Graphical abstract
Keywords: Islet biology, Cell and tissue engineering, Biomaterials, Implantation devices, Cell therapies
Abbreviations: 3D, 3-Dimensional; Ad-MSC, Adipose-derived Mesenchymal Stromal Cell; AIS, Adaptive Immune System; Arx, Aristaless Related Homeobox; ATP, Adenosine Triphosphate; β2M, β2 Microglobulin; BCR, B-Cell Receptor; BMP, Bone Morphogenetic Protein; Cas, CRISPR Associated Protein; CCL, Chemokine (C-C motif) Ligand; CD, Cluster of Differentiation; CIITA, Class II Major Histocompatibility Complex Transactivator; CO, Carbon Monoxide; CPO, Calcium Peroxide; CRISPR, Clustered Regularly Interspaced Short Palindromic Repeats; CTLA4, Cytotoxic T-lymphocyte-associated Protein 4; CXCL, Chemokine (C-X-C motif) Ligand; DDA, 1,12-Dodecanedioic Acid; EC, Endothelial Cell; ECM, Extracellular Matrix; Erk, Extracellular signal-Related Kinases; ERRy, Estrogen-Related Receptor gamma; ESC, Embryonic Stem Cell; FAK, Focal Adhesion Kinase; FBR, Foreign Body Response; FGF, Fibroblast Growth Factor; Gal, Galactose-α1,3-galactose; GAPDH, Glyceraldehyde 3-Phosphate Dehydrogenase; GCK, Glucokinase; GFP, Green Fluorescent Protein; GLUT, Glucose Transporter; GMP, Good Manufacturing Practice; GSIS, Glucose-Stimulated Insulin Secretion; HA, Hyaluronic Acid; hAEC, Human Amniotic Epithelial Cell; Hb, Hemoglobin; hESC, Human Embryonic Stem Cell; HGF, Hepatocyte Growth Factor; HIF, Hypoxia-Inducible Factor; HILO, Human Islet-Like Organoid; hiPSC, Human Induced Pluripotent Stem Cell; HLA, Human Leukocyte Antigen; hPSC, Human Pluripotent Stem Cell; HUVEC, Human Umbilical Vein Endothelial Cell; IBMIR, Instant Blood Mediated Inflammatory Reaction; ICC, Islet-like Cell Clusters; IEQ, Islet Equivalent; IFN, Interferon; IIS, Innate Immune System; IL, Interleukin; iPSC, Induced Pluripotent Stem Cell; Isl1, ISL LIM Homeobox 1; KLF4, Krüppel-Like Factor 4; KO, Knockout; Maf, MAF BZIP Transcription Factor; MHC, Major Histocompatibility Complex; MSC, Mesenchymal Stromal Cell; NeuroD1, Neuronal Differentiation 1; Ngn3, Neurogenin 3; NHP, Non-Human Primates; NID1, Nidogen-1; NK, Natural Killer; Nkx6.1, NK6 Homeobox 1; NOD, Nonobese Diabetic; OCT4, Octamer-binding Transcription Factor 4; Pax4, Paired Box 4; PDGF, Platelet-Derived Growth Factor; PD-L1, Programmed Death Ligand 1; PDMS, Polydimethylsiloxane; Pdx1, Pancreatic And Duodenal Homeobox 1; PEG, Polyethylene Glycol; PEGDA, Polyethylene Glycol) Diacrylate; PERV, Porcine Endogenous Retrovirus; PFC, Perfluorocarbon; PGA, Poly-Glycolic Acid; PGK, Phosphoglycerate Kinase; PLA, Poly(Lactic Acid); PLGA, Poly(Lactic-co-Glycolic Acid); PolyHb, Polymerized Hemoglobin; PSC, Pluripotent Stem Cell; Ptf1a, Pancreas transcription factor 1 subunit alpha; RNA, Ribonucleic Acid; SC, Stem Cell; scRNA-seq, Single Cell RNA-Sequencing; SOX2, Sex-determining region Y-box 2; STZ, Streptozotocin; T1D, Type 1 Diabetes; T2D, Type 2 Diabetes; TCR, T-Cell Receptor; TGF, Transforming Growth Factor; TNF, Tumour Necrosis Factor; Tregs, Regulatory T-Cells; Ucn3, Urocortin-3; USP, United States Pharmacopeia; VEGF, Vascular Endothelial Growth Factor; WNT, Wingless-related Integration Site
Abstract
The development of new therapeutic approaches to treat type 1 diabetes mellitus (T1D) relies on the precise understanding and deciphering of insulin-secreting β-cell biology, as well as the mechanisms responsible for their autoimmune destruction. β-cell or islet transplantation is viewed as a potential long-term therapy for the millions of patients with diabetes. To advance the field of insulin-secreting cell transplantation, two main research areas are currently investigated by the scientific community: (1) the identification of the developmental pathways that drive the differentiation of stem cells into insulin-producing cells, providing an inexhaustible source of cells; and (2) transplantation strategies and engineered transplants to provide protection and enhance the functionality of transplanted cells. In this review, we discuss the biology of pancreatic β-cells, pathology of T1D and current state of β-cell differentiation. We give a comprehensive view and discuss the different possibilities to engineer enhanced insulin-secreting cell/islet transplantation from a translational perspective.
1. Introduction
Diabetes mellitus is a chronic disease that is caused by the body’s inability to effectively produce or use insulin to process glucose in the bloodstream. It affects 463 million people worldwide and is projected to affect 700 million people by 2045, making it the fastest growing health challenge in the world [1]. Diabetes mellitus is classified into two categories: type 1 diabetes (T1D) and type 2 diabetes (T2D). T1D results from the autoimmune attack and death of β-cells that produce insulin in response to glucose in the islets of Langerhans of the pancreas; while T2D is caused by the inability of β-cells to produce insulin and/or other cells’ ability to respond to insulin.
Currently, most patients with T1D, and some patients with T2D, maintain glucose levels through the continuous monitoring of glucose and exogenous administration of the appropriate dose of insulin. While there have been significant advances in glucose sensors, insulin pumps and so called ‘closed-loop systems’ for more precise control of glucose levels, disease progression still occurs [2]. Insufficient blood glucose homeostasis puts patients at risk for ketoacidosis or hypoglycaemic episodes, which can cause cardiovascular complications, seizures, and death [3]; and long-term insulin replacement therapy can cause several adverse effects, such as partial lipodystrophy at the administration sites as well as weight gain, further worsening the course of the disease [4]. Furthermore, there are many obstacles in the adoption rates of new technical advancements. Patient accessibility or tolerance of the lifestyle adjustments required for new technologies has been shown to be a hindrance and new technologies can take decades for patient adoption [5]. Even when adopted, dropout rates have been shown to be as high as 32 % for well-accepted technologies such as insulin pumps [5]. As such, scientists are in pursuit of a treatment that has a more innate glucose response. Over the past few decades, many transplantation procedures have been proposed as endocrine replacement therapy for T1D.
Whole-pancreas transplants have been successfully performed with relatively high graft survival rates; however, the procedure is limited by the number of eligible donors and still poses risk as an invasive surgery. Pancreatic islet transplants from donor tissues hold great promise for the treatment of patients with T1D [6]. Recently, stem cell (SC)-derived β-cell or insulin-producing cell implantation has received considerable focus. This new direction has required deeper investigation into islet and β-cell development to understand their mechanisms of differentiation. As some of these cell types have unproven safety profiles, new technologies to encapsulate and support cells post-transplantation are being vigorously investigated.
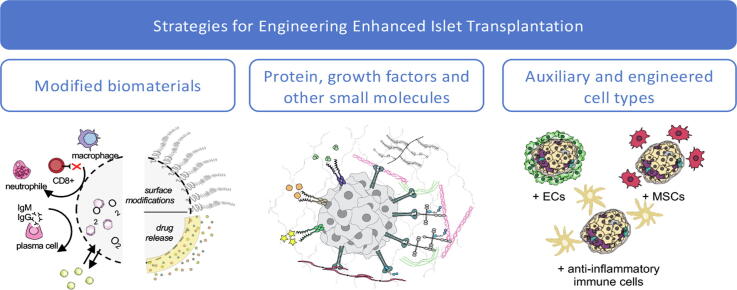
Islet transplantation is hindered by the ischemic conditions of the transplant site and an immune reaction to the foreign islets, which destroys approximately half of the islets one-week post-transplantation [7], [8]. These problems are compounded by the donor shortage issue by requiring considerably more donor pancreases for one patient. There has been intense research in the public and private area towards the development of biomedical devices to house islets and islet cells. These devices can contain only islets, but many have been developed to contain molecules and gels to support islet function in the first weeks of transplantation. Devices have been developed to enhance the vascularization of the implant as well as protect islets from the host’s immune system. Taken together, and with the right transplantation site, researchers have developed many promising methods to improve islet transplantation for the millions for diabetes sufferers worldwide (Fig. 1).
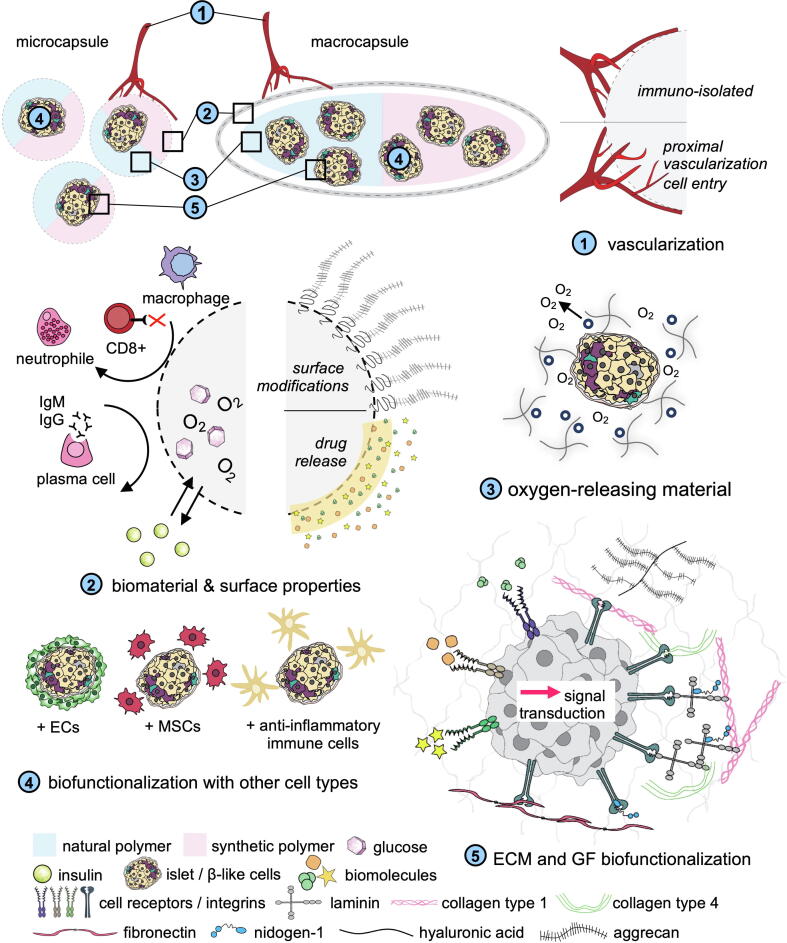
Fig. 1.
Strategies for islet and insulin-producing cell encapsulation. Encapsulation strategies can be divided into two main approaches: macroencapsulation or micro- nanoencapsulation. A number of research areas are currently being investigated with the aim to develop immunosuppressant-free therapies for islet transplantation with biocompatible biomaterials and improve the overall long-term transplant function and viability. (1) Transplants can be immune-isolated and allowing proximal vascularization within; (2) transplants can be optimized based on the biomaterials used or by tuning its surface properties; (3) Integration of oxygen-releasing materials surrounding the islets can provide gradual oxygenation to reduce the hypoxic conditions; (4) biofunctionalization is of interest to improve the transplanted cells by biological interactions; (5) ECM and GF can stimulate specific cellular pathways resulting in an increase of cellular function and or survival.
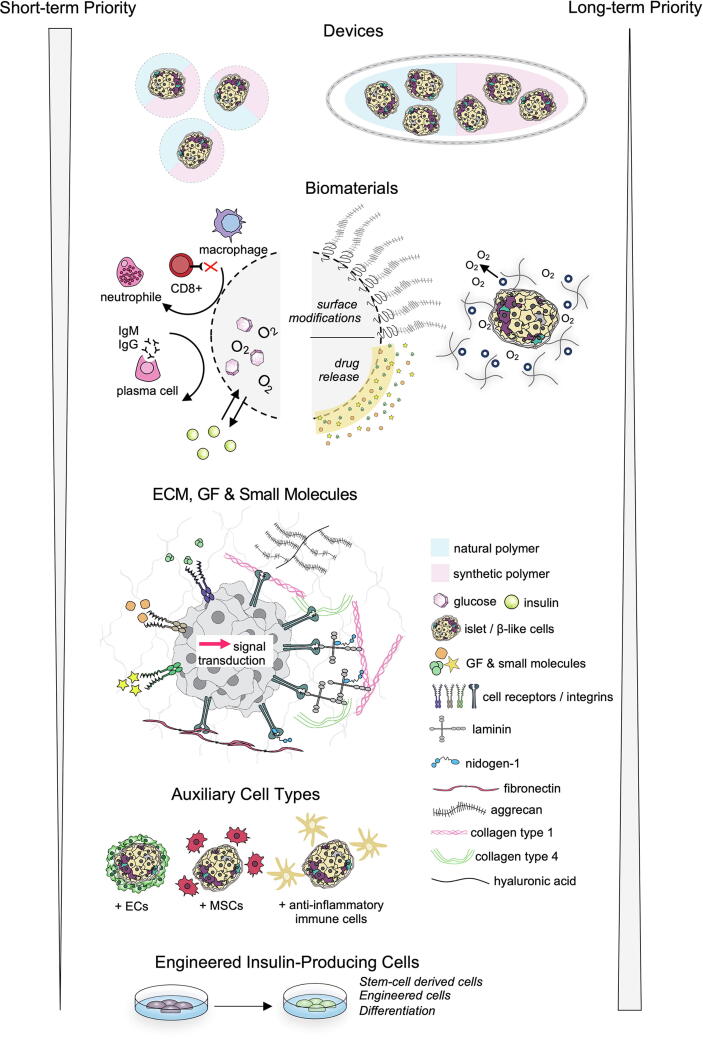
In this review, we provide a short overview of T1D pathophysiology, pancreas development and discuss topics regarding islet/cell transplantation, as well as current clinical treatments. The bulk of this review will have a strong focus on pre-clinical developments in new cell types, biomaterials, biomolecules, and devices for the advancement of transplanting insulin-producing cells. The conclusion will discuss the short-term and long-term future perspectives for islet transplantation and identify which of the reviewed methods and technologies have the greatest potential to help the largest stratification of patients with T1D.
2. Pancreatic islet development and biology
Genetic studies in mice have proven to be useful to study the development of the pancreas and β-cells. Detailed studies of human pancreatic development are difficult; however, many observational articles were reviewed by Jennings et al. [9]. During development, pancreatic endoderm cells, marked by Ptf1a and Pdx1 expression, become specified towards the exocrine or endocrine lineages of the pancreas. Ptf1a is a regulator of acinar cells of the exocrine lineage, while Pdx1 is a regulator of cells of the endocrine lineage. The majority of the pancreas functions as the exocrine gland consisting of acinar and pancreatic ductal epithelial cells that secrete digestive enzymes and bicarbonate. These enzymes are secreted into the pancreatic duct to the duodenum to aid in the digestion of food. Approximately 7–10 % of the cells in the pancreas are responsible for the endocrine role of the organ and are found in clusters called the islets of Langerhans. They consist largely of α-, β-, and δ-cells that produce and secrete glucagon, insulin, and somatostatin, respectively, to maintain blood glucose levels. These islets arise from precursor cells expressing Ngn3, and their further specification into α- and β-cells can be attributed to expression of Arx or Pax4. Ngn3 is an upstream activator of both Arx and Pax4, which transcriptionally repress one another to promote either α- or β-cell specification. Islets of mice mutant for Pax4 lack β-cells and show a greater proportion of α-cells [10], [11]; while those of a mice mutant for Arx lack α-cells and have a greater proportion of β-cells [11]. Furthermore, Arx-mutant mice had increased numbers of Pax4 transcripts in their pancreatic cells. Interestingly, in double-mutants of Arx and Pax4, there was a dramatic increase in the proportion of δ-cells, and no α- or β-cells. In all mutant backgrounds, the number of cells within the islets were relatively unchanged, with only changes in the proportion of pancreatic endocrine cell types.
2.1. Islet composition and architecture
While human islets and cells show similar markers and suggest similar developmental patterns to that of mice, its cellular organization is quite different. Human islets show no clear pattern or organization of the endocrine cells, while in rodents, the β- and α-cells appear as distinct masses within the islet. The islets have a mean diameter of around 100 μm and consist of endocrine cells including β-cells (70–80 %), α-cells (15–20 %), δ-cells (5–10 %), pancreatic polypeptide cells (5–10 %) and ε-cells (∼1%) [12], [13]. Islets are highly vascularized structures with a dense network of blood vessels and capillaries supporting the exchange of molecules such as the hormones, oxygen, and growth factors. Islets are also innervated with nerve fibers, which are found between the capillary and islet cells, regulating insulin production and secretion [14]. Immune cells, including macrophages, dendritic cells, and T-cells [15], are also present. These cells play a major role in the onset and progression of diabetes mellitus, due to their inflammatory and cytotoxic effects. Furthermore, human islets have a unique organization of extracellular matrix (ECM) proteins to create a double basement membrane, compared to rodent islets and the majority of other human tissues that only have a single basement membrane [16]. Both the peripheral ECM surrounding the islet and the peri-islet ECM that surrounds the endocrine cells are primarily composed of laminins [17], collagen IV [18], and collagen VI [19]. The sequestered growth factors, particularly VEGF-A, in the ECM supports (re)vascularization of the cells, playing a key role in islet survival [20]. ECM components, integrin receptor activity, and islet-ECM interactions have been detailed in other reviews [21], [22], [23].
2.2. β-cells and the immune system
T1D is a result of the body targeting and destroying its own insulin-producing β-cells. Normally, through the cooperation of the innate and adaptive immune systems, the body recognizes and targets foreign bodies that it deems harmful to the individual. Together, these two components of the immune system fight off infections and maintain homeostasis within the body. The innate immune system (IIS) provides an immediate response to invading pathogens and acts as the first line of defense [24], [25]. The IIS acts through multiple types of cells, including macrophages, natural killer (NK) cells, and dendritic cells, that recognize conserved features of pathogens such as prokaryotic peptides produced during translation of bacterial proteins or viral double-stranded RNA. The host’s immune cells recognize the pathogen-specific features by Toll-like receptor proteins, which activate a cascade of pathways to initiate a phagocytic or inflammatory response [26]. These responses also signal to the adaptive immune system (AIS) that there is a foreign body posing a threat to the individual [27].
AIS responds to foreign objects in a targeted manner, producing antigen-specific antibodies. The major players of the AIS are two types of lymphocytes, B-cells and T-cells. While they both originate from the bone marrow, B-cells mature and assemble their B-cell receptor (BCR) and cell surface immunoglobulins in the bone marrow, while T-cells mature and assemble their T-cell receptors (TCRs) in the thymus. Upon recognition of an antigen, B-cells become activated into antigen-specific plasma cells. These cells then secrete antibodies specific to the detected antigen to remove it from the host system. T-cells recognize degraded pathogen peptides presented by major histocompatibility complexes (MHCs) on surfaces of the diseased cells [28]. MHCs exist in two classes: MHC-I-class are found on all cells while MHC-II-class molecules are found on antigen-presenting cells of the immune system including macrophages, dendritic cells, and B-cells [29]. When T-cells come into direct contact with a MHC-I-class molecule with the pathogenic peptide, it becomes a CD8+ cytotoxic-T-cell, killing the diseased cell. MHC-II-class protein are presented along with pathogen peptides to be recognized by CD4+ helper-T-cells. Helper-T-cells activate B-cells, macrophages, and CD8+ cytotoxic-T-cells to induce a greater immune response and eventual inactivation of the pathogens [30], [31].
In the case of autoimmune disorders, T-cells recognize peptides of the host as foreign bodies and induce an immune response. Normally, in a process called central tolerance, T-cells that react to self-antigens are killed. During the maturation of TCRs in the thymus, self-antigens from tissues all over the body are presented by the medullary thymic epithelial cells, and the T-cells that react to these antigens undergo apoptosis [32]. T-cells have multiple types of receptors and ligands, such that to stimulate an immune response, multiple signals must be sensed. When a T-cell recognizes a self-antigen, but does not receive any other signals, it undergoes anergy where it becomes unresponsive to that self-antigen. When the central tolerance mechanism fails and autoreactive T-cells remain in the body, peripheral tolerance is employed through mechanisms such as anergy, inhibitory receptors, and using regulatory T-cells (Tregs) [33]. Tregs are a part of the helper-T-cell population that modulate the immune response. When T-cells are responding to a self-antigen, Tregs in the area would act to suppress the immune response [34]. Autoimmune disorders arise when these tolerance mechanisms fail and autoreactive T-cells remain throughout the body [35], [36].
Histologically, T1D is characterized by the inflammation of the islets and lack of healthy β-cells, due to the infiltration of autoreactive lymphocytes and macrophages [37]. The autoimmune nature of T1D is due to β-cells being targeted by autoreactive T-cells. Studies have identified a few genetic predispositions of T1D, particularly those with specific alleles for the MHC-II-class, and some MHC-I-class proteins; however, there is no clear marker linked to the disease or its progression [38]. The combination of different MHC proteins allows for the presentation of β-cell auto-antigens, which can result in the activation of T-cells and the immune response.
Due to the limited availability of pancreases and islets from patients with T1D, the peptides presented as auto-antigens remain inconclusive. Antibodies against islet-specific targets have been identified as potential targets of autoreactive T-cells through both in vitro studies using patient blood samples and in vivo studies using animal models. Most of these islet-specific targets are involved in the secretory pathway of islet cells, including glutamic acid decarboxylase, zinc transporter 8, islet-cell auto-antigen 69, and proinsulin. Assays for antibodies against these proteins are often a measure for T1D diagnosis as results from multiple studies suggest that the number of antibodies, rather than the antibody type, is more telling of disease development [39]. Interestingly, many of the identified auto-antigens are found in all cells of the islets, with proinsulin being the only β-cell-specific antigen. T-cells should not be autoreactive due to the unique MHC combinations and presentation of the islet-specific antigens, suggesting impairment of the tolerance mechanisms. This could be due to low levels of islet antigens in the thymus resulting in the escape of the autoreactive T-cells that do not come in contact with the antigen. While studies in diabetic mice have shown that most T-cells targeting insulin are anergic, this peripheral tolerance was not enough to prevent the onset or development of T1D; however, introducing more Tregs in the pancreas prevents the progression of diabetes in mice and is a possible therapeutic measure for patients. Kreiner et al. [40] provide a thorough summary and update on antigen-specific immunotherapy for the treatment of T1D. Through the exposure of islet-specific antigens, the goal is to establish immune tolerance to the peptides potentially inducing an immune attack against the β-cells; however, this is still limited due to the lack of knowledge regarding these peptides. Recently, Sona et al. [41] found increased presence of cell adhesion molecule 1 along with T-cells in pancreatic sections of patients with T1D when compared to healthy patients. They suggest this adhesion molecule may allow intercellular interactions between the T-cells and the islet, leading to the loss of β-cells. While specific genes, auto-antigens, or impairments in immune tolerance have not yet been identified as necessary or sufficient for the progression of T1D, research has advanced greatly in identifying key players involved in the development of the disease, creating more therapeutic targets to explore. More in-depth information on T1D can be found in Rogal et al. [42].
2.3. β-cell maturation
β-cells differentiate from their progenitors into an immature state where they can detect glucose and secrete insulin. Mature adult β-cells have increased glucose sensitivity and are able to regulate their insulin production and secretion depending on the amount of glucose in the bloodstream [43]. An ideal glucose-responsive cell for implantation would be able to regulate insulin secretion in response to the amount of glucose in the bloodstream while maintaining its self-renewable capacities for long-term efficacy. In vitro, this is assessed through a glucose-stimulated insulin secretion (GSIS) assay where the islets are challenged with low and high concentrations of glucose to determine their insulin secretion. Analysis of insulin secretion of islets from infants showed an increased basal secretion of insulin compared to those of adults until one year of age. These results suggested that the islets were only functionally mature after the first year [44], [45], [46]. Transcriptomic studies differentially analyzing immature and mature β-cells found key differences in the expression of transcription factors. Mature β-cells from mice showed increased RNA expression levels of Nkx6.1, NeuroD1, and MafA, and significantly reduced levels of MafB compared to immature β-cells. Ucn3 showed the greatest increase in mature β-cells; however, its introduction to immature β-cells in vitro was not sufficient to support their maturation [47]. scRNA-seq of human adult islets showed that β-cells expressing both MAFA and MAFB had higher expression levels of genes involved in glucose sensing and had greater electrophysiological activity than those expressing only one of the two transcription factors [48].
Studies have also investigated the pathways involved in glucose metabolism to determine how mature β-cells tune their response to the amount of glucose. This was analyzed by how glucose enters the cell, how glucose is recognized or processed within the cell, or how insulin is produced and secreted. Normally, glucose enters the cells passively through glucose transporters (GLUT) on the cell surface. GLUT-2 was shown to be the primary transporter; however, analysis of human islets and β-cells in vitro showed greater expression of GLUT-1 and GLUT-3, suggesting a larger role for these transporters in humans [49], [50]. A study exploring the genetic mutations in 104 patients with neonatal diabetes mellitus found that 5 % of the patients had a homozygous mutation in the gene encoding GLUT-2. These patients were unable to secrete insulin and were dependent on insulin replacement therapy, showing that GLUT-2 played a key role in the transport of glucose in humans as well. Within the cell, glucose enters the glycolytic pathway, eventually producing ATP and depolarizing the membrane, leading to the exocytosis of insulin granules. The change in glucose sensitivity between the immature and mature β-cells can be attributed to differences in the glucose-sensing and metabolic pathways.
Mature β-cells have been shown to have a heterogenous population in terms of metabolic redox rates, where cells have different response levels. When β-cells from adult rats were sorted based on low- and high-metabolic rates, Heimberg et al. [51], found that the differences in glucose metabolism was not because of changes in expression or activity of the glucose transporter, but of the phosphorylation of the glucose by hexokinase during the first step of glycolysis. Of the four mammalian hexokinases, mature β-cells only express hexokinase 4, glucokinase, which has a low-affinity for glucose, while repressing expression of the hexokinases with high-affinity for glucose [52], [53], [54], [55].
While highly-responsive adult rat β-cells also showed high levels of hexokinase 1 and glucokinase activity, the low-response cells, similar to mature β-cells, showed only glucokinase-dependent glucose phosphorylation [51]. These results suggested that the switch from hexokinase to glucokinase regulated the maturation of the β-cells. This was further supported by in vitro cultures of fetal rat islets where initial cultures of the fetal islets expressed hexokinase 1 which was then lost upon further culture, and only glucokinase was present, in line with the maturation of the β-cells [52]. Furthermore, upon culture of mouse islets from healthy and undernourished pups, it was found that there was increased hexokinase activity in the undernourished pups compared to the healthy pups, while glucokinase activity was similar [56]. This suggested that the β-cells did not mature postnatally as expected due to the poor diet, supporting the idea that β-cell development and postnatal maturation were also dependent on external factors; however, these measures of hexokinase-1 are disputed due to potential contamination of the tissue isolation with fetal pancreatic acinar cells, which were not supported by the culture medium and died [57].
Tu and Tuch [58] compared glucokinase activity in islet-like cell clusters (ICCs) from human fetal tissue with human adult islets and found that while the ICCs had similar Michaelis coefficients (Km) of glucokinase to that of the islets, the maximal velocity (Vmax) of the enzyme was significantly lower in the ICCs. When the ICCs were cultured for a week, the Vmax of the glucokinase had almost a 4-fold increase; however, this did not result in an increase in insulin secretion, suggesting differences not only in the glucokinase enzyme presence and kinetics between immature and mature β-cells, but also downstream in the signalling pathway for insulin secretion.
These studies shed light on the complexity of β-cell maturation at each step of sensing and responding to glucose through different types of glucose receptors and different enzyme kinetics during glucose metabolism, and how these factors need to be considered during the treatment of T1D.
3. Current state of islet transplantation
3.1. The Edmonton protocol and islet allotransplantation
The Edmonton Protocol was the first successful protocol for islet transplantation and is still the basis of the current gold standard for islet transplantation. First published in the year 2000, it greatly advanced the field of islet transplantation. Improvements on the protocol has giving patients insulin-independency for up to 5 years [59], [60]. It consists of islet isolation through enzymatic digestion and infusion of the islets into the portal vein. Induction agents are added before the procedure, a maintenance therapy is induced and, often, the addition of steroid-sparing anti-inflammatory agents, such as etanercept or anakinra, follows to protect the islets from the immune system. The aim of the protocol initially was to achieve independence of external insulin supplementation for treated patients. Yet, over time, it has changed to eliminating hypoglycaemia and restoring the patient’s awareness of hypoglycaemia. In the rather rare cases, in which independence of insulin supplementation can be reached, transplant function progressively declines over time, which nevertheless allows long-term satisfactory metabolic control [59]. In line with these goals, a delay of complications of diabetes is another objective of the procedure. Studies suggest a beneficial effect of islet transplantation compared to sole basal-bolus insulin regimens on the decline in kidney function [61], retinopathy [62], as well as neuropathy [63], [64]. More data are currently needed to better understand the effect on macrovascular events in patients with islet transplantation. Of note, islet transplantation has been shown to lead to an improvement in quality of life likely owing to the resolution of hypoglycaemic events, the reduction or freedom from external insulin supplementation, better glucose control and reduction in microvascular complications [65]. The risks, however, include complications for the minimally invasive surgery and side effects from long-term immunotherapy. Furthermore, the beforementioned shortage of islet donors is very restrictive.
