Abstract
The liver is the largest internal organ in the human body with largest mass of glandular tissue. Modeling the liver has been challenging due to its variety of major functions, including processing nutrients and vitamins, detoxification, and regulating body metabolism. The intrinsic shortfalls of conventional two-dimensional (2D) cell culture methods for studying pharmacokinetics in parenchymal cells (hepatocytes) have contributed to suboptimal outcomes in clinical trials and drug development. This prompts the development of highly automated, biomimetic liver-on-a-chip (LOC) devices to simulate native liver structure and function, with the aid of recent progress in microfluidics. LOC offers a cost-effective and accurate model for pharmacokinetics, pharmacodynamics, and toxicity studies. This review provides a critical update on recent developments in designing LOCs and fabrication strategies. We highlight biomimetic design approaches for LOCs, including mimicking liver structure and function, and their diverse applications in areas such as drug screening, toxicity assessment, and real-time biosensing. We capture the newest ideas in the field to advance the field of LOCs and address current challenges.
I. INTRODUCTION
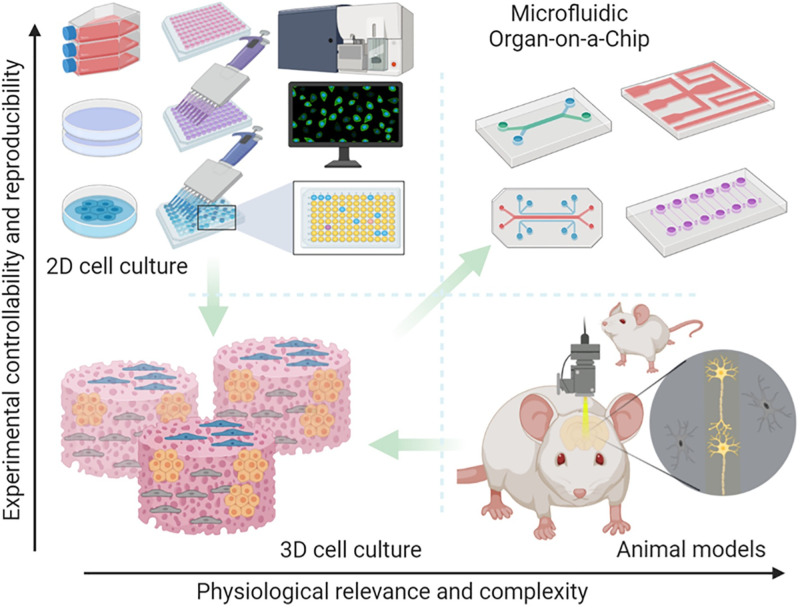
The ability to simulate human physiology outside of the body is invaluable in medical research, enabling the study of disease mechanisms and drug development. The traditional practice is to use a conventional two-dimensional (2D) cell culturing system (i.e., cells in a Petri dish). 2D culture only permits cells to grow in a flat monolayer1 but has, nevertheless, become an essential platform for studying cellular mechanisms and interactions due to its simplicity and ease of operation.2 Despite their higher controllability and reproducibility, 2D cultures have much lower physiological relevance and complexity compared to three-dimensional (3D) cultures, as shown in Fig. 1.3–5
FIG. 1.
Preclinical studies rely on major tools, including 2D or 3D in vitro cell cultures, and in vivo animal models, for drug development. 2D culture offers a rapid and reproducible way to analyze drug response; however, they lack the 3D physiological tissue environment. Conventional 3D culture can provide a 3D environment but still falls short of controllably recapitulating the in vivo physiology and pathology of the human body. Animal models enable in vivo analysis, yet the species differences between animal and human physiological mechanisms and the complexity of in vivo physiology weakens the accuracy and reproducibility of experimental results. A microfluidic organ-on-a-chip platform that enables controllable cell culture within an organotypic microarchitectural environment provides a simple yet more physiologically relevant platform to controllably and systematically interrogate human biology. Figure generated in BioRender (BioRender.com). Reproduced with permission from Ma et al., Trends Pharmacol. Sci. 42(2), 119–133 (2021). Copyright 2021 Elsevier Ltd.
Conventional cell culturing methods and organ-on-a-chip (OOC) are both considered in vitro models.1–6 A comprehensive analysis between 2D and 3D cultures is presented in Table I. 2D cultured cells experience nutrient depletion over time since there is no fluid flow.3 Replenishment of cell culture medium is required for the continuous growth of cells. In addition, 2D cell culture often only permits the study of a single cell type. Compared to the native in vivo environment where cells are normally located, 2D cell cultures on a flat surface pose a clear disadvantage. In comparison, 3D culturing and OOC can encourage cell–cell communications and produce physiologically relevant data.3–8 OOC is a microfluidic-based platform that bridges between 2D cell culture and animal models, mimicking the critical aspects of human physiology. Since the development of the first mechanically actuatable lung-on-a-chip device in 2010,9 there has been a plethora of work on OOC for different organs, such as the intestine,10,11 brain,12,13 blood–brain-barrier,14,15 and multi-organ system.16,17
TABLE I.
Comparison between conventional two-dimensional cell culturing technique and three-dimensional cell culturing technique.1–76
| Parameters | Two-dimensional | Three-dimensional | |
|---|---|---|---|
| Monolayer Petri dish culture | Liver organoids | Liver-on-a-chip | |
| General characteristics | Cells grow on rigid tissue culture plastic in monolayer fashion | Usually cultured with hydrogel or as suspension culture where the cell mass is clustered | Gas permeable polymeric membrane usually used as culturing chamber material where cells are encouraged to reconstruct extracellular matrix (ECM); multicellular interactions can be easily encouraged |
| Cell morphologies | Unnatural cell spreading, limited ECM secretion and limited cell–cell interaction | Possess similar hepatic physiological architecture and excellent cell–cell and ECM interactions | Can achieve close resemblance of physiological and pathological hepatic architecture, high level of ECM production and cell–cell/cell–surface interactions |
| Flow characteristics | Cannot induce flow parameters; regular replenishing of culture media is required | Can induce flow depending on the design of the bioreactor | Controllable flow parameters, such as shear stress and recirculation of metabolites |
| Signaling molecules and mass transfer | Short range | Due to formation of spheroids, inner cell mass experience limited mass transfer which could result in cell apoptosis | Accurate control of signaling molecules and nutrients spatially and over time. Incorporations of non-parenchymal cells can more accurately mimic physiological conditions and produce clinically relevant data |
| High throughput screening (HTS) | A widespread model for HTS | Depending on the platforms used, the throughput levels may vary | Depending on the designed platform, it could be achieved but is limited by the technical challenges |
| Experimental data | Ease of operation but single time-point data | Difficult to obtain homogenous data | Able to perform real-time monitoring of metabolites over the entire course of the experiment |
In this review, we will focus on liver-on-a-chip (LOC), which is a sub-group of OOC. LOC has attracted increasing attention over the years, with recent developments aimed at recapitulating the in vivo tissue structure, functions, biochemical cues, and microenvironment of the liver, which conventional 2D culturing has failed to achieve.6–18 lOC allows the study of drug metabolism in models relevant to human physiology and offers an alternative approach to animal models.19,20 Although animal studies are still required during drug development to assess drug efficacy and toxicity before human trials, increasing evidence has suggested that animal models may not sufficiently reflect human physiological conditions, and numerous failed trials are becoming an increasing source of concern.21–23 lOC can be a solution that minimizes ethical hurdles while producing accurate and high throughput scientific data, especially in the context of drug toxicity predictions.23–27 Besides drug development, nanomedicine toxicity assessment can also be a rising application of LOC.28–32 With mRNA vaccines and lipid-based nanotechnology for vaccine manufacture, nanomedicine has been brought to the public on an unprecedented scale.33 However, the lack of policies and protocols for nanotoxicity evaluation has raised concerns.32 With a handful of Food and Drug Administration (FDA) approved nanomedicine solutions available on the market, it is time to rethink methods that could safely bring nanomedicine into critical care.34 lOC could find application in the assessment of nanomaterials and other drugs in the context of treatment-induced liver injuries.35,36
We will critically discuss these applications and provide fresh insights into the future of LOCs. We will highlight the recent developments in designing LOCs and fabrication strategies, with a focus on the various approaches to achieve a biomimetic design of LOCs. Furthermore, our review critically highlights the gap in evaluating nanomedicine toxicity, and how LOCs can be used to address these gaps. Finally, we conclude with a forward look into the challenges and novel aspects of the advancement of LOCs. We hope to provide fresh perspectives and new application ideas for the next generation LOCs, particularly modeling-based work, such as pharmacometrics, fluid dynamics, and machine learning-aided designs, which supplements a number of recent reviews on different aspects of LOC technology.26–39
II. DEVELOPMENT OF LIVER-ON-A-CHIP (LOC) SYSTEMS
Cell–cell and cell–extracellular interactions are critical factors in influencing cellular behavior.40,41 2D cell cultures do not allow many of these interactions and are often ineffective in predicting physiologically relevant drug efficacy and toxicity profile. This eventually may lead to failure in drug validation and approval processes for clinical application.42 Thus, it is essential to recognize that although 2D cell culturing could offer greater flexibility and has been a pivotal part of modern scientific advances, state-of-the-art technologies such as 3D cultures, and OOC platforms are more physiologically relevant evaluation strategies.3 To overcome the drawbacks of conventional culturing methods, efforts have been made to engineer OOCs that consider the spatial organization of cells.40–45
A. In vitro cell study methods
Cells in their native environment are intrinsically surrounded by other cells in an interconnected 3D matrix. 2D cell culture fails to address the complex spatial, biochemical, and mechanical requirements of in vivo architecture and microenvironments.46 There is growing evidence that 3D-cultured cells can better recapitulate or even completely resemble in vivo cellular responses. One of the advantages of 3D culture is the ability to recapitulate the native tissue environment.2–43 Such systems permit the study of cell responses to mechanical cues, cell–cell interactions, and extracellular matrix (ECM) communication. Unlike 2D monolayer adherent cell culture, 3D cell culture takes into account the spatial organization of cellular structures.43 This is an extra dimension compared to 2D culture that significantly impacts molecular signal transduction, allowing physiologically relevant gene expression, morphological changes, and even directing stem cell lineage in vitro.
Over the last decades, different in vitro models have been developed to simulate liver physiology. One of these techniques is to produce 3D spheroids. Spheroids are “multicellular spherical structures composed of aggregated cells that do not adhere to a substrate but adhere to each other.”47 Primary human hepatocytes (PHH) can give invaluable information for studies on cell metabolism, inflammation, preclinical drug screening, toxicology screenings, and the development of bioartificial liver devices.48 Despite the view of PHH as a gold-standard cellular model, its drawbacks include a short lifetime during in vitro culture, rapid loss of liver-specific function and morphology, the tendency to undergo fibrosis, and weak proliferation capabilities.49,50 It has been shown that cultivation of PHH in 3D spheroids can more closely recapitulate human liver function. The main advantage of such methods is the ability to retain cell–cell contacts, cell viability, and mature hepatic phenotypes.51
In recent studies, the emergence of spheroids and commercially available spheroid culture plates has led to their use in OOC. The combination of OOC and liver spheroids have shown great promise in recapitulating liver physiology,52–54 liver pathology,47–55 liver xenobiotic/drug metabolism,6–56 and liver regeneration mechanism.57 To properly define LOC, there are three characteristic requirements: (1) 3D cell culturing environment;58 (2) the integration of multiple cell types;27–59 and (3) the presence of biochemical and biomechanical forces that are native to the designed organ or tissue.41–60 OOC systems allows precise, systematic control (intensity, duration, and pattern) and cyclic strains on a cell culture substrate to mimic the mechanical forces a cell may experience in its native environment.61 These platforms can be beneficial for studying mechanotransduction, where modular control of different types of mechanical forces is critical to understanding cellular behavior.62–64 To create a native niche for liver cells, there are several non-parenchymal cells (non-hepatocytes) that perform critical functions, which should be included with in vitro cultures of hepatocytes. Many multi-cellular or co-culturing systems have been developed to recreate such organic interactions between different cell types to promote cross-talk of cytokines and signaling molecules.
B. A brief history of LOCs
The first LOC was reported in 2007 by Philip Lee et al., 3 years before the first lung-on-a-chip.52 Much attention was given to Huh et al. due to the novel introduction of tunable mechanical forces in the lung-on-a-chip design, mimicking human breathing patterns.9 The introduction of mechanical forces was not incorporated in early LOC devices due to the static nature of the liver. Following this, further understanding of mechanotransduction in cell development and function has broadened the definition of mechanical forces from macroscopic stretching to microscopic shearing forces.62 Recent LOCs have paid greater attention to such aspects, making them an intricate and desired platform for pathophysiological, mechanistic, and drug toxicity studies. Typical LOC devices contain the parenchymal cells (hepatocytes) as the main or sole cell type. It is now generally recognized by the scientific community that monocellular culturing of hepatocytes does not accurately reflect physiological conditions2–4 or produce accurate results compared to in vivo models.3 In recent advancements of LOCs, many have incorporated multicellular culturing or co-culturing to better recapitulate the physiological state of the human liver.25–67
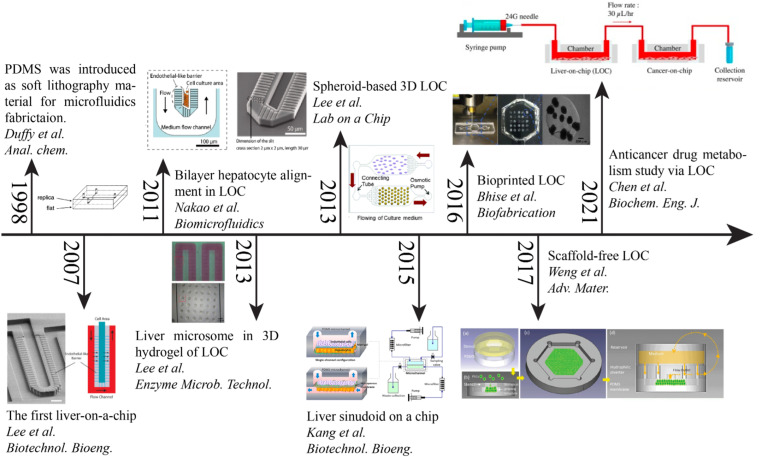
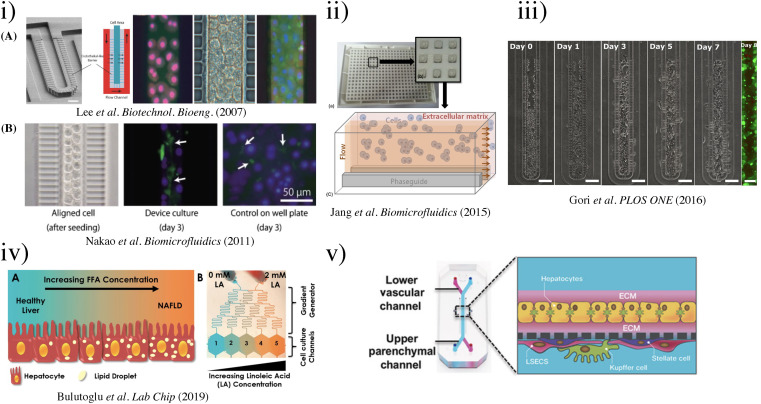
As shown in Fig. 2, LOC designs have advanced from the simplistic monocellular single-channel device model to a multiple-chip multi-cellular system for studying anticancer drug metabolism effects. In 1998, the introduction of poly(dimethylsiloxane) (PDMS) as an elastomer for rapid prototyping accelerated the field of LOCs and OOCs in general.68 In 2007, Lee et al. published their design of LOC to encourage biliary formation using a perfusion-based system.52 Then, Nakao et al. took Lee's model further to precisely control the number of cells allowed in the channel by modifying the design into asymmetric patterns.69 Nakao et al. restricted two-line cell seeding in the channel and promoted biliary formation. Since numerous studies have suggested that 3D scaffolding affects cellular responses, a 3D hydrogel was designed to study the interactions between liver microsomes and hepatocytes by Lee et al.70 They utilized polyethyleneglycol (PEG) pillars within a weaving channel and rat liver microsomes enclosed in a 3D hydrogel matrix. Seven different substrates were tested for the P450 reaction in a microsome solution, creating a metabolic liver model. In the same year, another group showed that a continuous supply of oxygen and nutrients, and removal of wastes using an osmotic pump could assist the long-term maintenance of hepatocyte spheroids.71 Kang et al. in 2015 co-cultured hepatocytes with endothelial cells, and demonstrated long-term maintenance of normal cellular morphology and urea production for up to 30 days.72 A direct-write bioprinter was used to create the bioreactor by Bhise et al. in 2016.73 The tissue-like construct was assessed over 4 weeks in conjunction with the cellular response of acute acetaminophen (APAP) exposure for predicting drug toxicity. Rather than making direct interaction with the substrate, a scaffold-free technique that encouraged the formation of organoids was demonstrated by Weng et al. in 2017.74 This design took inspiration from liver anatomy where the portal inlets flow radially into the hepatic central outlet, mimicking the structure of liver lobules. It was found that this biomimicry design approach achieved the reconstruction of hepatic cord-like architecture and the formation of fenestrated window-like nanostructures after 7 days of incubation. Recent efforts have been dedicated to drug metabolism screening with multi-chip systems, such as the work by Chen et al. in 2021.75 The LOC was combined with a cancer-on-a-chip (PC3, HepG2, A549, and MCF-7) system to study the liver metabolite effects on cancer cells. Chen et al. observed an improvement in hepatocyte synthesis and metabolism, which improved the effects of cancer drugs in this multi-chip system. The field of LOCs is moving into a multi-system, multi-cellular, and integrated sensing era, evidenced by the plethora of works in multi-sensing platforms of LOCs, which are discussed in Sec. V E.
FIG. 2.
Timeline of the development of microfluidics-based liver-on-a-chip (LOC) technology. Evolution of the field from the early concept of endothelial barrier-like micropillar by Lee et al. in 2007 to the more complex multi-purpose LOC for drug efficacy study reported by Chen et al. in 2021. Reproduced with permission from [Copyright permissions: 1998—Reproduced with permission from Duff et al., Anal. Chem. 70(23), 4974–4984 (1998). Copyright 1998, American Chemical Society. 2007—Reproduced with permission from Lee et al., Biotechnol. Bioeng. 97(5), 1340–1346 (2007). Copyright 2007 Wiley Periodicals, Inc. 2011—Reproduced with permission from Nakao et al., Biomicrofluidics 5(2), 022212 (2011). Copyright 2011 AIP Publishing LLC. 2013—Reproduced with permission from Lee et al., Enzyme Microb. Technol. 53(3), 159–164 (2013). Copyright 2013 Elsevier Inc. 2013—Reproduced with permission from Lee et al., Lab Chip 13(18), 3529–3537 (2013).Copyright 2013 the Royal Society of Chemistry. 2015—Reproduced with permission from Kang et al., Biotechnol. Bioeng. 112(12), 2571–2582 (2015). Copyright 2015 Wiley Periodicals, Inc. 2016—Reproduced with permission from Bhise et al., Biofabrication 8(1), 014101 (2016). Copyright 2016 IOP Publishing Ltd. 2017—Reproduced with permission from Weng et al., Adv. Mater. 29(36), 1701545 (2017). Copyright 2017 John Wiley & Sons. 2021—Reproduced with permission from Chen et al., Biochem. Eng. J. 165, 107831 (2021). Copyright 2021 Elsevier B.V.].
III. LOC DESIGNS THAT MIMIC LIVER CHARACTERISTICS
As the largest gland in the human body, the liver weighs about 1.4 kg (2.5% body weight) in the average adult,77,78 making it impractical to mimic the liver mass in a LOC device. Nevertheless, blood flow characteristics provide a practical approach for in vitro hepatic mimicry. Dimensionless parameters can be used to describe the fluid flow and mass transport characteristics, such as Reynold's number (Re, inertial/viscosity),79 Péclet number (Pe, convective/diffusive transport),80 and Damköhler number (Da, diffusion/reaction timescale).81 LOCs provide the ability to control and manipulate these dimensionless numbers to mimic human physiological conditions. Unfortunately, only a handful of studies have specifically reported these parameters, although they are considered essential for ensuring the reproducibility of LOC designs. Other features of LOCs should also be reported, such as shear stress (induced by flow over tissue surface), effective culture time, characteristics of culture media (particularly for co-culture systems), and surface coating of the culture substrate. We have provided an overview of current studies on LOCs which have reported some of these essential parameters (Table II) and hope that this can serve as a guide for future research to consider the inclusion of such data.
TABLE II.
