Abstract
In the SoUth Korean study to PrEvent cognitive impaiRment and protect BRAIN health through lifestyle intervention in at-risk elderly people (SUPERBRAIN), we evaluated the impact of a 24-week facility-based multidomain intervention (FMI) and home-based MI (HMI) on cortical thickness, brain volume, and the serum brain-derived neurotrophic factor (BDNF). Totally, 152 participants, aged 60–79 years without dementia but with ≥ 1 modifiable dementia risk factor, were randomly assigned to the FMI, HMI, or control groups. Among them, 55 participants (20 FMI, 19 HMI, and 16 controls) underwent brain MRI at baseline and 24 weeks. We compared changes in global/regional mean cortical thickness at the region-of-interest (ROI) between the intervention and control groups. The changes in the total cortical gray matter volume and global mean cortical thickness were compared using analysis of covariance with age, sex, and education as covariates. ComBat site harmonization was applied for cortical thickness values across the scanners. ROI-based analysis was controlled for multiple comparisons, with a false discovery rate threshold of p < 0.05. Serum BDNF levels were significantly higher in the FMI group than in the control group (p = 0.029). Compared with the control group, the mean global cortical thickness increased in the FMI group (0.033 ± 0.070 vs. − 0.003 ± 0.040, p = 0.013); particularly, cortical thickness of the bilateral frontotemporal lobes, cingulate gyri, and insula increased. The increase in cortical thickness and serum BDNF in the FMI group suggests that group preventive strategies at the facility may be beneficial through structural neuroplastic changes in brain areas, which facilitates learning and neurotrophic factors.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13311-022-01276-x.
Keywords: Cortical thickness, Dementia, Prevention, Lifestyle, Intervention
Introduction
Recent studies have shown that lifestyle modifications can prevent the occurrence of dementia in at-risk individuals [1–3]. The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), which included dietary counseling, physical exercise, cognitive training, and vascular and metabolic risk monitoring, was the first large randomized controlled trial to report beneficial effects on cognition for a 2-year multidomain lifestyle intervention among older individuals with an increased risk of dementia [4]. Although the French Multidomain Alzheimer Preventive Trial (MAPT) [5] and the Dutch Prevention of Dementia by Intensive Vascular Care (PreDIVA) trial [6] reported a lack of effect on the primary outcomes, exploratory subgroup analyses in both studies have provided evidence that interventions yielded cognitive benefits in subpopulations of participants with increased risk of dementia [6, 7]. Furthermore, the SoUth Korean study to PrEvent cognitive impaiRment and protect BRAIN health through lifestyle intervention in at-risk elderly people (SUPERBRAIN) proved for the first time that the multidomain intervention based on the FINGER study, modified according to the culture and circumstances of the other country, is feasible and may be effective [8–10].
In contrast to the cognitive benefits, the effects of multidomain lifestyle interventions on neural substrates underling the cognitive benefits are still not fully clear. The possible mechanisms of dementia prevention in at-risk individuals by multidomain lifestyle intervention involve enhancing or maintaining cognitive reserve as well as risk reduction of potentially modifiable risk factors [1]. Cognitive reserve is related to the efficiency of the brain functions and its neural substrate is referred to brain volume, the intensity of cerebral metabolism, or the connectivity of neural networks such as density of synapses and dendrites branching [11]. Therefore, enhanced or maintained cognitive reserve by multidomain lifestyle interventions could be evaluated by changes in structural or functional brain imagings or concentration of neurotrophic factors such as the brain-derived neurotrophic factor (BDNF). As such, physical activity has been associated with slower rates of cognitive decline and lower risk of dementia [12], and with increased levels of peripheral serum or plasma BDNF [13], and social interactions [14] and environmental enrichment [15] improve memory deficits in AD-like animal models through BDNF-dependent hippocampal neurogenesis, the exploratory analysis of the SUPERBRAIN [10] also showed that serum BDNF levels were significantly increased in the facility-based multidomain intervention (FMI) compared to the control group. However, benefits on cortical thickness or brain volumes of multidomain lifestyle interventions remain unclear.
A few lifestyle-based trials have so far included brain MRI markers for cortical thickness, or brain volumes. While randomized controlled trials assessing 6- to 24-month single-domain lifestyle interventions including physical activity [16, 17], a multimodal social engagement program [18], or nutrition-related interventions [19, 20] have reported promising effects on various gray matter measures including Alzheimer’s disease (AD) signature regions such as entorhinal, inferior temporal, middle temporal, and fusiform regions on MRI. However, the FINGER MRI exploratory sub-study did not show significant differences between the intervention and control groups in terms of changes in brain volumes or regional cortical thickness including AD signature regions after 2 years in at-risk elderly individuals without substantial impairment [21].
In this study, we aimed to evaluate the impact of a 6-month multidomain lifestyle intervention on the neural substrates related to the cognitive reserve through exploratory analyses of changes in global and regional cortical thickness, brain volumes, and the serum BDNF in the SUPERBRAIN.
Methods
Study Population
The SUPERBRAIN trial protocol (ClinicalTrials. gov: NCT03980392) [8] and primary findings [10] have been previously described in detail. In brief, this study was a 24-week, multicenter, outcome assessor-blinded, randomized controlled trial with a three-parallel-arm design performed in three hospitals and five public health centers across South Korea. The facility-based multidomain intervention (FMI) and home-based multidomain intervention (HMI) groups were the two experimental arms, and the control group was used as a comparator. The participants were 152 individuals aged 60–79 years who had no dementia and one or more modifiable dementia risk factors such as hypertension [22], diabetes mellitus [23], dyslipidemia [24], smoking [25], obesity [26], abdominal obesity [27], metabolic syndrome [28], educational level of ≤ 9 years, social isolation [8], and physical inactivity [8]. In addition, they had a Mini-Mental State Examination [29] z score of ≥ − 1.5, were able to perform independent activities of daily living and pass a literacy test [30], and had a reliable informant who can provide investigators with the requested information. Individuals with dementia, conditions affecting safe participation or cooperation, or concurrent participation in another trial were excluded. This study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice Guidelines. The institutional review boards of all institutions approved the protocol and consent forms before the start of the study. Written informed consent was obtained from all potential participants by a doctor before enrollment in the study.
