Abstract
Background and objective:
The reduction of forced expiratory volume in 1 s (FEV1) in response to methacholine challenge in asthma may reflect two components: airway narrowing, assessed by the change in FEV1/forced vital capacity (FVC), and airway closure, assessed by the change in FVC. The purpose of this study was to determine the degree and determinants of airway closure in response to methacholine in a large group of asthmatic patients participating in studies conducted by the American Lung Association-Airways Clinical Research Centers (ALA-ACRC).
Methods:
We used the methacholine challenge data from participants in five studies of the ALA-ACRC to determine the closing index, defined as the contribution of airway closure to the decrease in FEV1ʹ and calculated as %ΔFVC/%ΔFEV1.
Results:
There were a total of 936 participants with asthma, among whom the median closing index was 0.67 relative to that of a published healthy population of 0.54. A higher closing index was associated with increased age (10-year increments) (0.04, 95% CI = 0.02, 0.05, P < 0.005) and obesity (0.07, 95% CI = 0.03, 0.10, P < 0.001). There was no association between the closing index and asthma control.
Conclusion:
Our findings confirm that airway closure in response to methacholine occurs in a large, diverse population of asthmatic participants, and that increased airway closure is associated with older age and obesity. These findings suggest that therapies targeting airway closure may be important in patients with a high closing index.
Keywords: airway closure, airway hyperresponsiveness, asthma, methacholine, obesity
INTRODUCTION
The process of airway hyperresponsiveness (AHR) in asthma is complex, involving elements of both airway narrowing and airway closure.1,2 Incremental doses of inhaled methacholine cause a reduction in forced expiratory volume in 1 s (FEV1), but the decrease in FEV1 can be thought of as having two components: airway narrowing, assessed by a reduction in FEV1/forced vital capacity (FVC), and airway closure, estimated indirectly by a decrease in FVC. A decrease in FVC in response to methacholine corresponds with changes in small airway function and gas trapping,3–5 implicating airway closure. The relative contribution of airway narrowing versus airway closure to the decrease in FEV1 can be assessed by calculating %ΔFVC/%ΔFEV1, which has been called the closing index.6 Airway closure promotes air trapping and hyperinflation that contribute significantly to dyspnoea and exercise intolerance.7 Airway closure has implications for treatment as well, as inhaled drugs may not penetrate to areas of the lung distal to closed airways.8 Although excessive airway closure has been described in asthma, its prevalence among a large population of asthmatic individuals is not known.
The American Lung Association-Airways Clinical Research Centers (ALA-ACRC) is a multicentre network formed to evaluate a range of asthma therapies. Study participants routinely undergo standardized bronchoprovocation testing with inhaled methacholine. Although airway closure has been described in asthma, the studies involved have been smaller investigations that did not encompass the wide variety of asthma. Therefore, it is important to assess airway closure in response to methacholine in a large, diverse population of asthmatic participants. Accordingly, we calculated the closing index from a large group of participants with asthma who were involved in different studies conducted by the ALA-ACRC in order to determine the contribution of airway closure to the response to methacholine, and to assess clinical features associated with airway closure.
METHODS
We analysed methacholine challenge data from both paediatric and adult participants involved in one of the five different studies of the ALA-ACRC9–14 (ClinicalTrials.gov Identifiers: NCT00069823, NCT00442013, NCT01118312, NCT00705341 and NCT01629823). More information about each study is provided in Table S1 (Supplementary Information). Each original study had been individually approved by each centre’s local institutional review board (IRB), but the current study was exempt from IRB review because it involved analysis of aggregate, de-identified data only. Methacholine challenge study protocols were standardized using the dosimeter method, and spirometric methods included exhalation for at least 6 s and development of a 1-s plateau in exhaled volume over time, as per the American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines.15,16 All research coordinators performing methacholine challenge testing were formally trained and certified, and test results were periodically audited for quality. From the methacholine challenge data, we extracted the individual changes in FEV1 and FVC at the maximal concentration of methacholine and calculated the closing index as %ΔFVC/%ΔFEV1, which reflects the contribution of airway closure to the reduction in FEV1.6 We also calculated the closing index using the %ΔFVC at the provocative concentration of methacholine causing a 20% decrease in FEV1 (PC20), in order to assess the degree of airway closure standardized for the same level of change in FEV1. We not only examined the data from all participants, but also analysed the subset of participants who demonstrated AHR, defined by a PC20 ≤ 8 mg/mL, to determine whether there were any unique features relating AHR to the closing index.
Data analysis
We describe continuous data using median (Q1 and Q3) and categorical data using counts with proportions. Continuous variables were compared using Kruskal–Wallis test, and categorical variables were compared using chi-square test. To compare the closing index calculated using the maximal dose of methacholine versus using the PC20, we constructed a plot of the difference in the closing index calculated by both methods versus the maximal change in FEV1 from the maximal concentration challenge. We used multivariable linear regression to determine the association of factors with the magnitude of the closing index, and expressed the results as the coefficient estimate (95% CI). These factors included age, sex, body mass index (BMI), race, baseline use of inhaled corticosteroid/long-acting beta-agonist therapy (ICS/LABA), Asthma Control Test (ACT) score17 and Asthma Symptom Utility Index (ASUI).18 Using negative binomial regression, taking into account age, sex, race, obesity (binary) and study, we analysed if the closing index was associated with the rate of episodes of poor asthma control (EPAC), offset for the length of study follow-up. EPAC were defined as any one of the following occurring within 1 week by diary account: decreased peak flow by more than 30% from personal best for two or more consecutive days, increased beta-agonist use over baseline by more than four inhalations of meter dose inhaler or two nebulizer treatments, increased oral corticosteroid use or unscheduled healthcare visits.19 We also used intraclass correlation to examine the stability of the closing index over time using data from the one study (MeCIS, Methacholine Bronchoprovocation – Influence of High-Potency Inhaled Corticosteroids in Asthma) that involved up to three repeated assessments of PC20 over time without any change in intervention.14
RESULTS
The characteristics of all participants stratified by study are listed in Table 1. There were a total of 936 participants across a broad range of age, ethnicity and disease characteristics. Most participants had poorly controlled asthma. The overall median closing index among all participants was 0.67 (Table 1, Fig. 1). We censored the data from six participants who had a post-methacholine FEV1/FVC ratio of >1, which is not physiologically reasonable, and likely reflects technical error. The characteristics of the subset of participants who achieved a PC20 < 8 mg/mL are shown in Table S2 (Supplementary Information). Overall, these characteristics were similar to those of the total population, as was the distribution of the closing index (Fig. S1, Supplementary Information). In both populations, we used the closing index calculated from the maximal concentration of methacholine to be consistent with the methodology of Chapman et al.,6 who found a similar closing index among asthmatic patients (0.60) as we did in our study (0.67). As shown in Figure S2 (Supplementary Information), the closing index was slightly higher when calculated from the maximal concentration than when calculated at the PC20, especially when FEV1 had decreased by more than about 30% during the methacholine challenge ending at the maximal concentration.
