Abstract
Objective
The current diagnostic criteria for periprosthetic joint infection (PJI) are diverse and controversial, leading to delayed diagnosis. This study aimed to evaluate and unify their diagnostic accuracy and the threshold selection of serum and synovial routine tests for PJI at an early stage.
Methods
We searched the MEDLINE and Embase databases for retrospective or prospective studies which reported preoperative‐available assays (serum, synovial, or culture tests) for the diagnosis of chronic PJI among inflammatory arthritis (IA) or non‐IA populations from January 1, 2000 to June 30, 2022. Threshold effective analysis was performed on synovial polymorphonuclear neutrophils (PMN%), synovial white blood cell (WBC), serum C‐reactive protein (CRP), and erythrocyte sedimentation rate (ESR) to find the relevant cut‐offs.
Results
Two hundred and sixteen studies and information from 45,316 individuals were included in the final analysis. Synovial laboratory‐based α‐defensin and calprotectin had the best comprehensive sensitivity (0.91 [0.86–0.94], 0.95 [0.88–0.98]) and specificity (0.96 [0.94‐0.97], 0.95 [0.89–0.98]) values. According to the threshold effect analysis, the recommended cut‐offs are 70% (sensitivity 0.89 [0.85–0.92], specificity 0.90 [0.87–0.93]), 4100/μL (sensitivity 0.90 [0.87–0.93], specificity 0.97 [0.93–0.98]), 13.5 mg/L (sensitivity 0.84 [0.78–0.89], specificity 0.83 [0.73–0.89]), and 30 mm/h (sensitivity 0.79 [0.74–0.83], specificity 0.78 [0.72–0.83]) for synovial PMN%, synovial WBC, serum CRP, and ESR, respectively, and tests seem to be more reliable among non‐IA patients.
Conclusions
The laboratory‐based synovial α‐defensin and synovial calprotectin are the two best independent preoperative diagnostic tests for PJI. A cut off of 70% for synovial PMN% and tighter cut‐offs for synovial WBC and serum CRP could have a better diagnostic accuracy for non‐IA patients with chronic PJI.
Keywords: Diagnosis, Meta‐analysis, Periprosthetic joint infection, Serum and synovial test, Threshold effect, Total joint arthroplasty
This work provides additional insights and brand‐new evidences to the diagnostic accuracy of traditional and novel tests in early diagnosis of periprosthetic joint infection (PJI), especially the threshold selection. The results could attract more attentions on finding reliable cut‐offs and further influence the update of present criteria, which might provide significant meanings to the diagnosis and therapy strategy of PJI.
Introduction
Different guidelines, criteria, and articles recommend diverse tests for periprosthetic joint infection (PJI), and the research on this subject is continuously updated, creating further confusion. 1 , 2 Positive cultures of tissues or joint fluids extracted from patients are the most intuitionistic evidence supporting direct PJI diagnosis, but the high rates of delayed or missed diagnosis is the main constraint. The Musculoskeletal Infection Society (MSIS) criteria, 3 , 4 one of the most globally accepted PJI diagnostic strategies, selects aspiration culture and the presence of the sinus tract as the major criteria and chooses other routine serum and synovial tests like serum C‐reactive protein (CRP), erythrocyte sedimentation rate (ESR), synovial white blood cell (WBC), and synovial polymorphonuclear neutrophils (PMN%) as minor criteria, similar to the diagnostic criteria published by the Infectious Diseases Society of America 5 and the European Bone and Joint Infection Society criteria. 6 Although the MSIS criteria present a reliable definition of PJI, it does not aid diagnosis at the very early stage of disease when infection occurs, because the tissue and aspiration culture require days or even weeks for the pathogen to grow.
The tests that can be performed before revision surgery have also been discussed widely, their various cut‐offs, especially the routine tests like serum CRP and synovial WBC, showed controversial diagnostic performance in different studies. In addition, the different gold standards selected in various articles may also lead to the over‐ or under‐estimation of the preoperative diagnostic accuracy of histological markers. 1 , 2 , 7 , 8 Furthermore, patients with inflammatory arthritis (IA), including inflammatory autoimmune arthritis, such as rheumatoid arthritis, who are undergoing immunosuppressive treatment have not only a higher risk of PJI, but the level of their inflammatory markers, especially synovial WBC and PMN%, have also been reported to be extremely similar with those of ordinary PJI patients, further complicating PJI diagnosis. 9 , 10 Moreover, the most important facts are probably interfering with the diagnostic accuracy of these tests, and the cut‐offs selected before are based mainly on experts' consensus, without scientific evidence or evaluation. 11 Several experts have previously noticed this problem. 12 After the 2018 International Consensus Meeting (ICM), the updated consensus on PJI has added new tests and altered the cut‐offs of PMN% from 80% to 70%, which verified that the cut‐offs selection had received enough attention. 13 , 14 Herein, a systematic review by Carli et al. 1 tried to unify these ambiguities, but reported only a composite conclusion that synovial tests have a better holistic diagnostic capability than serum‐ or tissue‐based tests, maintaining the study with excess IA population and ignoring the mistiness of thresholds. Additionally, their work did not refer the results to a unified gold standard like the MSIS criteria.
The primary objective of this study was to identify the independent tests and their reliable cut‐offs for the diagnosis of chronic PJI with satisfactory diagnostic capability. The secondary objective was to separately evaluate the diagnostic performance of the tests among non‐IA and IA populations.
Methods and Materials
Search Strategy and Selection Criteria
This meta‐analysis and systematic review was performed according to the Cochrane Collaboration guidelines. 15 Prospective and retrospective studies were included if they provided information on the diagnostic test accuracy of one or more preoperative biomarkers for PJI diagnosis. We included studies published from January 2000 to June 2022 by systematically searching MEDLINE and EMBASE databases, using a search string based on MeSH (Appendix S1). Inclusion criteria were: (i) studies with data on specific information of preoperative accessible biomarkers from patients who needed revision surgery for the treatment of PJI after knee or hip joint arthroplasty; (ii) studies with sufficient data to calculate the number of true‐positive (TP), false‐negative (FN), false‐positive (FP), and true‐negative (TN) patients with and without IA in the study; (iii) studies that reported a clear “gold standard”; and (iv) the cutoffs reported. Exclusion criteria were: (i) studies demonstrated the invalid information including patients with shoulder or other arthroplasties; (ii) studies reported the diagnostic biomarkers accessible only from revision surgery progress, such as periprosthetic tissue or sonicate fluid for culture; (iii) studies without extractive data for diagnostic test accuracy, such as TP, FP, FN, and TN; (iv) studies that provided equivocal gold standard or reference diagnostic methods; (v) research included suspicious same group of the patients; (vi) cadaver studies; and (vii) studies not in English.
Data Extraction
All independently extracted articles were screened and reviewed independently by two researchers (TH and YH), and any controversy was resolved by a third reviewer (QX). One investigator (TH) extracted the data, including patient characteristics and biomarker details. To maximize the number of the studies and retain more calculable information, we retained all studies which reported the partial information mentioned above, and recorded the details for further subgroup analysis. The suspected duplicated data was deleted and retained only once in the end.
Data Analysis and Threshold Effective Analysis
Statistical analyses were performed using the Stata/SE version 17.1 (Stata Corp, College Station, TX, USA). 16 R software (The R Project, Vienna, Austria) was used for additional statistical analysis. Two reviewers (TH and JX) calculated the TP, FP, FN, and TN using relevant data extracted from included studies to independently evaluate the subsequent diagnostic accuracy of PJI. 17 The forest plot analysis, positive and negative likelihood ratios, diagnostic odds ratio (DOR), and the area under the curve of the summary receiver operating characteristic curves (AUCs) were analyzed among different diagnostic markers. 18 , 19 Fagan's nomograms were used to demonstrate the post‐test probability, and likelihood ratio scattergrams were used to evaluate the clinical application value of different markers. Publication bias was estimated using the Deek's funnel plot asymmetry test. 20
The threshold level was determined by trial and error, including the selection of turning points along a predefined interval, after which the turning point that gave the maximum model likelihood was chosen. We conducted a log likelihood ratio test comparing the one‐line linear regression model with the two‐piecewise linear model. 21 Heterogeneity calculation was conducted using Cochran's Q‐test. The random effects model was used to evaluate the diagnostic accuracy when the result showed significant heterogeneity (I 2 > 50%), while the fixed effects model was used when the result showed minor heterogeneity (I 2 < 50%). 22 We divided the original sample into different subgroups based on the IA comorbidities, types of arthroplasty, and gold standards for heterogeneity analysis. 18 We used the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS‐2) tool to evaluate the quality of the articles. 23
Results
Study Selection
Final analysis was performed on 215 articles with six additional studies searched manually, as shown in Fig. Fig. 1, and the QUADAS‐2 score criteria revealed that most of the extracted studies had high risks. Excluding brand new markers that only appeared recently and have limited data, 129 records reported diagnostic information of serum markers (serum CRP, ESR, procalcitonin, IL‐6, D‐Dimer, WBC, platelet count [PLT], neutrophil to lymphocyte ratio [NLR], monocyte to lymphocyte ratio [MLR], platelet to lymphocyte ratio [PLR], platelet to mean platelet volume ratio [PVR], FDP [fibrin degradation product] and Fibrinogen), 9 , 10 , 11 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 114 studies reported synovial markers (synovial WBC, PMN%, IL‐6, IL‐1β, CRP, TNF‐α, Calprotectin, and leucocyte esterase [LE] and laboratory or lateral‐flow based α‐defensin test), 10 , 29 , 31 , 34 , 36 , 43 , 49 , 51 , 52 , 58 , 59 , 61 , 62 , 64 , 65 , 72 , 73 , 74 , 76 , 77 , 79 , 80 , 81 , 84 , 85 , 86 , 87 , 88 , 90 , 95 , 97 , 99 , 117 , 118 , 122 , 124 , 125 , 127 , 129 , 132 , 135 , 136 , 137 , 138 , 142 , 144 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 , 186 , 187 , 188 , 189 , 190 , 191 , 192 , 193 , 194 , 195 , 196 , 197 , 198 , 199 , 200 , 201 , 202 , 203 , 204 , 205 , 206 , 207 , 208 , 209 , 210 , 211 , 212 , 213 , 214 , 215 , 216 , 217 and 36 studies reported aspiration culture. 24 , 25 , 26 , 31 , 35 , 36 , 40 , 44 , 62 , 81 , 82 , 83 , 84 , 96 , 97 , 115 , 130 , 138 , 197 , 198 , 209 , 210 , 218 , 219 , 220 , 221 , 222 , 223 , 224 , 225 , 226 , 227 , 228 , 229 , 230 , 231 , 232 , 233 for chronic PJI (Appendix S2). All 24 biomarkers tests were evaluated on the basis of ≥2 articles, with only synovial TNF‐α evaluated on the basis of only two articles.
Fig. 1.
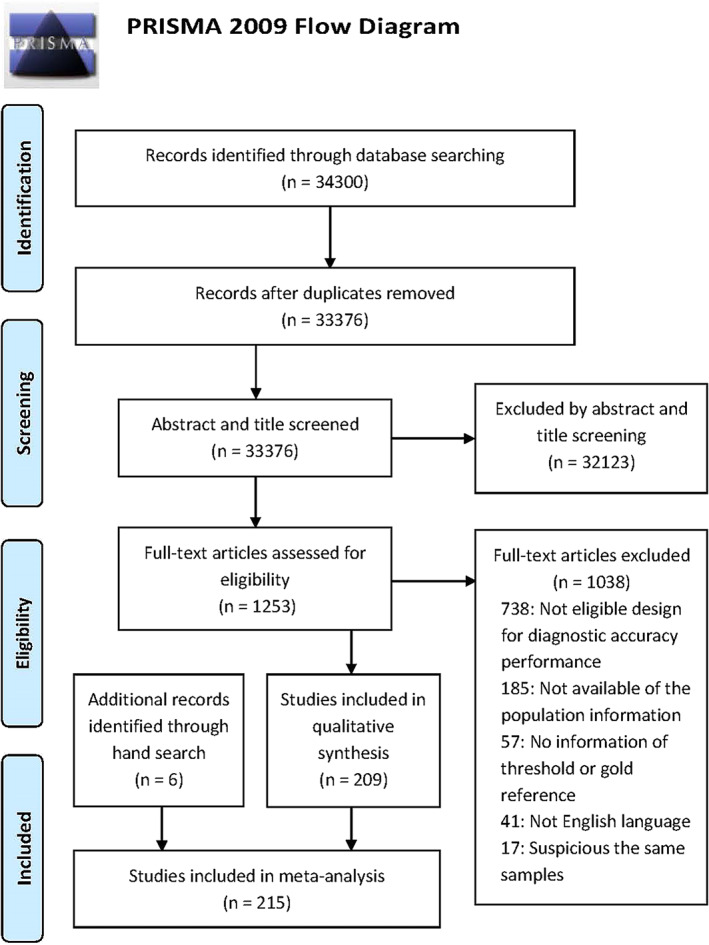
Flow diagram of the included studies
Main Characteristics
All synovial biomarkers but TNF‐α showed AUC ≥ 0.9. The diagnostic accuracy of most synovial tests performed better than serum tests and aspiration culture, while laboratory‐based α‐defensin test (ELISA) and calprotectin test are the two best among the 10 synovial tests in the chronic PJI population. Among all 13 serum tests, serum IL‐6 has the highest DOR and AUC. We then maintained only the studies containing IA patients lower than 5% (chronic PJI without IA population) for subsequent analysis, achieving similar results (Table 1 and Figs [Link], [Link]).
TABLE 1.
