Abstract
This systematic review and meta-analysis aimed at evaluating acute and chronic effects of physical exercise on IgA and IgG levels, as well as its relationship with the susceptibility to develop upper respiratory tract infections (URTI). This systematic review and meta-analysis was conducted and reported in accordance with PRISMA statement. A systematic search of PubMed, Web of Science, and EMBASE was performed in July 2020. This systematic review and meta-analysis included studies in which participants performed acute exercise or chronic physical training and were subjected to analyses of URTI incidence and concentrations of IgA and IgG. The selected studies for systematic review were divided into the following three groups: (I) trials that evaluated the effects of acute exercise in sedentary subjects, (II) trials that evaluated the effects of acute exercise in athletes/trained individuals, and (III) trials that evaluated the effects of chronic physical training on the incidence of URTI, as well as on the levels of IgA and IgG. Acute exercise increases the IgA levels in trained subjects but does not affect its levels in untrained subjects. Such increase in IgA levels induced by acute exercise is greater in trained individual that performed ultramarathon. On the other hand, chronic physical training reduces IgA levels in both trained and untrained subjects, does not change IgA levels in non-military subjects, besides from not affecting IgG levels. The present systematic review and meta-analysis indicates that acute exercise positively influences IgA levels in trained individuals, being this effect pronounced when a strenuous exercise such as ultramarathon is executed. Chronic physical training, in turn, does not affect IgG levels.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00424-022-02760-1.
Keywords: Immunoglobulins, Immune system, Physical training, Respiratory tract
Introduction
The COVID-19 pandemic is a global crisis of unprecedented scale in modern times [83]. The social distancing measures employed to curtail the impact of the infection are likely to reduce the amount of usual physical activity being performed by most individuals [2, 28]. However, regular practice of physical exercise is known to induce multiple benefits on physical and mental health, including the improvement of the body’s ability to combat infections [2]. Thus, in addition to personal hygiene and social distancing, maintaining a healthy and active lifestyle can reduce the risk of COVID-19 infection [82]. A very important question emerges on the topic in this context, the regular practice of moderate physical exercise or even vigorous chronic physical training can increase the susceptibility to severe cases of COVID-19?
The immune system protects the body against infectious diseases through the recognition, attack and destruction of foreign elements to the body, being the resistance to infections associated with the effectiveness of such immune actions [21]. Physical exercise influences the immune system in a dual way, as illustrated by the “J”-shaped curve that models the relationship between exercise and susceptibility to infection [50]. While several evidences indicate that moderate intensity physical training is associated with several benefits to the immune system, such as reduction of chronic inflammation and elevation of immunological markers related to various diseases [14, 22, 76], others indicate that high-intensity physical exercise can result in mucosal and cellular immunosuppression, associated with increased incidence of upper respiratory tract infections (URTI) [55, 65].
URTI episodes result from the invasion of microorganisms (i.e., viruses, bacteria or fungi), specifically in the airways above the glottis or vocal cords, that can manifest itself as tonsillitis, pharyngitis, laryngitis, sinusitis, otitis media, flu, and cold [25, 78]. Studies indicate that during periods of intense training and competitions, athletes may experience immunosuppression and, consequently, increased susceptibility to URTI [16, 27]. One body’s immune response to exercise is the production and secretion of immunoglobulins, whose serum levels and secretion, especially the antigen-specific antibodies IgA and IgG, are associated with resistance to infection [54].
IgA is the most abundant antimicrobial protein that protects the mucosal surfaces [6], region where most infections are initiated [5, 32, 42, 49]. It is proposed that secretory IgA provides an immunological barrier by neutralizing and preventing viral pathogens from penetrating the body through the mucosal surfaces [32, 42, 49]. Based on the above mentioned, low concentrations of SIgA in athletes may in fact be associated with the increased susceptibility to URTI [23, 46, 49]. However, in sedentary individuals, moderate chronic exercise training has been shown to increase SIgA levels, which may have contributed to the apparent reduced susceptibility to URTI [23].
Immunoglobulin G (IgG) is the most common type of antibody found in blood circulation. It is also one of the most abundant proteins in the human serum, accounting for about 10–20% of all plasma proteins. IgG appears during the first and second immune responses by activating the complement system and macrophages [6, 80]. A recent study demonstrated that serum IgG levels were increased in pre-frailty elderly women after 12-weeks of aquatic moderate exercise [34]. Previous study also reported that acute moderate exercise, such as 45 min bout of walking, is associated with a transient rise in serum immunoglobulin (IgA, IgG, and IgM) levels [54].
The studies that have examined exercise-associated changes in the levels of immunoglobulin isotypes often have contradictory results. As such, this interesting research area, which could potentially impact the practical guidelines for recommending exercise, remains open. While some studies have shown that exercise may decrease immunoglobulins in athletes [19, 20], others have reported, instead, an increase in its levels [44, 75]. No association between immunoglobulins and exercise has been described as well [35]. In face of such inconsistences, and taking into account the apparent value of IgA and IgG as a potential biomarkers of URTI susceptibility in the context of exercise, the present systematic review intended to update the knowledge about the effects of acute exercise and exercise training, the two situations in which URTI mostly often occur, on IgA and IgG levels. The relationship between secretory immunoglobulins and the susceptibility to develop the diseases was also evaluated. Taking into account that COVID-19 is an infection that also affects the respiratory tract, it seems relevant to better understand the beneficial effects of exercise in fighting URTI through modulation of immunoglobulin levels. This analysis could eventually be useful as guidance on prescribing physical exercise safely.
Methods
Search strategy
This systematic review and meta-analysis was conducted and reported according to the guidelines outlined in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) statement [36, 43, 64]. A systematic search in electronic databases, including PubMed, Web of Science and EMBASE was performed in July 2020 without any date restrictions. The search strategy used combinations of the following keywords: respiratory tract, exertion, physical effort, firefighters, military, immune system, infection, exercise, physical training, and sport. This review was not registered.
Study selection
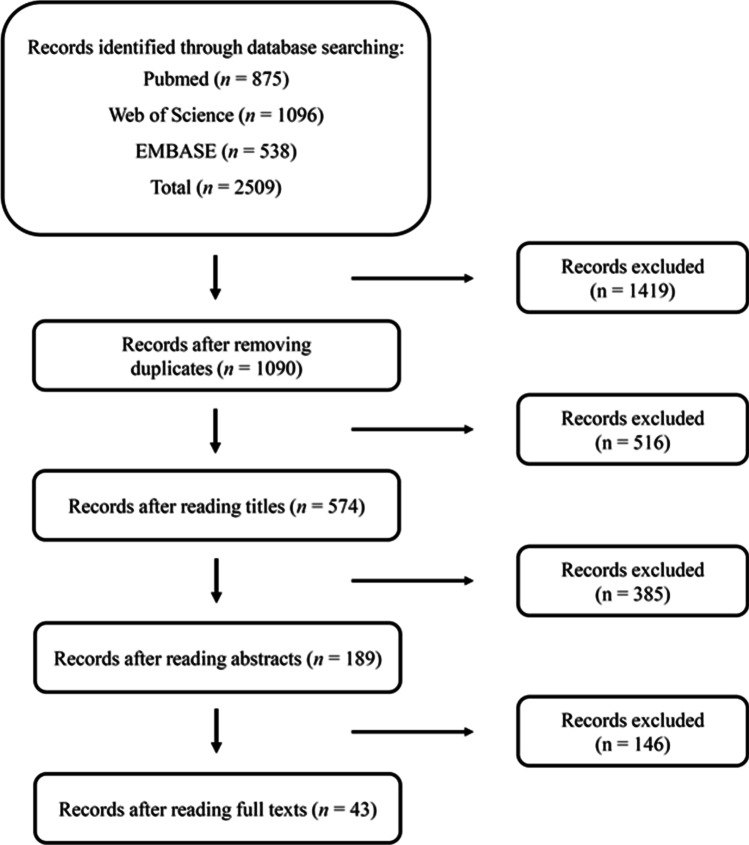
This systematic review and meta-analysis included studies in which participants performed acute exercise or chronic physical training and were subjected to analyses of URTI incidence and concentrations of IgA and IgG. Furthermore, all included studies were written in English. Reviews, summaries, case studies, and letters were disregarded, although this bibliography was consulted. Based on the search and inclusion/exclusion criteria, 43 studies (112 trials, n = 1291 individuals) were selected for inclusion in this systematic review (Fig. 1). For inclusion in the meta-analysis, only the 29 studies (85 trials, n = 662 individuals) that assessed IgA and IgG concentrations after acute exercise or chronic physical training were selected. Notably, several studies measured more than one physical performance parameter. Therefore, since all data addressing the effect of exercise on each parameter were included, the number of trials was greater than the number of studies.
Fig. 1.
Summary of the study selection process
Data grouping
The selected studies for systematic review were divided into the following three groups: (I) trials that evaluated the effects of acute exercise in sedentary subjects (11 trials; 83 individuals, Table 1), (II) trials that evaluated the effects of acute exercise in athletes/trained individuals (64 trials; 345 individuals, Table 2), and (III) trials that evaluated the effects of chronic exercise training (42 trials; 748 individuals, Table 3)on the incidence of URTI, as well as on the levels of IgA and IgG.
Table 1.
Studies characteristics — acute exercise in sedentary subjects
| Reference | Nº of subjects(♂♀) | Characteristics of subjects | Exercise protocol | Variable of infection | Result | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Duration | Intensity | Days with exercise | Exercise environment | Exercise protocol | Modality | |||||
| Eda et al. [15] |
23 (0/23) |
Healthy women | 90 min | – | 1 | – | Yoga stretching for 90 min | Yoga | sIgA (μg·mL−1) |
Pre: 26.3 ± 10.3 Post: 31.5 ± 13.4* |
|
Gleeson et al (1) [19] |
26 (11/15) |
Healthy people involved in regular physical activities | – | High intensity | 1 | – | – | Swimming | sIgA (μg·mL−1) |
Pre: 41.7 ± 1.8 Post: 46.1 ± 1.8 |
| Gleeson et al. (2) [19] |
26 (11/15) |
Healthy people involved in regular physical activities | – | High intensity | 1 | – | – | Swimming | sIgG (μg·mL−1) |
Pre: 3.9 ± 20.9 Post: 5.8 ± 14.7 |
| Mackinnon et al. (1) [38] |
10 (–/–) |
Recreational joggers | 40 min | 55% VO2peak | 1 | 22–23 °C | Treadmill runs 55% VO2peak | Running | IgA secretion rate (μg·min−1) |
Pre: 52.2 ± 9.0 Post: 40.5 ± 9.0 |
| Mackinnon et al. (2) [38] |
10 (–/–) |
Recreational joggers | 40 min | 75% VO2peak | 1 | 22–23 °C | Treadmill runs 75% VO2peak | Running | IgA secretion rate (μg·min−1) |
Pre: 61.2 ± 10.0 Post: 48.8 ± 9.7 |
| Murase et al. (1) [47] |
16 (16/0) |
Sedentary young | 59 min | 75% VO2max | 1 | 25 ºC | Cycling exercise | Cycling | sIgA secretion rate (μg·min−1) |
Pre: 26.9 ± 12.6 Post: 20.3 ± 10.4 |
| Murase et al. (2) [47] |
16 (16/0) |
Sedentary young | 59 min | 75% VO2max | 1 | 25 ºC | Cycling exercise | Cycling | sIgA (μg·mL−1) |
Pre: 22.8 ± 8.5 Post: 18.5 ± 7.6 |
| Sari-Sarraf et al. (1) [72] |
8 (8/0) |
Healthy | 90 min |
VO2max: 56.6 ± 12.0% RPE: 11.9 ± 0.8 |
1 |
16 ± 1 °C 47 ± 8% RH |
Intermittent exercise specific to soccer | Soccer | sIgA secretion rate (μg·min−1) |
Pre: 78.7 ± 44.5 Post: 113.9 ± 85.7 |
| Sari-Sarraf et al. (2) [72] |
8 (8/0) |
Healthy | 90 min |
VO2max: 56.6 ± 12.0% RPE: 11.9 ± 0.8 |
1 |
16 ± 1 °C 47 ± 8% RH |
Intermittent exercise specific to soccer | Soccer | sIgA (mg L−1) |
Pre:131.6 ± 61.2 Post: 148.4 ± 82.5 |
| Sari-Sarraf et al. (3) [72] |
8 (8/0) |
Healthy | 90 min |
VO2max: 56.6 ± 12.0% RPE: 11.9 ± 0.8 |
1 |
16 ± 1 °C 47 ± 8% RH |
45-min exercise + 15-min half-time + 45-min exercise | Soccer | sIgA secretion rate (μg·min−1) |
Pre:78.9 ± 56.3 Post:101.8 ± 70.1 |
| Sari-Sarraf et al. (4) [72] |
8 (8/0) |
Healthy | 90 min |
VO2max: 56.6 ± 12.0% RPE: 11.9 ± 0.8 |
1 |
16 ± 1 °C 47 ± 8% RH |
45-min exercise + 15-min half-time + 45-min exercise | Soccer | sIgA (mg L−1) |
Pre: 146.4 ± 07.6 Post: 229.2 ± 159.6 |
♂, Male; ♀, female. VO2peak peak oxygen uptake, VO2max maximal oxygen uptake, RPE rate of perceived exertion, RH relative humidity. Results showed as mean values and standard deviations
*Statistical difference
Table 2.