Table 1.
Demographics and baseline pulmonary function of all study participants
| Characteristic | Total (n = 936) |
CPAP (n = 194) |
MeCIS (n = 102) |
SARA (n = 175) |
SARCA (n = 212) |
STAN (n = 253) |
P-value† |
|---|---|---|---|---|---|---|---|
| Age (years), median (Q1,Q3) | 24 (14, 41) | 31 (20, 41) | 41 (26, 50) | 37 (26, 47) | 11 (9, 14) | 22 (12, 38) | <0.001 |
| Male, n (%) | 410 (44) | 81 (42) | 37 (36) | 47 (27) | 131 (62) | 114 (45) | <0.001 |
| Race, n (%) | |||||||
| White | 425 (45) | 85 (44) | 57 (56) | 98 (56) | 76 (36) | 109 (43) | <0.001 |
| Black | 346 (37) | 62 (32) | 34 (33) | 63 (36) | 95 (45) | 92 (36) | |
| Hispanic | 122 (13) | 36 (19) | 9 (9) | 11 (6) | 25 (12) | 41 (16) | |
| Other | 43 (5) | 11 (6) | 2 (2) | 3 (2) | 16 (8) | 11 (4) | |
| Age at asthma onset (years), median (Q1, Q3) | 5 (2, 13) | 8 (3, 15) | 13 (4, 30) | 9 (3, 24) | 2 (1, 5) | 5 (1, 12) | <0.001 |
| BMI for adults (kg/m2), median (Q1, Q3) | 28 (25, 33) | 26 (23, 30) | 28 (25, 34) | 30 (25, 35) | 30 (25, 36) | <0.001 | |
| BMI percentile for children (<20 years old), median (Q1, Q3) | 84 (52, 96) | 68 (42, 90) | 65 (44, 95) | 98 (92, 99) | 85 (54, 97) | 87 (57, 97) | 0.008 |
| Obese‡, n (%) | 344 (37) | 41 (21) | 41 (40) | 86 (49) | 70 (33) | 106 (42) | <0.001 |
| On ICS/LABA, n (%) | 446 (48) | 54 (28) | 57 (56) | 134 (77) | 121 (57) | 80 (32) | <0.001 |
| Questionnaires, median (Q1, Q3) | |||||||
| Asthma Control Questionnaire | 1.6 (1.1, 2.1) | 1.0 (0.6, 1.6) | 1.9 (1.6, 2.4) | <0.001 | |||
| ACT score | 19 (16, 21) | 22 (20, 23) | 19 (16, 21) | 16 (13, 18) | <0.001 | ||
| ACT ≤ 19 | 300 (62%) | 46 (24%) | 63 (64%) | 191 (100%) | <0.001 | ||
| cACT score | 18 (15, 20) | 19 (16, 22) | 17 (13, 18) | <0.001 | |||
| cACT ≤ 19 | 119 (69%) | 57 (52%) | 62 (100%) | <0.001 | |||
| ASUI | 0.83 (0.69, 0.90) | 0.92 (0.84, 0.98) | 0.78 (0.63, 0.85) | 0.83 (0.72, 0.89) | 0.75 (0.60, 0.87) | <0.001 | |
| Spirometry, median(Q1, Q3) | |||||||
| % Predicted FEV1, pre-BD | 90 (82, 100) | 91 (83, 98) | 85 (78, 94) | 87 (81, 96) | 94 (86, 105) | 90 (81, 102) | <0.001 |
| % Predicted FVC, pre-BD | 99 (90, 108) | 101 (91, 108) | 96 (87, 105) | 95 (87, 102) | 102 (92, 111) | 100 (91, 110) | <0.001 |
| FEV1/FVC ratio, pre-BD | 0.77 (0.72, 0.82) | 0.76 (0.71, 0.81) | 0.75 (0.69, 0.81) | 0.77 (0.71, 0.81) | 0.81 (0.76, 0.86) | 0.77 (0.72, 0.81) | <0.001 |
| % Change FEV1 from MC, last challenge | 25 (22, 29) | 24 (22, 28) | 23 (21, 27) | 26 (22, 31) | 25 (23, 29) | 24 (22, 28) | <0.001 |
| % Change FVC from MC, last challenge | 17 (12, 22) | 17 (12, 22) | 17 (11, 21) | 21 (16, 26) | 16 (12, 22) | 15 (10, 20) | <0.001 |
| % Change FEV1 from MC, at PC20 | 20 (20, 20) | 20 (20, 20) | 20 (20, 20) | 20 (20, 20) | 20 (20, 20) | 20 (20, 20) | 0.219 |
| % Change FVC from MC, at PC20 | 13 (10, 17) | 13 (10, 16) | 14 (11, 17) | 15 (12, 18) | 13 (10, 16) | 13 (9, 16) | <0.001 |
| Other respiratory measures, median (Q1, Q3) | |||||||
| PC20 | 0.89 (0.29, 2.97) | 0.75 (0.24, 1.90) | 1.16 (0.40, 3.55) | 1.39 (0.38, 4.24) | 1.26 (0.34, 3.70) | 0.65 (0.19, 2.50) | <0.001 |
| Closing index at last challenge | 0.67 (0.49, 0.81) | 0.68 (0.51, 0.82) | 0.70 (0.49, 0.83) | 0.75 (0.62, 0.89) | 0.62 (0.48, 0.79) | 0.62 (0.43, 0.78) | <0.001 |
| Closing index at PC20 | 0.67 (0.50, 0.83) | 0.66 (0.48, 0.80) | 0.69 (0.53, 0.83) | 0.75 (0.59, 0.89) | 0.65 (0.48, 0.82) | 0.63 (0.46, 0.80) | <0.001 |
Missing data: ACT was not done in MeCIS, or SARA or children in SARCA or STAN (total n = 483); cACT was only done in children in SARCA and STAN (total n = 172); ASUI was not done in MeCIS, missing one subject from SARCA (total n = 833).
P-values are based upon chi-square and Kruskal–Wallis tests for categorical and continuous characteristics, respectively.
Obesity defined as BMI > 30 kg/m2 in adults (≥20 years), and BMI > 95th percentile in children (<20 years).
ACT, Asthma Control Test (low scores indicate better health); ASUI, Asthma Symptom Utility Index (high scores indicate better health); BD, bronchodilator; BMI, body mass index; cACT, child ACT; CPAP, Effect of Positive Airway Pressure on Reducing Airway Reactivity in Patients with Asthma; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; ICS, inhaled corticosteroid; LABA, long-acting beta-agonist; MeCIS, Methacholine Bronchoprovocation – Influence of High-Potency Inhaled Corticosteroids in Asthma; MC, methacholine challenge; PC20, provocative concentration of methacholine causing a 20% decrease in FEV1; SARA, Study of Acid Reflux and Asthma; SARCA, Study of Acid Reflux in Childhood Asthma; STAN, Study of Asthma and Nasal Steroids.
