This study evaluates the association between COVID-19 vaccination during pregnancy and peripartum outcomes.
Key Points
Question
Is COVID-19 vaccination during pregnancy associated with increased risks of peripartum adverse outcomes?
Findings
In this systematic review and meta-analysis, COVID-19 vaccination during pregnancy was not associated with increased risks of peripartum adverse outcomes, including preterm birth, small size for gestational age, low Apgar score at 5 minutes, cesarean delivery, postpartum hemorrhage, and chorioamnionitis. Furthermore, COVID-19 vaccination during pregnancy was associated with lower risks of neonatal intensive care unit admission, intrauterine fetal death, and maternal SARS-CoV-2 infection.
Meaning
In this study, COVID-19 vaccination appeared to be safe and beneficial to pregnant individuals.
Abstract
Importance
The risk and benefits of COVID-19 vaccination during pregnancy are under investigation. Pooled evidence regarding neonatal and maternal outcomes in association with COVID-19 vaccination during pregnancy is scarce.
Objective
To evaluate the association between COVID-19 vaccination during pregnancy and peripartum outcomes.
Data Sources
PubMed and EMBASE databases were searched on April 5, 2022. Language restrictions were not applied.
Study Selection
Prospective trials and observational studies comparing the individuals who received at least 1 COVID-19 vaccination during pregnancy with those who did not and reporting the neonatal outcomes, including preterm birth, small for gestational age, low Apgar score, neonatal intensive care units (NICU) admission, and intrauterine fetal death (IFD).
Data Extraction and Synthesis
Two independent investigators extracted relevant data from each study. Odds ratios (ORs) were calculated using a random-effects model. This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines.
Main Outcomes and Measures
The primary outcomes were the neonatal outcomes, including preterm birth, small for gestational age, low Apgar score, NICU admission, and IFD. The secondary outcomes were maternal outcomes, including maternal SARS-CoV-2 infection, cesarean delivery, postpartum hemorrhage, and chorioamnionitis.
Results
Nine observational studies involving 81 349 vaccinated (mean age, 32-35 years) and 255 346 unvaccinated individuals during pregnancy (mean age, 29.5-33 years) were included. COVID-19 vaccination during pregnancy was associated with lower risk of NICU admission (OR, 0.88; 95% CI, 0.80-0.97) and IFD (OR, 0.73; 95% CI, 0.57-0.94), whereas there was no statistically significant association with preterm birth (OR, 0.89; 95% CI, 0.76-1.04), small for gestational age (OR, 0.99; 95% CI, 0.94-1.04), and low Apgar score (OR, 0.94; 95% CI, 0.87-1.02). COVID-19 vaccination during pregnancy was associated with a lower risk of maternal SARS-CoV-2 infection (OR, 0.46; 95% CI, 0.22-0.93), whereas it was not associated with increased risk of cesarean delivery (OR, 1.05; 95% CI, 0.93-1.20), postpartum hemorrhage (OR, 0.95; 95% CI, 0.83-1.07), and chorioamnionitis (OR, 1.06; 95% CI, 0.86-1.31).
Conclusions and Relevance
COVID-19 vaccination during pregnancy was not associated with an increase in the risk of peripartum outcomes, was associated with a decreased risk of NICU admission, IFD, and maternal SARS-CoV-2 infection. Thus, COVID-19 vaccination should be encouraged for pregnant individuals.
Introduction
SARS-CoV-2 infection during pregnancy is associated with increased risks of maternal morbidity and adverse perinatal outcomes, such as hospitalization, intensive care unit admission, and death.1,2 The association between COVID-19 infection in pregnancy and adverse neonatal events has also been reported, including preterm birth, stillbirth, and neonatal or perinatal morbidity.3,4,5 Since the approval of COVID-19 messenger RNA (mRNA) vaccines, vaccination during pregnancy has been recommended to prevent illness in pregnant individuals and newborns.6 However, vaccine hesitancy during pregnancy may still exist owing to safety concerns.7,8
Initial data on COVID-19 vaccines were limited because pregnant individuals were not included in the phase 3 trials of mRNA COVID-19 vaccines that were approved in the US and the European Union.9,10 Preliminary studies for pregnant individuals did not show the increased risk of adverse neonatal outcomes, including miscarriage, preterm birth, small size for gestational age (SGA), and fetal/neonatal death, associated with mRNA COVID-19 vaccination.11,12,13 In addition, emerging evidence from large epidemiological studies has indicated that COVID-19 vaccination during pregnancy was not associated with increased risks of adverse maternal and neonatal outcomes, such as miscarriage, preterm birth, and SGA.14,15,16,17 Recently, 2 population-based observational studies from Canada and Sweden/Norway have provided further reassuring evidence regarding the safety of COVID-19 vaccination during pregnancy, using large cohort data on more than 250 000 pregnancies.18,19 However, pooled evidence from large studies regarding neonatal and maternal outcomes of COVID-19 vaccination during pregnancy is scarce. Furthermore, comparative outcomes after COVID-19 vaccines in the first, second, or third trimester are unclear.
Therefore, we conducted a systematic review and meta-analysis to investigate neonatal and maternal outcomes associated with COVID-19 vaccination during pregnancy for a better understanding of the benefits and safety of COVID-19 vaccines in pregnant individuals.
Methods
This research was conducted under Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines and registered in the International Prospective Register of Systematic Reviews (CRD42022323318).20 Because our study does not include individual patient data, an informed consent waiver and an ethics exemption were granted.
Data Sources and Search
We used a 2-level strategy to search for all prospective trials and observational studies that investigated the neonatal outcomes in association with COVID-19 vaccination during pregnancy. First, a comprehensive literature search was conducted using the PubMed and EMBASE databases on April 5, 2022. The search terms included (“COVID-19” or “SARS-CoV-2”) and (“vaccination” or “vaccine”) and (during pregnancy) and (“neonates” or “neonatal,” or “birth” or “baby”). Second, we performed an additional manual search of secondary sources, such as references of initially identified studies, to collect relevant articles comprehensively. No restrictions on language, publication date, and publication status were applied.
