Abstract
Extracellular matrices (ECMs), such as the cell walls and biofilms, are important for supporting cell integrity, function, and regulating intercellular communication. These biomaterials are also of significant interest to the production of biofuels and the development of antimicrobial treatment. Solid-state nuclear magnetic resonance (ssNMR) and Magic Angle Spinning-Dynamic Nuclear Polarization (MAS-DNP) are uniquely powerful for understanding the conformational structure, dynamical characteristics, and supramolecular assemblies of carbohydrates and other biomolecules in ECMs. This review highlights the recent high-resolution investigations of intact ECMs and native cells in many organisms spanning across plants, bacteria, fungi, and algae. We spotlight the structural principles identified in ECMs, discuss the current technical limitation and underexplored biochemical topics, and point out the promising opportunities enabled by the recent advances of the rapidly evolving ssNMR technology.
Graphical Abstract

1. Introduction
1.1. Current Status of NMR Research on Extracellular Matrices
Exposed to environmental stresses, living organisms have developed the extracellular matrix (ECM) to regulate the integrity and communication of cells. Although the plasma membrane determines the cell border, bacterial, fungal, plant, and algal cells are further covered by a carbohydrate-rich layer called cell wall, which plays pivotal roles in protecting the cell cytoplasm from contaminants and other stresses and in supporting cellular growth and differentiation1–4. Bacterial and fungal ECMs, such as cell walls and biofilms, are associated with toxicity and virulence and serve as the targets of many antifungals and antibiotics5–7. The ECM materials are highly diverse in molecular composition and ultrastructure, accommodating carbohydrates, proteins, lipids, polyphenols, and occasionally some inorganic components. A large collection of methods based on vibrational spectroscopy, biochemical assays, chromatography coupled with mass spectrometry, neutron, and X-ray diffraction, as well as imaging methods have been employed to analyze the composition and architecture of these biocomposites on different length scales, from covalent linkage patterns to microscopic-level arrangement of polymer meshes8–12.
Benefited from the nondestructive nature and atomic-level resolution, nuclear magnetic resonance (NMR) has proven to be a useful tool for analyzing biomolecules in many organisms13. The number of annual NMR publications on topics related to plants, bacteria, algae, and fungi has increased by 9-fold over the past three decades, from slightly over 300 papers in 1991 to more than 2,700 studies in 2020, with around 37,000 accumulated studies over this period (Figure 1). The number of studies decreases sequentially in the research fields of plants, bacteria, fungi, and then algae. Most solution NMR investigations focus on metabolite identification and structural determination, but isolation, chemical modification, and solubilization procedures are often required before ECM material could be subjected to structural characterization, which can compromise the physical properties and chemical structures of biomacromolecules14,15. In response to this technical obstacle, solid-state NMR (ssNMR) has emerged as a promising technique for investigating intact ECM material using intact insoluble materials and even whole cells and organisms that are free from chemical treatments16–20. Since 1991, around 1,700 ssNMR studies have been conducted on bacteria, fungi, algae, and plants, with the majority (more than 1,000 articles) published after 2011. These ssNMR investigations were mainly focused on plants (47%) and bacteria (32%), while fungi (17%) and algae (4%) are under-investigated. Expedited by the development of high-field ssNMR and magic-angle spinning dynamic nuclear polarization (MAS-DNP) techniques21–24, a rapid expansion of ssNMR applications on ECM materials is expected in the near future.
Figure 1. NMR studies of various organisms.

A simplified phylogenetic tree of organisms based on genetic sequencing presents the diversity and evolutionary relationships between biological species25,26. The organisms described in this review are in bold, with their associated number of NMR publications summarized as four separate panels. The annual number of publications between 1991 and 2020 has been plotted using data indexed by Web of Science. Asterisks in the central plot indicate other organisms that have also been categorized. Most of the published works were focused on plants or bacteria.
This review emphasizes the cell walls and biofilms of two pathogenic microbes, namely bacteria and fungi, as well as the biomass of two photosynthetic organisms, i.e., algae and plants. We will focus on the panorama of ssNMR research published in the last decade, which has combined structural and dynamical angles to describe the nanoscale architecture of cell walls. Specific consideration is devoted to the applications of multidimensional (2D/3D) correlation methods to isotopically enriched cell wall materials17,18, providing sufficient sensitivity and resolution for understanding such complex biosystems. We will highlight the experimental protocols and data interpretation pathways for deriving the varied compositions and polymorphic structures of biomacromolecules along with their nanoscale assembly and the formation of integrated networks. We will also discuss the possibility of investigating unlabeled biomaterials as enabled by technical advances in MAS-DNP and proton-detection techniques27,28. The methodology and structural schemes summarized here will guide the ongoing investigations of the modes of action and structural effects of antimicrobial therapies and facilitate the development of cost-effective production of bioenergy and biopolymers.
1.2. Structural Components of Extracellular Matrices
ECM biomaterials are hierarchical assemblies of covalently linked and/or physically stacked polysaccharides and glycoconjugates, which are formed by an array of relatively simple building blocks: the monosaccharides (Figure 2). These supramolecular networks are highly complex and diverse in structures and functions. The organization of bacterial cell walls can substantially differ in the gram-positive (such as Staphylococcus aureus) and gram-negative (such as Escherichia coli) species, leading to distinct cell morphologies and different mechanisms of action for antibiotics2,29. Underneath the capsule of gram-positive bacteria, there is a cell-wall layer rich in peptidoglycan (PG) anchored in the plasma membrane via lipoteichoic acid (LTA), which assemble into a thick and compact crust covering the cell surface. On the contrary, gram-negative bacteria have a thin layer of periplasmic peptidoglycan that is sandwiched between the permeable lipopolysaccharide (LPS)-rich outer membrane and the internal phospholipid-rich plasma membrane. A fundamental molecule present in both groups is the PG, which is made up of linear polysaccharides (typically 50–250 disaccharide long) and linked to small peptides (usually up to five amino acid residues)7,30.
Figure 2. Simplified representation of cell walls and biomolecules studied by ssNMR.

Built from very fundamental monosaccharide units (central panel), the polymerized carbohydrate components exhibit highly diverse structures and can associate with each other and other molecules to form the cell wall materials in bacteria (top left), plants (top right), fungi (bottom left), and algae (bottom right). The structural schemes of molecules and ECMs are briefly summarized in section 1.2 and will guide the detailed discussions in later sections.
Bacteria infect animal tissues not only as a single cell but also through a multicellular arrangement called biofilm. Biofilms enable the cohabitation of cells and preserve a safe environment for bacteria communities to evolve and resist chemical and physiological reactions, thus becoming a major contributor to the globally increasing multidrug resistance31,32. On the other hand, such biomaterial also has promising applications in aquatic biofouling33 and medical sterilization32. Biofilms are mostly made of proteins (e.g., functional amyloids) but can also be accompanied by other biomolecules including polysaccharides (such as cellulose derivatives) and lipid components34–36.
The cell wall is also present in another pathogenic microorganism, the fungus. Over three million patients are exposed to invasive fungal infections annually and around half of them die despite antifungal treatment5,37,38. Fungal infections can also cause morbidities such as asthma, allergy, chronic skin infections, and keratitis5. In addition, immunosuppressive medical interventions, the widespread cases of AIDS, and the outbreak of the COVID-19 pandemic rampantly elevate the risk of fungi infections39,40. The mechanical strength and plasticity of fungal cell walls are essential for preserving cellular viability, adjusting cellular permeability, and enduring osmotic stresses41,42. Some of the cell wall components, such as β-glucans and mannans, are the main targets for antibodies in the diagnosis of fungal infection43. Benefited from their unique chemical identities and absence in the host cells, fungal polysaccharides are also ideal targets for antifungal agents. Recently, three inhibitors of β-1,3-glucan biosynthesis, all of which are lipopeptide compounds from the echinocandin family, have been clinically approved and are currently used in medical therapies44,45.
As mentioned, fungal cell walls are primarily made up of polysaccharides and glycoproteins, together with a low amount of pigments. These molecules are often immunogenic and promote cellular and humoral distortions when infections occur46. The major carbohydrate components include three commonly found polysaccharides, namely the partially crystalline and rigid chitin (a long-chain polymer of N-acetylglucosamine), the crosslinking β-glucans, and the surface-exposed and protein-associated mannans (Figure 2)47. In addition, galactosaminogalactan (GAG) and α-1,3-glucans represent two under-investigated molecules unique to certain fungal species48. The fungal cell wall is structurally dynamic, with a variable composition depending on the species, cell types (for example, hyphae or conidia), age, and environmental stresses. Previous ssNMR studies have primarily focused on three pathogenic fungi, Cryptococcus neoformans, Aspergillus fumigatus, and Candida albicans49–52, but the territory is rapidly expanding to other fungal species.
Plants and algae share a history of over 1 billion years through their ability to perform photosynthesis, with cell walls being the product of energy conversion and carbon capture53. As ancestors of higher plants, most microalgae are protected by a plant-like cell wall. However, a glycoprotein-rich cell wall is frequently found in Volvocales (e.g., Chlamydomonas sp.), while partially mineralized fungi-like matrices with a high amount of chitin protect some diatoms such as Cyclotella and Thalassiosira (Figure 2)54–56.
Land plants evolved from Charophyceae microalgae57, which conquered freshwater habitats, but both are thought to be the result of endosymbiosis with a cyanobacterium53,58,59. While displaying a diverse geometry according to their function60, plant cells are typically made of two distinct walls. Present in all plants and formed during cytokinesis, the primary cell wall is characterized by a high extendibility to accommodate plant growth61,62. The secondary cell wall is usually deposited after cell expansion has ceased, further strengthening the resilience and mechanical integrity63. Both cell walls contain rigid scaffolds of cellulose microfibrils enclosed in a soft matrix, which is a mesh formed by hemicellulose and pectin in primary cell walls but changes to a hemicellulose and lignin mixture in secondary cell walls64–67. Understanding the polymer network in such lignocellulosic biomass will facilitate the development of more digestible crops and cost-effective conversion technology for biofuel production68–70.
2. Solid-State NMR Spectroscopy and MAS-DNP Methodology
2.1. A Practical Guide of ssNMR Techniques for ECM Characterization
The use of ssNMR technique minimizes and sometimes eliminates the need for purification and isolation, allowing for the characterization of intact ECM materials or native cells. However, these advantages come with two challenges: the intrinsically low sensitivity of NMR and the often-insufficient resolution for cellular studies. Here we briefly discuss the fundamental phenomena underlying the technical difficulties in investigating ECM materials. More in-depth descriptions of NMR spectroscopy can be found in multiple references71–73.
Many of the ssNMR studies of ECMs are using intact cells or tissues. In-cell ssNMR studies can be conducted on four categories of samples, including purified molecules or simplified and reconstituted systems (in vitro), extracted but structurally preserved macromolecular complexes such as the cell envelope (ex vivo/ex situ), intact but metabolically compromised cells (in situ/in cell/whole cell; often without awareness of the cell viability), and recently, living cells directly packed into MAS rotors (in vivo)49,50,74–82. Studying living cells using ssNMR presents an attractive research avenue, but careful consideration should be given regarding the sample preparation and experimental conditions. Recently, Frederick et al. have proposed the use of viability measurements before and after the experiment to closely monitor the cell state under MAS-DNP investigations83. Since the spinning of the sample can induce centrifugal and dehydrating effects, the experimental time and spinning frequency should be moderated to maintain cell integrity and viability. Other factors include the physiological concentration of cells, the heating effect due to radiofrequency pulses, and the limited availability of media and nutrients in MAS rotors.
Solid-state NMR detects signals coming from the excitation of nuclear spins ½ and above. Nuclear spins are sensitive to local chemical environments and atomic interactions, and therefore enable studying structural and dynamical parameters13,84. A large variety of nuclei are used in the investigation of biomolecules and functional materials, from protein structure determination to the functional scheme of catalysts and batteries (Figure 3A). Carbon-13 (13C) is usually the standard nucleus for cellular ssNMR as its chemical shift spans a sufficiently large range to resolve a huge number of chemically inequivalent carbon sites in carbohydrates, lipids, proteins, and polyphenol polymers85. Since 13C isotope has a small gyromagnetic ratio and a low natural abundance of 1.1%, isotopic enrichment is generally required to circumvent the challenges of limited sensitivity80,86. Proton (1H) and oxygen-17 (17O) are also pertinent nuclei for most biomolecules considering their elemental composition, but they are still underused in ECM research mainly due to the limited dispersion of chemical shifts or low abundance of these isotope87–89. Nitrogen-15 (15N) is useful for characterizing protein constituents and nitrogenated polysaccharides (such as chitin, chitosan, and GAG), and 31P and 2H are the key isotopes for characterizing lipid molecules such as glycolipids and phospholipids90–92. 2H and 19F are potential replacements of 1H with minimal structural perturbation93–97, thus exhibiting an increasing interest in biomolecular ssNMR. Multiple exotic nuclei, such as 29Si, 11B, 43Ca, and 33S, might be of potential applications to the bioinorganic centers of ECMs, such as the borate esters and calcium egg boxes in pectin complexes as well as algal biosilica and sulfated molecules.
Figure 3. Technical aspects of ssNMR spectroscopy.
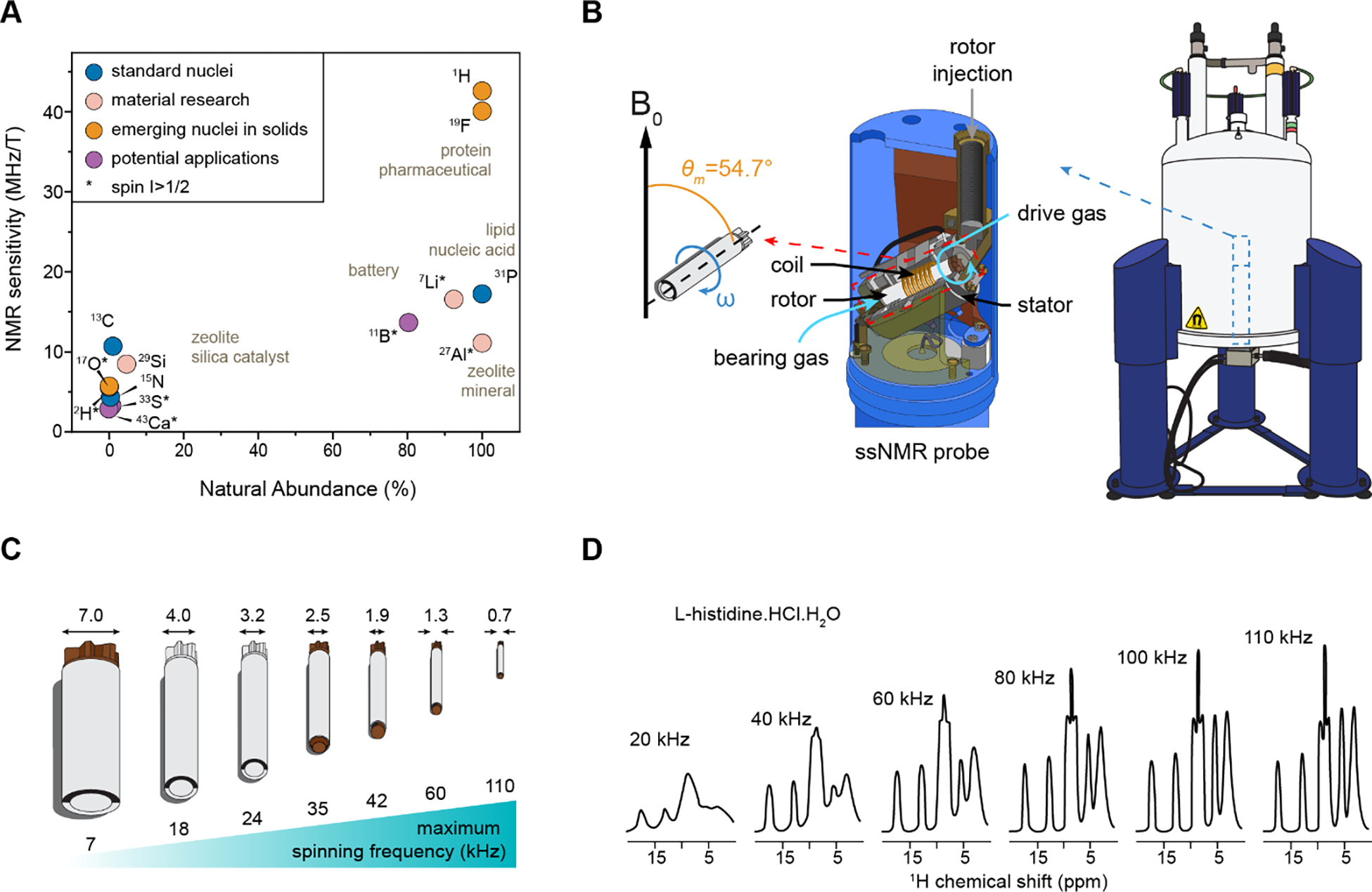
(A) Typical NMR nuclei exploited in biomolecular and material research showing different sensitivities and natural abundances. 13C, 15N, and 31P are the most common nuclei used for ECM research while 1H, 19F, and 17O are still underused. Quadrupolar nuclei are indicated using an asterisk. Common nuclei used in material research are also shown. (B) Solid-state NMR probe with radiofrequency emission and detection (coil), as well as bearing and driving gas for the MAS of the rotor containing the sample. (C) Various rotor diameters (mm) containing different sample quantities for reaching different MAS frequencies (kHz). (D) Proton detection in solids enabled by ultrafast MAS. The resolution increases with spinning speed. Panel D is adapted with permission from reference98. Copyright 2016 Elsevier.
For solid-state samples, a complication appears: the chemical shift and many spin-spin interactions are orientation-dependent, which leads to broad spectra under static and non-oriented conditions, limiting both resolution and sensitivity. Magic-Angle Spinning (MAS) has thus been developed to average these anisotropic interactions99,100. Nowadays, static experiments are used only for specific cases and most ssNMR studies are conducted under MAS conditions. From the experimental perspective, a biological sample is packed into an MAS rotor to spin at an angle of 54.74° relative to the static magnetic field direction (Figure 3B). The outer diameter of commercial rotors typically varies from 0.7 to 7 mm, which can contain 0.5 to 500 mg of materials (Figure 3C). The MAS frequencies are mainly restricted by the rotor size; for example, a 0.7 mm rotor enables stable ultrafast spinning up to 111 kHz, which efficiently averages out 1H-1H dipolar couplings and enables high-resolution 1H detection of protonated solids (Figure 3D)88,101–103.
The NMR signal originates from the non-uniform distribution of the spin population. In particular, the population difference for spins distributed in different energy levels split by the Zeeman interactions (and further by inter-spin interactions) is the prime origin of the magnetic resonance phenomenon. The smaller the population difference the lower the detected signal, and the lower the sensitivity. Unfortunately, the energy diagram spans in the tens to hundreds of MHz regime which leads to a very small population difference when compared with the total number of spins in each state (in the range of 10−5-10−4). This energy scale is what makes NMR a high-resolution technique but also one of the least-sensitive spectroscopic methods. The sensitivity of ssNMR is often evaluated using the signal-to-noise (S/N) ratio that can be calculated using collected spectra104,105:
| (1) |
Better S/N ratios can be obtained by packing more sample or isotopically labeling the material to increase the number of spins (n), elongating the experimental time (t) to collect more scans if the interscan delay time (recycle delays) is fixed, lowering the temperature (T), measuring the sample on a larger magnet with higher field strength (B0), optimizing the 1H-13C cross polarization efficiency (fCP), and building better probes with optimized parameters for coil filling (η), coil volume (V), and coil quality (Q). The signal-to-noise ratio will be increased, ideally in proportion to the square root of the number of scans. Two additional factors are the gyromagnetic ratios of the excited (γe) and detected nucleus (γd), which inspired the development of many sensitivity-enhancing methods, including cross polarization (CP) and DNP, to transfer polarization from the “more-sensitive” nuclei or even electrons to the “less-sensitive” nuclei.
We choose 13C as the standard nucleus for ECM studies for its abundance, nontoxic enrichment, reasonable sensitivity, spin ½ nature (Figure 3A), and more importantly, for its well-dispersed chemical shifts. Chemical shifts (δ) are sensitive indicators of chemical environments and are typically reported in parts per million (ppm) as defined by:
| (2) |
where the difference of observed and reference frequencies are normalized by the reference frequency after considering a 106 factor. The reference frequency can be obtained using the standard resonance frequency of nuclei in a standard compound such as tetramethylsilane (TMS; most used for ECM studies) and sodium trimethylsilylpropanesulfonate (DSS; more applicable to protein research). Resonance frequencies of given nuclei slightly vary depending on the local chemical environments, resulting in resolvable peaks in an NMR spectrum. The smaller and larger chemical shifts are sometimes described as “upfield” (or more shielded) and “downfield” (or less shielded), respectively. These terms describe the effective field experienced by a nucleus, which has been reduced (shielded) or increased (deshielded) by the additional magnetic field created by the surrounding electrons when compared to a reference compound.
To rapidly screen a large collection of cells and ECM materials, one-dimensional 13C spectra are typically measured using different ways of creating the initial magnetization (Figure 4A, B)80. J-coupling-mediated 1H-13C INEPT spectra are commonly used to select the highly dynamic polymers and largely solvated molecules106. Direct polarization (DP) of 13C can either give quantitative detection of all carbons by using sufficiently long recycle delays (the interscan delay) or provide selective detection of mobile molecules by using short recycle delays. For a carbon site, a long recycle delay set equivalent to 4–5 times of its 13C spin-lattice (T1) relaxation time constants can efficiently detect 98.2–99.3% of the carbons (thus giving quantitative detection) while a short recycle delay of a quarter of the 13C-T1 can only detect 22% of the signal (thus being suppressed and filtered). The dipolar-mediated 1H-13C CP preferentially detects the rigid molecules with stronger 1H–13C dipolar coupling or the chemical motifs with higher proton densities107. A MultiCP method has been developed recently to provide quantitative detection through repetitive 1H-13C cross-polarization and 1H repolarization steps, providing a time-efficient replacement for 13C-DP experiment108,109. These 1D 13C experiments are frequently used to assess the characteristics of the dynamically heterogeneous ECM or cellular materials to guide the design of more sophisticated experiments.
Figure 4. Representative ssNMR pulse sequences and spectra.
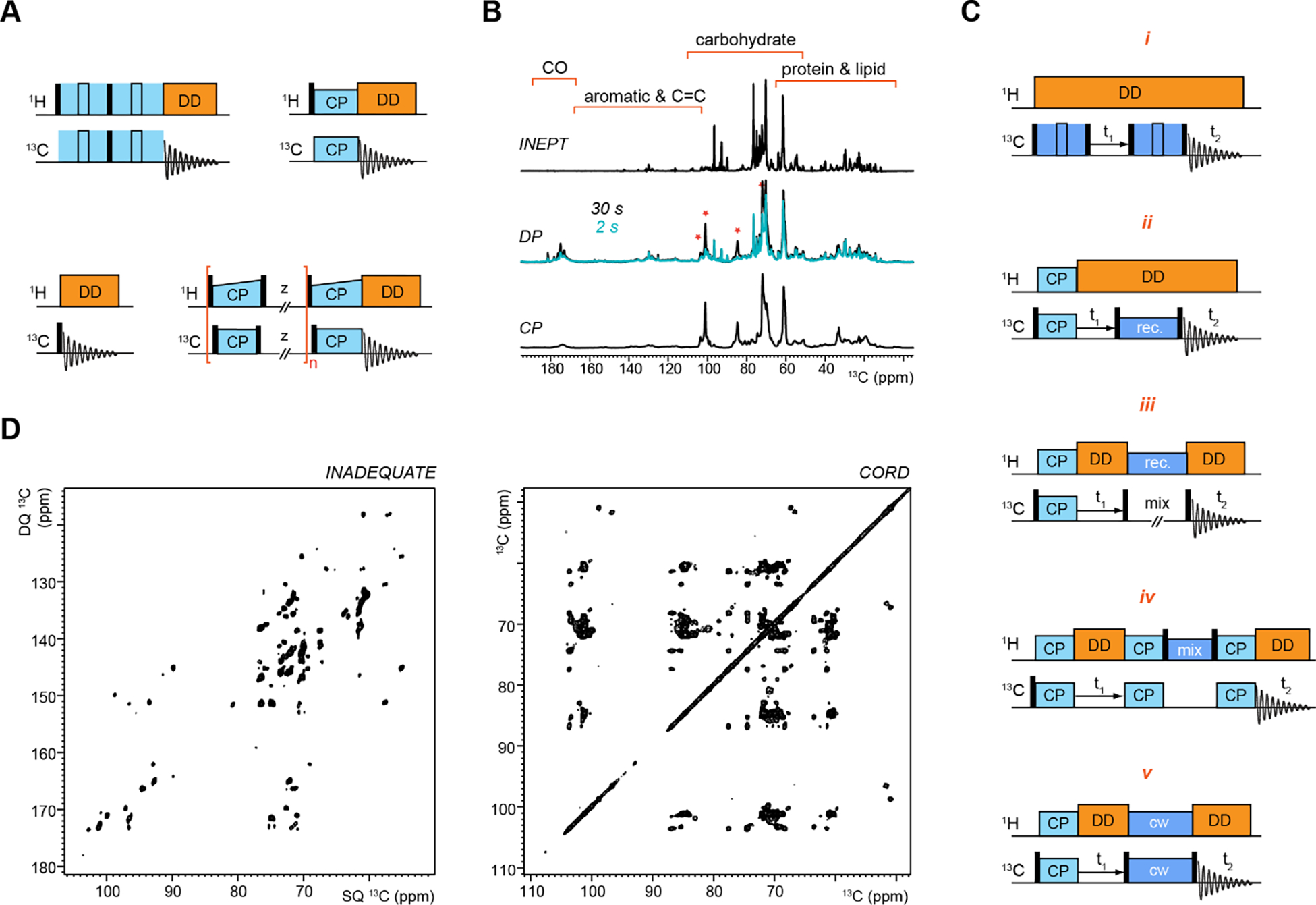
(A) 1D 13C experiments of refocused INEPT (top left), CP (top right), DP (bottom left), and MultiCP (bottom right). (B) Representative spectra collected on the living cells of A. fumigatus. From top to bottom are 13C spectra measured using refocused INEPT, 13C DP with long (30 s; black spectrum) and short (2 s; blue spectrum) recycle delays, and 13C CP. Asterisks show the dephasing of signals from rigid components in the 2s DP spectrum, which were enhanced in CP. (C) Five types (i to v) of ssNMR pulse sequences for measuring 13C-13C homonuclear correlation experiments. Abbreviations are used for the pulse sequences: cross polarization (CP); dipolar decoupling (DD); recoupling (rec.); continuous wave (cw). (D) Representative 2D 13C-13C spectra measured using J-INADEQUATE (left) and CORD (right) experiments. All spectra shown here were collected on an 800 MHz NMR on intact A. fumigatus cells49. Panels B and D are adapted with permission from reference49. Copyright 2018 Springer Nature.
Multidimensional (typically 2D or 3D) correlation spectra are often required for adequately resolving the large number of chemically different biomolecules and their variable conformational structures in a cellular system. The conventional 2D 13C-13C correlation experiments in solids could be grouped into five categories depending on the mechanisms used for 13C-13C polarization transfer (Figure 4C, D): (i) J-coupling based experiments that enable through-bond polarization transfer such as the J-INADEQUATE experiment110–113, (ii) direct recoupling of 13C-13C homonuclear dipolar couplings using recoupling blocks such as RFDR or symmetry-based sequences114–116, (iii) assisting 13C-13C spin diffusion by either passively (without recoupling pulses, for example, PDSD) or actively (with recoupling pulses, for example, DARR, CORD, and PARIS) recoupling the 13C-1H dipolar couplings117–119, (iv) 1H-1H spin diffusion preceded and followed by 13C-1H and 1H-13C heteronuclear polarization transfer, such as CHHC120,121, and (v) recently developed third-spin assisted recoupling (TSAR) schemes such as PAR122–124.
The introduction of more dimensions provides additional resolution and enables structurally enlightening methods by engineering the polarization transfer pathway. A straightforward way is to combine 2D sequences with different polarization blocks (INEPT, CP, DP, etc.) to distinguish components with different mobilities. Spectral editing methods can be applied to select molecules based on their chemical structures or dynamics. Examples include the selection of aromatics or non-protonated carbons through dipolar-gated sequences, the suppression of signals from rigid components via relaxation filters, the highlight of nitrogenated carbohydrates against other glycans using their 15N-13C dipolar couplings, and the detection of hydrated molecules using water-edited experiments125–128. In addition, the homonuclear spatial proximities (e.g., 13C-13C and 1H-1H) probed using 2D correlation experiments and the heteronuclear distances (e.g., 13C-19F and 13C-15N) determined by Rotational-Echo DOuble-Resonance (REDOR) provide structural restraints for understanding intermolecular packing129.
NMR is a powerful tool for investigating the dynamics of biomolecules, which can be correlated to their structural roles. For example, ECM polymers are often found to form rigid scaffolds or distributed in soft matrices as observed in plant and fungal cell walls16,130–132. The dynamical characteristics of polymers can be studied using a variety of relaxation experiments that probe motions on different timescales, as well as measurements of dipolar couplings and chemical shift anisotropy133,134. It should be noted that there is still a missing connection between the mechanical compliance of ECM materials and the molecular dynamics of polymers measured by NMR, which requires further investigations61.
Non-Uniform Sampling (NUS) acquisition methods have been introduced to reduce the experimental time by skipping a fraction of the data that can be deduced or extrapolated from the rest of the experiment135. In general, NUS refers to any pattern of data acquisition that is performed at irregular time intervals or with variable numbers of transients taken at different time points136. NUS has become a standard approach in solution NMR and emerges as a frontier of ssNMR137–139. Studies of ECM and cells present promising future NUS applications. Paramagnetic Relaxation Enhancement (PRE) is another important method that has been employed for the structure determination of proteins140–143 and recently applied to carbohydrate and ECMs144,145. PRE substantially extends the reach of distance measurement (to 10–35 Å)146; therefore, it is uniquely capable of providing long-distance restraints that are important to the structure determination of large biomolecular systems.
The ssNMR versatile toolbox can serve a variety of purposes, from resonance assignment to investigations of polymer dynamics, water association, and supramolecular organization147. Methodological advances in 1H-detection148,149, sensitivity-enhancing MAS-DNP147,150–153, and ultra-high-field magnets (1.0–1.5 GHz)154–157 have further extended the technical capability of ssNMR.
2.2. Sensitivity-Enhancing MAS-DNP as an Emerging Technique
At natural isotopic abundance, 13C represents only ~1.1% of all the carbon atoms. To measure a 2D 13C-13C correlation, the first 13C nucleus must be neighbored with another 13C nucleus, meaning that only 1.1%×1.1% or ~1/10,000 of carbon pairs can generate a correlation. To overcome this sensitivity limitation, a solution is to “hyperpolarize” the nuclear spins. The most common approach nowadays is MAS-DNP21,158, which consists of doping the sample with a polarizing agent (PA), a molecule with unpaired electron spins, then cooling and spinning the sample while irradiating with an appropriate microwave frequency. The gyromagnetic ratio of the unpaired electron spins leads to a polarization that is theoretically ~658 times greater than the 1H’s and ~2600-fold greater than 13C polarization under identical experimental conditions. Typically, the electron spin resonant frequencies are in the hundreds of GHz.
This large electron polarization can be transferred to the surrounding nuclei by applying appropriate microwave irradiation that depends on the PA’s structure147. The nature of the transfer, at the quantum level, can be classified as Solid Effect (SE), Cross Effect (CE), Thermal Mixing or Overhauser Effect21,151,159–169. The type of active mechanism depends on the PA’s nature, its concentration, the magnetic field, the microwave power, and the temperature.
A naive analogy to explain the SE DNP process is illustrated in Figure 5A using buckets, pumps, valves, and liquid. The polarization (liquid) of the electron spin is pumped up toward the proton bucket under microwave irradiation. In the SE mechanism, the microwave drives a forbidden electron-nuclei transition that enables the transfer of polarization from the electron spin towards the protons. The stronger the microwave the faster the transfer, but a short electron longitudinal relaxation T1,e plays against this transfer by preventing the polarization from being transferred efficiently. Once transferred to the proton bucket, the polarization can subsequently be transferred to an X nucleus (e.g., 13C or 15N) via CP for example. In both cases, the 1H and X-nucleus buckets can leak polarization via their corresponding longitudinal relaxation mechanisms T1,H and T1,X, respectively. The analogy clarifies the role of the relaxation times: T1,e and T1,H must be long to favor a high level of hyperpolarization. This is often the case when the sample temperature is lowered.
Figure 5. Mechanism, radical, and instrumentation of MAS-DNP.

(A) Scheme simplifying the DNP process. The microwave irradiation enables the transfer of electron spin polarization to the protons via the electron-nucleus interaction, and the proton polarization is then transferred to the less sensitive X nuclei via CP. The proton polarization level depends on the nuclear and the electron relaxation times relaxation T1,H and T1,e. Short T1,e prevents efficient polarization transfer, while short T1,H reduces the ability of protons to retain polarization. (B) Examples of biradicals used for ECM characterizations170,171. (C) The MAS-DNP setup at the National High Magnetic Field Laboratory, USA. The microwave irradiation is generated by a second harmonic gyrotron emitting a Gaussian beam at 395 GHz. The beam’s power, polarization, and gating are controlled by a quasi-optical bridge and directed towards the probe in the NMR magnet where the sample is irradiated and spun at 3–15 kHz as regulated by the console. Cooling to ~100 K is achieved using a dedicated cooling cabinet172.
The analogy has its limitation: infinite microwave does not lead to infinitely fast or infinitely high polarization transfer as the hyperpolarization plateaus beyond a certain microwave power. In addition, the polarization of the electrons, protons, and X spin systems are in constant equilibrium processes with their environment. This means that the electron spins polarization is repopulated at a rate proportional to 1/T1,e, which was omitted to keep the analogy more accessible.
For MAS-DNP, CE is the most used mechanism while the Overhauser effect is anecdotal166,173–175 and Thermal Mixing under MAS has not been demonstrated. The CE generates a high degree of 1H polarization in a very short amount of time, enabling high 13C polarization and quick experiment repetitions for signal averaging and phase cycling. An accurate analogy for CE is harder to establish but the general concepts shown in the SE analogy (Figure 5A) remain applicable: the microwave irradiation helps “activate” a transfer of polarization from electron spins to proton spins, the relaxation times must be long enough to enable the mechanism and maintain significant nuclear polarization levels. More detailed explanations of the CE mechanism under MAS can be found in many recent articles169,176–185.
The CE is obtained when the PA possesses two interacting unpaired electron spins, and the span of their Larmor frequency must be greater than the Larmor frequency of the targeted nuclei. This is easily reached for bis-nitroxides that were the first biradicals designed to efficiently polarize 1H186,187. These biradicals are dubbed “homo-biradicals” as they consist of the same electron spin species. AMUPol drawn in Figure 5B belongs to that category170. Another class called “hetero-biradicals” are made of different electron spin species, such as a nitroxide and a Trityl for TEMPTriPol188, and have been introduced more recently to overcome limitations at high magnetic fields. In general, these biradicals are dissolved in a glass-forming matrix, like the mixture of glycerol/water or DMSO/water, and the resulting solution is used to wet the sample of interest.
Since their early inception, these biradicals have been massively improved. Experimentalists and chemists have designed ever better performing biradicals by educated guess170,188–197. Their efforts illustrated the crucial role of biradical’s solubility, the molecular weights’ impact on the electron spin relaxation times, and the importance of the coupling between the two electrons spins. In parallel, theoretical analyses revealed how complex the mechanism of polarization transfer is under MAS, identified the parameters determining the polarization transfer efficiency, and revealed the rate at which it occurs166,171,176–178,180–182,184,198–202. Nowadays, theoretical models have reached the capability for the in-silico design of efficient PAs, for instance the AsymPolPOK (Figure 5B), and the prediction of nuclear hyperpolarization and buildup rate171,181.
Today’s best biradicals deliver nuclear polarization levels that are ~100 times greater than the thermal equilibrium171,178,181,198,199, with polarization transfer happening on the time scale of seconds170,171,193,195,196, additionally offering massive time saving.
MAS-DNP requires special instrumentation, which consists of the NMR magnet and consoles, a dedicated probe, a powerful microwave source, an optional microwave control system, and a heat exchanger (Figure 5C). The microwave source is typically a gyrotron that generates powerful coherent microwave irradiation203–206. The cooling and spinning are carried out with cold nitrogen gas to secure a low sample temperature of ~100 K, which enables long relaxation times of electron and nuclear spins for efficient MAS-DNP. The probe and rotor are designed to ensure that the microwave irradiation can reach the sample as well as to enable MAS at low temperature.
MAS-DNP instrumentation has been improved since its commercialization. MAS-DNP spectrometers are commercially available for magnetic fields of 9.4 T/400 MHz/263 GHz and up to 21.1 T/900 MHz/590 GHz205–207. In recent years, probes using smaller rotors (0.7–1.9 mm) have been developed to join the originally commercialized 3.2 mm probes. High-resolution 1H capabilities are now accessible thanks to the 0.7 mm rotors that can spin up to 65 kHz in an MAS-DNP instrument205,207,208. Smaller rotors seem to have additional benefits at high magnetic fields. Indeed, a drastic loss of hyperpolarization performance for bis-nitroxides in 3.2 mm rotors at high magnetic fields (>14.1 T) has been observed. This loss seems less pronounced for smaller rotors, which may indicate that high-field DNP is more sensitive to microwave absorption206,209. This has been circumvented by using hetero-biradicals which are optimal with weaker microwave power198.
Low temperatures make the spinning slower by ~50% as compared to room temperature conditions205,206, limiting the resolution and pulse sequence library that can be used. To enable both higher nuclear hyperpolarization and faster spinning, closed-loop helium spinning systems have been recently developed210–216. Temperature reduction also lengthens relaxation times, therefore reducing the need for stronger microwave irradiation217,218. A closed-circuit helium system can reach dramatically lower temperatures, enabling these benefits. In addition, the speed of sound in He is much higher than for N2 and results in faster spinning at a given temperature210,211.
The low temperature required for MAS-DNP often leads to less-resolved spectra for more dynamic molecules. This is not the case for rigid carbohydrates, and the cell-wall in general, as illustrated by recent work on ECMs82,219,220, and the sensitivity gains are sufficient to forgo isotope sample enrichment altogether as demonstrated recently on rice stems221,222. For complementary information on the topic, we refer the reader to other reviews21,151,158,167,223–226. In sum, experiments that were deemed impossible previously, such as natural abundance 13C-13C correlation, can now be executed on a time scale of a few hours to a day27,227–231.
3. Solid-State NMR Studies of Plant Material
3.1. Plant Cell Wall: A Heterogeneous Network of Biopolymers
Three classes of polysaccharides coexist in the plant cell wall, i.e., cellulose, hemicellulose, and pectin67,232. Cellulose microfibrils can be viewed as the stiff scaffold of the polymer network65. Synthesized by cellulose synthase (CesA) proteins in the plasma membrane using UDP-glucose substrates, eighteen linear chains of β-(1,4)-glucose residues are held together by hydrogen bonds to form a partially crystalline microfibril with a diameter of 3–5 nm233. As for hemicellulose, it is typically a branched polysaccharide with high diversity in monosaccharide composition and branching pattern234,235. Common hemicelluloses include xyloglucan, xylans, mixed-linkage glucan (MLG), and mannan (Figure 2). Many types of xylans and mannans are present with variable patterns of substitutions, including arabinoxylan (AX), glucuronoarabinoxylan (GAX), galactomannan, glucomannan, and galactoglucomannan (GGM). The structure of pectin is difficult to define as it includes a large collection of sugar units, mainly uronic acids but also galactose, rhamnose, arabinose, and xylose236,237. Hence, no single technique can provide complete resolution or separation of all these sugars. Pectin is perceived as an integrated matrix formed by covalently interconnected structural domains, including homogalacturonan (HG) and rhamnogalacturonan-I (RG-I), together with a low percentage of RG-II and proteoglycans238,239. Finally, lignin is an important non-carbohydrate polyphenolic component, and is polymerized from monolignols through radical coupling reactions240–242.
Plants cells contain primary and secondary cell walls. The primary wall covers the outside of the membrane of a growing cell, and the secondary wall starts to accumulate once the growth has ceased. The extent of cellulose coalescence, namely the bundling of elementary microfibrils, is significantly higher in the secondary cell wall, resulting in large bundles of up to tens of nanometers across243. The primary cell wall hemicellulose is xyloglucan in dicots, such as Arabidopsis thaliana, but is made of GAX and MLG in grasses (a type of commelinid monocots), such as Zea mays244. In the secondary cell wall, xylan becomes the dominant form of hemicellulose, although mannan and mixed-linkage glucans are occasionally found in certain plants. While pectin is negligible in the secondary cell wall, it is abundant in the primary one. Pectin is also the major component of the middle lamella that joins adjacent cells together, and where the lignification occurs. From the middle lamella, lignification will progressively reach and dominate the secondary cell wall245. Its chemistry relies on three basic phenylpropanoid monomers, p-hydroxyphenyl (H), guaiacyl (G) and syringyl (S), differentiated by their number of methoxy groups (Figure 2). They are heterogeneously crosslinked to form a polymer network that strengthens and waterproofs the cell wall and increases biomass recalcitrance246,247.
It is a formidable challenge to understand the structural arrangement of a polymer composite as heterogeneous and complex as the cell wall using conventional analytical methods. Therefore, the following section will briefly summarize the past decades of ssNMR efforts in characterizing cellulose structure and then present the more recent breakthroughs in understanding polymer interactions using intact cell walls and whole-cell materials.
