Abstract
Context:
Polycystic ovary syndrome (PCOS) is one of the commonest endocrine disorders affecting women of reproductive age, and metformin is a widely used medication in managing this condition.
Aim:
To review the available literature comprehensively on the therapeutic impact of metformin on the clinical and metabolic parameters of women with PCOS.
Data source:
We searched PubMed, MEDLINE, Scopus, Embase, Cochrane Library and the Web of Science and selected sources for grey literature from their inception to April 2020. An updated search in PubMed was performed in June 2022.
Data synthesis:
Two reviewers selected eligible studies and extracted data, and the review is reported following the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Results:
In 24 eligible randomised controlled trials (RCTs) involving 564 participants who received metformin therapy, metformin was associated with significant reduction in body weight by 3.13 kg (95% CI: −5.33, −0.93), body mass index (BMI) by 0.82 kg/m² (95% CI: −1.22, −0.41), fasting blood glucose [standardised mean difference (SMD): −0.23; 95% CI: −0.40, −0.06], low-density lipoprotein cholesterol (LDL-C) (SMD: −0.41; 95% CI: −0.85, 0.03), total testosterone (SMD: −0.33; 95% CI: −0.49, −0.17), androstenedione (SMD: −0.45; 95% CI: −0.70, −0.20), 17-hydroxyprogesterone (17-OHP) (SMD: −0.58; 95% CI: −1.16, 0.00) and increase the likelihood of clinical pregnancy rate [odds ratio (OR): 3.00; 95% CI: 1.95, 4.59] compared with placebo.
Conclusion:
In women with PCOS, metformin use has shown a positive impact in reducing body weight, BMI, total testosterone, androstenedione, 17-OHP, LDL-C, fasting blood glucose and increasing the likelihood of pregnancy in women with PCOS.
Keywords: DHEAS, FAI, FSH, LH, metformin, PCOS, pharmacological therapy, polycystic ovary syndrome
Introduction
Polycystic ovary syndrome (PCOS) is a heterogeneous endocrine condition that affects women of reproductive age, with a prevalence of up to 20%.1 PCOS is characterised by biochemical and clinical features of excess androgen, menstrual irregularities and polycystic ovarian morphology.2 In PCOS, high insulin contributes to excess ovarian androgen production,3 and insulin enhances steroid hormone release in the ovaries.4 High androgen levels are the drive for hirsutism and reduced fertility levels in women with PCOS.5 Women with PCOS have a significantly higher rate of impaired glucose tolerance and insulin resistance, risk factors for type 2 diabetes mellitus (T2DM).6 In addition, nearly 70% of women with PCOS will develop the metabolic syndrome (MS), characterised by the constellation of dyslipidaemia, central adiposity, hypertension and impaired glucose tolerance, all predisposing factors to coronary heart disease and diabetes.7,8 A therapeutic approach targeting weight loss and improving insulin resistance is the cornerstone in managing PCOS and preventing its related complications,9 however, significant weight loss is still challenging in PCOS. Lifestyle intervention is the first-line therapy causing significant though minimal weight reduction.10 Pharmacological options for managing PCOS exist; however, their actual impact in clinical practice is still relatively unexplored.11 Metformin is a member of the biguanide family primarily used to manage T2DM.12 Metformin is also widely used in managing women with PCOS; it reduces androgen levels by improving insulin sensitivity and reducing the cardiometabolic risks associated with hyperinsulinemia in PCOS.13,14
Metformin inhibits hepatic glucose production by activating the AMP-activated protein kinase (AMPK), a major glucose and lipid homeostasis cell regulator. The activation of AMPK is associated with glucose inhibition in the hepatocytes.15 Metformin is transported to hepatocytes mainly via organic transporter 1 (OCT1) and mitochondrial respiratory-chain-complex 1 (NADPH), resulting in the reduction of adenosine triphosphate (ATP) and the increase in adenosine monophosphate (AMP)/ATP and adenosine diphosphate (ADP)/ATP ratios which subsequently activate AMPK.16,17 The reduction of ATP and the accumulation of AMP reduce gluconeogenesis by reducing key gluconeogenic enzymes such as fructose 1,6-bisphosphatase. In addition, high AMP inhibits adenylate cyclase, thus reducing cyclic AMP (cAMP) and inhibiting glycerol conversion to glucose.18 Metformin has also been shown to improve lipid metabolism by reducing hepatic steatosis.19 It was also reported that metformin exerts beneficial effects by reducing circulating plasma TGs by selectively increasing the VLDL-TGs uptake and FFA oxidation in the adipose tissues.20 Metformin-induced lipid storage reduction is mediated by both increases in FFA oxidation and the inhibition of lipid synthesis via its activation of AMPK.20 However, controversy exists on the beneficial impact of metformin during pregnancy and in offspring.21,22 This review aimed to comprehensively assess and appraise the existing evidence and provide in-depth analyses of the impact of metformin on clinical and metabolic parameters in women with PCOS.
Methods
This systematic review was prospectively registered in the PROSPERO international prospective register of systematic reviews (CRD42020178783) as reported previously.23 In a recently published systematic review and meta-analysis we reported the effects of the various pharmacological interventions on the anthropometric indices of women with PCOS.23 However, the current study only evaluated the effect of metformin on the differing outcomes in women with PCOS. In addition, this study was reported following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) 2020 statement24 and informed by Cochrane Handbook for Systematic Reviews of Interventions.25 Ethical permission was not required to undertake this study.
Search and identification of the studies
The eligibility criteria for the included studies are presented in Table 1. Only randomised clinical trials (RCTs) that included women diagnosed with PCOS were eligible to be included in this review. Eligible RCTs employed a randomised design to measure the effect of metformin compared with placebo or no treatment. Accordingly, we accepted all methods of randomisation and design, including cross-over, double-blinded, single-blinded, open-label and parallel-group trials. In addition, to be deemed eligible, an RCT should have reported quantitative data on the effect of metformin, compared with placebo or no treatment, on body weight, body mass index (BMI), waist circumference (WC), waist-to-hip ratio (WHR), fasting blood glucose (FBG), fasting insulin (FI), homeostatic model assessment of insulin resistance (HOMA-IR), homeostatic model assessment of B-cell (HOMA-B), low-density lipoprotein (LDL), total cholesterol (TC), triglycerides (TGs), high-density lipoprotein (HDL), C-reactive protein (CRP), total testosterone, follicular stimulating hormone (FSH), androstenedione (A4), 17-hyderoxyepianderstendione (17-OHP), free testosterone (FT), free androgen index (FAI), sex-hormone-binding globulin (SHBG), dehydroepiandrosterone sulphate (DHEAS), luteinising hormone (LH), estradiol and pregnancy and ovulation rate.
Table 1.
The inclusion/exclusion criteria for the included studies in this systematic review.
| Inclusion criteria |
|---|
|
Study design: randomised controlled trials, including (randomised open-label trials, double-blind controlled trials, cross-over randomised trials and parallel randomised trials). Population: adult females aged 18 and over with a diagnosis of PCOS based on a robust diagnostic criterion. Comparator: studies reported metformin compared with placebo or other treatment. Outcomes: reported outcomes such as BMI, body weight, waist circumference and waist-to-hip ratio, CRP, LDL-C, HDL-C, TC, TGs, TT, FT, FAI, A4, 17-OHP, LH, FSH, FBG, FI, HMOA-IR, HOMA-B, SHBG, DHEAS, pregnancy rate and ovulation rate. |
| Exclusion criteria |
|
Study design: case studies, cross-sectional studies and animal studies. Patient population: paediatric and adolescents, females, postmenopausal women and women without PCOS. Comparators: non-metformin intervention, pharmacological interventions versus dietary interventions, pharmacological intervention versus physical activity or surgery |
PCOS: polycystic ovary syndrome; BMI: body mass index; CRP: C-reactive protein; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; TC: total cholesterol; Tg: triglycerides; T: total testosterone; FT: free testosterone; FAI: free androgen index; A4: androstenedione; 17-OHP: 17-hydroxepianderostendione; LH: luteinising hormone; FSH: follicular; Stimulating hormone; FBG: fasting blood glucose; FI: fasting insulin; HOMA-IR: homeostatic model of insulin resistance; HOMA-B: homeostatic model of B-cell; SHBG: sex hormone-binding globulin; DHEAS: dehydroepiandrosterone sulphate.
