Abstract
Background:
Anti–IL-5 therapy is a potential treatment for patients with hypereosinophilic syndrome (HES), although its clinical efficacy is unclear.
Objective:
We sought to investigate the clinical efficacy and safety of mepolizumab versus placebo in patients with HES.
Methods:
This randomized, multicenter, double-blind, placebo-controlled, phase III trial was conducted across 39 centers in 13 countries. Eligible patients had FIP1L1-PDGFRA-negative HES, experienced 2 or more flares (worsening of HES-related symptoms or blood eosinophil count requiring therapeutic escalation) in the previous 12 months, and had a screening blood eosinophil count greater than or equal to 1000 cells/μL. Patients were randomized (1:1) to subcutaneous mepolizumab (300 mg) or placebo every 4 weeks for 32weeks, plus existing HES therapy. The primary outcome was the proportion of patients with 1 or more flares (worsening of HES-related symptoms necessitating therapy escalation or ≥2 courses of blinded rescue oral corticosteroids) during the study; in addition, patients who withdrew early from the study were counted as having a flare. Safety end points were also assessed.
Results:
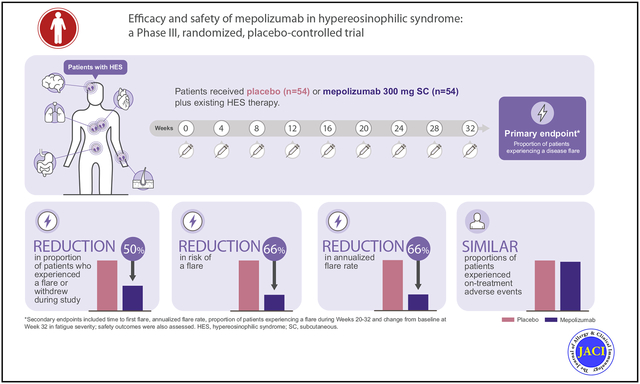
The proportion of patients experiencing 1 or more flares/withdrawing from the study was 50% lower with mepolizumab versus placebo (15 of 54 [28%] vs 30 of 54 [56%]; P = .002). Logistic regression analysis was consistent with the primary analysis (odds ratio, 0.28; 95% CI, 0.12–0.64; P = .003). Similar proportions of patients in the mepolizumab and placebo groups experienced on-treatment adverse events (48 of 54 [89%] vs 47 of 54 [87%]).
Conclusions:
Compared with placebo, mepolizumab significantly reduced the occurrence of flares in patients with HES, with no new safety signals identified.
Keywords: Hypereosinophilic syndrome, mepolizumab, flare, efficacy, safety
GRAPHICAL ABSTRACT
Hypereosinophilic syndrome (HES) is a rare group of disorders characterized by elevated eosinophil levels in blood and/or tissues.1 The clinical presentation of the disease is highly variable, but dermatological, pulmonary, gastrointestinal, and cardiovascular symptoms are frequently reported.1,2 The goal of treatment for patients with HES is the long-term reduction of blood and tissue eosinophil levels to reverse and prevent end-organ damage.3 With the exception of patients with imatinib-sensitive HES variants (including those associated with the FIP1-like-1-platelet-derived growth factor receptor a fusion gene [FIP1L1-PDGFRA]), the standard of care consists of glucocorticoids and cytotoxic/immunosuppressive therapy.3 However, these have variable efficacy and are often associated with significant morbidity and adverse side effects.2,4 The heterogeneous nature of the disease also makes clinical management challenging, with patients typically displaying different patterns of disease activity (eg, symptom worsening/relapse).5
IL-5 is a key regulator of eosinophil biology6; therefore, therapy directed against the IL-5 pathway has been explored as a potential treatment for patients with HES. Mepolizumab, a targeted, humanized mAb that selectively binds to IL-5, is approved for use in patients with eosinophilic diseases such as severe eosinophilic asthma and eosinophilic granulomatosis with polyangiitis.7,8 Previous clinical studies in patients with HES have shown reduced blood eosinophil counts and oral corticosteroid (OCS) sparing with mepolizumab treatment (750 mg administered intravenously).9–14 Although small open-label studies suggest that mepolizumab may reduce disease activity in HES, this has not been explored in the setting of a randomized, double-blind, placebo-controlled trial. The aim of this study was to investigate the clinical efficacy and safety of a mepolizumab dose (300 mg administered subcutaneously every 4 weeks), identified through blood eosinophil modeling, versus placebo in patients with FIP1L1-PDGFRA–negative HES.
METHODS
Trial design and procedures
This was a randomized, placebo-controlled, double-blind, parallel-group, multicenter, phase III trial (GlaxoSmithKline [GSK] ID: 200622; ClinicalTrials.gov No. NCT02836496) conducted at 39 sites across 13 countries (see this article’s Online Repository at www.jacionline.org). The trial protocol is available via the GSK Clinical Studies Register.15 After screening (1–4 weeks), patients were randomized (1:1) to receive mepolizumab (300 mg subcutaneous) or placebo every 4 weeks, plus their existing HES therapy, for 32 weeks (Fig 1, A). Baseline HES therapy dosing was maintained throughout the treatment period unless there was symptom worsening that required a dose increase. Where possible, patients who discontinued study treatment prematurely continued in the study until week 32.
FIG 1.