3.2. Structural Investigations of Cellulose
3.2.1. Cellulose: A Chemically Simple but Structurally Complex Polymer
The assembly and crystallization of cellulose microfibrils, as well as their coalescence into fibrils on a larger scale, are structurally stabilized by numerous intra- and inter-molecular hydrogen bonds that chemically involve the hydroxide groups attached to C2, C3 and C6 carbon sites of β-D-glucose units248. Crystalline cellulose found in nature was generally considered to be a mixture of two allomorphs249, Iα and Iβ, evidenced with distinct 13C CP ssNMR spectra (Figure 6A)250,251. In higher plants, the thermodynamically more stable Iβ allomorph dominates, whereas Iα is preponderant in algae and bacteria. From diffractograms, it has been established that Iα has a one-chain triclinic unit cell (P1 space group), while Iβ has a two-chain monoclinic unit (P21 space group)252–254. 2D 13C-13C ssNMR spectra fully resolved two magnetically inequivalent glucopyranose units in each of the two allomorphs: A and A’ units in Iα and B and B’ residues in Iβ255–257. Inter-residue cross peaks have been observed for A-A’, B-B, and B’-B’ linkages, which were estimated to be at least up to 3.6 Å due to the long recoupling time used to record 2D RFDR spectra256. Together, NMR and diffraction results have revealed that Iα has identical chains formed alternating A and A’ units, while Iβ has two types of chains arranged in alternating layers248.
Figure 6. An evolving view of cellulose structure from ssNMR.
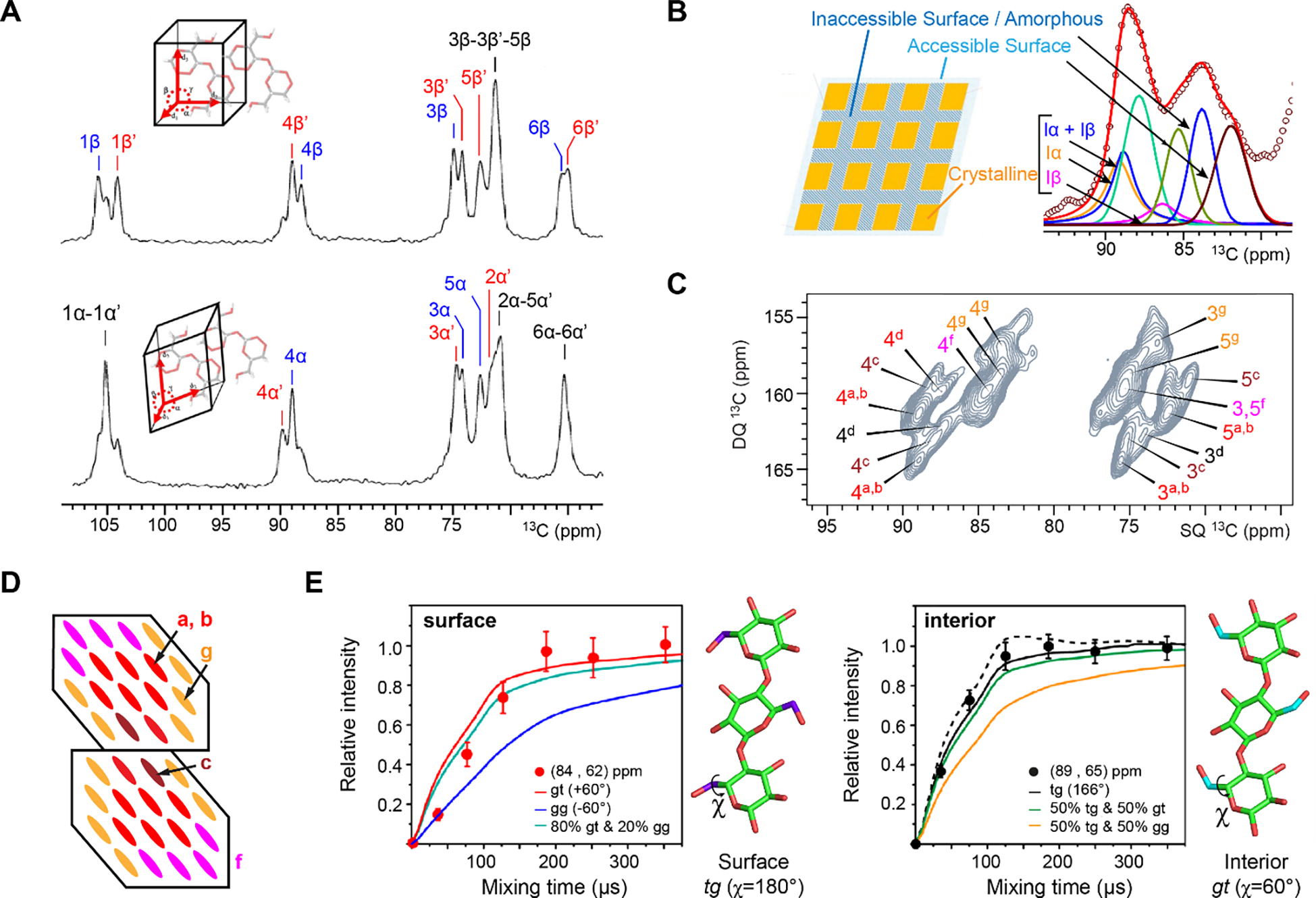
(A) Reference 13C spectra of two cellulose samples dominated by either Iβ (top) or Iα allomorphs (bottom). They both have two magnetically inequivalent carbon sites and the splitting of lines into doublets. Panel A is adapted with permission from references249,256. Copyright 2021 Elsevier and 2006 Springer Nature. (B) Core-shell model of cellulose fibrils with a crystalline interior enveloped by an amorphous inaccessible surface and then an accessible surface. Panel B is adapted with permission from references258,259. Copyrights 2020 Springer Nature and 2019 Elsevier. (C) 2D 13C INADEQUATE spectrum (C4-C3/5 region) resolving a variety of glucose conformers (types a to g) in intact maize stems. Panel C is adapted with permission from reference260. Copyright 2019 Springer Nature. (D) Scheme of two elementary microfibrils bundled together. Each microfibril has 18 glucan chains in a possible 2-3-4-4-3-2 hexagonal pattern. Bundling might lead to the embedded type-c conformer260,261. (E) Hydroxymethyl conformation restrained by 1H-1H distance measurements. Interior cellulose and surface glucan chains feature different χ (O5–C5–C6–O6) torsion angles as shown by the 1H-1H spin diffusion curves obtained on the cellulose of intact Brachypodium primary cell walls. Panel E is adapted with permission from reference262. Copyright 2018 American Chemical Society.
In addition, ssNMR has been applied to distinguish multiple artificial crystalline forms, including cellulose-II irreversibly generated by cellulose-I mercerization (treatment with strong alkaline solutions) or regeneration procedures (solubilization and recrystallization), as well as metastable form III (obtained from evaporation of amines in an ammonia-cellulose complex), and form IV (following heat-treatment of cellulose III)263–269.
Despite the well-defined crystallographic structures of model allomorphs, the structure of cellulose in the plant cell wall is not yet fully understood. For decades, a 36-chain model has been widely used to comprehend the organization of a microfibril, based on a hypothesized arrangement of cellulose synthase complex (CSC) as a hexamer of hexamer270,271. However, recent analyses of CSC and cellulose microfibrils support a hexamer of trimer organization where each elementary microfibril should contain 18 glucan chains272–275. Regarding the formation of larger fibrils, a simplistic model has proposed that the crystalline core may be wrapped by an outer amorphous layer (which can be either accessible or not), with the addition of an intermediate para-crystalline layer between the core and amorphous layers (Figure 6B)276–278. This model was based on deconvolution procedures applied to the different C4 signals and the paramagnetic relaxation enhancement (PRE) effect after adding 4-OH-TEMPO radical species, but the limited resolution could introduce significant uncertainty144,279.
Since 2016, the combination of 2D 13C-13C correlation spectra, isotopically enriched cell wall samples, and high-field NMR magnets, have provided unprecedented resolution and sensitivity for investigating cellulose structure. Four conceptual advances have thus been achieved. First, native cellulose microfibrils in intact plant cell walls are not a simple superposition of the Iα and Iβ allomorph. Seven types of glucose units (types a-g) are resolved and consistently found in the 2D 13C-13C correlation spectra of a large variety of plant species (Figure 6C)261,280. Their chemical shifts substantially deviate from those determined with highly crystalline and purified model cellulose samples. Second, the spatial organization of plant cellulose is more sophisticated than the crystallographic structures. These seven types of glucose units coexist in cellulose microfibrils (Figure 6D), with residues residing on the surface (types f and g), underneath the surface (types a and b), embedded in the core (type c), and on the interaction site with hemicellulose (type d). An increase in the number of embedded chains (type-c) has been observed from primary to secondary cell-wall samples281, likely due to the enhanced coalescence of microfibrils, but we still lack a way to correlate it with microscopic observation for quantitatively assessing the organization of cellulose microfibrils in a large bundle, namely the macrofibrils. Third, the surface and internal residues, which are important for the stability of hydrogen-bonding network and the chemical reactivity of cellulose, were found to adapt gauche-trans (gt) and trans-gauche (tg) conformations, respectively (Figure 6E) 262. The hydroxymethyl conformation is restrained by 1H4-1H6 distances. Fourth, the existence of Iα and Iβ structures was confirmed but only in the fibrils with large crystallites. Natural-abundance 13C-13C correlation DNP spectra collected on cotton cellulose have revealed chemical shifts data that agree with the literature values collected on these model allomorphs281. These findings have exposed the unexpected complexity of cellulose. The improved spectroscopic resolution and sensitivity provided by the current NMR technologies allow us to pursue many unresolved aspects of the microfibrils and macrofibrils.
3.2.2. Degree of Crystallization: A Well-Defined Parameter?
The structural trait of cellulosic material is often presented by its degree of crystallization, also denoted as crystallinity index (CrI)282–284. This key parameter can be correlated with the mechanical and structural properties as well as the chemical and enzymatical reactivity of cellulose285. Amorphous and crystalline phases coexist in cellulose, but their spatial organization is still debated as they might be distributed longitudinally along the fibril axis or form the disordered surface and ordered core in the cross-section of a fibril. 13C ssNMR has provided a robust and efficient method to determine the CrI, which relies on either direct integration or spectral deconvolution of the C4 chemical shift region, followed by calculating the ratio of intensities from the crystalline domain against the amorphous component. Spectral deconvolution partially alleviates the peak overlap issue, thus it is considered to be more accurate than direct peak integration259,283. A recent protocol of CrI determination also includes the use of 13C T1 filters for differentiating amorphous and crystalline sites in cellulose285,286. Furthermore, by assuming a cross-sectional distribution of these phases, the NMR-measured CrI value can be converted to a surface-to-interior ratio that can be extrapolated to the fibril diameter or the number of glucan chains enclosed in a microfibril287–289. These straightforward protocols are well illustrated in the studies of cellulosic materials from different sources and processed with different procedures, for example, ball-milling, solvent treatment, and mutants for higher digestibility258,287,290.
Unfortunately, it is impossible to align the CrI values measured using ssNMR and other experimental methods such as X-ray diffraction, Fourier-transform infrared (FTIR) and Raman, as well as vibrational sum frequency generation (SFG) spectroscopy291. Indeed, a single CrI value for a cellulosic sample cannot be provided due to the difference in the chemical motifs and physical arrangements being examined by different methods, which has been recently discussed using data collected on cotton cellulose255. We must better understand the connection between NMR chemical shifts and cellulose structure in order to precisely evaluate the crystallinity of cellulosic materials.
3.3. Interactions Between Cellulose and Matrix Polysaccharides in Primary Cell Walls
As mentioned before, matrix polysaccharides include hemicellulose and pectin corresponding to the acidic polymers in the mobile gel-like matrix that can be extracted from primary cell walls by alkali treatments. Hemicellulose and pectin are produced in the Golgi apparatus, and then secreted through the plasma membrane to the cell wall via vesicles 236,237. This process happens concomitantly to cellulose synthesis and assembly, which might have contributed to the association of matrix polysaccharides to cellulose by surface deposition or chain entrapment. Hemicellulose was long thought to crosslink multiple cellulose microfibrils, and thus assumed a central role in load-bearing, while pectin was typically considered as phase-separated from the cellulose-hemicellulose network 244. Our understanding of these polymers’ interactions and their contributions to cell wall mechanical properties has been revised following recent biophysical and biochemical studies 232.
In 2021, a groundbreaking study of primary cell walls using mesoscale coarse-grained molecular dynamics (CGMD) simulations (Figure 7A)292 allowed the establishment of a nanoscale-to-mesoscale correlation for the polymer assembly293,294. Demonstrated on onion epidermal cell walls, this work showed that tensile forces are mostly transmitted by cellulose microfibrils through sliding between microfibrils, thus contributing to the irreversible extension and regulating the plastic deformation of the cell walls. The role of hemicellulose and pectin in the cell wall mechanical properties was proposed to be indirect, primarily by influencing the arrangement of cellulose microfibrils in the networks and affecting the accessibility of cellulose-modifying proteins. This latest model incorporates some key conceptual contributions from ssNMR studies as will be briefly discussed below.
Figure 7. From macro- to micro-scale primary cell wall models.

(A) Coarse-grain model of onion epidermal cell wall with multiple lamellae featured. Purple spheres represent cellulose, green for xyloglucan (XyG) and yellow for pectin. Panel A is adapted with permission from reference292. Copyright 2021 The American Association for the Advancement of Science. (B) Scheme of primary cell walls with pectin-cellulose and XyG-cellulose interactions restrained by NMR data298. Panel B is adapted with permission from reference298. Copyright 2021 Elsevier. (C) Expansin-bound region of carbohydrates viewed by MAS-DNP. In Arabidopsis, the protein-targeted molecules include XyG as well as the surface chain and interior type-d chain of cellulose microfibrils. Panel C is adapted with permission from reference297. Copyright 2013 United States National Academy of Sciences. (D) Representative pectin-cellulose ssNMR cross peak as highlighted using yellow circle as observed in Arabidopsis. Panel D is adapted with permission from reference299. Copyright 2015 Oxford University Press. (E) Model of β-expansin (EXPB) loosening the junctions between highly substituted GAX (hsGAX) and lowly substituted GAX (lsGAX). (F) A difference spectrum obtained from 13C-labeled maize cell walls with and without paramagnetically tagged EXPB proteins. Only matrix polymers showed up and no cellulose signals were observed, revealing that EXPB does not bind cellulose. Panels E and F are adapted with permission from reference145. Copyright 2016 Oxford University Press.
By applying ssNMR, the hemicellulose xyloglucan (XyG) was found to interact with cellulose at specific regions (Figure 7B), based on the limited XyG-cellulose cross peaks observed in a 3D 13C correlation spectrum of Arabidopsis seedling cell walls - the first high-resolution ssNMR dataset collected on intact primary cell wall material295. This finding was supported by subsequent biomechanical studies61,296 and Field Emission Scanning Electron Microscope (FESEM) observations294. The “biomechanical hotspots” of Arabidopsis primary cell walls, where xyloglucan is entrapped in the type-d conformer of cellulose, were also found to be the binding target of a wall-loosening protein named expansin (Figure 7C)261,297. This result is in agreement with the equilibrated CGMD model, in which XyG exists as either an extended chain or a locally clustered coil, and is attached to cellulose microfibrils with very limited sites of contacts292.
Unexpectedly, many intense cross peaks have been observed between cellulose and pectin, more specifically, with the galacturonic acid (GalA) and rhamnose (Rha) residues that form HG and RG-I backbones (Figure 7D)298–300. A quarter to half of the cellulose surface was estimated to be spatially proximal to pectic polymers based on the spin-diffusion buildup data300. Pectin-cellulose contacts were consistently observed in a large number of wild-type and mutant Arabidopsis samples, and fully retained even when bulk portions of HG were extracted from the cell wall130,298,299,301–304. This suggests that some pectic backbones are physically entrapped between glucan chains in a cellulose microfibril or sandwiched between multiple microfibrils when they are being assembled and bundled in the cell wall. Notably, in a study of Arabidopsis inflorescence stems, pectic polymers in the top segments were found to have more branched structure, reduced interactions with cellulose, and increased molecular mobility (probed by measuring 13C-1H dipolar couplings) than in the bottom segments130. These NMR results are in good agreement with the observed apical-to-basal decrease in elongation rate as well as elastic and plastic compliances. This notable advance presents an effort to correlate the molecular-level structure and dynamics of polymers with the mechanical properties of cell walls and the plant growth.
The primary cell wall of grass (a commelinid monocot) has also been investigated. In Brachypodium, the major hemicellulose, glucuronoarabinoxylan (GAX), was shown to be partitioned into two dynamically distinct domains, as revealed by their different 13C-T1 and 1H-T1ρ relaxation parameters305. The rigid domain is interacting with cellulose while the mobile region may be filling the interfibrillar space. In a study of maize primary cell walls, the junctions between highly- and weakly-substituted GAX were identified as the binding target of β-expansins that are native to the grasses (Figure 7E). This study used extracted expansin proteins from the grass pollen that have been bound to paramagnetic Mn(II) tags to examine the transverse PRE effect on the carbohydrate signals in grass cell walls (Figure 7F)145. The PRE method, together with the differential labeling and DNP approach, demonstrated its usefulness in the examination of the binding targets of many other proteins or enzymes containing carbohydrate-binding modules (CBM). The role of other matrix polysaccharides, such as mixed-linkage glucan and pectin in grasses, were not evaluated using these simplistic model samples, and require further investigation.
3.4. Polymer Contacts in Secondary Cell Walls
Recent efforts have been progressively shifted to the understanding of the spatial organization of cellulose, hemicellulose, and lignin in secondary plant cell walls. The first breakthrough is the identification of the conformational selectivity of xylan309,310. Complemented by Density Function Theory (DFT) calculation, the NMR-observed peak multiplicity was associated to the conformational heterogeneity of this hemicellulose309. As illustrated in Figure 8A for maize stem, two sets of xylan cross peaks could be identified, i.e., 2-fold xylan (Xn2f) and 3-fold xylan (Xn3f), with a 360° helical rotation occurring every two or three xylose units, respectively260. Xn2f has a flat ribbon structure, while Xn3f has a helical shape and is the one observed in solution and presumably in the more disordered matrix of the secondary cell walls. In the vicinity of cellulose, mutual interaction leads xylan to adapt a two-fold conformation to match the conformation of cellulose – a form that is abolished in a cellulose-deficient mutant307. Quantification of adsorbed xylan on birch pulp kraft has validated the conformational selectivity of xylan in the presence of cellulose and demonstrated its reversibility after xylan removal or re-adsorption311.
Figure 8: Polymer structure and interactions in secondary plant cell walls.

(A) 13C CP INADEQUATE spectrum of maize stem resolving two-fold (Xn2f) and three-fold (Xn3f) xylan domains. (B) Intermolecular contacts between lignin units (H, S, G, and ferulate FA) and polysaccharides (xylan and cellulose) in maize. The overlay of dipolar-gated PDSD spectra collected using short (0.1 s) and long (1.0 s) mixing times showed numerous lignin-carbohydrate cross peaks. Abbreviations were also used for the internal (i) and surface (s) glucan chains and the acetyl group of xylan (Ac). (C) Polymer hydration level revealed by water-edited NMR intensities presented using violin plots. Lignin (L) has the lowest level of water retention in maize secondary cell walls. (D) Proposed model of secondary cell walls based on high-resolution ssNMR data collected using maize stems. Panels A, B, C, and D are adapted with permission from reference260. Copyright 2019 Springer Nature. (E) Comparative models of Brachypodium stem and leaf secondary cell walls. A higher proportion of xylan functional groups (ferulic acid, FA, and acetyl, Ac) promotes its 2-fold conformation and contacts with cellulose fibrils. Panel E is adapted with permission from reference306. Copyright 2021 American Chemical Society. (F) Limited interactions between 3-fold xylan and amorphous cellulose in Sorghum secondary cell walls. Panel F is adapted with permission from reference307. Copyright 2020 Springer Nature. (G) A microfibril of Spruce softwood formed by many 18-chains cellulose microfibrils and other associated molecules including GGM, xylan, and lignin. Panel G is adapted with permission from reference308. Copyright 2019 Springer Nature.
Xylan also interacts with lignin, primarily through its 3-fold conformation. This is evidenced with maize stems by the xylan-lignin cross peaks observed in a set of spectral editing experiments that selectively detect signals of aromatic molecules and their associated components (Figure 8B)16,260,312. Xylan-lignin interactions would occur through surface contacts between different polymer domains as each type of molecule efficiently retained its unique hydration and dynamics (Figure 8C). Altogether, these results led to a structural scheme of secondary cell walls where xylan uses its 2-fold and 3-fold conformations to respectively bridge the cellulose surface and lignin nanodomains (Figure 8D)260.
The conformational dependence of xylan’s function was also confirmed in a recent study on 7-week-old Brachypodium (a model grass)306. The structure and interactions of xylans were compared between the leaves and the stems, the latter containing more secondary cell walls. Xylan chains were found to have twice as much acetylation and 60% more ferulation in the stems than in the leaves. Also, a high content of 2-fold xylan was observed in the stems, as well as more extensive xylan-cellulose interactions (Figure 8E). Surprisingly, a ssNMR study of the grass species Sorghum bicolor revealed a unique and weak interaction between three-fold xylan and cellulose. This grass sample lacks two-fold xylan and relies on the three-fold conformers to interact with cellulose, likely at the amorphous region of the microfibrils (Figure 8F)307. A recent study on softwood conifer secondary cell walls further reports that galactoglucomannan (GGM), the major hemicellulose of softwood, binds the surface of cellulose microfibrils in a semi-crystalline manner, with lignin in association with the cellulose-bound polysaccharides (Figure 8G)308,313. In addition, the physical and mechanical properties of hemicellulose have been assessed by incorporating extracted softwood or hardwood hemicellulose to bacterial cellulose hydrogels to closely imitate plant secondary cell walls before lignification314. Despite these major advances, many structural aspects of the secondary cell walls, such as cellulose-lignin contacts and the covalent and physical interactions regulating polymer associations, still require in-depth investigations by ssNMR methods.
3.5. Limitations and Perspectives of Solid-State NMR in Plant Research
3.5.1. Lignin Structure and Lignified Cell Walls
Lignin is commonly found in the secondary cell walls of certain specialized plant cells, where it confers high rigidity and resistance to microbial degradation315. There is a paradox between the simplicity of monolignol basic units and the complexity of the polymerized network, which is a result of the variety of linkages present in lignin240,316. Solution NMR is the most employed analytical technique to characterize lignin (Figure 9A)14,15,317,318, and only a few ssNMR experiments have been carried out to investigate the lignin structure. 1D ssNMR approaches have been used to determine how well lignin fractions were solubilized prior to solution NMR measurements, and to avoid the chemically destructive acidic hydrolysis methods (like Klason lignin analysis) for quantification319–324. Efforts have also been made to estimate the ratio between the G and S units in poplar and other woody plants325–327, but insufficient resolution prevents accurate determination of the concentration of individual lignin units and linkage patterns328. In addition, all deconvolution procedures applied in the spectral analysis are affected by considerable uncertainty, especially when broad peaks overlap (Figure 9B). Lignin units can be better resolved in 2D 13C-13C correlation spectra (Figure 9C)260,324. DNP has also been applied to wild-type and mutant poplar samples to unravel the structural changes of lignin, and the resolution enabled distinguishing the three types of fundamental units and partially probing their linkages329. More efforts are needed to improve the ssNMR capability for lignin structure characterization to par with solution NMR methods, with the additional merit of using native samples free of chemical treatment, ball-milling (which restructures biomolecules as shown recently281), and solubilization procedures. The covalent linkers bridging monolignol units and the putative lignin-carbohydrate complex313,330 are also of special interest for ssNMR investigations.
Figure 9. Difficulty of lignin ssNMR and compositional analysis.
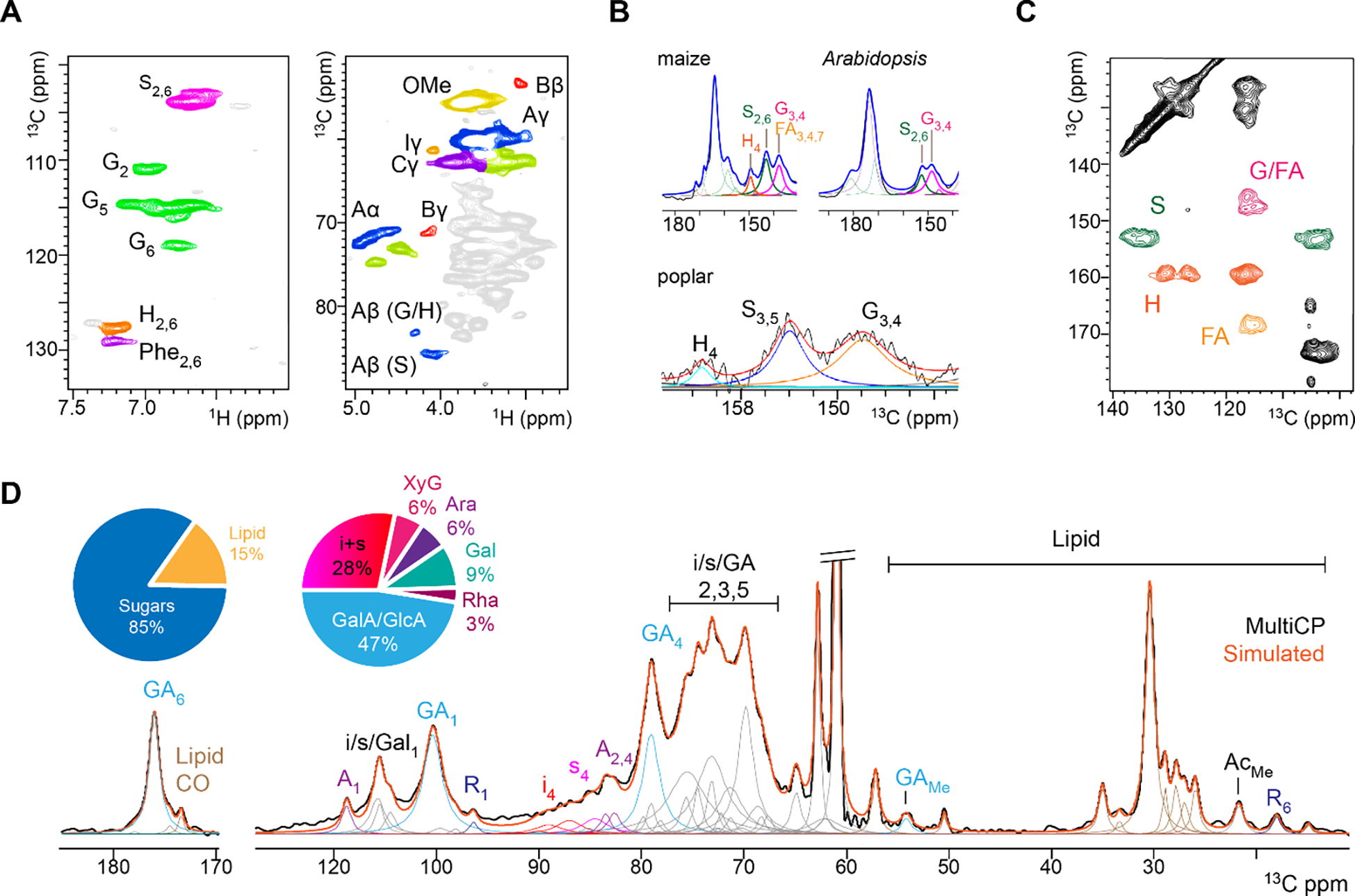
(A) Representative solution-state HSQC spectra with high resolution of both monolignol units and linkages for poplar wood316. Panel A is adapted with permission from reference316. Copyright 2020 American Chemical Society. (B) Representative 1D 13C ssNMR spectra of various plants showing broad lignin signals. Panel B is adapted with permission from references260,327. Copyrights 2019 Springer Nature and 2020 Elsevier. (C) 2D 13C ssNMR resolving the signals of lignin units. Panel C is adapted with permission from reference260. Copyright 2019 Springer Nature. (D) Quantitative MultiCP spectrum of onion cell walls reconstructed from 59 deconvoluted peaks. Sugar-to-lipid ratio and carbohydrate composition are obtained, but with considerable uncertainty. Panel D is adapted with permission from reference331. Copyright 2021 Springer Nature.
3.5.2. Quantification of Cell Wall Composition
To account for the diversity of plant species, their evolving traits during growth, and the development of genetically modified organisms, statistical analysis approaches, more commonly undertaken in metabolomics and 1H HR-MAS studies of natural products, begin to extend to ssNMR research of lignocellulose. For example, such strategies have been applied to monitor water deficiency effects in order to better understand drought stress on plants332. Unfortunately, ssNMR lacks the time-efficiency to survey a large number of samples — a mandatory step to match the success of metabolomics in solution NMR333,334.
Since NMR resolves differences in chemical environments, changes that occur on biomaterials after they are subjected to any form of exterior physical or chemical perturbation can be easily detected using well-established chemical shifts335–337, with the assistance of an online chemical shift database338. Therefore, modifications of the plant cell wall structure, composition, dynamical properties, and polymeric interactions during their development130,302 or under external factors339, such as heat340, physical stress, enzymatic digestion259, chemical treatment341–343 or any combination of these could be better understood339,344.
To bypass exceedingly long 13C T1 relaxation times, CP is preferred to DP as it is well adapted for rigid cellulose and lignin, notably when internal references are used to quickly calibrate the polarization transfer efficiency between samples345. For example, celery is a model plant with two types of primary cell walls (parenchyma and collenchyma). This plant has been used in comparative NMR studies to elaborate 1D CP and DP methods for discriminating different sugars346–350. We recently delved into sugar quantification on a chemically similar system, i.e., the epidermal cell wall of onion, by combining quantitative Multi-CP spectrum and spectral deconvolution (Figure 9D)331. The results were in relatively good agreement with chromatography techniques (±8% for major cellulose and homogalacturonan units, and ±4% for minor sugars). However, quantifying the polymer composition in ECMs remains a challenging task.
3.5.3. Is Isotopic Labeling Still Mandatory?
Multiple emerging approaches allow to either entirely or partially alleviate the need for uniform isotopic enrichment using 13CO2 or 13C6-glucose86. The “natural-abundance DNP” approach227,229,230 has been applied to a large variety of unlabeled plant samples. For example, MAS-DNP provided a 23-fold signal enhancement using cotton samples, with the linewidths largely preserved (0.8–0.9 ppm) despite cryogenic temperature281. The 2D spectra assignment agreed with cellulose I references, but substantial modifications of cellulose structure by ball-milling procedures have been noticed. Recently, a DNP protocol for systematically investigating plant cell walls has been developed and demonstrated on the wild-type sample of Oryza sativa (rice) as well as a ctl1 ctl2 double mutant showing brittleness phenotypes221. A 22–57-fold signal enhancement allowed resolving different conformers of cellulose and xylan using unlabeled stems, with 2D INADEQUATE and CHHC spectra resembling those collected with classic 13C-labeled samples (Figure 10A). The structural information provided by cryogenic temperature DNP (circa 100 K) was complemented by room-temperature natural abundance relaxation measurements, revealing higher polysaccharide mobility in the mutant cell walls221. Since the samples used in this study were rice stems directly collected from the field, the toolbox is thus generally applicable to all lignocellulose materials.
Figure 10. Natural-abundance MAS-DNP spectra and 1H-detection of plant cell walls.

(A) Refocused-INADEQUATE spectra and assignment of polysaccharides and their conformers using unlabeled rice stems taken from the field221. The resolved carbohydrates include the 2-fold (Xn2f) and 3-fold (Xn3f) xylan, and the many types of glucose units from surface (s) and interior (i) chains of cellulose. Top panel represents the non-reducing carbon (C6/C5 for cellulose/xylan, respectively) directly linked to the central carbon (C5/C4 for cellulose/xylan, respectively). While bottom panel indicates the chemically similar central carbons. Panel A is adapted with permission from reference221. Copyright 2021 Springer Nature. (B) Refocused-INADEQUATE spectrum of wild-type poplar and high-S mutant paving the way for assignment of lignin signals using unlabeled samples. Panel B is adapted with permission from reference329. Copyright 2017 American Chemical Society. (C) Fast MAS (50 kHz) INEPT spectrum of 13C-Arabidopsis primary cell walls resolving the signals of sugar units in the mobile matrix polysaccharides. Dash line indicates the position of the 1H cross section for highlighting the narrow linewidth. Panel C is adapted with permission from reference89. Copyright 2019 Springer Nature.
Natural-abundance DNP has also been used to determine the extent of delignification in wood material subjected to pretreatment (lignin extraction)351, and to analyze the monolignol units and linkages in wild-type poplar and a mutant with a high content of S-units (Figure 10B)329. DNP has also been employed to characterize cellulose derivatives and understand the regioselectivity of cellulose methylation352. The DNP-enabled 2D 13C-13C INADEQUATE spectra of unlabeled cellulose derivatives provided sufficient resolution for identifying the methylation site. Alternatively, the unique mobility of CH3 groups, which was retained at the DNP cryogenic temperature, was utilized to locate their closest neighbors in space, here cellulose C3, by monitoring NOE polarization transfers352.
Another promising direction is the proton detection under ultrafast MAS. In 2019, a series of 2D and 3D 1H-13C correlation experiments were conducted on the model plant Arabidopsis under slow to moderately fast spinning frequencies (10–50 kHz)89. With 1H linewidth of ~50 Hz (0.06 ppm on an 800 MHz spectrometer), resonance assignment has been successfully achieved for matrix polysaccharides (Figure 10C), and a good agreement was found between the 1H chemical shifts of these mobile molecules measured in the solid and solution states. Long-range correlations between matrix polysaccharides and cellulose were also reported, based on 1H-1H spin diffusion instead of the more conventional 13C-13C polarization transfer. These experiments have been demonstrated using either 1H or 13C detection; however, it should be noted that the broad 1H signals of cellulose are typically filtered out to ensure sufficient resolution, and cellulose is typically detected only using 13C. Almost at the same time, another 1H detection study has been conducted on bacterial materials under 100 kHz MAS to investigate the structure of peptidoglycans88. The 1H chemical shifts of polysaccharides reported by these studies certainly pave the way to investigate unlabeled plant materials exploiting the 1H nucleus with high natural abundance (99.98%).
Another strategy worth mentioning is the selective or sparse labeling approach. It does not evade the demanding procedures involved in isotope labeling but can provide a way to tackle specific molecules of interest. For example, from 2H-labeled UDP-glucose (precursor for cellulose synthesis) and 13C-coniferin (a lignin precursor), cellulose and lignin have been tracked in the ginkgo tree to determine their mutual interactions, the linkages they form, as well as follow where labeling occurs in newly formed xylem353. Selective labeling has also been coupled with 13C{2H} REDOR experiments to identify the tyrosine-tyrosine crosslinking occurring in plant cell walls and measure the strength of the associated dipolar coupling, whereas 13C{15N} REDOR enables exploring steric hindrance from neighboring lysine peptides354.
At this stage, information on the polymorphic structure (with chemical shifts as a sensitive reporter of the linkages and conformations), dynamics, and hydration of polysaccharides can be efficiently obtained using unlabeled tissues221. Another structurally important aspect - intermolecular interactions - could possibly be addressed by the establishment of 1H-detection techniques. Therefore, we expect a new era of research on unlabeled materials for addressing key biochemical questions related to ECMs. After sample optimization, each DNP-enhanced 13C-13C correlation spectrum can be recorded within tens of hours using unlabeled materials221. The experimental time, however, is still noticeably longer than the typical NMR experiment conducted on isotope-enriched samples. DNP and proton-detection approaches should primarily serve as enabling methods for samples that are either difficult to label or impractical to replicate. With constantly improving radicals and instrumentation, the combination of the natural-abundance MAS-DNP and 1H detection techniques and isotope-labeling should substantially expand the ssNMR playground in biomolecular and biomaterial research.
4. Resolving the Molecular Architecture of Fungal Cell Walls
4.1. Fungal Cell Wall as a Target for Drug Development
Fungi are a class of eukaryotic microorganisms widespread in nature where some of them cause invasive infections or systemic diseases in humans5. Candida, Aspergillus, and Cryptococcus species are responsible for more than 1.6 million life-threatening infections annually, with 20–95% mortality355–362. Azoles and amphotericin B (AmB) are prevalent agents used in antifungal treatment, exhibiting a broad spectrum of activity against most clinically relevant unicellular non-sporous yeasts and multicellular sporous molds. These compounds either directly bind ergosterol molecules in fungal membranes or inhibit enzymes involved in the biosynthesis of ergosterol. Similarly in human membranes, they can exhibit disadvantageous interactions with cholesterol and enzymes in human membranes, leading to severe side effects363–365. In addition, drug resistance has been growing significantly over the past decades357,364.
Since the fungal cell wall contains polysaccharides that are absent in human cells, significant attention has been devoted to the identification of antifungal compounds targeting fungal polysaccharides. Rewarding these efforts, a class of β-(1,3)-glucan synthase inhibitors named echinocandins have been clinically approved and are currently used to treat fungal infections366–368. Nevertheless, echinocandins have a limited spectrum of activities: these compounds have high efficacy against invasive candidiasis (infections caused by Candida species) but not for invasive aspergillosis, which is due to the adaptive mechanisms developed by Aspergillus species369–371. An in-depth understanding of the molecular organization of cell walls across fungal species and the structural effects from antifungal compounds could pave the way for developing wall-targeting drugs with higher efficacy and broader spectrum of antifungal activities.
4.2. Incomplete Understanding of Fungal Cell Wall Structure
The polymer composition of the fungal cell wall is highly heterogeneous, but chitin and β-glucans are two components commonly found in many species. Chitin is a highly elastic polymer of β-1,4-linked N-acetylglucosamine monomers (Figure 2), which can be stabilized through hydrogen bonds to form microfibrils372. Chitin accounts for 10–20% of the polysaccharides in Aspergillus fumigatus and Candida albicans373,374 and becomes deacetylated chitosan in Cryptococcus neoformans. Glucans are the most abundant polysaccharides in fungal cell walls, accounting for 50–60% of the dry mass373. β-1,3-glucans comprise 65–90% of all β-glucans in the cell walls, where their content varies from 25–30% in the filamentous fungus Aspergillus fumigatus to 50–55% in Saccharomyces cerevisiae373,375–377. β-1,6-glucan is also important, with a proportion of 5–12% in S. cerevisiae cell walls and around 20% in C. albicans cell walls. There are no linear β-1,6- or β-1,4-glucans in A. fumigatus, however, branched β-1,3/1,6-glucans (with β-1,6-linkage as the branching point) and linear β-1,3/1,4-glucans have been identified. Compared to β-glucans and chitin, mannans are less rigid and constitute ~10–20% of the cell wall in A. fumigatus and C. albicans378. In C. neoformans, cell walls are embedded in a capsule that contains up to 90% of glucuronoxylomannan (GXM) and galactoxylomannan (GalXM)379.
A conceptual understanding of the fungal cell wall includes an inner layer made up of branched β-1,3-glucans, which covalently connect to chitin and other glycan components, as well as an outer layer rich in glycoproteins380–382. Such supramolecular organization was based on biochemical assays that include initial chemical/enzymatic digestion, fractional solubilization, and isolation of cell wall components followed by sugar compositional and linkage analysis381,383. This method allows for the assessment of biopolymers’ susceptibility to chemicals (such as alkali) and enables the identification of key connections between different cell wall components. The cumulative results lead to the structural scheme in which the chitin-β-glucan center (and further complexed with mannan), an alkali-resistant fraction stabilized by intermolecular covalent bonds, is proposed as the mechanical core (Figure 11A)3,384. Mass spectrometry techniques, with a focus on the surface layer, have identified the presence of mannans, proteins, α-glucans, and GAGs385,386. These molecules are often proposed to regulate fungal virulence by covering the inner cell wall components and evading the detection of immune receptors.
Figure 11. Fungal cell wall architecture viewed by ssNMR.
