Abstract
Primary ovarian insufficiency (POI) is a clinical syndrome of ovarian dysfunction characterized by premature exhaustion of primordial follicles. POI causes infertility, severe daily life disturbances and long-term health risks. However, the underlying mechanism remains largely unknown. We previously identified a Basonuclin 1 (BNC1) mutation from a large Chinese POI pedigree and found that mice with targeted Bnc1 mutation exhibit symptoms of POI. In this study, we found that BNC1 plays key roles in ovarian reserve and maintaining lipid metabolism and redox homeostasis in oocytes during follicle development. Deficiency of BNC1 results in premature follicular activation and excessive follicular atresia. Mechanistically, BNC1 deficiency triggers oocyte ferroptosis via the NF2-YAP pathway. We demonstrated that pharmacologic inhibition of YAP signaling or ferroptosis significantly rescues Bnc1 mutation-induced POI. These findings uncover a pathologic mechanism of POI based on BNC1 deficiency and suggest YAP and ferroptosis inhibitors as potential therapeutic targets for POI.
Subject terms: Genetics research, Infertility, Oogenesis, Apoptosis
Primary ovarian insufficiency (POI) is a clinical syndrome of ovarian dysfunction that results in infertility. Here they show that BCN1 mutation results in premature ovarian follicle activation and atresia through dysregulation of ferroptosis.
Introduction
Primary ovarian insufficiency (POI) is a clinical syndrome defined as premature exhaustion of the resting pool of primordial follicles before the age of 40 years and characterized by oligo-/amenorrhea for at least 4 months with elevated gonadotrophins (FSH level >25 IU/l on two occasions over 4 weeks apart). Approximately 1% of women under 40 years of age are affected1,2. POI causes severe daily life disturbances in these patients, including hot flashes, sweating, sleep disorders, vaginal dryness, depression, and compromised mental health3. Another major concern related to POI is the reproductive health impairment and even infertility among women of reproductive age. Potential etiologies for POI can be categorized as genetic, autoimmune, or iatrogenic. Although the immediate contributions of POI are unknown in most cases, this disorder generally arises from defects in primordial follicle pool formation, follicular recruitment/maturation, or follicular atresia4–6.
Past studies have focused on genes involved in POI pathogenesis. To date, genetic factors have been found to be associated with approximately 7% of POI cases7. Candidate gene screening has indicated that BMP15, GDF9, NR5A1, FMR1, FIGLA, and PTEN are closely related to POI. Defects in follicle recruitment and maturation have been studied in the context of POI. A lack of phosphatase and tensin homolog (PTEN) in oocytes can result in excessively activated primordial follicles and a subsequent decrease in the size of the primordial follicle pool8,9, while a lack of NOBOX accelerates postnatal oocyte loss and abolishes the transition from primordial to growing follicles10. However, the mechanism of follicular depletion is not well characterized. In our previous study, we found a large Chinese POI pedigree in which a heterozygous 5 bp deletion was identified in patients. The deletion led to frameshift mutation of BNC1, a premature TGA stop codon, loss of the nuclear localization signal (NLS) domain and three pairs of zinc finger (ZF) domains11. Basonuclin 1 (BNC1), a cell-type-specific transcription factor, is mainly expressed in the human basal keratinocytes of stratified epithelia, human testes, reproductive germ cells of mouse testes and ovaries and mouse one-cell embryos12–14. It has been found that knockdown of BNC1 inhibits oocyte development. In addition, targeted Bnc1 truncation mutation causes the phenotype of POI in mice11. We hypothesize that BNC1 deficiency may induce POI by perturbing oocyte development and thereby causing follicular atresia.
Oocyte death is closely connected with POI. Previous studies have revealed that apoptosis serves as a major type of cell death in oocyte loss. Primordial oocytes are most likely activated and then undergo apoptosis after cyclophosphamide injection, and their apoptosis can be inhibited by checkpoint 2 inhibitor treatment15. Severe DNA damage induces oocyte death upon TAp63α activation16,17. This evidence highlights the significance of oocyte death in the pathogenesis of POI.
Ferroptosis is a form of iron- and reactive oxygen species (ROS)-dependent regulated cell death. Lipid peroxidation products and lethal ROS have been found to accumulate during ferroptosis18. Recently, ferroptosis has become a popular research topic in the contexts of a variety of diseases, especially cancer14,19. Several recent findings reveal that cell density can affect the sensitivity of cells to ferroptosis via the Merlin (NF2)-YAP-Hippo pathway20–22. NF2 and/or LATS2 mutations lead to increased nuclear localization of YAP and promote proliferation and migration of cancer cells23,24. Some studies have reported that Hippo-YAP1 regulates primordial follicular activation25,26. However, activation of YAP can promote ferroptosis by upregulating several ferroptosis modulators, including ACSL4 and TFRC, which sensitize cancer cells to ferroptosis20,27,28. In patients with polycystic ovary syndrome (PCOS), iron-mediated mitophagy has been found to promote ferroptosis by activating TFRC/PINK1 signaling29. In addition, ovarian single-cell RNA sequencing has revealed an association between oocyte loss and ferroptosis around the perinatal period30. However, the role of ferroptosis in POI remains unknown.
In this work, we find that BNC1 is involved in lipid metabolism and maintaining redox homeostasis in oocytes. Dysfunction of BNC1 activates ferroptosis through the NF2-Hippo pathway in oocytes and ultimately leads to POI.
Results
Bnc1 truncation mutation induces excessive activation and atresia of follicles
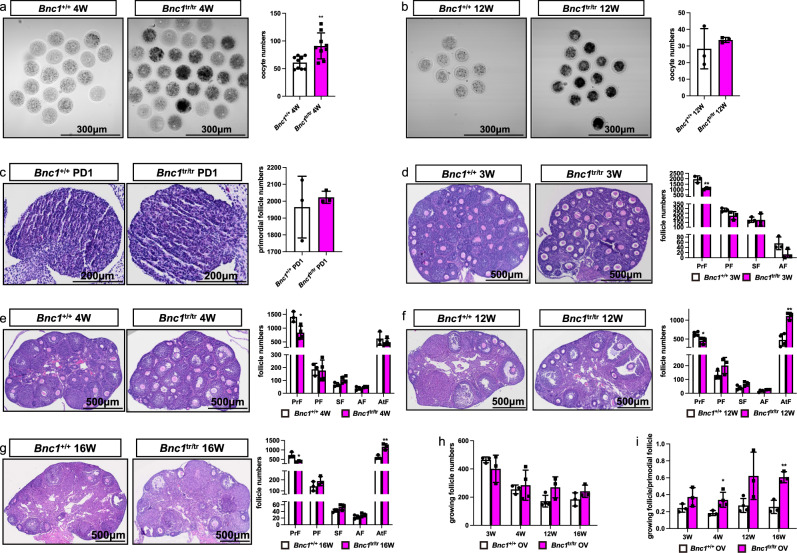
To determine the effect of Bnc1 frameshift mutation on folliculogenesis, germinal vesicle (GV) oocytes were collected from 4- and 12-week-old mice superovulated with 10 IU of pregnant mare serum gonadotropin (PMSG). The dynamic changes in folliculogenesis in Bnc1tr/tr mice (homozygous mutations) were analyzed and compared with those in wild-type (WT) Bnc1+/+ mice (Fig. 1a–b). We found an increase in oocyte retrieval in 4-week-old Bnc1tr/tr mice compared to WT mice (Fig. 1a). However, the ovarian response of the Bnc1tr/tr mice declined with increasing age (Fig. 1b). We hypothesized that the ovarian reserve of the Bnc1tr/tr mice also declined with age. To test this hypothesis, we compared follicle numbers at postnatal day 1 (PD1) and weeks 3, 4, 12, and 16 in Bnc1tr/tr and Bnc1+/+ mice. These data suggested that primordial follicle numbers in Bnc1tr/tr mice were comparable to those in Bnc1+/+ mice at PD1 (Fig. 1c). At 3 weeks of age, Bnc1tr/tr mice showed decreased numbers of primordial follicles and follicular overactivation, but apparent follicular atresia was not observed in Bnc1tr/tr mice compared with Bnc1+/+ mice (Fig. 1d). At 4 weeks of age, compared with Bnc1+/+ mice, Bnc1tr/tr mice also showed decreased numbers of primordial follicles and follicular overactivation (Fig. 1e). The phenotypes of 12-week-old and 16-week-old Bnc1tr/tr mice were similar to those of 4-week-old mice. However, the numbers of atretic follicles increased significantly, while the primordial follicle depletion rate declined at 12 and 16 weeks of age (Fig. 1f, g). Overall, these data showed that follicles were overactivated (Fig. 1h–i) and that atretic follicle numbers increased with age in the mouse POI model with Bnc1 truncation mutation (Fig. 1e–g).
Fig. 1. Bnc1 mutation affects follicle development.
a GV oocytes from Bnc1+/+ (n = 9) and Bnc1tr/tr (n = 9) mice at 4 weeks old (p value = 0.0032). b GV oocytes from Bnc1+/+ (n = 3) and Bnc1tr/tr (n = 3) mice at 12 weeks old (p value=0.4909). c Comparison of the numbers of primordial follicles (PrFs) of mice (n = 3) at PD1 (p value = 0.6174). d Comparison of the numbers of PrFs, primary follicles (PFs), secondary follicles (SFs) and antral follicles (AFs) of mice (n = 3) at 3 weeks old (p value = 0.0093 for PrFs, p value = 0.0903 for PFs, p value = 0.9641 for SFs, p value = 0.0804 for AFs). e Comparison of the follicle numbers of mice (n = 3) at 4 weeks old (p value = 0.0183 for PrFs, p value = 0.8694 for PFs, p value = 0.0769 for SFs, p value = 0.1996 for AFs, p value = 0.3926 for AtFs). f Comparison of the follicle numbers of mice (n = 4 for Bnc1+/+, n = 3 for Bnc1tr/tr) at 12 weeks old (p value = 0.0211 for PrFs, p value = 0.0910 for PFs, p value = 0.0560 for SFs, p value = 0.0571 for Afs, p value = 0.0021 for AtFs). g Comparison of the follicle numbers of mice (n = 3) at 16 weeks old (p value = 0.01810 for PrFs, p value = 0.2460 for PFs, p value = 0.1346 for SFs, p value = 0.2720 for AFs, p value = 0.0077 for AtFs). Scale bar = 500 μm. h Comparison of the growing follicle numbers of mice (n = 3) at 3, 4, 12, and 16 weeks old (p value = 0.3265 for 3 weeks old, p value = 0.6693 for 4 weeks old, p value = 0.0750 for 12 weeks old, p value = 0.1923 for 16 weeks old). i Comparison of the ratios of growing follicle numbers to PrFs of mice (n ≥ 3) at 3, 4, 12, and 16 weeks old (p value = 0.1352 for 3 weeks old, p value = 0.0341 for 4 weeks old, p value = 0.0592 for 12 weeks old, p value = 0.0040 for 16 weeks old). The error bars indicate the mean values ± SDs, unpaired t test, two-tailed, *p value < 0.05, **p value < 0.01 and ***p value < 0.001. Source data are provided as a Source Data file.
