Abstract
Inadequate sleep duration and quality are associated with reduced cardiovascular health and increased mortality. Experimental evidence points to the sympathetic nervous system as a key mediator in the observed relationship between poor sleep and cardiovascular dysfunction. However, brain mechanisms underpinning the impaired sympathetic function associated with poor sleep remain unclear. Recent evidence suggests the central orexin system, particularly orexins A and B and their receptors, have a key regulatory role for sleep in animal and human models. While orexin system activity has been observed to significantly impact sympathetic regulation in animals, the extension of these findings to humans has been difficult due to an inability to directly assess orexin system activity in humans. However, direct measures of sympathetic activity in populations with narcolepsy and chronic insomnia, two sleep disorders associated with deficient and excessive orexin neural activity, have allowed indirect assessment of the relationships between orexin, sleep, and sympathetic regulation. Further, the recent pharmaceutical development of dual orexin receptor antagonists for use in clinical insomnia populations offers an unprecedented opportunity to examine the mechanistic role of orexin in sleep and cardiovascular health in humans. The current review assesses the role of orexin in both sleep and sympathetic regulation from a translational perspective, spanning animal and human studies. The review concludes with future research directions necessary to fully elucidate the mechanistic role for orexin in sleep and sympathetic regulation in humans.
Keywords: Sympathetic nervous system, narcolepsy, insomnia, cardiovascular health, orexin antagonist
Introduction
A significant proportion of the general population suffers from poor and/or insufficient sleep.1–3 Reduced sleep quantity and quality is associated with a number of cardiovascular and metabolic disorders, including an increased risk of hypertension as reported in cross-sectional1 and longitudinal analyses.4 Sleep disorders such as insomnia are associated with cardiometabolic dysfunction.5, 6 While the preponderance of epidemiological studies indicate a clear role for poor sleep efficiency and quantity in the pathogenesis of cardiovascular dysfunction, the mechanism(s) underlying these associations remain elusive.
One posited mechanism by which poor sleep contributes to cardiovascular dysfunction is dysregulation of the sympathetic nervous system. Experimental sleep deprivation and sleep restriction paradigms have reported elevated levels of sympathetic activity following acute sleep impairment.7–9 Dysfunction of the sympathetic nervous system is similarly observed within sleep disorders,10, 11 as well as cardiometabolic disorders such as hypertension and12 heart failure.13 Despite these findings, the central regulatory mechanisms underpinning these associations has not been established in humans.
Recent attention has shifted towards the potential role for the central orexin system in sleep and cardiovascular regulation.14–17 In an attempt to build upon prior findings, the current review assesses the crucial role that central orexinergic activity has on poor sleep and sympathetic dysregulation within animal and human models. Further, the potential for pharmaceutical interventions targeting the orexin system to more adequately assess its impact in human models, and to improve upon the translational efficacy of studies to date, will be discussed. Specifically, the recent development of dual orexin receptor antagonists offers a unique opportunity to assess the dynamic impact of orexin function on sleep and sympathetic neural control, not only in rodents and small animals, but also in humans.
Anatomical Situation of Orexin Neurons and Axonal Projections
The orexin peptides, also called hypocretins, were discovered simultaneously by two research groups just over two decades ago.18, 19 The orexin peptides consist of two types, orexin-A and B, which are encoded by the hypocretin neuropeptide precursor (HCRT) gene, and subsequently produced from the same precursor, prepro-orexin. Orexin producing neurons are contained within the lateral, dorsomedial, posterior and perifornical areas of the hypothalamus.18, 19 The isolation of orexin-producing neurons within specific hypothalamic regions suggests a limited scope of influence by the orexin system on other brain regions and physiological processes. However, orexin neurons extend a network of axonal projections which innervate numerous brain regions,20, 21 allowing a profound effect on complex physiological processes.
Some of the densest orexinergic axonal projections synapse in wake-promoting brain areas such as the noradrenergic locus coeruleus (LC), among others.20, 21 Orexin neurons receive additional input from the sleep-promoting regions,22, 23 which contribute to the modulatory role of orexin in sleep/wake transitions. In addition to its clear impact on sleep, orexin impacts areas involved in sympathetic autonomic control, including the nucleus of the solitary tract (NTS), rostral ventrolateral medulla (RVLM), and the parvocellular neurons of the paraventricular nucleus of the hypothalamus (PVN).20, 24–26 Many of these projections are reciprocated via afferent neural synapses on orexin-producing neurons from numerous autonomic and sleep/wakefulness related brain nuclei.22, 23 The interconnectedness of orexin producing neurons within both sleep and autonomic related brain regions indicate a crucial role of orexin as a link between insufficient sleep and impaired sympathetic control.27
Activity of Orexin at Cellular Targets
Orexin neuropeptides A and B bind selectively to the orexin receptors 1 and 2 (OX1R and OX2R). OX1R and OX2R are seven-transmembrane G-protein coupled receptors with differing affinities for orexin-A and orexin-B. Specifically, orexin A has equal affinity for both receptors, while orexin B has higher affinity for OX2R.19 This is particularly important as the expression of OX1R and OX2R differs throughout brain regions. For example, OX1R is more heavily distributed within arousal promoting areas such as the LC, while OX2R is more prominent in the histaminergic tuberomammillary nucleus (TMN).28 Conversely, other regions of the brain, including the serotonergic dorsal raphe (DR), the PVN, and the RVLM, express both OX1R and OX2R.28–33
In the brain, orexin receptors are primary distributed in neurons.32 One recent study reported that PVN OX1Rs are also co-localized with astrocytes.32 In vitro studies have consistently observed a robust cyclic AMP (cAMP) production in rat cerebral cortex astrocyte cultures upon stimulation with orexin A, and this increase in cAMP is mediated by OX1R but not OX2R, suggesting that OX1R is expressed in the brain astrocyte and its activation activates Gs-adenylyl cyclase-cAMP signaling.34 Whether these astrocytes expressing OX1R are involved in blood pressure and sleep regulation remains unknown.
Orexins A and B have been observed to elicit primarily excitatory influences on brain regions involved in sleep/wake35–42 and sympathetic43–46 regulation. However, the mechanisms by which orexins carry out this excitatory response are vast and complex (for reviews see Kukkonen et al.47, Scammell et al.33, and Dale et al.48). Orexin receptors have been shown to act variably through coupling to G-protein families including Gq/11, Gi/o, and Gs,33, 47 whereby binding of orexins subsequently regulate phospholipases, ion channels, protein kinases, or adenylyl cyclase, ultimately triggering the activation of various downstream signaling pathways. One of the primary means of orexin-mediated neuronal excitation is through a rise in intracellular Ca2+ at the target cell.19 Orexin administration results in an augmented intracellular Ca2+ concentration that appears to be primarily mediated through extracellular Ca2+ influx into the cytosol.49, 50 A role for transient receptor potential canonical channels (TRPCs) in facilitating the entry of extracellular Ca2+ into the cytosol has been proposed.51 While Ca2+ influx from extracellular space appears to be the primary mechanism for cellular activation, intracellular release of Ca2+ from the endoplasmic reticulum via activation of the Gq/11 mediated phospholipase C inositol trisphosphate (IP3) pathway may serve as a secondary mediator of neuronal excitability.49, 50 Ca2+ is an important secondary messenger, which can in turn activate many Ca2+-sensitive enzymes including calcium/calmodulin-dependent protein kinase II (CaMKII).32 Previous studies report that orexin-mediated CaMKII activation within the rat PVN can result in elevations of sympathetic nerve activity and blood pressure.32, 52 Orexins may also act through activation of the sodium-calcium exchanger53 or suppression of potassium efflux54 to further post-synaptically modify neuronal excitability. Many orexin neurons are glutamatergic, and can also release glutamate to facilitate neuronal excitation.55 Lastly, orexin neurons have been observed to modify neuronal excitability through pre-synaptic modulation of glutamate and γ-amino-butyric acid (GABA) release,50, 53 outlining a complex regulatory role for orexin in neuronal excitability through both pre- and post-synaptic mechanisms (Figure 1).
