Abstract
Introduction
Previous randomized controlled trials (RCTs) and meta-analyses of RCTs evaluating vitamin D supplementation for the prevention of cancer incidence and mortality have found inconsistent results and no meta-analysis has assessed the quality of the evidence available. We, therefore, aimed to perform an updated meta-analysis by including recent large-scale RCTs and assessing the quality of the pooled evidence.
Methods
We searched several databases and trial registers from inception to April 2022. We used a random-effects model to estimate pooled risk ratios (RRs) and 95% confidence intervals (CIs). We used the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) considerations to evaluate the certainty of evidence.
Results
We included 13 RCTs in our study. Vitamin D supplementation had no effect on the risk of total cancer incidence (RR 0.99, 95% CI: 0.94–1.04; I2= 0%), total cancer mortality (RR 0.93, 95% CI: 0.84–1.03; I2= 24%) and total mortality (RR 0.92, 95% CI: 0.82–1.04; I2= 36%). The overall quality of evidence was high for all outcomes.
Discussion
Vitamin D supplementation is ineffective in reducing total cancer incidence and mortality in largely vitamin D-replete older adult populations. Future research should be based on populations with a higher prevalence of vitamin D deficiency and should involve more extended follow-up periods.
Study protocol
PROSPERO database, CRD42021285401.
Keywords: Meta-analysis, Vitamin D supplements, Cancer incidence, Cancer mortality
Highlights
-
•
We assessed the effect of vitamin D supplementation on total cancer incidence and mortality.
-
•
We included 13 randomized controlled trials (RCTs) with 109,543 participants.
-
•
Vitamin D is ineffective in reducing total cancer incidence and mortality in largely vitamin D-replete older adults.
-
•
Future RCTs should focus on populations with a higher prevalence of vitamin D deficiency.
Meta-analysis; Vitamin D supplements; Cancer incidence; Cancer mortality.
1. Introduction
The effects of 25-hydroxyvitamin-D [25(OH)D], also known as calcitriol, have been previously investigated and several roles have been attributed to it. 25(OH)D has been found to affect cell differentiation and apoptosis, and inhibit angiogenesis and the proliferation of cancer cells [1]. Several studies have suggested a negative association between 25(OH)D serum levels and all-cause, cardiovascular and cancer mortality [2, 3, 4]. However, reverse causality could be a potential explanation for these observations.
A previous meta-analysis of cohort studies suggested that higher serum levels of 25(OH)D are associated with lower cancer incidence and cancer mortality [4]. Additionally, higher survival rates were reported among patients with higher levels of circulating 25(OH)D [5, 6, 7]. A previous meta-analysis of RCTs published in 2014 suggested a positive impact of vitamin D supplementation on cancer mortality but no effect on cancer incidence [8]. However, no evidence to support these findings was found in another meta-analysis published in 2018 [9]. The most recent meta-analysis of RCTs published in 2019 suggested that vitamin D supplementation significantly reduced cancer mortality but had no effect on cancer incidence [10]. However, it is worth noting that the beneficial effect seen on mortality was largely influenced by the results of the US VITAL Trial [11] which is contrary to the results of the D-Health Trial [12], a large population-based trial which has been recently published. Therefore, the role of vitamin D in cancer remains to be established. Moreover, no prior meta-analysis has assessed the quality of the existing evidence base.
Several RCTs, including the D-Health Trial, have been published after the most recently published meta-analysis. Therefore, this meta-analysis aims to update the literature and address the inconsistencies regarding the role of vitamin D supplementation compared with placebo in the prevention of the onset of cancer and cancer mortality.
2. Materials and methods
Our meta-analysis conforms to the guidelines presented in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement and the Cochrane Handbook for Systematic Reviews of Interventions (Supplementary Table 1) [13, 14]. Our protocol has been registered with The International Prospective Register of Systematic Reviews, PROSPERO (CRD42022308968).
2.1. Data sources and searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, via The Cochrane Library), MEDLINE (via PubMed), Embase (via Ovid), and ClinicalTrials.gov. from inception till April 2022 for any RCTs that involved a comparison of vitamin D at any dose with a control group. We used ProQuest Dissertations and Theses Global (PQDT) and OpenGrey to identify relevant grey literature. Bibliographies of previous meta-analyses and included trials were also screened to identify further RCTs related to our topic. No filter was applied in terms of language or publication period of the included studies.
The complete search strategy for each database can be found in Supplementary Table 2.
2.2. Eligibility criteria
We included all RCTs, regardless of the study population, that evaluated the effect of vitamin D supplementation (administered as ergocalciferol or cholecalciferol, regardless of whether in combination with other nutrients such as calcium or not) compared with placebo on total cancer incidence or mortality. Study designs other than RCTs such as observational studies were excluded. We also excluded those RCTs in which the total number of outcome events was ≤10 or the length of follow-up was ≤1 year.
2.3. Study selection and data abstraction
All identified records were imported to Mendeley Desktop 1.19.8 (Mendeley Ltd., Amsterdam, The Netherlands) where two authors (AS and MF) working independently screened them, first on the basis of titles and abstracts and then on the basis of full texts in accordance with the predefined eligibility criteria. Any disagreements were resolved by a third author (HAC).