Conditional knockout of Bnc1 in oocytes induces POI through excessive activation and atresia of follicles
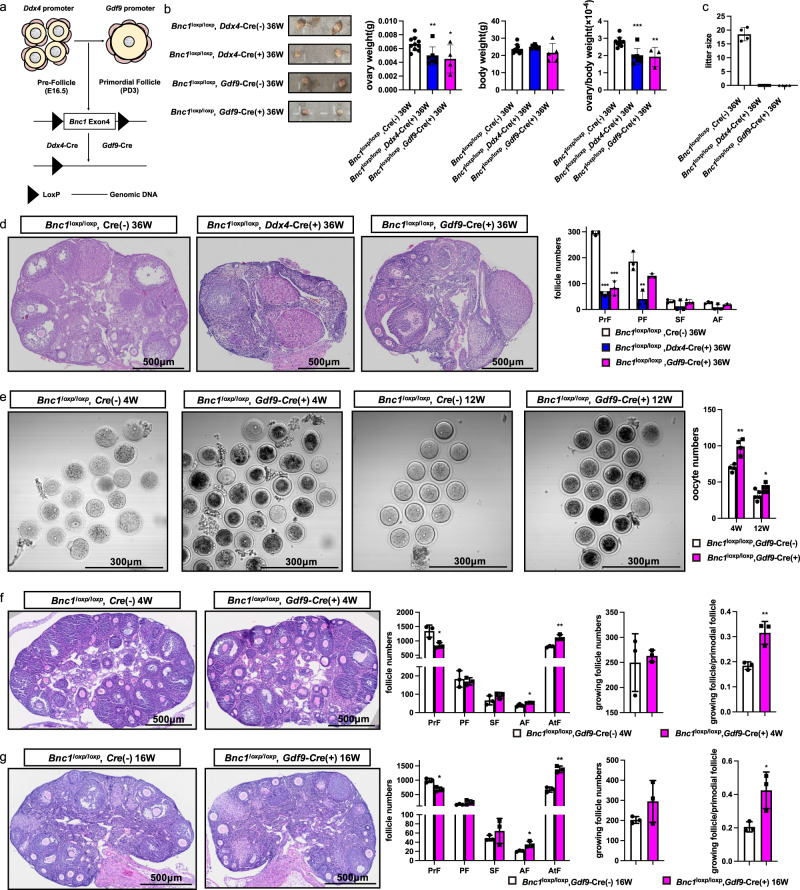
To examine the role of Bnc1 in oocytes, we deleted the Bnc1 gene from mouse oocytes by crossing Bnc1loxP/loxP mice with transgenic mice expressing growth differentiation factor 9 (Gdf-9) promoter-mediated Cre recombinase (referred to as Gdf9-Cre mice) and DEAD-box helicase 4 (Ddx4) promoter-mediated Cre recombinase (referred to as Ddx4-Cre mice) (Fig. 2a). According to the ovary weight/body weight ratio (i.e., the relative ovary weight) and ovary size, the ovaries of Bnc1loxP/loxP, Ddx4-Cre (+) and Bnc1loxP/loxP, Gdf9-Cre (+) mice were significantly smaller than those of WT mice at 36 weeks (Fig. 2b). Bnc1loxP/loxP, Ddx4-Cre (+) and Bnc1loxP/loxP, Gdf9-Cre (+) female mice were infertile (Fig. 2c). Histological and morphometric analyses revealed decreased follicle numbers in ovaries of Bnc1loxP/loxP, Ddx4-Cre (+) and Bnc1loxP/loxP, Gdf9-Cre (+) female mice compared with the ovaries of WT mice (Fig. 2d). This phenotype suggested that BNC1 played a role in the oocytes and functioned at the primordial follicle stage. Loss of BNC1 function could induce POI. GV oocytes were collected from 4- and 12-week-old mice superovulated with 10 IU of PMSG, which revealed dynamic changes in folliculogenesis in Bnc1loxP/loxP, Gdf9-Cre (+) mice compared with WT mice, and the ovarian response was consistent with that in Bnc1tr/tr mice (Fig. 2e). We then counted follicles at 4 and 16 weeks in Bnc1loxP/loxP, Gdf9-Cre (−) and Bnc1loxP/loxP, Gdf9-Cre (+) mice. In comparison with Bnc1loxP/loxP, Cre (−) mice, Bnc1loxP/loxP, Gdf9-Cre (+) mice showed decreased numbers of primordial follicles, follicular overactivation and apparent follicular atresia at 4 and 16 weeks (Fig. 2f, g). Overall, these data showed that follicles were overactivated and that the numbers of atretic follicles were increased in oocyte-specific Bnc1-knockout mice.
Fig. 2. Bnc1 affects follicle development by influencing oocytes.
a Schematic representation of the deletion of Bnc1 exon 4 in oocytes of pre-follicles, PrFs by using Ddx4 and Gdf9-Cre transgenic. b Ovary weight, body weight and ovary weight/body weight ratios of Bnc1loxP/loxP, Cre (−) (n = 9), Bnc1loxP/loxP, Ddx4-Cre (+) (n = 7, p value = 0.0069 for ovary weight, p value = 0.2647 for body weight, p value = 0.0003 for ovary weight/body weight) and Bnc1loxP/loxP, Gdf9-Cre (+) (≥3, p value = 0.0179 for ovary weight, p value = 0.2835 for body weight, p value = 0.0029 for ovary weight/body weight) mice at 36 weeks. c Average litter sizes of Bnc1loxP/loxP, Cre (−) (n = 4), Bnc1loxP/loxP, Ddx4-Cre (+) (n = 5, p value < 0.0001) and Bnc1loxP/loxP, Gdf9-Cre (+) (n = 4, p value < 0.0001) female mice. d Comparison of follicle numbers of Bnc1loxP/loxP, Cre (−) (n = 3), Bnc1loxP/loxP, Ddx4-Cre (+) (n = 3, p value < 0.0001 for PrFs, p value = 0.0060 for PFs, p value = 0.2081 for SFs and p value = 0.0667 for AFs) and Bnc1loxP/loxP, Gdf9-Cre (+) (n = 3, p value = 0.0002 for PrFs, p value = 0.0620 for PFs, p value = 0.7357 for SFs and p value = 0.1135 for AFs) mice at 36 weeks. Scale bar = 500 μm. e GV oocytes obtained from Bnc1loxP/loxP, Cre (−) and Bnc1loxP/loxP, Gdf9-Cre (+) mice at 4 (n = 4) and 12 (n = 4) weeks old (p value = 0.0022 for 4 weeks old, p value = 0.0398 for 12 weeks old). f Comparison of follicle numbers of Bnc1loxP/loxP, Cre (−) and Bnc1loxP/loxP, Gdf9-Cre (+) mice at 4 weeks (n = 3, p value = 0.7145 for growing follicles, p value = 0.0091 for growing follicles/PrFs, p value = 0.0206 for PrFs, p value = 0.6640 for PFs, p value = 0.1959 for SFs, p value = 0.03219 for AFs and p value = 0.0065 for AtFs). g Comparison of follicle numbers of Bnc1loxP/loxP, Cre (−) and Bnc1loxP/loxP, Gdf9-Cre (+) mice at 16 weeks (n = 3, p value = 0.2046 for growing follicles, p value = 0.0287 for growing follicles/PrFs, p value = 0.0074 for PrFs, p value = 0.1718 for PFs, p value = 0.3858 for SFs, p value = 0.0219 for AFs and p value = 0.0011 for AtFs). Scale bar = 500 μm. The error bars indicate the mean values ± SDs, unpaired t test, two-tailed, *p value < 0.05, **p value < 0.01 and ***p value < 0.001. Source data are provided as a Source Data file.
BNC1 deficiency in oocytes leads to follicular atresia through nonapoptotic cell death
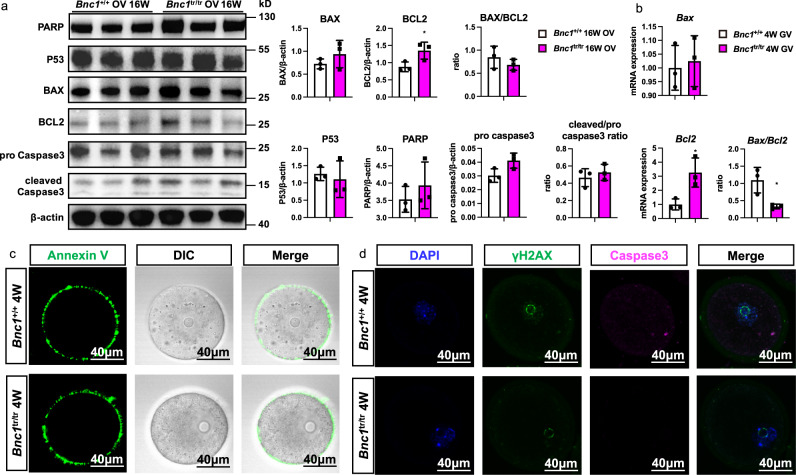
Since apoptosis is involved in follicular atresia, we determined whether apoptosis contributed to ovary follicular atresia in the ovaries of Bnc1+/+ and Bnc1tr/tr mice. However, the western blot analysis showed that the levels of apoptotic markers did not differ between Bnc1+/+ and Bnc1tr/tr mouse ovaries (OV) at either 16 weeks (Fig. 3a) or 36 weeks (Supplementary Fig.1b). In addition, we assessed whether apoptosis occurred in oocytes. However, the results showed that Bnc1 mutation and knockout in oocytes could induce antiapoptotic effects to some extent (Fig. 3b and Supplementary Fig. 1c). Annexin V staining also showed that there was no difference in early-stage apoptosis in Bnc1tr/tr and Bnc1loxP/loxP, Gdf9-Cre (+) mouse oocytes compared with WT mouse oocytes (Fig. 3c and Supplementary Fig. 1d). Similarly, the DNA damage marker γH2AX and apoptosis marker caspase3 did not differ between the Bnc1+/+ and Bnc1tr/tr mouse oocytes and the Bnc1loxP/loxP, Gdf9-Cre (−) and Bnc1loxP/loxP, Gdf9-Cre (+) mouse oocytes (Fig. 3d and Supplementary Fig. 1e). These results suggested that the process of follicular atresia in Bnc1tr/tr and Bnc1loxP/loxP, Gdf9-Cre (+) mice was mediated not by apoptosis but rather by a nonapoptotic cell death pathway.
Fig. 3. Bnc1 mutation induces follicular atresia through nonapoptotic cell death.
a Ovaries were obtained from Bnc1+/+ (n = 3) and Bnc1tr/tr mice (n = 3) at 16 weeks old for western blotting (WB). The expression levels of P53, Bcl2, Bax, PARP, Caspase3 and cleaved-caspase3 are shown (p value = 0.6585 for P53, p value = 0.0457 for Bcl2, p value = 0.3090 for Bax, p value = 0.3467 for BAX/BCL2, p value = 0.0624 for Caspase3, p value = 0.4892 for cleaved-Caspase3/Caspase3 and p value = 0.4162 for PARP). b GV oocytes were obtained from Bnc1+/+ (n = 3) and Bnc1tr/tr (n = 3) mice at 4 weeks old for real-time PCR. The mRNA expression of Bax and Bcl2 is shown (p value = 0.7446 for Bax, p value = 0.0241 for Bcl2 and p value = 0.0247 for Bax/Bcl2). c GV oocytes obtained from Bnc1+/+ and Bnc1tr/tr mice at 4 weeks old were used for detection of early apoptosis (3 independent experiments with total oocyte numbers >30 oocytes). d GV oocytes obtained from Bnc1+/+ and Bnc1tr/tr mice at 4 weeks old were used for detection of Caspase3 (CAS3) and the DNA damage marker γ-H2AX (3 independent experiments with total oocyte numbers >30 oocytes). The error bars indicate the mean values ± SDs, unpaired t test, two-tailed, *p value < 0.05, **p value < 0.01 and ***p value < 0.001. Source data are provided as a Source Data file.
Abnormal lipid metabolism and ferroptosis are involved in follicular atresia
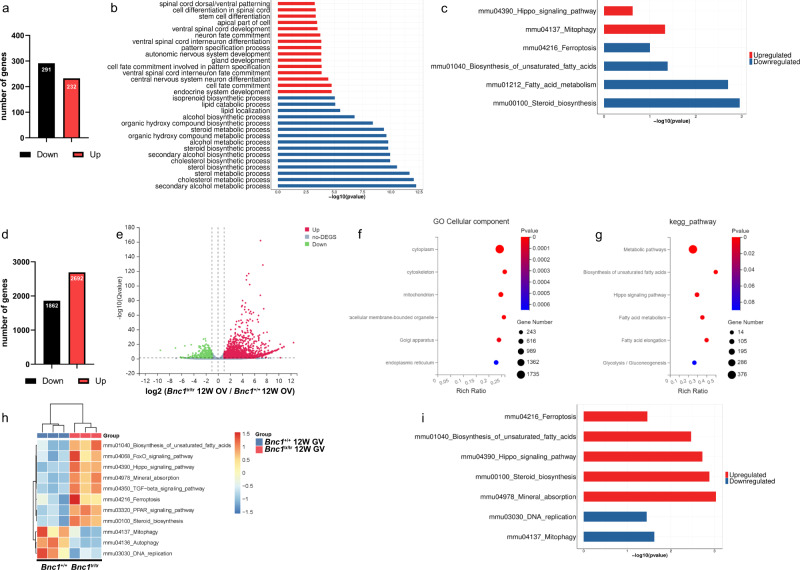
To uncover the molecular mechanism by which Bnc1 mutation led to follicular atresia, we conducted RNA sequencing (RNA-seq) of ovary tissues. There were 523 differentially expressed genes (DEGs) between the Bnc1+/+ and Bnc1tr/tr mouse ovaries (Fig. 4a). Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis and Gene set variation analysis (GSVA) showed disrupted lipid metabolism in the ovaries of Bnc1tr/tr mice compared with those of WT mice (Fig. 4b, c). Moreover, the results of oocyte Smart-seq (with a switching mechanism at the 5ʹ end of the RNA transcript) were consistent with those of RNA-seq. There were 4554 DEGs between the Bnc1+/+ and Bnc1tr/tr mouse oocytes (Fig. 4d, e). Gene Ontology (GO) analysis and KEGG enrichment analysis suggested the involvement of abnormal mitochondrial function and impaired lipid metabolism (Fig. 4f, g). Gene set variation analysis (GSVA) of the Smart-seq transcriptome data revealed several important pathways involved in follicular atresia in Bnc1tr/tr mice, including the Hippo pathway and ferroptosis (Fig. 4h, i). Since mitochondria-mediated lipid peroxidation is crucial in ferroptosis31,32 and the Hippo pathway promotes ferroptosis20,33, these findings indicated that ferroptosis might be involved in the process of Bnc1 mutation leading to POI.