A medical librarian specialising in systematic reviews (L.Ö.) developed and performed the literature search in close collaboration with subject experts (M.A and T.S). The biomedical databases PubMed, EMBASE, MEDLINE, Scopus, Cochrane Central Library and Web of Science are covered in the search performed in April 2020, with an update in PubMed in June 2022. Sources for grey literature [European Union Drug Regulating Authorities Clinical Trials Database (EudraCT), Open Grey and ClinicalTrial.gov] were also included. Initially developed in PubMed, the search strategy was replicated in each database without publication year or language restrictions. A combination of Medical Subject Headings (MeSH)/Thesaurus terms and searches in the Title and Abstract fields (alternatively ‘Topic’ or ‘Title, Abstract & Keywords’) were applied to ensure the best possible search outcome. All records located in the literature search were exported to the Covidence systematic review software26 for automatic deduplication and blinded screening. Cabell’s Predatory Report27 was used to verify the quality of the open-access publications reported in this review.
Study selection
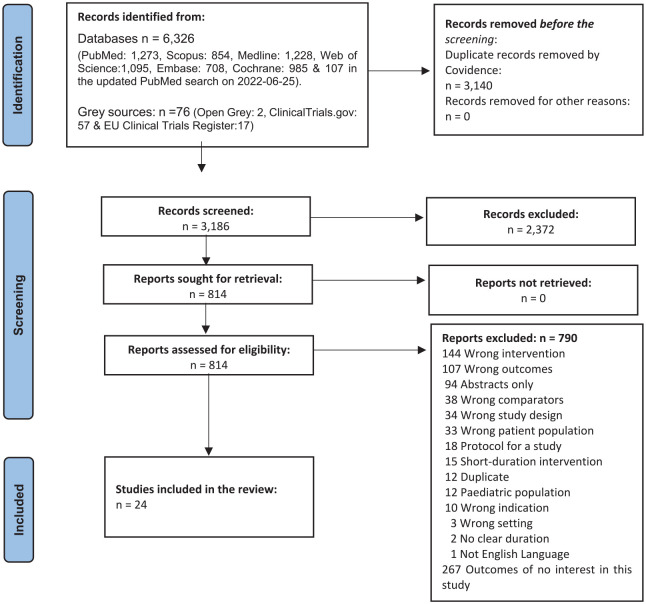
Two reviewers (M.A and N.S) initially screened the titles and abstracts of all retrieved studies for their potential eligibility using the blinded screening module in Covidence. Subsequently, the full text of those eligible studies was retrieved for further independent detailed evaluation (M.A and N.S). Finally, any disagreement between the reviewers was resolved by either discussion, consensus or mediation of a third reviewer (T.S). The detailed study selection process is presented in Figure 1- PRISMA flow diagram.24
Figure 1.
PRISMA flow diagram.
Data extraction
Using a preprepared data extraction form and a list of variables to be extracted, two reviewers (M.A. and N.S.) extracted information from all RCTs deemed eligible. Data extraction covered the basic characteristics of the RCT in addition to other data related to the objectives of the present review. Data extraction covered the country where the RCT was performed, methodological aspects of the RCT, baseline characteristics of the included study population, PCOS diagnostic criteria, trial’s duration and the reported outcomes. All reported outcomes were considered for inclusion, but the primary outcomes of interest were anthropometric parameters, indices of insulin resistance, lipid profiles and CRP, androgen hormones, and fertility outcomes.
Risk of bias assessment in the included studies
The Cochrane collaboration’s tool for assessing the risk of bias in randomised trials (RoB) was used as recommended by Higgins et al.28 Six domains, including (selection bias, performance bias, detection bias, attrition bias, reporting bias and other biases) were assessed by two reviewers (M.A. and N.S.), and a third reviewer (T.S.) arbitrated any conflict that arose between the two reviewers. We followed the recommendation from the Cochrane handbook,25 and any RoB was graded as either ‘high RoB’, ‘low RoB’ or ‘unclear RoB’. The overall RoB of the included RCTs is presented in Figure 1 in Supplementary Material.
GRADE scoring
We assessed the strength of evidence for each outcome using the Grade of Recommendations Assessment, Development, and Evaluation (GRADE) system.29 GRADEpro GDT software was used to summarise the findings for each outcome, which is presented in Table 1 in Supplementary Material. Four points were given for each outcome, and then we assessed factors reducing the quality of the evidence. For each outcome, points were reduced based on the presence of the following; the overall RoB for each RCT, inconsistency (significant heterogeneity), indirectness (significant differences in the population, comparisons and outcomes), imprecision [size of the cohort, width and significance of the confidence intervals (CIs)]. Accordingly, we graded the evidence in four categories based on the overall GRADE scores for each intervention: high-grade evidence (at least 4 points), moderate-grade evidence (3 points), low-grade evidence (2 points) and very low–grade evidence (1 point). All grades of evidence are shown in Table 1 in Supplementary Materials.
Investigation for heterogeneity
The I2 statistic was used to evaluate the statistical heterogeneity for each outcome across the RCTs. We described heterogeneity as insignificant heterogeneity (I2 = 0-40%), moderate heterogeneity (I2 = 30–60%), substantial heterogeneity (I2 = 50–90%) and considerable heterogeneity (I2 = 75–100%). Heterogeneity with p < 0.1 was statistically significant; if this was the case, the source of the heterogeneity was investigated by observing and removing the largest outlier. Where significant heterogeneity was not resolved, subgroup analysis was performed using the random-effect model of the analysis.
Statistical analyses
All meta-analyses were conducted using the statistical methods outlined in the Cochrane Handbook for Systematic Reviews and Meta-analysis.25 Where data from two or more RCTs were available, their pooled estimates and 95% confidence intervals (95% CIs) were presented. For outcomes reported using the same scale, continuous data were pooled using (unstandardised) mean difference (MD) with inverse variance (IV) and random-effects model of analysis based on Cochrane recommendation.25 Dichotomous data were combined using odds ratios (ORs) with 95% CIs to pool the estimated effects. Where the scales were different, if possible, the unit of measurement was converted to the most common unit. If this was not possible, the standardised mean difference (SMD) was used to pool the estimated effect of the same outcomes measured using different scales. We used data reported as changes from-baseline as this removes any between-person variability. Postintervention values were also included, which eliminates the possibility of selective reporting. Where necessary, data presented as standard error (SE), CIs, and p values were converted into SD using the RevMan calculator. For RCTs with more than one intervention arm, the desired outcome was pooled by combining data of the same outcome in all arms. When an RCT was used a cross-over design, data were used only from the cross-over point. All meta-analyses were performed using the Review Manager software (RevMan version 5.4, The Cochrane collaboration) and differences with two-tailed p values of ⩽0.05 were considered statistically significant.
Subgroup analysis
Subgroup analysis was performed at different levels where data from at least two RCTs were available on the same outcome. Subgroup analysis was performed based on the administered dosage of metformin (e.g. 500, 750, 1000, 1500 and 2000 mg), frequency of administration (once a day-QD twice a day-BID or three times a day-TDS) and duration of the intervention (weeks/months/years). Moreover, outcome-specific weighted effect estimates regardless of the dosage of metformin and frequency and duration of administration were quantified and reported. Data collected immediately after the intervention or at follow-up were included in the analysis. The funnel plot of the RevMan with standard error (SE) was used to assess publication bias where more than 10 RCTs were meta-analysed.
Results
Literature search
A total of 6326 unique records were identified in electronic databases and grey sources in the literature search. As a result, 2372 were excluded after the title and abstract screening with the pre-set inclusion and exclusion criteria. Of the 814 studies screened in full text, 24 RCTs involving 564 individuals met the eligibility criteria and were included in the systematic review and meta-analysis Figure 1.
Characteristics of the included RCTs
The 24 RCTs included were published until 2020, of which 13 RCTs30–42 diagnosed PCOS based on the Rotterdam criteria 2003.43 Two RCTs44,45 diagnosed PCOS based on the National Institute of Health (NIH/NICHD) criteria.46 No diagnostic criteria were specified for the remaining RCTs. The characteristics of the included RCTs are presented in Table 2.
