Abstract
STUDY QUESTION
Did the first wave of the COVID-19 pandemic have an impact on monthly birth rates in Europe?
SUMMARY ANSWER
Using datasets on live births per month in Europe, collected from the Human Fertility Database, we found a −14.1% decline in live births in January 2021 (i.e. 9–10 months after the epidemic peaks and first lockdowns), compared to the average number of live births in January 2018 and 2019.
WHAT IS KNOWN ALREADY
Previous pandemics in the 20th and 21st centuries have been associated with a decline in birth rates 9 months after their peak, and a rebound in births over time. Lockdowns were necessary to control the first wave of the COVID-19 pandemic and may have had an impact on subsequent birth rates.
STUDY DESIGN, SIZE, DURATION
Monthly time series data on live births from January 2018 to March 2021 were extracted to provide a time-series analysis of birthrates during and after the first wave of the COVID-19 pandemic in 24 European countries.
PARTICIPANTS/MATERIALS, SETTING, METHODS
We conducted a random-effect generalized least squares regression to assess the seasonality of births from January 2018 to March 2021, and to identify potential differences in monthly live births after the first wave of the COVID-19 pandemic, considering the seasonality of births. To quantify these potential differences, we estimated the variation rate between the monthly live births observed during 2020 and 2021 and the mean of the 2018–2019 monthly live births in Europe. Factors potentially associated with a variation in monthly birth rates were assessed using univariable and multivariable generalized linear regressions.
MAIN RESULTS AND THE ROLE OF CHANCE
When considering the seasonality of births, January 2021 was the only month with a significant difference in live births. A drop of −14.1% was observed compared to the average number of live births in January 2018 and 2019. At the national level, this drop was observed 9–10 months after the epidemic peaks in 13 countries. The duration of lockdowns was the variable that had the stronger association with this decrease, whereas higher incomes per capita could be a factor limiting this decline. A rebound in births compared to the previous years occurred in March 2021 in 13 countries.
LIMITATIONS, REASONS FOR CAUTION
Our data are based on national data, limiting the power in the multivariable models used and the identification of other potential factors contributing to a decrease or an increase in birth rates. In addition, we collected only live births up to April 2021, which precludes the identification of a difference in births seasonality in 2021.
WIDER IMPLICATIONS OF THE FINDINGS
As with previous pandemics, the COVID-19 outbreak was associated with a decline in births 9 months after its first wave. This trend may be associated with the duration of the lockdowns. Although there was a rebound in births in the following months, it does not seem to compensate for this decline.
STUDY FUNDING/COMPETING INTEREST(S)
The authors receive no external funding and have no conflict of interest to declare.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: COVID-19, SARS-COV-2, birthrates, fertility, pregnancy, lockdown, birth, demographics
Introduction
The coronavirus disease 2019 (COVID-19) pandemic and social distancing measures established to control its spread have brought significant changes to almost every aspect of life worldwide (Haleem et al., 2020). While the rapid increase in the number of cases leading to intensive care hospitalizations and high mortality in at-risk groups remain priority indicators for pandemic control, the demographic consequences are also important to assess (WHO, 2021). Increased mortality is not the only demographic consequence of such a pandemic, as fertility and birth rates may also be affected either through direct exposure or indirect effects related to social fears and distancing measures (Aassve et al., 2020). Previous pandemics that occurred in the 20th and 21st centuries, such as the 1918 H1N1 Influenza, the 2013 Ebola and the 2016 Zika virus outbreaks were associated with a decline in birth rates 9 months after their peaks (Pomar, 2020). The reasons for this decline were high parental mortality (H1N1 and Ebola) (Dahal et al., 2018) or high fetal morbi-mortality (Zika) in cases of direct exposure (Coelho et al., 2017), but also the desire of couples to postpone pregnancy in a time of crisis (Vrachnis et al., 2014). These may have major consequences on the demographic pyramids, especially in countries with an already low number of children per couple. Economic concerns as well as the lack of information on the potential teratogenic effect and maternal morbidity and mortality associated with SARS-CoV-2 infection during pregnancy may have played a role in the decision of couples to postpone pregnancies. Furthermore, it is known that parental stress is associated with a fertility decrease, which may also prevent conception during a crisis period (Li et al., 2020). The cessation of non-emergency activities in hospitals to allow for the management of COVID-19 patients may also have reduced fertility during the first wave of the COVID-19 pandemic, particularly for pregnancies resulting from IVF (Smith et al., 2020).
A cohort study in the USA using electronic medical record surveillance found an initial decline in pregnancy events during and after the first epidemic peak of the COVID-19 outbreak, and predicted an increase in births remote from the outbreak (Stout et al., 2021). A first analysis of the European datasets found a drop of −0.5% to −11.4% in livebirths after the initiation of containments in 11 of 14 countries included, and the authors associated this decrease to high excess mortality during the first wave of the COVID-19 pandemic (De Geyter et al., 2022). Based on national statistics on birth rates available, we aim to investigate if a similar trend is observed by including 24 European countries in the analysis. As the number of births varies markedly by season, with typically higher rates during the spring and lower rates during the last quarter of the year (Dahlberg and Andersson, 2018), we aimed to use a time-series analysis based on monthly birth rates before, during and after the first wave of the COVID-19 pandemic. Finally, we aimed to include factors other than mortality, such as the duration and the stringency of lockdowns, to investigate whether a substantial difference in live births could be associated with these factors.
Materials and methods
Data collection
Datasets on live births per month before, during and after the first wave of the COVID-19 pandemic were collected from the Human Fertility Database (Jasilioniene et al., 2016) (collected on 9 September 2021, publicly available at https://www.humanfertility.org/cgi-bin/stff.php). To be included, national datasets needed to provide information on live birth rates per month for at least 2 years pre-pandemic (2018–2019) and up to March 2021. Datasets that were provisional or lacked birthrates per month were not included in the present study.
For the included datasets, information on the timing of the first wave and epidemic peak, the total number of severe acute respiratory syndrome-corona virus-2 (SARS-CoV-2) cases and deaths at the end of the first wave, the date and duration of lockdown associated with the first wave, the maximum occupancy rate of intensive care units (ICUs), the pre-pandemic incomes per capita and the stringency index were collected for each country, based on national data available in the WHO situation reports (WHO, 2021), national statistics offices and UN databases (UN, UN Databases, 2021).
