Social processing difficulties and higher oxytocin (OT) levels were more common in people living with HIV (PWH) exposed to early life trauma (ELT) compared with ELT-unexposed PWH. An OT/C-reactive protein factor moderated ELT-performance associations, whereas a myeloid migration factor was associated with reduced social processing accuracy regardless of ELT. Interventions that target OT/C-reactive protein and myeloid migration factors may alter social processing for PWH including ELT-exposed individuals.
Key words/Abbreviations: early life trauma, HIV, inflammation, oxytocin, vasopressin, cortisol, social processing, AVP = arginine vasopressin, CRP = C-reactive protein, CTQ = Childhood Trauma Questionnaire, EIA = enzyme immunoassay, ELT = early life trauma, FEPT = Facial Emotion Perception Test, HPA = hypothalamic-pituitary-adrenal axis, IL = interleukin, IP-10 = interferon γ–induced protein, MCP-1 = monocyte chemotactic protein-1, MIG = monokine induced by interferon, MMP = matrix metalloproteinase, OT = oxytocin, PTSD = posttraumatic stress disorder, PWH = people with HIV, RT = reaction time, TNF-α = tumor necrosis factor α, SCID = Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition)
ABSTRACT
Objective
Early life trauma (ELT) and HIV are associated with social processing deficits. In people with HIV (PWH), we examined whether facial emotion identification accuracy differs by ELT and whether neuroendocrine factors including cortisol, oxytocin (OT), and arginine vasopressin, and/or immune system measures play a role in the ELT-performance association.
Methods
We used secondary data from the placebo condition of a pharmacologic challenge study in PWH. Presence of ELT was measured with the Childhood Trauma Questionnaire (at least moderate experiences of sexual, physical, and/or emotional abuse). Social processing was measured with the Facial Emotion Perception Test (FEPT). Salivary immune system measures and cortisol were sampled across a 5-hour study session. Blood was collected at study session start (12 pm) to measure OT and arginine vasopressin. We examined the association of ELT with FEPT and five biological moderators (from principal components analysis of 12 biomarkers) of ELT-FEPT associations.
Results
Of 58 PWH (42 men; mean [standard deviation] age = 33.7 [8.9] years), 50% endorsed ELT. ELT-exposed PWH demonstrated lower identification accuracy across all emotional expressions (unstandardized β [B] = 0.13; standard error [SE] = 0.05; p = .021, d = 0.63) and had higher OT levels compared with ELT-unexposed PWH (t(1,56) = 2.12, p = .039; d = 0.57). For total accuracy, an OT/C-reactive protein factor moderated the ELT-FEPT association (B = 0.14; SE = 0.05; p = .014); accuracy was lower in ELT-exposed PWH versus ELT-unexposed PWH when the factor was low but not when high. Similar results were obtained for fearful, neutral, and happy faces (p values < .05). Regardless of ELT, a myeloid migration (MCP-1/MMP-9) factor was associated with reduced accuracy (p values < .05).
Conclusions
Our pilot findings suggest that ELT may alter social processing in PWH, and OT and C-reactive protein may be a target for improving social processing in ELT-exposed PWH, and myeloid migration markers may be a target in PWH more generally.
INTRODUCTION
Compared with the general US population, people with HIV (PWH) disproportionately experience stressful and traumatic life experiences including physical, sexual, and emotional abuse (1–3). The prevalence rate of childhood sexual abuse in PWH ranges from 32% to 53% (2,3) versus 7.5% to 11.7% in the US population (4). Notably, most trauma exposure occurs before the age of 13 years (5), a neurodevelopmental period for brain areas (e.g., temporal-limbic and prefrontal regions) critical for social processing (6,7). Normative neurodevelopment of social processing circuits contributes to adaptive social skills and peer relationships, whereas alterations are associated with increased rates of psychiatric disorders including mood and anxiety (8–10).
According to the National Institute of Mental Health‘s Research Domain Criteria, one important aspect of social processing is the ability to accurately identify facial emotions, a crucial skill for effective social interactions (11,12). Deficits in this function are commonly observed in PWH (13–17) and early life trauma (ELT)–exposed adults (18,19). PWH demonstrate reduced accuracy and slower response times in identifying and discriminating facial expressions (13,15,16), and demonstrate particular difficulties identifying negative emotions including sadness, fear, and anger (13,14,17,20,21). Similar to PWH, ELT-exposed adults also demonstrate difficulties identifying negative emotions and a bias to identifying neutral faces as negative (18,19). To date, little is known about the effect of ELT on facial emotion identification in PWH and on biological factors associated with altered performance in this population.
Two closely related neuroendocrine factors altered in ELT-exposed individuals and associated with facial emotion identification are cortisol and oxytocin (OT). Cortisol is a steroid hormone secreted by adrenal glands and plays a major role in the regulation of the hypothalamic-pituitary-adrenal (HPA) axis. OT is a neurohormone synthesized in the hypothalamus and released into systemic circulation by the posterior pituitary or into the brain (projections into temporal-limbic and prefrontal regions) (22). There is a strong interplay between OT and cortisol; both are released in response to stressors in animal models (23) and in anticipation of and after a variety of stressors (pharmacologic, psychosocial, physical) in humans (24,25). Given the relationship, it is not surprising that OT and cortisol are altered among ELT-exposed compared with ELT-unexposed adults (26). ELT-exposed compared with ELT-unexposed adults show lower basal cortisol levels, a blunted cortisol response to psychosocial stressors (27,28), lower cerebrospinal fluid OT levels (29), and alterations in OT levels, sometimes higher (30,31) and sometimes lower (32). These neuroendocrine factors are reliably associated with facial emotion identification (33), but these associations have not been investigated in HIV.
Arginine vasopressin (AVP) is a neuroendocrine factor likely to be perturbed by ELT and contribute to social processing deficits (34). AVP is closely related to OT differing by only two of nine amino acids; consequently, OT receptors recognize AVP and OT with high affinity (35). Similar to OT, AVP is synthesized in the hypothalamus and transported to the posterior pituitary, where it is released peripherally. AVP also stimulates the release of adrenocorticotropic hormone into the blood stream—linking AVP to the HPA axis—and AVP is also synthesized in the limbic system in areas implicated in defensive responses (22), including (36) regions important for facial emotion identification (37). Strong evidence from animal models shows that early life experiences influence AVP and social recognition (34,38,39). In humans, less is known about the relationship between ELT and peripheral AVP (40). However, ELT was previously shown to moderate the association between genetic variants in the AVP receptor AVPR1A and self-reported measures of social attachment in a population-based cohort (41). In addition, alterations in AVP occur in mental health disorders such as psychosis where social processing deficits, including facial emotion identification, are a prominent feature (42–44).
Although ELT may alter neuroendocrine factors in PWH and contribute to facial emotion identification, immune markers warrant consideration given the strong bidirectional communication pathways between the HPA axis, OT, and inflammation (45,46). ELT has been examined as a contributor to peripheral inflammatory dysregulation in adults (47). The most common markers examined include C-reactive protein (CRP), interleukin (IL) 6, and tumor necrosis factor (TNF) α. Although other immune markers have been examined, these predominately proinflammatory markers are reliably elevated in ELT-exposed adults (47–50). With respect to facial emotion identification, immune markers (composite score of CRP, TNF-α, IL-6, IL-8, and IL-10) related to greater amygdala responsivity to angry facial expressions (51). In addition, pharmacologic challenge studies administering immune-activating drugs (e.g., endotoxin versus placebo) indicate that drug-related increases in inflammation are associated with neural circuits involved in threat processing (52). Finally, a meta-analysis of 24 neuroimaging studies indicated consistent associations between peripheral inflammation and temporal-limbic and prefrontal regions critical for facial emotion identification, as well as the hypothalamus (53).
The overarching goal of these analyses is twofold. First, we aimed to examine the association of ELT with emotion processing in PWH. As alterations in social processing in PWH may be more evident in ELT-exposed adults because of HIV-specific vulnerabilities such as legacy effects (potential irreversible central nervous system injury before ART), we hypothesized that ELT-exposed PWH would show reduced accuracy and slower response times identifying and discriminating facial expressions irrespective of emotional expression compared with ELT-unexposed PWH. We also predicted that ELT-exposed PWH would demonstrate difficulties specifically in identifying negative emotions (e.g., sad, fear, anger) compared with ELT-unexposed PWH. Lastly, we aimed to understand whether the association between neuroendocrine, systemic immune markers and facial emotion identification differs by reported ELT exposure in PWH. We hypothesized that OT, AVP, cortisol, and commonly identified immune markers altered in ELT (CRP, IL-6, TNF-α) would be biological moderators of the ELT–Facial Emotion Perception Test (FEPT) performance associations in PWH. Thus, varying levels of these markers would modify the relationship between ELT and FEPT performance in PWH.
