Abstract
Differentiated thyroid cancer and breast cancer account for a significant portion of endocrine-related malignancies and predominately affect women. As hormonally responsive tissues, the breast and thyroid share endocrine signaling. Breast cells are responsive to thyroid hormone signaling and are affected by altered thyroid hormone levels. Thyroid cells are responsive to sex hormones, particularly estrogen, and undergo protumorigenic processes upon estrogen stimulation. Thyroid and sex hormones also display significant transcriptional crosstalk that influences oncogenesis and treatment sensitivity. Obesity-related adipocyte alterations—adipocyte estrogen production, inflammation, feeding hormone dysregulation, and metabolic syndromes—promote hormonal alterations in breast and thyroid tissues. Environmental toxicants disrupt endocrine systems, including breast and thyroid homeostasis, and influence pathologic processes in both organs through hormone mimetic action. In this brief review, we discuss the hormonal connections between the breast and thyroid and perspectives on hormonal therapies for breast and thyroid cancer. Future research efforts should acknowledge and further explore the hormonal crosstalk of these tissues in an effort to further understand the prevalence of thyroid and breast cancer in women and to identify potential therapeutic options.
Keywords: thyroid cancer, breast cancer, estrogen, thyroid hormones, obesity
Breast cancer (BC) and thyroid cancer (TC) are among the most common malignancies in women, with respectively, 84.8 and 23.1 new cases per 100 000 annually in North America (1, 2). Of cancers in women worldwide, breast and thyroid malignancies are the first and eleventh respective leading diagnoses (1, 3). Both cancers have a significant sex disparity: women have a 100 times greater BC risk and 2.9 times greater TC risk than men (4, 5). Incidence rates for both BC and TC have increased in the last several decades with a 1.9-fold increase in BC (women 25 to 39 years of age) and 2.9-fold increase in TC (3, 6). Combined morbidity and increasing prevalence of these malignancies warrant continued investigation of causes and underlying biological susceptibilities.
A growing body of literature supports a link between BC and TC. The observed co-occurrence of TC and BC is significant; observational and epidemiological studies identify a bidirectional risk increase between BC and thyroid dysfunction or TC (7-10). Survivorship studies suggest a 1.55-fold increased risk for developing secondary TC among BC survivors and a 1.32-fold increased risk for developing BC among TC survivors, relative to the general population (10). Hormone receptor and HER2 receptor positivity are elevated in these metachronous cases, suggesting a hormonal connection between breast and thyroid malignancies (11-13). In rare cases, TC and BC present synchronously, prompting debate as to the role of hormones, genetics, and radiation exposure in BC and TC co-development (14, 15).
Likely, several biological mechanisms influence mutual risk of BC and TC. Radiation and alkylating chemotherapeutics for treatment of primary cancers are well-established risk factors for secondary malignancy (16-18). Particularly, triple-negative BC is not responsive to hormonal therapies, requiring radiation and chemotherapy. Other theories, including shared genetic drivers, account for BC and TC co-occurrence irrespective of prior interventional risk (eg, patients with PTEN hamartoma tumor syndrome, previously referred to as Cowden disease, named after the index, eponymous patient Rachel Cowden by Lloyd and Dennis) (19, 20). However, despite sharing many hormonal components within hypothalamic-pituitary pathways, the thyroid and breast are commonly viewed as separate endocrine tissues. Discussion of breast and thyroid hormone crosstalk within the setting of carcinogenesis is notably lacking, yet some recent reviews have incorporated hormone action within conventional risk factors (21-24). Here, we discuss the thyroid and breast systems as connected endocrine systems in which hormonal crosstalk influences tissue-specific oncologic processes.
Clinical and Physiological Connections
Thyroid Endocrine Signaling
The hypothalamic-pituitary-thyroid (HPT) axis is a negative feedback loop that regulates production of the thyroid hormones triiodothyronine (T3) and thyroxine (T4). Upon thyroid-stimulating hormone (TSH) activation, the thyroid releases thyroid hormones—primarily the prohormone T4—with tissue-specific conversion into T3 by deiodinase enzymes in target cells (Fig. 1). Both benign and malignant thyroid disorders alter the HPT axis and thyroid hormone signaling. Hypothyroidism (ie, congenital and acquired) and hyperthyroidism present with alterations to serum thyroid hormone levels and TSH, while TC is most commonly associated with normal thyroid hormone production and asymptomatic nodules detected on physical exam or thyroid ultrasound (25). Disruption to any portion of the HPT axis, whether from benign or malignant pathologies, alters physiological thyroid equilibria and often requires lifelong, exogenous thyroid hormone supplementation (26).
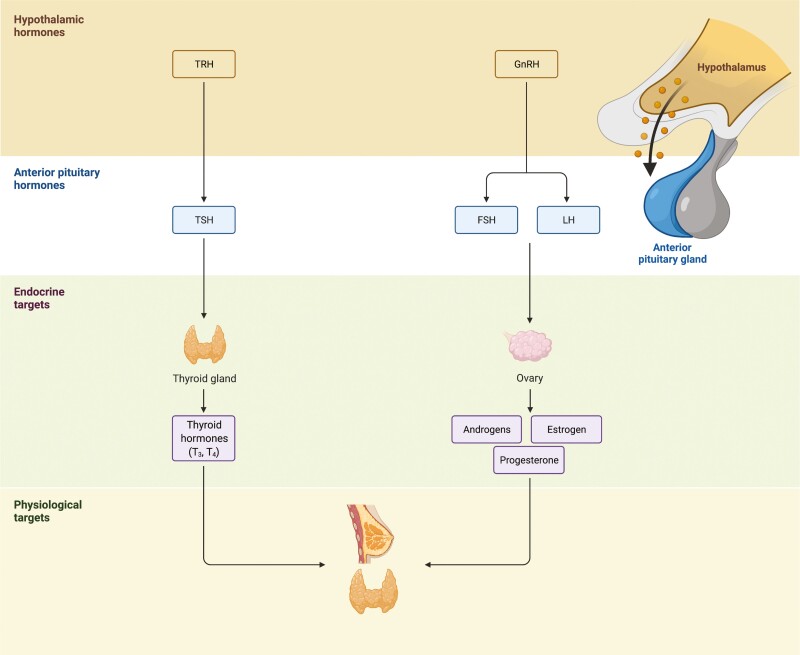
Figure 1.
Breast and thyroid within the hypothalamic-pituitary hormone system. The hypothalamus is a part of the brain that secretes gonadotropin (GnRH)- and thyrotropin (TRH)- releasing hormones, which stimulate the anterior pituitary gland. In response to these hormones, the pituitary gland activates the thyroid via thyroid-stimulating hormone (TSH) and the gonadal axes via luteinizing hormone (LH) and follicle-stimulating hormone (FSH). Endocrine targets downstream of the anterior pituitary gland, such as the thyroid and ovaries, release thyroid hormones (T3 and T4) or androgens, estrogen, and progesterone to regulate various physiological targets, including the thyroid and breast. Figure created with BioRender.com.
While not often considered a primary HPT target, breast tissue is responsive to thyroid hormone (Fig. 1) (27). Thyroid hormone dysfunction, particularly hyperthyroidism, is associated with increased risk of BC, yet the effects of hypothyroidism are unclear (7, 8, 28-30). Elevated free T4 is associated with risk of several solid cancers, including BC (31, 32). Even in the absence of hormonal or HER2 receptor positivity, T4 influences native immunologic response to BC (33). Additionally, several studies report a general association between all benign thyroid diseases, including non-autoimmune and autoimmune-related thyroid dysfunction, and BC (34-38). Autoantibodies in autoimmune thyroid disorders are independent predictors of BC, especially those directed against thyroid peroxidase and the TSH receptor (TSHR) (34, 39-45). Normal and cancerous breast tissues express structurally similar thyroid peroxidase and TSHR analogs that may be cross-reactive to thyroid-associated autoantibodies (46, 47). Benign breast tissue expresses sodium-iodine symporters (NIS) similar to thyroid tissue, and high dose radioactive iodine (RAI) for treatment of thyroid disease is associated with RAI uptake, with mixed data on whether there is an increased risk of BC (48-52). These clinical observations substantiate an evolutionary and functional connection of the breast to thyroid physiology.
Breast Endocrine Signaling
Breast tissue is a primary target for estrogen and progesterone within the hypothalamic-pituitary-gonadal endocrine axis. Follicle-stimulating hormone (FSH) and luteinizing hormone (LH) are released from the anterior pituitary in pulsed cycles and regulated by gonadotropin-releasing hormone from the hypothalamus (53). In premenopausal women, estrogen and progesterone are produced primarily from the ovaries and are sensitive to dynamic daily and monthly rhythmic cycles of FSH and LH during the menstrual cycle (Fig. 1). Following menopause, estrogen production declines and shifts to extragonadal tissues, primarily adipocytes, which express aromatase enzymes for conversion of androgens to local estradiol (54-56). Elevated endogenous sex hormones—estrogen, progesterone, androgens—are associated with an increased risk of developing BC both pre- and postmenopause (57-62). Hormone replacement therapy (HRT) was a common treatment in the United States for postmenopausal symptoms with mixed data on a link between HRT and BC risk, dependent on the type of HRT (increased risk if the HRT contains norethisterone) and duration of therapy (62-65). Due to significant hormone sensitivity, BC development and progression involves dynamic alterations to estrogen, progesterone, and HER2/neu receptors (66, 67). Tumor subtypes that express estrogen and/or progesterone receptors are classified as hormone receptor (HR) positive or HER2/neu receptor positivity as HER2+ (absence of receptors is termed triple-negative) (67, 68). Knowledge of the receptor status is crucial for selecting hormonal/endocrine therapies with receptor-specific action (69).
Similar to breast tissue, both benign and malignant thyroid tissue are highly responsive to circulating estrogen (Fig. 1) (70). Indeed, elevated circulating sex hormone levels are associated with TC (71, 72). Hyperestrogenism (elevated endogenous estrogen) during reproductive years is associated with an increased prevalence of TC in women of reproductive age; however, HRT or other exogenous estrogen exposure are not linked to TC (72-74). As the use of hormonal contraceptives has steadily increased, its connection to BC and TC development is under debate (73, 75-79). Additionally, estrogen may serve as a link between the co-occurrence of autoimmune thyroid disorders and BC, which predominantly affect women (80). Immune tolerance and the development of autoimmunity is largely controlled by the AIRE gene (81); estrogen is a key regulator of this gene, and reduced activity from elevated estrogen contributes to autoimmune susceptibility (81-85). Overall, sex hormones, primarily estrogen, appear to have a connection to not only BC, but also thyroid dysfunction and malignancy.
Obesity Endocrine Link
In parallel with rising BC and TC incidences, obesity has risen 11.9% among adults from 1999 to 2018 (86). Body weight and fat composition in the setting of obesity are associated with both BC and TC risk (87-93). Perspectives on the causes and impacts of obesity have become increasingly endocrine-focused in recent decades (94). Many hormonally driven physiological changes—including adipocyte aromatase activity, aberrant chemokine signaling, low-grade inflammation, and metabolic alterations—are linked to obesity and are associated with BC and TC (90, 95, 96).
Elevated circulating estrogen from adipocytes may contribute to an increase in BC and TC risk (94). Postmenopausal women produce significant levels of estrone, estradiol, and free estradiol from aromatase-dependent sterol conversion in adipocyte tissue (97-99). Obesity is also associated with enhanced macrophage cyclooxygenase-2 (COX2) expression, which further promotes adipocyte-dependent estrogen production and elevated circulating estrogen (100-102). Obesity-related hypothyroidism reduces serum sex hormone-binding globulin (SHBG) and elevates free estradiol and testosterone (subsequently converted to estradiol by aromatase enzymes) (103-105). This apparent rise in estrogen due to high adipocyte accumulation is connected to BC and TC risk in postmenopausal women (71, 99, 104, 106, 107). Obesity-related changes to tissue-specific and circulating sex hormones support obesity as a potential hormonal mediator between thyroid and BC.
Beyond their role in postmenopausal estrogen production, adipocytes are hormonally active cells that produce and respond to numerous adipochemokines (adipokines). Adipokine dysregulation alters a variety of physiological processes—feeding psychology, inflammatory equilibria, and dysmetabolic syndrome—and is associated with BC and TC risk (108, 109). Leptin is a peptide hormone that signals satiety upon binding to ventromedial hypothalamic nuclei cells and is overproduced by adipocytes in obesity (110). Binding of leptin in nonphysiologic target tissues promotes cellular proliferation and carcinogenesis (111, 112). Proinflammatory adipokines—resistin, tumor necrosis factor alpha (TNFα), interleukin-6 (IL-6), interleukin-18 (IL-18), CXCL5, and CCL2—are also overproduced in obesity (109, 113). These signaling molecules promote BC and TC development through recruitment of cancer-associated fibroblasts (CAF), microenvironment remodeling, and direct cellular effects (109, 114-117). Finally, metabolism-altering adipokines—lipocalin-2, CXCL5, resistin—promote hyperinsulinemia and insulin resistance and directly interact with thyroid and breast tissue to promote metabolic changes and carcinogenic processes (118-124). Considered together, obesity alters physiological functioning of many adipocyte signaling molecules with notable impact on the breast and thyroid.
