Abstract
Retinal ischemia/reperfusion (I/R) injury can cause severe vision impairment. Retinal I/R injury is associated with pathological increases in reactive oxygen species and inflammation, resulting in retinal neuronal cell death. To date, effective therapies have not been developed. Nicotinamide mononucleotide (NMN), a key nicotinamide adenine dinucleotide (NAD+) intermediate, has been shown to exert neuroprotection for retinal diseases. However, it remains unclear whether NMN can prevent retinal I/R injury. Thus, we aimed to determine whether NMN therapy is useful for retinal I/R injury-induced retinal degeneration. One day after NMN intraperitoneal (IP) injection, adult mice were subjected to retinal I/R injury. Then, the mice were injected with NMN once every day for three days. Electroretinography and immunohistochemistry were used to measure retinal functional alterations and retinal inflammation, respectively. The protective effect of NMN administration was further examined using a retinal cell line, 661W, under CoCl2-induced oxidative stress conditions. NMN IP injection significantly suppressed retinal functional damage, as well as inflammation. NMN treatment showed protective effects against oxidative stress-induced cell death. The antioxidant pathway (Nrf2 and Hmox-1) was activated by NMN treatment. In conclusion, NMN could be a promising preventive neuroprotective drug for ischemic retinopathy.
Keywords: nicotinamide mononucleotide, retinal ischemia/reperfusion, oxidative stress, neuroprotection, inflammation
1. Introduction
Retinal ischemia/reperfusion (I/R) injury is involved in various retinal ischemic diseases, including diabetic retinopathy, glaucoma, and vascular ischemic retinopathy. Retinal I/R injury can cause pathological events, such as the induction of reactive oxygen species and retinal inflammation, ultimately leading to retinal neuronal cell death [1,2,3]. As effective therapeutics have not yet been found or developed for retinal I/R injury, research on searching for promising neuroprotective drugs to prevent and/or suppress retinal I/R injury has been attempted at the preclinical stage [4,5].
Nicotinamide mononucleotide (NMN) is one of the major intermediates of nicotinamide adenine dinucleotide (NAD+), a crucial co-enzyme for various cellular redox metabolisms, including cellular proliferation, DNA repair, and cell death/survival [6,7]. In this aspect, boosting NAD+ biosynthesis has been nominated as beneficial for age-related metabolic disorders and diseases, including obesity, insulin resistance, and diabetes [8]. When it comes to the eye, recent studies have demonstrated that NMN administration could protect against photoreceptor cell damage in rodent models of light-induced retinopathy or retinal detachment [9,10]. However, little is known about the preventive role of NMN for retinal I/R injury in mice.
Thus, in the present study, we aimed to determine whether NMN treatment could exert retinal protection in a mouse model of retinal I/R injury induced by acute transient elevation of intraocular pressure. Furthermore, we attempted to investigate how NMN treatment could show therapeutic effects using a retinal 661W in vitro system.
2. Results
2.1. NMN Treatment Prevents Retinal Dysfunction in a Mouse Model of Retinal I/R Injury Induced by Acute Elevation of Intraocular Pressure
To examine whether NMN treatment could prevent retinal dysfunction in a mouse model of retinal I/R injury, NMN was intraperitoneally injected into the mice one day before retinal I/R injury (Figure 1A). After injury, NMN was continuously intraperitoneally injected into the mice every day according to our experimental scheme. The dose of NMN was determined based on previous studies that evaluated various effects of NMN treatment [9,10,11,12]. We found that the amplitude of b-wave significantly decreased five days after retinal I/R injury, and its reduction was lessened by NMN treatment (Figure 1B).
Figure 1.
Retinal functional changes by nicotinamide mononucleotide (NMN) treatment in mice. (A) The schematic illustration shows whole experimental plans for NMN injection, retinal ischemia/reperfusion (I/R) injury, and the termination day of the whole experiment. i.p., intraperitoneal; D, day; ERG, electroretinography. (B) Representative waveforms (50 cd·s/m2) of ERG (n = 5–6 per group) demonstrated that the ERG amplitudes decreased 5 days after retinal I/R injury. NMN injection suppressed reductions in the ERG amplitude (especially b-wave), flashed with various intensities (0.5, 2, 10, or 50 cd·s/m2). * p < 0.05, *** p < 0.001, # p < 0.05, and ## p < 0.01. ns, not significant. The data were analyzed using one-way ANOVA followed by a Bonferroni post-hoc test. The data are drawn as the mean ± standard deviation.
