Abstract
Aims
The purpose of the work was to investigate bacterial levels and diversity as well as survival of Salmonella in used dish washing sponges and brushes and identify consumer practices that can potentially explain bacterial status of these items.
Methods and Results
Used washing up utensils were collected from consumers. The bacterial numbers (TVC) were very variable with an extremely high median level (10.3 log cfu/item) in Portuguese sponges and lower levels in Norwegian items (7.3 and 7.0 cfu/item for sponges and brushes). No self‐reported practices or household composition could explain differences found in TVC levels among the collected sponges. Lower mean TVC levels were found in unworn brushes and brushes regularly cleaned with soap, but the differences were modest (1.5 log or less). A common set of bacteria was found in brushes and sponges, dominated by Acinetobacter, Chryseobacterium, Enhydrobacter, Enterobacteriaceae and Pseudomonas. There was no difference in TVC or bacterial diversity between conventional and antimicrobial sponges containing silver after 4 weeks of use. For used brushes inoculated with Salmonella and allowed to dry overnight, a significant reduction in Salmonella numbers was observed. No reduction was observed for brushes stored in humid conditions (in a plastic bag) or for sponges regardless of storing conditions.
Conclusions
Overall, lower bacterial levels were observed in used brushes than in sponges, and Salmonella died more rapidly in brushes. A common set of non‐pathogenic bacteria dominated in brushes and sponges.
Significance and Impact of Study
The study demonstrates that the use of brushes may be more hygienic than the use of sponges.
Keywords: bacteriota, brush, cleaning, consumer practice, Salmonella, sponge
INTRODUCTION
High numbers of bacteria, and occasionally also pathogens have been found in sponges used in kitchens (Cardinale et al., 2017; Cogan et al., 2002; Møretrø, Nguyen‐The, et al., 2021). There is a concern that sponges may spread pathogenic bacteria to kitchen surfaces and hands, thus representing a threat to the consumer rather than a means to reduce cross‐contamination to food or mouth. The use of sponges and other cleaning utensils varies between countries. We previously reported that sponges were commonly used for cleaning in kitchens in the majority of 10 European countries surveyed, while brushes were the dominant cleaning utensil for washing up in two countries (Norway and Denmark) (Møretrø, Moen, et al., 2021).
Two former studies showed that used sponges were dominated by non‐pathogenic bacteria. Cardinale et al. (2017) found that 14 used sponges collected in Germany were heavily colonized by Acinetobacter, Moraxella and Chryseobacterium, while in another German study with 20 sponges, Acinetobacter, Enhydrobacter, Agrobacterium, Pseudomonas and Chryseobacterium dominated them (Jacksch et al., 2020). To our knowledge, no data on bacterial diversity in used kitchen brushes has been reported. In a previous laboratory study with new brushes and sponges, to which a mixture of bacteria isolated from kitchen surfaces and kitchen cloths as well as Salmonella and Campylobacter and a food soil mixture were added, it was found that Serratia and Pseudomonas were dominant (Møretrø, Moen, et al., 2021).
There is limited information available on how different usage routines of cleaning utensils affect bacterial levels and diversity; however, bacterial levels are reported to be higher in humid than in dry cleaning utensils (Cogan et al., 2002; Mattick et al., 2003; Møretrø, Moen, et al., 2021). In their study, Cardinale et al. (2017) claimed that regular cleaning of sponges leads to a higher abundance of opportunistic pathogens. However, a limitation of the study was the low number (14) of sponges analysed.
In earlier laboratory experiments using new brushes and sponges, we found lower growth and survival of bacteria, including the pathogens Salmonella and Campylobacter in brushes than in sponges (Møretrø, Moen, et al., 2021). It was hypothesized that the rapid drying rate of brushes compared to sponges leads to higher bacterial reduction in the former. In the same study, we also observed lower survival of Salmonella and Campylobacter and lower growth of total bacteria in a type of antimicrobial sponge containing silver than in two other types of sponges. However, since no inhibition zones around the silver‐containing sponge were observed when its antibacterial activity was assessed by agar diffusion tests, it was not clear whether the effects on growth/survival were due to an antibacterial effect by silver or not. All these experiments were performed with new sponges and brushes, and further studies are necessary to evaluate whether the results also are relevant for bacterial growth/survival in brushes and sponges used in kitchens (Møretrø, Moen, et al., 2021).
The aim of the present study was to get an overview of microbial loads and microbiota in washing up brushes and sponges that had been used by consumers. Secondly, consumer practices that may affect the microbial status and survival of Salmonella were investigated.
MATERIALS AND METHODS
Collection of used sponges and brushes and quantitative bacterial analyses
Convenience samples of used sponges and brushes were collected from consumers (colleagues and students at our institutions or their family members) in Norway and Portugal (Table 1 and Figure S1). In Norway, the consumers were asked to bring brushes and/or sponges, while in Portugal, they were asked to bring sponges. Based on the reported low use of brushes for washing up in Portugal in a previous study (6.3% used brushes (Møretrø, Moen, et al., 2021)), we chose not to try to collect brushes in Portugal. The consumers filled out a form with information about the history of the collected items (age, use, storage, cleaning, etc.), and their households (Table S1). All collected items were weighted and visually inspected (humid vs. dry, clean vs. dirty, worn vs. new and foam vs. no foam).
TABLE 1.
Overview of experiments and analyses on used brushes and sponges collected from consumers
| Item | Immediately a (n b ) | Humid storage a (n) | Dry storage a (n) | Country |
|---|---|---|---|---|
| Brushes | TVC c , Bacteriota c (15) | TVC, S c (10) | TVC, S (11) | Norway |
| Sponges | TVC, Bacteriota (6) | TVC, S (7) | TVC, S (7) | Norway |
| Sponges | TVC, Bacteriota (20) | TVC, S (20) | TVC, S (20) | Portugal |
| Sponges d | TVC, Bacteriota (18) | Portugal |
Sampling stage; immediately; direct analysis, humid storage; stored in a plastic bag overnight, dry storage: Stored by hanging (brushes) or on an open tray (sponges).
n; number of items.
Analyses performed: TVC, total viable count; S, Salmonella; Bacteriota.
Consumers used an antimicrobial sponge for 4 weeks.
For brushes, some were sampled immediately, for analysis of total viable counts (TVC) and bacteriota, while to the others a mixture of two Salmonella strains (S. Enteritidis MF6974 from hen's egg (Portugal) and S. Infantis MF6976 from poultry (Hungary)) (Møretrø, Moen, et al., 2021) were added to study the survival of Salmonella during storage. The parts of the brushes with bristles were immersed in 20 ml of a mixed suspension of the two strains (overnight cultures grown in TSB [Tryptic Soy Broth, Oxoid] in test tubes at 30°C with 150 rpm agitation, diluted 10,000 times in sterile dH2O), leading to an inoculum level of about 6 log cfu of Salmonella per brush, and stored either in a plastic bag or hanging for 20 h at 19 ± 1°C, before analyses. The brushes were sampled by transferring them to a bag and adding 100 ml buffered peptone water (BPW), hand‐massaging the bag with the brush for 60 s, before enumeration of bacteria by serial dilution and plating on PCA (TVC, 30°C, 2 days). Brushes that had Salmonella added to them were also plated on XLD (Oxoid) (37°C, 24 h).