Aberrant adipokine signaling in obesity causes alterations in endocrine metabolism and energy homeostasis. Obesity gives rise to a global low-grade inflammatory phenotype due to immune remodeling of adipose tissue and elevated proinflammatory cytokines (125). Alongside chronic inflammation, metabolism-altering adipokines in obese states detrimentally affect energy utilization in many organs, resulting in metabolic syndrome, hyperinsulinemia, and insulin resistance (126). Elevated insulin levels and cellular resistance to insulin are associated with the development of BC and TC (127-130). Insulin-like growth factor-1 (IGF-1) signaling, which is critically integrated within hypothalamic-pituitary development of the breast and thyroid, is also altered in obese states, and elevated serum IGF-1 is associated with BC and TC risk (131-134). Aberrant IGF-1 signaling causes excessive activation of mitogen-activated protein kinase (MAPK) and protein kinase B (AKT) pathways in breast and thyroid cells, leading to proliferation and migration of cancerous cells; several reviews are dedicated to describing the cellular changes that occur due to this disruption (133, 134). The interplay between obesity, hormones, and metabolic dysfunction alongside cancer risk is an active field of research.
Endocrine-Disrupting Chemicals
Endocrine-disrupting chemicals (EDC) are a class of environmental toxicants that interfere with hormonal pathways (135, 136). Estrogen-mimetic EDCs, xenoestrogens, effectively alter physiological estrogen signaling. Bisphenol A and genistein are well-established estrogen-mimetic, thyroid-disruptive EDCs associated with increased risk of BC and TC (137-142). Many additional chemicals, such as biphenyl ether flame retardants, polychlorinated biphenyls, phthalates, polyfluoroalkyls, and organo-pesticides, have thyroid-disrupting effects with TC risk (143). As previously described, thyroid endocrine disruption is associated with BC development; indeed, polychlorinated biphenyls, dioxins, and other thyroid-disrupting EDCs are associated with increased risk of BC (144, 145). The landscape of EDC disruption is complex and continually expanding, and the apparent effects of EDCs in thyroid and breast carcinogenesis give credence to a hormonal connection between these malignancies.
Stimulatory Capacity of Thyroid and Sex Hormones
Thyroid Hormones and Breast Cancer
Thyroid hormones elicit genomic actions by binding to nuclear receptors that subsequently exert transcriptional effects on target cells (146). There are multiple splice variants of the thyroid hormone nuclear receptor genes, TRα and TRβ, including TRα1, TRα2, TRβ1, TRβ2 (146). T3 has significant bioactivity on 3 of these receptor isoforms—TRα1, TRβ1, TRβ2—and induces expression of genes associated with cell growth and movement (147). Thyroid hormones also interact with target tissues independent of transcriptional action via the membrane-bound integrin αvβ3 protein (148). Both T3 and T4 exert cellular effects via the integrin αvβ3 pathway; T3 and T4 binding to the S1 subunit activates the PI3K/Akt pathway, and T4 binding to the S2 subunit activates MAPK/ERK1/2 pathways (27, 149, 150). Activation of the integrin αvβ3 protein is associated with cellular migration, proliferation, and angiogenesis via the MAPK and ERK1/2 pathways (148, 151-153). TH-mediated activation of integrin αvβ3 is implicated in protumorigenic features of many cancers, including glioma, ovarian, oral, colorectal, and BC cells (154-159).
All thyroid hormone nuclear receptor splice variants—TRα1, TRα2, TRβ1, and TRβ2—have been detected in BC cells (160-162). While not inducible by T3, TRα2 downregulates TRα1 such that high expression of TRα2 reduces the actions of TRα1 (163). In BC, TRα2 expression is a positive prognostic marker compared with diminished disease-free survival among TRα1-expressing BC tissue (164-166). Contrary to TRα1, TRβ1 expression in BC is associated with protective effects in vitro and in vivo through regulation of genes associated with aberrant cellular growth (161, 167-171). While TRβ2-specific studies and discussion are limited, Davidson and Gillis et al provide a comprehensive review of the antitumor effects of the TRβ protein class on solid tumor oncogenesis, particularly through JAK-STAT and PI3K pathway inhibition (172). Within BC studies, TRβ protein activation via T3 is associated with attenuation of JAK-STAT and RUNX2 signaling pathways, leading to reduced metastatic potential (173, 174). Taken together, these findings suggest that T3 has both pro- and antitumorigenic effects on breast tissue through transcriptional action, although the dominant effect is not well understood (Fig. 2).
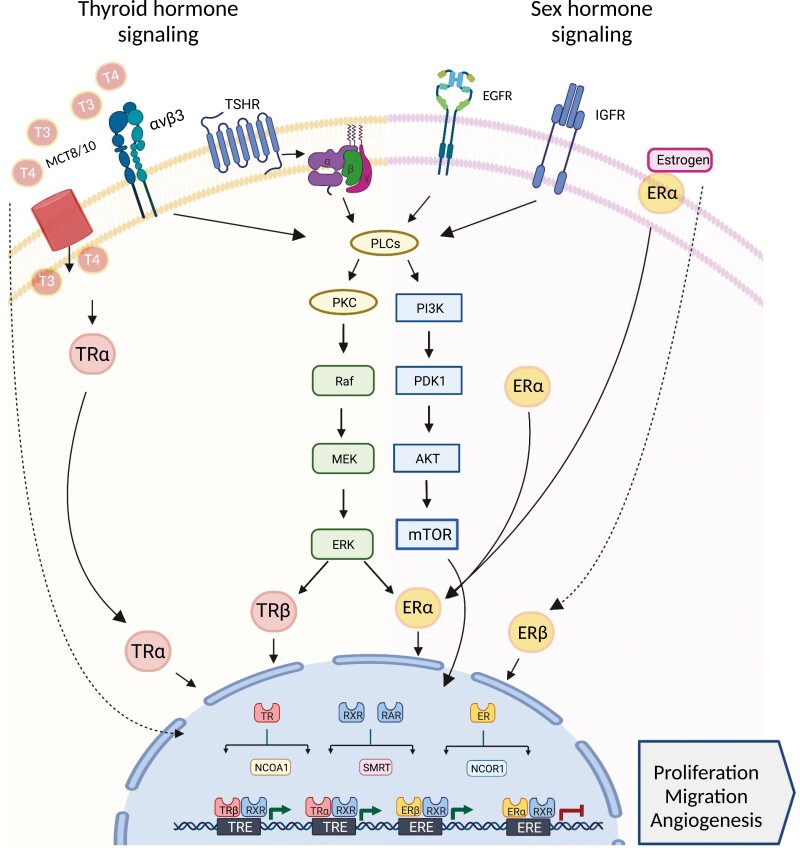
Figure 2.
Hormone signaling crosstalk between thyroid and breast. Thyroid and sex hormone signaling is driven either by interactions with membrane receptors (eg, MCT8/10, integrin avb3, TSHR, EGRF, IGFR, or ER among others) or direct access to the cytoplasm or the nucleus. The mechanisms of action of these hormones are often overlapping between thyroid and breast cells, ultimately resulting in transcriptional changes at the nucleus. While genomic actions involve the interaction of the thyroid (T3 or T4) or sex hormones (eg, estrogen) to nuclear receptor (TR or ER), they can also regulate target tissues by transcription-independent mechanisms. Nongenomic actions are initiated in the cytoplasm and include the activation of signal transduction kinases in the MAPK or PI3K/AKT pathway prior the stimulation of TR and ER. Thyroid hormones and estrogen also display cytoplasmic and nuclear crosstalk through MAPK-induced estrogen receptor modification, shared nuclear coregulators (eg, NCOA1, SMART, and NCOR1), and hormone response element ambiguity. Figure created with BioRender.com.
Thyroid hormones also influence BC risk via integrin αvβ3 independent of genomic activity. Primary cell cultures of human BC express epithelial integrin αvβ3, which is associated with lymph node and distant metastasis (175-178). T4 enhances ERK1/2 activity and promotes PD-L1 expression, presumably through integrin αvβ3 binding, and T3 binds and activates integrin αvβ3 to promote cell migration via downstream actin pathways and PI3K activation (Fig. 2) (33, 179, 180). However, despite these observations, the impact of thyroid dysfunction within the multistep metastatic process in BC is not well understood (177, 178). In addition to the physiologically abundant thyroid hormones, T3 and T4 metabolism yields a variety of bioactive metabolites—notably reverse triiodothyronine (rT3), 3,5-diiodothyronine (3,5-T2), 3-iodothyronamine (3T1AM), and tetraiodothyroacetic acid (tetrac) – with varying effects on integrin αvβ3 (181). 3,5-T2 and rT3 bind and activate integrin αvβ3, leading to proliferation in breast and ovarian cancer cell lines (182-184). Cancer cells in general, including BC cells, overexpress the deiodinase-3 enzyme for conversion of T4 and T3 into rT3 and 3,5-T2 respectively, which promote local protumor conditions (152, 185, 186). 3T1AM and tetrac counteract BC invasiveness—3T1AM via trace amine-associated receptor 1 (TAAR1) activation, and tetrac via inhibition of integrin αvβ3 (187-189). In particular, tetrac has notable antitumorigenic effects on BC cells by reducing cellular proliferation, angiogenesis, and PD-L1 activity and increasing apoptosis, suggesting promising therapeutic action (33, 189-192).
Sex Hormones and Thyroid Cancer
Several studies and reviews report estrogen’s effects on TC development and progression (70, 193-195). Estrogen binds to 2 nuclear receptors—ERα and ERβ (196). Both ERα and ERβ are overexpressed by TC cells with estrogen-activated ERα inducing proliferation, angiogenesis, and migration in TC cells (197-203). In contrast, ERβ activation is associated with antiproliferative properties with a higher ratio of ERα to ERβ thought to influence estrogen’s dominant effect on thyroid tumorigenesis (204-207). ERα can be localized to either the cell membrane/cytoplasm or the nucleus, with differential cellular effects upon activation (208). Estrogen stimulation of membrane-associated ERα impacts many cellular pathways within TC cells with notable activation of AKT/mTOR, MEK1/2, and MAPK pathways (Fig. 2) (208-213). Nuclear activity of ERα in thyroid cells, alongside epigenetic alterations and chromatin decondensation, induces transcription of proliferation-associated proteins and regulation of PPARγ transcriptional activity (208, 209, 214-218). The cellular effects of membrane-associated and nuclear ERα are significantly interconnected; Manole and colleagues provide a detailed review of these cellular actions and overlap in thyroid cells (208). Finally, estrogen receptor signaling also has an indirect proliferative effect on thyroid cells by increasing thyroid cell sensitivity to TSH signaling (202).
Estrogen’s apparent activation of MAPK signaling pathways in TC cells has implications for iodine avidity and RAI therapy. BRAFV600E− and fusion-driven differentiated TCs display marked MAPK and MEK/ERK activation that alters epigenetic protein activity and sodium-iodine symporter (NIS) promoter transcription (219). Therefore, it stands to reason that MAPK activation via estrogen stimulation in TC cells may account for reduced NIS expression in estrogen-stimulated TC cell lines (208, 220). Few confirmatory reports of this phenomenon exist, yet nuclear receptors similar to estrogen receptor affect iodine avidity. Particularly, estrogen-related receptor gamma (ERRγ), a protein in the nuclear receptor superfamily, enhances MAPK signaling and causes reduced NIS expression, providing indirect evidence of estrogen nuclear receptor involvement in NIS expression (221). Considering the importance of NIS expression for RAI therapy in TCs, the interactions between estrogen signaling and iodine avidity must be further defined.
Progesterone and androgens (ie, testosterone and dihydrotestosterone) also interact with nuclear receptors to elicit cellular responses. As mentioned, progesterone receptor (PR) positivity is an important prognostic factor in BC. While progesterone is expressed in some TC tissue, studies have not shown a significant correlation between progesterone receptor status and TC progression (200). Similarly, androgen receptors (Ars) are expressed in TC tissue, yet there is little consensus as to the oncologic effects of AR activation. Some studies report an association between AR expression and capsular invasion and extrathyroidal extension, while others suggest a protective role via growth arrest and reduction of PD-L1 expression (222-224). Interestingly, testosterone exhibits a direct protumorigenic effect on TC progression and severity via attenuation of tumor suppressor genes (225, 226). More research is needed to further define the potential role of progesterone and androgen signaling in TC tumorigenesis and progression.
Thyroid and Steroid Hormone Nuclear Receptor Cross-Reactivity and Crosstalk
Sex steroids and thyroid hormones bind to nuclear receptors belonging to a structurally related protein superfamily. Estrogen and progesterone interact with steroid nuclear receptors to allow for binding to estrogen response elements and subsequent gene transcription (227). Thyroid hormones interact with nuclear receptors that heterodimerize with retinoid X receptors (RXR) and bind to thyroid response elements to lift epigenetic restraints on gene transcription (227). These nuclear receptors regulate gene transcription alongside numerous gene coactivators and corepressors; many of these coregulators—such as SRC-1/NCOA-1, NCOR-1, SMRT, retinoid receptor proteins—are shared between steroid and RXR-heterodimeric thyroid nuclear receptor complexes (227, 228). Additionally, hormone response elements—including estrogen and thyroid response elements—have low binding specificity within the nuclear receptor superfamily such that hormone-specific nuclear complexes have overlapping binding potential with broad hormone response elements (229, 230).
