Abstract
Background
Despite improved medical treatment strategies, postoperative pain, nausea, and vomiting remain major challenges. This systematic review investigated the relationship between perioperative respiratory and hemodynamic interventions and postoperative pain, nausea, and vomiting.
Methods
PubMed and Embase were searched on March 8, 2021 for randomized clinical trials investigating the effect of perioperative respiratory or hemodynamic interventions in adults undergoing non‐cardiac surgery. Investigators reviewed trials for relevance, extracted data, and assessed risk of bias. Meta‐analyses were performed when feasible. GRADE was used to assess the certainty of the evidence.
Results
This review included 65 original trials; of these 48% had pain, nausea, and/or vomiting as the primary focus. No reduction of postoperative pain was found in meta‐analyses when comparing recruitment maneuvers with no recruitment, high (80%) to low (30%) fraction of oxygen, low (5–7 ml/kg) to high (9–12 ml/kg) tidal volume, or goal‐directed hemodynamic therapy to standard care. In the meta‐analysis comparing recruitment maneuvers with no recruitment maneuvers, patients undergoing laparoscopic gynecological surgery had less shoulder pain 24 h postoperatively (mean difference in the numeric rating scale from 0 to 10: −1.1, 95% CI: −1.7, −0.5). In meta‐analyses, comparing high to low fraction of inspired oxygen and goal‐directed hemodynamic therapy to standard care in patients undergoing abdominal surgery, the risk of postoperative nausea and vomiting was reduced (odds ratio: 0.45, 95% CI: 0.24, 0.87 and 0.48, 95% CI: 0.27, 0.85). The certainty in the evidence was mostly very low to low. The results should be considered exploratory given the lack of prespecified hypotheses and corresponding risk of Type 1 errors.
Conclusion
There is limited evidence regarding the impact of intraoperative respiratory and hemodynamic interventions on postoperative pain or nausea and vomiting. More definitive trials are needed to guide clinical care within this area.
Keywords: anesthesia, hemodynamic, respiratory, review, pain, nausea, vomiting, PONV
Editorial Comment.
This systematic review assessed published clinical trials, which provide results concerning controlled intraoperative treatments for ventilation and circulatory management, and nausea/vomiting and pain outcomes after surgery. The study design was explorative—it did not include prospective hypotheses for specific treatments and outcomes, but rather general categories of interventions and outcomes. The authors found limited evidence, but some which might suggest that there could be a relation between inspired oxygen levels as well as goal‐directed circulatory therapy and postoperative nausea or vomiting.
1. INTRODUCTION
Severe postoperative pain, nausea, and vomiting remain major challenges. Despite improvements in treatment strategies, patients often experience these symptoms following surgery. Postoperative pain is associated with serious complications including increased incidence of pulmonary and cardiac complications, development of chronic pain, prolonged hospitalization, and increased mortality. 1 Postoperative nausea and vomiting (PONV) is associated with prolonged time to hospital discharge. 2
Pre‐, intra‐, and postoperatively, various medical interventions are used to minimize pain, nausea, and vomiting in the postoperative setting and avoid severe complications. 3 Recent reviews have shown limited effects of specific intra‐operative respiratory and hemodynamic interventions on “hard” endpoints such as hospital length of stay and mortality. 4 , 5 , 6 , 7 However, identification of intraoperative respiratory and hemodynamic interventions and specific targets that can influence the development and intensity of postoperative pain, nausea, and vomiting would be valuable additions in a multimodal approach.
The aim of this hypothesis‐generating systematic review was to describe the literature regarding perioperative respiratory and hemodynamic interventions potentially affecting postoperative development of pain, nausea, and vomiting and explore any associations between these interventions and outcomes in meta‐analyses when possible.
2. METHODS
2.1. Protocol and registration
This paper is part of a larger systematic review project including clinical trials assessing various respiratory and hemodynamic interventions and targets for patients undergoing non‐cardiac surgery under general anesthesia. Previous manuscripts from this review project have focused on the outcomes mortality, length of stay, and postoperative complications. 4 , 5 , 6 , 7 In this manuscript, we focus on the outcomes of postoperative pain, nausea, and vomiting.
The protocol was uploaded to Figshare.com on June 11, 2020 and updated on August 19, 2020. The protocol is provided in the Supplementary Content. Reporting of this review followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. 8 The PRISMA checklist is provided in the Supplementary Content.
2.2. Eligibility criteria and population identification
The population of interest was adult patients under general anesthesia and invasive mechanical ventilation undergoing non‐cardiac surgery. Trials including caesarean sections, interventional radiology, very short duration of anesthesia (e.g., for electroconvulsive therapy), and surgery requiring one‐lung ventilation were excluded. Only English language publications were included, and there was no limitation regarding year of publication.
All specific intraoperative respiratory and hemodynamic interventions and targets (e.g., fraction of inspired oxygen, end‐tidal or arterial carbon dioxide level, tidal volume, positive end‐expiratory pressure, recruitment maneuver, goal‐directed hemodynamic therapy [GDHT], blood pressure, and different ventilation modes) were included. The comparator could be a different target or standard of care. Interventions occurring only pre‐ or postoperatively were not included. This manuscript includes all trials from the original search reporting the following outcomes: (1) postoperative pain, (2) administration of analgesics, and/or (3) nausea and vomiting.
2.3. Search strategy
PubMed and Embase were searched on July 24, 2020, and again on March 8, 2021. The full search strategies for both databases are provided in the protocol. The search included a combination of various text and indexing search terms for general anesthesia or surgery and the various respiratory and hemodynamic targets. The Cochrane sensitivity‐maximizing search strategy was used to identify randomized trials. 9 The bibliographies of included articles were reviewed for additional relevant articles.
2.4. Study selection and data collection
Reviewers in pairs independently screened titles and abstracts retrieved from the systematic searches. Subsequently, articles were assessed in full text. Using a predefined standardized form, reviewers extracted data from the individual manuscripts. During all steps, any disagreement regarding eligibility and the extracted data was resolved via discussion between the reviewers and a third investigator if needed.