Data from included studies were extracted by two authors (AUR and MMH) into a pre-piloted Excel sheet. A third author (HAC) rechecked the completed extraction sheet and resolved any discrepancies and ensured the accuracy of the data. The following data items were extracted: study characteristics including the year of publication, study location, and follow-up duration, population characteristics such as age and gender, interventions (including co-interventions, types, dosages, and duration of interventions), comparators, and outcomes (primary and secondary outcomes).
2.4. Outcome measures
The primary outcomes were total cancer incidence and mortality. The additional outcome was all-cause mortality.
2.5. Risk of bias assessment
The methodological quality of our included studies was assessed by two authors (OB and AS) working independently using the revised Cochrane Risk of Bias Tool for randomized controlled trials (RoB 2.0) [15]. The authors assessed bias across five domains: (1) randomisation process; (2 deviations from intended interventions; (3) missing outcome data; (4) measurement of the outcome; and (5) selection of the reported result.
2.6. Data synthesis
Statistical analysis was performed using Review Manager (RevMan, Version 5.4; The Cochrane Collaboration, Copenhagen, Denmark). DerSimonian and Laird random-effects models were used to perform meta-analyses. Outcomes were reported as relative risk (RR) along with 95% confidence intervals. The Chi2 test and I2 statistic were calculated for each group or subgroup analysis to evaluate and quantify the heterogeneity present. I2 values were interpreted according to the Cochrane Handbook for Systematic Reviews of Interventions. [13] The Chi2 test was deemed statistically significant when P < 0.10 [13].
Funnel plots were constructed to assess publication bias and Egger's test was used to check funnel plot asymmetry using Jamovi (version 1.8) [16]. A P-value less than 0.10 indicated publication bias.
2.7. Subgroup and sensitivity analyses
We conducted subgroup analyses, on our primary outcomes, on the basis of the regimen of vitamin D supplementation, baseline and attained circulating 25(OH)D levels, and the different population groups (postmenopausal women versus general population versus diseased population). For the test for subgroup differences, P < 0.05 was deemed statistically significant.
For the primary outcomes, we also conducted a sensitivity analysis by excluding trials that tested the combination of vitamin D and calcium against a placebo.
2.8. Certainty of evidence assessment
Two authors (AS and HAC) separately evaluated the certainty of the evidence by using the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) tool which is based on five domains (risk of bias in individual studies, heterogeneity, imprecision, indirectness, and publication bias) [17, 18]. We judged the pooled effects as imprecise if the optimal information size (OIS) criterion was not met, or the associated 95% CIs included the null effect as well as appreciable benefit or harm [19]. The GRADE approach rates the quality of evidence as one of the four grades: high (the true effect lies close to that of the estimate of the effect), moderate (the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different), low (true effect may be substantially different from the estimate of the effect), and very low (the true effect is likely to be substantially different from the estimate of effect).
3. Results
3.1. Study selection and characteristics
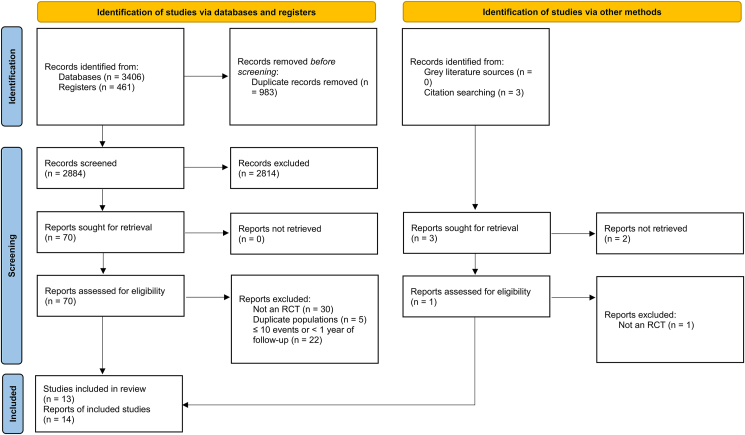
A comprehensive database search yielded a total of 3867 records while 3 articles were retrieved via citation searching. After removing duplicates (n = 983), the remaining articles were subjected to screening, leaving behind 14 reports of 13 RCTs, comprising 109,543 participants and spanning from 2003 to 2022, that were deemed eligible to be included in this meta-analysis (Figure 1) [11, 12, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30]. Among the included trials, six RCTs were conducted in the USA, two in the UK, two in Australia, one in Finland, one in New Zealand, and one in Norway. In terms of the regimen of Vitamin D intake, eight trials provided Vitamin D3 (400–2000 IU/day) to the participants daily [11, 20, 23, 25, 26, 28, 29, 30], whereas five trials provided an intermittent large dose of Vitamin D3 to the participants (20 000 IU/week to 500 000 IU/year) [12, 21, 22, 24, 27]. At baseline, circulating levels of 25(OH)D in the included trials ranged between 38 and 83 nmol/l, with the intervention group's range reaching between 54 and 135 nmol/l at some point during the follow-up. The post-intervention follow-up duration was approximately 3–10 years. The detailed characteristics of the studies are presented in Table 1.
Figure 1.
PRISMA 2020 flow chart. Flow chart of included and excluded trials. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 1.