Summary of the current literature on liver-on-a-chip (LOC): (1) type of device: solid organ chip—does not contain a membrane structure to mimic endothelial interactions, or scaffold-free, or barrier tissue—contains a membrane structure; (2) chip material; (3) substrate surface coating, (4) co-culturing (YES/NO); (5) cell lines used in the study; (6) length of the study; (7) operation flow rate and/or shear stress—also contains the values of the dimensionless parameters where mentioned; (8) application.
| Reference | Type of LOC | Chip material | Substrate surface coating | Co-culturing (YES/NO) | Cell lines | Length of the study | Operation flow rate or/and shear stress | Application |
|---|---|---|---|---|---|---|---|---|
| Bhise et al.73 | Solid organ chip | Multilayers of PDMS and PMMA glass——bottom | 3-(trimethoxysilyl)propyl methacrylate | NO | HepG2 and C3A spheroids | Up to 30 days | 200 μl/h | 15 mM acute acetaminophen (APAP) exposure |
| Bonanini et al.93 | Solid organ chip——OrganoPlate® Graft | Top plate: virgin polystyrene. Bottom plate: optical quality 150 μm glass (1H coverslip thickness). Microfluidics: glass, proprietary polymers | Collagen I Matrigel GFR | YES | Cryopreserved Upcyte® human hepatocytes human primary RFP-labeled HUVECs (Alphabioregen, #RFP4) | 7 days | On a rocker at + 14° and −14° inclination every 8 min | Vascularisation and experimentation of tissues in vitro |
| Boos et al.94 | Scaffold free—hanging drop | PDMS | 0.1% gelatin solution Biolipidure | YES | Primary human liver microtissues mouse embryonic-stem-cell line ES-D3 | Up to 10 days | Spheroids generation on a rocker at ± 2° standing stop on a rocker at ±4° | Embryotoxic prodrug cyclophosphamide study |
| Bulutoglu et al.95 | Solid organ chip | PDMS glass slide | 50 μg/ml fibronectin | NO | Primary rat hepatocytes | Not mentioned | Not mentioned | Hypothesis testing about NAFLD progression |
| Chen et al.92 | Solid organ chip | PDMS with glass slide | Not mentioned | YES | Hepa1–6 tumor spheroids JS-1 stellate cells | 3 days | 1 μl/min | Cancer cell stellate interactions under drug stimulation in a microchannel plate |
| Chen et al.75 | Solid organ chip | PDMS | Collagen | YES | Human prostatic cancer cell (PC3) Mouse NIH/3T3 fibroblasts Human lung cancer cells (A549) Human breast cancer cells (MCF-7) Human liver cancer cells (HepG2) Primary rat hepatocytes | Not mentioned | 30 μl/h flow rate 4.7 × 10−4 dyne/cm2 shear stress | Testing for statin using prodrug simvastatin and active drug atorvastatin testing |
| Chhabra et al.57 | Solid organ chip | Not mentioned | ECM | YES | Human primary hepatocytes | Not mentioned | On a rocker at ±25° at a frequency of 1 Hz 6.21 dyn/cm2 shear stress | Not mentioned |
| Choi et al.7 | Solid organ chip | PDMS | Pluronic F127 | No | Primary rat hepatocytes | Up to 4 weeks | Static | Hepatic functions improvement |
| Corrado et al.96 | Solid organ chip | Glass and polydimethylsiloxane | Not mentioned | NO | HepG2 μTPs HepG2 spheroids | 14 days | Not mentioned | μTPs may be used to study the cytotoxic effects of xenobiotics |
| Delalat et al.97 | Solid organ chip | Silicon and silicon dioxide | Potassium hydroxide | NO | Rat primary hepatocytes | 4 weeks | 90 μl/h | Microtrenches allow for maintenance of hepatocytes |
| Frey et al.98 | Scaffold-free—hanging drop | PDMS | trichloro(1H,1H,2H,2H-per-fluorooctyl)silane | YES | Human colorectal carcinoma cells (HCT-116) Primary cell isolates from rat liver | Not mentioned | Maximal shear stress of 1.2 mPa Flow rate 10 μl/min Drop height 0.5 mm | Not mentioned |
| Gori et al.99 | Solid organ chip | PDMS | Not mentioned | NO | HepG2 | 8 days | 18 μl/day Negligible shear stress | Development of on-chip non-alcoholic fatty liver disease (NAFLD) models |
| Jang et al.100 | Solid organ chip | OrganoPlate from MIMETAS and Leiden University | Matrigel | NO | HepG2 | 3 weeks | 0.3 dyn/cm2 shear stress | Cultivation of HepG2 cells |
| Jang et al.101 | Solid organ chip | Not mentioned | ECM | NO | Primary rat, human, or dog hepatocytes Sinusoidal rat, human, or dog liver endothelial cells with or without Kupffer and/or stellate cells | 14 days | 10 μl/h | Not mentioned |
| Kang et al.72 | Solid organ chip | PDMS | Collagen I 30% (v/v) Matrigel for RAMECs | YES | Rat primary hepatocytes and Endothelial cells (primary rat adrenal medullary and bovine aortic) | Up to 30 days | 30–40 μl/h | Urea synthesis using diacetylmonoxime Viral replication for hepatotropic hepatitis B virus (HBV) and analysis |
| Khetani et al.102 | Scaffold free—micropatterned | Tissue culture-treated polystyrene omnitrays (Nunc) | Collagen I | YES | 3T3-J2 fibroblasts : Primary rat hepatocytes = 4:1 | Up to 6 weeks | Static | Hepatic functions improvement |
| Kim et al.103 | Scaffold-free—culture plate | PMMA | Pluronic127 | YES | HepG2 HS68 fibroblasts Primary HUVEC | Up to 7 days | Mimetas Rocker kept at 7° angle and six rotation cycles per hour | Pro-inflammatory protein, IL-1β, used to induce inflammation |
| Lee et al.104 | Solid organ chip | PMMA | Gelatin and liver dECM | YES | Human HepaRG cells Human umbilical vein endothelial cells (HUVEC) | Not mentioned | 25 μl/min final flow rate | APAP used to check hepatotoxicity |
| Lee et al.52 | Solid organ chip | Acrylic—- top PDMS—- middle Glass—bottom | Collagen I (10 μg/well) for mutiwell No coating for the LOC | NO | Rat and human primary hepatocytes | Up to 7 days | 10–20 nl/min Re < 0.01 Pe = 56 in flow channel Pe = 0.8 in the barrier channel | Hepatotoxicity of the anti-inflammatory drug diclofenac |
| Lee et al.70 | Solid organ chip | PDMS—- top PEGDA-microsomes—- middle Glass—bottom | Not mentioned | NO | Rat primary hepatocytes | Not mentioned | Tilt angle not mentioned 5 μl/min flow rate of substrate solution Pe > 1000 | P450 reaction with microsome in solution phase tested with different substrate concentrations ranging from 2 to 80 μM |
| Lee et al.71 | Solid organ chip | PDMS | 3% (w/v) BSA Collagen I | YES | Rat primary hepatocytes and HSCs | Up to 13 days | 5.53 mm/h flow speed | Live/Dead assay Albumin and urea secretion analyzed by measuring concentration in medium |
| Li et al.105 | Barrier tissue | Polyethylene terephthalate—- middle glass-bottom | Fibronectin (100 μg/ml) Collagen (100 μg/ml) | NO | Human primary hepatocytes Human dermal microvascular endothelial cells Human stellate cells | Not mentioned | 80 μl/h in heptic channel 100 μl/h in vascular channel | Induction of inflammation in liver diseases |
| Mazari-Arrighi et al.106 | Scaffold-free-cell fibres | Glass bovine collagen I 10% Matrigel 1.0% Na-alginate 100 mM CaCl2 3% w/w sucrose solution | Not mentioned | NO | Primary rat hepatocytes | Up to 30 days | 20 μl/min in the core 80 μl/min in the shell 3.6 ml/min in the sheath | Hepatotoxicity of the drugs acetaminophen and diclofenac |
| Moya et al.107 | Barrier tissue | Glass-bottom | Collagen | NO | Rat and human hepatocytes | Not mentioned | Not mentioned | OOC devices including printed sensors allowing for real-time physiological measurements |
| Nakao et al.69 | Solid organ chip | PDMS—- top Glass-bottom | Collagen Matrigel (150 μg/ml) | NO | Rat primary hepatocytes | Up to 4 days | 1.36 mm/s at center of medium flow channel No flow in cell culture area 1.3 Pa (shear stress) in medium flow channel No shear stress in cell culture area | CDF excreted into bile canaliculi by MRP2 protein |
| Rennert et al.91 | Solid organ chip | Multi-Organ-Tissue-Flow (MOTiF) biochip Cyclic olefin copolymers (COC)—TOPAS | COC-TOPAS® Plasma treatment | YES | HepaRG cells HUVECs Peripheral Blood Mononuclear Cells (PBMCs) | Up to 4 weeks | 50 mPa⋅(0.5 dyn/cm2) shear stress 50 μl/min perfusion rate | Not mentioned |
| Roth et al.108 | Scaffold free—micropatterned | Polystyrene micropillar and microwell chip by MBD Korea | 0.01% (w/v) PMA-OD attached PuraMatrix spots onto a micropillar chip | No | Human hepatoma (Hep3B) | Up to 3 days | Static | Adenoviral transduction and drug toxicity |
| Weng et al.74 | Scaffold-free—micropatterned | PDMS | Collagen | NO | Primary liver cells (PLCs) and Primary HSCs | Up to 14 days | 30 μl/min | APAP-induced hepatotoxicity |
| Yu et al.109 | Barrier tissue | PDMS—- top Glass coverslip and parylene membrane—- middle Silicon—bottom | 3,4-dihydroxy-l-phenylalanine (DOPA) | YES | Top—vascular cells Bottom—rat primary hepatocyte spheroids | Not mentioned | 0.1 ml/h | Alternating flow microfluidic assisted co-culture model |
| Yu et al.110 | Solid organ chip | Glass/silicon chip PDMS/glass cover | Collagen 1 (40 μl) | YES | Rat hepatocyte spheroids | 24 days | Working range: between 0.06 and 0.2 ml/h Optimum flow rate: 0.1 ml/h | Not mentioned |
| Zhou et al.8 | Solid organ chip | PDMS—top and middle Glass—bottom | Collagen 1 | YES | Stellate cells Rat primary hepatocytes | Up to 3 weeks | Not mentioned | Aptamer-based biosensors for detecting secreted proteins in parallel |
APAP, acetaminophen; BSA, bovine serum albumin; CDF, 5-(and-6)-carboxy-2′,7′-dichloro-fluorescein; COC, cyclic olefin copolymers; dECM, Decellularized extracellular matrix; ECM, extracellular matrix; GFR, growth factor reduced; HBV, hepatitis B virus; HSCs, hepatic stellate cells; LOC, liver-on-a-chip; NAFLD, non-alcoholic fatty liver disease; PDMS, poly(dimethylsiloxane); PEGDA, poly(ethylene glycol) diacrylate; PMA-OD, poly(maleic anhydride-alt-1-octadecene); PMMA, poly(methyl methacrylate); RAMECs, primary rat adrenal medullary endothelial cells; μTPs, microtissue precursors.
In this section, we discuss the progress of LOC development to mimic different liver characteristics, including models of different liver lobules and their microstructures.82 This leads into a discussion of mimicking liver heterogeneity and drug metabolism using LOCs, followed by a discussion of multicellular co-culturing in LOC designs.
A. Blood supply of the liver results in heterogeneity
The liver is supplied with both nutrient-rich and nutrient-depleted blood, and its functions are supported by interconnected networks of veins and arteries. The liver has an extremely high metabolic rate and is responsible for nutrient uptake, protein synthesis, detoxification, and bile production. The minute structures of liver lobules, a hexagonal shape with a central vein in the center, were introduced in 1833 by Kierman.83 The nutritious blood from the hepatic portal vein mixes with oxygenated blood (from hepatic arteries) in the sinusoids, a conduit for blood flow from the portal tract toward the central vein. The sinusoids are lined by endothelial cells wherein the space of Disse resides between the endothelial cells and the hepatic plates (cords of hepatocytes). The hepatocytes secrete bile which flows to bile ducts and ultimately leaves the liver to travel toward the duodenum.78 These three components, the bile duct, hepatic artery, and hepatic portal vein, collectively form the portal triad, which is located at the six corners of the hexagon. Lymphatic vessels could also be observed at this location, making it the portal tetrad or portal tracts. Two other models have subsequently been introduced: portal lobules and hepatic acinus lobules. Both are commonly used to define the structural and functional units of the liver.84 Each of these hepatic models has inspired different types of LOC designs to replicate liver characteristics.
1. Portal lobules
The portal lobule can be identified as the basic unit of the liver by centering at the portal triad and connecting the three adjacent central veins, forming a triangular region [Fig. 3(i)]. The direction of blood flow at the portal lobule “diverges” from the portal triad to the central vein. Conversely, the flow of bile “converges” at the center. Such classification of liver units can be particularly useful when considering the exocrine (bile secretion) function of the cells.85
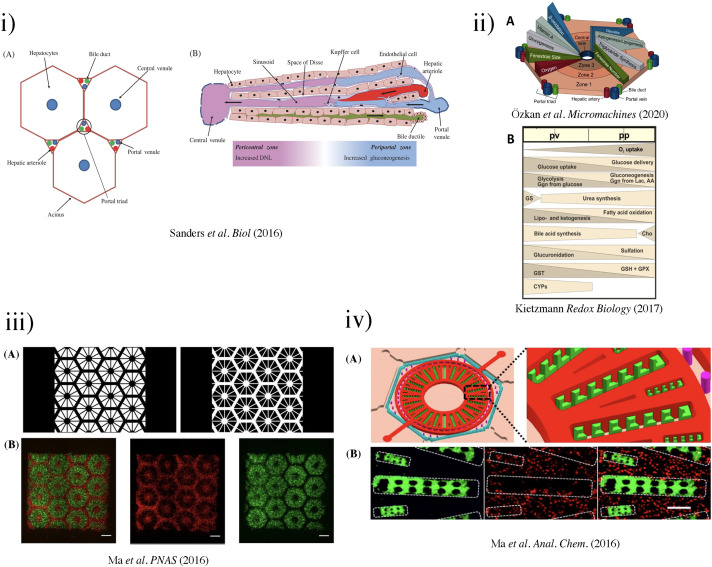
FIG. 3.
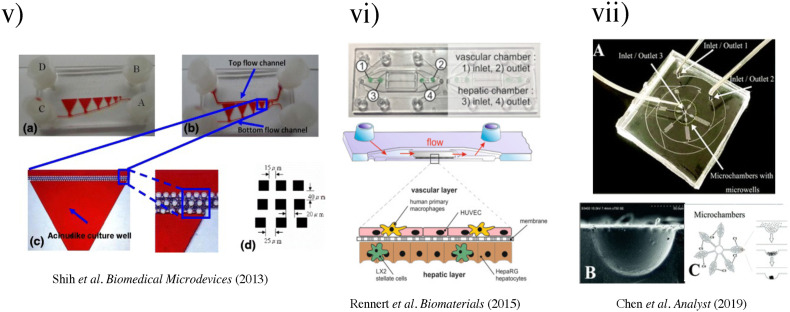
(i) The liver acinus and zonation of metabolic processes. (a) The gross cytoarchitecture of the hepatic parenchyma. (b) A cross section of liver tissue along the portocentral axis demonstrates the proposed zonation of metabolic processes, with the pericentral zone as the primary site of de novo lipogenesis (DNL) and the periportal zone as the primary site for gluconeogenesis. As indicated by the arrows, blood flows from the portal area via the sinusoid into the hepatic venule. Bile flows in the opposite direction from hepatocytes to the bile duct through the bile canaliculi.87 Reproduced with permission from Sanders et al. Biol. Rev. 91(2), 452–468 (2016). Copyright 2015 John Wiley & Sons Ltd on behalf of Cambridge Philosophical Society. (ii) (a) Biochemical pathways, gradients, and endothelial properties alternation across the zones of the liver lobule. Reproduced with permission from Özkan et al. Micromachines, 11(5), 487 (2020). Copyright 2020 MDPI.251 (b) Distribution of major metabolic pathways. (pp, periportal; pv, perivenous; AA, amino acids; Cho, cholesterol synthesis; CYP, cytochrome P450 enzymes; Ggn, glycogen; Lac, lactate; GPX, glutathione peroxidase; GS, glutamin synthesis; GST, glutathione transferase.)88 Reproduced with permission from Kietzmann et al., Redox Biology, Vol. 11. Copyright 2017 Elsevier B.V. (iii) (a) Grayscale digital masks corresponding to polymerizing lobule structure (left) and vascular structure (right) are designed for two-step bioprinting. The white patterns represent the light reflecting patterns for photo-polymerization. (b) Images (5×) taken under fluorescent showing patterns of fluorescently labeled hiPSC-HPCs (green) in 5% (wt./vol) GelMA and supporting cells (red) in 2.5% (wt./vol) GelMA with 1% GMHA on day 0 (Scale bars, 500 μm).86 (iv) Biomimetic microfluidic device with liver microarchitecture. (a) Schematic of the biomimetic microfluidic device with a hexagonal cell culture chamber, (b) morphological images of a single hepatic sinusoid-like structure with HepG2 cells and human aortic endothelial cell line.89 Reprinted with permission from Ma et al., Anal. Chem. 88(3), 1719–1727 (2016). Copyright 2016 American Chemical Society. (v) (a) The microfluidic device prototype with four medium inlets/outlets (ABCD) and six cell culture wells. The width of the cell culture well ranges from 1 to 6 mm. (b) The top channel of the microfluidic device prototype was filled with the red dyes. (c) The zoom-in picture of the acinus-like culture well with the multi-row square-pillar PDMS microstructure with the trapped air. (d) The design sketch of the three rows microstructure.90 Reproduced with permission from Shin et al., Biomed. Microdevices 15(5), 767–780 (2013). Copyright 2013 Springer Nature. (vi) Measurement of oxygen saturation in the cell culture medium by fluorescence emitting sensor spots. Top: Integration of oxygen sensor spots in the microfluidic biochip. Sensor spots were integrated at the inlets (1, 3) and the outlets (2, 4) of the upper and lower channel systems, respectively. Establishment of a three-dimensional liver model in a microfluidic biochip. Middle: Cross section of the biochip-embedded liver model.91 Reproduced with permission from Rennert et al., Biomaterials 71, 119–131 (2015). Copyright 2015 Elsevier Ltd. All rights reserved. (vii) (a) The photograph of the fabricated microchip. (b) Scanning electron microscope (SEM) images of the concave microwell. (c) The scheme of cell aggregation and spheroid formation in the microwell. C1–C6, cell clture chamber 1–cell culture chamber 6.92 Reproduced with permission from Chen et al., Analyst 144(14), 4233–4240 (2019). Copyright 2019 the Royal Society of Chemistry.
Ma et al. designed a chip [Fig. 3(iii)] that simulated the natural complexity of the liver microenvironment by integrating rapid 3D bioprinting with tissue engineering to construct physiologically relevant hexagonal units of liver cells and supporting cells (HUVECs and adipose-derived stem cells).86 This hexagonal pattern directly imitated the classic lobule and portal lobule, which enabled improvements in the structure and function of hiPSCs-derived hepatic progenitor cells (HPCs). Both hiPSC-HPCs and supporting cells were found to recognize the designated lobular pattern and achieve cell–cell interactions in 3D tri-culture mode. The study suggested that this LOC could be used for early personalized drug screening and in vitro studies of liver pathophysiology.
2. Zoning of hepatic acinus
In 1954, another classification of liver units called hepatic acinus was proposed by Rappaport.83 The hepatic acinus is identified by connecting central veins of two adjacent classic lobules with the adjacent portal triad, forming a diamond structure. Despite the apparent homogenous appearance on the histological level, the hepatic acini are regarded as heterogeneous at the subcellular level, as well as for biochemical and physiological functions. The hepatic acinus can be zoned into three tiers, Zones I, II, and III. The zonings are categorized by their proximity to the portal triad. Zone I is the closest to the blood supply, the first zone for receiving oxygen, nutrients, and toxins. Zone III is the furthest to the blood supply and the closest to the terminal hepatic central vein. Zone II lies between Zones I and III. The possibility of creating chemical gradients in LOCs to better recapitulate liver physiology inspires many designs.
Due to large variations in the distances of hepatocytes to the portal triad, a chemical and nutrient gradient can be observed in the hepatic acinus [Fig. 3(ii)(A)]. Variations such as enzyme activity and the size and number of cytoplasmic organelles are observed between Zone I and III. Cells in Zone II have intermediate functional responses and morphological characteristics compared to those in Zones I and III. Cells in Zone I are more resistant to the effects of nutritional deficiencies or circulatory compromises.111 Upon circulation impairment in the hepatic lobules, cells in Zone I are the last to die and the first to regenerate. However, after bile duct occlusion (bile stasis), these cells are the first to show morphological changes.112 Zone I is primarily responsible for ammonia detoxification and glucose metabolism processes.113 Under reduced perfusion, cells in Zone III are the first to exhibit centrilobular necrosis and accumulate fat.111
Zonal differences could be seen in liver sinusoidal endothelial cells (LSECs). With the aid of transmission electron microscopy (TEM), the features of fenestrae were determined (Table III).114–117 Wisse et al. reported the diameter of fenestrae to be around 107 ± 1.5 nm on the endothelial surface.117,118 However, Zapotoczny et al. showed that different treatment of the tissue sample might affect the measured sizes. Most studies have shown that the average diameters of fenestrae in Zone I are higher than in Zone III, and the number of fenestrae increases from Zone I to Zone III. The exact measurements are unknown due to technical difficulties, such as limited access to tissue samples and changes in the diameter and number of fenestrations after treatment with various agents (hormones, drugs, and toxins). Despite this, the zonal variations of fenestrae are proven to play critical roles in gene therapy.118 These physical measurements could be a guide for constructing in vitro models of the fenestrae of liver sinusoids.
TABLE III.
Compiled data from various sources on the measurement of fenestrae.
| Diameter (nm) | Density (per area, μm2) | Sample | Reference | ||
|---|---|---|---|---|---|
| Zone I | Zone III | Zone I | Zone III | ||
| 150–175 | NA | 9 | 13 | Unknown | Gebhardt 1992 Pharmacol. Ther.119 |
| 111 ± 0.25 | 105 ± 0.22 | 9 | 13 | Rat liver | Wisse et al. 1985 Hepatology120 |
| NA | NA | 19 | 23 | Human liver; non-alcoholic | Horn et al. 1987 Hepatology121 |
| NA | NA | 8.5 | 12 | Human liver; alcoholic | Horn et al. 1987 Hepatology121 |
| 107 ± 1.5 | NA | NA | NA | Human liver | Wisse et al. 2008 Gene Ther.118 |
| 180 ± 41 | NA | NA | NA | Murine liver | Zapotoczny et al. 2017 Sci. Rep.122 |
In 2013, Shih et al. designed a gradient microphysiological system that mimicked liver acinus by adopting three mechanisms.90 First, PDMS was used to host the cells as a hydrophobic and gas-permeable material. Second, cell–cell interaction was encouraged by reducing the drag force during cell seeding, where the chip contained a multi-row square pillar [Fig. 3(v)] to balance the shear stress and mass transfer perfusion of the culture medium. Third, a reduced flow speed was enabled by connecting the top and bottom flow channels to achieve a concentration gradient. A non-linear concentration gradient was achieved by semi-circle flow design where the crossflow and the expansion phenomenon were balanced. Shih et al. inspired the creation of the biomimicry approach for LOCs.
3. Metabolic zonation
The concept of “metabolic zonation” was formally acknowledged by the work of Jungermann and Sasse in 1978.123 This was derived from previous studies of carbohydrate metabolism, suggesting opposing metabolic pathways such as gluconeogenesis (or glucogenesis) and glycolysis are simultaneously occurring in hepatocytes in the periportal and perivenous regions, respectively.119–126 Key chemical/metabolic gradients are present across the three zones [Fig. 3(ii)]. For example, oxygen and nutrient concentrations decrease from Zone I to Zone III, directly impacting the liver's functional adaptation to meet the various nutritional and energetic requirements for supporting metabolic pathways. Ma et al. described a biomimetic design to recapitulate the liver's metabolic zonation, by mimicking the hepatic cord network and hepatic sinusoid network [Fig. 3(iv)].89 Using hepatic enzyme assay, it was found that the chip maintained high basal CYP-1A1/2 and UGT activities, indicative of great drug metabolism capacity. The chip also successfully predicted potential adverse reactions from clinical pharmaceuticals causing drug-induced liver injury, suggesting an application in the in vitro assessment of drug-induced hepatoxicity.
