Summary
Background
The COVID-19 pandemic has substantially affected the antibiotic stewardship activities in most hospitals of India.
Aims
We conducted an antibiotic point prevalence survey (PPS) immediately after the decline of a major COVID-19 wave at a dedicated COVID-19 hospital. By doing so we aimed to identify the antibiotic prescription patterns, identify factors influencing the choice of antibiotics, and identify/develop strategies to improve the antibiotic stewardship program in such setups.
Methods
The PPS was single-centred, cross-sectional, and retrospective in nature. Patients admitted in various wards and intensive care units (ICUs) between September 2021 to October 2021 were included in our PPS.
Results
Of the included 460 patients, 192 were prescribed antibiotics. Of these 192 patients, ICU-admitted patients had the highest number of antibiotics prescribed i.e. 2.09 ± 0.92. Only a minor fraction (7.92 %) of antibiotics prescriptions were on the basis of culture reports. Most of the antibiotics were prescribed empirically by the parenteral route. The most common group of antibiotics prescribed were third-generation cephalosporins. Carbapenems were the most common designated antibiotics prescribed. A large number of patients (22.40 %) were prescribed a double anaerobic coverage.
Conclusion
The strategies that we identified to improve the antibiotic stewardship program at our institute included reviving the culture of sending culture reports to prescribe antibiotics, improving surgical prophylaxis guidelines, training resident doctors to categorize antibiotic prescriptions appropriately, closely monitoring prescriptions providing double anaerobic coverage, and improving the electronic medical record system for improving prescription auditing.
Keywords: Antibiotic stewardship program, COVID-19, Prescription auditing, Point prevalence survey
Introduction
Antimicrobial resistance is a growing public health challenge worldwide that has been identified as one of the top ten threats to global health by the World Health Organization (WHO) [1]. Antimicrobial resistance has caused an increase in the duration of hospitalization, increased disease-related complications, a substantial increase in economic load, and an overall deleterious impact on health [2]. The major consumers of antibiotics are low- and middle-income countries, including India [3].
During the COVID-19 pandemic there has been a massive upsurge in the usage of antibiotics in hospital settings [[4], [5], [6]]. Also, antimicrobial stewardship activities in tertiary care hospitals have been derailed due to the lack of manpower and pooling of resources towards tackling the COVID-19 crisis [7]. Therefore, considering the negative impact of the COVID-19 pandemic on antibiotic stewardship activities, it is imperative for tertiary care hospitals, specifically dedicated to cater COVID-19 patients to closely monitor their usage of antibiotics and resistance patterns. In the Indian scenario, wherein there is a lack of a national registry system for documenting antimicrobial consumption, local antibiotic surveillance becomes even more crucial [8].
There have been a few point prevalence surveys (PPSs) in India to estimate antibiotic consumption patterns in major government-run hospitals. However, most of these studies pre-date the COVID-19 pandemic, [9,10] and certainly none were conducted immediately after a major COVID-19 wave.
Considering all the above-mentioned factors, we conducted this point prevalence survey (PPS) to assess the community-level antibiotic consumption pattern immediately after a major COVID-19 wave in India. We conducted this PPS at our hospital, which exclusively catered only COVID-19 patients during the COVID-19 pandemic, using the WHO Access, Watch and Reserve (AWaRe) classification. The aims of this PPS were to identify antibiotic prescription patterns, factors influencing the choice of antibiotics, and to assist in improving our local antibiotic stewardship program. Nationally, our experience should assist with formulating guidelines for promoting rational therapeutics in dedicated COVID-19 hospitals.
Methods
Ethical considerations
Ethical clearance was obtained from the institutional ethics committee (letter No. 134X/11/13/2021-IEC/34 dated 12.08.2021). The study was in accordance with the revised Helsinki, 2006 and ICMR guidelines for biomedical research on human participants 2017. Each patient was allotted a sequential subject number, and data anonymity was maintained throughout.
Study design and settings
The study was a single-centre, cross-sectional, point prevalence, retrospective study carried over four weeks in September–October 2021 at Employee's State Insurance Corporation Medical College and Hospital, Faridabad which is a tertiary care teaching hospital in India. The institute was a dedicated COVID-19 hospital during major COVID-19 waves in India. Dedicated COVID-19 hospitals cater exclusively for COVID-19 patients during major COVID-19 waves, providing outpatient and inpatient services, including ICUs. After the wave declines, these hospitals resume their services to non-COVID-19 patients. The delta variant COVID-19 wave started in India around the first week of March 2021 and ended at the end of August 2021. Our PPS was conducted immediately after the decline of this wave. The PPS was part of the antibiotic stewardship program of our hospital, and followed the standard methodology as described by the Global Point Prevalence Survey of Antimicrobial Consumption and Resistance (January 2019 version) [11].
Study population and sample size
The accessible population comprised all patients admitted in wards or ICUs during the surveillance period. A single ward or ICU was covered on a single day. The ward or ICU to be surveyed on a particular day was decided by the lottery method on the previous day. There was no formal sample size calculation.