Table 2.
Characteristics of the studies included in the systematic review and meta-analysis.
| Author | Year of publication | Sample size (PCOS patients) | Country | PCOS diagnostic criteria | PCOS patient’s Characteristics | Duration | Outcomes | |
|---|---|---|---|---|---|---|---|---|
| Age (Mean ± SD) | BMI (Mean ± SD) | |||||||
| Gambineri et al.47 | 2004 | 40 | Italy | N/A | 27·1 ± 3·6 | 37·6 ± 4·1 | 6 months | FBG, FI, Wt, BMI, HOMA-IR |
| Trolle et al.48 | 2010 | 52 | Denmark | N/A | 31 ± 0 | 32 ± 0 | 6 months | Wt, FBG, FI, HOMA-IR, LDL, HDL |
| Eisenhardt et al.31 | 2006 | 45 | Germany | Rotterdam | 27 ± 0 | 28.9 ± 0 | 12 weeks | FBG.FI, HOMA-IR |
| Heidari et al.32 | 2019 | 48 | USA | Rotterdam | 32.4 ± 7.5 | 37.1 ± 9.1 | 3 months | BMI, WC, WHR, Wt |
| Vandermolen et al.49 | 2001 | 45 | USA | N/A | 29 6 ± 1.2 | 37.6 ± 4.3 | 7 weeks | Wt, BMI, FBG, FI |
| Zain et al.41 | 2009 | 115 | Australia | Rotterdam | 27.8 ± 3.6 | N/A | 6 months | TG, TC, HOMA-IR, HOMA-B, BMI |
| Lingaiah et al. 35 | 2019 | 118 | Finland | Rotterdam | 27.6 ± 4.0 | 26.5 ± 6.0 | 3 months | FI, FBG, WC, WHR |
| Morin-Papunen et al.36 | 2012 | 160 | Finland | Rotterdam | 28.4 ± 3.9 | 27.1 ± 6.3 | 3 months | Wt, WC, BMI, WHR |
| Sova et al.38 | 2012 | 50 | Denmark | Rotterdam | 29.5 ± 3.9 | 28.7 ± 6.9 | N/A | Wt, WC, WHR, BMI, FBG, FI |
| Underdal et al.39 | 2018 | 239 | Australia | Rotterdam | 26 ± 4 | 24.2 ± 5.3 | 6 months | Wt, BMI, WC, WHR |
| Chen et al.50 | 2016 | 156 | Turkey | Rotterdam | 26.2 ± 3.7 | 31.91 ± 5.38 | 2 months | Wt, BMI, WC, WHR, LDL, HDL |
| Kocak et al.34 | 2002 | 56 | Turkey | N/A | 29.7 ± 5.6 | 28.6 ± 4 | 6 weeks | TG, TC, HOMA-IR, HOMA-B |
| Yarali et al.51 | 2002 | 16 | Brazil | N/A | 24 ± 5 | 35.6 ± 4.9 | 3 months | BMI, FI, FBG, WHR |
| Chou et al.42 | 2003 | 30 | Iran | Rotterdam | 25.6 ± 4.32 | 28.52 ± 1.61 | 12 weeks | WHR, BMI, FBG, FI, TG, TC, HOMA-IR, HOMA-B |
| Kazerooni et al.33 | 2010 | 84 | UK | N/A | 27.76 ± 4.89 | 33.74 ± 6.74 | 3 months | BMI, WHR, FBG, FI, TG, TC, HDL, LDL |
| Lord et al.52 | 2006 | 40 | UK | N/A | 30.5 | N/A | 3 months | BMI, WHR, LDL, HDL, TC |
| Ng et al.53 | 2001 | 20 | China | Rotterdam | 25.6 ± 4.02 | 28.9 ± 5 | 3 months | BMI, WHR, LDL, HDL, TC |
| Amiri et al.30 | 2014 | 120 | Iran | Rotterdam | 24.3 ± 3.1 | 22.2 ± 2.0 | 6 months | BMI, DHEAS |
| Palomba et al.54 | 2007 | 30 | Italy | Rotterdam | 24.7 ± 4.4 | 22.2 ± 2.2 | 24 months | WC, BMI, FI, FBG, TC, TG, WHR, LDL, HDL |
| Romualdi et al.37 | 2010 | 28 | Italy | N/A | 28.9 ± 4.8 | 30.6 ± 7.3 | 6 months | WC, BMI, FI, FBG, TC, TG, WHR, LDL, HDL |
| Vanky et al.40 | 2004 | 40 | Norway | Rotterdam | 23.3 ± 4.9 | 28.7 ± 5.5 | 36 weeks | TG, TC, HOMA-IR, HOMA-B |
| Naka et al.55 | 2011 | 43 | Greece | N/A | 29 ± 4.5 | 38 ± 7.8 | 6 months | WHR, BMI, FBG, FI |
| Ladson et al.44 | 2011 | 22 | U.S.A | NIH | 23.9 ± 6 1.2 | 27.1 ± 6 1.5 | 6 months | WC, BMI, FI, FBG, TC, TG, WHR, LDL, HDL |
| Moghetti et al.45 | 2000 | 23 | Italy | NICHD | 25.6 ± 4.02 | 35.6 ± 4.9 | 6 months | BMI, WHR, LDL, HDL, TC |
RCT: randomised clinical trial; N/A: not available; BMI: body mass index; Wt: weight; WHR: waist-to-hip ratio; WC: waist circumference; FBG: fasting blood glucose; FI: fasting insulin; HDL: high-density lipoprotein; LDL: low-density lipoprotein; TGs: triglycerides; TC: total cholesterol; HOMA-IR: homeostatic model of insulin resistance; NIH: national institute of health; NICHD: national institute of child health and development.
Sensitivity analysis
Small sample-sized RCTs and those with high RoB were eliminated from the analysis while monitoring their impact on the final results. No significant effect was found, and hence, no RCT was removed from the meta-analysis.
Assessment of publication bias
The funnel plot did not reveal significant asymmetry (Supplementary Materials), which indicates no publication bias. Egger’s test was not statistically significant for publication bias (regression intercept = 0.456, SE = 0.584, p = 0.745).
Effects of metformin on anthropometric parameters
Body weight
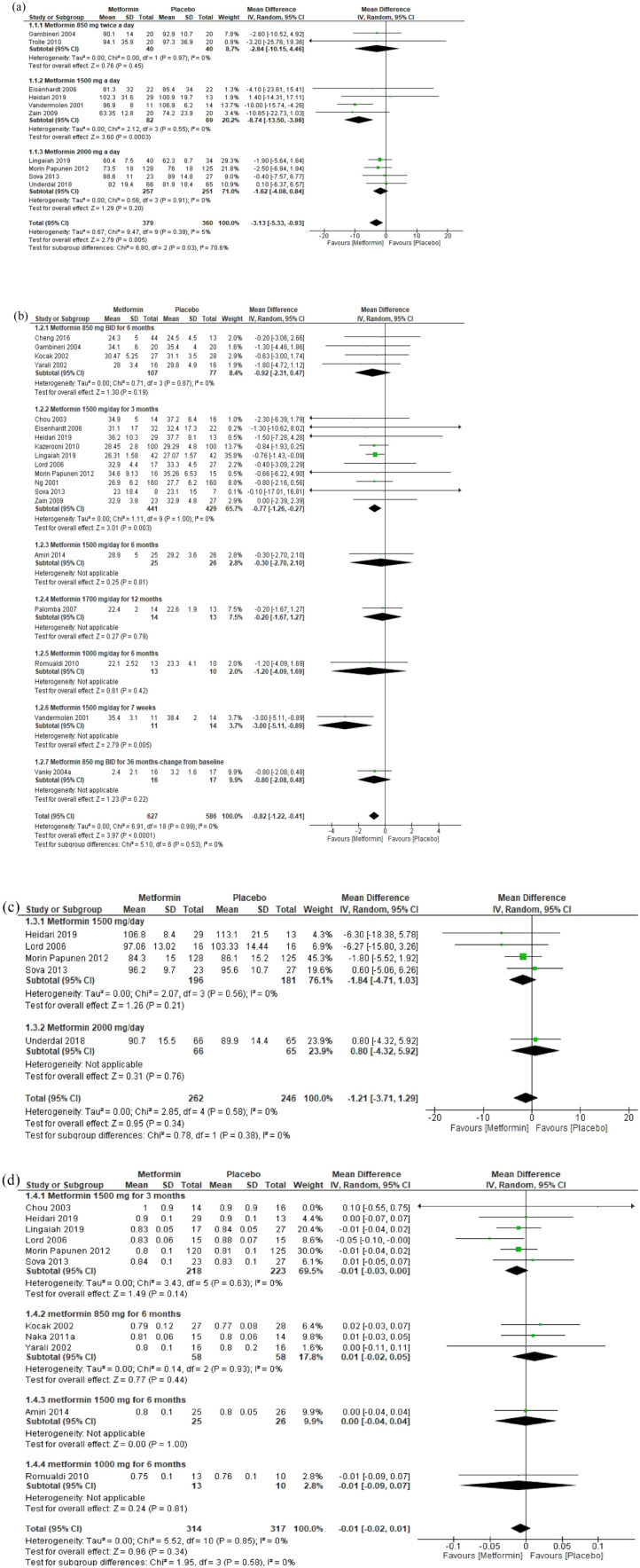
Ten RCTs included 739 women with PCOS; of them, 379 were assigned in the metformin group and 360 in the placebo group. In two RCTs, metformin 850 mg had no effect on body weight (MD: −2.84 kg; 95% CI: −10.15, 4.46). In four RCTs, metformin 1500 mg had significantly reduced body weight by 8.74 kg (95% CI: −13.50, −3.98). In four RCTs, metformin 2000 mg had no effect on body weight compared with placebo (MD: −1.62 kg; 95% CI: −4.08, 0.84). Overall, regardless of the administered dosage and duration, metformin significantly reduced body weight by 3.13 kg (95% CI: −5.33, −0.93, 739 participants, p < 0.005) [Figure 2(a)] (moderate-grade evidence, Table 1 in Supplementary Materials).