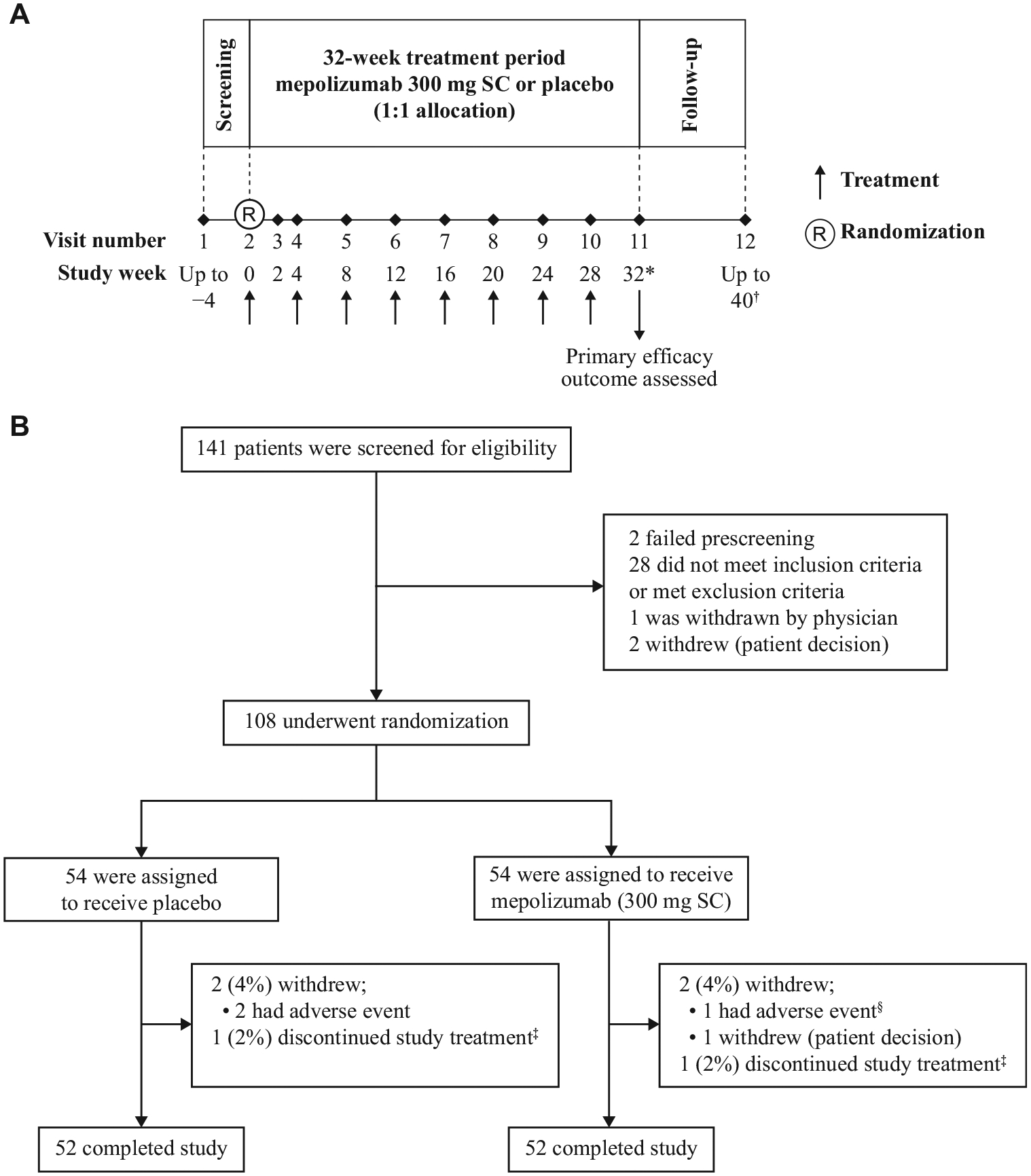
Study design and enrolment and follow-up of patients. A, The design of the study. B, The screening, enrolment, randomization, treatment, and follow-up of patients. *Following study completion, patients could be entered in an open-label extension (mepolizumab 300 mg SC, every 4 weeks; GSK study ID: 205203; ClinicalTrials.gov No. NCT03306043). Patients who continued with open-label mepolizumab had their last assessment at week 32. †Patients who did not continue with open-label mepolizumab had an additional 8-week follow-up period, concluding with a final visit 12 weeks after their last dose. ‡Two patients (1 in the placebo group, 1 in the mepolizumab group) discontinued treatment and remained in the study off-treatment until week 32. The patient in the placebo group discontinued owing to a lack of willingness to regularly fill out the eDiary; the patient in the mepolizumab group discontinued owing to patient/proxy decision. §The patient in the mepolizumab group who discontinued owing to AE experienced 4 serious AEs (HES flare, pneumonia, respiratory failure, and septic shock). These were fatal, and were not considered by the investigator to be treatment-related. SC, Subcutaneous.
The trial was conducted in accordance with the ethical principles of the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice guidelines, and applicable country-specific regulatory requirements. All patients provided written informed consent.
Patients
Enrolled patients were 12 years or older at screening, had received a diagnosis of FIP1L1-PDGFRA–negative HES 6 or more months previously, had uncontrolled HES (defined as a history of ≥2 flares within the past 12 months and a blood eosinophil count ≥1000 cells/μL at screening), and had been receiving stable background HES therapy (which could include, but was not limited to, OCS, immunosuppressive, and/or cytotoxic therapy) for 4 or more weeks before and including randomization. HES diagnosis was based on organ system involvement and/or dysfunction that could be directly related to a blood eosinophil count more than 1500 cells/μL on 2 or more occasions, and/or tissue eosinophilia, without a discernible secondary cause.16 Historical flares were defined as a worsening of HES-related clinical symptoms or a blood eosinophil count requiring an escalation in therapy; 1 or more flares within the past 12 months had to be unrelated to a decrease in HES therapy in the preceding 4 weeks. All patients required OCS/cytotoxic/immunosuppressive therapy for HES flares in the 12 months before screening. Patients with life-threatening HES or life-threatening HES comorbidities were excluded. Additional information regarding inclusion/exclusion criteria and withdrawal/stopping criteria is provided in this article’s Online Repository at www.jacionline.org.
Randomization and masking
Randomization was performed using an interactive response system. The randomization sequence was computer-generated centrally using a permuted block design of block size 4 and stratified by region (United States; Argentina, Mexico, and Brazil; rest of world). Mepolizumab and placebo were prepared by staff aware of the trial group assignments but not involved in trial assessments; preparations were identical in appearance and administered in a blinded fashion. Investigators, GSK staff involved in the study, and patients were blinded to study treatment and results of absolute blood eosinophil counts, total white blood cell counts, and white blood cell differentials; blinding was maintained for the full trial duration. Separate GSK staff who were unblinded to blood eosinophil counts (but not involved in other aspects of the trial) monitored blood eosinophil counts throughout the study and initiated rescue blinded OCS treatment if necessary (see below and this article’s Online Repository at www.jacionline.org).
