Abstract
Background
Surgical resection for early‐stage non‐small cell lung cancer (NSCLC) offers the best chance of cure, but it is associated with a risk of postoperative pulmonary complications. It is unclear if preoperative exercise training, and the potential resultant improvement in exercise capacity, may improve postoperative outcomes. This review updates our initial 2017 systematic review.
Objectives
1. To evaluate the benefits and harm of preoperative exercise training on postoperative outcomes, such as the risk of developing a postoperative pulmonary complication and the postoperative duration of intercostal catheter, in adults scheduled to undergo lung resection for NSCLC.
2. To determine the effect on length of hospital stay (and costs associated with postoperative hospital stay), fatigue, dyspnoea, exercise capacity, lung function and postoperative mortality.
Search methods
We used standard, extensive Cochrane search methods. The latest search date was from 28 November 2016 to 23 November 2021.
Selection criteria
We included randomised controlled trials (RCTs) in which study participants who were scheduled to undergo lung resection for NSCLC were allocated to receive either preoperative exercise training or no exercise training.
Data collection and analysis
We used standard Cochrane methods. Our primary outcomes were 1. risk of developing a postoperative pulmonary complication; 2. postoperative duration of intercostal catheter and 3. safety. Our secondary outcomes were 1. postoperative length of hospital stay; 2. postintervention fatigue; 3. postintervention dyspnoea; 4. postintervention and postoperative exercise capacity; 5. postintervention lung function and 6. postoperative mortality. We used GRADE to assess the certainty of evidence for each outcome.
Main results
Along with the five RCTs included in the original version, we identified an additional five RCTs, resulting in 10 RCTs involving 636 participants. Preoperative exercise training results in a large reduction in the risk of developing a postoperative pulmonary complication compared to no preoperative exercise training (risk ratio (RR) 0.45, 95% CI 0.33 to 0.61; I2 = 0%; 9 studies, 573 participants; high‐certainty evidence). The evidence is very uncertain about its effect on postoperative intercostal catheter duration (MD −2.07 days, 95% CI −4.64 to 0.49; I2 = 77%, 3 studies, 111 participants; very low‐certainty evidence). Preoperative exercise training is likely safe as studies reported no adverse events. Preoperative exercise training likely results in a reduction in postoperative length of hospital stay (MD −2.24 days, 95% CI −3.64 to −0.85; I2 = 85%; 9 studies, 573 participants; moderate‐certainty evidence). Preoperative exercise training likely increases postintervention exercise capacity measured by peak oxygen consumption (MD 3.36 mL/kg/minute, 95% CI 2.70 to 4.02; I2 = 0%; 2 studies, 191 participants; moderate‐certainty evidence); but the evidence is very uncertain about its effect on postintervention exercise capacity measured by the 6‐minute walk distance (MD 29.55 m, 95% CI 12.05 to 47.04; I2 = 90%; 6 studies, 474 participants; very low‐certainty evidence). Preoperative exercise training may result in little to no effect on postintervention lung function (forced expiratory volume in one second: MD 5.87% predicted, 95% CI 4.46 to 7.28; I2 = 0%; 4 studies, 197 participants; low‐certainty evidence).
Authors' conclusions
Preoperative exercise training results in a large reduction in the risk of developing a postoperative pulmonary complication compared to no preoperative exercise training for people with NSCLC. It may also reduce postoperative length of hospital stay, and improve exercise capacity and lung function in people undergoing lung resection for NSCLC. The findings of this review should be interpreted with caution due to risk of bias. Research investigating the cost‐effectiveness and long‐term outcomes associated with preoperative exercise training in NSCLC is warranted.
Keywords: Adult; Humans; Carcinoma, Non-Small-Cell Lung; Carcinoma, Non-Small-Cell Lung/surgery; Dyspnea; Fatigue; Forced Expiratory Volume; Lung Neoplasms; Lung Neoplasms/surgery; Postoperative Complications; Postoperative Complications/epidemiology
Plain language summary
Exercise training before lung surgery in people with non‐small cell lung cancer
Review questions
What is the benefit of exercise undertaken before surgery for lung cancer and how safe is exercise at this time?
Background
Lung surgery for non‐small cell lung cancer offers people a chance of cure; however, lung surgery is associated with a risk of complications. Exercise training before surgery, through its improvement in fitness, may reduce the risk of lung complications and improve other outcomes, such as number of days people need a chest drain (a plastic tube inserted into the chest to drain off fluid or air that might be collecting after the operation), and length of hospital stay. In the 2017 version of this review, we found that exercise training was associated with a reduced risk of developing lung complications after surgery, shorter time people needed a chest drain, shorter hospital stay, and improved fitness and lung function before surgery. However, the quality of evidence was low.
Study characteristics
The evidence is current to November 2021. This review included data from 636 people in 10 studies.
Key results
Exercise training for people with lung cancer before surgery results in a large reduction (55%) in their risk of developing a lung complication after surgery compared to people who do no exercise before surgery. There were no side effects reported during exercise. Exercise before surgery is likely safe. Preoperative exercise likely reduces length of hospital stay after surgery (by about two days) and increases fitness levels upon completion of the exercise programme. The evidence is very uncertain for its effects on chest drain time.
Quality of the evidence
The overall quality of evidence ranged from very low to high, mainly because of limitations in the studies' methods, the small number of participants in the included studies and variability in the results.
Summary of findings
Summary of findings 1. Summary of findings table ‐ Preoperative exercise training compared to no exercise training for people scheduled to undergo lung resection for non‐small cell lung cancer.
| Preoperative exercise training compared to no exercise training for people scheduled to undergo lung resection for non‐small cell lung cancer | ||||||
| Patient or population: people scheduled to undergo lung resection for non‐small cell lung cancer Setting: USA, China, Brazil, Turkey, Italy, Spain and Switzerland Intervention: preoperative exercise training Comparison: no exercise training | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no exercise training | Risk with preoperative exercise training | |||||
| Risk of developing a postoperative pulmonary complication – total | 35 per 100 | 16 per 100 (12 to 21) | RR 0.45 (0.33 to 0.61) | 573 (9 RCTs) | ⊕⊕⊕⊕ Higha,b | Preoperative exercise training results in large reduction in risk of developing a postoperative pulmonary complication. |
| Postoperative intercostal catheter duration | The mean postoperative intercostal catheter duration ranged from 3.33 to 8.8 days | MD 2.07 days lower (4.64 lower to 0.49 higher) | ‐ | 111 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,c,d | The evidence is very uncertain about the effect of preoperative exercise training on postoperative intercostal catheter duration. |
| Safety of the intervention assessed with: number of adverse events related to the intervention assessed postintervention (preoperative) | No adverse events reported in all 3 studies | 188 (3 RCTs) | ⊕⊕⊕⊝ Moderatea,b,e | Preoperative exercise training is likely safe. | ||
| Postoperative length of hospital stay | The mean postoperative length of hospital stay ranged from 3.75 to 12.2 days | MD 2.24 days lower (3.64 lower to 0.85 lower) | ‐ | 573 (9 RCTs) | ⊕⊕⊕⊝ Moderatea,b,f | Preoperative exercise training likely results in a reduction in postoperative length of hospital stay. |
| Postintervention (preoperative) exercise capacity assessed with peak oxygen consumption | The mean postintervention (preoperative) exercise capacity assessed with peak oxygen consumption ranged from 14.5 to 19.0 mL/kg/minute | MD 3.36 mL/kg/minute higher (2.7 higher to 4.02 higher) | ‐ | 191 (2 RCTs) | ⊕⊕⊕⊝ Moderatea,b,g | Preoperative exercise training likely increases postintervention (preoperative) exercise capacity (peak oxygen consumption). |
| Postintervention (preoperative) exercise capacity assessed with 6‐minute walk distance | The mean postintervention (preoperative) exercise capacity assessed with 6‐minute walk distance ranged from 335 to 557 metres | MD 29.55 metres higher (12.05 higher to 47.04 higher) | ‐ | 474 (6 RCTs) | ⊕⊝⊝⊝ Very lowa,h,i | The evidence is very uncertain about the effect of preoperative exercise training on postintervention (preoperative) exercise capacity (6‐minute walk distance). |
| Postintervention (preoperative) forced expiratory volume in 1 second | The mean postintervention (preoperative) forced expiratory volume in 1 second ranged from 57.5 to 90.5 % predicted | MD 5.87 % predicted higher (4.46 higher to 7.28 higher) | ‐ | 197 (4 RCTs) | ⊕⊕⊝⊝ Lowa,j | Preoperative exercise training may result in little to no difference in postintervention (preoperative) lung function (forced expiratory volume in 1 second). |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_429907492607292218. | ||||||
a Serious risk of bias: the proportion of information from studies at high risk of bias was sufficient to affect the interpretation of results – downgraded one level. b Large magnitude of effect – upgraded one level. c Some inconsistency exists: there was little overlap of confidence intervals associated with the effect estimates and statistical tests suggest there was substantial heterogeneity (I2 = 77%) – downgraded one level. d Some imprecision exists: sample size (n = 111) was not large enough to calculate a precise effect estimate; and the 95% confidence interval around the estimate of effect included both appreciable benefit and harm – downgraded one level. e Some imprecision exists: sample size (n = 188) was not large enough to calculate a precise effect estimate – downgraded one level. f Some inconsistency exists: statistical tests suggest there was considerable heterogeneity (I2 = 85%) – downgraded one level. g Some imprecision exists: sample size (n = 191) was not large enough to calculate a precise effect estimate – downgraded one level. h Some inconsistency exists: statistical tests suggest there was considerable heterogeneity (I2 = 90%) – downgraded one level. i Some imprecision exists: the 95% confidence interval around the estimate of effect included both little or no effect and appreciable benefit based on the minimal important difference of 22 to 42 metres – downgraded one level. j Some imprecision existed: sample size (n = 197) was not large enough to calculate a precise effect estimate – downgraded one level.
Background
Description of the condition
Lung cancer is the leading cause of cancer death worldwide (Ferlay 2021). Despite improvements in the medical treatment of lung cancer over recent decades, the five‐year survival rate remains poor, at approximately 19% to 21% (AIHW 2019; SEER 2018). Lung cancer is the second most commonly diagnosed cancer worldwide (Ferlay 2021), and non‐small cell lung cancer (NSCLC) accounts for 85% of cases (Siegel 2019).
Surgical resection of the tumour provides the best chance of cure for NSCLC (Rosen 2016), with five‐year survival rates for people with localised disease at approximately 60% (SEER 2018). Lung resection is suitable for people with early‐stage disease, and those with sufficient cardiopulmonary reserve to withstand the surgery (NCCN 2021). International clinical practice guidelines recommend that patients undergo routine preoperative evaluation, consisting of lung function tests and additional exercise tests, if forced expiratory volume in one second (FEV1) or diffusing capacity of the lung for carbon monoxide are reduced (Brunelli 2013). For people assessed to be unfit for surgery, or those with advanced disease, alternative treatments include chemotherapy, radiotherapy, targeted agents, immunotherapy or a combination (NCCN 2021). Although lung resection offers a chance of cure, it also results in an immediate insult to the cardiorespiratory system. There is a known immediate reduction in peak oxygen consumption (VO2peak) of approximately 12% postlobectomy and 18% postpneumonectomy (Brunelli 2009). Postoperative pulmonary complications are common. These include: respiratory failure (such as prolonged mechanical ventilation, re‐intubation, or acute respiratory distress syndrome), pneumonia, atelectasis requiring bronchoscopy, lung emboli, myocardial infarction and arrhythmias (Benzo 2007). The incidence of postoperative pulmonary complications is higher in people treated with an open thoracotomy approach (4% to 15%) than minimally invasive video‐assisted thoracic surgery (VATS) (2%) (Agostini 2010; Lugg 2016; McKenna 2006; Reeve 2010). Lower VO2peak, poorer performance on field walking tests such as the six‐minute walk test (6MWT), and lower levels of physical activity preoperatively are associated with a higher risk of postoperative complications or postoperative mortality, or both (Billé 2021; Voorn 2021). Other independent risk factors for the development of postoperative pulmonary complications after lung resection include: age over 75 years, body mass index over 30 kg/m², a diagnosis of chronic obstructive pulmonary disease (COPD) and being a current smoker (Agostini 2010; Lugg 2016). Postoperative pulmonary complications following lung resection are associated with longer length of hospital stay, higher rate of intensive care unit admissions, higher 30‐day readmissions and reduced overall survival (Lugg 2016); hence, prevention is important.
People with lung cancer experience a high disease burden, physical hardship and morbidity over the disease trajectory. The adverse physical and psychological impairments in lung cancer occur as a result of multiple processes, including the disease, the cancer treatment, and individual patient factors such as multiple comorbidities and a history of poor lifestyle behaviours (Jones 2009; Schmitz 2010). Common symptoms in lung cancer include dyspnoea, cough, fatigue and pain; these commonly occur as complex symptom clusters, and are particularly debilitating to the patient (Cooley 2000; Hung 2011; Pan 2012). Most (85% to 90%) cases of lung cancer are caused by voluntary or involuntary exposure to cigarette smoke (NCCN 2021), and not surprisingly, 40% to 70% of people also have COPD (Dela Cruz 2011). Many people have a history of sedentary behaviour. At time of diagnosis, prior to treatment, people with lung cancer are generally worse than their healthy, age‐matched peers in physical activity levels, exercise capacity, muscle strength and health‐related quality of life (HRQoL) (Coups 2009; Granger 2014; Novoa 2009). Following diagnosis and treatment, the subsequent vicious cycle of inactivity and functional decline is common (Granger 2014; Novoa 2009). Activity limitations, participation restrictions* and reduced HRQoL commonly ensue (Cavalheri 2015; Hung 2011; Pan 2012; Schmitz 2010; Tanaka 2002).
Description of the intervention
Exercise training is the intervention in this review. Exercise training is "a subset of physical activity that is planned, structured, and repetitive, and has as a final or an intermediate objective, the improvement or maintenance of physical fitness" (Caspersen 1985). This includes aerobic training, resistance training or a combination of these with or without inspiratory muscle training. Exercise training was not commonly prescribed in the preoperative management of people with NSCLC (Cavalheri 2013), possibly due to uncertain evidence of its feasibility and effectiveness (Cavalheri 2020). However, the evidence for the effectiveness of preoperative exercise training in people with NSCLC has substantially grown since the early 2010s. Our original Cochrane Review in 2017 demonstrated initial evidence (low quality) that preoperative exercise training may reduce the risk of postoperative pulmonary complications, intercostal catheter duration and length of hospital stay, and may improve preoperative exercise capacity and forced vital capacity (FVC) (Cavalheri 2017).
How the intervention might work
Postulated mechanisms linking exercise with improved survival in lung cancer include: the modulation of circulating metabolic and sex‐steroid hormone concentrations, immune surveillance, and reduced systemic inflammation and oxidative damage (McTiernan 2008). Further, exercise training is standard clinical practice for people with many other chronic respiratory diseases, as part of their pulmonary rehabilitation (McCarthy 2015; Spruit 2013). Exercise training, the cornerstone of pulmonary rehabilitation programmes, includes aerobic and resistance training, delivered in a supervised environment. For people with COPD, it has been demonstrated to improve exercise capacity, HRQoL, dyspnoea and fatigue (McCarthy 2015). Given many similar features between COPD and lung cancer, and the common co‐occurrence of these two conditions, it is possible that exercise training may result in similar outcomes for those undergoing lung resection for NSCLC.
Why it is important to do this review
The original version of this review in 2017 was undertaken to evaluate the effects of preoperative exercise training in adults scheduled to undergo lung resection for NSCLC, and to identify the strengths and limitations of the published studies in this area and gaps in the literature (Cavalheri 2017). The original review included five randomised controlled trials (RCTs) and, in addition to demonstrating the effects of the intervention on preoperative and postoperative clinical and patient‐related outcomes, we also suggested the direction of future research by mapping the evidence gaps, and highlighting areas of critical limitations. Some suggestions for upcoming studies were to: 1. investigate the effect of preoperative exercise training on mortality, and the cost‐effectiveness of the intervention; 2. minimise bias by undertaking intention‐to‐treat analysis, attempting to blind participants, improving the reporting of attrition and reporting full outcome data; and 3. adding longer‐term follow‐up measures. In the current review, we investigated whether our suggestions have helped inform the methodology of new RCTs and whether the certainty of the evidence for the effectiveness of preoperative exercise training in adults scheduled to undergo lung resection for NSCLC has improved.
Objectives
1. To evaluate the benefits and harm of preoperative exercise training on postoperative outcomes, such as the risk of developing a postoperative pulmonary complication and the postoperative duration of intercostal catheter, in adults scheduled to undergo lung resection for NSCLC.
2. To determine the effect on length of hospital stay (and costs associated with postoperative hospital stay), fatigue, dyspnoea, exercise capacity, lung function and postoperative mortality.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs of preoperative exercise training compared with no exercise training for people with NSCLC. We considered studies published in any language.