Figure 1.
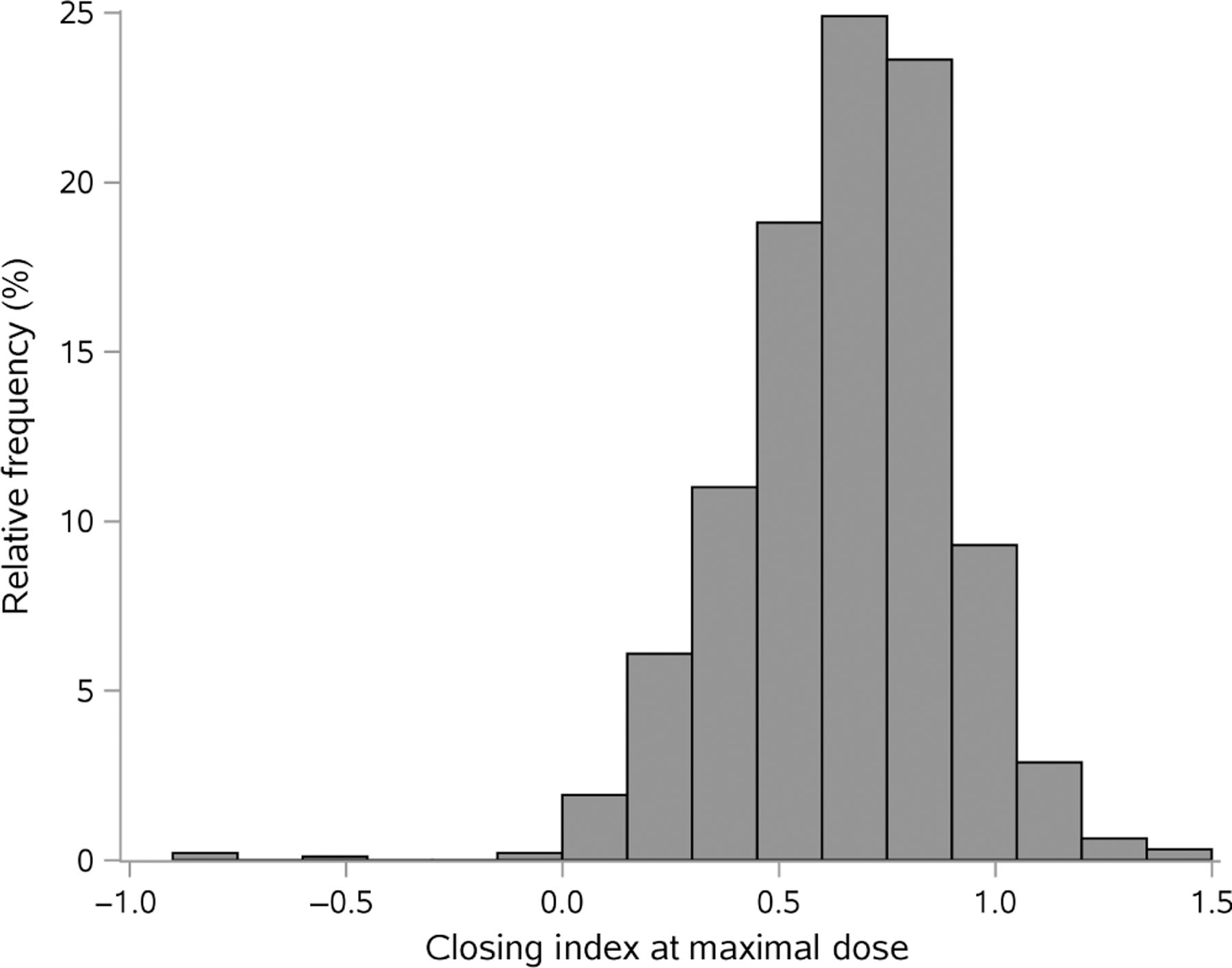
Distribution of closing index among all participants.
Univariate analysis of the closing index among all participants showed that a higher closing index was associated with older age, female sex, obesity, later onset of asthma, use of ICS/LABA, asthma control in children, lower % predicted FEV1 and FVC and higher PC20 (Table 2). In the multivariable linear regression model, a higher closing index was associated with higher age (10 year increments) (0.04, 95% CI = 0.02, 0.05, P < 0.005) and obesity (0.07, 95% CI = 0.03, 0.10, P < 0.001) after controlling for race, sex and study (Table 3). Age of asthma onset and baseline use of ICS/LABA therapy were dropped from the model due to lack of significance, after adding each specific study to the model (Table S5, Supplementary Information). Lung function variables (FEV1, FVC and PC20) were not included due to their being directly linked to the closing index. Child ACT (cACT) score was only measured in SARCA (Study of Acid Reflux in Childhood Asthma) and STAN (Study of Asthma and Nasal Steroids), and was required to be ≤19 in STAN. In this subset of patients, a cACT of 19 or less was associated with a higher closing index (Table S7, Supplementary Information).
Table 2.
Results of univariate regression modelling of closing index for all participants
| Characteristics | Regression coefficient (95% CI) | P-value |
|---|---|---|
| Age (10-year intervals) | 0.036 (0.026, 0.046) | <0.001 |
| Male (vs female) | −0.049 (−0.082, −0.017) | 0.003 |
| Black (vs white) | −0.017 (−0.051, 0.016) | 0.316 |
| Hispanic (vs white) | −0.027 (−0.075, 0.021) | 0.274 |
| Other race (vs white) | 0.057 (−0.021, 0.134) | 0.150 |
| Age of asthma onset | 0.004 (0.002, 0.005) | <0.001 |
| BMI for adults 20 years or older | 0.006 (0.003, 0.009) | <0.001 |
| BMI percentile for children <20 years | 0.002 (0.001, 0.003) | 0.001 |
| Obese (vs not) | 0.086 (0.052, 0.119) | <0.001 |
| On ICS/LABA (vs not) | 0.047 (0.015, 0.079) | 0.004 |
| Asthma Control Questionnaire | 0.021 (−0.014, 0.057) | 0.236 |
| ACT score | 0.003 (−0.003, 0.008) | 0.339 |
| ACT score ≤ 19 (vs 20 or more) | −0.016 (−0.063, 0.031) | 0.506 |
| cACT score | 0.006 (−0.002, 0.015) | 0.116 |
| cACT score ≤ 19 (vs 20 or more) | −0.08 (−0.158, −0.003) | 0.043 |
| Adult or cACT score ≤ 19 (vs 20 or more) | −0.032 (−0.072, 0.008) | 0.115 |
| Asthma symptom utility index (ASUI) | 0.016 (−0.084, 0.115) | 0.757 |
| % Predicted FEV1, pre-BD | −0.002 (−0.003, −0.001) | 0.004 |
| % Predicted FVC, pre-BD | −0.003 (−0.004, −0.002) | <0.001 |
| FEV1/FVC ratio, pre-BD | 0.016 (−0.193, 0.224) | 0.884 |
| PC20 | 0.006 (0.001, 0.011) | 0.014 |
| Study, ref. = STAN | ||
| CPAP | 0.006 (−0.034, 0.046) | 0.757 |
| MeCIS | −0.0003 (−0.052, 0.052) | 0.991 |
| SARA | 0.117 (0.076, 0.158) | <0.001 |
| SARCA | −0.028 (−0.067, 0.011) | 0.156 |
Missing data: ACT was not done in MeCIS, or SARA or children in SARCA or STAN (total n = 483); cACT was only done in children in SARCA and STAN (total n = 172); ASUI was not done in MeCIS, missing one subject from SARCA (total n = 833).