Fig. 4. Bnc1 mutation may induce follicular atresia through ferroptosis.
a DEGs of Bnc1+/+ (n = 3) and Bnc1tr/tr (n = 3) mouse ovaries. b KEGG analysis of Bnc1+/+ and Bnc1tr/tr mouse ovary RNA-seq data (Top significantly (FDR < 0.05, Benjamini–Hochberg p adjustment)). c GSVA of Bnc1+/+ and Bnc1tr/tr mouse ovary RNA-seq data (Wilcox test p value). d, e DEGs of Bnc1+/+ (n = 3) and Bnc1tr/tr (n = 3) mouse GV oocytes through Smart-seq2 (significantly (FDR < 0.05) up- and downregulated genes (|log2FC| > 0.5)). f, g GO and KEGG analyses of Bnc1+/+ and Bnc1tr/tr mouse GV oocytes (Top significantly (P value < 0.05)). h, i GSVA of Bnc1+/+ and Bnc1tr/tr mouse GV oocytes (Wilcox test p value). Three biological replicates per group were analyzed in duplicate.
BNC1 deficiency-induced follicular atresia is modulated by ferroptosis
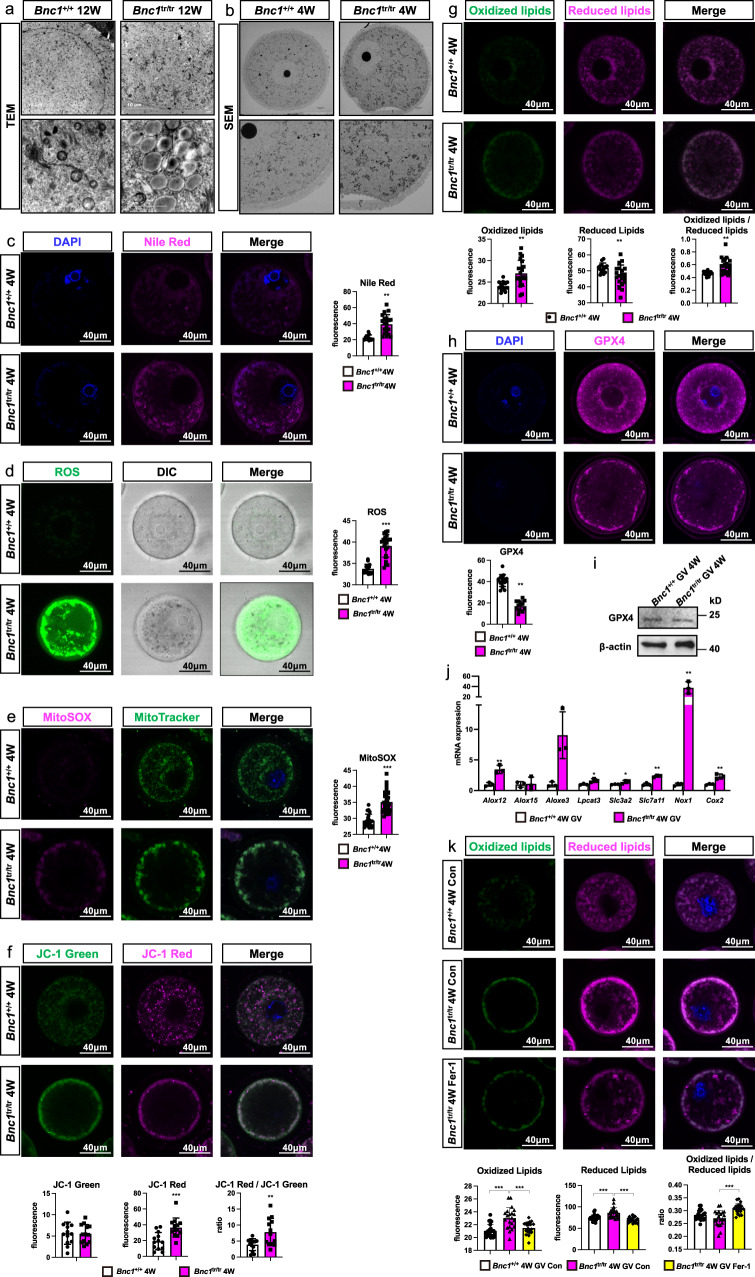
To address whether BNC1 deficiency triggers ferroptosis in oocytes, we assessed well-characterized morphological markers of ferroptosis in Bnc1-mutant mice. We performed transmission electron microscopy (TEM) of mouse ovaries and scanning electron microscopy (SEM) of mouse GV oocytes. We found that the Bnc1 truncation mutation induced excessive lipid accumulation (Fig. 5a). In Bnc1tr/tr mouse oocytes, abnormal mitochondrial distribution with abnormal aggregation was observed; an increased density of the mitochondrial membrane was also observed (Fig. 5b). Nile red staining of GV oocytes and mouse ovaries also confirmed that lipids accumulated in oocytes after Bnc1 mutation and knockout (Fig. 5c, Supplementary Fig. 2a and Supplementary Fig. 3a, b). Furthermore, we examined mitochondrial function and the levels of oxidative stress markers, including ROS, MitoSOX, MitoTracker and JC-1. We found that ROS and MitoSOX levels were significantly increased in both Bnc1tr/tr mouse oocytes and Bnc1loxP/loxP, Gdf9-Cre (+) mouse oocytes compared with WT mouse oocytes (Fig. 5d, e and Supplementary Fig. 3c, d). In addition, defects in mitochondrial distribution, with mitochondria localized beneath the cell membrane, were found in oocytes from Bnc1tr/tr mice and Bnc1loxP/loxP, Gdf9-Cre (+) mice (Fig. 5e and Supplementary Fig. 3d). JC-1 staining showed that mitochondrial membrane potential was significantly increased in Bnc1tr/tr and Bnc1loxP/loxP, Gdf9-Cre (+) mouse oocytes (Fig. 5f and Supplementary Fig. 3e), further suggesting that BNC1 deficiency was associated with ferroptosis32. Oocytes from Bnc1tr/tr and Bnc1loxP/loxP, Gdf9-Cre (+) mice also exhibited significantly increased lipid ROS production (Fig. 5g and Supplementary Fig. 3f), a key driver in ferroptosis2. In addition, the decreased expression level of glutathione peroxidase 4 (GPX4) in Bnc1tr/tr and Bnc1loxP/loxP, Gdf9-Cre (+) mouse oocytes further suggested that the Bnc1 mutation and knockout sensitized oocytes to ferroptosis (Fig. 5h and Supplementary Fig. 3g). The level of GPX4 was also decreased in both the ovaries and oocytes from Bnc1tr/tr mice (Fig. 5i, Supplementary Fig. 2b and Supplementary Fig. 3h). Nox1 and Cox2 were highly expressed (Fig. 5j and Supplementary Fig. 3i), which suggested the existence of dysregulated oxidative stress in Bnc1tr/tr mouse ovaries and oocytes. The expression of ferroptosis marker genes, such as Alox12, Aloxe3, Lpcat3, Slc3a2, Slc7a11, was significantly abnormal in oocytes from Bnc1tr/tr and Bnc1loxP/loxP, Gdf9-Cre (+) mice (Fig. 5j and Supplementary Fig. 3i). Furthermore, the ferroptosis agonist RLS3 significantly enhanced lipid peroxidation in Bnc1tr/tr and Bnc1loxP/loxP, Gdf9-Cre (+) mouse oocytes (Supplementary Fig. 2c and Supplementary Fig. 3j). In addition, the ferroptosis antagonist ferrostatin-1 (Fer-1) partially reversed BNC1 deficiency-induced lipid peroxidation in Bnc1tr/tr and Bnc1loxP/loxP, Gdf9-Cre (+) mouse oocytes (Fig. 5k and Supplementary Fig. 3k). Collectively, these results suggested that Bnc1 mutation and knockout in oocytes induced ferroptosis.
Fig. 5. Oocytes affected by Bnc1 mutation are more sensitive to ferroptosis.
a TEM of mouse ovaries (n = 3). b SEM of GV oocyte (n = 1). c Nile red of GV oocytes (p value < 0.0001). d ROS in GV oocytes (p value < 0.0001). e MitoSOX and MitoTracker in oocytes (p value < 0.0001). f JC-1of GV oocytes (p value = 0.9010 for JC-1 green, p value = 0.0009 for JC-1 red and p value = 0.0055 for JC-1 red/green). g Lipid ROS in GV oocytes (p value = 0.0020 for oxidized lipids, p value = 0.0206 for reduced lipids and p value = 0.0013 for oxidized/reduced lipids). h GPX4 in GV oocytes (p value < 0.0001). i WB of GPX4 in GV oocytes (3 independent experiments). j RT–PCR of ferroptosis-associated markers in GV oocytes (n = 3) (p value = 0.0048 for Alox12, p value = 0.8962 for Aloxe15, p value = 0.0221 for Aloxe3, p value = 0.0307 for Lpcat3, p value = 0.0498 for Slc3a2 and p value = 0.0010 for Slc7a11, p value = 0.0074 for Cox2 and p value = 0.0055 for Nox1). k Lipid ROS in GV oocytes after Fer-1 treatment (for oxidized lipids p value < 0.0001 for Bnc1tr/tr Con, p value = 0.0007, for Bnc1tr/tr Fer-1, for reduced lipids p value = 0.0002 for Bnc1tr/tr Con, p value < 0.0001, for Bnc1tr/tr Fer-1, for oxidized to reduced lipids ratio p value = 0.0923 for Bnc1tr/tr Con and p value < 0.0001 for Bnc1tr/tr Fer-1). The error bars indicate the mean values ± SDs, unpaired t test, two-tailed, 3 independent experiments with total oocyte numbers >30 oocytes, *p value < 0.05, **p value < 0.01 and ***p value < 0.001. Source data are provided as a Source Data file.