Inclusion criteria
We included admitted patients in wards or ICUs before 08.00 hours (the start of the morning shift) on the intended day of the survey, who were prescribed at least one antibiotic.
Exclusion criteria
Patients admitted after 08.00 hours, patients in outpatient and emergency departments, patients intended to be discharged on the day of the survey, and patients posted for surgery on the day of the survey, were excluded.
Data collection
The medical record files, bedside treatment charts, and culture reports included in the case files were referred to by the study team to extract the data. Inputs from the treating clinician (ward-resident or consultant-on-duty) were taken while extracting the data.
Working definitions
An empiric antibiotic prescription was defined as the initial antibiotic prescribed in absence of culture reports [12]. A prophylactic antibiotic prescription was defined as an antibiotic prescribed for prevention of an infectious complication of a disease or preventing an infectious complication arising from a non-surgical (medical) or surgical intervention [13,14]. A lab-based antibiotic prescription was defined as an antibiotic prescribed after the pathogen was identified and reported [12].
Community acquired infections (CAIs) were defined as infections that were developing outside hospital, or diagnosed within 48 h of admission without any previous health care encounter [15]. Hospital-acquired infections (HAIs) were defined as infections not present or incubating at the time of admission to a hospital [16].
Designated antibiotics were selected by the study team based on the drugs mentioned in the hospital antibiotic policy and antibiotics mentioned in the ‘Watch’ and ‘Reserve’ categories of the WHO AWaRe classification. The antibiotics considered as designated antibiotics in our study included carbapenems, polymyxins, vancomycin/teicoplanin, tigecycline, minocycline, linezolid, systemic antifungals, systemic antivirals and antitubercular drugs.
Parameters calculated
The following parameters were calculated in our PPS: (a) calculation of the number/percentage of patients prescribed antimicrobials; (b) categorization of antimicrobial prescriptions as empiric, prophylactic or lab-based; (c) calculation of the number/percentage of antibiotics administered by the oral or parenteral route; (d) categorization of antimicrobial prescriptions based on the indication i.e. for CAIs, HAIs, medical prophylaxis, surgical prophylaxis, unknown infections, or others; (e) calculation of the number/percentage of patients prescribed a double coverage for anaerobes or Gram-negative organisms; (f) calculation of the number/percentage of patients on designated antimicrobials; (g) overall consumption of antimicrobials by class according to the WHO Anatomical Therapeutic Chemical (ATC) classification.
Statistical analysis
Data were collected in case record forms and was then entered onto Microsoft Excel. Most descriptive data were expressed as numbers or percentages. Means ± standard deviation (SD) were calculated using SPSS version 27 (IBM Corporation, USA).
Results
The PPS included 460 patients on ten different wards, of whom 192 (41.73%) were prescribed antibiotics (Table I). Patients admitted to the obstetrics and gynecology (OBG) ward had the highest percentage of antibiotics prescribed (75%); those on the Eye and Psychiatry wards received no antibiotics.
Table I.
Percentage of patients on antimicrobials
| Name of ward | Total number of beds occupied | Number (%) of patients on antimicrobials |
|---|---|---|
| Medicine | 80 | 41 (51) |
| Surgery | 80 | 36 (45) |
| OBG | 40 | 30 (75) |
| Paediatric | 40 | 13 (33) |
| Oncology | 40 | 11 (28) |
| ENT/Mucormycosis | 40 | 12 (30) |
| Eye | 15 | 0 |
| Psychiatry | 25 | 0 |
| ICU | 60 | 35 (58) |
| Orthopaedics | 40 | 14 (35) |
| Overall | 460 | 192 (42) |
OBG, Obstetrics and gynecology; ENT, Ear Nose Throat; ICU, Intensive care unit.
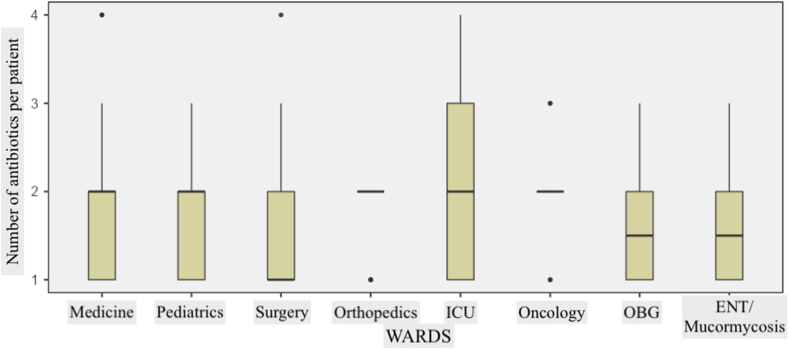
43% of treated patients were receiving 1 antibiotic, 40% 2 antibiotics, and 18 % patients ≥3 antibiotics (Table II). Three or more antibiotics were prescribed predominantly for ICU patients. Overall, the average number of antibiotics prescribed per patient was 1.78 ± 0.80, the highest average number being on the ICU (2.09 ± 0.92) (Figure 1). The majority of antibiotic prescriptions were empirical (72.14%); 19.94% were prophylactic, and only 7.92% of prescription were based on laboratory reports. The vast majority (81.23%) of antibiotics were administered by the parenteral route. 32.59% of antibiotic prescriptions were for unknown infections, the other indications for antibiotics being CAIs (30.17%), surgical prophylaxis (22.37%), medical prophylaxis (9.63%), and HAIs (5.24%).