2.2. NMN Treatment Reduces Retinal Inflammation in a Mouse Model of Retinal I/R Injury Induced by Acute Elevation of Intraocular Pressure
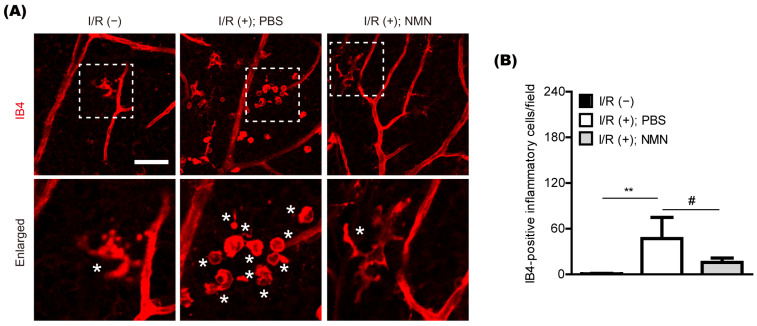
Previously, we found pathologic increases in isolectin GS-IB4 (IB4)-positive inflammatory cells in the retina five days after retinal I/R injury [13]. Therefore, we examined whether the likelihood of its occurrence could be lessened by NMN treatment (Figure 2A,B). In our current system, the number of IB4-positive inflammatory cells in the ischemic retina was markedly reduced by NMN treatment.
Figure 2.
General modulation of retinal inflammation by nicotinamide mononucleotide (NMN) treatment in mice. (A,B) Representative pictures and quantitative analyses (n = 5–6 per group) demonstrated that the number of retinal I/R injury-induced IB4 positive inflammatory cells in the retina was reduced by NMN injection 5 days after injury. Scale bar: 50 μm. White boxes, enlarged pictures; Stars (*) in enlarged pictures, IB4 positive inflammatory cells. ** p < 0.01 and # p < 0.05. One-way ANOVA followed by a Bonferroni post-hoc test was used for the data analysis. Bar graphs are shown as the mean ± standard deviation. IB4, isolectin GS-IB4 from Griffonia simplicifolia.
2.3. NMN Treatment Exerts Neuroprotection in Retinal 661W Cells under CoCl2-Induced Oxidative Stress Conditions
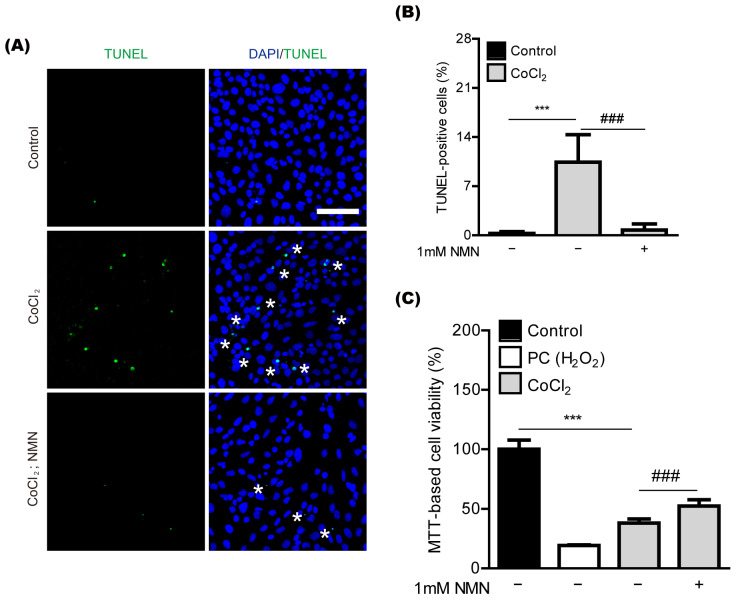
To further determine whether NMN treatment could show protective effects in retinal neuronal cells, we applied an in vitro system to our current study (Figure 3). 661W cells, a mouse-derived photoreceptor cell line possessing features of retinal ganglion precursor-like cells, were used, as they are generally used as one of the in vitro models for studying retinal degeneration [14,15,16]. Previously, we found that CoCl2-induced oxidative stress could cause 661W cell death [17]. In our current system, we also found 661W cell death by CoCl2-induced oxidative stress, analyzed with TUNEL assay (Figure 3A,B). NMN treatment significantly reduced oxidative stress-induced 661W cell death. This outcome was further confirmed with MTT assay (Figure 3C).