2.5. Outcome definitions and timeframes
Nausea, vomiting, PONV, and pain were the main outcomes in this manuscript. These outcomes were not prespecified in the protocol. Nausea is a subjectively unpleasant sensation associated with the awareness of the urge to vomit, whereas vomiting is an objective symptom; both are in general reported as incidences (i.e., yes/no) within a given timeframe. In some studies, nausea is quantified using a numeric rating scale (NRS), visual analogue scale (VAS), or categorized (e.g., none/mild/severe). VAS is an analog scale frequently presented as a 10 cm long line. The patients are asked to make a mark on the line corresponding to a particular symptom. NRS is a discrete numerical scale from 0 to 10 requiring that the patient choose a specific number to rate their symptoms. PONV is a composite outcome including nausea or vomiting.
Pain is a subjective symptom and is mostly reported using NRS or VAS (ranging from 0 to 10 or 0 to 100), where 0 is no pain and 10/100 is the worst pain imaginable. We report pain on the 0 to 10 scale. We also collected data on postoperative analgesic use. However, this data were very inconsistently reported and was therefore not considered further for meta‐analyses.
Various time points for the outcomes were reported in the included publications. Further details are provided in the Supplementary Content.
2.6. Risk of bias in individual trials
Using version 2 of the Cochrane risk‐of‐bias tool for randomized trials, risk of bias in the individual trials was independently assessed by two reviewers. 10 Disagreements were resolved via discussion. Risk of bias was assessed for each outcome within a trial but is reported at the trial level as the highest risk of bias score across all outcomes. However, in most included trials, the risk of bias was the same across all outcomes. If the bias was different for the various outcomes, this was noted. Additional considerations about bias assessment are provided in the Supplementary Content.
2.7. Statistical analyses
Trials were assessed for clinical (i.e., participants, interventions, comparators, and outcomes) and methodological (i.e., study design or risk of bias) heterogeneity. If major heterogeneity was identified, no meta‐analyses were performed, and a descriptive summary of the trials was provided. A minimum of three trials reporting relevant events was required to perform meta‐analyses if there was no major heterogeneity. Meta‐analyses were performed using Review Manager 5.4.1 (Cochrane Collaboration, Nordic Cochrane Centre, Copenhagen, Denmark). Given that these meta‐analyses were not clearly specified in the protocol, they should be considered exploratory. For binary outcomes, we conducted meta‐analyses using Mantel–Haenszel random effects models. Results from these analyses are reported as odds ratios (ORs) with 95% confidence intervals (CI) with values below 1 indicating better outcomes in the intervention group. DerSimonian and Laird random effects meta‐analyses were used for continuous outcomes. Results from these analyses are presented as mean differences with 95% CI with values below zero indicating better outcomes in the intervention group. To allow for meta‐analyses, any continuous outcome reported as a median with a measure of variance (e.g., quartiles, range) was transformed to a mean and a standard deviation using the method described by Shi et al. 11 Statistical heterogeneity was assessed using forest plots and I‐squared statistics. 12 Based on the available data, exploratory subgroup analyses according to surgical characteristics were conducted.
2.8. Cumulative evidence
The certainty of the overall evidence for a given comparison and outcome was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology and classified within one of four categories: very low, low, moderate, or high certainty of evidence. 13 Additional details are provided in the Supplementary Content. GRADE evaluation was only performed when a meta‐analysis was feasible. GRADEpro (McMaster University, 2020) was used for drafting of the GRADE tables.
3. RESULTS
Overall, the search identified 23,454 unique records of which 535 full‐text articles were assessed for eligibility. Of 209 manuscripts included in previous reviews, 4 , 5 , 6 , 7 63 manuscripts including relevant outcomes for the current manuscript were identified. Review of references and previous reviews resulted in identification of two additional manuscripts, yielding a total of 65 manuscripts (Figure S1). An overview of the trials is provided in Table S1. Pain and/or nausea and vomiting were the primary focus in 31 of the manuscripts (48%). There was a large degree of heterogeneity among the included trials, for example in the type of surgery and the definition of the reported outcomes.
3.1. Fraction of inspired oxygen
Eighteen publications investigated a higher vs. a lower fraction of oxygen. 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 In the majority of the trials, a FiO2 of 80% was compared with a FiO2 of 30%. 14 , 16 , 17 , 18 , 19 , 20 , 21 , 23 , 24 , 26 , 27 , 29 , 30 , 31 A few trials investigated different mixtures of the combination of oxygen and nitrous oxide 19 , 21 , 25 of which two were eligible for meta‐analyses. 19 , 21 Five trials contained more than 500 patients including two trials of 2012 and 4702 patients. 17 , 18 , 19 , 20 , 31 The majority of the trials included surgery within the abdominal region (i.e., gastrointestinal, gynecologic, and urologic surgery). Additional information is reported in Tables S2–S4. All trials were assessed as having an intermediate risk of bias (Table S5).
In a meta‐analysis including 12 trials and 5583 patients, there was no clear difference in PONV (OR: 0.75; 95% CI: 0.52, 1.07; Figure 1) between a high and a low fraction of inspired oxygen. In the subgroup analysis according to surgery type (Figure 1), a FiO2 of 80% resulted in a decrease in PONV in trials primarily including patients having abdominal surgery (OR: 0.45, 95% CI: 0.24, 0.87), whereas there was no significant difference in PONV for laparoscopic gynecological surgery patients (OR 0.74, 95% CI: 0.41, 1.32), or other surgeries (OR: 1.31, 95%CI: 0.97, 1.77). The p value for a subgroup difference was 0.02. Table S6 summarizes meta‐analyses for nausea and vomiting separately and at different time points.
FIGURE 1.
