Sickle cell disease (SCD) is one of the most common and clinically severe inherited diseases in the world, estimated to affect at least 3 to 6 million people. Greater than 90% of affected individuals live in Africa and India and have very limited access to appropriate health care.
Sickle cell anemia, first described in 1910 by Dr. Herrick in Chicago, was identified as the first human molecular disease in 1949 by Linus Pauling et al. (1). This monogenic disease results from a single nucleotide change from A to T in the codon (GAG) of the gene encoding the beta globin chain, resulting in the replacement of a negatively charged glutamate with a neutral, hydrophobic valine in the contact regions of hemoglobin tetramers. This modification leads to polymerization of Sickle hemoglobin (Hb S) upon deoxygenation. Since its original description over a century ago, SCD has been the most extensively studied human disorder by geneticists, structural biologists, biophysicists, biochemists, cell biologists, and clinical scientists. While these efforts have led to some improvements in the clinical management of SCD, significant gaps remain in the effective prevention, management, and cure of this complex human disease. These gaps are of particular concern in low-resource countries where the vast majority of SCD patients live. Specifically, there is a great paucity of drugs to effectively treat this important global disease. In PNAS, Metaferia et al. outline a screening strategy for discovery of drug targets for treatment of SCD, a major and clinically severe global health problem (2).
Clinical manifestations of SCD are highly variable from individual to individual. Typical complications include chronic anemia, acute and chronic pain, increased infections, cerebrovascular disease, cardiopulmonary dysfunction, renal impairment, hepatopathy, retinopathy, priapism, and leg ulcers (3, 4). Life expectancy of SCD individuals is shortened by ∼20 y even with optimal clinical care, and in low-income countries many infants with SCD die in childhood.
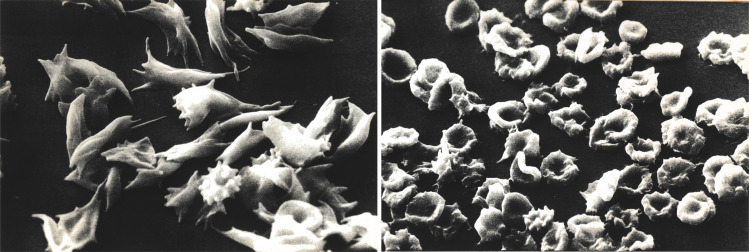
The polymerization of Hb S in red blood cells upon deoxygenation is the root cause of SCD pathology, as Hb S fibers stiffen and distort (“sickle”) erythrocytes (Fig. 1), inducing significant cell rigidity, which in turn compromises the ability of these red blood cells to deliver oxygen to tissues (5–7). In fact, SCD is a disease of lifelong chronic tissue hypoxia. The morphological changes induced following deoxygenation depend on intracellular hemoglobin concentration (mean corpuscular hemoglobin concentration, MCHC) (8). Sickle cells with normal MCHC undergo extensive morphological changes (Fig. 1A), while dehydrated red blood cells that are a feature of SCD undergo much less extensive changes (Fig. 1B) despite extensive fiber formation. Vaso-occlusion induced by rigid sickle cells in association with their increased adhesion to vascular endothelial cells reduces blood flow and decreases oxygen delivery to almost every tissue. The chronic hypoxia results in long-term tissue organ damage and in intermittent episodes of acute severe pain, referred to as a sickle cell crisis. Furthermore, the irreversible structural changes in the red blood cell membrane associated with sickling in the tissues and unsickling in the lungs induces membrane vesiculation and irreversible membrane structural alterations and leads to hemolysis. Decreased life span of circulating red blood cells in conjunction with decreased red blood cell production from ineffective erythropoiesis in the bone marrow leads to chronic anemia (9, 10). Recurrent vaso-occlusion and chronic hemolysis trigger and sustain a persistent inflammatory response, leading to vasculopathy and acute and chronic damage to the brain, lungs, kidney, liver, and other organs. The best strategy for treatment of SCD is development of drugs that inhibit Hb S polymerization
Fig. 1.
Scanning electron micrographs of deoxygenated sickle red cells. Extensive morphological changes of discoid sickle red cells with normal cell hemoglobin concentration following deoxygenation (Left). In contrast, less extensive morphological changes of dehydrated discoid sickle red cells with elevated MCHC concentration following deoxygenation, despite extensive Hb S polymer formation (Right).
A number of potential strategies can be exploited to either inhibit or alter the kinetics of Hb S polymerization (11–14). These include disruption of intermolecular contacts in the sickle fiber, sickle fiber destabilization by decreasing 2,3-diphosphoglycerate, altering the kinetics of hemoglobin polymerization by decreasing the intracellular hemoglobin concentration through increased hydration and swelling of sickle red cells, decreasing the amount of oxygen released by cells to tissues by altering the oxygen affinity of Hb S, and reactivation of fetal hemoglobin (Hb F) synthesis, a potent inhibitor of Hb S polymerization during erythroid differentiation.
Hydroxyurea (HU) is currently the most widely used antisickling drug; developed almost three decades ago, it was initially approved for use in adults and subsequently for use in children (15–18). It is reasonably inexpensive. The efficacy of HU is due to its effect on various aspects of sickle pathophysiology (15), with induction of Hb F being a dominant factor. Based on clinical response and changes in hematological parameters, the individual responses to HU can be highly variable and the increase in Hb F is not evenly distributed in all cells. Consequently, while it is clearly efficacious and has a good long-term safely profile, it is not curative.