Hormone nuclear receptor crosstalk has implications for BC and TC development and co-occurrence. Indeed, mutations in sex hormone and thyroid hormone nuclear receptor coregulators, like NCOR-1, serve as drivers for some BC and TCs (231-233). Shared coregulators regulate sex and thyroid hormone-induced transcription, which may influence aberrant cellular processes and mutual oncogenesis (234, 235). In BC cells, thyroid hormone and estrogen signaling also display significant crosstalk through promoter cross-reactivity, both with estrogen-induced thyroid hormone response element-binding and thyroid hormone-induced estrogen response element-binding (236-240). Additionally, nongenomic thyroid hormone action via integrin αvβ3 influences cellular localization and activity of estrogen receptors by activating MAPK-dependent phosphorylation of ERα nuclear receptors (241). Cellular thyroid hormone signaling directly impacts estrogen-related therapeutics in BC cells through shared nuclear coregulators, promoter ambiguity, and nongenomic overlap (242-246). Considering analogous structural and functional features within the nuclear receptor superfamily, research into the cellular overlap of estrogen and thyroid signaling is important for both a greater understanding of breast-thyroid interplay as well as for the anticipation of unintended effects of hormonal therapies.
Therapeutic Considerations
Thyroid Hormone Signaling Targets in Breast Cancer
Thyroid-based therapeutics provide potential adjuvant options for the treatment of BC (22). Considering the genomic actions of thyroid hormones, thyroid hormone nuclear receptor isoforms present several therapeutic targets. Dronedarone has been shown to antagonize the protumorigenic activity of TRα1 nuclear receptors with cytotoxic effects on BC cell lines (247). The antiproliferative effects of TRβ activity have been targeted with many TRβ-specific agonists over the past few decades for metabolic and musculoskeletal disorders (248). Resmetirom has proved most successful with recent FDA approval for nonalcoholic fatty liver disease; such agents may have efficacy in BC (249). Considering the role of retinoic acid receptors within sex and thyroid hormone nuclear crosstalk, retinoic acid and retinoid receptor agonists are being considered for adjuvant therapy in BC (250-252).
Integrin αvβ3 inhibition presents a nongenomic therapeutic target for reducing the proliferative effects of thyroid hormones on BC cells. Tetrac displays considerable integrin αvβ3 antagonism with broad anticancer properties. So far, in vitro and in vivo evidence suggests that tetrac has the potential to reduce proliferation and angiogenesis of BC cells (191, 192, 253). Synthetic inhibitors of integrin αvβ3, like cilengitide, may prove efficacious in BC despite recent failures in glioblastoma clinical trials (254). With particular interest in receptor-targeted radionuclides, integrin αvβ3 radionuclides are also being explored as theragnostic agents for BC and other cancers (255). Despite documented action of T3 on breast cell integrin αvβ3 proteins, T3 replacement of T4 supplementation has been anecdotally used to reduce integrin αvβ3 activity in other cancer cells and may be applicable to BC (152). Integrin αvβ3 signaling has garnered significant attention in a variety of cancers, and future work may translate laboratory findings to clinical practice for BC and TC.
Sex Hormone Signaling Targets in Thyroid Cancer
The effects of estrogen and androgens on TC cells suggest potential benefit of sex hormone targeted therapies for the treatment of hormone-responsive TC. ERβ could be therapeutically activated to elicit antitumorigenic response in TC. Erteberel, a selective ERβ agonist, is currently under investigation for the treatment of schizophrenia and has gained recent attention for use in glioblastoma (256). Similarly, ERα antagonists may prove efficacious for reducing proliferation in TC. However, no studies to our knowledge have investigated the potential of repurposing estrogen receptor antagonists (ie, fulvestrant) or selective estrogen receptor modulators (ie, tamoxifen, raloxifene) in ERα-positive TC. While a study by Hoelting and colleagues shows in vitro and in vivo suppression of thyroid function and proliferation by tamoxifen, it is unclear whether selective estrogen modulators would produce unintended effects compared with full estrogen antagonists (257). Finally, androgen antagonists are successfully utilized in androgen-responsive cancers, like prostate cancer, yet greater understanding of androgen signaling in the thyroid is required before androgen-targeted therapeutics should be investigated for repurposing in TC.
Targeting NIS Expression in Breast and Thyroid Cancer
In TC, iodine avidity can be therapeutically induced by tyrosine kinase (TK) inhibitors (219, 258). Vermafenib, a BRAFV600E-specific inhibitor, and selumetinib, a MEK1/2 inhibitor, restore iodine avidity in RAI-refractory TC by attenuating RAS-RAF-MEK-ERK signaling (259, 260). With likely effects of estrogen receptor and ERRγ on MAPK signaling, estrogen antagonists and selective estrogen receptor modulators may restore iodine avidity in estrogen-responsive TCs similar to TK inhibitors. Indeed, ERRγ inverse agonists have been shown to increase NIS expression in anaplastic TC cell lines and may be clinically efficacious for restoring iodine avidity clinically (221, 261).
While conventionally used to treat TC, RAI may be clinically beneficial for treatment of advanced BC. Malignant breast tissue, including triple-negative BC, expresses NIS proteins and functionally uptakes iodine (262, 263). Developing therapeutics that differentially increase NIS expression in breast tissue over benign thyroid tissue could spare a healthy thyroid from breast-targeted RAI (258). Retinoic acid has been shown to increase functional NIS expression in BC cells and TC cells while reducing NIS activity in nonmalignant thyroid cells (264, 265). Further exploration of such therapeutics may allow for practical RAI therapy in the setting of advanced BC.
Metabolic Signaling Targets in Breast and Thyroid Cancer
Obesity-related pathophysiology promotes BC and TC, and therapeutics targeting these alterations have garnered recent attention. Adipocyte aromatase activity in obesity has notable effects on circulating estrogen that can be targeted by aromatase inhibitors (ie, letrozole and anastrozole). Such agents show significant clinical efficacy in postmenopausal patients with BC, but no studies have explored their use for TC (266, 267). Adipokines present several additional therapeutic targets. For example, anti-lipocalin-2 and anti-IL-6-receptor antibodies reduce BC metastasis in murine models (120, 268). However, the intersection of adipokines, metabolism, and BC/TC risk is extremely complex, contributing to mixed clinical trial results and unintended side effects. To the surprise of academic and industry investigators, studies evaluating efficacy of diabetes medications, like metformin, as adjuvant agents for BC treatment have reported little clinical benefit; however, there are data supporting an antitumorigenic effect of metformin in TC (269, 270). Similarly, teprotumumab, an anti-IGF-1 receptor antibody, achieved greater clinical success for the treatment of thyroid eye disease than as a cancer therapeutic (271). Clinical trials for many of these targets are ongoing, and future research is needed to better explain the cellular and endocrine consequences of obesity in relation to breast and thyroid tumorigenesis.
Conclusions
Numerous hormones, including thyroid, reproductive/sex, adipocyte, and hormone-like EDCs, influence BC and TC pathogenesis. As endocrine organs, the breast and thyroid are responsive to their respective hypothalamic-pituitary axes as well as hormones from the broader endocrine physiologic pathways. While many therapeutic opportunities present from this hormonal crosstalk, one must be cognizant of the potential unintended consequences of clinical therapeutics directed at one organ system that may negatively impact the other. For example, with the increased use of TK inhibitory therapy to re-sensitize TC to RAI therapy, clinicians and researchers must also consider the potential impact of TK inhibitor therapy on NIS expression in breast tissue and the potential impact of increased radiation burden to the breast. Future collaborations and discussions are needed to improve our understanding of the interconnected signaling pathways between the breast and thyroid systems. Doing so may enable the development of novel hormonal treatment strategies, reduce unintended physiological disruption, and limit adverse effects of conventional cytotoxic therapy.
Glossary
Abbreviations
- 3,5-T2
3,5-diiodothyronine
- 3T1AM
3-iodothyronamine
- AKT
protein kinase B
- AR
androgen receptor
- BC
breast cancer
- EDC
endocrine-disrupting chemical
- ERα/β
estrogen receptor α/β
- ERRγ
estrogen-related receptor gamma
- FSH
follicle stimulating hormone
- HPT
hypothalamus-pituitary-thyroid
- HRT
hormone replacement therapy
- IGF-1
insulin-like growth factor 1
- IL-
interleukin
- LH
luteinizing hormone
- MAPK
mitogen-activated protein kinase
- NIS
sodium-iodine symporter
- RAI
radioactive iodine
- rT3
reverse triiodothyronine
- T3
triiodothyronine
- T4
thyroxine
- TC
thyroid cancer
- tetrac
tetraiodothyroacetic
- TK
tyrosine kinase
- TRα/β
thyroid hormone receptor α/β
- TSH
thyrotropin (thyroid-stimulating hormone)
- TSHR
thyroid-stimulating hormone receptor
Contributor Information
Stephen Halada, Division of Endocrinology and Diabetes, Children’s Hospital of Philadelphia, Philadelphia, PA 19104, USA.
Victoria Casado-Medrano, Division of Endocrinology and Diabetes, Children’s Hospital of Philadelphia, Philadelphia, PA 19104, USA.
Julia A Baran, Division of Endocrinology and Diabetes, Children’s Hospital of Philadelphia, Philadelphia, PA 19104, USA.
Joshua Lee, Division of Endocrinology and Diabetes, Children’s Hospital of Philadelphia, Philadelphia, PA 19104, USA.
Poojita Chinmay, Division of Endocrinology and Diabetes, Children’s Hospital of Philadelphia, Philadelphia, PA 19104, USA.
Andrew J Bauer, Division of Endocrinology and Diabetes, Children’s Hospital of Philadelphia, Philadelphia, PA 19104, USA; Department of Pediatrics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA.
Aime T Franco, Division of Endocrinology and Diabetes, Children’s Hospital of Philadelphia, Philadelphia, PA 19104, USA; Abramson Cancer Center, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA; Department of Pediatrics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA.
Supporting Grants/Fellowships
National Institutes of Health, R01 CA214511, Children’s Hospital of Philadelphia Intramural Frontier Grant
Disclosures
S.H., V.C.M., J.A.B., J.L., P.C., and J.A.B. have nothing to declare. A.T.F. is on the Board of Directors for the American Thyroid Association.