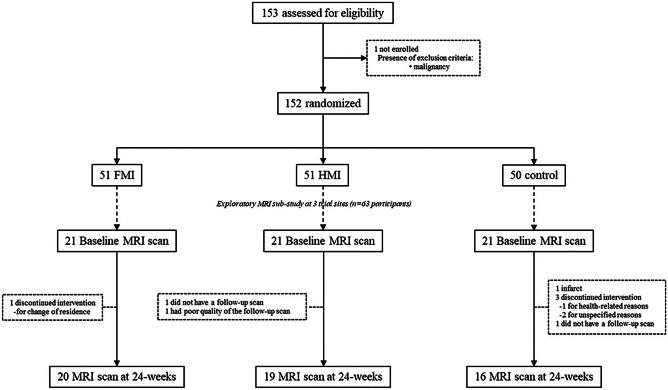
The SUPERBRAIN MRI exploratory sub-study included 63 participants from three trial sites (Ajou University Hospital, Ewha Woman’s University Medical Center, and Inha University Hospital), where MRI resources were available (Fig. 1). Brain scans were conducted at the baseline and 24-week visits. The present study included 55 participants with both baseline and repeat scans of good quality for brain cortical thickness analysis, which was evaluated by a subjective step and one participant’s follow-up scan was excluded because the gray matter surfaces were shown to be overlapped by the skull during the MRI processing for the MRI structural analysis.
Fig. 1.
Diagram for exploratory MRI analyses in the SUPERBRAIN trial. FMI facility-based multidomain intervention, HMI home-based multidomain intervention
Randomization and Intervention
The participants were randomly assigned to the FMI, HMI, and control groups at baseline in a 1:1:1 ratio. Randomization was performed using a permuted block randomization method with block sizes of three and six using SAS macro programming and stratified by the participating center. Therefore, there were three groups at each center. The allocation sequence was known only to an independent statistical specialist. To randomize participants, a file containing the participant’s research identification number was emailed to the statistical specialist by the principal investigator or study coordinator at the participating center, and a file containing the participant’s assignment information was received via email from the statistical specialist. Cognitive outcome assessors were blinded to the assigned groups and were not involved in the intervention activities. The participants were advised not to discuss the intervention during the testing sessions.
The participants from the FMI and HMI groups received interventions of the five components previously described in detail [8, 10], including monitoring and management of metabolic and vascular risk factors, cognitive training and social activity, physical exercise [9], nutritional guidance, and motivational enhancement.
Participants in the FMI group visited a facility three times per week to participate in all intervention programs in the group or individual sessions. The contents of the cognitive training and physical exercise programs in the HMI group were identical to those in the FMI group. Those in the HMI arm participated in one group of cognitive training sessions (each lasting 50 min) and one home-based cognitive training session (each lasting 30–40 min) per week and one group of exercise sessions (each lasting 60 min) and two home-based exercise sessions (each lasting 60 min) per week during the first 2 months of the trial. For the remainder of the 6-month study, participants in the HMI arm attended one group cognitive training session and one group exercise session every two weeks. For the weeks that included group sessions, the participants performed one cognitive training session and two exercise sessions alone at home each week. For weeks that did not include group sessions, participants performed two cognitive training sessions and three exercise sessions alone at home each week. The control group received regular health advice according to the established guidelines.
Cognitive Outcomes and Serum Brain-Derived Neurotrophic Factor
The total scale index score of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) was assessed at baseline and within 4 weeks after the last intervention in all participants. The RBANS consists of tests A, B, C, and D, with an identical degree of difficulty. Each test included 12 subtests and evaluated the following five cognitive domains: attention, language, visuospatial/constructional abilities, immediate memory, and delayed memory [31]. The participants performed tests A and D of the RBANS at baseline and post-intervention, respectively. A standard score based on same-aged peers for each of the five cognitive domains was provided. These indices were combined to compute the total scale scores of cognitive functioning. Higher scores indicated better cognitive functioning.
Changes in serum BDNF levels after multidomain intervention were investigated. Fasting blood samples were collected at around 9 a.m. in serum separator tubes (SSTs) within 4 weeks before the intervention and within 4 weeks after the last intervention. The SSTs were maintained at room temperature for 30 min and then centrifuged for 10 min at 3000 rpm. In 0.5 mL aliquots, serum samples were stored in cryovial tubes at ≤ −70 °C until analysis. Serum BDNF levels were measured using a quantitative sandwich enzyme-linked immunosorbent assay (ELISA) using DBD00 (R&D Systems, Inc., Minneapolis, MN, USA), according to the manufacturer’s instructions.
MRI Acquisition and Processing
Brain MRI scans at baseline and follow-up were acquired at three clinical sites: Ajou University Hospital, Ewha Woman’s University Medical Center, and Inha University Hospital. The following MR systems and 3D T1 imaging parameters were used: 3 T Achieva, Philips (sagittal slice thickness, 1.2 mm; no gap; repetition time (TR), 6.9 ms; echo time (TE), 3.2 ms; flip angle, 9°; voxel size 1.2 × 1.0 × 1.0 mm3 and a matrix size of 256 × 256 pixels) at the Ajou University Hospital, 3 T Achieva, Philips (sagittal slice thickness, 1.2 mm; no gap; TR, 7.9 ms; TE, 3.7 ms; flip angle, 9°; voxel size 1.2 × 1.0 × 1.0 mm3 and a matrix size of 256 × 256 pixels) at the Ewha Woman’s University Medical Center, and 3 T Signa Architect, GE, (sagittal slice thickness, 1.2 mm; no gap; TR, 7.4 ms; TE, 2.7 ms; flip angle, 11°; voxel size 1.2 × 1.0 × 1.0 mm3 and a matrix size of 256 × 256 pixels) at the Inha University Hospital. The same imaging parameters and MRI scanners were used for both the baseline and 24-week scans at each site. Regular phantom scans were performed within each site, although the phantom was not shared among three centers. Before quantitative analysis, an experienced neurologist visually inspected the T1-weighted images at each site. Images were excluded if there were brain lesions potentially affecting volumetry and/or scanning issues, such as no full-brain coverage, artifacts, intensity inhomogeneity, and adequate gray/white matter contrast.
We used the CIVET v2.1 pipeline (https://wiki.bic.mni.mcgill.ca/ServicesSoftware/CIVET) developed by the Montreal Neurological Institute (MNI) to measure cortical thickness, as described in detail elsewhere [32, 33]. The native T1-weighted images were corrected for intensity inhomogeneity and spatially normalized to the MNI-152 symmetric template [34, 35]. The corrected and registered images were segmented into gray matter, white matter, cerebrospinal fluid, and background using a 3D stereotaxic brain mask and the Intensity-Normalized Stereotaxic Environment for Classification of Tissues (INSECT) algorithm.