B. Multicellular co-culturing
A rich population of specialized cells supports the functions of the liver, classified into two categories: parenchymal cells (hepatocytes) and non-parenchymal cells (all other cells).26 To build more physiologically relevant LOCs, co-culturing of parenchymal and non-parenchymal cells has been established for liver function evaluation and mechanistic studies. Monocellular culturing of hepatocytes can lead to shortened preservation of cellular morphology and functionality, finite perfusion duration, diminished ECM-derived biochemical signals, and loss of cell polarity. The inclusion of non-parenchymal cells creates biomimetic tissue structures that allow hepatocytes to be subjected to native biophysiological–biophysical cues, enabling better modeling of liver homeostasis and functions.18 Multi-cellular seeded chips have been shown to provide a more realistic physiological or disease model of the liver, and higher accuracy in predicting toxicity.65–75
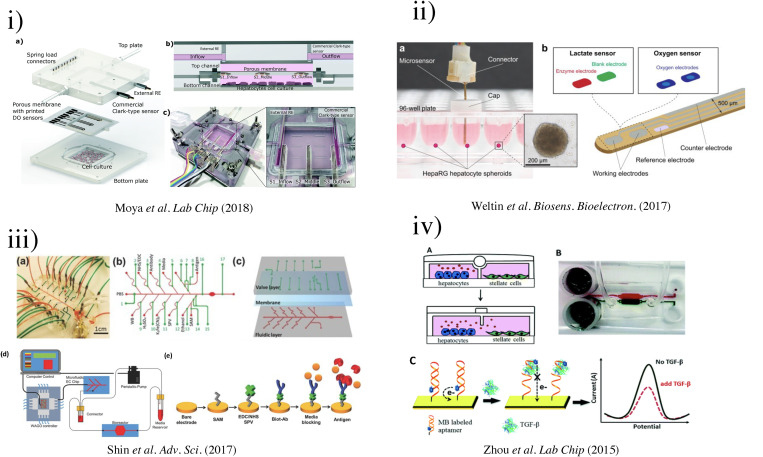
To overcome the rapid loss of hepatocyte cell polarization and unwanted differentiation in static culture, Rennert et al. established a Multi-Organ-Tissue-Flow (MOTiF) perfusion microfluidic system together with co-culturing [Fig. 3(vi)].91 By incorporating HUVECs (to mimic liver sinusoidal endothelial cells), monocyte-derived macrophages (to mimic Kupffer cells), and immortalized human hepatic stellate cells (LX-2) with HepaRG (immortalized hepatic cell line), the perfused liver organoid showed close resemblance to the morphological and functional characteristics of the human liver. This system could provide a continuous supply of oxygen and nutrients, and real-time monitoring of cellular oxygen consumption through the use of luminescence-based sensor spots. The oxygen consumption of HepaRG cells was studied at different media perfusion rates (1–3 μl/min) and found to increase at higher perfusion rates. These dynamic responses suggested that the MOTiF biochip could mimic in vivo conditions, making it a valuable tool for studying liver physiology, metabolism, and underlying molecular processes.
It has been reported that HSCs are a major component of the hepatic tumor microenvironment, and play critical roles in cancer progression as well as drug resistance. To elucidate the effects of HSCs (JS-1) on hepatic tumor spheroids (Hepa1–6), Chen et al. developed a microchannel plate-based co-culture model and used it to study drug resistance and cellular interactions.92 The in vivo tumor microenvironment was set up using 3D concave microwells, recapitulating epithelial-mesenchymal transition and chemoresistance. The design incorporated a concentration generator to study the spatial and temporal stability of the LOC [Fig. 3(vii)]. This system facilitated the formation of hepatic tumor spheroids with simultaneous ability to monitor cell morphology, behavior, and other physiological changes under continuous flow.
IV. FABRICATION TECHNIQUES FOR LOC
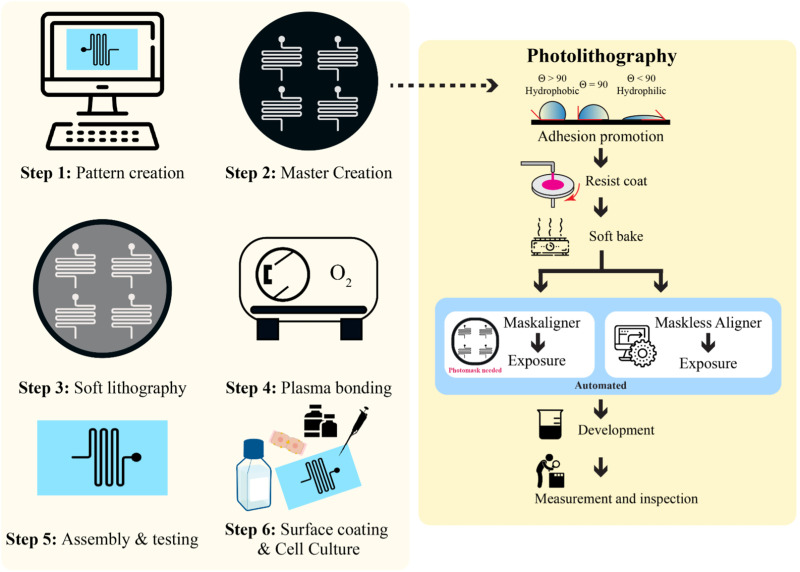
To benefit readers from a wide range of disciplines interested in self-fabricating LOCs, we have provided a summary of the general procedures in fabricating LOC devices, drawing on recently published protocols and other comprehensive reviews. We hereby provide a graphical overview in Fig. 4, including recent advances in lithographic techniques and LOC-specific procedures. For detailed protocols, readers are encouraged to seek further details in other reviews.39–127
FIG. 4.
A general procedure for creating a microfluidic LOC device in six steps. Step 1 is pattern creation, usually performed using computer-aided design software, such as AutoCAD. Step 2 is creating a master template on a silicon wafer where there is a choice of mask photolithography or maskless photolithography. Step 3 is the fabrication of the device by soft lithography. Step 4 is the plasma bonding of the chip materials. Step 5 involves the tube insertion and testing of the device before cell culturing. Step 6 shows the coating of the chip channels and the seeding of cells.
A. The general procedure to produce LOC devices
LOC devices have been combined with biosensors to achieve real-time monitoring of hepatocyte morphology and functions.128,129 For continuous monitoring, LOCs need to sustain the phenotype of hepatocytes, particularly primary cells, and liver-specific functions in long-term culture.130 In most LOC devices, multi-cellular or co-culturing has seen better and more accurate responses to drug testing and toxicological evaluations compared to 2D in vitro models.131 The versatility of designing microfluidics patterns meets the needs of different research focuses, such as pathological studies and drug development.55–59 In this section, we give an overview of the common procedures for fabricating LOC devices. Then, we specifically discuss emerging trends in cell fabrication techniques that have been incorporated for LOC technologies.
The general procedure for producing LOCs devices is outlined below:
-
(1)
Create the desired pattern using computer-aided design (CAD) software with consideration of positive or negative imprint.
-
(2)
Use photolithography techniques to create a master (or mold). The alternative method, maskless lithography, is discussed in Sec. IV B.
-
(3)
Adopt the soft lithography technique to create a polymer-based LOC.
-
(4)
Device bonding by plasma treatment.
-
(5)
Microfluidics device evaluation and testing.
-
(6)
Surface treatment of the materials for cell culturing to provide a functional LOC.
B. Photomask lithography and maskless lithography
Photomask lithography is solely dependent on the use of photomasks. Hereby, we summarized the different types of photomasks and their characteristics in Table IV. The typical costs of a photomask can range from the most expensive quartz (∼USD$500, 5 in.) down to the least expensive plastic film (∼USD$100, 9 × 12 in.) per photomask. The cost increases with size and resolution, as well as other handling expenses such as shipping (also extends the time needed for such fabrication process). One photomask may accommodate multiple designs at once, thus bringing down the cost per design. Typically, several iterations are required to produce an optimal LOC device. With any modification to the design, a new photomask needs to be procured. Efforts have been made to shorten the processing time and lower the cost by adopting techniques mentioned in other studies and reviews.132–135 Here, we discuss a recent advance in photolithography using a maskless aligner, which completely abandons the use of photomasks.
TABLE IV.
| Type of photomask | Advantages | Disadvantages |
|---|---|---|
| Quartz photomask | High resolution (>= 1.0 μm) Very stable Low thermal expansion coefficient Easy to clean Non-restricted wavelength range (> 180 nm) | Could break Very expensive Time consuming |
| Glass (soda lime) photomask | Cost-effective solution Stable Easy to clean High resolution (>= 2 μm) | Fragile Limited wavelength range (> 350 nm) Time consuming |
| Plastic photomask | Cheapest option of all Very easy to handle Rapid prototyping method Flexible Thin | Weak stability Low resolution (> 6 μm) Limited wavelength range (> 350 nm) |
With advances in instrumental designs, maskless lithography has become an option to perform rapid prototyping of microfluidics.138 Maskless lithography relies on the use of a maskless aligner (MLA), such as Heidelberg MLA150.139 The MLA150 can pattern features down to 0.6 μm with topside and backside alignment features. The maximum exposure area of MLA150 is 150 × 150 mm2. Using MLA150, microfluidic devices can be fabricated without the need to produce or outsource photomasks. In a recent study by Kasi et al.,139 maskless photolithography was used to produce an OOC device capable of culturing human-induced pluripotent stem cell (hiPSC)-derived vascular cells and neuron cells. They also achieved a cleanroom-free microfabrication process, one step closer to an accessible and practical method for making microfluidic devices. Rapid prototyping with maskless photolithography offers opportunities for researchers to adopt it as a regular practice to produce high-fidelity and high-resolution microfluidic devices with shortened time frames and without the expenses of photomasks. The use of MLA is ideal for rapid prototyping during design-testing iteration cycles.
C. Soft lithography and surface functionalization
Duffy and Whiteside et al. introduced soft lithography in 1998.68 This procedure is defined as micromolding an elastomeric polymer to generate a pattern, by replica-molding from a microfabricated master typically produced by photolithography. Hence, soft lithography is an auxiliary microfabrication technique to produce microfluidics devices.61–142 Soft lithography is endorsed by researchers as an inexpensive way of fabricating microfluidics.
Over the years, PDMS has become one of the most commonly used materials in soft lithography. PDMS can achieve fidelity of below 0.1 μm.143 Since PDMS is optically transparent above 240 nm, it is a suitable material for optical detection between 240 AND 1100 nm. PDMS is considered an insulator that allows circuits to be embedded, and its elasticity is tunable with a typical value of ∼750 kPa. Due to its excellent biocompatibility, PDMS is the preferred material for rapid prototyping of microfluidic devices. Nevertheless, PDMS can absorb hydrophobic biological molecules and be incompatible with certain organic solvents.144 To overcome these issues, alternative elastomers have been adopted, such as polyester elastomers, tetrafluoroethylene-propylene elastomers, and thermoplastic elastomers.145
The general steps to produce a microfluidic chip using soft lithography are described as follows: (i) PDMS casting and thermal annealing at 40–70 °C or at higher temperature for a shorter curing time; (ii) PDMS chip peeled off from the mold and cut into the desired shape; (iii) creation of inlets and outlets of the assembled device using biopsy puncher; (iv) sealing the device by plasma bonding where different layers of design and a glass slide are joined together.127 PDMS can be sealed to another PMDS block or other surfaces reversibly or irreversibly, making multi-layered device design possible. An alternative method, such as corona discharge, can be performed to achieve the same result.146
Surface treatment of the device may be necessary to promote cell adhesion or other processes such as spheroid production, enhance biocompatibility, and reduce chemical diffusion into the polymer material. In 3D spheroid cultures, pluronic acid is usually used to prevent undesired spheroid dissociation due to potential cell attachment to the surface. Other coatings such as proteins and ECM can promote cell attachment to the surface. Readers are encouraged to seek further information from other recently published reviews.39–148
D. Long-term cell culturing techniques
Primary human hepatocytes are considered the gold standard for in vitro cell culture to study liver characteristics and function. However, they are prone to rapid death in vitro in 2D culturing platforms. A key characteristic of LOCs is to achieve long-term culture of hepatocytes (over two weeks of continuous culturing) to address common issues such as contamination, clogging, and bubble accumulation in the chips, which can cause deterioration of cell function. This section reviews some of the current techniques to enable long-term cell culture in LOCs.
1. Production of 3D spheroids
As conventional spheroid culture is performed in static conditions, the depletion of nutrients around the spheroid periphery is inevitable, causing cell death at the center of the spheroids. Liquid overlay techniques have been used to produce a non-adherent surface for cells to form 3D spheroids, such as the use of poly-2-hydroxyethyl methacrylate (Poly-HEMA),149 pluronic acid,150 or 1–2% agarose coating on the substrate surface.151 To improve the throughput, spheroids have been cultured with various techniques and in conjunction with LOCs. Spheroid LOCs can be realized by two general approaches: (1) produce spheroids using cell culturing techniques and then transfer the cell aggregates into LOCs and (2) direct production of spheroids in the LOCs. Several methods have been explored to produce spheroids, such as the use of ultra-low attachment (ULA) surfaces, bioreactors, hanging drops, microarrays, and the most recent developments in microfluidic spheroid formation chips—a technique for on-chip formation of spheroids.152
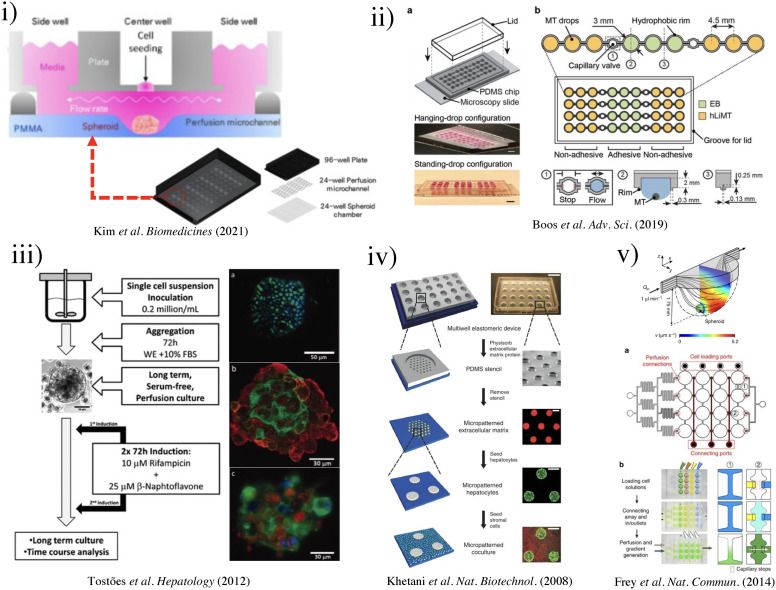
Kim et al. designed and fabricated a polymethyl methacrylate (PMMA) based hemispherical well-shaped cell culture chamber that was functionalized with a pluronic coating (1% w/v in water) to encourage cell aggregation [Fig. 5(i)].103 The hepatocyte cell line HepG2, primary human umbilical vein endothelial cells (HUVECs), and fibroblast cell line H368 were adopted in the ratio of 5:4:1. The spheroids, with dynamic fluid flow caused by a rocker at 7° tilt and a rate of six rotations per hour, were exposed to IL-1β (1, 5, 10, and 20 ng/ml) over 5 days to cause cellular stress and produce an inflammatory disease model. Although this model demonstrated the possibility of simulating the hepatic microenvironment and human liver physiology in disease, there was lack of control over the dynamic fluid flow. Precise control of fluid flow could be introduced by the use of syringe pumps, peristaltic pumps, pressure controllers, or other types of fluid control devices.
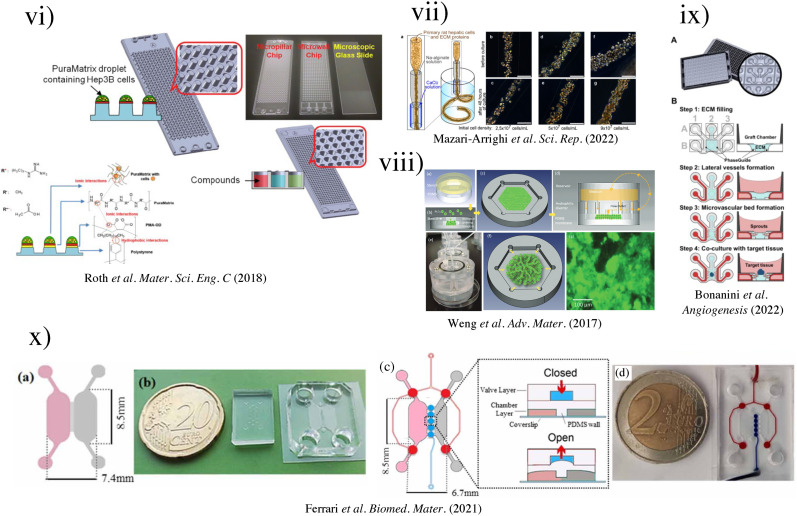
FIG. 5.
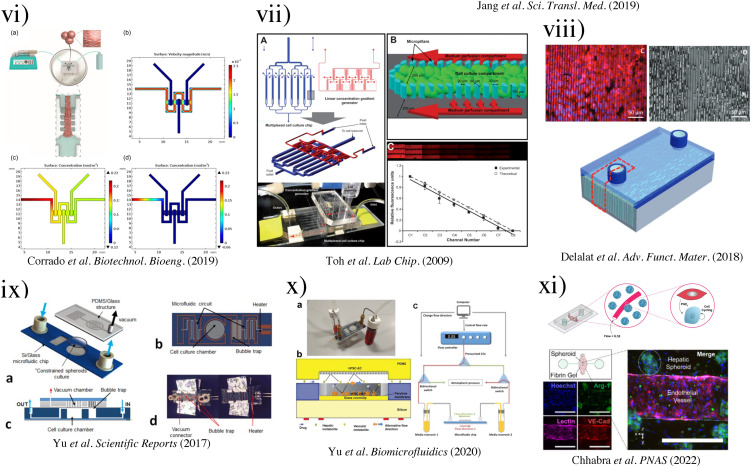
(i) Schematic of three-well array for the gravity-based spheroid culture.103 Reproduced with permission from Kim et al., Biomedicines 9(10), 1369 (2021). Copyright 2021 MDPI. (ii) Microfluidic multi-tissue hanging-drop platform. (a) The microfluidic network is patterned on the surface of a PDMS substrate. Photographs of the chip show its operation in hanging-drop and standing-drop configuration. Scale bar: 5 mm. (b) The chip layout consists of four individual lanes of nine interconnected drops.94 Reproduced with permission from Boos et al., Adv. Sci. (Weinh) 6(13), 1900294 (2019). Copyright 2019 John Wiley& Sons. (iii) Experimental design of the induction of the CYP450 enzymes in primary cultures of hepatocyte spheroids in the bioreactor (left). Scale bar = 50 μm. Immunofluorescence microscopy of liver-specific antigens in human hepatocyte spheroids after two weeks of bioreactor culture (right).153 Reproduced with permission from Tostões et al., Hepatology 55(4), 1227–1236 (2012). Copyright 2011 John Wiley & Sons; (iv) schematic of the process flow aside photomicrographs taken at each step. A reusable PDMS stencil is seen consisting of membranes with through-holes at the bottom of each well in a 24-well mold. Primary hepatocytes selectively adhere to matrix-coated domains, allowing supportive stromal cells to be seeded into the remaining bare areas (hepatocytes labeled green and fibroblasts orange; scale bar is 500 μm).102 Reproduced with permission from Khetani et al., Nat. Biotechnol. 26(1), 120–126 (2008). Copyright 2007 Springer Nature. (v) Numerically simulated streamlines and flow velocities of a perfused drop containing a 400-μm-diameter spheroid (the applied flow rate is 1 μl min−1; hanging drop has maximal size and a height of 1.75 mm). The same flow rate is applied at the outlet. Gray areas indicate contact walls with the no-slip condition (v = 0 μm s−1). (a) Layout of the four-by-four drop array, showing the added features for array reconfiguration (marked in red). (c) Three handling steps are required during an experiment (close-up views show key areas).98 Reproduced with permission from Frey et al., Nat. Commun. 5(1), 4250 (2014). Copyright 2014 Springer Nature. (vi) Schematic representation of the micropillar and microwell chip platform for use in Hep3B cell encapsulation in PuraMatrix and compound toxicity assessment.108 Reproduced with permission from Roth et al., Mater. Sci. Eng. C 90, 634–644 (2018). Copyright 2018 Elsevier. (vii) Encapsulating primary rat hepatocytes within core–shell hydrogel microfibers by applying cell fiber technology. (a) Schematic drawing of the fabrication of core–shell hydrogel microfibers encapsulating freshly isolated rat hepatocytes through a double-coaxial microfluidic device. (b)–(g) Representative dark-field images (n = 12 cell fibers for each group) of primary rat hepatocytes encapsulated in cell fibers before culture and after 48 h of culture in three experimental groups possessing different initial cell seeding densities: 2.5, 5, and 9 × 107 cells ml−1. Scale bars; 100 μm.106 Reproduced with permission from Mazari-Arrighi et al., Sci. Rep. 12(1), 1–12 (2022). Copyright 2022 Springer Nature. (viii) Design principles of tissue incubator. (a)–(d) Schematic diagram of design principles. (e) Photo of entire device. (f) Schematic diagram of radial flow. (g) LOC shows good cell viability with Calcein AM staining (green).74 Reproduced with permission from Weng et al., Adv. Mater. 29(36), 1701545 (2017). Copyright 2017 John Wiley& Sons. (ix) The OrganoPlate Graft allows for the generation of robust microvascular beds. (a) Top and bottom views of the OrganoPlate Graft with 64 microfluidic units positioned underneath a 384 microtiter plate. Each microfluidic unit makes use of a 2 × 3 array of wells from the microtiter plate (the inset image). (b) Sequence of steps for generating a microvascular bed.93 Reproduced with permission from Bonanini et al., Angiogenesis 25(4), 455–470 (2022). Copyright 2022 Springer Nature. (x) Developed micropatterned devices. (a) Schematic of the microgrooves-based platform with pink: liver compartment and gray: tumor compartment; (b) picture of the microgrooves-based device and stamp for micropatterning; (c) concept of the valve-based platform with pink: liver compartment and gray: tumor compartment. In the zoom is shown the valve operating principle; (d) a picture of the valve-based device.154 Reproduced with permission from Ferrari et al., Biomed. Mater. 16(4), 045032 (2021). Copyright 2022 IOP Publishing, Ltd.