Studies characteristics—acute exercise in trained (athletes) subjects
| Reference | Nº of subjects (♂♀) | Characteristics of subjects | Training protocol | Session protocol | Variable of infection | Result | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Duration of protocol | Frequency | Intensity | Duration of session | Exercise environment | Exercise protocol | |||||
| Canto et al. (1) [11] |
47 (28/19) |
Healthy non-elite marathon runners | – | – | – | Mean finishing time was 3 h and 38 min (± 41 min) | – | Marathon | sIgA (mg/mL) |
Pre: 2.34 ± 1.16 Post: 2.37 ± 0.37 |
| Canto et al. (2) [11] |
47 (28/19) |
Healthy non-elite marathon runners | – | – | – | Mean finishing time was 3 h and 38 min (± 41 min) | – | Marathon | sIgA (normalized to total salivary protein) |
Pre: 0.39 ± 0.25 Post: 0.35 ± 0.24 |
| Costa et al. (1) [13] |
32 (32/0) |
Healthy triathletes (Olympic and Iroman Triathlon) | 20.9 ± 5,8 h/week | 6 day/week | 70% VO2max | 1 h | – | Run for 1 h/day | sIgA (mg L) |
Pre:184.6 ± 67.4 Post: 167.9 ± 62.1 |
| Costa et al. (2) [13] |
32 (32/0) |
Healthy triathletes (Olympic and Iroman Triathlon) | 20.9 ± 5,8 h/week | 6 day/week | 70% VO2max | 1 h | – | Run for 1 h/day | sIgA (mg L) |
Pre: 153.4 ± 45.7 Post: 165.9 ± 82.4 |
| Costa et al. (3) [13] |
32 (32/0) |
Healthy triathletes (Olympic and Iroman Triathlon) | 20.9 ± 5,8 h/week | 6 day/week | 70% VO2max | 1 h | – | Run for 1 h/day | sIgA (mg L) |
Pre: 168.8 ± 55.3 Post: 157.1 ± 73.7 |
| Gleeson et al.(1) [19] |
26 (11/15) |
Elite swimmers | 6 months | 20–25 h of training in the pool and 5 h of training on dry land per week | High intensity | – | – | – | sIgA (mg L) |
Pre: 50.4 ± 1.6 Post: 45.2 ± 1.7 |
| Gleeson et al.(2) [19] |
26 (11/15) |
Elite swimmers | 6 months | 20–25 h of training in the pool and 5 h of training on dry land per week | High intensity | – | – | – | sIgG (mg L) |
Pre: 8.1 ± 13.2 Post: 5.2 ± 18.5 |
| Gleeson et al.(1) [20] |
22 (12/10) |
Elite swimmers | 12 weeks | 10 to 25 h/week | High intensity | – | Mean air temperature 24 °C, mean water temperature 27 °C | Training in the pool and of training on land (resistance, flexibility and circuits) | sIgA (mg L) |
Pre: 55.3 Post: 37.11 |
| Gleeson et al. (2) [20] |
22 (12/10) |
Elite swimmers | 12 weeks | 10 to 25 h/week | High intensity | – | Mean air temperature 24 °C, mean water temperature 27 °C | Training in the pool and of training on land (resistance, flexibility and circuits) | sIgG (mg L) |
Pre: 15.8 Post: 13.9 |
| Kunz et al. (1) [30] |
17 (13/4) |
Healthy and experienced cyclists | Minimum period of 12 months | At least three times per week | − 5% blood lactate threshold | 30 min | 10-min warm-up + 30 min of exercise | Cycling | sIgA. (µg/mL) |
Pre: 290 ± 219 Post: 297 ± 169* |
| Kunz et al. (2) [30] |
17 (13/4) |
Healthy and experienced cyclists | Minimum period of 12 months | At least three times per week | + 5% blood lactate threshold | 30 min | 10-min warm-up + 30 min of exercise | Cycling | sIgA. (µg/mL) |
Pre: 283 ± 204 Post: 319 ± 202* |
| Kunz et al. 2015 (3) [30] |
17 (13/4) |
Healthy and experienced cyclists | Minimum period of 12 months | At least three times per week | + 15% blood lactate threshold | 30 min | 10-min warm-up + 30 min of exercise | Cycling | sIgA. (µg/mL) |
Pre: 264 ± 268 Post: 386 ± 260* |
| Libicz et al. (1) [37] |
8 (8/0) |
Elite triathletes | – | – | Extended intense exercise | 40 min | 14.5 – 27,2 °C |
Run 2.5 km, bike 20 km, run 2.5 km |
sIgA (µg /ml) |
Pre: 61.9 ± 36.5 Post: 88.1 ± 53.3 |
| Libicz et al. (2) [37] |
8 (8/0) |
Elite triathletes | – | – | Extended intense exercise | 20 min | 14.5 – 27,2 °C | Swim 400 m, bike 10 km, run 2,5 km | sIgA (µg /ml) |
Pre: 77.3 ± 47.5 Post: 82.2 ± 50.8 |
| Libicz et al. (3) [37] |
8 (8/0) |
Elite triathletes | – | – | Extended intense exercise | 1 h 40 min | 14.5 – 27,2 °C | Swim 1500 m, bike 40 km, run 10 km | sIgA (µg /ml) |
Pre: 101.0 ± 51.6* Post: 85.6 ± 82.0 |
| Libicz et al. (4) [37] |
8 (8/0) |
Elite triathletes | – | – | Extended intense exercise | 40 min | 14.5 – 27,2 °C | Swim 750 m, bike 20 km, run 5 km | sIgA (µg /ml) |
Pre: 56.8 ± 30.6 Post: 69.2 ± 32.1 |
| Libicz et al. (5) [37] |
8 (8/0) |
Elite triathletes | – | – | Extended intense exercise | 1 h | 14.5 – 27,2 °C | Swim 750 m, bike 20 km, run 5 km | sIgA (µg /ml) |
Pre: 75.8 ± 54.9 Post: 58.3 ± 14.3 |
| Libicz et al. (6) [37] |
8 (8/0) |
Elite triathletes | – | – | Extended intense exercise | 2 h 20 min | 14.5 – 27,2 °C | 51.5 km Tri | sIgA (µg /ml) |
Pre: 53.6 ± 22.5 Post: 70.1 ± 39.8 |
| Libicz et al. (7) [37] |
8 (8/0) |
Elite triathletes | – | – | Extended intense exercise | 40 min | 14.5 – 27,2 °C |
Run 2.5 km, bike 20 km, run 2.5 km |
sIgA (relative to total protein) (µg/ml) |
Pre: 20.7 ± 15.7 Post: 12.8 ± 6.0 |
| Libicz et al. (8) [37] |
8 (8/0) |
Elite triathletes | – | – | Extended intense exercise | 20 min | 14.5 – 27,2 °C | Swim 400 m, bike 10 km, run 2,5 km | sIgA (relative to total protein) (µg/ml) |
Pre: 31.4 ± 21.6 Post: 8.4 ± 4.0 |
| Libicz et al. (9) [37] |
8 (8/0) |
Elite triathletes | – | – | Extended intense exercise | 1 h 40 min | 14.5 – 27,2 °C | Swim 1500 m, bike 40 km, run 10 km | sIgA (relative to total protein) (µg/ml) |
Pre: 47.3 ± 40.7 Post: 11.1 ± 2.6 |
| Libicz et al. (10) [37] |
8 (8/0) |
Elite triathletes | – | – | Extended intense exercise | 40 min | 14.5 – 27,2 °C | Swim 750 m, bike 20 km, run 5 km | sIgA (relative to total protein) (µg/ml) |
Pre: 14.9 ± 9.9 Post: 16.1 ± 7.4 |
| Libicz et al. (11) [37] |
8 (8/0) |
Elite triathletes | – | – | Extended intense exercise | 1 h | 14.5 – 27,2 °C | Swim 750 m, bike 20 km, run 5 km | sIgA (relative to total protein) (µg/ml) |
Pre: 14.6 ± 7.1 Post: 15.8 ± 7.7 |
| Libicz et al. (12) [37] |
8 (8/0) |
Elite triathletes | – | – | Extended intense exercise | 2 h 20 min | 14.5 – 27,2 °C | 51.5 km Tri | sIgA (relative to total protein) (µg/ml) |
Pre: 21.6 ± 13.4 Post: 12.7 ± 3.4 |
| Mackinnonet al. (1) [40] |
8 (8/0) |
Elite kayakers | – | – | 7.5 (1 to 10) | 30 min |
2-min efforts of increasing intensity, at 70%, 80%, 90%, and 100% (HRmax), with 30-s rest |
Kayaking | sIgA (relative to total protein) (µg /mg) |
Pre: 41.1 ± 5.3 Post: 33.4 ± 5.2 |
| Mackinnonet al. (2) [40] |
8 (8/0) |
Elite kayakers | – | – | 7.5 (1 to 10) | 30 min |
2-min efforts of increasing intensity, at 70%, 80%, 90%, and 100% (HRmax), with 30-s rest |
Kayaking | sIgG (relative to total protein) (µg /mg) |
Pre: 25.3 ± 9.5 Post: 28.7 ± 7.1 |
| Mackinnonet al. (3) [40] |
8 (8/0) |
Elite kayakers | – | – | 8.0 (1 to 10) | 24 min |
Three 8-min efforts, at 70%, 80% and 90% (HRmax) with 30-s rest |
Kayaking | sIgA (relative to total protein) (µg /mg) |
Pre: 37.4 ± 6.7 Post: 32.2 ± 5.0 |
| Mackinnonet al. (4) [40] |
8 (8/0) |
Elite kayakers | – | – | 8.0 (1 to 10) | 24 min | Three 8-min efforts, at 70%, 80%, and 90% (HRmax) with 30-s rest | Kayaking | sIgG (relative to total protein) (µg /mg) |
Pre: 30.7 ± 9.9 Post: 36.8 ± 9.9 |
| Mackinnonet al. (5) [40] |
8 (8/0) |
Elite kayakers | – | – | 8.5 (1 to 10) | 40 min | Two 20-min efforts at an intensity eliciting FC determined in critical power testing sessions | Kayaking | sIgA (relative to total protein) (µg/mg) |
Pre: 27.1 ± 3.7 Post: 25.8 ± 5.3 |
| Mackinnonet al. (6) [40] |
8 (8/0) |