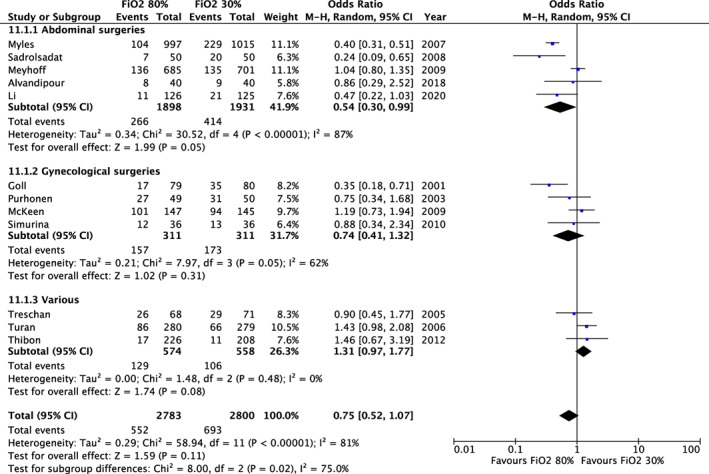
Fraction of inspired oxygen and postoperative nausea and vomiting. Results from random‐effects meta‐analyses of trials assessing fraction of inspired oxygen. Results are displayed as odds ratios (dots) with 95% confidence intervals (error bars). Values below 1 indicate reduced postoperative nausea and vomiting with 80% inspired oxygen. Subgroup analysis by abdominal surgeries, gynecological surgeries, and various surgeries.
There was no difference in pain early postoperatively (0–6 h) nor late postoperatively (2–24 h), mean difference 0.0 (95% CI: −0.1, 0.0) and mean difference − 0.1 (95% CI: −0.3, 0.1), Table S6.
Details of the GRADE evaluation are provided in Table S7. The certainty in the evidence was assessed as very low for PONV and low to moderate for postoperative pain.
3.2. End‐tidal or arterial carbon dioxide level
Seven publications investigated different end‐tidal or arterial carbon dioxide level. 32 , 33 , 34 , 35 , 36 , 37 , 38 Four trials compared PetCO2 levels ranging from 26 to 50 mmHg. 34 , 35 , 36 , 38 Two trials compared PaCO2 levels ranging from 35 to 65 mmHg. One trial compared an individualized PetCO2 with a fixed PetCO2. 33 Additional information of the trials, including bias assessment, is reported in Tables S8–S11. Besir et al. found beneficial effects of hypocapnia on PONV in laparoscopic gynecological operations, whereas Saghaei et al. found a protective effect of mild hypercapnia on PONV in percutaneous nephrolithotomy operations. 35 , 38 Due to the heterogeneity in the intervention and the small number of trials, meta‐analyses were not considered meaningful.
3.3. Tidal volume and positive end‐expiratory pressure
Six publications investigated the effect of different tidal volumes. 39 , 40 , 41 , 42 , 43 , 44 The trials were grouped in higher (9–12 ml/kg) vs. lower tidal volumes (5–7 ml/kg). Five of the trials included an additional intervention of high positive end‐expiratory pressure (PEEP 6–10 cm H20 vs. PEEP 0–4 cm H20). 39 , 41 , 42 , 43 , 44 Two of the trials included recruitment maneuvers in the intervention group. 39 , 43 Additional information is reported in Tables S12–S15.
Three trials investigated high (5–12 cm H20) vs. low PEEP (0–4 cm H20), one including recruitment maneuvers. 45 , 46 , 47 Bluth et al. included 989 patients; the two other trials included ≤150 patients.
None of the six trials found a difference in postoperative pain. Due to the heterogeneity in type of surgery and intervention, no meta‐analysis was conducted for postoperative pain.
PONV was not reported in any of the trials regarding tidal volume or positive end‐expiratory pressure.
3.4. Recruitment maneuver
Eleven publications investigated the effect of recruitment maneuvers on pain and/or PONV. 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 All trials included ≤150 patients with all patients undergoing surgery in the abdominal region. Ten of the trials compared recruitment maneuvers consisting of 2–6 manual pulmonary inflations with pressure up to 60 cmH2O with no recruitment maneuvers. The patients were placed in the Trendelenburg position during the maneuver, except in one trial with the patients in the supine position. 53 One small trial compared recruitment maneuvers of different pressure, 15 vs. 30–40 cmH2O and was not considered further. 56 Additional information is reported in Tables S16–S19.
In a meta‐analysis including seven trials and 735 patients, there was no clear difference in pain 24 h postoperatively (mean difference: −0.2, 95% CI: −0.5, 0.2) with recruitment maneuvers vs. no recruitment maneuvers, Figure 2. In a subgroup analysis, a significant reduction of pain was seen when considering the trials including abdominal surgery (mean difference −0.7, 95% CI: −0.9, −0.4), whereas no significant reduction of pain was found in gynecological surgery patients (mean difference: 0.0, 95% CI: −0.3, 0.3). The p value for the subgroup difference was 0.001. No significant difference in pain was found 48 h postoperatively, Figure S2.
FIGURE 2.
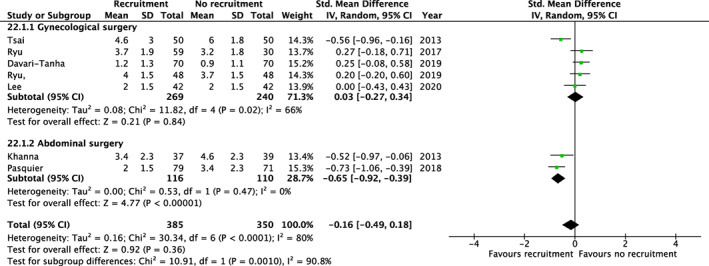
Recruitment maneuvers and pain 24 h postoperatively. Results from random‐effects meta‐analyses of trials assessing recruitment maneuvers. Results are displayed as mean differences (dots) with 95% confidence intervals (error bars). Values below 0 indicate reduced pain 24 h postoperatively with recruitment maneuvers.
Only laparoscopic gynecological surgery trials specifically investigated shoulder pain. In a meta‐analysis including nine trials and 864 patients, there was a significant reduction in shoulder pain 24 h postoperatively in the intervention group (mean difference: −1.1, 95% CI: −1.7, −0.5), Figure 3. Furthermore, in five of the trials including 500 patients the reduction in shoulder pain was maintained in the intervention group 48 h postoperatively (mean difference −0.8, 95% CI −1.0, −0.6), Figure S3. Results for shoulder pain at 4–6 h postoperatively is presented in Figure S4.
