its de Novo Synthesis in the Apicoplast.” The EMBO Journal 25 (13): 3214–22. 10.1038/sj.emboj.7601189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado Ana, and Nebreda Angel R.. 2010. “Mechanisms and Functions of P38 MAPK Signalling.” The Biochemical Journal 429 (3): 403–17. 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- Curt-Varesano Aurélie, Braun Laurence, Ranquet Caroline, Hakimi Mohamed-Ali, and Bougdour Alexandre. 2016. “The Aspartyl Protease TgASP5 Mediates the Export of the Toxoplasma GRA16 and GRA24 Effectors into Host Cells.” Cellular Microbiology 18 (2): 151–67. 10.1111/cmi.12498. [DOI] [PubMed] [Google Scholar]
- Cygan Alicja M., Jean Beltran Pierre M., Mendoza Alma G., Branon Tess C., Ting Alice Y., Carr Steven A., and Boothroyd John C.. 2021. “ Proximity-Labeling Reveals Novel Host and Parasite Proteins at the Toxoplasma Parasitophorous Vacuole Membrane.” Edited by Blader Ira J. and Coyne Carolyn B.. MBio 12 (6): 1–56. 10.1128/mbio.00260-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cygan Alicja M., Theisen Terence C., Mendoza Alma G., Marino Nicole D., Panas Michael W., and Boothroyd John C.. 2020. “Coimmunoprecipitation with MYR1 Identifies Three Additional Proteins within the Toxoplasma gondii Parasitophorous Vacuole Required for Translocation of Dense Granule Effectors into Host Cells.” MSphere 5 (1): 1–17. 10.1128/msphere.00858-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deffieu Maika S., Alayi Tchilabalo Dilezitoko, Slomianny Christian, and Tomavo Stanislas. 2019. “The Toxoplasma gondii Dense Granule Protein TgGRA3 Interacts with Host Golgi and Dysregulates Anterograde Transport.” Biology Open 8 (3). 10.1242/bio.039818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esra Demircioglu F, Burkhardt Pawel, and Fasshauer Dirk. 2014. “The SM Protein Sly1 Accelerates Assembly of the ER-Golgi SNARE Complex.” Proceedings of the National Academy of Sciences 111 (38): 13828–33. 10.1073/pnas.1408254111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di-Cristina Manlio, Marocco Daniela, Galizi Roberto, Proietti Carla, Spaccapelo Roberta, and Crisanti Andrea. 2008. “Temporal and Spatial Distribution of Toxoplasma gondii Differentiation into Bradyzoites and Tissue Cyst Formation In Vivo.” Infection and Immunity 76 (8): 3491–3501. 10.1128/IAI.00254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos-Santos-Pacheco Nicolas, Tosetti Nicolò, Koreny Ludek, Waller Ross F, and Soldati-Favre Dominique. 2020. “Evolution, Composition, Assembly, and Function of the Conoid in Apicomplexa.” Trends in Parasitology 36 (8): 688–704. 10.1016/j.pt.2020.05.001. [DOI] [PubMed] [Google Scholar]
- Dou Zhicheng, McGovern Olivia L., Di Cristina Manlio, and Carruthers Vern B.. 2014. “Toxoplasma gondii Ingests and Digests Host Cytosolic Proteins.” MBio 5 (4): 1–12. 10.1128/mBio.01188-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubremetz Jean François, Achbarou Abderrahim, Bermudes David, and Joiner Keith A.. 1993. “Kinetics and Pattern of Organelle Exocytosis DuringToxoplasma gondii/Host-Cell Interaction.” Parasitology Research 79 (5): 402–8. 10.1007/BF00931830. [DOI] [PubMed] [Google Scholar]
- Dunn Joe Dan, Ravindran Sandeep, Kim Seon-Kyeong, and Boothroyd John C.. 2020. “The Toxoplasma gondii Dense Granule Protein GRA7 Is Phosphorylated upon Invasion and Forms an Unexpected Association with the Rhoptry Proteins ROP2 and ROP4.” Infection and Immunity 76 (12): 5853–61. 10.1128/IAI.01667-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsworth Brendan, Matthews Kathryn, Nie Catherine Q., Kalanon Ming, Charnaud Sarah C., Sanders Paul R., Chisholm Scott A., Counihan Natalie A., Shaw Philip J., Pino Paco, Chan Jo-Anne, Azevedo Mauro F., Rogerson Stephen J., Beeson James G., Crabb Brendan S., Gilson Paul R., and de Koning-Ward Tania F.. 2014. “PTEX Is an Essential Nexus for Protein Export in Malaria Parasites.” Nature 511 (7511): 587–91. 10.1038/nature13555. [DOI] [PubMed] [Google Scholar]
- Engelberg Klemens, Chen Chun-Ti, Bechtel Tyler, Sánchez Guzmán Victoria, Drozda Allison A., Chavan Suyog, Weerapana Eranthie, and Gubbels Marc-Jan. 2020. “The Apical Annuli of Toxoplasma gondii Are Composed of Coiled-coil and Signalling Proteins Embedded in the Inner Membrane Complex Sutures.” Cellular Microbiology 22 (1). 10.1111/cmi.13112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasshauer D, Sutton RB, Brunger AT, and Jahn R. 1998. “Conserved Structural Features of the Synaptic Fusion Complex: SNARE Proteins Reclassified as Q- and R-SNAREs.” Proceedings of the National Academy of Sciences of the United States of America 95 (26): 15781–86. 10.1073/pnas.95.26.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliu Virginie, Vasseur Virginie, Grover Harshita S., Chu H. Hamlet, Brown Mark J., Wang Jeremy, Boyle Jon P., Robey Ellen A., Shastri Nilabh, and Blanchard Nicolas. 2013. “Location of the CD8 T Cell Epitope within the Antigenic Precursor Determines Immunogenicity and Protection against the Toxoplasma gondii Parasite.” Edited by Denkers Eric Y.. PLoS Pathogens 9 (6): e1003449. 10.1371/journal.ppat.1003449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox Barbara A., Guevara Rebekah B., Rommereim Leah M., Falla Alejandra, Bellini Valeria, Pètre Graciane, Rak Camille, Cantillana Viviana, Dubremetz Jean-François, Cesbron-Delauw Marie-France, Taylor Gregory A., Mercier Corinne, and Bzik David J.. 2019. “Toxoplasma gondii Parasitophorous Vacuole Membrane-Associated Dense Granule Proteins Orchestrate Chronic Infection and GRA12 Underpins Resistance to Host Gamma Interferon.” MBio 10 (4). 10.1128/mBio.00589-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francia Maria E., and Striepen Boris. 2014. “Cell Division in Apicomplexan Parasites.” Nature Reviews. Microbiology 12 (2): 125–36. 10.1038/nrmicro3184. [DOI] [PubMed] [Google Scholar]
- Franco Magdalena, Panas Michael W., Marino Nicole D., Lee Mei-Chong Wendy Chong Wendy Chong Wendy, Buchholz Kerry R., Kelly Felice D., Bednarski Jeffrey J., Sleckman Barry P., Pourmand Nader, and Boothroyd John C.. 2016. “A Novel Secreted Protein, MYR1, Is Central to Toxoplasma ‘s Manipulation of Host Cells.” Edited by Casadevall Arturo. MBio 7 (1). 10.1128/mBio.02231-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco Magdalena, Shastri Anjali J., and Boothroyd John C.. 2014. “Infection by Toxoplasma gondii Specifically Induces Host C-Myc and the Genes This Pivotal Transcription Factor Regulates.” Eukaryotic Cell 13 (4): 483–93. 10.1128/EC.00316-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frickel Eva-Maria, and Hunter Christopher A.. 2021. “Lessons from Toxoplasma : Host Responses That Mediate Parasite Control and the Microbial Effectors That Subvert Them.” Journal of Experimental Medicine 218 (11). 10.1084/jem.20201314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay Gabrielle, Braun Laurence, Brenier-Pinchart Marie-Pierre, Vollaire Julien, Josserand Véronique, Bertini Rose-Laurence, Varesano Aurélie, Touquet Bastien, De Bock Pieter-Jan, Coute Yohann, Tardieux Isabelle, Bougdour Alexandre, and Hakimi Mohamed-Ali. 2016. “Toxoplasma gondii TgIST Co-Opts Host Chromatin Repressors Dampening STAT1-Dependent Gene Regulation and IFN-γ–Mediated Host Defenses.” Journal of Experimental Medicine 213 (9): 1779–98. 10.1084/jem.20160340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendrin Claire, Mercier Corinne, Braun Laurence, Musset Karine, Dubremetz Jean-François, and Cesbron-Delauw Marie-France. 2008. “Toxoplasma gondii Uses Unusual Sorting Mechanisms to Deliver Transmembrane Proteins into the Host-Cell Vacuole.” Traffic (Copenhagen, Denmark) 9 (10): 1665–80. 10.1111/j.1600-0854.2008.00793.x. [DOI] [PubMed] [Google Scholar]