Characteristics of included RCTs.
| Author | Location | Study population; Mean age |
Gender distribution (Male %) | Primary endpoint of trial; Follow-up period |
Experimental group (Intervention) | Control group | Baseline Vitamin D change (nmol/L)a (Baseline-- > Follow-up) |
Inclusion/Exclusion criteria regarding Vitamin D supplementation |
|---|---|---|---|---|---|---|---|---|
| Trivedi [24] 2003 |
UK | General population; 75 years |
76% | Fracture, Total mortality; 5 years |
Vitamin D3 where Vitamin. D3: 100,000 IU/4 m (∼833 IU/d) |
Placebo | Intervention: NA--> 74 at 4y Control: NA--> 53 at 4y |
Excluded current users of Vitamin D supplement |
| Wactawski-Wende [29] 2006 LaCroix [40] 2009 |
USA | Postmenopausal women; 62 years |
0% | Hip fracture; 7 years (up to 9.7 years) |
Vitamin D3+Ca where Vitamin. D3: 400 IU/d and Ca (carbonate): 1000 mg/d |
Placebo | Intervention: 42--> 54 at 2 years Control: 42-- > NA |
Excluded current users of Vitamin D supplement (≥600 IU/d); Calcitriol |
| Lappe [28] 2007 |
USA | Postmenopausal women; 67 years |
0% | Fracture; 4 years |
Vitamin D3+Ca where Vitamin. D3: 1100 IU/d and Ca (carbonate) 1500 mg/d or (citrate) 1400 mg/d |
Calcium | Intervention: 72--> 96 at 1 year Control: 72--> 71 at 1 year |
NR |
| Sanders [21] 2010 |
Australia | postmenopausal women; 76 years |
0% | Fracture; 3–5 years (with 1-year post-intervention follow-up) |
Vitamin D3 where Vitamin. D3: 500 000 IU/year (∼1370 IU/d) |
Placebo | Intervention: 53--> 58 at trial end Control: 45--> 38 at trial end |
Excluded current users of Vitamin D supplement (≥400 IU/d); calcitriol |
| Avenell [26] 2012 |
UK | General population; 77 years |
15% | Fracture; 2–5.2 years (with 3 years post-intervention follow-up) |
Vitamin D3 (w, w/o Ca) where Vitamin D3: 800 IU/d and Ca (carbonate): 1000 mg/d |
No Vitamin D3 (w, w/o Ca) | Intervention: 38--> 62 at 1 year Control: 38--> 44 at 1 year |
Excluded current users of Vitamin D supplement (200 IU/d); those with past treatment with Vitamin D metabolite or Vitamin D injection |
| Baron [23] 2015 |
USA | Individuals with a history of colorectal adenomas; 58 years |
63% | Colorectal adenomas; 3.7 years (Range: 3–5 years) |
Vitamin D3 (w, w/o Ca) where Vit. D3: 1000 IU/d and Ca (carbonate): 1200 mg/d |
No Vitamin D3 (w, w/o Ca) | Intervention: 61--> 81 at trial end Control: 61-- > NA |
Limited non-protocol supplement of Vitamin D up to 1000 IU/d |
| Jorde [22] 2016 |
Norway | Individuals with impaired fasting glucose and/or impaired glucose tolerance; 62 years |
NR | Type 2 diabetes; 5 years |
Vitamin D3 where Vitamin D3: 20 000 IU/week (∼2857 IU/d) |
Placebo | Intervention: 60--> 122 at trial end Control: 61--> 67 at trial end |
Limited non-protocol supplement of Vitamin D (including cod liver oil) up to 400 IU/d |
| Lappe [25] 2017 |
USA | Postmenopausal women; 65 years |
0% | Total cancer excluding non-melanoma skin cancers; 4 years |
Vitamin D3+Ca where Vitamin D3: 2000 IU/d and Ca (carbonate): 1500 mg/d |
Placebo | Intervention: 83--> 106 at trial end Control: 82--> 77 at trial end |
Limited non-protocol supplement of Vitamin D up to 800 IU/d |
| Manson [11] 2018 |
USA | General population including African-Americans by 20%; 67 years |
49% | Total invasive cancer, Major cardiovascular events; 5.3 years (Range: 3.8–6.1 years) |
Vitamin D3 (w, w/o omega-3 fatty acids) where Vitamin D3: 2000 IU/d and Omega-3 fatty acids: 1 g/d |
No vitamin D3 (w, w/o omega-3 fatty acids) | Intervention: 75--> 105 at 1 year Control: 75--> 73 at 1 year |
Limited non-protocol supplement of Vitamin D up to 800 IU/d |
| Scragg [27] 2018 |
New Zealand | General population; 66 years |
58% | CVD; 3.3 years (Range: 2.5–4.2 years) |
Vitamin D3 where Vitamin D3: Initial bolus of 200 000 IU followed by 100 000 IU/m (∼3279 IU/d) |
Placebo | Intervention: 64--> 120–135 at 0.5–3 years Control: 63--> 70–85 at 0.5–3 years |
Excluded current users of Vitamin D supplements including cod liver oil (>600 IU/d if aged 50–70 years; >800 IU/d if aged 71–84 years) |
| Chatterjee [30] 2021 |
USA | Overweight and pre-diabetic patients; 60 years |
55.50% | Development of cancer 2.9 years (Range: 2–3.5 years) |
Vitamin D3 where Vitamin D3: 4000 IU/d |
Placebo | Intervention: 69.4-->135.5 at 2 years Control: 70.4-->71.9 at 2 years |
Excluded current users of Vitamin D supplements containing vitamin D (1000 IU/d) or Users of medications or conditions that could interfere with the absorption or metabolism of vitamin D |
| Neale [12] 2022 |
Australia | General population aged 60–84; 69.3 years |
54.10% | Mortality; 5.7 years (Range: 5.4–6.7) |
Vitamin D3 where Vitamin D3: 60,000 IU/month |
Placebo | Intervention: NA-->115 Control: NA-->77 |
Excluded current users of Vitamin D supplements >500 IU/d |
| Virtanen [20] 2022 |
Finland | General population of males ≥60; Postmenopausal females (≥65); 68.2 years |
57% | CVD, Invasive cancer 4.3 years |
Vitamin D3 where Vitamin D3: either 1600 IU/d or 3200 IU/d |
Placebo | Intervention: 74.9-->120.4 at 1 year Control: 73.7-->72.7 at 1 year |
Excluded current users of Vitamin D supplements >800 IU/d |
Abbreviations: Ca, calcium; d, day; IU, international unit, M, male; m, month; n, number; NA, not available; Vit, vitamin; w, with; w/o, without.