FIGURE 3.
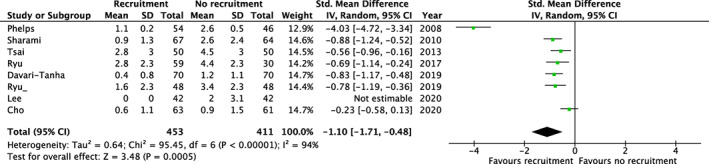
Recruitment maneuvers and shoulder pain 24 h postoperatively. Results from random‐effects meta‐analyses of trials assessing recruitment maneuvers. Results are displayed as mean differences (dots) with 95% confidence intervals (error bars). Values below 0 indicate reduced shoulder pain 24 h postoperatively with recruitment maneuvers.
In a meta‐analysis including six trials and 620 patients, there was no difference in PONV (OR: 0.72, 95% CI: 0.31, 1.71), Figure S5.
Details of the GRADE evaluation are provided in Table S20. The certainty in the evidence was very low for pain, low for shoulder pain, and low for PONV.
3.5. Goal‐directed hemodynamic therapy
Sixteen publications investigated the effect of GDHT on pain and/or PONV, Tables S21–S24. 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74
In a meta‐analysis including eleven trials and 740 patients, GDHT resulted in a significantly lower risk of PONV (OR: 0.44, 95% CI 0.24, 0.78), Figure 4. All but one trial included patients undergoing surgery in the abdominal region. When excluding the trial with surgery in the central nervous system, GDHT still resulted in a significant lower risk of PONV (OR: 0.48, 95% CI: 0.27, 0.85).
FIGURE 4.
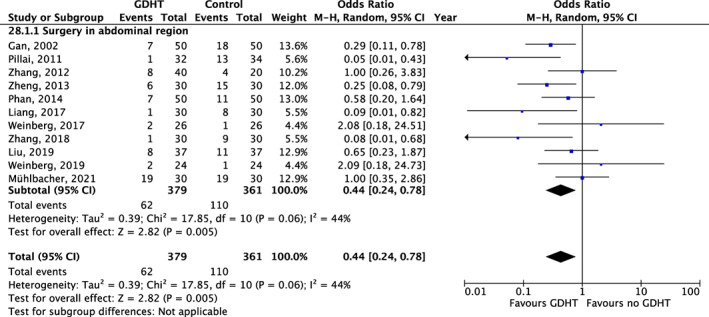
Goal‐directed hemodynamic therapy and postoperative nausea and vomiting. Results from random‐effects meta‐analyses of trials assessing goal‐directed hemodynamic therapy. Results are displayed as odds ratios (dots) with 95% confidence intervals (error bars). Values below 1 indicate reduced postoperative nausea and vomiting with goal‐directed hemodynamic therapy.
Pain was only reported in two trials. 63 , 73
Details of the GRADE evaluation are provided in Table S25. The certainty in the evidence was very low for PONV.
3.6. Other interventions
Two publications investigated the effect on pain of different blood pressures. 75 , 76 Delfino et al. found reduced pain score with mild hypertension during laparoscopic cholecystectomy, whereas the other trial found no difference. Two publications reported the effect on pain of different ventilation modes and found no difference in postoperative pain. 77 , 78 These trials are only reported descriptively (Tables S1 and S26–S29).
4. DISCUSSION
In this manuscript, we have systematically identified and described clinical trials assessing various intra‐operative respiratory and hemodynamic interventions reporting pain, nausea, and/or vomiting as outcomes. The main conclusions are: (1) Many trials did not report postoperative pain, nausea, or vomiting as an outcome or only reported these outcomes as secondary findings, (2) most of the included trials were small, (3) there was heterogeneity in the included trials limiting the interpretability of meta‐analyses, and (4) despite some positive findings there is limited evidence to support that intraoperative respiratory or hemodynamic interventions can meaningfully influence postoperative pain, nausea, and vomiting.
Postoperative pain, nausea, and vomiting are important outcomes for multiple reasons. First, these outcomes are by themselves important to patients. Avoidance of pain and PONV are important for a satisfactory recovery. 79 Second, the development of pain and PONV are associated with an increased risk of complications and prolonged hospital stay. 1 , 2 Given these factors, it seems relevant to collect and report these outcomes for trials evaluating intra‐operative interventions. The current review shows that pain and PONV were infrequently and inconsistently reported.
A major limitation to the literature was the infrequent and inconsistent reporting of prophylaxis and treatment for PONV and pain. Given the unblinded nature of the various interventions, it is possible that such treatments could have differed between groups, potentially resulting in bias. Given this concern, all trials were assessed as having an intermediate risk of bias.
Exploratory meta‐analyses were conducted when considered feasible, that is, when there was a sufficient number of trials for a given treatment and outcome and when the trials were considered comparable. Although most of the analyses found no clear relationship between the intra‐operative intervention and the development of postoperative pain or PONV, we did identify some positive findings. There was some indication that a high FiO2 decreased PONV, especially in patients undergoing abdominal surgery. However, the certainty in the evidence was very low. Similarly, we found that the use of GDHT resulted in lower odds of PONV. The certainty of this evidence was also rated as very low. Given this, additional trials are needed before a definitive effect can be concluded. Moreover, it is important to consider these results in the context of other outcomes, such as mortality, length of stay, and postoperative complications as reported previously. 4 , 6
There was limited evidence that any of the intra‐operative hemodynamic or respiratory interventions affected postoperative pain. The one exception was the use of recruitment maneuvers at the end of laparoscopic gynecological surgery to reduce postoperative shoulder pain. This finding is consistent with previous reviews and the mechanism is considered to involve removal of air from the abdomen. 80 However, the average effect was relatively small corresponding to a difference of approximately 1 on a pain scale from 0 to 10 and the certainty in the evidence was low. Although the minimally clinical important difference in pain varies substantially depending on the setting, most studies consider a change of 1 near or below the minimally clinical important difference. 81 However, given the safety of recruitment maneuvers, this intervention can be considered. 5
This systematic review has multiple strengths. We provide a comprehensive overview of hemodynamic and respiratory interventions that might affect postoperative pain and PONV. Trials were identified based on a broad search. The review follows recommended methodology including bias assessment and GRADE evaluation. There are also certain limitations. The review was limited to English language articles and relevant non‐English articles may be missed. The low number of relevant trials and heterogeneity among trials limited the conduct of meta‐analyses. Some articles only reported limited information on prophylactic PONV medication and treatment strategies for PONV and pain. The current manuscript reports multiple comparisons and there is therefore a risk of Type I errors. The specific outcomes reported in this manuscript were not prespecified in the protocol. Given these limitations, our findings should be considered hypothesis‐generating and not definitive.