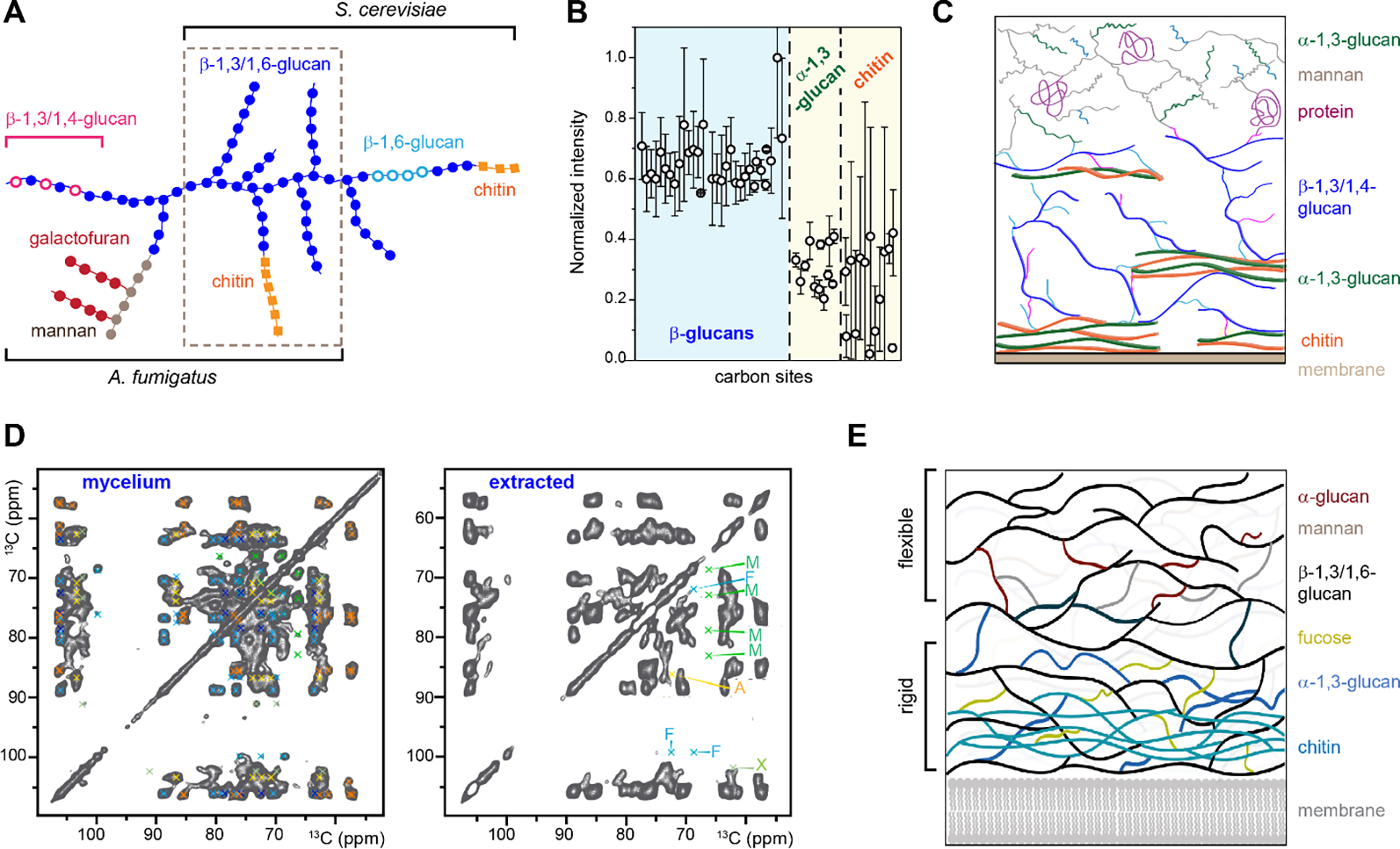
(A) Carbohydrate connectivity of the alkaline-insoluble core of fungal cell walls as reported using biochemical assays. The common module found in many fungal species is boxed using dash lines. The structural features in A. fumigatus and S. cerevisiae are distinguished3. Panel A is adapted with permission from reference3. Copyright 2007 John Wiley and Sons.(B) Water-edited intensity as an indicator of polymer hydration measured using A. fumigatus mycelium. For instance, chitin and α-1,3-glucan are poorly hydrated when compared to β-glucans. (C) Structural scheme proposed for A. fumigatus, emphasizing the packing between chitin and α-1,3-glucan to form the hydrophobic and rigid core. Panel B and C are adapted with permission from reference49. Copyright 2018 Springer Nature. (D) 2D 13C PARIS spectra collected on the hydrated mycelium of S. commune (left) and the same material after PBS-washing, SDS-extraction, and alkali-extraction (right). Many carbohydrate components are missing after extractions. (E) Proposed model for cell walls in S. commune based on ssNMR combined with HPLC and GC–MS. Panel D and E are adapted with permission from reference82. Copyright 2020 Elsevier.
However, most biochemical techniques are detrimental to the sample, and the imaging methods lack sufficient resolution for precisely disclosing polymer interactions in this complex biomaterial. Therefore, ssNMR methods have been recently developed to investigate polymer composites or whole-cell samples of major fungal pathogens, thus complementing the information collected using conventional methods to advance our understanding of cell wall architecture.
4.3. Function and Assembly of Biopolymers in Fungal Cell Walls
Recently, we combined ssNMR with MAS-DNP to disclose the cell wall organization of uniformly 13C,15N-labeled A. fumigatus mycelia49,80,387. Different from the conventional vision of biochemical assays, which are mostly focused on covalent linkages and the resistance of polymer assembly to chemicals, now we are considering the mobility, hydration, and physical packing of these molecules. Structural polymorphism is found to be a conserved feature of polysaccharides in the native cellular environment. This insight is enabled by the high resolution, typically within 0.7 ppm 13C linewidth, provided by high-field NMR (800 MHz or above) at room temperature49. With the unprecedented resolution, it was found that α-1,3-glucan molecules in A. fumigatus mycelia have a structural function beyond expectation. Water-editing and relaxation-based experiments revealed its hydrophobicity and rigidity, respectively, just like chitin (Figure 11B), while 2D 13C–13C/15N intermolecular cross peaks, many of which were enabled by the sensitivity enhancement of DNP, directly revealed the sub-nanometer association of α-1,3-glucan and chitin. This concept had never been reported before because the bulk of α-1,3-glucan can be easily digested by alkali treatment, thus lacking the covalent linkages to the chitin-β-glucan core. However, physical associations allow for the coexistence of α-glucans and chitin to form a rigid and water-proof scaffold around the cell, protecting it from external stresses. These results provide a picture in which the β-glucans serve as tethers between multiple chitin-α-1,3-glucans domains underneath the outer layer of proteins and mannans (Figure 11C)49.
Ehren et al. investigated the cell wall organization in the basidiomycete Schizophyllum commune82, which is one of the most commonly found fungi and can be pathogen388. In this work, they extracted cell wall components from uniformly 13C, 15N-labeled mycelia by sequentially subjecting the mycelia to PBS-washing to remove the cytoplasm, SDS-extraction to deplete hot-SDS-soluble components such as β-1,3/1,6-glucans, and alkali extraction treatment to remove α-1,3-glucan. Over the course of these steps, an increasing number of molecules have been removed from the cell wall as shown by the 2D 13C–13C INEPT-TOBSY and PARIS spectra (Figure 11D). The mobile part of β-1,3/1,6-glucan is left behind as the primary molecule found in the J-based spectra of the final, alkali-treated sample. Signals from lipids and amino acids were exclusively found in the hydrated mycelium but not the treated samples, indicating that they are not part of the cell wall but rather the inside of the cell. SsNMR analysis showed that various forms of glucans, mannose, chitin, and surprisingly fucose constitute the primary components of S. commune cell walls (Figure 11E). The rigid part of the cell wall is made of chitin that is likely branched to glucans while the mobile part comprises the terminal hexoses included in α- and β-linked glucans. Distinct types of mannose species were observed in the mobile region, indicating mannose as being part of an extended, mobile component that is either branched or integrated into mannosylated proteins. These findings confirmed the previously reported arrangement of the cell wall389–391 and further provided new insights that the rigid part of S. commune cell wall contains α-(1,3)-glucan and polymeric fucose82. In contrary to what has been reported for S. commune392, Ehren et al. found no evidence of peptides engaging in polysaccharide cross-linkage82.
4.4. Reorganization of Fungal Cell Walls in Mutants
It is noteworthy that ssNMR and biochemical analysis have remarkable synergism. Molecules are distinguished by their distribution in the mobile and rigid phase using ssNMR, which complements the view of their covalent linkages and alkali solubility as probed by chemical approaches. In a recent study, the functional diversity of α-1,3-glucan, the molecule overlooked in biochemical assays, was confirmed through its heterogeneous distribution in the alkali-soluble (AS) and alkali-insoluble (AI) portions of the inner and outer domains of cell walls (Figure 12) 387. This study also evaluated the function of GM and GAG, two molecules not discussed in the previous study49, and both were found to mainly reside in the mobile phase of the cell wall. An unexpected finding is the colocalization of valine and the alkali-insoluble core of the cell wall. The resistance of valine to hot alkali extraction suggested a putative role of this amino acid in stabilizing the macromolecular complex of cell walls.
Figure 12. Restructuring of cell walls in the mutant strains of A. fumigatus.

The mobile and rigid domains are shown as pale yellow and pale blue regions, respectively. The alkali-soluble (AS) and alkali-insoluble (AI) parts are labeled for the parental strain. The molar fractions of polymers in each phase and the average cell wall thickness observed by TEM were taken into consideration for depicting the schemes. Adapted with permission from reference387. Copyright 2021 Springer Nature.
The combination of ssNMR with functional genomics allowed us to compare the cell wall organization in a widely used strain (ΔakuBKU80) of A. fumigatus and four mutants constructed based on this parental strain387. Each mutant selectively eliminates a major structural polysaccharide, including chitin, α-1,3-glucan, galactomannan (GM), and GAG. Using this strategy, we evaluated the structural function of each polysaccharide and monitored the compensatory changes of the cell wall in response to internal stresses. Comparison of different strains indicated that gene deletion significantly reshuffled the molecular composition and spatial organization of cell wall polysaccharides (Figure 12). These changes were correlated with the sophisticated modification of polymer dynamics and the decreased water-retention of cell wall polysaccharides to rationalize the reorganization of macromolecules in each mutant. The results suggest that fungi tend to produce denser and more hydrophobic cell walls as a mechanism to address structural defects, and further studies are ongoing to validate if this is a generally applicable compensatory mechanism for different fungal species and internal and external stresses.
4.5. Structural Polymorphism of Chitin and Chitosan in Different Fungal Species
Chitin is one of the most important carbohydrate components of fungal cell walls and has a prominent role in protecting and supporting the cell wall3. Widely found in almost all fungal species, chitin naturally serves as a promising target for novel antifungal agents393. Three model allomorphs (α, β, and γ) of chitin, with major variations in the chain orientation and the hydrogen-bonding pattern have already been reported by crystallographic studies372. In these allomorphs, adjacent chains are positioned in an antiparallel or parallel arrangement, namely, the α- and β-forms, respectively (Figure 13A). Recently, high-resolution ssNMR was combined with statistical methods, such as principal component analysis (PCA) and linear discriminant analysis (LDA), to compare the 13C chemical shifts of chitin using uniformly 13C-labeled cells of six fungal strains from Aspergillus, Rhizopus, and Candida species394. The structure of chitin was found to be intrinsically heterogeneous, with peak multiplicity observed in each sample, and with distinct features across fungal species (Figure 13B). Partial similarity was found between fungal chitin and the model structures of α- and γ-allomorphs (Figure 13C). It was also shown that the addition of salt and commercially available antifungal drugs did not significantly perturb chitin’s structure, emphasizing the structural resistance of chitin to external stresses. In the same study, the structure of fungal chitosan, a deacetylated form of chitin, was found to resemble a relaxed two-fold helix conformation. This study provided a promising strategy for analyzing the structure of many other polysaccharides and their numerous conformers across a broad spectrum of fungal species.
Figure 13. Chitin structure analyzed by ssNMR and PCA.
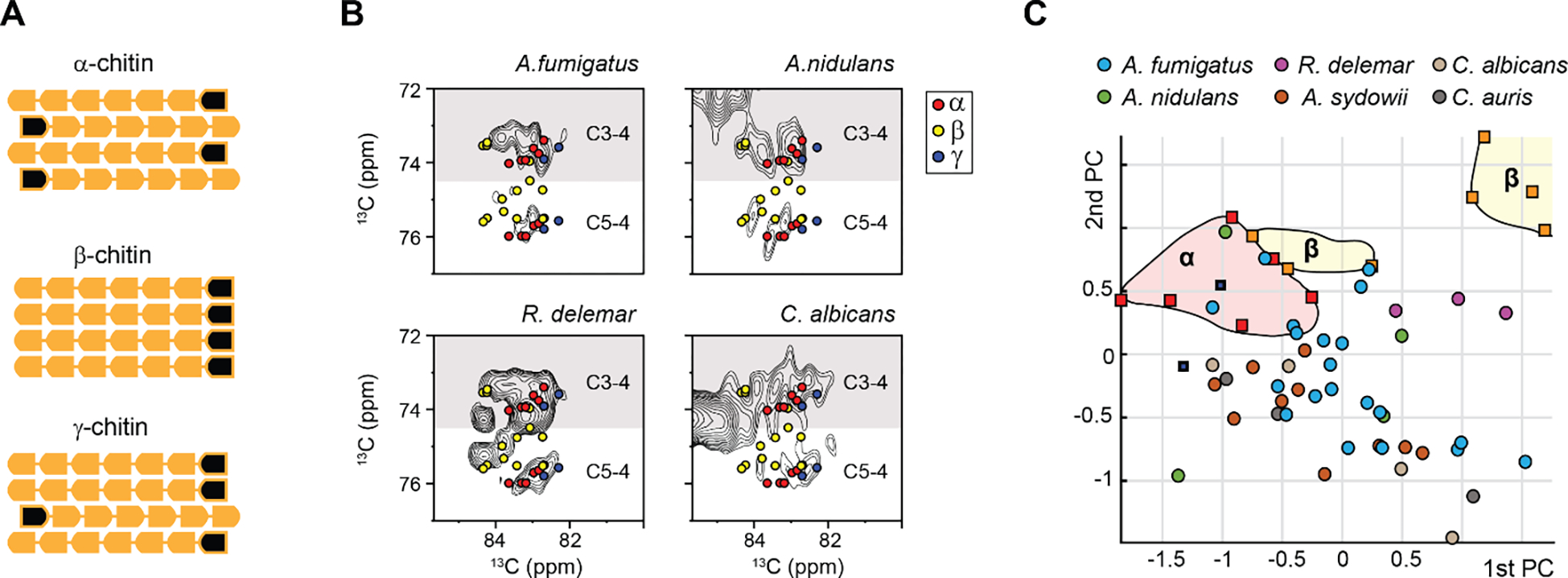
(A) Chitin allomorphs (α, β, and γ) showing different chain arrangements. Black marks represent the non-reducing ends. (B) Comparison of chitin signals in different fungi. Colored dots denote the data from three crystalline forms of chitin: α-chitin (red), β-chitin (yellow), and γ-chitin (blue). (C) PCA scores for chitin NMR chemical shifts projected onto two principal components (PC). Model chitin allomorphs (α, β, and γ-types) are illustrated using squares while chitin forms identified in the fungi cell walls are displayed by circles. Data from different species are color-coded. The figure is adapted with permission from reference394. Copyright 2021 Frontiers Media S. A.
4.6. Melanization of Fungal Cell Walls
Another important feature of fungus cell walls that can be described by ssNMR is the pigment deposition in C. neoformans, which is both a pathogen and a key model system for understanding fungal virulence395. This basidiomycete fungus is a cause of meningoencephalitis, inflammation of the brain and associated tissues, in immunodeficient individuals396. C. neoformans grown in a nutrient-deficient medium will polymerize and oxidize external phenolic compounds (such as catecholamines), depositing a group of structurally different pigments into cell walls397. For example, 3,4-dihydroxyphenylalanine (DOPA) melanin, also called eumelanin, can be synthesized using external precursors398. Despite its contribution to cell wall integrity and microbial pathogenesis399,400, the molecular organization of melanin and its association with carbohydrates are still not well understood.
Isolating melanins from the fungi preserve the morphology of the cells, causing hollow spheres called melanin “ghosts”. Using NMR cryoporometry on C. neoformans melanin extract, Eisenman et al. determined that this assembly is porous, with pore diameters mainly ranging from 1 to 4 nm in diameter, with only a few larger particles around 30 nm401. Interestingly, the available volume inside the pore was reduced once antibodies were attached to melanin. This observation led to a proposed antifungal mechanism. Melanin granules are held together in layers and small nutrient molecules, such as sugars and amino acids, can pass through the pores between the granules, while larger antifungal molecules get blocked (Figure 14A)401. Camacho et al. further combined ssNMR with EPR, microscopy, and proteomics to determine whether the melanin granules are also associated with non-pigment cellular constituents. In this study, melanin is found to accumulate into much larger spheres of ~ 200 nm in diameter, called melanin granules, each of which is made up of nanospheres with diameters ~ 30 nm402. Therefore, they uncovered the connection of crude melanin granules to polysaccharides and provided evidence that melanin granules are also connected to proteins.
Figure 14. Melaninization of C. neoformans cell walls viewed by ssNMR.

(A) L-DOPA precursors oxidized by laccase to make dopaquinone, then 5,6-dihydroxyindol (DHI) or 5,6-dihydroxyindole-2-carboxylic acid (DHICA) is formed by further oxidation. This expanding process continues when DHI/DHICA builds locally-ordered oligomers402. The accumulation of these smaller units results in the creation of the melanin granular, these melanin granules in turn interact with lipids, proteins, and polysaccharide components in the cell membrane and cell-wall. Panel A is adapted with permission from references401. Copyright 2005 American Chemical Society. (B) 13C spectra of catecholamine precursor (L-dopa here; left), corresponding melanins from cell-free autopolymerization (middle), and melanin ghosts produced by fungal biosynthesis (right). Panel B is adapted with permission from references404. Copyright 2018 Elsevier. (C) Lipid composition in whole-cell and melanin ghost. Panel C is adapted with permission from references50. Copyright 2020 Elsevier.
The biosynthesis of melanin was investigated using the catecholamine precursors, with significant differences observed between the synthetic and isolated melanins due to the requirement of exogenous substrates for melanization (Figure 14B)403. As revealed by 2D 13C-13C ssNMR and DNP-assisted 15N-13C correlation spectra of C. neoformans cell walls, different precursors not only yield distinct melanins but also alter the structural scaffold of melanized cell walls404. Chatterjee et al. applied a set of 1D 13C CP/MAS and 2D 13C-13C correlation techniques to elucidate the mechanism of melanin deposition in C. neoformans398,405. They studied the development of melanin conformation over time in the cell wall and identified an early-stage developing aliphatic scaffold comprised of polysaccharides and acylglycerides and a late-developing aromatic pigment together with indole and pyrrole structural moieties. They were also able to observe a structural network that contains close indole-indole connections and pyrrole-chitin covalent bonds. The observed development of multicomponent melanized assemblies confirmed and substantiated an interlayer deposition model that was proposed previously406,407, which will practically guide the therapeutic drug delivery targeting melanoma fungi398,408,409.
Zhong et al. developed labeling protocols to enable more detailed investigations of C. neoformans melanin using CP-MAS and HR-MAS methods410–412. Briefly, implementation of 2,3-13C2-L-dopa and ring-13C6-L-dopa precursors resulted in an indole structure formed from an L-dopa side chain, which revealed the site and structural modification involved in the cross-linking and polymer chain elongation of melanin. With the use of 1-13C-mannoses, it was further demonstrated that purified melanin ghosts contain mannose as β-pyranose, which indicates the integration of 1-13C-mannoses into the pigment-linked fraction of polysaccharides. In addition to mannose, D-glucose-13C6 was found to be integrated into the polysaccharide and aliphatic groups that may be linked to melanin412.
The presence of lipid components in C. neoformans melanin ghosts was discovered using J-coupling-based 2D 13C-1H INEPT and 13C-13C INADEQUATE experiments, which resolved the signals from triglycerides (TGs), sterol esters (SEs), and polyisoprenoids (PPs). Although the polymer composition (quantified using 1D 13C DP spectra) is comparable in the melanized and non-melanized cells, the melanized cells are particularly rich in SEs and PPs (Figure 14C). Also, the identification of these lipids in the non-melanized cells prepared in nutrient-limited media for a long time period (10–14 days) indicates that they could be produced even under nutrient stress, likely in anticipation of melanin synthesis.
Chrissian et al. compared two Cryptococcus species, C. neoformans and Cryptococcus gattii that share some phenotypic traits. They found that the high content of chitosan in C. gattii is associated with the distribution of melanin across the cell walls413. It was also shown that lipids and polysaccharides, especially chitosan, play important roles in attaching and layering melanin across the cell walls of C. gattii and C. neoformans413. Interestingly, the spore cell wall from a different fungus, S. cerevisiae, which lacks melanin414, bears a composition similar to the melanized cells in C. neoformans397. Indeed, both species use chitosan as a backbone to host either dityrosine or melanin. Similarities observed in these two evolutionarily separated species indicate that chitosan, polyaromatics, and triglycerides are basic elements in the formation of cell walls. These observations indicate that the composition and architecture of fungal cell walls are crucial factors in hosting and organizing pigments.
4.7. Understanding Structural Dynamics and Antifungal Mechanism
Solid-state NMR has been and is currently being employed to understand the mechanisms of actions of antifungal drugs. Recently, Anderson et al. proposed a new vision of the functional mechanism of Amphotericin B (AmB), which is one of the mainstays agents in the antifungal armamentarium (Figure 15A)415. In this study, the long 13C-T1 relaxation times of AmB suggest the formation of rigid aggregates. AmB is also far from lipids as shown by the slow 1H polarization transfer from the lipid acyl chains of phospholipid membrane (Figure 15B) and the lack of PRE effect from the paramagnetic DOXYL motifs covalently linked to the C5 and C16 of the lipids. These results lead to a structural model where AmBs dwell mainly in the form of large extramembranous accumulations (sponges), which extract ergosterol from the membranes and eventually lead to cell death (Figure 15C). The extraction of this polyfunctional lipid underlies the resistance-refractory antifungal action of AmB and opens a new perspective for describing its cytocidal and membrane-permeabilizing activities415. These structural findings revised the prevailing hypotheses that AmB forms ion channels for permeabilizing the membrane and deforming the fungal cell416–423. The results also challenge the models where AmB deposits in the intermediate/headgroup region of membranes for sequestering ergosterols419,424.
Figure 15. Structure and function of antifungal compound AmB in lipid bilayers.

(A) Representative structures of AmB and ergosterol (Erg). (B) 1H spin diffusion buildup curves revealing slow polarization transfer from lipid acyl chain to AmBs but fast equilibrium from water to AmBs. (C) The new sterol sponge model based on ssNMR data, disagreeing with the ion channel and the surface adoption models. Purple dots indicate the positions where paramagnetic tags (DOXYL) were covalently linked to lipids to check the PRE effects on AmBs. Panels A, B, and C are adapted with permission from reference415. Copyright 2014 Springer Nature. (D) 13C{19F} REDOR data for restraining the parallel and antiparallel orientations between AmB (yellow) and Erg. Left panel: Dephasing values for the Erg C26/27 signal at a 32-F-AmB:26,27-13C2-Erg ratio of 1:1. The dashed line is an average of the two solid lines simulated for 13C-19F distances of 5.2 and 6.8 Å. Right panel: dephasing of the Erg C4 signal at a 14-F-AmB:4-13C-Erg ratio of 1:1, with a fit to 13C–19F distance of 6.0 Å. Panels D is adapted with permission from reference425. Copyright 2016 American Chemical Society.
AmBs also exhibited considerable toxicity to human cells, which originates from the binding of this molecule to undesired sterols due to the similarity between fungal ergosterols and human cholesterols426,427. Nakagawa et al. used two labeled ergosterols (on the methyl end-group carbons 26/27 or on carbon 4) and two labeled AmB molecules (on carbon 32 or carbon 14) to study AmB–ergosterol and AmB–AmB interactions using 13C{19F} REDOR measurements425. The data revealed that AmB attaches to ergosterols in both parallel and antiparallel orientations at a ratio of 7:3 (Figure 15D). Intermolecular distances showed that the mycosamine part of the macrolide of AmB and the flat α-face of ergosterol directly interact, promoting a face-to-face van der Waals interaction in both parallel and antiparallel alignments. The described van der Waals interaction might help us better understand the sterol recognition mechanism and develop ways to reduce the undesired interaction with human cholesterols.
The exploratory studies discussed in Section 4 have demonstrated the capability of ssNMR for deciphering the supramolecular organization of biopolymers in native-state fungal cell walls. Interestingly, the unexpected function of α-1,3-glucan in A. fumigatus echoes with the omitted role of pectin in plant primary cell walls as discussed in Section 3.3. Despite the dramatic difference in their dynamics, both molecules are partially extractable by alkali, and therefore their structural contributions have been overlooked in biochemical analysis. Besides cell walls, ssNMR was also used to characterize the biofilms formed by fungi, for example, in A. fumigatus428 by quantifying different carbon pools inside the extracted ECM. We expect that the methods will result in a wide range of applications, from the systematic assessment of antifungal compounds targeting the cell walls to the structural principles underlying pathogenesis and the emerging drug resistance429,430.
5. The Highly Diverse Cell Wall Complexes of Ubiquitous Algae
5.1. Algal Cell Walls Are Structurally Diverse but Under-Investigated
Alga is an informal term regrouping different representatives in the Eukarya as well as in the Bacteria domains (Figure 1). They are a puzzlingly varied group of species, including multicellular and unicellular organisms, which are usually categorized primarily based on their photosynthetic pigment used for light harvesting during photosynthesis. The 70,000 species of microalgae (small and unicellular aquatic photosynthetic organisms) and 20,000 types of macroalgae (large multicellular aquatic photosynthetic plant-like organisms) include, among others, 8,000 green-blue algae, 14,000 red algae, 2,000 brown algae, and 10,000–30,000 diatoms431,432. They are ubiquitously found in almost all biomes and are adapted to different environments. Algae have a size ranging from less than a micron in diameter (e.g., microalga Prochlorococcus) to ~60 m long (e.g., macroalga Macrosystis)433,434. Strong from their 2.5 billion years of evolution, microalgae not only populate shallow oceans but are also found as deep as 300 m (e.g., Navicula pennata diatom), in polar ice, and even in aerial environments435–438. Due to the extremely high diversity of algae, most studies only focus on the production of molecules of biotechnological interest, usually by microalgae because of their rapid growth, rather than the structural principles of cell wall assembly for example. Indeed, some natural or artificially designed algae can be optimized to overproduce fatty acids for biofuels439,440, or minerals, proteins, vitamins, and polysaccharides for human health and nutrition441.
The morphology of algal cell walls can also be very different, with assemblies being simple “scales” coating the cell surface, more organized organic assemblies, or even almost purely crystalline mineral phases such as the silicon or bicarbonate crystals found in diatoms442. As for the composition, the main structural elements are polysaccharides, although highly rigid glycoprotein granules and biosillica shells are also found (Figure 2)4. Typically, algal cell wall polysaccharides include cellulose, mannan, xylan, alginic acid, chitin, sulfated glycans443,444, agar, keto sugars, extensin, lignin, and homogalacturonan4. Research on algal cell wall composition and structure started as early as in the 50’s using enzymatic reactions and chemical titration, with a focus on the chain length and branching of xylan in red algae and polysaccharide metabolism in marine species445–447.
Structural models have been proposed for some algal cell walls, although a considerable variation should exist within each specific group (Figure 16). Most related studies focus on polymer crosslinking and cation complexation448–450. Water molecules are important to the organization of cell walls, where hydrogel binding and ion interactions play key roles in maintaining the wall integrity. Green algae are the most studied systems due to their rapid growth and universal availability in nature. They usually have a fibrous cell wall, sometimes including cellulose fibrils associated with more dynamic xyloglucan and glucoronan (Figure 16A). Green algae also contain long polymers called ulvans, and a higher protein content compared to plant cell walls. Compared to green algae, red algae contain more sulphated glycans including glucans and xylogalactans and usually have more and thicker cellulose (Figure 16B). Red algae also have mannan-rich polymers crosslinking with other glucomannan or cellulose fibrils. As for brown algae, they have a thicker cell wall with cellulose microfibrils and a hydrated complex formed by alginate, in which glycoproteins can be found associated with other xylan/glucan-rich polysaccharides and fucans (Figure 16C).
Figure 16. Cell wall architecture of three major types of algae.

On the top of each panel, structural models were proposed for the cell walls of (A) green, (B) red, and (C) brown algae455,456. At the bottom of each panel, 13C NMR spectra (right) and SEM/TEM images (left) were shown for (A) Ulva armoricana457, (B) Sargassum sp.458, and (C) Chondria macropcarpa459. Panel A is adapted with permission from references455,460. Copyright 2007 American Chemical Society and copyright 2020 Egyptian Society for the Development of Fisheries and Human Health. Panel B is adapted with permission from references458,461. Copyright 1997 Canadian Science Publishing and copyright 2015 Springer Nature. Panel C is adapted with permission from references456,459,462. Copyright 2010 John Wiley and Sons and Copyright 2005 WALTER/DE GRUYTER GMBH & CO. KG and 2015 Springer Nature.
The complexity of the algal cell wall requires a variety of solution and ssNMR methods as well as nuclei to characterize its different organic and inorganic components. Solution 1H NMR was employed to detect the cell wall components of several brown algae including Fucales and Himanthalia elongata, which revealed the interaction between fucose-containing sulfated polysaccharides, proteins, and cellulose in the cell wall, and the association of alginate with phenolic compounds in the surrounding matrix451. They also managed to track the modification of glycan linkages and metabolomic dynamics of H. elongata, in response to a changing habitat. Besides the 13C methods for identifying carbohydrate components (Figure 16), ssNMR has also exploited 15N signals to track the cellular degradation of Botryococcus barunii green algae452, 31P to study phosphate-calcium complexes in Ulva lactuca cell walls453, and 29Si to characterize the mineral components in diatoms454. However, the algal cell wall remains substantially under-investigated (Figure 1). Solid-state NMR has been mostly used to identify algal cell wall components but never as intensively as for plants or even the fungal or bacterial cell walls.
5.2. Plant-Like Extracellular Matrices
Most NMR publications dedicated to algal cell walls report organisms whose constituents are similar to the carbohydrates found in higher plants463. Cellulose is an important polysaccharide found in almost all types of algae, and its structure, degree of polymerization, production, and extraction have been especially studied in green algae, mostly Cladophorales and Siphonocladales orders464,465. For example, Valonia ventricosa has been investigated because this large unicellular organism (diameter up to 5 cm) produces square-shaped cellulose crystallites466–468, which allowed the identification of the biphasic character of type-I cellulose (Iα and Iβ) by 13C CP-MAS data469.
Plant/liverwort-like470 mannose-rich cell surface and cell walls have been reported in Codium fragile - the giant-cell alga - based on 13C ssNMR data471. This work identified two types of 1,4-linked β-D-mannans in the cell wall replacing cellulose fibrils: mannan type-I alkali soluble-fibrils and mannan type-II alkali-resistant fibrils. Similar to cellulose, ssNMR has also been used to describe the coexistence of crystalline and non-crystalline mannan and orientational order/dynamics in the cell wall of the alga Acetabularia, known as mannan weed472. Specifically, the transition of the less-crystalline mannan-rich cell wall in diplophase to the cellulose-dominant cell wall in haplophase has been examined, which goes with mannan being the scaffold for the early stage of alga prior to cellulose. In this work, other molecules have also been identified in these flexible assemblies, including xyloglucan, pectic polysaccharides, as well as a small number of homorhamnan, galactomannan, glucogalactomannan, and also xylan.
Similar to the plant counterpart, algal cellulose is perceived as stiff fibrils mixed with other polysaccharides such as the insoluble portion of xylan473,474. This insoluble complex coexists with soluble fractions mostly containing other types of xylan, sulfated carbohydrates, and xyloglucan, as identified by 13C ssNMR in Nemaliales, Palmariales, and in Caulerpa species475–479. Some algal species (such as Charophyceae) even contain HG, RG-I, and hemicellulosic polymers, as well as MLG and lignin-like molecules4. Finally, another important constituent found in algae is arabinogalactan-rich proteins called AGPs, which are usually found in the extensin-like portion of photosynthetic organism cell walls480 including in plants481. In algae, AGPs are typically associated with hydroxyproline-rich glycoproteins (HRGPs) or N-glycosylation.
5.3. Sulfated Polysaccharide and Other Aliphatic Components
Other glycans have been reported in algal cell walls, most notably a wide range of sulfated saccharides470. Sulfate moieties are often described in soluble extracts and therefore characterized using solution-NMR, and these molecules are found decorating cell wall polysaccharides in native samples, mainly on galactans in red algae (Rhodophyta)482,483 and on fucans of brown algae (Phaeophyta)484. Oceanic green algae of the genus Codium have also been demonstrated to have a large amount of sulfated galactan. In a study of Codium isthmocladum, Farias et al.485 extracted and characterized 4-sulfated 3-β-D-galactopyranosyl units using solution NMR. Sulfated fucans crosslink different elements in the brown alga Fucales, leading to complex NMR spectra. Therefore, cell wall extraction and purification are necessary to recover the structural information and, for example, to confirm the presence of sulfated fucans and unique α-1,3-L-fucans451.
Several fucose-containing sulfated polysaccharides called fucoidans have been reported in brown algae, and exhibited antioxidant or anti-inflammatory activities486,487. Agar units can also be sulfated or methylated at different positions, giving furcellaran, funoran, porphyran and carrageaenan, which have been differentiated using 13C ssNMR488–490. Solid-state NMR has also been conducted to separate agars and carrageenans extracted from red algae488.
Alginate is the most abundant gel-forming molecule in the cell walls of brown algae and is considered as the main component for the control of ECM rigidity. The regulation of the gel strength is based on the frequency of polyguluronate blocks in the polymeric chains of alginate through the enzymatic conversion of β-(1–4)-mannuronate to α-(1–4)-guluronate491. Ulvan is a water-soluble sulfated polysaccharide found in green seaweeds and is the only one predominantly rich in rhamnose. Ulvan has been identified in different seaweeds using 13C NMR, together with other polysaccharides such as xyloglucan, cellulose, and glucoronan492.
Algaenan is a highly aliphatic molecule widely distributed in unicellular algae cell walls493. This biopolymer coexists with polysaccharides in many algal cell walls. For example, 13C CP/MAS characterization of cell wall extracts revealed the coexistence of algaenan with proteins and other polysaccharides such as cellulose and β-1,4-mannan in Coelastrum sphaericum green algae494. Algaenan is thought to induce local heterogeneity by creating gaps in crystalline materials, thus providing the flexibility needed for cell division495. Furthermore, this work reports the assignment of 13C signals from algaenan and fibrillar polysaccharides as well as the linkages within algeanan or between algaenan and cellulose. More recently, HR-MAS NMR and ESI-MS have been used to describe the structural features of Botryoccocu braunii algaenan-rich cell wall, where hexane-insoluble botryals is found to reticulate and form algaenan496.
5.4. A Dilemma of Non-Cellulosic, Fungi-Like Cell Walls
Chitin is the second most available polysaccharide in the biosphere but is far less studied compared to cellulose. Usually, chitin is found in arthropods, nematodes, and fungi, but has been reported in some microalgae, mostly diatoms. The studies of chitin using XRD and FTIR497,498 are typically associated with biomineralization happening in diatoms497,499. NMR studies of diatoms will be reported below in Section 5.5. Nonetheless, 1H NMR data has also reported chitin in a freshwater green microalgae Chlorella56; however, chitin is also associated with algal pathogens, which might explain the presence of chitinases in many microalgae. Chitinase from Volvox carteri has been isolated, and its crystal structure was determined by X-ray crystallography. Solution 1H and 15N NMR, in combination with isothermal titration calorymetry (ITC), allowed to determine the interaction strength of chitinase with chitin oligosaccharide500. Therefore, caution is needed when referring to older publications mentioning chitin as an algal cell wall component. However, microalgae can be genetically modified to produce chitin, as shown with Chlorella using chloroviruses501. Solid-state NMR might be a suitable tool for exploring this type of algal cell walls as it can resolve the signals of chitin and related cell wall molecules using intact cells49,82.
5.5. The Diatom Mineral Shell
Diatoms include over 20,000 species431,432 and are among the most common form of phytoplankton, being responsible for more than 25% of the world’s net primary production (NPP). These microorganisms are primary producers mostly found in fresh water and oceans, where they support the survival of most higher organisms. Diatoms are covered with a silicified skeleton (frustule), which is a natural hybrid of organic and mineral material. Its external shell is made of two distinct regions, i.e., the valves (on top) separated by the girdle bands (on the side) (Figure 17A). The valves are the nanoscale-sculpted parts of the diatom shell that allow molecular transport and efflux, while the girdle bands are the structural elements holding the valves together. Diatom cell walls are rich in silica and also contain organic components such as proteins, long chains of polyamines, and polysaccharides502. Silica production in diatoms has been proposed to be initiated in silica deposit vesicles (SDV) where the valves and girdle bands are built. However, mobilization and trafficking precursors, exocytosis, and even associated protein are still either poorly understood or unknown503. Current knowledge on the synthesis mechanisms involved in diatom cell division and cell wall building is summarized by Shresta et al.504.
Figure 17. Characterization of diatom cell wall and frustule using ssNMR.

(A) SEM images of the diatom showing valve biosilica that have organic threads (white arrows)505. Panel A is adapted with permission from references505,506. Copyright 2018 Frontiers Media S.A and copyright 2011 Springer Nature. (B) DNP-assisted 15N-edited 13C-13C PDSD correlation (top) and N(CA)CX experiments (bottom) detecting amino acid signals as mostly in β-sheet conformation in S. turris220. The structural model contains a 3 nm layer of carbohydrates and proteins covering the 40–80 nm silica domain with LCPAs enclosed. Organic matter and silicon can be detected within (C) whole cell and (D) extracted diatom shells (frustule) as adapted from Tesson et al.507. (C) 31P NMR detects lipids, glycerol 1,2-cyclic phosphate and polyphosphates, while 29Si data reports Q2, Q3, and Q4 silicon environments. (D) 13C ssNMR reporting organic molecules including proteins and carbohydrates. Extracted shells with SDS conserve their overall native structure as seen by SEM508. The valve costa (green line), cross-connections (orange lines), areola pore (red circle) and fultoportula (yellow/blue circles, tube-like structures) can be observed. Panel B is adapted with permission from reference220.Copyright 2015 John Wiley and Sons. Panel C is adapted with permission from reference507. Copyright 2008 Springer Nature. Panel D is adapted with permission from reference507,508. Copyright 2008 Springer Nature and copyright 2019 Springer Nature.
Baldus, Brunner, and coworkers have employed MAS-DNP methods to characterize [13C, 15N, 29Si]-enriched biosilica from Stephanopyxis turris220 and T. pseudonana509. 2D 13C-13C/15N experiments distinguish the secondary structure of the proteins involved in the diatom cell wall of Stephanopyxis turris220, showing mainly β-sheet structure (Figure 17B). The low DNP enhancement observed on LCPA suggests these molecules as being included in the thick (40–80 nm) biosilica matrix instead of the thin (3 nm) protein layer in which the polarizing agent should penetrate and diffuse easily.
Little is known about the frustule biosynthesis, but our understanding of the cell-wall architecture and association of organic molecules has been improving. Two major proteins, namely silaffins/silacidins and long-chain polyamines (LCPAs), have been identified in the organic phase of the diatom cell wall510, while cingulins were found to be associated with the girdle bands and involved in the 3D organization of the frustule in Thalassiosira pseudonana511. Interestingly, chitin has also been described in T. pseudonana cell walls, where it would be associated with valve formation512, suggesting that the architecture of the biosilica cell wall is solidified with a chitin network. In addition to these soluble materials, insoluble microtubule and actin microfilament networks were found in the cell and associated with the SDVs during valve formation, and being important for SDV positioning and mesoscale organization of silica, respectively513,514.
Silica is the most abundant component in the diatom matrix, therefore the 29Si spin ½ nucleus is of interest for ssNMR characterization despite their ~5% natural abundance (Figure 3). The ensuing low sensitivity makes it difficult to characterize silica in cellular systems. Using isotopically enriched 29SiO2, NaH13CO3, and Na15NO3, Tesson et al.507 and Kolbe et al.515 produced labeled samples of T. pseudonana and Cyclotella cryptica diatoms. The combination of 31P, 13C, 15N and 29Si ssNMR methods allowed the identification of signals from carbohydrates, nucleic acids, biosilica, lipids and proteins using a single sample (Figure 17C, D). Three 29Si broad peaks were detected, namely Q2, Q3, and Q4 corresponding to different levels of condensation of SiOH moieties, referring to different numbers of hydroxyl groups converted to Si groups in the building block of biosilica (Figure 2). Therefore, 1D 29Si spectra are sufficient for determining the degree of condensation of SiOH using the Q4/Q3 ratio (Figure 17C)516. Tesson et al.507, together with other groups517, further showed that changing the medium salinity modifies the material density and packing, and increases the amount of organic component in the frustule, even if the organic phase is made of the same elements. 13C and 29Si ssNMR results confirmed the presence of proteins220 and polysaccharides within the silica network507. Comparison between 31P DP and CP differentiated the mobile polyphosphates from the more rigid inorganic phosphates, phospholipids, and glycerol-1,2-cyclic phosphates (20–30% of the diatom’s phosphorous).
Tesson and Hildebrand also reported the cell wall composition of many other diatoms518, such as Amphora salinam, Stephanopyxis turris or Navicula cryptocephala, in which glucans were identified. The major form contains β-1,3-linkages, and is called chrysolaminarin as an energy storage molecule or callose when in a fibrillar form associated with the cell wall. Mannan-rich carbohydrates and more recently chitin have also been reported. Chitin occurs in numerous calcium-based biominerals519 where it forms insoluble compartments within the cell wall that is associated with mineralization. This phenomenon has been studied in detail only recently using ssNMR512, although it has been reported almost 60 years ago520. Indeed, most recent works report both surface507 and interior512 chitin molecules that can be important within the cell wall of T. pseudonana. 13C labeling allowed the identification of α- and β-chitin allomorphs in C. cryptica515 and T. pseudonana512, respectively associated with silica and structural chitin. Finally, glucoronomannan was found to be of high significance to cell-wall biogenesis in some diatoms. In Phaeodactylum tricornutum521, 1H and 13C NMR allowed the resonance assignment of the α-1,3-mannans that are decorated with sulfate ester groups and β-D-glucuronic residues442.
The contacts between biosilica and organic molecules in Thalassoria pseudonana and Cyclotella cryptica cell walls have been assessed using both {1H}−13C-29Si double CP-based HETCOR and 13C{29Si} REDOR expriments515,522. Contacts were observed between the 29Si in silica and the 13C sites of long-chain polyamine (LPA) and chitin.
5.6. Trends in Whole-Cell Investigations of Microalgae
Multiple solution NMR methods have been developed to allow the use of whole-cell samples for characterizing the dissolved components or gels made of very mobile aquatically available molecules523. This has been achieved using suspensions of intact 13C-labeled cells of Chlamydomonas reinhardtii, Chlorella vulgaris, and Synechocystis sp. green microalgae placed in solution NMR tubes or through more complex set-ups. For example, Farjon and coworkers have flown microalgae suspension through a benchtop solution NMR spectrometer to allow live-cell metabolomic studies focusing on lipid metabolism524,525. Based on a more solid-like methodology, Simpson and coworkers are developing comprehensive multiphase NMR (CMP-NMR) to probe the dynamically distinct phases in intact cells, which allows to benefit from the spectral improvement of MAS, but not only. Indeed, MAS is combined with magnetic field gradients and also lock, full susceptibility matching, all that built with a solid-state circuitry to allow high power pulses needed for characterizing gel/solid samples526. Application of this method to microalgae led to the identification of several cell elements in native Hyalella azteca, such as different amino acids, glucosamine, glucose, and trehalose526. Using these methods, multidimensional NMR experiments reported the signals of polysaccharides from the very mobile, gel-like, and even rigid fractions of cell walls as well as metabolites. It should be noted that the same multiphase NMR approach has also been applied to higher organisms such as Daphnia magna527.