Authorship Contributions
S.H., J.L., A.J.B., and A.T.F. devised the project. S.H. wrote the manuscript, V.C.M. and A.T.F. created the figures, and V.C.M., J.A.B., P.C., A.J.B., and A.T.F. provided support and critical review. S.H., V.C.M., J.A.B., J.L., P.C., A.J.B., and A.T.F. discussed the review and provided editorial feedback of the manuscript.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Bray F, Ferlay J, Laversanne M, et al. Cancer incidence in five continents: inclusion criteria, highlights from volume X and the global status of cancer registration. Int J Cancer. 2015;137(9):2060-2071. doi: 10.1002/ijc.29670 [DOI] [PubMed] [Google Scholar]
- 3. Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140(4):317-322. doi: 10.1001/jamaoto.2014.1. [DOI] [PubMed] [Google Scholar]
- 4. Rahbari R, Zhang L, Kebebew E. Thyroid cancer gender disparity. Future Oncol. 2010;6(11):1771-1779. doi: 10.2217/fon.10.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anderson WF, Jatoi I, Tse J, Rosenberg PS. Male breast cancer: a population-based comparison with female breast cancer. J Clin Oncol. 2010;28(2):232-239. doi: 10.1200/JCO.2009.23.8162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johnson RH, Chien FL, Bleyer A. Incidence of breast cancer with distant involvement among women in the United States, 1976 to 2009. JAMA. 2013;309(8):800-805. doi: 10.1001/jama.2013.776 [DOI] [PubMed] [Google Scholar]
- 7. Joseph KR, Edirimanne S, Eslick GD. The association between breast cancer and thyroid cancer: a meta-analysis. Breast Cancer Res Treat. 2015;152(1):173-181. doi: 10.1007/s10549-015-3456-6 [DOI] [PubMed] [Google Scholar]
- 8. Tran TV, Kitahara CM, de Vathaire F, Boutron-Ruault MC, Journy N. Thyroid dysfunction and cancer incidence: a systematic review and meta-analysis. Endocr Relat Cancer. 2020;27(4):245-259. doi: 10.1530/ERC-19-0417 [DOI] [PubMed] [Google Scholar]
- 9. Jung HK, Park S, Kim NW, et al. Development of second primary cancer in Korean breast cancer survivors. Ann Surg Treat Res. 2017;93(6):287-292. doi: 10.4174/astr.2017.93.6.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nielsen SM, White MG, Hong S, et al. The breast-thyroid cancer link: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2016;25(2):231-238. doi: 10.1158/1055-9965.EPI-15-0833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. An JH, Hwangbo Y, Ahn HY, et al. A possible association between thyroid cancer and breast cancer. Thyroid. 2015;25(12):1330-1338. doi: 10.1089/thy.2014.0561 [DOI] [PubMed] [Google Scholar]
- 12. Hu ZY, Xiao H, Xiao M, et al. Inducing or preventing subsequent malignancies for breast cancer survivors? double-edged sword of estrogen receptor and progesterone receptor. Clin Breast Cancer. 2018;18(5):e1149-e1163. doi: 10.1016/j.clbc.2018.04.009 [DOI] [PubMed] [Google Scholar]
- 13. Kuo JH, Chabot JA, Lee JA. Breast cancer in thyroid cancer survivors: an analysis of the Surveillance, Epidemiology, and End Results-9 database. Surgery. 2016;159(1):23-29. doi: 10.1016/j.surg.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 14. Zhong J, Lei J, Jiang K, Li Z, Gong R, Zhu J. Synchronous papillary thyroid carcinoma and breast ductal carcinoma: a rare case report and literature review. Medicine (Baltim). 2017;96(7):e6114. doi: 10.1097/MD.0000000000006114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kong H, Chen J, Tang SC. Synchronous papillary thyroid carcinoma and breast ductal carcinoma. J Int Med Res. 2020;48(8):300060520948710. doi: 10.1177/0300060520948710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bhatti P, Veiga LH, Ronckers CM, et al. Risk of second primary thyroid cancer after radiotherapy for a childhood cancer in a large cohort study: an update from the childhood cancer survivor study. Radiat Res. 2010;174(6):741-752. doi: 10.1667/RR2240.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bhakta N, Liu Q, Ness KK, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet 2017;390(10112):2569-2582. doi: 10.1016/S0140-6736(17)31610-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rheingold SR, Neugut AI, Meadows AT. Therapy-Related Secondary Cancers. In: Kufe DW, Pollock RE, Weichselbaum RR, et al. , editors. Holland-Frei Cancer Medicine. 6th edition. Hamilton (ON): BC Decker; 2003. [Google Scholar]
- 19. Liaw D, Marsh DJ, Li J, et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet. 1997;16(1):64-67. doi: 10.1038/ng0597-64 [DOI] [PubMed] [Google Scholar]
- 20. Lloyd KM, Dennis M. Cowden’s disease. A possible new symptom complex with multiple system involvement. Ann Intern Med. 1963;58:136-142. doi: 10.7326/0003-4819-58-1-136 [DOI] [PubMed] [Google Scholar]
- 21. Bolf EL, Sprague BL, Carr FE. A linkage between thyroid and breast cancer: a common etiology? Cancer Epidemiol Biomarkers Prev. 2019;28(4):643-649. doi: 10.1158/1055-9965.EPI-18-0877 [DOI] [PubMed] [Google Scholar]
- 22. Krashin E, Piekiełko-Witkowska A, Ellis M, Ashur-Fabian O. Thyroid hormones and cancer: a comprehensive review of preclinical and clinical studies. Front Endocrinol (Lausanne) 2019;10:59. doi: 10.3389/fendo.2019.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smyth PP. The thyroid and breast cancer. Curr Opin Endocrinol Diabetes Obes. 2016;23(5):389-393. doi: 10.1097/MED.0000000000000273 [DOI] [PubMed] [Google Scholar]
- 24. Hercbergs A, Mousa SA, Leinung M, Lin HY, Davis PJ. Thyroid hormone in the clinic and breast cancer. Horm Cancer. 2018;9(3):139-143. doi: 10.1007/s12672-018-0326-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1-133. doi: 10.1089/thy.2015.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jongejan RMS, van Velsen EFS, Meima ME, et al. Change in thyroid hormone metabolite concentrations across different thyroid states. Thyroid. 2022;32(2):119-127. doi: 10.1089/thy.2021.0453 [DOI] [PubMed] [Google Scholar]
- 27. Liu YC, Yeh CT, Lin KH. Molecular functions of thyroid hormone signaling in regulation of cancer progression and anti-apoptosis. Int J Mol Sci. 2019;20(20). doi: 10.3390/ijms20204986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Søgaard M, Farkas DK, Ehrenstein V, Jørgensen JO, Dekkers OM, Sørensen HT. Hypothyroidism and hyperthyroidism and breast cancer risk: a nationwide cohort study. Eur J Endocrinol. 2016;174(4):409-414. doi: 10.1530/EJE-15-0989 [DOI] [PubMed] [Google Scholar]
- 29. Yang H, Holowko N, Grassmann F, Eriksson M, Hall P, Czene K. Hyperthyroidism is associated with breast cancer risk and mammographic and genetic risk predictors. BMC Med. 2020;18(1):225. doi: 10.1186/s12916-020-01690-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fang Y, Yao L, Sun J, et al. Does thyroid dysfunction increase the risk of breast cancer? A systematic review and meta-analysis. J Endocrinol Invest. 2017;40(10):1035-1047. doi: 10.1007/s40618-017-0679-x [DOI] [PubMed] [Google Scholar]
- 31. Khan SR, Chaker L, Ruiter R, et al. Thyroid function and cancer risk: the rotterdam study. J Clin Endocrinol Metab. 2016;101(12):5030-5036. doi: 10.1210/jc.2016-2104 [DOI] [PubMed] [Google Scholar]
- 32. Ortega-Olvera C, Ulloa-Aguirre A, Ángeles-Llerenas A, et al. Thyroid hormones and breast cancer association according to menopausal status and body mass index. Breast Cancer Res. 2018;20(1):94. doi: 10.1186/s13058-018-1017-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lin HY, Chin YT, Nana AW, et al. Actions of l-thyroxine and Nano-diamino-tetrac (Nanotetrac) on PD-L1 in cancer cells. Steroids. 2016;114:59-67. doi: 10.1016/j.steroids.2016.05.006 [DOI] [PubMed] [Google Scholar]
- 34. Hardefeldt PJ, Eslick GD, Edirimanne S. Benign thyroid disease is associated with breast cancer: a meta-analysis. Breast Cancer Res Treat. 2012;133(3):1169-1177. doi: 10.1007/s10549-012-2019-3 [DOI] [PubMed] [Google Scholar]
- 35. Prinzi N, Baldini E, Sorrenti S, et al. Prevalence of breast cancer in thyroid diseases: results of a cross-sectional study of 3,921 patients. Breast Cancer Res Treat. 2014;144(3):683-688. doi: 10.1007/s10549-014-2893-y [DOI] [PubMed] [Google Scholar]
- 36. Muller I, Pinchera A, Fiore E, et al. High prevalence of breast cancer in patients with benign thyroid diseases. J Endocrinol Invest. 2011;34(5):349-352. doi: 10.1007/BF03347458 [DOI] [PubMed] [Google Scholar]
- 37. Dobrinja C, Scomersi S, Giudici F, et al. Association between benign thyroid disease and breast cancer: a single center experience. BMC Endocr Disord. 2019;19(1):104. doi: 10.1186/s12902-019-0426-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Giani C, Fierabracci P, Bonacci R, et al. Relationship between breast cancer and thyroid disease: relevance of autoimmune thyroid disorders in breast malignancy. J Clin Endocrinol Metab. 1996;81(3):990-994. doi: 10.1210/jcem.81.3.8772562 [DOI] [PubMed] [Google Scholar]
- 39. Smyth PP, Shering SG, Kilbane MT, et al. Serum thyroid peroxidase autoantibodies, thyroid volume, and outcome in breast carcinoma. J Clin Endocrinol Metab. 1998;83(8):2711-2716. doi: 10.1210/jcem.83.8.5049 [DOI] [PubMed] [Google Scholar]
- 40. Chen S, Wu F, Hai R, et al. Thyroid disease is associated with an increased risk of breast cancer: a systematic review and meta-analysis. Gland Surg. 2021;10(1):336-346. doi: 10.21037/gs-20-878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Szychta P, Szychta W, Gesing A, Lewiński A, Karbownik-Lewińska M. TSH receptor antibodies have predictive value for breast cancer - retrospective analysis. Thyroid Res. 2013;6(1):8. doi: 10.1186/1756-6614-6-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Giustarini E, Pinchera A, Fierabracci P, et al. Thyroid autoimmunity in patients with malignant and benign breast diseases before surgery. Eur J Endocrinol. 2006;154(5):645-649. doi: 10.1530/eje.1.02108 [DOI] [PubMed] [Google Scholar]
- 43. Jiskra J, Barkmanova J, Limanova Z, et al. Thyroid autoimmunity occurs more frequently in women with breast cancer compared to women with colorectal cancer and controls but it has no impact on relapse-free and overall survival. Oncol Rep. 2007;18(6):1603-1611. [PubMed] [Google Scholar]
- 44. Rasmusson B, Feldt-Rasmussen U, Hegedüs L, Perrild H, Bech K, Høier-Madsen M. Thyroid function in patients with breast cancer. Eur J Cancer Clin Oncol. 1987;23(5):553-556. doi: 10.1016/0277-5379(87)90319-1 [DOI] [PubMed] [Google Scholar]
- 45. Ditsch N, Liebhardt S, Von Koch F, et al. Thyroid function in breast cancer patients. Anticancer Res. 2010;30(5):1713-1717. [PubMed] [Google Scholar]
- 46. Muller I, Giani C, Zhang L, et al. Does thyroid peroxidase provide an antigenic link between thyroid autoimmunity and breast cancer? Int J Cancer. 2014;134(7):1706-1714. doi: 10.1002/ijc.28493 [DOI] [PubMed] [Google Scholar]
- 47. Godlewska M, Krasuska W, Czarnocka B. Biochemical properties of thyroid peroxidase (TPO) expressed in human breast and mammary-derived cell lines. PLoS One. 2018;13(3):e0193624. doi: 10.1371/journal.pone.0193624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Goldman MB, Maloof F, Monson RR, Aschengrau A, Cooper DS, Ridgway EC. Radioactive iodine therapy and breast cancer. A follow-up study of hyperthyroid women. Am J Epidemiol. 1988;127(5):969-980. doi: 10.1093/oxfordjournals.aje.a114900 [DOI] [PubMed] [Google Scholar]
- 49. Ryan J, Curran CE, Hennessy E, et al. The sodium iodide symporter (NIS) and potential regulators in normal, benign and malignant human breast tissue. PLoS One. 2011;6(1):e16023. doi: 10.1371/journal.pone.0016023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pasqual E, Schonfeld S, Morton LM, et al. Association between radioactive iodine treatment for pediatric and young adulthood differentiated thyroid cancer and risk of second primary malignancies. J Clin Oncol. 2022:JCO2101841. doi: 10.1200/JCO.21.01841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nappi C, Klain M, Cantoni V, et al. Risk of primary breast cancer in patients with differentiated thyroid cancer undergoing radioactive iodine therapy: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2021. doi: 10.1007/s00259-021-05625-4 [DOI] [PubMed] [Google Scholar]
- 52. Kim BW. Does radioactive iodine therapy for hyperthyroidism cause cancer? J Clin Endocrinol Metab. 2022;107(2):e448-e457. doi: 10.1210/clinem/dgab700 [DOI] [PubMed] [Google Scholar]
- 53. Draper CF, Duisters K, Weger B, et al. Menstrual cycle rhythmicity: metabolic patterns in healthy women. Sci Rep. 2018;8(1):14568. doi: 10.1038/s41598-018-32647-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. Production and actions of estrogens. N Engl J Med. 2002;346(5):340-352. doi: 10.1056/NEJMra000471 [DOI] [PubMed] [Google Scholar]
- 55. Blakemore J, Naftolin F. Aromatase: Contributions to physiology and disease in women and men. Physiology (Bethesda). 2016;31(4):258-269. doi: 10.1152/physiol.00054.2015 [DOI] [PubMed] [Google Scholar]
- 56. Ishikawa T, Glidewell-Kenney C, Jameson JL. Aromatase-independent testosterone conversion into estrogenic steroids is inhibited by a 5 alpha-reductase inhibitor. J Steroid Biochem Mol Biol. 2006;98(2-3):133-138. doi: 10.1016/j.jsbmb.2005.09.004 [DOI] [PubMed] [Google Scholar]