In conclusion, although the overall certainty in the evidence was generally low, there was some indication that a high FiO2 and the use of GDHT might result in lower odds of PONV. Although most interventions did not affect pain, the use of recruitment maneuvers at the end of laparoscopic gynecological surgery likely results in a small reduction in postoperative shoulder pain. The results should be considered exploratory given the lack of prespecified hypotheses and corresponding risk of Type 1 errors. More definitive trials are needed to guide clinical care within this area.
AUTHOR CONTRIBUTIONS
Lars W. Andersen, Mathias J. Holmberg, and Asger Granfeldt designed the review. All authors substantial contributed to the acquisition of the data. Johanne M. Holst and Lars W. Andersen analyzed the data. Johanne M. Holst and Lars W. Andersen drafted the manuscript and all authors revised it critically. All authors approved the final manuscript and agreed to be accountable for all aspects of the work.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Appendix S1 Supporting Information
ACKNOWLEDGMENTS
We would like to thank the following for their contributions to the overall systematic review project: Frederik B. Hansen, Andreas Magnussen, Niklas S. Hansen, Cecilie M. Johannsen, Johannes Enevoldsen, Thomas H. Jensen, Lara L. Roessler, Mark A. Eggertsen, Philip Caap, Caroline Boye, Karol M. Dabrowski, Lasse Vormfenne, Carl M. Karlsson, Ida R. Balleby, Marie S. Rasmussen, and Kim Pælestik.
Holst JM, Klitholm MP, Henriksen J, et al. Intraoperative respiratory and hemodynamic strategies for reducing nausea, vomiting, and pain after surgery: Systematic review and meta‐analysis. Acta Anaesthesiol Scand. 2022;66(9):1051‐1060. doi: 10.1111/aas.14127
DATA AVAILABILITY STATEMENT
The datasets used are based on publicly available data from the included studies.
REFERENCES
- 1. Gerbershagen HJ, Aduckathil S, van Wijck AJM, Peelen LM, Kalkman CJ, Meissner W. Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology. 2013;118(4):934‐944. [DOI] [PubMed] [Google Scholar]
- 2. White PF, Elvir‐Lazo OL, Yumul R, Cruz EH. Management strategies for the treatment and prevention of postoperative/postdischarge nausea and vomiting: an updated review. F1000Research. 2020;9:F1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weibel S, Rücker G, Eberhart LH, et al. Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta‐analysis. Cochrane Database Syst Rev. 2020;10(10):CD012859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jessen MK, Vallentin MF, Holmberg MJ, et al. Goal‐directed haemodynamic therapy during general anaesthesia for noncardiac surgery: a systematic review and meta‐analysis. Br J Anaesth. 2021;S0007–0912(21):733‐739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bolther M, Henriksen J, Holmberg MJ, et al. Ventilation strategies during general anesthesia for non‐cardiac surgery: a systematic review and meta‐analysis. Anesth Analg. 2022. [DOI] [PubMed] [Google Scholar]
- 6. Høybye M, Lin PC, Holmberg MJ, et al. Fraction of inspired oxygen during general anesthesia for non‐cardiac surgery: systematic review and meta‐analysis. Acta Anaesthesiol Scand. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bolther M, Henriksen J, Holmberg MJ, Granfeldt A, Andersen LW. Blood pressure targets during general anesthesia for non‐cardiac surgery: a systematic review of clinical trials. Eur J Anaesthesiol. 2022; (In press). [DOI] [PubMed] [Google Scholar]
- 8. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA . Cochrane Handbook for Systematic Reviews of Interventions , version 6.3 (updated February 2022). Cochrane, 2022. Accessed August 13, 2022. www.training.cochrane.org/handbook.
- 10. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 11. Shi J, Luo D, Weng H, et al. Optimally estimating the sample standard deviation from the five‐number summary. Res Synth Methods. 2020;11(5):641‐654. [DOI] [PubMed] [Google Scholar]
- 12. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011. [Google Scholar]
- 13. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Purhonen S, Niskanen M, Wüstefeld M, Hirvonen E, Hynynen M. Supplemental 80% oxygen does not attenuate post‐operative nausea and vomiting after breast surgery. Acta Anaesthesiol Scand. 2006;50(1):26‐31. [DOI] [PubMed] [Google Scholar]
- 15. Purhonen S, Turunen M, Ruohoaho U‐M, Niskanen M, Hynynen M. Supplemental oxygen does not reduce the incidence of postoperative nausea and vomiting after ambulatory gynecologic laparoscopy. Anesth Analg. 2003;96(1):91‐96. [DOI] [PubMed] [Google Scholar]
- 16. Li XF, Jiang D, Jiang YL, et al. Comparison of low and high inspiratory oxygen fraction added to lung‐protective ventilation on postoperative pulmonary complications after abdominal surgery: a randomized controlled trial. J Clin Anesth. 2020;67:110009. [DOI] [PubMed] [Google Scholar]
- 17. Cohen B, Ahuja S, Schacham YN, et al. Intraoperative hyperoxia does not reduce postoperative pain: subanalysis of an alternating cohort trial. Anesth Analg. 2019;128(6):1160‐1166. [DOI] [PubMed] [Google Scholar]
- 18. Turan A, Apfel CC, Kumpch M, et al. Does the efficacy of supplemental oxygen for the prevention of postoperative nausea and vomiting depend on the measured outcome, observational period or site of surgery? Anaesthesia. 2006;61(7):628‐633. [DOI] [PubMed] [Google Scholar]