Types of participants
We included studies with participants who were scheduled to undergo lung resection for NSCLC. We included lung resection of any extent, that is, wedge resection, segmentectomy, lobectomy, bi‐lobectomy or pneumonectomy. We also included studies with participants who underwent both VATS or open thoracotomy (or both).
Types of interventions
Preoperative exercise training was the experimental intervention and was compared to no preoperative exercise training (usual care). We included studies if the intervention group received a minimum of seven exercise sessions completed over a minimum of one week in the preoperative setting. We set up this short arbitrary cut‐off point because long exercise programmes are unlikely to be conducted, because of concerns from both patients and multidisciplinary medical teams related to delaying lung resection for long periods of time following the diagnosis of cancer (Benzo 2011; Morano 2013). The exercise sessions could be supervised, unsupervised, or both, and include aerobic, resistance or respiratory muscle training, or a combination. We recorded specific details of the exercise programme, including type of exercise, setting of exercise, supervision, frequency, duration, monitoring and safety.
Types of outcome measures
Our primary and secondary outcome measures are described below.
Primary outcomes
Risk of developing a postoperative pulmonary complication (i.e. pneumonia (new infiltrate coupled with either fever (greater than 38 ºC) and purulent secretions, or fever and white cell count greater than 11,000), bronchopleural fistula, severe atelectasis that required chest physiotherapy, or bronchoscopy and prolonged mechanical ventilation (greater than 48 hours)).
Number of days participants needed an intercostal catheter following surgery.
Safety of the intervention as measured by numbers of adverse events postintervention (preoperative).
Secondary outcomes
Postoperative length of hospital stay and costs associated with postoperative hospital stay.
Postintervention (preoperative) fatigue (e.g. the Functional Assessment of Chronic Illness Therapy – Fatigue Subscale).
Postintervention (preoperative) dyspnoea (e.g. the BORG scale or Medical Research Council scale).
Postintervention (preoperative) and postoperative exercise capacity (e.g. six‐minute walk distance (6MWD), performance during the stair climbing test, maximum work rate (Wmax), or VO2peak).
Postintervention (preoperative) lung function (e.g. volumes – FEV1 and FVC, flows and diffusing capacity).
Postoperative mortality.
Search methods for identification of studies
Electronic searches
We searched the following databases to identify RCTs:
Central Register of Controlled Trials (CENTRAL) (Cochrane Library Issue 11, 2021; searched 23 November 2021);
MEDLINE (PubMed; 28 November 2016 to 23 November 2021);
Embase (www.embase.com; 28 November 2016 to 23 November 2021);
PEDro (Physiotherapy Evidence database; 28 November 2016 to 23 November 2021); and
SciELO (the Scientific Electronic Library Online; 28 November 2016 to 23 November 2021).
We listed the search terms and strategies used to search for studies using the CENTRAL, MEDLINE and Embase in Appendix 1, Appendix 2, and Appendix 3. The MEDLINE search string was developed according to the Cochrane Highly Sensitive Search Strategy, sensitivity‐maximising version as referenced in Chapter 6.4.11.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We adapted the strategy for Embase. We also adapted both the terms and the strategies for use in PEDro and SciELO. We placed no restrictions on language. Date restrictions were placed to search databases from the date of our last search in our original review (Cavalheri 2017).
Searching other resources
In the original review, we screened reference lists of all RCTs included in the review; contacted experts in the field for additional references; and handsearched abstracts from the Thoracic Society of Australia and New Zealand, European Respiratory Society and American Thoracic Society scientific meetings (2010 to March 2016).
For this update, we screened the reference lists of all RCTs included in the review to search for other sources.
Data collection and analysis
Selection of studies
The two review authors independently examined the studies identified in the literature search using Covidence (Covidence). First, we excluded studies based on their title and abstract and recorded the reason for exclusion. Subsequently, the two review authors independently examined the full text of the remaining studies and coded them as 'include', 'unclear' or 'exclude', based on the review criteria. We discussed and resolved studies coded as 'unclear' and any disagreements by consensus and kept a full record of the decisions. We listed studies excluded at this stage in the Characteristics of excluded studies table. We attempted to contact authors of any potential overlapping reports from a study to avoid multiple counts of the study.
Data extraction and management
The two review authors independently extracted data from the included studies using a standardised form. We resolved any discrepancies by consensus. We attempted to contact authors of the included studies to provide any missing data detected during the process. During online meetings, one of the review authors (VC) then entered data into Review Manager Web whilst being guided by the other review author (CG) (Review Manager Web 2022).
Assessment of risk of bias in included studies
Two review authors independently appraised the risk of bias of the included studies using the Cochrane 'seven evidence‐based domains' tables. We resolved disagreements by consensus. We judged risk of bias as high, low or unclear for selection bias (i.e. random sequence generation and allocation concealment), performance bias (i.e. blinding of participants and personnel), detection bias (i.e. blinding of outcome assessor), attrition bias (i.e. incomplete outcome data), reporting bias (i.e. selective outcome reporting) and other potential sources of bias. The judgement was accompanied by a direct quote, specific details of the study, or both, in the risk of bias table. We contacted study authors, where applicable, to seek clarification on issues regarding bias. We also contacted authors of unpublished studies to provide us with information pertaining to bias, and we added notes in the risk of bias table. We generated both the risk of bias graph (i.e. bar chart) and the risk of bias summary (i.e. traffic lights).
Measures of treatment effect
For the primary outcome 'risk of developing a postoperative pulmonary complication', we used the risk ratio (RR). We also used the risk difference (RD), in order to calculate the number needed to treat for an additional beneficial outcome (NNTB). For continuous outcomes, we used the mean difference (MD). We calculated 95% confidence intervals (CIs). If studies reported median and interquartile ranges (IQR), we converted them to mean and standard deviation (SD) (Wan 2014).
Unit of analysis issues
For studies that randomly allocated individual participants to study groups, we considered the participant as the unit of analysis. For cluster‐randomised studies, we intended to consider the cluster as the unit of analysis, but this approach was not required.
Dealing with missing data
We attempted to contact authors of the included studies for missing data. When our attempts to contact a study author were unsuccessful, we limited presentation of the outcome(s) of that specific study to a narrative discussion.
Assessment of heterogeneity
We assessed statistical heterogeneity across the studies using the I² statistic. We considered values of the I² statistic greater than 50% as substantial heterogeneity (Higgins 2021). If there was substantial statistical heterogeneity detected, we investigated whether clinical or methodological heterogeneity were the potential causes. If there was substantial statistical heterogeneity in meta‐analyses, we undertook sensitivity analyses.
Assessment of reporting biases
We searched online trial registries to investigate potential publication bias and to assess potential outcome reporting bias in the included studies.
Data synthesis
We used Review Manager Web for statistical analyses and to generate forest plots (Review Manager Web 2022). For studies published by the same research group that used the same sample of participants, we only included data from one of the published studies in meta‐analyses. We meta‐analysed the results of studies using the inverse variance DerSimonian and Laird method (DerSimonian 1986). We analysed pooled data using a random‐effects model and if, the studies did not have substantial heterogeneity, applied a fixed‐effect model. For I² values ranging between 50% and 75%, data aggregation was kept if the magnitude and direction of the studies' effects were not conflicting. Where data aggregation was not possible, due to clinical, methodological or statistical heterogeneity, we used narrative discussion. We checked skewness of data for the outcomes number of days participants needed an intercostal catheter postoperative and length of hospital stay by calculating the observed mean minus the lowest possible value, and dividing this by the SD. A ratio less than two was used to define skewed data (Altman 1996). A ratio less than one was used to define strong evidence of a skewed distribution.
Subgroup analysis and investigation of heterogeneity
Where possible, we had planned to conduct subgroup analysis for the primary outcomes to evaluate the effect of the intervention in the following groups: 1. different exercise training regimens (e.g. aerobic versus resistance training; or varying exercise training programme duration); 2. extent of lung resection (e.g. lobectomy versus pneumonectomy); 3. type of surgical approach (e.g. open thoracotomy versus VATS); 4. stage of NSCLC (e.g. stage I NSCLC versus stage II NSCLC) and 5. comorbidities (e.g. participants diagnosed with COPD versus participants not diagnosed with COPD, or participants with coronary artery disease versus participants without coronary artery disease). Approaches 2, 3 and 5 were not required. We used the formal test for subgroup interactions in Review Manager Web (Review Manager Web 2022).
We assessed heterogeneity and the extent of inconsistency between studies by visual inspection of the forest plots, and using the Chi² test, and the I² statistic.
Sensitivity analysis
We performed sensitivity analyses where we found significant heterogeneity amongst the studies. We investigated the effects of methodological differences or of data that we had to calculate (i.e. calculation of mean and SD based on median and IQR reported in the include RCTs) on the results.
Summary of findings and assessment of the certainty of the evidence
In order to interpret the findings, we created a GRADE summary of findings table (Atkins 2004; Guyatt 2008) including the outcomes 1. risk of developing a postoperative pulmonary complication; 2. number of days participants needed an intercostal catheter; 3. safety of the intervention; 4. postoperative length of hospital stay; 5. preoperative exercise capacity (6MWD); 6. preoperative exercise capacity (VO2peak); and 7. FEV1. We used both the 'summary of findings' screen for numerical data and the 'quality assessment' screen to grade the evidence. We assessed the certainty of the evidence for each outcome by downgrading or upgrading the evidence according to the GRADE criteria. We used the methods and recommendations described in the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 14; Higgins 2021).
Results
Description of studies
Results of the search
We searched the databases to 23 November 2021. The search yielded 610 new records: 237 from CENTRAL; 52 from MEDLINE; 275 from Embase; 23 from PEDro and 23 from SciELO. After removing duplicates, we had 494 records. We excluded 469 based on the title and abstract. We assessed 25 full‐text articles and conference abstracts for eligibility. We excluded 13 studies: 11 did not meet the review criteria and 2 were conference abstracts (Figure 1). We included five studies (12 references) identified in this current version of the review, in addition to the five studies (eight references) identified in the original review (Cavalheri 2017); totalling 10 studies (20 references). We were able to contact the authors of four studies eligible for this review to obtain missing data (two studies in the original review and two studies for this update). We were unable to contact authors from one previous and five new included references arising from one research group; in this instance, the six references were cross‐matched with trial identification numbers, author names, location and settings, details of the interventions, number of participants and baseline data, and data and duration of the study to avoid multiple counts of data; and these six references contributed to three study counts, one in our original review (Lai 2017a) and two newly included studies (Lai 2017b; Lai 2019).
1.
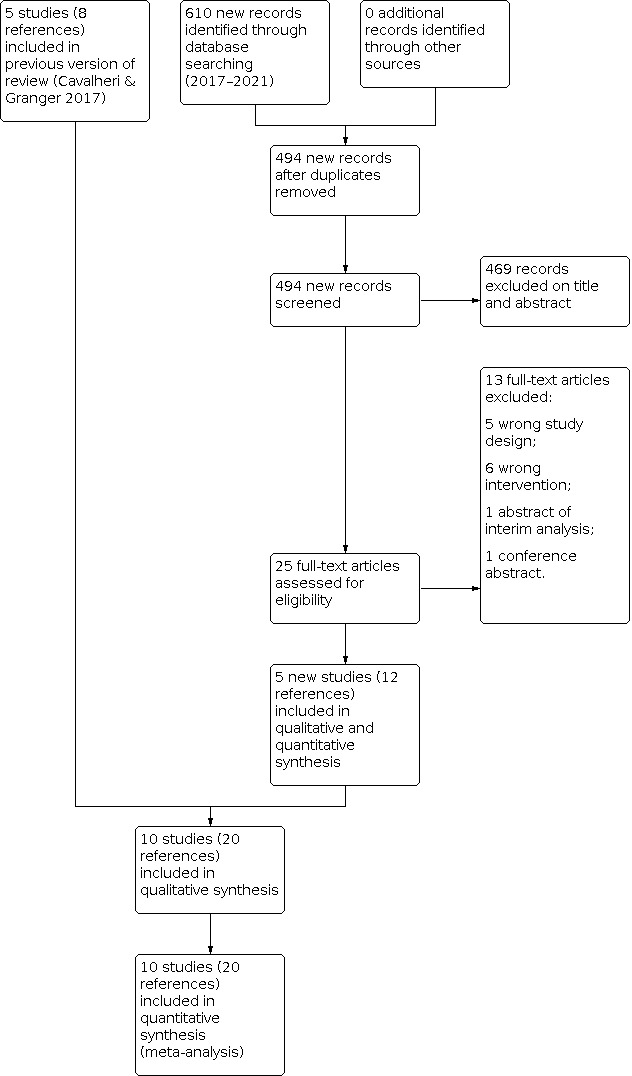
Flow diagram of references identified, excluded and included in review.
Included studies
See Characteristics of included studies table for further details.
Study
We included five RCTs (eight references) (Benzo 2011; Lai 2017a; Morano 2013; Pehlivan 2011; Stefanelli 2013) from the 2017 version of this review (Cavalheri 2017). We included five new RCTs (12 references) for this update (Garcia 2017; Lai 2017b; Lai 2019; Licker 2016; Liu 2020). Therefore, 10 RCTs (20 references), including 636 participants, contributed data to the meta‐analyses.
Population
Nine of the 10 studies only included participants with NSCLC undergoing lung resection (Garcia 2017; Lai 2017a; Lai 2017b; Lai 2019; Licker 2016; Liu 2020; Morano 2013; Pehlivan 2011; Stefanelli 2013). One study did not specify the type of lung cancer of the participants (Benzo 2011). Two studies specifically included participants with NSCLC and a diagnosis of COPD (Benzo 2011; Stefanelli 2013). Two studies specifically included participants with at least one prespecified risk factor for postoperative pulmonary complications (Garcia 2017; Lai 2017b). Three studies only included participants undergoing lung resection via VATs (Garcia 2017; Lai 2019; Liu 2020). Five studies included participants undergoing lung resection via either open thoracotomy or VATS (Benzo 2011; Lai 2017a; Lai 2017b; Licker 2016; Morano 2013). Stefanelli 2013 only included participants undergoing lung resection via open thoracotomy. One study did not specify the type of surgical technique used for the lung resection (Pehlivan 2011). The sample sizes ranged from 19 to 151, with the mean age of the participants ranging from 54 to 72.5 years.
Setting
The studies were based in Brazil (Morano 2013), China (Lai 2017a; Lai 2017b; Lai 2019; Liu 2020), Italy (Stefanelli 2013), Spain (Garcia 2017), Turkey (Pehlivan 2011), Switzerland (Licker 2016), and the USA (Benzo 2011).
Intervention
The type, frequency and intensity of the exercise programmes varied across the included studies. The frequency and duration of exercise training programmes varied from three times per day for one week (Pehlivan 2011), to five times per week for four weeks (Morano 2013). In six studies the duration of the exercise programme was two weeks or less (Benzo 2011; Lai 2017a; Lai 2017b; Lai 2019; Liu 2020; Pehlivan 2011). All 10 studies prescribed aerobic exercise training. Four studies included resistance training (Benzo 2011; Garcia 2017; Licker 2016; Liu 2020); two studies included inspiratory muscle training (Benzo 2011; Morano 2013); eight studies included breathing exercises (Benzo 2011; Garcia 2017; Lai 2017a; Lai 2017b; Lai 2019; Liu 2020; Pehlivan 2011; Stefanelli 2013); and two studies included stretches (Liu 2020; Morano 2013). The control groups received usual care with no formal exercise training. In one study, participants in the control group received instructions about lung expansion breathing techniques (Morano 2013). In another study, participants in the control group received usual care consisting of preoperative advice regarding active mobilisation (Licker 2016).
Outcomes
Nine studies reported the number of participants who developed a postoperative pulmonary complication (Benzo 2011; Garcia 2017; Lai 2017a; Lai 2017b; Lai 2019; Licker 2016; Liu 2020; Morano 2013; Pehlivan 2011). Three studies reported the number of days participants needed an intercostal catheter following surgery (Benzo 2011; Liu 2020; Morano 2013). Three studies reported data on safety of the intervention (Benzo 2011; Garcia 2017; Licker 2016). Nine studies reported postoperative length of hospital stay (Benzo 2011; Garcia 2017; Lai 2017a; Lai 2017b; Lai 2019; Licker 2016; Liu 2020; Morano 2013; Pehlivan 2011). Two of these studies also reported costs associated with the hospital stay (Lai 2017b; Lai 2019). Two studies reported postintervention fatigue (Lai 2017b; Lai 2019) and three studies reported postintervention dyspnoea on exertion (Lai 2017b; Lai 2019; Stefanelli 2013). Seven studies reported postintervention exercise capacity (Lai 2017a; Lai 2017b; Lai 2019; Licker 2016; Liu 2020; Morano 2013; Stefanelli 2013) and three studies reported postoperative exercise capacity (Garcia 2017; Liu 2020; Stefanelli 2013). Six studies reported postintervention lung function (Lai 2017a; Lai 2017b; Liu 2020; Morano 2013; Pehlivan 2011; Stefanelli 2013). Four studies reported mortality, three reported in‐hospital mortality (Lai 2019; Liu 2020; Pehlivan 2011), and one reported mortality to both 30 days and 12 months postoperative (Licker 2016).
