Abstract
Introduction
The persistent erythema and flushing seen in some cases of rosacea do not respond effectively to, or may easily relapse after, oral medication or light-based therapies (laser or intense pulsed light). Intradermal botulinum toxin A (BTX-A) injection can be used to treat intractable erythema and flushing, but studies with large samples and long-term observation have not been conducted to determine its effectiveness and safety. The aim of this study is thus to investigate the effective duration and safety of intradermal BTX-A injection for intractable erythema and flushing.
Methods
Sixteen patients with rosacea with erythema telangiectasia were injected with BTX-A at 1-cm intervals between each point. Clinician Erythema Assessment (CEA) scores were obtained at baseline and 1 month after injection. Flushing assessment and survey using the Dermatological Quality of Life Index (DLQI) questionnaire were conducted at baseline and at 1, 3, and 6 months after injection.
Results
At 1 month after injection, CEA scores revealed significant improvements in erythema and flushing; the results of the questionnaire on flushing and DLQI indicated that the improvement of flushing usually lasted for 3–6 months, but the effect decreased significantly at 6 months, and individual patients needed another treatment.
Conclusions
BTX-A significantly improves the symptoms and quality of life of patients with refractory rosacea with few adverse effects.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-022-00784-0.
Keywords: Botulinum toxin A, Erythema, Flushing, Rosacea
Key Summary Points
| Oral medication or light-based therapies for persistent erythema and flushing in rosacea may be ineffective in some cases. |
| Studies with large samples and long-term observations have not been conducted to determine the effectiveness and safety of intradermal botulinum toxin-A injection for intractable erythema and flushing. |
| This study aims to investigate the effective duration and safety of intradermal BTX-A injection for intractable erythema and flushing. |
| The improvement after intradermal BTX-A injection for intractable erythema and flushing usually lasts for 3–6 months, but the effect decreases significantly at 6 months. |
| BTX-A significantly improves the symptoms and quality of life of patients with refractory rosacea with few adverse effects. |
Introduction
Rosacea is a chronic recurrent inflammatory disease that often affects the middle of the face and mainly involves facial blood vessels and nerves. The global prevalence rate of rosacea is 5.5%, which is increasing annually [1]. Rosacea is characterized by paroxysmal flushing, persistent erythema or papules, pustules, and telangiectasia in the face. A few patients experience phymatous and eye changes, which may be accompanied by skin sensitive symptoms such as burning, tingling, tension, and dry skin. Rosacea is induced by multiple factors, dominated by abnormal natural immunity and neurovascular regulation with unknown etiology and complex pathogenesis. According to its different clinical manifestations, rosacea can be divided into four types, namely erythrotelangiectatic, papulopustular, phymatous, and ocular rosacea [2]. Moreover, the life and work of such patients are often greatly affected by lingering disease that is difficult to heal [3]. Persistent facial erythema and flushing are common complaints and the most troubling symptoms of the disease. Therefore, safe and effective treatment methods should be developed to deal with this condition.
Erythema telangiectasia rosacea (ETR) is prone to skin sensitive symptoms such as dryness, burning, or tingling in the middle of the face and high reactivity of blood vessels (paroxysmal flushing and erythema) caused by the relatively thin cuticle of the cheek, the reduced distribution of sebaceous glands, and the abundant distribution of blood vessels in the dermis. These patients have impaired skin barrier function and sensitive skin at the same time, and they cannot tolerate treatment by pulsed dye laser and intense pulsed light [4, 5]. Considering that the symptoms of paroxysmal flushing can easily occur repeatedly, conventional methods for treatment of rosacea-related erythema and flushing include systematic drugs, topical drugs, and physical therapy. Although many methods are available, they are not effective in all patients. Indeed, some patients do not experience alleviation of their symptoms after using these methods [6]. This condition belongs to the category of refractory rosacea. Therefore, many dermatologists have attempted to develop new treatments to improve or treat this kind of paroxysmal flushing and persistent erythema in rosacea.