Further, the hemispherical gray matter and white matter surfaces, consisting of 40,962 vertices, were extracted from each MR volume using the constrained Laplacian-based automated segmentation with Proximities (CLASP) algorithm [36, 37]. Finally, the cortical thickness was measured in native space by calculating the Euclidean distance between the corresponding vertices of the inner and outer surfaces [37–39]. The global cortical thickness was calculated as the average cortical thickness of all 81,924 vertices. For the region-of-interest (ROI)-based analysis, two types of the atlas were used: the cortex was divided into eight regions using a lobe atlas (https://www.bic.mni.mcgill.ca/ServicesSoftware/VisualGuides) and 76 regions using the automated anatomical labeling (AAL) atlas. The average values of the thickness of the entire vertex in each region were used for our analysis.
Statistical Analyses
To verify if there were differences between groups at baseline, we performed a t-test for independent samples, and the chi-square test for comparison of categorical variables (FMI vs. control; HMI vs. control; FMI vs. HMI). Analysis of covariance (ANCOVA), with a baseline score as a covariate, was used to compare changes from baseline to the study endpoint in the RBANS and serum BDNF levels between each intervention group and the control group. The groups were considered similar when p > 0.05. In addition, correlation analyses between changes of the RBANS and serum BDNF levels were done by the Pearson analyses.
Changes in total cortical gray matter volume, mean cortical thickness at each ROI, and global mean cortical thickness were compared between each group pair (FMI vs. control; HMI vs. control; FMI vs. HMI) using ANCOVA with changes as dependent variables and age, sex, and education as covariates. In addition, changes of cortical thickness in AD signature regions calculated as the average of cortical thickness in entorhinal, inferior temporal, middle temporal, and fusiform regions as previously described [40] was also compared. Finally, correlation analyses between changes of the RBANS or the serum BDNF levels and changes in total cortical gray matter volume, mean cortical thickness at each ROI, and global mean cortical thickness were done by the Pearson analyses, respectively. ComBat site harmonization was also applied to combine and harmonize cortical thickness values across the scanners [41–43]. Specifically, the statistical analyses were conducted using statsmodels.api.OLS package (https://www.statsmodels.org/dev/generated/statsmodels.regression.linear_model.OLS.html). We used SurfStat (http://www.math.mcgill.ca/keith/surfstat/) for cortical thickness visualization and analysis with a false discovery rate threshold of p < 0.05 to control for multiple comparisons.
Results
The population undergoing MRI was younger, more educated, and had a higher baseline RBANS total scale index than the population not undergoing MRI in SUPERBRAIN (Supplementary Table 1). In contrast, their adherence to the intervention or distribution of sex, APOE ε4 carriers, or distribution of groups was not different. The intervention and control groups in the SUPERBRAIN exploratory MRI sub-study were not significantly different in terms of demographic, clinical, cognitive, and MRI characteristics at the baseline (Table 1). The adherence rates in the FMI and HMI groups were 96.0% and 97.0%, respectively. The total cortical gray matter volume, mean cortical thickness at each ROI, global, and AD signature mean cortical thickness at the baseline were not different among groups (Tables 1, 2, and 3).
Table 1.
Baseline clinical characteristics and their changes after 24 weeks in the intervention and control groups
| FMI (n = 20) | HMI (n = 19) | Control (n = 16) | p, FMI vs. control | p, HMI vs. control | p, FMI vs. HMI | |
|---|---|---|---|---|---|---|
| Baseline | ||||||
| Age, y | 68.6 ± 4.9 | 68.0 ± 4.4 | 67.1 ± 4.4 | 0.367 | 0.607 | 0.687 |
| Education, y | 11.3 ± 4.2 | 11.8 ± 4.4 | 9.8 ± 4.3 | 0.294 | 0.161 | 0.696 |
| Female, n (%) | 14 (70.0) | 12 (63.2) | 14 (87.5) | 0.257 | 0.135 | 0.676 |
| APOE ε4 carriers, n (%) | 3 (15.0) | 2 (10.5) | 3 (18.8) | 1.000 | 0.642 | 0.352 |
| Total intracranial volume, mL | 1356.7 ± 1353.9 | 1345.7 ± 1299.8 | 1314.0 ± 1468.4 | 0.358 | 0.499 | 0.804 |
| RBANS indexes | ||||||
| Total | 109.6 ± 19.1 | 110.4 ± 14.1 | 105.5 ± 18.8 | 0.488 | 0.411 | 0.884 |
| Attention | 112.3 ± 16.5 | 112.9 ± 14.5 | 104.8 ± 18.1 | 0.179 | 0.152 | 0.910 |
| Immediate recall | 106.1 ± 15.7 | 104.1 ± 16.4 | 101.1 ± 15.2 | 0.356 | 0.584 | 0.697 |
| Delayed recall | 99.7 ± 18.3 | 98.2 ± 11.8 | 99.9 ± 17.4 | 0.965 | 0.761 | 0.782 |
| Visuospatial/Construction | 99.1 ± 11.1 | 101.5 ± 14.2 | 95.6 ± 13.4 | 0.436 | 0.186 | 0.553 |
| Language | 108.7 ± 15.2 | 111.8 ± 10.3 | 111.2 ± 15.1 | 0.582 | 0.899 | 0.478 |
| BDNF, ng/mL | 29.9 ± 11.4 | 33.5 ± 15.6 | 40.5 ± 22.1 | 0.062 | 0.215 | 0.508 |
| Changes over 24-weeks | ||||||
| RBANS indexes | ||||||
| Total | 7.1 ± 6.4 | 5.2 ± 9.7 | −2.3 ± 9.8 | 0.002 | 0.013 | 0.491 |
| Attention | 1.5 ± 8.2 | −0.5 ± 9.0 | −2.7 ± 9.1 | 0.156 | 0.460 | 0.475 |
| Immediate memory | 6.2 ± 9.4 | 7.3 ± 14.1 | 3.3 ± 10.4 | 0.461 | 0.319 | 0.774 |
| Delayed memory | 10.6 ± 10.3 | 11.0 ± 9.3 | 2.9 ± 7.9 | 0.017 | 0.014 | 0.908 |
| Visuospatial/Construction | 3.0 ± 9.3 | −0.9 ± 16.4 | −8.4 ± 13.4 | 0.013 | 0.103 | 0.359 |
| Language | 2.1 ± 12.1 | 0.1 ± 11.5 | −3.1 ± 11.1 | 0.187 | 0.411 | 0.605 |
| BDNF, ng/mL | 14.7 ± 24.6 | 4.2 ± 2.2 | −3.7 ± 2.6 | 0.029 | 0.338 | 0.188 |
FMI facility-based multidomain intervention, HMI home-based multidomain intervention, RBANS Repeatable Battery for the Assessment of Neuropsychological Status
Table 2.