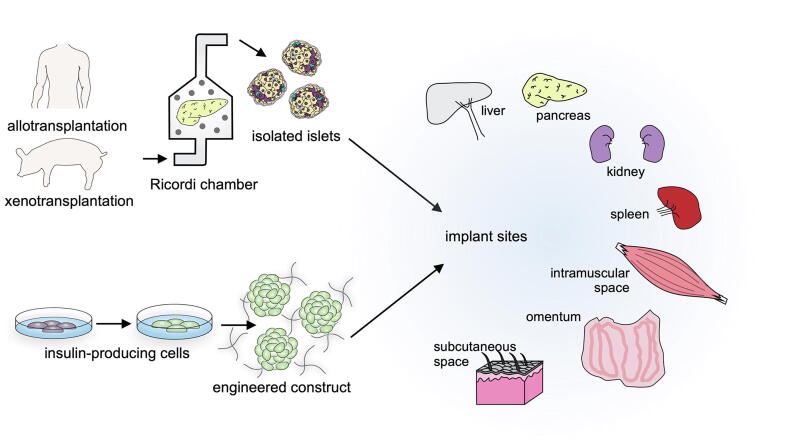
Over the years, the isolation of islets has been optimized to obtain the greatest quantity and purity from donor pancreases. Standard practice is to treat the donor tissue with collagenase along with mechanical disruption of the tissue to free the islet clusters while the acinar tissue is digested. This is followed by a filtration and purification process. The islets are then cultured for 72 h to assess their viability and functionality through their response to glucose levels. Often, the quality of the islets was poor due to damage from the mechanical disruption. The isolation was a laborious and abrasive process that risked contamination of the tissue between the steps. In 1988, Ricordi et al. [66] developed an isolation chamber, commonly referred to as the Ricordi chamber, that allowed for most of the islet isolation to be automated. The tissue is placed in the chamber with proteases and stainless-steel balls for breakdown of the fibrous tissue. The islets and tissue fragments then pass through the filtration chamber where they are no longer digested, increasing the yield of viable islets. The chamber design has been modified over the years to reduce rough handling of the tissue. An interesting adaptation was the use of hooks inside the chamber walls to capture and more gently tear apart the tissue instead of the stainless-steel balls. A general standard for number of islets to be transplanted should be at least 5000 islet equivalents (IEQ: islets of 150 μm diameter) per kilogram of body weight (5000 IEQ/kg) [67]. While many advances in isolation have allowed for more and more numbers of healthy islets to be procured, it still does not allow for 100 % isolation of the islets, such that, on average, two donor pancreases are needed to meet the transplant requirement [68], [69]. Other islet cell sources including porcine islets, and differentiation of stem cells into β-cells have been explored to overcome this limitation.
3.2. Xenotransplantation
With the limited number of donor pancreatic islets, the use of porcine islets has shown great potential for islet cell replacement therapy. Porcine insulin was one of the first types of insulin to be derived and used as treatment for T1D. Porcine and human insulin differ only by one amino acid, and the islets are also structurally and functionally very similar. The transplant and treatment regimen of porcine islets have been modified since the first porcine pancreatic xenotransplantation in the late 19th century, such that in 1994, Groth et al. [70] showed functional grafts of porcine ICCs in patients with T1D up to one year post-transplant.
When considering any transplant, the immunological response of the host to the foreign tissue must be addressed. The biggest obstacle in such xenotransplants is overcoming immune attacks against xenoantigens such as porcine endothelial cell-specific galactose-α1,3-galactose (Gal) and N-glycolyl neuraminic acid (Neu5Gc) [71]. Previous attempts in suppressing or removing anti-Gal antibodies in the non-human primates (NHPs) only delayed rejection and did not improve the outcome of the transplant; however, deletion of the genes encoding Gal and Neu5Gc in the pigs significantly reduced human serum antibody binding. When blood samples from the edited pigs, humans, and chimpanzees were analyzed for human antibody binding, it was found that the blood from pigs lacking Gal and Neu5Gc were a better match to the human samples than the chimpanzees [72]. In addition, porcine xenotransplants come with the risk of transmission of porcine endogenous retroviruses (PERVs). Earlier studies tried to select for pigs with a low copy number of the proviruses, but it proved to be difficult and was still transmitted to mice with porcine xenotransplants. Though there are no reported instances of PERV transmission in NHP studies yet, many measures are being taken to ensure there is no risk to humans. With the advent of genetic engineering, the genomes of the pigs used for tissue procurement were modified to delete the Gal antigen, and to inactivate the retrovirus. In studies looking at porcine heart xenotransplants into baboons, hearts from pigs with the Gal gene deleted had longer survival and function of the graft [73], [74]. Furthermore, multiple clinical studies in NHPs, where diabetes was chemically induced, showed that when paired with an immunosuppressive regimen, the xenotransplanted porcine islets survived and were functional for at least a year. Through measurements of blood glucose and C-peptide levels, researchers found that normoglycemia was maintained for greater than six months, with the longest survival for almost two years [75]. The recent heart transplant performed by surgeons at the University of Maryland of a porcine heart into a human further demonstrated the applicability of xenotransplantation from pig donors. Revivicor™ genetically modified ten genes in the pigs to make them more compatible with humans. Three genes responsible for the production of the galactose that triggers an immune response were knocked out [76], [77]. A growth hormone receptor was also knocked out to prevent the growth of the pig organ following transplantation. Six human genes were introduced into the pigs to support anti-inflammatory responses, blood coagulation, blood vessel integrity, and immune modulation [78]. The transplant of the heart from these “clinical-grade pigs” [79] into a human recipient has also encouraged similar strategies to be investigated more rigorously. Interestingly, there exists the “cleanest pigs” [80] in New Zealand that were introduced there centuries ago which were found to be disease-free. The lack of infections in this population of pigs makes them an attractive choice as organ donors for humans, and are being studied by scientists to determine which genetic modifications they may require to make them more suitable for human recipients [80]. With genetic modifications to limit the risk of immune attack and disease transmission, and similar islet physiology to humans, pigs are a promising source of islet cells to meet the growing demands for islet cell replacements.
As previously mentioned, islet transplantation, whether it be the Edmonton Protocol or newly developed encapsulation devices, has two major challenges: hypoxia and the immune reaction to the transplant. Throughout this review, we will discuss a very broad-spectrum of methods to overcome these hurdles, a considerable amount only investigated in animal models. Despite all of the research that will be presented in this review, the Edmonton Protocol is still the gold standard and may continue to be so for a number of years to come. A short summary of the key challenges and potential solutions can be found in Table 1.
Table 1.
Summary of key challenges and recent strategies regarding islets encapsulation.
| Key challenges | Strategies | Methods |
|---|---|---|
| Acute hypoxia | Hypoxia preconditioning |
|
| Oxygen-releasing molecules |
|
|
| External oxygen supply |
|
|
| ||
| ||
| Chronic hypoxia | Pre-vascularization |
|
| ||
| Re-vascularization |
|
|
| Foreign body response | Immunoisolation |
|
| Implant topography modification |
|
|
| ||
| ||
| Anti-fouling materials |
|
|
| Coating techniques |
|
|
| ||
| Delivery system for immunomodulatory cells and immunomodulators |
|
|
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| Robotic drug delivery technique |
4. Advanced insulin-producing cells for transplantation
4.1. Stem cells
Stem cells are a renewable source of cells defined by their potency to differentiate into specific cell types. Stem cells can be isolated at different stages of development ranging from embryonic tissue to specific organs, where their potency to produce different cells becomes more restricted. Those derived from the pre-implantation embryo are considered pluripotent, meaning they can produce all cell lineages of the body, while somatic stem cells are multipotent. Somatic stem cells can be from different tissues of the fetal and adult body where they are from a particular germline or organ, and are restricted to produce cell types of that lineage [81]. These cells can be cultured, expanded, and differentiated into the desired cells in vitro, allowing for rapid generation of cells needed for cellular therapies [82], [83]. The differentiation of stem cells into pancreatic islets and/or insulin-producing β-cells has been of great interest in the recent decade to meet the high demand of endocrine replacement therapy for patients with T1D.
4.1.1. Embryonic stem cells
Embryonic stem cells (ESCs), derived from the pre-implantation embryo, are examples of pluripotent stem cells (PSCs) as they have the capacity to produce cells of all lineages. Greater understanding of the development of the pancreas and its β-cells has allowed for better control of the signals ESCs need to differentiate into insulin-producing β-cells. Through sequential activation and inhibition of growth factors and signalling molecules, studies have shown that a stage-based differentiation procedure most effectively produces β-cells that function similarly to those found in islets in vivo. Progressive differentiation of ESCs into the definitive endoderm, primitive gut tube, pancreatic and endocrine progenitors, and finally β-cells proved to be more efficient in generating functional insulin-producing β-cells [84].
Protocols from different labs use similar medium cocktails and timing with TGF-β-family molecules, retinoic acid, and fibroblast growth factors (FGFs) playing key roles in directed differentiation. Each stage is defined and confirmed through expression of stage-specific proteins, and the final culture of β-cell function is assessed by insulin production and secretion in response to glucose. While differentiation protocols from various labs are similar in terms of pathways targeted, some protocols are unique with respect to specific molecules and cell culture systems used to produce glucose-responsive β-cells.
In a study by Rezania et al. [85], they assessed specific signalling molecules and changed the cell culture method from adherent to suspension cultures within the protocol to obtain a greater number of β-cells from human ESCs (hESCs). Building on earlier studies, the group targeted the same pathways using different proteins, such as GDF8 in place of the commonly used activin A, both of which are members of the TGF-β-family. They found that such replacements in the culture medium increased the pancreatic progenitor population. In addition, the group introduced vitamin C to the medium at the beginning of the process and identified that it supported the expansion of the pancreatic progenitor cell population before it became further differentiated. Furthermore, this group also emphasized the advantage of switching culture systems from a planar culture at the start to an air-liquid interface culture at the stage of endocrine progenitors. This change allowed for the cells to be exposed to oxygen and obtain apical-basal polarity. This resulted in a greater population of cells expressing β-cell-specific markers and increased expression of insulin compared to cells on a planar culture system throughout the entire differentiation process. These cells, when transplanted into diabetic mice, reversed diabetes much faster than the pancreatic progenitor cells described by previous studies. Recently, Liu et al. [86] used this protocol to differentiate hESCs into pancreatic progenitors following which they included different combinations of small molecules and growth factors to the medium recipe to increase the efficiency of β-cell differentiation to greater than 60 %. This massive screen showed that some of the factors were similar to that of the original protocol; however, the combination and timing of their inclusion after the pancreatic progenitor cell stage were key to increase the differentiation efficiency. The effectiveness of these modifications was shown in the original hESC line and may need to be further optimized for other cell lines.
Over the years, slight modifications to the stepwise protocol have been made to reduce culture period and improve efficiency of β-cell differentiation. These modifications are often made to the protein supplement and/or its concentration. A major limiting factor of the differentiated β-cells is the lack of maturation of these cells. Developmentally, β-cells mature within the islets of Langerhans postnatally. As such, most of the knowledge on β-cell maturation is from animal models. While the molecular mechanisms driving this functional maturation are still under investigation, comparisons of fetal and adult islets have allowed for the identification of the differences between immature and mature β-cells [87]. While both immature and mature β-cells metabolize glucose, mature cells show increased activity of the Krebs cycle as they metabolize the glucose and have increased number of insulin secretory granules. In addition to β-cell markers, Pdx1, Nkx6.1, and Isl1, upon maturation, β-cells also express MafA, Ucn3, and Errγ. Functional maturation of the β-cells allows them to regulate their insulin secretion in response to glucose levels in the environment and has been a challenge to achieve in hPSC-derived β-cells [47], [52].
Nair et al. [88] achieved around 90 % efficiency of β-cells by implementing cell cluster dissociation, sorting, and reaggregation steps. In line with clustering of islet cells as β-cells mature in human development, these additional steps attempted to mimic the environment in which β-cells mature. hESCs engineered with GFP-tagged insulin were differentiated into β-cells using a stage-wise protocol. To increase the yield and purity of the β-cell population, this group dissociated the aggregates of differentiated β-cells, and then sorted and cultured only the GFP+ ve β-cells. This resulted in the formation of islet-sized clusters of only β-cells which functioned similarly to mature human β-cells.
Another study published around the same time also emphasized the importance of regulating cluster size in differentiation of PSCs into β-cells [89]. They achieved an almost-pure population of β-cells by modulating TGF-β signalling throughout the differentiation process while also restricting cluster size for uniform differentiation within the cluster. These β-cell clusters showed greater insulin production and glucose-responsiveness than previously published studies using similar protocols and cell numbers [90]. Furthermore, this group also showed that the β-cell population could be effectively isolated from the pool of differentiated cells using the intracellular zinc content. The process of insulin-packaging and secreting involves the organization of multiple zinc ions and transporters, allowing for the sorting of zinc-enriched cells to be a strong selection tool for β-cells [91].
Recently, Balboa et al. [92] used different aspects of previously published protocols together including medium cocktails and culture conditions and provided an in-depth analysis of the hPSC-derived β-cells and primary islets using scRNA-sequencing, electrophysiological analyses, calcium and metabolite imaging, and animal studies. They found that the hPSC-derived β-cells and primary islets had similar biphasic glucose-responsiveness and action potential and exocytotic behaviour; however, had lower mitochondrial respiratory activity and Krebs cycle turnover in response to glucose compared to the primary islets, indicating an immature state of the cells despite their glucose-responsiveness. Engraftment of the hPSC-derived β-cells in mice further allowed for the maturation of the cells as shown by the transcriptomic analyses [92], which is also in line with the concept of requiring the pancreatic microenvironment for proper maturation of these cells [93]. This study highlighted the need for thorough analysis of hPSC-derived β-cells and evaluations against primary human islets to understand what modifications must be made to current differentiation protocols to better generate functional β-cells.
As our knowledge of human islet development expands, differentiation protocols are adapted to mimic the cues the cells experience in vivo to efficiently differentiate ESCs into β-cells for islet cell replacement therapy. Though there has been great progress in such technical aspects, there still exists ethical issues of harvesting human embryos. As such, the use of induced-pluripotent stem cells (iPSCs) and adult stem cells is of great interest to produce β-cells for islet replacement therapy.
4.1.2. Induced-pluripotent stem cells
A renewable source of pluripotent cells is available through the generation of iPSCs from adult cells [94], [95], [96]. By exposing adult somatic cells to the classical Yamanaka factors OCT4, KLF4, SOX2, and MYC in culture conditions similar to that of ESCs, the adult cells obtain ESC-like characteristics. iPSCs have similar morphological and transcriptomic characteristics to PSCs, with their ability to differentiate into cells of all three lineages. As such, these cells also present the risk of teratoma formation like ESCs, but the use of iPSCs overcomes ethical issues as no embryos are destroyed. More interestingly, the use of iPSCs for cellular replacement therapy overcomes the issue of immune rejection as the patient’s own cells can be reprogrammed into iPSCs; however, in the case of T1D, this will likely still pose a problem as T1D is a result of an autoimmune attack against the patient’s own β-cells.
iPSCs can be differentiated into β-cells using similar protocols to those established for ESC differentiation [85], [90], [97], [98], [99]; however, a couple of studies have also claimed that differences in epigenetic profiles and genetic instability of iPSCs affect their differentiation requirements and efficiency. Rezania et al. [85] show greater efficiency of ESC to β-cell differentiation when cultured with vitamin C; however, they also report fewer β-cells derived from iPSCs using the same protocol. Furthermore, when Yabe et al. [100] used the same protocol with another hESC and hiPSC line, they were unable to achieve similar efficiencies. These results emphasize much more work is needed to find optimal conditions for each cell type and cell line to determine which has the greatest efficiency for differentiation into β-cells and greatest survival rate in the patient.
Hogrebe et al. [101] achieved greater β-cell differentiation efficiencies using hiPSCs and hESCs by modulating the cytoskeleton. They reasoned that actin polymerization had been shown to influence endodermal lineage specification, and as such, used Latrunculin A to depolymerize the actin network for endocrine specification. This depolymerization allowed cytoskeletal rearrangement and cell polarization, supporting the maturation of β-cells that displayed similar GSIS to human islets and were able to maintain blood glucose levels in diabetic mice [101]. As the basement membrane is a crucial trigger for cell polarization, Singh et al. [102] studied the ECM components of differentiated islet-like spheroids and found that this was not sufficient for cell polarity establishment of the cells. When hPSC-derived islet-like spheroids were dispersed and seeded as a monolayer on basement membrane proteins laminin 511, collagen IV, or fibronectin, they showed significantly lower basal insulin secretions with a greater GSIS index. Furthermore, this cytoskeletal rearrangement has also allowed for the differentiation of pancreatic endocrine progenitors towards mature β-cells when in planar culture [103], reducing the complexity of the clustering of cells, and increasing the number of cells differentiated.
The improvements in differentiation efficiencies of stem cells into β-cells are largely based on expression of β-cell-specific gene expression and their ability to respond to glucose; however, this response is still inferior to islets from donors. Davis et al. [104] investigated the metabolic differences between donor islets and SC- derived β-cells in terms of glucose sensing and response. They found that both donor islets and SC- derived β-cells were capable of similar levels of glucose uptake and produced similar levels of metabolites during early glycolysis. SC-derived β-cells were unable to maintain electron transport chain activity of late glycolysis in the mitochondria due to inefficient replenishing of the glycolytic metabolites, particularly following the PGK1 and GAPDH reactions, in high glucose conditions [104], [105]. When metabolites from the late glycolysis reactions were supplemented to the cultures, SC- derived β-cells were able to maintain mitochondrial electron transport chain activity and secrete insulin in response to glucose at levels comparable to donor islets. However, a question remains as to why the SC-derived β-cells have this inhibition in their glycolytic pathway and how this can be prevented during the differentiation process.
4.1.3. Stem cell-derived β-cells and the immune system
The mis-matching of human leukocyte antigens (HLA) of hiPSCs limits their effectiveness as a therapeutic solution to the autologous use of the cells rather than from a cell bank [106], [107], where characterizing and banking hiPSCs homozygous for different HLAs is important [108]. Furthermore, it has also been shown that the in vitro culture of hiPSCs and differentiation affects their immunogenicity and increases their HLA profiles [106]. The in vitro conditions results in the expression of immune ligands that normally would have been presented to the body during development to build central tolerance [109]. These factors still pose the risk of stem cell-derived β-cells to be identified and eliminated by the immune system. Current strategies in protecting the transplanted β-cells from the immune system include encapsulating them with biomaterials and cells such as MSCs in order to modulate the immune response which we discuss further in this review. Nevertheless, researchers are trying to minimize the need for further devices and cells for a successful islet transplant. In this regard, scientists have generated stem cell lines deficient of HLA genes encoded by MHCs so that cells differentiated using these lines are not recognized or targeted by the immune system. Riolobos et al. [110] used adeno-associated viruses to knockout β2 Microgloblulin (β2M) in hESCs to create HLA-I-deficient stem cells. The modified SCs, in their naïve and embryoid body state, did not stimulate T-cells or become targets of NK cells, compared to their wild-type counterparts. More recently, β2M-deficient hiPSCs were generated using CRISPR/Cas9 technology, showing similar immune-modulating effects when differentiated towards the hematopoietic lineage [111]. These results are in line with a previous study by Prange et al. [112] where transplantation of β2M-deficient islets in the immune-privileged kidney capsule [113], [114] of nonobese diabetic (NOD) mice showed increased viability compared to wild-type islets.
The idea of universal stem cell donors is picking up traction amongst cell therapies as ready-to-use stem cells for differentiation into any lineage for any patient. hiPSCs engineered to lack both β2M of MHC-I-class and MHC-II-class transactivator (CIITA) through CRISPR/Cas9 maintained their pluripotency and ability to differentiate into cardiomyocytes, while activating T-cells at significantly lower levels compared to wild-type hiPSCs [115]. The engineered hiPSCs were not recognized by NK cells and the cardiomyocyte spheroid was maintained compared to the wild-type spheroids which decreased in size and showed irregular contractile behaviour.
Han et al. [116] employed a slightly different strategy in creating a universal stem cell donor. They used CRISPR/Cas9 in hESCs to knockout multiple HLAs of the MHC-I class and CIITA of MHC-II-class, while also overexpressing HLA-G, programmed death ligand 1 (PD-L1), and CD47 which are involved in protecting cells from NK cells, suppressing T-cell response, and preventing macrophage phagocytosis, respectively. They engineered two hESC lines, one with just the knockout (KO cells) and one with both the KO and overexpression (KI cells). These cells retained their pluripotent capacities and were able to differentiate in to the three germ layers effectively and comparably to wild-type cells. The engineering hESCs were differentiated into endothelial cells (ECs) or vascular smooth muscle cells to act as MHC-presenting cells. They were co-cultured with T-cells and T-cell activation was assessed. The KO cells showed reduced T-cell proliferation, activation, and T-cell cytotoxicity, where the KI cells were significantly more protective in terms of reducing T-cell proliferation and cytotoxicity. When cultured with NK cells, the KO cells showed similar NK cell degranulation levels to the wild-type cells, while the KI cells showed significantly reduced NK cell degranulation and cytotoxicity compared to the KO cells. Similarly, Parent et al. [117] used CRISPR/Cas9 in a stem cell line with an insulin-GFP reporter to delete most HLA- I genes and CIITA, while retaining HLA-A2. These cells were then successfully differentiated into insulin-producing cells and assessed for their immunogenicity. Insulin-producing cells differentiated from HLA-KO with retention of HLA-A2, compared to wild-type cells or cells without any HLA genes, activated significantly lower levels of NK cells. Furthermore, when transplanted into the spleen of humanized mice, these cells showed significantly greater cell mass and survival four weeks post-transplant than those derived from the unmodified stem cell line. Engineering of stem cells to create these universal stem cell donors allow for the targeting of both the adaptive and innate immune responses to create a universal stem cell donor for patients also suffering from autoimmune disorders such as T1D.
The transplantation of human islet-like organoids (HILOs), that are more similar to native islets in terms of cellular composition and architecture, have been proposed as a treatment for T1D [118]. hiPSCs overexpressing PD-L1, a suppressor of the AIS, were differentiated into pancreatic endocrine progenitors and co-cultured with human adipose-derived stem cells and human umbilical vein endothelial cells (HUVECs). They all formed a spheroid structure in a 3D cell culture system, resulting in the upregulation of WNT4 and ERRγ, known to improve SC-derived β-cell maturation and GSIS [119]. This also resulted in the upregulation of mitochondrial genes, improving the oxidative respiration and glycolysis, and consequently improving insulin production in response to glucose. Furthermore, when transplanted into immune-competent mice, the HILOs were able to evade T-cells and NK cells and maintain glucose homeostasis longer than the HILOs without the PD-L1 overexpression. Recently, Leite et al. [120] generated hPSC-derived β-cells that were then transduced with lentiviral small hairpin RNA to knockdown genes involved in cellular stress and immune recognition. Following knockdown of XBP1, CDKN1A, NLRC5, and β2M reduced apoptosis was observed upon stress induction while there were no effects on the β-cell function. Interestingly, the knockdown also protected the cells from recognition and apoptosis by allogeneic T1D PBMCs with reduced activation and proliferation of T-cells, and a decrease in proinflammatory cytokines.
While these immuno-evasive cells are an attractive solution for cell replacement therapies, they do pose a risk should their differentiation not be complete or homogenous, and the cells become tumorigenic post-transplantation. Modulating their own antigen presentation or T-cell activity are strategies tumour cells use to evade the immune system, leading to tumour progression [121]. Immune system engineering to overcome these evasion techniques and target the tumour cells is under intense research, with much progress still required to eradicate the cancer. Genetic engineering of stem cells to generate hypoimmunogenic stem cells through deletion of the antigen-presenting molecules and expression of proteins that can modulate T cells, makes it easier for these cells to become cancer if they are tumorigenic post-transplantation. The inclusion of safety switches in the engineered cells [122], or antibodies targeting an overexpressed immune modulator are possible strategies for overcoming this issue. These cells are engineered to not be detected by the host’s immune system to prevent immune rejection in cell replacement strategies, but there are considerable implications if these cells eventually become cancerous.
4.2. Stromal cells
4.2.1. Pancreatic stromal cells
Multipotent adult stem cells residing in the pancreas have been shown in mice. These cells were found near the ducts and when cultured, had the ability to self-renew and differentiate into endocrine cells of the pancreas [123], [124], [125], [126]; however, the presence of such cells in humans remained unclear for decades. Isolation of cells near ducts of the human pancreas quickly entered senescence and failed to remain as a renewable source of cells [127]. This may have been due to inadequate culture conditions to support cell proliferation and stem-ness. Interestingly, the transduction of PDX1 and NeuroD proteins allowed these cells to be differentiated into insulin-producing cells. Bonner-Weir et al. [128] cultured and expanded human pancreatic ductal tissue discarded following islet isolation. Upon addition of external ECM cues by adding Matrigel on top, the cells organized into 3D duct-like cysts with pancreatic endocrine cells. These islet cells budded from the cyst structures and upon further culturing, differentiated into mature β-cells that produced insulin in response to glucose. Work published by Seeberger et al. [129] suggested that the cells from the exocrine pancreas contain mesenchymal stromal cells (MSCs). Ductal tissue cells remaining post-islet isolation were capable, with appropriate medium, of differentiating into osteogenic, adipogenic, and hepatic lineages, and presented cell surface antigens like that seen in MSCs. While these studies suggested the presence of multipotent MSCs residing in pancreatic tissue, this cell population was still not well identified or characterized. Using lineage-tracing, Domínguez-Bendala’s group from the University of Miami showed a progenitor-like cell population within the pancreas [130]. They found that cells of the pancreas’ exocrine tissue can be expanded and directed towards multiple pancreatic cell types, including endocrine cells, through modulation of BMP7 signalling. This study described potential markers of identifying progenitor cells within the pancreas and how to expand this cell population ex vivo to produce sufficient cells for β-cell replacement therapy. A major advantage of using adult, organ-specific stem cells compared to ESCs is their low affinity to form teratomas, and their commitment towards a lineage already reduces the in vitro manipulation and, perhaps, greater efficiency of differentiation for the desired cells.
4.2.2. Mesenchymal stromal cells
Found within adult tissues, multipotent MSCs, have been characterized by the ability to self-renew and differentiate into cells of multiple lineages. MSCs can be isolated from bone marrow, umbilical cord, and adipose tissue, where the most common cellular therapy is bone marrow transplantation for autoimmune and hematologic disorders. Similar to iPSCs, MSCs also offer the advantage of overcoming the risk of immune rejection and can be expanded in vitro. In addition, MSCs do not tend to form teratomas and secrete many growth factors which help the growth and survival of surrounding cells. They have been shown to have immunomodulatory properties through the suppression of T-cell proliferation by inhibiting IFN-γ and TNF-α, and upregulating IL-10 [131], [132]. MSCs are also pro-angiogenic through production and secretion of VEGF, HGF, IL-6, and TGF-β1 [133], [134].
MSCs and their characterization as a “stem cell” has been misinterpreted in the field of stem cell biology. While these cells act as multipotent stem cells maintaining tissue homeostasis their differentiation potential is limited depending on their tissue of origin [135], [136]. They were considered for β-cell differentiation in vitro; however, with poor efficiency thus far [137], [138], [139]. Nevertheless, due to their immunomodulatory and pro-angiogenic properties. MSCs still show significant impact on β-cell replacement therapy when transplanted with β-cells, improving graft survival and GSIS [134], [140], [141], [142]. Co-culture of human adipose-derived MSCs (Ad-MSCs) with either murine or human islets reduced islet cell death and improved GSIS in vitro. When the Ad-MSCs and islets co-cultures were transplanted into mice, they were able to achieve normoglycemia compared to islets alone or Ad-MSC and islet co-transplants that were not cultured together beforehand [143]. This improved insulin secretion was dependent on the direct cell contact between the MSCs and islets through N-cadherin [144]. Co-transplantation of murine MSCs and islets through the portal vein of mice showed that the MSCs through their secretion of prostaglandin E2 was able to inhibit NK cell activity in the liver improving survival of the transplant [145]. A similar study where the cells were also co-encapsulated in microcapsules and transplanted into the intraperitoneal cavity of the mice found that the MSCs helped reduce fibrotic overgrowth around the capsule through increased expression of anti-inflammatory cytokines [146].
Furthermore, Villard et al. [147] isolated the stromal cells surrounding human islets. Through isolation and culturing of human islets, this group was able to identify and maintain the adherent cells of these cultures. While these cells exhibited similar markers, and proliferation and immunomodulatory capabilities to bone-marrow MSCs, they showed increased expression of ECM proteins enriched within the pancreas, including type I, IV, and VI collagens, fibronectin, and laminin. When co-transplanted with β-cells, this would further support the survival and function of the β-cells by creating a pancreatic-like environment while reducing peripheral blood mononuclear cell activation and encouraging angiogenesis at the transplant site. Recently, Wang et al. [148] engineered MSCs (eMSCs) to express PD-L1 and cytotoxic T-lymphocyte-associated protein 4 (CTLA4) to further enhance their immunoprotective effects. The eMSCs offered a local protective effect and significantly improved islet viability and function for up to 100 days when co-transplanted into the kidney capsule of diabetic mice without immunosuppression compared to wild-type MSCs or no MSC control groups. The eMSCs rescued blood glucose levels in diabetic mice sooner than the control groups while significantly reducing effector T cell presence at the transplant site, delaying graft rejection. These results show potential of modifying accessory cells to modulate the immune system and reduce the need for immunosuppressants in such transplants.