Outcomes
The primary end point was the proportion of patients who experienced a flare during the 32-week study period. An HES flare was defined as either (a) an HES-related clinical manifestation, based on a physician-documented change in clinical signs or symptoms, necessitating an increase in the maintenance OCS dose by greater than or equal to 10 mg prednisone equivalent/day for 5 days or an increase in/addition of any cytotoxic and/or immunosuppressive HES therapy; or (b) receipt of 2 or more courses of blinded OCS during the treatment period. An increase in blood eosinophil count above the predefined threshold level (in the absence of a clinical manifestation requiring an escalation of HES therapy by the investigator) led to the administration of blinded OCS treatment for approximately 2 weeks after which blood eosinophil count was reassessed. Blinded OCS was discontinued if this blood eosinophil count was no longer above the predefined threshold, or a new blinded OCS course was started if it remained above the predefined threshold (in the absence of a clinical manifestation requiring an escalation of HES therapy by the investigator since the first blinded OCS course). When a patient received a second course of blinded OCS during the 32-week treatment period, the patient was considered to be experiencing a flare according to definition (b). To be considered a discrete flare, the onset date of a new flare must have been at least 14 days after the resolution of the most recent flare.
Secondary end points were time to first flare (allowing assessment of the probability of first flare over time); the proportion of patients with a flare during weeks 20 to 32; the annualized rate of flares and change from baseline at week 32 in fatigue severity (assessed by daily completion of the Brief Fatigue Inventory [BFI] item 3 using an eDiary: BFI range 0–10; higher score indicates worse fatigue severity).17 Blood samples were taken at each visit to determine blood eosinophil counts. Safety assessments included monitoring for adverse events (AEs) and serious AEs, including systemic and local injection-site reactions. Immunogenicity was also assessed (see this article’s Online Repository at www.jacionline.org).
Statistical analysis
An initial sample size of 80 patients (40 per treatment arm) was estimated to provide at least 90% power to detect an absolute reduction of 38% (at a 2-sided significance level of .05) in the proportion of patients experiencing a flare during the study period, assuming the true proportion of patients experiencing a flare on placebo is 60%. Because there were little previous data to support this estimate of 60%, a preplanned blinded sample size reestimation was conducted using a noncomparative analysis; this permitted an increase in sample size if the blinded overall flare rate was less than 30%. Sample size reestimation based on noncomparative interim results has been shown to have a negligible effect on the type I error probability.18 Because the reestimation was blinded, only the overall event rate (proportion of patients with a physician-documented flare) was used for the reassessment. The sample size reestimation retained power to detect an absolute risk reduction of 38% and a relative risk of 0.28, subject to a maximum sample size of 60 per group (120 patients overall). The proportion of patients who had a flare was monitored. When 60 patients had been randomized, the estimated blinded overall proportion of patients who would have a flare by the end of the 32-week treatment period was between 25% and 27.5%, and the sample size (rounded to the nearest 10 patients) required to maintain 90% power was calculated to be 50 patients per arm; a decision was therefore made to increase the sample size to 50 patients per arm (100 patients overall).
Efficacy end points were assessed in the intent-to-treat population (all patients who were randomized; analyzed by randomized trial groups); safety end points were assessed in the safety population (all patients who were randomized and received ≥1 dose of mepolizumab or placebo; analyzed by treatment received for >50% of injections administered). Baseline OCS dose and region were included as stratification factors or covariates in all statistical analyses, unless otherwise stated. Data for patients continuing in the study after treatment discontinuation were included in efficacy analyses.
Proportions of patients experiencing a flare were analyzed using a Cochran-Mantel-Haenszel test, supplemented by a logistic regression model. Patients who withdrew prematurely from the study were included in the analysis as having a flare; sensitivity analyses to assess the impact of missing data were performed. Time to first flare was analyzed using a Cox proportional hazards regression model; cumulative event rates were calculated using the Kaplan-Meier method. The annualized rate of flares was analyzed using a negative binomial generalized linear model with a log link-function including the log of the observed time (as an offset variable). A post hoc subgroup analysis by baseline OCS dose (0 mg, >0-≤5 mg, >5-≤10 mg, >10 mg prednisone equivalent daily dose) was conducted for both the proportion of patients experiencing 1 or more flares and the rate of flares, using logistic regression and negative binomial generalized linear regression, respectively; owing to the small sample size in each subgroup, region was not included as a covariate in these analyses. Change from baseline in fatigue severity at week 32 was analyzed using a Wilcoxon rank-sum test with an additional stratification factor of baseline fatigue severity; patients with missing data were included in this analysis with the largest value observed for any patient. A supportive repeated-measures analysis in which missing data were assumed to be missing at random was also performed. For strong control of type I error, a hierarchical, closed testing procedure was used for primary and secondary end points. Reported P values are 2-sided and not adjusted for multiplicity. Further information on statistical methods is provided in this article’s Online Repository at www.jacionline.org.
RESULTS
Patient population
Patients were recruited from March 7, 2017, to October 18, 2018; follow-up continued until August 8, 2019. Overall, 141 patients were screened for eligibility and 108 were randomized, of whom 54 received mepolizumab and 54 received placebo (Fig 1, B). A total of 4 patients (2 per treatment group) withdrew from the study before week 32; 2 additional patients (1 per treatment group) discontinued treatment but continued in the study until week 32. Patients’ demographic and clinical characteristics are presented in Table I and Table E1 in this article’s Online Repository at www.jacionline.org.
TABLE I.