Table II.
Number of antibiotics per patients
| Parameter | No. (%) of patients in different specialties receiving different numbers of antibiotics |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Medicine | Paediatric | Surgery | Orthopaedics | ICU | Oncology | OBG | ENT/Mucormycosis | Total | |
| Number of patients on 1 antibiotic | 20 (49) | 6 (46) | 19 (53) | 3 (21) | 11 (31) | 2 (18) | 15 (50) | 6 (50) | 82 (43) |
| Number of patients on 2 antibiotics | 14 (34) | 6 (46) | 13 (36) | 11 (79) | 12 (34) | 7 (64) | 9 (30) | 4 (33) | 76 (40) |
| Number of patients on 3 antibiotics | 5 (12) | 1 (8) | 3 (8) | 0 (0) | 10 (29) | 2 (18) | 6 (20) | 2 (17) | 29 (15) |
| Number of patients on ≥ 4 antibiotics | 2 (5) | 0 (0) | 1 (3) | 0 (0) | 2 (6) | 0 (0) | 0 (0) | 0 (0) | 5 (3) |
| Average number of antibiotics per patient | 1.73 ± 0.87 | 1.62 ± 0.65 | 1.61 ± 0.77 | 1.79 ± 0.43 | 2.09 ± 0.92 | 2.00 ± 0.63 | 1.70 ± 0.79 | 1.67 ± 0.78 | 1.78 ± 0.80 |
Number of patients on antibiotics are expressed as n (%); Average number of antibiotics per patient are expressed as n ± SD. ICU, Intensive care unit; OBG, Obstetrics and gynaecology; ENT, Ear Nose Throat.
Figure 1.
Distribution of average number of antibiotics prescribed per patient according to the surveyed ward/ICU.
Forty-three (22.40 %) patients were prescribed a double anaerobic coverage, most commonly piperacillin/tazobactam with metronidazole (9 cases) or with meropenem (7 cases). A total of 32 (16.67%) patients were prescribed designated antibiotics, predominantly carbapenems (24) and glycopeptides (5). One patient each received tigecycline, linezolid and bacitracin. Nineteen (9.90%) patients were receiving antifungal drugs, 9 (4.69%) antitubercular drugs and 3 (1.56%) antiviral drugs.
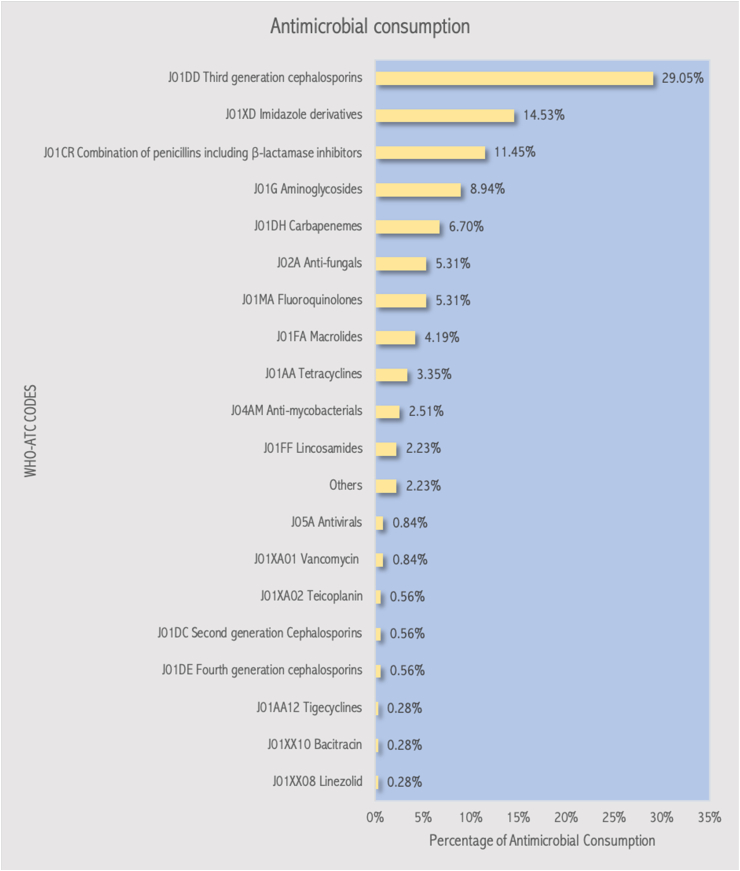
The overall consumption of antimicrobials by WHO ATC class is shown in Figure 2. Third generation cephalosporins (29.05%) were the most common antimicrobials prescribed, followed by imidazoline derivatives (14.53%) and penicillin-beta-lactamase inhibitor combinations (11.45%). The majority (78.56 %) of the antibiotics prescribed were from the ‘Watch’ category.
Figure 2.