Excluded studies
In this update, we excluded 13 records. See Characteristics of excluded studies table.
Risk of bias in included studies
One of the seven domains included in the Cochrane 'seven evidence‐based domains' table was identical across the 10 studies (blinding of participants and personnel). None of the studies reported blinding participants or personnel. Five studies reported an intention‐to‐treat analysis (Lai 2017a; Lai 2019; Licker 2016; Liu 2020; Morano 2013). See risk of bias section within the Characteristics of included studies table with summaries in Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Four studies were at low risk for random sequence generation (Garcia 2017; Lai 2017b; Lai 2019; Liu 2020). We rated one study at high risk of selection bias (random sequence generation) because their allocation was based on hospital record number (Pehlivan 2011). We judged five studies at unclear risk, since they failed to report sufficient information about the random sequence generation process to permit judgement (Benzo 2011; Lai 2017a; Licker 2016; Morano 2013; Stefanelli 2013).
Three studies were at low risk of allocation concealment (Garcia 2017; Lai 2017b; Liu 2020). We judged seven studies at unclear risk of selection bias (allocation concealment) since they failed to report sufficient information about allocation concealment to permit judgement (Benzo 2011; Lai 2017a; Lai 2019; Licker 2016; Morano 2013; Pehlivan 2011; Stefanelli 2013).
Blinding
We rated all studies at high risk of performance bias, since neither the participants nor the personnel responsible for delivering the intervention were blinded to group allocation in any of the studies. Therefore, some of our results may be influenced by a placebo effect.
Seven studies were at low risk of detection bias as they ensured blinding of outcome assessors (Benzo 2011; Garcia 2017; Lai 2017a; Lai 2017b; Lai 2019; Licker 2016; Liu 2020). Two studies did not describe blinding of outcome assessors and at unclear risk of detection bias (Pehlivan 2011; Stefanelli 2013). Postoperative outcomes were obtained by a physical therapist blinded to the treatment assignment in Morano 2013. However, it was unclear whether postintervention outcome measures were taken by a blinded assessor, therefore, we judged the risk unclear.
Incomplete outcome data
We rated six studies at low risk of attrition bias because missing outcome data were balanced in numbers between the experimental and control groups with low overall rates of missing data (Lai 2017b; Lai 2019); because missing outcome data were balanced in numbers between the intervention and control groups with similar reasons for missing data across groups (Benzo 2011; Licker 2016; Liu 2020); or because missing outcome data were balanced in numbers between the intervention and control groups and all participants successfully completed the training programme and assessments (Lai 2017a). Two studies were at unclear risk of attrition bias due to insufficient reporting of attrition and exclusions (Pehlivan 2011; Stefanelli 2013). Two studies were at high risk of attrition bias due mainly to a large loss to follow‐up despite giving reasons for the attrition (45% attrition in Garcia 2017; 25% attrition in the control group in Morano 2013).
Selective reporting
Two studies were at unclear risk of reporting bias because of insufficient information (Pehlivan 2011; Stefanelli 2013). We rated eight studies at high risk of reporting bias as reported outcomes were not all prespecified in the trial registration (Garcia 2017; Lai 2017b; Lai 2019; Liu 2020; Morano 2013); not all prespecified outcomes were reported (Lai 2017a; Licker 2016; Liu 2020; Morano 2013); the trial was registered retrospectively (Lai 2017a; Lai 2017b); and outcomes of interest were reported incompletely (Benzo 2011).
Other potential sources of bias
Four studies were at low risk as they appeared free of other sources of bias (Lai 2017a; Licker 2016; Morano 2013; Pehlivan 2011). Six studies were at high risk of other sources of bias. The prespecified sample size at registration was greater than the sample size reported in the publications (Lai 2017b; Lai 2019; Liu 2020). The inclusion criteria varied between trial register and published reports (Lai 2017a; Lai 2017b). Benzo 2011 reported findings of two studies they had undertaken; one study was stopped early due to poor recruitment. Stefanelli 2013 did not report numbers of participants allocated to each group. Garcia 2017 reported an extra assessment time point in the experimental group in the results which was not mentioned in the study methods.
Effects of interventions
See: Table 1
See: Table 1.
1. Primary outcome: risk of developing a postoperative pulmonary complication
Nine studies reported the number of participants who developed a postoperative pulmonary complication (Benzo 2011; Garcia 2017; Lai 2017a; Lai 2017b; Lai 2019; Licker 2016; Liu 2020; Morano 2013; Pehlivan 2011; Table 2). Preoperative exercise training results in a large reduction in the risk of developing a postoperative pulmonary complication compared to no preoperative exercise training. Compared to the non‐exercise group, the relative risk of developing a postoperative pulmonary complication was reduced by 55% in the exercise group (RR 0.45, 95% CI 0.33 to 0.61; I2 = 0%; 9 studies, 573 participants; high‐certainty evidence; Analysis 1.1; Figure 4). It is expected that one fewer person will develop a postoperative pulmonary complication for every five participants receiving preoperative exercise training rather than usual care (RD −0.19, 95% CI −0.26 to −0.13; NNTB 5, 95% CI 4 to 8). Subgroup analysis was conducted to investigate the effects of interventions that were two weeks or less in duration and the effects of interventions that were more than two weeks in duration. There was no difference between the subgroups (test for subgroup difference; P = 0.39; Analysis 1.1; Figure 4).
1. Results of included studies.
| Study | Results |
| Benzo 2011 |
Number of participants who developed a postoperative pulmonary complication IG: 3/9 (33%); CG: 5/8 (63%) P = 0.23 (between‐group) Number of days participants needed a chest tube IG: 4.3 (SD 2.1) days; CG: 8.8 (SD 5.3) days P = 0.03 (between‐group) Postoperative length of hospital stay IG: 6.3 (SD 3.0) days; CG: 11.0 (SD 6.3) days P = 0.058 (between‐group) |
| Garcia 2017 |
Number of participants who developed a postoperative pulmonary complication IG: 5/10 (50%); CG: 8/12 (66%) P = 0.361 (between‐group) Postoperative length of hospital stay IG: 5.5 (SD 4.85) daysa; CG: 3.75 (SD 1.9) daysa P = 0.539 (between‐group difference reported in paper calculated with median days) Exercise capacity 6MWD IG: 10 participants completed; CG: 12 participants completed Preoperative measurements: baseline; and postoperative measurements: postsurgery: mean: IG: 507.7 (SD 9) m decrease by 15.55 (SD 47.73) m; CG: 420.2 (SD 116.3) m decrease by 27.7 (SD 33.7) m P = 0.500 (between‐group) |
| Lai 2017a |
Number of participants who developed a postoperative pulmonary complication IG: 4/30 (13%); CG: 11/30 (37%) P = 0.037 (between‐group) Postoperative length of hospital stay IG: 6.9 (SD 4.4) days; CG: 10.7 (SD 6.4) days P = 0.01 (between‐group) Exercise capacity 6MWD IG: 30 participants completed; CG: 30 participants completed Preoperative measurements: baseline and postintervention: mean: IG: 431.7 (SD 102.8) m to 460.3 (SD 93.6) m; CG: 434.5 (SD 86.2) m to 443.9 (SD 88.4) m P = 0.029 (between‐group) Lung function PEF IG: 30 participants completed; CG: 30 participants completed Preoperative measurements: baseline and postintervention: IG: 351.7 (SD 132.3) L/minute to 377.8 (SD 130.5) L/minute (no P value reported); CG: 372.0 (SD 101.2) L/minute to 380.1 (SD 102.8) L/minute (no P value reported) P < 0.001 (between‐group) |
| Lai 2017b |
Number of participants who developed a postoperative pulmonary complication IG: 5/51 (10%); CG: 14/50 (28%) P = 0.019 (between‐group) Postoperative length of hospital stay IG: 6.1 (SD 3.0) days; CG: 8.7 (SD 4.6) days P = 0.001 (between‐group) Costs Cost of hospital stay IG: EUR 7550.7 (SD 1351.9); CG: EUR 8466.4 (SD 2441.2) P = 0.023 (between‐group) Medication cost IG: EUR 1235.5 (SD 564.5); CG: EUR 1817.6 (SD 1443.8) P = 0.010 (between‐group) Dyspnoea BORG scale during the 6MWT IG: 51 participants completed; CG: 50 participants completed Preoperative measurements: baseline and postintervention: mean: IG: 1.2 (SD 1.7) to 1.0 (SD 1.5) (no P value reported); CG: 1.1 (SD 0.8) to 1.2 (SD 0.6) (no P value reported) P = 0.065 (between‐group) Exercise capacity 6MWD IG: 51 participants completed; CG: 50 participants completed Preoperative measurements: baseline and postintervention: mean: IG: 476.4 (SD 102.7) m to 499.6 (SD 105.0) m (no P value reported); CG: 485.4 (SD 83.1) m to 489.6 (SD 81.4) m (no P value reported) P < 0.001 (between‐group) Lung function PEF IG: 51 participants completed; CG: 50 participants completed Preoperative measurements: baseline and postintervention: IG: 359.0 (SD 127.2) L/minute to 384.2 (SD 122) L/minute (no P value reported); CG: 381.0 (SD 90.4) L/minute to 388.0 (SD 89.7) L/minute (no P value reported) P = 0.003 (between‐group) |
| Lai 2019 |
Number of participants who developed a postoperative pulmonary complication IG: 4/34 (12%); CG: 12/34 (35%) P = 0.022 (between‐group) Postoperative length of hospital stay Median: IG: 5.0 (IQR 4.0 to 7.0) days; CG: 8.0 (IQR 7.0 to 10.0) days P < 0.001 (between‐group) Costs Cost of hospital stay IG: median: CNY 48,588.7 (IQR 44,999.1 to 52,693.3); CG: CNY 52,445.3 (IQR 49,002.9 to 61,994.0) P = 0.016 (between‐group) Medication cost IG: median medication cost: CNY 7230.0 (IQR 6661.9 to 8347.4); CG: CNY 11,388.6 (IQR 7963.0 to 16,314.3) P < 0.001 (between‐group) Exercise capacity 6MWD IG: 34 participants completed; CG: 34 participants completed Preoperative measurements: baseline and postintervention: mean: IG: 454.6 (SD 100.9) m baseline; change postintervention 22.6 (SD 27.0) m; CG: 464.4 (SD 83.0) m baseline; change postintervention 2.7 (SD 27.6) m P = 0.004 (between‐group) |
| Licker 2016 |
Number of participants who developed a postoperative pulmonary complication IG: 17/74 (23%); CG: 33/77 (43%) P = 0.018 (between‐group) Postoperative length of hospital stay IG: 9.7 (SD 3.4) daysa; CG: 10.5 (SD 4.5) daysa P < 0.05 (between‐group) Exercise capacity 6MWD IG: 74 participants completed; CG: 77 participants completed Preoperative measurements: baseline and postintervention: mean: IG: 398 (SD 167) m to 462 (SD 206) m (no P value reported); CG: 368 (SD 143) m to 362 (SD 172) m (no P value reported)a P = 0.804 (between‐group) Exercise capacity VO2peak IG: 74 participants completed; CG: 77 participants completed Preoperative measurements: baseline and postintervention: IG: 19.9 (SD 5.7) mL/kg/minute to 21.9 (SD 6.2) mL/kg/minute (no P value reported); CG: 20.4 (SD 5.7) mL/kg/minute to 19.0 (SD 5.8) mL/kg/minute (no P value reported)a P = 0.004 (between‐group) |
| Liu 2020 |
Number of participants who developed a postoperative pulmonary complication IG: 4/37 (11%); CG: 5/36 (14%) Between‐group difference not calculated for this grade of complications Number of days participants needed a chest tube Median: IG: 3 (IQR 3 to 4) days; CG: 3 (IQR 3 to 4.8) days P = 0.762 (between‐group) Postoperative length of hospital stay Median: IG: 5 (IQR 4 to 6) days; CG: 5 (IQR 4 to 6) days P = 0.973 (between‐group) Exercise capacity 6MWD IG: 37 participants completed; CG: 36 participants completed Preoperative measurements: baseline and postintervention; and postoperative measurement: 30 days postoperative: mean: IG: 564.6 (SD 67.8) m, increased by 45.1 m at postintervention (P < 0.05), and increased (from baseline) by 21.5 m at 30 days postoperative (P < 0.05); CG: 553.2 (SD 56.3) m, increased by 3.8 m at postintervention (P < 0.05), and decreased (from baseline) by 36.1 m at 30 days postoperative P < 0.001 (between‐group) Lung function FEV1 IG: 37 participants completed; CG: 36 participants completed Preoperative measurements: baseline and postintervention: IG: 90.2 (SD 15.0) % predicted to 95.6 (SD 13.6) % predicted; CG: 92.1 (SD 16.2) % predicted to 90.5 (SD 12.4) % predicted Between‐group difference was not calculated FVC IG: 37 participants completed; CG: 36 participants completed Preoperative measurements: baseline and postintervention: IG: 98.9 (SD 11.8) % predicted to 104.1 (SD 13.0) % predicted (no P value reported); CG: 96.2 (SD 14.0) % predicted to 95.1 (SD 13.7) % predicted (no P value reported) Between‐group difference was not calculated PEF IG: 37 participants completed; CG: 36 participants completed Preoperative measurements: baseline and postintervention: IG: 316.1 (SD 112.4) L/minute to 377.9 (SD 89.4) L/minute (no P value reported); CG: 335.1 (SD 106.9) L/minute to 368.3 (SD 110.1) L/minute (no P value reported) P = 0.339 (between‐group) |
| Morano 2013 |
Number of participants who developed a postoperative pulmonary complication IG: 2/12 (17%); CG: 7/9 (78%) P = 0.01 (between‐group) Number of days participants needed a chest tube IG: 4.5 (SD 2.9) days; CG: 7.4 (SD 2.6) days P = 0.03 (between‐group) Postoperative length of hospital stay IG: 7.8 (SD 4.8) days; CG: 12.2 (SD 3.6) days P = 0.04 (between‐group) Exercise capacity 6MWD IG: 12 participants completed; CG: 12 participants completed Preoperative measurements: baseline and postintervention: mean: IG: 425.5 (SD 85.3) m to 475 (SD 86.5) m (P < 0.01); CG: 339.6 (SD 107) m to 335 (SD 107) m (P > 0.05) P < 0.001 (between‐group) Lung function FEV1 IG: 12 participants completed; CG: 12 participants completed Preoperative measurements: baseline and postintervention: IG: 48.1 (SD 13.9) % predicted to 54.8 (SD 22.4) % predicted (P = 0.08); CG: 51.7 (SD 9.8) % predicted to 58.8 (SD 13.0) % predicted (P = 0.23) Between‐group difference not calculated FVC IG: 12 participants completed; CG: 12 participants completed Preoperative measurements: baseline and postintervention: median: IG: 62.5% (IQR 49 to 71) to 76% (IQR 65 to 79.7); P = 0.02; CG: 62.5% (IQR 56 to 92) to 71% (IQR 63.2 to 89); P = 0.37 Between‐group difference not calculated |
| Pehlivan 2011 |
Number of participants who developed a postoperative pulmonary complication IG: 1/30 (3%); CG: 5/30 (17%) P = 0.04 (between‐group) Postoperative length of hospital stay IG: 5.4 (SD 2.7) days; CG: 9.7 (SD 3.1) days P < 0.001 (between‐group) Lung function FEV1 IG: 30 participants completed; CG: 30 participants completed Preoperative measurements: change from baseline to postintervention: IG: 15.84 (SD 2.10) % predicted; CG: 9.92 (SD 3.5) % predicted P = 0.3 (between‐group) FVC IG: 30 participants completed; CG: 30 participants completed Preoperative measurements: baseline and postintervention: IG: 19.26 (SD 2.33) % predicted; CG: 16.3 (SD 2.4) % predicted P = 0.6 (between‐group) |
| Stefanelli 2013 |
Dyspnoea BORG scale at end of 6MWT IG: 20 participants completed; CG: 20 participants completed Preoperative measurements: baseline and postintervention: mean: IG: 1.7 (SD 2.2) to 0.9 (SD 1.0) (P < 0.05); CG: 1.9 (SD 0.6) to 1.8 (SD 0.7) (P > 0.05) Between‐group difference not calculated Exercise capacity VO2peak IG: 20 participants completed; CG: 20 participants completed Preoperative measurements: baseline and postintervention: IG: 14.9 (SD 2.3) mL/kg/minute to 17.8 (SD 2.1) mL/kg/minute (no P value reported); CG: 14.8 (SD 1.4) mL/kg/minute to 14.5 (SD 1.82) mL/kg/minute (no P value reported) P < 0.001 (between‐group) Lung function FEV1 IG: 20 participants completed; CG: 20 participants completed Preoperative measurements: baseline and postintervention: IG: 57.4 (SD 19.1) % predicted to 59.8 (SD 19.2) % predicted; CG: 57.6 (SD 16.9) % predicted to 57.5 (SD 17.0) % predicted P > 0.05 (between‐group) |
6MWD: six‐minute walk distance; CG: control group; CNY: Chinese Yuan; EUR: Euro; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; IG: intervention group (exercise); IQR: interquartile range; PEF: peak expiratory flow; SD: standard deviation; VO2peak: peak oxygen consumption.
aData provided by the study author.