Elite kayakers | – | – | 8.5 (1 to 10) | 40 min | Two 20-min efforts at an intensity eliciting FC determined in critical power testing sessions | Kayaking | sIgG (relative to total protein) (µg/mg) |
Pre: 19.7 ± 5.8 Post: 24.6 ± 6.3 |
| Mackinnonet al. (1) [38] |
8 (–/–) |
Competitive endurance athletes | – | – | 55% VO2peak | 90 min | 22 – 23 °C | Treadmill running | sIgA secretion rate (µg/min) |
Pre: 45.3 ± 9.6 Post: 46.6 ± 8.0 |
| Mackinnonet al. (2) [38] |
8 (–/–) |
Competitive endurance athletes | – | – | 75% VO2peak | 90 min | 22 – 23 °C | Treadmill running | sIgA secretion rate (µg/min) |
Pre: 62.0 ± 12.6 Post: 51.5 ± 12.2 |
| Mackinnonet al. (1) [38] |
7 (–/–) |
Competitive marathon runners and triathletes | – | – | 75% VO2peak | 90 min | 22 – 23 °C | Treadmill running | sIgA secretion rate (µg/min) |
Pre: 59.1 ± 9.8 Post: 48.9 ± 12.5 |
| Mackinnonet al. (2) [38] |
7 (–/–) |
Competitive marathon runners and triathletes | – | – | 75% VO2peak | 90 min | 22 – 23 °C | Treadmill running | sIgAsecretion rate (µg/min) |
Pre: 53.2 ± 5.0 Pós: 30.8 ± 9.6* |
| Mackinnonet al. (3) [38] |
7 (–/–) |
Competitive marathon runners and triathletes | – | – | 75% VO2peak | 90 min | 22 – 23 °C | Treadmill running | sIgAsecretion rate (µg/min) |
Pre: 52.3 ± 10.6 Pós: 36.2 ± 7.5 |
| Moreira et l al. (1) [44] |
10 (10/–) |
Professional top-level Brazilian futsal players | – | – | Highly competitive games | 40 min | – | Futsal match |
sIgA (µg mL−1) |
Pre: 175 ± 43 Post: 99 ± 17* |
| Moreira et al. (2) [44] |
10 (10/–) |
Professional top-level Brazilian futsal players | – | – | Highly competitive games | 40 min | – | Futsal match | sIgA secretion rate (µg min–1) |
Pre: 14 ± 2 Post: 7 ± 1* |
| Nieman et al. (1) [58] |
45 (–/–) |
Ultramarathon runners | – | – | – | 27 ± 0.4 h | Running 90 km | Ultramarathon | sIgA secretion rate (μg·min−1) |
Pre: 508 ± 40 Post: 287 ± 39* |
| Nieman et al. (2) [58] |
45 (–/–) |
Ultramarathon runners | – | – | – | 27 ± 0.4 h | Running 160 km | Ultramarathon | sIgA secretion rate (μg·min−1) |
Pre: 508 ± 40 Post: 254 ± 30* |
| Nieman et al. (3) [58] |
45 (–/–) |
Ultramarathon runners | – | – | – | 27 ± 0.4 h | Running 90 km | Ultramarathon | sIgA (µg·mL−1) |
Pre: 530 ± 37 Post: 577 ± 59 |
| Nieman et al. (4) [58] |
45 (–/–) |
Ultramarathon runners | – | – | – | 27 ± 0.4 h | Running 160 km | Ultramarathon | sIgA(µg·mL−1) |
Pre: 530 ± 37 Post: 562 ± 75 |
| Nieman et al. (5) [58] |
45 (–/–) |
Ultramarathon runners | – | – | – | 27 ± 0.4 h | Running 90 km | Ultramarathon | sIgA relative to total protein (µg·mg−1) |
Pre: 611 ± 57 Post: 567 ± 59 |
| Nieman et al. (6) [58] |
45 (–/–) |
Ultramarathon runners | – | – | – | 27 ± 0.4 h | Running 160 km | Ultramarathon | sIgA relative to total protein (µg·mg−1) |
Pre: 611 ± 57 Post: 523 ± 51 |
| Nieman et al. (7) [58] |
45 (–/–) |
Ultramarathon runners | – | – | – | 27 ± 0.4 h | Running 160 km | Ultramarathon | Incidence URTI (15 days post-race) | Post: 26% |
| Novas et al. (1) [60] |
17 (0/17) |
Tennis players | 12 weeks | – |
RPE: 6.7 ± 1.5 HR: 145 ± 13 bpm %HRR: 61 ± 9% |
60 min | – | Tennis | sIgA secretion rate (μg·min−1) |
Pre: 308.3 ± 150 Post: 326.8 ± 156.3 |
| Novas et al. (2) [60] |
17 (0/17) |
Tennis players | 12 weeks | – |
RPE: 6.7 ± 1.5 HR: 145 ± 13 bpm %HRR: 61 ± 9% |
60 min | – | Tennis | sIgA absolute concentration (µg·mL−1) |
Pre: 369 ± 167 Post: 361.1 ± 185.1 |
| Novas et al. (3) [60] |
17 (0/17) |
Tennis players | 12 weeks | – |
RPE: 6.7 ± 1.5 HR: 145 ± 13 bpm %HRR: 61 ± 9% |
60 min | – | Tennis | sIgA secretion rate (μg·min−1) |
Pre: 287 ± 165 Post: 222 ± 152* |
| Novas et al. (4) [60] |
17 (0/17) |
Tennis players | 12 weeks | – |
RPE: 6.7 ± 1.5 HR: 145 ± 13 bpm %HRR: 61 ± 9% |
60 min | – | Tennis | sIgA absolute concentration (µg·mL−1) |
Pre: 395 ± 223 Post: 398 ± 211 |
| Owen et al. (1) [62] |
10 (10/–) |
Elite professional soccer players | 1 week | 4 session/week |
High-intensity RPE: 15.1 – 16.3 |
– | – | Tactical and technical soccer training | sIgA (µU/mL) |
Pre: 67.89 ± 41.53 Post: 58.11 ± 44.98 |
| Owen et al. (2) [62] |
10 (10/-) |
Elite professional soccer players | 1 week | 4 session/week |
High-intensity RPE: 15.1 – 16.3 |
– | – | Tactical and technical soccer training | sIgA (µU/mL) |
Pre: 156.11 ± 111.01 Post: 101.11 ± 38.76 |
| Owen et al. (3) [62] |
10 (10/–) |
Elite professional soccer players | 1 week | 4 session/week |
High-intensity RPE: 15.1 – 16.3 |
– | – | Tactical and technical soccer training | sIgA (µU/mL) |
Pre: 125.00 ± 87.30 Post: 85.33 ± 42.53 |
| Owen et al. (4) [62] |
10 (10/–) |
Elite professional soccer players | 1 week | 4 session/week |
High-intensity RPE: 15.1 – 16.3 |
– | – | Tactical and technical soccer training | sIgA (µU/mL) |
Pre: 64.44 ± 43.50 Post: 59.15 ± 54.92 |
| Owen et al. (5) [62] |
10 (10/–) |
Elite professional soccer players | 1 week | 4 session/week |
Low-intensity RPE: 9.4 – 10.3 |
– | – | Tactical and technical soccer training | sIgA (µU/mL) |
Pre: 132.44 ± 71.26 Post: 86,56 ± 63,78 |
| Owen et al. (6) [62] |
10 (10/–) |
Elite professional soccer players | 1 week | 4 session/week |
Low-intensity RPE: 9.4 – 10.3 |
– | – | Tactical and technical soccer training | sIgA (µU/mL) |
Pre: 127.33 ± 43.68 Post: 124.56 ± 49.21 |
| Owen et al. (7) [62] |
10 (10/–) |
Elite professional soccer players | 1 week | 4 session/week |
Low-intensity RPE: 9.4 – 10.3 |
– | – | Tactical and technical soccer training | sIgA (µU/mL) |
Pre: 121.00 ± 52.22 Post: 113.56 ± 56.52 |
| Owen et al. (8) [62] |
10 (10/–) |
Elite professional soccer players | 1 week | 4 session/week |
Low-intensity RPE: 9.4 – 10.3 |
– | – | Tactical and technical soccer training | sIgA (µU/mL) |
Pre: 119.44 ± 57.02* Post: 129.00 ± 58.60* |
| Pacque et al. (1) [63] |
17 (13/4) |
Healthy race participants | – | 4,5 – 15 h/week | – | 11h25min ± 1h32min |
5 – 18 ºC Minimal rain |
Ultramarathon | sIgA secretion rate (μg·min−1) |
Pre: 252 Post: 106* |
| Pacque et al. (2) [63] |
17 (13/4) |
Healthy race participants | – | 4,5 – 15 h/week | – | 11h25min ± 1h32min |
5 – 18 ºC Minimal rain |
Ultramarathon | Serum IgA (mg· −1) |
Pre: 2142 ± 817 Post: 1640 ± 766* |
| Pacque et al. (3) [63] |
17 (13/4) |
Healthy race participants | – | 4,5 – 15 h/week | – | 11h25min ± 1h32min |
5 – 18 ºC Minimal rain |
Ultramarathon | URTI incidence |
Pre: 4 Post: 3 |
| Peters et al. (1) [68] |
14 (0/14) |
Ultramarathon runners | Until 5 km/week | – | – |
9:33 ± 1:04 h |
– | 86.5 km | sIgA(µg·mL−1) |
Pre: 27.24 ± 17.86 Post: 44.18 ± 19.47 |
| Peters et al. (2) [68] |
14 (0/14) |
Ultramarathon runners | Until 5 km/week | – | – |
9:33 ± 1:04 h |
– | 86.5 km | URTI incidence | Post: 71% |
| Steerenberg et al. [75] |
42 (36/6) |
Triathletes | – | – | – | 2–2.5 h |
24.5 °C 45% RH |
1000–1500 m swimming, 40 km cycling and 10 km running | sIgA secretion in 5 min (μg) |
Pre: 500 Post: 308 |
| Zakovska et al. [84] |
15 (12/3) |
Ultra-marathoners |
91.4 ± 27.0 h/week or 10.7 ± 3.7 h/week |
– | 6:18 ± 1:42 min·km | – |
− 1– + 1 °C snow and rain |
100 km ultramarathon running (completing 60 km at least) | Serum IgA (mg.dl−1) |
Pre: 21.09 ± 90.52 Post: 24.0 ± 13.56 |
♂, Male; ♀, female. VO2peak peak oxygen uptake, VO2max maximal oxygen uptake, HR heart rate, HRmax maximum heart rate, HRR heart rate reserve, RPE rate of perceived exertion, RH relative humidity. Results showed as mean values and standard deviations
*Statistical difference
Table 3.