In an early study by Tostões et al.,153 a perfusion-stirred tank bioreactor was used to promote the formation of primary human hepatocyte spheroids (81 ± 4 μm diameter, week 2) during the first 72 h of culture. With proper controls, the bioreactor [Fig. 5(ii)] achieved convective mass transfer and environmental control appropriate for the robust formation of hepatic-like microtissue units. In this study, the primary human hepatocyte spheroids could maintain hepatic liver-specific synthesis, drug-metabolizing enzyme gene expression and activity, and liver-like architecture inside the spheroids for 2–4 weeks.
2. Hanging drop for LOC
The hanging drop method is an elegant use of the Young–Laplace equation155
| (1) |
where p denotes the pressure, denotes the interfacial energy (air–liquid), and r denotes the droplet's radius. This cell fabrication technique relies on accumulating cells at the liquid–air interface to develop spheroids. There is an emergence of commercial hanging drop plates (HDPs) on the market, allowing the streamlined production of spheroids.156 Disadvantages of such methods include the inability to use large liquid droplets (>50 μl) and the inability to change media without adversely affecting the spheroids.
To tackle the inability of media exchange during spheroid formation, Frey et al. presented a highly versatile analytical platform for forming multi-cellular spheroids.98 The microfluidic system is composed of hydrophobic rims and circular chambers. Capillary forces drive the liquid from the inlet to the outlet, and as the pressure increases, the droplet builds up in size. As the bottom surface is the liquid–air interface, gas exchange and fluid dynamic profiles differ from closed microfluidic channels. The maximal flow velocity was found at the liquid–air interface [Fig. 5(v)]. This configuration offers the advantage of washing away unwanted single cells and debris. Frey et al. also achieved parallel spheroid formation by designing connecting ports (capillary valving), enabling the liquid flow without crossover. By introducing a small volume of liquid, the capillary valve broke, and the liquid would infuse together to create connected streamlines. By incorporating gradient microfluidics, different cell culturing media could be prepared. This array configuration allowed the rapid production of individual hanging drops with metabolic cross-communication between spheroids. They successfully demonstrated the ability to culture primary rat liver microtissues (rLiMT) in parallel with human colon cancer cell line HCT-116.
In a recently developed device by Boos et al.,94 the hanging drop method produced embryonic bodies (EBs) and primary human liver microtissues (hLiMTs) to integrate liver metabolism into the embryonic stem cell test (EST). The hanging-drop-network [Fig. 5(ii)] was used to co-culture EBs with hLiMTs in immediate proximity to each other, named “metaEST.” During EB formation, hLiMTs were disconnected from EB due to the presence of the hydrophobic rim and capillary valve under static culturing conditions. After 24 h, the wetting of the capillary valve established liquid exchange between the compartments, and fluid flow was induced by slightly titling the chip by ±2° every 15 s with an average turnover of 4.5 μl per tilting cycle for 5 days. On day 5, the chip was flipped upside-down to obtain a standing-drop configuration and tilted at ±4° every 15 s with an average medium turnover of 8.7 μl per tilting cycle. Due to different coatings of the substrate, hLiMTs remained spheroids, whereas the EBs adhered and spread on the adhesive surface. As proof of concept, the drug cyclophosphamide was used, which showed a fourfold lower ID50 concentration after biotransformation, demonstrating the metaEST as a promising tool for EST.
3. Microarrays
Another emerging technique to produce spheroids for LOCs is using microarrays. The aforementioned techniques for spheroid production by ultralow attachment 96-well plates and hanging drops have the disadvantages of low throughput, challenging operations, and being labor intensive.157,158 To improve throughput, a microwell microarray has been proposed by Chao et al. by engineering the cell attachment surfaces with agarose gel.159 A negative mold with microwell patterns of 250 μm diameter and 400 μm center-to-center spacing was created; approximately 160 microwells could fit into the bottom surface of a single microwell of the plate. A PDMS stamp with microarrayed pillars was created using the mold and subsequently set in the molten agarose over GelBond film (Lonza, 53761) for 15 min. By removing the PDMS mold, an array of microwells was created. The agarose gel was clamped between a 96-well bottomless plate and a glass plate while the cell loading was done. With the removal of the 96-well bottomless plate, the patterned agarose microwell microarray trapped HepG2 cells and encouraged the formation of HepG2 spheroids within 1–2 days. With this technique, Chao et al. further modified the assay to show that intact HepG2 spheroids cultured in microwells could be electrophoresed to reveal the extent of DNA damage following exposure to inflammatory chemicals, such as H2O2 and SIN-1.
The use of hydrogel scaffolding can accelerate and maintain cell growth in microarray systems. For instance, incorporating hydrogels on top of the micropillars in the microarray can improve their surface chemistry. Key advantages of coating the pillars with hydrogels are the improved high-throughput screening of potential drug candidates, diffusion of nutrients, and imaging of cells. Roth et al. explored the use of PuraMatrix as the hydrogel matrix,108 which made the study of viral transduction possible [Fig. 5(vi)]. The surface chemistry of micropillars was optimized by a coating of poly(maleic anhydride-alt-1-octadecene) (PMA-OD, 0.01% w/v in ethanol) and subsequently printed PuraMatrix (0.25% in water) using the S + microarrayer at 60 nl/micropillar. Six model compounds, acetaminophen, lovastatin, rotenone, tamoxifen, menadione, and sodium citrate, were tested on 3D-cultured Hep3B cells for rapid toxicity assessment by obtaining IC50 values. With the improved surface chemistry, Roth et al. demonstrated the suitability of the PuraMatrix hydrogel for 3D cell encapsulation, gene expression, and rapid toxicity assessment.
4. Microfluidic spheroid formation chips (μSFCs)
μSFCs refer to microfluidic chips that could promote the formation of spheroids in chips with the additional ability to maintain the culture of spheroids.152 μSFCs have shown the ability to prolong the lifetime of hepatic cell lines (such as HepaRG, HepG2, and Fa2N-4) in culture and help preserve their phenotype, making them a promising in vitro model for evaluating hepatic metabolism and cytotoxicity.160 To exploit the use of 3D spheroids in constructing LOC, Choi et al. produced a microfluidic device with a microstructured floor, containing pyramidal wells to promote primary rat hepatocytes in forming uniform assemblies of hepatic spheroids (∼100 μm in diameter).7 Two different sizes of pyramidal wells, a small size (4.25 μl with 100-μm headspace) and a large size (40 μl with 1600-μm headspace), and a 2D culturing platform were used, showing that hepatic spheroids in LOCs can enhance cell phenotype and function. More importantly, Choi et al. demonstrated that the geometry and mass transport of the cell culture system played an unequivocal role in the accumulation of autocrine signals and maintenance of primary hepatocytes. It was found that small-volume spheroid cultures were more robust. Compact spheroids formed over the course of 24–48 h showed significantly higher production of albumin, higher cytochrome P450 2A1 expression and the formation of bile canalicular networks within individual spheroids in small-volume microfluidic spheroid culture.
Another bottleneck in modeling human hepatic tissues in vitro is the ability to produce vasculatures in liver organogenesis and regeneration models. Bonanini et al. presented a viable approach to promote vasculatures for in vitro models, using an innovative design combining spheroids and a microfluidic vascular bed.93 They created an OrganoPlate Graft, comprising patterned 64 microfluidic chips underneath a standard 384-well plate. Each chip had two perfusion channels and a gel channel [Fig. 5(ix)]. Two phaseguides at the bottom of the microfluidic channel served as a capillary pressure barrier and assisted in the filling of ECM gel. Collagen I was used to pattern the phasedguide at inlet A2 [Fig. 5(ix)(B)] for improved cell viability. A four-step procedure was adopted for in vitro tissue grafting, which achieved co-culture of HUVECs and cryopreserved Upcyte® human hepatocytes.
5. Matrix embedding
Hydrogels are the most common choice for cell scaffolding in constructing LOC because of their space-filling ability, controllable porosity and topography, and biocompatibility.18–162 Novel methods such as electrospinning nanofibers have also been studied. 3D scaffolds made from a range of natural and synthetic materials have been used to help promote hepatocyte growth.163,164 Using native ECM is another method for mimicking the native microenvironment of hepatocytes,165 such as a decellularized liver scaffold by removing hepatocytes and non-parenchymal cells.166 Studies have shown that decellularized ECM could provide a physiologically relevant environment with suitable biophysical and biochemical factors to encourage hepatocyte growth and function.53–168
To improve the longevity of primary hepatocytes during in vitro culture, Mazari-Arrighi et al. developed cell-laden core–shell hydrogel microfibers, named “cell fibers”, that could maintain primary rat hepatocyte viability and liver-specific functions for up to 30 days in culture.106 The cell fibers were generated using a double-coaxial laminar-flow microfluidics device, where the core contained both cells and ECM proteins (mixture of collagen I and Matrigel), while the shell was an alginate hydrogel. By varying the initial cell seeding concentration, cellular clusters would vary their morphology, whereby a lower cell density increased the likelihood of the cell cluster becoming spheroid shaped [Fig. 5(vii)]. Mazari-Arrighi et al. tested the performance of cell fibers in detecting drug hepatotoxicity using acetaminophen and diclofenac. Cell fibers allowed accurate estimation of the 50% inhibitory effect (IC50) for up to 30 days for these two drugs, representing a significant improvement compared to cells cultured in a 24-well plate. These cell fibers showed the possibility of offering scalability and handleability over long culture periods.
E. Other techniques
The fabrication of microfluidics devices is complicated and requires state-of-the-art facilities, such as a cleanroom, increases the relative cost of a LOC. Additive manufacturing including 3D printing allows complex structures to be built from various materials, for example, through layer-by-layer deposition of material ink.169 Building up from this, 3D bioprinting enables the production of microfluidic devices incorporating cells in a one-step procedure.170,171 This dramatically reduces the cost per chip and shortens the production time, making 3D bioprinting an attractive technique for rapid prototyping and proof-of-concept validation in constructing LOC.
Recent advances in 3D bioprinting allow the direct printing of viable cells with 3D tissue structures in a single continuous procedure with great accuracy.172,173 3D bioprinting techniques can be categorized further into stereolithography, inkjet, extrusion, and laser-assisted bioprinting. Different techniques can achieve varying degrees of cell viability, resolution, and printing speed. Two-step fabrication has been recently reported where the microfluidic chip is fabricated using a conventional microfabrication technique, followed by bioprinting cells into the prefabricated chip. Different bioinks have been developed to accommodate various OOCs including LOCs, as captured in recent reviews.174,175
Bhise et al. provided a novel model where human HepG2/C3A spheroids encapsulated in a hydrogel scaffold were bioprinted into a microfluidic device for hepatotoxicity testing.73 The primary device comprised PDMS and PMMA, and spheroids were formed using microwell technique followed by suspending the cell clusters in gelatin methacryloyl (GelMA) hydrogel scaffold. Unlike other devices, this platform could be easily disassembled and reassembled during the experiment to allow access to the cells in culture. The tissue-like construct was assessed over 4 weeks, in conjunction with cellular response to acute acetaminophen exposure over 1 week for predicting drug toxicity. Results indicated a significant decrease in metabolic activity over 6 days in cultures with acetaminophen, similar to the hepatotoxicity response reported in animal models. This device was thought to provide a 3D culture environment to study drug-induced toxicity in vitro, with high throughput and prediction capability comparable to in vivo conditions.
To reproduce the biliary system of the liver, Lee et al. used 3D bioprinting to create a fluidic structure with decellularized ECM bioink.104 The device consisted of an upper channel for nutrient supply and a lower channel for bile salt secretion and waste removal. HepaRG cell line was used for the differentiation of hepatocytes and liver biliary-epithelial cells. HUVECs were incorporated to create liver sinusoids in the 3D bioprinted structure between the two channels. This LOC device was able to incorporate multiple cell types and create biomimetic vascular and biliary systems, and also showed an effective drug response when evaluated using acetaminophen.
Despite the potential of 3D bioprinting in creating biomimetic LOC designs, the success of this technique is limited by the printing resolution. More complex features, such as capillary networks, could be a challenging feature to be incorporated into the bioprinted structure. Increasing the printing resolution would also extend the printing time, and a balance needs to be considered to ensure cell viability during a prolonged printing process. Although high-fidelity 3D bioprinters exist, they could be expensive, which might defeat the purpose of rapid and economical prototyping.176,177 Further advances in cell sources and printing technologies are required to realize the full potential of using bioprinting to create LOCs.
Recent developments in micropatterning also show promise as an alternative method for OOC fabrication. Khetani and Bhatia used an elastomeric PMDS stencil to culture human liver cells in multi-well format.102 The stencil contained 300-μm-thick membranes with through-holes at the bottom of each well in a 24-well mold. The multi-well mold was sealed against a polystyrene plate. After applying collagen I to the exposed polystyrene plate, the stencil was removed, and the coated area could selectively support the growth of hepatocytes. After varying the diameter of collagen islands over several orders of magnitude, hepatocyte functions were found to be maximized using 500 μm islands with 1200 μm center–center spacing [Fig. 5(iv)]. The microscale architecture, where the hepatocytes were surrounded by fibroblasts, remained stable for several weeks in culture. Dose- and time-dependent chronic toxicity was demonstrated using troglitazone, characterized by TC50 (the concentration that produced a 50% reduction in mitochondrial activity after acute exposure) and morphological changes. This study successfully showed that micropatterning could be used for drug toxicity screening.
To further investigate the micropatterning capability, a multi-organs-on-a-chip was developed by Ferrari et al. based on the micropatterned technique proposed by Khetani and Bahtia.154 Ferrari et al. created a micropatterned dual-compartment microfluidic device to co-culture human Caucasian hepatocyte cell line (HepG2) and murine embryonic fibroblasts (NIH–3T3) in the liver compartment and colon cancer cell line (HCT-166) in the tumor chamber, as shown in Fig. 5(x). Instead of a stencil, plasma ablation was used to create the coating patterns of collagen I (50 μg/ml, 300 μl) on glass slides using a PDMS stamp mask. Two different systems were developed, microgrooves (5 μm high, 3 μm wide, 1 mm long, and inter-channel gap 30 μm) and a valve system actuated by vacuum. Tegafur, a prodrug of 5-fluorouracil, was injected into the liver compartment to test the dual-compartment systems. This platform reproduced the metabolism of Tegafur in the liver and demonstrated the killing of colon cancer cells.
Using a different approach, Weng et al. developed a scaffold-free model by introducing primary hepatic stellate cells (HSCs) as a physiologically relevant organotypic culture [Fig. 5(viii)].74 The cells synthesized physiological ECM within a specially designed tissue incubator device made using PDMS, which was later removed, enabling the construction of scaffold-free multiscale and hierarchical tissue structures. The formation of tissues showed close resemblance to natural hepatic morphogenesis. Nevertheless, these tissues lacked biliary structures which were essential for studying metabolic mechanisms.
V. APPLICATIONS OF LOCS AS MODELS OF LIVER FUNCTIONS
The translation of new drugs can often fail during clinical testing due to the absence of physiologically relevant and cost-effective models to predict drug toxicity and therapeutic effects. From data collected in the United States in 2014, the high failure rate of drug development at a striking 90% amounted to a cost of USD 2.59 billion,178 due to inadequate screening in preclinical trials.179 lOC could be a solution that mimics liver function to enable adequate preclinical testing of hepatic drugs. In this section, we surveyed recent advances in LOC designs for measuring drug metabolism and assessing nanotoxicity, and as pharmacokinetic models and multi-component sensing platforms for hepatic biomarkers.
A. Pathophysiological models
A number of LOCs were developed over the years as models to study liver pathophysiology. In one of the first models, Lee et al. designed a biomimicry microfluidic device that modeled hepatic sinusoids using primary rat and human hepatocytes.52 It could use 100 times less cells per experiment compared to macroscopic hepatic organoids and achieve improved hepatocyte viability over 7 days. Endothelial-like barriers were designed to reduce shear stress by preventing direct fluid flow to the hepatocytes [Fig. 6(i)(A)]. This device encouraged cell–cell interactions and allowed mass transport of liver sinusoids to be closely studied. This study guided the design of future devices.
FIG. 6.
(i) (a) SEM image depicting the microfluidic sinusoid unit. Scale bar represents 50 μm. Microfluidic culture of hepatocytes.52 (Copyright 2007 Wiley Periodicals, Inc.); (b) results of cell alignment and culture. Left, aligned cells in two lines similar to a hepatic cord. Bile canaliculi were formed randomly in the well plate.69 Reproduced with permission from Nakao et al., Biomicrofluidics 5(2), 022212 (2011). Copyright 2011 AIP Publishing LLC. (ii) Schematic presentation of the microfluidic device. (a) The microfluidic structure is embedded on the bottom of 364-well plates. (b) Each culture chamber has three lanes with an inlet and an outlet, respectively. (c) Culture model for HepG2 cells in an extracellular matrix separated from flow without any physical barrier using phaseguides. HepG2 cells have indirect contact to flow. Reproduced with permission from Jang et al., Biomicrofluidics 9(3), 034113 (2015). Copyright 2015 AIP Publishing LLC. (iii) Phase contrast micrographs of HepG2 cell growth inside the microfluidic sinusoid over a week in culture (Day 0, 1, 3, 5, and 7 are shown). Scalebar: 100 μm. On the right, fluorescence micrograph of live/dead assay performed at Day 8 (living cells in green, calcein dye; dead cells in red, EthD-1 dye; scalebar: 50 μm).99 Reproduced with permission from Gori et al., PLOS ONE 11(7), e0159729 (2016). Copyright 2016 PLOS ONE. (iv) Schematics of the NAFLD development process and gradient microfluidics with the corresponding concentrations.95 Reproduced with permission from Bulutoglu et al., Lab Chip 19(18), 3022–3031 (2019). Copyright 2019 the Royal Society of Chemistry. (v) Schematic of the liver-chip that recapitulates complex liver cytoarchitecture. Primary hepatocytes are grown in the upper parenchymal channel in ECM sandwich format, and non-parenchymal cells are grown on the opposite side of the same membrane in the lower vascular channel.101 Reproduced with permission from Jang et al., Sci. Transl. Med. 11(517), eaax5516 (2019). Copyright 2019 The American Association for the Advancement of Science. (vi) Representation of the HepG2-μTPs loading procedure and fluid dynamic simulation.96 (Copyright 2018 Wiley Periodicals, Inc.). (vii) 3D HepaTox Chip for the simultaneous administration of multiple drug concentrations. (a) Microfluidic design and assembly of the linear concentration gradient generator and multiplexed cell culture chip. (b) Magnified view of a single cell culture channel of the multiplexed cell culture chip. An array of 30 × 50 mm micropillars separated the channel into three compartments. (c) Characterization of the concentration gradient profile in the 3D HepaTox Chip coupled with a linear concentration gradient generator.187 Reproduced with permission from Toh et al., Lab Chip 9(14), 2026–2035 (2009). Copyright 2009 the Royal Society of Chemistry. (viii) Functional maintenance of rat hepatocytes cell line (H-4-II-E) at a density of 1 × 105 cells ml−1 on silicon microtrenches (11 mm in length × 10 mm in width) with different depths of 10 and 20 μm, with and without heparin coating, under static conditions. Representative bioartificial liver (bottom)97 (Copyright 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim). (ix) Schematic of the perfusion-incubator-liver-chip (PIC). (a) 3D view with the PIC. (b) Bottom view of the chip's layout illustrating the microfluidic circuit, the cell culture chamber, the bubble trap, and the heater. (c) Cross section of the PIC illustrating the structure of the bubble trap. It consists of a 70 μm-thick PDMS membrane (gas permeable) bonded to a PDMS molded chamber with pillars that support the membrane. (d) Top and bottom views of the PIC.110 Reproduced with permission from Yu et al., Sci. Rep. 7(1), 14528 (2017). Copyright 2017 Scientific Reports. (x) Vascular-liver PIC: (a) photo of the PIC, showing the glass–silicon chip, connectors, tubing, and media reservoirs and (b) cross-section schematic of the cell co-culture chamber. (c) Schematic of the bidirectional perfusion culture testing setup.109 Reproduced with permission from Yu et al., Biomicrofluidics 14(3), 034108 (2020).Copyright 2020 AIP Publishing LLC. (xi) Model of molecular interactions in SHEAR. Cytokine stimulation combined with flow and IL-1β stimulates endothelial cells to produce PGE2, which plays an important role in the signaling cascade that leads to primary human hepatocyte cell-cycle entry in SHEAR devices.57 Reproduced with permission from Chhabra et al., Proc. Natl. Acad. Sci. U.S.A. 119(28), e2115867119 (2022). Copyright 2022 PNAS.
Nakao et al. focused on mimicking the microscopic structure of hepatic cords.69 As freshly isolated human hepatocytes rapidly lose membrane polarity, they proposed a new technique for maintaining the cells based on Lee's design. The cell culturing channel was altered to become asymmetric and promoted the cells to self-organize into two lines [Fig. 6(i)(B)], creating a separate channel for bile secretion. This system was considered by the authors to have flow distribution similar to in vivo structures.
In an improved design by Jang et al., separation of cell culture area and perfusion flow was achieved without physical barriers using OrganoPlateTM, developed by MIMETAS and Leiden University [Fig. 6(ii)].100 This microfluidic device had 40 culture chambers, where each chamber consisted of three lanes separated by phaseguides, allowing the generation of continuous passive perfusion without the need for an extra pump or tubing. Improved function of HepG2 cells was observed in the microfluidic chip compared to static 2D and 3D cultures. Capillary force directed the flow of HepG2 and Matrigel mixture in the inner channel along the phaseguide, which ultimately formed hepatic spheroids. The system was capable of preserving cellular viability and integrity for at least 15 days, demonstrated by the constant low lactate dehydrogenase activity in the cells and substantial increase of albumin production over this time. The LOC showed higher sensitivity for measuring the toxicity of acetaminophen than the monolayer control.
Other than mimicking physiological liver structure, LOCs can be used to create hepatic disease models. A prime example is non-alcoholic fatty liver disease (NAFLD), affecting millions of people worldwide.180 This chronic disease can range from simple steatosis to non-alcoholic steatohepatitis, which may even progress to cirrhosis and eventually hepatocellular carcinoma (HCC).181 The underlying cellular and molecular mechanisms of NAFLD pathogenesis and progression remain largely debatable and having LOCs which provide a reproducible and accurate disease model would accelerate discoveries of potential treatments.
To this front, Gori et al. followed the work of Lee et al. and designed LOCs with an augmented cell culture chamber.99 HepG2/C3A cells (CRL-10741) were seeded, and subjected to fluid flow of 18 μl/day for 8 days through a gravity-operated mass transport channel [Fig. 6(iii)]. To induce steatosis, cells were treated with free fatty acids (FFAs), palmitic acid (PA; 16:0), and oleic acid (OA; 18:1 cis-9) in methanol. This model maintained higher hepatic cell viability and minimal oxidative stress compared to 2D static cultures. This was one of the first in vitro LOC models of human NAFLD in a sinusoid-like fashion.