BNC1 deficiency triggers oocyte ferroptosis via the NF2-YAP pathway
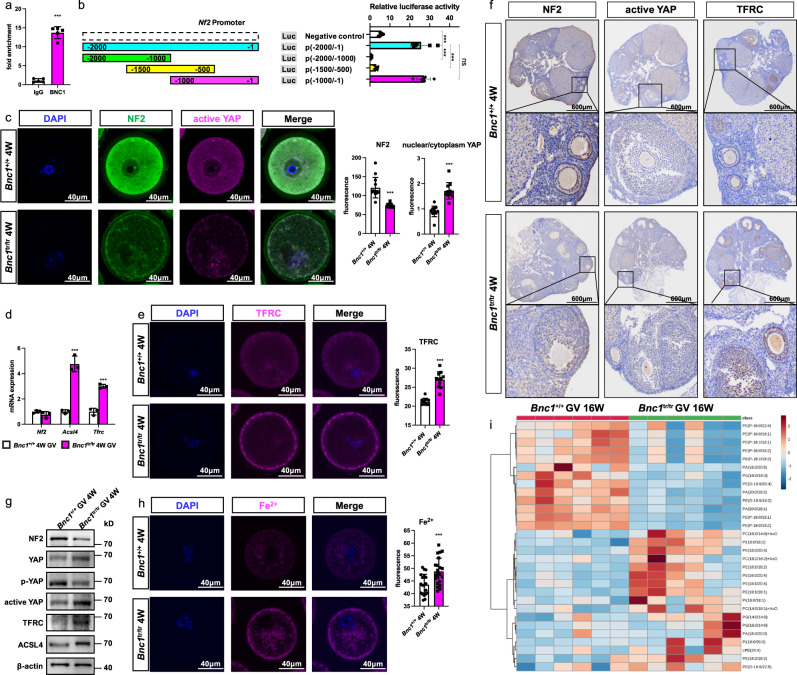
To study the mechanism underlying the regulation of ferroptosis induced by BNC1 deficiency, we performed chromatin immunoprecipitation (ChIP) with sequencing (ChIP-seq). We found that NF2 might be a target gene of BNC1 (Supplementary Fig. 4). Knockdown of NF2 and activation of the Hippo pathway have been found to promote ferroptosis20,22. Thus, we determined whether BNC1 deficiency induced ferroptosis through the regulation of NF2 in oocytes. ChIP–qPCR (Fig. 6a) and luciferase activity (Fig. 6b) assays confirmed the binding of BNC1 to the promoter region of Nf2. Furthermore, a promoter deletion assay identified a fragment (bp −1 to −500) as the region required for BNC1 regulation of Nf2 (Fig. 6b). Then, we examined the expression levels of NF2, active YAP, and transferrin receptor (TFRC) through immunofluorescence in oocytes and immunohistochemistry (IHC) in ovaries. We found that NF2 expression was significantly decreased in oocytes of Bnc1tr/tr and Bnc1loxP/loxP, Gdf9-Cre (+) mice compared with those of WT mice (Fig. 6c, f and Supplementary Fig. 5a). Consistently, the nuclear localization of YAP and the expression of the downstream genes TFRC and ACSL4 were significantly increased in the oocytes of Bnc1tr/tr and Bnc1loxP/loxP, Gdf9-Cre (+) mice (Fig. 6c, e–g and Supplementary Fig. 5a, b, d). The mRNA expression of Nf2, Tfrc, Acsl4 and the protein expression of NF2, active YAP, p-YAP, YAP, ACSL4 and TFRC in oocytes from Bnc1tr/tr and Bnc1loxP/loxP, Gdf9-Cre (+) mice showed the same trends as the above results (Fig. 6d, g and Supplementary Fig. 5c-d). TFRC has been identified as a key iron transporter on the plasma membrane34. Given the role of Fe2+ in lipid peroxidation and ferroptosis, we sought to determine the level of Fe2+ using the Fe2+-selective fluorescent probe FerroOrange. Confocal imaging revealed that FerroOrange intensity was significantly greater in oocytes of Bnc1tr/tr mice than in oocytes of WT mice (Fig. 6h). Excessive iron-dependent peroxidation of polyunsaturated fatty acid (PUFA)-containing phospholipids (PUFA-PLs) induces ferroptosis35–37. ACSL4 is a key enzyme involved in PUFA-PL biosynthesis. Targeted lipidomics showed that phosphatidylethanolamine (PE) (18:1/18:2), PE (16:0/20:4), PE (18:2/18:2), PE (18:1/20:4), phosphatidylserine (PS) (14:0/20:4), and phosphatidylinositol (PI) (18:0/18:2) levels were higher in Bnc1tr/tr mice than in WT mice (Fig. 6i and Supplementary Fig. 6a–f), suggesting that the levels of oocyte lipid substrates for peroxidation were increased after Bnc1 mutation. In addition, increased lyso-phosphatidylethanolamine (LPE) (20:4) levels and decreased phosphatidic acid (PA) (20:0/18:1) levels were observed (Supplementary Fig. 6g, h); these molecules are related to ferroptosis36,38. These results suggested that BNC1 targeted NF2 to mediate the Hippo-YAP pathway, which was involved in the regulation of ferroptosis.
Fig. 6. Bnc1 truncation mutation sensitizes oocytes to ferroptosis by regulating NF2-YAP signaling.
a ChIP–qPCR for BNC1 at Nf2 promoter using mouse ovaries (n = 5, p value < 0.0001, 3 independent experiments). b Dual luciferase reporter assay of Bnc1 at the Nf2 promoter (n = 6, the error bars indicate the mean values ± SDs, unpaired t test, two-tailed, p value < 0.0001, 3 independent experiments). c NF2 and active YAP in GV oocytes at 4 weeks old (the error bars indicate the mean values ± SDs, unpaired t test, two-tailed, p value < 0.0001 for NF2, p value < 0.0001 for nuclear/cytoplasmic YAP, 3 independent experiments with total oocyte numbers >30 oocytes). d RT–PCR of Nf2, Tfrc and Acsl4 in GV oocytes (n = 3) at 4 weeks old (p value = 0.3555 for Nf2, p value < 0.0001 for Tfrc and p value < 0.0001 for Acsl4). e TFRC of GV oocytes at 4 weeks old (the error bars indicate the mean values ± SDs, unpaired t test, two-tailed, p value < 0.0001, 3 independent experiments with total oocyte numbers >30 oocytes). f IHC of NF2, active YAP and TFRC in mouse ovaries at 8 weeks old (n = 3). g WB of NF2, p-YAP, active YAP, YAP, ACSL4 and TFRC in GV oocytes at 4 weeks old (3 independent experiments). h Fe2+ in GV oocytes at 4 weeks old (p value = 0.0010, the error bars indicate the mean values ± SDs, unpaired t test, two-tailed, p value < 0.0001, 3 independent experiments with total oocyte numbers >30 oocytes). i Heatmap of targeted lipidomics of Bnc1+/+ and Bnc1tr/tr GV oocytes(n = 6). Source data are provided as a Source Data file.
Targeting NF2-Hippo/YAP signaling rescues Bnc1 mutation-induced ferroptosis
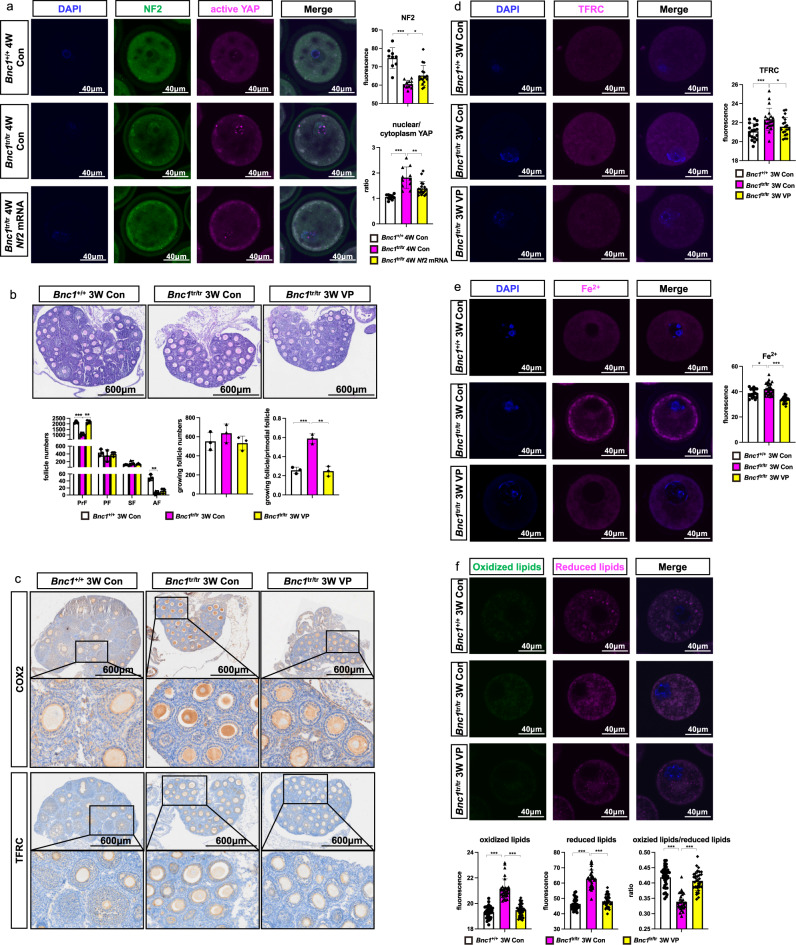
Since Bnc1 mutation induced ferroptosis through NF2/YAP signaling, we determined whether targeting NF2/YAP could reverse BNC1 deficiency induced ferroptosis. To further study the regulation of Nf2 by BNC1, we microinjected Nf2 mRNA into GV oocytes and cultured them for 24 h in vitro. Then, we examined the expression levels of NF2 and active-YAP through immunofluorescence in oocytes. We found that NF2 expression in oocytes of Bnc1tr/tr mice was significantly increased after Nf2 mRNA supplementation (Fig. 7a). Consistently, the level of YAP in nucleus was significantly decreased after Nf2 mRNA injection (Fig. 7a). Next, we examined the effect of targeting YAP by its specific inhibitor verteporfin (VP). Mice were injected with either VP (100 mg/kg) or vehicle (corn oil) from the postnatal 7 days at 3-day intervals for 14 days. Then, we collected the ovaries at 3 weeks for follicle number counting. Compared with Bnc1tr/tr mice, Bnc1tr/tr mice injected with VP showed increased numbers of primordial follicles and decreased follicular overactivation, these results were comparable to those observed in Bnc1+/+ mice (Fig. 7b). The immunohistochemical assay showed that the expression of TFRC and COX2 was higher in Bnc1tr/tr control mouse ovaries than in Bnc1+/+ control mouse ovaries (Fig. 7c). Intriguingly, VP treatment partially rescued TFRC and COX2 expression in Bnc1tr/tr mouse ovaries (Fig. 7c). We subsequently detected TFRC expression and Fe2+ levels in GV oocytes, and found that TFRC expression was decreased in Bnc1tr/tr mice treated with VP (Fig. 7d). Consistently, Fe2+ level was also decreased in VP-treated Bnc1tr/tr mouse oocytes (Fig. 7e). Oocytes from Bnc1tr/tr mice treated with VP exhibited significantly decreased lipid ROS level compared with those from Bnc1tr/tr control mice (Fig. 7f). In vitro, knockdown of BNC1 in ES-2 cells resulted in the down regulation of NF2, and phosphorylated YAP, and the upregulation of active-YAP, TFRC and ACSL4 (Supplementary Fig. 7a, b). These results further confirmed that BNC1 targeted the NF2 -Hippo-YAP-TFRC/ACSL4 pathway, deficiency of which induced ferroptosis.
Fig. 7. Targeting NF2-Hippo/YAP signaling pathway rescues Bnc1 mutation induced ferroptosis.
a Immunofluorescence of NF2 and active YAP in GV oocytes (p value < 0.0001 for NF2 of Bnc1tr/tr Con, p value = 0.0120 for NF2 of Bnc1tr/tr Nf2 mRNA, p value < 0.0001 for nuclear/cytoplasmic YAP of Bnc1tr/tr Con, p value = 0.0023 for nuclear/cytoplasmic YAP of Bnc1tr/tr Nf2 mRNA, 3 independent experiments with total oocyte numbers >30 oocytes). b HE staining and PrFs, PFs, SFs and AFs of Bnc1+/+ Con (n = 3), Bnc1tr/tr Con (n = 3, p value = 0.0008 for PrFs, p value = 0.4975 for PFs, p value = 0.1942 for SFs, p value = 0.0019 for AFs, p value = 0.3379 for growing follicles and p value = 0.0007 for growing follicles/PrFs) and Bnc1tr/tr VP (n = 3, p value = 0.0013 for PrFs, p value = 0.7104 for PFs, p value = 0.3955 for SFs, p value = 0.3796 for AFs, p value = 0.2134 for growing follicles and p value = 0.0012 for growing follicles/PrFs) mice at 3 weeks old. Scale bar = 600 μm. c IHC of COX2 and TFRC in Bnc1+/+ Con (n = 3), Bnc1tr/tr Con (n = 3) and Bnc1tr/tr VP (n = 3) mouse ovaries. d TFRC in mouse GV oocytes of Bnc1+/+ Con, Bnc1tr/tr Con (p value = 0.0005) and Bnc1tr/tr VP (p value = 0.0315) group at 3 weeks old. e Fe2+ concentration in Bnc1+/+ Con, Bnc1tr/tr Con (p value=0.0115) and Bnc1tr/tr VP (p value < 0.0001) mouse GV oocytes at 3 weeks old. f Lipid ROS in Bnc1+/+ Con, Bnc1tr/tr Con (p value < 0.0001 for oxidized lipids, p value < 0.0001 for reduced lipids and p value < 0.0001 for oxidized/reduced lipids) and Bnc1tr/tr VP (p value < 0.0001 for oxidized lipids, p value < 0.0001 for reduced lipids and p value < 0.0001 for oxidized/reduced lipids) mouse GV oocytes at 3 weeks old. The error bars indicate the mean values ± SDs, unpaired t test, two-tailed, 3 independent experiments with total oocyte numbers >30 oocytes. Source data are provided as a Source Data file.