1.1. Analysis.

Comparison 1: Exercise versus no exercise, Outcome 1: Risk of developing a postoperative pulmonary complication
4.

Forest plot of comparison: 1 Intervention group versus control group, outcome: 1.1 Risk of developing a postoperative pulmonary complication.
2. Primary outcome: number of days participants needed an intercostal catheter following surgery
Three studies reported the number of days participants needed an intercostal catheter following surgery (Benzo 2011; Liu 2020; Morano 2013; Table 2). The evidence is very uncertain about the effect of preoperative exercise training on postoperative intercostal catheter duration (MD −2.07 days, 95% CI −4.64 to 0.49; I2 = 77%; 3 studies, 111 participants; very low‐certainty evidence; Analysis 1.2; Figure 5). Skewness was calculated (observed mean minus the lowest possible value, divided by SD) and the result was a ratio of 0.30, showing strong evidence of a skewed distribution. Heterogeneity was substantial (I2 = 77%); therefore, we undertook sensitivity analyses. When we removed Liu 2020 (the only study that we had to calculate mean and SD based on the reported median and IQR) from the analysis, the I2 statistic reduced to 0%. Sensitivity analysis demonstrated that, compared to the non‐exercise group, the number of days participants in the exercise group needed an intercostal catheter following surgery was lower (MD −3.33 days, 95% CI −5.35 to −1.30).
1.2. Analysis.

Comparison 1: Exercise versus no exercise, Outcome 2: Number of days participants needed an intercostal catheter following surgery
5.

Forest plot of comparison: 1 Intervention group versus control group, outcome: 1.2 Number of days patients needed an intercostal catheter.
3. Primary outcome: safety of the intervention
Three studies reported data on safety of the intervention (i.e. adverse events related to the intervention) (Benzo 2011; Garcia 2017; Licker 2016; Table 2). Preoperative exercise training is likely safe. There were no adverse events in all three studies (moderate‐certainty evidence).
4. Secondary outcome: postoperative length of hospital stay
Nine studies reported postoperative length of hospital stay (Benzo 2011; Garcia 2017; Lai 2017a; Lai 2017b; Lai 2019; Licker 2016; Liu 2020; Morano 2013; Pehlivan 2011; Table 2). Preoperative exercise training likely results in a reduction in postoperative length of hospital stay (MD −2.24 days, 95% CI −3.64 to −0.85; I2 = 85%; 9 studies, 573 participants; moderate‐certainty evidence; Analysis 1.3; Figure 6). Skewness was calculated (observed mean minus the lowest possible value, divided by SD) and the result was a ratio of 0.26, showing strong evidence of a skewed distribution. Heterogeneity was substantial (I2 = 85%); therefore, we undertook sensitivity analyses. The shorter postoperative length of hospital stay in the exercise group was maintained when we excluded the two studies (Lai 2019; Liu 2020) that we had to calculate mean and SD based on the reported median and IQR (MD −2.56 days, 95% CI −4.15 to −0.97) and the I2 statistic reduced to 73%.
1.3. Analysis.

Comparison 1: Exercise versus no exercise, Outcome 3: Postoperative length of hospital stay (days)
6.

Forest plot of comparison: 1 Intervention group versus control group, outcome: 1.3 Postoperative length of hospital stay.
Two studies that reported postoperative length of hospital stay also reported the costs associated with hospital stay (Lai 2017b; Lai 2019). In Lai 2017b, both the cost associated with hospital stay and the cost associated with medication use (in Euro (EUR)) were lower in the exercise group than in the non‐exercise group (mean cost of hospital stay: EUR 7550.7 (SD 1351.9) in exercise group versus EUR 8466.4 (SD 2441.2) in non‐exercise group; P = 0.023; mean medication cost: EUR 1235.5 (SD 564.5) in exercise group versus EUR 1817.6 (SD 1443.8) in non‐exercise group; P = 0.010). Lai 2019 reported similar findings (median cost of hospital stay in Chinese Yuan (CNY): CNY 48,588.7 (IQR 44,999.1 to 52,693.3) in exercise group versus CNY 52,445.3 (IQR 49,002.9 to 61,994.0) in non‐exercise group; P = 0.016; median medication cost: CNY 7230.0 (IQR 6661.9 to 8347.4) in exercise group versus CNY 11,388.6 (IQR 7963.0 to 16,314.3); P < 0.001).
5. Secondary outcome: postintervention (preoperative) fatigue
There were no data for postintervention fatigue.
6. Secondary outcomes: postintervention (preoperative) dyspnoea
Two studies reported postintervention dyspnoea on exertion measured using the BORG scale during (Lai 2017b) or after (Stefanelli 2013) the 6MWT (Table 2). The evidence is very uncertain about the effect of preoperative exercise training on postintervention exertional dyspnoea (MD −0.53, 95% CI −1.22 to 0.15; I2 = 74%; 2 studies, 141 participants; very low‐certainty evidence; Analysis 1.4).
1.4. Analysis.

Comparison 1: Exercise versus no exercise, Outcome 4: Postintervention (preoperative) dyspnoea (BORG scale)
7. Secondary outcome: postintervention (preoperative) and postoperative exercise capacity
Six studies reported postintervention 6MWD as their measure of exercise capacity (Lai 2017a; Lai 2017b; Lai 2019; Licker 2016; Liu 2020; Morano 2013; Table 2). The evidence is very uncertain about the effect of preoperative exercise training on postintervention exercise capacity measured using the 6MWT (MD 29.55 m, 95% CI 12.05 to 47.04; I2 = 90%; 6 studies, 474 participants; very low‐certainty evidence; Analysis 1.5). Heterogeneity was substantial (I2 = 90%); therefore, we undertook sensitivity analyses. The higher postintervention 6MWD in the exercise group was maintained after excluding the three studies rated at high risk of selection bias (random allocation) (Lai 2017a; Licker 2016; Morano 2013) (MD 20.89 m, 95% CI 12.81 to 28.98), and the I2 statistic reduced to 8%.
1.5. Analysis.

Comparison 1: Exercise versus no exercise, Outcome 5: Postintervention (preoperative) exercise capacity (6‐minute walk distance in m)
Two studies reported postintervention VO2peak as their measure of exercise capacity (Licker 2016; Stefanelli 2013; Table 2). Preoperative exercise training likely increases postintervention exercise capacity measured by VO2peak (MD 3.36 mL/kg/minute, 95% CI 2.70 to 4.02; I2 = 0%; 2 studies, 191 participants; moderate‐certainty evidence; Analysis 1.6).
1.6. Analysis.

Comparison 1: Exercise versus no exercise, Outcome 6: Postintervention (preoperative) exercise capacity (VO2peak in mL/kg/minute)
Two studies reported postoperative 6MWD (Garcia 2017; Liu 2020; Table 2). In Garcia 2017, the median time to postoperative assessment was 3.5 weeks (IQR 1.5 to 4.9). Liu 2020 conducted postoperative assessment 30 days after surgery. The evidence is very uncertain about its effect on postoperative 6MWD (MD 37.77 m, 95% CI −10.30 to 85.84; I2 = 78%; 2 studies, 95 participants; very low‐certainty evidence; Analysis 1.7).
1.7. Analysis.

Comparison 1: Exercise versus no exercise, Outcome 7: Postoperative exercise capacity (6‐minute walk distance in m)
One study reported postoperative VO2peak (Stefanelli 2013). The study found that exercise capacity decreased from immediately before surgery (postintervention time point) to 60 days postoperatively in both groups (VO2peak: exercise group: from 17.8 (SD 2.1) mL/kg/minute to 15.1 (SD 2.4) mL/kg/minute; P < 0.01; non‐exercise group: from 14.5 (SD 1.2) mL/kg/minute to 11.4 (SD 1.2) mL/kg/minute; P < 0.01), however there was no significant between‐group difference.
8. Secondary outcome: postintervention (preoperative) lung function
Four studies reported postintervention FEV1 (Liu 2020; Morano 2013; Pehlivan 2011; Stefanelli 2013; Table 2). Preoperative exercise training may result in little to no effect on postintervention FEV1 (MD 5.87% predicted, 95% CI 4.46 to 7.28; I2 = 0%; 4 studies, 197 participants; low‐certainty evidence; Analysis 1.8).
1.8. Analysis.

Comparison 1: Exercise versus no exercise, Outcome 8: Postintervention (preoperative) forced expiratory volume in 1 second (% predicted)
Three studies reported postintervention FVC (Liu 2020; Morano 2013; Pehlivan 2011; Table 2). The evidence is very uncertain about its effect on postintervention FVC (MD 5.42% predicted, 95% CI 0.73 to 10.11; I2 = 55%; 3 studies, 157 participants; very low‐certainty evidence; Analysis 1.9).
1.9. Analysis.

Comparison 1: Exercise versus no exercise, Outcome 9: Postintervention (preoperative) forced vital capacity (% predicted)
Three studies reported postintervention peak expiratory flow (PEF) (Lai 2017a; Lai 2017b; Liu 2020; Table 2). The evidence is very uncertain about its effect on postintervention PEF (MD 21.52 L/minute, 95% CI −7.11 to 50.16; I2 = 0%; 3 studies, 234 participants; very low‐certainty evidence; Analysis 1.10).
1.10. Analysis.

Comparison 1: Exercise versus no exercise, Outcome 10: Postintervention (preoperative) peak expiratory flow (L/minute)
9. Secondary outcome: postoperative mortality
Four studies reported postoperative mortality (Lai 2019; Licker 2016; Liu 2020; Pehlivan 2011; Table 2). Three studies reported no inhospital postoperative mortality in either the exercise or the non‐exercise group (Lai 2019; Liu 2020; Pehlivan 2011). One study reported 30‐day mortality (Licker 2016). They reported four deaths, with no between‐group difference (two deaths in each group; P = 0.64).
Discussion
Summary of main results
Our review provided high‐certainty evidence that preoperative exercise training results in a large reduction in the risk of developing a postoperative pulmonary complication compared to no preoperative exercise training. Preoperative exercise training confers a 55% relative reduction in the risk of developing a postoperative pulmonary complication. We also found that it likely reduces postoperative hospital length of stay and increases postintervention (preoperative) peak exercise capacity (VO2peak). Regarding postoperative pulmonary complications, we found an NNTB of 5, meaning that it is expected that one fewer person will develop a postoperative pulmonary complication for every five participants receiving preoperative exercise training rather than usual care. The ability to reduce postoperative pulmonary complications is of significant value to patients and the healthcare system.
Our review demonstrated that the evidence is very uncertain about the effect of preoperative exercise training on postoperative intercostal catheter duration, dyspnoea on exertion, preoperative or postoperative exercise capacity measured by the 6MWD and lung function. Data were insufficient to comment on the effect on fatigue. Only four studies reported on mortality. Three reported no inhospital postoperative mortality in either group, and one study reported 30‐day mortality with no between‐group difference (two deaths in each group). Two studies reported costs associated with the hospital stay and reported lower costs in the exercise group than in the non‐exercise group. Only three studies reported adverse events and there were none reported. Preoperative exercise training is likely safe.
Overall completeness and applicability of evidence
The update of this systematic review was warranted as we were able to include five additional RCTs and increase our sample size from 167 to 636. Of critical importance, we were able to include the RCT by Licker and colleagues, with a sample size of 151, which on its own almost doubled the sample size of our original Cochrane Review (Licker 2016). The updated meta‐analyses, investigating the effects of preoperative exercise training were able to provide novel, moderate‐certainty evidence for the effect of preoperative exercise training on preoperative VO2peak and reduced postoperative hospital length of stay, and strengthen the certainty of the effect on postoperative pulmonary complications. Our original review was limited with only one of the included studies reporting data on VO2peak (Stefanelli 2013), and, whilst data are still lacking, we were able to include the Licker 2016 data in this meta‐analysis to demonstrate an MD of 3.36 mL/kg/minute in favour of the intervention group. These findings are essential to support the theoretical principles behind the intervention and the hypothesis that the reduction in postoperative pulmonary complications seen, conferred through preoperative exercise training, is generated in part by improvements in preoperative exercise capacity and improvement in physiological reserves (Licker 2016).
Measurement of peak exercise capacity (i.e. VO2peak) is recommended before lung resection in high‐risk patients (i.e. those with FEV1 or diffusing capacity for carbon monoxide less than 80% of predicted values, or both) to determine their eligibility for surgery (Brunelli 2013). People with a VO2peak greater than 20 mL/kg/minute are considered operable, those with a VO2peak between 10 mL/kg/minute and 20 mL/kg/minute are borderline operable and those with a VO2peak less than 10 mL/kg/minute are considered inoperable. People with a VO2peak less than 16 mL/kg/minute are at higher risk for perioperative or postoperative complications (Loewen 2007). In Stefanelli 2013, the mean VO2peak of participants in the intervention group was 14.9 (SD 2.3) mL/kg/minute and the control group was 14.8 (SD 1.4) mL/kg/minute. That is, according to the cut‐off proposed by Loewen et al (Loewen 2007), they were at higher risk for perioperative or postoperative complications. Importantly, Stefanelli 2013 demonstrated that participants in the intervention group significantly improved their VO2peak to 17.8 (SD 2.1) mL/kg/minute, a value that is higher than the cut‐off for increased risk of perioperative or postoperative complications. Licker 2016 included participants with higher baseline VO2peak closer or above the operable category (intervention group: 19.9 (SD 5.7) mL/kg/minute; control group: 20.4 (SD 5.7) mL/kg/minute) and demonstrated improvements of similar magnitude to those in the higher risk cohort of Stefanelli 2013. Additionally, our meta‐analysis demonstrated an improvement in 6MWD in the intervention group that over and above changes seen in the control group (although this was with very low‐certainty evidence). The MD of 30 m in the 6MWD is greater than the reported minimal clinically important difference (range 22 to 42 m) for people with lung cancer (Granger 2015). Further studies are needed to investigate relationships between a significant improvement in exercise capacity following preoperative exercise training and better postoperative outcomes. However, based on our findings, people within the lower range of VO2peak (10 mL/kg/minute to 15 mL/kg/minute) should be referred to preoperative exercise training as an attempt to decrease their risk of postoperative pulmonary complications.
Our earlier systematic review observed low‐certainty evidence that preoperative exercise training may reduce the number of days people need an intercostal catheter following surgery. The updated meta‐analysis which tripled the sample size (albeit with only the addition of one extra study) instead observed an uncertain effect with very low‐certainty evidence. With the recent development and increased use of digital chest drainage systems, the clinical importance of this outcome may become less significant in the future. In many settings, the digital systems have replaced conventional chest drainage, with the preliminary evidence suggesting digital drains are associated with shorter drainage times and shorter hospital length of stay (Evans 2019; Pompili 2011). We do not believe any studies in our review used digital chest drainage systems and this will be an important consideration for future preoperative exercise RCTs wishing to use this as an outcome of interest.
The interventions provided in the studies included in our review varied in the types of exercise training. All studies included aerobic exercise training and supplemented this with resistance training (Benzo 2011; Garcia 2017; Licker 2016; Liu 2020), inspiratory muscle training (Benzo 2011; Morano 2013), breathing exercises (Benzo 2011; Garcia 2017; Lai 2017a; Lai 2017b; Lai 2019; Liu 2020; Pehlivan 2011; Stefanelli 2013), or stretches (Liu 2020; Morano 2013). The specific aerobic exercise prescribed also varied, from moderate to high intensity, and delivered as either continuous or interval‐based training. The frequency and duration of training varying across studies (the least frequent programme was two or three times per week for three or four weeks (Licker 2016), most frequent programme was three times per day for only one week (Pehlivan 2011)). However, overall the programmes were delivered more frequently and for a shorter duration than in typical pulmonary rehabilitation programmes for people with COPD (McCarthy 2015) or postoperative programmes for people with lung cancer (Cavelheri 2019). Half the studies included programmes that were only one or two weeks in duration (Benzo 2011; Lai 2017b; Lai 2019; Liu 2020; Pehlivan 2011); and no studies provided programmes that were longer than four weeks, which is shorter than the maximal waiting time for surgery as recommended in international guidelines (Institute of Medicine 2015; The NHS Cancer Plan 2000). We were able to conduct a subgroup analysis for the primary outcome of risk of developing a postoperative pulmonary complication comparing intervention programmes that were two weeks or less in duration and those that were more than two weeks in duration. Although the greater than two‐week subgroup was small (3 studies, 194 participants), there was no difference between subgroups. Further studies investigating the optimal duration of programmes are warranted. We had also planned to conduct a subgroup analysis to evaluate the effect of different exercise training regimens. However, because of the lack of studies and small sample sizes, this was not possible. We cannot attribute the benefits observed to any particular component of the exercise training, and, therefore, until further studies are completed comparing different types of exercise training, or study numbers increase significantly to allow us to undertake subgroup analyses, the optimal preoperative exercise prescription remains unknown. The studies included in the review did not report harm associated with preoperative exercise training. There is the possibility that patients may experience short‐term temporary general muscle soreness after exercising, especially if they are unaccustomed to the specific types of exercises undertaken (Armstrong 1984). However, this is a usual response to exercise and not associated with permanent impairment.