Figure 3.
Neuroprotection in vitro by nicotinamide mononucleotide (NMN) treatment. (A,B) Representative pictures and quantitative analyses (n = 5 per group) demonstrated that the amount of oxidative stress-induced 661W cell death stained by TUNEL assay was reduced by 1 mM NMN treatment after 24 h of 400 μM of CoCl2 incubation. Scale bar: 100 μm. DAPI (blue); TUNEL (green); Stars (*) in pictures, TUNEL positive cells. *** p < 0.001 and ### p < 0.001. One-way ANOVA followed by a Bonferroni post-hoc test was used for the data analysis. Graphs are depicted as the mean ± standard deviation. (C) Quantitative analyses (n = 9 per group) demonstrated that MTT-based 661W cell viability increased by 1 mM NMN treatment after 10 h of 400 μM of CoCl2 incubation. *** p < 0.001 and ### p < 0.001. One-way ANOVA followed by a Bonferroni post-hoc test was used for the data analysis. Bar graphs are shown as the mean ± standard deviation. PC, positive control (250 μM of H2O2).
2.4. NMN Treatment Upregulates the Antioxidant Genes in Retinal 661W Cells under CoCl2-Induced Oxidative Stress Conditions
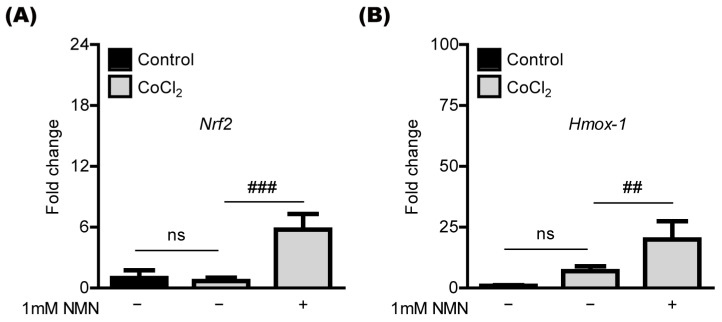
NMN treatment has been shown to increase antioxidant genes such as Nrf2 and Hmox-1 in various cell types [9,10,11,18,19]. Thus, we applied its concept to our current system (Figure 4). Under the same conditions of CoCl2-induced oxidative stress above, we found that the expression of Nrf2 and Hmox-1 mRNA was markedly increased by NMN treatment in 661W cells (Figure 4A,B).
Figure 4.
Antioxidant gene regulation by nicotinamide mononucleotide (NMN) treatment. (A,B) Quantitative analyses (n = 5 per group) demonstrated that NMN treatment (6 h) increases mRNA expression in the antioxidant genes (Nrf2 and Hmox-1) in 661W cells under 400 μM CoCl2-induced oxidative stress conditions. ## p < 0.01 and ### p < 0.001. ns, not significant. The data were analyzed using one-way ANOVA followed by a Bonferroni post-hoc test and drawn as the mean ± standard deviation.
3. Discussion
The current study demonstrated that retinal neuronal cells could be damaged by retinal I/R injury, and consecutive NMN treatment could show preventive effects against such injury. The therapeutic effects of NMN were further examined using the 661W cell line. Oxidative stress-induced 661W cell death was markedly decreased by NMN treatment, with increases in antioxidant gene expression (Nrf2 and Hmox-1). Although NMN therapy has gradually been reported to be beneficial for eye diseases [20,21], as far as we know, this report is the first to expand the role of NMN in a mouse model of retinal I/R injury induced by acute increases in intraocular pressure.
NMN is a key intermediate of NAD+, an important metabolic redox co-enzyme in most eukaryotic cells. NMN supplements could be involved in various biological processes such as aging, cell growth, cell death and protection, and DNA repair [22,23]. To date, the therapeutic outcomes of treating NMN or targeting its related NAD+ pathways have been unraveled in various experimental models of metabolic diseases and disorders, including diabetes, obesity, ischemia/reperfusion injury, heart failure, vascular dysfunction, hemorrhage, cognitive dysfunction, kidney injury, and alcoholic liver disease [22,23,24,25,26]. This indicates that NMN and its related NAD+ pathways might be crucial for regulating multiple cellular functions in the body.