- Germanos Mark, Gao Andy, Taper Matthew, Yau Belinda, and Kebede Melkam A.. 2021. “Inside the Insulin Secretory Granule.” Metabolites 11 (8): 515. 10.3390/metabo11080515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold Daniel A., Kaplan Aaron D., Lis Agnieszka, Bett Glenna C.L., Rosowski Emily E., Cirelli Kimberly M., Bougdour Alexandre, Sidik Saima M., Beck Josh R., Lourido Sebastian, Egea Pascal F., Bradley Peter J., Hakimi Mohamed-Ali, Rasmusson Randall L., and Saeij Jeroen P.J.. 2015. “The Toxoplasma Dense Granule Proteins GRA17 and GRA23 Mediate the Movement of Small Molecules between the Host and the Parasitophorous Vacuole.” Cell Host & Microbe 17 (5): 642–52. 10.1016/j.chom.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondré-Lewis Marjorie C., Park Joshua J., and Loh Y. Peng. 2012. “Cellular Mechanisms for the Biogenesis and Transport of Synaptic and Dense-Core Vesicles.” International Review of Cell and Molecular Biology 299: 27–115. 10.1016/B978-0-12-394310-1.00002-3. [DOI] [PubMed] [Google Scholar]
- Gregg Beth, Dzierszinski Florence, Tait Elia, Jordan Kimberly A., Hunter Christopher A., and Roos David S.. 2011. “Subcellular Antigen Location Influences T-Cell Activation during Acute Infection with Toxoplasma gondii.” Edited by Kwaik Yousef Abu. PLoS ONE 6 (7): e22936. 10.1371/journal.pone.0022936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover Harshita Satija, Chu H. Hamlet, Kelly Felice D., Yang Soo Jung, Reese Michael L., Blanchard Nicolas, Gonzalez Federico, Chan Shiao Wei, Boothroyd John C., Shastri Nilabh, and Robey Ellen A.. 2014. “Impact of Regulated Secretion on Antiparasitic CD8 T Cell Responses.” Cell Reports 7 (5): 1716–28. 10.1016/j.celrep.2014.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevara Rebekah B., Fox Barbara A., and Bzik David J.. 2020. “Toxoplasma gondii Parasitophorous Vacuole Membrane-Associated Dense Granule Proteins Regulate Maturation of the Cyst Wall.” Edited by Moreno Silvia N. J.. MSphere 5 (1): 1–17. 10.1128/mSphere.00851-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevara Rebekah B., Fox Barbara A., and David J 2021. “A Family of Toxoplasma gondii Genes Related to GRA12 Regulate Cyst Burdens and Cyst Reactivation.” MSphere 6 (2): 1–20. 10.1128/msphere.00182-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevara Rebekah B, Fox Barbara A., Falla Alejandra, and Bzik David J.. 2019. “Toxoplasma gondii Intravacuolar-Network-Associated Dense Granule Proteins Regulate Maturation of the Cyst Matrix and Cyst Wall.” Edited by Moreno Silvia N. J.. MSphere 4 (5): 1–25. 10.1128/mSphere.00487-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Huanping, Gao Yang, Jia Honglin, Moumouni Paul Franck Adjou, Masatani Tatsunori, Liu Mingming, Lee Seung Hun, Galon Eloiza May, Li Jixu, Li Yongchang, Tumwebaze Maria Agnes, Benedicto Byamukama, and Xuan Xuenan. 2019. “Characterization of Strain-Specific Phenotypes Associated with Knockout of Dense Granule Protein 9 in Toxoplasma gondii.” Molecular and Biochemical Parasitology 229 (September 2018): 53–61. 10.1016/j.molbiopara.2019.01.003. [DOI] [PubMed] [Google Scholar]
- Gupta Nishith, Hartmann Anne, Lucius Richard, and Voelker Dennis R.. 2012. “The Obligate Intracellular Parasite Toxoplasma gondii Secretes a Soluble Phosphatidylserine Decarboxylase.” Journal of Biological Chemistry 287 (27): 22938–47. 10.1074/jbc.M112.373639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakamada Kazuaki, Nakamura Manami, Midorikawa Rio, Shinohara Kyosuke, Noguchi Keiichi, Nagaoka Hikaru, Takashima Eizo, Morishima Ken, Inoue Rintaro, Sugiyama Masaaki, Kawamoto Akihiro, and Yohda Masafumi. 2020. “PV1 Protein from Plasmodium falciparum Exhibits Chaperone-Like Functions and Cooperates with Hsp100s.” International Journal of Molecular Sciences 21 (22): 8616. 10.3390/ijms21228616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakimi Mohamed-Ali, Olias Philipp, and Sibley David L.. 2017. “Toxoplasma Effectors Targeting Host Signaling and Transcription.” Clinical Microbiology Reviews 30 (3): 615–45. 10.1128/CMR.00005-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halonen Sandra K., and Weidner Earl. 1994. “Overcoating of Toxoplasma Parasitophorous Vacuoles with Host Cell Vimentin Type Intermediate Filaments.” The Journal of Eukaryotic Microbiology 41 (1): 65–71. 10.1111/j.1550-7408.1994.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Hammer John A., and Sellers James R.. 2011. “Walking to Work: Roles for Class V Myosins as Cargo Transporters.” Nature Reviews. Molecular Cell Biology 13 (1): 13–26. 10.1038/nrm3248. [DOI] [PubMed] [Google Scholar]
- Hammoudi Pierre-Mehdi Mehdi, Jacot Damien, Mueller Christina, Manlio Di Cristina, Dogga Sunil Kumar, Baptiste Marq Jean-Baptiste, Romano Julia, Tosetti Nicolò, Dubrot Juan, Emre Yalin, Lunghi Matteo, Coppens Isabelle, Yamamoto Masahiro, Sojka Daniel, Pino Paco, and Soldati-Favre Dominique. 2015. “Fundamental Roles of the Golgi-Associated Toxoplasma Aspartyl Protease, ASP5, at the Host-Parasite Interface.” Edited by Knoll Laura J. PLOS Pathogens 11 (10): e1005211. 10.1371/journal.ppat.1005211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Huan, Brenier-Pinchart Marie-Pierre, Braun Laurence, Kraut Alexandra, Touquet Bastien, Couté Yohann, Tardieux Isabelle, Hakimi Mohamed-Ali, and Bougdour Alexandre. 2018. “Characterization of a Toxoplasma Effector Uncovers an Alternative GSK3/β-Catenin-Regulatory Pathway of Inflammation.” ELife 7 (October). 10.7554/eLife.39887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaslip Aoife T., Nelson Shane R., and Warshaw David M.. 2016. “Dense Granule Trafficking in Toxoplasma gondii Requires a Unique Class 27 Myosin and Actin Filaments.” Edited by Steinberg Gero. Molecular Biology of the Cell 27 (13): 2080–89. 10.1091/mbc.E15-12-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiber Arlett, Kruse Florian, Pick Christian, Grüring Christof, Flemming Sven, Oberli Alexander, Schoeler Hanno, Retzlaff Silke, Mesén-Ramírez Paolo, Hiss Jan A., Kadekoppala Madhusudan, Hecht Leonie, Holder Anthony A., Gilberger Tim Wolf, and Spielmann Tobias. 2013. “Identification of New PNEPs Indicates a Substantial Non-PEXEL Exportome and Underpins Common Features in Plasmodium falciparum Protein Export.” PLoS Pathogens. 10.1371/journal.ppat.1003546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller N. Luisa, Bhattacharjee Souvik, van Ooij Christiaan, Liolios Konstantinos, Harrison Travis, Lopez-Estraño Carlos, and Haldar Kasturi. 2004. “A Host-Targeting Signal in Virulence Proteins Reveals a Secretome in Malarial Infection.” Science 306 (5703): 1934–37. 10.1126/science.1102737. [DOI] [PubMed] [Google Scholar]