ACT, Asthma Control Test (low scores indicate better health); ASUI, Asthma Symptom Utility Index (high scores indicate better health); BD, bronchodilator; BMI, body mass index; cACT, child ACT; CPAP, Effect of Positive Airway Pressure on Reducing Airway Reactivity in Patients with Asthma; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; ICS, inhaled corticosteroid; LABA, long-acting beta-agonist; MeCIS, Methacholine Bronchoprovocation – Influence of High-Potency Inhaled Corticosteroids in Asthma; PC20, provocative concentration of methacholine causing a 20% decrease in FEV1; SARA, Study of Acid Reflux and Asthma; SARCA, Study of Acid Reflux in Childhood Asthma; STAN, Study of Asthma and Nasal Steroids.
Table 3.
Results of multivariable regression modelling of closing index for all participants
| Characteristics | Estimate (95% CI) | P-value |
|---|---|---|
| Age (10-year units) | 0.035 (0.023, 0.047) | <0.001 |
| Male (vs female) | −0.014 (−0.046, 0.019) | 0.408 |
| Black (vs white) | −0.015 (−0.05, 0.019) | 0.382 |
| Hispanic (vs white) | 0.002 (−0.047, 0.052) | 0.926 |
| Other race (vs white) | 0.073 (−0.003, 0.149) | 0.061 |
| Obese (vs not) | 0.066 (0.033, 0.099) | <0.001 |
| Study, ref. = STAN | ||
| CPAP | 0.049 (0.003, 0.095) | 0.038 |
| MeCIS | 0.009 (−0.048, 0.066) | 0.758 |
| SARA | 0.101 (0.053, 0.15) | <0.001 |
| SARCA | 0.089 (0.041, 0.136) | <0.001 |
CPAP, Effect of Positive Airway Pressure on Reducing Airway Reactivity in Patients with Asthma; MeCIS, Methacholine Bronchoprovocation – Influence of High-Potency Inhaled Corticosteroids in Asthma; SARA, Study of Acid Reflux and Asthma; SARCA, Study of Acid Reflux in Childhood Asthma; STAN, Study of Asthma and Nasal Steroids.
For the 861 patients with diary card data, there was no association between the closing index and asthma control as shown by the number of EPAC (rate ratio (RR) = 1.002, 95% CI = 0.65, 1.54, P = 0.99, Table 4). Males had significantly fewer EPAC than females (RR = 0.76, 95% CI = 0.61, 0.95, P = 0.015). Black subjects had significantly more EPAC compared to white subjects (RR = 1.41, 95% CI = 1.13, 1.77, P = 0.002).
Table 4.
Results of multivariable negative binomial regression modelling of EPAC for all participants with diary card data (n = 861)
| Characteristics | Risk ratio (95% CI) | P-value |
|---|---|---|
| Closing index | 1.002 (0.65, 1.54) | 0.994 |
| Age (10-year units) | 0.92 (0.85, 1.01) | 0.067 |
| Male (vs female) | 0.76 (0.61, 0.95) | 0.015 |
| Black (vs white) | 1.41 (1.13, 1.77) | 0.002 |
| Hispanic (vs white) | 0.82 (0.58, 1.17) | 0.277 |
| Other race (vs white) | 1.5 (0.92, 2.44) | 0.101 |
| Obese (vs not) | 0.97 (0.77, 1.21) | 0.792 |
| Study, ref. = STAN | ||
| CPAP | 0.67 (0.47, 0.94) | 0.021 |
| MeCIS | 0.55 (0.31, 0.99) | 0.046 |
| SARA | 1.16 (0.86, 1.56) | 0.347 |
| SARCA | 1.26 (0.94, 1.7) | 0.119 |
CPAP, Effect of Positive Airway Pressure on Reducing Airway Reactivity in Patients with Asthma; EPAC, episode of poor asthma control; MeCIS, Methacholine Bronchoprovocation – Influence of High-Potency Inhaled Corticosteroids in Asthma; SARA, Study of Acid Reflux and Asthma; SARCA, Study of Acid Reflux in Childhood Asthma; STAN, Study of Asthma and Nasal Steroids.
Among those participants who had repeat methacholine challenge testing while on stable treatment in the MeCIS trial (n = 27),14 there was poor reproducibility of the closing index between study visits, with an intraclass correlation coefficient (ICC) of 0.25, (95% CI = 0.10, 0.48).
All of these findings were similar in the subset of the participants who had AHR (Tables S3, S4, S6, S9, Supplementary Information), except for there being no association of cACT <19 and a higher closing index among children with AHR (Table S8, Supplementary Information). In addition, the findings were similar whether the closing index was calculated at the maximal concentration or at the PC20 concentration of methacholine.
DISCUSSION
This is the first study to demonstrate that airway closure occurs in response to methacholine, and is associated with increased age and obesity, in a large, diverse cohort of patients with asthma participating in clinical trials. The demographics and physiological characteristics of the participants in the five different studies were statistically different from each other, highlighting the diversity of the total population, although the absolute differences in many variables (e.g. FEV1 % predicted) were not necessarily clinically different. Nevertheless, given that our results are comparable to prior studies, but in a much larger, diverse group of asthmatic participants, we believe this study supports the closing index as a measure of enhanced airway closure in response to methacholine among patients with asthma, and is associated with older age and obesity.
We believe the closing index is an accurate indicator of airway closure. Since we did not directly measure airway closure, the change in FVC was used as a surrogate for airway closure, whether it be actual anatomic closure or functional closure due to extreme airway narrowing. Assessing the change in FVC following methacholine challenge has been described previously and is thought to reflect the degree of maximal airway response with subsequent airway closure leading to gas trapping.20–24 Of note, while Chapman et al. calculated the closing index at the maximal dose of methacholine,6 other studies measured changes in FVC at the same relative change in FEV1, that is, at the PC20.3,7,21,23,25,26 For this reason, we analysed our data both ways and found no significant differences in the results. This finding is consistent with that of Chapman et al. who found a linear relationship between the %ΔFVC and %FEV1,6 and supports the view that the closing index may be calculated by either method within the usual range of change in FEV1 (<30%) during a typical methacholine challenge test (as supported by the data in Fig. S2, Supplementary Information).