To systematically study different microenvironmental conditions and their impacts on cell behavior in liver disease, single channeled LOCs presented above can become laborious and impractical. Bulutoglu et al. utilized a microfluidic gradient generator, shown in Fig. 6(iv), to create highly reproducible chemical gradients that allowed continuous media flow.95 In this model, Metabolic Patterning on a Chip (MPOC) platform with the ability to generate five defined gradients of FFA concentrations was used. The platform was capable of inducing oxygen deprivation in incremental changes. Using rat primary hepatocytes, the oxygen gradient was shown to impose the highest effect on fat storage at the lowest FFA concentration. This LOC was useful for simulating a spectrum of disease progression, as well as aiding in dissecting the effects of spatial heterogeneity, which previous models have not been able to achieve.
B. Models for studying drug metabolism and nanotoxicity
LOCs have been developed to mimic the metabolic functions of the liver, for instance, the two phases of drug metabolism.182 Phase I (non-synthetic) involves oxidation, reduction, hydrolysis, and hydroxylation, while Phase II (synthetic) is the conjugation with an endogenous ligand (e.g., glycine, glucuronide, glutathione, or sulfate).183 When a drug undergoes metabolism in the liver, it could experience one of the two phases or a combination of the two. Phases I and II are performed mainly by hepatocytes. Cytochrome P450 (CYP) family enzymes from phase I and transferases from phase II could transform the insoluble toxic substances from phase I into less toxic and soluble substances.184 Thus, the measurement of CYP could be a biomarker for high metabolic activity of hepatocytes. Long-term hepatic cell culture is an important tool for studying drug metabolism in vitro. The use of a microfluidics device or perfusion-based device can collect and recirculate hepatic biomarkers, which was impossible to achieve using conventional methods. LOC platforms can help sustain the phenotype of hepatocytes and liver-specific functions in long-term culture.130
The primary cause of drug attrition in preclinical testing is drug-induced liver injury. For this reason, it is essential to develop a reliable, accurate and reproducible in vitro hepatic platform for predicting drug toxicity. The current method of characterizing absorption, distribution, metabolism, excretion, and toxicity of new drugs relies on animal models, which often fail to predict human responses due to species-specific differences in drug metabolism pathways.185,186 As an example, a study led by the company Emulate [Fig. 6(v)]101 showed species-specific drug toxicity responses when modeled using a Liver-Chip, highlighting the need to use human primary cell lines in the model. The Emulate chip had an interesting design that could further benefit from incorporating organ-specific features. For instance, the membrane structure could have been replaced with other types of barrier structures, such as pillars, to encourage biliary structure formation. Another consideration was to vary the membrane pores sizes to simulate fenestrae structures as listed in Table III. Furthermore, an investigation of the changes in dimensionless numbers with different flow conditions and channel geometry designs could be beneficial to better understand the mechano-responses of liver cells in vitro.
In another study, Corrado et al. developed a LOC with HepG2 cell line that showed prolonged functional performance and capacity to be used as a predictive platform for studying the cytotoxic effects of xenobiotics and drugs [Fig. 6(vi)].96 They evaluated the distribution of oxygen under a flow rate of 5 μl/min in the chip, showing close to 100% of oxygen equilibrium concentration (0.22 mol/m3) in the first compartment with progressively lower percentage and the lowest at 55% (0.124 mol/m3) in the final compartment. As a proof-of-concept study, ethanol (100 and 300 mM) was used to study alcoholic liver injury for 2 or 4 days. Through damage quantification (albumin and urea decreased production), the system was shown to be suitable for sensing hepatotoxicity.
A microfluidic 3D hepatocyte chip (3D HepaTox chip) with a different design was used to recapitulate in vivo liver functions for in vitro assessment of drug toxicity [Fig. 6(vii)].187 Toh et al. designed this chip with eight parallel cell culture channels independently controlled by a concentration generator. Each cell culture channel was separated into three distinct compartments by an array of elliptical micropillars, with one central cell culture compartment and two side perfusion compartments. The HepaTox chip accurately predicted hepatotoxicity using five model drugs in a dose-dependent manner. The IC50 values obtained using this in vitro platform showed a positive correlation to in vivo experiments, suggesting its usefulness in drug testing and screening. However, the study also noted that the required volume of conventional assays for cytotoxicity testing exceeds the typical operating volume of the chip, and a highly sensitive micro-biosensor was needed to accurately reflect the IC50 value using this LOC.
In another interesting design, 3D heparin-coated microtrenches were produced to mimic the architecture of hepatic sinusoids [Fig. 6(viii)].97 This design recapitulated 3D microarchitecture, cord-like arrangement of hepatocytes, and physiological microcirculation, with an optimal microtrench of 20 μm depth. This model was able to maintain cell viability and hepatocyte function over 4 weeks, verified by measuring basal levels of cytochrome P450 activity. The model also produced IC50 values for the model drugs acetaminophen, tacrine, and chlorpromazine in agreement with LD50 values in vivo, suggesting its capability for evaluating liver response to hepatotoxins.
A typical problem faced by OOCs including LOCs is air bubble contaminants, which could cause detrimental effects once they burst. Although a bubble trap can prevent large bubbles from entering the culture chamber, the accumulation of tiny bubbles or the formation of dissociated gas is difficult to suppress. To overcome this problem, Yu et al. created a perfusion-incubator-liver-chip (PIC), which maintained hepatic cell viability over 3 weeks and their functions over 2 weeks [Fig. 6(ix)].110 The design criteria for this chip to be used in chronic liver toxicity testing were: organotypic 3D cellular architecture, good mass transfer, maintenance of mechanical forces and limited shear stress to the cells, stable cell culture conditions, and ease of handling cells and replenishing media. A hydrophilic surface of the microfluidic elements prevented rapid adsorption of proteins and drug molecules. The use of silicon structures with good thermal conductivity helped to maintain uniform media temperature. The PIC operated at an optimal flow rate of 0.1 ml/h, and showed higher sensitivity in evaluating chronic drug response to repeated dosing of diclofenac and acetaminophen compared to the static culture control.
To further improve the performance of the PIC, Yu et al. recirculated the culture media in a follow-up study to better predict drug toxicity due to the accumulation of metabolites in the solution [Fig. 6(x)].109 The PIC was a parallel culturing chamber where the top had human pluripotent stem cells (hPSC)-derived vascular cells, and the bottom hPSC-derived hepatocyte spheroids. Better attachment of hPSC-derived endothelial cells was achieved by modifying the PDMS surface using 3,4-dihydroxy-l-phenylalanine (DOPA). This recirculating perfusion chip co-culturing system demonstrated superior performance over the conventional static culture platform.
LOCs have also found interesting applications in nanotoxicity evaluation as another type of metabolic study. The increasing use of nanomedicine therapeutics, such as mRNA vaccines, can undergo different routes of clearance depending on their (organic or inorganic) compositions.188 Synthetic nanomaterials might introduce nanotoxicity during their clearance and biodegradation by the body, largely through the liver. For instance, nanoparticles (NPs) with a hydrodynamic diameter (HD) less than 5 nm are found to result in rapid renal clearance, but the same clearance mechanism is prohibited with NPs over 15 nm in HD.189 New nanomedicine therapeutics have faced barriers to translation partly due to the potential of nanomaterials to trigger immune responses or accumulate in the mononuclear phagocyte system.35–190 Currently, our knowledge of the long-term toxicity responses of new nanomaterials remain limited, giving rise to a growing concern about the health risks of using nanotherapeutics or medical implants that produce nanoparticles from wearing. Animal models do not always provide accurate or reliable prediction of human physiological response in the assessment of nanotoxicity due to inter-species differences.22–191 lOCs could provide a solution to fill this gap, which can be constructed with human cells for screening the safety of nanomedicine, and provide physiologically relevant data to predict in vivo behavior of nanomaterials as well as associated biological response.
In a recent study, Li et al. developed a 3D hepatocyte chip that recapitulated key physiological responses in the hepatotoxicity response of superparamagnetic iron oxide nanoparticles (SPION).29 Rat hepatocytes were freshly isolated and used for tissue culture with collagen gel. Three-day (short-term) and one-week (long-term) liver-specific functions were evaluated in the presence of different doses of SPION. Analyses performed using the LOC suggested that cumulative exposure to nanoparticles resulted in more dramatic hepatocyte damage. Surface patterning is another technique that can be used to maintain long-term cell culture.192 To evaluate the safety of silver nanoparticles (AgNPs), Liu et al. developed a LOC culturing system with patterned electrospun poly-dl-lactide fibers.193 They investigated the impacts of flow rates on hepatocyte viability and found the optimal flow rate to be 10 μl/min, which was able to maintain cells for up to 15 days. Primary rat hepatocytes were cultured in the perfusion system and exposed to 120 μg/ml AgNPs for 24 h. The damage induced by AgNPs was assessed by measuring the activity of lactate dehydrogenase. The LOC system showed a higher sensitivity for measuring AgNPs induced toxicity compared to cells grown under static conditions. After treatment with AgNPs, 50% of cells died after 24 h, which was in agreement with the findings of Faedmaleki et al., who reported an AgNPs IC50 value of 121.7 μg/ml in primary rat hepatocytes.194 In these studies, LOCs provided a robust platform for investigating the hepatotoxicity profiles of nanomaterials, which could provide important data for their translation into clinical use.
C. Pharmacokinetic models
In recent developments, LOCs have been integrated with other OOC systems (e.g., gut, lung, kidney) to form microphysiological systems (MPS) for quantitatively analyzing and predicting human drug pharmacokinetics (PK) in vitro, specifically drug absorption, distribution, metabolism, and excretion (ADME). Compared to traditional animal models and cell cultures that often fail to correctly predict drug toxicity and efficacy due to inter-species differences,195,196 MPS offers human multi-organ models with greater physiological relevance, yielding results that better match with human clinical data.57–200 A multi-species (rat, dog, and human) LOC system has also been reported, where Jang et al. incorporated hepatocytes, sinusoidal endothelial cells, Kupffer cells, and hepatic stellate cells for predicting species-specific drug metabolism and toxicity.101
A recent study has found that the quantitative prediction of PK parameters for oral nicotine can be achieved by computational simulation of fluidically coupled gut, liver, and kidney organ chips with an arteriovenous reservoir for drug mixing.198 The whole system is modeled as a set of ordinary differential equations (ODEs) that describe drug transport across different compartments. The general transport equation for the drug is defined as
| (2) |
where C, D, v, and S denote the drug concentration, diffusion coefficient, fluid velocity, and the source term, respectively. Equation (2) is further derived into a set of fluxes equations for each model compartment,
| (3) |
| (4) |
| (5) |
where the subscripts B, M, and A refer to the basal channel, membrane, and apical channel, respectively. J represents the transmembrane diffusive fluxes and Q is the flow rate in the microchannels. The predicted maximum concentration of nicotine (0.050 μM) showed a close match with the clinically measured value in patient blood (0.052 μM). Moreover, this model showed a better fit to human PK parameters than ones measured from rodents.
In addition, a multi-organ model has been proposed that integrates seven organ-chips, including the brain, heart, pancreas, liver, lung, gut, and endometrium to study the PK parameters of diclofenac.199 The predicted drug concentration using this model agreed with experimentally measured values. The reader is referred to a review on more multi-organ chip studies for PK of certain drugs.201
D. Multi-component sensing platforms
LOCs can be used in conjunction with sensors for real-time monitoring and analysis of in vitro hepatic responses. In one of the first studies in this area, Schober et al. incorporated biosensors in conjunction with LOC to study in vitro cell culture.202 AlGaN/GaN nanosensors were used due to their high sensitivity, chemical stability, biocompatibility, and label-free detection. Albumin was used as the metabolic marker to compare the in vitro cultures and their in vivo counterparts. More recently, Zhang et al. constructed a platform for accurate drug screening using LOCs, seeded with Hep-G2 (liver cancer cells).203 They incorporated a real-time monitoring system consisting of electrochemical impedance and near-infrared spectroscopy measurements over five days, which provided quick sensing of liver state turnover. The system maintained 100% viability of hepatocytes over seven days, and allowed continuous real-time sensing of responses to paracetamol during drug resistance testing. More sophisticated designs have been reported,204–206 and practical guides on fabricating integrated sensors for LOCs are captured in a recent protocol.207
Oxygen concentration in LOCs is a critical parameter that should be monitored in most applications, important for regulating cellular behavior and influencing cell differentiation and function.24–210 However, the enclosed nature of microfluidic chips makes monitoring oxygen concentration in the culture media difficult. Although open microfluidics can provide easy access to cultured cells, it also requires precise and reliable liquid pumping equipment.208 Changes in oxygen tension above (hyperoxia) or below (hypoxia) the physiological level, or complete absence (anoxia) need to be closely monitored and controlled. Moya et al. presented an integrated amperometric oxygen sensor in a LOC system using an ultrathin porous cell culture membrane, achieving in situ real-time oxygen monitoring of the cell culture chamber [Fig. 7(i)].107 As proof of concept, three electrochemical dissolved oxygen (DO) sensors were integrated with the porous membrane of the LOC device (ExoLiver). In cultures of primary rat and human hepatocytes, the DO sensors could detect an oxygen gradient up to 17.5% and 32.5% for respective cell populations. This study also demonstrated the feasibility of inkjet printing in sensor fabrication for use with LOCs, which could be extended for applications in other OOCs.
FIG. 7.
(i) (a) Schematic of the OOC system with modifications to incorporate the control with external elements and the spring load for the connectors on the top plate, (b) cross section of the bioreactor showing the position of the three DO printed sensors, and (c) real picture of the ExoLiver system with all fluidic and electrical connections.107 Reproduced with permission from Moya et al., Lab Chip 18(14), 2023–2035 (2018). Copyright 2018 the Royal Society of Chemistry. (ii) (a) Measurement setup with microsensor device placed in the 96-well cell culture plate. (b) Close-up scheme of microsensor tip with electrode layout.208 Reproduced with permission from Weltin et al., Biosens. Bioelectron. 87, 941–948 (2017). Copyright 2016 Elsevier. (iii) Design, fabrication, and control of the automated microfluidic EC biosensor. (a) Photograph of the lectrochemical (EC) microfluidic chip bonded with a microelectrode. (b) Labeling of the microfluidic channels and the valves with corresponding flowing solutions for fully automated biosensing measurements. (c) Three-layered microfluidic chip consisting of the microfluidic channel, a thin membrane, and the valve channel layer. (d) Schematic of the microfluidic EC biosensing system integrated with organ-on-a-chip for continual monitoring of a target biomarker in an automated manner. (e) A schematic illustration for immobilization of antibodies using streptavidin on the surface of the microelectrodes.209 Reproduced with permission from Shin et al., Adv. Sci. (Weinh) 4(5), 1600522 (2017). Copyright 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (iv) (a) Design of reconfigurable microfluidic co-culture system. (b) A typical microfluidic device used in a co-culture experiment. The wall separating microchannels was lowered to demonstrate the food dye did not mix. (c) Principle of TGF-β detection. The binding of cytokine molecules causes aptamer molecules to change conformation.8 Reproduced with permission from Zhou et al., Lab Chip 15(23), 4467–4478 (2015). Copyright 2015 the Royal Society of Chemistry.
Other metabolic biomarkers can be important to characterize using LOCs for mechanistic studies. For instance, there is a strong need for high-resolution real-time analysis of biochemical and metabolic activities of living cells in LOCs. Weltin et al. demonstrated the use of an electrochemical microsensor that achieved label-free, in situ and continuous measurement of lactate and oxygen levels from single human HepaRG (HPR116) hepatocyte spheroids [Fig. 7(ii)].208 The lactate sensor was immobilized with lactate oxidase in a hydrogel matrix and showed a sensitivity up to 134 nA/mm2. This design sustained up to 70 h of continuous metabolite monitoring. To further test the sensor performance, cells were exposed to antimycin A, which suppressed the aerobic metabolic pathway, and Bosentan, a dual endothelin receptor antagonist that could induce liver injury. Measurements showed clear dose-dependent metabolic behavior, confirming the ability of this LOC system for continuous monitoring of hepatocyte metabolism and toxicological applications.
In a similar approach, Shin et al. focused on monitoring albumin and GTS- using electrochemical sensors [Fig. 7(iii)].209 They developed on-chip valves to manipulate the fluid flow, and the ability to perform repeated measurements and surface cleaning cycles in an automated fashion using a WAGO controller. The metabolic activity of primary human hepatocyte spheroids was measured for up to 7 days. The limit of detection was 0.09 ng/ml for albumin and of 50 ng/ml for GTF- . This could be a unique universal strategy for constructing automated microfluidic-based sensors for continuous monitoring of drug toxicity using OOCs.
Liver injury is a complicated process that involves secreted signaling molecules, such as cytokines and growth factors, which could be impractical to measure in co-cultured cells grown in conventional cell culture. LOCs can be designed to have actuating compartments for studying individual cell-secreted signaling molecules. Zhou et al. recently presented reconfigurable microfluidics integrated with a miniaturized aptamer-based biosensor [Fig. 7(iv)].8 The LOC contained a pneumatic actuatable wall, which could be raised or lowered to create different device configurations. The reconfigurable compartments were used to co-culture primary rat hepatocytes with human stellate cells (LX2). This model revealed that alcohol injury would cause hepatocytes to secrete FGF-β molecules and trigger neighboring stellate cells to release more. Similar designs could be useful for studying other hepatic paracrine signals.
VI. SUMMARY AND FUTURE PERSPECTIVES
LOCs have provided new means for studying liver physiology, disease progression, and associated mechanisms, as well as been used in a variety of applications related to drug screening. With continuous developments in nanomedicine, LOCs are gaining increasing value as platforms for nanotoxicity studies to advance the translation of new nanotherapeutics. By providing a more physiologically relevant and cost-effective in vitro model of liver responses, LOCs may accelerate the progression of policymaking and approval of nanomedicine strategies.211 Multi-chip LOC systems could further accelerate drug development and provide insights to a multi-organ or cross-species response, bringing immense economic benefits. It was estimated that the utility of OOCs, and especially LOCs in pharmaceutical industries and preclinical development could lead to a 26% reduction in research and development cost for new medical therapeutics.212 Furthermore, OOC technology in general offers the hope to replace, reduce, and refine (the “3Rs”) the use of animal models in research, which is particularly important for LOCs since the majority of studies relating to the liver are still performed using animal models. Nevertheless, many gaps and challenges remain.5–213 Here, we provide further insights into potential future developments in LOCs for their broader applications.
A. Fit-for-purpose LOC model
Although multi-organ chips have been used in preclinical studies of drug development to test toxicity and PK in vitro (discussed in Sec. V E), one of their primary disadvantages is that such models need to be tuned to suit specific target drugs or species. This is time consuming and not cost-effective but can be potentially overcome by developing a unified OOC system that could be implemented in screening different drugs without redefining the overall modeling of the system.105–214
Li et al. developed an adaptable model of the liver acinus using a glass-based microfluidic device, which could be customized by tuning the shear stress and flow rate to optimize liver zonation.105 Other efforts have been taken to mimic the architectural features of the liver organization and provide adaptable models that could potentially be incorporated into multi-organ chip systems.215,216 However, further work is required to fully deliver a model that could accurately depict the portal triad, central vein, sinusoid, and bile canaliculi.
B. Recapitulation of liver heterogeneity
Zonal differences exhibit clinical significance in drug-induced liver injury.217 Thus, the ability to recapitulate hepatic zonal physiology is critical for predicting drug toxicity using LOCs. Current models have not been able to fully capture complex and multifaceted hepatic physiology, especially in long-term culture. Drug-induced hepatotoxicity and zonal effects need to be studied using improved LOCs, for instance, using a modular system that couples biochemical and biophysical cues, and incorporates the essential roles of mechanical forces in cellular development and signal transduction.218
C. Mechanistic study of liver regeneration
The liver is unique as it is the only organ in the human body that has a high regenerative capacity and still performs complex functions. Loss of liver mass will trigger liver regeneration, which occurs in all vertebrate organisms from fish to mammals.219 Stable liver function is critical for optimal brain function, otherwise leading to chronic “hepatic encephalopathy” and eventual coma.220 The liver also dynamically responds to changes in physiological conditions by changing its size.221 For example, the liver increases in size during pregnancy and decreases in size from severe loss in body weight.
To understand liver regeneration and these complex physiological processes using an in vitro model is inarguably difficult. Recently, Chhabra et al. designed LOCs which successfully mimicked hepatocyte regeneration, using a microfluidic model named structurally vascularized hepatic ensembles for analyzing regeneration (SHEAR).57 SHEAR captured the flow-dependent paracrine aspects of human liver regeneration by linking a parenchymal compartment and a biomaterial lumen [Fig. 6(xi)]. The parenchymal compartment consisted of 3D spheroids composed of primary human hepatocytes and human dermal fibroblasts, while the lumen of the channel was embedded within an extracellular matrix and lined with human endothelial cells. Using this model, they identified IL-1β as a key molecule that could be responsible for initiating hepatocyte proliferation.
A few improvements may be made to the above model to more accurately capture physiological liver regeneration, such as (i) using natural ECM-derived biomaterials that better replicate liver physiological conditions; (ii) investigating the effects of fluid flow on various pathways implicated in liver regeneration, as well as pathological changes involved in cirrhosis, and (iii) adding physical perturbation (e.g., heat) or hepatotoxins to the model for studying liver regeneration in response to injury.
D. Real-time biosensing
OOC devices have been coupled with a variety of biosensing approaches to track the temperature, pH, cell metabolism, and responses to the external environment. Apart from recent developments in LOC-integrated biosensing platforms discussed in Sec. V E, LOCs could also be used in multi-organ sensing systems for studying liver injury induced by factors such as environmental pollution or pesticides.222,223 Additionally, OOC sensing systems have been used to perform long-term (28 days) monitoring of different cell functions.224 A future goals for LOC sensing platforms would be to integrate multi-sensors with multi-organ chips to create a unified system for detecting various parameters such as metabolic indicators (e.g., oxygen concentration), physical activities (e.g., cardiac beating rates), and biomarkers, for in vitro drug screening and disease modeling. Since different types of sensors (electrical, electrochemical, and optical) are needed for monitoring different biochemical activities, the main challenge of such a system would be the miniaturization and integration of various biosensors into a single platform with minimal interference among them. The complexity of output data from a multi-sensor system could create obstacles in design, which might benefit from machine learning algorithms for data analysis.225–227
E. Human-on-a-chip systems
Human-on-a-chip refers to the total mimicry of human physiochemical responses using OOC technology, often considered the ultimate in vitro model for drug screening and disease studies.228,229 The human body is intricate and functions through closely interconnected organ systems, with many organ functions being highly dependent on each other. Studying individual organs using in vitro models will not provide the same physiological relevance as a systematic model, which is the greatest shortcoming for OOC devices modeling specific organs compared to animal models. By incorporating LOC devices with other organs of interest, such as gut,230 skin,231 cardiac-muscle-neuronal,232 pancreas,233,234 lung,24 and kidney,235 human disease models could be more readily recapitulated through in vitro systems to produce highly relevant physiological data. The main advantage of connecting these MPS is that complicated modeling of drug absorption, distribution, metabolism and excretion (ADME) and physiological-based pharmacokinetics could be achieved,236 ultimately serving as a predictive tool for drug development.237 A prime example is the study by Sung and Shuler,238 where a LOC device was fabricated to mimic multi-organ interactions by co-culturing liver cells (HepG2/C3A), tumor cells (HCT-116), and bone marrow cells (Kasumi-1) in separate chambers. The pharmacokinetic and pharmacodynamic profiles of the anticancer drug, Tegafur, were analyzed. This system was able to metabolize tegafur into its active component which led to cell death, proving the physiological relevance of such MPS platforms.