4.2.3. Amniotic epithelial cells
Human amniotic tissue is an underrated reservoir of stem cells. Isolated from the placenta, human amniotic epithelial cells (hAECs) express stem cell markers including OCT4, NANOG, and SOX2, and can differentiate into all three germ layers with low risk of teratoma formation [149]. These cells pose no ethical issues as placental tissue is considered a waste product following birth. Similar to MSCs, hAECs have been shown to not elicit an immune response and secrete proteins supporting angiogenesis such as VEGF and angiogenin. In addition, these cells also prevent fibrosis of surrounding tissue through its secretion of hyaluronic acid [150]. The pluripotent and immunomodulatory effects of hAECs make them a strong candidate for cell transplant therapies [151], [152]. hAECs have been differentiated into hepatic [153], neuronal [154], and osteogenic [155] lineages.
hAECs have successfully been differentiated into cells of the pancreas in vitro [156]. Using a 3D culture system in basement membrane extract, Okere et al. [156] differentiated hAECs into pancreatic spheroids consisting of glucagon- and insulin-producing cells in an islet-like cluster. They also showed that these clusters secreted insulin in response to glucose levels in the culture medium. hAECs transplanted into the spleen of mice with streptozotocin-induced diabetes were able to regulate blood glucose levels within a month post-transplantation [157], emphasizing the in vivo capacity of hAECs to integrate into host tissue and differentiate into insulin-secreting cells. The immunomodulatory properties of hAECs and their effects on insulin secretion were assessed in a co-culture of human islets and hAECs [158]. The in vitro study found that the co-culture with hAECs did not impact the insulin secreted by the islets. When this human islet/hAEC co-culture was exposed to peripheral blood lymphocytes, the proliferation of the peripheral blood lymphocytes was dramatically reduced compared to their proliferative behaviour when cultured with the human islets alone, suggesting an immunomodulatory advantage with no impairment of β-cell function. Lebreton et al. [159] showed that islet-like organoids produced from dissociated islet cells and hAECs improved transplant and engraftment success of these organoids in mouse models of T1D, while maintaining β-cell function. A similar study, in vitro, employing rat islet cells and hAECs to make spheroids showed that the spheroids with both cell types had higher levels of insulin and were more robust in hypoxic conditions such that there was little cell death compared to spheroids of the rat islet cells alone [160]. hAECs are a potential source of PSCs; however, with their immunomodulatory and pro-angiogenic qualities, they play a more effective role as an auxiliary cell for β-cell replacement therapy.
4.3. Reprogramming/trans-differentiation
In addition to classical protocols using small molecules and growth factors in cell culture medium for differentiation, some labs have also reprogrammed or trans-differentiated adult cells into their cells of interest using viral vectors. Zhou et al. [161] transfected mouse pancreatic exocrine tissues in vivo with adenoviral vectors to express β-cell-specific transcription factors Ngn3, Pdx1, and MafA. Interestingly, these markers were seen as early as ten days post-transfection, generating β-like cells. The induced cells were morphologically similar to endogenous β-cells and expressed insulin. When the viral vector was introduced into diabetic mice, the induced β-cells were able to prevent hyperglycaemia and the mice had a significantly higher glucose tolerance compared to the control group. Furthermore, they effectively secreted VEGF and induced angiogenesis for insulin release into the blood. Interestingly, though the introduced vectors were no longer seen two months post-transfection, the cells still expressed PDX1 and MAFA but not NGN3, comparable to endogenous β-cells, suggesting this was sufficient to reprogram the pancreatic exocrine cells into β-cells.
When a similar strategy with the same three factors was attempted in a rat pancreatic exocrine cell line, there was also a change in morphology similar to β-cells, a downregulation of exocrine genes, and an upregulation of most β-cell-specific genes, including insulin, three days post-transfection; however, apart from Abcc8, the other β-cell membrane markers, namely Glut2, were not significantly affected [162]. Furthermore, functional GSIS assays showed that these cells secreted lower levels of insulin than mature β-cells and not in a glucose-dependent manner. Interestingly, the introduction of these cells into the kidney capsule of diabetic mice was able to restore blood glucose levels within twenty days. This suggested that the rat exocrine cells were not completely reprogrammed, and further maturation of these cells was required. The same group then tried introducing this vector into the liver of diabetic mice through the tail vein [163]. They saw protein expression of the introduced genes in cells of the liver after one week. The cells were able to restore and maintain blood glucose levels for at least four months; however, 10 % of the mice did become hypoglycaemic and died within two weeks, suggesting a similar issue to the previous study where insulin secretion was continuous and not glucose-dependent. The insulin-positive cells in the liver were found to change morphology into an epithelial duct-like manner infused with blood capillaries. Isolation of the insulin-positive cells in the liver and gene expression analysis found increased transcript levels of β-cell markers including that of the β-cell membrane markers. GSIS analysis showed that though the cells secreted lower levels of insulin compared to mouse islets, they were still glucose-responsive. Furthermore, analysis of four rat and four mouse cell types including hepatocytes and fibroblasts showed that the rat pancreatic exocrine cell line, rat primary hepatocytes, rat multipotent adult progenitor cells, and mouse hepatocyte small cells most effectively activated β-cell-specific genes upon infection [164]. Though there was not a complete reprogramming of these cells into β-cells, when the mouse hepatocyte small cells were infected and cultured with the Notch inhibitor DAPT, histone methyltransferase inhibitor BIX, and adenosine agonist NECA, there was an increased number of insulin-positive cells suggesting the infection and reprogramming efficiencies can be greatly improved by adding these chemical compounds to the medium.
Saxena et al. [165] designed a synthetic lineage-control network to control the expression of Pdx1, Ngn3, and MafA to represent their dynamic expression patterns seen during development. The three genes are not expressed simultaneously, but rather act as an activator and repressor within each other. Normally, Pdx1 is one of the first genes expressed for pancreatic specification which then moves towards the pancreatic endocrine lineage through expression of NGN3 [166]. During this time, PDX1 expression is downregulated. Upon upregulation of PDX1 again, NGN3 is silenced, and MAFA is activated for the maturation of β-cells [167], [168], [169]. To mimic this, Saxena et al. [165] designed a synthetic network where the genes are under promoters activated by different concentrations of vanillic acid. hiPSCs were differentiated into pancreatic progenitors where these genetic switches were activated with vanillic acid in the medium to drive the cells towards mature β-cells. Transcriptomic analyses were comparable to cells differentiated with classical differentiation protocols, and functional assays showed glucose-stimulated insulin secretion levels similar to that of the human islets. This synthetic network allows for control over the temporal expression patterns of key transcription factors driving the differentiation and maturation of β-cells.
With increasing understanding of β-cell identity and function, hiPSC-derived β-cells seem to hold great potential for islet transplantation. Scientists are able to assess these cells through many different techniques as shown by Balboa et al. [92] while also overcoming the issue of immune rejection. Engineering of the cells to not be recognized by the host’s autoimmune system would further improve the efficacy of the transplant. A limitation of the differentiation protocols is the heterogenous generation of pancreatic endocrine cells. Strategies to address these include cell sorting at each stage using their respective markers, or sorting the cells at the final stage using β-cell-specific membrane markers. Sorting cells at the anterior definitive endoderm stage using CD177 generates a greater proportion of pancreatic progenitor cells than CD275 which promotes specification towards the hepatic lineages [170]. Recent studies have identified markers that are β-cell-specific, including GP-2 [171] and ITGA-1 [172], ENTPD-3 [173] as well as antibodies developed for the sorting of β-cells [174]. Interestingly, the specificity of such antibodies seemed to be specific to the origin of the hPSC, where certain antibodies worked better for hESC-derived β-cells than hiPSC-derived β-cells, and vice versa. Further work is still required for the translatability of these cells, particularly with respect to recreating the β-cell niche in terms of surrounding cells and ECM to support their survival. Once functional β-cells are established, they need to be transplanted in the patient. As with most transplants, many obstacles remain such as overcoming immune rejection, and supporting the transplant in terms of oxygen and nutrients until it is vascularized and can obtain these resources from the host. Major obstacles in islet transplant are immune attack, particularly through the host’s instant blood mediated inflammatory reaction (IBMIR) resulting in fibrosis and supporting cell survival until engraftment. Some common strategies to overcome these challenges are to encapsulate the islets within biomaterials along with other cell types and growth factors, protecting them from the host immune system and supporting them until the transplant is vascularized.
5. Accessory cells
To improve vascularization or immunological response to the islet transplant, studies have looked at the incorporation of specific molecules; however, this is limited by the molecules’ half-life, stability, and diffusion throughout the device [175], [176]. As such, cells that produce these factors can be co-transplanted with the islets for a regulated production and secretion of the molecules. Through paracrine signalling, these molecules will be produced by the co-transplanted cells when needed. The secreted molecules from the co-transplanted cells aid the success of the islet transplant through promotion of vascularization and modulation of the immune system. In addition to the aforementioned MSCs and hAECs, below, we discuss some cell types that have been useful for these purposes.
5.1. Endothelial cells (ECs)
Pancreatic islets are well-vascularized tissues. The endothelial cells lining the vascular network are in close contact with the insulin-secreting β-cells. The vascular network is lost during the islet isolation procedure; however, the capillaries remaining within the islets have been shown to undergo angiogenesis when exposed to exogenous stimuli such as fibrin, FGF-2, and VEGF [177]. The co-transplantation of rat islets and aortic ECs in diabetic rats resulted in healthy glucose levels three days post-transplantation [178], [179]. When human islets were coated with primary human aortic ECs pre-transplantation, it resulted in reduced coagulation and infiltration of macrophages compared to uncoated islets. The coated islets were functional and survived for at least seven weeks post-transplant [180]. Similar results were also found when porcine islets coated with human endothelial colony-forming cells were transplanted into nude mice [181]. Interestingly, Li et al. [263] showed that the efficiency of coating islets with ECs was significantly improved when done on PGA scaffolds as shown by increased VEGF, angiogenesis, and prolonged graft survival post-transplantation. These results are supported by recent studies from our group showing a spatial relationship between ECs and human β-cells. Magnetic levitation was used to create different spatial distributions in pseudo-islets and we identified that surrounding a core of β-cells with ECs had a significant effect on insulin secretion in response to glucose when compared to other distributions [182]. In further studies, we demonstrated that a collagen I and EC co-culture could improve β-cell function and recovering ECM molecules lost in hypoxic, transplant-like environments [183]. Studies transplanting islets with endothelial progenitor cells have been shown to reduce engraftment time and improved vascularization compared to islets transplanted alone, owing to the increased VEGF-A production. Higher insulin levels were found in the co-transplanted animals due to the greater vasculature through the islets, and the islets themselves were able to maintain their morphology and structural integrity [184], [185], [186], [187].
5.2. Fibroblasts
Fibroblasts are the most abundant cell type found in connective tissue and are key players in maintaining tissue homeostasis through the production and organization of ECM and growth factors. ECM proteins support tissue organization while the various growth factors, including VEGF, PDGF, and HIF-1α, support cell survival in stressed conditions such as hypoxia [188]. Embedding islets in a fibroblast-laden collagen I gel for transplantation in mice significantly improved islet survival and proliferation when compared with just the collagen I gel or no gel controls [189]. Furthermore, dermal fibroblasts have also been suggested to have immunomodulatory properties similar to the MSCs discussed earlier. Haniffa et al. [190] demonstrated that dermal fibroblasts were capable of suppressing T-cell proliferation and activation to a similar extent to that of MSCs. Temporary exposure of T-cells to fibroblasts was sufficient to modify the T-cells to an anti-inflammatory phenotype. A co-culture system of fibroblasts and T-cells on either side of a mesh membrane showed that fibroblasts modulate and respond to the T-cells through soluble factors. In response to IFN-γ secreted by T-cells, fibroblasts showed increased tryptophan degradation, preventing T-cell proliferation [190]. Perez-Basterrechea et al. [191] transplanted rat islets in a plasma-based scaffold subcutaneously and found that the incorporation of fibroblasts in the scaffold significantly improved engraftment and was able to achieve long-term glycemic control. Gene expression analysis showed that this effect of the fibroblasts on the β-cells could be attributed to the overexpression of proliferative, angiogenic, and anti-inflammatory genes [191], [192]. Fibroblasts are relatively easy to isolate from patients to further prevent immune rejection and IBMIR response and present themselves as strong candidates to support islet graft survival.
5.3. Regulatory T-cells (Tregs)
Tregs are involved in the suppression of effector T-cells, NK cells, B-cells and dendritic cells [193]. They are a subset for CD4+ cells that originate from the thymus and are identified by intracellular FoxP3 or the surface marker CD25. Tregs are responsible for preventing autoimmunity and stopping the immune response to foreign organisms [194], [195], [196]. Tregs/islet co-transplantation increased islet survival compared to islets transplanted alone [197]. Tregs have also shown to support islet survival when they are used to coat human islets [198], have been injected into the transplant location prior to surgery [199], and co-transplanted within a PLG scaffold in the liver and kidney [200]. Islets within the PLG/Treg scaffold were able to maintain blood glucose levels for at least 100 days in a mouse model. Interestingly, this protective effect was only observed when both cell types were co-localized within the scaffold and not when the Tregs were systemically injected. Furthermore, the transplanted Tregs recruited host Tregs which continued protecting both the scaffolded-islets and islets from another transplant site, suggesting the host developed immune tolerance to the islets [200]. To improve the efficacy of a Treg co-transplantation, Hull et al. [201] described lentiviral gene transfer of islet-specific TCRs to Tregs expanded ex vivo, which resulted in increased Treg potency for suppression of T-cells attacking the islets.
6. Unprotected and encapsulated islet transplantation
Transplanted unprotected islets can initiate the IBMIR, which is a non-specific inflammatory response by the innate immune system that is initiated when the islets are exposed to blood. The response of the IBMIR can be characterized by the activation of the coagulation and complement systems, platelets, and the consumption of neutrophils and monocytes [202], [203], [204], [205]. It can rapidly destroy approximately 60 % of the islets in a graft [206], [207], [60]. It has been shown that proinflammatory cytokines such as TNF-α and MCP-1 and thrombin-anti-thrombin III complex (TAT) significantly increase 15 min post-transplantation [208]. Significant increases in cytokines and chemokines including IL-6, IL-8 and IP-10 have been shown 60 min post-transplantation [208]. The Edmonton Protocol established a regimen utilizing sirolimus, tacolimus and daclizumab for regular administration to alleviate the IBMIR; however, they alone are not a sufficient solution [209], [210], [211]. To overcome the IBMIR, islet encapsulation devices have been developed for islet transplantation. The devices themselves however can induce other immune system responses, such as the foreign body response (FBR) [203]. The process of the FBR is generally described by the overlapping events of protein adsorption, leukocyte recruitment and infiltration, macrophage activation, angiogenesis, the formation of foreign body giant cells, and fibrogenesis, which lead to chronic inflammation and device encapsulation through fibroblast deposition of collagen I and collagen III [203], [212], [213], [214], [215], [216]. The resulting fibrotic capsule can significantly reduce oxygen, nutrient, glucose and insulin diffusion between the host and the transplanted islets, leading to cell death and transplant failure [217]. Therefore, the regulation of the FBR is an area of intense research. Here, we will discuss the unprotected and encapsulated strategies for islet transplantation.
6.1. Unprotected islet transplantation
The requirement for chronic immunosuppression has limited the current indication for islet transplantation to mainly adult patients with severe hypoglycaemia complicated by impaired hypoglycaemia awareness; these patients represent less than 10 % of the population with T1D. Generally, islets are either transplanted as image-guided percutaneous transhepatic islet infusion or image-guided surgical transmesenteric islet infusion into the liver. Immunosuppression needed for this procedure consists of an induction phase and a maintenance phase during the entire lifespan of the islet transplant. Once islets are installed, post-transplantation monitoring in a weekly and then monthly manner is critical. Islet rejection is indicated by a decrease in C-peptide levels alongside with worsened metabolic control. The liver is the most efficient site for islet engraftment but an alternative site giving the possibility to monitor immune responses is desirable. Case reports indicate islet transplantation into the omental pocket [218], and the brachioradialis muscle [219], as well as subcutaneously in a mouse model [220]. Metabolic results and survival of the transplant using this procedure have been discussed in this review in Section 3) Current state of Islet Transplantation. Future research needs to focus on the refinement of the immunosuppressive therapeutic strategy as well as a more accessible transplantation site.
6.2. Encapsulated islet transplantation
Islet encapsulation devices can be classified as either extravascular or intravascular based on their proximity to the bloodstream [146]. Extravascular devices are transplanted near a vascularized region, but not connected to the vascular system directly. Intravascular devices are integrated within the host vascular system, allowing for the distribution of nutrients, oxygen, and waste through the blood flow [221]. For the purposes of this review, we are focusing on extravascular encapsulation strategies in terms of macro- and micro-encapsulation devices.
6.2.1. Macroencapsulation
Macroencapsulation devices are typically planar or tubular in shape and involve the incorporation of many islets within a single device. Their large size requires design considerations for the transport of oxygen, nutrients, glucose, insulin, and metabolic waste, while blocking the entry of immune cells and modulating their response to the device, as well as considerations for implant location, operative simplicity, and retrievability [222]. Implant thickness plays a major role for human implants due to the large volume of islets required, which are issues that may not be correctly addressed in small animal models where diffusion is not as much of an issue. The lack of revascularization post-macroencapsulation device implantation is one of the biggest hurdles for the technology as local hypoxia and the lack of nutrients destroy transplanted islets within the device in the first one to three days [223]. Therefore, it is essential that device design strategies are considered that enhance revascularization and support islets in the first days post-transplantation. Such strategies are addressed later in this review.
As mentioned, the FBR and fibrosis are other hurdles in islet encapsulation. To address the fibrotic encapsulation problem, Dolan et al. [224] developed a robotic device that mechanically modulates the biotic-abiotic interface in the peri-implant tissue, thereby altering strain and flow and affecting cellular activity. In a preclinical rodent model, the device showed a significant reduction in fibrotic capsule thickness and number of myofibroblasts surrounding the device. To address the issue of cell viability, Duffy et al. [225] developed the Regenervoir implant system designed of a flexible thermoplastic reservoir device capable of delivering transplant material via fill lines, which would allow the easy replenishment of islets over the device’s lifetime.
Current examples of macroencapsulation devices that are being driven towards commercialization are the devices from BetaO2 Technologies and ViaCyte Inc. BetaO2 Technologies is commercializing the βAirTM islet transplant device that incorporates a refillable oxygen tank to support cell oxygenation and survival. They showed that their device supported the survival of porcine islets with a bioartificial pancreas device in diabetic primates without any immune suppression for up to six months [226], [227] and allogeneic transplant for several months with no safety issues reported; however, there was an indication of impaired islet function [228], [229]. ViaCyte Inc. is currently developing the PEC-Encap™, PEC-Direct™, and PEC-QT™ devices. These devices are made from expanded polytetrafluroethylene. Each device has different advantages in terms of vascularization and immune-protection [230]. PEC-Encap™ utilizes their Encaptra® cell delivery system as an immuno-protective device that supports vascularization on its surface to allow gas and nutrient exchange through diffusion. PEC-Direct™ enables direct vascularization into the device and interaction with the cells. To overcome the need for oxygen to diffuse throughout the device and the use of immunosuppressants they are also working on PEC-QT™. ViaCyte uses their stem cell-derived PEC-01™ pancreatic progenitor cells as their cell source. Using the PEC-Direct™ device, ViaCyte Inc. is also developing a gene-edited immune-evasive cell line for this device (CyT49 pluripotent human stem cell) that might reduce or eliminate the need for immunosuppressants, while supporting vascularization of the transplant [228].
Different materials, coatings, molecules, and cell types have all been explored to be incorporated within macroencapsulation devices to encourage vascularization and immune protection while supporting islet survival and function. Despite these advancements and the aforementioned commercial efforts, there are still major hurdles to be addressed, including severe destruction of insulin-producing cells due to acute hypoxia at the initial transplantation stage, fibrosis surrounding devices, and inefficient long-term oxygen delivery. Further macroencapsulation strategies and requirements for islet transplantation were reviewed by Goswami et al. [231].
6.2.2. Microencapsulation
Microencapsulation involves surrounding a gas, liquid, or solid materials with a continuous polymer shell. The typical microencapsulated material ranges from 1 to 1000 µm in size [232]. In islet transplantation, microencapsulation incorporates a single or small number of islets or β-cells within a single capsule transplanted near a vascularized region [233], [234]. These devices have a relatively high surface to volume ratio, which overcomes the issues of poor diffusion. Lim and Sun [235] were one of the first to microencapsulate islets. They demonstrated islet survival three weeks post-transplantation in comparison to eight days without encapsulation. This was followed by O’Shea et al. [236] who used an alginate microcapsule which was shown to protect islets for one year in one animal. Work from the Paul de Vos group, who have a strong focus on the FBR, looked at the effect different formulations of alginate-polycation capsules have on the immune response and discovered that it was the interaction between the alginate and polycation, and not the alginates alone, that elicited different immune responses, which was an important step towards creating a translatable microencapsulation device [237]. Recently, Bansal et al. [238] used an alginate-chitosan based microcapsule to incorporate β-TC-6 cells to overcome immune rejection. Compared to the not encapsulated β-TC-6 cells, the encapsulated group was able to retain glycemic control due to relatively low immune reactions, which included low levels of CD8+, CD62L and CD4+ T-cells. Kogawa et al. [142] proposed transplanting microencapsulated islets and MSCs within a mesh bag made of a bioresorbable recombinant protein produced by Pichia pastoris. Once transplanted into the peritoneal cavity in diabetic mice, it showed good cell viability and functionality. In order to further suppress fibrosis, Alagpulinsa et al. [239] demonstrated that the incorporation of the immunomodulatory chemokine CXCL12 into an alginate-based microsphere, which isolated the device from the immune system and reduced the FBR, allowed for better long-term glycemic control without systemic immunosuppression. Interestingly, there is a correlation between fibrotic response and capsule geometry [240]. Chen et al. [241] compared toroid, rod and spheroid geometries of insulin-secreting microtissues by using agarose and alginate hydrogels and characterized the structural properties and cell viability. They identified that the morphology of toroid microtissues supports structure integrity and mechanical stability, which can prevent gel leakage from the device and improve the long-term survival of encapsulated insulin-producing cells.
Nanotechnology has also been employed for islet microencapsulation. The oxygen supply to islets in many devices relies on passive diffusion through the capsule. The diameter of the islet has been recommended to be below 100 µm to avoid central necrosis due to hypoxia [242]. Therefore, the concept of nanoencapsulation is to coat the pancreatic islets with nano-scale polymer film to minimize the size of the capsule [243]. Nanoencapsulation size has been shown to enhance the bioavailability, targeting and release of bioactive compounds making it a very interesting delivery technology for new therapeutics and regenerative medicine [244], [245]. Furthermore, nanoencapsulation coatings allow for a more precise control over islet aggregation [243], [246], [247]. A deeper review of the technical considerations and differences between micro- and nano-encapsulation of complementary materials for applications in regenerative medicine can be found in Suganya et al. [248], and an in-depth review on islet nanoencapsulation can be found in Ernst et al. [249]. Microencapsulation devices are often composed of hydrogels such as alginate, agarose, or collagen. We discuss the use of different scaffolds later in this review, which is also detailed by Wu et al. [250]. A thorough review of microencapsulation from the viewpoint of vascularization and the immune response can be found at Barkai et al. [251].
7. Biomaterials
The use of biomaterials in islet transplantation can play a key role in supporting cell survival. Biomaterials are developed from natural sources or from synthetic polymers that can be tuned for specific mechanical properties or drug release profiles. Regardless of the application, biocompatibility is one of the most important concerns when developing a new biomaterial [252]. In 1970, Homsy et al. [253] was one of the first to mention the importance of biomaterial biocompatibility. He proposed multiple factors related to the physical and chemical interactions between implant materials and the host response. Moreover, his team developed in vitro protocols to screen the implantations to reduce the in vivo studies. The general definition for biomaterial biocompatibility has been described as the capability of a biomaterial to induce a mild and appropriate host response in a particular condition. A biomaterial with good biocompatibility possesses minimum adverse effects such as irritation, toxicity, and immune rejection. In 1990, the United States Pharmacopeia (USP) [254] first standardized the guidance of the in vitro and in vivo assessment of biological reactivity for polymeric implants. These include evaluating interactions between the biomaterial and mammalian cells, and then animal studies to examine the systemic effects in response to the biomaterial. Several biological endpoints such as toxicity, sensitization, irritation, intracutaneous reactivity, material-mediated pyrogenicity, implantation, hemocompatibility, carcinogenicity, and degradation of the device components are monitored and assessed for approval. More generally, ISO 10993 also provides a guidance for risk management and biological assessment of the medical devices (ISO 10993-18:2020). Currently there is no specific guidance for biomaterials in islet encapsulation; however, GMP-grade and GRAS-approved biomaterials are available for clinical translation. In the following sections we will address the different types of biomaterials, their biocompatibility, their effects on islet viability, and challenges that they are facing in this field, which includes the lack of clinical translation in islet transplantation.
7.1. Natural polymers
Alginate is one of the most used biomaterials for islet transplantation research. As previously mentioned, Lim and Sun [235] showed that islets encapsulated in alginate enhanced cell survival rates, glycemic control, and immuno-protective effects post-transplantation in STZ-induced diabetes rats. Sodium alginate is composed of 1,4-linked-d-mannuronic acid and l-guluronate residues [255]. Different ratios of these two residues, different order of the residues and the final molecular weight of the polymers can build various chemical compositions of alginates. This means the variations can render the alginate compositions with different properties [251]. However, the apparent downside is the materials in each batch are difficult to standardize. Increased guluronic acid content creates a stiffer gel; whereas mannuronic acid-rich alginate is more flexible [256]. Alginate with higher percentage of guluronic acid has greater mechanical strength or viscosity [257]. Low viscosity alginate has been reported to allow the loading of more islets per volume of biomaterial, thus reducing transplant size [258]. This change in composition can influence alginate sphere and pore size. Pore size influences the permeability of inflammatory agents as well. Paul de Vos’s group proposed that a selective membrane can be designed based on the electrical charges of alginate under physiological conditions [251]. The charges of every cytokine in the physiological condition depend on their own isoelectric points (pI), which means the opposite charges of the membrane can be designed to repulse specific agents [251]. Recently, Takaichi et al. [259] proposed a fiber-shaped hydrogel from alginate with a diameter of 1 mm. The hydrogel can be retrieved after long-term transplantation, which overcomes a challenge of bead-shaped hydrogels. In vivo, the gel showed promising glycemic control in mice and the FBR was reduced without the use of immunosuppressive agents. Moreover, several studies reported that modifications of alginates can circumvent or mitigate the FBR after implantation into the body [260], [261], [262]. Vegas et al. [263] encapsulated cells within a derivative of alginate, triazole-thiomorpholine dioxide, which achieved long-term glycemic control and suppressed the FBR without immunosuppression. Another group utilized ARG-GLY-ASP-rich alginate with high viscosity to encapsulate MSCs and pancreatic islets, which increased viability and VEGF secretion [264]. A study investigating different FBR to modified alginate formulations found that formulation Z1-Y15 was the best performer in terms of reduced fibrosis in large animal macaque model studies [265], [260], [266]. Liu et al. [267] modified alginate with a zwitterionic polymer, sulfobetaine, to encapsulate rat islets. The hydrogel was implanted in different species of animals, including mice, dogs, and pigs. The results indicated that their formulation reduced fibrosis and cellular overgrowth around the implant.
Chitosan is a very common biomaterial in the field of tissue engineering. Chitosan is a linear polysaccharide, consists of β-(1 → 4)-linked d-glucosamine and N-acetyl-d-glucosamine and has been reported to be non-toxic, biocompatible, and biodegradable [268]. Moreover, chitosan has been reported to suppress the expression of inflammatory cytokines such as TNF-α, IL-6, TL-4 receptors, as well as T-cell proliferation. Many studies related to islet transplantation used chitosan to fully or partially modify the compositions of the encapsulation device to mitigate immunological reactions. In 2010, Yang et al. [269] showed that chitosan hydrogels protect implants from immune cell infiltration. Chitosan is also often fabricated with alginate to form novel biomaterials. Yang et al. [270] produced a chitosan-coated alginate microcapsule and found that it had better graft survival and induced significantly less pericapsular fibrosis compared to that of alginate microcapsules. Najafikhah et al. [271] designed a three-layer device composed of layers of alginate, chitosan, and polyethylene glycol that induced a downregulation of IL-2 secretion. Kim et al. [272] fabricated delivery devices with a mixture of chitosan with either alginate or hyaluronic acid (HA) and showed promising long-term glycemic control. They encapsulated β-cells in a chitosan-hyaluronic based hydrogel nanoflim delivery system, which prevented immune cells such as NK cells from passing through the barrier without affecting insulin secretion between the control and the hydrogel implant groups. Recently, Perikamana et al. [273] developed a macroencapsulation pouch made of chitosan and coated with 1,12-dodecanedioic acid (DDA) to prevent the attachment of cells that can induce fibrosis and the FBR. When loaded with primary hepatocytes and transplanted subcutaneously into mice, the device was able to support cell viability and improved cell function for up to six months compared to the cells transplanted in the chitosan pouch without the DDA modification. Furthermore, the tissue surrounding the DDA-modified pouch showed reduced fibrotic tissue and increased vascularization compared to the tissue around the unmodified pouch.
Agarose was first used as a microencapsulation biomaterial in 1987 where the authors demonstrated non-toxicity and normal islet function in vitro [274]. Iwata et al. [275] demonstrated sustained normoglycemia for 53 days in mice transplanted with hamster islets microencapsulated in agarose. This was followed by a study macroencapsulating islets within a mixture of agarose, collagen, and Gelfoam® which could sustain normoglycemia for over 170 days in mice [276]. In recent work, agarose-based FGF rods were implanted subcutaneously into an implant site pre-transplantation to induce angiogenesis. After the removal of the rods, the islets were transplanted successfully in the vascularized pocket without any immunosuppressive agents. All the animals showed long-termed glycemic control with good survival rate for over 100 days [277]. More recently, an agarose rod containing cyclic oligopeptide SEK-1005 was generated for allogeneic islet transplantation. The combination of components had an immuno-isolation effect, but also induced pre-vascularization of the subcutaneous site [277].