Baseline demographic and clinical characteristics in the intent-to-treat population*
| Characteristic | Placebo (N = 54) | Mepolizumab 300 mg SC (N = 54) |
|---|---|---|
| Age (y), mean (range) | 45.4 (15–80) | 46.6 (12–82) |
| Female sex, n (%) | 27 (50) | 30 (56) |
| BMI (kg/m2), mean ± SD | 26.20 ± 5.934 | 26.38 ± 5.885 |
| Duration of HES (y), mean ± SD | 5.66 ± 8.035 | 5.45 ± 5.079 |
| Baseline HES therapy, n (%) | ||
| Any | 49 (91) | 50 (93) |
| OCS | 38 (70) | 40 (74) |
| Prednisone ≤20 mg/d or equivalent | 37 (69) | 35 (65) |
| Prednisone >20 mg/d or equivalent | 1 (2) | 5 (9) |
| Cytotoxic/immunosuppressive therapy† | 9 (17) | 14 (26) |
| Other‡ | 19 (35) | 22 (41) |
| Not taking OCS or cytotoxic/immunosuppressive therapy | 14 (26) | 11 (20) |
| Prednisone equivalent daily dose (mg),§ median (range) | ||
| Patients with a baseline prednisone equivalent daily dose >0 mg only | 10.0 (3–25) | 10.0 (3–50) |
| All patients | 5.6 (0–25) | 5.6 (0–50) |
| Number of flares in 12 mo before screening, mean ± SD | 2.7 ± 1.02 | 2.7 ± 1.28 |
| Geometric mean ± SD of log blood eosinophil count§ (cells/μL) | 1350 ± 0.708 | 1460 ± 0.946 |
| Most bothersome HES-related symptoms,‖ n (%) | ||
| Breathing symptoms | 30 (56) | 30 (56) |
| Skin symptoms | 28 (52) | 25 (46) |
| Muscle or joint pain | 20 (37) | 24 (44) |
| Nasal or sinus symptoms | 19 (35) | 22 (41) |
| Abdominal pain or bloating | 24 (44) | 16 (30) |
| Chills or sweats | 5 (9) | 10 (19) |
BMI, Body mass index; SC, subcutaneous.
Statistical significance testing between groups was not performed.
Examples of cytotoxic/immunosuppressive therapy include but are not limited to hydroxyurea, cyclosporine, imatinib, methotrexate, tacrolimus, and azathioprine.
Examples of “other” HES therapy include but are not limited to beclometasone dipropionate, formoterol fumarate, omeprazole, salbutamol, tiotropium bromide, triamcinolone acetonide, and cetirizine.
Values of zero were replaced with 0.005 before log transformation.
As reported by patients; at baseline/randomization, patients reported up to 3 HES-related symptoms that they considered most bothersome.
Primary end point
Overall, 65 flares were reported in 42 patients during the study; most (50 of 65 [77%]) met flare definition (a) (physician-documented change in clinical signs or symptoms requiring OCS therapy escalation or an increase in/addition of cytotoxic and/or immunosuppressive therapy) (see Table E2 in this article’s Online Repository at www.jacionline.org; Fig 2, A). Three patients withdrew prematurely from the study before experiencing a flare. The proportion of patients who experienced 1 or more flares during the study or who withdrew from the study was 50% lower for patients receiving mepolizumab versus placebo (15 of 54 [28%] vs 30 of 54 [56%]; P = .002; Table II). Logistic regression analysis was consistent with the primary analysis (odds ratio, 0.28; 95% CI, 0.12–0.64; P = .003); similar results were obtained in sensitivity analyses to assess the impact of missing data (see Fig E1 in this article’s Online Repository at www.jacionline.org).
FIG 2.
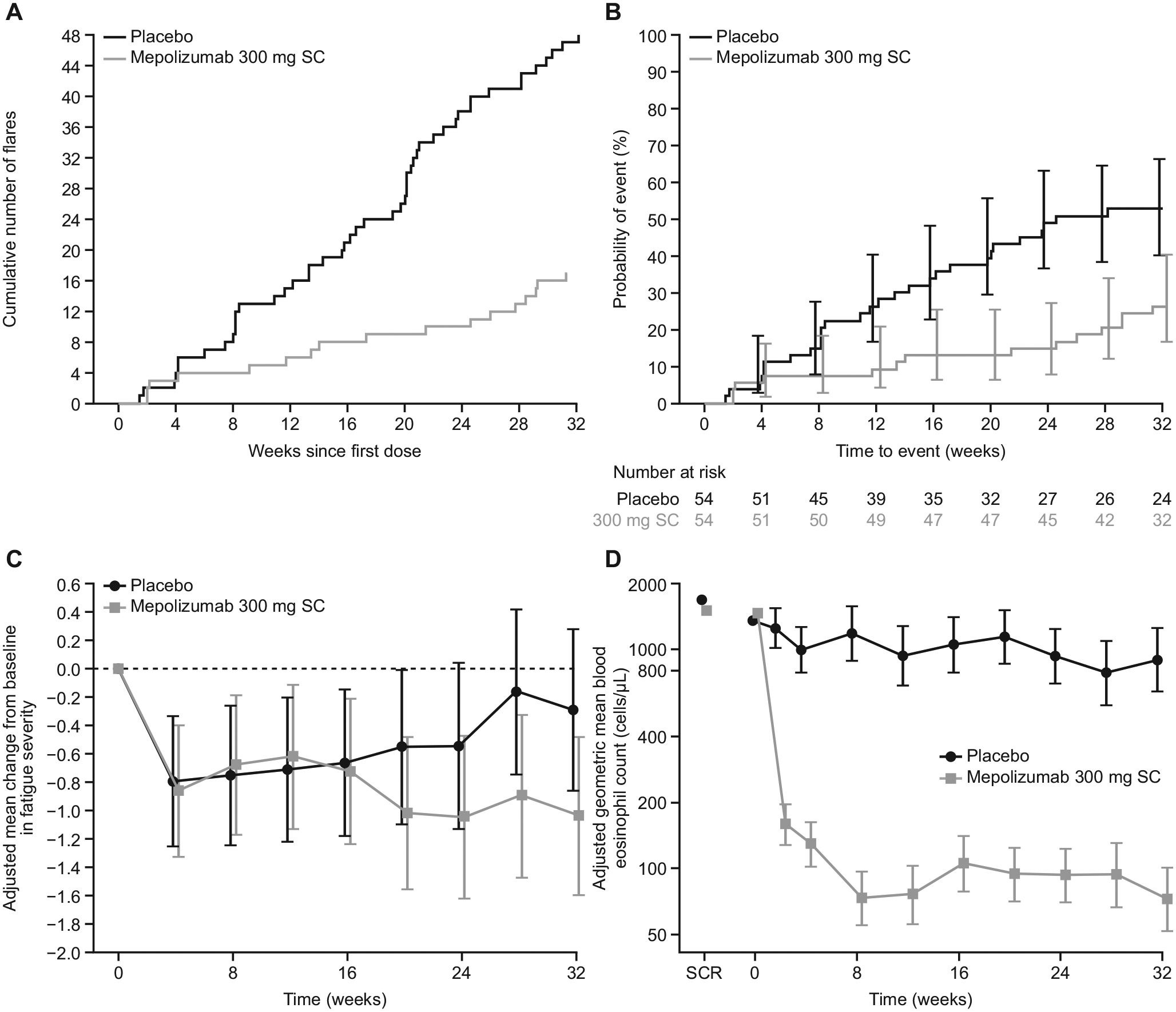
Flares, fatigue severity, and blood eosinophil counts in the intent-to-treat population. A, The cumulative number of flares over the study period. B, A Kaplan-Meier cumulative incidence curve for probability of first flare over time. C, Adjusted mean change from baseline in fatigue severity.* D, The adjusted geometric mean blood eosinophil count over time. Vertical bars in Fig 2, B, C, and D, represent 95% CI. *Fatigue severity assessed on the basis of BFI item 3 recorded daily; for each patient the mean score over the 7 days before each time point was analyzed (range 0–10; higher score indicates worse fatigue severity; minimal clinically important difference for patients in HES not determined). SC, Subcutaneous; SCR, screening.
TABLE II.
Summary of efficacy outcomes
| Primary end point | Placebo (N = 54) | Mepolizumab 300 mg SC (N = 54) | Odds ratio (95% CI) | P value (CMH/logistic regression) |