Effects of the intervention on the mean cortical thickness of the global or lobar areas
| Baseline, mm | Changes (from baselines to study end), mm | p-value* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cortical areas | FMI (n = 20) | HMI (n = 19) | Control (n = 16) | FMI (n = 20) | HMI (n = 19) | Control (n = 16) | FMI vs. Control | HMI vs. Control | FMI vs.HMI |
| Global mean | 3.095 (0.090) | 3.108 (0.080) | 3.126 (0.110) | 0.033 (0.070) | −0.004 (0.050) | −0.003 (0.040) | 0.013 | 0.732 | 0.050 |
| AD signature region | 3.529 (0.253) | 3.529 (0.246) | 3.578 (0.272) | 0.031 (0.140) | −0.001 (0.101) | −0.000 (0.117) | 0.054 | 0.726 | 0.192 |
| 8 ROI using lobe atlas* | |||||||||
| Parietal | 2.885 (0.117) | 2.93 (0.104) | 2.949 (0.119) | 0.026 (0.077) | −0.001 (0.065) | 0.002 (0.047) | 0.0723 | 0.4548 | 0.158 |
| Occipital | 2.951 (0.151) | 2.925 (0.142) | 2.921 (0.157) | 0.009 (0.124) | −0.044 (0.081) | −0.04 (0.063) | 0.0418 | 0.4548 | 0.120 |
| Frontal | 3.095 (0.093) | 3.106 (0.108) | 3.129 (0.108) | 0.039 (0.063) | 0.004 (0.042) | 0.006 (0.028) | 0.0095 | 0.4548 | 0.048 |
| Isthmus | 3.325 (0.19) | 3.315 (0.117) | 3.358 (0.207) | 0.026 (0.107) | 0.001 (0.069) | −0.006 (0.083) | 0.0583 | 0.4548 | 0.199 |
| Parahippocampal | 3.32 (0.134) | 3.339 (0.078) | 3.402 (0.126) | 0.035 (0.088) | 0.01 (0.043) | 0.007 (0.06) | 0.0723 | 0.4548 | 0.161 |
| Cingulate | 3.115 (0.139) | 3.098 (0.084) | 3.144 (0.133) | 0.043 (0.073) | 0.011 (0.04) | 0.008 (0.039) | 0.0085 | 0.4548 | 0.048 |
| Temporal | 3.354 (0.097) | 3.369 (0.094) | 3.391 (0.121) | 0.039 (0.101) | −0.006 (0.055) | −0.006 (0.048) | 0.0145 | 0.4548 | 0.081 |
| Insula | 3.635 (0.108) | 3.638 (0.139) | 3.682 (0.174) | 0.048 (0.047) | −0.004 (0.048) | −0.0 (0.052) | 0.0085 | 0.4548 | 0.002 |
Data are presented as the mean (standard deviation)
AD Alzheimer’s disease, FMI facility-based multidomain intervention, HMI home-based multidomain intervention
*Analysis with a false discovery rate threshold of p < 0.05 to control for multiple comparisons
Table 3.
Areas showing beneficial effects of the intervention on mean cortical thickness among the AAL regions
| Baseline, mm | Changes (from baselines to study end), mm | p-value* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| FMI (n = 20) | HMI (n = 19) | Control (n = 16) | FMI (n = 20) | HMI (n = 19) | Control (n = 16) | FMI vs Control | HMI vs Control | FMI vs HMI | |
| Dorsolateral part of left superior frontal gyrus | 3.049 (0.096) | 3.057 (0.141) | 3.082 (0.128) | 0.034 (0.072) | 0.001 (0.073) | -0.023 (0.04) | 0.0443 | 0.8444 | 0.1737 |
| Orbital part of left middle frontal gyrus | 3.076 (0.119) | 3.093 (0.102) | 3.154 (0.171) | 0.05 (0.061) | 0.003 (0.058) | -0.025 (0.066) | 0.0429 | 0.8444 | 0.1092 |
| Medial orbital part of left superior fontal gyrus | 3.186 (0.109) | 3.141 (0.095) | 3.227 (0.154) | 0.077 (0.166) | 0.02 (0.057) | -0.012 (0.094) | 0.0429 | 0.8444 | 0.0671 |
| Left middle occipital gyrus | 3.057 (0.173) | 3.027 (0.178) | 3.08 (0.186) | 0.023 (0.167) | -0.036 (0.109) | -0.076 (0.085) | 0.0429 | 0.8444 | 0.1854 |
| Left middle temporal gyrus | 3.268 (0.112) | 3.328 (0.107) | 3.351 (0.173) | 0.033 (0.119) | -0.026 (0.085) | -0.058 (0.103) | 0.0429 | 0.8444 | 0.0452 |
| Orbital part of right superior frontal gyrus | 3.142 (0.154) | 3.165 (0.111) | 3.191 (0.127) | 0.077 (0.131) | 0.014 (0.068) | 0.028 (0.081) | 0.0429 | 0.8901 | 0.1718 |
| Medial part of right superior frontal gyrus | 3.245 (0.115) | 3.258 (0.153) | 3.284 (0.114) | 0.068 (0.086) | -0.004 (0.051) | -0.003 (0.059) | 0.0429 | 0.8571 | 0.0671 |
| Medial orbital part of right superior frontal gyrus | 3.191 (0.145) | 3.233 (0.12) | 3.257 (0.13) | 0.073 (0.129) | -0.01 (0.065) | 0.023 (0.08) | 0.0429 | 0.9332 | 0.1727 |
| Right olfactory cortex | 2.934 (0.196) | 2.938 (0.113) | 2.979 (0.156) | 0.108 (0.157) | 0.053 (0.117) | 0.022 (0.108) | 0.0429 | 0.8444 | 0.0671 |
| Right gyrus rectus | 3.135 (0.201) | 3.158 (0.114) | 3.193 (0.129) | 0.109 (0.196) | 0.037 (0.126) | 0.034 (0.117) | 0.0429 | 0.8444 | 0.2278 |
| Right anterior cingulate and paracingulate gyri | 3.101 (0.215) | 3.077 (0.152) | 3.125 (0.143) | 0.074 (0.091) | 0.011 (0.054) | 0.011 (0.083) | 0.0429 | 0.8501 | 0.1276 |
| Right middle temporal gyrus | 3.31 (0.101) | 3.319 (0.129) | 3.309 (0.162) | 0.08 (0.094) | 0.008 (0.074) | 0.022 (0.068) | 0.0491 | 0.8901 | 0.0671 |
Data are presented as the mean (standard deviation)
FMI facility-based multidomain intervention, HMI home-based multidomain intervention, L left, R right
*Analysis with a false discovery rate threshold of p < 0.05 to control for multiple comparisons