The change in FVC, and thus the degree to which airways narrow and close, appears to be an important physiological determinant of the clinical expression of AHR. For example, among subjects with equal degrees of AHR, those with no asthma symptoms had smaller changes in FVC in response to methacholine compared to those with mild, symptoms of asthma.20,22,23 In addition, the change in FVC per dose of methacholine has been found to be correlated with asthma severity as judged by FEV1, symptoms, requirement for ICS and risk of near death.26 The change in FVC in response to methacholine has also been found to correlate with measures of small airway dysfunction by the forced oscillation technique,3 and we have previously reported that changes in peripheral airway resistance are associated with airway closure based on computational modelling.5 Recently, Downie et al. provided direct evidence of an independent correlation between change in FVC following methacholine and increase in trapped gas as measured by multiple-breath nitrogen washout.4 Thus, there are strong data to support that a change in FVC following methacholine is an indicator of airway closure and has important clinical implications in asthma.
Multiple other studies also implicate airway closure as an important component of asthma. Airway closure occurs during bronchial challenge6,21,24 and is associated with more severe asthma,27 risk of exacerbations28 and poor control.29,30 Peripheral airway closure in asthma and in response to bronchial challenge has been documented directly by imaging studies.31–34 The data from imaging studies combined with those from direct measurement of lung mechanics using the forced oscillation technique or multiple-breath nitrogen washout indicate that airway closure is due to heterogeneous, extreme narrowing or closure of small, peripheral airways, with or without concomitant narrowing of larger central airways,35,36 and is associated with AHR.4,31,37,38 However, our data demonstrate that there is significant inter-subject variability in the closing index among patients with asthma and AHR. This likely reflects that airway closure is but one component of AHR.1,2
Airway closure may also have important implications for asthma treatment. As convective flow is the primary determinant of aerosol deposition in the lung,8 inhaled drugs would not be able to access poorly ventilated areas of the lung distal to closed or extremely narrowed airways and thus be ineffective for treating the entire lung. Such altered flow patterns and aerosol deposition have been demonstrated using computational fluid dynamics in lung models.39,40 Methods to reduce airway closure, such as application of positive expiratory pressure, may thus have benefit for improving inhaled drug deposition.41
Our data reveal a median closing index of 0.67 among a large, diverse group of patients with asthma. This value is very similar to the mean value (0.60) found by Chapman et al. in a much smaller (n = 62) groups of patients with asthma.6 In addition, we found a higher closing index among the asthmatic patients in the current study (0.67) compared to the non-asthmatic individuals in the study by Chapman et al.6 (0.54), suggesting that, on average, more asthmatic patients respond with airway closure to methacholine than do non-asthmatic individuals.
Importantly, we found that a high closing index is more common among older and obese individuals. The association with age is consistent with previous studies that have documented increased closing volume with age.25,42 Age-related changes in lung function are well documented and commonly indicate increased airway closure during bronchoconstriction. While usually thought of as due to loss of lung elastic recoil with age,43 increased airway closure may also be due, in part, to increased neutrophilic, as opposed to eosinophilic, inflammation in older asthmatic patients,44 or differences in patterns and regions of ventilation heterogeneity in older persons.45 The association with obesity is consistent with the study by Chapman et al., who demonstrated that obese, non-asthmatic subjects have increased airway closure in response to methacholine.6 In recent work, we have shown that airway closure is elevated in obese asthmatic patients and decreases following weight loss surgery.46,47 Airway closure was found to account for 43% of the effect of BMI on AHR in a large population study by Burgess et al.48 Obesity is known to result in low lung volumes, particularly functional residual capacity (FRC) and expiratory reserve volume (ERV), once BMI > 30 kg/m2.49 Interestingly, increased airway closure in obesity is not solely a function of chronic low lung volume.50 In fact, multiple other abnormalities have been described that may enhance airway closure in obese asthmatic patients, including increased peripheral bronchomotor tone,51 decreased peripheral airway compliance,46 impaired response to deep inspiration,52 inflammation in serum and adipose tissue,53,54 airway remodelling55 and pulmonary vascular remodelling.56
Our data do not support an association between the closing index and asthma control, as defined by either the number of EPAC or the ACT or ASUI. This contradicts findings from previous studies,28–30 but these studies used different methods than the closing index to assess airway closure and tended to involve patients with more severe or poorly controlled asthma.
Our data also do not show an association between the closing index and PC20; thus, while airway closure is an important component of AHR, the degree of closure does not appear to correspond to the degree of AHR measured by the PC20. This finding is consistent with the data of Gibbons et al.21 and Chapman et al.6 The reason for this lack of association is likely because the closing index reflects reactivity to methacholine in terms of relative change in FVC and FEV1, whereas the PC20 measures both reactivity and sensitivity to methacholine.6 In addition, this lack of association further supports the argument that airway closure and airway narrowing are likely due to separate mechanisms.2,21
Although data on patients with stable treatment were limited to a subset of a single study (MeCIS), we also found that the closing index response was poorly reproducible over time. This finding is consistent with imaging studies that demonstrate that not all asthmatic patients have stable ventilatory defects, which may reflect both waxing and waning inflammation, as well as underlying airway remodelling or airway closure.57,58 Thus, in asthmatic patients, the closing index may be more useful to identify airway closure as a component of AHR rather than be used longitudinally as a measure of disease activity or treatment response. Interestingly, the intraclass correlation for the log PC20 among the same participants in this trial was 0.53,14 indicating that methacholine challenge reproducibility is only slightly better than that of the closing index over the same period of time, again reflecting the dynamic and variable nature of asthma.
Our study has some limitations. First, it is a retrospective, cross-sectional analysis of the closing index among different cohorts of patients. Therefore, even though the methacholine challenge methodology was highly standardized within studies, there may be some variability across studies. Second, airway closure was assessed in response to methacholine, a direct bronchial challenge agent with certain aerosol characteristics. We cannot be sure that the results would be the same had we used a different bronchial challenge agent that would act indirectly and have different aerosol properties, such as inhaled mannitol. Third, we made the common assumption that the TLC did not change in response to methacholine, which has recently been challenged.59 Fourth, we did not perform other measures of gas trapping, such as residual volume (RV)/total lung capacity (TLC), in order to directly link the closing index with airway closure. Finally, the closing index might have been underestimated by a greater than expected apparent change in FEV1 at maximal challenge due to the effects of gas compression,60 which were not measured in this study.
In conclusion, airway closure in response to methacholine is common among asthmatic participants in the ALA-ACRC, especially among those who are older and obese. This finding supports airway closure as an important feature of AHR, and suggests that therapies directed at airway closure may be important in patients with a high closing index.
Supplementary Material
Figure S1 Distribution of closing index among participants with airway hyperresponsiveness (PC20 ≤ 8 mg/mL).