Antimicrobial consumption by class according to the WHO ATC classification.
Discussion
In order to rationalize antibiotic prescription and in turn to control the emergence of multidrug-resistant microbes, effective surveillance of antibiotic use is essential. Antibiotic surveillance aids in observing the effectiveness of policies, identifying targets for quality improvement, and informing policymakers [17,18]. More irrational antibiotic use during the COVID-19 pandemic makes ongoing surveillance of antibiotic prescription crucial [4,5,8,17,18].
A multicentre PPS conducted prior to a COVID-19 wave in five major Indian hospitals, reported an antibiotic prescription rate of 50.03% [9]. Another pre-COVID-19 wave PPS conducted in 16 private-sector hospitals in India reported an antibiotic prescription rate of 57.4% [10]. Another pre-COVID-19 wave PPS in a major tertiary care Indian hospital reported an antibiotic prescription rate of 50.9 % [19]. Even higher rates of antibiotic prescription were reported in pre-COVID-19 studies from neighbouring countries: 78% in Bangladesh, 77.16% in Pakistan, and 63.4% in Myanmar [[20], [21], [22]]. Data on antibiotic prescribing in India during major COVID-19 waves are sparse [18]. However, PPSs conducted in neighbouring countries found prescribing rates of 100% in Bangladesh and 97.3% and 89.67% in Pakistan, far higher than at other times [[23], [24], [25]]. Our PPS, conducted immediately after a COVID-19 wave, found an antibiotic prescription rate of 41.73 %, which was much lower than the antibiotic prescription rates reported in regional PPSs conducted during a COVID-19 wave and was comparable to the antibiotic prescription rates reported in regional PPSs conducted prior to a COVID-19 wave [9,10,[19], [20], [21], [22],[22], [22], [23], [24], [25]]. In contrast to the developing nations of the Indian subcontinent, most developed nations of Europe and America had substantial lower antimicrobial prescription rates (25–34%) in the pre-COVID-19 period [[26], [27], [28]]. Similarly, antibiotic prescription rates during the COVID-19 pandemic in developed countries (63.1–64.8%) have been lower than in developing Southeast Asian countries (87.5 %) [29]. A number of factors have been identified for this wide variation in the antibiotic use between developing and developed nations, including variations in social determinants of health, volume of infectious diseases, differences in healthcare systems, prescription policies of hospitals, and marketing strategies of pharmaceutical companies [8,17,18].
A PPS conducted in India prior to a COVID-19 wave reported that the proportion of patients receiving ≥3 antibiotics ranged from 6.5% to 30.9% [9]. In a PPS conducted in Pakistan during a COVID-19 wave, 29.24% of patients were receiving ≥3 antibiotics [25]. In our study, conducted after a COVID-19 wave, 15.10% were receiving ≥3 antibiotics, and 2.6% ≥4 antibiotics. The majority of these patients were on the ICU, where combination therapy may be advised for polymicrobial infections, to broaden the antimicrobial spectrum, obtain a synergistic effect or improving synergy between different antibiotics, and preventing antibiotic resistance [30]. An Indian PPS conducted prior to a COVID-19 wave, reported the average number of antibiotics prescribed per patient was 1.62 [9]. Two PPSs conducted in the Indian subcontinent during a COVID-19 wave reported average numbers of 1.66 and 2.21, respectively [23,25]. In our post-COVID-19 wave study the average number of antibiotics prescribed per patient was 1.78 ± 0.80, indicating that the number of antibiotics prescribed per patient has been unaffected by the pandemic.
In a pre-COVID wave PPS conducted in India, 40.1% of antibiotics were prescribed empirically, 37.1% prophylactically, and 22.8% on the basis of culture reports [9]. In a PPS conducted during a COVID-19 wave in Pakistan, 85.5% antibiotics were prescribed prophylactically, probably to prevent secondary bacterial infections in COVID-19 [24]. By contrast, in our study the majority (72.14%) of antibiotic prescriptions were empiric, and only 19.94% antibiotics were prescribed prophylactically. There are several possible reasons why only 7.92% of antibiotic prescriptions were based on laboratory results. First, during the COVID-19 wave our institute was a dedicated COVID-19 hospital, and the practice of prescribing antibiotics including fluoroquinolones and macrolides without culture reports became the norm [18,29]. This practice may have continued even after the COVID-19 wave had declined. Second, as a tertiary care hospital, we receive many patients already receiving antibiotics prescribed in the referring hospitals. Third, a delay in results turnaround times may have impeded de-escalation and/or targeting therapy based on culture results. Reviving the habit among clinicians to send cultures and review culture reports when COVID-19 waves decline may be an effective strategy in improving antimicrobial stewardship [31].