To convert to ng/ml, divide by 2.5.
3.2. Risk of bias in included studies
Overall, two studies were found to have some concerns of bias, one in the domain of randomization and the other in the domains of randomization and deviations from intended interventions [11, 28], and one study was judged to be at a high risk of bias due to missing outcome data (Supplementary Figure 1) [22]. The remaining studies (76.9%) were found to be at low risk of bias.
3.3. Effects of interventions
3.3.1. Primary outcomes
3.3.1.1. Vitamin D supplementation and total cancer incidence
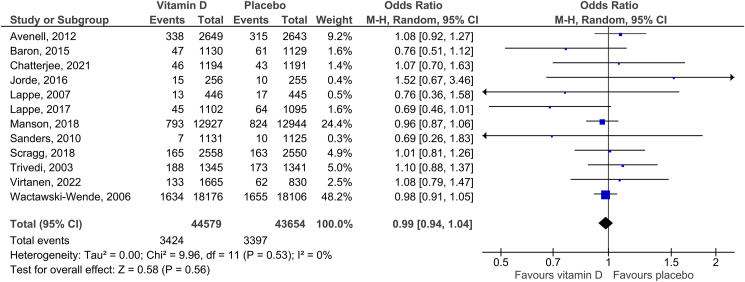
Twelve RCTs were included in the analysis of total cancer incidence. The summary RR for intervention versus control group was 0.99 (95% CI: 0.94–1.04, P = 0.56). There was no heterogeneity reported (I2 = 0%; Figure 2). No funnel plot asymmetry was detected with Egger’s test (P = 0.651; Supplementary Figure 2). The overall quality of evidence was rated as high (Supplementary Table 3). There was no statistically significant difference between any of the subgroups: the regimen of vitamin D intake (Pinteraction = 0.38), attained 25(OH)D level (Pinteraction = 0.64), baseline 25(OH)D level (Pinteraction = 0.55), and type of study population (Pinteraction = 0.47) (Table 2). The sensitivity analysis reported an RR of 1.00 (95% CI: 0.94–1.07, P = 0.94; I2 = 0%) after excluding two trials that reported a combined effect of vitamin D and calcium against a placebo [25, 29].
Figure 2.
Effect of vitamin D supplementation on total cancer incidence.
Table 2.
Subgroup analysis.
| Subgroups | No. of Studies | RR (95% CI) | I2 (%) | Pinteraction (between subgroups) |
|---|---|---|---|---|
| Total Cancer Incidence | ||||
| By Regimen Intake | ||||
| Daily intake | 8 | 0.98 (0.93–1.03) | 0 | 0.38 |
| Nondaily intake in a large bolus | 4 | 1.05 (0.90–1.23) | 0 | |
| By Attained 25(OH)D | ||||
| >100 nmol/L | 6 | 0.97 (0.90–1.04) | 0 | 0.64 |
| ≤100 nmol/L | 6 | 0.99 (0.93–1.06) | 0 | |
| By baseline 25(OH)D | ||||
| >50 nmol/L | 9 | 0.96 (0.89–1.03) | 0 | 0.55 |
| ≤50 nmol/L | 2 | 1.00 (0.93–1.08) | 20 | |
| Total Cancer Mortality | ||||
| By Regimen Intake | ||||
| Daily intake | 4 | 0.87 (0.79–0.97) | 0 | 0.05 |
| Nondaily intake in a large bolus | 3 | 1.06 (0.90–1.25) | 8 | |
| By Attained 25(OH)D | ||||
| >100 nmol/L | 4 | 0.99 (0.80–1.23) | 46 | 0.25 |
| ≤100 nmol/L | 3 | 0.88 (0.79–0.98) | 0 | |
| By baseline 25(OH)D | ||||
| >50 nmol/L | 5 | 0.97 (0.82–1.15) | 34 | 0.16 |
| ≤50 nmol/L | 2 | 0.88 (0.78–0.99) | 0 | |
| By study population | ||||
| Postmenopausal women | 4 | 0.89 (0.73–1.08) | 26 | 0.47 |
| General population | 5 | 1.01 (0.94–1.08) | 0 | |
| Diseased population | 3 | 0.97 (0.70, 1.34) | 29 | |
3.3.1.2. Vitamin D supplementation and total cancer mortality
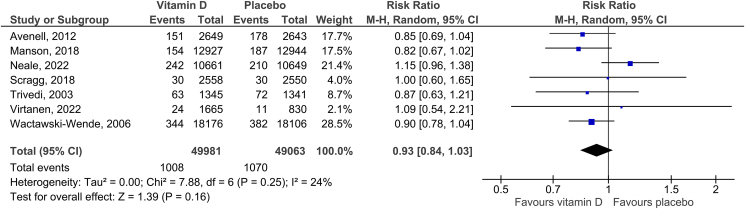
We included seven RCTs in the meta-analysis of total cancer mortality. The summary RR for intervention versus control group was 0.93 (95% CI: 0.84–1.03, P = 0.16). There was slight heterogeneity reported (I2 = 24%; Figure 3). No funnel plot asymmetry was detected with Egger’s test (P = 0.813; Supplementary Figure 3). The certainty of the evidence was rated as high (Supplementary Table 3). The sensitivity analysis reported an RR of 0.94 (95% CI: 0. 82–1.08, P = 0.38; I2 = 34%) after excluding one trial that reported a combined effect of vitamin D and calcium against a placebo [29]. There was no statistically significant difference between the subgroups regarding attained 25(OH)D level (Pinteraction = 0.25). Vitamin D decreased the risk of cancer mortality in participants with low baseline 25(OH)D levels (≤50 nmol/L) but not in those with baseline 25(OH)D levels >50 nmol/L; however, the test for subgroup differences was not significant (Pinteraction = 0.16; Table 2). When stratified according to the regimen of vitamin D intake, there was a borderline statistically significant difference between the two subgroups (Pinteraction = 0.05); daily intake of vitamin D was found to reduce cancer mortality (RR = 0.87; 95% CI: 0.79–0.96, P = 0.008, I2 = 0%) but no statistically significant association was observed between infrequent intake of regimen in the form of bolus and cancer mortality (Table 2). The subgroup by type of study population was not conducted due to a lack of data.