Fibrin, also known as Factor Ia, is commonly used in tissue engineering applications [278], [279]. During wound clotting, soluble fibrinogen circulating in the blood is converted by thrombin into insoluble strands of fibrin which then bind to platelets at the wound site to form a clot [138]. The clots partially can induce the IBMIR and normally are degraded gradually during wound healing, which translates to fibrin being an unstable and degradable biomaterial in physiological conditions [280]. Therefore, several improvements have been investigated to enhance its properties, including combination of other materials and crosslink modifications [280], [281], [282], [283]. Studies have indicated that embedding islets in fibrin could improve cell function, viability, and maintain their morphology [284], [285]. Riopel et al. [286] suggested that one mechanism driving the effect of fibrin on β-cells is through αvβ3 integrin increasing phosphorylated FAK, Erk1/2 and Akt, which prevents cell apoptosis and supports proliferation. Kuehn et al. [287] encapsulated porcine pancreatic islets into fibrin hydrogels to investigate the immuno-protective effects of monocytes. While there were high levels of pro-inflammatory cytokines (TNF-α, IL-1 and IL-6) secreted from the monocytes, the encapsulated cells showed less apoptosis compared with the cells without any physical barriers. Recently, a 22-day study showed that the subcutaneous transplant of islets with fibrin can increase the survival without the need for the pre-vascularization of the transplant space, despite delayed vascularization [288].
Hyaluronic acid (HA), is a non-sulfated glycosaminoglycan found throughout connective tissue and is a major part of the ECM [289]. It is often used in the pharmaceutical, cosmetic, and biomaterial industries as it is known to be non-toxic, anti-inflammatory, biodegradable and easy to modify [290], [291]. The anti-inflammatory response might be related to CD44 receptors that are expressed on the cell membrane of many human cells [292], [293]. Another well-known receptor that is associated with wound healing is called hyaluronan-mediated motility or CD168 which is presented on the cell surface, especially fibroblasts, or intracellular space such as cytoplasm and nucleus [294], [295]. The interaction between HA and these receptors plays a vital role in tissue repair process [296]. As a biomaterial, HA has been used for wound healing [297], bone and cartilage tissue engineering [298], [299], nerve regeneration [300], and cell survival [301]. Unfortunately, there has been little investigation into HA as a biomaterial for islet transplantation. Using core-shell spherification, Harrington et al. [302] produced HA-based islet microencapsulation devices that transiently restored normoglycemia in diabetic mice for 3–4 weeks. This was an improvement in comparison to non-encapsulated islet controls; however, this was not as promising as the poly(ethylene glycol) diacrylate, PEGDA, devices tested in the study which showed the reversal of diabetes for up to 16 weeks [302]. Despite the lack of islet transplantation-specific research using HA, it has been successfully used in other therapeutic applications [303], [304], [305], [306], [307], indicating that it may be a promising option for islet transplantation.
Silk is a fibrous protein generated by spiders and silkworms [308]. It is biocompatible, tunable, and durable [309], [310]. Raw silk is mainly composed of fibroin and sericin. Fibroin is the main structural constituent serving as the core of fibers, whereas sericin, is the minor component which plays a role in coating or bonding the fiber threads [311], [312]. Silk is a relatively new biomaterial in the area of islet transplantation, but has been employed for decades in other fields [313]. Silk sericin was first used in 2009 as biomaterial for serum-free islet culture [314], and in 2012 for islet cryopreservation [315]. These studies were followed by the first use of a collagen IV and laminin enhanced silk fibroin as a transplant biomaterial. When encapsulating MSCs and islets, silk increased cell viability and insulin secretion when compared to the non-encapsulated controls [316], [317]. Kumar et al. [309] designed an injectable silk hydrogel derived from two varieties of silk, mulberry B. mori and non-mulberry A. assama, and loaded the hydrogel with IL-4 and Dexamethasone for anti-inflammatory and immunosuppressive effects. Biocompatibility tests in vivo demonstrated that the hydrogels could modulate the local inflammatory response; nevertheless, diabetic animal studies will be required to demonstrate proof of concept.
Important islet ECM proteins are lost during islet isolation and implantation. Therefore, natural polymers are often biofunctionalized with ECM proteins to replace lost endogenous proteins and recapitulate the native islet microenvironment [318], [319]. Recent work by our group demonstrated the protective or regenerative effect of nidogen-1 (NID1) on multiple cell types in hypoxic conditions, including β-cells and endothelial cells. We demonstrated that NID1 modulates the immune system in vitro, increases innervation and vascularization, and reduces fibrosis in a mouse myocardial infarction and reperfusion model. We argued that utilizing the synergic effect of NID1 on the multiple problems of islet transplantation, such as immune intolerance, lack of vasculature, and high rates of initial cell death post-transplantation, may be a future solution in the field of islet transplantation [320]. Furthermore, Brandhorst et al. [321] demonstrated that the supplementation of NID1 during islet isolation significantly improved islet viability when compared with the current gold standard procedure. Predictive extrapolation of their data on islet isolation results from over 100 processed human pancreases showed that the use of NID1 in those isolation could have rescued 90 % of the suboptimal pancreases for clinical islet transplantation, which would have enabled 15 more islet transplantations. Further research on the effect of ECM molecules on islet transplantation was reviewed by Cheng et al. [23] and Llacua et al. [22].
7.2. Synthetic materials
PEG is composed of non-biodegradable and synthetic polymers [322]. PEG-modified (pegylation) delivery systems and device coatings have been shown to evade several immunological responses and foster islet viability [323]. Yang et al. [324] designed a nano-coating composed of chondroitin sulfate and star-shaped PEG for the surface of the implanted islets that was shown to ameliorate the IBMIR and maintain islet functionality. Kim et al. [325] formulated a novel delivery system based on PEG to protect neonatal porcine islet-like cell clusters from the immune system for 14 days post-implantation. PEG hybrids can be used to release biomolecules, such as was done by Scheiner et al. [326] to release VEGF from a macroencapsulation device. Coronel et al. [327] demonstrated the effect of a PD-LI-presenting PEG material in a diabetic mouse model, which is a different strategy than the overexpression of PD-LI in insulin-producing cells that was previously discussed in this review. The PD-LI-presenting materials supported allograft acceptance and led to an increase in Tregs at the delivery site when compared to unmodified controls.
Polydimethylsiloxane (PDMS) is a silicon-based polymeric compound with high cytocompatibility and stability. It is commonly used as a biomaterial for several tissue engineering purposes, especially in cornea replacement due to its excellent oxygen solubility [278], [328]. Nevertheless, the hydrophobic surface of PDMS hinders cell adhesion. Thus, fibronectin is often coated on PDMS scaffolds in order to increase the material hydrophilicity, cell adhesion, spreading, migration and proliferation [278]. Brady et al. [329] loaded fibrin-based pro-angiogenic hydrogel into PDMS scaffolds for in vivo islet engraftment and showed increased vascularization around the implants and significant glycemic control when compared to controls [329]. Anti-inflammatory agents are often embedded into PDMS due to its controlled drug releasing properties. For instance, Jiang et al. [330] incorporated 0.1 %–0.25 % dexamethasone into a PDMS-based 3D scaffold to suppress the immune response, ultimately enhancing blood glucose levels and suppressing inflammatory pathways on M2 macrophages. Several recent studies utilized PDMS scaffolds as an oxygen vehicle for transplanted islets [331], [332]. To mitigate the negative effects induced by hypoxic conditions post-transplantation at the extra-hepatic site, Liang et al. [333] incorporated calcium peroxide in the PDMS scaffold. They found that the device increased local oxygenation for 20 days compared to controls. Tokito et al. [334] demonstrated that a PDMS-based scaffold can also provide sufficient oxygen to support the viability and function of rat β-cells seeded at a high density in a 3D tissue-like manner.
PLGA (poly(lactic-co-glycolic acid)) is a biodegradable biomaterial consisting of poly-lactic acid (PLA) and poly-glycolic acid (PGA). Different PLA: PGA ratios modify biomaterial characteristics such as degradation rate and hydrophilicity. [278], [335]. Similar to PDMS, the main drawback of PLGA is poor hydrophilicity. Surface hydrophobicity prevents cell attachment, affecting cell proliferation, migration, and differentiation [336]. PLGA degradation causes a decrease in the pH which can induce immunological responses and impact insulin release [337], [338]. To overcome these issues, several modifications of the PLGA gel have been developed for islet encapsulation. Salvay et al. [339] developed PLGA-based scaffolds embedded with other ECM proteins such as collagen IV, fibronectin, and laminin. Their study showed the scaffolds could support islet architecture after long-term transplantation and found an increase in vascular density around the graft [339]. PLGA could also act as a drug carrier. Lew et al. [340] co-fabricated exentide, a GLP-1 receptor agonist, with PLGA and alginate, demonstrating that exentide could be sustainably released over 21 days. The study showed that the PLGA-based drug releasing microcapsules enhanced GSIS and islet survival. More recent research also used the sustained releasing property of PLGA for immunomodulatory agents. Li et al. [341] used PLGA to develop a delivery system with a controlled release of TGF-β1 at the transplantation site to recruit Treg cells without affecting islet function. Nguyen et al. [342] locally delivered tacrolimus through PLGA microspheres at the transplantation site of PEG-coated pancreatic islets.
8. Oxygenation strategies
β-cells require a considerable amount of oxygen for the metabolization of glucose and insulin secretion [343], [344], [345]. While islets only make up a very small percentage of the total pancreas, they require between 5 % and 20 % of the oxygen supplied to the whole pancreas. Depending on the size of an islet, its vascularization may develop through the islet for larger islets or around smaller islets [346]. The oxygen tension around the native islets is normally 30–40 mmHg and it can be elevated to 80–100 mmHg if the islets are adjacent to arterial capillaries [347]. In the Edmonton Protocol, islets are transplanted through the portal vein to the liver. The pO2 of the portal blood is approximately 40 mmHg [348]. Large islet size and the lack of vasculature leads to hypoxia and massive cell death after a day [349]. Studies demonstrated that the formation of microvascular networks and observable blood flow are the first signs of development approximately 4–10 days post-transplantation [350], [351], [352], [353], which correspond with studies demonstrating that islet survival, insulin releasing function, and islet mass decrease in the first three days post-transplantation [354], [355], [356]. However, the development of a glomerulum-like structure of microvessels and regeneration of the vascular density can require 30 days [350], [353]. Taken together, the need to address acute hypoxia at a minimum of the first three days, preferably 15 days post-transplantation is one of the most significant hurdles for islet transplantation. Below, we discuss some techniques used to improve islet viability in hypoxic conditions.
8.1. Hypoxic preconditioning
Prior to transplantation, islets can be “preconditioned” in culture at low oxygen levels in a process called hypoxic preconditioning [357], [358]. Lo et al. [357] demonstrated that the hypoxia-induced reduction in insulin secretion could be recovered through the modulation of intermittent oxygen levels between 5 % and 21 % in their culture system. Islets preconditioned in carbon monoxide (CO) have been reported to provide protective effects from the hypoxia-induced cell death after transplantation [359]. Studies revealed that patients who received CO-treated islets had reduced serum levels of CCL23, a chemokine that can recruit immune cells and upregulate several inflammatory cytokines, and increased levels of CXCL12, which can induce angiogenesis and mitigate oxidative stress [359].
8.2. Oxygen-releasing molecules
The incorporation of oxygen-releasing molecules within transplantation devices is a promising oxygenation strategy. Perfluorocarbons (PFCs) can store oxygen within their structure and release oxygen depending on the partial pressure of oxygen in their environment. PFC-derived materials including PVA, PVDF and ePTFE have been utilized in numerous biomedical applications, which was thoroughly reviewed by Grainger [360]. In the context of islet transplantation, PFCs have been used as hard materials for islet encapsulation devices [361], [362], [363]. PFCs can also be used for the sole purpose of releasing stored oxygen. PFCs have been incorporated in encapsulation devices and scaffolds for cell transplantation in liver [364], bone [365], and neural [366] purposes within a variety of biomaterials including alginate, fibrin, chitosan, and PDMS gels. PFCs had been used in the past for pancreas organ transport. Dr. Camillo Ricordi has highlighted the tremendous beneficial effects of PFC on pancreatic cells preservation from pancreases [367]. However, the first attempt to culture rat islets with PFC was unsuccessful as it resulted in reduced insulin secretion [368]. This was followed by a study in 2012 showing that PFC protects islet viability in hypoxia; however, with the same reduction in insulin stimulation that was seen in previous studies [369]. Lee et al. [370] transplanted a 20 % perfluorodecalin, 80 % alginate gel/islet material into streptozotocin-induced mice and showed an improvement in islet survival.
Calcium peroxide (CPO) is a common oxygen-releasing compound that can be incorporated in biomaterials. These compounds release oxygen and water upon hydrolytic activation. CPO was incorporated into a PDMS gel and assessed for islet survival in hypoxic conditions using the mouse MIN6 cell line and pancreatic rat islets in vitro [371]. This study demonstrated that CPO-loaded gels could support islet viability and function, comparable to the controls. This was followed by in vivo studies using a separate gel [372] and an encapsulation device [373] in a streptozotocin-induced rat model. Both techniques showed improved cell survival and function. CPO has also been loaded into alginate microspheres containing either rat or porcine islets. After seven days in hypoxic conditions in vitro, islets showed improved glucose response and cell survival [373]. Coronel et al. [374] designed a CPO-loaded collagen with pores designed to enhance vascularization at the transplant site which also showed increased cell survival and function in vivo. More recently, an analog lithium peroxide recycling system [375] approach for islet encapsulation was developed to recycle the waste product, carbon dioxide, released from the islets and regenerate oxygen by the chemical reaction with lithium peroxide.
Hemoglobin (Hb) is a natural carrier of oxygen throughout our bodies, and polymerized hemoglobin (PolyHb) is considered a blood substitute. When co-transplanted with islets into mice, low-levels of PolyHb reduced β-cell apoptosis; however, high levels induced an inflammatory reaction. The low dose PolyHb co-transplantation supported islet survival up to a month post-transplant and maintained glucose tolerance in the mice compared to islets transplanted alone [376]. It has been shown that exposing rat pancreas to PolyHb during islet isolation improves islet yield and when transplanted into diabetic mice, islets had a higher survival rate and were able to restore normoglycemia [377]. Mouré et al. [378] combined silicone-encapsulated CPO with hemoglobin to generate and carry oxygen for the incorporated islets. Although the study showed that encapsulation of hemoglobin alone was unsuccessful at maintaining transplanted islet survival and insulin secretion under hypoxia, the combination of both compounds protected the islets from a pro-inflammatory reaction.
9. Growth factors
Growth factors play key roles in maintaining tissue homeostasis through regulating cell survival, proliferation, and functionality. Often, these cytokines are incorporated within the encapsulation device to support engraftment until the host’s body can sustain the transplant. Like other pharmaceutical products, growth factor pharmacokinetics determine the dosage and the duration of the exposure time to their targets. Wound healing is a complex process involving many cell types and growth factors, the complexity of which is very difficult to mimic in a therapeutic setting, particularly due to the changing presence of growth factors in different tissues over different time points. Nevertheless, there has been interesting scientific progress in the use of growth factors in islet transplantation. Below, we discuss some cytokines that have been considered for co-transplantation strategies.
9.1. Vascular endothelial growth factor (VEGF)
VEGF is one of the most potent pro-angiogenic factors involved in vascularization. It plays roles in the inhibition of apoptosis, vascular permeability elevation, and immune cell recruitment [379], [380]. Post-transplantation, islets transfected with VEGF for elevated expression survived longer and achieved normoglycemia [381], [382] when compared with islets without VEGF overexpression; however, this comes with the risk of continuous, unregulated VEGF production, which might cause hemangiomas [383]. As such, labs incorporated VEGF in the encapsulation device to support vascularization, allowing the use of poorly vascularized transplantation sites [384], [385]. Hydrogels can be engineered to release VEGF as needed [386], or can be tuned for gradual release of VEGF to support vascularization of the transplant site with a reduced risk of attracting immune cells [385], [387]. Farina et al. [388] co-transplanted islets with either a low (0.5 μg/mL) or high (5.0 μg/mL) concentration of VEGF in a 3D-printed PLA device. While both concentrations supported vascularization within four weeks, the high concentration of VEGF also promoted an inflammatory response and calcification on the implants. Interestingly, Cabric et al. [389], [390] showed that conjugation of the pancreatic islets with heparin and VEGF-A effectively inhibited IBMIR and improved graft vascularization without affecting islet function. Similar results with VEGF, FGF-2, and heparin were also observed when incorporated within nanofiber gels [391], [392]. Recently, Scheiner et al. [393] established innovative 3D-printed PDMS microspheres that can be loaded with VEGF prior to the loading of islets. The in vitro study indicated that the device was able to release bioactive VEGF for at least four weeks, suggesting that this could be a promising vascularization strategy for future pancreatic islet transplantation. While the incorporation of VEGF had a positive effect on islet viability in post-transplantation studies, there still exists the risk of angioma formation in such a strategy. The concentration and timing of VEGF release needs to be tightly regulated in order to effectively support angiogenesis and not angioma formation [394]. Furthermore, new vasculature requires vasculogenesis or arteriogenesis as much as angiogenesis, and VEGF alone does not drive healthy angiogenesis. Other growth factors and cell types are required to develop a mature vasculature that connects to the native vasculature in vivo without initiating pathological processes. A detailed review regarding the vascular biodesign concepts specific for islet transplantation can be found in Bowers et al. [395].
9.2. Hepatocyte growth factor (HGF)
HGF is produced by stromal cells and is involved in promoting cell proliferation and angiogenesis [396], [397]. HGF has been reported to be a potential growth factor to improve transplantation outcomes in animal studies [398]. Moreover, in vivo studies have shown that HGF can suppress fibrosis in organ transplantation, including liver [399], cardiac allografts [400], and skeletal myoblasts [401]. When HGF was overexpressed in murine islets, García-Ocaña et al. [402] found increased insulin content and secretion upon glucose stimulation, with greater GLUT-2 and GCK expression. They also showed prolonged survival and function through activation of pro-survival signalling cascades when transplanted into diabetic mice compared to those without the HGF overexpression [403], [404]. Interestingly, this enhanced islet survival and function was also seen when non-human primate islets were transplanted into mice [405]. The survival rate of the transplanted islets was significantly higher in the HGF group compared to the control group.
9.3. Fibroblast growth factor (FGF)
FGFs have several functions in tissue regeneration, such as cell migration, differentiation, and angiogenesis [406]. In clinical practice, FGFs have been utilized at injured sites to support wound healing [407]. In previous studies, supplementation of FGF2 in isolated murine islet cultures supported insulin secretion in response to glucose, and when co-transplanted into diabetic mice, assisted in the reduction of blood glucose levels and improved vascularization compared to islets transplanted without FGF2 [408]. FGF1 encapsulated in alginate has been shown to successfully allow for the vascularization of the implant site [409]. FGF21 administration to diabetic mice following an islet transplant improved transplant survival and restored glucose levels compared to those without FGF21 [410]. Nevertheless, FGFs are not stable in vivo [411]. Therefore, some studies have focused on the encapsulation of FGFs to increase their half-life. Smink et al. [412] proposed the use of a liposomal formulation with acidic FGF and basic FGF. When these liposomes were introduced to the transplant site, there was no effect on the long-term survival rate of islets, but the liposomes with acidic FGF enhanced vascularization of the scaffold [412]. Yang et al. [413] investigated the role of basic FGF in pre-vascularizing transplant sites. They implanted an FGF-releasing collagen-based sheet into mice for ten days, and then removed this sheet before transplanting rat islets contained in an immune-isolation device. They found that the pre-vascularization step with basic FGF was necessary for maintaining blood glucose levels post transplantation when compared to the controls [413].
9.4. Transforming growth factor-β1 (TGF-β1)
TGF-β1 is a key cytokine involved in many cellular processes including cell proliferation, cell survival, and promoting angiogenesis [414], [415]. It is involved in the suppression of inflammatory cytokines such as interleukins, IFN-γ, and TNF-α, and cytotoxic-T-cells [414]. The dose of TGF-β1 can exert different cellular effects. Animal studies investigating TGF-β1 showed that a limited dose of TGF-β1 (5 μg/mL) can maintain islet integrity and prevent islet apoptosis [416]. The TGF-β1 pathway has been shown to be important in maintaining β-cell populations both in vitro and in vivo [416], [417]. Administration of TGF-β1 inhibitors to mice transplanted with murine and human β-cells promoted β-cell replication [418] and prevented β-cell apoptosis [419]. When human islets were transduced to express TGF-β1, there were no adverse effects on C-peptide production or cell viability, suggesting this could be used to evade immune reaction [420]. Researchers have co-transplanted TGF-β1 with islets for its immunosuppressive benefits. When donor islets from transgenic mice expressing islet-specific TGF-β1 were transplanted into diabetic mice, they demonstrated prolonged graft survival, and reduced presence of T-cells and inflammatory cytokines [421]. Liu et al. [422] co-transplanted islets and TGF-β1 in a multi-layered PLG scaffold. TGF-β1 was encapsulated within the scaffold, while islets were seeded on an outer layer. Transplantation of these devices to the epididymal fat pad of diabetic mice achieved normoglycemia within a week and found significantly decreased cytokine expression of TNF-α, and IL-12, and leukocyte infiltration. This study showed that TGF-β1 can be co-transplanted with islets to delay graft rejection and an immune response, while not impairing islet function [422].
10. Transplantation site and delivery
Choosing an appropriate transplantation site is crucial for the survival and function of an islet graft. Transplant size is often not an issue in small animal studies as the number of islets and/or the required device to house the islets is not a limiting factor in terms of space and diffusion. However, humans require a considerably large number of islets, and this can have a great effect on the transplant site, and device design and size. The site should have minimal innate and adaptive immune responses, allow for neovascularization, and be minimally invasive for transplant and monitoring purposes. Many of the common sites such as liver, kidney, pancreas, and subcutaneous space have been extensively studied and reviewed [113], [423], [424], [425] (Fig. 2). Below we give an overview of some transplantation sites considered for islet replacement therapy.
Fig. 2.
Insulin-secreting cell sources and transplant locations. The main sources of pancreatic islets for patients can be potentially obtained from the pancreases of cadavers or pigs. The isolation procedures and the Ricordi chamber have been optimized to acquire sufficient and high-quality islets from the doners. In the recent years, insulin-producing cells which can be differentiated from stem cells has been considered as a new source for the transplanted islets. Islets isolated through manual procedures or derived from other cell sources can be transplanted into areas of the body such as the liver, pancreas, kidney, spleen, omentum, and intramuscular and subcutaneous spaces.
10.1. Liver
As one of the most vascularized organs in the body, infusion of islets through the portal vein to the liver is the most common site for experimental and clinical implantation. The liver is the organ that insulin mainly targets physiologically and is currently the accepted site for the Edmonton Protocol [426], [427], [428]. The procedure itself is minimally invasive as it is aided by interventional radiology and ultrasound guidance. The islets are infused into the portal vein using a catheter and transfusion bag [429], [430]. When comparing the liver to the subcutaneous space for the transplantation of islets embedded within a poly(N-isopropylacrylamide) sheet device, it was shown that the liver surface supported vascularization of the transplanted site significantly better than at the subcutaneous space [431]. Nevertheless, the liver has been reported to be not an ideal transplant site as it tends to induce an IBMIR. The liver is the main source of complement proteins secreted by hepatocytes, which produces up to 15 times more complement C3 than macrophages [204], [432] and inflammatory cells [204], [433]. Furthermore, the implanted cells are difficult to monitor for survival and function (inaccessible for graft biopsy), bleeding, portal hypertension, and thrombosis. To overcome the IBMIR and thrombosis problems, anticoagulation agents such as heparin are used for the treatment [434]. The implanted islets are also hard to completely retrieve from the patient if safety concerns arise [113], [435]. Nevertheless, the liver is still the main site for islet replacement therapy and considered to be the location which can provide long-term survival for transplanted islets.
10.2. Kidney
Kidney subcapsular space provides good vascularization for transplanted islets. Compared to the liver, implanted islets can be retrieved and monitored easily after transplantation. Moreover, the site was reported to be immunologically privileged [113], [114]. Recent research on rodents indicated that islets transplanted in the kidney subcapsular space have the best result compared to portal vein and muscle [436], [437], though further investigation is still required. Most studies utilized naked islets for transplantation in the kidney due to limited space, resulting in several disadvantages of this site. The first drawback is the low oxygen partial pressure [438], which may cause substantial cell number loss because of necrosis. The second drawback is the subcapsular space only possesses limited volume for the islet graft, though some studies demonstrated that fewer islets are required in the renal subcapsular site to reverse diabetes in comparison to the portal vein [438].
10.3. Omentum and peritoneal cavity
Encapsulated islets are generally implanted into the peritoneal cavity and omentum because of relatively large spaces [439]. In addition, there are high lymphatic vessels and vascularization, which allows for oxygen and nutrient supply to the transplanted islets [440]. Although these sites are still under early-stage research, the good plasticity, high capacity, and vascular networks make these potential sites for medical device implantation [441]. Kim et al. [442] showed that the glycemic control for omentum islet transplantation in mice was better than kidney, liver, and muscle. Interestingly, a peritoneal pouch to deliver a large amount of islets (about 1000 islets) was designed for this transplantation site [443]. The pouch delivery device resulted in proper vascularization and high insulin secretion; however, major disadvantages of this site are the invasive surgery required compared to the other sites [222], [442] and cytokine-mediated damage to encapsulated islets which is induced by peritoneal macrophages [444], [445], [446].
10.4. Subcutaneous and intramuscular spaces
The subcutaneous and intramuscular spaces are some of the simplest operative locations for islet implantation with minimal complications and reduced IBMIR [447]. Islet viability and function can be monitored due to the superficial implantation location below the skin, and can be easily retrieved from the patients; however, they offer insufficient oxygen supply without a neovascular network, leading to poor outcomes [113]. Furthermore, the insulin release patterns at these sites are not as favourable as locations like the liver. Physiologically, about 50 % of insulin secreted from the pancreas flows into the liver through the portal vein and then undergoes the first-pass metabolism [448]. Islet transplantation into intramuscular and subcutaneous sites leads to insulin secretion directly to the peripheral vessels which ultimately results in poor glycemic control [449]. Several medical devices that can be implanted subcutaneously are in clinical trials. Recently, the Shapiro group developed a subcutaneous “device-less” technique for transplantation [220], [450], [451]. They designed a special catheter and implanted it at the subcutaneous site to induce controllable FBR. The purpose was to promote neovascularization at the location before transplantation.
10.5. Pancreas
The pancreas appears to be an obvious site for islet replacement therapy, and several animal studies also indicated that the site could offer a well-vascularized microenvironment with minimal inflammatory and fibrotic response [452], [453]; however, the surgical procedure for the pancreas is technically difficult with a high risk of complications such as hemorrhage [454]. Therefore, the pancreas is unlikely to be an ideal islet transplantation site. For the same reason, so far, we are unaware of any studies on implanting large medical devices in the pancreas.
10.6. Spleen
The spleen has been suggested to be an optimal site for islet transplantation because of its high vascularization and blood flow into the portal venous system. The spleen is involved in immune tolerance, playing a role in the suppression of cytokine secretion, T-cell proliferation, and antibody production. The presence of Tregs in the spleen further supports that this site might be immunosuppressed [449]. Conversely, some research also demonstrated islets transplanted in the spleen are more accessible by lymphocytes, leading to an immune response. Transplantation of islets in a composite fibroblast-populated collagen matrix in the spleen of diabetic mice showed a better survival rate of the islet allograft when compared to controls [455]. Compared to other transplant sites, the spleen has an increased risk of hemorrhaging [438]. When comparing the splenic parenchyma site and the hepatic sinus tract for naked islet transplantation, it was found that the grafts in the splenic parenchyma group possessed a better outcome of glycemic control [456].
11. Reimbursement
The cost of islet transplantation varies from country to country. A 2015 UK study comparing lifelong insulin therapy and unprotected islet and hiPSC derived β-cell transplantation reported that islet transplantation costs GB£60,000 in total for the one-off procedure with a 20-year cost of GB£321,704 [457]. A US study showed that islet transplantation can have a total 20-year cost of US$519,000 [458]. The Swiss-French GRAGIL network reported a 2013 cost of US$38,000 for the procedure and a 1 year post-transplantation cost of US$72,000 [459]. These initial costs are high when compared to the gold standard insulin therapy. Nevertheless, studies did show that islet transplantation can be more cost effective than insulin therapy after 9 years [459]. Interestingly, the same study showed that hiPSC derived β-cell transplantation could be more cost effective than insulin therapy after 8–11 years, depending on a few manufacturing and immunosuppressive variables. Despite these promising economic estimations, clinical trial data reviews have shown that only a third of islet transplantation recipients are insulin independent after 5 years and very few after 10 years. However, the purely economic variables are not the only consideration for health care provider calculations for reimbursement. Quality adjusted life years, life years gained, and the prevention of severe hypoglycemic events are major considerations with their own calculations, all of which favour islet transplantation.