Definition of variables
Birth rates included all live births. Miscarriages, intra-uterine fetal demises and stillbirths were excluded.
The timing of the epidemic peak in all European countries was estimated based on the WHO situation reports (WHO, 2021) and the evolution of daily new cases was summarized in the ourworldindata database (Hannah Ritchie, 2020). The total numbers of COVID-19 cases and deaths were estimated using the 132nd situation report of the WHO published 31 May 2020 (WHO, 2021), and morbidity and mortality rates were estimated for each country. The impact of the first wave on healthcare systems was estimated using the European CDC database (ECDC, 2020) and classified as low (occupancy rate in ICU <80%), moderate (occupancy rate in ICU 80–100%) or high (occupancy rate in ICU >100%). The duration of lockdown associated with the first wave of the pandemic was calculated based on the WHO situation reports for all countries (WHO, 2021). The stringency of lockdowns was evaluated through a composite measure developed by researchers of the University of Oxford based on nine indicators including school closures, workplace closures and travel bans, rescaled to a value from 0 to 100 (100 the strictest and 0 the least strict) (https://www.bsg.ox.ac.uk/research/research-projects/oxford-covid-19-government-response-tracker) (Hale et al., 2021). The pre-pandemic income per capita in all countries was assessed using the World Bank classification for 2019 (https://blogs.worldbank.org/opendata/new-world-bank-country-classifications-income-level-2020-2021).
Statistical analyses
For all countries included, we described the average annual rate of change in live births between 2015 and 2019, to assess the general trend before the pandemic: growth (>1.0% per year), stability (−1.0% to 1.0% per year) or decline (>−1.0% per year).
Using a time series analysis between January 2018 and March 2021, we assessed the seasonality of births in countries included in a random-effect generalized least squares (GLS) regression. This regression permitted the identification of potential differences in monthly live births after the first wave of the COVID-19 pandemic, considering the seasonality of births. To quantify these potential differences, we estimated the variation rate between the monthly live births observed during 2020 and 2021 and the mean of the 2018–2019 monthly live births in Europe. We then calculated variation rates for all European countries with data available.
Factors potentially associated with a variation in monthly birth rates were assessed using univariable and multivariable generalized linear regressions. The multivariable model included variables with a P-value <0.10 in univariable analysis. As the overoccupancy of ICUs may be related to the decision to increase the duration and the stringency of the first lockdown, we included only one of these variables (i.e. the lockdown’s duration) in the multivariable model in case of collinearity. A factor was considered to be independently associated with the variation of monthly birth rates when it had a P-value <0.05 in multivariable analysis. To test the validity of our findings, we performed a second analysis where a substantial drop in monthly birth rates was defined as a variation rate >−10% (i.e. three times the European decrease observed in previous years) and a rebound as a variation rate >+1%. Statistical analyses were done using Stata 15.
Ethics approval was not required for this study.
Results
Monthly birth rates between January 2018 and April 2021 were available for 24 European countries (24/27, 88.9%): Austria, Belgium, Croatia, Czechia, Denmark, Estonia, Finland, France, Germany, Hungary, Italy, Latvia, Lithuania, the Netherlands, Portugal, Romania, Russia, Slovenia, Spain, Sweden, Switzerland, England and Wales, Scotland and Ukraine. Data available for these countries were extracted and included in the analysis (Supplementary Table SI). Data for monthly births in Bulgaria, Iceland and Norway stop in December 2020, so these three countries were not included.
Evolution of birthrates during the pre-pandemic period
Overall, an average decline in live births of −2.9% per year (py) was observed in Europe between 2015 and 2019. A progressive decline in annual birth rates was observed between 2015 and 2019 in Finland (−4.8% py), France (−1.6% py), Italy (−2.9% py), Latvia (−3.8% py), Lithuania (−3.4% py), Russia (−6.4% py), Slovenia (−1.6% py), Spain (−3.7% py), the UK (−2.1% py in England and Wales, and −2.7% py in Scotland) and Ukraine (−6.9% py). During the same period, a progressive growth was observed in Denmark (+1.3% py) and Germany (+1.4%). Birth rates were stable in the other countries during this period (Supplementary Table SII).
Seasonality of live births
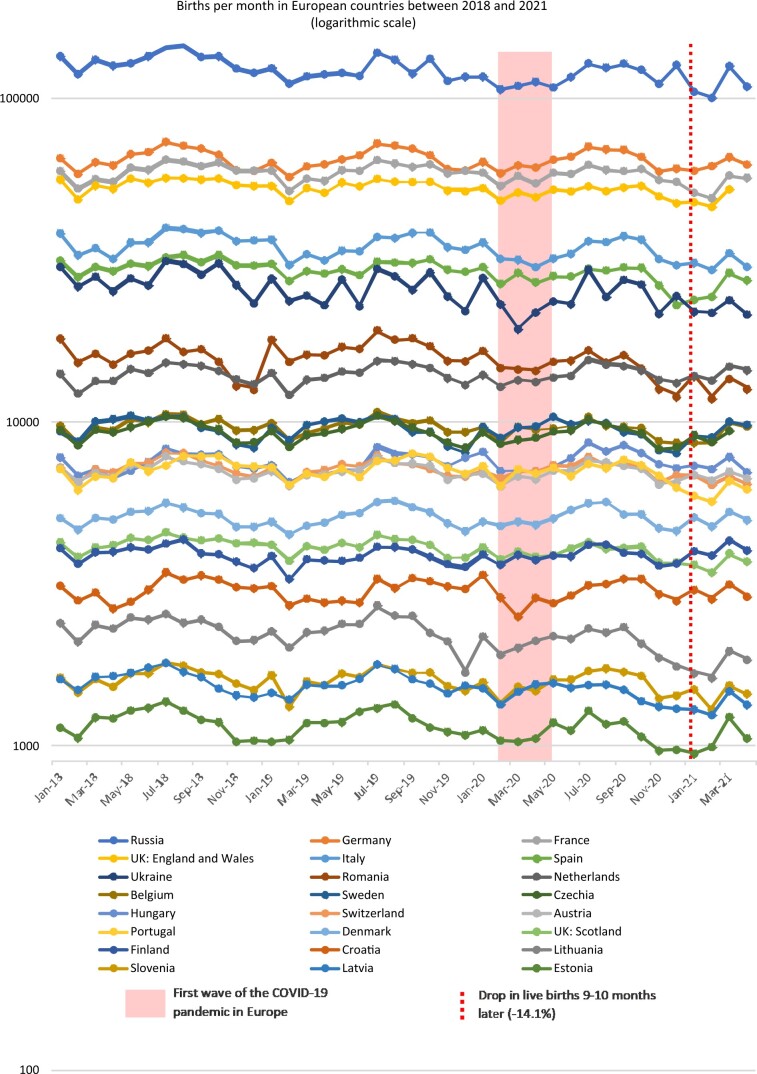
Between January 2018 and March 2021, a seasonality of live births was observed in the European countries included, with more births between March and October than between November and February (Fig. 1). Raw data are presented in Supplementary Table SIII, and a random-effects GLS regression clustered by country to assess seasonality is in Supplementary Table SIV. When considering this seasonality, January 2021 was the only month with a significant difference in live births compared to the previous years: the adjusted coefficient of the GLS regression clustered by country was −1467 (95% CI −2329 to −605), P = 0.001 (Table I).