BTX-A injection is an effective method for the treatment of ETR [7, 8]. A clinical trial that included 25 ETR patients revealed that erythema was significantly improved at 1, 2, and 3 months after 15–45 U BTX-A injection [9]. In a prospective study [10], an open randomized controlled trial of 24 patients with facial flushing showed that the symptoms were improved within 2–3 weeks after BTX-A injection, and satisfaction remained high after 2 months. However, these two studies lacked long-term follow-up and cannot provide an understanding of the effective duration of BTX-A treatment. Twenty cases of ETR were treated with pulsed dye laser combined with BTX-A intradermal injection for three times [11]. The symptoms of erythema, telangiectasia, flushing, pruritus, and burning sensation were improved. The patients were followed up at 3 and 9 months after treatment. The condition of erythema improved continuously. A few patients experienced recurrence of flushing symptoms, but the severity was lower than before treatment. In another study [12], esthetic therapy combined with BTX-A was used to treat intractable telangiectasia and papular pustular rosacea, having a significant effect, but the patients required repeated treatment after 4–5 months. The application of these two methods is limited by their high cost and short curative time. A retrospective study [13] treated 16 ETR patients with BTX-A twice at an interval of 1 month. The erythema score was high at 1 month after treatment but showed slight recurrence at 3 and 6 months, albeit better than at baseline before treatment, while the patients’ self-evaluation satisfaction was high.
BTX-A is a safe and effective drug that can reduce the severity of rosacea symptoms and improve patient satisfaction. However, previous studies employed BTX-A combined with phototherapy or shorter observation times and thus cannot provide an understanding of the effective duration of BTX-A alone. The aim of the current work is thus to carry out a clinical retrospective study to reveal the time window of BTX-A, thereby providing a safe and effective ETR treatment scheme for clinical doctors.
Patients and Methods
Ethics
All subjects provided written informed consent to participate in the study, which was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Photographs are published with patient consent. The study was approved by the Ethics Committee of The First Affiliated Hospital of Xi’an Jiaotong University (no. KYLLSL-2022-227).
Patients
Sixteen female ETR patients aged 30 ± 13 years were enrolled from the dermatology clinic of the First Affiliated Hospital of Xi'an Jiaotong University from January 2020 to March 2021. The inclusion criteria were as follows: (1) patients diagnosed by two experienced dermatologists separately according to the criteria for the clinical classification of rosacea [14] and meeting the diagnostic criteria for patients with ETR or ETR with a small amount of papules and pustules; (2) before treatment, the patients were treated according to the guidelines for 2–3 months, and the symptoms of erythema and flushing were not relieved; (3) patients participated voluntarily in the trial and signed informed consent; and (4) patients were willing to receive BTX treatment and agreed to complete follow-up.
The exclusion criteria were as follows: (1) pregnant or lactating women, and those preparing for pregnancy during treatment; (2) never received drugs or physical therapy recommended by the guidelines; (3) received facial cosmetic surgery or BTX-A treatment within 6 months before the treatment; (4) other infectious or neoplastic diseases of the face; and (5) allergic to any component of BTX-A.
Flushing assessment and life quality assessment were carried out through a questionnaire survey for patients with ETR before treatment and then at 1, 3, and 6 months after treatment. The questionnaires were completed in the hospital before and at 1 month after the injection, then questionnaires were sent to the patients through WeChat at 3 and 6 months. Whether they were completed in the hospital or through WeChat, all patients completed the questionnaires independently. The results resulting from the two methods were consistent.