- 19. Myles PS, Leslie K, Chan MTV, et al. Avoidance of nitrous oxide for patients undergoing major surgery: a randomized controlled trial. Anesthesiology. 2007;107(2):221‐231. [DOI] [PubMed] [Google Scholar]
- 20. Meyhoff CS, Wetterslev J, Jorgensen LN, et al. Effect of high perioperative oxygen fraction on surgical site infection and pulmonary complications after abdominal surgery: the PROXI randomized clinical trial. JAMA – J Am Med Assoc. 2009;302(14):1543‐1550. [DOI] [PubMed] [Google Scholar]
- 21. Alvandipour M, Mokhtari‐Esbuie F, Baradari AG, Firouzian A, Rezaie M. Effect of hyperoxygenation during surgery on surgical site infection in colorectal surgery. Ann Coloproctol. 2019;35(1):9‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sadrolsadat SH, Shoroghi M, Farahbakhsh F, Moharreri RS, Sheikhvatan M, Abbasi A. The effect of supplemental 70% oxygen on postoperative nausea and vomiting in patients undergoing inguinal hernia surgery. Hernia. 2008;12(2):167‐171. [DOI] [PubMed] [Google Scholar]
- 23. Treschan TA, Zimmer C, Nass C, Stegen B, Esser J, Peters J. Inspired oxygen fraction of 0.8 does not attenuate postoperative nausea and vomiting after strabismus surgery. Anesthesiology. 2005;103:6‐10. [DOI] [PubMed] [Google Scholar]
- 24. Thibon P, Borgey F, Boutreux S, Hanouz JL, Le Coutour X, Parienti JJ. Effect of perioperative oxygen supplementation on 30‐day surgical site infection rate in abdominal, gynecologic, and breast surgery: the ISO2 randomized controlled trial. Anesthesiology. 2012;117:504‐511. [DOI] [PubMed] [Google Scholar]
- 25. Mraovic B, Šimurina T, Sonicki Z, Skitarelić N, Gan TJ. The dose‐response of nitrous oxide in postoperative nausea in patients undergoing gynecologic laparoscopic surgery: a preliminary study. Anesth Analg. 2008;107(3):818‐823. [DOI] [PubMed] [Google Scholar]
- 26. Goll V, Akça O, Greif R, et al. Ondansetron is no more effective than supplemental intraoperative oxygen for prevention of postoperative nausea and vomiting. Anesth Analg. 2001;92(1):112‐117. [DOI] [PubMed] [Google Scholar]
- 27. Šimurina T, Mraović B, Mikulandra S, et al. Effects of high intraoperative inspired oxygen on postoperative nausea and vomiting in gynecologic laparoscopic surgery. J Clin Anesth. 2010;22(7):492‐498. [DOI] [PubMed] [Google Scholar]
- 28. Purhonen S, Niskanen M, Wüstefeld M, Mustonen P, Hynynen M. Supplemental oxygen for prevention of nausea and vomiting after breast surgery. Br J Anaesth. 2003;91(2):284‐287. [DOI] [PubMed] [Google Scholar]
- 29. Joris JL, Poth NJ, Djamadar AM, et al. Supplemental oxygen does not reduce postoperative nausea and vomiting after thyroidectomy. Br J Anaesth. 2003;91:857‐861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McKeen DM, Arellano R, O'Connell C. Supplemental oxygen does not prevent postoperative nausea and vomiting after gynecological laparoscopy. Can J Anesth. 2009;56(9):651‐657. [DOI] [PubMed] [Google Scholar]
- 31. Kurz A, Fleischmann E, Sessler DI, Buggy DJ, Apfel C, Akcą O. Effects of supplemental oxygen and dexamethasone on surgical site infection: a factorial randomized trial. Br J Anaesth. 2015;115(3):434‐443. [DOI] [PubMed] [Google Scholar]
- 32. Wang L, Yang L, Yang J, Shan S. Effects of permissive hypercapnia on laparoscopic surgery for rectal carcinoma. Gastroenterol Res Pract. 2019;2019:3903451. doi: 10.1155/2019/3903451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mäkinen MT, Heinonen PO, Klemola UM, Yli‐Hankala A. Gastric air tonometry during laparoscopic cholecystectomy: a comparison of two PaCO2 levels. Can J Anaesth. 2001;48(2):121‐128. doi: 10.1007/BF03019723 [DOI] [PubMed] [Google Scholar]
- 34. Murphy GS, Szokol JW, Avram MJ, et al. Effect of ventilation on cerebral oxygenation in patients undergoing surgery in the beach chair position: a randomized controlled trial. Br J Anaesth. 2014;113(4):618‐627. doi: 10.1093/bja/aeu109 [DOI] [PubMed] [Google Scholar]
- 35. Saghaei M, Matin G, Golparvar M. Effects of intra‐operative end‐tidal carbon dioxide levels on the rates of post‐operative complications in adults undergoing general anesthesia for percutaneous nephrolithotomy: a clinical trial. Adv Biomed Res. 2014;3(1):84. doi: 10.4103/2277-9175.127997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Akça O, Kurz A, Fleischmann E, et al. Hypercapnia and surgical site infection: a randomized trial. Br J Anaesth. 2013;111(5):759‐767. doi: 10.1093/bja/aet233 [DOI] [PubMed] [Google Scholar]
- 37. Son J‐S, Oh J‐Y, Ko S. Effects of hypercapnia on postoperative nausea and vomiting after laparoscopic surgery: a double‐blind randomized controlled study. Surg Endosc. 2017;31(11):4576‐4582. doi: 10.1007/s00464-017-5519-8 [DOI] [PubMed] [Google Scholar]
- 38. Besir A, Tugcugil E. Comparison of different end‐tidal carbon dioxide levels in preventing postoperative nausea and vomiting in gynaecological patients undergoing laparoscopic surgery. J Obstet Gynaecol (Lahore). 2021;41(5):755‐762. [DOI] [PubMed] [Google Scholar]
- 39. Soh S, Shim JK, Ha Y, Kim YS, Lee H, Kwak YL. Ventilation with high or low tidal volume with PEEP does not influence lung function after spinal surgery in prone position: a randomized controlled trial. J Neurosurg Anesthesiol. 2018;30(3):237‐245. [DOI] [PubMed] [Google Scholar]
- 40. Shin HY, Kim SH, Lee YJ, Kim DK. The effect of mechanical ventilation tidal volume during pneumoperitoneum on shoulder pain after a laparoscopic appendectomy. Surg Endosc. 2010;24(8):2002‐2007. [DOI] [PubMed] [Google Scholar]