Figure 1.
Live births between January 2018 and April 2021 in the 24 European countries included, according to a logarithmic scale.
Table I.
Evolution of monthly live births in 2020–2021 compared to the mean monthly rates in 2018–2019 and according to birth seasonality.
| Months | Evolution of monthly birth rates compared to 2018–2019 |
Random-effects GLS regression adjusted on births seasonality, clustered by country |
||
|---|---|---|---|---|
| Total (min to max) | Coef. | [95% CI] | P | |
| January 2020 | −3.99% (−9.82% to +8.18%) | −1043.76 | [−2654.23 to 566.71] | 0.243 |
| February 2020 | −1.50% (−7.36% to +5.46%) | 194.11 | [−225.52 to 613.74] | 0.365 |
| March 2020 | −5.39% (−26.47% to +4.30%) | −793.29 | [−2784.12 to 1197.54] | 0.478 |
| April 2020 | −4.63% (−11.82% to +5.72%) | −164.61 | [−847.53 to 518.31] | 0.753 |
| May 2020 | −6.74% (−14.73% to +2.72%) | −441.46 | [−1220.70 to 337.78] | 0.267 |
| June 2020 | −4.38% (−13.73% to +5.84%) | 38.27 | [−131.17 to 207.72] | 0.989 |
| July 2020 | −5.12% (−14.10% to +3.96%) | −831.67 | [−2252.85 to 589.51] | 0.271 |
| August 2020 | −6.87% (−17.41% to +1.99%) | −1003.88 | [−2420.83 to 413.08] | 0.165 |
| September 2020 | −1.45% (−7.89% to +6.95%) | −207.32 | [−1687.37 to 1272.73] | 0.548 |
| October 2020 | −5.20% (−11.34% to +1.86%) | −1095.19 | [−2587.45 to 397.07] | 0.158 |
| November 2020 | −6.24% (−15.23% to +1.55%) | −1053.12 | [−2172.32 to 66.08] | 0.063 |
| December 2020 | −2.26% (−22.99% to +8.40%) | −525.97 | [−2075.00 to 1023.06] | 0.506 |
| January 2021 | −14.12% (−28.06% to +1.94%) | −1466.93 | [−2329.14 to −604.71] | 0.001 |
| February 2021 | −5.17% (−22.88% to +11.79%) | −504.17 | [−2118.24 to 1109.90] | 0.689 |
| March 2021 | +0.64% (−16.13% to +11.33%) | 1030.85 | [−378.80 to 2440.49] | 0.152 |
Coefficients with 95% CIs and P-values were estimated with random-effects GLS regressions adjusted on births seasonality, clustered by countries. These coefficients correspond to the average difference in live births for each country, taking into account variations due to the seasonality of births since 2018.
Potential impact of the first wave of COVID-19 on birth rates
When considering the total of live births in the 24 countries included, a drop of −14.1% was observed in January 2021 compared to the average number of live births in January 2018 and 2019 (Table I, Fig. 2). This drop occurred 9–10 months after the epidemic peaks and lockdowns related to the first wave of COVID-19 in Europe. This drop was observed at the national level in Belgium (−12.2%), Estonia (−12.8%), France (−14.4%), Italy (−17.2%), Latvia (−15.5%), Lithuania (−28.1%), Portugal (−17.8%), Romania (−23.3%), Russia (−19.1%), Spain (−23.5%), Ukraine (−24.4%) and the UK (−13.0% in England and Wales; −14.0% in Scotland).
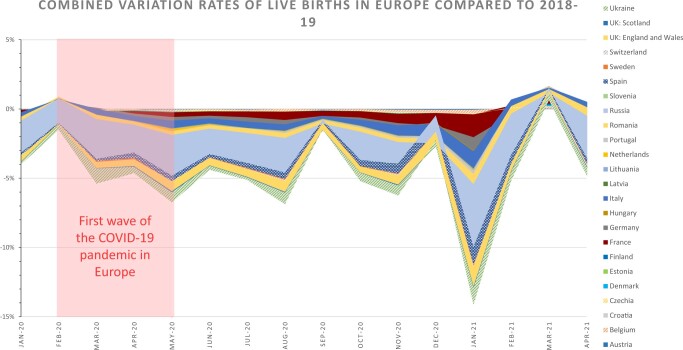
Figure 2.
Combined variation rates of live births in Europe compared to 2018–2019. Variation rate between the monthly live births observed during 2020 and 2021 and the mean of the 2018–2019 monthly live births were weighted by the number of births in each country to present cumulative rates of change, corresponding to the total variation observed in Europe. Raw data are presented in the Supplementary Table SIV.
March 2021 was the only month with a rate of live births similar to the pre-pandemic monthly rate (+0.6%), corresponding to a rebound 9–10 months after the end of lockdowns. A rebound in March 2021 was observed in Austria (+1.8%), Belgium (+6.0%), Croatia (+8.1%), Czechia (1.2%), Denmark (7.2%), Estonia (2.3%), Finland (+11.3%), France (+2.5%), Germany (+5.2%), Hungary (+10.9%), the Netherlands (+10.2%), Russia (+1.4%) and Sweden (+1.2%, Supplementary Table SV).