For sponges, some were analysed immediately for TVC, while others were divided in two or three parts (Table 1). To two parts, a mixture of the two Salmonella strains (as described for brushes but with immersion and absorption of 10 ml bacterial suspension made by diluting the overnight cultures 100,000 times in dH2O) were added, leading to an inoculum level of about 6 log cfu of Salmonella per sponge, to study survival during storage. One part was incubated on an open tray, and the other in a closed plastic bag to test the effect of drying versus humid conditions. If sponges were divided in three parts, the third part was analysed directly for TVC. Microbial analysis of sponges was performed similarly to brushes; however, sponges were placed into a bag with 50 ml BPW before stomacher treatment for 60 s followed by the enumeration of bacteria. In parallel with the used items, four new brushes (Jordan Trend, Lilleborg AS, Norway) and two new sponges (First Price, Norgesgruppen) from Norway, and two new sponges from Portugal (Auchan, Lisboa) (chosen as these products were the most common type collected from consumers) were acquired and the pathogens were added to them and were treated in a similar procedure (i.e. two brushes hanging and two in bags, sponges divided in two pieces; one in tray and one in a bag) as the used items. After about 20 h at 19 ± 1°C, the concentration of bacteria was determined as described above.
In Portugal, the 20 consumers providing their used sponge, received a new sponge in return and were asked to use the sponge as normally. All new sponges were of type no. 21 (Ultrafresh, Vileda, Portugal) as presented in Møretrø, Moen, et al. (2021), containing silver and claimed on the package to have antibacterial effect (the consumers were not informed about details of the sponge). After 4 weeks, 18 of the consumers returned the sponges, which were analysed for TVC of the whole sponge as described above (i.e. using 100 ml BPW in the stomacher procedure) and sampled for bacteriota analysis.
Qualitative bacteriota analysis
Sampling and DNA extraction
For bacteriota analyses, 4 ml of samples were collected from the homogenate (after hand‐massaging for brushes or stomacher treatment for sponges), subjected to centrifugation at 13,000g for 5 min, the supernatant was poured off, and the tube with the pellet was frozen at −20°C until analysis. Further, DNA extraction, 16S rDNA amplification and sequencing (MiSeq) were performed as described previously (Møretrø, Moen, et al., 2021).
Bacteriota analysis
The samples were sequenced in two separate Illumina MiSeq runs (Run1: the Norwegian samples and Run2: the Portuguese samples). For all samples, PCR was performed in triplicates and paired end sequencing (2 × 150 bp) was performed as described previously (Caporaso et al., 2012). Briefly, the V4 region of the 16S rRNA gene was amplified with region‐specific primers (515F, 806R) that included the Illumina flowcell adapter sequences (Apprill et al., 2015; Parada et al., 2016), as described previously (Møretrø, Moen, et al., 2021). The reverse amplification primer also contained a 12 base barcode sequence that supports pooling of different samples. Samples were purified with Ampure (Agencourt Bioscience Corporation) and quantified using the Quant‐iT Picogreen ds DNA with picogreen before pooling. The sample pool was diluted to 4 nM and sequenced using the MiSeq Reagent Kit v3 on a MiSeq (Illumina) following the protocol provided by Illumina. In addition to the experimental samples, the MiSeq run also contained a control library made from phiX Control v3, which, in this run, accounted for 10% of the reads. The library quantification and sequencing were performed at Nofima. The MiSeq Control Software (MCS) version used was RTA v1.18.54.
To verify the presence/absence of Campylobacter sequences from the MiSeq analysis, a Campylobacter real‐time PCR assay (modified from NordVal International/NMKL, 2019) was performed on 19 of the Norwegian brushes/sponges. The forward/reverse primers and Campylobacter probe were as described in the NMKL method. The reactions were performed on a QuantStudio 5 Real‐Time PCR System (Applied Biosystems) using the TaqMan® Fast Advanced Master Mix following the manufacturer's recommendations. DNA from C. jejuni NCTC 11168 was used to generate a standard curve and as a positive control.
Calculations and statistics
The MiSeq 16S rRNA amplicon sequences from the two runs were processed separately in QIIME2 (qiime2‐2019.1 and qiime2‐2020.2) (Bolyen et al., 2019). Briefly, the data were demultiplexed using demux, the paired ends were joined using vsearch, the quality was filtered based on a q‐score above 30, were denoised using deblur, the taxonomy was achieved using classify‐sklearn with the Greengenes 16S 13_8 database, and the alpha diversity (population richness) and beta diversity (structure variation of bacteriota between samples/environments) and significance were calculated (Amir et al., 2017; Bokulich et al., 2018; Bolyen et al., 2019; McDonald et al., 2012; Pedregosa et al., 2011). Sequences originating from mitochondria and chloroplast (very few) were filtered out of the final dataset. Samples were rarefied to 30,000 reads per sample before diversity analysis, population richness and permutational multivariate analysis of variance (PERMANOVA). Observed OTUs (operational taxonomic unit) group significance were checked for all reported consumer practices and household information, and the beta diversity plots were visually inspected for all practices/information before PERMANOVA was used on selected practices/information. PERMANOVA analysis was based on Bray–Curtis dissimilarity (non‐phylogenetic), weighted‐ and unweighted Unifrac and was used to test the hypothesis that distances between samples within one group (within‐group distances) differ from the distances to samples in another group (across‐group distances). UniFrac takes into account the evolutionary relationship between sequences, and unweighted (qualitative) Unifrac is more sensitive to differences in low‐abundance features compared to weighted Unifrac (quantitative). The number of permutations were 999 for all analyses.
The taxonomy tables were collapsed to level 6 (genus), converted to relative values and exported to text files and further processed in Excel. Taxa below an average of 1% across all samples were represented as “Other.” Core‐features were calculated on feature table (sub‐OTUs) and data from level 6 table. When comparing the bacteriota with TVC levels, the data were divided into the following categories (log TVC per brush or sponge): Low, <6.5; medium, 6.5–8.0; high, 8.1–9.5; ex‐high, >9.5. To determine genera significantly different between brushes and sponges one‐way ANOVA was performed on selected genera from the level 6 table from the five households with both brushes and sponges (Minitab 19 Statistical Software (2020) [Computer software]).
To determine connection patterns between consumer practices and utensil contamination levels, sample brushes (N = 40, Norway only) and sample sponges (N = 102, wherein 78 from Portugal and 24 from Norway) (new unused control brushes and sponges were included) were modelled in different partial least squares regression (PLSR) models. For each type of utensil, we built three predictive models: (i) utensils as collected from consumers modelled for TVC log, (ii) incubated utensils modelled for TVC log and (iii) incubated utensils modelled for Salmonella. In each of these models, the independent variables included consumer responses (usage habits, cleaning and storage habits, and socio‐demographics), observed utensil condition upon collection (e.g. clean, dry, worn and/or foam) and experimental setting of incubation (stored dry (hanging (brushes)/on an open tray (sponges)) or stored humid in a closed plastic bag, and used or control (new) utensil). All variables were standardized. In all models, full cross‐validation and uncertainty testing based on jack‐knifing were used for model validation and variable selection (Martens & Martens, 2000). Cross‐validation results were utilized to determine the appropriate number of factors in each model, where a drop or stagnation in validated variance indicated the first non‐validated factor. Fitted variance for the retained models varied from 39.9% (1‐factor model for TVC log on 43 used sponges) to 87.8% (4‐factor model for TVC log on 58 used and new sponges after incubation and storage). The results section presents the predictive models for TVC log and Salmonella, where weighted regression coefficients (B) and associated p‐values from uncertainty testing are reported. For each of the independent variables, the p‐value indicates whether the specific practice or household characteristic significantly led to higher (positive B‐coefficient) or lower (negative B‐coefficient) bacterial count. The models were run in The Unscrambler® X 10.4.1 (Camo Software AS).