Figure 3.
Effect of vitamin D supplementation on total cancer mortality.
3.3.2. Secondary outcome
3.3.2.1. Vitamin D supplementation and total mortality
The summary RR for intervention versus control group was 0.92 (95% CI: 0.82–1.04, P = 0.19). There was moderate heterogeneity reported (I2 = 36%; Supplementary Figure 4). No funnel plot asymmetry was detected with Egger’s test (P = 0.925; Supplementary Figure 5). The overall certainty of the evidence was rated as high (Supplementary Table 3).
4. Discussion
To the best of our knowledge, this is the most comprehensive meta-analysis to date evaluating the effect of vitamin D supplementation on cancer incidence and mortality. We found no significant association between vitamin D supplementation and total cancer incidence, cancer mortality and all-cause mortality. The overall certainty of the evidence was high for all outcomes suggesting that further research is very unlikely to change our confidence in the estimate of effect in largely vitamin D-replete older adult populations. The results also remained consistent across the subgroup analyses. Daily vitamin D intake was found to be associated with reduced cancer mortality compared to bolus intake; however, this observation could be due to chance as the P-value for subgroup differences was borderline nonsignificant (P = 0.05).
Regarding total cancer incidence, our results are coherent with those reported by recent meta-analyses, which also showed no benefit of vitamin D supplementation in preventing cancer incidence [10, 31]. The evidence from epidemiologic studies regarding the preventive effects of vitamin D based on circulating 25(OH)D levels is limited to the incidence of colorectal and ovarian cancer [32, 33, 34] Anti-neoplastic effects of vitamin D, including anti-proliferation, pro-differentiation, pro-apoptosis, anti-angiogenesis, immune modulation, and miR regulation, provide a possible explanation for these findings [1]. Wei et al. reported an inverse association between circulating levels of circulating 25(OH)D and total vitamin D intake with the incidence of colorectal adenoma and recurrent adenomas. However, the analysis did not find any association with supplemental vitamin D indicating that the supplemental sources of vitamin D may not be effective [35]. Meta-analyses of RCTs have also returned no benefit of vitamin D supplementation on colorectal cancer incidence [31, 36]. Results from the D-Health Trial on total and colorectal cancer incidence are forthcoming and may help clarify this further [12].
More importantly, our findings disagree with those reported by recent meta-analyses, which suggested that vitamin D supplementation reduced cancer mortality [10, 31]. However, it is worth pointing out that their pooled estimates were significantly affected by the results of the VITAL trial [11]. Findings from the trials published since then, especially the D-Health Trial, negate these results and our updated meta-analysis also found no beneficial effect of vitamin D on mortality. Indeed, the D-Health Trial found an increased risk of cancer mortality with vitamin D supplementation after excluding the follow-up of the first two years, which was in direct contrast to the VITAL trial [11, 12]. It could be speculated that this disparity might be due to the intermittent dosing regimen used in the D-Health Trial (60 000 IU per month), which might not provide the same benefits as daily dosing regimens [37]. Our meta-analysis also suggested benefits only in trials with daily dosing; however, this needs to be substantiated by trials directly comparing the different dosing regimens.