The reimbursement for islet transplantation varies in each country. Japan, Australia, UK, France, Sweden, Norway, Poland, Czech, Switzerland, Italy and Canada, have approved islet transplantation as a safe and efficient therapy, which allows patients with T1D who undergo this treatment to receive reimbursement from national health systems or third-party insurance [460]. Nevertheless, the situation in the US is much more complicated. Although whole pancreas transplantation is reimbursable for patients with T1D by health insurance, current allogeneic islet transplantation is not reimbursable and is only permitted for clinical trials [460]. In the US, islets are regarded and regulated as biological drugs instead of organs [460], [461], which requires a Biological License Application (BLA) for further use. The requirement of BLA approval impedes reimbursement from insurance companies. In 2021, the Am. Diabetes Assoc. published a perspective article to assert that the criteria used for biologic drugs based on BLA are not suitable for the quality reassurance system of the transplanted islets. The misconceived regulation resulted in the decline of the activities of islet transplantation over decades and this might be why this treatment has been stuck in the pre-clinical or clinical trial stages in the US [460], [461]. Therefore, the current regulations urgently need to be re-evaluated by the FDA.
12. Future perspectives
Insulin-secreting cell transplantation is an exciting therapeutic approach for the treatment of diabetes mellitus. As demonstrated throughout this review, there has been intense and broad research into the enhancement or replacement of the Edmonton Protocol. Topics of interest such as insulin-producing cell sources, cell encapsulation with materials and devices, biomolecule supplementation, and implant location have been investigated to solve important problems such as immune rejection, lack of vascularization, and cell viability. Despite a large body of science and therapeutic potential, the commercial viability of islet transplantation does limit the robustness of what can be delivered to patients today. In other words, we may have 100 different options to improve islet transplantation, but cost issues in clinical translation and reimbursement on the payer side limit most commercial strategies because the cost benefit analysis must be better than the clinical gold standard and financial margins for the developer. Considering the complexity of Advanced Therapy Medicinal Product regulation, it is wise to keep products simple. Our proposal of the future of insulin-secreting cell transplantation is a mixture of short-term and long-term goals that will serve patients currently suffering greatly from T1D, but provides an accessible solution for a greater stratification of patients in the long-term (Fig. 3).
Fig. 3.
Research and translational priorities for islet transplantation. The short-term priorities for islet transplantations that can help current patients in need are placed from top to bottom. Devices and biomaterials are already either clinically approved for other applications or currently under clinical trials. As you move down, the proteins, growth factors, and other small molecules become more effective and specific; however, the cost and safety profiles of these options make them long-term possibilities. Towards the bottom, other cell types, particularly engineered cell types, become more powerful in their efficacy; however, many are still in pre-clinical translation.
Transplantable islets can be acquired from multiple sources. Allogeneic islets from cadavers have been the main source of islets and insulin-producing β-cells. Although the cost of the islet isolation process is similar in the xeno- and allo-transplantation, donor limitations of the latter always exist. Therefore, SC-derived β-cells have been regarded as a promising source of implantable insulin-secreting cells. Different stem cell types and cell lines have different differentiation efficiencies and transcription profiles, which greatly influence the viability and function of the final implanted cells. The future insulin-secreting cell should be engineered to genetically evade the host immune response and resist hypoxia-induced apoptosis. The pre-differentiation cell source, whether it be an iPSC source or primary cell for trans-differentiation, should be commercially viable and widely accepted as safe. Recently, strategies to facilitate large-scale production of functional islets from stem cells have been described [174]. Importantly, the differentiation process should be as short and simple as possible. 30+ day complex multi-step differentiation protocols are great science and an exceptional step towards the future, but they may not be commercially viable as a long-term solution for a large population of patients.
Encapsulation devices can help resolve current issues in islet transplantation. The general pros and cons of macro- and micro-encapsulation devices have been discussed. In the short-term, macroencapsulation is the current trend in commercialization as they are easy to retrieve and therefore have a much stronger safety profile than the smaller devices. However, microencapsulation devices may be the best option in the long-term as cell sources become more trustworthy and retrievability is not a requirement. Oxygen and nutrient diffusion are not an issue with these devices, which somewhat mitigates the need for oxygenation strategies. The operative procedures of the smaller devices are minimally invasive which is an important advantage for doctors considering whether to adopt new technologies.
Biomaterials can be both short-term and long-term solutions. There are many GMP quality biomaterials currently on the market, which can be modified for islet transplantation purposes. However, there is not much guidance on the clinical translation side. Simple collagen type I or alginate biomaterials have been shown to have positive effects on islet viability and function. Biomaterials become a more long-term solution when they are modified to induce a desired immune response, release drugs into the transplant environment, or act upon transplanted cells or islets. However, a microencapsulated and properly engineered insulin-secreting cell line may not require a biomaterial at all. Biomaterials in islet transplantation may become obsolete in the long-term due to cost and efficacy considerations. Nevertheless, oxygen-producing and highly permeable biomaterials may be a relatively straightforward solution for acute hypoxia at the transplant location.
ECM proteins, growth factors, and other small molecules all have their potential; however, the options seem almost endless. Our group demonstrated how the ECM protein NID1 alone can protect β-cell viability, modulate the immune response, increase insulin secretion, angiogenesis and neurogenesis, and reduce fibrosis. Nevertheless, the cost of the GMP production and clinical trials to bring NID1 to the market still needs commercial validation. Short-term, already GMP available and validated growth factors like VEGF may be the best available options to support islet transplantation, if there is an established dosage and release kinetic criteria available, as well as a proper biodesign that mitigates pathological remodelling. Molecules to increase oxygenation will remain important until microencapsulation techniques can be employed with engineered cell lines. Macroencapsulation devices still suffer from fibrotic encapsulation and therefore molecules to modulate the immune system are very relevant.
Accessory cells co-transplanted with islets support islet survival and function through paracrine signaling. While the co-transplantation of growth factors and small molecules suffer from their short half-life, accessory cells exist as a continuous source of such proteins on a need basis; however, the issue arises when considering how many different cell types are to be transplanted for islet transplantation purposes. Endothelial cells are pro-angiogenic and secrete ECM proteins to support cell survival while Tregs modulate the host’s immune response to prevent immune rejection. MSCs and hAECs have both pro-angiogenic and immunomodulatory properties to reduce the host’s immune response. Different cell types confer different advantages and disadvantages, complicating the decision on which cell type and in what ratio they should be introduced to support islet survival and function post-transplantation. Furthermore, the addition of another living cell type complicates the regulatory process and increases costs. Here, we propose that Tregs are the most promising co-transplantation option due to the importance of modulating the immune system.
The idea of universal stem cells holds great potential for cell replacement therapies as they can be used for multiple patients. Stem cells that are engineered to lack MHC-I-class and MHC-II-class proteins and express PD-L1 evade immune detection and attract protective Tregs. In the scope of cell replacement strategies for an autoimmune disorder such as T1D, this holds even greater promise as there would be no ligands for the host’s immune response to detect. Glucose-responsive insulin-producing cells differentiated from such a stem cell line can be successfully transplanted without an encapsulation device, immunomodulatory cells/proteins, or an immunosuppressive regimen. Furthermore, as differentiation protocols have been shown to vary between stem cell types, a universal stem cell line would allow for prior optimization of the protocols. It would also open doors to modify other aspects of the cells such as ECM production and angiogenic potential to further support their survival in transplantation settings. While universal stem cells seem to be an ideal approach for cell replacement therapies, much remains unknown on how such genetic modifications can affect long-term function of the differentiated cells.
The currently strategies for islet transplantation are dependent on safety, ease of regulatory approval and health care reimbursement, and therefore the current future remains very device focused. However, with all new technologies, costs will decrease, enabling a more robust product for a cost that the reimbursement agencies are willing to pay. Here, we see a very bright future in the field of islet transplantation as new technologies, molecules, and cell types will be able to provide greater efficacy for a more accessible price. Once these obstacles have been successfully tackled, islet transplantation may also become an attractive treatment option for patients with longstanding T2D and severe β-cell failure [462], [463], [464].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors would like to thank Prof. Dr. Katja Schenke-Layland for proofreading the article.
Funding sources
This work was financially supported by the European Union's Horizon 2020 framework programme under grant agreement 812865 (DELIVER). Swiss National Science Foundation (SNSF; P500PB_203146) to A.Z.
Data availability
No data was used for the research described in the article.
References
- 1.R. Williams, S. Colagiuri, IDF Diabetes Atlas, ninth ed., S. Karuranga, et al., editors, 2019.
- 2.Berget C., Messer L.H., Forlenza G.P. A clinical overview of insulin pump therapy for the management of diabetes: past, present, and future of intensive therapy. Diabetes Spectrum. 2019;32(3):194–204. doi: 10.2337/ds18-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinstock R.S., et al. Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D exchange clinic registry. J. Clin. Endocrinol. Metab. 2013;98(8):3411–3419. doi: 10.1210/jc.2013-1589. [DOI] [PubMed] [Google Scholar]
- 4.Gentile S., Strollo F., Ceriello A. Lipodystrophy in insulin-treated subjects and other injection-site skin reactions: are we sure everything is clear? Diabetes Ther. 2016;7(3):401–409. doi: 10.1007/s13300-016-0187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonder-Frederik L.A., et al. Psychology, technology, and diabetes management. Am. Psychol. 2016;71(7):577–589. doi: 10.1037/a0040383. [DOI] [PubMed] [Google Scholar]
- 6.R.P. Robertson, I.B. Hirsch, J.E. Mulder, Pancreas and islet transplantation in diabetes mellitus (2021) 1767.
- 7.Lammert E., et al. Role of VEGF-A in vascularization of pancreatic islets. Curr. Biol. 2003;13(12):1070–1074. doi: 10.1016/s0960-9822(03)00378-6. [DOI] [PubMed] [Google Scholar]
- 8.Barra J.M., Tse H.M. Redox-dependent inflammation in islet transplantation rejection. Front. Endocrinol. 2018 doi: 10.3389/fendo.2018.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jennings R.E., et al. Human pancreas development. Development. 2015;142(18):3126–3137. doi: 10.1242/dev.120063. [DOI] [PubMed] [Google Scholar]
- 10.Beatriz Sosa-Pineda B., et al. The Pax4 gene is essential for differentiation of insulin-producing β-cells in the mammalian pancreas.pdf. Nature. 1997;386:399–402. doi: 10.1038/386399a0. [DOI] [PubMed] [Google Scholar]
- 11.Collombat P., et al. The simultaneous loss of Arx and Pax4 genes promotes a somatostatin-producing cell fate specification at the expense of the alpha- and beta-cell lineages in the mouse endocrine pancreas. Development. 2005;132(13):2969–2980. doi: 10.1242/dev.01870. [DOI] [PubMed] [Google Scholar]
- 12.Book The islets of Langerhans. Adv. Exp. Med. Biol. 2010;654 [PubMed] [Google Scholar]
- 13.Xavier G. The cells of the islets of Langerhans. J. Clin. Med. 2018;7(3):54. doi: 10.3390/jcm7030054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koma Y., et al. Cell adhesion molecule 1 is a novel pancreatic-islet cell adhesion molecule that mediates nerve-islet cell interactions. Gastroenterology. 2008;134(5):1544–1554. doi: 10.1053/j.gastro.2008.01.081. [DOI] [PubMed] [Google Scholar]
- 15.Vagesjo E., et al. Immunological shielding by induced recruitment of regulatory T-lymphocytes delays rejection of islets transplanted in muscle. Cell Transplant. 2015;24(2):263–276. doi: 10.3727/096368914X678535. [DOI] [PubMed] [Google Scholar]
- 16.Virtanen I., et al. Blood vessels of human islets of Langerhans are surrounded by a double basement membrane. Diabetologia. 2008;51(7):1181–1191. doi: 10.1007/s00125-008-0997-9. [DOI] [PubMed] [Google Scholar]
- 17.Jiang F.X., et al. Laminin-1 promotes differentiation of fetal mouse pancreatic beta-cells. Diabetes. 1999;48(4):722–730. doi: 10.2337/diabetes.48.4.722. [DOI] [PubMed] [Google Scholar]
- 18.van Deijnen J.H., et al. Distribution of collagens type I, type III and type V in the pancreas of rat, dog, pig and man. Cell Tissue Res. 1994;277(1):115–121. doi: 10.1007/BF00303087. [DOI] [PubMed] [Google Scholar]
- 19.Hughes S.J., et al. Characterisation of collagen VI within the islet-exocrine interface of the human pancreas: implications for clinical islet isolation? Transplantation. 2006;81(3):423–426. doi: 10.1097/01.tp.0000197482.91227.df. [DOI] [PubMed] [Google Scholar]
- 20.Brissova M., et al. Pancreatic islet production of vascular endothelial growth factor–a is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55(11):2974–2985. doi: 10.2337/db06-0690. [DOI] [PubMed] [Google Scholar]
- 21.Stendahl J.C., Kaufman D.B., Stupp S.I. Extracellular matrix in pancreatic islets: Relevance to scaffold design and transplantation. Cell Transplant. 2009;18(1):1–12. doi: 10.3727/096368909788237195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Llacua L.A., Faas M.M., de Vos P. Extracellular matrix molecules and their potential contribution to the function of transplanted pancreatic islets. Diabetologia. 2018;61(6):1261–1272. doi: 10.1007/s00125-017-4524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng J., et al. Matrix components and scaffolds for sustained islet function. Tissue Eng. Part B, Rev. 2011;17(4):235–247. doi: 10.1089/ten.TEB.2011.0004. [DOI] [PubMed] [Google Scholar]
- 24.Bayersdorf R., Fruscalzo A., Catania F. Linking autoimmunity to the origin of the adaptive immune system. Evol. Med. Public Health. 2018;2018(1):2–12. doi: 10.1093/emph/eoy001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kubelkova K., Macela A. Innate immune recognition: an issue more complex than expected. Front. Cell. Infect. Microbiol. 2019;9:241. doi: 10.3389/fcimb.2019.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar H., Kawai T., Akira S. Toll-like receptros and innate immunity. Biochem. Biophys. Res. Commun. 2009;388(4):621–625. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 27.Iwasaki A., Medshitov R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015;16:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lukasch B., et al. Genes of the major histocompatibility complex highlight interactions of the innate and adaptive immune system. PeerJ. 2017;5(e3679) doi: 10.7717/peerj.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wieczorek M., et al. Major histocompatibility complex (MHC) class I and MHC class II proteins: conformational plasticity in antigen presentation. Front. Immunol. 2017;8:292. doi: 10.3389/fimmu.2017.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miceli M.C., Parnes J.R. The roles of CD4 and CD8 in T cell activation. Semin. Immunol. 1991;3(3):133–141. [PubMed] [Google Scholar]
- 31.Li Y., Yin Y., Mariuzza R.A. Structural and biophysical insights into the role of CD4 and CD8 in T cell activation. Front. Immunol. 2013;4:206. doi: 10.3389/fimmu.2013.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hogguist K.A., Baldwin T.A., Jameson S.C. Central tolerance: learning self-control in the thymus. Nat. Rev. Immunol. 2005;5:772–785. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- 33.Mueller D.L. Mechanisms maintaining peripheral tolerance. Nat. Immunol. 2009;11:21–27. doi: 10.1038/ni.1817. [DOI] [PubMed] [Google Scholar]
- 34.Sakaguchi S., Wing K., Yamaguchi T. Dynamics of peripheral tolerance and immune regulation mediated by Treg. Eur. J. Immunol. 2009;39(9):2331–2336. doi: 10.1002/eji.200939688. [DOI] [PubMed] [Google Scholar]
- 35.Mueller D.L., Jenkins M.K. Autoimmunity: when self-tolerance breaks down. Curr. Biol. 1997;7(4):R255–R257. doi: 10.1016/s0960-9822(06)00115-1. [DOI] [PubMed] [Google Scholar]
- 36.Dornmair K., et al. T-cell-mediated autoimmunity. Am. J. Pathol. 2003;163(4):1215–1225. doi: 10.1016/S0002-9440(10)63481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Culina S., et al. Islet-reactive CD8+ T cell frequencies in the pancreas, but not in blood, distinguish type 1 diabetic patients from healthy donors. Sci. Immunol. 2018;3 doi: 10.1126/sciimmunol.aao4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Erlich H., et al. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes. 2008;57(4):1084–1092. doi: 10.2337/db07-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taplin C.E., Barker J.M. Autoantibodies in type 1 diabetes. Autoimmunity. 2008;41(1):11–18. doi: 10.1080/08916930701619169. [DOI] [PubMed] [Google Scholar]
- 40.Kreiner F.F., et al. Current state of antigen-specific immunotherapy for type 1 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2021;28(4):411–418. doi: 10.1097/MED.0000000000000647. [DOI] [PubMed] [Google Scholar]
- 41.Sona C., et al. Evidence of islet CADM1-mediated immune cell interactions during human type 1 diabetes. JCI Insight. 2022;7(6) doi: 10.1172/jci.insight.153136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rogal J., et al. Stem-cell based organ-on-a-chip models for diabetes research. Adv. Drug Deliv. Rev. 2019;140:101–128. doi: 10.1016/j.addr.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 43.Stranescu D.E., et al. Functional maturation of pancreatic ß cells during development. Am. Diabetes Assoc. 2018:A558. [Google Scholar]
- 44.Henquin J.C., Nenquin M. Dynamics and regulation of insulin secretion in pancreatic islets from normal young children. PLoS ONE. 2016;11(11) doi: 10.1371/journal.pone.0165961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henquin J.C., Nenquin M. Immaturity of insulin secretion by pancreatic islets isolated from one human neonate. J. Diabetes Investig. 2018;9(2):270–273. doi: 10.1111/jdi.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaestner K.H., et al. What is a beta cell? - Chapter I in the Human Islet Research Network (HIRN) review series. Mol Metab. 2021 doi: 10.1016/j.molmet.2021.101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blum B., et al. Functional beta-cell maturation is marked by an increased glucose threshold and by expression of urocortin 3. Nat. Biotechnol. 2012;30(3):261–264. doi: 10.1038/nbt.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shrestha S., et al. Combinatorial transcription factor profiles predict mature and functional human islet alpha and beta cells. JCI Insight. 2021 doi: 10.1172/jci.insight.151621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Vos A., et al. Human and rat beta cells differ in glucose transporter but not in glucokinase gene expression. J. Clin. Invest. 1995;96:2489–2495. doi: 10.1172/JCI118308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCulloch L.J., et al. GLUT2 (SLC2A2) is not the principal glucose transporter in human pancreatic beta cells: implications for understanding genetic association signals at this locus. Mol. Genet. Metab. 2011;104(4):648–653. doi: 10.1016/j.ymgme.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 51.Heimberg H., et al. Heterogeneity in glucose sensitivity among pancreatic β-cells is correlated to differences in glucose phosphorylation rather than glucose transport. EMBO. 1993;12:2873–2879. doi: 10.1002/j.1460-2075.1993.tb05949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu J.S.E., Hebrok M. All mixed up- defining roles for β-cell subtypes in mature islets. Genes Dev. 2017:228–240. doi: 10.1101/gad.294389.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liang Y., et al. In situ glucose uptake and glucokinase activity of pancreatic islets in diabetic and obese rodents. J. Clin. Invest. 1994;93:2473–2481. doi: 10.1172/JCI117256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matschinsky F., et al. Glucokinase as pancreatic B cell glucose sensor and diabetes gene. J. Clin. Invest. 1993;92:2092–2098. doi: 10.1172/JCI116809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shimzu T., et al. Control of glucose metabolism in pancreatic beta-cells by glucokinase, hexokinase, and phosphofructokinase. Model study with cell lines derived from beta-cells. Diabetes. 1988;11:1524–1530. doi: 10.2337/diab.37.11.1524. [DOI] [PubMed] [Google Scholar]
- 56.Jimenez-Chillaron J.C., et al. β-cell secretory dysfunction in the pathogenesis of low birth weight-associated diabetes. Diabetes. 2005;54:702–711. doi: 10.2337/diabetes.54.3.702. [DOI] [PubMed] [Google Scholar]
- 57.Schuit F., et al. Cellular origin of hexokinase in pancreatic islets. J. Biol. Chem. 1999;274:32803–32809. doi: 10.1074/jbc.274.46.32803. [DOI] [PubMed] [Google Scholar]
- 58.Tu J., Tuch B.E. Expression of glucokinase in glucose-unresponsive human fetal pancreatic islet-like cell clusters. J. Clin. Endocrinol. Metab. 1997;82 doi: 10.1210/jcem.82.3.3837. [DOI] [PubMed] [Google Scholar]
- 59.Brennan D.C., et al. Long-term follow-up of the Edmonton protocol of islet transplantation in the United States. Am. J. Transplant. 2015;16(2):509–517. doi: 10.1111/ajt.13458. [DOI] [PubMed] [Google Scholar]
- 60.Shapiro A.M.J., et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 2000;343(4):230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 61.Senior P.A., et al. Changes in renal function after clinical islet transplantation: four-year observational study. Am. J. Transplant. 2007;7:91–98. doi: 10.1111/j.1600-6143.2006.01573.x. [DOI] [PubMed] [Google Scholar]
- 62.Thompson D.M., et al. Reduced progression of diabetic microvascular complications with islet cell transplantation compared with intensive medical therapy. Transplantation. 2011;91(3):373–378. doi: 10.1097/TP.0b013e31820437f3. [DOI] [PubMed] [Google Scholar]
- 63.Fensom B., et al. Islet cell transplantation improves nerve conduction velocity in type 1 diabetes compared with intensive medical therapy over six years. Diabetes Res. Clin. Pract. 2016;122:101–105. doi: 10.1016/j.diabres.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 64.Vantyghem M.-C., et al. Improvement of electrophysiological neuropathy after islet transplantation for type 1 diabetes: a 5-year prospective study. Diabetes Care. 2014;37(6):e141–e142. doi: 10.2337/dc14-0320. [DOI] [PubMed] [Google Scholar]
- 65.Foster E.D., et al. Improved health-related quality of life in a phase 3 islet transplantation trial in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care. 2018;41(5):1001–1008. doi: 10.2337/dc17-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ricordi C., et al. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37(7):413–420. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 67.Bottino R., et al. The future of islet transplantation is now. Front. Med. 2018;5:202. doi: 10.3389/fmed.2018.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gray D.W., et al. Development of a novel digestion chamber for human and porcine islet isolation. Transplant. Proc. 2004;36(4):1135–1138. doi: 10.1016/j.transproceed.2004.04.050. [DOI] [PubMed] [Google Scholar]
- 69.Shapiro A.M., Pokrywczynska M., Ricordi C. Clinical pancreatic islet transplantation. Nat. Rev. Endocrinol. 2017;13(5):268–277. doi: 10.1038/nrendo.2016.178. [DOI] [PubMed] [Google Scholar]
- 70.Groth C.G., et al. Transplantation of porcine fetal pancreas to diabetic patients. Lancet. 1994;344:1402–1404. doi: 10.1016/s0140-6736(94)90570-3. [DOI] [PubMed] [Google Scholar]
- 71.Tector A.J., et al. The possible role of Anti-Neu5Gc as an obstacle in xenotransplantation. Front. Immunol. 2020;11:622. doi: 10.3389/fimmu.2020.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Burlak C., et al. Reduced binding of human antibodies to cells from GGTA1/CMAH KO pigs. Am. J. Transplant. 2014;14(8):1895–1900. doi: 10.1111/ajt.12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuwaki K., et al. Heart transplantation in baboons using α1,3-galactosyltransferase gene-knockout pigs as donors- initial experience. Nat. Med. 2004;11 doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 74.Tseng Y.L., et al. alpha1,3-Galactosyltransferase gene-knockout pig heart transplantation in baboons with survival approaching 6 months. Transplantation. 2005;80(10):1493–1500. doi: 10.1097/01.tp.0000181397.41143.fa. [DOI] [PubMed] [Google Scholar]
- 75.Shin J.S., et al. Long-term control of diabetes in immunosuppressed nonhuman primates (NHP) by the transplantation of adult porcine islets. Am. J. Transplant. 2015;15(11):2837–2850. doi: 10.1111/ajt.13345. [DOI] [PubMed] [Google Scholar]
- 76.Dolgin E. First GM pigs for allergies. Could xenotransplants be next? Nat. Biotechnol. 2021;39:397–400. doi: 10.1038/s41587-021-00885-9. [DOI] [PubMed] [Google Scholar]
- 77.Dai Y., et al. Targeted disruption of the α1,3-galactosyltransferase gene in cloned pigs. Nat. Biotechnol. 2002;20:251–255. doi: 10.1038/nbt0302-251. [DOI] [PubMed] [Google Scholar]
- 78.Servick K. Here’s how scientists pulled off the first pig-to-human heart transplant. Science. 2022 [Google Scholar]
- 79.Reardon S. First pig-to-human heart transplant: what can scientists learn? Nature. 2022;601:305–306. doi: 10.1038/d41586-022-00111-9. [DOI] [PubMed] [Google Scholar]
- 80.Carr R. 100% pure pigs: New Zealand and the cultivation of pure Auckland Island pigs for xenotransplantation. Anim. Stud. J. 2016;5(2):78–100. [Google Scholar]
- 81.Romito A., Cobellis G. Pluripotent stem cells: current understanding and future directions. Stem Cells Int. 2016;2016:9451492. doi: 10.1155/2016/9451492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Johnston P.V., et al. Not all stem cells are created equal. Circ. Res. 2018;123(8):944–946. doi: 10.1161/CIRCRESAHA.118.313425. [DOI] [PubMed] [Google Scholar]
- 83.Forbes S., Rosenthal N. Preparing the ground for tissue regeneration: from mechanism to therapy. Nat. Med. 2014;20:857–869. doi: 10.1038/nm.3653. [DOI] [PubMed] [Google Scholar]
- 84.Mayhew C.N., Wells J.M. Converting human pluripotent stem cells into beta-cells: recent advances and future challenges. Curr. Opin. Organ Transplant. 2010;15(1):54–60. doi: 10.1097/MOT.0b013e3283337e1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rezania A., et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 2014;32(11):1121–1133. doi: 10.1038/nbt.3033. [DOI] [PubMed] [Google Scholar]
- 86.Liu H., et al. Chemical combinations potentiate human pluripotent stem cell-derived 3D pancreatic progenitor clusters toward functional beta cells. Nat. Commun. 2021;12(1):3330. doi: 10.1038/s41467-021-23525-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chmielowiec J., Borowiak M. In vitro differentiation and expansion of human pluripotent stem cell-derived pancreatic progenitors. Rev. Diabet. Stud. 2014;11(1):19–34. doi: 10.1900/RDS.2014.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nair G.G., et al. Recapitulating endocrine cell clustering in culture promotes maturation of human stem-cell-derived beta cells. Nat. Cell Biol. 2019;21(2):263–274. doi: 10.1038/s41556-018-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Velazco-Cruz L., et al. Acquisition of dynamic function in human stem cell-derived beta cells. Stem Cell Rep. 2019;12(2):351–365. doi: 10.1016/j.stemcr.2018.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pagliuca F.W., et al. Generation of functional human pancreatic beta cells in vitro. Cell. 2014;159(2):428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Davis J.C., et al. Live cell monitoring and enrichment of stem cell-derived β cells using intracellular zinc content as a population marker. Curr. Protocols Stem Cell Biol. 2019;51 doi: 10.1002/cpsc.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Balboa D., et al. Functional, metabolic and transcriptional maturation of human pancreatic islets derived from stem cells. Nat. Biotechnol. 2022 doi: 10.1038/s41587-022-01219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chmielowiec J., et al. Human pancreatic microenvironment promotes beta-cell differentiation via non-canonical WNT5A/JNK and BMP signaling. Nat. Commun. 2022;13(1):1952. doi: 10.1038/s41467-022-29646-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takahashi K., et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 95.Nakagawa M., et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol. 2008;26(1):101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 96.Yu J., et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 97.Shahjalal H.M., et al. Generation of insulin-producing beta-like cells from human iPS cells in a defined and completely xeno-free culture system. J. Mol. Cell. Biol. 2014;6(5):394–408. doi: 10.1093/jmcb/mju029. [DOI] [PubMed] [Google Scholar]