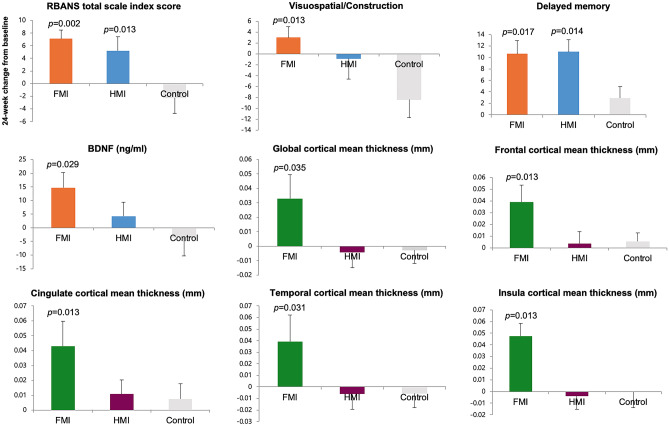
Compared with the control group, the total scale index score of the RBANS was significantly improved in both the FMI (p = 0.002) and HMI groups (p = 0.013) at the end of the study (Table 1; Fig. 2). In the FMI group, compared with the control group, delayed memory (p = 0.017) and visuospatial/construction (p = 0.013) revealed significantly greater improvement among the components of the RBANS. In the HMI group, only delayed memory showed significantly greater improvement than in the control group (p = 0.014). Serum BDNF levels were significantly higher in the FMI group than in the control group (p = 0.029; Table 1; Fig. 2). There was no significant difference in the change of the total scale index and five cognitive domains scores of the RBANS as well as serum BDNF levels between the FMI and HMI groups. The correlation analyses did not show any significant correlation between changes of the RBANS and serum BDNF levels.
Fig. 2.
Mean changes from baseline at study end in the RBANS index score, a blood biomarker and cortical thickness. The bars and lines represent the mean and standard error of the mean. The p values represent the results of comparison between the intervention and control groups using analysis of covariance. FMI facility-based multidomain intervention, HMI home-based multidomain intervention
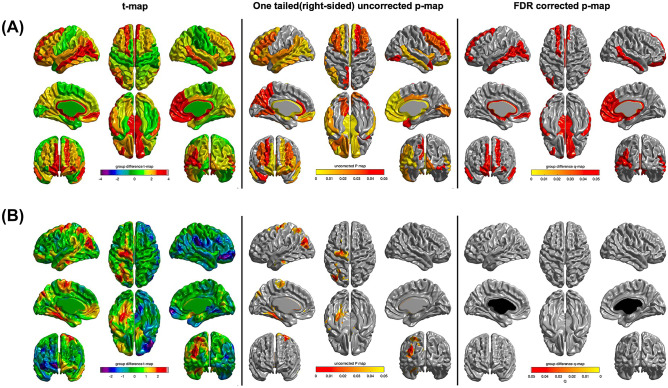
The global cortical thickness as well as regional cortical thickness in the occipital, frontal, cingulate, temporal, and insular regions among the eight ROIs using the lobe atlas increased more significantly only in the FMI group than in the control group, whereas change of the total cortical gray matter volume were not different between the groups (Fig. 2; Table 2). However, the changes in the mean global cortical thickness and cortical thickness among the eight ROIs using the lobe atlas were not significantly different between the HMI and control groups. Among the AAL regions, compared with the control group, the FMI group yielded significantly more beneficial effects on the cortical thickness of the dorsolateral part of the left superior frontal gyrus, orbital part of left middle frontal gyrus, medial orbital part of left superior frontal gyrus, left middle occipital gyrus, left middle temporal gyrus, orbital part of right superior frontal gyrus, medial part of right superior frontal gyrus, medial orbital part of right superior frontal gyrus, right olfactory cortex, right gyrus rectus, right anterior cingulate and paracingulate gyri, and right middle temporal gyrus (Table 3; Fig. 3). Finally, mean cortical thickness in AD signature regions tended to be more increased after the intervention in the FMI group than the control (Table 2; p = 0.054).
Fig. 3.
Effects of intervention on cortical thickness among AAL regions (analysis with a false discovery rate threshold of p < 0.05 to control for multiple comparisons) in each intervention group in comparison to the control group. The FMI group (A) yielded significantly more beneficial effects on the cortical thickness of the dorsolateral part of the left superior frontal gyrus, orbital part of the left middle frontal gyrus, medial orbital part of left superior frontal gyrus, left middle occipital gyrus, left middle temporal gyrus, orbital part of right superior frontal gyrus, the medial part of right superior frontal gyrus, medial orbital part of right superior frontal gyrus, right olfactory cortex, right gyrus rectus, right anterior cingulate and paracingulate gyri, and right middle temporal gyrus. However, the changes in the mean regional cortical thickness were not significantly different between the HMI and control groups (B). FMI facility-based multidomain intervention, HMI home-based multidomain intervention
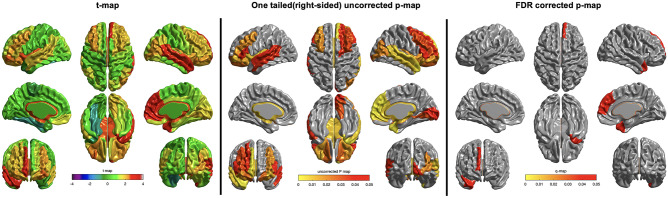
In comparison between the FMI and HMI groups, mean global cortical thickness tended to be more increased after the intervention in the FMI group than the HMI group (Table 2; p = 0.050). In addition, compared with the HMI, the FMI group yielded significantly more beneficial effects on the cortical thickness of frontal, cingulate, and insular regions among the eight ROIs using the lobe atlas (Table 2) and right medial superior frontal gyrus (p = 0.045), right temporal pole of superior temporal gyrus (p = 0.045), and middle temporal gyrus (p = 0.045) among the AAL regions (Table 3; Fig. 4). Finally, changes of the mean cortical thickness in AD signature regions were not different between two intervention groups (Table 2; p = 0.192).