We also found no reduction in all-cause mortality with vitamin D supplementation, which is congruent with a recent comprehensive meta-analysis [38] but contrary to the findings of Keum et al., which showed a 7% reduction in mortality [10]. Our updated meta-analysis and evidence from large community-based trials indicate that routine vitamin D supplementation is unlikely to confer any mortality benefits in largely vitamin D-replete populations and hence, should be avoided.
The strengths of our study include it being the first meta-analysis to use the GRADE certainty of evidence considerations to inform its conclusions as well as being the largest meta-analysis to date, therefore, having a significantly increased statistical power compared to prior meta-analyses. We also assessed the effects of treatment on cancer mortality and incidence over a period of 3–10 years and excluded studies with few numbers of outcomes to increase the reliability of our results. Moreover, due to the variation in the designs of the studies, we were able to assess several pre-specified subgroups of interest. We did not investigate a large number of subgroups as the rate of type I error increases rapidly with the number of subgroup comparisons [13]. We also used formal statistical tests to compare the subgroups and to inform our conclusions instead of using the different levels of statistical significance within subgroups, which are potentially highly misleading [13], as prior meta-analyses have attempted to do [10, 31]. Our results have little heterogeneity and no evidence of publication bias allowing us to place a high degree of confidence in them.
We would like to acknowledge a few limitations, however. First, there were a limited number of RCTs available and most of the included trials did not have cancer incidence and mortality as primary endpoints. Second, the majority of study participants consisted of Caucasians, and racial differences might modify the intervention effects [39]. Third, we could not assess the intervention effect on organ-specific malignancies as the majority of the trials did not provide site-specific data, and performing subgroup analyses in the absence of a substantial amount of data is not recommended [13]. Moreover, our study was restricted by variable reporting of several important demographic variables such as age and sex by RCTs, precluding any attempt to run a subgroup analysis on them. Lastly, there exists significant heterogeneity in the included RCTs in terms of interventions and inclusion and exclusion criteria of patients. However, we used multiple subgroup and sensitivity analyses to address this heterogeneity.
The null findings of our meta-analysis preclude any recommendations regarding the use of vitamin D supplements as a chemo-preventive measure. Importantly, however, most of the trials had few participants with a 25(OH)D concentration below 50 nmol/L, which is the population that is most likely to benefit. Our subgroup analysis suggested a reduction in cancer mortality in trials with baseline 25(OH)D less than 50 nmol/L; however, the scarcity of studies and a nonsignificant Pinteraction preclude any firm conclusions. Moreover, longer follow-ups may be required for cancers that progress over decades to demonstrate any benefit. Future large-scale RCTs should be conducted in populations with a higher prevalence of vitamin D deficiency to elicit any potential anticancer benefits of vitamin D supplementation.
In conclusion, vitamin D supplementation is ineffective in reducing total cancer incidence and mortality in largely vitamin D-replete older adult populations. Future research should be based on populations with a higher prevalence of vitamin D deficiency and should involve more extended follow-up periods.
Declarations
Author contribution statement
Huzaifa Ahmad Cheema: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Maurish Fatima; Anas Elgenidy: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Abia Shahid: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Oumnia Bouaddi; Mohammad Mehedi Hasan: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Aqeeb Ur Rehman; Salah Eddine Oussama Kacimi: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ka Yiu Lee, PhD: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
N/A.
Contributor Information
Huzaifa Ahmad Cheema, Email: huzaifacheema@kemu.edu.pk.