Data classifying antibiotic usage according to the route of administration is sparse in PPSs conducted in the Indian subcontinent during major COVID-19 waves [[23], [24], [25]]. However, in our post-COVID-19 wave PPS, the percentage of antibiotics prescribed parenterally (81.23%) was comparable to the corresponding value found in a pre-COVID-19 wave PPS (77.9%) [9]. Widespread use of third generation cephalosporins especially ceftriaxone, for which there is currently no oral equivalent available in the market, was identified as one of the factors which could have influenced this finding. Also, many physicians and patients believe that parenteral therapy is superior and more effective than oral administration [32]. The use of parenteral antibiotics is unavoidable in cases of critical infections, infants, unconscious patients and, in absence of oral alternatives. However, hospitals should actively pursue early iv-oral switch whenever possible, recognizing the clinical and economic benefits from this approach [32,33].
Two pre-COVID-19 wave PPSs conducted in India demonstrated CAIs as the most common indication for prescribing antibiotics to patients, followed by surgical prophylaxis [9,10]. Indications for antibiotics in most PPSs conducted in the Indian subcontinent during a COVID-19 wave are poorly documented [[23], [24], [25]]. In our post-COVID-19 wave PPS, the majority of antibiotic prescriptions were for an unknown indication (32.59%), followed by CAIs (30.17%) and surgical prophylaxis (22.37%). HAIs constituted a small fraction (5.24 %) of the indications for antimicrobial use. Indian hospitals have widely varying proportions of antimicrobials prescribed for HAIs (0.9 %–32.6 %) [9]. A reason for this variation may be differences in the clinical assessment by treating doctors, who are unable to identify the infection to be a HAI when it is initially presented to them. Hence, training doctors vigorously to correctly diagnose and categorize infections broadly as HAIs or CAIs would not only aid in rational prescription of antibiotics but also prevent antibiotic resistance [9]. The percentage of patients to whom antibiotics were indicated for surgical prophylaxis was consistently high in all PPSs conducted in India before and after a COVID-19 wave [9,10]. There are several reasons for the large number of patients on surgical prophylaxis at our institute. First, a large number of patients operated in smaller hospitals are referred to our hospital with post-operative complications. Some of these patients require re-operation, and their antibiotics may therefore be categorized as prophylactic when they were in fact therapeutic Second, fear of being sued for post-operative infections is also a motivator for clinicians to prescribe surgical prophylaxis [9]. In the OBG ward of our institute, which had the highest rate of antibiotic prescription in any ward, most antibiotics were administered as surgical prophylaxis. Likewise, in pre-COVID-19 times, 81.4% of admitted patients in the OBG ward were prescribed antibiotics [20]. Also, many patients (78.4%) in our PPS were continued with surgical prophylaxis for more than a day, as reported in previous PPSs [9,10]. Surgical prophylaxis guidelines in most Indian hospitals, including ours, discourage continuing surgical prophylaxis for more than one day [31]. Most surgeons quoted presence of post-operative complications as the rationale for continuing antibiotics longer post-operatively [34]. Whether COVID-19 has increased the proportion of patients receiving prolonged surgical prophylaxis requires further investigation, but it seems that in our institution at least attention needs to be paid to improve compliance with surgical prophylaxis guidelines [35,36]. This could be done by restricting the availability of broad-spectrum antibiotics and/or strictly limiting the duration of surgical prophylaxis to a day [31,37].
In a multicentre pre-COVID-19 wave Indian PPS the incidence of double anaerobic coverage ranged between 3% to 15.9% [9]. In our study the rate was rather higher at 22.40%; although regional PPSs conducted during COVID-19 waves have described the use of double anaerobic coverage, they do not specify the frequency [[23], [24], [25]]. In cases of Gram-negative infections, there are a very few indications when more than one antibiotic may be appropriate [38]. However, it seems that there is likewise over-prescription of antibiotics with anti-Gram-negative activity.
The wide use of designated antimicrobials at our institute is a matter of concern. In our post-COVID-19 wave PPS, the most common designated antibiotics used included carbapenems (12.50 %), followed by glycopeptides (2.60 %). These findings are similar to previous Indian PPSs [9,10]. The highest number of such prescriptions were on the ICU. During major COVID-19 waves the prescription of designated antibiotics was prevalent in ICUs due to the lack of proper treatment guidelines, the unknown nature of the disease and a lack of antibiotic surveillance [18,39,40]. This practice may have continued even after the decline of a major COVID-19 wave. However, one limitation of our study is that we had no instituitional baseline pre-COVID-19 data for comparison. One encouraging observation was the extremely limited use of agents such as tigecycline, linezolid and bacitracin.
As described in a pre-COVID-19 wave Indian PPS, the commonest drug classes prescribed were third generation cephalosporins (24.48 %), imidazole derivatives (11.19 %), and beta-lactam- beta-lactamase-inhibitor combinations (10.17 %) [9]. A PPS conducted during the five major COVID-19 waves of Pakistan reported piperacillin-tazobactam (20.66 %), azithromycin (17.37 %), meropenem (15.45 %), and ceftriaxone (13.99 %) as the most commonly prescribed antibiotics [25]. In Bangladesh a PPS during a COVID-19 wave reported ceftriaxone (53.88 %), meropenem (40.9 %) and doxycycline (25.4 %) as the most common antibiotics prescribed [23]. Our results aligned with the findings of pre-COVID-19 wave PPSs. All PPSs conducted before, during and after a COVID-19 wave revealed that over 75 % of prescribed antibiotics belonged to the ‘Watch’ category of the WHO AWaRe classification [9,10,23,25]. Differences in the classes of antibiotics prescribed in the PPSs conducted during a COVID-19 wave from those prescribed before and after a COVID-19 wave reflect use of azithromycin and doxycycline as anti-COVID-19 treatments in some countries [40].