Fig. 4.
Effects of intervention on cortical thickness among AAL regions (analysis with a false discovery rate threshold of p < 0.05 to control for multiple comparisons) in the FMI group in comparison to the HMI group. Compared with the HMI, the FMI group yielded significantly more beneficial effects on the cortical thickness of right medial superior frontal gyrus (p = 0.045), right temporal pole of superior temporal gyrus (p = 0.045) and middle temporal gyrus (p = 0.045) among the AAL regions. FMI facility-based multidomain intervention, HMI home-based multidomain intervention
The correlation analyses did not show any significant correlation between changes of the RBANS or the serum BDNF levels and changes in total cortical gray matter volume, mean cortical thickness at each ROI, and global mean cortical thickness.
Discussion
To the best of our knowledge, this study is the first to show that multidomain lifestyle intervention for dementia prevention could be beneficial for global and regional cortical thickness. Compared with the control group, the FMI group showed significantly increased cortical thickness after the 6-month intervention.
Increased global and regional cortical thickness in the FMI group suggests that FMI programs, such as those used in the current study, may counteract age-associated structural changes in the elderly. In particular, a previous study showed that the lateral and medial prefrontal cortices, as well as the superior and middle temporal gyri, were consistently affected during the aging process [44]. The precise neurobiological mechanisms that are responsible for the changes in cortical thickness in older adults after the FMI program are not known. However, previous studies have suggested several explanations, such as changes in the size of neurons or glial cells, as well as the genesis of neurons or glial cells or changes in vascularization [45–48]. This is thought to be mediated by increased serum levels of BDNF, insulin-like growth factor type-1, and vascular endothelial growth factors, often noted after physical exercise [49]. In our study, compared with the control group, the FMI group showed an increase in the serum BDNF levels and cortical thickness, whereas the HMI group revealed no significant difference in the serum BDNF levels and cortical thickness. Because there was no significant correlation between the serum BDNF levels and changes in the mean cortical thickness, our study could not reveal that increased cortical thickness after the FMI program in the current study was related to increased serum BDNF levels. However, increased cortical thickness as well as serum BDNF after our FMI program showed that our FMI program could be neuroplastic. Further, the population undergoing MRI was younger, more educated, and had a higher baseline RBANS total scale index than the population not undergoing MRI in the SUPERBRAIN. In younger and/or high-performing populations, a more powerful intervention might be necessary to provoke plastic changes in the cortices. The FMI program involved more face-to-face intervention with interaction within group meetings, and therefore, this FMI could provide more stimuli for plastic changes in the brain than HMI as shown in comparison of the changes in the mean cortical thickness at each ROI between the FMI and HMI groups.
In the analyses of the change in regional cortical thickness, compared with the control group, the FMI group yielded significantly more beneficial effects on the cortical thickness of the occipital, frontal, cingulate, temporal, or insula among eight ROIs using the lobe atlas and the dorsolateral part of the left superior frontal gyrus, orbital part of the left middle frontal gyrus, medial orbital part of the left superior frontal gyrus, left middle occipital gyrus, left middle temporal gyrus, orbital part of the right superior frontal gyrus, the medial part of the right superior frontal gyrus, medial orbital part of the right superior frontal gyrus, right olfactory cortex, right gyrus rectus, right anterior cingulate and paracingulate gyri, and right middle temporal gyrus among the AAL regions. These areas are heteromodal association cortices responsible for integrating information from the unimodal association cortex and the paralimbic areas, which are important in facilitating learning [50, 51]. Compared with the rest of the cortex, these regions exhibit a distinct gene expression profile characterized by relative upregulation of gene sets implicated in ionotropic and metabotropic neurotransmission as well as activation of immune response and underlie a higher capacity for plastic changes in response to lifetime intellectual enrichment and a potential higher resilience to age-related pathologic brain changes [51]. The programs used in this study may require strong integration of information as well as active interactions between neuronal networks because these interventions target multiple cognitive domains, which require activation and processing by the heteromodal association cortices. In addition, these areas include regions which are known to be AD signature areas and our study showed that mean cortical thickness in AD signature regions tended to be more increased after the intervention in the FMI group than the control; therefore, increased cortical thickness in these areas might be beneficial in the prevention of cognitive decline in the AD.
In contrast to the cognitive benefits of multidomain lifestyle interventions, the effects of lifestyle interventions on cortical thickness or brain volumes have not yet been fully clarified. While the FINGER trial revealed significant cognitive benefits of a multidomain lifestyle intervention [4], the FINGER MRI exploratory sub-study did not show significant differences between the intervention and control groups in terms of changes in brain volume or regional cortical thicknesses after 2 years in at-risk elderly without substantial impairment [21]. It had been thought that the negative results on the cortical thickness in the FINGER MRI exploratory sub-study might be due to the characteristics of the study population. The participants were at-risk segments of the general elderly population (not patients in a clinical setting), and their cortical thickness was approximately decreased by 0.1 mm during 2 years in both the intervention and control groups. The structural brain changes in this at-risk population were not very pronounced over 2 years, and this interval may not have been sufficient to observe significant effects on structural brain changes. In our study, the intervention lasted for 6 months, during which the global cortical thickness in the FMI group increased by approximately 0.033 mm, whereas the control group showed a decrease in global cortical thickness by 0.003 mm. It is impossible to directly compare the results from two studies, the FINGER and SUPERBRAIN, due to the difference in the trial duration. Smaller (N < 160 participants) and/or shorter trials (up to 24 weeks) seemed more likely to report intervention benefits on overall cognition and some specific domains (e.g., spatial working memory, executive functioning) [52]. Therefore, the distinct beneficial impact of the 6-month FMI of the SUPERBRAIN on the cortical thickness could not guarantee longer-term effects of multidomain lifestyle interventions, especially as some older at-risk people may start to develop brain pathology. Therefore, further investigation is needed to determine whether the impact of multidomain lifestyle interventions with high adherence on brain volume or cortical thickness would simply disappear or be sustainable in the longer-term.