- Hirokawa Nobutaka, Noda Yasuko, Tanaka Yosuke, and Niwa Shinsuke. 2009. “Kinesin Superfamily Motor Proteins and Intracellular Transport.” Nature Reviews. Molecular Cell Biology 10 (10): 682–96. 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- Ho Chi Min, Beck Josh R., Lai Mason, Cui Yanxiang, Goldberg Daniel E., Egea Pascal F., and Zhou Z. Hong. 2018. “Malaria Parasite Translocon Structure and Mechanism of Effector Export.” Nature. 10.1038/s41586-018-0469-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe HC, Ngô HM, Yang M, and Joiner KA. 2000. “Targeting to Rhoptry Organelles of Toxoplasma gondii Involves Evolutionarily Conserved Mechanisms.” Nature Cell Biology 2 (7): 449–56. 10.1038/35017090. [DOI] [PubMed] [Google Scholar]
- Hsiao Chia Hung Christine, Hiller N. Luisa, Haldar Kasturi, and Knoll Laura J.. 2013. “A HT/PEXEL Motif in Toxoplasma Dense Granule Proteins Is a Signal for Protein Cleavage but Not Export into the Host Cell.” Traffic 14 (5): 519–31. 10.1111/tra.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Ke, Johnson Jeff, Florens Laurence, Fraunholz Martin, Suravajjala Sapna, DiLullo Camille, Yates John, Roos David S., and Murray John M.. 2006. “Cytoskeletal Components of an Invasion Machine—The Apical Complex of Toxoplasma gondii.” Edited by Boothroyd John. PLoS Pathogens 2 (2): e13. 10.1371/journal.ppat.0020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Xiaoyu, Binns Derk, and Reese Michael L.. 2017. “The Coccidian Parasites Toxoplasma and Neospora Dysregulate Mammalian Lipid Droplet Biogenesis.” Journal of Biological Chemistry 292 (26): 11009–20. 10.1074/jbc.M116.768176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones Thomas C., Yeh Shirley, and Hirsch James G.. 1972. “THE INTERACTION BETWEEN TOXOPLASMA GONDII AND MAMMALIAN CELLS.” Journal of Experimental Medicine 136 (5): 1157–72. 10.1084/jem.136.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsten V, Qi H, Beckers CJ, Reddy A, Dubremetz JF, Webster P, and Joiner KA. 1998. “The Protozoan Parasite Toxoplasma gondii Targets Proteins to Dense Granules and the Vacuolar Space Using Both Conserved and Unusual Mechanisms.” The Journal of Cell Biology 141 (6): 1323–33. 10.1083/jcb.141.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katris Nicholas J., Ke Huiling, McFadden Geoffrey I., van Dooren Giel G., and Waller Ross F.. 2019. “Calcium Negatively Regulates Secretion from Dense Granules in Toxoplasma gondii.” Cellular Microbiology 21 (6): e13011. 10.1111/cmi.13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil Ibrahim A., Troeger Christopher, Rao Puja C., Blacker Brigette F., Brown Alexandria, Brewer Thomas G., Colombara Danny V., De Hostos Eugenio L., Engmann Cyril, Guerrant Richard L., Haque Rashidul, Houpt Eric R., Kang Gagandeep, Korpe Poonum S., Kotloff Karen L., Lima Aldo A.M., Petri William A., Platts-Mills James A., Shoultz David A., Forouzanfar Mohammed H., Hay Simon I., Reiner Robert C., and Mokdad Ali H.. 2018. “Morbidity, Mortality, and Long-Term Consequences Associated with Diarrhoea from Cryptosporidium Infection in Children Younger than 5 Years: A Meta-Analyses Study.” The Lancet. Global Health 6 (7): e758–68. 10.1016/S2214-109X(18)30283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Ye-Ram, Kim Jae-Sung, Yun Jin-Seung, Kim Sojin, Kim Sun Young, Jang Kiseok, and Yang Chul-Su. 2018. “Toxoplasma gondii GRA8 Induces ATP5A1–SIRT3-Mediated Mitochondrial Metabolic Resuscitation: A Potential Therapy for Sepsis.” Experimental & Molecular Medicine 50 (3): e464–e464. 10.1038/emm.2017.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Koning-Ward Tania F., Gilson Paul R., Boddey Justin A., Rug Melanie, Smith Brian J., Papenfuss Anthony T., Sanders Paul R., Lundie Rachel J., Maier Alexander G., Cowman Alan F., and Crabb Brendan S.. 2009. “A Newly Discovered Protein Export Machine in Malaria Parasites.” Nature 459 (7249): 945–49. 10.1038/nature08104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug Ulrike, Zebisch Matthias, Krauss Michel, and Sträter Norbert. 2012. “Structural Insight into Activation Mechanism of Toxoplasma gondii Nucleoside Triphosphate Diphosphohydrolases by Disulfide Reduction.” The Journal of Biological Chemistry 287 (5): 3051–66. 10.1074/jbc.M111.294348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Külzer Simone, Charnaud Sarah, Dagan Tal, Riedel Jan, Mandal Pradipta, Pesce Eva R., Blatch Gregory L., Crabb Brendan S., Gilson Paul R., and Przyborski Jude M.. 2012. “Plasmodium falciparum -Encoded Exported Hsp70/Hsp40 Chaperone/Co-Chaperone Complexes within the Host Erythrocyte.” Cellular Microbiology 14 (11): 1784–95. 10.1111/j.1462-5822.2012.01840.x. [DOI] [PubMed] [Google Scholar]
- Labruyere E, Lingnau M, Mercier C, and Sibley LD. 1999. “Differential Membrane Targeting of the Secretory Proteins GRA4 and GRA6 within the Parasitophorous Vacuole Formed by Toxoplasma gondii.” Molecular and Biochemical Parasitology 102 (2): 311–24. 10.1016/s0166-6851(99)00092-4. [DOI] [PubMed] [Google Scholar]
- LaFavers Kaice A., Márquez-Nogueras Karla M., Coppens Isabelle, Moreno Silvia N. J., and Arrizabalaga Gustavo. 2017. “A Novel Dense Granule Protein, GRA41, Regulates Timing of Egress and Calcium Sensitivity in Toxoplasma gondii.” Cellular Microbiology 19 (9): e12749. 10.1111/cmi.12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecordier L, Mercier C, Sibley LD, and Cesbron-Delauw MF. 1999. “Transmembrane Insertion of the Toxoplasma gondii GRA5 Protein Occurs after Soluble Secretion into the Host Cell.” Molecular Biology of the Cell 10 (4): 1277–87. 10.1091/mbc.10.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemgruber Leandro, Lupetti Pietro, Martins-Duarte Erica S., De Souza Wanderley, and Vommaro Rossiane C.. 2011. “The Organization of the Wall Filaments and Characterization of the Matrix Structures of Toxoplasma gondii Cyst Form.” Cellular Microbiology 13 (12): 1920–32. 10.1111/j.1462-5822.2011.01681.x. [DOI] [PubMed] [Google Scholar]
- Leriche Marie Anne, and Dubremetz Jean François. 1991. “Characterization of the Protein Contents of Rhoptries and Dense Granules of Toxoplasma gondii Tachyzoites by Subcellular Fractionation and Monoclonal Antibodies.” Molecular and Biochemical Parasitology 45 (2): 249–59. 10.1016/0166-6851(91)90092-K. [DOI] [PubMed] [Google Scholar]