RESULTS
Consumer practices and microbial status
Bacterial levels
The bacterial numbers in Portuguese sponges (median TVC 10.3 log10 cfu per sponge [range < 4.5–13.3 log]) were higher than in Norwegian sponges (7.3 log10 cfu per sponge (range < 4.5–10.7 log) (Figures 1 and 2)). The median TVC of brushes (only Norwegian) was 7.0 log10 cfu per brush (range < 3.9–9.2 log) (Figure 1). A similar pattern of usage type and usage frequency was reported for Portuguese sponges and Norwegian brushes. In Portugal, all consumers reported that the sponges were used for washing up (including scrubbing pots, pans and similar), and 19 of the 20 sponges were used 5–6 times a week or more often. Thirty‐two of the 35 brushes collected in Norway were reported to be commonly used for washing up and 31 used their brush 5–6 times a week or more often. The remaining brushes were from households that had two brushes and used the second brush for very dirty dishes. Seven of the 14 Norwegian sponges were exclusively used to clean very dirty dishes, scrub casseroles, etc., and not for general washing up and eight of the sponges were used once a week or less often. All of the Norwegian sponges, except one, were from households that also provided a brush that was commonly used for washing up.
FIGURE 1.
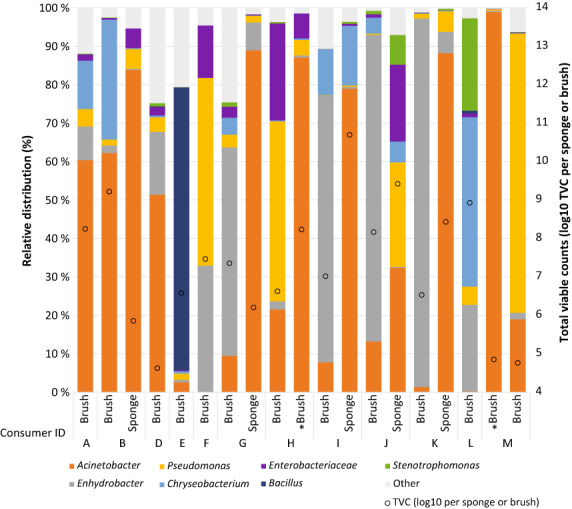
Bacterial diversity in brushes and sponges collected from Norwegian consumers (A–M). Only taxa that were above 0.1% across all items are indicated, the remaining taxa are shown together as “Other.” Some consumers provided two cleaning utensils. Consumers H and M provided two brushes where one (marked *) was used to clean very dirty dishes. Total viable counts (log10 TVC) per brush/sponge are shown as circles. One brush and one sponge are not included in the figure due to non‐successful amplification.
FIGURE 2.
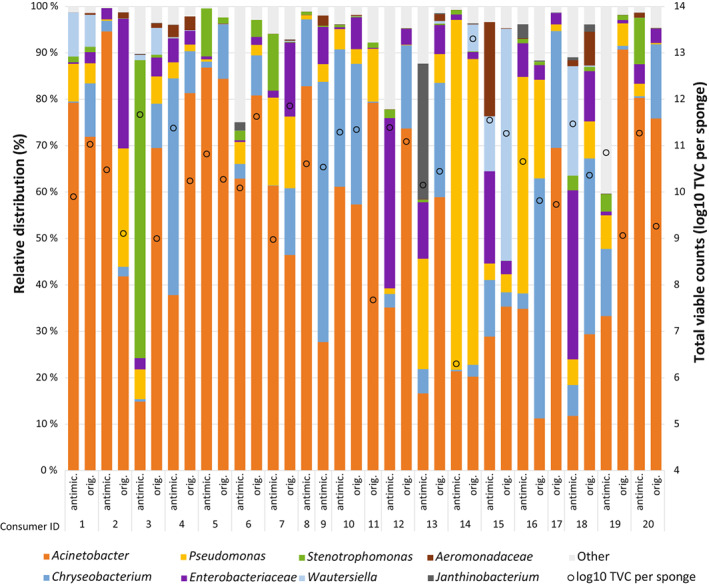
Bacterial diversity in used sponges from Portuguese consumers (1–20). Only taxa that were above 0.1% across all items are indicated, the remaining taxa are shown together as “Other.” “Orig.” indicates the original sponges collected, and these were of several different brands. “Antimic.” indicates sponges of a type containing silver, which were given to all consumers and collected after 4 weeks. Total viable counts (log10 TVC) per sponge are shown as circles. Consumer nos. 11 and 17 did not return the antimicrobial sponge. For consumer nos. 8 and 9, the original sponges are not included in the figure due to non‐successful amplification.
The consumers also reported about how they kept their dishwashing utensils clean. The use of dishwasher was more common for brushes than sponges. Of the 35 collected Norwegian brushes, 23 were reportedly cleaned in dishwasher (some in combination with rinse in water and use of soap), three with chlorine, four with water and soap, and five rinsed in water only. Among the 14 collected Norwegian sponges, seven were reported to be cleaned by rinsing with water, four with soap and two in dishwasher. One sponge was disinfected using chlorine. Among the 20 sponges collected from Portuguese consumers in the first round, 13 were reported to be cleaned with water and soap, one with water only and six were disinfected with chlorine. Considering the consumers using chlorine, three used it in combination with soap and one with dishwashing machine.
The time of use before donating the utensils to microbial analysis varied considerably between consumers, the type of utensil and the two countries. The Norwegian brushes had been used for the longest time, as 18 of 35 Norwegian brushes had been used for 2 months or more, while the majority (13/20) of the Portuguese sponges had been in use for 2–4 weeks and only three for more than a month. For the Norwegian sponges, the reported usage time was between 3 days and 6 months, with no clear distribution pattern. At the collection time, 75%, 50% and 36% of Portuguese sponges, Norwegian brushes and Norwegian sponges, respectively, were observed to look worn.
Potential relationships between TVC levels and reported usage and observations of the cleaning utensils were investigated in PLS regressions. Brushes that looked new rather than worn (p = 0.044, Table 2) had lower TVC (mean TVC new = 6.1 log (eight items), worn = 7.7 log (seven items)). Also, brushes reported to be cleaned with soap as a routine, rather than in the dishwasher, had lower TVC (p = 0.022, Table 2) (mean TVC soap = 5.6 log (seven items), dishwasher = 6.9 log (eight items)). Only three brushes were cleaned with chlorine, with no observable effect on TVC. There were no statistically significant differences (p > 0.05) in bacterial counts between different types/brands of brushes, or other reported information about use. For sponges collected from Portuguese and Norwegian consumers, none of the consumer practices investigated, such as cleaning and storage routines or when the sponge was changed, impacted the TVC results significantly (p > 0.05). In the study where Portuguese consumers used sponge no. 21 (containing silver) for 4 weeks, the median TVC was 10.7 log (range 6.3–11.7 log), not significantly different (p > 0.05) from the level in the sponges collected in the first round (Figure 2).
TABLE 2.