Eligibility Criteria
Studies meeting the following criteria were included in our review: (1) the study was published in a peer-reviewed journal, (2) the study compared pregnant individuals who received at least 1 COVID-19 vaccination during pregnancy with those who did not, (3) the study reported at least 1 of the following neonatal outcomes, preterm birth (delivery at <37 weeks’ gestation), SGA (birth weight below the 10th percentile standardized for gestational age and sex), low Apgar score (Apgar score at 5 minutes <7), neonatal intensive care unit (NICU) admission, and intrauterine fetal death (IFD). Articles without original patient data (eg, guidelines, correspondence, research letters, and reviews) were excluded. The risk of bias of the included studies was evaluated using a tool for assessing risk of bias in nonrandomized studies (ROBINS).21 The overall quality of each study was assessed using GRADE approach.22
Data Extraction
Two investigators (A.W. and J.Y.) reviewed the search results separately to identify the studies based on the inclusion and exclusion criteria and assessed the eligibility for each study. After screening the articles based on title and abstract, we then retrieved the full texts of potentially eligible studies for further review. Disagreements were resolved through consensus or the third investigator (T.K.).
Data Items
Baseline characteristics, such as age, comorbidities, smoking status, the proportion of twins, obesity (BMI >30), and nulliparous were extracted. Regarding the COVID-19 vaccine, we collected the type of vaccine (eg, mRNA and viral vector), doses, and the timing of the first injection (first, second, and third trimester [<14 weeks, 14-28 weeks, and >28 weeks gestation, respectively]). The primary outcomes of this study were preterm birth, SGA, NICU admission, low Apgar score, and IFD. Secondary outcomes included maternal SARS-CoV-2 infection, postpartum hemorrhage, cesarean delivery, and chorioamnionitis. The definition of postpartum hemorrhage followed each study.
Data Synthesis and Analysis
The unadjusted and adjusted (whenever available) odds ratios (ORs) of each study were extracted. For studies that used propensity score analyses, we extracted the outcomes estimated by propensity score matching or inverse probability treatment weighting. The OR with a 95% CI of each outcome was calculated using the Review Manager (RevMan) version 5.4 (Nordic Cochrane Center, the Cochrane Collaboration) with a random-effects model. Heterogeneity was assessed using I2, with more than 50% indicating substantial heterogeneity. As secondary analyses, we compared the frequency of preterm birth and SGA in the 2 subgroups: (1) pregnant individuals who received the first vaccination during the first trimester vs those who did not receive vaccination during pregnancy, (2) pregnant individuals who received the first vaccination during the second and third trimester vs those who did not receive vaccination during pregnancy. Publication bias was assessed by Egger linear regression tests and funnel plots of the primary outcomes in each study using ProMeta 3.0.23
Results
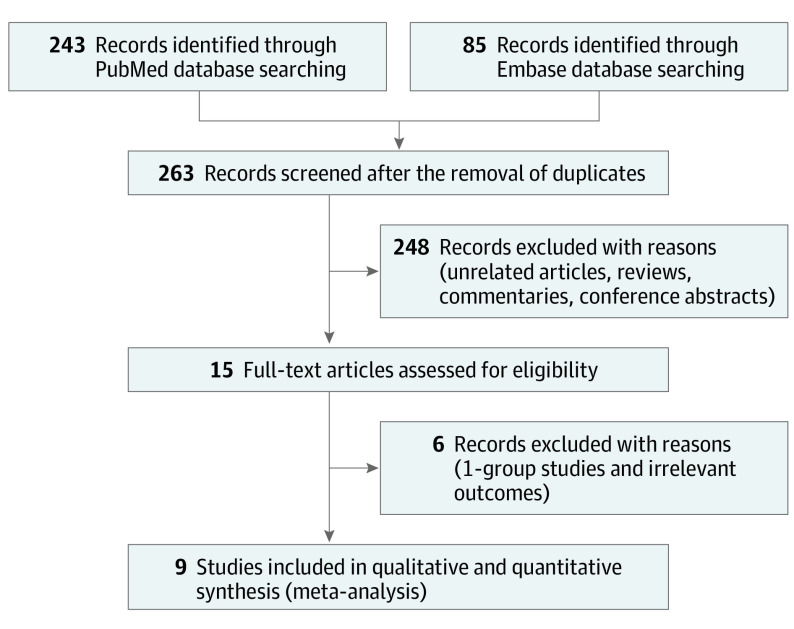
The study team identified 263 articles by the initial database search and subsequent manual search. After removing 248 items based on the title and abstract, the study team retrieved the full text of 15 articles. Six were excluded either because they did not have a comparison between vaccinated pregnant individuals and those unvaccinated or they reported irrelevant outcomes. Ultimately, the study team included 81 349 pregnant individuals who received at least 1 COVID-19 vaccination during pregnancy (vaccinated group) and 255 346 pregnant individuals who did not (unvaccinated group) from 9 observational studies15,17,18,19,24,25,26,27,28 (Figure 1). The risk of bias assessment and the overall quality of each study were summarized in eFigure 1 and eTable 1 in the Supplement. The overall quality of evidence of the most studies was graded low or moderate level of certainty (Table 1).
Figure 1. Flowchart of Study Selection.
Of the 263 identified articles, 9 studies comparing neonatal/maternal outcomes in individuals with vs without COVID-19 vaccination during pregnancy.