Precursors and degradation products can be analyzed using multidimensional solution NMR, which allowed to assign amino acids and glycans released by microalgae in the medium after natural enzymatic hydrolysis (Figure 18A)523. HR-MAS data have been associated with statistical tools such as PCA to differentiate microalgal species (Figure 18B)528. In fact, HR-MAS is of interest for gel-like materials, even for those included in whole-cell samples. Chauton et al. focused on the characterization of P. tricornutum, Chaetoceros muelleri, T. pseudomona, Dunaliella sp., and many other marine microalgae using both ssNMR and HR-MAS techniques529–531. PCA results of the 1H and 13C data of a large number of metabolites and middle-sized molecules provided reproducible differences between strains and species, which are further linked to the nutritional values of those samples.
Figure 18. High-resolution solution and solid-state NMR on whole-cell samples of algae.

(A) Solution NMR of whole-cell C. reinhardtii resolving carbohydrate and amino acid signals. Panel A is adapted with permission from references523. Copyright 2016 Springer Nature. (B) HR-MAS allowing to make principal component analysis to distinguish algal species based on the metabolite composition. Panel B is adapted with permission from references528. Copyright 2018 Springer Nature. (C) Morphological diversity in multiple green microalgae recently studied by ssNMR. Differences are mostly based on cell-wall type, lipid head groups, and storage molecules. (D) 1D 13C ssNMR spectra probing polymers in different regimes of dynamics in C. reinhardtii. The dynamic and rigid molecules were probed using RINEPT and CP, respectively. Quantitative detection was achieved using DP with long recycle delays. Panel D is adapted with permission from references532. Copyright 2015 Elsevier. (E) Summary of glycosylated components in different regimes of dynamics. (F) 2D 13C spectra of C. reinhardtii showing rigid carbohydrates and proteins in CP-DARR and mobile lipids and proteins in a RINEPT-TOBSY spectrum. Panel F is adapted with permission from references533. Copyright 2018 Springer Nature.
Systematic ssNMR characterization of whole microalgae has been conducted recently74,532,533. In 2015 and 2018, Arnold et al. employed spectral editing methods to selectively detect cell constituents based on their overall dynamics. Three organisms have been investigated including the freshwater C. reinhardtii (wild type and cell-wall depleted strains) and marine microalgae Nannochloropsis oculata and Pavlova lutheri (Figure 18C). Combination of dynamic filters and 1D 13C ssNMR spectra proved a valuable strategy for differentiating cell compartments (Figure 18D) with sequentially increasing dynamics: the rigid energy-storing starch grains, the intermediately dynamic polysaccharides in the cell wall, and the very mobile saccharides in the galactolipids found in membranes (Figure 18D, E). A range of different magnetization transfer techniques and spin-diffusion filters (i.e. long PDSD mixing time) have been combined to highlight starch, the most abundant and most crystalline element of microalgae (Figure 18F). In C. reinhardtii, the rigid fraction is dominated by starch, whose crystal type has been determined directly in the cell79, while the mobile fraction includes lipids and several polysaccharides. 2D ssNMR experiments, such as TOBSY, DARR and PDSD, enabled the unambiguous identification of galactofuranose and arabinofuranose, which are likely associated with cell wall glycoproteins.
Recently, we have introduced a broadly applicable protocol for quantifying the content and linkages of glycans in microalgae using ssNMR spectroscopy534. The method was demonstrated on a naturally cellulose-deficient strain (CK-5) of the green microalga Parachlorella beijerinckii, which is a promising candidate for producing high-value fermentable carbohydrates. 2D 13C−13C correlation spectra of uniformly 13C-labeled cells revealed that most of the carbohydrates in the glycolipids and cell walls are highly mobile; therefore, we can use 2D 13C DP INADEQUATE with short recycle delays (Figure 19A), an experimental scheme preferentially detecting mobile molecules, to quantify the glycan composition. Starch was found to dominate the rigid phase of this microalga, and its content was separately calibrated using a calibration factor, which was obtained by comparing the starch intensities in two 1D 13C spectra with either selective detection of mobile components or quantitative detection of all molecules. This non-destructive method provides accurate quantification of the amount and linkage of glycans using whole-cell samples, as verified by the good agreement with MS results.
Figure 19. Structure and packing of microalgal carbohydrates resolved by 2D ssNMR of whole cells.
(A) 13C refocused DP-based J-INADEQUATE spectrum of uniformly 13C-labeled P. beijerinckii cells with a 2 s recycle delay selecting the mobile polysaccharides. (B) Difference spectrum obtained from two parental spectra (PAR and CORD) showing only long-range intermolecular or inter-residue contacts. Representation of identified inter-residue contacts of starch is illustrated on the top. (C) 1D 13C cross sections of the 2D INADEQUATE spectrum showing splittings based on 1JCC couplings for the Sa residue. Carbon numbers are labeled on the sides of each panel. The deconvoluted (blue) peaks are plotted underneath the simulated (magenta) and measured (black) spectra. Adapted with permission from reference. Copyright 2021 American Chemical Society534.
In addition, starch was found to adopt a well-organized structure in the cell as shown by the extensive intermolecular contacts between its constituent molecules (Figure 19B)534. Some xyloses were found to exist in both the mobile and rigid regions of the cell wall, with their chemical shifts partially aligned with the flat-ribbon 2-fold xylan that was initially recognized in plants. Therefore, some structural components of algal cell walls, probably the glycoproteins, provide the even surface required for accommodating the flat-ribbon structure of 2-fold xylan. For the first time, one-bond scalar couplings (1JCC) were systematically observed for almost all carbohydrate components in cellular samples, enabling future explorations using J-coupling in ssNMR to facilitate structural determination (Figure 19C).
In conclusion, the ssNMR studies reported the remarkable complexity of microalgal cells architecture and synthesis. Due to the incredible diversity of microalgae, only a small fraction of these organisms has been studied in detail so far. Further developments are to be expected, with interesting insights from a variety of nuclei that can be detected and incorporated into this kind of organism.
6. Solid-State NMR Investigations of Bacterial Extracellular Matrices
6.1. Bacterial Cell Walls and Biofilms
Bacteria are universal species found in all biomes and can survive extreme conditions535. Commonly considered as the simplest living organisms, bacteria may closely resemble the Earth’s original living organisms due to their prokaryotic cellular organization. Bacteria play key roles in atmospheric nitrogen capture and degradation of organic matter536. They are also associated with toxic or contaminated environments where uncontrolled bacterial growth can be devastating, as well as with many diseases found in humans, animals, or plants. Exposure to antibiotics has been dated back to 350–550 Common Era, as revealed by traces of tetracycline found in human skeletal remains537,538. Today, antibiotics target a broad spectrum of metabolic pathways in bacteria, such as depolarizing cell membranes and inhibiting the synthesis of specific proteins, nucleic acids, and cell wall components539. However, bacteria readily adapt to antibiotics and develop resistance mechanisms540, the timeline of which can be as short as one year as seen with daptomycin541. The resistance mechanisms developed by bacteria include efflux pumps, limitation of drug uptake by cell surface modification, modification of cell wall precursors or target molecules, and direct degradation of antibiotics542,543. This is why bacterial infection and multidrug resistance will continue their significant impacts on all living organisms31,32.
Nowadays, most antibiotics are targeting bacterial cell walls. From a structural point of view, gram-positive bacteria have a thick peptidoglycan (PG) cell wall, with lipoteichoic acid (LTA) crosslinking different PG layers and outer components such as glycoprotein-rich S-layer and the polysaccharide capsule (Figure 2). Gram-negative bacteria have a thinner peptidoglycan layer protected by a second outer membrane mainly made of lipopolysaccharides (LPS), with further coverage by entangled S-layer and capsule components. Bacteria can also be found in a highly organized multicellular arrangement called biofilms34. Once bacteria adhere to a surface, they can adopt a sedentary behavior and grow in a complex organic viscoelastic material. This extracellular polymer substance (EPS) consists of an assembly of curli amyloids and different polysaccharides such as cellulosic derivatives. The carbohydrate components facilitate surface adherence and act like glue to help curli association and coherence. Biofilms protect the microorganisms from chemical and mechanical clearance processes, making bacterial communities a threat to human health, as seen with medical devices and implants544,545. It has been estimated that 60 to 80% of chronic human microbial infections are associated with bacterial biofilms546. Understanding the overall architecture, synthesis, and remodeling of cell walls and biofilms is thus of great interest.
An increasing number of research groups have been using ssNMR to elucidate cell wall architecture and the structural factors driving cell adherence and biofilm assembly19,547–549. We will highlight the recent advances in understanding bacterial ECMs using bacterial cells or extracts. We will also briefly describe the remodeling process of ECMs in response to antibiotics or other bioactive molecules.
6.2. Bacterial Cell Architecture and Antibiotics
6.2.1. Structural Components of Bacterial Cell Walls
Since the cell wall is a relatively rigid surface presenting many microscopic elevations, its ultrastructure has been examined by atomic force microscopy (AFM) and other imaging techniques. For example, AFM 3D reconstitution and other imaging techniques550,551 have been used to identify the surface pores formed by loosely packed peptidoglycans (PGs) and make a comparison with the much denser material of the inner cell wall where polysaccharide strands are only apart by less than 7 nm (Figure 20A)550. Such microscopic arrangements can be substantiated by the atomistic description of polymer structure and packing provided by ssNMR of extracts and even intact cells.
Figure 20. Composition and architecture of PG and teichoic acids in gram-positive bacteria.
(A) AFM images of PG in S. aureus cells revealing ring architecture made of concentric peptidoglycan fibers. The strand width and inner-strand distances of PG were also shown. Panel A is adapted with permission from references550. Copyright 2020 Springer Nature. (B) Representative structures of PGs in S. aureus and B. subtilis, with different stems and bridges. (C) 1H-detection ssNMR of PG amino acids under ultrafast MAS of 100 kHz. Overlay of 1H-13C correlation (red) and 2D hHcH-TOCSY correlation (black) spectra showed through-bond correlations as indicated using dashed lines. Panel C is adapted with permission from references88. Copyright 2019 Elsevier. (D) WTA as another important component of the gram-positive cell wall. 31P and 13C spectra detect the different structural motifs in WTA. Panel D is adapted with permission from references552,553. Copyright 2009 American Chemical Society and copyright 2018 American Chemical Society.
Bacterial cell walls are mainly made of PG, which contains a linear polymer of two alternating sugars: N-acetylglucosamine (GlcNAc or NAG) and N-acetylmuramic acid (MurNAc or NAM). NAM units from different chains are linked via short oligopeptide bridges (3–5 amino acids), creating a compact and strong material covering the bacteria surface. Variations exist in the bridge structure and the amino acid sequence of the stem (Figure 20B). For example, a penta-glycine bridge is present in S. aureus but absent in Bacillus subtilis where diaminopimelic acid (DAP), a non-protein amino acid, is found in the stem. Our current ssNMR methods for PG characterization are mainly designed for 13C and 15N-labeled materials. However, the recent development of ultrafast MAS and 1H detection might have paved up the way for rapidly and cost-effectively investigating the structure of multi-gigadalton PGs using unlabeled bacterial cells (Figure 20C)88.
The building blocks of PGs are synthesized on the external region of the cytoplasm and involve a precursor called lipid II - a lipid covalently linked with a disaccharide connected to a pentapeptide. It is therefore not surprising that lipid II also serves as a target of antimicrobial drugs554. To initiate PG building, from the inner membrane leaflet, lipid II is translocated outside of the cell by flipases, where the PG building blocks are progressively encapsulating the cell with the assistance of the well-known penicillin-binding proteins and other partners555–557.
PGs can be covalently bound to other cell wall molecules. Depending on the bacteria species, they can bind to a polysaccharides capsule (the glycocalyx) or to the glycoprotein-rich S-layer (Figure 2)558–561. In gram-positive bacteria, the polyphosphate-rich LTA and wall teichoic acid (WTA) (Figure 20D) are associated with the thick PG layers (more than 30 layers corresponding to tens of nanometer thickness)562. In gram-negative strains, thinner PG sheets (less than ten layers) are covered with an outer membrane made of O-glycosylated lipids called lipid-A, giving the well-known LPS2,563. These components make both types of assemblies harder for antibiotics to penetrate564, and provide the mechanical properties needed for handling external and osmotic stresses and accommodating cell growth, communication, and multiplication565.
6.2.2. Investigations of Bacterial Cell Walls
Simorre and colleagues have compared the organization, metal coordination, crosslinking, and flexibility of PGs across gram-negative (E. coli) and gram-positive (B. subtilis and S. aureus) strains219,549. In E. coli strains, a tetrapeptide is linked to the NAM residue of the PG with the sequence of D-Ala–X–D-Glu–L-Ala–MurNAc, where X is a non-protein amino acid (e.g., meso-A2pm in many gram-negative bacteria and Mycobacteria) (Figure 21A)30. This structural scheme differs notably from that of PGs in gram-positive bacteria presented in Figure 20B. Overall, the dynamics in PG, associated with the degree of peptide cross-linking, is the highest in S. aureus, followed by B. subtilis and then E. coli (Figure 21B). Therefore, E. coli has a thin PG made of short chains, with reduced crosslinking and therefore more pronounced dynamics.
Figure 21. Peptidoglycan and lipopolysaccharide in gram-negative bacterial cell walls.
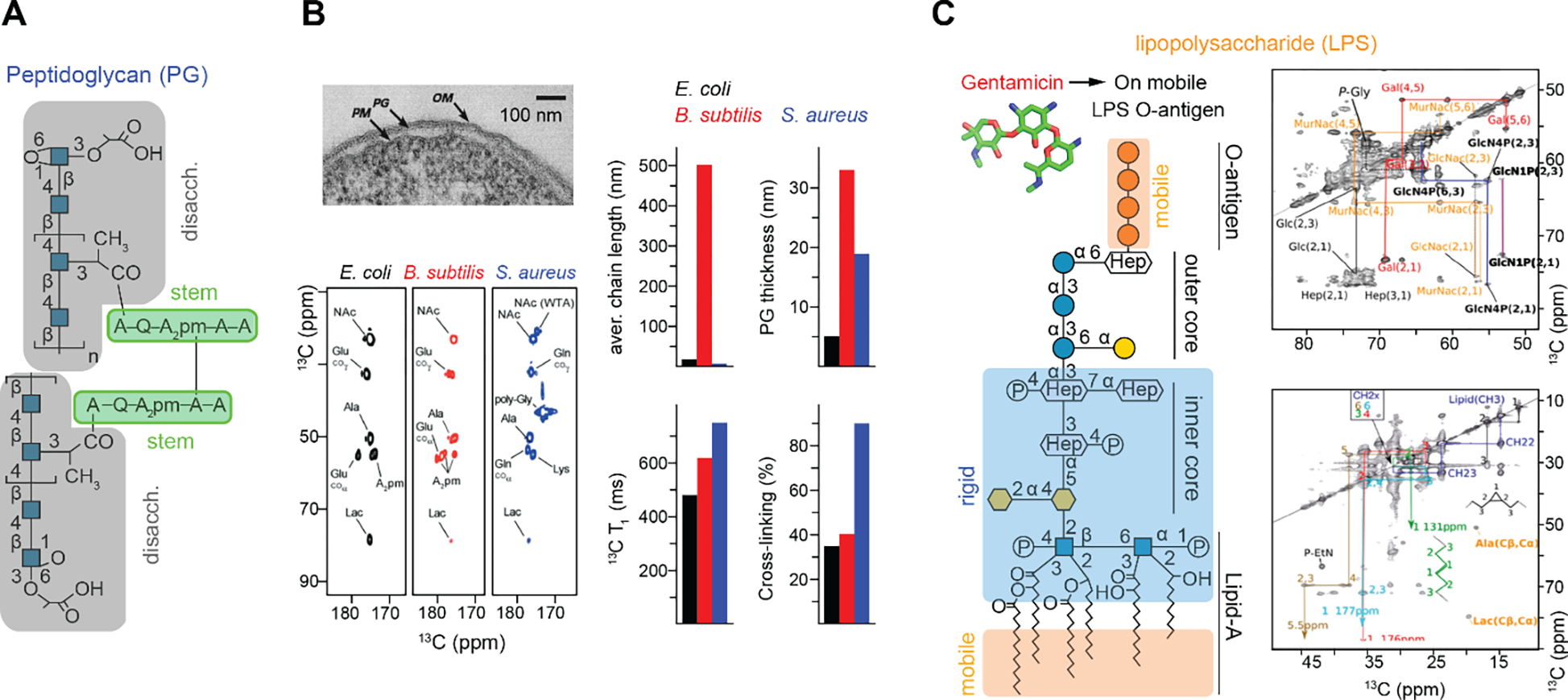
(A) Representative E. coli PG architecture with a non-protein amino acid A2pm. (B) Solid-state NMR spectra (bottom left) resolving the different amino acid composition in the PG stems of E. coli (gram-negative) and two gram-positive bacteria (B. subtilis and S. aureus)549. Chain length, PG average thickness, 13C-T1 relaxation, and cross-linking were compared across the three species (right). The PG layer can be resolved from the outer membrane (OM) and plasma membrane (PM) using TEM (top left). Panel B is adapted with permission from references549,569. Copyright 2020 American Society for Microbiology and copyright 2010 American Chemical Society. (C) LPS as another cell wall component in gram-negative bacteria. Its lipid A (bottom) and glycan (top) regions were assigned using ssNMR 13C-13C DARR spectra (right). Figure is adapted with permission from reference570. Copyright 2018 American Chemical Society.
The 3D arrangement of S. aureus PG has also been discussed for a long time and ssNMR identified a 4-fold helical glycan symmetry that allows PG stems to organize into parallel and antiparallel orientations in the PG mesh566. In S. aureus, the PG architecture and the cell wall morphology were found to be modulated by the variations in medium composition and growth stage, highlighting the plasticity of bacterial cell walls567. Based on high-resolution ssNMR data, Schanda et al. also proposed a model of PG complexed with a transpeptidase enzyme that is responsible for PG synthesis in B. subtilis568. Chemical shift perturbation was used to identify the region of interaction between this enzyme and PG568.
Gram-negative bacteria also have LPS that decorates the surface of the outer membrane571. This molecule is made of different sections, as shown in Figure 21C. Jachymek et al.572 reported 1D 13C/1H HR-MAS, and 2D HSQC/HMBC results, assisted by MALDI-TOF MS, to assign the glycan component of LPS. They showed that the O-glycan pattern of LPS called antigen remains identical in different strains of the gram-negative bacterium Yokenella regensburgei, even if the level and position of acetylation can vary. Simorre, Schanda, and coworkers applied ssNMR on extracted or reconstituted LPS endotoxin in E. coli and P. aeruginosa570, proving that the LPS O-antigen is the structural domain for interacting with antibiotic gentamicin (Figure 21C). Other components were also identified in this extract, mainly PGs or enzymes involved in PG synthesis found in the outer membrane573.
The negatively charged teichoic acids are crucial to surface adhesion and metal binding in gram-positive strains574. 31P ssNMR spectra and T1ρ relaxation have been used to detect the binding of the LTA to different surfaces. For example, the LTA from S. aureus could use its glucosamine and alanine groups to bind PGs and the solid surface, respectively575,576. This organization can help localize water molecules between the PG and the surface. At high metal concentrations, 31P chemical shift anisotropy (CSA) data suggested a bidentate coordination model for both S. aureus LTA and B. subtilis WTA with magnesium552. However, at a low concentration of metal, 111Cd-31P distances measured by REDOR experiments suggested inequivalent monodentate P–Cd interactions that lead to a linear Cd–P–Cd arrangement577.
Notably, cell-associated components such as the lipoproteins found in E. coli cell envelopes have also been studied by ssNMR578. Using mostly INEPT-based methods, they examined the structure of the recombinant outer membrane protein (PagL) and other inner components of the cell envelope such as lipids, PGs, and lipoproteins. They emphasized the effect of the surrounding compartment on the molecular arrangement of PagL and established ssNMR protocols for investigating cell-associated molecules.
Nordmark et al. identified exopolysaccharides and acidic polysaccharides from Pseudoalteromonas aliena cell walls579. Using 13C and 31P ssNMR, Bui et al. have examined Streptococcus pneumoniae, revealing glycan chains with approximately 25 disaccharide units in the water-insoluble fraction (probably from PG fractions). 31P resonances allowed the assignment of WTA ribitol and phosphocholine lipid head groups, while 2D 13C ssNMR assigned protein side-chains and resolved glycan configurations580. The structure of the S-layer was also examined, which was found to be sensitive to the temperature and pH581.
Many studies used HR-MAS methods to examine oligosaccharides in the bacterial cell wall assembly. For example, signals of arabinogalactan (AG) and lipoarabinomannan (LAM) were assigned using 1H-13C HR-MAS HSQC and 3D HCCH-TOCSY experiments. Also, a bottom-up approach was used to reconstitute the signals, where the spectra of a whole-cell sample were reconstructed from the signals of isolated fractions582. 2D and 3D HR-MAS methods were also used to monitor the cell composition and characterize the abundant osmoregulated periplasmic glucans in Ralstonia solanacearum583,584.
6.2.3. Interactions of Peptidoglycans and Other Molecules with Antimicrobial Agents
Schaefer, Cegelski, Kim, and colleagues have immensely contributed to the development of ssNMR methods, involving the versatile use of 15N, 13C, 19F, and 31P nuclei in the REDOR technique to understand the bacterial cell wall and biofilm architecture as well as the effects of antibiotics548,585–588. The modes of action of many different antibiotics have been examined, including lipoglycopeptides such as vancomycin and its derivatives548,585–588. In these studies, bacteria were typically labeled using site-specifically labeled 13C-alanine and 15N-glycine or 13C-glycine and 15N-lysine, and treated with specifically 19F-labeled antibiotics, thus enabling the use of the REDOR pulse sequence for probing PG structure and drug binding585,587,589. For example, a vancomycin derivative named amphomycin was found to cause thinner cell walls in S. aureus590. Also, the observed inhibition of WTA synthesis is consistent with Park’s nucleotide accumulation. The added lipoglycopeptide binds the mature PG template to reduce the crosslinking in the cell walls and deconstruct the PG template566,591. Fighting against resistance, another derivative (DFPBV) was found to use its hydrophobic sidechain to stabilize the binding to PGs in a vancomycin-resistant strain of S. aureus592.
Similar approaches have been applied to Enterococcus faecium and were further coupled with LC/MS analysis587,593. MAS-DNP was also employed to detect interactions between antibiotics and lipid II in situ594. The mechanism of antibiotic resistance involving the cell walls of several bacteria such as B. subtilis, S. aureus, E. coli, and Pseudomonas aeruginosa has also been described88,549,568,595. In addition, antimicrobial peptides (AMPs) often interact with the PG and other cell wall components, although they are not the final target of AMPs. AMPs, such as the human cationic polypeptide ECP, have been proved to interact strongly with the PG layer and indirectly interfere with cell replication by interacting with genetic materials596–598.
6.3. The Biofilm: An Organized Complex Supporting Bacterial Communities
6.3.1. The Formation of Biofilms
Biofilms ensure a safe environment in which bacterial communities can cooperate (such as synergistic microconsortia), evolve, and resist chemical and physiological reactions. The density of cells in these communities is high, ranging from 108 to 1010 cells per gram of biofilm599, with possible mixing of different species. To form biofilms, microorganisms need to switch from individual cells (planktonic) to a multicellular form (sessile) where microcolonies can be clustered600. This process involves several metabolism transitions601,602 and leads to the production of very different biofilms. For example, to protect the internal material of bacteria from desiccation, E. coli and B. subtilis can form a dry surface layer603,604, while P. aeruginosa produces a liquid-crystal sponge structure to maintain internal moisture605.
Biofilm formation can be divided into five main stages, through which it becomes progressively more differentiated and more strongly attached to the surface (Figure 22)606,607. First, individual planktonic cells settle and adhere to a substrate. Then, under favorable conditions, bacteria are still capable of some motion using pilus motility, and start secreting EPS, resulting in more tightly attached cells to their substrate. Third, the biofilm maturation begins: microcolonies start to appear, connected through water channels that allow communication, nutrient flow, transversal gene exchange and quorum sensing608. Simultaneously, bacteria are creating a multi-layered assembly and a heterogeneous matrix with gradients of small molecules or oxygen availability between the cells’ surface and interior609. Stage four consists of a mature biofilm where many coherent layers of cells form a 3D assembly. Quorum sensing orchestrates the overall biofilm development, mainly using water channels that irrigate the colonies. Lastly, microcolonies from the biofilm - that can be 50 (aerobic) to 400 (anaerobic) μm thick610 - are released in the medium to colonize other surfaces and start a new biofilm when physiological conditions are optimal, for example, when an excess of nutrients is available611.
Figure 22. Schematic model of biofilm formation and maturation.

Cells in solution first attach to a surface (stage 1) where it will grow (stage 2) and produce EPS if conditions are favorable (stage 3). The bacteria microcolonies will mature (stage 3) and slowly develop a complex communication system through the organized, spatially heterogeneous, and multilayered cell biofilm (stage 4). Bacteria colonies can then be released and dispersed in the medium to colonize other space and repeat the cycle (stage 5). Representative SEM images of biofilms are also shown. Colorized low magnification perspective view SEM image of E. coli macrocolony. Higher magnification showed that the biofilm consists of bacteria covered with cellulose and curli fibers on the surface (magenta), while all the bacteria flagella form a dense mesh at the bottom (blue). SEM images are taken from Serra et al.603. The SEM images are adapted with permission from reference603. Copyright 2013 American Society for Microbiology.
For a long time, ssNMR methods, together with EM, AFM, and solution NMR techniques, have been combined to address the composition and architecture of mature biofilms, to understand the transition between the single cell and the community, and to identify the response to antibiotics.
6.3.2. Determination of the Composition and Architecture of Biofilms
Biofilms are made of cells immobilized in EPS made of proteins, polysaccharides, lipids, and nucleic acids. The EPS is the backbone of a biofilm612. It provides mechanical stability and has a significant role in retaining water, absorbing nutrients, and protecting from external stress. Water represents up to 97% of the biofilm material while polysaccharides (including modified cellulose), proteins (including enzymes and curli amyloids), DNA and lipids each represents only 1–2% or less613.
Despite their biomedical significance, there are few reliable quantitative methods to determine the composition and structure of a biofilm matrix547. Quantitative analyses of EPS are typically achieved using HPLC and MS methods while Fourier transform infrared (FTIR) and Raman spectroscopy can qualitatively probe their chemical composition. Interactions and affinity of EPS with other molecules have been probed with surface plasmon resonance and small-angle X-ray scattering, while spatial architecture can be imaged using confocal scanning light, electron or atomic force microscopy. Solution NMR studies of soluble extracts were used to quantify the metabolites present in the biofilm614. The interactions between EPS and cerium oxide nanoparticles have been examined by 31P NMR since these particles create metabolic stress that leads to phosphorous ejection615. Phosphorous integration was also monitored as a function of temperature and oxygen availability by detecting DNA and poly/pyro-phosphate within the EPS616. Unfortunately, extraction and purification procedures, such as filtration, heating, blending, and sonication, are often required when characterizing biofilms, which might perturb the structure and state of the biomaterial617–619.
The biofilm of E. coli contains curli functional amyloid fibrils and phosphoethanolamine (pEtN) cellulose as shown by ssNMR (Figure 23A)625,626. This intriguing type of cellulose has initially been described by Cegelski and colleagues using a combination of ssNMR and EM imaging19,547,548,620,621,625,627,628. This modified cellulose is key to the formation of long cellulose chains and helps the biofilm packing. This likely confers resistance of this biofilm to cellulose-degrading enzymes and might prevent amyloid curli fibers from overstimulating an immune response when invading a living organism. The fractions of cellulose and amyloids in whole-cell samples of E. coli were also examined using ssNMR in combination with EM and biochemical titration. Inhibition of curli synthesis led to the production of a purely cellulosic biofilm (top of Figure 23B)621. Later, Jeffries et al.622 employed 13C ssNMR to assign, quantify and characterize both the cellulose and curli amyloids from biofilms of several strains of E. coli (bottom of Figure 23B). They proposed that curli amyloids were associated with adhesion, while pEtN cellulose favors cohesion. They also studied how biofilm formation could be affected by the surface where bacteria settle, and the data revealed that curli became dominant in the biofilm formed on plastic surface. Other groups also reported interactions between amyloidogenic fragments and the cell surface during the bacteria virulence phase629–631.
Figure 23. Investigations of bacterial biofilms by ssNMR.
(A) E. coli growing on YESCA nutrient agar has biofilm with the hallmark wrinkled colony morphology. Phosphoethanolamine (pEtN) cellulose enhances the adhesion of E. coli on surfaces and also packs with curli amyloid. Panel A is adapted with permission from reference620. Copyright 2015 Elsevier. (B) E. coli biofilm mutants monitored by TEM and ssNMR621 (top). Reconstitution of whole-cell spectra from the individual spectra collected on cell extracts (curli or pEtN cellulose) to enable compositional analysis with a bottom-up approach (bottom). Panel B is adapted with permission from reference621 and 622. Copyright 2013 Elsevier and copyright 2020 John Wiley and Sons. (C) 13C{15N} REDOR spectrum (top) of Vibrio cholerae specifically detecting the one-bond C-N pairs, allowing simplification over the control spectrum (bottom). The selected signals are from the carbon alpha of amino acids, phospholipids or glycine modified from lipid A. Panel C is adapted with permission from reference623. Copyright 2015 Elsevier. (D) Amyloid component of Bacillus biofilm investigated using 50 ms PDSD spectra, differentiating TasA proteins from B. cereus (black) and B. subtilis (red). Panel D is adapted with permission from reference624. Copyright 2019 John Wiley and Sons.
13C-CP along with REDOR methods involving 13C, 15N, 31P, and 19F nuclei, were applied to another biofilm extracted from Vibrio cholerae623 (Figure 23C). A top-down approach was used to describe the complex carbon pool of this bacterium, with quantification of the carbohydrates (58%), lipids (33%), and proteins (9%). They observed many amine moieties originating from the lysine sidechains and phospholipids, and from molecular modification of the free amine groups associated with glucosamine as shown by REDOR results. They also described possible modifications of amino acids and lipids that can be glycosylated when involved in cell walls.
Biofilms in P. aeruginosa are mainly composed of alginate, a polysaccharide made of 1,4-linked α-guluronate and β-mannuronate residues, with acetyl groups on the mannuronate residues. The T1 relaxation and spectral lineshapes of the EPS isolated from P. aeruginosa have been monitored using 13C ssNMR, which has revealed two different mechanisms for binding of Ca2+ and Mg2+: calcium ions would create a specifically chelated complex with mannuronate-guluronate blocks in alginate, while the binding of magnesium is much weaker and non-specific632. Lithium chloride was also found to strongly interact with alginate of the cell external matrix633. Mobility measurements using relaxometry revealed a very mobile assembly, where alginate undergoes molecular tumbling comparable to molecules in solution.
Bacillus subtilis is another important bacterium, studied by Loquet and coworkers, with exopolysaccharides, hydrophobins and several functional amyloid proteins identified. A recent model proposed that a major protein called TasA is surrounded by polysaccharides in the biofilm. The TasA protein is a soluble monomer with different oligomers characterized by X-ray, EM and solution NMR. Surprisingly, ssNMR on live biofilms gave different structural insights. Once in biofilms, the TasA protein has only one form that is protease-resistant with several β-sheet fibrils 634. El Mammeri et al. also characterized the structure of functional amyloids in both B. subtilis and B. cereus (Figure 23D)624. Their data revealed both β-sheet and α-helical conformations in the TasA protein, consistent with the functional amyloid structure that is different from the commonly reported β-sheet disease-associated amyloids. Although these two Bacillus bacteria shared a largely conserved TasA architecture, minor differences were detected using ssNMR and FTIR in terms of conformation and polymorphism. The data also led to a model where the two bacteria have different synthesis pathways for biofilm amyloids624.
6.3.3. Metabolism Flexibility and Remodeling of Biofilm in Response to Stress
Solid-state NMR has been used to describe the viscoelasticity of the biofilm in different conditions including sub-lethal concentrations of vitamin C or inactivation of the eps operon responsible for EPS synthesis635. Jiao et al.636 reported the use of FTIR, MS and ssNMR, among others, to characterize the EPS of bacteria found in acid mine drainage, and proved that the growth stage affects the EPS composition. They showed that mature biofilms have more carbohydrates and metals but fewer proteins and almost no DNA. The insufficient resolution could not differentiate β-O-4 and β-O-3 linkages, which are important in PG, but the problem was partially resolved in another study using extracted material from V. cholerae623. It was also shown that DMSO in the growth media enhances the amount of curli amyloids in E. coli biofilm637.
Interestingly, magnetic resonance imaging (MRI) methods have been employed to investigate bacterial biofilms. Using high-resolution spectroscopy/microscopy, with MRI combined to confocal laser scanning microscopy (NMR/CLSM), McLean et al.638 managed to build a 3D model of the biofilms of Shewanella oneidensis strain and Streptococcus mutants, which are associated with dissimilatory metal-reduction and caries disease, respectively. NMR and MRI techniques have also been employed to study biofilms in porous environments and flow cells to investigate water dynamics and biofilm expansion on various time and length scales. For example, 1H T2 and velocity MRI imaging were used in porous media that can be invaded by biofilms, which gave information with spatio-temporal resolution on this kind of assembly. This can be applied to mass transfer and flow abnormalities in porous materials639.
Caudill et al. used ssNMR to examine the interactions of biofilms with external molecules640,641. Using 31P and 13C ssNMR, they showed that gold nanoparticles decorated with cationic branched polyethylenimines interact electrostatically with biofilms and form hydrogen bonds with the oxygen atoms of phosphate groups of the teichoic acid residues in LPS. Such interactions will be altered by variation in teichoic acid composition (modification of the glucose or alanine quantities in LPS), suggesting the important function of these moieties.
Magnetic resonance techniques were also used to reveal the function of biofilms in preventing invasion of other bacterial species. MRI and ssNMR data on B. subtilis mutants showed that the absence of EPS in this bacterium leads to the production of less dense colonies, thus favoring the invasion by Pseudomonas chlororaphis642. Transcriptomics and genomics helped identify the main participants of the interactions between these two bacteria: a lipopeptide surfactin in B. subtilis and T6SS components in P. chlororaphis. This interaction between the two bacteria has also been monitored on melon leaves and seeds to understand the molecular phenomena orchestrating the concomitant invasion of both bacteria on plant materials.
Righi et al. used HR-MAS and transcriptomics to describe the metabolomics of biofilm, assigning and quantifying several amino acids and comparing biofilms before and after treatment by 2-amino acetophenone643. Solution NMR has been used to characterize other soluble fractions and monitor metabolites in medically relevant bacterial biofilms546. Although with improved resolution compared to ssNMR, 1D 1H HR-MAS spectra were still insufficient for differentiating P. aeruginosa biofilms or planktonic cells using PCA analysis644.
6.4. Other Bacterial Parts Exposed to the Medium
Other appendices can be found on the surface of bacterial cells. These extracellular assemblies include flagella, fimbriae, needles and pili, which are essential for bacterial infection and adhesion. Associated to cell motility, the cytoplasmic protein FliK is the main protein of flagella. Solution NMR experiments645 showed that FliK is mainly a disordered protein in which only the C- and N- termini are structured. The needles in P. aeruginosa have been examined by 13C ssNMR646. As for pili, they have rod-like polymeric structures and can be categorized by the secretion and adherence mechanisms: type I for surface attachment that can be associated to adhesion647, type III for secretion of toxin outside of the cytoplasm, and type IV for another kind of secretion and attachment system. Type II secretion system is often associated with toxins, degradative enzymes, proteases or lipases648. Unfortunately, the pilus secretion has only been reported in a few NMR publications, such as the work of Lopez-Castilla et al., which used solution NMR to examine interactions between calcium and type II secretion system from Klebsiella oxytoca649. NMR combined with other techniques, such as X-ray, provided information on the orientation and structure of these extracellular protein assemblies650,651. The type-I helical pilus in E. coli was characterized using ssNMR, detailing the interfaces between their FimA subunits652. Similar approaches were also used to characterize other extracellular fractions, such as the type-III secretion systems purified from different strains including Salmonella646,653–656.
6.5. Probing the Cell Surface Using DNP
Starting from 2012, MAS-DNP has been applied by Baldus, Hediger, Simorre, and others219,657 to investigate E. coli and B. subtilis. It was shown that radicals tend to accumulate in the cell wall, therefore improving the signal of the cell envelope only and simplifying the overall spectra. Nevertheless, internal elements seemed to be detected to some extent, including RNAs by 15N or lipids by 13C approaches. The preferential partitioning and non-uniform distribution of radicals in bacterial cell walls are probably attributable to their strong interactions with PG layer likely via hydrogen bonding. On the contrary, the carbohydrate-dominant cell walls of plants typically have uniform polarization under MAS-DNP, as evidenced by identical spectral patterns with and without microwave irradiation221. In bacteria, PG signals can be selectively increased or suppressed in a whole-cell sample by changing the radical concentration. This selectivity allows for specifically monitoring certain components of interest using intact bacterial cells.
7. Concluding Remarks and Perspectives
Undoubtedly, ssNMR has demonstrated its unique capability to investigate carbohydrates and associated biomolecules from the ECM materials (including cell walls) in physically relevant and chemically unperturbed states. Spanning across the plants, fungi, algae, and bacteria, the identified structural principles underlying the construction of ECMs should guide the development of technology for better using their constituent molecules for the production of biofuel, functionalized materials, nutrition, and high-value bioproducts. In-depth knowledge of microbial cell walls also serves as the basis for developing efficient antibiotics and antifungals.
Despite the recent landmark studies highlighted in this review, many structural aspects of ECMs remain unexplored as discussed in previous sections. There are promising opportunities in the characterization of algal cell walls (Section 5.1) and plant secondary cell walls (Section 3.4), the supramolecular organization of which have not been extensively investigated using ssNMR. High-resolution methods need to be developed to analyze the lignin structure, quantify molecular composition, and screen unlabeled materials (Section 3.5). SsNMR could also emerge as a standard strategy for assessing the action mechanism of antifungal drugs and antibiotics on bacteria or fungi, especially for those compounds targeting the ECM components (Section 4.7).
Projecting forward, numerous grand challenges are awaiting our efforts. What computational, statistical, and machine learning techniques should we use to promptly convert ssNMR observables to averaged structures of polymer complexes? Are we prepared for high-throughput and automatic analyses of unlabeled ECMs and carbohydrate-rich biomaterials? Could we obtain high-resolution views of the sugar components in mammalian and human cells81,658? How should we handle cellular mixtures like the communication and interface between microbes and plants/humans? When could we completely get rid of isotope labeling? The recent development of high- and ultrahigh-field magnets, ssNMR cryoprobes, MAS-DNP approaches and biradicals, proton detection in solids, and database-oriented tools might be the key to addressing these questions. Uniquely placed in this rapidly evolving era of ssNMR and DNP technology, we are eager to explore the many under-investigated research directions related to ECMs and cellular carbohydrates.
ACKNOWLEDGMENTS
This work was primarily supported as part of the Center for Lignocellulose Structure and Formation, an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, Basic Energy Sciences under award number DE-SC0001090. N.G. thanks the support by the National Institutes of Health grant AI149289 for fungal cell wall research. I.M. wishes to thank the financial support of the Natural Sciences and Engineering Research Council (NSERC) of Canada (grant RGPIN-2018-06200). A.P. would like to thank the Fonds de recherche du Québec – Nature et technologies (FRQNT), as well as the Quebec Network for Research on Protein Function, Engineering, and Applications (PROTEO) for the award of scholarships. F.M.V. thanks the National Science Foundation (DMR-1644779), the State of Florida, and NIH P41 GM122698.