Figure 1:
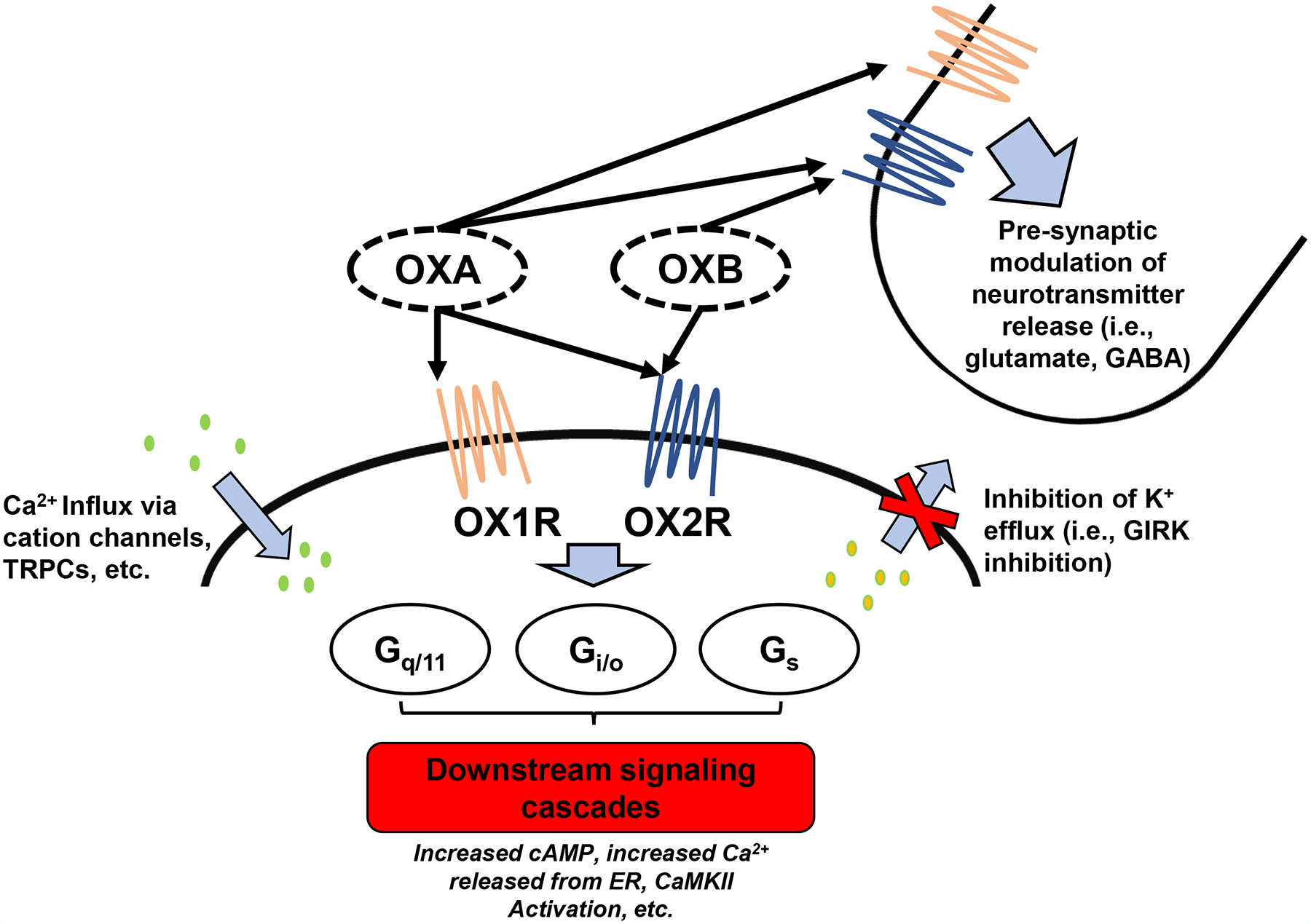
Actions of orexins A and B at cellular targets. Orexins elicit cellular excitation at targets through numerous means. Orexin receptors have been shown to act variably through coupling to G-protein families including Gq/11, Gi/o, and Gs which ultimately lead to numerous downstream signaling cascades. Orexin binding also leads to an influx of Ca2+ through cation channels, an inhibition of K+ efflux, and pre-synaptic modulation of glutamate and GABA release. OXA, orexin A; OXB, orexin B; OX1R, orexin receptor 1; OX2R, orexin receptor 2; TRPC, transient receptor potential canonical channels; GIRK, G protein-activated inwardly rectifying K+ channels; GABA, γ-amino butyric acid; cAMP, cyclic adenosine monophosphate; ER, endoplasmic reticulum; CaMKII, calcium/calmodulin-dependent protein kinase II.
Natural Orexin Oscillations in Sleep and Wakefulness
Strong evidence of orexin’s behavior-state dependent activity come from two studies utilizing single-unit recordings of orexin neurons in head-fixed56 and freely moving, unrestrained rats.57 Both studies reported similar findings, namely that orexin neuronal discharge is at its peak during wakefulness in freely moving rodents, and subsequently decreases when entering resting wakefulness, slow wave sleep (SWS), and rapid eye movement (REM) sleep.56, 57 Orexin activities are further elevated during active exploration.57 Similar findings have reported that orexin neuronal discharge levels are highest during goal-oriented tasks, such as foraging behavior and in response to food restriction,58, 59 indicating a role for orexin neuronal activation in achieving central arousal levels necessary for motivated behaviors based on external demands and energy balance. However, discharge of orexin neurons is abruptly reduced upon goal attainment. For instance, in the case of food consumption, orexin neuron activity in freely moving mice was diminished less than 1 second after contact was made with the food source.59 As sleep ensues, orexin discharge rates are reduced with increased depth of sleep.56, 57 However, during REM sleep, while orexin neuronal activation is nearly absent in tonic REM, brief bursts of orexin neuronal firing occur in tandem with phasic events and muscle twitches.57
This pattern of reduced orexin neuronal firing is similarly observed in other wake-promoting nuclei, such as the noradrenergic LC that is virtually silenced during REM sleep.60 The activity of orexin neurons precedes muscle movement and arousals causing transitions from REM to wakefulness,56 suggesting an important role in transitions from sleep to wakefulness. This is relevant to disorders such as insomnia, where fragmented or poor REM sleep serve as a key underlying characteristic associated with the perceived poor sleep61–63 and subsequent emotional disturbance.64, 65 The findings of orexin activation preceding arousal during REM sleep, as well as the role for REM disruption in the manifestation of insomnia, suggest a link between orexin hyperactivity and the clinical traits observed within insomnia disorder.
Causal Role for Orexin in Sleep-Wake Transitions
A key role for orexin stabilization of wake and sleep states was inferred by the discovery of orexin neuron destruction in the development of narcolepsy in animals66, 67 and humans.68, 69 Experimental manipulations of orexin activity have advanced the causal role for orexin in sleep, wakefulness, and sleep state transitions. De Lecea and colleagues have shown that selective, optogenetic stimulation of orexin producing neurons evokes transitions from SWS and REM to wakefulness.70, 71 Activation of orexin neurons selectively reduces the latency to awakening from sleep, indicating a key role for orexin neurons in sleep-state transitons,72 potentially through indirect effects on other arousal promoting brain regions.71, 73 Due to the differential expression of OX1R and OX2R in nuclei involved in sleep/wake regulation, it is likely that OX1R and OX2R exhibit differing effects on sleep regulation. Intracerebroventricular injection of orexin-A, which binds to both receptor types, promotes wakefulness in wildtype mice, although this effects is diminished to a greater extent following selective OX2R versus OX1R depletion.30 Similarly, antagonism of OX2R, but not OX1R, prior to optogenetic stimulation of orexin-producing neurons results in a significant reduction in orexin-mediated wakefulness.74
In the case of narcolepsy, the impact of orexin on maintaining wakefulness via projections to wake-promoting brain nuclei is thought to be a primary means by which narcolepsy occurs. In the ‘flip-flop’ model of sleep-state switching proposed by Saper and colleagues,72 orexin neurons send excitatory input to wake-promoting neurons, which act through a negative feedback mechanism to inhibit orexin neuronal activity. In narcolepsy, due to the removal of orexin neurons from this system, wake and sleep promoting brain centers exhibit a mutual inhibitory circuit, whereby any slight increase in activity in one system exceeding the other causes abrupt changes in arousal state due to self-disinhibition, leading to transitions from wakefulness to sleep.72 Orexin receptor restoration in orexin-receptor deficient mice,75 as well as intranasal orexin administration in narcoleptic humans76, 77 improve narcoleptic symptomology, supporting the role of proper orexin signaling in maintained sleep/wake regulation.
Conversely, mice with transgenic overexpression of prepro-orexin experience augmented levels of sleep fragmentation, particularly during REM sleep.78 Further, abnormalities are observed in muscle tone, with chronic overexpression of orexin resulting in intrusive muscle activation during REM sleep when atonia is normally observed.78 These findings are relevant given the associations between REM fragmentation and sleep disturbance observed in individuals with chronic insomnia.61–63 Thus, experimental evidence supports a causal role for orexin hyperactivity in the pathogenesis of insomnia.
Recently, there has been a surge in pharmaceutical development of dual orexin receptor antagonists for the treatment of insomnia,79–81 which have been shown to improve sleep outcomes while not drastically impacting sleep architecture. While invasive measures investigating the impact of orexin neuronal activity on sleep in humans are not feasible, the use of dual orexin receptor antagonists to improve sleep offers evidence of a key role for proper orexin signaling to maintain adequate sleep quality and offers a new research avenue to assess mechanistic roles of orexin in sleep and wakefulness in humans.
Orexin’s Role in Autonomic Nervous System Function
Orexin Regulation of Cardiac Activity
While the primary topic of this review surrounds the key role for orexin in sympathetic function and dysfunction, evidence has shown an additional role for the orexin system in parasympathetic regulation. Orexin-producing neurons synapse on brain regions involved in vagal control of the heart.82–84 Orexin axonal projections synapse on preganglionic cardiac vagal neurons, and primarily exhibit excitatory influences through activation of glutamatergic post-synaptic currents.84 In support of this, acute microinjection of orexin-A into regions involved in parasympathetic control of cardiac function such as the nucleus ambiguus (NAmb)82 and certain subnuclei of the NTS83 results in a robust dose-dependent bradycardic response which is abolished following muscarinic blockade or surgical vagotomy. However, this response is dependent on the brain region effected. For instance, orexin-A administration into the commissural nucleus of the NTS elicits a paradoxical pressor and tachycardic response.83, 85 Similarly, orexin-A injection into the rostral ventromedial medulla elicits tachycardia, a response which is partially abolished by muscarinic blockade, and fully ameliorated following nicotinic receptor inhibition,24 highlighting the complex role for orexin in the regulation of both parasympathetic and sympathetic influences at the level of the heart.
Orexin Regulation of Peripheral Sympathetic Outflow
Orexin axonal projections synapse in numerous areas involved with central regulation of sympathetic outflow to the periphery,20, 24–26 and receptor expression appears to be primarily found in autonomic neurons rather than glial populations.32 Early studies established a clear pressor response and associated augmented peripheral sympathetic outflow following central injection of orexin peptides in healthy animals,86, 87 likely through interactions at the level of pre-sympathetic neurons within the RVLM26, 88, 89 or indirectly through input to cardiovascular relevant regions such as the PVN.31, 46, 90, 91 In both brain regions, orexin-A and orexin-B are effective in eliciting membrane depolarization on target cells,44–46 although this effect within the RVLM may be facilitated primarily through the OX2R.45 Recent studies have additionally observed augmented baroreflex gain or sensitivity in response to orexin administration,29, 92, 93 indicating that orexin not only elicits a sympathoexcitatory response, but also enhances sympathetic responsiveness to blood pressure fluctuations. Finally, orexin hyperactivity has been observed in numerous models of hypertension including salt-sensitive,94, 95 obesity related,90, 91 stress-induced,96 and others (for review see Huber et al.16).