|---|---|---|---|---|
| Proportion of patients who experienced ≥1 HES flare during the 32-wk study period | ||||
| Patients who experienced ≥1 HES flare during the 32-wk study period or who withdrew from the study, n (%) | 30 (56) | 15 (28) | 0.28 (0.12–0.64) | .002/.003 |
| Patients who experienced ≥1 HES flare during the 32-wk study period, n (%) | 28 (52) | 14 (26) | — | — |
| Patients who withdrew from the study, n (%) | 2 (4) | 1 (2) | — | — |
| Secondary end point | Placebo (N = 54) | Mepolizumab 300 mg SC (N = 54) | Hazard ratio (95% CI) | P value |
| Time to first flare | ||||
| Probability of flare by week 32, % (95% CI) | 52.7 (40.1–66.5) | 26.3 (16.5–40.3) | 0.34 (0.18–0.67) | .002 |
| Secondary end point | Placebo (N = 54) | Mepolizumab 300 mg SC (N = 54) | Odds ratio (95% CI) | P value (CMH/logistic regression) |
| Proportion of patients who experienced ≥1 HES flare during weeks 20–32 | ||||
| Patients who experienced ≥1 HES flare during weeks 20–32 or who withdrew from the study, n (%) | 19 (35) | 9 (17) | 0.33 (0.13–0.85) | .02/.02 |
| Patients who experienced ≥1 HES flare during weeks 20–32, n (%) | 17 (31) | 7 (13) | — | — |
| Patients who withdrew from the study, n (%) | 2 (4) | 2 (4) | — | — |
| Secondary end point | Placebo (N = 54) | Mepolizumab 300 mg SC (N = 54) | Rate ratio (95% CI) | P value |
| Annualized rate of HES flares | ||||
| Adjusted mean rate of HES flares per year | 1.46 | 0.50 | 0.34 (0.19–0.63) | <.001 |
| Secondary end point | Placebo (N = 54) | Mepolizumab 300 mg SC (N = 54) | P value | |
| Change from baseline at week 32 in fatigue severity * | ||||
| Median change from baseline | 0.32 | −0.66 | — | .04 |
CMH, Cochran-Mantel-Haenszel test; SC, subcutaneous.
Based on BFI item 3 recorded daily; for each patient, the mean score over the 7 d before baseline and week 32 was analyzed (range 0–10; higher score indicates worse fatigue severity; minimal clinically important difference for patients in HES not determined); patients (7 placebo, 4 mepolizumab) with missing data were included in this analysis with the largest (ie, worst) value observed for any patient.
A similar benefit was seen in a post hoc analysis of the proportion of patients who experienced 1 or more flares meeting flare definition (a); the proportion of patients who experienced 1 or more flares meeting definition (a) was significantly lower in the mepolizumab group than in the placebo group (12 of 54 [22%] vs 23 of 54 [43%]; P = .024; odds ratio, 0.35; 95% CI, 0.15–0.85; P = .020). Owing to the small number of patients experiencing 1 or more flare meeting definition (b) (receipt of ≥2 courses of blinded OCS), statistical analysis of flares meeting definition (b) was not performed.
The post hoc subgroup analysis of the primary end point by baseline OCS dose showed a slight trend toward increasing mepolizumab benefit in flare occurrence compared with placebo with increasing baseline OCS dose category (see Fig E2, A, in this article’s Online Repository at www.jacionline.org).
Secondary end points
The Kaplan-Meier estimate of the probability of first flare over time is presented in Fig 2, B; the risk of experiencing a first flare during the treatment period was 66% lower for patients treated with mepolizumab versus placebo (hazard ratio: 0.34; 95% CI, 0.18–0.67; P = .002) (Table II). Fewer patients experienced a flare or withdrew from the study during weeks 20 to 32 when receiving mepolizumab compared with placebo (9 of 54 [17%] vs 19 of 54 [35%]; P = .02; odds ratio, 0.33; 95% CI, 0.13–0.85; Table II). The adjusted annualized rate of flares was also 66% lower with mepolizumab compared with placebo (0.50 vs 1.46 flares per year, respectively; P <.001) (Table II); results of the post hoc analysis by baseline OCS dose showed that this between-treatment difference was similar regardless of baseline OCS dose category (Fig E2, B). In addition, fatigue severity at week 32 improved with mepolizumab compared with placebo (median change, −0.66 vs 0.32, respectively; P = .04) (Table II; Fig 2, C).