Extracted results from partial least squares regression models connecting consumers' usage habits, cleaning and storage habits, socio‐demographics, utensil condition and experimental settings with measures of total bacteria (TVC log) and Salmonella counts in sponges and brushes collected from consumers, before and after Salmonella addition and incubation. See Table S2 for the full models.
| Treatment | Sponges (N = 102) | Brushes (N = 40) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No incubation | Incubated with Salmonella | No incubation | Incubated with Salmonella | |||||||||
| Predicted variable | TVC log (N = 43) | TVC log (N = 58) | Salmonella (N = 54) | TVC log (N = 15) | TVC log (N = 25) | Salmonella (N = 25) | ||||||
| Independent variables a | Weighted B‐coefficient | p‐value | Weighted B‐coefficient | p‐value | Weighted B‐coefficient | p‐value | Weighted B‐coefficient | p‐value | Weighted B‐coefficient | p‐value | Weighted B‐coefficient | p‐value |
| Usage habits | ||||||||||||
| Change_worn/old | 0.016 | 0.831 | −0.133 | 0.252 | −0.124 | 0.038* | −0.001 | 0.985 | 0.044 | 0.605 | −0.082 | 0.375 |
| Last changed | 0.058 | 0.300 | 0.113 | 0.371 | 0.113 | 0.064 | 0.142 | 0.149 | 0.069 | 0.038* | 0.098 | 0.126 |
| WashUp_running water | 0.044 | 0.508 | −0.072 | 0.494 | −0.124 | 0.028* | −0.069 | 0.312 | −0.043 | 0.153 | −0.029 | 0.655 |
| Cleaning and storage habits | ||||||||||||
| Clean_chlorine | 0.063 | 0.088 | 0.225 | 0.023* | −0.082 | 0.331 | 0.061 | 0.361 | −0.148 | 0.125 | −0.144 | 0.034* |
| Clean_dishwasher | 0.032 | 0.264 | −0.171 | 0.039* | 0.107 | 0.176 | 0.006 | 0.939 | 0.072 | 0.304 | 0.061 | 0.563 |
| Clean_soap | 0.048 | 0.281 | 0.127 | 0.336 | 0.014 | 0.812 | −0.190 | 0.022* | −0.053 | 0.476 | −0.021 | 0.792 |
| Sink | 0.015 | 0.598 | 0.103 | 0.500 | 0.105 | 0.013* | 0.066 | 0.343 | 0.058 | 0.107 | 0.030 | 0.719 |
| Utensil dries | −0.077 | 0.107 | −0.338 | 0.004** | 0.065 | 0.346 | −0.176 | 0.119 | 0.029 | 0.424 | −0.044 | 0.672 |
| Socio‐demographics | ||||||||||||
| Norway | −0.144 | 0.003** | NA b | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Portugal | 0.144 | 0.003** | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Young > 18 years | 0.033 | 0.361 | −0.093 | 0.341 | 0.024 | 0.683 | NA | NA | 0.100 | 0.003** | 0.056 | 0.500 |
| Utensil condition | ||||||||||||
| New | −0.098 | 0.093 | −0.073 | 0.323 | 0.003 | 0.966 | −0.141 | 0.044* | NA | NA | NA | NA |
| Worn | 0.098 | 0.093 | 0.073 | 0.323 | −0.003 | 0.966 | 0.141 | 0.044* | NA | NA | NA | NA |
| Experimental settings | ||||||||||||
| Air | NA | NA | −0.029 | 0.610 | −0.135 | 0.054 | NA | NA | −0.093 | 0.010* | −0.280 | 0.001*** |
| Bag | NA | NA | 0.029 | 0.610 | 0.135 | 0.054 | NA | NA | 0.093 | 0.010* | 0.280 | 0.001*** |
| Control utensil | NA | NA | −0.354 | 0.015* | 0.077 | 0.068 | NA | NA | −0.135 | 0.039* | −0.085 | 0.181 |
The following independent variables are not reported in this table (p > 0.05 in all models); Usage habits: Usage frequency, change_dirty, change_schedule, cleaning and storage habits: washup_sink filled with water, container, countertop, hanging, socio‐demographics: child/teen uses, child<12 year, child 13–17 years, microbiologist, pet, utensil condition: clean, dirty, dry, humid, experimental settings: antibacterial (silver) (Table S2).
Not applicable.
p‐value <0.05
p‐value <0.01
p‐value <0.001.
Microbiota
In total, the bacteriota was identified in 14 used brushes and 41 sponges. Core‐feature analysis (genus level) of the bacteriota in sponges and brushes showed that the two countries had five core genera/families (taxa present in all items) in common: Acinetobacter, Chryseobacterium, Enhydrobacter, Enterobacteriaceae and Pseudomonas. Among these, Acinetobacter, Chryseobacterium, Enterobacteriaceae and Pseudomonas were also among the five most abundant taxa in both countries (Figures 1 and 2, Table 3). The major difference between the two countries was a lower relative abundance of Enhydrobacter in Portuguese items compared to Norwegian items, and this was majorly due to a lower abundance of Enterobacter in sponges than in brushes. For the five Norwegian households that provided both a brush and a sponge, the relative abundance of Enhydrobacter was significantly higher (p = 0.007) in brushes than in the sponges, while Acinetobacter was higher (p = 0.007) in sponges than in brushes (Figure 1). Core‐feature analysis on sub‐OTUs (sOTUs) showed that one sOTU representing Enhydrobacter was present in all items (Table 3). However, manual BLASTn search of the representative sequence yielded both Moraxella osloensis and Enhydrobacter aerosaccus as the closest match (both 100%). This sOTU was also the most abundant in the Norwegian items. Overall, Acinetobacter was the most abundant genus, represented by seven different sOTUs (among the core‐features above 90% shown in Table 3), where three were among the 10 most abundant in both collections. Two of the households provided two brushes each, where one of the brushes was older and downgraded from normal use and used only on very dirty dishes. The two brushes used only for very dirty dishes had a higher abundance of Acinetobacter and lower of Pseudomonas, than the brushes used for ordinary dishwashing (Figure 1).
TABLE 3.
Core and top 10 abundant taxa. The table shows the taxa of the core sOTUs (prevalent in >90% of items and the 10 most abundant sOTUs) in brushes and sponges from Norway and sponges from Portugal
| Taxa (sOTUs) | Norway a | Portugal b | ||
|---|---|---|---|---|
| top_10 c | % of items | top_10 | % of items | |
| Acinetobacter 1 d | 5 | 73.7 | 2 | 80.6 |
| Acinetobacter 2 | 9 | 5.3 | ||
| Acinetobacter 3 | 2 | 73.7 | 1 | 94.4 |
| Acinetobacter guillouiae 1 | 8 | 21.1 | ||
| Acinetobacter guillouiae 2 | 5 | 30.6 | ||
| Acinetobacter johnsonii | 4 | 68.4 | 4 | 77.8 |
| Acinetobacter rhizosphaerae | 3 | 78.9 | ||
| Aeromonadaceae | 94.4 | |||
| Brevundimonas vesicularis | 94.7 | 100.0 | ||
| Chryseobacterium | 6 | 78.9 | 3 | 97.2 |
| Comamonas terrigena | 100.0 | |||
| Enhydrobacter | 1 | 100.0 | 100.0 | |
| Enterobacteriaceae 1 | 97.2 | |||
| Enterobacteriaceae 2 | 7 | 72.2 | ||
| Enterobacteriaceae 3 | 10 | 55.6 | ||
| Pseudomonas | 7 | 73.7 | ||
| Pseudomonas fragi | 10 | 84.2 | ||
| Pseudomonas veronii | 8 | 88.9 | ||
| Roseomonas mucosa | 100.0 | |||
| Sphingobium yanoikuyae | 91.7 | |||
| Stenotrophomonas geniculata 1 | 94.7 | |||
| Stenotrophomonas geniculata 2 | 9 | 47.2 | ||
| Wautersiella | 6 | 88.9 | ||
Norway: 14 brushes and 5 sponges.
Portugal: 36 sponges.
The top 10 sOTUs are ranked from 1–10 and the percent of items that have this sOTU is shown in “% of items.” Missing values are due to sOTU either not being among the top 10 abundant or among core >90%.
The corresponding feature IDs are shown in Table S3.