Studies characteristics—chronic exercise training
| Reference | Nº of subjects (♂/♀) | Characteristics of subjects | Exercise training protocol | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exercise protocol | Duration of protocol | Intensity | Duration of session | Frequency | Exercise environment | Variable of infection | Result | |||
| Blume et al. [3] |
274 (175/99) |
Young athletes (cross-country skiing, cycling, figure skating, gymnastics, high diving, soccer, speed skating, swimming, tennis and volleyball) |
– | 2010–2014 | – | – | 14.9 ± 5.6 h weekly | – | URTI | 30,7% |
| Borges et al. [4] |
12 (6/6) |
Elite kayakers | Kayaking (60–140 km/week), running (20–35 km/week), swimming (4–6 km/week), strength (90–140 tons/week) and calisthenics (100–220 min/week) | November to June | – | – | – | – | URTI | 9 episodes |
| Brisola et al. [8] |
20 (0/20) |
Young female water polo players | First session of the day was only for dry and training and second session of the day included swimming routine and specific water polo exercises | January to April of the competitive season | – |
General cycle: 118.6 ± 27.0 min Specific cycle: 118.8 ± 17.0 min Competitive cycle: 109.9 ± 13.6 min |
6 times a week with 2 daily sessions | – | URTI |
General cycle: 4.00 ± 2.0 a.u* Specific cycle: 2.00 ± 1.2 a.u Competitive cycle: 2.00 ± 1.3 a.u |
| Broadbent et al. [9] |
15 (15/0) |
Ironman triathletes |
3–4 swim sessions per week (12–16 km) 3–4 cycle sessions per week (300–400 km) 3–5 run sessions per week (60–90 km) |
12 months | – | – | 9–13 sessions per week | – | URTI | 2 episodes |
| Brunelli et al. [10] |
11 (11/0) |
Adolescent basketball athletes | Technical and functional skills according to the objectives of training | 20 weeks | – | 98.7 ± 4.5 min | Three weekly sessions | – | URTI |
Preparatory period: 28,8% Competitive period: 61,7%* |
| Fahlman et al. (1) [16] |
75 (75/0) |
University football team | – | 12 months | Moderate | – | – | – | URTI |
Pre: 6% Post: 59% |
| Fahlman et al. (2) [16] |
75 (75/0) |
University football team | – | 12 months | Moderate | – | – | – |
IgA to protein (µg mg−1) |
Pre: 96 ± 7 Post: 92 ± 10 |
| Fahlman et al. (3) [16] |
75 (75/0) |
University football team | – | 12 months | Moderate | – | – | – | IgA to osmolality (mg mOsmol−1) |
Pre: 2.8 ± 0.8 Post: 1.9 ± 1.8 |
| Fahlman et al. (4) [16] |
75 (75/0) |
University football team | – | 12 months | Moderate | – | – | – |
Secretion rate of sIgA (µg min−1) |
Pre: 54.9 ± 1.3 Post: 38.3 ± 1.5 |
| Filaire et al. (1) [17] |
20 (20/0) |
Professional football players | Running (70–75% VO2max), physical training, and technical training sessions | 12 months | High intensity | 27 h a week | – | – | IgA |
Pre: 2.3 ± 0.2 Post: 2.3 ± 0.1 |
| Filaire et al. (2) [17] |
20 (20/0) |
Professional football players | Running (70–75% VO2max), physical training, and technical training sessions | 12 months | High intensity | 27 h a week | – | – | IgG |
Pre: 11.9 ± 0.4 Post: 11.8 ± 0.2 |
| Gleeson et al. (1) [19] |
26 (11/15) |
Elite swimmers | 20–25 h of training in the pool and 5 h of training on dry land per week | 6 months (April to October) | High intensity | 6 h/day | – | – | Serum IgA |
Athletes: 1.51 ± 1.39 Control: 1.73 ± 1.62* |
| Gleeson et al. (2) [19] |
26 (11/15) |
Elite swimmers | 20–25 h of training in the pool and 5 h of training on dry land per week | 6 months (April to October) | High intensity | 6 h/day | – | – | Serum IgG |
Athletes: 10.28 ± 1.26 Control: 10.80 ± 1.21* |
| Gomez-Merino et al. [24] |
21 (21/0) |
Cadets |
1 week – sea phase 2 and 3 week – mountain phase 5 days combat course – rough terrain |
3 weeks of training and 5 days combat course | High | – | – | 12 – 25 °C | URTI | 14 episodes |
| Ihalainen et al. [29] |
25 (25/0) |
Recreational male endurance runners | Running | 12 weeks |
1 to 2 incremental run: 65–85% HRmax, 1 long run: 60–65% HRmax 1 interval run: 80–85%HRmax 1 to 2 light run: 60–65% HRmax |
1 to 2 incremental run: 35 – 45 min 1 long run: 70 – 120 min 1 interval run: 20 – 25 min 1 to 2 light run 35 – 40 min |
4–6 sessions/week | Average temperature: –7 to –1 °C | URTI | 13 episodes |
| Mackinnon et al. [39] |
24 (8/16) |
Elite swimmers | Intensified training | 4 weeks | > 80% HRmax | – |
6 x/week 2x /day |
– | URTI | 10 episodes |
| Moreira et al. (1) [45] |
15 (15/0) |
Basketball players under-19 | – | – | – | 90–120 min |
5 x/week 2x /day |
– | sIgA (µg ml−1) |
Pre: 587 ± 94 Post: 720 ± 153 |
| Moreira et al. (2) [45] |
15 (15/0) |
Basketball players under-19 | – | – | – | 90–120 min |
5 x/week 2x /day |
– | sIgA secretion rate (µg min−1) |
Pre: 106 ± 20 Post: 92 ± 21* |
| Martins et al. (1) [41] |
21 (8/13) |
Elderly | Aerobic exercise training | 16 weeks | 40 – 85% HRR | 45 min | 3x/week | – | sIgA (mg dL−1) |
Pre: 8.40 ± 7.09 Post: 6.25 ± 4.25 |
| Martins et al. (2) [41] |
21 (8/13) |
Elderly | Aerobic exercise training | 16 weeks | 40 – 85% HRR | 45 min | 3x/week | – | IgA (g L−1) |
Pre: 1.08 ± 0.51 Post: 2.40 ± 0.86* |
| Martins et al. (3) [41] |
21 (8/13) |
Elderly | Aerobic exercise training | 16 weeks | 40 – 85% HRR | 45 min | 3x/week | – | IgG (g L−1) |
Pre: 4.95 ± 2.09 Post: 12.44 ± 2.91* |
| Nehlsen-Cannarella et al., 2000 (1) [48] |
20 (0/20) |
Elite rowers | – | – | – | 90–120 min training/week | – | – | Days with URTI symptoms |
Rowers: 5.2 ± 1.2 Nonathletes: 3.3 ± 1.1 |
| Nehlsen-Cannarella et al. (2) [48] |
20 (0/20) |
Elite rowers | – | – | – | 90–120 min training/week | – | – | sIgG (µg ml−1) |
Rowers: 14.7 ± 1.9 Nonathletes: 11.4 ± 1.6 |
| Nehlsen-Cannarella et al. (3) [48] |
20 (0/20) |
Elite rowers | – | – | – | 90–120 min training/week | – | – | Saliva protein IgA concentration (µg mg1) |
Rowers: 425 ± 20 Nonathletes: 409 ± 96 |
| Nehlsen-Cannarella et al. (4) [48] |
20 (0/20) |
Elite rowers | – | – | – | 90–120 min training/week | – | – | Saliva protein IgG concentration (µg mg1) |
Rowers: 15.4 ± 1.6 Nonathletes: 21.6 ± 6.7 |
| Nehlsen-Cannarella et al. (5) [48] |
20 (0/20) |
Elite rowers | – | – | – | 90–120 min training/week | – | – | sIgA secretion rate (µg min−1) |
Rowers: 63.0 ± 12.3 Nonathletes: 47.1 ± 7.6 |
| Nehlsen-Cannarella et al. (6) [48] |
20 (0/20) |
Elite rowers | – | – | – | 90–120 min training/week | – | – | sIgG secretion rate (µg min−1) |
Rowers: 2.3 ± 0.5 Nonathletes: 2.7 ± 0.5 |
| Nieman et al. [56] |
32 (0/32) |
Healthy elderly women | Walking | 12 weeks | 60% heart rate reserve | 30–40 min | 5 days /week | – | URTI |
Calisthenic: 8/16 (50%) Walking: 3/14 (21%) Highly conditioned: 1/12 (8,3%)* |
| Nieman et al. [57] |
39 (0/39) |
Elite female rowers | Rower | – | – | 90–120 min | 12–13 sessions/week | – | URTI days |
Rowers: 5.2 ± 1.2 Nonathletes: 3.3 ± 1.1 |
| Novas et al. (1) [60] |
17 (0/17) |
Female tennis player | – | 12 weeks | – | 104 ± 32 min | – | – | IgA (µg ml−1) |
Pre: 395 ± 223 Post: 398 ± 211 |
| Novas et al. (2) [60] |
17 (0/17) |
Female tennis player | – | 12 weeks | – | 104 ± 32 min | – | – | sIgA rate (µg min−1) |
Pre: 287 ± 165 Post: 222 ± 152* |
| Novas et al. (3) [60] |
17 (0/17) |
Female tennis player | – | 12 weeks | – | 104 ± 32 min | – | – | URTI episodes | 1.5 ± 1.4 |
| Novas et al. (4) [60] |
17 (0/17) |
Female tennis player | – | 12 weeks | – | 104 ± 32 min | – | – | URTI days | 10 ± 10.3 |
| Orysiak et al. (1) [61] |
12 (12/0) |
Young ice hockey players | Strength training, training on ice and friendly match | 17 days | – | – | – | – | URTI |
Training camp 42% During the tournament 0% After the World Championship 8% |
| Orysiak et al. (2) [61] |
12 (12/0) |
Young ice hockey players | Strength training, training on ice and friendly match | 17 days | – | – | – | – | sIgA concentration (g/ml) |
Pre: 116.17 ± 51.66 Post: 122.84 ± 82.67 |
| Orysiak et al. (3) [61] |
12 (12/0) |
Young ice hockey players | Strength training, training on ice and friendly match | 17 days | – | – | – | – | sIgA secretion rate (µg/min) |
Pre: 84.93 ± 32.30 Post: 73.69 ± 45.75 |
| Rama et al. [70] |
19 (13/6) |
Elite swimmers | – | 29 weeks | – | – | 8–11 sessions/week | – | URTI | 31 episodes |
| Tiollier et al. (1) [79] |
21 (21/0) |
Male cadets from the French Military | – | 3 weeks of commando training followed by a 5-day combat course | – | – | – |
Minimum temperature: 1 – 12.7 °C, maximum temperature: 7 – 27.2 °C |
URTI | 14 episodes |
| Tiollier et al. (2) [79] |
21 (21/0) |
Male cadets from the French Military | – | 3 weeks of commando training followed by a 5-day combat course | – | – | – |
Minimum temperature: 1 – 12.7 °C, maximum temperature: 7 – 27.2 °C |
IgA |
Pre: 113 ± 11 Post: 120 ± 14 |
| Whitham et al. (1) [81] | 14 | PARA recruits | 10- and 20-mile foot races carrying a 35-lb bergan and weapon, a 1.8-mile log race in teams of six to nine, and a 5-mile stretcher race | 19 weeks | – | – | – | Ambient temperatures of > 25 °C | URTI | 4.1 ± 0.3 |
| Whitham et al. (2) [81] | 14 | PARA recruits | 10- and 20-mile foot races carrying a 35-lb bergan and weapon, a 1.8-mile log race in teams of six to nine, and a 5-mile stretcher race | 19 weeks | – | – | – | Ambient temperatures of > 25 °C | sIgA concentration (mg/L) |
Pre: 66 ± 15 Post: 347 ± 79 |
| Whitham et al. (3) [81] | 14 | PARA recruits | 10- and 20-mile foot races carrying a 35-lb bergan and weapon, a 1.8-mile log race in teams of six to nine, and a 5-mile stretcher race | 19 weeks | – | – | – | Ambient temperatures of > 25 °C | sIgA secretion rate (µg/min) |
Pre: 14 ± 3 Post: 27 ± 6 |
♂, male; ♀, female. HRmax maximum heart rate, HRR heart rate reserve. Results showed as mean values and standard deviations
*Statistical difference
The studies included in the meta-analysis were divided into studies that evaluated the concentrations of IgA or IgG. IgA analysis was performed in the following groups: (I) untrained individuals that performed acute exercise, (II) trained individuals that performed acute exercise, (III) trained individuals that performed acute exercise in ultramarathon, (IV) trained individuals that performed acute exercise in triathlon, and (V) non-military trained individuals that performed acute exercise. IgG analysis was only performed in chronic physical trained individuals.