Potential determinants of birth drop or rebound during the COVID-19 pandemic
Table II presents the pre-pandemic birth rate trend, the estimated number and prevalence of COVID-19 cases and deaths at the end of the first wave, the occupancy of ICUs during the first wave, the duration and stringency index of the first lockdown and the pre-pandemic income per capita for each country included. In univariate analysis, the birthrate trend before the pandemic, the lockdown length and the income per capita were significantly correlated to the difference in live births between January 2021 and January of the pre-pandemic years (2018–2019). In a multivariable analysis, the duration of lockdowns was the only factor associated with a negative difference in live births between January 2021 and January of the pre-pandemic years (2018–2019), whereas the income per capita was the only factor associated with a positive difference: adjusted coefficients (log OR (odds ratio)) −0.0014 (95% CI −0.0026 to −0.0001) and 3.2e−6 (0.3e−6 to 6.0e−6), respectively (Table III). The secondary analysis confirmed that the duration of lockdowns was higher in countries that experienced a difference >−10% of live births in January 2021 compared to those that did not experience this drop: average duration of 54 days versus 27 days, adjusted coefficient 0.0076 (95% CI 0.0016 to 0.0137, Table IV); and tended to be lower in countries that experienced a rebound (>+1%) in live births in March 2021: average duration of 31 days versus 55 days, adjusted coefficient 0.0066 (95% CI −0.0135 to 0.0003, Table V).
Table II.
COVID-19 cases and deaths, impact on intensive care units (ICUs), lockdowns duration and stringency, variation in birth rates and pre-pandemic incomes in the countries included.
| Country | Date of epidemic peaks | Total cases (31 May 2020) | Total deaths (31 May 2020) | Impact on ICUs* | Date of lockdown | Lockdown length (days) | Stringency index | Income per capita ($/year)** | Birth rate trend before the pandemic (2015–2019) | Variation in births in January 2021 compared to January 2018–2019 |
|---|---|---|---|---|---|---|---|---|---|---|
| Lithuania | April 3 | 1670 (0.61‰) | 70 (0.03‰) | Low | March 16 | 94 | 81.48 | High, 37 420 | −3.4% | −28.1% |
| Ukraine | Mai 7 | 23 672 (0.54‰) | 708 (0.02‰) | Low | March 17 | 38 | 88.89 | High, 13 260 | −6.9% | −24.4% |
| Spain | March 31 | 239 600 (5.12‰) | 29 043 (0.62‰) | High | March 14 | 56 | 85.19 | High, 42 250 | −3.8% | −23.5% |
| Romania | April 21 | 19 133 (0.99‰) | 1253 (0.06‰) | Low | March 25 | 48 | 87.04 | High, 31 410 | 0.1% | −23.3% |
| Russia | May 12 | 405 843 (2.78‰) | 4693 (0.03‰) | Moderate | March 28 | 43 | 78.24 | High, 27 550 | −6.4% | −19.1% |
| Portugal | April 3 | 32 203 (3.16‰) | 1396 (0.14‰) | Moderate | March 19 | 14 | 82.41 | High, 33 980 | 0.3% | −17.8% |
| Italy | March 27 | 232 664 (3.85‰) | 33 340 (0.55‰) | High | March 9 | 70 | 91.67 | High, 42 270 | −2.9% | −17.1% |
| Latvia | April 1 | 1065 (0.56‰) | 24 (0.01‰) | Low | No lockdown | 69.44 | High, 31 590 | −3.8% | −15.5% | |
| France | April 17 | 148 436 (2.24‰) | 28 717 (0.43‰) | High | March 17 | 55 | 87.96 | High, 50 400 | −1.6% | −14.4% |
| UK | April 10 | 272 830 (4.01‰) | 38 376 (0.56‰) | High | March 23 | 103 | 79.63 | High, 47 620 | −2.4% | −13.5% |
| Estonia | April 5 | 1865 (1.41‰) | 67 (0.05‰) | Low | March 11 | 31 | 77.78 | High, 37 940 | 0.4% | −12.8% |
| Belgium | April 12 | 58 186 (5.02‰) | 9453 (0.81‰) | High | March 18 | 47 | 81.48 | High, 55 370 | −0.9% | −12.2% |
| Slovenia | April 2 | 1473 (0.72‰) | 108 (0.05‰) | Low | March 15 | 35 | 89.81 | High, 40 530 | −1.6% | −8.8% |
| Germany | March 30 | 181 482 (2.23‰) | 8500 (0.42‰) | Moderate | March 22 | 29 | 76.85 | High, 55 220 | 1.4% | −7.1% |
| Switzerland | March 25 | 30 762 (3.64%) | 1656 (0.23‰) | Moderate | March 17 | 41 | 73.15 | High, 73 620 | −0.1% | −5.4% |
| Sweden | June 28 | 37 113 (3.73‰) | 4395 (0.41‰) | Moderate | No lockdown | 59.26 | High, 56 270 | 0.0% | −4.5% | |
| Austria | March 28 | 16 638 (1.85‰) | 668 (0.07‰) | Moderate | March 16 | 28 | 81.48 | High, 58 940 | 0.2% | −3.4% |
| Czechia | March 31 | 9230 (0.86‰) | 319 (0.03‰) | Low | March 16 | 27 | 82.41 | High, 40 360 | 0.3% | −3.2% |
| Hungary | April 14 | 3867 (0.40‰) | 524 (0.05‰) | Moderate | March 28 | 13 | 76.85 | High, 33 070 | −0.7% | −3.2% |
| Croatia | April 13 | 2246 (0.55‰) | 103 (0.03‰) | Low | March 18 | 54 | 96.3 | High, 28 630 | −0.9% | −2.9% |
| Netherlands | April 14 | 46 257 (2.70‰) | 5951 (0.34%) | Moderate | March 15 | 22 | 78.7 | High, 59 700 | −0.1% | −1.8% |
| Finland | March 30 | 6826 (1.23‰) | 316 (0.06‰) | Moderate | March 08 | 20 | 71.3 | High, 51 150 | −4.8% | 0.5% |
| Denmark | April 8 | 11 633 (2.01‰) | 571 (0.10‰) | High | March 12 | 31 | 72.22 | High, 62 180 | 1.3% | 1.9% |
Low = no overload in ICU (occupancy rate <80%), Moderate: overload in ICU (occupancy rate 80–100%) requiring changes in healthcare activities, High: outdated healthcare systems (occupancy rate of ICUs >100%), during the epidemic peak. Data are available at: https://www.ecdc.europa.eu/en/publications-data/download-data-response-measures-covid-19.