Injection Design
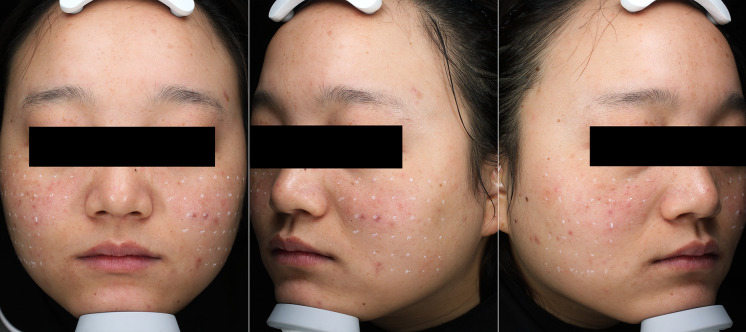
The patient’s facial image was obtained using a VISIA Canfield imaging system (Fairfield, NJ, USA), and the skin lesions were recorded objectively. After assessment, the patient’s face was cleaned. Lidocaine cream (5%, Tongfang Pharmaceutical Group Co., Ltd., Beijing, China) was applied to the skin lesion for 40 min and then washed off. After the skin at the injection point had been sterilized, 100 U BTX-A (Lanzhou Institute of Biological Products Co., Ltd., Lanzhou, China) was added to 6.25 ml sterile 0.9% sodium chloride to achieve a final concentration of 16 U/ml BTX-A solution. By using a 1-ml syringe with a 34-gauge needle having a length of 4 mm, 0.05 ml of (0.8 U) BTX-A solution was injected at each point in the erythema area. The injection points were staggered 1 cm apart (Fig. 1). A total of 40–60 units of BTX was used for each patient. The injected area covered the erythema and flushing areas of the patients (such as cheeks, eyebrows, nose, and mandible). The injected area and the total number of injection points were determined according to the areas of the erythema and flushing. There were approximately 50–70 points on both cheeks, 9–12 points on the nose, 8–12 points on the mandible, and 6–9 points between the eyebrows. Ice pack pressure was applied for 20 min after injection. The patients were told that the injection site should not touch water for 24 h after injection.
Fig. 1.
Sketch marking the injected area
CEA Assessment
Before and at 1 month after treatment, the subjects’ facial erythema was assessed by two independent nontreating dermatologists using the CEA scale at each visit (0 = clear, 1 = almost clear, 2 = mild, 3 = moderate, and 4 = severe; Table 1) [15].
Table 1.
Clinician Erythema Assessment scale
| Scale | CEA |
|---|---|
| 0 = clear | Clear skin with no signs of erythema |
| 1 = almost clear | Almost clear; slight redness |
| 2 = mild | Mild erythema, definite redness |
| 3 = moderate | Moderate erythema; marked redness |
| 4 = severe | Severe erythema; fiery redness |
Facial photographs (front, left 45°, and right 45°) of the patients were obtained using a VISIA Canfield imaging system (Fairfield, NJ, USA) before and at 1 month after treatment by using a fixed wooden frame in the same place.
Flushing Assessment
The flushing of the patients was assessed using our modified questionnaire of flushing symptoms [16] at baseline and at 1, 3, and 6 months after treatment. The questionnaire at baseline and at 1 month after treatment was answered in the hospital, while that at 3 and 6 months after treatment was answered via WeChat.
Ten items were included in the flushing assessment questionnaire. The options for each item were divided into five levels, with corresponding scores of 0, 1–3, 4–6, 7–9, and 10 (except for items 1, 3, and 9). For the ninth item, we used Yes = 1 and No = 0. For items 1 and 3, the corresponding scores were 0, 1, 2, 3, and 4. The scores for the ten options were summed to obtain the final flushing score of the patient (Supplementary Material).
Life Quality Assessment
The Dermatology Life Quality Index (DLQI) [17] is a quality-of-life questionnaire for adults that is used to evaluate the effect of skin condition on patients’ life in the past 7 days, including ten questions. Each question has four options, namely, “no,” “slight,” “serious,” and “extremely serious,” corresponding to 0, 1, 2, and 3 points, respectively. The scores from all the questions were added. The minimum DLQL score is 0, while the maximum score is 30. The higher the score, the greater the effect of skin diseases on quality of life (Supplementary Table S1).
Safety Evaluation
At baseline and at 1, 3, and 6 months after treatment, the same investigator asked the patients whether they had pain, burning, redness, and swelling during injection treatment and objectively evaluated the skin bruising, facial expression stiffness, asymmetry, skin tension, and other conditions after treatment. The severity was recorded.
Statistical Analysis
Data were analyzed using SPSS 18 (IBM Corporation, Armonk, NY, USA). T test was used for comparison between two groups. One-way analysis of variance (ANOVA) was used for comparison among three groups. All data are presented as mean ± standard deviation (SD). Difference was considered significant at P < 0.05.