In the current review, we investigated whether the certainty of the evidence for the effectiveness of preoperative exercise training in adults scheduled to undergo lung resection for NSCLC has improved. Some concerns outlined in our original review (Cavalheri 2017) have been addressed in the newly added studies and the certainty of evidence for the effectiveness of preoperative exercise on our primary outcome of risk of developing a postoperative pulmonary complication has improved from low‐certainty to high‐certainty. One of our recommendations was to investigate the effect of preoperative exercise training on mortality. Three new studies measured mortality (Lai 2019; Licker 2016; Liu 2020), adding data to the one original study (Pehlivan 2011), and these four studies found no difference between groups. Another recommendation was the need to investigate the cost‐effectiveness of the intervention. Since our original review, we were able to include two new studies reporting costs. Both Lai 2017b and Lai 2019 reported lower costs (hospital costs and medication costs) in the intervention groups in their preoperative exercise training programmes of one‐week duration. Lai 2017b delivered this as an inpatient programme (it was unclear whether Lai 2019 delivered an in‐ or outpatient programme). Both also reported reduced postoperative hospital length of stay in favour of the intervention group, which may account for the cost savings. These are promising cost‐effectiveness findings, but further studies are needed to strengthen these findings and address methodological limitations in the studies to date.
We considered the addition of longer‐term follow‐up measures was needed but only one new study adopted this (Licker 2016 measured outcomes to 12 months). Interestingly most of the data in our meta‐analyses were for outcomes measured preoperatively (postintervention) or very short term (inhospital) postoperatively. The addition of postoperative outcomes and longer‐term follow‐up in further research will be important to strengthen our understanding of the medium‐ and longer‐term benefit of this intervention. Finally, none of the studies used induction/neoadjuvant therapies such as chemotherapy, radiotherapy, immunotherapy or a combination of these. Therefore, further RCTs are needed to investigate the feasibility, role and effects of exercise training programmes delivered before, during or after induction/neoadjuvant therapies.
Quality of the evidence
The certainty of the evidence ranged from very low (e.g. postintervention exercise capacity 6MWD) to high (e.g. risk of developing a postoperative pulmonary complication), mainly due to significant risk of bias, small sample sizes (the largest study included only 151 participants) and imprecision of results. We rated all studies at high risk of performance bias, since none of them blinded study personnel or participants. Of note, blinding of personnel and participants cannot be achieved in studies of exercise training, as the personnel are required to deliver the exercise intervention, and participants are often aware of whether they are receiving usual care or exercise training. We rated eight studies at high risk of selective reporting, since these studies reported outcomes that were not prespecified in the trial registration, not all the prespecified outcomes were reported, the trial was registered retrospectively, outcomes of interest were reported incompletely or a combination of these. Two studies were at unclear risk of reporting bias because of insufficient information. Lastly, only five studies used intention‐to‐treat analyses.
In the current review, we investigated whether our considerations from the original review helped inform the methodology of new studies (Cavalheri 2017). Pertinent methodological limitations of prior studies included lack of intention‐to‐treat analysis, lack of participant blinding, poor reporting of attrition and poor reporting of full outcome data. The newly added studies addressed several methodological concerns. Overall, the new studies have less risk of bias, especially with improving reporting of attrition. However, only three new studies reported intention‐to‐treat analysis (Lai 2019; Licker 2016; Liu 2020); none of the studies reported blinding participants or personnel; and reporting bias remains a concern. Reporting of safety was incomplete in most trials. To address these concerns, further research should prospectively register and subsequently report the trial in full, attempt to blind participants, conduct intention‐to‐treat analyses and provide detailed information on safety of the intervention.
Our review only included 10 RCTs, and since not all outcomes were measured in every study, each meta‐analysis included data from only two to nine studies. Therefore, the low number of small studies impacted the overall certainty of the evidence. The low number of studies also prevented us from undertaking all the planned subgroup analyses. Further RCTs are required to add data to improve the certainty of the evidence.
Potential biases in the review process
Our review was strengthened by the systematic processes followed to ensure rigour and completeness. This included the registration and publication of our protocol prior to starting the search for the original review (Cavalheri 2017); the use of broad search terms not restricted to language; the inclusion of two review authors to independently determine study inclusion, as well as assessing their agreement for study inclusion; and multiple attempts to contact authors of studies to clarify their suitability for inclusion, methodological details for assessment of risk of bias and missing or unpublished outcome data. The limitation of this review was the exclusion of two studies where authors could not be contacted to clarify details required for inclusion, which added potential selection bias. Another limitation of this review is the fact that despite multiple attempts, we were unable to contact the research group who published six of the included references (one from the previous review and five newly added). We wished to seek clarification of data sets to avoid multiple counts of data, especially since some studies had methodological limitations including lack of registration. As we were unable to contact the authors, the six references were cross‐matched with trial identification numbers, author names, location and settings, details of the interventions, number of participants and baseline data, and data and duration of the study to avoid multiple counts of data; and these six references contributed to three study counts, one in our original review (Lai 2017a), and two newly included studies (Lai 2017b; Lai 2019). There is a possibility that additional patient data from some of these references could have contributed to meta‐analyses.
Agreements and disagreements with other studies or reviews
In 2017, we published the first Cochrane Review of preoperative exercise training in lung cancer (Cavalheri 2017). Before our original review, there were five published systematic reviews investigating exercise training in lung cancer (Crandall 2014; Granger 2011; Pouwels 2015; Rodriguez‐Larrad 2014; Sebio Garcia 2016). Two of these reviews also included studies examining exercise in the postoperative period for people following lung resection (Crandall 2014; Granger 2011), and one review also included perioperative physiotherapy interventions (i.e. not limited to exercise training; Rodriguez‐Larrad 2014). In contrast to our review, these previously published reviews included a wide range of study designs (i.e. RCTs, non‐RCTs, single group studies and retrospective cohort studies), and therefore, their results should be interpreted with caution. Two of these systematic reviews specifically investigated the effectiveness of preoperative exercise training in people scheduled to undergo lung resection for NSCLC (Pouwels 2015; Sebio Garcia 2016). Pouwels 2015 did not include one RCT included in our review and did not undertake meta‐analyses. Sebio Garcia 2016 included all the RCTs included in our review, and undertook meta‐analyses for lung function, length of hospital stay and postoperative pulmonary complications. Consistent with our findings, Sebio Garcia 2016 reported a significant reduction of postoperative pulmonary complications (MD 0.55, 95% CI 0.34 to 0.89), and hospital length of stay (MD −4.83 days, 95% CI −5.90 to −3.76) with preoperative exercise training. The magnitude of difference of their findings was different to our findings, and this was likely because they included prospective non‐RCTs and retrospective cohort studies, in addition to RCTs.
Since our original review (Cavalheri 2017), there have been several published systematic reviews investigating preoperative exercise training in lung cancer (Gravier 2022; Li 2019; Rosero 2019; Xu 2022). None of these past reviews included the same data as our review. Previous reviews 1. duplicated studies from the same research groups (that had different first authors) in their meta‐analysis (whereas we attempted to group citations according to studies and only reported the main study in the meta‐analysis), 2. included studies that did both preoperative and immediate postoperative exercise training (which might affect the rate of postoperative pulmonary complications and other postoperative outcomes including length of hospital stay), 3. included studies that used interventions that are typically not classified as exercise training (i.e. breathing exercises and incentive spirometry), or a combination of these. However, overall, our findings our consistent with the findings of these reviews and in line with the conclusions from the most recent review (Gravier 2022), which showed preoperative exercise training to be associated with reduced risk of postoperative pulmonary complications (RR 0.58, 95% CI 0.45 to 0.75; 10 studies, 617 participants).
Authors' conclusions
Implications for practice.
Preoperative exercise training for people with non‐small cell lung cancer (NSCLC) results in a large reduction in the risk of developing a postoperative pulmonary complication compared to no preoperative exercise training and is likely safe. Preoperative exercise training likely results in a reduction in postoperative length of hospital stay and increases exercise capacity measured by peak oxygen consumption after completion of the exercise programme. The evidence is very uncertain about its effect on postoperative intercostal catheter duration or postintervention exercise capacity measured by the six‐minute walk distance. Based on these results, people with NSCLC awaiting lung resection should be referred to a preoperative exercise programme.
Implications for research.
Research is warranted to investigate the cost‐effectiveness of this intervention. This information will assist in implementation of preoperative exercise programmes into clinical services. Research into the long‐term outcomes of preoperative exercise training is also needed given the current gap in the literature. The feasibility, role and effects of exercise training delivered before, during or after induction/neoadjuvant therapies are unknown and should be investigated in future studies. The methodological limitations found in many of the current studies should also be addressed and minimised in future studies. This includes intention‐to‐treat analysis, attempts to blind participants, complete preregistration of trial outcomes and reporting full prespecified outcome data, and improved reporting of intervention safety.
What's new
| Date | Event | Description |
|---|---|---|
| 28 September 2022 | New search has been performed | Background, objectives (to report on safety of the intervention was added as a primary aim), outcomes (safety of the intervention added as a primary outcome), analysis, summary of findings table, discussion and conclusions updated. |
| 28 September 2022 | New citation required and conclusions have changed | New literature search ran on 23 November 2021. Five new studies included (Garcia 2017; Lai 2017b; Lai 2019; Licker 2016; Liu 2020), resulting in 10 included studies. Conclusions changed |
History
Protocol first published: Issue 12, 2015 Review first published: Issue 6, 2017
| Date | Event | Description |
|---|---|---|
| 8 June 2017 | Amended | Correction in figure 4 |
Acknowledgements
The authors thank:
Corynne Marchal, Managing Editor of the Cochrane Lung Cancer Group, for her feedback and support.
Paul E Van Schil, MD, PhD; Department of Thoracic and Vascular Surgery, Antwerp University Hospital, Belgium; Fergus Macbeth, Cochrane Lung Cancer Group; Marta Roqué i Figuls, Cochrane Iberoamerica and Anne‐Claire Toffart, CHU Grenoble, France, for their comments on the review.
Anne Lawson, Central Production Service, Cochrane, for copy editing the review.
This research was supported by a Cancer Council WA Postdoctoral Fellowship (VC) and a Victorian Cancer Agency Clinical Research Fellowship (CG).
Appendices
Appendix 1. CENTRAL search strategy
#1 lung cancer*
#2 non‐small cell*
#3 non small cell*
#4 nonsmall cell*
#5 MeSH descriptor: [Lung Neoplasms] explode all trees
#6 MeSH descriptor: [Carcinoma, Non‐Small‐Cell Lung] explode all trees
#7 nsclc
#8 #1 or #2 or #3 or #4 or #5 or #6 or #7
#9 exercis*
#10 rehabilitat*
#11 aerobic*
#12 endurance
#13 treadmill
#14 walking
#15 physiother*
#16 physical there*
#17 #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16
#18 #8 and #17
#19 preoperat*
#20 pre‐operat*
#21 pre operat*
#22 presurg*
#23 pre‐surg*
#24 pre surg*
#25 before surg*
#26 before operat*
#27 #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26
#28 #18 and #27
Appendix 2. MEDLINE (PubMed) search strategy
#1, Carcinoma, Non‐Small‐Cell Lung[MeSH]
#2, nsclc[Title/Abstract]
#3, lung cancer*[Title/Abstract]
#4, lung carcinoma*[Title/Abstract]
#5, lung neoplasm*[Title/Abstract]
#6, lung tumor*[Title/Abstract]
#7, lung tumour*[Title/Abstract]
#8, non‐small cell*[Title/Abstract]
#9, nonsmall cell*[Title/Abstract]
#10, (#3 OR #4 OR #5 OR #6 OR #7) AND (#8 OR #9)
#11, #1 OR #2 OR #10
#12, exercise[MeSH Terms]
#13, exercis*[Title/Abstract]
#14, rehabilitation[MeSH Terms]
#15, rehabilitat*[Title/Abstract]
#16, aerobic*[Title/Abstract]
#17, endurance[Title/Abstract]
#18, treadmill[Title/Abstract]
#19, walking[MeSH Terms]
#20, walk*[Title/Abstract]
#21, breathing exercises[MeSH Terms] OR respiratory muscle training[Text Word]
#22, bicycl*[Title/Abstract] OR cycling*[Title/Abstract]
#23, physiotherap*[Title/Abstract] OR physical therap*[Title/Abstract]
#24, #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23
#25, #11 AND #24
#26, preop*[Title/Abstract] OR pre‐op*[Title/Abstract]
#27, presurg*[Title/Abstract] OR pre‐surg*[Title/Abstract]
#28, before surg*[Title/Abstract] OR before operat*[Title/Abstract]
#29, #26 or #27 or #28
#30, #25 and #29
Appendix 3. Embase (www.embase.com) search strategy
#32 #22 AND #31
#31 #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30
#30 'before operat*':tn,lnk,ab,ti
#29 'before surg*':tn,lnk,ab,ti
#28 'pre surg*':tn,lnk,ab,ti
#27 'presurg*':tn,lnk,ab,ti
#26 'presurg*':tn,lnk,ab,ti
#25 'pre operat*':tn,lnk,ab,ti
#24 'pre‐operat*':tn,lnk,ab,ti
#23 'preoperat*':tn,lnk,ab,ti
#22 #10 AND #21
#21 #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20
#20 'physical activit*':tn,lnk,ab,ti
#19 'physical therapy':tn,lnk,ab,ti
#18 'physiotherapy'/exp
#17 'walking'/exp
#16 'treadmill'/exp
#15 'endurance'/exp
#14 'aerobic*':tn,lnk,ab,ti
#13 'rehabil*':tn,lnk,ab,ti
#12 'rehabilitation'/exp
#11 'exercise'/exp
#10 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9
#9 'nsclc':tn,lnk,ab,ti
#8 'thoracic cancer':tn,lnk,ab,ti
#7 'lung neoplasm':tn,lnk,ab,ti
#6 'lung carcinoma'/exp
#5 'lung tumor'/exp
#4 'nonsmall cell':tn,lnk,ab,ti
#3 'non ‐ small cell':tn,lnk,ab,ti
#2 'non small cell lung cancer'/exp
#1 'lung cancer'/exp
Data and analyses
Comparison 1. Exercise versus no exercise.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Risk of developing a postoperative pulmonary complication | 9 | 573 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.33, 0.61] |
| 1.1.1 Duration of intervention: ≤ 2 weeks | 6 | 379 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.25, 0.62] |
| 1.1.2 Duration of intervention: > 2 weeks | 3 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.35, 0.76] |
| 1.2 Number of days participants needed an intercostal catheter following surgery | 3 | 111 | Mean Difference (IV, Random, 95% CI) | ‐2.07 [‐4.64, 0.49] |
| 1.3 Postoperative length of hospital stay (days) | 9 | 573 | Mean Difference (IV, Random, 95% CI) | ‐2.24 [‐3.64, ‐0.85] |
| 1.4 Postintervention (preoperative) dyspnoea (BORG scale) | 2 | 141 | Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.22, 0.15] |
| 1.5 Postintervention (preoperative) exercise capacity (6‐minute walk distance in m) | 6 | 474 | Mean Difference (IV, Random, 95% CI) | 29.55 [12.05, 47.04] |
| 1.6 Postintervention (preoperative) exercise capacity (VO2peak in mL/kg/minute) | 2 | 191 | Mean Difference (IV, Random, 95% CI) | 3.36 [2.70, 4.02] |
| 1.7 Postoperative exercise capacity (6‐minute walk distance in m) | 2 | 95 | Mean Difference (IV, Random, 95% CI) | 37.77 [‐10.30, 85.84] |
| 1.8 Postintervention (preoperative) forced expiratory volume in 1 second (% predicted) | 4 | 197 | Mean Difference (IV, Fixed, 95% CI) | 5.87 [4.46, 7.28] |
| 1.9 Postintervention (preoperative) forced vital capacity (% predicted) | 3 | 157 | Mean Difference (IV, Random, 95% CI) | 5.42 [0.73, 10.11] |
| 1.10 Postintervention (preoperative) peak expiratory flow (L/minute) | 3 | 234 | Mean Difference (IV, Fixed, 95% CI) | 21.52 [‐7.11, 50.16] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Benzo 2011.