Inhibition of ferroptosis alleviates Bnc1 mutation-induced POI in mice
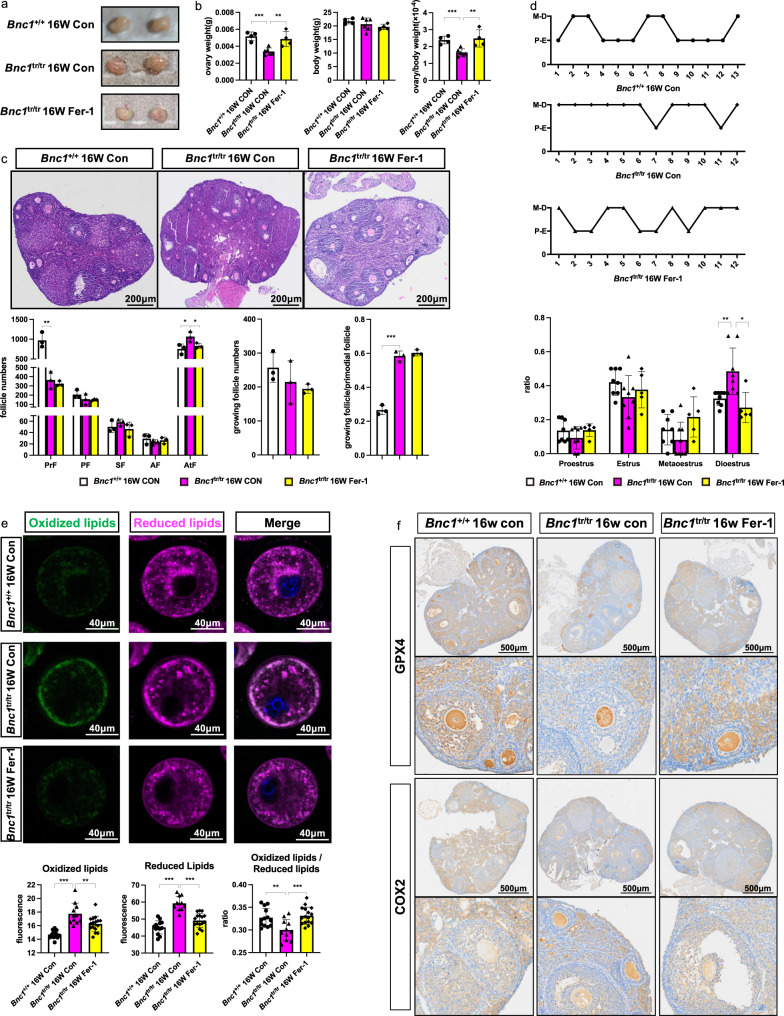
Next, we explored whether ferroptosis in Bnc1tr/tr mice directly induced POI and whether such impairment could be alleviated via the ferroptosis inhibitor Fer-1. We injected intraperitoneally (i.p.) Fer-1 into Bnc1+/+ mice and Bnc1tr/tr mice every day and injected an equal dose of DMSO as a control. The ovary weight of Bnc1tr/tr mice was lower (0.0034 ± 0.00042 g) than that of Bnc1+/+ mice (0.0052 ± 0.00051 g). Intriguingly, Fer-1 treatment significantly restored the ovary weight of Bnc1tr/tr mice to normal levels (0.0049 ± 0.00090 g) (Fig. 8a, b). Moreover, the numbers of atretic follicles in Bnc1tr/tr mice after Fer-1 treatment were comparable to those in the Bnc1+/+ control mice (Fig. 8c). In addition, on assessment of estrous cycle, Bnc1tr/tr mice exhibited disordered estrous cycle with prolonged diestrus compared with Bnc1+/+ mice, but Fer-1 treatment partially improved this disorder and significantly shortened the duration of diestrus (Fig. 8d). Furthermore, the levels of oxidized lipids and reduced lipids were significantly lower in Fer-1-treated Bnc1tr/tr mice than in Bnc1tr/tr control mice, suggesting that lipid metabolism was improved with the inhibition of ferroptosis (Fig. 8e). The immunohistochemical assay showed that GPX4 expression was significantly lower and that COX2 expression was higher in Bnc1tr/tr control mouse ovary follicles than in Bnc1+/+ mouse ovary follicles; however, Fer-1 treatment of Bnc1tr/tr mice attenuated these differences in GPX4 and COX2 expression (Fig. 8f). Therefore, these results indicate an important role of ferroptosis in POI induced by BNC1 deficiency (Fig. 9) and suggest that block of ferroptosis might be a potential treatment for POI.
Fig. 8. Fer-1 partially ameliorates the POI phenotype of the Bnc1 truncation mutation.
a Gross of Bnc1+/+ Con (n = 3), Bnc1tr/tr Con (n = 3) and Bnc1tr/tr Fer-1 (n = 3) mice at 16 weeks old. b The ovary weights, body weights and ovarian weight coefficients of Bnc1+/+ Con (n = 4), Bnc1tr/tr Con (n = 6, p value = 0.0003 for ovary weight, p value = 0.4137 for body weight and p value = 0.0009 for ovary weight/body weight) and Bnc1tr/tr Fer-1 (n = 4, p value = 0.0077 for ovary weight, p value = 0.4395 for body weight and p value = 0.0068 for ovary weight/body weight) mice at 16 weeks old. c HE staining of PrFs, PFs, SFs, AFs and AtFs of Bnc1+/+ Con (n = 3), Bnc1tr/tr Con (n = 3, p value = 0.0073 for PrFs, p value = 0.3055 for PFs, p value = 0.3015 for SFs, p value = 0.3501 for AFs, p value = 0.0382 for AtFs, p value = 0.3957 for growing follicles and p value = 0.0002 for growing follicles/PrFs) and Bnc1tr/tr Fer-1 (n = 3, p value = 0.4860 for PrFs, p value = 0.8158 for PFs, p value = 0.2028 for SFs, p value = 0.4977 for AFs, p value = 0.0471 for AtFs, p value = 0.6138 for growing follicles and p value = 0.4052 for growing follicles/PrFs) mice at 16 weeks old. Scale bar = 200 μm. d Estrous cycles of Bnc1+/+ Con (n = 8), Bnc1tr/tr Con (n = 8, p value = 0.2186 for proestrus, p value = 0.1167 for estrus, p value = 0.2377 for metestrus and p value = 0.0073 for diestrus) and Bnc1tr/tr Fer-1 (n = 5, p value = 0.1956 for proestrus, p value = 0.5312 for estrus, p value = 0.0536 for metestrus and p value = 0.0108 for diestrus) mice. e Lipid ROS in Bnc1+/+ Con, Bnc1tr/tr Con (p value < 0.0001 for oxidized lipids, p value < 0.0001 for reduced lipids and p value = 0.0052 for oxidized/reduced lipids) and Bnc1tr/tr Fer-1 (p value = 0.0083 for oxidized lipids, p value < 0.0001 for reduced lipids and p value = 0.0006 for oxidized/reduced lipids) mouse GV oocytes at 16 weeks old. 3 independent experiments with total oocyte numbers >30 oocytes). f IHC of GPX4 and COX2 in Bnc1+/+ Con (n = 3), Bnc1tr/tr Con (n = 3) and Bnc1tr/tr Fer-1 (n = 3) mouse ovaries. The error bars indicate the mean values ± SDs, unpaired t test, two-tailed, *p value < 0.05, **p value < 0.01 and ***p value < 0.001. Source data are provided as a Source Data file.
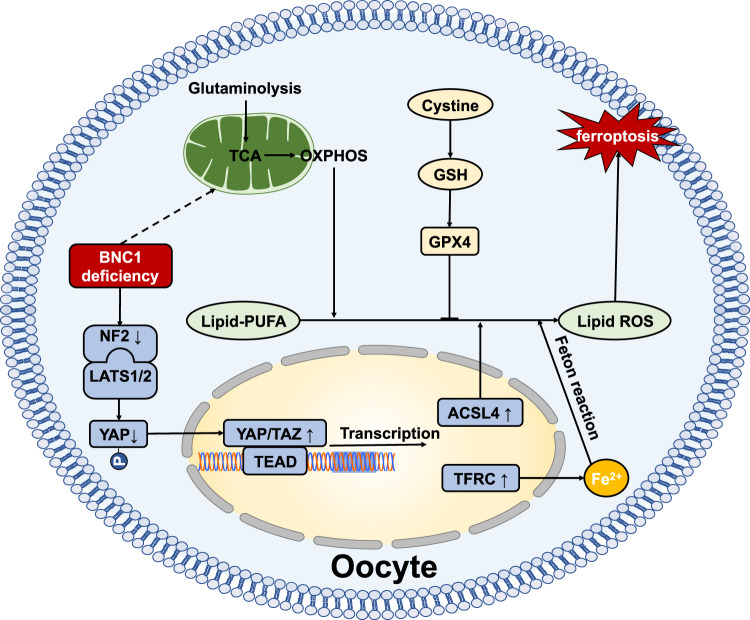
Fig. 9. BNC1-Merlin-Hippo-YAP signaling -Ferroptosis axis.
BNC1 directly regulates Nf2 expression. BNC1 deficiency downregulates NF2 expression, which reduces YAP phosphorylation and promote YAP nuclear accumulation. YAP activation upregulates Tfrc and Acsl4 expression, followed by increased iron uptake and enhanced lipid ROS production, which ultimately leads to oocyte ferroptosis.
Discussion
POI is caused by defective primordial follicle pool formation, follicular recruitment/maturation, and accelerated follicular atresia. It has long been considered that granulosa cell death pathways, including apoptosis, necrosis and autophagy, are involved in follicular atresia39–44. However, detailed information on oocyte death during POI remains limited. Ferroptosis, a recently recognized form of programmed cell death, has rarely been investigated in the context of the female reproductive system. BNC1, a transcription factor, is involved in oogenesis and folliculogenesis. We have found that the Bnc1 truncation mutation leads to high FSH, reduced follicle numbers and fertility loss, which are typical phenotypes of POI11. In this study, we identified BNC1 as a factor that helps to maintain the ovarian reserve, deficiency of which results in POI by inducing oocyte ferroptosis. Regarding the mechanism, BNC1 deficiency in oocytes downregulates NF2, activates YAP, upregulates TFRC and ASCL4, and ultimately sensitizes oocytes to ferroptosis, which results in oocyte death and follicular atresia.
The expression of BNC1 in oocyte nuclei and the fact that POI results from BNC1 deficiency led us to investigate whether oocyte death contributes to follicular atresia19. We hypothesized that follicular atresia may be caused not by granulosa cell death but rather by oocyte death45. Oocyte-specific knockout of Bnc1 induces the phenotype of POI, which suggests that Bnc1 plays a crucial role in oocytes. Bnc1 mutation leads to progressive fertility loss and testicular premature aging by inducing germ cell apoptosis in the testes46,47. However, Bnc1 mutation results in POI not by mediating apoptosis. Intriguingly, ferroptosis induced by Bnc1 mutation contributes to POI. These findings suggest that BNC1 functions in a tissue-specific manner.
During oogenesis, mitochondrial numbers increase rapidly, while the rate of oxygen consumption is maintained at a low level48. POI patients exhibit increases in both mitochondria mutations and ROS levels49,50. Many studies have shown that oxidative stress and mitochondrial dysfunction induce ovarian cell apoptosis to contribute to POI and ovarian aging51,52. Our findings are consistent with previous findings that some Bnc1-knockdown oocytes are morphologically and biochemically abnormal, evidenced by dark granules in the cytoplasm, and some oocytes degenerated whereas others could survive53. In this study, we found that the Bnc1 mutation or oocyte-specific knockout model showed increased mitochondrial membrane potential and elevated ROS and MitoSOX levels in oocytes, which suggested the existence of an oxidative stress state in the oocytes. An increase in ROS level beyond a certain threshold may sensitize oocytes, particularly to lipid peroxidation induced ferroptosis, while low concentration of ROS may act as signal inducer. Intriguingly, the oocytes in Bnc1tr/tr mice showed increased lipid peroxidation, which is a hallmark of ferroptosis. Our transcriptomic data also showed that ferroptosis is involved in Bnc1-induced POI. Several clinical lines of evidence support our findings. For example, women with POI exhibit abnormalities of lipid metabolism and metabolic profiles54,55. Dysregulation of follicle fatty acids is a potential driver of human POI, as free fatty acid treatment reduces the proliferation rate and induces obvious apoptosis signals in granulosa cells isolated from the ovaries56. Iron overload-induced ferroptosis may suppress the meiotic process during porcine oocyte maturation and lead to oxidative stress in each stage of the follicles, thus disturbing follicle development57–59. Here, we found that BNC1 is required for maintaining mitochondrial function and lipid metabolism in oocytes and deficiency of BNC1 triggers ferroptosis-dependent POI.