Diagnostic test accuracy of serum, synovial tests and aspiration culture
| Studies Number | SEN (95% CI) | SPE (95% CI) | AUC (95% CI) | Positive‐LR (95% CI) | Negative‐LR (95% CI) | DOR (95% CI) | I 2 (P value) | |
|---|---|---|---|---|---|---|---|---|
| Chronic PJI | ||||||||
| Serum tests | ||||||||
| CRP | 120 | 0.82 (0.80, 0.84) | 0.79 (0.76, 0.81) | 0.87 (0.84, 0.90) | 3.9 (3.5, 4.4) | 0.23 (0.20, 0.26) | 17 (14, 21) |
100% (P < 0.001) |
| ESR | 92 | 0.79 (0.76, 0.82) | 0.78 (0.74, 0.81) | 0.85 (0.82, 0.88) | 3.5 (3.1, 4.0) | 0.27 (0.24, 0.31) | 13 (10, 16) |
100% (P < 0.001) |
| IL‐6 | 19 | 0.86 (0.78, 0.92) | 0.83 (0.76, 0.88) | 0.91 (0.88, 0.93) | 5.1 (3.6, 7.3) | 0.17 (0.10, 0.27) | 31 (16, 60) |
99% (P < 0.001) |
| D‐dimer | 24 | 0.77 (0.69, 0.83) | 0.75 (0.66, 0.82) | 0.82 (0.79, 0.85) | 3.0 (2.3, 4.0) | 0.31 (0.24, 0.41) | 10 (6, 15) |
99% (P < 0.001) |
| PCT | 12 | 0.57 (0.43, 0.70) | 0.90 (0.79, 0.95) | 0.81 (0.77, 0.84) | 5.6 (2.9, 10.8) | 0.48 (0.36, 0.63) | 12 (6, 23) |
99% (P < 0.001) |
| WBC | 25 | 0.45 (0.35, 0.56) | 0.86 (0.78, 0.92) | 0.71 (0.67, 0.75) | 3.3 (2.3, 4.8) | 0.63 (0.54, 0.74) | 5 (3, 8) |
100% (P < 0.001) |
| PLT | 5 | 0.63 (0.59, 0.67) | 0.73 (0.67, 0.78) | 0.70 (0.66, 0.74) | 2.3 (1.9, 2.8) | 0.51 (0.45, 0.57) | 5 (4, 6) |
70% (P = 0.019) |
| NLR | 8 | 0.70 (0.65, 0.74) | 0.74 (0.70, 0.77) | 0.78 (0.74, 0.81) | 2.7 (2.2, 3.2) | 0.41 (0.34, 0.49) | 6 (4, 9) |
0% (P = 0.500) |
| MLR | 4 | 0.69 (0.57, 0.79) | 0.80 (0.77, 0.82) | 0.80 (0.77, 0.84) | 3.4 (2.8, 4.1) | 0.39 (0.27, 0.56) | 9 (5, 15) |
91% (P < 0.001) |
| PLR | 4 | 0.77 (0.73, 0.80) | 0.72 (0.57, 0.83) | 0.79 (0.75, 0.82) | 2.7 (1.7, 4.3) | 0.32 (0.25, 0.42) | 8 (4, 17) |
92% (P < 0.001) |
| PVR | 10 | 0.71 (0.61, 0.80) | 0.75 (0.70, 0.79) | 0.79 (0.75, 0.82) | 2.8 (2.4, 3.3) | 0.38 (0.28, 0.53) | 7 (5, 11) |
99% (P < 0.001) |
| Fibrinogen | 15 | 0.80 (0.74, 0.85) | 0.84 (0.80, 0.87) | 0.89 (0.86, 0.91) | 4.9 (4.1, 5.8) | 0.24 (0.19, 0.31) | 20 (16, 26) |
95% (P < 0.001) |
| FDP | 7 | 0.73 (0.67, 0.78) | 0.70 (0.63, 0.75) | 0.77 (0.73, 0.81) | 2.4 (1.9, 3.0) | 0.39 (0.30, 0.51) | 6 (4, 10) |
0% (P = 0.464) |
| Synovial tests | ||||||||
| sWBC | 50 | 0.87 (0.84, 0.90) | 0. 90 (0.87, 0.92) | 0.95 (0.92, 0.96) | 8.7 (6.8, 11.2) | 0.14 (0.11, 0.18) | 62 (42, 92) |
99% (P < 0.001) |
| PMN% | 50 | 0.88 (0.85, 0.89) | 0.88 (0.85, 0.90) | 0.94 (0.91, 0.95) | 7.1 (5.7, 8.9) | 0.14 (0.12, 0.17) | 50 (36, 69) |
99% (P < 0.001) |
| sCRP | 25 | 0.87 (0.84, 0.90) | 0.93 (0.89, 0.95) | 0.94 (0.91, 0.96) | 12.0 (8.2, 17.7) | 0.14 (0.11, 0.17) | 85 (59, 123) |
99% (P < 0.001) |
| sIL‐1b | 7 | 0.88 (0.70, 0.96) | 0.92 (0.85, 0.96) | 0.95 (0.93, 0.97) | 10.6 (5.2, 21.8) | 0.13 (0.04, 0.38) | 83 (15, 456) |
23% (P = 0.136) |
| sIL‐6 | 11 | 0.82 (0.74, 0.88) | 0.95 (0.87, 0.98) | 0.93 (0.90, 0.95) | 15.0 (6.2, 36.3) | 0.19 (0.13, 0.28) | 80 (27, 239) |
96% (P < 0.001) |
|
α‐defensin Lab‐based |
16 | 0.91 (0.86, 0.94) | 0.96 (0.94, 0.97) | 0.98 (0.96, 0.99) | 21.3 (14.4, 31.4) | 0.10 (0.06, 0.14) | 221 (114, 428) |
88% (P = 0.211) |
|
α‐defensin Lateral Flow |
17 | 0.85 (0.78, 0.90) | 0.96 (0.95, 0.97) | 0.98 (0.96, 0.99) | 22.5 (15.8, 31.9) | 0.15 (0.10, 0.23) | 146 (92, 232) |
98% (P < 0.001) |
| LE | 19 | 0.88 (0.81, 0.92) | 0.94 (0.91, 0.96) | 0.97 (0.95, 0.98) | 14.0 (9.3, 20.9) | 0.13 (0.09, 0.21) | 105 (58, 189) | 99% (P < 0.001) |
| sTNF‐α | 4 | 0.76 (0.69, 0.82) | 0.81 (0.69, 0.89) | 0.80 (0.75, 0.84) | 4.1 (2.4, 6.8) | 0.29 (0.22, 0.38) | 14 (7, 27) |
86% (P < 0.001) |
| Calprotectin | 6 | 0.95 (0.88, 0.98) | 0.95 (0.89, 0.98) | 0.99 (0.97, 0.99) | 20.1 (7.9, 50.8) | 0.05 (0.02, 0.14) | 371 (70, 1959) |
0% (P = 0.494) |
| Aspiration culture | ||||||||
| Joint fluid | 36 | 0.65 (0.58, 0.72) | 0.97 (0.95, 0.98) | 0.92 (0.89, 0.94) | 20.5 (12.8, 32.9) | 0.36 (0.30, 0.44) | 57 (33, 98) |
98% (P < 0.001) |
| Chronic PJI without IA Population | ||||||||
| Serum tests | ||||||||
| CRP | 106 | 0.81 (0.79, 0.84) | 0.79 (0.76, 0.81) | 0.87 (0.84, 0.90) | 3.8 (3.4, 4.3) | 0.24 (0.21, 0.27) | 16 (13, 20) |
100% (P < 0.001) |
| ESR | 82 | 0.78 (0.75, 0.81) | 0.78 (0.75, 0.81) | 0.85 (0.82, 0.88) | 3.6 (3.2, 4.1) | 0.28 (0.24, 0.32) | 13 (11, 16) |
100% (P < 0.001) |
| IL‐6 | 16 | 0.84 (0.73, 0.92) | 0.84 (0.78, 0.89) | 0.91 (0.88, 0.93) | 5.4 (3.8, 7.7) | 0.19 (0.10, 0.34) | 29 (13, 65) |
98% (P < 0.001) |
| D‐dimer | 23 | 0.76 (0.69, 0.82) | 0.75 (0.66, 0.82) | 0.82 (0.79, 0.85) | 3.1 (2.3, 4.1) | 0.32 (0.24, 0.41) | 10 (6, 15) |
99% (P < 0.001) |
| PCT | 9 | 0.58 (0.40, 0.75) | 0.84 (0.69, 0.93) | 0.79 (0.75, 0.82) | 3.7 (2.1, 6.6) | 0.49 (0.35, 0.70) | 8 (4, 14) |
99% (P < 0.001) |
| WBC | 23 | 0.45 (0.34, 0.57) | 0.87 (0.79, 0.92) | 0.72 (0.68, 0.76) | 3.5 (2.3, 5.3) | 0.63 (0.53, 0.74) | 6 (3, 9) |
100% (P < 0.001) |
| PLT | 5 | 0.63 (0.59, 0.67) | 0.73 (0.67, 0.78) | 0.70 (0.66, 0.74) | 2.3 (1.9, 2.8) | 0.51 (0.45, 0.57) | 5 (4, 6) |
70% (P = 0.019) |
| NLR | 8 | 0.70 (0.65, 0.74) | 0.74 (0.70, 0.77) | 0.78 (0.74, 0.81) | 2.7 (2.2, 3.2) | 0.41 (0.34, 0.49) | 6 (4, 9) |
0% (P = 0.500) |
| MLR | 4 | 0.69 (0.57, 0.79) | 0.80 (0.77, 0.82) | 0.80 (0.77, 0.84) | 3.4 (2.8, 4.1) | 0.39 (0.27, 0.56) | 9 (5, 15) |
91% (P < 0.001) |
| PLR | 4 | 0.77 (0.73, 0.80) | 0.72 (0.57, 0.83) | 0.79 (0.75, 0.82) | 2.7 (1.7, 4.3) | 0.32 (0.25, 0.42) | 8 (4, 17) |
92% (P < 0.001) |
| PVR | 9 | 0.73 (0.62, 0.82) | 0.74 (0.69, 0.78) | 0.79 (0.75, 0.82) | 2.8 (2.4, 3.3) | 0.37 (0.26, 0.52) | 8 (5, 12) |
99% (P < 0.001) |
| Fibrinogen | 14 | 0.80 (0.74, 0.85) | 0.83 (0.79, 0.87) | 0.89 (0.86, 0.91) | 4.8 (4.0, 5.7) | 0.24 (0.18, 0.31) | 20 (16, 26) |
95% (P < 0.001) |
| FDP | 7 | 0.73 (0.67, 0.78) | 0.70 (0.63, 0.75) | 0.77 (0.73, 0.81) | 2.4 (1.9, 3.0) | 0.39 (0.30, 0.51) | 6 (4, 10) |
0% (P = 0.464) |
| Synovial tests | ||||||||
| sWBC | 47 | 0.87 (0.83, 0.90) | 0.90 (0.87, 0.92) | 0.95 (0.92, 0.96) | 8.7 (6.5, 11.4) | 0.15 (0.11, 0.19) | 59 (38, 93) |
99% (P < 0.001) |
| PMN% | 45 | 0.88 (0.85, 0.90) | 0.89 (0.86, 0.92) | 0.94 (0.91, 0.96) | 8.0 (6.1, 10.3) | 0.14 (0.12, 0.17) | 58 (41, 82) |
99% (P < 0.001) |
| sCRP | 19 | 0.88 (0.85, 0.91) | 0.94 (0.90, 0.96) | 0.95 (0.93, 0.97) | 14.6 (9.1, 23.2) | 0.12 (0.09, 0.16) | 118 (76, 182) |
98% (P < 0.001) |
| sIL‐1b | 4 | 0.91 (0.52, 0.99) | 0.94 (0.82, 0.98) | 0.97 (0.95, 0.98) | 15.8 (4.2, 59.6) | 0.09 (0.01, 0.77) | 167 (7, 4271) |
0% (P = 0.214) |
| sIL‐6 | 7 | 0.80 (0.63, 0.90) | 0.95 (0.80, 0.99) | 0.93 (0.91, 0.95) | 17.4 (3.4, 88.9) | 0.21 (0.10, 0.42) | 83 (10, 674) |
93% (P < 0.001) |
|
α‐defensin Lab‐based |
16 | 0.91 (0.88, 0.94) | 0.96 (0.93, 0.97) | 0.98 (0.96, 0.99) | 20.7 (13.6, 31.4) | 0.09 (0.06, 0.13) | 230 (116, 454) |
0% (P = 0.433) |
|
α‐defensin Lateral Flow |
17 | 0.85 (0.78, 0.90) | 0.96 (0.95, 0.97) | 0.98 (0.96, 0.99) | 22.5 (15.8, 31.9) | 0.15 (0.10, 0.23) | 146 (92, 232) |
98% (P < 0.001) |
| LE | 22 | 0.88 (0.82, 0.93) | 0.94 (0.90, 0.96) | 0.97 (0.95, 0.98) | 14.5 (9.3, 22.6) | 0.12 (0.08, 0.20) | 117 (63, 218) | 99% (P < 0.001) |
| sTNF‐α | 2 | 0.81 (0.70, 0.89) | 0.72 (0.64, 0.78) | 0.84 (0.78, 0.88) | 2.9 (2.2, 3.8) | 0.26 (0.16, 0.44) | 11 (5, 22) |
100% (P < 0.001) |
| Calprotectin | 5 | 0.95 (0.85, 0.98) | 0.95 (0.87, 0.98) | 0.99 (0.97, 0.99) | 20.3 (6.8, 60.7) | 0.06 (0.02, 0.17) | 364 (51, 2604) |
0% (P = 0.492) |
| Aspiration culture | ||||||||
| Joint fluid | 35 | 0.65 (0.58, 0.72) | 0.97 (0.95, 0.98) | 0.92 (0.89, 0.94) | 19.7 (12.3, 31.5) | 0.36 (0.30, 0.44) | 55 (32, 94) |
98% ( p < 0.001) |
Abbreviations: AUC, area under the ROC curve; DOR, diagnostic odds ratio; FDP, fibrin degradation product; IA, inflammatory arthritis; LE, leucocyte esterase; LR, likelihood ratio; MLR, monocyte to lymphocyte ratio; NLR, neutrophil to lymphocyte ratio; PCT, serum procalcitonin; PLR, platelet to lymphocyte ratio; PLT, platelet count; PMN%, proportion of neutrophils in synovial fluid; PVR, platelet to mean platelet volume ratio; sCRP, synovial CRP; sIL‐6, synovial IL‐6; sIL‐1β, synovial IL‐1β; SEN, sensitivity; SPE, specificity; sTNF‐α, synovial TNF‐α; sWBC, synovial WBC.
Subgroup and Threshold Analysis
Threshold effect regression analysis on four routine markers (serum CRP, ESR, synovial WBC, and synovial PMN%) for chronic PJI diagnosis among the non‐IA population revealed different results, with three having new cut‐offs: 13.5 mg/L, 4100/μL and 70% for serum CRP, synovial WBC and synovial PMN%, respectively (Table 2). We also conducted subgroup analysis and hierarchical analysis with groups by thresholds to verify the newly updated recommendation, and the results proved their validity (Appendices S3 and S4). However, ESR showed quasi‐linear performance during the analysis. The results after grouping the cut‐offs showed that the traditional cut‐off, 30 mm/h, appeared to have the best performance. In the subgroup with MSIS criteria as the gold standard, new cut‐offs were proved to have better diagnostic capability in some situations. (Appendix S5).
TABLE 2.