To investigate differences in number of bacterial species and composition between brushes and sponges and potential correlation with reported consumer practices and household information, alpha diversity (population richness) and beta diversity (variation of bacteriota between items/environments) analyses were performed. There was no significant difference (p > 0.05) in population richness (observed OTUs) or beta diversity between Norwegian brushes (n = 14) and sponges (n = 5)). A significant higher population richness (observed OTUs) was found in products from households not having children <12 years (p = 0.02), and in items with low‐ or medium TVC (low (n = 5); medium (n = 6)) (p = 0.02) compared to items with high bacterial counts (n = 7). There was also a significant difference in community structure (beta diversity) between the TVC categories (p = 0.001); high versus low (p = 0.004); high versus medium (p = 0.002) when abundance information was not weighted (unweighted unifrac).
There was a significant higher population richness (observed OTUs) in visually dirty (n = 9) than visually clean (n = 27) sponges (p = 0.02) from Portugal. There was no significant difference (p > 0.05) in population richness (observed OTUs) or community structure between original and antimicrobial (S21) sponges, or between the different TVC categories.
The bacteriota analysis indicated that sequences classified to Campylobacter were present in nine of the cleaning utensils, at a low abundance (0.002–0.098%). However, a Campylobacter real‐time PCR analysis (targeting C. jejuni, C. coli and C. lari) of four of the Miseq‐positive items and 15 negative items, did not result in any positive signals. Sequences classified to Salmonella or Listeria were not found in any sponge or brush.
Consumer practices and survival of Salmonella
Effect of drying items
Brushes and sponges collected from consumers and newly purchased (control utensils) were inoculated with Salmonella and either kept in humid condition in a plastic bag or allowed to dry overnight on an open tray (sponges) or by hanging (brushes). About 1.5 log lower (p = 0.01) TVC and 3.3 log lower (p = 0.001) Salmonella counts were found in brushes stored hanging compared to those stored in a bag (Table 4). For used sponges, there was no significant effect on Salmonella or TVC levels by storing sponges on a tray compared to storing in a plastic bag (Table 4). It was observed that the sponges laying on trays were not completely dry at sampling after 1 day of storage. The Portuguese sponges were weighted after 1 day of storage, and the weight of the sponges in the bags was on average 2.1 times higher (14.3 g) than for the sponges in trays (6.8 g).
TABLE 4.
Salmonella and total viable count in sponges/brushes stored on a tray/hanging or in a bag overnight after Salmonella was added
| Number tested |
Total viable count (log cfu per brush/sponge) |
Salmonella (log cfu per brush/sponge) |
|||
|---|---|---|---|---|---|
| Tray/hanging a | Bag | Tray/hanging | Bag | ||
| Norway | |||||
| New brush | 2 | 3.3 b | 5.9 (0.1) c | <3.0 | 5.9 (0.1) |
| Used brush | 11/10 d | 6.8 (0.4) | 8.3 (0.2) | 3.9 (0.2) | 7.2 (0.2) |
| New sponge | 2 | 5.6 (0.2) | 6.5 (0.2) | 5.6 (0.2) | 6.6 (0.2) |
| Used sponge | 7 | 8.6 (0.4) | 9.1 (0.3) | 5.8 (0.3) | 6.6 (0.5) |
| Portugal | |||||
| New sponge | 2 | <5.7 e | <5.7 | <4.7 | <4.7 |
| Used sponge | 20/15 f | 9.7 (0.2) | 10.0 (0.2) | 6.1 (0.2) | 6.3 (0.2) |
Brushes were hanging and sponges stored openly on a tray.
One of the replicates below detection limit (<3.0 log per brush). Standard error not calculated.
Means of log cfu per brush/sponge with standard errors in parentheses.
11 brushes hanging, 10 brushes in plastic bag.
Below detection limit.
Number of sponges were 20 for TVC and 15 for Salmonella. For Salmonella, data from sponges below the detection limit for one or both storage conditions were removed.
Effect of the consumers' practices
The only reported consumer practice that affected survival of Salmonella in brushes according to the statistical analysis was the use of chlorine to disinfect the brush (p = 0.034, Table 2). However, it must be noted that this practice involved two brushes only, where both were hanging to dry; therefore, it is difficult to conclude from this limited sample size. Higher total bacterial numbers after incubation and storage were found in brushes that had been used for a long time (‘Last changed’ median 4.3 weeks, p = 0.038) (mean TVC last changed <4.3 weeks = 6.8 log cfu/item (15 items), >4.3 weeks = 7.6 log cfu/item (10 items)) and those from households with children above 18 years (p = 0.003, Table 2) (mean TVC children >18 = 7.7 log cfu/item (12 items), no children >18 = 6.1 log cfu/item (11 items)). The brushes were of five different types/brands, and there was no difference in bacterial levels (TVC or Salmonella) between different types of used brushes (see Figure S1 for photos of brushes). Also, there was no difference in water uptake (range 1.7–6.2 g per brush) in different types of used brushes or between new and used brushes.
Several self‐reported practices significantly (p < 0.05, Table 2) affected the survival of Salmonella in sponges. Levels of Salmonella were on average 0.9 log higher in sponges from households that routinely stored sponges in the sink (mean = 6.3 log [8 items]) than sponges reportedly stored in a container (30 items) or on the countertop (10 items). Sponges from households which primarily changed sponges when they were worn or old had on average 0.7 log lower Salmonella levels (mean = 5.4 log [40 items]) compared to households that changed sponges when they looked dirty (8 items). After incubation and storage, sponges that were reported to be cleaned in chlorine (21% of the total households) presented higher TVC (p = 0.023, mean = 10.4 log (12 items)) than sponges from other households (mean = 9.3 log [42 items]). Further, two reported practices gave a significant lower TVC; sponges cleaned in the dishwasher (p = 0.039, mean = 8.8 log [4 items]) and sponges reported to dry in‐between usage events (p = 0.004, mean = 8.9 log [38 items]), with the latter having the largest effect size and highest statistical significance due to a broader representation of the practice in the data material.
The background data used in this publication has been deposited in a data repository (Møretrø et al. 2022).
DISCUSSION
The results in the present study showed that in general, high bacterial numbers were observed in cleaning utensils used by consumers, and no clear link was found between bacterial levels and most consumer practices. An exception was the use of brushes instead of sponges for washing up. The collected sponges in Portugal and brushes in Norway were majorly used for washing up. A previous survey confirmed that sponges and brushes were the most common cleaning utensils for washing up in Portugal and Norway, respectively (Møretrø, Moen, et al., 2021). The results in the present study indicated that cleaning utensils that dried between use would have lower numbers of bacteria including pathogens. This was supported by the experiment where Salmonella was added to the brushes and which were either kept humid in a plastic bag or allowed to dry by hanging, for which the bacterial numbers where highest in brushes kept humid in plastic bags. The difference in bacterial numbers between sponges in plastic bags and sponges stored on a tray was not significant. This is in line with the fact that brushes dry faster than sponges, and it was observed that some of the sponges on the trays were not completely dry even after the overnight incubation, which may explain the lower difference in bacterial numbers between sponges in plastic bags and on trays than between brushes in plastic bags and hanging brushes. On the other hand, for sponges reported by consumers to dry in between use, lower TVC but not Salmonella counts were observed for sponges to which Salmonella was added. Therefore, it cannot be ruled out that drying may lower bacterial numbers also in sponges, but the effect on food safety is unclear as Salmonella was not affected. In used brushes, it was observed that Salmonella was more sensitive to drying than the other bacteria present. The brushes likely dry up completely between use and this may select for bacteria that are more adapted to dry conditions than Salmonella.The bacterial levels found in brushes in the present study were in the same range as in a UK study among elderly (3–8 log cfu per brush) (Evans & Redmond, 2019). There were higher bacterial numbers in the used brushes collected from consumers in the present study, compared to bacterial levels obtained after simulated usage in new brushes in a previous laboratory study (Møretrø, Moen, et al., 2021). In the previous laboratory experiment where bacteria and a food soil mix were added to new brushes, there was a reduction in total bacterial levels from 6 log to 4 log cfu per brush after 1 day, and the level remained at 3–4 log cfu per brush for the rest of the 7 days study. It is not clear whether this lower bacterial level was due to the brushes being new or differences in for example humidity, food soils, adaptation to stress, type of bacteria between the laboratory model system and conditions during use in kitchens. In the present study, the levels of Salmonella after addition and overnight incubation were lower for new than used brushes, both for brushes kept humid and allowed to dry. Also, when used brushes were analysed directly, brushes that looked new had lower TVC levels. Evans and Redmond (2019) found a positive correlation between Enterobacteriaceae concentrations and usage length of brushes. Together this indicates that used brushes may support higher bacterial growth and survival compared to new brushes. Most of the used brushes were reported to have been in use for several months and many of them were visibly worn, and it is possible that scratches, degeneration of fibres or accumulation of soil in the used brushes facilitate colonization of bacteria and/or make the brushes more difficult to clean compared to new brushes. However, since we found no significant differences in levels of the pathogen Salmonella between new and used brushes, and no increased Salmonella numbers in old/worn brushes, we cannot conclude based on data in the present study that consumers should be advised to change their brushes regularly from a safety standpoint. Further work may be done to test whether survival of other pathogens, for example Campylobacter, is higher in worn brushes. We chose not to include Campylobacter in the experiments as we observed in initial tests that the direct enumeration of Campylobacter on mCCDA plates failed due to the overgrowth of background flora from used brushes and sponges. A modified experiment combining a Campylobacter enrichment step with MPN enumeration may be an alternative approach in further research.