Despite several studies examining the role of orexin in sympathetic regulation within animal models, only one study has assessed orexin’s sympathomimetic effects in healthy humans using intranasal administation.97 Orexin administered intravenously does not appear to significantly impact cardiovascular measures, indicating a primarily central impact.88 For this reason, a significant barrier exists in assessing the effect of orexin on sympathetic regulation in humans. However, intranasal administration of orexin-A results in elevations of cerebrospinal fluid (CSF) orexin-A concentrations in non-human primates,98 indicating that nasal administration of orexins offers a feasible option to assess the effects of central orexin neuronal activity on peripheral sympathetic activation. In the only study of its kind, Meusel et al.97 utilized intranasal administration of orexin-A in healthy adults to assess its effects on muscle sympathetic nerve activity (MSNA), a direct measure of post-ganglionic efferent sympathetic outflow in humans.99–101 In response to intranasal orexin-A, the authors observed a significant, albeit modest, increase in MSNA independent of changes in other cardiovascular measures.97 In contrast to studies in rodents,29, 92, 93 orexin-A administration did not impact baroreflex sensitivity,97 although it appeared to elevate the set-point of sympathetic outflow to the periphery. In tandem with results from animal models, the findings of Meusel et al.97 suggest a sympathomimetic effect of central orexin, supporting the concept that hyperactive orexinergic activity is a key player in the exaggerated sympathetic activation observed in human sleep disorders.10
Orexin as a Link Between Sleep Disorders and Sympathetic Dysregulation in Humans
Sleep disruption and discontinuity are associated with sympathetic dysregulation in humans.27 While blood pressure is elevated in response to sleep deprivation, experimental evidence has shown that total sleep deprivation significantly increases peripheral sympathetic outflow dependent upon age and sex.7, 102 Similarly, levels of urinary and plasma norepinephrine have been observed to increase following semi-chronic models of sleep restriction with9 and without8 circadian misalignment in otherwise healthy adults. In an observational study assessing correlates of sleep disruption and corresponding associations with MSNA, Taylor et al.103 observed that the frequency of nocturnal arousals, or sleep disruptions, was most highly associated with peripheral sympathetic outflow in individuals with and without obstructive sleep apnea. These findings suggest an association between inadequate sleep quality/quantity and excessive sympathetic activation in humans. However, the role that orexin plays in these associations remains difficult to disentangle in humans due to methodological limitations. While acute intranasal administration of orexin-A evokes a modest increase in sympathetic activity in healthy adults,97 the mechanisms of its action on central regulatory centers of peripheral sympathetic outflow can only be inferred from animal models. However, human models of chronic sleep disorders known to be impacted by orexin activity (i.e., narcolepsy and insomnia) offer further insight into the role of orexin in chronic sympathetic disturbance.
Narcolepsy Types 1 and 2.
Perhaps the most compelling evidence for a role of orexin in human sympathetic function comes from a study by Donadio et al.11 where MSNA was monitored in a group of patients with narcolepsy type 1 (NT1) and healthy controls. The authors reported a reduced level of peripheral sympathetic outflow in participants with NT1. These findings are consistent with early studies in orexin-knockout mice,104 whereby blood pressure and cardiovascular reactivity to stress were significantly reduced compared to wild-type mice. Further, these differences were abolished following systemic application of an α-adrenergic receptor antagonist, offering evidence of reduced peripheral sympathetic outflow in orexin knockout mice.104 The concentration of circulating orexin-A within the cerebrospinal fluid (CSF) of the patients with NT1 was positively associated with MSNA levels, indicating that lower levels of orexin expression was associated with blunted sympathetic activity11 (Figure 2).
Figure 2:
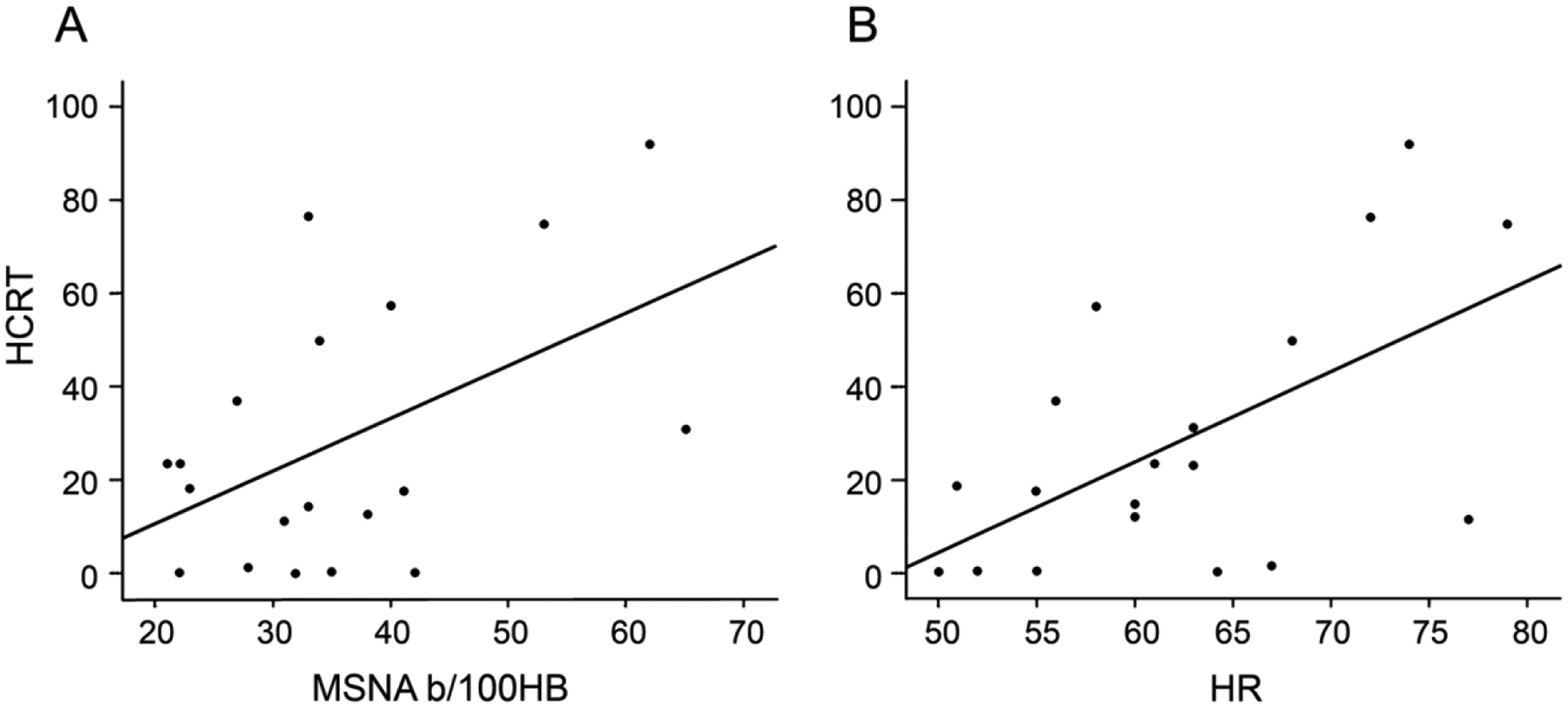
Relationships between orexin cerebrospinal fluid concentrations and resting A) muscle sympathetic nerve activity (MSNA) and B) heart rate (HR) in individuals with narcolepsy. HCRT, hypocretin; MSNA, muscle sympathetic nerve activity (bursts/100 heart beats); HR, heart rate.Obtained with permission from Donadio et al.11
While sympathetic outflow during wakefulness mimics what might be expected based on findings in animals, nocturnal sympathetic activity does not appear to be disturbed in individuals with NT1.105 Rather, MSNA in NT1 patients is reduced as NREM sleep is initiated, and increased during REM sleep,105 similar to healthy adults.106 Despite the maintained reductions in nocturnal NREM sympathetic activity, narcolepsy is associated with elevated nocturnal blood pressure in humans105, 107 and animals108 alike. However, the mechanisms underlying non-dipping status are inconsistent between animal and human studies.105, 108 Administration of prazosin, an α1-receptor antagonist, alleviates blunted blood pressure dipping patterns in orexin-knockout mice,108 hinting at a role for augmented peripheral sympathetic nerve activity and thus increased peripheral vasoconstriction as a key determinant underlying the impaired blood pressure dipping pattern. However, this hypothesized sympathetic augmentation is not observed in humans.105 It is notable that Alvente et al.108 also observed a normalized blood pressure dipping pattern after application of atenolol, a β1-adrenergic antagonist in orexin knockout mice, which reduces adrenergic influence at the level of the heart. This finding suggests that while reductions in orexin levels may lead to reduced sympathetic outflow to regional arteries, including muscle sympathetic nerves, augmented regional sympathetic outflow to other organs such as the heart may be responsible for maintained elevations in nocturnal blood pressure. However, assessment of cardiac sympathetic activity in humans with narcolepsy via cardiac scintigraphy does not support this hypothesis.109 These discrepant findings of nocturnal sympathetic function and elevated blood pressure in NT1 patients infer that the nocturnal cardiovascular dysfunction is secondary to disturbed sleep, not necessarily orexin deficiency. Nocturnal arousals are associated with elevations in sympathetic outflow and blood pressure.110, 111 Importantly, the assessment by Donadio et al.105 only sampled MSNA during periods of undisturbed sleep, potentially limiting the extension of their findings to durations of sleep characterized by frequent arousals commonly associated with narcolepsy.112 Grimaldi et al.107 have shown that fluctuations in nocturnal blood pressure in narcolepsy are temporally related to sleep disruption caused by arousals, periodic limb movements, etc., indicating that the apparent contrary increases in nocturnal blood pressure in orexin-deficient humans may be more closely associated with sleep disruption, and not orexin-deficiency alone (Figure 3).