Table 1. Baseline Characteristics.
| Source | Vaccination status | Country | Observational period | Cohort size, No. | Vaccine type, No. (2 doses during pregnancy)a | Vaccination timing, No. | Age, mean (SD), y | No. (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nulliparous | Comorbiditiesb | Smoking historyc | Obesity | Twins | ||||||||
| Mayo et al,24 2021 | Vaccinated | Israel | April 2020-June 2021 | 125 | Vaccine type unavailable (125) | 1st, 9; 2nd, 80; 3rd, 36 | 31.4 (6.1) | NA | HTN, 5 (4.0); diabetes, 9, (7.2); asthma, 1 (0.8); thyroid disease, 8 (6.4) | 5 (4.0) | NA | NA |
| Unvaccinated | 369 | NA | NA | 29.5 (5.5) | NA | HTN, 12 (3.3); diabetes, 19 (5.1); asthma, 7 (1.9); thyroid disease, 14 (3.8) | 11 (3.0) | NA | NA | |||
| Theiler et al,25 2021 | Vaccinated | US | December 2020-April 2021 | 140 | Pfizer-BioNTech, 127 (97), Moderna, 12 (6), Janssen/Johnson & Johnson (1) | NA | 31.8 (3.7) | 56 (40) | HTN, 6 (4.3); diabetes, 2 (1.4); asthma, 15 (10.7); infertility, 6 (4.3) | 0 | 33 (23.6) | 2 (1.4) |
| Unvaccinated | 1862 | NA | NA | 30.5 (5.2) | 546 (29.3) | HTN, 64 (3.4); diabetes, 11 (0.6); asthma, 206 (11.1); infertility, 14 (0.8) | 196 (10.5) | 464 (24.9) | 22 (1.2) | |||
| Rottenstreich et al,26 2022 | Vaccinated | Israel | January 2021-April 2021 | 712 | Pfizer-BioNTech (712) | 1st, 0; 2nd, 0; 3rd, 712 | 30.6 (5.8) | 122 (17.1) | Previous miscarriage, 240 (33.7); HTN, 10 (1.4); diabetes, 45 (6.3); infertility, 33 (4.6) | NA | 101 (14.2) | 16 (2.2) |
| Unvaccinated | 1063 | NA | NA | 29.5 (6) | 211 (19.8) | Previous miscarriage, 296 (27.8); HTN, 19 (1.8); diabetes, 45 (4.2); infertility, 24 (2.3) | NA | 140 (13.2) | 15 (1.4) | |||
| Lipkind et al,17 2022 | Vaccinated | US | December 2020-July 2021 | 10 064 | Pfizer-BioNTech, 5478, Moderna, 4162 (7881), Janssen/ Johnson & Johnson, 424 | 1st, 172; 2nd, 3668; 3rd, 6224 | 32.3 (4.5) | NA | HTN, 552 (5.5); diabetes, 167 (1.7); asthma, 802 (8.0); cancer, 28 (0.3); SLE, 20 (0.2); liver disease, 97 (1.0); cardiovascular disease, 43 (0.4); | 1786 (17.7) | 2407 (23.9) | 0 |
| Unvaccinated | 36 015 | NA | NA | 29.8 (5.3) | NA | HTN, 1732 (4.8); diabetes, 611 (1.7); asthma, 2733 (7.6); cancer, 120 (0.3); SLE, 103 (0.3); liver disease, 417 (1.2); cardiovascular disease, 104 (0.3) | 7242 (20.1) | 10 426 (28.9) | 0 | |||
| Blakeway et al,27 2022 | Vaccinated | United Kingdom | March 2021-April 2021 | 140 | Pfizer-BioNTech, 109 (NA), Moderna, 18 (NA), AstraZeneca, 13 | 1st, 0; 2nd, 20; 3rd, 120 | 35 (31.7-37) | 78 (55.7) | HTN, 13 (9.3); diabetes, 26 (18.6); cardiovascular disease, 1 (0.7) | 1 (0.7) | 15 (10.7) | 4 (2.9) |
| Unvaccinated | 1188 | NA | NA | Median (range), 33 (30-36) | 593 (49.9) | HTN, 46 (3.9); diabetes, 153 (12.9); cardiovascular disease, 10 (0.8) | 27 (2.3) | 173 (14.6) | 24 (2.0) | |||
| Goldshtein et al,15 2022 | Vaccinated | Israel | March 2021-September 2021 | 16 697 | Pfizer-BioNTech, 16 697 (NA) | 1st, 2134; 2nd, 9364; 3rd, 5199 | 31.6 (5.2) | 5555 (33.3) | HTN, 159 (1.0); diabetes, 145 (0.9); infertility, 304 (1.8); cancer, 168 (1.0); CKD, 118 (0.7) | 798 (4.8) | 1768 (10.6) | 0 |
| Unvaccinated | 7591 | NA | NA | 30.5 (5.7) | 2484 (32.7) | HTN, 76 (1.0); diabetes, 59 (0.8); infertility, 84 (1.1); cancer, 55 (0.7); CKD, 67 (0.9) | 441 (5.8) | 862 (11.4) | 0 | |||
| Dick et al,28 2022 | Vaccinated | Israel | December 2020-July 2021 | 2305 | Pfizer-BioNTech, Moderna (NA) | 1st, 12; 2nd, 964; 3rd, 1329 | Median (range), 30 (26-34) | 611 (26.5) | HTN, 25 (1.1); diabetes, 222 (9.6) | 79 (3.4) | NA | 0 |
| Unvaccinated | 3313 | NA | NA | Median (range), 30 (26-34) | 838 (25.3) | HTN, 44 (1.3); diabetes, 275 (8.3) | 88 (2.7) | NA | 0 | |||
| Fell et al,18 2022 | Vaccinated | Canada | December 2020-September 2021 | 22 660 | Pfizer-BioNTech, 18 101, Moderna, 4507 (21 894),c others, 52 | NA | 32.8 (4.3) | 10 382 (46.1) | HTN, 202 (0.9); diabetes, 234 (1.0); asthma, 935 (4.1); thyroid disease, 1531 (6.8); cardiovascular disease, 43 (0.2) | 723 (3.3) | 4096 (20.0) | 328 (1.4) |
| Unvaccinated | 74 930 | NA | NA | 32.0 (4.8) | 31 965 (42.8) | HTN, 729 (3.2); diabetes, 836 (1.1); asthma, 2886 (3.9); thyroid disease, 3977 (5.3); cardiovascular disease, 66 (0.1) | 5657 (7.7) | 14 043 (21.1) | 1064 (1.4) | |||
| Magnus et al,19 2022 | Vaccinated | Sweden | January 2021-January 2022 | 28 506 | Pfizer-BioNTech, 20 424, Moderna, 7607 (21 580),c AstraZeneca, 475 | 1st, 1125; 2nd, 13 012; 3rd, 1468 | 32.2 (4.6) | 12 450 (43.7) | HTN, 185 (0.6); diabetes, 417 (1.5); asthma, 2456 (8.6); CKD, 129 (0.5); cardiovascular disease, 433 (1.5); VTE, 219 (0.8) | 657 (2.3) | NA | 0 |
| Unvaccinated | 129 015 | NA | NA | 30.5 (4.8) | 54 306 (42.1) | HTN, 685 (0.5); diabetes, 1201 (0.9); asthma, 8826 (6.8); CKD, 627 (0.5); cardiovascular disease, 1563 (1.2); VTE, 775 (0.6) | 5268 (4.1) | NA | 0 | |||
Abbreviations: CKD, chronic kidney disease; HTN, hypertension; NA, not available; SLE, systemic lupus erythematosus; VTE, venous thromboembolism; 1st, during the first trimester; 2nd, during the second trimester; 3rd, during the third trimester.