- Li Muyang, Chen Delin, Shiloh Ariel, Luo Jianyuan, Nikolaev Anatoly Y., Qin Jun, and Gu Wei. 2002. “Deubiquitination of P53 by HAUSP Is an Important Pathway for P53 Stabilization.” Nature 416 (6881): 648–53. 10.1038/nature737. [DOI] [PubMed] [Google Scholar]
- Li Xianhe, Straub Julian, Medeiros Tânia Catarina, Mehra Chahat, den Brave Fabian, Peker Esra, Atanassov Ilian, Stillger Katharina, Michaelis Jonas Benjamin, Burbridge Emma, Adrain Colin, Christian Münch Jan Riemer, Becker Thomas, and Pernas Lena F.. 2022. “Mitochondria Shed Their Outer Membrane in Response to Infection-Induced Stress.” Science 375 (6577). 10.1126/science.abi4343. [DOI] [PubMed] [Google Scholar]
- Lopez Jodie, Bittame Amina, Massera Céline, Vasseur Virginie, Effantin Grégory, Valat Anne, Buaillon Célia, Allart Sophie, Fox Barbara A A., Rommereim Leah M M., Bzik David J J., Schoehn Guy, Weissenhorn Winfried, Dubremetz Jean François, Gagnon Jean, Mercier Corinne, Cesbron-Delauw Marie France, and Blanchard Nicolas. 2015. “Intravacuolar Membranes Regulate CD8 T Cell Recognition of Membrane-Bound Toxoplasma gondii Protective Antigen.” Cell Reports 13 (10): 2273–86. 10.1016/j.celrep.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Lourido Sebastian, Shuman Joel, Zhang Chao, Shokat Kevan M., Hui Raymond, and Sibley L David. 2010. “Calcium-Dependent Protein Kinase 1 Is an Essential Regulator of Exocytosis in Toxoplasma.” Nature 465 (7296): 359–62. 10.1038/nature09022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourido Sebastian, Tang Keliang, and Sibley L David. 2012. “Distinct Signalling Pathways Control Toxoplasma Egress and Host-Cell Invasion.” The EMBO Journal 31 (24): 4524–34. 10.1038/emboj.2012.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Ji Su, Sasai Miwa, Ohshima Jun, Lee Youngae, Bando Hironori, Takeda Kiyoshi, and Yamamoto Masahiro. 2014. “Selective and Strain-Specific NFAT4 Activation by the Toxoplasma gondii Polymorphic Dense Granule Protein GRA6.” Journal of Experimental Medicine 211 (10): 2013–32. 10.1084/jem.20131272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino Nicole D., Panas Michael W., Franco Magdalena, Theisen Terence C., Naor Adit, Rastogi Suchita, Buchholz Kerry R., Lorenzi Hernan A., and Boothroyd John C.. 2018. “Identification of a Novel Protein Complex Essential for Effector Translocation across the Parasitophorous Vacuole Membrane of Toxoplasma gondii.” Edited by Coppens Isabelle. PLOS Pathogens 14 (1): e1006828. 10.1371/journal.ppat.1006828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti Matthias, and Spielmann Tobias. 2013. “Protein Export in Malaria Parasites: Many Membranes to Cross.” Current Opinion in Microbiology 16 (4): 445–51. 10.1016/j.mib.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews Kathryn, Kalanon Ming, Chisholm Scott A., Sturm Angelika, Goodman Christopher D., Dixon Matthew W. A., Sanders Paul R., Nebl Thomas, Fraser Fiona, Haase Silvia, McFadden Geoffrey I., Gilson Paul R., Crabb Brendan S., and de Koning-Ward Tania F.. 2013. “The Plasmodium Translocon of Exported Proteins (PTEX) Component Thioredoxin-2 Is Important for Maintaining Normal Blood-Stage Growth.” Molecular Microbiology 89 (6): 1167–86. 10.1111/mmi.12334. [DOI] [PubMed] [Google Scholar]
- Matz Joachim M., Matuschewski Kai, and Kooij Taco W.A.. 2013. “Two Putative Protein Export Regulators Promote Plasmodium Blood Stage Development in Vivo.” Molecular and Biochemical Parasitology 191 (1): 44–52. 10.1016/j.molbiopara.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Mayoral Joshua, Guevara Rebekah B., Rivera-Cuevas Yolanda, Tu Vincent, Tadakimi Tomita, Romano Julia D., Gunther-Cummins Leslie, Sidoli Simone, Coppens Isabelle, Carruthers Vernon B., and Weiss Louis M.. 2021. “Dense Granule Protein, GRA64 Interacts with Host Cell ESCRT Proteins during Toxoplasma gondii Infection.” BioRxiv. 10.1101/2021.11.02.467042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayoral Joshua, Shamamian Peter, and Weiss Louis M.. 2020. “In Vitro Characterization of Protein Effector Export in the Bradyzoite Stage of Toxoplasma gondii.” Edited by Boothroyd John C.. MBio 11 (2). 10.1128/mBio.00046-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayoral Joshua, Tomita Tadakimi, Tu Vincent, Aguilan Jennifer T., Sidoli Simone, and Weiss Louis M.. 2020. “Toxoplasma gondii PPM3C, a Secreted Protein Phosphatase, Affects Parasitophorous Vacuole Effector Export.” Edited by Blader Ira J.. PLOS Pathogens 16 (12): e1008771. 10.1371/journal.ppat.1008771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer Heather L., Snyder Lindsay M., Doherty Claire M., Fox Barbara A., Bzik David J., and Denkers Eric Y.. 2020. “Toxoplasma gondii Dense Granule Protein GRA24 Drives MyD88-Independent P38 MAPK Activation, IL-12 Production and Induction of Protective Immunity.” Edited by Gazzinelli Ricardo T.. PLOS Pathogens 16 (5): e1008572. 10.1371/journal.ppat.1008572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier Corinne, Adjogble Koku D Z, and Da Walter. 2005. “Dense Granules : Are They Key Organelles to Help Understand the Parasitophorous Vacuole of All Apicomplexa Parasites?” 35: 829–49. 10.1016/j.ijpara.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Mercier Corinne, and Cesbron-Delauw Marie-France. 2015. “Toxoplasma Secretory Granules: One Population or More?” Trends in Parasitology 31 (2): 60–71. 10.1016/j.pt.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Mercier Corinne, Dubremetz Jean-François, Rauscher Béatrice, Lecordier Laurence, Sibley L. David, and Cesbron-Delauw Marie-France. 2002. “Biogenesis of Nanotubular Network in Toxoplasma Parasitophorous Vacuole Induced by Parasite Proteins.” Edited by Lippincott-Schwartz Jennifer. Molecular Biology of the Cell 13 (7): 2397–2409. 10.1091/mbc.e02-01-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelin Adeline, Bittame Amina, Bordat Yann, Travier Laetitia, Mercier Corinne, François Dubremetz Jean-François, and Lebrun Maryse. 2009. “GRA12, a Toxoplasma Dense Granule Protein Associated with the Intravacuolar Membranous Nanotubular Network.” International Journal for Parasitology 39 (3): 299–306. 10.1016/j.ijpara.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Morano Alexander A., and Dvorin Jeffrey D.. 2021. “The Ringleaders: Understanding the Apicomplexan Basal Complex Through Comparison to Established Contractile Ring Systems.” Frontiers in Cellular and Infection Microbiology 11: 656976. 10.3389/fcimb.2021.656976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordue Dana G., Håkansson Sebastian, Niesman Ingrid, and Sibley L. David. 1999. “Toxoplasma gondii Resides in a Vacuole That Avoids Fusion with Host Cell Endocytic and Exocytic Vesicular Trafficking Pathways.” Experimental Parasitology 92 (2): 87–99. 10.1006/expr.1999.4412. [DOI] [PubMed] [Google Scholar]