Results
Erythema was Significantly Improved by BTX-A
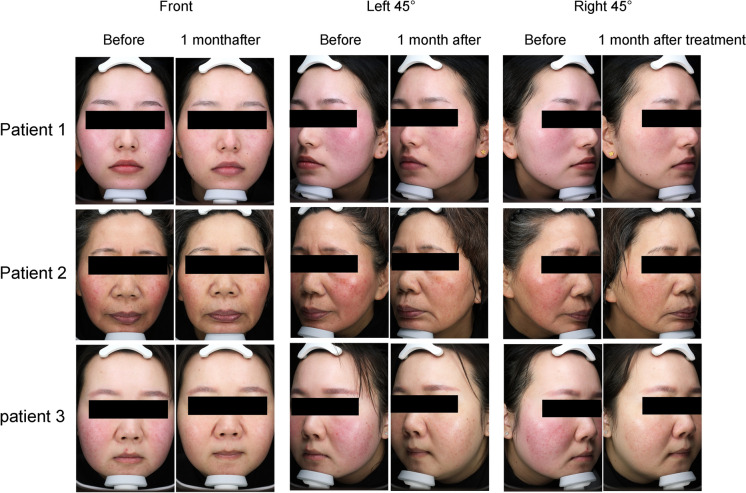
The CEA scores were 2.88 ± 0.62 and 1.00 ± 0.37 at baseline and 1 month after treatment, respectively. The average CEA scores at 1 month after treatment were significantly improved compared with baseline (P = 0.000, Fig. 2). The facial erythema of ETR patients showed significant appearance improvement after BTX-A injection (Fig. 3).
Fig. 2.
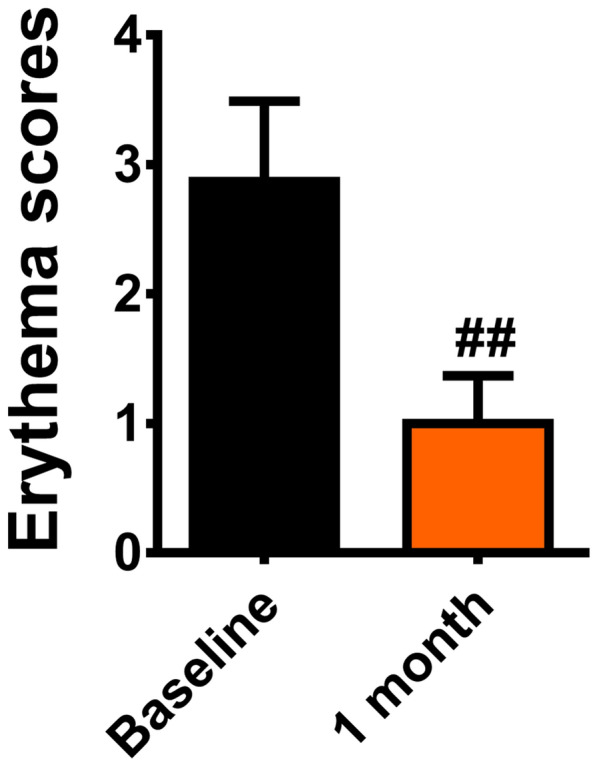
CEA scores before and 1 month after treatment (##P < 0.01)
Fig. 3.
Representative rosacea status at baseline and 1 month after treatment for three patients
Flushing Symptom Was Alleviated by BTX-A
The mean flushing scores were 47.81 ± 8.68, 18.75 ± 3.86, 20.19 ± 4.26, and 26.50 ± 7.93 on the cheek at baseline and 1, 3, and 6 months after treatment, respectively. In comparison with baseline, the flushing score at 1, 3, or 6 months after treatment was significantly decreased (P = 000). Notably, from 1 to 6 months after treatment, the mean flushing scores continued to increase until 6 months after treatment, indicating that the curative effect decreased to some extent. However, no difference was observed between the flushing scores at 1 and 3 months (P = 0.521), suggesting that the curative effect of BTX-A remained unchanged. However, significant difference was observed in the efficacy between 3 and 6 months (P = 0.011), suggesting that the efficacy of BTX-A decreased sharply. However, no values returned to the initial baseline values, suggesting that the role of BTX-A was significantly weakened in some patients, and an additional injection of BTX-A is needed (Fig. 4).
Fig. 4.