Figure S2 Difference between closing index calculated using the maximal dose versus the PC20 dose relative to the percent change in FEV1 at the maximal dose of methacholine.
Table S1 Number of participants in each phase of each study.
Table S2 Demographics and baseline pulmonary function of study participants with airway hyperresponsiveness (PC20 ≤ 8).
Table S3 Results of univariate regression modelling of closing index for participants with airway hyperresponsiveness.
Table S4 Results of multivariable regression modelling of closing index for participants with airway hyperresponsiveness.
Table S5 Covariates removed from multivariable regression modelling of closing index for all participants.
Table S6 Covariates removed from multivariable regression modelling of closing index for participants with airway hyperresponsiveness.
Table S7 Results of multivariable regression modelling of closing index for all child participants (n = 172).
Table S8 Results of multivariable regression modeling of closing index for child participants with airway hyperresponsiveness (n = 149).
Table S9 Results of multivariable negative binomial regression modeling of EPAC for participants with airway hyperresponsiveness who had diary card data (n = 791).
Visual Abstract What factors are associated with airway closure in response to methacholine challenge in asthma?
SUMMARY AT A GLANCE.
Airway closure during methacholine challenge contributes importantly to airway hyperresponsiveness in asthma and is associated with older age and obesity.
Acknowledgement
This study was funded by the American Lung Association-Airways Clinical Research Centers.
Abbreviations:
- ACT
Asthma Control Test
- AHR
airway hyperresponsiveness
- ALA-ACRC
American Lung Association-Airways Clinical Research Centers
- ASUI
Asthma Symptom Utility Index; BD, bronchodilator
- cACT
child ACT
- CPAP
Effect of Positive Airway Pressure on Reducing Airway Reactivity in Patients with Asthma
- EPAC
episode of poor asthma control
- ERV
expiratory reserve volume
- FEV1
forced expiratory volume in 1 s
- FVC
forced vital capacity
- ICS
inhaled corticosteroid
- IRB
institutional review board
- LABA
long-acting beta-agonist
- MeCIS
Methacholine Bronchoprovocation – Influence of High-Potency Inhaled Corticosteroids in Asthma
- PC20
provocative concentration of methacholine causing a 20% decrease in FEV1
- RR
rate ratio
- RV
residual volume
- SARA
Study of Acid Reflux and Asthma
- SARCA
Study of Acid Reflux in Childhood Asthma
- STAN
Study of Asthma and Nasal Steroids
- TLC
total lung capacity
Footnotes
Supplementary Information
Additional supplementary information can be accessed via the html version of this article at the publisher’s website.
Data availability statement
Individual participant data, including data that underlie the results reported in this paper, the study protocol and data forms, will be available for data sharing. In line with the current ALA-ACRC policies, data requests from IRB-approved investigators are provided with a HIPPA compliant limited use data set conditioned on the acceptance of a simple data use agreement that protects the confidentiality and integrity of the data. There is no limited time frame for release of data. Data sharing requests may be sent to jhsph.ala-acrc@jhu.edu.
REFERENCES
- 1.Berend N, Salome C, King G. Mechanisms of airway hyperresponsiveness in asthma. Respirology 2008; 13: 624–31. [DOI] [PubMed] [Google Scholar]
- 2.Chapman D, Irvin C. Mechanisms of airway hyper-responsiveness in asthma: the past, present and yet to come. Clin. Exp. Allergy 2015; 45: 706–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alfieri V, Aiello M, Pisi R, Tzani P, Mariani E, Marangio E, Olivieri D, Nicolini G, Chetta A. Small airway dysfunction is associated to excessive bronchoconstriction in asthmatic patients. Respir. Res 2014; 15: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Downie S, Salome C, Verbanck S, Thompson B, Berend N, King G. Effect of methacholine on peripheral lung mechanics and ventilation heterogeneity in asthma. J. Appl. Physiol 2013; 114: 770–7. [DOI] [PubMed] [Google Scholar]
- 5.Kaminsky D, Bates J, Irvin C. Effects of cool, dry air stimulation on peripheral lung mechanics in asthma. Am. J. Respir. Crit. Care Med 2000; 162: 179–86. [DOI] [PubMed] [Google Scholar]
- 6.Chapman D, Berend N, King G, Salome C. Increased airway closure is a determinant of airway hyperresponsiveness. Eur. Respir. J 2008; 32: 1563–9. [DOI] [PubMed] [Google Scholar]
- 7.Lougheed D, Fisher T, O’Donnell D. Dynamic hyperinflation during bronchoconstriction in asthma: implications for symptom perception. Chest 2006; 130: 1072–81. [DOI] [PubMed] [Google Scholar]
- 8.Darquenne C, van Ertbruggen C, Prisk G. Convective flow dominates aerosol delivery to the lung segments. J. Appl. Physiol 2011; 111: 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dixon A, Castro M, Cohen R, Gerald L, Holbrook J, Irvin C, Mohapatra S, Peters S, Rayapudi S, Sugar E et al. Efficacy of nasal mometasone for the treatment of chronic sinonasal disease in patients with inadequately controlled asthma. J. Allergy Clin. Immunol 2015; 135: 701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holbrook J, Sugar E, Brown R, Drye L, Schwartz A, Tepper R, Wise R, Yasin R, Busk M. Effect of continuous positive airway pressure on reducing airway reactivity in patients with asthma: a multi-center, randomized, sham-controlled clinical trial. Ann. Am. Thorac. Soc 2016; 13: 194–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holbrook J, Wise R, Gold B, Blake K, Brown E, Castro M, Dozor A, Lima J, Mastronade J, Sockrider M et al. Lansoprazole for children with poorly controlled asthma. JAMA 2012; 307: 373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mastronade J, Anthonisen N, Castro M, Holbrook J, Leone F, Teague W, Wise R. Efficacy of esomeprazole for treatment of poorly controlled asthma. N. Engl. J. Med 2009; 360: 1487–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sumino K, Sugar E, Irvin C, Kaminsky D, Shade D, Wei C, Holbrook J, Wise R, Castro M. Methacholine challenge test: diagnostic characteristics in asthmatic patients receiving controller medications. J. Allergy Clin. Immunol 2012; 130: 69–75. [DOI] [PubMed] [Google Scholar]
- 14.Sumino K, Sugar EA, Irvin CG, Kaminsky DA, Shade D, Wei CY, Holbrook JT, Wise RA, Castro M. Variability of methacholine bronchoprovocation and the effect of inhaled corticosteroids in mild asthma. Ann. Allergy Asthma Immunol 2014; 112: 354–60.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller M, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten C, Gustafsson P et al. Standardisation of spirometry. Eur. Respir. J 2005; 26: 319–38. [DOI] [PubMed] [Google Scholar]