Based on our current data, retinal I/R-induced retinal dysfunction and oxidative stress-induced cell death were reduced by NMN treatment. Other groups have reported similar effects. Chen et al. demonstrated that NMN supplementation reduces photoreceptor cell loss in the early phase of retinal detachment [10]. They further found that NMN treatment exerts neuroprotection in 661W cells under tBuOOH-induced oxidative stress conditions (tBuOOH; one of the most widely used inducers of oxidative stress [27,28,29]). Lin et al. showed that NMN treatment protects against retinal dysfunction in mice lacking nicotinamide phosphoribosyltransferase (Nampt; a rate-limiting enzyme in the NAD+ salvage biosynthesis pathway [30,31]) and mice with light-induced retinopathy [9]. They further found that NAMPT inhibitor FK866-induced cell death was decreased by NMN treatment in 661W cells. In this regard, our current results are consistent with those of other previous reports, which implies that NMN could have therapeutic effects against retinal cell damage.
Previous reports from us and others have suggested that reductions in pathologic retinal inflammatory cells could be beneficial for retinal neuronal protection in a murine model of retinal I/R injury [13,32,33]. The therapeutic strategy of modulating pathologic inflammatory cells for neuroprotection can be seen in brain injury, rather than just retinal I/R injury [34,35,36]. Our current system detected reductions in pathologic retinal inflammatory cells in the NMN-treated retinas. Chen et al. also found that NMN supplementation could suppress retinal inflammation in retinal detachment [10]. Wei et al. demonstrated that NMN treatment could suppress neuroinflammation to exert neuroprotection in intracerebral hemorrhage [18]. Although the way in which NMN treatment is involved in modulating inflammation requires further investigation, its effect might contribute to retinal protection against retinal I/R injury.
NMN is widely known to be associated with antioxidants to protect cells against free radicals [37]. Based on previous reports, the antioxidant function of NMN has been linked to the Nrf2/Hmox-1;Ho-1 antioxidant signaling pathway [9,10,11,18,19,38]. Pu et al. demonstrated that NMN treatment could increase cell viability and restore tight junctions in human corneal epithelial cells through the Nrf2/Hmox-1 pathway [19]. Luo et al. showed that NMN administration could restore redox homeostasis in a murine model of oxidative stress-induced liver injury through the Nrf2 pathway [39]. Wei et al. showed that NMN treatment could attenuate brain damage induced by intracerebral hemorrhage through the Nrf2/Hmox-1 pathway [18]. Chen et al. demonstrated that NMN treatment could increase HO-1 protein expression in 661W cells under tBuOOH-induced oxidative stress conditions [10]. Taken together, our current data also support the notion that the therapeutic role of NMN could be involved in the Nrf2/Hmox-1 antioxidant signaling pathway in various eukaryotic cells.
In summary, we applied the promising NMN therapy to retinal I/R injury in the current study. We further found that NMN treatment could protect against oxidative stress-induced retinal cell death and could upregulate the antioxidant pathway (Nrf2 and Hmox-1) in retinal cells. Although more studies (such as unraveling the clear mode of action of NMN treatment in ischemic eyes, including the retina, choroid, and retinal pigment epithelium) are needed, we suggest a promising NMN therapy for ischemic retinopathy based on our short current summary.
4. Materials and Methods
4.1. Animal and Retinal Ischemia/Reperfusion (I/R) Injury
The mouse experimental processes of the Ethics Committee on Animal Research of Keio University School of Medicine (#16017), the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and the International Standards of Animal Care and Use, Animal Research: Reporting in Vivo Experiments were followed. Adult male mice (C57BL/6, 6–8 weeks old) were bought from CLEA Japan (Tokyo, Japan). After mouse randomization, retinal I/R injury was induced in their eyes, as described in our previous paper [13]. Briefly, anterior chamber cannulation was performed using a 35-gauge needle, and high intraocular pressure was maintained by PBS solution for 40 min. After injury, the mice were recovered with hot pads to maintain their body temperature and then moved back to their cages for further experiments.
4.2. Electroretinography (ERG)
General scotopic ERG was performed as described in our previous retinal I/R injury study [13]. After 24 h of dark adaptation, the mice were placed on a Ganzfeld dome table under dimly lit conditions. After pupil dilation followed by anesthesia (midazolam, medetomidine, and butorphanol tartrate, called MMB [40]), active ERG electrodes, connected to a PuREC acquisition system (MAYO, Inazawa, Japan), were softly contacted with the mouse cornea. Then, the general scotopic ERG amplitudes (a- and b-waves) were obtained at standard flash intensities.