The total number of individuals who received the second dose of messenger RNA vaccines during pregnancy regardless of brands.
Hypertension and diabetes include both pregestational and gestational conditions.
Smoking history includes ever-smoker and smoking during pregnancy.
Baseline Characteristics
The mean or median age ranged from 32 to 35 years in the vaccinated group and from 29.5 to 33 years in the unvaccinated group. The proportion of comorbidities were as follows: pregestational/gestational diabetes, 1267 of 81 349 (1.6%) and 3210 of 255 346 (1.3%); pregestational/gestational hypertension, 1176 of 81 349 (1.4%) and 3632 of 255 346 (1.4%); obesity, 8420 of 48 231 (17.5%) and 26 108 of 114 355 (22.8%); smoking history, 4049 of 80 035 (5.1%) and 7.5% 18 930 of 252 990 (7.5%) in vaccinated and unvaccinated pregnant individuals, respectively. Nulliparous consisted 29 254 of 71 031 (41.2%) and 90 943 of 218 666 (41.6%) births in vaccinated and unvaccinated pregnant individuals, respectively. Of the included births, 350 of 81 224 (0.4%) vaccinated and 1125 of 254 997 (0.4%) unvaccinated pregnant individuals were nonsingletons. For vaccinated pregnant individuals, 98.2% received mRNA vaccines (Pfizer-BioNTech, 61 288; Moderna, 16 036; unstipulated, 2575), 1.1% received viral vector vaccine (AstraZeneca, 488; Janssen/Johnson & Johnson, 425), and 0.7% were not clearly documented. Six studies reported the number of doses; 52 295 of 61 255 (85.4%) received 2 doses of mRNA vaccines during pregnancy. Seven studies reported the timing of the first vaccination; 3452 of 58 548 (5.9%), 27 108 of 58 548 (46.3%), and 27 988 of 58 548 (47.8%) of the pregnant individuals received the first injection during the first, second, and third trimester, respectively.
Neonatal Outcomes
The COVID-19 vaccination during pregnancy was associated with lower risks of NICU admission (OR, 0.88; 95% CI, 0.80-0.97) and IFD (OR, 0.73; 95% CI, 0.57-0.94) (Figure 2A and B). The other primary outcomes did not show statistically significant differences between the 2 groups: preterm birth (OR, 0.89; 95% CI 0.76-1.04), SGA (OR, 0.99; 95% CI, 0.94-1.04) (Figure 2C and D), and low Apgar score (OR, 0.94; 95% CI, 0.87-1.02) (eFigure 3 in the Supplement). The outcome of each study is summarized in eTable 2 in the Supplement. Significant publication bias was not detected (eTable 3 and eFigure 2 in the Supplement), although the Egger test did not have enough power to fully detect the publication bias because the study team included only 9 studies.
Figure 2. Forest Plots Showing the Odds Ratio (OR) of Neonatal Outcomes.
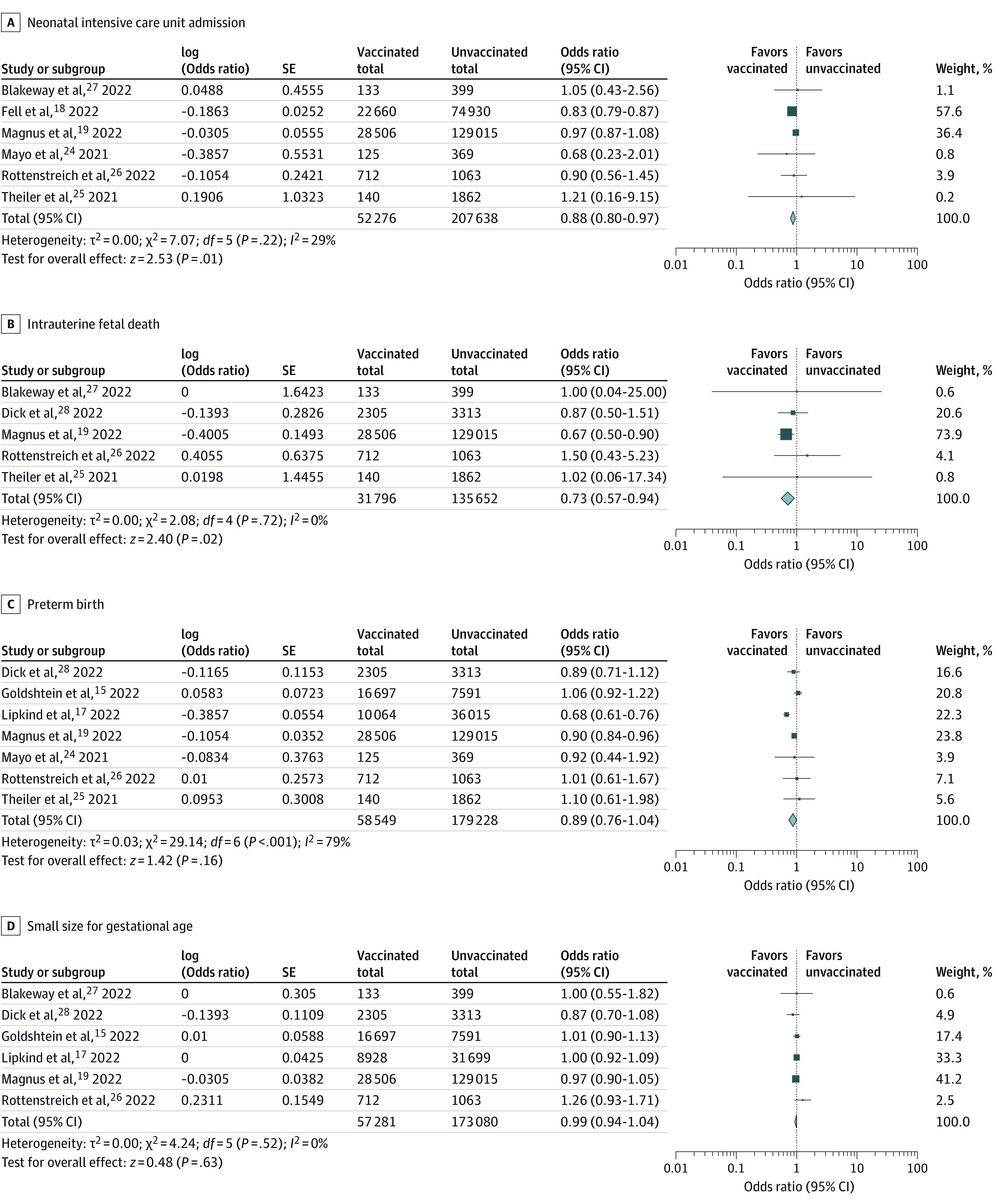
COVID-19 vaccination during pregnancy was associated with lower risks of neonatal intensive care unit admission and intrauterine fetal death. There were no associations between COVID-19 vaccination during pregnancy and preterm birth and small size for gestational age. ORs were calculated using random-effects model.