METHODS
Participants
This secondary analysis included data from PWH during the placebo condition of a randomized, double-blind, placebo-controlled crossover pharmacologic challenge (10 mg of hydrocortisone versus placebo) (54,55) who provided blood and saliva samples and also completed the FEPT. Data were collected between March 2013 and March 2017. Participants were recruited through Chicago-based HIV primary care clinics and the surrounding community using advertisements and Web site postings. Participants were aged 18 to 45 years with English as their first language and used the same ART regimen for >3 months and did not have any of the following: lifetime history of psychosis, neurological condition (e.g., loss of consciousness >1 hour), body mass index >40 kg/m2, history of substance use disorder in the past 6 months (excluding alcohol/nicotine), or an inability to abstain from illicit substances 24 hours before study assessments (confirmed via urine toxicology screen). Participants were compensated for their participation.
Of the 81 participants completing the placebo condition, 63 (78%) completed the FEPT, as the task was introduced into the study after the other 18 participants had completed the study. Of those 63 with FEPT data, OT data on 58 participants (92%) were available. Thus, 72% (58 of 81) of participants were included in the analysis. Importantly, the sociodemographic characteristics of the 58 individuals were similar to the 81 participants in age (mean = 33.7 versus 34.3 years), race (93.1% Black versus 93.8%), and ELT exposure to the larger sample (50% versus 51.9%) (54,55).
Procedures
During session 1, participants provided written informed consent after institution review board approval and then completed a urine toxicology screen, blood draw, the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (SCID), and self-report questionnaires. As sensitive information was being collected (mental health, childhood trauma), all procedures occurred in a private room. Participants were told that they could choose to decline to answer any question, which they were uncomfortable answering. Participants were provided with a comprehensive list of mental health resources before the administration of the SCID-IV. If it was discovered that participants had a diagnosis that they were unaware of, the study psychiatrist informed them of this and provided them with a referral for further psychiatric evaluation and treatment. A procedure was also in place so that in the event that we uncovered a diagnosis that made the individual a threat to herself or others, we would immediately refer them to someone at the University of Illinois at Chicago and/or escort them to the emergency department. During either session 2 or 3, masked placebo was given (data points used for this analysis) followed by cognitive assessments at two time points (30 minutes and 4 hours) and social processing assessments at one time point (4 hours). Saliva was sampled throughout the session (12 pm–6 pm).
Measures
At session 1, participants completed a number of self-report questionnaires, including the Schedule of Life Events Checklist (56), Posttraumatic Stress Disorder (PTSD) Checklist—Civilian version (57), Perceived Stress Scale (58), Center for Epidemiologic Studies Depression Scale (59), Pittsburgh Sleep Quality Index (60), Medication Adherence Self-Report Inventory (61), and Childhood Trauma Questionnaire (CTQ) (62), which is described in detail hereinafter because it is the primary exposure variable of interest in the present analysis.
Childhood Trauma
The CTQ was used to assess childhood sexual, physical, and emotional abuse (62). Statements such as “When I was growing up, someone tried to touch me in a sexual way, or tried to make me touch them” or “When I was growing up, I got hit so hard by someone in my family that I had to see a doctor or go to the hospital” corresponded to a specific type of trauma (e.g., sexual, physical) and were rated on a 5-point Likert scale ranging from “never true” to “very often true.” Each trauma subscale includes five questions. Severity of sexual, physical, and emotional childhood abuse was categorized used standard CTQ scoring guidelines with ratings of statements that corresponded to each abuse subscale on the CTQ added. A score of 8 or higher in the sexual abuse category was defined as moderate to extreme abuse, a score of 10 or higher in the physical abuse category was defined as moderate to extreme abuse, and a score of 13 or higher in the emotional abuse category was defined as moderate to extreme abuse. Participants meeting the cutoff in any of the three categories were defined as ELT exposed.
Blood Collection and Serum Hormone Assays
Blood samples were drawn at the beginning of each study session, between 11 am and 12 pm. Samples were spun at 4°C, divided into aliquots (300 μl), and stored at −80°C. Serum aliquots were thawed and subsequently batch tested in duplicates after dilution in an assay buffer to give reliable results within the linear portion of the standard curve (OT, 1:4; AVP, 1:2). Enzyme immunoassay (EIA) kits (Enzo Life Sciences/Assay Designs) were used to quantify OT and AVP (63), and both hormones were assayed simultaneously. Samples were not extracted before performing the EIA because studies using mass spectrometry demonstrate high levels of OT in plasma and serum and indicate that methods involving extraction of samples removes much of this peptide (64,65). These EIAs are highly sensitive (minimal detection levels, <12 pg/ml for OT and 4 pg/ml for AVP), and cross-reactivity between OT and AVP is low (<0.04%). All OT values >2000 pg/ml and AVP values >400 pg/ml were rerun to confirm sample accuracy. This was only the case for OT level; no cases had AVP values >400 pg/ml. For both assays, intra-assay coefficients of variation were <15%. OT and AVP levels are only being used from the placebo session day. Details associated with the measurement of peptide hormones are found in the study by MacLean et al. (66).
Saliva Collection, Cortisol, and Cytokines
During sessions 2 or 3, salivary samples from the placebo session day were obtained at 10 time points (35 and 20 minutes before placebo pill administration and 30, 60, 90, 180, 210, 240, 270, and 300 minutes after placebo pill administration). See Refs. (54,55) for details regarding saliva collection procedures. Samples were assayed for cortisol with an EIA kit by Salimetrics. Area under the curve with respect to ground was computed to assess basal afternoon cortisol concentrations (67). Salivary cytokines were assessed at three time points (20 minutes before pill administration and 30 and 240 minutes after pill administration). For the present analysis, we used salivary cytokines from the time point that was 240-minute after pill administration on the placebo day because that coincided with when the FEPT was completed. Nine cytokines were assessed in the original panel of markers, including IL-6, IL-8, IL-1β, TNF-α, CRP, interferon γ–induced protein (IP-10), monocyte chemotactic protein (MCP)-1, monokine induced by interferon (MIG), and matrix metalloproteinase (MMP)-9. See cytokine assay details (54,55). Samples below the level of detection were assigned one-half of the lowest detectable value for that analyte. This was needed for IL-6 (15% of values), IL-1β (10% of values), TNF-α (10% of values), and MMP-9 (2% of values). Because cytokine levels were not normally distributed, all markers were log transformed. All markers were then z-scored so that they were on the same scale for data visualization. Table S1 (Supplemental Digital Content, http://links.lww.com/PSYMED/A863) provides the median and interquartile range for each of the raw cytokine values for reference.
Facial Emotion Perception Test
The FEPT is a computerized task that assesses the ability to categorize facial expressions, a social processing task (68). The FEPT involves briefly showing participants pictures of faces on a laptop screen with one of four primary target emotions (fear, anger, happiness, sadness) or a neutral expression, or pictures of animals that fall into one of four categories (primate, dog, cat, bird). Each stimulus presentation (faces and animals) began with an orienting cross in the center of the computer screen that was presented for 500 milliseconds, followed by the stimulus presentation for 300 milliseconds, a visual mask for 100 milliseconds, and then a response period of 2600 milliseconds. Thus, each trial lasted for 3500 milliseconds, and there was no intertrial interval. The primary outcome measure was accuracy of affect identification across all faces and for each type of facial expression. Secondary outcomes included reaction time (RT) for correct affect identification responses.