- Nadipuram Santhosh M., Kim Elliot W., Vashisht Ajay A., Lin Andrew H., Bell Hannah N., Coppens Isabelle, Wohlschlegel James A., and Bradley Peter J.. 2016. “In Vivo Biotinylation of the Toxoplasma Parasitophorous Vacuole Reveals Novel Dense Granule Proteins Important for Parasite Growth and Pathogenesis.” MBio 7 (4): 1–15. 10.1128/mBio.00808-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadipuram Santhosh Mukund, Thind Amara Cervantes, Rayatpisheh Shima, Wohlschlegel James Akira, and Bradley Peter John. 2020. “Proximity Biotinylation Reveals Novel Secreted Dense Granule Proteins of Toxoplasma gondii Bradyzoites.” PLoS ONE 15 (5): 1–20. 10.1371/journal.pone.0232552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaar Valerian, Samuel Benjamin U., Ngo Emily O., and Joiner Keith A.. 1999. “Targeted Reduction of Nucleoside Triphosphate Hydrolase by Antisense RNA Inhibits Toxoplasma gondii Proliferation.” Journal of Biological Chemistry 274 (8): 5083–87. 10.1074/jbc.274.8.5083. [DOI] [PubMed] [Google Scholar]
- Nam Ho-Woo Woo. 2009. “GRA Proteins of Toxoplasma gondii: Maintenance of Host-Parasite Interactions across the Parasitophorous Vacuolar Membrane.” The Korean Journal of Parasitology 47 Suppl (SUPPL.): S29–37. 10.3347/kjp.2009.47.S.S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naor Adit, Panas Michael W., Marino Nicole, Coffey Michael J., Tonkin Christopher J., and Boothroyd John C.. 2018. “MYR1-Dependent Effectors Are the Major Drivers of a Host Cell’s Early Response to Toxoplasma, Including Counteracting MYR1-Independent Effects.” MBio 9 (2): 1–17. 10.1128/mBio.02401-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngô Huân M., Yang Mei, Paprotka Kerstin, Pypaert Marc, Hoppe Heinrich, and Joiner Keith A.. 2003. “AP-1 in Toxoplasma gondii Mediates Biogenesis of the Rhoptry Secretory Organelle from a Post-Golgi Compartment.” The Journal of Biological Chemistry 278 (7): 5343–52. 10.1074/jbc.M208291200. [DOI] [PubMed] [Google Scholar]
- Nichols Barbara A., Chiappino Mary Louise, and Pavesio Carlos E. N.. 1994. “Endocytosis at the Micropore OfToxoplasma gondii.” Parasitology Research 80 (2): 91–98. 10.1007/BF00933773. [DOI] [PubMed] [Google Scholar]
- Ning Hong-Rui, Huang Si-Yang, Wang Jin-Lei, Xu Qian-Ming, and Zhu Xing-Quan. 2015. “Genetic Diversity of Toxoplasma gondii Strains from Different Hosts and Geographical Regions by Sequence Analysis of GRA20 Gene.” The Korean Journal of Parasitology 53 (3): 345–48. 10.3347/kjp.2015.53.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan Sabrina J., Romano Julia D., and Coppens Isabelle. 2017. Host Lipid Droplets: An Important Source of Lipids Salvaged by the Intracellular Parasite Toxoplasma gondii. PLoS Pathogens. Vol. 13. 10.1371/journal.ppat.1006362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyonda Mary Akinyi, Hammoudi Pierre-Mehdi, Ye Shu, Maire Jessica, Marq Jean-Baptiste, Yamamoto Masahiro, and Soldati-Favre Dominique. 2021. “Toxoplasma gondii GRA60 Is an Effector Protein That Modulates Host Cell Autonomous Immunity and Contributes to Virulence.” Cellular Microbiology 23 (2). 10.1111/cmi.13278. [DOI] [PubMed] [Google Scholar]
- Odell Anahi V., Tran Fanny, Foderaro Jenna E., Poupart Séverine, Pathak Ravi, Westwood Nicholas J., and Ward Gary E.. 2015. “Yeast Three-Hybrid Screen Identifies TgBRADIN/GRA24 as a Negative Regulator of Toxoplasma gondii Bradyzoite Differentiation.” PloS One 10 (3): e0120331. 10.1371/journal.pone.0120331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Tadashi, Marmansari Dini, Li Zeng Mei, Adilbish Altanchimeg, Canko Shishenkov, Ueno Akio, Shono Haruhi, Furuoka Hidefumi, and Igarashi Makoto. 2013. “A Novel Dense Granule Protein, GRA22, Is Involved in Regulating Parasite Egress in Toxoplasma gondii.” Molecular and Biochemical Parasitology 189 (1–2): 5–13. 10.1016/j.molbiopara.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Olias Philipp, Etheridge Ronald D., Zhang Yong, Holtzman Michael J., and Sibley L. David. 2016. “Toxoplasma Effector Recruits the Mi-2/NuRD Complex to Repress STAT1 Transcription and Block IFN-γ-Dependent Gene Expression.” Cell Host & Microbe 20 (1): 72–82. 10.1016/j.chom.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Storch M-J, Anderson RGW, Vassalli J-D, and Perrelet A. 1987. “Proteolytic Maturation of Insulin Is a Post-Golgi Event Which Occurs in Acidifying Clathrin-Coated Secretory Vesicles.” Cell 49 (6): 865–68. 10.1016/0092-8674(87)90624-6. [DOI] [PubMed] [Google Scholar]
- Ossig R, Dascher C, Trepte HH, Schmitt HD, and Gallwitz D. 1991. “The Yeast SLY Gene Products, Suppressors of Defects in the Essential GTP-Binding Ypt1 Protein, May Act in Endoplasmic Reticulum-to-Golgi Transport.” Molecular and Cellular Biology 11 (6): 2980–93. 10.1128/mcb.11.6.2980-2993.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panas Michael W., and Boothroyd John C.. 2020. “Toxoplasma Uses GRA16 To Upregulate Host C-Myc.” Edited by Ralston Katherine S.. MSphere 5 (3). 10.1128/mSphere.00402-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panas Michael W., and Boothroyd John C.. 2020. 2021. “Seizing Control: How Dense Granule Effector Proteins Enable Toxoplasma to Take Charge.” Molecular Microbiology 115 (3): 466–77. 10.1111/mmi.14679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panas Michael W., Ferrel Abel, Naor Adit, Tenborg Elizabeth, Lorenzi Hernan A., and Boothroyd John C.. 2019. “ Translocation of Dense Granule Effectors across the Parasitophorous Vacuole Membrane in Toxoplasma- Infected Cells Requires the Activity of ROP17, a Rhoptry Protein Kinase.” MSphere 4 (4): 1–15. 10.1128/msphere.00276-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panas Michael W., Naor Adit, Cygan Alicja M., and Boothroyd John C.. 2019. “Toxoplasma Controls Host Cyclin E Expression through the Use of a Novel MYR1-Dependent Effector Protein, HCE1.” Edited by Weiss Louis M.. MBio 10 (2): 299–306. 10.1128/mBio.00674-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes-Santos TC, Martins-Duarte ES, Vitor RWA, de Souza W, Attias M, and Vommaro RC. 2013. “Spontaneous Cystogenesis in Vitro of a Brazilian Strain of Toxoplasma gondii.” Parasitology International 62 (2): 181–88. 10.1016/j.parint.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Paredes-Santos Tatiana Christina, de Souza Wanderley, and Attias Márcia. 2012. “Dynamics and 3D Organization of Secretory Organelles of Toxoplasma gondii.” Journal of Structural Biology 177 (2): 420–30. 10.1016/j.jsb.2011.11.028. [DOI] [PubMed] [Google Scholar]