Comparation between traditional cutoffs and new cutoffs based on threshold effect analysis (chronic PJI without IA population)
| Studies number | SEN (95% CI) | SPE (95% CI) | AUC (95% CI) | Positive‐LR (95% CI) | Negative‐LR (95% CI) | DOR (95% CI) | I 2 (P value) | |
|---|---|---|---|---|---|---|---|---|
| PMN (%) | ||||||||
| 80 | 16 | 0.84 (0.80, 0.88) | 0.94 (0.90, 0.97) | 0.93 (0.90, 0.95) | 14.6 (8.4, 25.4) | 0.17 (0.13, 0.21) | 88 (50, 155) | 98% (P < 0.001) |
| 70 (69–73) | 12 | 0.89 (0.85, 0.92) | 0.90 (0.87, 0.93) | 0.95 (0.93, 0.97) | 9.1 (6.7, 12.3) | 0.12 (0.09, 0.17) | 75 (46, 120) | 66% (P = 0.027) |
| sWBC (/μL) | ||||||||
| 3000 | 19 | 0.88 (0.85, 0.90) | 0.92 (0.88, 0.95) | 0.94 (0.91, 0.96) | 11.1 (7.2, 17.2) | 0.13 (0.11, 0.17) | 83 (47, 145) | 97% (P < 0.001) |
| 4100 (3966–4450) | 3 | 0.90 (0.87, 0.93) | 0.97 (0.93, 0.98) | 0.91 (0.89, 0.94) | 27.1 (13.3, 55.2) | 0.10 (0.07, 0.13) | 274 (121, 624) | 86% (P < 0.001) |
| CRP (mg/L) | ||||||||
| 10 | 56 | 0.81 (0.77, 0.85) | 0.78 (0.74, 0.82) | 0.87 (0.83, 0.89) | 3.7 (3.2, 4.4) | 0.24 (0.20, 0.29) | 16 (12, 20) | 100% (P < 0.001) |
| 13.5 (12.5–14.5) | 6 | 0.84 (0.78, 0.89) | 0.83 (0.73, 0.89) | 0.90 (0.87, 0.92) | 4.8 (3.1, 7.6) | 0.19 (0.13, 0.27) | 26 (14, 46) | 86% (P < 0.001) |
| ESR (mm/h) | ||||||||
| 25–29 | 7 | 0.73 (0.65, 0.80) | 0.82 (0.74, 0.88) | 0.82 (0.79, 0.85) | 4.2 (2.9, 6.0) | 0.33 (0.26, 0.41) | 13 (9, 9) | 92% (P < 0.001) |
| 30 | 47 | 0.79 (0.74, 0.83) | 0.78 (0.72, 0.83) | 0.85 (0.82, 0.88) | 3.5 (2.8, 4.4) | 0.27 (0.22, 0.33) | 13 (10, 18) | 100% (P < 0.001) |
| 30–39 | 15 | 0.77 (0.69, 0.84) | 0.78 (0.72, 0.83) | 0.84 (0.81, 0.87) | 3.5 (2.7, 4.5) | 0.29 (0.21, 0.40) | 12 (7, 20) | 96% (P < 0.001) |
| 40 | 3 | 0.56 (0.42, 0.68) | 0.79 (0.65, 0.88) | 0.71 (0.68, 0.77) | 2.6 (1.6, 4.2) | 0.56 (0.43, 0.75) | 5 (2, 9) | 91% (P < 0.001) |
| 40–49 | 12 | 0.71 (0.64, 0.77) | 0.79 (0.74, 0.84) | 0.82 (0.79, 0.85) | 3.4 (2.7, 4.4) | 0.37 (0.29, 0.47) | 9 (6, 15) | 97% (P < 0.001) |
Abbreviations: AUC, area under the ROC curve; DOR, diagnostic odds ratio; IA, inflammatory arthritis; LR, likelihood ratio; PMN%, proportion of neutrophils in synovial fluid; SEN, sensitivity; SPE, specificity; sWBC, synovial WBC.
Among the articles included, those with information regarding the independent IA population available were also isolated 9 , 10 , 49 , 86 , 133 , 144 , 203 for the subgroup analysis. The diagnostic accuracy of the four routine tests in two groups revealed that all four markers had better diagnostic performance in non‐IA (Table 3). In addition, following the suggestion from previous reviews, we performed a subgroup analysis using the MSIS criteria as the gold standard. The performance of laboratory‐based α‐defensin test was corroborated by the significant diagnostic capability with MSIS as the gold standard (Table 4).
TABLE 3.
Comparation between inflammatory and non‐inflammatory Arthritis
| Studies Number | SEN (95% CI) | SPE (95% CI) | AUC (95% CI) | Positive‐LR (95% CI) | Negative‐LR (95% CI) | DOR (95% CI) | I 2 (P value) | |
|---|---|---|---|---|---|---|---|---|
| PMN% | ||||||||
| Non‐inflammatory | 18 | 0.90 (0.87, 0.92) | 0.91 (0.87, 0.94) | 0.95 (0.93, 0.97) | 10.2 (6.9, 15.0) | 0.11 (0.09, 0.14) | 92 (56, 152) |
97% (P < 0.001) |
| Inflammatory | 5 | 0.93 (0.82, 0.98) | 0.85 (0.80, 0.89) | 0.89 (0.86, 0.91) | 6.1 (4.6, 8.2) | 0.08 (0.03, 0.22) | 74 (26, 214) | 22% (P = 0.138) |
| sWBC | ||||||||
| Non‐inflammatory | 14 | 0.89 (0.86, 0.91) | 0.93 (0.89, 0.95) | 0.94 (0.92, 0.96) | 12.4 (8.2, 18.6) | 0.12 (0.10, 0.15) | 100 (61, 163) |
96% (P < 0.001) |
| Inflammatory | 4 | 0.90 (0.83, 0.94) | 0.81 (0.75, 0.85) | 0.92 (0.90, 0.94) | 4.6 (3.5, 5.8) | 0.12 (0.07, 0.21) | 37 (19, 73) | 100% (P = 0.500) |
| CRP | ||||||||
| Non‐inflammatory | 48 | 0.84 (0.82, 0.87) | 0.78 (0.74, 0.82) | 0.89 (0.85, 0.91) | 3.8 (3.2, 4.6) | 0.20 (0.17, 0.24) | 19 (14, 25) | 100% (P < 0.001) |
| Inflammatory | 6 | 0.84 (0.78, 0.90) | 0.75 (0.46, 0.88) | 0.87 (0.84, 0.89) | 3.4 (1.4, 8.5) | 0.21 (0.15, 0.28) | 17 (6, 48) | 96% (P < 0.001) |
| ESR | ||||||||
| Non‐inflammatory | 38 | 0.81 (0.76, 0.84) | 0.79 (0.76, 0.82) | 0.86 (0.83, 0.89) | 3.9 (3.4, 4.4) | 0.24 (0.20, 0.30) | 16 (12, 20) | 99% (P < 0.001) |
| Inflammatory | 6 | 0.80 (0.71, 0.87) | 0.71 (0.56, 0.83) | 0.83 (0.80, 0.86) | 2.8 (1.6, 4.8) | 0.28 (0.17, 0.47) | 10 (4, 28) | 55% (P = 0.054) |
Abbreviations: AUC, area under the ROC curve; DOR, diagnostic odds ratio; LR, likelihood ratio; PMN%, proportion of neutrophils in synovial fluid; SEN, sensitivity; SPE, specificity; sWBC, synovial WBC
TABLE 4.
Diagnostic test accuracy of the tests with MSIS criteria as gold standard
| Studies Number | SEN (95% CI) | SPE (95% CI) | AUC (95% CI) | Positive‐LR (95% CI) | Negative‐LR (95% CI) | DOR (95% CI) | I 2 (P value) | |
|---|---|---|---|---|---|---|---|---|
| Serum test | ||||||||
| CRP | 53 | 0.83 (0.80, 0.85) | 0.78 (0.73, 0.81) | 0.87 (0.84, 0.90) | 3.7 (3.1, 4.4) | 0.22 (0.20, 0.26) | 17 (13, 22) | 100% (P < 0.001) |
| ESR | 47 | 0.77 (0.73, 0.81) | 0.81 (0.78, 0.84) | 0.86 (0.83, 0.89) | 4.1 (3.6, 4.7) | 0.28 (0.24, 0.33) | 15 (12, 19) |
99% (P < 0.001) |
| D‐dimer | 20 | 0.75 (0.66, 0.81) | 0.78 (0.69, 0.85) | 0.83 (0.79, 0.86) | 3.4 (2.4, 4.8) | 0.33 (0.25, 0.44) | 10 (6, 17) | 99% (P < 0.001) |
| Synovial test | ||||||||
| sWBC | 18 | 0.86 (0.83, 0.88) | 0.90 (0.86, 0.94) | 0.91 (0.89, 0.94) | 8.9 (6.0, 13.3) | 0.16 (0.13, 0.19) | 56 (34, 93) |
97% (P < 0.001) |
| PMN % | 17 | 0.87 (0.84, 0.90) | 0.87 (0.81, 0.91) | 0.92 (0.89, 0.94) | 6.5 (4.5, 9.4) | 0.15 (0.12, 0.19) | 43 (26, 71) |
97% (P < 0.001) |
| LE | 12 | 0.85 (0.76, 0.91) | 0.94 (0.90, 0.96) | 0.96 (0.94, 0.97) | 13.6 (8.5, 22.0) | 0.16 (0.10, 0.26) | 85 (43, 170) |
99% (P < 0.001) |
|
α‐defensin Lab‐test |
12 | 0.92 (0.86, 0.95) | 0.96 (0.94, 0.98) | 0.98 (0.97, 0, 99) | 24.1 (15.3, 37.8) | 0.09 (0.05, 0.14) | 283 (129, 622) | 33% (P = 0.113) |
|
α‐defensin Lateral flow |
16 | 0.89 (0.84, 0.93) | 0.96 (0.94, 0.97) | 0.98 (0.96, 0.99) | 21.3 (13.9, 32.6) | 0.11 (0.08, 0.17) | 188 (110, 321) | 97% (P < 0.001) |
| sCRP | 15 | 0.88 (0.84, 0.91) | 0.92 (0.86, 0.95) | 0.94 (0.92, 0.96) | 10.6 (6.2, 18.2) | 0.13 (0.10, 0.18) | 80 (49, 133) |
99% (P < 0.001) |
| Preoperative Culture | ||||||||
| Aspiration culture | 10 | 0.66 (0.50, 0.79) | 0.97 (0.95, 0.98) | 0.97 (0.95, 0.98) | 22.5 (11.1, 45.7) | 0.35 (0.23, 0.55) | 64 (23, 178) |
77% (P = 0.007) |
Abbreviations: AUC, area under the ROC curve; DOR, diagnostic odds ratio; LE, leucocyte esterase; LR, likelihood ratio; PCT, serum procalcitonin; PMN%, proportion of neutrophils in synovial fluid; sIL‐6, synovial IL‐6; sTNF‐α, synovial TNF‐α; sIL‐1β, synovial IL‐1β; SEN, sensitivity; SPE, specificity; sWBC, synovial white blood cell; sCRP, synovial C‐reactive protein.
Discussion
Our study not only systematic reviewed the biomarkers with preoperative diagnosis of PJI which were reported since 2000, but also reviewed cut‐off value of multiple commonly used clinical biomarkers such as CRP via curve fitting method for the first time. The results showed that the synovial fluid tests performed better in PJI diagnosis than serum tests and aspiration culture. And among the plethora of synovial fluid tests, the lab‐based synovial α‐defensin test is the most satisfying independent preoperative diagnostic test for PJI, especially in the non‐inflammatory group. Furthermore, the statistical analysis underpinning the threshold effect analysis of PJI diagnosis was based on a liner regression model. We analyzed the cut‐off values of four common tests and provided more satisfactory new thresholds of them. Among them, we reduced the threshold of PMN% to 70%, which is completely consistent with the adjustment in MSIS 2018.
Currently, an independent test to definitively diagnose PJI has not yet been discovered. The updated definition of PJI (2018 ICM criteria) has been broadly recognized as a reliable guideline for diagnosis of PJI. This combines multiple evidence‐based tests to rate and then decide whether infection has occurred. However, the major criteria, pathogen culture, takes a long time to yield a result, and is vulnerable to being contaminated during the traumatic operation. Furthermore, the sinus tract with evidence of communication to the joint or visualization of the prosthesis often appears only in the late stage of PJI. 13 Consequently, it is challenging to diagnose chronic PJI in the early period of infection. For chronic PJI, an earlier diagnosis is strongly linked to the development of less sequelae. Therefore, the importance of developing an accurate and convenient mechanism of early diagnosis for chronic PJI is obvious, and our study aimed to achieve this.
Accuracy of Diagnostic Tests
In this comprehensive and systematic study, based mainly on the threshold effect analysis for preoperatively accessible chronic PJI diagnostic tests, laboratory‐based α‐defensin test (ELISA) with a regular threshold of 5.2 mg/L (equal to a semiquantitative signal‐to‐cut = off [S/CO] ratio of 1.0) 163 was identified as having the highest recommended evaluation among the 24 evaluated tests. Moreover, the results from continuous subgroup analysis with MSIS criteria as the unified gold standard proved this conclusion (Tables 1 and 4). The superior performance of laboratory‐based α‐defensin test corresponds well with previous randomized studies and systematic reviews. 1 , 186 , 234 However, low penetration and high price limit further popularization of this test in many countries. In contrast, calprotectin, the new synovial biomarker has the advantages of price and availability. Calprotectin is an innate immune protein mainly originated from neutrophils and macrophages. 235 It will be secreted to resist bacterial infection when the inflammatory response occurs. Calprotectin has the promising potential of being an independent diagnostic biomarker for diagnosing preoperative PJI with its excellent sensitivity (0.95) and specificity (0.95). However, the included six studies about calprotectin test have high heterogeneity within diagnostic methods (ELISA, lateral flow assay, immunoturbidimetric immunoassay) and cut‐off values.
Cut‐off Values and Threshold Effect Analysis
Consequently, we conducted the threshold effect analysis to the four routine tests, serum CRP, ESR, synovial WBC, and synovial PMN%, as the most popular and convenient tests for early PJI diagnosis. This is thus the first systematic review to explore the diagnostic cut‐offs of PJI. The results revealed that the updated cut‐offs of serum CRP (13.5 mg/L), synovial WBC (4100/μL), and synovial PMN% (70.0%) have better diagnostic accuracy than traditional values among non‐IA population, while the cut‐off of ESR remains at 30 mm/h (Table 2). This result also partially supported the newly updated guideline from the consensus of 2018 ICM. 14 Although the sensitivity and specificity of the routine tests with updated cut‐offs cannot compare favorably with novel tests such as the laboratory‐based α‐defensin and calprotectin tests, this discovery nevertheless showed that the thresholds of the existing tests for PJI diagnosis are far from perfect.
Researchers continuously attempt to find more convenient and reliable methods to diagnose PJI at an early stage, given the multiple disadvantages of the current mainstream diagnostic methods, such as the insufficient diagnostic accuracy or delayed definition, while our work has also proved that the traditional bacterial culture has an unacceptable high FN rate. When the pre‐test probability of the PJI is at 80% (high clinical suspicion), the post‐test probability of PJI, provided that the culture is negative, is still 57% (Figure S23). Comprehensive diagnostic strategies like the MSIS criteria have provided solutions to these problems by promoting the combined use of the valuable serum and synovial tests. The newly updated MSIS criteria, one of the most popular diagnostic strategies, has added some novel reliable tests like d‐dimer and α‐defensin and modified the cut‐off of synovial PMN to 70%. 14 In spite of this, the status of joint aspiration or pathological tissue culture appears to have never been threatened in the clinics, which is most likely because of the lack of dependable and objective evidence supporting the regulation including both additional tests and updated threshold. Based on the results of this, we supposed that the novel tests with significantly higher diagnostic accuracy will provide help better, as the tighter serum CRP and WBC counting might also enhance the specificity and reduce the misdiagnosis rate, while the looser PMN% could increase the sensitivity of early diagnosis of PJI at a very early stage instead.
This evidence‐based analysis aimed to evaluate and identify reliable tests with satisfying thresholds to provide reliable early diagnosis for chronic PJI. The results confirmed that synovial tests have better holistic diagnostic accuracy than both the serum tests and aspiration culture, while laboratory‐based α‐defensin tests showed the best performance with or without the unified MSIS criteria as the gold standard. First, we strongly recommended using the synovial α‐defensin ELISA test as a reliable diagnostic test for chronic PJI after excluding the IA diagnosis; while other synovial tests (except synovial IL‐6 and TNF‐α) could also be treated as surrogates if the α‐defensin ELISA test is unavailable in some areas. Some studies have previously evaluated the point‐of‐care lateral‐flow testing technique, Synovasure™ (Zimmer Biomet, Warsaw, IN, USA), which could provide results of synovial α‐defensin tests in only 10 min; however, its lower diagnostic capability and higher price restrict its widespread use. 189 Second, use of the α‐defensin ELISA test might also be interfered by low‐virulence organisms caused infection, further studies focusing on this project should be promoted as current studies are still limited. 199 Third, the synovial calprotectin test requires more prospective randomized trials to determine its diagnostic ability and the best diagnostic method.