Targeting the Hippo pathway mediating ferroptosis has been shown to be a promising strategy in cancer therapy20,21,33. Inactivation of NF2 enables activation of the proto-oncogenic transcriptional coactivator YAP to promote ferroptosis via the upregulation of several ferroptosis modulators20,22. The Hippo-YAP/TAZ pathway can induce the expression of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 4 (NOX4), ALOXES, and SKP2, which contribute to YAP-promoted ferroptosis21,33,60. Given these findings, we focused on the NF2-YAP-Hippo pathway. We demonstrated that BNC1 directly targets NF2 expression, and that BNC1 deficiency leads to YAP activation and TFRC and ACSL4 upregulation. TFRC has been identified as a key iron transporter on the plasma membrane, and can lead to iron overload and lipid peroxidation. Upregulation of TFRC triggers ferroptosis34. ACSL4 is a key enzyme involved in PUFA-PL biosynthesis35–37. Our results show that increased ACSL4 promotes the accumulation of lipid peroxidation products. We also proved that in vitro, BNC1 regulated the NF2-YAP-TFRC/ACSL4 signaling axis in the ES-2 cell line. In breast cancer cell and renal cell carcinoma lines, loss of BNC1 expression increases cell motility, which is associated with a poorer prognosis61,62. In some studies, loss of function of NF2 has been reported to programmatically influences the redox imbalance that orchestrates malignant attributes of mammary/breast cancer63,64. These results support our finding that low NF2 expression in Bnc1-mutant mice results in a dysregulated cellular redox management system.
Here, we demonstrate that BNC1 is required for oocyte lipid metabolism and the redox system and affects the NF2-YAP signaling pathway. BNC1 deficiency results in ferroptosis-dependent follicular atresia. Our findings provide insights regarding the occurrence of ferroptotic oocyte death in POI and shed light on ferroptosis-targeted therapies for patients with POI.
Methods
Ethics approval for the research
The care and experimental procedures for the mice were in accordance with the Institutional Guidelines of the Animal Care and Use Committee (ACUC) and were approved by the ACUC of the Zhejiang University School of Medicine.
Animal care
The mice were housed under controlled environmental conditions with free access to water and food. The mice were reared in standard conditions with controlled temperature (21–25 °C), humidity (40–70%) and the housing facility was illuminated between 7:00 am and 7:00 pm. All comparisons were made between littermates. All procedures were conducted according to the guidelines of the ACUC of the Zhejiang University School of Medicine Laboratory Animal Research Center. The experimental protocols were approved by the Regional Ethical Committee of Zhejiang University.
Generation of Bnc1-targeted mutant mice and oocyte-conditional knockout mice
Mice with the targeted Bnc1 mutation on a C57BL/6 J background were generated by the Nanjing Biomedical Research Institute of Nanjing University (Nanjing, China). The targeting vector was constructed with a 5 bp (CCGGG) deletion in exon 4 homologous to the identified human mutation, which leads to a frameshift downstream of the mutant site and a premature stop codon in the Bnc1 transcript11. Male and female mice of the Bnc1+/tr genotype were mated to produce Bnc1+/+, Bnc1+/tr and Bnc1tr/tr mice.
Ddx4-Cre and Gdf9-Cre knock-in mice were maintained on a C57BL/6 J background and obtained from Nanjing Biomedical Research Institute of Nanjing University (Nanjing, China). Dicer conditional null (flox) mice were maintained as a flox/flox stock on a C57BL/6 J background and generated by Innovative Cellular Therapeutics (Shanghai, China). Ddx4-Cre mice were genotyped by PCR using Cre primers (Cre forward: 5′ CACGTGCAGCCGTTTAAGCCGCGT 3′, Cre reverse: 5′ TTCCCATTCTAAACAACACCCTGAA 3′) that yielded a 240-bp DNA fragment. Gdf9-Cre mice were genotyped by PCR using Cre primers (Cre forward: 5′ TCTGATGAAGTCAGGAAGAACC 3′, Cre reverse: 5′ GAGATGTCCTTCACTCTGATTC 3′) that yielded a 500-bp DNA fragment. Dicer flox mice were genotyped with the PCR forward primer 5′ GACCTGGAAAGGCATGATGC 3′ and the PCR reverse primer 5′ GATCTCTCAGACTAGAAAGG 3′, yielding a 270-bp DNA fragment for the homozygous strain and a 177-bp DNA fragment for the WT strain. Initially, Ddx4-Cre and Gdf9-Cre heterozygotes were bred with Bnc1flox/flox mice. The resulting male Bnc1flox/+, Ddx4-Cre and Gdf9-Cre mice were bred to female Cre (−), Bnc1flox/flox mice to generate Bnc1flox/flox, Ddx4-Cre and Gdf9-Cre males and females. Bnc1flox/flox, Ddx4-Cre and Gdf9-Cre males were normal and fertile. Therefore, for the final cross, Bnc1flox/flox, Ddx4-Cre and Gdf9-Cre males were bred with, Bnc1flox/flox, Cre (−) females to generate Bnc1flox/flox, Ddx4-Cre and Gdf9-Cre females. Bnc1flox/flox, Cre (−) female littermates served as controls.
Cell culture
The human ovarian cancer cell line ES-2 (CL-0079, Procell, China) was grown in McCoy’s 5A medium (C3020-0500, VivaCell) supplemented with 10% fetal bovine serum (FBS) (Serana, Germany), while the human embryonic kidney (HEK) 293 T cell line (ATCC, CRL-1573, USA) was cultured in high-glucose DMEM (Gibco, USA) supplemented with 10% FBS. All cells were cultured in a humidified atmosphere containing 5% CO2 at 37 °C, authenticated by Short tandem repeat (STR). The cells were plated into 6- or 24-well plates at a concentration of 100,000 cells per milliliter (20,000 cells/cm2). Cell growth was monitored, and treatment was initiated when cells reached 50% confluence.
Gene silencing
Specific small-interfering RNAs (siRNAs) targeting human BNC1 were generated by Gene Pharma (Shanghai, China). The sequences are listed in Supplementary Table 1. SiRNA was administered to cells with jetPRIME® Reagent (101000046, Polyplus transfection, France) according to the manufacturer’s instructions at 50 nM. The transfected cells were incubated at 37 °C and harvested at the indicated time points (24 h or 48 h) for the following assays.
Histological analysis of ovarian tissues and counting of follicles
Ovarian tissues were collected at PD1 and at 3, 4, 12, and 16 weeks after the mice were born. The tissues were fixed in 4% paraformaldehyde, dehydrated in graded alcohol and xylene, and embedded in paraffin. The paraffin-embedded ovaries were serially sectioned at 5 μm thickness and stained with hematoxylin and eosin (HE) for morphological observation. As defined in a previous work11, the primordial, primary, secondary, and antral follicles were identified based on the well-accepted standards established by Pedersen and Peters were counted in sections at least 25 µm apart (each fifth section) spanning the entire ovary. The exact numbers of primordial follicles were determined by multiplying the raw numbers by a correction factor, which was 5 in this study.
GV oocyte collection
To study ovarian responses to exogenous gonadotropins, female mice at 4 weeks and 12 weeks were injected i.p. with 10 IU of PMSG (Hong Kong Cen industrial, China). After 44–48 h, oocytes were collected from antral follicles by puncturing with a 26.5-gauge needle.
mRNA synthesis and overexpression analysis
A T7 promoter vector encoding Nf2 was generated by GuanNan Co., Ltd. (Hangzhou, China). For mRNA synthesis, the pcDNA3.1-Nf2 (mouse) plasmids were linearized by BamHI-HF (R3136, NEB). Capped complementary RNAs (cRNAs) were made with an mMESSAGE mMACHINE T7 ULTRA Transcription Kit Invitrogen (AM1345, Thermo Fisher, USA) and purified with RNA extraction reagent (P1011, Solarbio, China). Nf2 mRNA solution was injected into fully grown oocytes. To facilitate mRNA translation, oocytes were arrested in medium with 25 μmol/L 3-Isobutyl-1-methylxanthine (IBMX) for 24 h and then cultured in M16 medium for further experiments.
Estrous cycle examination
A vaginal smear was taken every day with normal saline for 2–3 cycles of estrus. Approximately 0.1 ml of saline was drawn into the pipette, which was gently inserted into the vaginal canal. Then, the vaginal fluid was smeared on slides, stained with methylene blue after drying, and observed under a light microscope. The estrous cycle was classified into four stages, namely, the proestrus (P), estrus (E), metestrus (M), and diestrus (D) phases. The exact phase was determined by evaluating the major cell type59. Proestrus is characterized by low numbers of neutrophils, or large and nucleated epithelial cells, estrus is exhibited by predominantly anucleated keratinized epithelial cells, metestrus shows a combination of anucleated keratinized epithelial cells and neutrophils and diestrus is characterized by higher neutrophil numbers.
Fertility test
Female mice were continuously mated with fertile male mice from the age of 8 weeks to 32 weeks (female:male = 1:1, n = 5 per genotype). The numbers of pups and litters were recorded.
In vitro oocyte culture
GV oocytes from 4-week-old mice were treated with 5 µM RSL3 (T3646, Topscience, China) for 3 h in vitro. GV oocytes from 4-week-old mice were treated with 2 µM Fer-1 (S7243, Selleck Chemicals, USA) for 8 h in vitro.
Immunofluorescence
Oocytes were fixed in 4% paraformaldehyde for 30 min at room temperature and washed 3 times with 1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS). The oocytes were incubated in permeabilization buffer (1× PBS, 0.2% Triton-X 100 (Sigma–Aldrich, USA)) at 4 °C overnight and then blocked with 1% BSA in PBS for 1 h at room temperature. Then, the oocytes were incubated with primary antibodies at 4 °C overnight. After being washed three times with 1% BSA in PBS, the oocytes were incubated for 1 h at room temperature with secondary antibodies (Thermo Fisher) and DAPI (ab228549, Abcam, U.K.). Negative controls included parallel oocytes that were treated without primary antibodies. The oocytes were observed using a fluorescence microscope (Olympus IX81-FV1000).
The antibodies employed were as follows: anti-Caspase3 (1:50, #9664, Cell Signaling), anti-γH2AX (1:50, 05-636-I, Sigma–Aldrich), anti-NF2 (1:50, ab88957, Abcam), anti-active YAP (1:50, ab205270, Abcam), anti-TFRC (1:50, ab214039, Abcam), and anti-GPX4 (1:50, A13309, ABclonal, China).
RNA isolation and sequencing
For preparation of ovary samples, total RNA was extracted with TRIzol reagent (Invitrogen, USA). The complementary DNA (cDNA) libraries for single-end sequencing were prepared using an Ion Total RNA-Seq Kit v2.0 (Life Technologies) according to the manufacturer’s instructions. The cDNA libraries were then processed for the Proton Sequencing process with an Ion PI Sequencing 200 Kit v2.0 (Life Technologies) by NovelBio Corp. Laboratory (Shanghai, China).
Single oocyte cDNAs were processed following a previously reported modified Smart-seq2 protocol65,66. Briefly, a single oocyte was transferred to 2 μl of cell lysis buffer with 1 μl of oligo-dT primer and 1 μl of dNTP mix (Fermentas). The tube was quickly vortexed to mix and incubated at 72 °C for 3 min. Then, the first-strand cDNA was reverse-synthesized using oligo(dT) and template-switching oligonucleotides (TSO). Reverse transcription was carried out by incubating the mixture at 42 °C for 90 min, 50 °C for 2 min, 42 °C for 2 min and 70 °C for 15 min. PCR preamplification was performed using the ISPCR primer. The reaction was incubated at 95 °C for 3 min; cycled 18 times at 98 °C for 20 s, 67 °C for 15 s, and 72 °C for 1 min; and then subjected to a final extension step at 72 °C for 5 min. Then, the cDNA was purified using VAHTS DNA Clean Beads N411. The primers were as follows: oligo(dT), 5′-AAGCAGTGGTATCAACGCAGAGTACT30VN-3′; TSO, 5′-AAGCAGTGGTATCAACGCAGAGTACATrGrG+G-3′; and ISPCR primer, 5′-AAGCAGTGGTATCAACGCAGAGT-3′. The cDNA library was constructed using a TruePrep DNA Library Prep Kit V2 for Illumina TD503 and a TruePrep Index Kit V2 for Illumina TD202. The Illumina® High-throughput sequencing platform (Novogene, China) was used.