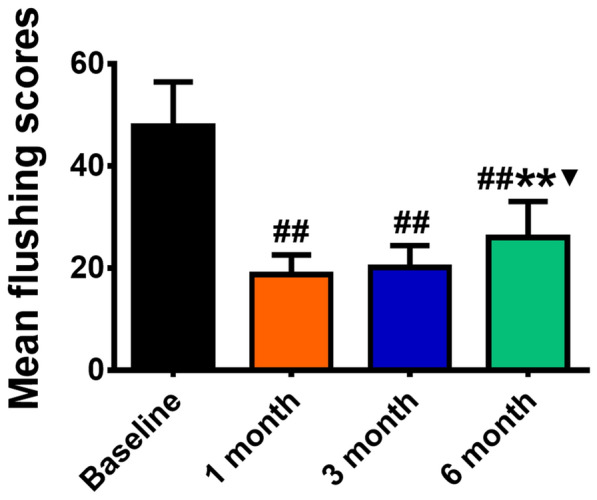
Flushing assessment scores at baseline and 1, 3, and 6 months after treatment (##P < 0.01 versus baseline, **P < 0.01 versus 1 months, ▼P < 0.05 versus 3 months)
Dermatology Life Quality Index
The DLQI values were 22.25 ± 5.25, 7.69 ± 2.47, 7.13 ± 2.16, and 10.56 ± 3.53 at baseline and 1, 3, and 6 months after treatment, respectively. In comparison with baseline, the DLQI scores at 1, 3, or 6 months after treatment decreased significantly (P = 000). Notably, from 1 to 3 months after treatment, the DLQI gradually increased, indicating that the curative effect decreased. No difference was observed between the flushing scores at 1 and 3 months (P = 0.657), suggesting that quality of life remained unchanged. However, a significant difference was observed in the efficacy between 3 and 6 months (P = 0.008), indicating that quality of life decreased sharply at 6 months, that the role of BTX-A was significantly weakened in some patients, and that additional injection of BTX-A is needed (Fig. 5).
Fig. 5.
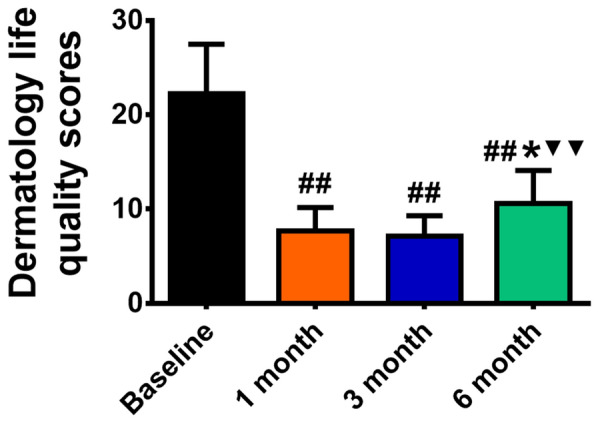
Dermatology life quality scores before and 1, 3, and 6 months after treatment (##P < 0.01 versus baseline, *P < 0.05 versus 1 months, ▼▼P < 0.01 versus 3 months)
Side Effects
All 16 patients experienced slight pain during BTX-A injection, but the pain was relieved immediately after the injection and was tolerable. The erythema in the injection area increased in four cases and decreased after 20 min of cold compress. Bruises were observed at the injection point in five cases, while the number of bruise sites did not exceed five in each patient. The bruises gradually subsided after 1 week. Three patients felt tightness in the injection area at 1 week after injection, but the symptoms disappeared after 1 month. One patient had a slightly asymmetric facial expression at 2 weeks after injection, but only when making laughter expression. The symptom disappeared after 1 month. All patients had no serious adverse effects such as allergic reaction, muscle weakness, respiratory distress, or fatigue. At 6 months, five patients experienced recurrent symptoms, but the symptoms of erythema and flushing were lighter than before treatment. BTX-A injection results in white patches all over the face [18], but this problem did not occur in the patients in our study.
Discussion
In the present study, BTX-A was injected into erythema skin of patients with ETR. Based on an evaluation of erythema, flushing, and quality of life after injection, intradermal injection BTX-A considerably improved the symptoms of refractory rosacea and the quality of life of patients with ETR. The application of BTX-A in ETR is very safe and effective. According to the follow-up for 6 months after treatment, the effectiveness of the BTX-A treatment lasted for approximately 6 months. After 6 months, the effect began to decline, and individual patients needed additional injection. Importantly, no patients showed serious side effects.