Under this circumstance, although the diagnostic performance of routine tests like serum CRP, ESR, synovial WBC counting or PMN% is unsatisfactory, they will not be replaced in the near future, due to their ease‐of‐use. Based on the threshold effect analysis in this study, we believe that the sensitivity/specificity of the synovial PMN, WBC, and serum CRP can be elevated from 0.84/0.94 to 0.89/0.90, 0.88/0.92 to 0.90/0.97, and 0.81/0.78 to 0.84/0.83, respectively, while the threshold of ESR remains at 30 mm/h (Table 2). Especially, the results of synovial PMN first supported the revision that the cut‐off of PMN was changed from 80% to 70% in the 2018 MSIS criteria. Verification of the importance of the biomarkers' threshold is a significant finding. Although the other two new cut‐offs reported in this work still require verifications from further studies, but they purposed those tighter cut‐offs of serum CRP and synovial WBC counting could show better diagnostic performance. Further, updated recommendation of the thresholds was based mainly on the non‐IA population, and has also been confirmed by the hierarchical analysis (Appendices S3 and S4). With improved cut‐off values, the clinical practice of PJI diagnosis will be benefited by the threshold effect. Like looser cut‐offs hint more potential patients. In contrast, tighter cut‐offs will make diagnosis more accurate. Compared to the non‐IA group, the accuracy and sensitivity of four routine diagnostic tests for the IA patients are very low. There are several limitations that may limit the availability of diagnostic tests, including fluctuations of inflammatory factors and the influence of undergoing drug treatment for IA. Therefore, evaluating the cut‐off values for threshold effect is currently difficult among IA patients.
Efforts to Reliable Diagnostic Tests for IA Patients
In clinical practice, the discrimination between PJI and the flare state of IA by histological tests is another challenge, as several inflammatory indicators of patients with IA in their acute flare stage will also be considerably increased. Additionally, immunosuppressive agents may increase the risk of infection and block the production of several inflammatory markers (serum CRP, IL‐6, etc.), which could impair accurate diagnosis of PJI. By ignoring this key problem, prior diagnostic guidelines and systematic reviews provided few recommendations; therefore, we also tried to evaluate the PJI diagnostic performance of four routine tests among patients with IA (Table 3). We found that the diagnostic accuracy of routine tests for chronic PJI was generally lower in the IA population. We think that the study included an excessive IA population, which could interfere with the interpretation of the systematic analysis of the diagnostic accuracy of the biomarkers; thus, future studies on the diagnosis of the PJI should exclude IA populations. Studies on PJI diagnosis targeting IA patients should also be conducted. Unfortunately, we were unable to provide more information due to the limited number of publications in this work. More information on this point is needed, and we believe that the ambiguity will be clarified in the near future.
Strengths and Limitations
This study has several strengths. First, this is not only the most comprehensive and systematic evaluation of preoperative diagnostic biomarkers for PJI in this field, with the largest number of articles included, but is also the first to review cut‐off value of multiple commonly used clinical biomarkers, such as CRP, using the curve fitting method. Meanwhile, our work explored the diagnostic cut‐offs of PJI in a systematic review for the first time. Overall, we concluded that the cut‐offs selected previously for the majority of traditional testing biomarkers were inappropriate, which may be the ultimate cause of the continued inefficiency and confusing diagnostic efficacy of PJI diagnosis. In addition, with the method of threshold effect analysis, we confirmed the rationalization of the PMN% threshold revision in the 2018 MSIS. We found that lowering of the threshold for PMN from 80% to 70% increased the sensitivity/specificity and AUC of the synovial PMN from 0.84/0.94 to 0.89/0.90, 0.93 to 0.95. The widespread acceptance of the ICM criteria have been attributed to its universal popularity for PJI diagnosis. Nevertheless, this criteria has an unavoidable disadvantage for such criteria, in that the threshold of every test has to be renewed in conjunction with the most recent research. Our research showed that some criteria from the newly updated guidelines from the consensus of 2018 ICM have room for improvement. It is a significant finding to verify the importance of the biomarkers' threshold. We also provided a subgroup analysis of the studies using the MSIS criteria as the only gold standard. The results are also the same with and supporting the former conclusion. The activity period of IA frequently along with enhancement of inflammatory factors and effects from anti‐inflammatory treatment. Therefore, we divided patients into the IA and non‐IA groups to compare the discrepancies in commonly used biomarkers between these two kinds of patients.
Nevertheless, this study has some limitations which should be mentioned. First, this work only contained studies that reported data from chronic PJI‐suspicious patients who underwent total knee arthroplasty or total hip arthroplasty; hence, all conclusions from this study cannot be applied to the diagnosis of acute PJI and patients with shoulder or elbow arthroplasty. Second, many details or further analysis cannot be collected and conducted, such as whether or not IA patients were in active flare or the threshold effective analysis in non‐IA patients, due to the meta‐analysis design of this study. Further research providing patient‐based data or large samples of individuals based on a registration database on PJI diagnosis should be performed to verify our results. Third, there are many other potential candidates, such as serum toll‐like receptor 2, 236 synovial d‐lactate, 237 synovial lipocalin 2, 164 and high‐sensitivity CRP, 63 that may provide independent diagnostic capability of PJI diagnosis before revision surgery but require further investigation. Fourth, this study includes 215 articles, which would unavoidably introduce heterogeneity, as indicated by the high I 2 value shown in Table 1. To combat this, we tried to minimize diagnosis bias by performing subgroup analysis. Further, although the risk of diagnosis bias cannot be completely avoided, statistically significant evidence of publication bias was not found in this study. Further, as the only study used the newly updated 2018 MSIS criteria as the good standard, 202 further investigations should take special note of studies that use the new guidelines.
Conclusion
According to the results of this study, synovial fluid tests have better diagnostic accuracy for PJI than serum indicators and aspiration culture, and the laboratory‐based α‐defensin has the potential to be an independent chronic PJI diagnostic biomarker among non‐IA population when a diagnostic cut‐off of 5.2 mg/L (1 S/CO) is selected. This was confirmed when compared with the results of the analysis that used the MSIS criteria as the single gold standard. The synovial calprotectin test also has outstanding diagnostic accuracy and the advantage of low cost compared with other tests. The best cut‐off value and diagnostic tool of it need further research to determine.
Overall, we suggest using newly updated thresholds for synovial PMN% (70%), as well as tighter suggested cut‐offs of synovial WBC (4100/μL), serum CRP (13.5 mg/L), which could improve the diagnostic performance of these four routine tests. We believe that this study could contribute to the preoperative diagnosis of PJI and provide relevant insights for future diagnostic strategies for PJI.
Author Contributions
All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization: HT and JX. Methodology: WY and YW. Validation: XQ and BY. Investigation: BY. Formal Analysis: HT and JX. Formal Analysis: WY, YW, BY and XQ. Supervision: HT and QX. Project Administration: WY and YW. Funding Acquisition: BY.
Supporting information
Appendix S1 Systematic review search strategy
Appendix S2 Information on the 215 studies included
Appendix S3 Subgroup analysis of chronic PJI without IA population
Appendix S4 Hierarchical analysis of thresholds (chronic PJI without IA population)
Appendix S5 Hierarchical analysis of thresholds with MSIS criteria as gold standard (chronic PJI without IA population)
Fig. S1 Alpha‐defensin Laboratory test
Fig. S2 Alpha‐defensin Lateral Flow
Fig. S3 Calprotectin
Fig. S4 LE
Fig. S5 Synovial WBC
Fig. S6 Synovial PMN%
Fig. S7 Synovial CRP
Fig. S8 Synovial IL‐6
Fig. S9 Synovial IL‐1β
Fig. S10 Serum CRP
Fig. S11 Serum ESR
Fig. S12 Serum IL‐6
Fig. S13 Serum D‐Dimer
Fig. S14 Serum PCT
Fig. S15 Serum WBC
Fig. S16 Serum PLT
Fig. S17 Serum NLR
Fig. S18 Serum MLR
Fig. S19 Serum PLR
Fig. S20 Serum PVR
Fig. S21 Serum Fibrinogen
Fig. S22 Serum FDP
Fig. S23 Aspiration Culture
Disclosure: All authors have completed the ICMJE uniform disclosure and declare no competing interests remaining.
Grant Sources: This work was supported by the National Natural Science Foundation of China (Grant No. 82172464, 82172453, 81972086); National Key Research and Development Project of China (Grant No. 2020YFC1107500, 2020YFC1107503). “Technology Innovation Action Plan” Key Project of Shanghai Science and Technology Commission (Grant No. 19411962800); The Shanghai Rising‐Star Program (21QA1405500);
Haozheng Tang and Jialian Xu contributed equally to this work.
Contributor Information
Bing Yue, Email: advbmp2@163.com.
Xinhua Qu, Email: xinhua_qu@126.com.
References
- 1. Carli AV, Abdelbary H, Ahmadzai N, Cheng W, Shea B, Hutton B, et al. Diagnostic accuracy of serum, synovial, and tissue testing for chronic periprosthetic joint infection after hip and knee replacements: a systematic review. J Bone Joint Surg Am. 2019;101(7):635–49. [DOI] [PubMed] [Google Scholar]
- 2. Lee YS, Koo KH, Kim HJ, Tian S, Kim TY, Maltenfort MG, et al. Synovial fluid biomarkers for the diagnosis of periprosthetic joint infection: a systematic review and meta‐analysis. J Bone Joint Surg Am. 2017;99(24):2077–84. [DOI] [PubMed] [Google Scholar]
- 3. Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, et al. New definition for periprosthetic joint infection: from the workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469(11):2992–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parvizi J, Gehrke T. International consensus group on periprosthetic joint I. Definition of periprosthetic joint infection. J Arthroplasty. 2014;29(7):1331. [DOI] [PubMed] [Google Scholar]
- 5. Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1–e25. [DOI] [PubMed] [Google Scholar]
- 6. Parvizi J, Gehrke T, Chen AF. Proceedings of the international consensus on periprosthetic joint infection. Bone Joint J. 2013;95‐B(11):1450–2. [DOI] [PubMed] [Google Scholar]
- 7. Bian T, Shao H, Zhou Y, Huang Y, Song Y. Tests for predicting reimplantation success of two‐stage revision for periprosthetic joint infection: a systematic review and meta‐analysis. Orthop Traumatol Surg Res. 2018;104(7):1115–23. [DOI] [PubMed] [Google Scholar]
- 8. De Fine M, Giavaresi G, Fini M, Illuminati A, Terrando S, Pignatti G. The role of synovial fluid analysis in the detection of periprosthetic hip and knee infections: a systematic review and meta‐analysis. Int Orthop. 2018;42(5):983–94. [DOI] [PubMed] [Google Scholar]
- 9. George J, Jawad M, Curtis GL, Samuel LT, Klika AK, Barsoum WK, et al. Utility of serological markers for detecting persistent infection in two‐stage revision arthroplasty in patients with inflammatory arthritis. J Arthroplasty. 2018;33(7S):S205–S8. [DOI] [PubMed] [Google Scholar]
- 10. Tahta M, Simsek ME, Isik C, Akkaya M, Gursoy S, Bozkurt M. Does inflammatory joint diseases affect the accuracy of infection biomarkers in patients with periprosthetic joint infections? A prospective comparative reliability study. J Orthop Sci. 2019;24(2):286–9. [DOI] [PubMed] [Google Scholar]
- 11. Kheir MM, Tan TL, Shohat N, Foltz C, Parvizi J. Routine diagnostic tests for periprosthetic joint infection demonstrate a high false‐negative rate and are influenced by the infecting organism. J Bone Joint Surg Am. 2018;100(23):2057–65. [DOI] [PubMed] [Google Scholar]
- 12. Saleh A, George J, Sultan AA, Samuel LT, Mont MA, Higuera‐Rueda CA. The quality of diagnostic studies in periprosthetic joint infections: can we do better? J Arthroplasty. 2019;34(11):2737–43. [DOI] [PubMed] [Google Scholar]
- 13. Parvizi J, Tan TL, Goswami K, Higuera C, Della Valle C, Chen AF, et al. The 2018 definition of periprosthetic hip and knee infection: an evidence‐based and validated criteria. J Arthroplasty. 2018;33(5):1309–14.e2. [DOI] [PubMed] [Google Scholar]
- 14. Amanatullah D, Dennis D, Oltra EG, Marcelino Gomes LS, Goodman SB, Hamlin B, et al. Hip and knee section, diagnosis, definitions: proceedings of international consensus on orthopedic infections. J Arthroplasty. 2019;34(2S):S329–S37. [DOI] [PubMed] [Google Scholar]
- 15. Cochrane Methods . Cochrane Handbook for DTA Reviews. 2013. Available from: https:// methods.cochrane.org/sdt/handbook-dta-reviews.