- Paredes-Santos Tatiana, Wang Yifan, Waldman Benjamin, Lourido Sebastian, and Saeij Jeroen P.. 2019. “The GRA17 Parasitophorous Vacuole Membrane Permeability Pore Contributes to Bradyzoite Viability.” Frontiers in Cellular and Infection Microbiology 9 (September): 1–11. 10.3389/fcimb.2019.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Joshua J., and Loh Y. Peng. 2008. “How Peptide Hormone Vesicles Are Transported to the Secretion Site for Exocytosis.” Molecular Endocrinology (Baltimore, Md.) 22 (12): 2583–95. 10.1210/me.2008-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmley Stephen F., Yang Shumin, Harth Guenter, Sibley L. David, Sucharczuk Anita, and Remington Jack S.. 1994. “Molecular Characterization of a 65-Kilodalton Toxoplasma gondii Antigen Expressed Abundantly in the Matrix of Tissue Cysts.” Molecular and Biochemical Parasitology 66 (2): 283–96. 10.1016/0166-6851(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Peixoto Lucia, Chen Feng, Harb Omar S., Davis Paul H., Beiting Daniel P., Brownback Catie Small, Ouloguem Dinkorma, and Roos David S.. 2010. “Integrative Genomic Approaches Highlight a Family of Parasite-Specific Kinases That Regulate Host Responses.” Cell Host and Microbe 8 (2): 208–18. 10.1016/j.chom.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernas Lena, Adomako-Ankomah Yaw, Shastri Anjali J., Ewald Sarah E., Treeck Moritz, Boyle Jon P., and Boothroyd John C.. 2014. “Toxoplasma Effector MAF1 Mediates Recruitment of Host Mitochondria and Impacts the Host Response.” PLoS Biology 12 (4). 10.1371/journal.pbio.1001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernas Lena, Bean Camilla, Boothroyd John C., and Scorrano Luca. 2018. “Mitochondria Restrict Growth of the Intracellular Parasite Toxoplasma gondii by Limiting Its Uptake of Fatty Acids.” Cell Metabolism 27 (4): 886–897.e4. 10.1016/j.cmet.2018.02.018. [DOI] [PubMed] [Google Scholar]
- Pieperhoff Manuela S., Schmitt Miriam, Ferguson David J. P., and Meissner Markus. 2013. “The Role of Clathrin in Post-Golgi Trafficking in Toxoplasma gondii.” Edited by Hakimi Mohamed Ali. PLoS ONE 8 (10): e77620. 10.1371/journal.pone.0077620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pszenny Viviana, Ehrenman Karen, Romano Julia D., Kennard Andrea, Schultz Aric, Roos David S., Grigg Michael E., Carruthers Vern B., and Coppens Isabelle. 2016. “A Lipolytic Lecithin:Cholesterol Acyltransferase Secreted by Toxoplasma Facilitates Parasite Replication and Egress.” Journal of Biological Chemistry 291 (8): 3725–46. 10.1074/jbc.M115.671974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese ML, Zeiner GM, Saeij JPJ, Boothroyd JC, and Boyle JP. 2011. “Polymorphic Family of Injected Pseudokinases Is Paramount in Toxoplasma Virulence.” Proceedings of the National Academy of Sciences 108 (23): 9625–30. 10.1073/pnas.1015980108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Cuevas Yolanda, Mayoral Joshua, Di Cristina Manlio, Lawrence Anna-Lisa E., Olafsson Einar B., Patel Romir K., Thornhill Dishari, Waldman Benjamin S., Ono Akira, Sexton Jonathan Z., Lourido Sebastian, Weiss Louis M., and Carruthers Vern B.. 2021. “Toxoplasma gondii Exploits the Host ESCRT Machinery for Parasite Uptake of Host Cytosolic Proteins.” PLOS Pathogens 17 (12): e1010138. 10.1371/journal.ppat.1010138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano Julia D., de Beaumont Catherine, Carrasco Jose A., Ehrenman Karen, Bavoil Patrik M., and Coppens Isabelle. 2013. “Fierce Competition between Toxoplasma and Chlamydia for Host Cell Structures in Dually Infected Cells.” Eukaryotic Cell 12 (2): 265–77. 10.1128/EC.00313-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano Julia D., Nolan Sabrina J., Porter Corey, Ehrenman Karen, Hartman Eric J., Hsia Ru ching, and Coppens Isabelle. 2017. “The Parasite Toxoplasma Sequesters Diverse Rab Host Vesicles within an Intravacuolar Network.” Journal of Cell Biology 216 (12): 4235–54. 10.1083/jcb.201701108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano Julia D., Sonda Sabrina, Bergbower Emily, Smith Maria Elisa, and Coppens Isabelle. 2013. “Toxoplasma gondii Salvages Sphingolipids from the Host Golgi through the Rerouting of Selected Rab Vesicles to the Parasitophorous Vacuole.” Molecular Biology of the Cell 24 (12): 1974–95. 10.1091/mbc.E12-11-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommereim Leah M., Bellini Valeria, Fox Barbara A., Pètre Graciane, Rak Camille, Touquet Bastien, Aldebert Delphine, Dubremetz Jean François, Cesbron-Delauw Marie France, Mercier Corinne, and Bzik David J.. 2016. “Phenotypes Associated with Knockouts of Eight Dense Granule Gene Loci (GRA2–9) in Virulent Toxoplasma gondii.” PLoS ONE 11 (7): 1–21. 10.1371/journal.pone.0159306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommereim Leah M., Fox Barbara A., Butler Kiah L., Cantillana Viviana, Taylor Gregory A., and Bzik David J.. 2019. “Rhoptry and Dense Granule Secreted Effectors Regulate CD8+ T Cell Recognition of Toxoplasma gondii Infected Host Cells.” Frontiers in Immunology 10 (September). 10.3389/fimmu.2019.02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg Alex, and Sibley L. David. 2021. “Toxoplasma gondii Secreted Effectors Co-Opt Host Repressor Complexes to Inhibit Necroptosis.” Cell Host and Microbe 29 (7): 1186–1198.e8. 10.1016/j.chom.2021.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosowski Emily E., Lu Diana, Julien Lindsay, Rodda Lauren, Gaiser Rogier A., Jensen Kirk D.C., and Saeij Jeroen P.J.. 2011. “Strain-Specific Activation of the NF-KB Pathway by GRA15, a Novel Toxoplasma gondii Dense Granule Protein.” Journal of Experimental Medicine 208 (1): 195–212. 10.1084/jem.20100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudzki Elizabeth N., Ander Stephanie E., Coombs Rachel S., Alrubaye Hisham S., Cabo Leah F., Blank Matthew L., Gutiérrez-Melo Nicolás, Dubey JP, Coyne Carolyn B., and Boyle Jon P.. 2021. “Toxoplasma gondii GRA28 Is Required for Placenta-Specific Induction of the Regulatory Chemokine CCL22 in Human and Mouse.” MBio 12 (6): e0159121. 10.1128/mBio.01591-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo Ilaria, Babbitt Shalon, Muralidharan Vasant, Butler Tamira, Oksman Anna, and Goldberg Daniel E.. 2010. “Plasmepsin V Licenses Plasmodium Proteins for Export into the Host Erythrocyte.” Nature 463 (7281): 632–36. 10.1038/nature08726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeij JPJ, Boyle JP, Coller S, Taylor S, Sibley LD, Brooke-Powell ET, Ajioka JW, and Boothroyd JC. 2006. “Polymorphic Secreted Kinases Are Key Virulence Factors in Toxoplasmosis.” Science 314 (5806): 1780–83. 10.1126/science.1133690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeij Jeroen P., and Frickel Eva-Maria. 2017. “Exposing Toxoplasma gondii Hiding inside the Vacuole: A Role for GBPs, Autophagy and Host Cell Death.” Current Opinion in Microbiology 40 (December): 72–80. 10.1016/j.mib.2017.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders Paul R., Dickerman Benjamin K., Charnaud Sarah C., Ramsland Paul A., Crabb Brendan S., and Gilson Paul R.. 2019. “The N-Terminus of EXP2 Forms the Membrane-Associated Pore of the Protein Exporting Translocon PTEX in Plasmodium falciparum.” The Journal of Biochemistry 165 (3): 239–48. 10.1093/jb/mvy099. [DOI] [PubMed] [Google Scholar]