Ka Yiu Lee, Email: kyle.lee@miun.se.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Feldman D., Krishnan A.V., Swami S., Giovannucci E., Feldman B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer. 2014;14:342–357. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 2.Gaksch M., Jorde R., Grimnes G., Joakimsen R., Schirmer H., Wilsgaard T., Mathiesen E.B., Njølstad I., Løchen M.-L., März W., et al. Vitamin D and mortality: individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium. PLoS One. 2017;12 doi: 10.1371/journal.pone.0170791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aspelund T., Grübler M.R., Smith A.V., Gudmundsson E.F., Keppel M., Cotch M.F., Harris T.B., Jorde R., Grimnes G., Joakimsen R., et al. Effect of genetically low 25-hydroxyvitamin D on mortality risk: Mendelian randomization analysis in 3 large European cohorts. Nutrients. 2019;11:74. doi: 10.3390/nu11010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han J., Guo X., Yu X., Liu S., Cui X., Zhang B., Liang H. 25-Hydroxyvitamin D and total cancer incidence and mortality: a meta-analysis of prospective cohort studies. Nutrients. 2019;11:2295. doi: 10.3390/nu11102295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song Z.Y., Yao Q., Zhuo Z., Ma Z., Chen G. Circulating vitamin D level and mortality in prostate cancer patients: a dose–response meta-analysis. Endocr. Connect. 2018;7:R294–R303. doi: 10.1530/EC-18-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maalmi H., Ordóñez-Mena J.M., Schöttker B., Brenner H. Serum 25-hydroxyvitamin D levels and survival in colorectal and breast cancer patients: systematic Review and meta-analysis of prospective cohort studies. Eur. J. Cancer. 2014;50:1510–1521. doi: 10.1016/j.ejca.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Vrieling A., Seibold P., Johnson T.S., Heinz J., Obi N., Kaaks R., Flesch-Janys D., Chang-Claude J. Circulating 25-hydroxyvitamin D and postmenopausal breast cancer survival: influence of tumor characteristics and lifestyle factors? Int. J. Cancer. 2014;134:2972–2983. doi: 10.1002/ijc.28628. [DOI] [PubMed] [Google Scholar]
- 8.Keum N., Giovannucci E. Vitamin D supplements and cancer incidence and mortality: a meta-analysis. Br. J. Cancer. 2014;111:976–980. doi: 10.1038/bjc.2014.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goulão B., Stewart F., Ford J.A., MacLennan G., Avenell A. Cancer and vitamin D supplementation: a systematic Review and meta-analysis. Am. J. Clin. Nutr. 2018;107:652–663. doi: 10.1093/ajcn/nqx047. [DOI] [PubMed] [Google Scholar]
- 10.Keum N., Lee D.H., Greenwood D.C., Manson J.E., Giovannucci E. Vitamin D supplementation and total cancer incidence and mortality: a meta-analysis of randomized controlled trials. Ann. Oncol. 2019;30:733–743. doi: 10.1093/annonc/mdz059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manson J.E., Cook N.R., Lee I.-M., Christen W., Bassuk S.S., Mora S., Gibson H., Gordon D., Copeland T., D’Agostino D., et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N. Engl. J. Med. 2019;380:33–44. doi: 10.1056/NEJMoa1809944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neale R.E., Baxter C., Romero B.D., McLeod D.S.A., English D.R., Armstrong B.K., Ebeling P.R., Hartel G., Kimlin M.G., O’Connell R., et al. The D-health trial: a randomised controlled trial of the effect of vitamin D on mortality. Lancet Diabetes Endocrinol. 2022;10:120–128. doi: 10.1016/S2213-8587(21)00345-4. [DOI] [PubMed] [Google Scholar]
- 13.Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A., editors. Cochrane Handbook for Systematic Reviews of Interventions. second ed. Wiley Blackwell; Hoboken, New Jersey: 2019. [Google Scholar]
- 14.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic Reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.-Y., Corbett M.S., Eldridge S.M., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 16.Viechtbauer W. Conducting meta-analyses in R with the Metafor package. J. Stat. Software. 2010;36:1–48. [Google Scholar]
- 17.Guyatt G.H., Oxman A.D., Vist G.E., Kunz R., Falck-Ytter Y., Alonso-Coello P., Schünemann H.J. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guyatt G.H., Oxman A.D., Vist G.E., Kunz R., Falck-Ytter Y., Schünemann H.J. GRADE: what is “quality of evidence” and why is it important to clinicians? BMJ. 2008;336:995–998. doi: 10.1136/bmj.39490.551019.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guyatt G.H., Oxman A.D., Kunz R., Brozek J., Alonso-Coello P., Rind D., Devereaux P.J., Montori V.M., Freyschuss B., Vist G., et al. GRADE guidelines 6. Rating the quality of evidence—imprecision. J. Clin. Epidemiol. 2011;64:1283–1293. doi: 10.1016/j.jclinepi.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Virtanen J.K., Nurmi T., Aro A., Bertone-Johnson E.R., Hyppönen E., Kröger H., Lamberg-Allardt C., Manson J.E., Mursu J., Mäntyselkä P., et al. Vitamin D supplementation and prevention of cardiovascular disease and cancer in the Finnish vitamin D trial: a randomized controlled trial. Am. J. Clin. Nutr. 2022 doi: 10.1093/ajcn/nqab419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanders K.M., Stuart A.L., Williamson E.J., Simpson J.A., Kotowicz M.A., Young D., Nicholson G.C. Annual high-dose oral vitamin D and falls and fractures in older women. JAMA. 2010;303:1815. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- 22.Jorde R., Sollid S.T., Svartberg J., Schirmer H., Joakimsen R.M., Njølstad I., Fuskevåg O.M., Figenschau Y., Hutchinson M.Y.S. Vitamin D 20 000 IU per week for five years does not prevent progression from prediabetes to diabetes. J. Clin. Endocrinol. Metab. 2016;101:1647–1655. doi: 10.1210/jc.2015-4013. [DOI] [PubMed] [Google Scholar]