Lastly, electronic medical record systems in hospitals dealing with COVID-19 patients would not only increase the accuracy of documentation, lead to efficient retrieval of patient records, and but also reduce the risk of COVID-19 to the data auditing team while handling patient files [41,42].
Our study had numerous limitations. First, although the WHO recommends completion of a PPS within seven days, we could complete the data collection process of our PPS within ten days. Second, our study was restricted to one institute and included a small population size. Third, baseline antibiotic prescription data at our institute prior to and during a COVID-19 wave could not be retrieved. Larger multicentric PPSs conducted before, during and after a major COVID-19 wave could generate more reliable and robust data to identify the impact of COVID-19 on antibiotic prescription and help develop strategies to rationalize antibiotic use in dedicated COVID-19 hospitals.
Conclusions
Our PPS showed a high use of antimicrobials in admitted patients to our institute. The findings of our PPS were helpful in generating a baseline data at our institute for identifying strategies to rationalize antimicrobial prescription, especially after a major COVID-19 wave. Some of the strategies identified for improving the antibiotic stewardship program at our institute included improving surgical prophylaxis guidelines by restricting certain broad spectrum antibiotics for the same and restricting surgical prophylaxis duration to one day, training residents to categorize antibiotic prescriptions appropriately, decreasing prescriptions with a double anaerobic coverage, reviving the culture of sending culture reports, and improving the electronic medical record system for improving prescription auditing. Larger multicentre PPSs are required to further validate our findings.
Credit author statement
Sheikh S: Conceptualization, Methodology, Validation, Supervision Vishwas G: Investigation, Validation, Data Curation, Visualization, Formal analysis Aggarwal M: Project administration, Supervision, Resources Bhattacharya S: Investigation Kumari P: Investigation Parashar L: Investigation Meshram GG: Supervision, Writing - Original Draft, Writing - Reviewing and Editing.
Conflicts of interest statement
All authors have no conflicts of interest.
Funding statement
The study was not funded by any external agency or grant.
References
- 1.Ten Health Issues WHO Will Tackle This Year. Available online at: https://www.who.int/emergencies/ten-threats-to-global-health-in-2019 (accessed October 1, 2019).
- 2.O’Neill J. World Health Organization; London, UK: 2014. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. [Google Scholar]
- 3.Klein E.Y., Van Boeckel T.P., Martinez E.M., Pant S., Gandra S., Levin S.A., et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci USA. 2018;115:E3463–E3470. doi: 10.1073/pnas.1717295115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King L.M., Lovegrove M.C., Shehab N., Tsay S., Budnitz D.S., Geller A.I., et al. Trends in U.S. outpatient antibiotic prescriptions during the COVID-19 pandemic. Clin Infect Dis. 2021;73:e652–e660. doi: 10.1093/cid/ciaa1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buehrle D.J., Nguyen M.H., Wagener M.M., Clancy C.J. Impact of the coronavirus disease 2019 pandemic on outpatient antibiotic prescriptions in the United States. Open Forum Infect Dis. 2020;7:ofaa575. doi: 10.1093/ofid/ofaa575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abelenda-Alonso G., Padullés A., Rombauts A., Gudiol C., Pujol M., Alvarez-Pouso C., et al. Antibiotic prescription during the COVID-19 pandemic: A biphasic pattern [published correction appears in Infect Control Hosp Epidemiol. 2020 Nov;41(11):1373] Infect Control Hosp Epidemiol. 2020;41(11):1371–1372. doi: 10.1017/ice.2020.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghosh S., Moledina N., Hasan M.M., Jain S., Ghosh A. Colossal challenges to healthcare workers combating the second wave of coronavirus disease 2019 (COVID-19) in India [published online ahead of print, 2021 Jun 2] Infect Control Hosp Epidemiol. 2021:1–2. doi: 10.1017/ice.2021.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walia K., Ohri V.C., Madhumathi J., Ramasubramanian V. Policy document on antimicrobial stewardship practices in India. Indian J Med Res. 2019;149(2):180–184. doi: 10.4103/ijmr.IJMR_147_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panditrao A.M., Shafiq N., Chatterjee S., Pathak A., Trivedi N., Sadasivam B., et al. A multicentre point prevalence survey (PPS) of antimicrobial use amongst admitted patients in tertiary care centres in India. J Antimicrob Chemother. 2021 Apr 1;76(4):1094–1101. doi: 10.1093/jac/dkaa533. [DOI] [PubMed] [Google Scholar]