Risk of bias assessment
Two independent reviewers assessed the risk of bias using an adapted Grading of Recommendations Assessment, Development and Evaluation (GRADE) instrument [26]. Discrepant evaluations were settled via discussion with a third reviewer. Using this approach, it was possible to evaluate the risk of bias in each study included in the present systematic review. Domains reflecting sequence generation, allocation concealment, blinding of participants and personnel, incomplete outcome data, selective outcome reporting and other sources of bias were evaluated. The lack of one of these domains characterizes the reduction in the individual methodological quality of each study in the following sequence: high, moderate, low and very low.
Furthermore, the GRADE approach was used to assess the overall quality with levels of evidence. This analysis was performed using a GRADE profiler software, available online (https://gdt.gradepro.org/app/), which was used to summarize our findings on the certainty of the evidence. Thus, for each outcome analyzed in the meta-analysis, the global assessment of the level of evidence was performed and classified into four levels: high (GRADE A), moderate (GRADE B), low (GRADE C), and very low (GRADE D) [26].
Statistical analysis
The mean and SD values of the IgA and IgG levels after acute and chronic exercise were obtained from the data provided in the consulted research papers. Heterogeneity was evaluated using the χ2 test for homogeneity and the I2 statistic. The effect size (Cohen’s d or Hedges’ g) was calculated for the physical performance indexes in each study. Then, a weighted-mean estimate of the effect size was calculated to account for differences in the sample sizes. The mean unweighted effect size and associated 95% CI were also calculated. We used Cohen’s classification of the effect size magnitude, where d < 0.20 = negligible effect; d = 0.20–0.49 = small effect; d = 0.50–0.79 = moderate effect; and d > 0.8 = large effect [12].
Results
Systematic review
In total, 2509 studies were identified through the database and reference searches. After removing the duplicates and excluding papers that did not meet the eligibility criteria following a review of their titles, abstracts and full texts, 43 studies (117 trials, n = 1176 individuals) were selected for inclusion in the systematic review (Fig. 1). The characteristics of the subjects, the exercise/physical training protocols and the infection parameter evaluated in each study are summarized in Tables 1, 2, and 3.
Meta-analyses
Twenty-nine studies (85 trials, n = 662 individuals) were included in the meta-analysis.
Untrained: acute exercise – IgA levels (GRADE C)
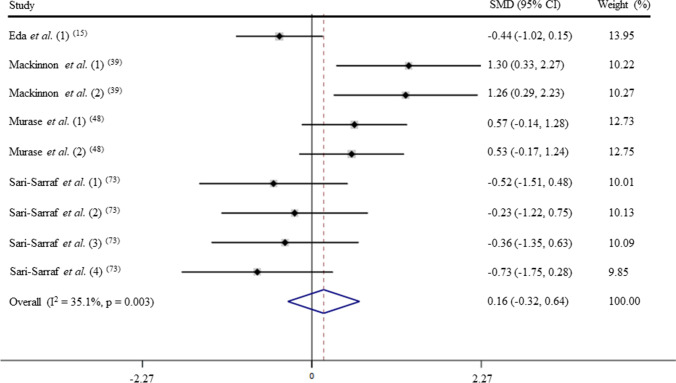
After pooling the data from 9 trials, the mean effect size was 0.16 (95% CI: − 0.32 to 0.64), which indicates that acute exercise has a non-significant effect on IgA levels (p > 0.05; Fig. 2). According to a random effects analysis, medium heterogeneity was observed among these studies (I2 = 62.1%; Q = 21.1, df = 8, p = 0.07).
Fig. 2.
Forest plot of IgA levels in response of acute exercise in untrained subjects. SMD: standardized mean difference
Trained: acute exercise – IgA levels (GRADE B)
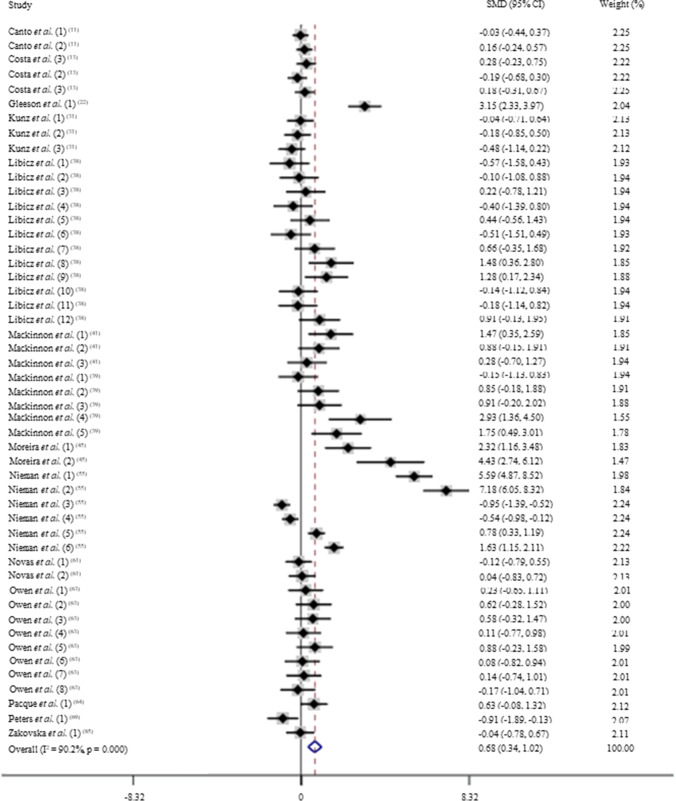
After pooling the data from 50 trials, the mean effect size was 0.68 (95% CI: 0.34 to 1.02), which indicates that acute exercise induces a moderate and significant increase on IgA levels (p < 0.05; Fig. 3). According to a random effects analysis, high heterogeneity was observed among these studies (I2 = 90.2%; Q = 500.8, df = 49, p = 0.00). The subsequent analysis consisted of subdividing the trained subjects into two different sports: ultramarathon and triathlon. These sports were chosen based on their higher frequency on the trials. In addition, these sports are known to impose a high burden on the organism, challenging the immune system.
Fig. 3.
Forest plot of IgA levels in response of acute exercise in trained subjects. SMD: standardized mean difference
Trained: acute exercise—IgA levels in ultramarathon (GRADE A)
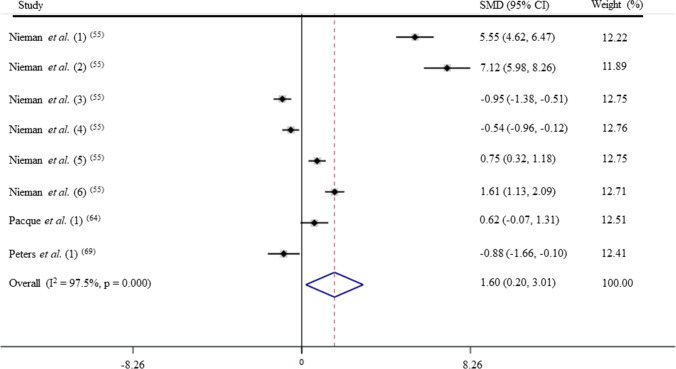
After pooling the data from 8 trials, the mean effect size was 1.60 (95% CI: 0.20 to 3.01), which indicates that ultramarathon induces a large and significant increase on IgA levels (p < 0.05; Fig. 4). According to a random effects analysis, high heterogeneity was observed among these studies (I2 = 97.5%; Q = 358.8, df = 9, p = 0.00).
Fig. 4.
Forest plot of IgA levels in response of acute exercise in ultramarathoner. SMD: standardized mean difference
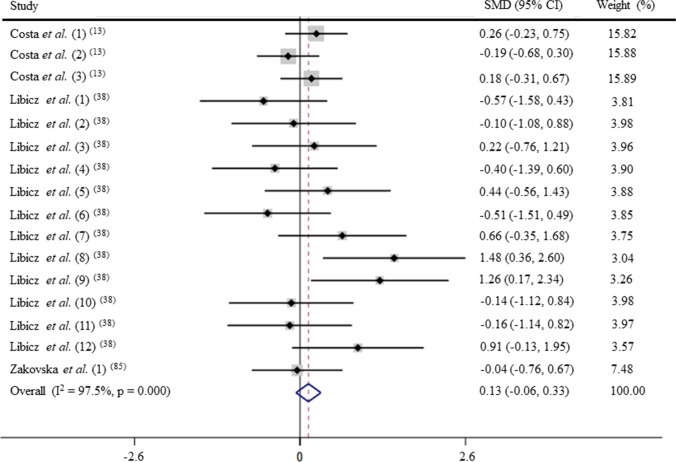
Trained: acute exercise—IgA levels in triathlon (GRADE C)
After pooling the data from 16 trials, the mean effect size was 0.13 (95% CI: − 0.06 to 0.33), which indicates that triathlon has a non-significant effect on IgA levels (p > 0.05; Fig. 5). According to a fixed effects analysis, no heterogeneity was observed among these studies (I2 = 28.1%; Q = 20.87, df = 15, p = 0.14).
Fig. 5.
Forest plot of IgA levels in response of acute exercise in triathletes. SMD: standardized mean difference
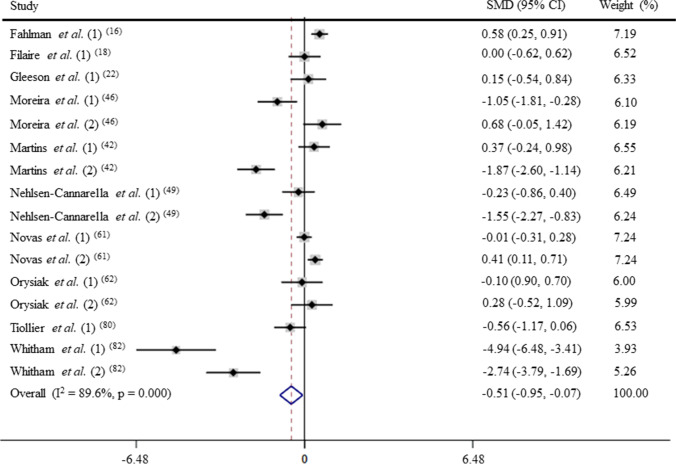
Chronic physical training and IgA levels (GRADE B)
After pooling the data from 16 trials, the mean effect size was − 0.51 (95% CI: − 0.95 to − 0.07), which indicates that the chronic physical training induces a moderate and significant decrease on IgA levels (p < 0.05; Fig. 6). According to a random effects analysis, high heterogeneity was observed among these studies (I2 = 89.6%; Q = 144.1, df = 15, p = 0.00).
Fig. 6.