Figure 3:
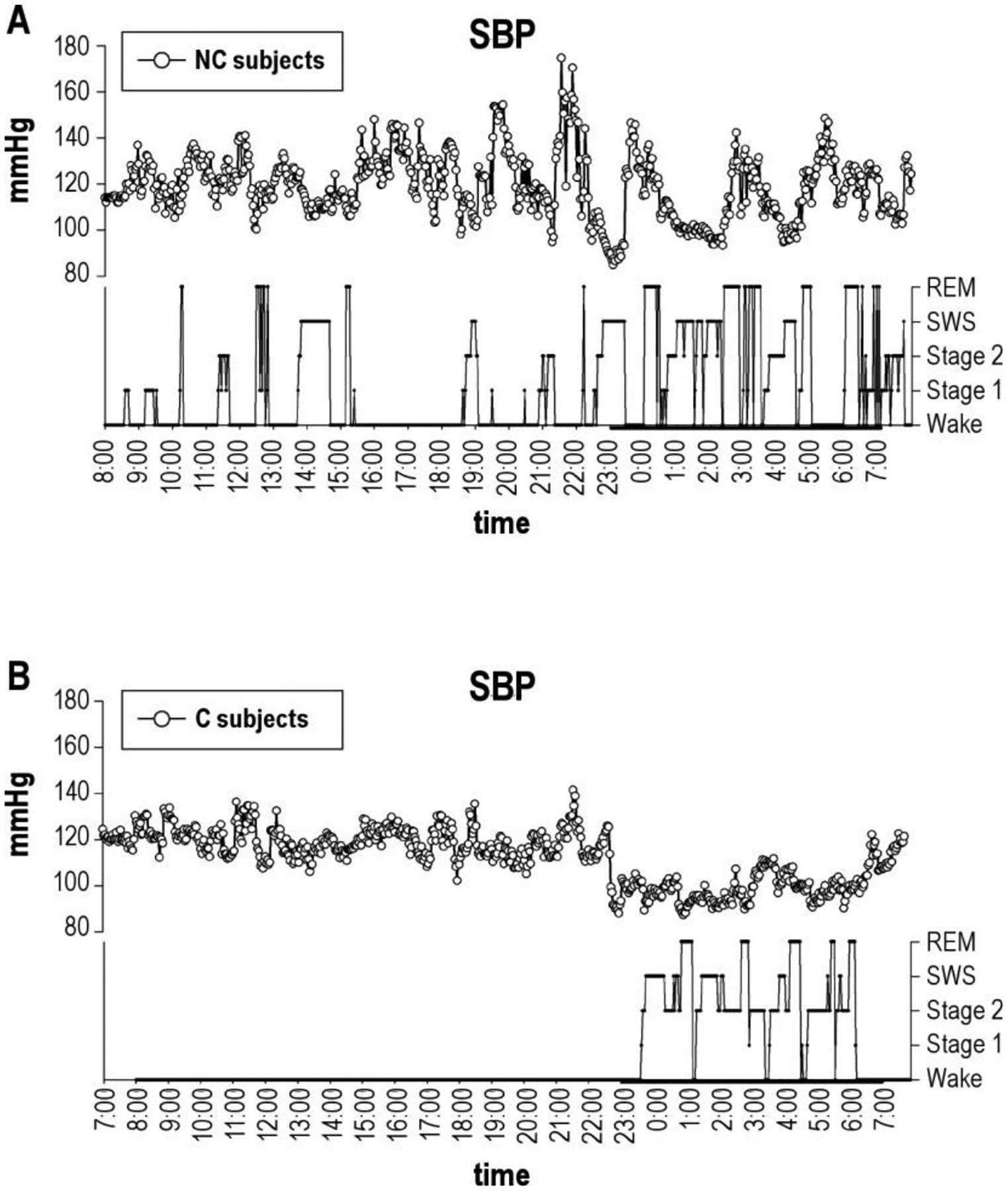
Nocturnal blood pressure fluctuations in a representative individual with narcolepsy (A) and a healthy control (B). Nocturnal fluctuations in systolic blood pressure (SBP) in individuals with narcolepsy are temporally related to changes in wake/sleep state. NC, narcolepsy with cataplexy; SBP, systolic blood pressure; C, control subject. Obtained with permission from Grimaldi et al.107
Based on these findings, it is plausible that increased sympathetic and cardiovascular reactivity to frequent arousals may lead to the augmented nocturnal blood pressure in NT1.107 Conversely, during the daytime when orexin neurons should be active,56, 57 the absence of these neurons in narcoleptic patients results in reduced sympathetic activity.11 This is further supported by a blunted morning blood pressure surge in narcoleptic patients,107 given the well-characterized relationship between augmented sympathetic reactivity and elevated blood pressure surge in the morning.113
It is worth noting, however, that a recent study examining heart rate reactivity to nocturnal arousal and sleep disruption reported that NT1 patients with low levels of CSF orexin-A exhibited blunted reactivity.114 Given the findings that orexin neuronal activation precedes arousal,56 and in turn facilitates shifts from sleep to wakefulness,70, 71 deficient orexin neural activity may blunt the cardiovascular response to sleep disruption through inadequate orexin signaling. While blunted cardiovascular reactivity to nocturnal arousal may not support the augmented nocturnal blood pressure observed in NT1, an increased sleep fragmentation may lead to frequent arousals, whereby cardiovascular parameters remain elevated throughout the night107 despite acutely depressed reactivity to singular arousal events.114
There has been limited research assessing autonomic control in populations with narcolepsy type 2 (NT2), though this is likely due to its etiology being less understood than that of NT1, making diagnosis and further assessment challenging. NT2 is phenotypically similar to NT1, although it is not associated with additional cataplexy. While NT2 has been associated with partial loss of orexin neurons within the hypothalamus,115 CSF orexin levels are more often within normal ranges when compared to narcolepsy with cataplexy.116, 117 The similarities in symptomology, yet differing levels of orexin deficiency between NT1 and NT2, make comparative analysis of autonomic dysfunction between the two disease subtypes an applicable avenue of future research to delineate the unique roles of orexin dysfunction versus sleep impairment on sympathetic control in humans.
In summary, while research within NT1 patients supports a role for orexin in maintaining wake basal sympathetic tone, discrepant findings exist regarding its role in nocturnal autonomic control, making it difficult to definitively determine the nocturnal role of orexin on sympathetic outflow. Future work assessing the diurnal impacts of orexin-deficiency on sympathetic regulation of the vasculature are warranted to disentangle the complex mechanisms underlying cardiovascular health concerns in individuals with narcolepsy.
Insomnia
In contrast with narcolepsy, insomnia has been suggested to result from excessive orexin neural activity.78 In a large sample of 228 humans with chronic insomnia, significant elevations in plasma orexin-A levels were reported when compared to controls.118 Orexin-A is highly lipophilic,119 allowing its access across the blood brain barrier and subsequent monitoring in the bloodstream. Further, plasma orexin-A levels were exacerbated both in the duration and self-reported severity of the disorder,118 outlining a key role for excessive orexin neural activity as an underlying factor of chronic insomnia.
While assessment of sympathetic activity in humans with chronic insomnia has been a variable of interest in numerous studies, the findings are often controversial and inconsistent with one another (for review120), although this may be primarily due to differing methodologies utilized to assess and/or estimate sympathetic activity. While early assessment of sympathetic activity in chronic insomnia reported elevated plasma norepinephrine levels,121 a recent study by Grimaldi et al.122 reported the opposite. These findings are beneficial given existing scientific gaps, but the use of plasma catecholamine sampling is subject to numerous limitations123 that limit the interpretability of the data.
To date, only one study has directly assessed sympathetic neural activity via microneurography in participants with chronic insomnia.10 In a cross-sectional analysis of 12 individuals with diagnosed chronic insomnia and 12 healthy controls, Carter et al.10 reported that baseline sympathetic outflow in insomnia participants did not differ compared to controls, although sympathetic baroreflex sensitivity was reduced. Tang et al.118 reported that orexin concentrations differ based upon disease severity and the course of the disorder. It is possible that lack of differences in baseline sympathetic outflow reported by Carter et al.10 were due, in part, to differing levels of insomnia symptom severity and time-course, thus differing levels of central orexin neural activation. Evidence in support of this notion can be observed in both rats and rabbits, whereby dose-dependent increases in peripheral sympathetic activity were observed following central administration of orexin peptides.86, 87
Although baseline MSNA was not different between insomnia and controls, MSNA reactivity to the cold pressor test was augmented in chronic insomnia10 (Figure 4). In animal models, activity of orexin neurons can differ based upon the type of stress.104, 124 Kayaba et al.104 reported a significantly blunted cardiovascular and locomotor response to social stress in orexin knockout mice. Conversely, the cardiovascular response to noxious stimuli was not impacted by orexin neuronal depletion, suggesting that orexin-mediated cardiovascular reactivity primarily occurs in response to social stressors that require active vigilance of the environment.104 These findings were supported by subsequent research reporting that pharmaceutical dual orexin receptor antagonism blunts the cardiovascular response to fear stress (i.e., re-exposure to previous foot-shock box), but not to restraint or cold stress.124 These findings in animal models are not concordant with those in humans with chronic insomnia,10 perhaps in part due to the differing forms of cold stress.10, 124 In the study by Carter et al.,10 participants were required to submerge their hand voluntarily up to the wrist in cold ice water for 2 minutes, while the animals studied by Furlong et al.124 were placed in a 4°C refrigerator. The differences in the modality of the cold stress utilized makes translation of the findings between the two studies challenging. Further, previous work has shown that acute total sleep deprivation leads to an augmented pain perception in healthy adults exposed to cold pressor test.125 The chronic insomnia participants in the study by Carter et al.10 received, on average, just over 6 hours of sleep per night based on 2-week actigraphy wristwatch monitoring in their home environment. As such, the insomnia participants tested likely had concurrent chronic sleep restriction that may have influenced how the cold stress and its associated perceived pain.125 The combined difficulties in the translatability of stressor methodologies between animal104, 124 and human10 studies, as well as the added perceptual/psychological influence on reactivity to cold stress in humans10, 125 makes assessment of orexin on sympathetic regulation at rest and in response to stress difficult to interpret. Future work is needed to assess stressor-specific sympathetic reactivity in individuals with chronic insomnia, ideally in tandem with assessment of plasma or CSF orexin concentrations.