Statistical Analyses
To examine group differences (ELT exposed versus ELT unexposed) on FEPT performance (accuracy, RT), we conducted three linear mixed models with repeated effects. Primary predictor variables included group, type of facial expression (happy, sad, etc.), and the interaction. Models controlled for age, sex, and current cannabis use. We controlled for current cannabis use because this factor differed by ELT status and related to the outcome measures. Next, to understand whether the association between neuroendocrine and immune markers and performance differs by ELT exposure, we did the following. First, in the total sample, we conducted a principal components factor analysis (varimax rotation) of the 12 neuroendocrine and immune markers to address correlations between markers and to minimize the number of statistical analyses. Five factors emerged (Supplemental Table S2, http://links.lww.com/PSYMED/A863) and were defined based on the markers demonstrating the highest factor loadings: factor 1-proinflammatory cytokines (TNF-α, IL-1β, IL-8, IL-6); factor 2-myleoid migration (MCP-1, MMP-9); factor 3-OT/CRP (OT, CRP), factor 4-chemokines regulating immune cell trafficking (MIG, IP-10); and factor 5-HPA axis hormones (AVP, cortisol). The extracted factor scores from the principal components were then used as predictor variables in subsequent multivariable linear regression models along with ELT-exposure status and the ELT-exposure status by factor score interactions and covariates (same as the mixed models mentioned previously). Interactions at p < .10 were removed from the models. To facilitate interpretation and guide subsequent research, we conducted Pearson correlations to identify which individual neuroendocrine and immune markers were driving the associations between the component and FEPT performance. Significance was defined as p < .05 (two-sided; trends at p < .10). Cohen d (reported as d) effect sizes are reported where relevant (small effect, 0.2; medium effect, 0.5; large effect, 0.8) (69). Analyses were performed using SAS (version 9.4; SAS Institute Inc., Cary, North Carolina).
RESULTS
The 58 PWH ranged in age from 18 to 45 years (mean [standard deviation] = 33.7 [8.9]), 93% were non-Hispanic African American, 72% were male, and 28% had less than 12 years of education. Of the 58 PWH, 50% were ELT exposed to moderate to severe physical, sexual, and/or emotional abuse. Table 1 shows sociodemographic, behavioral, and clinical characteristics by ELT-exposure status. ELT-exposed PWH reported higher depressive and PTSD symptoms, and cannabis use and were more likely to have an undetectable viral loads (p values < .05). However, the proportion of PWH diagnosed with major depressive disorder (via SCID-IV) was similar by ELT exposure status, and none of the individuals in either group met the criteria for a lifetime diagnosis of PTSD. In addition, ELT-exposed PWH had higher OT levels compared with ELT-unexposed PWH (p = .039; d = 0.57; Table 2). Serum AVP levels, salivary basal afternoon cortisol concentrations, and all salivary immune marker levels as well as the related factor scores did not differ by ELT exposure status.
TABLE 1.
Demographic and Clinical Characteristics by Early Life Trauma in People With HIV at the Enrollment Visit, Session 1
| Early Life Trauma | |||
|---|---|---|---|
| Exposed (n = 29), n (%) | Unexposed (n = 29), n (%) | p | |
| Sociodemographic factors | |||
| Age, mean (SD), y | 32.7 (9.1) | 34.6 (8.7) | .43 |
| Male | 21 (72) | 21 (72) | 1.00 |
| Education | .95 | ||
| <High school | 8 (28) | 8 (28) | |
| High school graduate | 9 (31) | 8 (28) | |
| >High school | 12 (41) | 13 (44) | |
| Black, not Hispanic | 28 (97) | 26 (90) | .30 |
| Unemployed | 18 (62) | 19 (65) | .78 |
| Risky health behaviors | |||
| Currently smoking | 20 (69) | 14 (48) | .11 |
| No. alcohol drinks/wk, median (IQR) | 1 (1) | 1 (2) | .38 |
| Current cannabis use (via urine toxicology) | 17 (60) | 8 (30) | .035 |
| Psychological profile | |||
| CES-D, mean (SD) | 14.3 (7.7) | 10.2 (7.1) | .041 |
| PCL-C, mean (SD) | 31.6 (11.4) | 22.6 (6.4) | .001 |
| PSS-10, mean (SD) | 21.6 (6.4) | 19.4 (3.9) | .12 |
| CTQ, mean (SD) | |||
| ≥Moderate sexual abuse | 21 (72) | 0 (0) | <.001 |
| ≥Moderate physical abuse | 18 (62) | 0 (0) | <.001 |
| ≥Moderate emotional abuse | 15 (52) | 0 (0) | <.001 |
| PSQI total score, mean (SD) | 7.5 (3.3) | 5.6 (2.3) | .011 |
| Lifetime diagnosisa | |||
| MDD PTSD |
12 (41) 0 (0) |
8 (28) 0 (0) |
.27 — |
| Clinical characteristics | |||
| Body mass index, mean (SD), kg/m2 | 24.1 (5.2) | 24.4 (4.0) | .83 |
| Years living with HIV, mean (SD) | 8.9 (7.0) | 10.4 (6.9) | .44 |
| Efavirenz use | 10 (34) | 12 (41) | .58 |
| Dolutegravir use | 0 (0) | 1 (3) | .31 |
| Raltegravir use | 5 (17) | 4 (14) | .71 |
| Elvitegravir use | 2 (7) | 6 (21) | .13 |
| Medication (MASRI) missing ≥1 dose in the last monthc | 10 (34) | 13 (45) | .42 |
| CD4 count, median (IQR), cells/μl | 567 (384) | 476 (335) | .50 |
| Viral load (HIV RNA), copies/mlb | .021 | ||
| Undetectable | 17 (61) | 7 (26) | |
| Lowest detectable limit (20) | 6 (21) | 5 (19) | |
| <500 | 2 (7) | 9 (33) | |
| >500 | 3 (11) | 6 (22) | |
SD = standard deviation; IQR = interquartile range; Current use = use in the last month; CES-D = Center for Epidemiologic Studies Depression Scale; PCL-C = PTSD Checklist—-Civilian Version; PSS-10 = Perceived Stress Scale-10; CTQ = Childhood Trauma Questionnaire; PSQI = Pittsburgh Sleep Quality Index; MDD = major depressive disorder; MASRI = Medication Adherence.
a Major depressive disorder based on the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition).
b Data for viral load are missing for one participant in the exposed group and for two participants in the unexposed group.
c Visual analog scale for the proportion of doses taken in the last month.
TABLE 2.
Neuroendocrine and Inflammatory Markers by Early Life Trauma in People With HIV
| Early Life Trauma | ||||
|---|---|---|---|---|
| Exposed, Median (IQR) | Unexposed, Median (IQR) | p | Cohen d | |
| Blood-based markers | ||||
| OT | 1693.9 (436.9) | 1465.1 (508.6) | .039 | 0.57 |
| AVP | 146.3 (75.1) | 168.3 (56.9) | .46 | 0.17 |
| Saliva-based markers | ||||
| Basal afternoon cortisola | 51.4 (29.1) | 46.2 (26.5) | .69 | 0.06 |
| IL-6 | 0.17 (1.1) | 0.21 (1.9) | .75 | 0.05 |
| IL-8 | −0.14 (0.9) | 0.04 (1.3) | .30 | 0.25 |
| IL-1β | 0.30 (1.4) | 0.26 (1.5) | .79 | 0.09 |
| TNF-α | −0.8 (1.4) | −0.8 (2.0) | .53 | 0.15 |
| CRP | −0.2 (1.7) | 0.3 (1.7) | .71 | 0.13 |
| IP-10 | 0.2 (0.9) | 0.1 (0.9) | .83 | 0.13 |
| MCP-1 | 0.2 (1.1) | 0.3 (1.4) | .54 | 0.18 |
| MMP-9 | 0.6 (1.2) | 0.2 (1.1) | .87 | 0.37 |
| MIG | −0.0 (0.3) | −0.1 (1.3) | .62 | 0.08 |
| Mean (SD) | Mean (SD) | |||
| Factor scoresb | ||||
| 1: Proinflammatory cytokines (TNF-α, IL-1β, IL-8, IL-6; 31%) | −0.02 (1.0) | 0.02 (0.9) | .89 | 0.03 |
| 2: Myeloid migration (MCP-1, MMP-9; 13%) | −0.15 (1.0) | 0.15 (0.9) | .24 | 0.31 |
| 3: OT/CRP (OT, CRP; 12%) | −0.14 (1.0) | 0.14 (0.9) | .27 | 0.29 |
| 4: Chemokines regulating immune cell trafficking (MIG, IP-10; 10%) | 0.01 (1.0) | −0.1 (0.9) | .91 | 0.03 |
| 5: HPA axis hormones (AVP, cortisol; 8%) | −0.11 (0.9) | 0.11 (1.0) | .38 | 0.23 |
IQR = interquartile range; OT = oxytocin; AVP = arginine vasopressin; IL = interleukin; TNF-α = tumor necrosis factor α; CRP = C-reactive protein; IP-10 = interferon γ–induced protein; MCP-1 = monocyte chemotactic protein-1; MMP-9 = matrix metalloproteinase-9; MIG = monokine induced by interferon; SD = standard deviation; HPA = hypothalamic-pituitary-adrenal.