Figure 2.
Forest plots of anthropometric parameters. (a) body weight, (b) BMI, (c) WC and (d) WHR.
BMI
Nineteen RCTs reported the effect of various dosages and duration of metformin compared with placebo on the BMI of women with PCOS. Four RCTs evaluated metformin 850 mg BID for 6 months and showed nonsignificant reduction in BMI (MD: -0.92 kg/m²; 95% CI: −2.31, 0.47). In 10 RCTs, metformin 1500 mg QD for 3 months significantly reduced the BMI by 0.77 kg/m² (95% CI: −1.26, −0.27) compared with placebo. In one RCT, metformin 1500 mg QD for 6 months had no effect on BMI (MD: −0.30 kg/m²; 95% CI: −2.70, 2.10). In one RCT, metformin 1700 mg QD had no effect on BMI (MD: −0.20 kg/m²; 95% CI: −1.67, 1.27). In one RCT, metformin 1000 mg QD for 6 months had no effect on BMI (MD: −1.20 kg/m²; 95% CI: −4.09, 1.69). One RCT of metformin 1500 mg QD for 7 weeks had no effect on BMI (MD: −3.00 kg/m²; 95% CI: −5.11, −0.89). Overall, regardless of the administered dosages, frequency and duration, metformin significantly reduced the mean BMI by 0.82 kg/m² (95% CI: −1.22, −0.41, 1213 participants, p < 0.0001) [Figure 2(b)] (moderate-grade evidence, Table 1 in Supplementary Materials).
WC and WHR
The five RCTs reported on the effect of metformin on WC showed a nonsignificant reduction in the WC of women with PCOS [Figure 2(c)]. Similarly, in 11 RCTs reported on the effect of various dosages, frequencies, and duration of metformin, there was a nonsignificant reduction in the WHR of women with PCOS [Figure 2(d)].
Effects of metformin on insulin resistance
FBG
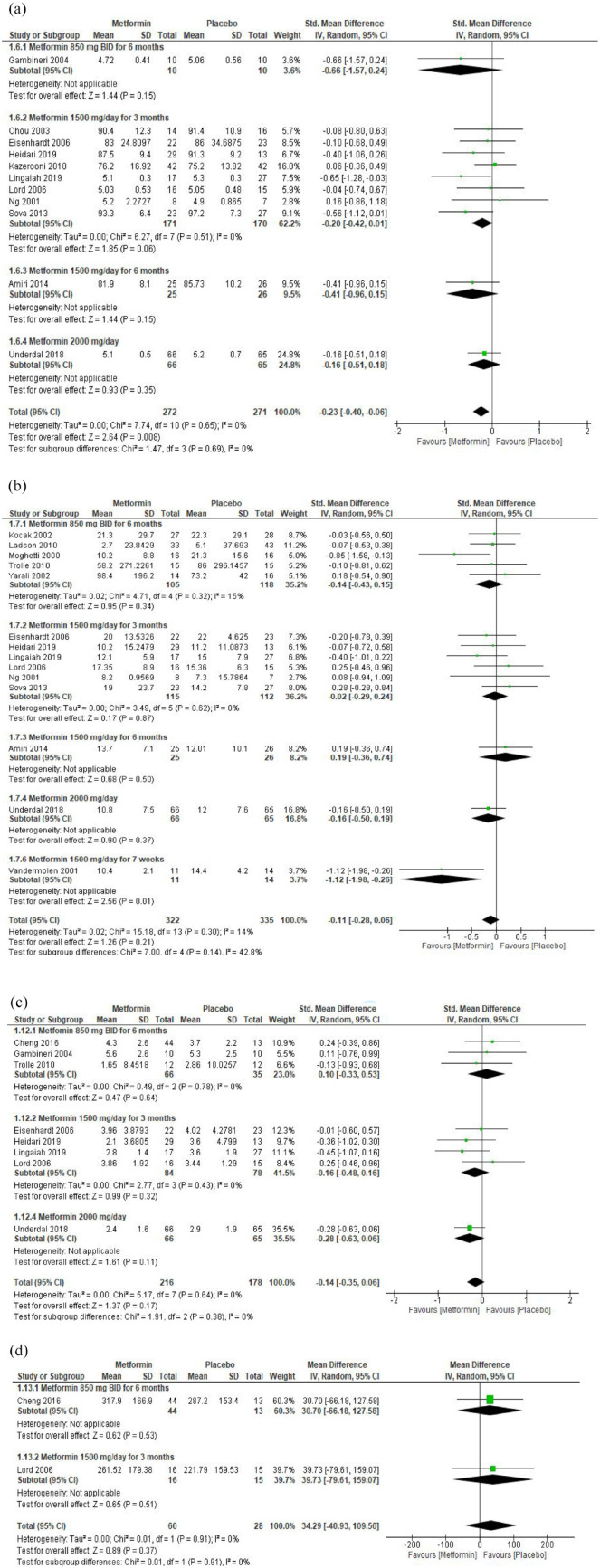
Eleven RCTs investigated the effect of metformin on FBG in 272 women with PCOS compared with 271 in the placebo group. In one RCT, metformin 850 mg BID for 6 months did not affect fasting blood glucose compared with placebo (SMD: −0.66; 95% CI: −1.57, 0.24). In eight RCTs, metformin 1500 mg QD for 6 months significantly reduced the mean fasting blood glucose compared with placebo (SMD: −0.20; 95% CI: −0.42, 0.01). In one RCT, metformin 1500 mg QD for 6 months did not affect the mean fasting blood glucose (SMD: −0.41; 95% CI: −0.96, 0.15). In one RCT, metformin 2000 mg QD did not affect the mean blood glucose (SMD: −0.16; 95% CI: −0.51, 0.18). Overall, regardless of the duration and administered dosages, metformin significantly reduced the mean fasting blood glucose compared with placebo (SMD: −0.23; 95% CI: −0.40, −0.06; 543 participants, p = 0.008) [Figure 3(a)] (moderate-grade evidence, Table 1 in Supplementary Materials).
Figure 3.
Forest plots of the effect of metformin on insulin resistance: (a) FBG, (b) FI, (c) HOMA-IR and (d) HOMA-B.