Blood eosinophil count
At week 2, the geometric mean blood eosinophil count in patients receiving mepolizumab was markedly reduced compared with baseline (1460 cells/μL to 170 cells/μL). This reduction reached a nadir by week 8 and was sustained to week 32 (Fig 2, D; see Fig E3 in this article’s Online Repository at www.jacionline.org). At week 32, patients receiving mepolizumab had a 92% reduction from baseline in blood eosinophil count compared with those receiving placebo (least squares mean blood eosinophil counts at week 32: 70 and 900 cells/μL, respectively; see Table E3 in this article’s Online Repository at www.jacionline.org).
Safety
The proportion of patients who experienced on-treatment AEs was similar between groups (48 of 54 [89%] with mepolizumab and 47 of 54 [87%] with placebo; Table III). The most frequently reported AEs were bronchitis, diarrhea, headache, nasopharyngitis, pain in extremity, pruritis, rhinitis, and upper respiratory tract infection (Table III). AEs considered by the investigator to be drug-related were reported in 12 of 54 (22%) patients receiving mepolizumab and 7 of 54 (13%) of those receiving placebo (Table III). Local injection-site reactions occurred in 4 of 54 (7%) patients receiving mepolizumab and 2 of 54 (4%) of those receiving placebo (Table III). On-treatment serious AEs occurred in 10 of 54 (19%) patients receiving mepolizumab and 8 of 54 (15%) of those receiving placebo; none were considered drug-related (Table III; see Table E4 in this article’s Online Repository at www.jacionline.org). One death was reported in the mepolizumab group (owing to HES flare, pneumonia, respiratory failure, and septic shock), which was not considered by the investigator to be treatment-related (see this article’s Online Repository at www.jacionline.org). One patient receiving mepolizumab had positive antimepolizumab antibodies at week 32; however, these were not neutralizing.
TABLE III.
Summary of AEs
| AEs | Placebo (N = 54) | Mepolizumab 300 mg SC (N = 54) |
|---|---|---|
| Event | ||
| All AEs | 47 (87) | 48 (89) |
| Any on-treatment event | 47 (87) | 48 (89) |
| Drug-related event* | 7 (13) | 12 (22) |
| Leading to treatment discontinuation | 2 (4) | 0 |
| Leading to study withdrawal | 2 (4) | 1 (2) |
| All SAEs | 9 (17) | 10 (19) |
| Any on-treatment event | 8 (15) | 10 (19) |
| Drug-related event* | 0 | 0 |
| Fatal | 0 | 1 (2)† |
| Anaphylaxis | 1 (2)‡ | 0 |
| On-treatment systemic or local injection-site reactions | ||
| Systemic reactions§ | 0 | 1 (2) |
| Local injection-site reactions‖ | 2 (4) | 4 (7) |
| Malignancies¶ | 1 (2) | 0 |
| Most common on-treatment AEs# | ||
| Bronchitis | 10 (19) | 8 (15) |
| Diarrhea | 7 (13) | 5 (9) |
| Headache | 7 (13) | 7 (13) |
| Nasopharyngitis | 7 (13) | 7 (13) |
| Pain in extremity | 2 (4) | 6 (11) |
| Pruritis | 7 (13) | 4 (7) |
| Rhinitis | 6 (11) | 5 (9) |
| Upper respiratory tract infection | 2 (4) | 8 (15) |
| On-treatment cardiovascular AEs** | ||
| Arrhythmia | 0 | 1 (2) |
| Atrial fibrillation | 1 (2) | 0 |
| Bundle branch block left | 0 | 1 (2) |
| Palpitations | 0 | 2 (4) |
| Restrictive cardiomyopathy | 1 (2) | 0 |
| Tachycardia | 1 (2) | 0 |
eCRF, Electronic case report form; MedDRA, Medical Dictionary for Regulatory Activities; SAE, serious adverse event; SC, subcutaneous.
Values are n (%).
Statistical significance testing between groups was not performed.
Status assigned by the investigators while masked to the treatment group.
One fatality was reported (owing to HES, pneumonia, respiratory failure, and septic shock), but this was not considered related to treatment.
The investigator did not consider this event to represent a systemic reaction meeting Sampson’s criteria for anaphylaxis.
Identified by the investigator in the eCRF designed to collect data on systemic reactions.
Identified by the investigator in the eCRF designed to collect data on local injection-site reactions.
Identified from prespecified standardized MedDRA queries.
Reported in ≥10% of the patients in any treatment group.
AEs in the Cardiac Disorders system organ class.