Independent-samples Mann-Whitney U test used for blood- and saliva-based markers; independent t tests used for factor scores.
a Basal afternoon concentrations computed as area under the curve with respect to ground.
b Scores from the principal components factor analysis (varimax rotation) of the blood- and saliva-based markers listed in this table, and the markers with the strongest factor loading on each factor and the percent of the total variance explained. All inflammatory markers were log transformed and z-scored.
Does Facial Emotion Identification Differ by ELT in PWH?
ELT-exposed PWH performed worse compared with ELT-unexposed PWH in total recognition accuracy (p = .021, d = 0.63; Figure 1A). This pattern did not significantly differ by facial expression (p = .94). However, examination of the pairwise group comparisons indicated that the differences in overall recognition accuracy were strongest for neutral (p = .067, d = 0.48) followed by sad (p = .096, d = 0.44), angry (p = .10, d = 0.43), fearful (p = .13, d = 0.39), and happy (p = .36, d = 0.24). When examining ELT exposure and errors misidentifing neutral faces (descriptive), the proportion of errors in identifying neutral faces as happy, angry, fearful, or sad was evenly distributed. For the ELT-unexposed group, neutral faces were more likely to be identified as fearful followed by angry (data not shown). There were no group differences in the RT (for total correct only; p = .31), and there were no group differences by emotion (p = .33; Figure 1B).
FIGURE 1.
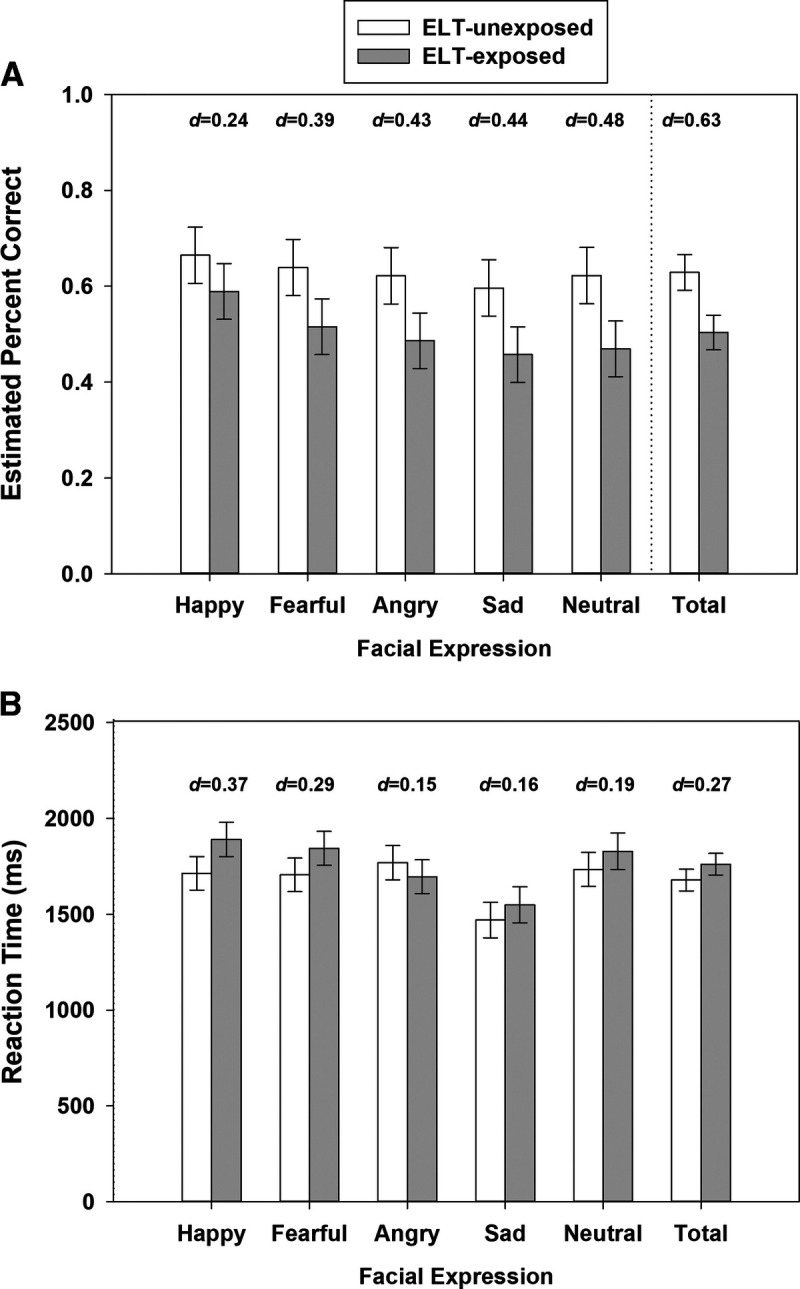
FEPT performance (error bars indicate the standard error of the mean estimates) for (A) percent accuracy and (B) reaction time for correct trials by ELT among people with HIV. Model controlled for age, sex, and current cannabis use. ELT = early life trauma; FEPT = Facial Emotion Perception Test.
Do Associations Between Neuroendocrine Markers, Immune Markers, and Facial Emotion Identification Performance Differ by ELT Exposure in PWH?
Two consistent patterns of results emerged. First, the relationship between ELT and performance (total recognition accuracy, neutral, fearful, and happy faces) was moderated by factor 3-OT/CRP (p values < .05 for the ELT by factor interactions; Figure 2). Specifically, ELT-exposed PWH performed worse than ELT-unexposed PWH among individuals with low factor 3-OT/CRP scores. This group difference was no longer significant among individuals with high scores on this factor. OT was the primary biological moderator of the ELT-FEPT performance relationship (Supplemental Table S3, http://links.lww.com/PSYMED/A863). Second, irrespective of ELT exposure, higher scores on factor 2-myeloid migration were associated with poorer total recognition accuracy (β = −0.39, p = .0026) as well as on happy (β = −0.33, p = .017), angry (β = −0.32, p = .018), sad (β = −0.32, p = .022), and fearful faces (β = −0.28, p = .038; trend on neutral faces: β = −0.24, p = .061; Table 3). The third finding was a trend for factor 5-HPA axis hormones to moderate the ELT-FEPT performance association on neutral faces (p = .099 for the ELT by factor interaction). Specifically, ELT-exposed PWH performed worse than ELT-unexposed PWH among individuals with high factor 5-HPA axis hormones scores (Figure 3). This group difference was no longer significant among individuals with low scores on this factor. Factor 1-proinflammatory cytokines and factor 4-chemokines regulating immune cell trafficking were not associated with FEPT performance and did not moderate the relationship between ELT and FEPT performance.
FIGURE 2.
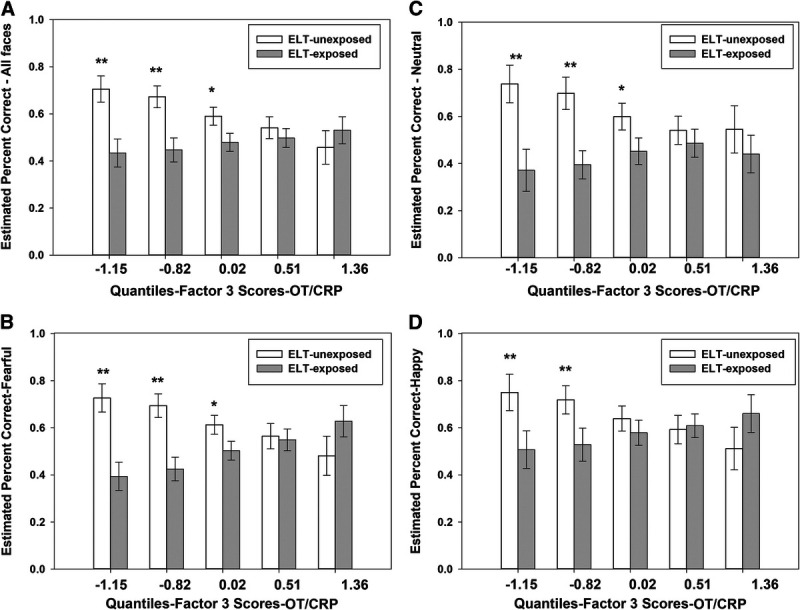
Factor 3-OT/CRP is a biological moderator of the association between ELT and FEPT performance for (A) percent accuracy across all faces, (B) faces expressing fear, (C) neutral faces, and (D) happy faces. CRP = C-reactive protein; ELT = early life trauma; FEPT = Facial Emotion Perception Test; OT = oxytocin.