- 16.American Thoracic Society. Guidelines for methacholine and exercise challenge testing – 1999. Am. J. Respir. Crit. Care Med 1999; 161: 309–29. [DOI] [PubMed] [Google Scholar]
- 17.Schatz M, Kosinski M, Yarlas A, Hanlon J, Watson M, Jhingran P. The minimally important difference of the Asthma Control Test. J. Allergy Clin. Immunol 2009; 124: 719–23. [DOI] [PubMed] [Google Scholar]
- 18.Bime C, Wei C, Holbrook J, Sockrider M, Revicki D, Wise R. Asthma symptoms utility index: reliability, validity, responsiveness and the minimal important difference in adult asthmatic patients. J. Allergy Clin. Immunol 2012; 130: 1078–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irvin C, Kaminsky D, Anthonisen N, Castro M, Hanania N, Holbrook J, Lima J, Wise R. Clinical trial of low-dose theophylline and montelukast in patients with poorly controlled asthma. Am. J. Respir. Crit. Care Med 2007; 175: 235–42. [DOI] [PubMed] [Google Scholar]
- 20.Choi S, Kim D, Yoo Y, Yu J, Koh Y. Comparison of deltaFVC between patients with allergic rhinitis and airway hypersensitivity and patients with mild asthma. Ann. Allergy Asthma Immunol 2007; 98: 128–33. [DOI] [PubMed] [Google Scholar]
- 21.Gibbons W, Sharma A, Lougheed D, Macklem P. Detection of excessive bronchoconstriction in asthma. Am. J. Respir. Crit. Care Med 1996; 153: 582–9. [DOI] [PubMed] [Google Scholar]
- 22.Yoo Y, Choung J, Yu J, Kim D, Choi S, Koh Y. Comparison of percentage fall in FVC at the provocative concentration of methacholine causing a 20% fall in FEV1 between patients with asymptomatic bronchial hyperresponsiveness and mild asthma. Chest 2007; 132: 106–11. [DOI] [PubMed] [Google Scholar]
- 23.Yoo Y, Yu J, Kim D, Koh Y. Percentage fall in FVC at the provocative concentration of methacholine causing a 20% fall in FEV1 in asymptomatic asthma and clinical remission during adolescence. Chest 2006; 129: 272–7. [DOI] [PubMed] [Google Scholar]
- 24.Milanese M, Crimi E, Scordamaglia A, Riccio A, Pellegrino R, Canonica G, Brusasco V. On the functional consequences of bronchial basement membrane thickening. J. Appl. Physiol 2001; 91: 1035–40. [DOI] [PubMed] [Google Scholar]
- 25.Cuttitta G, Cibella F, Bellia V, Grassi V, Cossi S, Bucchieri S, Bonsignore G. Changes in FVC during methacholine-induced bronchoconstriction in elderly patients with asthma. Chest 2001; 119: 1685–90. [DOI] [PubMed] [Google Scholar]
- 26.Lee P, Abisheganaden J, Chee C, Wang Y. A new asthma severity index: a predictor of near-fatal asthma? Eur. Respir. J 2001; 18: 272–8. [DOI] [PubMed] [Google Scholar]
- 27.Sorkness R, Bleeker E, Busse W, Calhoun W, Castro M, Chung K, Curran-Everett D, Erzurum S, Gaston B, Israel E et al. Lung function in adults with stable but severe asthma: air trapping and incomplete reversal of obstruction with bronchodilation. J. Appl. Physiol 2008; 104: 394–403. [DOI] [PubMed] [Google Scholar]
- 28.in’tveen J, Beekman A, Bel E, Sterk P. Recurrent exacerbations in severe asthma are associated with enhanced airway closure during stable episodes. Am. J. Respir. Crit. Care Med 2000; 161: 1902–6. [DOI] [PubMed] [Google Scholar]
- 29.Kelly V, Sands S, Harris R, Venegas J, Brown N, Stuart-Andrews C, King G, Thompson B. Respiratory system reactance is an independent determinant of asthma control. J. Appl. Physiol 2013; 115: 1360–9. [DOI] [PubMed] [Google Scholar]
- 30.Perez T, Chanez P, Dusser D, Devillier P. Prevalence and reversibility of lung hyperinflation in adult asthmatics with poorly controlled disease or significant dyspnea. Allergy 2016; 71: 108–14. [DOI] [PubMed] [Google Scholar]
- 31.Farrow C, Salome C, Harris B, Bailey D, Bailey E, Berend N, Young I, King G. Airway closure on imaging relates to airway hyperresponsiveness and peripheral airway disease in asthma. J. Appl. Physiol 2012; 113: 958–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris R, Winkler T, Tgavalekos N, Musch G, Melo M, Schroeder T, Chang Y, Venegas J. Regional pulmonary perfusion, inflation and ventilation defects in bronchoconstricted patients with asthma. Am. J. Respir. Crit. Care Med 2006; 174: 245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.King G, Eberl S, Salome C, Young I, Woolcock A. Differences in airway closure between normal and asthmatic subjects measured with single-photon emission computed tomography and technegas. Am. J. Respir. Crit. Care Med 1998; 158: 1900–6. [DOI] [PubMed] [Google Scholar]
- 34.Samee S, Altes T, Powers P, de Lange E, Knight-Scott J, Rakes G, Mugler J, Ciambotti J, Alford B, Brookeman J et al. Imaging the lungs in asthmatic patients by using hyperpolarized helium-3 magnetic resonance: assessment of response to methacholine and exercise challenge. J. Allergy Clin. Immunol 2003; 111: 1205–11. [DOI] [PubMed] [Google Scholar]
- 35.Tgavalekos N, Musch G, Harris B, Melo M, Winkler T, Schroeder T, Callahan R, Lutchen K, Venegas J. Relationship between airway narrowing, patchy ventilation and lung mechanics in asthmatics. Eur. Respir. J 2007; 29: 1174–81. [DOI] [PubMed] [Google Scholar]
- 36.Tgavalekos N, Tawhai M, Harris B, Mush G, Vidal-Melo M, Venegas J, Lutchen K. Identifying airways responsible for heterogeneous ventilation and mechanical dysfunction in asthma: an image functional modeling approach. J. Appl. Physiol 2005; 99: 2388–97. [DOI] [PubMed] [Google Scholar]
- 37.Downie S, Salome C, Verbanck S, Thompson B, Berend N, King G. Ventilation heterogeneity is a major determinant of airway hyper-responsiveness in asthma, independent of airway inflammation. Thorax 2007; 62: 684–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lui J, Parameswaran H, Albert M, Lutchen K. Linking ventilation heterogeneity quantified via hyperpolarized 3He MRI to dynamic lung mechanics and airway hyperresponsiveness. PLoS One 2015; 10: e0142738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verbanck S, Ghorbaniasl G, Biddiscombe MF, Dragojlovic D, Ricks N, Lacor C, Ilsen B, de Mey J, Schuermans D, Underwood SR et al. Inhaled aerosol distribution in human airways: a scintigraphy-guided study in a 3D printed model. J. Aerosol Med. Pulm. Drug Deliv 2016; 29: 525–33. [DOI] [PubMed] [Google Scholar]