4.3. Immunohistochemistry (IHC)
IHC was performed as described in our previous retinal I/R injury study [13]. Briefly, after paraformaldehyde (4% PFA) fixation for more than 3 h, the mouse eyeballs were moved to a Petri dish with cold PBS. After the retinas were collected from the eyeballs, the retinal samples were flat-mounted using micro-scissors and incubated in IB4 solution for 24 h. After washing with cold PBS several times, the retinal samples were mounted and examined by an LSM710 fluorescent microscope (Carl Zeiss, Jena, Germany).
4.4. Cell Culture
Retinal 661W cells were generally cultured in DMEM (Cat #08456-36, Nacalai Tesque, Kyoto, Japan) media with 10% FBS and 1% streptomycin–penicillin as described in our previous papers [41,42]. The cell culture was maintained under atmospheric conditions containing 5% CO2 at 37 °C.
4.5. Terminal Deoxynucleotidyl Transferase dUTP Nick end Labeling (TUNEL) and MTT Assays
A TUNEL assay was conducted as described in our previous papers [13,43]. We basically followed the manufacturer’s manual instructions (in situ Apoptosis Detection Kit, Cat #MK500, Takara Bio, Japan). PFA (4%)-fixed 661W cells were subjected to permeabilization using a permeabilization buffer supplied from the assay kit for 5 min on ice. A labeling reaction mixture with TdT enzymes was added to the cells for 1 h. Then, the DAPI solution was incubated for 1 min to stain the nuclei. The samples were mounted and investigated using an LSM710 fluorescent microscope (Carl Zeiss, Jena, Germany).
An MTT assay was also performed as described in our previous report [41]. The MTT solution (Cat #M2128, Sigma, St. Louis, MO, USA) was given to each well for 2 h. After removing the supernatant, DMSO was added to each well. Then, color absorbance was measured and determined using a microplate reader (Synergy HT Multi-Mode, Winooski, VT, USA).
4.6. Quantitative PCR (qPCR)
All steps for quantitative PCR (qPCR) were described in our previous reports [42,44]. Briefly, RNA extraction, cDNA synthesis, and qPCR were performed with each kit (Qiagen, Velno, Netherlands; TOYOBO, Osaka, Japan; Applied Biosystems, Waltham, MA, USA, respectively). The primer information used in our current study is outlined in Table 1. The general ΔΔCT calculation method was applied for our qPCR analysis.
Table 1.
Primer list.
| Name | Direction | Sequence (5′→3′) | Accession Number |
|---|---|---|---|
| Hprt | Forward | TCAGTCAACGGGGGACATAAA | NM_013556.2 |
| Reverse | GGGGCTGTACTGCTTAACCAG | ||
| Hmox-1 | Forward | CACTCTGGAGATGACACCTGAG | NM_010442.2 |
| Reverse | GTGTTCCTCTGTCAGCATCACC | ||
| Nrf2 | Forward | TAGATGACCATGAGTCGCTTGC | NM_010902.4 |
| Reverse | GCCAAACTTGCTCCATGTCC |
4.7. Statistical Analysis
All values in our current data were depicted as the mean ± standard deviation. Statistical significance was determined using one-way ANOVA followed by a Bonferroni post-hoc test. Statistical significance was considered when the p-value < 0.05.
Author Contributions
D.L. designed the whole study, performed the experiments, analyzed the data, and wrote the manuscript. Y.T., A.S., N.B., Y.M., S.Y., K.N. (Ken Nishioka) and J.Y. provided experimental support. K.N. (Kazuno Negishi) reviewed and revised the manuscript. T.K. supervised the entire project. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
All mouse procedures of the Ethics Committee on Animal Research of the Keio University School of Medicine (#16017), the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and the International Standards of Animal Care and Use, Animal Research: Reporting in Vivo Experiments guidelines were followed.
Informed Consent Statement
Not applicable.
Data Availability Statement
The current study’s data are available upon request from the corresponding author.
Conflicts of Interest
Y.M. is employed by Aichi Animal Eye Clinic. The other authors declare no conflicts of interest.