Low <1045, lower-middle income [1045–4095], upper-middle income [4096–12 695], high income >12 695 (Gross National Income per capita, US$, according to the World Bank classifications of countries).
Table III.
Factors associated with the differences observed in livebirths after the first wave of COVID-19 compared to the pre-pandemic period (2018–2019).
| Difference in live births between January 2021 and January of pre-pandemic years (2018–2019) |
||||
|---|---|---|---|---|
| Univariable analysis |
Multivariable analysis |
|||
| Coef (95% CI) | P | Coef (95% CI) | P | |
| Birthrates trend before the pandemic | 2.0 (0.6 to 3.3) | 0.004 | 0.5 (−1.0 to 2.1) | 0.502 |
| Deaths related to COVID-19, per 1000 | −0.035 (−0181 to 0.11.0) | 0.637 | ||
| Overocupancy in ICUs (>100%) | −0.025 (−0.102 to 0.510) | 0.513 | ||
| Lockdown length | −0.0013 (−0.0024 to −0.0002) | 0.019 | −0.0014 (−0.0026 to −0.0001) | 0.032 |
| Stringency index | −0.0039 (−0.0081 to 0.0002) | 0.066 | 0.0017 (−0.0051 to 0.0086) | 0.611 |
| Income per capita | 3.6e−6 (1.4e−6 to 5.8e−6) | 0.001 | 3.2e−6 (0.3e−6 to 6.0e−6) | 0.031 |
Coefficients with 95% CIs and P-values were estimated with generalized linear models. These coefficients correspond to the mean difference in birth rates for every additional unit increase in the independent variables. All variables are continuous, except for the overoccupancy of intensive care units (ICUs) (binary variable). Variables with a P < 0.10 in the univariable analysis were included in the multivariable analysis.
Table IV.
Factors associated with a drop >−10% in livebirths in January 2021 compared to January 2018–2019.
| Countries with a substantial drop (>−10%) in livebirths in January 2021 | Countries without substantial drop in livebirths in January 2021 | Univariable analysis |
Multivariable analysis |
|||
|---|---|---|---|---|---|---|
| n = 13 | n = 11 | Coef [95% CI] | P | Coef [95% CI] | P | |
| Birthrates trend before the pandemic—mean (min–max) | −2.6% (−6.9% to 0.4%) | −0.5% (−4.8% to 1.4%) | −10.5 [−18.6 to −2.4] | 0.011 | −3.9 [−12.7 to 5.0] | 0.393 |
| COVID-19 mortality—mean (min–max) | 0.29‰ (0.01‰ to 0.81‰) | 0.16‰ (0.03‰ to 0.42‰) | 0.6 [−0.3 to 1.4] | 0.172 | ||
| Occupancy of ICUs >100%—n (%) | 6 (46.2%) | 1 (9.1%) | 0.4 [0.1 to 0.9] | 0.037 | * | |
| Lockdown length (d)—mean (min–max) | 54 (0 to 103) | 27 (0 to 54) | 0.0086 [0.0020 to 0.0152] | 0.010 | 0.0076 [0.0016 to 0.0137] | 0.014 |
| Stringency index—mean (min–max) | 82.4 (69.4 to 91.7) | 78.0 (59.3 to 96.3) | 0.017 [−0.008 to 0.152] | 0.180 | ||
| Income per capita—mean (min–max) | 38 360$/y (13 260 to 55 370) | 50 879$/y (28 630 to 73 620) | −1.7e−5 [−3.1e−5 to 3.6−6] | 0.013 | −1.3e−5 [−2.8e−5 to 10.4e−5] | 0.069 |
Coefficients with 95% CIs and P-values were estimated with generalized linear models. These coefficients correspond to the mean difference in birth rates for every additional unit increase in the independent variables. All variables are continuous, except for the overoccupancy of intensive care units (ICUs) (binary variable). Variables with a P < 0.10 in the univariable analysis were included in the multivariable analysis.
Variable not included in the multivariable analysis due to collinearity with the lockdown length.
Table V.
Factors associated with a rebound (>+1%) in livebirths in March 2021 compared to March 2018–2019.
| Countries with a substantial rebound (>+1%) in livebirths in March 2021 | Countries without substantial rebound in livebirths in March 2021 | Univariable analysis |
Multivariable analysis |
|||
|---|---|---|---|---|---|---|
| n = 13 | n = 11 | Coef [95% CI] | P | Coef [95% CI] | P | |
| Birthrates trend before the pandemic—mean (min–max) | −0.1% (−6.4% to 1.4%) | −2.7% (−4.77% to 1.4%) | 7.5 [−1.2 to 16.2] | 0.090 | 5.4 [−3.1 to 14.0] | 0.210 |
| COVID-19 mortality—mean (min–max) | 0.22‰ (0.03‰ to 0.81‰) | 0.26‰ (0.01‰ to 0.62‰) | −0.2 [−1.0 to 0.7] | 0.699 | ||
| Occupancy of ICUs > 100%—n (%) | 3 (23.1%) | 4 (36.4%) | −0.2 [−0.6 to 0.3] | 0.490 | ||
| Lockdown length (d)—mean (min–max) | 31 (0 to 55) | 55 (0 to 103) | −0.0077 [−0.0145 to −0.0009] | 0.025 | 0.0066 [−0.0135 to 0.0003] | 0.061 |
| Stringency index—mean (min–max) | 78.5 (59.3 to 96.3) | 82.6 (69.4 to 91.7) | −0.016 [−0.041 to 0.009] | 0.213 | ||
| Income per capita—mean (min–max) | 47 444$/y (27 550 to 62 180) | 40 142$/y (13 260 to 73 620) | 1.0e−5 [−4.8e−6 to 2.5−6] | 0.185 | ||
Coefficients with 95% CIs and P-values were estimated with generalized linear models. These coefficients correspond to the mean difference in birth rates for every additional unit increase in the independent variables. All variables are continuous, except for the overoccupancy of intensive care units (ICUs) (binary variable). Variables with a P < 0.10 in the univariable analysis were included in the multivariable analysis.