- 98.Pellegrini S., et al. Differentiation of sendai virus-reprogrammed iPSC into beta cells, compared with human pancreatic islets and immortalized beta cell line. Cell Transplant. 2018;27(10):1548–1560. doi: 10.1177/0963689718798564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Toyoda T., et al. Cell aggregation optimizes the differentiation of human ESCs and iPSCs into pancreatic bud-like progenitor cells. Stem Cell Res. 2015;14(2):185–197. doi: 10.1016/j.scr.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 100.Yabe S.G., et al. Induction of functional islet-like cells from human iPS cells by suspension culture. Regen. Ther. 2019;10:69–76. doi: 10.1016/j.reth.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hogrebe N.J., et al. Targeting the cytoskeleton to direct pancreatic differentiation of human pluripotent stem cells. Nat. Biotechnol. 2020;38(4):460–470. doi: 10.1038/s41587-020-0430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Singh R., et al. Enhanced structure and function of human pluripotent stem cell-derived beta-cells cultured on extracellular matrix. Stem Cells Transl. Med. 2021;10(3):492–505. doi: 10.1002/sctm.20-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hogrebe N.J., et al. Generation of insulin-producing pancreatic beta cells from multiple human stem cell lines. Nat. Protoc. 2021;16(9):4109–4143. doi: 10.1038/s41596-021-00560-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Davis J.C., et al. Glucose response by stem cell-derived beta cells in vitro is inhibited by a bottleneck in glycolysis. Cell Rep. 2020;31(6) doi: 10.1016/j.celrep.2020.107623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kibbey R.G., et al. Mitochondrial GTP regulates glucose-stimulated insulin secretion. Cell Metab. 2007;5(4):253–264. doi: 10.1016/j.cmet.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tang C., Weissman I.L., Drukker M. Immunogenicity of in vitro maintained and matured populations: potential barriers to engraftment of human pluripotent stem cell derivatives. Methods Mol. Biol. 2013;1029:17–31. doi: 10.1007/978-1-62703-478-4_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pappas D.J., et al. Proceedings: human leukocyte antigen haplo-homozygous induced pluripotent stem cell haplobank modeled after the California population: evaluating matching in a multiethnic and admixed population. Stem Cells Transl. Med. 2015;4(5):413–418. doi: 10.5966/sctm.2015-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sullivan S., et al. Haplobanking induced pluripotent stem cells for clinical use. Stem Cell Res. 2020;49 doi: 10.1016/j.scr.2020.102035. [DOI] [PubMed] [Google Scholar]
- 109.Rossbach B., et al. Human iPSC-derived renal cells change their immunogenic properties during maturation: implications for regenerative therapies. Cells. 2022;11(8):1328. doi: 10.3390/cells11081328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Riolobos L., et al. HLA engineering of human pluripotent stem cells. Mol. Ther. 2013;21(6):1232–1241. doi: 10.1038/mt.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Norbnop P., et al. Generation and characterization of HLA-universal platelets derived from induced pluripotent stem cells. Sci. Rep. 2020;10(1):8472. doi: 10.1038/s41598-020-65577-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Prange S., et al. Transplanted MHC class I-deficient nonobese diabetic mouse islets are protected from autoimmune injury in diabetic nonobese recipients. Transplantation. 2001;71 doi: 10.1097/00007890-200104150-00025. [DOI] [PubMed] [Google Scholar]
- 113.Rajab A. Islet transplantation: alternative sites. Curr. Diab.Rep. 2010;10(5):332–337. doi: 10.1007/s11892-010-0130-6. [DOI] [PubMed] [Google Scholar]
- 114.Ng T.F., et al. Allogeneic neonatal neuronal retina grafts display partial immune privilege in the subcapsular space of the kidney. J. Immunol. 2002;169(10):5601. doi: 10.4049/jimmunol.169.10.5601. [DOI] [PubMed] [Google Scholar]
- 115.Mattapally S., et al. Human leukocyte antigen class I and II knockout human induced pluripotent stem cell-derived cells: universal donor for cell therapy. J. Am. Heart Assoc. 2018;7(23) doi: 10.1161/JAHA.118.010239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Han X., et al. Generation of hypoimmunogenic human pluripotent stem cells. Proc. Natl. Acad. Sci. USA. 2019;116(21):10441–10446. doi: 10.1073/pnas.1902566116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Parent A.V., et al. Selective deletion of human leukocyte antigens protects stem cell-derived islets from immune rejection. Cell Rep. 2021;36(7) doi: 10.1016/j.celrep.2021.109538. [DOI] [PubMed] [Google Scholar]
- 118.Yoshihara E., et al. Immune-evasive human islet-like organoids ameliorate diabetes. Nature. 2020 doi: 10.1038/s41586-020-2631-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yoshihara E., et al. ERRgamma is required for the metabolic maturation of therapeutically functional glucose-responsive beta cells. Cell Metab. 2016;23(4):622–634. doi: 10.1016/j.cmet.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Leite N.C., Pelayo G.C., Melton D.A. Genetic manipulation of stress pathways can protect stem-cell-derived islets from apoptosis in vitro. Stem Cell Rep. 2022;17(4):766–774. doi: 10.1016/j.stemcr.2022.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vinay D.S., et al. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015;35:S185–S198. doi: 10.1016/j.semcancer.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 122.Eissenberg L.G., et al. Suicide genes: monitoring cells in patients with a safety switch. Front. Pharmacol. 2014;5 doi: 10.3389/fphar.2014.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ramiya V.K., et al. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat. Med. 2000;6 doi: 10.1038/73128. [DOI] [PubMed] [Google Scholar]
- 124.Yamamoto T., et al. Stimulation of cAMP signalling allows isolation of clonal pancreatic precursor cells from adult mouse pancreas. Diabetologia. 2006;49(10):2359–2367. doi: 10.1007/s00125-006-0372-7. [DOI] [PubMed] [Google Scholar]
- 125.Noguchi H., et al. Establishment of mouse pancreatic stem cell line. Cell Transplant. 2009;18 doi: 10.1177/096368970901805-612. [DOI] [PubMed] [Google Scholar]
- 126.Bonner-Weir S., et al. Transdifferentiation of pancreatic ductal cells to endocrine β-cells. Biochem. Soc. Trans. 2008;36:353–356. doi: 10.1042/BST0360353. [DOI] [PubMed] [Google Scholar]
- 127.Noguchi H., et al. Characterization of human pancreatic progenitor cells. Cell Transplant. 2010;19(6):879–886. doi: 10.3727/096368910X509004. [DOI] [PubMed] [Google Scholar]
- 128.Bonner-Weir S., et al. In vitro cultivation of human islets from expanded ductal tissue. Proc. Natl. Acad. Sci. 2000;97 doi: 10.1073/pnas.97.14.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Seeberger K.L., et al. Expansion of mesenchymal stem cells from human pancreatic ductal epithelium. Lab. Invest. 2006;86(2):141–153. doi: 10.1038/labinvest.3700377. [DOI] [PubMed] [Google Scholar]
- 130.Qadir M.M.F., et al. P2RY1/ALK3-expressing cells within the adult human exocrine pancreas are BMP-7 expandable and exhibit progenitor-like characteristics. Cell Rep. 2018;22(9):2408–2420. doi: 10.1016/j.celrep.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Beyth S., et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105(5):2214–2219. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 132.Jung K.H., et al. Human bone marrow-derived clonal mesenchymal stem cells inhibit inflammation and reduce acute pancreatitis in rats. Gastroenterology. 2011;140(3):998–1008. doi: 10.1053/j.gastro.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 133.Kwon H.M., et al. Multiple paracrine factors secreted by mesenchymal stem cells contribute to angiogenesis. Vasc. Pharmacol. 2014;63(1):19–28. doi: 10.1016/j.vph.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 134.Hess D., et al. Bone marrow–derived stem cells initiate pancreatic regeneration. Nat. Biotechnol. 2003;21 doi: 10.1038/nbt841. [DOI] [PubMed] [Google Scholar]
- 135.Sipp D., Robey P.G., Turner L. Clear up this stem-cell mess. Springer Nature. 2018;561 doi: 10.1038/d41586-018-06756-9. [DOI] [PubMed] [Google Scholar]
- 136.Soliman H., et al. Multipotent stromal cells: one name, multiple identities. Cell Stem Cell. 2021;28:1690–1707. doi: 10.1016/j.stem.2021.09.001. [DOI] [PubMed] [Google Scholar]
- 137.Taneera J., et al. Failure of transplanted bone marrow cells to adopt a pancreatic beta-cell fate. Diabetes. 2006;55 doi: 10.2337/diabetes.55.02.06.db05-1212. [DOI] [PubMed] [Google Scholar]
- 138.Dave S.D., Vanikar A.V., Trivedi H.L. Extrinsic factors promoting in vitro differentiation of insulin-secreting cells from human adipose tissue-derived mesenchymal stem cells. Appl. Biochem. Biotechnol. 2013;170(4):962–971. doi: 10.1007/s12010-013-0250-y. [DOI] [PubMed] [Google Scholar]
- 139.Lechner A., et al. No evidence for significant transdifferentiation of bone marrow into pancreatic β-cells in vivo. Diabetes. 2004;53:616–623. doi: 10.2337/diabetes.53.3.616. [DOI] [PubMed] [Google Scholar]
- 140.Hayward J.A., et al. Cotransplantation of mesenchymal stem cells with neonatal porcine islets improve graft function in diabetic mice. Diabetes. 2017;66(5):1312–1321. doi: 10.2337/db16-1068. [DOI] [PubMed] [Google Scholar]
- 141.Wang H., et al. Autologous mesenchymal stem cell and islet cotransplantation: safety and efficacy. Stem Cells Transl. Med. 2018;7(1):11–19. doi: 10.1002/sctm.17-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kogawa R., Nakamura K., Mochizuki Y. A new islet transplantation method combining mesenchymal stem cells with recombinant peptide pieces, microencapsulated islets, and mesh bags. Biomedicines. 2020;8(9) doi: 10.3390/biomedicines8090299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gamble A., et al. Improved islet recovery and efficacy through co-culture and co-transplantation of islets with human adipose-derived mesenchymal stem cells. PLoS ONE. 2018;13(11) doi: 10.1371/journal.pone.0206449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Montanari E., et al. Multipotent mesenchymal stromal cells enhance insulin secretion from human islets via N-cadherin interaction and prolong function of transplanted encapsulated islets in mice. Stem Cell Res. Ther. 2017;8(1):199. doi: 10.1186/s13287-017-0646-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ishida N., et al. Cotransplantation of preactivated mesenchymal stem cells improves intraportal engraftment of islets by inhibiting liver natural killer cells in mice. Am. J. Transplant. 2019;19(10):2732–2745. doi: 10.1111/ajt.15347. [DOI] [PubMed] [Google Scholar]
- 146.Vaithilingam V., et al. Co-encapsulation and co-transplantation of mesenchymal stem cells reduces pericapsular fibrosis and improves encapsulated islet survival and function when allografted. Sci. Rep. 2017;7(1):10059. doi: 10.1038/s41598-017-10359-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Villard O., et al. Characterization of immortalized human islet stromal cells reveals a MSC-like profile with pancreatic features. Stem Cell Res. Ther. 2020;11(1):158. doi: 10.1186/s13287-020-01649-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.X. Wang et al., Engineered immunomodulatory accessory cells improve experimental allogeneic islet transplantation without immunosuppression, Sci. Adv. 8(29) (2022) eabn0071. [DOI] [PMC free article] [PubMed]
- 149.Ilancheran S., et al. Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol. Reprod. 2007;77(3):577–588. doi: 10.1095/biolreprod.106.055244. [DOI] [PubMed] [Google Scholar]
- 150.Cargnoni A., et al. Effect of human amniotic epithelial cells on pro-fibrogenic resident hepatic cells in a rat model of liver fibrosis. J. Cell Mol. Med. 2018;22(2):1202–1213. doi: 10.1111/jcmm.13396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Miki T. Stem cell characteristics and the therapeutic potential of amniotic epithelial cells. Am. J. Reprod. Immunol. 2018;80(4) doi: 10.1111/aji.13003. [DOI] [PubMed] [Google Scholar]
- 152.Miki T. Amnion-derived stem cells- in quest of clinical applications. Stem Cells Res. Ther. 2011;2 doi: 10.1186/scrt66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Marongiu F., et al. Hepatic differentiation of amniotic epithelial cells. Hepatology. 2011;53(5):1719–1729. doi: 10.1002/hep.24255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Garcia-Castro I.L., et al. Markers of pluripotency in human amniotic epithelial cells and their differentiation to progenitor of cortical neurons. PLoS ONE. 2015;10(12) doi: 10.1371/journal.pone.0146082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhou J., et al. Bone morphogenetic protein-7 promotes chondrogenesis in human amniotic epithelial cells. Int. Orthop. 2011;35(6):941–948. doi: 10.1007/s00264-010-1116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Okere B., et al. In vitro differentiation of human amniotic epithelial cells into insulin-producing 3D spheroids. Int. J. Immunopathol. Pharmacol. 2015;28(3):390–402. doi: 10.1177/0394632015588439. [DOI] [PubMed] [Google Scholar]
- 157.Wei J.P., et al. Human amnion-isolated cells normalize blood glucose in streptozotocin-induced diabetic mice. Cell Transplant. 2003;12:545–552. doi: 10.3727/000000003108747000. [DOI] [PubMed] [Google Scholar]
- 158.Qureshi K.M., et al. Human amniotic epithelial cells induce localized cell-mediated immune privilege in vitro: implications for pancreatic islet transplantation. Cell Transplant. 2011;20(4):523–534. doi: 10.3727/096368910X528111. [DOI] [PubMed] [Google Scholar]
- 159.Lebreton F., et al. Insulin-producing organoids engineered from islet and amniotic epithelial cells to treat diabetes. Nat. Commun. 2019;10(1):4491. doi: 10.1038/s41467-019-12472-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lavallard V., et al. Human amniotic epithelial cells integrated into the islet heterospheroids enhance insulin secretion and protect islet cells from hypoxic injury. Transplantation. 2018 [Google Scholar]
- 161.Zhou Q., et al. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455(7213):627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Akinci E., et al. Reprogramming of pancreatic exocrine cells towards a beta (beta) cell character using Pdx1, Ngn3 and MafA. Biochem. J. 2012;442(3):539–550. doi: 10.1042/BJ20111678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Banga A., et al. In vivo reprogramming of Sox9+ cells in the liver to insulin-secreting ducts. Proc. Natl. Acad. Sci. USA. 2012;109(38):15336–15341. doi: 10.1073/pnas.1201701109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Akinci E., et al. Reprogramming of various cell types to a beta-like state by Pdx1, Ngn3 and MafA. PLoS ONE. 2013;8(11) doi: 10.1371/journal.pone.0082424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Saxena P., et al. A programmable synthetic lineage-control network that differentiates human IPSCs into glucose-sensitive insulin-secreting beta-like cells. Nat. Commun. 2016;7:11247. doi: 10.1038/ncomms11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Johansson K.A., et al. Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev. Cell. 2007;12(3):457–465. doi: 10.1016/j.devcel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 167.Schwitzgebel V.M., et al. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 2000;127:3533–3542. doi: 10.1242/dev.127.16.3533. [DOI] [PubMed] [Google Scholar]
- 168.Nishimura W., Bonner-Weir S., Sharma A. Expression of MafA in pancreatic progenitors is detrimental for pancreatic development. Dev. Biol. 2009;333(1):108–120. doi: 10.1016/j.ydbio.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhu Y., et al. PDX1, Neurogenin-3, and MAFA: critical transcription regulators for beta cell development and regeneration. Stem Cell Res. Ther. 2017;8(1):240. doi: 10.1186/s13287-017-0694-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mahaddalkar P.U., et al. Generation of pancreatic β cells from CD177+ anterior definitive endoderm. Nat. Biotechnol. 2020;38:1061–1072. doi: 10.1038/s41587-020-0492-5. [DOI] [PubMed] [Google Scholar]
- 171.Aghazadeh Y., et al. GP2-enriched pancreatic progenitors give rise to functional beta cells in vivo and eliminate the risk of teratoma formation. Stem Cell Rep. 2022;17(4):964–978. doi: 10.1016/j.stemcr.2022.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Veres A., et al. Charting cellular identity during human in vitro beta-cell differentiation. Nature. 2019;569(7756):368–373. doi: 10.1038/s41586-019-1168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Docherty F.M., et al. ENTPD3 marks mature stem cell-derived β-cells formed by self-aggregation in vitro. Diabetes. 2021;70(11):2554–2567. doi: 10.2337/db20-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Parent A.V., et al. Development of a scalable method to isolate subsets of stem cell-derived pancreatic islet cells. Stem Cell Rep. 2022;17(4):979–992. doi: 10.1016/j.stemcr.2022.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ren X., et al. Growth factor engineering strategies for regenerative medicine applications. Front. Bioeng. Biotechnol. 2020;7 doi: 10.3389/fbioe.2019.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Niu Y., et al. Engineered delivery strategies for enhanced control of growth factor activities in wound healing. Adv. Drug Deliv. Rev. 2019;146:190–208. doi: 10.1016/j.addr.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 177.Linn T., et al. Angiogenic capacity of endothelial cells in islets of Langerhans. FASEB. 2003;17 doi: 10.1096/fj.02-0615fje. [DOI] [PubMed] [Google Scholar]
- 178.Song H.J., et al. Prolongation of islet graft survival using concomitant transplantation of islets and vascular endothelial cells in diabetic rats. Transplant. Proc. 2010;42(7):2662–2665. doi: 10.1016/j.transproceed.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 179.Pan X., et al. Islet graft survival and function: concomitant culture and transplantation with vascular endothelial cells in diabetic rats. Transplantation. 2011;92:1208–1214. doi: 10.1097/TP.0b013e3182356ca7. [DOI] [PubMed] [Google Scholar]
- 180.Johansson U., et al. Composite islet-endothelial cell grafts: a novel approach to counteract innate immunity in islet transplantation. Am. J. Transplant. 2005 doi: 10.1111/j.1600-6143.2005.01076.x. [DOI] [PubMed] [Google Scholar]
- 181.Jung H.S., et al. The potential of endothelial colony-forming cells to improve early graft loss after intraportal islet transplantation. Cell Transplant. 2014;23(3):273–283. doi: 10.3727/096368912X661364. [DOI] [PubMed] [Google Scholar]
- 182.Zbinden A., et al. Collagen and endothelial cell coculture improves beta-cell functionality and rescues pancreatic extracellular matrix. Tissue Eng. Part A. 2020 doi: 10.1089/ten.TEA.2020.0250. [DOI] [PubMed] [Google Scholar]
- 183.Urbanczyk M., et al. Controlled heterotypic pseudo-islet assembly of human beta-cells and human umbilical vein endothelial cells using magnetic levitation. Tissue Eng. Part A. 2020;26(7–8):387–399. doi: 10.1089/ten.tea.2019.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kang S., et al. Endothelial progenitor cell cotransplantation enhances islet engraftment by rapid revascularization. Diabetes. 2012;61:866–876. doi: 10.2337/db10-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Oh B.J., et al. Co-Transplantation of bone marrow-derived endothelial progenitor cells improves revascularization and organization in islet grafts. Am. J. Transplant. 2013;13 doi: 10.1111/ajt.12222. [DOI] [PubMed] [Google Scholar]
- 186.Penko D., et al. Endothelial progenitor cells enhance islet engraftment, influence beta-cell function, and modulate islet connexin 36 expression. Cell Transplant. 2015;24(1):37–48. doi: 10.3727/096368913X673423. [DOI] [PubMed] [Google Scholar]
- 187.Barba-Gutierrez D.A., et al. Facilitated engraftment of isolated islets coated with expanded vascular endothelial cells for islet transplantation. Transplant. Proc. 2016;48(2):669–672. doi: 10.1016/j.transproceed.2016.02.036. [DOI] [PubMed] [Google Scholar]
- 188.Wong T., McGrath J.A., Navsaria H. The role of fibroblasts in tissue engineering and regeneration. Br. J. Dermatol. 2007;156:1149–1155. doi: 10.1111/j.1365-2133.2007.07914.x. [DOI] [PubMed] [Google Scholar]
- 189.Jalili R.B., et al. Fibroblast populated collagen matrix promotes islet survival and reduces the number of islets required for diabetes reversal. J. Cell. Physiol. 2011;226:1813–1819. doi: 10.1002/jcp.22515. [DOI] [PubMed] [Google Scholar]
- 190.Haniffa M.A., et al. Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J. Immunol. 2007;179(3):1595–1604. doi: 10.4049/jimmunol.179.3.1595. [DOI] [PubMed] [Google Scholar]
- 191.Perez-Basterrechea M., et al. Fibroblasts accelerate islet revascularization and improve long-term graft survival in a mouse model of subcutaneous islet transplantation. PLoS ONE. 2017 doi: 10.1371/journal.pone.0180695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Perez-Basterrechea M., et al. Cooperation by fibroblasts and bone marrow-mesenchymal stem cells to improve pancreatic rat-to-mouse islet xenotransplantation. PLoS ONE. 2013;8(8) doi: 10.1371/journal.pone.0073526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Shevach E.M. Foxp3(+) T regulatory cells: still many unanswered questions-a perspective after 20 years of study. Front. Immunol. 2018;9:1048. doi: 10.3389/fimmu.2018.01048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Krzystyniak A., et al. Islet cell transplant and the incorporation of Tregs. Curr Opin Organ Transplant. 2014;19(6):610–615. doi: 10.1097/MOT.0000000000000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lu J., et al. Unique features of pancreatic-resident regulatory T cells in autoimmune type 1 diabetes. Front. Immunol. 2017;8:1235. doi: 10.3389/fimmu.2017.01235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chhabra P., Brayman K.L. Current status of immunomodulatory and cellular therapies in preclinical and clinical islet transplantation. J Transplant. 2011;2011 doi: 10.1155/2011/637692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ikemoto T., et al. Donor-specific tolerance induced by simultaneous allogeneic islet transplantation with CD4+CD25+ T-cells into hepatic parenchyma in mice. J. Med. Invest. 2004;51:178–185. doi: 10.2152/jmi.51.178. [DOI] [PubMed] [Google Scholar]
- 198.N. Marek et al., Coating human pancreatic islets with CD4(+)CD25(high)CD127(-) regulatory T cells as a novel approach for the local immunoprotection, Ann. Surg. 254(3) (2011) 512–518; discussion 518–519. [DOI] [PubMed]
- 199.Gagliani N., et al. Transplant tolerance to pancreatic islets is initiated in the graft and sustained in the spleen. Am. J. Transplant. 2013;13 doi: 10.1111/ajt.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Graham J.G., et al. PLG scaffold delivered antigen-specific regulatory T cells induce systemic tolerance in autoimmune diabetes. Tissue Eng. Part A. 2013;19(11–12):1465–1475. doi: 10.1089/ten.tea.2012.0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hull C.M., et al. Generation of human islet-specific regulatory T cells by TCR gene transfer. J. Autoimmun. 2017;79:63–73. doi: 10.1016/j.jaut.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 202.Bennet W., et al. Incompatibility between human blood and isolated islets of Langerhans: a finding with implications for clinical intraportal islet transplantation? Diabetes. 1999;48(10):1907–1914. doi: 10.2337/diabetes.48.10.1907. [DOI] [PubMed] [Google Scholar]
- 203.Kastellorizios M., Tipnis N., Burgess D.J. Springer International Publishing; Cham: 2015. Foreign Body Reaction to Subcutaneous Implants. [DOI] [PubMed] [Google Scholar]
- 204.Lee C.A., et al. Instant blood-mediated inflammatory reaction in hepatocyte transplantation: current status and future perspectives. Cell Transplant. 2016;25(7):1227–1236. doi: 10.3727/096368916X691286. [DOI] [PubMed] [Google Scholar]
- 205.X. Li, Q. Meng, L. Zhang, The fate of allogeneic pancreatic islets following intraportal transplantation: challenges and solutions, J. Immunol. Res. 2018 (2018) 2424586. [DOI] [PMC free article] [PubMed]
- 206.Barshes N.R., Wyllie S., Goss J.A. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: implications for intrahepatic grafts. J. Leukoc. Biol. 2005;77(5):587–597. doi: 10.1189/jlb.1104649. [DOI] [PubMed] [Google Scholar]
- 207.Vivot K., et al. Instant blood-mediated inflammatory reaction during islet transplantation: the role of Toll-like receptors signaling pathways. Transplant. Proc. 2011;43(9):3192–3194. doi: 10.1016/j.transproceed.2011.09.056. [DOI] [PubMed] [Google Scholar]
- 208.Naziruddin B., et al. Evidence for instant blood-mediated inflammatory reaction in clinical autologous islet transplantation. Am. J. Transplant. 2014;14(2):428–437. doi: 10.1111/ajt.12558. [DOI] [PubMed] [Google Scholar]
- 209.Ryan E.A., et al. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes. 2001;50(4):710–719. doi: 10.2337/diabetes.50.4.710. [DOI] [PubMed] [Google Scholar]
- 210.Ryan E.A., et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54(7):2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 211.Brennan D.C., et al. Long-term follow-up of the Edmonton protocol of islet transplantation in the United States. Am. J. Transplant. 2016;16(2):509–517. doi: 10.1111/ajt.13458. [DOI] [PubMed] [Google Scholar]
- 212.J.S. Roh, D.H. Sohn, Damage-associated molecular patterns in inflammatory diseases. Immune Network 18(4) (2018) e27. [DOI] [PMC free article] [PubMed]
- 213.Moyano D.F., et al. Nanoparticle hydrophobicity dictates immune response. J. Am. Chem. Soc. 2012;134(9):3965–3967. doi: 10.1021/ja2108905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Mariani E., et al. Biomaterials: foreign bodies or tuners for the immune response? Int. J. Mol. Sci. 2019;20(3):636. doi: 10.3390/ijms20030636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zaveri T.D., et al. Integrin-directed modulation of macrophage responses to biomaterials. Biomaterials. 2014;35(11):3504–3515. doi: 10.1016/j.biomaterials.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sheikh Z., et al. Macrophages, foreign body giant cells and their response to implantable biomaterials. Materials (Basel, Switzerland) 2015;8(9):5671–5701. doi: 10.3390/ma8095269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bartoli C.R., Nadar M.M., Godleski J.J. Capsule thickness correlates with vascular density and blood flow within foreign-body capsules surrounding surgically implanted subcutaneous devices. Artif. Organs. 2010;34(10):857–861. doi: 10.1111/j.1525-1594.2010.01024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Baidal D.A., et al. Bioengineering of an intraabdominal endocrine pancreas. N. Engl. J. Med. 2017;376(19):1887–1889. doi: 10.1056/NEJMc1613959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Pattou F., Kerr-Conte J., Wild D. GLP-1-receptor scanning for imaging of human beta cells transplanted in muscle. N. Engl. J. Med. 2010;363(13) doi: 10.1056/NEJMc1004547. [DOI] [PubMed] [Google Scholar]
- 220.Pepper A.R., et al. A prevascularized subcutaneous device-less site for islet and cellular transplantation. Nat. Biotechnol. 2015;33(5):518–523. doi: 10.1038/nbt.3211. [DOI] [PubMed] [Google Scholar]
- 221.Sun A.M., et al. The use, in diabetic rats and monkeys, of artificial capillary units containing cultured islets of Langerhans (artificial endocrine pancreas) Diabetes. 1977;26(12):1136–1139. doi: 10.2337/diab.26.12.1136. [DOI] [PubMed] [Google Scholar]
- 222.Song S., Roy S. Progress and challenges in macroencapsulation approaches for type 1 diabetes (T1D) treatment: cells, biomaterials, and devices. Biotechnol. Bioeng. 2016;113(7):1381–1402. doi: 10.1002/bit.25895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Brissova M., et al. Intraislet endothelial cells contribute to revascularization of transplanted pancreatic islets. Diabetes. 2004;53(5):1318–1325. doi: 10.2337/diabetes.53.5.1318. [DOI] [PubMed] [Google Scholar]
- 224.E.B. Dolan et al., An actuatable soft reservoir modulates host foreign body response, Sci. Robot 28(4) (2019) eaax7043. [DOI] [PubMed]
- 225.Duffy G.P., et al. Implantable therapeutic reservoir systems for diverse clinical applications in large animal models. Adv. Healthc Mater. 2020;11 doi: 10.1002/adhm.202000305. [DOI] [PubMed] [Google Scholar]
- 226.Ludwig B., et al. Favorable outcome of experimental islet xenotransplantation without immunosuppression in a nonhuman primate model of diabetes. PNAS. 2017;114(44):11745–11750. doi: 10.1073/pnas.1708420114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Bornstein S.R., Ludwig B., Steenblock C. Progress in islet transplantation is more important than ever. Nat. Rev. Endocrinol. 2022 doi: 10.1038/s41574-022-00689-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Carlsson P.-O., et al. Transplantation of macroencapsulated human islets within the bioartificial pancreas betaAir to patients with type 1 diabetes mellitus. Am. J. Transplant. 2018;18(7):1735–1744. doi: 10.1111/ajt.14642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Evron Y., et al. Long-term viability and function of transplanted islets macroencapsulated at high density are achieved by enhanced oxygen supply. Sci. Rep. 2018;8(1):6508. doi: 10.1038/s41598-018-23862-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Desai T., Shea L.D. Advances in islet encapsulation technologies. Nat. Rev. Drug Discovery. 2016;16:338–350. doi: 10.1038/nrd.2016.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Goswami D., et al. Design considerations for macroencapsulation devices for stem cell derived islets for the treatment of type 1 diabetes. Adv. Sci. 2021;8(16) doi: 10.1002/advs.202100820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Umer H., et al. Microencapsulation: process, techniques and applications. Int. J. Res. Pharm. Biomed. Sci. 2011;2(2):474–481. [Google Scholar]
- 233.Schweicher J., Nyitray C., Desai T.A. Membranes to achieve immunoprotection of transplanted islets. Front. Biosci. 2014;19:49–76. doi: 10.2741/4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Orive G., et al. Engineering a clinically translatable bioartificial pancreas to treat type I diabetes. Trends Biotechnol. 2018;36:445–456. doi: 10.1016/j.tibtech.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 235.Lim F., Sun A.M. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210(4472):908–910. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 236.O’Shea G.M., Goosen M.F., Sun A.M. Prolonged survival of transplanted islets of Langerhans encapsulated in a biocompatible membrane. Biochim. Biophys. Acta, Mol. Cell. Biol. Lipids. 1984;804:133–136. doi: 10.1016/0167-4889(84)90107-1. [DOI] [PubMed] [Google Scholar]
- 237.Ponce S., et al. Chemistry and the biological response against immunoisolating alginate-polycation capsules of different composition. Biomaterials. 2006;27(28):4831–4839. doi: 10.1016/j.biomaterials.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 238.Bansal A., D’Sa S., D’Souza M.J. Biofabrication of microcapsules encapsulating beta-TC-6 cells via scalable device and in-vivo evaluation in type 1 diabetic mice. Int. J. Pharm. 2019;572 doi: 10.1016/j.ijpharm.2019.118830. [DOI] [PubMed] [Google Scholar]
- 239.Alagpulinsa D.A., et al. Alginate-microencapsulation of human stem cell-derived beta cells with CXCL12 prolongs their survival and function in immunocompetent mice without systemic immunosuppression. Am. J. Transplant. 2019;19(7):1930–1940. doi: 10.1111/ajt.15308. [DOI] [PubMed] [Google Scholar]
- 240.King A. Microencapsulation of islets of Langerhans: impact of cellular overgrowth. Ups. J. Med. Sci. 2001;106(3):161–174. doi: 10.3109/2000-1967-140. [DOI] [PubMed] [Google Scholar]
- 241.Chen Y., et al. Microencapsulated islet-like microtissues with toroid geometry for enhanced cellular viability. Acta Biomater. 2019;97 doi: 10.1016/j.actbio.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 242.Buchwald P. A local glucose-and oxygen concentration-based insulin secretion model for pancreatic islets. Theor. Biol. Med. Model. 2011;8:20. doi: 10.1186/1742-4682-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Syed F., et al. Conformal coating by multilayer nano-encapsulation for the protection of human pancreatic islets: in-vitro and in-vivo studies. Nanomedicine. 2018;14(7):2191–2203. doi: 10.1016/j.nano.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 244.Mozafari M.R., et al. Recent trends in the lipid based nanoencapsulation of antioxidants and their role in food. J. Sci. Food Agric. 2006;86(13):2038–2045. [Google Scholar]
- 245.Soppimath K.S., et al. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control Rel. 2001;70:1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 246.Hoesli C.A., et al. Pancreatic cell immobilization in alginate beads produced by emulsion and internal gelation. Biotechnol. Bioeng. 2011;108(2):424–434. doi: 10.1002/bit.22959. [DOI] [PubMed] [Google Scholar]
- 247.K.E. Markwick et al., Microchannel emulsification: a novel approach to cell encapsulation, in: XXIV International Conference on Bioencapsulation, 2016.