DISCUSSION
This randomized, placebo-controlled, phase III study demonstrated that treatment with mepolizumab (300 mg subcutaneous) was associated with a 50% reduction in the proportion of patients with HES who experienced 1 or more flares during the 32-week treatment period, compared with placebo. Patients included in the study had experienced 2 or more flares requiring an escalation in treatment in the 12 months before the study and had blood eosinophil counts of greater than or equal to 1000 cells/μL at baseline, indicating uncontrolled disease. Consistent with the previous clinical trial of intravenous mepolizumab in patients with HES, the frequencies of AEs were generally similar between patients receiving mepolizumab and placebo,11 and the types of AEs reported in patients receiving mepolizumab treatment were also similar to those previously reported.11,14
Intravenous administration of mepolizumab (750 mg) has been shown to be associated with reduced OCS use in patients with HES,11,13,14 and improvements in symptoms such as pruritis, skin lesions, nasal congestion, and polyposis and dysphagia.9,10 However, given the heterogeneous nature of the disease, identification of a suitable end point for assessing the impact of treatment on disease activity is challenging. For this reason, we focused on flares, using a definition that captured changes in signs or symptoms necessitating an increase in standard therapy and/or a sustained increase in blood eosinophil counts (both of which indicate a lack of disease control). Although it could be argued that inclusion of an increase in blood eosinophil count as part of the flare definition could potentially favor mepolizumab given its mode of action, approximately three-quarters of reported flares in our study were identified by physicians on the basis of a change in clinical signs or symptoms resulting in an increase in therapy. For each definition (a) flare, the physician documented the symptoms that were present and provided a narrative; all such data were consistent with an HES flare. The clinical signs and symptoms reported during these flares varied between patients, but included dermatologic, gastrointestinal, respiratory, and neurologic symptoms, as well as pain and fatigue. It is also worth noting that although flares have not been used previously as a formal outcome measure in HES, the flare definition we used allowed investigators to capture the worsening in clinical signs and symptoms across a broad range of organ systems typically seen by physicians treating patients with HES in the clinic. Moreover, these flares could occur at any time during the study, whereas definition (b) flares, which were based on an increase in blood eosinophil count, were detected at treatment visits. The assessment of worsening based on a clinical need for an increase in treatment is similar to the efficacy end point used in mepolizumab trials for other eosinophil-mediated conditions. For example, in severe eosinophilic asthma, clinically significant exacerbations were defined as a worsening of asthma requiring the use of OCS, hospital admission, or emergency department visit,19 and in eosinophilic granulomatosis with polyangiitis, relapse was defined as active vasculitis, asthma symptoms, or nasal and/or sinus disease leading to an increase in OCS or immunosuppressive therapy or hospitalization.20
Despite heterogeneous organ involvement in patients with HES, fatigue has been reported as a sign or symptom in several studies and improves with mepolizumab treatment.2,9 Consistent with this, we observed an improvement in fatigue severity (based on results from BFI item 3) compared with baseline in patients receiving mepolizumab versus placebo. Use of the BFI in patients with HES has not been reported previously; therefore, the minimal clinically important difference in this patient population has not been defined. Nonetheless, the mitigation of fatigue symptoms may be an important patient-reported benefit in patients receiving novel treatment options for HES.
Mepolizumab treatment was also associated with a 92% reduction from baseline in blood eosinophil count compared with placebo, which is similar to earlier clinical studies of mepolizumab in patients with HES.10,11,13,14 Because increased blood eosinophil count is a surrogate marker for tissue eosinophilia and organ damage in patients with HES, the substantial reduction in blood eosinophil count that we observed with mepolizumab is a relevant finding. Taken together with the observation that patients receiving mepolizumab experienced fewer flares than those receiving placebo, our results suggest that mepolizumab would likely be beneficial to patients with uncontrolled HES, particularly because this would likely lead to a reduced need for OCS/cytotoxic/immunosuppressive therapy. Although a post hoc subgroup analysis of the proportion of patients who experienced 1 or more flares by baseline OCS dose suggested a slight trend toward a greater mepolizumab treatment effect with increasing OCS dose category, there was no evidence of a difference in treatment effect by baseline OCS dose category when analyzing the rate of flares. As such, our data suggest that mepolizumab is an important treatment option for patients with uncontrolled HES, regardless of their prior maintenance dose of OCS.
There are several study limitations to consider. First, the eligibility criteria were broad and although most patients were receiving chronic OCS or immunosuppressant therapy at baseline, some patients were only treated as needed in the event of a flare to avoid the long-term toxicity associated with standard of care maintenance therapy. Second, all patients had a blood eosinophil count greater than or equal to 1000 cells/μL at screening, although this requirement may not be appropriate in clinical practice because patients’ existing therapy may have already reduced blood eosinophil counts to below this threshold. Third, one of the definitions used to identify flares was based on an increase in the maintenance OCS dose by greater than or equal to 10 mg/day for 5 days. It is possible that there may have been instances where the OCS dose increased but not enough to have been counted as a flare; as a result, the number of flares may have been underestimated in both treatment arms. Finally, the treatment period was limited to 32 weeks, because physicians were not comfortable with blood eosinophil count blinding for a longer time period. However, the ongoing open-label extension (ClinicalTrials.gov No. NCT03306043) will provide longer-term information (up to 52 weeks) on the efficacy and safety of mepolizumab in patients with HES. In addition, results from a compassionate use program in patients with HES indicate that long-term treatment (up to 11 years) is well tolerated in those who are nonresponsive or intolerant to other therapies.21
Conclusions
Mepolizumab is the first treatment shown to reduce disease flares in patients with FIP1L1-PDGFRA–negative HES, with no new safety signals identified. As such, the findings from this study represent an important advance for the management of this rare, debilitating disease.
Supplementary Material
Clinical implications:
Mepolizumab is the first treatment shown to reduce disease flares in patients with FIP1L1-PDGFRA–negative HES. These findings represent an important advance for the management of this rare, debilitating disease.
Disclosure of potential conflict of interest:
F. Roufosse reports consultancy fees from AstraZeneca, GlaxoSmithKline (GSK), and Knopp Biosciences. J.-E. Kahn reports consulting fees for advisory boards from AstraZeneca and GSK, research funding from AstraZeneca and GSK, and participation in clinical trials sponsored by AstraZeneca. M. E. Rothenberg is a consultant for Allakos, Arena Pharmaceuticals, AstraZeneca, Serpin Pharma, and Celgene; owns stocks/shares in ClostrBio, PulmOne Advanced Medical Devices, Serpin Pharma, and Spoon Guru; has received royalties from reslizumab (Teva Pharmaceuticals), PEESSv2 (Mapi Research Trust), and UpToDate; and is an inventor of patents owned by Cincinnati Children’s Hospital Medical Center. A. J. Wardlaw reports fees for participation in advisory boards from GSK, and participation in clinical trials sponsored by AstraZeneca, GSK, and Pulmocide. A. D. Klion reports funding to cover time reviewing the trial protocol and results from the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. S. Y. Kirby was an employee of GSK when this research was conducted and holds stocks/shares in GSK. E. S. Bradford is a former employee of GSK and is currently employed by Aeglea BioTherapeutics, Austin, Texas, and has stocks/shares in both companies. M. J. Gilson, J. H. Bentley, S. W. Yancey, and J. Steinfeld are all employees of GSK and own stocks/shares. G. J. Gleich is currently an employee of NexEos Diagnostics; has acted as a consultant for Genentech, GSK, and Knopp Biosciences; has received royalties from the Mayo Foundation; and has a royalty sharing agreement with Teva.