- 41. Mølsted M, Ekeløf P, Bech JN, Wessels J, Jensen JB. Effects of lung protective ventilation on postoperative respiratory parameters in patients undergoing robot‐assisted radical prostatectomy. J Robot Surg. 2020;14(3):509‐516. [DOI] [PubMed] [Google Scholar]
- 42. Haliloglu M, Bilgili B, Ozdemir M, Umuroglu T, Bakan N. Low tidal volume positive end‐expiratory pressure versus high tidal volume zero‐positive end‐expiratory pressure and postoperative pulmonary functions in robot‐assisted laparoscopic radical prostatectomy. Med Princ Pract. 2018;26(6):573‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Severgnini P, Selmo G, Lanza C, et al. Protective mechanical ventilation during general anesthesia for open abdominal surgery improves postoperative pulmonary function. Anesthesiology. 2013;118(6):1307‐1321. [DOI] [PubMed] [Google Scholar]
- 44. Asida SM, Badawy MS. Effect of low tidal volume during general anesthesia for urological procedures on lung functions. Egypt J Anaesth. 2015;31(2):127‐134. [Google Scholar]
- 45. Wetterslev J, Hansen EG, Roikjaer O, Kanstrup IL, Heslet L. Optimizing peroperative compliance with PEEP during upper abdominal surgery: effects on perioperative oxygenation and complications in patients without preoperative cardiopulmonary dysfunction. Eur J Anaesthesiol. 2001;18(6):358‐365. [DOI] [PubMed] [Google Scholar]
- 46. Seo H, Do Son J, Lee HC, Oh HM, Jung CW, Park HP. Effects of positive end‐expiratory pressure on intraoperative core temperature in patients undergoing posterior spine surgery: prospective randomised trial. J Int Med Res. 2018;46(3):984‐995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bluth T, Serpa Neto A, Schultz MJ, et al. Effect of intraoperative high positive end‐expiratory pressure (PEEP) with recruitment maneuvers vs low PEEP on postoperative pulmonary complications in obese patients: a randomized clinical trial. JAMA – J Am Med Assoc. 2019;321(23):2292‐2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tsai HW, Wang PH, Yen MS, Chao KC, Hsu TF, Chen YJ. Prevention of postlaparoscopic shoulder and upper abdominal pain: a randomized controlled trial. Obstet Gynecol. 2013;121(3):526‐531. [DOI] [PubMed] [Google Scholar]
- 49. Ryu KH, Lee SH, Cho EA, Kim JA, Lim GE, Song T. Comparison of impacts of intraperitoneal saline instillation with and without pulmonary recruitment maneuver on post‐laparoscopic shoulder pain prevention: a randomized controlled trial. Surg Endosc. 2019;33(3):870‐878. [DOI] [PubMed] [Google Scholar]
- 50. Khanna A, Sezen E, Barlow A, Rayt H, Finch JG. Randomized clinical trial of a simple pulmonary recruitment manoeuvre to reduce pain after laparoscopy. Br J Surg. 2013;100(10):1290‐1294. [DOI] [PubMed] [Google Scholar]
- 51. Cho M, Kim CJ, Hahm TS, et al. Combination of a pulmonary recruitment maneuver and intraperitoneal bupivacaine for the reduction of postoperative shoulder pain in gynecologic laparoscopy: a randomized, controlled trial. Obstet Gynecol Sci. 2020;63(2):187‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Phelps P, Cakmakkaya OS, Apfel CC, Radke OC. A simple clinical maneuver to reduce laparoscopy‐induced shoulder pain: a randomized controlled trial. Obstet Gynecol. 2008;111(5):1155‐1160. [DOI] [PubMed] [Google Scholar]
- 53. Pasquier EK, Andersson E. Pulmonary recruitment maneuver reduces pain after laparoscopic bariatric surgery: a randomized controlled clinical trial. Surg Obes Relat Dis. 2018;14(3):386‐392. [DOI] [PubMed] [Google Scholar]
- 54. Lee J, Park C, Kim J, Ki Y, Cha SH, Kim JY. Effect of low‐pressure pulmonary recruitment maneuver on postlaparoscopic shoulder pain: randomized controlled trial. J Minim Invasive Gynecol. 2020;27(1):173‐177. [DOI] [PubMed] [Google Scholar]
- 55. Davari‐Tanha F, Samimi S, Khalaj Z, Bastanhagh E. Comparison of intraperitoneal normal saline infusion with pulmonary recruitment maneuver in reducing shoulder and upper abdomen pain following gynecologic laparoscopic procedures: a randomized, controlled, triple‐blind trial. Anesth Pain Med. 2019;9(3):e92444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yilmaz G, Kiyak H, Akca A, Salihoglu Z. Low‐pressure pulmonary recruitment maneuver: equal to or worse than moderate‐pressure pulmonary recruitment maneuver in preventing postlaparoscopic shoulder pain? A randomized controlled trial of 72 patients. Wideochir Inne Tech Maloinwazyjne. 2020;15(3):519‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sharami SH, Sharami MB, Abdollahzadeh M, Keyvan A. Randomised clinical trial of the influence of pulmonary recruitment manoeuvre on reducing shoulder pain after laparoscopy. J Obstet Gynaecol (Lahore). 2010;30(5):505‐510. [DOI] [PubMed] [Google Scholar]
- 58. Ryu K, Choi W, Shim J, Song T. The impact of a pulmonary recruitment maneuver to reduce post‐laparoscopic shoulder pain: a randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. 2017;208:55‐60. [DOI] [PubMed] [Google Scholar]
- 59. Noblett SE, Snowden CP, Shenton BK, Horgan AF. Randomized clinical trial assessing the effect of Doppler‐optimized fluid management on outcome after elective colorectal resection. Br J Surg. 2006;93(9):1069‐1076. [DOI] [PubMed] [Google Scholar]