Forest plot of IgA levels in response of physical training. SMD: standardized mean difference
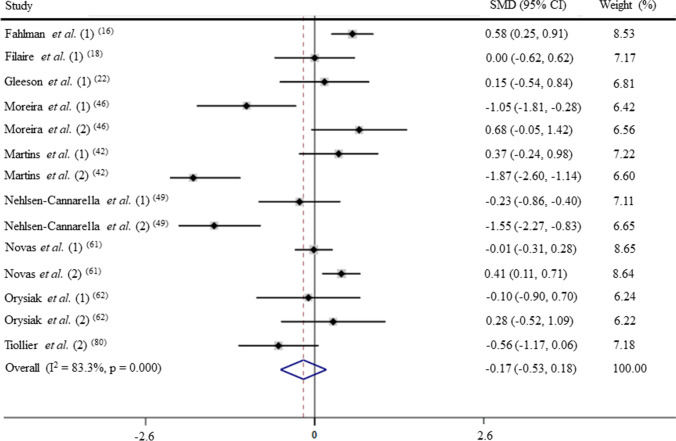
Chronic physical training and IgA levels non-military personnel (GRADE C)
Due to the fact that the IgA response to chronic physical training was greatly affected by military coaching, IgA levels were analysed in non-military trained individual separately. After pooling the data from 14 trials, the mean effect size was − 0.17 (95% CI: − 0.53 to 0.18), which indicates that the chronic physical training has a non-significant effect on IgA levels (p > 0.05; Fig. 7). According to a random effects analysis, high heterogeneity was observed among these studies (I2 = 83.3%; Q = 78.0, df = 13, p = 0.00).
Fig. 7.
Forest plot of IgA levels in response of physical training in non-military personnel. SMD: standardized mean difference
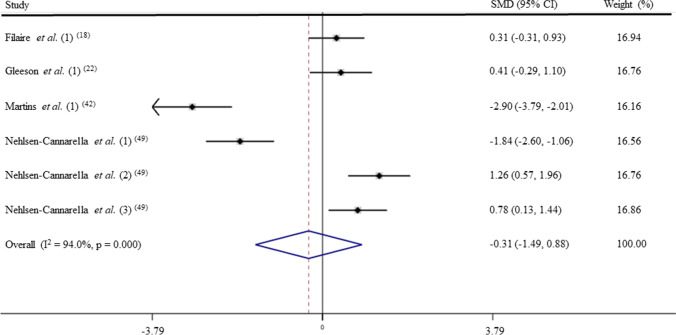
Chronic physical training and IgG levels (GRADE C)
After pooling the data from 6 trials, the mean effect size was − 0.31 (95% CI: − 1.49 to 0.88), which indicates that the chronic physical training has a and non-significant effect on IgG levels (p > 0.05; Fig. 8). According to a random effects analysis, high heterogeneity was observed among these studies (I2 = 94.0%; Q = 83.5, df = 5, p = 0.00).
Fig. 8.
Forest plot of IgG levels in response of physical training. SMD: standardized mean difference
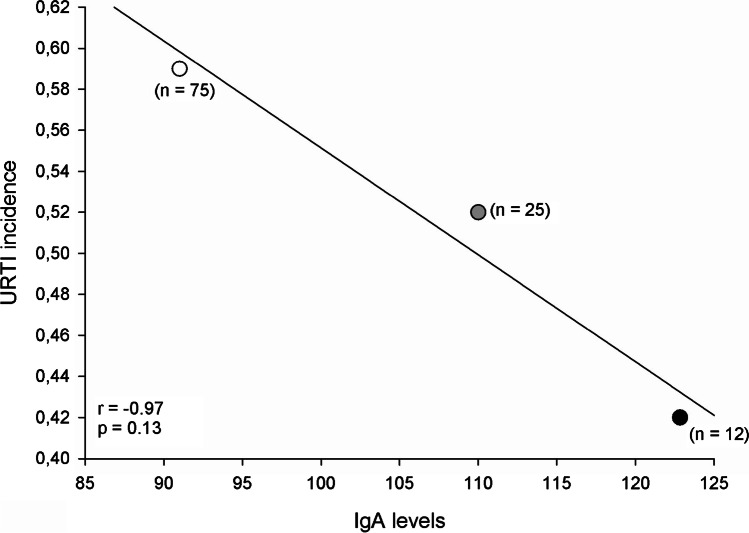
Relation between IgA levels and URTI incidences
A correlation between the URTI incidence and IgA levels in response to exercise was performed with data from three studies that simultaneously analyzed these variables. A strong negative correlation between the variables was found (r = − 0.97. p = 0.13; Fig. 9).
Fig. 9.
Correlation between IgA levels and the incidence of URTI in athletes
Risk of bias
The risk of bias was assessed in 43 studies in the systematic review. Approximately 25% (11 studies) of them did not blind the participants or researchers. This limitation seems to be related to the characteristics of the studies’ experimental protocols. Nevertheless, most of the studies evaluated in the present systematic review consistently controlled the risk of bias and, therefore, were deemed moderate to high quality (Supplementary Table 1).
Discussion
The present systematic review and meta-analysis demonstrates that acute exercise increases the IgA levels in trained subjects, but does not affect its levels in untrained subjects. Such increase in IgA levels induced by acute exercise is greater in trained individual that performed ultramarathon in comparison with those that executed triathlon. Although, trained individuals’ present elevated IgA response to acute exercise, these individuals have decreased IgA baseline. On the other hand, the basal IgA levels were reduced by chronic physical training. However, when data from non-military personnel are excluded from the trained individuals group, the remaining data reveals that chronic physical training does not alter IgA levels. In addition, chronic physical training does not change IgG levels. These data indicate that acute exercise positively influences IgA levels in trained individuals, being this effect pronounced when a strenuous exercise such as ultramarathon is executed. Moreover, chronic physical training response over IgA levels seems to depend on the characteristics of the subject’s chronic physical training since non-military personnel presented unchanged IgA levels. The present analysis brings important information for exercise practitioners and athletes and provide support for the better understanding of the beneficial effects of exercise in fighting infections such as URTI through modulation of immunoglobulin levels.
The execution of acute, moderate and vigorous aerobic exercise of less than 60 min duration is known to enhance the recirculation of agents that play critical roles in immune defense activity and metabolic health, including immunoglobulins [55]. Thus, this type of exercise is now seen as an immune system adjuvant. However, in response to prolonged and intensive exercise, and in competition events, salivary IgA output and other biomarkers of immune function are altered for several hours to days [52, 59]. These immune changes occur in different body sites such as the upper respiratory tract mucosal tissue and the lungs [55]. Secretory IgA plays an important role in the organism’s defense against respiratory illness through immune exclusion at mucosal surfaces, intra-epithelial viral naturalization and immune elimination across mucosal surfaces [7, 20, 42].
The results of the present study indicate that acute exercise does not modify IgA levels in untrained individuals. It does only when acute exercise is performed by trained subjects, particularly after a strenuous bout of exercise. It is difficult to provide a definitive description of the acute effects of exercise on salivary IgA concentration [20]. Evidence demonstrated that athletes seem to experience a transitory decrease in s-IgA for up to 24 h post strenuous training sessions or competition [66]. It is during this “open window” period of immune depression that athletes are thought to be at greatest risk of URTI [49]. In fact, low IgA concentration and secretion rate have been associated with increased incidence of URTI in athletes [16, 20]. On the other hand, basketball players have shown an increase of IgA after competitive games [77]. This increase in IgA levels possibly represents a beneficial effect of chronic physical training on immunity [20].
Upper respiratory tract infection during a critical training period may have a deleterious effect on the athlete’s ability to train and compete [20, 69].The increase in the incidence of URTI in athletes is still under debate in the literature. However, it is known that URTIs are the most common infection in highly trained athletes [67, 71]. Therefore, it is plausible to predict that chronic exercise training protocols that cause a reduction in IgA levels increase the incidence of URTI mostly in athletes than in the general sedentary population. In the present study, trained athletes present such a reduction of IgA levels. Interestingly, this response is unaffected in non-military trained individuals. Among the studies evaluated in the present review, only three simultaneously assessed the incidence of URTI and IgA levels. Despite of the few studies, the relationship between IgA levels and the incidence of URTI seems clear (Fig. 9). Even though no significant response was found, probably as a consequence of the small number of available studies, the sample of individuals (n = 125) is substantial. Therefore, to better establish this relationship between URTI incidence and IgA levels, a higher number of trials would be necessary.
Military training environment is a situation associate with several stressors such as intense physical training, energy deficit, psychological pressure, and sleep deprivation. The combination of these stressors enhances the susceptibility of detrimental effects on the immune system [79]. In the present study, military trained individuals show a greater reduction in IgA levels compared to non-military ones, probably due to an immune reaction to the combined actions of these stressors.
Small changes in IgG levels may be relevant for mucosal resistance to infections in the respiratory tract [7, 20]. The results of the meta-analysis indicate that chronic physical training does not alter baseline IgG levels. However, it should be noted that the number of studies and trials that carried out this type of evaluation was also small.
Exercise-induced immunomodulation appears to be dependent on the relationship between exercise intensity, duration and frequency [74]. As suggested by Nieman [53], the risk of upper tract infection is lower in the case of moderate-intensity exercise. This is supported by the concept that the relationship between the exercise load and the risk of URTI has the shape of the letter “J.” This means that both too little and too much physical activity may increase the risk of upper respiratory tract infection [51]. Therefore, while regular practice of moderate physical exercise can be considered as a tool to prevent respiratory infections, vigorous intensity physical exercise is usually associated with adverse events [31, 73]. Such scenario implies that the effect of physical exercise on immunoglobulin levels may take into account the subject’s physical training. In fact, elite athletes frequently report upper respiratory symptoms associated with decreased efficiency of humoral immune response on a mucosal level, manifesting predominantly as lowered secretory IgA levels [31].
In this context, the present study demonstrated that military trained individuals showed a greater reduction in IgA levels compared to non-military ones. We propose that this is probably due to the military training environment, which mimics a strenuous training load. Similarly, to elite athletes, who have an increased chance of URTIs associated with decreased IgA [31], this creates the possibility that military personnel may experience URTI symptoms as a result of greater infection susceptibility due to vigorous intensity physical exercise. Still, the issue of exercise load-related infection susceptibility requires further research.
Leandro et al. [33] state that exercise of long duration and/or intense exercise (> 2 h and/or > 80% of maximal oxygen uptake, VO2max) is associated with markers of immunosuppression, including reduced production of salivary IgA and plasma IgM and IgG. Therefore, long duration and/or intense exercise can make people more susceptible to infections (mainly URTI), which can increase the risk of contamination and worsening of COVID-19 symptoms [33]. COVID-19’s high morbidity and mortality is more prevalent in older people (> 60 years), but the health of a young and well-fit population or even athletes should also be noted since there are still many answers to be given in relation to COVID 19 [28]. Thus, understanding whether acute exercise or even chronic physical training increases the susceptibility to URTI or even COVID is very important. And for that, it is necessary that adequate guidance becomes available [28].
Although we found it interesting/important to compare gender and age, this was not possible in the present study, because the number of studies was insufficient for this analysis to be carried out. We point out that Fondell et al. [18], whose study included 1509 subjects divided into groups of men and women aged 20–60 years, found that physical activity reduces the incidence of upper respiratory tract illness in both gender, regardless of age. In another study, Akimoto et al. [1] demonstrated that regular physical training increases salivary IgA levels in healthy subjects (18 males and 27 females) over 60 years of age.
Conclusion
The present systematic review and meta-analysis indicates that acute exercise positively influences IgA levels in trained individuals, being this effect pronounced when a strenuous exercise such as ultramarathon is executed. Moreover, chronic physical training response over IgA levels seems to depend on the characteristics of the subject´s physical training since non-military personnel present unchanged IgA levels.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
All authors have contributed to the development of the research question and study design. LRD, HOC, FRD, and GMO developed the literature search. LRD, HOC, FRD, GMO, JGRPF, RPA, MCM, HFGL, LHRL, and CCC performed the study selection. LRD, HOC, and FRD analyzed the data. LRD, HOC, FRD, GMO, JGRPF, RPA, MCM, HFGL, LHRL, and CCC performed the study selection. LRD, HOC, and FRD interpret the results and wrote the manuscript. All authors reviewed and approved the manuscript.