The bacterial counts in used Portuguese sponges were considerably higher than in Norwegian used sponges. The Portuguese sponges were used more frequently than Norwegian sponges. The higher usage frequency may result in Portuguese sponges being humid more often, which may explain the higher bacterial levels. The highest bacterial count (9 log cfu/sponge) among the Norwegian used sponges was found for a sponge reportedly used several times a day. In both countries, the sponges were reportedly used for washing up casseroles, pots, pans, etc. and very dirty dishes. However, Norwegian consumers reportedly used brushes more often than sponges for such purposes, which probably leads to less usage of the sponges than in Portugal. There are several other studies from other countries (Brazil, Netherlands, UK) on bacterial levels in used kitchen sponges with a reported bacterial count in the range 6–9 log (Hilton & Austin, 2000; Ikawa & Rossen, 1999; Kusumaningrum et al., 2002; Rossi et al., 2013), meaning that the levels in the present Portuguese study were higher than in other studies. However, as very limited information about the use of the sponges was provided in the other studies, it is not known why higher bacterial numbers were observed in the Portuguese sponges.Virtually, no consumer practices investigated had a significant effect on the bacterial numbers in sponges or brushes when analysed at the point of delivery. One exception was cleaning of brushes in soap, which led to lower total numbers of bacteria. Regarding bacterial numbers after addition of Salmonella, sponges that had reportedly been cleaned with a dishwasher at the consumers’ homes showed lower TVC and those cleaned with chlorine higher TVC than the sample average. These results were in general not completely in line with previous results, as cleaning of sponges by rinsing in water, or cleaning with soap are not very effective, while cleaning with chlorine is effective (Møretrø, Moen, et al., 2021; Sharma et al., 2009). It should be noted that the present study was very unbalanced in terms of number of households reporting using chlorine; thus, the present sample does not allow concluding on the benefits and drawbacks of using chlorine.
The finding that used brushes cleaned in dishwasher had higher TVC than when cleaned with soap was surprising, as cleaning of new brushes in dishwasher was effective in the previous laboratory study (Møretrø, Moen, et al., 2021). It may be speculated that repeated cleaning in dishwasher can contribute to the brush being worn and this may increase bacterial colonization; however, the frequency of the cleaning procedure is not known and may also affect the results.
There was no difference in bacterial levels or diversity between the antimicrobial sponges containing silver (S21), and the other sponges used by consumers in Portugal. Previously, in laboratory experiments over 7 days with new sponges (not used by consumers) inoculated with kitchen associated bacteria, Salmonella and Campylobacter and a food soil mix, slower total bacterial growth and higher reduction of Salmonella and Campylobacter were found in the antimicrobial S21 sponge than in two other types of sponges, but no inhibition zones were seen in agar diffusion tests. It was not clear whether the lower bacterial levels were due to the addition of silver to the sponge or other factors (Møretrø, Moen, et al., 2021). Based on the results from the two studies, it cannot be excluded that there may be an initial antibacterial effect in new antimicrobial S21 sponges. However, such an effect was not seen for sponges used for 4 weeks by consumers, indicating that the addition of silver to the sponge had no effect during long‐term practical use. It is known that products with added antibacterial compounds often show limited antibacterial effects during practical use: this may be due to factors such as too low concentration of antibacterial compound, decreasing concentration over time and neutralization of the antibacterial effect by food soil (DeFlorio et al., 2021; Møretrø & Langsrud, 2011).
Although the bacterial levels between different used brushes and sponges varied with several magnitudes, the bacterial diversity was more uniform (Figures 1 and 2, Table 3), with five core genera/family that were present in all items: Acinetobacter, Chryseobacterium, Enhydrobacter, Enterobacteriaceae and Pseudomonas. Also, in a German study on 14 used sponges, the dominating OTUs were rather ubiquitous, as the 10 most commonly isolated OTUs represented 69% of all sequences and 9 of the 10 most common OTUs were found in between 11 and 14 of the sponges (Cardinale et al., 2017). In both the present (Figures 1 and 2, Table 3) and the German studies, the dominating bacterial class were Gammaproteobacteria, and many of the same genera (Acinetobacter, Enhydrobacter, Pseudomonas, Chryseobacterium) dominated in both studies. Also, in another study on 20 German sponges, the dominating bacteria were similar as in the two other studies as the most commonly detected genera were Acinetobacter (22%), Enhydrobacter (8%), Agrobacterium (6%), Pseudomonas (5%) and Chryseobacterium (2%) (Jacksch et al., 2020). Based on the present and the two German studies, this suggests that there may be a set of common bacteria, with Acinetobacter, Enhydrobacter, Pseudomonas and Chryseobacterium as the most dominating, in used sponges and brushes across different countries in Europe. These bacteria seem to be present regardless of variations in utensil type, storage conditions, frequency of use, cleaning procedures and household composition. It is not clear whether these bacteria are constantly introduced and/or persisting in the brushes and sponges. Pseudomonas and Acinetobacter are widely distributed in nature (e.g. soil and water), are among the most commonly isolated bacteria in relatively humid food processing environments and are also frequently isolated from foods. These bacteria have low nutrient requirements, grow fast at a wide temperature range and may form biofilms (Møretrø & Langsrud, 2017). Chryseobacterium has been found in salmon and small‐scale cheese production environments and in raw foods (de Beer et al., 2005; Møretrø et al., 2016; Schirmer et al., 2013; Tsôeu et al., 2016). Enhydrobacter is not frequently found in food processing environments, but was reported to dominate in Norwegian kitchen sinks in a previous study (Moen et al., 2016). The cleaning utensils are majorly used and commonly stored in the sink, but it is not clear if they are contaminated with Enhydrobacter from the sink, or opposite. As the kitchen environment is generally drier than many food processing environments and that Enhydrobacter was especially associated with brushes (that dry fast), this may indicate that Enhydrobacter is especially adapted to dry environments. Enhydrobacter/Moraxella are among the dominating bacteria on human skin (Li et al., 2021), which is a very dry environment. Also, other explanations for the dominance of Enhydrobacter in kitchen environments (brushes, sponges, sinks), but not in food processing environments, are possible. Their common presence on human skin may lead to more frequent transfers to surfaces and cleaning utensils in kitchens than in the food industry, where fewer surfaces are in direct contact with human skin. Another hypothesis could be that Enhydrobacter is sensitive to sanitation agents used in the food industry; however, more research is necessary to conclude on why Enhydrobacter is dominating in cleaning utensils in kitchens (and not in food processing environments). The sOTU representing Enhydrobacter was the only sOTU that was present in all items in the current study. Manual BLASTn search of the representative sequence for Enhydrobacter yielded Moraxella osloensis and Enhydrobacter aerosaccus as the closest relative. This sequence match was also observed by Cardinale et al. (2017). Moraxella osloensis is also known for generating malodor in laundry (Kubota et al., 2012) and Cardinale et al. (2017) speculated that the abundant occurrence of this bacterium might be responsible for bad smelling kitchen sponges.