Funding Statement
The current research was supported by Grants-in-Aid for Scientific Research (KAKENHI, number 15 K10881, and 18 K09424) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) to T.K. and JST SPRING (number JPMJSP2123) to D.L.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hangai M., Yoshimura N., Hiroi K., Mandai M., Honda Y. Inducible nitric oxide synthase in retinal ischemia-reperfusion injury. Exp. Eye Res. 1996;63:501–509. doi: 10.1006/exer.1996.0140. [DOI] [PubMed] [Google Scholar]
- 2.Neufeld A.H., Kawai S., Das S., Vora S., Gachie E., Connor J.R., Manning P.T. Loss of retinal ganglion cells following retinal ischemia: The role of inducible nitric oxide synthase. Exp. Eye Res. 2002;75:521–528. doi: 10.1006/exer.2002.2042. [DOI] [PubMed] [Google Scholar]
- 3.Wei Y., Gong J., Yoshida T., Eberhart C.G., Xu Z., Kombairaju P., Sporn M.B., Handa J.T., Duh E.J. Nrf2 has a protective role against neuronal and capillary degeneration in retinal ischemia-reperfusion injury. Free. Radic. Biol. Med. 2011;51:216–224. doi: 10.1016/j.freeradbiomed.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minhas G., Morishita R., Anand A. Preclinical models to investigate retinal ischemia: Advances and drawbacks. Front. Neurol. 2012;3:75. doi: 10.3389/fneur.2012.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah M., Cabrera-Ghayouri S., Christie L.A., Held K.S., Viswanath V. Translational Preclinical Pharmacologic Disease Models for Ophthalmic Drug Development. Pharm. Res. 2019;36:58. doi: 10.1007/s11095-019-2588-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shade C. The Science Behind NMN-A Stable, Reliable NAD+Activator and Anti-Aging Molecule. Integr. Med. 2020;19:12–14. [PMC free article] [PubMed] [Google Scholar]
- 7.Braidy N., Berg J., Clement J., Khorshidi F., Poljak A., Jayasena T., Grant R., Sachdev P. Role of Nicotinamide Adenine Dinucleotide and Related Precursors as Therapeutic Targets for Age-Related Degenerative Diseases: Rationale, Biochemistry, Pharmacokinetics, and Outcomes. Antioxid. Redox Signal. 2019;30:251–294. doi: 10.1089/ars.2017.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nadeeshani H., Li J., Ying T., Zhang B., Lu J. Nicotinamide mononucleotide (NMN) as an anti-aging health product—Promises and safety concerns. J. Adv. Res. 2022;37:267–278. doi: 10.1016/j.jare.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin J.B., Kubota S., Ban N., Yoshida M., Santeford A., Sene A., Nakamura R., Zapata N., Kubota M., Tsubota K., et al. NAMPT-Mediated NAD(+) Biosynthesis Is Essential for Vision in Mice. Cell Rep. 2016;17:69–85. doi: 10.1016/j.celrep.2016.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X., Amorim J.A., Moustafa G.A., Lee J.J., Yu Z., Ishihara K., Iesato Y., Barbisan P., Ueta T., Togka K.A., et al. Neuroprotective effects and mechanisms of action of nicotinamide mononucleotide (NMN) in a photoreceptor degenerative model of retinal detachment. Aging. 2020;12:24504–24521. doi: 10.18632/aging.202453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshino J., Mills K.F., Yoon M.J., Imai S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14:528–536. doi: 10.1016/j.cmet.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramanathan C., Lackie T., Williams D.H., Simone P.S., Zhang Y., Bloomer R.J. Oral Administration of Nicotinamide Mononucleotide Increases Nicotinamide Adenine Dinucleotide Level in an Animal Brain. Nutrients. 2022;14:300. doi: 10.3390/nu14020300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee D., Nakai A., Miwa Y., Tomita Y., Kunimi H., Chen J., Ikeda S.I., Tsubota K., Negishi K., Kurihara T. Retinal degeneration induced in a mouse model of ischemia-reperfusion injury and its management by pemafibrate treatment. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2022;36:e22497. doi: 10.1096/fj.202200455RRR. [DOI] [PubMed] [Google Scholar]