In this multivariable analysis, countries that experienced a difference >−10% of live births in January 2021 also tended to have lower per capita incomes: average income of 38 360$/y versus 50 879$/y, adjusted coefficient −1.3e−5 (95% CI −2.8e−5 to 10.4e−5, Table IV).
Discussion
The first wave of COVID-19 appears to have resulted in a 14% decrease in live births in Europe 9–10 months after the epidemic peak (January 2021). This decline at the European level appears to be the result of a substantial drop of 12–28% in 13 European countries. Within a few months (March 2021), corresponding to 9 months after the end of the first lockdown, a rebound in births also occurred in 13 countries. This trend in birth rates seems to be similar to what has been described in other high-income countries or following previous crises (Aassve et al., 2020; Ullah et al., 2020; Aassve et al., 2021; Stout et al., 2021). The decline we found is larger than the one previously described by De Geyter et al. (2022) in 11 European countries, which is mainly explained by a difference in the methods used to assess the variation in births in Europe. De Geyter et al. (2022) used P-scores to compare a 3-month period (October to December) with a previous reference for that period, whereas we used a comparison of monthly rates to identify the month with the largest decline in birth rate (January 2021). They also found an association between an excess of mortality in the general population and a decline in births after the first wave of the pandemic (De Geyter et al., 2022). In our study, the mortality related to COVID-19 was higher in the countries that experienced a decline in births >10% in January (Table IV), but this difference did not reach statistical significance. Once again, this difference seems to be explained by the methods used, as we preferred not to consider the variation in mortality compared to the previous years, but the estimated number of deaths related to COVID-19, which may be underestimated and less reliable than the P-scores used by De Geyter et al.
Monthly live births in 2020 in Europe varied between −1% and −7% compared to the pre-pandemic period, with an annual variation rate of −3.1%, which is close to the average annual variation of −2.9% found in the pre-pandemic period. Thus, the COVID-19 pandemic does not appear to have changed the trend of live births in Europe in the early months of the pandemic. This suggests that direct exposure of pregnant women to COVID-19 at the beginning of the pandemic does not seem to be the primary factor resulting in a decline in births 9 months later. If direct exposure had a significant negative impact, it would have led to a drop in live births a few weeks/months after exposure, related to severe maternal complications or fetal/neonatal adverse outcomes. The incidence of COVID-19 during the first wave varied from 0.5‰ to 5.1‰ in the countries included. The case fatality rate among pregnant women with COVID-19 was estimated to be 1.3% (Karimi et al., 2021) and the rate of stillbirth among infected mothers was 0.5–5.0% (Hcini et al., 2021; Vouga et al., 2021; Wei et al., 2021). These rates are much lower than those described as having directly impacted birth rates in previous pandemics (Bloom-Feshbach et al., 2011; Dahal et al., 2018; Pomar et al., 2018; Foeller et al., 2020). Even if we cannot exclude that direct exposure could have increased the rate of miscarriage, partially contributing to a decrease 9 months later in births expected to reach term (Baud et al., 2020), it seems unlikely that direct exposure to COVID-19 during pregnancy is the only factor responsible for the observed decline in live births 9 months after the epidemic peak.
A decline in births 9 months after the epidemic peak appears to be more common in countries where the health system capacity was exceeded during the pandemic. Six of the seven countries with the higher rates of ICU overoccupancy (>100%) during the first wave encountered a decline in births 9 months later, while none of the countries and only two of the nine countries where health systems were slightly or moderately impacted, respectively, experienced a decline in births 9 months later. The overoccupancy of ICUs led to lockdowns and social distancing measures to contain the pandemic. Data from the included countries suggest that the longer the containment, the fewer pregnancies occurred during this period, even in countries not severely affected by the COVID-19 crisis. Conversely, Sweden, which had a high number of deaths but no lockdown, did not show a drop in live births. We hypothesize that the political decision about the lockdowns were taken according to the overoccupancy of ICUs. From our data, it seems that a decrease in birth rates was more associated with the duration of lockdowns (which directly impact the couples) than with the overoccupancy of ICUs, explaining why we chose to include only the duration of the lockdowns in our multivariable model due to collinearity between these two variables.
At the beginning of the lockdowns, the media suggested there was an increase in intercourse frequency for couples who worked from home, however, this period instead seems to be associated with a decrease in sexual desire (Li et al., 2020; Schiavi et al., 2020), even more in unemployed women (Fuchs et al., 2020). The stress related to the lack of information available on the maternal and fetal consequences of SARS-CoV-2 infection at the beginning of the pandemic, and a potential social, health and economic crisis at the end of the pandemic could be one of the main factors influencing the choice of couples to postpone pregnancies until after the end of the first wave (Luppi et al., 2020; Puig-Barrachina et al., 2020; Ceulemans et al., 2021). The decision by couples to delay conception during a health crisis may also be a consequence of health recommendations (the American Society for Reproductive Medicine and the European Society of Human Reproduction and Embryology released an advisory to avoid reproductive care at the beginning of the pandemic), although these recommendations were controversial and temporary (Rasmussen et al., 2020; Townsend et al., 2021).
Finally, the rebound observed 2 months later does not seem to compensate for decline in birth rates observed in January 2021. According to Aassve et al. (2020), the consequences of a drop in births could result in socioeconomic concerns in high-income countries, related to population aging and long-term decline. Advances in economic and social development, however, reinforcing the well-being and reinsurance of the population could have the potential to reverse a fertility decline during a crisis (Myrskyla et al., 2009). This could explain why the countries without a decline that did have a rebound in births in March 2021, are mostly those with the highest income per capita in Europe (Austria, Denmark, Finland, Germany, the Netherlands and Sweden). It should be noted that maternity services’ anticipation of a rebound in births is crucial as health care providers, who are already at higher risk of anxiety, depression and sleep disturbances due to the increased workload during the pandemic, may now have to cope with an increase in births (Marvaldi et al., 2021).