LOC devices may perform more accurately than animal models in toxicity assessment in the early stages of drug development, but it is currently impossible to entirely replace animal testing using isolated LOCs or those integrated into more complex multi-organ or human-on-a-chip systems. Nevertheless, LOCs have already exhibited potential to dramatically reduce the number of animals used in preclinical testing, particularly for drug toxicity assessment and preclinical screening.200–239
F. Numerical simulation and machine learning-aided design
In silico modeling of microfluidics devices by COMSOL has been widely used in designing microchannel geometries and investigating fluid behavior for achieving optimal performance.240 Computational simulation can reduce the design cost and shorten the design cycle by virtually testing the system with a combination of parameters before actual fabrication.241,242 In OOC devices, COMSOL has been used to analyze the impact of changes in microchannel dimensions and flow rate on the wall shear stress and fluid velocity, which may impact cell responses.240 This software can be similarly utilized to quantitatively monitor the metabolic function of cells within LOC devices.243 Building the 3D model in COMSOL and tuning the parameters to achieve the desired outcome can complement or validate the experimentally-obtained data.
Machine learning has taken off tremendously in recent years due to rapid advances in computing power, which could aid in creating intelligent structural designs of microfluidic chips.244 For instance, machine learning could be used to validate microfluidic designs prior to fabrication, or used to calculate the required flow speed for the media to achieve physiologically relevant experimental conditions.245–247 The adoption of machine learning into OOC design requires data training and collaboration across multiple fields, which hopefully in the future will inform desired model chip parameters such as the number of cells per channel, shear force on cells, cell density, and cell type.248,249 Adapting existing algorithms to produce intelligent designs for OOCs or specifically LOCs could be an incredibly cost-effective approach to generate physiologically relevant models of organ function.250 This could be an emerging strategy to create new LOC devices that may also contribute to the development of automated human-on-a-chip, enhancing the throughput of future drug development, disease studies, and personalized medicine at a low cost.
AUTHOR DECLARATIONS
Conflict of Interest
The authors have no conflicts to disclose.
Author Contributions
Zhenxu Yang: Conceptualization (equal); Formal analysis (equal); Methodology (equal); Project administration (equal); Validation (equal); Writing – original draft (lead); Writing – review & editing (equal). Xiaochen Liu: Conceptualization (supporting); Formal analysis (equal); Project administration (equal); Resources (supporting); Supervision (supporting); Validation (equal); Writing – original draft (equal); Writing – review & editing (equal). Elise M. Cribbin: Formal analysis (supporting); Investigation (supporting); Validation (supporting); Writing – review & editing (supporting). Alice M. Kim: Formal analysis (supporting); Investigation (supporting); Validation (supporting); Writing – review & editing (supporting). Jiao Jiao Li: Conceptualization (supporting); Formal analysis (equal); Investigation (equal); Project administration (equal); Supervision (equal); Validation (equal); Writing – review & editing (equal). Ken-Tye Yong: Conceptualization (equal); Formal analysis (equal); Funding acquisition (equal); Project administration (equal); Resources (lead); Supervision (equal); Validation (equal); Visualization (equal); Writing – review & editing (equal).
DATA AVAILABILITY
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1.Breslin S. and O’Driscoll L., Drug Discovery Today 18(5), 240–249 (2013). 10.1016/j.drudis.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 2.Godoy P., Hewitt N. J., Albrecht U., Andersen M. E., Ansari N., Bhattacharya S., Bode J. G., Bolleyn J., Borner C., Böttger J., Braeuning A., Budinsky R. A., Burkhardt B., Cameron N. R., Camussi G., Cho C. S., Choi Y. J., Craig Rowlands J., Dahmen U., Damm G., Dirsch O., Donato M. T., Dong J., Dooley S., Drasdo D., Eakins R., Ferreira K. S., Fonsato V., Fraczek J., Gebhardt R., Gibson A., Glanemann M., Goldring C. E. P., Gómez-Lechón M. J., Groothuis G. M. M., Gustavsson L., Guyot C., Hallifax D., Hammad S., Hayward A., Häussinger D., Hellerbrand C., Hewitt P., Hoehme S., Holzhütter H. G., Houston J. B., Hrach J., Ito K., Jaeschke H., Keitel V., Kelm J. M., Kevin Park B., Kordes C., Kullak-Ublick G. A., Lecluyse E. L., Lu P., Luebke-Wheeler J., Lutz A., Maltman D. J., Matz-Soja M., McMullen P., Merfort I., Messner S., Meyer C., Mwinyi J., Naisbitt D. J., Nussler A. K., Olinga P., Pampaloni F., Pi J., Pluta L., Przyborski S. A., Ramachandran A., Rogiers V., Rowe C., Schelcher C., Schmich K., Schwarz M., Singh B., Stelzer E. H. K., Stieger B., Stöber R., Sugiyama Y., Tetta C., Thasler W. E., Vanhaecke T., Vinken M., Weiss T. S., Widera A., Woods C. G., Xu J. J., Yarborough K. M., and Hengstler J. G., Arch. Toxicol. 87, 1315–1530 (2013). 10.1007/s00204-013-1078-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jensen C. and Teng Y., Front. Mol. Biosci. 7, 33 (2020). 10.3389/fmolb.2020.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapałczyńska M., Kolenda T., Przybyła W., Zajączkowska M., Teresiak A., Filas V., Ibbs M., Bliźniak R., Łuczewski Ł, and Lamperska K., Arch. Med. Sci. 14(4), 910–919 (2018). 10.5114/aoms.2016.63743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma C., Peng Y., Li H., and Chen W., Trends Pharmacol. Sci. 42(2), 119–133 (2021). 10.1016/j.tips.2020.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bale S. S., Vernetti L., Senutovitch N., Jindal R., Hegde M., Gough A., McCarty W. J., Bakan A., Bhushan A., Shun T. Y., Golberg I., DeBiasio R., Usta O. B., Taylor D. L., and Yarmush M. L., Exp. Biol. Med. 239(9), 1180–1191 (2014). 10.1177/1535370214531872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi J. H., Loarca L., De Hoyos-Vega J. M., Dadgar N., Loutherback K., Shah V. H., Stybayeva G., and Revzin A., Am. J. Physiol.-Cell. Physiol. 319(3), C552–C560 (2020). 10.1152/ajpcell.00094.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Q., Patel D., Kwa T., Haque A., Matharu Z., Stybayeva G., Gao Y., Diehl A. M., and Revzin A., Lab Chip 15(23), 4467–4478 (2015). 10.1039/C5LC00874C [DOI] [PubMed] [Google Scholar]
- 9.Huh D., Matthews B. D., Mammoto A., Montoya-Zavala M., Hsin H. Y., and Ingber D. E., Science 328(5986), 1662–1668 (2010). 10.1126/science.1188302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasendra M., Tovaglieri A., Sontheimer-Phelps A., Jalili-Firoozinezhad S., Bein A., Chalkiadaki A., Scholl W., Zhang C., Rickner H., Richmond C. A., Li H., Breault D. T., and Ingber D. E., Sci. Rep. 8(1), 2871 (2018). 10.1038/s41598-018-21201-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bein A., Shin W., Jalili-Firoozinezhad S., Park M. H., Sontheimer-Phelps A., Tovaglieri A., Chalkiadaki A., Kim H. J., and Ingber D. E., Cell. Mol. Gastroenterol. Hepatol. 5(4), 659–668 (2018). 10.1016/j.jcmgh.2017.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mofazzal Jahromi M. A., Abdoli A., Rahmanian M., Bardania H., Bayandori M., Moosavi Basri S. M., Kalbasi A., Aref A. R., Karimi M., and Hamblin M. R., Mol. Neurobiol. 56(12), 8489–8512 (2019). 10.1007/s12035-019-01653-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhalerao A., Sivandzade F., Archie S. R., Chowdhury E. A., Noorani B., and Cucullo L., Fluids Barriers CNS 17(1), 22 (2020). 10.1186/s12987-020-00183-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park T.-E., Mustafaoglu N., Herland A., Hasselkus R., Mannix R., FitzGerald E. A., Prantil-Baun R., Watters A., Henry O., Benz M., Sanchez H., McCrea H. J., Goumnerova L. C., Song H. W., Palecek S. P., Shusta E., and Ingber D. E., Nat. Commun. 10(1), 2621 (2019). 10.1038/s41467-019-10588-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herland A., van der Meer A. D., FitzGerald E. A., Park T.-E., Sleeboom J. J. F., and Ingber D. E., PLoS One 11(3), e0150360 (2016). 10.1371/journal.pone.0150360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skardal A., Murphy S. V., Devarasetty M., Mead I., Kang H.-W., Seol Y.-J., Shrike Zhang Y., Shin S.-R., Zhao L., Aleman J., Hall A. R., Shupe T. D., Kleensang A., Dokmeci M. R., Jin Lee S., Jackson J. D., Yoo J. J., Hartung T., Khademhosseini A., Soker S., Bishop C. E., and Atala A., Sci. Rep. 7(1), 8837 (2017). 10.1038/s41598-017-08879-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maoz B. M., Herland A., FitzGerald E. A., Grevesse T., Vidoudez C., Pacheco A. R., Sheehy S. P., Park T. E., Dauth S., Mannix R., Budnik N., Shores K., Cho A., Nawroth J. C., Segrè D., Budnik B., Ingber D. E., and Parker K. K., Nat. Biotechnol. 36(9), 865–874 (2018). 10.1038/nbt.4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caplin J. D., Granados N. G., James M. R., Montazami R., and Hashemi N., Advanced Healthcare Materials (Wiley-VCH Verlag, 2015), Vol. 4, pp. 1426–1450. [DOI] [PubMed] [Google Scholar]
- 19.Saleh A., DeBiasio R., Wilschut K., Vernetti L., Taylor D. L., Vulto P., Gough A., and Sharma A., Drug Metab. Pharmacokinet. 34(1), S50 (2019). 10.1016/j.dmpk.2018.09.178 [DOI] [Google Scholar]
- 20.Zakharyants A. A., Burmistrova O. A., and Poloznikov A. A., Bull. Exp. Biol. Med. 162(4), 515–519 (2017). 10.1007/s10517-017-3651-z [DOI] [PubMed] [Google Scholar]
- 21.Hingorani A. D., Kuan V., Finan C., Kruger F. A., Gaulton A., Chopade S., Sofat R., MacAllister R. J., Overington J. P., Hemingway H., Denaxas S., Prieto D., and Casas J. P., Sci. Rep. 9(1), 18911 (2019). 10.1038/s41598-019-54849-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bracken M. B., J. R. Soc. Med. 102(3), 120–122 (2009). 10.1258/jrsm.2008.08k033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilkinson J. M., Organ-on-a-Chip (Elsevier, 2020), pp. 1–11. [Google Scholar]
- 24.Bovard D., Sandoz A., Luettich K., Frentzel S., Iskandar A., Marescotti D., Trivedi K., Guedj E., Dutertre Q., Peitsch M. C., and Hoeng J., Lab Chip 18(24), 3814–3829 (2018). 10.1039/C8LC01029C [DOI] [PubMed] [Google Scholar]
- 25.Deng J., Chen Z., Zhang X., Luo Y., Wu Z., Lu Y., Liu T., Zhao W., and Lin B., Biomed. Microdevices 21(3), 57 (2019). 10.1007/s10544-019-0414-9 [DOI] [PubMed] [Google Scholar]
- 26.Deng J., Wei W., Chen Z., Lin B., Zhao W., Luo Y., and Zhang X., Micromachines (Basel) 10(10), 676 (2019). 10.3390/mi10100676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes D. J., Kostrzewski T., and Sceats E. L., Experimental Biology and Medicine (SAGE Publications Inc., 2017), Vol. 242, pp. 1593–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh K. K., Patenting Nanomedicines: Legal Aspects, Intellectual Property and Grant Opportunities (Springer-Verlag, Berlin, 2012), pp. 401–434. [Google Scholar]
- 29.Li L., Gokduman K., Gokaltun A., Yarmush M. L., and Usta O. B., Nanomedicine 14(16), 2209–2226 (2019). 10.2217/nnm-2019-0086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mvango S., Matshe W. M. R., Balogun A. O., Pilcher L. A., and Balogun M. O., Pharm. Res. 35(12), 237 (2018). 10.1007/s11095-018-2517-z [DOI] [PubMed] [Google Scholar]
- 31.Rahman K., Khan S. U., Fahad S., Chang M. X., Abbas A., Khan W. U., Rahman L., Ul Haq Z., Nabi G., and Khan D., Int. J. Nanomed. 14, 1401–1410 (2019). 10.2147/IJN.S190692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soares S., Sousa J., Pais A., and Vitorino C., Front. Chem. 6, 360 (2018). 10.3389/fchem.2018.00360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tenchov R., Bird R., Curtze A. E., and Zhou Q., ACS Nano 15(11), 16982–17015 (2021). 10.1021/acsnano.1c04996 [DOI] [PubMed] [Google Scholar]
- 34.Anselmo A. C. and Mitragotri S., Bioeng. Transl. Med. 4(3), e10143 (2019). 10.1002/btm2.10143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ventola C. L., Pharm. Ther. 42(12), 742–755 (2017). [PMC free article] [PubMed] [Google Scholar]
- 36.Halwani A. A., Pharmaceutics 14(1), 106 (2022). 10.3390/pharmaceutics14010106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Underhill G. H. and Khetani S. R., Cell. Mol. Gastroenterol. Hepatol. 5(3), 426–439.e1 (2018). 10.1016/j.jcmgh.2017.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fowler S., Chen W. L. K., Duignan D. B., Gupta A., Hariparsad N., Kenny J. R., Lai W. G., Liras J., Phillips J. A., and Gan J., Lab Chip 20(3), 446–467 (2020). 10.1039/C9LC00857H [DOI] [PubMed] [Google Scholar]
- 39.Leung C. M., de Haan P., Ronaldson-Bouchard K., Kim G.-A., Ko J., Rho H. S., Chen Z., Habibovic P., Jeon N. L., Takayama S., Shuler M. L., Vunjak-Novakovic G., Frey O., Verpoorte E., and Toh Y.-C., Nat. Rev. Methods Primers 2(1), 33 (2022). 10.1038/s43586-022-00118-6 [DOI] [Google Scholar]
- 40.Engler A. J., Sen S., Sweeney H. L., and Discher D. E., Cell 126(4), 677–689 (2006). 10.1016/j.cell.2006.06.044 [DOI] [PubMed] [Google Scholar]
- 41.Gerardo H., Lima A., Carvalho J., Ramos J. R. D., Couceiro S., Travasso R. D. M., Pires das Neves R., and Grãos M., Sci. Rep. 9(1), 9086 (2019). 10.1038/s41598-019-45352-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friedrich J., Ebner R., and Kunz-Schughart L. A., Int. J. Radiat. Biol. 83(11–12), 849–871 (2007). 10.1080/09553000701727531 [DOI] [PubMed] [Google Scholar]
- 43.Haycock J. W., Methods in Molecular Biology (Humana Press, Clifton, NJ, 2011), Vol. 695, pp. 1–15. [DOI] [PubMed] [Google Scholar]
- 44.Chan B. P. and Leong K. W., Eur. Spine J. 17(Suppl 4), 467–479 (2008). 10.1007/s00586-008-0745-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y., Wu D., Zhao X., Pakvasa M., Tucker A. B., Luo H., Qin K. H., Hu D. A., Wang E. J., Li A. J., Zhang M., Mao Y., Sabharwal M., He F., Niu C., Wang H., Huang L., Shi D., Liu Q., Ni N., Fu K., Chen C., Wagstaff W., Reid R. R., Athiviraham A., Ho S., Lee M. J., Hynes K., Strelzow J., He T. C., and El Dafrawy M., Front. Bioeng. Biotechnol. 8, 598607 (2020). 10.3389/fbioe.2020.598607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edmondson R., Broglie J. J., Adcock A. F., and Yang L., Assay Drug Dev. Technol. 12(4), 207–218 (2014). 10.1089/adt.2014.573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kozyra M., Johansson I., Nordling Å, Ullah S., Lauschke V. M., and Ingelman-Sundberg M., Sci. Rep. 8(1), 14297 (2018). 10.1038/s41598-018-32722-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guillouzo A., Environ. Health Perspect. 106(Suppl 2), 511–532 (1998). 10.1289/ehp.98106511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shulman M. and Nahmias Y., in Epithelial Cell Culture Protocols, 2nd ed., edited by Randell S. H. and Fulcher M. L. (Humana Press, Totowa, NJ, 2013), pp. 287–302. [Google Scholar]
- 50.Godoy P., Hewitt N. J., Albrecht U., Andersen M. E., Ansari N., Bhattacharya S., Bode J. G., Bolleyn J., Borner C., Böttger J., Braeuning A., Budinsky R. A., Burkhardt B., Cameron N. R., Camussi G., Cho C. S., Choi Y. J., Craig Rowlands J., Dahmen U., Damm G., Dirsch O., Donato M. T., Dong J., Dooley S., Drasdo D., Eakins R., Ferreira K. S., Fonsato V., Fraczek J., Gebhardt R., Gibson A., Glanemann M., Goldring C. E., Gómez-Lechón M. J., Groothuis G. M., Gustavsson L., Guyot C., Hallifax D., Hammad S., Hayward A., Häussinger D., Hellerbrand C., Hewitt P., Hoehme S., Holzhütter H. G., Houston J. B., Hrach J., Ito K., Jaeschke H., Keitel V., Kelm J. M., Kevin Park B., Kordes C., Kullak-Ublick G. A., LeCluyse E. L., Lu P., Luebke-Wheeler J., Lutz A., Maltman D. J., Matz-Soja M., McMullen P., Merfort I., Messner S., Meyer C., Mwinyi J., Naisbitt D. J., Nussler A. K., Olinga P., Pampaloni F., Pi J., Pluta L., Przyborski S. A., Ramachandran A., Rogiers V., Rowe C., Schelcher C., Schmich K., Schwarz M., Singh B., Stelzer E. H., Stieger B., Stöber R., Sugiyama Y., Tetta C., Thasler W. E., Vanhaecke T., Vinken M., Weiss T. S., Widera A., Woods C. G., Xu J. J., Yarborough K. M., and Hengstler J. G., Arch. Toxicol. 87(8), 1315–1530 (2013). 10.1007/s00204-013-1078-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.LeCluyse E. L., Witek R. P., Andersen M. E., and Powers M. J., Critical Reviews in Toxicology (Taylor & Francis, 2012), Vol. 42, pp. 501–548. 10.3109/10408444.2012.682115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee P. J., Hung P. J., and Lee L. P., Biotechnol. Bioeng. 97(5), 1340–1346 (2007). 10.1002/bit.21360 [DOI] [PubMed] [Google Scholar]
- 53.Lee H., Han W., Kim H., Ha D.-H., Jang J., Kim B. S., and Cho D.-W., Biomacromolecules 18(4), 1229–1237 (2017). 10.1021/acs.biomac.6b01908 [DOI] [PubMed] [Google Scholar]
- 54.Banaeiyan A. A., Theobald J., Paukstyte J., Wolfl S., Adiels C. B., and Goksor M., Biofabrication 9(1), 015014 (2017). 10.1088/1758-5090/9/1/015014 [DOI] [PubMed] [Google Scholar]
- 55.Hassan S., Sebastian S., Maharjan S., Lesha A., Carpenter A. M., Liu X., Xie X., Livermore C., Zhang Y. S., and Zarrinpar A., Hepatology (John Wiley and Sons Inc., 2020), Vol. 71, pp. 733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kang Y. B. A., Sodunke T. R., Lamontagne J., Cirillo J., Rajiv C., Bouchard M. J., and Noh M., Biotechnol. Bioeng. 112, 2571–2582 (2015). 10.1002/bit.25659 [DOI] [PubMed] [Google Scholar]
- 57.Chhabra A., Song H.-H. G., Grzelak K. A., Polacheck W. J., Fleming H. E., Chen C. S., and Bhatia S. N., Proc. Natl. Acad. Sci. U.S.A. 119(28), e2115867119 (2022). 10.1073/pnas.2115867119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Horejs C., Nat. Rev. Mater. 6(5), 372–373 (2021). 10.1038/s41578-021-00313-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dickson I., Nature Reviews Gastroenterology and Hepatology (Nature Research, 2020), Vol. 17, p. 4. [DOI] [PubMed] [Google Scholar]