- 16. Liu L, Jyu J, Wang X, Lyu F, Zhang H. How close are we to diagnosing polycystic ovary syndrome with miRNAs: a meta‐analysis and review. Research Square. 2021. https://orcid.org/0000-0002-4381-536
- 17. Cai Q, Wu Y, Guo Z, Gong R, Tang Y, Yang K, et al. Urine BLCA‐4 exerts potential role in detecting patients with bladder cancers: a pooled analysis of individual studies. Oncotarget. 2015;6(35):37500–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McGrath TA, Alabousi M, Skidmore B, Korevaar DA, PMM B, Moher D, et al. Recommendations for reporting of systematic reviews and meta‐analyses of diagnostic test accuracy: a systematic review. Syst Rev. 2017;6(1):194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang SM, Wang YJ, Zhang ST. Accuracy of artificial intelligence‐assisted detection of esophageal cancer and neoplasms on endoscopic images: a systematic review and meta‐analysis. J Dig Dis. 2021;22(6):318–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moraitis AA, Shreeve N, Sovio U, Brocklehurst P, Heazell AEP, Thornton JG, et al. Universal third‐trimester ultrasonic screening using fetal macrosomia in the prediction of adverse perinatal outcome: a systematic review and meta‐analysis of diagnostic test accuracy. PLoS Med. 2020;17(10):e1003190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Long J, Lin H, Cao G, Wang MZ, Huang XJ, Xia J, et al. Relationship between intracranial pressure and phase‐contrast cine MRI‐derived measures of cerebrospinal fluid parameters in communicating hydrocephalus. Quant Imaging Med Surg. 2019;9(8):1413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen B, Benedetti A. Quantifying heterogeneity in individual participant data meta‐analysis with binary outcomes. Syst Rev. 2017;6(1):243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36. [DOI] [PubMed] [Google Scholar]
- 24. Teller RE, Christie MJ, Martin W, Nance EP, Haas DW. Sequential indium‐labeled leukocyte and bone scans to diagnose prosthetic joint infection. Clin Orthop Relat Res. 2000;373:241–7. [DOI] [PubMed] [Google Scholar]
- 25. Itasaka T, Kawai A, Sato T, Mitani S, Inoue H. Diagnosis of infection after total hip arthroplasty. J Orthop Sci. 2001;6(4):320–6. [DOI] [PubMed] [Google Scholar]
- 26. Bernard L, Lubbeke A, Stern R, Bru JP, Feron JM, Peyramond D, et al. Value of preoperative investigations in diagnosing prosthetic joint infection: retrospective cohort study and literature review. Scand J Infect Dis. 2004;36(6–7):410–6. [DOI] [PubMed] [Google Scholar]
- 27. Di Cesare PE, Chang E, Preston CF, Liu CJ. Serum interleukin‐6 as a marker of periprosthetic infection following total hip and knee arthroplasty. J Bone Joint Surg Am. 2005;87(9):1921–7. [DOI] [PubMed] [Google Scholar]
- 28. Bare J, MacDonald SJ, Bourne RB. Preoperative evaluations in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;446:40–4. [DOI] [PubMed] [Google Scholar]
- 29. Nilsdotter‐Augustinsson A, Briheim G, Herder A, Ljunghusen O, Wahlstrom O, Ohman L. Inflammatory response in 85 patients with loosened hip prostheses: a prospective study comparing inflammatory markers in patients with aseptic and septic prosthetic loosening. Acta Orthop. 2007;78(5):629–39. [DOI] [PubMed] [Google Scholar]
- 30. Greidanus NV, Masri BA, Garbuz DS, Wilson SD, McAlinden MG, Xu M, et al. Use of erythrocyte sedimentation rate and C‐reactive protein level to diagnose infection before revision total knee arthroplasty. A prospective evaluation. J Bone Joint Surg Am. 2007;89(7):1409–16. [DOI] [PubMed] [Google Scholar]
- 31. Della Valle CJ, Sporer SM, Jacobs JJ, Berger RA, Rosenberg AG, Paprosky WG. Preoperative testing for sepsis before revision total knee arthroplasty. J Arthroplasty. 2007;22(6 Suppl 2):90–3. [DOI] [PubMed] [Google Scholar]
- 32. Simonsen L, Buhl A, Oersnes T, Duus B. White blood cell scintigraphy for differentiation of infection and aseptic loosening: a retrospective study of 76 painful hip prostheses. Acta Orthop. 2007;78(5):640–7. [DOI] [PubMed] [Google Scholar]
- 33. Bottner F, Wegner A, Winkelmann W, Becker K, Erren M, Gotze C. Interleukin‐6, procalcitonin and TNF‐alpha: markers of peri‐prosthetic infection following total joint replacement. J Bone Joint Surg Br. 2007;89(1):94–9. [DOI] [PubMed] [Google Scholar]
- 34. Schinsky MF, Della Valle CJ, Sporer SM, Paprosky WG. Perioperative testing for joint infection in patients undergoing revision total hip arthroplasty. J Bone Joint Surg Am. 2008;90(9):1869–75. [DOI] [PubMed] [Google Scholar]
- 35. Muller M, Morawietz L, Hasart O, Strube P, Perka C, Tohtz S. Diagnosis of periprosthetic infection following total hip arthroplasty—evaluation of the diagnostic values of pre‐ and intraoperative parameters and the associated strategy to preoperatively select patients with a high probability of joint infection. J Orthop Surg Res. 2008;3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Austin MS, Ghanem E, Joshi A, Lindsay A, Parvizi J. A simple, cost‐effective screening protocol to rule out periprosthetic infection. J Arthroplasty. 2008;23(1):65–8. [DOI] [PubMed] [Google Scholar]
- 37. Fink B, Makowiak C, Fuerst M, Berger I, Schafer P, Frommelt L. The value of synovial biopsy, joint aspiration and C‐reactive protein in the diagnosis of late peri‐prosthetic infection of total knee replacements. J Bone Joint Surg Br. 2008;90(7):874–8. [DOI] [PubMed] [Google Scholar]
- 38. Chevillotte CJ, Ali MH, Trousdale RT, Larson DR, Gullerud RE, Berry DJ. Inflammatory laboratory markers in periprosthetic hip fractures. J Arthroplasty. 2009;24(5):722–7. [DOI] [PubMed] [Google Scholar]
- 39. Ghanem E, Antoci V Jr, Pulido L, Joshi A, Hozack W, Parvizi J. The use of receiver operating characteristics analysis in determining erythrocyte sedimentation rate and C‐reactive protein levels in diagnosing periprosthetic infection prior to revision total hip arthroplasty. Int J Infect Dis. 2009;13(6):e444–9. [DOI] [PubMed] [Google Scholar]
- 40. Panousis K, Grigoris P, Butcher I, Rana B, Reilly JH, Hamblen DL. Poor predictive value of broad‐range PCR for the detection of arthroplasty infection in 92 cases. Acta Orthop. 2009;76(3):341–6. [PubMed] [Google Scholar]
- 41. Morgan PM, Sharkey P, Ghanem E, Parvizi J, Clohisy JC, Burnett RSJ, et al. The value of intraoperative Gram stain in revision total knee arthroplasty. J Bone Joint Surg Am. 2009;91(9):2124–9. [DOI] [PubMed] [Google Scholar]
- 42. Buttaro MA, Tanoira I, Comba F, Piccaluga F. Combining C‐reactive protein and interleukin‐6 may be useful to detect periprosthetic hip infection. Clin Orthop Relat Res. 2010;468(12):3263–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Deirmengian C, Hallab N, Tarabishy A, Valle CD, Jacobs JJ, Lonner J, et al. Synovial fluid biomarkers for periprosthetic infection. Clin Orthop Relat Res. 2010;468(8):2017–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tohtz SW, Muller M, Morawietz L, Winkler T, Perka C. Validity of frozen sections for analysis of periprosthetic loosening membranes. Clin Orthop Relat Res. 2010;468(3):762–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Piper KE, Fernandez‐Sampedro M, Steckelberg KE, Mandrekar JN, Karau MJ, Steckelberg JM, et al. C‐reactive protein, erythrocyte sedimentation rate and orthopedic implant infection. PLOS One. 2010;5(2):e9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Worthington T, Dunlop D, Casey A, Lambert R, Luscombe J, Elliott T. Serum procalcitonin, interleukin‐6, soluble intercellular adhesin molecule‐1 and IgG to short‐chain exocellular lipoteichoic acid as predictors of infection in total joint prosthesis revision. Br J Biomed Sci. 2010;67(2):71–6. [DOI] [PubMed] [Google Scholar]
- 47. Randau TWM, Kuberra D, Reichert B, Stoffel‐Wagner B, Wirtz DC, Gravius S. Detection of periprosthetic joint infections: blood infection markers in patients undergoing revision arthroplasty. Eur Cell Mater. 2011;21(Suppl 2):36. [Google Scholar]
- 48. Johnson AJ, Zywiel MG, Stroh A, Marker DR, Mont MA. Serological markers can lead to false negative diagnoses of periprosthetic infections following total knee arthroplasty. Int Orthop. 2011;35(11):1621–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cipriano CA, Brown NM, Michael AM, Moric M, Sporer SM, Della Valle CJ. Serum and synovial fluid analysis for diagnosing chronic periprosthetic infection in patients with inflammatory arthritis. J Bone Joint Surg Am. 2012;94(7):594–600. [DOI] [PubMed] [Google Scholar]
- 50. Toossi N, Adeli B, Rasouli MR, Huang R, Parvizi J. Serum white blood cell count and differential do not have a role in the diagnosis of periprosthetic joint infection. J Arthroplasty. 2012;27(8 Suppl):51–4 e1. [DOI] [PubMed] [Google Scholar]
- 51. Parvizi J, Jacovides C, Adeli B, Jung KA, Hozack WJ, Mark B. Coventry Award: synovial C‐reactive protein: a prospective evaluation of a molecular marker for periprosthetic knee joint infection. Clin Orthop Relat Res. 2012;470(1):54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schwartz AJ, Education SoURaC . Diagnosis of periprosthetic joint infection after unicompartmental knee arthroplasty. J Arthroplasty. 2012;27(Suppl 1):46–50. [DOI] [PubMed] [Google Scholar]
- 53. Glehr M, Friesenbichler J, Hofmann G, Bernhardt GA, Zacherl M, Avian A, et al. Novel biomarkers to detect infection in revision hip and knee arthroplasties. Clin Orthop Relat Res. 2013;471(8):2621–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fink B, Gebhard A, Fuerst M, Berger I, Schafer P. High diagnostic value of synovial biopsy in periprosthetic joint infection of the hip. Clin Orthop Relat Res. 2013;471(3):956–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Alijanipour P, Bakhshi H, Parvizi J. Diagnosis of periprosthetic joint infection: the threshold for serological markers. Clin Orthop Relat Res. 2013;471(10):3186–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Abou El‐Khier NT, El Ganainy AR, Elgeidy A, Rakha SA. Assessment of interleukin‐6 and other inflammatory markers in the diagnosis of Egyptian patients with periprosthetic joint infection. Egypt J Immunol. 2013;20(2):93–9. [PubMed] [Google Scholar]
- 57. Miyamae Y, Inaba Y, Kobayashi N, Choe H, Yukizawa Y, Ike H, et al. Different diagnostic properties of C‐reactive protein, real‐time PCR, and histopathology of frozen and permanent sections in diagnosis of periprosthetic joint infection. Acta Orthop. 2013;84(6):524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gollwitzer H, Dombrowski Y, Prodinger PM, Peric M, Summer B, Hapfelmeier A, et al. Antimicrobial peptides and proinflammatory cytokines in periprosthetic joint infection. J Bone Joint Surg Am. 2013;95(7):644–51. [DOI] [PubMed] [Google Scholar]
- 59. Wyles CC, Larson DR, Houdek MT, Sierra RJ, Trousdale RT. Utility of synovial fluid aspirations in failed metal‐on‐metal total hip arthroplasty. J Arthroplasty. 2013;28(5):818–23. [DOI] [PubMed] [Google Scholar]
- 60. Elgeidi A, Elganainy AE, Abou Elkhier N, Rakha S. Interleukin‐6 and other inflammatory markers in diagnosis of periprosthetic joint infection. Int Orthop. 2014;38(12):2591–5. [DOI] [PubMed] [Google Scholar]
- 61. Randau TM, Friedrich MJ, Wimmer MD, Reichert B, Kuberra D, Stoffel‐Wagner B, et al. Interleukin‐6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PLOS One. 2014;9(2):e89045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha defensin‐1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472(12):4006–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Milone MT, Kamath AF, Israelite CL. Converting between high‐ and low‐sensitivity C‐reactive protein in the assessment of periprosthetic joint infection. J Arthroplasty. 2014;29(4):685–9. [DOI] [PubMed] [Google Scholar]
- 64. Ronde‐Oustau C, Diesinger Y, Jenny JY, Antoni M, Gaudias J, Boeri C, et al. Diagnostic accuracy of intra‐articular C‐reactive protein assay in periprosthetic knee joint infection—a preliminary study. Orthop Traumatol Surg Res. 2014;100(2):217–20. [DOI] [PubMed] [Google Scholar]
- 65. Tetreault MW, Wetters NG, Moric M, Gross CE, Della Valle CJ. Is synovial C‐reactive protein a useful marker for periprosthetic joint infection? Clin Orthop Relat Res. 2014;472(12):3997–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liu JZ, Saleh A, Klika AK, Barsoum WK, Higuera CA. Serum inflammatory markers for periprosthetic knee infection in obese versus non‐obese patients. J Arthroplasty. 2014;29(10):1880–3. [DOI] [PubMed] [Google Scholar]
- 67. Cansu EDC, Erdogan F, Babacan M. Comparison of cultures immediately incubated intraoperatively with cultures incubated postoperatively in the laboratory for causes of periprosthetic loosening. Marmara Med J. 2014;27(4):102–6. [Google Scholar]
- 68. Claassen L, Radtke K, Ettinger M, Plaass C, von Lewinski G. Preoperative diagnostic for periprosthetic joint infection prior to total knee revision arthroplasty. Orthop Rev (Pavia). 2014;6(3):5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Friedrich MJ, Randau TM, Wimmer MD, Reichert B, Kuberra D, Stoffel‐Wagner B, et al. Lipopolysaccharide‐binding protein: a valuable biomarker in the differentiation between periprosthetic joint infection and aseptic loosening? Int Orthop. 2014;38(10):2201–7. [DOI] [PubMed] [Google Scholar]
- 70. Wu C, Qu X, Mao Y, Li H, Dai K, Liu F, et al. Utility of intraoperative frozen section in the diagnosis of periprosthetic joint infection. PLOS One. 2014;9(7):e102346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yuan K, Li WD, Qiang Y, Cui ZM. Comparison of procalcitonin and C‐reactive protein for the diagnosis of periprosthetic joint infection before revision total hip arthroplasty. Surg Infect (Larchmt). 2015;16(2):146–50. [DOI] [PubMed] [Google Scholar]