TABLE 3.
Adjusted Associations Between Neuroendocrine and Inflammatory Factor Scores and FEPT Performance in People With HIV
| Factor | Total Accuracy, B (SE) | Neutral, B (SE) | Sad, B (SE) | Anger, B (SE) | Fear, B (SE) | Happy, B (SE) |
|---|---|---|---|---|---|---|
| Early life trauma (versus unexposed) | — | — | −0.13 (0.09) | −0.12 (0.08) | — | — |
| 1: Proinflammatory cytokines (TNF-α, IL-1β, IL-8, IL-6) | −0.03 (0.03) | −0.01 (0.04) | 0.02 (0.04) | −0.07 (0.04)† | −0.02 (0.03) | −0.04 (0.04) |
| 2: Myeloid migration (MCP-1, MMP-9) | −0.09 (0.03)** | −0.08 (0.04)† | −0.11 (0.04)* | −0.10 (0.04)* | −0.07 (0.03)* | −0.09 (0.04)* |
| 3: OT/CRP (OT, CRP) | — | — | −0.05 (0.04) | −0.04 (0.04) | — | — |
| 4: Chemokines regulating immune cell trafficking (MIG, IP-10) | 0.03 (0.03) | 0.06 (0.04) | −0.04 (0.04) | 0.05 (0.04) | 0.03 (0.03) | 0.03 (0.03) |
| 5: HPA axis hormones (AVP, cortisol) | −0.04 (0.03) | — | −0.02 (0.04) | −0.01 (0.04) | −0.04 (0.03) | −0.01 (0.03) |
FEPT = Facial Emotion Perception Test; B = unstandardized β coefficients; SE = standard error; TNF-α = tumor necrosis factor α; IL = interleukin; MCP-1 = monocyte chemotactic protein-1; MMP-9 = matrix metalloproteinase-9; OT = oxytocin; CRP = C-reactive protein; MIG = monokine induced by interferon; IP-10 = interferon γ–induced protein; HPA = hypothalamic-pituitary-adrenal.
The dash (—) indicates that the factor was a biological moderator of the early life trauma–performance association at p < .10.
* p < .05.
** p < .01.
*** p < .001.
†p > .05 and p < .10.
FIGURE 3.
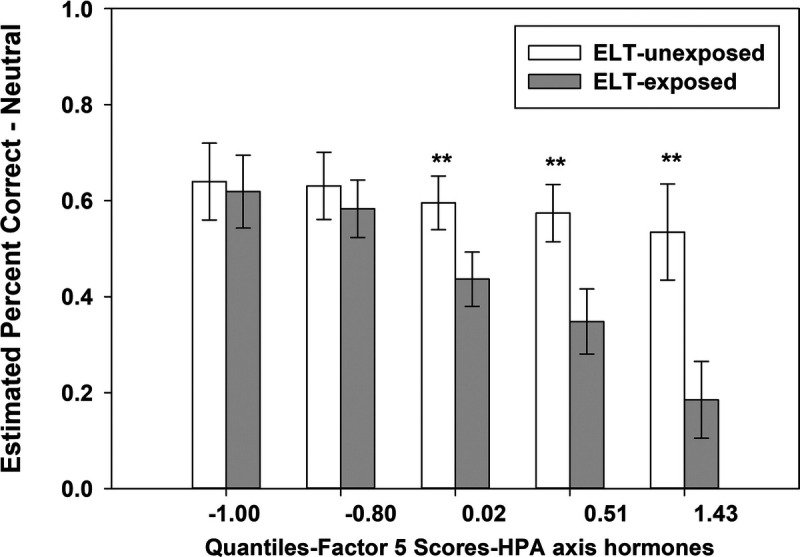
Factor 5-HPA axis hormones (AVP, cortisol) is a biological moderator of the association between ELT and FEPT performance for neutral faces. AVP = arginine vasopressin; ELT = early life trauma; FEPT = Facial Emotion Perception Test; HPA = hypothalamic-pituitary-adrenal axis; OT = oxytocin.
DISCUSSION
In our pilot study of 58 PWH, we examined the hypothesis that social processing difficulties in PWH would be more evident in adults reporting ELT and that key neuroendocrine (OT, AVP, cortisol) and immune markers (CRP, IL-6, TNF-α) would be biological moderators of the ELT-performance associations. Several key findings emerged from our analyses in this study of PWH, where 50% reported ELT, defined as childhood exposure ranging from moderate to severe sexual, physical, and/or emotional abuse. First, ELT-exposed PWH compared with ELT-unexposed PWH demonstrated difficulties with social processing, specifically with respect to their ability to accurately identify all emotional expressions, with the greatest difficulty on neutral and negative emotions. This finding aligns with prior studies demonstrating that ELT-exposed adults show difficulties in accurate facial emotion identification particularly negative emotions (18,19). Of note, ELT-exposed PWH did not show a negative bias on neutral faces, identifying them as fearful, sad, or angry. Rather, they incorrectly identified these faces as happy, sad, fearful, or angry suggesting that PWH and ELT exposure have a general difficulty identifying correct facial expressions rather than a particular pattern of bias. The finding that ELT exposure in PWH is associated with decreased social processing is important because the prevalence of ELT among PWH is common, and difficulties reading emotional expressions in others can have negative impacts on interpersonal relationships. Furthermore, rates of depression and anxiety disorders are high in PWH, and difficulties in interpersonal relationships can lead to, perpetuate and/or exacerbate symptoms of these disorders (8–10).
Second, consistent with some (30,31) but not all studies (32), ELT-exposed PWH had significantly higher OT levels compared with those ELT-unexposed PWH. One of the two biological factors moderating the relationships between ELT and performance was the factor (factor 3) where OT and CRP were the markers of greatest relevance. Specifically, the greatest performance gap between those reporting and not reporting ELT was when factor scores were lower, that is, lower levels of OT and CRP detected in ELT-exposed compared with ELT-unexposed PWH. At higher factor scores, that is, higher levels of OT and CRP detected in ELT-exposed compared with ELT-unexposed PWH, the performance gap was no longer significant between the two groups. Analysis of individual biomarkers revealed that OT, not CRP, moderated the ELT-FEPT performance relationship. One possible explanation is that OT is acting in its traditional role as a neurohormone to promote social processing and social adaptation by modulating emotion reaction and regulation networks (e.g., reward and saliency networks) (70,71). Another explanation for the present findings is that higher OT levels among ELT-exposed PWH may reflect health-promoting OT effects including OT acting as an anti-inflammatory (72–74) and have restorative actions for aspects of social processing. CRP is an adaptive molecule and not simply a “marker” for inflammation; CRP seems to be primarily anti-inflammatory and capable of moderating various effects of inflammatory processes. However, complicating its interpretation, isoforms of CRP can be proinflammatory (75). Interactions between OT and CRP are not well studied. However, data from our analysis suggest that, separately or in combination, these molecules may serve adaptive and potentially protective functions. The finding that the ELT-related decrease in social processing was not apparent when levels of OT were high may inform efforts to identify therapeutic targets for improving emotional processing in PWH, a skill that has been shown in many studies to be worse among PWH compared with those without HIV (13–17,20,21).