- 40.Walenga RL, Longest PW. Current inhalers deliver very small doses to the lower tracheobronchial airways: assessment of healthy and constricted lungs. J. Pharm. Sci 2016; 105: 147–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alcoforado L, Brandao S, Rattes C, Brandao D, Lima V, Lima G, Fink J, de Andrade A. Evaluation of lung function and deposition of aerosolized bronchodilators carried by heliox associated with positive expiratory pressure in stable asthmatics: a randomized clinical trial. Respir. Med 2013; 107: 1178–85. [DOI] [PubMed] [Google Scholar]
- 42.Janssens J, Pache J, Nicod L. Physiological changes in respiratory function associated with ageing. Eur. Respir. J 1999; 13: 197–205. [DOI] [PubMed] [Google Scholar]
- 43.Mead J, Turner JM, Macklem PT, Little JB. Significance of the relationship between lung recoil and maximum expiratory flow. J. Appl. Physiol 1967; 22: 95–108. [DOI] [PubMed] [Google Scholar]
- 44.Porsbjerg CM, Gibson PG, Pretto JJ, Salome CM, Brown NJ, Berend N, King GG. Relationship between airway pathophysiology and airway inflammation in older asthmatics. Respirology 2013; 18: 1128–34. [DOI] [PubMed] [Google Scholar]
- 45.Hardaker KM, Downie SR, Kermode JA, Farah CS, Brown NJ, Berend N, King GG, Salome CM. Predictors of airway hyperresponsiveness differ between old and young patients with asthma. Chest 2011; 139: 1395–401. [DOI] [PubMed] [Google Scholar]
- 46.Al-Alwan A, Bates J, Chapman D, Kaminsky D, SeSarno M, Irvin C, Dixon A. The nonallergic asthma of obesity. A matter of distal lung compliance. Am. J. Respir. Crit. Care Med 2014; 189: 1494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chapman D, Irvin C, Kaminsky D, Forgione P, Bates J, Dixon A. Influence of distinct asthma phenotypes on lung function following weight loss in the obese. Respirology 2014; 9: 1170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burgess JA, Matheson MC, Diao F, Johns DP, Erbas B, Lowe AJ, Gurrin LC, Lodge CJ, Thomas PS, Morrison S et al. Bronchial hyperresponsiveness and obesity in middle age: insights from an Australian cohort. Eur. Respir. J 2017; 50: 1602181. [DOI] [PubMed] [Google Scholar]
- 49.Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest 2006; 130: 827–33. [DOI] [PubMed] [Google Scholar]
- 50.Mahadev S, Salome C, Berend N, King G. The effect of low lung volume on airway function in obesity. Respir. Physiol. Neurobiol 2013; 188: 192–9. [DOI] [PubMed] [Google Scholar]
- 51.Desai AG, Togias A, Schechter C, Fisher B, Parow A, Skloot G. Peripheral airways dysfunction in obesity reflects increased bronchomotor tone. J. Allergy Clin. Immunol 2015; 135: 820–2. [DOI] [PubMed] [Google Scholar]
- 52.Skloot G, Schechter C, Desai A, Togias A. Impaired response to deep inspiration in obesity. J. Appl. Physiol (1985) 2011; 111: 726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bates JHT, Poynter ME, Frodella CM, Peters U, Dixon AE, Suratt BT. Pathophysiology to phenotype in the asthma of obesity. Ann. Am. Thorac. Soc 2017; 14(Suppl. 5): S395–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sideleva O, Suratt BT, Black KE, Tharp WG, Pratley RE, Forgione P, Dienz O, Irvin CG, Dixon AE. Obesity and asthma: an inflammatory disease of adipose tissue not the airway. Am. J. Respir. Crit. Care Med 2012; 186: 598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barton JH, Ireland A, Fitzpatrick M, Kessinger C, Camp D, Weinman R, McMahon D, Leader JK, Holguin F, Wenzel SE et al. Adiposity influences airway wall thickness and the asthma phenotype of HIV-associated obstructive lung disease: a cross-sectional study. BMC Pulm. Med 2016; 16: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oppenheimer BW, Berger KI, Ali S, Segal LN, Donnino R, Katz S, Parikh M, Goldring RM. Pulmonary vascular congestion: a mechanism for distal lung unit dysfunction in obesity. PLoS One 2016; 11: e0152769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Lange E, Altes T, Patrie J, Battiston J, Juersivich A, Mugler J, Platts-Mills T. Changes in regional airflow obstruction over time in the lungs of patients with asthma: evaluation with 3He MR imaging. Radiology 2009; 250: 567–75. [DOI] [PubMed] [Google Scholar]
- 58.Svenningsen S, Kirby M, Starr D, Coxson H, Paterson N, McCormack D, Parraga G. What are ventilation defects in asthma? Thorax 2013; 69: 63–71. [DOI] [PubMed] [Google Scholar]
- 59.Brown R, Pearse D, Pyrgos G, Liu M, Togias L, Permutt S. The structural basis of airways hyperresponsiveness. J. Appl. Physiol 2006; 101: 30–9. [DOI] [PubMed] [Google Scholar]
- 60.Krowka MJ, Enright PL, Rodarte JR, Hyatt RE. Effect of effort on measurement of forced expiratory volume in one second. Am. Rev. Respir. Dis 1987; 136: 829–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Distribution of closing index among participants with airway hyperresponsiveness (PC20 ≤ 8 mg/mL).
Figure S2 Difference between closing index calculated using the maximal dose versus the PC20 dose relative to the percent change in FEV1 at the maximal dose of methacholine.
Table S1 Number of participants in each phase of each study.
Table S2 Demographics and baseline pulmonary function of study participants with airway hyperresponsiveness (PC20 ≤ 8).
Table S3 Results of univariate regression modelling of closing index for participants with airway hyperresponsiveness.
Table S4 Results of multivariable regression modelling of closing index for participants with airway hyperresponsiveness.
Table S5 Covariates removed from multivariable regression modelling of closing index for all participants.
Table S6 Covariates removed from multivariable regression modelling of closing index for participants with airway hyperresponsiveness.
Table S7 Results of multivariable regression modelling of closing index for all child participants (n = 172).
Table S8 Results of multivariable regression modeling of closing index for child participants with airway hyperresponsiveness (n = 149).
Table S9 Results of multivariable negative binomial regression modeling of EPAC for participants with airway hyperresponsiveness who had diary card data (n = 791).
Visual Abstract What factors are associated with airway closure in response to methacholine challenge in asthma?
Data Availability Statement
Individual participant data, including data that underlie the results reported in this paper, the study protocol and data forms, will be available for data sharing. In line with the current ALA-ACRC policies, data requests from IRB-approved investigators are provided with a HIPPA compliant limited use data set conditioned on the acceptance of a simple data use agreement that protects the confidentiality and integrity of the data. There is no limited time frame for release of data. Data sharing requests may be sent to jhsph.ala-acrc@jhu.edu.