- 72. Omar M, Ettinger M, Reichling M, Petri M, Guenther D, Gehrke T, et al. Synovial C‐reactive protein as a marker for chronic periprosthetic infection in total hip arthroplasty. Bone Joint J. 2015;97‐B(2):173–6. [DOI] [PubMed] [Google Scholar]
- 73. Frangiamore SJ, Gajewski ND, Saleh A, Farias‐Kovac M, Barsoum WK, Higuera CA. Alpha‐defensin accuracy to diagnose periprosthetic joint infection‐best available test? J Arthroplasty. 2016;31(2):456–60. [DOI] [PubMed] [Google Scholar]
- 74. Hoell S, Moeller A, Gosheger G, Hardes J, Dieckmann R, Schulz D. Two‐stage revision arthroplasty for periprosthetic joint infections: what is the value of cultures and white cell count in synovial fluid and CRP in serum before second stage reimplantation? Arch Orthop Trauma Surg. 2016;136(4):447–52. [DOI] [PubMed] [Google Scholar]
- 75. Claassen L, Ettinger S, Pastor MF, Budde S, Windhagen H, Floerkemeier T. The value of arthroscopic neosynovium biopsies to diagnose periprosthetic knee joint low‐grade infection. Arch Orthop Trauma Surg. 2016;136(12):1753–9. [DOI] [PubMed] [Google Scholar]
- 76. Kwon YM, Antoci V Jr, Leone WA, Tsai TY, Dimitriou D, Liow MH. Utility of serum inflammatory and synovial fluid counts in the diagnosis of infection in taper corrosion of dual taper modular stems. J Arthroplasty. 2016;31(9):1997–2003. [DOI] [PubMed] [Google Scholar]
- 77. Shah RP, Plummer DR, Moric M, Sporer SM, Levine BR, Della Valle CJ. Diagnosing infection in the setting of periprosthetic fractures. J Arthroplasty. 2016;31(9 Suppl):140–3. [DOI] [PubMed] [Google Scholar]
- 78. Lindsay CP, Olcott CW, Del Gaizo DJ. ESR and CRP are useful between stages of 2‐stage revision for periprosthetic joint infection. Arthroplast Today. 2017;3(3):183–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shahi A, Kheir MM, Tarabichi M, Hosseinzadeh HRS, Tan TL, Parvizi J. Serum D‐dimer test is promising for the diagnosis of periprosthetic joint infection and timing of reimplantation. J Bone Joint Surg Am. 2017;99(17):1419–27. [DOI] [PubMed] [Google Scholar]
- 80. Zmistowski BM, Clyde CT, Ghanem ES, Gotoff JR, Deirmengian CA, Parvizi J. Utility of synovial white blood cell count and differential before reimplantation surgery. J Arthroplasty. 2017;32(9):2820–4. [DOI] [PubMed] [Google Scholar]
- 81. Rothenberg AC, Wilson AE, Hayes JP, O'Malley MJ, Klatt BA. Sonication of arthroplasty implants improves accuracy of periprosthetic joint infection cultures. Clin Orthop Relat Res. 2017;475(7):1827–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Fernandez‐Sampedro M, Farinas‐Alvarez C, Garces‐Zarzalejo C, Alonso‐Aguirre MA, Salas‐Venero C, Martínez‐Martínez L, et al. Accuracy of different diagnostic tests for early, delayed and late prosthetic joint infection. BMC Infect Dis. 2017;17(1):592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Pohlig F, Muhlhofer HM, Lenze U, Lenze FW, Suren C, Harrasser N, et al. Diagnostic accuracy of arthroscopic biopsy in periprosthetic infections of the hip. Eur J Med Res. 2017;22(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Berger P, Van Cauter M, Driesen R, Neyt J, Cornu O, Bellemans J. Diagnosis of prosthetic joint infection with alpha‐defensin using a lateral flow device: a multicentre study. Bone Joint J. 2017;99‐B(9):1176–82. [DOI] [PubMed] [Google Scholar]
- 85. Shahi A, Tan TL, Kheir MM, Tan DD, Parvizi J. Diagnosing periprosthetic joint infection: and the winner is? J Arthroplasty. 2017;32(9S):S232–S5. [DOI] [PubMed] [Google Scholar]
- 86. Shohat N, Goswami K, Fillingham Y, Tan TL, Calkins T, Della Valle CJ, et al. Diagnosing periprosthetic joint infection in inflammatory arthritis: assumption is the enemy of true understanding. J Arthroplasty. 2018;33(11):3561–6. [DOI] [PubMed] [Google Scholar]
- 87. Gallo J, Svoboda M, Zapletalova J, Proskova J, Juranova J. Serum IL‐6 in combination with synovial IL‐6/CRP shows excellent diagnostic power to detect hip and knee prosthetic joint infection. PLOS One. 2018;13(6):e0199226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Erdemli B, Ozbek EA, Basarir K, Karahan ZC, Ocal D, Biriken D. Proinflammatory biomarkers' level and functional genetic polymorphisms in periprosthetic joint infection. Acta Orthop Traumatol Turc. 2018;52(2):143–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Stylianakis A, Schinas G, Thomaidis PC, Papaparaskevas J, Ziogas DC, Gamaletsou MN, et al. Combination of conventional culture, vial culture, and broad‐range PCR of sonication fluid for the diagnosis of prosthetic joint infection. Diagn Microbiol Infect Dis. 2018;92(1):13–8. [DOI] [PubMed] [Google Scholar]
- 90. Renz N, Yermak K, Perka C, Trampuz A. Alpha defensin lateral flow test for diagnosis of periprosthetic joint infection: not a screening but a confirmatory test. J Bone Joint Surg Am. 2018;100(9):742–50. [DOI] [PubMed] [Google Scholar]
- 91. Sebastian S, Malhotra R, Sreenivas V, Kapil A, Chaudhry R, Dhawan B. Sonication of orthopaedic implants: a valuable technique for diagnosis of prosthetic joint infections. J Microbiol Methods. 2018;146:51–4. [DOI] [PubMed] [Google Scholar]
- 92. Sa‐Ngasoongsong P, Wongsak S, Jarungvittayakon C, Limsamutpetch K, Channoom T, Kawinwonggowit V. Comparison of synovial fluid and serum Procalcitonin for diagnosis of periprosthetic joint infection: a pilot study in 32 patients. Biomed Res Int. 2018;2018:8351308–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wouthuyzen‐Bakker M, Ploegmakers JJW, Ottink K, Kampinga GA, Wagenmakers‐Huizenga L, Jutte PC, et al. Synovial calprotectin: an inexpensive biomarker to exclude a chronic prosthetic joint infection. J Arthroplasty. 2018;33(4):1149–53. [DOI] [PubMed] [Google Scholar]
- 94. Tani S, Lepetsos P, Stylianakis A, Vlamis J, Birbas K, Kaklamanos I. Superiority of the sonication method against conventional periprosthetic tissue cultures for diagnosis of prosthetic joint infections. Eur J Orthop Surg Traumatol. 2018;28(1):51–7. [DOI] [PubMed] [Google Scholar]
- 95. Dwyer MK, Damsgaard C, Wadibia J, Wong G, Lazar D, Smith E, et al. Laboratory tests for diagnosis of chronic Periprosthetic joint infection can help predict outcomes of two‐stage exchange. J Bone Joint Surg Am. 2018;100(12):1009–15. [DOI] [PubMed] [Google Scholar]
- 96. Fink B, Steurer M, Hofacker S, Schäfer P, Sandow D, Schuster P, et al. Preoperative PCR analysis of synovial fluid has limited value for the diagnosis of periprosthetic joint infections of total knee arthroplasties. Arch Orthop Trauma Surg. 2018;138(6):871–8. [DOI] [PubMed] [Google Scholar]
- 97. Kuo FC, Lu YD, Wu CT, You HL, Lee GB, Lee MS. Comparison of molecular diagnosis with serum markers and synovial fluid analysis in patients with prosthetic joint infection. Bone Joint J. 2018;100‐B(10):1345–51. [DOI] [PubMed] [Google Scholar]
- 98. Fu J, Ni M, Chai W, Li X, Hao L, Chen J. Synovial fluid viscosity test is promising for the diagnosis of periprosthetic joint infection. J Arthroplasty. 2019;34(6):1197–200. [DOI] [PubMed] [Google Scholar]
- 99. Ding BTK, Tan KG, Kau CY, Chan HYH, Fadil MFB. Accuracy of the alpha‐defensin lateral flow assay for diagnosing periprosthetic joint infection in Asians. J Orthop Surg. 2019;27(1):230949901982845. [DOI] [PubMed] [Google Scholar]
- 100. Schiffner E, Latz D, Thelen S, Grassmann JP, Karbowski A, Windolf J, et al. Normal CRP and WBC values in total hip arthroplasty (THA) with signs of loosening. Do we need a joint aspiration? J Clin Orthop Dent Traumatol. 2019;10(3):566–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Huang J, Zhang Y, Wang Z, Dong Y, Zhao Y, Zheng J, et al. The serum level of D‐dimer is not suitable for distinguishing between prosthetic joint infection and aseptic loosening. J Orthop Surg Res. 2019;14(1):407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Li R, Shao HY, Hao LB, Yu BZ, Qu PF, Zhou YX, et al. Plasma fibrinogen exhibits better performance than plasma D‐dimer in the diagnosis of periprosthetic joint infection: a multicenter retrospective study. J Bone Joint Surg Am. 2019;101(7):613–9. [DOI] [PubMed] [Google Scholar]
- 103. Xiong L, Li S, Dai M. Comparison of D‐dimer with CRP and ESR for diagnosis of periprosthetic joint infection. J Orthop Surg Res. 2019;14(1):240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Xu H, Xie J, Huang Q, Lei Y, Zhang S, Pei F. Plasma fibrin degradation product and D‐dimer are of limited value for diagnosing periprosthetic joint infection. J Arthroplasty. 2019;34(10):2454–60. [DOI] [PubMed] [Google Scholar]
- 105. Qin L, Li F, Gong X, Wang J, Huang W, Hu N. Combined measurement of D‐dimer and C‐reactive protein levels: highly accurate for diagnosing chronic periprosthetic joint infection. J Arthroplasty. 2020;35(1):229–34. [DOI] [PubMed] [Google Scholar]
- 106. Fink B, Schlumberger M, Beyersdorff J, Schuster P. C‐reactive protein is not a screening tool for late periprosthetic joint infection. J Orthop Traumatol. 2020;21(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bin G, Xinxin Y, Fan L, Shenghong W, Yayi X. Serum fibrinogen test performs well for the diagnosis of periprosthetic joint infection. J Arthroplasty. 2020;35(9):2607–12. [DOI] [PubMed] [Google Scholar]
- 108. Wu H, Meng Z, Pan L, Liu H, Yang X, Yongping C. Plasma fibrinogen performs better than plasma d‐dimer and fibrin degradation product in the diagnosis of periprosthetic joint infection and determination of reimplantation timing. J Arthroplasty. 2020;35(8):2230–6. [DOI] [PubMed] [Google Scholar]
- 109. Xu H, Xie J, Yang J, Chen G, Huang Q, Pei F. Plasma fibrinogen and platelet count are referable tools for diagnosing periprosthetic joint infection: a single‐center retrospective cohort study. J Arthroplasty. 2020;35(5):1361–7. [DOI] [PubMed] [Google Scholar]
- 110. Bingham JS, Hassebrock JD, Christensen AL, Beauchamp CP, Clarke HD, Spangehl MJ. Screening for periprosthetic joint infections with ESR and CRP: the ideal cutoffs. J Arthroplasty. 2020;35(5):1351–4. [DOI] [PubMed] [Google Scholar]
- 111. Qin L, Hu N, Li X, Chen Y, Wang J, Huang W. Evaluation of synovial fluid neutrophil CD64 index as a screening biomarker of prosthetic joint infection. Bone Joint J. 2020;102‐b(4):463–9. [DOI] [PubMed] [Google Scholar]
- 112. Klim SM, Amerstorfer F, Glehr G, Hauer G, Smolle MA, Leitner L, et al. Combined serum biomarker analysis shows no benefit in the diagnosis of periprosthetic joint infection. Int Orthop. 2020;44(12):2515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Paziuk T, Rondon AJ, Goswami K, Tan TL, Parvizi J. A novel adjunct indicator of periprosthetic joint infection: platelet count and mean platelet volume. J Arthroplasty. 2020;35(3):836–9. [DOI] [PubMed] [Google Scholar]
- 114. Ackmann T, Mollenbeck B, Gosheger G, Schwarze J, Schmidt‐Braekling T, Schneider KN, et al. Comparing the diagnostic value of serum D‐dimer to CRP and IL‐6 in the diagnosis of chronic prosthetic joint infection. J Clin Med. 2020;9(9);2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wang CX, Huang Z, Fang X, Li W, Yang B, Zhang W. Comparison of broad‐range polymerase chain reaction and metagenomic next‐generation sequencing for the diagnosis of prosthetic joint infection. Int J Infect Dis. 2020;95:8–12. [DOI] [PubMed] [Google Scholar]
- 116. Ye Y, Chen W, Gu M, Liu Q, Xian G, Pan B, et al. Limited value of serum neutrophil‐to‐lymphocyte ratio in the diagnosis of chronic periprosthetic joint infection. J Orthop Traumatol. 2021;22(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Tirumala V, Klemt C, Xiong L, Chen W, van den Kieboom J, Kwon YM. Diagnostic utility of platelet count/lymphocyte count ratio and platelet count/mean platelet volume ratio in periprosthetic joint infection following Total knee arthroplasty. J Arthroplasty. 2021;36(1):291–7. [DOI] [PubMed] [Google Scholar]
- 118. Li R, Li X, Ni M, Fu J, Xu C, Chai W, et al. What is the performance of novel synovial biomarkers for detecting periprosthetic joint infection in the presence of inflammatory joint disease? Bone Joint J. 2021;103‐b(1):32–8. [DOI] [PubMed] [Google Scholar]
- 119. Xu C, Qu PF, Chai W, Li R, Chen JY. Plasma fibrinogen may predict persistent infection before reimplantation in two‐stage exchange arthroplasty for periprosthetic hip infection. J Orthop Surg Res. 2019;14(1):133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Pannu TS, Villa JM, Patel PD, Riesgo AM, Barsoum WK, Higuera CA. The utility of serum d‐dimer for the diagnosis of periprosthetic joint infection in revision total hip and knee arthroplasty. J Arthroplasty. 2020;35(6):1692–5. [DOI] [PubMed] [Google Scholar]
- 121. Wang Y, Li Y, Qiao L, Sun S. Comparison of a comprehensive set of fibrinolytic markers with C‐reactive protein and erythrocyte sedimentation rate for the diagnosis of periprosthetic joint infection. J Arthroplasty. 2020;35(9):2613–8. [DOI] [PubMed] [Google Scholar]
- 122. Levent A, Neufeld ME, Piakong P, Lausmann C, Gehrke T, Citak M. Which international consensus meeting preoperative minor criteria is the most accurate marker for the diagnosis of periprosthetic joint infection in hip and knee arthroplasty? J Arthroplasty. 2021;36(11):3728–33. [DOI] [PubMed] [Google Scholar]
- 123. Yu BZ, Li R, Li X, Chai W, Zhou YG, Chen JY. The relationship of C‐reactive protein/interleukin‐6 concentrations between serum and synovial fluid in the diagnosis of periprosthetic joint infection. J Orthop Surg Res. 2021;16(1):733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Deirmengian C, Madigan J, Kallur Mallikarjuna S, Conway J, Higuera C, Patel R. Validation of the alpha defensin lateral flow test for periprosthetic joint infection. J Bone Joint Surg Am. 2021;103(2):115–22. [DOI] [PubMed] [Google Scholar]