Figure 4:
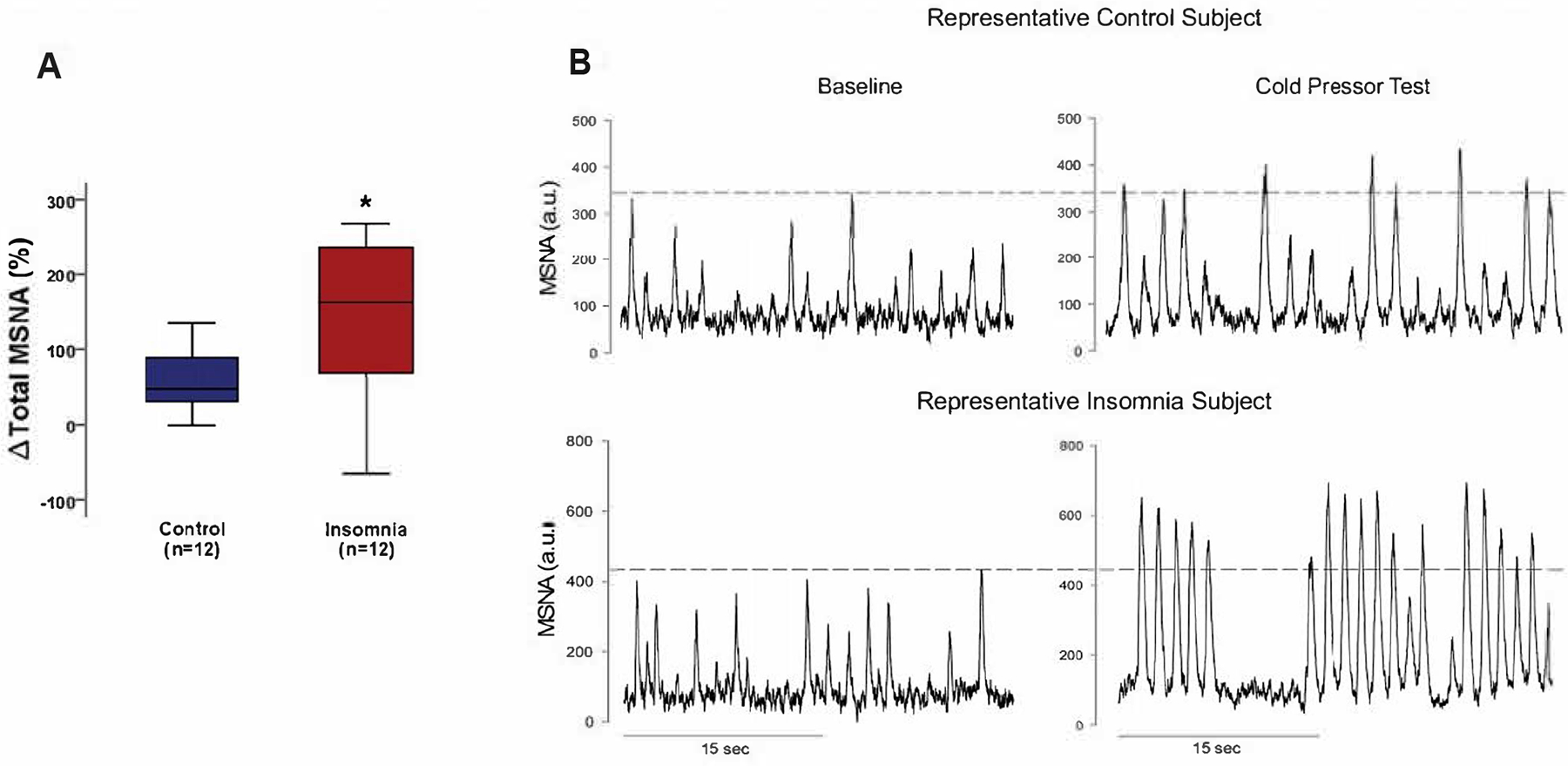
Muscle sympathetic nerve (MSNA) reactivity to the cold pressor test in healthy adults and individuals with chronic insomnia. Individuals with chronic insomnia showed augmented total MSNA reactivity to cold pressor stress (A). This is similarly depicted in the representative neurogram (B) showing healthy controls (B, Top) and individuals with chronic insomnia (B, bottom) during resting baseline and a 2-minute cold pressor test. The dotted lines (B) represent amplitude normalization to the largest sympathetic burst during baseline recording. MSNA, muscle sympathetic nerve activity. Obtained with permission from Carter et al.10
Dual Orexin Receptor Antagonism and Sympathetic Cardiovascular Control
Despite the difficulties regarding direct assessment of orexin neural activity in individuals with chronic insomnia, the recent utilization of dual orexin receptor antagonists for insomnia treatment80, 81 offer a unique opportunity to assess the role of orexin in sympathetic regulation. In hypertensive animal models, systemic dual orexin receptor antagonism reduced blood pressure.126, 127 Specifically, oral administration of almorexant in spontaneously hypertensive rats reduced daytime and nocturnal arterial pressure levels, and reduced norepinephrine sampled from cerebrospinal fluid and plasma.127 Similarly, in genetically hypertensive BPH/2J mice, Jackson et al.126 observed reduced blood pressure following intraperitoneal almorexant administration. Following almorexant administration, sympathetic ganglionic blockade only marginally reduced blood pressure in BPH/2J mice, indicating a significant sympathoinhibitory effect of pharmaceutical orexin receptor antagonism alone.126 To date, few studies have assessed the cardiovascular and sympathetic consequences of orexin receptor antagonism in humans. In a study of treated hypertensive subjects with insomnia symptoms that persisted for at least one month, 2 weeks of treatment with Suvorexant did not improve ambulatory blood pressure compared to the placebo condition.128
Patel et al.129 examined the acute impact of low-dose SB-649868, a dual orexin-receptor antagonist, on neuroendocrine and sympathetic responsiveness to hypoglycemia in healthy young men. While hypoglycemia elicited an increase in circulating epinephrine and norepinephrine, orexin antagonism did not impact these responses.129 However, in a population of psychiatric patients treated with Suvorexant, plasma norepinephrine levels tended to decrease following 8-weeks of treatment,130 although plasma norepinephrine sampling is once again subject to numerous limitations in interpretability, and does not directly assess regional differentiation in sympathetic outflow.123
There is presently a lack of studies assessing the impact of orexin antagonism on subsequent sympathetic regulation in humans. Of the studies that have been conducted in humans,128–130 differing concentrations and length of medication, as well as the lack of direct sympathetic recordings, make conclusions difficult. Further, differing populations and comorbidities, including hypertension128 and psychiatric disorders,130 add ambiguity. The presence of dual orexin receptor antagonists that have shown efficacy in clinical trials80, 81 provide a unique opportunity to assess direct sympathetic recordings in healthy and disordered populations to fully elucidate the impacts of orexin receptor antagonism on high fidelity markers of peripheral sympathetic activity.
Conclusions and Future Directions
While orexin producing neurons are locally produced within the lateral hypothalamic area, widespread projections to numerous brain regions allow them to have an important role in orchestrating complex physiological processes. Orexin axonal projections exhibit dense innervation in brain regions responsible for sleep/wakefulness, as well as central sympathetic regulatory brain regions, suggesting a potential mechanistic link between sleep disruption and sympathetic dysregulation. Studies performed in animals have documented a clear role for orexin in the regulation of sleep and wakefulness, as well as sympathetic outflow to the periphery in healthy and diseased models. The translation of these findings into humans has been challenging due to numerous methodological and ethical considerations. Assessment of direct sympathetic recordings in response to acute intranasal orexin administration demonstrated a moderate sympathomimetic effect of orexin, corresponding to findings observed in animals.97 Studies assessing autonomic control in populations with sleep disorders, including narcolepsy and insomnia, have supported a role for deficient or excessive orexin neural activity as a mechanism underlying the autonomic impairments observed in these populations.10, 11, 107
Despite the important conclusions taken from studies to date, additional work remains to determine how orexin mediates sleep and sympathetic control in humans. Direct measurements of sympathetic neural activity via microneurography are necessary to assess how acute and chronic treatment with oral dual orexin receptor antagonists impact sympathetic regulation and sleep parameters in healthy and disordered populations. How these treatments impact daytime versus nocturnal cardiovascular control will help to elucidate the impact of orexin versus secondary mechanisms, such as sleep fragmentation, on sympathetic regulation in humans. This is particularly important because sleep fragmentation alone has been associated with sympathoexcitation.103 Second, attentiveness to stressors employed in laboratory studies appears warranted. In animal models, cardiovascular responses differ in response to social versus physical stress following orexin knockdown,104, 124 whereas in humans, individuals with chronic insomnia and presumed augmented orexin activity118 exhibit hyperreactivity to cold stress.10 Additional studies assessing different stressor types in humans is necessary. Third, attention to symptom severity in human sleep-disordered populations is warranted. Sympathetic activity is related to CSF orexin levels in narcolepsy,11 and plasma orexin levels correspond to the severity of insomnia symptomology,10 indicating an association between present orexin levels and physiological dysfunction. Finally, age and sex need to be accounted for in research moving forward. Previous research from has shown that older, post-menopausal women are more susceptible to sympathoexcitation following sleep deprivation,7 supporting a greater association between poor sleep and hypertension in women compared to men.1 Orexin antagonists were recently shown to reduce subjective vasomotor symptoms in older women,131 suggesting that treatment with orexin receptor antagonists may improve sleep quality surrounding the menopausal transition and into menopause. How these treatments impact subsequent cardiovascular and sympathetic function have yet to be determined. Further studies assessing the impact of orexin on sleep and sympathetic function may lead to a more robust understanding of mechanisms underlying the relationship between poor sleep and sympathetic dysfunction, and may lead to increased preventative care measures and improved the cardiovascular outlook for individuals impacted by chronic sleep disturbance.