Two additional drugs have recently been approved by the Food and Drug Administration (FDA) for treatment of SCD: voxelotor and l-glutamine. Voxelotor acts by inhibiting sickling by shifting oxygen affinity and increases circulating hemoglobin levels but does not seem to reduce the frequency of sickle cell crises. l-glutamine, on the other hand, apparently decreases the frequency of sickle cell crises by reducing oxidative stress, one of the sequelae of sickling. As with HU, neither of these two FDA-approved drugs is curative. Hematopoietic stem cell transplantation is effective and curative, but its use is limited to select patients in high-resource settings. Various forms of gene therapy, which can also be curative, are being explored (19). It is possible gene therapy–based approaches may eventually become the treatment of choice, but technical and economic issues could limit its availability globally.
As such, there is a critical need for inexpensive oral drugs that inhibit sickling in all red blood cells to provide improved therapeutic responses. To achieve this objective Metaferia et al. (19) developed a relatively high-throughput drug screening strategy of relatively large libraries of compounds that have been either previously approved by the FDA or have been tested in clinical trials to identify drugs that inhibit sickling at serum concentrations achievable in humans.
In the work described in PNAS they outline their findings from the first phase of their phenotypic screen of the 12,657 compounds of the Scripps ReFRAME drug repurposing library using their recently developed high-throughput assay to measure sickling times following deoxygenation to 0% oxygen of red cells from sickle trait individuals (2). Based on dose–response measurements, they showed that 106 of the 12,657 compounds exhibit antisickling at concentrations ranging from 31 nM to 10 μM. Comparing inhibitory concentrations with free concentrations of these oral drugs in human serum, 21 of the 106 identified antisickling compounds appear to be potentially effective for the treatment of SCD. The identification of new candidate drug targets with clearly defined safety and toxicity profiles could lead to development of novel treatments for SCD that are relatively inexpensive. It is exciting to imagine one or more of these compounds could be repurposed for treatment of SCD patients around the globe. Furthermore, it is likely that identifying classes of drugs with different mechanisms of action and using them in combination could result in improved therapeutic benefits.
Footnotes
The authors declare no competing interest.
See companion article, “Phenotypic screening of the ReFRAME drug repurposing library to discover new drugs for treating sickle cell disease,” 10.1073/pnas.2210779119.
References
- 1.Pauling L., Itano H. A., Singer S. J., Wells I. C., Sickle cell anemia a molecular disease. Science 110, 543–548 (1949). [DOI] [PubMed] [Google Scholar]
- 2.Metaferia B., et al. , Phenotypic screening of the ReFRAME drug repurposing library to discover new drugs for treating sickle cell disease. Proc. Natl. Acad. Sci. U.S.A. 119, e2210779119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunn H. F., Pathogenesis and treatment of sickle cell disease. N. Engl. J. Med. 337, 762–769 (1997). [DOI] [PubMed] [Google Scholar]
- 4.Ware R. E., de Montalembert M., Tshilolo L., Abboud M. R., Sickle cell disease. Lancet 390, 311–323 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Eaton W. A., Bunn H. F., Treating sickle cell disease by targeting HbS polymerization. Blood 129, 2719–2726 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark M. R., Mohandas N., Shohet S. B., Deformability of oxygenated irreversibly sickled cells. J. Clin. Invest. 65, 189–196 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans E., Mohandas N., Leung A., Static and dynamic rigidities of normal and sickle erythrocytes. Major influence of cell hemoglobin concentration. J. Clin. Invest. 73, 477–488 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark M. R., Guatelli J. C., Mohandas N., Shohet S. B., Influence of red cell water content on the morphology of sickling. Blood 55, 823–830 (1980). [PubMed] [Google Scholar]
- 9.Quinn C. T., et al. , Biochemical surrogate markers of hemolysis do not correlate with directly measured erythrocyte survival in sickle cell anemia. Am. J. Hematol. 91, 1195–1201 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Hoss S., et al. , Fetal hemoglobin rescues ineffective erythropoiesis in sickle cell disease. Haematologica 106, 2707–2719 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark M. R., Mohandas N., Shohet S. B., Hydration of sickle cells using the sodium ionophore Monensin. A model for therapy. J. Clin. Invest. 70, 1074–1080 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldberg M. A., Husson M. A., Bunn H. F., Participation of hemoglobins A and F in polymerization of sickle hemoglobin. J. Biol. Chem. 252, 3414–3421 (1977). [PubMed] [Google Scholar]
- 13.Poillon W. N., Kim B. C., 2,3-Diphosphoglycerate and intracellular pH as interdependent determinants of the physiologic solubility of deoxyhemoglobin S. Blood 76, 1028–1036 (1990). [PubMed] [Google Scholar]
- 14.Li Q., et al. , Kinetic assay shows that increasing red cell volume could be a treatment for sickle cell disease. Proc. Natl. Acad. Sci. U.S.A. 114, E689–E696 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charache S., et al. ; Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia, Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. N. Engl. J. Med. 332, 1317–1322 (1995). [DOI] [PubMed] [Google Scholar]
- 16.Ware R. E., How I use hydroxyurea to treat young patients with sickle cell anemia. Blood 115, 5300–5311 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinberg M. H., et al. ; Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia and MSH Patients’ Follow-Up, The risks and benefits of long-term use of hydroxyurea in sickle cell anemia: A 17.5 year follow-up. Am. J. Hematol. 85, 403–408 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eaton W. A., Hofrichter J., The biophysics of sickle cell hydroxyurea therapy. Science 268, 1142–1143 (1995). [DOI] [PubMed] [Google Scholar]
- 19.Abraham A. A., Tisdale J. F., Gene therapy for sickle cell disease: Moving from the bench to the bedside. Blood 138, 932–941 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]