- 57. Khan SA. Progesterone exposure and breast cancer risk-addressing barriers. JAMA Netw Open 2020;3(4):e203608. doi: 10.1001/jamanetworkopen.2020.3608 [DOI] [PubMed] [Google Scholar]
- 58. Asi N, Mohammed K, Haydour Q, et al. Progesterone vs. synthetic progestins and the risk of breast cancer: a systematic review and meta-analysis. Syst Rev. 2016;5(1):121. doi: 10.1186/s13643-016-0294-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Key TJ, Appleby PN, Reeves GK, et al. Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol. 2013;14(10):1009-1019. doi: 10.1016/S1470-2045(13)70301-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vinogradova Y, Coupland C, Hippisley-Cox J. Use of hormone replacement therapy and risk of breast cancer: nested case-control studies using the QResearch and CPRD databases. BMJ. 2020;371:m3873. doi: 10.1136/bmj.m3873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Marjoribanks J, Farquhar C, Roberts H, Lethaby A. Long term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2012(7):CD004143. doi: 10.1002/14651858.CD004143.pub4 [DOI] [PubMed] [Google Scholar]
- 62. Olsson HL, Ingvar C, Bladström A. Hormone replacement therapy containing progestins and given continuously increases breast carcinoma risk in Sweden. Cancer. 2003;97(6):1387-1392. doi: 10.1002/cncr.11205 [DOI] [PubMed] [Google Scholar]
- 63. Pizot C, Boniol M, Mullie P, Koechlin A, Boyle P, Autier P. Physical activity, hormone replacement therapy and breast cancer risk: a meta-analysis of prospective studies. Eur J Cancer. 2016;52:138-154. doi: 10.1016/j.ejca.2015.10.063 [DOI] [PubMed] [Google Scholar]
- 64. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Collaborative Group on Hormonal Factors in Breast Cancer. [published correction appears in Lancet 1997 Nov 15;350(9089):1484]. Lancet. 1997;350(9084):1047-1059. [PubMed] [Google Scholar]
- 65. Saul H, Gursul D, Cassidy S, Vinogradova Y. Risk of breast cancer with HRT depends on therapy type and duration. BMJ. 2022;376:o485. doi: 10.1136/bmj.o485 [DOI] [PubMed] [Google Scholar]
- 66. Brisken C, O’Malley B. Hormone action in the mammary gland. Cold Spring Harb Perspect Biol. 2010;2(12):a003178. doi: 10.1101/cshperspect.a003178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Harbeck N, Penault-Llorca F, Cortes J, et al. Breast cancer. Nat Rev Dis Primers. 2019;5(1):66. doi: 10.1038/s41572-019-0111-2 [DOI] [PubMed] [Google Scholar]
- 68. Zubair M, Wang S, Ali N. Advanced approaches to breast cancer classification and diagnosis. Front Pharmacol. 2020;11:632079. doi: 10.3389/fphar.2020.632079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Russnes HG, Lingjærde OC, Børresen-Dale AL, Caldas C. Breast cancer molecular stratification: from intrinsic subtypes to integrative clusters. Am J Pathol. 2017;187(10):2152-2162. doi: 10.1016/j.ajpath.2017.04.022 [DOI] [PubMed] [Google Scholar]
- 70. Santin AP, Furlanetto TW. Role of estrogen in thyroid function and growth regulation. J Thyroid Res. 2011;2011:875125. doi: 10.4061/2011/875125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Liu J, Chen G, Meng XY, Liu ZH, Dong S. Serum levels of sex hormones and expression of their receptors in thyroid tissue in female patients with various types of thyroid neoplasms. Pathol Res Pract. 2014;210(12):830-835. doi: 10.1016/j.prp.2014.09.002 [DOI] [PubMed] [Google Scholar]
- 72. Moleti M, Sturniolo G, Di Mauro M, Russo M, Vermiglio F. Female reproductive factors and differentiated thyroid cancer. Front Endocrinol (Lausanne). 2017;8:111. doi: 10.3389/fendo.2017.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. La Vecchia C, Ron E, Franceschi S, et al. A pooled analysis of case-control studies of thyroid cancer. III. Oral contraceptives, menopausal replacement therapy and other female hormones. Cancer Causes Control. 1999;10(2):157-166. doi: 10.1023/a:1008832513932 [DOI] [PubMed] [Google Scholar]
- 74. Uygur MM, Yoldemir T, Yavuz DG. Thyroid disease in the perimenopause and postmenopause period. Climacteric. 2018;21(6):542-548. doi: 10.1080/13697137.2018.1514004 [DOI] [PubMed] [Google Scholar]
- 75. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347(9017):1713-1727. doi: 10.1016/s0140-6736(96)90806-5 [DOI] [PubMed] [Google Scholar]
- 76. Khoo SK, Chick P. Sex steroid hormones and breast cancer: is there a link with oral contraceptives and hormone replacement therapy? Med J Aust. 1992;156(2):124-132. [PubMed] [Google Scholar]
- 77. Hedayati M, Rajabi S, Nikzamir A. Papillary thyroid cancer-promoting activities of combined oral contraceptive components. Galen Med J. 2020;9:e1648. doi: 10.31661/gmj.v9i0.1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. White ND. Hormonal contraception and breast cancer risk. Am J Lifestyle Med. 2018;12(3):224-226. doi: 10.1177/1559827618754833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wu L, Zhu J. Linear reduction in thyroid cancer risk by oral contraceptive use: a dose-response meta-analysis of prospective cohort studies. Hum Reprod. 2015;30(9):2234-2240. doi: 10.1093/humrep/dev160 [DOI] [PubMed] [Google Scholar]
- 80. Moulton VR. Sex hormones in acquired immunity and autoimmune disease. Front Immunol. 2018;9:2279. doi: 10.3389/fimmu.2018.02279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Pitkänen J, Peterson P. Autoimmune regulator: from loss of function to autoimmunity. Genes Immun. 2003;4(1):12-21. doi: 10.1038/sj.gene.6363929 [DOI] [PubMed] [Google Scholar]
- 82. Brown MA, Su MA. An inconvenient variable: sex hormones and their impact on T cell responses. J Immunol. 2019;202(7):1927-1933. doi: 10.4049/jimmunol.1801403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chailurkit LO, Aekplakorn W, Ongphiphadhanakul B. The relationship between circulating estradiol and thyroid autoimmunity in males. Eur J Endocrinol. 2014;170(1):63-67. doi: 10.1530/EJE-13-0455 [DOI] [PubMed] [Google Scholar]
- 84. Chen Y, Xia F, Wang N, et al. A higher ratio of estradiol to testosterone is associated with autoimmune thyroid disease in males. Thyroid. 2017;27(7):960-966. doi: 10.1089/thy.2016.0661 [DOI] [PubMed] [Google Scholar]
- 85. Dragin N, Bismuth J, Cizeron-Clairac G, et al. Estrogen-mediated downregulation of AIRE influences sexual dimorphism in autoimmune diseases. J Clin Invest. 2016;126(4):1525-1537. doi: 10.1172/JCI81894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief. 2020;( 360):1-8. [PubMed] [Google Scholar]
- 87. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625-1638. doi: 10.1056/NEJMoa021423 [DOI] [PubMed] [Google Scholar]
- 88. Schapira DV, Kumar NB, Lyman GH, Cox CE. Abdominal obesity and breast cancer risk. Ann Intern Med. 1990;112(3):182-186. doi: 10.7326/0003-4819-112-3-182 [DOI] [PubMed] [Google Scholar]
- 89. Zhao ZG, Guo XG, Ba CX, et al. Overweight, obesity and thyroid cancer risk: a meta-analysis of cohort studies. J Int Med Res. 2012;40(6):2041-2050. doi: 10.1177/030006051204000601 [DOI] [PubMed] [Google Scholar]
- 90. Pappa T, Alevizaki M. Obesity and thyroid cancer: a clinical update. Thyroid. 2014;24(2):190-199. doi: 10.1089/thy.2013.0232 [DOI] [PubMed] [Google Scholar]
- 91. Xu L, Port M, Landi S, et al. Obesity and the risk of papillary thyroid cancer: a pooled analysis of three case-control studies. Thyroid. 2014;24(6):966-974. doi: 10.1089/thy.2013.0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body fatness and cancer--viewpoint of the IARC working group. N Engl J Med. 2016;375(8):794-798. doi: 10.1056/NEJMsr1606602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Keum N, Greenwood DC, Lee DH, et al. Adult weight gain and adiposity-related cancers: a dose-response meta-analysis of prospective observational studies. J Natl Cancer Inst. 2015;107(2). doi 10.1093/jnci/djv088 [DOI] [PubMed] [Google Scholar]
- 94. Xue J, Ideraabdullah FY. An assessment of molecular pathways of obesity susceptible to nutrient, toxicant and genetically induced epigenetic perturbation. J Nutr Biochem. 2016;30:1-13. doi: 10.1016/j.jnutbio.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Brown KA. Metabolic pathways in obesity-related breast cancer. Nat Rev Endocrinol. 2021;17(6):350-363. doi: 10.1038/s41574-021-00487-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mauro L, Naimo GD, Ricchio E, Panno ML, Andò S. Cross-talk between adiponectin and IGF-IR in breast cancer. Front Oncol. 2015;5:157. doi: 10.3389/fonc.2015.00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Richardson H, Ho V, Pasquet R, et al. Baseline estrogen levels in postmenopausal women participating in the MAP.3 breast cancer chemoprevention trial. Menopause. 2020;27(6):693-700. doi: 10.1097/GME.0000000000001568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Tin Tin S, Reeves GK, Key TJ. Body size and composition, physical activity and sedentary time in relation to endogenous hormones in premenopausal and postmenopausal women: findings from the UK Biobank. Int J Cancer. 2020;147(8):2101-2115. doi: 10.1002/ijc.33010 [DOI] [PubMed] [Google Scholar]
- 99. Cleary MP, Grossmann ME. Minireview: obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150(6):2537-2542. doi: 10.1210/en.2009-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bowers LW, deGraffenried LA. Targeting the COX-2 pathway to improve therapeutic response in the obese breast cancer patient population. Curr Pharmacol Rep. 2015;1(5):336-345. doi: 10.1007/s40495-015-0041-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Johnston SR, Dowsett M. Aromatase inhibitors for breast cancer: lessons from the laboratory. Nat Rev Cancer. 2003;3(11):821-831. doi: 10.1038/nrc1211 [DOI] [PubMed] [Google Scholar]
- 102. Zatterale F, Longo M, Naderi J, et al. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front Physiol. 2019;10:1607. doi: 10.3389/fphys.2019.01607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sanyal D, Raychaudhuri M. Hypothyroidism and obesity: an intriguing link. Indian J Endocrinol Metab. 2016;20(4):554-557. doi: 10.4103/2230-8210.183454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Santini F, Marzullo P, Rotondi M, et al. Mechanisms in endocrinology: the crosstalk between thyroid gland and adipose tissue: signal integration in health and disease. Eur J Endocrinol. 2014;171(4):R137-R152. doi: 10.1530/EJE-14-0067 [DOI] [PubMed] [Google Scholar]
- 105. Gambineri A, Pelusi C. Sex hormones, obesity and type 2 diabetes: is there a link? Endocr Connect 2019;8(1):R1-R9. doi: 10.1530/EC-18-0450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Liu E, Samad F, Mueller BM. Local adipocytes enable estrogen-dependent breast cancer growth: role of leptin and aromatase. Adipocyte. 2013;2(3):165-169. doi: 10.4161/adip.23645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Savolainen-Peltonen H, Vihma V, Leidenius M, et al. Breast adipose tissue estrogen metabolism in postmenopausal women with or without breast cancer. J Clin Endocrinol Metab. 2014;99(12):E2661-E2667. doi: 10.1210/jc.2014-2550 [DOI] [PubMed] [Google Scholar]
- 108. Christodoulatos GS, Spyrou N, Kadillari J, Psallida S, Dalamaga M. The role of adipokines in breast cancer: current evidence and perspectives. Curr Obes Rep. 2019;8(4):413-433. doi: 10.1007/s13679-019-00364-y [DOI] [PubMed] [Google Scholar]
- 109. Zhao J, Wen J, Wang S, Yao J, Liao L, Dong J. Association between adipokines and thyroid carcinoma: a meta-analysis of case-control studies. BMC Cancer. 2020;20(1):788. doi: 10.1186/s12885-020-07299-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Obradovic M, Sudar-Milovanovic E, Soskic S, et al. Leptin and obesity: role and clinical implication. Front Endocrinol (Lausanne). 2021;12:585887. doi: 10.3389/fendo.2021.585887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sánchez-Jiménez F, Pérez-Pérez A, de la Cruz-Merino L, Sánchez-Margalet V. Obesity and breast cancer: role of leptin. Front Oncol. 2019;9:596. doi: 10.3389/fonc.2019.00596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Di Cristofano A. Obesity and thyroid cancer: is leptin the (only) link? Endocrinology. 2013;154(8):2567-2569. doi: 10.1210/en.2013-1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Khan S, Shukla S, Sinha S, Meeran SM. Role of adipokines and cytokines in obesity-associated breast cancer: therapeutic targets. Cytokine Growth Factor Rev. 2013;24(6):503-513. doi: 10.1016/j.cytogfr.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 114. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85-97. doi: 10.1038/nri2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Esquivel-Velázquez M, Ostoa-Saloma P, Palacios-Arreola MI, Nava-Castro KE, Castro JI, Morales-Montor J. The role of cytokines in breast cancer development and progression. J Interferon Cytokine Res. 2015;35(1):1-16. doi: 10.1089/jir.2014.0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Caperton CO, Jolly LA, Massoll N, Bauer AJ, Franco AT. Development of novel follicular thyroid cancer models which progress to poorly differentiated and anaplastic thyroid cancer. Cancers (Basel). 2021;13(5). doi 10.3390/cancers13051094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Jolly LA, Novitskiy S, Owens P, et al. Fibroblast-mediated collagen remodeling within the tumor microenvironment facilitates progression of thyroid cancers driven by BrafV600E and Pten loss. Cancer Res. 2016;76(7):1804-1813. doi: 10.1158/0008-5472.CAN-15-2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Tai J, Wang S, Zhang J, et al. Up-regulated lipocalin-2 in pediatric thyroid cancer correlated with poor clinical characteristics. Eur Arch Otorhinolaryngol. 2018;275(11):2823-2828. doi: 10.1007/s00405-018-5118-x [DOI] [PubMed] [Google Scholar]