Supplementary Material
Funding Sources:
The current research project was funded in part by the National Institutes of Health (JRC: AA-024892, U54GM115371, P20GM103474; ZS: R15HL150703).
Disclosure Statement:
JRC has previously received research funding from the Merck Investigators Studies Program (MISP) to study the effects of Suvorexant, and FDA-approved drug for insomnia treatment, on sympathetic and cardiovascular control. However, JRC does not receive any personal financial benefits from Merck Pharmaceutical.
Footnotes
Competing Interests: The authors declare that they have no competing interests.
References
- 1.Grandner M, Mullington JM, Hashmi SD, Redeker NS, Watson NF, Morgenthaler TI. Sleep duration and hypertension: Analysis of > 700,000 adults by age and sex. J Clin Sleep Med. 2018;14:1031–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grandner MA, Chakravorty S, Perlis ML, Oliver L, Gurubhagavatula I. Habitual sleep duration associated with self-reported and objectively determined cardiometabolic risk factors. Sleep Med. 2014;15:42–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krueger PM, Friedman EM. Sleep duration in the united states: A cross-sectional population-based study. Am J Epidemiol. 2009;169:1052–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cappuccio FP, Stranges S, Kandala NB, Miller MA, Taggart FM, Kumari M, et al. Gender-specific associations of short sleep duration with prevalent and incident hypertension: The whitehall ii study. Hypertension. 2007;50:693–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: The most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17:241–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32:491–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter JR, Fonkoue IT, Greenlund IM, Schwartz CE, Mokhlesi B, Smoot CA. Sympathetic neural responsiveness to sleep deprivation in older adults: Sex differences. Am J Physiol Heart Circ Physiol. 2019;317:H315–H322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Covassin N, Bukartyk J, Singh P, Calvin AD, St Louis EK, Somers VK. Effects of experimental sleep restriction on ambulatory and sleep blood pressure in healthy young adults: A randomized crossover study. Hypertension. 2021;78:859–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimaldi D, Carter JR, Van Cauter E, Leproult R. Adverse impact of sleep restriction and circadian misalignment on autonomic function in healthy young adults. Hypertension. 2016;68:243–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter JR, Grimaldi D, Fonkoue IT, Medalie L, Mokhlesi B, Cauter EV. Assessment of sympathetic neural activity in chronic insomnia: Evidence for elevated cardiovascular risk. Sleep. 2018;41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donadio V, Liguori R, Vandi S, Pizza F, Dauvilliers Y, Leta V, et al. Lower wake resting sympathetic and cardiovascular activities in narcolepsy with cataplexy. Neurology. 2014;83:1080–1086 [DOI] [PubMed] [Google Scholar]
- 12.Grassi G, Pisano A, Bolignano D, Seravalle G, D’Arrigo G, Quarti-Trevano F, et al. Sympathetic nerve traffic activation in essential hypertension and its correlates: Systematic reviews and meta-analyses. Hypertension. 2018;72:483–491 [DOI] [PubMed] [Google Scholar]
- 13.Grassi G, Seravalle G, Cattaneo BM, Lanfranchi A, Vailati S, Giannattasio C, et al. Sympathetic activation and loss of reflex sympathetic control in mild congestive heart failure. Circulation. 1995;92:3206–3211 [DOI] [PubMed] [Google Scholar]
- 14.Mullington JM, Cunningham TJ, Haack M, Yang H. Causes and consequences of chronic sleep deficiency and the role of orexin. Front Neurol Neurosci. 2021;45:128–138 [DOI] [PubMed] [Google Scholar]
- 15.Sieminski M, Szypenbejl J, Partinen E. Orexins, sleep, and blood pressure. Curr Hypertens Rep. 2018;20:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huber MJ, Chen QH, Shan Z. The orexin system and hypertension. Cell Mol Neurobiol. 2018;38:385–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuwaki T. Orexin (hypocretin) participates in central autonomic regulation during fight-or-flight response. Peptides. 2021;139:170530. [DOI] [PubMed] [Google Scholar]
- 18.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: A family of hypothalamic neuropeptides and g protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585 [DOI] [PubMed] [Google Scholar]
- 20.Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827:243–260 [DOI] [PubMed] [Google Scholar]
- 21.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, et al. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46:297–308 [DOI] [PubMed] [Google Scholar]
- 23.Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciriello J, Li Z, de Oliveira CV. Cardioacceleratory responses to hypocretin-1 injections into rostral ventromedial medulla. Brain Res. 2003;991:84–95 [DOI] [PubMed] [Google Scholar]
- 25.Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, et al. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci U S A. 1999;96:748–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Machado BH, Bonagamba LG, Dun SL, Kwok EH, Dun NJ. Pressor response to microinjection of orexin/hypocretin into rostral ventrolateral medulla of awake rats. Regul Pept. 2002;104:75–81 [DOI] [PubMed] [Google Scholar]
- 27.Greenlund IM, Carter JR. Sympathetic neural responses to sleep disorders and insufficiencies. Am J Physiol Heart Circ Physiol. 2022;322:H337–H349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25 [DOI] [PubMed] [Google Scholar]
- 29.Shahid IZ, Rahman AA, Pilowsky PM. Orexin a in rat rostral ventrolateral medulla is pressor, sympatho-excitatory, increases barosensitivity and attenuates the somato-sympathetic reflex. Br J Pharmacol. 2012;165:2292–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mieda M, Hasegawa E, Kisanuki YY, Sinton CM, Yanagisawa M, Sakurai T. Differential roles of orexin receptor-1 and −2 in the regulation of non-rem and rem sleep. J Neurosci. 2011;31:6518–6526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan Y, Jiang E, Hahka T, Chen QH, Yan J, Shan Z. Orexin a increases sympathetic nerve activity through promoting expression of proinflammatory cytokines in sprague dawley rats. Acta Physiol (Oxf). 2018;222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan Y, Jiang E, Gao H, Bigalke J, Chen B, Yu C, et al. Activation of orexin system stimulates camkii expression. Front Physiol. 2021;12:698185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scammell TE, Winrow CJ. Orexin receptors: Pharmacology and therapeutic opportunities. Annu Rev Pharmacol Toxicol. 2011;51:243–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woldan-Tambor A, Bieganska K, Wiktorowska-Owczarek A, Zawilska JB. Activation of orexin/hypocretin type 1 receptors stimulates camp synthesis in primary cultures of rat astrocytes. Pharmacol Rep. 2011;63:717–723 [DOI] [PubMed] [Google Scholar]
- 35.Bayer L, Eggermann E, Serafin M, Saint-Mleux B, Machard D, Jones B, et al. Orexins (hypocretins) directly excite tuberomammillary neurons. Eur J Neurosci. 2001;14:1571–1575 [DOI] [PubMed] [Google Scholar]
- 36.Brown RE, Sergeeva O, Eriksson KS, Haas HL. Orexin a excites serotonergic neurons in the dorsal raphe nucleus of the rat. Neuropharmacology. 2001;40:457–459 [DOI] [PubMed] [Google Scholar]
- 37.Brown RE, Sergeeva OA, Eriksson KS, Haas HL. Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/hypocretin, histamine and noradrenaline). J Neurosci. 2002;22:8850–8859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burlet S, Tyler CJ, Leonard CS. Direct and indirect excitation of laterodorsal tegmental neurons by hypocretin/orexin peptides: Implications for wakefulness and narcolepsy. J Neurosci. 2002;22:2862–2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eggermann E, Serafin M, Bayer L, Machard D, Saint-Mleux B, Jones BE, et al. Orexins/hypocretins excite basal forebrain cholinergic neurones. Neuroscience. 2001;108:177–181 [DOI] [PubMed] [Google Scholar]
- 40.Eriksson KS, Sergeeva O, Brown RE, Haas HL. Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci. 2001;21:9273–9279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, et al. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415:145–159 [PubMed] [Google Scholar]
- 42.Liu RJ, van den Pol AN, Aghajanian GK. Hypocretins (orexins) regulate serotonin neurons in the dorsal raphe nucleus by excitatory direct and inhibitory indirect actions. J Neurosci. 2002;22:9453–9464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dergacheva O, Yamanaka A, Schwartz AR, Polotsky VY, Mendelowitz D. Optogenetic identification of hypothalamic orexin neuron projections to paraventricular spinally projecting neurons. Am J Physiol Heart Circ Physiol. 2017;312:H808–H817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Follwell MJ, Ferguson AV. Cellular mechanisms of orexin actions on paraventricular nucleus neurones in rat hypothalamus. J Physiol. 2002;545:855–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang SC, Dai YW, Lee YH, Chiou LC, Hwang LL. Orexins depolarize rostral ventrolateral medulla neurons and increase arterial pressure and heart rate in rats mainly via orexin 2 receptors. J Pharmacol Exp Ther. 2010;334:522–529 [DOI] [PubMed] [Google Scholar]