Four studies separately reported the incidence of preterm birth and SGA according to the time of the first vaccination.15,17,19,28 Between the pregnant individuals who received the first vaccination during the first trimester vs those who did not receive COVID-19 vaccination during pregnancy, the incidence of preterm birth (OR, 1.81; 95% CI, 0.94-3.46) and SGA (OR, 1.09; 95% CI, 0.95-1.27) were not significantly different (Table 2; eFigures 4 and 5 in the Supplement). In contrast, COVID-19 vaccination during the second or third trimester was associated with lower risks of preterm birth (OR, 0.80; 95% CI, 0.69-0.92) and SGA (OR, 0.94; 95%, CI 0.88-1.00) vs those who did not receive COVID-19 vaccination during pregnancy (eFigures 5 and 6 in the Supplement).
Table 2. Neonatal Outcomes and the Timing of the First COVID-19 Vaccination.
| Outcomes | No. | OR (95% CI) | |
|---|---|---|---|
| Vaccinated during pregnancy | Unvaccinated during pregnancy | ||
| Preterm birth | |||
| 1st Trimester vs unvaccinated | 3443 | 171 927 | 1.81 (0.94-3.46) |
| 2nd & 3rd Trimester vs unvaccinated | 54 218 | 171 927 | 0.875 (0.63-0.90) |
| SGA | |||
| 1st Trimester vs unvaccinated | 3249 | 165 741 | 1.09 (0.95-1.27) |
| 2nd & 3rd Trimester vs unvaccinated | 52 000 | 165 741 | 0.94 (0.88-1.00) |
Abbreviations: OR, odds ratio; SGA, small for gestational age.
Maternal Outcomes
The COVID-19 vaccination during pregnancy was significantly associated with a lower risk of maternal SARS-CoV-2 infection over the follow-up periods (OR, 0.46; 95% CI, 0.22-0.93) (Figure 3A). In contrast, the COVID-19 vaccinations during pregnancy were not associated with higher risks of cesarean delivery (OR, 1.05; 95% CI, 0.93-1.20), postpartum hemorrhage (OR, 0.95; 95% CI, 0.83-1.07) (the definition in each study is available in eTable 4 in the Supplement), and chorioamnionitis (OR, 1.06; 95% CI, 0.86-1.31) (Figure 3B-D).
Figure 3. Forest Plots Showing the Odds Ratio (OR) of Maternal Outcomes.
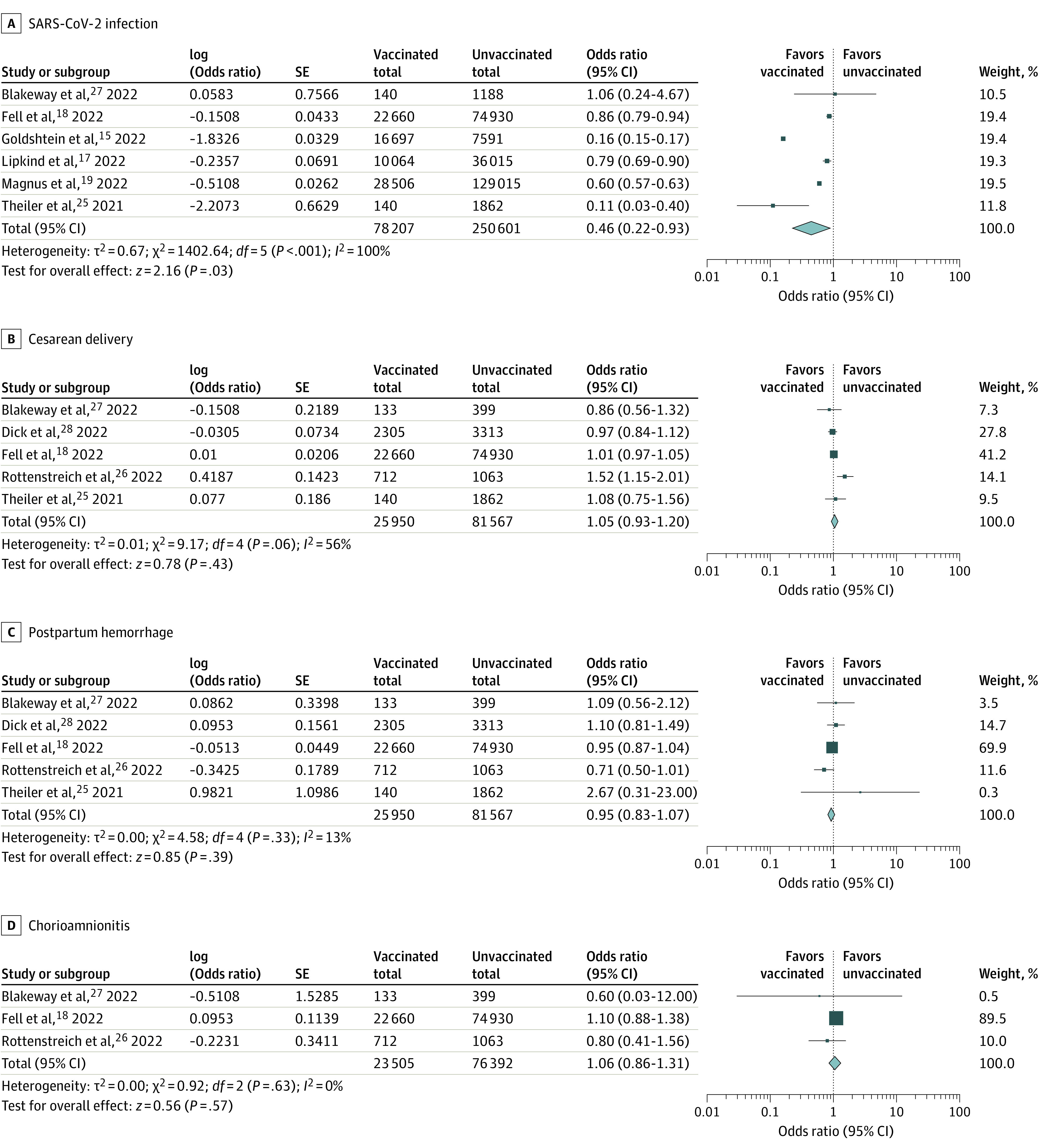
COVID-19 vaccination was associated with a lower risk of maternal SARS-CoV-2 infection. There was no association between COVID-19 vaccination during pregnancy and cesarean delivery, postpartum hemorrhage, and chorioamnionitis. ORs were calculated using random-effects model.
Discussion
In this meta-analysis of 9 studies including 81 349 pregnant individuals who received COVID-19 vaccination during pregnancy and 255 346 of those who did not, we demonstrated that COVID-19 vaccination was not associated with increased risk of neonatal and maternal adverse outcomes, regardless of the timing of the first dose.
COVID-19 vaccination during pregnancy was not associated with increased risks of neonatal outcomes, including preterm birth, SGA, and low Apgar score. Furthermore, it was associated with lower risks of NICU admission and IFD. This positive association between COVID-19 vaccination during pregnancy and neonatal outcomes is plausible because SARS-CoV-2 infection in pregnant individuals may be associated with higher risks of NICU admission, IFD, and perinatal mortality.4,5 According to previous multicenter studies, the COVID-19 severity appeared to be related to worse maternal and neonatal outcomes.4,29 In particular, since most pregnant individuals with COVID-19 who required intensive care were unvaccinated,29 maternal protection against SARS-CoV-2 is paramount. Moreover, it should also be noted that even asymptomatic SARS-CoV-2 infection was associated with higher risks of maternal outcomes, including preeclampsia and preterm labor.4 Given the promising efficacy of COVID-19 vaccination in preventing maternal SARS-CoV-2 infection and the critical association between COVID-19 and neonatal/maternal outcomes, our findings further underlined the importance of maternal protection against SARS-CoV-2 infection.