| Study characteristics | ||
| Methods | 2 randomised controlled trials Setting: USA (University of Pittsburgh and Mayo Clinic) Study duration Study 1: 18 months. Exercise training for 4 weeks Study 2: 1 year. Exercise training for 1 week |
|
| Participants | Participants undergoing lung cancer resection by open thoracotomy (segmentectomy, lobectomy or pneumonectomy) or by VATS (at least lobectomy), with moderate‐to‐severe chronic obstructive pulmonary disease. Study 1: 9 participants randomised in 18 months from a large surgical practice (5 hospitals: academic (3) and community (2)). Study 2: 19 participants (mean age: control group: 72 (SD 7) years: exercise group: 70 (SD 9) years) were randomised in 1 year from 1 site (Mayo Clinic). 2 were considered inoperable and therefore, postoperative data were missing. |
|
| Interventions |
Study 1 Control (4 participants): usual care, details not reported. Exercise (5 participants): 4 weeks of preoperative pulmonary rehabilitation that followed American Thoracic Society/ European Respiratory Society guidelines on exercise prescription (details on exercise training programme not reported). Study 2 Control (9 participants): usual care, details not reported. Exercise (10 participants): twice‐daily, 10‐session preoperative pulmonary rehabilitation that included 20 minutes of lower extremity endurance training on a treadmill, upper extremity endurance training on an arm ergometer, strengthening exercises for upper and lower limbs with Thera‐band (every second day), 15–10 minutes of inspiratory muscle training, 10 minutes of pursed‐lip breathing and prescription of weekend exercises based on their performance during the pulmonary rehabilitation programme. |
|
| Outcomes | Both studies
|
|
| Notes | Study 1 had poor recruitment (providers were unwilling to delay the curative surgery for 4 weeks) and was stopped, due to low likelihood of meaningful accrual during the funding period. Therefore, only data from study 2 were extracted for this systematic review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Outcomes were obtained using chart review by a nurse trained in the abstraction of the desired outcomes from the medical records and blinded to the treatment". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Two patients (one on each arm) were missing length of stay data; because they were considered inoperable once they were in the operating room and were excluded from the outcome analysis". Comment: missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | High risk | Quote: "Patients did not improve the shuttle walk test after the short term PR [pulmonary rehabilitation] (P = NS [not significant])". Comment: 1 outcome of interest in the review (exercise capacity) was reported incompletely, so it could not be entered in a meta‐analysis. |
| Other bias | High risk | Comment: study 1 ceased early due to poor recruitment. |
Garcia 2017.
| Study characteristics | ||
| Methods | Randomised controlled trial Setting: University Hospital of A Coruña, Spain Study duration: October 2013 to April 2015. Exercise training conducted in time before surgery (mean time between baseline assessment and surgery 54 (SD 15) days). Assessments performed before intervention, after intervention (intervention group only), at hospital discharge and 3 months' postoperative. |
|
| Participants | 40 participants (mean age: control group: 69 (SD 9) years; exercise group: 71 (SD 6) years) with suspected or confirmed NSCLC who were considered for lung resection surgery via VATS, and had ≥ 1 of: FEV1 ≤ 80% of predicted value; body mass index ≥ 30; age ≥ 75 years; ≥ 2 comorbidities identified in the Colinet Comorbidity Score; and lived within 80 km of centre. 46 participants recruited; 6 withdrew before randomisation. |
|
| Interventions | Control (20 participants): usual care consisting of no exercise training. Exercise (20 participants): 1‐hour supervised preoperative pulmonary rehabilitation programme 3–5 times per week depending on surgery date. Outpatient programme occurred in rehabilitation room at hospital. Pulmonary rehabilitation programme included moderate‐intensity endurance training with a cycle ergometer (initial target 30 minutes' duration) and resistance training with elastic bands targeting major muscle groups. Participants also completed 2 sessions per day of incentive spirometry with a volume‐oriented device (Coach 2 Incentive Spirometer 22‐4000 HD, Smith Medicals, USA). |
|
| Outcomes | Postintervention (in the intervention group only)
Postoperatively
After hospital discharge and 3 months postoperatively
|
|
| Notes | Study registered (NCT01963923). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was undertaken using a random based computer programme (Epidat® v3.1 Xunta de Galicia, 2005) with an allocation ratio of 1:1". |
| Allocation concealment (selection bias) | Low risk | Quote: "Individual allocations were placed in consecutively numbered and sealed opaque envelopes by a third person not involved in the study". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Assessor‐blind"…"Group allocation was only revealed to the physiotherapist after the initial evaluations were completed, ensuring the blindness of the assessment. Postoperative evaluations were performed by two independent physiotherapists who were specifically trained to perform the outcome measurements and who were unaware of the patients' allocation". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "46 (67.6%) signed the informed consent. Six patients (13%) withdrew before randomization, therefore 40 patients were randomized and 22 (55%) completed at least one postoperative evaluation and were analysed (10 patients in the prehabilitation group and 12 in the control group). At three months, two patients in the control group and one in the prehabilitation group were lost to follow‐up". Comment: significant attrition rate (40 participants randomised and only 22 analysed). |
| Selective reporting (reporting bias) | High risk | Comment: study registration available and all study's prespecified (primary and secondary) outcomes that were of interest in the review were reported in the prespecified way. Some extra outcome measures that were not included in the study registration were reported. This included functional exercise capacity measured with the 6MWT, length of hospital stay and postoperative pulmonary complications. |
| Other bias | High risk | Comment: 1 extra assessment time point for the intervention group was reported in results but not mentioned in methods. |
Lai 2017a.
| Study characteristics | ||
| Methods | Randomised controlled trial Setting: Department of Thoracic Surgery, West China Hospital, China Study duration: 1 week before lung resection until hospital discharge |
|
| Participants | 60 participants aged ≥ 70 years (mean age: control group: 71.6 (SD 1.9) years; exercise group: 72.5 (SD 3.4) years), with NSCLC, referred for lung resection via VATS or open approach | |
| Interventions | Control (30 participants): conventional preoperative respiratory management, and no formal preoperative exercise training Exercise (30 participants): abdominal breathing training, expiration exercises and aerobic training using the NuStep (NuStep Inc, Ann Arbor, MI, USA) |
|
| Outcomes | Postintervention
Postoperatively
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "…randomly allocated into the PR [pulmonary rehabilitation] or control (non‐pulmonary rehabilitation, NPR) group". Comment: insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All participants were assessed, and data were recorded by a physiotherapist who was blinded to the grouping and the study purpose". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "During the study, four patients in the PR group suspended the training because they could not endure the highly intensive regimen, one perceived a lack of benefit, and one suffered from knee pain. According to the intention‐to‐treat principle, we included those who did not complete the regimen in the final analysis". |
| Selective reporting (reporting bias) | High risk | Comment: protocol registered retrospectively (ChiCTR‐IOR‐16008109). Age inclusion criterion on registration was different (> 60 years) and 2 outcome measures (cardiopulmonary function and blood gas analysis) listed on registration were not reported in published study. |
| Other bias | Low risk | Comment: study appeared free of other sources of bias. |
Lai 2017b.
| Study characteristics | ||
| Methods | Randomised controlled trial Setting: West China Hospital, China Study duration: 1 January 2015 to 30 December 2015. Exercise training performed 1 week preoperatively. Assessments before and after preoperative intervention and complications assessed to 30 days postoperative. |
|
| Participants | 101 participants (mean age: control group: 65 (SD 7) years; exercise group: 64 (SD 8) years) with NSCLC and presence of selected risk factors for postoperative pulmonary complications (> 20 pack‐year smoking history, aged > 75 years, body mass index > 30, postoperative predicted percentage FEV1 < 60%, postoperative predicted diffusing capacity of the lungs for carbon monoxide < 60% or chronic obstructive pulmonary disease) who were scheduled to undergo lung resection via VATS or open approach. | |
| Interventions | Control (50 participants): usual care consisted of routine care, including preoperative inhospital education, preoperative preparation (relevant examinations and arrangements) and essential encouragement or psychological caring. Exercise (51 participants): 1‐week high‐intensity pulmonary rehabilitation preoperative programme run as inpatients. This included 1. thoracic expansion and incentive spirometry exercises (performed with visual feedback device – HUDSON RCI 2500, Teleflex, Temecula, CA, USA), 20 breaths per session for 3 sessions per day; 2. abdominal breathing exercises twice daily for 15–30 minutes per session while seated or recumbent with knees bent and shoulders relaxed; 3. aerobic endurance exercises performed daily for 30 minutes per session using a Nu‐Step device (NuStep Inc, Ann Arbor, MI, USA). |
|
| Outcomes | Postintervention
Postoperatively
|
|
| Notes | Study registered as a parallel group trial (ChiCTRIOR‐16008109). Under this trial registry number, study was published as 3 different papers. 1 paper published in 2017 (Lai et al 2017b) included 2 groups (control and exercise) and included 101 participants; another paper published in 2017 (Huang et al) included 3 groups (control, exercise with inspiratory muscle training and inspiratory muscle training alone) and 90 participants; another paper was published in 2016 (Lai et al) in Chinese with 48 participants. For this purpose of this Cochrane Review, we used the Lai et al 2017b paper for data retrieval and analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were allocated to a treatment or a control group, based on a randomization table generated by a computer by an independent person". |
| Allocation concealment (selection bias) | Low risk | Quote: "group allocation placed in sequential opaque envelopes. Group allocation was revealed by a research assistant". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "It is impossible for the patients to be blinded to their treatment group allocation due to the nature of the exercise intervention". Comment: no blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "all study personnel collecting data and conducting statistical analyses were blinded to patient allocation". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "6 patients did not finish the 7‐day regimen. Two of them required an operation before completing the 7‐day regimen; 2 requested withdrawal from the study because of a perceived lack of benefit; and 2 requested withdrawal because they felt they could not endure the regimen". Comment: 6 participants did not complete the intervention; however, both Figure 1 and Table 2 of the paper suggest that all participants were included in the final analysis. |
| Selective reporting (reporting bias) | High risk | Quote: "Our study showed improvements in PEF and 6‐min walk distance and reductions in the total/postoperative length of stay, in‐hospital costs and occurrence of PPCs [postoperative pulmonary complications]". Comment: study registered retrospectively (ChiCTRIOR‐16008109; www.chictr.org.cn/showprojen.aspx?proj=13593). Study included results on hospital length of stay and inhospital expenses, which were not registered as outcomes on the registry. |
| Other bias | High risk | Comment: the registry reported a sample size of 144 (not 101 as reported in this publication). The registry included different inclusion and exclusion criteria to the study. |
Lai 2019.
| Study characteristics | ||
| Methods | Randomised controlled trial Setting: West China Hospital, China Study duration: exercise training: 1 week preoperatively. Assessments performed before and after the preoperative intervention and complications assessed until hospital discharge. |
|
| Participants | 68 participants (mean age: control group: 63 (SD 8) years; exercise group: 64 (SD 7) years; eligibility age range 45–80 years) with NSCLC, scheduled to undergo lung cancer lobectomy via VATS, with a predicted postoperative FEV1 < 60%. | |
| Interventions | Control (34 participants): usual care consisting of routine preoperative preparation, including laboratory and radiological examinations and preoperative education. Exercise (34 participants): 1 week of preoperative physical training combining aerobic and breathing exercises which included: breathing exercises, 20 breaths/session for 3 sessions/day via a volumetric Incentive spirometer (Hudson RCI 2500, Teleflex Inc, USA) under the guidance of specialised nurses. The spirometer was a visual feedback device that encourages maximal inspiration, including breath‐holding; aerobic exercise, 30 minutes/session every day via a Nu‐Step instrument (NuStep Inc, Ann Arbor, MI, USA) under the supervision of physical therapists. |
|
| Outcomes | Postintervention
Postoperatively
|
|
| Notes | Study registered (ChiCTR1800014512). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were randomly assigned into one of two treatment types using a random number table after baseline assessments were completed". |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement of low risk or high risk. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Due to the nature of this research, we could not keep the participants blind to group assignment". Comment: no blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from paper: "The study staff and statistical analyses were all blind to patient allocation during data collection". Trial registration: "All study personnel collecting data and conducting statistical analyses were blinded to patient allocation". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "2 IG [exercise group] patients did not complete the training programme, as they could not endure the training intensity (one stopped after the third training day and one at the fourth). Two IG patients and 3 controls were non‐NSCLC, and were all inflammatory nodules except for 1 small cell lung cancer patient in the CG [control] group. However, according to the intention of the treatment principle, all the randomized patients were included in the analysis". Comment: low attrition rate and reasons for attrition stated. |
| Selective reporting (reporting bias) | High risk | Comment: trial was prospectively registered (ChiCTR1800014512). The primary outcome reported in the paper and the trial registration did not match. The primary outcome in the paper was not listed in the study registration. The only outcomes on the registry listed were lung‐related complications, quality of life and lung function. The publication also included the outcomes of functional exercise capacity (6MWT), length of hospital stay and inhospital costs. |
| Other bias | High risk | Comment: sample size on registry was 200, which varies to the publication including 68 participants. |
Licker 2016.
| Study characteristics | ||
| Methods | Randomised controlled trial Setting: University Hospital of Geneva and the Hospital of Valais, Switzerland Study duration: October 2011 to October 2014. Exercise: time before surgery (median 26 days, IQR 21–33 in the exercise group). Assessments performed before and after intervention, after surgery and 12 months postoperative |
|
| Participants | 151 participants (mean age: control group: 64 (SD 10) years; exercise group: 64 (SD 13) years) scheduled for lung resection with suspected or confirmed NSCLC stage IIIA or less; via VATS or open approach 164 participants recruited, 13 excluded (control group: 3 withdrew and 3 had operation cancelled; exercise group: 3 withdrew and 2 had operation cancelled |
|
| Interventions | Control (77 participants): usual care consisting of preoperative advice regarding active mobilisation (≥ 4 × 30‐minute walks per week) and risk factor management (e.g. healthy nutrition and smoking and alcohol cessation). Exercise (74 participants): usual care plus preoperative rehabilitation based on high‐intensity interval training delivered in an outpatient clinic. Exercise on a cycling ergometer 2 or 3 times a week under supervision physiotherapists. High‐intensity interval training: 5‐minute warm‐up period at 50% at peak work rate; then 2 × 10‐minute series of 15‐second sprint intervals (at 80–100% peak work rate) interspersed by 15‐second pauses and a 4‐minute rest between the 2 series; then 5‐minute active recovery period at 30% peak work rate cool down. Additional exercises, such as leg press, leg extension, back extension, seat row, biceps curls, or chest and shoulder press, were proposed on an individual basis. Outpatient programme run in an outpatient clinic. |
|
| Outcomes | Postintervention
Postoperatively
12 months postoperatively
|
|
| Notes | Study published as 3 different papers. First paper published in 2017 (Licker et al) focused on early postoperative outcomes, second paper published in 2017 (Karenovics et al) focused on 12‐month postoperative outcomes and third paper published in 2019 (Bhatia et al) focused on proof‐of‐concept of the specific exercise protocol. Study registered (NCT01258478). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Consenting patients were randomized on a 1:1 basis into a rehabilitation (Rehab) arm and UC arm by using a permuted block of four patients". Comment: insufficient information to permit judgement of low risk or high risk. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The randomization sequence was developed before initiation of the trial and concealed until after enrolment". Comment: insufficient information to permit judgement of low risk or high risk. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Study was a prospective randomized, open, blinded end point controlled trial using assessor blinding". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 77/81 participants in control group and 74/83 in exercise group. 8 participants withdrew and 5 participants had operations cancelled. Missing outcome data balanced in numbers across groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | High risk | Comment: trial registry (NCT01258478) included secondary outcomes that were not reported in publications: quality of life scores, electrocardiogram, nutritional status and smoking behaviour (changes baseline to 3 weeks). |
| Other bias | Low risk | Comment: study appeared free of other sources of bias. |
Liu 2020.
| Study characteristics | ||
| Methods | Randomised controlled trial Setting: Peking Union Medical College Hospital, China Study duration: between March 2017 to December 2017. Exercise for 2 weeks preoperative. Assessments performed before and after the intervention, and 30 days postoperative. |
|
| Participants | 73 participants (mean age: control group: 56 (SD 9) years; exercise group: 56 (SD 10) years; eligibility was aged < 70 years) with newly suspected or confirmed NSCLC, clinical stage I–III, who were scheduled for lobectomy via VATS. 85 participants recruited, 12 were excluded because of not receiving lobectomy surgery (6), failure of follow‐up (5) or final pathology was not NSCLC (1). |
|
| Interventions | Control (36 participants): usual care consisting of a comprehensive preoperative anaesthesia assessment, perioperative drug recommendations for chronic diseases, smoking cessation and abstinence. No specific recommendations for preoperative exercise, diet or mental health. Exercise (37 participants): 2‐week preoperative multimodal intervention programme. Exercises performed in the home. Programme included:
|
|
| Outcomes | Postintervention and 30 days postoperatively
Postoperatively
|
|
| Notes | Study registered (NCT03068507). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were then assigned randomly to the prehabilitation or control group according to computer‐generated random numbers". |
| Allocation concealment (selection bias) | Low risk | Quote: "assigned randomly to the prehabilitation or control group according to computer‐generated random numbers concealed in sealed envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A doctor blinded to group allocation assessed all patients". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quotes: "85 were enrolled and randomized. Twelve patients were excluded because of not receiving lobectomy surgery (n = 6), failure of follow‐up (n = 5), or the final pathology was not NSCLC (n = 1). The number of patients excluded in the analyses was balanced, and the reasons for dropping out were comparable between groups". "There were no significant differences between the analyzed population and the patients who were randomized but excluded in terms of demographic and physiological characteristics". Comment: missing outcome data balanced in numbers across groups, with similar reasons for missing data across groups. Attrition rate low (14%). |
| Selective reporting (reporting bias) | High risk | Comment: trial registered (NCT03068507). The registry states quality of life was measured but this was not reported in the publication. The publication included data on extra outcomes not specified in the trial registry. The publication included data on lung function, short‐term recovery quality, length of hospital stay, chest tube duration, postoperative complications and mortality, which were not recorded on the registry. |
| Other bias | High risk | Quote: "A sample size of 70 (35 per group) was required to detect a statistically significant difference at a 2‐sided significance level of.05 and 90% statistical power. To account for patient dropouts and missing data, we planned to recruit a total of 85 patients". Comment: sample size on trial registry (100 participants) differed to the publication (70 participants). |
Morano 2013.