ABBREVIATIONS
- AFM
atomic force microscope
- AG
arabinogalactan
- AmB
amphotericin B
- AMP
antimicrobial peptide
- AX
arabinoxylan
- CBM
carbohydrate-binding module
- CE
cross effect
- CesA
cellulose synthase
- CGMD
coarse-grained molecular dynamics
- CLSM
confocal laser scanning microscopy
- CMP-NMR
comprehensive multiphase NMR
- CORD
combined R2nυ-driven
- CP
cross polarization
- CrI
crystallinity index
- CSA
chemical shift anisotropy
- cw
continuous wave
- DARR
dipolar assisted rotational resonance
- DD
dipolar decoupling
- DFT
density-functional theory
- DNP
dynamic nuclear polarization
- DOPA
dihydroxyphenylalanine
- DP
direct polarization
- ECM
extracellular matrix
- ECMs
extracellular matrices
- EPS
extracellular polymer substances
- EXPB
β-expansin
- FESEM
field emission scanning electron microscope
- FTIR
Fourier transform infrared
- GAG
galactosaminogalactan
- GalA
galacturonic acid
- GalXM
galactoxylomannan
- GAX
glucuronoarabinoxylan
- GGM
galactoglucomannan
- GM
galactomannan
- gt
gauche-trans
- GXM
glucuronoxylomannan
- HG
homogalacturonan
- HRGP
hydroxyproline-rich glycoprotein
- INADEQUATE
incredible natural abundance double quantum transfer experiment
- INEPT
insensitive nuclei enhanced by polarization transfer
- LAM
lipoarabinomannan
- LCPA
long chain polyamine
- LDA
linear discriminant analysis
- LPS
lipopolysaccharide
- LTA
lipoteichoic acid
- MAS
magic-angle spinning
- MD
molecular dynamics
- MLG
mixed-linkage glucan
- NMR
nuclear magnetic resonance
- OM
outer membrane
- PA
polarizing agent
- PAR
proton assisted recoupling
- PARIS
phase-alternated recoupling irradiation scheme
- PASS
phase-adjusted spinning sidebands
- PCA
principal component analysis
- PDSD
proton-driven spin diffusion
- pEtN
phosphoethanolamine
- PG
peptidoglycan
- PM
plasma membrane
- PRE
paramagnetic relaxation enhancement
- PPs
polyisoprenoids
- REDOR
rotational echo double-resonance
- RFDR
radiofrequency driven dipolar recoupling
- RG-I
rhamnogalacturonan-I
- Rha
rhamnose
- SDV
silica deposit vesicle
- SE
solid effect
- SEs
sterol esters
- SFG
sum frequency generation
- S/N
signal-to-noise
- ssNMR
solid-state nuclear magnetic resonance
- TEM
transmission electron microscopy
- TGs
triglycerides
- tg
trans-gauche
- TSAR
third-spin assisted recoupling
- WTA
wall teichoic acids
- XyG
xyloglucan
Biographies
Nader Ghassemi obtained his B.S. (2011) and M.Sc. (2013) degrees in Physics from Sharif University of Technology, Iran. In 2020, he received his Ph.D. degree in Physics under the supervision of Dr. Joseph Ross at Texas A&M University where his research was focused on applying NMR spectroscopy to study electronic materials. Shortly after, he joined Dr. Tuo Wang’s group at Louisiana State University as a postdoctoral researcher. His current research focuses on applying ssNMR to understand cell wall structure in biological systems.
Alexandre Poulhazan obtained a double diploma from AgroParisTech and the Pierre and Marie Curie University in Paris in 2017. After a 6-month internship at the University of Melbourne where he studied antimicrobial peptide synergy by ssNMR, he became a biochemistry Ph.D. candidate at the Université du Québec à Montréal under the supervision of Drs. Isabelle Marcotte and Dror Warschawski. His research focuses on in situ ssNMR studies of microalgae and other systems such as velvet worm slime and carbon dots. In 2020, he had a 6-month visit at Louisiana State University (Dr. Tuo Wang) to collaborate on the characterization of microalgae cells and extracts.
Fabien Deligey received his Ph.D. degree in 2019 from Université de Lorraine. Under the supervision of Drs. Bouguet-Bonnet and Gansmüller, he characterized nano-systems engineered for drug-delivery with solid-state NMR. Since 2020, he is a postdoctoral associate in Dr. Tuo Wang’s group at Louisiana State University, where he is investigating plant-based biomaterials under natural-abundance DNP methods.
Frederic Mentink-Vigier received his M.Sc. in 2008 and PhD in 2011 from Ecole Nationale Supérieure de Chimie ParisTech, France, under the supervision of Dr. L. Binet, working on paramagnetic materials for quantum computing. He undertook two postdoctoral stays, first at the Weizmann Institute, Israel, with Drs. D. Goldfarb and S. Vega and then back in France at CEA Grenoble (Dr. Gaël De Paëpe). In 2016, he joined the National High Magnetic Field Laboratory as a research faculty to continue his research in MAS-DNP and high-field EPR methodology, which includes the design of new radicals using numerical simulations.
Isabelle Marcotte obtained her M.Sc. in chemistry at the Université de Montréal in Canada and Ph.D. at the Université Laval (with Dr. Michèle Auger) in 2003. She did her postdoctoral research on spider silk at the ETH-Zurich in Switzerland between 2003 and 2006 (with Dr. Beat Meier). She came back to Canada in 2006 at the Université du Québec à Montréal (UQAM) in the Department of Chemistry as a faculty member. Since 2017, she has been serving as Associate-Dean of research of the faculty of sciences at UQAM. Her biomolecular research covers a large variety of systems including mussel byssus, model membranes, as well as living organisms such as red blood cells, bacteria, and microalgae.
Tuo Wang received his B.S. from Nankai University (China) in 2010 and started his graduate research at Iowa State University. He moved to Massachusetts Institute of Technology (MIT) in 2014 and received his PhD in Physical Chemistry from MIT in 2016 under the supervision of Dr. Mei Hong. After the postdoctoral training in Hong lab, he joined the Department of Chemistry at Louisiana State University as an assistant professor in 2017 and became an associate professor in 2021. His research focuses on the structural elucidation of carbohydrate-rich biosystems.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Cosgrove DJ Growth of the plant cell wall. Nat. Rev. Mol. Cell. Biol 2005, 6, 850–961. [DOI] [PubMed] [Google Scholar]
- (2).Silhavy TJ; Kahne D; Walker S The bacterial cell envelope. Cold Spring Harb Perspect Biol 2010, 2, a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Latgé J-P The cell wall: a carbohydrate armour for the fungal cell. Mol. Microbiol 2007, 66, 279–290. [DOI] [PubMed] [Google Scholar]
- (4).Domozych DS; Ciancia M; Fangel JU; Mikkelsen MD; Ulvskov P; Willats WGT The cell walls of green algae: a journey through evolution and diversity. Front. Plant Sci 2012, 3, 82–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Brown GD; Denning DW; Gow NAR; Levitz SM; Netea MG; White TC Hidden killers: human fungal infections. Sci. Transl. Med 2012, 4, 165rv13. [DOI] [PubMed] [Google Scholar]
- (6).Roy R; Tiwari M; Donelli G; Tiwari V Strategies for combating bacterial biofilms: a focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Kohanski MA; Dwyer DJ; Collins JJ How antibiotics kill bacteria: from targets to networks. Nat. Rev. Microbiol 2010, 8, 423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).York WS; Darvill AG; McNeil M; Stevenson TT; Albersheim P Isolation and characterization of plant cell walls and cell wall components, Methods Enzymol 1986, 118, 3–40. [Google Scholar]
- (9).François JM A simple method for quantitative determination of polysaccharides in fungal cell walls. Nat. Protoc 2006, 1, 2995–3000. [DOI] [PubMed] [Google Scholar]
- (10).Mouille G; Robin S; Lecomte M; Pagant S; Höfte H Classification and identification of Arabidopsis cell wall mutants using Fourier-Transform InfraRed (FT-IR) microspectroscopy. Plant J 2003, 35, 393–404. [DOI] [PubMed] [Google Scholar]
- (11).Martinez-Sanz M; Gidley MJ; Gilbert EP Application of X-ray and neutron small angle scattering techniques to study the hierarchical structure of plant cell walls: A review. Carbohydr. Polym 2015, 125, 120–134. [DOI] [PubMed] [Google Scholar]
- (12).Desmarais SM; De Pedro MA; Cava F; Huang KC Peptidoglycan at its peaks: how chromatographic analyses can reveal bacterial cell wall structure and assembly. Mol. Microbiol 2013, 89, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Reif B; Ashbrook SE; Emsley L; Hong M Solid-state NMR spectroscopy. Nat. Rev. Methods Primers 2021, 1, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Mansfield SD; Kim H; Lu F; Ralph J Whole plant cell wall characterization using solution-state 2D NMR. Nat. Protoc 2012, 7, 1579–1589. [DOI] [PubMed] [Google Scholar]
- (15).Foston M; Samuel R; He J; Ragauskas AJ A review of whole cell wall NMR by the direct-dissolution of biomass. Green Chem 2016, 18, 608–621. [Google Scholar]
- (16).Zhao W; Fernando LD; Kirui A; Deligey F; Wang T Solid-state NMR of plant and fungal cell walls: a critical review. Solid State Nucl. Magn. Reson 2020, 107, 101660. [DOI] [PubMed] [Google Scholar]
- (17).Wang T; Phyo P; Hong M Multidimensional solid-state NMR spectroscopy of plant cell walls. Solid State Nucl. Magn. Reson 2016, 78, 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Kelly JE; Chrissian C; Stark RE Tailoring NMR experiments for structural characterization of amorphous biological solids: A practical guide. Solid State Nucl. Magn. Reson 2020, 109, 101686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Reichhardt C; Cegelski L Solid-state NMR for bacterial biofilms. Mol. Phys 2014, 112, 887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Poulhazan A; Arnold AA; Warschawski DE; Marcotte I Solid-state NMR study of microalgal membranes and cell walls. Solid-State NMR; IOP Publishing, 2020, 1–23. [Google Scholar]
- (21).Rossini AJ; Zagdoun A; Lelli M; Lesage A; Copéret C; Emsley L Dynamic nuclear polarization surface enhanced NMR spectroscopy. Acc. Chem. Res 2013, 46, 1942–1951. [DOI] [PubMed] [Google Scholar]
- (22).Corzilius B High-field dynamic nuclear polarization. Annu. Rev. Phys 2020, 71, 143–170. [DOI] [PubMed] [Google Scholar]
- (23).Su Y; Andreas L; Griffin RG Magic angle spinning NMR of proteins: high-frequency dynamic nuclear polarization and 1H detection. Annu. Rev. Biochem 2015, 84, 465–497. [DOI] [PubMed] [Google Scholar]
- (24).Gan Z; Hung I; Wang X; Paulino J; Wu G; Litvak IM; Gor’kov PL; Brey WW; Lendi P; Schiano JL et al. NMR spectroscopy up to 35.2T using a series-connected hybrid magnet. J. Magn. Reson 2017, 284, 125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Ciccarelli FD; Doerks T; von Mering C; Creevey CJ; Snel B; Bork P Toward automatic reconstruction of a highly resolved tree of life. Science 2006, 311, 1283–1287. [DOI] [PubMed] [Google Scholar]
- (26).Letunic I; Bork P Interactive Tree Of Life (iTOL): an online tool for phylogenetic tree display and annotation. Bioinformatics 2007, 23, 127–128. [DOI] [PubMed] [Google Scholar]
- (27).Takahashi H; Lee D; Dubois L; Bardet M; Hediger S; De Paëpe G Rapid natural-abundance 2D 13C–13C correlation spectroscopy using dynamic nuclear polarization enhanced solid-state NMR and matrix-free sample preparation. Angew. Chem. Int. Ed 2012, 51, 11766–11769. [DOI] [PubMed] [Google Scholar]
- (28).Zhou DH; Shah G; Mullen C; Sandoz D; Rienstra CM Proton-detected solid-state NMR spectroscopy of natural-abundance peptide and protein pharmaceuticals. Angew. Chem. Int. Ed 2009, 48, 1253–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Sohlenkamp C; Geiger O Bacterial membrane lipids: diversity in structures and pathways. FEMS Microbiol. Rev 2015, 40, 133–159. [DOI] [PubMed] [Google Scholar]
- (30).Vollmer W; Blanot D; De Pedro MA Peptidoglycan structure and architecture. FEMS Microbiol. Rev 2008, 32, 149–167. [DOI] [PubMed] [Google Scholar]
- (31).Li B; Webster TJ Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. J. Orthop. Res 2018, 36, 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Del Pozo J; Patel R The challenge of treating biofilm-associated bacterial infections. Clin. Pharmacol. Ther 2007, 82, 204–209. [DOI] [PubMed] [Google Scholar]
- (33).Flemming H-C Biofouling in water systems–cases, causes and countermeasures. Appl. Microbiol. Biotechnol 2002, 59, 629–640. [DOI] [PubMed] [Google Scholar]
- (34).Hall-Stoodley L; Costerton JW; Stoodley P Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol 2004, 2, 95–108. [DOI] [PubMed] [Google Scholar]
- (35).Sand W; Gehrke T Extracellular polymeric substances mediate bioleaching/biocorrosion via interfacial processes involving iron(III) ions and acidophilic bacteria. Res. J. Microbiol 2006, 157, 49–56. [DOI] [PubMed] [Google Scholar]
- (36).Benamara H; Rihouey C; Jouenne T; Alexandre S Impact of the biofilm mode of growth on the inner membrane phospholipid composition and lipid domains in Pseudomonas aeruginosa. Biochim. Biophys. Acta 2011, 1808, 98–105. [DOI] [PubMed] [Google Scholar]
- (37).Armstrong-James D; Meintjes G; Brown GD A neglected epidemic: fungal infections in HIV/AIDS. Trends Microbiol 2014, 22, 120–127. [DOI] [PubMed] [Google Scholar]
- (38).Beardsley J; Halliday CL; Chen SC-A; Sorrell TC Responding to the emergence of antifungal drug resistance: perspectives from the bench and the bedside. Future Microbiol 2018, 13, 1175–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Fekkar A; Lampros A; Mayaux J; Poigon C; Demeret S; Constantin J-M; Marcelin A-G; Monsel A; Luyt C-E; Blaize M Occurrence of invasive pulmonary fungal infections in patients with severe COVID-19 admitted to the ICU Am. J. Respir. Crit. Care Med 2021, 203, 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Eades CP; Armstrong-James DPH Invasive fungal infections in the immunocompromised host: Mechanistic insights in an era of changing immunotherapeutics. Med. Mycol 2019, 57, S307–S317. [DOI] [PubMed] [Google Scholar]
- (41).Agustinho DP; Miller LC; Li LX; Doering TL Peeling the onion: the outer layers of Cryptococcus neoformans. Mem. Inst. Oswaldo Cruz 2018, 113, e180040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Gow NAR; Latge JP; Munro CA The fungal cell wall: structure, biosynthesis, and function. Microbiol Spectr 2017, 5, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Pazos C; Moragues M-D; Quindós G; Pontón J; del Palacio A Diagnostic potential of (1→3)-β-D-glucan and anti-Candida albicans germ tube antibodies for the diagnosis and therapeutic monitoring of invasive candidiasis in neutropenic adult patients. Rev. Iberoam. Micol 2006, 23, 209–215. [DOI] [PubMed] [Google Scholar]
- (44).Perlin DS Mechanisms of echinocandin antifungal drug resistance. Ann. N. Y. Acad. Sci 2015, 1354, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Denning DW Echinocandin antifungal drugs. Lancet 2003, 362, 1142–1151. [DOI] [PubMed] [Google Scholar]
- (46).Erwig LP; Gow NAR Interactions of fungal pathogens with phagocytes. Nat. Rev. Microbiol 2016, 14, 163–176. [DOI] [PubMed] [Google Scholar]
- (47).Latgé J-P; Beauvais A; Chamilos G The cell wall of the human fungal pathogen Aspergillus fumigatus: biosynthesis, organization, immune response, and virulence. Annu. Rev. Microbiol 2017, 71, 99–116. [DOI] [PubMed] [Google Scholar]
- (48).Briard B; Fontaine T; Samir P; Place DE; Muszkieta L; Malireddi RKS; Karki R; Christgen S; Bomme P; Vogel P et al. Galactosaminogalactan activates the inflammasome to provide host protection. Nature 2020, 588, 688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Kang X; Kirui A; Muszynski A; Widanage MCD; Chen A; Azadi P; Wang P; Mentink-Vigier F; Wang T Molecular architecture of fungal cell walls revealed by solid-state NMR. Nat. Commun 2018, 9, 2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Chrissian C; Camacho E; Kelly JE; Wang H; Casadevall A; Stark RE Solid-state NMR spectroscopy identifies three classes of lipids in Cryptococcus neoformans melanized cell walls and whole fungal cells. J. Biol. Chem 2020, 295, 15083–15096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Engel A; Shewmaker F; Edskes HK; Dyda F; Wickner RB Amyloid of the Candida albicans Ure2p prion domain is infectious and has an in-register parallel β-sheet structure. Biochemistry 2011, 50, 5971–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Fernando LD; Zhao WC; Widanage MCD; Mentink-Vigier F; Wang T Solid-state NMR and DNP investigations of carbohydrates and cell-wall biomaterials. eMagRes 2020, 9, 251–258. [Google Scholar]
- (53).Lewis LA; McCourt RM Green algae and the origin of land plants. Am. J. Bot 2004, 91, 1535–1556. [DOI] [PubMed] [Google Scholar]
- (54).LeDuff P; Rorrer GL Formation of extracellular β-chitin nanofibers during batch cultivation of marine diatom Cyclotella sp. at silicon limitation. J. Appl. Phycol 2019, 31, 3479–3490. [Google Scholar]
- (55).Wustmann M; Poulsen N; Kröger N; van Pée K-H Chitin synthase localization in the diatom Thalassiosira pseudonana. BMC Materials 2020, 2, 10. [Google Scholar]
- (56).Kapaun E; Reisser W A chitin-like glycan in the cell wall of a Chlorella sp. (Chlorococcales, Chlorophyceae). Planta 1995, 197, 577–582. [Google Scholar]
- (57).Lee J-H; Waffenschmidt S; Small L; Goodenough U Between-species analysis of short-repeat modules in cell wall and sex-related hydroxyproline-rich glycoproteins of Chlamydomonas Plant Physiol 2007, 144, 1813–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Popper ZA; Michel G; Hervé C; Domozych DS; Willats WG; Tuohy MG; Kloareg B; Stengel DB Evolution and diversity of plant cell walls: from algae to flowering plants. Annu. Rev. Plant Biol 2011, 62, 567–590. [DOI] [PubMed] [Google Scholar]
- (59).Popper ZA Evolution and diversity of green plant cell walls. Curr. Opin. Plant Biol 2008, 11, 286–292. [DOI] [PubMed] [Google Scholar]
- (60).Höfte H; Voxeur A Plant cell walls. Curr. Biol 2017, 27, R865–R870. [DOI] [PubMed] [Google Scholar]
- (61).Cosgrove DJ Plant cell wall extensibility: connecting plant cell growth with cell wall structure, mechanics, and the action of wall-modifying enzymes. J. Exp. Bot 2016, 67, 463–476. [DOI] [PubMed] [Google Scholar]
- (62).Cosgrove DJ Diffuse growth of plant cell walls. Plant Physiol 2017, 176, 16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Zhong R; Cui D; Ye ZH Secondary cell wall biosynthesis. New Phytol 2019, 221, 1703–1723. [DOI] [PubMed] [Google Scholar]
- (64).Somerville C; Bauer S; Brininstool G; Facette M; Hamann T; Milne J; Osborne E; Paredez A; Persson S; Raab T Toward a systems approach to understanding plant cell walls. Science 2004, 306, 2206–2211. [DOI] [PubMed] [Google Scholar]
- (65).Lampugnani ER; Khan GA; Somssich M; Persson S Building a plant cell wall at a glance. J. Cell Sci 2018, 131, 1–6. [DOI] [PubMed] [Google Scholar]
- (66).Petridis L; Smith JC Molecular-level driving forces in lignocellulosic biomass deconstruction for bioenergy. Nat. Rev. Chem 2018, 2, 382–389. [Google Scholar]
- (67).Jarvis M; Cosgrove DJ Comparative structure and biomechanics of plant primary and secondary cell walls. Front. Plant Sci 2012, 3, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Somerville C; Youngs H; Taylor C; Davis SC; Long SP Feedstocks for lignocellulosic biofuels. Science 2010, 329, 790–792. [DOI] [PubMed] [Google Scholar]
- (69).Carroll A; Somerville C Cellulosic biofuels. Annu. Rev. Plant Biol 2009, 60, 165–182. [DOI] [PubMed] [Google Scholar]
- (70).Pauly M; Keegstra K Cell-wall carbohydrates and their modification as a resource for biofuels. Plant J 2008, 54, 559–568. [DOI] [PubMed] [Google Scholar]
- (71).Levitt M Spin dynamics: Basics of Nuclear Magnetic Resonance, Second Edition; Wiley-Blackwell, 2008, 1–744. [Google Scholar]
- (72).Schmidt-Rohr K; Spiess HW Multidimensional Solid-State NMR and Polymers; Academic Press: San Diego, 1994, 1–496. [Google Scholar]
- (73).McDermott AE; Polenova T Solid state NMR studies of biopolymers; John Wiley & Sons, 2010, 1–592. [Google Scholar]
- (74).Warnet XL; Arnold AA; Marcotte I; Warschawski DE In-cell solid-state NMR: an emerging technique for the study of biological membranes. Biophys. J 2015, 109, 2461–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Sarou-Kanian V; Joudiou N; Louat F; Yon M; Szeremeta F; Meme S; Massiot D; Decoville M; Fayon F; Beloeil J-C Metabolite localization in living drosophila using high resolution magic angle spinning NMR. Sci. Rep 2015, 5, 9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Zandomeneghi G; Ilg K; Aebi M; Meier BH On-cell MAS NMR: physiological clues from living cells. J. Am. Chem. Soc 2012, 134, 17513–17519. [DOI] [PubMed] [Google Scholar]
- (77).Warnet XL; Laadhari M; Arnold AA; Marcotte I; Warschawski DEA 2H magic-angle spinning solid-state NMR characterisation of lipid membranes in intact bacteria. BBA-Biomembranes 2016, 1858, 146–152. [DOI] [PubMed] [Google Scholar]
- (78).Ghosh R; Xiao Y; Kragelj J; Frederick KK In-cell sensitivity-enhanced NMR of intact living mammalian cells. J. Am. Chem. Soc in press. DOI: 10.1021/jacs.1c06680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Poulhazan A; Arnold AA; Warschawski DE; Marcotte I Unambiguous ex situ and in cell 2D 13C solid-state NMR characterization of starch and its constituents. Int. J. Mol. Sci 2018, 19, 3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Kirui A; Dickwella Widanage MC; Mentink-Vigier F; Wang P; Kang X; Wang T Preparation of fungal and plant materials for structural elucidation using dynamic nuclear polarization solid-state NMR. J. Vis. Exp 2019, e59152. [DOI] [PubMed] [Google Scholar]
- (81).Albert BJ; Gao C; Sesti EL; Saliba EP; Alaniva N; Scott FJ; Sigurdsson ST; Barnes AB Dynamic nuclear polarization nuclear magnetic resonance in human cells using fluorescent polarizing agents. Biochemistry 2018, 57, 4741–4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Ehren HL; Appels FVW; Houben K; Renault MAM; Wösten HAB; Baldus M Characterization of the cell wall of a mushroom forming fungus at atomic resolution using solid-state NMR spectroscopy. Cell Surface 2020, 6, 100046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Ghosh R; Kragelj J; Xiao Y; Frederick KK Cryogenic sample loading into a magic angle spinning nuclear magnetic resonance spectrometer that preserves cellular viability. J. Vis. Exp 2020, 163, e61733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Laws DD; Bitter HML; Jerschow A Solid-state NMR spectroscopic methods in chemistry. Angew. Chem. Int. Ed 2002, 41, 3096–3129. [DOI] [PubMed] [Google Scholar]
- (85).Damman R; Narasimhan S; Weingarth M; Baldus M Cellular solid-state NMR spectroscopy. In-cell NMR spectroscopy: from molecular sciences to cell biology. The Royal Society of Chemistry, 2020, 131–151. [Google Scholar]
- (86).Gao Y; Mortimer JC Unlocking the architecture of native plant cell walls via solid-state nuclear magnetic resonance. Methods Cell Biol 2020, 160, 121–143. [DOI] [PubMed] [Google Scholar]
- (87).Sefzik TH; Houseknecht JB; Clark TM; Prasad S; Lowary TL; Gan Z; Grandinetti PJ Solid-state 17O NMR in carbohydrates. Chem. Phys. Lett 2007, 434, 312–315. [Google Scholar]
- (88).Bougault C; Ayala I; Vollmer W; Simorre JP; Schanda P Studying intact bacterial peptidoglycan by proton-detected NMR spectroscopy at 100 kHz MAS frequency. J. Struct. Biol 2019, 206, 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Phyo P; Hong M Fast MAS 1H–13C correlation NMR for structural investigations of plant cell walls. J. Biomol. NMR 2019, 73, 661–674. [DOI] [PubMed] [Google Scholar]
- (90).Haya K; Makino Y; Kikuchi-Kinoshita A; Kawamura I; Naito A 31P and 13C solid-state NMR analysis of morphological changes of phospholipid bilayers containing glucagon during fibril formation of glucagon under neutral condition. BBA-Biomembranes 2020, 1862, 183290. [DOI] [PubMed] [Google Scholar]
- (91).Molugu TR; Lee S; Brown MF Concepts and methods of solid-state NMR spectroscopy applied to biomembranes. Chem. Rev 2017, 117, 12087–12132. [DOI] [PubMed] [Google Scholar]
- (92).Tardy-Laporte C; Arnold AA; Genard B; Gastineau R; Morançais M; Mouget J-L; Tremblay R; Marcotte IA 2H solid-state NMR study of the effect of antimicrobial agents on intact Escherichia coli without mutating. BBA-Biomembranes 2013, 1828, 614–622. [DOI] [PubMed] [Google Scholar]
- (93).Lu M; Sarkar S; Wang M; Kraus J; Fritz M; Quinn CM; Bai S; Holmes ST; Dybowski C; Yap GPA et al. 19F magic angle spinning NMR spectroscopy and density functional theory calculations of fluorosubstituted tryptophans: integrating experiment and theory for accurate determination of chemical shift tensors. J. Phys. Chem. B 2018, 122, 6148–6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Lu M; Wang M; Sergeyev IV; Quinn CM; Struppe J; Rosay M; Maas W; Gronenborn AM; Polenova T 19F dynamic nuclear polarization at fast magic angle spinning for NMR of HIV-1 capsid protein assemblies. J. Am. Chem. Soc 2019, 141, 5681–5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Lane Gilchrist M; Monde K; Tomita Y; Iwashita T; Nakanishi K; McDermott AE Measurement of interfluorine distances in solids. J. Magn. Reson 2001, 152, 1–6. [DOI] [PubMed] [Google Scholar]
- (96).Roos M; Wang T; Shcherbakov AA; Hong M Fast magic-angle-spinning 19F spin exchange NMR for determining nanometer 19F–19F distances in proteins and pharmaceutical compounds. J. Phys. Chem. B 2018, 122, 2900–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Gelenter MD; Wang T; Liao S-Y; O’Neill H; Hong M 2H–13C correlation solid-state NMR for investigating dynamics and water accessibilities of proteins and carbohydrates. J. Biomol. NMR 2017, 68, 257–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Nishiyama Y Fast magic-angle sample spinning solid-state NMR at 60–100 kHz for natural abundance samples. Solid State Nucl. Magn. Reson 2016, 78, 24–36. [DOI] [PubMed] [Google Scholar]
- (99).Andrew ER; Bradbury A; Eades RG Nuclear magnetic resonance spectra from a crystal rotated at high speed. Nature 1958, 182, 1659–1659. [Google Scholar]
- (100).Lowe IJ Free induction decays of rotating solids. Phys. Rev. Lett 1959, 2, 285–287. [Google Scholar]
- (101).Andreas LB; Jaudzems K; Stanek J; Lalli D; Bertarello A; Le Marchand T; Cala-De Paepe D; Kotelovica S; Akopjana I; Knott B et al. Structure of fully protonated proteins by proton-detected magic-angle spinning NMR. Proc. Natl. Acad. Sci. USA 2016, 113, 9187–9192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Struppe J; Quinn CM; Lu M; Wang M; Hou G; Lu X; Kraus J; Andreas LB; Stanek J; Lalli D et al. Expanding the horizons for structural analysis of fully protonated protein assemblies by NMR spectroscopy at MAS frequencies above 100 kHz. Solid State Nucl. Magn. Reson 2017, 87, 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Zhang R; Mroue KH; Ramamoorthy A Proton-based ultrafast magic angle spinning solid-state NMR spectroscopy. Acc. Chem. Res 2017, 50, 1105–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).Ishii Y; Tycko R Sensitivity enhancement in solid state 15N NMR by indirect detection with high-speed magic angle spinning. J. Magn. Reson 2000, 142, 199–204. [DOI] [PubMed] [Google Scholar]
- (105).Webb A Increasing the sensitivity of magnetic resonance spectroscopy and imaging. Anal. Chem 2012, 84, 9–16. [DOI] [PubMed] [Google Scholar]
- (106).Elena B; Lesage A; Steuernagel S; Böckmann A; Emsley L Proton to carbon-13 INEPT in solid-state NMR spectroscopy. J. Am. Chem. Soc 2005, 127, 17296–17302. [DOI] [PubMed] [Google Scholar]
- (107).Kolodziejski W; Klinowski J Kinetics of cross-polarization in solid-state NMR: a guide for chemists. Chem. Rev 2002, 102, 613–628. [DOI] [PubMed] [Google Scholar]
- (108).Johnson RL; Schmidt-Rohr K Quantitative solid-state 13C NMR with signal enhancement by multiple cross polarization. J. Magn. Reson 2014, 239, 44–49. [DOI] [PubMed] [Google Scholar]
- (109).Duan P; Schmidt-Rohr K Composite-pulse and partially dipolar dephased multiCP for improved quantitative solid-state 13C NMR. J. Magn. Reson 2017, 285, 68–78. [DOI] [PubMed] [Google Scholar]
- (110).Cadars S; Lesage A; Emsley L Chemical shift correlations in disordered solids. J. Am. Chem. Soc 2005, 127, 4466–4476. [DOI] [PubMed] [Google Scholar]
- (111).Cadars S; Sein J; Duma L; Lesage A; Pham TN; Baltisberger JH; Brown SP; Emsley L The refocused INADEQUATE MAS NMR experiment in multiple spin-systems: Interpreting observed correlation peaks and optimising lineshapes. J. Magn. Reson 2007, 188, 24–34. [DOI] [PubMed] [Google Scholar]
- (112).Chen L; Kaiser JM; Lai J; Polenova T; Yang J; Rienstra CM; Mueller LJ J-based 2D homonuclear and heteronuclear correlation in solid-state proteins. Magn. Reson. Chem 2007, 45, S84–S92. [DOI] [PubMed] [Google Scholar]
- (113).Chen L; Olsen RA; Elliott DW; Boettcher JM; Zhou DH; Rienstra CM; Mueller LJ Constant-time through-bond 13C correlation spectroscopy for assigning protein resonances with solid-state NMR spectroscopy. J. Am. Chem. Soc 2006, 128, 9992–9993. [DOI] [PubMed] [Google Scholar]
- (114).Bayro MJ; Ramachandran R; Caporini MA; Eddy MT; Griffin RG Radio frequency-driven recoupling at high magic-angle spinning frequencies: homonuclear recoupling sans heteronuclear decoupling. J. Chem. Phys 2008, 128, 052321. [DOI] [PubMed] [Google Scholar]
- (115).Hughes CE; Luca S; Baldus M Radio-frequency driven polarization transfer without heteronuclear decoupling in rotating solids. Chem. Phys. Lett 2004, 385, 435–440. [Google Scholar]
- (116).Levitt MH; Grant DM; Harris RK Symmetry-based pulse sequences in magic-angle spinning solid-state NMR. Encyclopedia of Nuclear Magnetic Resonance Chichester, UK. Wiley, 2007, 9, 165–196. [Google Scholar]
- (117).Hou G; Yan S; Trébosc J; Amoureux J-P; Polenova T Broadband homonuclear correlation spectroscopy driven by combined R2nv sequences under fast magic angle spinning for NMR structural analysis of organic and biological solids. J. Magn. Reson 2013, 232, 18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (118).Weingarth M; Demco DE; Bodenhausen G; Tekely P Improved magnetization transfer in solid-state NMR with fast magic angle spinning. Chem. Phys. Lett 2009, 469, 342–348. [Google Scholar]
- (119).Hou G; Yan S; Sun S; Han Y; Byeon I-JL; Ahn J; Concel J; Samoson A; Gronenborn AM; Polenova T Spin diffusion driven by R-symmetry sequences: applications to homonuclear correlation spectroscopy in MAS NMR of biological and organic solids. J. Am. Chem. Soc 2011, 133, 3943–3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).Aluas M; Tripon C; Griffin JM; Filip X; Ladizhansky V; Griffin RG; Brown SP; Filip C CHHC and 1H–1H magnetization exchange: Analysis by experimental solid-state NMR and 11-spin density-matrix simulations. J. Magn. Reson 2009, 199, 173–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (121).Lange A; Luca S; Baldus M Structural constraints from proton-mediated rare-spin correlation spectroscopy in rotating solids. J. Am. Chem. Soc 2002, 124, 9704–9705. [DOI] [PubMed] [Google Scholar]
- (122).Paul S; Takahashi H; Hediger S; De Paëpe G Chapter three - third spin-assisted recoupling in SSNMR: theoretical insights and practicable application to biomolecular structure determination. Annual Reports on NMR Spectroscopy 2015, 85, 93–142. [Google Scholar]
- (123).Paëpe GD; Lewandowski JR; Loquet A; Böckmann A; Griffin RG Proton assisted recoupling and protein structure determination. J. Chem. Phys 2008, 129, 245101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (124).Gelenter MD; Dregni AJ; Hong M Pulsed third-spin-assisted recoupling NMR for obtaining long-range 13C–13C and 15N–13C distance restraints. J. Phys. Chem. B 2020, 124, 7138–7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (125).Ader C; Schneider R; Seidel K; Etzkorn M; Becker S; Baldus M Structural rearrangements of membrane proteins probed by water-edited solid-state NMR spectroscopy. J. Am. Chem. Soc 2009, 131, 170–176. [DOI] [PubMed] [Google Scholar]
- (126).Williams JK; Schmidt-Rohr K; Hong M Aromatic spectral editing techniques for magic-angle-spinning solid-state NMR spectroscopy of uniformly 13C-labeled proteins. Solid State Nucl. Magn. Reson 2015, 72, 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (127).Schmidt-Rohr K; Fritzsching KJ; Liao SY; Hong M Spectral editing of two-dimensional magic-angle-spinning solid-state NMR spectra for protein resonance assignment and structure determination. J. Biomol. NMR 2012, 54, 343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).Komatsu T; Kikuchi J Selective signal detection in solid-state NMR using rotor-synchronized dipolar dephasing for the analysis of hemicellulose in lignocellulosic biomass. J. Phys. Chem. Lett 2013, 4, 2279–2283. [Google Scholar]
- (129).Shcherbakov AA; Medeiros-Silva J; Tran N; Gelenter MD; Hong M From angstroms to nanometers: measuring interatomic distances by solid-state NMR. Chem. Rev 2021, in press. DOI: 10.1021/acs.chemrev.1c00662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).Phyo P; Wang T; Kiemle SN; O’Neill H; Pingali SV; Hong M; Cosgrove DJ Gradients in wall mechanics and polysaccharides along growing inflorescence stems. Plant Physiol 2017, 175, 1593–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Dick-Perez M; Wang T; Salazar A; Zabotina OA; Hong M Multidimensional solid-state NMR studies of the structure and dynamics of pectic polysaccharides in uniformly 13C-labeled Arabidopsis primary cell walls. Magn. Reson. Chem 2012, 50, 539–550. [DOI] [PubMed] [Google Scholar]
- (132).Wang T; Hong M Structure and dynamics of polysaccharides in plant cell walls from solid-state NMR. NMR in Glycoscience and Glycotechnology; Royal Society of Chemistry 2017, 290–304. [Google Scholar]
- (133).Ishima R; Torchia DA Protein dynamics from NMR. Nat. Struct. Biol 2000, 7, 740–743. [DOI] [PubMed] [Google Scholar]
- (134).Mittermaier A; Kay LE New tools provide new insights in NMR studies of protein dynamics. Science 2006, 312, 224–228. [DOI] [PubMed] [Google Scholar]
- (135).Barna JCJ; Laue ED; Mayger MR; Skilling J; Worrall SP Exponential sampling, an alternative method for sampling in two-dimensional NMR experiments. J. Magn. Reson 1969, 73, 69–77. [Google Scholar]
- (136).Kaur M; Lewis CM; Chronister A; Phun GS; Mueller LJ Non-uniform sampling in NMR spectroscopy and the preservation of spectral knowledge in the time and frequency domains. J. Phys. Chem. A 2020, 124, 5474–5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (137).Burakova E; Vasa SK; Klein A; Linser R Non-uniform sampling in quantitative assessment of heterogeneous solid-state NMR line shapes. J. Biol. NMR 2020, 74, 71–82. [DOI] [PubMed] [Google Scholar]
- (138).Sun S; Yan S; Guo C; Li M; Hoch JC; Williams JC; Polenova T A timesaving strategy for MAS NMR spectroscopy by combining non-uniform sampling and paramagnetic relaxation assisted condensed data collection. J. Phys. Chem. B 2012, 116, 13585–13596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).Linser R; Bardiaux B; Andreas LB; Hyberts SG; Morris VK; Pintacuda G; Sunde M; Kwan AH; Wagner G Solid-state NMR structure determination from diagonal-compensated, sparsely nonuniform-sampled 4D proton–proton restraints. J. Am. Chem. Soc 2014, 136, 11002–11010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (140).Bertini I; Luchinat C; Parigi G; Pierattelli R NMR spectroscopy of paramagnetic metalloproteins ChemBioChem 2005, 6, 1536–1549. [DOI] [PubMed] [Google Scholar]
- (141).Sengupta I; Nadaud PS; Helmus JJ; Schwieters CD; Jaroniec CP Protein fold determined by paramagnetic magic-angle spinning solid-state NMR spectroscopy. Nat. Chem 2012, 4, 410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (142).Nadaud PS; Helmus JJ; Kall SL; Jaroniec CP Paramagnetic ions enable tuning of nuclear relaxation rates and provide long-range structural restraints in solid-state NMR of proteins. J. Am. Chem. Soc 2009, 131, 8108–8120. [DOI] [PubMed] [Google Scholar]
- (143).Oster C; Kosol S; Hartlmuller C; Lamley JM; Luga D; Oss A; Org M-L; Vanatalu K; Samoson A; Madl T et al. Characterization of protein–protein interfaces in large complexes by solid-state NMR solvent paramagnetic relaxation enhancements. J. Am. Chem. Soc 2017, 139, 12165–12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (144).Zuckerstätter G; Terinte N; Sixta H; Schuster KC Novel insight into cellulose supramolecular structure through 13C CP-MAS NMR spectroscopy and paramagnetic relaxation enhancement. Carbohydr. Polym 2013, 93, 122–128. [DOI] [PubMed] [Google Scholar]
- (145).Wang T; Chen Y; Tabuchi A; Cosgrove DJ; Hong M The target of β-expansin EXPB1 in maize cell walls from binding and solid-state NMR studies. Plant Physiol 2016, 172, 2107–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (146).Clore MG; Iwahara J Theory, practice, and applications of paramagnetic relaxation enhancement for the characterization of transient low-population states of biological macromolecules and their complexes. Chem. Rev 2009, 109, 4108–4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (147).Chakraborty A; Deligey F; Quach J; Mentink-Vigier F; Wang P; Wang T Biomolecular complex viewed by dynamic nuclear polarization solid-state NMR spectroscopy. Biochem. Soc. Trans 2020, 48, 1089–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (148).Reif B Proton-detection in biological MAS solid-state NMR spectroscopy. Modern Magnetic Resonance Springer International Publishing: Cham, 2017, 1–33. [Google Scholar]
- (149).Ishii Y; Wickramasinghe A; Matsuda I; Endo Y; Ishii Y; Nishiyama Y; Nemoto T; Kamihara T Progress in proton-detected solid-state NMR (ssNMR): super-fast 2D ssNMR collection for nano-mole-scale proteins. J. Magn. Reson 2018, 286, 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (150).Lee D; Hediger S; Paëpe GD High-field solid-state NMR with dynamic nuclear polarization. Modern Magnetic Resonance Springer International Publishing: Cham, 2017, 861–877. [Google Scholar]
- (151).Lilly Thankamony AS; Wittmann JJ; Kaushik M; Corzilius B Dynamic nuclear polarization for sensitivity enhancement in modern solid-state NMR. Prog. Nucl. Mag. Res. Sp 2017, 102–103, 120–195. [DOI] [PubMed] [Google Scholar]