In addition, a recent study showed that COVID-19 vaccination during early pregnancy was not associated with an increased risk of ultrasound-detectable congenital fetal structural anomalies. They detected fetal anomalies in 27 of 534 unvaccinated pregnant individuals (5.1%) and 109 of 2622 pregnant individuals (4.2%) who received at least 1 dose of vaccine and suggested no significant risk of congenital fetal anomalies stratified by COVID-19 vaccine exposure within teratogenic periods, although the generalizability of this quaternary center’s finding may be limited.30 Notwithstanding, their data were compatible with our results, showing no significant difference in the risk of preterm birth between COVID-19 vaccination during the first trimester vs nonvaccination. Our meta-analysis could contribute to establishing the safety of COVID-19 vaccines during pregnancy for newborns and will serve a critical role when counseling pregnant patients regarding the COVID-19 vaccination’s teratogenicity.
Our study also demonstrated that COVID-19 vaccination during pregnancy was not associated with adverse maternal outcomes, including cesarean delivery, postpartum hemorrhage, and chorioamnionitis. Not surprisingly, COVID-19 vaccines exhibited a significant association with a decreased risk of maternal SARS-CoV-2 infection. Two doses of COVID-19 vaccines have been shown to induce comparable immune responses in pregnant individuals vs nonpregnant individuals.31 Likewise, COVID-19 vaccines have provided high protection against documented SARS-CoV-2 infection in pregnant individuals.32 Moreover, no evidence has been shown indicating an associated with increased risk of miscarriage after COVID-19 vaccination during early pregnancy.14,16 These findings support both the safety and effectiveness of COVID-19 vaccination during pregnancy for pregnant individuals.
Although emerging data have revealed the efficacy and safety of COVID-19 vaccination during pregnancy in neonatal and maternal outcomes, vaccination rates among pregnant individuals remain low worldwide.33 Increasing vaccination rates in pregnant individuals are of paramount importance because they are at high risk for maternal morbidity and adverse perinatal outcomes. However, many pregnant individuals hesitate to receive COVID-19 vaccination despite global vaccination campaigns.29 The previous studies reported that unvaccinated individuals against SARS-CoV-2 vaccination during pregnancy were more likely to be younger and non-White, smoke during pregnancy, use illicit drugs, have a lower income, and have a lower proportion of higher education.18,19,25,26,28 Vaccine communication comprising education and recommendations can increase COVID-19 vaccine acceptance among pregnant individuals, as reported for tetanus-diphtheria-pertussis and influenza vaccines.34,35 Additionally, our findings are reassuring and encouraging for pregnant individuals to consider COVID-19 vaccination. Although vaccinated and unvaccinated populations were not precisely matched, our findings should be widely disseminated to address the disparity and vaccine hesitancy. Further studies with tailored strategies are needed to validate our findings and achieve the acceptance of COVID-19 vaccines.