| Study characteristics | ||
| Methods | Randomised controlled trial Setting: teaching hospital in Ceara, Brazil Study duration: March 2008 to March 2011. Exercise training: 4 weeks preoperatively. Assessments performed before and after preoperative intervention. Postoperative outcomes obtained from medical records. |
|
| Participants | 24 participants (mean age: control group: 69 (SD 7) years; exercise group: 65 (SD 8) years) with NSCLC, who were undergoing lung resection via open thoracotomy or VATS, and had impaired lung function. 31 participants recruited, 7 excluded, 5 of whom refused participation, and 2 did not meet inclusion criteria because of normal pulmonary function. |
|
| Interventions | Control (12 participants): usual care consisting of instructions about lung expansion techniques Exercise (12 participants): 5 sessions/week for 4 weeks of upper and lower limb endurance training (prescribed at 80% of maximum work rate achieved during a treadmill incremental test); inspiratory muscle training; and flexibility, stretching and balance exercises. Both groups had education: classes about the importance of preoperative and postoperative care and knowledge of the surgical process, energy conservation techniques, relaxation and stress management techniques, focus on nutrition, and the need to seek health services when necessary. |
|
| Outcomes | Postintervention
Postoperatively
|
|
| Notes | Study published in 2 different papers. Paper published in 2013 focused on postoperative outcomes, whereas paper published in 2014 focused on postintervention (preoperative) outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned to undergo a preoperative PR [pulmonary rehabilitation] or CPT [chest physical therapy] programme. The randomisation was done in blocks of 4". Comment: insufficient information about the sequence generation process to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The randomisation was done in blocks of 4, and individual allocations were placed in sealed envelopes. An external investigator blinded to the allocation sequence picked the envelopes". Comment: insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Single‐blinded". Comment: no blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Postoperative outcomes were obtained from the medical records by a physical therapist blinded to the treatment assignment". Comment: although postoperative outcomes were obtained by a physical therapist blinded to the treatment assignment, it is unclear whether postintervention outcome measures were taken by a blind assessor. Therefore, there was insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: 2013 study: "Three patients in the CPT [chest physical therapy] arm were not submitted to lung resection because of inoperable cancer". Quote: 2014 study: "All 24 participants successfully completed the training assignments". Comment: 2013 study: all participants accounted for. Greater dropouts in control group but reasons given. |
| Selective reporting (reporting bias) | High risk | Comment: some reported outcomes were not prespecified in the study protocol (UTN number: U1111‐1122‐2906) and not all the study's prespecified outcomes were reported. |
| Other bias | Low risk | Comment: study appeared free of other sources of bias. |
Pehlivan 2011.
| Study characteristics | ||
| Methods | Randomised controlled trial Setting: not described. Study undertaken in Turkey Study duration: January 2007 to August 2008. Exercise training: 1 week before lung resection until hospital discharge |
|
| Participants | 60 participants (mean age: control group: 55 (SD 8) years; exercise group: 54 (SD 9) years, with NSCLC (stages I–IIIB), referred for lung resection | |
| Interventions | Control (30 participants): usual care with no formal preoperative exercise training. Exercise (30 participants): intensive physical therapy (chest physiotherapy and walking exercise). Chest physiotherapy consisted of diaphragmatic, pursed lip, segmental breathing exercise, usage of incentive spirometry and coughing exercise. Walking exercise performed on a treadmill 3 times a day, according to the participant's tolerance to exercise speed and time. Postoperatively: routine physical therapy performed until discharge in both groups. |
|
| Outcomes | Postintervention
Postoperatively
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Comment: different quotes in 2 parts of the paper. Both methods described were at high risk of failure. Quotes: "… randomly allocated (according to hospital record number) to control or study group"; "Allocation was based on hospital record number". |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to permit judgement. |
| Other bias | Low risk | Comment: study appeared free of other sources of bias. |
Stefanelli 2013.
| Study characteristics | ||
| Methods | Randomised controlled trial Setting: outpatient clinic. Study undertaken in Naples, Italy Study duration: February 2010 to December 2011 |
|
| Participants | 40 participants (23 men; mean age 65 (SD 7) years) with chronic obstructive pulmonary disease, undergoing lobectomy (via open thoracotomy) for stage I/II NSCLC were enrolled in study. | |
| Interventions | Control (number not reported): usual care with no formal exercise training. Exercise (number not reported): 3‐week (15 × 3‐hour sessions, from Monday to Friday) preoperative outpatient intensive pulmonary rehabilitation programme based on high‐intensity training of both upper‐ and lower‐limb muscles (the upper limbs with the rowing ergometer, and the lower limbs by means of the treadmill and the ergometric bicycle). The exercise work load for each participant was set according to results of cardiopulmonary exercise test, starting with 70% of maximum work rate and increased by 10 watts when the participant was able to tolerate the set load for 30 minutes. The programme also included respiratory exercises on the bench, mattress pad and wall bars. |
|
| Outcomes | Postintervention and 60 days postoperatively
|
|
| Notes | Study did not report on length of hospital stay or postoperative pulmonary complications. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to two groups". Comment: insufficient information about sequence generation process to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: insufficient reporting of attrition/exclusions to permit judgement. |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information to permit judgement. |
| Other bias | High risk | Comment: number of participants in each group not reported. |
6MWT: 6‐minute walk test; EORTC QLQ C30: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire; EORTC LC13 CN: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire – Lung Cancer 13; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; ICU: intensive care unit; IQR: interquartile range; NSCLC: non‐small cell lung cancer; PEF: peak expiratory flow; SD: standard deviation; SF‐36: Medical Outcomes Study 36‐Item Short Form Health Survey; VATS: video‐assisted thoracic surgery; VO2peak: peak oxygen consumption.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chen 2016 | No preoperative exercise training |
| Ferreira 2021 | Wrong intervention (multimodal intervention with nutritional supplementation) |
| Garofano 2018 | Wrong study design (prognostic study using data of Licker et al – already included) |
| Han 2016 | Wrong intervention |
| Horváth 2017 | Conference abstract, not a randomised controlled trial |
| Hsiao 2018 | Wrong intervention (included early postoperative exercise) |
| Jonsson 2019 | Wrong intervention (no preoperative exercise training, only education) |
| Laurent 2020 | Wrong intervention (intervention was respiratory muscle training) |
| Meng 2018 | Not a randomised controlled trial |
| Patel 2021 | Conference abstract |
| Tenconi 2017 | Conference abstract – interim analysis of randomised controlled trial |
| Vagvolgyi 2017 | Not a randomised controlled trial |
| Zhou 2017 | Not a randomised controlled trial |
Differences between protocol and review
We added a subgroup analysis for the primary outcome 'risk of developing a postoperative pulmonary complication' to investigate the effects of interventions that were two weeks or less in duration and the effects of interventions that were more than two weeks in duration.
We added 'safety of the intervention' as a primary outcome and 'costs associated with postoperative hospital stay' as part of the first secondary outcome.
We presented the results of the secondary outcome 'postintervention exercise capacity' in a separate meta‐analysis: one that included six‐minute walk distance and a second one that included peak oxygen consumption (VO2peak). Due to these changes and new meta‐analyses that were not included in the original version of the review (Cavalheri 2017), we included new outcomes in the summary of findings table. These include: safety of the intervention; postintervention dyspnoea; postintervention exercise capacity (as measured by VO2peak); forced expiratory volume in one second (FEV1) and peak expiratory flow.
Contributions of authors
CG: initiation, writing of protocol and protocol development, selection of studies, extraction of data from studies and writing of the final review paper.
VC: initiation, writing of protocol, organisation of protocol into Review Manager 5, selection of studies, extraction of data from studies, conduct of the analysis and writing of the final review paper.
Sources of support
Internal sources
-
Curtin School of Allied Health, Faculty of Health Sciences, Curtin University, Australia
Source of support
-
Department of Physiotherapy, The University of Melbourne, Australia
Source of support
-
Department of Physiotherapy, Royal Melbourne Hospital, Australia
Source of support
-
Allied Health, South Metropolitan Health Service, Perth, Australia
Source of support
External sources
-
Cancer Council Western Australia, Australia
Vinicius Cavalheri is supported by a Cancer Council Western Australia Postdoctoral Fellowship
-
Victorian Cancer Agency, Australia
Catherine Granger is supported by a Victorian Cancer Agency Clinical Research Fellowship
Declarations of interest
CG: none.
VC: none.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Benzo 2011 {published data only}
- Benzo R, Wetztein M, Novotny P, Wigle D, Shen RS, Nichols F, et al. Randomized study of pulmonary rehabilitation before lung cancer resection in severe COPD. American Journal of Respiratory and Critical Care Medicine 2011;183:A3973. [Google Scholar]
- Benzo R, Wigle D, Novotny P, Wetztein M, Nichols F, Shen RS, et al. Preoperative pulmonary rehabilitation before lung cancer resection: results from two randomized studies. Lung Cancer 2011;74:441-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garcia 2017 {published and unpublished data}
- Garcia RS, Paz AL, Brage MI, Moolhuyzen EG, Rioboo MS, Mate JM. Preliminary efficacy of preoperative exercise training in patients with lung malignancies undergoing VATS. European Respiratory Journal 2016;48(60):PA541. [DOI: 10.1183/13993003.congress-2016.PA541] [DOI] [Google Scholar]
- Sebio García R, Yáñez-Brage MI, Giménez Moolhuyzen E, Salorio Riobo M, Lista Paz A, Borro Mate JM. Preoperative exercise training prevents functional decline after lung resection surgery: a randomized, single-blind controlled trial. Clinical Rehabilitation 2017;31(8):1057-67. [DOI: 10.1177/0269215516684179] [DOI] [PubMed] [Google Scholar]
Lai 2017a {published data only}
- Lai Y, Huang J, Yang M, Su J, Liu J, Che G. Seven-day intensive preoperative rehabilitation for elderly patients with lung cancer: a randomized controlled trial. Journal of Surgical Research 2017;209:30-6. [DOI] [PubMed] [Google Scholar]
Lai 2017b {published data only (unpublished sought but not used)}
- Huang J, Lai Y, Zhou X, Li S, Su J, Yang M, Che G. Short-term high-intensity rehabilitation in radically treated lung cancer: a three-armed randomized controlled trial. Journal of thoracic disease 2017;9(7):1919-29. [DOI: 10.21037/jtd.2017.06.15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Su J, Qui P, Wang M, Zhou K, Tang Y, et al. Systematic short-term pulmonary rehabilitation before lung cancer lobectomy: a randomized trial. Interactive CardioVascular and Thoracic Surgery 2017;25(3):476-83. [DOI: 10.1093/icvts/ivx141] [DOI] [PubMed] [Google Scholar]
- Lai Y, Su J, Yang M, Zhou K, Che G. Impact and effect of preoperative short-term pulmonary rehabilitation training on lung cancer patients with mild to moderate chronic obstructive pulmonary disease: a randomized trial. Chinese Journal of Lung Cancer 2016;19(11):746-54. [DOI: 10.3779/j.issn.1009-3419.2016.11.05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Che G, Liu L. A short-term high-intensive pattern of preoperative rehabilitation better suits surgical lung cancer patients. Interactive Cardiovascular and Thoracic Surgery 2017;25:i11. [DOI: 10.1093/icvts/ivx280.037] [DOI] [PubMed] [Google Scholar]
Lai 2019 {published data only (unpublished sought but not used)}
- Lai Y, Wang X, Zhou K, Su J, Che G. Impact of one-week preoperative physical training on clinical outcomes of surgical lung cancer patients with limited lung function: a randomized trial. Annals of Translational Medicine 2019;7(20):544. [DOI: 10.21037/atm.2019.09.151] [DOI] [PMC free article] [PubMed] [Google Scholar]
Licker 2016 {published data only}
- Bhatia C, Kayser B. Preoperative high-intensity interval training is effective and safe in deconditioned patients with lung cancer: a randomized clinical trial. Journal of Rehabilitation Medicine 2019;51(9):712-8. [DOI: 10.2340/16501977-2592] [DOI] [PubMed] [Google Scholar]
- Karenovics W, Licker M, Christodoulou M, Diaper J, Bhatia C, Bridevaux P, et al. Does short-term preoperative exercise therapy influence long term lung functional outcome following lung cancer surgery? Interactive Cardiovascular and Thoracic Surgery 2016;23:i2. [DOI: 10.1093/icvts/ivw260.04] [DOI] [Google Scholar]
- Karenovics W, Licker M, Ellenberger C, Christodoulou M, Diaper J, Bhatia C, et al. Short-term preoperative exercise therapy does not improve long-term outcome after lung cancer surgery: a randomized controlled study. European Journal of Cardio-thoracic Surgery 2017;52(1):47-54. [DOI: 10.1093/ejcts/ezx030] [DOI] [PubMed] [Google Scholar]
- Licker M, Karenovics W, Diaper J, Fresard I, Triponez F, Ellenberger C, et al. Short-term preoperative high-intensity interval training in patients awaiting lung Cancer surgery: a randomized controlled trial. Journal of Thoracic Oncology 2016;12(2):323-33. [DOI: 10.1016/j.jtho.2016.09.125] [DOI] [PubMed] [Google Scholar]
Liu 2020 {published data only}
- Liu Z, Qiu T, Pei L, Zhang Y, Xu L, Cui Y, et al. Two-week multimodal prehabilitation program improves perioperative functional capability in patients undergoing thoracoscopic lobectomy for lung cancer: a randomized controlled trial. Anesthesia and Analgesia 2020;131(3):840-9. [DOI: 10.1213/ANE.0000000000004342] [DOI] [PubMed] [Google Scholar]
Morano 2013 {published data only}
- Morano MT, Araujo AS, Nascimento FB, da Silva GF, Mesquita R, Pinto JS, et al. Preoperative pulmonary rehabilitation versus chest physical therapy in patients undergoing lung cancer resection: a pilot randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2013;94:53-8. [UTN Number: U1111-1122-2906] [DOI] [PubMed] [Google Scholar]
- Morano MT, Mesquita R, da Silva GF, Araujo AS, Pinto JS, Neto AG, et al. Comparison of the effects of pulmonary rehabilitation with chest physical therapy on the levels of fibrinogen and albumin in patients with lung cancer awaiting lung resection: a randomized clinical trial. BMC Pulmonary Medicine 2014;14:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira ED, Morano M, Araujo A, Nascimento F, Pinheiro G, Mesquita R, et al. Preoperative pulmonary rehabilitation versus chest physical therapy in patients undergoing lung cancer resection: a pilot randomized clinical trial. American Journal of Respiratory and Critical Care Medicine 2013;187:A5119. [DOI] [PubMed] [Google Scholar]
Pehlivan 2011 {published data only}
- Pehlivan E, Turna A, Gurses A, Gurses HN. The effects of preoperative short-term intense physical therapy in lung cancer patients: a randomized controlled trial. Annals of Thoracic and Cardiovascular Surgery 2011;17:461-8. [DOI] [PubMed] [Google Scholar]
Stefanelli 2013 {published data only}
- Stefanelli F, Meoli I, Cobuccio R, Curcio C, Amore D, Casazza D, et al. High-intensity training and cardiopulmonary exercise testing in patients with chronic obstructive pulmonary disease and non-small-cell lung cancer undergoing lobectomy. European Journal of Cardio-thoracic Surgery 2013;44:e260-5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Chen 2016 {published data only}
- Chen HM, Tsai CM, Wu YC, Lin KC, Lin CC. Effect of walking on circadian rhythms and sleep quality of patients with lung cancer: a randomised controlled trial. British Journal of Cancer 2016;115(11):1304-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferreira 2021 {published data only}
- Ferreira V, Minnella EM, Awasthi R, Gamsa A, Ferri L, Mulder D, et al. Multimodal prehabilitation for lung cancer surgery: a randomized controlled trial. Annals of Thoracic Surgery 2021;112(5):1600-8. [DOI] [PubMed] [Google Scholar]
Garofano 2018 {published data only}
- Ellenberger C, Garofano N, Reynaud T, Triponez F, Diaper J, Bridevaux PO, et al. Patient and procedural features predicting early and mid-term outcome after radical surgery for non-small cell lung cancer. Journal of Thoracic Disease 2018;10(11):6020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofano N, Ellenberger C, Reynaud T, Triponez F, Diaper J, Bridevaux PO, et al. Patient and procedural features predicting early and mid-term outcome after radical surgery for non-small cell lung cancer. Swiss Medical Weekly 2018;148(5S):-. [DOI] [PMC free article] [PubMed] [Google Scholar]
Han 2016 {published data only}
- Han R, Lin HS. A clinical study on therapeutic effects of fitness Qigong Baduanjin on pulmonary function and quality of life of post-operative non-small cell lung cancer patients. Tianjin Journal of Traditional Chinese Medicine 2016;33(12):715-8. [Google Scholar]
Horváth 2017 {published data only}
- Horváth K, Bán B, Szántó Z, Ács P, Boncz I, Molics B. Physiotherapeutic methods aimed to improve the cardiorespiratory state before and after operation in patients with lung cancer. Value in Health 2017;20:A464. [Google Scholar]
Hsiao 2018 {published data only}
- Hsiao WL, Wang TJ. Testing efficacy of a pulmonary rehabilitation program for post lung cancer resection surgery. Supportive Care in Cancer 2018;26(2):S283. [Google Scholar]
Jonsson 2019 {published data only}
- Jonsson M, Hurtig-Wennlof A, Ahlsson A, Vidlund M, Cao Y, Westerdahl E. In-hospital physiotherapy and physical recovery 3 months after lung cancer surgery: a randomized controlled trial. Integrative Cancer Therapies 2019;18:1534735419876346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson M, Hurtig-Wennlof A, Ahlsson A, Vidlund M, Cao Y, Westerdahl E. In-hospital physiotherapy improves physical activity level after lung cancer surgery: a randomized controlled trial. Physiotherapy 2019;105(4):434-41. [DOI] [PubMed] [Google Scholar]
Laurent 2020 {published data only}
- Laurent H, Aubreton S, Galvaing G, Pereira B, Merle P, Richard R, et al. Preoperative respiratory muscle endurance training improves ventilatory capacity and prevents pulmonary postoperative complications after lung surgery: a randomized controlled trial. European Journal of Physical and Rehabilitation Medicine 2020;56(1):73-81. [DOI] [PubMed] [Google Scholar]
Meng 2018 {published data only}
- Meng SL, Yang F, Dai FQ, Chen S, Huang C, Tan Q, et al. Effect of a high intensive preoperative rehabilitation on the perioperative complications in patients with chronic obstructive pulmonary disease eligible for lung cancer surgery. Chinese Journal of Lung Cancer 2018;21(11):841-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2021 {published data only}
- Patel YS, Churchill IF, Sullivan KA Beauchamp M, Wald J, Mbuagbaw L, et al. OA04.01 Move for surgery – a novel preconditioning program to optimize health before thoracic surgery: a randomized controlled trial. Journal of Thoracic Oncology 2021;10:S852-3. [Google Scholar]
Tenconi 2017 {published data only}
- Tenconi S, Galeone C, Fugazzaro S, Rapicetta C, Piro R, Formisano D. Perioperative and long-term effects of comprehensive pulmonary rehabilitation on exercise capacity, postoperative outcome and quality of life in patients undergoing lung resection: a randomized controlled trial granted by the ministry of health. Interactive Cardiovascular and Thoracic Surgery 2017;25:i25. [Google Scholar]
Vagvolgyi 2017 {published data only}
- Vagvolgyi A, Rozgonyi Z, Kerti M, Vadasz P, Varga J. Effectiveness of perioperative pulmonary rehabilitation in thoracic surgery. Journal of Thoracic Disease 2017;9(6):1584-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2017 {published data only}
- Zhou K, Su J, Lai Y, Li P, Li S, Che G. Short-term inpatient-based high-intensive pulmonary rehabilitation for lung cancer patients: is it feasible and effective? Journal of Thoracic Disease 2017;9(11):4486-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Agostini 2010
- Agostini P, Cieslik H, Rathinam S, Bishay E, Kalkat M, Rajesh P, et al. Postoperative pulmonary complications following thoracic surgery: are there any modifiable risk factors? Thorax 2010;65:815-8. [DOI] [PubMed] [Google Scholar]
AIHW 2019
- Australian Institute of Health and Welfare. Cancer in Australia 2019. Cancer series no.119. Cat. no. CAN 123. Canberra: AIHW. www.aihw.gov.au/getmedia/8c9fcf52-0055-41a0-96d9-f81b0feb98cf/aihw-can-123.pdf.aspx (accessed 25 August 2021).