- 60.Huh D., Leslie D. C., Matthews B. D., Fraser J. P., Jurek S., Hamilton G. A., Thorneloe K. S., McAlexander M. A., and Ingber D. E., Sci. Transl. Med. 4(159), 159ra147 (2012). 10.1126/scitranslmed.3004249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferreira D. A., Rothbauer M., Conde J. P., Ertl P., Oliveira C., and Granja P. L., Adv. Sci. 8(8), 2003273 (2021). 10.1002/advs.202003273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ergir E., Bachmann B., Redl H., Forte G., and Ertl P., Front. Physiol. 9, 1417 (2018). 10.3389/fphys.2018.01417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walther B. K., Rajeeva Pandian N. K., Gold K. A., Kiliç E. S., Sama V., Gu J., Gaharwar A. K., Guiseppi-Elie A., Cooke J. P., and Jain A., Lab Chip 21(9), 1738–1751 (2021). 10.1039/D0LC01283A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thompson C. L., Fu S., Knight M. M., and Thorpe S. D., Front. Bioeng. Biotechnol. 8, 602646 (2020). 10.3389/fbioe.2020.602646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bale S. S., Geerts S., Jindal R., and Yarmush M. L., Sci. Rep. 6(1), 25329 (2016). 10.1038/srep25329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Troutman T. D., Bennett H., Sakai M., Seidman J. S., Heinz S., and Glass C. K., STAR Protoc. 2(1), 100363 (2021). 10.1016/j.xpro.2021.100363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deng J., Cong Y., Han X., Wei W., Lu Y., Liu T., Zhao W., Lin B., Luo Y., and Zhang X., Biomicrofluidics 14(6), 064107 (2020). 10.1063/5.0024767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Duffy D. C., McDonald J. C., Schueller O. J. A., and Whitesides G. M., Anal. Chem. 70(23), 4974–4984 (1998). 10.1021/ac980656z [DOI] [PubMed] [Google Scholar]
- 69.Nakao Y., Kimura H., Sakai Y., and Fujii T., Biomicrofluidics 5(2), 022212 (2011). 10.1063/1.3580753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee J., Kim S. H., Kim Y. C., Choi I., and Sung J. H., Enzyme Microb. Technol. 53(3), 159–164 (2013). 10.1016/j.enzmictec.2013.02.015 [DOI] [PubMed] [Google Scholar]
- 71.Lee S.-A., No D. Y., Kang E., Ju J., Kim D.-S., and Lee S.-H., Lab Chip 13(18), 3529–3537 (2013). 10.1039/c3lc50197c [DOI] [PubMed] [Google Scholar]
- 72.Kang Y. B. A., Sodunke T. R., Lamontagne J., Cirillo J., Rajiv C., Bouchard M. J., and Noh M., Biotechnol. Bioeng. 112(12), 2571–2582 (2015). 10.1002/bit.25659 [DOI] [PubMed] [Google Scholar]
- 73.Bhise N. S., Manoharan V., Massa S., Tamayol A., Ghaderi M., Miscuglio M., Lang Q., Shrike Zhang Y., Shin S. R., Calzone G., Annabi N., Shupe T. D., Bishop C. E., Atala A., Dokmeci M. R., and Khademhosseini A., Biofabrication 8(1), 014101 (2016). 10.1088/1758-5090/8/1/014101 [DOI] [PubMed] [Google Scholar]
- 74.Weng Y. S., Chang S. F., Shih M. C., Tseng S. H., and Lai C. H., Adv. Mater. 29(36), 1701545 (2017). 10.1002/adma.201701545 [DOI] [PubMed] [Google Scholar]
- 75.Chen P.-Y., Hsieh M.-J., Liao Y.-H., Lin Y.-C., and Hou Y.-T., Biochem. Eng. J. 165, 107831 (2021). 10.1016/j.bej.2020.107831 [DOI] [Google Scholar]
- 76.Engel M., Belfiore L., Aghaei B., and Sutija M., SLAS Technol. 27(1), 32–38 (2022). 10.1016/j.slast.2021.10.002 [DOI] [PubMed] [Google Scholar]
- 77.Wang H., Liang X., Gravot G., Thorling C. A., Crawford D. H. G., Xu Z. P., Liu X., and Roberts M. S., J. Biophotonics 10(1), 46–60 (2017). 10.1002/jbio.201600083 [DOI] [PubMed] [Google Scholar]
- 78.Marieb E. N. and Hoehn K., Human Anatomy & Physiology (Pearson Education, 2007). [Google Scholar]
- 79.Stone H. A. and Kim S., AIChE J. 47(6), 1250–1254 (2001). 10.1002/aic.690470602 [DOI] [Google Scholar]
- 80.Shirure V. S. and George S. C., Lab Chip 17(4), 681–690 (2017). 10.1039/C6LC01401A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sung J. H., Wang Y. I., Kim J. H., Lee J. M., and Shuler M. L., AIChE J. 64(12), 4351–4360 (2018). 10.1002/aic.16448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kmieć Z., Cooperation of Liver Cells in Health and Disease (Springer, 2001), pp. 1–6. [DOI] [PubMed] [Google Scholar]
- 83.Guandalini S., Dhawan A., and Branski D., Textbook of Pediatric Gastroenterology, Hepatology and Nutrition (Springer, 2016). 10.1007/978-3-319-17169-2 [DOI] [Google Scholar]
- 84.Arias I. M., Alter H. J., Boyer J. L., Cohen D. E., Shafritz D. A., Thorgeirsson S. S., and Wolkoff A. W., The Liver: Biology and Pathobiology (John Wiley & Sons, 2020). [Google Scholar]
- 85.Mescher A. L., Junqueira's Basic Histology: Text and Atlas, 13th ed. (McGraw-Hill Medical, New York, 2013). [Google Scholar]
- 86.Ma X., Qu X., Zhu W., Li Y.-S., Yuan S., Zhang H., Liu J., Wang P., Lai Cheuk Sun E., Zanella F., Feng G.-S., Sheikh F., Chien S., and Chen S., Proc. Natl. Acad. Sci. U.S.A. 113(8), 2206–2211 (2016). 10.1073/pnas.1524510113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sanders F. W. B. and Griffin J. L., Biol. Rev. 91(2), 452–468 (2016). 10.1111/brv.12178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kietzmann T., Redox Biology (Elsevier B.V., 2017), Vol. 11, pp. 622–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ma C., Zhao L., Zhou E.-M., Xu J., Shen S., and Wang J., Anal. Chem. 88(3), 1719–1727 (2016). 10.1021/acs.analchem.5b03869 [DOI] [PubMed] [Google Scholar]
- 90.Shih M.-C., Tseng S.-H., Weng Y.-S., Chu I. M., and Liu C.-H., Biomed. Microdevices 15(5), 767–780 (2013). 10.1007/s10544-013-9762-z [DOI] [PubMed] [Google Scholar]
- 91.Rennert K., Steinborn S., Gröger M., Ungerböck B., Jank A. M., Ehgartner J., Nietzsche S., Dinger J., Kiehntopf M., Funke H., Peters F. T., Lupp A., Gärtner C., Mayr T., Bauer M., Huber O., and Mosig A. S., Biomaterials 71, 119–131 (2015). 10.1016/j.biomaterials.2015.08.043 [DOI] [PubMed] [Google Scholar]
- 92.Chen Y., Sun W., Kang L., Wang Y., Zhang M., Zhang H., and Hu P., Analyst 144(14), 4233–4240 (2019). 10.1039/C9AN00612E [DOI] [PubMed] [Google Scholar]
- 93.Bonanini F., Kurek D., Previdi S., Nicolas A., Hendriks D., de Ruiter S., Meyer M., Clapes Cabrer M., Dinkelberg R., Garcia S. B., Kramer B., Olivier T., Hu H., Lopez-Iglesias C., Schavemaker F., Walinga E., Dutta D., Queiroz K., Domansky K., Ronden B., Joore J., Lanz H. L., Peters P. J., Trietsch S. J., Clevers H., and Vulto P., Angiogenesis 25(4), 455–470 (2022). 10.1007/s10456-022-09842-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boos J. A., Misun P. M., Michlmayr A., Hierlemann A., and Frey O., Adv. Sci. (Weinh) 6(13), 1900294 (2019). 10.1002/advs.201900294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bulutoglu B., Rey-Bedón C., Kang Y. B., Mert S., Yarmush M. L., and Usta O. B., Lab Chip 19(18), 3022–3031 (2019). 10.1039/C9LC00354A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Corrado B., De Gregorio V., Imparato G., Attanasio C., Urciuolo F., and Netti P. A., Biotechnol. Bioeng. 116(5), 1152–1163 (2019). 10.1002/bit.26902 [DOI] [PubMed] [Google Scholar]
- 97.Delalat B., Cozzi C., Rasi Ghaemi S., Polito G., Kriel F. H., Michl T. D., Harding F. J., Priest C., Barillaro G., and Voelcker N. H., Adv. Funct. Mater. 28(28), 1801825 (2018). 10.1002/adfm.201801825 [DOI] [Google Scholar]
- 98.Frey O., Misun P. M., Fluri D. A., Hengstler J. G., and Hierlemann A., Nat. Commun. 5(1), 4250 (2014). 10.1038/ncomms5250 [DOI] [PubMed] [Google Scholar]
- 99.Gori M., Simonelli M. C., Giannitelli S. M., Businaro L., Trombetta M., and Rainer A., PLoS One 11(7), e0159729 (2016). 10.1371/journal.pone.0159729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jang M., Neuzil P., Volk T., Manz A., and Kleber A., Biomicrofluidics 9(3), 034113 (2015). 10.1063/1.4922863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jang K. J., Otieno M. A., Ronxhi J., Lim H. K., Ewart L., Kodella K. R., Petropolis D. B., Kulkarni G., Rubins J. E., Conegliano D., Nawroth J., Simic D., Lam W., Singer M., Barale E., Singh B., Sonee M., Streeter A. J., Manthey C., Jones B., Srivastava A., Andersson L. C., Williams D., Park H., Barrile R., Sliz J., Herland A., Haney S., Karalis K., Ingber D. E., and Hamilton G. A., Sci. Transl. Med. 11(517), eaax5516 (2019). 10.1126/scitranslmed.aax5516 [DOI] [PubMed] [Google Scholar]
- 102.Khetani S. R. and Bhatia S. N., Nat. Biotechnol. 26(1), 120–126 (2008). 10.1038/nbt1361 [DOI] [PubMed] [Google Scholar]
- 103.Kim Y. S., Asif A., Chethikkattuveli Salih A. R., Lee J. W., Hyun K. N., and Choi K. H., Biomedicines 9(10), 1369 (2021). 10.3390/biomedicines9101369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee H., Chae S., Kim J. Y., Han W., Kim J., Choi Y., and Cho D. W., Biofabrication 11(2), 025001 (2019). 10.1088/1758-5090/aaf9fa [DOI] [PubMed] [Google Scholar]
- 105.Li X., George S. M., Vernetti L., Gough A. H., and Taylor D. L., Lab Chip 18(17), 2614–2631 (2018). 10.1039/C8LC00418H [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mazari-Arrighi E., Okitsu T., Teramae H., Aoyagi H., Kiyosawa M., Yano M., Chatelain F., Fuchs A., and Takeuchi S., Sci. Rep. 12(1), 1–12 (2022). 10.1038/s41598-022-12679-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moya A., Ortega-Ribera M., Guimerà X., Sowade E., Zea M., Illa X., Ramon E., Villa R., Gracia-Sancho J., and Gabriel G., Lab Chip 18(14), 2023–2035 (2018). 10.1039/C8LC00456K [DOI] [PubMed] [Google Scholar]
- 108.Roth A. D., Lama P., Dunn S., Hong S., and Lee M. Y., Mater. Sci. Eng. C 90, 634–644 (2018). 10.1016/j.msec.2018.04.092 [DOI] [PubMed] [Google Scholar]
- 109.Yu F., Goh Y. T., Li H., Chakrapani N. B., Ni M., Xu G. L., Hsieh T. M., Toh Y. C., Cheung C., Iliescu C., and Yu H., Biomicrofluidics 14(3), 034108 (2020). 10.1063/5.0004286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yu F., Deng R., Hao Tong W., Huan L., Chan Way N., IslamBadhan A., Iliescu C., and Yu H., Sci. Rep. 7(1), 14528 (2017). 10.1038/s41598-017-13848-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Boron W. F. and Boulpaep E., Medical Physiology: A Cellular and Molecular Approach, updated 2nd ed. (Elsevier Saunders, Philadelphia, PA, 2009). [Google Scholar]
- 112.Pawlina W. and Ross M. H., Histology: A Text and Atlas: With Correlated Cell and Molecular Biology (Lippincott Williams & Wilkins, 2018). [Google Scholar]
- 113.Fu X., Sluka J. P., Clendenon S. G., Dunn K. W., Wang Z., Klaunig J. E., and Glazier J. A., PLoS One 13(9), e0198060 (2018). 10.1371/journal.pone.0198060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wisse E., J. Ultrastruct. Res. 31(1–2), 125–150 (1970). 10.1016/S0022-5320(70)90150-4 [DOI] [PubMed] [Google Scholar]
- 115.Ogawa K., Minase T., Enomoto K., and Onoé T., Tohoku J. Exp. Med. 110(1), 89–101 (1973). 10.1620/tjem.110.89 [DOI] [PubMed] [Google Scholar]
- 116.Widmann J. J., Cotran R. S., and Fahimi H. D., J. Cell Biol. 52(1), 159–170 (1972). 10.1083/jcb.52.1.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Braet F. and Wisse E., Comp. Hepatol. 1(1), 1 (2002). 10.1186/1476-5926-1-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wisse E., Jacobs F., Topal B., Frederik P., and De Geest B., Gene Ther. 15(17), 1193–1199 (2008). 10.1038/gt.2008.60 [DOI] [PubMed] [Google Scholar]
- 119.Gebhardt R., Pharmacol. Ther. 53(3), 275–354 (1992). 10.1016/0163-7258(92)90055-5 [DOI] [PubMed] [Google Scholar]
- 120.Wisse E., de Zanger R. B., Charels K., van der Smissen P., and McCuskey R. S., Hepatology 5(4), 683–692 (1985). 10.1002/hep.1840050427 [DOI] [PubMed] [Google Scholar]
- 121.Horn T., Christoffersen P., and Henriksen J. H., Hepatology 7(1), 77–82 (1987). 10.1002/hep.1840070117 [DOI] [PubMed] [Google Scholar]
- 122.Zapotoczny B., Szafranska K., Owczarczyk K., Kus E., Chlopicki S., and Szymonski M., Sci. Rep. 7(1), 7994 (2017). 10.1038/s41598-017-08555-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jungermann K. and Sasse D., Trends Biochem. Sci. 3(3), 198–202 (1978). 10.1016/S0968-0004(78)91764-4 [DOI] [Google Scholar]
- 124.Katz N., Teutsch H. F., Jungermann K., and Sasse D., FEBS Lett. 69(1), 23–26 (1976). 10.1016/0014-5793(76)80645-X [DOI] [PubMed] [Google Scholar]
- 125.Sasse D., Katz N., and Jungermann K., FEBS Lett. 57(1), 83–88 (1975). 10.1016/0014-5793(75)80157-8 [DOI] [PubMed] [Google Scholar]
- 126.Guder W. G. and Schmidt U., Hoppe Seylers Z. Physiol. Chem. 357(12), 1793–1800 (1976). 10.1515/bchm2.1976.357.2.1793 [DOI] [PubMed] [Google Scholar]
- 127.Huh D., Kim H. J., Fraser J. P., Shea D. E., Khan M., Bahinski A., Hamilton G. A., and Ingber D. E., Nat. Protoc. 8(11), 2135–2157 (2013). 10.1038/nprot.2013.137 [DOI] [PubMed] [Google Scholar]
- 128.Bavli D., Prill S., Ezra E., Levy G., Cohen M., Vinken M., Vanfleteren J., Jaeger M., and Nahmias Y., Proc. Natl. Acad. Sci. U.S.A. 113(16), E2231–E2240 (2016). 10.1073/pnas.1522556113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Farooqi H. M. U., Khalid M. A. U., Kim K. H., Lee S. R., and Choi K. H., J. Micromech. Microeng. 30(11), 115013 (2020). 10.1088/1361-6439/ababf4 [DOI] [Google Scholar]
- 130.Zhang J., Zhao X., Liang L., Li J., Demirci U., and Wang S. Q., Biomaterials (Elsevier Ltd, 2018), Vol. 157, pp. 161–176. [DOI] [PubMed] [Google Scholar]
- 131.Kojima N., Tao F., Mihara H., and Aoki S., in Stem Cells and Cancer in Hepatology, edited by Zheng Y.-W. (Academic Press, 2018), pp. 145–158. [Google Scholar]
- 132.Rothbauer M., Eilenberger C., Spitz S., Bachmann B. E. M., Kratz S. R. A., Reihs E. I., Windhager R., Toegel S., and Ertl P., Front. Bioeng. Biotechnol. 10, 837087 (2022). 10.3389/fbioe.2022.837087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hosic S., Bindas A. J., Puzan M. L., Lake W., Soucy J. R., Zhou F., Koppes R. A., Breault D. T., Murthy S. K., and Koppes A. N., ACS Biomater. Sci. Eng. 7(7), 2949–2963 (2021). 10.1021/acsbiomaterials.0c00190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kratz S. R. A., Eilenberger C., Schuller P., Bachmann B., Spitz S., Ertl P., and Rothbauer M., Sci. Rep. 9(1), 9287 (2019). 10.1038/s41598-019-45633-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li Q., Niu K., Wang D., Xuan L., and Wang X., Lab Chip 22(14), 2682–2694 (2022). 10.1039/D1LC00767J [DOI] [PubMed] [Google Scholar]
- 136.French R. H. and Tran H. V., Annu. Rev. Mater. Res. 39(1), 93–126 (2009). 10.1146/annurev-matsci-082908-145350 [DOI] [Google Scholar]
- 137.Rizvi S., Handbook of Photomask Manufacturing Technology (CRC Press, 2018). [Google Scholar]
- 138.Jose Varghese R., Sakho E. h. M., Parani S., Thomas S., Oluwafemi O. S., and Wu J., in Nanomaterials for Solar Cell Applications, edited by Thomas S., Sakho E. H. M., Kalarikkal N., Oluwafemi S. O., and Wu J. (Elsevier, 2019), pp. 75–95. [Google Scholar]
- 139.Kasi D. G., de Graaf M. N. S., Motreuil-Ragot P. A., Frimat J.-P. M. S., Ferrari M. D., Sarro P. M., Mastrangeli M., van den Maagdenberg A. M. J. M., Mummery C. L., and Orlova V. V., Micromachines 13(1), 49 (2022). 10.3390/mi13010049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Morbioli G. G., Speller N. C., and Stockton A. M., Anal. Chim. Acta 1135, 150–174 (2020). 10.1016/j.aca.2020.09.013 [DOI] [PubMed] [Google Scholar]
- 141.Qin D., Xia Y., and Whitesides G. M., Nat. Protoc. 5(3), 491–502 (2010). 10.1038/nprot.2009.234 [DOI] [PubMed] [Google Scholar]
- 142.Xia Y. and Whitesides G. M., Angew Chem. Int. Ed. Engl. 37(5), 550–575 (1998). [DOI] [PubMed] [Google Scholar]
- 143.McDonald J. C. and Whitesides G. M., Acc. Chem. Res. 35(7), 491–499 (2002). 10.1021/ar010110q [DOI] [PubMed] [Google Scholar]
- 144.Campbell S. B., Wu Q., Yazbeck J., Liu C., Okhovatian S., and Radisic M., ACS Biomater. Sci. Eng. 7(7), 2880–2899 (2021). 10.1021/acsbiomaterials.0c00640 [DOI] [PubMed] [Google Scholar]
- 145.Nielsen J. B., Hanson R. L., Almughamsi H. M., Pang C., Fish T. R., and Woolley A. T., Anal. Chem. 92(1), 150–168 (2020). 10.1021/acs.analchem.9b04986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Scott S. M. and Ali Z., Micromachines 12(3), 319 (2021). 10.3390/mi12030319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Danku A. E., Dulf E. H., Braicu C., Jurj A., and Berindan-Neagoe I., Front. Bioeng. Biotechnol. 10, 840674 (2022). 10.3389/fbioe.2022.840674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wu Q., Liu J., Wang X., Feng L., Wu J., Zhu X., Wen W., and Gong X., BioMed. Eng. OnLine 19(1), 9 (2020). 10.1186/s12938-020-0752-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Friedrich J., Seidel C., Ebner R., and Kunz-Schughart L. A., Nat. Protoc. 4(3), 309–324 (2009). 10.1038/nprot.2008.226 [DOI] [PubMed] [Google Scholar]
- 150.Azizipour N., Avazpour R., Sawan M., Rosenzweig D. H., and Ajji A., Sens. Diagn. 1(4), 750–764 (2022). 10.1039/D2SD00004K [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Costa E. C., de Melo-Diogo D., Moreira A. F., Carvalho M. P., and Correia I. J., Biotechnol. J. 13(1), 1700417 (2018). 10.1002/biot.201700417 [DOI] [PubMed] [Google Scholar]
- 152.Moshksayan K., Kashaninejad N., Warkiani M. E., Lock J. G., Moghadas H., Firoozabadi B., Saidi M. S., and Nguyen N.-T., Sens. Actuators, B 263, 151–176 (2018). 10.1016/j.snb.2018.01.223 [DOI] [Google Scholar]
- 153.Tostões R. M., Leite S. B., Serra M., Jensen J., Björquist P., Carrondo M. J., Brito C., and Alves P. M., Hepatology 55(4), 1227–1236 (2012). 10.1002/hep.24760 [DOI] [PubMed] [Google Scholar]
- 154.Ferrari E., Ugolini G. S., Piutti C., Marzorati S., and Rasponi M., Biomed. Mater. 16(4), 045032 (2021). 10.1088/1748-605X/ac0454 [DOI] [PubMed] [Google Scholar]
- 155.Behroozi F., Phys. Teach. 60(5), 358–361 (2022). 10.1119/5.0045605 [DOI] [Google Scholar]
- 156.Liu D., Chen S., and Win Naing M., Biotechnol. Bioeng. 118(2), 542–554 (2021). 10.1002/bit.27620 [DOI] [PubMed] [Google Scholar]
- 157.Wang X. and Yang P., J. Vis. Exp. 17, e825 (2008). 10.3791/825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pinto B., Henriques A. C., Silva P. M. A., and Bousbaa H., Pharmaceutics (MDPI, 2020), Vol. 12. 10.3390/pharmaceutics12121186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chao C., Ngo L. P., and Engelward B. P., ACS Biomater. Sci. Eng. 6(4), 2427–2439 (2020). 10.1021/acsbiomaterials.9b01951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bell C. C., Hendriks D. F. G., Moro S. M. L., Ellis E., Walsh J., Renblom A., Fredriksson Puigvert L., Dankers A. C. A., Jacobs F., Snoeys J., Sison-Young R. L., Jenkins R. E., Nordling Å, Mkrtchian S., Park B. K., Kitteringham N. R., Goldring C. E. P., Lauschke V. M., and Ingelman-Sundberg M., Sci. Rep. 6(1), 25187 (2016). 10.1038/srep25187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.El-Sherbiny I. M. and Yacoub M. H., Global Cardiol. Sci. Pract. 2013(3), 316–342 (2013). 10.5339/gcsp.2013.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mantha S., Pillai S., Khayambashi P., Upadhyay A., Zhang Y., Tao O., Pham H. M., and Tran S. D., Materials (Basel) 12(20), 3323 (2019). 10.3390/ma12203323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ali M. and Payne S. L., Biomater. Res. 25(1), 5 (2021). 10.1186/s40824-021-00206-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.van de Kamp J., Jahnen-Dechent W., Rath B., Knuechel R., and Neuss S., Stem Cells Int. 2013, 1 (2013). 10.1155/2013/892065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bual R. P. and Ijima H., Regen. Ther. 11, 258–268 (2019). 10.1016/j.reth.2019.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Grant R., Hallett J., Forbes S., Hay D., and Callanan A., Sci. Rep. 9(1), 6293 (2019). 10.1038/s41598-019-42627-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hussein K. H., Park K.-M., Yu L., Kwak H.-H., and Woo H.-M., Mater. Sci. Eng. C 116, 111160 (2020). 10.1016/j.msec.2020.111160 [DOI] [PubMed] [Google Scholar]