Although a recent study revealed the efficacy of vaccination against SARS-CoV-2 among adolescents,36 there still remains hesitancy against vaccination among adolescents or reproductive ages.8 However, our data support the safety and efficacy of COVID-19 vaccination during pregnancy, facilitating the vaccination rates among pregnant individuals even if they do not get vaccinated before pregnancy.
Limitations
This study had several limitations. First, no clear distinction could be made between emergency and scheduled procedures for cesarean delivery and preterm birth from available data. Although clinical indications for emergency cesarean delivery (eg, placental abruption, hypertensive disorders of pregnancy, and preterm prelabor rupture of membranes) differ from elective procedures, only 1 study reported them separately.18 Further studies distinguishing this factor are warranted. Second, maternal obstetric histories were not extensively available. While more than half of the included individuals were multiparous, histories of previous cesarean delivery, hypertensive disorders of pregnancy, gestational diabetes, postpartum hemorrhage, miscarriage, preterm birth, and SGA were unobtainable, leading to the uncertainty of the baseline perinatal maternal risks. Third, the outcomes according to the timing of COVID-19 vaccination were not always reported in the included studies. As the number of vaccinated individuals during the first trimester was low, the results should be interpreted with caution. However, combining the existing publications allowed for a reliable analysis and provided no significant increased risk of neonatal and maternal outcomes associated with COVID-19 vaccination during the first trimester. Fourth, we could not assess the effect of variant types of SARS-CoV-2, such as Delta or Omicron, which may affect the effectiveness of COVID-19 vaccination.37,38 Fifth, since all included articles were observational studies, our meta-analysis does not confirm the effect of COVID-19 vaccination in randomly assigned cohorts. Additionally, despite the vaccines’ placental transportability and possible protective effect for newborns against SARS-CoV-2, long-term outcomes remain unelucidated.39 Further large cohort studies with longer follow-up periods will help investigate long-term outcomes of COVID-19 vaccination during pregnancy.
Conclusions
In this systematic review and meta-analysis, COVID-19 vaccination during pregnancy was not associated with increased adverse peripartum outcomes. Our findings suggest that COVID-19 vaccination during pregnancy is safe and beneficial to mothers and newborns.
eTable 1. GRADE evidence profile for the included studies
eTable 2. Outcomes of all studies
eTable 3. The results of Egger’s linear regression tests of the primary outcomes
eTable 4. Definitions of postpartum hemorrhage in each study
eFigure 1. Risk of bias summary
eFigure 2. Funnel plots of the primary outcomes
eFigure 3. Forest plot showing the odds ratio of low Apgar score (<7 at 5 min of birth)
eFigure 4. Forest plots showing the odds ratio of preterm birth
eFigure 5. Forest plots showing the odds ratio of small size for gestational age
eFigure 6. Forest plots showing the odds ratio of small size for gestational age (a: women who received COVID-19 vaccination during the first trimester vs. women who did not receive COVID-19 vaccination during pregnancy, b: women who received COVID-19 vaccination during the second or third trimester vs. women who did not receive COVID-19 vaccination during pregnancy)
References
- 1.Allotey J, Stallings E, Bonet M, et al. ; for PregCOV-19 Living Systematic Review Consortium . Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellington S, Strid P, Tong VT, et al. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22-June 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):769-775. doi: 10.15585/mmwr.mm6925a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karasek D, Baer RJ, McLemore MR, et al. The association of COVID-19 infection in pregnancy with preterm birth: a retrospective cohort study in California. Lancet Reg Health Am. 2021;2:100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID Multinational Cohort Study. JAMA Pediatr. 2021;175(8):817-826. doi: 10.1001/jamapediatrics.2021.1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pérez-López FR, Savirón-Cornudella R, Chedraui P, et al. Obstetric and perinatal outcomes of pregnancies with COVID 19: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2022; March 13:1-17. doi: 10.1080/14767058.2022.2051008 [DOI] [PubMed] [Google Scholar]
- 6.The American College of Obstetricians and Gynecologists . COVID-19 vaccination considerations fr obstetric-gynecologic care. Accessed April 6, 2022. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care
- 7.Skjefte M, Ngirbabul M, Akeju O, et al. COVID-19 vaccine acceptance among pregnant women and mothers of young children: results of a survey in 16 countries. Eur J Epidemiol. 2021;36(2):197-211. doi: 10.1007/s10654-021-00728-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Battarbee AN, Stockwell MS, Varner M, et al. Attitudes toward COVID-19 illness and COVID-19 vaccination among pregnant women: a cross-sectional multicenter study during August-December 2020. Am J Perinatol. 2022;39(1):75-83. doi: 10.1055/s-0041-1735878 [DOI] [PubMed] [Google Scholar]
- 9.Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. doi: 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall VJ, Foulkes S, Saei A, et al. ; SIREN Study Group . COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397(10286):1725-1735. doi: 10.1016/S0140-6736(21)00790-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimabukuro TT, Kim SY, Myers TR, et al. ; CDC v-safe COVID-19 Pregnancy Registry Team . Preliminary findings of mRNA COVID-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384(24):2273-2282. doi: 10.1056/NEJMoa2104983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zauche LH, Wallace B, Smoots AN, et al. ; CDC v-safe COVID-19 Pregnancy Registry Team . Receipt of mRNA COVID-19 vaccines and risk of spontaneous abortion. N Engl J Med. 2021;385(16):1533-1535. doi: 10.1056/NEJMc2113891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bookstein Peretz S, Regev N, Novick L, et al. Short-term outcome of pregnant women vaccinated with BNT162b2 mRNA COVID-19 vaccine. Ultrasound Obstet Gynecol. 