The main limitation of the present study is that it is based on currently available data and did not allow the inclusion of some European countries for which data are not yet available. However, it is unlikely that the evolution of births in these countries will change our conclusions at the European level given the inclusion of the most populous countries. Our data are based on national data, limiting the power in the multivariable models used and the identification of other potential factors contributing to a decrease or an increase in births. In addition, we collected only live births up to April 2021, which precludes the identification of a difference in births seasonality in 2021. The seasonality we observed in the pre-pandemic years suggests that the first and last quarters of the year are those with the fewest births in Europe. But this seasonality could be impacted by the pandemic and containment, and result in an increase in births in the last months of 2021, which would minimize the decline of births over the whole year. This variation in birth seasonality might be more expected in countries with high socioeconomic and educational levels (Bobak and Gjonca, 2001). The health crisis associated with the COVID-19 pandemic, and the choice of measures used to control its spread, are complex phenomena based on many health, but also social, economic and political variables. Identifying the factors associated with a decline in births in such a crisis is limited by the complexity of the phenomenon, but also by the limited data yet available on some factors. Thus, our study seems to show an association between a decrease in birth rates and the duration of lockdowns, but we cannot exclude that other factors interfere in this association, and we cannot conclude a causal link.
Future studies should be undertaken as more data become available to assess the consequences of the various waves of the pandemic on fertility and the impact of public health policies based on national and individual data. Individual questionnaires on the determinants of the decisions about pregnancy timing during the COVID-19 crisis may provide a deeper understanding of the trends in birthrates.
Conclusion
The COVID-19 pandemic seems to have contributed to a drop in birthrates 9–10 months after its first wave in Europe. This drop could particularly affect countries with a declining birth rate prior to the pandemic and those which were severely affected by the first wave, requiring long and harsh lockdowns to contain the pandemic. Social distancing measures, fears related to the pathogen and the social/economic crisis may be indirect factors that played a role in the decision of couples to postpone pregnancies and could be investigated in future research.
Supplementary Material
Contributor Information
Léo Pomar, Department Woman-Mother-Child, Lausanne University Hospital and Lausanne University, Lausanne, Switzerland; School of Health Sciences (HESAV), HES-SO University of Applied Sciences and Arts Western Switzerland, Lausanne, Switzerland.
Guillaume Favre, Department Woman-Mother-Child, Lausanne University Hospital and Lausanne University, Lausanne, Switzerland.
Claire de Labrusse, School of Health Sciences (HESAV), HES-SO University of Applied Sciences and Arts Western Switzerland, Lausanne, Switzerland.
Agathe Contier, Department Woman-Mother-Child, Lausanne University Hospital and Lausanne University, Lausanne, Switzerland.
Michel Boulvain, Faculty of Medicine, University of Geneva, Geneva, Switzerland.
David Baud, Department Woman-Mother-Child, Lausanne University Hospital and Lausanne University, Lausanne, Switzerland.
Data Availability
Previously published data were used for this work (https://www.humanfertility.org/cgi-bin/stff.php). The raw data used for the figures, including rates of change, are presented in the supplementary tables.
Authors’ roles
Conceptualization, L.P., C.d.L. and D.B.; methodology, L.P., G.F. and M.B.; investigation and data management: L.P. and A.C.; data analysis: L.P. and G.F.; writing—original draft preparation, L.P., C.d.L. and M.B.; writing—review and editing, G.F., A.C. and D.B. All authors have read and agreed to the published version of the manuscript.
Funding
No funding was secured for this study. The authors have no financial relationships relevant to this article to disclose.
Conflict of interest
The authors have no conflicts of interest to disclose.
References
- Aassve A, Cavalli N, Mencarini L, Plach S, Livi Bacci M.. The COVID-19 pandemic and human fertility. Science 2020;369:370–371. [DOI] [PubMed] [Google Scholar]
- Aassve A, Cavalli N, Mencarini L, Plach S, Sanders S.. Early assessment of the relationship between the COVID-19 pandemic and births in high-income countries. Proc Natl Acad Sci USA 2021;118:e2105709118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud D, Greub G, Favre G, Gengler C, Jaton K, Dubruc E, Pomar L.. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA 2020;323:2198–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom-Feshbach K, Simonsen L, Viboud C, Molbak K, Miller MA, Gottfredsson M, Andreasen V.. Natality decline and miscarriages associated with the 1918 influenza pandemic: the Scandinavian and United States experiences. J Infect Dis 2011;204:1157–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobak M, Gjonca A.. The seasonality of live birth is strongly influenced by socio-demographic factors. Hum Reprod 2001;16:1512–1517. [DOI] [PubMed] [Google Scholar]
- Ceulemans M, Foulon V, Ngo E, Panchaud A, Winterfeld U, Pomar L, Lambelet V, Cleary B, O'Shaughnessy F, Passier A. et al. Mental health status of pregnant and breastfeeding women during the COVID-19 pandemic—a multinational cross-sectional study. Acta Obstet Gynecol Scand 2021;100:1219–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho FC, Armstrong M, Saraceni V, Lemos C.. Can Zika account for the missing babies? Front Public Health 2017;5:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahal S, Mizumoto K, Bolin B, Viboud C, Chowell G.. Natality decline and spatial variation in excess death rates during the 1918-1920 influenza pandemic in Arizona, United States. Am J Epidemiol 2018;187:2577–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg J, Andersson G.. Changing seasonal variation in births by sociodemographic factors: a population-based register study. Hum Reprod Open 2018;2018:hoy015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Geyter C, Masciocchi M, Gobrecht-Keller U.. Excess mortality caused by the COVID-19 pandemic negatively impacts birth numbers in European countries. Hum Reprod 2022;37:822–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECDC. Data on Country Response Measures to COVID-19. Data on country response measures to COVID-19 (europa.eu). 2020. https://www.ecdc.europa.eu/en/publications-data/download-data-response-measures-covid-19 (11 October 2021, date last accessed).