- 248.Suganya V., Anuradha V. Microencapsulation and nanoencapsulation: a review. Int. J. Pharm. Clin. Res. 2017;9(3):233–239. [Google Scholar]
- 249.Ernst A.U., et al. Nanotechnology in cell replacement therapies for type 1 diabetes. Adv. Drug Deliv. Rev. 2019;139:116–138. doi: 10.1016/j.addr.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Wu S., et al. Advances in encapsulation and delivery strategies for islet transplantation. Adv. Healthc Mater. 2021 doi: 10.1002/adhm.202100965. [DOI] [PubMed] [Google Scholar]
- 251.Barkai U., Rotem A., de Vos P. Survival of encapsulated islets: more than a membrane story. World J. Transpl. 2016;6(1):69–90. doi: 10.5500/wjt.v6.i1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Masaeli R., Zandsalimi K., Tayebi L. Biomaterials evaluation: conceptual refinements and practical reforms. Ther. Innov. Regul. Sci. 2019;53(1):120–127. doi: 10.1177/2168479018774320. [DOI] [PubMed] [Google Scholar]
- 253.C.A. Homsy, Bio-compatibility in selection of materials for implantation, J. Biomed. Mater. Res. 4(3) (1970) 341–356. [DOI] [PubMed]
- 254.R.S. Tirumalai, 87. Biological Reactivity Tests, in vitro, U. S. Pharmacopeia [cited 2022 January 13]; USP29-NF24 Page 2525], Available from: <http://www.pharmacopeia.cn/v29240/usp29nf24s0_c87.html>.
- 255.Lee K.Y., Mooney D.J. Alginate: properties and biomedical applications. Prog. Polym. Sci. 2012;37(1):106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.M. Shimoda, S. Matsumoto, Microencapsulation in clinical islet xenotransplantation, in: E.C. Opara (Ed.), Cell Microencapsulation: Methods and Protocols, Springer New York, New York, NY, 2017, pp. 335–345. [DOI] [PubMed]
- 257.Bhujbal S.V., et al. Factors influencing the mechanical stability of alginate beads applicable for immunoisolation of mammalian cells. J. Mech. Behav. Biomed. Mater. 2014;37:196–208. doi: 10.1016/j.jmbbm.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 258.Vériter S., et al. In vivo selection of biocompatible alginates for islet encapsulation and subcutaneous transplantation. Tissue Eng. Part A. 2009;16(5):1503–1513. doi: 10.1089/ten.TEA.2009.0286. [DOI] [PubMed] [Google Scholar]
- 259.Watanabe T., et al. Millimeter-thick xenoislet-laden fibers as retrievable transplants mitigate foreign body reactions for long-term glycemic control in diabetic mice. Biomaterials. 2020;255 doi: 10.1016/j.biomaterials.2020.120162. [DOI] [PubMed] [Google Scholar]
- 260.Veiseh O., et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat. Mater. 2015;14(6):643–651. doi: 10.1038/nmat4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Lebre F., et al. The shape and size of hydroxyapatite particles dictate inflammatory responses following implantation. Sci. Rep. 2017;7:2922. doi: 10.1038/s41598-017-03086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Acarregui A., et al. Multifunctional hydrogel-based scaffold for improving the functionality of encapsulated therapeutic cells and reducing inflammatory response. Acta Biomater. 2014;10(10):4206–4216. doi: 10.1016/j.actbio.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 263.Vegas A.J., et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat. Med. 2016;22(3):306–311. doi: 10.1038/nm.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Laporte C., et al. Improved human islets’ viability and functionality with mesenchymal stem cells and arg-gly-asp tripeptides supplementation of alginate micro-encapsulated islets in vitro. Biochem. Biophys. Res. Commun. 2020;528 doi: 10.1016/j.bbrc.2020.05.107. [DOI] [PubMed] [Google Scholar]
- 265.Bochenek M.A., et al. Alginate encapsulation as long-term immune protection of allogeneic pancreatic islet cells transplanted into the omental bursa of macaques. Nat. Biomed. Eng. 2018;2(11):810–821. doi: 10.1038/s41551-018-0275-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Lim F., Sun A.M. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210 doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 267.Liu Q., et al. Zwitterionically modified alginates mitigate cellular overgrowth for cell encapsulation. Nat. Commun. 2019;10(1):5262. doi: 10.1038/s41467-019-13238-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Bakshi P.S., et al. Chitosan as an environment friendly biomaterial – a review on recent modifications and applications. Int. J. Biol. Macromol. 2020;150:1072–1083. doi: 10.1016/j.ijbiomac.2019.10.113. [DOI] [PubMed] [Google Scholar]
- 269.Yang K.-C., et al. The cytoprotection of chitosan based hydrogels in xenogeneic islet transplantation: an in vivo study in streptozotocin-induced diabetic mouse. Biochem. Biophys. Res. Commun. 2010;393(4):818–823. doi: 10.1016/j.bbrc.2010.02.089. [DOI] [PubMed] [Google Scholar]
- 270.Yang H.K., et al. Long-term efficacy and biocompatibility of encapsulated islet transplantation with chitosan-coated alginate capsules in mice and canine models of diabetes. Transplantation. 2016;100(2):334–343. doi: 10.1097/TP.0000000000000927. [DOI] [PubMed] [Google Scholar]
- 271.N. Najafikhah et al., Normal insulin secretion from immune-protected islets of langerhans by PEGylation and encapsulation in the alginate-chitosan-PEG, Iran. J. Biotechnol. 16(4) (2018) e1669. [DOI] [PMC free article] [PubMed]
- 272.Kim M., et al. Novel enzymatic cross-linking–based hydrogel nanofilm caging system on pancreatic beta cell spheroid for long-term blood glucose regulation. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abf7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Perikamana S.K.M., et al. Molecularly tailored interface for long-term xenogeneic cell transplantation. Adv. Funct. Mater. 2021 doi: 10.1002/adfm.202108221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Gin H., et al. Agarose encapsulation of islets of Langerhans: reduced toxicity in vitro. J. Microencap. 1987;4(3):239–242. doi: 10.3109/02652048709021817. [DOI] [PubMed] [Google Scholar]
- 275.Iwata H., et al. Evaluation of microencapsulated islets in agarose gel as bioartificial pancreas by studies of hormone secretion in culture and by xenotransplantation. Diabetes. 1989;38 doi: 10.2337/diab.38.1.s224. [DOI] [PubMed] [Google Scholar]
- 276.Jain K., et al. Retrievable, replaceable, macroencapsulated pancreatic islet xenografts. Long-term engraftment without immunosuppression. Transplantation. 1995;59(3):319–324. [PubMed] [Google Scholar]
- 277.Kuwabara R., et al. Long-term functioning of allogeneic islets in subcutaneous tissue pretreated with a novel cyclic peptide without immunosuppressive medication. Transplantation. 2018;102(3):417–425. doi: 10.1097/TP.0000000000001923. [DOI] [PubMed] [Google Scholar]
- 278.Kumar N., Joisher H., Ganguly A. Polymeric scaffolds for pancreatic tissue engineering: a review. Rev. Diabetic Stud.: RDS. 2018;14(4):334–353. doi: 10.1900/RDS.2017.14.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Ozmen L., et al. Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: possible application of the thrombin inhibitor melagatran in clinical islet transplantation. Diabetes. 2002;51(6):1779–1784. doi: 10.2337/diabetes.51.6.1779. [DOI] [PubMed] [Google Scholar]
- 280.Riopel M., Trinder M., Wang R. Fibrin, a scaffold material for islet transplantation and pancreatic endocrine tissue engineering. Tissue Eng. Part B Rev. 2015;21(1):34–44. doi: 10.1089/ten.TEB.2014.0188. [DOI] [PubMed] [Google Scholar]
- 281.Ahmed T.A.E., Dare E.V., Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng. Part B: Rev. 2008;14(2):199–215. doi: 10.1089/ten.teb.2007.0435. [DOI] [PubMed] [Google Scholar]
- 282.Haugh M.G., et al. The application of plastic compression to modulate fibrin hydrogel mechanical properties. J. Mech. Behav. Biomed. Mater. 2012;16:66–72. doi: 10.1016/j.jmbbm.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 283.Helms C.C., et al. α-α Cross-links increase fibrin fiber elasticity and stiffness. Biophys. J. 2012;102(1):168–175. doi: 10.1016/j.bpj.2011.11.4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Beattie G.M., et al. A novel approach to increase human islet cell mass while preserving β-cell function. Diabetes. 2002;51(12):3435–3439. doi: 10.2337/diabetes.51.12.3435. [DOI] [PubMed] [Google Scholar]
- 285.Kim S.J., et al. A fibrin gel carrier system for islet transplantation into kidney subcapsule. Acta Diabetol. 2009;46(3):243–248. doi: 10.1007/s00592-008-0073-4. [DOI] [PubMed] [Google Scholar]
- 286.Riopel M., Stuart W., Wang R. Fibrin improves beta (INS-1) cell function, proliferation and survival through integrin αvβ3. Acta Biomater. 2013;9(9):8140–8148. doi: 10.1016/j.actbio.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 287.Kuehn C., et al. Young porcine endocrine pancreatic islets cultured in fibrin and alginate gels show improved resistance towards human monocytes. Pathol. Biol. (Paris) 2014;62(6):354–364. doi: 10.1016/j.patbio.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 288.Salama B.F., Seeberger K.L., Korbutt G.S. Fibrin supports subcutaneous neonatal porcine islet transplantation without the need for pre-vascularization. Xenotransplantation. 2020;4 doi: 10.1111/xen.12575. [DOI] [PubMed] [Google Scholar]
- 289.I.S. Bayer, Hyaluronic acid and controlled release: a review, Molecules 25(11) (2020). [DOI] [PMC free article] [PubMed]
- 290.Kogan G., et al. Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2007;29(1):17–25. doi: 10.1007/s10529-006-9219-z. [DOI] [PubMed] [Google Scholar]
- 291.Birajdar M.S., et al. Natural bio-based monomers for biomedical applications: a review. Biomater. Res. 2021;25(1):8. doi: 10.1186/s40824-021-00208-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Huang G., Huang H. Application of hyaluronic acid as carriers in drug delivery. Drug Deliv. 2018;25(1):766–772. doi: 10.1080/10717544.2018.1450910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Krolikoski M., Monslow J., Puré E. The CD44-HA axis and inflammation in atherosclerosis: a temporal perspective. Matrix Biol. 2019;78–79:201–218. doi: 10.1016/j.matbio.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Cui Z., et al. The Receptor for Hyaluronan-Mediated Motility (CD168) promotes inflammation and fibrosis after acute lung injury. Matrix Biol. 2019;78–79:255–271. doi: 10.1016/j.matbio.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Mele V., et al. The hyaluronan-mediated motility receptor RHAMM promotes growth, invasiveness and dissemination of colorectal cancer. Oncotarget. 2017;8(41):70617–70629. doi: 10.18632/oncotarget.19904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Dovedytis M., Liu Z.J., Bartlett S. Hyaluronic acid and its biomedical applications: a review. Eng. Regener. 2020;1:102–113. [Google Scholar]
- 297.Moseley R., et al. Comparison of the antioxidant properties of wound dressing materials–carboxymethylcellulose, hyaluronan benzyl ester and hyaluronan, towards polymorphonuclear leukocyte-derived reactive oxygen species. Biomaterials. 2003;24:1549–1557. doi: 10.1016/s0142-9612(02)00540-9. [DOI] [PubMed] [Google Scholar]
- 298.Ulery B.D., Nair L.S., Laurencin C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. B Polym. Phys. 2011;49(12):832–864. doi: 10.1002/polb.22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Hamlet S.M., et al. 3-Dimensional functionalized polycaprolactone-hyaluronic acid hydrogel constructs for bone tissue engineering. J. Clin. Periodontol. 2016;44(4):428–437. doi: 10.1111/jcpe.12686. [DOI] [PubMed] [Google Scholar]
- 300.Jensen G., Holloway J.L., Stabenfeldt S.E. Hyaluronic acid biomaterials for central nervous system regenerative medicine. Cells. 2020;9(9) doi: 10.3390/cells9092113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Gallagher L.B., et al. Pre-culture of mesenchymal stem cells within RGD-modified hyaluronic acid hydrogel improves their resilience to ischaemic conditions. Acta Biomater. 2020;107:78–90. doi: 10.1016/j.actbio.2020.02.043. [DOI] [PubMed] [Google Scholar]
- 302.Harrington S., et al. A versatile microencapsulation platform for hyaluronic acid and polyethylene glycol. Tissue Eng. Part A. 2021;27(3–4):153–164. doi: 10.1089/ten.tea.2019.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Mudhol R., Bansal R. Cross-linked hyaluronic acid viscoelastic scleral implant in trabeculectomy. Indian J. Ophtalmol. 2021;69(5):1135–1141. doi: 10.4103/ijo.IJO_2462_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.He T., et al. Hyaluronic acid-based shape-memory cryogel scaffolds for focal cartilage defect repair. Tissue Eng. Part A. 2021;27(11–12) doi: 10.1089/ten.TEA.2020.0264. [DOI] [PubMed] [Google Scholar]
- 305.Hinsenkamp Á., et al. Crosslinked hyaluronic acid gels with blood-derived protein components for soft tissue regeneration. Tissue Eng. Part A. 2021;27(11–12) doi: 10.1089/ten.TEA.2020.0197. [DOI] [PubMed] [Google Scholar]
- 306.Guarise C., et al. Titanium implant coating based on dopamine-functionalized sulphated hyaluronic acid: in vivo assessment of biocompatibility and antibacterial efficacy. Mater. Sci. Eng., C. 2021;128 doi: 10.1016/j.msec.2021.112286. [DOI] [PubMed] [Google Scholar]
- 307.Chen C.-H., et al. Functional hyaluronic acid-polylactic acid/silver nanoparticles core-sheath nanofiber membranes for prevention of post-operative tendon adhesion. Int. J. Mol. Sci. 2021;22(16):8781. doi: 10.3390/ijms22168781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Vepari C., Kaplan D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007;32(8–9):991–1007. doi: 10.1016/j.progpolymsci.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Kumar M., et al. Immunomodulatory injectable silk hydrogels maintaining functional islets and promoting anti-inflammatory M2 macrophage polarization. Biomaterials. 2018;187:1–17. doi: 10.1016/j.biomaterials.2018.09.037. [DOI] [PubMed] [Google Scholar]
- 310.Hamilton D.C., et al. A silk-based encapsulation platform for pancreatic islet transplantation improves islet function in vivo. J. Tissue Eng. Regen. Med. 2017;11(3):887–895. doi: 10.1002/term.1990. [DOI] [PubMed] [Google Scholar]
- 311.Kwak H.W., et al. Sericin promotes fibroin silk I stabilization across a phase-separation. Biomacromolecules. 2017;18(8):2343–2349. doi: 10.1021/acs.biomac.7b00549. [DOI] [PubMed] [Google Scholar]
- 312.Lee O.J., et al. Recent advances in fluorescent silk fibroin. Front. Mater. 2020;7 [Google Scholar]
- 313.Wang Y., et al. Stem cell-based tissue engineering with silk biomaterials. Biomaterials. 2006;27(36):6064–6082. doi: 10.1016/j.biomaterials.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 314.Morikawa M., et al. Rat islet culture in serum-free medium containing silk protein sericin. J. Hepatobiliary Pancreat. Surg. 2009;16(2):223–228. doi: 10.1007/s00534-009-0049-y. [DOI] [PubMed] [Google Scholar]
- 315.Ohnishi K., et al. Effect of the silk protein sericin on cryopreserved rat islets. J. Hepatobiliary Pancreat. Surg. 2012;19(4):354–360. doi: 10.1007/s00534-011-0415-4. [DOI] [PubMed] [Google Scholar]
- 316.Davis N.E., et al. Enhanced function of pancreatic islets co-encapsulated with ECM proteins and mesenchymal stromal cells in a silk hydrogel. Biomaterials. 2012;33(28):6691–6697. doi: 10.1016/j.biomaterials.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Perteghella S., et al. Stromal vascular fraction loaded silk fibroin mats effectively support the survival of diabetic mice after pancreatic islet transplantation. Macromol. Biosci. 2017;17(9) doi: 10.1002/mabi.201700131. [DOI] [PubMed] [Google Scholar]
- 318.Cheng J., et al. Matrix components and scaffolds for sustained islet function. Tissue Eng. Part B, Rev. 2011;17 doi: 10.1089/ten.TEB.2011.0004. [DOI] [PubMed] [Google Scholar]
- 319.J. Crisóstomo et al., ECM-enriched alginate hydrogels for bioartificial pancreas: an ideal niche to improve insulin secretion and diabetic glucose profile, J. Appl. Biomater. Funct. Mater. 17(4) (2019) 2280800019848923. [DOI] [PubMed]
- 320.Zbinden A., et al. Nidogen-1 mitigates ischemia and promotes tissue survival and regeneration. Adv. Sci. (Weinh) 2021;8(4):2002500. doi: 10.1002/advs.202002500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Brandhorst D., et al. Basement membrane proteins improve human islet survival in hypoxia: implications for islet inflammation. Acta Biomater. 2022;137:92–102. doi: 10.1016/j.actbio.2021.10.013. [DOI] [PubMed] [Google Scholar]
- 322.Ghosh B., Biswas S. Polymeric micelles in cancer therapy: state of the art. J. Control. Release. 2021;332:127–147. doi: 10.1016/j.jconrel.2021.02.016. [DOI] [PubMed] [Google Scholar]
- 323.Weaver J.D., et al. Design of a vascularized synthetic poly(ethylene glycol) macroencapsulation device for islet transplantation. Biomaterials. 2018;172:54–65. doi: 10.1016/j.biomaterials.2018.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Yang J., et al. Surface engineering of pancreatic islets with a heparinized StarPEG nanocoating. J. Visual. Exp.: JoVE. 2018;136:56879. doi: 10.3791/56879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Kim H.-O., et al. Cell-mimic polymersome-shielded islets for long-term immune protection of neonatal porcine islet-like cell clusters. J. Mater. Chem. B. 2020;8(12):2476–2482. doi: 10.1039/c9tb02270h. [DOI] [PubMed] [Google Scholar]
- 326.Scheiner K.C., et al. Vascular endothelial growth factor-releasing microspheres based on poly(epsilon-caprolactone-PEG-epsilon-caprolactone)-b-poly(L-Lactide) multiblock copolymers incorporated in a three-dimensional printed poly(dimethylsiloxane) cell macroencapsulation device. J. Pharm. Sci. 2020;109(1):863–870. doi: 10.1016/j.xphs.2019.10.028. [DOI] [PubMed] [Google Scholar]
- 327.Coronel M.M., et al. Immunotherapy via PD-L1–presenting biomaterials leads to long-term islet graft survival. Sci. Adv. 2020;6 doi: 10.1126/sciadv.aba5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Teferra M. Poly-dimethylsiloxane (PDMS) an ideal biomaterial for cornea replacement. Int. J. Latest Res. Eng. Technol. 2017;3:77–82. [Google Scholar]
- 329.Brady A.C., et al. Proangiogenic hydrogels within macroporous scaffolds enhance islet engraftment in an extrahepatic site. Tissue Eng. Part A. 2013;19(23–24):2544–2552. doi: 10.1089/ten.tea.2012.0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Jiang K., et al. Local release of dexamethasone from macroporous scaffolds accelerates islet transplant engraftment by promotion of anti-inflammatory M2 macrophages. Biomaterials. 2017;114:71–81. doi: 10.1016/j.biomaterials.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 331.Rojas-Canales D.M., et al. Oxygen-permeable microwell device maintains islet mass and integrity during shipping. Endocr. Connect. 2018;7 doi: 10.1530/EC-17-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Myasnikova D., et al. Synergic effects of oxygen supply and antioxidants on pancreatic beta-cell spheroids. Sci. Rep. 2019;9(1):1802. doi: 10.1038/s41598-018-38011-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Liang J.P., et al. Engineering a macroporous oxygen-generating scaffold for enhancing islet cell transplantation within an extrahepatic site. Acta Biomater. 2021 doi: 10.1016/j.actbio.2021.05.028. [DOI] [PubMed] [Google Scholar]
- 334.Tokito F., et al. High density culture of pancreatic islet-like 3D tissue organized in oxygen-permeable porous scaffolds with external oxygen supply. J. Biosci. Bioeng. 2021;131(5):543–548. doi: 10.1016/j.jbiosc.2020.12.009. [DOI] [PubMed] [Google Scholar]
- 335.Gentile P., et al. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014;15(3):3640–3659. doi: 10.3390/ijms15033640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Zhao W., et al. Fabrication of functional PLGA-based electrospun scaffolds and their applications in biomedical engineering. Mater. Sci. Eng., C. 2016;59:1181–1194. doi: 10.1016/j.msec.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 337.Hu S., de Vos P. Polymeric approaches to reduce tissue responses against devices applied for islet-cell encapsulation. Front. Bioeng. Biotechnol. 2019;7:134. doi: 10.3389/fbioe.2019.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Jiskoot W., et al. Immunological risk of injectable drug delivery systems. Pharm. Res. 2009;26(6):1303–1314. doi: 10.1007/s11095-009-9855-9. [DOI] [PubMed] [Google Scholar]
- 339.Salvay D.M., et al. Extracellular matrix protein-coated scaffolds promote the reversal of diabetes after extrahepatic islet transplantation. Transplantation. 2008;85(10):1456–1464. doi: 10.1097/TP.0b013e31816fc0ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Lew B., et al. Sustained exenatide delivery via intracapsular microspheres for improved survival and function of microencapsulated porcine islets. Drug Deliv. Transl. Res. 2018;8(3):857–862. doi: 10.1007/s13346-018-0484-x. [DOI] [PubMed] [Google Scholar]
- 341.Li Y., et al. Immunosuppressive PLGA TGF-beta1 microparticles induce polyclonal and antigen-specific regulatory T cells for local immunomodulation of allogeneic islet transplants. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.653088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Nguyen T.T., et al. The impact of locally-delivered tacrolimus-releasing microspheres and polyethylene glycol-based islet surface modification on xenogeneic islet survival. J. Control. Release. 2021;336:274–284. doi: 10.1016/j.jconrel.2021.06.020. [DOI] [PubMed] [Google Scholar]
- 343.Wiederkehr A., Wollheim C.B. Minireview: implication of mitochondria in insulin secretion and action. Endocrinology. 2006;147(6):2643–2649. doi: 10.1210/en.2006-0057. [DOI] [PubMed] [Google Scholar]
- 344.Maechler P., Wollheim C.B. Mitochondrial function in normal and diabetic β-cells. Nature. 2001;414:807–812. doi: 10.1038/414807a. [DOI] [PubMed] [Google Scholar]
- 345.Sato Y., et al. Cellular hypoxia of pancreatic beta-cells due to high levels of oxygen consumption for insulin secretion in vitro. J. Biol. Chem. 2011;286(14):12524–12532. doi: 10.1074/jbc.M110.194738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Kampf C., Mattsson G., Carlsson P.-O. Size-dependent revascularization of transplanted pancreatic islets. Cell Transplant. 2006;15(2):205–209. doi: 10.3727/000000006783982124. [DOI] [PubMed] [Google Scholar]
- 347.Komatsu H., et al. Oxygen environment and islet size are the primary limiting factors of isolated pancreatic islet survival. PLoS ONE. 2017;12(8) doi: 10.1371/journal.pone.0183780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.T.M. Suszynski, E.S. Avgoustiniatos, K.K. Papas, oxygenation of the intraportally transplanted pancreatic islet, J. Diabet. Res. 2016 (2016) 7625947. [DOI] [PMC free article] [PubMed]
- 349.Olsson R., et al. Increased numbers of low-oxygenated pancreatic islets after intraportal islet transplantation. Diabetes. 2011;60(9):2350–2353. doi: 10.2337/db09-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Sterkers A., et al. Islet survival and function following intramuscular autotransplantation in the minipig. Am. J. Transplant. 2013;13(4):891–898. doi: 10.1111/ajt.12136. [DOI] [PubMed] [Google Scholar]
- 351.Strieth S., et al. A new animal model to assess angiogenesis and endocrine function of parathyroid heterografts in vivo. Transplantation. 2005;79(4):392–400. doi: 10.1097/01.tp.0000151633.92173.75. [DOI] [PubMed] [Google Scholar]
- 352.Ander S.J., et al. Revascularisation of human parathyroid tissue transplanted to athymic mice. Apmis. 1997;105(12):931–940. doi: 10.1111/j.1699-0463.1997.tb05104.x. [DOI] [PubMed] [Google Scholar]
- 353.Menger M.D., et al. Angiogenesis and hemodynamics of microvasculature of transplanted islets of Langerhans. Diabetes. 1989;38(Suppl. 1):199–201. doi: 10.2337/diab.38.1.s199. [DOI] [PubMed] [Google Scholar]
- 354.Davalli A.M., et al. Function, mass, and replication of porcine and rat islets transplanted into diabetic nude mice. Diabetes. 1995;44(1):104–111. doi: 10.2337/diab.44.1.104. [DOI] [PubMed] [Google Scholar]
- 355.Davalli A.M., et al. Vulnerability of islets in the immediate posttransplantation period. Dynamic changes in structure and function. Diabetes. 1996;45(9):1161–1167. doi: 10.2337/diab.45.9.1161. [DOI] [PubMed] [Google Scholar]
- 356.Davalli A.M., et al. Early changes in syngeneic islet grafts: effect of recipient's metabolic control on graft outcome. Transplant. Proc. 1995;27(6):3238–3239. [PubMed] [Google Scholar]
- 357.Lo J.F., et al. Islet preconditioning via multimodal microfluidic modulation of intermittent hypoxia. Anal. Chem. 2012;84(4):1987–1993. doi: 10.1021/ac2030909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Ma Z., et al. Preconditioning with associated blocking of Ca2+ inflow alleviates hypoxia-induced damage to pancreatic beta-cells. PLoS ONE. 2013;8(7) doi: 10.1371/journal.pone.0067498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Dugbartey G.J. Carbon monoxide in pancreatic islet transplantation: a new therapeutic alternative to patients with severe type 1 diabetes mellitus. Front. Pharmacol. 2021 doi: 10.3389/fphar.2021.750816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.W.R. Wagner et al., Fluorinated biomaterials, in: D.W. Grainger (Ed.), Biomaterials Science (Fourth Edition) An Introduction to Materials in Medicine, 2020.