- 10.Singh S.K., Sengupta S., Antony R., Bhattacharya S., Mukhopadhyay C., Ramasubramanian V., et al. Variations in antibiotic use across India: multi-centre study through Global Point Prevalence survey. J Hosp Infect. 2019 Nov;103(3):280–283. doi: 10.1016/j.jhin.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Global Point Prevalence Survey (PPS) – Year 2019 (Global-PPS). http://www.global-pps.com/wp-content/uploads/2019/01/Data-collection form-Global-PPS-2019.pdf. [PubMed]
- 12.Leekha S., Terrell C.L., Edson R.S. General principles of antimicrobial therapy. Mayo Clin Proc. 2011;86(2):156–167. doi: 10.4065/mcp.2010.0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crader M.F., Varacallo M. StatPearls Publishing; 2022 Jan. Preoperative antibiotic prophylaxis. [Updated 2022 feb 12]. In: StatPearls [Internet]. Treasure Island (FL)https://www.ncbi.nlm.nih.gov/books/NBK442032/ Available from: [PubMed] [Google Scholar]
- 14.Deelen J.W.T., Visser C.E., Prins J.M., van Hest R.M. Antimicrobial prophylaxis outside the operating theatre, an audit in a university hospital. BMC Infect Dis. 2017 Apr 21;17(1):296. doi: 10.1186/s12879-017-2354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cardoso T., Almeida M., Friedman N.D., Aragão I., Costa-Pereira A., Sarmento A.E., et al. Classification of healthcare-associated infection: a systematic review 10 years after the first proposal. BMC Med. 2014 Mar 6;12:40. doi: 10.1186/1741-7015-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monegro A.F., Muppidi V., Regunath H. StatPearls Publishing; 2022 Jan. Hospital acquired infections. [Updated 2022 may 2]. In: StatPearls [Internet]. Treasure Island (FL)https://www.ncbi.nlm.nih.gov/books/NBK441857/ Available from: [PubMed] [Google Scholar]
- 17.Saleem Z., Hassali M.A., Godman B., Versporten A., Hashmi F.K., Saeed H., et al. Point prevalence surveys of antimicrobial use: a systematic review and the implications. Expert Rev Anti Infect Ther. 2020 Sep;18(9):897–910. doi: 10.1080/14787210.2020.1767593. [DOI] [PubMed] [Google Scholar]
- 18.Seethalakshmi P.S., Charity O.J., Giakoumis T., Kiran G.S., Sriskandan S., Voulvoulis N., et al. Delineating the impact of COVID-19 on antimicrobial resistance: An Indian perspective. Sci Total Environ. 2022 Apr 20;818 doi: 10.1016/j.scitotenv.2021.151702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garg R., Singh G., Kumar S., Verma M., Podder L., Ingle V., et al. Impact of an Anti-Microbial Stewardship Program on Targeted Antimicrobial Therapy in a Tertiary Care Health Care Institute in Central India. Cureus. 2021 Oct 5;13(10) doi: 10.7759/cureus.18517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rashid M.M., Akhtar Z., Chowdhury S., Islam M.A., Parveen S., Ghosh P.K., et al. Pattern of Antibiotic Use among Hospitalized Patients according to WHO Access, Watch, Reserve (AWaRe) Classification: Findings from a Point Prevalence Survey in Bangladesh. Antibiotics. 2022 Jun 16;11(6):810. doi: 10.3390/antibiotics11060810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saleem Z., Hassali M.A., Versporten A., Godman B., Hashmi F.K., Goossens H., et al. A multicenter point prevalence survey of antibiotic use in Punjab, Pakistan: findings and implications. Expert Rev Anti Infect Ther. 2019 Apr;17(4):285–293. doi: 10.1080/14787210.2019.1581063. [DOI] [PubMed] [Google Scholar]
- 22.AT. Oo K.M., Lwin K.T., Win H.H., Crump J.A. Point-prevalence surveys of antimicrobial consumption and resistance at a paediatric and an adult tertiary referral hospital in Yangon, Myanmar. Infect Prev Pract. 2021 Dec 11;4(1) doi: 10.1016/j.infpip.2021.100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molla M.M.A., Yeasmin M., Islam M.K., Sharif M.M., Amin M.R., Nafisa T., et al. Antibiotic Prescribing Patterns at COVID-19 Dedicated Wards in Bangladesh: Findings from a Single Center Study. Infect Prev Pract. 2021 Jun;3(2) doi: 10.1016/j.infpip.2021.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mustafa Z.U., Saleem M.S., Ikram M.N., Salman M., Butt S.A., Khan S., et al. Co-infections and antimicrobial use among hospitalized COVID-19 patients in Punjab, Pakistan: findings from a multicenter, point prevalence survey. Pathog Glob Health. 2021 Nov 16:1–7. doi: 10.1080/20477724.2021.1999716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramzan K., Shafiq S., Raees I., Mustafa Z.U., Salman M., Khan A.H., et al. Co-Infections, Secondary Infections, and Antimicrobial Use in Patients Hospitalized with COVID-19 during the First Five Waves of the Pandemic in Pakistan; Findings and Implications. Antibiotics. 2022 Jun 9;11(6):789. doi: 10.3390/antibiotics11060789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Versporten A., Zarb P., Caniaux I., Gros M.-F., Drapier N., Miller M., et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: results of an internet-based point prevalence survey. Lancet Glob Health. 2018 Jun;6(6):e619–e629. doi: 10.1016/S2214-109X(18)30186-4. [DOI] [PubMed] [Google Scholar]