FI
Fourteen RCTs investigated the effect of different dosages, frequency and duration of metformin on FI in 657 (322 metformin group, 335 placebo group) women with PCOS. Only two studies reported a significant reduction in FI (SMD: 0.85; 95 % CI: −1.58, −0.13) and (−1.12; 95% CI: −1.98, −0.26). Overall, within each subgroup, based on and regardless of the dosage, frequency and duration of metformin, metformin was associated with a nonsignificant reduction in FI (SMD: −0.11; 95% CI: −0.28, 0.06) [Figure 3(b)].
Homeostatic model assessment of insulin resistance (HOMA-IR) and B-cells (HOMA-B)
Eight RCTs investigated the effect of different dosages, frequency, and duration of metformin on HOMA-IR in 394 women with PCOS (216 in metformin and 178 in placebo group). None of those eight RCTs, individually or collectively, achieved a significant impact of metformin on HOMA-IR [Figure 3(b)]. This is similar to the two RCTs that investigated the effect of metformin on HOMA-B [Figure 3(c)].
Effects of metformin on the lipid profiles and CRP
LDL-C
In three RCTs, metformin 850 mg BID had no effect on the mean LDL-C (SMD: −0.65; 95% CI: −1.53, 0.22). In four RCTs, metformin 1500 mg QD had no effect on the mean LDL-C (SMD: −0.23; 95% CI: −0.71, 0.24). Overall, regardless of the administered dosages, metformin significantly reduced the mean LDL-C compared with placebo (SMD: -0.41; 95% CI: -0.85, 0.03, 226 participants, p = 0.06) [Figure 4(a)] (moderate-grade evidence, Table 1 in Supplementary Materials).
Figure 4.
Forest plots of lipid profiles and CRP: (a) LDL, (b) TC, (c) TGs, (d) HDL and (e) CRP.
The meta-analysis did not show any effects of metformin on the mean total cholesterol, triglycerides, HDL-C and CRP when metformin was compared with placebo [Figure 4(b)–(e)].
Effects of metformin on androgen hormones
TT
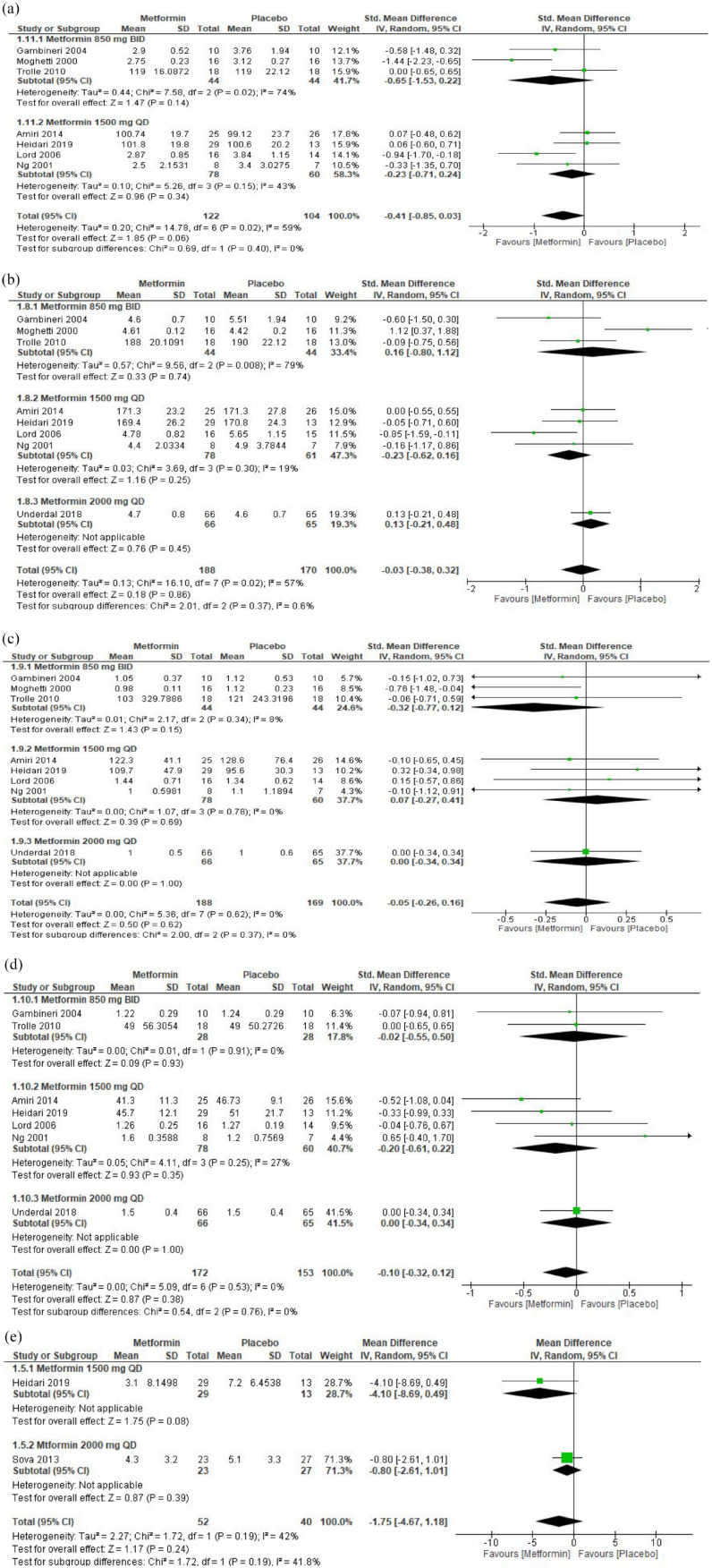
In three RCTs, metformin 850 mg BID for 6 months had no significant effect on the mean total testosterone (SMD: −0.28; 95% CI: −0.74, 0.17). In eight RCTs, metformin 1500 mg QD for 3 months significantly reduced the mean total testosterone compared with placebo (SMD: −0.32; 95% CI: −0.58, −0.07). One RCT of metformin 1500 mg QD for 6 months showed no effect on total testosterone compared with placebo (SMD: −0.35; 95% CI: −0.90, 0.20). One RCT compared metformin 1700 mg QD with placebo and showed no effect on the mean total testosterone (SMD: 0.00; 95% CI: −0.75, 0.75). One RCT compared metformin 850 mg BID for 36 months showed no effect on total testosterone (SMD: −0&x46;19; 95% CI: −0.86, 0.49). One RCT compared metformin 1500 mg QD for 7 weeks and showed a significant reduction in the mean total testosterone (SMD: −0.93; 95% CI: −1.81, −0.05). One RCT compared metformin 1000 mg QD with placebo for 6 months and showed a significant reduction in the total testosterone (SMD: −0.46; 95% CI: −1.30, 0.37). Overall, regardless of the administered dosages and duration, metformin significantly reduced the mean total testosterone compared with placebo (SMD: −0.33; 95% CI: −0.49, −0.17, 690 participants, p < 0.0001) [Figure 3(a) in Supplementary Materials] (moderate grade evidence, Table 1 in Supplementary Materials).
FSH
In two RCTs, metformin 850 mg BID for 6 months had no effect on the mean FSH compared with placebo (MD: 0.24 IU/L; 95% CI: −0.34, 0.83). In four RCTs, metformin 1500 mg QD for 3 months had no effect on the mean FSH compared with placebo (MD: −0.07 IU/L; 95% CI: −0.38, 0.24). In one RCT, metformin 1000 mg QD for 6 months had no effect on the mean FSH compared with placebo (MD: −0.55 IU/L; 95% CI: −2.30, 1.20). However, in one RCT, metformin 1500 mg QD for 7 weeks significantly increased the mean FSH compared with placebo (MD: 0.40 IU/L; 95% CI: 0.15, 0.65). Overall, regardless of the administered dosages and duration, metformin significantly increased the mean level of FSH compared with placebo (MD: 0.20 IU/L; 95% CI: −0.00, 0.40, 319 participants, p = 0.06) [Figure 3(b) in Supplementary Materials] (moderate-grade evidence, Table 1 in Supplementary Materials)
Androstenedione
In one RCT, metformin 850 mg BID for 6 months had no effect on androstenedione (SMD: −0.18; 95%; CI: −0.88, 0.51). Three RCTs comparing metformin 1500 mg QD for 3 months showed a significant reduction in androstenedione (SMD: −0.58; 95% CI: −0.92, −0.23). One RCT comparing metformin 1700 mg QD for 12 months showed no effect on androstenedione (SMD: −0.32; 95% CI: −1.08, 0.44). One RCT compared metformin 1000 mg QD for 6 months and showed no effect on androstenedione. However, another RCT that compared metformin 1500 mg QD for 7 weeks showed a significant reduction in androstenedione (SMD: −1.25; 95% CI: −2.13, −0.38). One RCT compared metformin 850 mg BID for 36 months and showed no reduction in the level of androstenedione (SMD: −0.17; 95% CI: −0.84, 0.51). Overall, metformin at various dosages significantly reduced the level of androstenedione when compared with placebo (SMD: −0.45; 95% CI: −0.70, −0.20; 275 participants, p = 0.0005) [Figure 3(c) in Supplementary Materials] (very low–grade evidence, Table 1 in Supplementary Materials).