- 168.Shimoda H., Yagi H., Higashi H., Tajima K., Kuroda K., Abe Y., Kitago M., Shinoda M., and Kitagawa Y., Sci. Rep. 9(1), 12543 (2019). 10.1038/s41598-019-48948-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang J., Shao C., Wang Y., Sun L., and Zhao Y., Engineering 6(11), 1244–1257 (2020). 10.1016/j.eng.2020.10.001 [DOI] [Google Scholar]
- 170.Miri A. K., Mostafavi E., Khorsandi D., Hu S. K., Malpica M., and Khademhosseini A., Biofabrication 11(4), 042002 (2019). 10.1088/1758-5090/ab2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Thakare K., Jerpseth L., Pei Z., Elwany A., Quek F., and Qin H., J. Manuf. Mater. Process. 5(3), 91 (2021). 10.3390/jmmp5030091 [DOI] [Google Scholar]
- 172.Vijayavenkataraman S., Yan W. C., Lu W. F., Wang C. H., and Fuh J. Y. H., Adv. Drug Delivery Rev. 132, 296–332 (2018). 10.1016/j.addr.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 173.Murphy S. V. and Atala A., Nat. Biotechnol. 32(8), 773–785 (2014). 10.1038/nbt.2958 [DOI] [PubMed] [Google Scholar]
- 174.Ashammakhi N., Ahadian S., Xu C., Montazerian H., Ko H., Nasiri R., Barros N., and Khademhosseini A., Mater. Today Bio 1, 100008 (2019). 10.1016/j.mtbio.2019.100008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hoeng J., Bovard D., and Peitsch M., Organ-on-a-Chip: Engineered Microenvironments for Safety and Efficacy Testing (Academic Press, 2019). [Google Scholar]
- 176.Janani G., Priya S., Dey S., and Mandal B. B., ACS Appl. Mater. Interfaces 14(8), 10167–10186 (2022). 10.1021/acsami.2c00312 [DOI] [PubMed] [Google Scholar]
- 177.Lyden T., Hultgren H., Beethe N., Jaskens R., LeBeau M., and Thrun L., FASEB J. 36, 1 (2022). [Google Scholar]
- 178.Evens R. P., AAPS J. 18(1), 281–285 (2016). 10.1208/s12248-015-9833-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sun D., Gao W., Hu H., and Zhou S., Acta Pharm. Sin. B 12(7), 3049–3062 (2022). 10.1016/j.apsb.2022.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Brunt E. M., Wong V. W. S., Nobili V., Day C. P., Sookoian S., Maher J. J., Bugianesi E., Sirlin C. B., Neuschwander-Tetri B. A., and Rinella M. E., Nat. Rev. Dis. Primers 1(1), 15080 (2015). 10.1038/nrdp.2015.80 [DOI] [PubMed] [Google Scholar]
- 181.Klein S. and Dufour J. F., Hepatic Oncol. 4(3), 83–98 (2017). 10.2217/hep-2017-0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Almazroo O. A., Miah M. K., and Venkataramanan R., Clin. Liver Dis. 21(1), 1–20 (2017). 10.1016/j.cld.2016.08.001 [DOI] [PubMed] [Google Scholar]
- 183.Marcdante K. and Kliegman R. M., Nelson Essentials of Pediatrics, e-Book (Elsevier Health Sciences, 2014). [Google Scholar]
- 184.Gebhardt R., Hengstler J. G., Müller D., Glöckner R., Buenning P., Laube B., Schmelzer E., Ullrich M., Utesch D., Hewitt N., Ringel M., Hilz B. R., Bader A., Langsch A., Koose T., Burger H.-J., Maas J., and Oesch F., Drug Metab. Rev. 35(2–3), 145–213 (2003). 10.1081/DMR-120023684 [DOI] [PubMed] [Google Scholar]
- 185.Graham M. J. and Lake B. G., Toxicology 254(3), 184–191 (2008). 10.1016/j.tox.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 186.Martignoni M., Groothuis G. M. M., and de Kanter R., Expert Opin. Drug Metab. Toxicol. 2(6), 875–894 (2006). 10.1517/17425255.2.6.875 [DOI] [PubMed] [Google Scholar]
- 187.Toh Y.-C., Lim T. C., Tai D., Xiao G., van Noort D., and Yu H., Lab Chip 9(14), 2026–2035 (2009). 10.1039/b900912d [DOI] [PubMed] [Google Scholar]
- 188.Picollet-D’hahan N., Zuchowska A., Lemeunier I., and Le Gac S., Trends Biotechnol. 39(8), 788–810 (2021). 10.1016/j.tibtech.2020.11.014 [DOI] [PubMed] [Google Scholar]
- 189.Longmire M., Choyke P. L., and Kobayashi H., Nanomedicine (London) 3(5), 703–717 (2008). 10.2217/17435889.3.5.703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Anselmo A. C. and Mitragotri S., Bioeng. Transl. Med. 4(3), e10143 (2019). 10.1002/btm2.10143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Akhtar A., Camb. Q. Healthcare Ethics 24(4), 407–419 (2015). 10.1017/S0963180115000079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Qiu S., Ji J., Sun W., Pei J., He J., Li Y., Li J. J., and Wang G., Smart Mater. Med. 2, 65–73 (2021). 10.1016/j.smaim.2020.12.002 [DOI] [Google Scholar]
- 193.Liu Y., Wang S., and Wang Y., Polymers 8(11), 402 (2016). 10.3390/polym8110402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Faedmaleki F., A H. S. F., Salarian A., Ahmadi Ashtiani H., and Rastegar H., Iran J. Pharm. Res. 13(1), 235–242 (2014). [PMC free article] [PubMed] [Google Scholar]
- 195.Shanks N., Greek R., and Greek J., Philos., Ethics, Humanities Med. 4(1), 2 (2009). 10.1186/1747-5341-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sung J. H., Wang Y. I., Narasimhan Sriram N., Jackson M., Long C., Hickman J. J., and Shuler M. L., Anal. Chem. 91(1), 330–351 (2019). 10.1021/acs.analchem.8b05293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Docci L., Milani N., Ramp T., Romeo A. A., Godoy P., Franyuti D. O., Krähenbühl S., Gertz M., Galetin A., Parrott N., and Fowler S., Lab Chip 22(6), 1187–1205 (2022). 10.1039/D1LC01161H [DOI] [PubMed] [Google Scholar]
- 198.Herland A., Maoz B. M., Das D., Somayaji M. R., Prantil-Baun R., Novak R., Cronce M., Huffstater T., Jeanty S. S. F., Ingram M., Chalkiadaki A., Benson Chou D., Marquez S., Delahanty A., Jalili-Firoozinezhad S., Milton Y., Sontheimer-Phelps A., Swenor B., Levy O., Parker K. K., Przekwas A., and Ingber D. E., Nat. Biomed. Eng. 4(4), 421–436 (2020). 10.1038/s41551-019-0498-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Edington C. D., Chen W. L. K., Geishecker E., Kassis T., Soenksen L. R., Bhushan B. M., Freake D., Kirschner J., Maass C., Tsamandouras N., Valdez J., Cook C. D., Parent T., Snyder S., Yu J., Suter E., Shockley M., Velazquez J., Velazquez J. J., Stockdale L., Papps J. P., Lee I., Vann N., Gamboa M., LaBarge M. E., Zhong Z., Wang X., Boyer L. A., Lauffenburger D. A., Carrier R. L., Communal C., Tannenbaum S. R., Stokes C. L., Hughes D. J., Rohatgi G., Trumper D. L., Cirit M., and Griffith L. G., Sci. Rep. 8(1), 4530 (2018). 10.1038/s41598-018-22749-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Esch E. W., Bahinski A., and Huh D., Nat. Rev. Drug Discovery 14(4), 248–260 (2015). 10.1038/nrd4539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sung J. H., Expert Opin. Drug Metab. Toxicol. 17(8), 969–986 (2021). 10.1080/17425255.2021.1908996 [DOI] [PubMed] [Google Scholar]
- 202.Schober A., Fernekorn U., Lübbers B., Hampl J., Weise F., Schlingloff G., Gebinoga M., Worgull M., Schneider M., Augspurger C., Hildmann C., Kittler M., and Donahue M., Mater. Werkst. 42(2), 139–146 (2011). 10.1002/mawe.201100746 [DOI] [Google Scholar]
- 203.Zhang Y., Yang N., Xie L., Shu F., Shi Q., and Shaheen N., Micromachines 11(12), 1–11 (2020). 10.3390/mi11121118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Clarke G. A., Hartse B. X., Niaraki Asli A. E., Taghavimehr M., Hashemi N., Abbasi Shirsavar M., Montazami R., Alimoradi N., Nasirian V., Ouedraogo L. J., and Hashemi N. N., Sensors (Basel) 21(4), 1367 (2021). 10.3390/s21041367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Fuchs S., Johansson S., Tjell AØ, Werr G., Mayr T., and Tenje M., ACS Biomater. Sci. Eng. 7(7), 2926–2948 (2021). 10.1021/acsbiomaterials.0c01110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhang Y. S., Aleman J., Shin S. R., Kilic T., Kim D., Mousavi Shaegh S. A., Massa S., Riahi R., Chae S., Hu N., Avci H., Zhang W., Silvestri A., Sanati Nezhad A., Manbohi A., De Ferrari F., Polini A., Calzone G., Shaikh N., Alerasool P., Budina E., Kang J., Bhise N., Ribas J., Pourmand A., Skardal A., Shupe T., Bishop C. E., Dokmeci M. R., Atala A., and Khademhosseini A., Proc. Natl. Acad. Sci. U. S. A. 114(12), E2293–E2302 (2017). 10.1073/pnas.1612906114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Aleman J., Kilic T., Mille L. S., Shin S. R., and Zhang Y. S., Nat. Protoc. 16(5), 2564–2593 (2021). 10.1038/s41596-021-00511-7 [DOI] [PubMed] [Google Scholar]
- 208.Weltin A., Hammer S., Noor F., Kaminski Y., Kieninger J., and Urban G. A., Biosens. Bioelectron. 87, 941–948 (2017). 10.1016/j.bios.2016.07.094 [DOI] [PubMed] [Google Scholar]
- 209.Shin S. R., Kilic T., Zhang Y. S., Avci H., Hu N., Kim D., Branco C., Aleman J., Massa S., Silvestri A., Kang J., Desalvo A., Hussaini M. A., Chae S. K., Polini A., Bhise N., Hussain M. A., Lee H., Dokmeci M. R., and Khademhosseini A., Adv. Sci. (Weinh) 4(5), 1600522 (2017). 10.1002/advs.201600522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Jungermann K. and Katz N., Physiol. Rev. 69(3), 708–764 (1989). 10.1152/physrev.1989.69.3.708 [DOI] [PubMed] [Google Scholar]
- 211.Low L. A., Mummery C., Berridge B. R., Austin C. P., and Tagle D. A., Nat. Rev. Drug Discovery 20(5), 345–361 (2021). 10.1038/s41573-020-0079-3 [DOI] [PubMed] [Google Scholar]
- 212.Franzen N., van Harten W. H., Retèl V. P., Loskill P., van den Eijnden-van Raaij J., and IJzerman M., Drug Discovery Today 24(9), 1720–1724 (2019). 10.1016/j.drudis.2019.06.003 [DOI] [PubMed] [Google Scholar]
- 213.Marx U., Akabane T., Andersson T. B., Baker E., Beilmann M., Beken S., Brendler-Schwaab S., Cirit M., David R., Dehne E. M., Durieux I., Ewart L., Fitzpatrick S. C., Frey O., Fuchs F., Griffith L. G., Hamilton G. A., Hartung T., Hoeng J., Hogberg H., Hughes D. J., Ingber D. E., Iskandar A., Kanamori T., Kojima H., Kuehnl J., Leist M., Li B., Loskill P., Mendrick D. L., Neumann T., Pallocca G., Rusyn I., Smirnova L., Steger-Hartmann T., Tagle D. A., Tonevitsky A., Tsyb S., Trapecar M., Van de Water B., Van den Eijnden-van Raaij J., Vulto P., Watanabe K., Wolf A., Zhou X., and Roth A., Altex 37(3), 365–394 (2020). 10.14573/altex.2001241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Candarlioglu P. L., Dal Negro G., Hughes D., Balkwill F., Harris K., Screen H., Morgan H., David R., Beken S., Guenat O., Rowan W., and Amour A., Biochem. Soc. Trans. 50(2), 665–673 (2022). 10.1042/BST20200661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ya S., Ding W., Li S., Du K., Zhang Y., Li C., Liu J., Li F., Li P., Luo T., He L., Xu A., Gao D., and Qiu B., ACS Appl. Mater. Interfaces 13(28), 32640–32652 (2021). 10.1021/acsami.1c00794 [DOI] [PubMed] [Google Scholar]
- 216.Lim J. T. C., Kwang L. G., Ho N. C. W., Toh C. C. M., Too N. S. H., Hooi L., Benoukraf T., Chow P. K.-H., Dan Y. Y., Chow E. K.-H., Toh T. B., and Fong E. L. S., Biomaterials 284, 121527 (2022). 10.1016/j.biomaterials.2022.121527 [DOI] [PubMed] [Google Scholar]
- 217.Zhou Y., Shen J. X., and Lauschke V. M., Front. Pharmacol. 10, 1093 (2019). 10.3389/fphar.2019.01093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wang A. J., Allen A., Sofman M., Sphabmixay P., Yildiz E., and Griffith L. G., Adv. NanoBiomed. Res. 2(1), 2100049 (2022). 10.1002/anbr.202100049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Michalopoulos G. K., J. Cell. Physiol. 213, 286–300 (2007). 10.1002/jcp.21172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ferenci P., Gastroenterol. Rep. 5(2), 138–147 (2017). 10.1093/gastro/gox013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Michalopoulos G. K. and Bhushan B., Nature Reviews Gastroenterology and Hepatology (Nature Research, 2021), pp. 40–55. [DOI] [PubMed] [Google Scholar]
- 222.Yang S., Chen Z., Cheng Y., Liu T., Yin L., Pu Y., and Liang G., Environ. Pollut. 268, 115861 (2021). 10.1016/j.envpol.2020.115861 [DOI] [PubMed] [Google Scholar]
- 223.Kumaran A., Vashishth R., Singh S., U S., James A., and Velayudhaperumal Chellam P., Microchem. J. 178, 107420 (2022). 10.1016/j.microc.2022.107420 [DOI] [Google Scholar]
- 224.Oleaga C., Lavado A., Riu A., Rothemund S., Carmona-Moran C. A., Persaud K., Yurko A., Lear J., Narasimhan N. S., Long C. J., Sommerhage F., Bridges L. R., Cai Y., Martin C., Schnepper M. T., Goswami A., Note R., Langer J., Teissier S., Cotovio J., and Hickman J. J., Adv. Funct. Mater. 29(8), 1970049 (2019). 10.1002/adfm.201970049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ferrari E., Palma C., Vesentini S., Occhetta P., and Rasponi M., Biosensors (Basel) 10(9), 110 (2020). 10.3390/bios10090110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Rothbauer M. and Ertl P., Adv. Biochem. Eng. Biotechnol. 179, 343–354 (2022). 10.1007/10_2020_129 [DOI] [PubMed] [Google Scholar]
- 227.Zhu Y., Mandal K., Hernandez A. L., Kawakita S., Huang W., Bandaru P., Ahadian S., Kim H.-J., Jucaud V., Dokmeci M. R., and Khademhosseini A., Curr. Opin. Biomed. Eng. 19, 100309 (2021). 10.1016/j.cobme.2021.100309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Marx U., Walles H., Hoffmann S., Lindner G., Horland R., Sonntag F., Klotzbach U., Sakharov D., Tonevitsky A., and Lauster R., Altern. Lab Anim. 40(5), 235–257 (2012). 10.1177/026119291204000504 [DOI] [PubMed] [Google Scholar]
- 229.Jacob E. M., Borah A., and Sakthi Kumar D., in Microfluidics and Multi Organs on Chip, edited by Mohanan P. V. (Springer Nature Singapore, Singapore, 2022), pp. 261–288. [Google Scholar]
- 230.Jeon J.-w., Lee S. H., Kim D., and Sung J. H., Biotechnol. Prog. 37(3), e3121 (2021). 10.1002/btpr.3121 [DOI] [PubMed] [Google Scholar]
- 231.Kühnl J., Tao T. P., Brandmair K., Gerlach S., Rings T., Müller-Vieira U., Przibilla J., Genies C., Jaques-Jamin C., Schepky A., Marx U., Hewitt N. J., and Maschmeyer I., Toxicology 448, 152637 (2021). 10.1016/j.tox.2020.152637 [DOI] [PubMed] [Google Scholar]
- 232.Wagner I., Materne E.-M., Brincker S., Süßbier U., Frädrich C., Busek M., Sonntag F., Sakharov D. A., Trushkin E. V., Tonevitsky A. G., Lauster R., and Marx U., Lab Chip 13(18), 3538–3547 (2013). 10.1039/c3lc50234a [DOI] [PubMed] [Google Scholar]
- 233.Shik Mun K., Arora K., Huang Y., Yang F., Yarlagadda S., Ramananda Y., Abu-El-Haija M., Palermo J. J., Appakalai B. N., Nathan J. D., and Naren A. P., Nat. Commun. 10(1), 3124 (2019). 10.1038/s41467-019-11178-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Essaouiba A., Okitsu T., Kinoshita R., Jellali R., Shinohara M., Danoy M., Legallais C., Sakai Y., and Leclerc E., Biochem. Eng. J. 164, 107783 (2020). 10.1016/j.bej.2020.107783 [DOI] [Google Scholar]
- 235.Hwang S.-H., Lee S., Park J. Y., Jeon J. S., Cho Y.-J., and Kim S., Micromachines 12(2), 215 (2021). 10.3390/mi12020215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Benedetti M. S., Whomsley R., Poggesi I., Cawello W., Mathy F.-X., Delporte M.-L., Papeleu P., and Watelet J.-B., Drug Metab. Rev. 41(3), 344–390 (2009). 10.1080/10837450902891295 [DOI] [PubMed] [Google Scholar]
- 237.Ishida S., Drug Metab. Pharmacokinet. 33(1), 49–54 (2018). 10.1016/j.dmpk.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 238.Sung J. H. and Shuler M. L., Lab Chip 9(10), 1385–1394 (2009). 10.1039/b901377f [DOI] [PubMed] [Google Scholar]
- 239.Ewart L., Apostolou A., Briggs S. A., Carman C. V., Chaff J. T., Heng A. R., Jadalannagari S., Janardhanan J., Jang K.-J., Joshipura S. R., Kadam M. M., Kanellias M., Kujala V. J., Kulkarni G., Le C. Y., Lucchesi C., Manatakis D. V., Maniar K. K., Quinn M. E., Ravan J. S., Rizos A. C., Sauld J. F. K., Sliz J. D., Tien-Street W., Trinidad D. R., Velez J., Wendell M., Irrechukwu O., Mahalingaiah P. K., Ingber D. E., Scannell J. W., and Levner D., “Qualifying a human liver-chip for predictive toxicology: Performance assessment and economic implications,” bioRxiv (2022). 10.1101/2021.12.14.472674 [DOI] [PMC free article] [PubMed]
- 240.Pisapia F., Balachandran W., and Rasekh M., Appl. Sci. 12(8), 3829 (2022). 10.3390/app12083829 [DOI] [Google Scholar]
- 241.Menezes P. D., Gadegaard N., Natal Jorge R. M., and Pinto S. I. S., Int. J. Numer. Methods Biomed. Eng. 37(5), e3445 (2021). 10.1002/cnm.3445 [DOI] [PubMed] [Google Scholar]
- 242.Wang J., Rodgers V. G., Brisk P., and Grover W. H., PLoS One 12(12), e0189429 (2017). 10.1371/journal.pone.0189429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Li Z., Hui J., Yang P., and Mao H., Biosensors 12(6), 370 (2022). 10.3390/bios12060370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ballard Z., Brown C., Madni A. M., and Ozcan A., Nat. Mach. Intell. 3(7), 556–565 (2021). 10.1038/s42256-021-00360-9 [DOI] [Google Scholar]
- 245.Mahdi Y. and Daoud K., J. Dispersion Sci. Technol. 38(10), 1501–1508 (2017). 10.1080/01932691.2016.1257391 [DOI] [Google Scholar]
- 246.Stoecklein D., Lore K. G., Davies M., Sarkar S., and Ganapathysubramanian B., Sci. Rep. 7(1), 46368 (2017). 10.1038/srep46368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hong S. H., Yang H., and Wang Y., Microfluid. Nanofluid. 24(6), 44 (2020). 10.1007/s10404-020-02349-z [DOI] [Google Scholar]
- 248.Comes M. C., Casti P., Mencattini A., Di Giuseppe D., Mermet-Meillon F., De Ninno A., Parrini M. C., Businaro L., Di Natale C., and Martinelli E., Sci. Rep. 9(1), 6789 (2019). 10.1038/s41598-019-42475-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Parlato S., De Ninno A., Molfetta R., Toschi E., Salerno D., Mencattini A., Romagnoli G., Fragale A., Roccazzello L., Buoncervello M., Canini I., Bentivegna E., Falchi M., Bertani F. R., Gerardino A., Martinelli E., Natale C., Paolini R., Businaro L., and Gabriele L., Sci. Rep. 7(1), 1093 (2017). 10.1038/s41598-017-01013-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Comes M. C., Filippi J., Mencattini A., Casti P., Cerrato G., Sauvat A., Vacchelli E., De Ninno A., Di Giuseppe D., D’Orazio M., Mattei F., Schiavoni G., Businaro L., Di Natale C., Kroemer G., and Martinelli E., Neural Comput. Appl. 33(8), 3671–3689 (2021). 10.1007/s00521-020-05226-6 [DOI] [Google Scholar]
- 251.Özkan A., Stolley D., Cressman E. N., McMillin M., DeMorrow S., Yankeelov T. E., and Rylander M. N., “The influence of chronic liver diseases on hepatic vasculature: A liver-on-a-chip review,” Micromachines 11(5), 487 (2020). 10.3390/mi11050487 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.