Flushing refers to a state in which the skin is obviously red, accompanied by fever. It is a transient vasodilation phenomenon and is part of the physiological temperature regulation response. Flushing can be intermittent or continuous. In general, endogenous vasoactive mediators or drugs cause the intermittent onset of flushing, while long-term repeated onset of flushing will cause fixed erythema on the face with telangiectasia [19]. Some erythema and flushing are persistent, and difficult to improve using conventional treatment measures.
Although BTX-A microinjection has been used to treat rosacea for many years, it has not been widely used in clinic. This may be related to doctors’ insufficient understanding of its mechanism in the treatment of ETR. No unified standard has been defined for BTX-A solution preparation and injection methods. Long-term follow-up and evaluation of the efficacy and safety of BTX-A for the treatment of ETR have not been studied. In China, injection of 0.5–1U BTX is recommended at each injection point of a lesion area, with injection points staggered 1 cm apart [20]. The treatment scheme used in this study confirmed that most patients showed a significant curative effect at 1 month after injection, which can be maintained for 6 months, but the effect decreased significantly in 6 months. Some patients need repeated treatment.
This study observed the effect of BTX-A injection on ETR refractory erythema and flushing at 1, 3, and 6 months, revealing that the effectiveness of BTX-A can extend to approximately 6 months, while patient satisfaction was high. Notably, patient satisfaction showed a constant trend with erythema and flushing assessment, indicating that patient satisfaction mainly results from the self-feeling for erythema and flushing. This study also provides a reference for the additional injection of BTX-A in clinical practice. The observation time in previous studies was short. For example, in a clinical trial involving 25 patients with ETR, erythema was observed at only 1, 2, and 3 months after BTX-A injection [9]. Another prospective study [10] including 24 patients observed the improvement of flushing symptoms within 2–3 weeks and conducted a satisfaction survey at 2 months after BTX-A injection. In addition, the present study revealed the effect of BTX-A without combination with other light-based therapies (laser or intense pulsed light). In another study [11], 20 cases of ETR were treated with BTX-A intradermal injection combined with pulsed dye laser, which improved the symptoms of erythema, telangiectasia, flushing, pruritus, and burning. The erythema of patients was continuously improved at 3 and 9 months after treatment. A few patients experienced recurrence of flushing symptoms, but with lower severity than before treatment. In another study, esthetic therapy combined with BTX-A was used to treat intractable telangiectasia and papular pustular rosacea. The effect was significant, but the treatment had to be repeated after 4–5 months [12], which is consistent with the treatment effect in this study, although the cost was high.
Facial flushing, telangiectasia, and associated tingling and burning sensation of rosacea are signs of active nerve function. Drummond [21] believed that the activation of pain nerve fibers increases skin sensitivity, and axonal reflex increases facial flushing in patients with rosacea. Skin blood flow is increased through the sympathetic cholinergic nerves and the release of one or more signal transmitters of acetylcholine [22]. Vasoactive intestinal peptide and pituitary adenylate cyclase activating peptide exist in the skin as active transmitters for vasodilation and can be co-located with acetylcholine. Considering this pathogenesis and the role of BTX-A, BTX-A plays a corresponding therapeutic role and fundamentally alleviates the symptoms of these patients with ETR.
We also introduce herein some suggestions regarding the treatment and application of BTX-A in patients with severe erythema and obvious flushing. Especially for patients in whom topical and systemic treatment is ineffective, intradermal injection of BTX-A alone in erythema area can be considered. The duration of the efficacy of BTX-A varies from person to person. Therefore, the duration of treatment is generally determined according to the wishes and expectations of patients. However, we learned through WeChat that the onset time of efficiency is about 14 days. Two of the 16 patients received a second injection at 5–6 months, while the rest also showed a significant decline in treatment efficacy, so the appropriate time for repeat treatment should be about 5–6 months.