- 60. Luo J, Xue J, Liu J, Liu B, Liu L, Chen G. Goal‐directed fluid restriction during brain surgery: a prospective randomized controlled trial. Ann Intensive Care. 2017;7(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Peng K, Li J, Cheng H, Ji FH. Goal‐directed fluid therapy based on stroke volume variations improves fluid management and gastrointestinal perfusion in patients undergoing major orthopedic surgery. Med Princ Pract. 2014;23(5):413‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pillai P, McEleavy I, Gaughan M, et al. A double‐blind randomized controlled clinical trial to assess the effect of Doppler optimized intraoperative fluid management on outcome following radical cystectomy. J Urol. 2011;186(6):2201‐2206. [DOI] [PubMed] [Google Scholar]
- 63. Mühlbacher J, Luf F, Zotti O, Herkner H, Fleischmann E, Kabon B. Effect of intraoperative goal‐directed fluid management on tissue oxygen tension in obese patients: a randomized controlled trial. Obes Surg. 2021;31(3):1129‐1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang J, Qiao H, He Z, Wang Y, Che X, Liang W. Intraoperative fluid management in open gastrointestinal surgery: goal‐directed versus restrictive. Clinics. 2012;67(10):1149‐1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Weinberg L, Ianno D, Churilov L, et al. Goal directed fluid therapy for major liver resection: a multicentre randomized controlled trial. Ann Med Surg. 2019;45:45‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Weinberg L, Ianno D, Churilov L, et al. Restrictive intraoperative fluid optimisation algorithm improves outcomes in patients undergoing pancreaticoduodenectomy: a prospective multicentre randomized controlled trial. PLoS ONE. 2017;12(9):e0183313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Phan TD, D'Souza B, Rattray MJ, Johnston MJ, Cowie BS. A randomised controlled trial of fluid restriction compared to oesophageal Doppler‐guided goal‐directed fluid therapy in elective major colorectal surgery within an Enhanced Recovery After Surgery program. Anaesth Intensive Care. 2014;42(6):752‐760. [DOI] [PubMed] [Google Scholar]
- 68. Gan TJ, Soppitt A, Maroof M, et al. Goal‐directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology. 2002;97(4):820‐826. [DOI] [PubMed] [Google Scholar]
- 69. Zheng H, Guo H, Ye JR, Chen L, Ma HP. Goal‐directed fluid therapy in gastrointestinal surgery in older coronary heart disease patients: randomized trial. World J Surg. 2013;37(12):2820‐2829. [DOI] [PubMed] [Google Scholar]
- 70. Zhang N, Liang M, Zhang DD, et al. Effect of goal‐directed fluid therapy on early cognitive function in elderly patients with spinal stenosis: a case‐control study. Int J Surg. 2018;54(Pt A):201‐205. [DOI] [PubMed] [Google Scholar]
- 71. Liu F, Lv J, Zhang W, Liu Z, Dong L, Wang Y. Randomized controlled trial of regional tissue oxygenation following goal‐directed fluid therapy during laparoscopic colorectal surgery. Int J Clin Exp Pathol. 2019;12(12):4390‐4399. [PMC free article] [PubMed] [Google Scholar]
- 72. Ramsingh DS, Sanghvi C, Gamboa J, Cannesson M, Applegate RL. Outcome impact of goal directed fluid therapy during high risk abdominal surgery in low to moderate risk patients: a randomized controlled trial. J Clin Monit Comput. 2013;27(3):249‐257. [DOI] [PubMed] [Google Scholar]
- 73. Bundgaard‐Nielsen M, Jans Ø, Müller RG, et al. Does goal‐directed fluid therapy affect postoperative orthostatic intolerance? A randomized trial. Anesthesiology. 2013;119(4):813‐823. [DOI] [PubMed] [Google Scholar]
- 74. Liang M, Li Y, Lin L, et al. Effect of goal‐directed fluid therapy on the prognosis of elderly patients with hypertension receiving plasma kinetic energy transurethral resection of prostat. Int J Clin Exp Med. 2017;10(1):1290‐1296. [Google Scholar]
- 75. Delfino AE, De La Fuente N, Echevarría GC, Altermatt FR, Cortinez LI. Effect of acute arterial hypertension on morphine requirements and postsurgical pain. J Clin Anesth. 2015;27(3):226‐232. [DOI] [PubMed] [Google Scholar]
- 76. Xu XM, Hu XW, Wu Y, et al. Effects of different BP management strategies on postoperative delirium in elderly patients undergoing hip replacement: a single center randomized controlled trial. J Clin Anesth. 2020;62:109730. [DOI] [PubMed] [Google Scholar]
- 77. Kim YS, Won YJ, Lee DK, et al. Lung ultrasound score‐based perioperative assessment of pressure‐controlled ventilationvolume guaranteed or volume‐controlled ventilation in geriatrics: a prospective randomized controlled trial. Clin Interv Aging. 2019;14:1319‐1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Le Guen M, Paternot A, Declerck A, et al. Impact of the modality of mechanical ventilation on bleeding during pituitary surgery: a single blinded randomized trial. Medicine (Baltimore). 2019;98(38):e17254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hocking G, Weightman WM, Smith C, Gibbs NM, Sherrard K. Measuring the quality of anaesthesia from a patient's perspective: development, validation, and implementation of a short questionnaire. Br J Anaesth. 2013;111(6):979‐989. [DOI] [PubMed] [Google Scholar]
- 80. Kaloo P, Armstrong S, Kaloo C, Jordan V. Interventions to reduce shoulder pain following gynaecological laparoscopic procedures. Cochrane Database Syst Rev. 2019;2019(1):CD011101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Olsen MF, Bjerre E, Hansen MD, et al. Pain relief that matters to patients: systematic review of empirical studies assessing the minimum clinically important difference in acute pain. BMC Med. 2017;15(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information
Data Availability Statement
The datasets used are based on publicly available data from the included studies.