- 119. Yang J, Bielenberg DR, Rodig SJ, et al. Lipocalin 2 promotes breast cancer progression. Proc Natl Acad Sci USA. 2009;106(10):3913-3918. doi: 10.1073/pnas.0810617106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Leng X, Ding T, Lin H, et al. Inhibition of lipocalin 2 impairs breast tumorigenesis and metastasis. Cancer Res. 2009;69(22):8579-8584. doi: 10.1158/0008-5472.CAN-09-1934 [DOI] [PubMed] [Google Scholar]
- 121. Cui D, Zhao Y, Xu J. Activation of CXCL5-CXCR2 axis promotes proliferation and accelerates G1 to S phase transition of papillary thyroid carcinoma cells and activates JNK and p38 pathways. Cancer Biol Ther. 2019;20(5):608-616. doi: 10.1080/15384047.2018.1539289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Romero-Moreno R, Curtis KJ, Coughlin TR, et al. The CXCL5/CXCR2 axis is sufficient to promote breast cancer colonization during bone metastasis. Nat Commun. 2019;10(1):4404. doi: 10.1038/s41467-019-12108-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Benomar Y, Gertler A, De Lacy P, et al. Central resistin overexposure induces insulin resistance through Toll-like receptor 4. Diabetes. 2013;62(1):102-114. doi: 10.2337/db12-0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Wang CH, Wang PJ, Hsieh YC, et al. Resistin facilitates breast cancer progression via TLR4-mediated induction of mesenchymal phenotypes and stemness properties. Oncogene. 2018;37(5):589-600. doi: 10.1038/onc.2017.357 [DOI] [PubMed] [Google Scholar]
- 125. McArdle MA, Finucane OM, Connaughton RM, McMorrow AM, Roche HM. Mechanisms of obesity-induced inflammation and insulin resistance: insights into the emerging role of nutritional strategies. Front Endocrinol (Lausanne). 2013;4:52. doi: 10.3389/fendo.2013.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9(5):367-377. doi: 10.1038/nrm2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Harikrishna A, Ishak A, Ellinides A, et al. The impact of obesity and insulin resistance on thyroid cancer: a systematic review. Maturitas. 2019;125:45-49. doi: 10.1016/j.maturitas.2019.03.022 [DOI] [PubMed] [Google Scholar]
- 128. Pan K, Chlebowski RT, Mortimer JE, et al. Insulin resistance and breast cancer incidence and mortality in postmenopausal women in the Women’s Health Initiative. Cancer. 2020;126(16):3638-3647. doi: 10.1002/cncr.33002 [DOI] [PubMed] [Google Scholar]
- 129. Aschebrook-Kilfoy B, Sabra MM, Brenner A, et al. Diabetes and thyroid cancer risk in the National Institutes of Health-AARP Diet and Health Study. Thyroid. 2011;21(9):957-963. doi: 10.1089/thy.2010.0396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Boyle P, Boniol M, Koechlin A, et al. Diabetes and breast cancer risk: a meta-analysis. Br J Cancer. 2012;107(9):1608-1617. doi: 10.1038/bjc.2012.414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Endogenous Hormones and Breast Cancer Collaborative Group; Key TJ, Appleby PN, Reeves GK, Roddam AW. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2010;11(6):530-542. doi: 10.1016/S1470-2045(10)70095-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Schmidt JA, Allen NE, Almquist M, et al. Insulin-like growth factor-i and risk of differentiated thyroid carcinoma in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2014;23(6):976-985. doi: 10.1158/1055-9965.EPI-13-1210-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Christopoulos PF, Msaouel P, Koutsilieris M. The role of the insulin-like growth factor-1 system in breast cancer. Mol Cancer. 2015;14:43. doi: 10.1186/s12943-015-0291-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Smith TJ. Insulin-like growth factor pathway and the thyroid. Front Endocrinol (Lausanne). 2021;12:653627. doi: 10.3389/fendo.2021.653627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293-342. doi: 10.1210/er.2009-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Zoeller RT, Brown TR, Doan LL, et al. Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology. 2012;153(9):4097-4110. doi: 10.1210/en.2012-1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Zhang J, Zhang X, Li Y, et al. Low dose of Bisphenol A enhance the susceptibility of thyroid carcinoma stimulated by DHPN and iodine excess in F344 rats. Oncotarget. 2017;8(41):69874-69887. doi: 10.18632/oncotarget.19434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Zhang Y, Wei F, Zhang J, et al. Bisphenol A and estrogen induce proliferation of human thyroid tumor cells via an estrogen-receptor-dependent pathway. Arch Biochem Biophys. 2017;633:29-39. doi: 10.1016/j.abb.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 139. Matsushima A, Kakuta Y, Teramoto T, et al. Structural evidence for endocrine disruptor bisphenol A binding to human nuclear receptor ERR gamma. J Biochem. 2007;142(4):517-524. doi: 10.1093/jb/mvm158 [DOI] [PubMed] [Google Scholar]
- 140. Kim JY, Choi HG, Lee HM, Lee GA, Hwang KA, Choi KC. Effects of bisphenol compounds on the growth and epithelial mesenchymal transition of MCF-7 CV human breast cancer cells. J Biomed Res. 2017;31(4):358-369. doi: 10.7555/JBR.31.20160162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Thambirajah AA, Wade MG, Verreault J, et al. Disruption by stealth - Interference of endocrine disrupting chemicals on hormonal crosstalk with thyroid axis function in humans and other animals. Environ Res. 2022;203:111906. doi: 10.1016/j.envres.2021.111906 [DOI] [PubMed] [Google Scholar]
- 142. Divi RL, Chang HC, Doerge DR. Anti-thyroid isoflavones from soybean: isolation, characterization, and mechanisms of action. Biochem Pharmacol. 1997;54(10):1087-1096. doi: 10.1016/s0006-2952(97)00301-8 [DOI] [PubMed] [Google Scholar]
- 143. Alsen M, Sinclair C, Cooke P, Ziadkhanpour K, Genden E, van Gerwen M. Endocrine disrupting chemicals and thyroid cancer: an overview. Toxics. 2021;9(1). doi: 10.3390/toxics9010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Eve L, Fervers B, Le Romancer M, Etienne-Selloum N. Exposure to endocrine disrupting chemicals and risk of breast cancer. Int J Mol Sci. 2020;21(23). doi: 10.3390/ijms21239139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Calaf GM, Ponce-Cusi R, Aguayo F, Muñoz JP, Bleak TC. Endocrine disruptors from the environment affecting breast cancer. Oncol Lett. 2020;20(1):19-32. doi: 10.3892/ol.2020.11566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev. 2010;31(2):139-170. doi: 10.1210/er.2009-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Sinha R, Yen PM. Cellular action of thyroid hormone. Jun 20, 2018. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, Dungan K, Hershman JM, Hofland J, Kalra S, Kaltsas G, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, Morley JE, New M, Purnell J, Sahay R, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, eds. Cellular Action of Thyroid Hormone. Endotext [Internet]. South Dartmouth (MA), MDText.com, Inc.; 2000, PMID: 25905423. [Google Scholar]
- 148. Bergh JJ, Lin HY, Lansing L, et al. Integrin alphaVbeta3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology. 2005;146(7):2864-2871. doi: 10.1210/en.2005-0102 [DOI] [PubMed] [Google Scholar]
- 149. Cody V, Davis PJ, Davis FB. Molecular modeling of the thyroid hormone interactions with alpha v beta 3 integrin. Steroids. 2007;72(2):165-170. doi: 10.1016/j.steroids.2006.11.008 [DOI] [PubMed] [Google Scholar]
- 150. Davis PJ, Goglia F, Leonard JL. Nongenomic actions of thyroid hormone. Nat Rev Endocrinol. 2016;12(2):111-121. doi: 10.1038/nrendo.2015.205 [DOI] [PubMed] [Google Scholar]
- 151. Lin HY, Su YF, Hsieh MT, et al. Nuclear monomeric integrin αv in cancer cells is a coactivator regulated by thyroid hormone. FASEB J. 2013;27(8):3209-3216. doi: 10.1096/fj.12-227132 [DOI] [PubMed] [Google Scholar]
- 152. Hercbergs A. Clinical implications and impact of discovery of the thyroid hormone receptor on integrin αvβ3-A review. Front Endocrinol (Lausanne). 2019;10:565. doi: 10.3389/fendo.2019.00565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Davis PJ, Davis FB, Lin HY, Mousa SA, Zhou M, Luidens MK. Translational implications of nongenomic actions of thyroid hormone initiated at its integrin receptor. Am J Physiol Endocrinol Metab. 2009;297(6):E1238-E1246. doi: 10.1152/ajpendo.00480.2009 [DOI] [PubMed] [Google Scholar]
- 154. Davis FB, Tang HY, Shih A, et al. Acting via a cell surface receptor, thyroid hormone is a growth factor for glioma cells. Cancer Res. 2006;66(14):7270-7275. doi: 10.1158/0008-5472.CAN-05-4365 [DOI] [PubMed] [Google Scholar]
- 155. Shinderman-Maman E, Cohen K, Weingarten C, et al. The thyroid hormone-αvβ3 integrin axis in ovarian cancer: regulation of gene transcription and MAPK-dependent proliferation. Oncogene. 2016;35(15):1977-1987. doi: 10.1038/onc.2015.262 [DOI] [PubMed] [Google Scholar]
- 156. Yang YSH, Ko PJ, Pan YS, et al. Role of thyroid hormone-integrin αvβ3-signal and therapeutic strategies in colorectal cancers. J Biomed Sci. 2021;28(1):24. doi: 10.1186/s12929-021-00719-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Chin YT, He ZR, Chen CL, et al. Tetrac and NDAT induce anti-proliferation via integrin αvβ3 in colorectal cancers with different. Front Endocrinol (Lausanne). 2019;10:130. doi: 10.3389/fendo.2019.00130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Huang CH, Huang TY, Chang WJ, et al. Combined treatment of heteronemin and tetrac induces antiproliferation in oral cancer cells. Mar Drugs. 2020;18(7). doi: 10.3390/md18070348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Felding-Habermann B, O’Toole TE, Smith JW, et al. Integrin activation controls metastasis in human breast cancer. Proc Natl Acad Sci USA. 2001;98(4):1853-1858. doi: 10.1073/pnas.98.4.1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Ditsch N, Toth B, Himsl I, et al. Thyroid hormone receptor (TR)alpha and TRbeta expression in breast cancer. Histol Histopathol. 2013;28(2):227-237. doi: 10.14670/HH-28.227 [DOI] [PubMed] [Google Scholar]
- 161. Silva JM, Domínguez G, González-Sancho JM, et al. Expression of thyroid hormone receptor/erbA genes is altered in human breast cancer. Oncogene. 2002;21(27):4307-4316. doi: 10.1038/sj.onc.1205534 [DOI] [PubMed] [Google Scholar]
- 162. Burstein MD, Tsimelzon A, Poage GM, et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res. 2015;21(7):1688-1698. doi: 10.1158/1078-0432.CCR-14-0432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Guissouma H, Ghaddab-Zroud R, Seugnet I, Decherf S, Demeneix B, Clerget-Froidevaux MS. TR alpha 2 exerts dominant negative effects on hypothalamic Trh transcription in vivo. PLoS One. 2014;9(4):e95064. doi: 10.1371/journal.pone.0095064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Zehni AZ, Batz F, Vattai A, et al. The prognostic impact of retinoid X receptor and thyroid hormone receptor alpha in unifocal vs. multifocal/multicentric breast cancer. Int J Mol Sci. 2021;22(2). doi 10.3390/ijms22020957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Jerzak KJ, Cockburn J, Pond GR, et al. Thyroid hormone receptor α in breast cancer: prognostic and therapeutic implications. Breast Cancer Res Treat. 2015;149(1):293-301. doi: 10.1007/s10549-014-3235-9 [DOI] [PubMed] [Google Scholar]
- 166. Sandsveden M, Borgquist S, Rosendahl AH, Manjer J. Low thyroid hormone receptor alpha-2 (THRα-2) tumor expression is associated with unfavorable tumor characteristics and high breast cancer mortality. Breast Cancer Res. 2021;23(1):117. doi: 10.1186/s13058-021-01496-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Li Z, Meng ZH, Chandrasekaran R, et al. Biallelic inactivation of the thyroid hormone receptor beta1 gene in early stage breast cancer. Cancer Res. 2002;62(7):1939-1943. [PubMed] [Google Scholar]
- 168. Martínez-Iglesias O, Garcia-Silva S, Tenbaum SP, et al. Thyroid hormone receptor beta1 acts as a potent suppressor of tumor invasiveness and metastasis. Cancer Res. 2009;69(2):501-509. doi: 10.1158/0008-5472.CAN-08-2198 [DOI] [PubMed] [Google Scholar]