Funding
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) and Universidade do Estado de Minas Gerais.
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Disclaimer
The funding institutions had no role in the study design, data analysis, decision to publish or preparation of the article.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Akimoto T, Kumai Y, Akama T, Hayashi E, Murakami H, Soma R, Kuno S, Kono I. Effects of 12 months of exercise training on salivary secretory IgA levels in elderly subjects. Br J Sports Med. 2003;37:76–79. doi: 10.1136/bjsm.37.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatia RT, Marwaha S, Malhotra A, Iqbal Z, Hughes C, Borjesson M, Niebauer J, Pelliccia A, Schmied C, Serratosa L, Papadakis M, Sharma S. Exercise in the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) era: a question and answer session with the experts endorsed by the section of Sports Cardiology & Exercise of the European Association of Preventive Cardiology (EAPC) Eur J Prev Cardiol. 2020;27:1242–1251. doi: 10.1177/2047487320930596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blume K, Korber N, Hoffmann D, Wolfarth B. Training load, immune status, and clinical outcomes in young athletes: a controlled, prospective, longitudinal study. Front Physiol. 2018;9:120. doi: 10.3389/fphys.2018.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borges GF, Rama L, Carvalho HM, Gaspar J, Santos A, Massart A, Gomes B, Minuzzi LG, Paiva A, Teixeira AM. Variation in plasma cytokine concentration during a training season in elite kayakers. J Sports Med Phys Fit. 2018;58:1519–1524. doi: 10.23736/s0022-4707.17.07202-4. [DOI] [PubMed] [Google Scholar]
- 5.Bosch JA, Ring C, de Geus EJ, Veerman EC, Amerongen AV. Stress and secretory immunity. Int Rev Neurobiol. 2002;52:213–253. doi: 10.1016/s0074-7742(02)52011-0. [DOI] [PubMed] [Google Scholar]
- 6.Brandtzaeg P. Role of secretory antibodies in the defence against infections. Intl J Med Microbiol: IJMM. 2003;293:3–15. doi: 10.1078/1438-4221-00241. [DOI] [PubMed] [Google Scholar]
- 7.Brandtzaeg P, Farstad IN, Haraldsen G. Regional specialization in the mucosal immune system: primed cells do not always home along the same track. Immunol Today. 1999;20:267–277. doi: 10.1016/s0167-5699(99)01468-1. [DOI] [PubMed] [Google Scholar]
- 8.Brisola GMP, Claus GM, Dutra YM, Malta ES, de Poli RAB, Esco MR, Zagatto AM. Effects of seasonal training load on performance and illness symptoms in water polo. J Strength Cond Res. 2020;34:406–413. doi: 10.1519/JSC.0000000000003358. [DOI] [PubMed] [Google Scholar]
- 9.Broadbent S. Seasonal changes in haematology, lymphocyte transferrin receptors and intracellular iron in Ironman triathletes and untrained men. Eur J Appl Physiol. 2011;111:93–100. doi: 10.1007/s00421-010-1635-z. [DOI] [PubMed] [Google Scholar]
- 10.Brunelli DT, Rodrigues A, Lopes WA, Gaspari AF, Bonganha V, Montagner PC, Borin JP, Cavaglieri CR. Monitoring of immunological parameters in adolescent basketball athletes during and after a sports season. J Sports Sci. 2014;32:1050–1059. doi: 10.1080/02640414.2013.878806. [DOI] [PubMed] [Google Scholar]
- 11.Canto E, Roca E, Perea L, Rodrigo-Troyano A, Suarez-Cuartin G, Giner J, Feliu A, Soria JM, Nescolarde L, Vidal S, Sibila O. Salivary immunity and lower respiratory tract infections in non-elite marathon runners. PLoS ONE. 2018;13:e0206059. doi: 10.1371/journal.pone.0206059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 13.Costa RJ, Jones GE, Lamb KL, Coleman R, Williams JH. The effects of a high carbohydrate diet on cortisol and salivary immunoglobulin A (s-IgA) during a period of increase exercise workload amongst Olympic and Ironman triathletes. Int J Sports Med. 2005;26:880–885. doi: 10.1055/s-2005-837467. [DOI] [PubMed] [Google Scholar]
- 14.Duggal NA, Niemiro G, Harridge SDR, Simpson RJ, Lord JM. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat Rev Immunol. 2019;19:563–572. doi: 10.1038/s41577-019-0177-9. [DOI] [PubMed] [Google Scholar]
- 15.Eda N, Ito H, Shimizu K, Suzuki S, Lee E, Akama T. Yoga stretching for improving salivary immune function and mental stress in middle-aged and older adults. J Women Aging. 2018;30:227–241. doi: 10.1080/08952841.2017.1295689. [DOI] [PubMed] [Google Scholar]
- 16.Fahlman MM, Engels HJ. Mucosal IgA and URTI in American college football players: A year longitudinal study. Med Sci Sports Exerc. 2005;37:374–380. doi: 10.1249/01.mss.0000155432.67020.88. [DOI] [PubMed] [Google Scholar]
- 17.Filaire E, Lac G, Pequignot JM. Biological, hormonal, and psychological parameters in professional soccer players throughout a competitive season. Percept Mot Skills. 2003;97:1061–1072. doi: 10.2466/pms.2003.97.3f.1061. [DOI] [PubMed] [Google Scholar]
- 18.Fondell E, Lagerros YT, Sundberg CJ, Lekander M, Balter O, Rothman KJ, Balter K. Physical activity, stress, and self-reported upper respiratory tract infection. Med Sci Sports Exerc. 2011;43:272–279. doi: 10.1249/MSS.0b013e3181edf108. [DOI] [PubMed] [Google Scholar]
- 19.Gleeson M, McDonald WA, Cripps AW, Pyne DB, Clancy RL, Fricker PA. The effect on immunity of long-term intensive training in elite swimmers. Clin Exp Immunol. 1995;102:210–216. doi: 10.1111/j.1365-2249.1995.tb06658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gleeson M, McDonald WA, Pyne DB, Clancy RL, Cripps AW, Francis JL, Fricker PA. Immune status and respiratory illness for elite swimmers during a 12-week training cycle. Int J Sports Med. 2000;21:302–307. doi: 10.1055/s-2000-313. [DOI] [PubMed] [Google Scholar]
- 21.Gleeson M, McFarlin B, Flynn M. Exercise and Toll-like receptors. Exerc Immunol Rev. 2006;12:34–53. [PubMed] [Google Scholar]
- 22.Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11:607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 23.Gleeson M, Bishop N, Oliveira M, McCauley T, Tauler P, Muhamad AS. Respiratory infection risk in athletes: association with antigen-stimulated IL-10 production and salivary IgA secretion. Scand J Med Sci Sports. 2012;22:410–417. doi: 10.1111/j.1600-0838.2010.01272.x. [DOI] [PubMed] [Google Scholar]
- 24.Gomez-Merino D, Drogou C, Chennaoui M, Tiollier E, Mathieu J, Guezennec CY. Effects of combined stress during intense training on cellular immunity, hormones and respiratory infections. NeuroImmunoModulation. 2005;12:164–172. doi: 10.1159/000084849. [DOI] [PubMed] [Google Scholar]
- 25.Grande AJ, Keogh J, Silva V, Scott AM. Exercise versus no exercise for the occurrence severity and duration of acute respiratory infections. Cochrane Database System Rev. 2020;4:CD010596. doi: 10.1002/14651858.CD010596.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guyatt AD, Oxman GE, Vist R, Kunz Y, Falck-Ytter P, Alonso-Coello H, Schunemann GW, GRADE Working Group (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336:924–926. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed]
- 27.Heath GW, Macera CA, Nieman DC. Exercise and upper respiratory tract infections. Is there a relationship? Sports Med. 1992;14:353–365. doi: 10.2165/00007256-199214060-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hull JH, Loosemore M, Schwellnus M. Respiratory health in athletes: facing the COVID-19 challenge. Lancet Respir Med. 2020;8:557–558. doi: 10.1016/S2213-2600(20)30175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ihalainen JK, Schumann M, Hakkinen K, Mero AA. Mucosal immunity and upper respiratory tract symptoms in recreational endurance runners. Appl Physiol Nutr Metab. 2016;41:96–102. doi: 10.1139/apnm-2015-0242. [DOI] [PubMed] [Google Scholar]
- 30.Kunz H, Bishop NC, Spielmann G, Pistillo M, Reed J, Ograjsek T, Park Y, Mehta SK, Pierson DL, Simpson RJ. Fitness level impacts salivary antimicrobial protein responses to a single bout of cycling exercise. Eur J Appl Physiol. 2015;115:1015–1027. doi: 10.1007/s00421-014-3082-8. [DOI] [PubMed] [Google Scholar]
- 31.Kurowski M, Seys S, Bonini M, Del Giacco S, Delgado L, Diamant Z, Kowalski ML, Moreira A, Rukhadze M, Couto M. Physical exercise, immune response, and susceptibility to infections-current knowledge and growing research areas. Allergy. 2022;77:2653–2664. doi: 10.1111/all.15328. [DOI] [PubMed] [Google Scholar]
- 32.Lamm ME. Interaction of antigens and antibodies at mucosal surfaces. Annu Rev Microbiol. 1997;51:311–340. doi: 10.1146/annurev.micro.51.1.311. [DOI] [PubMed] [Google Scholar]
- 33.Leandro CG, Ferreira ESWT, Lima-Silva AE. Covid-19 and Exercise-Induced Immunomodulation. NeuroImmunoModulation. 2020;27:75–78. doi: 10.1159/000508951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee E, Lim ST, Kim WN (2020) Aquatic exercise for improving immune function and mental stress in pre-frailty elderly women. J Women Aging:1–9. 10.1080/08952841.2020.1735287 [DOI] [PubMed]
- 35.Li TL, Cheng PY. Alterations of immunoendocrine responses during the recovery period after acute prolonged cycling. Eur J Appl Physiol. 2007;101:539–546. doi: 10.1007/s00421-007-0529-1. [DOI] [PubMed] [Google Scholar]
- 36.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 37.Libicz S, Mercier B, Bigou N, Le Gallais D, Castex F. Salivary IgA response of triathletes participating in the French Iron Tour. Int J Sports Med. 2006;27:389–394. doi: 10.1055/s-2005-865747. [DOI] [PubMed] [Google Scholar]
- 38.Mackinnon LT, Hooper S. Mucosal (secretory) immune system responses to exercise of varying intensity and during overtraining. Int J Sports Med. 1994;15(Suppl 3):S179–183. doi: 10.1055/s-2007-1021134. [DOI] [PubMed] [Google Scholar]
- 39.Mackinnon LT, Hooper SL. Plasma glutamine and upper respiratory tract infection during intensified training in swimmers. Med Sci Sports Exerc. 1996;28:285–290. doi: 10.1097/00005768-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 40.MacKinnon LT, Jenkins DG. Decreased salivary immunoglobulins after intense interval exercise before and after training. Med Sci Sports Exerc. 1993;25:678–683. doi: 10.1249/00005768-199306000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Martins RA, Cunha MR, Neves AP, Martins M, Teixeira-Verissimo M, Teixeira AM. Effects of aerobic conditioning on salivary IgA and plasma IgA, IgG and IgM in older men and women. Int J Sports Med. 2009;30:906–912. doi: 10.1055/s-0029-1237389. [DOI] [PubMed] [Google Scholar]