With the exception of OT, there were no other differences in the levels of salivary or systemic neuroendocrine and immune markers or factors by ELT exposure. This was unexpected for cortisol and proinflammatory cytokines because these levels are typically altered with ELT in HIV-uninfected individuals (27,28,47–50). Rather, findings with these markers were most notable in their associations with performance on the facial emotion identification task. Important to the ELT-performance association was factor 5-HPA axis hormones where AVP and afternoon basal cortisol levels had the highest factor loadings. This factor was a biological moderator of the relationship between ELT and FEPT performance where group differences in performance were evident among individuals with high, but not low, levels of factor 5. In the context of high HPA axis hormone levels, one possibility is that ELT may have a greater capacity to modulate performance. This pattern suggests alterations in downstream processes at the level of AVP and glucocorticoid receptors or in the integrity of brain regions dense in these receptors that may be impacted by ELT rather than an abnormality in levels.
One additional finding consistently emerged from the data was that irrespective of ELT: higher levels of myeloid migration markers (MCP-1, MMP-9) were associated with poorer performance on identifying facial expressions. Because this was observed in both groups, it is likely a general marker of HIV infection that is not ELT specific. MCP-1, also known as CCL-2, is a marker of myeloid migration and known to be upregulated in PWH and result in enhanced migration of monocytes across the blood-brain barrier (BBB), which may perpetuate HIV neuropathogenesis (76,77). MMP-9 is a gelatinase that influences BBB permeability (78). For example, elevated cerebral spinal fluid levels of MMP-9 are associated with HIV dementia (79,80), with activated microglia and monocyte migration in brain tissue (81), and with increased BBB permeability and HIV-infected leukocyte migration in an in vitro system (76). Clearly, MCP-1 and MMP-9 have strong associations with HIV and central nervous system dysfunction; however, they have not been previously linked to a deficiency in social processing in PWH, a novel finding of this study.
The present study had a number of strengths including the assessment of numerous neuroendocrine and immune markers concurrent with behavioral performance on a social processing measure. Limitations include the cross-sectional analysis, small sample size for ELT status subgroups, and the number of analyses that were conducted without adjustment for family-wise error rate because these analyses are hypothesis generating for future studies. In addition, a majority of participants in the analysis were men with HIV, and thus, these findings may not necessarily generalize to women with HIV given reported sex differences in social processing and biological markers including OT. We used saliva as a noninvasive measure for assessing inflammation, but the reliability of saliva as an index of systemic inflammation is debated and fluctuation in values can occur because of factors such as oral hygiene (82). However, 35 of the 58 participants in this analysis (60%) participated in an oral examination as part of a substudy, the majority (71%) did not have oral lesions, and this did not differ by ELT status. Because our goal was generalizability, we did not exclude individuals based on smoking or cannabis use, which can alter the HPA axis (83). Controlling for cannabis did not change the pattern of results, and current smoking status did not differ by ELT. Future larger-scale studies are warranted to determine whether the same patterns of results would emerge with serum or cerebral spinal fluid markers of inflammation.
One additional point to note is that OT and AVP were measured without extraction and controversy remains around this method. The decision to measure these hormones without extraction was based on tests for parallelism, spike recovery, and cross-reactivity/specificity validating the present study’s EIA procedure and suggesting that EIA without extraction produces useful measurements of these hormones in human blood plasma (63,66). Although nonextracted samples yield higher concentrations than extracted samples (84), unextracted samples of OT and AVP are highly familial (42) and repeatedly relate to neurobehavioral outcomes (measured using behavioral tasks and neuroimaging) in our studies in healthy individuals as well as psychiatric populations (42–44,85–87) and in studies conducted by others (88). Interesting, in a study using both methods, unextracted OT but not extracted OT related to social exclusion in young adults with a suicide attempt history (89). A second study demonstrated similar associations of extracted and unextracted OT with depressive symptoms; however, only unextracted levels were associated with anxiety symptoms (90). Thus, although differences in methods do not enable levels to be compared across studies, unextracted measures of OT and AVP seem to have important associations with clinically relevant behavioral measures.
In sum, our findings provide preliminary support that ELT exposure is associated with decreased social processing as measured by a facial expression identification task in PWH. The OT/CRP factor (driven primarily by OT) moderates the relationship between ELT and total facial emotion identification including neutral, happy, and fearful faces. Irrespective of ELT, higher levels of myeloid migration markers (MCP-1, MMP-9) were associated with poorer performance on identifying facial expressions.
Supplementary Material
Acknowledgments
We would like to thank Bruni Hirsch, Alana Aziz-Bradley, Jacob Ellis, Sheila D’Sa, Shannon Dowty, Lauren Drogos, Lacey Wisslead, Aleksa Anderson, and Preet Dhillon for their assistance with this study. We would also like to thank Kathleen Weber and the CORE Center at John H. Stroger Jr Hospital of Cook County for help in recruiting participants to the present study. We would also like to thank all of our participants, for without you this work would not be possible.
Source of Funding and Conflicts of Interest: Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Numbers K01MH098798 (L.H.R.), R21MH099978 (L.H.R.), and R01MH113512 (L.H.R.). The project described was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR000050 and in part by a University of Illinois at Chicago Campus Review Board Grant (L.H.R.) and a Chicago Developmental Center for AIDS Research pilot grant. The authors report no conflicts of interest.
Open Access publication for this article, which is part of a special themed issue of Psychosomatic Medicine, was funded by the National Institute of Mental Health.
Footnotes
Supplemental Digital Content
Contributor Information
Leah H. Rubin, Email: lrubin@jhu.edu.
Deeya Bhattacharya, Email: dbhatta4@jhmi.edu.
Joelle Fuchs, Email: fuchsj15@gmail.com.
Abigail Matthews, Email: amatthews1429@gmail.com.
Sarah Abdellah, Email: sabdell3@jhu.edu.
Rebecca T. Veenhuis, Email: rterill1@jhmi.edu.
Scott A. Langenecker, Email: s.langenecker@hsc.utah.edu.
Kathleen M. Weber, Email: kathleenmweber1@gmail.com.
Hans P. Nazarloo, Email: nazarloo@yahoo.com.
Sheila M. Keating, Email: smkeats410@gmail.com.
C. Sue Carter, Email: suecarterporges@gmail.com.
Pauline M. Maki, Email: pmaki1@uic.edu.