- (152).Gauto D; Dakhlaoui O; Marin-Montesinos I; Hediger S; De Paëpe G Targeted DNP for biomolecular solid-state NMR. Chem. Sci 2021, 12, 6223–6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (153).Narasimhan S; Scherpe S; Lucini Paioni A; van der Zwan J; Folkers GE; Ovaa H; Baldus M DNP-Supported Solid-state NMR spectroscopy of proteins inside mammalian cells. Angew. Chem. Int. Ed 2019, 58, 12969–12973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (154).Quinn CM; Wang M; Polenova T NMR of macromolecular assemblies and machines at 1 GHz and beyond: new transformative opportunities for molecular structural biology. Methods Mol Biol 2018, 1688, 1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (155).Nimerovsky E; Movellan KT; Zhang XC; Forster MC; Najbauer E; Xue K; Dervişoǧlu R; Giller K; Griesinger C; Becker S et al. Proton detected solid-state NMR of membrane proteins at 28 Tesla (1.2 GHz) and 100 kHz magic-angle spinning. Biomolecules 2021, 11, 752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (156).Mance D; Weingarth M; Baldus M Solid-state NMR on complex biomolecules: methods and applications. Modern Magnetic Resonance Springer International Publishing: Cham, 2016, 487–503. [Google Scholar]
- (157).van der Wel PCA New applications of solid-state NMR in structural biology. Emerging Top. Life Sci 2018, 2, 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (158).Ni QZ; Daviso E; Can TV; Markhasin E; Jawla SK; Swager TM; Temkin RJ; Herzfeld J; Griffin RG High frequency dynamic nuclear polarization. Acc. Chem. Res 2013, 46, 1933–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (159).Abragam A; Goldman M Principles of dynamic nuclear polarisation. Rep. Prog. Phys 1978, 41, 395–467. [Google Scholar]
- (160).Wenckebach WT Dynamic nuclear polarization via the cross effect and thermal mixing: A. The role of triple spin flips. J. Magn. Reson 2019, 299, 124–134. [DOI] [PubMed] [Google Scholar]
- (161).Serra SC; Rosso A; Tedoldi F On the role of electron–nucleus contact and microwave saturation in thermal mixing DNP. Phys. Chem. Chem. Phys 2013, 15, 8416–8428. [DOI] [PubMed] [Google Scholar]
- (162).Jannin S; Comment A; van der Klink JJ Dynamic nuclear polarization by thermal mixing under partial saturation. Appl. Magn. Reson 2012, 43, 59–68. [Google Scholar]
- (163).Hovav Y; Feintuch A; Vega S Theoretical aspects of dynamic nuclear polarization in the solid state – The cross effect. J. Magn. Reson 2012, 214, 29–41. [DOI] [PubMed] [Google Scholar]
- (164).Wind RA; Duijvestijn MJ; van der Lugt C; Manenschijn A; Vriend J Applications of dynamic nuclear polarization in 13C NMR in solids. Prog. Nucl. Mag. Res. Sp 1985, 17, 33–67. [Google Scholar]
- (165).Can TV; Ni QZ; Griffin RG Mechanisms of dynamic nuclear polarization in insulating solids. J. Magn. Reson 2015, 253, 23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (166).Can TV; Caporini MA; Mentink-Vigier F; Corzilius B; Walish JJ; Rosay M; Maas WE; Baldus M; Vega S; Swager TM et al. Overhauser effects in insulating solids. J. Chem. Phys 2014, 141, 064202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (167).Maly T; Debelouchina GT; Bajaj VS; Hu K-N; Joo C-G; Mak–Jurkauskas ML; Sirigiri JR; Wel P. C. A. v. d.; Herzfeld J; Temkin RJ.et al. Dynamic nuclear polarization at high magnetic fields. J. Chem. Phys 2008, 128, 052211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (168).Smith AA; Corzilius B; Barnes AB; Maly T; Griffin RG Solid effect dynamic nuclear polarization and polarization pathways. J. Chem. Phys 2012, 136, 015101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (169).Hediger S; Lee D; Mentink-Vigier F; De Paepe G MAS-DNP enhancements: hyperpolarization, depolarization, and absolute sensitivity. eMagRes 2018, 7, 105–116. [Google Scholar]
- (170).Sauvée C; Rosay M; Casano G; Aussenac F; Weber RT; Ouari O; Tordo P Highly efficient, water-soluble polarizing agents for dynamic nuclear polarization at high frequency. Angew. Chem. Int. Ed 2013, 52, 10858–10861. [DOI] [PubMed] [Google Scholar]
- (171).Mentink-Vigier F; Marin-Montesinos I; Jagtap AP; Halbritter T; van Tol J; Hediger S; Lee D; Sigurdsson ST; De Paëpe G Computationally assisted design of polarizing agents for dynamic nuclear polarization enhanced NMR: the AsymPol family. J. Am. Chem. Soc 2018, 140, 11013–11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (172).Dubroca T; Smith AN; Pike KJ; Froud S; Wylde R; Trociewitz B; McKay J; Mentink-Vigier F; van Tol J; Wi S et al. A quasi-optical and corrugated waveguide microwave transmission system for simultaneous dynamic nuclear polarization NMR on two separate 14.1 T spectrometers. J. Magn. Reson 2018, 289, 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (173).Chaudhari SR; Wisser D; Pinon AC; Berruyer P; Gajan D; Tordo P; Ouari O; Reiter C; Engelke F; Copéret C et al. Dynamic nuclear polarization efficiency increased by very fast magic angle spinning. J. Am. Chem. Soc 2017, 139, 10609–10612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (174).Lesage A; Lelli M; Gajan D; Caporini MA; Vitzthum V; Miéville P; Alauzun J; Roussey A; Thieuleux C; Mehdi A et al. Surface enhanced NMR spectroscopy by dynamic nuclear polarization. J. Am. Chem. Soc 2010, 132, 15459–15461. [DOI] [PubMed] [Google Scholar]
- (175).DeHaven BA; Tokarski JT 3rd; Korous AA; Mentink-Vigier F; Makris TM; Brugh AM; Forbes MDE; van Tol J; Bowers CR; Shimizu LS Persistent radicals of self-assembled benzophenone bis-urea macrocycles: characterization and application as a polarizing agent for solid-state DNP MAS spectroscopy. Chem. Eur.J 2017, 23, 8315–8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (176).Mentink-Vigier F; Akbey Ü; Hovav Y; Vega S; Oschkinat H; Feintuch A Fast passage dynamic nuclear polarization on rotating solids. J. Magn. Reson 2012, 224, 13–21. [DOI] [PubMed] [Google Scholar]
- (177).Mentink-Vigier F; Akbey Ü; Oschkinat H; Vega S; Feintuch A Theoretical aspects of magic angle spinning - dynamic nuclear polarization. J. Magn. Reson 2015, 258, 102–120. [DOI] [PubMed] [Google Scholar]
- (178).Mentink-Vigier F; Paul S; Lee D; Feintuch A; Hediger S; Vega S; De Paëpe G Nuclear depolarization and absolute sensitivity in magic-angle spinning cross effect dynamic nuclear polarization. Phys. Chem. Chem. Phys 2015, 17, 21824–21836. [DOI] [PubMed] [Google Scholar]
- (179).Mentink-Vigier F; Vega S; De Paëpe G Fast and accurate MAS–DNP simulations of large spin ensembles. Phys. Chem. Chem. Phys 2017, 19, 3506–3522. [DOI] [PubMed] [Google Scholar]
- (180).Mentink-Vigier F Optimizing nitroxide biradicals for cross-effect MAS-DNP: the role of g-tensors’ distance. Phys. Chem. Chem. Phys 2020, 22, 3643–3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (181).Mentink-Vigier F; Dubroca T; Van Tol J; Sigurdsson ST The distance between g-tensors of nitroxide biradicals governs MAS-DNP performance: The case of the bTurea family. J. Magn. Reson 2021, 329, 107026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (182).Thurber KR; Tycko R Theory for cross effect dynamic nuclear polarization under magic-angle spinning in solid state nuclear magnetic resonance: The importance of level crossings. J. Chem. Phys 2012, 137, 084508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (183).Thurber KR; Tycko R On Mechanisms of dynamic nuclear polarization in solids. Isr. J. Chem 2014, 54, 39–46. [Google Scholar]
- (184).Thurber KR; Tycko R Perturbation of nuclear spin polarizations in solid state NMR of nitroxide-doped samples by magic-angle spinning without microwaves. J. Chem. Phys 2014, 140, 184201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (185).Kundu K; Mentink-Vigier F; Feintuch A; Vega S DNP mechanisms. eMagRes 2019, 8, 295–337. [Google Scholar]
- (186).Hu K-N; Yu H-H; Swager TM; Griffin RG Dynamic nuclear polarization with biradicals. J. Am. Chem. Soc 2004, 126, 10844–10845. [DOI] [PubMed] [Google Scholar]
- (187).Song C; Hu K-N; Joo C-G; Swager TM; Griffin RG TOTAPOL: A biradical polarizing agent for dynamic nuclear polarization experiments in aqueous media. J. Am. Chem. Soc 2006, 128, 11385–11390. [DOI] [PubMed] [Google Scholar]
- (188).Mathies G; Caporini MA; Michaelis VK; Liu Y; Hu K-N; Mance D; Zweier JL; Rosay M; Baldus M; Griffin RG Efficient dynamic nuclear polarization at 800 MHz/527 GHz with trityl-nitroxide biradicals. Angew. Chem. Int. Ed 2015, 54, 11770–11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (189).Sauvée C; Casano G; Abel S; Rockenbauer A; Akhmetzyanov D; Karoui H; Siri D; Aussenac F; Maas W; Weber RT et al. Tailoring of polarizing agents in the bTurea series for cross-effect dynamic nuclear polarization in aqueous media. Chem. Eur. J 2016, 22, 5598–5606. [DOI] [PubMed] [Google Scholar]
- (190).Kubicki DJ; Casano G; Schwarzwälder M; Abel S; Sauvée C; Ganesan K; Yulikov M; Rossini AJ; Jeschke G; Copéret C et al. Rational design of dinitroxide biradicals for efficient cross-effect dynamic nuclear polarization. Chem. Sci 2016, 7, 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (191).Stevanato G; Casano G; Kubicki DJ; Rao Y; Esteban Hofer L; Menzildjian G; Karoui H; Siri D; Cordova M; Yulikov M et al. Open and closed radicals: local geometry around unpaired electrons governs magic-angle spinning dynamic nuclear polarization performance. J. Am. Chem. Soc 2020, 142, 16587–16599. [DOI] [PubMed] [Google Scholar]
- (192).Zagdoun A; Casano G; Ouari O; Lapadula G; Rossini AJ; Lelli M; Baffert M; Gajan D; Veyre L; Maas WE et al. A slowly relaxing rigid biradical for efficient dynamic nuclear polarization surface-enhanced NMR spectroscopy: expeditious characterization of functional group manipulation in hybrid materials. J. Am. Chem. Soc 2012, 134, 2284–2291. [DOI] [PubMed] [Google Scholar]
- (193).Lund A; Casano G; Menzildjian G; Kaushik M; Stevanato G; Yulikov M; Jabbour R; Wisser D; Renom-Carrasco M; Thieuleux C et al. TinyPols: a family of water-soluble binitroxides tailored for dynamic nuclear polarization enhanced NMR spectroscopy at 18.8 and 21.1 T. Chem. Sci 2020, 11, 2810–2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (194).Wisser D; Karthikeyan G; Lund A; Casano G; Karoui H; Yulikov M; Menzildjian G; Pinon AC; Purea A; Engelke F et al. BDPA-nitroxide biradicals tailored for efficient dynamic nuclear polarization enhanced solid-state NMR at magnetic fields up to 21.1 T. J. Am. Chem. Soc 2018, 140, 13340–13349. [DOI] [PubMed] [Google Scholar]
- (195).Jagtap AP; Geiger M-A; Stöppler D; Orwick-Rydmark M; Oschkinat H; Sigurdsson ST bcTol: a highly water-soluble biradical for efficient dynamic nuclear polarization of biomolecules. Chem. Commun 2016, 52, 7020–7023. [DOI] [PubMed] [Google Scholar]
- (196).Geiger M-A; Jagtap AP; Kaushik M; Sun H; Stöppler D; Sigurdsson ST; Corzilius B; Oschkinat H Efficiency of water-soluble nitroxide biradicals for dynamic nuclear polarization in rotating solids at 9.4 T: bcTol-M and cyolyl-TOTAPOL as new polarizing agents. Chem. Eur. J 2018, 24, 13485–13494. [DOI] [PubMed] [Google Scholar]
- (197).Zhai W; Lucini Paioni A; Cai X; Narasimhan S; Medeiros-Silva J; Zhang W; Rockenbauer A; Weingarth M; Song Y; Baldus M et al. Postmodification via thiol-click chemistry yields hydrophilic trityl-nitroxide biradicals for biomolecular high-field dynamic nuclear polarization. J. Phys. Chem. B 2020, 124, 9047–9060. [DOI] [PubMed] [Google Scholar]
- (198).Mentink-Vigier F; Mathies G; Liu Y; Barra A-L; Caporini MA; Lee D; Hediger S; G. Griffin R; De Paëpe G Efficient cross-effect dynamic nuclear polarization without depolarization in high-resolution MAS NMR. Chem. Sci 2017, 8, 8150–8163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (199).Mentink-Vigier F; Barra A-L; van Tol J; Hediger S; Lee D; De Paëpe G De novo prediction of cross-effect efficiency for magic angle spinning dynamic nuclear polarization. Phys. Chem. Chem. Phys 2019, 21, 2166–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (200).Perras FA; Sadow A; Pruski M In Silico Design of DNP polarizing agents: can current dinitroxides be improved? ChemPhysChem 2017, 18, 2279–2287. [DOI] [PubMed] [Google Scholar]
- (201).Perras FA; Pruski M Large-scale ab initio simulations of MAS DNP enhancements using a Monte Carlo optimization strategy. J. Chem. Phys 2018, 149, 154202. [DOI] [PubMed] [Google Scholar]
- (202).Perras FA; Raju M; Carnahan SL; Akbarian D; van Duin ACT; Rossini AJ; Pruski M Full-scale ab initio simulation of magic-angle-spinning dynamic nuclear polarization. J. Phys. Chem. Lett 2020, 11, 5655–5660. [DOI] [PubMed] [Google Scholar]
- (203).Becerra LR; Gerfen GJ; Temkin RJ; Singel DJ; Griffin RG Dynamic nuclear polarization with a cyclotron resonance maser at 5 T. Phys. Rev. Lett 1993, 71, 3561–3564. [DOI] [PubMed] [Google Scholar]
- (204).Hall DA; Maus DC; Gerfen GJ; Inati SJ; Becerra LR; Dahlquist FW; Griffin RG Polarization-enhanced NMR spectroscopy of biomolecules in frozen solution. Science 1997, 276, 930–932. [DOI] [PubMed] [Google Scholar]
- (205).Rosay M; Tometich L; Pawsey S; Bader R; Schauwecker R; Blank M; Borchard PM; Cauffman SR; Felch KL; Weber RT et al. Solid-state dynamic nuclear polarization at 263 GHz: spectrometer design and experimental results. Phys. Chem. Chem. Phys 2010, 12, 5850–5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (206).Rosay M; Blank M; Engelke F Instrumentation for solid-state dynamic nuclear polarization with magic angle spinning NMR. J. Magn. Reson 2016, 264, 88–98. [DOI] [PubMed] [Google Scholar]
- (207).Berruyer P; Björgvinsdóttir S; Bertarello A; Stevanato G; Rao Y; Karthikeyan G; Casano G; Ouari O; Lelli M; Reiter C et al. Dynamic nuclear polarization enhancement of 200 at 21.15 T enabled by 65 kHz magic angle spinning. J. Phys. Chem. Lett 2020, 11, 8386–8391. [DOI] [PubMed] [Google Scholar]
- (208).Chaudhari SR; Berruyer P; Gajan D; Reiter C; Engelke F; Silverio DL; Copéret C; Lelli M; Lesage A; Emsley L Dynamic nuclear polarization at 40 kHz magic angle spinning. Phys. Chem. Chem. Phys 2016, 18, 10616–10622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (209).Purea A; Reiter C; Dimitriadis AI; de Rijk E; Aussenac F; Sergeyev I; Rosay M; Engelke F Improved waveguide coupling for 1.3 mm MAS DNP probes at 263 GHz. J. Magn. Reson 2019, 302, 43–49. [DOI] [PubMed] [Google Scholar]
- (210).Bouleau E; Saint-Bonnet P; Mentink-Vigier F; Takahashi H; Jacquot JF; Bardet M; Aussenac F; Purea A; Engelke F; Hediger S et al. Pushing NMR sensitivity limits using dynamic nuclear polarization with closed-loop cryogenic helium sample spinning. Chem. Sci 2015, 6, 6806–6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (211).Lee D; Bouleau E; Saint-Bonnet P; Hediger S; De Paëpe G Ultra-low temperature MAS-DNP. J. Magn. Reson 2016, 264, 116–124. [DOI] [PubMed] [Google Scholar]
- (212).Matsuki Y; Ueda K; Idehara T; Ikeda R; Ogawa I; Nakamura S; Toda M; Anai T; Fujiwara T Helium-cooling and -spinning dynamic nuclear polarization for sensitivity-enhanced solid-state NMR at 14T and 30K. J. Magn. Reson 2012, 225, 1–9. [DOI] [PubMed] [Google Scholar]
- (213).Matsuki Y; Idehara T; Fukazawa J; Fujiwara T Advanced instrumentation for DNP-enhanced MAS NMR for higher magnetic fields and lower temperatures. J. Magn. Reson 2016, 264, 107–115. [DOI] [PubMed] [Google Scholar]
- (214).Sugishita T; Matsuki Y; Fujiwara T Absolute 1H polarization measurement with a spin-correlated component of magnetization by hyperpolarized MAS-DNP solid-state NMR. Solid State Nucl. Magn. Reson 2019, 99, 20–26. [DOI] [PubMed] [Google Scholar]
- (215).Matsuki Y; Kobayashi T; Fukazawa J; Perras FA; Pruski M; Fujiwara T Efficiency analysis of helium-cooled MAS DNP: case studies of surface-modified nanoparticles and homogeneous small-molecule solutions. Phys. Chem. Chem. Phys 2021, 23, 4919–4926. [DOI] [PubMed] [Google Scholar]
- (216).Matsuki Y; Fujiwara T Cryogenic platforms and optimized DNP sensitivity. eMagRes 2018, 7, 9–23. [Google Scholar]
- (217).Thurber KR; Potapov A; Yau W-M; Tycko R Solid state nuclear magnetic resonance with magic-angle spinning and dynamic nuclear polarization below 25K. J. Magn. Reson 2013, 226, 100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (218).Thurber K; Tycko R Low-temperature dynamic nuclear polarization with helium-cooled samples and nitrogen-driven magic-angle spinning. J. Magn. Reson 2016, 264, 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (219).Takahashi H; Ayala I; Bardet M; De Paëpe G; Simorre JP; Hediger S Solid-state NMR on bacterial cells: selective cell wall signal enhancement and resolution improvement using dynamic nuclear polarization. J. Am. Chem. Soc 2013, 135, 5105–5110. [DOI] [PubMed] [Google Scholar]
- (220).Jantschke A; Koers E; Mance D; Weingarth M; Brunner E; Baldus M Insight into the supramolecular architecture of intact diatom biosilica from DNP-supported solid-state NMR spectroscopy. Angew. Chem. Int. Ed 2015, 54, 15069–15073. [DOI] [PubMed] [Google Scholar]
- (221).Zhao W; Kirui A; Deligey F; Mentink-Vigier F; Zhou Y; Zhang B; Wang T Solid-state NMR of unlabeled plant cell walls: high-resolution structural analysis without isotopic enrichment. Biotechnol. Biofuels 2021, 14, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (222).Zhang L; Gao C; Mentink-Vigier F; Tang L; Zhang D; Wang S; Cao S; Xu Z; Liu X; Wang T et al. Arabinosyl deacetylase modulates the arabinoxylan acetylation profile and secondary wall formation. Plant Cell 2019, 31, 1113–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (223).Lee D; Hediger S; De Paëpe G Is solid-state NMR enhanced by dynamic nuclear polarization? Solid State Nucl. Magn. Reson 2015, 66–67, 6–20. [DOI] [PubMed] [Google Scholar]
- (224).Equbal A; Leavesley A; Jain SK; Han S Cross-effect dynamic nuclear polarization explained: polarization, depolarization, and oversaturation. J. Phys. Chem. Lett 2019, 10, 548–558. [DOI] [PubMed] [Google Scholar]
- (225).Rankin AGM; Trébosc J; Pourpoint F; Amoureux J-P; Lafon O Recent developments in MAS DNP-NMR of materials. Solid State Nucl. Magn. Reson 2019, 101, 116–143. [DOI] [PubMed] [Google Scholar]
- (226).Akbey Ü; Franks WT; Linden A; Orwick-Rydmark M; Lange S; Oschkinat H Dynamic nuclear polarization enhanced NMR in the solid-state. Top. Curr. Chem 2013, 338, 181–228. [DOI] [PubMed] [Google Scholar]
- (227).Takahashi H; Hediger S; De Paëpe G Matrix-free dynamic nuclear polarization enables solid-state NMR 13C–13C correlation spectroscopy of proteins at natural isotopic abundance. Chem. Commun 2013, 49, 9479–9481. [DOI] [PubMed] [Google Scholar]
- (228).Rossini AJ; Zagdoun A; Hegner F; Schwarzwälder M; Gajan D; Copéret C; Lesage A; Emsley L Dynamic nuclear polarization NMR spectroscopy of microcrystalline solids. J. Am. Chem. Soc 2012, 134, 16899–16908. [DOI] [PubMed] [Google Scholar]
- (229).Smith AN; Märker K; Hediger S; De Paëpe G Natural isotopic abundance 13C and 15N multidimensional solid-state NMR enabled by dynamic nuclear polarization. J. Phys. Chem. Lett 2019, 10, 4652–4662. [DOI] [PubMed] [Google Scholar]
- (230).Märker K; Paul S; Fernández-de-Alba C; Lee D; Mouesca J-M; Hediger S; De Paëpe G Welcoming natural isotopic abundance in solid-state NMR: probing π-stacking and supramolecular structure of organic nanoassemblies using DNP. Chem. Sci 2017, 8, 974–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (231).Märker K; Pingret M; Mouesca J-M; Gasparutto D; Hediger S; De Paëpe G A new tool for NMR crystallography: complete 13C/15N assignment of organic molecules at natural isotopic abundance using DNP-enhanced solid-state NMR. J. Am. Chem. Soc 2015, 137, 13796–13799. [DOI] [PubMed] [Google Scholar]
- (232).Cosgrove DJ Re-constructing our models of cellulose and primary cell wall assembly. Curr. Opin. Plant Biol 2014, 22, 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (233).Purushotham P; Ho R; Zimmer J Architecture of a catalytically active homotrimeric plant cellulose synthase complex. Science 2020, 369, 1089–1094. [DOI] [PubMed] [Google Scholar]
- (234).Scheller HV; Ulvskov P Hemicelluloses. Annu. Rev. Plant Biol 2010, 61, 263–289. [DOI] [PubMed] [Google Scholar]
- (235).Pauly M; Gille S; Liu L; Mansoori N; de Souza A; Schultink A; Xiong G Hemicellulose biosynthesis. Planta 2013, 238, 627–642. [DOI] [PubMed] [Google Scholar]
- (236).Mohnen D Pectin structure and biosynthesis. Curr. Opin. Plant Biol 2008, 11, 266–277. [DOI] [PubMed] [Google Scholar]
- (237).Anderson CT We be jammin’: an update on pectin biosynthesis, trafficking and dynamics. J. Exp. Bot 2015, 67, 495–502. [DOI] [PubMed] [Google Scholar]
- (238).Atmodjo MA; Hao Z; Mohnen D Evolving views of pectin biosynthesis. Annu. Rev. Plant Biol 2013, 64, 747–779. [DOI] [PubMed] [Google Scholar]
- (239).Biswal AK; Atmodjo MA; Li M; Baxter HL; Yoo CG; Pu Y; Lee YC; Mazarei M; Black IM; Zhang JY et al. Sugar release and growth of biofuel crops are improved by downregulation of pectin biosynthesis. Nat. Biotechnol 2018, 36, 249–257. [DOI] [PubMed] [Google Scholar]
- (240).Ralph J; Lapierre C; Boerjan W Lignin structure and its engineering. Curr. Opin. Biotechnol 2019, 56, 240–249. [DOI] [PubMed] [Google Scholar]
- (241).Ralph J; Lundquist K; Brunow G; Lu F; Kim H; Schatz PF; Marita JM; Hatfield RD; Ralph SA; Christensen JH et al. Lignins: Natural polymers from oxidative coupling of 4-hydroxyphenyl- propanoids. Phytochem. Rev 2004, 3, 29–60. [Google Scholar]
- (242).Bonawitz ND; Chapple C The genetics of lignin biosynthesis: connecting genotype to phenotype. Annu. Rev. Genet 2010, 44, 337–363. [DOI] [PubMed] [Google Scholar]
- (243).Fernandes AN; Thomas LH; Altaner CM; Callow P; Forsyth VT; Apperley DC; Kennedy CJ; Jarvis MC Nanostructure of cellulose microfibrils in spruce wood. Proc. Natl. Acad. Sci. USA 2011, 108, 18863–18864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (244).Carpita NC; Gibeaut DM Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J 1993, 3, 1–30. [DOI] [PubMed] [Google Scholar]
- (245).Donaldson LA Lignification and lignin topochemistry — an ultrastructural view. Phytochemistry 2001, 57, 859–873. [DOI] [PubMed] [Google Scholar]
- (246).Himmel ME; Ding S-Y; Johnson DK; Adney WS; Nimlos MR; Brady JW; Foust TD Biomass Recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. [DOI] [PubMed] [Google Scholar]
- (247).Vanholme R; Morreel K; Ralph J; Boerjan W Lignin engineering. Curr. Opin. Plant Biol 2008, 11, 278–285. [DOI] [PubMed] [Google Scholar]
- (248).Jarvis M Cellulose stacks up. Nature 2003, 426, 611–612. [DOI] [PubMed] [Google Scholar]
- (249).Hernández-Varela JD; Chanona-Pérez JJ; Sodi FC; Vicente-Flores M Effect of ball milling on cellulose nanoparticles structure obtained from garlic and agave waste. Carbohydr. Polym 2021, 255, 117347. [DOI] [PubMed] [Google Scholar]
- (250).Atalla RH; Vanderhart DL Native cellulose: a composite of two distinct crystalline forms. Science 1984, 223, 283–285. [DOI] [PubMed] [Google Scholar]
- (251).VanderHart DL; Atalla RH Studies of microstructure in native celluloses using solid-state carbon-13 NMR. Macromolecules 1984, 17, 1465–1472. [Google Scholar]
- (252).Nishiyama Y; Sugiyama J; Chanzy H; Langan P Crystal structure and hydrogen bonding system in cellulose Iα from synchrotron X-ray and neutron fiber diffraction. J. Am. Chem. Soc 2003, 125, 14300–14306. [DOI] [PubMed] [Google Scholar]
- (253).Nishiyama Y; Langan P; Chanzy H Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J. Am. Chem. Soc 2002, 124, 9074–9082. [DOI] [PubMed] [Google Scholar]
- (254).Kono H; Yunoki S; Shikano T; Fujiwara M; Erata T; Takai M CP/MAS 13C NMR study of cellulose and cellulose derivatives. 1. complete assignment of the CP/MAS 13C NMR spectrum of the native cellulose. J. Am. Chem. Soc 2002, 124, 7506–7511. [DOI] [PubMed] [Google Scholar]
- (255).Ling Z; Wang T; Makarem M; Cintrón MS; Cheng H; Kang X; Bacher M; Potthast A; Rosenau T; King H Effects of ball milling on the structure of cotton cellulose. Cellulose 2019, 26, 305–328. [Google Scholar]
- (256).Kono H; Numata Y Structural investigation of cellulose Iα and Iβ by 2D RFDR NMR spectroscopy: determination of sequence of magnetically inequivalent D-glucose units along cellulose chain. Cellulose 2006, 13, 317–326. [Google Scholar]
- (257).Kono H; Erata T; Takai M Determination of the Through-bond carbon−carbon and carbon− proton connectivities of the native celluloses in the solid state. Macromolecules 2003, 36, 5131–5138. [Google Scholar]
- (258).Nomura S; Kugo Y; Erata T 13C NMR and XRD studies on the enhancement of cellulose II crystallinity with low concentration NaOH post-treatments. Cellulose 2020, 27, 3553–3563. [Google Scholar]
- (259).Ghosh M; Prajapati BP; Suryawanshi RK; Dey KK; Kango N Study of the effect of enzymatic deconstruction on natural cellulose by NMR measurements. Chem. Phys. Lett 2019, 727, 105–115. [Google Scholar]
- (260).Kang X; Kirui A; Dickwella Widanage MC; Mentink-Vigier F; Cosgrove DJ; Wang T Lignin-polysaccharide interactions in plant secondary cell walls revealed by solid-state NMR. Nat. Commun 2019, 10, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (261).Wang T; Yang H; Kubicki JD; Hong M Cellulose structural polymorphism in plant primary cell walls investigated by high-field 2D solid-state NMR spectroscopy and density functional theory calculations. Biomacromolecules 2016, 17, 2210–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (262).Phyo P; Wang T; Yang Y; O’Neill H; Hong M Direct determination of hydroxymethyl conformations of plant cell wall cellulose using 1H polarization transfer solid-state NMR. Biomacromolecules 2018, 19, 1485–1497. [DOI] [PubMed] [Google Scholar]
- (263).Kono H; Numata Y; Erata T; Takai M 13C and 1H resonance assignment of mercerized cellulose II by two-dimensional MAS NMR spectroscopies. Macromolecules 2004, 37, 5310–5316. [Google Scholar]
- (264).Nagarajan S; Skillen NC; Irvine JT; Lawton LA; Robertson PK Cellulose II as bioethanol feedstock and its advantages over native cellulose. Renew. Sust. Energ. Rev 2017, 77, 182–192. [Google Scholar]
- (265).Idström A; Schantz S; Sundberg J; Chmelka BF; Gatenholm P; Nordstierna L 13C NMR assignments of regenerated cellulose from solid-state 2D NMR spectroscopy. Carbohydr. Polym 2016, 151, 480–487. [DOI] [PubMed] [Google Scholar]
- (266).Kita Y; Kusumi R; Kimura T; Kitaoka M; Nishiyama Y; Wada M Surface structural analysis of selectively 13C-labeled cellulose II by solid-state NMR spectroscopy. Cellulose 2020, 27, 1899–1907. [Google Scholar]
- (267).Heinze T Cellulose: structure and properties. Cellulose chemistry and properties: fibers, nanocelluloses and advanced materials Springer, Cham. 2015, 271, 1–52. [Google Scholar]
- (268).Chen Q; Zheng K; Fan Q; Wang K; Yang H; Jiang J; Liu S Solvability and thermal response of cellulose with different crystal configurations. Front. Eng. Manag 2019, 6, 62–69. [Google Scholar]
- (269).Wu Q; Xu J; Zhu S; Kuang Y; Wang B; Gao W Crystalline stability of cellulose III nanocrystals in the hydrothermal treatment and NaOH solution. Carbohydr. Polym 2020, 249, 116827. [DOI] [PubMed] [Google Scholar]
- (270).Somerville C Cellulose Synthesis in Higher Plants. Annu. Rev. Cell Dev. Biol 2006, 22, 53–78. [DOI] [PubMed] [Google Scholar]
- (271).Ding S-Y; Zhao S; Zeng Y Size, shape, and arrangement of native cellulose fibrils in maize cell walls. Cellulose 2014, 21, 863–871. [Google Scholar]
- (272).Yang H; Kubicki JD A density functional theory study on the shape of the primary cellulose microfibril in plants: effects of C6 exocyclic group conformation and H-bonding. Cellulose 2020, 27, 2389–2402. [Google Scholar]
- (273).Hill JL Jr.; Hammudi MB; Tien M The Arabidopsis cellulose synthase complex: a proposed hexamer of CESA trimers in an equimolar stoichiometry. Plant Cell 2014, 26, 4834–4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (274).Sethaphong L; Haigler CH; Kubicki JD; Zimmer J; Bonetta D; DeBolt S; Yingling YG Tertiary model of a plant cellulose synthase. Proc. Natl. Acad. Sci. USA 2013, 110, 7512–7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (275).Jarvis MC Cellulose biosynthesis: counting the chains. Plant Physiol 2013, 163, 1485–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (276).Foston M Advances in solid-state NMR of cellulose. Curr. Opin. Biotechnol 2014, 27, 176–184. [DOI] [PubMed] [Google Scholar]
- (277).Peciulyte A; Karlström K; Larsson PT; Olsson L Impact of the supramolecular structure of cellulose on the efficiency of enzymatic hydrolysis. Biotechnol. Biofuels 2015, 8, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (278).Wickholm K; Hult E-L; Larsson PT; Iversen T; Lennholm H Quantification of cellulose forms in complex cellulose materials: a chemometric model. Cellulose 2001, 8, 139–148. [Google Scholar]
- (279).Wickholm K; Larsson PT; Iversen T Assignment of non-crystalline forms in cellulose I by CP/MAS 13C NMR spectroscopy. Carbohydr. Res 1998, 312, 123–129. [Google Scholar]
- (280).Yang H; Wang T; Oehme D; Petridis L; Hong M; Kubicki JD Structural factors affecting 13C NMR chemical shifts of cellulose: a computational study. Cellulose 2018, 25, 23–36. [Google Scholar]
- (281).Kirui A; Ling Z; Kang X; Widanage MCD; Mentink-Vigier F; French AD; Wang T Atomic resolution of cotton cellulose structure enabled by dynamic nuclear polarization solid-state NMR. Cellulose 2019, 26, 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (282).Daicho K; Fujisawa S; Kobayashi K; Saito T; Ashida J Cross-polarization dynamics and conformational study of variously sized cellulose crystallites using solid-state 13C NMR. J. Wood Sci 2020, 66, 62. [Google Scholar]
- (283).Ferreira JC; Evtuguin DV; Prates A Effect of cellulose structure on reactivity of Eucalyptus acid sulphite dissolving pulp. Cellulose 2020, 27, 4763–4772. [Google Scholar]
- (284).Ioelovich MY Models of supramolecular structure and properties of cellulose. Polym. Sci. Ser. A 2016, 58, 925–943. [Google Scholar]
- (285).Sparrman T; Svenningsson L; Sahlin-Sjövold K; Nordstierna L; Westman G; Bernin D A revised solid-state NMR method to assess the crystallinity of cellulose. Cellulose 2019, 26, 8993–9003. [Google Scholar]
- (286).Ghosh M; Kango N; Dey KK Investigation of the internal structure and dynamics of cellulose by 13C-NMR relaxometry and 2DPASS-MAS-NMR measurements. J. Biomol. NMR 2019, 73, 601–616. [DOI] [PubMed] [Google Scholar]
- (287).Harris DM; Corbin K; Wang T; Gutierrez R; Bertolo AL; Petti C; Smilgies D-M; Estevez JM; Bonetta D; Urbanowicz BR Cellulose microfibril crystallinity is reduced by mutating C-terminal transmembrane region residues CESA1A903V and CESA3T942I of cellulose synthase. Proc. Natl. Acad. Sci. USA 2012, 109, 4098–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (288).Newman RH; Hill SJ; Harris PJ Wide-angle x-ray scattering and solid-state nuclear magnetic resonance data combined to test models for cellulose microfibrils in mung bean cell walls. Plant Physiol 2013, 163, 1558–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (289).Wang T; Hong M Solid-state NMR investigations of cellulose structure and interactions with matrix polysaccharides in plant primary cell walls. J. Exp. Bot 2016, 67, 503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (290).Zhang X; Qu T; Mosier NS; Han L; Xiao W Cellulose modification by recyclable swelling solvents. Biotechnol. Biofuels 2018, 11, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (291).Barnette AL; Lee C; Bradley LC; Schreiner EP; Park YB; Shin H; Cosgrove DJ; Park S; Kim SH Quantification of crystalline cellulose in lignocellulosic biomass using sum frequency generation (SFG) vibration spectroscopy and comparison with other analytical methods. Carbohydr. Polym 2012, 89, 802–809. [DOI] [PubMed] [Google Scholar]
- (292).Zhang Y; Yu J; Wang X; Durachko DM; Zhang S; Cosgrove DJ Molecular insights into the complex mechanics of plant epidermal cell walls. Science 2021, 372, 706–711. [DOI] [PubMed] [Google Scholar]
- (293).Zhang T; Tang H; Vavylonis D; Cosgrove DJ Disentangling loosening from softening: insights into primary cell wall structure. Plant J 2019, 100, 1101–1117. [DOI] [PubMed] [Google Scholar]
- (294).Zheng Y; Wang X; Chen Y; Wagner E; Cosgrove DJ Xyloglucan in the primary cell wall: assessment by FESEM, selective enzyme digestions and nanogold affinity tags. Plant J 2018, 93, 211–226. [DOI] [PubMed] [Google Scholar]
- (295).Dick-Pérez M; Zhang Y; Hayes J; Salazar A; Zabotina OA; Hong M Structure and interactions of plant cell-wall polysaccharides by two-and three-dimensional magic-angle-spinning solid-state NMR. Biochemistry 2011, 50, 989–1000. [DOI] [PubMed] [Google Scholar]
- (296).Kafle K; Park YB; Lee CM; Stapleton JJ; Kiemle SN; Cosgrove DJ; Kim SH Effects of mechanical stretching on average orientation of cellulose and pectin in onion epidermis cell wall: A polarized FT-IR study. Cellulose 2017, 24, 3145–3154. [Google Scholar]
- (297).Wang T; Park YB; Caporini MA; Rosay M; Zhong L; Cosgrove DJ; Hong M Sensitivity-enhanced solid-state NMR detection of expansin’s target in plant cell walls. Proc. Natl. Acad. Soc. USA 2013, 110, 16444–16449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (298).Kirui A; Du J; Zhao W; Barnes W; Kang X; Anderson CT; Xiao C; Wang T A pectin Methyltransferase modulates polysaccharide dynamics and interactions in Arabidopsis primary cell walls: Evidence from solid-state NMR. Carbohydr. Polym 2021, 270, 118370. [DOI] [PubMed] [Google Scholar]
- (299).Wang T; Park YB; Cosgrove DJ; Hong M Cellulose-pectin spatial contacts are inherent to never-dried Arabidopsis primary cell walls: evidence from solid-state nuclear magnetic resonance. Plant Physiol 2015, 168, 871–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (300).Wang T; Zabotina O; Hong M Pectin–cellulose interactions in the Arabidopsis primary cell wall from two-dimensional magic-angle-spinning solid-state nuclear magnetic resonance. Biochemistry 2012, 51, 9846–9856. [DOI] [PubMed] [Google Scholar]
- (301).Phyo P; Wang T; Xiao C; Anderson CT; Hong M Effects of pectin molecular weight changes on the structure, dynamics, and polysaccharide interactions of primary cell walls of Arabidopsis thaliana: insights from solid-state NMR. Biomacromolecules 2017, 18, 2937–2950. [DOI] [PubMed] [Google Scholar]
- (302).Phyo P; Gu Y; Hong M Impact of acidic pH on plant cell wall polysaccharide structure and dynamics: insights into the mechanism of acid growth in plants from solid-state NMR. Cellulose 2019, 26, 291–304. [Google Scholar]
- (303).Wang T; Williams JK; Schmidt-Rohr K; Hong M Relaxation-compensated difference spin diffusion NMR for detecting 13C–13C long-range correlations in proteins and polysaccharides. J. Biomol. NMR 2015, 61, 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (304).White PB; Wang T; Park YB; Cosgrove DJ; Hong M Water–polysaccharide interactions in the primary cell wall of Arabidopsis thaliana from polarization transfer solid-state NMR. J. Am. Chem. Soc 2014, 136, 10399–10409. [DOI] [PubMed] [Google Scholar]
- (305).Wang T; Salazar A; Zabotina OA; Hong M Structure and dynamics of Brachypodium primary cell wall polysaccharides from two-dimensional 13C solid-state nuclear magnetic resonance spectroscopy. Biochemistry 2014, 53, 2840–2854. [DOI] [PubMed] [Google Scholar]
- (306).Duan P; Kaser SJ; Lyczakowski JJ; Phyo P; Tryfona T; Dupree P; Hong M Xylan structure and dynamics in native Brachypodium grass cell walls investigated by solid-state NMR spectroscopy. Acs Omega 2021, 6, 15460–15471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (307).Gao Y; Lipton AS; Wittmer Y; Murray DT; Mortimer JC A grass-specific cellulose–xylan interaction dominates in sorghum secondary cell walls. Nat. Commun 2020, 11, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (308).Terrett OM; Lyczakowski JJ; Yu L; Iuga D; Franks WT; Brown SP; Dupree R; Dupree P Molecular architecture of softwood revealed by solid-state NMR. Nat. Commun 2019, 10, 4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (309).Simmons TJ; Mortimer JC; Bernardinelli OD; Pöppler A-C; Brown SP; Deazevedo ER; Dupree R; Dupree P Folding of xylan onto cellulose fibrils in plant cell walls revealed by solid-state NMR. Nat. Commun 2016, 7, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (310).Dupree R; Simmons TJ; Mortimer JC; Patel D; Iuga D; Brown SP; Dupree P Probing the molecular architecture of Arabidopsis thaliana secondary cell walls using two- and three-dimensional 13C solid state nuclear magnetic resonance spectroscopy. Biochemistry 2015, 54, 2335–2345. [DOI] [PubMed] [Google Scholar]
- (311).Falcoz-Vigne L; Ogawa Y; Molina-Boisseau S; Nishiyama Y; Meyer V; Petit-Conil M; Mazeau K; Heux L Quantification of a tightly adsorbed monolayer of xylan on cellulose surface. Cellulose 2017, 24, 3725–3739. [Google Scholar]
- (312).Addison B; Stengel D; Bharadwaj VS; Happs RM; Doeppke C; Wang T; Bomble YJ; Holland GP; Harman-Ware AE Selective one-dimensional 13C–13C spin-diffusion solid-state nuclear magnetic resonance methods to probe spatial arrangements in biopolymers including plant cell walls, peptides, and spider silk. J. Phys. Chem. B 2020, 124, 9870–9883. [DOI] [PubMed] [Google Scholar]
- (313).Terrett OM; Dupree P Covalent interactions between lignin and hemicelluloses in plant secondary cell walls. Curr. Opin. Biotechnol 2019, 56, 97–104. [DOI] [PubMed] [Google Scholar]
- (314).Berglund J; Mikkelsen D; Flanagan BM; Dhital S; Gaunitz S; Henriksson G; Lindström ME; Yakubov GE; Gidley MJ; Vilaplana F Wood hemicelluloses exert distinct biomechanical contributions to cellulose fibrillar networks. Nat. Commun 2020, 11, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (315).Vanholme R; Demedts B; Morreel K; Ralph J; Boerjan W Lignin biosynthesis and structure. Plant Physiol 2010, 153, 895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (316).Wang H-M; Ma C-Y; Li H-Y; Chen T-Y; Wen J-L; Cao X-F; Wang X-L; Yuan T-Q; Sun R-C Structural variations of lignin macromolecules from early growth stages of poplar cell walls. ACS Sustain. Chem. Eng 2019, 8, 1813–1822. [Google Scholar]
- (317).Capanema EA; Balakshin MY; Kadla JF A comprehensive approach for quantitative lignin characterization by NMR spectroscopy. J. Agric. Food Chem 2004, 52, 1850–1860. [DOI] [PubMed] [Google Scholar]
- (318).Cheng K; Sorek H; Zimmermann H; Wemmer DE; Pauly M Solution-state 2D NMR spectroscopy of plant cell walls enabled by a dimethylsulfoxide-d6/1-ethyl-3-methylimidazolium acetate solvent. Anal. Chem 2013, 85, 3213–3221. [DOI] [PubMed] [Google Scholar]
- (319).Haw JF; Maciel GE; Schroeder HA Carbon-13 nuclear magnetic resonance spectrometric study of wood and wood pulping with cross polarization and magic-angle spinning. Anal. Chem 1984, 56, 1323–1329. [Google Scholar]
- (320).Love G; Snape C; Jarvis M Determination of the aromatic lignin content in oak wood by quantitative solid state 13C-NMR. Biopolymers 1992, 32, 1187–1192. [Google Scholar]