17-hydroxyprogesterone (17-OHP)
In one RCT, metformin 1000 mg QD for 6 months did not affect 17-OHP compared with placebo (SMD: −0.51; 95% CI: −1.35, 0.33). In another RCT, metformin 1500 mg QD for 7 weeks did not affect 17-OHP (SMD: −0.64; 95% CI: −1.46, 0.17). However, the pooled estimate showed that metformin significantly reduced 17-OHP when compared with placebo (SMD: −0.58; 95% CI: −1.16, 0.00; 48 participants, p = 0.05) [Figure 3(d) in Supplementary Materials] (very low–grade evidence, Table 1 in Supplementary Materials).
The meta-analysis did not show any effect on free testosterone, free androgen index, SHBG, DHEAS, LH, and estradiol when metformin was compared with placebo [Figure 3(e)–(j), Supplementary Materials].
Effects of metformin on pregnancy rate
Pregnancy rate
In two RCTs, metformin 1500 mg QD for 3 months significantly increased the pregnancy rate (OR: 2.76; 95% CI: 1.78, 4.30). In one RCT, metformin 850 mg BID for 6 months did not affect the pregnancy rate (OR: 6.0; 95% CI: 0.52, 68.72). In one RCT, metformin 1500 mg QD for 7 weeks significantly increased the pregnancy rate (OR: 15.60; 95% CI: 1.48, 164.38). Overall, regardless of the administered dosage or duration, metformin significantly increased the rate of pregnancy (OR: 3.00; 95% CI: 1.95, 4.59, I2 = 0%, p < 0.00001) [Figure 4(a), Supplementary Materials] (very low-grade evidence, Table 1, Supplementary Material).
The meta-analysis did not show any effect on the ovulation rate when metformin was compared with placebo [Figure 4(b), Supplementary Materials].
Discussion
This systematic review has outlined the up-to-date evidence supporting metformin’s effectiveness in managing PCOS. To our knowledge, this is the first comprehensive systematic review to report the effects of metformin on anthropometric outcomes, insulin resistance indices, lipid profiles and CRP, androgen hormones and fertility outcomes of women with PCOS. When metformin was administered at various therapeutic doses and compared with placebo, there were statistically significant reductions in mean body weight, BMI, WC, fasting blood glucose, total testosterone, 17-OHP and LDL-C, and an increase in the pregnancy rate in women with PCOS. However, we should acknowledge that metformin is not a fertility drug such as clomiphene citrate (CC); it indirectly induces ovulation by reducing the insulin level and is less effective in ovulation induction than CC. However, CC acts directly by inhibiting the negative feedback on HPO-axis and induces ovulation.41 However, using metformin as an add-on therapy to CC significantly increases ovulation and the pregnancy rate in PCOS.56 These findings are in line with the findings of previous studies. Legro et al.57 in an RCT of 626 infertile women with PCOS, participants were randomised to receive metformin plus placebo, CC plus placebo, or the combination for 6 months. There was a high pregnancy rate and live birth rate with CC than with metformin. In an RCT of obese women with PCOS evaluating the effect of metformin on body weight, a significant decrease in BMI independent of lifestyle changes was reported.58 Women with PCOS are also at a higher risk of developing CVD due to hyperinsulinemia, high androgen levels, obesity and dyslipidaemia.59 There is evidence that obesity and PCOS independently affect the vascular endothelial function,60 however, the associations between hyperinsulinemia and CVD are independent of body weight.61,62 Women with PCOS also have dyslipidaemia,63 manifested as a low HDL and high triglyceride levels, a strong CVD predictor of CVD.64 Thus, the management of dyslipidaemia is crucial in PCOS. Metformin improves dyslipidaemia by directly affecting the hepatic metabolism of free fatty acids or indirectly by reducing hyperinsulinemia by enhancing the insulin senseitivty;65 however, there was no beneficial effect of metformin on total cholesterol levels.66 A recent network meta-analysis of 101 RCTs assessed the effects of 55 interventions in obese women with PCOS and found superior efficacy for flutamide and cyproterone acetate on hormonal and metabolic paratmeters.67
Metformin is usually administered at a starting dose of 500 mg QD, increasing to the highest dose of 2000 mg QD if tolerated; sustained-release (SR) metformin is a preferred alternative for metformin intolerance. Although the duration of metformin treatment is unclear, the studies presented here were not less than 2 months with the longest being at over 7 years.
This study followed a comprehensive and systematic method to search for relevant databases and grey sources and only included RCTs. Steps were taken to minimise the risk of bias, and we excluded observational studies and non-randomised clinical trials. To the authors’ knowledge, this review is the most comprehensive and up-to-date systematic review and meta-analysis on the effect of metformin in women with PCOS.
The limitations of this study include that the majority of the RCTs were small, and the statistical power used to calculate the sample size was not fully reported. Moreover, all trials were of short duration; therefore, the long-term effects of metformin in women with PCOS are not apparent.
Conclusion
Metformin, alone and irrespective of the dosage and duration of therapy, significantly reduces the mean body weight, BMI, LDL-C, total testosterone, androstenedione, 17-OHP, fasting blood glucose and increases the pregnancy rate in women with PCOS compared with placebo.
Supplemental Material
Supplemental material, sj-docx-1-tae-10.1177_20420188221127142 for Impact of metformin on the clinical and metabolic parameters of women with polycystic ovary syndrome: a systematic review and meta-analysis of randomised controlled trials by Mohammed Altigani Abdalla, Najeeb Shah, Harshal Deshmukh, Amirhossein Sahebkar, Linda Östlundh, Rami H. Al-Rifai, Stephen L. Atkin and Thozhukat Sathyapalan in Therapeutic Advances in Endocrinology and Metabolism
Acknowledgments
None.
Footnotes
ORCID iDs: Mohammed Altigani Abdalla  https://orcid.org/0000-0002-6016-3157
https://orcid.org/0000-0002-6016-3157
Thozhukat Sathyapalan  https://orcid.org/0000-0003-3544-2231
https://orcid.org/0000-0003-3544-2231
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Mohammed Altigani Abdalla, Allam Diabetes Centre, Academic Diabetes, Endocrinology and Metabolism, Hull York Medical School (HYMS), University of Hull, Hull, UK.
Najeeb Shah, Allam Diabetes Centre, Academic Diabetes, Endocrinology and Metabolism, Hull York Medical School (HYMS), University of Hull, Hull, UK.
Harshal Deshmukh, Allam Diabetes Centre, Academic Diabetes, Endocrinology and Metabolism, Hull York Medical School (HYMS), University of Hull, Hull, UK.
Amirhossein Sahebkar, Biotechnology Research Center, Pharmaceutical Technology Institute, Mashhad University of Medical Sciences, Mashhad, Iran; Applied Biomedical Research Center, Mashhad University of Medical Sciences, Mashhad, Iran; School of Medicine, The University of Western Australia, Perth, WA, Australia.
Linda Östlundh, National Medical Library, College of Medicine and Health Sciences, United Arab Emirates University, Al Ain, United Arab Emirates.