The main limitation of the SUPERBRAIN MRI exploratory sub-study is the small sample size together with the short trial because this study was a SUPERBRAIN MRI exploratory sub-study, and the results should be interpreted with caution. Future studies including cortical thickness as a primary outcome measure are necessary to confirm the impact of multidomain lifestyle intervention on cortical thickness in the longer-term. In addition, MRI scanners differed among sites; however, this was adjusted for in all analyses.
In conclusion, significant differences between the FMI and control groups in changes in cortical thickness in at-risk elderly individuals without substantial impairment in the SUPERBRAIN suggest that group preventive strategies at the facility may be beneficial for cognition through structural neuroplastic changes in the brain areas, which play a crucial role in facilitating learning. Future studies, including cortical thickness as a primary outcome measure, are necessary to confirm the impact of multidomain lifestyle intervention on cortical thickness.
Supplementary Information
Below is the link to the electronic supplementary material.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Funding
This work was supported by grants from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) and Korea Dementia Research Center (KDRC), funded by the Ministry of Health and Welfare and Ministry of Science and ICT, Republic of Korea (HI18C0479, HU20C0198, and HU21C0016), and from the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT, Republic of Korea (NRF- 2019R1F1A1059660).
Declarations
Conflict of Interest
SYM receives a research grant from Hyundai Pharmaceutical Co. Ltd. CHH receives research support from Eisai Korea Inc. JHJ receives research grants from Chong Kun Dang pharmaceutical Corp., Jeil Pharmaceutical Co. Ltd., and Kuhnil Pharmaceutical Co. Ltd., and consults for PeopleBio Co. Ltd. SYM, CHH, JHJ, YKP, HRN, and SHC are shareholders of Rowan Inc. YKP consults for Pulmuone Co. Ltd. HRN consults for Hyundai Pharmaceutical Co. Ltd. SHC consults for Hyundai Pharmaceutical Co. Ltd. and PeopleBio Co. Ltd. All other authors declare no conflicts of interest.
Disclaimer
The funders had no role in the study design, data collection, analysis, interpretation of data, writing of the report, or decision to submit the manuscript for publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
So Young Moon and Sohui Kim contributed equally to this work.
Contributor Information
Jong-Min Lee, Email: ljm@hanyang.ac.kr.
Jee Hyang Jeong, Email: jjeong@ewha.ac.kr.
References
- 1.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matthews FE, Arthur A, Barnes LE, et al. A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet. 2013;382:1405–1412. doi: 10.1016/S0140-6736(13)61570-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Satizabal CL, Beiser AS, Chouraki V, Chêne G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med. 2016;374:523–532. doi: 10.1056/NEJMoa1504327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 5.Andrieu S, Guyonnet S, Coley N, et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol. 2017;16:377–389. doi: 10.1016/S1474-4422(17)30040-6. [DOI] [PubMed] [Google Scholar]
- 6.Moll van Charante EP, Richard E, Eurelings LS, et al. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster-randomised controlled trial. Lancet. 2016;388:797–805. doi: 10.1016/S0140-6736(16)30950-3. [DOI] [PubMed] [Google Scholar]
- 7.Chhetri JK, de Souto BP, Cantet C, et al. Effects of a 3-year multidomain intervention with or without omega-3 supplementation on cognitive functions in older subjects with increased CAIDE dementia scores. J Alzheimers Dis. 2018;64:71–78. doi: 10.3233/JAD-180209. [DOI] [PubMed] [Google Scholar]
- 8.Park HK, Jeong JH, Moon SY, et al. South Korean study to prevent cognitive impairment and protect brain health through lifestyle intervention in at-risk elderly people: protocol of a multicenter, randomized controlled feasibility trial. J Clin Neurol. 2020;16:292–303. doi: 10.3988/jcn.2020.16.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SM, Song HS, Chun BO, et al. Feasibility of a 12 week physical intervention to prevent cognitive decline and disability in the at-risk elderly population in Korea. J Clin Med. 2020;9:3135. doi: 10.3390/jcm9103135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moon SY, Hong CH, Jeong JH, et al. Facility-based and home-based multidomain interventions including cognitive training, exercise, diet, vascular risk management, and motivation for older adults: a randomized controlled feasibility trial. Aging (Albany NY) 2021;13:15898–15916. doi: 10.18632/aging.203213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steffener J, Stern Y. Exploring the neural basis of cognitive reserve in aging. Biochim Biophys Acta. 2012;1822:467–473. doi: 10.1016/j.bbadis.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown BM, Peiffer JJ, Martins RN. Multiple effects of physical activity on molecular and cognitive signs of brain aging: can exercise slow neurodegeneration and delay Alzheimer’s disease? Mol Psychiatry. 2013;18:864–874. doi: 10.1038/mp.2012.162. [DOI] [PubMed] [Google Scholar]
- 13.Currie J, Ramsbottom R, Ludlow H, Nevill A, Giler M. Cardio-respiratory fitness, habitual physical activity and serum brain derived neurotrophic factor (BDNF) in men and women. Neurosci Lett. 2009;451:152–155. doi: 10.1016/j.neulet.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 14.Hsiao YH, Hung HC, Chen SH, Gean PW. Social interaction rescues memory deficit in animal model of Alzheimer’s disease by increasing BDNF-dependent hippocampal neurogenesis. J Neurosci. 2014;34:16207–16219. doi: 10.1523/JNEUROSCI.0747-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossi C, Angelucci A, Costantin L, et al. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichement. Eur J Neurosci. 2006;24:1850–1856. doi: 10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- 16.Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ten Brinke LF, Bolandzadeh N, Nagamatsu LS, et al. Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: a 6-month randomized controlled trial. Br J Sports Med. 2015;49:248–254. doi: 10.1136/bjsports-2013-093184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlson MC, Kuo JH, Chuang YF, et al. Impact of the Baltimore Experience Corps Trial on cortical and hippocampal volumes. Alzheimers Dement. 2015;11:1340–1348. doi: 10.1016/j.jalz.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith AD, Smith SM, de Jager CA, et al. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PLoS ONE. 2010;5:e12244. doi: 10.1371/journal.pone.0012244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soininen H, Solomon A, Visser PJ, et al. 24-month intervention with a specific multinutrient in people with prodromal Alzheimer’s disease (LipiDiDiet): a randomised, double-blind, controlled trial. Lancet Neurol. 2017;16:965–975. doi: 10.1016/S1474-4422(17)30332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stephen R, Liu Y, Ngandu T, et al. Brain volumes and cortical thickness on MRI in the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) Alzheimers Res Ther. 2019;11:53. doi: 10.1186/s13195-019-0506-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chalmers J, MacMahon S, Mancia G, et al. 1999 World Health Organization-International Society of Hypertension Guidelines for the management of hypertension. Guidelines sub-committee of the World Health Organization. Clin Exp Hypertens. 1999;21:1009–1060. doi: 10.3109/10641969909061028. [DOI] [PubMed] [Google Scholar]