Altman 1996
- Altman DG, Bland JM. Statistics notes: detecting skewness from summary information. BMJ 1996;313(7066):1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Armstrong 1984
- Armstrong RB. Mechanisms of exercise-induced delayed onset muscular soreness: a brief review. Medicine and Science in Sports and Exercise 1984;16(6):529-38. [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Eccles M, Flottorp S, Guyatt GH, Henry D, Hill S, et al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches the GRADE Working Group. BMC Health Services Research 2004;4(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benzo 2007
- Benzo R, Kelley G, Recchi L, Hofman A, Sciurba F. Complications of lung resection and exercise capacity: a meta-analysis. Respiratory Medicine 2007;101(8):1790-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Billé 2021
- Billé A, Buxton J, Viviano A, Gammon D, Veres L, Routledge T, et al. Preoperative physical activity predicts surgical outcomes following lung cancer resection. Integrative Cancer Therapies 2021;20:1534735420975853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brunelli 2009
- Brunelli A, Belardinelli R, Refai M, Salati M, Socci L, Pompili C, et al. Peak oxygen consumption during cardiopulmonary exercise test improves risk stratification in candidates to major lung resection. Chest 2009;135(5):1260-7. [DOI] [PubMed] [Google Scholar]
Brunelli 2013
- Brunelli A, Kim AW, Berger KI, Addrizzo-Harris DJ. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143(5):e166S-90S. [DOI] [PubMed] [Google Scholar]
Caspersen 1985
- Caspersen C. Physical activity, exercise and physical fitness: definitions and distinctions for health related research. Public Health Reports 1985;100(2):126-31. [PMC free article] [PubMed] [Google Scholar]
Cavalheri 2013
- Cavalheri V, Jenkin S, Hill K. Physiotherapy practice patterns for patients undergoing surgery for lung cancer: a survey of hospitals in Australia and New Zealand. Internal Medicine Journal 2013;43(4):394-401. [DOI] [PubMed] [Google Scholar]
Cavalheri 2015
- Cavalheri V, Jenkins S, Cecins N, Gain K, Phillips M, Sanders LH, et al. Impairments after curative intent treatment for non-small cell lung cancer: a comparison with age and gender-matched healthy controls. Respiratory Medicine 2015;109(10):1332-9. [DOI] [PubMed] [Google Scholar]
Cavalheri 2020
- Cavalheri V, Granger CL. Exercise training as part of lung cancer therapy. Respirology 2020;25(S2):80-7. [DOI] [PubMed] [Google Scholar]
Cavelheri 2019
- Cavalheri V, Burtin C, Formico VR, Nonoyama ML, Jenkins S, Spruit MA, et al. Exercise training undertaken by people within 12 months of lung resection for non-small cell lung cancer. Cochrane Database of Systematic Reviews 2019, Issue 6. Art. No: CD009955. [DOI: 10.1002/14651858.CD009955.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooley 2000
- Cooley ME. Symptoms in adults with lung cancer. A systematic research review. Journal of Pain and Symptom Management 2000;19(2):137-53. [DOI] [PubMed] [Google Scholar]
Coups 2009
- Coups E, Park B, Feinstein M, Steingart R, Egleston B, Wilson D, et al. Physical activity among lung cancer survivors: changes across the cancer trajectory and associations with quality of life. Cancer Epidemiology, Biomarkers & Prevention 2009;18(2):664-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed 25 November 2021. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Dela Cruz 2011
- Dela Cruz C, Tanoue L, Matthay R. Lung cancer: epidemiology, etiology, and prevention. Clinics in Chest Medicine 2011;32(4):605-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [DOI] [PubMed] [Google Scholar]
Evans 2019
- Evans JM, Ray A, Dale M, Morgan H, Dimmock P, Carolan-Rees G. Thopaz+ Portable Digital System for managing chest drains: a NICE medical technology Guidance. Applied Health Economics and Health Policy 2019;17(3):285-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferlay 2021
- Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, et al. Cancer statistics for the year 2020: an overview. International Journal of Cancer 2021;149(4):778-89. [DOI] [PubMed] [Google Scholar]
Granger 2014
- Granger C, McDonald C, Irving L, Clark R, Gough K, Murnane A, et al. Low physical activity levels and functional decline in individuals with lung cancer. Lung Cancer 2014;83(2):292-9. [DOI] [PubMed] [Google Scholar]
Granger 2015
- Granger CL, Holland AE, Gordon IR, Denehy L. Minimal important difference of the 6-minute walk distance in lung cancer. Chronic Respiratory Disease 2015;12(2):146-54. [DOI] [PubMed] [Google Scholar]
Gravier 2022
- Gravier F-E, Smondack P, Prieur G, Medrinal C, Combret Y, Muir J-F, et al. Effects of exercise training in people with non-small cell lung cancer before lung resection: a systematic review and meta-analysis. Thorax 2022;77(5):486-496(5):486-96. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Available from training.cochrane.org/handbook/archive/v6.2.
Hung 2011
- Hung R, Krebs P, Coups E, Feinstein M, Park B, Burkhalter J, et al. Fatigue and functional impairment in early-stage non-small cell lung cancer survivors. Journal of Pain and Symptom Management 2011;41(2):426-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Institute of Medicine 2015
- Institute of Medicine (IOM). Transforming Health Care Scheduling and Access: Getting to Now. Washington (DC): The National Academies Press, 2015. [PubMed] [Google Scholar]
Jones 2009
- Jones L, Eves N, Haykowsky M, Freedland S, Mackey J. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncology 2009;10(6):598-605. [DOI] [PubMed] [Google Scholar]
Li 2019
- Li X, Li S, Yan S, Wang Y, Wang X, Sihoe AD, et al. Impact of preoperative exercise therapy on surgical outcomes in lung cancer patients with or without COPD: a systematic review and meta-analysis. Cancer Management and Research 2019;11:1765-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Loewen 2007
- Loewen GM, Watson D, Kohman L, Herndon JE, Shennib H, Kernstine K, et al. Preoperative exercise VO2 measurement for lung resection candidates: results of Cancer and Leukemia Group B Protocol 9238. Journal of Thoracic Oncology 2007;2(7):619-25. [DOI] [PubMed] [Google Scholar]
Lugg 2016
- Lugg ST, Agostini PJ, Tikka T, Kerr A, Adams K, Bishay E, et al. Long-term impact of developing a postoperative pulmonary complication after lung surgery. Thorax 2016;71(2):171-6. [DOI] [PubMed] [Google Scholar]
McCarthy 2015
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2015, Issue 2. Art. No: CD003793. [DOI: 10.1002/14651858.CD003793.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
McKenna 2006
- McKenna R, Houck W, Fuller C. Video-assisted thoracic surgery lobectomy: experience with 1100 cases. Annals of Thoracic Surgery 2006;81(2):421-5. [DOI] [PubMed] [Google Scholar]
McTiernan 2008
- McTiernan A. Mechanisms linking physical activity with cancer. Nature Reviews. Cancer 2008;8(3):205-11. [DOI] [PubMed] [Google Scholar]
NCCN 2021
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): non-small cell lung cancer. 2021. www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (accessed 25 August 2021).
Novoa 2009
- Novoa N, Varela G, Jimenez M, Aranda J. Influence of major pulmonary resection on postoperative daily ambulatory activity of the patients. Interactive Cardiovascular and Thoracic Surgery 2009;9(6):934-8. [DOI] [PubMed] [Google Scholar]
Pan 2012
- Pan HH, Lin KC, Ho ST, Liang CY, Lee SC, Wang KY. Factors related to daily life interference in lung cancer patients: a cross-sectional regression tree study. European Journal of Oncology Nursing 2012;16(4):345-52. [DOI] [PubMed] [Google Scholar]
Pompili 2011
- Pompili C, Brunelli A, Salati M, Refai M, Sabbatini A. Impact of the learning curve in the use of a novel electronic chest drainage system after pulmonary lobectomy: a case-matched analysis on the duration of chest tube usage. Interactive Cardiovascular and Thoracic Surgery 2011;13(5):490-3. [DOI] [PubMed] [Google Scholar]
Pouwels 2015
- Pouwels S, Fiddelaers J, Teijink JA, Woorst JF, Siebenga J, Smeenk FW. Preoperative exercise therapy in lung surgery patients: a systematic review. Respiratory Medicine 2015;109(12):1495-504. [DOI] [PubMed] [Google Scholar]
Reeve 2010
- Reeve JC, Nicol K, Stiller K, McPherson KM, Birch P, Gordon IR, et al. Does physiotherapy reduce the incidence of postoperative pulmonary complications following pulmonary resection via open thoracotomy? A preliminary randomised single-blind clinical trial. European Journal of Cardio-thoracic Surgery 2010;37(5):1158-66. [DOI] [PubMed] [Google Scholar]
Review Manager Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.12.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Rodriguez‐Larrad 2014
- Rodriguez-Larrad A, Lascurain-Aguirrebena I, Abecia-Inchaurregui L, Seco J. Perioperative physiotherapy in patients undergoing lung cancer resection. Interactive Cardiovascular and Thoracic Surgery 2014;19:269-81. [DOI] [PubMed] [Google Scholar]
Rosen 2016
- Rosen JE, Keshava HB, Yao X, Kim AW, Detterbeck FC, Boffa DJ. The natural history of operable non-small cell lung cancer in the National Cancer Database. Annals of Thoracic Surgery 2016;101(5):1850-5. [DOI] [PubMed] [Google Scholar]
Rosero 2019
- Rosero ID, Ramírez-Vélez R, Lucia A, Martínez-Velilla N, Santos-Lozano A, Valenzuela PL, et al. Systematic review and meta-analysis of randomized, controlled trials on preoperative physical exercise interventions in patients with non-small-cell lung cancer. Cancers 2019;11(7):944. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmitz 2010
- Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Medicine and Science in Sports and Exercise 2010;42(7):1409-26. [DOI] [PubMed] [Google Scholar]
Sebio Garcia 2016
- Sebio Garcia R, Yanez Brage MI, Gimenez Moolhuyzen E, Granger CL, Denehy L. Functional and postoperative outcomes after preoperative exercise training in patients with lung cancer: a systematic review and meta-analysis. Interactive Cardiovascular and Thoracic Surgery 2016;23:486-97. [DOI] [PubMed] [Google Scholar]
SEER 2018
- National Cancer Institute Surveillance, Epidemiology, and End Results program. Cancer stat facts: lung and bronchus cancer. seer.cancer.gov/statfacts/html/lungb.html (accessed 25 August 2021).
Siegel 2019
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: a Cancer Journal for Clinicians 2019;69(1):7-34. [DOI] [PubMed] [Google Scholar]
Spruit 2013
- Spruit MA, Singh SJ, Garvey C, Zu Wallack R, Nici L, Rochester C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. American Journal of Respiratory and Critical Care Medicine 2013;188(8):e13-64. [DOI] [PubMed] [Google Scholar]
Tanaka 2002
- Tanaka K, Akechi T, Okuyama T, Nishiwaki Y, Uchitomi Y. Impact of dyspnea, pain, and fatigue on daily life activities in ambulatory patients with advanced lung cancer. Journal of Pain and Symptom Management 2002;23(5):417-23. [DOI] [PubMed] [Google Scholar]
The NHS Cancer Plan 2000
- Department of Health. The NHS cancer plan: a plan for investment, a plan for reform. www.thh.nhs.uk/documents/_Departments/Cancer/NHSCancerPlan.pdf (accessed prior to 12 September 2022).
Voorn 2021
- Voorn M, Franssen R, Verlinden J, Bootsma G, Ruysscher D, Bongers B, et al. Associations between pretreatment physical performance tests and treatment complications in patients with non-small cell lung cancer: a systematic review. Critical Reviews in Oncology/Hematology 2021;158:103207. [DOI] [PubMed] [Google Scholar]
Wan 2014
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 2014;14(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xu 2022
- Xu X, Cheung D, Smith R, Lai A, Lin C. The effectiveness of pre- and post-operative rehabilitation for lung cancer: a systematic review and meta-analysis on postoperative pulmonary complications and length of hospital stay. Clinical Rehabilitation 2022;36(2):172-89. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Cavalheri 2015b
- Cavalheri V, Granger C. Preoperative exercise training for patients with non-small cell lung cancer. Cochrane Database of Systematic Reviews 2015, Issue 12. Art. No: CD012020. [DOI: 10.1002/14651858.CD012020] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cavalheri 2017
- Cavalheri V, Granger C. Preoperative exercise training for patients with non-small cell lung cancer. Cochrane Database of Systematic Reviews 2017, Issue 6. Art. No: CD012020. [DOI: 10.1002/14651858.CD012020.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