The bacteriota analysis in the present study showed that the bacterial foodborne pathogens Salmonella spp. and Listeria monocytogenes were not present in detectable numbers in used brushes and sponges. Campylobacter was found in six sponges and three brushes, comprising up to 0.1% of the total sequences, however control studies with real‐time PCR failed to detect Campylobacter. The presence of sequences classified as Campylobacter could be an artefact of the MiSeq analysis. Due to complexities of next generation sequencing methodologies and sequence‐based microbial identification, there may be a risk of false‐positive and/or false‐negative results. However, we cannot rule out the presence of other Campylobacter species than the ones tested for (C. jejuni, C. coli and C. lari) in the real‐time PCR assay.
The low relative abundance of foodborne pathogenic bacteria is in line with the other studies on sponges (Cardinale et al., 2017; Jacksch et al., 2020). In some studies, safety concerns are raised due to the presence of high numbers of Enterobacteriaceae, Moraxella, Chryseobacterium, Acinetobacter spp., etc. in sponges, based on claims that these types of bacteria are potentially pathogens or opportunistic pathogens (Cardinale et al., 2017; Dey et al., 2020; Osaili et al., 2020). We think that the relevance for public health of the presence of these groups of bacteria in cleaning utensils, which were also confirmed in the present study, is difficult to evaluate based on amplicon based bacteriota analysis or other methodologies with low resolution. The pathogenic potential of a bacteria is often species‐dependent and sometimes strain‐dependent, and methods with high resolution are needed to identify the bacteria to the species level and/or to evaluate if for example virulence factors or antibiotic resistance genes are present to evaluate the pathogenic potential of the bacteria. Cardinale et al. (2017) previously reported that sponges that had been cleaned by consumers were associated with higher concentrations of opportunistic pathogens, but no effects of reported cleaning methods on the bacteriota were observed in the present study.
It should be noted that the consumer results in the present study present some limitations pertaining to the recruitment of a convenience sample limited in size and biased towards food scientists and microbiologists. However, the inclusion of two different cultures (Norway and Portugal) contributes to documenting a wide range of cleaning utensil practices and to uncover effects that are robust across household conditions and types or brands of cleaning utensils. Further, some of the studied practices were poorly represented while others were broadly represented in the participating households, leading to an unbalanced investigation of the different practices. Further research is recommended with larger consumer samples or in controlled intervention studies, to better investigate the effects of dish cleaning utensil practices on pathogenic bacterial growth/survival.
In general, the results on used sponges and brushes from the present study supported the conclusions and advice from our previous study on new sponges and brushes about the importance of drying and on using brushes instead of sponges for washing up (Møretrø, Moen, et al., 2021). Also, some food safety authorities do not recommend sponges for cleaning (BfR, 2020; WHO, 2006).
CONCLUSIONS
Non‐pathogenic bacteria dominated the used sponges and brushes, and a set of common bacteria comprised of Acinetobacter, Chryseobacterium, Enhydrobacter, Enterobacteriaceae and Pseudomonas were commonly abundant and seemed to be robust against variations in usage of the cleaning utensils. Practices found to prevent Salmonella growth in sponges in the case of a later contamination included: a habit of changing the sponge when it is worn and not storing the sponge in the sink. The results confirm that drying brushes can kill bacteria, including the pathogen Salmonella. While brushes will often dry up between usage occurrences, drying of sponges is difficult to obtain if sponges are used daily. Thus, to prevent health hazards in case of Salmonella contamination on the utensil, consumers should be advised to use brushes. Further research is recommended to investigate the role of consumer practices, and in particular, the role of cleaning routines on brushes as well as the lifetime of brushes from a hygienic viewpoint. Also, studies may be expanded with pathogens other than Salmonella.
CONFLICT OF INTEREST
No conflict of interest declared.
Supporting information
Figure S1
Table S1
Table S2
Table S3
ACKNOWLEDGEMENTS
This research was funded by the European Commission H2020–SFS–2016–2017: Project no. 727580 Safeconsume, the Norwegian research funding for agriculture and the food industry, Norway grant nos. 262306 and 314743, and the Research Council of Norway grant no. 296083. Tove Maugesten, Charlotte Nilsen, Anette Wold Åsli, Merete Rusås Jensen and Rui Magalhães are thanked for excellent technical assistance.
Møretrø, T. , Ferreira, V.B. , Moen, B. , Almli, V.L. , Teixeira, P. & Kasbo, I.M. et al. (2022) Bacterial levels and diversity in kitchen sponges and dishwashing brushes used by consumers. Journal of Applied Microbiology, 133, 1378–1391. Available from: 10.1111/jam.15621
REFERENCES
- Amir, A. , McDonald, D. , Navas‐Molina, J.A. , Kopylova, E. , Morton, J.T. , Xu, Z.Z. et al. (2017) Deblur rapidly resolves single‐nucleotide community sequence patterns. Msystems, 2, e00191‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apprill, A. , McNally, S. , Parsons, R. & Weber, L. (2015) Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquatic Microbial Ecology, 75, 129–137. [Google Scholar]
- BfR (2020) Frequently asked questions about protection against foodborne infections in private households . https://www.bfr.bund.de/cm/349/frequently‐asked‐questions‐about‐protection‐against‐foodborne‐infections‐in‐private‐households.pdf.