- 23.American Diabetes Association 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S13–S27. doi: 10.2337/dc18-S002. [DOI] [PubMed] [Google Scholar]
- 24.National Cholesterol Education Program (NCEP). Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed]
- 25.Centers for Disease Control and Prevention (CDC) Cigarette smoking among adults–United States, 1992, and changes in the definition of current cigarette smoking. MMWR Morb Mortal Wkly Rep. 1994;43:342–346. [PubMed] [Google Scholar]
- 26.Pan WH, Yeh WT. How to define obesity? Evidence-based multiple action points for public awareness, screening, and treatment: an extension of Asian-Pacific recommendations. Asia Pac J Clin Nutr. 2008;17:370–374. [PubMed] [Google Scholar]
- 27.Nam GE, Park HS. Perspective on diagnostic criteria for obesity and abdominal obesity in Korean adults. J Obes Metab Syndr. 2018;27:134–142. doi: 10.7570/jomes.2018.27.3.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alberti KG, Eckel RH, Grundy SM, et al. International diabetes federation task force on epidemiology and prevention, hational heart, lung, and blood institute, American heart association, world heart federation, international atherosclerosis society, and international association for the study of obesity. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120:1640–5. [DOI] [PubMed]
- 29.Han C, Jo SA, Jo I, Kim E, Park MH, Kang Y. An adaptation of the Korean mini-mental state examination (K-MMSE) in elderly Koreans: demographic influence and population-based norms (the AGE study) Arch Gerontol Geriatr. 2008;47:302–310. doi: 10.1016/j.archger.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Choi SH, Shim YS, Ryu SH, et al. Validation of the literacy independent cognitive assessment. Int Psychogeriatr. 2011;23:593–601. doi: 10.1017/S1041610210001626. [DOI] [PubMed] [Google Scholar]
- 31.Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20:310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- 32.Shin NY, Shin YS, Lee PH, et al. Different functional and microstructural changes depending on duration of mild cognitive impairment in Parkinson disease. AJNR Am J Neuroradiol. 2016;37:897–903. doi: 10.3174/ajnr.A4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin NY, Hong J, Choi JY, Lee SK, Lim SM, Yoon U. Retrosplenial cortical thinning as a possible major contributor for cognitive impairment in HIV patients. Eur Radiol. 2017;27:4721–4729. doi: 10.1007/s00330-017-4836-6. [DOI] [PubMed] [Google Scholar]
- 34.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- 35.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 36.Kim JS, Singh V, Lee JK, et al. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage. 2005;27:210–221. doi: 10.1016/j.neuroimage.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 37.MacDonald D, Kabani N, Avis D, Evans AC. Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI. Neuroimage. 2000;12:340–356. doi: 10.1006/nimg.1999.0534. [DOI] [PubMed] [Google Scholar]
- 38.Kabani N, Le Goualher G, MacDonald D, Evans AC. Measurement of cortical thickness using an automated 3-D algorithm: a validation study. Neuroimage. 2001;2001(13):375–380. doi: 10.1006/nimg.2000.0652. [DOI] [PubMed] [Google Scholar]
- 39.Lerch JP, Evans AC. Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage. 2005;24:163–173. doi: 10.1016/j.neuroimage.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 40.Jack CR, Jr, Wiste HJ, Weigand SD, et al. Different definitions of neurodegeneration produce similar amyloid/neurodegeneration biomarker group findings. Brain. 2015;138:3747–3759. doi: 10.1093/brain/awv283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fortin JP, Cullen N, Sheline YI, et al. Harmonization of cortical thickness measurements across scanners and sites. Neuroimage. 2018;167:104–120. doi: 10.1016/j.neuroimage.2017.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orlhac F, Frouin F, Nioche C, Ayache N, Buvat I. Validation of a method to compensate multicenter effects affecting CT radiomics. Radiology. 2019;291:53–59. doi: 10.1148/radiol.2019182023. [DOI] [PubMed] [Google Scholar]
- 43.Orlhac F, Eertink JJ, Cottereau A, et al. A guide to ComBat harmonization of imaging biomarkers in multicenter studies. J Nucl Med. 2022;63:172–179. doi: 10.2967/jnumed.121.262464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fjell AM, Westlye LT, Amlien I, et al. High consistency of regional cortical thinning in aging across multiple samples. Cereb Cortex. 2009;19:2001–2012. doi: 10.1093/cercor/bhn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Engvig A, Fjell AM, Westlye LT, et al. Effects of memory training on cortical thickness in the elderly. Neuroimage. 2010;52:1667–1676. doi: 10.1016/j.neuroimage.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 46.Wenger E, Schaefer S, Noack H, et al. Cortical thickness changes following spatial navigation training in adulthood and aging. Neuroimage. 2012;59:3389–3397. doi: 10.1016/j.neuroimage.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 47.Ilg R, Wohlschläger AM, Gaser C, et al. Gray matter increase induced by practice correlates with task-specific activation: a combined functional and morphometric magnetic resonance imaging study. J Neurosci. 2008;28:4210–4215. doi: 10.1523/JNEUROSCI.5722-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muotri AR, Gage FH. Generation of neuronal variability and complexity. Nature. 2006;441:1087–1093. doi: 10.1038/nature04959. [DOI] [PubMed] [Google Scholar]
- 49.Voss MW, Erickson KI, Prakash RS, et al. Neurobiological markers of exercise-related brain plasticity in older adults. Brain Behav Immun. 2013;28:90–99. doi: 10.1016/j.bbi.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mesulam MM. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- 51.Bartrés-Faz D, González-Escamilla G, Vaqué-Alcázar L, et al. Characterizing the molecular architecture of cortical regions associated with high educational attainment in older individuals. J Neurosci. 2019;39(4566–4575):52. doi: 10.1523/JNEUROSCI.2370-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Solomon A, Stephen R, Altomare D, et al. Multidomain interventions: state-of-the-art and future directions for protocols to implement precision dementia risk reduction. A user manual for brain health services-part 4 of 6. Alzheimers Res Ther. 2021;13:171. doi: 10.1186/s13195-021-00875-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.