- 46.Shirasaka T, Miyahara S, Kunitake T, Jin QH, Kato K, Takasaki M, et al. Orexin depolarizes rat hypothalamic paraventricular nucleus neurons. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1114–1118 [DOI] [PubMed] [Google Scholar]
- 47.Kukkonen JP, Leonard CS. Orexin/hypocretin receptor signalling cascades. Br J Pharmacol. 2014;171:314–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dale NC, Hoyer D, Jacobson LH, Pfleger KDG, Johnstone EKM. Orexin signaling: A complex, multifaceted process. Front Cell Neurosci. 2022;16:812359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lund PE, Shariatmadari R, Uustare A, Detheux M, Parmentier M, Kukkonen JP, et al. The orexin ox1 receptor activates a novel ca2+ influx pathway necessary for coupling to phospholipase c. J Biol Chem. 2000;275:30806–30812 [DOI] [PubMed] [Google Scholar]
- 50.van den Pol AN, Gao XB, Obrietan K, Kilduff TS, Belousov AB. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci. 1998;18:7962–7971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peltonen HM, Magga JM, Bart G, Turunen PM, Antikainen MS, Kukkonen JP, et al. Involvement of trpc3 channels in calcium oscillations mediated by ox(1) orexin receptors. Biochem Biophys Res Commun. 2009;385:408–412 [DOI] [PubMed] [Google Scholar]
- 52.Li DP, Zhou JJ, Zhang J, Pan HL. Camkii regulates synaptic nmda receptor activity of hypothalamic presympathetic neurons and sympathetic outflow in hypertension. J Neurosci. 2017;37:10690–10699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Acuna-Goycolea C, van den Pol AN. Neuroendocrine proopiomelanocortin neurons are excited by hypocretin/orexin. J Neurosci. 2009;29:1503–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoang QV, Bajic D, Yanagisawa M, Nakajima S, Nakajima Y. Effects of orexin (hypocretin) on girk channels. J Neurophysiol. 2003;90:693–702 [DOI] [PubMed] [Google Scholar]
- 55.Rosin DL, Weston MC, Sevigny CP, Stornetta RL, Guyenet PG. Hypothalamic orexin (hypocretin) neurons express vesicular glutamate transporters vglut1 or vglut2. J Comp Neurol. 2003;465:593–603 [DOI] [PubMed] [Google Scholar]
- 56.Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–6720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gonzalez JA, Jensen LT, Iordanidou P, Strom M, Fugger L, Burdakov D. Inhibitory interplay between orexin neurons and eating. Curr Biol. 2016;26:2486–2491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713 [DOI] [PubMed] [Google Scholar]
- 60.Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feige B, Al-Shajlawi A, Nissen C, Voderholzer U, Hornyak M, Spiegelhalder K, et al. Does rem sleep contribute to subjective wake time in primary insomnia? A comparison of polysomnographic and subjective sleep in 100 patients. J Sleep Res. 2008;17:180–190 [DOI] [PubMed] [Google Scholar]
- 62.Feige B, Nanovska S, Baglioni C, Bier B, Cabrera L, Diemers S, et al. Insomnia-perchance a dream? Results from a nrem/rem sleep awakening study in good sleepers and patients with insomnia. Sleep. 2018;41 [DOI] [PubMed] [Google Scholar]
- 63.Riemann D, Spiegelhalder K, Nissen C, Hirscher V, Baglioni C, Feige B. Rem sleep instability--a new pathway for insomnia? Pharmacopsychiatry. 2012;45:167–176 [DOI] [PubMed] [Google Scholar]
- 64.Wassing R, Benjamins JS, Dekker K, Moens S, Spiegelhalder K, Feige B, et al. Slow dissolving of emotional distress contributes to hyperarousal. Proc Natl Acad Sci U S A. 2016;113:2538–2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wassing R, Lakbila-Kamal O, Ramautar JR, Stoffers D, Schalkwijk F, Van Someren EJW. Restless rem sleep impedes overnight amygdala adaptation. Curr Biol. 2019;29:2351–2358 e2354 [DOI] [PubMed] [Google Scholar]
- 66.Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376 [DOI] [PubMed] [Google Scholar]
- 67.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, et al. Narcolepsy in orexin knockout mice: Molecular genetics of sleep regulation. Cell. 1999;98:437–451 [DOI] [PubMed] [Google Scholar]
- 68.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40 [DOI] [PubMed] [Google Scholar]
- 69.Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997 [DOI] [PubMed] [Google Scholar]
- 70.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carter ME, Adamantidis A, Ohtsu H, Deisseroth K, de Lecea L. Sleep homeostasis modulates hypocretin-mediated sleep-to-wake transitions. J Neurosci. 2009;29:10939–10949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68:1023–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carter ME, Brill J, Bonnavion P, Huguenard JR, Huerta R, de Lecea L. Mechanism for hypocretin-mediated sleep-to-wake transitions. Proc Natl Acad Sci U S A. 2012;109:E2635–2644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li SB, Nevarez N, Giardino WJ, de Lecea L. Optical probing of orexin/hypocretin receptor antagonists. Sleep. 2018;41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hasegawa E, Yanagisawa M, Sakurai T, Mieda M. Orexin neurons suppress narcolepsy via 2 distinct efferent pathways. J Clin Invest. 2014;124:604–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baier PC, Hallschmid M, Seeck-Hirschner M, Weinhold SL, Burkert S, Diessner N, et al. Effects of intranasal hypocretin-1 (orexin a) on sleep in narcolepsy with cataplexy. Sleep Med. 2011;12:941–946 [DOI] [PubMed] [Google Scholar]
- 77.Weinhold SL, Seeck-Hirschner M, Nowak A, Hallschmid M, Goder R, Baier PC. The effect of intranasal orexin-a (hypocretin-1) on sleep, wakefulness and attention in narcolepsy with cataplexy. Behav Brain Res. 2014;262:8–13 [DOI] [PubMed] [Google Scholar]
- 78.Willie JT, Takahira H, Shibahara M, Hara J, Nomiyama M, Yanagisawa M, et al. Ectopic overexpression of orexin alters sleep/wakefulness states and muscle tone regulation during rem sleep in mice. J Mol Neurosci. 2011;43:155–161 [DOI] [PubMed] [Google Scholar]
- 79.Janto K, Prichard JR, Pusalavidyasagar S. An update on dual orexin receptor antagonists and their potential role in insomnia therapeutics. J Clin Sleep Med. 2018;14:1399–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mignot E, Mayleben D, Fietze I, Leger D, Zammit G, Bassetti CLA, et al. Safety and efficacy of daridorexant in patients with insomnia disorder: Results from two multicentre, randomised, double-blind, placebo-controlled, phase 3 trials. Lancet Neurol. 2022;21:125–139 [DOI] [PubMed] [Google Scholar]
- 81.Herring WJ, Connor KM, Snyder E, Snavely DB, Zhang Y, Hutzelmann J, et al. Suvorexant in patients with insomnia: Pooled analyses of three-month data from phase-3 randomized controlled clinical trials. J Clin Sleep Med. 2016;12:1215–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ciriello J, de Oliveira CV. Cardiac effects of hypocretin-1 in nucleus ambiguus. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1611–1620 [DOI] [PubMed] [Google Scholar]
- 83.de Oliveira CV, Rosas-Arellano MP, Solano-Flores LP, Ciriello J. Cardiovascular effects of hypocretin-1 in nucleus of the solitary tract. Am J Physiol Heart Circ Physiol. 2003;284:H1369–1377 [DOI] [PubMed] [Google Scholar]
- 84.Dergacheva O, Yamanaka A, Schwartz AR, Polotsky VY, Mendelowitz D. Direct projections from hypothalamic orexin neurons to brainstem cardiac vagal neurons. Neuroscience. 2016;339:47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith PM, Connolly BC, Ferguson AV. Microinjection of orexin into the rat nucleus tractus solitarius causes increases in blood pressure. Brain Res. 2002;950:261–267 [DOI] [PubMed] [Google Scholar]
- 86.Matsumura K, Tsuchihashi T, Abe I. Central orexin-a augments sympathoadrenal outflow in conscious rabbits. Hypertension. 2001;37:1382–1387 [DOI] [PubMed] [Google Scholar]
- 87.Shirasaka T, Nakazato M, Matsukura S, Takasaki M, Kannan H. Sympathetic and cardiovascular actions of orexins in conscious rats. Am J Physiol. 1999;277:R1780–1785 [DOI] [PubMed] [Google Scholar]
- 88.Chen CT, Hwang LL, Chang JK, Dun NJ. Pressor effects of orexins injected intracisternally and to rostral ventrolateral medulla of anesthetized rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R692–697 [DOI] [PubMed] [Google Scholar]
- 89.Antunes VR, Brailoiu GC, Kwok EH, Scruggs P, Dun NJ. Orexins/hypocretins excite rat sympathetic preganglionic neurons in vivo and in vitro. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1801–1807 [DOI] [PubMed] [Google Scholar]
- 90.Zhou JJ, Ma HJ, Shao J, Wei Y, Zhang X, Zhang Y, et al. Downregulation of orexin receptor in hypothalamic paraventricular nucleus decreases blood pressure in obese zucker rats. J Am Heart Assoc. 2019;8:e011434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou JJ, Yuan F, Zhang Y, Li DP. Upregulation of orexin receptor in paraventricular nucleus promotes sympathetic outflow in obese zucker rats. Neuropharmacology. 2015;99:481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang C, AlMarabeh S, Cavers J, Abdulla MH, Johns EJ. Effects of intracerebroventricular leptin and orexin-a on the baroreflex control of renal sympathetic nerve activity in conscious rats fed a normal or high-fat diet. Clin Exp Pharmacol Physiol. 2021;48:585–596 [DOI] [PubMed] [Google Scholar]