- 23.Baron J.A., Barry E.L., Mott L.A., Rees J.R., Sandler R.S., Snover D.C., Bostick R.M., Ivanova A., Cole B.F., Ahnen D.J., et al. A trial of calcium and vitamin D for the prevention of colorectal adenomas. N. Engl. J. Med. 2015;373:1519–1530. doi: 10.1056/NEJMoa1500409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trivedi D.P., Doll R., Khaw K.T. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. Br. Med. J. 2003;326:469–472. doi: 10.1136/bmj.326.7387.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lappe J., Watson P., Travers-Gustafson D., Recker R., Garland C., Gorham E., Baggerly K., McDonnell S.L. Effect of vitamin D and calcium supplementation on cancer incidence in older women: a randomized clinical trial. JAMA, J. Am. Med. Assoc. 2017;317:1234–1243. doi: 10.1001/jama.2017.2115. [DOI] [PubMed] [Google Scholar]
- 26.Avenell A., MacLennan G.S., Jenkinson D.J., McPherson G.C., McDonald A.M., Pant P.R., Grant A.M., Campbell M.K., Anderson F.H., Cooper C., et al. Long-term follow-up for mortality and cancer in a randomized placebo-controlled trial of vitamin D3 and/or calcium (RECORD trial) J. Clin. Endocrinol. Metab. 2012;97:614–622. doi: 10.1210/jc.2011-1309. [DOI] [PubMed] [Google Scholar]
- 27.Scragg R., Khaw K.-T., Toop L., Sluyter J., Lawes C.M.M., Waayer D., Giovannucci E., Camargo C.A. Monthly high-dose vitamin D supplementation and cancer risk. JAMA Oncol. 2018;4 doi: 10.1001/jamaoncol.2018.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lappe J.M., Travers-Gustafson D., Davies K.M., Recker R.R., Heaney R.P. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am. J. Clin. Nutr. 2007;85:1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 29.Wactawski-Wende J., Kotchen J.M., Anderson G.L., Assaf A.R., Brunner R.L., O’Sullivan M.J., Margolis K.L., Ockene J.K., Phillips L., Pottern L., et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N. Engl. J. Med. 2006;354:684–696. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 30.Chatterjee R., Fuss P., Vickery E.M., Leblanc E.S., Sheehan P.R., Lewis M.R., Dolor R.J., Johnson K.C., Kashyap S.R., Nelson J., et al. Vitamin D supplementation for prevention of cancer: the D2d cancer outcomes (D2dCA) ancillary study. J. Clin. Endocrinol. Metab. 2021;106:2767–2778. doi: 10.1210/clinem/dgab153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo Z., Huang M., Fan D., Hong Y., Zhao M., Ding R., Cheng Y., Duan S. Association between vitamin D supplementation and cancer incidence and mortality: a trial sequential meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2022;1–15 doi: 10.1080/10408398.2022.2056574. [DOI] [PubMed] [Google Scholar]
- 32.Mantell D.J., Owens P.E., Bundred N.J., Mawer E.B., Canfield A.E. 1 alpha, 25-dihydroxyvitamin D(3) inhibits angiogenesis in vitro and in vivo. Circ. Res. 2000;87:214–220. doi: 10.1161/01.res.87.3.214. [DOI] [PubMed] [Google Scholar]
- 33.McCullough M.L., Zoltick E.S., Weinstein S.J., Fedirko V., Wang M., Cook N.R., Eliassen A.H., Zeleniuch-Jacquotte A., Agnoli C., Albanes D., et al. Circulating vitamin D and colorectal cancer risk: an international pooling project of 17 cohorts. J. Natl. Cancer Inst. 2019;111:158–169. doi: 10.1093/jnci/djy087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ong J.-S., Cuellar-Partida G., Lu Y., Fasching P.A., Hein A., Burghaus S., Beckmann M.W., Lambrechts D., Van Nieuwenhuysen E., Vergote I., et al. Association of vitamin D levels and risk of ovarian cancer: a Mendelian randomization study. Int. J. Epidemiol. 2016;45:1619–1630. doi: 10.1093/ije/dyw207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei M.Y., Garland C.F., Gorham E.D., Mohr S.B., Giovannucci E. Vitamin D and prevention of colorectal adenoma: a meta-analysis. Cancer Epidemiol. Biomarkers Prev. 2008;17:2958–2969. doi: 10.1158/1055-9965.EPI-08-0402. [DOI] [PubMed] [Google Scholar]
- 36.Bjelakovic G., Gluud L.L., Nikolova D., Whitfield K., Krstic G., Wetterslev J., Gluud C. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst. Rev. 2014;2014 doi: 10.1002/14651858.CD007469.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Griffin G., Hewison M., Hopkin J., Kenny R.A., Quinton R., Rhodes J., Subramanian S., Thickett D. Perspective: vitamin D supplementation prevents rickets and acute respiratory infections when given as daily maintenance but not as intermittent bolus: implications for COVID-19. Clin. Med. (Northfield. Il). 2021;21:e144–e149. doi: 10.7861/clinmed.2021-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y., Fang F., Tang J., Jia L., Feng Y., Xu P., Faramand A. Association between vitamin D supplementation and mortality: systematic Review and meta-analysis. BMJ. 2019;366 doi: 10.1136/bmj.l4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giovannucci E., Liu Y., Willett W.C. Cancer incidence and mortality and vitamin D in black and white male health professionals. Cancer Epidemiol. Biomarkers Prev. 2006;15:2467–2472. doi: 10.1158/1055-9965.EPI-06-0357. [DOI] [PubMed] [Google Scholar]
- 40.LaCroix A.Z., Kotchen J., Anderson G., Brzyski R., Cauley J.A., Cummings S.R., Gass M., Johnson K.C., Ko M., Larson J., et al. Calcium plus vitamin D supplementation and mortality in postmenopausal women: the women’s health initiative calcium-vitamin D randomized controlled trial. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2009;64A:559–567. doi: 10.1093/gerona/glp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.