- 14.Wheway G., Nazlamova L., Turner D., Cross S. 661W Photoreceptor Cell Line as a Cell Model for Studying Retinal Ciliopathies. Front. Genet. 2019;10:308. doi: 10.3389/fgene.2019.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sayyad Z., Sirohi K., Radha V., Swarup G. 661W is a retinal ganglion precursor-like cell line in which glaucoma-associated optineurin mutants induce cell death selectively. Sci. Rep. 2017;7:16855. doi: 10.1038/s41598-017-17241-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson A.F., Crowe M.E., Lieven C.J., Levin L.A. Induction of Neuronal Morphology in the 661W Cone Photoreceptor Cell Line with Staurosporine. PLoS ONE. 2015;10:e0145270. doi: 10.1371/journal.pone.0145270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunimi H., Lee D., Ibuki M., Katada Y., Negishi K., Tsubota K., Kurihara T. Inhibition of the HIF-1α/BNIP3 pathway has a retinal neuroprotective effect. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2021;35:e21829. doi: 10.1096/fj.202100572R. [DOI] [PubMed] [Google Scholar]
- 18.Wei C.C., Kong Y.Y., Li G.Q., Guan Y.F., Wang P., Miao C.Y. Nicotinamide mononucleotide attenuates brain injury after intracerebral hemorrhage by activating Nrf2/HO-1 signaling pathway. Sci. Rep. 2017;7:717. doi: 10.1038/s41598-017-00851-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pu Q., Guo X.X., Hu J.J., Li A.L., Li G.G., Li X.Y. Nicotinamide mononucleotide increases cell viability and restores tight junctions in high-glucose-treated human corneal epithelial cells via the SIRT1/Nrf2/HO-1 pathway. Biomed. Pharmacother. Biomed. Pharmacother. 2022;147:112659. doi: 10.1016/j.biopha.2022.112659. [DOI] [PubMed] [Google Scholar]
- 20.Westenskow P.D. Nicotinamide: A novel treatment for age-related macular degeneration? Stem Cell Investig. 2017;4:86. doi: 10.21037/sci.2017.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pîrvu A.S., Andrei A.M., Stănciulescu E.C., Baniță I.M., Pisoschi C.G., Jurja S., Ciuluvica R. NAD(+) metabolism and retinal degeneration (Review) Exp. Ther. Med. 2021;22:670. doi: 10.3892/etm.2021.10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Covarrubias A.J., Perrone R., Grozio A., Verdin E. NAD(+) metabolism and its roles in cellular processes during ageing. Nat. Reviews. Mol. Cell Biol. 2021;22:119–141. doi: 10.1038/s41580-020-00313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong W., Mo F., Zhang Z., Huang M., Wei X. Nicotinamide Mononucleotide: A Promising Molecule for Therapy of Diverse Diseases by Targeting NAD+ Metabolism. Front. Cell Dev. Biol. 2020;8:246. doi: 10.3389/fcell.2020.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lautrup S., Sinclair D.A., Mattson M.P., Fang E.F. NAD(+) in Brain Aging and Neurodegenerative Disorders. Cell Metab. 2019;30:630–655. doi: 10.1016/j.cmet.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshino J., Baur J.A., Imai S.I. NAD(+) Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metab. 2018;27:513–528. doi: 10.1016/j.cmet.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sasaki Y., Kakita H., Kubota S., Sene A., Lee T.J., Ban N., Dong Z., Lin J.B., Boye S.L., DiAntonio A., et al. SARM1 depletion rescues NMNAT1-dependent photoreceptor cell death and retinal degeneration. Elife. 2020;9:e62027. doi: 10.7554/eLife.62027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wenz C., Faust D., Linz B., Turmann C., Nikolova T., Bertin J., Gough P., Wipf P., Schröder A.S., Krautwald S., et al. t-BuOOH induces ferroptosis in human and murine cell lines. Arch. Toxicol. 2018;92:759–775. doi: 10.1007/s00204-017-2066-y. [DOI] [PubMed] [Google Scholar]
- 28.Takayama F., Egashira T., Yamanaka Y. Protective effect of Ninjin-yoei-to on damage to isolated hepatocytes following transient exposure to tert-butyl hydroperoxide. Jpn. J. Pharmacol. 2001;85:227–233. doi: 10.1254/jjp.85.227. [DOI] [PubMed] [Google Scholar]
- 29.Baysal E., Sullivan S.G., Stern A. Prooxidant and antioxidant effects of ascorbate on tBuOOH-induced erythrocyte membrane damage. Int. J. Biochem. 1989;21:1109–1113. doi: 10.1016/0020-711X(89)90051-7. [DOI] [PubMed] [Google Scholar]