This study was funded by GlaxoSmithKline (GSK study ID: 200622; NCT02836496). Editorial support (in the form of writing assistance, including development of the initial draft based on author direction, assembling tables and figures, collating authors’ comments, grammatical editing, and referencing) was provided by Laura Gardner, PhD, of Fishawack Indicia Ltd, UK, and was funded by GSK.
We thank the patients who participated in the trials, the trial staff, and Laura Gardner, PhD, of Fishawack Indicia Ltd, UK, for editorial support (in the form of writing assistance, including development of the initial draft based on author direction, assembling tables and figures, collating authors’ comments, grammatical editing, and referencing; funded by GlaxoSmithKline).
Abbreviations used
- AE
Adverse event
- BFI
Brief Fatigue Inventory
- FIP1L1-PDGFRA
FIP1-like-1-platelet-derived growth factor receptor α fusion gene
- GSK
GlaxoSmithKline
- HES
Hypereosinophilic syndrome
- OCS
Oral corticosteroid
Footnotes
The CrossMark symbol notifies online readers when updates have been made to the article such as errata or minor corrections
Data sharing statement:
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com. The trial protocol is available at https://www.gsk-studyregister.com/en/.
REFERENCES
- 1.Curtis C, Ogbogu P. Hypereosinophilic syndrome. Clin Rev Allergy Immunol 2016;50:240–51. [DOI] [PubMed] [Google Scholar]
- 2.Ogbogu PU, Bochner BS, Butterfield JH, Gleich GJ, Huss-Marp J, Kahn JE, et al. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol 2009;124: 1319–25.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shomali W, Gotlib J. World Health Organization-defined eosinophilic disorders: 2019 update on diagnosis, risk stratification, and management. Am J Hematol 2019;94:1149–67. [DOI] [PubMed] [Google Scholar]
- 4.Whitehouse MW. Anti-inflammatory glucocorticoid drugs: reflections after 60 years. Inflammopharmacology 2011;19:1–19. [DOI] [PubMed] [Google Scholar]
- 5.Kahn JE, Groh M, Lefevre G. (A critical appraisal of) classification of hypereosinophilic disorders. Front Med (Lausanne) 2017;4:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menzella F, Lusuardi M, Galeone C, Taddei S, Zucchi L. Profile of anti-IL-5 mAb mepolizumab in the treatment of severe refractory asthma and hypereosinophilic diseases. J Asthma Allergy 2015;8:105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mepolizumab (NUCALA) highlights of prescribing information. 2019. Available at: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Nucala/pdf/NUCALA-PI-PIL.PDF. Accessed February 24, 2020.
- 8.Mepolizumab (NUCALA) summary of product characteristics. 2019. Available at: https://www.ema.europa.eu/en/documents/product-information/nucala-epar-product-information_en.pdf. Accessed February 24, 2020.
- 9.Plotz SG, Simon HU, Darsow U, Simon D, Vassina E, Yousefi S, et al. Use of an anti-interleukin-5 antibody in the hypereosinophilic syndrome with eosinophilic dermatitis. N Engl J Med 2003;349:2334–9. [DOI] [PubMed] [Google Scholar]
- 10.Garrett JK, Jameson SC, Thomson B, Collins MH, Wagoner LE, Freese DK, et al. Anti-interleukin-5 (mepolizumab) therapy for hypereosinophilic syndromes. J Allergy Clin Immunol 2004;113:115–9. [DOI] [PubMed] [Google Scholar]
- 11.Rothenberg ME, Klion AD, Roufosse FE, Kahn JE, Weller PF, Simon HU, et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med 2008;358:1215–28. [DOI] [PubMed] [Google Scholar]
- 12.Stein ML, Villanueva JM, Buckmeier BK, Yamada Y, Filipovich AH, Assa’ad AH, et al. Anti-IL-5 (mepolizumab) therapy reduces eosinophil activation ex vivo and increases IL-5 and IL-5 receptor levels. J Allergy Clin Immunol 2008;121:1473–83, 483. e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roufosse F, de Lavareille A, Schandene L, Cogan E, Georgelas A, Wagner L, et al. Mepolizumab as a corticosteroid-sparing agent in lymphocytic variant hypereosinophilic syndrome. J Allergy Clin Immunol 2010;126:828–35.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roufosse FE, Kahn JE, Gleich GJ, Schwartz LB, Singh AD, Rosenwasser LJ, et al. Long-term safety of mepolizumab for the treatment of hypereosinophilic syndromes. J Allergy Clin Immunol 2013;131:461–7.e1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.GSK Clinical Studies Register 2020. Available at: https://www.gsk-studyregister.com/study/5185. Accessed January 17, 2020.
- 16.Valent P, Klion AD, Horny HP, Roufosse F, Gotlib J, Weller PF, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol 2012;130: 607–12.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer 1999;85:1186–96. [DOI] [PubMed] [Google Scholar]
- 18.Kieser M, Friede T. Simple procedures for blinded sample size adjustment that do not affect the type I error rate. Stat Med 2003;22:3571–81. [DOI] [PubMed] [Google Scholar]
- 19.Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 2012;380:651–9. [DOI] [PubMed] [Google Scholar]
- 20.Wechsler ME, Akuthota P, Jayne D, Khoury P, Klion A, Langford CA, et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med 2017;376:1921–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duncan EA, Ortega H, Gleich G, Price R, Yancey S, Klion A. Observational experience describing the use of mepolizumab in patients with hypereosinophilic syndrome. Am J Respir Crit Care 2015;191:A1365. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com. The trial protocol is available at https://www.gsk-studyregister.com/en/.