- 93.Shahid IZ, Rahman AA, Pilowsky PM. Intrathecal orexin a increases sympathetic outflow and respiratory drive, enhances baroreflex sensitivity and blocks the somato-sympathetic reflex. Br J Pharmacol. 2011;162:961–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bigalke JA, Gao H, Chen QH, Shan Z. Activation of orexin 1 receptors in the paraventricular nucleus contributes to the development of deoxycorticosterone acetate-salt hypertension through regulation of vasopressin. Front Physiol. 2021;12:641331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huber MJ, Fan Y, Jiang E, Zhu F, Larson RA, Yan J, et al. Increased activity of the orexin system in the paraventricular nucleus contributes to salt-sensitive hypertension. Am J Physiol Heart Circ Physiol. 2017;313:H1075–H1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xiao F, Jiang M, Du D, Xia C, Wang J, Cao Y, et al. Orexin a regulates cardiovascular responses in stress-induced hypertensive rats. Neuropharmacology. 2013;67:16–24 [DOI] [PubMed] [Google Scholar]
- 97.Meusel M, Voss J, Krapalis A, Machleidt F, Vonthein R, Hallschmid M, et al. Intranasal orexin a modulates sympathetic vascular tone: A pilot study in healthy male humans. J Neurophysiol. 2022;127:548–558 [DOI] [PubMed] [Google Scholar]
- 98.Deadwyler SA, Porrino L, Siegel JM, Hampson RE. Systemic and nasal delivery of orexin-a (hypocretin-1) reduces the effects of sleep deprivation on cognitive performance in nonhuman primates. J Neurosci. 2007;27:14239–14247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carter JR. Microneurography and sympathetic nerve activity: A decade-by-decade journey across 50 years. J Neurophysiol. 2019;121:1183–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shoemaker JK, Klassen SA, Badrov MB, Fadel PJ. Fifty years of microneurography: Learning the language of the peripheral sympathetic nervous system in humans. J Neurophysiol. 2018;119:1731–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vallbo AB. Microneurography: How it started and how it works. J Neurophysiol. 2018;120:1415–1427 [DOI] [PubMed] [Google Scholar]
- 102.Carter JR, Durocher JJ, Larson RA, DellaValla JP, Yang H. Sympathetic neural responses to 24-hour sleep deprivation in humans: Sex differences. Am J Physiol Heart Circ Physiol. 2012;302:H1991–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Taylor KS, Murai H, Millar PJ, Haruki N, Kimmerly DS, Morris BL, et al. Arousal from sleep and sympathetic excitation during wakefulness. Hypertension. 2016;68:1467–1474 [DOI] [PubMed] [Google Scholar]
- 104.Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M, Komuro I, et al. Attenuated defense response and low basal blood pressure in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R581–593 [DOI] [PubMed] [Google Scholar]
- 105.Donadio V, Liguori R, Vandi S, Giannoccaro MP, Pizza F, Leta V, et al. Sympathetic and cardiovascular changes during sleep in narcolepsy with cataplexy patients. Sleep Med. 2014;15:315–321 [DOI] [PubMed] [Google Scholar]
- 106.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993;328:303–307 [DOI] [PubMed] [Google Scholar]
- 107.Grimaldi D, Calandra-Buonaura G, Provini F, Agati P, Pierangeli G, Franceschini C, et al. Abnormal sleep-cardiovascular system interaction in narcolepsy with cataplexy: Effects of hypocretin deficiency in humans. Sleep. 2012;35:519–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Alvente S, Berteotti C, Bastianini S, Lo Martire V, Matteoli G, Silvani A, et al. Autonomic mechanisms of blood pressure alterations during sleep in orexin/hypocretin-deficient narcoleptic mice. Sleep. 2021;44 [DOI] [PubMed] [Google Scholar]
- 109.Barateau L, Lopez R, Chenini S, Evangelista E, Benkiran M, Mariano-Goulart D, et al. Exploration of cardiac sympathetic adrenergic nerve activity in narcolepsy. Clin Neurophysiol. 2019;130:412–418 [DOI] [PubMed] [Google Scholar]
- 110.Morgan BJ, Crabtree DC, Puleo DS, Badr MS, Toiber F, Skatrud JB. Neurocirculatory consequences of abrupt change in sleep state in humans. J Appl Physiol (1985). 1996;80:1627–1636 [DOI] [PubMed] [Google Scholar]
- 111.Xie A, Skatrud JB, Puleo DS, Morgan BJ. Arousal from sleep shortens sympathetic burst latency in humans. J Physiol. 1999;515 (Pt 2):621–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Roth T, Dauvilliers Y, Mignot E, Montplaisir J, Paul J, Swick T, et al. Disrupted nighttime sleep in narcolepsy. J Clin Sleep Med. 2013;9:955–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lambert EA, Chatzivlastou K, Schlaich M, Lambert G, Head GA. Morning surge in blood pressure is associated with reactivity of the sympathetic nervous system. Am J Hypertens. 2014;27:783–792 [DOI] [PubMed] [Google Scholar]
- 114.Sorensen GL, Knudsen S, Petersen ER, Kempfner J, Gammeltoft S, Sorensen HB, et al. Attenuated heart rate response is associated with hypocretin deficiency in patients with narcolepsy. Sleep. 2013;36:91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thannickal TC, Nienhuis R, Siegel JM. Localized loss of hypocretin (orexin) cells in narcolepsy without cataplexy. Sleep. 2009;32:993–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mignot E, Lammers GJ, Ripley B, Okun M, Nevsimalova S, Overeem S, et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59:1553–1562 [DOI] [PubMed] [Google Scholar]
- 117.Nakamura M, Kanbayashi T, Sugiura T, Inoue Y. Relationship between clinical characteristics of narcolepsy and csf orexin-a levels. J Sleep Res. 2011;20:45–49 [DOI] [PubMed] [Google Scholar]
- 118.Tang S, Huang W, Lu S, Lu L, Li G, Chen X, et al. Increased plasma orexin-a levels in patients with insomnia disorder are not associated with prepro-orexin or orexin receptor gene polymorphisms. Peptides. 2017;88:55–61 [DOI] [PubMed] [Google Scholar]
- 119.Kastin AJ, Akerstrom V. Orexin a but not orexin b rapidly enters brain from blood by simple diffusion. J Pharmacol Exp Ther. 1999;289:219–223 [PubMed] [Google Scholar]
- 120.Grimaldi D, Goldstein MR, Carter JR. Insomnia and cardiovascular autonomic control. Auton Neurosci. 2019;220:102551. [DOI] [PubMed] [Google Scholar]
- 121.Irwin M, Clark C, Kennedy B, Christian Gillin J, Ziegler M. Nocturnal catecholamines and immune function in insomniacs, depressed patients, and control subjects. Brain Behav Immun. 2003;17:365–372 [DOI] [PubMed] [Google Scholar]
- 122.Grimaldi D, Reid KJ, Papalambros NA, Braun RI, Malkani RG, Abbott SM, et al. Autonomic dysregulation and sleep homeostasis in insomnia. Sleep. 2021;44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Esler M, Jennings G, Lambert G, Meredith I, Horne M, Eisenhofer G. Overflow of catecholamine neurotransmitters to the circulation: Source, fate, and functions. Physiol Rev. 1990;70:963–985 [DOI] [PubMed] [Google Scholar]
- 124.Furlong TM, Vianna DM, Liu L, Carrive P. Hypocretin/orexin contributes to the expression of some but not all forms of stress and arousal. Eur J Neurosci. 2009;30:1603–1614 [DOI] [PubMed] [Google Scholar]
- 125.Larson RA, Carter JR. Total sleep deprivation and pain perception during cold noxious stimuli in humans. Scand J Pain. 2016;13:12–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jackson KL, Dampney BW, Moretti JL, Stevenson ER, Davern PJ, Carrive P, et al. Contribution of orexin to the neurogenic hypertension in bph/2j mice. Hypertension. 2016;67:959–969 [DOI] [PubMed] [Google Scholar]
- 127.Li A, Hindmarch CC, Nattie EE, Paton JF. Antagonism of orexin receptors significantly lowers blood pressure in spontaneously hypertensive rats. J Physiol. 2013;591:4237–4248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kario K, Yamasaki K, Yagi K, Tsukamoto M, Yamazaki S, Okawara Y, et al. Effect of suvorexant on nighttime blood pressure in hypertensive patients with insomnia: The super-1 study. J Clin Hypertens (Greenwich). 2019;21:896–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Patel AX, Miller SR, Nathan PJ, Kanakaraj P, Napolitano A, Lawrence P, et al. Neuroendocrine and sympathetic responses to an orexin receptor antagonist, sb-649868, and alprazolam following insulin-induced hypoglycemia in humans. Psychopharmacology (Berl). 2014;231:3817–3828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nakamura M, Nagamine T. Neuroendocrine, autonomic, and metabolic responses to an orexin antagonist, suvorexant, in psychiatric patients with insomnia. Innov Clin Neurosci. 2017;14:30–37 [PMC free article] [PubMed] [Google Scholar]
- 131.Rahman SA, Nathan MD, Wiley A, Crawford S, Cohn AY, Harder JA, et al. A double-blind, randomized, placebo-controlled trial of suvorexant for the treatment of vasomotor symptom-associated insomnia disorder in midlife women. Sleep. 2022;45 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