- 27.Metsvaht T., Nellis G., Varendi H., Nunn A.J., Graham S., Rieutord A., et al. High variability in the dosing of commonly used antibiotics revealed by a Europe-wide point prevalence study: implications for research and dissemination. BMC Pediatr. 2015 Apr 16;15:41. doi: 10.1186/s12887-015-0359-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Black E., Neville H., Losier M., Harrison M., Abbass K., Slayter K., et al. Antimicrobial Use at Acute Care Hospitals in Nova Scotia: A Point Prevalence Survey. Can J Hosp Pharm. 2018 Aug;71(4):234–242. [PMC free article] [PubMed] [Google Scholar]
- 29.Langford B.J., So M., Raybardhan S., Leung V., Soucy J.R., Westwood D., et al. Antibiotic prescribing in patients with COVID-19: rapid review and meta-analysis. Clin Microbiol Infect. 2021;27(4):520–531. doi: 10.1016/j.cmi.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmed A., Azim A., Gurjar M., Baronia A.K. Current concepts in combination antibiotic therapy for critically ill patients. Indian J Crit Care Med. 2014;18(5):310–314. doi: 10.4103/0972-5229.132495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Centre for Disease Control . Directorate general of health services MoHFW. Government of India; 2016. National treatment guidelines for antimicrobial use in infectious diseases version 1.0.http://pbhealth.gov.in/AMR_guideline7001495889.pdf Available from: [Google Scholar]
- 32.McCarthy K., Avent M. Oral or intravenous antibiotics? Aust Prescr. 2020 Apr;43(2):45–48. doi: 10.18773/austprescr.2020.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cyriac J.M., James E. Switch over from intravenous to oral therapy: A concise overview. J Pharmacol Pharmacother. 2014 Apr;5(2):83–87. doi: 10.4103/0976-500X.130042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Assadian O., Golling M., Krüger C.M., Leaper D., Mutters N.T., Roth B., et al. Surgical site infections: guidance for elective surgery during the SARS-CoV-2 pandemic - international recommendations and clinical experience. J Hosp Infect. 2021 May;111:189–199. doi: 10.1016/j.jhin.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salim S., Kumar M.N., Tripathi C.D., Arya S.V., Verma V., Ahmed K.B., et al. Pharmacological evaluation of prophylactic anti-microbial use in laparoscopic cholecystectomy; an open labelled study evaluating the concentrations of single dose intravenous ceftriaxone at serum and tissue level. Eur J Clin Pharmacol. 2021 Jul;77(7):1011–1016. doi: 10.1007/s00228-021-03093-1. [DOI] [PubMed] [Google Scholar]
- 36.Sheikh S., Majoka R., Tripathi C.D., Verma V., Bagga D., Karim B.A., et al. Variability in the serum and tissue concentrations of pre-incisional ceftriaxone for surgery in paediatric population and outcome of surgical-site infections; An open labelled, prospective, non-randomized, analytical study. Curr Res Pharmacol Drug Discov. 2022 Jan 18;3 doi: 10.1016/j.crphar.2022.100082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horumpende P.G., Mshana S.E., Mouw E.F., Mmbaga B.T., Chilongola J.O., de Mast Q. Point prevalence survey of antimicrobial use in three hospitals in North-Eastern Tanzania. Antimicrob Resist Infect Control. 2020 Sep 7;9(1):149. doi: 10.1186/s13756-020-00809-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ram K., Sheikh S., Bhati R.K., Tripathi C.D., Suri J.C., Meshram G.G. Steady-state pharmacokinetic and pharmacodynamic profiling of colistin in critically ill patients with multi-drug-resistant gram-negative bacterial infections, along with differences in clinical, microbiological and safety outcome. Basic Clin Pharmacol Toxicol. 2021 Jan;128(1):128–140. doi: 10.1111/bcpt.13482. [DOI] [PubMed] [Google Scholar]
- 39.Chedid M., Waked R., Haddad E., Chetata N., Saliba G., Choucair J. Antibiotics in treatment of COVID-19 complications: a review of frequency, indications, and efficacy. J Infect Public Health. 2021 May;14(5):570–576. doi: 10.1016/j.jiph.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan S., Hasan S.S., Bond S.E., Conway B.R., Aldeyab M.A. Antimicrobial consumption in patients with COVID-19: a systematic review and meta-analysis. Expert Rev Anti Infect Ther. 2022 May;20(5):749–772. doi: 10.1080/14787210.2022.2011719. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen L., Bellucci E., Nguyen L.T. Electronic health records implementation: an evaluation of information system impact and contingency factors. Int J Med Inf. 2014 Nov;83(11):779–796. doi: 10.1016/j.ijmedinf.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 42.Pryor R., Atkinson C., Cooper K., Doll M., Godbout E., Stevens M.P., et al. The electronic medical record and COVID-19: Is it up to the challenge? Am J Infect Control. 2020;48:966–967. doi: 10.1016/j.ajic.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]