- Foeller ME, Carvalho Ribeiro do Valle C, Foeller TM, Oladapo OT, Roos E, Thorson AE.. Pregnancy and breastfeeding in the context of Ebola: a systematic review. Lancet Infect Dis 2020;20:e149–e158. [DOI] [PubMed] [Google Scholar]
- Fuchs A, Matonog A, Pilarska J, Sieradzka P, Szul M, Czuba B, Drosdzol-Cop A.. The impact of COVID-19 on female sexual health. Int J Environ Res Public Health 2020;17:7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T, Angrist N, Goldszmidt R, Kira B, Petherick A, Phillips T, Webster S, Cameron-Blake E, Hallas L, Majumdar S. et al. A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker). Nat Hum Behav 2021;5:529–538. [DOI] [PubMed] [Google Scholar]
- Haleem A, Javaid M, Vaishya R.. Effects of COVID-19 pandemic in daily life. Curr Med Res Pract 2020;10:78–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah Ritchie EM, Rodés-Guirao L, Appel C, Giattino C, Ortiz-Ospina E, Hasell J, Macdonald B, Beltekian D, Roser M.. Coronavirus pandemic (COVID-19). Our World in Data 2020. https://ourworldindata.org/coronavirus. [Google Scholar]
- Hcini N, Maamri F, Picone O, Carod JF, Lambert V, Mathieu M, Carles G, Pomar L.. Maternal, fetal and neonatal outcomes of large series of SARS-CoV-2 positive pregnancies in peripartum period: a single-center prospective comparative study. Eur J Obstet Gynecol Reprod Biol 2021;257:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasilioniene A, Sobotka T, Jdanov DA, Zeman K, Kostova D, Andreev EM, Grigoriev P, Shkolnikov VM.. Data resource profile: the human fertility database. Int J Epidemiol 2016;45:1077–1078e. [DOI] [PubMed] [Google Scholar]
- Karimi L, Makvandi S, Vahedian-Azimi A, Sathyapalan T, Sahebkar A.. Effect of COVID-19 on mortality of pregnant and postpartum women: a systematic review and meta-analysis. J Pregnancy 2021;2021:8870129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Tang D, Song B, Wang C, Qunshan S, Xu C, Geng H, Wu H, He X, Cao Y.. Impact of the COVID-19 pandemic on partner relationships and sexual and reproductive health: cross-sectional, online survey study. J Med Internet Res 2020;22:e20961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Yin T, Fang F, Li Q, Chen J, Wang Y, Hao Y, Wu G, Duan P, Wang Y. et al. Potential risks of SARS-CoV-2 infection on reproductive health. Reprod Biomed Online 2020;41:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppi F, Arpino B, Rosina A.. The impact of COVID-19 on fertility plans in Italy, Germany, France, Spain, and the United Kingdom. Dem Res 2020;43:1399–1412. [Google Scholar]
- Marvaldi M, Mallet J, Dubertret C, Moro MR, Guessoum SB.. Anxiety, depression, trauma-related, and sleep disorders among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Neurosci Biobehav Rev 2021;126:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrskyla M, Kohler HP, Billari FC.. Advances in development reverse fertility declines. Nature 2009;460:741–743. [DOI] [PubMed] [Google Scholar]
- Pomar L. Potential consequences of Sars-Cov-2 pandemic on birth rates and subsequent demographics. Investig Gynecol Res Womens Health (IGRWH) 2020;3:295–296. [Google Scholar]
- Pomar L, Vouga M, Lambert V, Pomar C, Hcini N, Jolivet A, Benoist G, Rousset D, Matheus S, Malinger G. et al. Maternal-fetal transmission and adverse perinatal outcomes in pregnant women infected with Zika virus: prospective cohort study in French Guiana. BMJ 2018;363:k4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig-Barrachina V, Rodriguez-Sanz M, Dominguez-Berjon MF, Martin U, Luque MA, Ruiz M, Perez G.. Decline in fertility induced by economic recession in Spain. Gac Sanit 2020;34:238–244. [DOI] [PubMed] [Google Scholar]
- Rasmussen SA, Lyerly AD, Jamieson DJ.. Delaying pregnancy during a public health crisis—examining public health recommendations for Covid-19 and beyond. N Engl J Med 2020;383:2097–2099. [DOI] [PubMed] [Google Scholar]
- Schiavi MC, Spina V, Zullo MA, Colagiovanni V, Luffarelli P, Rago R, Palazzetti P.. Love in the time of COVID-19: sexual function and quality of life analysis during the social distancing measures in a group of Italian reproductive-age women. J Sex Med 2020;17:1407–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Gromski PS, Rashid KA, Tilling K, Lawlor DA, Nelson SM.. Population implications of cessation of IVF during the COVID-19 pandemic. Reprod Biomed Online 2020;41:428–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout MJ, Van De Ven CJM, Parekh VI, Pardo JL, Garifullin M, Xu M, Fenner DE, Smith RD.. Use of electronic medical records to estimate changes in pregnancy and birth rates during the COVID-19 pandemic. JAMA Netw Open 2021;4:e2111621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend R, Chmielewska B, Barratt I, Kalafat E, van der Meulen J, Gurol-Urganci I, O'Brien P, Morris E, Draycott T, Thangaratinam S. et al. Global changes in maternity care provision during the COVID-19 pandemic: a systematic review and meta-analysis. EClinicalMedicine 2021;37:100947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah MA, Moin AT, Araf Y, Bhuiyan AR, Griffiths MD, Gozal D.. Potential Effects of the COVID-19 Pandemic on Future Birth Rate. Front Public Health 2020;8:578438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UN. UN Databases. 2021. https://www.un.org/en/library/page/databases (11 October 2021, date last accessed).
- Vouga M, Favre G, Martinez-Perez O, Pomar L, Acebal LF, Abascal-Saiz A, Hernandez MRV, Hcini N, Lambert V, Carles G. et al. Maternal outcomes and risk factors for COVID-19 severity among pregnant women. Sci Rep 2021;11:13898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrachnis N, Vlachadis N, Iliodromiti Z, Vlachadi M, Creatsas G.. Greece's birth rates and the economic crisis. Lancet 2014;383:692–693. [DOI] [PubMed] [Google Scholar]
- Wei SQ, Bilodeau-Bertrand M, Liu S, Auger N.. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ 2021;193:E540–E548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Coronavirus Disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Update. Coronavirus Disease (COVID-19) Situation Reports (who.int). 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (11 October 2021, date last accessed).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Previously published data were used for this work (https://www.humanfertility.org/cgi-bin/stff.php). The raw data used for the figures, including rates of change, are presented in the supplementary tables.