2021;58(3):450-456. doi: 10.1002/uog.23729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magnus MC, Gjessing HK, Eide HN, Wilcox AJ, Fell DB, Håberg SE. COVID-19 vaccination during pregnancy and first-trimester miscarriage. N Engl J Med. 2021;385(21):2008-2010. doi: 10.1056/NEJMc2114466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldshtein I, Steinberg DM, Kuint J, et al. Association of BNT162b2 COVID-19 vaccination during pregnancy with neonatal and early infant outcomes. JAMA Pediatr. 2022;176(5):470-477. doi: 10.1001/jamapediatrics.2022.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kharbanda EO, Haapala J, DeSilva M, et al. Spontaneous abortion following covid-19 vaccination during pregnancy. JAMA. 2021;326(16):1629-1631. doi: 10.1001/jama.2021.15494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipkind HS, Vazquez-Benitez G, DeSilva M, et al. Receipt of COVID-19 vaccine during pregnancy and preterm or small-for-gestational-age at birth—eight integrated health care organizations, United States, December 15, 2020-July 22, 2021. MMWR Morb Mortal Wkly Rep. 2022;71(1):26-30. doi: 10.15585/mmwr.mm7101e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fell DB, Dhinsa T, Alton GD, et al. Association of COVID-19 vaccination in pregnancy with adverse peripartum outcomes. JAMA. 2022;327(15):1478-1487. doi: 10.1001/jama.2022.4255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magnus MC, Örtqvist AK, Dahlqwist E, et al. Association of SARS-CoV-2 vaccination during pregnancy with pregnancy outcomes. JAMA. 2022;327(15):1469-1477. doi: 10.1001/jama.2022.3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372(160):n160. doi: 10.1136/bmj.n160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayo RP, Raz T, David BB, et al. Waning of the humoral response to SARS-CoV-2 in pregnancy is variant-dependent. bioRxiv. Published online November 3, 2021. doi: 10.1101/2021.11.03.21265478 [DOI]
- 25.Theiler RN, Wick M, Mehta R, Weaver AL, Virk A, Swift M. Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy. Am J Obstet Gynecol MFM. 2021;3(6):100467. doi: 10.1016/j.ajogmf.2021.100467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rottenstreich M, Sela HY, Rotem R, Kadish E, Wiener-Well Y, Grisaru-Granovsky S. Covid-19 vaccination during the third trimester of pregnancy: rate of vaccination and maternal and neonatal outcomes, a multicentre retrospective cohort study. BJOG. 2022;129(2):248-255. doi: 10.1111/1471-0528.16941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blakeway H, Prasad S, Kalafat E, et al. COVID-19 vaccination during pregnancy: coverage and safety. Am J Obstet Gynecol. 2022;226(2):236.e1-236.e14. doi: 10.1016/j.ajog.2021.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dick A, Rosenbloom JI, Gutman-Ido E, Lessans N, Cahen-Peretz A, Chill HH. Safety of SARS-CoV-2 vaccination during pregnancy- obstetric outcomes from a large cohort study. BMC Pregnancy Childbirth. 2022;22(1):166. doi: 10.1186/s12884-022-04505-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stock SJ, Carruthers J, Calvert C, et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland. Nat Med. 2022;28(3):504-512. doi: 10.1038/s41591-021-01666-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruderman RS, Mormol J, Trawick E, et al. Association of COVID-19 vaccination during early pregnancy with risk of congenital fetal anomalies. JAMA Pediatr. 2022;176(7):717-719. doi: 10.1001/jamapediatrics.2022.0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atyeo C, DeRiso EA, Davis C, et al. COVID-19 mRNA vaccines drive differential antibody Fc-functional profiles in pregnant, lactating, and nonpregnant women. Sci Transl Med. 2021;13(617):eabi8631. doi: 10.1126/scitranslmed.abi8631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butt AA, Chemaitelly H, Al Khal A, et al. SARS-CoV-2 vaccine effectiveness in preventing confirmed infection in pregnant women. J Clin Invest. 2021;131(23):e153662. doi: 10.1172/JCI153662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall S. COVID vaccines safely protect pregnant people: the data are in. Nature. 2022;601(7893):308-309. doi: 10.1038/d41586-022-00031-8 [DOI] [PubMed] [Google Scholar]
- 34.Strassberg ER, Power M, Schulkin J, et al. Patient attitudes toward influenza and tetanus, diphtheria and acellular pertussis vaccination in pregnancy. Vaccine. 2018;36(30):4548-4554. doi: 10.1016/j.vaccine.2018.05.121 [DOI] [PubMed] [Google Scholar]
- 35.Yuen CYS, Tarrant M. Determinants of uptake of influenza vaccination among pregnant women—a systematic review. Vaccine. 2014;32(36):4602-4613. doi: 10.1016/j.vaccine.2014.06.067 [DOI] [PubMed] [Google Scholar]
- 36.Price AM, Olson SM, Newhams MM, et al. ; Overcoming Covid-19 Investigators . BNT162b2 Protection against the Omicron Variant in Children and Adolescents. N Engl J Med. 2022;386(20):1899-1909. 2022. doi: 10.1056/NEJMoa2202826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andrews N, Stowe J, Kirsebom F, et al. COVID-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med. 2022;386(16):1532-1546. doi: 10.1056/NEJMoa2119451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385(7):585-594. doi: 10.1056/NEJMoa2108891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gray KJ, Bordt EA, Atyeo C, et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;225(3):303.e1-303.e17. doi: 10.1016/j.ajog.2021.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. GRADE evidence profile for the included studies
eTable 2. Outcomes of all studies
eTable 3. The results of Egger’s linear regression tests of the primary outcomes
eTable 4. Definitions of postpartum hemorrhage in each study
eFigure 1. Risk of bias summary
eFigure 2. Funnel plots of the primary outcomes
eFigure 3. Forest plot showing the odds ratio of low Apgar score (<7 at 5 min of birth)
eFigure 4. Forest plots showing the odds ratio of preterm birth
eFigure 5. Forest plots showing the odds ratio of small size for gestational age
eFigure 6. Forest plots showing the odds ratio of small size for gestational age (a: women who received COVID-19 vaccination during the first trimester vs. women who did not receive COVID-19 vaccination during pregnancy, b: women who received COVID-19 vaccination during the second or third trimester vs. women who did not receive COVID-19 vaccination during pregnancy)