- Sauvola Chad W., and Littleton J Troy. 2021. “SNARE Regulatory Proteins in Synaptic Vesicle Fusion and Recycling.” Frontiers in Molecular Neuroscience 14: 733138. 10.3389/fnmol.2021.733138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz Aric J., and Carruthers Vern B.. 2018. “Toxoplasma gondii LCAT Primarily Contributes to Tachyzoite Egress.” MSphere 3 (1): 1–10. 10.1128/mspheredirect.00073-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab JC, Beckers CJM, and Joiner KA. 1994. “The Parasitophorous Vacuole Membrane Surrounding Intracellular Toxoplasma gondii Functions as a Molecular Sieve.” Proceedings of the National Academy of Sciences of the United States of America 91 (2): 509–13. 10.1073/pnas.91.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seizova Simona, Ruparel Ushma, Garnham Alexandra L., Bader Stefanie M., Uboldi Alessandro D., Coffey Michael J., Whitehead Lachlan W., Rogers Kelly L., and Tonkin Christopher J.. 2022. “Transcriptional Modification of Host Cells Harboring Toxoplasma gondii Bradyzoites Prevents IFN Gamma-Mediated Cell Death.” Cell Host and Microbe 30 (2): 232–247.e6. 10.1016/j.chom.2021.11.012. [DOI] [PubMed] [Google Scholar]
- Shastri Anjali J., Marino Nicole D., Franco Magdalena, Lodoen Melissa B., and Boothroyd John C.. 2014. “GRA25 Is a Novel Virulence Factor of Toxoplasma gondii and Influences the Host Immune Response.” Infection and Immunity 82 (6): 2595–2605. 10.1128/IAI.01339-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw MK, Roos DS, and Tilney LG. 1998. “Acidic Compartments and Rhoptry Formation in Toxoplasma gondii.” Parasitology 117 (5): 435–43. 10.1017/S0031182098003278. [DOI] [PubMed] [Google Scholar]
- Sheffield Harley G., and Melton Marjorie L.. 1968. “The Fine Structure and Reproduction of Toxoplasma gondii.” The Journal of Parasitology 54 (2): 209–26. 10.2307/3276925. [DOI] [PubMed] [Google Scholar]
- Shen Bang, Brown Kevin M., Lee Tobie D., and Sibley L. David. 2014. “Efficient Gene Disruption in Diverse Strains of Toxoplasma gondii Using CRISPR/CAS9.” Edited by Weiss Louis M.. MBio 5 (3). 10.1128/mBio.01114-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley LD, Niesman IR, Parmley SF, and Cesbron-Delauw MF. 1995. “Regulated Secretion of Multi-Lamellar Vesicles Leads to Formation of a Tubulovesicular Network in Host-Cell Vacuoles Occupied by Toxoplasma gondii.” Journal of Cell Science 108 (4): 1669–77. 10.1242/jcs.108.4.1669. [DOI] [PubMed] [Google Scholar]
- Sibley L. David, Niesman Ingrid R., Asai Takashi, and Takeuchi Tsutomu. 1994. “Toxoplasma gondii: Secretion of a Potent Nucleoside Triphosphate Hydrolase into the Parasitophorous Vacuole.” Experimental Parasitology. 10.1006/expr.1994.1093. [DOI] [PubMed] [Google Scholar]
- Sidik Saima M., Hackett Caroline G., Tran Fanny, Westwood Nicholas J., and Lourido Sebastian. 2014. “Efficient Genome Engineering of Toxoplasma gondii Using CRISPR/Cas9.” Edited by Blader Ira J.. PLoS ONE 9 (6): e100450. 10.1371/journal.pone.0100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidik Saima M., Triana Miryam A. Hortua, Paul Aditya S., El Bakkouri Majida, Hackett Caroline G., Tran Fanny, Westwood Nicholas J., Hui Raymond, Zuercher William J., Duraisingh Manoj T., Moreno Silvia N.J., and Lourido Sebastian. 2016. “Using a Genetically Encoded Sensor to Identify Inhibitors of Toxoplasma gondii Ca2+ Signaling.” Journal of Biological Chemistry 291 (18): 9566–80. 10.1074/jbc.M115.703546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidik Saima M., Huet Diego, Ganesan Suresh M., Huynh My-Hang, Wang Tim, Nasamu Armiyaw S., Thiru Prathapan, Saeij Jeroen P.J., Carruthers Vern B., Niles Jacquin C., and Lourido Sebastian. 2016. “A Genome-Wide CRISPR Screen in Toxoplasma Identifies Essential Apicomplexan Genes.” Cell 166 (6): 1423–1435.e12. 10.1016/j.cell.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinai Anthony P, Webster Paul, and Joiner Keith A. 1997. “Association of Host Cell Endoplasmic Reticulum and Mitochondria with the Toxoplasma gondii Parasitophorous Vacuole Membrane: A High Affinity Interaction.” Journal of Cell Science 110 (Pt 1 (September): 2117–28. http://www.ncbi.nlm.nih.gov/pubmed/9378762. [DOI] [PubMed] [Google Scholar]
- Sleebs Brad E., Lopaticki Sash, Marapana Danushka S., O’Neill Matthew T., Rajasekaran Pravin, Gazdik Michelle, Günther Svenja, Whitehead Lachlan W., Lowes Kym N., Barfod Lea, Hviid Lars, Shaw Philip J., Hodder Anthony N., Smith Brian J., Cowman Alan F., and Boddey Justin A.. 2014. “Inhibition of Plasmepsin V Activity Demonstrates Its Essential Role in Protein Export, PfEMP1 Display, and Survival of Malaria Parasites.” PLoS Biology. 10.1371/journal.pbio.1001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparvoli Daniela, and Lebrun Maryse. 2021. “Unraveling the Elusive Rhoptry Exocytic Mechanism of Apicomplexa.” Trends in Parasitology 37 (7): 622–37. 10.1016/j.pt.2021.04.011. [DOI] [PubMed] [Google Scholar]
- Striepen B, He CY, Matrajt M, Soldati D, and Roos DS. 1998. “Expression, Selection, and Organellar Targeting of the Green Fluorescent Protein in Toxoplasma gondii.” Molecular and Biochemical Parasitology 92 (2): 325–38. 10.1016/s0166-6851(98)00011-5. [DOI] [PubMed] [Google Scholar]
- Striepen B, Soldati D, Garcia-Reguet N, Dubremetz JF, and Roos DS. 2001. “Targeting of Soluble Proteins to the Rhoptries and Micronemes in Toxoplasma gondii.” Molecular and Biochemical Parasitology 113 (1): 45–53. 10.1016/s0166-6851(00)00379-0. [DOI] [PubMed] [Google Scholar]
- Taylor S, Barragan A, Su C, Fux B, Fentress SJ, Tang K, Beatty WL, El Hajj H, Jerome M, Behnke MS, White M, Wootton JC, and Sibley LD. 2006. “A Secreted Serine-Threonine Kinase Determines Virulence in the Eukaryotic Pathogen Toxoplasma gondii.” Science 314 (5806): 1776–80. 10.1126/science.1133643. [DOI] [PubMed] [Google Scholar]
- Tomita Tadakimi, Bzik David J., Ma Yan Fen, Fox Barbara A., Markillie Lye Meng, Taylor Ronald C., Kim Kami, and Weiss Louis M.. 2013. “The Toxoplasma gondii Cyst Wall Protein CST1 Is Critical for Cyst Wall Integrity and Promotes Bradyzoite Persistence.” Edited by Soldati-Favre Dominique. PLoS Pathogens 9 (12): e1003823. 10.1371/journal.ppat.1003823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita Tadakimi, Guevara Rebekah B., Shah Lamisha M., Afrifa Andrews Y., and Weiss Louis M.. 2021. “Secreted Effectors Modulating Immune Responses to Toxoplasma gondii.” Life 11 (9): 988. 10.3390/life11090988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita Tadakimi, Mukhopadhyay Debanjan, Han Bing, Yakubu Rama, Tu Vincent, Mayoral Joshua, Sugi Tatsuki, Ma Yanfen, Saeij Jeroen P.J., and Weiss Louis M.. 2021. “Toxoplasma gondii Matrix Antigen 1 Is a Secreted Immunomodulatory Effector.” MBio 12 (3): 1–16. 10.1128/mBio.00603-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travier Laetitia, Mondragon Ricardo, Dubremetz Jean-François, Musset Karine, Mondragon Monica, Gonzalez Sirenia, Cesbron-Delauw Marie-France, and Mercier Corinne. 