REFERENCES
- 1.Machtinger EL, Wilson TC, Haberer JE, Weiss DS. Psychological trauma and PTSD in HIV-positive women: a meta-analysis. AIDS Behav 2012;16:2091–100. [DOI] [PubMed] [Google Scholar]
- 2.Spies G, Afifi TO, Archibald SL, Fennema-Notestine C, Sareen J, Seedat S. Mental health outcomes in HIV and childhood maltreatment: a systematic review. Syst Rev 2012;1:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reisner SL, Falb KL, Mimiaga MJ. Early life traumatic stressors and the mediating role of PTSD in incident HIV infection among US men, comparisons by sexual orientation and race/ethnicity: results from the NESARC, 2004–2005. J Acquir Immune Defic Syndr 2011;57:340–50. [DOI] [PubMed] [Google Scholar]
- 4.Townsend C, Rheingold AA. Estimating a Child Sexual Abuse Prevalence Rate for Practitioners: A Review of Child Sexual Abuse Pervalence Studies. Charlston, SC: Darkness to Light; 2013. [Google Scholar]
- 5.Leserman J, Whetten K, Lowe K, Stangl D, Swartz MS, Thielman NM. How trauma, recent stressful events, and PTSD affect functional health status and health utilization in HIV-infected patients in the south. Psychosom Med 2005;67:500–7. [DOI] [PubMed] [Google Scholar]
- 6.Bick J, Nelson CA. Early adverse experiences and the developing brain. Neuropsychopharmacology 2016;41:177–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teicher MH, Samson JA. Annual research review: enduring neurobiological effects of childhood abuse and neglect. J Child Psychol Psychiatry 2016;57:241–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brady KT, Back SE. Childhood trauma, posttraumatic stress disorder, and alcohol dependence. Alcohol Res 2012;34:408–13. [PMC free article] [PubMed] [Google Scholar]
- 9.Brady KT, Sinha R. Co-occurring mental and substance use disorders: the neurobiological effects of chronic stress. Am J Psychiatry 2005;162:1483–93. [DOI] [PubMed] [Google Scholar]
- 10.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry 2001;49:1023–39. [DOI] [PubMed] [Google Scholar]
- 11.Cuthbert BN. The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry 2014;13:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Insel T Cuthbert B Garvey M Heinssen R Pine DS Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry 2010;167:748–51. [DOI] [PubMed] [Google Scholar]
- 13.Lane TA, Moore DM, Batchelor J, Brew BJ, Cysique LA. Facial emotional processing in HIV infection: relation to neurocognitive and neuropsychiatric status. Neuropsychology 2012;26:713–22. [DOI] [PubMed] [Google Scholar]
- 14.Clark US, Cohen RA, Westbrook ML, Devlin KN, Tashima KT. Facial emotion recognition impairments in individuals with HIV. J Int Neuropsychol Soc 2010;16:1127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heilman KJ, Harden ER, Weber KM, Cohen M, Porges SW. Atypical autonomic regulation, auditory processing, and affect recognition in women with HIV. Biol Psychol 2013;94:143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grabyan JM Morgan EE Cameron MV Villalobos J Grant I Paul Woods S, et al. Deficient emotion processing is associated with everyday functioning capacity in HIV-associated neurocognitive disorder. Arch Clin Neuropsychol 2018;33:184–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Baeza A Perez-Valero I Carvajal-Molina F Bayon C Montes-Ramirez M Bernardino JI, et al. Facial emotional processing deficits in long-term HIV-suppressed patients. J Int AIDS Soc 2014;17:19664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berube A, Turgeon J, Blais C, Fiset D. Emotion recognition in adults with a history of childhood maltreatment: a systematic review. Trauma Violence Abuse 2021;15248380211029403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Assed MM, Khafif TC, Belizario GO, Fatorelli R, Castanho de Ameida Rocca C, Serafim A. Facial emotion recognition in maltreated children: a systematic review. J Child Fam Stud 2020;29:1493–509. [Google Scholar]
- 20.Baldonero E Ciccarelli N Fabbiani M Colafigli M Improta E D’Avino A, et al. Evaluation of emotion processing in HIV-infected patients and correlation with cognitive performance. BMC Psychol 2013;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark US Walker KA Cohen RA Devlin KN Folkers AM Pina MJ, et al. Facial emotion recognition impairments are associated with brain volume abnormalities in individuals with HIV. Neuropsychologia 2015;70:263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grinevich V, Ludwig M. The multiple faces of the oxytocin and vasopressin systems in the brain. J Neuroendocrinol 2021;33:e13004. [DOI] [PubMed] [Google Scholar]
- 23.Carter CS. Developmental consequences of oxytocin. Physiol Behav 2003;79:383–97. [DOI] [PubMed] [Google Scholar]
- 24.Brown CA, Cardoso C, Ellenbogen MA. A meta-analytic review of the correlation between peripheral oxytocin and cortisol concentrations. Front Neuroendocrinol 2016;43:19–27. [DOI] [PubMed] [Google Scholar]
- 25.Cardoso C, Kingdon D, Ellenbogen MA. A meta-analytic review of the impact of intranasal oxytocin administration on cortisol concentrations during laboratory tasks: moderation by method and mental health. Psychoneuroendocrinology 2014;49:161–70. [DOI] [PubMed] [Google Scholar]
- 26.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 2009;10:434–45. [DOI] [PubMed] [Google Scholar]
- 27.Bunea IM, Szentagotai-Tatar A, Miu AC. Early-life adversity and cortisol response to social stress: a meta-analysis. Transl Psychiatry 2017;7:1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trickett PK, Noll JG, Susman EJ, Shenk CE, Putnam FW. Attenuation of cortisol across development for victims of sexual abuse. Dev Psychopathol 2010;22:165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heim C, Young LJ, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. Lower CSF oxytocin concentrations in women with a history of childhood abuse. Mol Psychiatry 2009;14:954–8. [DOI] [PubMed] [Google Scholar]
- 30.Olff M Frijling JL Kubzansky LD Bradley B Ellenbogen MA Cardoso C, et al. The role of oxytocin in social bonding, stress regulation and mental health: an update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology 2013;38:1883–94. [DOI] [PubMed] [Google Scholar]
- 31.Pierrehumbert B, Torrisi R, Laufer D, Halfon O, Ansermet F, Beck Popovic M. Oxytocin response to an experimental psychosocial challenge in adults exposed to traumatic experiences during childhood or adolescence. Neuroscience 2010;166:168–77. [DOI] [PubMed] [Google Scholar]
- 32.Opacka-Juffry J, Mohiyeddini C. Experience of stress in childhood negatively correlates with plasma oxytocin concentration in adult men. Stress 2012;15:1–10. [DOI] [PubMed] [Google Scholar]
- 33.Ellenbogen MA. Oxytocin and facial emotion recognition. Curr Top Behav Neurosci 2018;35:349–74. [DOI] [PubMed] [Google Scholar]
- 34.Veenema AH. Toward understanding how early-life social experiences alter oxytocin- and vasopressin-regulated social behaviors. Horm Behav 2012;61:304–12. [DOI] [PubMed] [Google Scholar]
- 35.Barberis C, Tribollet E. Vasopressin and oxytocin receptors in the central nervous system. Crit Rev Neurobiol 1996;10:119–54. [DOI] [PubMed] [Google Scholar]
- 36.Caffe AR, van Leeuwen FW. Vasopressin-immunoreactive cells in the dorsomedial hypothalamic region, medial amygdaloid nucleus and locus coeruleus of the rat. Cell Tissue Res 1983;233:23–33. [DOI] [PubMed] [Google Scholar]
- 37.Murray RJ, Brosch T, Sander D. The functional profile of the human amygdala in affective processing: insights from intracranial recordings. Cortex 2014;60:10–33. [DOI] [PubMed] [Google Scholar]
- 38.Carter CS, Boone EM, Pournajafi-Nazarloo H, Bales KL. Consequences of early experiences and exposure to oxytocin and vasopressin are sexually dimorphic. Dev Neurosci 2009;31:332–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kompier NF, Keysers C, Gazzola V, Lucassen PJ, Krugers HJ. Early life adversity and adult social behavior: focus on arginine vasopressin and oxytocin as potential mediators. Front Behav Neurosci 2019;13:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ellis BJ, Horn AJ, Carter CS, van IJzendoorn MH, Bakermans-Kranenburg MJ. Developmental programming of oxytocin through variation in early-life stress: four meta-analyses and a theoretical reinterpretation. Clin Psychol Rev 2021;86:101985. [DOI] [PubMed] [Google Scholar]
- 41.Liu JJ, Lou F, Lavebratt C, Forsell Y. Impact of childhood adversity and vasopressin receptor 1a variation on social interaction in adulthood: a cross-sectional study. PLoS One 2015;10:e0136436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubin LH Carter CS Bishop JR Pournajafi-Nazarloo H Drogos LL Hill SK, et al. Reduced levels of vasopressin and reduced behavioral modulation of oxytocin in psychotic disorders. Schizophr Bull 2014;40:1374–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubin LH Carter CS Drogos L Jamadar R Pournajafi-Nazarloo H Sweeney JA, et al. Sex-specific associations between peripheral oxytocin and emotion perception in schizophrenia. Schizophr Res 2011;130:266–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rubin LH Yao L Keedy SK Reilly JL Bishop JR Carter CS, et al. Sex differences in associations of arginine vasopressin and oxytocin with resting-state functional brain connectivity. J Neurosci Res 2017;95:576–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li T, Wang P, Wang SC, Wang YF. Approaches mediating oxytocin regulation of the immune system. Front Immunol 2016;7:693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang P Yang HP Tian S Wang L Wang SC Zhang F, et al. Oxytocin-secreting system: a major part of the neuroendocrine center regulating immunologic activity. J Neuroimmunol 2015;289:152–61. [DOI] [PubMed] [Google Scholar]