Mouse ovarian ChIP-seq, ChIP and qPCR
ChIP-Seq assays were performed as reported47. Each immunoprecipitation assay was performed using 25 mg of fresh ovary tissue with 2 µg anti-BNC1(ThermoFisher, PA5-85984) or anti-IgG control (2729, Cell Signaling, USA). The protocol of the SimpleChIP® Plus Enzymatic Chromatin IP Kit (Magnetic Beads) (9005, Cell Signaling) was followed. Briefly, 25 mg of fresh ovarian tissue was minced with a clean scalpel on ice. Then, the ovaries were fixed with 1.5% formaldehyde for in vivo crosslinking for 20 min at room temperature, which were terminated with glycine. Tissues were isolated into single cell suspensions using a Dounce homogenizer. Micrococcal Nuclease (10011, Cell Signaling) was added into each immunoprecipitation (IP) preparation, and the contents were mixed and incubated at 37 °C for 20 min with frequent agitation to digest the DNA to approximately 150-900 bp in length, followed by adding EDTA (7011, Cell Signaling) to stop digestion and then sonicated (Bioruptor Pico, Diagenode, Belgium) to disrupt the nuclear membrane. A total 10 µl of chromatin sample was removed as 2% input control before the antibodies were added. The IP samples were incubated at 4 °C with rotation overnight. Then, 30 μl of Protein G Magnetic Beads (9006, Cell Signaling) was added to each IP reaction, which was incubated at 4 °C for 2 h. After elution and reverse-crosslinking of chromatin from the antibodies/Protein G Magnetic Beads, the DNA was purified by spin columns. The primers used are listed in Supplementary Table 1.
TEM and SEM
Ovaries collected from 12-week-old mice were fixed in 2.5% glutaraldehyde at room temperature for 2 h and then at 4 °C overnight. The ovaries were washed with PBS three times for 10 min. Then, the ovaries were fixed with 1% osmic acid for 1 h and washed with PBS three times for 10 min each. The ovaries were fixed with 2% uranyl acetate for 30 min; dehydrated with 50%, 70%, 90% and 100% ethanol for 10 min each; and washed with 100% acetone twice for 15 min each. The ovaries were embedded with the embedding agent acetone (1:1) for 2 h at room temperature, transferred to an embedding agent, embedded at 37 °C and polymerized. Photographs were taken with a Leica UC7 and cryogenic electron microscope (Tecnai G2 Spirit 120 kV).
Oocytes were fixed in 2.5% glutaraldehyde at room temperature for 2 h and then at 4 °C overnight. The oocytes were washed with PBS three times for 10 min each. The oocytes were then placed in an ice bath in the dark for 1.5 h after adding 2% osmic acid and 3% potassium ferric oxide (1:1). The oocytes were washed with ddH2O three times for 10 min each, transferred into 1% thiocarbohydrazide at 37 °C for 2 min and washed with ddH2O three times for 10 min each. The oocytes were then incubated away from light for 1 h after adding 2% osmic acid, washed with ddH2O three times for 10 min each, transferred into 1% uranyl acetate, and incubated at 4 °C overnight before being washed with ddH2O three times for 10 min each. Aluminum nitrate (0.66%) and aspartic acid were added, and the oocytes were incubated at 60 °C for 30 min. After the incubation, the oocytes were washed with ddH2O three times for 10 min each; dehydrated with 30%, 50%, 70%, 90% and 100% ethanol for 15 min each; transferred to acetone:ethanol (1:1) for 15 min; and then transferred to acetone for 20 min. The oocytes were transferred into 7:3 acetone:resin, 3:7 acetone:resin, and acetone overnight. Photographs were taken via SEM (Teneo VS, Thermo Fisher).
Confocal microscopy of mouse oocytes
Oocytes were incubated with fluorescent dyes diluted with M16 medium (M7292, Sigma–Aldrich), including Reactive Oxygen Species Assay Kit (S0033S, Beyotime, China), MitoTracker® Green FM (40742ES50, Yeasen), MitoSOX Red Mitochondrial Superoxide Indicator (40778ES50, Yeasen), JC-1 (40705ES03, Yeasen), BODIPY™ 581/591 C11 (D3861, Thermo Fisher), FerroOrange (F374, Dojindo, Japan), and Annexin V-FITC (A211, Vazyme, China). The oocytes were observed using an Olympus IX81 fluorescence microscope (Olympus IX81-FV1000).
Targeted lipidomics
Oocytes were dissolved in 500 µl of methanol and sonicated at 67 Hz for 3 min (5 times). The samples were freeze–thawed 3 times with liquid nitrogen during beating. Each sample was then transferred to a 15 ml glass tube, and another 500 µl of methanol was added. Next, 2 ml of chloroform was added, and the mixture was vortexed vigorously for 30–60 s. The sample was spun down for 1 min at 4000 rpm, and the supernatant was transferred to a new 15 ml glass tube. Next, 400 µl of 50 mM citric acid and 800 µl of chloroform were added to the suspension, which was mixed well and spun to achieve phase separation. The upper phase was aspirated, and the bottom phase was separated into 2–3 EP tubes. A SpeedVac was used for concentration. The AB Sciex Triple Quad 6500+ System was used.
IHC and immunofluorescence
Paraffin-embedded ovarian tissue sections were deparaffinized, immersed in retrieval solution (10 mM sodium citrate), heated in an autoclave, blocked with 3% BSA (G5001, Servicebio), and then incubated overnight with the indicated primary antibodies. The antibodies employed were as follows: anti-NF2 (ab88957, Abcam), anti-active YAP (ab205270, Abcam), anti-transferrin receptor (ab214039, Abcam), anti-GPX4 (A13309, ABclonal), anti-COX2 (12375-1-AP, Proteintech), and anti-HRP secondary antibodies. Staining was performed using Vectastain ABC kits and DAB peroxidase substrate kits (G1211, Servicebio, China).
Ovaries were fixed in 4% paraformaldehyde for 2 h at room temperature, dehydrated with 30% sucrose overnight at room temperature, embedded with O.C.T. (4583, Sakura Finetek, Japan) and frozen-sectioned at a thickness of 10 μm. The ovary sections were dried at 37 °C for 30 min, washed with PBS and then incubated with Nile red (HY-D0718, MCE, USA) at 4 °C overnight. After being washed with PBS, the nuclei were stained using 1 μg/ml DAPI (Sigma). The slides were analyzed using a confocal microscope (Olympus IX81-FV1000).
Isolated oocytes were fixed with 4% paraformaldehyde for 1 hour at room temperature and washed 3 times with 1% BSA in PBS. Then, they were stained with 10 µg/ml Nile red at 4 °C overnight. The nuclei were stained using DAPI (ab228549, Abcam). Oocyte neutral lipids were observed using a confocal microscope (Olympus IX81-FV1000).
Animal treatments and related measurements
Thirteen-week-old mice were treated with Fer-1 (S7243, Selleck Chemicals, USA) for three weeks. The mice received daily intraperitoneal injections of either Fer-1 (1 mg/kg) or vehicle (corn oil). Then, estrous cycle detection, ovarian follicle counting, and oocyte staining with lipid ROS were performed. Mice at postnatal day 7 were treated with verteporfin (VP) (CL 318952, MCE) at 3-day intervals for 14 days and injected i.p. with either VP (100 mg/kg) or vehicle (corn oil).
RNA isolation, reverse transcription, and RT–qPCR
Total RNA was extracted with TRIzol reagent (Takara, Japan) according to the manufacturer’s protocol, and an equal amount of RNA from each sample was extracted with a reverse-transcription kit (R233, Vazyme, China). Real-time PCR was performed using SYBR® Green gene expression assays (R711, Vazyme, China). The primers are listed in Supplementary Table 1.
Western blotting
Tissue and cells were extracted in cold RIPA buffer (BL507A, Biosharp, China), which was supplemented with 1 mM phenylmethylsulfonyl fluoride (P0100, Biosharp) and protease inhibitor cocktail (HY-K0021, MCE). The protein lysates were resolved by SDS–polyacrylamide gel electrophoresis (SDS-PAGE). After protein transfer, the membrane was blocked with 5% skim milk and incubated with antibodies overnight. After three times washes, the membranes were incubated with HRP-conjugated secondary antibody at room temperature, and then washed three times with PBST. The images were captured with a chemiluminescence detection system (Bio-Rad). The antibodies employed were as follows: anti-NF2 (1:1000, ab88957, Abcam), anti-YAP (phospho-S127) (1:1000, ab76252, Abcam), anti-active YAP (1:1000, ab205270, Abcam), anti-YAP (1:1000, sc-101199, Santacruz, USA), anti-transferrin receptor (1:1000, ab214039, Abcam), anti-GPX4 (1:1000, A13309, ABclonal), anti-ACSL4 (1:1000, A20414, ABclonal), anti-β-actin (1:1000, 3700, Cell signaling), anti-P53 (1:1000, M1312-2, HuaBio, China), anti-PARP (1:1000, ET1608-56, HuaBio), anti-BAX (1:1000, ET1603-34, HuaBio), anti-BCL-2 (1:1000, ER1802-97, HuaBio), anti-Caspase3 (1:1000, 66470-2-Ig, Proteintech, USA) and anti-BNC1(1:1000, ARP33283_P050, Aviva, U.K.).
Luciferase assay
Expression vectors encoding pcDNA3.1-Bnc1 (mouse), the pGL3-Nf2 promoter (mouse) and the pGL3-Nf2-truncation mutation promoter (mouse) were cotransfected into HEK293T cells using Lipofectamine™ 3000 Transfection Reagent (L3000150, Thermo Fisher). After transfection for 24 h, the cultured cells were collected with passive lysis buffer and measured with the Dual-Luciferase® Reporter Assay System (Promega) according to the manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed with GraphPad Prism software (GraphPad Software, San Diego, CA). The results are given as the means and standard deviations (SDs). Each experiment included at least three independent samples and was repeated at least three times. Group comparisons were made by two-tailed unpaired Student’s t tests. P values of <0.05 were considered to indicate statistical significance. The sample sizes (“n”), the statistical tests and the exact p values were described in each figure legend.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We thank W.G. and H. L. for discussions of the manuscript. This work was supported by grants from the National Key Research and Development Program of China (Nos. 2018YFC1005003, D.Z., 2021YFC2700601, D.Z.), the National Natural Science Foundation of China (Nos. 81901448, Y.L., 81974224, D.Z.), the Key Research and Development Program of Zhejiang Province (No. 2021C03098, D.Z.), the Natural Science Foundation of Zhejiang Province (No. LZ18H040001, D.Z.), the China Postdoctoral Science Foundation Grant (No. 2019M652114, Y.L.), and Zhejiang University Global Partnership Fund (188170 + 194452205/001, D.Z.)
Source data
Author contributions
D.Z. designed the study. Y.L. and F.W. contributed to the conceptualization of the study. F.W., Y.L. and F.N. performed the experiments, analyzed the data, and wrote the draft of manuscript. J.J, Y.W., Y.H., X.Y., Y.Y., J.C., R.C., Y.Z., X.X., C.C. and J.G. performed experiments. X.Shen, X.Sun and S.W. analyzed the data. D.Z. supervised the study, reviewed and edited the manuscript.
Peer review
Peer review information
Nature Communications thanks Jen-Tsan Chi, Hang-soo Park and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Data availability
The data supporting the findings from this study are available within the article file and its supplementary information. Sequence data that support the findings of this study has been deposited in the Gene Expression Omnibus database (GEO) repository under accession code, GSE194194, GSE194195, GSE194196. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Feixia Wang, Yifeng Liu, Feida Ni.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-33323-8.