2008. “Functional Domains of the Toxoplasma GRA2 Protein in the Formation of the Membranous Nanotubular Network of the Parasitophorous Vacuole.” International Journal for Parasitology 38 (7): 757–73. 10.1016/j.ijpara.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Treeck Moritz, Sanders John L., Elias Joshua E., and Boothroyd John C.. 2011. “The Phosphoproteomes of Plasmodium falciparum and Toxoplasma gondii Reveal Unusual Adaptations Within and Beyond the Parasites’ Boundaries.” Cell Host & Microbe 10 (4): 410–19. 10.1016/j.chom.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Vincent, Mayoral Joshua, Sugi Tatsuki, Tomita Tadakimi, Han Bing, Ma Yan Fen, and Weissa Louis M.. 2019. “Enrichment and Proteomic Characterization of the Cyst Wall from in Vitro Toxoplasma gondii Cysts.” MBio 10 (2): 1–15. 10.1128/mBio.00469-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Vincent, Tomita Tadakimi, Sugi Tatsuki, Mayoral Joshua, Han Bing, Yakubu Rama R, Williams Tere, Horta Aline, Ma Yanfen, and Weiss Louis M. 2020. “The Toxoplasma gondii Cyst Wall Interactome.” Edited by Koshy Anita A.. MBio 11 (1): e02699–19. 10.1128/mBio.02699-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal Kannan, Chehade Sylia, Werkmeister Elisabeth, Barois Nicolas, Periz Javier, Lafont Frank, Tardieux Isabelle, Khalife Jamal, Langsley Gordon, Meissner Markus, and Marion Sabrina. 2020. “Rab11A Regulates Dense Granule Transport and Secretion during Toxoplasma gondii Invasion of Host Cells and Parasite Replication.” Edited by Blackman Michael J.. PLoS Pathogens 16 (5): e1008106. 10.1371/journal.ppat.1008106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal Kannan, and Marion Sabrina. 2018. “Secretory Organelle Trafficking in Toxoplasma gondii: A Long Story for a Short Travel.” International Journal of Medical Microbiology : IJMM 308 (7): 751–60. 10.1016/j.ijmm.2018.07.007. [DOI] [PubMed] [Google Scholar]
- Venugopal Kannan, Werkmeister Elisabeth, Barois Nicolas, Saliou Jean-Michel, Poncet Anais, Huot Ludovic, Sindikubwabo Fabien, Hakimi Mohamed Ali, Langsley Gordon, Lafont Frank, and Marion Sabrina. 2017. “Dual Role of the Toxoplasma gondii Clathrin Adaptor AP1 in the Sorting of Rhoptry and Microneme Proteins and in Parasite Division.” PLoS Pathogens 13 (4): e1006331. 10.1371/journal.ppat.1006331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhage Matthijs, and Sørensen Jakob B. 2008. “Vesicle Docking in Regulated Exocytosis.” Traffic (Copenhagen, Denmark) 9 (9): 1414–24. 10.1111/j.1600-0854.2008.00759.x. [DOI] [PubMed] [Google Scholar]
- Walker Margaret E., Hjort Elizabeth E., Smith Sherri S., Tripathi Abhishek, Hornick Jessica E., Hinchcliffe Edward H., Archer William, and Hager Kristin M.. 2008. “Toxoplasma gondii Actively Remodels the Microtubule Network in Host Cells.” Microbes and Infection 10 (14–15): 1440–49. 10.1016/j.micinf.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walworth NC, and Novick PJ. 1987. “Purification and Characterization of Constitutive Secretory Vesicles from Yeast.” The Journal of Cell Biology 105 (1): 163–74. 10.1083/jcb.105.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Peiyan, Li Siji, Zhao Yingchi, Zhang Baohuan, Li Yunfei, Liu Shengde, Du Hongqiang, Cao Lili, Ou Meiling, Ye Xiaohong, Li Peng, Gao Xiang, Wang Penghua, Jing Chunxia, Shao Feng, Yang Guang, and You Fuping. 2019. “The GRA15 Protein from Toxoplasma gondii Enhances Host Defense Responses by Activating the Interferon Stimulator STING.” Journal of Biological Chemistry 294 (45): 16494–508. 10.1074/jbc.RA119.009172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, and Hsu SC. 2006. “The Molecular Mechanisms of the Mammalian Exocyst Complex in Exocytosis.” Biochemical Society Transactions 34 (Pt 5): 687–90. 10.1042/BST0340687. [DOI] [PubMed] [Google Scholar]
- Wang Tuanlao, Li Liangcheng, and Hong Wanjin. 2017. “SNARE Proteins in Membrane Trafficking.” Traffic (Copenhagen, Denmark) 18 (12): 767–75. 10.1111/tra.12524. [DOI] [PubMed] [Google Scholar]
- Wang Yifan, Cirelli Kimberly M., Barros Patricio D.C., Sangaré Lamba Omar, Butty Vincent, Hassan Musa A., Pesavento Patricia, Mete Asli, and Saeij Jeroen P.J.. 2019. “Three Toxoplasma gondii Dense Granule Proteins Are Required for Induction of Lewis Rat Macrophage Pyroptosis.” MBio 10 (1): 1–20. 10.1128/mBio.02388-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Yifan, Sangaré Lamba Omar, Paredes-Santos Tatiana C., Hassan Musa A., Krishnamurthy Shruthi, Furuta Anna M., Markus Benedikt M., Lourido Sebastian, and Saeij Jeroen P.J. J. 2020. “Genome-Wide Screens Identify Toxoplasma gondii Determinants of Parasite Fitness in IFNγ-Activated Murine Macrophages.” Nature Communications 11 (1): 5258. 10.1038/s41467-020-18991-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts Elizabeth, Zhao Yihua, Dhara Animesh, Eller Becca, Patwardhan Abhijit, and Sinai Anthony P.. 2015. “Novel Approaches Reveal That Toxoplasma gondii Bradyzoites within Tissue Cysts Are Dynamic and Replicating Entities in Vivo.” MBio 6 (5). 10.1128/mBio.01155-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss Louis M., and Dubey Jitender P.. 2009. “Toxoplasmosis: A History of Clinical Observations.” International Journal for Parasitology 39 (8): 895–901. 10.1016/j.ijpara.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witola William H., Bauman Bretta, McHugh Mark, and Matthews Kwame. 2014. “Silencing of GRA10 Protein Expression Inhibits Toxoplasma gondii Intracellular Growth and Development.” Parasitology International 63 (5): 651–58. 10.1016/j.parint.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Wu Liang, Shen Jin, Tang Chenyu, Jiang Xugan, Wang Jiajian, Jin Xiaoling, Qiu Jinbo, Chen Shengxia, and Cao Jianping. 2017. “Increased Expression of Toxoplasma gondii GRA1 Suppresses Host Cell Apoptosis.” Journal of Bacteriology & Mycology: Open Access 4 (5). 10.15406/jbmoa.2017.04.00110. [DOI] [Google Scholar]
- Young Joanna C., Broncel Malgorzata, Teague Helena, Russell Matt R. G., McGovern Olivia L., Renshaw Matt, Frith David, Snijders Ambrosius P., Collinson Lucy, Carruthers Vern B., Ewald Sarah E., and Treeck Moritz. 2020. “ Phosphorylation of Toxoplasma gondii Secreted Proteins during Acute and Chronic Stages of Infection.” MSphere 5 (5): 1–21. 10.1128/msphere.00792-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Yi Wei, Halonen Sandra K., Ma Yan Fen, Wittner Murray, and Weiss Louis M.. 2020. “Initial Characterization of CST1, a Toxoplasma gondii Cyst Wall Glycoprotein.” Edited by Petri WA. Infection and Immunity 69 (1): 501–7. 10.1128/IAI.69.1.501-507.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION
Table S1. List of known GRA proteins. * indicates proteins shown to be cleaved by ASP5. Accession numbers and CRISPR scores were obtained from ToxoDB (www.ToxoDB.org) (Amos et al. 2022).