Rami H. Al-Rifai, Institute of Public Health, College of Medicine and Health Sciences, United Arab Emirates University, Al Ain, United Arab Emirates
Stephen L. Atkin, School of Postgraduate Studies and Research, RCSI Medical University of Bahrain, Busaiteen, Kingdom of Bahrain
Thozhukat Sathyapalan, Academic Diabetes, Endocrinology and Metabolism, Allam Diabetes Centre Hull Royal Infirmary Anlaby Road HU3 2JZ, Hull, UK.
Declarations
Ethics approval and consent to participate: Not needed as no patients were involved.
Consent for publication: Not applicable.
Author contribution(s): Mohammed Altigani Abdalla: Conceptualisation; Data curation; Formal analysis; Investigation; Methodology; Software; Writing – original draft.
Najeeb Shah: Data curation; Methodology; Writing – review & editing.
Harshal Deshmukh: Writing – review & editing.
Amirhossein Sahebkar: Writing – review & editing.
Linda Östlundh: Data curation; Methodology; Writing – review & editing.
Rami H. Al-Rifai: Methodology; Writing – review & editing.
Stephen L. Atkin: Writing – review & editing.
Thozhukat Sathyapalan: Project administration; Resources; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This systematic review was completed as part of a self-funded PhD project for MA, and no external fund was received.
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Availability of data and materials: The data sets generated and analysed for this review are available upon compelling request to the authors.
References
- 1. Barnard L, Ferriday D, Guenther N, et al. Quality of life and psychological well being in polycystic ovary syndrome. Hum Reprod 2007; 22: 2279–2286. [DOI] [PubMed] [Google Scholar]
- 2. Ndefo UA, Eaton A, Green MR. Polycystic ovary syndrome: a review of treatment options with a focus on pharmacological approaches. P T 2013; 38: 336–355. [PMC free article] [PubMed] [Google Scholar]
- 3. Witchel SF, Oberfield SE, Pena AS. Polycystic ovary syndrome: pathophysiology, presentation, and treatment with emphasis on adolescent girls. J Endocr Soc 2019; 3: 1545–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu S, Divall S, Wondisford F, et al. Reproductive tissues maintain insulin sensitivity in diet-induced obesity. Diabetes 2012; 61: 114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nisenblat V, Norman RJ. Androgens and polycystic ovary syndrome. Curr Opin Endocrinol Diabetes Obes 2009; 16: 224–231. [DOI] [PubMed] [Google Scholar]
- 6. Salley KE, Wickham EP, Cheang KI, et al. Glucose intolerance in polycystic ovary syndrome: a position statement of the Androgen Excess Society. J Clin Endocrinol Metab 2007; 92: 4546–4556. [DOI] [PubMed] [Google Scholar]
- 7. Garruti G, Depalo R, Vita MG, et al. Adipose tissue, metabolic syndrome and polycystic ovary syndrome: from pathophysiology to treatment. Reprod Biomed Online 2009; 19: 552–563. [DOI] [PubMed] [Google Scholar]
- 8. Essah PA, Wickham EP, Nestler JE. The metabolic syndrome in polycystic ovary syndrome. Clin Obstet Gynecol 2007; 50: 205–225. [DOI] [PubMed] [Google Scholar]
- 9. Wadden TA, Berkowitz RI, Womble LG, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med 2005; 353: 2111–2120. [DOI] [PubMed] [Google Scholar]
- 10. Wadden TA, Volger S, Sarwer DB, et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med 2011; 365: 1969–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abdalla MA, Shah N, Deshmukh H, et al. Effect of pharmacological interventions on lipid profiles and C-reactive protein in polycystic ovary syndrome: a systematic review and meta-analysis. Clin Endocrinol 2021; 96: 449–459. [DOI] [PubMed] [Google Scholar]
- 12. Jensterle M, Kravos NA, Ferjan S, et al. Long-term efficacy of metformin in overweight-obese PCOS: longitudinal follow-up of retrospective cohort. Endocr Connect 2020; 9: 44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev 2012; 33: 981–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sam S, Dunaif A. Polycystic ovary syndrome: syndrome XX. Trends Endocrinol Metab 2003; 14: 365–370. [DOI] [PubMed] [Google Scholar]
- 15. Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001; 108: 1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Viollet B, Guigas B, Sanz Garcia N, et al. Cellular and molecular mechanisms of metformin: an overview. Clin Sci 2012; 122: 253–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wheaton WW, Weinberg SE, Hamanaka RB, et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. Elife 2014; 3: e02242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miller RA, Chu Q, Xie J, et al. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature 2013; 494: 256–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Woo SL, Xu H, Li H, et al. Metformin ameliorates hepatic steatosis and inflammation without altering adipose phenotype in diet-induced obesity. PLoS ONE 2014; 9: e91111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Geerling JJ, Boon MR, van der Zon GC, et al. Metformin lowers plasma triglycerides by promoting VLDL-triglyceride clearance by brown adipose tissue in mice. Diabetes 2014; 63: 880–891. [DOI] [PubMed] [Google Scholar]
- 21. Kumar P, Khan K. Effects of metformin use in pregnant patients with polycystic ovary syndrome. J Hum Reprod Sci 2012; 5: 166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jorquera G, Echiburu B, Crisosto N, et al. Metformin during pregnancy: effects on offspring development and metabolic function. Front Pharmacol 2020; 11: 653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abdalla MA, Shah N, Deshmukh H, et al. Impact of pharmacological interventions on anthropometric indices in women with polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Clin Endocrinol 2022; 96: 758–780. [DOI] [PubMed] [Google Scholar]
- 24. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Higgins JP, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. New York: John Wiley & Sons, 2019. [Google Scholar]
- 26. Babineau J. Product review: covidence (systematic review software). J Can Health Libr Assoc/J l’Assoc Biblioth Santé Can 2014; 35: 68–71. [Google Scholar]
- 27. Das S, Chatterjee SS. Cabell’s blacklist: a new way to tackle predatory journals. Indian J Psychol Med 2018; 40: 197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Amiri M, Golsorkhtabaramiri M, Esmaeilzadeh S, et al. Effect of metformin and flutamide on anthropometric indices and laboratory tests in obese/overweight PCOS women under hypocaloric diet. J Reprod Infertil 2014; 15: 205–213. [PMC free article] [PubMed] [Google Scholar]
- 31. Eisenhardt S, Schwarzmann N, Henschel V, et al. Early effects of metformin in women with polycystic ovary syndrome: a prospective randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab 2006; 91: 946–952. [DOI] [PubMed] [Google Scholar]
- 32. Heidari B, Lerman A, Lalia AZ, et al. Effect of metformin on microvascular endothelial function in polycystic ovary syndrome. Mayo Clin Proc 2019; 94: 2455–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kazerooni T, Shojaei-Baghini A, Dehbashi S, et al. Effects of metformin plus simvastatin on polycystic ovary syndrome: a prospective, randomized, double-blind, placebo-controlled study. Fertil Steril 2010; 94: 2208–2213. [DOI] [PubMed] [Google Scholar]
- 34. Kocak M, Caliskan E, Simsir C, et al. Metformin therapy improves ovulatory rates, cervical scores, and pregnancy rates in clomiphene citrate-resistant women with polycystic ovary syndrome. Fertil Steril 2002; 77: 101–106. [DOI] [PubMed] [Google Scholar]
- 35. Lingaiah S, Morin-Papunen L, Risteli J, et al. Metformin decreases bone turnover markers in polycystic ovary syndrome: a post hoc study. Fertil Steril 2019; 112: 362–370. [DOI] [PubMed] [Google Scholar]
- 36. Morin-Papunen L, Rantala AS, Unkila-Kallio L, et al. Metformin improves pregnancy and live-birth rates in women with polycystic ovary syndrome (PCOS): a multicenter, double-blind, placebo-controlled randomized trial. J Clin Endocrinol Metab 2012; 97: 1492–1500. [DOI] [PubMed] [Google Scholar]