- 47.Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-alpha. Mol Psychiatry 2016;21:642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coelho R, Viola TW, Walss-Bass C, Brietzke E, Grassi-Oliveira R. Childhood maltreatment and inflammatory markers: a systematic review. Acta Psychiatr Scand 2014;129:180–92. [DOI] [PubMed] [Google Scholar]
- 49.Gouin JP, Glaser R, Malarkey WB, Beversdorf D, Kiecolt-Glaser JK. Childhood abuse and inflammatory responses to daily stressors. Ann Behav Med 2012;44:287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuhlman KR, Horn SR, Chiang JJ, Bower JE. Early life adversity exposure and circulating markers of inflammation in children and adolescents: a systematic review and meta-analysis. Brain Behav Immun 2020;86:30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller GE, White SF, Chen E, Nusslock R. Association of inflammatory activity with larger neural responses to threat and reward among children living in poverty. Am J Psychiatry 2021;178:313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Inagaki TK, Muscatell KA, Irwin MR, Cole SW, Eisenberger NI. Inflammation selectively enhances amygdala activity to socially threatening images. Neuroimage 2012;59:3222–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kraynak TE, Marsland AL, Wager TD, Gianaros PJ. Functional neuroanatomy of peripheral inflammatory physiology: a meta-analysis of human neuroimaging studies. Neurosci Biobehav Rev 2018;94:76–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rubin LH, Phan KL, Keating SM, Weber KM, Maki PM. Brief report: low-dose hydrocortisone has acute enhancing effects on verbal learning in HIV-infected men. J Acquir Immune Defic Syndr 2017;75:e65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rubin LH, Phan KL, Keating SM, Maki PM. A single low-dose of hydrocortisone enhances cognitive functioning in HIV-infected women. AIDS 2018;32:1983–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bieliauskas L, Counte M, Glandon G. Inventorying stressing life events as related to health change in the elderly. Stress Med 1995;11:93–103. [Google Scholar]
- 57.Ruggiero KJ, Del Ben K, Scotti JR, Rabalais AE. Psychometric properties of the PTSD Checklist—Civilian Version. J Trauma Stress 2003;16:495–502. [DOI] [PubMed] [Google Scholar]
- 58.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385–96. [PubMed] [Google Scholar]
- 59.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measur 1977;1:385–401. [Google Scholar]
- 60.Buysse DJ, Reynolds CF, 3rd, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI). Sleep 1991;14:331–8. [PubMed] [Google Scholar]
- 61.Walsh JC, Mandalia S, Gazzard BG. Responses to a 1 month self-report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. AIDS 2002;16:269–77. [DOI] [PubMed] [Google Scholar]
- 62.Bernstein D, Fink L. Childhood Trauma Questionnaire: A Retrospective Self-report. San Antonio, TX: The Psychological Coorporation; 1998. [Google Scholar]
- 63.Carter CS Pournajafi-Nazarloo H Kramer KM Ziegler TE White-Traut R Bello D, et al. Oxytocin: behavioral associations and potential as a salivary biomarker. Ann N Y Acad Sci 2007;1098:312–22. [DOI] [PubMed] [Google Scholar]
- 64.MacLean EL, Gesquiere LR, Gee N, Levy K, Martin WL, Carter CS. Validation of salivary oxytocin and vasopressin as biomarkers in domestic dogs. J Neurosci Methods 2018;293:67–76. [DOI] [PubMed] [Google Scholar]
- 65.Brandtzaeg OK Johnsen E Roberg-Larsen H Seip KF MacLean EL Gesquiere LR, et al. Proteomics tools reveal startlingly high amounts of oxytocin in plasma and serum. Sci Rep 2016;6:31693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.MacLean EL, Wilson SR, Martin WL, Davis JM, Nazarloo HP, Carter CS. Challenges for measuring oxytocin: the blind men and the elephant? Psychoneuroendocrinology 2019;107:225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 2003;28:916–31. [DOI] [PubMed] [Google Scholar]
- 68.Langenecker SA, Bieliauskas LA, Rapport LJ, Zubieta JK, Wilde EA, Berent S. Face emotion perception and executive functioning deficits in depression. J Clin Exp Neuropsychol 2005;27:320–33. [DOI] [PubMed] [Google Scholar]
- 69.Cohen J. A power primer. Psychol Bull 1992;112:155–9. [DOI] [PubMed] [Google Scholar]
- 70.Ma Y, Shamay-Tsoory S, Han S, Zink CF. Oxytocin and social adaptation: insights from neuroimaging studies of healthy and clinical populations. Trends Cogn Sci 2016;20:133–45. [DOI] [PubMed] [Google Scholar]
- 71.Wang D, Yan X, Li M, Ma Y. Neural substrates underlying the effects of oxytocin: a quantitative meta-analysis of pharmaco-imaging studies. Soc Cogn Affect Neurosci 2017;12:1565–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clodi M Vila G Geyeregger R Riedl M Stulnig TM Struck J Luger TA, et al. Oxytocin alleviates the neuroendocrine and cytokine response to bacterial endotoxin in healthy men. Am J Physiol Endocrinol Metab 2008;295:E686–91. [DOI] [PubMed] [Google Scholar]
- 73.Detillion CE, Craft TK, Glasper ER, Prendergast BJ, DeVries AC. Social facilitation of wound healing. Psychoneuroendocrinology 2004;29:1004–11. [DOI] [PubMed] [Google Scholar]
- 74.Carter CS Kenkel WM MacLean EL Wilson SR Perkeybile AM Yee JR, et al. Is oxytocin “nature’s medicine”? Pharmacol Rev 2020;72:829–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol 2018;9:754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)–infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci 2006;26:1098–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Williams DW, Veenstra M, Gaskill PJ, Morgello S, Calderon TM, Berman JW. Monocytes mediate HIV neuropathogenesis: mechanisms that contribute to HIV associated neurocognitive disorders. Curr HIV Res 2014;12:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Louboutin JP, Agrawal L, Reyes BA, Van Bockstaele EJ, Strayer DS. HIV-1 gp120-induced injury to the blood-brain barrier: role of metalloproteinases 2 and 9 and relationship to oxidative stress. J Neuropathol Exp Neurol 2010;69:801–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Conant K, McArthur JC, Griffin DE, Sjulson L, Wahl LM, Irani DN. Cerebrospinal fluid levels of MMP-2, 7, and 9 are elevated in association with human immunodeficiency virus dementia. Ann Neurol 1999;46:391–8. [DOI] [PubMed] [Google Scholar]
- 80.Suryadevara R Holter S Borgmann K Persidsky R Labenz-Zink C Persidsky Y, et al. Regulation of tissue inhibitor of metalloproteinase-1 by astrocytes: links to HIV-1 dementia. Glia 2003;44:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ghorpade A Persidskaia R Suryadevara R Che M Liu XJ Persidsky Y, et al. Mononuclear phagocyte differentiation, activation, and viral infection regulate matrix metalloproteinase expression: implications for human immunodeficiency virus type 1–associated dementia. J Virol 2001;75:6572–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Engeland CG, Bosch JA, Rohleder N. Salivary biomarkers in psychoneuroimmunology. Curr Opin Behav Sci 2019;28:58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van Leeuwen AP, Creemers HE, Greaves-Lord K, Verhulst FC, Ormel J, Huizink AC. Hypothalamic-pituitary-adrenal axis reactivity to social stress and adolescent cannabis use: the TRAILS study. Addiction 2011;106:1484–92. [DOI] [PubMed] [Google Scholar]
- 84.Szeto A McCabe PM Nation DA Tabak BA Rossetti MA McCullough ME, et al. Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosom Med 2011;73:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rubin LH, Carter CS, Drogos L, Pournajafi-Nazarloo H, Sweeney JA, Maki PM. Peripheral oxytocin is associated with reduced symptom severity in schizophrenia. Schizophr Res 2010;124:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rubin LH Carter CS Bishop JR Pournajafi-Nazarloo H Harris MS Hill SK, et al. Peripheral vasopressin but not oxytocin relates to severity of acute psychosis in women with acutely-ill untreated first-episode psychosis. Schizophr Res 2013;146:138–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rubin LH, Carter CS, Drogos LL, Pournajafi-Nazarloo H, Sweeney JA, Maki PM. Effects of sex, menstrual cycle phase, and endogenous hormones on cognition in schizophrenia. Schizophr Res 2015;166:269–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roels R, Rehman US, Carter CS, Nazarloo HP, Janssen E. The link between oxytocin plasma levels and observed communication behaviors during sexual and nonsexual couple discussions: an exploratory study. Psychoneuroendocrinology 2021;129:105265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chu C, Hammock EAD, Joiner TE. Unextracted plasma oxytocin levels decrease following in-laboratory social exclusion in young adults with a suicide attempt history. J Psychiatr Res 2020;121:173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saxbe D, Khaled M, Horton KT, Mendez AJ. Maternal prenatal plasma oxytocin is positively associated with prenatal psychological symptoms, but method of immunoassay extraction may affect results. Biol Psychol 2019;147:107718. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