- 125. Praz C, Gubbiotti L, Buia G, Chapus V, Dunet J, Grandhomme F, et al. Value of the synovial C‐reactive protein test in the diagnosis of total hip and knee periprosthetic joint infections: a case‐control study. Orthop Traumatol Surg Res. 2021;107(4):102903. [DOI] [PubMed] [Google Scholar]
- 126. Grzelecki D, Walczak P, Grajek A, Szostek M, Dudek P, Bartosz P, et al. Elevated plasma D‐dimer concentration has higher efficacy for the diagnosis of periprosthetic joint infection of the knee than of the hip‐a single‐center, retrospective study. J Orthop Res. 2021;39(2):291–8. [DOI] [PubMed] [Google Scholar]
- 127. Chisari E, Yacovelli S, Goswami K, Shohat N, Woloszyn P, Parvizi J. Leukocyte esterase versus ICM 2018 criteria in the diagnosis of periprosthetic joint infection. J Arthroplasty. 2021;36(8):2942–5 e1. [DOI] [PubMed] [Google Scholar]
- 128. Sahin E, Karaismailoglu B, Ozsahin MK, Guven MF, Kaynak G. Low value of platelet count to mean platelet volume ratio to diagnose chronic PJI: a case control study. Orthop Traumatol Surg Res. 2021;107(4):102899. [DOI] [PubMed] [Google Scholar]
- 129. Wang H, Qin L, Wang J, Huang W. Synovial fluid IL‐1beta appears useful for the diagnosis of chronic periprosthetic joint infection. J Orthop Surg Res. 2021;16(1):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Yin H, Xu D, Wang D. Diagnostic value of next‐generation sequencing to detect periprosthetic joint infection. BMC Musculoskelet Disord. 2021;22(1):252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Xu H, Xie J, Wang D, Huang Q, Huang Z, Zhou Z. Plasma levels of D‐dimer and fibrin degradation product are unreliable for diagnosing periprosthetic joint infection in patients undergoing re‐revision arthroplasty. J Orthop Surg Res. 2021;16(1):628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Sigmund IK, Holinka J, Staats K, Sevelda F, Lass R, Kubista B, et al. Inferior performance of established and novel serum inflammatory markers in diagnosing periprosthetic joint infections. Int Orthop. 2021;45(4):837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Huang JC, Chen X, Qiang S, Zheng WD, Zheng J, Jin Y. Exciting performance of plasma fibrinogen in periprosthetic joint infection diagnosis. Orthop Surg. 2021;13(3):812–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Qiao L, Sun S. A retrospective comparison of thromboelastography and conventional coagulation parameters for periprosthetic joint infection diagnosis and reimplantation timing. Clin Chim Acta. 2021;519:118–25. [DOI] [PubMed] [Google Scholar]
- 135. Wang H, Qin L, Wang J, Hu N, Huang W. Combined serum and synovial C‐reactive protein tests: a valuable adjunct to the diagnosis of chronic prosthetic joint infection. BMC Musculoskelet Disord. 2021;22(1):670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Klemt C, Tirumala V, Smith EJ, Xiong L, Kwon YM. Complete blood platelet and lymphocyte ratios increase diagnostic accuracy of periprosthetic joint infection following total hip arthroplasty. Arch Orthop Trauma Surg. 2022. 10.1007/s00402-021-04309-w. [DOI] [PubMed] [Google Scholar]
- 137. Baker CM, Goh GS, Tarabichi S, Shohat N, Parvizi J. Synovial C‐reactive protein is a useful adjunct for diagnosis of periprosthetic joint infection. J Arthroplasty. 2022. S0883‐5403(22)00678‐7. [DOI] [PubMed] [Google Scholar]
- 138. Kuo FC, Lin PC, Yen SH, Tan TL, Wu CT, Wang JW. Which minor criteria is the Most accurate predictor for the diagnosis of hip and knee periprosthetic joint infection in the Asian population? J Arthroplasty. 2022;S0883‐5403(22)00514‐9. [DOI] [PubMed] [Google Scholar]
- 139. Shang G, Fei Z, Xu H, Wang Y, Xiang S. Globulin and albumin to globulin ratio precisely diagnose periprosthetic joint infection and determine the timing of second‐stage reimplantation. J Orthop Surg Res. 2022;17(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Liu H, Yu Y, Niu Y. Utility of human neutrophil Lipocalin as a diagnosing biomarker of prosthetic joint infection: a clinical pilot study. Infect Drug Resist. 2022;15:2393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Xu H, Liu L, Xie J, Huang Q, Lai Y, Zhou Z. Plasma fibrinogen: a sensitive biomarker for the screening of periprosthetic joint infection in patients undergoing re‐revision arthroplasty. BMC Musculoskelet Disord. 2022;23(1):520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Huang J, Wang J, Qin L, Zhu B, Huang W, Hu N. Combination of synovial fluid IL‐4 and Polymorphonuclear cell percentage improves the diagnostic accuracy of chronic periprosthetic joint infection. Front Surg. 2022;9:843187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Jiao JB, Huang JC, Chen X, Jin Y. Albumin to globulin ratio, neutrophil to lymphocyte ratio, and globulin levels do not outperform ESR or CRP when diagnosing periprosthetic joint infection. BMC Musculoskelet Disord. 2022;23(1):404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Qin L, Wang H, Zhao C, Chen C, Chen H, Li X, et al. Serum and synovial biomarkers for distinguishing between chronic periprosthetic joint infections and rheumatoid arthritis: a prospective cohort study. J Arthroplasty. 2022;37(2):342–6. [DOI] [PubMed] [Google Scholar]
- 145. Xu H, Xie J, Zhang S, Wang D, Huang Z, Zhou Z. Potential blood biomarkers for diagnosing periprosthetic joint infection: a single‐center, retrospective study. Antibiotics (Basel). 2022;11(4):505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Fernandez‐Sampedro M, Sanles‐Gonzalez I, Garcia‐Ibarbia C, Fananas‐Rodriquez N, Fakkas‐Fernandez M, Farinas MC. The poor accuracy of D‐dimer for the diagnosis of prosthetic joint infection but its potential usefulness in early postoperative infections following revision arthroplasty for aseptic loosening. BMC Infect Dis. 2022;22(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Chen X, Xie J, Li Y, Jian Z, Li H, Yan Q. Limited value of coagulation parameters in diagnosing periprosthetic joint infection. Int Orthop. 2022. 10.1007/s00264-022-05495-x [DOI] [PubMed] [Google Scholar]
- 148. Chen Y, Wang H, Chen X, Ma H, Zheng J, Cao L. Serum D‐lactate, a novel serological biomarker, is promising for the diagnosis of periprosthetic joint infection. BMC Musculoskelet Disord. 2022;23(1):292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Maimaiti Z, Xu C, Fu J, Chai W, Zhou Y, Chen J. The potential value of monocyte to lymphocyte ratio, platelet to mean platelet volume ratio in the diagnosis of periprosthetic joint infections. Orthop Surg. 2022;14(2):306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Mason JB, Fehring TK, Odum SM, Griffin WL, Nussman DS. The value of white blood cell counts before revision total knee arthroplasty. J Arthroplasty. 2003;18(8):1038–43. [DOI] [PubMed] [Google Scholar]
- 151. Trampuz A, Hanssen AD, Osmon DR, Mandrekar J, Steckelberg JM, Patel R. Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am J Med. 2004;117(8):556–62. [DOI] [PubMed] [Google Scholar]
- 152. Parvizi J, Ghanem E, Menashe S, Barrack RL, Bauer TW. Periprosthetic infection: what are the diagnostic challenges? J Bone Joint Surg Am. 2006;88:138–47. [DOI] [PubMed] [Google Scholar]
- 153. Ghanem E, Parvizi J, Burnett RS, Ellsworth B, Karkare N. Cell count and differential of aspirated fluid in the diagnosis of infection at the site of total knee arthroplasty. J Bone Joint Surg Am. 2008;90(8):1637–43. [DOI] [PubMed] [Google Scholar]
- 154. Ghanem E, Houssock C, Pulido L, Han S, Jaberi FM, Parvizi J. Determining “true” leukocytosis in bloody joint aspiration. J Arthroplasty. 2008;23(2):182–7. [DOI] [PubMed] [Google Scholar]
- 155. Jacovides CL, Parvizi J, Adeli B, Jung KA. Molecular markers for diagnosis of periprosthetic joint infection. J Arthroplasty. 2011;26(6 Suppl):99–103 e1. [DOI] [PubMed] [Google Scholar]
- 156. Parvizi J, Jacovides C, Antoci V, Ghanem E. Diagnosis of periprosthetic joint infection: the utility of a simple yet unappreciated enzyme. J Bone Joint Surg Am. 2011;93(24):2242–8. [DOI] [PubMed] [Google Scholar]
- 157. Parvizi J, McKenzie JC, Cashman JP. Diagnosis of periprosthetic joint infection using synovial C‐reactive protein. J Arthroplasty. 2012;27(8 Suppl):12–6. [DOI] [PubMed] [Google Scholar]
- 158. Wetters NG, Berend KR, Lombardi AV, Morris MJ, Tucker TL, Della Valle CJ. Leukocyte esterase reagent strips for the rapid diagnosis of periprosthetic joint infection. J Arthroplasty. 2012;27(8 Suppl):8–11. [DOI] [PubMed] [Google Scholar]
- 159. Mihalic RTR, Tercic D. Synovial fluid cell count and neutrophils differential as a useful tool in intraoperative diagnosis of total hip arthroplasty infection. Hip Int. 2012;22(4):416. [Google Scholar]
- 160. Zmistowski B, Restrepo C, Huang R, Hozack WJ, Parvizi J. Periprosthetic joint infection diagnosis: a complete understanding of white blood cell count and differential. J Arthroplasty. 2012;27(9):1589–93. [DOI] [PubMed] [Google Scholar]
- 161. Dinneen A, Guyot A, Clements J, Bradley N. Synovial fluid white cell and differential count in the diagnosis or exclusion of prosthetic joint infection. Bone Joint J. 2013;95‐B(4):554–7. [DOI] [PubMed] [Google Scholar]
- 162. Vanderstappen C, Verhoeven N, Stuyck J, Bellemans J. Intra‐articular versus serum C‐reactive protein analysis in suspected periprosthetic knee joint infection. Acta Orthop Belg. 2013;79(3):326–30. [PubMed] [Google Scholar]
- 163. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid alpha‐Defensin and C‐reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am. 2014;96(17):1439–45. [DOI] [PubMed] [Google Scholar]
- 164. Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014;472(11):3254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Chalmers PN, Sporer SM, Levine BR. Correlation of aspiration results with periprosthetic sepsis in revision total hip arthroplasty. J Arthroplasty. 2014;29(2):438–42. [DOI] [PubMed] [Google Scholar]
- 166. Tischler EH, Cavanaugh PK, Parvizi J. Leukocyte esterase strip test: matched for musculoskeletal infection society criteria. J Bone Joint Surg Am. 2014;96(22):1917–20. [DOI] [PubMed] [Google Scholar]
- 167. Buttaro MA, Martorell G, Quinteros M, Comba F, Zanotti G, Piccaluga F. Intraoperative synovial C‐reactive protein is as useful as frozen section to detect periprosthetic hip infection. Clin Orthop Relat Res. 2015;473(12):3876–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Chalmers PN, Walton D, Sporer SM, Levine BR. Evaluation of the role for synovial aspiration in the diagnosis of aseptic loosening after total knee arthroplasty. J Bone Joint Surg Am. 2015;97(19):1597–603. [DOI] [PubMed] [Google Scholar]
- 169. Shafafy R, McClatchie W, Chettiar K, Gill K, Hargrove R, Sturridge S, et al. Use of leucocyte esterase reagent strips in the diagnosis or exclusion of prosthetic joint infection. Bone Joint J. 2015;97‐B(9):1232–6. [DOI] [PubMed] [Google Scholar]
- 170. Kasparek MF, Kasparek M, Boettner F, Faschingbauer M, Hahne J, Dominkus M. Intraoperative diagnosis of periprosthetic joint infection using a novel alpha‐defensin lateral flow assay. J Arthroplasty. 2016;31(12):2871–4. [DOI] [PubMed] [Google Scholar]
- 171. Frangiamore SJ, Siqueira MB, Saleh A, Daly T, Higuera CA, Barsoum WK. Synovial cytokines and the MSIS criteria are not useful for determining infection resolution after periprosthetic joint infection explantation. Clin Orthop Relat Res. 2016;474(7):1630–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Boettner F, Koehler G, Wegner A, Schmidt‐Braekling T, Gosheger G, Goetze C. The rule of histology in the diagnosis of periprosthetic infection: specific granulocyte counting methods and new immunohistologic staining techniques may increase the diagnostic value. Open Orthop J. 2016;10(1):457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Choi HR, Agrawal K, Bedair H. The diagnostic thresholds for synovial fluid analysis in late periprosthetic infection of the hip depend on the duration of symptoms. Bone Joint J. 2016;98‐B(10):1355–9. [DOI] [PubMed] [Google Scholar]
- 174. De Vecchi E, Villa F, Bortolin M, Toscano M, Tacchini L, Romanò CL, et al. Leucocyte esterase, glucose and C‐reactive protein in the diagnosis of prosthetic joint infections: a prospective study. Clin Microbiol Infect. 2016;22(6):555–60. [DOI] [PubMed] [Google Scholar]
- 175. Ruangsomboon P, Chinprasertsuk S, Khejonnit V, Chareancholvanich K. Effect of depth of centrifuged synovial fluid on leukocyte esterase test for periprosthetic joint infection. J Orthop Res. 2017;35(11):2545–50. [DOI] [PubMed] [Google Scholar]
- 176. Li R, Li X, Yu B, Li X, Song X, Li H, et al. Comparison of leukocyte esterase testing of synovial fluid with synovial histology for the diagnosis of Periprosthetic joint infection. Med Sci Monit. 2017;23:4440–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Koh IJ, Han SB, In Y, Oh KJ, Lee DH, Kim TK, et al. The leukocyte esterase strip test has practical value for diagnosing periprosthetic joint infection after total knee arthroplasty: a multicenter study. J Arthroplasty. 2017;32(11):3519–23. [DOI] [PubMed] [Google Scholar]
- 178. Wang C, Li R, Wang Q, Duan J, Wang C. Leukocyte esterase as a biomarker in the diagnosis of periprosthetic joint infection. Med Sci Monit. 2017;23:353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Kheir MM, Ackerman CT, Tan TL, Benazzo A, Tischler EH, Parvizi J. Leukocyte esterase strip test can predict subsequent failure following reimplantation in patients with Periprosthetic joint infection. J Arthroplasty. 2017;32(6):1976–9. [DOI] [PubMed] [Google Scholar]
- 180. Kawamura M KN, Inaba Y, Tomoyama A, Choe H, Tezuka T, Kubota S, et al. The usefulness of synovial fluid C‐reactive protein for periprosthetic hip joint infection. Presented as a Poster Exhibit at the Orthopaedic Research Society Annual Meeting 2017. pp. 19–22.