- 30.Audrito V., Messana V.G., Deaglio S. NAMPT and NAPRT: Two Metabolic Enzymes with Key Roles in Inflammation. Front. Oncol. 2020;10:358. doi: 10.3389/fonc.2020.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gardell S.J., Hopf M., Khan A., Dispagna M., Hampton Sessions E., Falter R., Kapoor N., Brooks J., Culver J., Petucci C., et al. Boosting NAD(+) with a small molecule that activates NAMPT. Nat. Commun. 2019;10:3241. doi: 10.1038/s41467-019-11078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abcouwer S.F., Shanmugam S., Muthusamy A., Lin C.M., Kong D., Hager H., Liu X., Antonetti D.A. Inflammatory resolution and vascular barrier restoration after retinal ischemia reperfusion injury. J. Neuroinflammation. 2021;18:186. doi: 10.1186/s12974-021-02237-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang R., Liang S., Fang L., Wu M., Cheng H., Mi X., Ding Y. Low-dose minocycline mediated neuroprotection on retinal ischemia-reperfusion injury of mice. Mol. Vis. 2018;24:367–378. [PMC free article] [PubMed] [Google Scholar]
- 34.Celorrio M., Shumilov K., Payne C., Vadivelu S., Friess S.H. Acute minocycline administration reduces brain injury and improves long-term functional outcomes after delayed hypoxemia following traumatic brain injury. Acta Neuropathol. Commun. 2022;10:10. doi: 10.1186/s40478-022-01310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schimmel S.J., Acosta S., Lozano D. Neuroinflammation in traumatic brain injury: A chronic response to an acute injury. Brain Circ. 2017;3:135–142. doi: 10.4103/bc.bc_18_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Begemann M., Leon M., van der Horn H.J., van der Naalt J., Sommer I. Drugs with anti-inflammatory effects to improve outcome of traumatic brain injury: A meta-analysis. Sci. Rep. 2020;10:16179. doi: 10.1038/s41598-020-73227-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamaura K., Mifune Y., Inui A., Nishimoto H., Kurosawa T., Mukohara S., Hoshino Y., Niikura T., Kuroda R. Antioxidant effect of nicotinamide mononucleotide in tendinopathy. BMC Musculoskelet. Disord. 2022;23:249. doi: 10.1186/s12891-022-05205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X., Dilxat T., Shi Q., Qiu T., Lin J. The combination of nicotinamide mononucleotide and lycopene prevents cognitive impairment and attenuates oxidative damage in D-galactose induced aging models via Keap1-Nrf2 signaling. Gene. 2022;822:146348. doi: 10.1016/j.gene.2022.146348. [DOI] [PubMed] [Google Scholar]
- 39.Luo C., Ding W., Yang C., Zhang W., Liu X., Deng H. Nicotinamide Mononucleotide Administration Restores Redox Homeostasis via the Sirt3-Nrf2 Axis and Protects Aged Mice from Oxidative Stress-Induced Liver Injury. J. Proteome Res. 2022;21:1759–1770. doi: 10.1021/acs.jproteome.2c00167. [DOI] [PubMed] [Google Scholar]
- 40.Miwa Y., Tsubota K., Kurihara T. Effect of midazolam, medetomidine, and butorphanol tartrate combination anesthetic on electroretinograms of mice. Mol. Vis. 2019;25:645–653. [PMC free article] [PubMed] [Google Scholar]
- 41.Ibuki M., Lee D., Shinojima A., Miwa Y., Tsubota K., Kurihara T. Rice Bran and Vitamin B6 Suppress Pathological Neovascularization in a Murine Model of Age-Related Macular Degeneration as Novel HIF Inhibitors. Int. J. Mol. Sci. 2020;21:8940. doi: 10.3390/ijms21238940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee D., Miwa Y., Wu J., Shoda C., Jeong H., Kawagishi H., Tsubota K., Kurihara T. A Fairy Chemical Suppresses Retinal Angiogenesis as a HIF Inhibitor. Biomolecules. 2020;10:1405. doi: 10.3390/biom10101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee D., Jeong H., Miwa Y., Shinojima A., Katada Y., Tsubota K., Kurihara T. Retinal dysfunction induced in a mouse model of unilateral common carotid artery occlusion. PeerJ. 2021;9:e11665. doi: 10.7717/peerj.11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee D., Tomita Y., Jeong H., Miwa Y., Tsubota K., Negishi K., Kurihara T. Pemafibrate Prevents Retinal Dysfunction in a Mouse Model of Unilateral Common Carotid Artery Occlusion. Int. J. Mol. Sci. 2021;22:9408. doi: 10.3390/ijms22179408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The current study’s data are available upon request from the corresponding author.