- Bokulich, N.A. , Kaehler, B.D. , Rideout, J.R. , Dillon, M. , Bolyen, E. , Knight, R. et al. (2018) Optimizing taxonomic classification of marker‐gene amplicon sequences with QIIME 2 ' s q2‐feature‐classifier plugin. Microbiome, 6, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolyen, E. , Rideout, J.R. , Dillon, M.R. , Bokulich, N. , Abnet, C.C. , Al‐Ghalith, G.A. et al. (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nature Biotechnology, 37, 852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso, J.G. , Lauber, C.L. , Walters, W.A. , Berg‐Lyons, D. , Huntley, J. , Fierer, N. et al. (2012) Ultra‐high‐throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME Journal, 6, 1621–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale, M. , Kaiser, D. , Lueders, T. , Schnell, S. & Egert, M. (2017) Microbiome analysis and confocal microscopy of used kitchen sponges reveal massive colonization by Acinetobacter, Moraxella and Chryseobacterium species. Scientific Reports, 7, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogan, T.A. , Slader, J. , Bloomfield, S.F. & Humphrey, T.J. (2002) Achieving hygiene in the domestic kitchen: the effectiveness of commonly used cleaning procedures. Journal of Applied Microbiology, 92, 885–892. [DOI] [PubMed] [Google Scholar]
- de Beer, H. , Hugo, C.J. , Jooste, P.J. , Willems, A. , Vancanneyt, M. , Coenye, T. et al. (2005) Chryseobacterium vrystaatense sp. nov., isolated from raw chicken in a chicken‐processing plant. International Journal of Systematic and Evolutionary Microbiology, 55, 2149–2153. [DOI] [PubMed] [Google Scholar]
- DeFlorio, W. , Liu, S. , White, A.R. , Taylor, T.M. , Cisneros‐Zevallos, L. , Min, Y. et al. (2021) Recent developments in antimicrobial and antifoulingcoatings to reduce or prevent contamination andcross‐contamination of food contact surfaces by bacteria. Comprehensive Reviews in Food Science and Food Safety, 20, 3093–3134. [DOI] [PubMed] [Google Scholar]
- Dey, N. , Farjees, F.A. , Shafina, F.S. & Sowjenya, M. (2020) Microbial evaluation of domestic kitchen sponge. International Journal of Advanced Research in Engineering and Technology, 11, 657–663. [Google Scholar]
- Evans, E.W. & Redmond, E.C. (2019) Domestic kitchen microbiological contamination and self‐reported food hygiene practices of older adult consumers. Journal of Food Protection, 82, 1326–1335. [DOI] [PubMed] [Google Scholar]
- Hilton, A.C. & Austin, E. (2000) The kitchen dishcloth as a source of and vehicle for foodborne pathogens in a domestic setting. International Journal of Environmental Research and Public Health, 10, 257–261. [Google Scholar]
- Ikawa, J.Y. & Rossen, J.S. (1999) Reducing bacteria in household sponges. Journal of Environmental Health, 62, 18–22. [Google Scholar]
- Jacksch, S. , Thota, J. , Shetty, S. , Smidt, H. , Schnell, S. & Egert, M. (2020) Metagenomic analysis of regularly microwave‐treated and untreated domestic kitchen sponges. Microorganisms, 8, 736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota, H. , Mitani, A. , Niwano, Y. , Takeuchi, K. , Tanaka, A. , Yamaguchi, N. et al. (2012) Moraxella species are primarily responsible for generating malodor in laundry. Applied and Environmental Microbiology, 78, 3317–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumaningrum, H.D. , van Putten, M.M. , Rombouts, F.M. & Beumer, R.R. (2002) Effects of antibacterial dishwashing liquid on foodborne pathogens and competitive microorganisms in kitchen sponges. Journal of Food Protection, 65, 61–65. [DOI] [PubMed] [Google Scholar]
- Li, Z. , Xia, J. , Jiang, L. , Tan, Y. , An, Y. , Zhu, X. et al. (2021) Characterization of the human skin resistome and identification of two microbiota cutotypes. Microbiome, 9, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens, H. & Martens, M. (2000) Modified Jack‐knife estimation of parameter uncertainty in bilinear modelling by partial least squares regression (PLSR). Food Quality and Preference, 11, 5–16. [Google Scholar]
- Mattick, K. , Durham, K. , Domingue, G. , Jorgensen, F. , Sen, M. , Schaffner, D.W. et al. (2003) The survival of foodborne pathogens during domestic washing‐up and subsequent transfer onto washing‐up sponges, kitchen surfaces and food. International Journal of Food Microbiology, 85, 213–226. [DOI] [PubMed] [Google Scholar]
- McDonald, D. , Clemente, J.C. , Kuczynski, J. , Rideout, J.R. , Stombaugh, J. , Wendel, D. et al. (2012) The biological observation matrix (BIOM) format or: how I learned to stop worrying and love the ome‐ome. Gigascience, 1, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moen, B. , Røssvoll, E. , Måge, I. , Møretrø, T. & Langsrud, S. (2016) Microbiota formed on attached stainless steel coupons correlates with the natural biofilm of the sink surface in domestic kitchens. Canadian Journal of Microbiology, 62, 148–160. [DOI] [PubMed] [Google Scholar]
- Møretrø, T. & Langsrud, S. (2011) Effects of materials containing antimicrobial compounds on food hygiene. Journal of Food Protection, 74, 1200–1211. [DOI] [PubMed] [Google Scholar]
- Møretrø, T. & Langsrud, S. (2017) Residential bacteria on surfaces in the food industry and their implications for food safety and quality. Comprehensive Reviews in Food Science and Food Safety, 16, 1022–1041. [DOI] [PubMed] [Google Scholar]
- Møretrø, T. , Moen, B. , Almli, V. & Ferreira, V. (2022) Bacterial levels and diversity in kitchen sponges and dishwashing brushes used by consumers. Mendeley Data, V1. 10.17632/7c49rz45c4.1 [DOI] [PMC free article] [PubMed]
- Møretrø, T. , Moen, B. , Almli, V.L. , Teixeira, P. , Ferreira, V.B. , Åsli, A.W. et al. (2021) Dishwashing sponges and brushes: consumer practices and bacterial growth and survival. International Journal of Food Microbiology, 337, 108928. [DOI] [PubMed] [Google Scholar]
- Møretrø, T. , Moen, B. , Heir, E. , Hansen, A.A. & Langsrud, S. (2016) Contamination of salmon fillets and processing plants with spoilage bacteria. International Journal of Food Microbiology, 237, 98–108. [DOI] [PubMed] [Google Scholar]
- Møretrø, T. , Nguyen‐The, C. , Didier, P. , Maître, I. , Izsó, T. , Kasza, G. et al. (2021) Consumer practices and prevalence of campylobacter, salmonella and norovirus in kitchens from six European countries. International Journal of Food Microbiology, 347, 109172. [DOI] [PubMed] [Google Scholar]
- NordVal International/NMKL . (2019) Campylobacter real‐time PCR . https://www.nmkl.org/wp‐content/uploads/2022/03/NordVal‐certificate‐017‐Campylobacter‐real‐time‐PCR_Eurofins_01June2021.pdf
- Osaili, T.M. , Obaid, R.S. , Alowais, K. , Almahmood, R. , Almansoori, M. , Alayadhi, N. et al. (2020) Microbiological quality of kitchens sponges used in university student dormitories. BMC Public Health, 20, 1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada, A.E. , Needham, D.M. & Fuhrman, J.A. (2016) Every base matters: assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environmental Microbiology, 18, 1403–1414. [DOI] [PubMed] [Google Scholar]
- Pedregosa, F. , Varoquaux, G. , Gramfort, A. , Michel, V. , Thirion, B. , Grisel, O. et al. (2011) Scikit‐learn: machine learning in python. Journal of Machine Learning Research, 12, 2825–2830. [Google Scholar]
- Rossi, E.M. , Scapin, D. & Tondo, E.C. (2013) Survival and transfer of microorganisms from kitchen sponges to surfaces of stainless steel and polyethylene. Journal of Infection in Developing Countries, 7, 229–234. [DOI] [PubMed] [Google Scholar]
- Schirmer, B.C.T. , Heir, E. , Møretrø, T. , Skaar, I. & Langsrud, S. (2013) Microbial background flora in small‐scale cheese production facilities does not inhibit growth and surface attachment of listeria monocytogenes . Journal of Dairy Science, 96, 6161–6171. [DOI] [PubMed] [Google Scholar]
- Sharma, M. , Eastridge, J. & Mudd, C. (2009) Effective household disinfection methods of kitchen sponges. Food Control, 20, 310–313. [Google Scholar]
- Tsôeu, L.I. , Jooste, P.J. , Charimba, G. & Hugo, C.J. (2016) Spoilage potential of a novel group of bacteria isolated from dairy products. South African Journal of Science, 112, 1–8. [Google Scholar]
- WHO (2006) Five keys to safer food manual . https://apps.who.int/iris/bitstream/handle/10665/43546/9789241594639_eng.pdf;jsessionid=576F1F0C053A9245028CC066A828193E?sequence=1.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Table S1
Table S2
Table S3