- (321).Yasuda S; Fukushima K; Kakehi A Formation and chemical structures of acid-soluble lignin I: sulfuric acid treatment time and acid-soluble lignin content of hardwood. J. Wood Sci 2001, 47, 69–72. [Google Scholar]
- (322).Matsushita Y; Kakehi A; Miyawaki S; Yasuda S Formation and chemical structures of acid-soluble lignin II: reaction of aromatic nuclei model compounds with xylan in the presence of a counterpart for condensation, and behavior of lignin model compounds with guaiacyl and syringyl nuclei in 72% sulfuric acid. J. Wood Sci 2004, 50, 136–141. [Google Scholar]
- (323).Fu L; McCallum SA; Miao J; Hart C; Tudryn GJ; Zhang F; Linhardt RJ Rapid and accurate determination of the lignin content of lignocellulosic biomass by solid-state NMR. Fuel 2015, 141, 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (324).Happs RM; Addison B; Doeppke C; Donohoe BS; Davis MF; Harman-Ware AE Comparison of methodologies used to determine aromatic lignin unit ratios in lignocellulosic biomass. Biotechnol. Biofuels 2021, 14, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (325).Evstigneyev EI; Mazur AS; Kalugina AV; Pranovich AV; Vasilyev AV Solid-State 13C CP/MAS NMR for Alkyl-O-Aryl bond determination in lignin preparations. J. Wood Chem. Technol 2018, 38, 137–148. [Google Scholar]
- (326).Porto DS; Forim MR; Costa ES; Fernandes JB; da Silva MF Evaluation of lignins of trunk and roots from Citrus sinensis L. Osbeck: a large available Brazilian biomass. J. Braz. Chem. Soc 2021, 32, 29–39. [Google Scholar]
- (327).Cipriano DF; Chinelatto LS; Nascimento SA; Rezende CA; de Menezes SMC; Freitas JCC Potential and limitations of 13C CP/MAS NMR spectroscopy to determine the lignin content of lignocellulosic feedstock. Biomass Bioenerg 2020, 142, 105792. [Google Scholar]
- (328).Gao X; Laskar DD; Zeng J; Helms GL; Chen SA 13C CP/MAS-based nondegradative method for lignin content analysis. ACS Sustain. Chem. Eng 2015, 3, 153–162. [Google Scholar]
- (329).Perras FA; Luo H; Zhang X; Mosier NS; Pruski M; Abu-Omar MM Atomic-level structure characterization of biomass pre-and post-lignin treatment by dynamic nuclear polarization-enhanced solid-state NMR. J. Phys. Chem. A 2017, 121, 623–630. [DOI] [PubMed] [Google Scholar]
- (330).Giummarella N; Pu Y; Ragauskas AJ; Lawoko M A critical review on the analysis of lignin carbohydrate bonds. Green Chem 2019, 21, 1573–1595. [Google Scholar]
- (331).Wilson L; Deligey F; Wang T; Cosgrove DJ Saccharide analysis of onion outer epidermal walls. Biotechnol. Biofuels 2021, 14, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (332).Coutinho ID; Moraes TB; Mertz-Henning LM; Nepomuceno AL; Giordani W; Marcolino-Gomes J; Santagneli S; Colnago LA Integrating high-resolution and solid-state magic angle spinning NMR spectroscopy and a transcriptomic analysis of soybean tissues in response to water deficiency. Phytochem. Anal 2017, 28, 529–540. [DOI] [PubMed] [Google Scholar]
- (333).Deborde C; Fontaine J-X; Jacob D; Botana A; Nicaise V; Richard-Forget F; Lecomte S; Decourtil C; Hamade K; Mesnard F Optimizing 1D 1H-NMR profiling of plant samples for high throughput analysis: Extract preparation, standardization, automation and spectra processing. Metabolomics 2019, 15, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (334).Tian H; Lam SM; Shui G Metabolomics, a powerful tool for agricultural research. Int. J. Mol. Sci 2016, 17, 1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (335).Ibragimova N; Mokshina N; Ageeva M; Gurjanov O; Mikshina P Rearrangement of the cellulose-enriched cell wall in flax phloem fibers over the course of the gravitropic reaction. Int. J. Mol. Sci 2020, 21, 5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (336).Bourmaud A; Siniscalco D; Foucat L; Goudenhooft C; Falourd X; Pontoire B; Arnould O; Beaugrand J; Baley C Evolution of flax cell wall ultrastructure and mechanical properties during the retting step. Carbohydr. Polym 2019, 206, 48–56. [DOI] [PubMed] [Google Scholar]
- (337).Reddy KO; Maheswari CU; Dhlamini M; Mothudi B; Kommula V; Zhang J; Zhang J; Rajulu AV Extraction and characterization of cellulose single fibers from native african napier grass. Carbohydr. Polym 2018, 188, 85–91. [DOI] [PubMed] [Google Scholar]
- (338).Kang X; Zhao W; Widanage MCD; Kirui A; Ozdenvar U; Wang T CCMRD: a solid-state NMR database for complex carbohydrates. J. Biomol. NMR 2020, 74, 239–245. [DOI] [PubMed] [Google Scholar]
- (339).Walker TW; Kuch N; Vander Meulen KA; Clewett CF; Huber GW; Fox BG; Dumesic JA Solid-state NMR studies of solvent-mediated, acid-catalyzed woody biomass pretreatment for enzymatic conversion of residual cellulose. ACS Sustainable Chem. Eng 2020, 8, 6551–6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (340).Tsuchida M; Yamaguchi H; Katayama N; Sato Y; Kawashima W; Kasai M Structural changes in cell wall of Japanese radish accompanied by release of rhamnogalacturonan during pressure cooker heating. Food Chem 2021, 349, 129117. [DOI] [PubMed] [Google Scholar]
- (341).Hossain A; Rahaman MS; Lee D; Phung TK; Canlas CG; Simmons BA; Renneckar S; Reynolds W; George A; Tulaphol S Enhanced softwood cellulose accessibility by H3PO4 pretreatment: high sugar yield without compromising lignin integrity. Ind. Eng. Chem. Res 2019, 59, 1010–1024. [Google Scholar]
- (342).Nishida M; Tanaka T; Miki T; Hayakawa Y; Kanayama K Instrumental analyses of nanostructures and interactions with water molecules of biomass constituents of Japanese cypress. Cellulose 2017, 24, 5295–5312. [Google Scholar]
- (343).Chen M-J; Zhang X-Q; Matharu A; Melo E; Li R-M; Liu C-F; Shi Q-S Monitoring the crystalline structure of sugar cane bagasse in aqueous ionic liquids. ACS Sustain. Chem. Eng 2017, 5, 7278–7283. [Google Scholar]
- (344).Nishida M; Tanaka T; Miki T; Hayakawa Y; Kanayama K Integrated analysis of modified Japanese cypress using solid-state NMR spectra and nuclear magnetic relaxation times. Cellulose 2019, 26, 3625–3642. [Google Scholar]
- (345).Jiang J; Hu Y; Tian Z; Chen K; Ge S; Xu Y; Tian D; Yang J Development of a rapid method for the quantification of cellulose in tobacco by 13C CP/MAS NMR. Carbohydr. Polym 2016, 135, 121–127. [DOI] [PubMed] [Google Scholar]
- (346).Thimm JC; Burritt DJ; Sims IM; Newman RH; Ducker WA; Melton LD Celery (Apium graveolens) parenchyma cell walls: cell walls with minimal xyloglucan. Physiol. Plant 2002, 116, 164–171. [DOI] [PubMed] [Google Scholar]
- (347).Chen D; Harris PJ; Sims IM; Zujovic Z; Melton LD Polysaccharide compositions of collenchyma cell walls from celery (Apium graveolens L.) petioles. BMC Plant Biol 2017, 17, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (348).Chen D; Melton LD; Zujovic Z; Harris PJ Developmental changes in collenchyma cell-wall polysaccharides in celery (Apium graveolens L.) petioles. BMC Plant Biol 2019, 19, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (349).Ha M-A; Viëtor RJ; Jardine GD; Apperley DC; Jarvis MC Conformation and mobility of the arabinan and galactan side-chains of pectin. Phytochemistry 2005, 66, 1817–1824. [DOI] [PubMed] [Google Scholar]
- (350).Zujovic Z; Chen D; Melton LD Comparison of celery (Apium graveolens L.) collenchyma and parenchyma cell wall polysaccharides enabled by solid-state 13C NMR. Carbohydr. Res 2016, 420, 51–57. [DOI] [PubMed] [Google Scholar]
- (351).Viger-Gravel J; Lan W; Pinon AC; Berruyer P; Emsley L; Bardet M; Luterbacher J Topology of pretreated wood fibers using dynamic nuclear polarization. J. Phys. Chem. C 2019, 123, 30407–30415. [Google Scholar]
- (352).Berruyer P; Gericke M; Moutzouri P; Jakobi D; Bardet M; Karlson L; Schantz S; Heinze T; Emsley L Advanced characterization of regioselectively substituted methylcellulose model compounds by DNP enhanced solid-state NMR spectroscopy. Carbohydr. Polym 2021, 262, 117944. [DOI] [PubMed] [Google Scholar]
- (353).Xie Y; Liu Y; Jiang C; Wu H; Bi S The Existence of Cellulose and Lignin Chemical Connections in Ginkgo Traced by 2H-13C Dual Isotopes. BioResources 2020, 15, 9028–9044. [Google Scholar]
- (354).Cegelski L; O’Connor RD; Stueber D; Singh M; Poliks B; Schaefer J Plant cell-wall cross-links by REDOR NMR spectroscopy. J. Am. Chem. Soc 2010, 132, 16052–16057. [DOI] [PubMed] [Google Scholar]
- (355).Denning DW Invasive aspergillosis. Clin. Infect. Dis 1998, 26, 781–803. [DOI] [PubMed] [Google Scholar]
- (356).Denning DW Therapeutic outcome in invasive aspergillosis. Clin. Infect. Dis 1996, 23, 608–615. [DOI] [PubMed] [Google Scholar]
- (357).Becksague CM; Jarvis WR Secular trends in the epidemiology of nosocomial fungal-infections in the United-States, 1980–1990. J. Infect. Dis 1993, 167, 1247–1251. [DOI] [PubMed] [Google Scholar]
- (358).Andriole VT Infections with Aspergillus Species. Clin. Infect. Dis 1993, 17, S481–S486. [DOI] [PubMed] [Google Scholar]
- (359).Latge JP Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev 1999, 12, 310–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (360).Marr KA; Boeckh M; Carter RA; Kim HW; Corey L Combination antifungal therapy for invasive aspergillosis. Ann. Intern. Med 2015, 162, 81–89. [DOI] [PubMed] [Google Scholar]
- (361).Andes DR; Safdar N; Baddley JW; Playford G; Reboli AC; Rex JH; Sobel JD; Pappas PG; Kullberg BJ; Group, f. t. M. S. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin. Infect. Dis 2012, 54, 1110–1122. [DOI] [PubMed] [Google Scholar]
- (362).Perfect JR The antifungal pipeline: a reality check. Nat. Rev. Drug Discov 2017, 16, 603–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (363).Le T; Van Kinh N; Cuc NTK; Tung NLN; Lam NT; Thuy PTT; Cuong DD; Phuc PTH; Vinh VH; Hanh DTH et al. A trial of itraconazole or amphotericin B for HIV-associated talaromycosis. New. Engl. J. Med 2017, 376, 2329–2340. [DOI] [PubMed] [Google Scholar]
- (364).Ghannoum MA; Rice LB Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev 1999, 12, 501–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (365).Gray KC; Palacios DS; Dailey I; Endo MM; Uno BE; Wilcock BC; Burke MD Amphotericin primarily kills yeast by simply binding ergosterol. Proc. Natl. Acad. Sci. USA 2012, 109, 2234–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (366).Marco F; Pfaller MA; Messer SA; Jones RN Antifungal activity of a new triazole, voriconazole (UK-109,496), compared with three other antifungal agents tested against clinical isolates of filamentous fungi. Med. Mycol 1998, 36, 433–436. [PubMed] [Google Scholar]
- (367).Odds FC; Brown AJP; Gow NAR Antifungal agents: mechanisms of action. Trends Microbiol 2003, 11, 272–279. [DOI] [PubMed] [Google Scholar]
- (368).Perlin DS Current perspectives on echinocandin class drugs. Future Microbiol 2011, 6, 441–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (369).Aruanno M; Glampedakis E; Lamoth F Echinocandins for the treatment of invasive aspergillosis: from laboratory to bedside. Antimicrob. Agents Chemother 2019, 63, e00399–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (370).Loiko V; Wagener J The paradoxical effect of echinocandins in Aspergillus fumigatus relies on recovery of the β-1,3-glucan synthase fks1. Antimicrob. Agents Chemother 2017, 61, e01690–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (371).Wagener J; Loiko V Recent insights into the paradoxical effect of echinocandins. J. Fungi 2017, 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (372).Rinaudo M Chitin and chitosan: properties and applications. Prog. Polym. Sci 2006, 31, 603–632. [Google Scholar]
- (373).Bowman SM; Free SJ The structure and synthesis of the fungal cell wall. Bioessays 2006, 28, 799–808. [DOI] [PubMed] [Google Scholar]
- (374).Latgé J-P; Chamilos G Aspergillus fumigatus and aspergillosis in 2019. Clin. Microbiol. Rev 2019, 33, e00140–00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (375).Gastebois A; Clavaud C; Aimanianda V; Latgé J-P Aspergillus fumigatus: cell wall polysaccharides, their biosynthesis and organization. Future Microbiol 2009, 4, 583–595. [DOI] [PubMed] [Google Scholar]
- (376).Orlean P Architecture and biosynthesis of the Saccharomyces cerevisiae cell wall. Genetics 2012, 192, 775–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (377).Lipke PN; Ovalle R Cell wall architecture in yeast: new structure and new challenges. J. Bacteriol 1998, 180, 3735–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (378).Brown JA; Catley BJ Monitoring polysaccharide synthesis in Candida albicans. Carbohydr. Res 1992, 227, 195–202. [Google Scholar]
- (379).Cherniak R; Sundstrom JB Polysaccharide antigens of the capsule of Cryptococcus neoforman. Infect. Immun 1994, 62, 1507–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (380).Cortés JCG; Curto MÁ; Carvalho VSD; Pérez P; Ribas JC The fungal cell wall as a target for the development of new antifungal therapies. Biotechnol. Adv 2019, 37, 107352. [DOI] [PubMed] [Google Scholar]
- (381).Latgé J-P; Beauvais A Functional duality of the cell wall. Curr. Opin. Microbiol 2014, 20, 111–117. [DOI] [PubMed] [Google Scholar]
- (382).Wang ZA; Li LX; Doering TL Unraveling synthesis of the cryptococcal cell wall and capsule. Glycobiology 2018, 28, 719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (383).Kollár R; Petrakova E; Ashwell G; Robbins PW; Cabib E Architecture of the yeast-cell wall: the linkage between chitin and β(1→3)-glucan. J. Biol. Chem 1995, 270, 1170–1178. [DOI] [PubMed] [Google Scholar]
- (384).Kollár R; Reinhold BB; Petrakova E; Yeh HJC; Ashwell G; Drgonova J; Kapteyn JC; Klis FM; Cabib E Architecture of the yeast cell wall - β(1→6)-glucan interconnects mannoprotein, β(1→3)-glucan, and chitin. J. Biol. Chem 1997, 272, 17762–17775. [DOI] [PubMed] [Google Scholar]
- (385).Latgé JP; Kobayashi H; Debeaupuis JP; Diaquin M; Sarfati J; Wieruszeski JM; Parra E; Bouchara JP; Fournet B Chemical and immunological characterization of the extracellular galactomannan of Aspergillus fumigatus. Infect. Immun 1994, 62, 5424–5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (386).Gressler M; Heddergott C; N’Go IC; Renga G; Oikonomou V; Moretti S; Coddeville B; Gaifem J; Silvestre R; Romani L et al. Definition of the anti-inflammatory oligosaccharides derived from the galactosaminogalactan (GAG) from Aspergillus fumigatus. Front. Cell. Infect. Microbiol 2019, 9, 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (387).Chakraborty A; Fernando LD; Fang W; Dickwella Widanage MC; Wei P; Jin C; Fontaine T; Latgé JP; Wang T A molecular vision of fungal cell wall organization by functional genomics and solid-state NMR. Nat. Commun 2021, 12, 6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (388).Ohm RA; de Jong JF; Lugones LG; Aerts A; Kothe E; Stajich JE; de Vries RP; Record E; Levasseur A; Baker SE et al. Genome sequence of the model mushroom Schizophyllum commune. Nat. Biotechnol 2010, 28, 957–963. [DOI] [PubMed] [Google Scholar]
- (389).Sietsma JH; Wessels JGH Chemical analysis of the hyphal walls of Schizophyllum commune. Biochim. Biophys. Acta 1977, 496, 225–239. [DOI] [PubMed] [Google Scholar]
- (390).Sietsma JH; Wessels JGH Solubility of (1→3)-β-D/(1→6)-β-D-glucan in fungal walls: importance of presumed linkage between glucan and chitin. Microbiology 1981, 125, 209–212. [DOI] [PubMed] [Google Scholar]
- (391).van der Valk P; Marchant R; Wessels JGH Ultrastructural localization of polysaccharides in the wall and septum of the basidiomycete Schizophyllum commune. Exp. Mycol 1977, 1, 69–82. [Google Scholar]
- (392).Sietsma JH; Wessels JGH Evidence for covalent linkages between chitin and β-glucan in a fungal wall. Microbiology 1979, 114, 99–108. [Google Scholar]
- (393).Li Y; Sun H; Zhu X; Bian C; Wang Y; Si S Identification of new antifungal agents targeting chitin synthesis by a chemical-genetic method. Molecules 2019, 24, 3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (394).Fernando LD; Dickwella Widanage MC; Penfield J; Lipton AS; Washton N; Latge J-P; Wang P; Zhang L; Wang T Structural polymorphism of chitin and chitosan in fungal cell walls from solid-state NMR and principal component analysis. Front. Mol. Biosci 2021, 8, 727053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (395).Nielsen K; Cox GM; Litvintseva AP; Mylonakis E; Malliaris SD; Benjamin DK; Giles SS; Mitchell TG; Casadevall A; Perfect JR et al. Cryptococcus neoformans α strains preferentially disseminate to the central nervous system during coinfection. Infect. Immun 2005, 73, 4922–4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (396).Rajasingham R; Smith RM; Park BJ; Jarvis JN; Govender NP; Chiller TM; Denning DW; Loyse A; Boulware DR Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect. Dis 2017, 17, 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (397).Chrissian C; Lin CP-C; Camacho E; Casadevall A; Neiman AM; Stark RE Unconventional constituents and shared molecular architecture of the melanized cell wall of C. neoformans and spore wall of S. cerevisiae. J. Fungi 2020, 6, 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (398).Eisenman HC; Casadevall A Synthesis and assembly of fungal melanin. Appl. Microbiol. Biotechnol 2012, 93, 931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (399).Ikeda R; Sugita T; Jacobson ES; Shinoda T Effects of melanin upon susceptibility of Cryptococcus to antifungals. Microbiol. Immunol 2003, 47, 271–277. [DOI] [PubMed] [Google Scholar]
- (400).Nosanchuk JD; Valadon P; Feldmesser M; Casadevall A Melanization of Cryptococcus neoformans in murine Iinfection. Mol. Cell. Biol 1999, 19, 745–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (401).Eisenman HC; Nosanchuk JD; Webber JBW; Emerson RJ; Camesano TA; Casadevall A Microstructure of cell wall-associated melanin in the human pathogenic fungus Cryptococcus neoformans. Biochemistry 2005, 44, 3683–3693. [DOI] [PubMed] [Google Scholar]
- (402).Camacho E; Vij R; Chrissian C; Prados-Rosales R; Gil D; O’Meally RN; Cordero RJB; Cole RN; McCaffery JM; Stark RE et al. The structural unit of melanin in the cell wall of the fungal pathogen Cryptococcus neoformans. J. Biol. Chem 2019, 294, 10471–10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (403).Chatterjee S; Prados-Rosales R; Frases S; Itin B; Casadevall A; Stark RE Using solid-state NMR To Monitor the Molecular Consequences of Cryptococcus neoformans Melanization with different catecholamine precursors. Biochemistry 2012, 51, 6080–6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (404).Chatterjee S; Prados-Rosales R; Tan S; Phan VC; Chrissian C; Itin B; Wang H; Khajo A; Magliozzo RS; Casadevall A et al. The melanization road more traveled by: Precursor substrate effects on melanin synthesis in cell-free and fungal cell systems. J. Biol. Chem 2018, 293, 20157–20168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (405).Chatterjee S; Prados-Rosales R; Itin B; Casadevall A; Stark RE Solid-state NMR reveals the carbon-based molecular architecture of Cryptococcus neoformans fungal eumelanins in the cell wall. J. Biol. Chem 2015, 290, 13779–13790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (406).Free SJ Chapter two - fungal cell wall organization and biosynthesis. Adv. Genet 2013, 81, 33–82. [DOI] [PubMed] [Google Scholar]
- (407).Crocker E; Patel AB; Eilers M; Jayaraman S; Getmanova E; Reeves PJ; Ziliox M; Khorana HG; Sheves M; Smith SO Dipolar assisted rotational resonance NMR of tryptophan and tyrosine in rhodopsin. J. Biomol. NMR 2004, 29, 11–20. [DOI] [PubMed] [Google Scholar]
- (408).Simon JD; Peles DN The red and the black. Acc. Chem. Res 2010, 43, 1452–1460. [DOI] [PubMed] [Google Scholar]
- (409).Della Vecchia NF; Avolio R; Alfè M; Errico ME; Napolitano A; d’Ischia M Building-block diversity in polydopamine underpins a multifunctional eumelanin-type platform tunable through a quinone control point. Adv. Funct. Mater 2013, 23, 1331–1340. [Google Scholar]
- (410).Schnitzer M; Chan YK Structural characteristics of a fungal melanin and a soil humic acid. Soil Sci. Soc. Am. J 1986, 50, 67–71. [Google Scholar]
- (411).Knicker H; Almendros G; González-Vila FJ; Lüdemann HD; Martin F 13C and 15N NMR analysis of some fungal melanins in comparison with soil organic matter. Org. Geochem 1995, 23, 1023–1028. [Google Scholar]
- (412).Zhong JY; Frases S; Wang H; Casadevall A; Stark RE Following fungal melanin biosynthesis with solid-state NMR: biopolyrner molecular structures and possible connections to cell-wall polysaccharides. Biochemistry 2008, 47, 4701–4710. [DOI] [PubMed] [Google Scholar]
- (413).Chrissian C; Camacho E; Fu MS; Prados-Rosales R; Chatterjee S; Cordero RJB; Lodge JK; Casadevall A; Stark RE Melanin deposition in two Cryptococcus species depends on cell-wall composition and flexibility. J. Biol. Chem 2020, 295, 1815–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (414).Neiman AM Sporulation in the budding yeast Saccharomyces cerevisiae. Genetics 2011, 189, 737–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (415).Anderson TM; Clay MC; Cioffi AG; Diaz KA; Hisao GS; Tuttle MD; Nieuwkoop AJ; Comellas G; Maryum N; Wang S et al. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat. Chem. Biol 2014, 10, 400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (416).Ermishkin LN; Kasumov KM; Potzeluyev VM Single ionic channels induced in lipid bilayers by polyene antibiotics amphotericin B and nystatine. Nature 1976, 262, 698–699. [DOI] [PubMed] [Google Scholar]
- (417).Cereghetti DM; Carreira EM Amphotericin B: 50 years of chemistry and biochemistry. Synthesis 2006, 2006, 0914–0942. [Google Scholar]
- (418).Fernandez-Lopez S; Kim H-S; Choi EC; Delgado M; Granja JR; Khasanov A; Kraehenbuehl K; Long G; Weinberger DA; Wilcoxen KM et al. Antibacterial agents based on the cyclic D,L-α-peptide architecture. Nature 2001, 412, 452–455. [DOI] [PubMed] [Google Scholar]
- (419).Mouri R; Konoki K; Matsumori N; Oishi T; Murata M Complex formation of amphotericin B in sterol-containing membranes as evidenced by surface plasmon resonance. Biochemistry 2008, 47, 7807–7815. [DOI] [PubMed] [Google Scholar]
- (420).Murata M; Kasai Y; Umegawa Y; Matsushita N; Tsuchikawa H; Matsumori N; Oishi T Ion channel complex of antibiotics as viewed by NMR. Pure Appl. Chem 2009, 81, 1123–1129. [Google Scholar]
- (421).Matsumori N; Sawada Y; Murata M Large molecular assembly of amphotericin B formed in ergosterol-containing membrane evidenced by solid-state NMR of intramolecular bridged derivative. J. Am. Chem. Soc 2006, 128, 11977–11984. [DOI] [PubMed] [Google Scholar]
- (422).Matsuoka S; Ikeuchi H; Umegawa Y; Matsumori N; Murata M Membrane interaction of amphotericin B as single-length assembly examined by solid state NMR for uniformly 13C-enriched agent. Bioorg. Med. Chem 2006, 14, 6608–6614. [DOI] [PubMed] [Google Scholar]
- (423).Umegawa Y; Nakagawa Y; Tahara K; Tsuchikawa H; Matsumori N; Oishi T; Murata M Head-to-tail interaction between amphotericin B and ergosterol occurs in hydrated phospholipid membrane. Biochemistry 2012, 51, 83–89. [DOI] [PubMed] [Google Scholar]
- (424).de Kruijff B; Demel RA Polyene antibiotic-sterol interactions in membranes of Acholeplasma laidlawii cells and lecithin liposomes. 3. Molecular structure of the polyene antibiotic-cholesterol complexes. Biochim. Biophys. Acta 1974, 339, 57–70. [DOI] [PubMed] [Google Scholar]
- (425).Nakagawa Y; Umegawa Y; Matsushita N; Yamamoto T; Tsuchikawa H; Hanashima S; Oishi T; Matsumori N; Murata M The structure of the bimolecular complex between amphotericin B and ergosterol in membranes is stabilized by face-to-face van der Waals interaction with their rigid cyclic cores. Biochemistry 2016, 55, 3392–3402. [DOI] [PubMed] [Google Scholar]
- (426).Ostrosky-Zeichner L; Casadevall A; Galgiani JN; Odds FC; Rex JH An insight into the antifungal pipeline: selected new molecules and beyond. Nat. Rev. Drug Discov 2010, 9, 719–727. [DOI] [PubMed] [Google Scholar]
- (427).Foglia F; Drake AF; Terry AE; Rogers SE; Lawrence MJ; Barlow DJ Small-angle neutron scattering studies of the effects of amphotericin B on phospholipid and phospholipid–sterol membrane structure. BBA-Biomembranes 2011, 1808, 1574–1580. [DOI] [PubMed] [Google Scholar]
- (428).Reichhardt C; Joubert LM; Clemons KV; Stevens DA; Cegelski L Integration of electron microscopy and solid-state NMR analysis for new views and compositional parameters of Aspergillus fumigatus biofilms. Med. Mycol 2019, 57, S239–s244. [DOI] [PubMed] [Google Scholar]
- (429).Pfaller MA; Diekema DJ Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev 2007, 20, 133–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (430).White TC; Marr KA; Bowden RA Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev 1998, 11, 382–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (431).Mann DG; Vanormelingen P An inordinate fondness? The number, distributions, and origins of diatom species. J. Eukaryot. Microbiol 2013, 60, 414–420. [DOI] [PubMed] [Google Scholar]
- (432).Guiry MD How many species of algae are there? J. Phycol 2012, 48, 1057–1063. [DOI] [PubMed] [Google Scholar]
- (433).Flombaum P; Gallegos J; Gordillo R; Rincón J; Zabala L; Jiao N; Karl D; Li W; Lomas M; Veneziano D et al. Present and future global distributions of the marine Cyanobacteria Prochlorococcus and Synechococcus. Proc. Natl. Acad. Sci. USA 2013, 110, 9824–9829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (434).Van Den Hoek C; Mann DG; Jahns HM Algae: an introduction to phycology; Cambridge University Press: Cambridge; New York, 1995. [Google Scholar]
- (435).Round FE The ecology of algae; Cambridge University Press: Cambridge, 1981, 1–653. [Google Scholar]
- (436).Lyon BR; Mock T Polar microalgae: new approaches towards understanding adaptations to an extreme and changing environment. Biology 2014, 3, 56–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (437).Cardon ZG; Gray DW; Lewis LA The green algal underground: evolutionary secrets of desert cells. BioScience 2008, 58, 114–122. [Google Scholar]
- (438).Tesson SVM; Skjøth CA; Šantl-Temkiv T; Löndahl J Airborne microalgae: insights, opportunities, and challenges. Appl. Environ. Microbiol 2016, 82, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (439).Radakovits R; Jinkerson RE; Darzins A; Posewitz MC Genetic engineering of algae for enhanced biofuel production. Eukaryot Cell 2010, 9, 486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (440).Goncalves EC; Koh J; Zhu N; Yoo M-J; Chen S; Matsuo T; Johnson JV; Rathinasabapathi B Nitrogen starvation-induced accumulation of triacylglycerol in the green algae: evidence for a role for ROC40, a transcription factor involved in circadian rhythm. Plant J 2016, 85, 743–757. [DOI] [PubMed] [Google Scholar]
- (441).Barkia I; Saari N; Manning SR Microalgae for high-value products towards human health and nutrition. Mar Drugs 2019, 17, 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (442).Le Costaouëc T; Unamunzaga C; Mantecon L; Helbert W New structural insights into the cell-wall polysaccharide of the diatom Phaeodactylum tricornutum. Algal Res 2017, 26, 172–179. [Google Scholar]
- (443).Berteau O; Mulloy B Sulfated fucans, fresh perspectives: structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29R–40R. [DOI] [PubMed] [Google Scholar]
- (444).Pomin VH; Mourão PAS Structure, biology, evolution, and medical importance of sulfated fucans and galactans. Glycobiology 2008, 18, 1016–1027. [DOI] [PubMed] [Google Scholar]
- (445).Barry VC; Dillon T; Hawkins B; O’Colla P The xylan of Rhodymenia palmata. Nature 1950, 166, 788–788. [DOI] [PubMed] [Google Scholar]
- (446).Björndal, Karl-Erik HE; Garegg Per J.; Lindberg Bengt; Swan Brita. Studies on the xylan from the red seaweed Rhodymenia palmata. Acta Chem. Scand 1965, 19, 2309–2315. [Google Scholar]
- (447).Percival E; McDowell RH; Husemann E Chemistry and enzymology of marine algal polysaccharides; Academic Press: London-New York, 1967. [Google Scholar]
- (448).Mikkelsen MD; Harholt J; Ulvskov P; Johansen IE; Fangel JU; Doblin MS; Bacic A; Willats WGT Evidence for land plant cell wall biosynthetic mechanisms in charophyte green algae. Ann. Bot 2014, 114, 1217–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (449).Fry SC Cell wall polysaccharide composition and covalent crosslinking. Annu. Plant Rev 2011, 41, 1–42 [Google Scholar]
- (450).Domozych DS; Sørensen I; Popper ZA; Ochs J; Andreas A; Fangel JU; Pielach A; Sacks C; Brechka H; Ruisi-Besares P et al. Pectin metabolism and assembly in the cell wall of the charophyte green alga Penium margaritaceum. Plant Physiol 2014, 165, 105–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (451).Deniaud-Bouët E; Kervarec N; Michel G; Tonon T; Kloareg B; Hervé C Chemical and enzymatic fractionation of cell walls from fucales: insights into the structure of the extracellular matrix of brown algae. Ann. Bot 2014, 114, 1203–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (452).Zang X; Nguyen RT; Harvey HR; Knicker H; Hatcher PG Preservation of proteinaceous material during the degradation of the green alga Botryococcus braunii: A solid-state 2D 15N 13C NMR spectroscopy study. Geochim. Cosmochim. Acta 2001, 65, 3299–3305. [Google Scholar]
- (453).Weich RG; Lundberg P; Vogel HJ; Jensén P Phosphorus-31 NMR studies of cell wall-associated calcium-phosphates in Ulva lactuca. Plant Physiol 1989, 90, 230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (454).La Vars SM; Johnston MR; Hayles J; Gascooke JR; Brown MH; Leterme SC; Ellis AV 29Si{1H} CP-MAS NMR comparison and ATR-FTIR spectroscopic analysis of the diatoms Chaetoceros muelleri and Thalassiosira pseudonana grown at different salinities. Anal. Bioanal. Chem 2013, 405, 3359–3365. [DOI] [PubMed] [Google Scholar]
- (455).Lahaye M; Robic A Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1774. [DOI] [PubMed] [Google Scholar]
- (456).Michel G; Tonon T; Scornet D; Cock JM; Kloareg B The cell wall polysaccharide metabolism of the brown alga Ectocarpus siliculosus. Insights into the evolution of extracellular matrix polysaccharides in eukaryotes. New Phytol 2010, 188, 82–97. [DOI] [PubMed] [Google Scholar]
- (457).Lahaye M; Alvarez-Cabal Cimadevilla E; Kuhlenkamp R; Quemener B; Lognoné V; Dion P Chemical composition and 13C NMR spectroscopic characterisation of ulvans from Ulva (Ulvales, Chlorophyta). J. Appl. Phycol 1999, 11, 1. [Google Scholar]
- (458).Llanes F; Sauriol F; Morin FG; Perlin AS An examination of sodium alginate from Sargassum by NMR spectroscopy. Can. J. Chem 1997, 75, 585–590. [Google Scholar]
- (459).Miller I; Blunt J Evaluation of the Structure of the Polysaccharides from Chondria macrocarpa and Ceramium rubrum as determined by 13C NMR Spectroscopy. Bot Mar. 2002, 45, 1–8. [Google Scholar]
- (460).Shams El Din N; Shaltout N; Ghazal M; Ali A; Beltagy D Chemical pretreatment of Ulva fasciata cell wall for enhancing biodiesel yield and properties. Egypt. J. Aquat. Biol. Fish 2020, 24, 103–125. [Google Scholar]
- (461).Soleymani F; Khani MH; Pahlavanzadeh H; Manteghian M Study of cobalt (II) biosorption on Sargassum sp. by experimental design methodology. Int. J. Environ. Sci. Technol 2015, 12, 1907–1922. [Google Scholar]
- (462).Chandler CJ; Wilts BD; Vignolini S; Brodie J; Steiner U; Rudall PJ; Glover BJ; Gregory T; Walker RH Structural colour in Chondrus crispus. Sci. Rep 2015, 5, 11645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (463).Suresh S Biosynthesis and assemblage of extracellular cellulose by bacteria. Handbook of environmental materials management Springer International Publishing: Cham, 2018, 1–43. [Google Scholar]
- (464).Mihranyan A Cellulose from cladophorales green algae: From environmental problem to high-tech composite materials. J. Appl. Polym. Sci 2011, 119, 2449–2460. [Google Scholar]
- (465).Samiee S; Ahmadzadeh H; Hosseini M; Lyon S Chapter 17 - algae as a source of microcrystalline cellulose. Advanced bioprocessing for alternative fuels, biobased chemicals, and bioproducts Woodhead Publishing, 2019, 331–350. [Google Scholar]
- (466).Revol J-F On the cross-sectional shape of cellulose crystallites in Valonia ventricosa. Carbohydr. Polym 1982, 2, 123–134. [Google Scholar]
- (467).Sugiyama J; Chanzy H; Revol JF On the polarity of cellulose in the cell wall of Valonia. Planta 1994, 193, 260–265. [Google Scholar]
- (468).Sugiyama J; Okano T; Yamamoto H; Horii F Transformation of Valonia cellulose crystals by an alkaline hydrothermal treatment. Macromolecules 1990, 23, 3196–3198. [Google Scholar]
- (469).Yamamoto H; Horii F CPMAS carbon-13 NMR analysis of the crystal transformation induced for Valonia cellulose by annealing at high temperatures. Macromolecules 1993, 26, 1313–1317. [Google Scholar]
- (470).Ibrahim R; Melton L; B.G s.; Schroeder R Mannans in primary and secondary plant cell walls. N. Z. J. For. Sci 2009, 39, 153–160. [Google Scholar]
- (471).Estevez JM; Fernández PV; Kasulin L; Dupree P; Ciancia M Chemical and in situ characterization of macromolecular components of the cell walls from the green seaweed Codium fragile. Glycobiology 2009, 19, 212–228. [DOI] [PubMed] [Google Scholar]
- (472).Dunn EK; Shoue DA; Huang X; Kline RE; MacKay AL; Carpita NC; Taylor IE; Mandoli DF Spectroscopic and biochemical analysis of regions of the cell wall of the unicellular ‘mannan weed’, Acetabularia acetabulum. Plant Cell Physiol 2007, 48, 122–133. [DOI] [PubMed] [Google Scholar]
- (473).George J; Sabapathi SN Cellulose nanocrystals: synthesis, functional properties, and applications. Nanotechnol Sci Appl 2015, 8, 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (474).Hsieh YSY; Harris PJ Xylans of red and green algae: what is known about their structures and how they are synthesised? Polymers 2019, 11, 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (475).Furneaux RH; Miller IJ Isolation and C-NMR spectral study of the water soluble polysaccharides from four south african red algae. Bot. Mar 1986, 29, 3–10. [Google Scholar]
- (476).Yamagaki T; Maeda M; Kanazawa K; Ishizuka Y; Nakanishi H Structural clarification of Caulerpa cell wall/M,3-xylan by NMR spectroscopy. Biosci. Biotechnol. Biochem 1997, 61, 1077–1080. [Google Scholar]
- (477).Ciancia M; Alberghina J; Arata P; Benavides H; Leliaert F; Verbruggen H; Estevez J Characterization of cell wall polysaccharides of the coencocytic green seaweed Bryopsis plumosa (Bryopsidaceae, Chlorophyta) from the argentine coast. J. Phycol 2012, 48, 326–35. [DOI] [PubMed] [Google Scholar]
- (478).Lahaye M; Rondeau-Mouro C; Deniaud E; Buléon A Solid-state 13C NMR spectroscopy studies of xylans in the cell wall of Palmaria palmata (L. Kuntze, Rhodophyta). Carbohydr. Res 2003, 338, 1559–1569. [DOI] [PubMed] [Google Scholar]
- (479).Deniaud E; Quemener B; Fleurence J; Lahaye M Structural studies of the mix-linked β-(1→3)/β-(1→4)-D-xylans from the cell wall of Palmaria palmata (Rhodophyta). Int. J. Biol. Macromol 2003, 33, 9–18. [DOI] [PubMed] [Google Scholar]
- (480).Hervé C; Siméon A; Jam M; Cassin A; Johnson KL; Salmeán AA; Willats WG; Doblin MS; Bacic A; Kloareg B Arabinogalactan proteins have deep roots in eukaryotes: identification of genes and epitopes in brown algae and their role in Fucus serratus embryo development. New Phytol 2016, 209, 1428–1441. [DOI] [PubMed] [Google Scholar]
- (481).Johnson KL; Cassin AM; Lonsdale A; Wong GK-S; Soltis DE; Miles NW; Melkonian M; Melkonian B; Deyholos MK; Leebens-Mack J et al. Insights into the evolution of hydroxyproline-rich glycoproteins from 1000 plant transcriptomes. Plant Physiol 2017, 174, 904–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (482).Lahaye M Developments on gelling algal galactans, their structure and physico-chemistry. J. Appl. Phycol 2001, 13, 173–184. [Google Scholar]
- (483).van de Velde F; Pereira L; Rollema HS The revised NMR chemical shift data of carrageenans. Carbohydr. Res 2004, 339, 2309–2313. [DOI] [PubMed] [Google Scholar]
- (484).Rocha HA; Moraes FA; Trindade ES; Franco CR; Torquato RJ; Veiga SS; Valente AP; Mourão PA; Leite EL; Nader HB et al. Structural and hemostatic activities of a sulfated galactofucan from the brown alga Spatoglossum schroederi. An ideal antithrombotic agent? J. Biol. Chem 2005, 280, 41278–41288. [DOI] [PubMed] [Google Scholar]
- (485).Farias EH; Pomin VH; Valente AP; Nader HB; Rocha HA; Mourão PA A preponderantly 4-sulfated, 3-linked galactan from the green alga Codium isthmocladum. Glycobiology 2008, 18, 250–259. [DOI] [PubMed] [Google Scholar]
- (486).Li B; Lu F; Wei X; Zhao R Fucoidan: structure and bioactivity. Molecules 2008, 13, 1671–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (487).Deniaud-Bouët E; Hardouin K; Potin P; Kloareg B; Hervé C A review about brown algal cell walls and fucose-containing sulfated polysaccharides: Cell wall context, biomedical properties and key research challenges. Carbohydr. Polym 2017, 175, 395–408. [DOI] [PubMed] [Google Scholar]
- (488).Gordon-Mills E; Tate M; Hounslow A Use of solid and gel state 13C NMR spectroscopy for differentiation between agarophytes and carrageenophytes. Hydrobiologia 1990, 204–205, 629–636. [Google Scholar]
- (489).Lahaye M; Rochas C Chemical structure and physico-chemical properties of agar. Hydrobiologia 1991, 221, 137–148. [Google Scholar]
- (490).Youngs HL; Gretz MR; West JA; Sommerfeld MR The cell wall chemistry of Bangia atropurpurea (Bangiales, Rhodophyta) and Bostrychia moritziana (Ceramiales, Rhodophyta) from marine and freshwater environments. Psychol. Res 1998, 46, 63–73. [Google Scholar]
- (491).Haug A; Larsen B; Smidsrød O Uronic acid sequence in alginate from different sources. Carbohydr. Res 1974, 32, 217–225. [Google Scholar]
- (492).Lahaye M; Robic A Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1774. [DOI] [PubMed] [Google Scholar]
- (493).Blokker P; Schouten S; van den Ende H; de Leeuw JW; Hatcher PG; Sinninghe Damsté JS Chemical structure of algaenans from the fresh water algae Tetraedron minimum, Scenedesmus communis and Pediastrum boryanum. Org. Geochem 1998, 29, 1453–1468. [Google Scholar]
- (494).Rodríguez MC; Cerezo AS The resistant ‘biopolymer’ in cell walls of Coelastrum sphaericum. Phytochemistry 1996, 43, 731–734. [Google Scholar]
- (495).Rodríguez MC; Noseda MD; Cerezo AS The Fibrillar Polysaccharides and their Linkage to Algaenan in the Trilaminar Layer of the Cell Wall of Coelastrum Sphaericum (Chlorophyceae). J. Phycol 1999, 35, 1025–1031. [Google Scholar]