- 169. Peng X, Zhang Y, Sun Y, et al. Overexpressing modified human TRβ1 suppresses the proliferation of breast cancer MDA-MB-468 cells. Oncol Lett 2018;16(1):785-792. doi: 10.3892/ol.2018.8764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Guigon CJ, Kim DW, Willingham MC, Cheng SY. Mutation of thyroid hormone receptor-β in mice predisposes to the development of mammary tumors. Oncogene. 2011;30(30):3381-3390. doi: 10.1038/onc.2011.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. López-Mateo I, Alonso-Merino E, Suarez-Cabrera C, et al. Thyroid hormone receptor β inhibits self-renewal capacity of breast cancer stem cells. Thyroid. 2020;30(1):116-132. doi: 10.1089/thy.2019.0175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Davidson CD, Gillis NE, Carr FE. Thyroid hormone receptor beta as tumor suppressor: untapped potential in treatment and diagnostics in solid tumors. Cancers (Basel). 2021;13(17). doi: 10.3390/cancers13174254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Park JW, Zhao L, Cheng SY. Inhibition of estrogen-dependent tumorigenesis by the thyroid hormone receptor β in xenograft models. Am J Cancer Res. 2013;3(3):302-311. [PMC free article] [PubMed] [Google Scholar]
- 174. Bolf EL, Gillis NE, Barnum MS, et al. The thyroid hormone receptor-RUNX2 Axis: a novel tumor suppressive pathway in breast cancer. Horm Cancer. 2020;11(1):34-41. doi: 10.1007/s12672-019-00373-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Havaki S, Kouloukoussa M, Amawi K, et al. Altered expression pattern of integrin alphavbeta3 correlates with actin cytoskeleton in primary cultures of human breast cancer. Cancer Cell Int. 2007;7:16. doi: 10.1186/1475-2867-7-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Beer AJ, Niemeyer M, Carlsen J, et al. Patterns of alphavbeta3 expression in primary and metastatic human breast cancer as shown by 18F-Galacto-RGD PET. J Nucl Med. 2008;49(2):255-259. doi: 10.2967/jnumed.107.045526 [DOI] [PubMed] [Google Scholar]
- 177. Zhao Y, Bachelier R, Treilleux I, et al. Tumor alphavbeta3 integrin is a therapeutic target for breast cancer bone metastases. Cancer Res. 2007;67(12):5821-5830. doi: 10.1158/0008-5472.CAN-06-4499 [DOI] [PubMed] [Google Scholar]
- 178. Liapis H, Flath A, Kitazawa S. Integrin alpha V beta 3 expression by bone-residing breast cancer metastases. Diagn Mol Pathol. 1996;5(2):127-135. doi: 10.1097/00019606-199606000-00008 [DOI] [PubMed] [Google Scholar]
- 179. Flamini MI, Uzair ID, Pennacchio GE, et al. Thyroid hormone controls breast cancer cell movement via Integrin αV/β3/SRC/FAK/PI3-Kinases. Horm Cancer. 2017;8(1):16-27. doi: 10.1007/s12672-016-0280-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Uzair ID, Conte Grand J, Flamini MI, Sanchez AM. Molecular actions of thyroid hormone on breast cancer cell migration and invasion via cortactin/N-WASP. Front Endocrinol (Lausanne). 2019;10:139. doi: 10.3389/fendo.2019.00139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Köhrle J. The colorful diversity of thyroid hormone metabolites. Eur Thyroid J. 2019;8(3):115-129. doi: 10.1159/000497141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Lin HY, Tang HY, Leinung M, Mousa SA, Hercbergs A, Davis PJ. Action of reverse T3 on cancer cells. Endocr Res. 2019;44(4):148-152. doi: 10.1080/07435800.2019.1600536 [DOI] [PubMed] [Google Scholar]
- 183. Adami HO, Rimsten A, Thorén L, Vegelius J, Wide L. Thyroid disease and function in breast cancer patients and non-hospitalized controls evaluated by determination of TSH, T3, rT3 and T4 levels in serum. Acta Chir Scand. 1978;144(2):89-97. [PubMed] [Google Scholar]
- 184. Martinez MB, Ruan M, Fitzpatrick LA. Altered response to thyroid hormones by breast and ovarian cancer cells. Anticancer Res. 2000;20(6B):4141-4146. [PubMed] [Google Scholar]
- 185. Ciavardelli D, Bellomo M, Crescimanno C, Vella V. Type 3 deiodinase: role in cancer growth, stemness, and metabolism. Front Endocrinol (Lausanne). 2014;5:215. doi: 10.3389/fendo.2014.00215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Nappi A, De Stefano MA, Dentice M, Salvatore D. Deiodinases and cancer. Endocrinology. 2021;162(4). doi: 10.1210/endocr/bqab016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Tremmel E, Hofmann S, Kuhn C, et al. Thyronamine regulation of TAAR1 expression in breast cancer cells and investigation of its influence on viability and migration. Breast Cancer (Dove Med Press). 2019;11:87-97. doi: 10.2147/BCTT.S178721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Vattai A, Akyol E, Kuhn C, et al. Increased trace amine-associated receptor 1 (TAAR1) expression is associated with a positive survival rate in patients with breast cancer. J Cancer Res Clin Oncol. 2017;143(9):1637-1647. doi: 10.1007/s00432-017-2420-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Glinskii AB, Glinsky GV, Lin HY, et al. Modification of survival pathway gene expression in human breast cancer cells by tetraiodothyroacetic acid (tetrac). Cell Cycle. 2009;8(21):3562-3570. doi: 10.4161/cc.8.21.9963 [DOI] [PubMed] [Google Scholar]
- 190. Davis PJ, Glinsky GV, Lin HY, et al. Cancer cell gene expression modulated from plasma membrane integrin αvβ3 by thyroid hormone and nanoparticulate tetrac. Front Endocrinol (Lausanne). 2014;5:240. doi: 10.3389/fendo.2014.00240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Davis PJ, Lin HY, Sudha T, et al. Nanotetrac targets integrin αvβ3 on tumor cells to disorder cell defense pathways and block angiogenesis. Onco Targets Ther. 2014;7:1619-1624. doi: 10.2147/OTT.S67393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Davis PJ, Davis FB, Lin HY, et al. Cell-surface receptor for thyroid hormone and tumor cell proliferation. Expert Rev Endocrinol Metab. 2006;1(6):753-761. doi: 10.1586/17446651.1.6.753 [DOI] [PubMed] [Google Scholar]
- 193. Liu J, Xu T, Ma L, Chang W. Signal pathway of estrogen and estrogen receptor in the development of thyroid cancer. Front Oncol. 2021;11:593479. doi: 10.3389/fonc.2021.593479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Derwahl M, Nicula D. Estrogen and its role in thyroid cancer. Endocr Relat Cancer. 2014;21(5):T273-T283. doi: 10.1530/ERC-14-0053 [DOI] [PubMed] [Google Scholar]
- 195. Zane M, Catalano V, Scavo E, et al. Estrogens and stem cells in thyroid cancer. Front Endocrinol (Lausanne). 2014;5:124. doi: 10.3389/fendo.2014.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Fuentes N, Silveyra P. Estrogen receptor signaling mechanisms. Adv Protein Chem Struct Biol. 2019;116:135-170. doi: 10.1016/bs.apcsb.2019.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Yane K, Kitahori Y, Konishi N, et al. Expression of the estrogen receptor in human thyroid neoplasms. Cancer Lett. 1994;84(1):59-66. doi: 10.1016/0304-3835(94)90358-1 [DOI] [PubMed] [Google Scholar]
- 198. Huang C, Cai Z, Huang M, et al. miR-219-5p modulates cell growth of papillary thyroid carcinoma by targeting estrogen receptor α. J Clin Endocrinol Metab. 2015;100(2):E204-E213. doi: 10.1210/jc.2014-2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Dong W, Zhang H, Li J, et al. Estrogen induces metastatic potential of papillary thyroid cancer cells through estrogen receptor α and β. Int J Endocrinol. 2013;2013:941568. doi: 10.1155/2013/941568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Sturniolo G, Zafon C, Moleti M, Castellví J, Vermiglio F, Mesa J. Immunohistochemical expression of estrogen receptor-α and progesterone receptor in patients with papillary thyroid cancer. Eur Thyroid J. 2016;5(4):224-230. doi: 10.1159/000452488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Inoue H, Oshimo K, Miki H, Kawano M, Monden Y. Immunohistochemical study of estrogen receptors and the responsiveness to estrogen in papillary thyroid carcinoma. Cancer. 1993;72(4):1364-1368. doi: [DOI] [PubMed] [Google Scholar]
- 202. Clark OH, Gerend PL, Davis M, Goretzki PE, Hoffman PG. Estrogen and thyroid-stimulating hormone (TSH) receptors in neoplastic and nonneoplastic human thyroid tissue. J Surg Res. 1985;38(2):89-96. doi: 10.1016/0022-4804(85)90012-5 [DOI] [PubMed] [Google Scholar]
- 203. Rubio GA, Catanuto P, Glassberg MK, Lew JI, Elliot SJ. Estrogen receptor subtype expression and regulation is altered in papillary thyroid cancer after menopause. Surgery. 2018;163(1):143-149. doi: 10.1016/j.surg.2017.04.031 [DOI] [PubMed] [Google Scholar]
- 204. Božović A, Mandušić V, Todorović L, Krajnović M. Estrogen receptor beta: the promising biomarker and potential target in metastases. Int J Mol Sci. 2021;22(4). doi: 10.3390/ijms22041656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Qiu YB, Liao LY, Jiang R, et al. PES1 promotes the occurrence and development of papillary thyroid cancer by upregulating the ERα/ERβ protein ratio. Sci Rep. 2019;9(1):1032. doi: 10.1038/s41598-018-37648-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Chen GG, Vlantis AC, Zeng Q, van Hasselt CA. Regulation of cell growth by estrogen signaling and potential targets in thyroid cancer. Curr Cancer Drug Targets. 2008;8(5):367-377. doi: 10.2174/156800908785133150 [DOI] [PubMed] [Google Scholar]
- 207. Mishra A, Kumari N, Jha CK, Bichoo RA, Mishra SK, Krishnani N. Distribution and prognostic significance of estrogen receptor. J Thyroid Res. 2020;2020:6935724. doi: 10.1155/2020/6935724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Manole D, Schildknecht B, Gosnell B, Adams E, Derwahl M. Estrogen promotes growth of human thyroid tumor cells by different molecular mechanisms. J Clin Endocrinol Metab. 2001;86(3):1072-1077. doi: 10.1210/jcem.86.3.7283 [DOI] [PubMed] [Google Scholar]
- 209. Meng D, Wu W, Li Z, Qin G. IQGAP1 modulates the proliferation and invasion of thyroid cancer cells in response to estrogen. Int J Mol Med. 2015;36(2):588-594. doi: 10.3892/ijmm.2015.2232 [DOI] [PubMed] [Google Scholar]
- 210. Kamat A, Rajoria S, George A, et al. Estrogen-mediated angiogenesis in thyroid tumor microenvironment is mediated through VEGF signaling pathways. Arch Otolaryngol Head Neck Surg. 2011;137(11):1146-1153. doi: 10.1001/archoto.2011.194 [DOI] [PubMed] [Google Scholar]
- 211. Fan D, Liu SY, van Hasselt CA, et al. Estrogen receptor α induces prosurvival autophagy in papillary thyroid cancer via stimulating reactive oxygen species and extracellular signal regulated kinases. J Clin Endocrinol Metab. 2015;100(4):E561-E571. doi: 10.1210/jc.2014-3257 [DOI] [PubMed] [Google Scholar]
- 212. Rajoria S, Suriano R, George A, et al. Estrogen induced metastatic modulators MMP-2 and MMP-9 are targets of 3,3’-diindolylmethane in thyroid cancer. PLoS One. 2011;6(1):e15879. doi: 10.1371/journal.pone.0015879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Rajoria S, Suriano R, Wilson YL, et al. Estradiol-mediated tumor neo-vascularization. Oncol Lett. 2011;2(3):453-457. doi: 10.3892/ol.2011.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Kumar A, Klinge CM, Goldstein RE. Estradiol-induced proliferation of papillary and follicular thyroid cancer cells is mediated by estrogen receptors alpha and beta. Int J Oncol. 2010;36(5):1067-1080. doi: 10.3892/ijo_00000588 [DOI] [PubMed] [Google Scholar]
- 215. Yang S, Gong Z, Liu Z, et al. Differential effects of estrogen receptor alpha and beta on endogenous ligands of peroxisome proliferator-activated receptor gamma in papillary thyroid cancer. Front Endocrinol (Lausanne). 2021;12:708248. doi: 10.3389/fendo.2021.708248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Chu R, van Hasselt A, Vlantis AC, et al. The cross-talk between estrogen receptor and peroxisome proliferator-activated receptor gamma in thyroid cancer. Cancer. 2014;120(1):142-153. doi: 10.1002/cncr.28383 [DOI] [PubMed] [Google Scholar]
- 217. Xue L, Yan H, Chen Y, et al. EZH2 upregulation by ERα induces proliferation and migration of papillary thyroid carcinoma. BMC Cancer. 2019;19(1):1094. doi: 10.1186/s12885-019-6306-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Pitto L, Gorini F, Bianchi F, Guzzolino E. New insights into mechanisms of endocrine-disrupting chemicals in thyroid diseases: the epigenetic way. Int J Environ Res Public Health. 2020;17(21). doi 10.3390/ijerph17217787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Zhang Z, Liu D, Murugan AK, Liu Z, Xing M. Histone deacetylation of NIS promoter underlies BRAF V600E-promoted NIS silencing in thyroid cancer. Endocr Relat Cancer. 2014;21(2):161-173. doi: 10.1530/ERC-13-0399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Furlanetto TW, Nguyen LQ, Jameson JL. Estradiol increases proliferation and down-regulates the sodium/iodide symporter gene in FRTL-5 cells. Endocrinology. 1999;140(12):5705-5711. doi: 10.1210/endo.140.12.7197 [DOI] [PubMed] [Google Scholar]
- 221. Singh TD, Jeong SY, Lee SW, et al. Inverse agonist of estrogen-related receptor γ enhances sodium iodide symporter function through mitogen-activated protein kinase signaling in anaplastic thyroid cancer cells. J Nucl Med. 2015;56(11):1690-1696. doi: 10.2967/jnumed.115.160366 [DOI] [PubMed] [Google Scholar]
- 222. Magri F, Capelli V, Rotondi M, et al. Expression of estrogen and androgen receptors in differentiated thyroid cancer: an additional criterion to assess the patient’s risk. Endocr Relat Cancer. 2012;19(4):463-471. doi: 10.1530/ERC-11-0389 [DOI] [PubMed] [Google Scholar]