Although there were some adverse effects (AEs) during treatment in this study, all the AEs were mild and also self-limited. In our study, three patients felt tightness in the injection area, but the symptoms disappeared 1 month later. One patient had a slightly asymmetric facial expression. This symptom disappeared after 1 month. The AEs were related to the large area and the total dosage of BTX-A injection, as well as the depth of injection. Individual injection points may extend into the muscle layer, causing tightness and asymmetric expression. Five patients had recurrent symptoms, but the erythema and flushing symptoms were reduced compared with baseline.
In our pilot tests, three different doses (0.5 U, 0.8 U, or 1 U) were selected for each point injection: (1) with 0.5 U at each point, the erythema and flushing improved poorly and the efficacy lasted for a shorter time; (2) with 1 U at each point, the cheek tightness and facial stiffness were obvious, and patient satisfaction was poor; (3) with 0.8 U at each point, the effect and satisfaction were better. Therefore, in our study, 0.05 ml (0.8 U) of BTX-A solution was injected at each point with a total dose of 40–60 U of BTX-A per patient. In previous studies, 1 U of BTA was injected intradermally per injection point, with a total dose of 30 units per session [10]; 0.5 U of BTX was administered intracutaneously in the hypervascular and telangiectatic centrofacial face with 0.5 cm spacing, but repeat sessions of BTX mesotherapy are required once every 4–5 months to maintain remission [12]. Our results are basically consistent with those studies.
The limitations of the current study include its small sample size and short follow-up period. Considering the lack of a unified standard for the injection dose, the clinical application of BTX-A for the treatment of erythema and flushing in ETR is still limited. Future research with larger sample sizes and long-term follow-up should determine the injection dose, injection frequency, efficacy duration, and safety and effectiveness of BTX-A for the treatment of facial erythema and flushing.
Conclusions
BTX-A alone can achieve the same efficacy as BTX-A combined with phototherapy. This is a safe and effective method for the treatment of persistent erythema and flushing in ETR.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the participants of the study.
Funding
This study was supported by the Natural Science Basic Research Program of Shaanxi (Program No. 2021JM278). The journal’s Rapid Service Fee was partially funded by the Natural Science Basic Research Program of Shaanxi (Program No. 2021JM278) and partially by the authors.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Rongli Yang, Chang Liu, Wenli Liu, Jintian Luo. The first draft of the manuscript was written by Dr Xin Mu. The manuscript was revised by Shaoli Cheng and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Rongli Yang, Chang Liu, Wenli Liu, Jintian Luo, Shaoli Cheng and Xin Mu have nothing to disclose.
Compliance with Ethics Guidelines
All subjects provided written informed consent to participate in the study, which was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Photographs were published with the consent of the patients. The study was approved by the Ethics Committee of The First Affiliated Hospital of Xi’an Jiaotong University (No. KYLLSL-2022-227).
Data Availability
The data that support the findings of this study are available from the corresponding author on reasonable request and with permission of the study’s sponsor.
Footnotes
The original online version of this article was revised: Error on Fig. 5 caption updated.
Change history
9/13/2022
A Correction to this paper has been published: 10.1007/s13555-022-00798-8
References
- 1.Gether L, et al. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179(2):282–289. doi: 10.1111/bjd.16481. [DOI] [PubMed] [Google Scholar]
- 2.Schaller M, et al. Recommendations for rosacea diagnosis, classification and management: update from the global ROSacea COnsensus 2019 panel. Br J Dermatol. 2020;182(5):1269–1276. doi: 10.1111/bjd.18420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huynh TT. Burden of disease: the psychosocial impact of rosacea on a patient's quality of life. Am Health Drug Benefits. 2013;6(6):348–354. [PMC free article] [PubMed] [Google Scholar]