- 181. Higuera CA, Zmistowski B, Malcom T, Barsoum WK, Sporer SM, Mommsen P, et al. Synovial fluid cell count for diagnosis of chronic periprosthetic hip infection. J Bone Joint Surg Am. 2017;99(9):753–9. [DOI] [PubMed] [Google Scholar]
- 182. Lausmann C, Zahar A, Citak M, Brañes J, Schmidl S, Frommelt L, et al. Are there benefits in early diagnosis of prosthetic joint infection with multiplex polymerase chain reaction? J Bone Jt Infect. 2017;2(4):175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Gallo J, Juranova J, Svoboda M, Zapletalova J. Excellent AUC for joint fluid cytology in the detection/exclusion of hip and knee prosthetic joint infection. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2017;161(3):310–9. [DOI] [PubMed] [Google Scholar]
- 184. Sousa R, Serrano P, Gomes Dias J, Oliveira JC, Oliveira A. Improving the accuracy of synovial fluid analysis in the diagnosis of prosthetic joint infection with simple and inexpensive biomarkers: C‐reactive protein and adenosine deaminase. Bone Joint J. 2017;99‐B(3):351–7. [DOI] [PubMed] [Google Scholar]
- 185. Suda AJ, Tinelli M, Beisemann ND, Weil Y, Khoury A, Bischel OE. Diagnosis of periprosthetic joint infection using alpha‐defensin test or multiplex‐PCR: ideal diagnostic test still not found. Int Orthop. 2017;41(7):1307–13. [DOI] [PubMed] [Google Scholar]
- 186. Bonanzinga T, Zahar A, Dutsch M, Lausmann C, Kendoff D, Gehrke T. How reliable is the alpha‐defensin immunoassay test for diagnosing periprosthetic joint infection? A prospective study. Clin Orthop Relat Res. 2017;475(2):408–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Plate A, Stadler L, Sutter R, Anagnostopoulos A, Frustaci D, Zbinden R, et al. Inflammatory disorders mimicking periprosthetic joint infections may result in false‐positive alpha‐defensin. Clin Microbiol Infect. 2018;24(11):1212 e1–6. [DOI] [PubMed] [Google Scholar]
- 188. Kelly MP, Darrith B, Hannon CP, Nam D, Courtney PM, Della Valle CJ. Synovial fluid alpha‐defensin is an adjunctive tool in the equivocal diagnosis of periprosthetic joint infection. J Arthroplasty. 2018;33(11):3537–40. [DOI] [PubMed] [Google Scholar]
- 189. Gehrke T, Lausmann C, Citak M, Bonanzinga T, Frommelt L, Zahar A. The accuracy of the alpha defensin lateral flow device for diagnosis of periprosthetic joint infection: comparison with a gold standard. J Bone Joint Surg Am. 2018;100(1):42–8. [DOI] [PubMed] [Google Scholar]
- 190. Balato G, Franceschini V, Ascione T, Lamberti A, Balboni F, Baldini A. Diagnostic accuracy of synovial fluid, blood markers, and microbiological testing in chronic knee prosthetic infections. Arch Orthop Trauma Surg. 2018;138(2):165–71. [DOI] [PubMed] [Google Scholar]
- 191. Balato G, Franceschini V, Ascione T, Lamberti A, D'Amato M, Ensini A, et al. High performance of alpha‐defensin lateral flow assay (synovasure) in the diagnosis of chronic knee prosthetic infections. Knee Surg Sports Traumatol Arthrosc. 2018;26(6):1717–22. [DOI] [PubMed] [Google Scholar]
- 192. Kanwar S, Al‐Mansoori AA, Chand MR, Villa JM, Suarez JC, Patel PD. What is the optimal criteria to use for detecting periprosthetic joint infections before total joint arthroplasty? J Arthroplasty. 2018;33(7S):S201–S4. [DOI] [PubMed] [Google Scholar]
- 193. Li R, Lu Q, Zhou YG, Chai W, Lu SB, Chen JY. Centrifugation may change the results of leukocyte esterase strip testing in the diagnosis of periprosthetic joint infection. J Arthroplasty. 2018;33(9):2981–5. [DOI] [PubMed] [Google Scholar]
- 194. Sigmund IK, Yermak K, Perka C, Trampuz A, Renz N. Is the enzyme‐linked immunosorbent assay more accurate than the lateral flow alpha defensin test for diagnosing periprosthetic joint infection? Clin Orthop Relat Res. 2018;476(8):1645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Stone WZ, Gray CF, Parvataneni HK, al‐Rashid M, Vlasak RG, Horodyski MB, et al. Clinical evaluation of synovial alpha defensin and synovial C‐reactive protein in the diagnosis of periprosthetic joint infection. J Bone Joint Surg Am. 2018;100(14):1184–90. [DOI] [PubMed] [Google Scholar]
- 196. Zahar A, Lausmann C, Cavalheiro C, Dhamangaonkar AC, Bonanzinga T, Gehrke T, et al. How reliable is the cell count analysis in the diagnosis of prosthetic joint infection? J Arthroplasty. 2018;33(10):3257–62. [DOI] [PubMed] [Google Scholar]
- 197. De Vecchi E, Romano CL, De Grandi R, Cappelletti L, Villa F, Drago L. Alpha defensin, leukocyte esterase, C‐reactive protein, and leukocyte count in synovial fluid for pre‐operative diagnosis of periprosthetic infection. Int J Immunopathol Pharmacol. 2018;32:2058738418806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Riccio G, Cavagnaro L, Akkouche W, Carrega G, Felli L, Burastero G. Qualitative alpha‐defensin versus the main available tests for the diagnosis of periprosthetic joint infection: best predictor test? J Bone Jt Infect. 2018;3(3):156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Kleiss S, Jandl NM, Novo de Oliveira A, Ruther W, Niemeier A. Diagnostic accuracy of alpha‐defensin enzyme‐linked immunosorbent assay in the clinical evaluation of painful hip and knee arthroplasty with possible prosthetic joint infection: a prospective study of 202 cases. Bone Joint J. 2019;101‐B(8):970–7. [DOI] [PubMed] [Google Scholar]
- 200. Di Benedetto P, Dalla Vecchia G, Dante F, Gisonni R, Cainero V, Causero A. Leukocyte esterase strip test as a reliable intraoperative PJIs biomarker. Our experience. Acta Biomed. 2019;90(12‐S):43–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Plate A, Anagnostopoulos A, Glanzmann J, Stadler L, Weigelt L, Sutter R, et al. Synovial C‐reactive protein features high negative predictive value but is not useful as a single diagnostic parameter in suspected periprosthetic joint infection (PJI). J Infect. 2019;78(6):439–44. [DOI] [PubMed] [Google Scholar]
- 202. Stone WZ, Gray CF, Parvataneni HK, Prieto HA. Clinical evaluation of alpha defensin test following staged treatment of prosthetic joint infections. J Arthroplasty. 2019;34(7):1446–51. [DOI] [PubMed] [Google Scholar]
- 203. Lazarides AL, Vovos TJ, Reddy GB, Kildow BJ, Wellman SS, Jiranek WA, et al. Traditional laboratory markers hold low diagnostic utility for immunosuppressed patients with periprosthetic joint infections. J Arthroplasty. 2019;34(7):1441–5. [DOI] [PubMed] [Google Scholar]
- 204. Zagra L, Villa F, Cappelletti L, Gallazzi E, Materazzi G, De Vecchi E. Can leucocyte esterase replace frozen sections in the intraoperative diagnosis of prosthetic hip infection? Bone Joint J. 2019;101‐B(4):372–7. [DOI] [PubMed] [Google Scholar]
- 205. Ettinger M, Savov P, Calliess T, Windhagen H, Lichtinghagen R, Lukasz A, et al. Improved diagnostic accuracy with the classification tree method for diagnosing low‐grade periprosthetic joint infections by quantitative measurement of synovial fluid alpha‐defensin and C‐reactive protein. Int Orthop. 2020;44(1):31–8. [DOI] [PubMed] [Google Scholar]
- 206. Trotter AJ, Dean R, Whitehouse CE, Mikalsen J, Hill C, Brunton‐Sim R, et al. Preliminary evaluation of a rapid lateral flow calprotectin test for the diagnosis of prosthetic joint infection. Bone Joint Res. 2020;9(5):202–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. de Saint VB, Martinot P, Pascal A, Senneville E, Loiez C, Pasquier G, et al. Does the alpha‐defensin lateral flow test conserve its diagnostic properties in a larger population of chronic complex periprosthetic infections? Enlargement to 112 tests, from 42 tests in a preliminary study, in a reference center. Orthop Traumatol Surg Res. 2021;107(4):102912. [DOI] [PubMed] [Google Scholar]
- 208. Dijkman C, Thomas AR, Koenraadt KLM, Ermens AAM, van Geenen RCI. Synovial neutrophilic gelatinase‐associated lipocalin in the diagnosis of periprosthetic joint infection after total knee arthroplasty. Arch Orthop Trauma Surg. 2020;140(7):941–7. [DOI] [PubMed] [Google Scholar]
- 209. Salari P, Grassi M, Cinti B, Onori N, Gigante A. Synovial fluid calprotectin for the preoperative diagnosis of chronic periprosthetic joint infection. J Arthroplasty. 2020;35(2):534–7. [DOI] [PubMed] [Google Scholar]
- 210. Zhang Z, Cai Y, Bai G, Zhang C, Li W, Yang B, et al. The value of calprotectin in synovial fluid for the diagnosis of chronic prosthetic joint infection. Bone Joint Res. 2020;9(8):450–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Yu BZ, Li R, Fu J, Chai W, Hao LB, Chen JY. Leukocyte esterase test and alpha‐defensin test have similar accuracy for the diagnosis of periprosthetic joint infection. Int Orthop. 2021;45(7):1677–82. [DOI] [PubMed] [Google Scholar]
- 212. Grzelecki D, Walczak P, Szostek M, Grajek A, Rak S, Kowalczewski J. Blood and synovial fluid calprotectin as biomarkers to diagnose chronic hip and knee periprosthetic joint infections. Bone Joint J. 2021;103‐b(1):46–55. [DOI] [PubMed] [Google Scholar]
- 213. Ivy MI, Sharma K, Greenwood‐Quaintance KE, Tande AJ, Osmon DR, Berbari EF, et al. Synovial fluid α defensin has comparable accuracy to synovial fluid white blood cell count and polymorphonuclear percentage for periprosthetic joint infection diagnosis. Bone Joint J. 2021;103‐b(6):1119–26. [DOI] [PubMed] [Google Scholar]
- 214. Shohat N, Yacovelli S, Chisari E, Clarkson S, Mann D, Parvizi J. Alpha‐defensin does not provide additional benefit over leukocyte esterase in the diagnosis of periprosthetic joint infection. Expert Rev Mol Diagn. 2021;21(8):845–9. [DOI] [PubMed] [Google Scholar]
- 215. Abdo RCT, Gobbi RG, Leite CBG, Pasoto SG, Leon EP, Lima ALLM, et al. Performance of alpha‐defensin lateral flow test after synovial fluid centrifugation for diagnosis of periprosthetic knee infection. World J Orthop. 2021;12(8):565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Warren J, Anis HK, Bowers K, Pannu T, Villa J, Klika AK, et al. Diagnostic utility of a novel point‐of‐care test of calprotectin for periprosthetic joint infection after Total knee arthroplasty: a prospective cohort study. J Bone Joint Surg Am. 2021;103(11):1009–15. [DOI] [PubMed] [Google Scholar]
- 217. Grassi M, Salari P, Farinelli L, D'Anzeo M, Onori N, Gigante A. Synovial biomarkers to detect chronic periprosthetic joint infection: a pilot study to compare calprotectin rapid test, calprotectin ELISA immunoassay and leukocyte esterase test. J Arthroplasty. 2022;37(4):781–6. [DOI] [PubMed] [Google Scholar]
- 218. Somme D, Ziza J‐M, Desplaces N, Chicheportiche V, Chazerain P, Leonard P, et al. Contribution of routine joint aspiration to the diagnosis of infection before hip revision surgery. Joint Bone Spine. 2003;70(6):489–95. [DOI] [PubMed] [Google Scholar]
- 219. Williams JL, Norman P, Stockley I. The value of hip aspiration versus tissue biopsy in diagnosing infection before exchange hip arthroplasty surgery. J Arthroplasty. 2004;19(5):582–6. [DOI] [PubMed] [Google Scholar]
- 220. Malhotra R, Morgan DAF. Role of core biopsy in diagnosing infection before revision hip arthroplasty. J Arthroplasty. 2004;19(1):78–87. [DOI] [PubMed] [Google Scholar]
- 221. Ali F, Wilkinson JM, Cooper JR, Kerry RM, Hamer AJ, Norman P, et al. Accuracy of joint aspiration for the preoperative diagnosis of infection in total hip arthroplasty. J Arthroplasty. 2006;21(2):221–6. [DOI] [PubMed] [Google Scholar]
- 222. Van den Bekerom MP, Stuyck J. The value of pre‐operative aspiration in the diagnosis of an infected prosthetic knee: a retrospective study and review of literature. Acta Orthop Belg. 2006;72(4):441–7. [PubMed] [Google Scholar]
- 223. Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, et al. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007;357(7):654–63. [DOI] [PubMed] [Google Scholar]
- 224. Gallo J, Kolar M, Dendis M, Loveckova Y, Sauer P, Zapletalova J, et al. Culture and PCR analysis of joint fluid in the diagnosis of prosthetic joint infection. New Microbiol. 2008;31(1):97–104. [PubMed] [Google Scholar]
- 225. Meermans G, Haddad FS. Is there a role for tissue biopsy in the diagnosis of periprosthetic infection? Clin Orthop Relat Res. 2010;468(5):1410–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Cross MC, Kransdorf MJ, Chivers FS, Lorans R, Roberts CC, Schwartz AJ, et al. Utility of percutaneous joint aspiration and synovial biopsy in identifying culture‐positive infected hip arthroplasty. Skeletal Radiol. 2014;43(2):165–8. [DOI] [PubMed] [Google Scholar]
- 227. Ryu SY, Greenwood‐Quaintance KE, Hanssen AD, Mandrekar JN, Patel R. Low sensitivity of periprosthetic tissue PCR for prosthetic knee infection diagnosis. Diagn Microbiol Infect Dis. 2014;79(4):448–53. [DOI] [PubMed] [Google Scholar]
- 228. Shen H, Tang J, Wang Q, Jiang Y, Zhang X. Sonication of explanted prosthesis combined with incubation in BD bactec bottles for pathogen‐based diagnosis of prosthetic joint infection. J Clin Microbiol. 2015;53(3):777–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Fang XY, Li WB, Zhang CF, Huang ZD, Zeng HY, Dong Z, et al. Detecting the presence of bacterial DNA and RNA by polymerase chain reaction to diagnose suspected periprosthetic joint infection after antibiotic therapy. Orthop Surg. 2018;10(1):40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Huang Z, Wu Q, Fang X, Li W, Zhang C, Zeng H, et al. Comparison of culture and broad‐range polymerase chain reaction methods for diagnosing periprosthetic joint infection: analysis of joint fluid, periprosthetic tissue, and sonicated fluid. Int Orthop. 2018;42(9):2035–40. [DOI] [PubMed] [Google Scholar]
- 231. Larsen LH, Khalid V, Xu Y, Thomsen TR, Schonheyder HC, the PSG . Differential contributions of specimen types, culturing, and 16S rRNA sequencing in diagnosis of prosthetic joint infections. J Clin Microbiol. 2018;56(5):e01351‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Morgenstern C, Cabric S, Perka C, Trampuz A, Renz N. Synovial fluid multiplex PCR is superior to culture for detection of low‐virulent pathogens causing periprosthetic joint infection. Diagn Microbiol Infect Dis. 2018;90(2):115–9. [DOI] [PubMed] [Google Scholar]
- 233. Fang X, Cai Y, Shi T, Huang Z, Zhang C, Li W, et al. Detecting the presence of bacteria in low‐volume preoperative aspirated synovial fluid by metagenomic next‐generation sequencing. Int J Infect Dis. 2020;99:108–16. [DOI] [PubMed] [Google Scholar]
- 234. Bonanzinga T, Ferrari MC, Tanzi G, Vandenbulcke F, Zahar A, Marcacci M. The role of alpha defensin in prosthetic joint infection (PJI) diagnosis: a literature review. EFORT Open Rev. 2019;4(1):10–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Wang S, Song R, Wang Z, Jing Z, Wang S, Ma J. S100A8/A9 in inflammation. Front Immunol. 2018;9:1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Galliera E, Drago L, Vassena C, Romanò C, Gioia Marazzi M, Salcito L, et al. Toll‐like receptor 2 in serum: a potential diagnostic marker of prosthetic joint infection? J Clin Microbiol. 2014;52(2):620–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Yermak K, Karbysheva S, Perka C, Trampuz A, Renz N. Performance of synovial fluid D‐lactate for the diagnosis of periprosthetic joint infection: a prospective observational study. J Infect. 2019;79:123–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Systematic review search strategy
Appendix S2 Information on the 215 studies included
Appendix S3 Subgroup analysis of chronic PJI without IA population
Appendix S4 Hierarchical analysis of thresholds (chronic PJI without IA population)
Appendix S5 Hierarchical analysis of thresholds with MSIS criteria as gold standard (chronic PJI without IA population)
Fig. S1 Alpha‐defensin Laboratory test
Fig. S2 Alpha‐defensin Lateral Flow
Fig. S3 Calprotectin
Fig. S4 LE
Fig. S5 Synovial WBC
Fig. S6 Synovial PMN%
Fig. S7 Synovial CRP
Fig. S8 Synovial IL‐6
Fig. S9 Synovial IL‐1β
Fig. S10 Serum CRP
Fig. S11 Serum ESR
Fig. S12 Serum IL‐6
Fig. S13 Serum D‐Dimer
Fig. S14 Serum PCT
Fig. S15 Serum WBC
Fig. S16 Serum PLT
Fig. S17 Serum NLR
Fig. S18 Serum MLR
Fig. S19 Serum PLR
Fig. S20 Serum PVR
Fig. S21 Serum Fibrinogen
Fig. S22 Serum FDP
Fig. S23 Aspiration Culture