- 37. Romualdi D, Giuliani M, Cristello F, et al. Metformin effects on ovarian ultrasound appearance and steroidogenic function in normal-weight normoinsulinemic women with polycystic ovary syndrome: a randomized double-blind placebo-controlled clinical trial. Fertil Steril 2010; 93: 2303–2310. [DOI] [PubMed] [Google Scholar]
- 38. Sova H, Puistola U, Morin-Papunen L, et al. Metformin decreases serum 8-hydroxy-2’-deoxyguanosine levels in polycystic ovary syndrome. Fertil Steril 2013; 99: 593–598. [DOI] [PubMed] [Google Scholar]
- 39. Underdal MO, Stridsklev S, Oppen IH, et al. Does metformin treatment during pregnancy modify the future metabolic profile in women with PCOS? J Clin Endocrinol Metab 2018; 103: 2408–2413. [DOI] [PubMed] [Google Scholar]
- 40. Vanky E, Salvesen KA, Heimstad R, et al. Metformin reduces pregnancy complications without affecting androgen levels in pregnant polycystic ovary syndrome women: results of a randomized study. Hum Reprod 2004; 19: 1734–1740. [DOI] [PubMed] [Google Scholar]
- 41. Zain MM, Jamaluddin R, Ibrahim A, et al. Comparison of clomiphene citrate, metformin, or the combination of both for first-line ovulation induction, achievement of pregnancy, and live birth in Asian women with polycystic ovary syndrome: a randomized controlled trial. Fert Steril 2009; 91: 514–521. [DOI] [PubMed] [Google Scholar]
- 42. Chou KH, von Eye Corleta H, Capp E, et al. Clinical, metabolic and endocrine parameters in response to metformin in obese women with polycystic ovary syndrome: a randomized, double-blind and placebo-controlled trial. Horm Metab Res 2003; 35: 86–91. [DOI] [PubMed] [Google Scholar]
- 43. Rotterdam EA-SPCWG. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004; 81: 19–25. [DOI] [PubMed] [Google Scholar]
- 44. Ladson G, Dodson WC, Sweet SD, et al. The effects of metformin with lifestyle therapy on polycystic ovary syndrome: a randomized double blind study. Endocr Rev 2010; 31:1059–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moghetti P, Castello R, Negri C, et al. Metformin effects on clinical features, endocrine and metabolic profiles, and insulin sensitivity in polycystic ovary syndrome: a randomized, double-blind, placebo-controlled 6-month trial, followed by open, long-term clinical evaluation. J Clin Endocrinol Metab 2000; 85: 139–146. [DOI] [PubMed] [Google Scholar]
- 46. Anaforoglu I, Algun E, Incecayir O, et al. Higher metabolic risk with National Institutes of Health versus Rotterdam diagnostic criteria for polycystic ovarian syndrome in Turkish women. Metab Syndr Relat Disord 2011; 9: 375–380. [DOI] [PubMed] [Google Scholar]
- 47. Gambineri A, Pelusi C, Genghini S, et al. Effect of flutamide and metformin administered alone or in combination in dieting obese women with polycystic ovary syndrome. Clin Endocrinol 2004; 60: 241–249. [DOI] [PubMed] [Google Scholar]
- 48. Trolle B, Lauszus FF, Frystyk J, et al. Adiponectin levels in women with polycystic ovary syndrome: impact of metformin treatment in a randomized controlled study. Fertil Steril 2010; 94: 2234–2238. [DOI] [PubMed] [Google Scholar]
- 49. Vandermolen DT, Ratts VS, Evans WS, et al. Metformin increases the ovulatory rate and pregnancy rate from clomiphene citrate in patients with polycystic ovary syndrome who are resistant to clomiphene citrate alone. Fertil Steril 2001; 75: 310–315. [DOI] [PubMed] [Google Scholar]
- 50. Chen Z, Zhang M, Qiao Y, et al. Effects of letrozole in combination with low-dose intramuscular injection of human menopausal gonadotropin on ovulation and pregnancy of 156 patients with polycystic ovary syndrome. Pak J Med Sci 2016; 32: 1434–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yarali H, Yildiz BO, Demirol A, et al. Co-administration of metformin during rFSH treatment in patients with clomiphene citrate-resistant polycystic ovarian syndrome: a prospective randomized trial. Hum Reprod 2002; 17: 289–294. [DOI] [PubMed] [Google Scholar]
- 52. Lord J, Thomas R, Fox B, et al. The effect of metformin on fat distribution and the metabolic syndrome in women with polycystic ovary syndrome: a randomised, double-blind, placebo-controlled trial. BJOG 2006; 113: 817–824. [DOI] [PubMed] [Google Scholar]
- 53. Ng EH, Wat NM, Ho PC. Effects of metformin on ovulation rate, hormonal and metabolic profiles in women with clomiphene-resistant polycystic ovaries: a randomized, double-blinded placebo-controlled trial. Hum Reprod 2001; 16: 1625–1631. [DOI] [PubMed] [Google Scholar]
- 54. Palomba S, Falbo A, Russo T, et al. Insulin sensitivity after metformin suspension in normal-weight women with polycystic ovary syndrome. J Clin Endocrinol Metab 2007; 92: 3128–3135. [DOI] [PubMed] [Google Scholar]
- 55. Naka KK, Kalantaridou SN, Kravariti M, et al. Effect of the insulin sensitizers metformin and pioglitazone on endothelial function in young women with polycystic ovary syndrome: a prospective randomized study. Fertil Steril 2011; 95: 203–209. [DOI] [PubMed] [Google Scholar]
- 56. Nestler JE. Metformin in the treatment of infertility in polycystic ovarian syndrome: an alternative perspective. Fertil Steril 2008; 90: 14–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Legro RS, Barnhart HX, Schlaff WD, et al. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med 2007; 356: 551–566. [DOI] [PubMed] [Google Scholar]
- 58. Harborne LR, Sattar N, Norman JE, et al. Metformin and weight loss in obese women with polycystic ovary syndrome: comparison of doses. J Clin Endocrinol Metab 2005; 90: 4593–4598. [DOI] [PubMed] [Google Scholar]
- 59. Akram T, Hasan S, Imran M, et al. Association of polycystic ovary syndrome with cardiovascular risk factors. Gynecol Endocrinol 2010; 26: 47–53. [DOI] [PubMed] [Google Scholar]
- 60. Kaya MG, Yildirim S, Calapkorur B, et al. Metformin improves endothelial function and carotid intima media thickness in patients with PCOS. Gynecol Endocrinol 2015; 31: 401–405. [DOI] [PubMed] [Google Scholar]
- 61. Mancini F, Cianciosi A, Reggiani GM, et al. Endothelial function and its relationship to leptin, homocysteine, and insulin resistance in lean and overweight eumenorrheic women and PCOS patients: a pilot study. Fertil Steril 2009; 91: 2537–2544. [DOI] [PubMed] [Google Scholar]
- 62. Mather KJ, Kwan F, Corenblum B. Hyperinsulinemia in polycystic ovary syndrome correlates with increased cardiovascular risk independent of obesity. Fertil Steril 2000; 73: 150–156. [DOI] [PubMed] [Google Scholar]
- 63. Kim JJ, Choi YM. Dyslipidemia in women with polycystic ovary syndrome. Obstet Gynecol Sci 2013; 56: 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wild RA. Dyslipidemia in PCOS. Steroids 2012; 77: 295–299. [DOI] [PubMed] [Google Scholar]
- 65. Sin HY, Kim JY, Jung KH. Total cholesterol, high density lipoprotein and triglyceride for cardiovascular disease in elderly patients treated with metformin. Arch Pharm Res 2011; 34: 99–107. [DOI] [PubMed] [Google Scholar]
- 66. Sharpe A, Morley LC, Tang T, et al. Metformin for ovulation induction (excluding gonadotrophins) in women with polycystic ovary syndrome. Cochrane Datab Syst Rev 2019; 12: CD013505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Abdel-Maboud M, Menshawy A, Hasabo EA, et al. The comparative effectiveness of 55 interventions in obese patients with polycystic ovary syndrome: a network meta-analysis of 101 randomized trials. PLoS ONE 2021; 16: e0254412. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tae-10.1177_20420188221127142 for Impact of metformin on the clinical and metabolic parameters of women with polycystic ovary syndrome: a systematic review and meta-analysis of randomised controlled trials by Mohammed Altigani Abdalla, Najeeb Shah, Harshal Deshmukh, Amirhossein Sahebkar, Linda Östlundh, Rami H. Al-Rifai, Stephen L. Atkin and Thozhukat Sathyapalan in Therapeutic Advances in Endocrinology and Metabolism