- 223. Chou CK, Chi SY, Chou FF, et al. Aberrant expression of androgen receptor associated with high cancer risk and extrathyroidal extension in papillary thyroid carcinoma. Cancers (Basel). 2020;12(5). doi: 10.3390/cancers12051109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. O’Connell TJ, Dadafarin S, Jones M, et al. Androgen activity is associated with PD-L1 downregulation in thyroid cancer. Front Cell Dev Biol. 2021;9:663130. doi: 10.3389/fcell.2021.663130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Banu KS, Govindarajulu P, Aruldhas MM. Testosterone and estradiol have specific differential modulatory effect on the proliferation of human thyroid papillary and follicular carcinoma cell lines independent of TSH action. Endocr Pathol. 2001;12(3):315-327. doi: 10.1385/ep:12:3:315 [DOI] [PubMed] [Google Scholar]
- 226. Zhang LJ, Xiong Y, Nilubol N, et al. Testosterone regulates thyroid cancer progression by modifying tumor suppressor genes and tumor immunity. Carcinogenesis. 2015;36(4):420-428. doi: 10.1093/carcin/bgv001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Shibata H, Spencer TE, Oñate SA, et al. Role of co-activators and co-repressors in the mechanism of steroid/thyroid receptor action. Recent Prog Horm Res. 1997;52:141-164; discussion 164-5. [PubMed] [Google Scholar]
- 228. Vasudevan N, Ogawa S, Pfaff D. Estrogen and thyroid hormone receptor interactions: physiological flexibility by molecular specificity. Physiol Rev. 2002;82(4):923-944. doi: 10.1152/physrev.00014.2002 [DOI] [PubMed] [Google Scholar]
- 229. Graupner G, Zhang XK, Tzukerman M, Wills K, Hermann T, Pfahl M. Thyroid hormone receptors repress estrogen receptor activation of a TRE. Mol Endocrinol. 1991;5(3):365-372. doi: 10.1210/mend-5-3-365 [DOI] [PubMed] [Google Scholar]
- 230. Penvose A, Keenan JL, Bray D, Ramlall V, Siggers T. Comprehensive study of nuclear receptor DNA binding provides a revised framework for understanding receptor specificity. Nat Commun. 2019;10(1):2514. doi: 10.1038/s41467-019-10264-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Rajendran BK, Deng CX. Characterization of potential driver mutations involved in human breast cancer by computational approaches. Oncotarget. 2017;8(30):50252-50272. doi: 10.18632/oncotarget.17225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Fozzatti L, Park JW, Zhao L, Willingham MC, Cheng SY. Oncogenic Actions of the Nuclear Receptor Corepressor (NCOR1) in a mouse model of thyroid cancer. PLoS One. 2013;8(6):e67954. doi: 10.1371/journal.pone.0067954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Zhang Z, Yamashita H, Toyama T, et al. NCOR1 mRNA is an independent prognostic factor for breast cancer. Cancer Lett. 2006;237(1):123-129. doi: 10.1016/j.canlet.2005.05.046 [DOI] [PubMed] [Google Scholar]
- 234. Bagamasbad P, Denver RJ. Mechanisms and significance of nuclear receptor auto- and cross-regulation. Gen Comp Endocrinol. 2011;170(1):3-17. doi: 10.1016/j.ygcen.2010.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Nisman B, Allweis TM, Carmon E, et al. Thyroid hormones, silencing mediator for retinoid and thyroid receptors and prognosis in primary breast cancer. Anticancer Res. 2020;40(11):6417-6428. doi: 10.21873/anticanres.14663 [DOI] [PubMed] [Google Scholar]
- 236. Dinda S, Sanchez A, Moudgil V. Estrogen-like effects of thyroid hormone on the regulation of tumor suppressor proteins, p53 and retinoblastoma, in breast cancer cells. Oncogene. 2002;21(5):761-768. doi: 10.1038/sj.onc.1205136 [DOI] [PubMed] [Google Scholar]
- 237. Figueiredo NB, Cestari SH, Conde SJ, et al. Estrogen-responsive genes overlap with triiodothyronine-responsive genes in a breast carcinoma cell line. ScientificWorldJournal. 2014;2014:969404. doi: 10.1155/2014/969404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Hall LC, Salazar EP, Kane SR, Liu N. Effects of thyroid hormones on human breast cancer cell proliferation. J Steroid Biochem Mol Biol. 2008;109(1-2):57-66. doi: 10.1016/j.jsbmb.2007.12.008 [DOI] [PubMed] [Google Scholar]
- 239. Shao ZM, Sheikh MS, Rishi AK, et al. Thyroid hormone enhancement of estradiol stimulation of breast carcinoma proliferation. Exp Cell Res. 1995;218(1):1-8. doi: 10.1006/excr.1995.1124 [DOI] [PubMed] [Google Scholar]
- 240. Wahdan-Alaswad RS, Edgerton SM, Salem H, et al. Exogenous thyroid hormone is associated with shortened survival and upregulation of high-risk gene expression profiles in steroid receptor-positive breast cancers. Clin Cancer Res. 2021;27(2):585-597. doi: 10.1158/1078-0432.CCR-20-2647 [DOI] [PubMed] [Google Scholar]
- 241. Tang HY, Lin HY, Zhang S, Davis FB, Davis PJ. Thyroid hormone causes mitogen-activated protein kinase-dependent phosphorylation of the nuclear estrogen receptor. Endocrinology. 2004;145(7):3265-3272. doi: 10.1210/en.2004-0308 [DOI] [PubMed] [Google Scholar]
- 242. Keeton EK, Brown M. Cell cycle progression stimulated by tamoxifen-bound estrogen receptor-alpha and promoter-specific effects in breast cancer cells deficient in N-CoR and SMRT. Mol Endocrinol. 2005;19(6):1543-1554. doi: 10.1210/me.2004-0395 [DOI] [PubMed] [Google Scholar]
- 243. Hong W, Chen L, Li J, Yao Z. Inhibition of MAP kinase promotes the recruitment of corepressor SMRT by tamoxifen-bound estrogen receptor alpha and potentiates tamoxifen action in MCF-7 cells. Biochem Biophys Res Commun. 2010;396(2):299-303. doi: 10.1016/j.bbrc.2010.04.085 [DOI] [PubMed] [Google Scholar]
- 244. Légaré S, Basik M. Minireview: The link between ERα corepressors and histone deacetylases in tamoxifen resistance in breast cancer. Mol Endocrinol. 2016;30(9):965-976. doi: 10.1210/me.2016-1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Peterson TJ, Karmakar S, Pace MC, Gao T, Smith CL. The silencing mediator of retinoic acid and thyroid hormone receptor (SMRT) corepressor is required for full estrogen receptor alpha transcriptional activity. Mol Cell Biol. 2007;27(17):5933-5948. doi: 10.1128/MCB.00237-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Conde SJ, Luvizotto RA, Síbio MT, Katayama ML, Brentani MM, Nogueira CR. Tamoxifen inhibits transforming growth factor-alpha gene expression in human breast carcinoma samples treated with triiodothyronine. J Endocrinol Invest. 2008;31(12):1047-1051. doi: 10.1007/BF03345650 [DOI] [PubMed] [Google Scholar]
- 247. Elliott MJ, Jerzak KJ, Cockburn JG, et al. The antiarrhythmic drug, dronedarone, demonstrates cytotoxic effects in breast cancer independent of thyroid hormone receptor Alpha 1 (THRα1) antagonism. Sci Rep. 2018;8(1):16562. doi: 10.1038/s41598-018-34348-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Saponaro F, Sestito S, Runfola M, Rapposelli S, Chiellini G. Selective Thyroid Hormone Receptor-Beta (TRβ) agonists: new perspectives for the treatment of metabolic and neurodegenerative disorders. Front Med (Lausanne). 2020;7:331. doi: 10.3389/fmed.2020.00331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Harrison SA, Bashir MR, Guy CD, et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2019;394(10213):2012-2024. doi: 10.1016/S0140-6736(19)32517-6 [DOI] [PubMed] [Google Scholar]
- 250. Lu M, Mira-y-Lopez R, Nakajo S, Nakaya K, Jing Y. Expression of estrogen receptor alpha, retinoic acid receptor alpha and cellular retinoic acid binding protein II genes is coordinately regulated in human breast cancer cells. Oncogene. 2005;24(27):4362-4369. doi: 10.1038/sj.onc.1208661 [DOI] [PubMed] [Google Scholar]
- 251. Mahalingam D, Wang JS, Hamilton EP, et al. Phase 1 open-label, multicenter study of first-in-class RORγ Agonist LYC-55716 (Cintirorgon): safety, tolerability, and preliminary evidence of antitumor activity. Clin Cancer Res. 2019;25(12):3508-3516. doi: 10.1158/1078-0432.CCR-18-3185 [DOI] [PubMed] [Google Scholar]
- 252. Luu T, Frankel P, Beumer JH, et al. Phase I trial of belinostat in combination with 13-cis-retinoic acid in advanced solid tumor malignancies: a California Cancer Consortium NCI/CTEP sponsored trial. Cancer Chemother Pharmacol. 2019;84(6):1201-1208. doi: 10.1007/s00280-019-03955-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Bharali DJ, Yalcin M, Davis PJ, Mousa SA. Tetraiodothyroacetic acid-conjugated PLGA nanoparticles: a nanomedicine approach to treat drug-resistant breast cancer. Nanomedicine (Lond). 2013;8(12):1943-1954. doi: 10.2217/nnm.12.200 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 254. Stupp R, Hegi ME, Gorlia T, et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1100-1108. doi: 10.1016/S1470-2045(14)70379-1. [DOI] [PubMed] [Google Scholar]
- 255. Chen H, Zhao L, Fu K, et al. Integrin α. Theranostics. 2019;9(25):7948-7960. doi: 10.7150/thno.39203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Sareddy GR, Li X, Liu J, et al. Selective estrogen receptor β agonist LY500307 as a novel therapeutic agent for glioblastoma. Sci Rep. 2016;6:24185. doi: 10.1038/srep24185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Hoelting T, Siperstein AE, Duh QY, Clark OH. Tamoxifen inhibits growth, migration, and invasion of human follicular and papillary thyroid cancer cells in vitro and in vivo. J Clin Endocrinol Metab. 1995;80(1):308-313. doi: 10.1210/jcem.80.1.7829632 [DOI] [PubMed] [Google Scholar]
- 258. Oh JM, Kalimuthu S, Gangadaran P, et al. Reverting iodine avidity of radioactive-iodine refractory thyroid cancer with a new tyrosine kinase inhibitor (K905-0266) excavated by high-throughput NIS (sodium iodide symporter) enhancer screening platform using dual reporter gene system. Oncotarget. 2018;9(6):7075-7087. doi: 10.18632/oncotarget.24159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Ho AL, Grewal RK, Leboeuf R, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 2013;368(7):623-632. doi: 10.1056/NEJMoa1209288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Dunn LA, Sherman EJ, Baxi SS, et al. Vemurafenib redifferentiation of BRAF Mutant, RAI-refractory thyroid cancers. J Clin Endocrinol Metab. 2019;104(5):1417-1428. doi: 10.1210/jc.2018-01478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Singh TD, Song J, Kim J, et al. A novel orally active inverse agonist of Estrogen-related Receptor Gamma (ERRγ), DN200434, a booster of NIS in anaplastic thyroid cancer. Clin Cancer Res. 2019;25(16):5069-5081. doi: 10.1158/1078-0432.CCR-18-3007 [DOI] [PubMed] [Google Scholar]
- 262. Renier C, Yao C, Goris M, et al. Endogenous NIS expression in triple-negative breast cancers. Ann Surg Oncol. 2009;16(4):962-968. doi: 10.1245/s10434-008-0280-9 [DOI] [PubMed] [Google Scholar]
- 263. Upadhyay G, Singh R, Agarwal G, et al. Functional expression of sodium iodide symporter (NIS) in human breast cancer tissue. Breast Cancer Res Treat. 2003;77(2):157-165. doi: 10.1023/a:1021321409159 [DOI] [PubMed] [Google Scholar]
- 264. Schmutzler C, Winzer R, Meissner-Weigl J, Köhrle J. Retinoic acid increases sodium/iodide symporter mRNA levels in human thyroid cancer cell lines and suppresses expression of functional symporter in nontransformed FRTL-5 rat thyroid cells. Biochem Biophys Res Commun. 1997;240(3):832-838. doi: 10.1006/bbrc.1997.7715 [DOI] [PubMed] [Google Scholar]
- 265. Willhauck MJ, Kane DJO, Wunderlich N, Göke B, Spitzweg C. Stimulation of retinoic acid-induced functional sodium iodide symporter (NIS) expression and cytotoxicity of ¹³¹I by carbamazepine in breast cancer cells. Breast Cancer Res Treat. 2011;125(2):377-386. doi: 10.1007/s10549-010-0835-x [DOI] [PubMed] [Google Scholar]
- 266. Gnant M, Pfeiler G, Stöger H, et al. The predictive impact of body mass index on the efficacy of extended adjuvant endocrine treatment with anastrozole in postmenopausal patients with breast cancer: an analysis of the randomised ABCSG-6a trial. Br J Cancer. 2013;109(3):589-596. doi: 10.1038/bjc.2013.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Baum M, Buzdar A, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: results of the ATAC (Arimidex, Tamoxifen Alone or in Combination) trial efficacy and safety update analyses. Cancer. 2003;98(9):1802-1810. doi: 10.1002/cncr.11745 [DOI] [PubMed] [Google Scholar]
- 268. Wakabayashi H, Hamaguchi T, Nagao N, et al. Interleukin-6 receptor inhibitor suppresses bone metastases in a breast cancer cell line. Breast Cancer. 2018;25(5):566-574. doi: 10.1007/s12282-018-0853-9 [DOI] [PubMed] [Google Scholar]
- 269. Nanni O, Amadori D, De Censi A, et al. Metformin plus chemotherapy versus chemotherapy alone in the first-line treatment of HER2-negative metastatic breast cancer. The MYME randomized, phase 2 clinical trial. Breast Cancer Res Treat. 2019;174(2):433-442. doi: 10.1007/s10549-018-05070-2 [DOI] [PubMed] [Google Scholar]
- 270. Klubo-Gwiezdzinska J, Costello J, Patel A, et al. Treatment with metformin is associated with higher remission rate in diabetic patients with thyroid cancer. J Clin Endocrinol Metab. 2013;98(8):3269-3279. doi: 10.1210/jc.2012-3799 [DOI] [PubMed] [Google Scholar]
- 271. Douglas RS, Kahaly GJ, Patel A, et al. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med. 2020;382(4):341-352. doi: 10.1056/NEJMoa1910434 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.