References
- 1.Webber L, et al. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum. Reprod. 2016;31:926–937. doi: 10.1093/humrep/dew027. [DOI] [PubMed] [Google Scholar]
- 2.Stockwell BR, Jiang X, Gu W. Emerging mechanisms and disease relevance of ferroptosis. Trends Cell Biol. 2020;30:478–490. doi: 10.1016/j.tcb.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang S, et al. Advances in biomaterials and regenerative medicine for primary ovarian insufficiency therapy. Bioact. Mater. 2021;6:1957–1972. doi: 10.1016/j.bioactmat.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goswami D, Conway GS. Premature ovarian failure. Hum. Reprod. Update. 2005;11:391–410. doi: 10.1093/humupd/dmi012. [DOI] [PubMed] [Google Scholar]
- 5.Persani L, Rossetti R, Cacciatore C. Genes involved in human premature ovarian failure. J. Mol. Endocrinol. 2010;45:257–279. doi: 10.1677/JME-10-0070. [DOI] [PubMed] [Google Scholar]
- 6.Rossetti R, Ferrari I, Bonomi M, Persani L. Genetics of primary ovarian insufficiency. Clin. Genet. 2017;91:183–198. doi: 10.1111/cge.12921. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi A, Yousif A, Hong L, Chefetz I. Premature ovarian insufficiency: pathogenesis and therapeutic potential of mesenchymal stem cell. J. Mol. Med (Berl.) 2021;99:637–650. doi: 10.1007/s00109-021-02055-5. [DOI] [PubMed] [Google Scholar]
- 8.Jagarlamudi K, et al. Oocyte-specific deletion of Pten in mice reveals a stage-specific function of PTEN/PI3K signaling in oocytes in controlling follicular activation. PLoS One. 2009;4:e6186. doi: 10.1371/journal.pone.0006186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddy P, et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611–613. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- 10.Qin Y, Jiao X, Simpson JL, Chen Z-J. Genetics of primary ovarian insufficiency: new developments and opportunities. Hum. Reprod. Update. 2015;21:787–808. doi: 10.1093/humupd/dmv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang D, et al. Basonuclin 1 deficiency is a cause of primary ovarian insufficiency. Hum. Mol. Genet. 2018;27:3787–3800. doi: 10.1093/hmg/ddy261. [DOI] [PubMed] [Google Scholar]
- 12.Teumer J, Tseng H, Green H. The human basonuclin gene. Gene. 1997;188:1–7. doi: 10.1016/S0378-1119(96)00659-2. [DOI] [PubMed] [Google Scholar]
- 13.Tseng H, Green H. Association of basonuclin with ability of keratinocytes to multiply and with absence of terminal differentiation. J. Cell Biol. 1994;126:495–506. doi: 10.1083/jcb.126.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahoney MG, et al. Translocation of the zinc finger protein basonuclin from the mouse germ cell nucleus to the midpiece of the spermatozoon during spermiogenesis. Biol. Reprod. 1998;59:388–394. doi: 10.1095/biolreprod59.2.388. [DOI] [PubMed] [Google Scholar]
- 15.Luan Y, Edmonds ME, Woodruff TK, Kim S-Y. Inhibitors of apoptosis protect the ovarian reserve from cyclophosphamide. J. Endocrinol. 2019;240:243–256. doi: 10.1530/JOE-18-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lena AM, et al. The p63 C-terminus is essential for murine oocyte integrity. Nat. Commun. 2021;12:383. doi: 10.1038/s41467-020-20669-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuppi M, et al. Oocyte DNA damage quality control requires consecutive interplay of CHK2 and CK1 to activate p63. Nat. Struct. Mol. Biol. 2018;25:261–269. doi: 10.1038/s41594-018-0035-7. [DOI] [PubMed] [Google Scholar]
- 18.Xie Y, et al. Ferroptosis: process and function. Cell Death Differ. 2016;23:369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang WS, Stockwell BR. Ferroptosis: death by lipid peroxidation. Trends Cell Biol. 2016;26:165–176. doi: 10.1016/j.tcb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu J, et al. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature. 2019;572:402–406. doi: 10.1038/s41586-019-1426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang, W.-H. et al. The Hippo pathway effector TAZ regulates ferroptosis in renal cell carcinoma. Cell Rep28 2501–2508 (2019). [DOI] [PMC free article] [PubMed]
- 22.Hou, L. et al. Targeted intervention of NF2-YAP signaling axis in CD24-overexpressing cells contributes to encouraging therapeutic effects in TNBC. ACS Nano. Preprint at https://pubs.acs.org/doi/10.1021/acsnano.1c10921 (2022). [DOI] [PubMed]
- 23.Tanaka K, et al. Statin suppresses Hippo pathway-inactivated malignant mesothelioma cells and blocks the YAP/CD44 growth stimulatory axis. Cancer Lett. 2017;385:215–224. doi: 10.1016/j.canlet.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 24.Lin C-C, et al. DDR2 upregulation confers ferroptosis susceptibility of recurrent breast tumors through the Hippo pathway. Oncogene. 2021;40:2018–2034. doi: 10.1038/s41388-021-01676-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao J, et al. Deficiency of Pdk1 contributes to primordial follicle activation via the upregulation of YAP expression and the pro‑inflammatory response. Int J. Mol. Med. 2020;45:647–657. doi: 10.3892/ijmm.2019.4437. [DOI] [PubMed] [Google Scholar]
- 26.Hu L-L, et al. Hippo pathway functions as a downstream effector of AKT signaling to regulate the activation of primordial follicles in mice. J. Cell Physiol. 2019;234:1578–1587. doi: 10.1002/jcp.27024. [DOI] [PubMed] [Google Scholar]
- 27.Zhu G, et al. O-GlcNAcylation enhances sensitivity to RSL3-induced ferroptosis via the YAP/TFRC pathway in liver cancer. Cell Death Disco. 2021;7:83. doi: 10.1038/s41420-021-00468-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye S, et al. Cytoglobin promotes sensitivity to ferroptosis by regulating p53-YAP1 axis in colon cancer cells. J. Cell Mol. Med. 2021;25:3300–3311. doi: 10.1111/jcmm.16400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Wang F, Li D, Yan Y, Wang H. Transferrin receptor-mediated reactive oxygen species promotes ferroptosis of KGN cells via regulating NADPH oxidase 1/PTEN induced kinase 1/acyl-CoA synthetase long chain family member 4 signaling. Bioengineered. 2021;12:4983–4994. doi: 10.1080/21655979.2021.1956403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J-J, et al. Single-cell transcriptome landscape of ovarian cells during primordial follicle assembly in mice. PLoS Biol. 2020;18:e3001025. doi: 10.1371/journal.pbio.3001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stockwell BR, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao, M. et al. Role of mitochondria in ferroptosis. Mol Cell73 354–363.e3 (2019). [DOI] [PMC free article] [PubMed]
- 33.Yang W-H, et al. The Hippo pathway effector YAP promotes ferroptosis via the E3 ligase SKP2. Mol. Cancer Res. 2021;19:1005–1014. doi: 10.1158/1541-7786.MCR-20-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu Y, et al. MYCN mediates TFRC-dependent ferroptosis and reveals vulnerabilities in neuroblastoma. Cell Death Dis. 2021;12:511. doi: 10.1038/s41419-021-03790-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tuo Q-Z, et al. Thrombin induces ACSL4-dependent ferroptosis during cerebral ischemia/reperfusion. Signal Transduct. Target Ther. 2022;7:59. doi: 10.1038/s41392-022-00917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang WS, et al. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl Acad. Sci. USA. 2016;113:E4966–E4975. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doll S, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017;13:91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xin S, et al. MS4A15 drives ferroptosis resistance through calcium-restricted lipid remodeling. Cell Death Differ. 2022;29:670–686. doi: 10.1038/s41418-021-00883-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X, et al. Long noncoding RNA HCP5 participates in premature ovarian insufficiency by transcriptionally regulating MSH5 and DNA damage repair via YB1. Nucleic Acids Res. 2020;48:4480–4491. doi: 10.1093/nar/gkaa127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun Z, et al. TMCO1 is essential for ovarian follicle development by regulating ER Ca store of granulosa cells. Cell Death Differ. 2018;25:1686–1701. doi: 10.1038/s41418-018-0067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blohberger J, et al. Readthrough acetylcholinesterase (AChE-R) and regulated necrosis: pharmacological targets for the regulation of ovarian functions? Cell Death Dis. 2015;6:e1685. doi: 10.1038/cddis.2015.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McEvoy, M. J. et al. Death processes in Bovine Theca and Granulosa cells modelled and analysed using a systems biology approach. Int J Mol Sci22 4888 (2021). [DOI] [PMC free article] [PubMed]
- 43.Liu T, et al. miR-15b induces premature ovarian failure in mice via inhibition of α-Klotho expression in ovarian granulosa cells. Free Radic. Biol. Med. 2019;141:383–392. doi: 10.1016/j.freeradbiomed.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 44.Shao T, Ke H, Liu R, Zhao S, Qin Y. Variation analysis of theTMEM150B gene in Chinese women with premature ovarian insufficiency. Reprod. Biomed. Online. 2019;38:407–412. doi: 10.1016/j.rbmo.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 45.Liu X-M, et al. Loss of oocyte Rps26 in mice arrests oocyte growth and causes premature ovarian failure. Cell Death Dis. 2018;9:1144. doi: 10.1038/s41419-018-1196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J-Y, et al. BNC1 promotes spermatogenesis by regulating transcription of Ybx2 and Papolb via direct binding to their promotor elements. Reprod. Sci. 2021;28:785–793. doi: 10.1007/s43032-020-00342-z. [DOI] [PubMed] [Google Scholar]
- 47.Li J-Y, et al. Basonuclin 1 deficiency causes testicular premature aging: BNC1 cooperates with TAF7L to regulate spermatogenesis. J. Mol. Cell Biol. 2020;12:71–83. doi: 10.1093/jmcb/mjz035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tiosano D, Mears JA, Buchner DA. Mitochondrial dysfunction in primary ovarian insufficiency. Endocrinology. 2019;160:2353–2366. doi: 10.1210/en.2019-00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Venkatesh S, et al. Oxidative stress and ATPase6 mutation is associated with primary ovarian insufficiency. Arch. Gynecol. Obstet. 2010;282:313–318. doi: 10.1007/s00404-010-1444-y. [DOI] [PubMed] [Google Scholar]
- 50.Kumar M, et al. Chromosomal abnormalities & oxidative stress in women with premature ovarian failure (POF) Indian J. Med. Res. 2012;135:92–97. doi: 10.4103/0971-5916.93430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tesarik, J., Galán-Lázaro, M. & Mendoza-Tesarik, R. Ovarian aging: molecular mechanisms and medical management. Int J Mol Sci22 1371 (2021). [DOI] [PMC free article] [PubMed]
- 52.Larsson N-G. Somatic mitochondrial DNA mutations in mammalian aging. Annu Rev. Biochem. 2010;79:683–706. doi: 10.1146/annurev-biochem-060408-093701. [DOI] [PubMed] [Google Scholar]
- 53.Ma J, Zeng F, Schultz RM, Tseng H. Basonuclin: a novel mammalian maternal-effect gene. Development. 2006;133:2053–2062. doi: 10.1242/dev.02371. [DOI] [PubMed] [Google Scholar]
- 54.Huang Y, et al. Metabolic profile of women with premature ovarian insufficiency compared with that of age-matched healthy controls. Maturitas. 2021;148:33–39. doi: 10.1016/j.maturitas.2021.04.003. [DOI] [PubMed] [Google Scholar]
- 55.Ates S, Yesil G, Sevket O, Molla T, Yildiz S. Comparison of metabolic profile and abdominal fat distribution between karyotypically normal women with premature ovarian insufficiency and age matched controls. Maturitas. 2014;79:306–310. doi: 10.1016/j.maturitas.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 56.Wang L, et al. Dysregulation of follicle fatty acid is a potential driver of human primary ovarian insufficiency. J. Mol. Cell Biol. 2020;12:817–819. doi: 10.1093/jmcb/mjaa044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu W, et al. Iron overload-induced ferroptosis impairs porcine oocyte maturation and subsequent embryonic developmental competence. Front Cell Dev. Biol. 2021;9:673291. doi: 10.3389/fcell.2021.673291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hayashi S, et al. Novel ovarian endometriosis model causes infertility via iron-mediated oxidative stress in mice. Redox Biol. 2020;37:101726. doi: 10.1016/j.redox.2020.101726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qin X, et al. Chronic overload of concentration-dependent iron exerts different effects on ovarian function in C57BL/6J mice†. Biol. Reprod. 2021;104:1347–1359. doi: 10.1093/biolre/ioab040. [DOI] [PubMed] [Google Scholar]
- 60.Qin Y, et al. Oncogenic activation of YAP signaling sensitizes ferroptosis of hepatocellular carcinoma ALOXE3-mediated lipid peroxidation accumulation. Front Cell Dev. Biol. 2021;9:751593. doi: 10.3389/fcell.2021.751593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pangeni RP, et al. The GALNT9, BNC1 and CCDC8 genes are frequently epigenetically dysregulated in breast tumours that metastasise to the brain. Clin. Epigenetics. 2015;7:57. doi: 10.1186/s13148-015-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morris MR, et al. Identification of candidate tumour suppressor genes frequently methylated in renal cell carcinoma. Oncogene. 2010;29:2104–2117. doi: 10.1038/onc.2009.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.White, S. M. et al. YAP/TAZ inhibition induces metabolic and signaling rewiring resulting in targetable vulnerabilities in NF2-deficient tumor cells. Dev Cell49 425–443.e9 (2019). [DOI] [PMC free article] [PubMed]
- 64.Mota M, et al. Merlin deficiency alters the redox management program in breast cancer. Mol. Oncol. 2021;15:942–956. doi: 10.1002/1878-0261.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Picelli S, et al. Smart-seq2 for sensitive full-length transcriptome profiling in single cells. Nat. Methods. 2013;10:1096–1098. doi: 10.1038/nmeth.2639. [DOI] [PubMed] [Google Scholar]
- 66.Miao Y, et al. BRCA2 deficiency is a potential driver for human primary ovarian insufficiency. Cell Death Dis. 2019;10:474. doi: 10.1038/s41419-019-1720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings from this study are available within the article file and its supplementary information. Sequence data that support the findings of this study has been deposited in the Gene Expression Omnibus database (GEO) repository under accession code, GSE194194, GSE194195, GSE194196. Source data are provided with this paper.