- (496).Simpson AJ; Zang X; Kramer R; Hatcher PG New insights on the structure of algaenan from Botryoccocus braunii race A and its hexane insoluble botryals based on multidimensional NMR spectroscopy and electrospray-mass spectrometry techniques. Phytochemistry 2003, 62, 783–796. [DOI] [PubMed] [Google Scholar]
- (497).Rahman MA; Halfar J First evidence of chitin in calcified coralline algae: new insights into the calcification process of Clathromorphum compactum. Sci. Rep 2014, 4, 6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (498).Rahman MA; Halfar J; Adey WH; Nash M; Paulo C; Dittrich M The role of chitin-rich skeletal organic matrix on the crystallization of calcium carbonate in the crustose coralline alga Leptophytum foecundum. Sci. Rep 2019, 9, 11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (499).Ehrlich H Chitin and collagen as universal and alternative templates in biomineralization. Int. Geol. Rev 2010, 52, 661–699. [Google Scholar]
- (500).Kitaoku Y; Fukamizo T; Numata T; Ohnuma T Chitin oligosaccharide binding to the lysin motif of a novel type of chitinase from the multicellular green alga, Volvox carteri. Plant Mol. Biol 2017, 93, 97–108. [DOI] [PubMed] [Google Scholar]
- (501).Kawasaki T; Tanaka M; Fujie M; Usami S; Sakai K; Yamada T Chitin synthesis in chlorovirus CVK2-infected Chlorella cells. Virology 2002, 302, 123–131. [DOI] [PubMed] [Google Scholar]
- (502).Hecky RE; Mopper K; Kilham P; Degens ET The amino acid and sugar composition of diatom cell-walls. Mar. Biol 1973, 19, 323–331. [Google Scholar]
- (503).Bedoshvili YD; Likhoshway YV Cellular mechanisms of diatom valve morphogenesis. Diatoms: fundamentals and applications 2019, 99–114. [Google Scholar]
- (504).Shrestha RP; Tesson B; Norden-Krichmar T; Federowicz S; Hildebrand M; Allen AE Whole transcriptome analysis of the silicon response of the diatom Thalassiosira pseudonana. BMC Genomics 2012, 13, 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (505).Hildebrand M; Lerch SJL; Shrestha RP Understanding diatom cell wall silicification—moving forward. Front. Mar. Sci 2018, 5. [Google Scholar]
- (506).Kroger N; Sandhage KH From diatom biomolecules to bioinspired syntheses of silica- and titania-based materials. MRS Bulletin 2010, 35, 122–126. [Google Scholar]
- (507).Tesson B; Masse S; Laurent G; Maquet J; Livage J; Martin-Jézéquel V; Coradin T Contribution of multi-nuclear solid state NMR to the characterization of the Thalassiosira pseudonana diatom cell wall. Anal. Bioanal. Chem 2008, 390, 1889–1898. [DOI] [PubMed] [Google Scholar]
- (508).Görlich S; Pawolski D; Zlotnikov I; Kröger N Control of biosilica morphology and mechanical performance by the conserved diatom gene Silicanin-1. Commun. Biol 2019, 2, 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (509).Ehren HL; Kolbe F; Paioni AL; Brunner E; Baldus M DNP-supported solid-state NMR studies of 13C,15N,29Si-enriched biosilica of Cyclotella cryptica and Thalassiosira pseudonan. Discov. Mater 2021, 1, 9. [Google Scholar]
- (510).Kröger N; Poulsen N Diatoms—from cell wall biogenesis to nanotechnology. Annu. Rev. Genet 2008, 42, 83–107 [DOI] [PubMed] [Google Scholar]
- (511).Scheffel A; Poulsen N; Shian S; Kröger N Nanopatterned protein microrings from a diatom that direct silica morphogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 3175–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (512).Brunner E; Richthammer P; Ehrlich H; Paasch S; Simon P; Ueberlein S; van Pée KH Chitin-based organic networks: an integral part of cell wall biosilica in the diatom Thalassiosira pseudonana. Angew. Chem. Int. Ed 2009, 48, 9724–9727. [DOI] [PubMed] [Google Scholar]
- (513).Tesson B; Hildebrand M Extensive and intimate association of the cytoskeleton with forming silica in diatoms: control over patterning on the meso- and micro-scale. PLOS One 2010, 5, e14300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (514).Tesson B; Hildebrand M Dynamics of silica cell wall morphogenesis in the diatom Cyclotella cryptica: Substructure formation and the role of microfilaments. J. Struct. Biol 2010, 169, 62–74. [DOI] [PubMed] [Google Scholar]
- (515).Kolbe F; Ehren HL; Kohrs S; Butscher D; Reiß L; Baldus M; Brunner E Solid-state NMR spectroscopic studies of 13C, 15N, 29Si-enriched biosilica from the marine diatom Cyclotella cryptica. Discov. Mater 2020, 1, 3. [Google Scholar]
- (516).Bertermann R; Kröger N; Tacke R Solid-state 29Si MAS NMR studies of diatoms: structural characterization of biosilica deposits. Anal. Bioanal. Chem 2003, 375, 630–634. [DOI] [PubMed] [Google Scholar]
- (517).Johnston MR; Gascooke JR; Ellis AV; Leterme SC Diatoms response to salinity changes: investigations using single pulse and cross polarisation magic angle spinning 29Si NMR spectra. Analyst 2018, 143, 4930–4935. [DOI] [PubMed] [Google Scholar]
- (518).Tesson B; Hildebrand M Characterization and localization of insoluble organic matrices associated with diatom cell walls: insight into their roles during cell wall formation. PLOS One 2013, 8, e61675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (519).Arias JL; Fernández MS Polysaccharides and proteoglycans in calcium carbonate-based biomineralization. Chem. Rev 2008, 108, 4475–4482. [DOI] [PubMed] [Google Scholar]
- (520).Falk M; Smith DG; McLachlan J; McInnes AG Studies on chitan (β-(1→4)-linked 2-acetamido-2-deoxy-D-glucan) fibers of the diatom Thalassiosira fluviatilis hustedt: II. proton magnetic resonance, Infrared, and X-ray studies. Can. J. Chem 1966, 44, 2269–2281. [Google Scholar]
- (521).Ford CW; Percival E The carbohydrates of phaeodactylum tricornutum. Part I. preliminary examination of the organism, and characterisation of low molecular weight material and of a glucan. J. Chem. Soc 1965, 7035–7041. [Google Scholar]
- (522).Wisser D; Brückner SI; Wisser FM; Althoff-Ospelt G; Getzschmann J; Kaskel S; Brunner E 1H-13C-29Si triple resonance and REDOR solid-state NMR-A tool to study interactions between biosilica and organic molecules in diatom cell walls. Solid State Nucl. Magn. Reson 2015, 66–67, 33–39. [DOI] [PubMed] [Google Scholar]
- (523).Akhter M; Dutta Majumdar R; Fortier-McGill B; Soong R; Liaghati-Mobarhan Y; Simpson M; Arhonditsis G; Schmidt S; Heumann H; Simpson AJ Identification of aquatically available carbon from algae through solution-state NMR of whole 13C-labelled cells. Anal. Bioanal. Chem 2016, 408, 4357–4370. [DOI] [PubMed] [Google Scholar]
- (524).Bouillaud D; Heredia V; Castaing-Cordier T; Drouin D; Charrier B; Gonçalves O; Farjon J; Giraudeau P Benchtop flow NMR spectroscopy as an online device for the in vivo monitoring of lipid accumulation in microalgae. Algal Res 2019, 43, 101624. [Google Scholar]
- (525).Bouillaud D; Drouin D; Charrier B; Jacquemmoz C; Farjon J; Giraudeau P; Gonçalves O Using benchtop NMR spectroscopy as an online non-invasive in vivo lipid sensor for microalgae cultivated in photobioreactors. Process Biochem 2020, 93, 63–68. [Google Scholar]
- (526).Mobarhan YL; Fortier-McGill B; Soong R; Maas WE; Fey M; Monette M; Stronks HJ; Schmidt S; Heumann H; Norwood W et al. Comprehensive multiphase NMR applied to a living organism. Chem. Sci 2016, 7, 4856–4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (527).Biswas R; Fortier-McGill B; Akhter M; Soong R; Ning P; Bastawrous M; Jenne A; Schmidig D; Castro P; Graf S et al. Ex vivo Comprehensive Multiphase NMR of whole organisms: a complementary tool to in vivo NMR. Analytica Chimica Acta: X 2020, 6, 100051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (528).Chauton MS; Størseth T HR MAS NMR spectroscopy of marine microalgae. Modern Magnetic Resonance Springer International Publishing: Cham, 2018, 1927–1935. [Google Scholar]
- (529).Chauton MS; Størseth TR; Krane J High-resolution magic angle spinning NMR analysis of whole cells of Chaetoceros muelleri (Bacillariophyceae) and comparison with 13C-NMR and distortionless enhancement by polarization transfer 13C-NMR analysis of lipophilic extracts. J. Phycol 2004, 40, 611–618. [Google Scholar]
- (530).Skogen Chauton M; Røvik Størseth T; Johnsen G High-resolution magic angle spinning 1H NMR analysis of whole cells of Thalassiosira pseudonana (Bacillariophyceae): broad range analysis of metabolic composition and nutritional value. J. Appl. Phycol 2003, 15, 533–542. [Google Scholar]
- (531).Matilde SC; Odd Inge O; Tone FB; Zsolt V; Ingrid SG; Geir J HR MAS 1H NMR spectroscopy analysis of marine microalgal whole cells. Mar. Ecol. Prog. Ser 2003, 256, 57–62. [Google Scholar]
- (532).Arnold A; Genard B; Zito F; Tremblay R; Warschawski D; Marcotte I Identification of lipid and saccharide constituents of whole microalgal cells by 13C solid-state NMR. BBA-Biomembranes 2015, 1848, 369–377. [DOI] [PubMed] [Google Scholar]
- (533).Arnold AA; Bourgouin JP; Genard B; Warschawski DE; Tremblay R; Marcotte I Whole cell solid-state NMR study of Chlamydomonas reinhardtii microalgae. J. Biomol. NMR 2018, 70, 123–131. [DOI] [PubMed] [Google Scholar]
- (534).Poulhazan A; Dickwella Widanage MC; Muszynski A; Arnold AA; Warschawski DE; Azadi P; Marcotte I; Wang T Identification and quantification of glycans in whole cells: architecture of microalgal polysaccharides described by solid-state nuclear magnetic resonance. J. Am. Chem. Soc 2021, in press. DOI: 10.1021/jacs.1c07429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (535).Magazù S; Migliardo F; Gonzalez MA; Mondelli C; Parker SF; Vertessy BG Molecular mechanisms of survival strategies in extreme conditions. Life (Basel) 2012, 2, 364–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (536).Gupta A; Gupta R; Singh RL Microbes and environment. Principles and applications of environmental biotechnology for a sustainable future Springer Singapore: Singapore, 2017, 43–84. [Google Scholar]
- (537).Nelson ML; Dinardo A; Hochberg J; Armelagos GJ Brief communication: mass spectroscopic characterization of tetracycline in the skeletal remains of an ancient population from Sudanese Nubia 350–550 CE. Am. J. Phys. Anthropol 2010, 143, 151–154. [DOI] [PubMed] [Google Scholar]
- (538).Aminov RI A brief history of the antibiotic era: lessons learned and challenges for the future. Front. Microbiol 2010, 1, 134–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (539).Kapoor G; Saigal S; Elongavan A Action and resistance mechanisms of antibiotics: A guide for clinicians. J Anaesthesiol Clin Pharmacol 2017, 33, 300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (540).Reygaert WC An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol 2018, 4, 482–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (541).Iwu CD; Korsten L; Okoh AI The incidence of antibiotic resistance within and beyond the agricultural ecosystem: A concern for public health. MicrobiologyOpen 2020, 9, e1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (542).Blair JM; Richmond GE; Piddock LJ Multidrug efflux pumps in Gram-negative bacteria and their role in antibiotic resistance. Future Microbiol 2014, 9, 1165–1177. [DOI] [PubMed] [Google Scholar]
- (543).Giedraitienė A; Vitkauskienė A; Naginienė R; Pavilonis A Antibiotic resistance mechanisms of clinically important bacteria. Medicina 2011, 47, 137–146. [PubMed] [Google Scholar]
- (544).Shunmugaperumal T The role of biofilms in device-related infections. J. Med. Devices 2011, 5. [Google Scholar]
- (545).Magana M; Sereti C; Ioannidis A; Mitchell CA; Ball AR; Magiorkinis E; Chatzipanagiotou S; Hamblin MR; Hadjifrangiskou M; Tegos GP Options and limitations in clinical investigation of bacterial biofilms. Clin. Microbiol. Rev 2018, 31, e00084–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (546).Zhang B; Powers R Analysis of bacterial biofilms using NMR-based metabolomics. Future Med. Chem 2012, 4, 1273–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (547).Reichhardt C; Ferreira JAG; Joubert L-M; Clemons KV; Stevens DA; Cegelski L Analysis of the Aspergillus fumigatus biofilm extracellular matrix by solid-state nuclear magnetic resonance spectroscopy. Eukaryot Cell 2015, 14, 1064–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (548).Romaniuk JAH; Cegelski L Bacterial cell wall composition and the influence of antibiotics by cell-wall and whole-cell NMR. Philos. Trans. R. Soc. B 2015, 370, 20150024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (549).Kern T; Giffard M; Hediger S; Amoroso A; Giustini C; Bui NK; Joris B; Bougault C; Vollmer W; Simorre J-P Dynamics characterization of fully hydrated bacterial cell walls by solid-state NMR: evidence for cooperative binding of metal ions. J. Am. Chem. Soc 2010, 132, 10911–10919. [DOI] [PubMed] [Google Scholar]
- (550).Pasquina-Lemonche L; Burns J; Turner RD; Kumar S; Tank R; Mullin N; Wilson JS; Chakrabarti B; Bullough PA; Foster SJ et al. The architecture of the Gram-positive bacterial cell wall. Nature 2020, 582, 294–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (551).Huang KC; Mukhopadhyay R; Wen B; Gitai Z; Wingreen NS Cell shape and cell-wall organization in Gram-negative bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 19282–19287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (552).Wickham JR; Halye JL; Kashtanov S; Khandogin J; Rice CV Revisiting magnesium chelation by teichoic acid with phosphorus solid-state NMR and theoretical calculations. J. Phys. Chem. B 2009, 113, 2177–2183. [DOI] [PubMed] [Google Scholar]
- (553).Romaniuk JAH; Cegelski L Peptidoglycan and Teichoic Acid Levels and Alterations in Staphylococcus aureus by cell-wall and whole-cell nuclear magnetic resonance. Biochemistry 2018, 57, 3966–3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (554).Bugg TDH; Braddick D; Dowson CG; Roper DI Bacterial cell wall assembly: still an attractive antibacterial target. Trends Biotechnol 2011, 29, 167–173. [DOI] [PubMed] [Google Scholar]
- (555).Taguchi A; Welsh MA; Marmont LS; Lee W; Sjodt M; Kruse AC; Kahne D; Bernhardt TG; Walker S FtsW is a peptidoglycan polymerase that is functional only in complex with its cognate penicillin-binding protein. Nat. Microbiol 2019, 4, 587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (556).Cho H; Wivagg CN; Kapoor M; Barry Z; Rohs PDA; Suh H; Marto JA; Garner EC; Bernhardt TG Bacterial cell wall biogenesis is mediated by SEDS and PBP polymerase families functioning semi-autonomously. Nat. Microbiol 2016, 1, 16172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (557).Meeske AJ; Riley EP; Robins WP; Uehara T; Mekalanos JJ; Kahne D; Walker S; Kruse AC; Bernhardt TG; Rudner DZ SEDS proteins are a widespread family of bacterial cell wall polymerases. Nature 2016, 537, 634–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (558).Fagan RP; Fairweather NF Biogenesis and functions of bacterial S-layers. Nat. Rev. Microbiol 2014, 12, 211–222. [DOI] [PubMed] [Google Scholar]
- (559).Sára M; Sleytr UB S-layer proteins. J. Bacteriol 2000, 182, 859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (560).Sleytr UB; Györvary E; Pum D Crystallization of S-layer protein lattices on surfaces and interfaces. Prog. Org. Coat 2003, 47, 279–287. [Google Scholar]
- (561).Messner P; Sleytr UB Crystalline bacterial cell-surface layers. Adv. Microb. Physiol 1992, 33, 213–275. [DOI] [PubMed] [Google Scholar]
- (562).Rajagopal M; Walker S Envelope structures of Gram-positive bacteria. Curr. Top. Microbiol. Immunol 2017, 404, 1–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (563).Caroff M; Novikov A LPS structure, function, and heterogeneity. Endotoxin detection and control in pharma, limulus, and mammalian systems Springer International Publishing: Cham, 2019, 53–93. [Google Scholar]
- (564).Jankute M; Cox JAG; Harrison J; Besra GS Assembly of the mycobacterial cell wall. Annu. Rev. Microbiol 2015, 69, 405–423. [DOI] [PubMed] [Google Scholar]
- (565).Mayer C; Kluj RM; Mühleck M; Walter A; Unsleber S; Hottmann I; Borisova M Bacteria’s different ways to recycle their own cell wall. Int. J. Med. Microbiol 2019, 309, 151326. [DOI] [PubMed] [Google Scholar]
- (566).Kim SJ; Chang J; Singh M Peptidoglycan architecture of Gram-positive bacteria by solid-state NMR. Biochim. Biophys. Acta 2015, 1848, 350–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (567).Zhou X; Cegelski L Nutrient-dependent structural changes in S. aureus peptidoglycan revealed by solid-state NMR spectroscopy. Biochemistry 2012, 51, 8143–8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (568).Schanda P; Triboulet S; Laguri C; Bougault CM; Ayala I; Callon M; Arthur M; Simorre J-P Atomic model of a cell-wall cross-linking enzyme in complex with an intact bacterial peptidoglycan. J. Am. Chem. Soc 2014, 136, 17852–17860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (569).Beveridge TJ Structures of Gram-negative cell walls and their derived membrane vesicles. J. Bacteriol 1999, 181, 4725–4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (570).Laguri C; Silipo A; Martorana AM; Schanda P; Marchetti R; Polissi A; Molinaro A; Simorre JP Solid state NMR studies of intact lipopolysaccharide endotoxin. ACS. Chem. Biol 2018, 13, 2106–2113. [DOI] [PubMed] [Google Scholar]
- (571).Di Lorenzo F; Duda KA; Lanzetta R; Silipo A; De Castro C; Molinaro A A journey from structure to function of bacterial lipopolysaccharides. Chem. Rev 2021, in press. DOI: 10.1021/acs.chemrev.0c01321 [DOI] [PubMed] [Google Scholar]
- (572).Jachymek W; Niedziela T; Petersson C; Lugowski C; Czaja J; Kenne L Structures of the O-specific polysaccharides from Yokenella regensburgei (Koserella trabulsii) strains PCM 2476, 2477, 2478, and 2494: high-resolution magic-angle spinning NMR investigation of the O-specific polysaccharides in native lipopolysaccharides and directly on the surface of living bacteria. Biochemistry 1999, 38, 11788–11795. [DOI] [PubMed] [Google Scholar]
- (573).Dekoninck K; Létoquart J; Laguri C; Demange P; Bevernaegie R; Simorre J-P; Dehu O; Iorga B; Elias B; Cho S-H et al. Defining the function of OmpA in the Rcs stress response. eLife 2020, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (574).Brown S; Santa Maria JP Jr.; Walker S Wall teichoic acids of Gram-positive bacteria. Annu. Rev. Microbiol 2013, 67, 313–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (575).Wickham JR; Rice CV Solid-state NMR studies of bacterial lipoteichoic acid adsorption on different surfaces. Solid State Nucl. Magn. Reson 2008, 34, 154–161. [DOI] [PubMed] [Google Scholar]
- (576).Rice CV; Wickham JR Heterogeneous binding of lipoteichoic acid to the surface of titanium dioxide as determined with 31P solid-state NMR spectroscopy. J. Am. Chem. Soc 2005, 127, 856–857. [DOI] [PubMed] [Google Scholar]
- (577).Halye JL; Rice CV Cadmium chelation by bacterial teichoic acid from solid-state nuclear magnetic resonance spectroscopy. Biomacromolecules 2010, 11, 333–340. [DOI] [PubMed] [Google Scholar]
- (578).Renault M; Boxtel R; Bos M; Post J; Tommassen J; Baldus M Cellular solid-state nuclear magnetic resonance spectroscopy. Proc. Natl. Acad. Sci. USA 2012, 109, 4863–4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (579).Nordmark EL; Yang Z; Huttunen E; Widmalm G Structural studies of the exopolysaccharide produced by Propionibacterium freudenreichii ssp. shermanii JS. Biomacromolecules 2005, 6, 521–523. [DOI] [PubMed] [Google Scholar]
- (580).Bui NK; Eberhardt A; Vollmer D; Kern T; Bougault C; Tomasz A; Simorre JP; Vollmer W Isolation and analysis of cell wall components from Streptococcus pneumoniae. Anal. Biochem 2012, 421, 657–666. [DOI] [PubMed] [Google Scholar]
- (581).De Sa Peixoto P; Roiland C; Thomas D; Briard-Bion V; Le Guellec R; Parayre S; Deutsch SM; Jan G; Guyomarc’h F Recrystallized S-layer protein of a probiotic Propionibacterium: structural and nanomechanical changes upon temperature or pH shifts probed by solid-state NMR and AFM. Langmuir 2015, 31, 199–208. [DOI] [PubMed] [Google Scholar]
- (582).Lee RE; Li W; Chatterjee D; Lee RE Rapid structural characterization of the arabinogalactan and lipoarabinomannan in live mycobacterial cells using 2D and 3D HR-MAS NMR: structural changes in the arabinan due to ethambutol treatment and gene mutation are observed. Glycobiology 2005, 15, 139–151. [DOI] [PubMed] [Google Scholar]
- (583).Wieruszeski JM; Bohin A; Bohin JP; Lippens G In vivo detection of the cyclic osmoregulated periplasmic glucan of Ralstonia solanacearum by high-resolution magic angle spinning NMR. J. Magn. Reson 2001, 151, 118–123. [DOI] [PubMed] [Google Scholar]
- (584).Li W; Lee RE; Lee RE; Li J Methods for acquisition and assignment of multidimensional high-resolution magic angle spinning NMR of whole cell bacteria. Anal. Chem 2005, 77, 5785–5792. [DOI] [PubMed] [Google Scholar]
- (585).Kim SJ; Cegelski L; Studelska DR; O’Connor RD; Mehta AK; Schaefer J Rotational-echo double resonance characterization of vancomycin binding sites in Staphylococcus aureus. Biochemistry 2002, 41, 6967–6977. [DOI] [PubMed] [Google Scholar]
- (586).Yang H; Singh M; Kim SJ; Schaefer J Characterization of the tertiary structure of the peptidoglycan of Enterococcus faecalis. BBA-Biomembranes 2017, 1859, 2171–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (587).Patti GJ; Kim SJ; Yu T-Y; Dietrich E; Tanaka KSE; Parr TR Jr.; Far AR; Schaefer J Vancomycin and oritavancin have different modes of action in Enterococcus faecium. J. Mol. Biol 2009, 392, 1178–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (588).Nygaard R; Romaniuk JAH; Rice DM; Cegelski L Spectral snapshots of bacterial cell-wall composition and the influence of antibiotics by whole-cell NMR. Biophys. J 2015, 108, 1380–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (589).Singh M; Kim SJ; Sharif S; Preobrazhenskaya M; Schaefer J REDOR constraints on the peptidoglycan lattice architecture of Staphylococcus aureus and its FemA mutant. BBA-Biomembranes 2015, 1848, 363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (590).Singh M; Chang J; Coffman L; Kim SJ Solid-state NMR characterization of amphomycin effects on peptidoglycan and wall teichoic acid biosyntheses in Staphylococcus aureus. Sci. Rep 2016, 6, 31757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (591).Kim SJ; Schaefer J Hydrophobic side-chain length determines activity and conformational heterogeneity of a vancomycin derivative bound to the cell wall of Staphylococcus aureus. Biochemistry 2008, 47, 10155–10161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (592).Kim SJ; Matsuoka S; Patti GJ; Schaefer J Vancomycin derivative with damaged D-Ala-D-Ala binding cleft binds to cross-linked peptidoglycan in the cell wall of Staphylococcus aureus. Biochemistry 2008, 47, 3822–3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (593).Patti GJ; Chen J; Schaefer J; Gross ML Characterization of structural variations in the peptidoglycan of vancomycin-susceptible Enterococcus faecium: understanding glycopeptide-antibiotic binding sites using mass spectrometry. J. Am. Soc. Mass. Spectrom 2008, 19, 1467–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (594).Medeiros-Silva J; Jekhmane S; Paioni AL; Gawarecka K; Baldus M; Swiezewska E; Breukink E; Weingarth M High-resolution NMR studies of antibiotics in cellular membranes. Nat. Commun 2018, 9, 3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (595).Kern T; Hediger S; Müller P; Giustini C; Joris B; Bougault C; Vollmer W; Simorre JP Toward the characterization of peptidoglycan structure and protein-peptidoglycan interactions by solid-state NMR spectroscopy. J. Am. Chem. Soc 2008, 130, 5618–5619. [DOI] [PubMed] [Google Scholar]
- (596).Torrent M; Navarro S; Moussaoui M; Nogués MV; Boix E Eosinophil cationic protein high-affinity binding to bacteria-wall lipopolysaccharides and peptidoglycans. Biochemistry 2008, 47, 3544–3555. [DOI] [PubMed] [Google Scholar]
- (597).Overall SA; Zhu S; Hanssen E; Separovic F; Sani M-A In Situ Monitoring of Bacteria under Antimicrobial Stress Using 31P Solid-State NMR. Int. J. Mol. Sci 2019, 20, 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (598).Separovic F; Keizer DW; Sani M-A In-cell solid-state NMR studies of antimicrobial peptides. Front. Med. Technol 2020, 2, 610203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (599).Balzer M; Witt N; Flemming HC; Wingender J Faecal indicator bacteria in river biofilms. Water Sci. Technol 2010, 61, 1105–1111. [DOI] [PubMed] [Google Scholar]
- (600).Berlanga M; Guerrero R Living together in biofilms: the microbial cell factory and its biotechnological implications. Microb Cell Fact 2016, 15, 165–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (601).Okada M; Sato I; Cho SJ; Iwata H; Nishio T; Dubnau D; Sakagami Y Structure of the Bacillus subtilis quorum-sensing peptide pheromone ComX. Nat. Chem. Biol 2005, 1, 23–24. [DOI] [PubMed] [Google Scholar]
- (602).Hentzer M; Eberl L; Givskov M Transcriptome analysis of Pseudomonas aeruginosa biofilm development: anaerobic respiration and iron limitation. Biofilms 2005, 2, 37–61. [Google Scholar]
- (603).Serra DO; Richter AM; Hengge R Cellulose as an architectural element in spatially structured Escherichia coli biofilms. J. Bacteriol 2013, 195, 5540–5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (604).Hobley L; Harkins C; MacPhee CE; Stanley-Wall NR Giving structure to the biofilm matrix: an overview of individual strategies and emerging common themes. FEMS Microbiol. Rev 2015, 39, 649–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (605).Secor PR; Sweere JM; Michaels LA; Malkovskiy AV; Lazzareschi D; Katznelson E; Rajadas J; Birnbaum ME; Arrigoni A; Braun KR et al. Filamentous bacteriophage promote biofilm assembly and function. Cell Host Microbe 2015, 18, 549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (606).Stoodley P; Sauer K; Davies DG; Costerton JW Biofilms as complex differentiated communities. Annu. Rev. Microbiol 2002, 56, 187–209. [DOI] [PubMed] [Google Scholar]
- (607).Verderosa AD; Totsika M; Fairfull-Smith KE Bacterial biofilm eradication agents: a current review. Front. Chem 2019, 7, 824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (608).Wu W; Jin Y; Bai F; Jin S Pseudomonas aeruginosa; Academic Press: Boston, 2015. [Google Scholar]
- (609).Shen Y; Stojicic S; Haapasalo M Antimicrobial efficacy of chlorhexidine against bacteria in biofilms at different stages of development. J. Endod 2011, 37, 657–661. [DOI] [PubMed] [Google Scholar]
- (610).Suarez C; Piculell M; Modin O; Langenheder S; Persson F; Hermansson M Thickness determines microbial community structure and function in nitrifying biofilms via deterministic assembly. Sci. Rep 2019, 9, 5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (611).Muhammad MH; Idris AL; Fan X; Guo Y; Yu Y; Jin X; Qiu J; Guan X; Huang T Beyond risk: bacterial biofilms and their regulating approaches. Front. Microbiol 2020, 11, 928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (612).Garny K; Neu TR; Horn H; Volke F; Manz B Combined application of 13C NMR spectroscopy and confocal laser scanning microscopy—Investigation on biofilm structure and physico-chemical properties. Chem. Eng. Sci 2010, 65, 4691–4700. [Google Scholar]
- (613).Sutherland IW The biofilm matrix—an immobilized but dynamic microbial environment. Trends Microbiol 2001, 9, 222–227. [DOI] [PubMed] [Google Scholar]
- (614).Smolinska A; Blanchet L; Buydens LMC; Wijmenga SS NMR and pattern recognition methods in metabolomics: from data acquisition to biomarker discovery: a review. Analytica Chimica Acta 2012, 750, 82–97. [DOI] [PubMed] [Google Scholar]
- (615).Xu Y; Wang C; Hou J; Wang P; You G; Miao L; Lv B; Yang Y Effects of cerium oxide nanoparticles on the species and distribution of phosphorus in enhanced phosphorus removal sequencing batch biofilm reactor. Bioresour. Technol 2017, 227, 393–397. [DOI] [PubMed] [Google Scholar]
- (616).Zeng F; Jin W; Zhao Q Temperature effect on extracellular polymeric substances (EPS) and phosphorus accumulating organisms (PAOs) for phosphorus release of anaerobic sludge. RSC Adv 2019, 9, 2162–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (617).Flemming H-C; Wingender J The biofilm matrix. Nat. Rev. Microbiol 2010, 8, 623–633. [DOI] [PubMed] [Google Scholar]
- (618).Mandakhalikar KD; Rahmat JN; Chiong E; Neoh KG; Shen L; Tambyah PA Extraction and quantification of biofilm bacteria: method optimized for urinary catheters. Sci. Rep 2018, 8, 8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (619).Chiba A; Sugimoto S; Sato F; Hori S; Mizunoe Y A refined technique for extraction of extracellular matrices from bacterial biofilms and its applicability. Microb. Biotechnol 2015, 8, 392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (620).Cegelski L Bottom-up and top-down solid-state NMR approaches for bacterial biofilm matrix composition. J. Magn. Reson 2015, 253, 91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (621).McCrate OA; Zhou X; Reichhardt C; Cegelski L Sum of the parts: composition and architecture of the bacterial extracellular matrix. J. Mol. Biol 2013, 425, 4286–4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (622).Jeffries J; Thongsomboon W; Visser JA; Enriquez K; Yager D; Cegelski L Variation in the ratio of curli and phosphoethanolamine cellulose associated with biofilm architecture and properties. Biopolymers 2021, 112, 23395. [DOI] [PubMed] [Google Scholar]
- (623).Reichhardt C; Fong JCN; Yildiz F; Cegelski L Characterization of the Vibrio cholerae extracellular matrix: a top-down solid-state NMR approach. BBA-Biomembranes 2015, 1848, 378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (624).El Mammeri N; Hierrezuelo J; Tolchard J; Cámara-Almirón J; Caro-Astorga J; Álvarez-Mena A; Dutour A; Berbon M; Shenoy J; Morvan E et al. Molecular architecture of bacterial amyloids in Bacillus biofilms. FASEB J 2019, 33, 12146–12163. [DOI] [PubMed] [Google Scholar]
- (625).Thongsomboon W; Serra DO; Possling A; Hadjineophytou C; Hengge R; Cegelski L Phosphoethanolamine cellulose: A naturally produced chemically modified cellulose. Science 2018, 359, 334–338. [DOI] [PubMed] [Google Scholar]
- (626).Olsén A; Jonsson A; Normark S Fibronectin binding mediated by a novel class of surface organelles on Escherichia coli. Nature 1989, 338, 652–655. [DOI] [PubMed] [Google Scholar]
- (627).Reichhardt C; Lim JY; Rice D; Fong JN; Cegelski L Structure and function of bacterial biofilms by solid-state NMR. Biophys. J 2014, 106, 192a. [Google Scholar]
- (628).Jeffries J; Fuller GG; Cegelski L Unraveling Escherichia coli’s cloak: identification of phosphoethanolamine cellulose, its functions, and applications. Microbiol. Insights 2019, 12, 1178636119865234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (629).Tang W; Bhatt A; Smith AN; Crowley PJ; Brady LJ; Long JR Specific binding of a naturally occurring amyloidogenic fragment of Streptococcus mutans adhesin P1 to intact P1 on the cell surface characterized by solid state NMR spectroscopy. J. Biomol. NMR 2016, 64, 153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (630).Van Gerven N; Van der Verren SE; Reiter DM; Remaut H The role of functional amyloids in bacterial virulence. J. Mol. Biol 2018, 430, 3657–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (631).Jordal PB; Dueholm MS; Larsen P; Petersen SV; Enghild JJ; Christiansen G; Højrup P; Nielsen PH; Otzen DE Widespread abundance of functional bacterial amyloid in mycolata and other gram-positive bacteria. Appl. Environ. Microbiol 2009, 75, 4101–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (632).Lattner D; Flemming HC; Mayer C 13C-NMR study of the interaction of bacterial alginate with bivalent cations. Int. J. Biol. Macromol 2003, 33, 81–88. [DOI] [PubMed] [Google Scholar]
- (633).Mayer C; Lattner D; Schürks N 13C nuclear magnetic resonance studies on selectively labeled bacterial biofilms. J. Ind. Microbiol. Biotechnol 2001, 26, 62–69. [DOI] [PubMed] [Google Scholar]
- (634).Diehl A; Roske Y; Ball L; Chowdhury A; Hiller M; Molière N; Kramer R; Stöppler D; Worth CL; Schlegel B et al. Structural changes of TasA in biofilm formation of Bacillus subtilis. Proc. Natl. Acad. Sci. USA 2018, 115, 3237–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (635).Pandit S; Fazilati M; Gaska K; Derouiche A; Nypelö T; Mijakovic I; Kádár R The exo-polysaccharide component of extracellular matrix is essential for the viscoelastic properties of Bacillus subtilis biofilms. Int. J. Mol. Sci 2020, 21, 6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (636).Jiao Y; Cody GD; Harding AK; Wilmes P; Schrenk M; Wheeler KE; Banfield JF; Thelen MP Characterization of extracellular polymeric substances from acidophilic microbial biofilms. Appl. Environ. Microbiol 2010, 76, 2916–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (637).Lim JY; May JM; Cegelski L Dimethyl sulfoxide and ethanol elicit increased amyloid biogenesis and amyloid-integrated biofilm formation in Escherichia coli. Appl. Environ. Microbiol 2012, 78, 3369–3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (638).McLean JS; Ona ON; Majors PD Correlated biofilm imaging, transport and metabolism measurements via combined nuclear magnetic resonance and confocal microscopy. ISME J 2008, 2, 121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (639).Zhang Y; Xu A; Lv X; Wang Q; Feng C; Lin J Non-invasive measurement, mathematical simulation and in situ detection of biofilm evolution in porous media: a review. Appl. Sci 2021, 11, 1391. [Google Scholar]
- (640).Caudill ER; Hernandez RT; Johnson KP; O’Rourke JT; Zhu L; Haynes CL; Feng ZV; Pedersen JA Wall teichoic acids govern cationic gold nanoparticle interaction with Gram-positive bacterial cell walls. Chem. Sci 2020, 11, 4106–4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (641).Joshi AS; Singh P; Mijakovic I Interactions of gold and silver nanoparticles with bacterial biofilms: molecular interactions behind inhibition and resistance. Int. J. Mol. Sci 2020, 21, 7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (642).Molina-Santiago C; Pearson JR; Navarro Y; Berlanga-Clavero MV; Caraballo-Rodriguez AM; Petras D; García-Martín ML; Lamon G; Haberstein B; Cazorla FM et al. The extracellular matrix protects Bacillus subtilis colonies from Pseudomonas invasion and modulates plant co-colonization. Nat. Commun 2019, 10, 1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (643).Righi V; Constantinou C; Kesarwani M; Rahme LG; Tzika AA Effects of a small, volatile bacterial molecule on Pseudomonas aeruginosa bacteria using whole cell high-resolution magic angle spinning nuclear magnetic resonance spectroscopy and genomics. Int. J. Mol. Med 2018, 42, 2129–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (644).Gjersing EL; Herberg JL; Horn J; Schaldach CM; Maxwell RS NMR metabolomics of planktonic and biofilm modes of growth in Pseudomonas aeruginosa. Anal. Chem 2007, 79, 8037–8045. [DOI] [PubMed] [Google Scholar]
- (645).Mizuno S; Amida H; Kobayashi N; Aizawa S; Tate S The NMR structure of FliK, the trigger for the switch of substrate specificity in the flagellar type III secretion apparatus. J. Mol. Biol 2011, 409, 558–573. [DOI] [PubMed] [Google Scholar]
- (646).Lombardi C; Tolchard J; Bouillot S; Signor L; Gebus C; Liebl D; Fenel D; Teulon JM; Brock J; Habenstein B et al. Structural and functional characterization of the type three secretion system (T3SS) needle of Pseudomonas aeruginosa. Front. Microbiol 2019, 10, 573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (647).Stones DH; Krachler AM Fatal attraction: how bacterial adhesins affect host signaling and what we can learn from them. Int. J. Mol. Sci 2015, 16, 2626–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (648).Korotkov KV; Sandkvist M; Hol WGJ The type II secretion system: biogenesis, molecular architecture and mechanism. Nat. Rev. Microbiol 2012, 10, 336–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (649).López-Castilla A; Thomassin J-L; Bardiaux B; Zheng W; Nivaskumar M; Yu X; Nilges M; Egelman EH; Izadi-Pruneyre N; Francetic O Structure of the calcium-dependent type 2 secretion pseudopilus. Nat. Microbiol 2017, 2, 1686–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (650).Shewmaker F The application of NMR techniques to bacterial adhesins. Adv. Exp. Med. Biol 2011, 715, 241–256. [DOI] [PubMed] [Google Scholar]
- (651).Ramboarina S; Garnett JA; Zhou M; Li Y; Peng Z; Taylor JD; Lee W.-c.; Bodey A; Murray JW; Alguel Y et al. Structural insights into serine-rich fimbriae from Gram-positive bacteria. J. Biol. Chem 2010, 285, 32446–32457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (652).Habenstein B; Loquet A; Hwang S; Giller K; Vasa SK; Becker S; Habeck M; Lange A Hybrid structure of the type 1 pilus of uropathogenic Escherichia coli. Angew. Chem. Int. Ed 2015, 54, 11691–11695. [DOI] [PubMed] [Google Scholar]
- (653).Demers J-P; Habenstein B; Loquet A; Kumar Vasa S; Giller K; Becker S; Baker D; Lange A; Sgourakis NG High-resolution structure of the Shigella type-III secretion needle by solid-state NMR and cryo-electron microscopy. Nat. Commun 2014, 5, 4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (654).Demers J-P; Sgourakis NG; Gupta R; Loquet A; Giller K; Riedel D; Laube B; Kolbe M; Baker D; Becker S et al. The common structural architecture of Shigella flexneri and Salmonella typhimurium type three secretion needles. PLoS Pathog 2013, 9, e1003245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (655).Loquet A; Sgourakis NG; Gupta R; Giller K; Riedel D; Goosmann C; Griesinger C; Kolbe M; Baker D; Becker S et al. Atomic model of the type III secretion system needle. Nature 2012, 486, 276–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (656).Guo EZ; Desrosiers DC; Zalesak J; Tolchard J; Berbon M; Habenstein B; Marlovits T; Loquet A; Galán JE A polymorphic helix of a Salmonella needle protein relays signals defining distinct steps in type III secretion. PLOS Biol 2019, 17, e3000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (657).Renault M; Pawsey S; Bos MP; Koers EJ; Nand D; Tommassen-van Boxtel R; Rosay M; Tommassen J; Maas WE; Baldus M Solid-state NMR spectroscopy on cellular preparations enhanced by dynamic nuclear polarization. Angew. Chem. Int. Ed 2012, 51, 2998–3001. [DOI] [PubMed] [Google Scholar]
- (658).Judge PT; Sesti EL; Price LE; Albert BJ; Alaniva N; Saliba EP; Halbritter T; Sigurdsson ST; Kyei GB; Barnes AB Dynamic nuclear polarization with electron decoupling in intact human cells and cell lysates. J. Phys. Chem. B 2020, 124, 2323–2330. [DOI] [PubMed] [Google Scholar]