- 42.Mazanec MB, Nedrud JG, Kaetzel CS, Lamm ME. A three-tiered view of the role of IgA in mucosal defense. Immunol Today. 1993;14:430–435. doi: 10.1016/0167-5699(93)90245-G. [DOI] [PubMed] [Google Scholar]
- 43.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(264–269):W264. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 44.Moreira A, Arsati F, de Oliveira Lima-Arsati YB, de Freitas CG, de Araujo VC. Salivary immunoglobulin A responses in professional top-level futsal players. J Strength Cond Res. 2011;25:1932–1936. doi: 10.1519/JSC.0b013e3181e7fbc0. [DOI] [PubMed] [Google Scholar]
- 45.Moreira A, Arsati F, Lima-Arsati YBD, Simoes AC, de Araujo VC. Monitoring stress tolerance and occurrences of upper respiratory illness in basketball players by means of psychometric tools and salivary biomarkers. Stress Health. 2011;27:E166–E172. doi: 10.1002/smi.1354. [DOI] [Google Scholar]
- 46.Moreira A, de Moura NR, Coutts A, Costa EC, Kempton T, Aoki MS. Monitoring Internal training load and mucosal immune responses in futsal athletes. J Strength Cond Res. 2013;27:1253–1259. doi: 10.1519/JSC.0b013e3182653cdc. [DOI] [PubMed] [Google Scholar]
- 47.Murase Y, Shimizu K, Tanimura Y, Hanaoka Y, Watanabe K, Kono I, Miyakawa S. Salivary extracellular heat shock protein 70 (eHSP70) levels increase after 59 min of intense exercise and correlate with resting salivary secretory immunoglobulin A (SIgA) levels at rest. Cell Stress Chaperones. 2016;21:261–269. doi: 10.1007/s12192-015-0656-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nehlsen-Cannarella SL, Nieman DC, Fagoaga OR, Kelln WJ, Henson DA, Shannon M, Davis JM. Saliva immunoglobulins in elite women rowers. Eur J Appl Physiol. 2000;81:222–228. doi: 10.1007/s004210050034. [DOI] [PubMed] [Google Scholar]
- 49.Neville V, Gleeson M, Folland JP. Salivary IgA as a risk factor for upper respiratory infections in elite professional athletes. Med Sci Sports Exerc. 2008;40:1228–1236. doi: 10.1249/MSS.0b013e31816be9c3. [DOI] [PubMed] [Google Scholar]
- 50.Nickel T, Emslander I, Sisic Z, David R, Schmaderer C, Marx N, Schmidt-Trucksass A, Hoster E, Halle M, Weis M, Hanssen H. Modulation of dendritic cells and toll-like receptors by marathon running. Eur J Appl Physiol. 2012;112:1699–1708. doi: 10.1007/s00421-011-2140-8. [DOI] [PubMed] [Google Scholar]
- 51.Nieman DC. Exercise, infection, and immunity. Int J Sports Med. 1994;15(Suppl 3):S131–141. doi: 10.1055/s-2007-1021128. [DOI] [PubMed] [Google Scholar]
- 52.Nieman DC. Risk of upper respiratory tract infection in athletes: an epidemiologic and immunologic perspective. J Athl Train. 1997;32:344–349. [PMC free article] [PubMed] [Google Scholar]
- 53.Nieman DC. Exercise effects on systemic immunity. Immunol Cell Biol. 2000;78:496–501. doi: 10.1046/j.1440-1711.2000.00950.x. [DOI] [PubMed] [Google Scholar]
- 54.Nieman DC, Nehlsen-Cannarella SL. The effects of acute and chronic exercise of immunoglobulins. Sports Med. 1991;11:183–201. doi: 10.2165/00007256-199111030-00003. [DOI] [PubMed] [Google Scholar]
- 55.Nieman DC, Wentz LM. The compelling link between physical activity and the body’s defense system. J Sport Health Sci. 2019;8:201–217. doi: 10.1016/j.jshs.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nieman DC, Henson DA, Gusewitch G, Warren BJ, Dotson RC, Butterworth DE, Nehlsencannarella SL. Physical-activity and immune function in elderly women. Med Sci Sports Exerc. 1993;25:823–831. doi: 10.1249/00005768-199307000-00011. [DOI] [PubMed] [Google Scholar]
- 57.Nieman DC, Nehlsen-Cannarella SL, Fagoaga OR, Henson DA, Shannon M, Hjertman JM, Schmitt RL, Bolton MR, Austin MD, Schilling BK, Thorpe R. Immune function in female elite rowers and non-athletes. Br J Sports Med. 2000;34:181–187. doi: 10.1136/bjsm.34.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nieman DC, Dumke CI, Henson DA, McAnulty SR, McAnulty LS, Lind RH, Morrow JD. Immune and oxidative changes during and following the western states endurance run. Int J Sports Med. 2003;24:541–547. doi: 10.1055/s-2003-42018. [DOI] [PubMed] [Google Scholar]
- 59.Northoff H, Berg A. Immunologic mediators as parameters of the reaction to strenuous exercise. Int J Sports Med. 1991;12(Suppl 1):S9–15. doi: 10.1055/s-2007-1024743. [DOI] [PubMed] [Google Scholar]
- 60.Novas AMP, Rowbottom DG, Jenkins DG. Tennis, incidence of URTI and salivary IgA. Int J Sports Med. 2003;24:223–229. doi: 10.1055/s-2003-39096. [DOI] [PubMed] [Google Scholar]
- 61.Orysiak J, Witek K, Malczewska-Lenczowska J, Zembron-Lacny A, Pokrywka A, Sitkowski D. Upper Respiratory Tract Infection and mucosal immunity in young ice hockey players during the pretournament training period. J Strength Cond Res. 2019;33:3129–3135. doi: 10.1519/JSC.0000000000002557. [DOI] [PubMed] [Google Scholar]
- 62.Owen AL, del Wong P, Dunlop G, Groussard C, Kebsi W, Dellal A, Morgans R, Zouhal H. High-Intensity training and salivary immunoglobulin a responses in professional top-level soccer players: effect of training intensity. J Strength Cond Res. 2016;30:2460–2469. doi: 10.1519/JSC.0000000000000380. [DOI] [PubMed] [Google Scholar]
- 63.Pacque PF, Booth CK, Ball MJ, Dwyer DB. The effect of an ultra-endurance running race on mucosal and humoral immune function. J Sports Med Phys Fitness. 2007;47:496–501. [PubMed] [Google Scholar]
- 64.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hrobjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10:89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peake JM, Neubauer O, Walsh NP, Simpson RJ. Recovery of the immune system after exercise. J Appl Physiol. 2017;122:1077–1087. doi: 10.1152/japplphysiol.00622.2016. [DOI] [PubMed] [Google Scholar]
- 66.Pedersen BK, Kappel M, Klokker M, Nielsen HB, Secher NH. The immune system during exposure to extreme physiologic conditions. Int J Sports Med. 1994;15(Suppl 3):S116–121. doi: 10.1055/s-2007-1021125. [DOI] [PubMed] [Google Scholar]
- 67.Peters EM. Exercise, immunology and upper respiratory tract infections. Int J Sports Med. 1997;18(Suppl 1):S69–77. doi: 10.1055/s-2007-972702. [DOI] [PubMed] [Google Scholar]
- 68.Peters EM, Shaik J, Kleinveldt N. Upper respiratory tract infection symptoms in ultramarathon runners not related to immunoglobulin status. Clin J Sport Med : Off J Can Acad Sport Med. 2010;20:39–46. doi: 10.1097/JSM.0b013e3181cb4086. [DOI] [PubMed] [Google Scholar]
- 69.Pyne DB, Baker MS, Fricker PA, McDonald WA, Telford RD, Weidemann MJ. Effects of an intensive 12-wk training program by elite swimmers on neutrophil oxidative activity. Med Sci Sports Exerc. 1995;27:536–542. doi: 10.1249/00005768-199504000-00011. [DOI] [PubMed] [Google Scholar]
- 70.Rama L, Teixeira AM, Matos A, Borges G, Henriques A, Gleeson M, Pedreiro S, Filaire E, Alves F, Paiva A. Changes in natural killer cell subpopulations over a winter training season in elite swimmers. Eur J Appl Physiol. 2013;113:859–868. doi: 10.1007/s00421-012-2490-x. [DOI] [PubMed] [Google Scholar]
- 71.Roberts JA. Viral illnesses and sports performance. Sports Med. 1986;3:298–303. doi: 10.2165/00007256-198603040-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sari-Sarraf V, Reilly T, Doran DA. Salivary IgA response to intermittent and continuous exercise. Int J Sports Med. 2006;27:849–855. doi: 10.1055/s-2006-923777. [DOI] [PubMed] [Google Scholar]
- 73.Scheffer DDL, Latini A. Exercise-induced immune system response: anti-inflammatory status on peripheral and central organs. Biochim Biophys Acta. 2020;1866:165823. doi: 10.1016/j.bbadis.2020.165823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Simpson RJ, Campbell JP, Gleeson M, Kruger K, Nieman DC, Pyne DB, Turner JE, Walsh NP. Can exercise affect immune function to increase susceptibility to infection? Exerc Immunol Rev. 2020;26:8–22. [PubMed] [Google Scholar]
- 75.Steerenberg PA, vanAsperen IA, Amerongen AV, Biewenga J, Mol D, Medema G. Salivary levels of immunoglobulin A in triathletes. Eur J Oral Sci. 1997;105:305–309. doi: 10.1111/j.1600-0722.1997.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 76.Suzuki K (2019) Chronic Inflammation as an Immunological Abnormality and Effectiveness of Exercise. Biomolecules 910.3390/biom9060223 [DOI] [PMC free article] [PubMed]
- 77.Tharp GD. Basketball exercise and secretory immunoglobulin A. Eur J Appl Physiol. 1991;63:312–314. doi: 10.1007/BF00233868. [DOI] [PubMed] [Google Scholar]
- 78.Thomas M. The management of acute lower respiratory tract infection in adults in primary care. Primary Care Respir J : J Gen Pract Airways Group. 2000;9:4–7. doi: 10.1038/pcrj.2000.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tiollier E, Chennaoui M, Gomez-Merino D, Drogou C, Filaire E, Guezennec CY. Effect of a probiotics supplementation on respiratory infections and immune and hormonal parameters during intense military training. Mil Med. 2007;172:1006–1011. doi: 10.7205/milmed.172.9.1006. [DOI] [PubMed] [Google Scholar]
- 80.Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520. doi: 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Whitham M, Laing SJ, Dorrington M, Walters R, Dunklin S, Bland D, Bilzon JL, Walsh NP. The influence of an arduous military training program on immune function and upper respiratory tract infection incidence. Mil Med. 2006;171:703–709. doi: 10.7205/milmed.171.8.703. [DOI] [PubMed] [Google Scholar]
- 82.Yamauchi R, Shimizu K, Kimura F, Takemura M, Suzuki K, Akama T, Kono I, Akimoto T. Virus activation and immune function during intense training in rugby football players. Int J Sports Med. 2011;32:393–398. doi: 10.1055/s-0031-1271674. [DOI] [PubMed] [Google Scholar]
- 83.Yeo TJ. Sport and exercise during and beyond the COVID-19 pandemic. Eur J Prev Cardiol. 2020;27:1239–1241. doi: 10.1177/2047487320933260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zakovska A, Knechtle B, Chlibkova D, Milickova M, Rosemann T, Nikolaidis PT. The Effect of a 100-km ultra-marathon under freezing conditions on selected immunological and hematological parameters. Front Physiol. 2017;8:638. doi: 10.3389/fphys.2017.00638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.
Not applicable.