- 361.Juang J.H., et al. Outcome of subcutaneous islet transplantation improved by polymer device. Transplantation. 1996;61(11):1557–1561. doi: 10.1097/00007890-199606150-00001. [DOI] [PubMed] [Google Scholar]
- 362.Hunter S.K., et al. Promotion of neovascularization around hollow fiber bioartificial organs using biologically active substances. ASAIO J. 1999;45(1):37–40. doi: 10.1097/00002480-199901000-00009. [DOI] [PubMed] [Google Scholar]
- 363.Krishnan L., et al. Cellular immunoisolation for islet transplantation by a novel dual porosity electrospun membrane. Transplant. Proc. 2011;43(9):3256–3261. doi: 10.1016/j.transproceed.2011.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Khattak S.F., et al. Enhancing oxygen tension and cellular function in alginate cell encapsulation devices through the use of perfluorocarbons. Biotechnol. Bioeng. 2007;96(1):156–166. doi: 10.1002/bit.21151. [DOI] [PubMed] [Google Scholar]
- 365.Kimelman-Bleich N., et al. The use of a synthetic oxygen carrier-enriched hydrogel to enhance mesenchymal stem cell-based bone formation in vivo. Biomaterials. 2009;30(27):4639–4648. doi: 10.1016/j.biomaterials.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 366.Li H., Wijekoon A., Leipzig N.D. Encapsulated neural stem cell neuronal differentiation in fluorinated methacrylamide chitosan hydrogels. Ann. Biomed. Eng. 2013;42:1456–1469. doi: 10.1007/s10439-013-0925-0. [DOI] [PubMed] [Google Scholar]
- 367.Fraker C.A., Alejandro R., Ricordi C. Use of oxygenated perfluorocarbon toward making every pancreas count. Transplantation. 2002;74(12):1811–1812. doi: 10.1097/00007890-200212270-00032. [DOI] [PubMed] [Google Scholar]
- 368.Bergert H., et al. Effect of oxygenated perfluorocarbons on isolated rat pancreatic islets in culture. Cell Transplant. 2005;14:441–448. doi: 10.3727/000000005783982873. [DOI] [PubMed] [Google Scholar]
- 369.Maillard E., et al. Perfluorocarbon emulsions prevent hypoxia of pancreatic beta-cells. Cell Transplant. 2012;21(4):657–669. doi: 10.3727/096368911X593136. [DOI] [PubMed] [Google Scholar]
- 370.Lee S.H., et al. Improvement of islet function and survival by integration of perfluorodecalin into microcapsules in vivo and in vitro. J. Tissue Eng. Regen. Med. 2018;12(4):e2110–e2122. doi: 10.1002/term.2643. [DOI] [PubMed] [Google Scholar]
- 371.Pedraza E., et al. Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proc. Natl. Acad. Sci. USA. 2012;109(11):4245–4250. doi: 10.1073/pnas.1113560109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Coronel M.M., Geusz R., Stabler C.L. Mitigating hypoxic stress on pancreatic islets via in situ oxygen generating biomaterial. Biomaterials. 2017;129:139–151. doi: 10.1016/j.biomaterials.2017.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.McQuilling J.P., et al. Applications of particulate oxygen-generating substances (POGS) in the bioartificial pancreas. Biomater. Sci. 2017;5(12):2437–2447. doi: 10.1039/c7bm00790f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Coronel M.M., et al. Oxygen generating biomaterial improves the function and efficacy of beta cells within a macroencapsulation device. Biomaterials. 2019;210:1–11. doi: 10.1016/j.biomaterials.2019.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.L.-H. Long-Hai Wang et al., An inverse-breathing encapsulation system for cell delivery, Sci. Adv. 7 (2021) eabd5835. [DOI] [PMC free article] [PubMed]
- 376.Espes D., et al. Cotransplantation of polymerized hemoglobin reduces beta-cell hypoxia and improves beta-cell function in intramuscular islet grafts. Transplantation. 2015;99(10):2077–2082. doi: 10.1097/TP.0000000000000815. [DOI] [PubMed] [Google Scholar]
- 377.Avila J.G., et al. Improved outcomes in islet isolation and transplantation by the use of a novel hemoglobin-based O2 carrier. Am. J. Transplant. 2006;6(12):2861–2870. doi: 10.1111/j.1600-6143.2006.01551.x. [DOI] [PubMed] [Google Scholar]
- 378.Moure A., et al. Extracellular hemoglobin combined with an O2-generating material overcomes O2 limitation in the bioartificial pancreas. Biotechnol. Bioeng. 2019;116(5):1176–1189. doi: 10.1002/bit.26913. [DOI] [PubMed] [Google Scholar]
- 379.S. Koch, L. Claesson-Welsh, Signal transduction by vascular endothelial growth factor receptors, Cold Spring Harbor Perspect. Med. 2(7) (2012) a006502. [DOI] [PMC free article] [PubMed]
- 380.C. Melincovici et al., Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis, Romanian J. Morphol. Embryol. = Revue roumaine de morphologie et embryologie 59 (2018) 455–467. [PubMed]
- 381.Zhang N., et al. Elevated vascular endothelial growth factor production in islets improves islet graft vascularization. Diabetes. 2004;53 doi: 10.2337/diabetes.53.4.963. [DOI] [PubMed] [Google Scholar]
- 382.Staels W., et al. Vegf-A mRNA transfection as a novel approach to improve mouse and human islet graft revascularisation. Diabetologia. 2018;61(8):1804–1810. doi: 10.1007/s00125-018-4646-7. [DOI] [PubMed] [Google Scholar]
- 383.Ozawa C.R., et al. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J. Clin. Investig. 2004;113(4):516–527. doi: 10.1172/JCI18420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Sigrist S., et al. Influence of VEGF on the viability of encapsulated pancreatic rat islets after transplantation in diabetic mice. Cell Transplant. 2003;12:627–635. doi: 10.3727/000000003108747109. [DOI] [PubMed] [Google Scholar]
- 385.Weaver J.D., et al. Vasculogenic hydrogel enhances islet survival, engraftment, and function in leading extrahepatic sites. Sci. Adv. 2017;3(6) doi: 10.1126/sciadv.1700184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Phelps E.A., et al. Engineered VEGF-releasing PEG-MAL hydrogel for pancreatic islet vascularization. Drug Deliv. Transl. Res. 2015;5:125–136. doi: 10.1007/s13346-013-0142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Phelps E.A., et al. Vasculogenic bio-synthetic hydrogel for enhancement of pancreatic islet engraftment and function in type 1 diabetes. Biomaterials. 2013;34(19):4602–4611. doi: 10.1016/j.biomaterials.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Farina M., et al. 3D printed vascularized device for subcutaneous transplantation of human islets. Biotechnol. J. 2017;12(9) doi: 10.1002/biot.201700169. [DOI] [PubMed] [Google Scholar]
- 389.Cabric S., et al. Islet surface heparinization prevents the instant blood-mediated inflammatory reaction in islet transplantation. Diabetes. 2007;56:2008–2015. doi: 10.2337/db07-0358. [DOI] [PubMed] [Google Scholar]
- 390.Cabric S., et al. Anchoring of vascular endothelial growth factor to surface-immobilized heparin on pancreatic islets: implications for stimulating islet angiogenesis. Tissue Eng. Part A. 2010;16:961–970. doi: 10.1089/ten.tea.2009.0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Stendahl J.C., et al. Growth factor delivery from self-assembling nanofibers to facilitate islet transplantation. Transplantation. 2008;86(3):478–481. doi: 10.1097/TP.0b013e3181806d9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Chow L.W., et al. Self-assembling nanostructures to deliver angiogenic factors to pancreatic islets. Biomaterials. 2010;31:6154–6161. doi: 10.1016/j.biomaterials.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Scheiner K.C., et al. Vascular endothelial growth factor-releasing microspheres based on poly(ε-caprolactone-PEG-ε-caprolactone)-b-poly(L-lactide) multiblock copolymers incorporated in a three-dimensional printed poly(dimethylsiloxane) cell macroencapsulation device. J. Pharm. Sci. 2020;109(1):863–870. doi: 10.1016/j.xphs.2019.10.028. [DOI] [PubMed] [Google Scholar]
- 394.Carmeliet P. VEGF gene therapy: stimulating angiogenesis or angioma-genesis? Nat. Med. 2000;6(10):1102–1103. doi: 10.1038/80430. [DOI] [PubMed] [Google Scholar]
- 395.Bowers D.T., et al. Engineering the vasculature for islet transplantation. Acta Biomater. 2019;95:131–151. doi: 10.1016/j.actbio.2019.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.T. Nakamura, S. Mizuno, The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine, Proc. Jpn. Acad. Ser. B, Phys. Biol. Sci. 86(6) (2010) 588–610. [DOI] [PMC free article] [PubMed]
- 397.Nakamura T., et al. Purification and subunit structure of hepatocyte growth factor from rat platelets. FEBS Lett. 1987;224(2):311–316. doi: 10.1016/0014-5793(87)80475-1. [DOI] [PubMed] [Google Scholar]
- 398.Fiaschi-Taesch N., Stewart A.F., Garcia-Ocaña A. Improving islet transplantation by gene delivery of hepatocyte growth factor (HGF) and its downstream target, protein kinase B (PKB)/Akt. Cell Biochem. Biophys. 2007;48(2–3):191–199. doi: 10.1007/s12013-007-0024-7. [DOI] [PubMed] [Google Scholar]
- 399.Tashiro H., et al. Hepatocyte growth factor prevents chronic allograft dysfunction in liver-transplanted rats. Transplantation. 2003;76(5):761–765. doi: 10.1097/01.TP.0000083040.50727.CE. [DOI] [PubMed] [Google Scholar]
- 400.Yamaura K., et al. Suppression of acute and chronic rejection by hepatocyte growth factor in a murine model of cardiac transplantation: induction of tolerance and prevention of cardiac allograft vasculopathy. Circulation. 2004;110(12):1650–1657. doi: 10.1161/01.CIR.0000143052.45956.71. [DOI] [PubMed] [Google Scholar]
- 401.Tambara K., et al. Administration of control-released hepatocyte growth factor enhances the efficacy of skeletal myoblast transplantation in rat infarcted hearts by greatly increasing both quantity and quality of the graft. Circulation. 2005;112(9 Suppl.):I129–I134. doi: 10.1161/CIRCULATIONAHA.104.526293. [DOI] [PubMed] [Google Scholar]
- 402.García-Ocaña A., et al. Transgenic overexpression of hepatocyte growth factor in the B-cell markedly improves islet function and islet transplant outcomes in mice. Diabetes. 2001;50:2752–2762. doi: 10.2337/diabetes.50.12.2752. [DOI] [PubMed] [Google Scholar]
- 403.Fiaschi-Taesch N., Stewart A.F., Garcia-Ocana A. Improving islet transplantation by gene delivery of hepatocyte growth factor (HGF) and its downstream target, protein kinase B (PKB)/Akt. Cell Biochem. Biophys. 2007;48(2–3):191–199. doi: 10.1007/s12013-007-0024-7. [DOI] [PubMed] [Google Scholar]
- 404.Rao P., et al. Hepatocyte growth factor gene therapy for islet transplantation. Expert Opin. Biol. Ther. 2004;4(4):507–518. doi: 10.1517/14712598.4.4.507. [DOI] [PubMed] [Google Scholar]
- 405.Fiaschi-Taesch N.M., et al. Hepatocyte growth factor enhances engraftment and function of nonhuman primate islets. Diabetes. 2008;57(10):2745–2754. doi: 10.2337/db08-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Y.-R. Yun et al., Fibroblast growth factors: biology, function, and application for tissue regeneration, J. Tissue Eng. 2010 (2010) 218142. [DOI] [PMC free article] [PubMed]
- 407.Kuroda Y., et al. Clinical application of injectable growth factor for bone regeneration: a systematic review. Inflamm. Regen. 2019;39(1):20. doi: 10.1186/s41232-019-0109-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Rivas-Carrillo J.D., et al. Amelioration of diabetes in mice after single-donor islet transplantation using the controlled release of gelatinized FGF-2. Cell Transplant. 2006;15 doi: 10.3727/000000006783981323. [DOI] [PubMed] [Google Scholar]
- 409.Moya M.L., et al. Fibroblast growth factor-1 (FGF-1) loaded microbeads enhance local capillary neovascularization. J. Surg. Res. 2010;160(2):208–212. doi: 10.1016/j.jss.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Uonaga T., et al. FGF-21 enhances islet engraftment in mouse syngeneic islet transplantation model. Islets. 2010;2:247–251. doi: 10.4161/isl.2.4.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Freudenberg U., et al. A star-PEG–heparin hydrogel platform to aid cell replacement therapies for neurodegenerative diseases. Biomaterials. 2009;30(28):5049–5060. doi: 10.1016/j.biomaterials.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 412.Smink A.M., et al. Stimulation of vascularization of a subcutaneous scaffold applicable for pancreatic islet-transplantation enhances immediate post-transplant islet graft function but not long-term normoglycemia. J. Biomed. Mater. Res. A. 2017;105(9):2533–2542. doi: 10.1002/jbm.a.36101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Yang S.Y., Yang K.C., Sumi S. Effect of basic fibroblast growth factor on xenogeneic islets in subcutaneous transplantation-a murine model. Transplant. Proc. 2019;51(5):1458–1462. doi: 10.1016/j.transproceed.2019.01.135. [DOI] [PubMed] [Google Scholar]
- 414.Oh S.A., Li M.O. TGF-beta: guardian of T cell function. J. Immunol. 2013;191(8):3973–3979. doi: 10.4049/jimmunol.1301843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Budi E.H., et al. Enhanced TGF-beta signaling contributes to the insulin-induced angiogenic responses of endothelial cells. iScience. 2019;11:474–491. doi: 10.1016/j.isci.2018.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Han B., et al. TGF-beta i promotes islet beta-cell function and regeneration. J. Immunol. 2011;186(10):5833–5844. doi: 10.4049/jimmunol.1002303. [DOI] [PubMed] [Google Scholar]
- 417.Brown M.L., Schneyer A.L. Emerging roles for the TGFbeta family in pancreatic beta-cell homeostasis. Trends Endocrinol. Metab. 2010;21(7):441–448. doi: 10.1016/j.tem.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Dhawan S., et al. Inhibition of TGF-beta signaling promotes human pancreatic beta-cell replication. Diabetes. 2016;65(5):1208–1218. doi: 10.2337/db15-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Lee J.H., et al. Protection from beta-cell apoptosis by inhibition of TGF-beta/Smad3 signaling. Cell Death Dis. 2020;11(3):184. doi: 10.1038/s41419-020-2365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Sabek O.M., et al. Expression of transforming growth factor-beta by human islets: impact on islet viability and function. Cell Transplant. 2007;16:775–785. doi: 10.3727/000000007783465217. [DOI] [PubMed] [Google Scholar]
- 421.Thomas D.C., et al. Protection of islet grafts through transforming growth factor-beta-induced tolerogenic dendritic cells. Diabetes. 2013;62(9):3132–3142. doi: 10.2337/db12-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Liu J.M.H., et al. Transforming growth factor-beta 1 delivery from microporous scaffolds decreases inflammation post-implant and enhances function of transplanted islets. Biomaterials. 2016;80:11–19. doi: 10.1016/j.biomaterials.2015.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.S. Pellegrini, Alternative transplantation sites for islet transplantation, in: Transplantation, Bioengineering, and Regeneration of the Endocrine Pancreas, 2020, pp. 833–847.
- 424.Perez-Basterrechea M., et al. Tissue-engineering approaches in pancreatic islet transplantation. Biotechnol. Bioeng. 2018;115(12):3009–3029. doi: 10.1002/bit.26821. [DOI] [PubMed] [Google Scholar]
- 425.van der Windt D.J., et al. The choice of anatomical site for islet transplantation. Cell Transplant. 2008;17:1005–1014. [PubMed] [Google Scholar]
- 426.Shapiro A.M.J., et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 2000;343 doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 427.Kemp C.B., et al. Effect of transplantation site on the results of pancreatic islet isografts in diabetic rats. Diabetologia. 1973;9:486–491. doi: 10.1007/BF00461694. [DOI] [PubMed] [Google Scholar]
- 428.Eaton R.P., Allen R.C., Schade D.S. Hepatic removal of insulin in normal man: dose response to endogenous insulin secretion. J. Clin. Endocrinol. Metabol. 1983;56:1294–1300. doi: 10.1210/jcem-56-6-1294. [DOI] [PubMed] [Google Scholar]
- 429.Pileggi A., et al. Twenty years of clinical islet transplantation at the Diabetes Research Institute-University of Miami. Clin. Transpl. 2004:177–204. [PubMed] [Google Scholar]
- 430.H. Liljebäck, D. Espes, P.-O. Carlsson, Unsurpassed intrahepatic islet engraftment – the quest for new sites for beta cell replacement, Cell Med. 11 (2019) 2155179019857662. [DOI] [PMC free article] [PubMed]
- 431.Fujita I., et al. The liver surface as a favorable site for islet cell sheet transplantation in type 1 diabetes model mice. Regen. Ther. 2018;8:65–72. doi: 10.1016/j.reth.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Ramadori G., et al. Quantitative determination of complement components produced by purified hepatocytes. Clin. Exp. Immunol. 1984;55(1):189–196. [PMC free article] [PubMed] [Google Scholar]
- 433.Pathak S., et al. Engineered islet cell clusters transplanted into subcutaneous space are superior to pancreatic islets in diabetes. FASEB J. 2017;31(11):5111–5121. doi: 10.1096/fj.201700490R. [DOI] [PubMed] [Google Scholar]
- 434.Park S., Lee D.Y. The anterior chamber of the eye as a site for pancreatic islet transplantation. J. Ind. Eng. Chem. 2017;50:29–35. [Google Scholar]
- 435.Vaithilingam V., Bal S., Tuch B.E. Encapsulated islet transplantation: where do we stand? The review of diabetic studies. RDS. 2017;14(1):51–78. doi: 10.1900/RDS.2017.14.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Stokes R.A., et al. Transplantation sites for human and murine islets. Diabetologia. 2017;60(10):1961–1971. doi: 10.1007/s00125-017-4362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Mellgren A., et al. The renal subcapsular site offers better growth conditions for transplanted mouse pancreatic islet cells than the liver or spleen. Diabetologia. 1986;29(9):670–672. doi: 10.1007/BF00869269. [DOI] [PubMed] [Google Scholar]
- 438.A.R. Pepper et al., Revascularization of transplanted pancreatic islets and role of the transplantation site, Clin. Dev. Immunol. 2013 (2013) 352315. [DOI] [PMC free article] [PubMed]
- 439.Damyar K., et al. An overview of current advancements in pancreatic islet transplantation into the omentum. Islets. 2021:1–6. doi: 10.1080/19382014.2021.1954459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Zweifach B.W., Lipowsky H.H. Quantitative studies of microcirculatory structure and function. III. Microvascular hemodynamics of cat mesentery and rabbit omentum. Circ. Res. 1977;41:380–390. doi: 10.1161/01.res.41.3.380. [DOI] [PubMed] [Google Scholar]
- 441.Svirskis D., et al. A non-opioid analgesic implant for sustained post-operative intraperitoneal delivery of lidocaine, characterized using an ovine model. Biomaterials. 2020;263 doi: 10.1016/j.biomaterials.2020.120409. [DOI] [PubMed] [Google Scholar]
- 442.Kim H.-I., et al. Comparison of four pancreatic islet implantation sites. J. Korean Med. Sci. 2010;25(2):203–210. doi: 10.3346/jkms.2010.25.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.K. Kumano et al., Grafting islets to a dissected peritoneal pouch to improve transplant survival and function, Transplantation, 9000. Online First. [DOI] [PubMed]
- 444.Weber C.J., et al. Evaluation of graft-host response for various tissue sources and animal models. Ann. N. Y. Acad. Sci. 1999;875:233–254. doi: 10.1111/j.1749-6632.1999.tb08507.x. [DOI] [PubMed] [Google Scholar]
- 445.Safley S.A., et al. Encapsulated piscine (tilapia) islets for Diabetes Ther.: studies in diabetic NOD and NOD-SCID mice. Xenotransplantation. 2014;21(2):127–139. doi: 10.1111/xen.12086. [DOI] [PubMed] [Google Scholar]
- 446.Kessler L., et al. Cytotoxicity of peritoneal murine macrophages against encapsulated pancreatic rat islets: in vivo and in vitro studies. J. Leukoc. Biol. 1996;60(6):729–736. doi: 10.1002/jlb.60.6.729. [DOI] [PubMed] [Google Scholar]
- 447.Hwa A.J., Weir G.C. Transplantation of macroencapsulated insulin-producing cells. Curr. Diab.Rep. 2018;18(8):50. doi: 10.1007/s11892-018-1028-y. [DOI] [PubMed] [Google Scholar]
- 448.Kalra S., Kalra B., Agrawal N. Oral insulin. Diabetol. Metabolic Syndrome. 2010;2:66. doi: 10.1186/1758-5996-2-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Sakata N., Yoshimatsu G., Kodama S. The spleen as an optimal site for islet transplantation and a source of mesenchymal stem cells. Int. J. Mol. Sci. 2018;19(5):1391. doi: 10.3390/ijms19051391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Pepper A.R., et al. Harnessing the foreign body reaction in marginal mass device-less subcutaneous islet transplantation in mice. Transplantation. 2016;100(7):1474–1479. doi: 10.1097/TP.0000000000001162. [DOI] [PubMed] [Google Scholar]
- 451.Pepper A.R., et al. Long-term function and optimization of mouse and human islet transplantation in the subcutaneous device-less site. Islets. 2016;8(6):186–194. doi: 10.1080/19382014.2016.1253652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Cantarelli E., Piemonti L. Alternative transplantation sites for pancreatic islet grafts. Curr. Diab.Rep. 2011;11(5):364. doi: 10.1007/s11892-011-0216-9. [DOI] [PubMed] [Google Scholar]
- 453.Stagner J., Rilo H., White K. The pancreas as an islet transplantation site. Confirmation in a syngeneic rodent and canine autotransplant model. JOP: J. Pancreas. 2007;8:628–636. [PubMed] [Google Scholar]
- 454.Ho C.-K., et al. Complications of pancreatic surgery. HPB: Off. J. Int. Hepato Pancreato Biliary Assoc. 2005;7(2):99–108. doi: 10.1080/13651820510028936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Hosseini-Tabatabaei A., et al. Immunoprotection and functional improvement of allogeneic islets in diabetic mice, using a stable indoleamine 2,3-dioxygenase producing scaffold. Transplantation. 2015;99(7) doi: 10.1097/TP.0000000000000661. [DOI] [PubMed] [Google Scholar]
- 456.F. Li et al., Comparative study of two different islet transplantation sites in mice: hepatic sinus tract vs splenic parenchyma, Cell Transplant 29 (2020) 963689720943576. [DOI] [PMC free article] [PubMed]
- 457.Archibald P.R.T., Williams D.J. Using the cost-effectiveness of allogeneic islet transplantation to inform induced pluripotent stem cell-derived β-cell therapy reimbursement. Regen. Med. 2015;10(8):959–973. doi: 10.2217/rme.15.59. [DOI] [PubMed] [Google Scholar]
- 458.Beckwith J., et al. A health economic analysis of clinical islet transplantation. Clin. Transplant. 2012;1:23–33. doi: 10.1111/j.1399-0012.2011.01411.x. [DOI] [PubMed] [Google Scholar]
- 459.G. Orlando et al., Transplantation, Bioengineering, and Regeneration of the Endocrine Pancreas, 2019.
- 460.Witkowski P., et al. Islets transplantation at a crossroads - need for urgent regulatory update in the United States: perspective presented during the scientific sessions 2021 at the Am. Diabetes Assoc. Congress. Front. Endocrinol. 2022;12 doi: 10.3389/fendo.2021.789526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Witkowski P., et al. The demise of islet allotransplantation in the United States: a call for an urgent regulatory update. Am. J. Transplant. 2021;21(4):1365–1375. doi: 10.1111/ajt.16397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Ahlqvist E., et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6(5):361–369. doi: 10.1016/S2213-8587(18)30051-2. [DOI] [PubMed] [Google Scholar]
- 463.Zaharia O.P., et al. Risk of diabetes-associated diseases in subgroups of patients with recent-onset diabetes: a 5-year follow-up study. Lancet Diabetes Endocrinol. 2019;7(9):684–694. doi: 10.1016/S2213-8587(19)30187-1. [DOI] [PubMed] [Google Scholar]
- 464.Wagner R., et al. Pathophysiology-based subphenotyping of individuals at elevated risk for type 2 diabetes. Nat. Med. 2021;27(1):49–57. doi: 10.1038/s41591-020-1116-9. [DOI] [PubMed] [Google Scholar]
- 465.Wang H., et al. Donor treatment with carbon monoxide can yield islet allograft survival and tolerance. Diabetes. 2005;54(5):1400–1406. doi: 10.2337/diabetes.54.5.1400. [DOI] [PubMed] [Google Scholar]
- 466.Günther L., et al. Carbon monoxide protects pancreatic β-cells from apoptosis and improves islet function/survival after transplantation. Diabetes. 2002;51(4):994–999. doi: 10.2337/diabetes.51.4.994. [DOI] [PubMed] [Google Scholar]
- 467.Ludwig B., et al. A novel device for islet transplantation providing immune protection and oxygen supply. Hormone Metabolic Res. 2010;42(13):918–922. doi: 10.1055/s-0030-1267916. [DOI] [PubMed] [Google Scholar]
- 468.Komatsu H., Rawson J., Barriga A., Gonzalez N., Mendez D., Li J., Omori K., Kandeel F., Mullen Y. Posttransplant oxygen inhalation improves the outcome of subcutaneous islet transplantation: a promising clinical alternative to the conventional intrahepatic site. Am. J. Transplant. 2018;18(4):832–842. doi: 10.1111/ajt.14497. [DOI] [PubMed] [Google Scholar]
- 469.Vériter S., Aouassar N., Adnet P.-Y., Paridaens M.-S., Stuckman C., Jordan B., Karroum O., Gallez B., Gianello P., Dufrane D. The impact of hyperglycemia and the presence of encapsulated islets on oxygenation within a bioartificial pancreas in the presence of mesenchymal stem cells in a diabetic Wistar rat model. Biomaterials. 2011;32(26):5945–5956. doi: 10.1016/j.biomaterials.2011.02.061. [DOI] [PubMed] [Google Scholar]
- 470.Scharp D.W., Swanson C.J., Olack B.J., Latta P.P., Hegre O.D., Doherty E.J., Gentile F.T., Flavin K.S., Ansara M.F., Lacy P.E. Protection of encapsulated human islets implanted without immunosuppression in patients with type I or type II diabetes and in nondiabetic control subjects. Diabetes. 1994;43(9):1167–1170. doi: 10.2337/diab.43.9.1167. [DOI] [PubMed] [Google Scholar]
- 471.Bridge M.J., Broadhead K.W., Hlady V., Tresco P.A. Ethanol treatment alters the ultrastructure and permeability of PAN-PVC hollow fiber cell encapsulation membranes. J. Membr. Sci. 2002;195(1):51–64. [Google Scholar]
- 472.George S., Nair P.D., Risbud M.V., Bhonde R.R. Nonporous polyurethane membranes as islet immunoisolation matrices–biocompatibility studies. J. Biomater Appl. 2002;16(4):327–340. doi: 10.1106/088532802024249. [DOI] [PubMed] [Google Scholar]
- 473.Kadam S.S., Sudhakar M., Nair P.D., Bhonde R.R. Reversal of experimental diabetes in mice by transplantation of neo-islets generated from human amnion-derived mesenchymal stromal cells using immuno-isolatory macrocapsules. Cytotherapy. 2010;12(8):982–991. doi: 10.3109/14653249.2010.509546. [DOI] [PubMed] [Google Scholar]
- 474.Suzuki K., et al. A method for estimating number and mass of islets transplanted within a membrane device. Cell Transplant. 1996;5(6):613–625. doi: 10.1177/096368979600500604. [DOI] [PubMed] [Google Scholar]
- 475.Suzuki K., et al. Number and volume of islets transplanted in immunobarrier devices. Cell Transplant. 1998;7(1):47–52. doi: 10.1177/096368979800700107. [DOI] [PubMed] [Google Scholar]
- 476.Vegas A.J., et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat. Med. 2016;22(3):306–311. doi: 10.1038/nm.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Desai T.A., et al. Microfabricated immunoisolating biocapsules. Biotechnol. Bioeng. 1998;57(1):118–120. doi: 10.1002/(sici)1097-0290(19980105)57:1<118::aid-bit14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 478.La Flamme K.E., et al. Nanoporous alumina capsules for cellular macroencapsulation: transport and biocompatibility. Diabetes Technol. Ther. 2005;7(5):684–694. doi: 10.1089/dia.2005.7.684. [DOI] [PubMed] [Google Scholar]
- 479.La Flamme K.E., et al. Biocompatibility of nanoporous alumina membranes for immunoisolation. Biomaterials. 2007;28(16):2638–2645. doi: 10.1016/j.biomaterials.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Kessler L., et al. Influence of corona surface treatment on the properties of an artificial membrane used for Langerhans islets encapsulation: permeability and biocompatibility studies. Biomaterials. 1995;16(3):185–191. doi: 10.1016/0142-9612(95)92116-n. [DOI] [PubMed] [Google Scholar]
- 481.A.A. Tomei et al., Device design and materials optimization of conformal coating for islets of Langerhans, Proc. Natl. Acad. Sci. U. S. A. 111(29) (2014) 10514–10519. [DOI] [PMC free article] [PubMed]
- 482.Teramura Y., et al. Surface modification of islets with PEG-lipid for improvement of graft survival in intraportal transplantation. Transplantation. 2009;88(5):624–630. doi: 10.1097/TP.0b013e3181b230ac. [DOI] [PubMed] [Google Scholar]
- 483.Park H.S., et al. Antifibrotic effect of rapamycin containing polyethylene glycol-coated alginate microcapsule in islet xenotransplantation. J. Tissue Eng. Regen. Med. 2017;11(4):1274–1284. doi: 10.1002/term.2029. [DOI] [PubMed] [Google Scholar]
- 484.Chae S.Y., et al. Protection of insulin secreting cells from nitric oxide induced cellular damage by crosslinked hemoglobin. Biomaterials. 2004;25(5):843–850. doi: 10.1016/s0142-9612(03)00605-7. [DOI] [PubMed] [Google Scholar]
- 485.Wijsman J., et al. Histological and immunopathological analysis of recovered encapsulated allogeneic islets from transplanted diabetic BB/W rats. Transplantation. 1992;54(4):588–592. doi: 10.1097/00007890-199210000-00004. [DOI] [PubMed] [Google Scholar]
- 486.Safley S.A., et al. Multiple clinically relevant immunotherapies prolong the function of microencapsulated porcine islet xenografts in diabetic NOD mice without the use of anti-CD154 mAb. Xenotransplantation. 2020;27(4):e12577. doi: 10.1111/xen.12577. [DOI] [PubMed] [Google Scholar]
- 487.Sremac M., et al. Preliminary studies of the impact of CXCL12 on the foreign body reaction to pancreatic islets microencapsulated in alginate in nonhuman primates. Transplant Direct. 2019;5(5):e447–e. doi: 10.1097/TXD.0000000000000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Pathak S., et al. Single synchronous delivery of FK506-loaded polymeric microspheres with pancreatic islets for the successful treatment of streptozocin-induced diabetes in mice. Drug Del. 2017;24(1):1350–1359. doi: 10.1080/10717544.2017.1377317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Hu S., et al. Toll-like receptor 2-modulating pectin-polymers in alginate-based microcapsules attenuate immune responses and support islet-xenograft survival. Biomaterials. 2021;266 doi: 10.1016/j.biomaterials.2020.120460. [DOI] [PubMed] [Google Scholar]
- 490.Headen D.M., et al. Local immunomodulation with Fas ligand-engineered biomaterials achieves allogeneic islet graft acceptance. Nat. Mater. 2018;17(8):732–739. doi: 10.1038/s41563-018-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Beatty R., Lu C.E., et al. The foreign body response to an implantable therapeutic reservoir in a diabetic rodent model. Tissue Eng. Part C Methods. 2021;27(10):515–528. doi: 10.1089/ten.TEC.2021.0163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.