- 4.Hofmann MA, Lehmann P. Physical modalities for the treatment of rosacea. J Dtsch Dermatol Ges. 2016;14(Suppl 6):38–43. doi: 10.1111/ddg.13144. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, et al. A decade retrospective study of light/laser devices in treating nasal rosacea. J Dermatolog Treat. 2020;31(1):84–90. doi: 10.1080/09546634.2019.1580669. [DOI] [PubMed] [Google Scholar]
- 6.van Zuuren EJ, et al. Interventions for rosacea based on the phenotype approach: an updated systematic review including GRADE assessments. Br J Dermatol. 2019;181(1):65–79. doi: 10.1111/bjd.17590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang H, Tang K, Wang Y, Fang R, Sun Q. Use of botulinum toxin in treating rosacea: a systematic review. Clin Cosmet Investig Dermatol. 2021;30(14):407–417. doi: 10.2147/CCID.S307013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scala J, Vojvodic A, Vojvodic P, Vlaskovic-Jovicevic T, Peric-Hajzler Z, Matovic D, Dimitrijevic S, Vojvodic J, Sijan G, Stepic N, Wollina U, Tirant M, Thuong NV, Fioranelli M, Lotti T. Botulin toxin use in rosacea and facial flushing treatment. Open Access Maced J Med Sci. 2019;7(18):2985–2987. doi: 10.3889/oamjms.2019.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bloom BS, et al. Impact of intradermal abobotulinumtoxinA on facial erythema of rosacea. Dermatol Surg. 2015;41(Suppl 1):S9–16. doi: 10.1097/DSS.0000000000000277. [DOI] [PubMed] [Google Scholar]
- 10.Eshghi G, Khezrian L, Alirezaei P. Botulinum toxin A in treatment of facial flushing. Acta Med Iran. 2016;54(7):454–457. [PubMed] [Google Scholar]
- 11.Al-Niaimi F, Glagoleva E, Araviiskaia E. Pulsed dye laser followed by intradermal botulinum toxin type-A in the treatment of rosacea-associated erythema and flushing. Dermatol Ther. 2020;33(6):e13976. doi: 10.1111/dth.13976. [DOI] [PubMed] [Google Scholar]
- 12.Bharti J, Sonthalia S, Jakhar D. Mesotherapy with botulinum toxin for the treatment of refractory vascular and papulopustular rosacea. J Am Acad Dermatol. 2018 doi: 10.1016/j.jaad.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Friedman O, et al. The toxic edge-A novel treatment for refractory erythema and flushing of rosacea. Lasers Surg Med. 2019;51(4):325–331. doi: 10.1002/lsm.23023. [DOI] [PubMed] [Google Scholar]
- 14.Gallo RL, et al. Standard classification and pathophysiology of rosacea: the 2017 update by the National Rosacea Society Expert Committee. J Am Acad Dermatol. 2018;78(1):148–155. doi: 10.1016/j.jaad.2017.08.037. [DOI] [PubMed] [Google Scholar]
- 15.Tan J, et al. Reliability of clinician erythema assessment grading scale. J Am Acad Dermatol. 2014;71(4):760–763. doi: 10.1016/j.jaad.2014.05.044. [DOI] [PubMed] [Google Scholar]
- 16.Norquist JM, et al. Validation of a questionnaire to assess niacin-induced cutaneous flushing. Curr Med Res Opin. 2007;23(7):1549–1560. doi: 10.1185/030079907X199637. [DOI] [PubMed] [Google Scholar]
- 17.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 18.Ballan A, Nasr M, Jabbour S. An unusual tell sign of botulinum toxin injection in patients with facial flushing: incorporating a new questionnaire in the evaluation of botulinum toxin patients. J Cosmet Laser Ther. 2021;23(1–2):24–25. doi: 10.1080/14764172.2021.1957114. [DOI] [PubMed] [Google Scholar]
- 19.Wilkin JK. The red face: flushing disorders. Clin Dermatol. 1993;11(2):211–223. doi: 10.1016/0738-081X(93)90057-J. [DOI] [PubMed] [Google Scholar]
- 20.Gu H, et al. Guidelines for the diagnosis and treatment of rosacea in China (2021 edition). Int J Dermatol Venereol. 2021;4.
- 21.Drummond PD, Su D. Endothelial and axon reflex vasodilatation to acetylcholine in rosacea-affected skin. Arch Dermatol Res. 2012;304(2):133–137. doi: 10.1007/s00403-011-1177-1. [DOI] [PubMed] [Google Scholar]
- 22.Kellogg DJ, et al. Nitric oxide and receptors for VIP and PACAP in cutaneous active vasodilation during heat stress in humans. J Appl Physiol. 2012;113(10):1512–1518. doi: 10.1152/japplphysiol.00859.2012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request and with permission of the study’s sponsor.